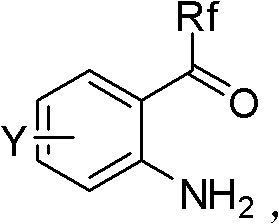
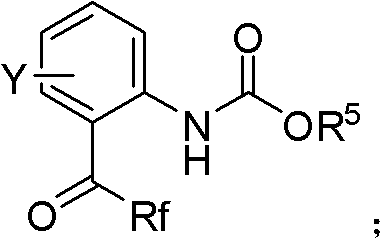
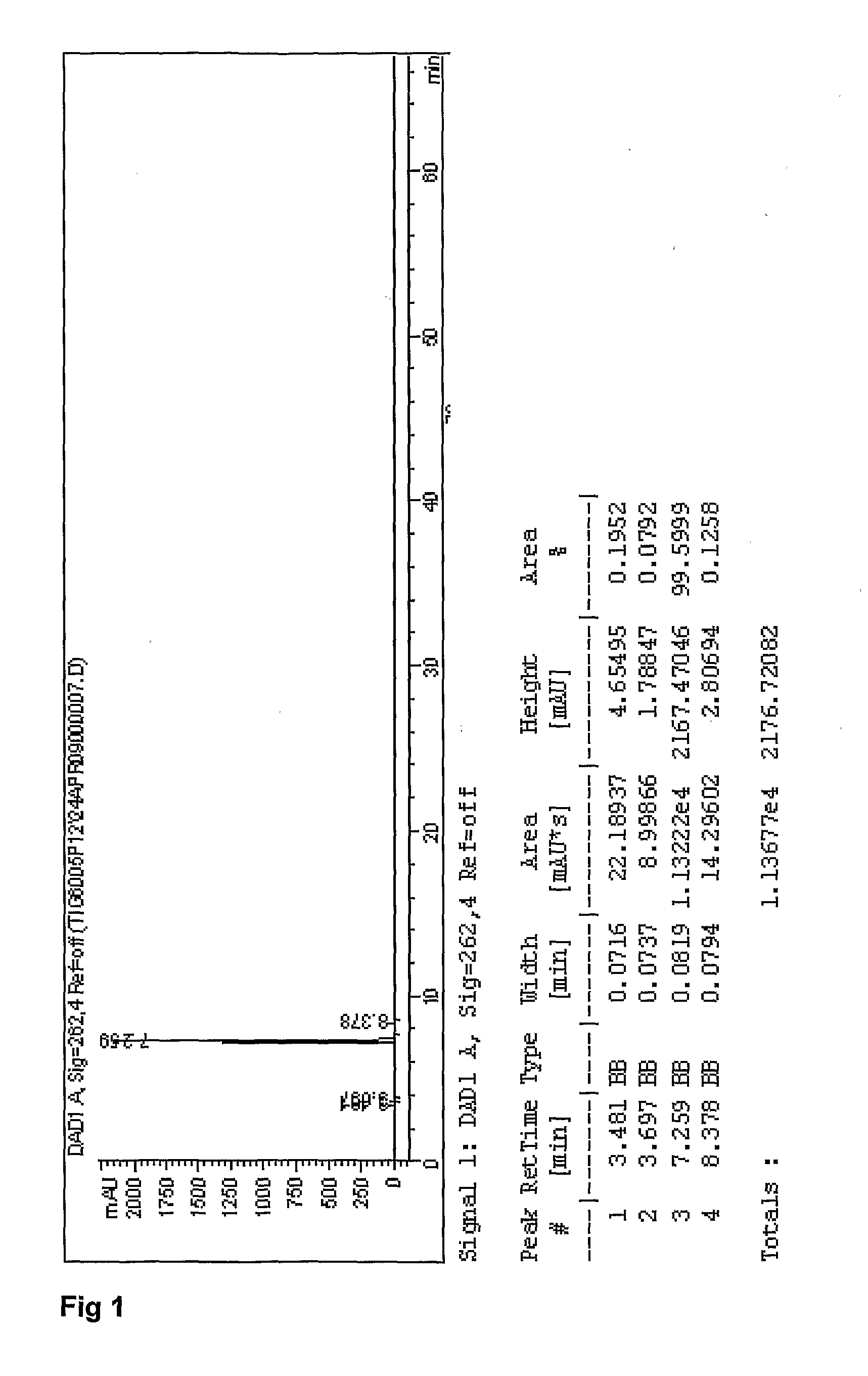
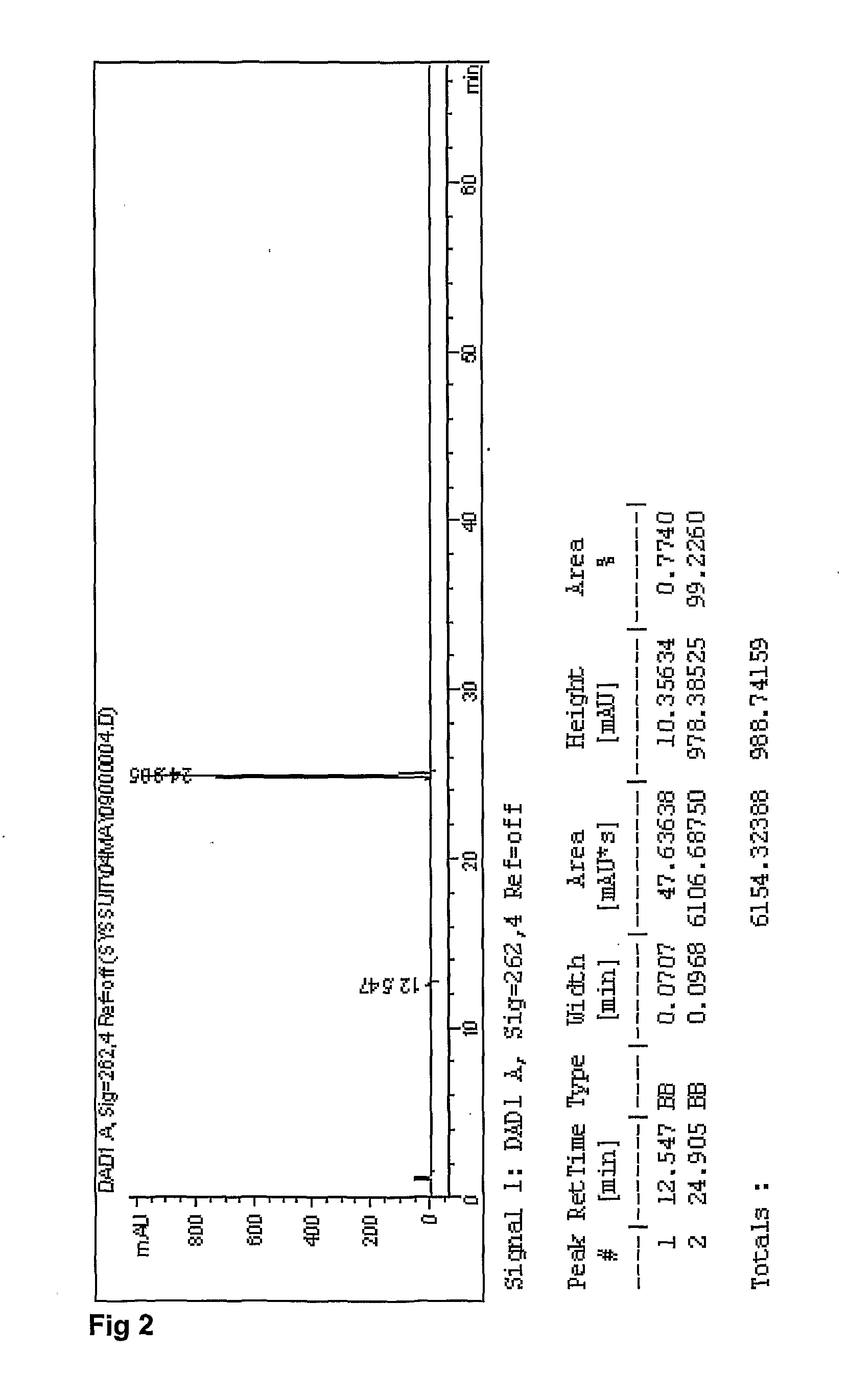
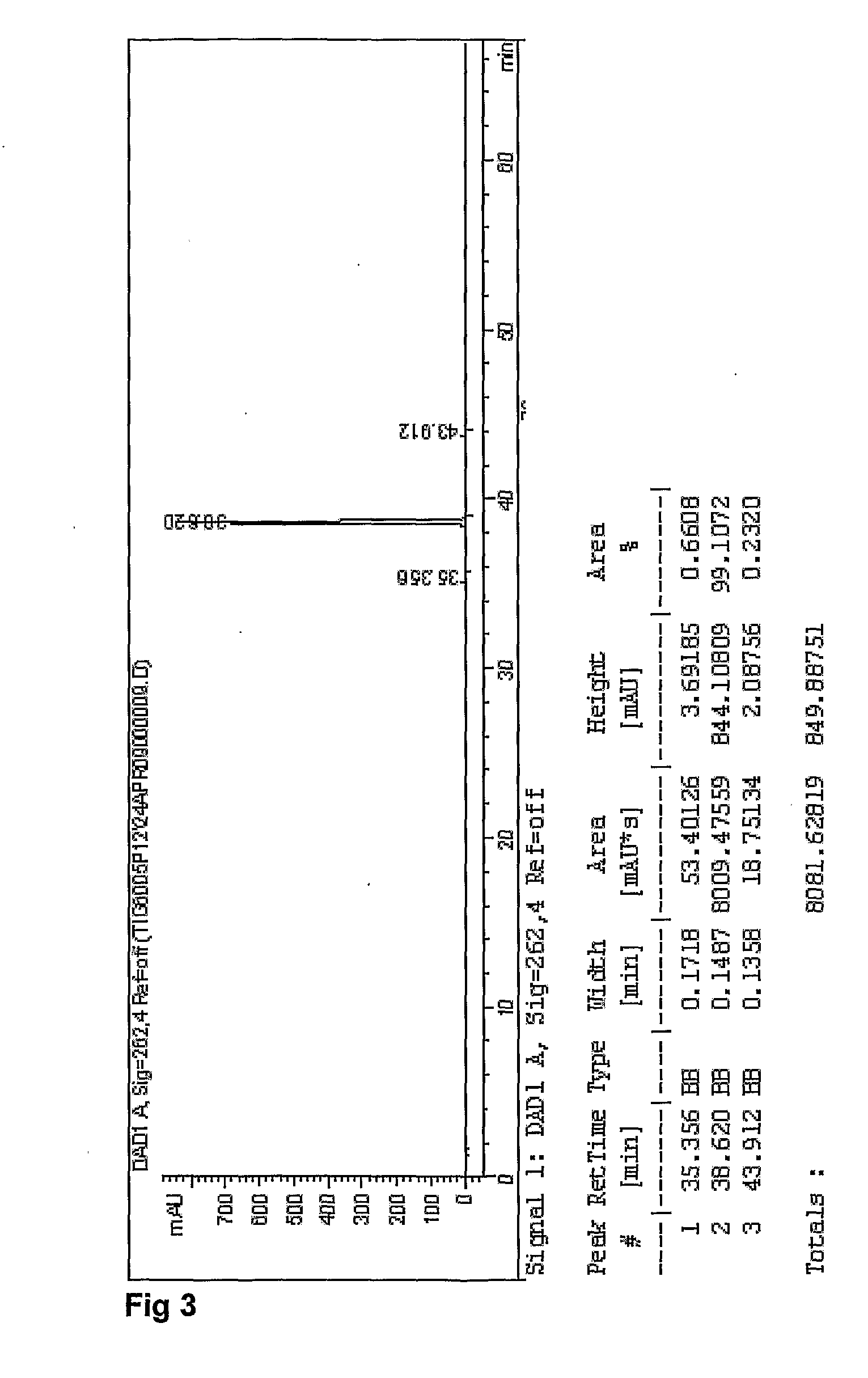
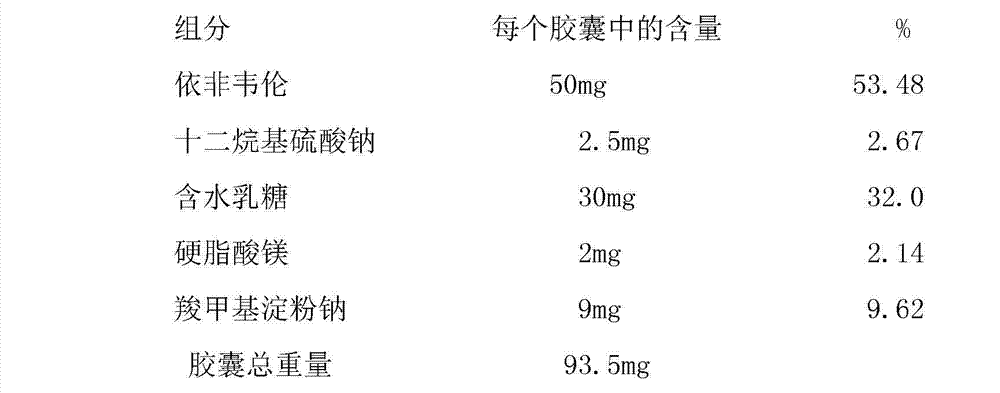
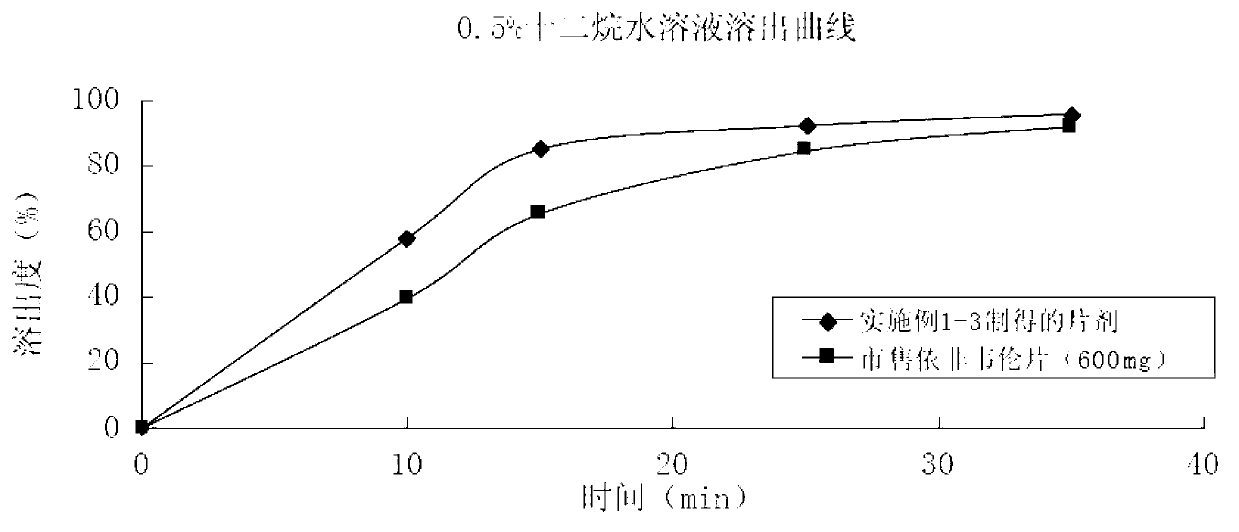
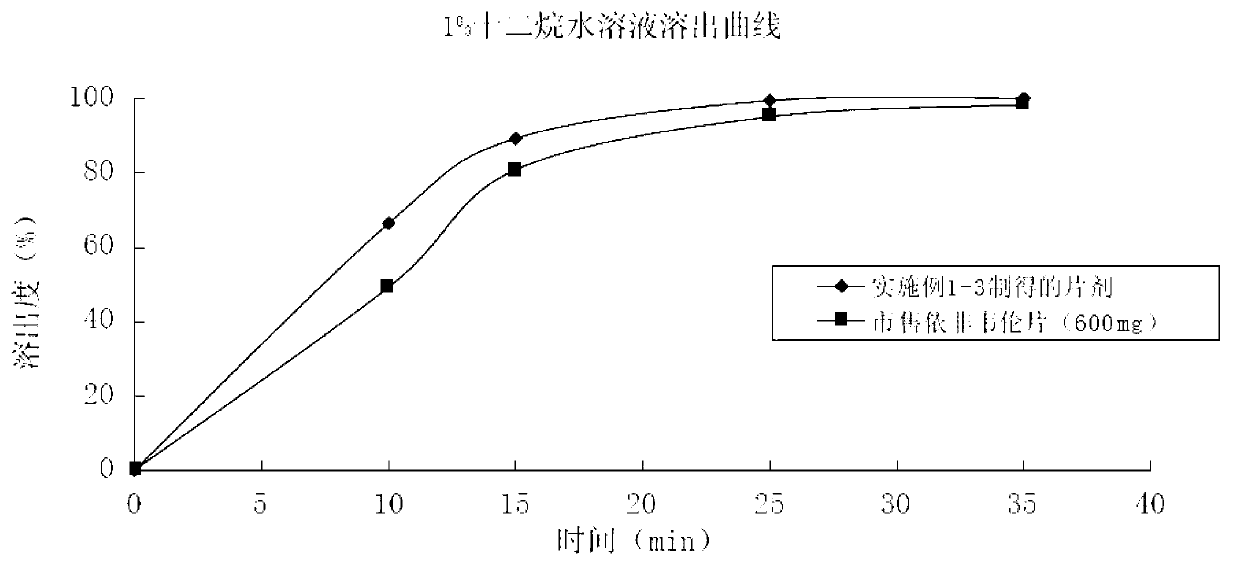
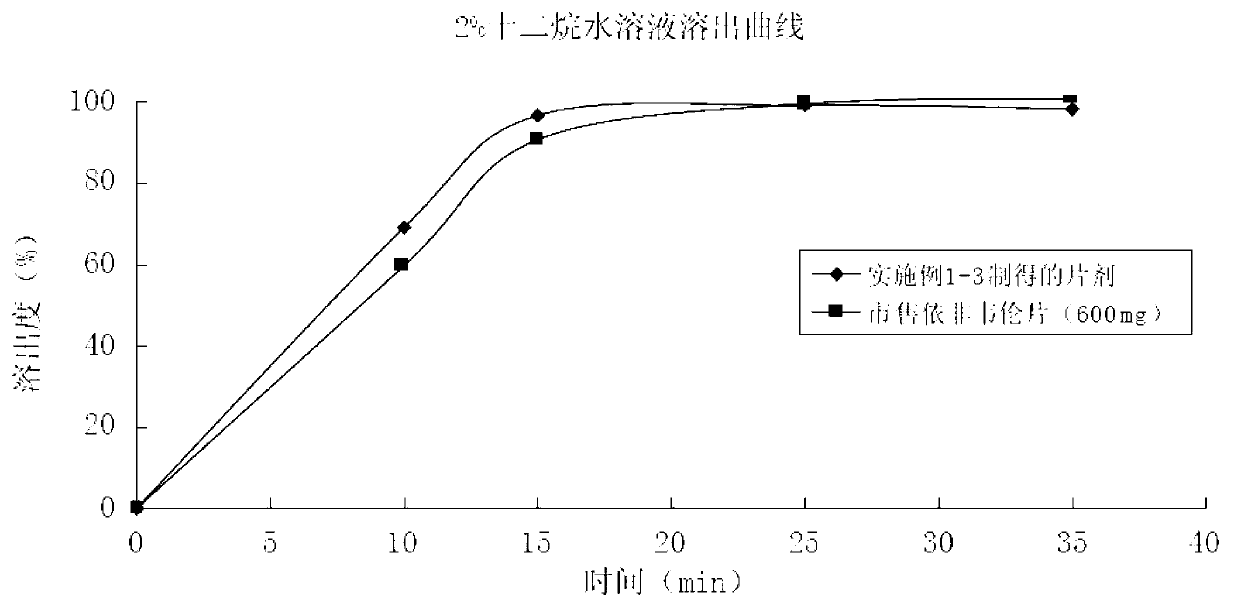
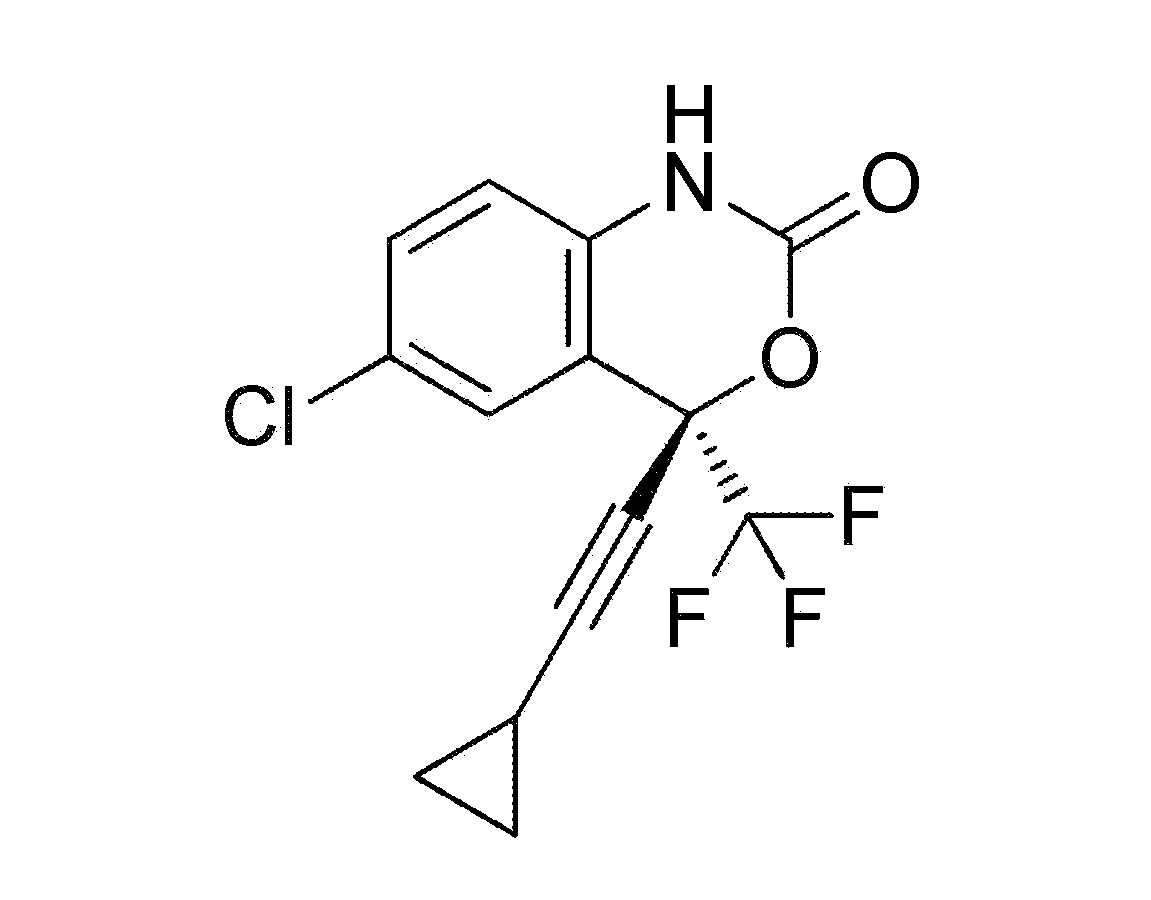
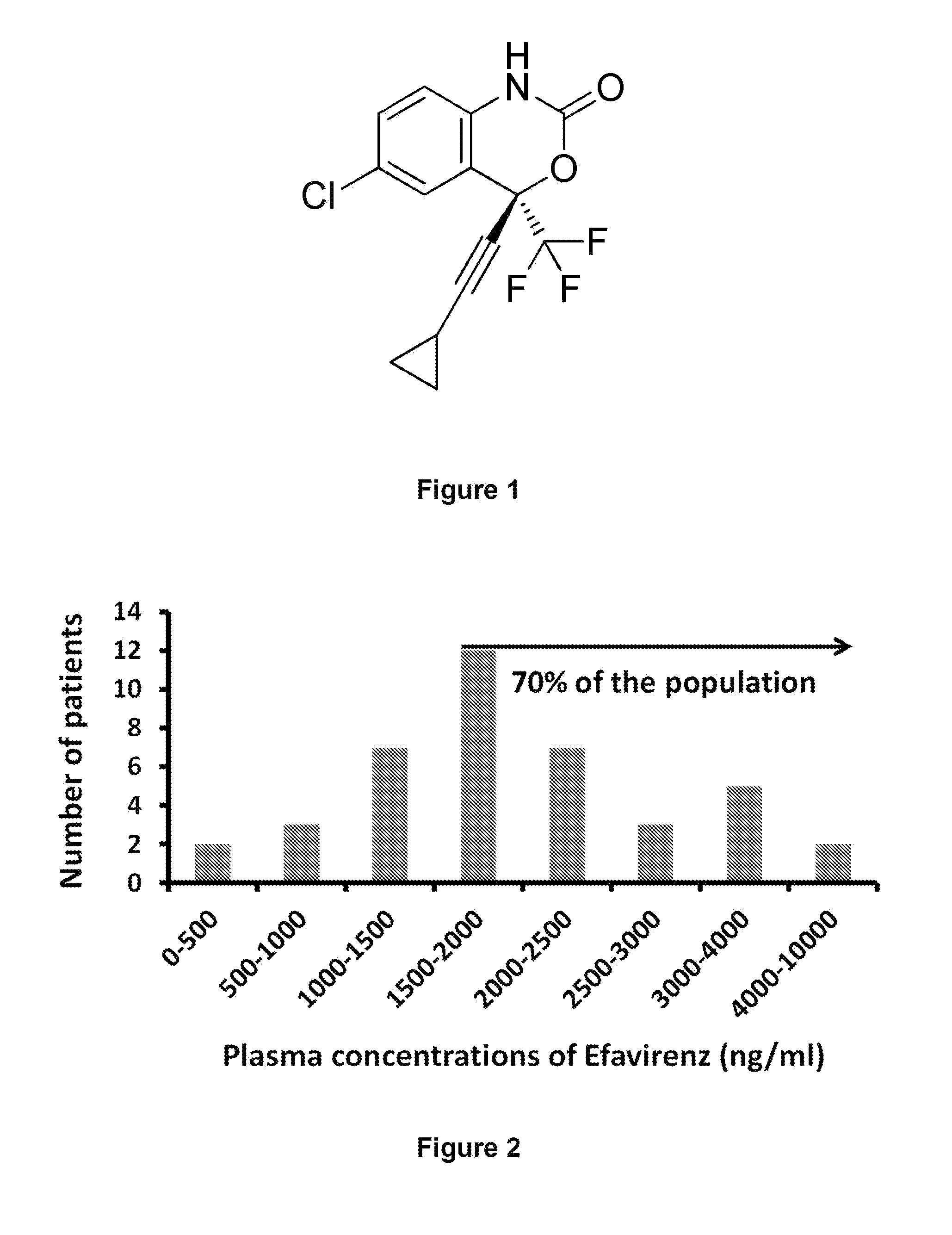
Patents
Literature
58 results about "Efavirenz" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This drug is used with other HIV medications to help control HIV infection.
Method and composition for pharmaceutical product
InactiveUS20070077295A1Improve stabilityFormulation stabilityOrganic active ingredientsBiocideMedicineEmtricitabine
This invention is directed to a composition comprising dry granulated tenofovir DF and emtricitabine, and a method for making same. Dry granulation was unexpectedly found to be important in preparing a tenofovir DF containing composition suitable for inclusion in a combination dosage form containing emtricitabine, efavirenz and tenofovir DF.
Owner:GILEAD SCI INC
Compressed tablet formulation
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
Compressed tablet formulation
InactiveUS7060294B2Organic active ingredientsCapsule deliveryNucleoside Reverse Transcriptase InhibitorClinical study
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
Combination therapy
ActiveUS20050288326A1Low effective doseLow cytotoxicityBiocideAntiviralsSide effectReverse transcriptase
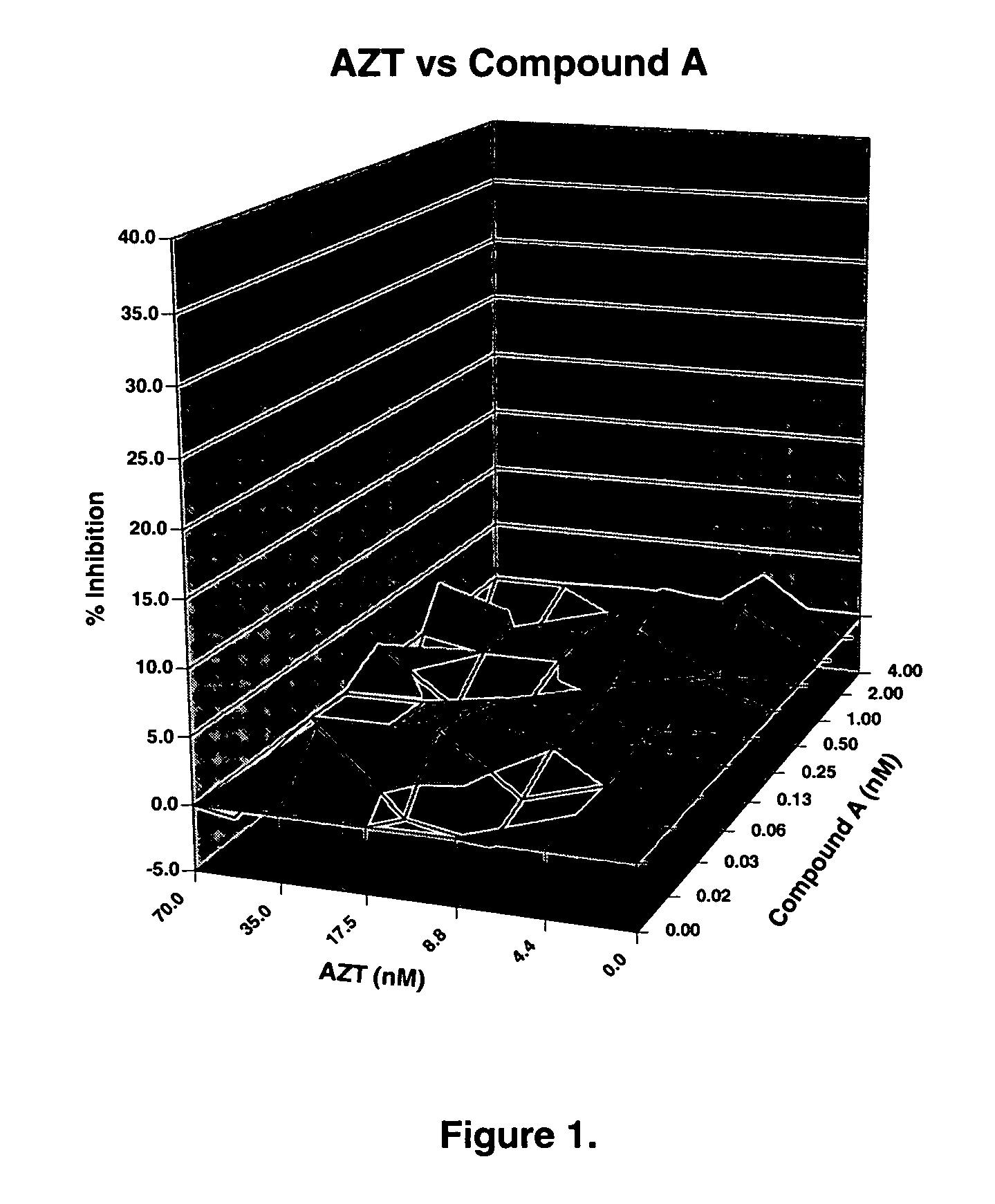
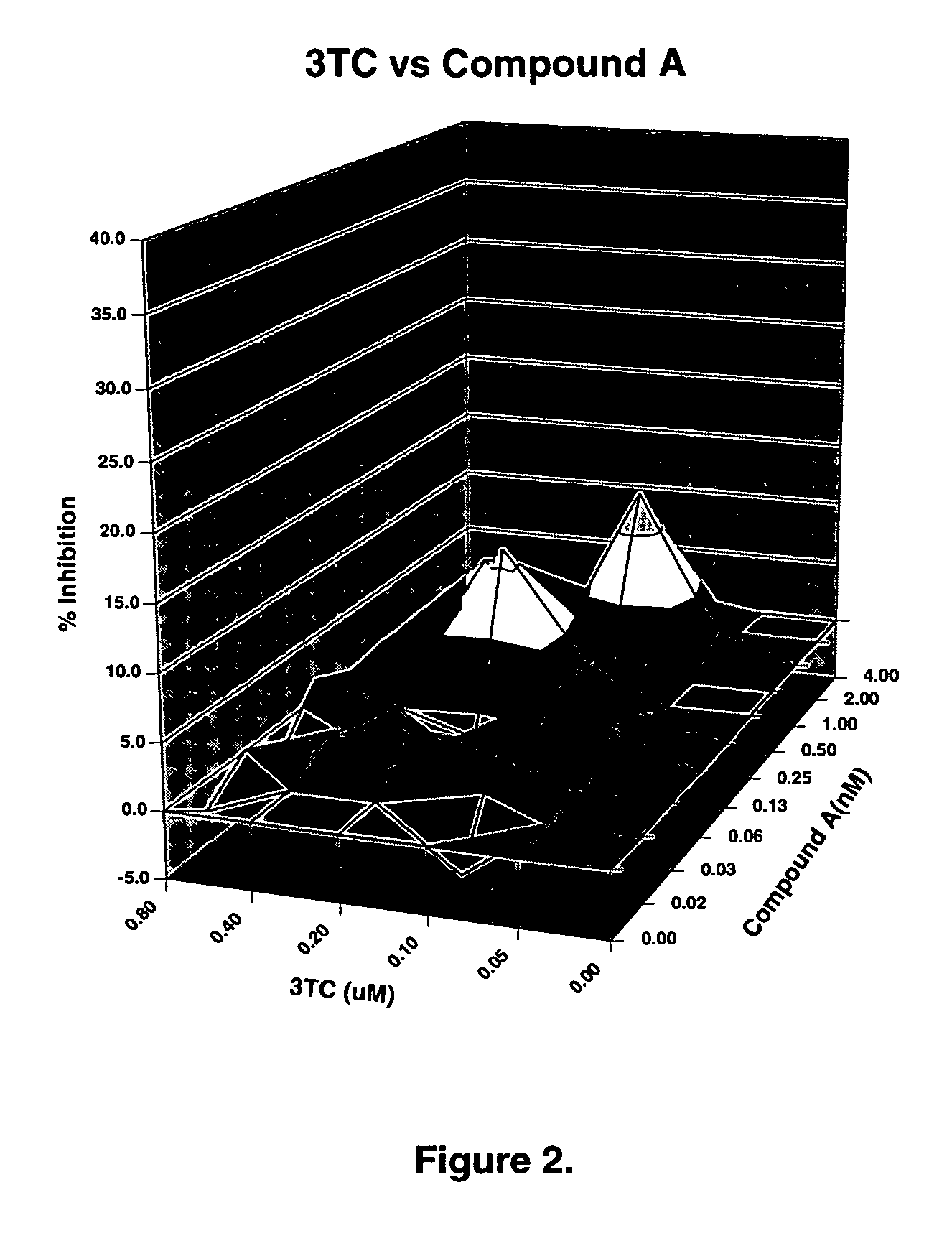
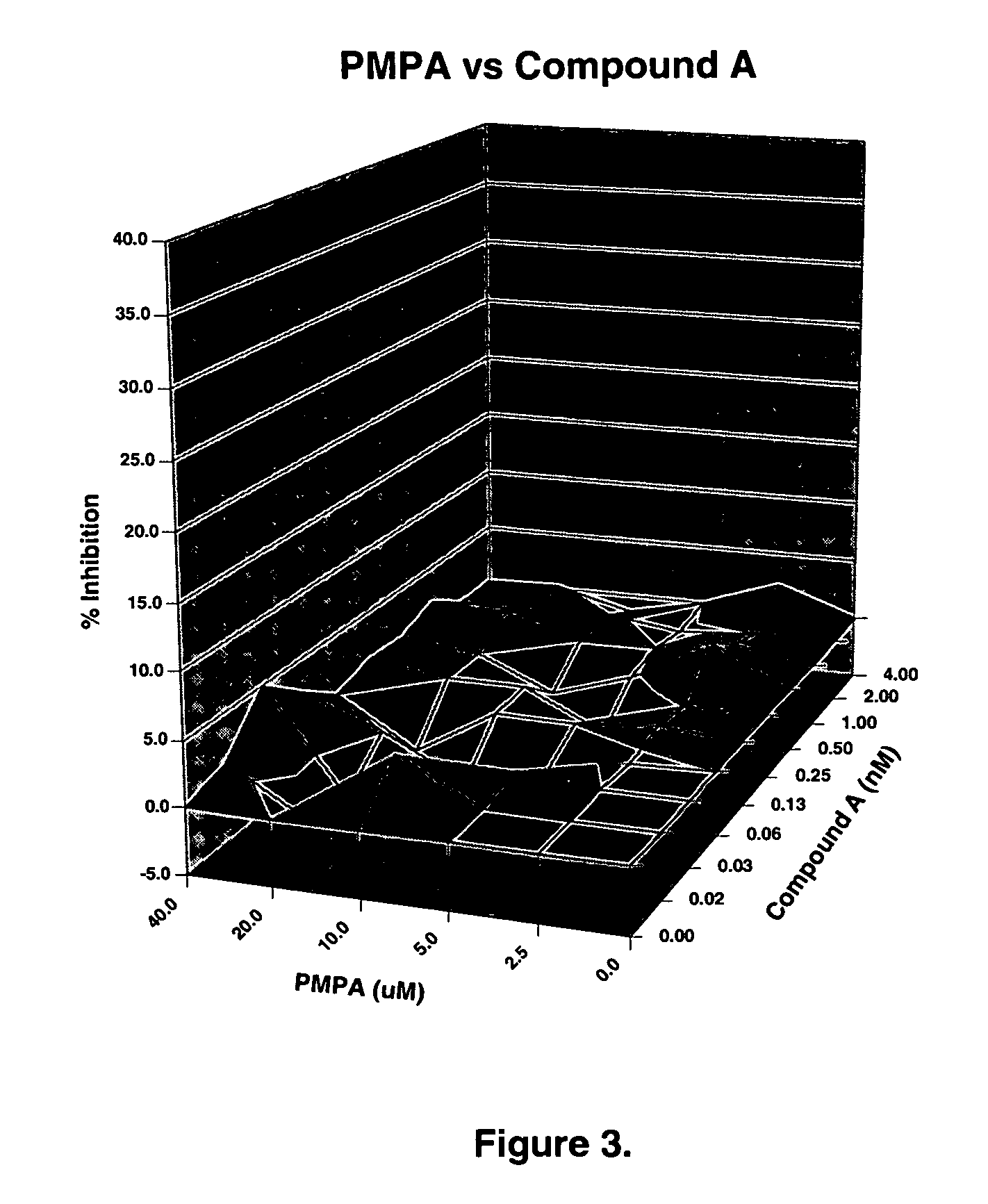
The present invention relates to a combination therapy for treating an HIV infection or inhibiting integrase comprising (S)-6-(3-Chloro-2-fluorobenzyl)-1-(1-hydroxymethyl-2-methylpropyl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (“Compound A”) or a pharmaceutically acceptable solvate or salt thereof in combination with at least one other anti-HIV agent. In some embodiments of the present invention, the other anti-HIV agents are chosen from reverse transcriptase inhibitors and protease inhibitors. In certain embodiments of the present invention, the other anti-HIV agents are chosen from AZT, 3TC, PMPA, efavirenz, indinavir, nelfinavir, a combination of AZT / 3TC, and a combination of PMPA / 3TC. Since Compound A has a high inhibitory activity specific for integrases, when used in combinations with other anti-HIV agents it can provide a combination therapy with fewer side effects for humans.
Owner:JAPAN TOBACCO INC
Combination therapy
ActiveUS8633219B2Low effective doseEffective treatmentBiocideOrganic chemistrySide effectCombined Modality Therapy
Owner:JAPAN TOBACCO INC
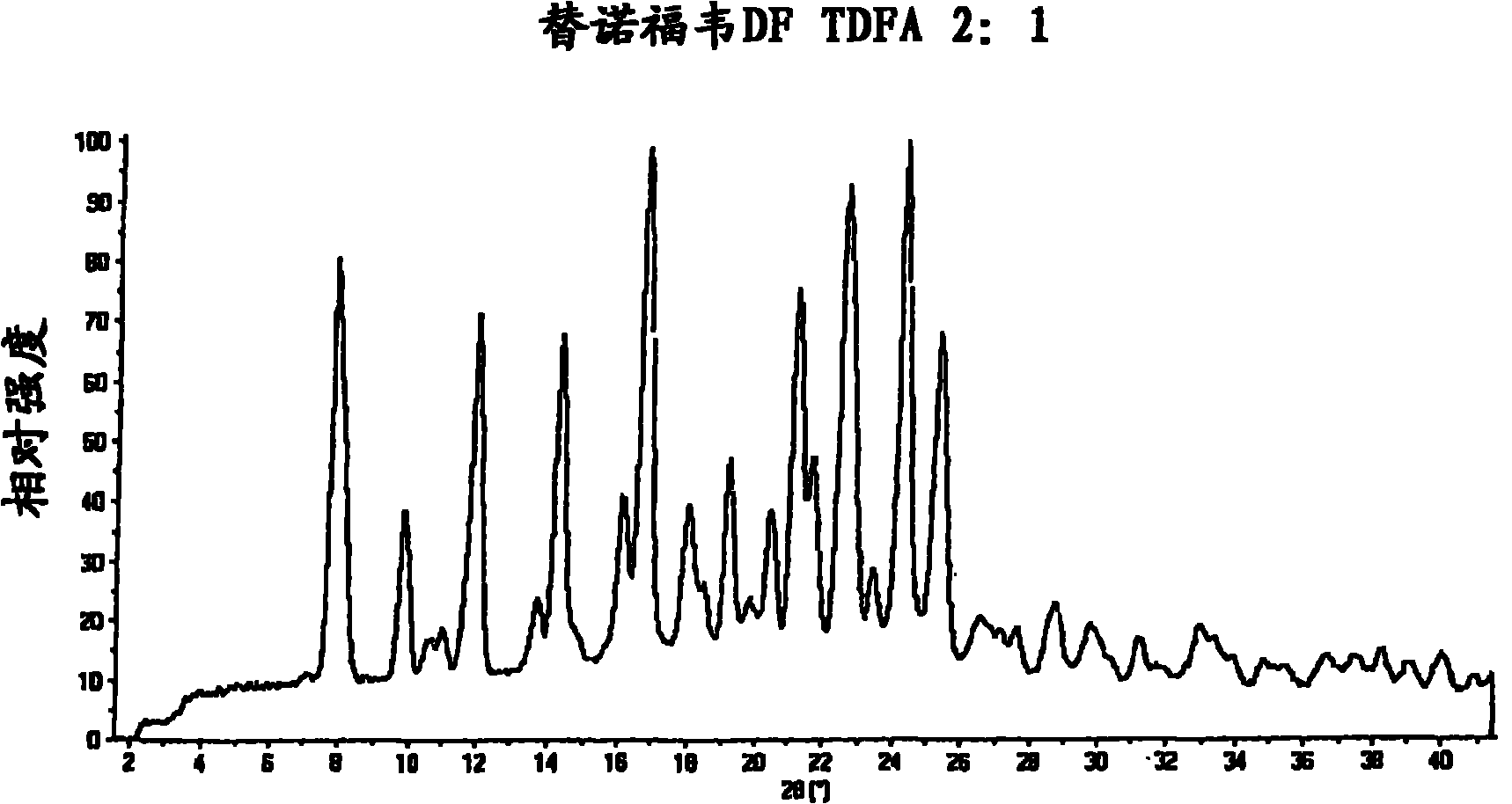
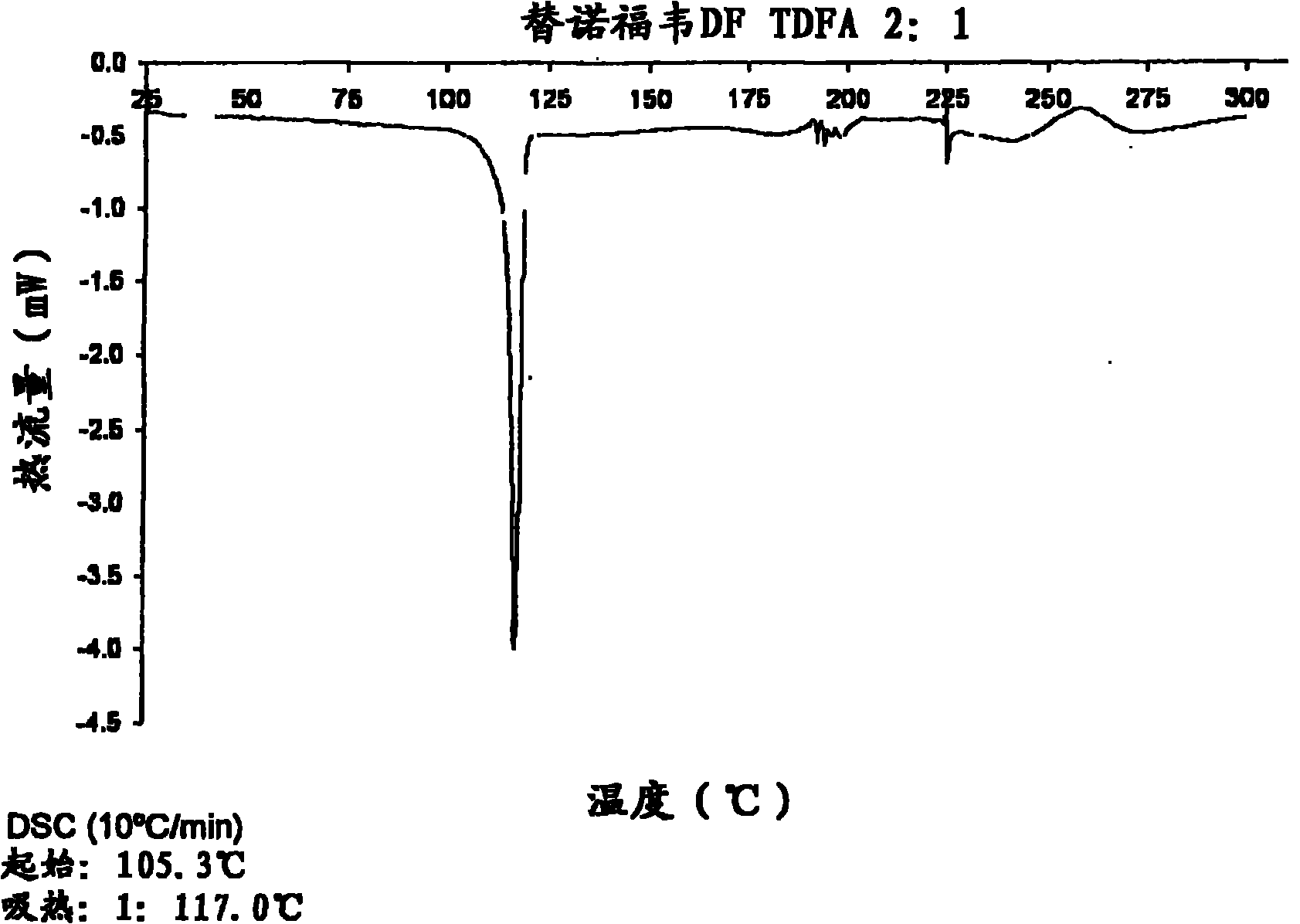
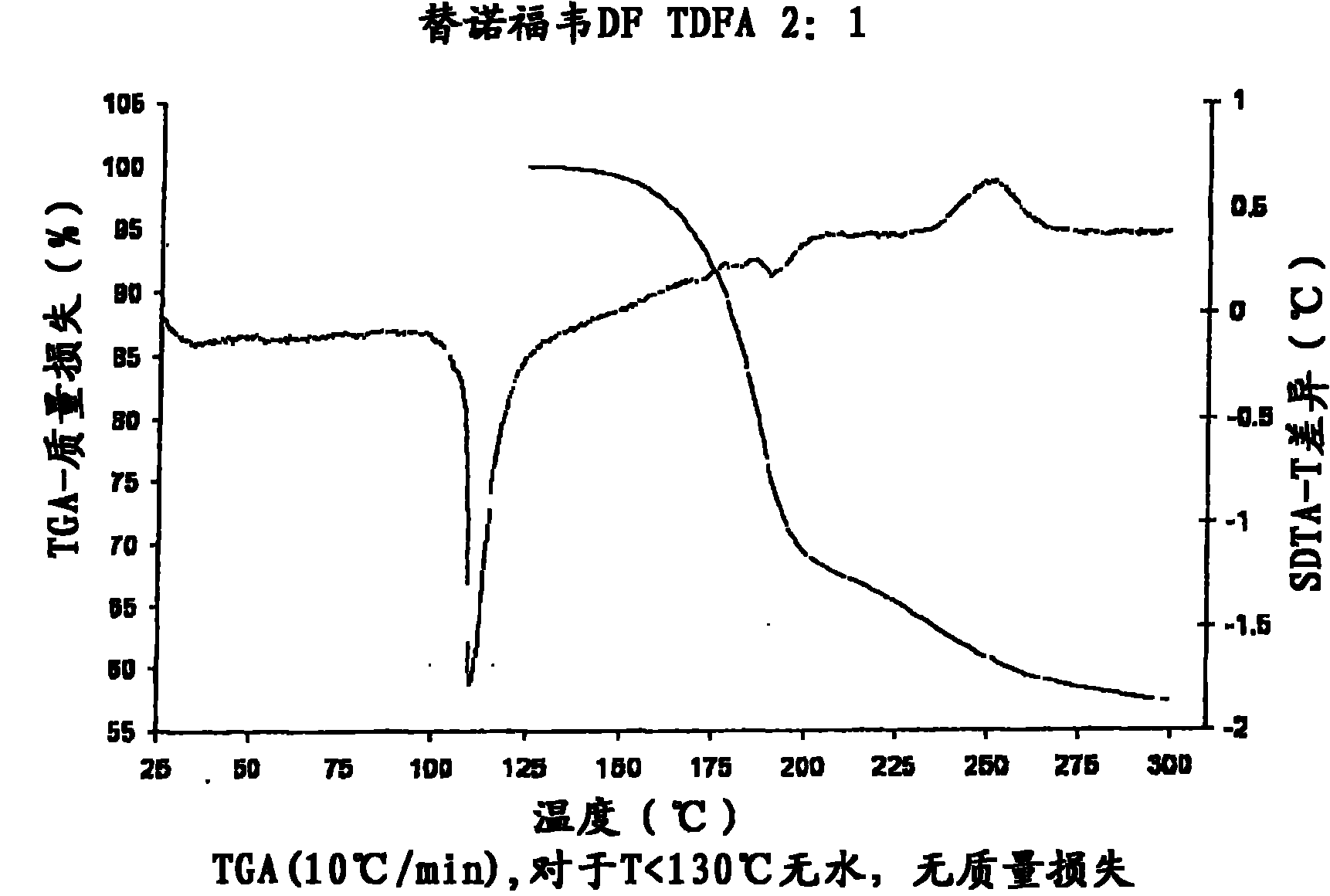
Tenofovir disoproxil hemi-fumaric acid co-crystal
InactiveCN101778855AOrganic active ingredientsGroup 5/15 element organic compoundsEmtricitabineTenofovir DF
The present invention provides a novel crystalline form of Tenofovir disoproxil fumarate (Tenofovir DF), designated Co-crystal TDFA 2:1, methods for the preparation thereof and its use in pharmaceutical applications, in particular in anti-HIV medicaments. The crystalline form TDFA 2:1 can be used in combination with other anti-HIV medicaments such as Efavirenz, Emtricitabine, Ritonavir and / or TMC114.
Owner:ULTIMORPHIX TECH
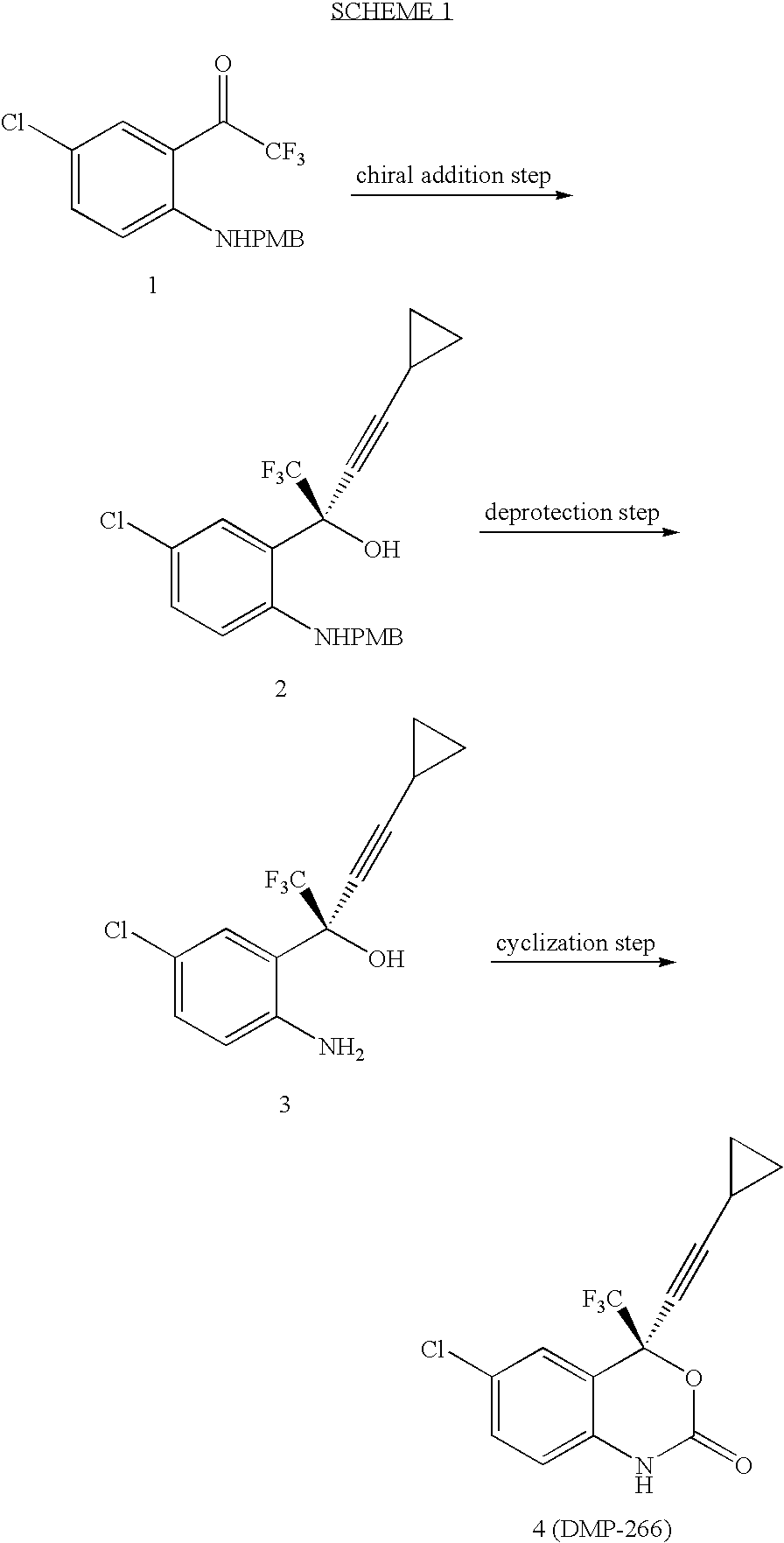
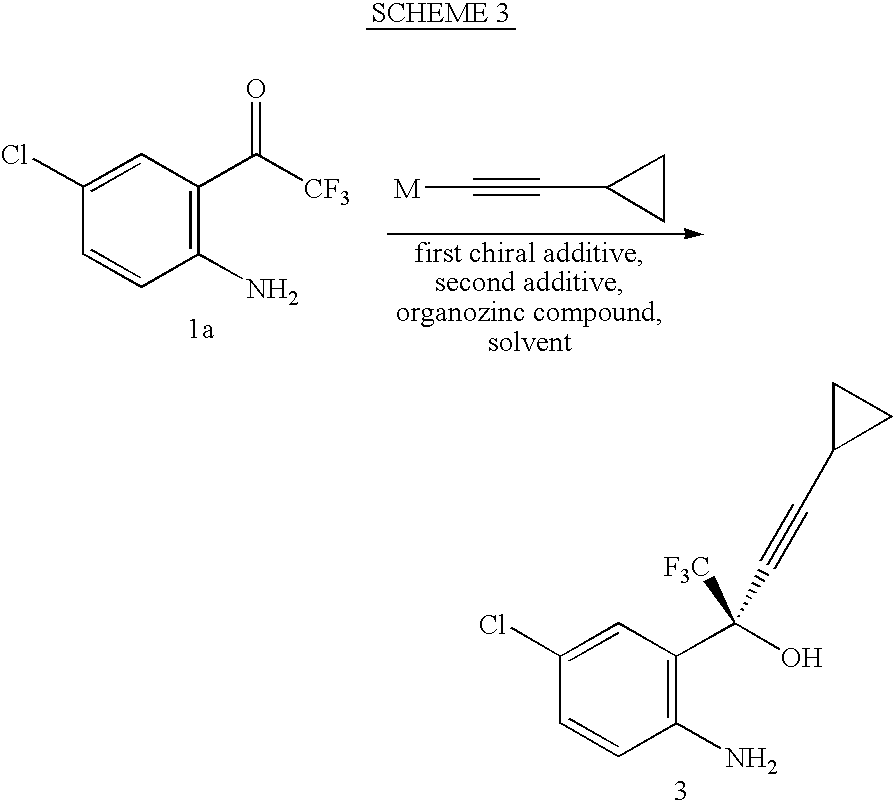
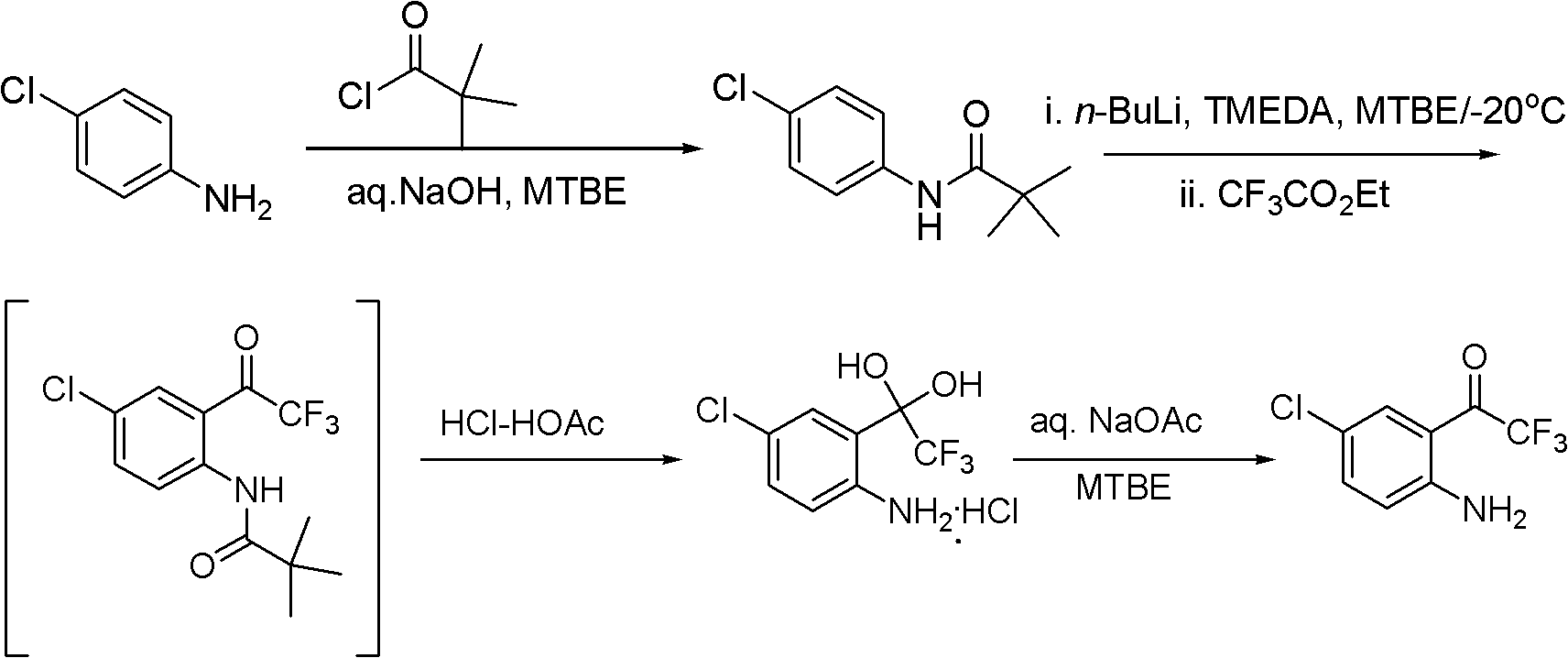
Method of Chiral alkamine ligand used as catalyst of asymmetric addition process for terminal alkyne to fluoroalkylaryl ketone
InactiveCN1449865AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCarboxyl radical
The present invention provides a kind of chiral ligand (1R, 2R)-2-N,N-substituted amino-1-(4-substituted phenyl)-1-ethanol or its enantiomorph and a method using the above-mentioned chiral ligand or its enantiomorph as catalyst for asymmetric addition of acetylene copper or acetylene zinc to trifluoromethylarylketone. Said invention provides its structural general formula. Said invention adopts the asymmetric addition process so as to can high-effectively and high enantiomeric-selectively create the chiral quanternary carbon center in HIV transfrase high-activity inhibitor Efavirenz (Sustiva TM), so that it can high-effectively synthesize Efavirenz (Sustiva TM).
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
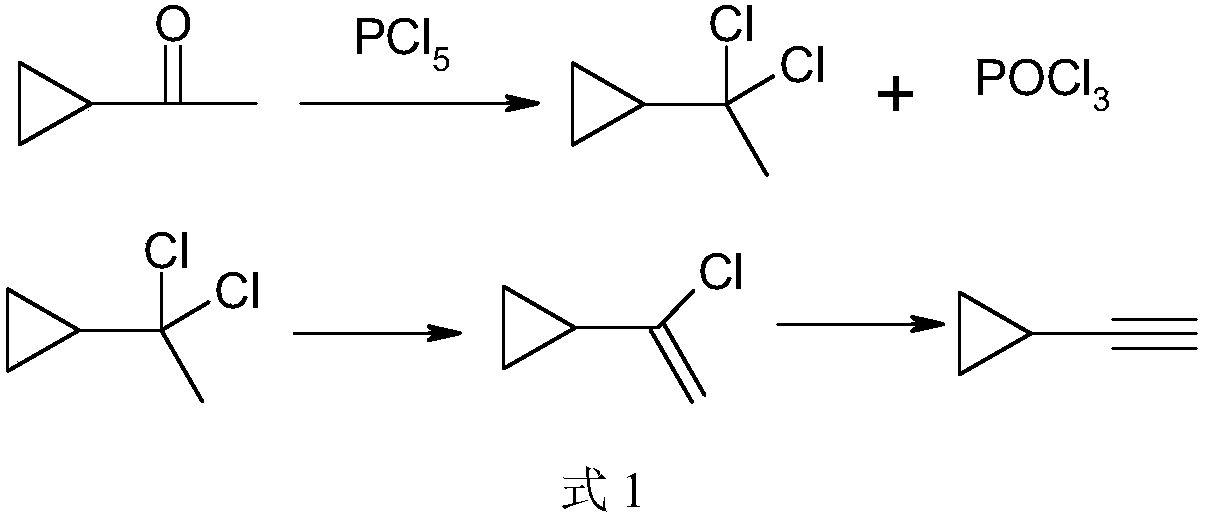
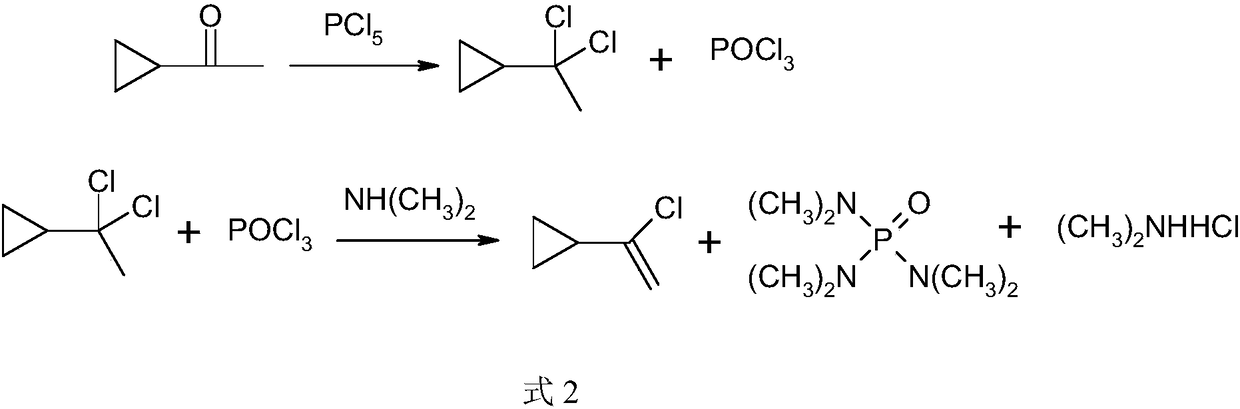
Preparation method of intermediate cyclopropyl acetylene of anti-AIDS (acquired immune deficiency syndrome) drug efavirenz
InactiveCN108440229AAvoid hydrolysisLow costPreparation by hydrogen halide split-offPhosphorus halides/oxyhalidesIce waterHydrolysis
The invention discloses a preparation method of intermediate cyclopropyl acetylene of anti-AIDS (acquired immune deficiency syndrome) drug efavirenz. The preparation method comprises the steps of performing a reaction in an organic solvent by taking cyclopropyl methyl ketone as a raw material and phosphorus pentachloride as a chlorinating agent by the action of a catalyst to generate alpha, alpha-dichloroethyl cyclopropane and phosphorus oxychloride, performing decompressed rectification to remove phosphorus oxychloride, removing part of hydrogen chloride from alpha, alpha-dichloroethyl cyclopropane by the action of triethylamine to generate alpha-chlorovinyl cyclopropane, and further removing part of hydrogen chloride by the action of strong base to generate cyclopropyl acetylene. Phosphorus oxychloride is removed by the decompressed rectification; the generation of much phosphorus wastewater due to hydrolysis of phosphorus oxychloride in ice water is avoided; recovered phosphorus oxychloride can be comprehensively utilized by simple rectification; waste gas, wastewater and industrial residue are reduced; the cost is lowered; cyclopropyl acetylene is prepared from alpha-chlorovinyl cyclopropane via a reaction rectification technology; and the method has the advantages of high conversion rate, good selectivity, low energy consumption and the like, and is suitable for industrialproduction.
Owner:JIANGSU YUXIANG CHEM
Disoproxil fumarate, lamivudine and efavirenz tri-combination compound mini-pill tablet and preparation method thereof
ActiveCN103908456AGuaranteed stabilityGood compressibilityOrganic active ingredientsAntiviralsDissolutionTableting
The invention discloses a tri-combination compound mini-pill tablet co-prepared by disoproxil fumarate DF coated mini-pills, lamivudine coated mini-pills and efavirenz mini-pills and a preparation method thereof. The invention solves the problems that effective component degradation and dissolution delaying phenomena are generated due to interaction among the tri-combination compound components, tabletting is not facilitated during preparation and patient swallowing is not facilitated; and the single-layer tablet having the three component drugs without mutual contact is prepared by a mini-pill tabletting method, so as to obtain good stability and fast dissolving rate.
Owner:ANHUI BIOCHEM BIO PHARMA
Synthetic method of intermediate cyclopropyl acetylene of anti-aids drug efavirenz
InactiveCN103664465AProcess raw materials are easy to getSimple processHydrocarbon from halogen organic compoundsPtru catalystEthyl group
The invention discloses a synthetic method of intermediate cyclopropyl acetylene of an anti-aids drug efavirenz, and relates to the technical field of synthesis of cyclopropyl acetylene. The synthetic method comprises the following steps: reacting in an organic solvent to produce alpha, alpha-ethyl dichloride cyclopropane by taking cyclopropyl methyl ketone as a raw material and taking phosphorus pentachloride as a chlorinating agent; and obtaining the cyclopropyl acetylene by adding the alpha, alpha-ethyl dichloride cyclopropane into strong base aqueous liquor through a phase transfer catalyst. The synthetic method has the beneficial effects that the organic solvent and inorganic strong base are not needed to be adopted, expensive reagents such as the organic solvent and potassium tert-butoxide are avoided; moreover, process raw materials are easily available, process is simple, operation is convenient, cost is lowered and the three wastes are reduced, and therefore, the synthetic method is suitable for industrial production.
Owner:JIUJIANG ZHONGTIAN PHARMA
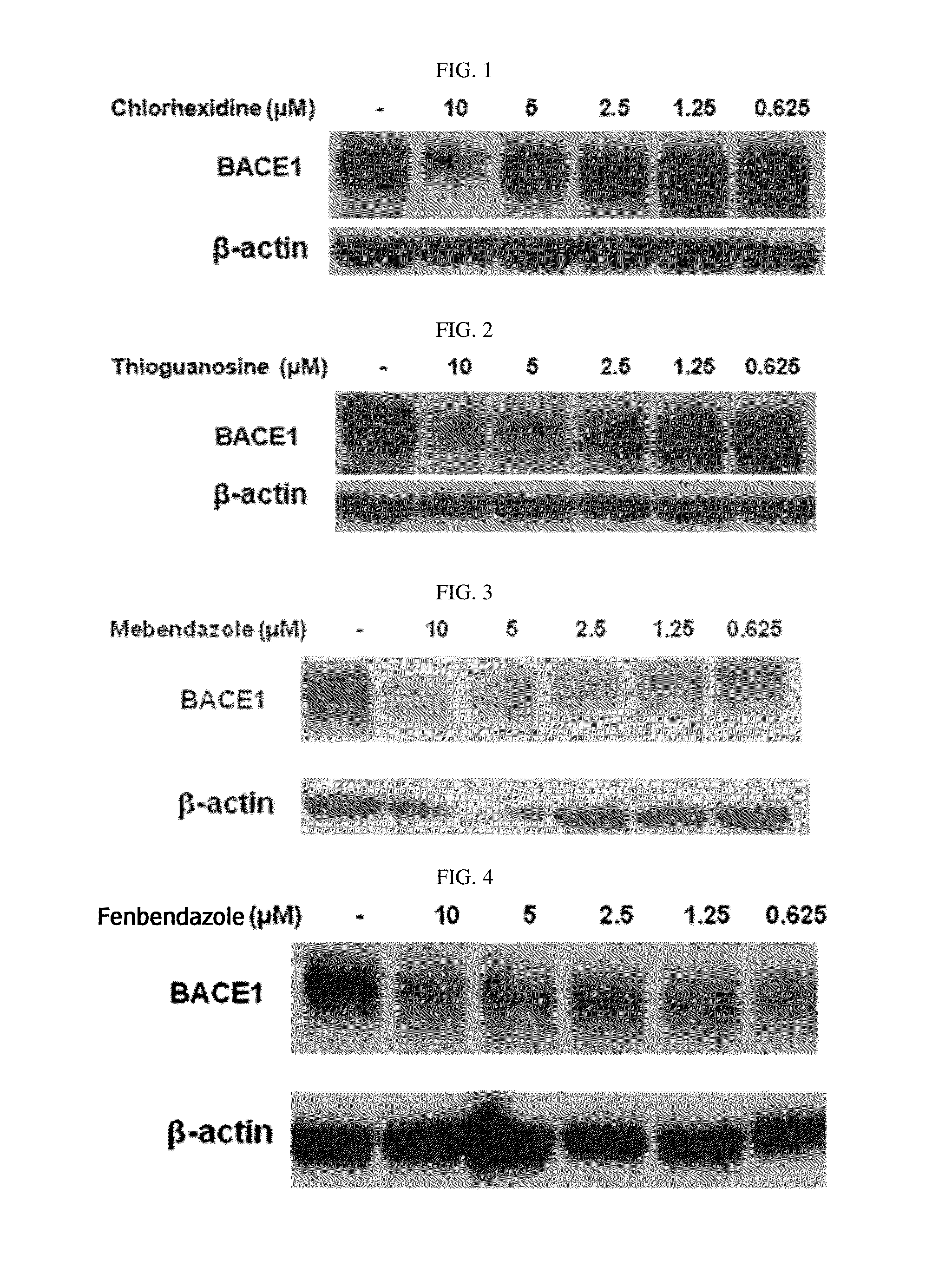
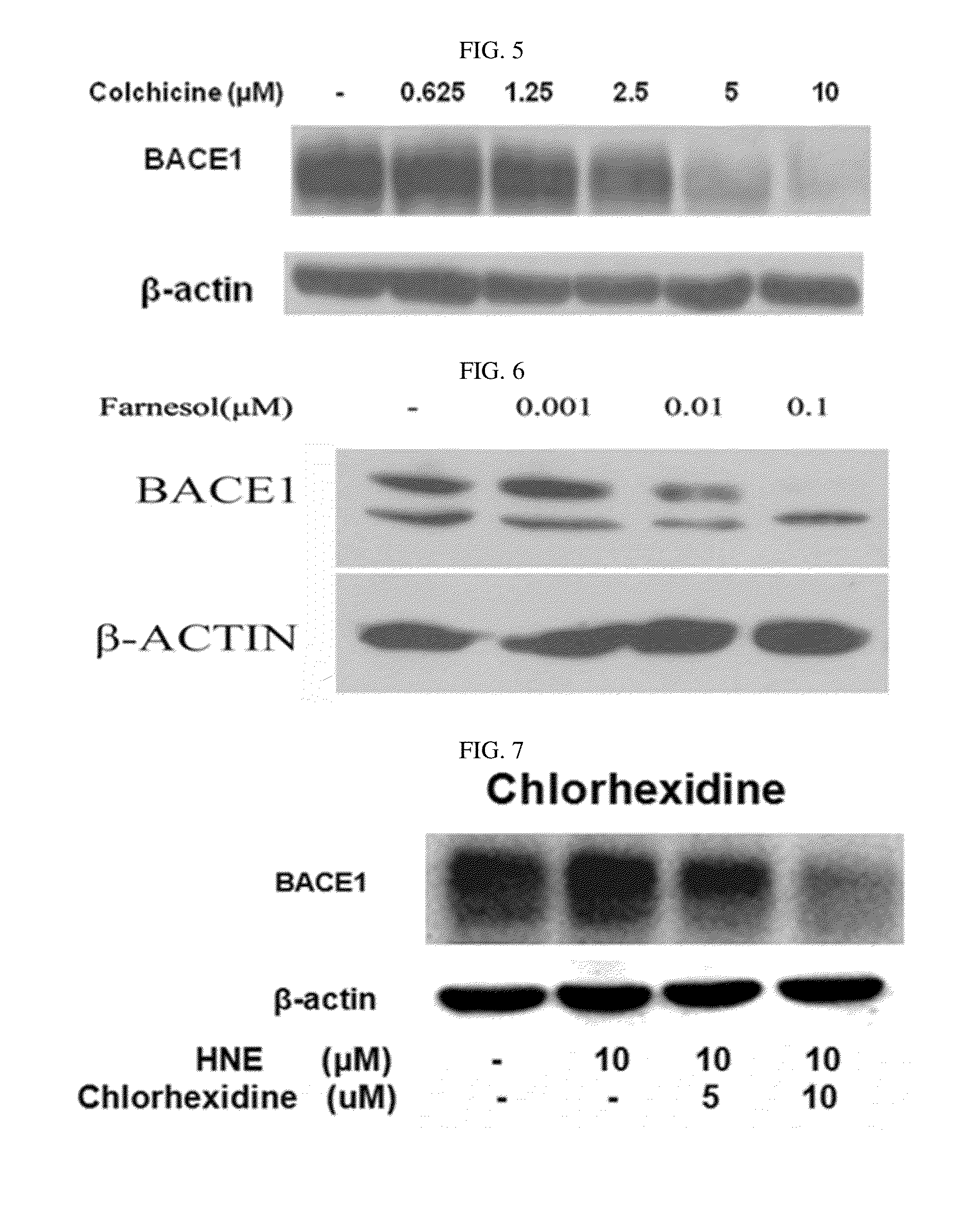
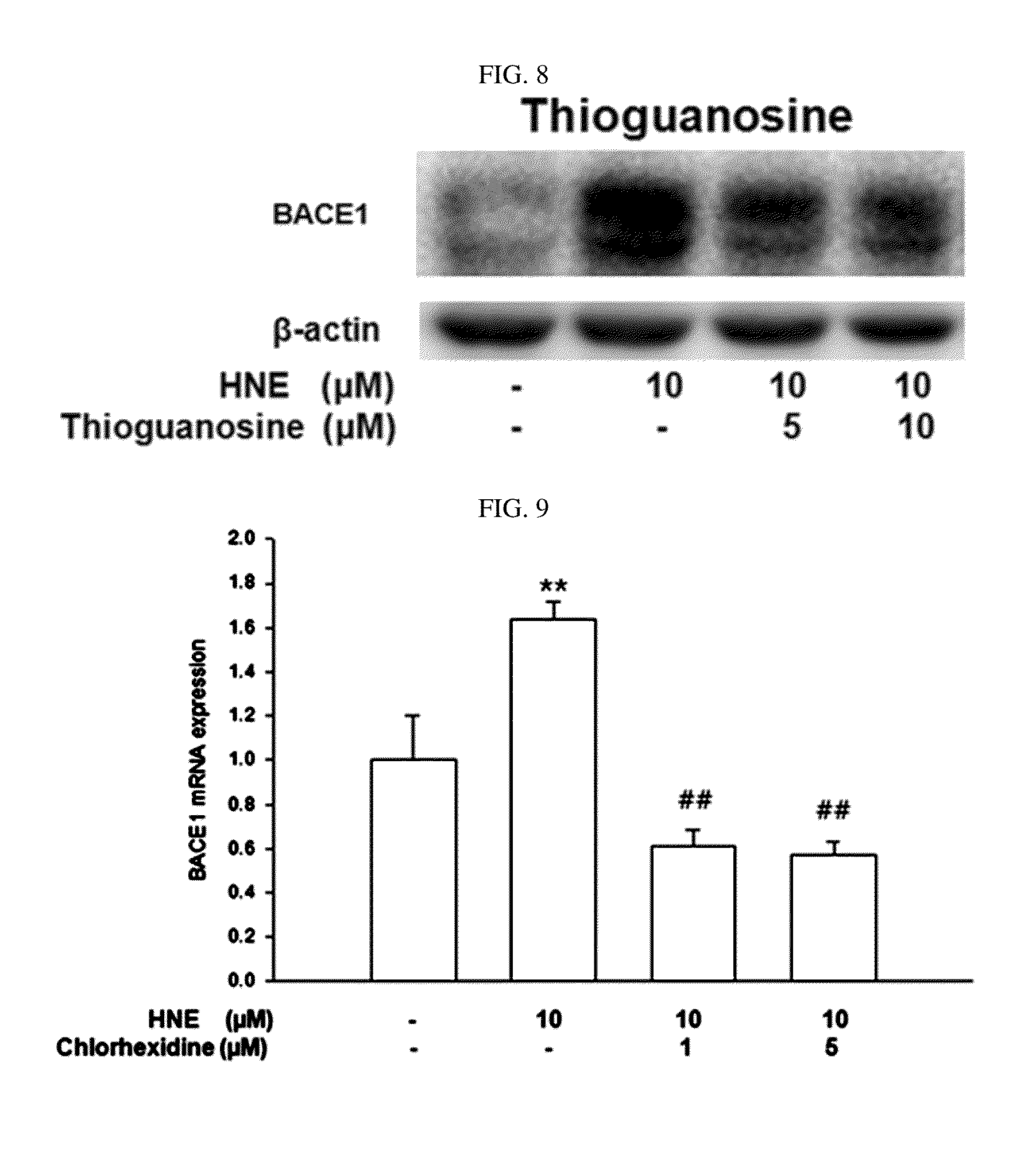
Composition for preventing or treating degenerative brain diseases including compound downregulating expression of BACE1 proteins
ActiveUS20150018297A1Reduce expressionSuppress generationBiocideSugar derivativesEfavirenzRaloxifene Hydrochloride
The present disclosure relates to a pharmaceutical composition, a health functional food composition, and a method for preventing or treating a brain disease or diabetes. The pharmaceutical composition, health functional food composition, and method includes at least one active ingredient that is chlorhexidine, thioguanosine, mebendazole, fenbendazole, colchicine, farnesol, trimethobenzamide hydrochloride, disulfuram, azathioprine, mebeverine hydrochloride, zaprinast, tosufloxacin hydrochloride, efavirenz, thiostrepton, probenecid, entacapone, harmine hydrochloride, flunisolide, thimerosal, hexestrol, sulfaquinoxaline sodium salt, monensin sodium salt, raloxifene hydrochloride, 2-chloropyrazine, or topotecan.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
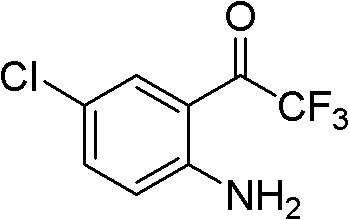
Simple, convenient and high-efficiency synthesis method of 4-chloro-2-trifluoroacetylaniline and analogs thereof
ActiveCN102675125BAvoid dangerSimple and fast operationOrganic compound preparationAmino compound preparationSynthesis methodsImmune deficiency syndrome
The invention relates to a simple, convenient and high-efficiency synthesis method of 4-chloro-2-trifluoroacetylaniline and analogs thereof. The 4-chloro-2-trifluoroacetylaniline can be used as a key midbody for synthesizing the anti-AIDS (Acquired Immune Deficiency Syndrome) drug efavirenz.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
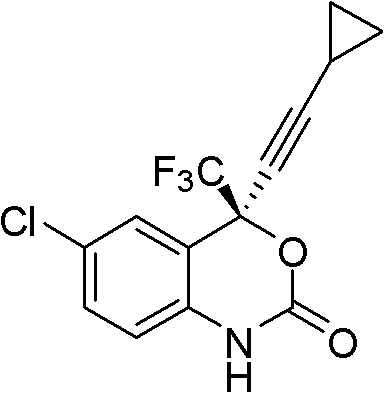
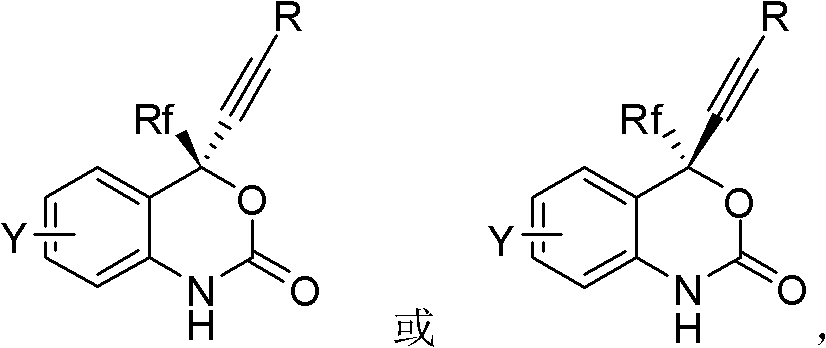
One pot method asymmetric synthesis process of HIV reverse transcriptase inhibitor Efavirenz compound
ActiveCN102584801AMild process conditionsSimple and fast operationGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsNucleoside Reverse Transcriptase InhibitorHydrogen
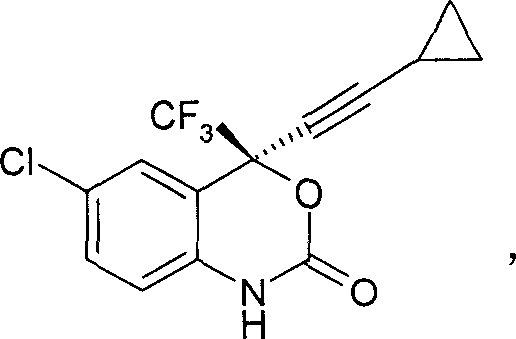
The invention relates to a novel one pot method asymmetric synthesis process of a (S)-6-chlorine-4-cylopropyl ethylnen-4-trifluoromethyl-1,4-dihydro-2H-1,3-benzoxazine-2-ketone (Efavirenz) compound, the compound can be used as an reverse transcriptase inhibitor for human immunodeficiency virus (HIV). The invention also relates to a novel aminoalcohol ligand used for the process.
Owner:然晟(上海)实业发展有限公司
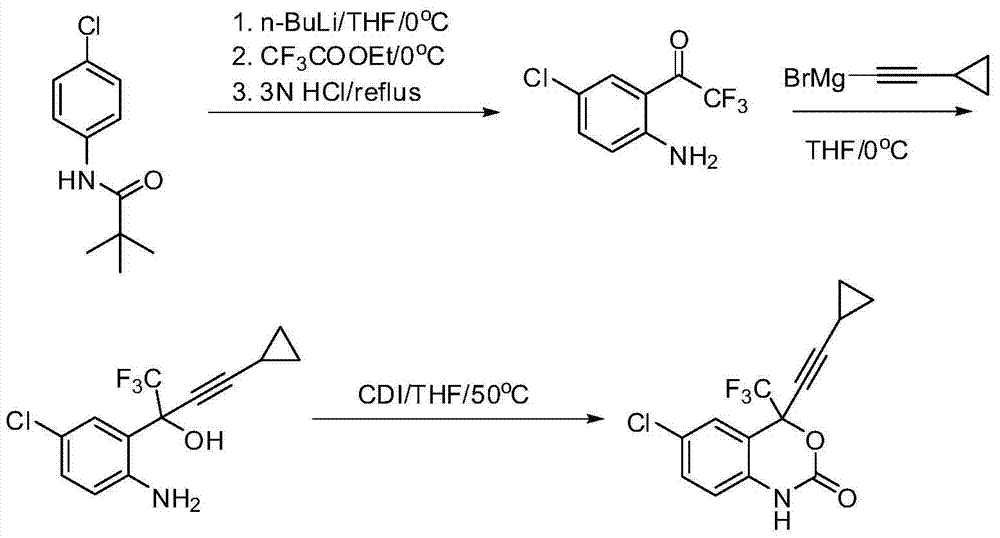
Method for synthesizing anti-AIDS pharmaceutical efavirenz
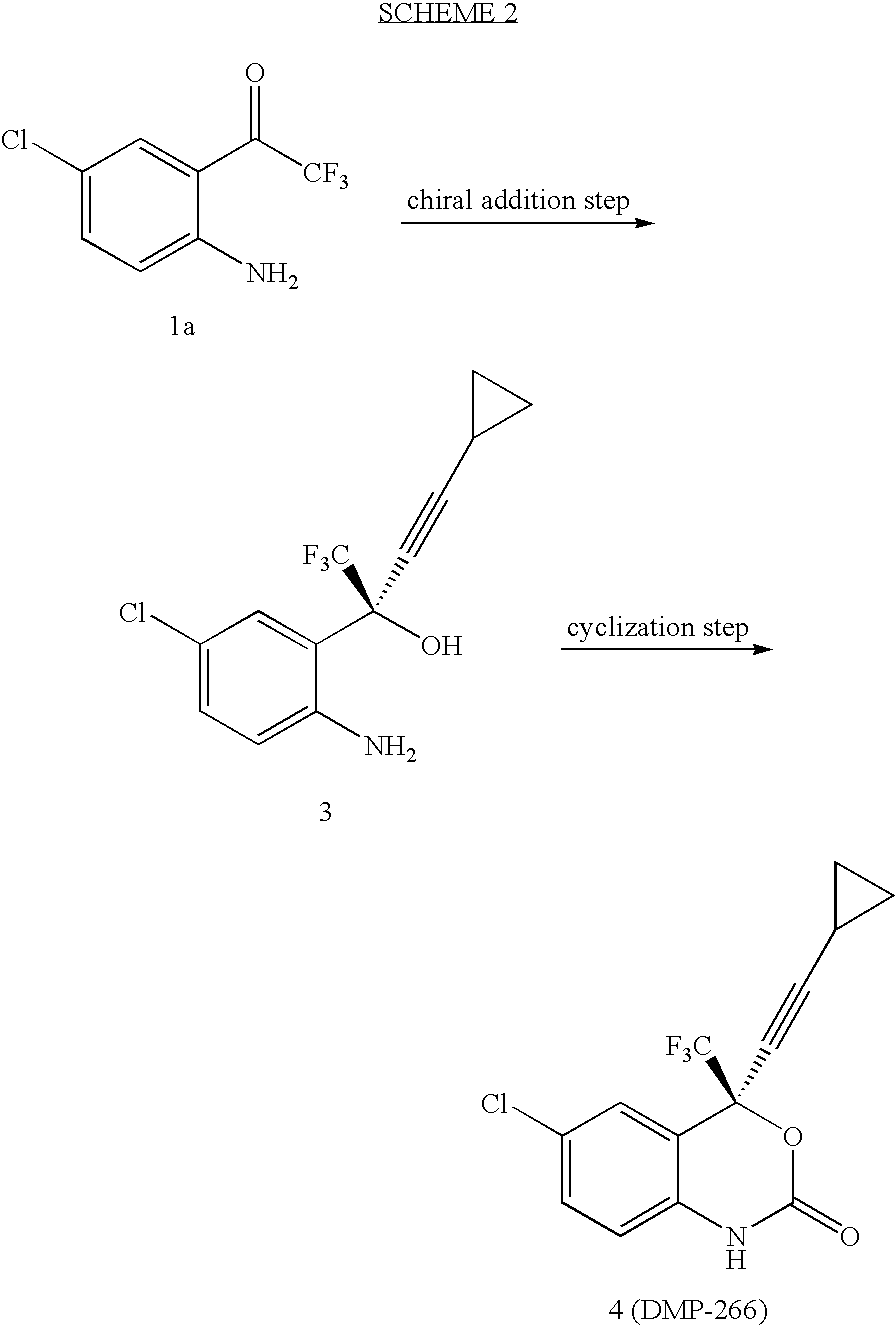
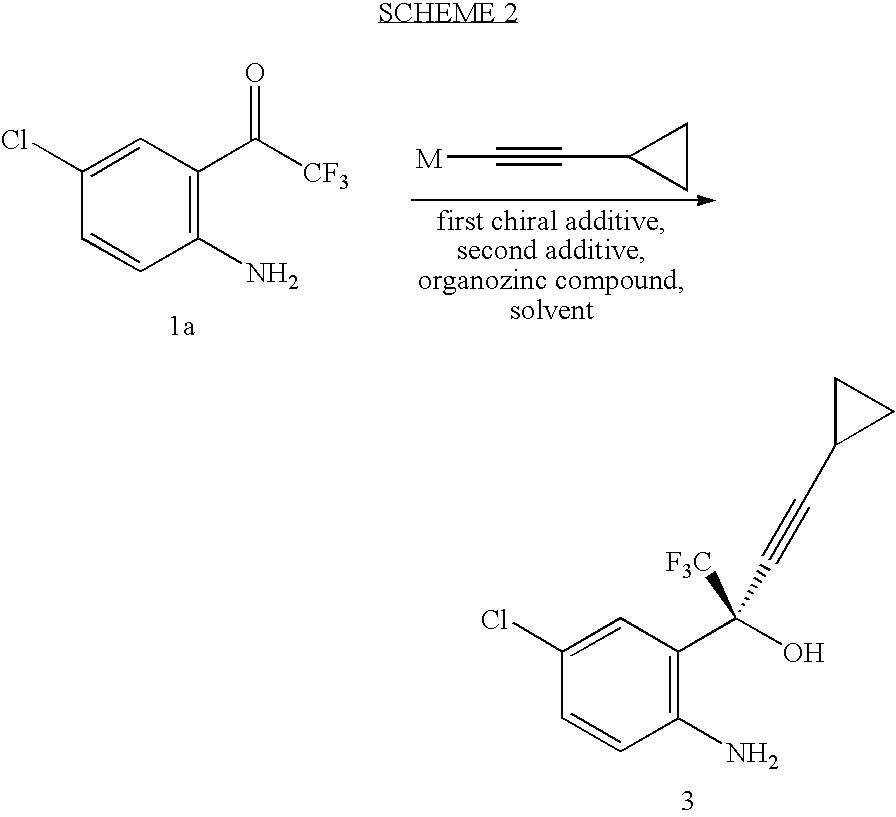
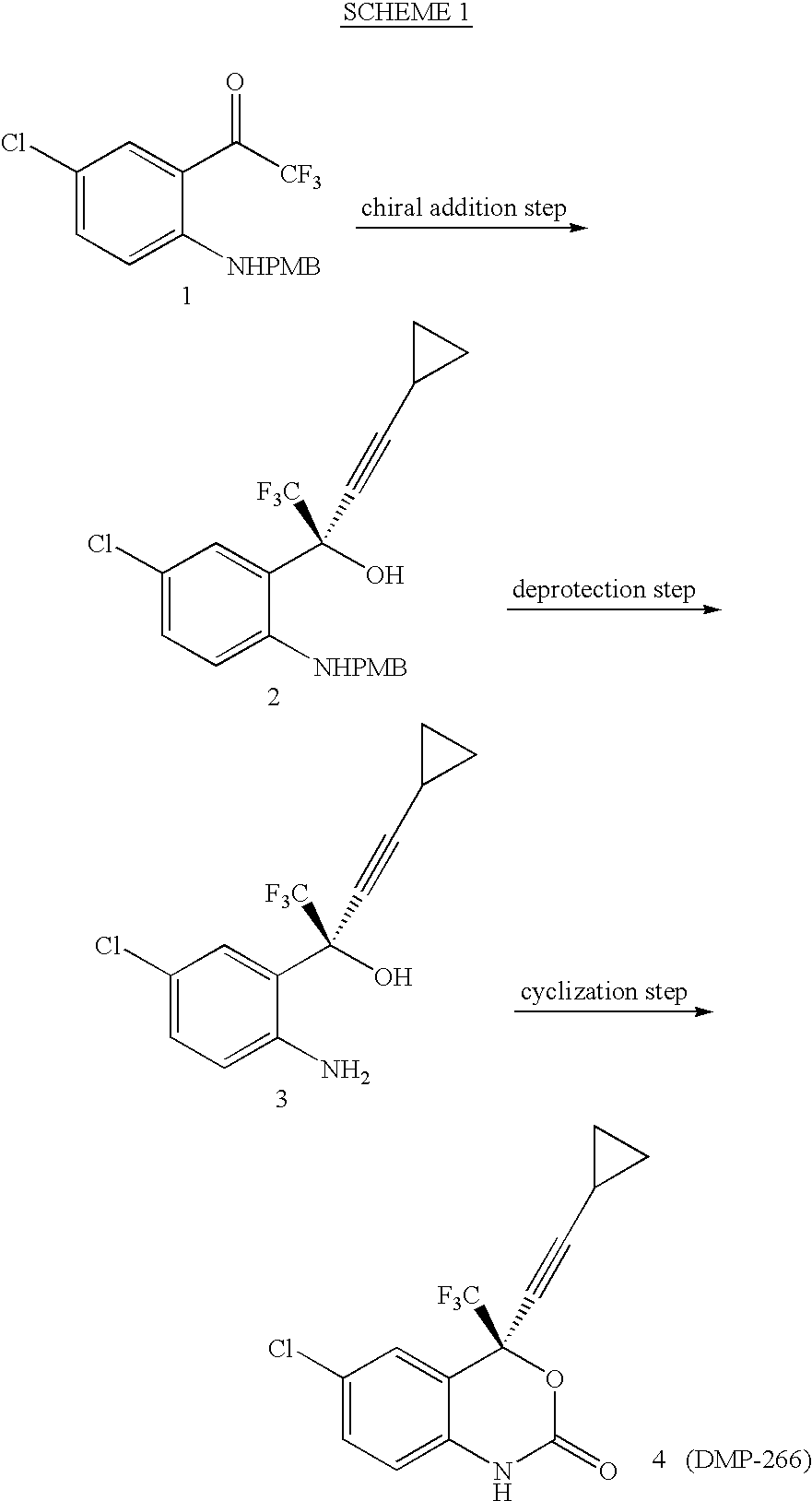
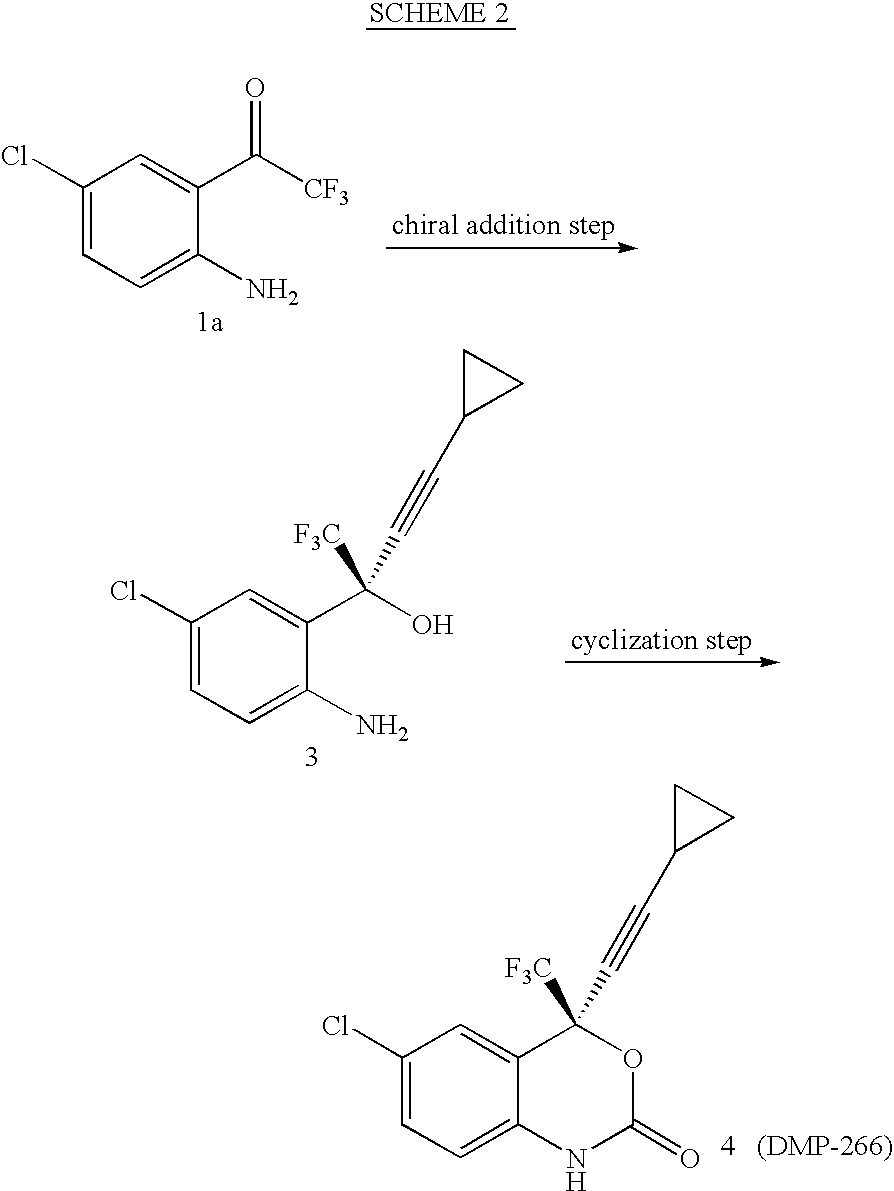
The invention discloses a method of synthesizing efavirenz which is an anti-AIDS medicine. The synthesizing steps are that: 4-chloro-2-trifluoroethylanilid is taken as raw material, after an h (hydrogen) on N being protected by protective group and the function of a ligand, the raw material is reacted with the substitute of cyclopropyl acetylene, thereby acquiring a chiral intermediate which is ring-closured and deprotected to obtain efavirenz product. The synthesizing method has the advantages that the cost is low, the reaction route is safe and environment protective and the chiral ee value of the product is high, etc.
Owner:SHANGHAI RECORD PHARM CO LTD
Simple, convenient and high-efficiency synthesis method of 4-chloro-2-trifluoroacetylaniline and analogs thereof
ActiveCN102675125AAvoid dangerSimple and fast operationOrganic compound preparationAmino compound preparationSynthesis methodsImmune deficiency syndrome
The invention relates to a simple, convenient and high-efficiency synthesis method of 4-chloro-2-trifluoroacetylaniline and analogs thereof. The 4-chloro-2-trifluoroacetylaniline can be used as a key midbody for synthesizing the anti-AIDS (Acquired Immune Deficiency Syndrome) drug efavirenz.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
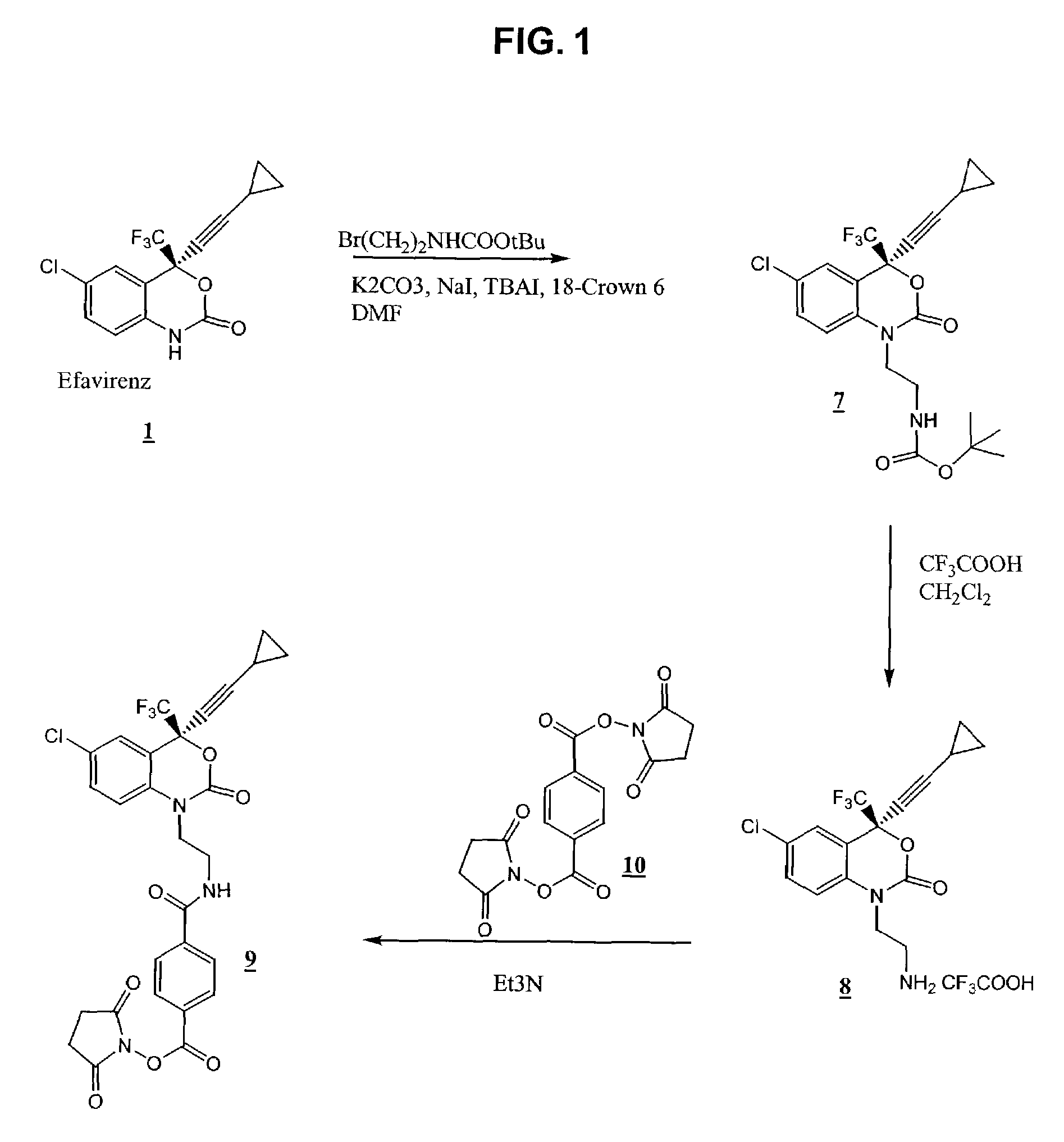
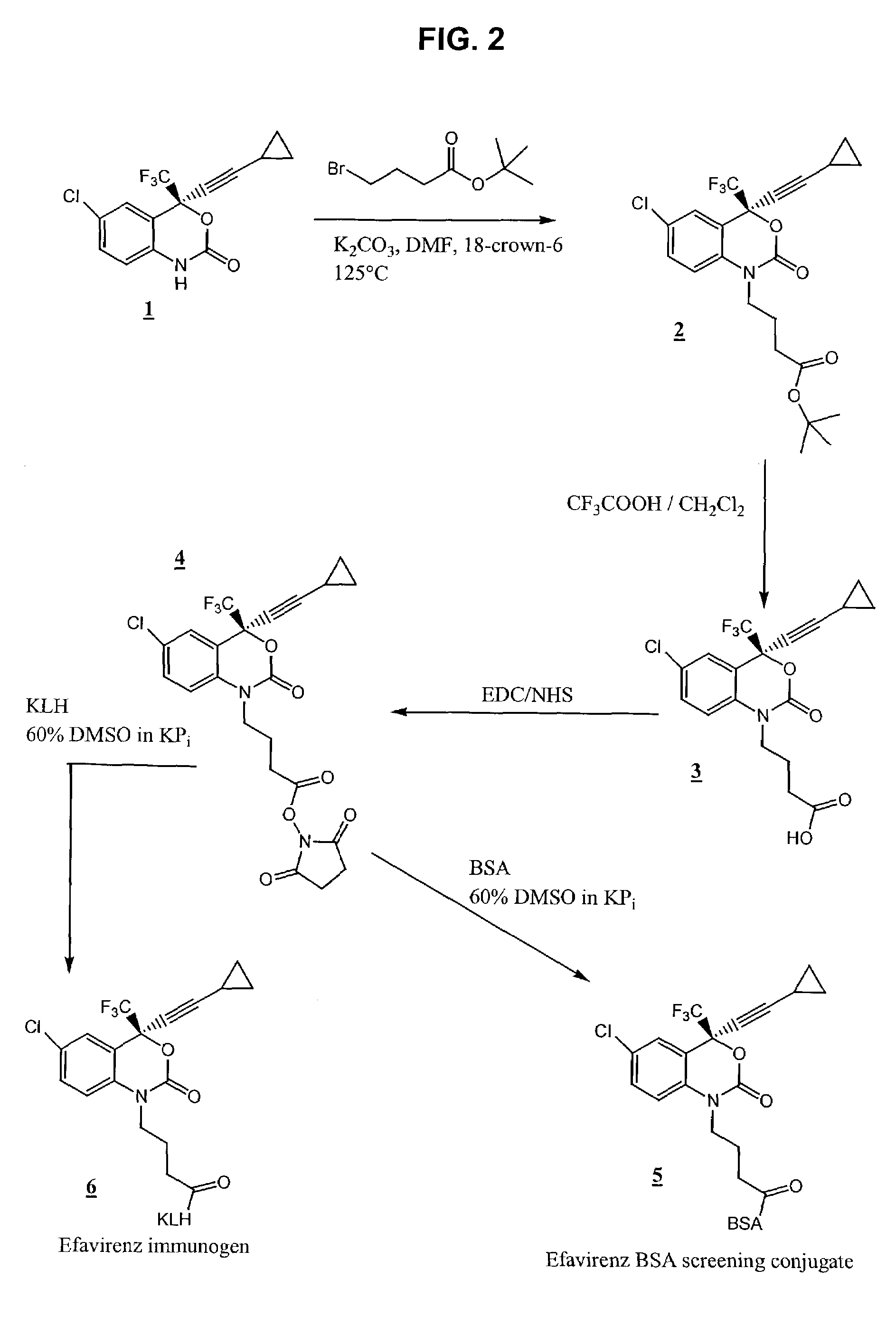
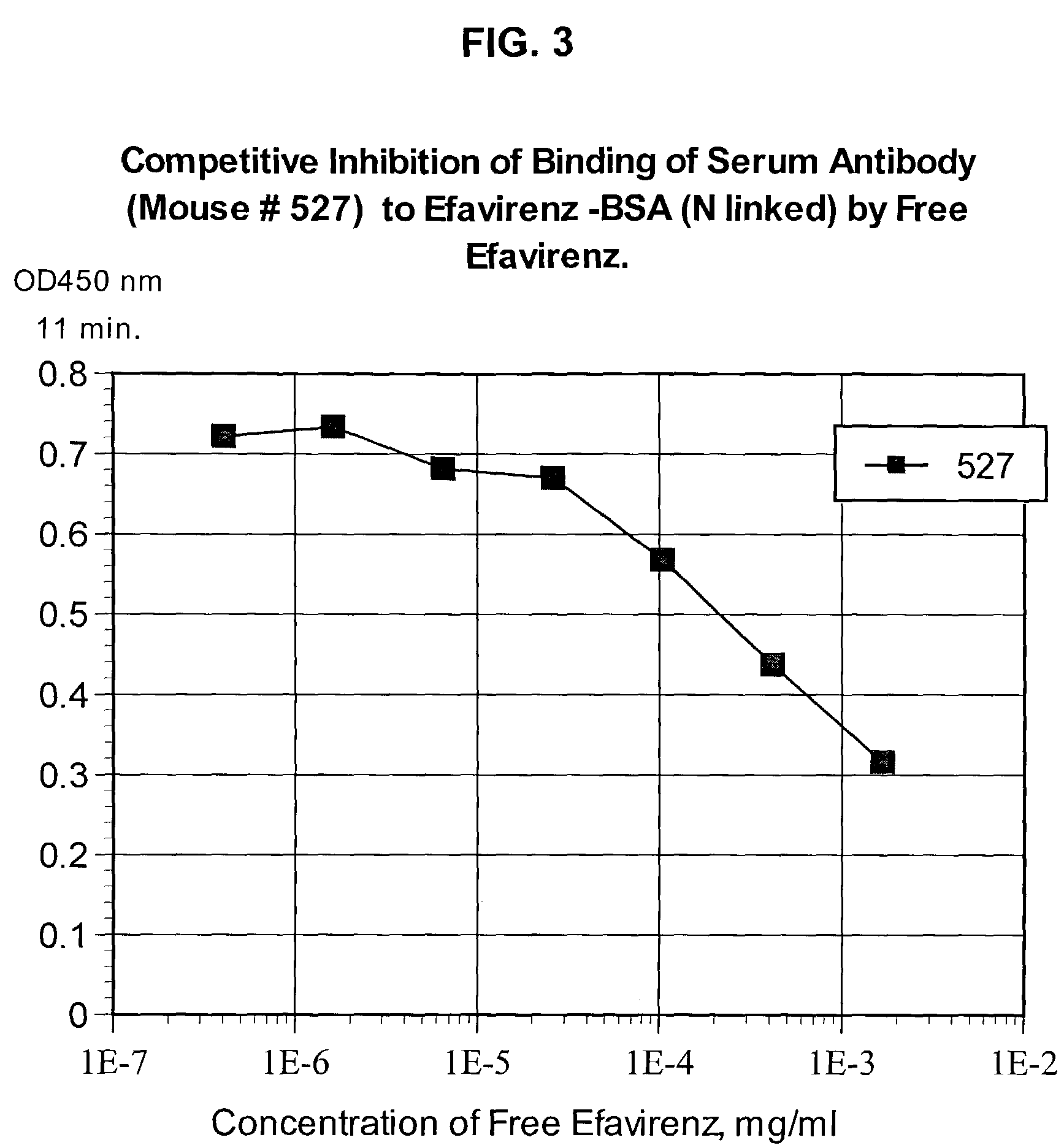
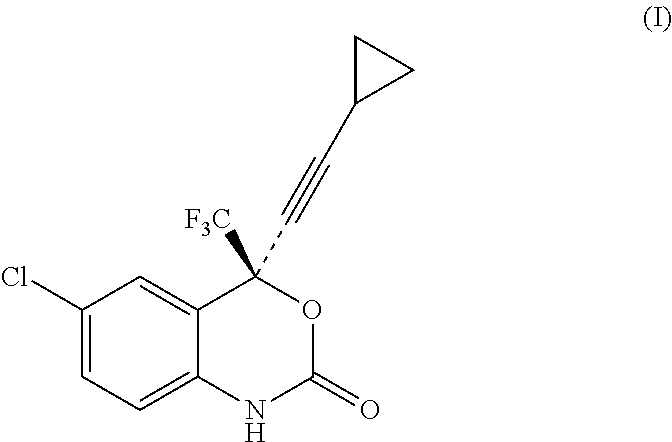
Reagents for detecting efavirenz
The invention provides derivatives of efavirenz and methods of making derivatives of efavirenz. The derivatives include immunogenic compounds for producing antibodies to efavirenz and labeled efavirenz tracers. These compounds are useful in immunoassay methods for determining efavirenz.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
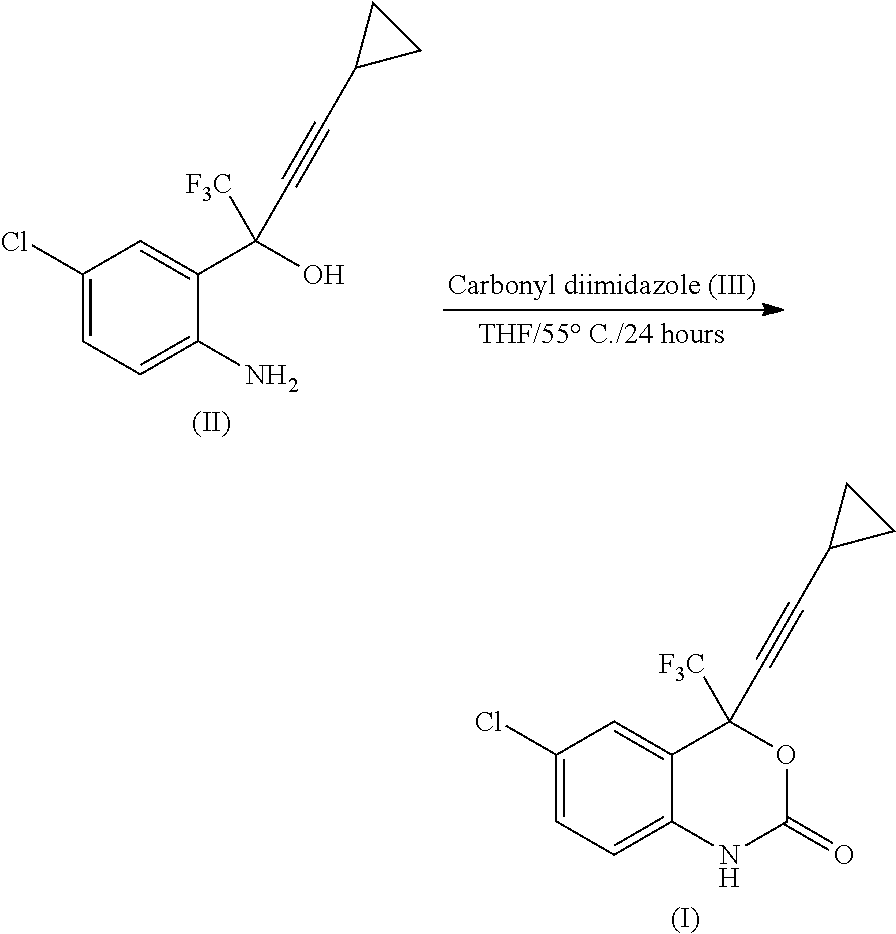
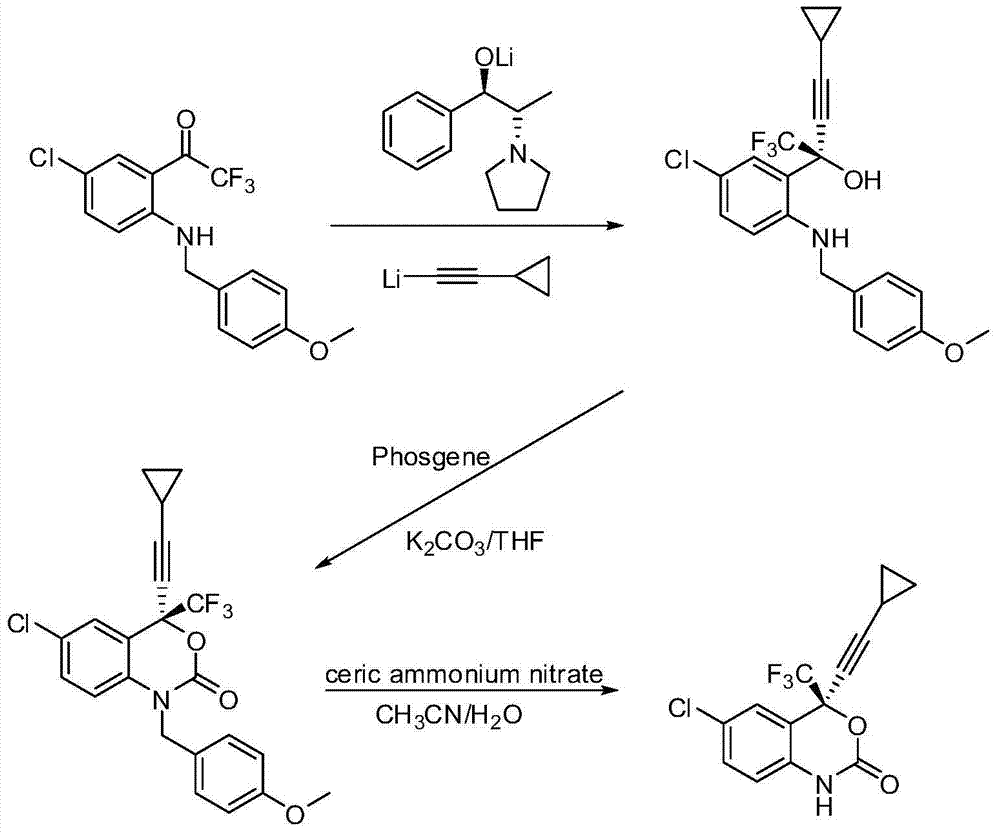
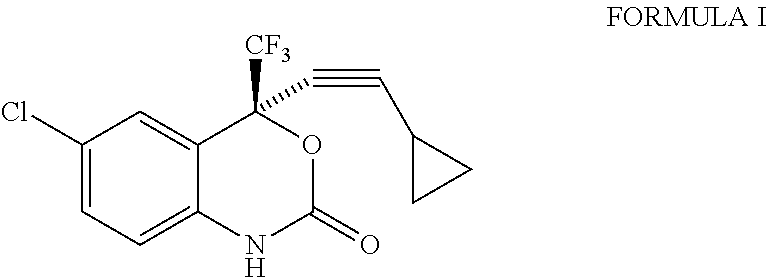
Process for the preparation of efavirenz
InactiveUS20110077397A1Simple and efficient and cost-effective processShorten the timeOrganic chemistryOrganic solventPhotochemistry
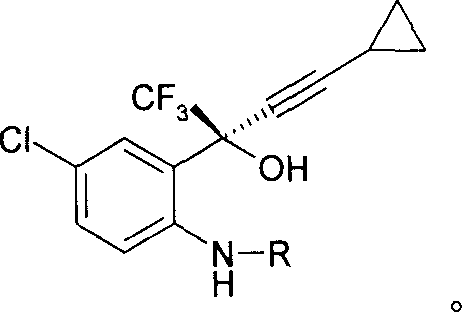
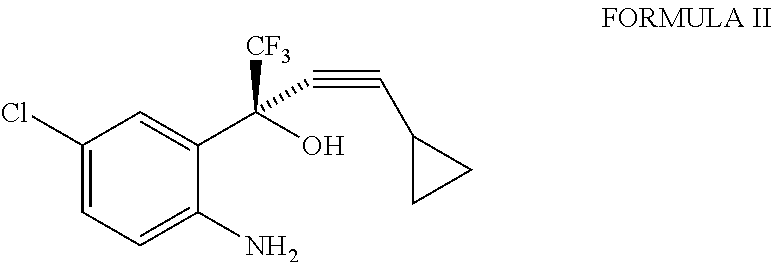
A simple, cost-effective process for preparation of efavirenz of formula (I) comprising reacting a solution of 5-chloro-α-(cyclopropylethynyl)-2-amino-α-trifluoromethyl) benzene methanol of formula (II) in an organic solvent with triphosgene in the presence of an inorganic base at a temperature range −5° C. to 25° C., adding water and isolating compound of formula (I).
Owner:EMCURE PHARAMACEUTICALS LTD
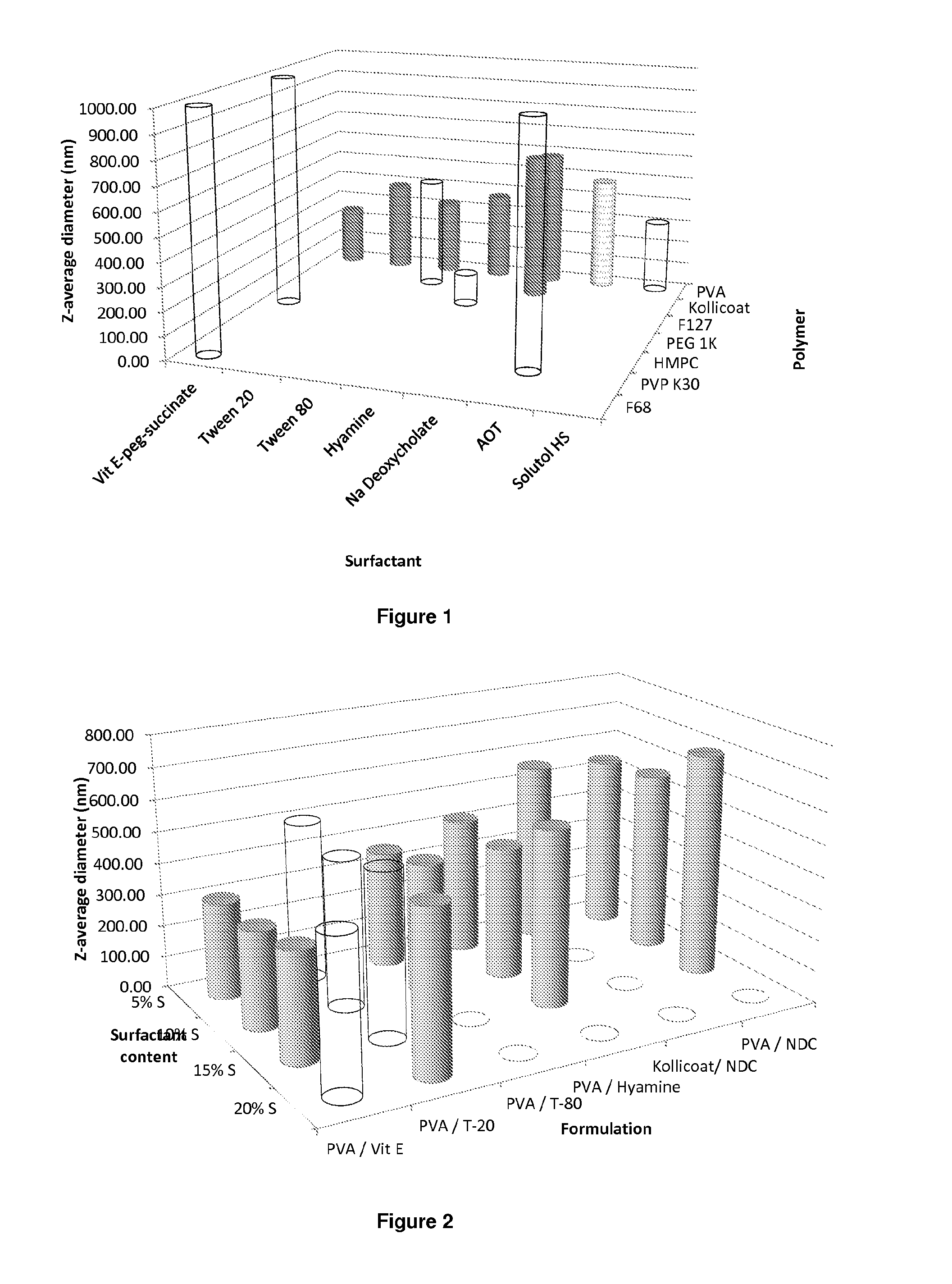
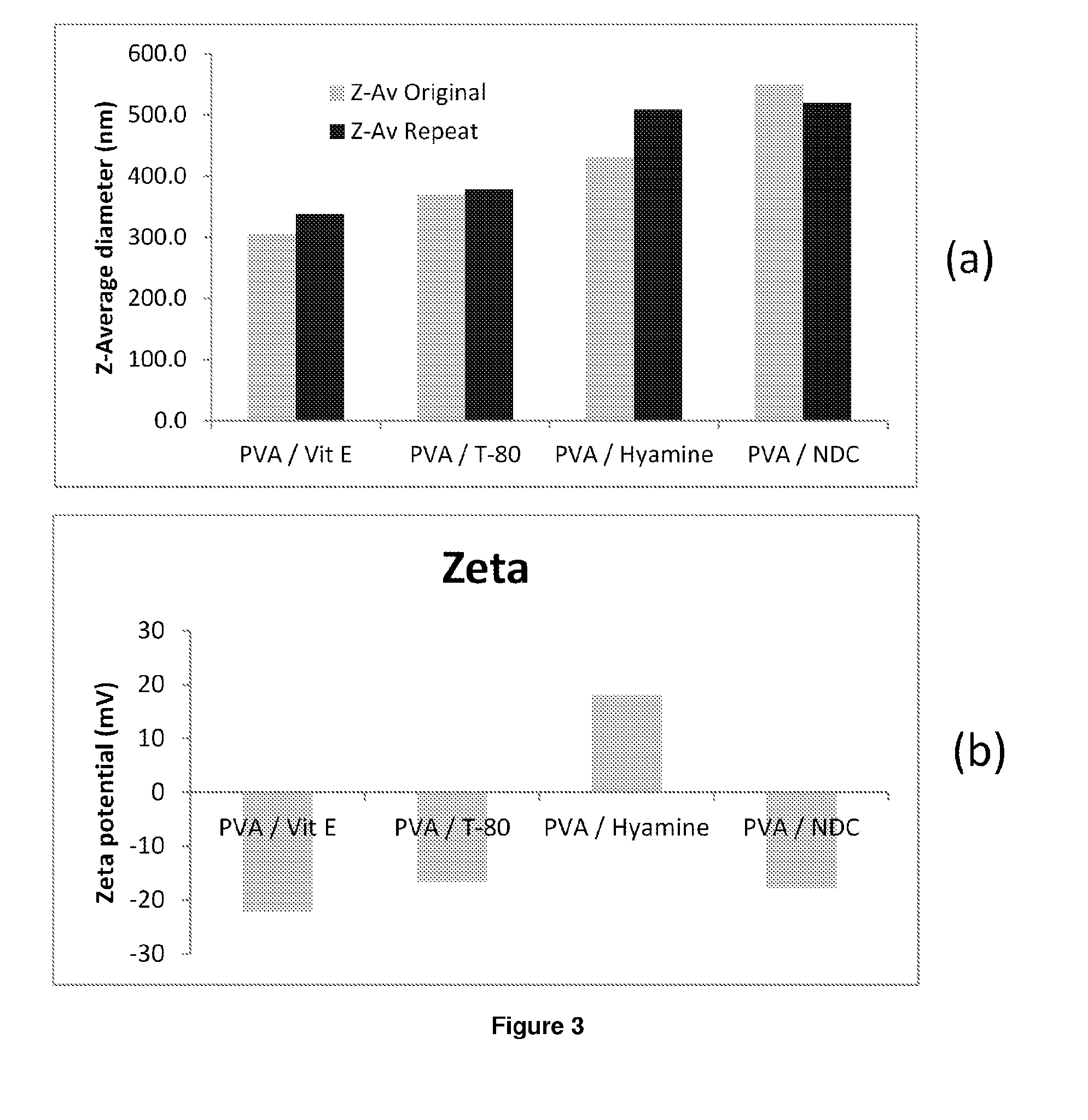
Compositions of efavirenz
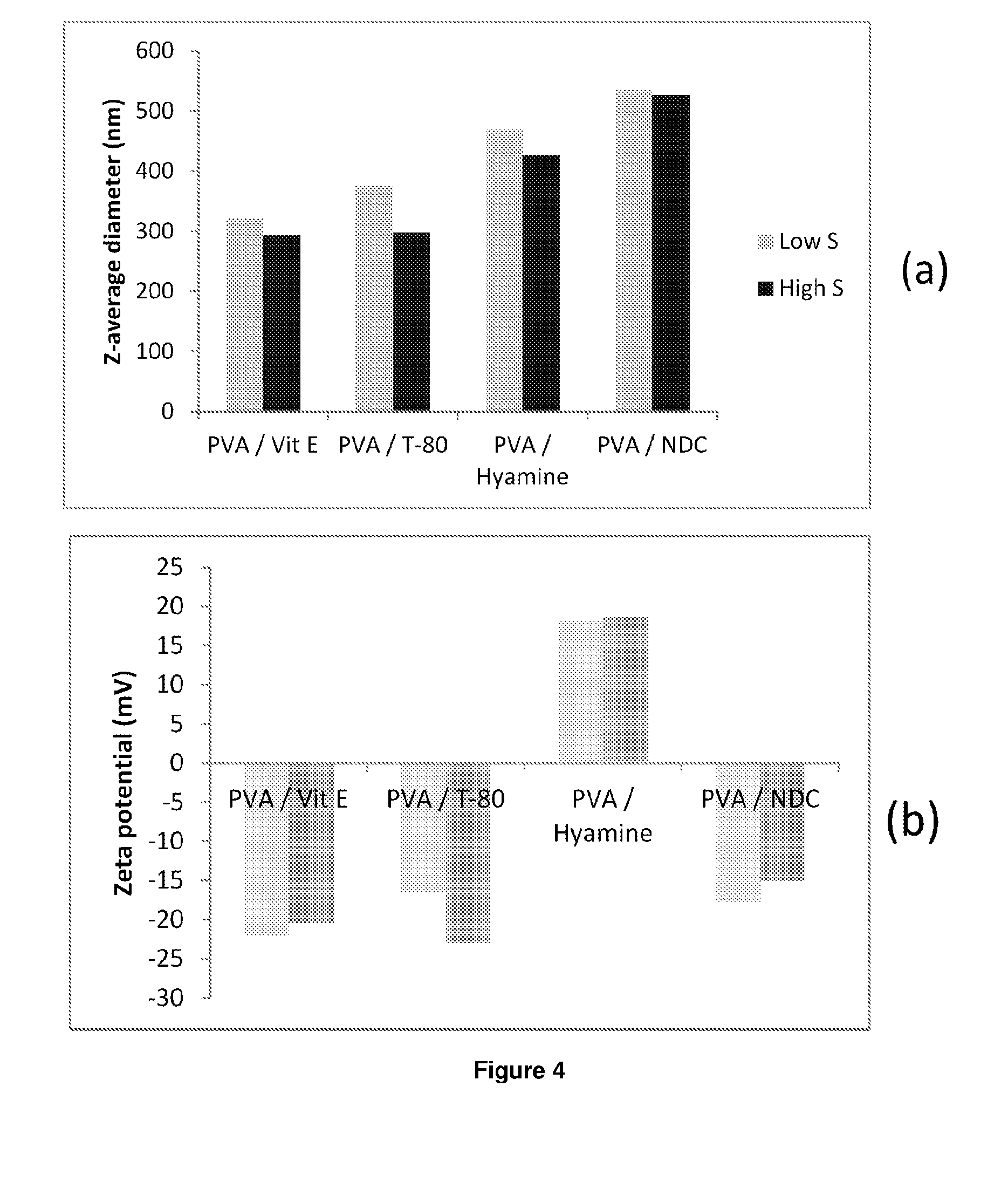
ActiveUS20140220140A1Improve distributionEffective treatmentOrganic active ingredientsPowder deliveryHydrophilic polymersRetroviral infection
The present inventions relates to a solid composition and an aqueous dispersion comprising nanoparticles of the anti-retroviral drug efavirenz. The solid composition and aqueous dispersion additionally comprise a mixture of a hydrophilic polymer and a surfactant. The surfactant is selected from vitamin-E-polyethylene glycol-succinate (Vit-E-PEG-succinate), a polyoxyethylene sorbitan fatty acid ester, N-alkyldimethylbenzylammonium chloride, sodium deoxycholate, dioctyl sodium sulfosuccinate, polyethyleneglycol-12-hydroxystearate, polyvinyl alcohol (PVA), and a block copolymer of polyoxyethylene and polyoxypropylene, or a combination thereof. The hydrophilic polymer is suitably selected from polyvinyl alcohol (PVA), a polyvinyl alcohol-polyethylene glycol graft copolymer, a block copolymer of polyoxyethylene and polyoxypropylene, polyethylene glycol, hydroxypropyl methyl cellulose (HPMC), and polyvinylpyrrolidone, or a combination thereof. The present invention also relates to processes for preparing both the solid composition and the aqueous dispersion, as well as to their use in therapy for the treatment and / or prevention of retroviral infections such as human immunodeficiency virus (HIV).
Owner:UNIV OF LIVERPOOL
One-pot asymmetric synthetic process of HIV (Human Immunodeficiency Virus) reverse transcriptase inhibitor efavirenz compound
ActiveCN103664816AMild process conditionsSimple and fast operationOrganic chemistryBulk chemical productionEnantioselective synthesisHuman immunodeficiency
The invention relates to a novel one-pot asymmetric synthetic process of (S)-6-chloro-4-cyclopropyl acetenyl-4-trifluoromethyl-1, 4-dihydro-2H-1, 3-benzoxazine-2-one (Efavirenz) compounds. The invention is the divisional application of a one-pot asymmetric synthetic process of HIV (Human Immunodeficiency Virus) reverse transcriptase inhibitor efavirenz compounds, wherein the application number is 201110001621.8 and the application date is 6th, January, 2011. The compounds can be used as the reverse transcriptase inhibitor of HIV. The invention further relates to a novel alkamine ligand for the process.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Wet granulation of tenofovir, emtricitabine and efavirenz
The present invention describes a method and composition for a pharmaceutical product based on Tenofovir disoproxil hemifumarate, Emtricitabine and Efavirenz. The composition can be prepared by a process comprising a wet granulation step to produce a stable dosage form suitable for the treatment of HIV in essential absence of known degradation products.
Owner:ULTIMORPHIX TECH
Efavirenz preparation method
The invention relates to an efavirenz preparation method. The method concretely comprises the following steps: 1, washing a separated chloroform layer by using brine, drying, and concentrating; 2, separating out an organic layer, washing the organic layer by using brine, drying, and concentrating to obtain 2-trifluoroacetyl-4-chloroaniline; and 3, adding 1mol / L of hydrochloric acid into n-butanol, reacting at 60DEG C for 72h, and splitting to obtain optically active efavirenz. The efavirenz preparation method has the advantages of simple preparation, high output and low cost.
Owner:储海燕
Efavirenz preparation adopting micronization technology
InactiveCN102872019AReduce usageImprove solubilityOrganic active ingredientsAntiviralsAcquired immunodeficiencyImmunodeficiency virus
The invention discloses an efavirenz preparation adopting a micronization technology. The invention provides a modified efavirenz orally-taken preparation which is used for inhibiting human immunodeficiency virus (HIV), preventing or treating HIV infection, and treating acquired immunodeficiency syndrome (AIDS) caused by the HIV infection, and in particular relates to tablets or a capsule which comprises efavirenz, wherein a micronization technology which can promote the dissolution velocity of the efavirenz in gastrointestinal tract, so that the absorption velocity and the absorption extent of the efavirenz in bodies is involved.
Owner:FUKANGREN BIO PHARMA
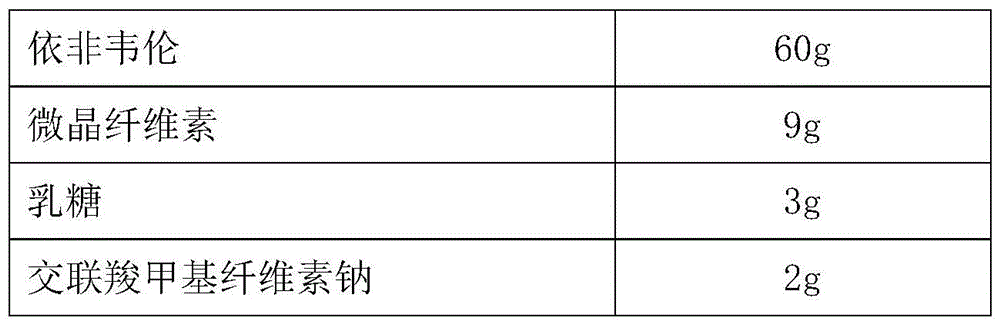
Efavirenz tablet and preparation method thereof
ActiveCN102988316APromote absorptionOrganic active ingredientsAntiviralsMethyl celluloseCroscarmellose sodium
The invention discloses an efavirenz tablet which is prepared from the following raw materials in parts by weight: 65-75 parts of efavirenz, 10-15 parts of croscarmellose sodium, 7-9 parts of microcrystalline cellulose and 7-12 parts of lauryl sodium sulfate, hydroxy propyl cellulose, magnesium stearate and hydroxypropyl methyl cellulose in total. The dispersibility of the efavirenz tablet in the intestinal tract is improved and the dissolution speed of efavirenz is improved by increasing the using amount of the croscarmellose sodium, and therefore the efavirenz can be rapidly absorbed and utilized.
Owner:ANHUI BIOCHEM BIO PHARMA
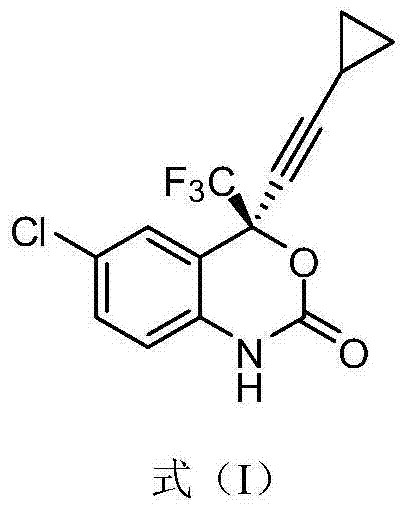
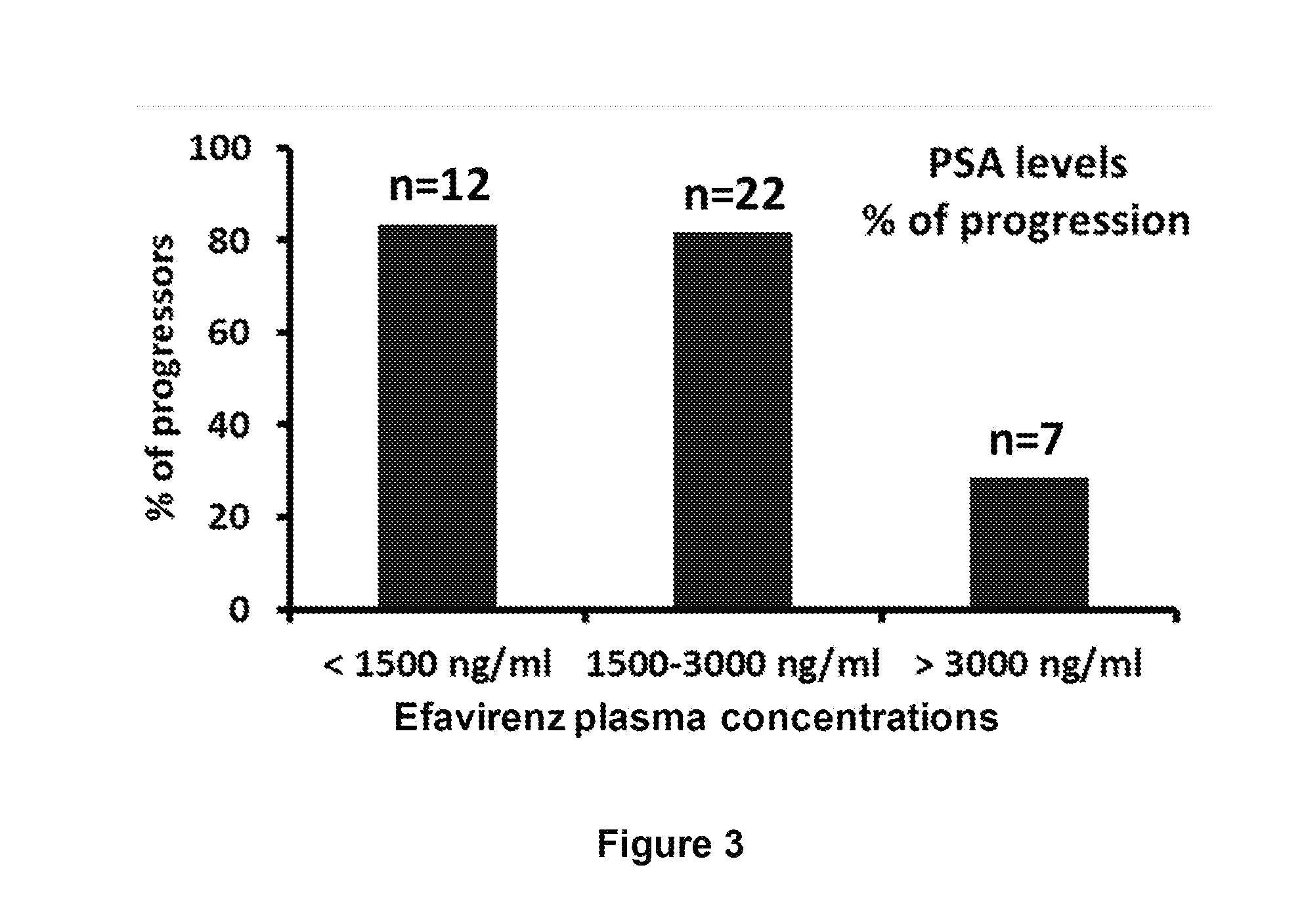
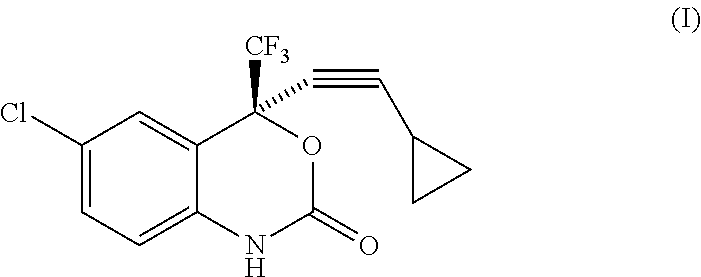
Increased Dosage of Efavirenz for the Treatment of Cancer
The invention is based on the finding that an increased daily dose of Efavirenz should be used for the treatment of cancer compared to the daily dose used in the treatment of AIDS. The invention thus relates to the use of Efavirenz for preparing a medicament intended for the treatment of cancer, wherein Efavirenz is administered at a dose generating an Efavirenz plasma concentration in said patient superior to 3000 ng / ml. It further relates to the use of Efavirenz for preparing a medicament intended for the treatment of cancer, wherein Efavirenz is administered at a daily dose of at least 1800 mg, in particular between 1800 and 2200 mg. It also relates to the use of Efavirenz for preparing a medicament intended for the treatment of cancer, in which the daily dose of Efavirenz is optimized after administration of a preliminary daily dose and measure or Efavirenz plasma concentration. It also relates to a unique dosage form of Efavirenz, comprising Efavirenz in an amount generating an Efavirenz plasma concentration in said patient superior to 3000 ng / ml, and a pharmaceutically acceptable carrier.
Owner:ALIENOR FARMA
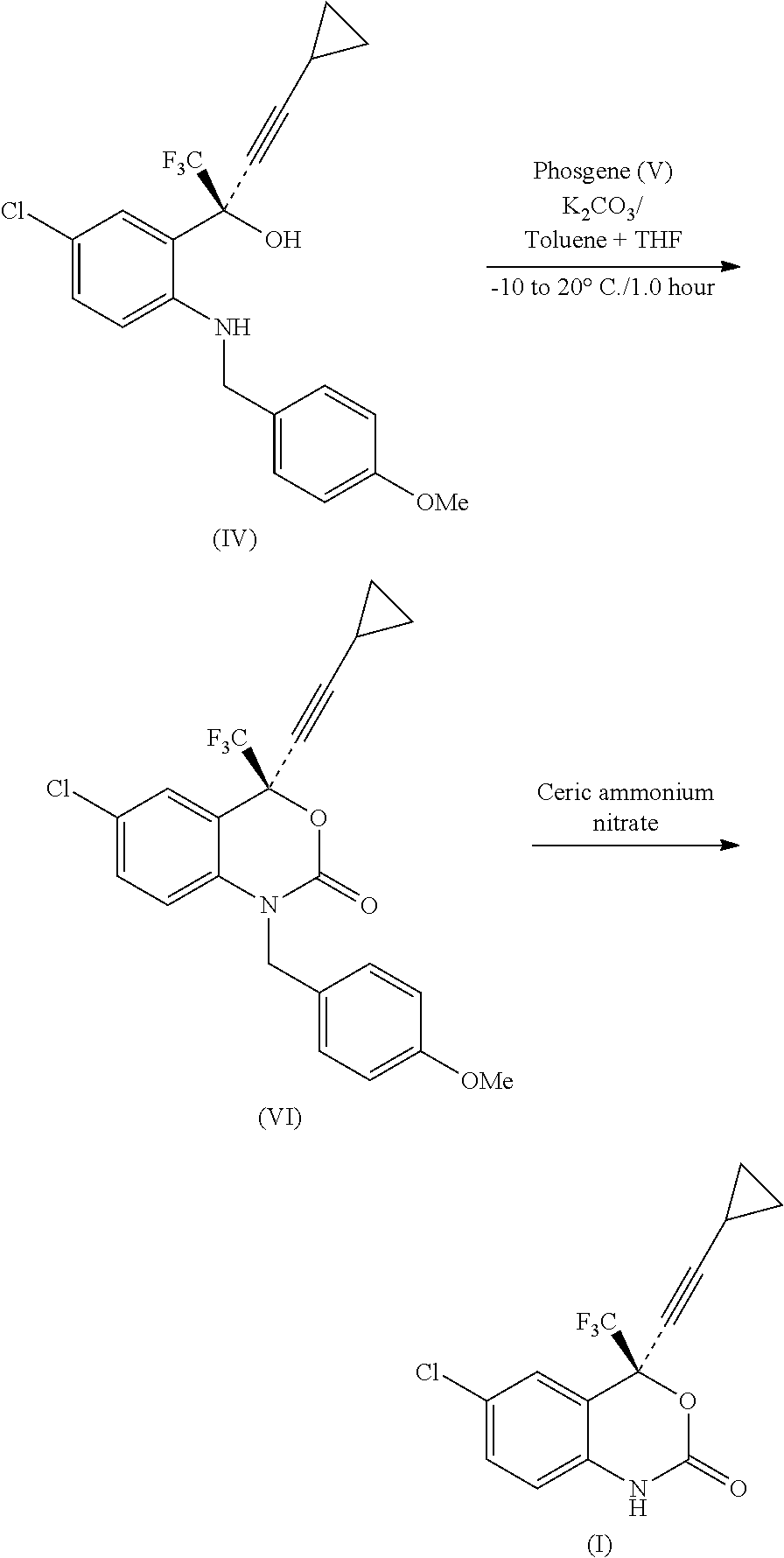
Process for the preparation of efavirenz
InactiveUS20110071287A1Minimizes by-product formationHigh chemical purityOrganic chemistryMedicinal chemistryEfavirenz
The present invention relates to a process for the preparation of Efavirenz (Formula I), wherein triphosgene is used as a cyclizing agent.
Owner:RANBAXY LAB LTD
Formulation of fast-dissolving efavirenz capsules or tablets using super-disintegrants
InactiveCN1296412AOrganic active ingredientsAntiviralsAcquired immunodeficiencyImmunodeficiency virus
The present invention provides improved oral dosage form formulations of efavirenz that are useful in the inhibition of human immunodeficiency virus (HIV), the prevention or treatment of infection by HIV, and in the treatment of the resulting acquired immune deficiency syndrome (AIDS). In particular, the present invention relates to compressed tablets or capsules comprising efavirenz that contain one or more disintegrants that enhance the dissolution rate of the efavirenz in the gastrointestinal tract thereby improving the rate and extent of absorption of efavirenz in the body. The present invention also relates to the process of making such tablets or capsules.
Owner:BRISTOL MYERS SQUIBB PHARMA CO
Tenofovir, lamivudine and efavirenz triple compound pellets and preparation method thereof
The invention discloses a tri-combination compound mini-pill tablet co-prepared by disoproxil fumarate DF coated mini-pills, lamivudine coated mini-pills and efavirenz mini-pills and a preparation method thereof. The invention solves the problems that effective component degradation and dissolution delaying phenomena are generated due to interaction among the tri-combination compound components, tabletting is not facilitated during preparation and patient swallowing is not facilitated; and the single-layer tablet having the three component drugs without mutual contact is prepared by a mini-pill tabletting method, so as to obtain good stability and fast dissolving rate.
Owner:ANHUI BIOCHEM BIO PHARMA
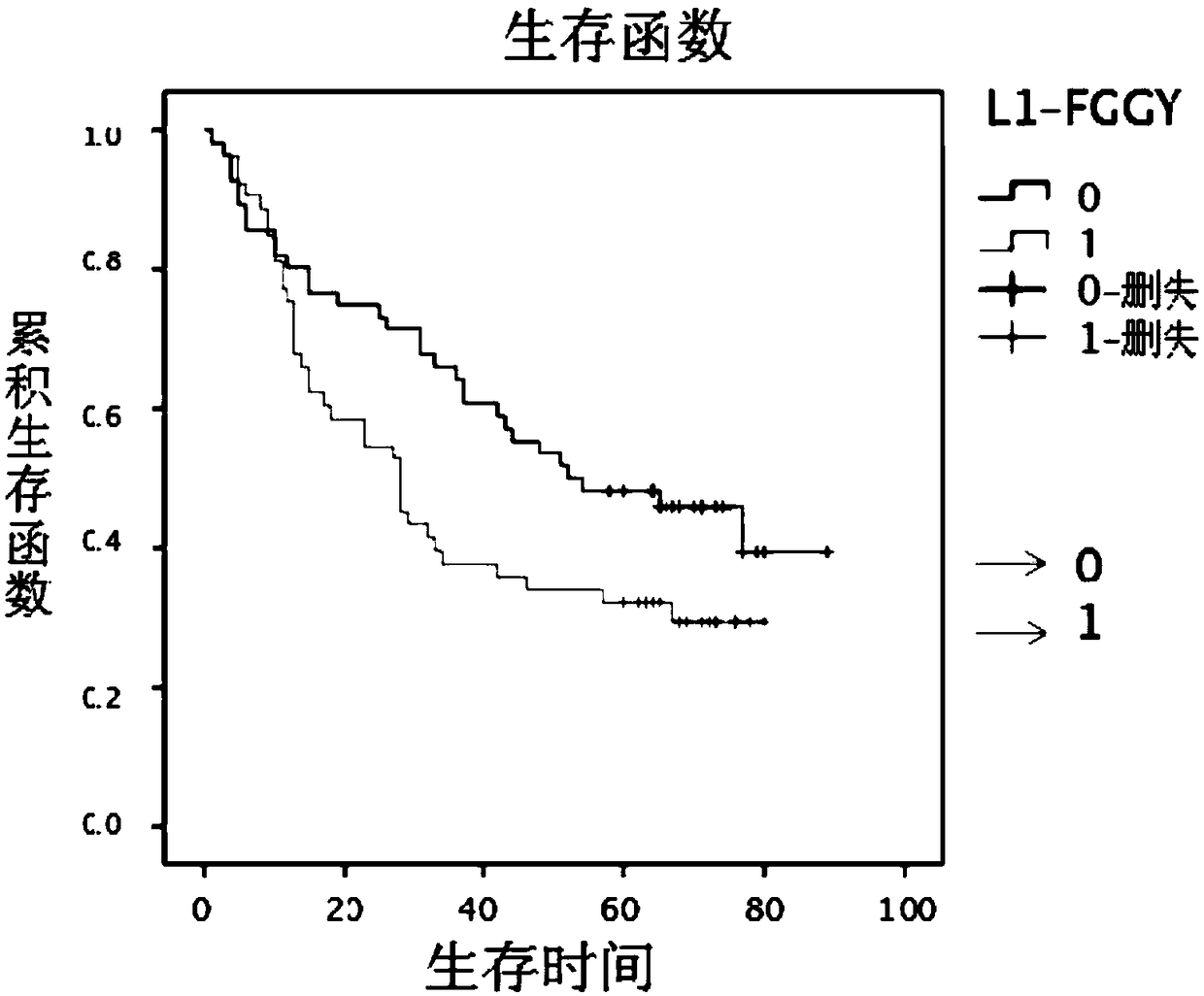
Retrotransposition gene L1-FGGY and application thereof as marker of lung squamous cell carcinoma
ActiveCN108796079AIncreased proliferationIncrease aggressivenessOrganic active ingredientsMicrobiological testing/measurementLung squamous cell carcinomaNucleic acid sequencing
The invention discloses retrotransposition gene L1-FGGY and application thereof as a marker of lung squamous cell carcinoma. The nucleic acid sequence of the retrotransposition gene L1-FGGY is SEQ IDNO. 1; the nucleic acid sequence of an upstream detection primer of the retrotransposition gene L1-FGGY is SEQ ID NO. 2; and the nucleic acid sequence of a downstream detection primer is SEQ ID NO. 3.A retrotransfer inhibitor nevirapine or efavirenz can inhibit the expression level of the retrotransposition gene L1-FGGY for treatment of lung squamous cell carcinoma. The retrotransposition gene L1-FGGY disclosed can be used as a new tumor marker, and the detection of L1-FGGY can be used for early diagnosis, molecular typing and prognosis evaluation of lung squamous cell carcinoma, and L1-FGGYcan also become a potential therapeutic target for clinical treatment of lung squamous cell carcinoma and has broad application prospects.
Owner:TIANJIN MEDICAL UNIV CANCER INST & HOSPITAL
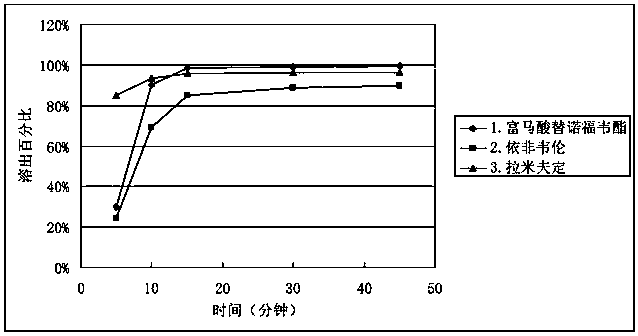
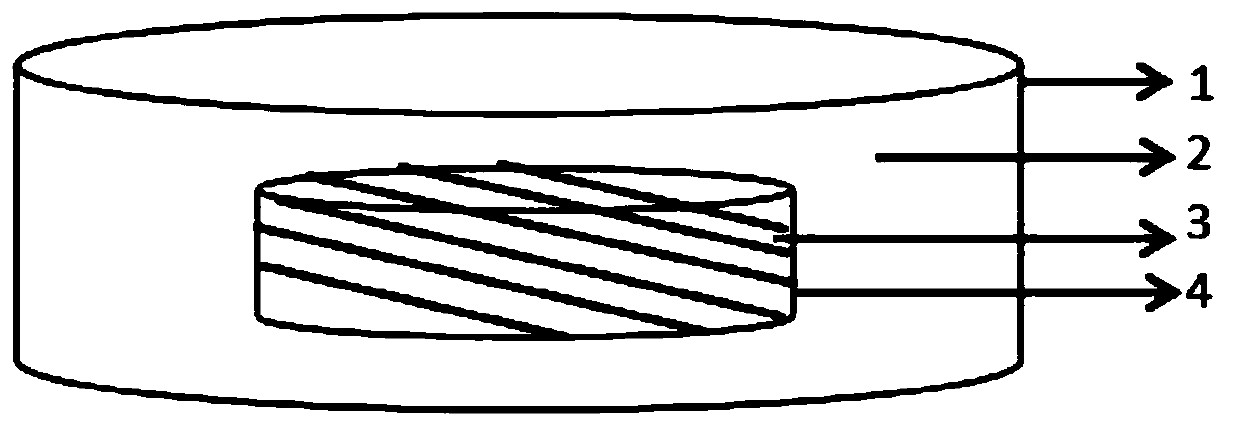
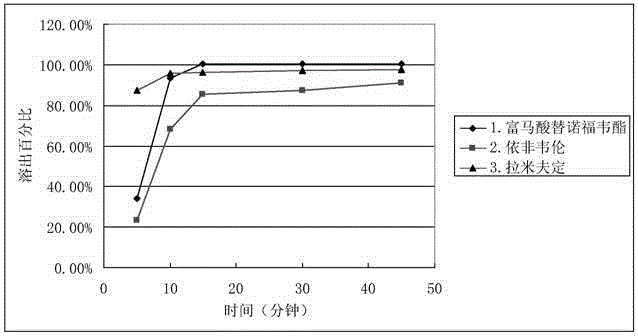
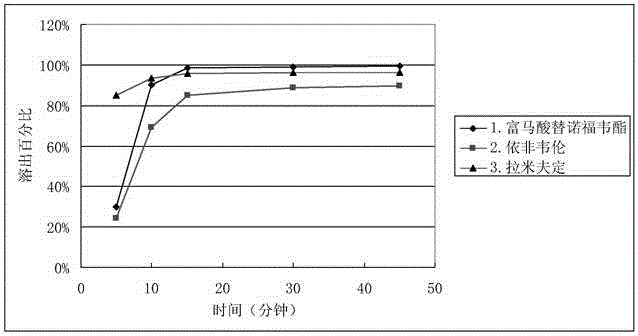
Efavirenz, lamivudine and tenofovir disoproxil fumarate triple compound tablet middle tablet and preparation method thereof
ActiveCN106822155BGood dissolution effectImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismActive ingredientCrude drug
The invention relates to a middle tablet of a triple compound tablet composed of tenofovir disoproxil fumarate, efavirenz and lamivudine in the field of pharmaceutical preparations and a preparation method thereof. The tablet-in-tablet contains a coated tablet core of tenofovir disoproxil fumarate, in which the core is contained by efavirenz, lamivudine and auxiliary materials. The invention solves the problems of degradation and slow dissolution caused by contacting tenofovir disoproxil fumarate with other two raw materials. The tablet-in-tablet preparation method adopted in the present invention can effectively avoid the degradation of tenofovir disoproxil fumarate due to contact with efavirenz and lamivudine, thereby obtaining a good product stability.
Owner:NORTHEAST PHARMA GRP
Efavirenz, lamivudine and tenofovir disoproxil fumarate triplex compound tablet in tablet and preparation method thereof
ActiveCN106822155AGood dissolution effectImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismActive ingredientCrude drug
The invention relates to the field of medicine preparations, and provides a tenofovir disoproxil fumarate, efavirenz and lamivudine triplex compound tablet in tablet and a preparation method thereof. The tablet in tablet comprises a tenofovir disoproxil fumarate coating tablet core, and the tablet core is coated by the efavirenz, the lamivudine and auxiliary materials. The preparation method of the tablet in tablet has the advantages that the problem of degrading and slow dissolving caused by contact with the tenofovir disoproxil fumarate and other two crude drugs is solved; the degrading of the tenofovir disoproxil fumarate caused by contact of the tenofovir disoproxil fumarate with the efavirenz and the lamivudine is effectively avoided, and the good product stability is obtained.
Owner:NORTHEAST PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com