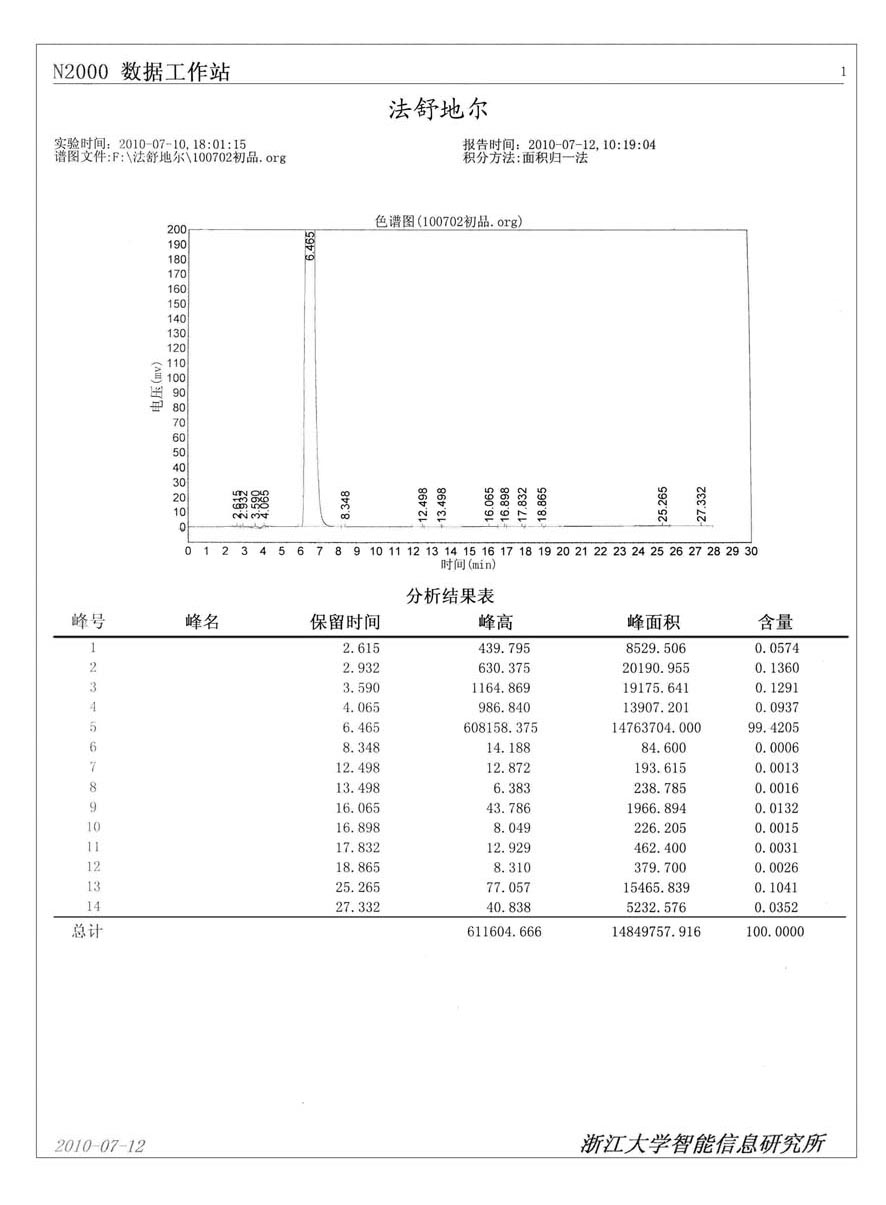
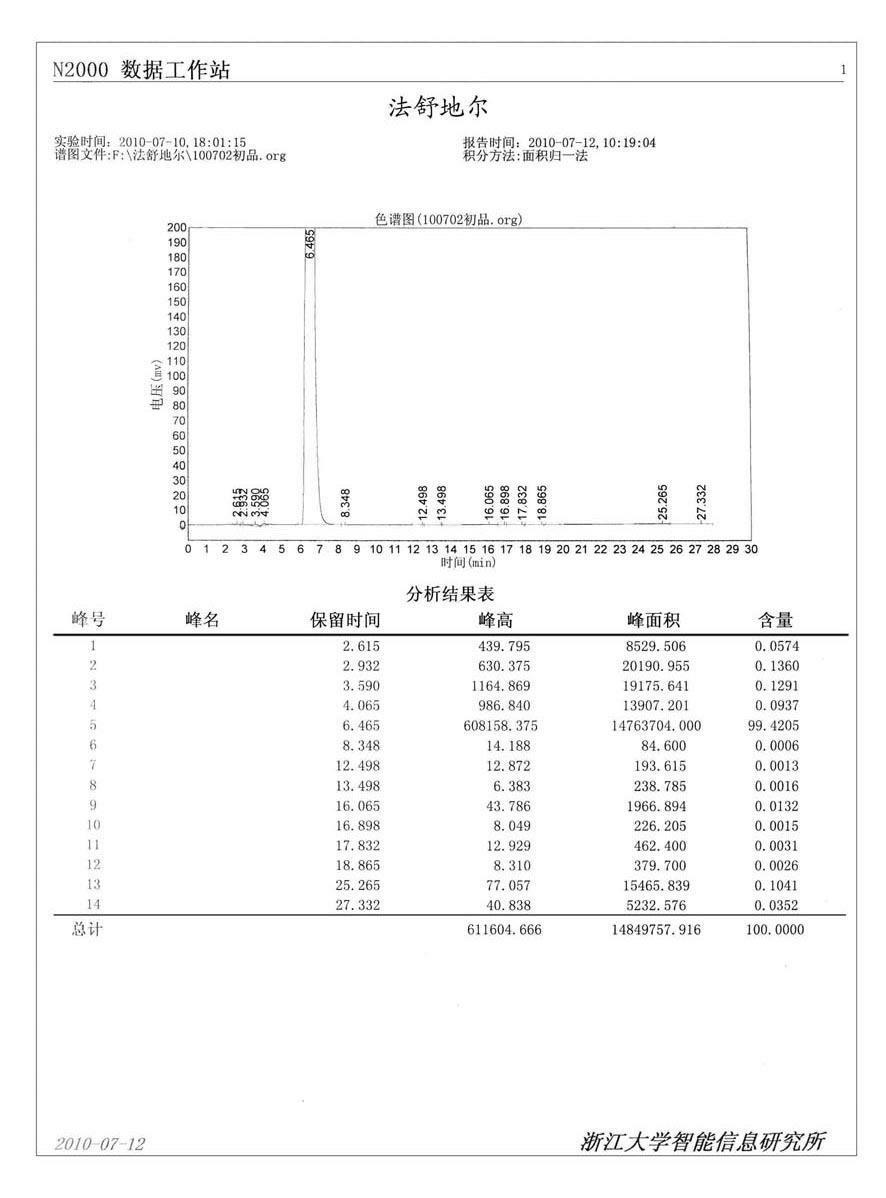
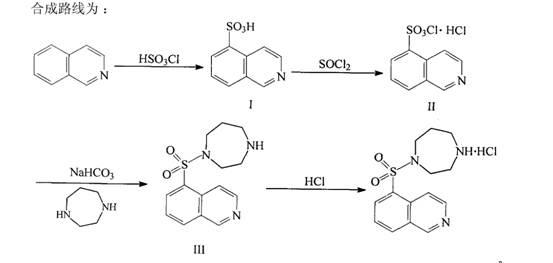
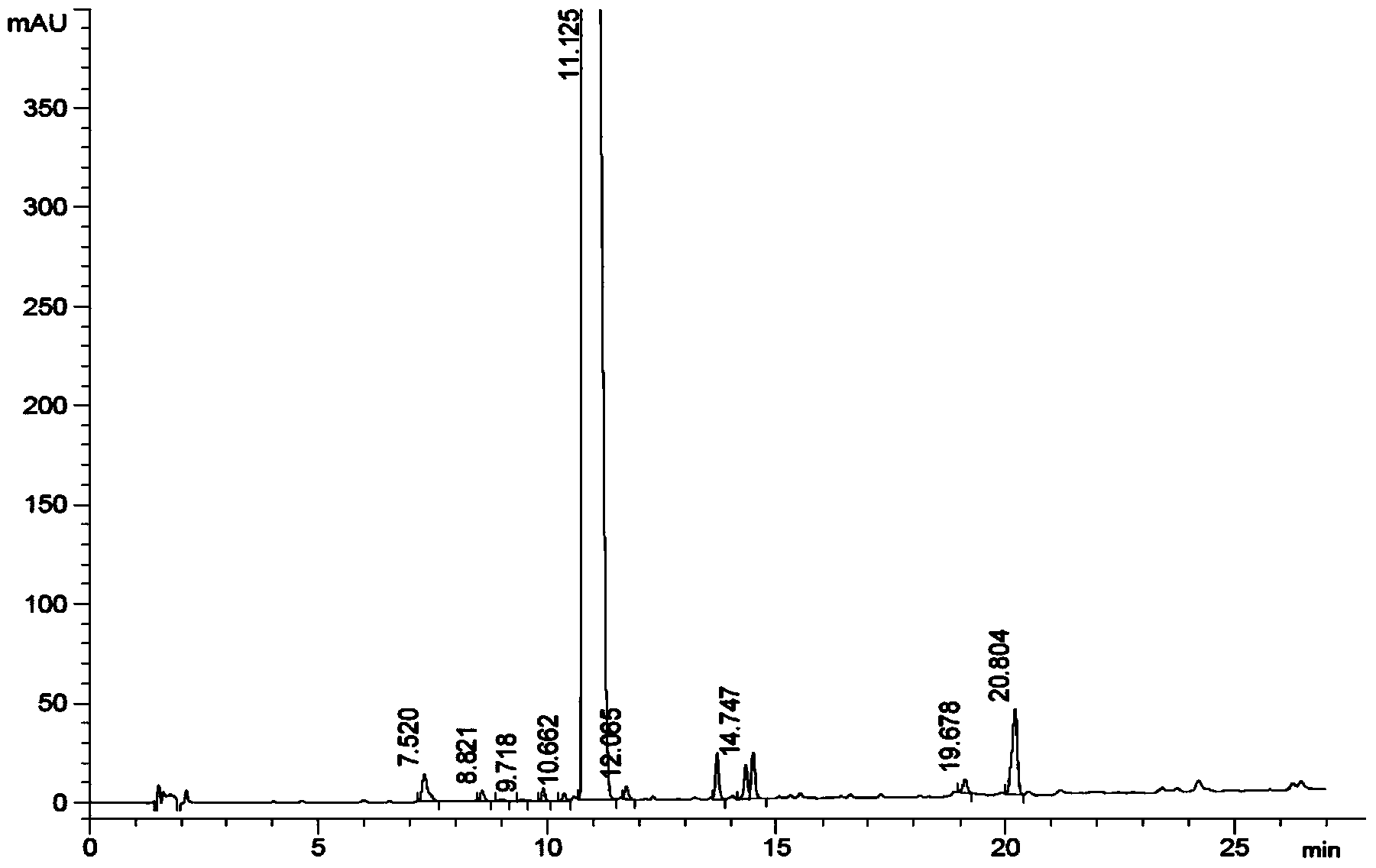
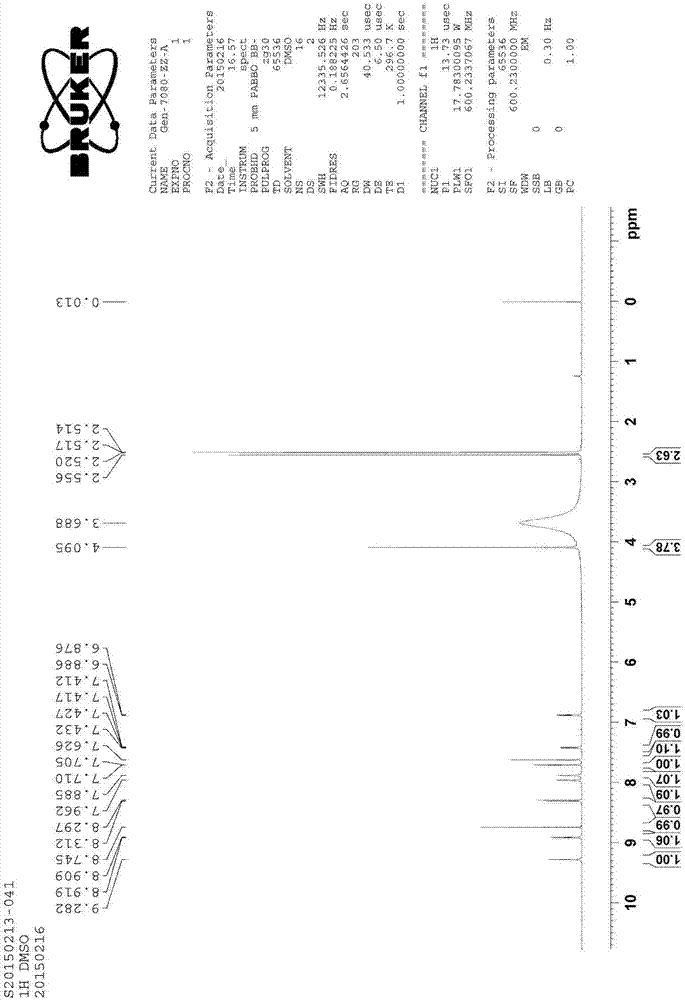
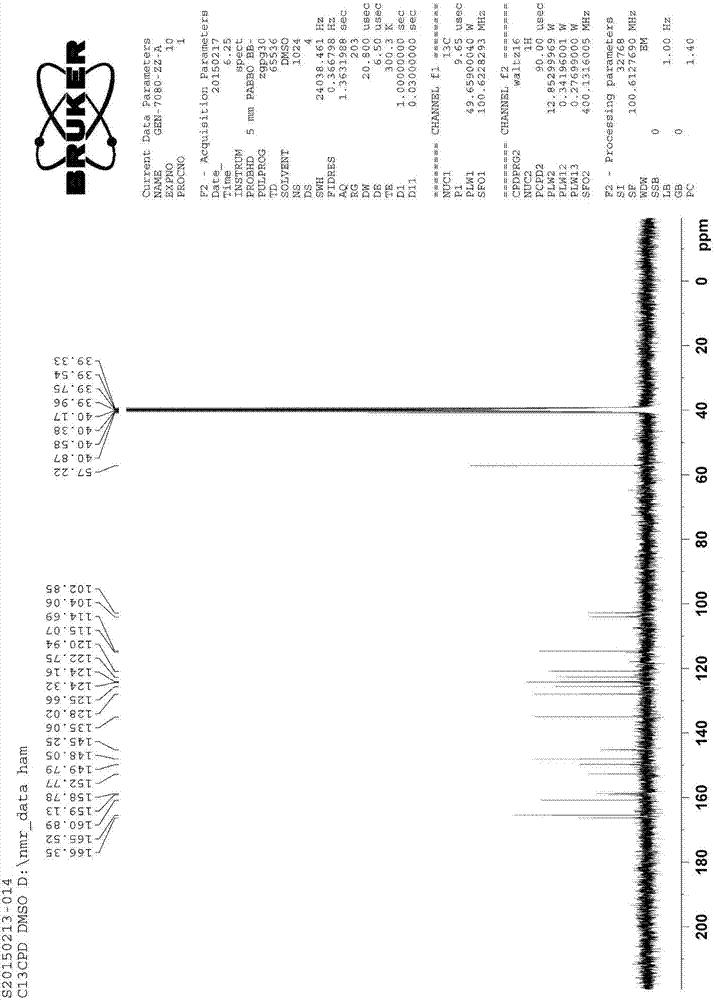
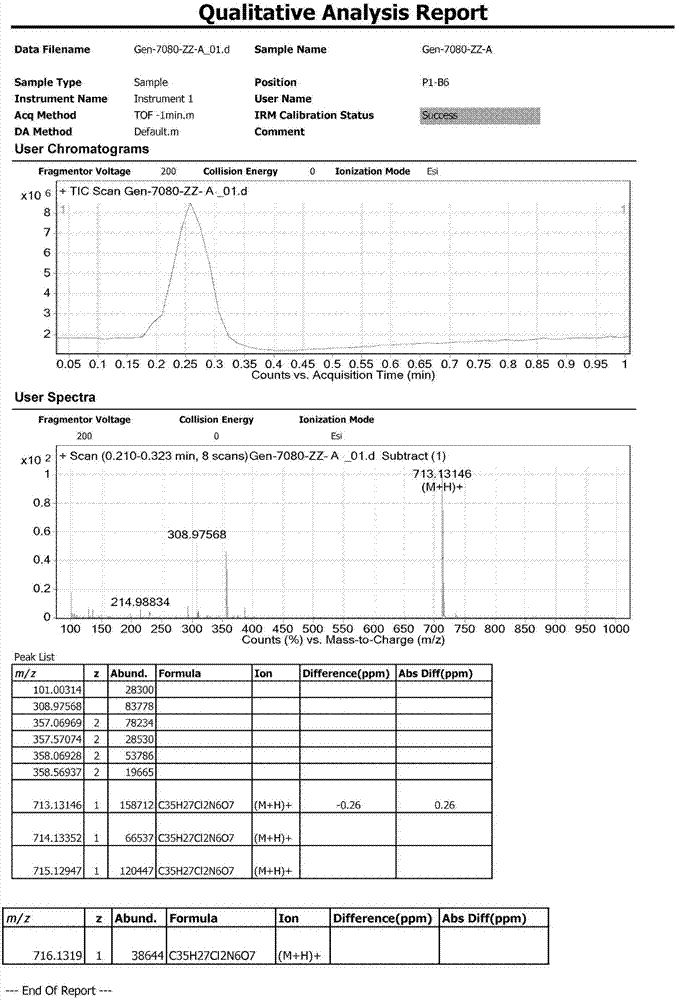
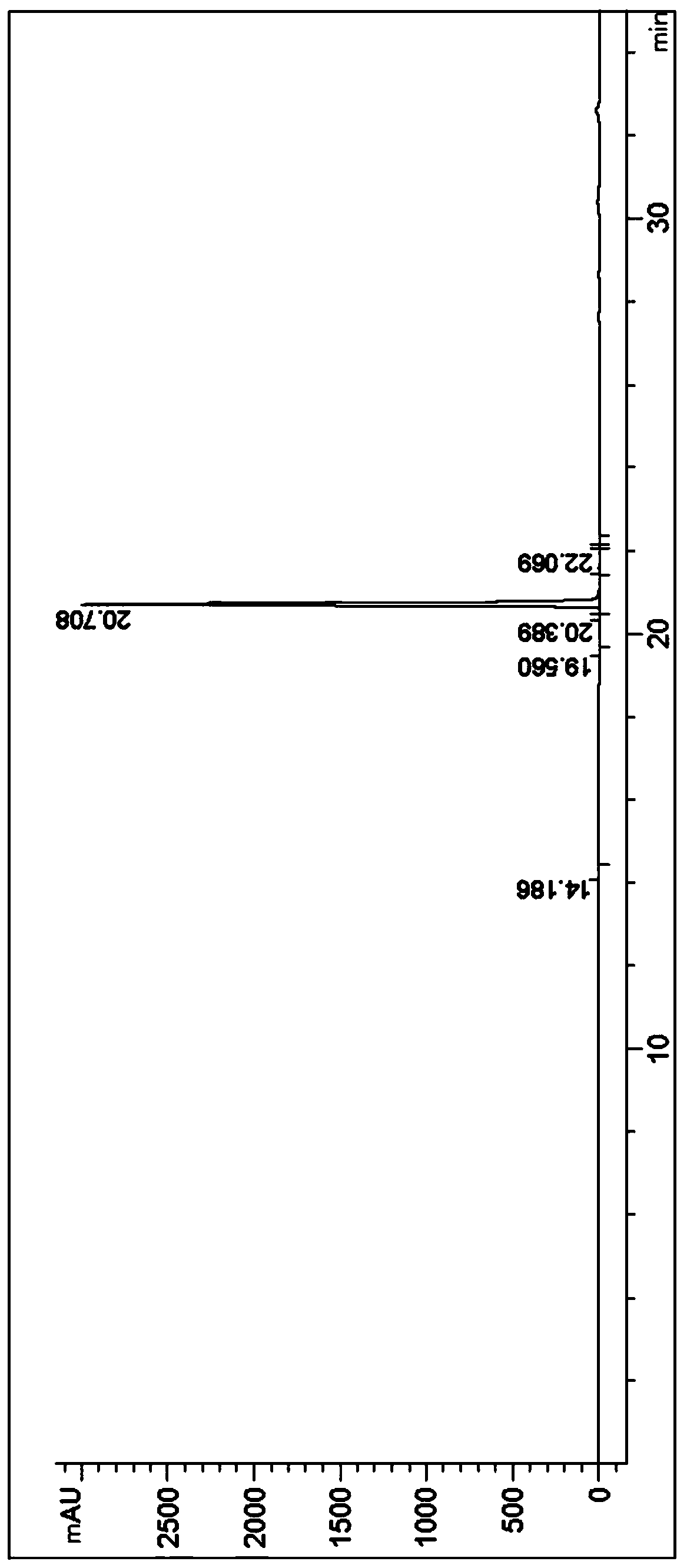
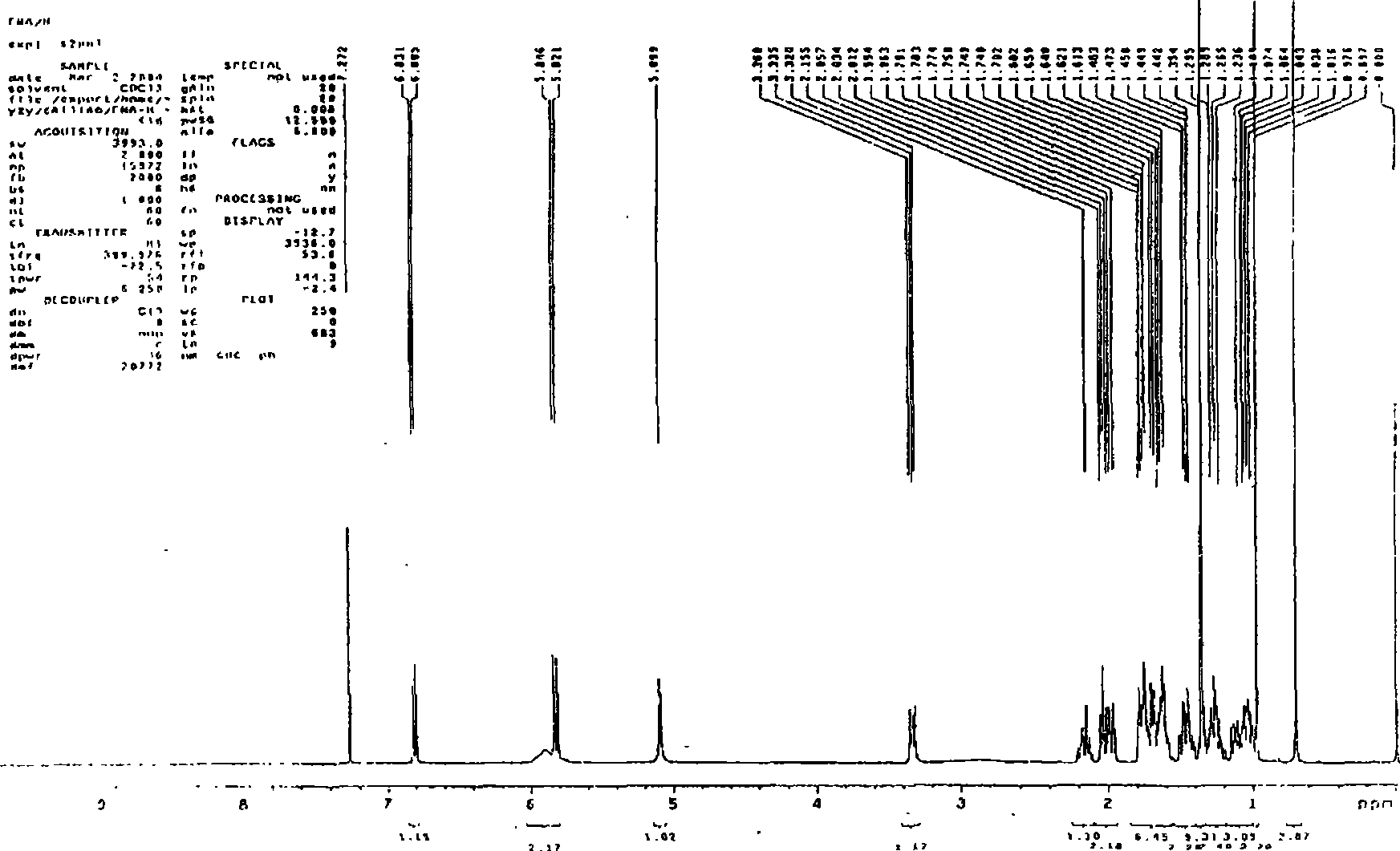
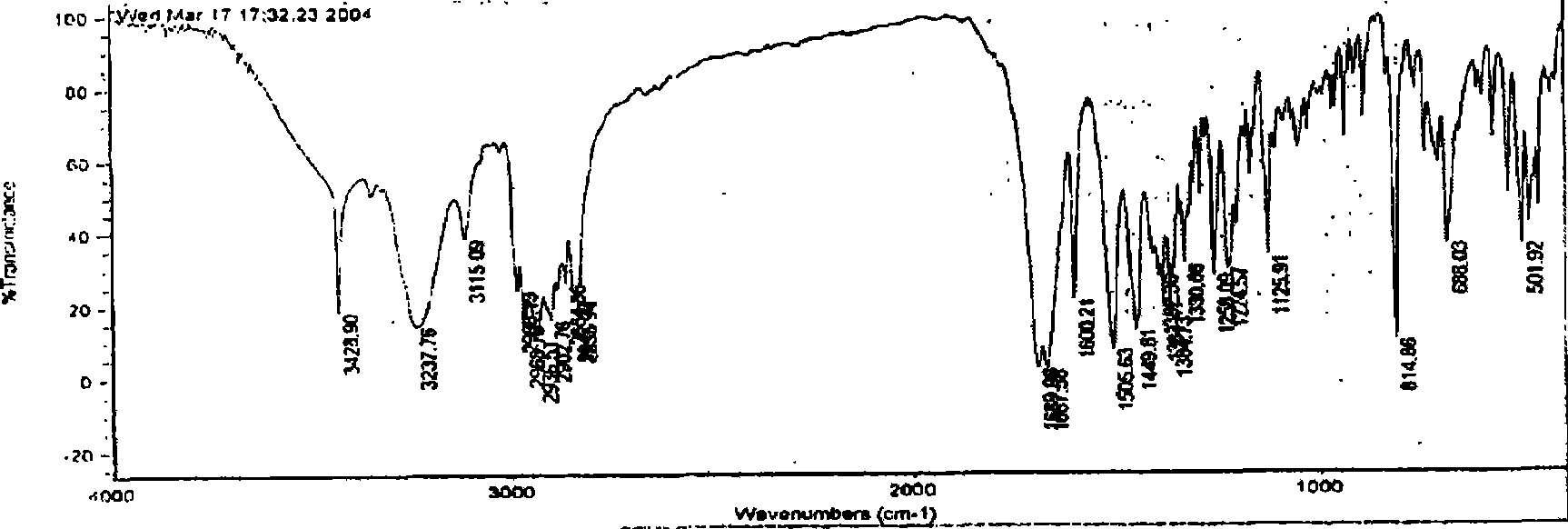
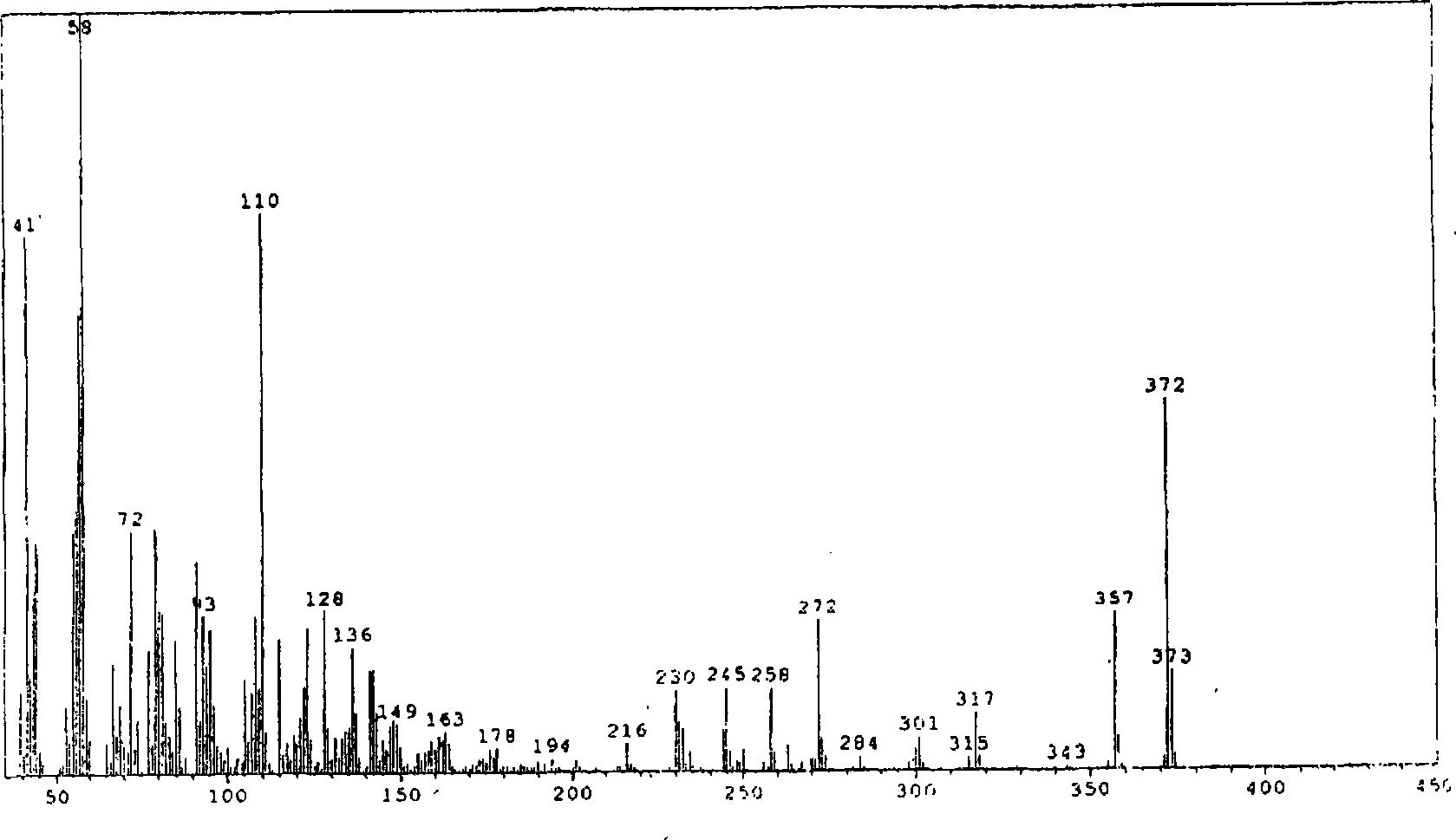
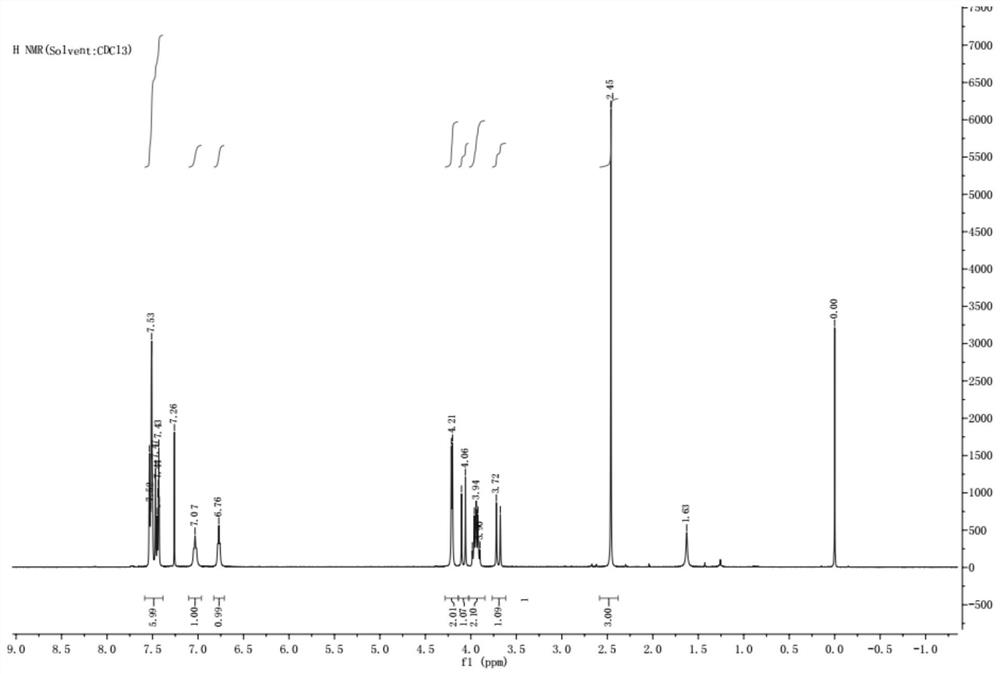
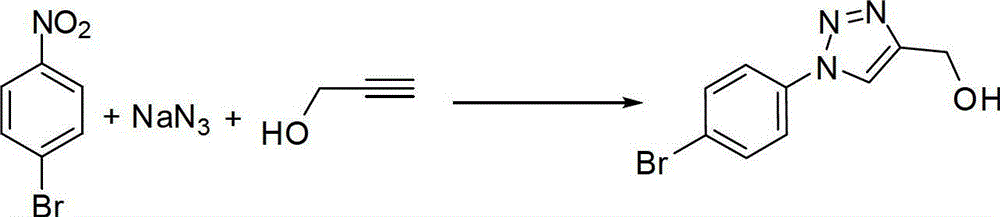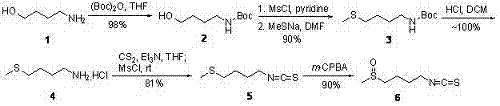Patents
Literature
1243 results about "Drugs synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ongoing production of designer drugs or synthesis drugs like PCP, methamphetamine, MDMA (ecstasy), and bath salts is a dangerous process. Each time a new synthesis drug is made, the ingredients used are made illegal by governing agencies responsible for taking those chemicals off the open market.
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Cyclodextrin polymer compositions for use as drug carriers
Owner:KOSAK KENNETH M
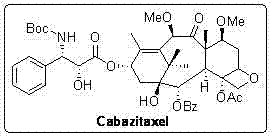
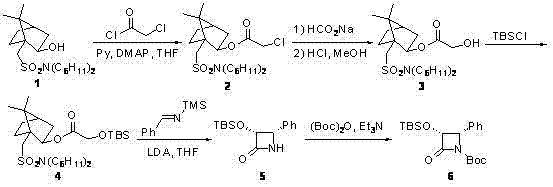
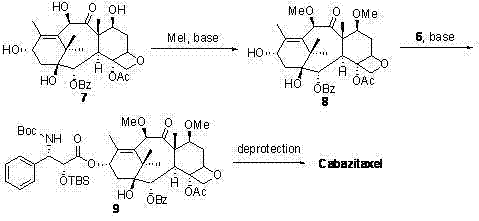
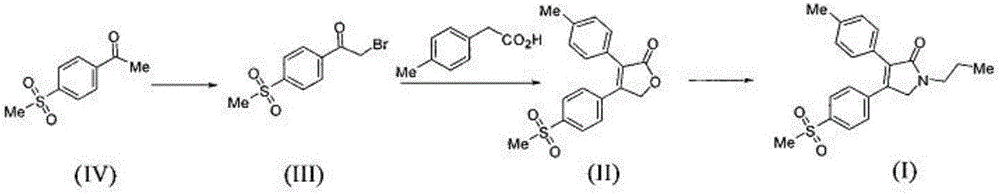
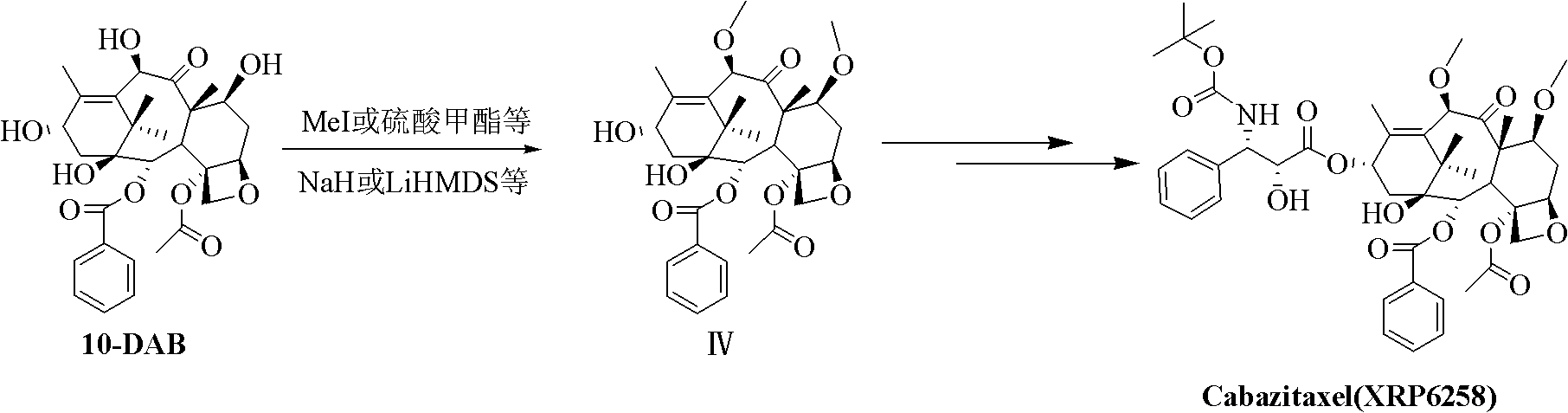
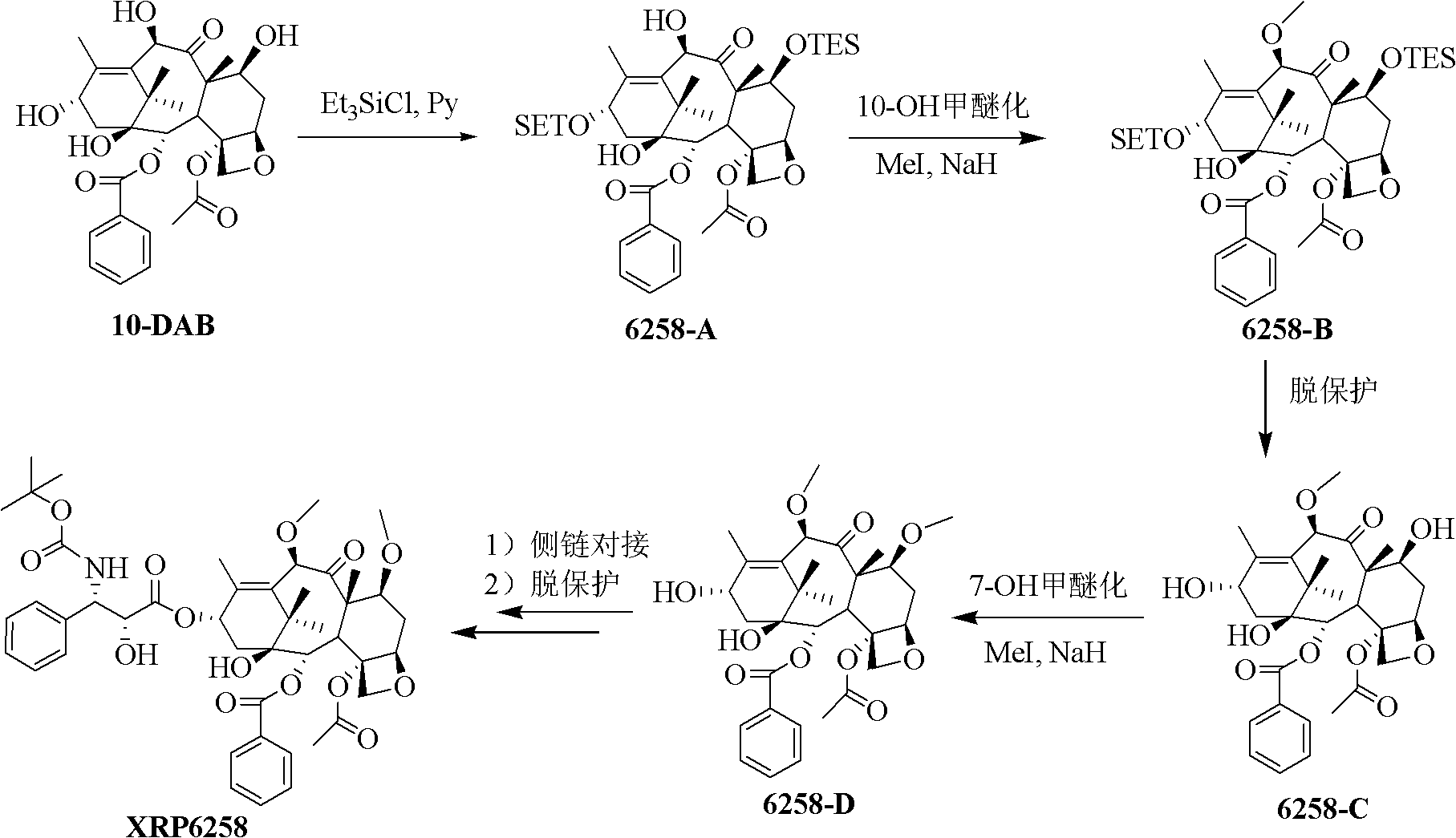
The synthetic method of cabazitaxel
ActiveCN102285947AShort stepsMild conditionsOrganic chemistryChemical recyclingCabazitaxelDrugs synthesis
The invention discloses a method for synthesizing cabazitaxel, and belongs to the field of medicine synthesis. In the method, oppolzer reagent is used as a chiral source, and (3R,4S)-beta-lactam with optical purity ee of 98 percent is obtained by five steps. Under the action of a proper alkali, hydroxyls on C-7 and C-10 in 10-baccatine III are methylated selectively to form 7,10-dimethoxy-10-baccatine III in one step. Then beta-lactam is used to esterify the 7,10-dimethoxy-10-baccatine III under an alkaline condition, and then cabazitaxel with a purity of 99 percent can be obtained by a step of removing protective groups. In the synthesis method, the steps are short and the conditions are mild.
Owner:江苏宁录科技股份有限公司
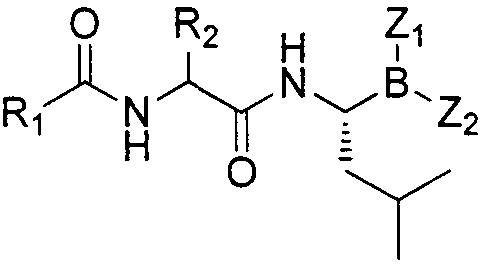
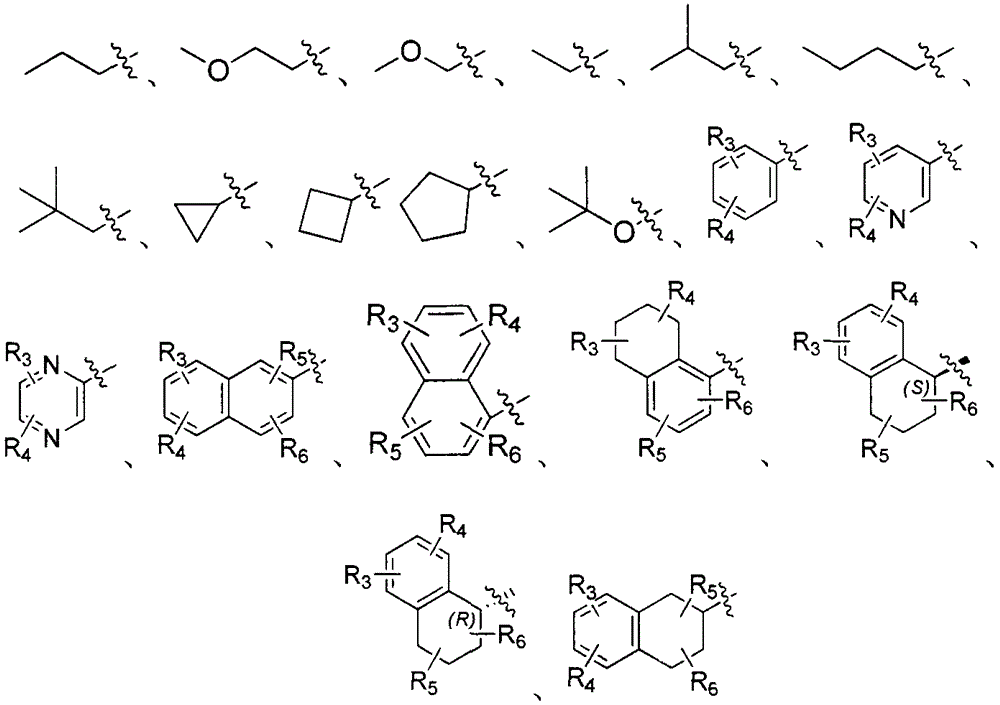
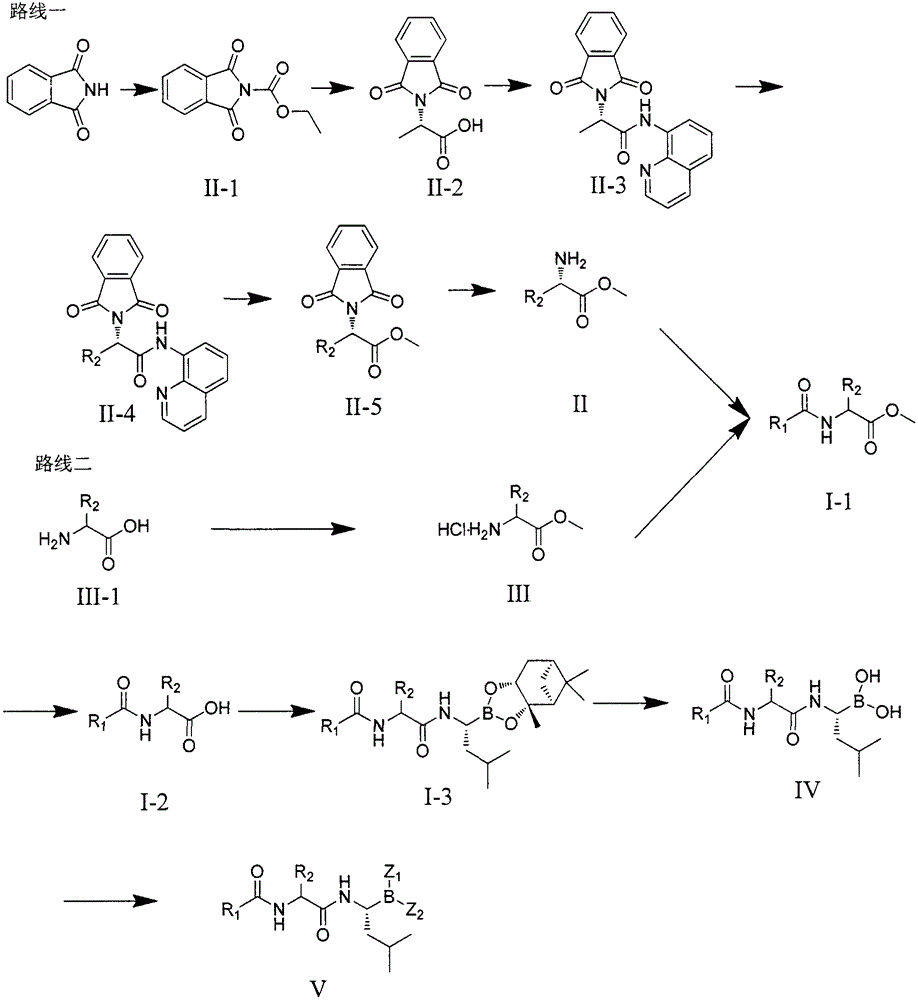
Dipeptide boric acid composed of carboxylic acid and alpha-amino acid as well as ester compound thereof, and preparation method and application of dipeptide boric acid and ester compound thereof
ActiveCN105732683AHigh yieldHigh activityBoron compound active ingredientsGroup 3/13 element organic compoundsProstate cancerProteasome inhibitor
The invention belongs to the field of drug synthesis and in particular relates to a series of novel peptide boric acids as well as an ester compound or pharmaceutical salt thereof, and a preparation method and application of the peptide boric acids as well as the ester compound or pharmaceutical salt thereof in pharmacodynamics. A structure of the peptide boric acid and the ester compound or pharmaceutical salt thereof is shown in a formula I (described in the specification). The compound provided by the invention can be used for preparing a proteasome inhibitor and can further be used for treating solid tumours and blood tumours, wherein the solid tumours are selected from non-small cell lung cancer, small cell lung cancer, lung adenocarcinoma, lung squamous carcinoma, pancreatic cancer, breast cancer, prostate cancer, liver cancer, skin cancer, epithelial cell cancer, gastrointestinal stromal tumor, nasopharynx cancer and leukemia; and the blood tumours are selected from multiple myeloma, mantle cell lymphoma and histiocytic lymphoma.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
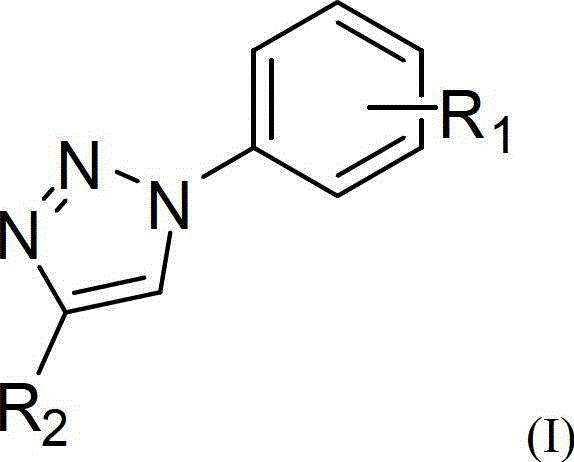
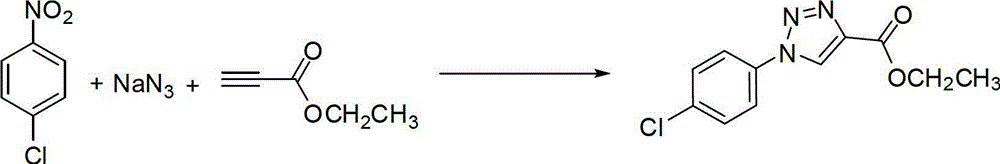
One-pot synthetic method for 1,2,3-triazole compounds
The invention provides a one-pot method for synthesizing 1,2,3-triazole compounds, and belongs to the technical field of organic synthesis. According to the one-pot synthetic method for the 1,2,3-triazole compounds, nitrobenzene compounds and alkyne compounds serve as starting materials, and a plurality of the 1,2,3-triazole compounds are synthesized conveniently in a one-pot mode under the catalysis of copper. The 1,2,3-triazole compounds synthesized with the one-pot synthetic method are important intermediates for drug synthesis, and have very important application prospects in the fields including biomedicine, medicines and the like. Certain components are already widely used for clinics. The method adopts one-pot preparation, so that diazotization reaction is avoided, organic azides are not directly used, and the one-pot synthetic method for the 1,2,3-triazole compounds is high in safety, simple in operation, mild in conditions, low in cost and easy to use for industrial production and has good application prospects.
Owner:THE CITY VOCATIONAL COLLEGE OF JIANGSU
Method for preparing hydroxyl fasudil compounds
The invention relates to a method for preparing hemangiectasis medicine, in particular to the method for preparing hydroxyl fasudil compounds, which belongs to the field of medicine synthesis. In the preparation process, the method of using column chromatography for elution and purification in a hydroxyl fasudil impurity removal method is omitted for realizing large-scale mass production.
Owner:珠海晨安医药有限公司
Synthetic method of kyprolis
ActiveCN103641890AHigh reaction yieldHigh yieldPeptidesBulk chemical productionAcetic acidMorpholine
The invention relates to the technical field of drug synthesis, and particularly relates to a synthetic method of kyprolis. The synthetic method comprises the following steps: carrying out condensation reaction on morpholine-4-base acetic acid, L-homophenylalanine ester and salt, and then carrying out decarboxylation protection to generate a compound V; carrying out the condensation reaction on the decarboxylation, the salt and N-Boc-L-leucine, and then carrying out deamination protection to generate a compound VI; carrying out the condensation reaction on the compound V and the compound VI, and then carrying out decarboxylation protection to generate a compound VII; carrying out the condensation reaction on the compound VII and a compound VIII to obtain the kyprolis. The method disclosed by the invention can be used for enhancing the reaction yield by adopting a converging synthetic method; the used reagent is easy, convenient, easy to obtain and less in pollution. The process disclosed by the invention only relates to the condensation and deprotection between amino acids and is simple and controllable in reaction and suitable for industrial production.
Owner:重庆兴泰濠制药有限公司
Process for preparing attapulgite based composite material
ActiveCN101469145AChange hydrophilicityGood compatibilityPigment treatment with organosilicon compoundsSolubilityChemical synthesis
The invention discloses a method for preparing an attapulgite / ionic liquid composite material. The method utilizes ionic liquid molecules with a designable molecular structure to modify attapulgite and provides a novel approach for increasing the compatibility between the attapulgite and diverse organic solvents. The method bonds a functionalized ionic liquid containing a carboxyl functional group on the surface of the attapulgite through a covalent bond, and utilizes anion exchange to change hydrophilic and hydrophobic properties of the attapulgite. The preparation process and the preparation method are simple. The attapulgite composite material with controllable solubility and is modified by the ionic liquid is expected to be applied in the field of catalysis, sensors, novel nanometer materials, chemosynthesis, water treatment, food processing, mining, drug synthesis and so on.
Owner:YANTAI ZHONGKE RES INST OF ADVANCED MATERIALS & GREEN CHEM ENG
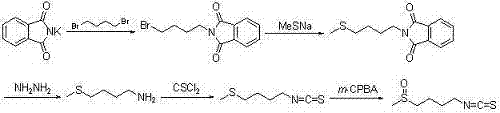
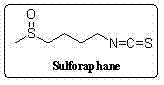
Synthetic method for sulforaphane
ActiveCN102249968AAvoid hydrazinolysisSimple and fast operationOrganic chemistryBulk chemical productionSulforaphaneSodium methanethiolate
The invention provides a synthetic method for sulforaphane and belongs to the field of drug synthesis. The method comprises the following steps that: after amino in 4-amino-1-butanol is protected by Boc groups, hydroxy in 4-amino-1-butanol is changed into methanesulfonyl ester by methanesulfonyl chloride, and then the resultant reacts with sodium methyl mercaptide to produce 4-methylthio butyl-1-tert-butoxycarbonylamide; Boc protective groups are removed under acidic condition to obtain 4-methylthio-1-butylamine; 4-methylthio-1-butylamine reacts with carbon disulfide for one hour in the presence of triethylamine and p-toluenesulfonyl chloride is added for treatment for half an hour to produce 4-methylthio butyl-1-isothiocyanate; and at last 4-methylthio butyl-1-isothiocyanate is oxidized by m-CPBA to produce sulforaphane. According to the invention, complex hydrazinolysis of phthalimide in aftertreatment is avoided and toxic thiophosgene is not needed in the preparation of isothiocyanate; overall yield of sulforaphane is 64%, substantially higher than the overall yield of 8% reported in literature; the whole preparation process is simple and time-saving and is suitable for large scale production.
Owner:江苏宁录科技股份有限公司
Preparation method for ibrutinib
ActiveCN105859728AHigh purityHigh yieldGroup 4/14 element organic compoundsFormamidine acetateDrugs synthesis
The invention discloses a preparation method for ibrutinib and belongs to the technical field of drug synthesis. The preparation method specifically includes the steps that 3-amino-4-cyano pyrazol and formamidine acetate serve as initial raw materials, and ibrutinib is obtained through a cyclization reaction, a halogenating reaction, a nucleophilic substitution reaction, a Mitsunobu reaction and an amidation reaction. According to the method, the raw materials are easy to obtain, conditions are mild, the process operability and controllability are high, cost is low, the yield is high, fewer side products are generated, purification is easy, and the high-quality product is obtained.
Owner:南京红太阳医药研究院有限公司
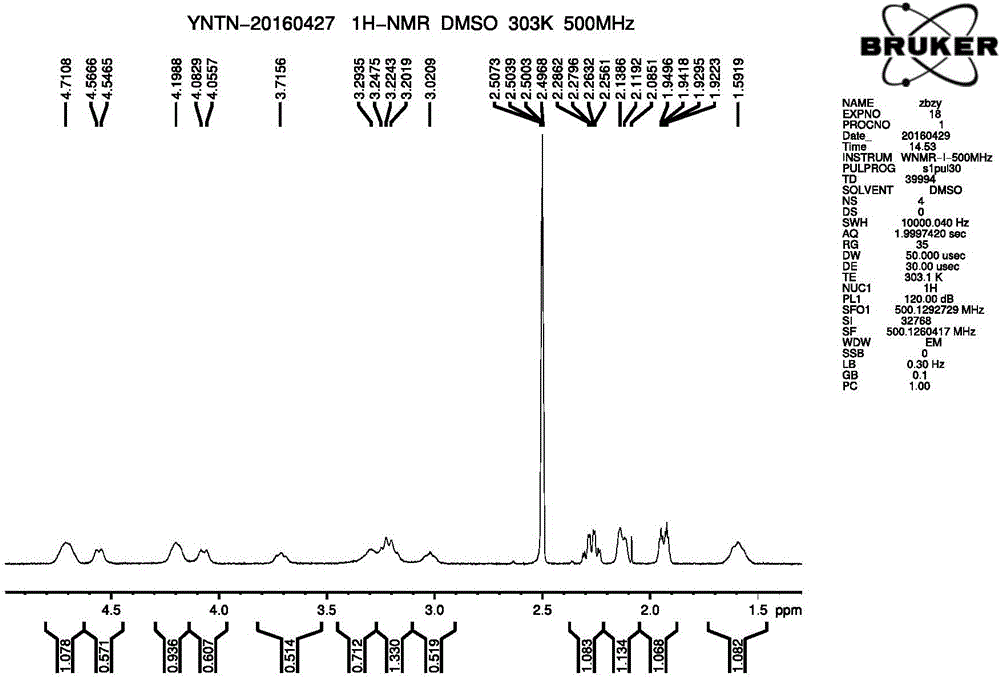
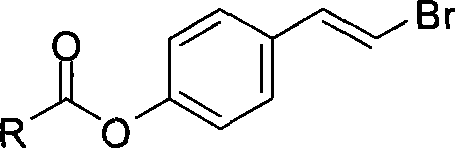
IDO restrainer containing (E)-4-(beta-bromo vinyl)benzoyloxy structure
The invention belongs to the technical field of new drug synthesis, and in particular relates to an IDO inhibitor containing (E)-4-(beta-bromovinyl)phenoxy acyl structure, as well as a preparation method thereof. The preparation method comprises the following steps: solvent benzene, (E)-4-(beta-bromovinyl) phenol, carboxylic acid and p-dimethylaminopyridine are added to a flask respectively and magnetically stirred for 5 to 20 minutes at room temperature; then N, N'-dicyclohexylcarbodiimide is added and reacts for 2 to 24 hours at room temperature; after the reaction is completed, the solvent benzene is steamed off after decompression; residue is subjected to column chromatography separation and purification by taking ethyl acetate / petroleum ether as leacheate, and then a needed product can be obtained, wherein the molar ratio of the solvent benzene to the (E)-4-(beta-bromovinyl) phenol is 50-200:1; the molar ratio of the carboxylic acid to the (E)-4-(beta-bromovinyl) phenol is 1-1.5:1; the molar ratio of the p-dimethylaminopyridine to the (E)-4-(beta-bromovinyl) phenol is 0.1-1.5:1; and the molar ratio of the N, N'-dicyclohexylcarbodiimide to the (E)-4-(beta-bromovinyl) phenol is 1-1.2:1. The method takes the (E)-4-(beta-bromovinyl) phenol as a synthesis building block and obtains a series of the novel IDO inhibitors containing the (E)-4-(beta-bromovinyl)phenoxy acyl structure through esterification reaction, and the IDO inhibitors can be used for treating the diseases with the pathological features of IDO mediated tryptophan metabolic pathway.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Preparation methods of lenvatinib mesylate drug impurities
The invention belongs to the field of pharmaceutical synthesis, and relates to impurities in a raw medical material production process and preparation methods of the impurities, in particular to preparation methods of process impurities A, B and C of lenvatinib mesylate, namely, 4-[3-chloro-4-(N'-cyclopropylureido) phenoxy]-7-methoxyquinoline-6-carboxamide mesylate), as a drug for treating radioiodine-refractory thyroid cancer and an application of the impurities to quality research of lenvatinib mesylate. With adoption of the methods, the process impurities A, B and C are obtained through chemical synthesis for the first time, and the target compounds shown in the description can be obtained through efficient and rapid separation.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +2
Synthesis method of dapoxetine hydrochloride
InactiveCN103664660ANo dangerThe reaction steps are simpleOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsNitrogen gas
The invention relates to a synthesis method of a dapoxetine hydrochloride, and belongs to the technical field of medicine synthesis. The synthesis method comprises the following steps: dispersing (S)-3-amino-3-phenylpropionic acid in a solvent, adding a reducing agent and an acid for reaction to obtain (S)-3-amino-3-phenylpropanol; dissolving the (S)-3-amino-3-phenylpropanol in formic acid, adding formaldehyde for reaction to obtain (S)-3-dimethylamino-3-phenylpropanol; dissolving the (S)-3-dimethylamino-3-phenylpropanol in an anhydrous solvent, under the protection of nitrogen and in ice bath, adding an alkali and 1-fluorinated naphthalene while stirring, heating up to 90-110 DEG C for reaction for 3 to 5 hours, leading into a dry hydrogen chloride gas to obtain the final product dapoxetine hydrochloride. The synthesis method has the advantages of less steps, simple reaction conditions, simple and practicable post processing, low production cost, high purity and yield, and suitability for industrialized mass production.
Owner:SUZHOU UUGENE BIOPHARMA
Novel synthesis method of mirabegron
ActiveCN103304511ALow costRealize safe and clean productionOrganic chemistryBulk chemical productionSynthesis methodsPhenethyl alcohol
The invention provides a novel synthesis method of mirabegron, and belongs to the technical field of drug synthesis. The problems that the yield of synthesized mirabegron is low in the prior art, and the method is unsuitable for large-scale industrial production are solved. The synthesis steps are as follows: 1) performing amino protection, namely reacting 2-aminothiazole-4-acetic acid with an amino protective agent to obtain a mirabegron intermediate product A; 2) performing condensation reaction, namely performing the condensation reaction on the mirabegron intermediate product A and the 4-aminophenethanol to obtain the mirabegron intermediate product B; 3) performing oxidizing reaction, namely performing the oxidizing reaction on the mirabegron intermediate product B and an oxidizing agent to obtain the mirabegron intermediate product C; 4) removing protective agent while performing reductive amination, namely reacting the mirabegron intermediate product C with (R)-2-amino-1-phenethanol under the effect of a reducing agent, and meanwhile removing the protective group on the mirabegron intermediate product C to obtain the mirabegron. The novel synthesis method of mirabegron provided by the invention is low in cost, high in product yield and suitable for large-scale industrial production.
Owner:江苏欣德瑞医药科技有限公司
Preparation method of antidepressant drug-vilazodone
InactiveCN103304547AReduce usageReaction raw materials are readily availableOrganic chemistryCarboxylic acidMethyl group
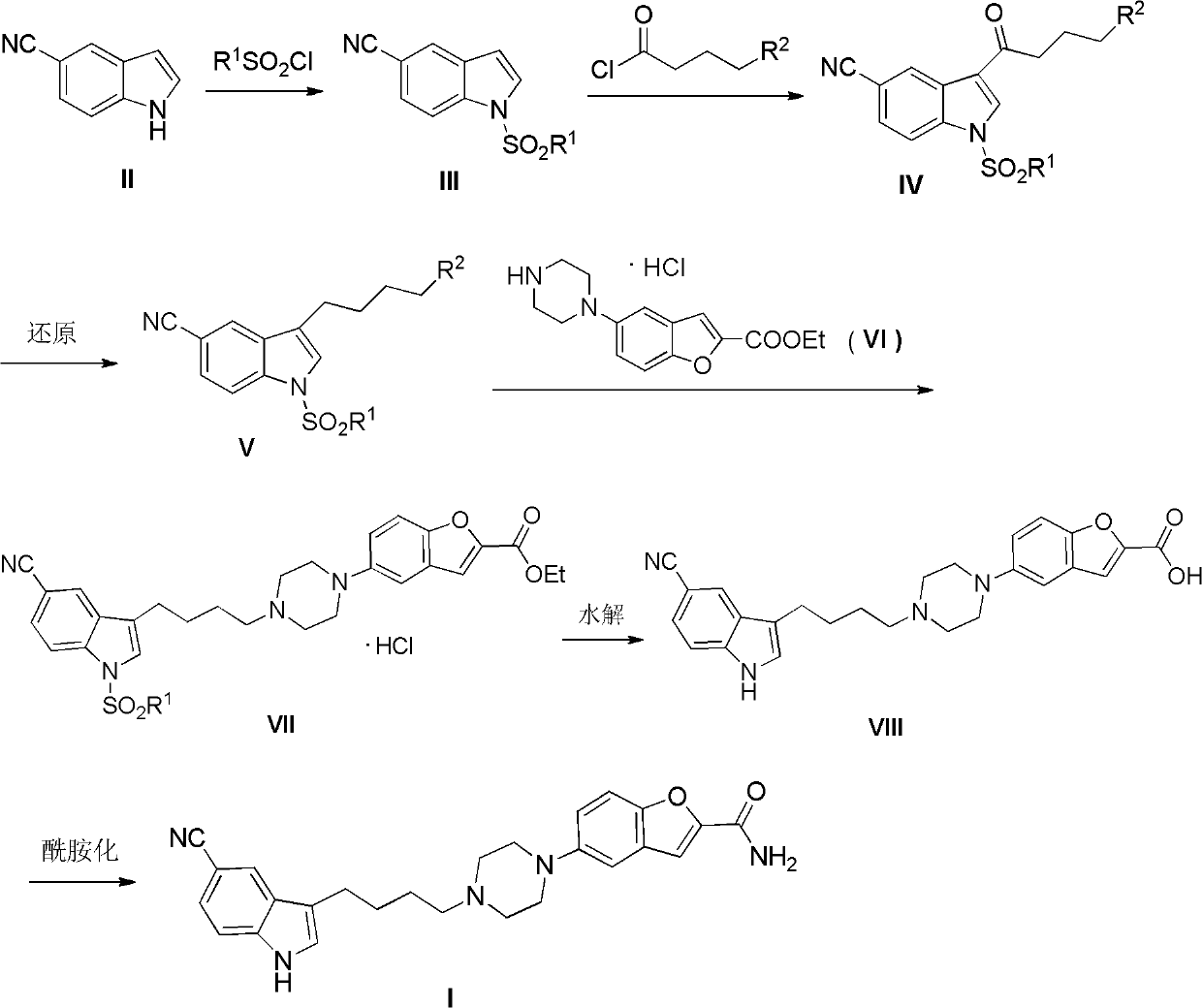
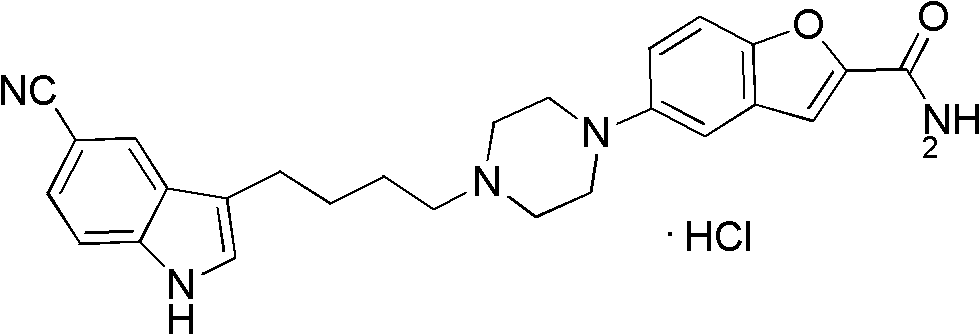
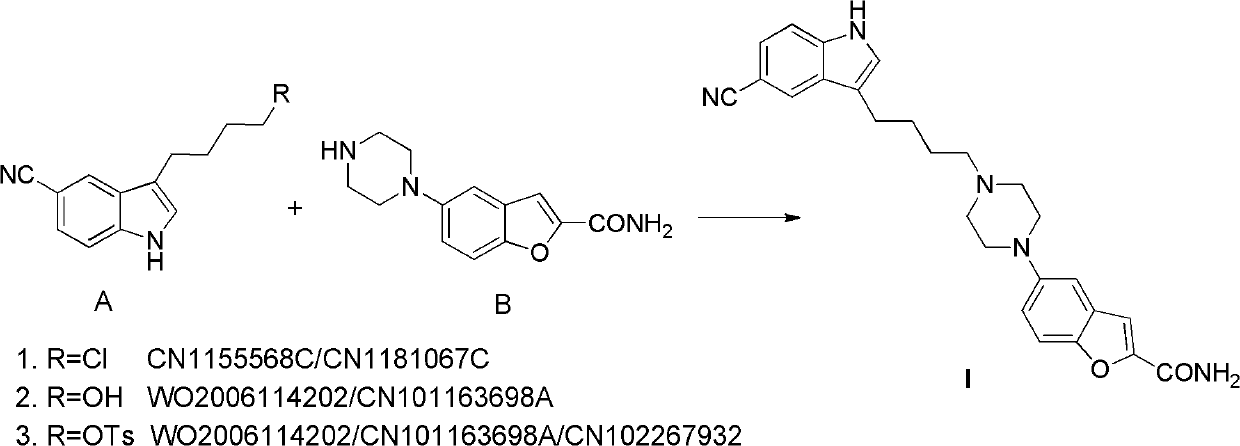
The invention relates to the field of drug synthesis, and in particular relates to a preparation method of an antidepressant drug-vilazodone. The preparation method is characterized by comprising the following steps of: protecting a 1-location nitrogen atom of 5-cyano benzopyrrole used as an initial raw material through utilizing toluenesulfonyl, carrying out 3-location Friedel-Carfts acylation, and selectively reducing carbonyl into methylidyne so as to obtain 1-tosyl-3-(4-chlorobutyl)-5-cyano benzopyrrole; reacting the 1-tosyl-3-(4-chlorobutyl)-5-cyano benzopyrrole with 5-(piperazine-1-radical) benzofuran-2-nonanoic acid-ethyl ester hydrochloride, hydrolyzing through one step, and removing tosyl and ethyl at the same time so as to obtain 5-(4-(4-(5-cyan-1H-benzopyrrole-3-radical) butyl) piperazine-1-radical) benzofuran-2-carboxylic acid; finally, amidating so as to obtain vilazodone, and salifying vilazodone with hydrogen chloride so as to obtain hydrochloric acid vilazodone. The method can be used for overcoming a plurality of defects of a conventional synthetic method, and has the advantages of easiness in raw material acquisition, high yield, good selectivity, convenience in operation, suitability for industrial production and high application value.
Owner:CHINA PHARM UNIV
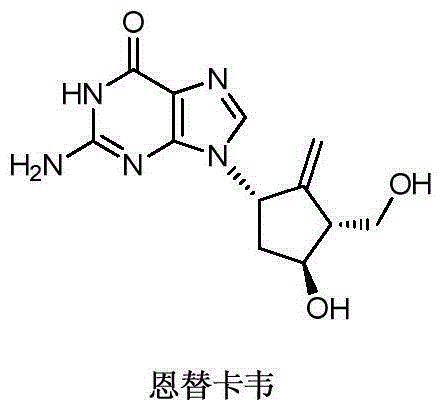
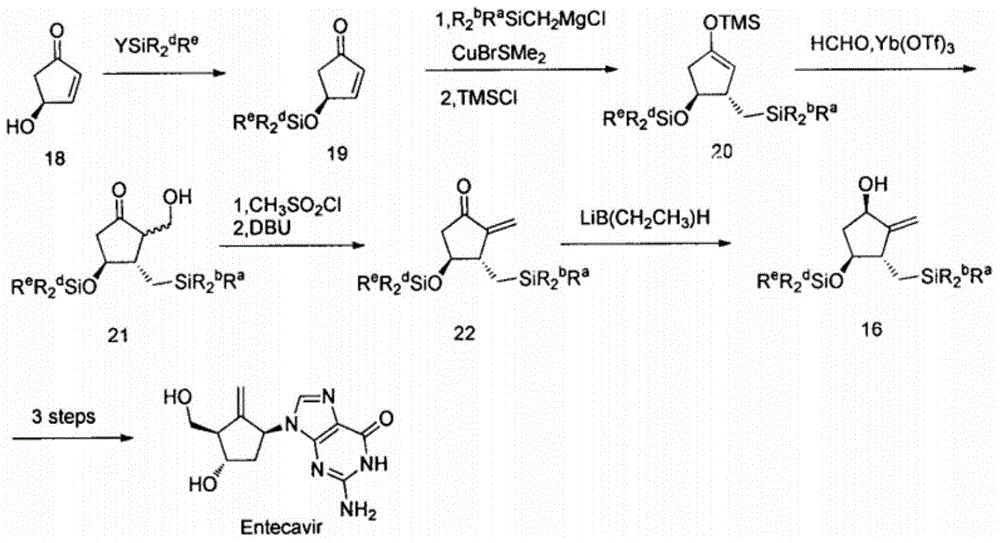
Novel synthetic method for entecavir compound
ActiveCN105037363ASolve rare problemsDosage controllableGroup 4/14 element organic compoundsKetoneAdipate
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Synthesis method of anti-influenza and avian influenza virus resistant medicine peramivir
InactiveCN102372657AMild reaction conditionsSimple and fast operationOrganic chemistryOrganic compound preparationSynthesis methodsKetone
The invention belongs to the synthesis technical field of organic medicine, and discloses a synthesis method of anti-influenza and avian influenza virus resistant medicine peramivir, which is characterized by adopting (1R, 4S)-2-azabicyclo[2.2.1]hept-5-en-3-one as a raw material and by comprising the following steps: step I: catalyzing, loop-opening and amino protection; step II: 1,3-dipolarcycloaddition; step II: sodium borohydride and nickel chloride hexahydrate reduction and acetylization; and step IV: amino protection removal, and chloroformamidine hydrochloride is adopted as reaction agent to generate guanidyl and hydrolysis methyl ester to prepare peramivir. The sodium borhydride and the nickelous chloride reduction system is used for substituting the expensive platinum dioxide to be used as reducing agent during the hydrogenation reduction process, and the self-produced chloroformamidine hydrochloride is used during the process for feeding the guanidyl reagent, so the cost is reduced, and the operation procedures are simplified. The synthesis method has short reaction routine and moderate reaction condition, is simple and convenient to operate, has low cost, and is suitable for the industrialized production.
Owner:JINAN UNIVERSITY
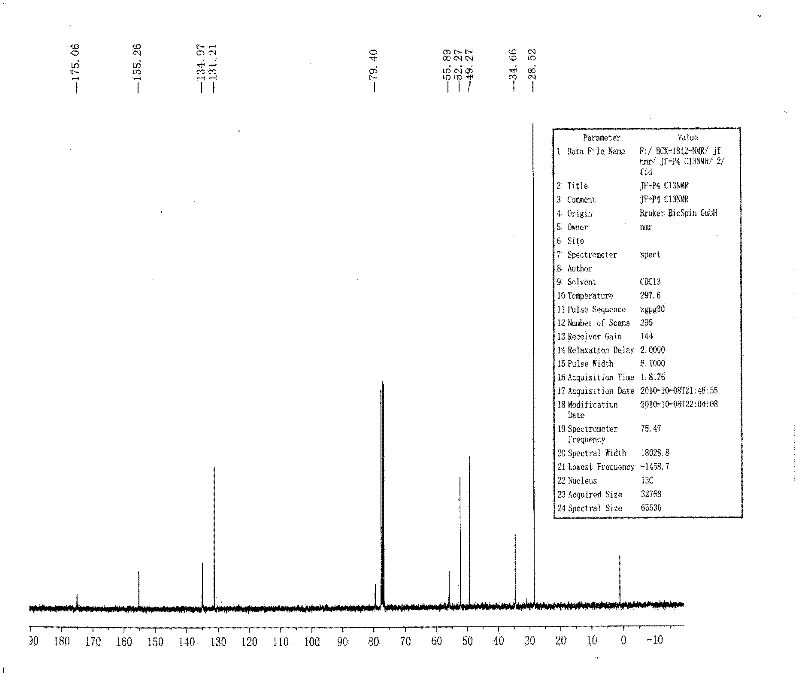
1-(3-indolyl)-1,2,3,4-tetrahydro-beta-carboline derivative and its prepn and use
The present invention belongs to the field of medicine synthesis technology, and is especially 1- (3-indolyl)-1, 2, 3, 4-tetrahydro-beta-carboline compounds and analogs in the general expression as shown and with substituted hydroxyl radical or acyl radical in the 2nd position, and their preparation process and medical application. Initial pharmacodynamic research of extracorporeal rice blast mold resisting experiment and extracorporeal antitumor experiment shows that these compounds has excellent antifungal activity and antitumor activity, so that they may be developed into antifungal medicine and antitumor medicine.
Owner:FUDAN UNIV
Preparation method of hepatitis C and novel coronavirus drug intermediate and salt thereof
ActiveCN114057627AShort reaction stepsShort production timeOrganic chemistry methodsReaction stepCarboxylate
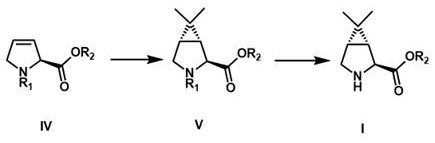
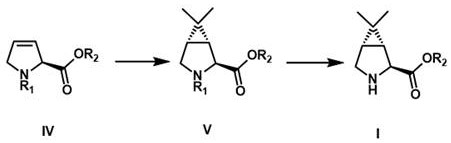
The invention relates to the technical field of drug synthesis, in particular to a preparation method of a hepatitis C and novel coronavirus drug intermediate and a salt thereof. The preparation method comprises the following steps: step 1, dissolving a compound shown in expression IV in a solvent, adding with 2-diazopropane or reacting with 2, 2-dihalogenated propane under the action of a metal reagent, and performing subsequent treatment and purification to obtain a compound as shown in expression V; 2, dispersing the compound as shown in expression V prepared in the step 1 in a solvent, and performing deprotection to obtain a compound as shown in expression I, namely (1R, 2S, 5S)-6, 6-dimethyl-3-azabicyclo [3, 1, 0] hexyl-2-carboxylate. Compared with the prior art, the synthesis method has the advantages of short reaction steps, easily available raw materials, simple reaction conditions, short production time and lower production cost.
Owner:NANJING CHEMPION BIOTECHNOLOGY CO LTD +1
A kind of synthetic method of Erecoxib
ActiveCN104193664BHarm reductionThe reaction steps are simpleOrganic chemistry2-PyrrolidoneDrugs synthesis
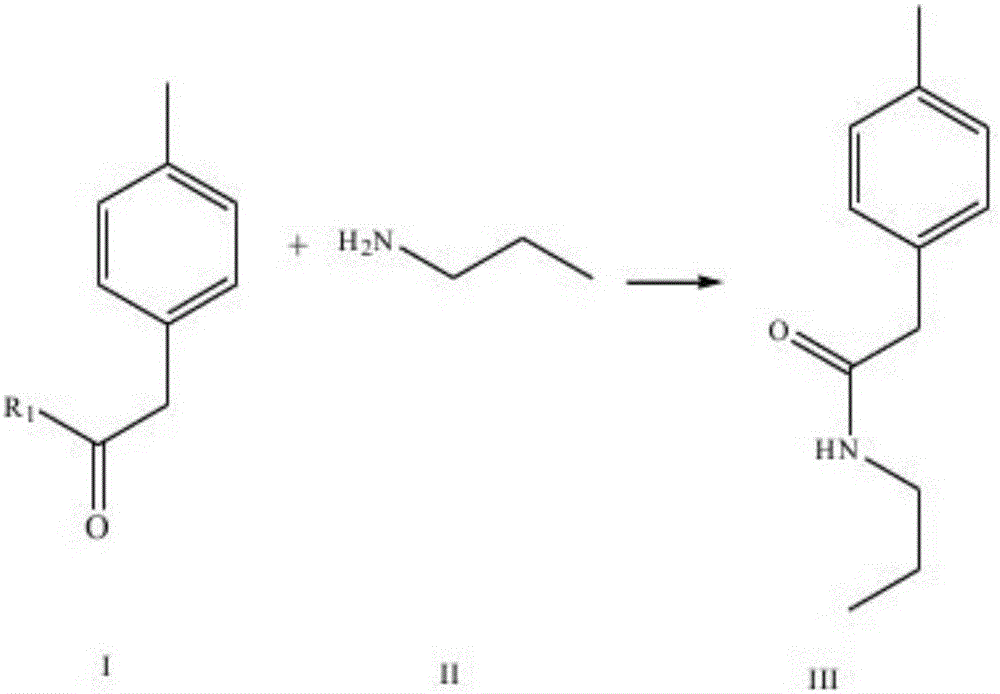
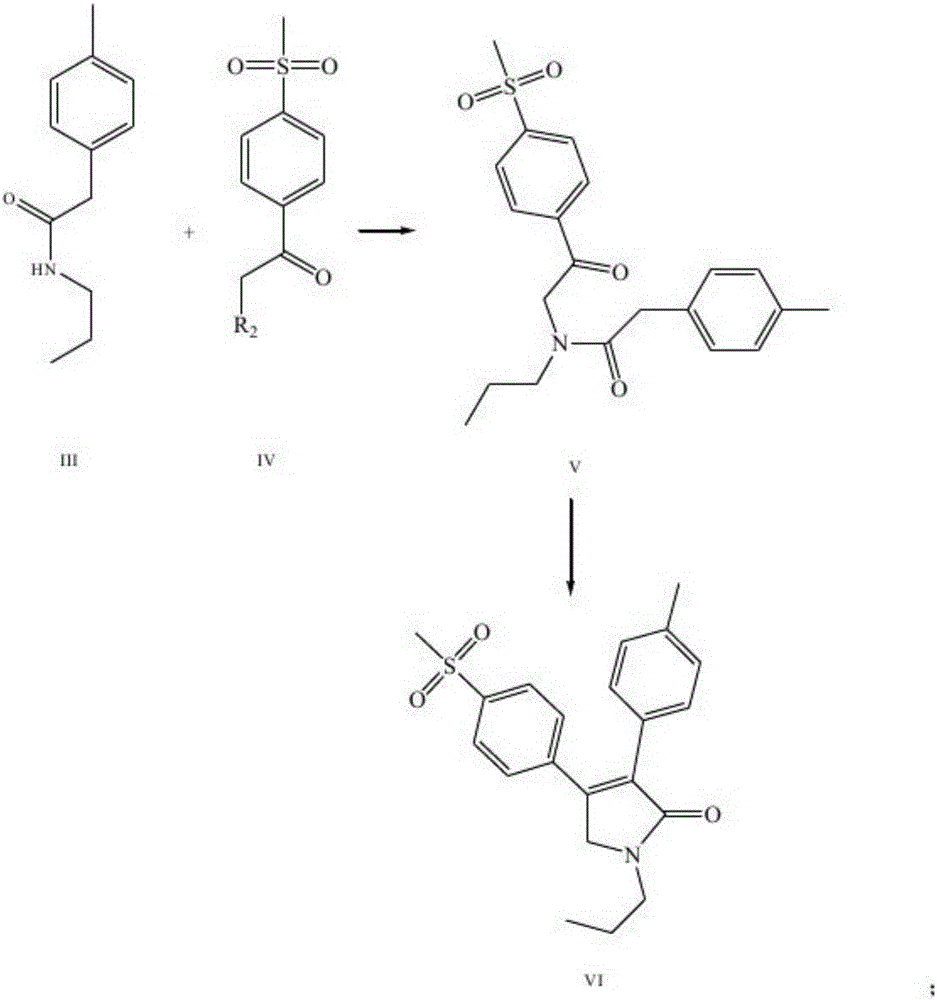
The invention discloses a synthesis method of imrecoxib and belongs to the field of drug synthesis. The method comprises the following steps: reacting p-toluene acetyl halide with propylamine to produce a compound III p-toluene levulinic amine; reacting the compound III with p-methylsulfonyl chloroacetophenone under an alkaline condition to produce a compound V; and then cyclizing to obtain a compound VII, namely n-propyl-3-(4-methyl phenyl)-4-(4-methylsulfonyl phenyl)-2,5-dihydro-1H-2-pyrrolidone. The method disclosed by the invention comprises simple reaction steps and can be put into industrial production easily.
Owner:SHANDONG BOYUAN PHARM CO LTD
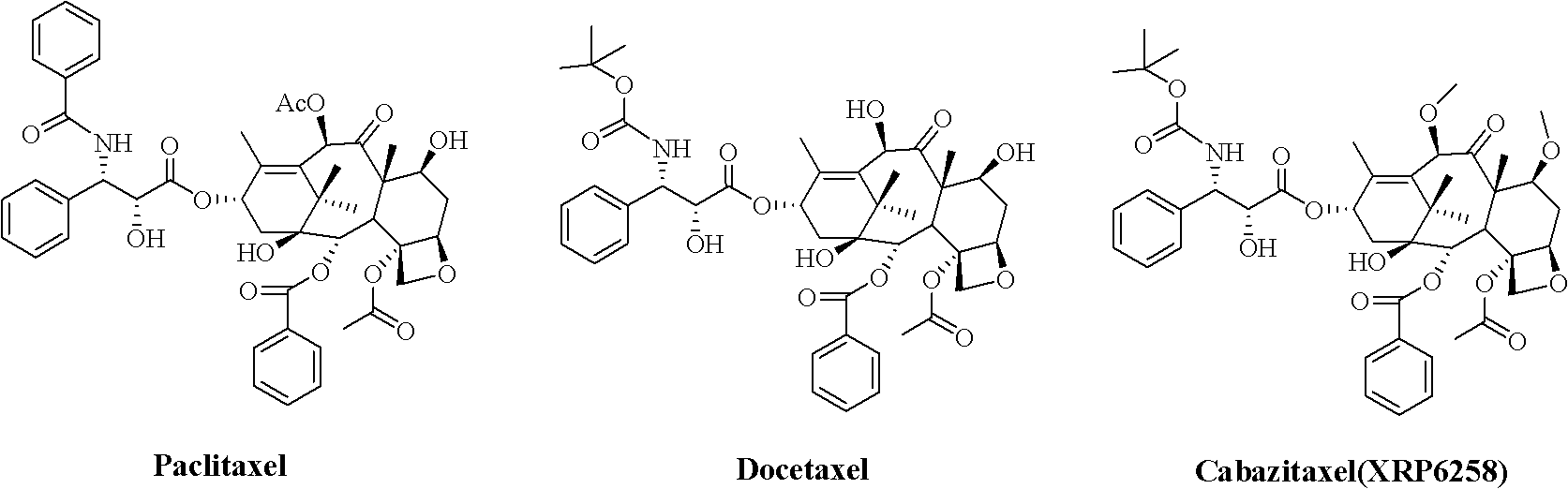
Preparation method of taxol anticancer drugs Cabazitaxel XRP6258
InactiveCN103012329AEasy to manufactureEasy to makeOrganic chemistryBulk chemical productionButt jointCabazitaxel
The invention belongs to the field of drug synthesis, and relates to a method for synthesizing a second-generation taxol anticancer drugs Cabazitaxel XRP6258. The method comprises the following steps of: obtaining C-7 and C-10 bit hydroxy methyl mercaptan methylene (MTM) and C-13 bit oxhydryl oxydic key intermediate (II) of 10-oxhydryl baccatin III(I) by high regioselectivity, reducing C-13 bit carbonyl to obtain a mother nucleus midbody (III), carrying out butt joint with various types of side chains to obtain butt joint product midbodies (IV-1) and (IV-2), and removing a side-chain protecting group after the di-methylthio is removed or removing the di-methylthio of the nuclear parent after the side-chain protecting group is removed to obtain a product Cabazitaxel XRP6258 (V). The method disclosed by the invention has the advantages of being simple in preparation process, high in yield, lower in cost, easy to operate and the like, so that the XRP6258 can be produced and prepared on a large scale.
Owner:FUDAN UNIV +1
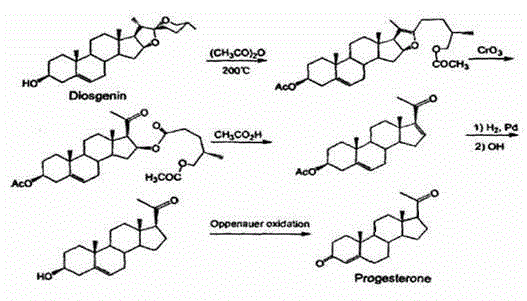
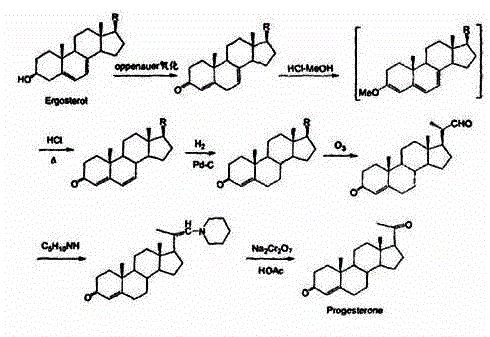
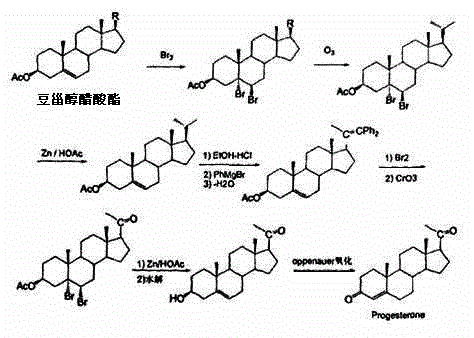
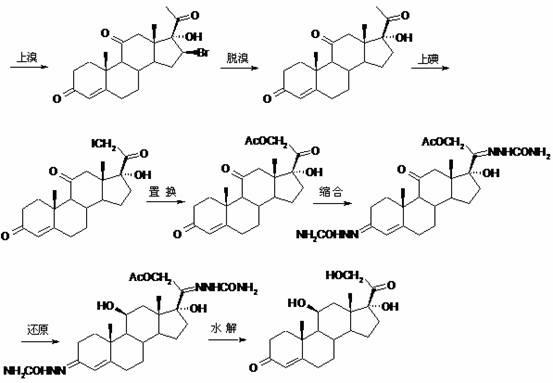
Method for preparing progesterone by taking 1,4-androstenedione as raw material
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
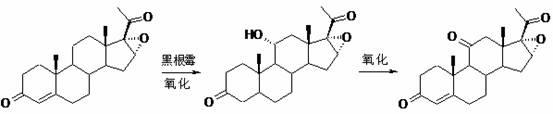
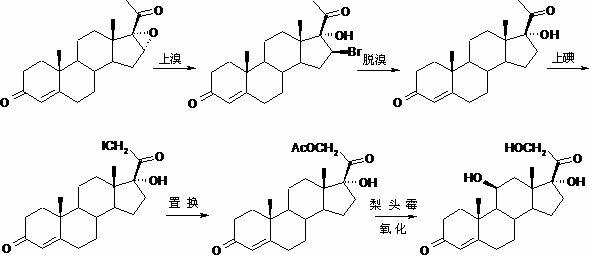
Preparation method of hydrocortisone
ActiveCN102367262AAvoid it happening againRaw materials are easy to getSteroidsDisplacement reactionsHydrocortisone acetate
The invention, relating to the field of pharmaceutical synthesis, particularly relates to a preparation method of hydrocortisone, comprising the following steps: using an intermediate 17 alfa-hydroxy-4-pregnene-3,11,20- triketone (II) in a Rhizopus nigricans method as raw material, successively carrying out ketal reaction, reduction reaction, hydrolysis reaction, iodine adding reaction, and displacement reaction to obtain hydrocortisone acetate (VII), and finally carrying out sodium hydroxide hydrolysis reaction to obtain the hydrocortisone (I). The invention has the advantages of easy obtainment of raw material, common auxiliary materials, and no need of using toxic, highly toxic, and carcinogenic reagents. The method is more efficient than the process in the prior art, and the products has good quality and yield.
Owner:ZHEJIANG XIANJU PHARMA
Test strip and method for fast quantitative detection of drug in blood
InactiveCN102890152AQuantitative detection concentrationRapid quantitationMaterial analysisAntigenDrugs synthesis
The invention discloses a test strip and a method for fast quantitative detection of a drug in blood. The test strip comprises a sample loading pad, a marker pad, a NC membrane, a sample suction pad and a support thin sheet. The sample loading pad, the marker pad, the NC membrane and the sample suction pad are orderly adhered to the support thin sheet which does not absorb water. The marker pad is coated with marked drug-resistant monoclonal antibodies. The NC membrane is coated with a detection line T composed of drug synthesis immunizing antigens, and a quality control line C composed of goat anti-mouse IgG antibodies. The test strip can be operated simply, is accurate, can realize fast quantitation, has high sensitivity and specificity, is economic and practical, and is suitable for on-site detection.
Owner:天津中新科炬生物制药股份有限公司
Fluorous synthesis method of betamethasone
InactiveCN102304163AReasonable designReduce chemical reaction timeSteroidsChemical reactionDrugs synthesis
The invention relates to a fluorous synthesis method of betamethasone, belonging to the technical field of steride medicament synthesis methods in pharmaceutical chemistry. The method comprises the following process steps: fluorinating and refining betamethasone epoxide which is used as a raw material so as to obtain betamethasone. According to the invention, the fluorous synthesis method of betamethasone is reasonable in design, thus chemical reaction time is greatly reduced, production period is shortened, a whole technical level of a product is greatly improved, cost is reduced by 15% as compared with the conventional process, and the fluorous yield of betamethasone reaches 85-90%.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
Di(7-hydrxyl-2,3-dihydro-1-1H-indeno)ether and the like, synthetic method and application
InactiveCN101058535AGood anti-HIV activityOrganic compound preparationDigestive systemDrugs synthesisEther
The invention discloses a di(7-hydroxy-2,3-dihydro-1H-inden-1-yl) ether and analogue, synthesizing method and application in the pharmacy, which is characterized by the following: the structure of the ether possesses formula I and formula II, which improves anti-HCV activity and better anti-HIV activity; the product can be used to study and develop new drug with obvious scientific meaning and using prospect.
Owner:FUDAN UNIV
Novel method for synthesizing finasteroid
The invention provides a new synthetic method of finasteride, belonging to the technical field of drug synthesis. The method comprises the following steps of: a. synthesis of dihydro finasteride iodo complex; and b. synthesis of finasteride; the synthesis method can transform dihydrotestosterone finasteride (F9) into the finasteride with a theoretic volume, avoids the use of harmful and toxic chemicals which are sensitive to the environment, has the advantages of total two-step reaction yield of more than 90%, purity of over 99.0%, good product yield, high purity, easy refining, simple operation, low cost, and less three-waste, conforms with the requirements of green chemical synthesis, and can achieve the requirements of high yield, high purity, green cleaning and industrial production of the finasteride.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD
Preparation method of isoxazoline insectifuge
InactiveCN111675667AHigh molar yieldEasy to operateBiocideOrganic chemistryAnthelmintic drugFluralaner
The invention belongs to the technical field of chemical drug synthesis, and particularly relates to a preparation method of an isoxazoline insectifuge. The preparation method is characterized in thatthe isoxazoline insectifuge is fluralaner; an intermediate I is sequentially subjected to an amidation condensation reaction, a substitution reaction and a ring closing reaction in the same reactioncontainer; and after the reactions are finished, a reaction product is poured into water, stirring and filtering are conducted to obtain a solid, and the obtained solid is recrystallized to obtain theisoxazoline insectifuge. The method has the beneficial effects that the method uses a solvent and one-pot method to synthesize fluralaner, technological operation of a synthesis process is reduced, treatment time and reaction time are shortened, product yield is improved, and the method is suitable for large-scale production.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
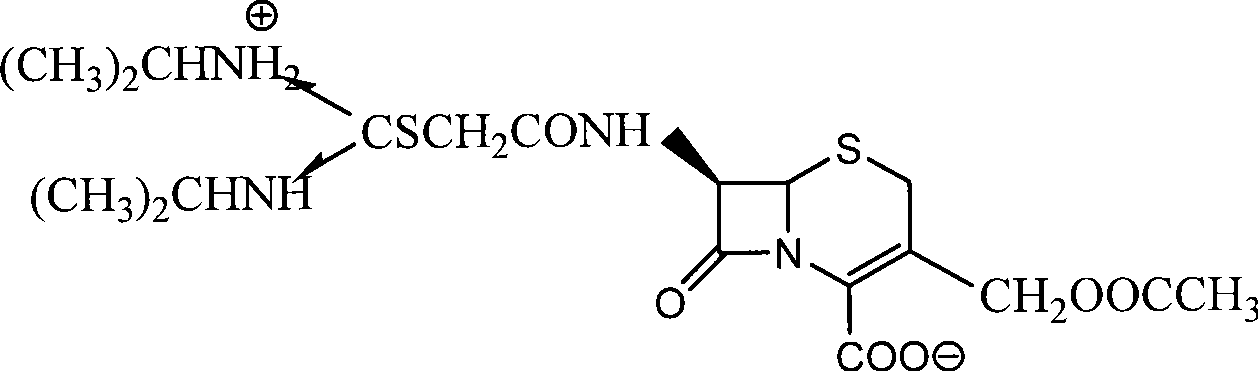
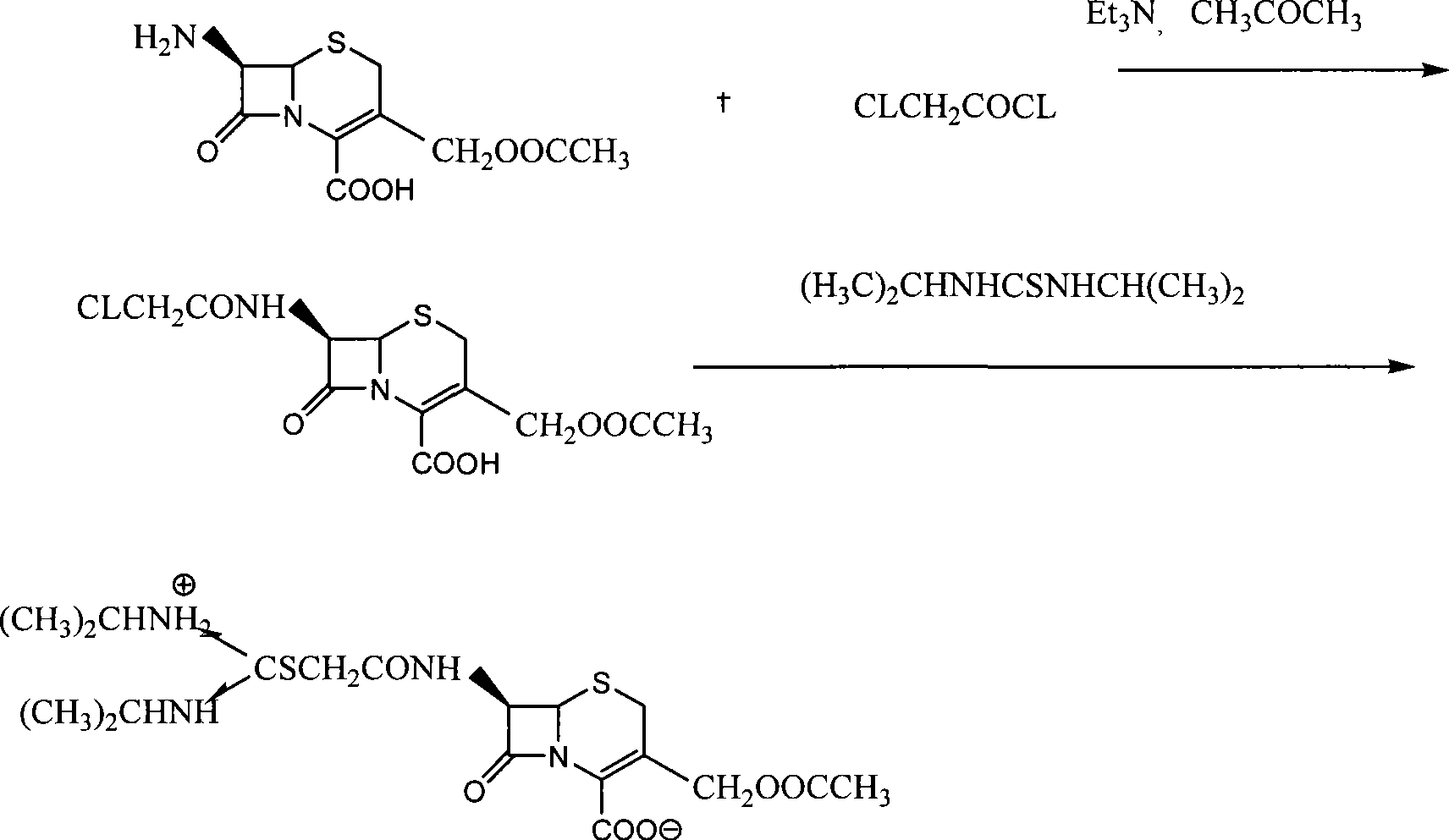
Process for preparing cefathiamidine
The invention relates to the field of the synthesis of chemical medicaments and discloses a preparation method of cefathiamidine; the method takes chloracetyl chloride as a raw material and comprises the following steps: (1) on the condition of the presence of a solvent, alkali is added so as to cause 7-ACA to be dissolved, and then the chloracetyl chloride is added for a condensation reaction; after the condensation reaction is finished, chloracetyl 7-ACA crystals are separated out with an acid; and (2) on the condition of the presence of both the solvent and a catalyst of a catalyzing amount, the chloracetyl 7-ACA reacts with N, N-di-isopropyl thiourea to produce the cefathiamidine. Besides the advantages of bromoacetyl-bromide preparation method of cefathiamidine, the technology of adopting chloracetyl chloride as the raw material to produce the cefathiamidine also has the advantages that: as no alkali is added into the reaction between the chloracetyl 7-ACA and the N, N-di-isopropyl thiourea, the produced cefathiamidine has lighter color, and better and more stable quality, is more beneficial to store and transport, improves the overall yield, lowers the cost and has broader prospects; and the price of the chloracetyl chloride is one sixth of that of the bromoacetyl bromide, which significantly reduce the cost.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
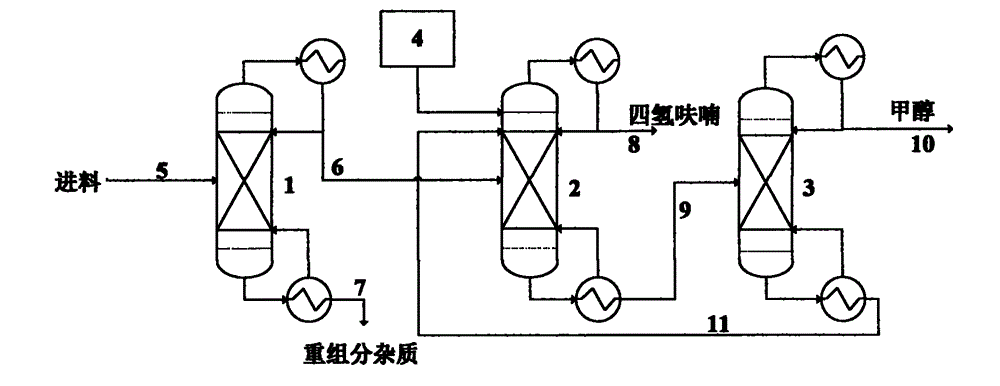
Recovery and separation method for solvent containing tetrahydrofuran-carbinol system
InactiveCN102911139AOrganic compound preparationHydroxy compound preparationLiquid wasteBoiling point
The invention relates to an extraction, rectification and separation method and an extraction, rectification and separation process for a solvent containing a tetrahydrofuran-carbinol system which take ethylene glycol as an extractions solvent, and take tetrahydrofuran and carbinol as products. In the drug synthesis process in the pharmaceutical industry, a great quantity of solvents containing the tetrahydrofuran, the carbinol and the like are used, and a mixed liquid waste is generated. Under the normal temperature and normal pressure, the boiling temperatures of the tetrahydrofuran and the carbinol are extremely similar (the boiling temperature of the tetrahydrofuran is 65.4 DEG C and the boiling temperature of the carbinol is 64.8 DEG C), so that the tetrahydrofuran and the carbinol are difficult to separate by adopting a common rectification operation. In addition, the tetrahydrofuran and the carbinol are good in intersolubility and can be well dissolved with great majority of organic or inorganic solvents so as to be incapable of being separated by an extraction operation. According to the method and the process, the waste solvent containing the tetrahydrofuran-carbinol system can be recovered by adopting an extraction and rectification manner, meanwhile, an extracting agent is recycled, so that the obtained tetrahydrofuran and carbinol products are high in purity, impurities are few, the recovered solvent can be recycled in the drug synthesis process, further not only is the cost of pharmacy process reduced, and also the pollution of the waste solvent to the environment is alleviated greatly.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com