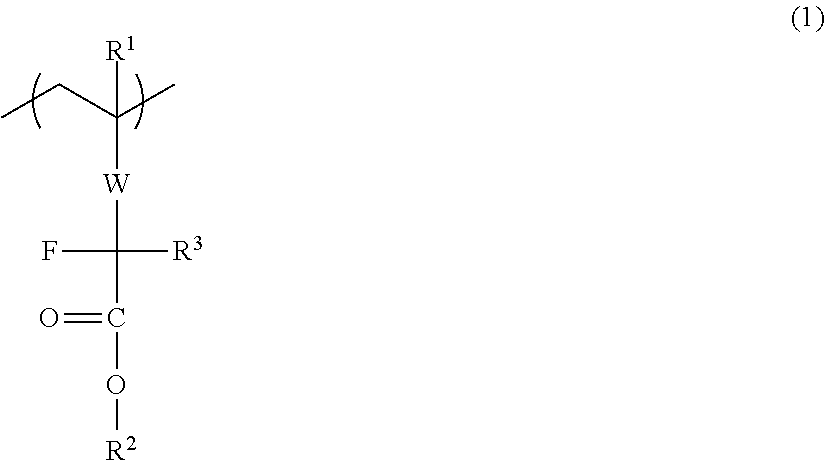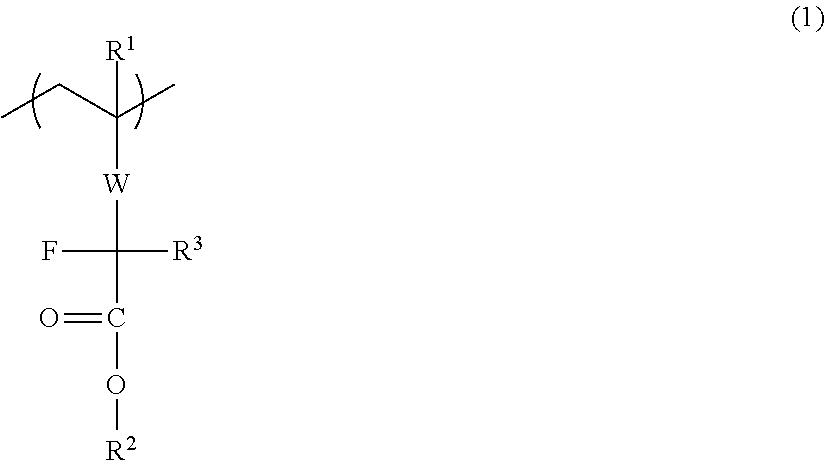Patents
Literature
435 results about "Improved solubility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
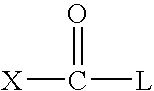
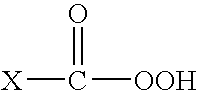
Improved solubility can be accomplished by reducing logP or melting point by increasing polarity or disrupting intermolecular interactions in the solid state. Tactics for increasing polarity include introducing a solubilizing appendage onto the drug or modifying the template or attached substituents.
Parenteral formulations of lipophilic pharmaceutical agents and methods for preparing and using the same
ActiveUS20120277249A1Improve solubilityImprove stabilityAntibacterial agentsOrganic active ingredientsOrganic solventAutoimmune disease
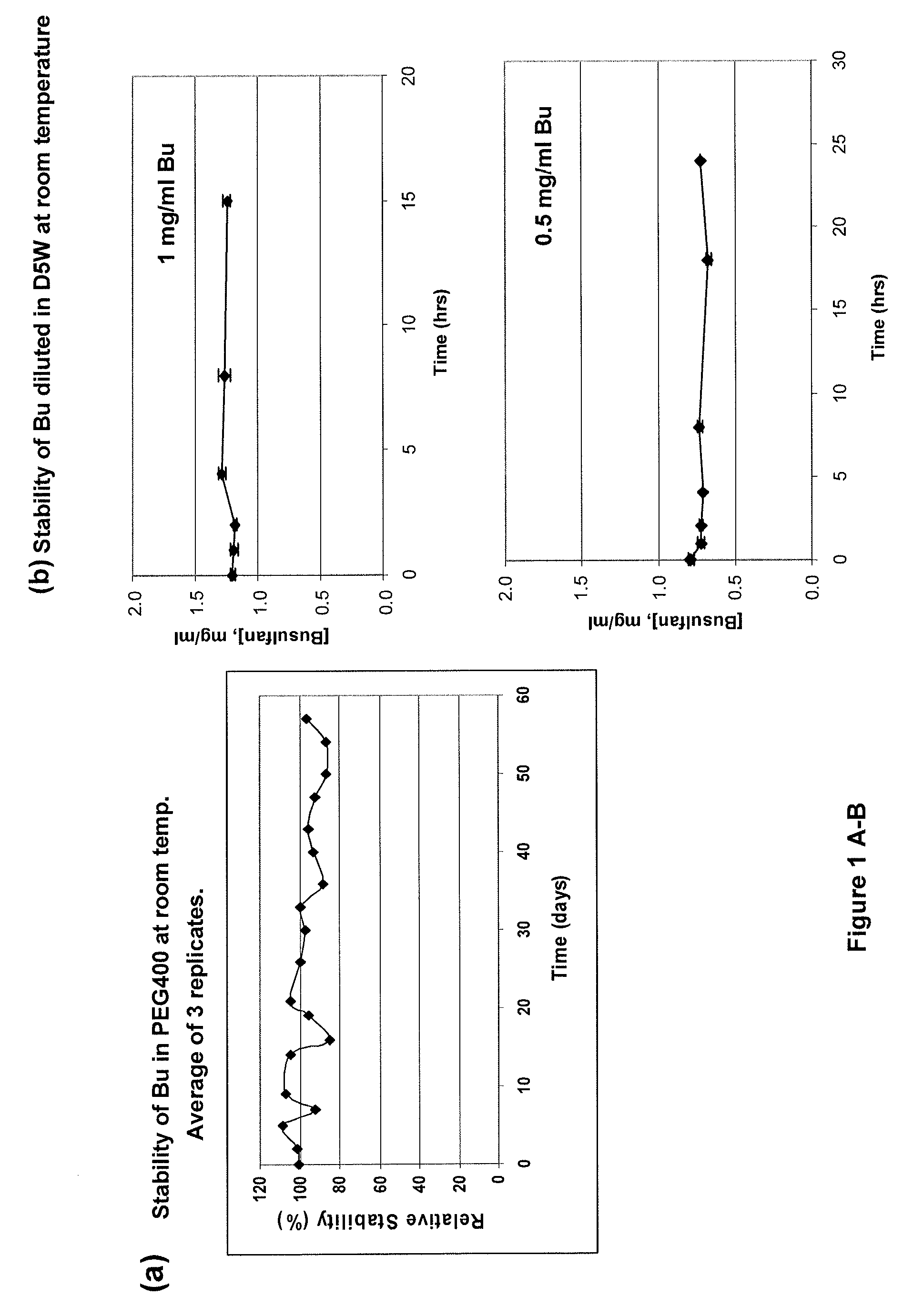
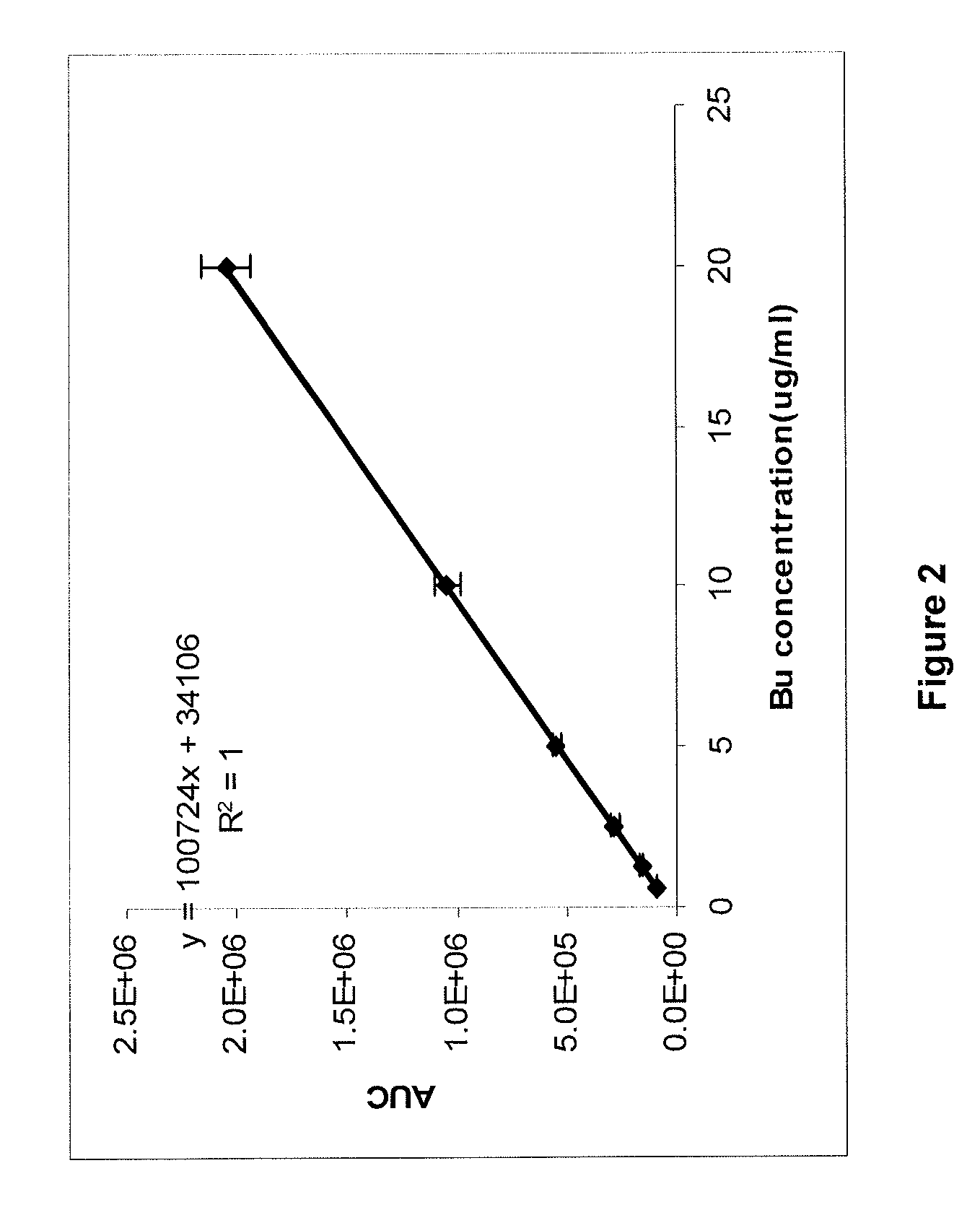
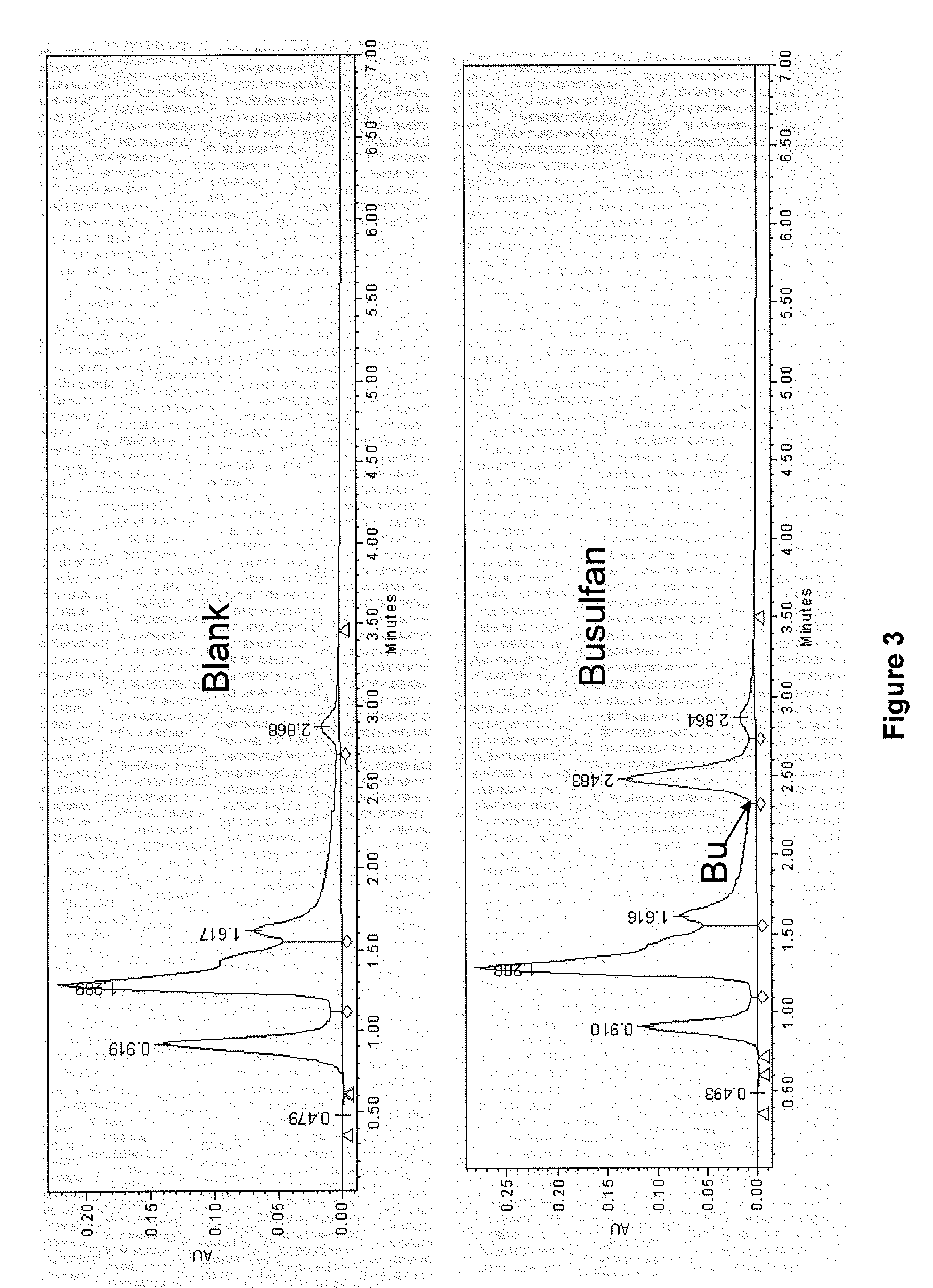
There may be provided compositions of lipophilic pharmaceutical agents with improved solubility and stability. For example, there may be provided a non-aqueous composition that comprises a lipophilic pharmaceutical agent, and an amphiphilic polymeric solvent such as PEG400 but essentially free of organic solvents and non-solubilized particles. The composition may be further diluted with a desired aqueous diluent such as an infusion fluid for parenteral administration to a subject such as a human. The compositions may be useful for the treatment for diseases or conditions that are sensitive to lipophilic agents, such as infectious diseases, malignant or autoimmune diseases.
Owner:GREENJAY THERAPEUTICS INC
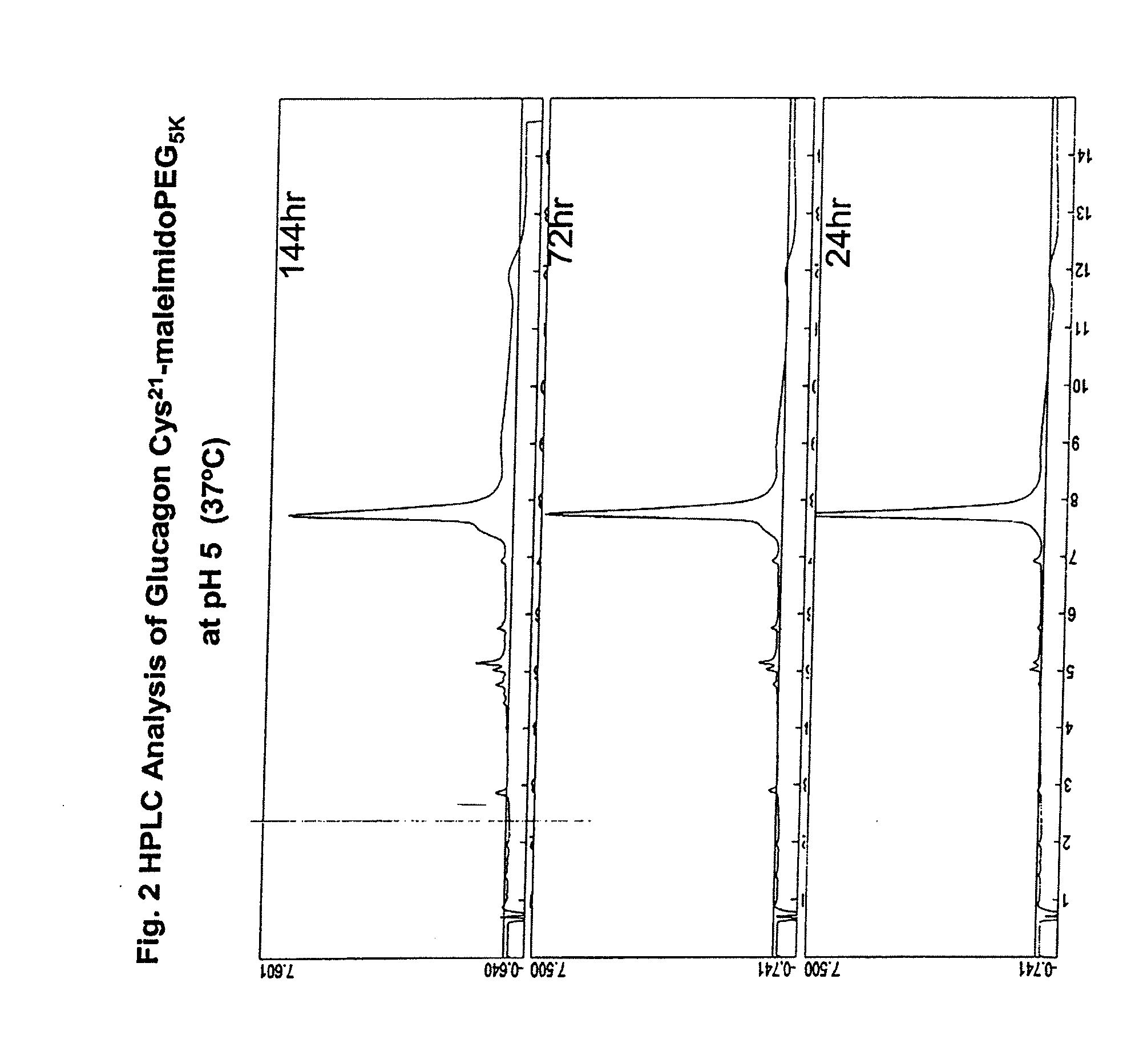
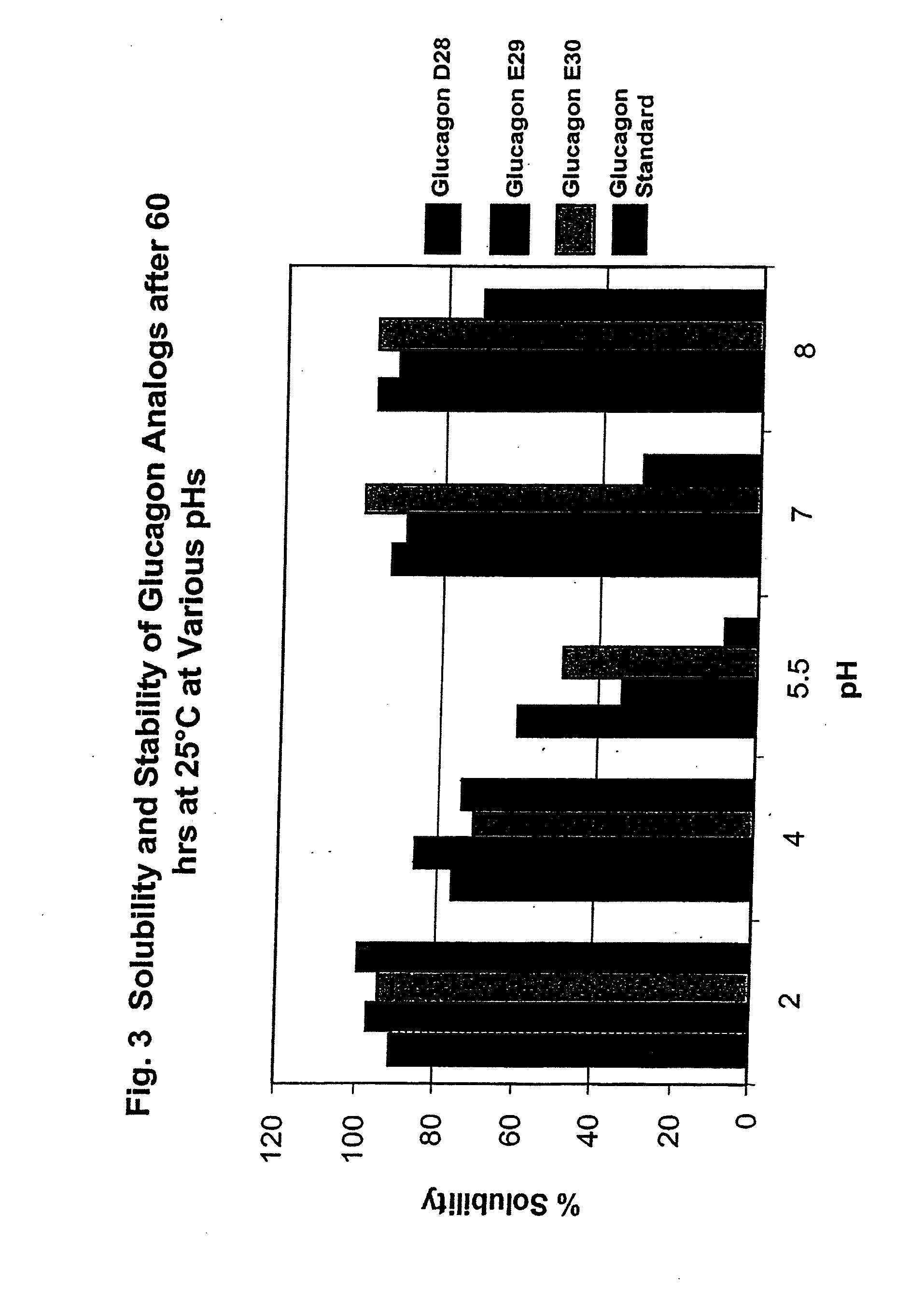
GLUCAGON ANALOGS EXHIBITING ENHANCED SOLUBILITY AND STABILITY IN PHYSIOLOGICAL pH BUFFERS
ActiveUS20110190200A1Rapidly increasing glucose levelNormalizing blood glucose levelAntimycoticsPeptide/protein ingredientsAmino acid substitutionHalf-life
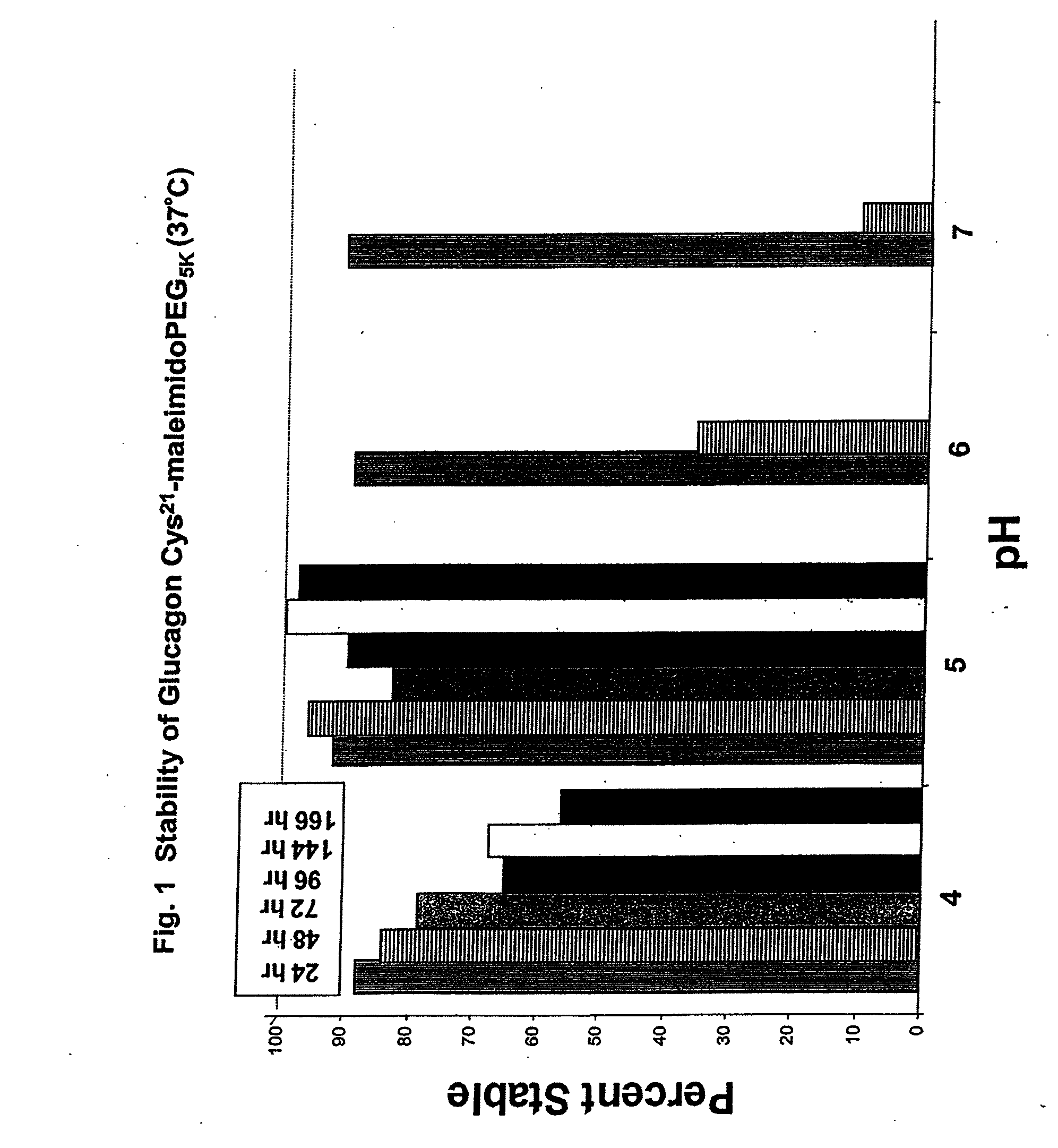
Modified glucagon peptides are disclosed having improved solubility and / or half-life while retaining glucagon agonist activity. The glycogen peptides have been modified by substitution of native amino acids with, and / or addition of, charged amino acids to the carboxy terminus of the peptide. The modified glucagon agonists can be further modified by pegylation, or the addition of a carboxy terminal peptide selected from the group consisting of SEQ ID NO: 20, SEQ ID NO: 21, SEQ ID NO: 23, or both to further enhance the solubility of the glucagon agonist analogs.
Owner:INDIANA UNIV RES & TECH CORP
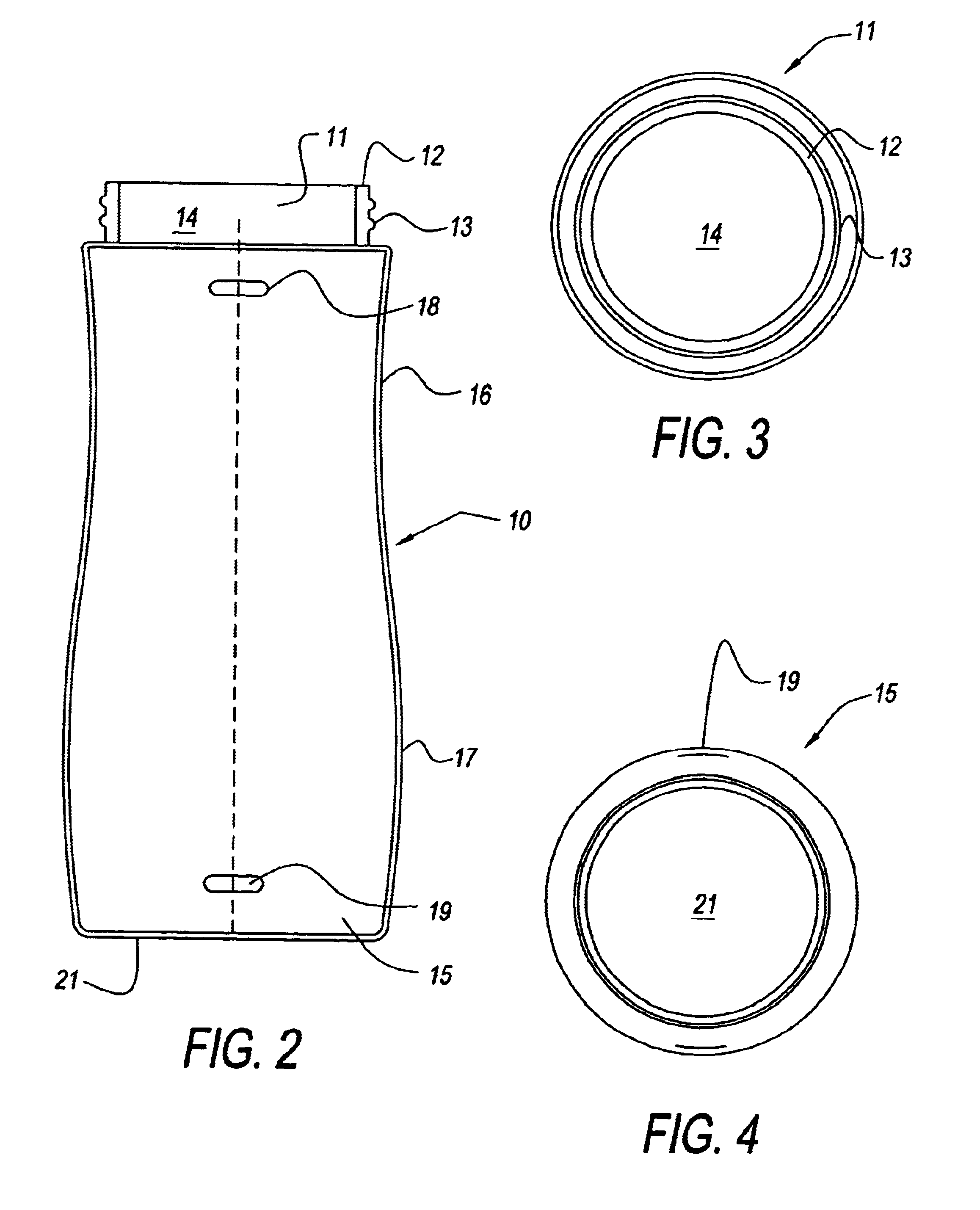
Bottle with mixing system
InactiveUS6616319B2Efficient mixingShaking/oscillating/vibrating mixersFlow mixersEngineeringMixing effect
There is provided an infant feeding bottle system having an agitator for mixing a powder substance with a liquid in a non-rigid disposable liner. The bottle system permits a user to mix powdered formula directly inside a non-rigid liner. The bottle system enhances the mixing effect, resulting in improved solubility, and provides for a self-contained uninterrupted mixing and feeding process.
Owner:JMBH HLDG LLC
Edible pet chew and method of making the same
InactiveUS20100003393A1Lower potentialImprovement ingredientsConfectioneryAnimal feeding stuffPlasticizerImproved solubility
An edible pet chew is disclosed that is comprised of fibrous protein, water absorbing polymer, plasticizer and water. The pet chew provides excellent textural properties and improved solubility in the stomach and intestinal environment for improved pet safety.
Owner:MARS INC
Hydrophilic complexes of lipophilic materials and an apparatus and method for their production
InactiveUS6878693B2Improve uniformitySimple processAntibacterial agentsPowder deliveryFood additiveWater insoluble
This invention provides a soluble inclusion complex formed of a water-insoluble lipophilic compound and an amphiphilic polymer and which demonstrated improved solubility and stability. The lipophilic compound within the inclusion complex may consist of pharmaceutical compounds, food additives, cosmetics, agricultural products and veterinary products. The invention also provides novel methods for preparing the inclusion complex, as well as a novel chemical reactor for forming the inclusion complex.
Owner:SOLUBEST
Tablets immediately disintegrating in the oral cavity
The present invention provides an intraoral quickly disintegrating tablet containing a phosphodiesterase inhibitor having an effect of improving the erectile dysfunction and a method for manufacturing the tablet. The present invention also provides an intraoral quickly disintegrating tablet containing a slightly soluble pharmaceutical agent having an improved solubility and a method for manufacturing the tablet. That is, it is an intraoral quickly disintegrating tablet containing a cyclic GMP phosphodiesterase inhibitor and a saccharide, and a method for manufacturing the tablet. Further, it is a method for manufacturing an intraoral quickly disintegrating tablet, which comprises dissolving a slightly soluble pharmaceutical agent in an organic solvent or an aqueous organic solvent together with a surfactant and / or a water-soluble polymer, coating the solution on a filler or granulating it with a filler to obtain molded products, mixing a saccharide with them, adding an organic solvent, water or an aqueous organic solvent thereto, followed by kneading, and subjecting it to a compression-molding.
Owner:EISIA R&D MANAGEMENT CO LTD
Water soluble nanoparticles of hydrophilic and hydrophobic active materials and an apparatus and method for their production
InactiveUS7081450B2Readily bioavailableReduce manufacturing costBiocidePowder deliveryFood additiveWater insoluble
This invention provides a soluble nano-sized particles formed of a core (water-insoluble lipophilic compound or hydrophilic compound) and an amphiphilic polymer and which demonstrated improved solubility and / or stability. The lipophilic compound within the soluble nano-sized soluble (“solu-nanoparticles”) may consist of pharmaceutical compounds, food additives, cosmetics, agricultural products and veterinary products. The invention also provides novel methods for preparing the nano-sized soluble particles, as well as a novel chemical reactor for manufacturing an inclusion complex comprising the nano-sized soluble particles.
Owner:SOLUBEST +1
Bottle with mixing system
InactiveUS7036975B2Efficient mixingPlace safeShaking/oscillating/vibrating mixersFlow mixersEngineeringMixing effect
There is provided an infant feeding bottle system having an agitator for mixing a powder substance with a liquid in a non-rigid disposable liner. The bottle system permits a user to mix powdered formula directly inside a non-rigid liner. The bottle system enhances the mixing effect, resulting in improved solubility, and provides for a self-contained uninterrupted mixing and feeding process.
Owner:JMBH HLDG LLC
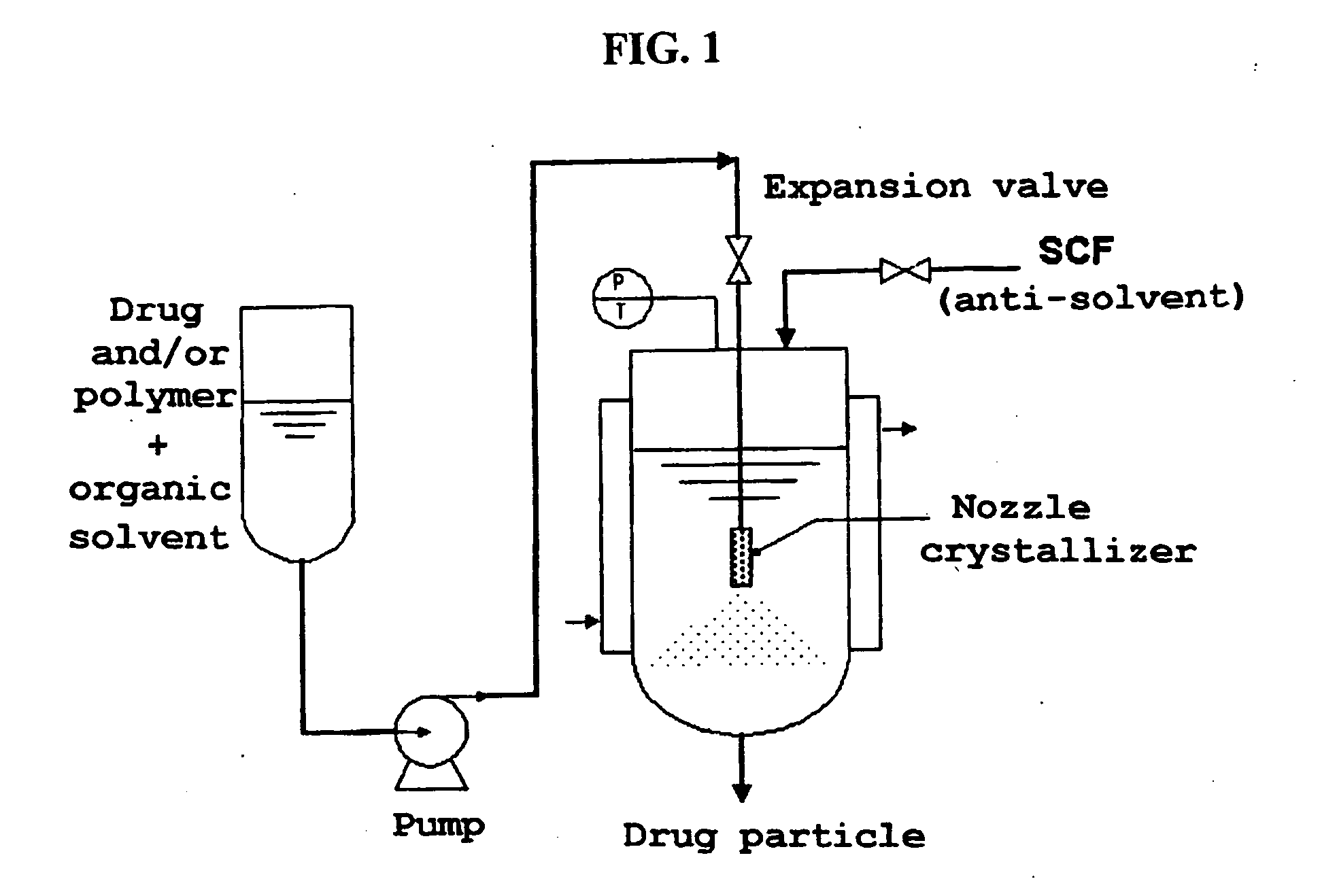
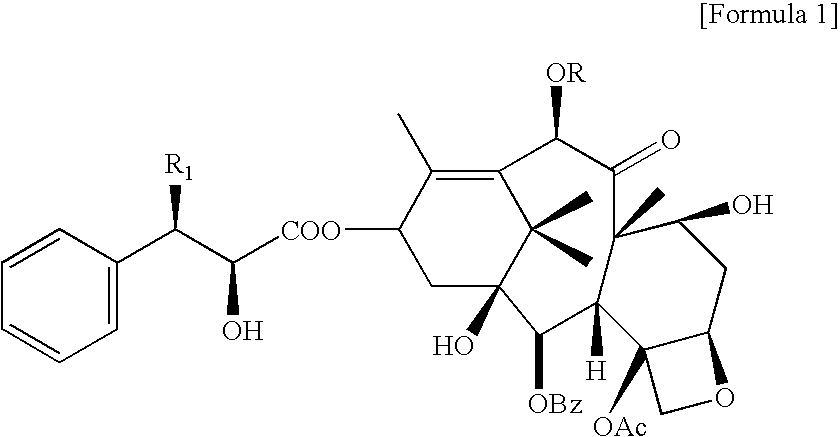
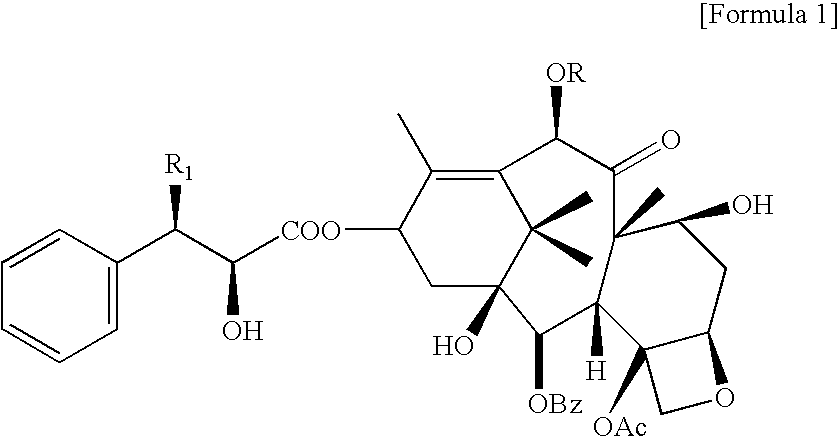
Method for the preparatin of paclitaxel solid dispersion by using the supercritical fluid process and paclitaxel solid dispersion prepared thereby
The present invention relates to a method for the preparation of paclitaxel solid dispersion by using the supercritical fluid process and paclitaxel solid dispersion prepared thereby, the paclitaxel solid dispersion being highly homogeneous and showing an improved solubility, thereby being effectively used for the preparation of paclitaxel injection and oral preparation having a high bioavailability.
Owner:HANMI PHARMA
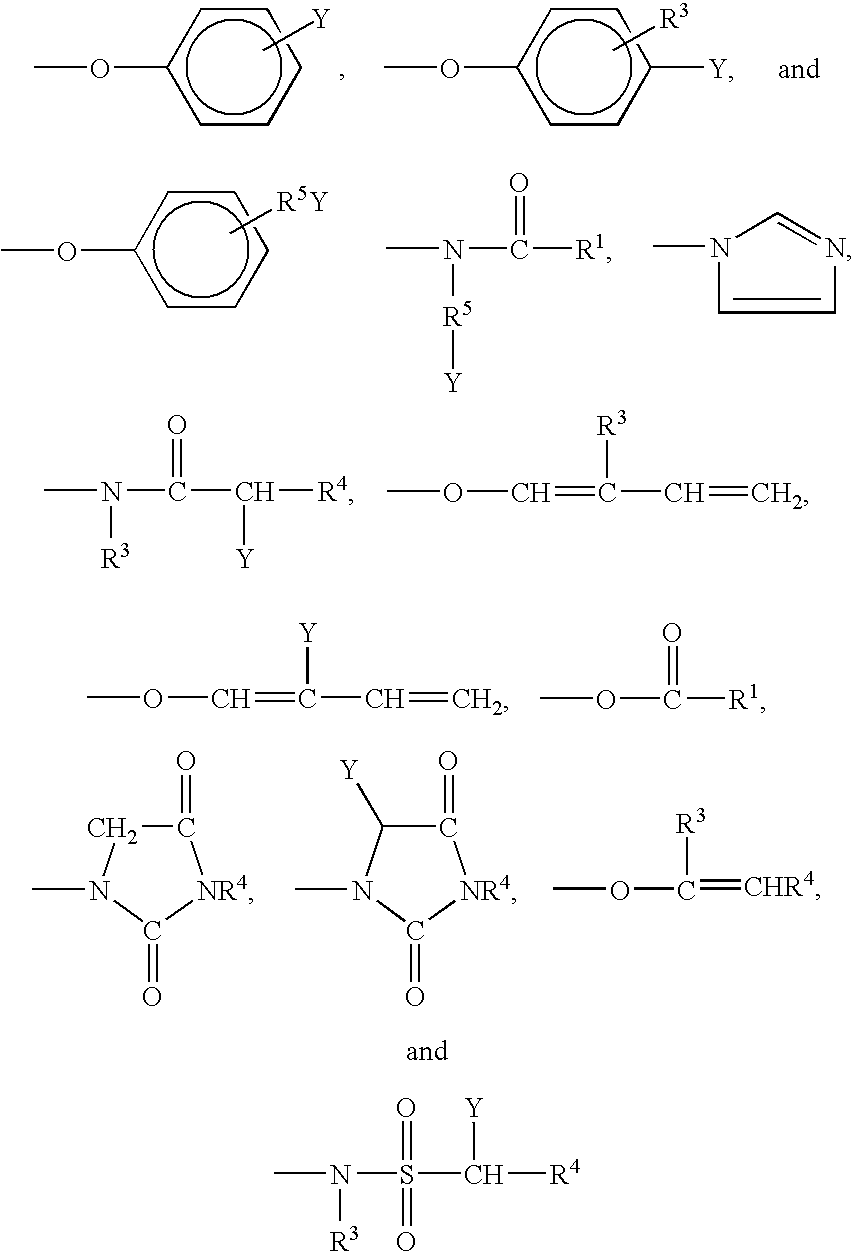
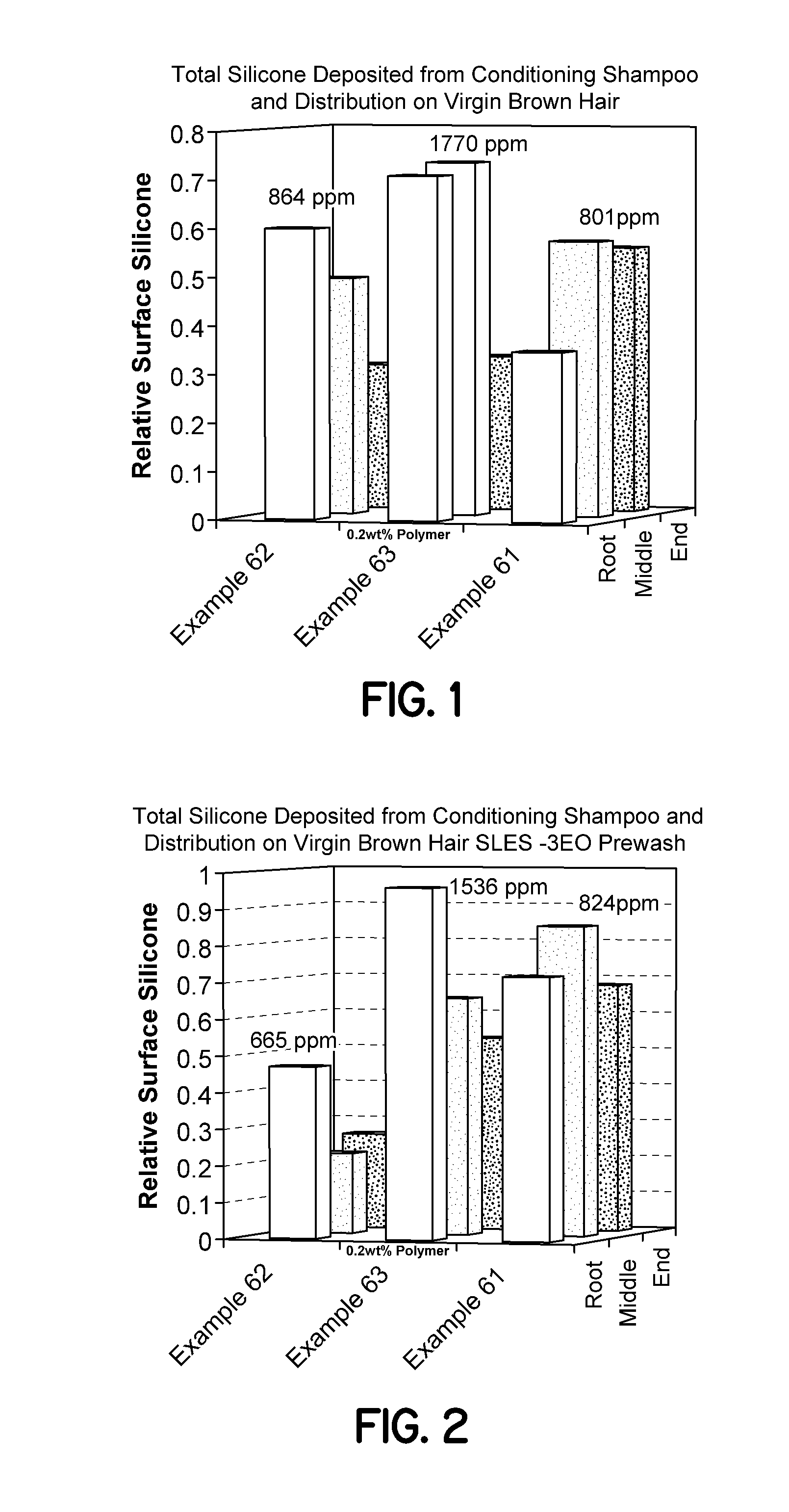
Cationic synthetic polymers with improved solubility and performance in surfactant-based systems and use in personal care and household applications
ActiveUS20110002868A1Improved of dispersed phaseReadily dispersibleBiocideOrganic active ingredientsPersonal carePolyelectrolyte
The present invention is related to surfactant-based formulations comprising the polyelectrolytes and blends of such polyelectrolytes with non-cellulosic cationic polysaccharide polymers. The surfactant-based formulations exhibit improved clarity of the resulting formulations, their improved conditioning of keratin substrates, textile substrates, and hard-surface substrates, their improved deposition of dispersed phase materials onto keratin substrates, textile substrates, and hard-surface substrates, their improved lather performance, and their improved rheology in applications such as personal care and household care products and textile applications.
Owner:HERCULES LLC
Soluble Fibrous Structures and Methods for Making Same
InactiveUS20160101204A1Improve solubilityRapid propagation rateInorganic/elemental detergent compounding agentsCosmetic preparationsActive agentFiber structure
Soluble fibrous structures and more particularly soluble fibrous structures that contain one or more fibrous elements, such as filaments, having one or more fibrous element-forming materials and one or more active agents present within the fibrous elements, wherein the fibrous structure exhibits improved dissolution properties compared to known soluble fibrous structures, and method for making such improved fibrous structures are provided.
Owner:THE PROCTER & GAMBLE COMPANY
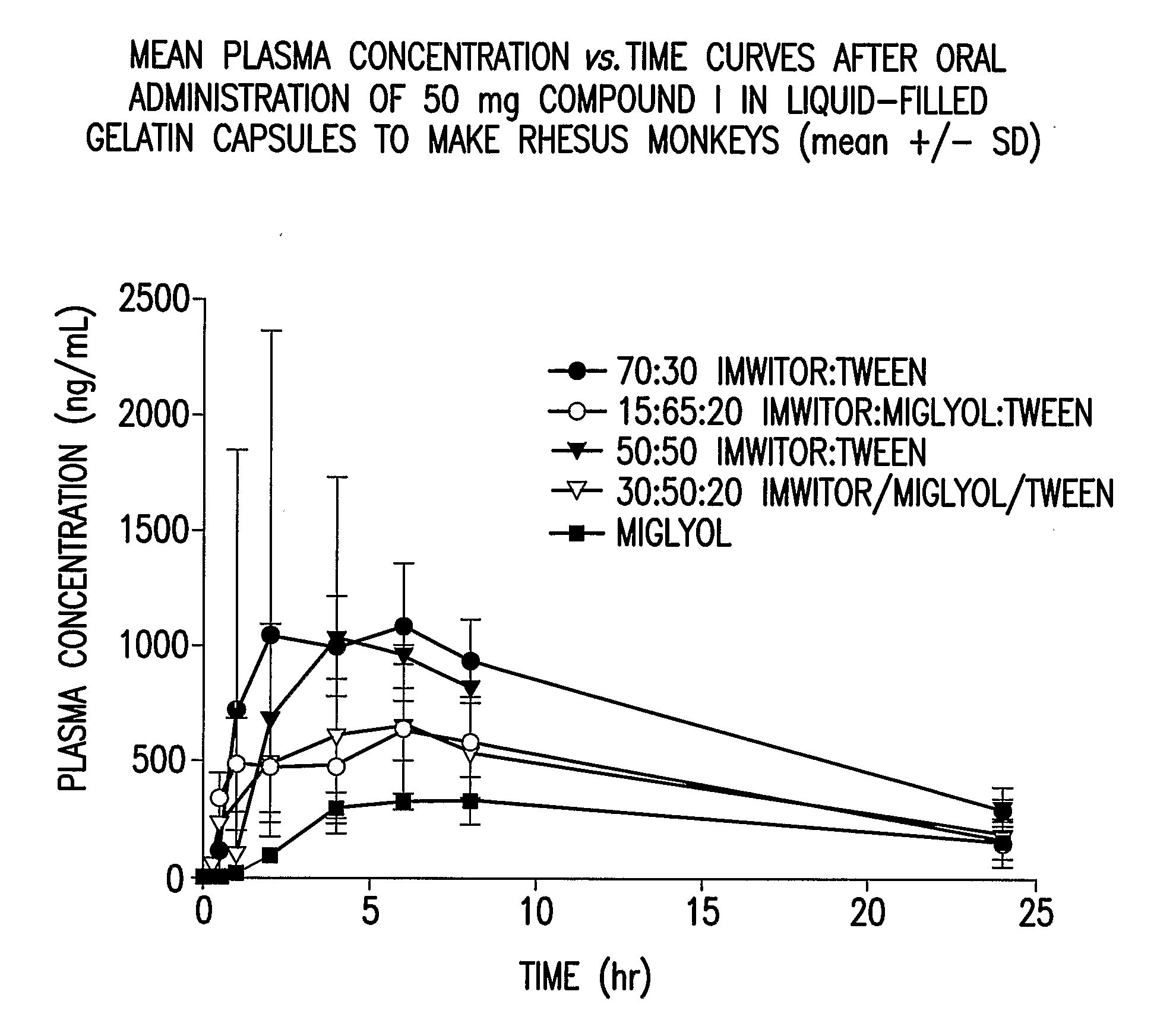
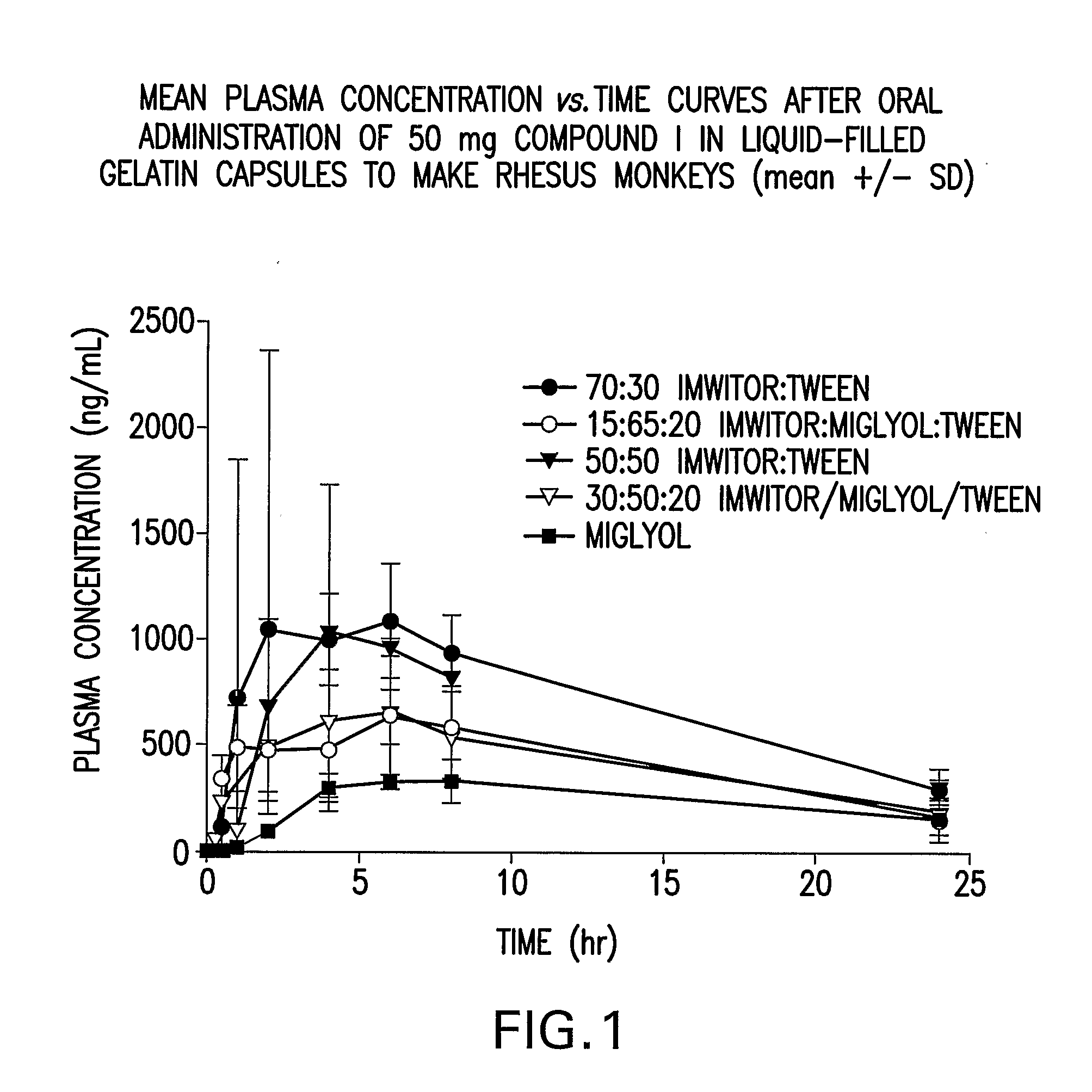
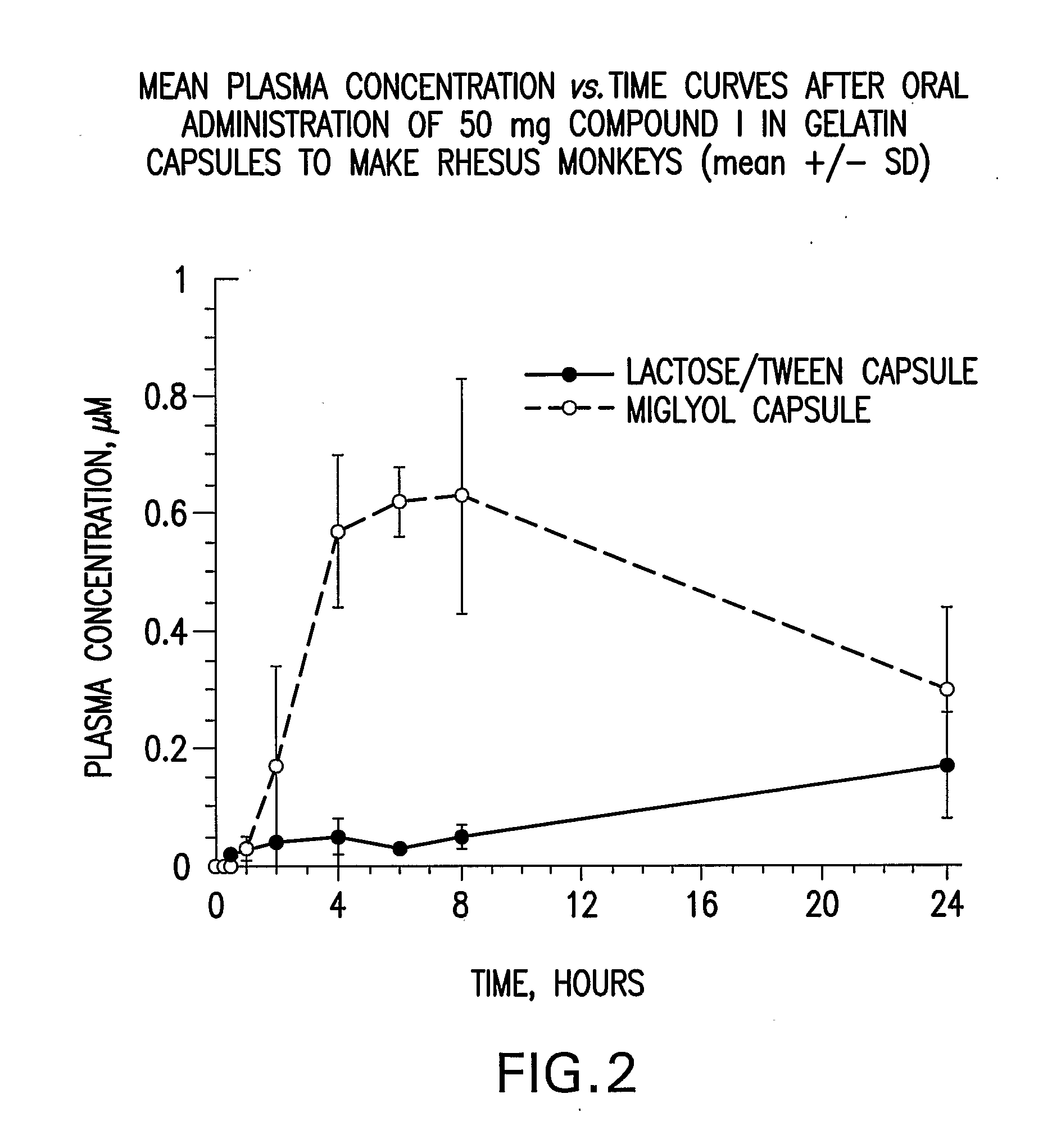
Liquid and Semi-Solid Pharmaceutical Formulations for Oral Administration of a Substituted Amide
InactiveUS20070298099A1Improve oral bioavailabilityImprove compoundBiocideNervous disorderAntioxidantSolvent
N-[1S,2S]-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-trifluoromethyl]pyridine-2-yl}oxy}propanamide (Compound I) has surprisingly improved solubility and bioavailability in a lipophilic vehicle comprising a pharmaceutically acceptable digestible oil, a surfactant, or a cosolvent, or a mixture of any two or more thereof. In one embodiment of the present invention are self-emulsifying or self-microemulsifying composition comprising 1) Compound I; 2) a surfactant having an HLB of 1 to 8; and 3) a surfactant having an HLB of over 8 to 20; and optionally, 4) a digestible oil and / or cosolvent and / or antioxidant or preservative.
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical formulations
InactiveUS20070032435A1Improve solubilityAntibacterial agentsBiocideLong chain fatty acidProteinase activity
Improved pharmaceutical compositions are provided comprising one or more solubilized HIV protease inhibiting compounds having improved solubility properties in a medium and / or long chain fatty acid, or mixtures thereof, a pharmaceutically acceptable alcohol, and water.
Owner:ABBVIE INC
Water soluble powders and tablets
InactiveUS6953592B2Partially compensateGood dispersionBiocidePowder deliveryPorosityWater dispersible
The invention relates to water soluble or water dispersible powders, tablets, or precursors therefor based on a carbohydrate matrix with improved dissolution properties in water. These components are subjected to treatment with a gas so that gas is entrapped therein, and sufficient closed porosity is provided so that gas entrapped therein promotes dissolution or dispersion upon contact with water. The powders or tablets may be pharmaceuticals or foods that optionally contain an active ingredient therein.
Owner:NESTEC SA
Detergent composition comprising an acid source with a specific particle size
InactiveUS6093218AInorganic/elemental detergent compounding agentsCationic surface-active compoundsParticulatesSURFACTANT BLEND
Owner:THE PROCTER & GAMBLE COMPANY
Quinone prodrug compositions and methods of use
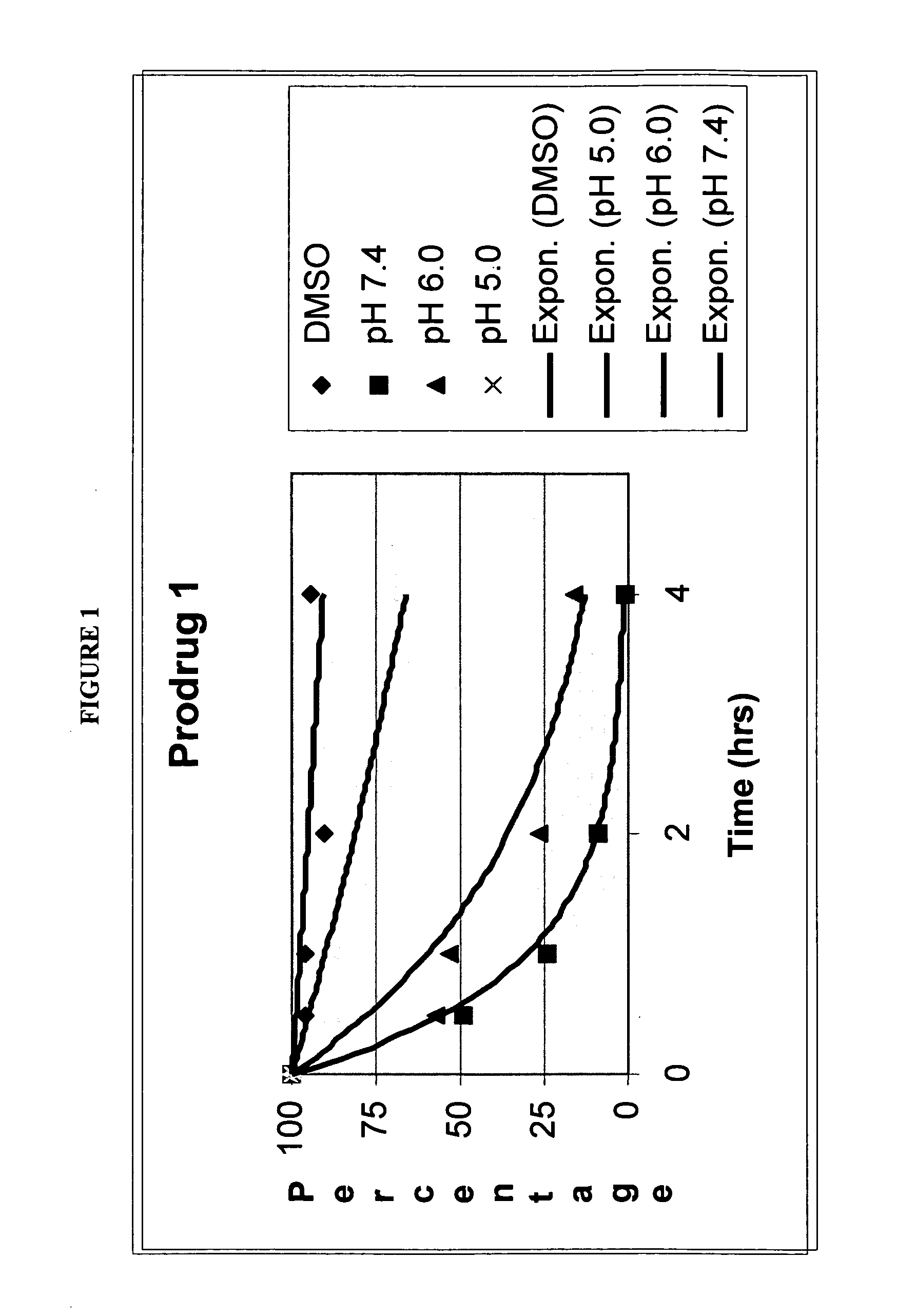
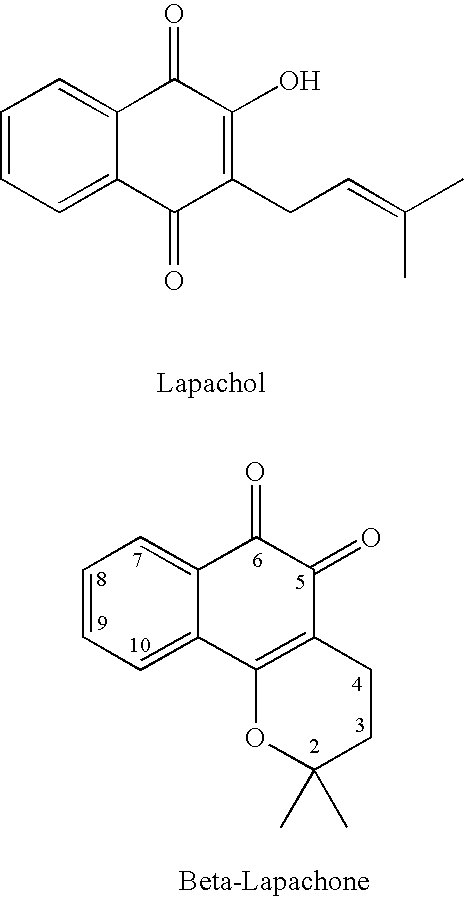
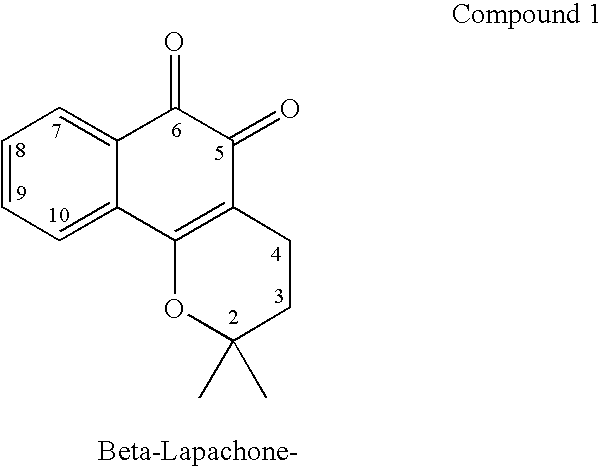
ActiveUS20060035963A1Good water solubilityImprove stabilityHeavy metal active ingredientsBiocideHalf-lifeCompound (substance)
The present invention relates to quinone prodrug compositions and therapeutic methods using such prodrug compositions. Preferably, the quinone compounds of the invention are napthoquinone compounds such as β-lapachone or β-lapachone analogs. The quinone prodrug compositions of the invention exhibit improved solubility, stability, bioavailability, and pharmacokinetic properties, as well as improved plasma half-life in vivo.
Owner:ARQULE INC
Acetaminophen compositions
InactiveUS7029698B2Maintaining clarity of appearanceSubject to degradationBiocideAmide active ingredientsAcetic acidCLARITY
The invention herein provides for an oral pharmaceutical composition adapted for use in capsular dosage forms comprising acetaminophen and a lactate salt alone or in combination with an acetate salt. Compositions of the invention exhibit improved solubility characteristics of the active ingredient per given fill volume, thereby permitting the use of smaller capsule sizes to deliver a given effective dose of the active ingredient. Compositions of the invention also exhibit improved clarity per concentration of active ingredient. The invention also provides for a capsular dosage form containing the composition.
Owner:R P SCHERER TECH INC
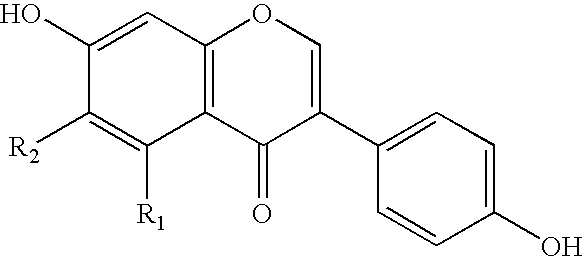
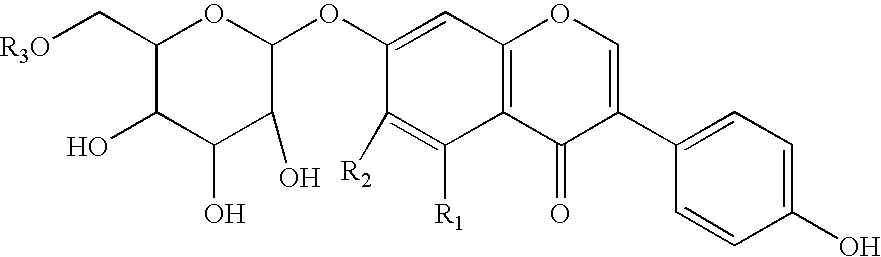
Soluble isoflavone compositions
ActiveUS6855359B2Good water solubilityImprove solubilityOrganic active ingredientsAnimal feeding stuffOptical propertyDietary supplement
The present invention provides isoflavone compositions exhibiting improved solubility (e.g., light transmittance), taste, color, and texture characteristics, and methods for making the same. The isoflavone compositions are useful for incorporation in a variety of foodstuffs, beverages, dietary supplements, and pharmaceutical compositions allowing for improved taste, texture, color, and optical properties of the same.
Owner:CARGILL INC
Fast dissolving water-soluble fertilizer formulations and methods and uses thereof
ActiveUS20100186471A1Fast preparationReadily and rapidly compoundBiocideAmmonium nitratesFree solutionWater soluble
Improved, solid water-soluble fertilizer (WSF) compositions are presented which comprise at least one acid (optionally nutritive) and at least one basic fertilizer component. In one or more embodiments of the present invention, the WSF compositions demonstrate improved solubility of one or more nutrients or additives in solution, do not require additional dissolution aids or anti-caking agents, demonstrate fast dissolution times, produce precipitate free solutions, are readily compounded without intermediate wetting or drying steps, do not generate gas, and demonstrate improved stability under typical usage conditions. Finally, the WSF compositions may be used in improved processes for the creation of stock solutions, optionally with cold water, and / or delivery of nutrients to plants.
Owner:EVERRIS INT
Detergent tablet
InactiveUS6974789B1Increase ratingsFacilitate water influx and/or effluxInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsParticulatesGranularity
A detergent tablet for use in a washing machine, the tablet having one or more phases at least one of which is in the form of a compressed particulate solid comprising a) a polymeric disintegrant having a particle size distribution such that at least 90% by weight thereof has a particle size below about 0.3 mm and at least 30% by weight thereof has a particle size below about 0.2 mm; and b) a water-soluble hydrated salt having a melting point in the range from about 30° C. to about 95° C. The detergent tablets display improved dissolution, strength and long-term storage characteristics.
Owner:THE PROCTER & GAMBLE COMPANY
Cationic synthetic polymers with improved solubility and performance in phosphate surfactant-based systems and use in personal care and household applications
ActiveUS20120076747A1Improve experienceIncrease depositionCosmetic preparationsHair removalCellulosePersonal care
The present invention is related to surfactant-based formulations comprising the polyelectrolytes and blends of such polyelectrolytes with non-cellulosic cationic polysaccharide polymers wherein the surfactant is a phosphate ester. The surfactant-based formulations exhibit improved clarity of the resulting formulations, their improved conditioning of keratin substrates, textile substrates, and hard-surface substrates, their improved deposition of dispersed phase materials onto keratin substrates, textile substrates, and hard-surface substrates, their improved lather performance, and their improved rheology in applications such as personal care and household care products and textile applications.
Owner:HERCULES LLC
Ezetimibe compositions
InactiveUS20100234342A1Improve bioavailabilityImprove solubilityBiocideOrganic active ingredientsEzetimibeDissolution
This invention is a novel pharmaceutical composition of ezetimibe or a pharmaceutically acceptable salt thereof comprising one or more pharmaceutically acceptable excipients having high bioavailability with improved solubility and dissolution rate which is stable throughout the shelflife, methods for their preparation, and methods for treatment using the same.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Formulations for non-parenteral use including hydrophobic cyclodextrins
InactiveUS20060025380A1Improve solubilityIncrease flexibilityBiocideHeavy metal active ingredientsAdditive ingredientCyclodextrin
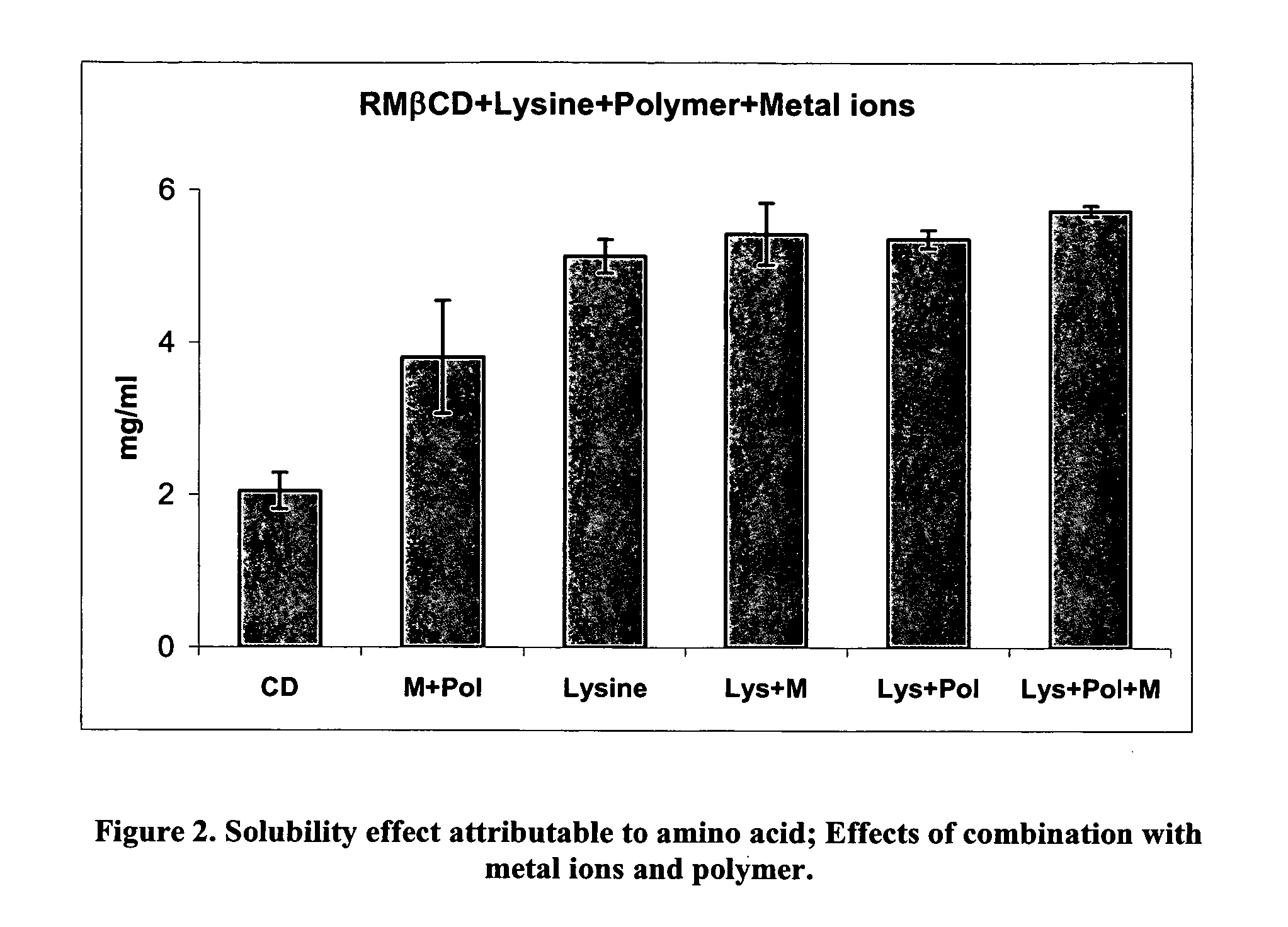
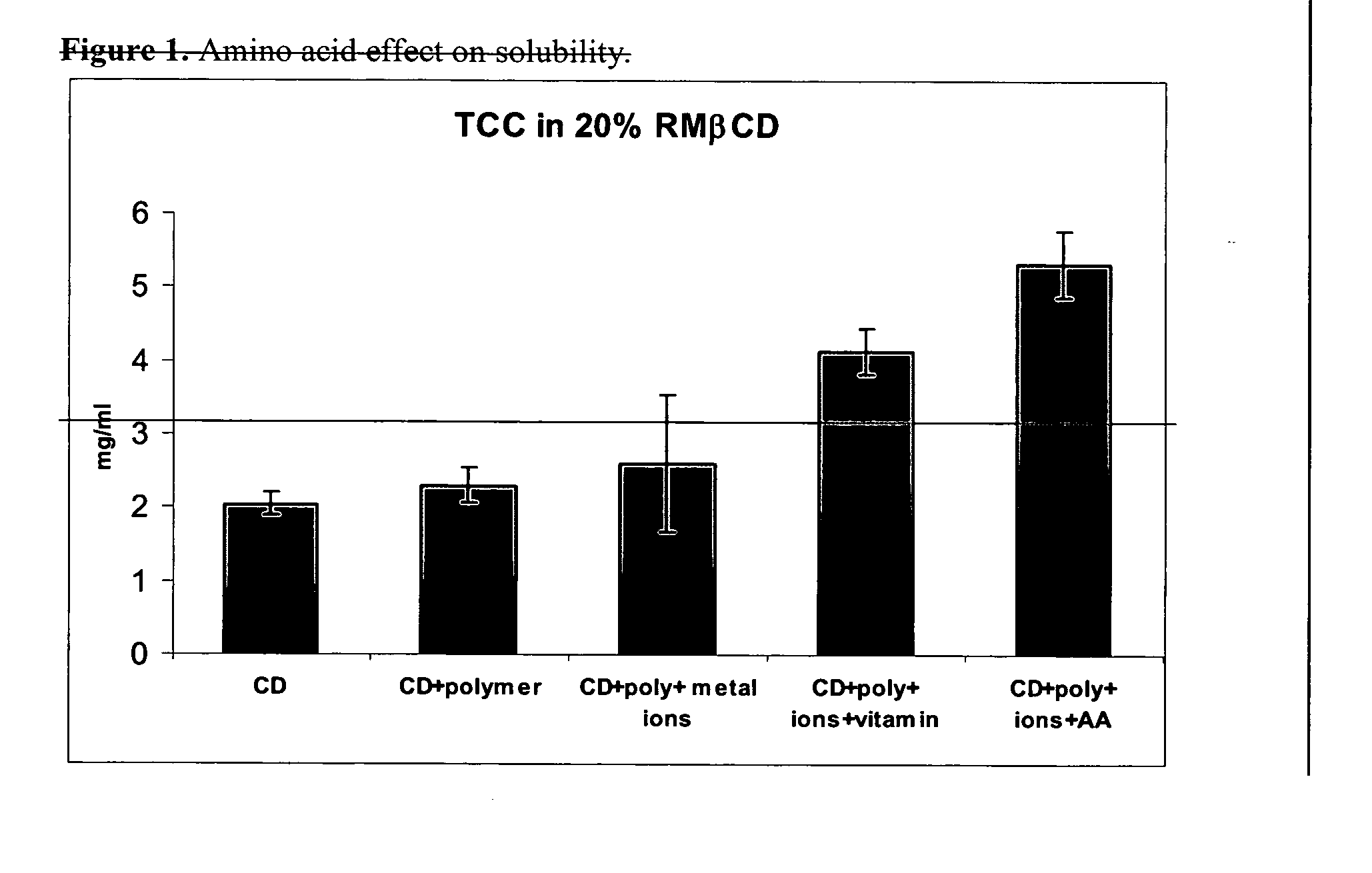
A novel system for non-parenteral formulations comprising cyclodextrins is disclosed. The system includes hydrophobic cyclodextrins and amino acids and homo- or co-polymers thereof. The cyclodextrins and amino acids form a complex with pharmaceutical and other ingredients to achieve greatly improved solubility and / or enhance stability. The complexes can be used for delivery to mammals in a wide variety of non-parenteral formulations.
Owner:DECODE CHEM
Water Repellent Additive for Immersion Resist
InactiveUS20120064459A1Improve water repellencyReduce solubilityPhotosensitive materialsSemiconductor/solid-state device manufacturingPolymer scienceFluoropolymer
Disclosed is a water repellent additive for an immersion resist, which is composed of a fluorine-containing polymer that has a repeating unit represented by general formula (1). By adding the water repellent additive to a resist composition, the resist composition can be controlled to have high water repellency during exposure and to exhibit improved solubility in a developing solution during development.[In the formula, R1 represents a hydrogen atom, a fluorine atom, a methyl group or a trifluoromethyl group; R2 represents a heat-labile protecting group; R3 represents a fluorine atom or a fluorine-containing alkyl group; and W represents a divalent linking group.]
Owner:CENT GLASS CO LTD
Novel glucagon analogues
InactiveUS20130035285A1Improve physical stabilityImprove solubilityNervous disorderPeptide/protein ingredientsAcute hyperglycaemiaDisease
The present invention relates to novel peptide compounds which have a protracted profile of action and improved solubility and stability, to the use of the compounds in therapy, to methods of treatment comprising administration of the compounds to patients in need thereof, and to the use of the compounds in the manufacture of medicaments. The compounds of the invention are of particular interest in relation to the treatment of hyperglycemia, diabetes and obesity, as well as a variety of diseases or conditions associated with hyperglycemia, diabetes and obesity.
Owner:NOVO NORDISK AS
Film with improved dissolution, cosmetic product
Owner:LOREAL SA
Solid dispersion pharamaceutical formulations
A pharmaceutical composition is disclosed which comprises a solid dispersion of an HIV protease inhibitor in a water soluble carrier, such as PEG, having enhanced bioavailability and improved dissolution properties. The solid dispersion may optionally be encapsulated in hard gelatin capsules, compressed into a tablet, or may be granulated with a pharmaceutically acceptable granulating agent. Also disclosed are methods of making said solid dispersion and methods of treating an HIV infection employing said solid dispersion.
Owner:ABBVIE INC
Organic solar cell material and preparation thereof
InactiveCN101525334AImprove solubilityImproved solar spectral responseOrganic chemistryFinal product manufactureOrganic solar cellDecomposition
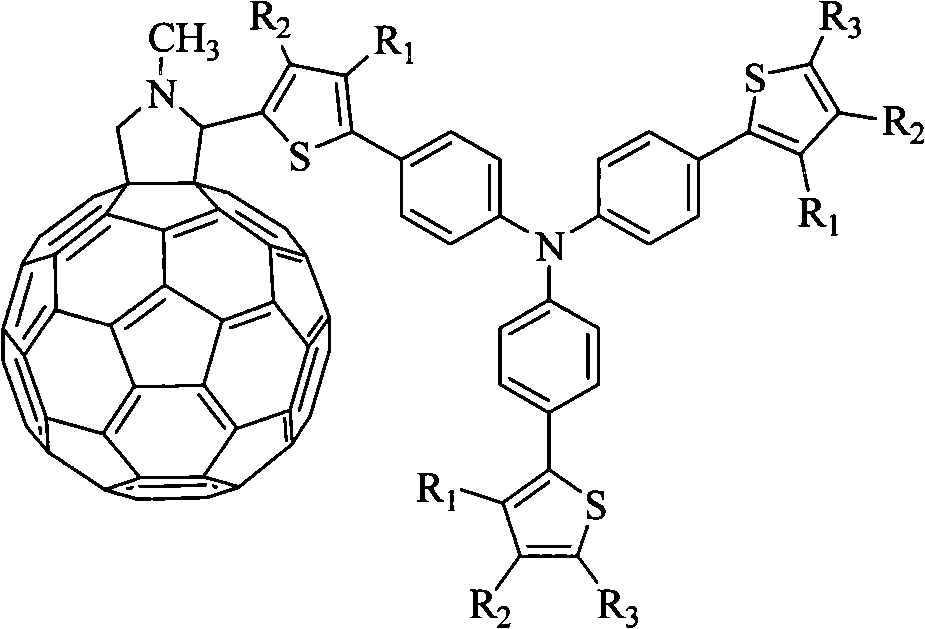
An organic solar cell material and the preparation thereof belong to the field of organic photoelectric materials. The invention discloses an organic solar cell material which contains C60-triphenylamine-thiofuran ternary system which is a fullerene-contained D-A (Donor-Accepter) type difunctional material, wherein the triphenylamine and the thiofuran are in stellated structure. Compared with the fullerene, the compound has greatly improved solubility in organic solvent, so that the process for manufacturing the large-area solar cell is simplified, stronger absorption is ensured in a visible region with a maximum of about 500 nm that is similar to the maximum of solar radiation energy of 475 nm, matching of the compound and solar spectrum radiation is enhanced, solar spectrum response of the material, as well as the photoelectric conversion efficiency is improved. In the invention, the compound can be polymerized on a macromolecule through simple ligand modification, and is made into PLED by spin coating, so as to overcome the defects of possible decomposition by heating and poor crystallization-resistant performance of the small-molecule luminescent material in the evaporation process.
Owner:JIANGNAN UNIV
Lyophilized pharmaceutical composition with improved stability containing taxane derivatives, and method of manufacturing the same
InactiveUS20100305202A1Good storage stabilityBiocideOrganic active ingredientsWater insolublePolyethylene glycol
The present invention relates to a lyophilized pharmaceutical composition for injection having superior storage stability comprising a taxoid, and a method thereof. More specifically, the present invention relates to a lyophilized pharmaceutical composition for injection having improved solubility and stability of dilution compared to the conventional preparations by dissolving a water-insoluble taxoid in distilled water added with a hydrophilic polymer such as hydroxypropylmethyl cellulose (HPMC), polyethylene glycol (PEG) or polyvinylpyrrolidone (PVP) cyclodextrin (CD), and lyophilizing the mixture and a method thereof.
Owner:SK CHEM CO LTD
Leucine/Peptide Composition and Method of Formulation
InactiveUS20110233469A1Low viscosityMore amenable to heat-treatmentPeptide/protein ingredientsOther chemical processesAmino acid compositionViscosity
Disclosed is a method for providing amino acids in a form having improved solubility and / or improved suspension properties, and a product made by the method. The method also provides protein and amino acid compositions having decreased viscosity following heat treatment.
Owner:GLANBIA NUTRITIONALS IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com