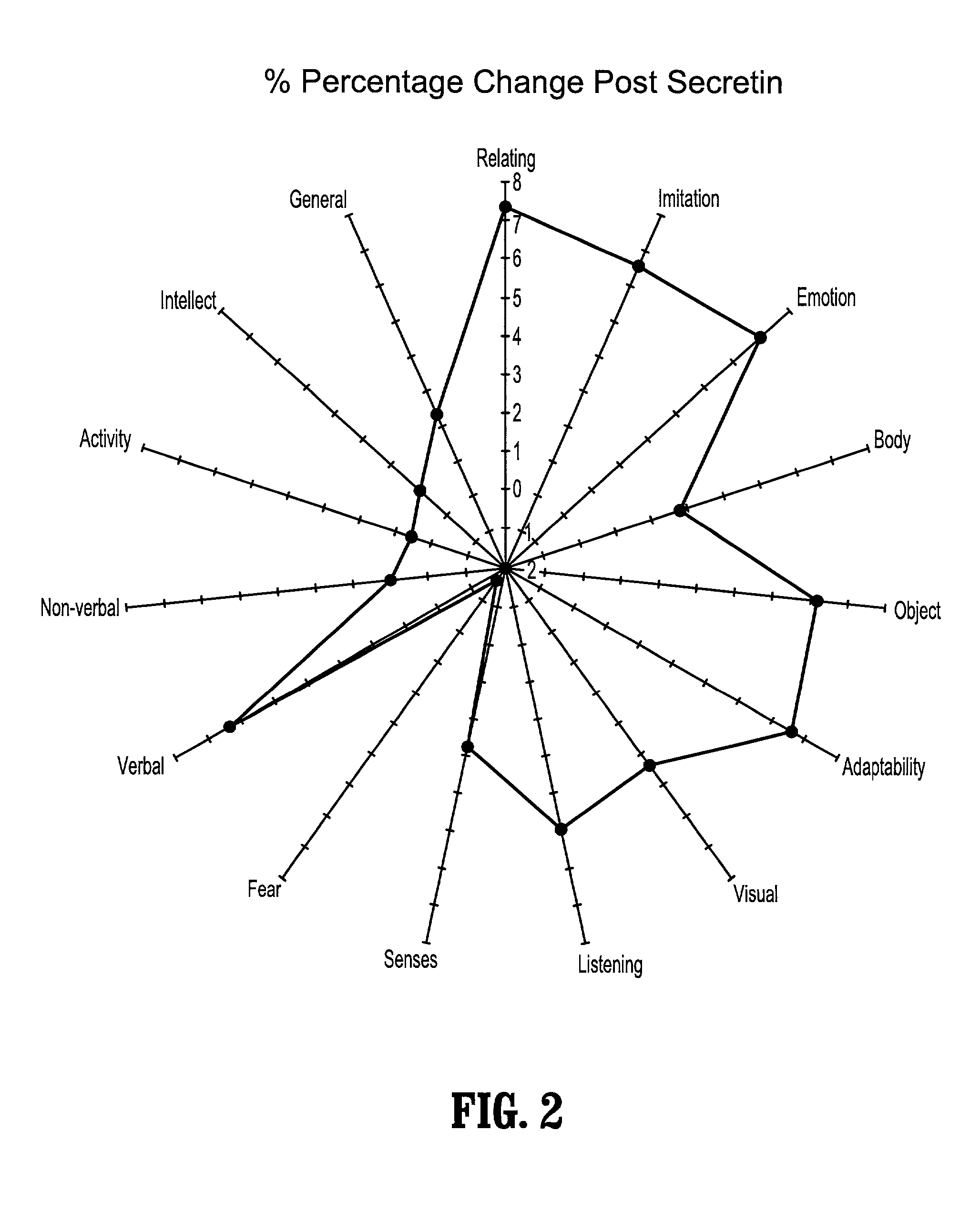
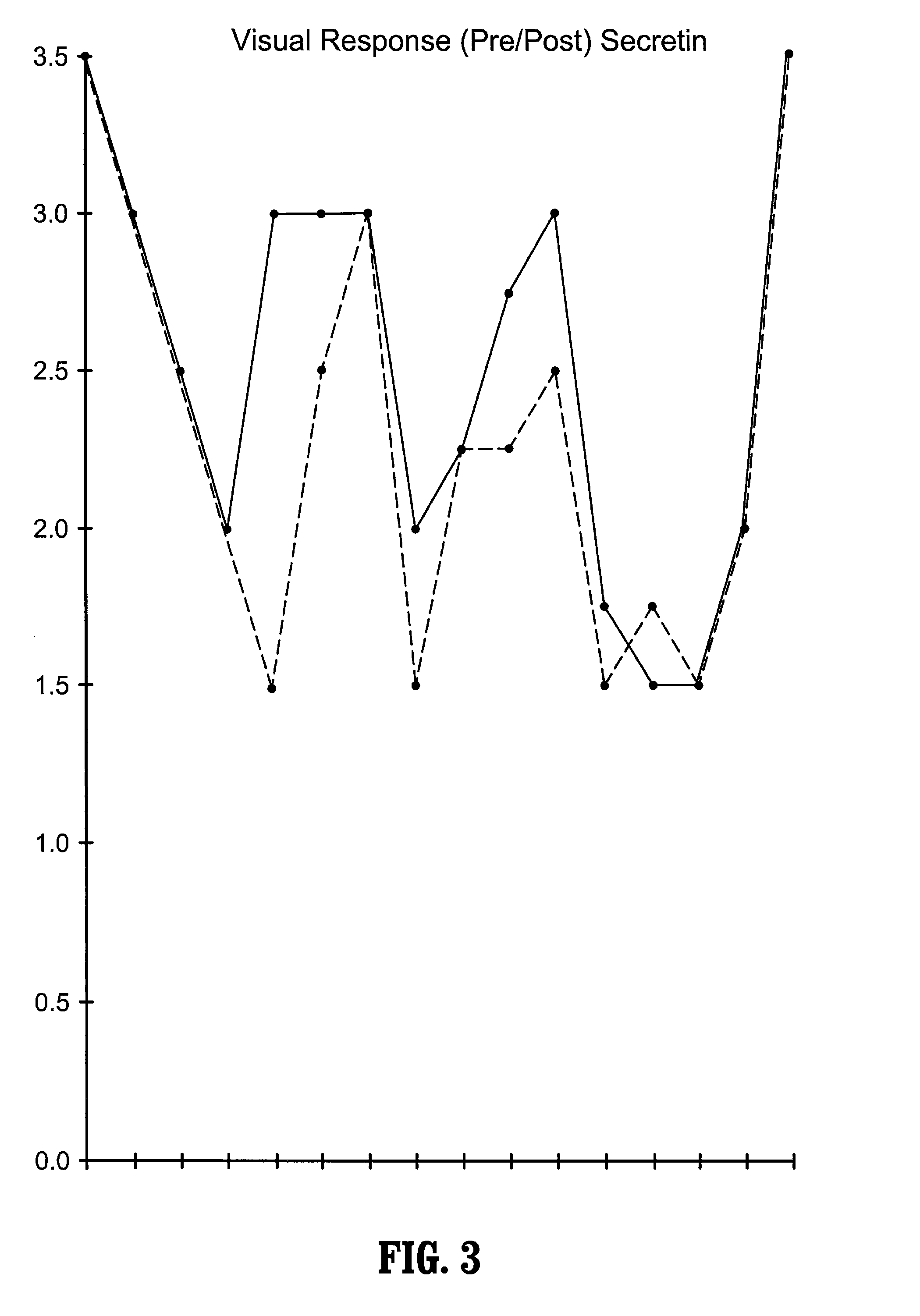
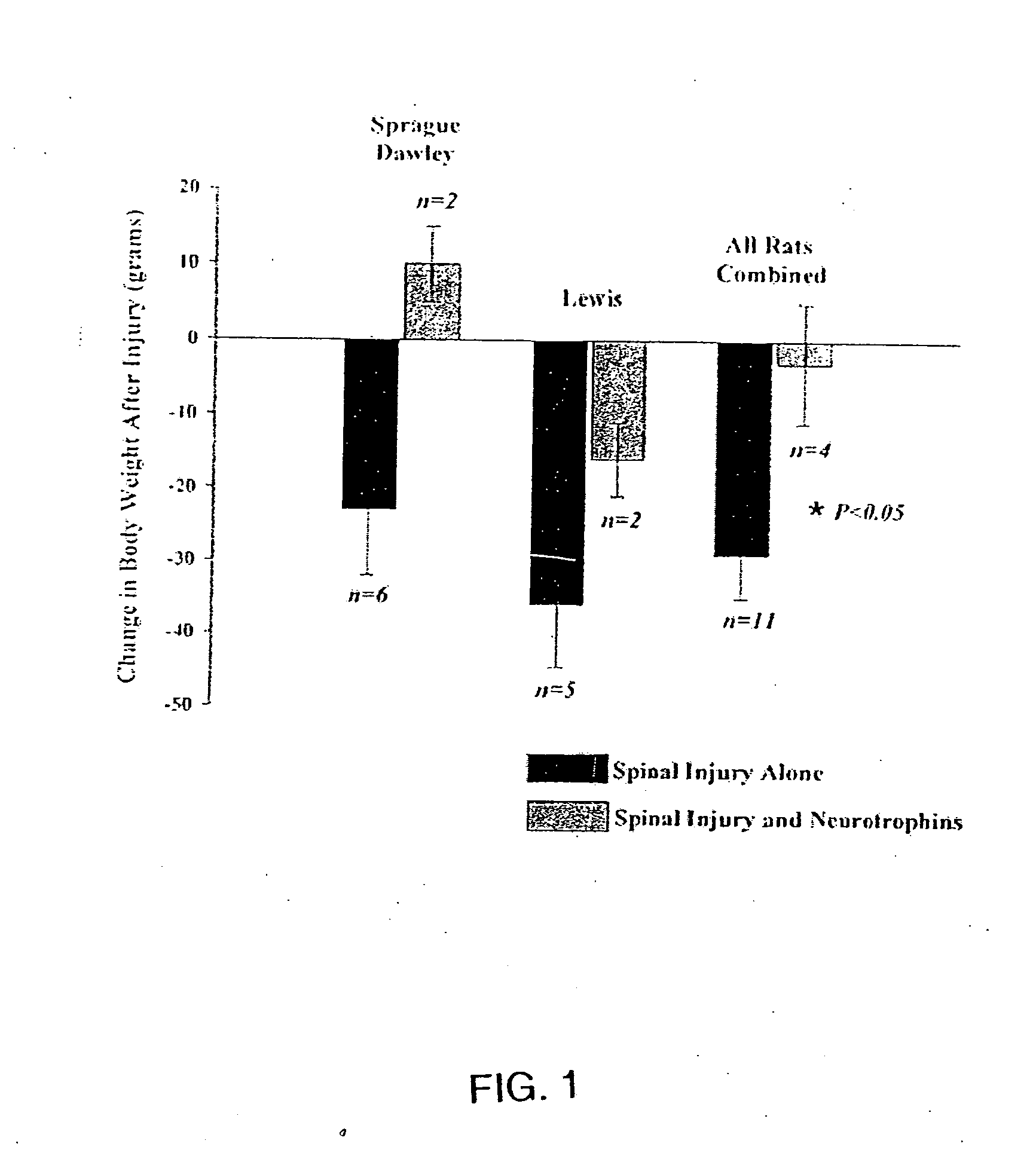
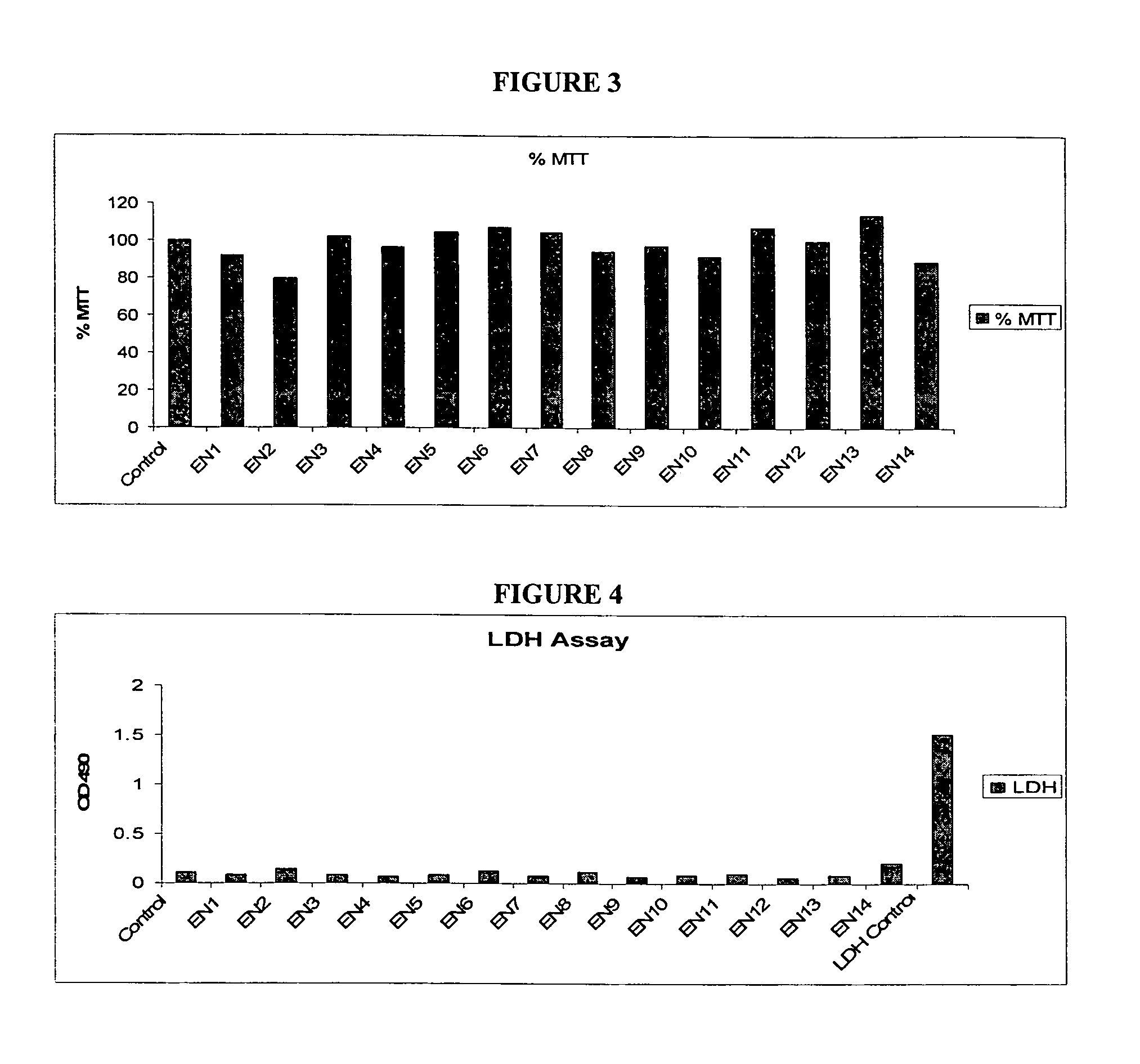
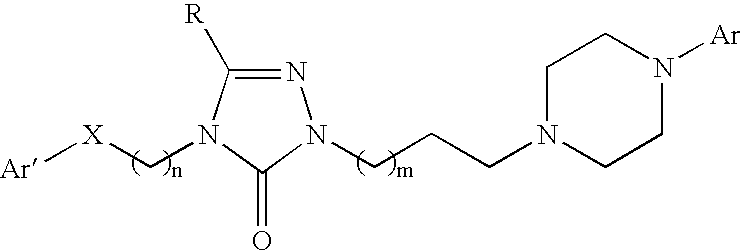
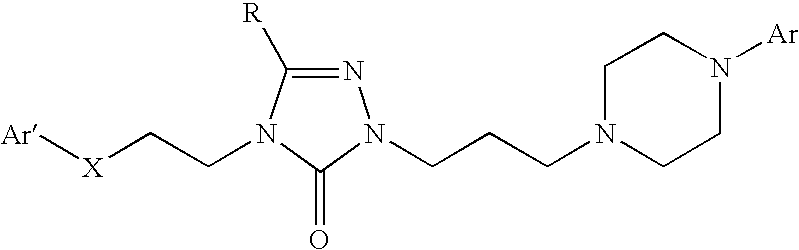
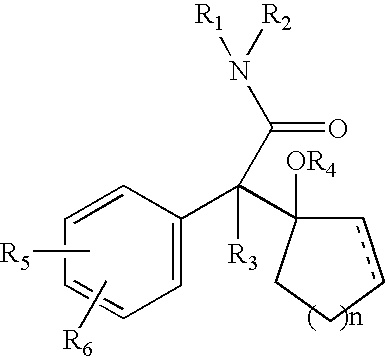
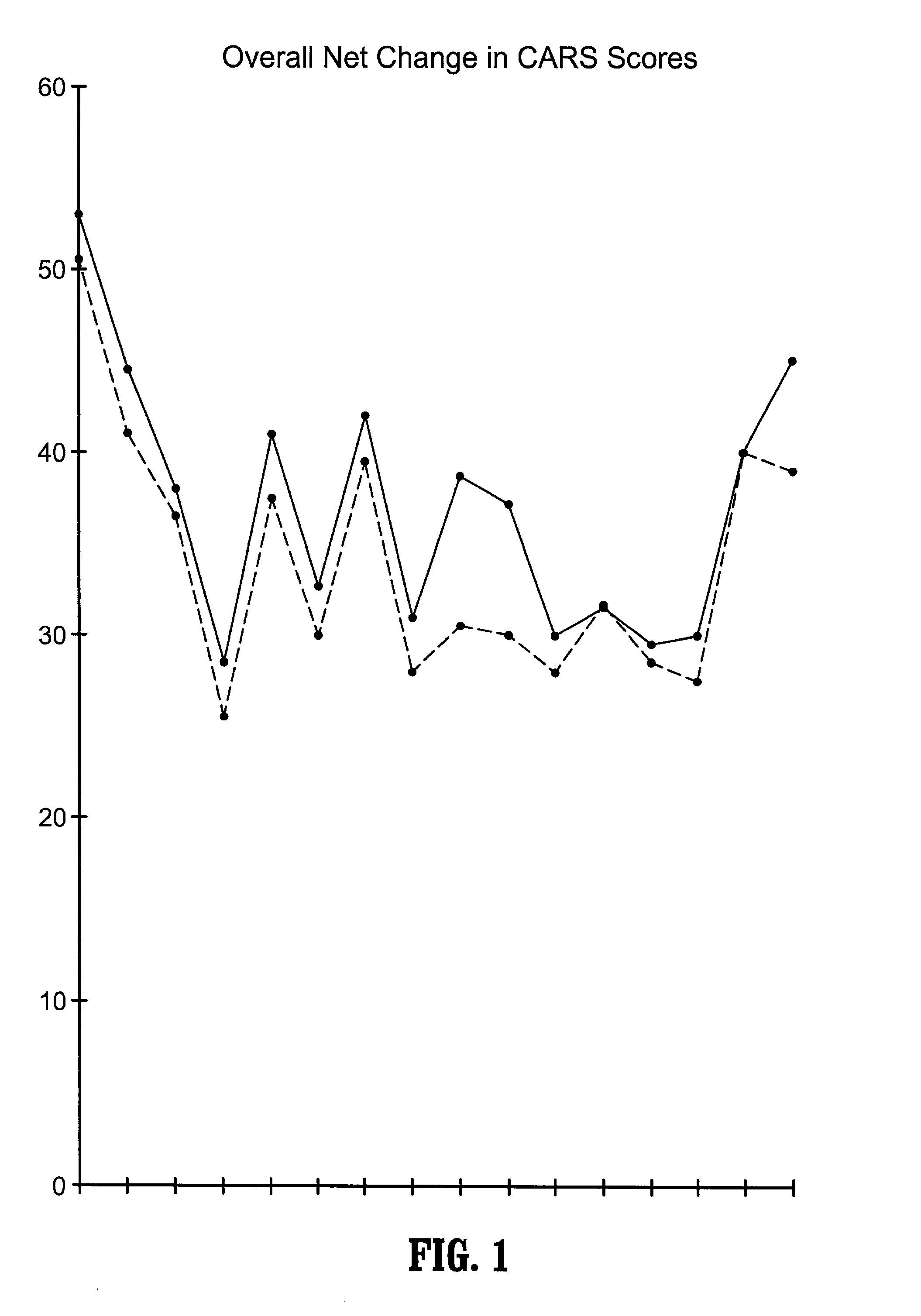Patents
Literature
177 results about "Neuropeptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neuropeptides are small protein-like molecules (peptides) used by neurons to communicate with each other. They are neuronal signalling molecules that influence the activity of the brain and the body in specific ways. Different neuropeptides are involved in a wide range of brain functions, including analgesia, reward, food intake, metabolism, reproduction, social behaviors, learning and memory.
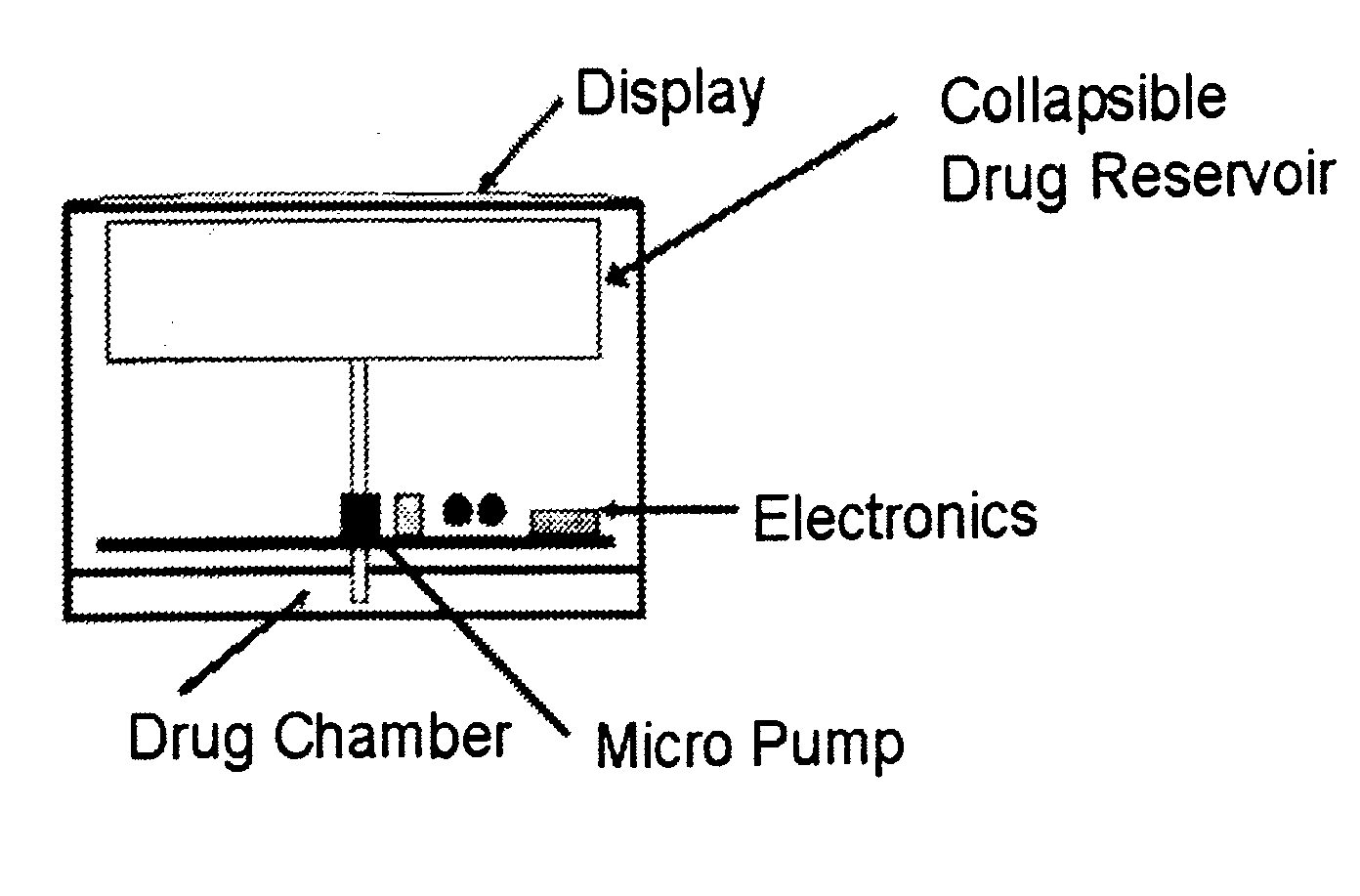
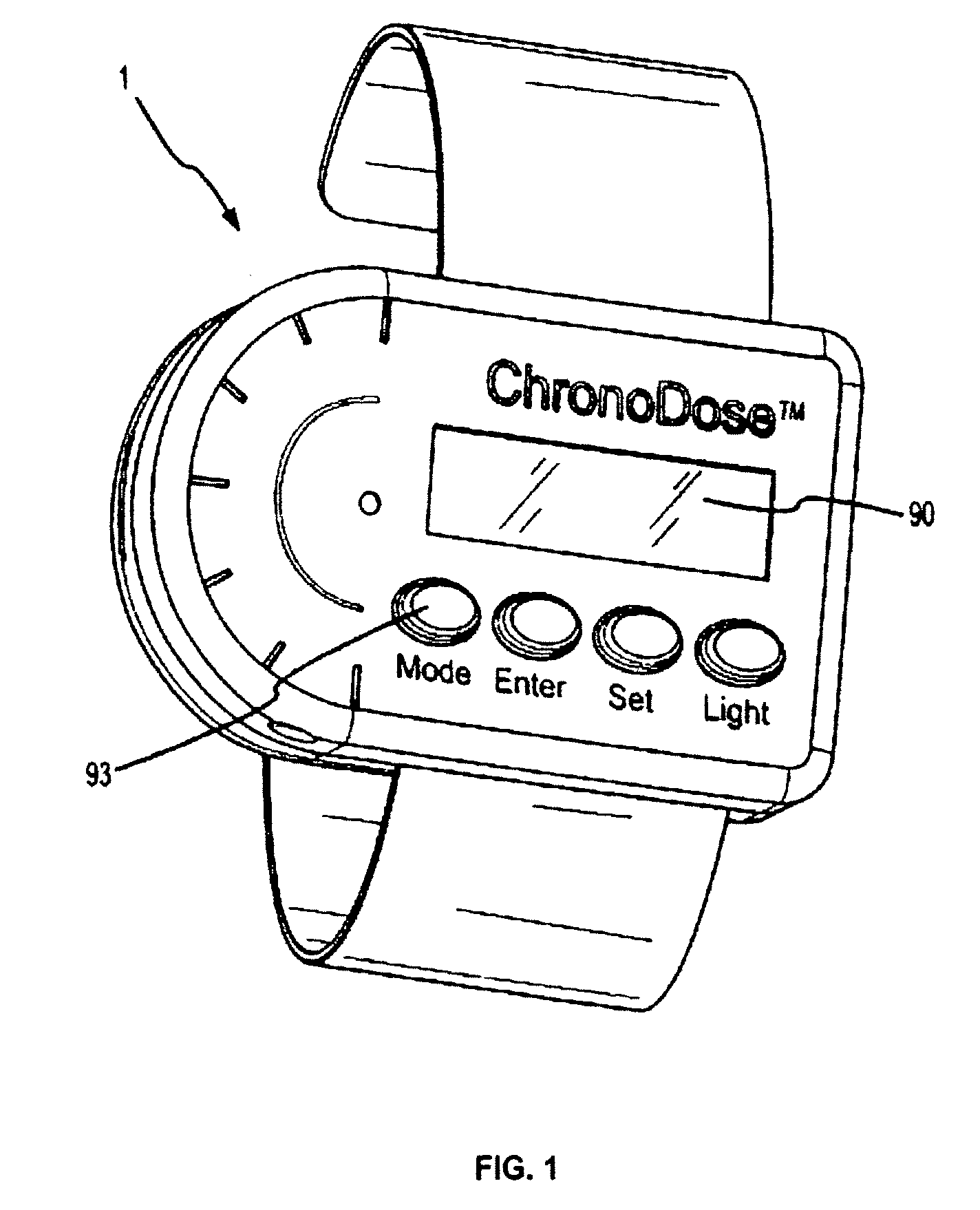
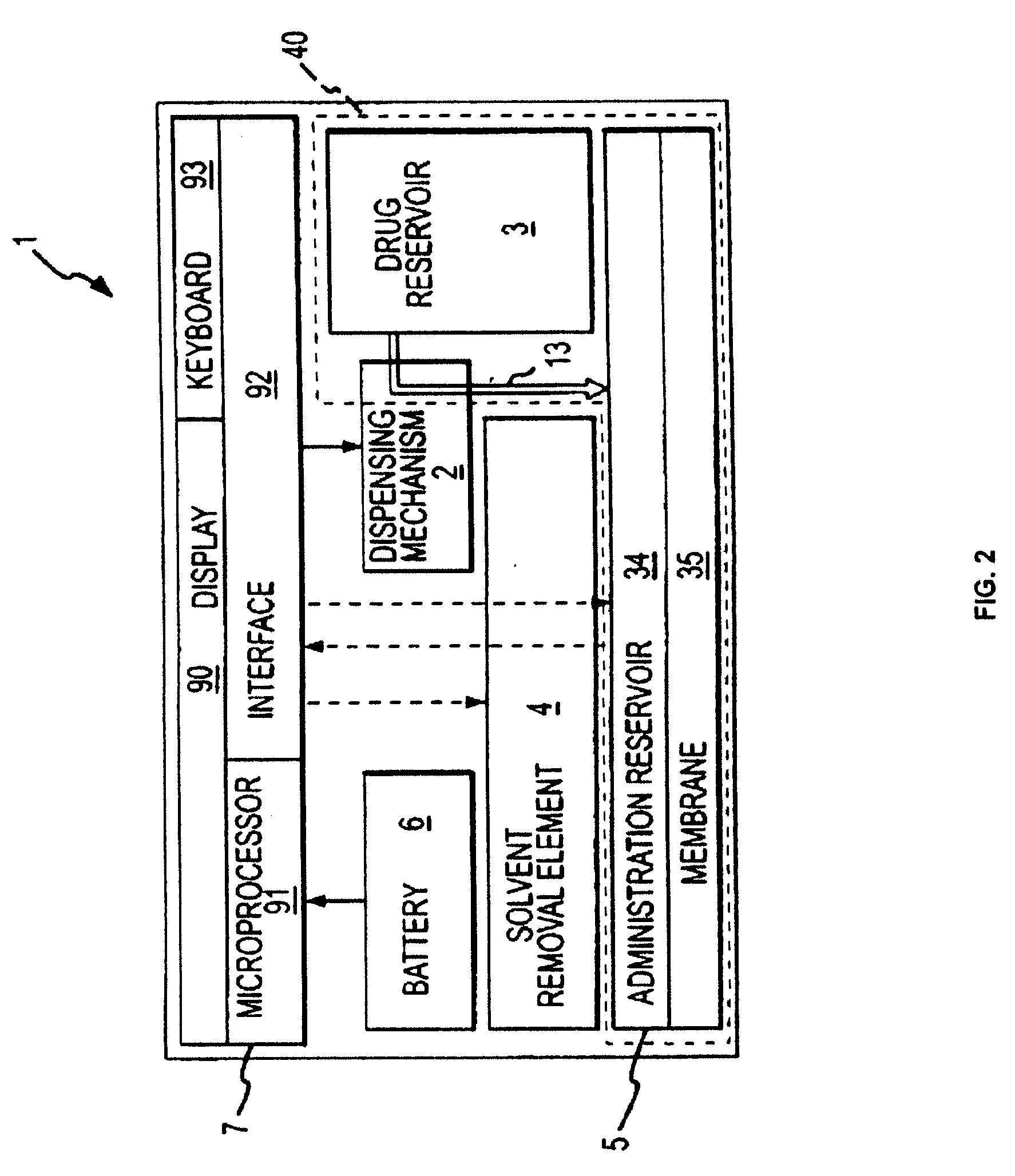
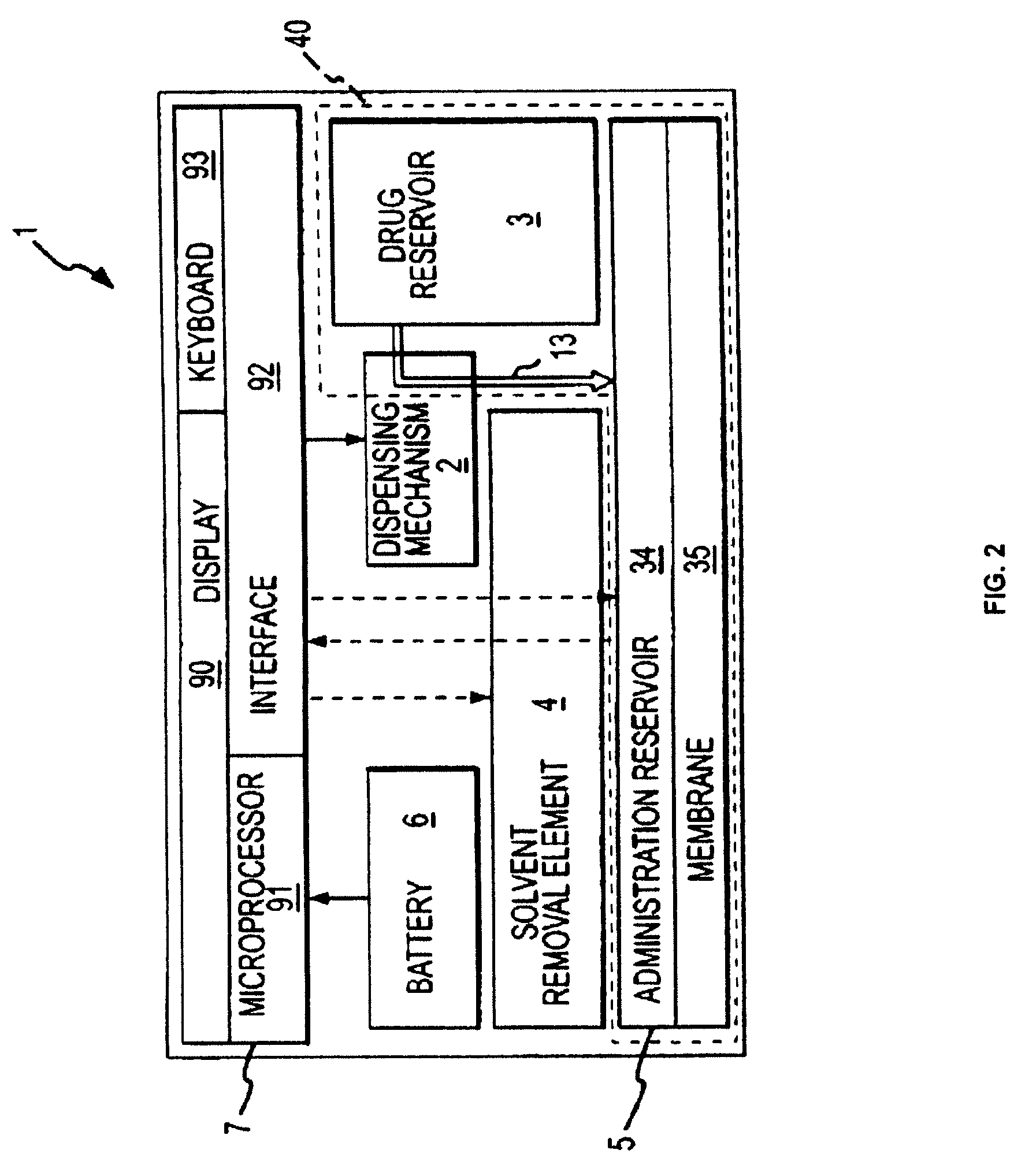
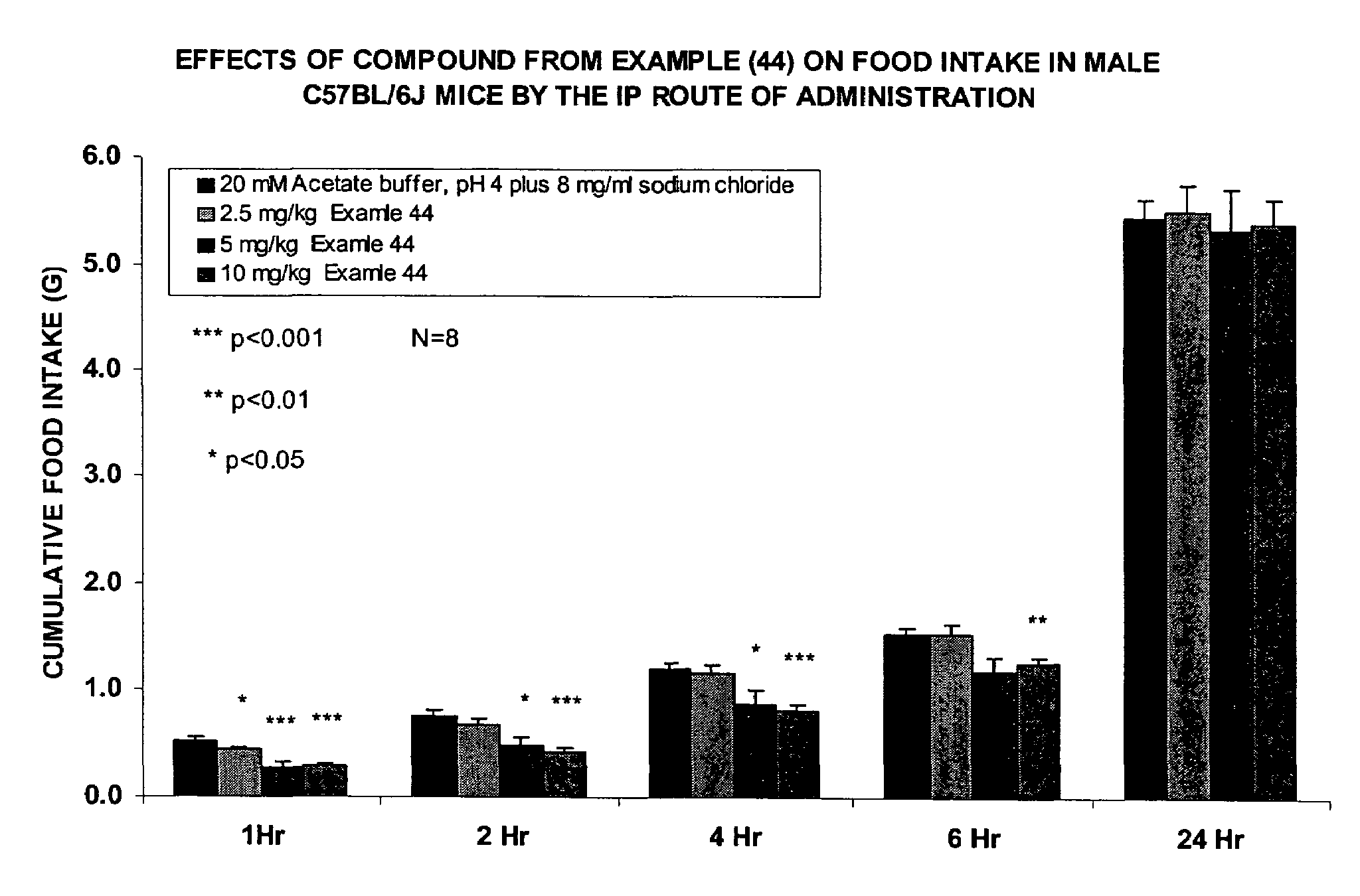
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
Methods for diagnosing and treating dysautonomia and other dysautonomic conditions
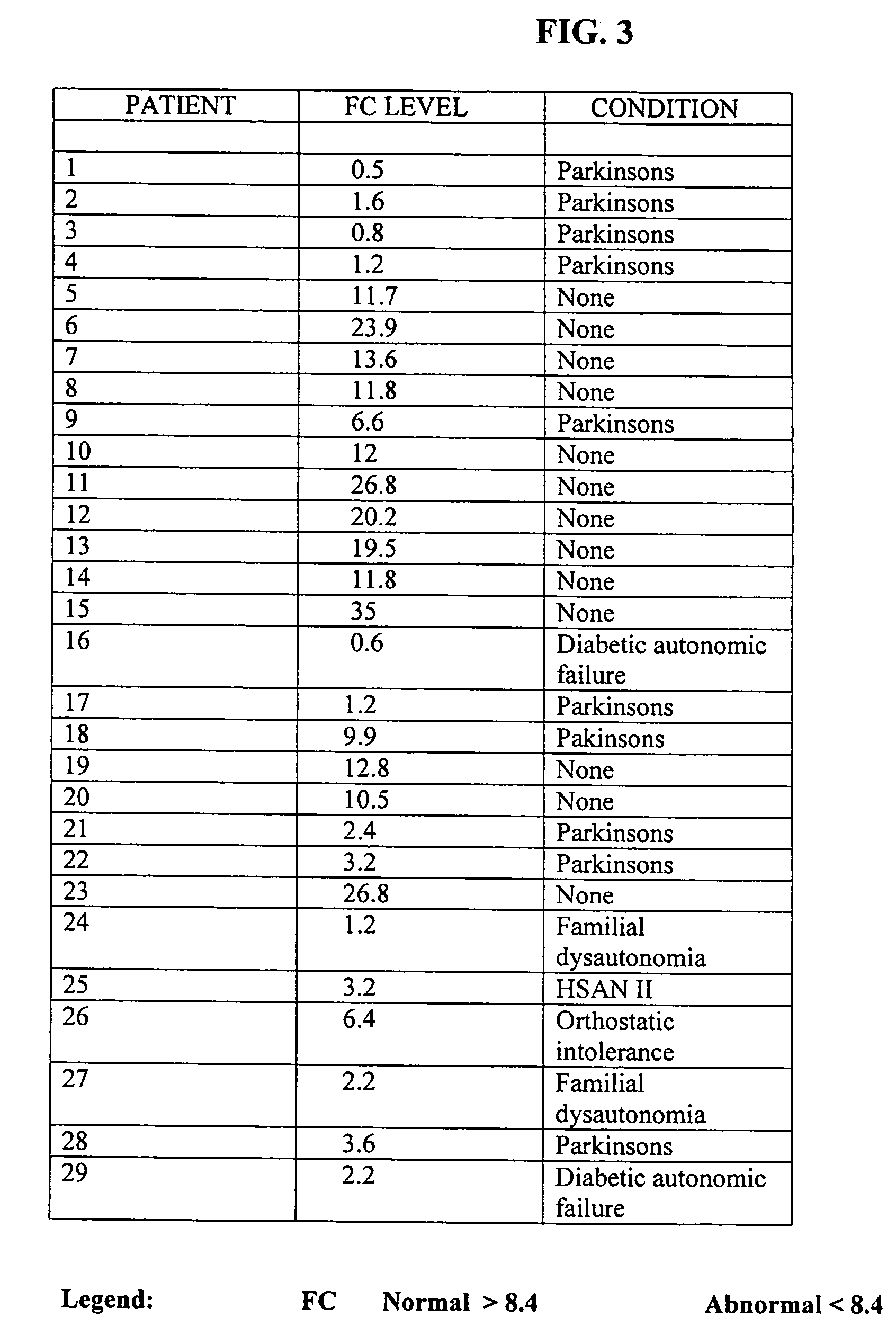
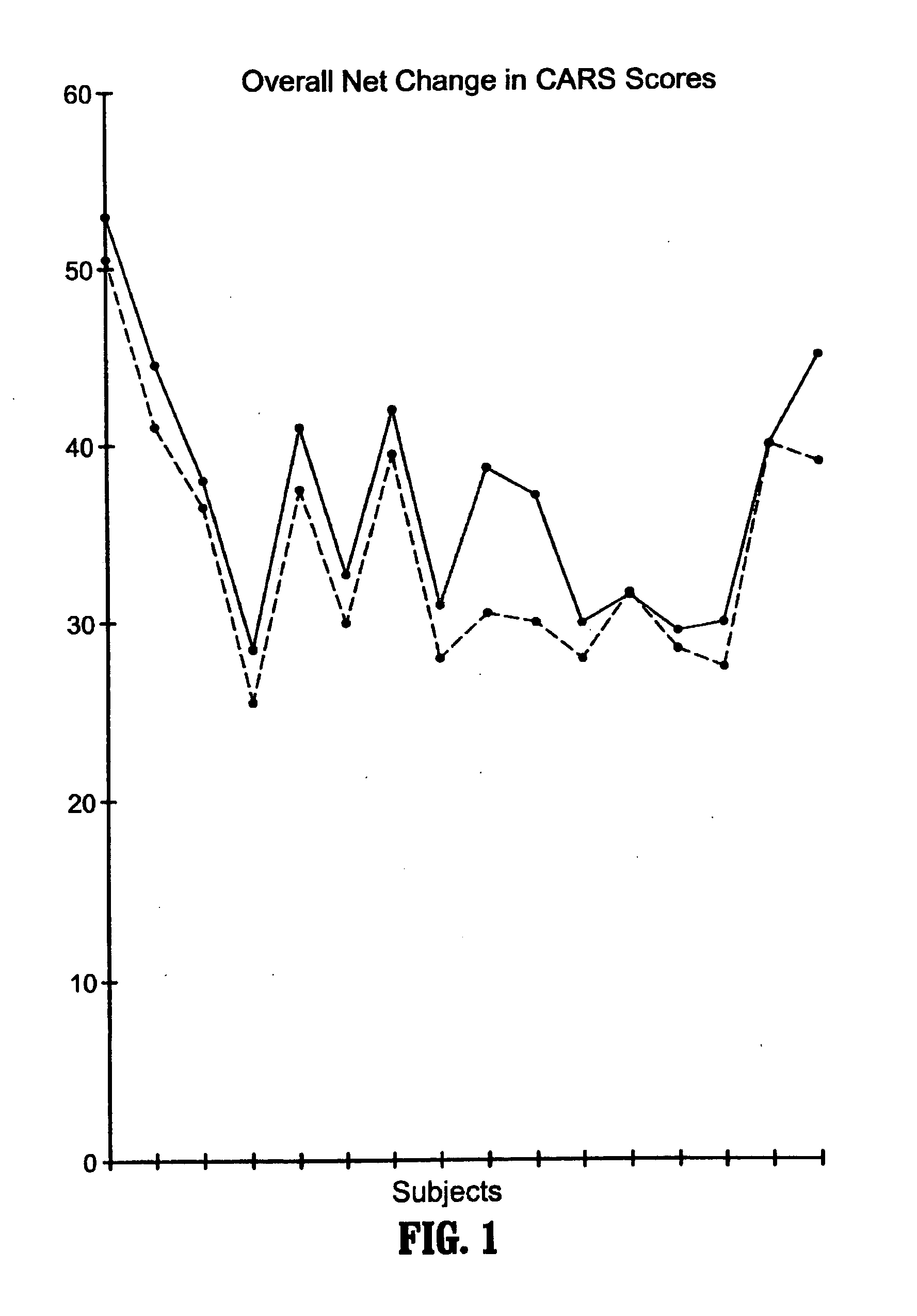
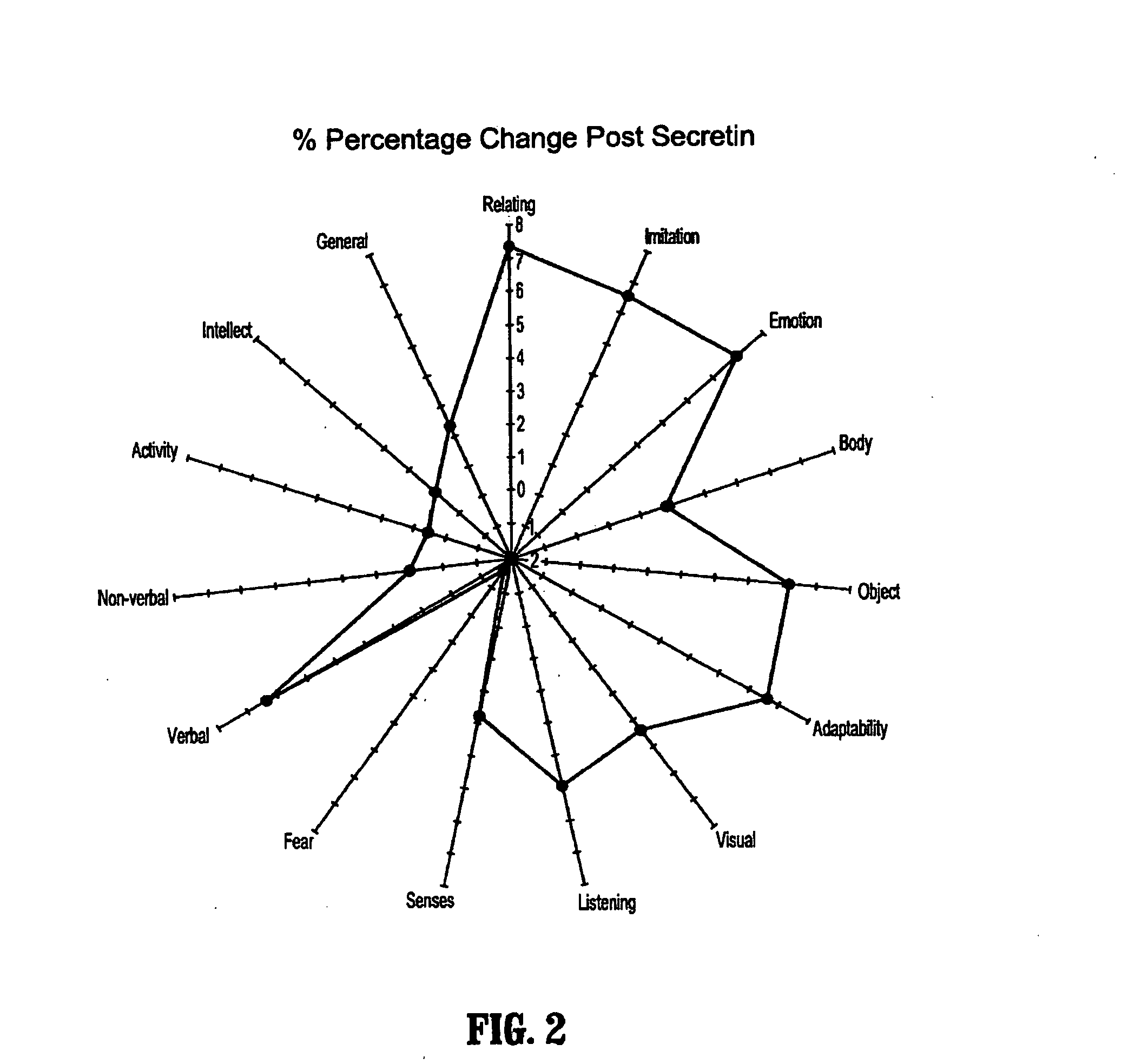
Methods for aiding in the diagnosis of dysautonomic disorders and dysautonomic conditions and methods for treating individuals diagnosed as having a dysautonomic disorder or a dysautonomic condition. In one aspect, a diagnosis method comprising analyzing a stool sample of an individual for the presence of a biological marker wherein the quantity of the biological marker is an indication of whether the individual has, or can develop, a dysautonomic disorder or dysautonomic condition, as well as a therapeutic method for treating a dysautonomic disorder or dysautonomic condition by administration of, e.g., secretin, neuropeptides, peptides and / or digestive enzymes.
Owner:CUREMARK
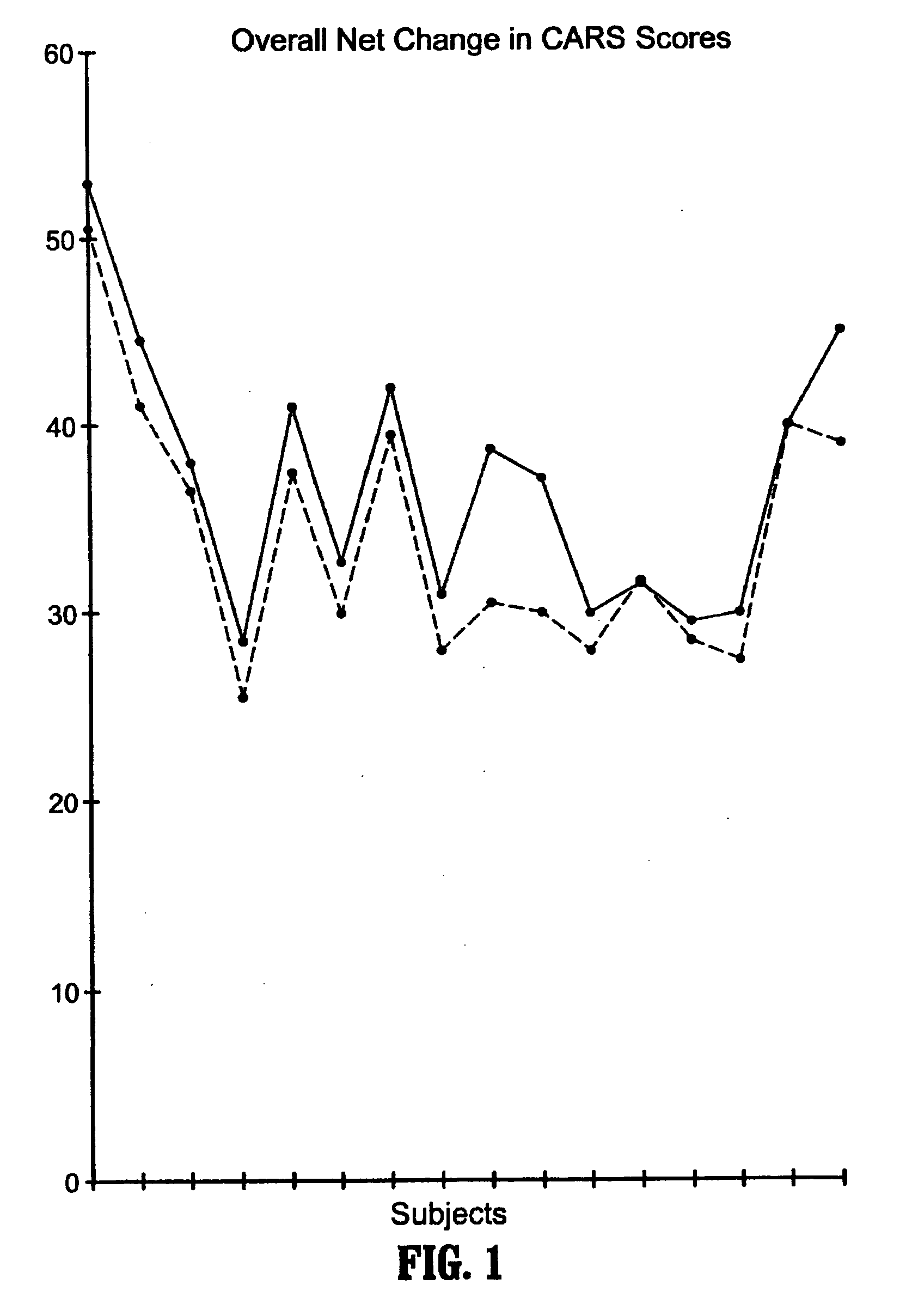
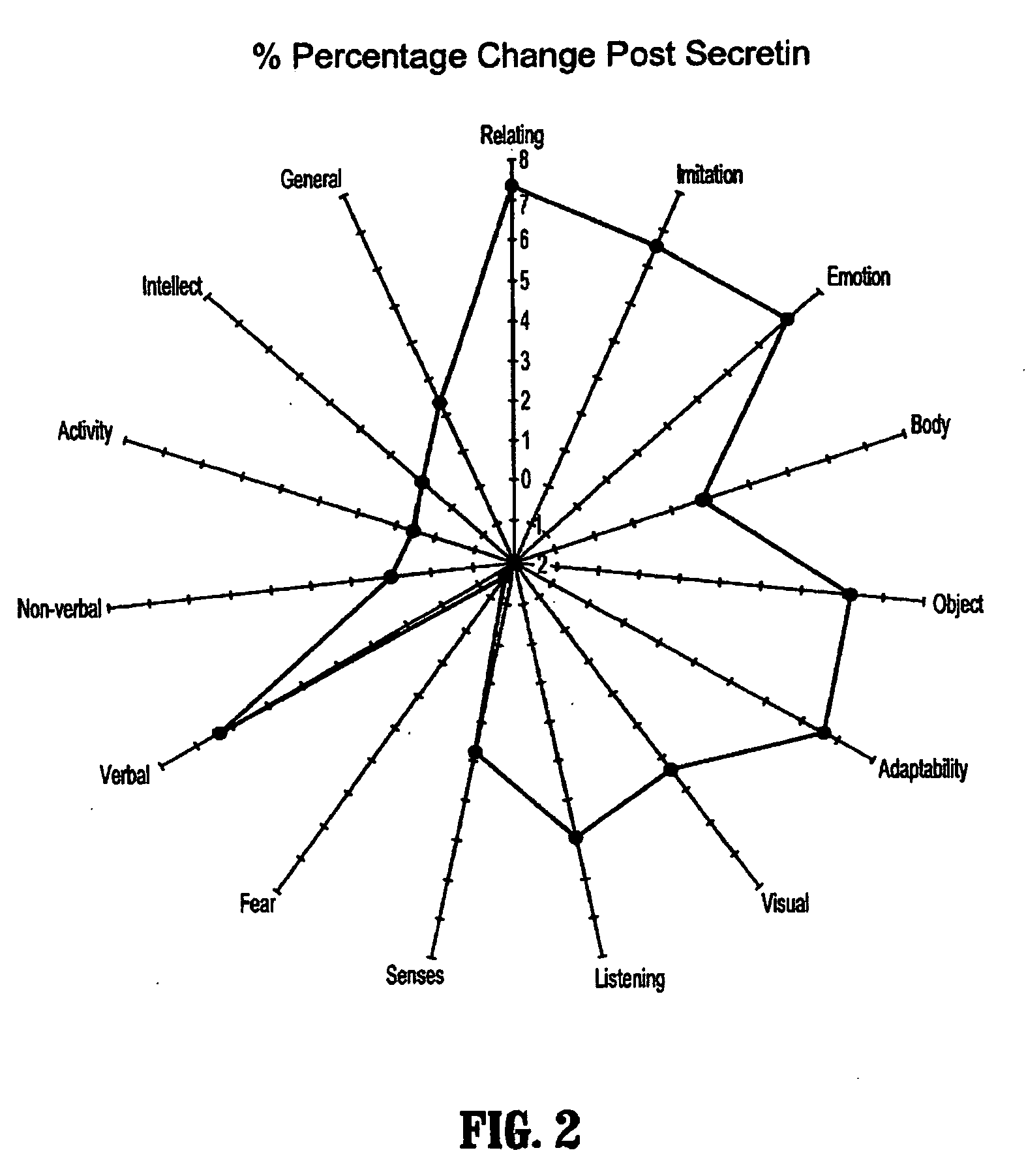
Methods for treating pervasive development disorders
InactiveUS20060183180A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
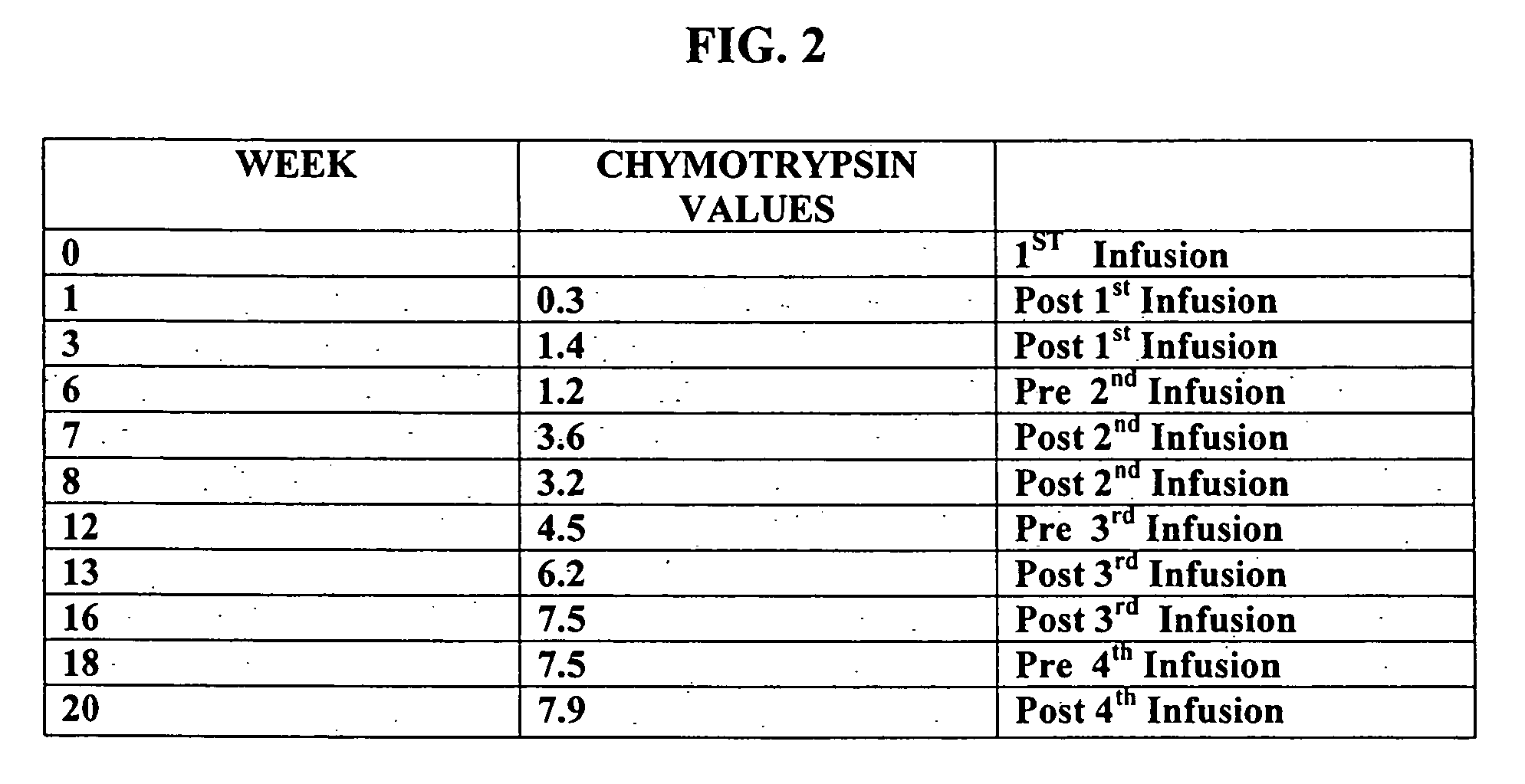
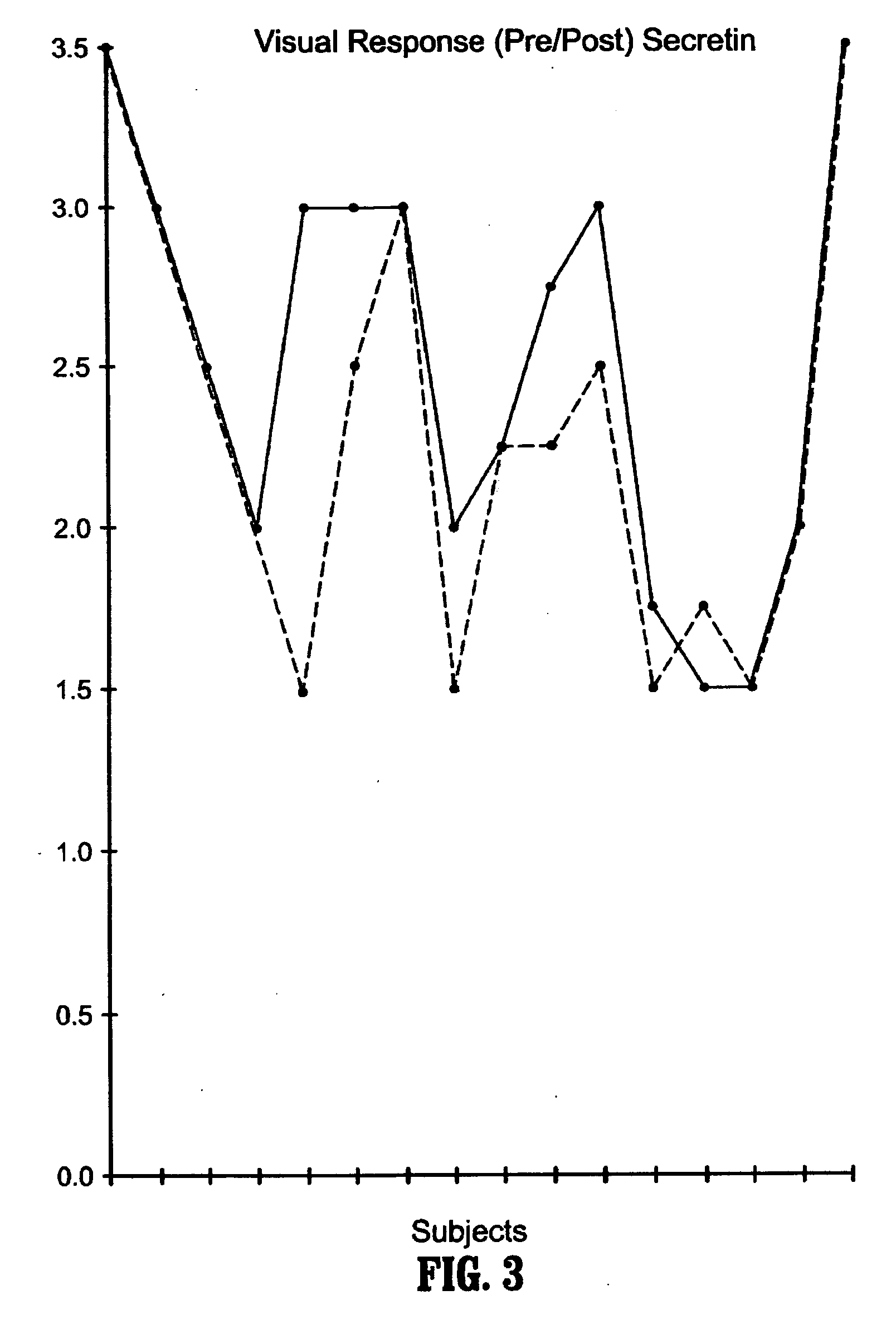
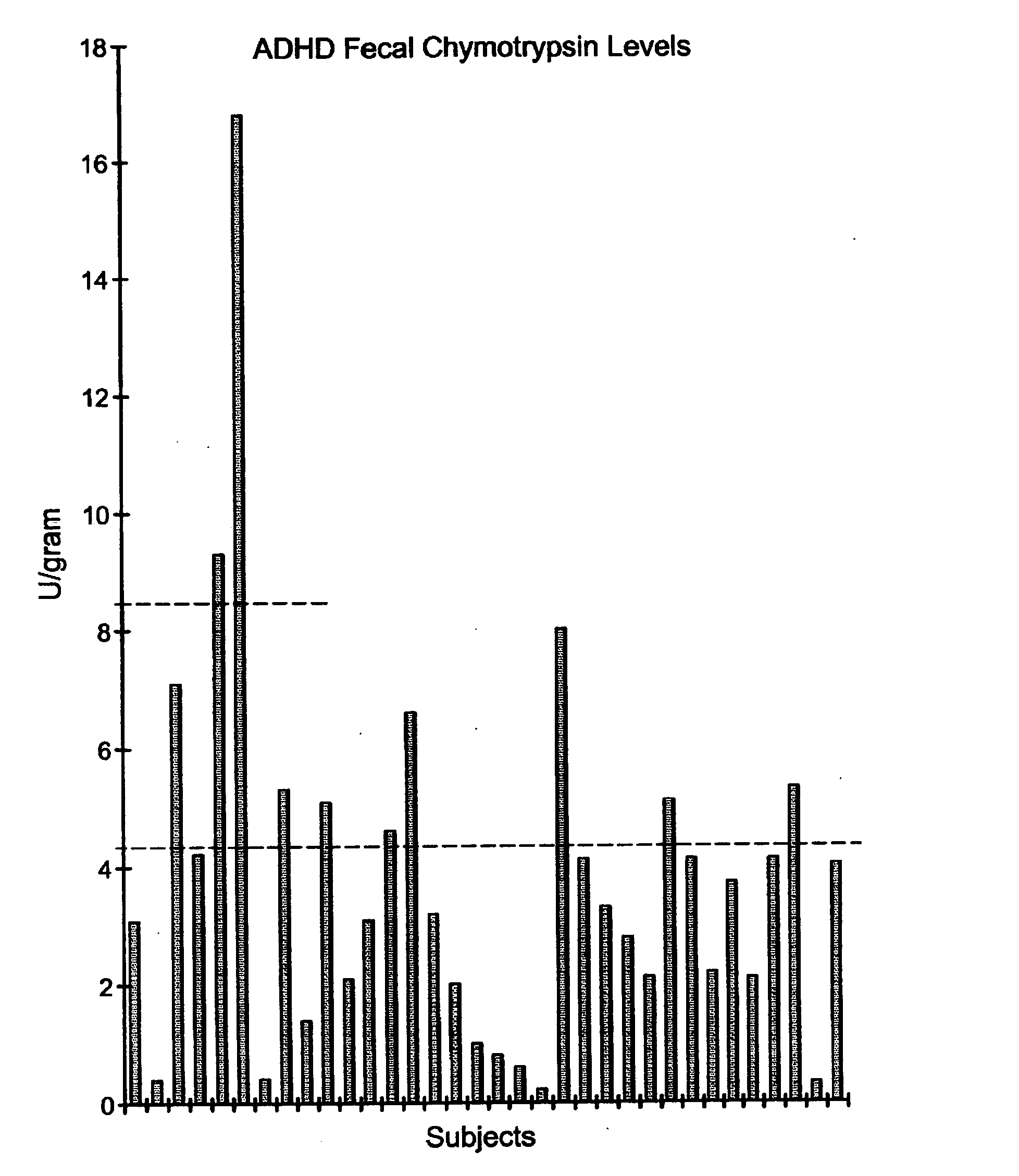
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods for treating pervasive development disorders
InactiveUS20060182728A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods of treating pervasive development disorders
InactiveUS20020090653A1Symptoms improvedPromote digestionPeptide/protein ingredientsMicrobiological testing/measurementDiseaseFeces
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADD, ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with a PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
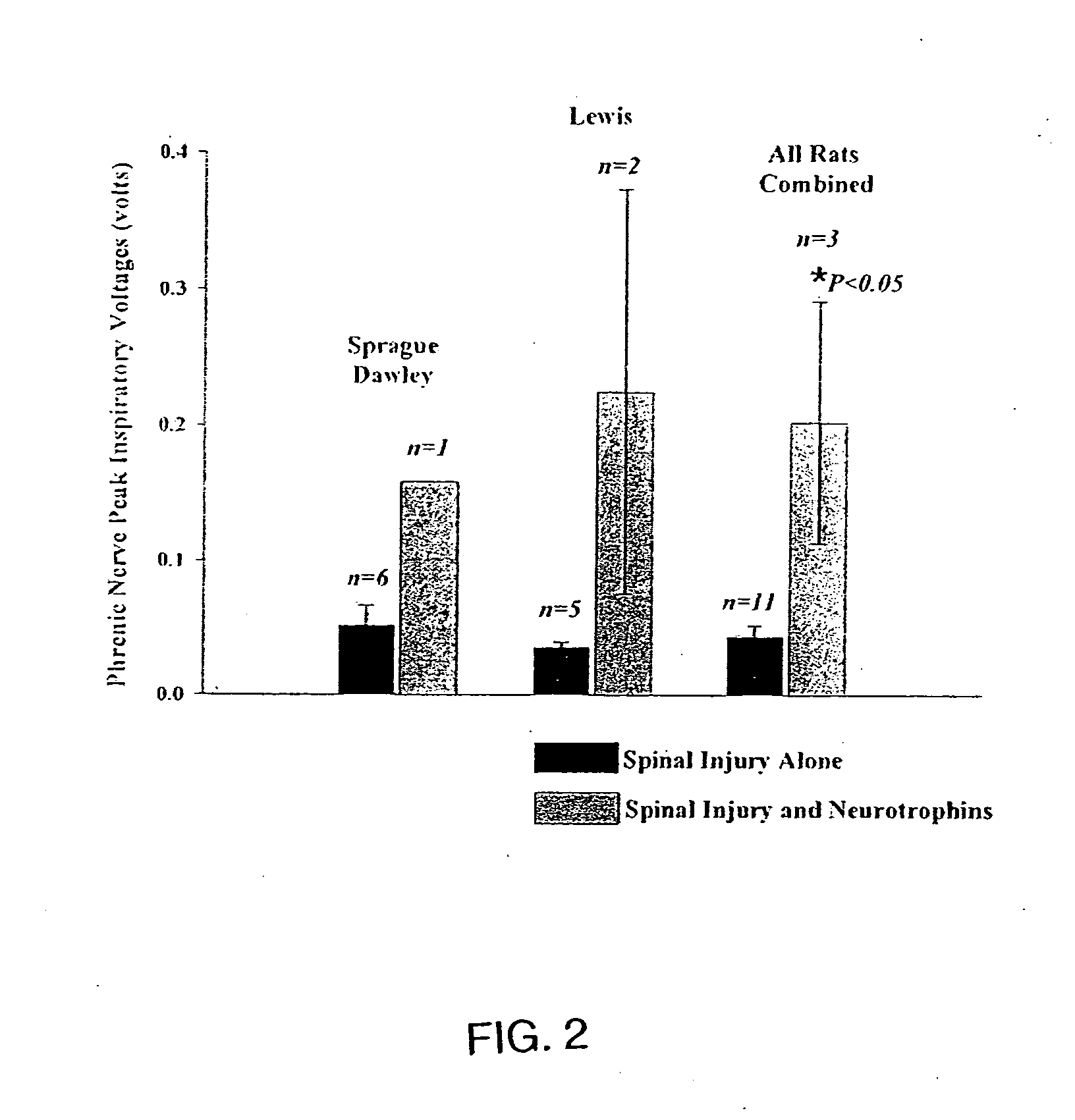
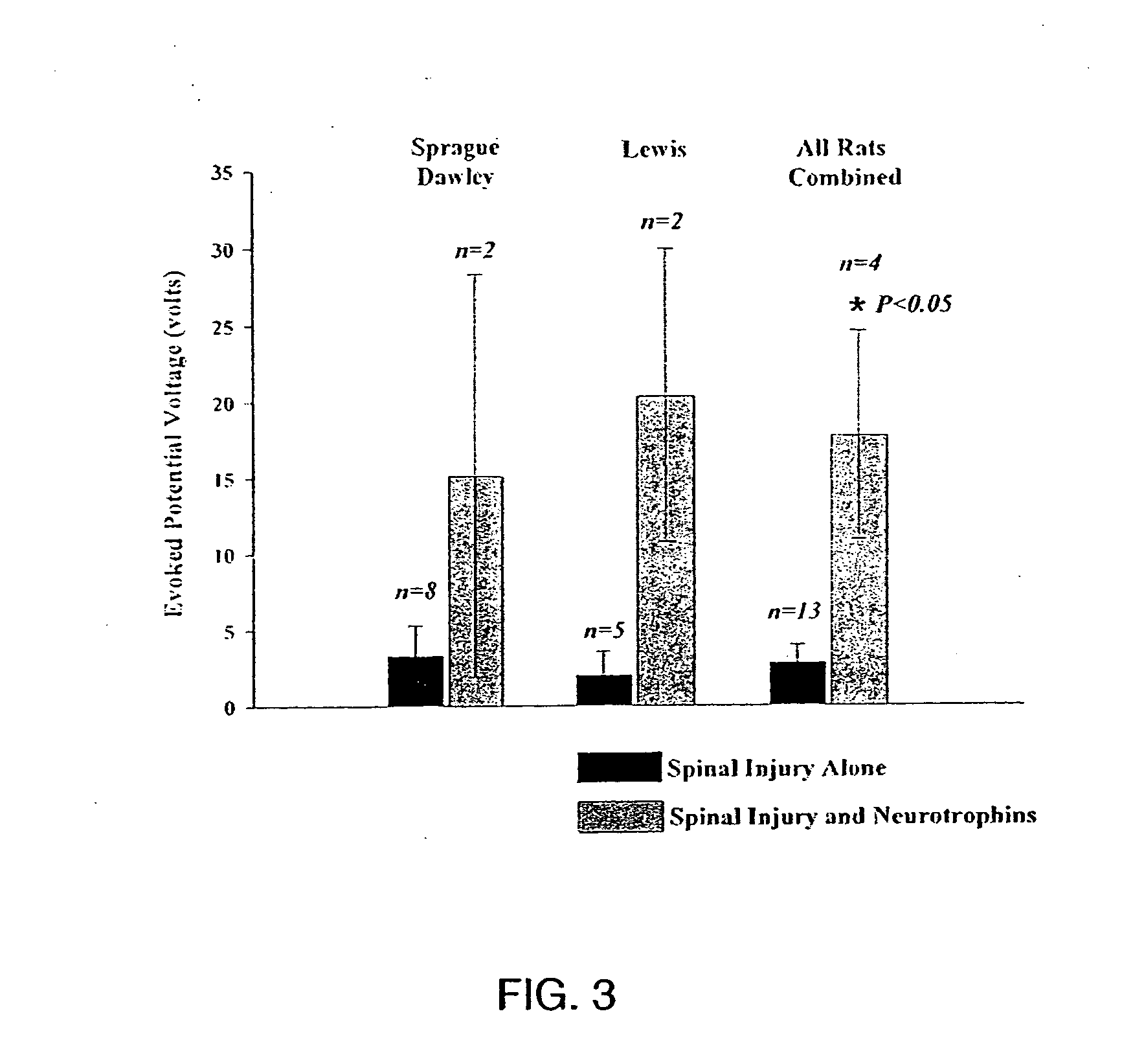
Trophic factor combinations for nervous system treatment
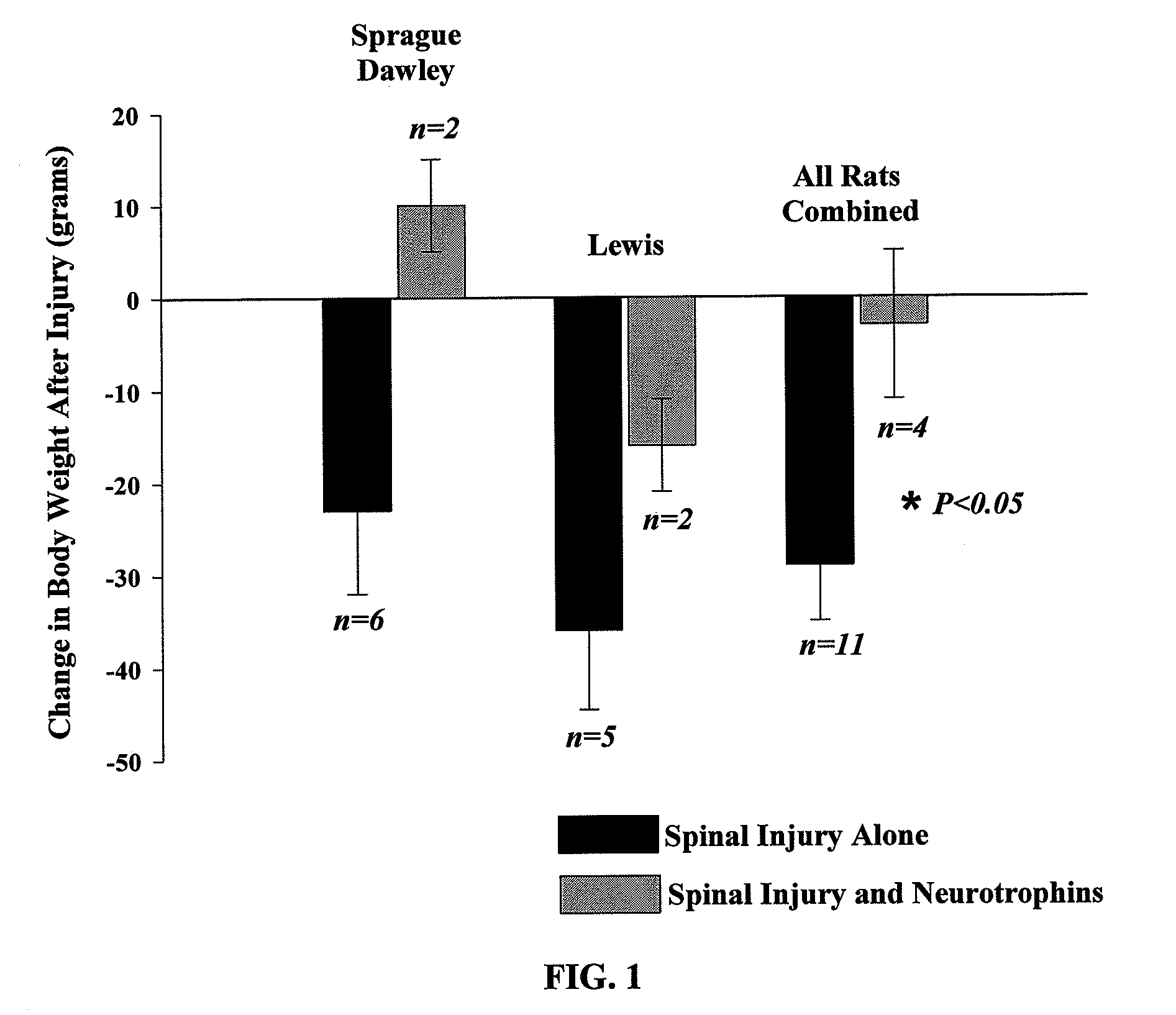
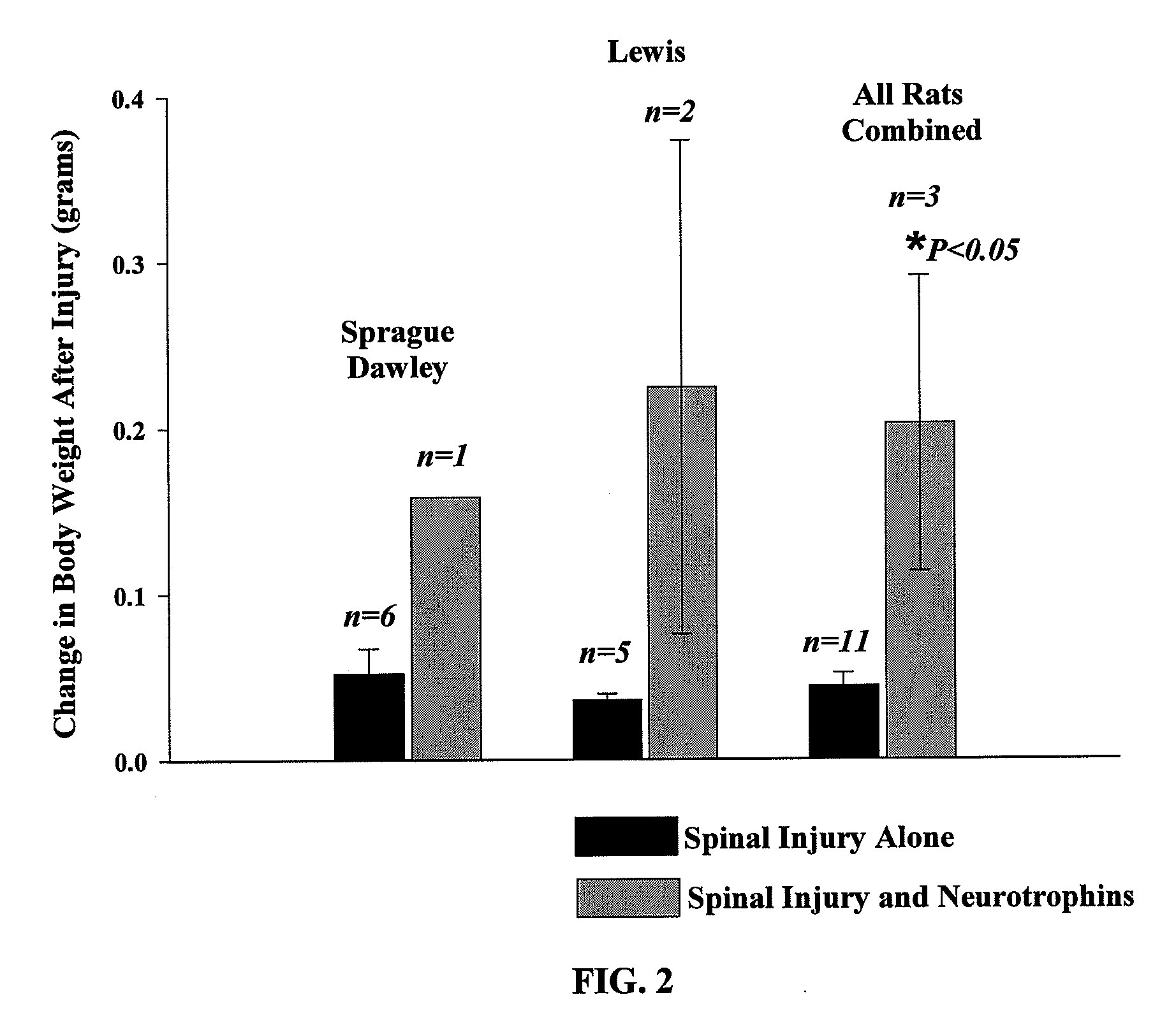
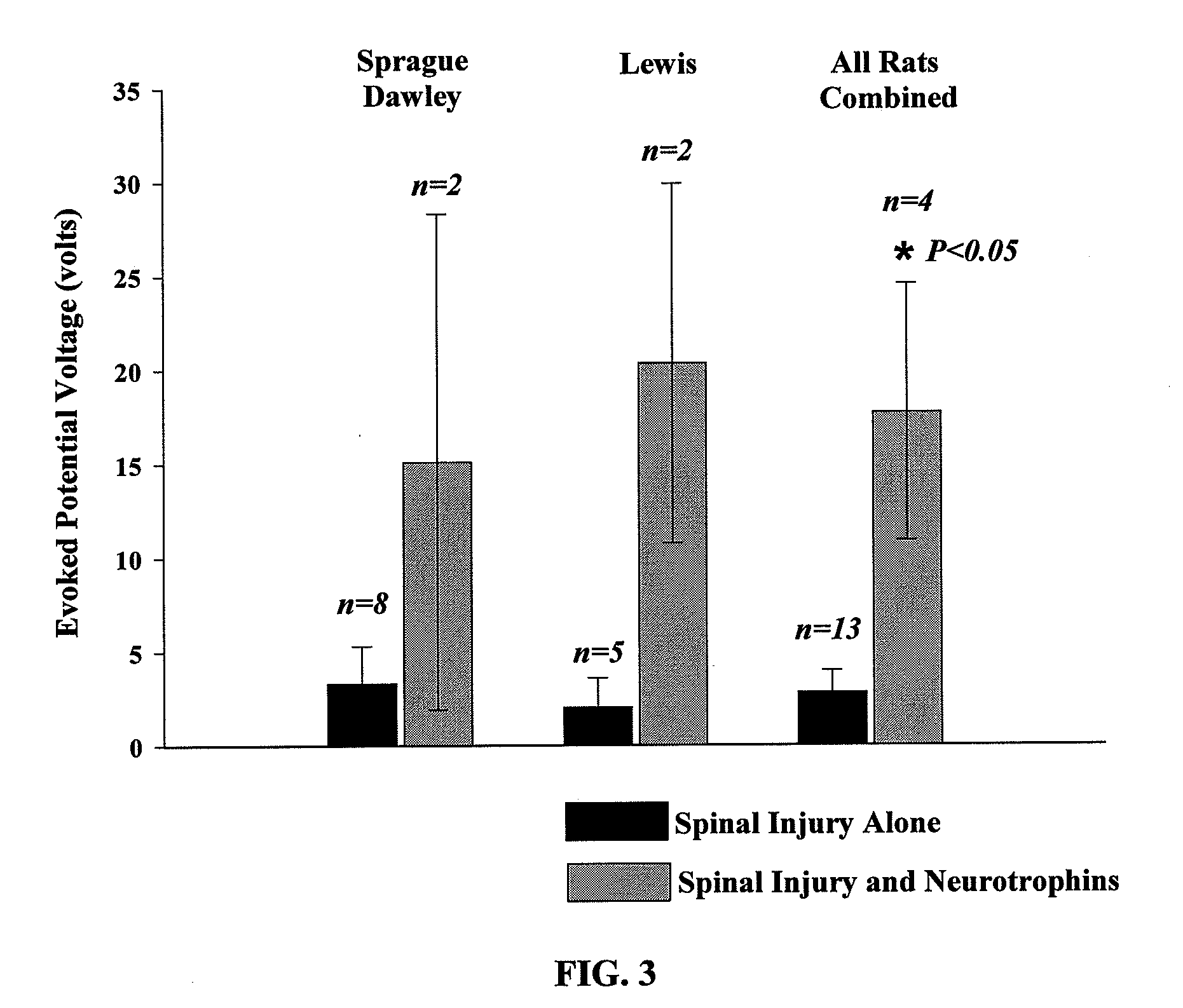
The present invention relates to a composition including an effective amount of at least one of an antimicrobial peptide and a substance having an antimicrobial peptide effect and an effective amount of a neurotrophin. The composition can also include an effective amount of at least one of a growth factor and a neuropeptide. The present invention also relates a method of treating an injury to a nervous system of an animal that includes the steps of identifying the injury to the nervous system and applying to the injury an effective amount of at least one of antimicrobial peptide and a substance having an antimicrobial peptide effect. The method can also include applying an effective amount of one or more trophic factors selected from the group consisting of a growth factor, a neurotrophin, and a neuropeptide to the injury.
Owner:WISCONSIN ALUMNI RES FOUND
Treatment of male sexual dysfunction
InactiveUS20030119714A1Reducing and eliminating prospectDrop in blood pressureBiocideDisease diagnosisNPY-Y1 receptorMale genitalia
The use of an inhibitor of a neuropeptide Y (NPY), preferably of a NPY Y1 receptor, which inhibitor is selective for an NPY or NPY Y1 receptor associated with male genitalia, in the preparation / manufacture of a medicament for the treatment or prevention of male erectile dysfunction (MED).
Owner:PFIZER INC
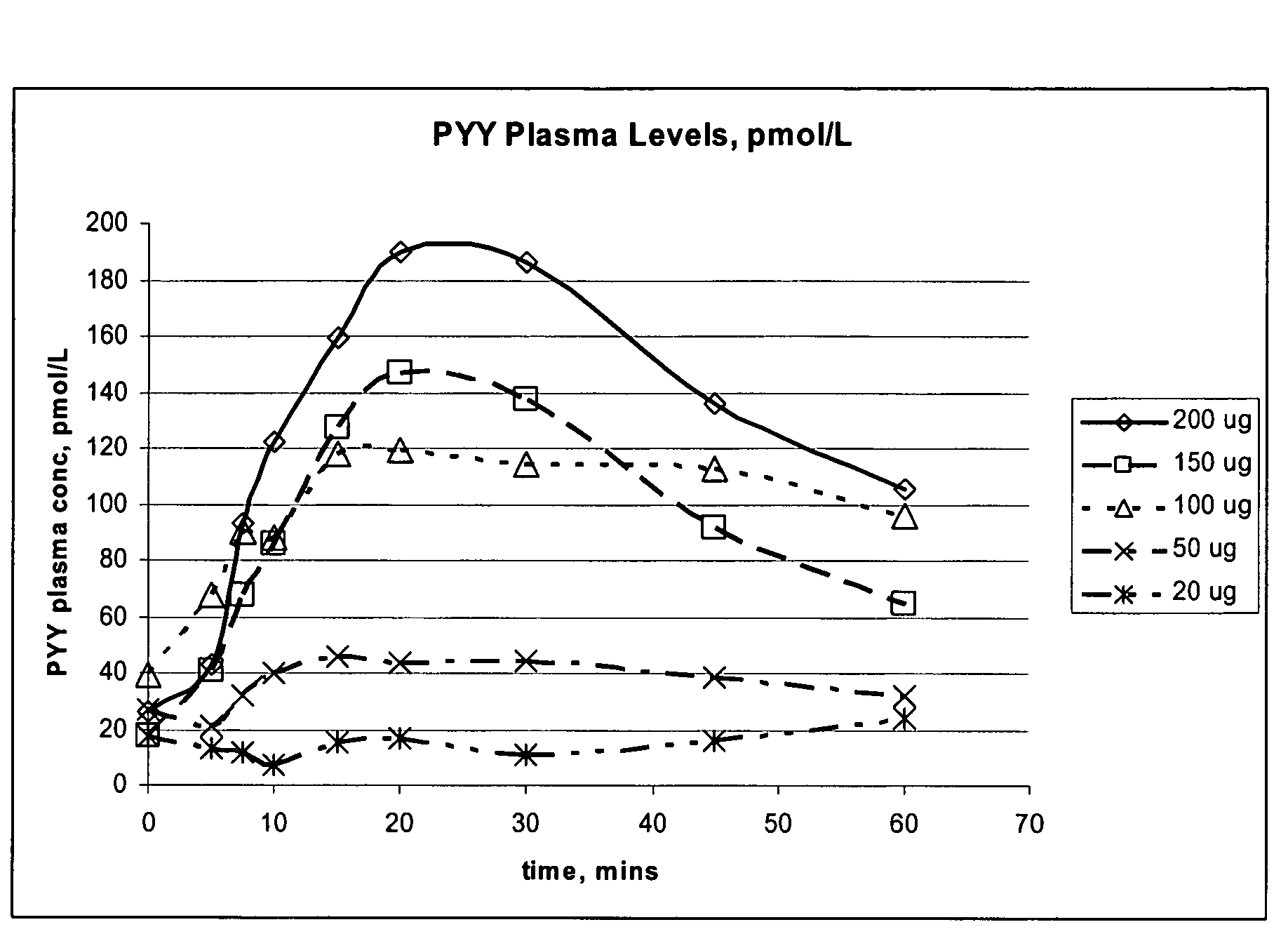
Compositions and methods for enhanced mucosal delivery of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7157426B2PromotePrevent and cure diabetesBiocideNervous disorderBinding peptideDense Core Vesicles
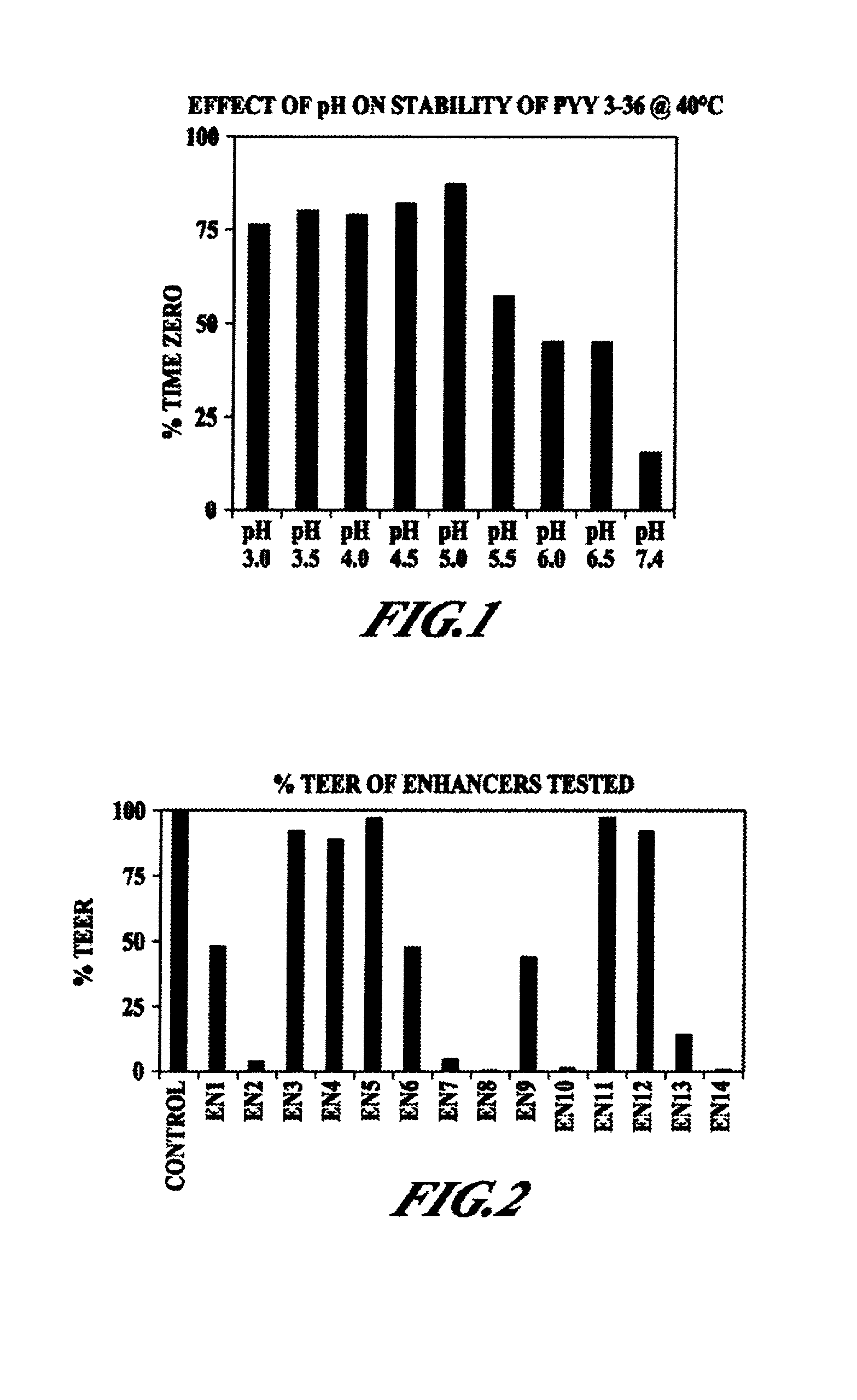
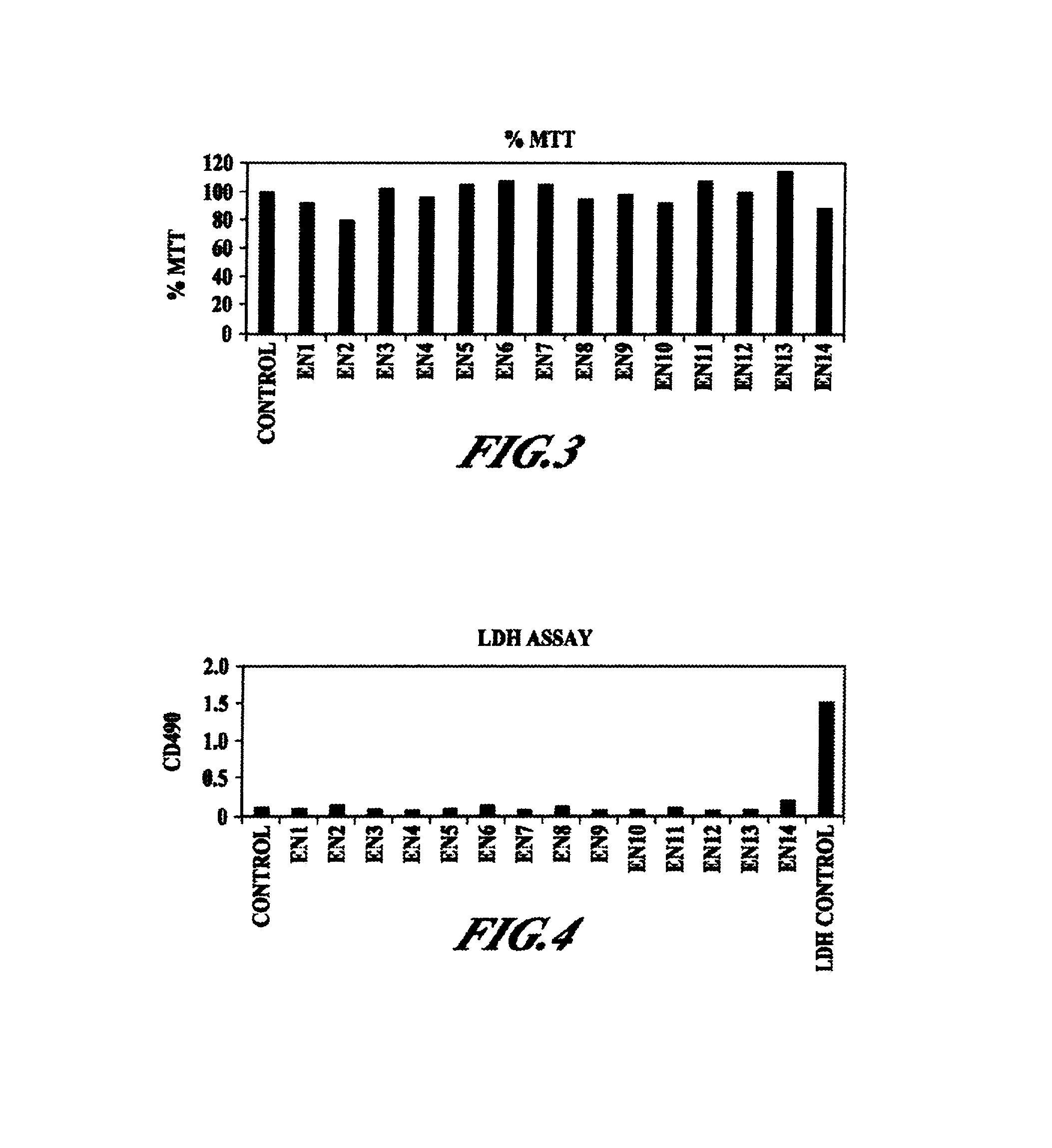
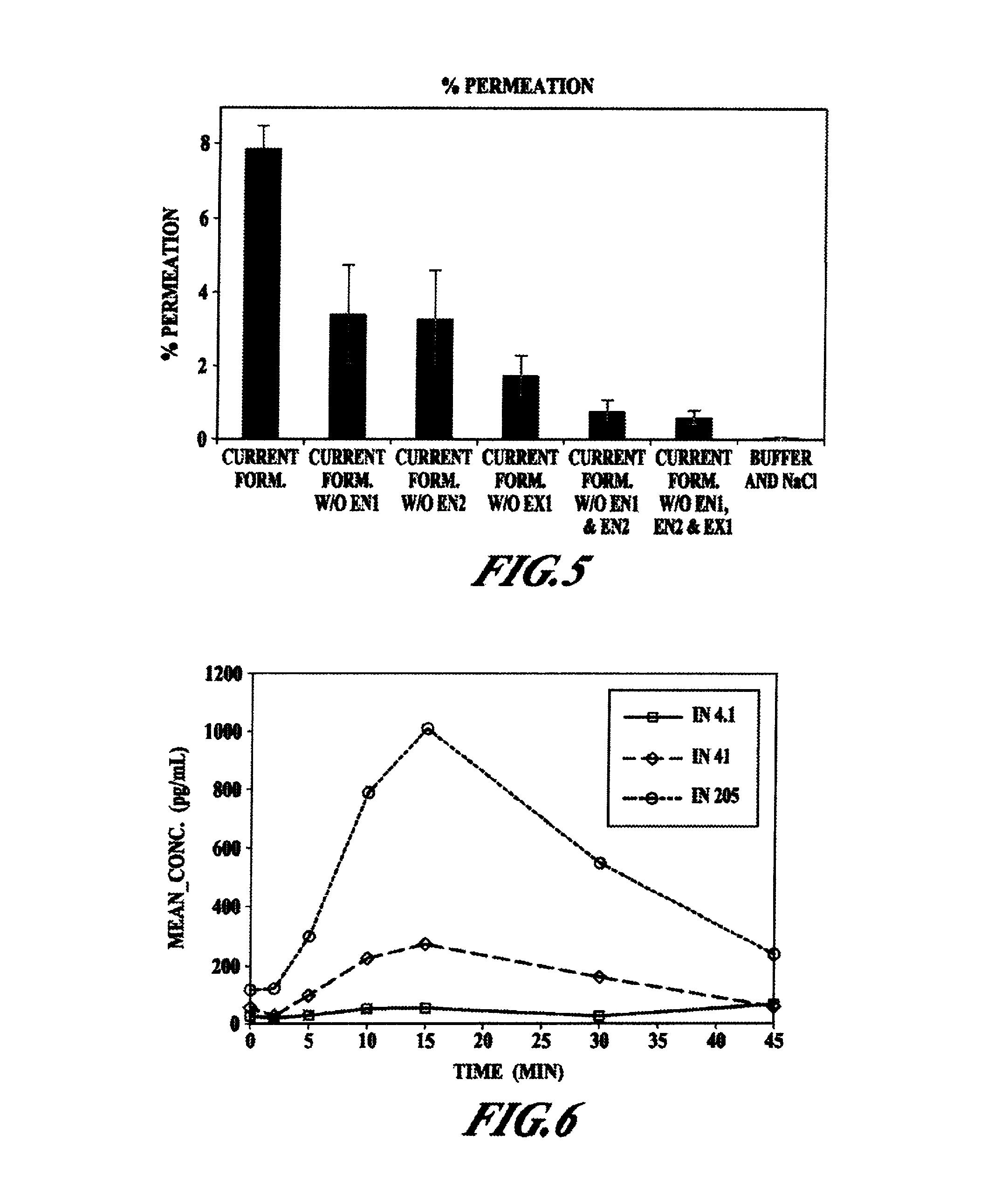
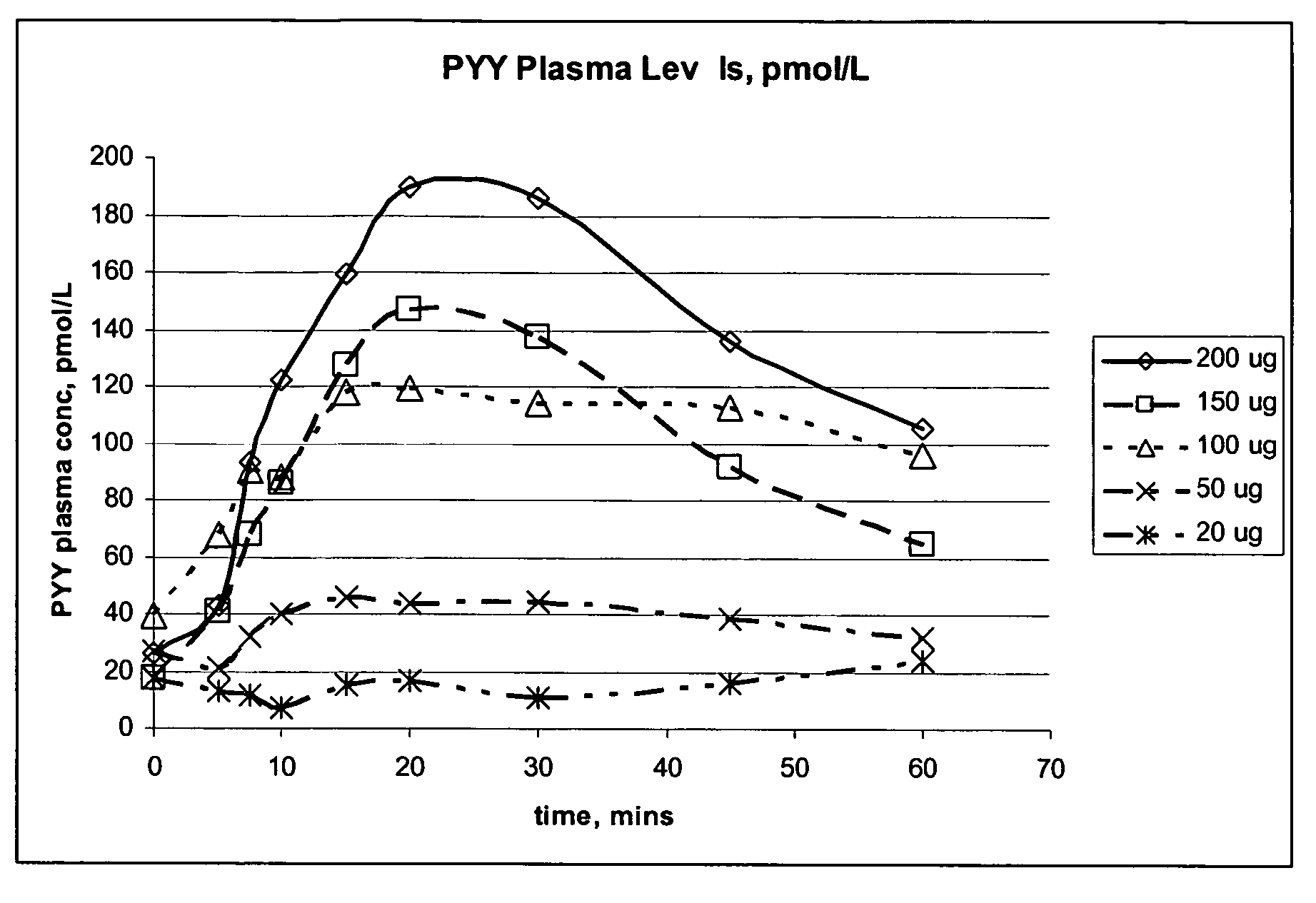
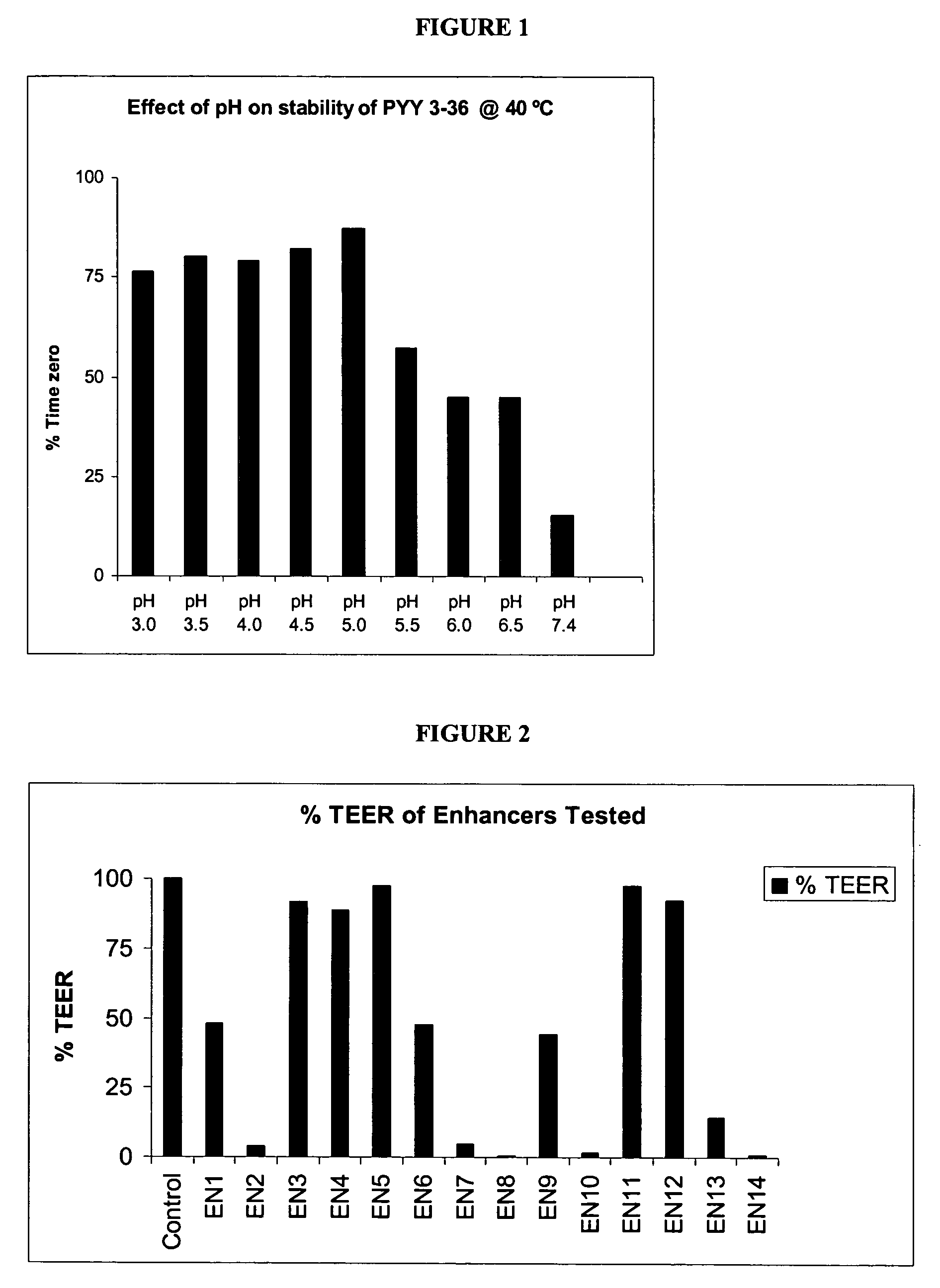
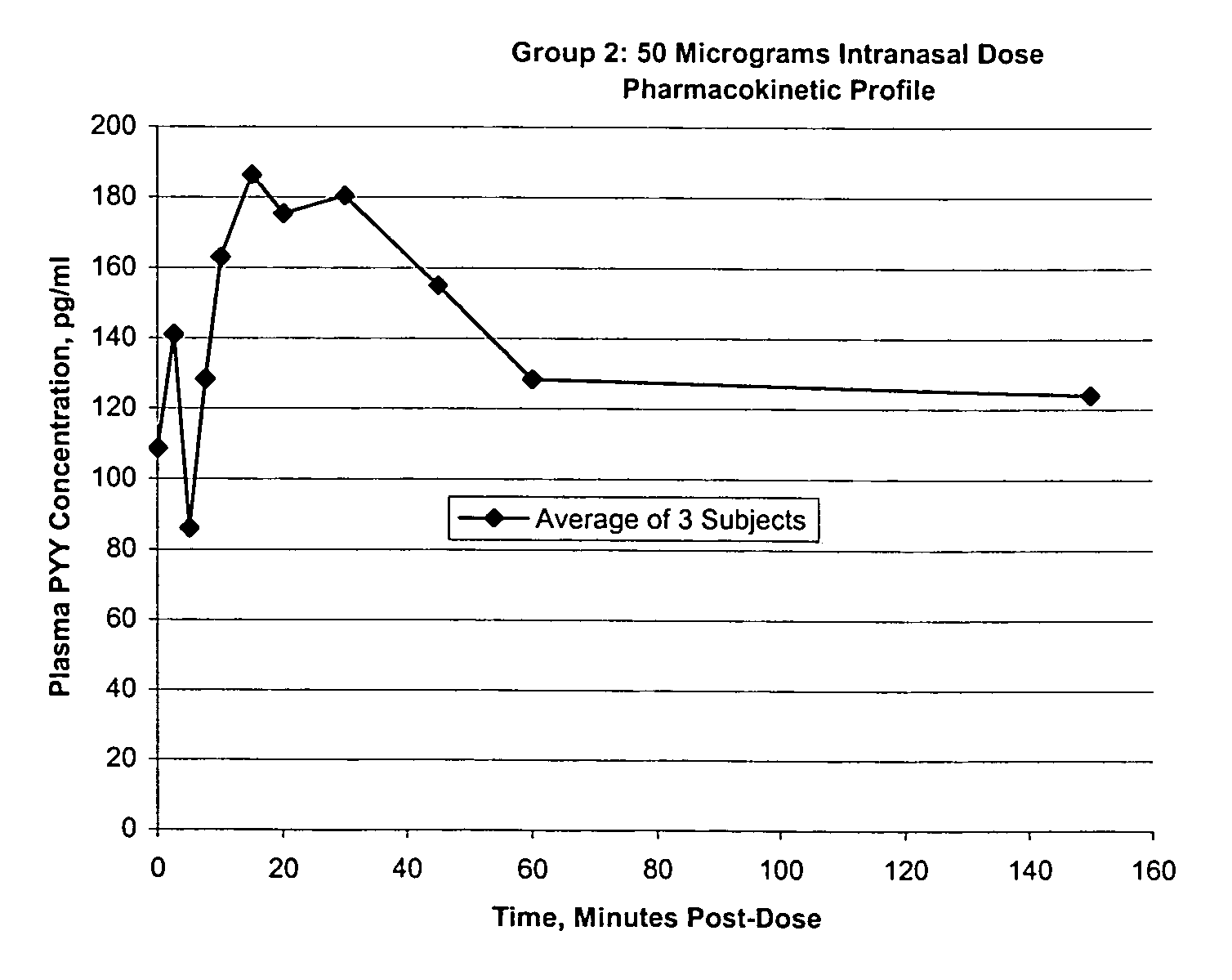
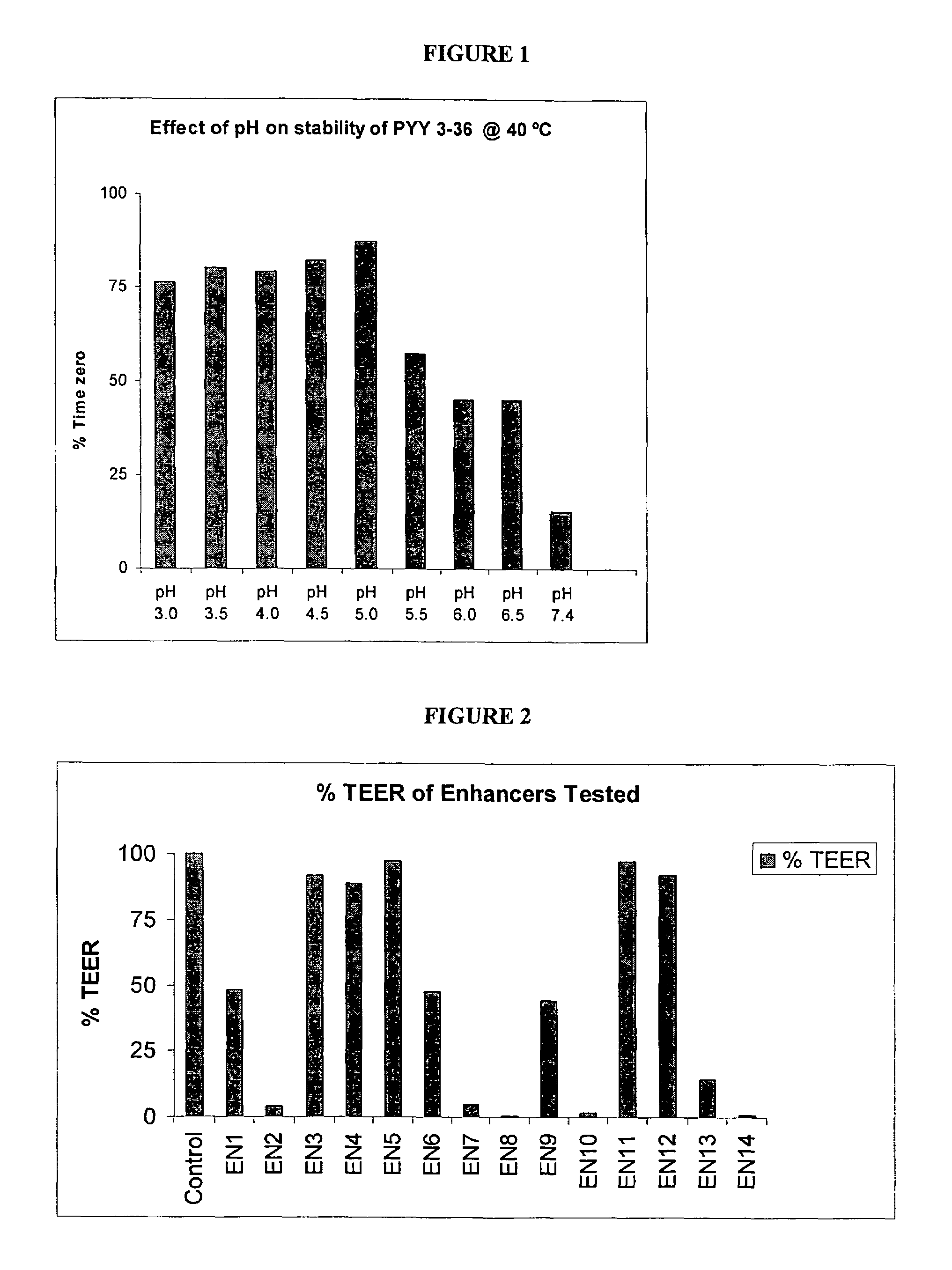
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Method and apparatus for stimulating the neurochemistry of the brain resulting in increased overall brain function, cognitive performance, and intelligence quota
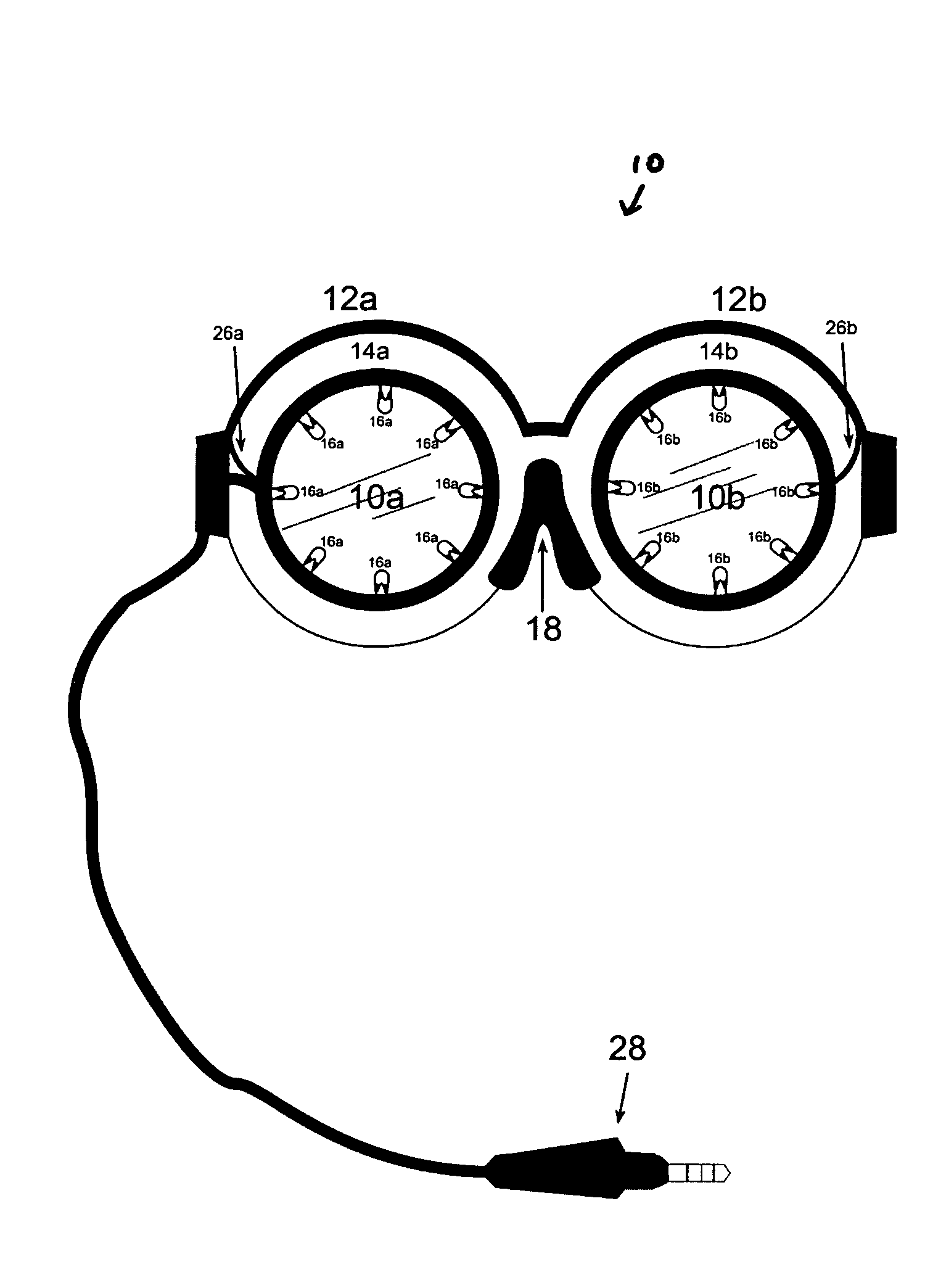
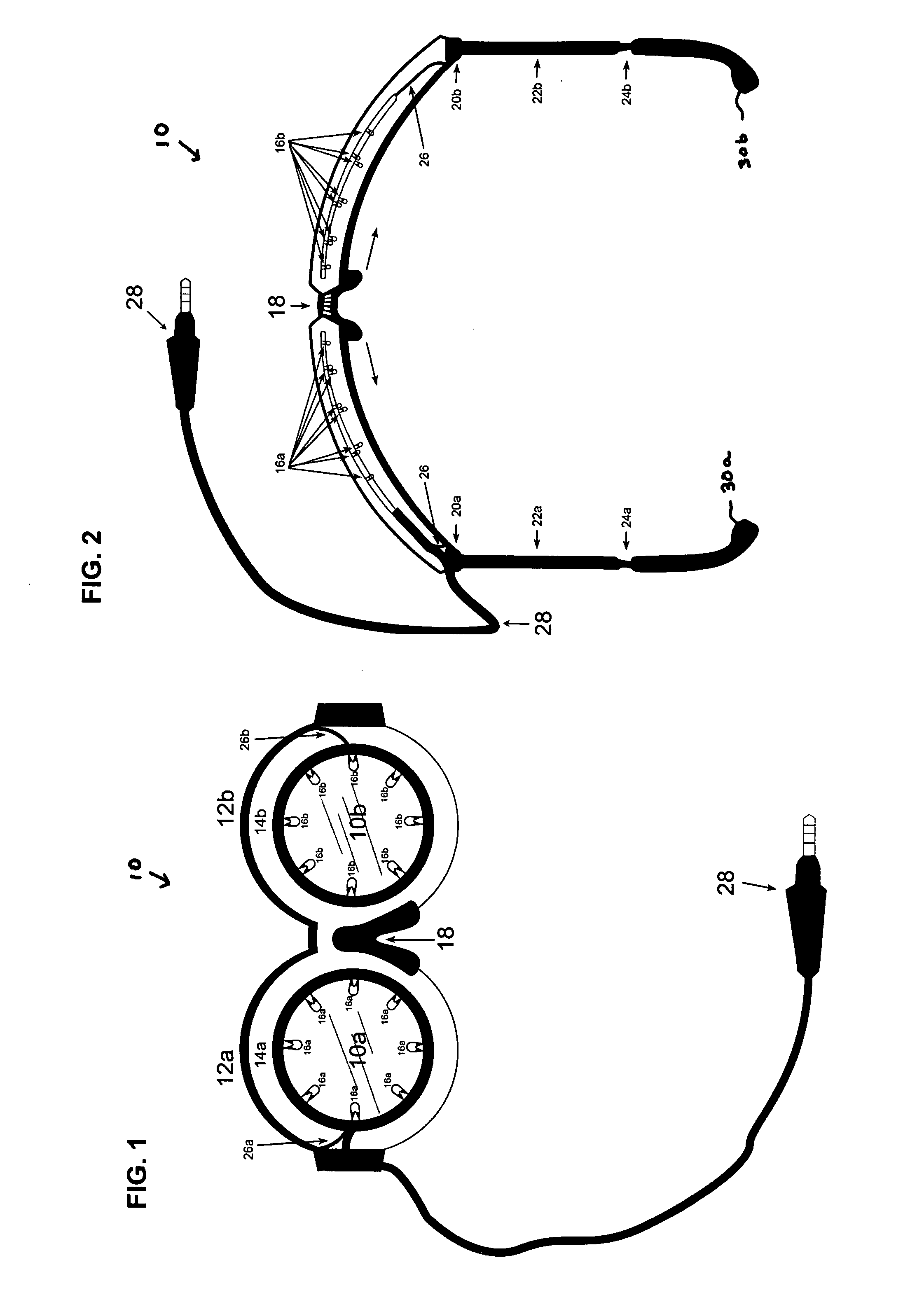
An apparatus and method for stimulating the neurochemistry of the brain including enzymes, nucleosides, nucleotides, neuropeptides, neurotransmitters, amino acids, brain glucose, steroids, and hormones, and more specifically, an apparatus and method for enhancing overall brain function, including cognitive abilities and intelligence quota (IQ). Other treatment interventions the apparatus and method can positively affect include neurological, physiological, and psychological disorders and diseases. The inventive method utilizes a specially designed goggle which contains two round eye pieces to which are attached the light assemblies. These light assemblies are comprised of 1-20 white full spectrum lights which are directed toward both eyes, within a two inch radius of the retina to avoid overstimulation. The goggle has an expandable nose piece with a mechanism to allow the eye pieces to swing outward and adjust over the eye of any individual, in accordance with the specified retinal placement. The inset lights flash or flicker utilizing intermittent “fuzzy” light, and encompass frequencies ranging from 1-1200 Hz (cycles per second), specifically excluding the range between 55-65 Hz to prevent any form of seizure. The light stimulus is accompanied by unmatched, intermittent sound stimulation, called “fuzzy” sound. The sound is audible through the use of any type headphone. The method is in the form of computerized programs, and includes a variety of formulas translated as “sessions” to be downloaded into any existing light / sound device or apparatus. These formulas are in the form of a variety of sessions, lasting no more than 40 minutes each, to address and or treat a variety of symptoms, disorders, and diseases. The sessions control the light goggle apparatus and sound. Neither the “fuzzy” light nor the “fuzzy” sound are synchronized.
Owner:OLMSTEAD RUTH
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS8252321B2Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocideDiseasePhytochemical
Owner:MORNINGSIDE VENTURE INVESTMENTS
Compositions and methods for enhanced mucosal delivery of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7229966B2Induce satiety in an individualPromote weight-loss in an individualBiocideNervous disorderDiseasePYY Peptide
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Compositions and methods for enhanced mucosal delivery of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7186691B2Induce satiety in an individualPromote weight-loss in an individualBiocideOrganic active ingredientsDiseaseBinding peptide
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Compositions for modulating growth of embryonic and adult kidney tissue and uses for treating kidney damage
InactiveUS20080090765A1Protection from damagePeptide/protein ingredientsBone-inducing factorNephronectinMammal
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Novel compositions comprising a phosphodiesterase-5 inhibitor and their use in methods of treatment
InactiveUS20130296324A1Enhance health and appearanceFacilitating accelerating healingBiocideNervous disorderDiseaseSynaptic cleft
The invention relates generally to novel pharmaceutical methods for the treatment of various conditions. Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; a serotonin-norepinephrine reuptake inhibitor; a cholinesterase inhibitor; a dopamine agonist; or a medication suitable to increase the chemical concentrations of the neurotransmitters, selected from amino acids, monoamines, neuropeptides and other agents capable of primary neurotransmission in the synaptic clefts, and their use for treating a neurodegenerative disease in a subject. The invention also relates to: Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; or a cholinesterase inhibitor, and their use for treating damaged skin in a subject.
Owner:HELD JERRY M
Modulation of central nervous system (CNS) dipeptidyl peptidase IV (DPIV) -like activity for the treatment of neurological and neuropsychological disorders
InactiveUS7132104B1Avoid rapid degradationGood effectBiocideNervous disorderHypertension medicationsNervous system
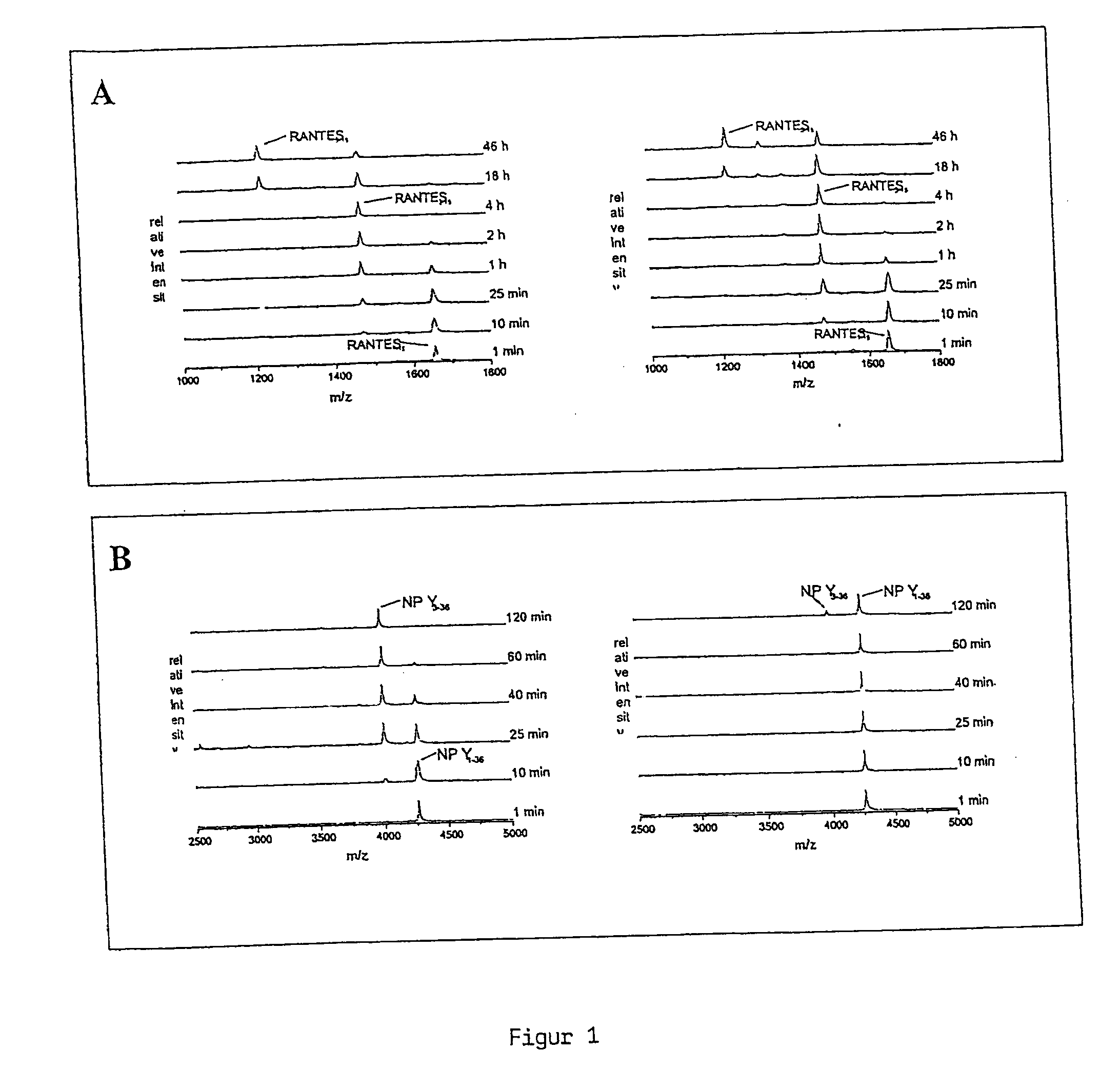
The present invention discloses a method for therapeutically treating an animal, including a human, for psychosomatic, depressive and neuropsychiatric diseases, such as anxiety, depression, insomnia, schizophrenia, epilepsy, spasm and chronic pain. Administration of a suitable DP IV inhibitor causes the reduction of activity in the enzyme dipeptidyl peptidase (DP IV or CD 26) or of DP IV-like enzyme activity in the brain of mammals and leads as a causal consequence to a reduced degradation of the neuropeptide Y (NPY) and similar substrates by DP IV and DP IV-like enzymes. Such treatment will result in a reduction or delay in the decrease of the concentration of functionally active neuronal NPY (1–36). As a consequence of the resulting enhanced stability of the endogenous NPY (1–36) caused by the inhibition of DP IV activity, NPY activity is prolonged thereby resulting among other things in functionally active NPY Y1 receptor activity thereby facilitating anti-depressive, anxiolytic, analgesic, anti-hypertension and other neurological effects.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Compositions and methods for enhanced mucosal delivery and non-infused administration of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7186692B2Induce satietyPromoteHormone peptidesPeptide/protein ingredientsDiseaseBinding peptide
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Lithium combinations, and uses related thereto
InactiveUS20080107756A1Prevent precipitating manic episodeLessening and preventing riskBiocideNervous disorderNorepinephrine reuptake inhibitorPsychoactive drug
The present invention relates to combinatorial therapies for treating anxiety, depression or psychotic conditions using a lithium salt and a psychoactive drug selected from the group consisting of serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an anticonvulsant, a norepinephrine reuptake inhibitor, an α-adrenoreceptor antagonist, an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A receptor antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase inhibitor, a sedative-hypnotic drug, and an atypical antipsychotic.
Owner:NOVEN THERAPEUTICS
Neuropeptide q as modulator of GPCR GALR2 and uses thereof
InactiveUS20130196348A1Reduces maximal activationCompound screeningApoptosis detectionNeuropeptide QG protein-coupled receptor
The invention relates to the identification of Neuropeptide Q as ligand of the GALR2 GPCR (G-protein coupled receptor). The invention encompasses the use of the interaction of GALR2 polypeptides and Neuropeptide Q polypeptides as the basis of screening assays for agents that modulate the activity of the GALR2 receptor. The invention also encompasses diagnostic assays based upon the GALR2 / Neuropeptide Q polypeptide interaction, as well as kits for performing diagnostic and screening assays.
Owner:IDORSIA PHARM LTD
Cytotoxic Conjugates Having Neuropeptide Y Receptor Binding Compound
InactiveUS20120010154A1Good curative effectReduce complicationsOrganic active ingredientsMetabolism disorderNPY-Y1 receptorDisease
There is provided a series of novel neuropeptide Y-cytotoxic conjugates, compositions comprising the same, and methods relating to their therapeutic use for the treatment of disease or condition states associated with aberrant or undesirable proliferation of cells that express NPY-Y1 receptors.
Owner:IPSEN PHARMA SAS
Methods and compositions for the treatment of neurological disease
InactiveUS20050136036A1Treatment effectRelieve symptomsBiocideNervous disorderNucleic acid sequencingBiology
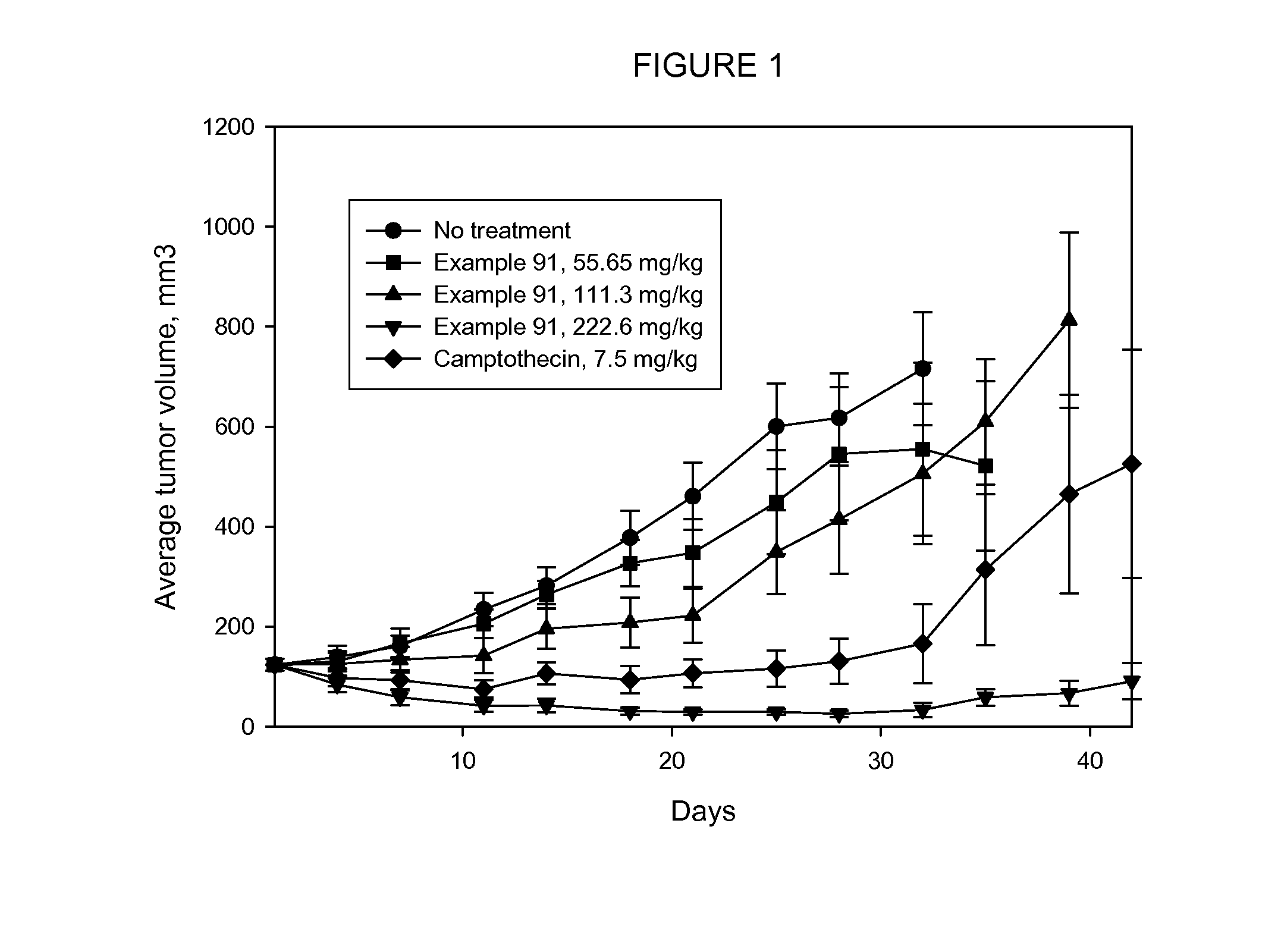
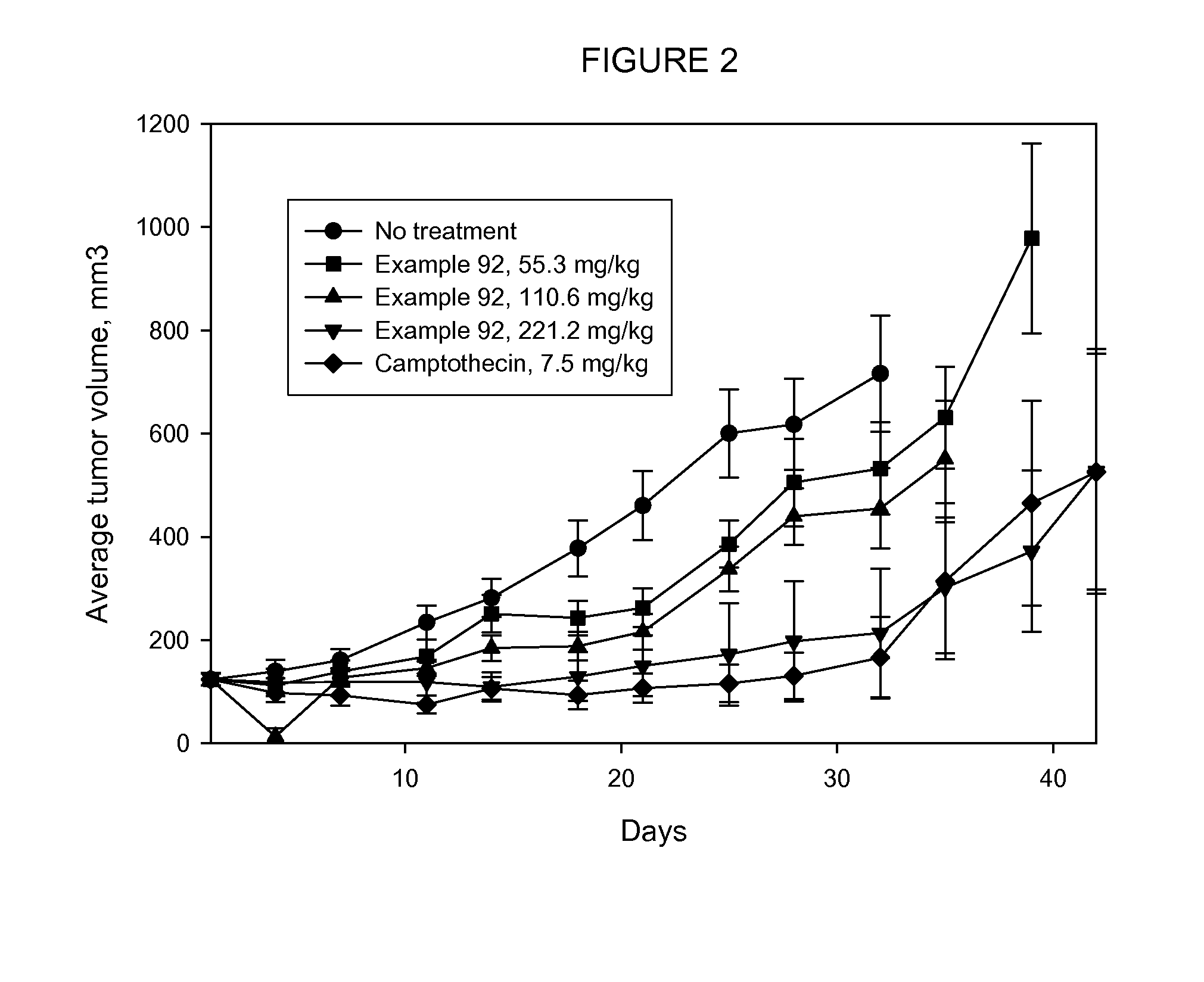
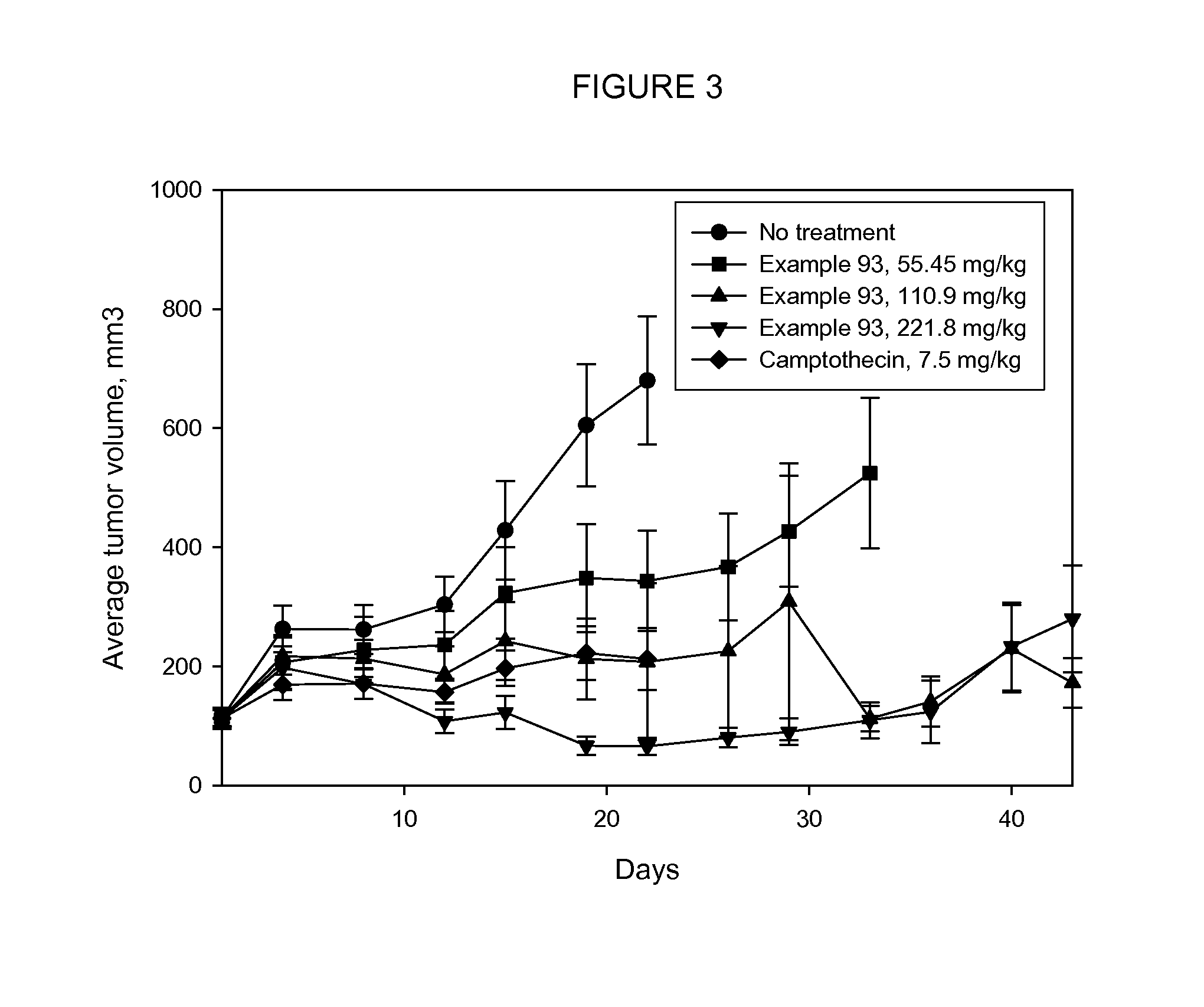
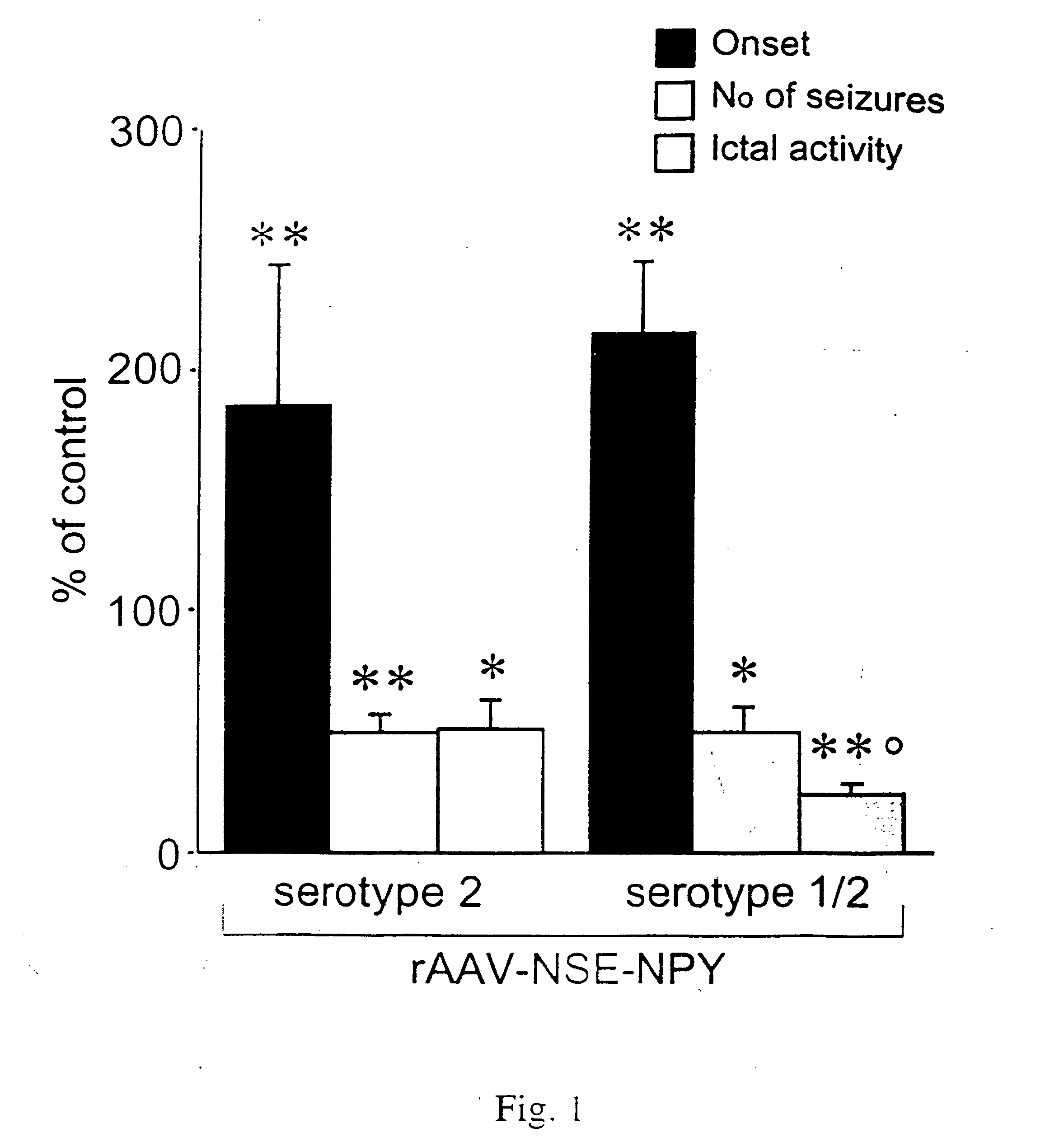
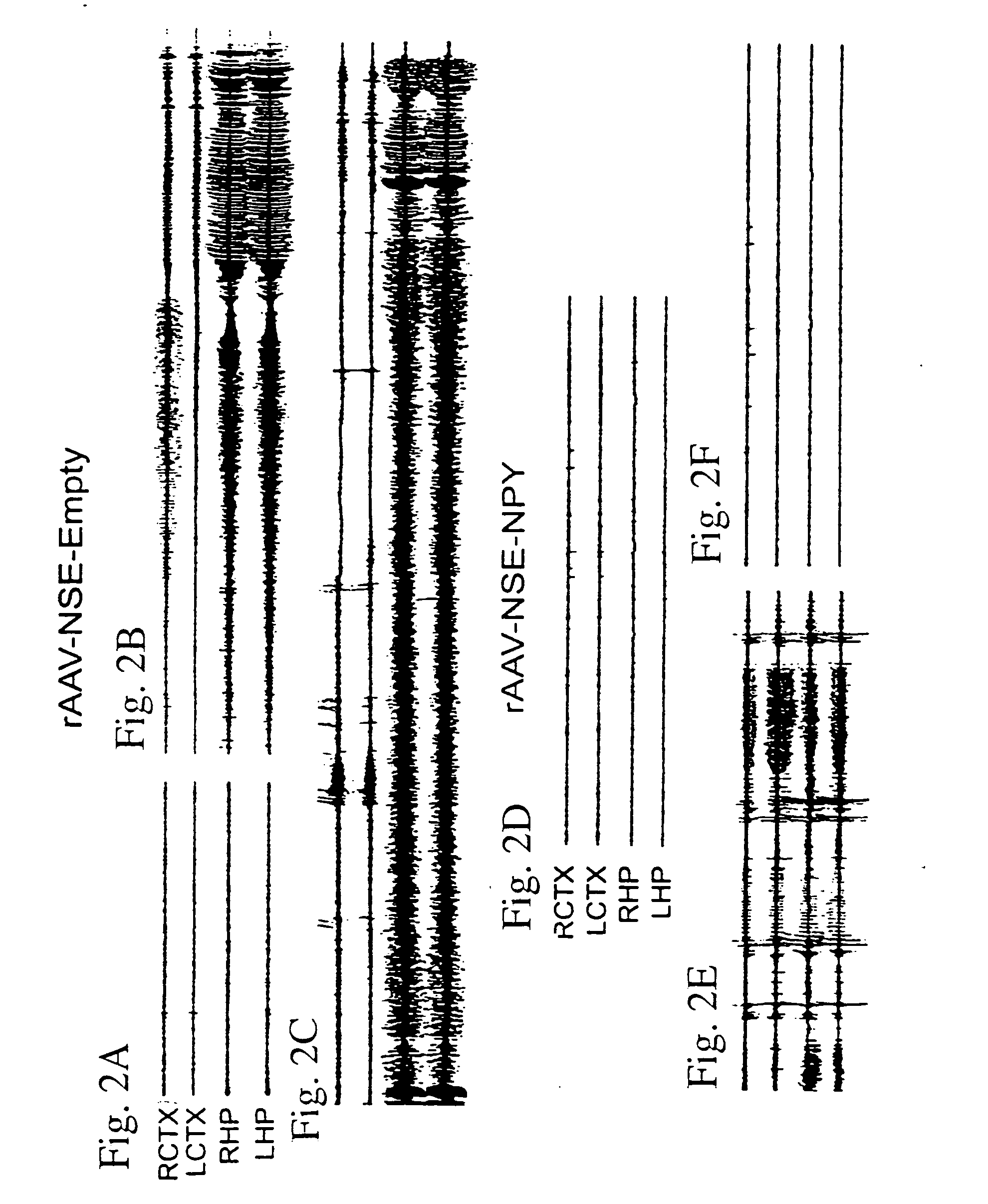
The present invention relates to a method for delivering a nucleic acid sequence encoding neuropeptide Y, or a derivative or functional fragment thereof, to a mammalian nervous system target cell. The expression of exogenous NPY, or a derivative or a functional fragment thereof in the target cell(s) provides therapeutic benefit for subjects afflicted with a neurological disorder.
Owner:NEUROLOGIX RESEARCH INC
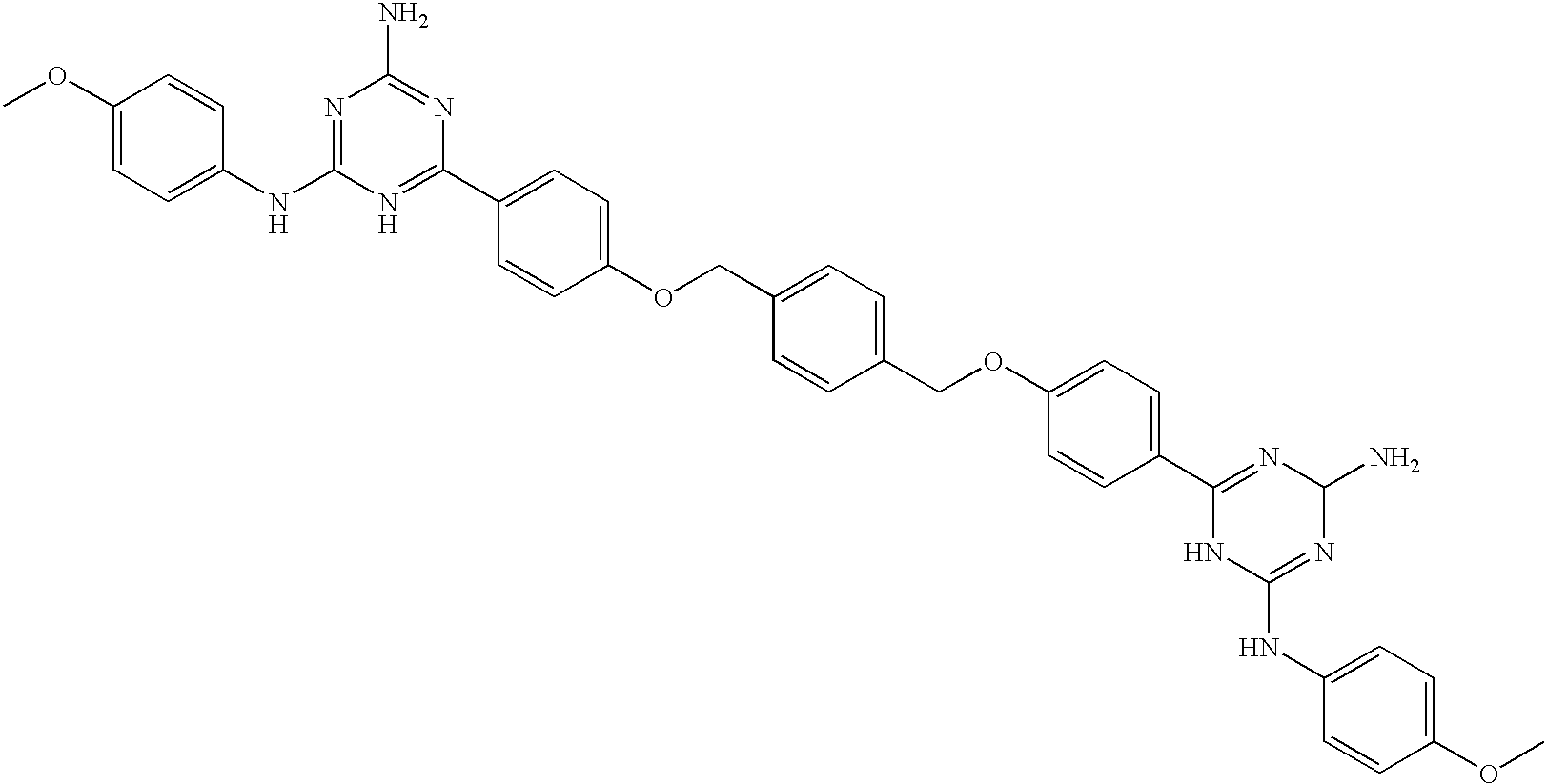
Neuropeptide-2 receptor (Y-2R) agonists and uses thereof
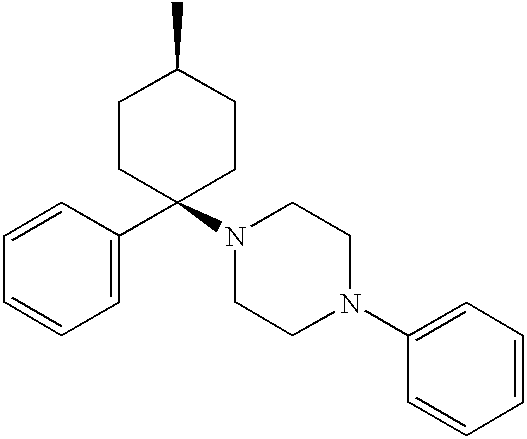
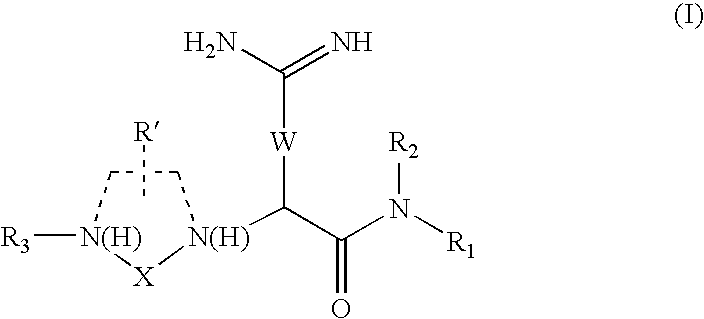
Provided herein are neuropeptide-2 receptor agonists of the formula (I): as well as pharmaceutically acceptable salts, derivatives and fragments thereof, wherein the substituents are as those disclosed in the specification. These compounds, and the pharmaceutical compositions containing them, are useful for the treatment of diseases such as, for example, obesity and diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for the treatment of neurological and neuropsychological disorders
The present invention discloses a method for therapeutically treating an animal, including a human, for psychosomatic, depressive and neuropsychiatric diseases, such as anxiety, depression, insomnia, schizophrenia, epilepsy, spasm and chronic pain. Administration of a suitable attractin inhibitor causes the reduction of activity in the enzyme attraction or in isoforms thereof in the brain of mammals and leads as a causal consequence to a reduced degradation of the neuropeptide Y (NPY) and similar substrates. Such treatment will result in a reduction or delay in the decrease of the concentration of functionally active neuronal NPY (1-36). As a consequence of the resulting enhanced stability of the endogenous NPY (1-36), NPY activity is prolonged thereby resulting among other things in functionally active NPY Y1 receptor activity thereby facilitating antidepressive, anxiolytic, analgesic, t antihypertension and other neurological effects.
Owner:VIVORYON THERAPEUTICS NV
Trophic Factor Combinations for Nervous System Treatment
The present invention relates to a composition including an effective amount of at least one of an antimicrobial peptide and a substance having an antimicrobial peptide effect and an effective amount of a neurotrophin. The composition can also include an effective amount of at least one of a growth factor and a neuropeptide. The present invention also relates a method of treating an injury to a nervous system of an animal that includes the steps of identifying the injury to the nervous system and applying to the injury an effective amount of at least one of antimicrobial peptide and a substance having an antimicrobial peptide effect. The method can also include applying an effective amount of one or more trophic factors selected from the group consisting of a growth factor, a neurotrophin, and a neuropeptide to the injury.
Owner:WISCONSIN ALUMNI RES FOUND
Antagonists of RF-amide neuropeptides
InactiveUS6960574B2Good curative effectAvoid drug dependenceBiocideNervous disorderOpiate dependenceDense Core Vesicles
Disclosed are compounds having the formula: whereR1=H, C1-C6 alkyl, cycloalkyl, R2=H, C1-C6 alkyl, cycloalkylW=CnH2n-m—NH (n=1-6, m=0, 2, or 4), X=R3=Y=Z=CONR8(CH2)n, CONR8(CH2)nCO, P(CH3)OCHR8OCOR9, SO2, SO2(CH2)n, SO2(CH2)nCO, SO2NR8(CH2)n, SO2NR8(CH2)nCO, n=1-4R4=H, (CH2)nOH, (CH2)nOCOR10, (CH2)nNR10R11, (CH2)nCONR10R11, n=0-4R5=H, (CH2)nNR12R13, n=0-4R6=H, (CH2)nNR14R15, n=0-4R7=H, C1-C6 alkyl, cycloalkyl; R8=H, C1-C6 alkyl, cycloalkyl; R9=H, C1-C6 alkyl, cycloalkyl;R10=H, C1-C6 alkyl, cycloalkyl; R11=H, C1-C6 alkyl, cycloalkyl; R12=H, C1-C6 alkyl, cycloalkyl;R13=H, C1-C6 alkyl, cycloalkyl; R14=H, C1-C6 alkyl, cycloalkyl; R15=H, C1-C6 alkyl, cycloalkyl.Dashed lines: optional; conformational constraint by (CH2)n, n=1-3, R′=H or O(═) as well as pharmaceuticals compositions and methods for the treatment of opiate addiction, opiate dependence, opiate tolerance, opiate related abstinence syndrome, nicotine addition and obesity based thereon.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Detection of neureopeptides associated with pelvic pain disorders and uses thereof
InactiveUS20080070239A1Effective treatmentRelieve symptomsOrganic active ingredientsBiocideDense Core VesiclesSensory neuron
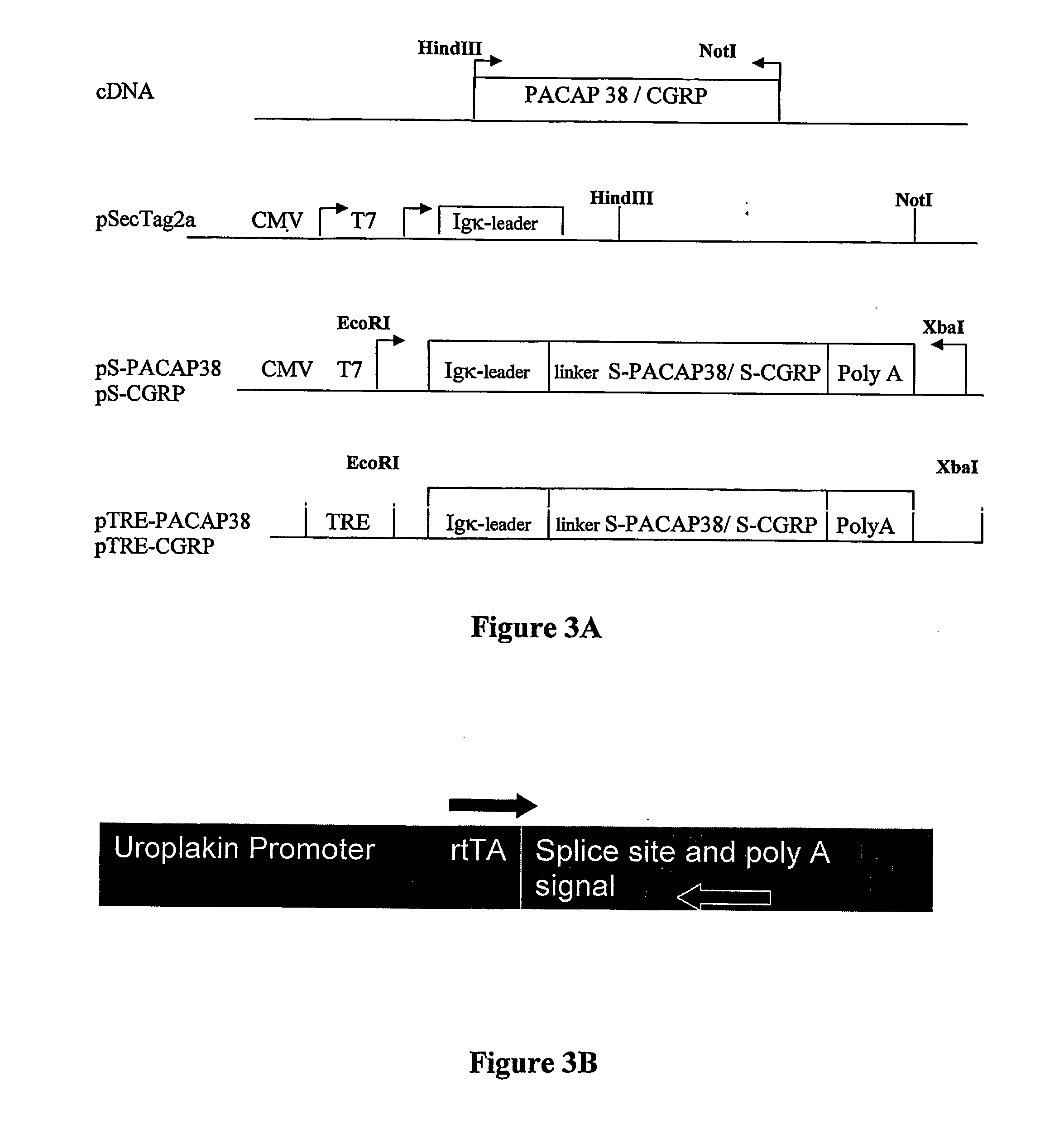
Diagnostic assessment and therapeutic treatment of pelvic pain disorders, including bladder disorders, bowel disorders, and / or reproductive tissue or organ disorders that are characterized by increased expression of the neuropeptides CGRP and / or PACAP. Additionally, applicants have developed a transgenic nonhuman model for pelvic pain disorders, where the transgenic animal expresses in bladder sensory neurons a recombinant neuropeptide implicated in the pelvic pain disorder.
Owner:UNIVERSITY OF VERMONT +1
Neuropeptide-2 receptor (Y-2R) agonists and uses thereof
Provided herein are neuropeptide-2 receptor agonists of the formula (I):Y—R1—R2—X—R3—R4—R5—R6—R7—R8—R9—R10—R11—R12—R13—R14—NH2, (I)as well as pharmaceutically acceptable salts, derivatives and fragments thereof, wherein the substituents are as those disclosed in the specification. These compounds, and the pharmaceutical compositions containing them, are useful for the treatment of diseases such as, for example, obesity.
Owner:F HOFFMANN LA ROCHE & CO AG
Sepia pharaonis neuropeptide GnPH procreation regulation preparation
InactiveCN106749587APrevent loss of activity due to dehydrationAvoid degradationPeptide preparation methodsAnimals/human peptidesDiseaseSepia pharaonis
The invention provides a sepia pharaonis neuropeptide GnPH procreation regulation preparation. The sepia pharaonis neuropeptide GnPH procreation regulation preparation is prepared by the steps: 1) pre-treatment: taking live sepia pharaonis, dissecting the live sepia pharaonis to take a brain tissue and preserving the brain tissue in a preservation solution at an ultra-low temperature; 2) taking of supernatant: taking the brain tissue, adding saline of the same volume to the brain tissue, homogenizing and centrifuging an obtained mixture to take supernatant A; adding distilled water of the same volume to the obtained mixture, centrifuging the mixture with the distilled water to take supernatant B; and combining the supernatant A and the supernatant B to obtain supernatant C; 3) column chromatography: carrying out column chromatography, chromatography and developing on the supernatant C and collecting components to obtain neuropeptide; and 4) catalysis: enabling the neuropeptide to have substitution reaction with a GnRH derivative under the catalysis of ammonium nitrate and cadmium metal to generate the sepia pharaonis neuropeptide GnPH procreation regulation preparation. The sepia pharaonis neuropeptide GnPH procreation regulation preparation, provided by the invention, has the advantages that the tissue can be preserved in a frozen manner and the preservation effect is excellent; the brain tissue degradation of the sepia pharaonis can be effectively prevented; the extraction rate of the neuropeptide is higher; the prepared control agent does not need to be continuously or repeatedly injected for a long time; and the preparation is applied to drugs for preventing or treating hormone-dependent diseases.
Owner:ZHEJIANG OCEAN UNIV
Use of neurotransmitters and neuropeptides for the treatment of dry eye diseases and related conditions
Owner:OUSLER GEORGE W +2
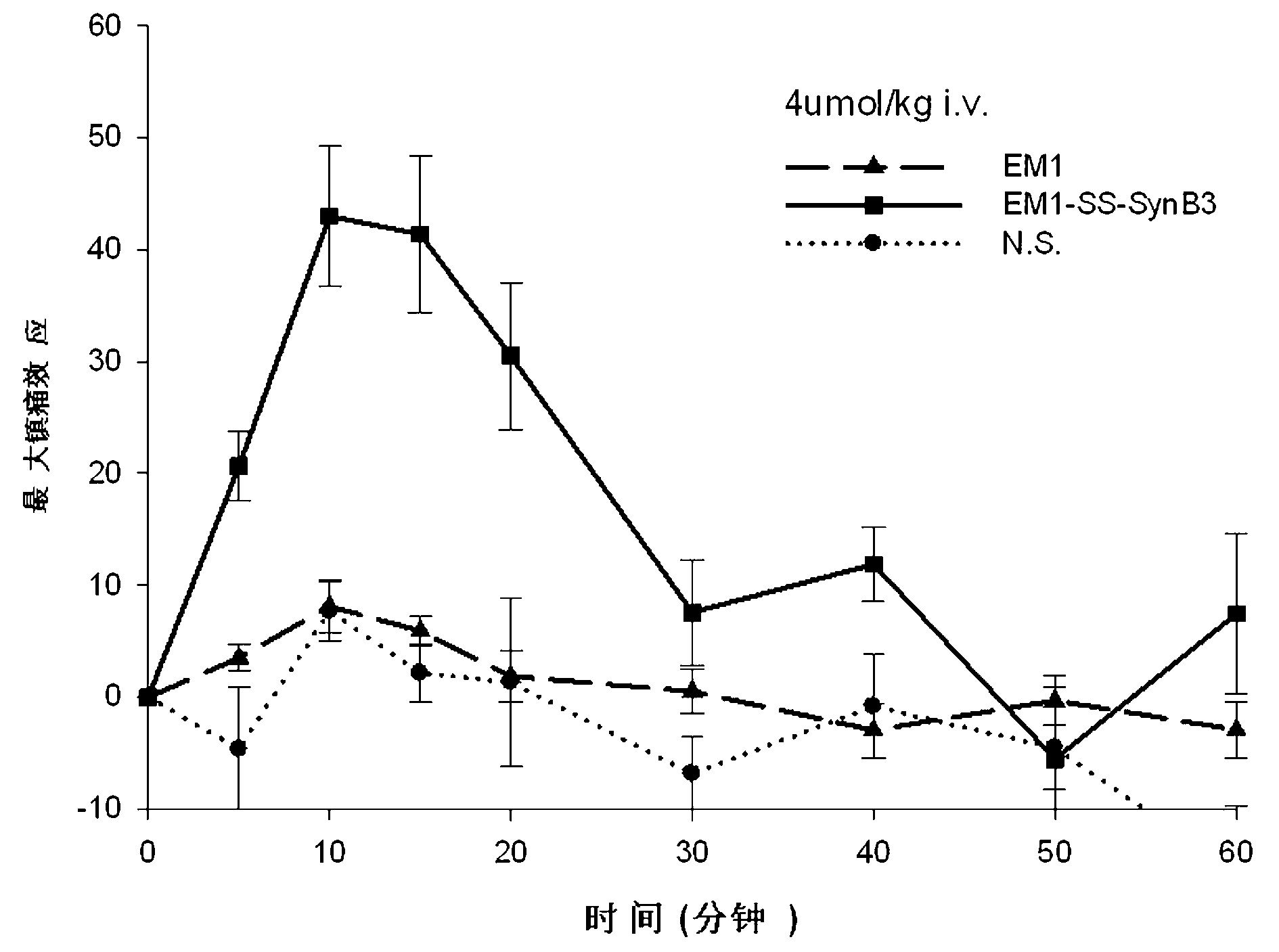
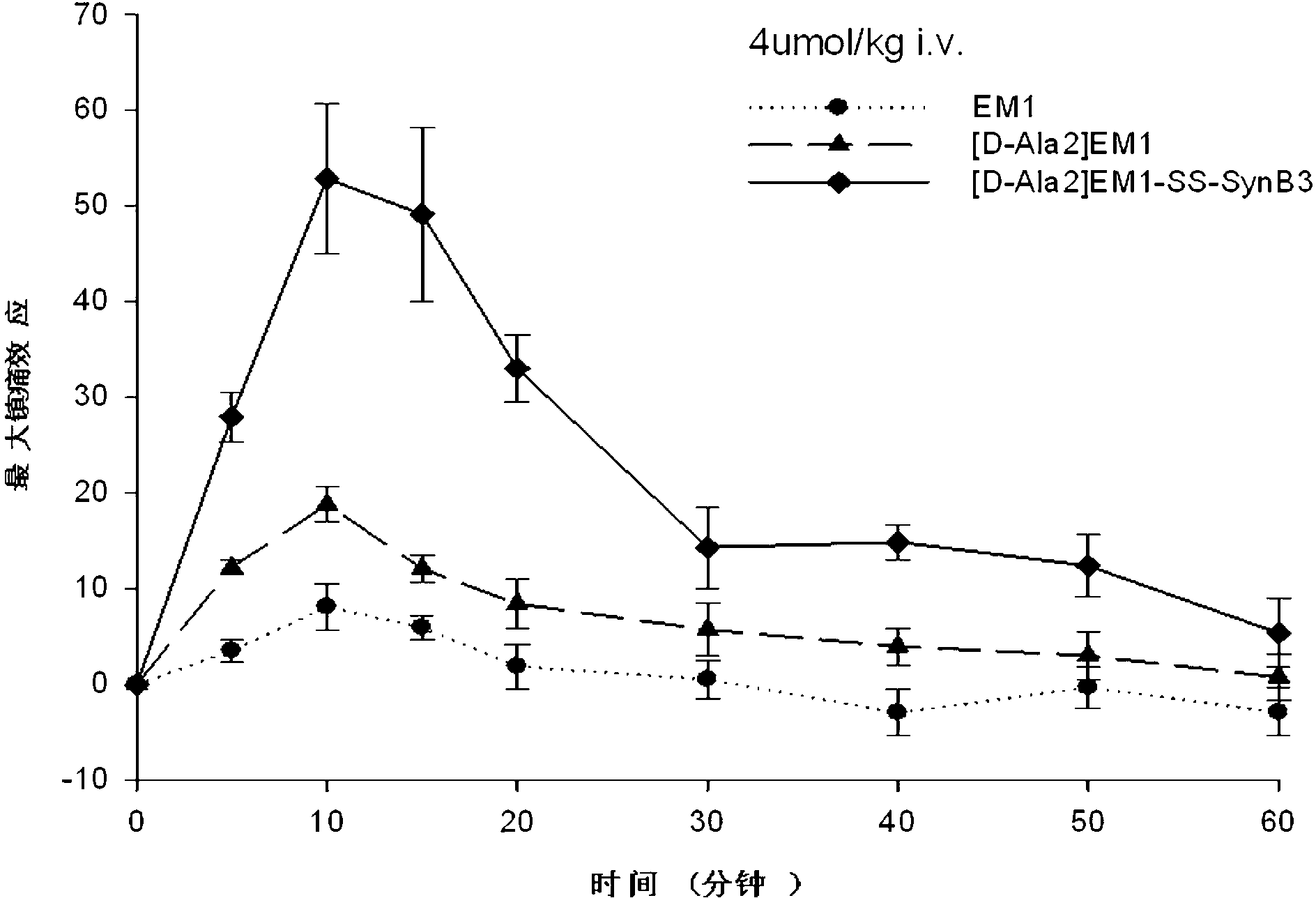
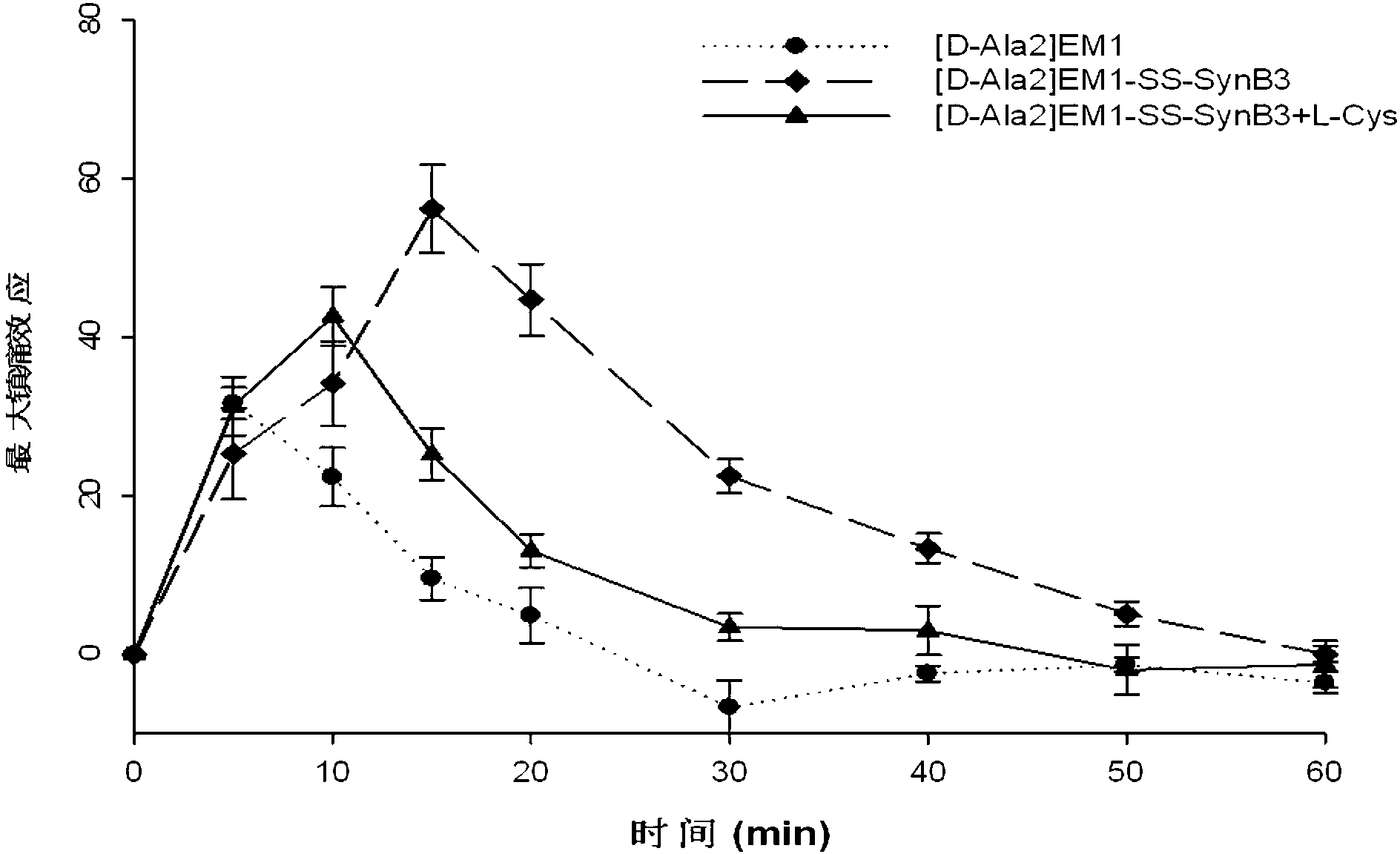
Endomorphin-derived peptide with blood-brain barrier permeability as well as synthesis and application of endomorphin-derived peptide
ActiveCN103012596AIncreased Brain UptakeWithout compromising integrityNervous disorderTetrapeptide ingredientsMeningesAdsorption effect
The invention discloses endomorphin-derived peptides with blood-brain barrier permeability. The endomorphin-derived peptides are formed by connecting cell-penetrating peptide SynB3 with the carbon terminal of EM1 (Endomorphin1) and analogue (D-A1a2) EM1 through a disulfide bond; good BBB (Blood-Brain Barrier) permeability of the SynB3 is used to mediate the EM1 and the analogue (D-A1a2) EM1 to permeate the blood-brain barrier to reach the center by means of adsorption effect; and the characteristics that the disulfide bond is stable in blood and is easily reduced to break in meninx are utilized, the disulfide bond is broken under the action of a meningeal reductase to release free endomorphin-derived peptide so that the pain killing effect of the endomorphin-derived peptide can be exerted. A pharmacodynamic experiment proves that the endomorphin-derived peptides can be peripherally administrated besides the obviously improved pain killing activity to provide a wide prospect for developing clinical application of a neuropeptide medicine.
Owner:LANZHOU UNIVERSITY
Expression system of tilapia neuropeptide Y recombinant protein
InactiveCN101824435AStable sourceLow costFungiPeptide/protein ingredientsBiotechnologyGrowth promoting
The invention discloses an expression system of tilapia neuropeptide Y recombinant protein. Tilapia neuropeptide Y gene with a digestion joint is cloned into a pichia expression vector to obtain a recombinant pichia expression vector; and then the recombinant pichia expression vector is converted into a pichia competent cell to obtain a pichia pastoris recombinant strain, and the recombinant strain expresses the tilapia neuropeptide Y recombinant protein. The tilapia neuropeptide Y recombinant protein expression system can be used for obtaining the tilapia neuropeptide Y recombinant protein with stable source and low cost, and the recombinant protein can be used for preparing fry growth promoting agent or additive, and can effectively promote the growth of fishes.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com