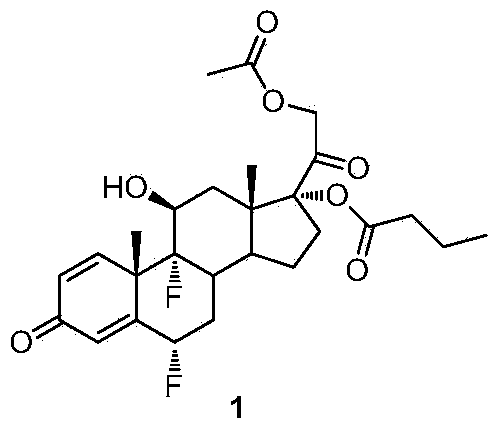
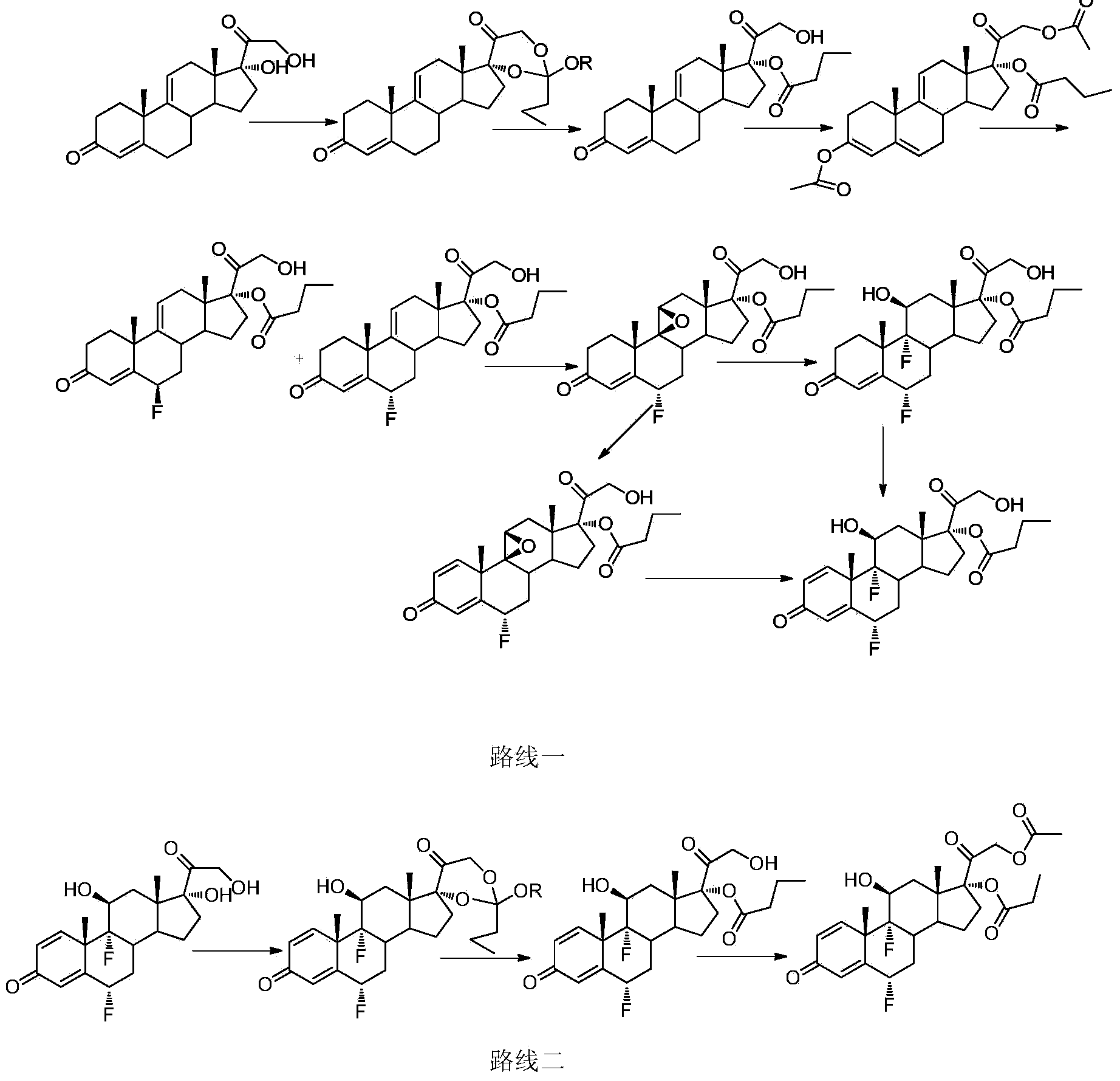
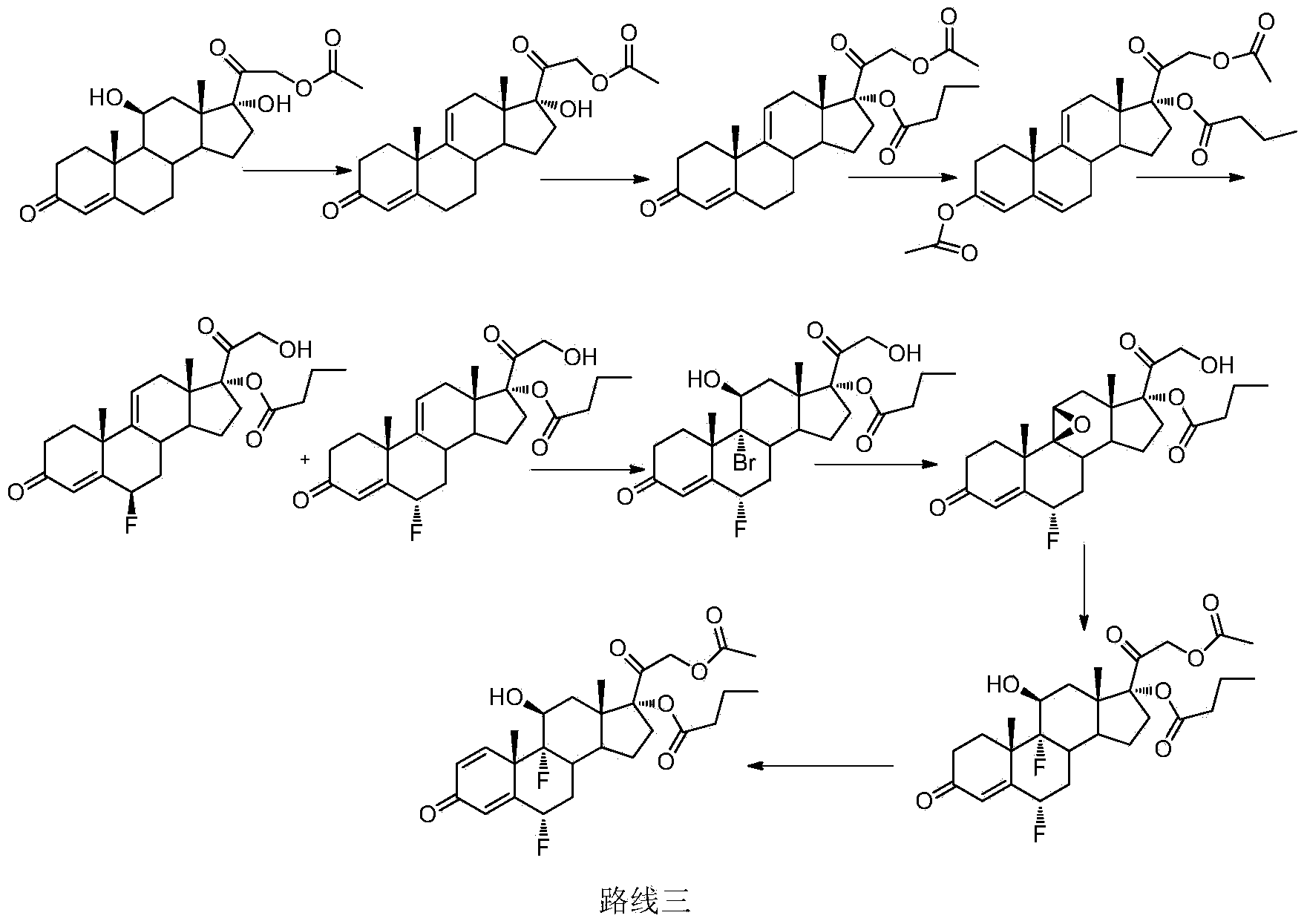
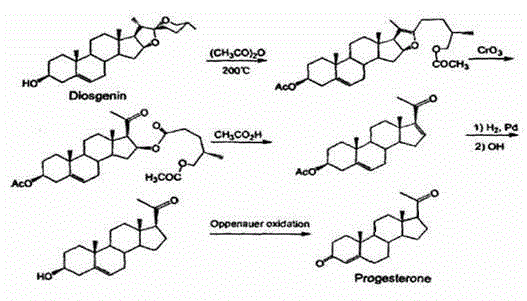
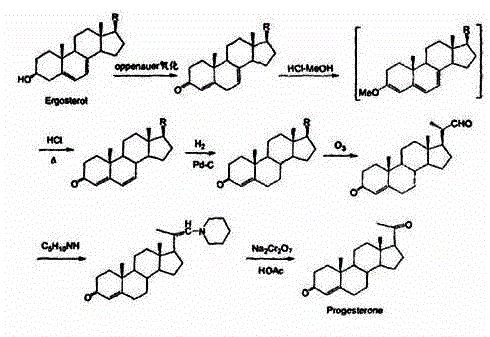
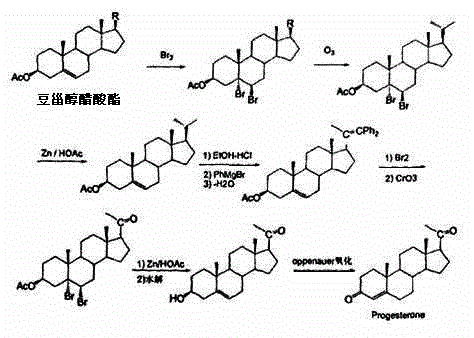
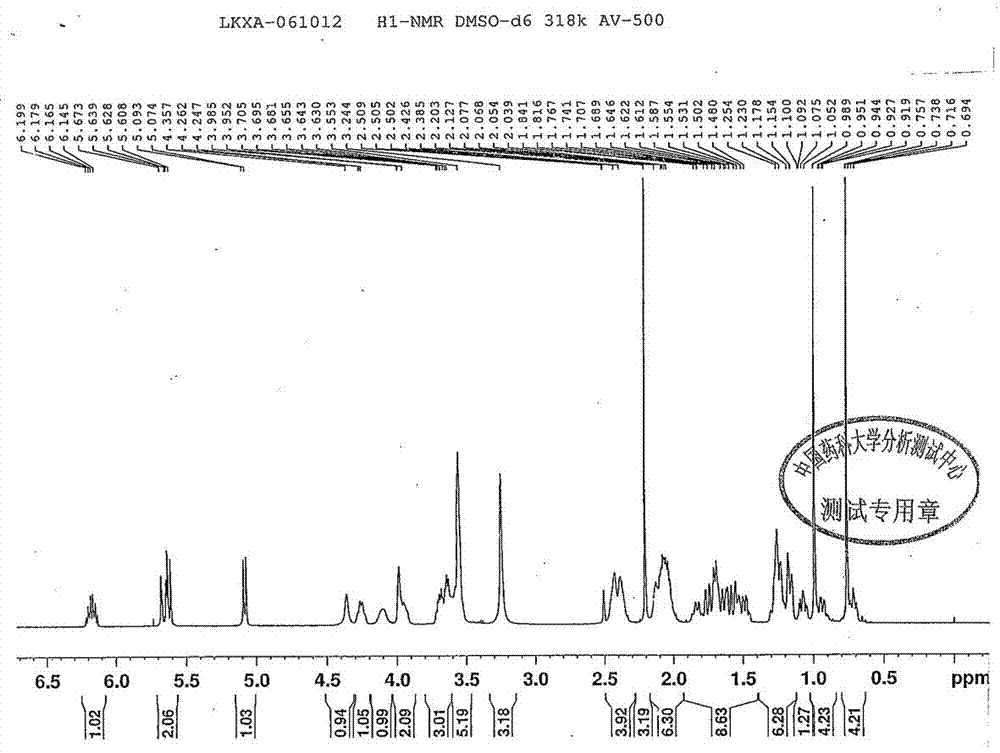
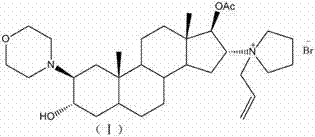
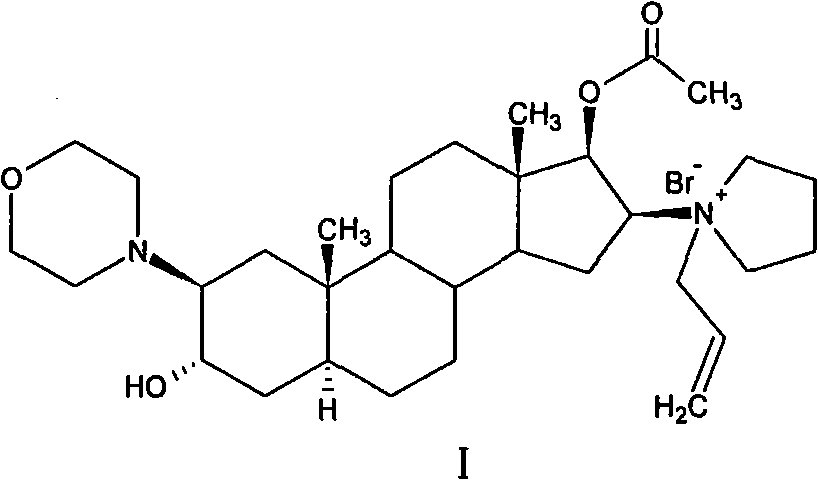
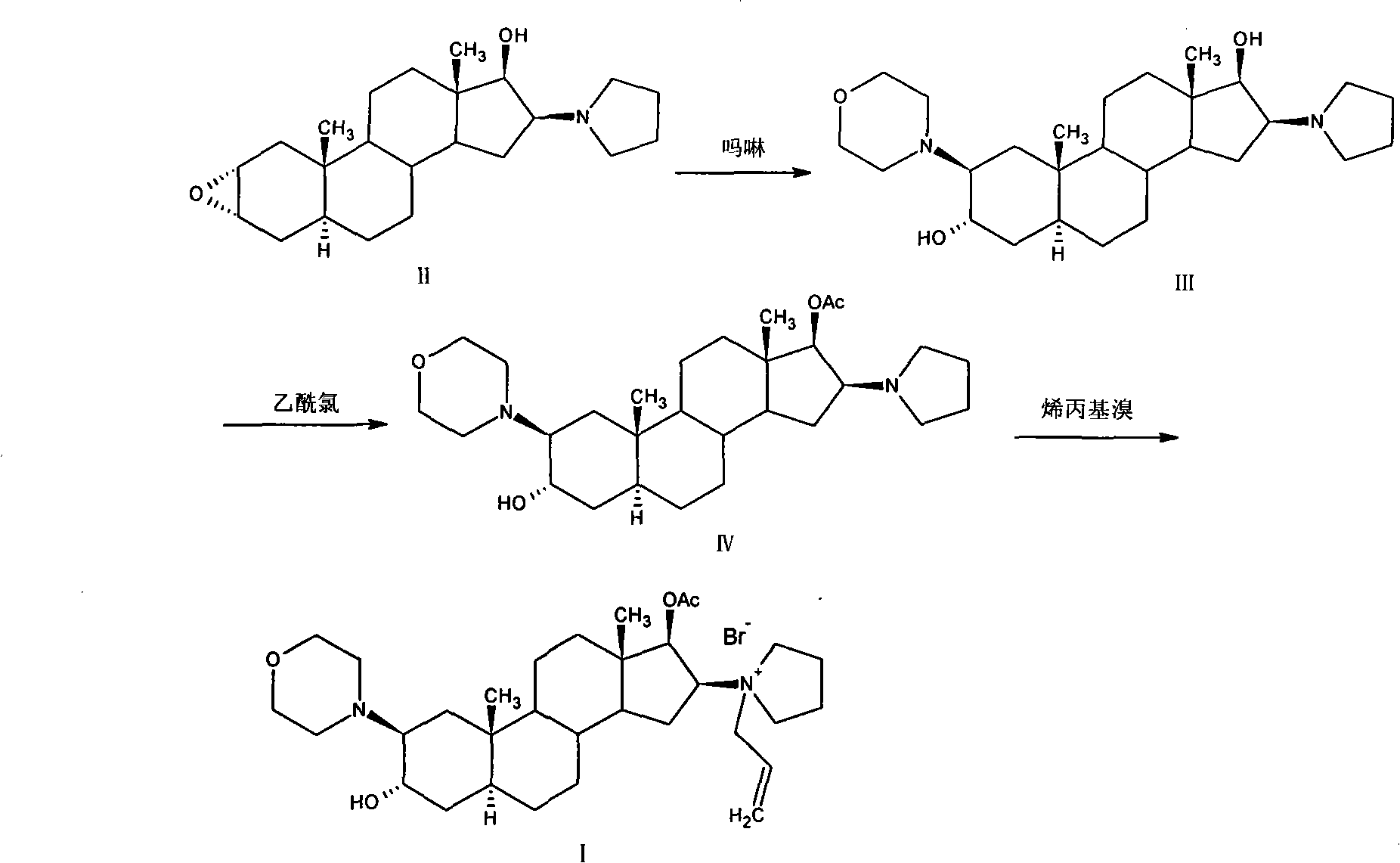
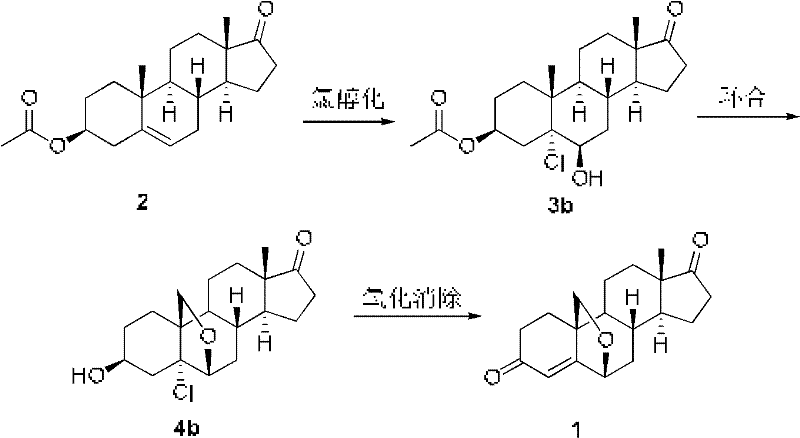
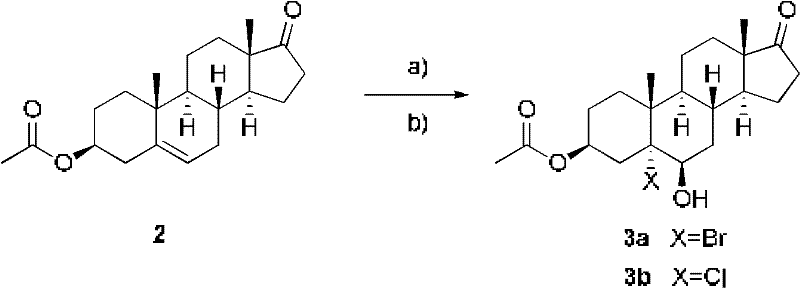
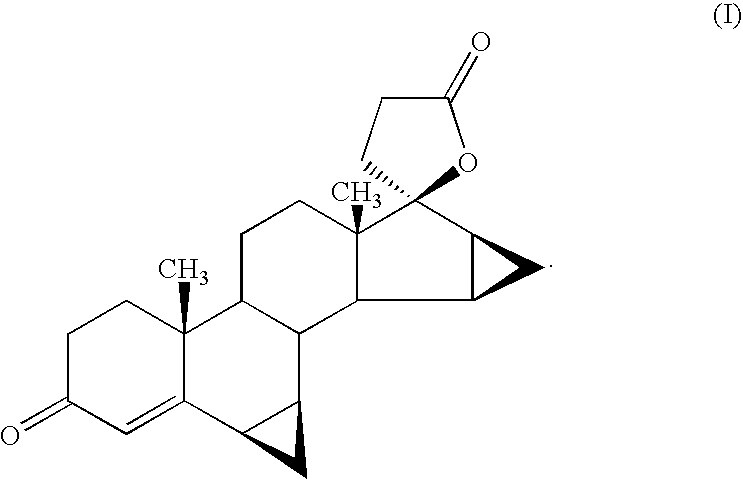
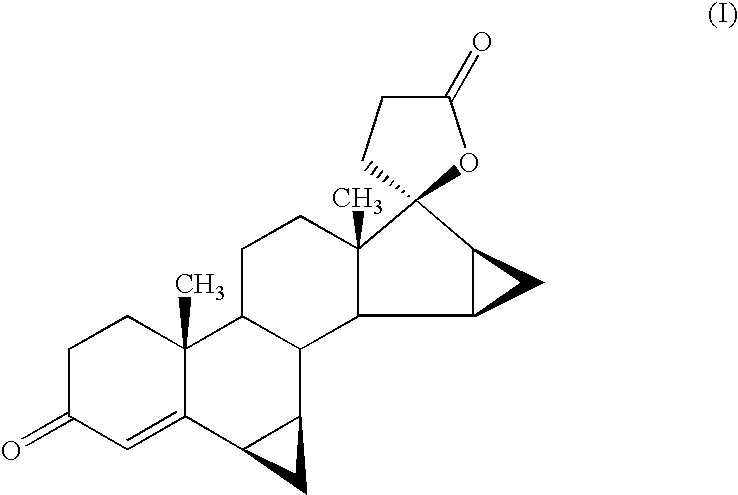
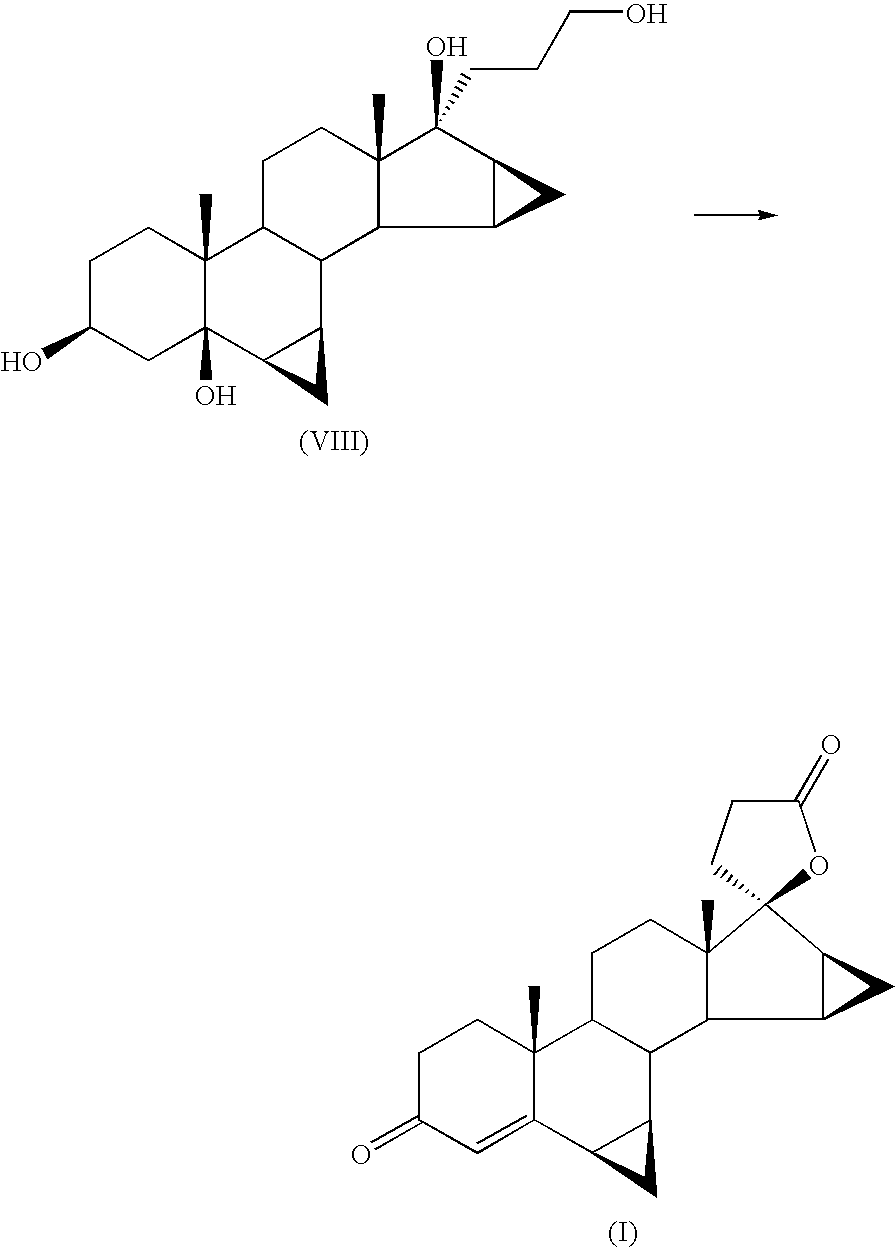
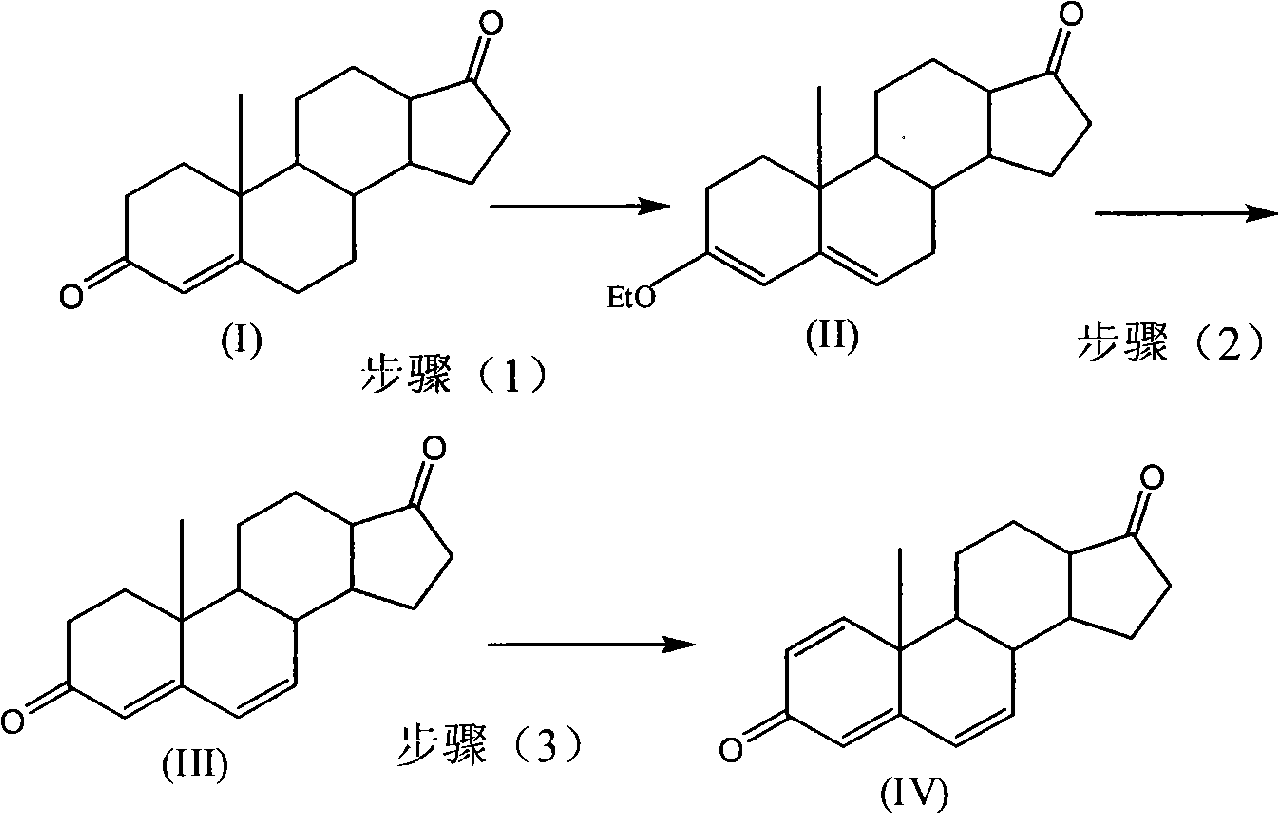
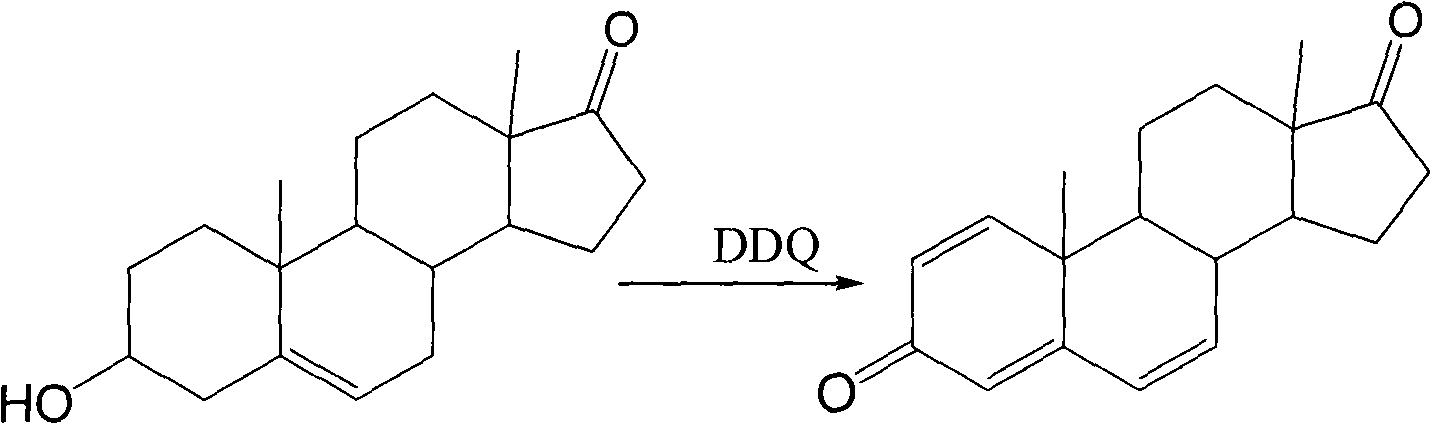
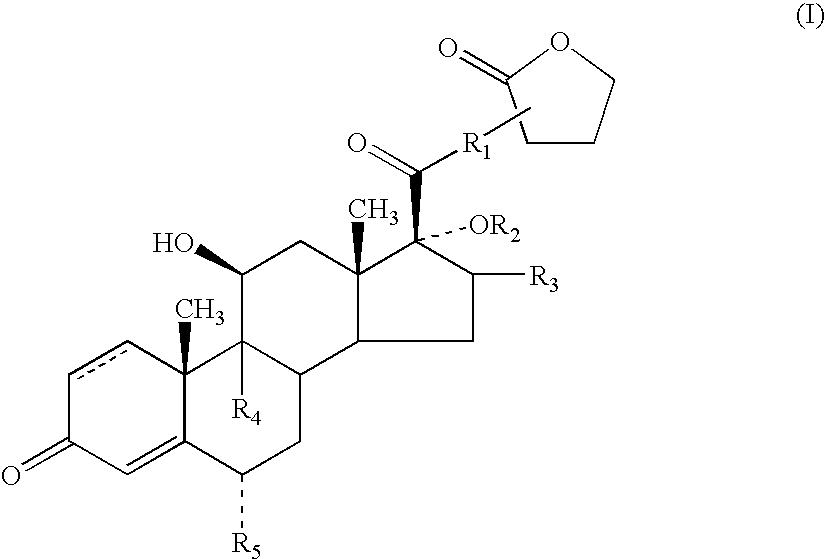
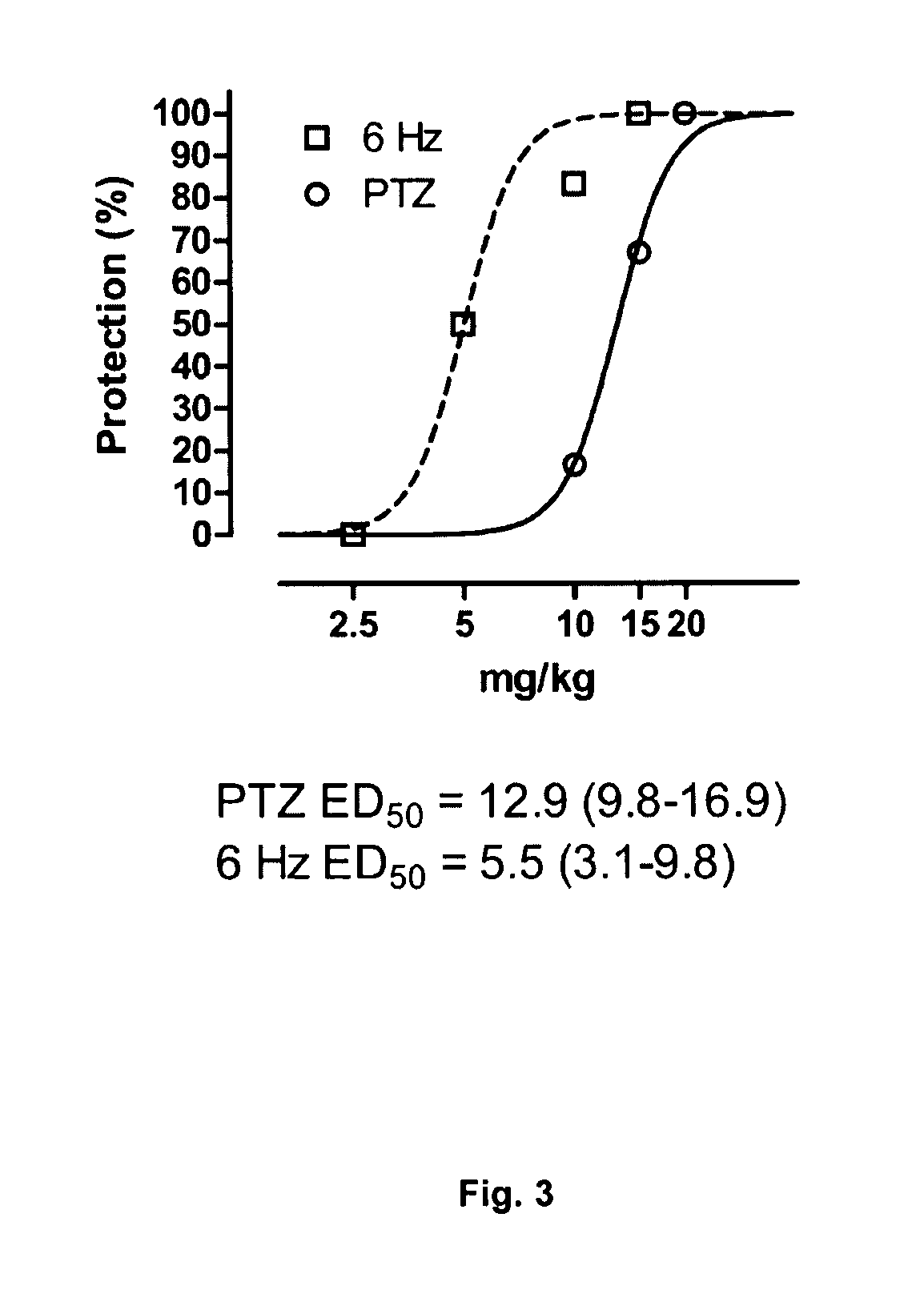
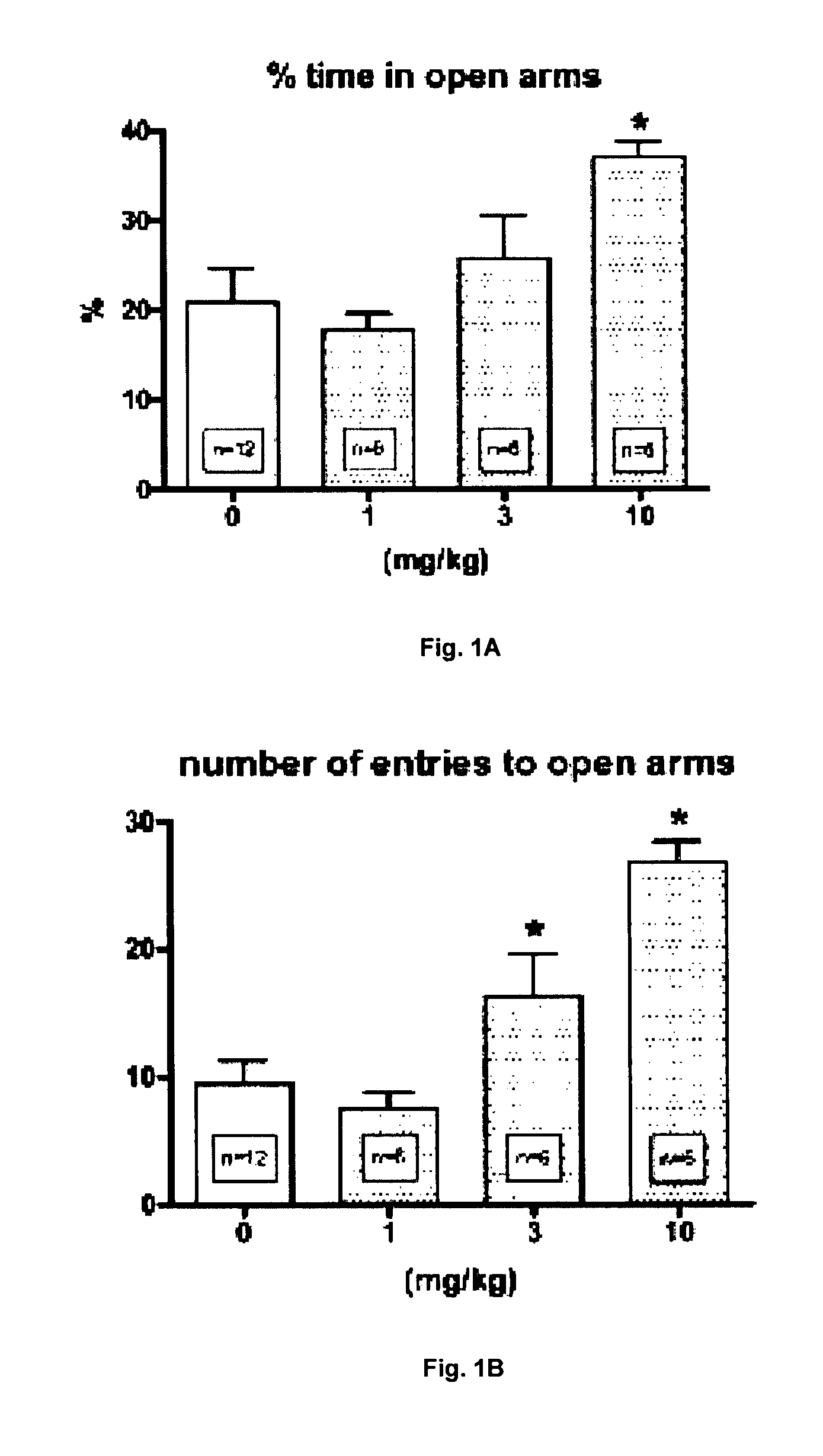
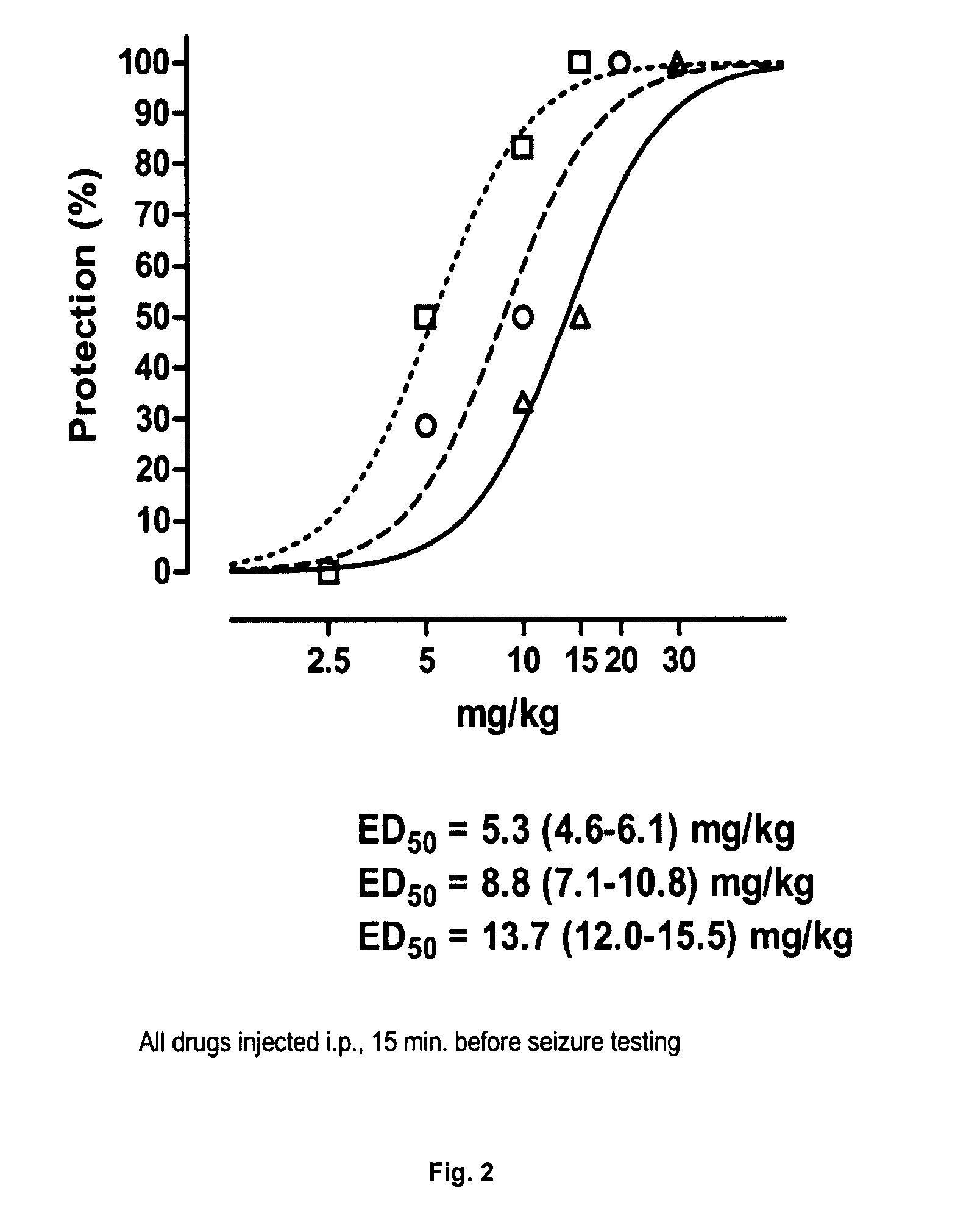
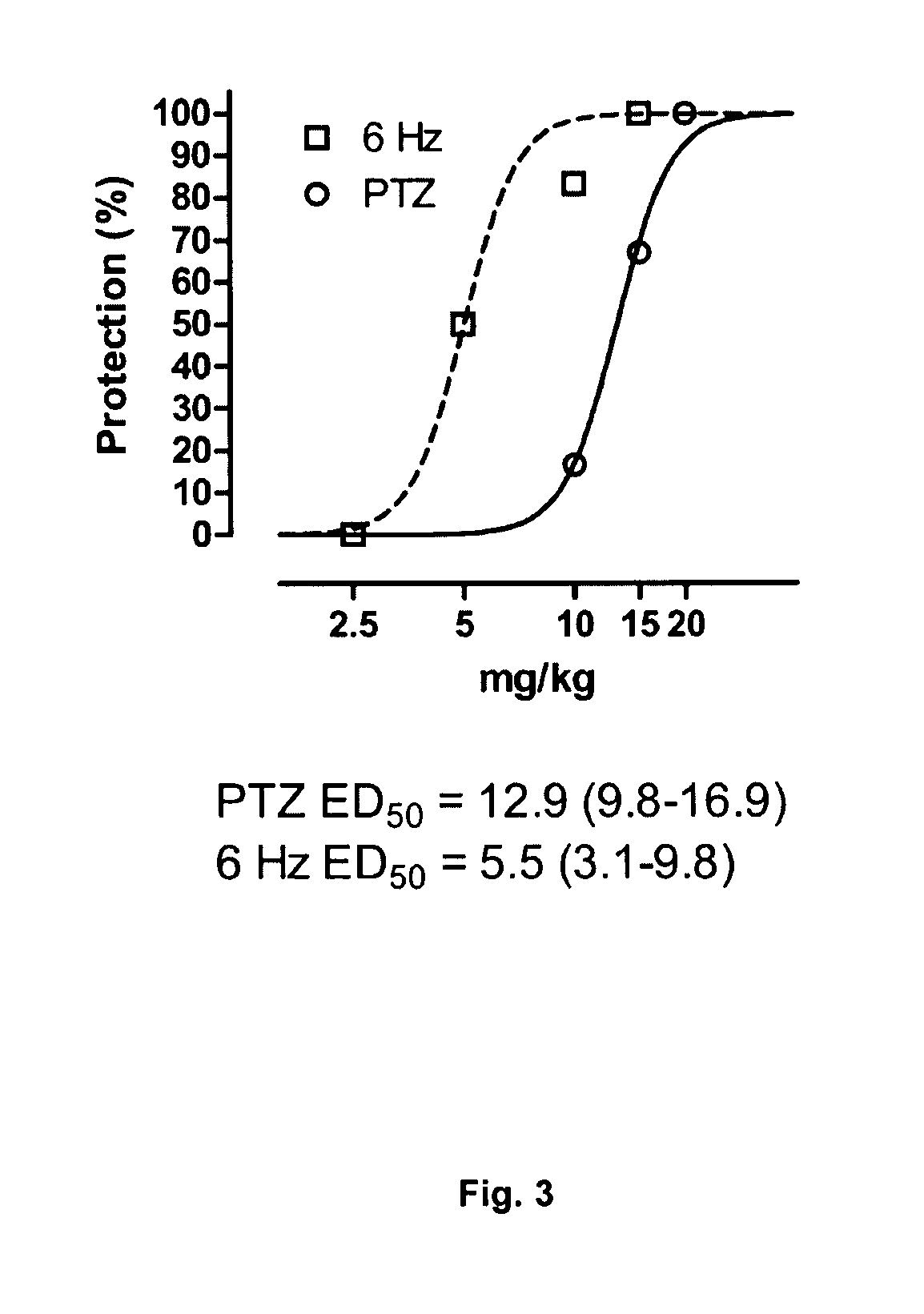
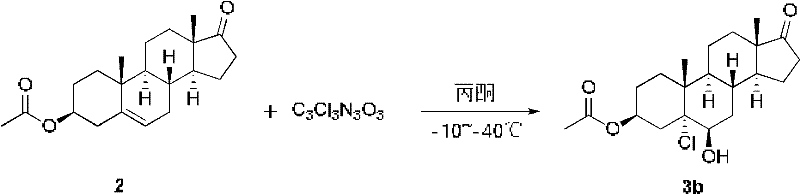Patents
Literature
280 results about "Androstane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Androstane is a C19 steroid with a gonane core. Androstane can exist as either of two isomers, known as 5α-androstane and 5β-androstane.
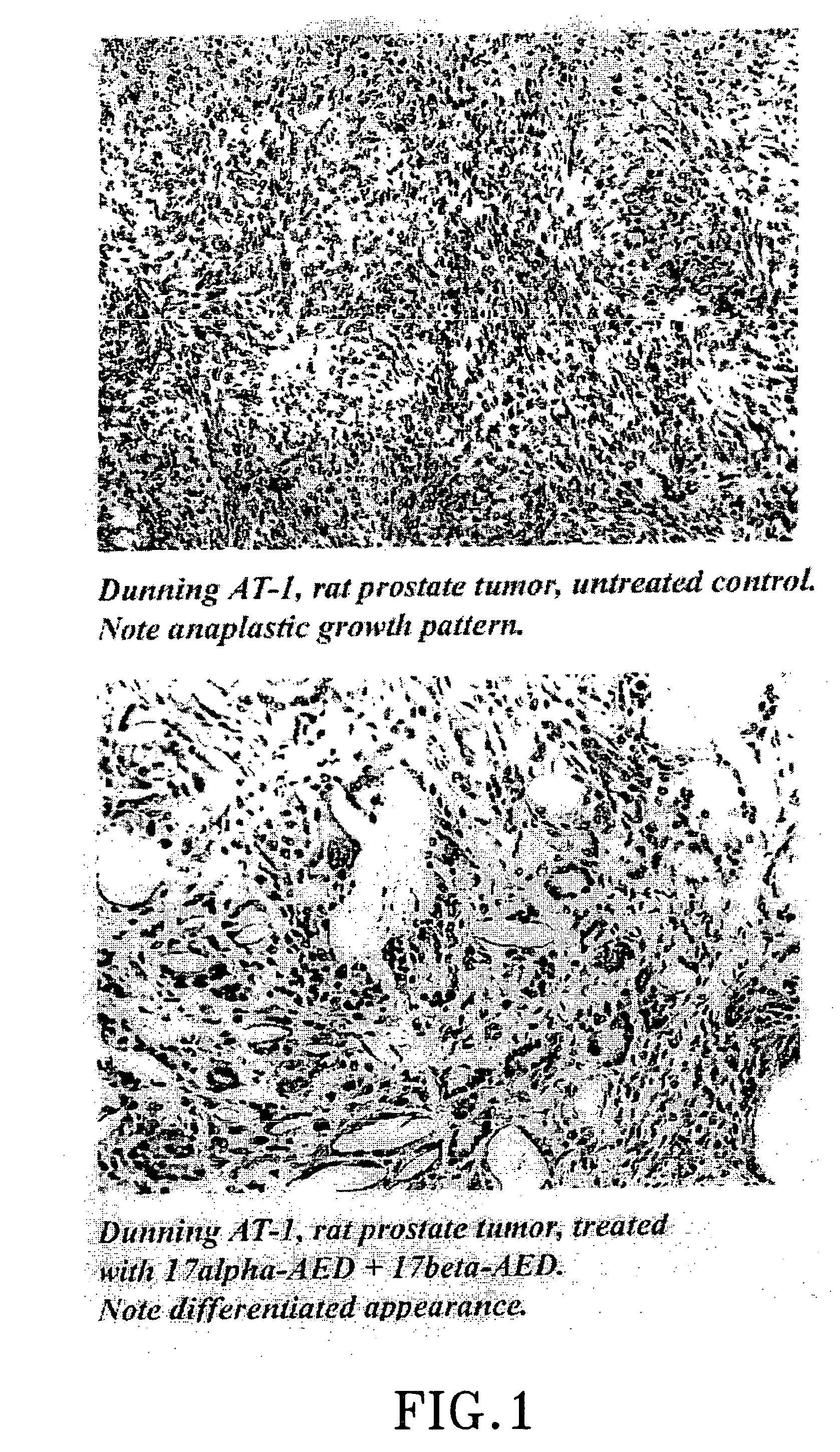
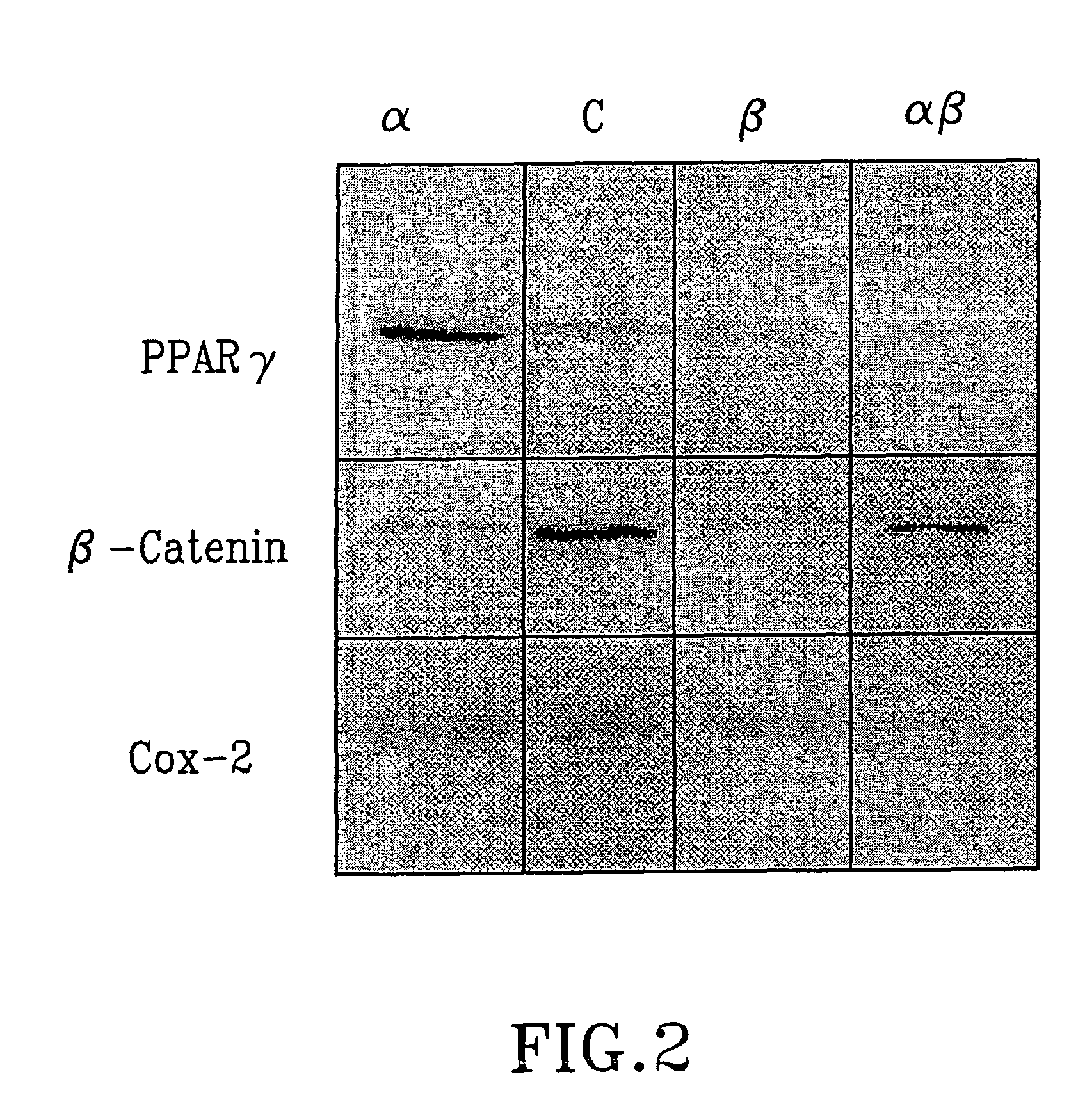
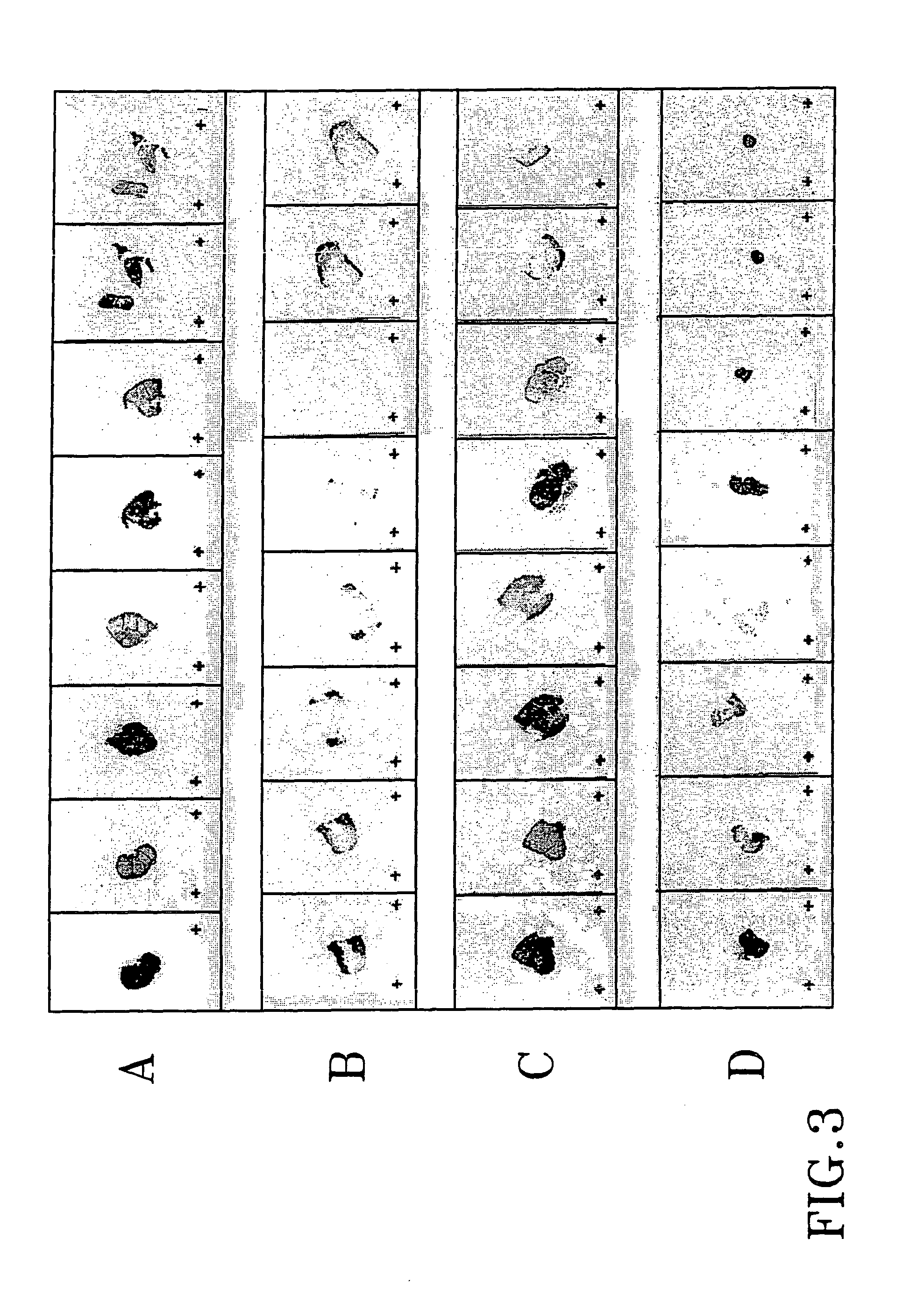
Treatment of tumours
InactiveUS20050192262A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsSteroidsDiseaseAndrostane
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wnt-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3β,17-diol or androstane-3β-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3β-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor-(PPAR) is produced.
Owner:HAGSTROM TOMAS
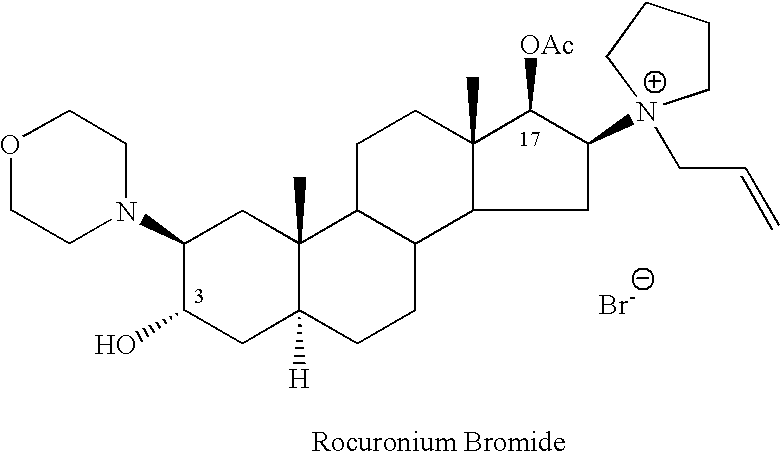
Processes for the preparation of rocuronium bromide and intermediates thereof
InactiveUS20050159398A1Efficient and cost-effectiveOrganic active ingredientsNervous disorderAndrostanesCombinatorial chemistry
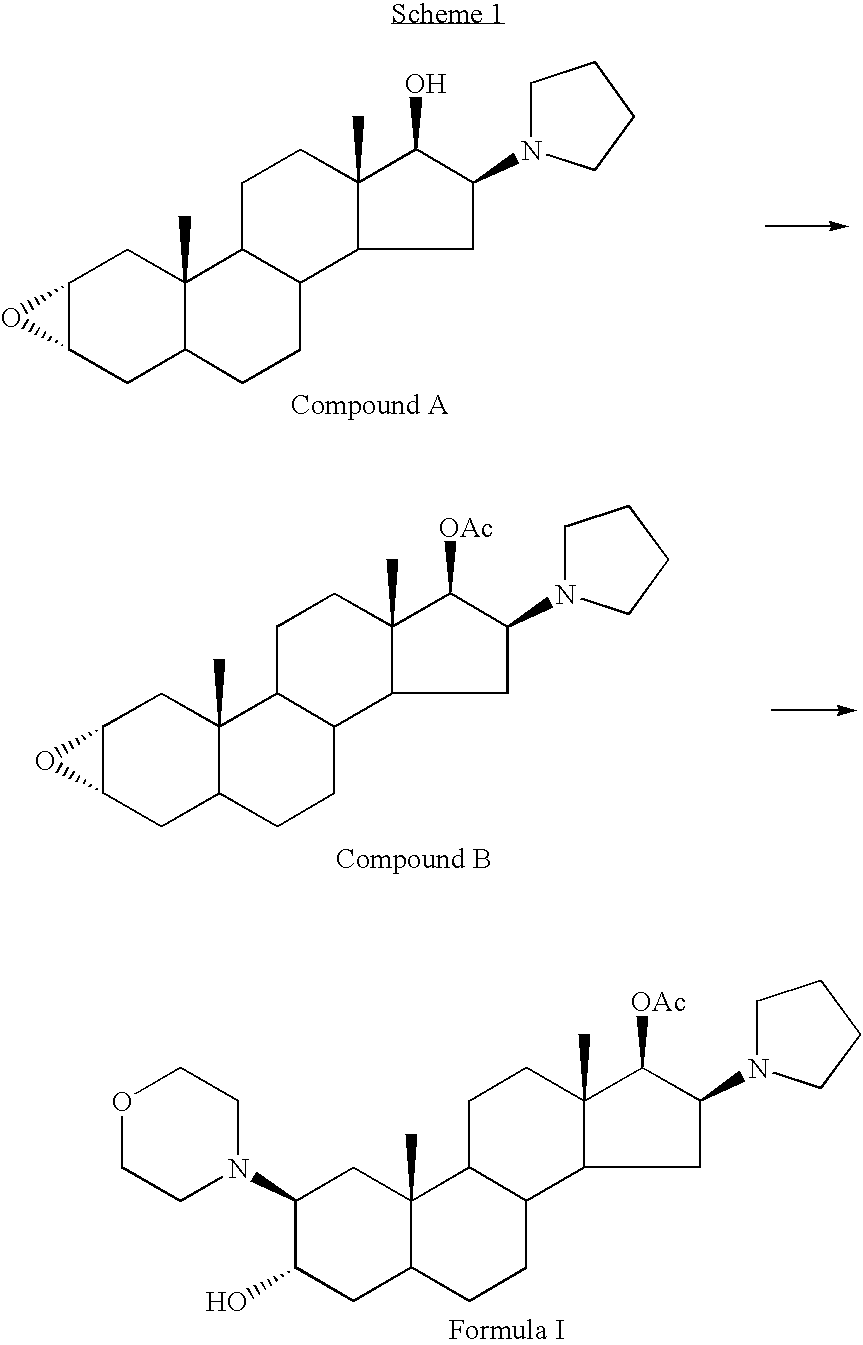
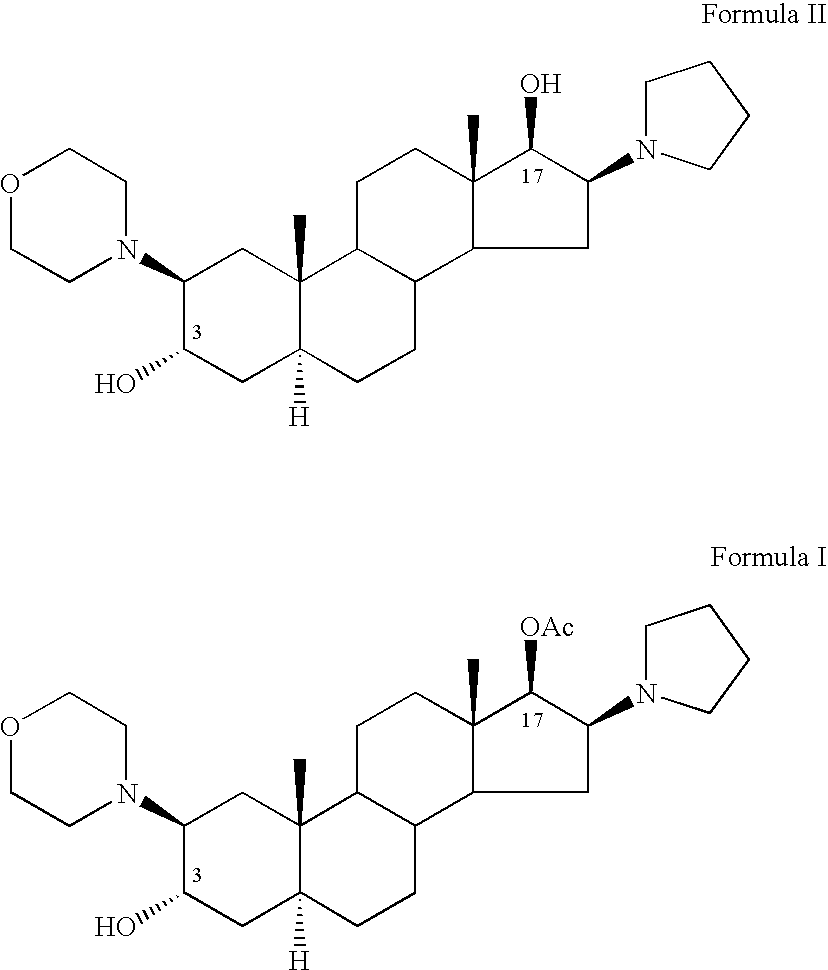
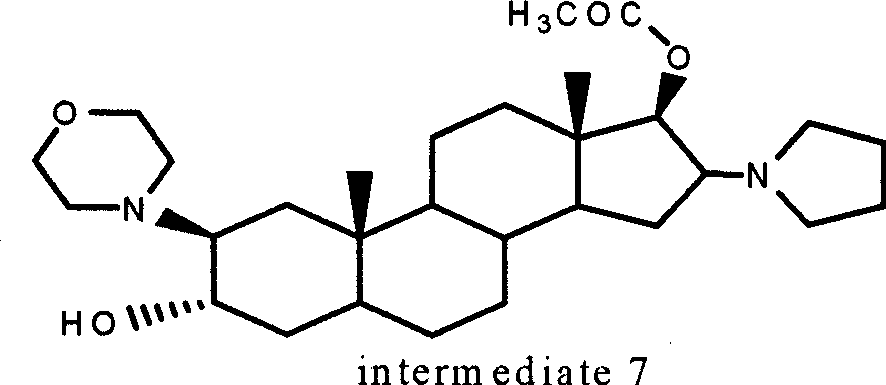
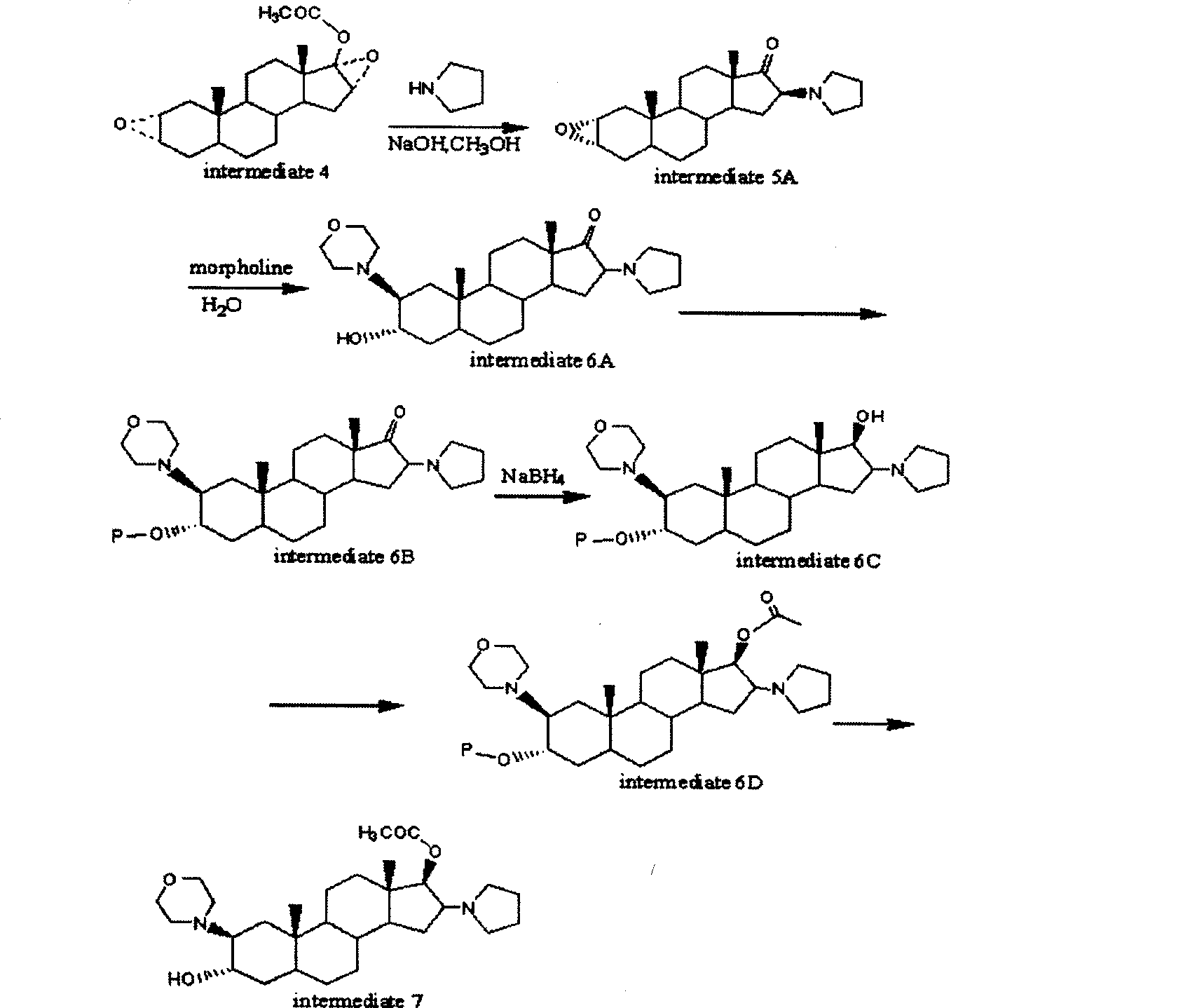
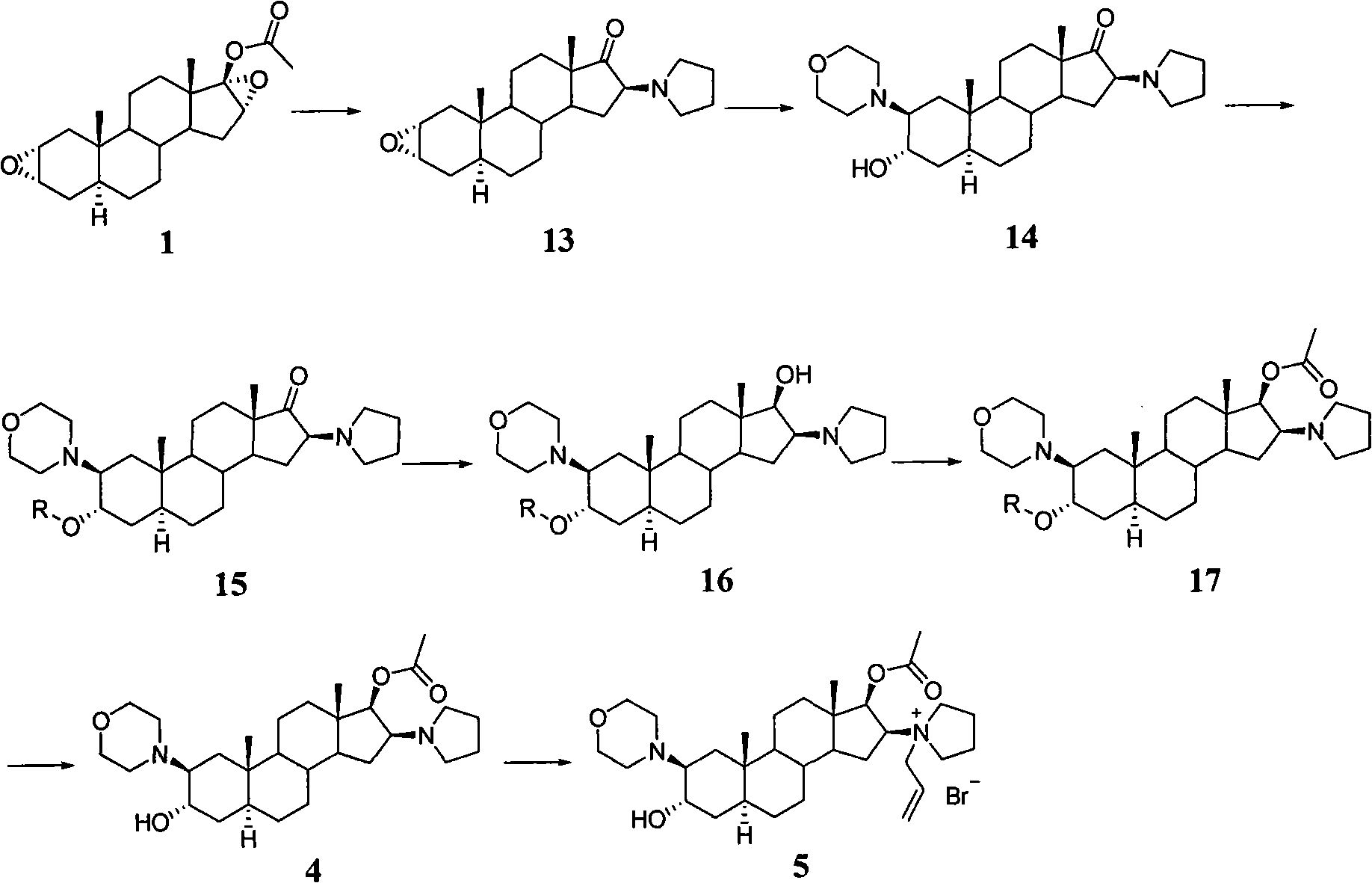
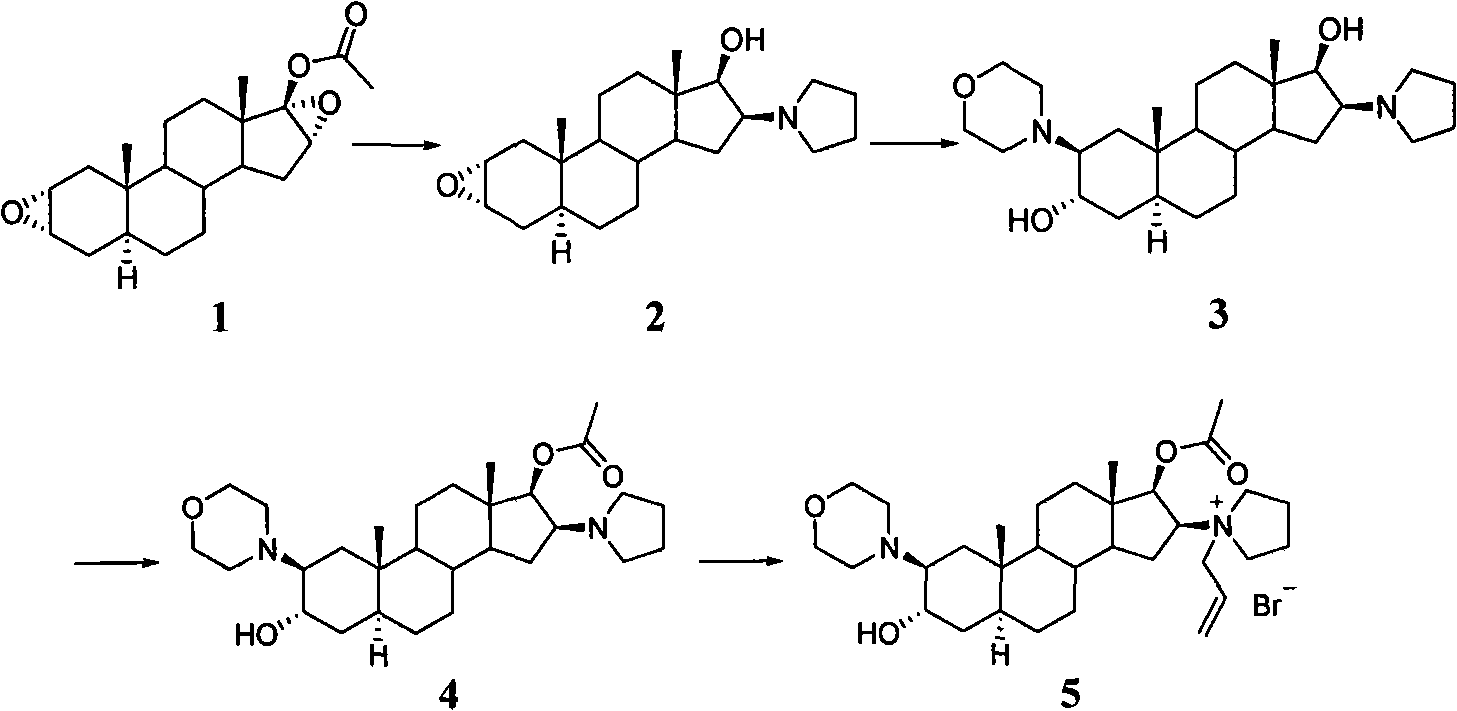
A novel process for preparing (2β,3α,5α,16β,17β)-17-acetoxy-3-hydroxy-2-(4-morpholinyl)-16-(1-pyrrolidinyl)androstane, a known intermediate in the synthesis of the skeletal muscle relaxant rocuronium bromide, is disclosed.
Owner:WAVELENGTH ENTERPRISES LTD
Synthetic method of bromamines muscle relaxant
The invention provides a bromide type muscle relaxant, which mainly comprises a method for synthesizing rocuronium bromide, vecuronium bromide, pancuronium bromide and pipecuronium bromide , and is mainly characterized by adopting a ketene as an acylating agent during the process of transforming 5Alpha-androstane -2-alkene-17-alkone to 17-acetoxyl group-5Alpha- androstane-2, and 16-diene, adopting a stress kettle as a reactor when realizing the step of ring-opening and condensation of the 2 Alpha and 3 Alpha-epoxy compound, and adding a solid catalyst and a desiccant in the last step of operation of rocuronium bromide to greatly improve the reaction yield and the quality of products. The technology provided by the invention can shorten the synthesis time of the bromide type muscle relaxant, simplify the operation steps, improve the quality of products and reduce the production cost.
Owner:王加旺
Method for preparing abiraterone acetate
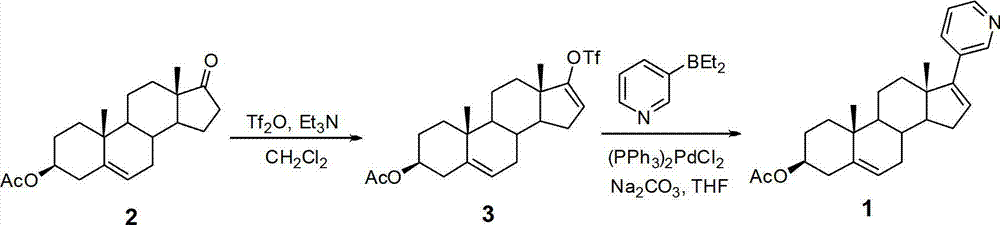
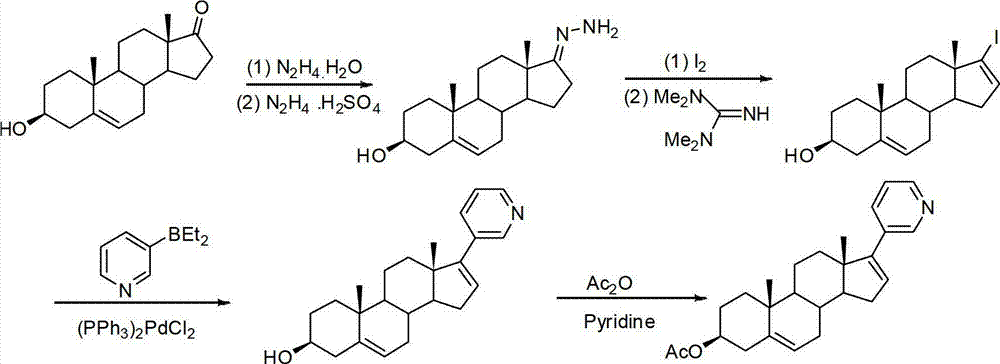
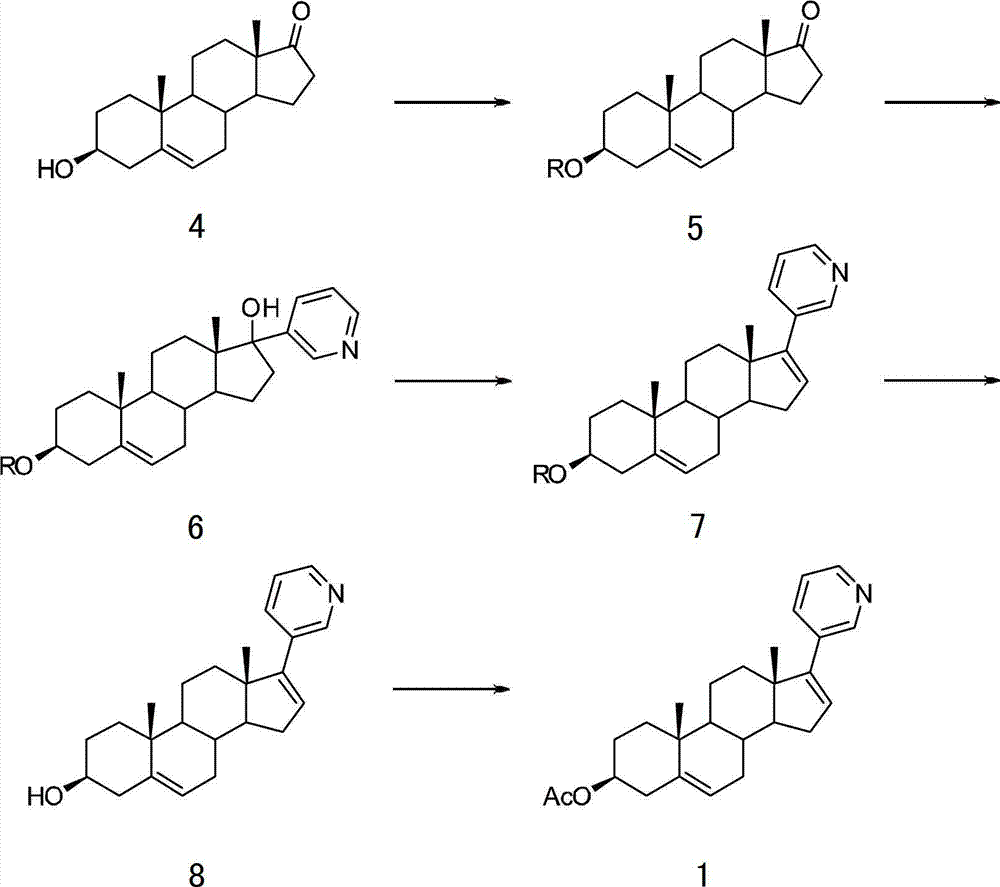
The invention provides a method for preparing (3beta)-17-(3-pyridyl)-androstane-5,16-diene-3-alcohol acetate. According to the method, the traditional Grignard addition reaction is adopted, pyridine groups are introduced in the presence of a catalyst such as cerous chloride, the method is low in raw material cost, available in raw materials, economical, practical, environment-friendly, convenient and simple in post-treatment, the reaction process is easily and continuously operated, the product quality and yield can be improved, and industrial production is promoted.
Owner:ZHEJIANG SHENZHOU PHARMA
Synthesis process of vecuronium bromide
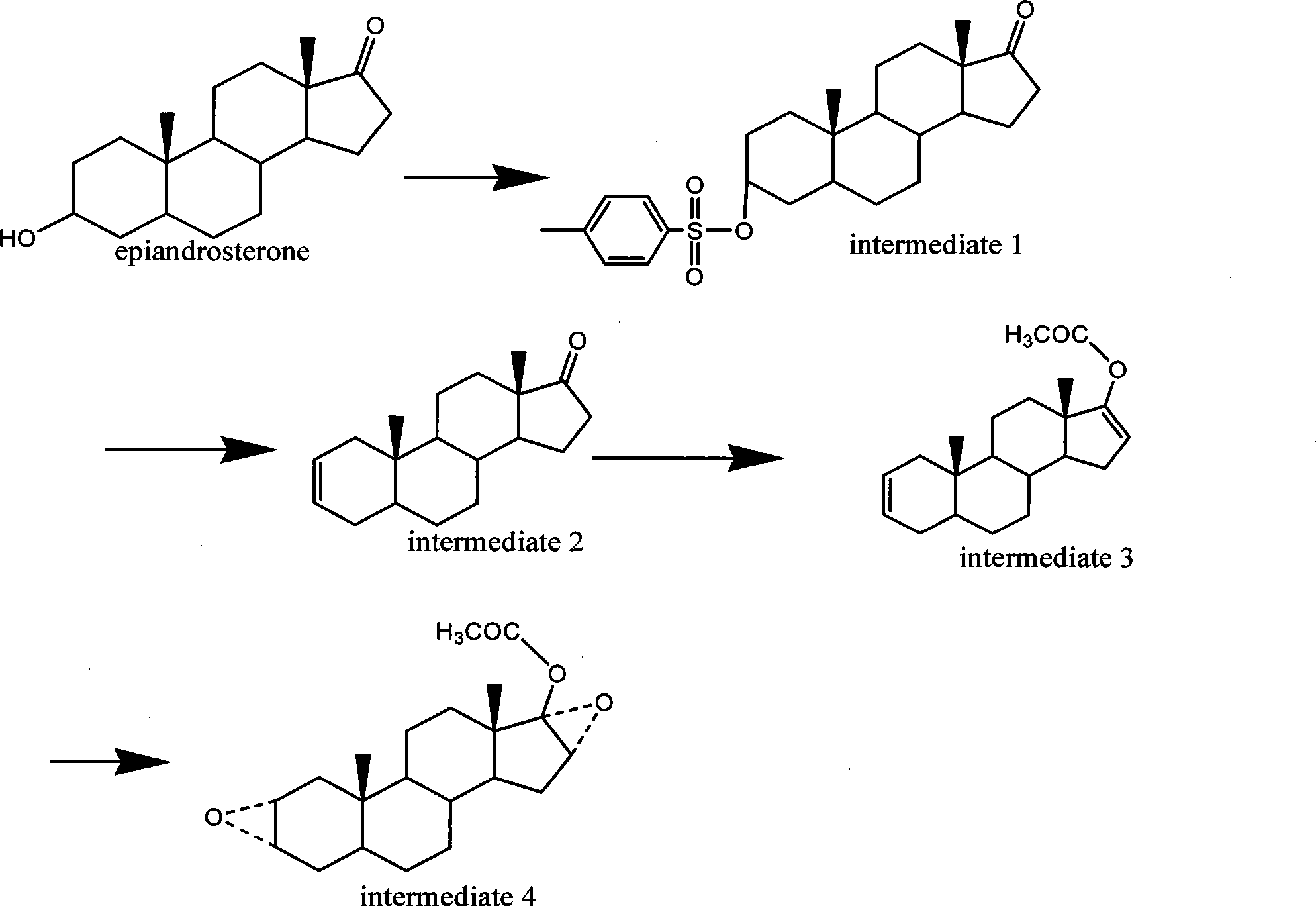
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
Method for synthesizing finasteride
InactiveCN101863956AStandards compliantThe synthesis process is simpleSteroidsSynthesis methodsAndrostanes
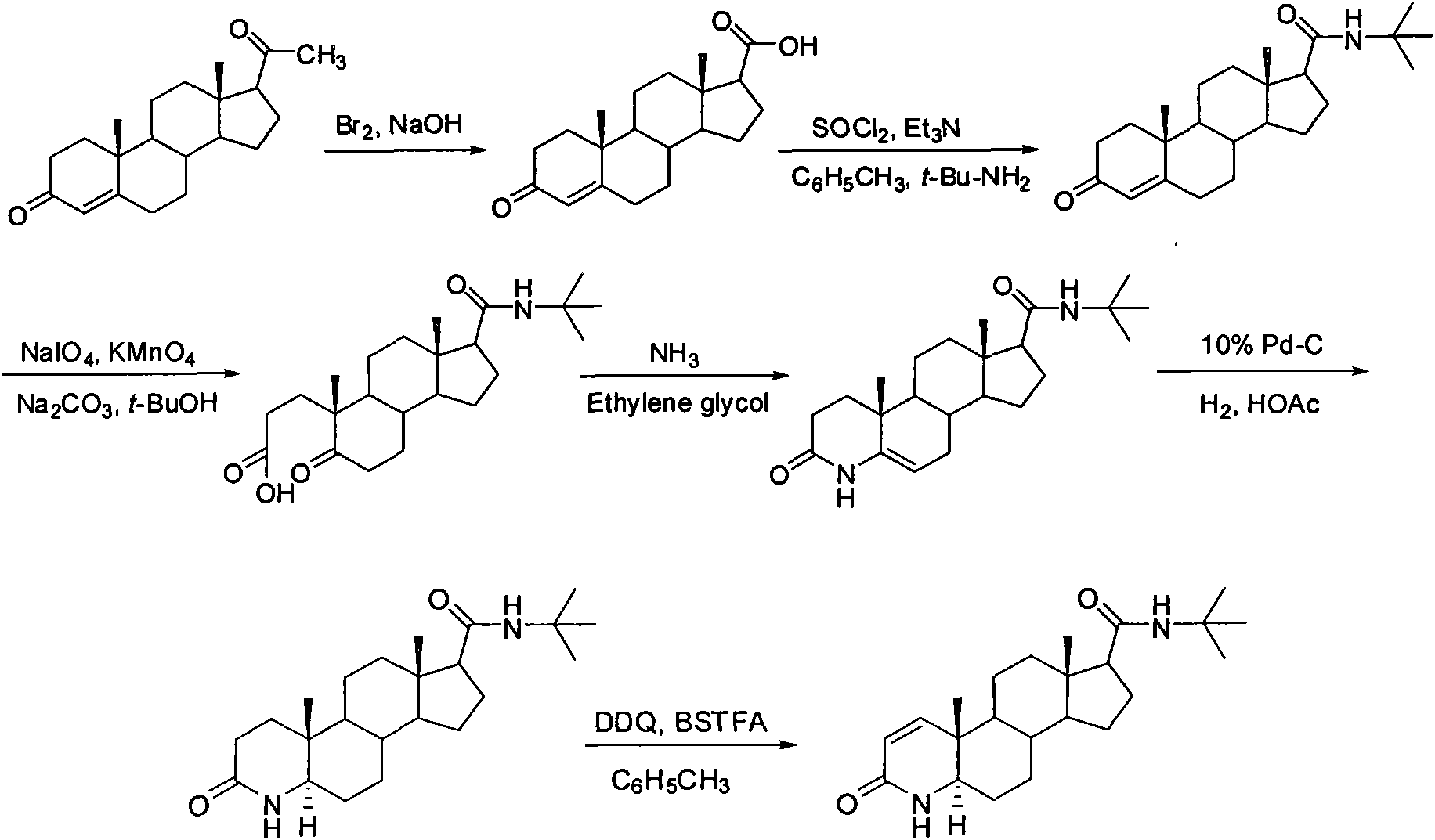
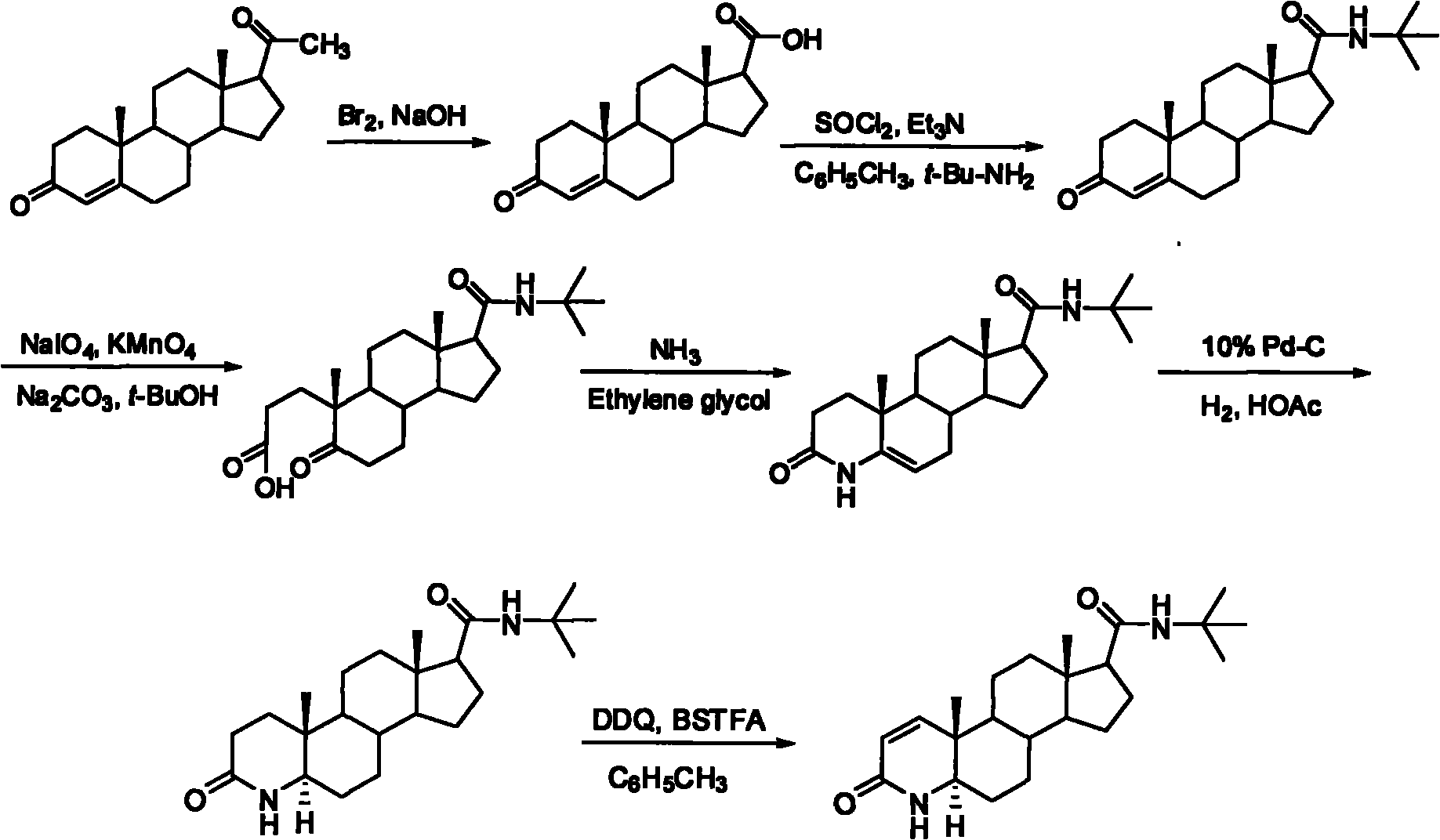
The invention discloses a synthesis method of finasteride, which comprises the following steps that: taking inexpensive luteosterone and the like as the raw materials to finally prepare finasteride through 6 steps of synthetic reaction, i.e. the synthesis of 3- carbonyl-4- androstene-17beta-carboxylic acid, the synthesis of N-tertiary-butyl-3- carbonyl-4- androstene-17beta-formamide, the synthesis of N-tertiary-butyl-5- carbonyl-17beta- carbamoyl-A-lost carbon-3,5-cracking- androstane-3-acid, the synthesis of N-tertiary-butyl-3-carbonyl-4- aza-5- androstene-17beta-formamide, the synthesis of N-tertiary-butyl-3- carbonyl-4- aza-5alpha- androstane-17beta- formamide, and the synthesis of finasteride. The synthesis process has the advantages of inexpensive and easily available raw materials and stable yield, is applicable to industrial production, and the product quality meets the pharmacopeia standards.
Owner:SHANGHAI INST OF TECH
Preparation method for progestin
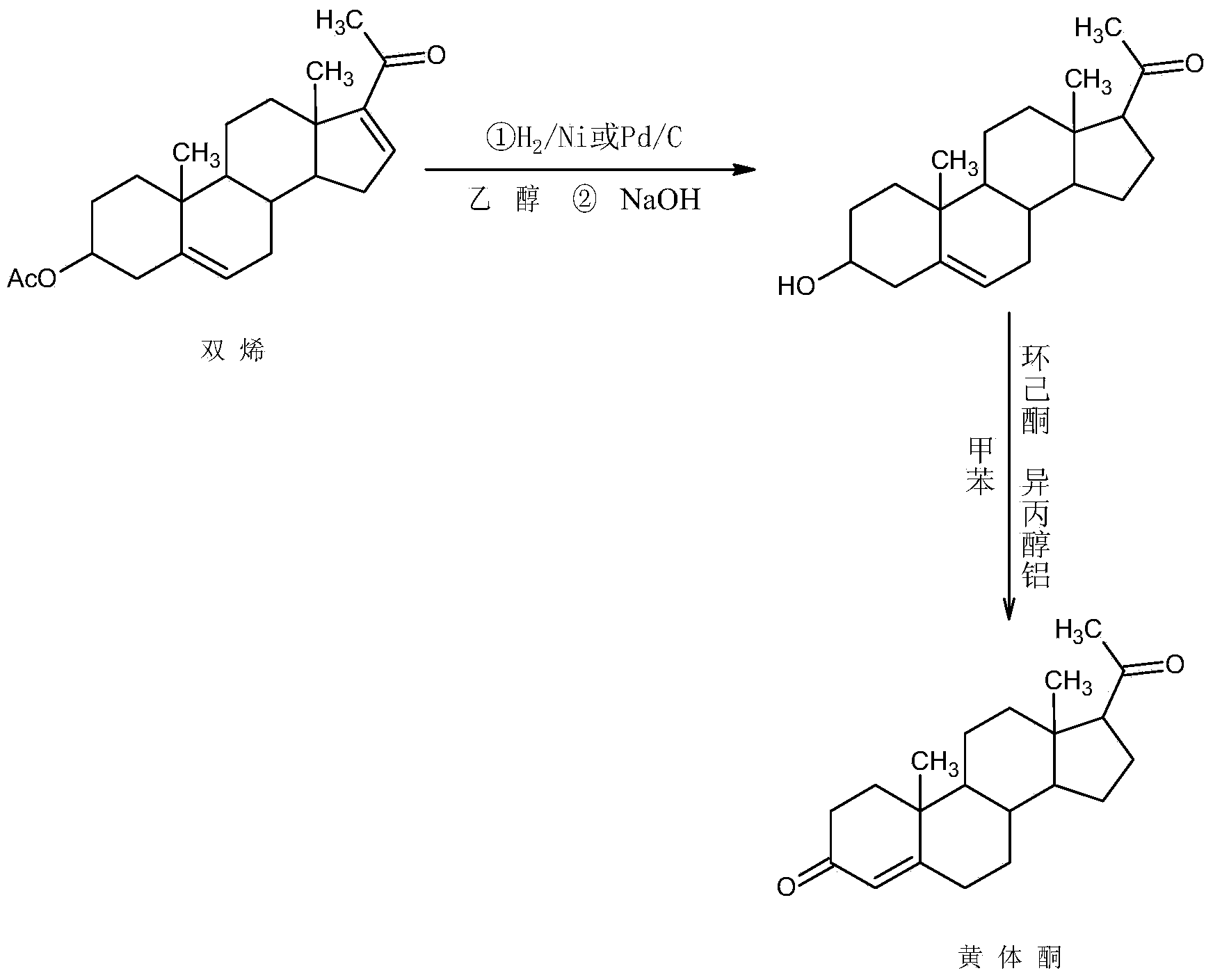
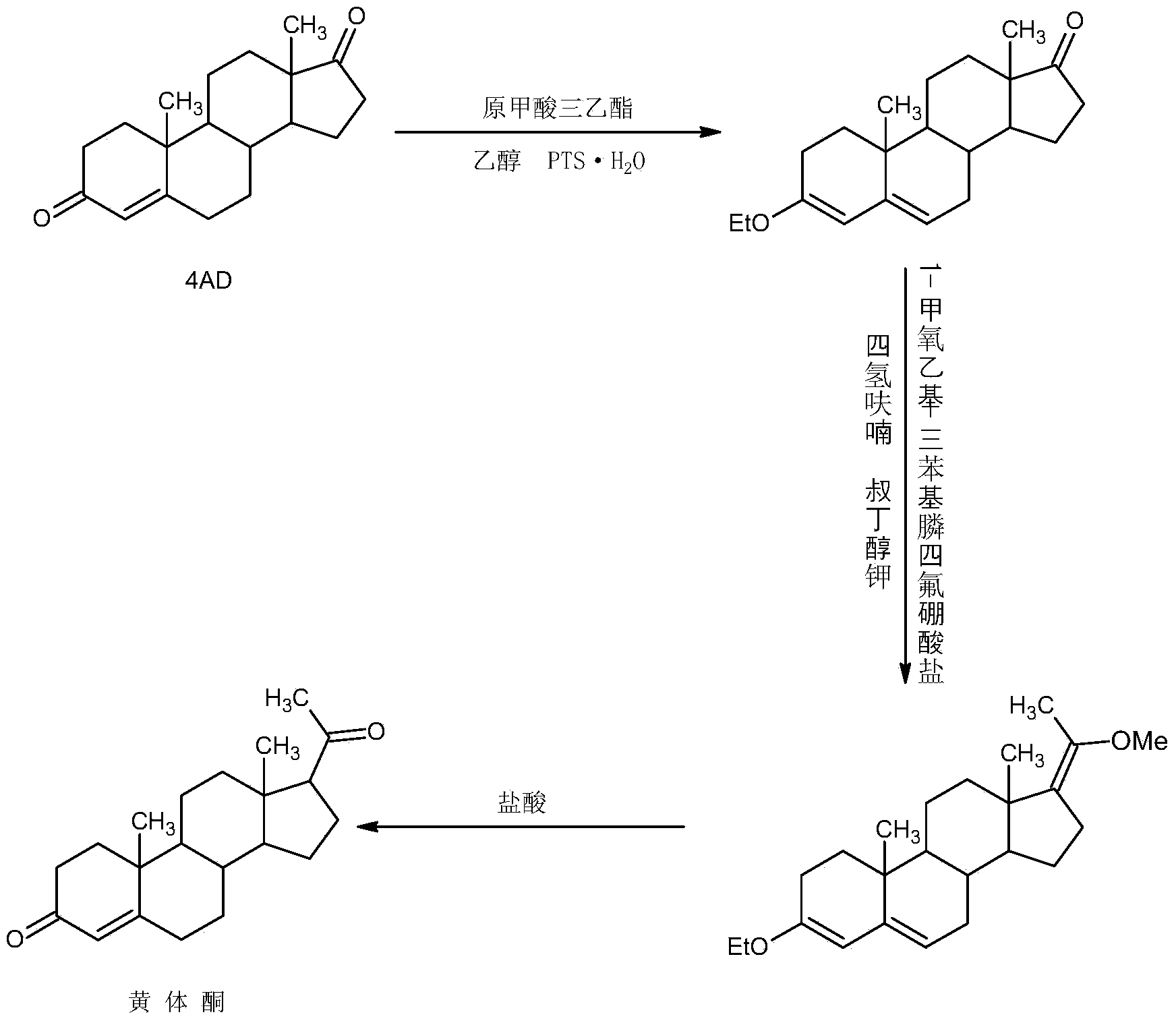
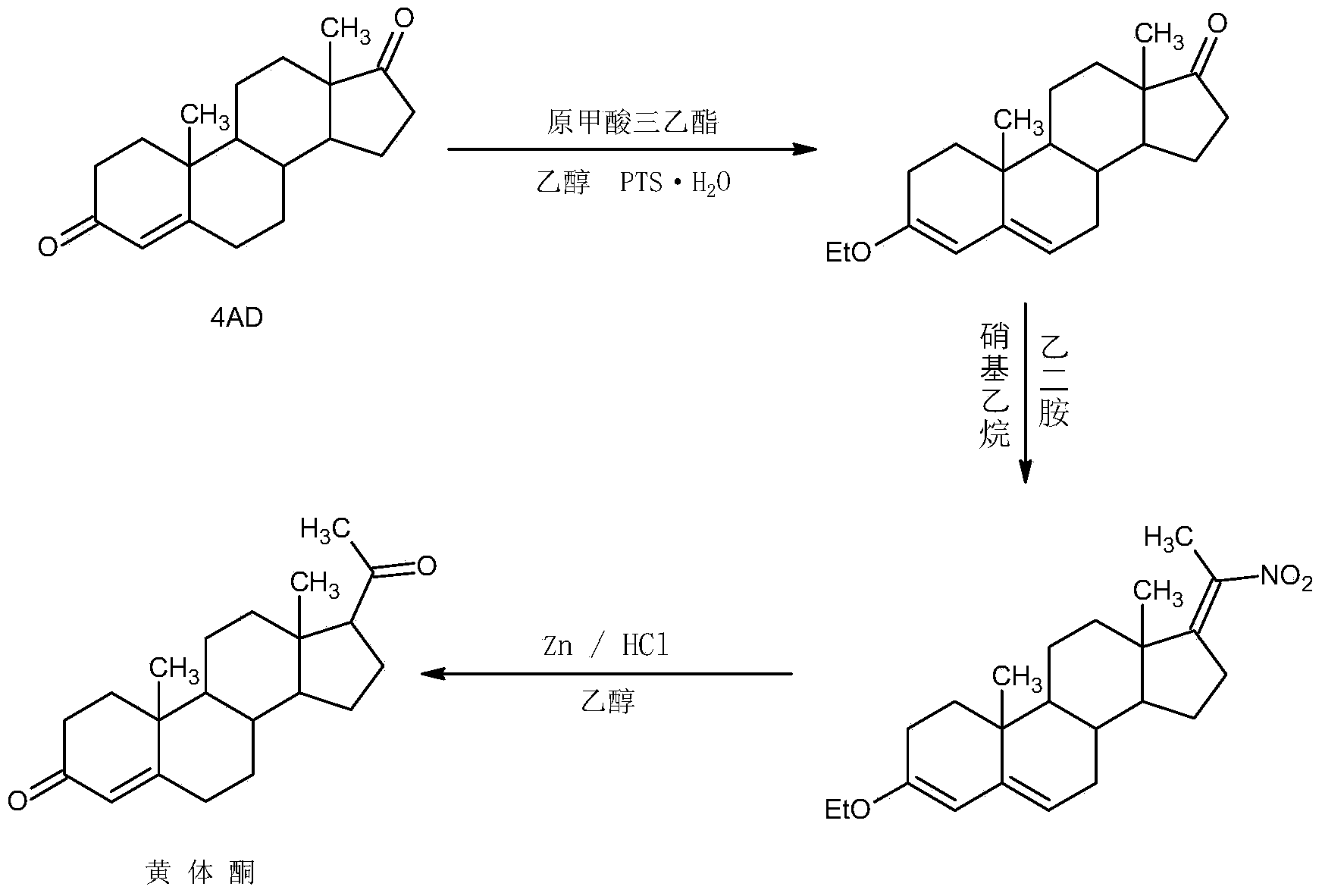
ActiveCN104262442AWide variety of sourcesProcess economy and environmental protectionSteroidsEthylenediamineKetone
The invention relates to a preparation method for progestin. 4-androstenedione is used as a raw material. The preparation method comprises the following steps: A, etherate is synthetized, wherein the 4-androstenedione and triethyl orthoformate perform an acid catalyzed reaction in organic solvents of dichloromethane, low-carbon alcohol and the like to obtain the etherate 3-ethoxy-androstane-3, 5-diolefin-17-ketone; B, a nitro substance is synthetized, wherein the etherate in the organic solvents and nitroethane perform 17-bit addition under the catalysis of ethylenediamine to obtain the nitro substance 3-ethoxy-20-nitro-pregnane-3, 5, 17 (20)-triene; and C, the progestin is synthetized, wherein the nitro substance is reduced by zinc powder in organic solvents of acetic acids, low-carbon alcohol and the like, acid hydrolysis is performed, so that semi-finished products of the progestin are obtained, the semi-finished products of the progestin are decolored and refined by alcohol and activated carbon to obtain the progestin, the content of HPLC is more than 99.5%, the melting point is 128-131 DEG C, and the total yield of synthetized weight is 83-87%. When the method disclosed by the invention is used for producing the progestin, the yield is high, the degree of purity is good, the quality is stable, the solvent recovering rate is high, and the method is economic and environment-friendly.
Owner:HUNAN KEREY BIOTECH
Method for synthesizing difluprednate from sterol fermentation product
The invention provides a method for synthesizing difluprednate from a sterol fermentation product. The sterol fermentation product, namely 9 Alpha-hydroxyl-androstane-1,4-diene-3,17-diketone (9 Alpha-OH-AD) obtained by fermenting phytosterol of which the content in byproducts of the grease industry is very high, serves as a starting raw material. The method comprises the following 15 reaction steps in total: dehydrating steride 9-hydroxyl to form a double-bond; adding 17-carbonyl with acetylene; dehydrating; producing 21-copper carbonyl under an acid condition; epoxidizing 16,17-double bond; oxidizing periodide and introducing 21-hydroxyl; performing ring opening on 16,17 Alpha-epoxy hydrobromate; hydrogenating for removing 16 Beta bromine; forming a ring on orthoester; performing ring opening; esterifying; epoxidizing 9,11-double bond-Beta; enolizing and esterifying; performing ring opening on 6-electrophilic fluoro; and performing ring opening on 9,11-epoxy fluoro. According to the method, steride 17 Alpha and 21-dyhydroxyl are efficiently built by means of periodide oxidization, epoxide ring-opening and debromination and an important intermediate type 11 compound is obtained; in the whole process, a large quantity of heavy metal pollutant chromium which is generated when producing corticoid medicines in the traditional industry is effectively avoided, so that the method is green, environment-friendly and suitable to industrialized production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Topical dermatological formulations and use thereof
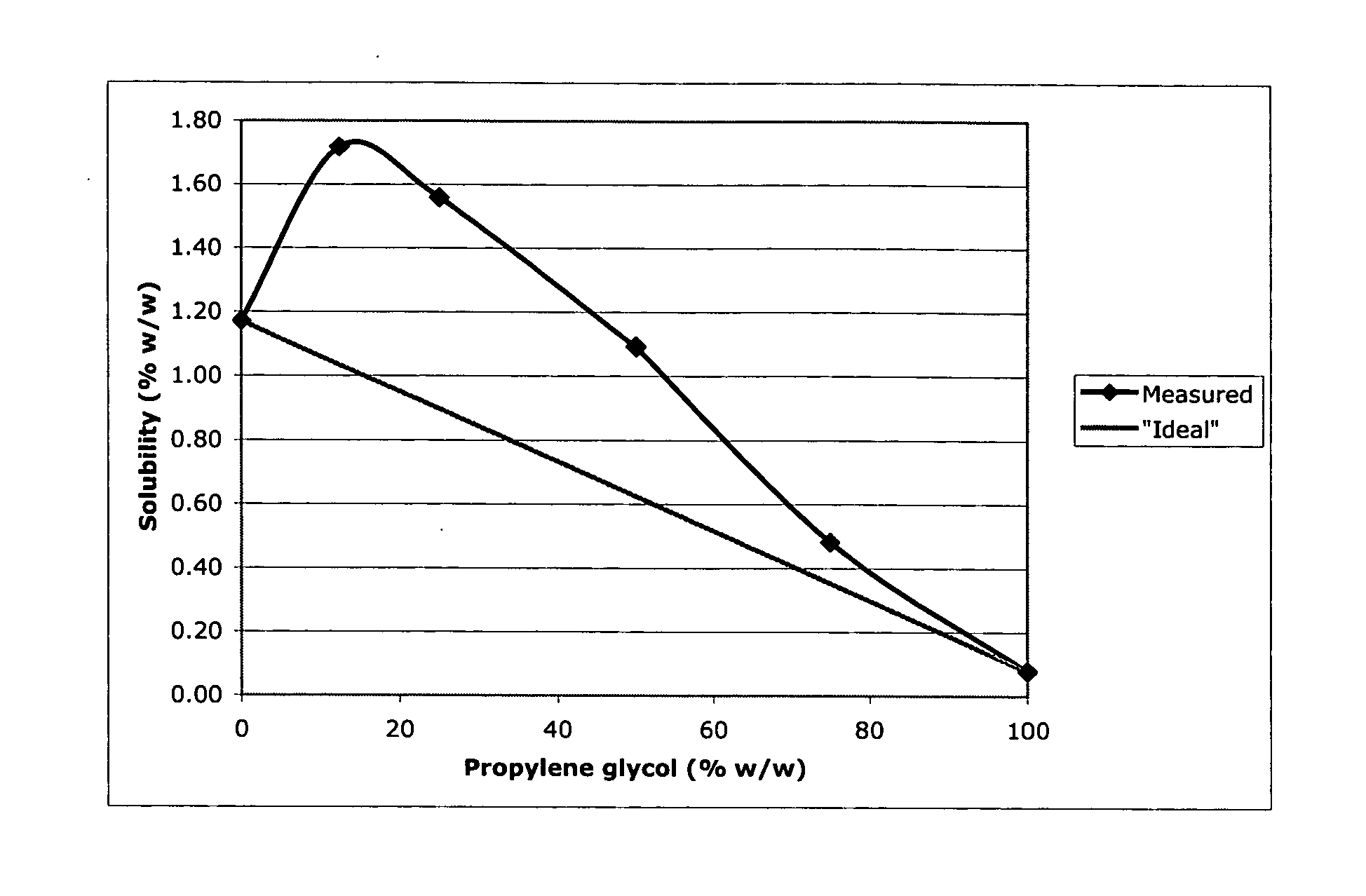
InactiveUS20060052353A1Improve solubilityImprove efficiencyOrganic active ingredientsSteroidsAndrostanesPropylene carbonate
A topical formulation of an androstane steroid compound of improved solubility in combinations of the solvents propylene glycol and propylene carbonate.
Owner:FOUGERA PHARM INC
3-ketosteroid -delta 1-dehydrogenase, engineering bacterium and application thereof
ActiveCN102168099AIncrease productivityImprove product qualityFungiMicroorganism based processesBiotechnologyProtein target
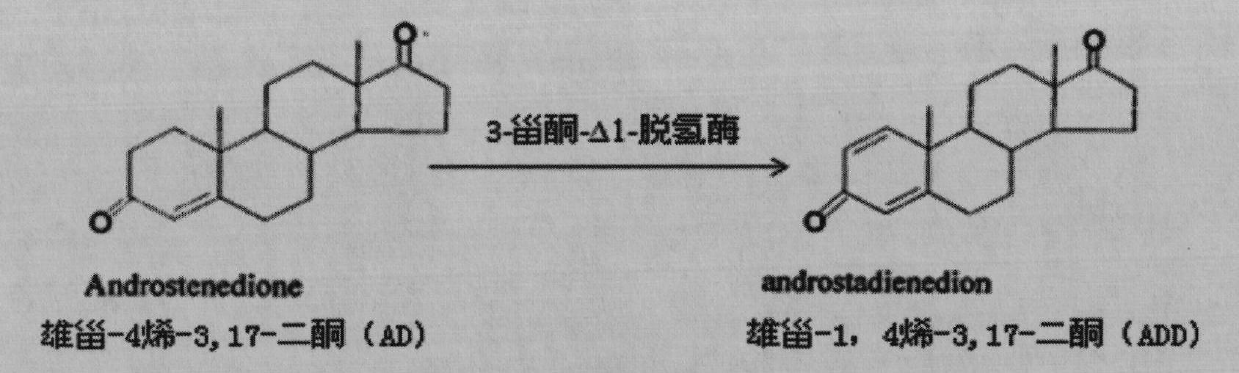
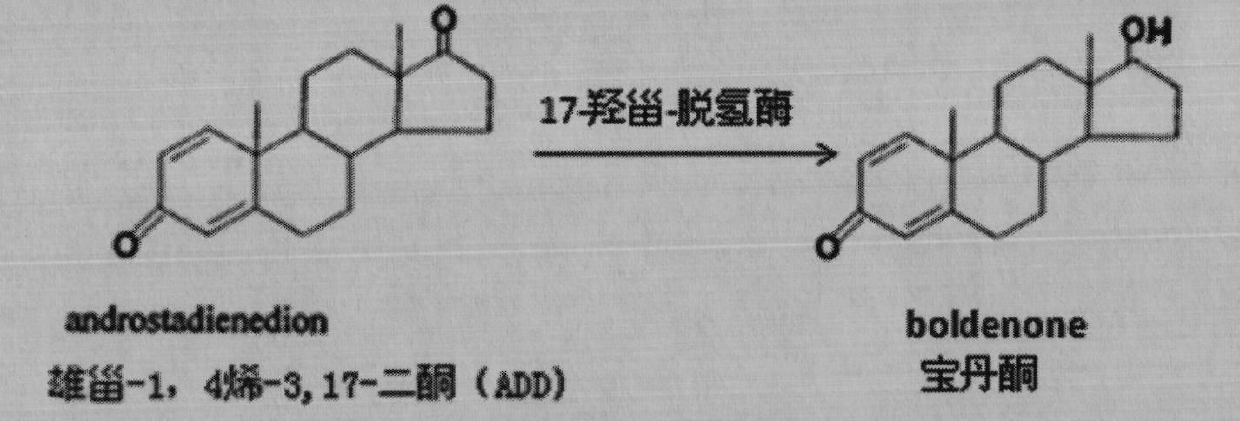
The invention provides a 3-ketosteroid-delta 1-dehydrogenase gene, cholesterol oxidase coded by the same, an expression vector containing a gene sequence of the 3-sterone-delta 1-dehydrogenase gene, a gene engineering recombinant strain containing the expression vector and a method for preparing 3-sterone-delta 1-dehydrogenase. The 3-sterone-delta 1-dehydrogenase provided by the invention is new 3-sterone-delta 1-dehydrogenase. The recombinant strain provided by the invention can be used for transforming 3-keto steroids, wherein the optimized recombinant strain can also be used for transforming androstane-4-alkenyl-3,17-diketone into boldenone, the expressed target protein is a soluble protein, the bottleneck of membrane protein in the traditional industrial production is broken through, and the recombinant strain has great significance in the industrial production of KSDD (3-ketosteroid -delta 1-dehydrogenase). Steroids are produced through microbial transformation, the production efficiency and the product quality of a steroid medicine production system are improved, and the production cost is reduced.
Owner:EAST CHINA UNIV OF SCI & TECH
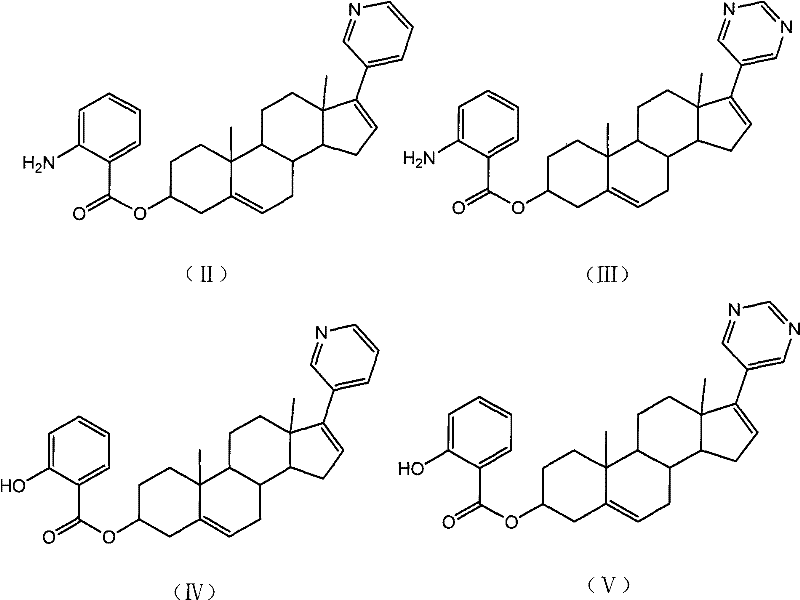
Pyridine androstane derivative and application thereof to preparing medicine for preventing and/or treating prostatic cancer
InactiveCN102477061AReduce first pass effectImprove effective bioavailabilityOrganic active ingredientsSteroidsTolerabilitySide effect
The invention relates to a novel pyridine androstane derivative and application thereof to preparing a medicine for preventing and / or treating prostatic cancer. The pyridine androstane derivative disclosed by the invention is an ideal selective cytochrome oxidase CYP450c17 inhibitor and can be used for treating or preventing various indications relative to male hormonal functions, in particular prostatic cancer. The pyridine androstane derivative has lower oxidative metabolism and higher effective bioavailability, therefore having the advantages of less application dosage, better tolerance and little side effect.
Owner:苏州波锐生物医药科技有限公司
Preparation method of compound 19-desmethyl-4-androstene-3,17 diketone
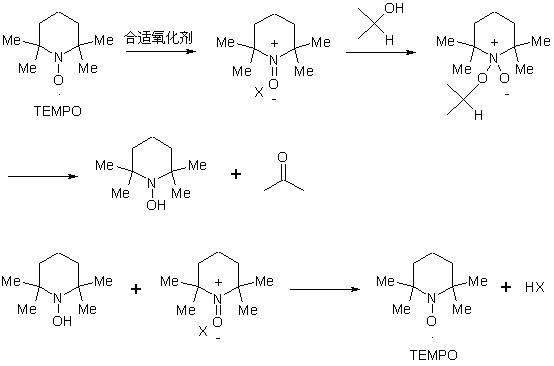
The invention discloses a preparation method of a compound 19-desmethyl-4-androstene-3,17 diketone, comprises the following step of by taking compounds of 5alpha-chloro-3beta-hydroxy-6beta and 19beta-epoxy-androstane-17-ketone as raw materials, and carrying out oxidation reaction on the compounds of 5alpha-chloro-3beta-hydroxy-6beta and 19beta-epoxy-androstane-17-ketone as well as an intermediate compound 19-hydroxy-4-androstene-3,17-diketone with N-halogenated amide oxidant in a mixed solution of an organic solvent and an alkaline buffer solution in the presence of a catalytic amount of 2,2,6,6-tetramethylpiperidine-N-oxide (TENPO) in the mild condition. In the two steps of oxidation reaction, the catalytic amount of 2,2,6,6-tetramethylpiperidine-N-oxide and the N-halogenated amide oxidant are both adopted to replace a mixed solution of chromium trioxide, sulfuric acid and water. In the oxidation method, without using the chromium trioxide, the use of carcinogenic substances are avoided being used, limitation by environmental protection is avoided and a great amount of wastes containing heavy metals in preparation are avoided being generated and accumulated, thereby no money and labor is consumed for removing the wastes.
Owner:ZHEJIANG XIANJU PHARMA
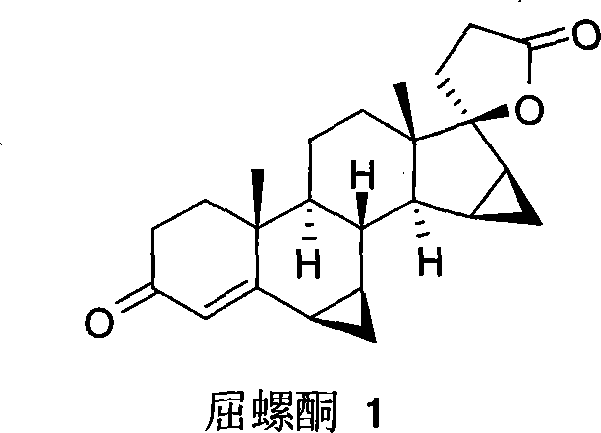
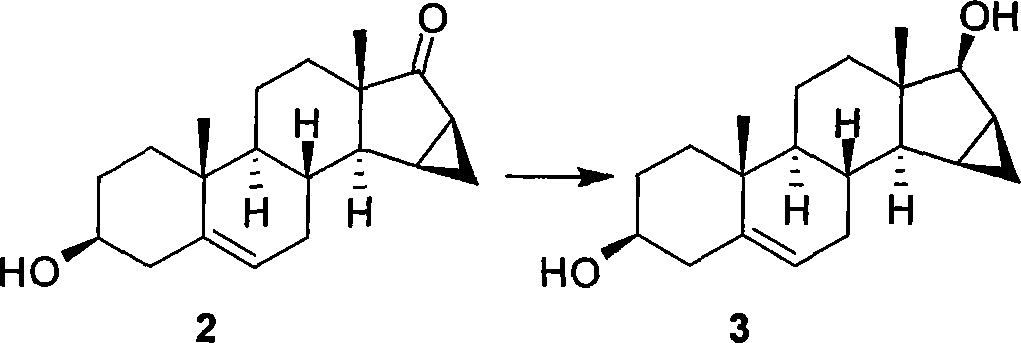
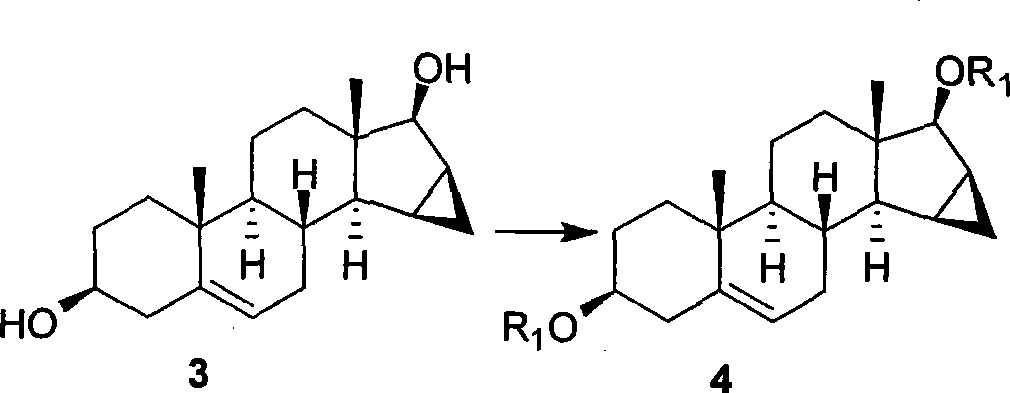
Method for preparing drospiroenonand intermediate
The invention relates to a new method for preparing drospirenone (6 beta, 7 beta; 15 beta, 16 beta- dimethylene-3-oxo-17 alpha- pregna-4-alkene-21, 17- carboxyl lactone) and an intermediate product obtained by the method, that is, 3- androstane protected by hydroxy group-5- alkene-15 beta, 16 beta- methylene-17-ketone. The invention uses the 3- androstane protected by hydroxy group-5- alkene-15 beta, 16 beta-methylene-17-ketone being available in the market as the starting material, which forms loop coils by the steps such as the hydroxy group protection, addition, hydrolysis, oxidation, and finally forms triatomic ring to prepare the drospirenone.
Owner:2Y CHEM
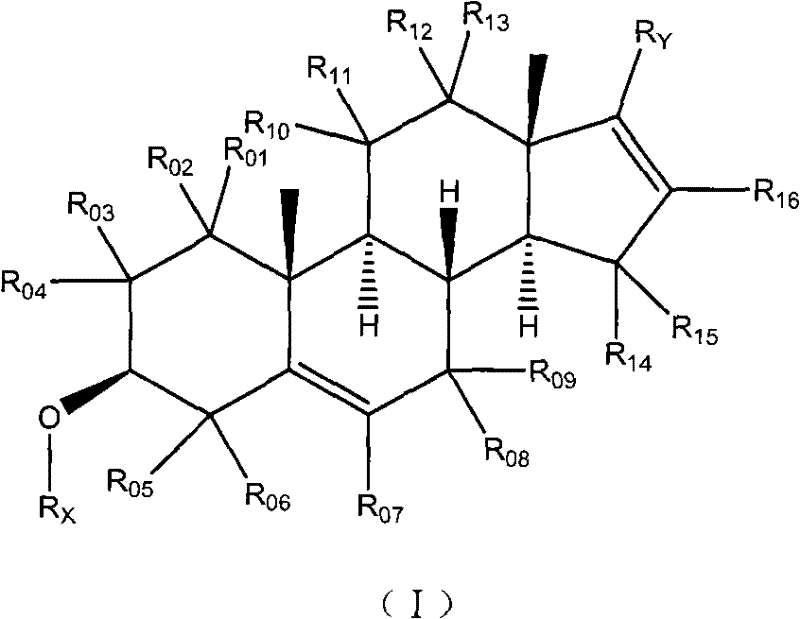
Anti-inflammatory androstane derivative compositions
InactiveUS6858593B2Minimize exposureImprove securityOrganic active ingredientsBiocideChemical compositionSpace group
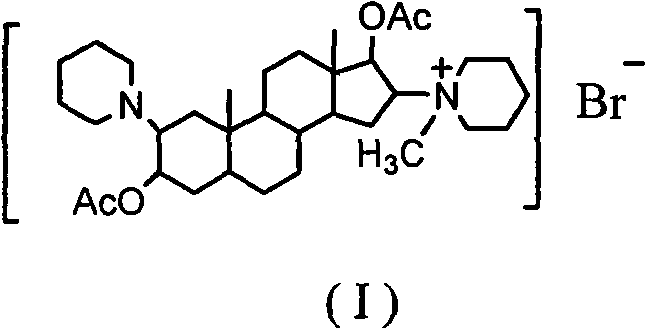
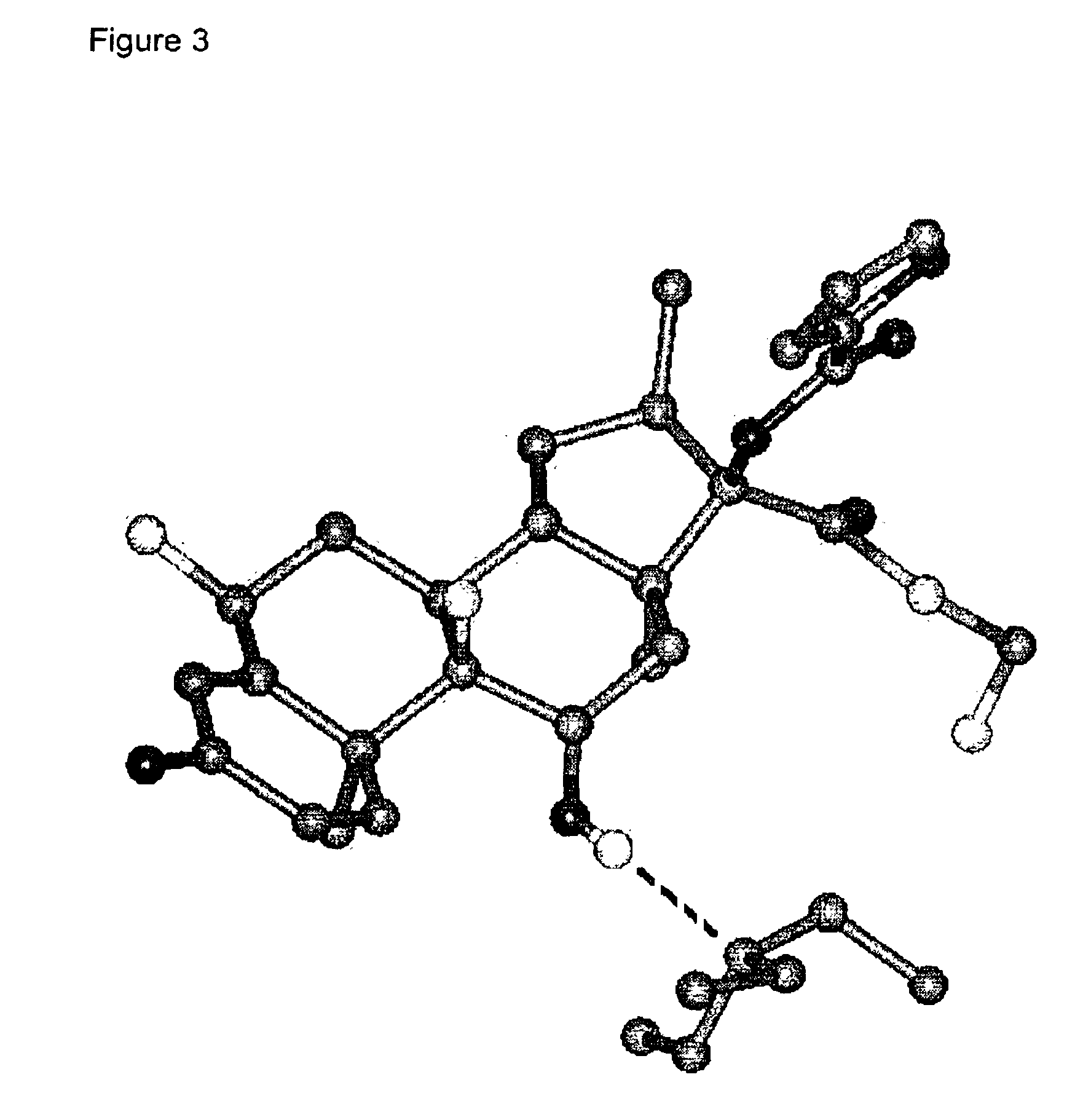
There is provided a crystalline chemical composition comprising a compound of formula (I) in which the crystal lattice is stabilized by the presence of a guest molecule, characterized in the crystalline composition is of space group P212121 having unit cell dimensions of about 7.6±0.6 Å, 12.7±0.7 Å, and 33±3 Å when determined at 120 K.
Owner:GLEKSOSMITKLAJN INTELLEKCHUAL PROPERTI MENEDZHMENT LTD
Novel method for stereo-selective chemosynthesis of drospirenone
The invention relates to a new method of stereo selectivity synthesis drospirenone and main intermediate in the synthesizing method; 15 Beta, 16 Beta-methylene dehydroepiandrosterone (3 Beta-hydroxy-15Beta, 16Beta-methylene -androstane -5-ene-17-ketone, 2) are adopted as starting raw material for the methods and the drospirenone is synthesized via fourteen steps. The invention has the advantages of higher yield and good stereo selectivity.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Method for preparing progesterone by taking 1,4-androstenedione as raw material
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
High-sensitivity nanometer cobalt oxide-doped amobarbital molecular imprinting electrochemical sensor and preparation method thereof
InactiveCN103926288AEasy to manufactureMaterial electrochemical variablesFunctional monomerAndrostane
The invention discloses a high-sensitivity nanometer cobalt oxide-doped amobarbital molecular imprinting electrochemical sensor and a preparation method thereof. Amobarbital serves as a template molecule, 20(s)-O-androstane-4-alkene-17beta-acyl camptothecin serves as a functional monomer, azodiisobutyronitrile serves as an initiator, nanometer cobalt oxide serves as a doping agent, and maleated rosin ethylene glycol acrylate synthesized by taking rosin as a raw material serves as a crosslinking agent, so as to prepare a high-sensitivity nanometer cobalt oxide-doped amobarbital molecular imprinting electrochemical sensor. The analytical method is simple and practical, and the defects that the previous analytical method is complex, expensive in equipment and low in sensitivity are overcome.
Owner:GUANGXI UNIV FOR NATITIES
Preparation of rocuronium
The invention pertains to the medicinal chemosynthesis field and relates to the preparation method of 1-square bracket 17Beta-acetoxyl-3Alpha-oxhydryl-2Beta-(4-morpholinyl)-androstane-16Beta-group square bracket-1-(2-propenyl) pyrrole bromide (rocuronium). The preparation method adopts 2Alpha, 3Alpha, 16Alpha, 17Alpha-diepoxy-5Alpha-androstane-17Beta-glycol acetate as the raw material from which the rocuronium is obtained after seven reaction steps including hydrolysis, pyrrole reaction, morpholinyl reaction, etherification, reduction, acetylation, hydrolysis and quaterisation. The preparation method of the invention has the advantages that the hydrolysis of 16Alpha, 17Alpha-epoxy-17Beta-acetoxyl takes place at a comparatively low temperature, which can avoid the ring opening of the epoxy and further the inhibition of the formation of 17Alpha-(1-pyrrolidine)-16-ketone; the synthesis avoids the optional acetylation of site 3 and site 17 oxhydryls and clearly simplifies the separation after reaction.
Owner:FUDAN UNIV
Novel method for preparing rocuronium bromide
The invention relates to a novel method for preparing rocuronium bromide 1-[17beta-acetoxyl-3alpha-hydroxyl-2beta-(4-morpholinyl)-androstane-16beta-yl]-1-(2-propenyl) pyrrole bromide, the problem of chemoselectivity of pyrrolidine open epoxy in an original line is solved, generation of byproducts is avoided, reaction yield is greatly improved, the production cost is reduced, column chromatography separation is avoided, and aftertreatment purification is implemented easily.
Owner:JIANGSU QINGJIANG PHARMA
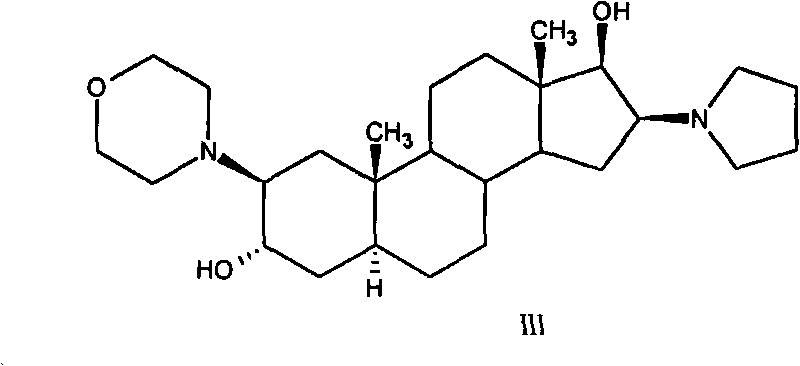
High-purity (2 beta, 3 alpha, 5 alpha, 16 beta, 17 beta)-2-(4-morpholinyl)-16-(1-pyrrolidinyl)-androstane-3,17-diol or composition thereof and preparation method thereof
The invention relates to a high-purity (2 beta, 3 alpha, 5 alpha, 16 beta, 17 beta)-2-(4- morpholinyl)-16-(1-pyrrolidinyl)-androstane-3,17-diol or a composition thereof, a preparation method thereof (a compound shown in a formula III) or a composition thereof and application thereof to preparing rocuronium (a compound shown in a formula I). The method has good effect, low cost, high quality and simple and convenient operation and is suitable for large-scale industrial production; and the obtained product has high purity and stable properties.
Owner:重庆凯林制药有限公司 +1
Preparation method of clobetasol and preparation method of clobetasol propionate
The invention discloses a preparation method of clobetasol and the preparation method of clobetasol propionate. The preparation method of the clobetasol comprises the following steps: by taking a compound I, namely 1,4,9(11)-triene androstane-3,17-diketone as an initial raw material, performing a methylation reaction, a cyan substitution reaction, a siloxy protection reaction, an intramolecular nucleophilic substitution reaction, a bromoepoxy reaction and a fluorination reaction to prepare a compound VII which is clobetasol. The compound VII is subjected to a propyl esterification reaction to prepare a compound VIII which is clobetasol propionate. According to the preparation method disclosed by the invention, since relatively basic initial raw materials which are cheap are used, each step of reaction is relatively easy to implement and high yield is achieved; the operation of multi-step protection and deprotection is simplified; moreover, 21 sites of fluorine are directly arranged in one step during arrangement of a side chain, and multiple steps of reaction for arranging the 21 sites of fluorine in the prior art are directly avoided, so that the synthetic route is greatly shortened, the total yield is increased, the product quality is improved and the production cost is greatly lowered.
Owner:江西赣亮医药原料有限公司
Method for preparing compound 6beta, 19beta-epoxy-4-androstene-3, 17-diketone
The invention relates to a method for preparing a major midbody of a 19-bit dishorn methyl steroid compound, in particular to a method for preparing a compound 6beta, 19beta-epoxy-4-androstene-3, 17-diketone, which comprises the following steps of: 1, halogen alcoholization reaction: 3beta-acetoxyl-androstane-5-alkene-17-ketone takes halogen alcoholization reaction or chlorine alcoholization reaction to generate 3beta-acetoxyl-5alpha-bromo / chloro-6beta-hydroxyl-17-ketone; 2, illumination cyclization reaction: compounds obtained in the first step take cyclization reaction under the illumination condition in the existence of iodine, halide and catalysis quantity of radical initiators to obtain 3beta-hydroxyl-5alpha-halogenate-6beta, 19beta-epoxy-17-ketone; and 3, oxidation elimination reaction: (1) the compounds obtained in the second step take oxidation reaction under the effect of oxidizing agents to generate corresponding oxides; and (2) the obtained oxides take elimination reaction through being heated under the alkaline condition to generate 6beta, 19beta-epoxy-4-androstene-3, 17-diketone.
Owner:ZHEJIANG SHENZHOU PHARMA
Process For The Preparation Of Drospirenone
A process is described for the preparation of drospirenone, a synthetic steroid with progestogenic, antimineralocorticoid and antiandrogenic activity, useful for preparing pharmaceutical compositions with contraceptive action; comprising the oxidation of 17α-(3-hydroxypropyl)-6β,7β,15β,16β-dimethylene-5β-androstane-3β,5,17β-triol.
Owner:IND CHEM SRL
Dermatological Formulation
InactiveUS20030216364A1Reduce the amount of solutionImproved VC potencyOrganic active ingredientsCosmetic preparationsFluoroethylDouble bond
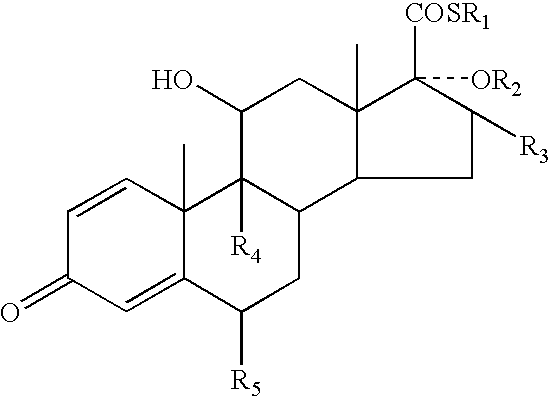
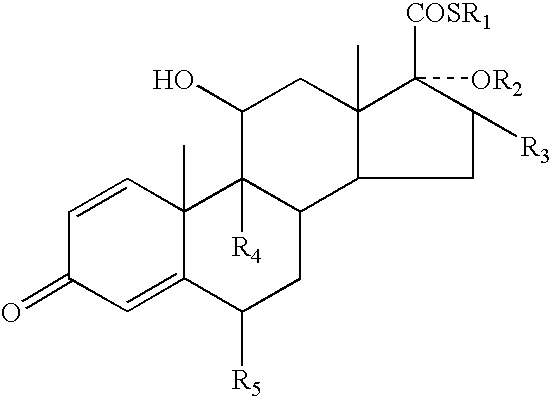
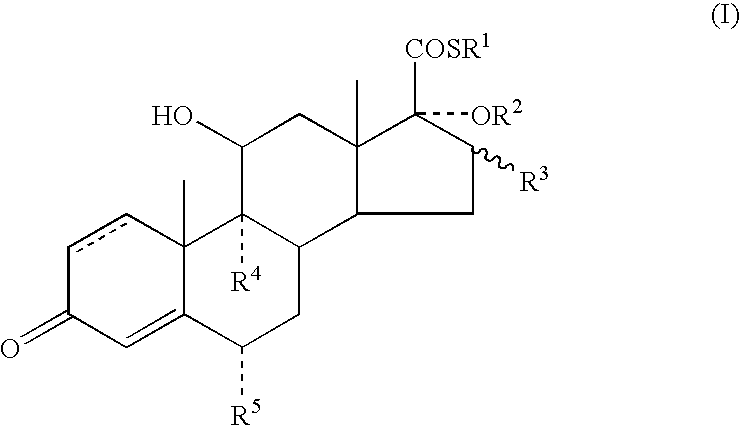
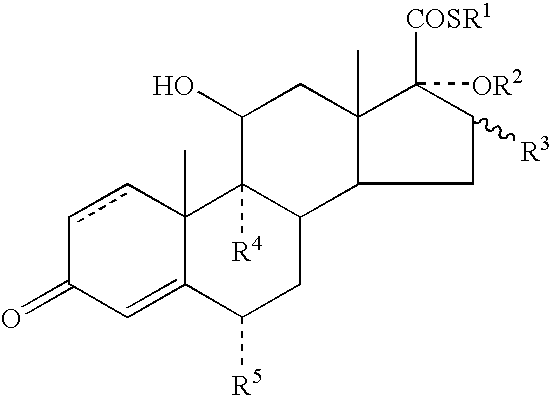
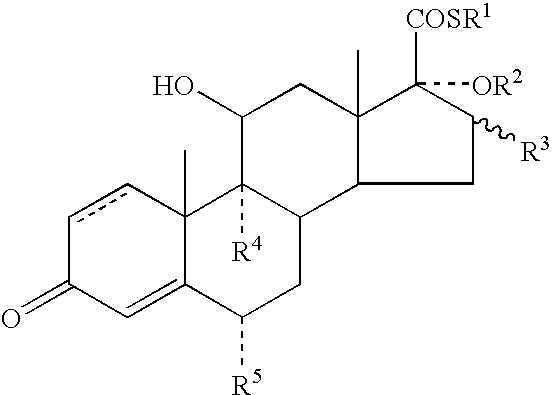
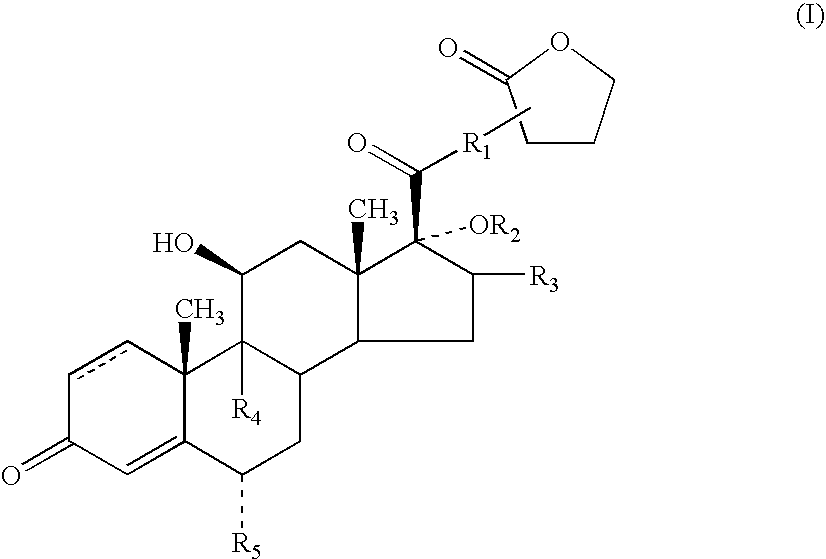
A topical formulation including a solvent, an occlusive agent, a surfactant system, an androstane steroid compound of formula (I) wherein R<1 >represents a fluoro-, chloro- or bromo-methyl group or a 2'-fluoroethyl group, R<2 >represents a group COR<6 >where R<6 >is a C1-3 alkyl group or OR<2 >and R<3 >together form a 16alpha, 17alpha-isopropylidenedioxy group; R<3 >represents a hydrogen atom, a methyl group (which may be in either the alpha- or beta-configuration) or a methylene group; R<4 >represents a hydrogen, chlorine or fluorine atom; R<5 >represents a hydrogen or fluorine atom and the symbol --- represents a single or double bond, and the balance being water.
Owner:SMITHKLINE BECKMAN CORP
Compounds useful in the manufacture of an anti-inflammatory androstane derivative
InactiveUS7125985B2Minimize exposureImprove securityOrganic active ingredientsSenses disorderAndrostanesAndrostane
The present invention relates to, for example, a compound of formula (X)useful in the manufacture of anti-inflammatory androstane derivatives.
Owner:GLEKSOSMITKLAJN INTELLEKCHUAL PROPERTI MENEDZHMENT LTD
Method for preparing androstane-1,4,6-triene-3,17-diketone
InactiveCN101659976AReduce pollutionImprove conversion rateMicroorganism based processesSteroidsDiketoneLithium
The invention provides a method for preparing androstane-1,4,6-triene-3,17-diketone. The method comprises the following steps: adding a compound (I) into a polar organic solvent, and adding an ester reagent and an acid catalyst into the mixture to preparing a compound (II); adding the compound (II) into the organic solvent, adding a halogen reagent and water into the mixture for halogenating reaction; after the reaction is completed, adding lithium salt for reaction to prepare a compound (III); and carrying out 1,2 position dehydrogenation of the compound (III) by a method for biofermentationby adopting an arthrobacter nicotinovorans to prepare the target product.
Owner:TIANJIN JINYAO GRP
Anti-inflammatory androstane derivatives
ActiveUS7405206B2Useful and long lasting anti-inflammatory activityLittle and no systemic activityOrganic active ingredientsAntipyreticArylHalogen
There are provided compounds of formula (I)whereinR1 represents O, S or NH;R2 represents —C(═O)-aryl or —C(═O)-heteroaryl;R3 represents hydrogen, methyl (which may be in either the α or β configuration) or methylene;R4 and R5 are the same or different and each represents hydrogen or halogen; and represents a single or a double bond;and salts and solvates thereof;process for preparing them, compositions containing them and their use in therapy.
Owner:GLAXO GROUP LTD
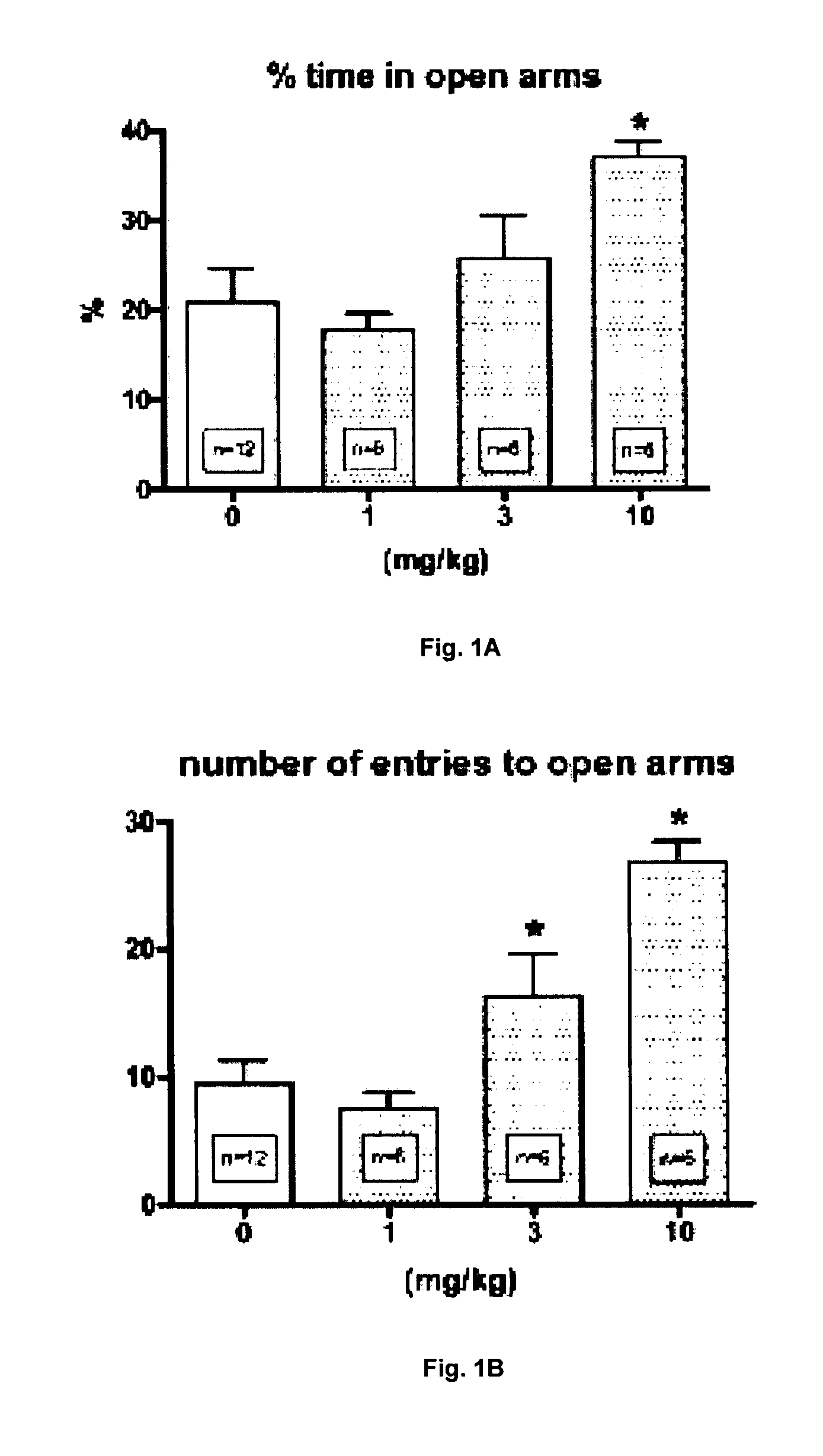
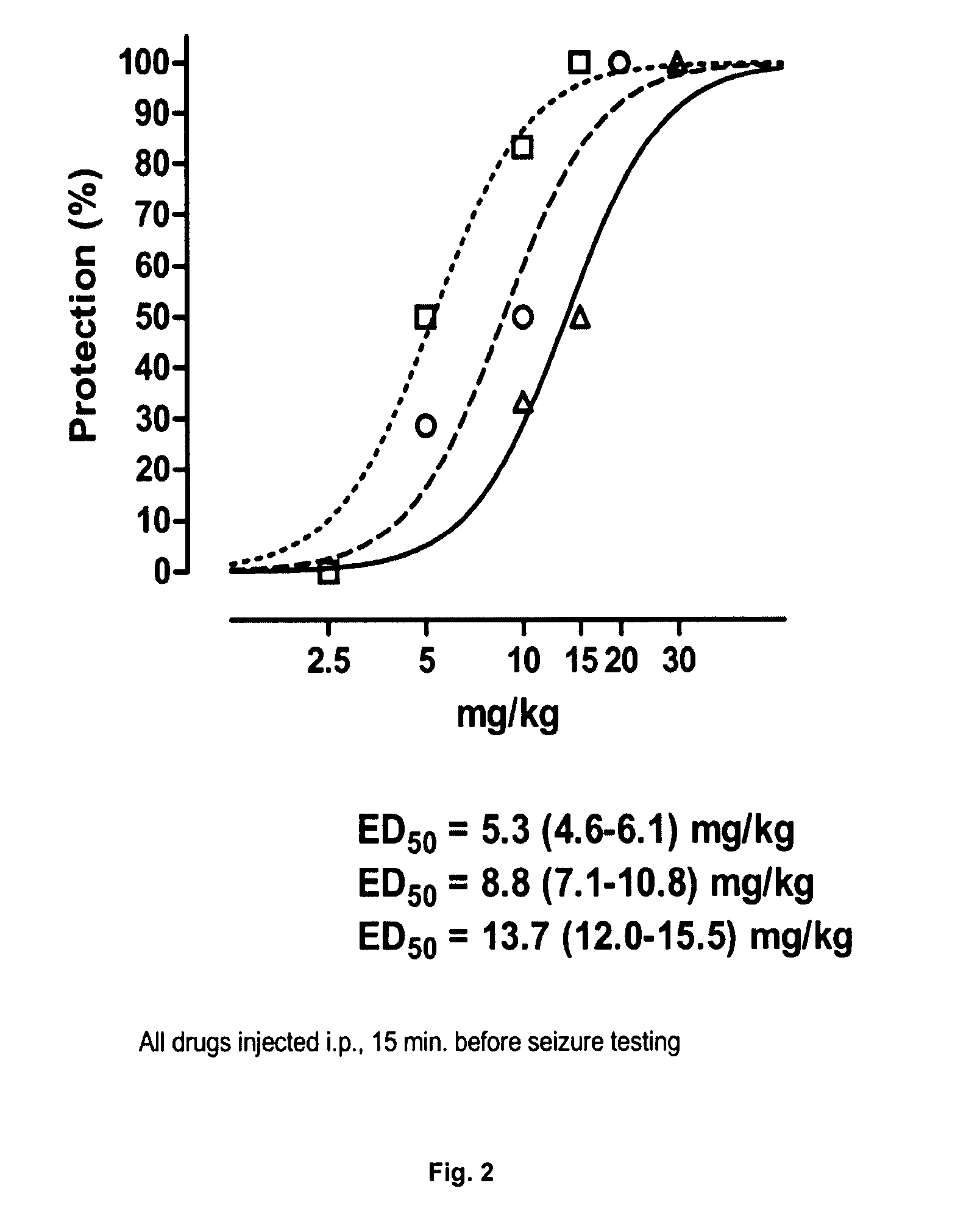
Androstane and pregnane steroids with potent allosteric gaba receptor chloride ionophore modulating properties
This invention describes compounds of Structures 1, 2, and 3 and their use as allosteric modulators of the GABA receptor chloride ionophore complex to alleviate stress, anxiety, mood disorders, seizures, depression, treatment of drug and alcohol abuse, memory, premenstrual disorders, and neural system damage.
Owner:UNITED STATES OF AMERICA +1
Androstane and pregnane steroids with potent allosteric GABA receptor chloride ionophore modulating properties
This invention describes compounds of Structures 1, 2, and 3 and their use as allosteric modulators of the GABA receptor chloride ionophore complex to alleviate stress, anxiety, mood disorders, seizures, depression, treatment of drug and alcohol abuse, memory, premenstrual disorders, and neural system damage.
Owner:UNITED STATES OF AMERICA +1
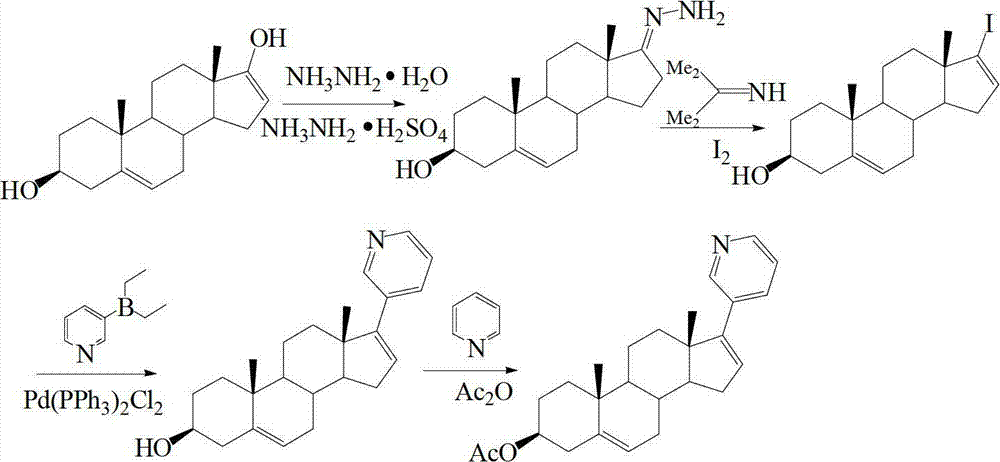
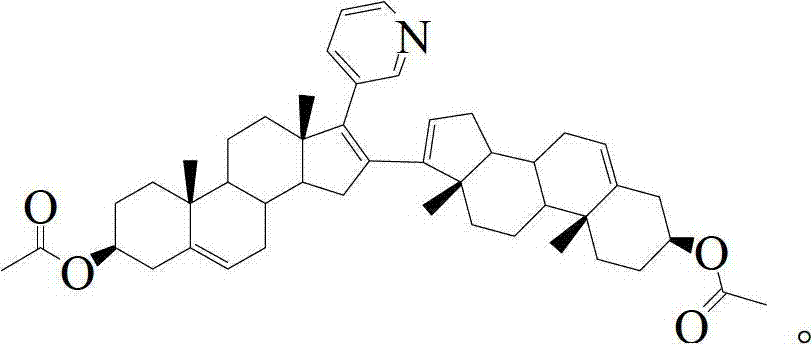
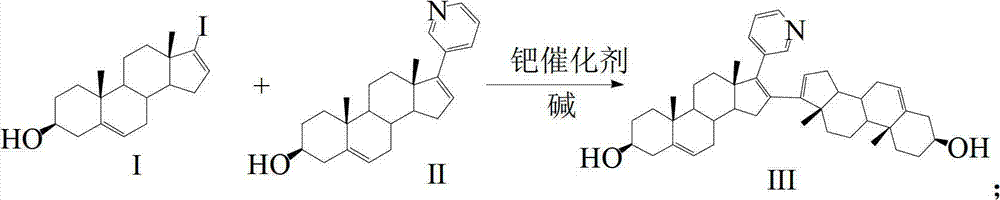
Preparation and detection method of abiraterone Acetate dimer compound
The invention discloses a preparation and detection method of an abiraterone acetate dimer compound, and relates to the of field pharmacy. The dimer compound is 3 beta-acetyl-16-(3 beta-acetyl androstane-5,16-diene-17-base)-17-(3-pyridyl) androsterone-5,16-diene. The method is as follows: adding compounds I and II into a reactor; dissolving the compounds and adding a palladium catalyst and alkali into the reactor; cooling to room temperature; filtering; concentrating; adding water; extracting; concentrating to obtain a compound III crude product; placing the crude product in the reactor; adding pyridine and an acetyl compound; stirring at room temperature till complete reaction; concentrating to remove pyridine and the acetyl compound; adding water, extracting and concentrating to obtain a dimer compound crude; and dissolving the dimer compound crude, washing, drying, filtering, concentrating and recrystallizing to obtain the abiraterone acetate dimer compound. According to high performance liquid chromatography detection and calculation by an area normalization method, the prepared dimer compound has a purity up to 98% and a yield higher than 80%.
Owner:武汉长联来福制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com