Expression system of tilapia neuropeptide Y recombinant protein
A technology of recombinant protein and expression system, which is applied in the field of expression system of tilapia neuropeptide Y recombinant protein, to achieve the effect of promoting fish growth, low cost and stable source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Cloning of Tilapia Neuropeptide Y Gene Fragment
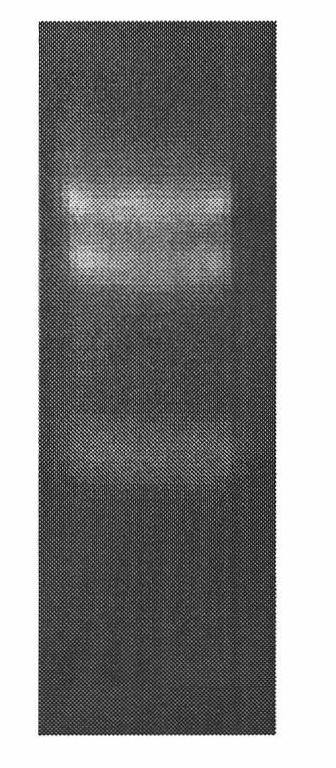
[0032] Total RNA was extracted from the hypothalamus of Gifu tilapia ( figure 1 ), reverse-transcribed into cDNA with AP primers. Using cDNA as a template, a pair of primers were designed according to the published sequence of Oreochromis sp.YC-2004 (red tilapia) neuropeptide Y gene (GenBank: AY779047.1). Primers: the upstream primer is shown in SEQ ID NO: 4, The downstream AUAP clones the gene sequence from the start codon of the neuropeptide Y gene to the PolyA tail. The PCR reaction conditions are: 95°C pre-denaturation for 3 minutes; the following is 35 cycles, 95°C denaturation for 15 seconds, 53°C annealing for 15 seconds, 72°C Extension for 60 seconds; final extension for 10 minutes at 72°C.
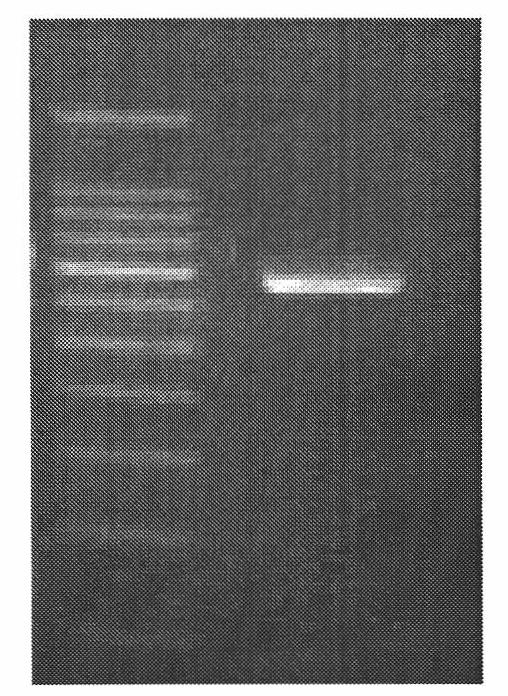
[0033] The electrophoresis identification chart of the PCR amplification product is shown in figure 2 . From figure 2 It can be seen that a sequence of about 630 bp was amplified by the first PCR, and the se...
Embodiment 2
[0034] Example 2 Gene synthesis of neuropeptide Y gene of Gifu tilapia with enzyme-cut linker
[0035]According to the above cloned nerve body Y gene sequence and the recognition sequences of restriction endonucleases XhoI and XbaI, design and synthesize two pairs of specific primers:
[0036] The upstream primer A is shown in SEQ ID NO: 1, with a total of 43 bp, which is added with an XhoI restriction recognition site and a signal peptide coding sequence behind it before the mature peptide sequence of the NPY gene.
[0037] The downstream primer B is shown as SEQ ID NO: 2, with a total of 45 bp, which is added with 6×His tag, stop codon and enzyme recognition site after the mature peptide sequence of NPY gene.
[0038] The downstream primer C is shown in SEQ ID NO:3. Use the carrier T-TiNPY obtained in Example 1 as a template to carry out a PCR reaction. The PCR reaction conditions are: 95°C pre-denaturation for 3 minutes; the following is 35 cycles, 95°C denaturation for 15...
Embodiment 3
[0040] Example 3 Construction of Pichia pastoris expression vectors D and E containing the neuropeptide Y gene of Gifu tilapia
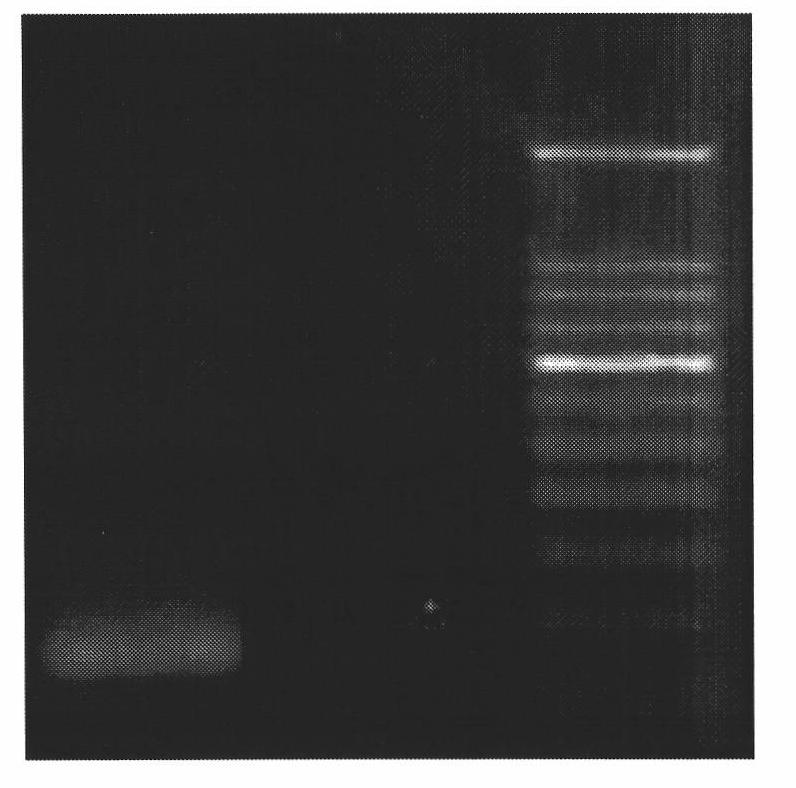
[0041] 1 and 2PCR products were double-digested with restriction endonucleases XhoI and XbaI, and the products were digested with Gel Extraction Kit recovery, agarose gel electrophoresis, separation and purification of about 160bp and about 140 tilapia neuropeptide Y gene fragments; vector plasmid pPICZ A was cut with restriction endonucleases Xba I and Xho I to isolate and purify a large fragment of 3.6 kb, mixed with two neuropeptide Y gene fragments at a ratio of 1:3, and ligated with NEB T4 ligase at 16°C for 16 hours Transform Escherichia coli DH5a into Escherichia coli DH5a using the standard calcium chloride transformation method, use a low-salt LB plate to screen transformants with Zeocin resistance, and extract the plasmid ( Figure 5 ), obtained a recombinant plasmid with a size of about 3.8kb, by using 5-AOX as primer sequencing, the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com