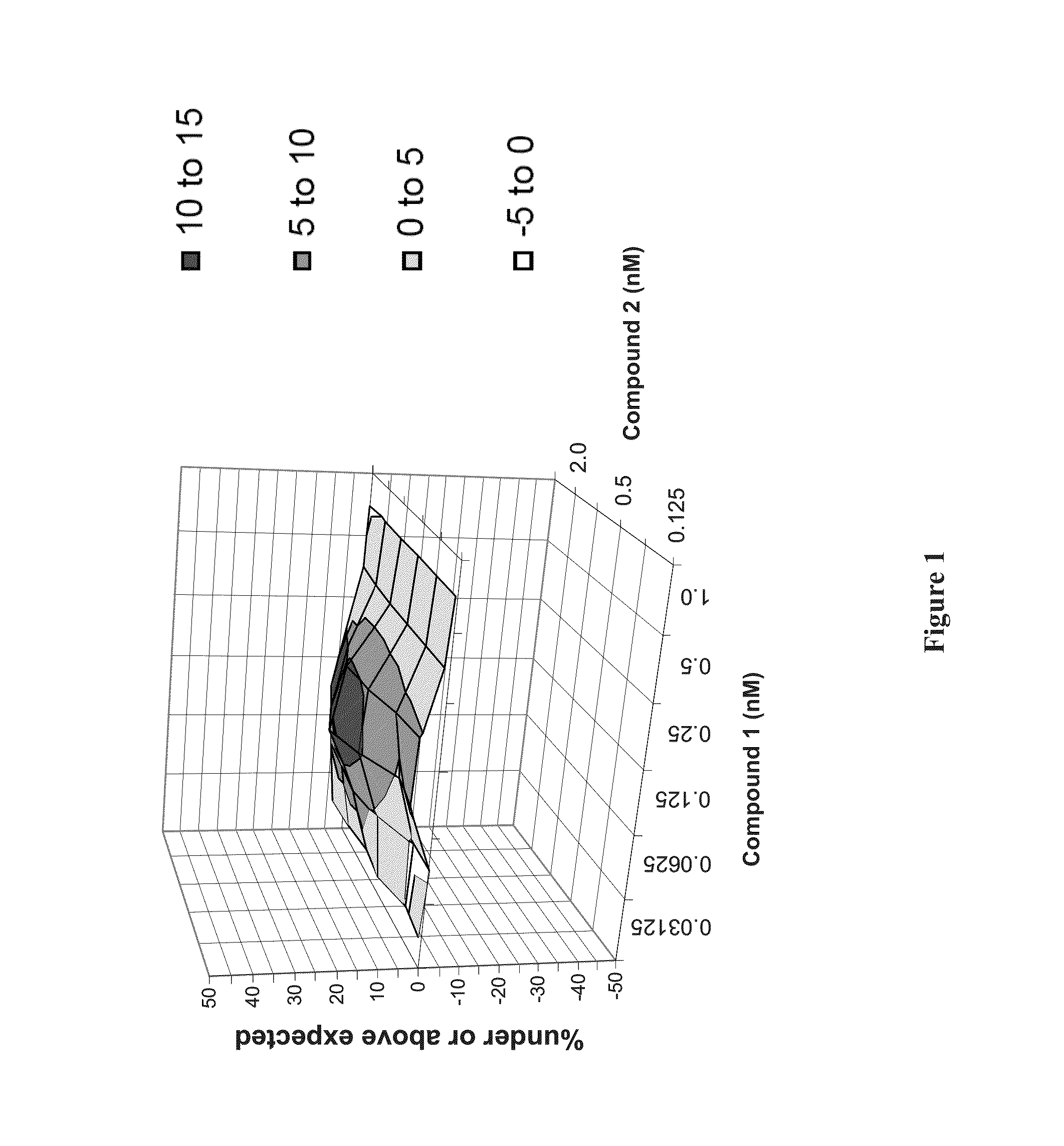
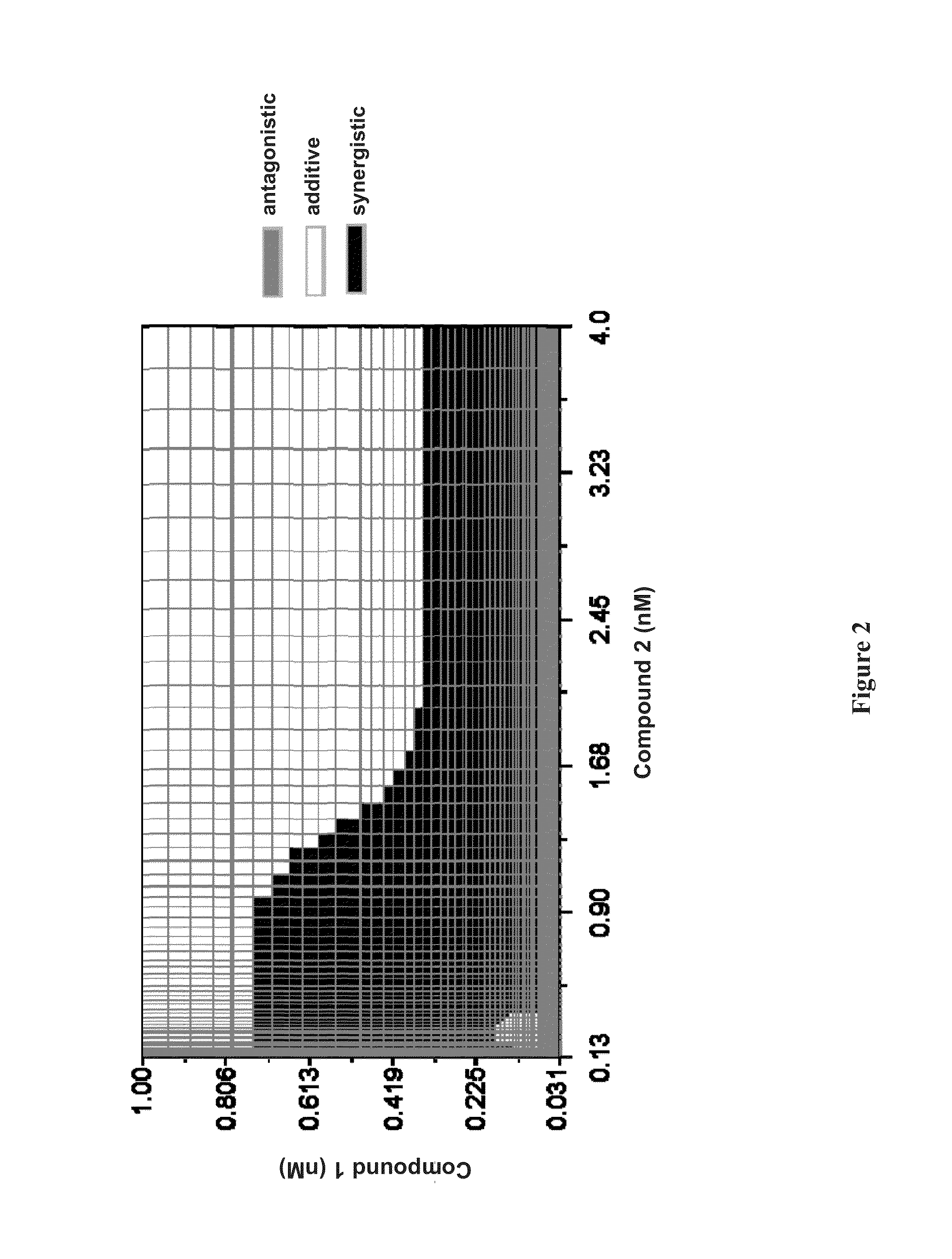
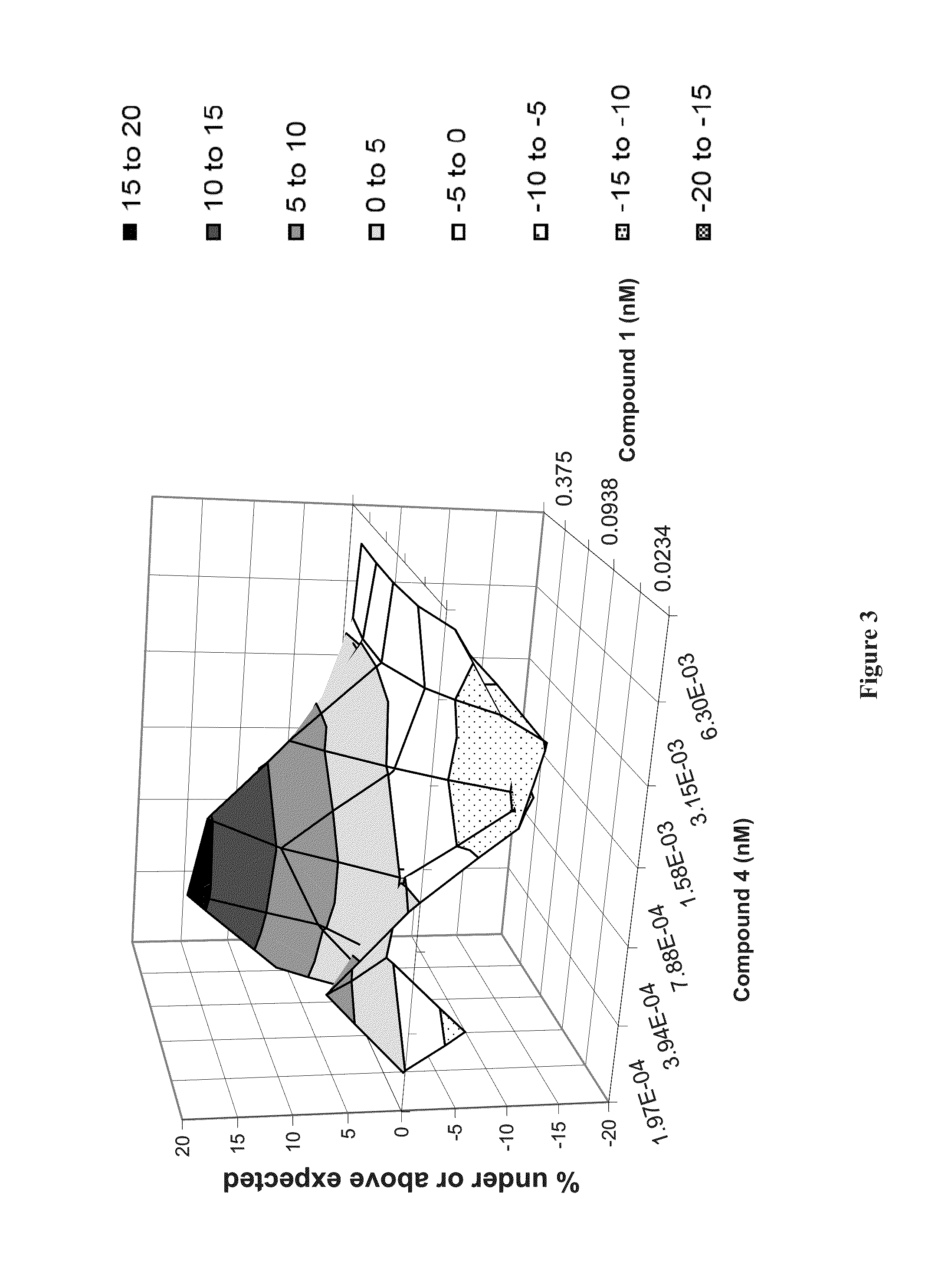
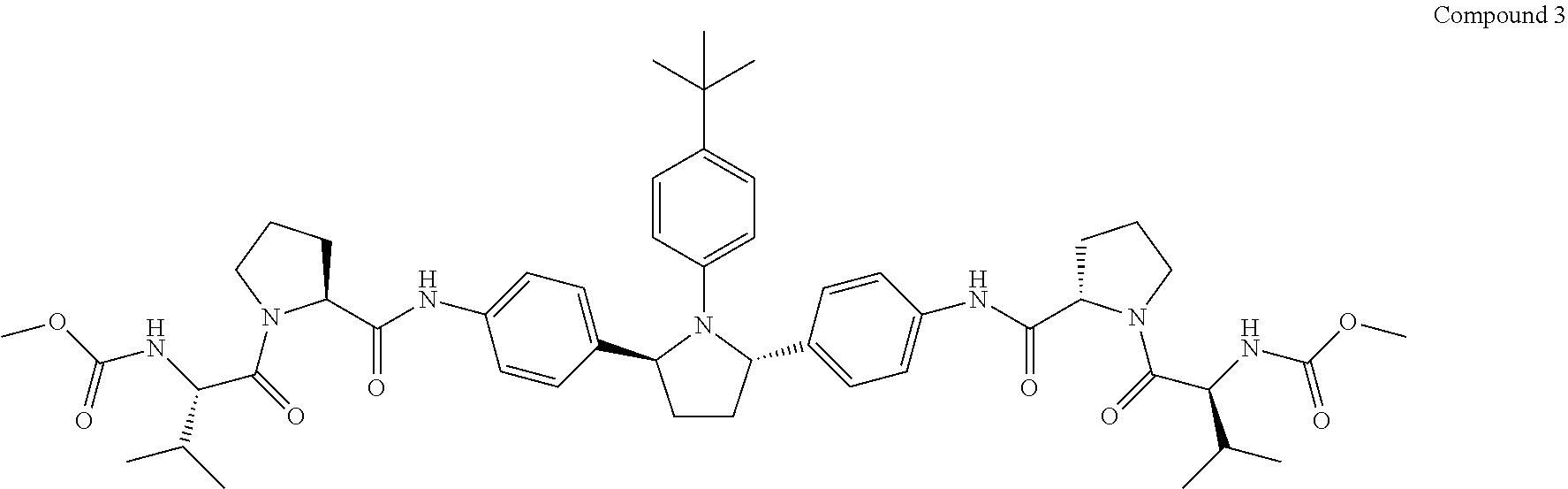
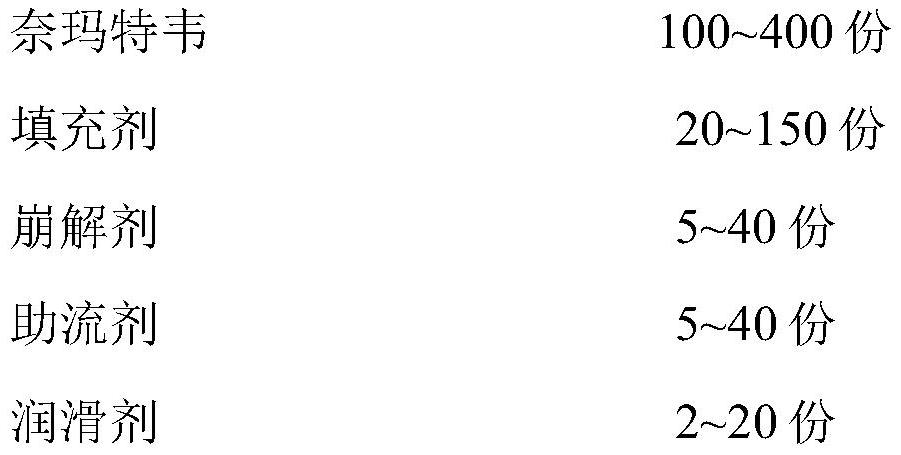Patents
Literature
58 results about "Ritonavir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This drug is used with other HIV medications to help control HIV infection.
Methods for Treating HCV
ActiveUS20130102526A1Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Methods for Treating HCV
ActiveUS20130102525A1Avoid side effectsImprove pharmacokineticsBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
Methods for treating HCV
ActiveUS8466159B2Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
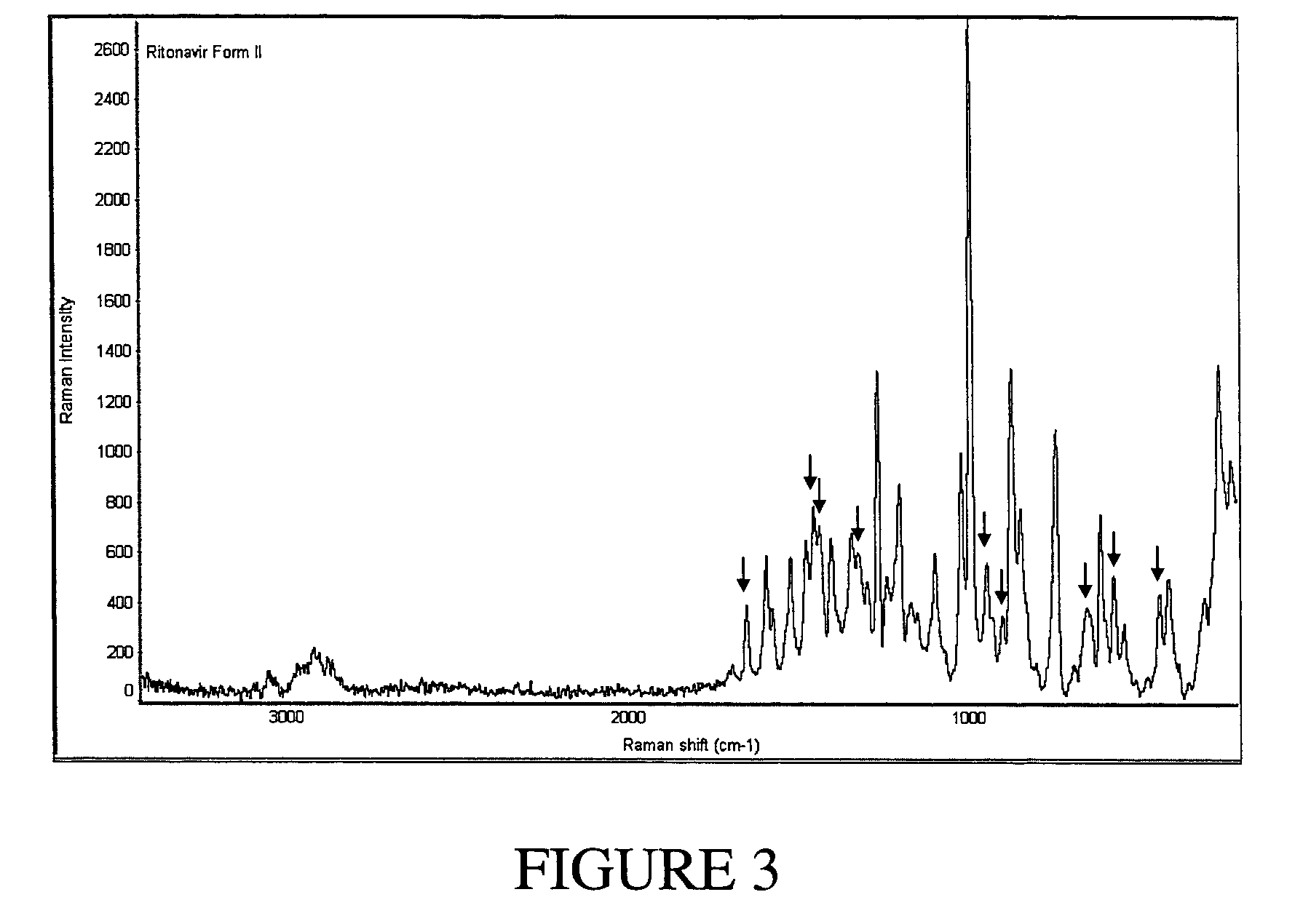
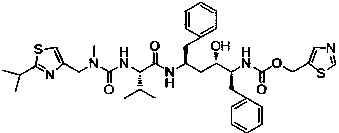
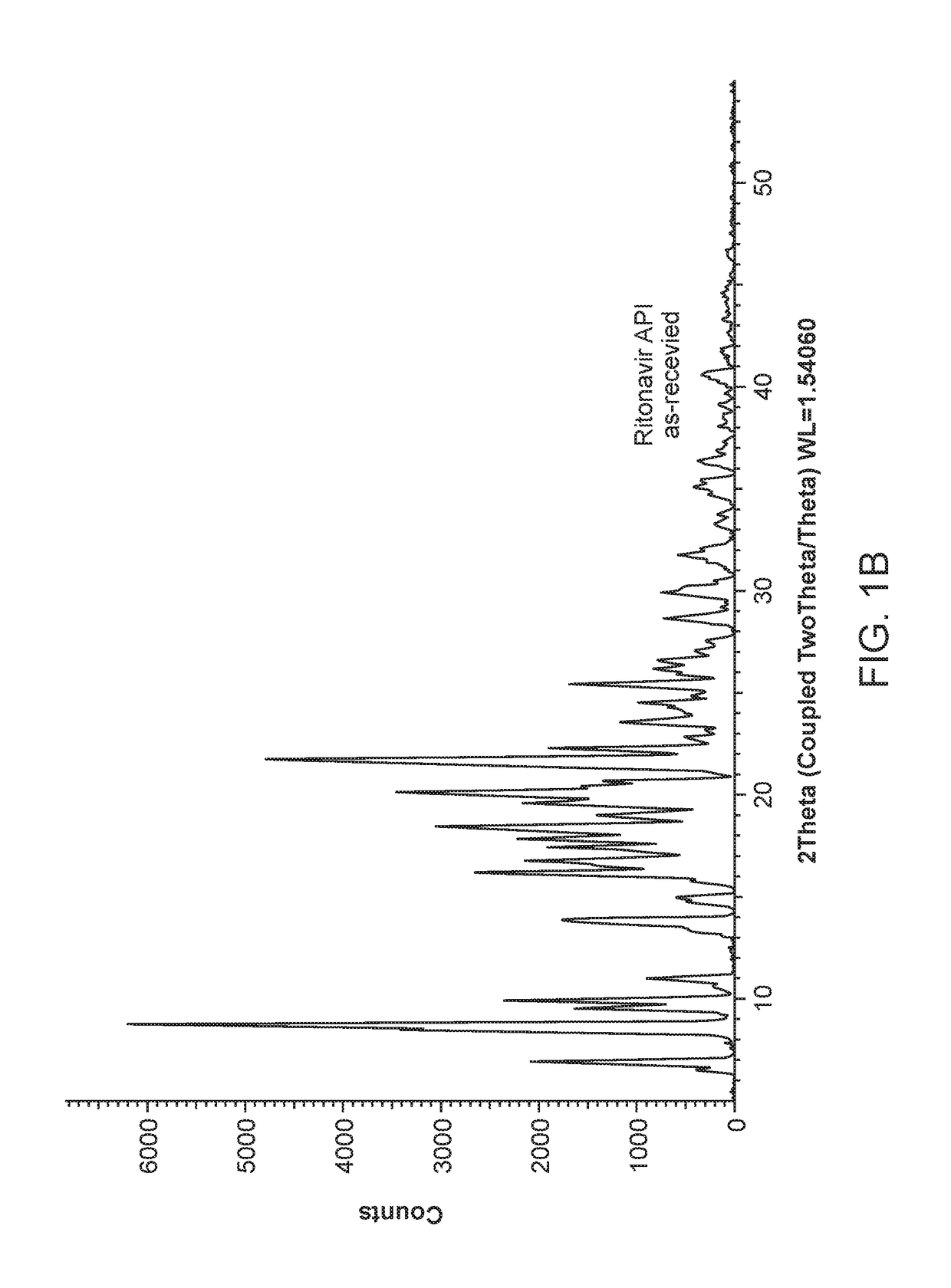
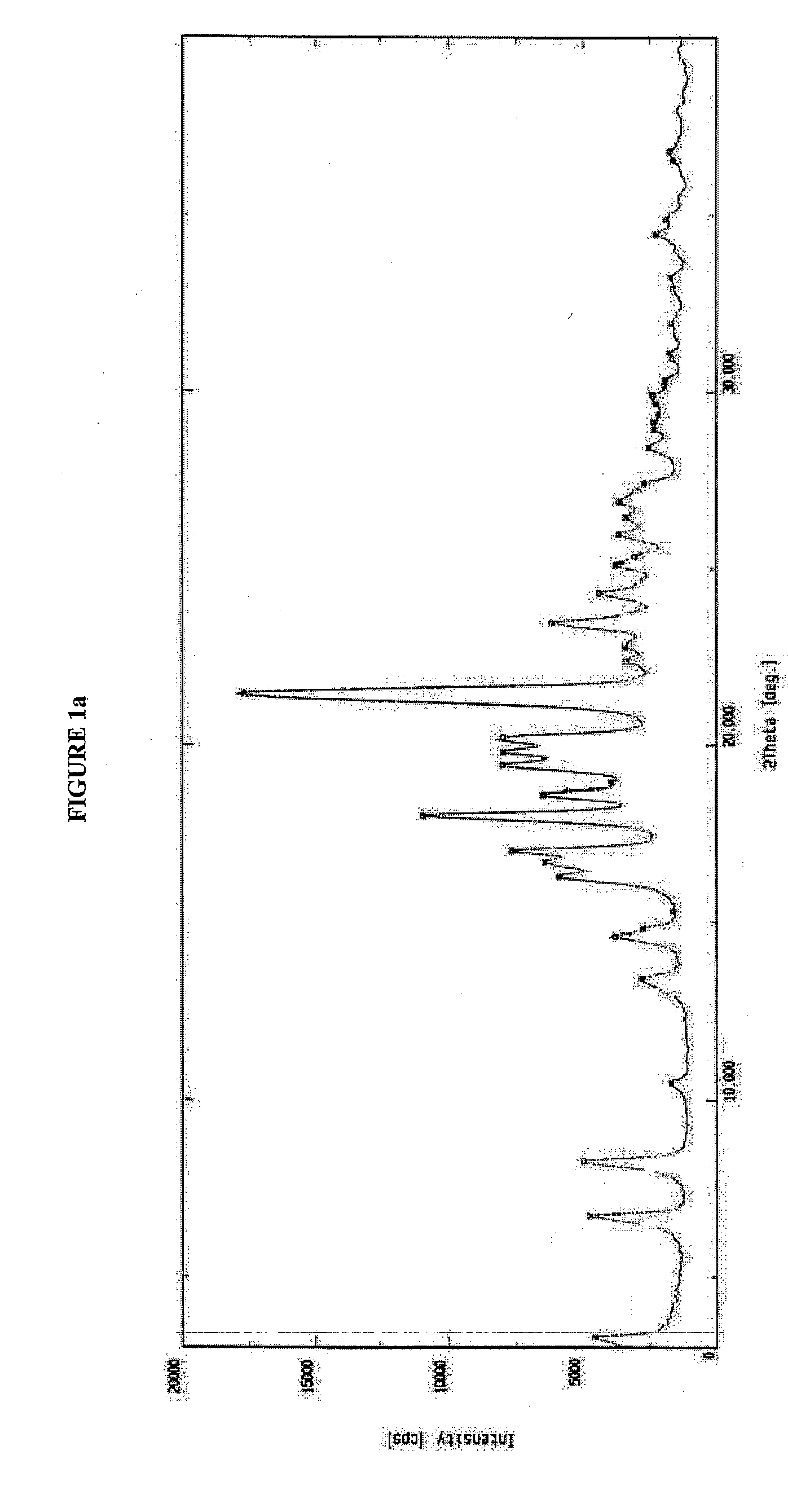
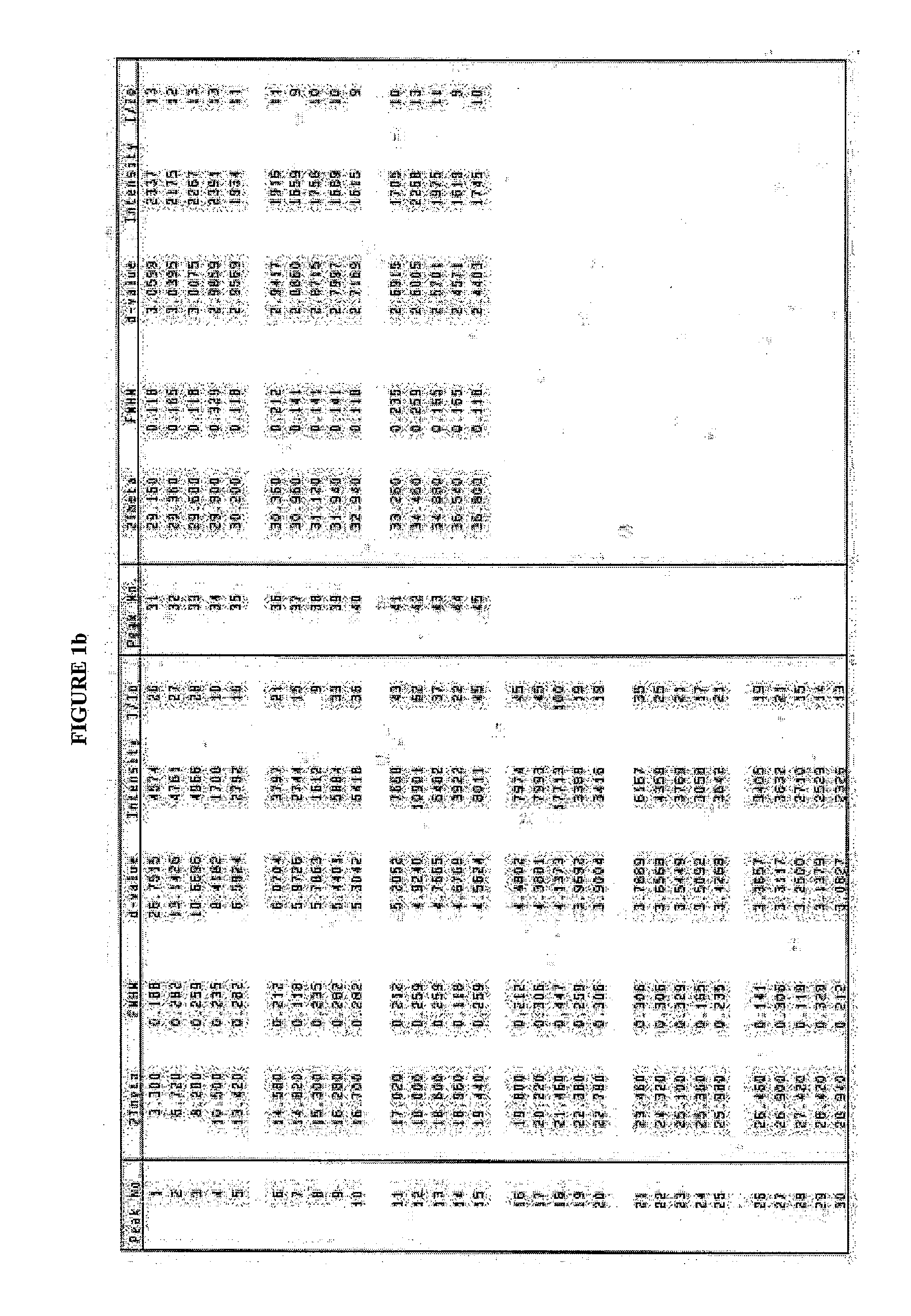
Solvates and polymorphs of ritonavir and methods of making and using the same
ActiveUS20040024031A1Improved pharmacokinetic profileOrganic active ingredientsBiocideDiseaseMedicinal chemistry
Novel solvates and crystal polymorphs of Ritonavir are disclosed, as well as methods of making them. Specific solvates of the compound include a formamide solvate and a partially desolvated solvate. Also disclosed are methods of making previously known forms of Ritonavir. Methods of using the novel forms of Ritonavir for the treatment of diseases, such as HIV-infection, are disclosed, as are pharmaceutical compositions and unit dosage forms comprising the novel forms of Ritonavir.
Owner:CENTOCOR ORTHO BIOTECH
Methods for treating HCV
ActiveUS8492386B2Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Tenofovir disoproxil hemi-fumaric acid co-crystal
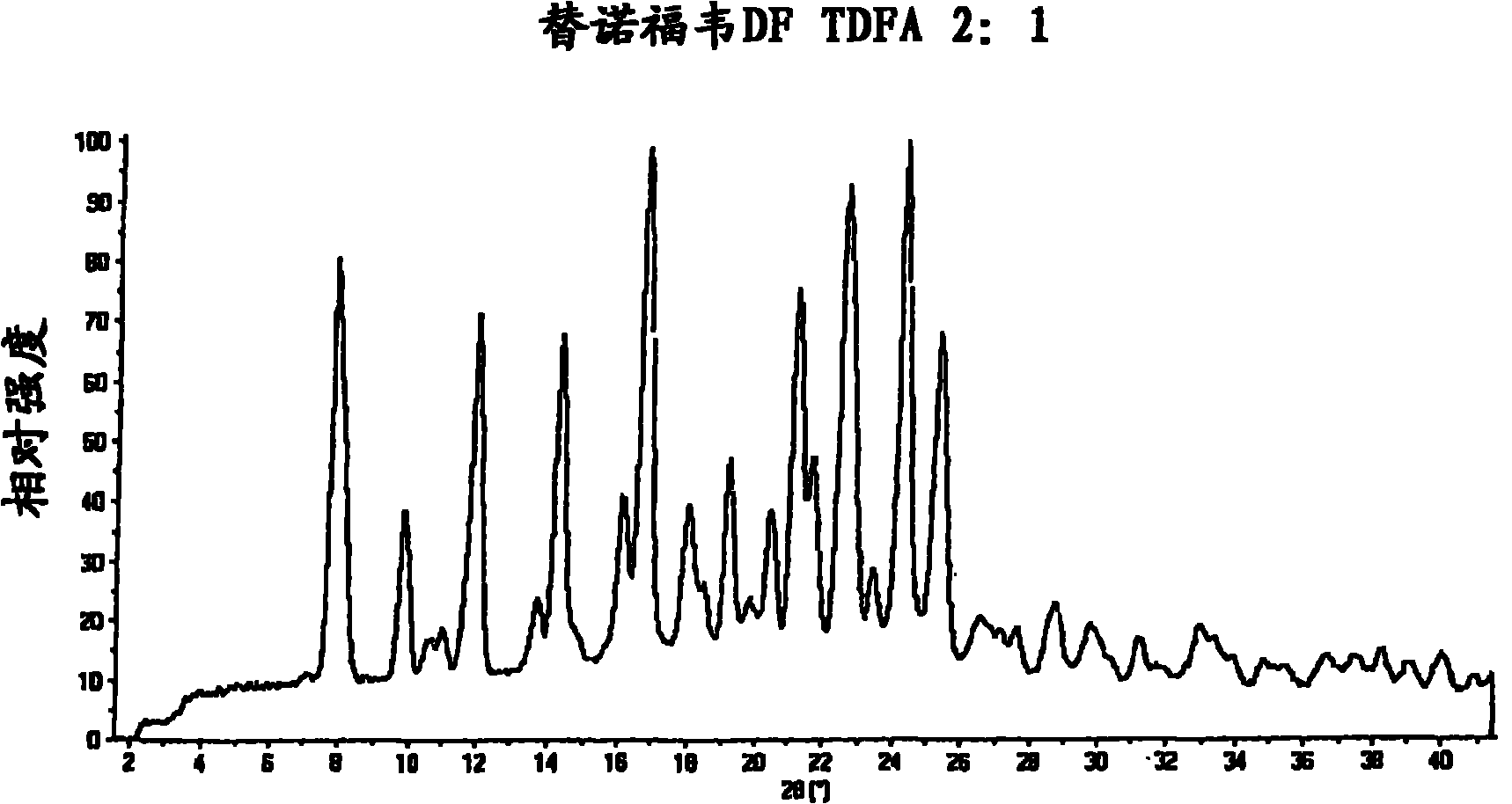
InactiveCN101778855AOrganic active ingredientsGroup 5/15 element organic compoundsEmtricitabineTenofovir DF
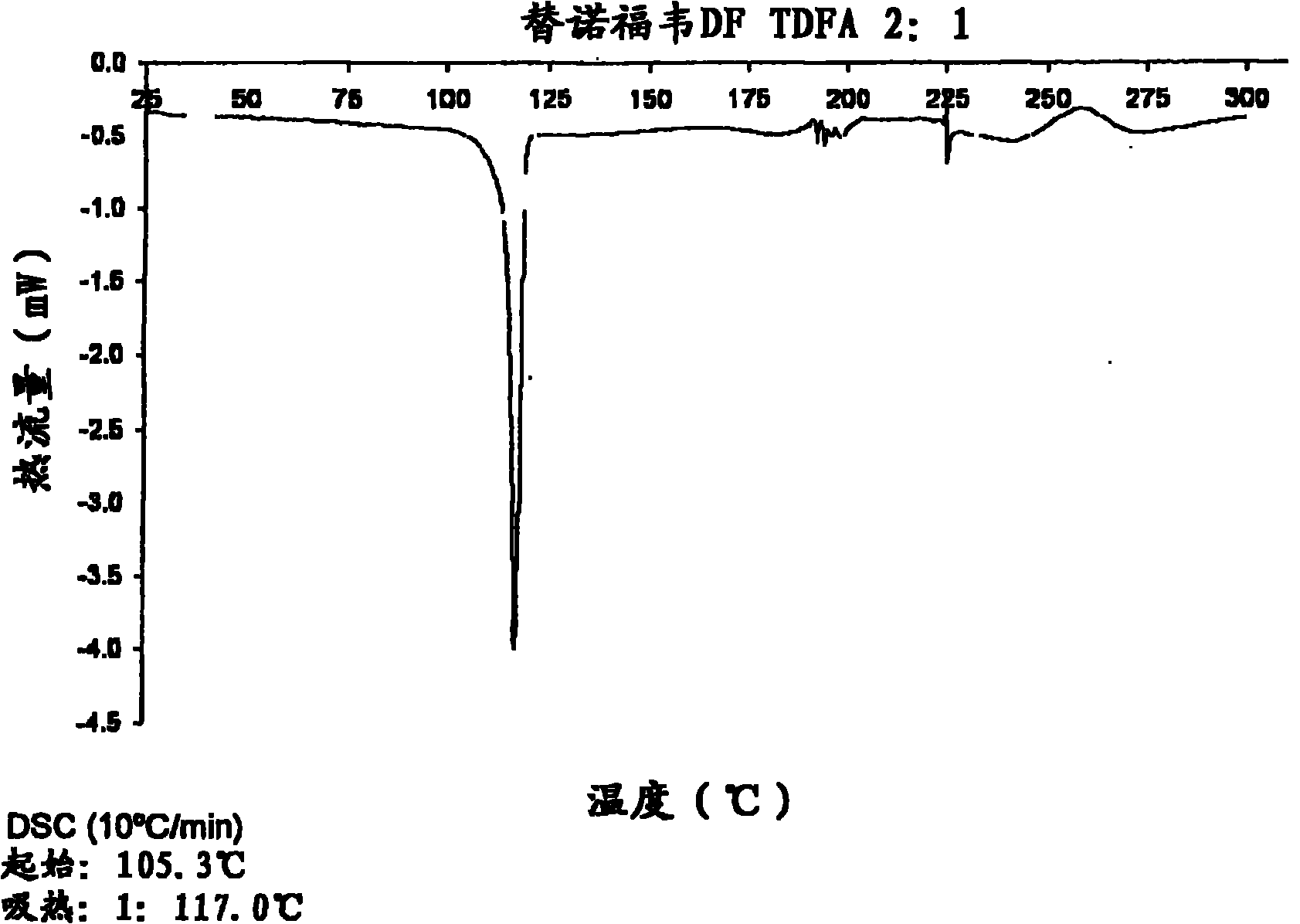
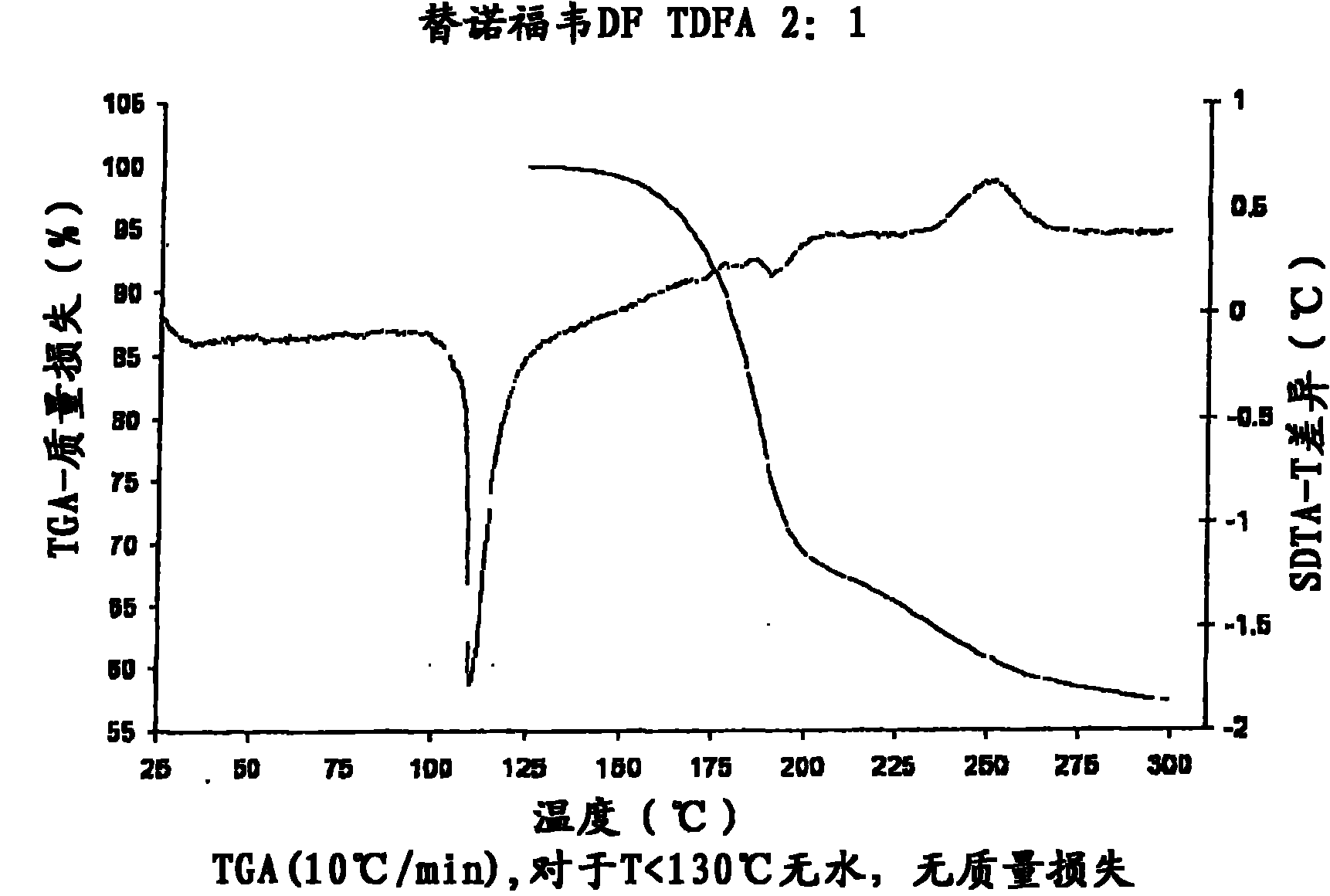
The present invention provides a novel crystalline form of Tenofovir disoproxil fumarate (Tenofovir DF), designated Co-crystal TDFA 2:1, methods for the preparation thereof and its use in pharmaceutical applications, in particular in anti-HIV medicaments. The crystalline form TDFA 2:1 can be used in combination with other anti-HIV medicaments such as Efavirenz, Emtricitabine, Ritonavir and / or TMC114.
Owner:ULTIMORPHIX TECH
Solvates and polymorphs of ritonavir and methods of making and using the same
Novel solvates and crystal polymorphs of Ritonavir are disclosed, as well as methods of making them. Specific solvates of the compound include a formamide solvate and a partially desolvated solvate. Also disclosed are methods of making previously known forms of Ritonavir. Methods of using the novel forms of Ritonavir for the treatment of diseases, such as HIV-infection, are disclosed, as are pharmaceutical compositions and unit dosage forms comprising the novel forms of Ritonavir.
Owner:CENTOCOR ORTHO BIOTECH INC
Methods for Treating HCV
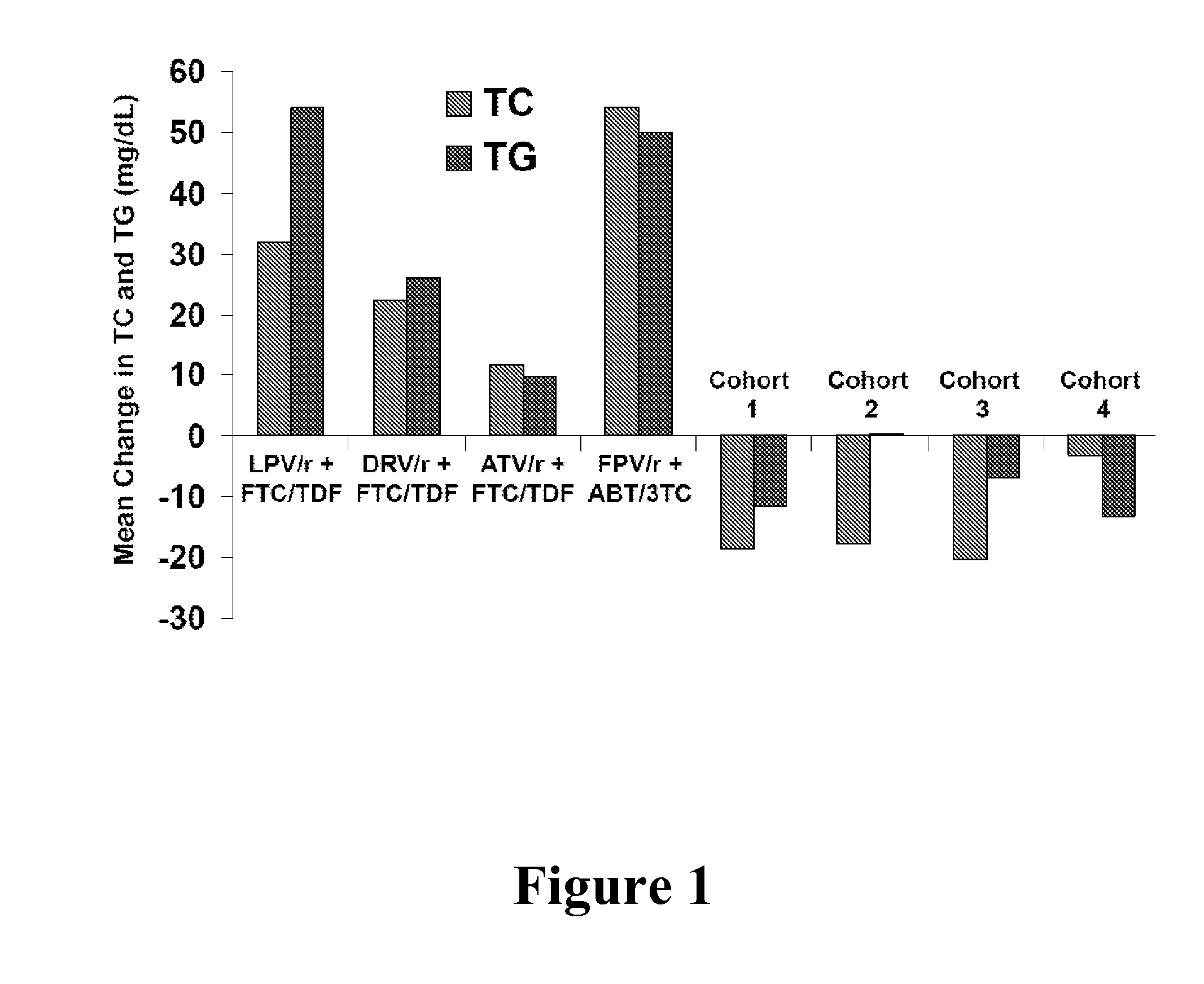
InactiveUS20140024613A1Improve pharmacokineticsDose lessBiocideDipeptide ingredientsTotal cholesterolHcv therapy
In one aspect, the present invention features HCV therapies comprising administering to a patient in need thereof an HCV protease inhibitor and ritonavir, wherein ritonavir is used as a pharmacokinetic booster to improve the pharmacokinetics of the HCV protease inhibitor. The HCV therapies do not require the testing of total cholesterol and triglyceride levels prior to and after the therapies.
Owner:ABBVIE INC
Combination of Anti-hiv reverse transcriptase and protease inhibitors
Owner:HOETELMANS RICHARD MARINUS WILHELMUS
Methods for treating HCV
ActiveUS8853176B2Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
Methods for Treating HCV
ActiveUS20130102558A1Avoid side effectsImprove pharmacokineticsBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
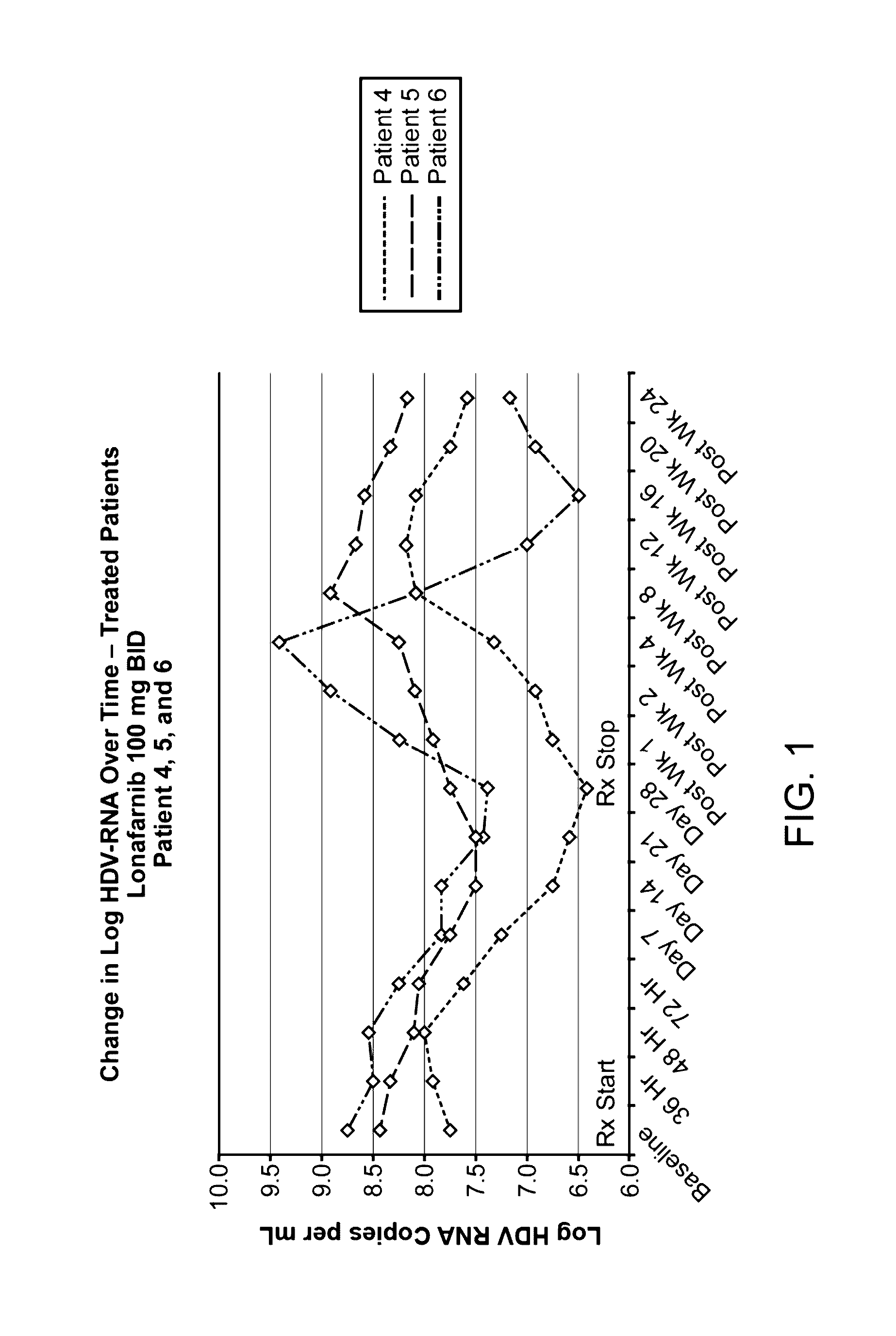
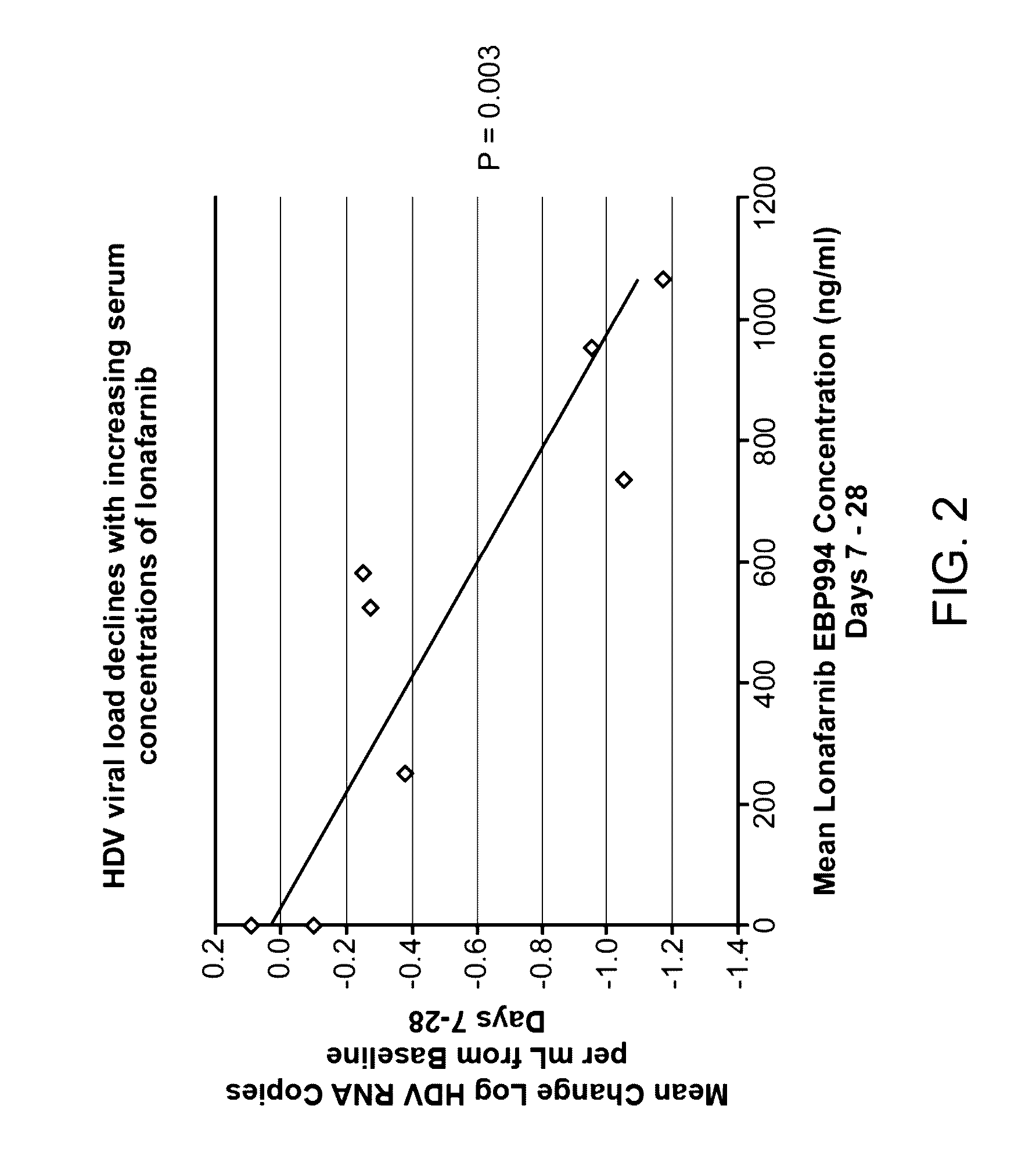
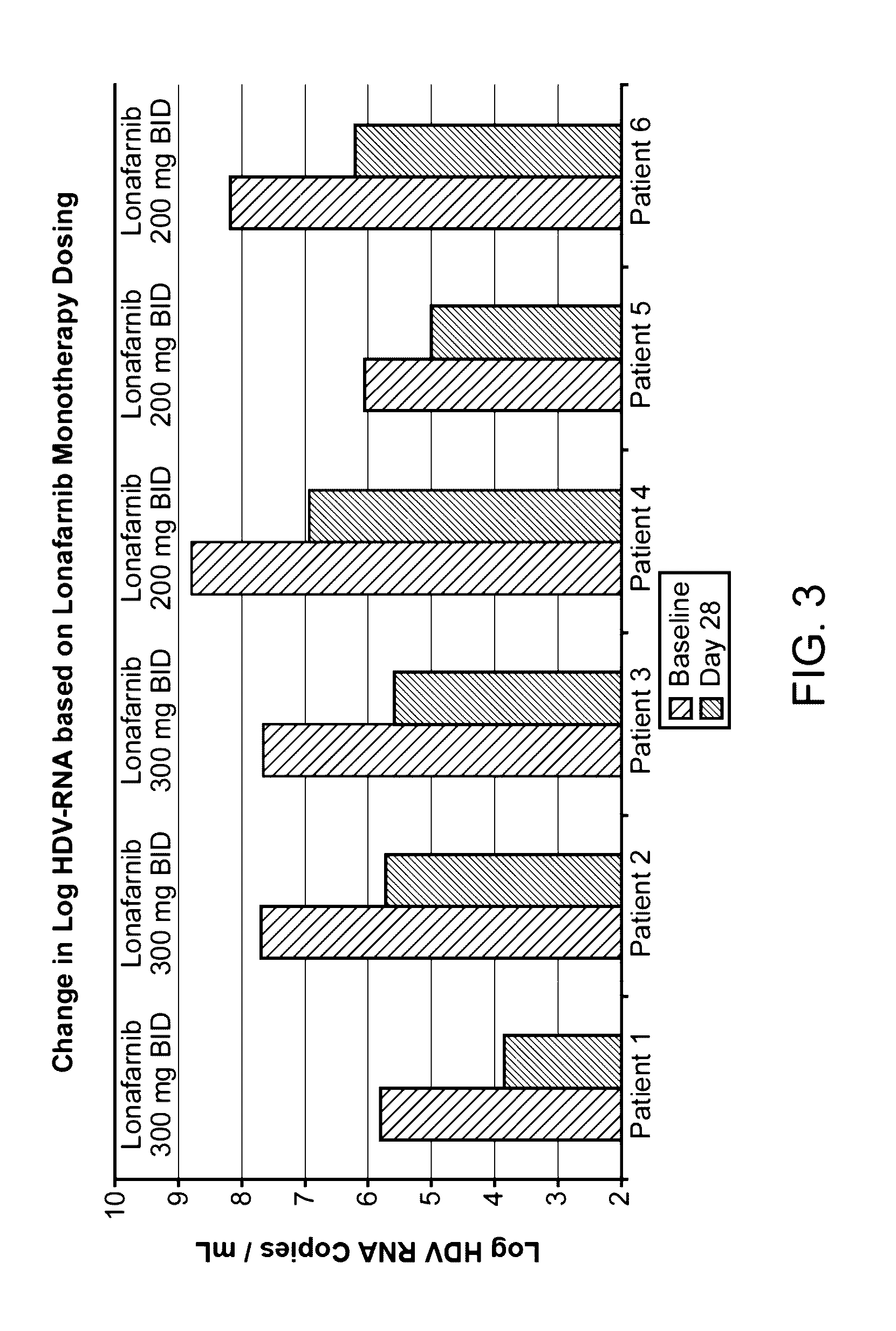
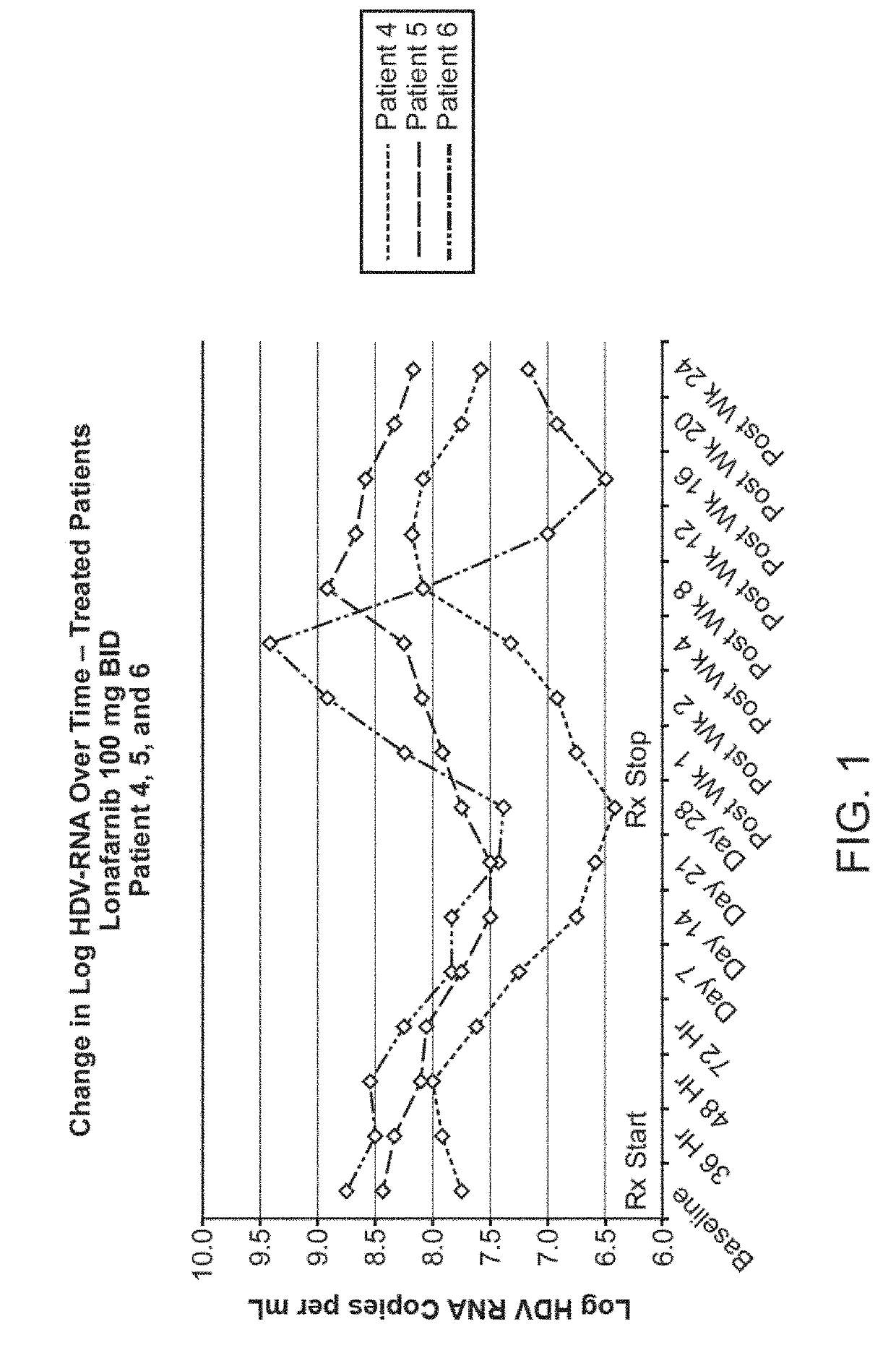
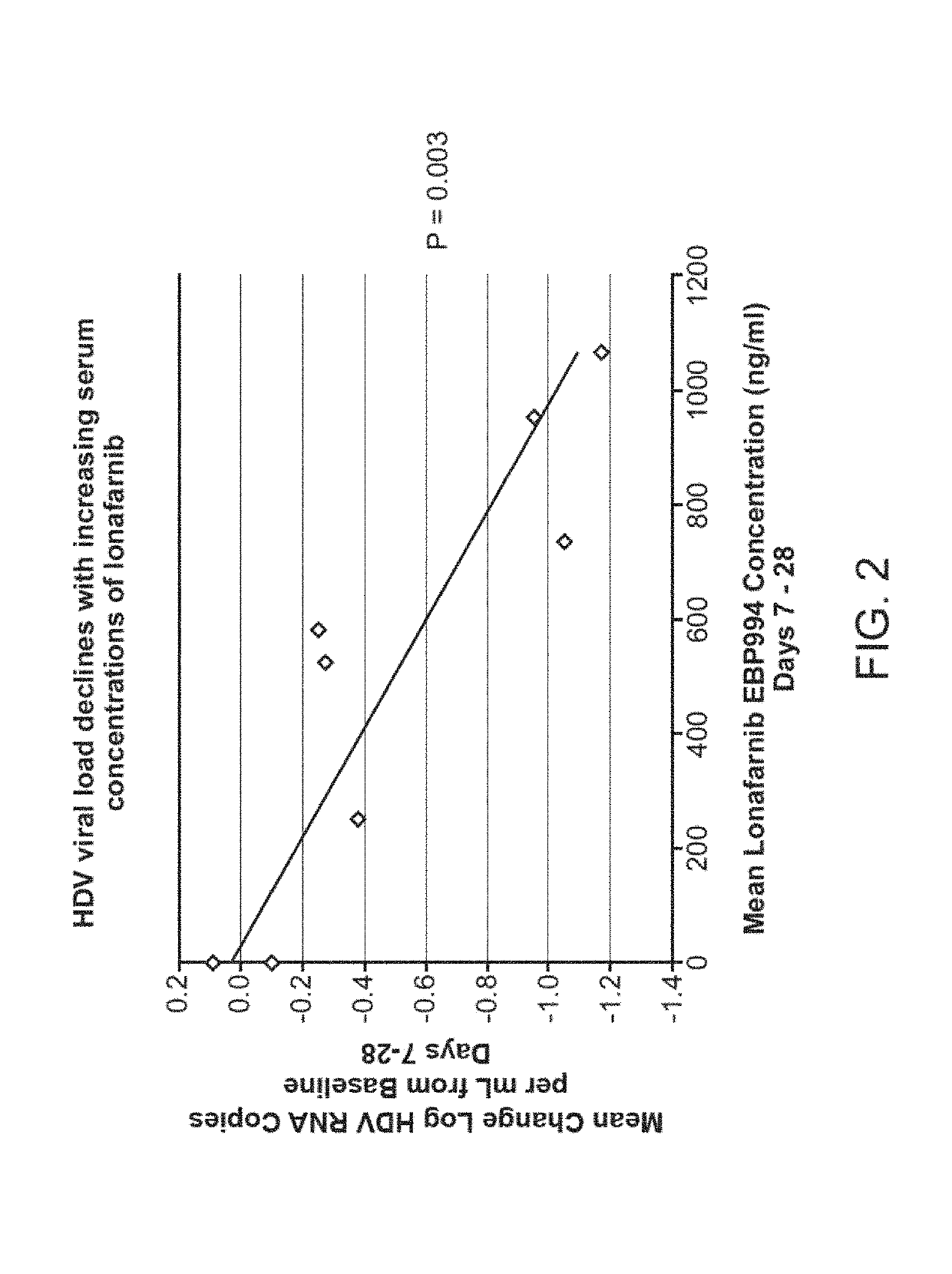
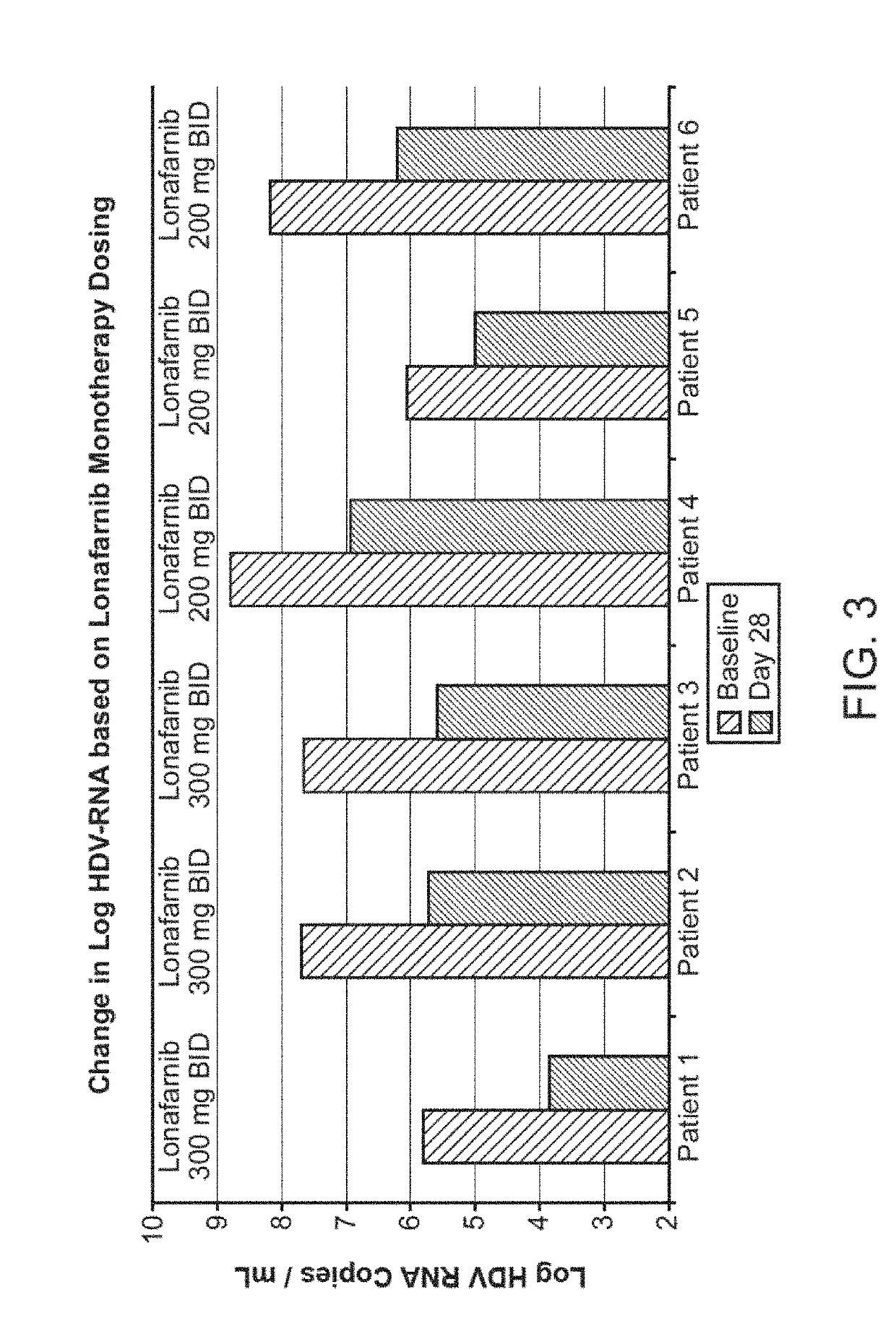
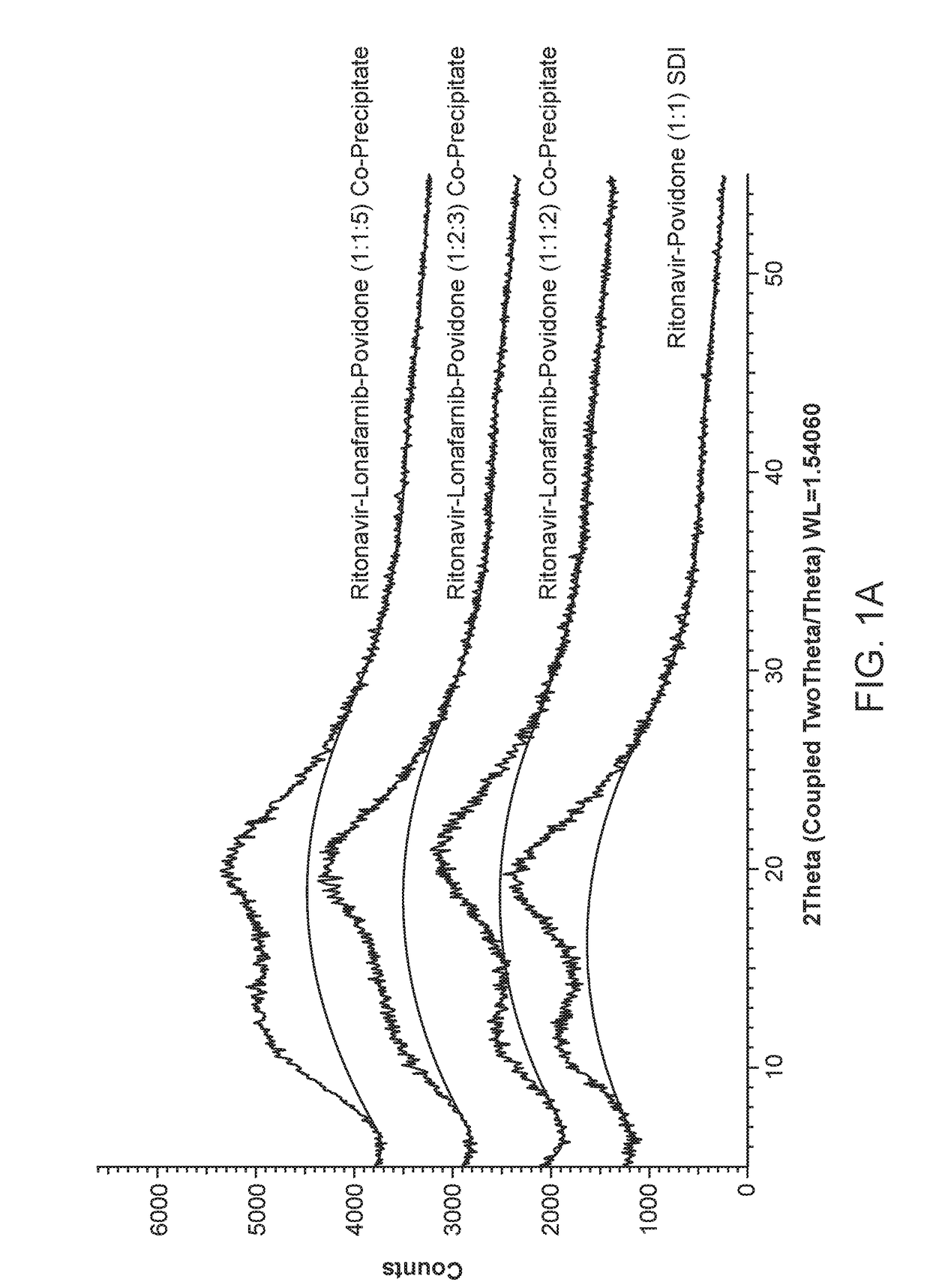
Treatment of hepatitis delta virus infection
ActiveUS20170042862A1Organic active ingredientsPowder deliveryInterferon therapyDeltaretrovirus Infections
Methods of reducing hepatitis delta virus (HDV) viral loads in a patient are provided. In some embodiments, the method comprises treating the patient with lonafarnib-ritonavir co-therapy. In some embodiments, the method further comprises treating the patient with an interferon.
Owner:EIGER BIOPHARMLS
Treatment of hepatitis delta virus infection
ActiveUS20190167646A1Powder deliveryOrganic active ingredientsInterferon therapyDeltaretrovirus Infections
Methods of reducing hepatitis delta virus (HDV) viral loads in a patient are provided. In some embodiments, the method comprises treating the patient with lonafarnib-ritonavir co-therapy. In some embodiments, the method further comprises treating the patient with an interferon.
Owner:EIGER BIOPHARMLS
Methods for Treating HCV
InactiveUS20150024999A1Improve pharmacokineticsImprove bioavailabilityOrganic active ingredientsBiocideCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
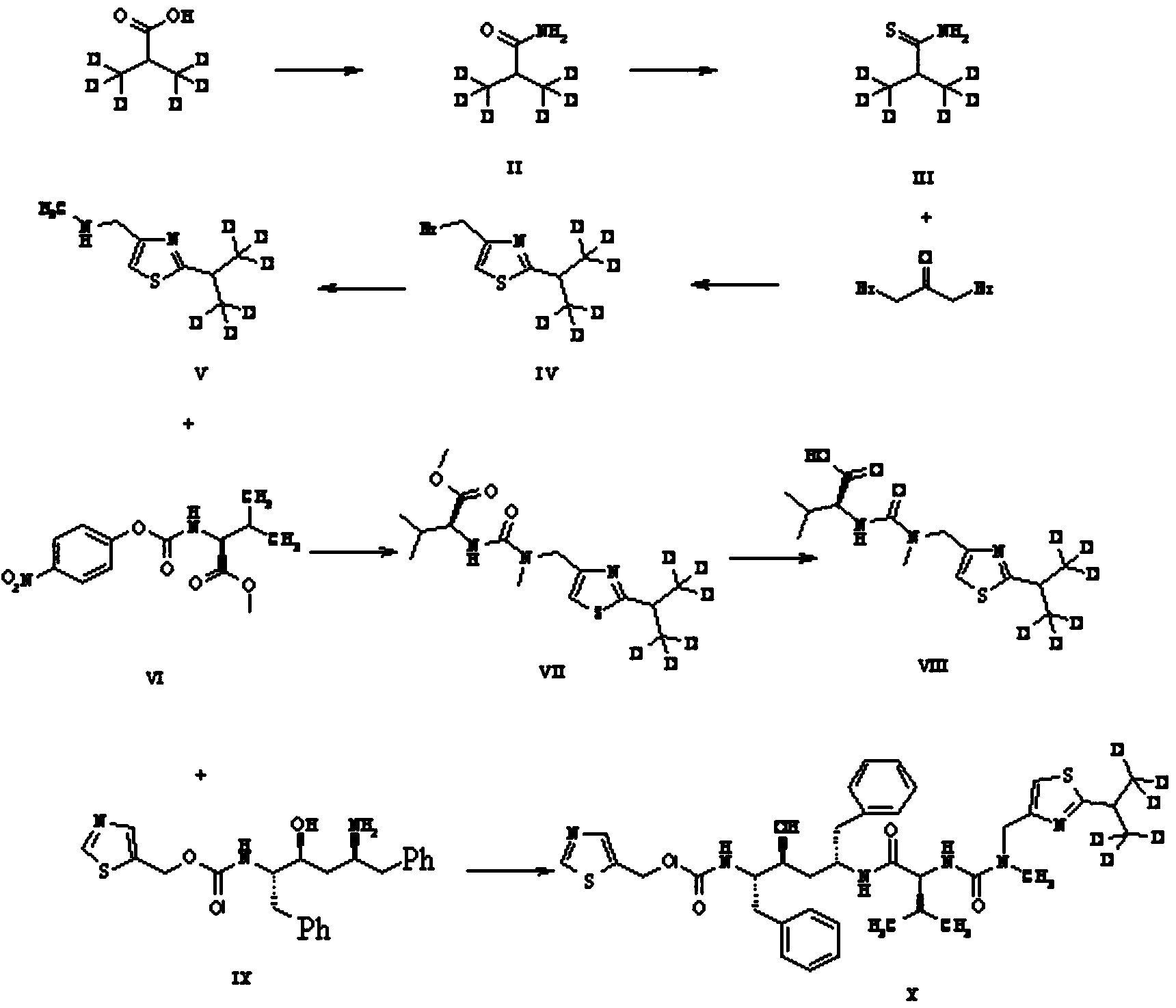
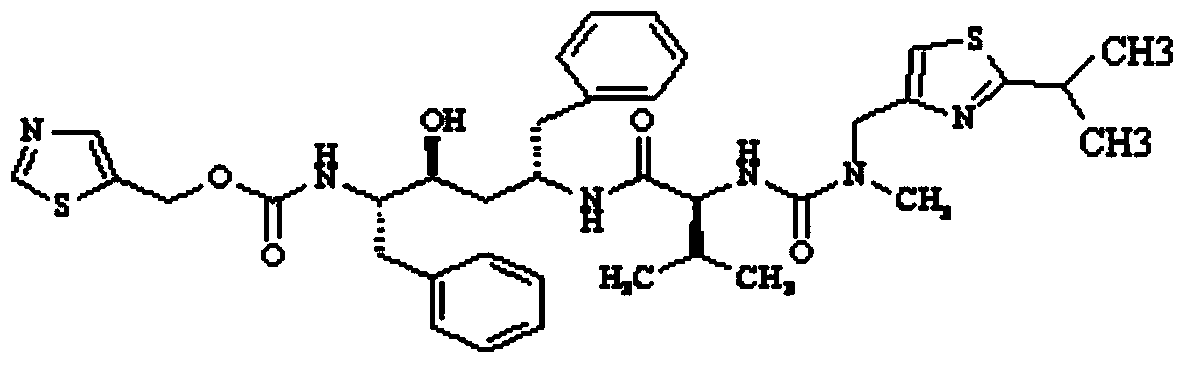
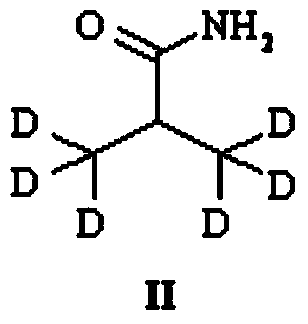
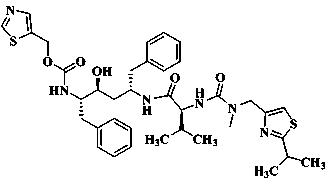
Preparation method of deuterium labeled Ritonavir
The invention discloses a preparation method of deuterium-labeled Ritonavir. According to the method, isobutyric acid-D6 is used as a starting raw material, and deuterium-labeled Ritonavir is synthesized through seven reactions. Optimal preparation steps and reaction conditions are screened through a lot of experiments, the whole process is reasonable in design and strong in operability; the purity of deuterium-labeled Ritonavir prepared by the preparation method provided by the invention is greater than 98 percent; the yield of the deuterium-labeled Ritonavir can be greater than 80 percent; and the isotope abundance is greater than 99 percent. The deuterium-labeled Ritonavir prepared by the preparation method provided by the invention can provide a test sample for research on metabolic mechanism of Ritonavir and has an important application value.
Owner:TLC NANJING PHARMA RANDD CO LTD
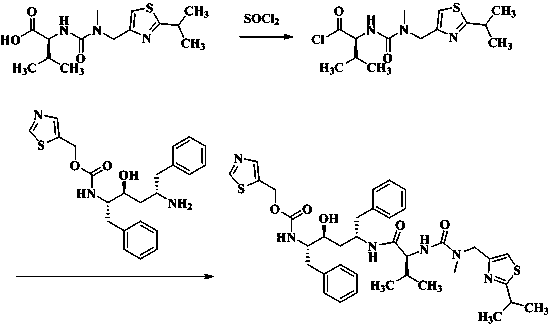
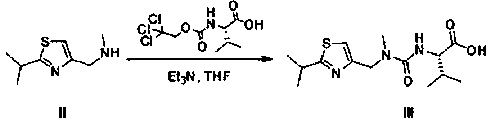
Method for preparing anti-HIV (human immunodeficiency virus) medicine ritonavir
The invention discloses a method for preparing anti-HIV medicine ritonavir and belongs to the technical field of medicines. In conditions of proper temperature, taking weak base as acid-binding agent and certain organic solvent, N-[N(-methyl-N-[(2-isopropyl-4-thiazolyl) methyl] amino-carbonyl]-L-valine and thionyl chloride are reacted to produice N(-[N-methyl-N-[(2-isopropyl-4-thiazolyl) methyl] amino-carbonyl]-L-valine acyl chloride, which is not required to be purified and can be directly subjected to amide reaction with (2s, 3s, 5s)-5-amino-2-((i)N( / i)-((5-thiazolyl)-methoxycarbonyl group) amino)-1, 6-diphenyl-3-hydroxy hexane at a room temperature, so as to obtain ritonavir; the mole ratio of the N-[N-methyl-N-[(2-isopropyl-4-thiazolyl) methyl] amino-carbonyl]-L-valine to thionyl chloride is 1:1 to 1:8; the mole ratio of the N-[N-methyl-N-[(2-isopropyl-4-thiazolyl) methyl] amino-carbonyl]-L-valine acyl chloride to weak base is 1:1 to 1:15. The method has the advantages that the price of thionyl chloride is low, the material cost is reduced, the production pollution is low, the pollution can be changed into soluble effluent brine, the method is simple to operate, the product yield is high, and the method is easy for separation and purification, and is applicable to industrial production.
Owner:NORTHEAST PHARMA GRP
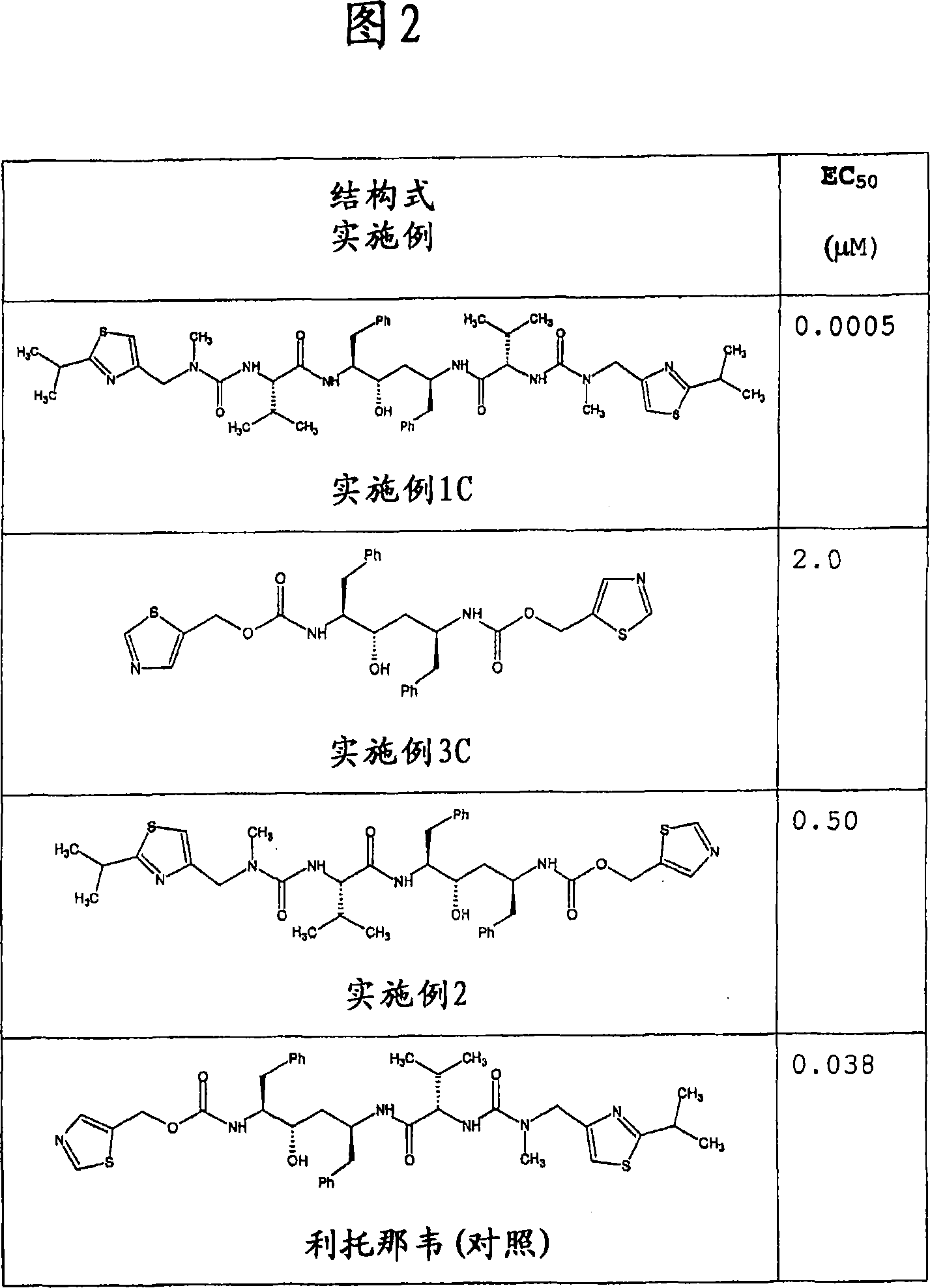
Ritonavir water-soluble derivatives, synthesizing method and use thereof
InactiveCN101440091AEnhanced inhibitory effectHigh yieldOrganic active ingredientsOrganic chemistrySolubilitySynthesis methods
The invention provides ritonavir water-soluble derivatives, a synthesizing method and application thereof, which relate to a ritonavir water-soluble derivative. Through improving a ritonavir synthesizing route, based on maintaining fundamental drug-effect structure of ritonavir, a pyridine ring is used to replace a benzene ring on a fork chain so as to improve the water solubility of anti-AIDS drugs, such as ritonavir. In the invention, 3 novel ritonavir water-soluble derivatives are synthesized, are estimated to overcome the defect of poor water solubility of the ritonavir based on maintaining the pharmaceutical activity of the ritonavior, and have excellent effect in inhibiting HIV prolease, and can be used for preparing the anti-AIDS drugs.
Owner:XIAMEN UNIV
Compositions and methods of use of ritonavir for treating hcv
InactiveUS20110091423A1Improve pharmacokineticsBiocidePeptide/protein ingredientsOxygenaseCytochrome P450
The present invention discloses compositions and a method of improving the pharmacokinetics of pharmaceutical agents (or pharmaceutically acceptable salts, esters, and prodrugs thereof) which are metabolized by cytochrome P450 monoxygenase comprising coadministering ritonavir or a pharmaceutically acceptable salt, ester, and prodrug thereof.
Owner:ABBVIE INC
Application of ritonavir in preparing medicines for preventing or treating acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis
InactiveCN104069104AOrganic active ingredientsRespiratory disorderApoptosisARDs - Acute respiratory distress syndrome
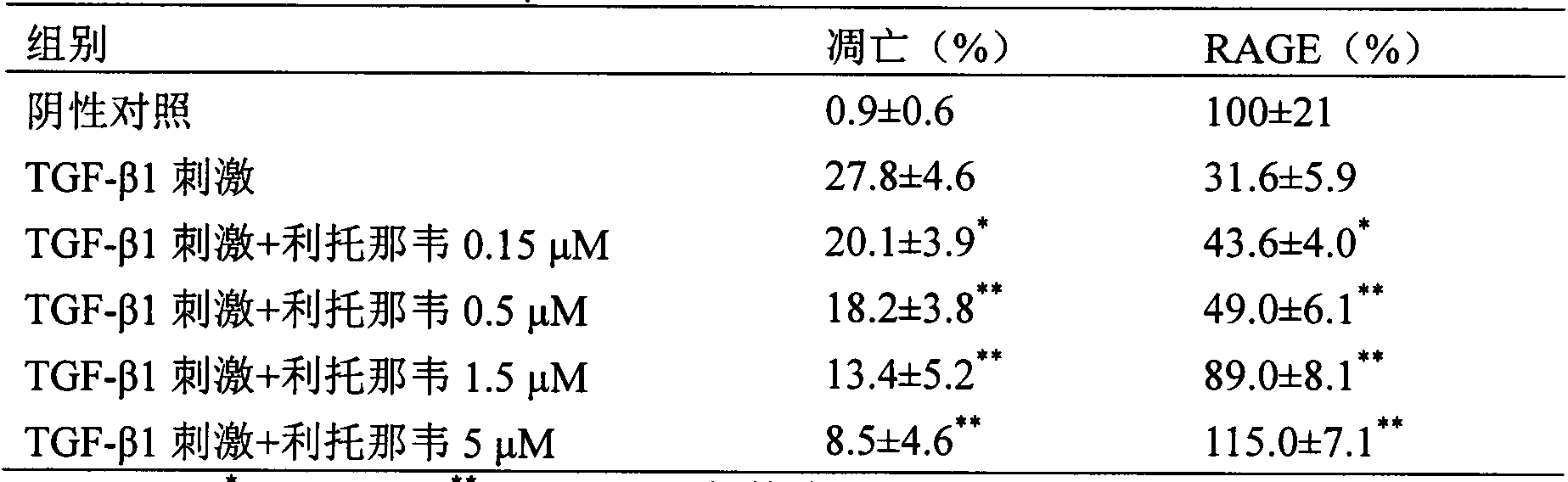
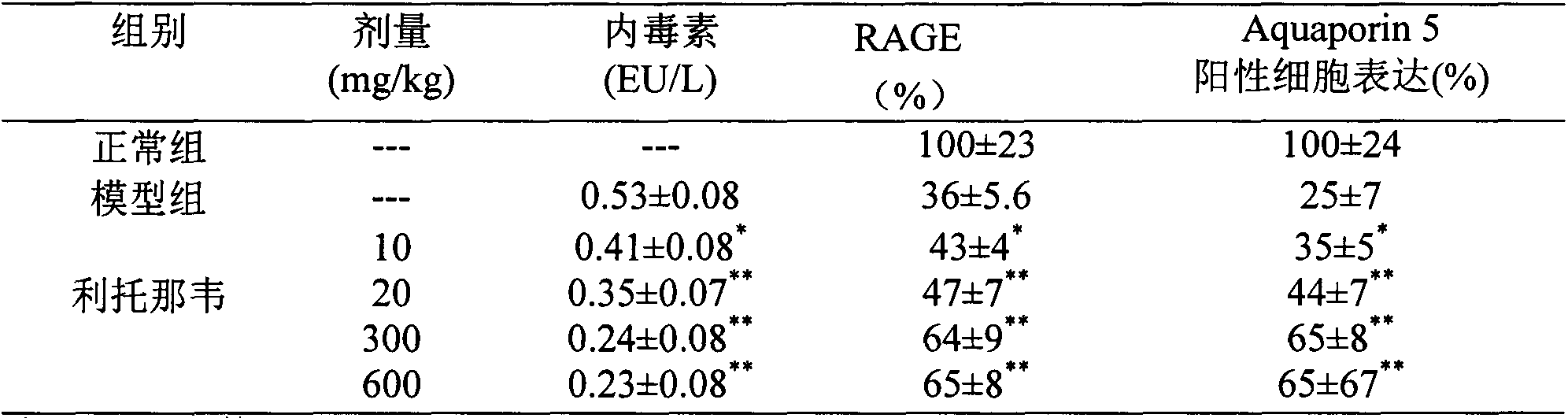
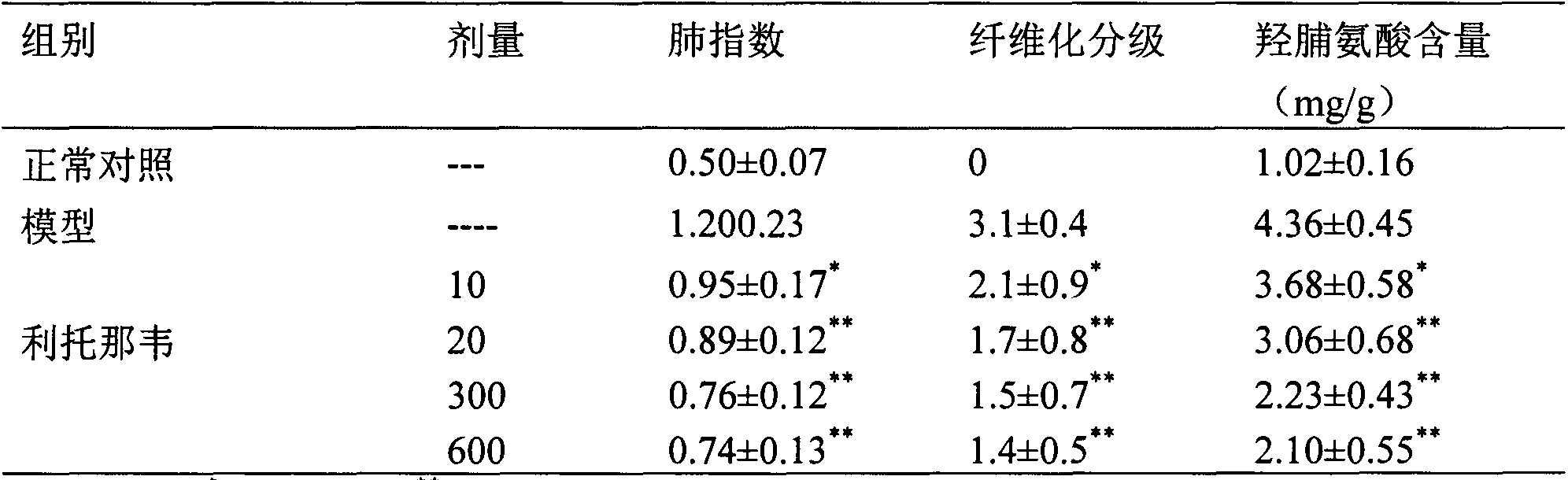
The invention provides a new medical application of ritonavir and in particular relates to an application of the ritonavir for preventing or treating Acute Lung Injury / Acute Respiratory Distress Syndrome (ALI / ARDS) and pulmonary fibrosis in such a manner of inhibiting the occurrence and development of the ALI / ARDS and the pulmonary fibrosis by inhibiting the apoptosis of type I alveolar epithelial cells and promoting the expression of the Receptor For Advanced Glycation Endproducts (RAGE) of the type I alveolar epithelial cells. When in use, the oral administration dosage of the ritonavir ranges from 100mg to 6000mg, preferably, from 200mg to 3000mg.
Owner:BINZHOU MEDICAL COLLEGE
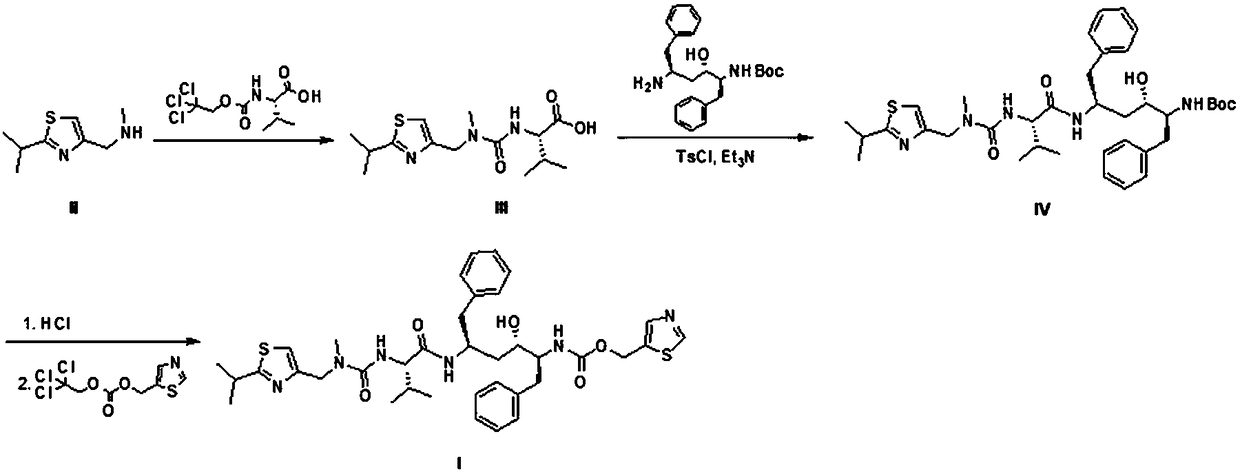
Preparation method of ritonavir
The invention relates to the technical field of medicine, in particular to a preparation method of ritonavir. According to the preparation method of the ritonavir, (2-isopropyl thiazole-4-yl)-nitrogen-methyl methylamine is taken as a raw material, and the ritonavir is synthesized through a three-step reaction; a urea bond is built by trichloroethanol chloroformate, paratoluensulfonyl chloride which is cheap and easy to obtain is adopted as a condensing agent for amide, the ritonavir is synthesized with the high yield, and the yield of the ritonavir is 79%; and compared with an existing preparation method, the preparation method has the advantages of low cost, environment friendliness, easy scale production and the like, and has good application prospects.
Owner:贵州永诺菲特生物制药有限公司
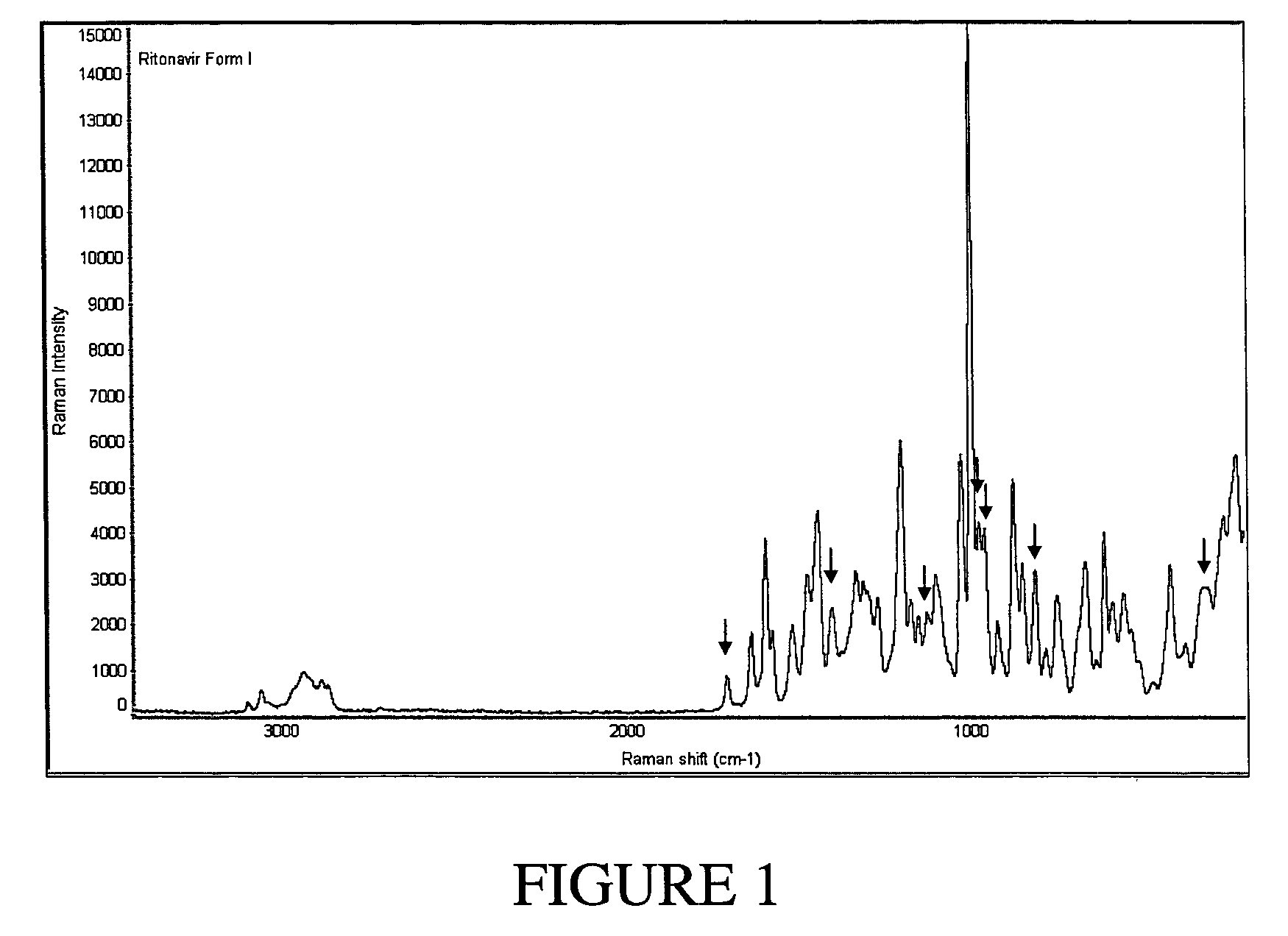
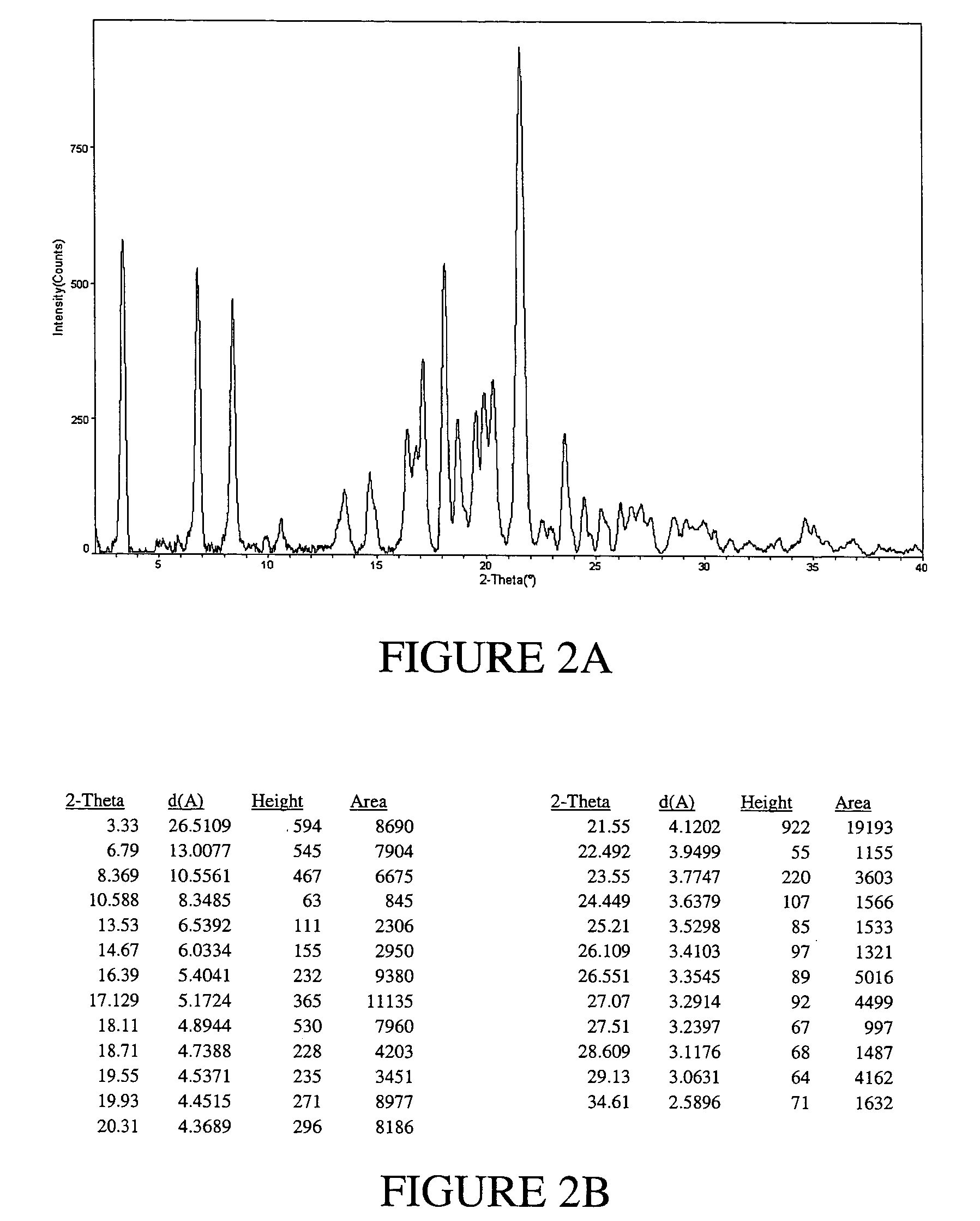
Processes for the Preparation of Stable Polymorphic Form I of Ritonavir
The invention relates to processes for the preparation of a polymorphic form of ritonavir. More particularly, it relates to the preparation of a stable polymorphic Form I of ritonavir. The invention also relates to pharmaceutical compositions that include the stable Form I of ritonavir and use of the compositions for treatment of HIV infections in combination with other antiretro viral agents.
Owner:RANBAXY LAB LTD
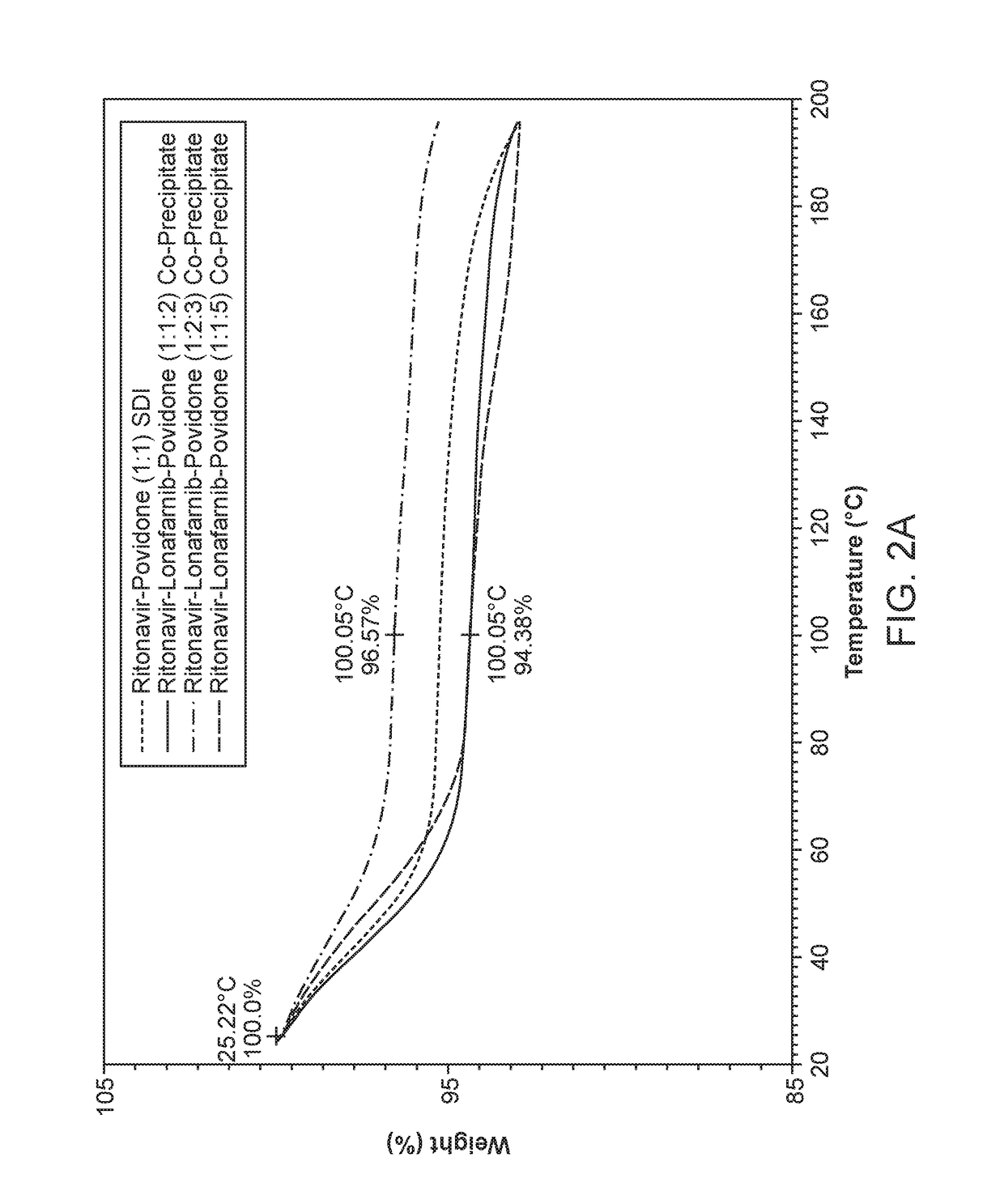
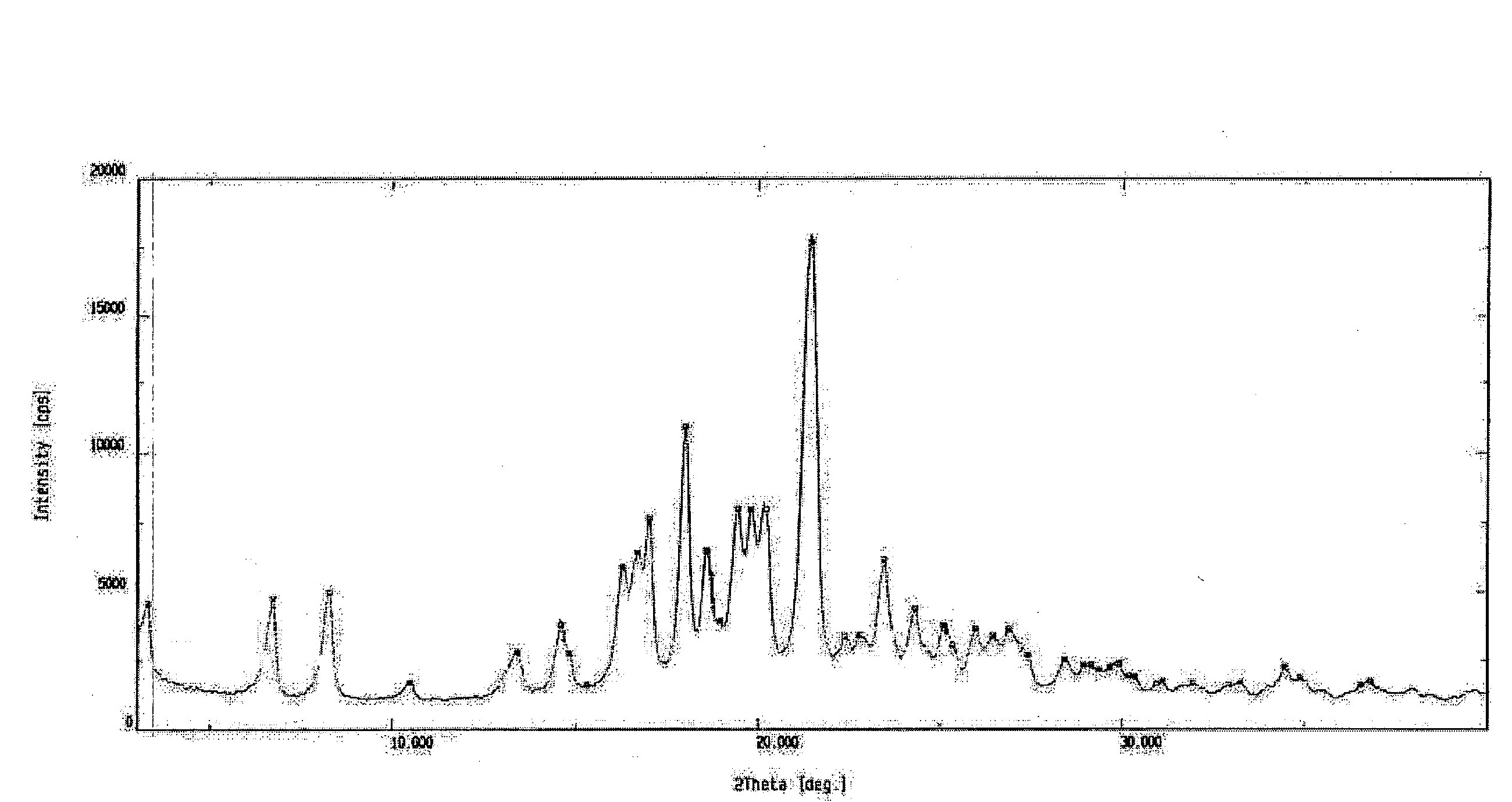
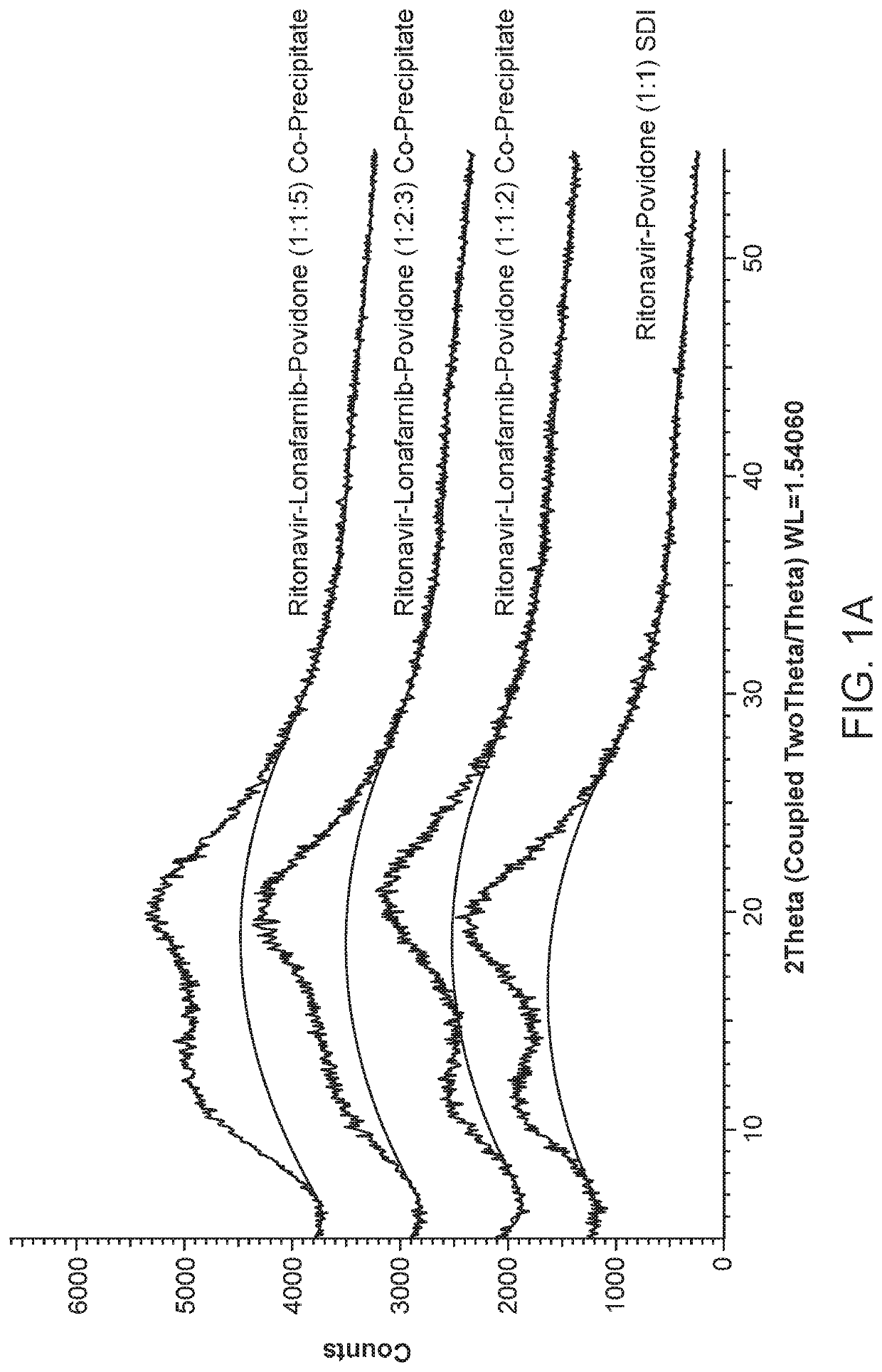
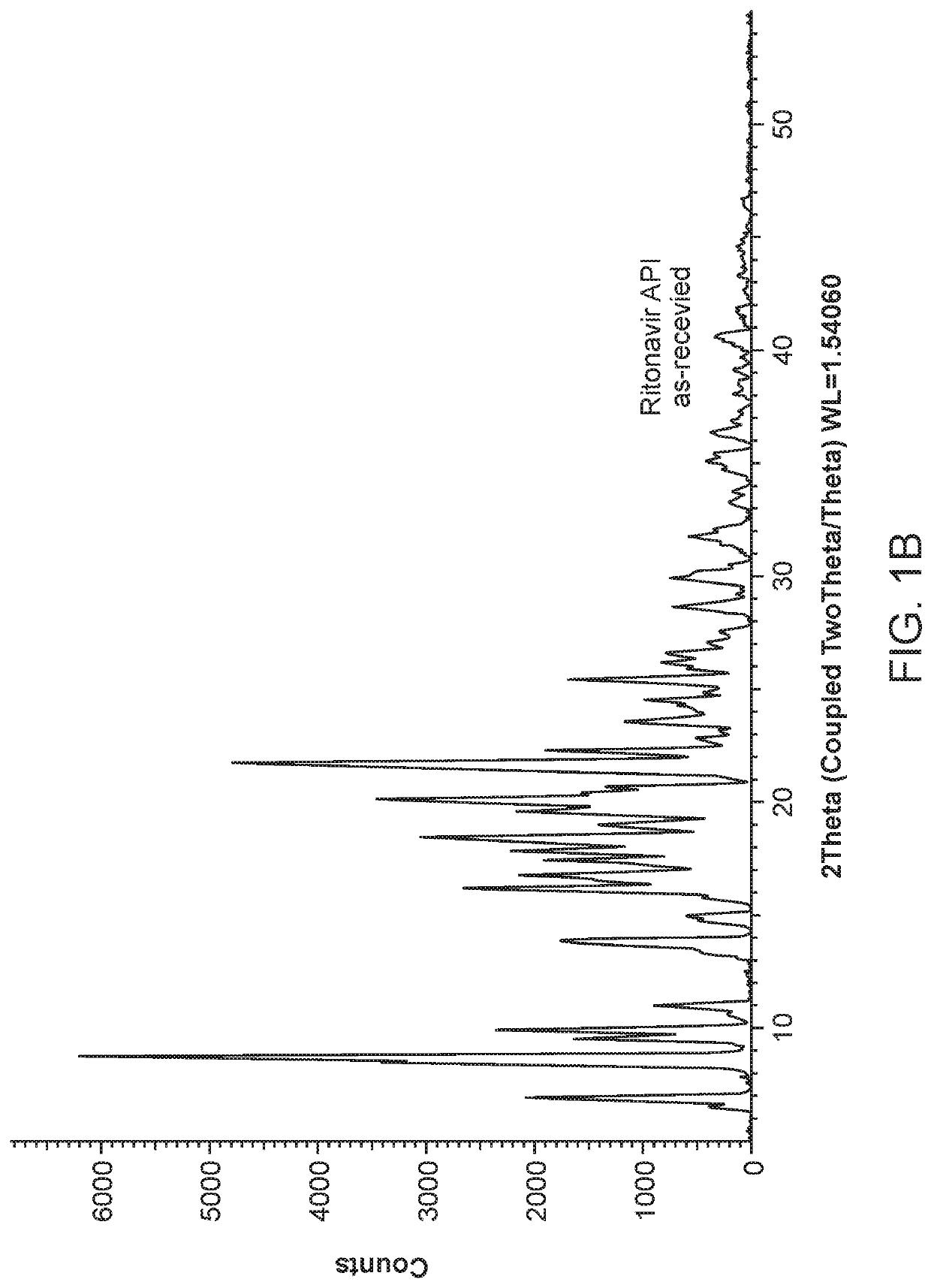
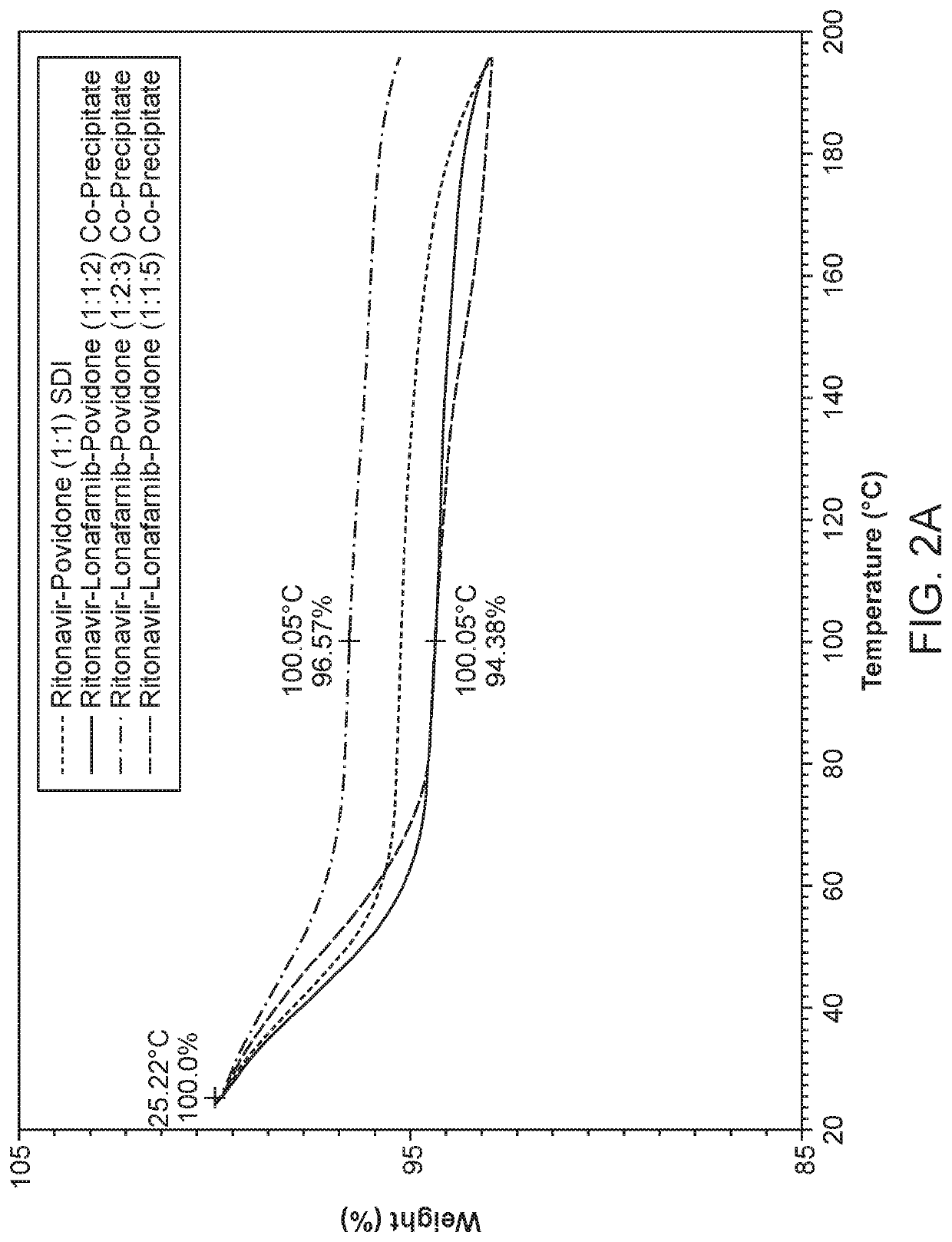
Pharmaceutical compositions comprising lonafarnib and ritonavir
ActiveUS10835496B2Organic active ingredientsPowder deliveryPharmaceutical drugPharmaceutical medicine
Owner:EIGER BIOPHARMLS
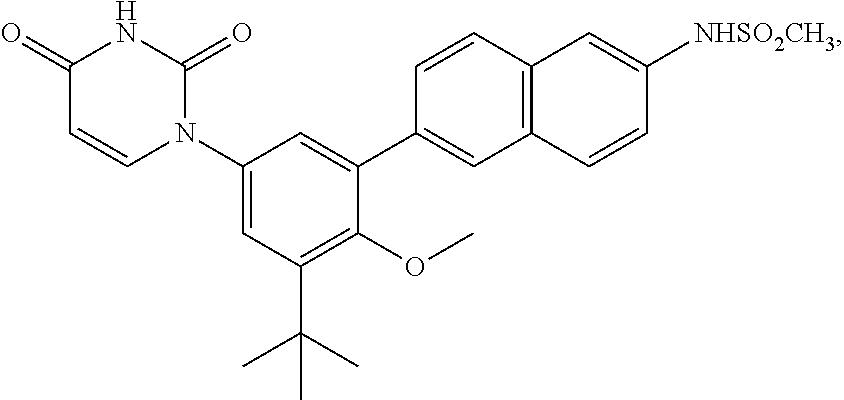
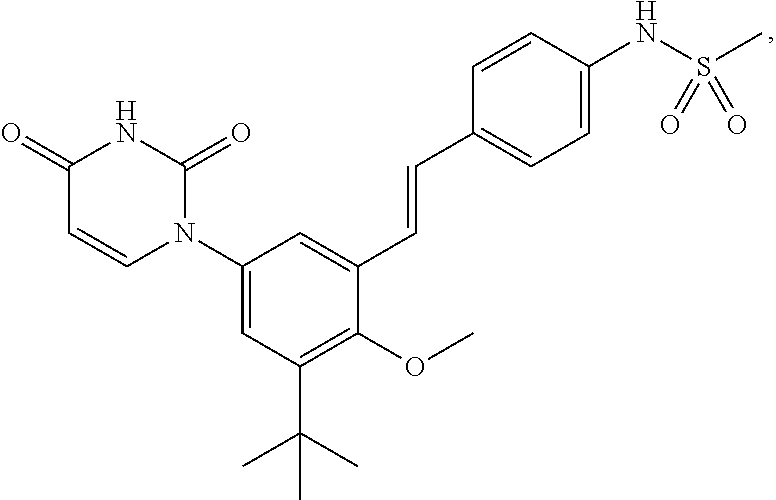
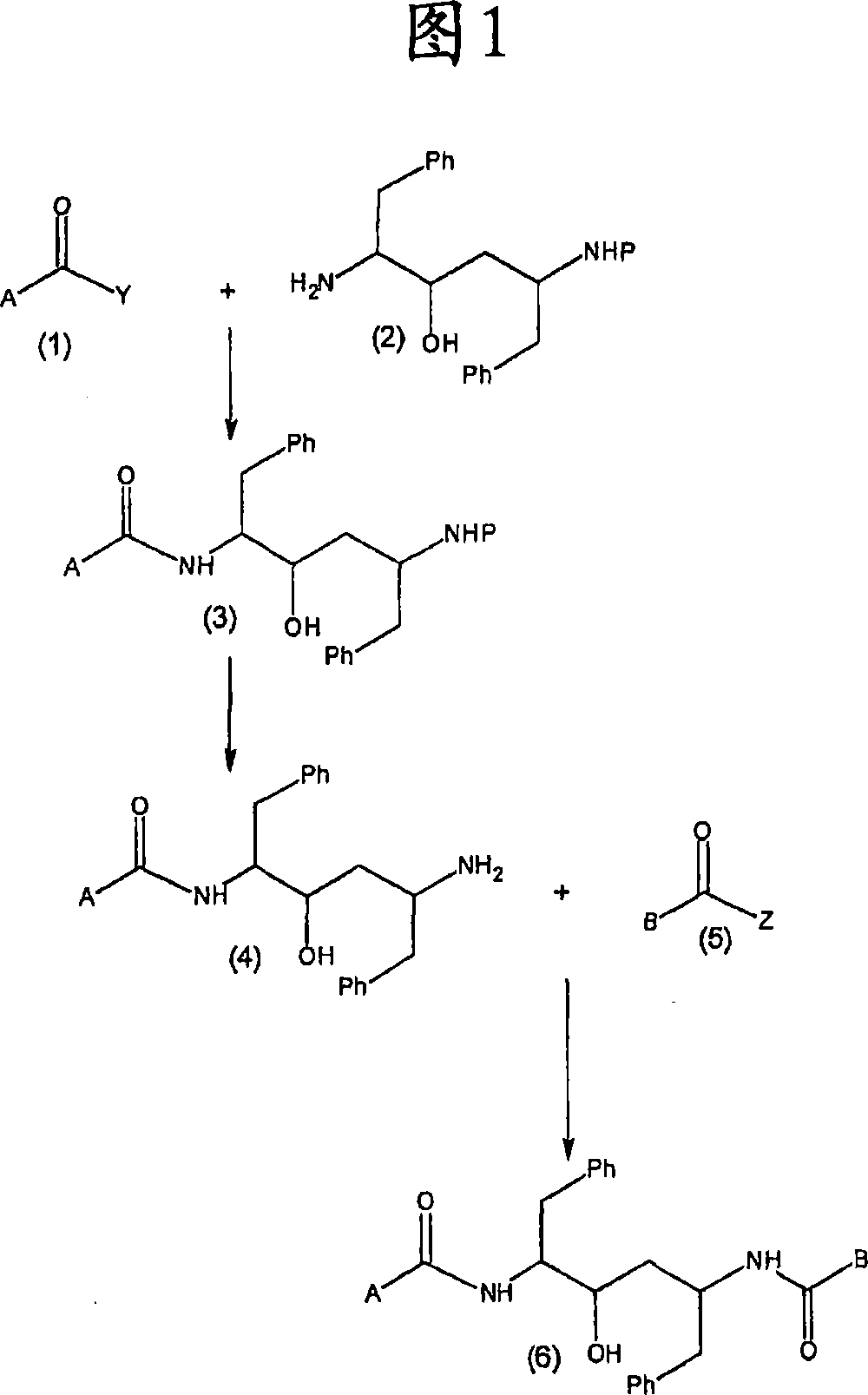
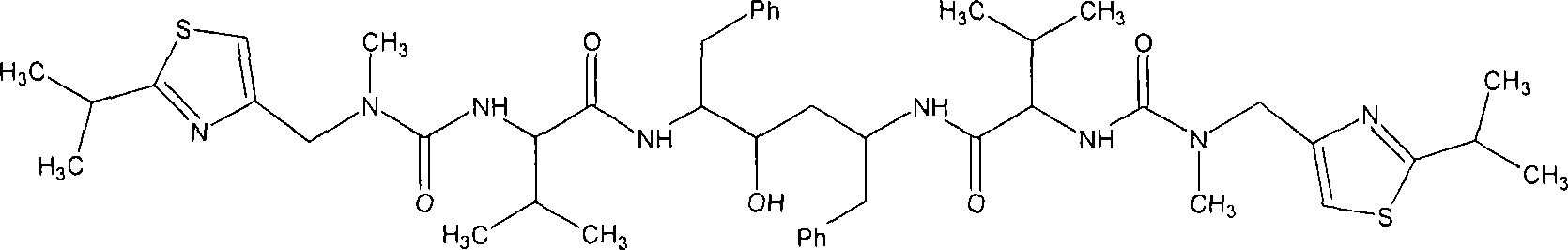
Ritonavir analogous compound useful as retroviral protease inhibitor, preparation of the ritonavir analogous compound and pharmaceutical composition for the ritonavir analogous compound.
The present invention describes a new one ritonavir analogous compound that presents significantly superior activity in inhibition of HIV protease. There are also described the usage of the ritonavir analogous compound of the present invention or salt, ester or prodrug thereof as well as the usage of the compound and its pharmaceutical compositions in medicine, particularly, in the treatment of HIV infection, by itself or in combination with others anti-HIV drugs.
Owner:CRISTALIA PROD QUI FARM LTDA
Methods for treating hcv
InactiveUS20160228496A1Improve pharmacokineticsImprove bioavailabilityOrganic active ingredientsDipeptide ingredientsMedicineCytochrome P450 Inhibitors
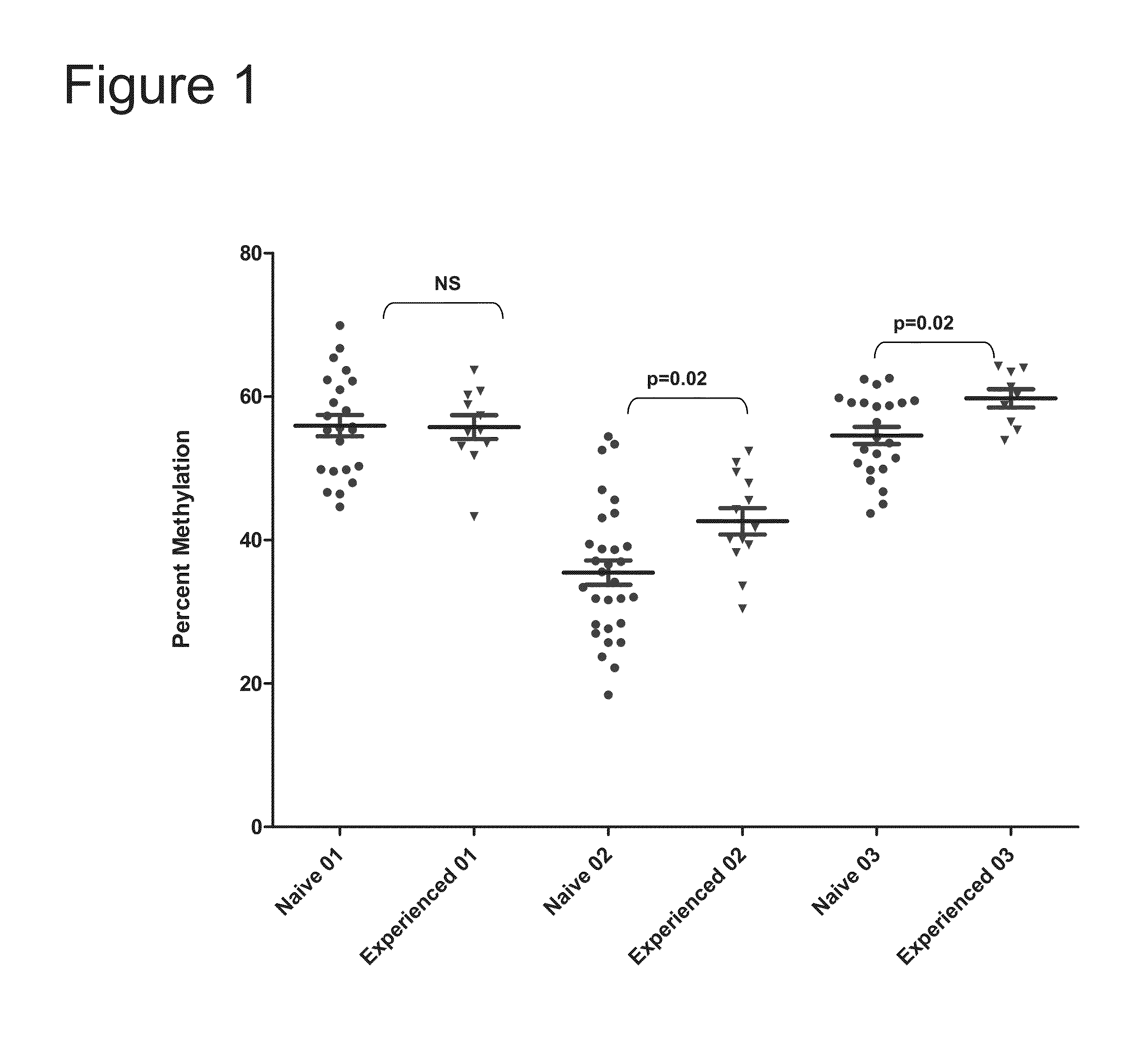
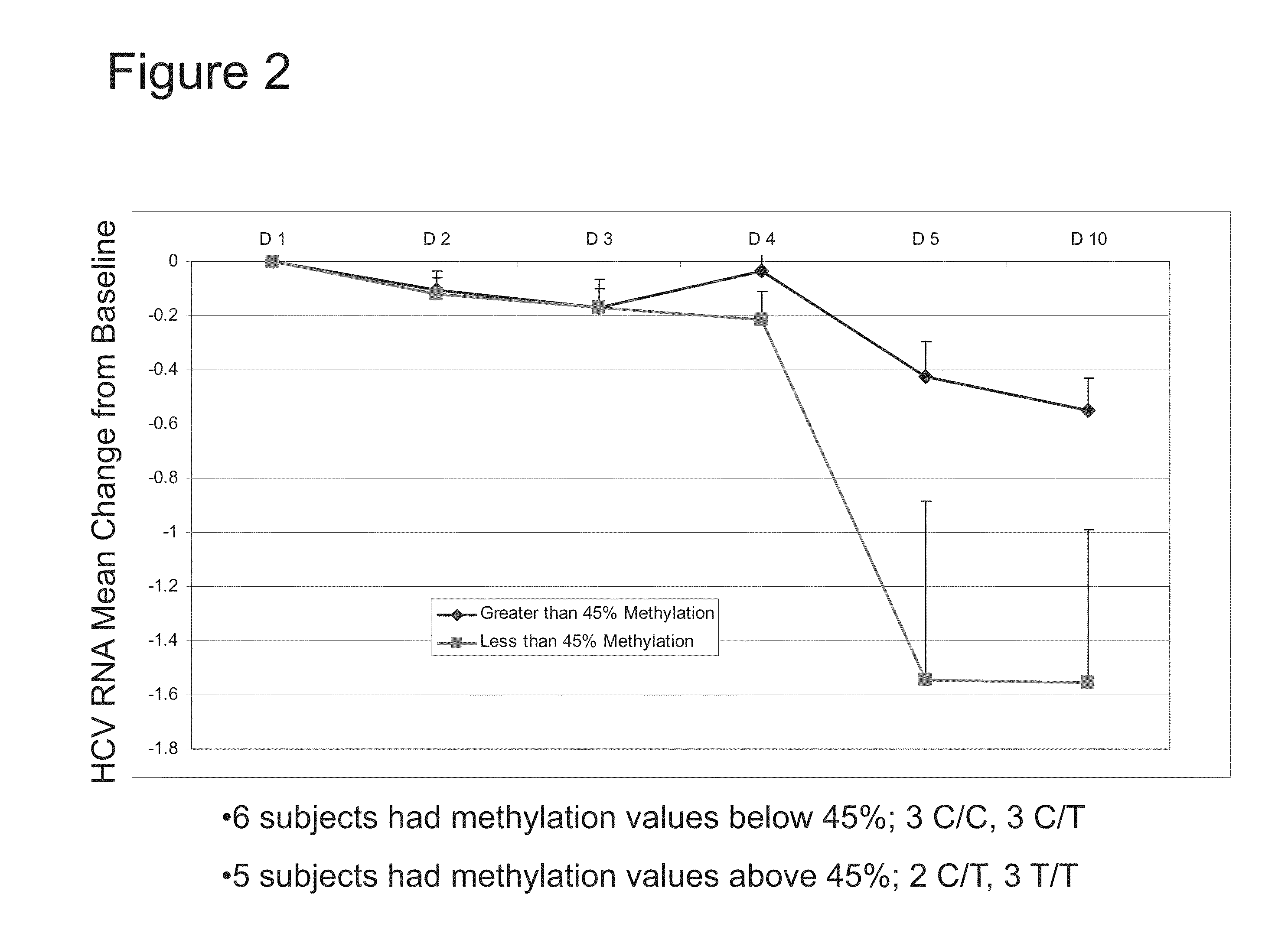
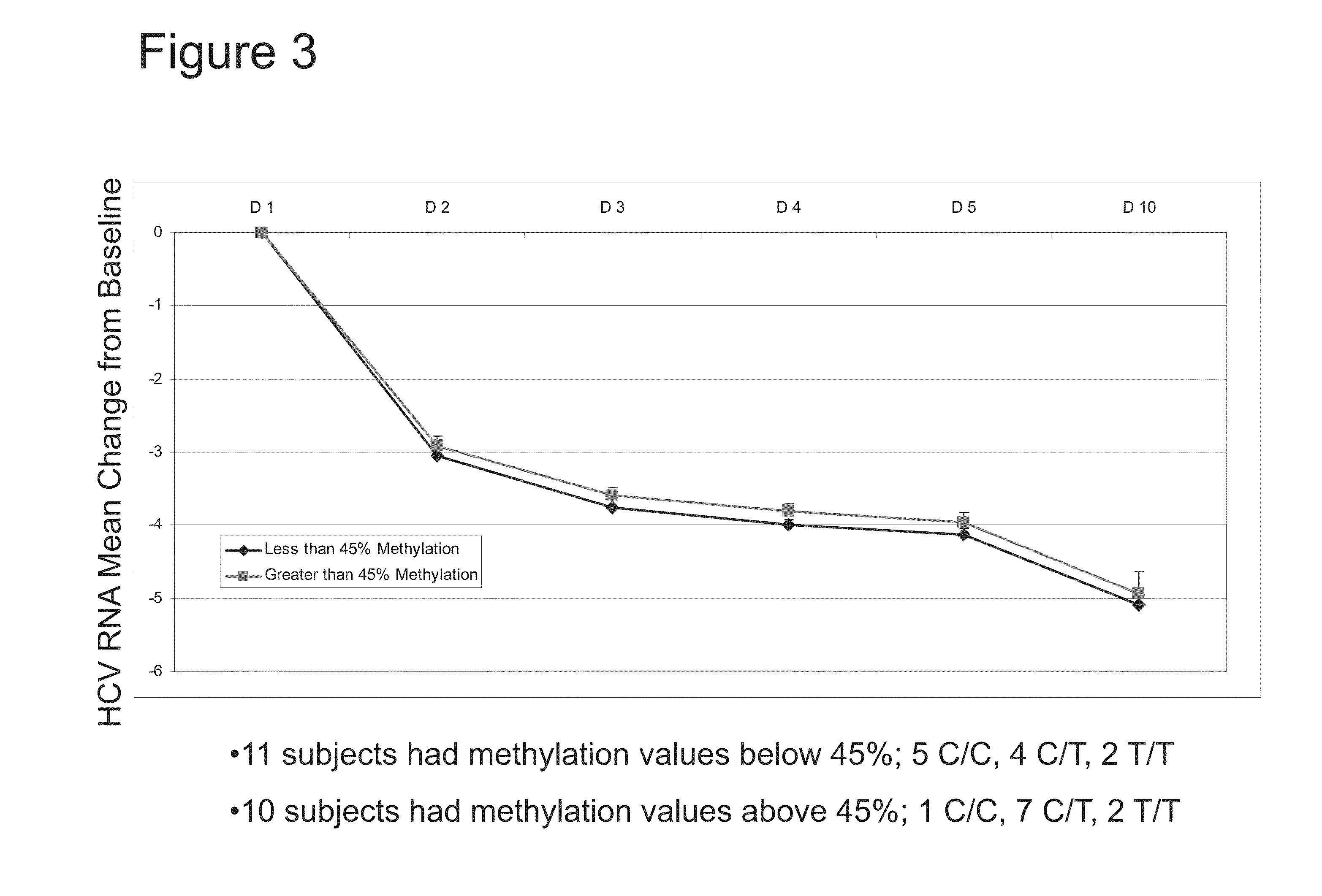
The present invention features therapies for the treatment of HCV comprising direct-acting antiviral agents. Preferably, the treatment is administered to an HCV-infected patient who has been tested to determine methylation status of a CpG island within a promoter region of the IL28B gene. In one aspect, the therapies comprise administering one or more direct acting antiviral agents and, optionally ribavirin, to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2, therapeutic agent 3, an inhibitor of cytochrome P450 (e.g., ritonavir), and / or ribavirin.
Owner:ABBVIE INC
Compound tablet containing nemategravir and ritonavir
PendingCN114652811ADissolution stabilityImprove utilizationOrganic active ingredientsDipeptide ingredientsDrug release rateBlood drug concentration
The invention discloses a compound tablet of nemategravir and ritonavir, the compound tablet is a coated chip, the drug delivery sequence is controlled by adopting an in-tablet technology, and through the combination of two layers of different drug release rates, a relatively ideal drug release rate can be obtained, an expected blood concentration can be achieved, and a stable and long-time effective concentration can be maintained; the toxic effect of the medicine is reduced, and adverse reactions such as gastric mucosa stimulation are avoided. The technology integrates the advantages of two single preparations. Medicine dissolution can be guaranteed, and meanwhile the stability of packaged medicine is guaranteed. Compared with the existing preparation which needs to take a plurality of tablets once and cannot ensure that the nemategravir can maintain the treatment concentration for a long time, the preparation disclosed by the invention can stably release medicine for a long time, greatly improves the in-vitro dissolution speed of the nemategravir and the ritonavir, solves the phenomena that the dissolution rate is low, the action time is short and a plurality of tablets need to be taken in the prior art, and can be used for preparing the nemategravir and the ritonavir. The medicine treatment effect is enhanced, the medicine treatment time is prolonged, and meanwhile the medicine taking compliance of a patient is improved.
Owner:乐普制药科技有限公司
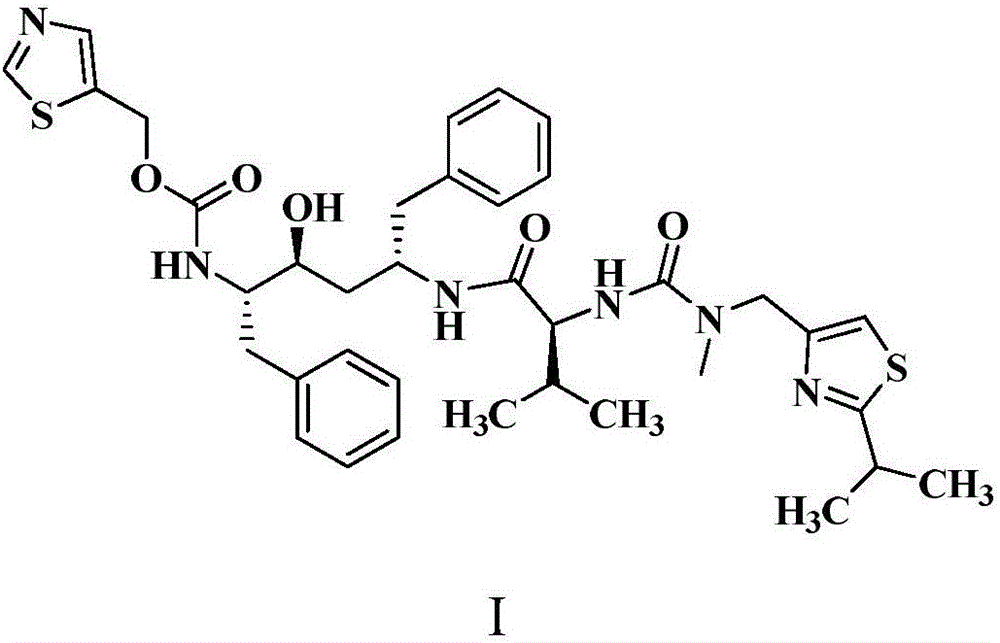
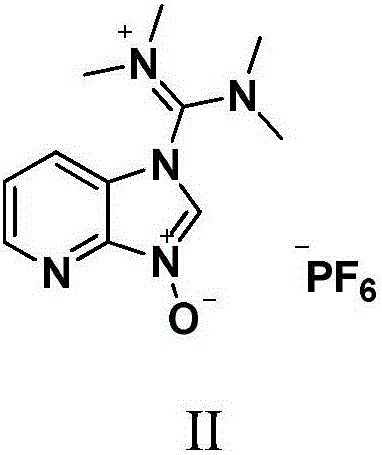
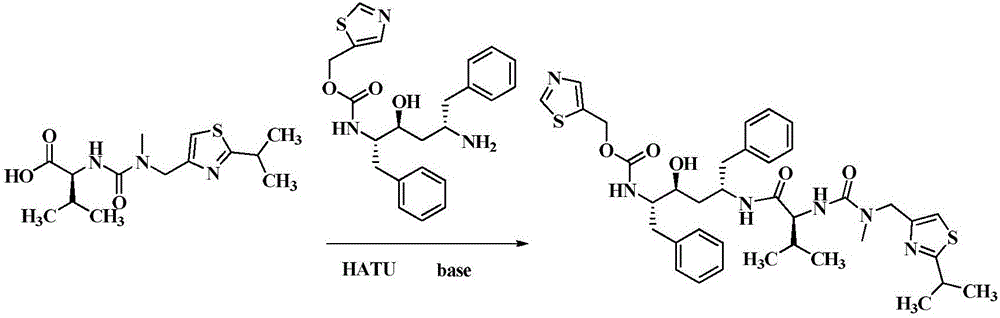
Method for preparing Ritonavir as anti-AIDS drug
The invention relates to a method for preparing Ritonavir applied to the technical field of pharmaceutical synthesis. According to the method, under the conditions of organic alkali and an organic solvent, HATU is used as a condensing agent, N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine and (2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane are subjected to an acidamide reaction, and then a certain post-treatment is performed, so that the Ritonavir is obtained. According to the method disclosed by the invention, the synthesis yield of the Ritonavir is increased, the purity is high, and the cost of raw materials is effectively reduced. Besides, the reaction time is short, the feed is simple, nitrogen protection is not needed, and feeding temperature needs to be appropriately controlled, so that by-products of HATU can be easy to wash away, the preparation time is greatly shortened, the working efficiency is improved, and the method is suitable for industrial production.
Owner:NORTHEAST PHARMA GRP
New medical application of ritonavir to enhancing anti-inflammation effect of glucocorticoid
The invention provides new medical application of ritonavir, and particularly relates to the medical application of ritonavir and glucocorticoid in cooperation to enhancing the anti-inflammation effect of glucocorticoid. In the application, the range of oral dosage is 100-2400 mg, preferentially 100-1200 mg.
Owner:BINZHOU MEDICAL COLLEGE
Methods for treating hcv
InactiveUS20160237491A1Improve pharmacokineticsImprove bioavailabilityOrganic active ingredientsDipeptide ingredientsInfected patientMedicine
The present invention features therapies for the treatment of HCV comprising direct-acting antiviral agents. Preferably, the treatment is administered to an HCV-infected patient who has been tested to determine methylation status of a CpG island within a promoter region of the IL28B gene. In one aspect, the therapies comprise administering one or more direct acting antiviral agents and, optionally ribavirin, to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2, therapeutic agent 3, an inhibitor of cytochrome P450 (e.g., ritonavir), and / or ribavirin.
Owner:ABBVIE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com