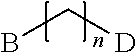Patents
Literature
476 results about "Glucagon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It works to raise the concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the GCG gene.
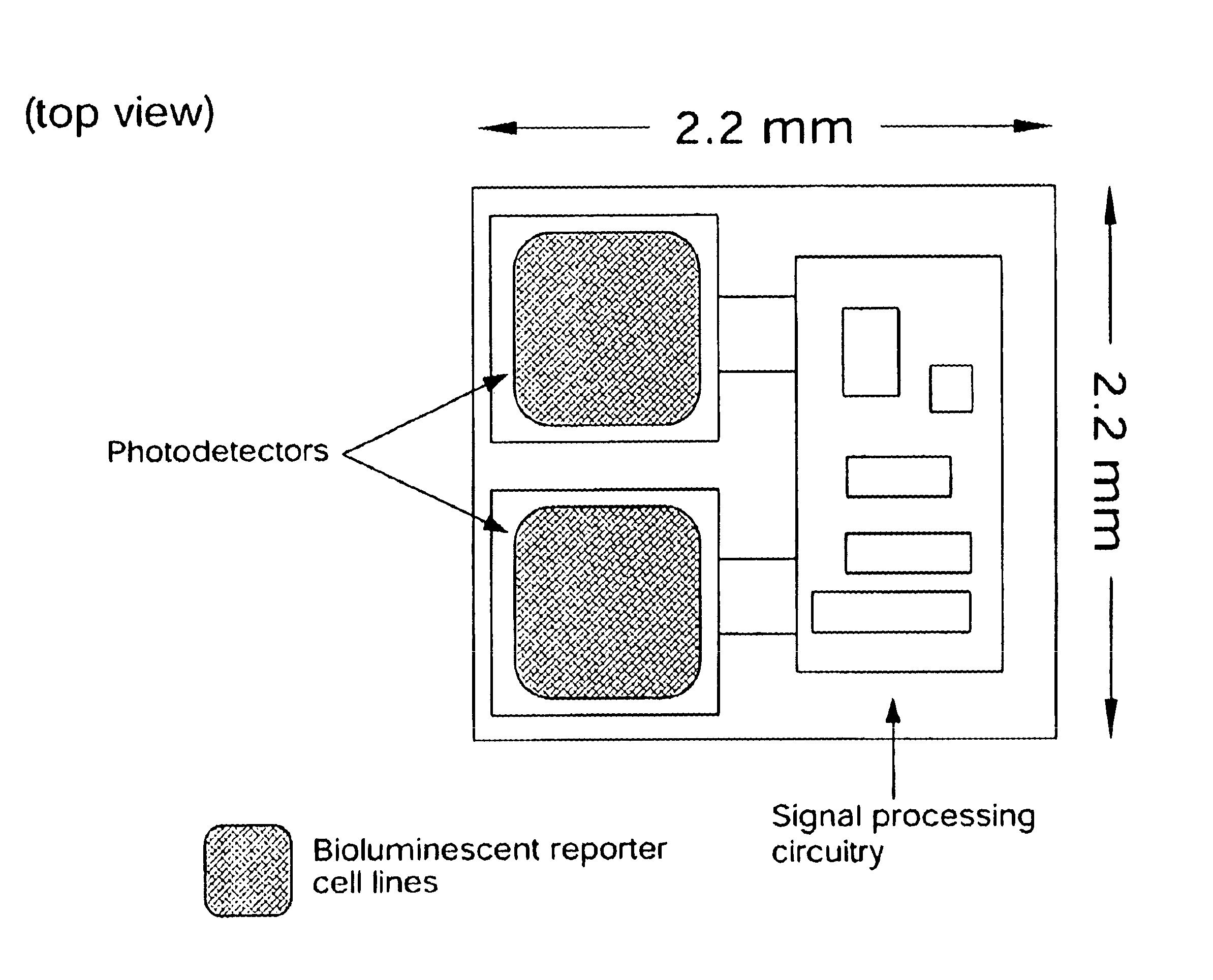
In vivo biosensor apparatus and method of use
InactiveUS6673596B1Less can be administeredCost-effective administration of drugBioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoGenetically engineered
Disclosed are bioluminescent bioreporter integrated circuit devices that detect selected analytes in fluids when implanted in the body of an animal. The device comprises a bioreporter that has been genetically engineered to contain a nucleic acid segment that comprises a cis-activating response element that is responsive to the selected substance operably linked to a gene encoding a bioluminescent reporter polypeptide. In preferred embodiments, the target analyte is glucose, glucagons, or insulin. Exposure of the bioreporter to the target substance causes the response element to up-regulate the nucleic acid sequence encoding the reporter polypeptide to produce a luminescent response that is detected and quantitated. In illustrative embodiments, the bioreporter device is encapsulated on an integrated circuit that is capable of detecting the emitted light, processing the resultant signal, and then remotely reporting the results. Also disclosed are controlled drug delivery systems capable of being directly or indirectly controlled by the detection device that provide drugs such as insulin to the animal in reponse to the amount of target analyte present in the body fluids.
Owner:UNIV OF TENNESSEE RES FOUND +1
Medication device
A dispenser for medicaments comprises a first metering pump for insulin and a second metering pump for glucose or glucagon. A controller for both pumps is programmed to maintain a basal supply of insulin, and is responsive to a signal from a separate glucometer to dispense additional insulin or glucose or glucagon as appropriate.
Owner:RICHARDS CYNTHIA C
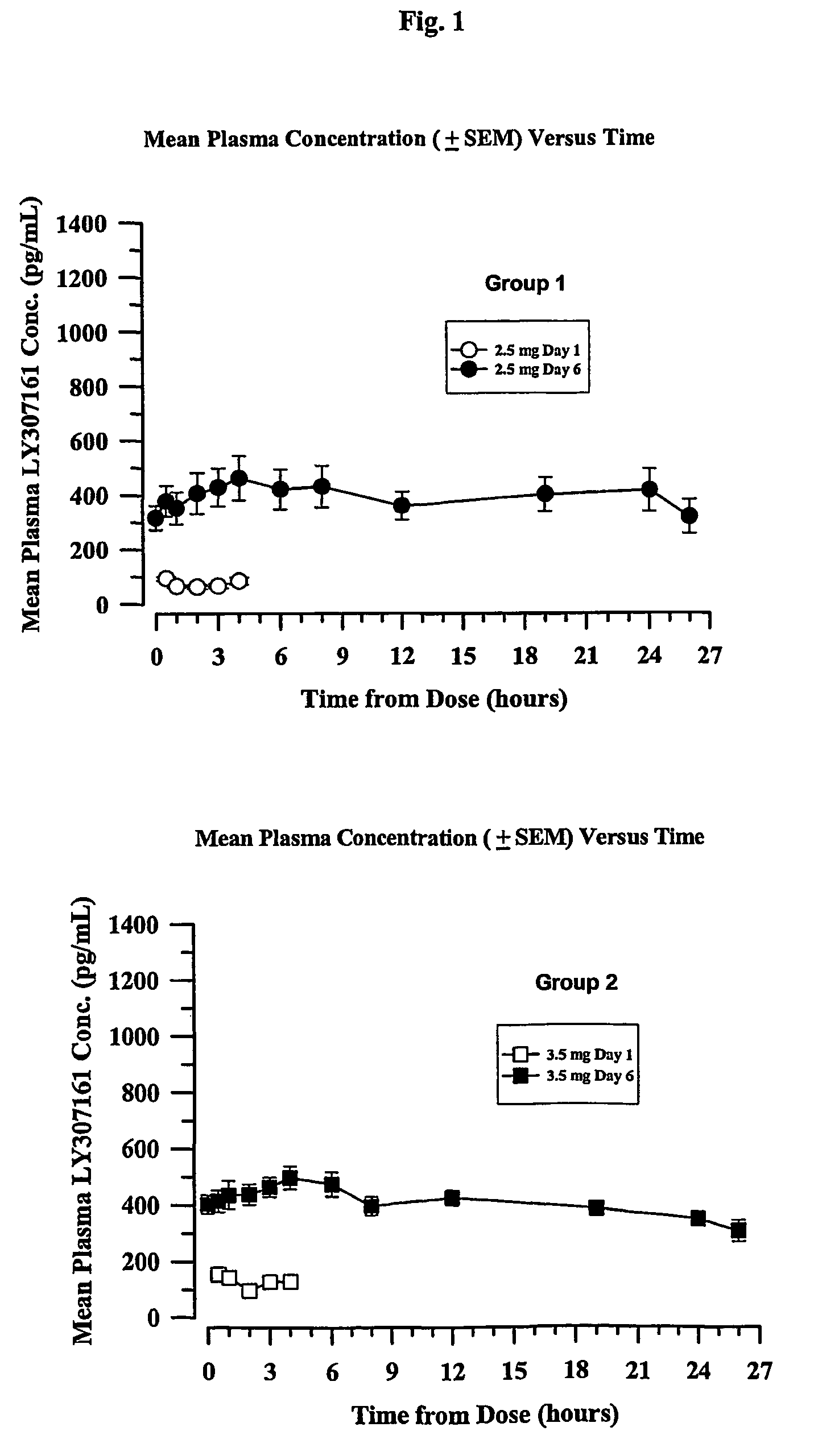
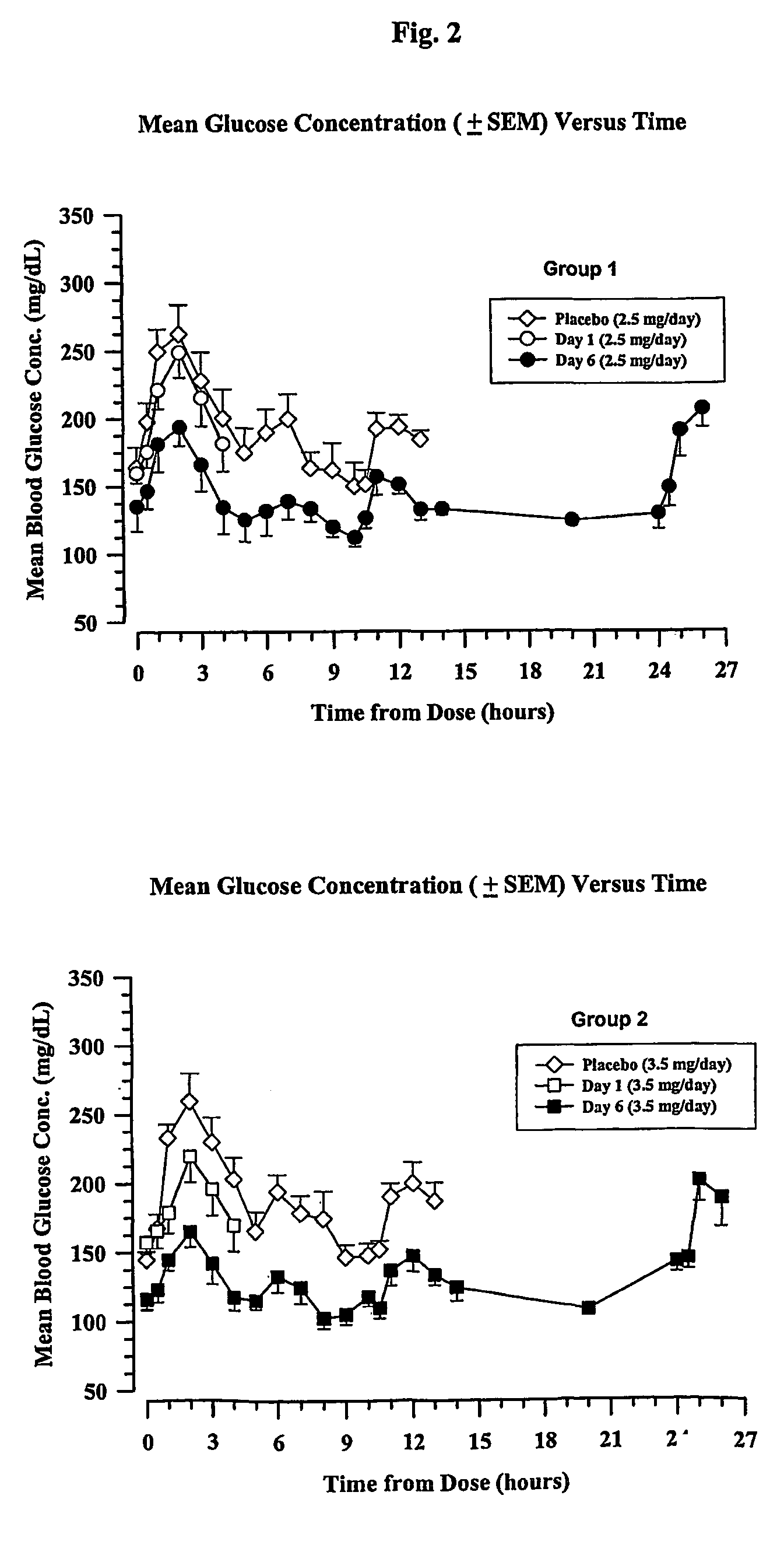
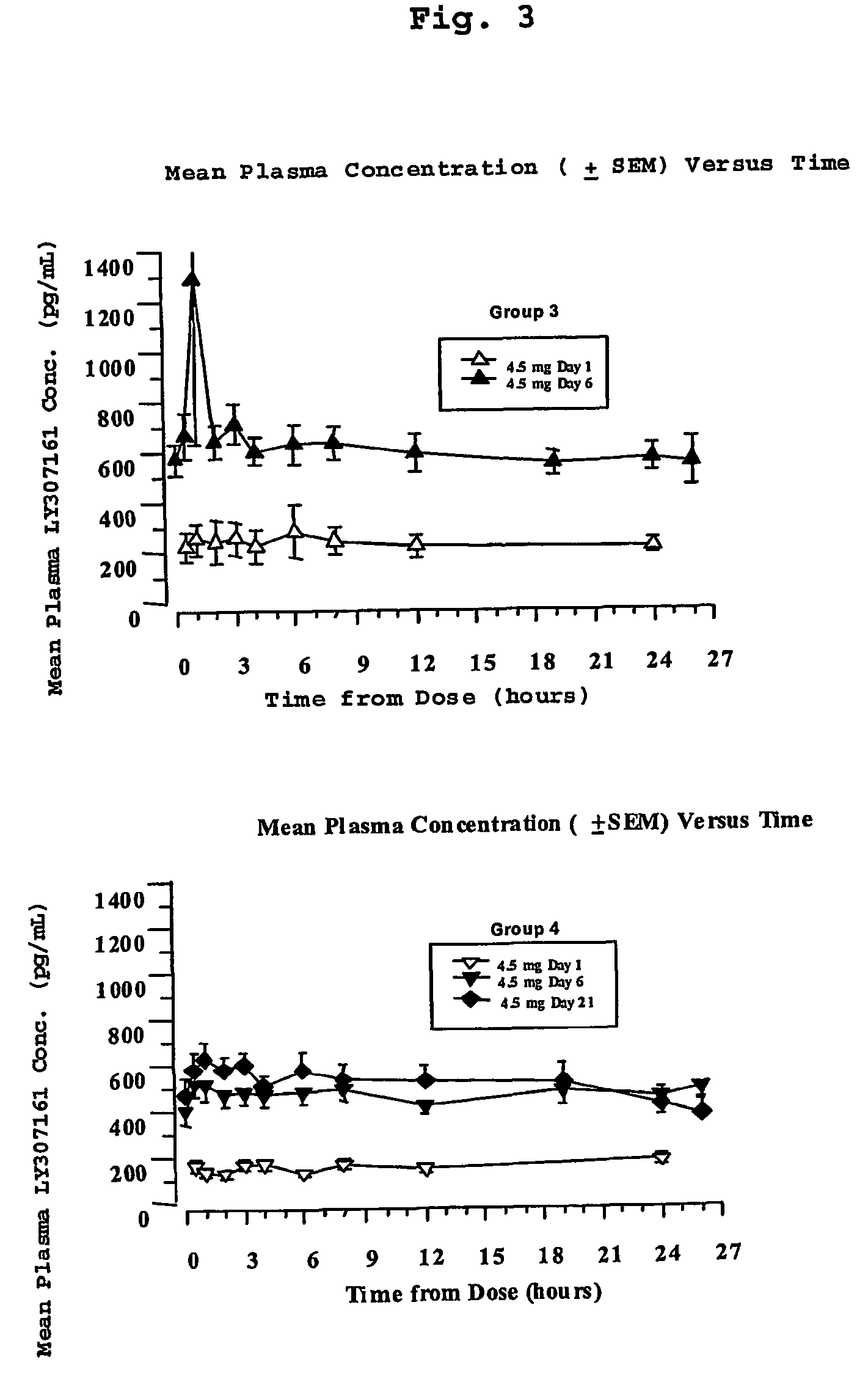
Chronic treatment regimen using glucagon-like insulinotropic peptides
InactiveUS7259233B2Avoids and minimizes side effectPeptide/protein ingredientsImmunoglobulinsDiseasePeptide
The present invention encompasses a method of treating a disease by maintaining chronic steady state serum levels of a GLP-1 compound within a specified range.
Owner:ELI LILLY & CO
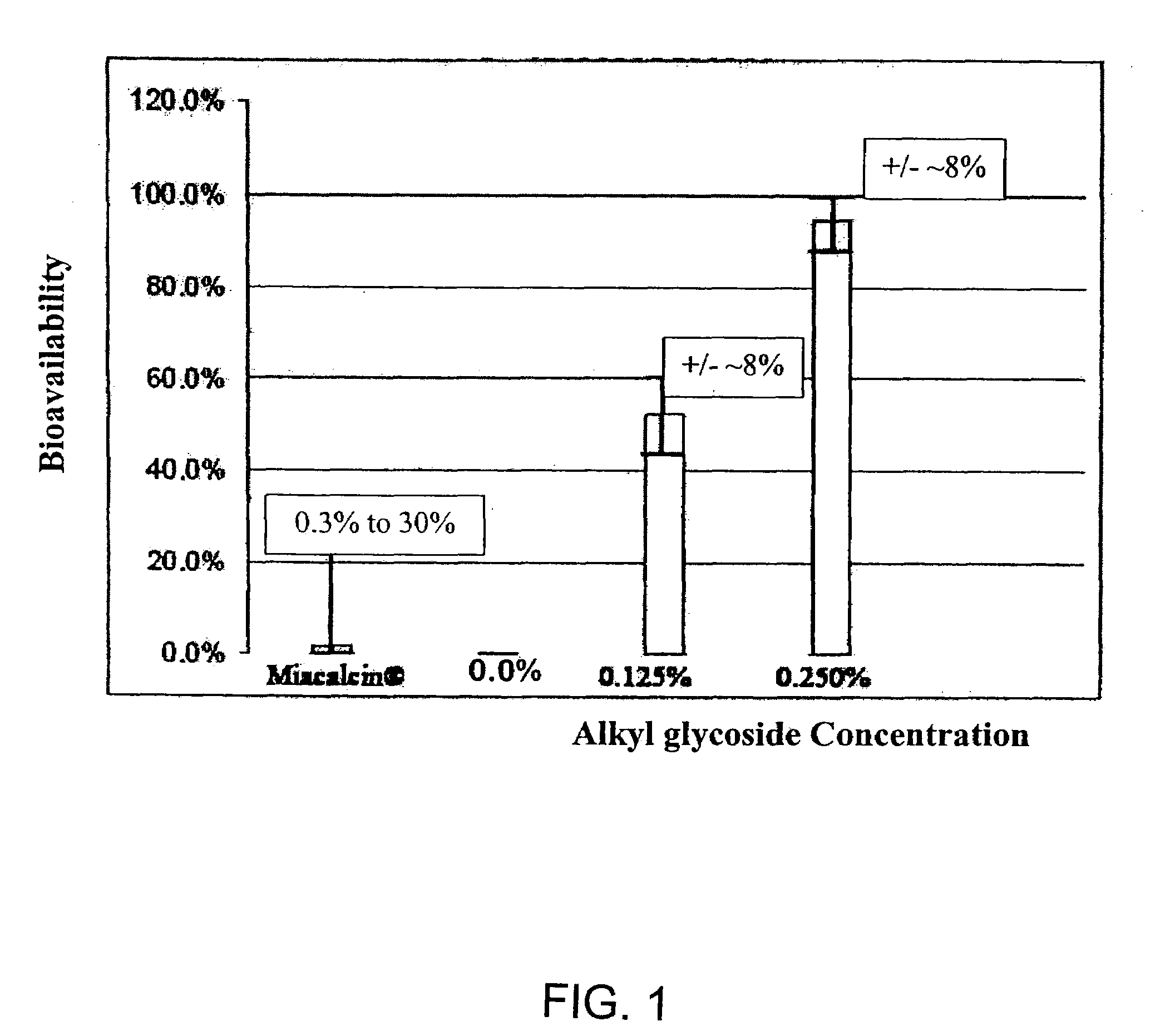
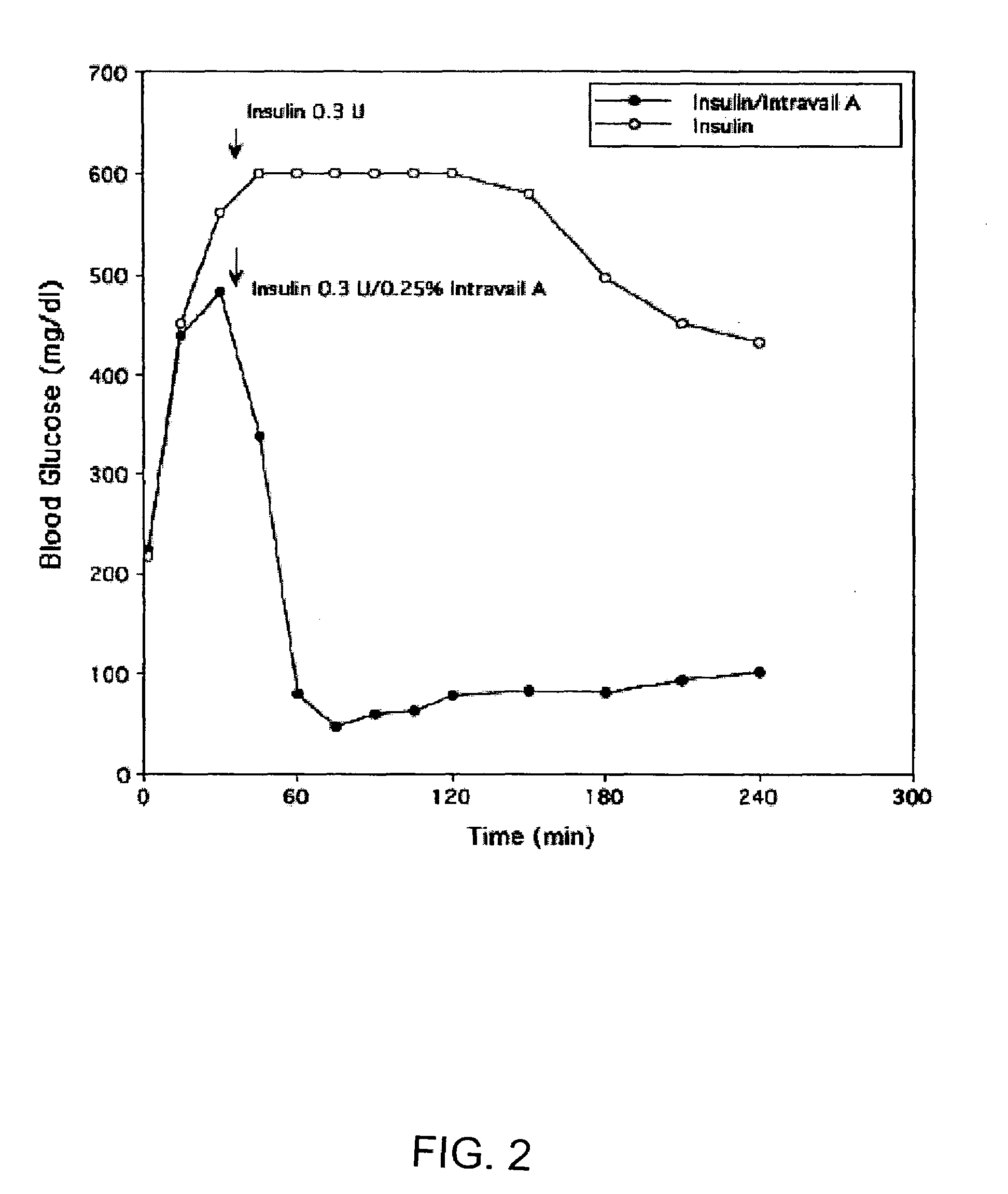
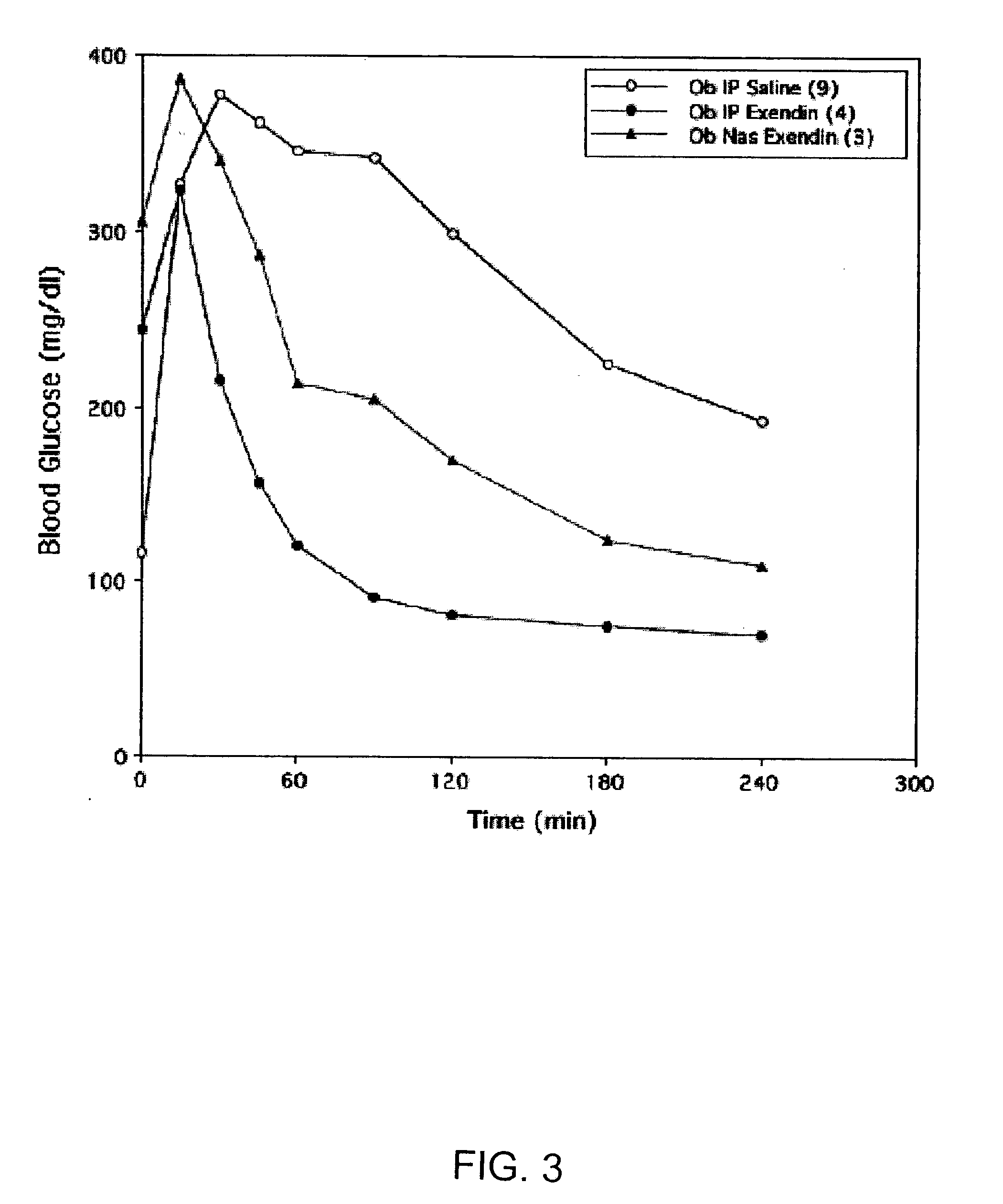
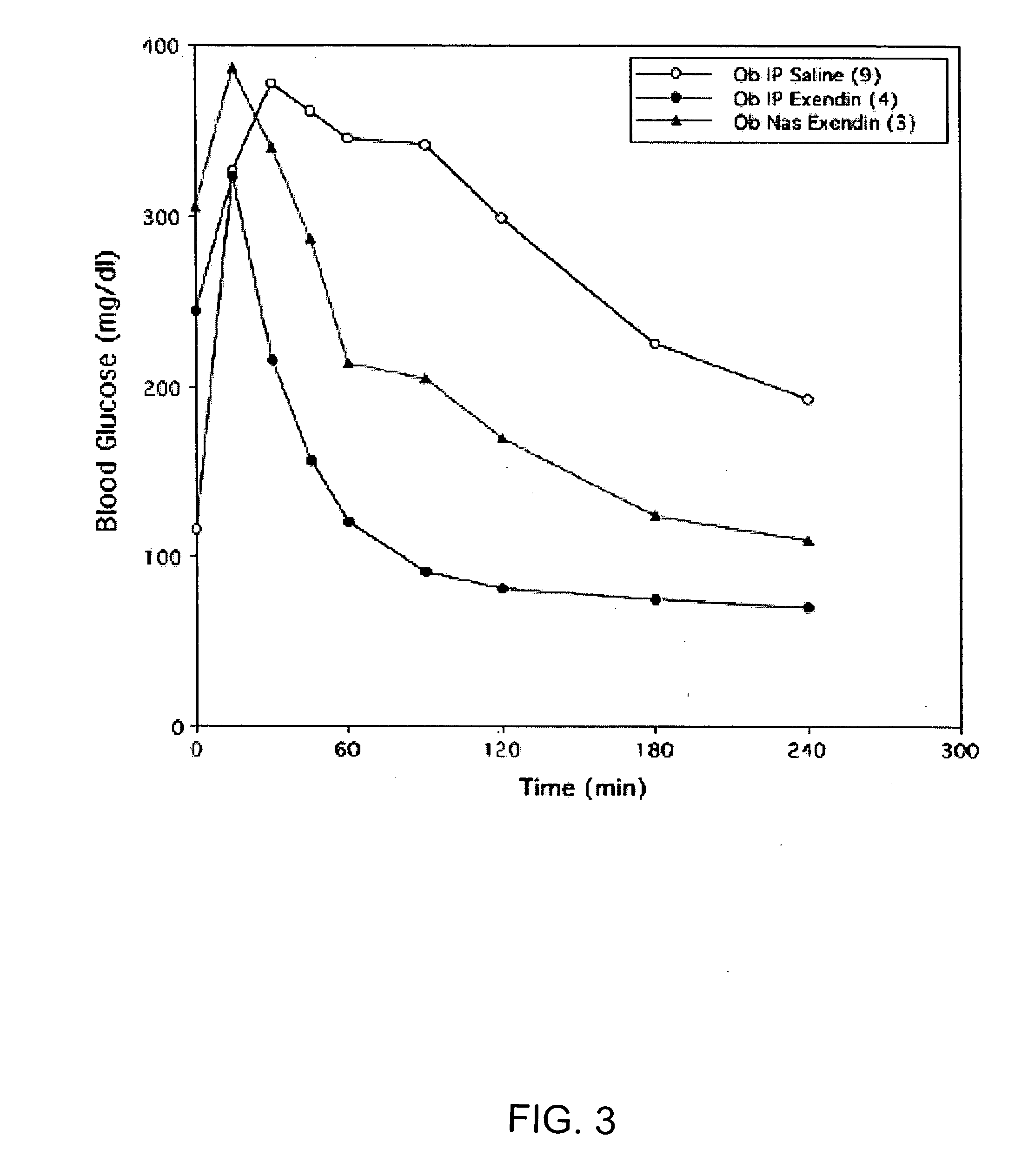
Absorption Enhancers for Drug Administration
ActiveUS20080299079A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderActive agentPancreatic hormone
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC
Method for the production of polypeptides
The present invention relates to a novel method for the production of short chain polypeptides, including polypeptides having up to 3 disulfide bonds and / or structures rich in basic amino acid residues, and open structured short chain polypeptides, e.g. glucagon, glucagon like peptides and their functional analogues, in genetically modified yeast cells, said genetically modified yeast cells, and a method for the preparation of said yeast cells.
Owner:NOVO NORDISK AS
Combination drug
InactiveUS20060094722A1Enhance pharmacological effectsPrevent degradationAntibacterial agentsBiocideDipeptidyl peptidasePharmacology
Owner:EISIA R&D MANAGEMENT CO LTD
Chronic treatment regimen using glucagon-like insulinotropic peptides
InactiveUS20040053819A1Avoids and minimizes side effectPeptide/protein ingredientsImmunoglobulinsDiseasePeptide
The present invention encompasses a method of treating a disease by maintaining chronic steady state serum levels of a GLP-1 compound within a specified range.
Owner:ELI LILLY & CO
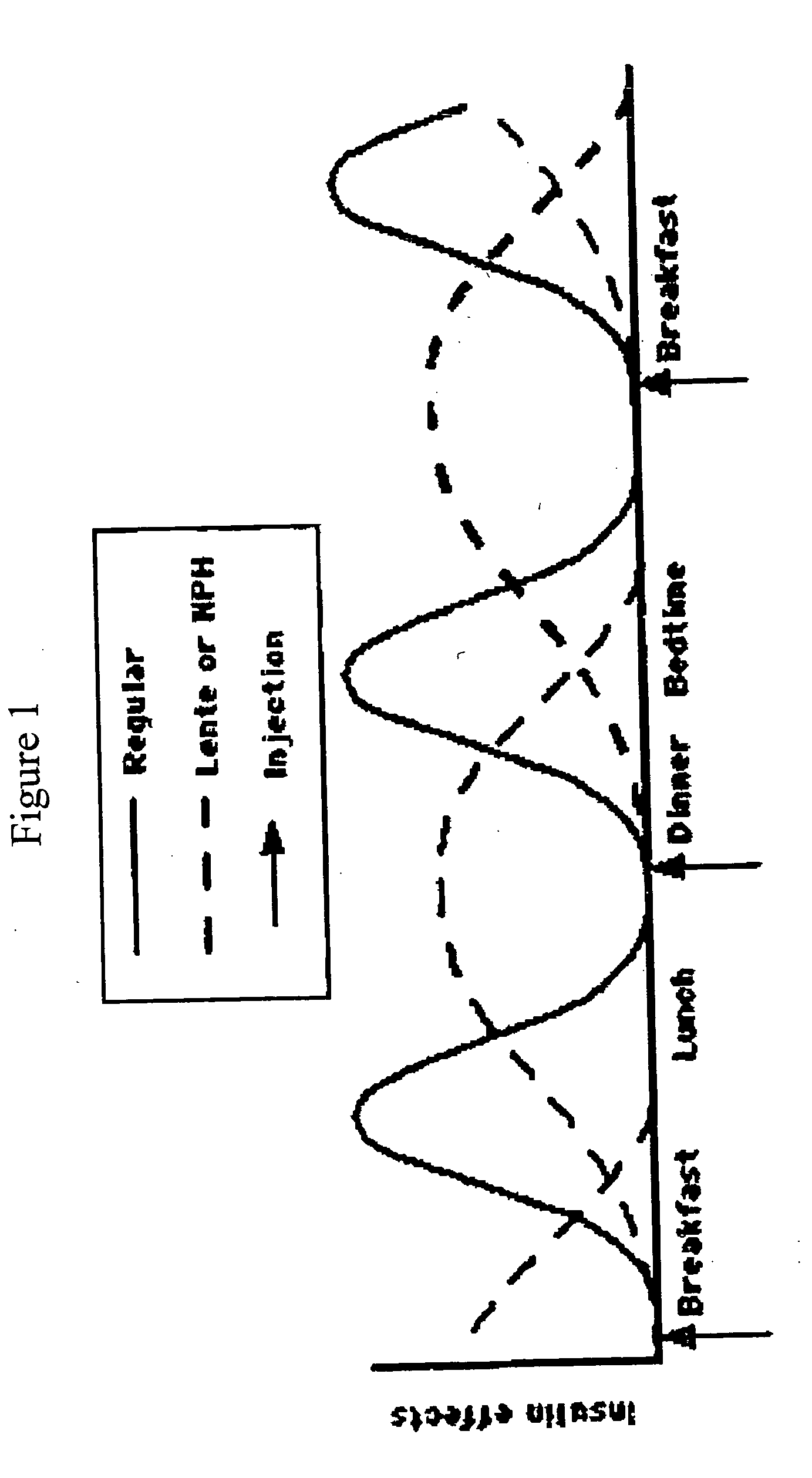
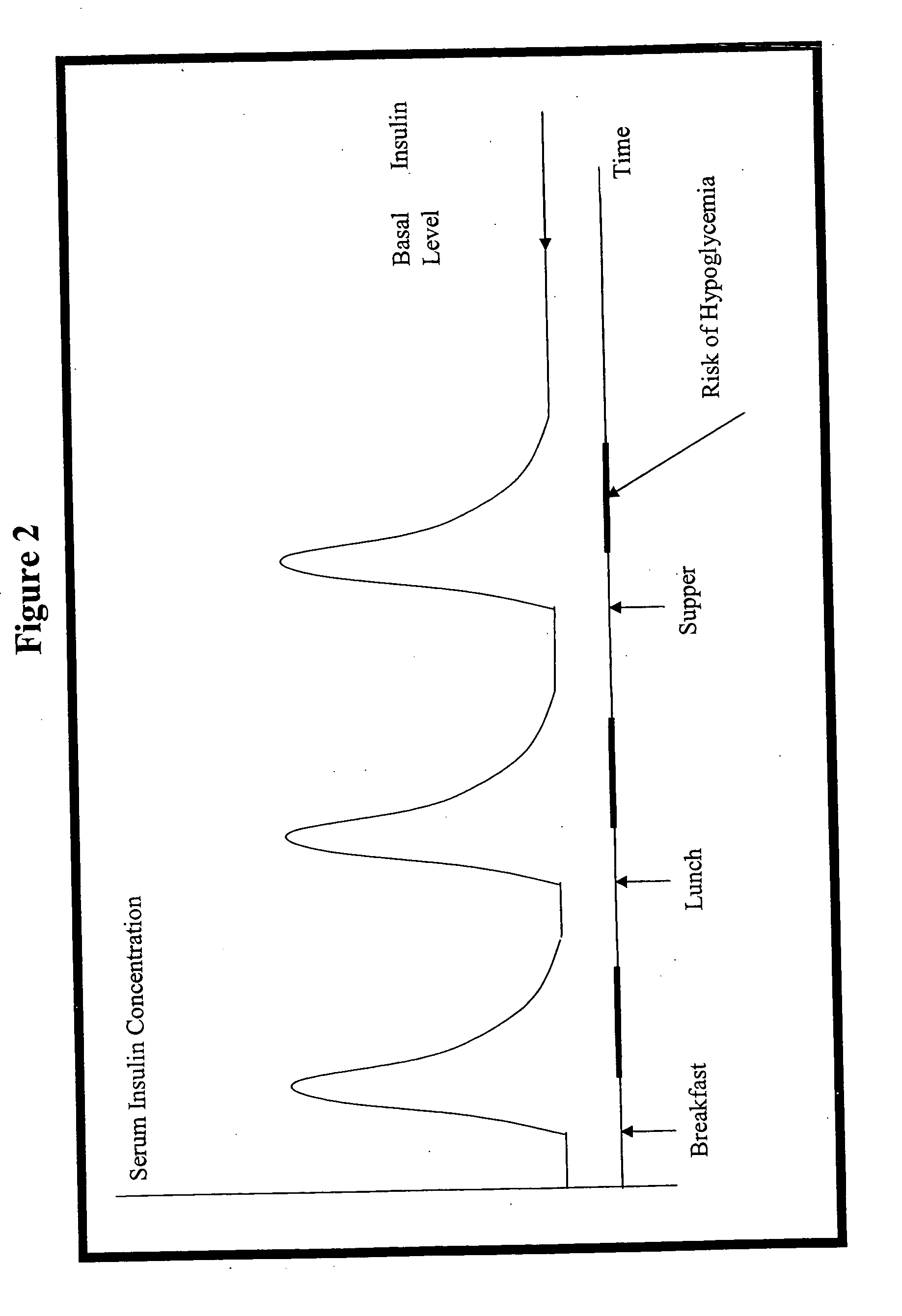
Compositions and methods for the prevention and control of insulin-induced hypoglycemia
InactiveUS7314859B2Extended half-lifePowder deliveryDispersion deliveryInsulin induced hypoglycemiaCvd risk
Pharmaceutical composition comprising both insulin and glucagon can be administered to control and treat diabetes while reducing or eliminating the risk of insulin-induced hypoglycemia.
Owner:DIOBEX INC
Absorption enhancers for drug administration
InactiveUS20060045868A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideInhalationDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
Glucagon-like insulinotropic peptides, compositions and methods
The present invention provides novel complexes consisting of certain GLP-1 molecules associated with a divalent metal cation that is capable of co-precipitating with a GLP-1 molecule. Pharmaceutical compositions and methods of using such complexes for enhancing the expression of insulin in B-type islet cells is claimed, as is a method for treating maturity onset diabetes mellitus in mammals, particularly humans.
Owner:ELI LILLY & CO
Stabilized pharmaceutical peptide compositions
ActiveUS20060178304A1Improve stabilityExtended shelf lifePeptide/protein ingredientsMetabolism disorderNeutral phMedicine
Method for increasing the shelf-life of a pharmaceutical composition for parenteral administration comprising a glucagon-like peptide which is prepared from a peptide product that has been subjected to treatment at a pH above neutral pH.
Owner:NOVO NORDISK AS
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
Compositions and methods for the prevention and control of insulin-induced hypoglycemia
InactiveUS20060014670A1Preventing hypoglycemiaPrevents a hypoglycemic eventIn-vivo radioactive preparationsPeptide/protein ingredientsMedicineInsulin induced hypoglycemia
Pharmaceutical compositions comprising both insulin and glucagon can be administered to control and treat diabetes while reducing or eliminating the risk of insulin-induced hypoglycemia.
Owner:ENJECT
Glp-1 and methods for treating diabetes
InactiveUS20060057137A1Prevent and treat diseasePrevent and treat and delay onset of and reduce symptomPeptide/protein ingredientsMetabolism disorderDiseaseDiabetes mellitus
The present invention relates to use of GLP-1 or a related molecule having GLP-effect for the manufacture of a medicament for preventing or treating diabetes in a mammal. The amount and timing of administration of said medicament are subsequently reduced to produce a “drug holiday”. Practice of the invention achieves effective therapy without continuous drug exposure and without continuous presence of therapeutic levels of the drug. The invention also discloses a method of treating diabetes and related disorders in a mammal by administering glucagons like peptide (GLP-1) or a related molecule having GLP-1 like effect and thereby providing a therapeutically effective amount of endogenous insulin.
Owner:ZEALAND PHARM AS
Therapeutic formulations for transmucosal administration that increase glucagon-like peptide-1 bioavailability
What is described is a pharmaceutical formulation for intranasal delivery of glucagon-like protein-1 (GLP-1), comprising an aqueous mixture of GLP-1, a solubilizing agent, a chelator, and a surface active agent.
Owner:AMYLIN PHARMA INC +1
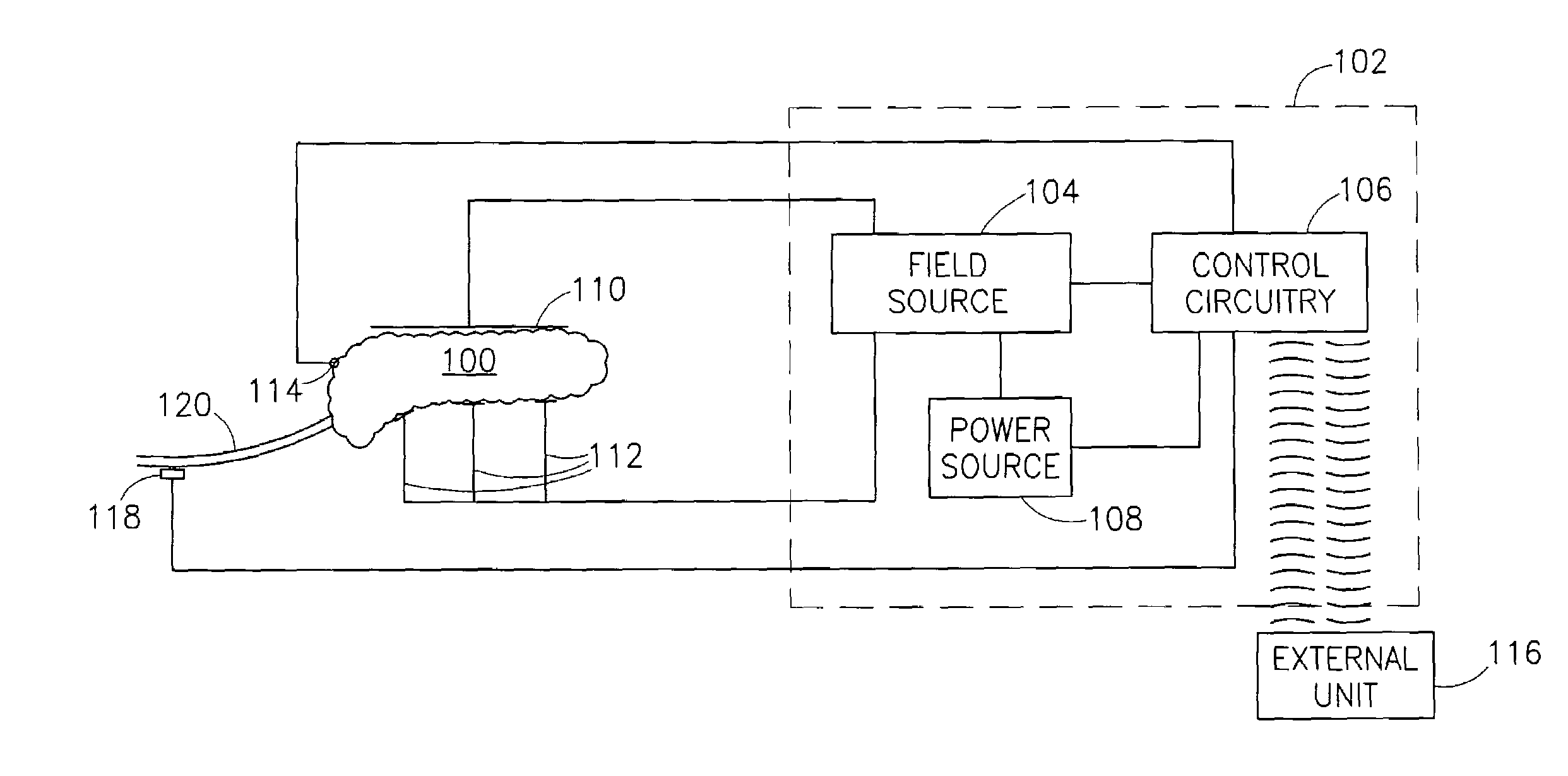
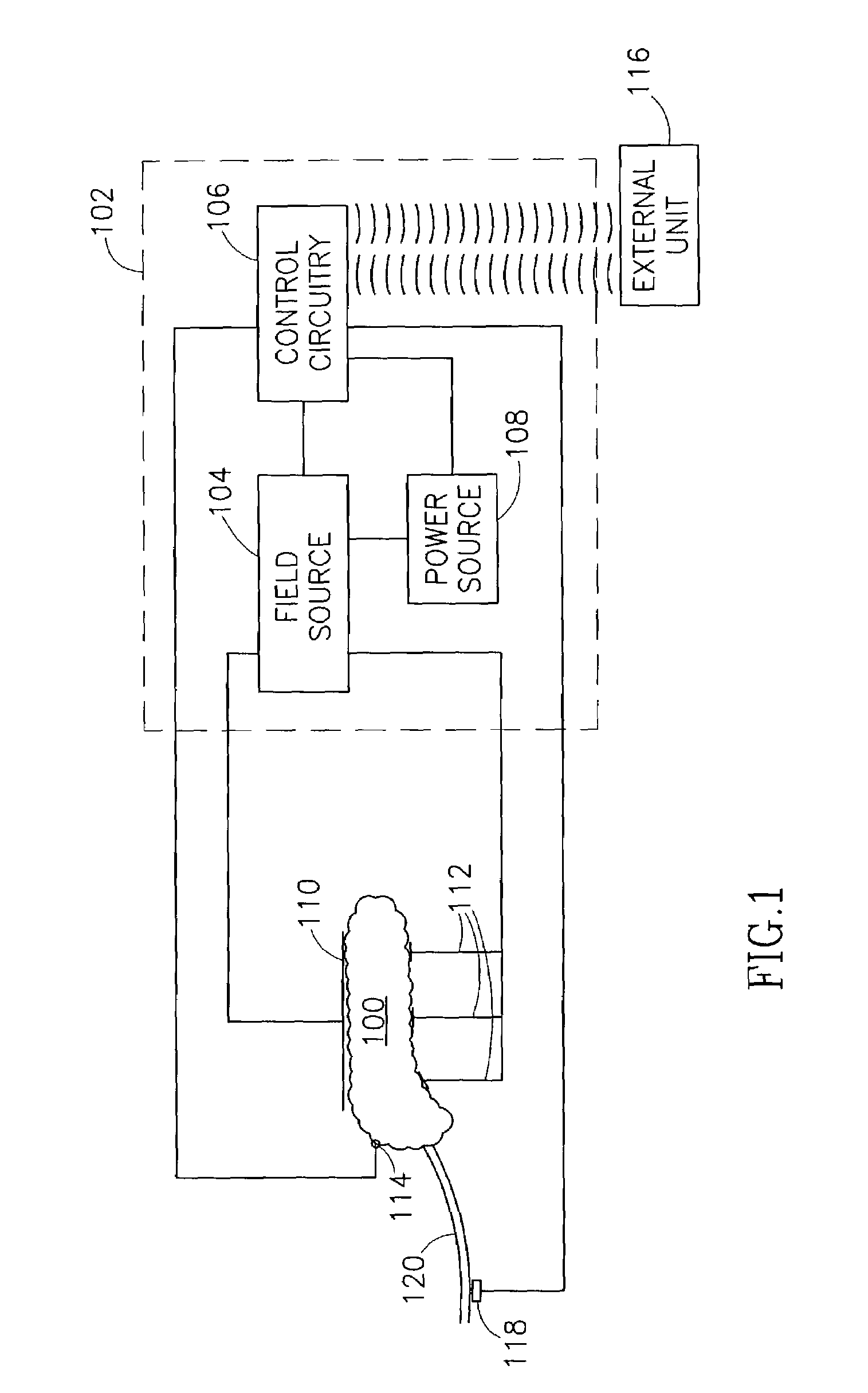
Blood glucose level control
InactiveUS8019421B2Reduced effectivenessOverworking pancreasInternal electrodesSurgical instrument detailsBlood levelBlood insulin
A pancreatic controller, comprising:at least one electrode adapted for electrifying at least a portion of a pancreas; anda controller programmed to electrify said electrode so as to positively control at least the effect of at least two members of a group consisting of blood glucose level, blood insulin level and blood level of another pancreatic hormone. In one example, the controller controls insulin, glucagon and / or glucose blood levels.
Owner:TYLERTON INT INC
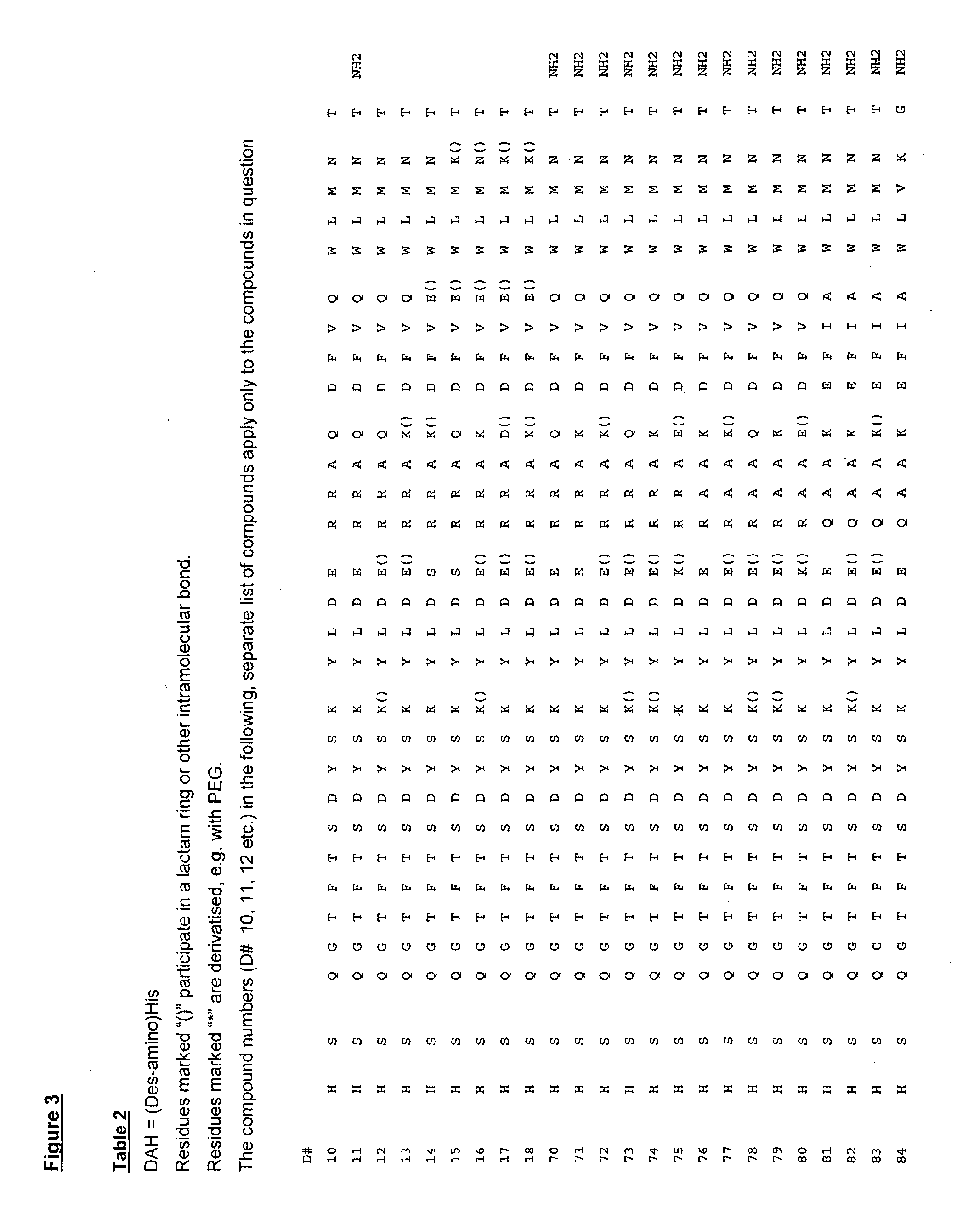
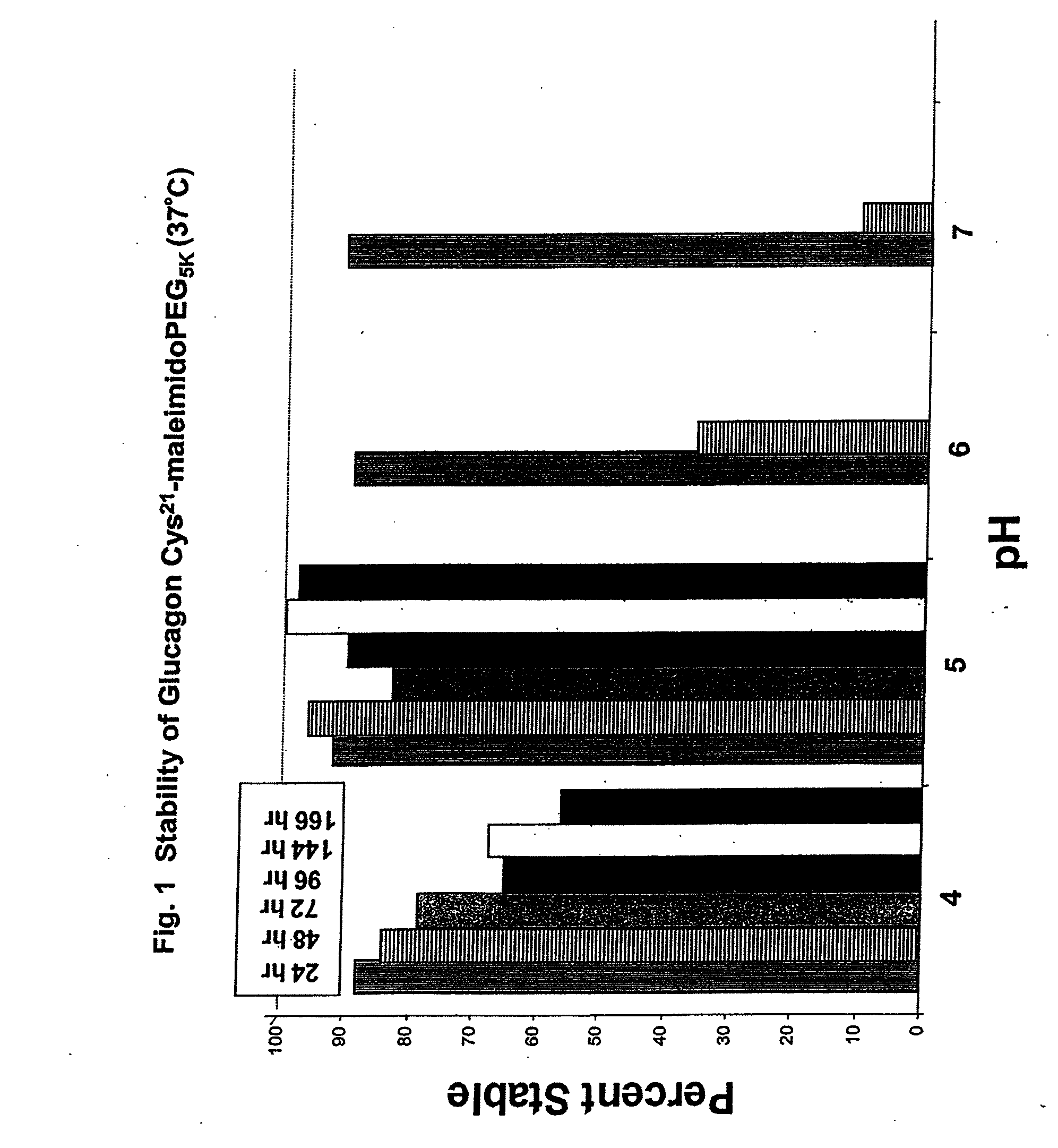
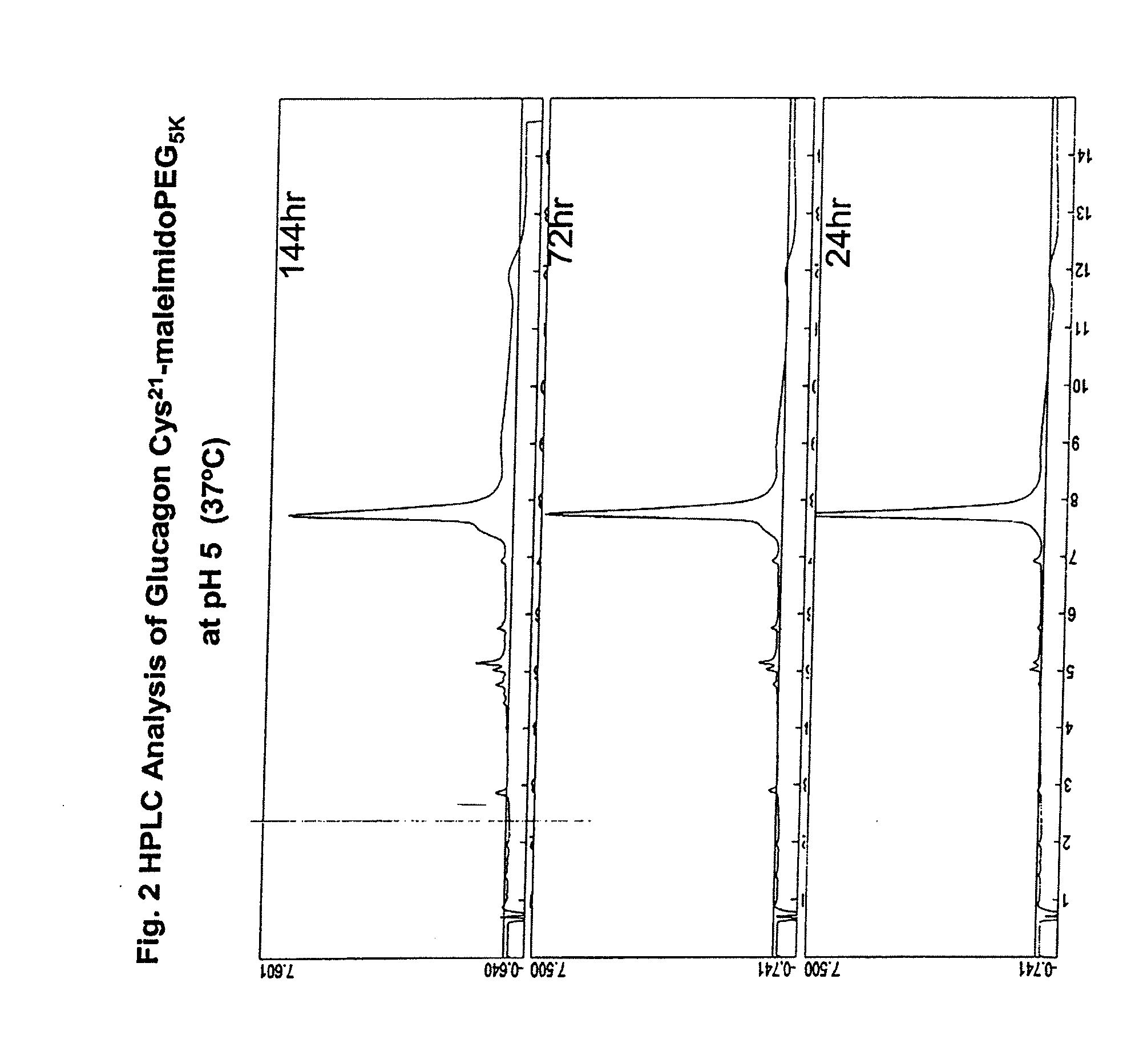
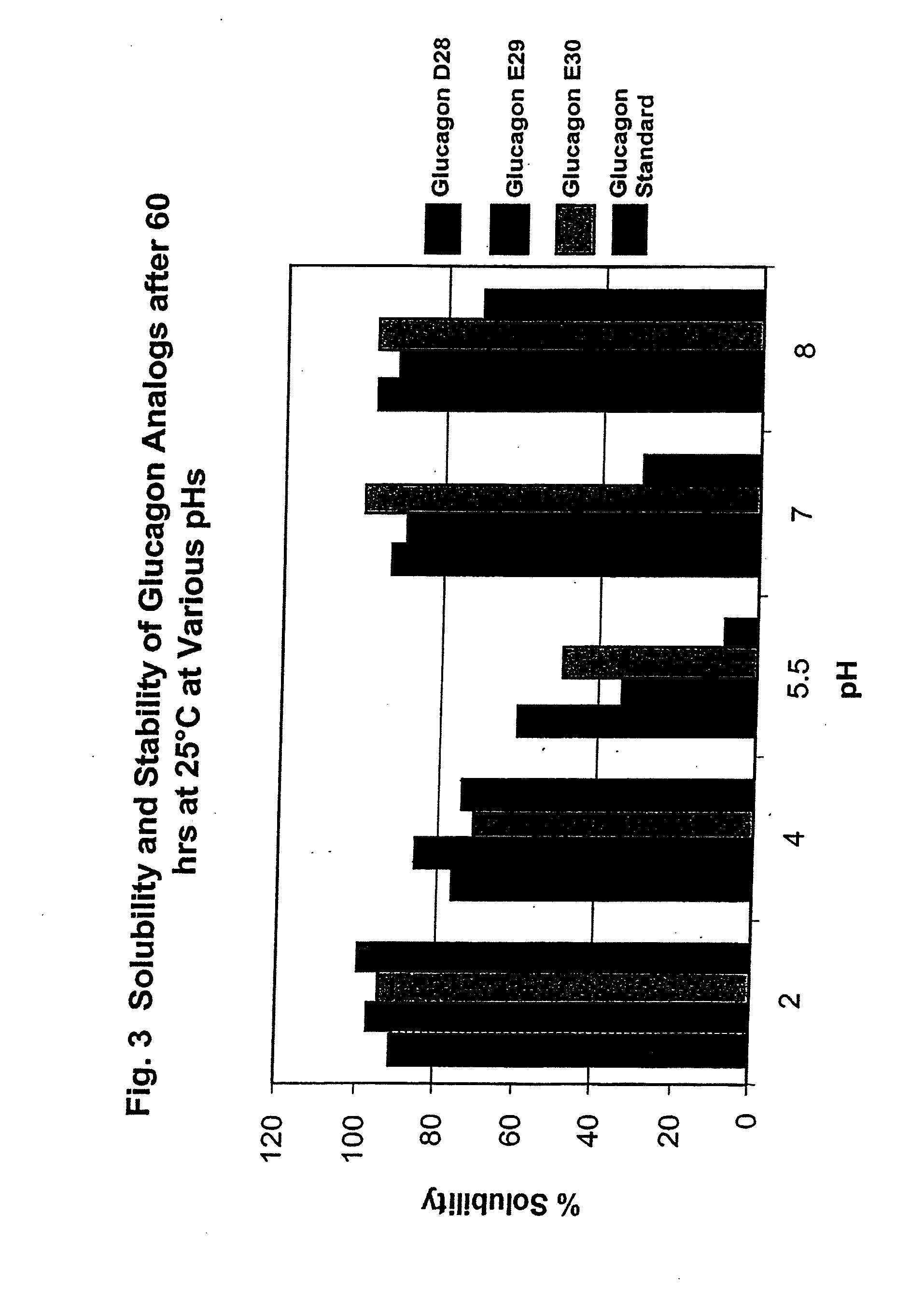
GLUCAGON ANALOGS EXHIBITING ENHANCED SOLUBILITY AND STABILITY IN PHYSIOLOGICAL pH BUFFERS
ActiveUS20110190200A1Rapidly increasing glucose levelNormalizing blood glucose levelAntimycoticsPeptide/protein ingredientsAmino acid substitutionHalf-life
Modified glucagon peptides are disclosed having improved solubility and / or half-life while retaining glucagon agonist activity. The glycogen peptides have been modified by substitution of native amino acids with, and / or addition of, charged amino acids to the carboxy terminus of the peptide. The modified glucagon agonists can be further modified by pegylation, or the addition of a carboxy terminal peptide selected from the group consisting of SEQ ID NO: 20, SEQ ID NO: 21, SEQ ID NO: 23, or both to further enhance the solubility of the glucagon agonist analogs.
Owner:INDIANA UNIV RES & TECH CORP
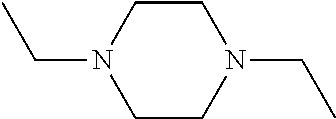
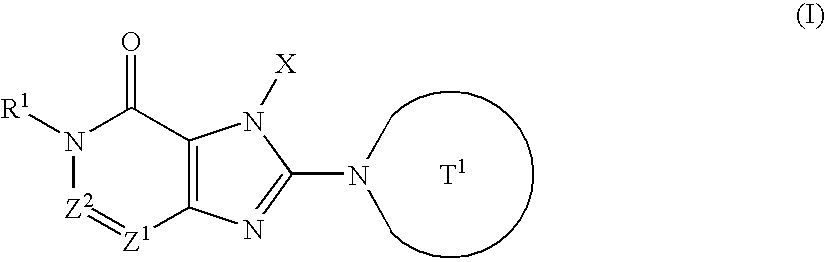
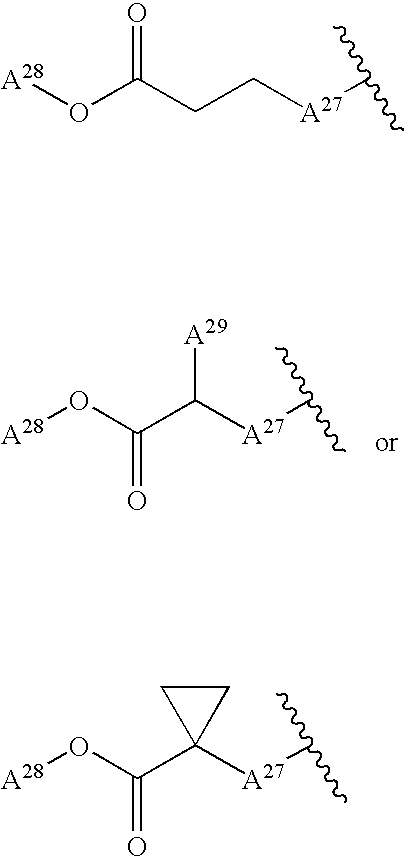
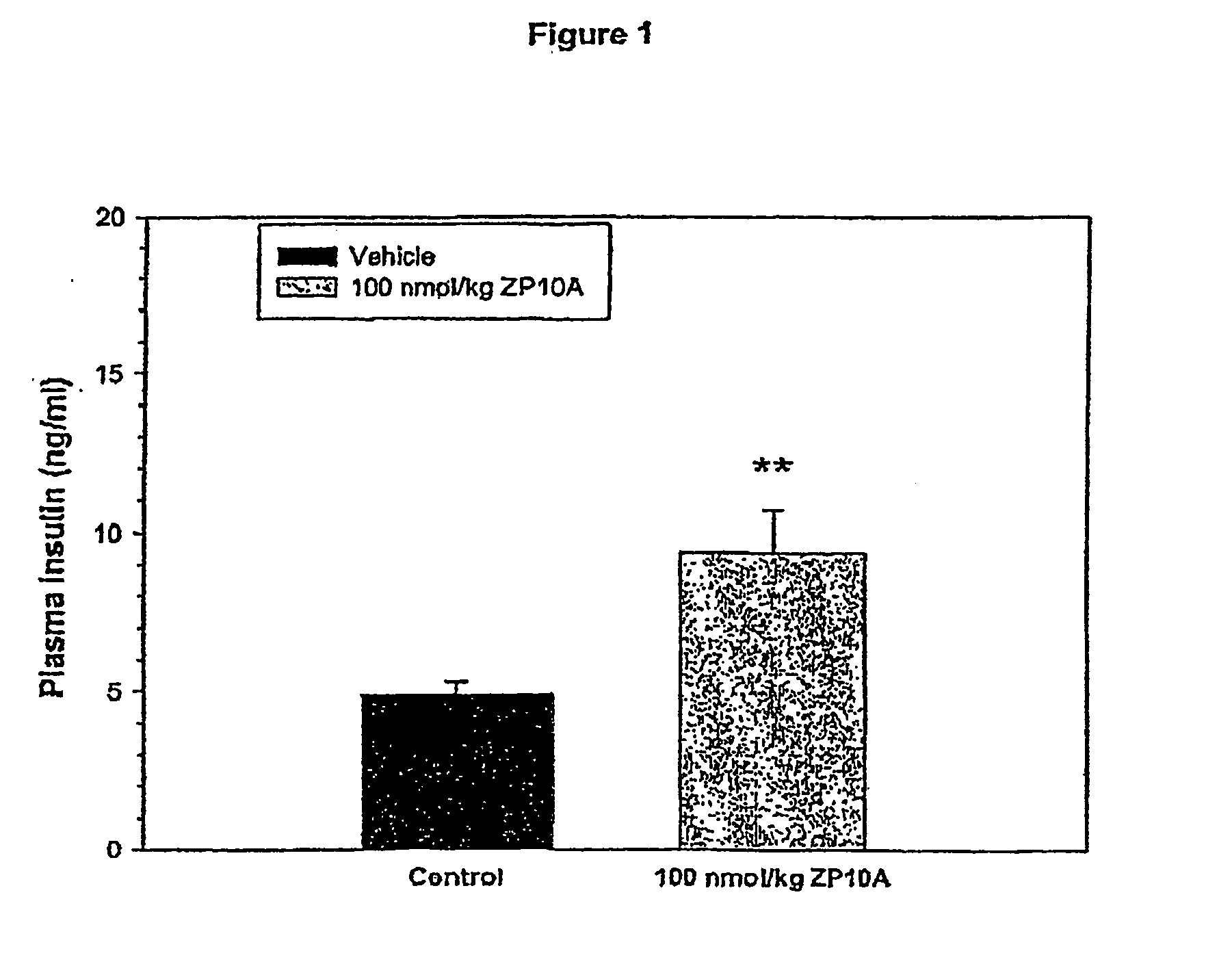
Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]
InactiveUS20080300173A1Improve blood sugar controlControlled more and lessSugar derivativesBacteriaMammalWild type
The present invention provides polypeptides that include an O-linked glycoconjugate in which a species such as a water-soluble polymer, a therapeutic agent of a biomolecule is covalently linked through an intact O-linked glycosyl residue to the polypeptide. The polypeptides of the invention include wild-type peptides and mutant peptides that include an O-linked glycosylation site that is not present in the wild-type peptide. Also provided are methods of making the peptides of the invention and methods, pharmaceutical compositions containing the peptides and methods of treating, ameliorating or preventing diseased in mammals by administering an amount of a peptide of the invention sufficient to achieve the desired response.
Owner:NOVO NORDISK AS
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Medical product for inhalation containing glucagon-like peptide-1 (GLP-1)
InactiveUS20060120969A1Reduce deliveryAccurate dosePowder deliverySpray deliveryPharmaceutical preservativesPulmonary inhalation
A medical product containing an accurately metered dose of a GLP-1 medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is preferably adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Synergistic use of thiazolidinediones with glucagon-like peptide-1 and agonists thereof to treat metabolic instability associated with non-insulin dependent diabetes
InactiveUS7223728B2Lower blood sugar levelsIncrease secretionPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesSide effect
Thiazolidinedione (TZD) and its pharmacologically active derivatives can be used, in combination with agonists of glucagon-like peptide-1 (GLP-1), to treat non-insulin dependent diabetes mellitus, optionally with other therapies, by improving glycemic control while minimizing side effects, such as heart hypertrophy and elevated fed-state plasma glucose, which are associate with both TZD and GLP-1 monotherapies. Thus, the co-administration of TZD and GLP-1 helps regulate glucose homeostasis in Type II diabetic patients.
Owner:ELI LILLY & CO
Acylated glucagon analogues
InactiveUS20120178670A1Avoid weight gainGood for weight lossPeptide/protein ingredientsAntipyreticDiabetes mellitusWeight gain prevention
The invention provides materials and methods for promoting weight loss or preventing weight gain, and in the treatment of diabetes and associated metabolic disorders. In particular, the invention provides novel acylated glucagon analogue peptides effective in such methods. The peptides may mediate their effect by having increased selectivity for the GLP-1 receptor as compared to human glucagon.
Owner:ZEALAND PHARM AS
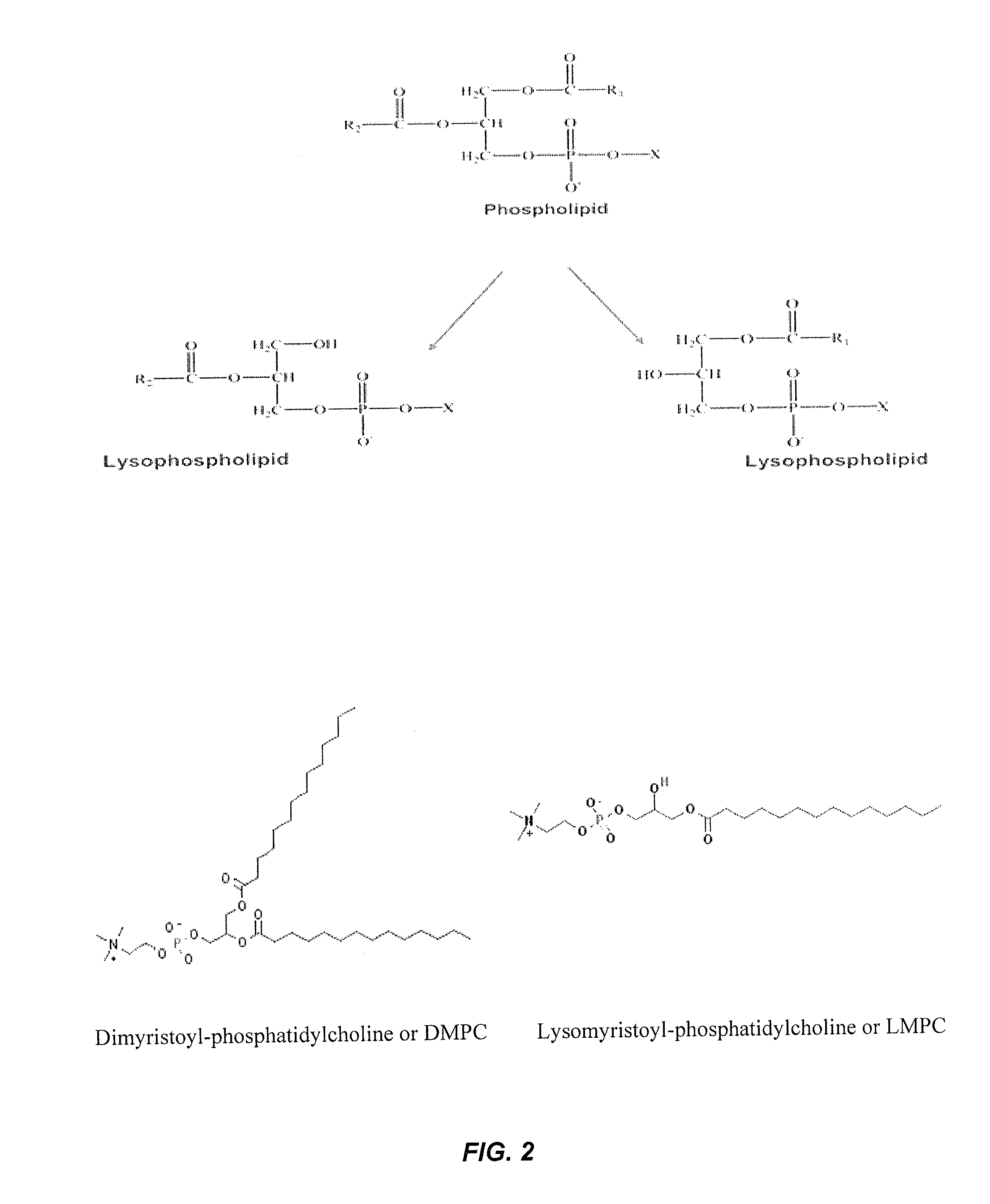
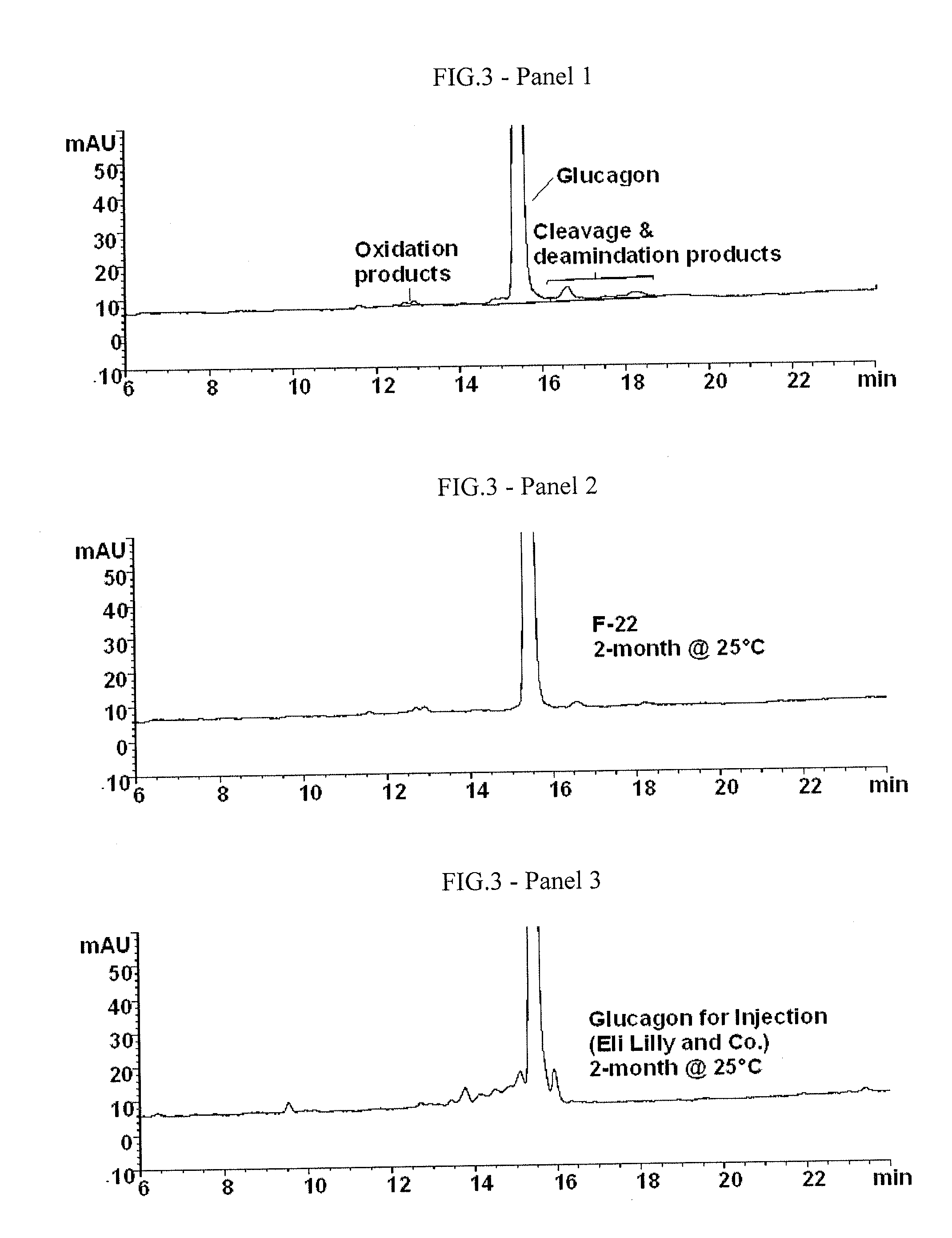
Stabilized glucagon solutions
InactiveUS20110097386A1Assist in elevationStabilize hydrophilic regionPeptide/protein ingredientsMetabolism disorderIrritationGlucose polymers
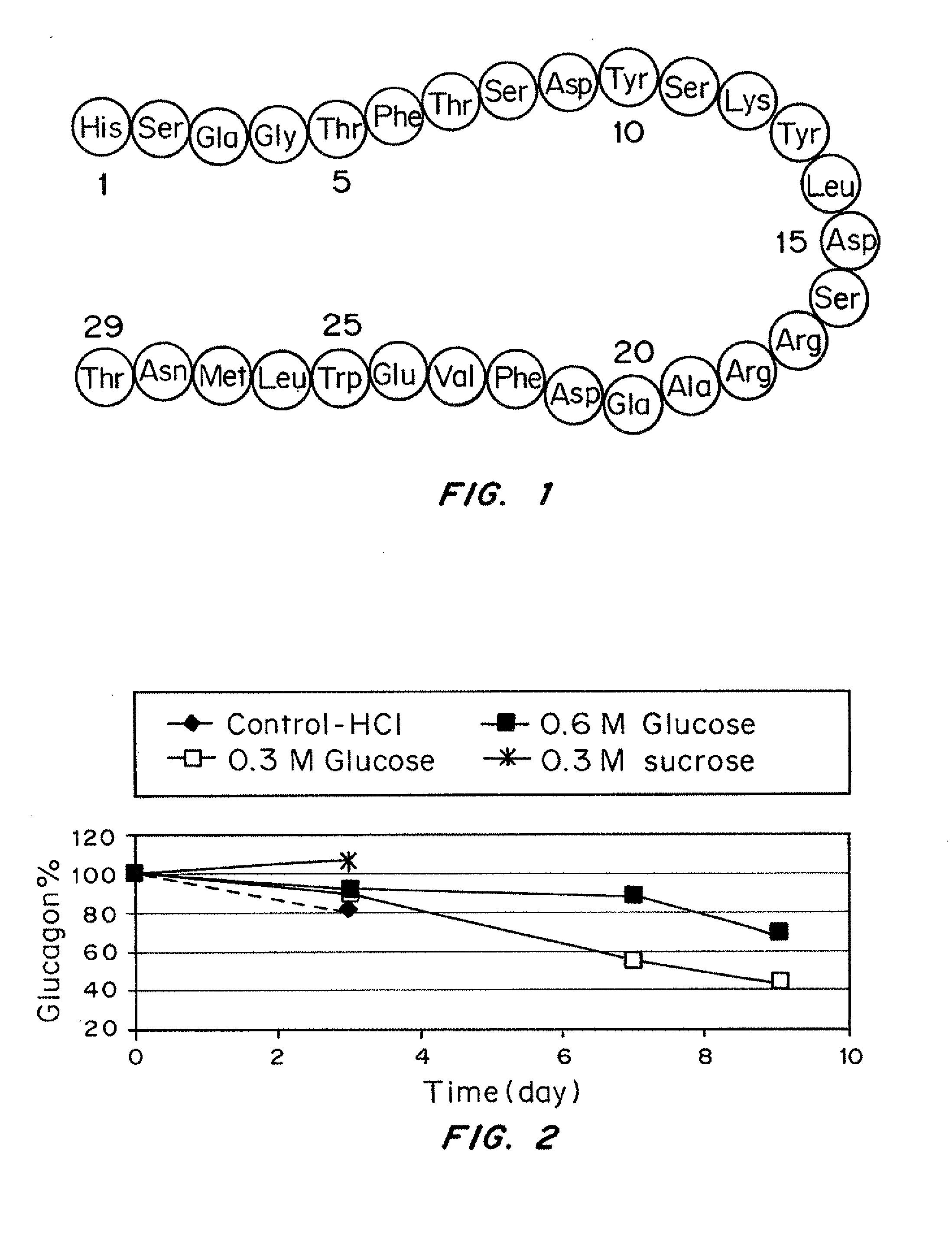
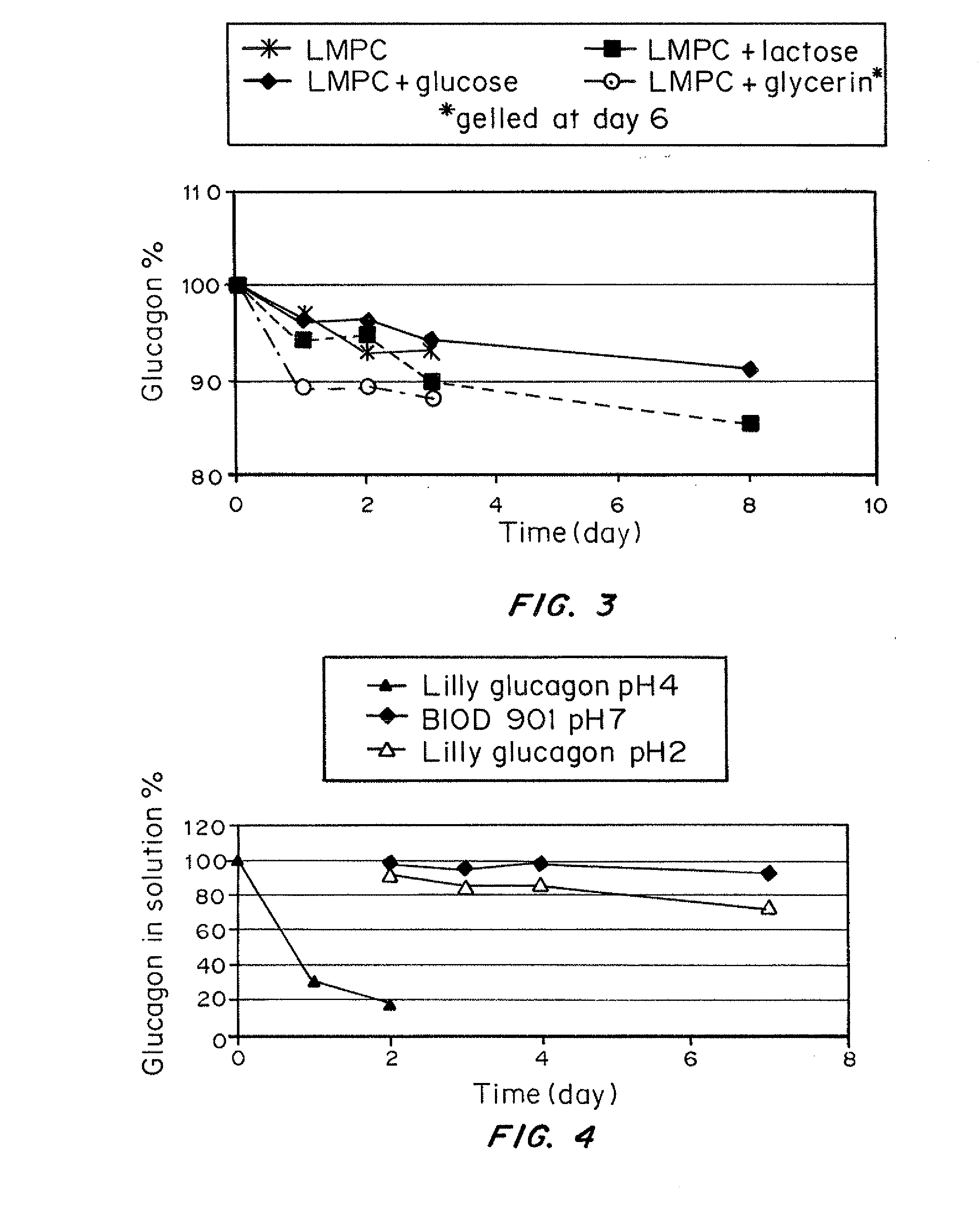
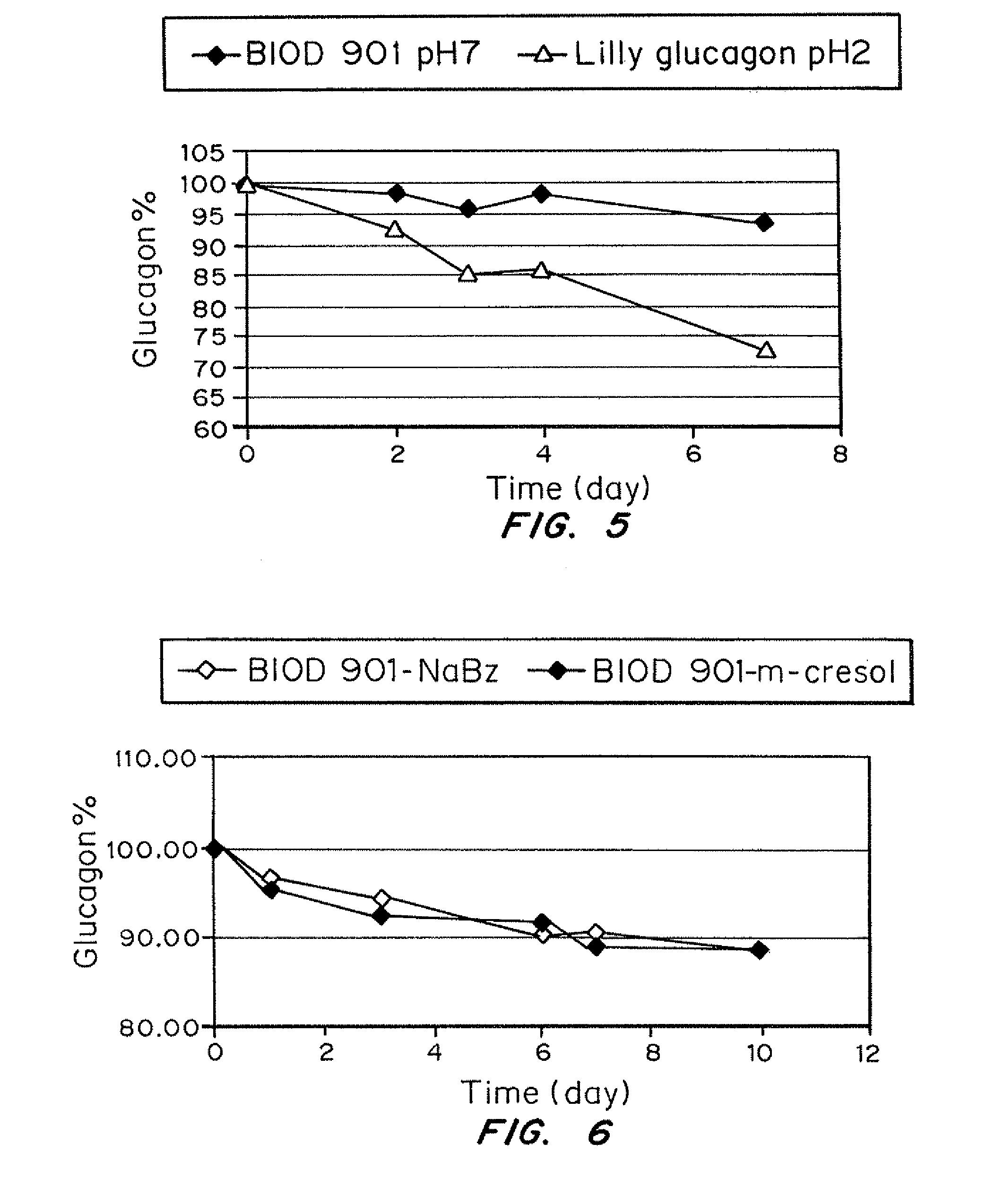
A formulation composed of a sugar such as glucose and a surfactant such as myristoyl lysophosphocholine (LMPC) has been designed to stabilize both hydrophilic and hydrophobic portions of the glucagon molecule, under prolonged physiological conditions, in a formulation that is sufficiently similar to the pH and osmolarity of plasma so as not to induce or to minimize site irritation. The combination of a simple sugar and an surfactant stabilizes the glucagon molecule in an aqueous solution for seven days at 37° C.
Owner:ALBIREO PHARMA INC
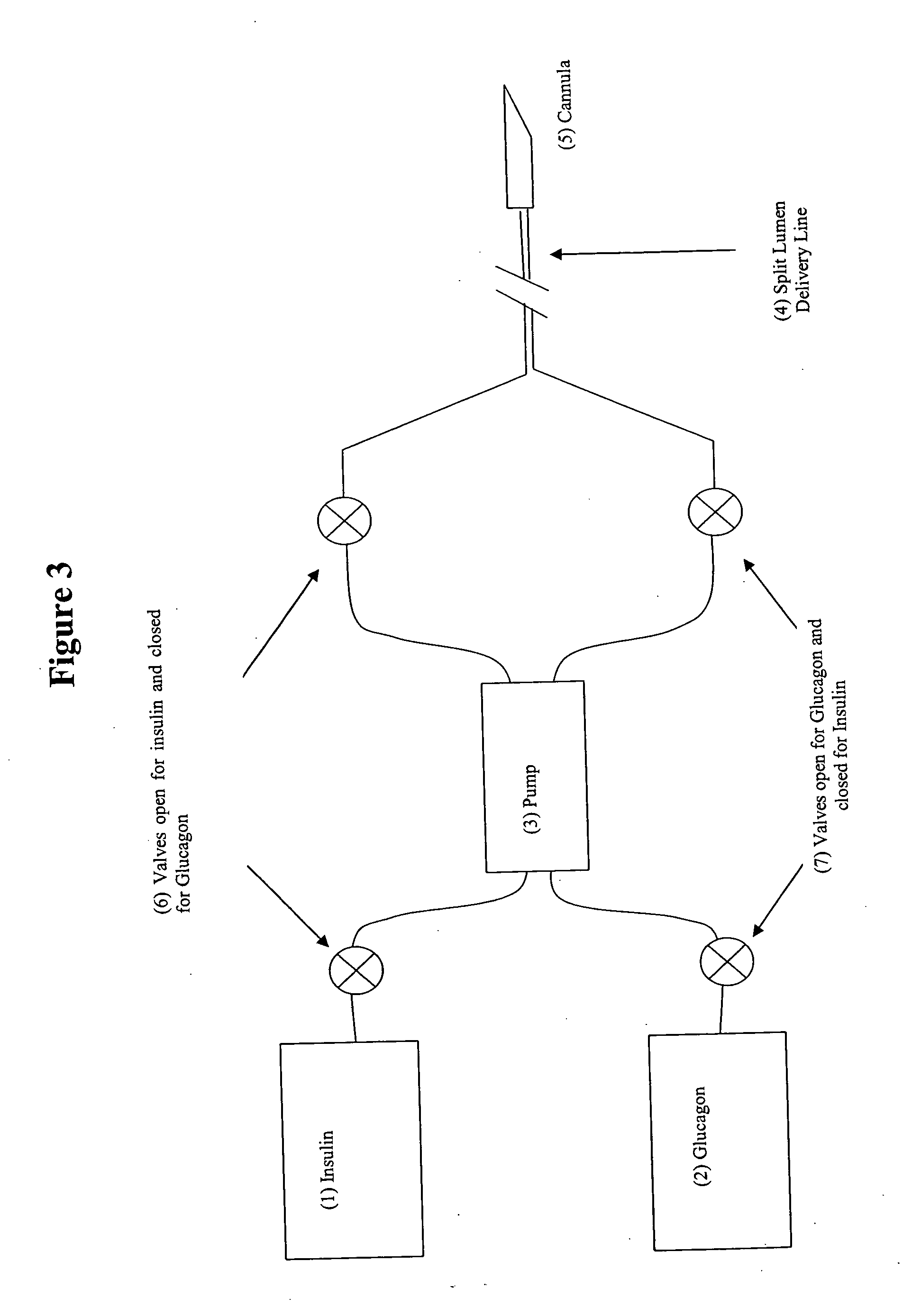
Homeostatic insulin pump
A permanently implanted insulin pump or combined insulin and glucagon pumps, controlled by a valve that expands and contracts due to osmosis as a result of changes in the blood sugar level. The valve is constructed from tissues harvested or grown from the patient's body tissues to prevent rejection, infection or thrombosis. It operates the pump(s) though a blood vessel via a magnetic strip within the valve tissue and a reed switch located outside the blood vessel. The pump battery is recharged by electromagnetic induction in response to low battery warnings. Glucagon and / or insulin are refilled in response to low-level warning indicators, using self-sealing septa located near to the surface of the skin.
Owner:WAGENER ROBERT JOSEPH
Treatment of diabetes
InactiveUS6899883B2Peptide/protein ingredientsMetabolism disorderDiabetes mellitusGlucagon-like peptide-1
Owner:LONDON HEALTH SCI CENT RES
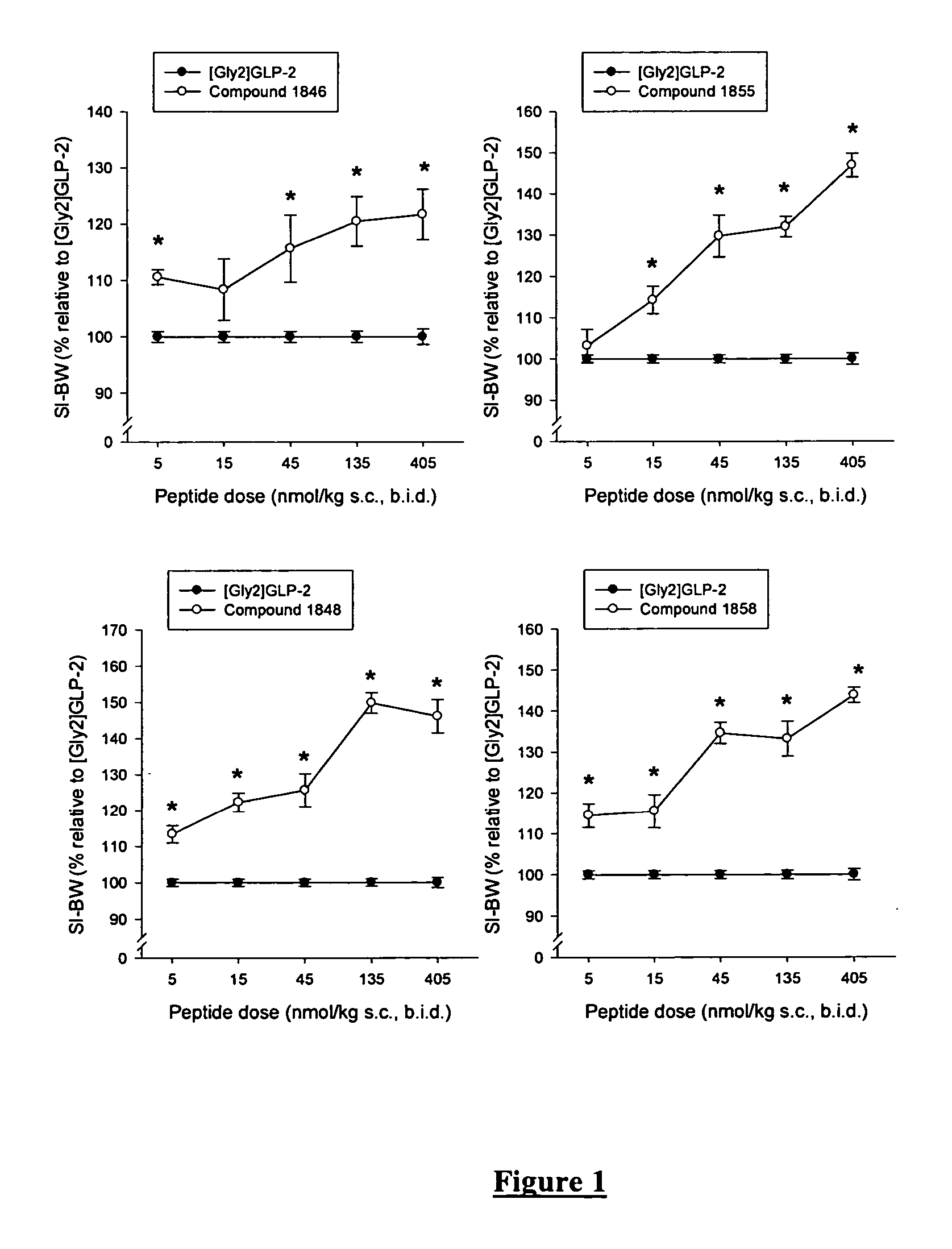
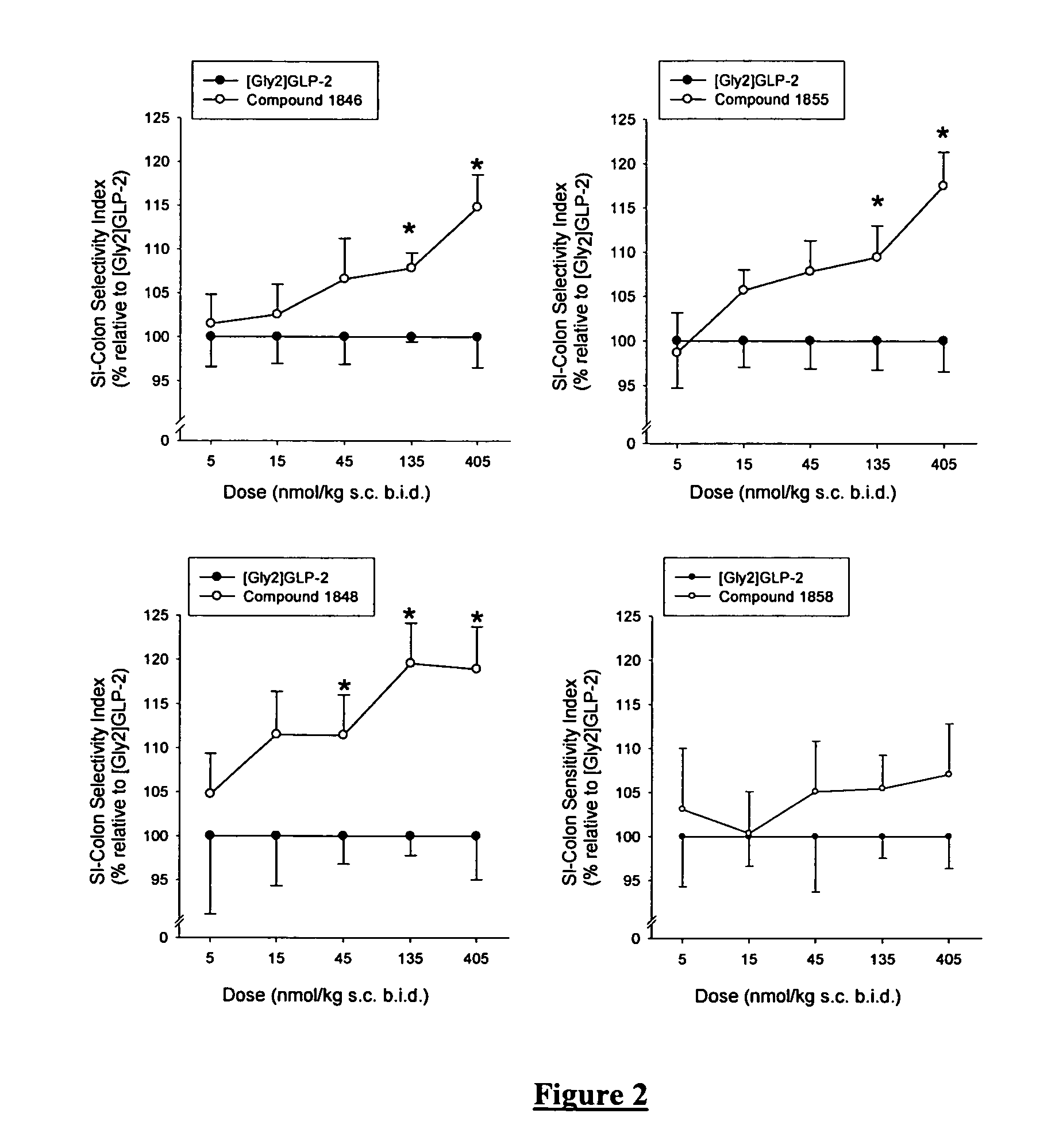
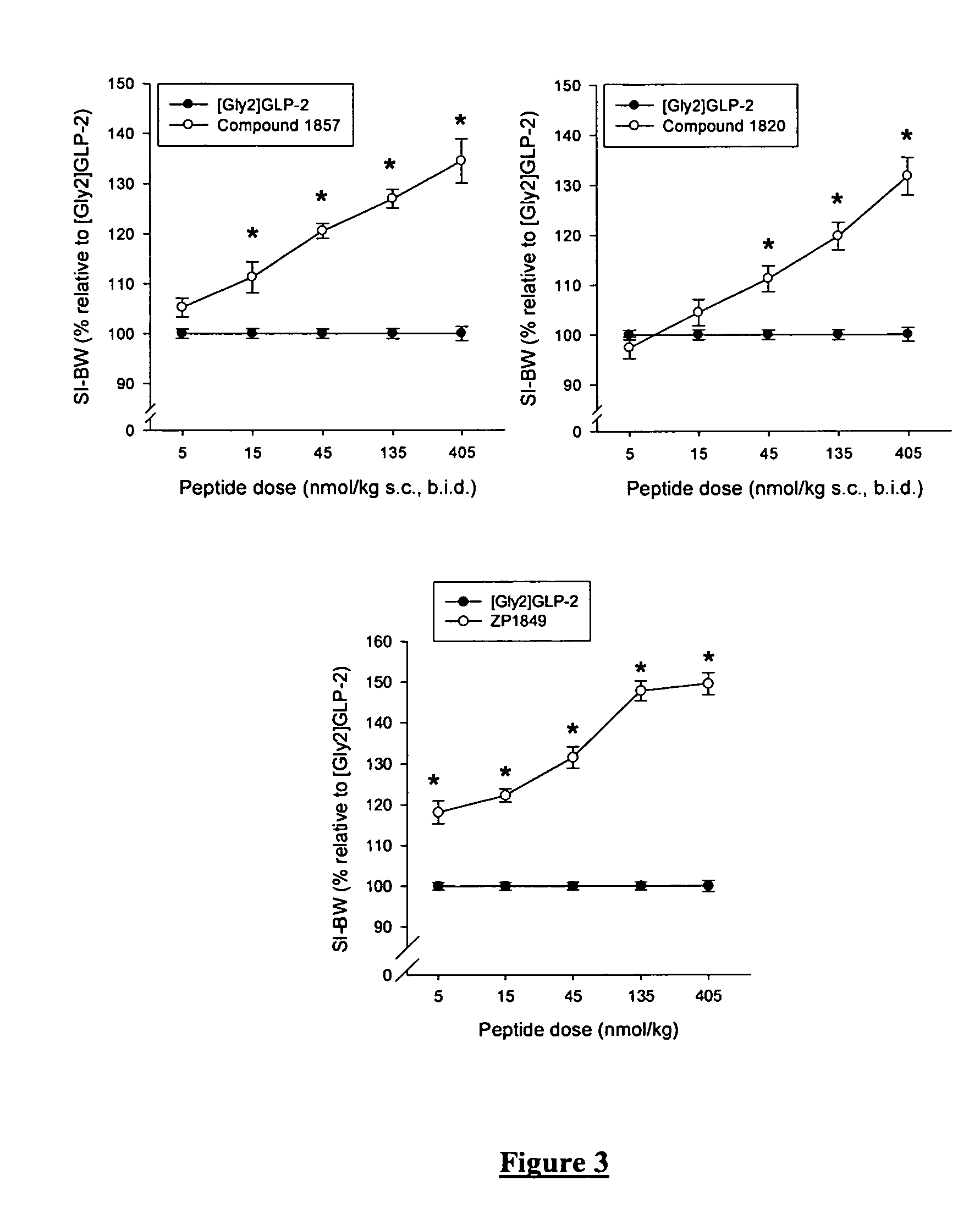
Glucagon-like-peptide-2 (GLP-2) analogues
ActiveUS20070117752A1Improve biological activityPreferential intestinal growth promoting activitySsRNA viruses negative-senseAntipyreticSide effectWild type
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to [hGly2]GLP-2 and which improved biological activity in vivo and / or improved chemical stability, e.g., as assessed in in vitro stability assays. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 8, 16, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 (as mentioned in the introduction) and one or more of positions 3, 5, 7, 10 and 11, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy. Also disclosed are methods and kits for selecting a patient from populations suited for treatment with GLP-2 analogues.
Owner:ZEALAND PHARM AS
Stabilized glucagon nanoemulsions
InactiveUS20140378381A1Reduce wastePeptide/protein ingredientsMetabolism disorderMedicineHypoglycemia
The present invention provides an oil-in-water nanoemulsion containing glucagon, an oily phase, and an aqueous phase, wherein the glucagon is physically and chemically stable and the nanoemulsion is suitable for administration by manual injection or by a pump to treat hypoglycemia.
Owner:LATITUDE PHARMA
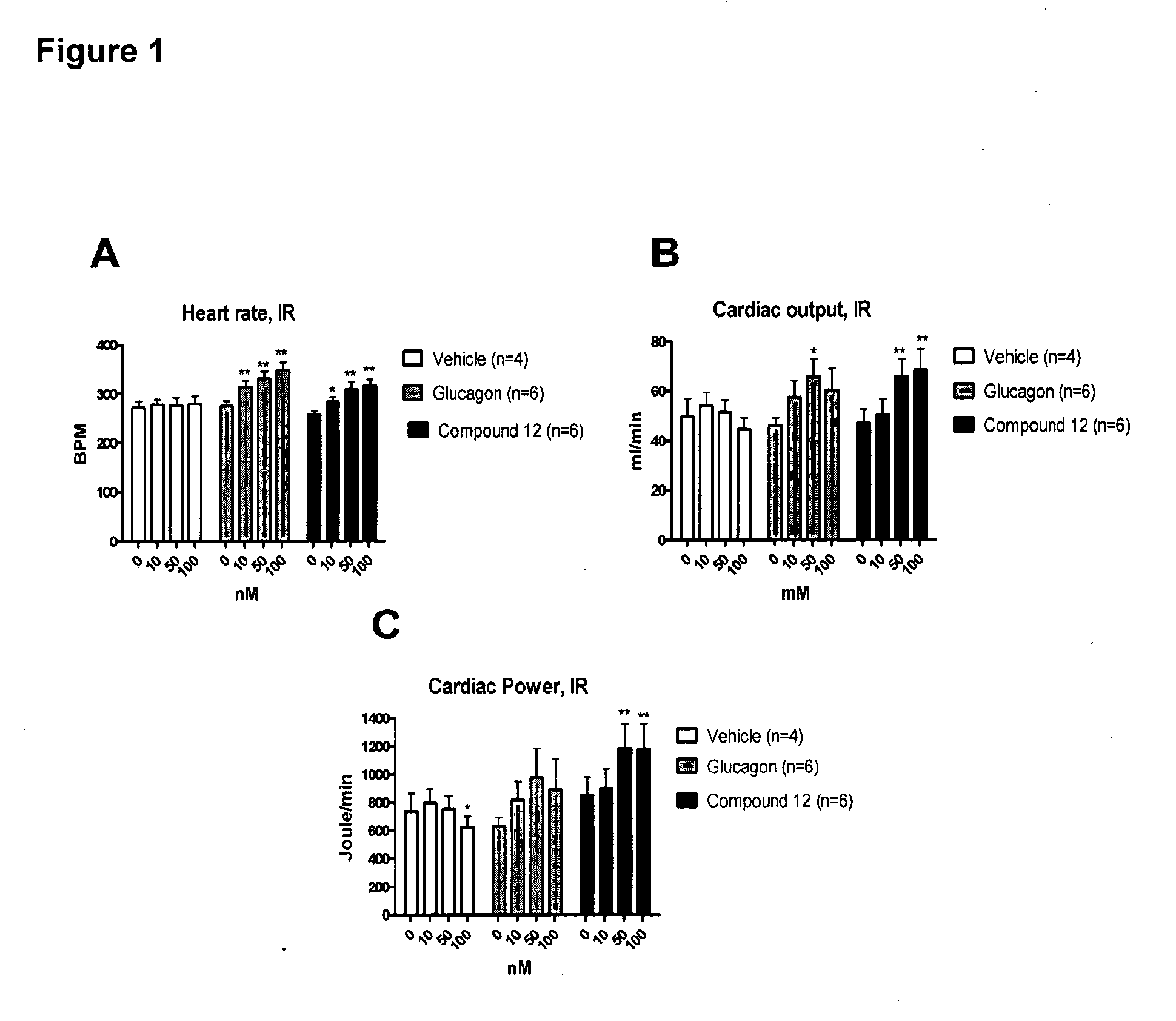
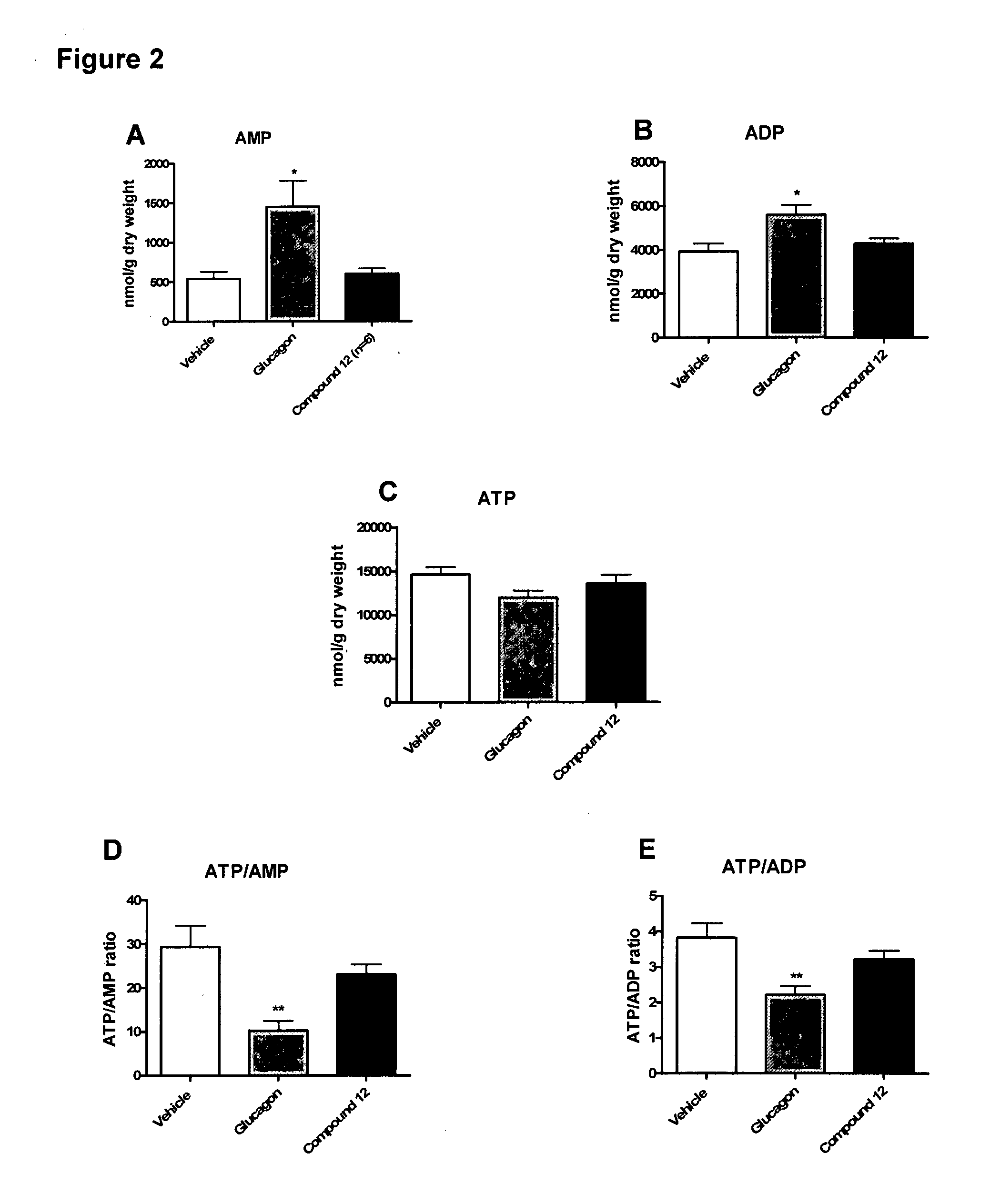
Treatment of cardiac conditions
InactiveUS20130157953A1Useful in therapyIncrease ratingsPeptide/protein ingredientsMetabolism disorderEnergy balancingCardiac dysfunction
The invention relates to the treatment of cardiac dysfunction. In particular, certain compounds, believed to be glucagon-GLP-1 dual agonist compounds, exert a positive inotropic effect while preserving the energy balance of the heart, and so may be superior to known inotropic agents such as dobutamine, norepinephrine and glucagon.
Owner:ZEALAND PHARM AS
Glucagon analogues
ActiveUS20140080757A1Avoid weight gainGood for weight lossBacteriaPeptide/protein ingredientsProviding materialObesity
The invention provides materials and methods for the treatment of obesity and excess weight, diabetes, and other associated metabolic disorders. In particular, the invention provides novel glucagon analogue peptides effective in such methods. The peptides may mediate their effect by having increased selectivity for the GLP-1 receptor as compared to human glucagon.
Owner:ZEALAND PHARM AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
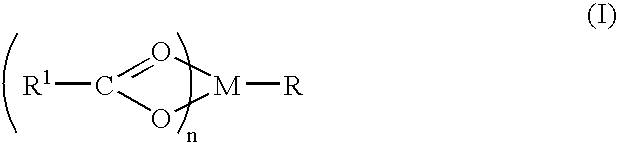
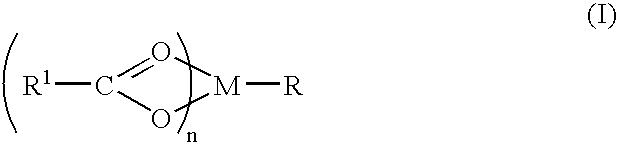

![Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1] Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]](https://images-eureka.patsnap.com/patent_img/4ee91e84-dc70-492d-8101-8e27c2003d31/US20080300173A1-20081204-D00001.png)
![Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1] Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]](https://images-eureka.patsnap.com/patent_img/4ee91e84-dc70-492d-8101-8e27c2003d31/US20080300173A1-20081204-C00001.png)
![Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1] Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]](https://images-eureka.patsnap.com/patent_img/4ee91e84-dc70-492d-8101-8e27c2003d31/US20080300173A1-20081204-C00002.png)