Method for preparing Ritonavir as anti-AIDS drug
A ritonavir, anti-AIDS technology, applied in the field of drug synthesis, can solve the problems of high price, unsuitable for industrialization, high price, etc., and achieve the effects of high synthesis reaction yield, easy separation and purification, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
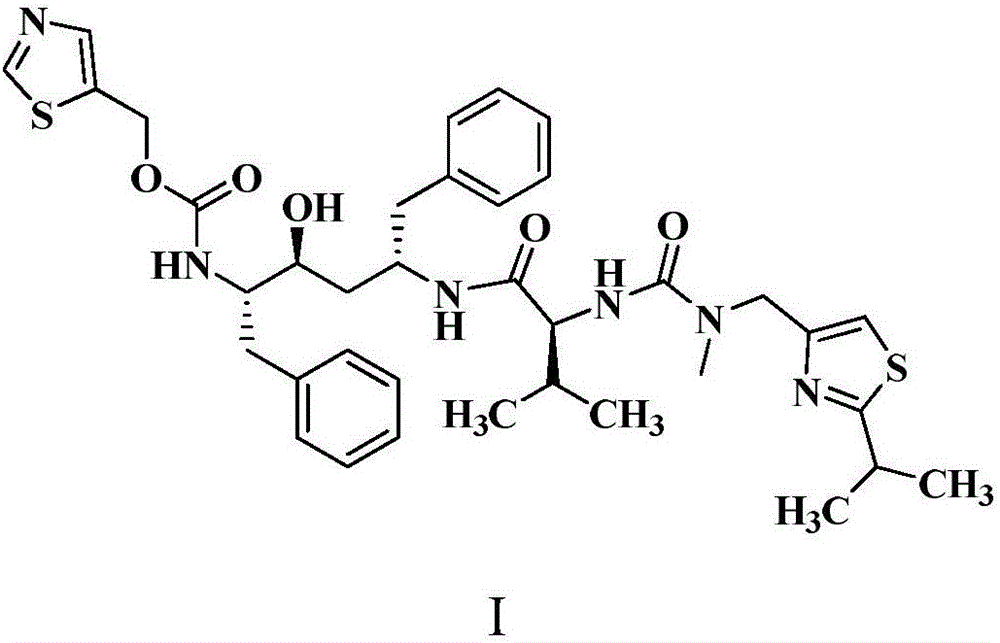
[0021] Embodiment one: the preparation of ritonavir
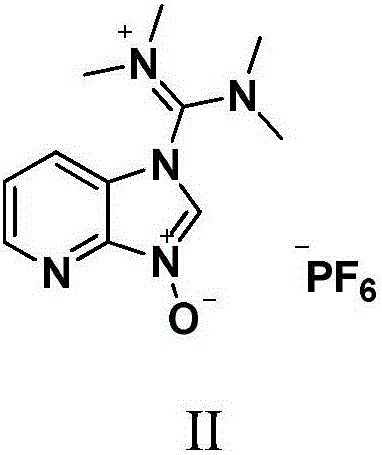
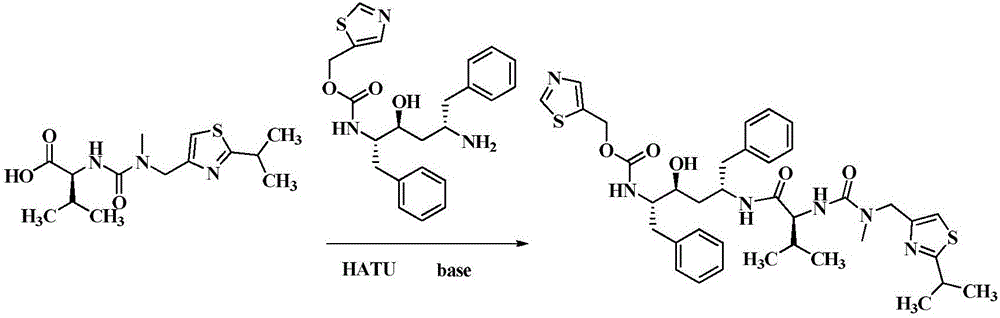
[0022] At 41°C, (2S,3S,5S)-5-amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexyl Alkane 42.6g (100mmol), N-[N-methyl-N-[(2-isopropyl-4-thiazolyl) methyl]aminocarbonyl]-L-valine 62.7g (200.0mmol), 76.0 g (200mmol) of HATU and 50.5g of diethylamine were placed in a 500mL eggplant-shaped bottle, added 200mL butanone and stirred for 40min, detected by TLC, the reaction was complete, and the reaction solution was washed with 10% sodium bicarbonate and purified water in 200mL ×2, discard the water layer, add anhydrous magnesium sulfate to the organic phase to dry for 6 hours, then filter, then heat the filtrate to 50°C, add 0.4g injection charcoal 767 for decolorization, stir for 20min, cool to 20-30°C, filter, vacuum Concentrate the organic phase to obtain oily ritonavir crude product, add 150mL ethyl acetate to the above oily product, heat to 70°C to dissolve it completely, then naturally cool to 20-30...
Embodiment 2
[0023] Embodiment two: the preparation of ritonavir
[0024]At 46°C, (2S,3S,5S)-5-amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexyl Alkane 72.4g (170mmol), N-[N-methyl-N-[(2-isopropyl-4-thiazolyl) methyl] aminocarbonyl]-L-valine 117.3g (374.0mmol), 142.2 g (374mmol) of HATU and 85.8g of diethylamine were placed in a 1L eggplant-shaped bottle, added 350mL of chloroform and stirred for 1h, detected by TLC, after the reaction was complete, the reaction solution was washed successively with 5% sodium bicarbonate and purified water for 200mL× 2. Discard the water layer, add anhydrous magnesium sulfate to the organic phase and dry it for 6 hours, then filter, then heat the filtrate to 50°C, add 0.7g activated carbon for decolorization, stir for 15min, cool to 20-30°C, filter, and concentrate the organic phase in vacuo To get oily ritonavir crude product, add 200mL ethyl acetate to the above oil, then heat to 65°C to make it completely dissolved, naturally...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com