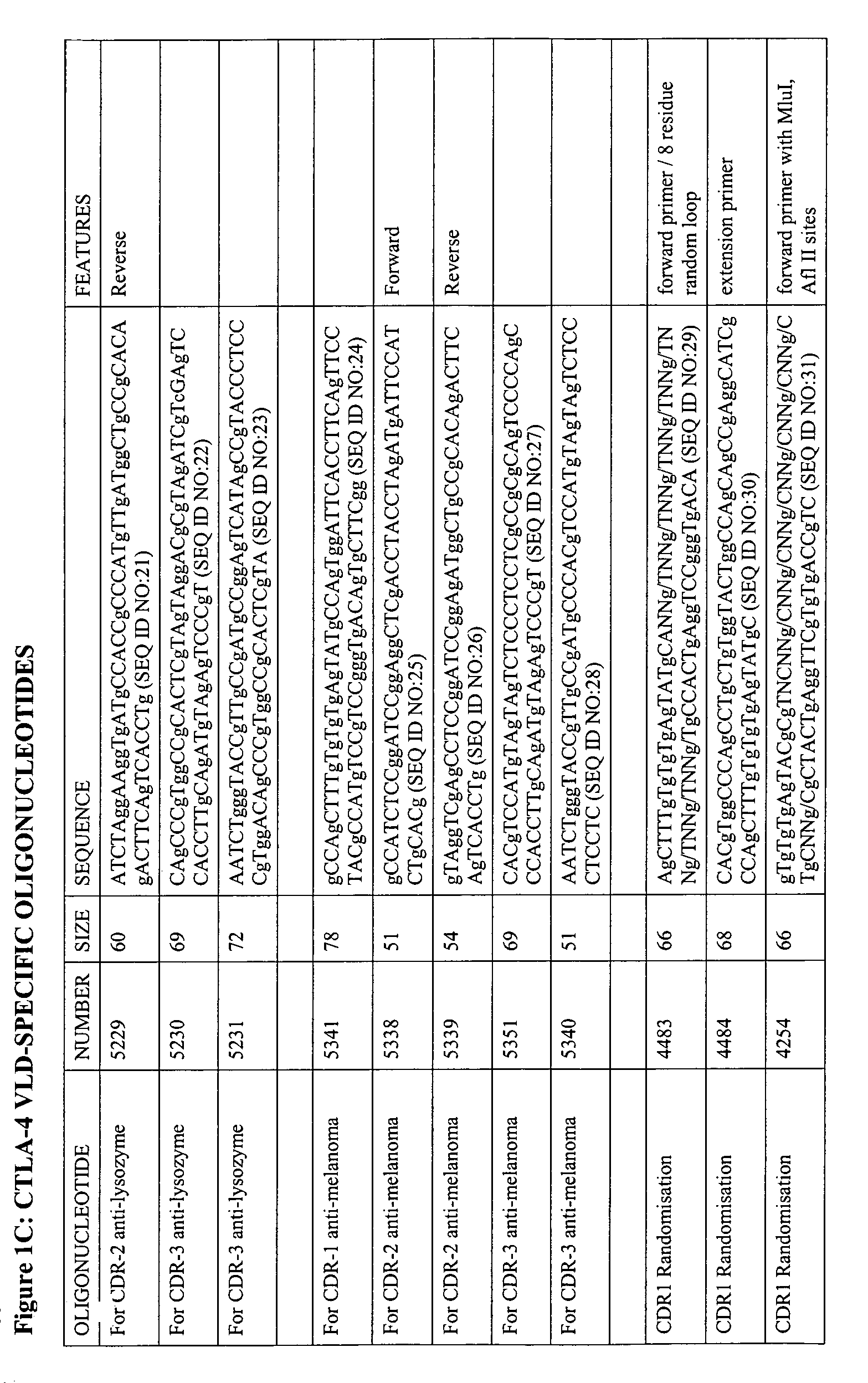
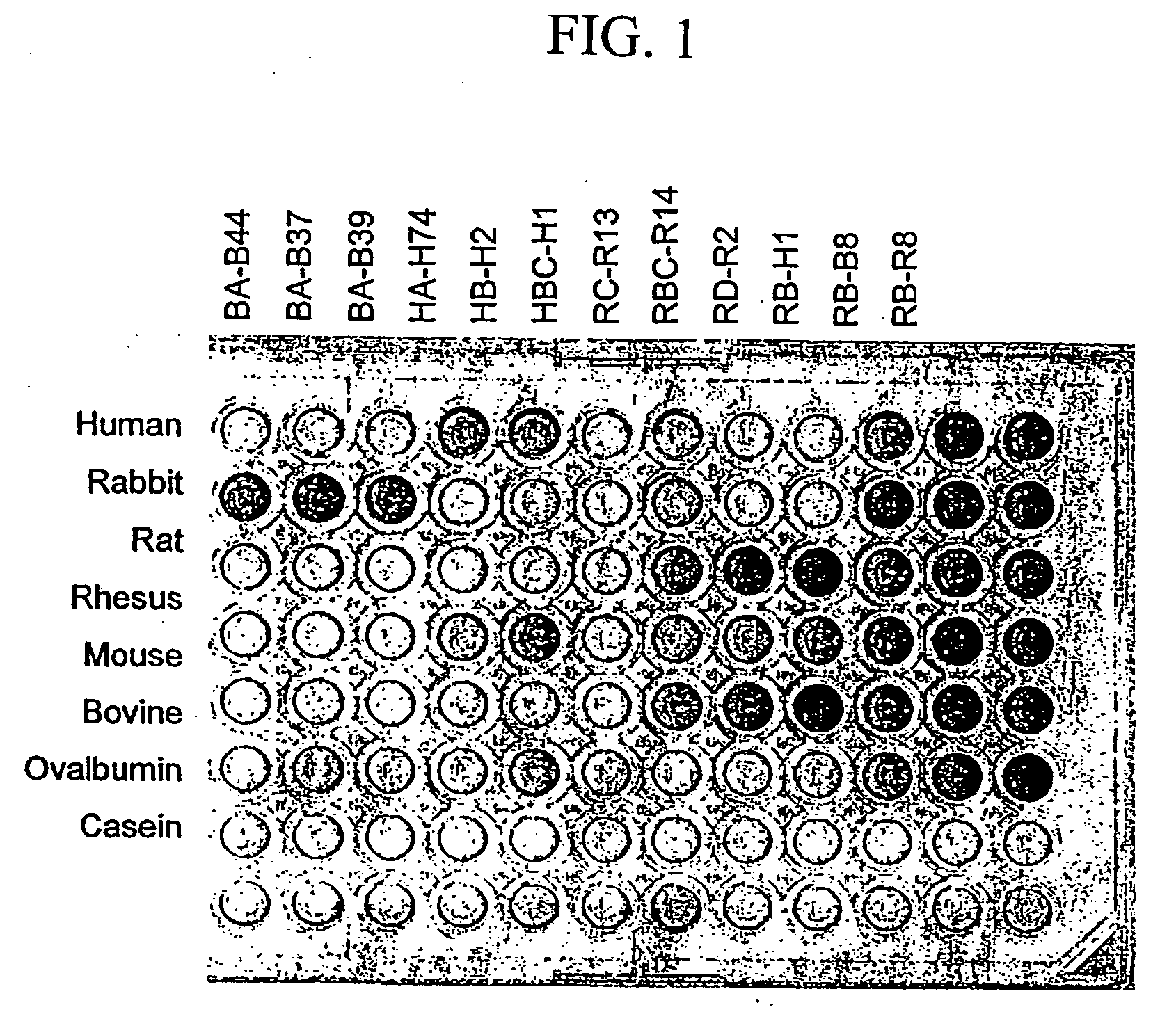
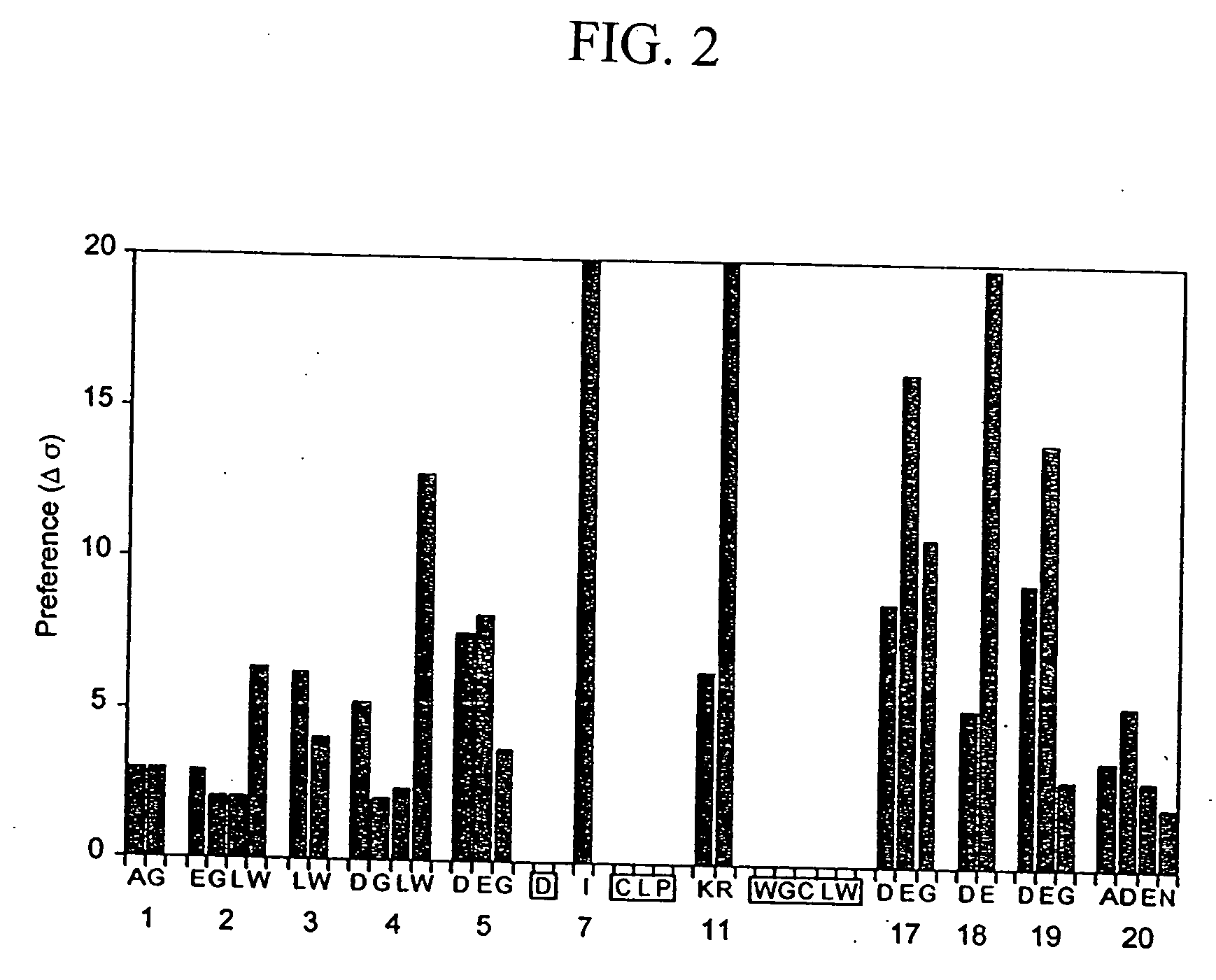
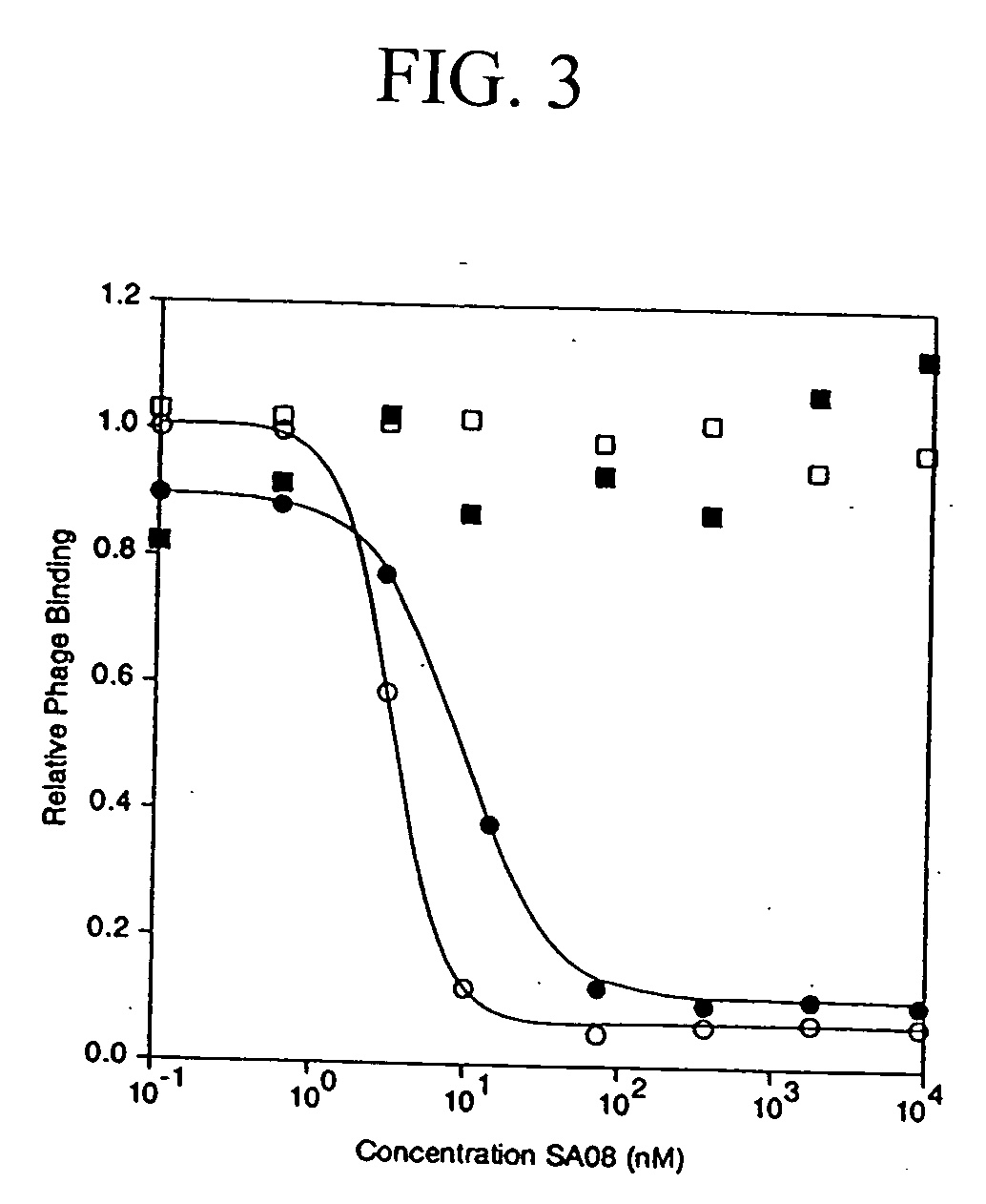
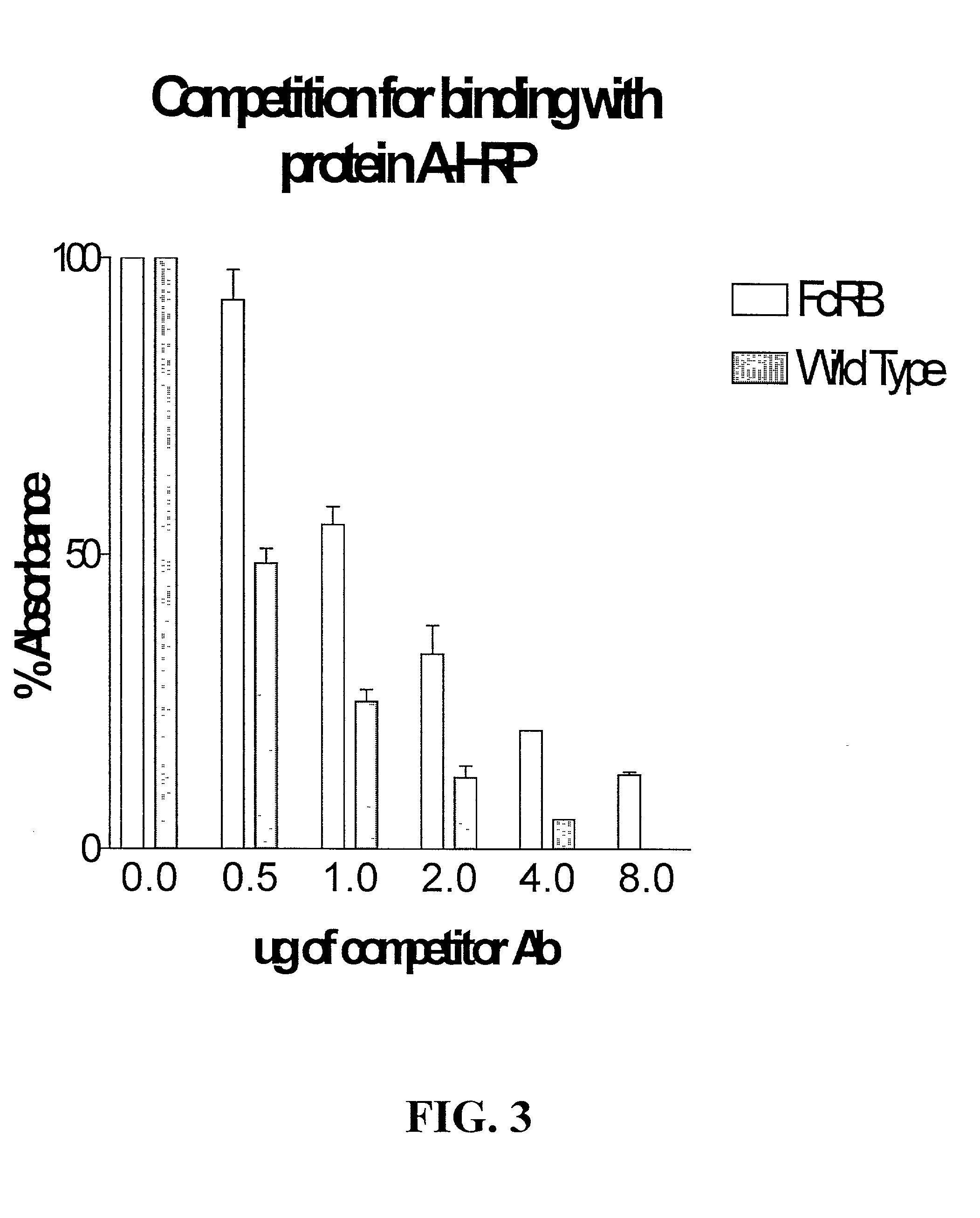
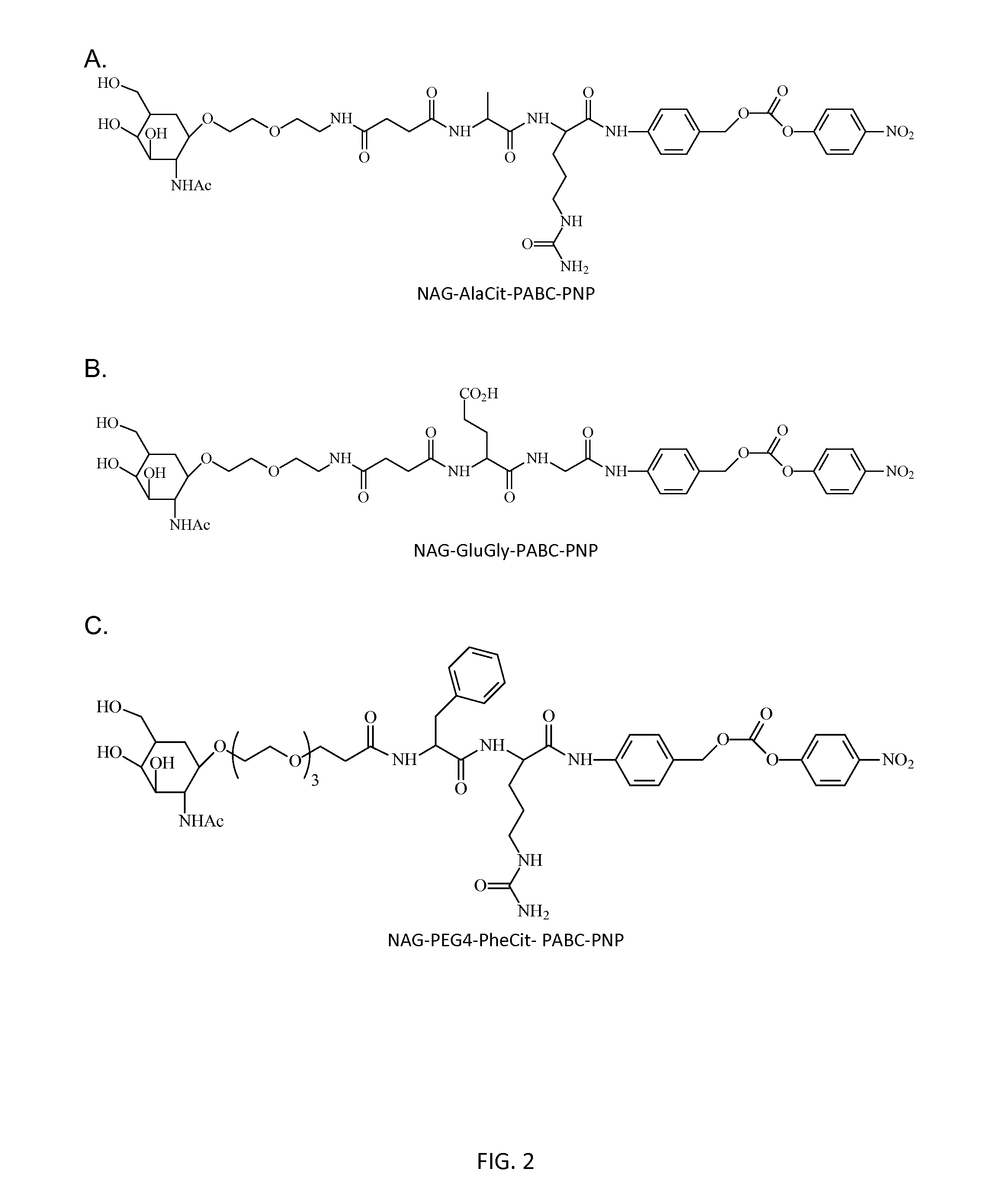
Patents
Literature
6583results about "Drug compositions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
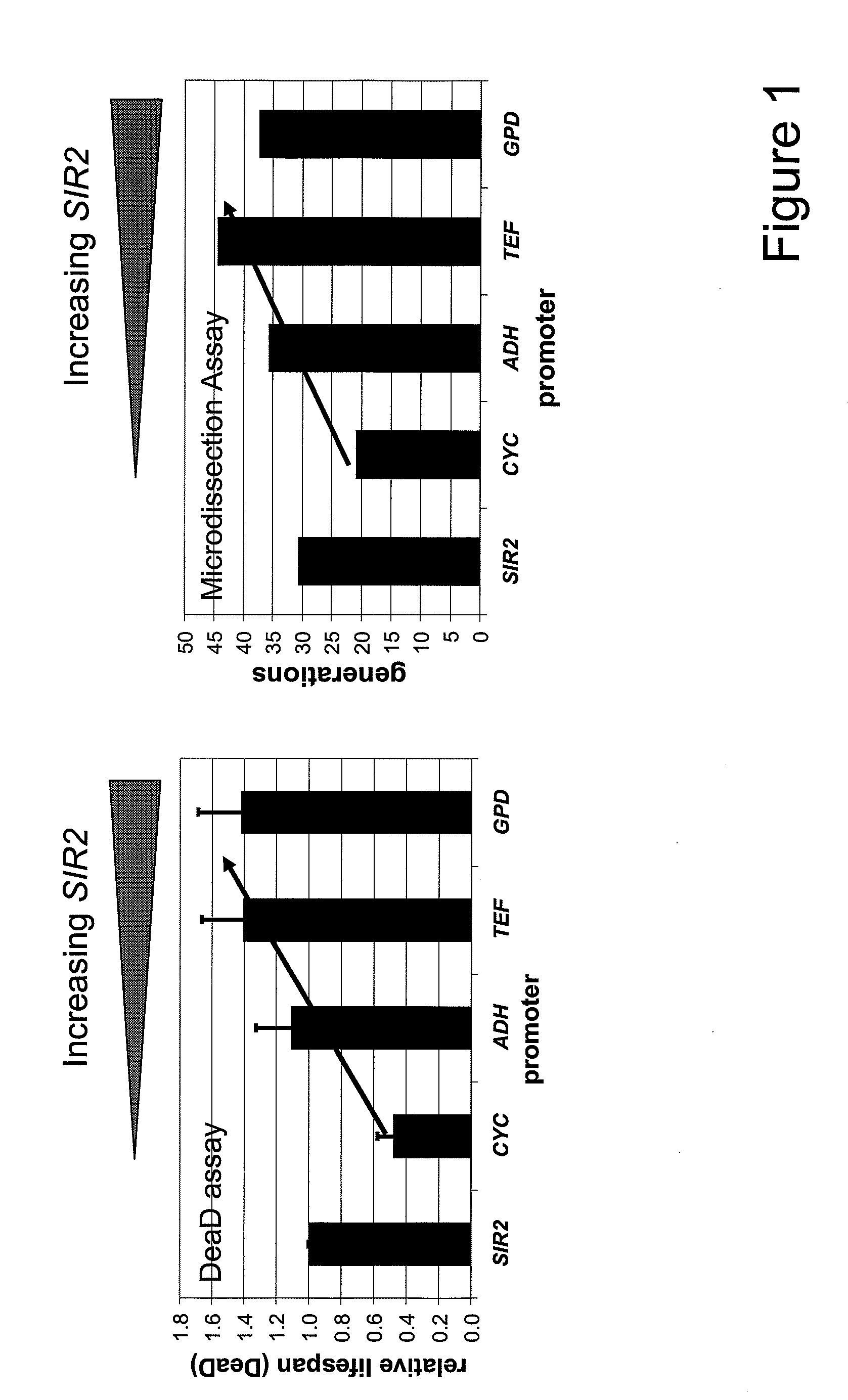
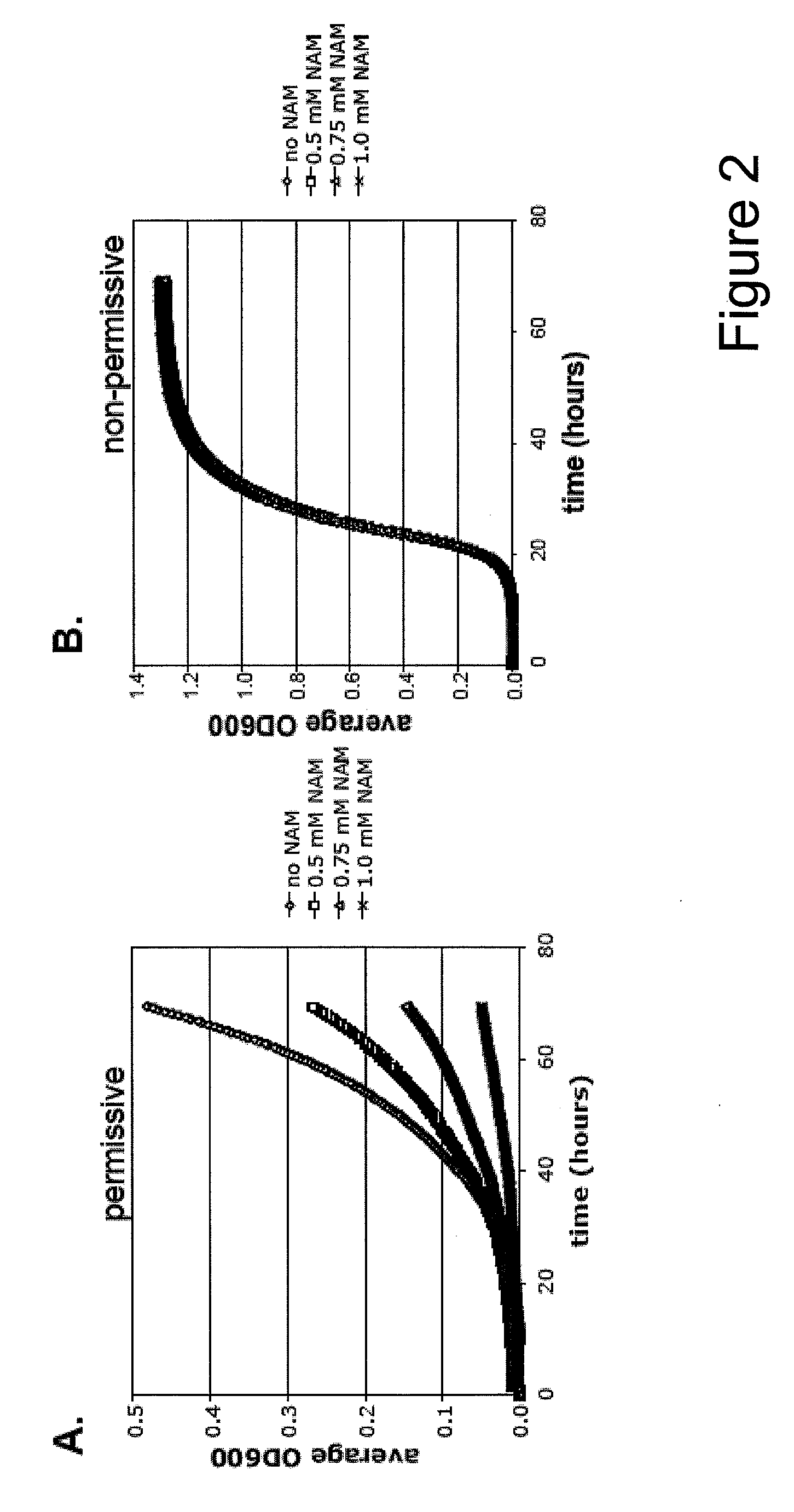
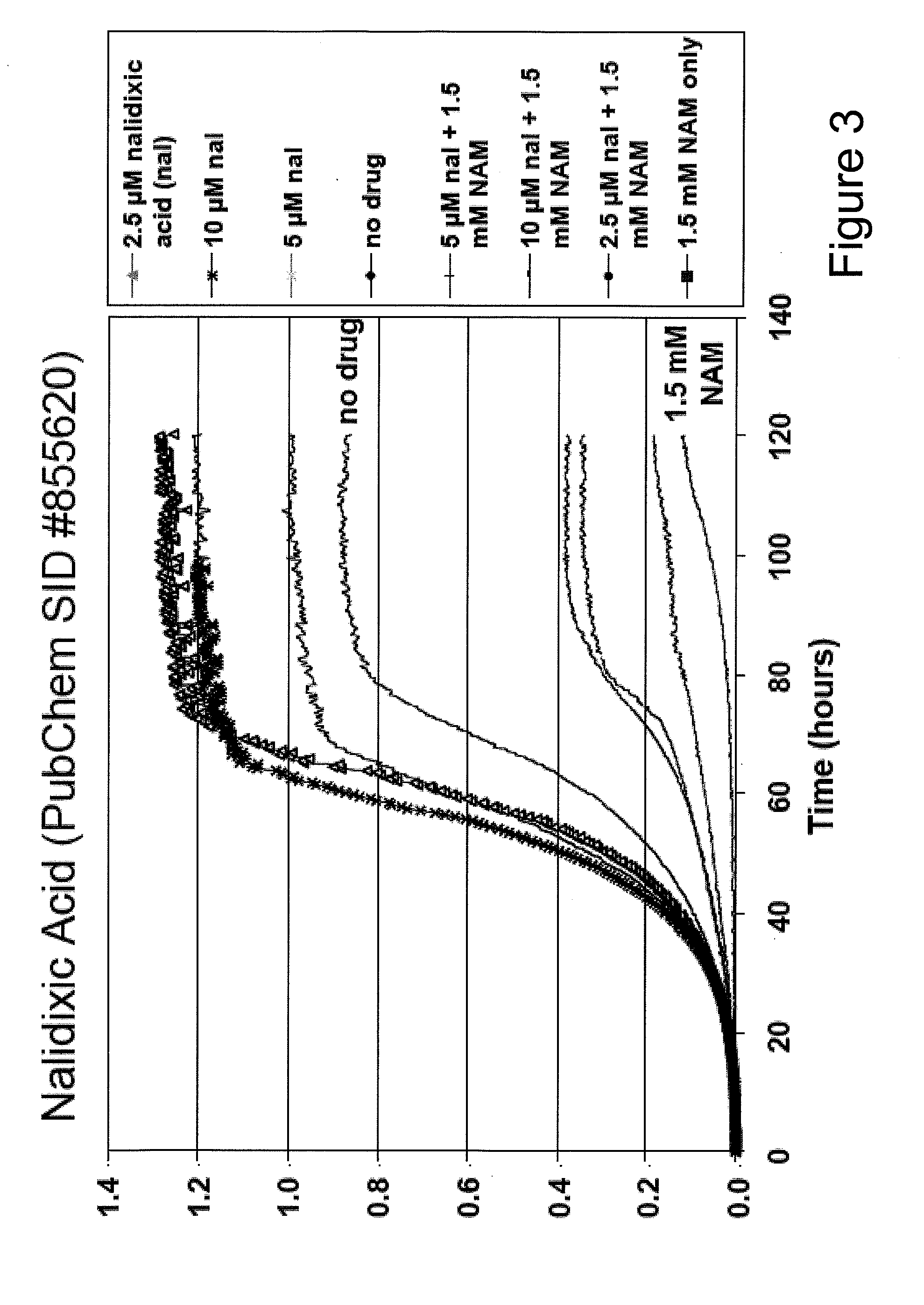
Method For Altering The Lifespan Of Eukaryotic Organisms
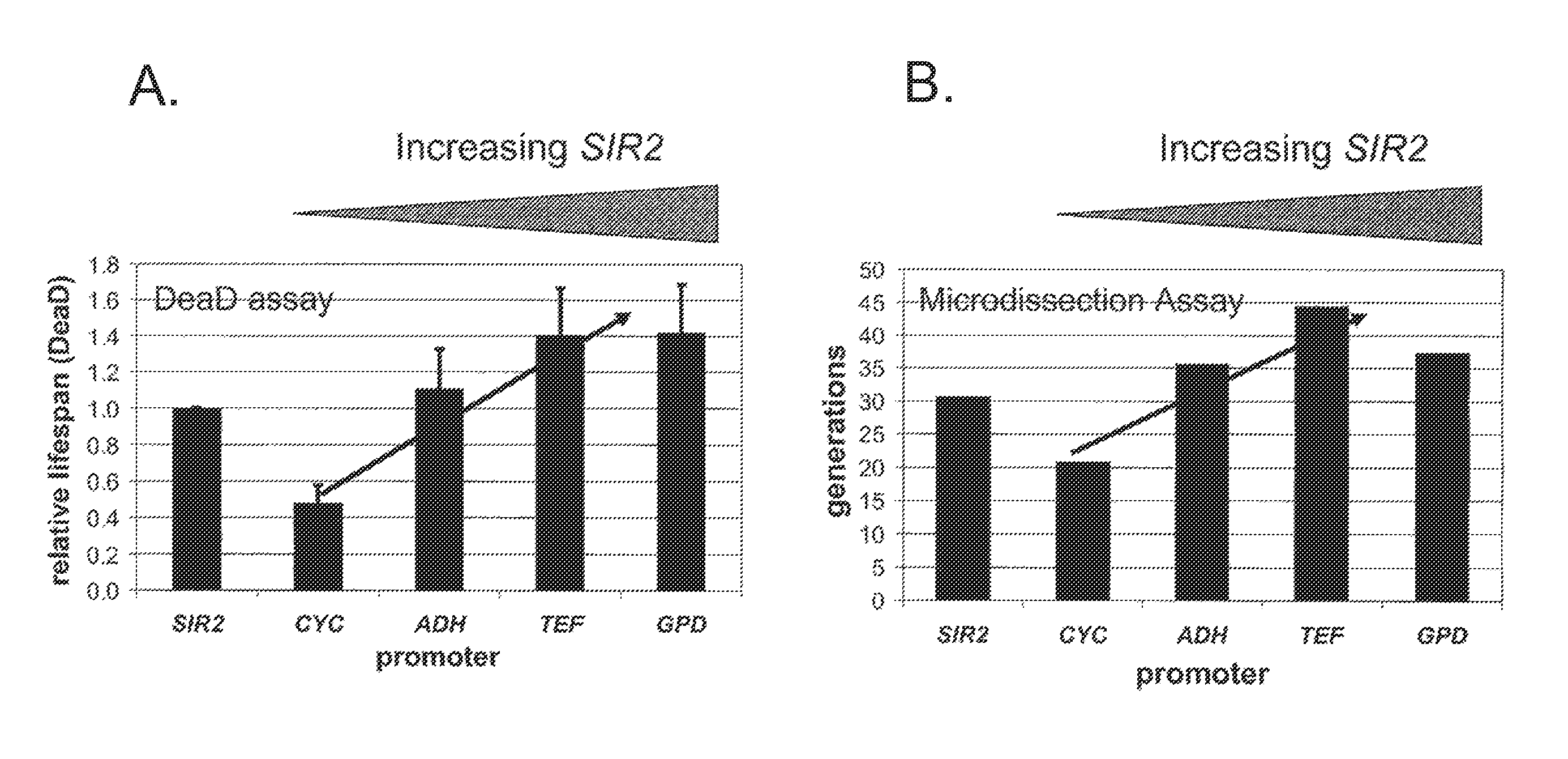
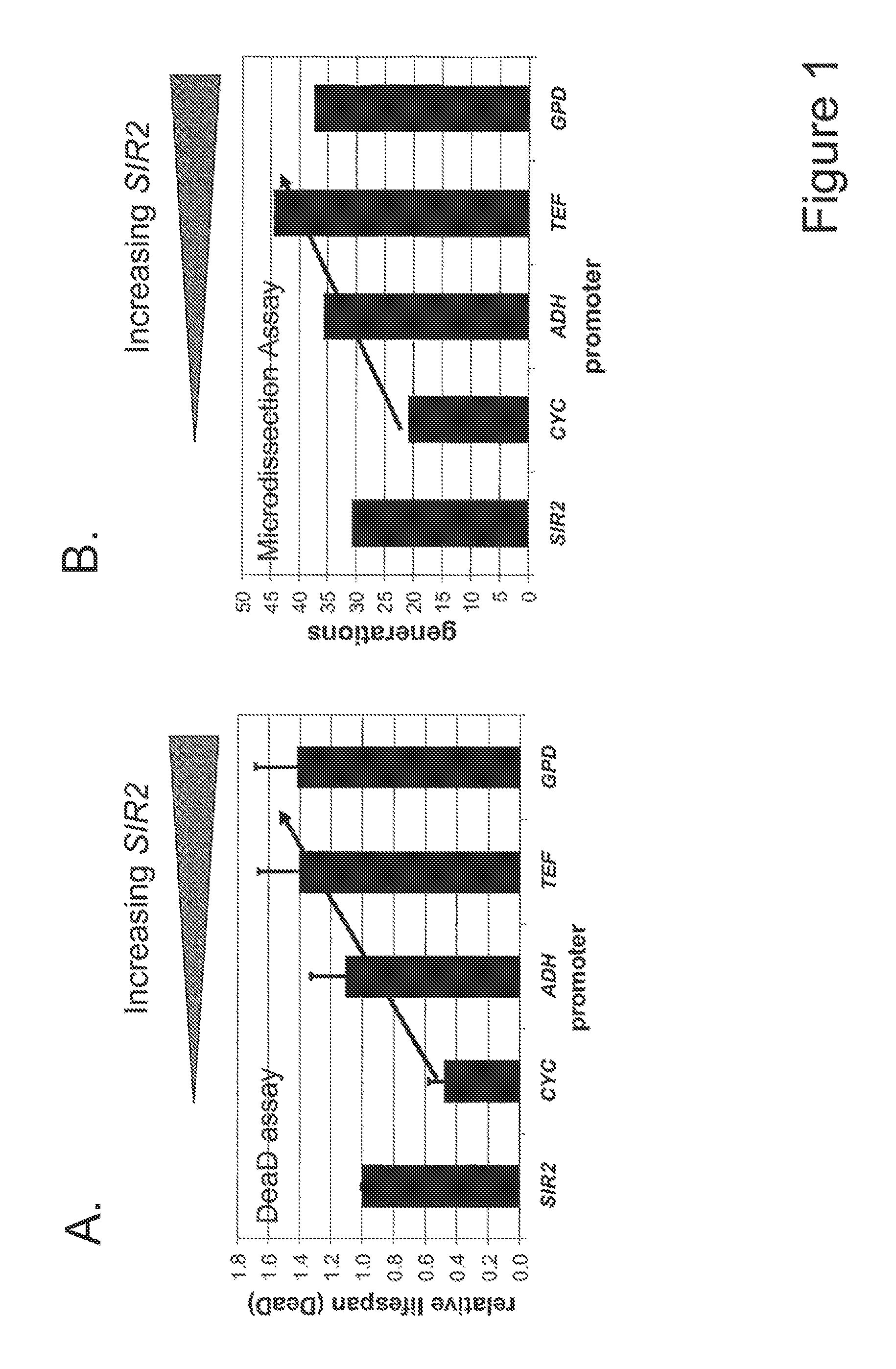
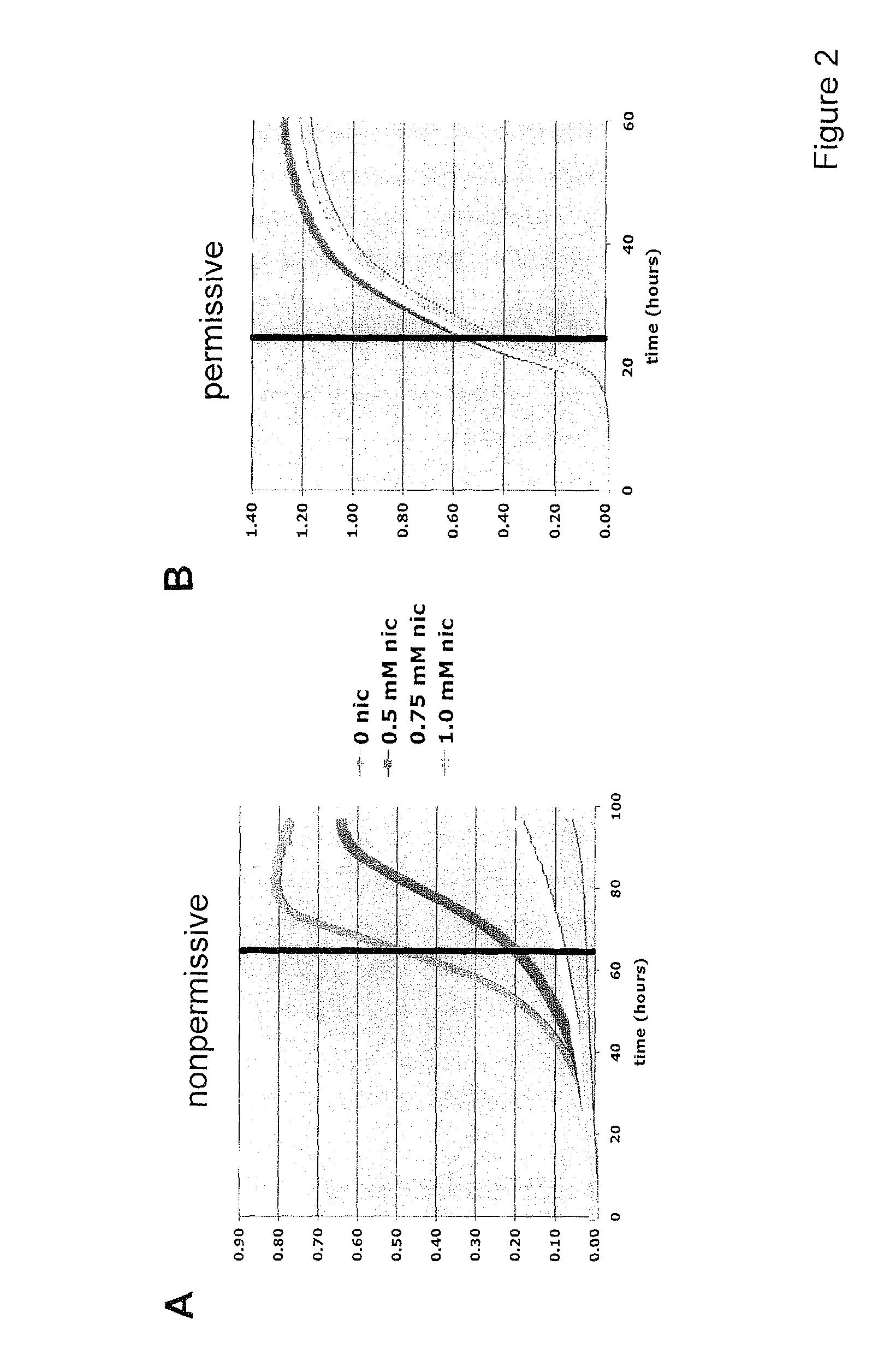
A method for altering the lifespan of a eukaryotic organism. The method comprises the steps of providing a lifespan altering compound, and administering an effective amount of the compound to a eukaryotic organism, such that the lifespan of the organism is altered. In one embodiment, the compound is identified using the DeaD assay.
Owner:UNIVERSITY OF ROCHESTER
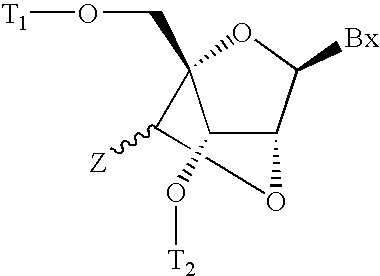
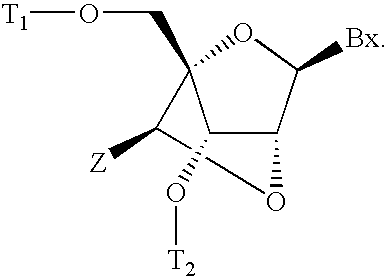
6-modified bicyclic nucleic acid analogs
The present invention provides 6-modified bicyclic nucleoside analogs and oligomeric compounds comprising these nucleoside analogs. In preferred embodiments the nucleoside analogs have either (R) or (S)-chirality at the 6-position. These bicyclicnucleoside analogs are useful for enhancing properties of oligomeric compounds including nuclease resistance.
Owner:IONIS PHARMA INC
Multispecific antibodies
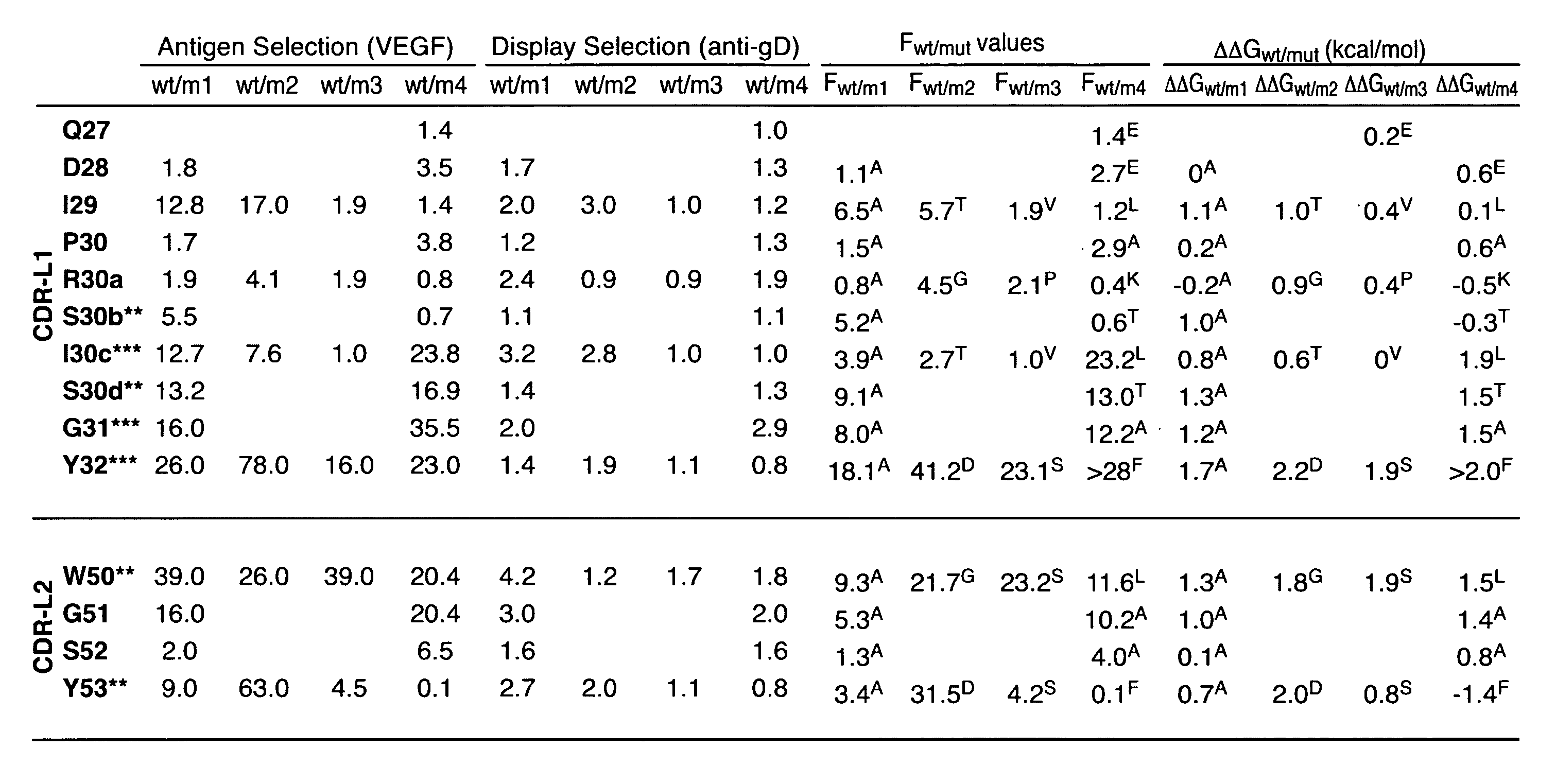
ActiveUS20080069820A1High binding affinityHigh affinityImmunoglobulins against growth factorsAntibody ingredientsSpecific antibody
Owner:GENENTECH INC
Humanization of antibodies
InactiveUS20050042664A1Limited diversityFast and less labor intensive productionHybrid immunoglobulinsMicrobiological testing/measurementAntigen bindingHumanized antibody
The present invention provides methods of re-engineering or re-shaping an antibody from a first species, wherein the re-engineered or re-shaped antibody does not elicit undesired immune response in a second species, and the re-engineered or re-shaped antibody retains substantially the same antigen binding-ability of the antibody from the first species. In accordance with the present invention, a combinatorial library comprising the CDRs of the antibody from the first species fused in frame with framework regions derived from a second species can be constructed and screened for the desired modified antibody. In particular, the present invention provides methods utilizing low homology acceptor antibody frameworks for efficiently humanizing an antibody or a fragment thereof. The present invention also provides antibodies produced by the methods of the invention.
Owner:MEDIMMUNE LLC
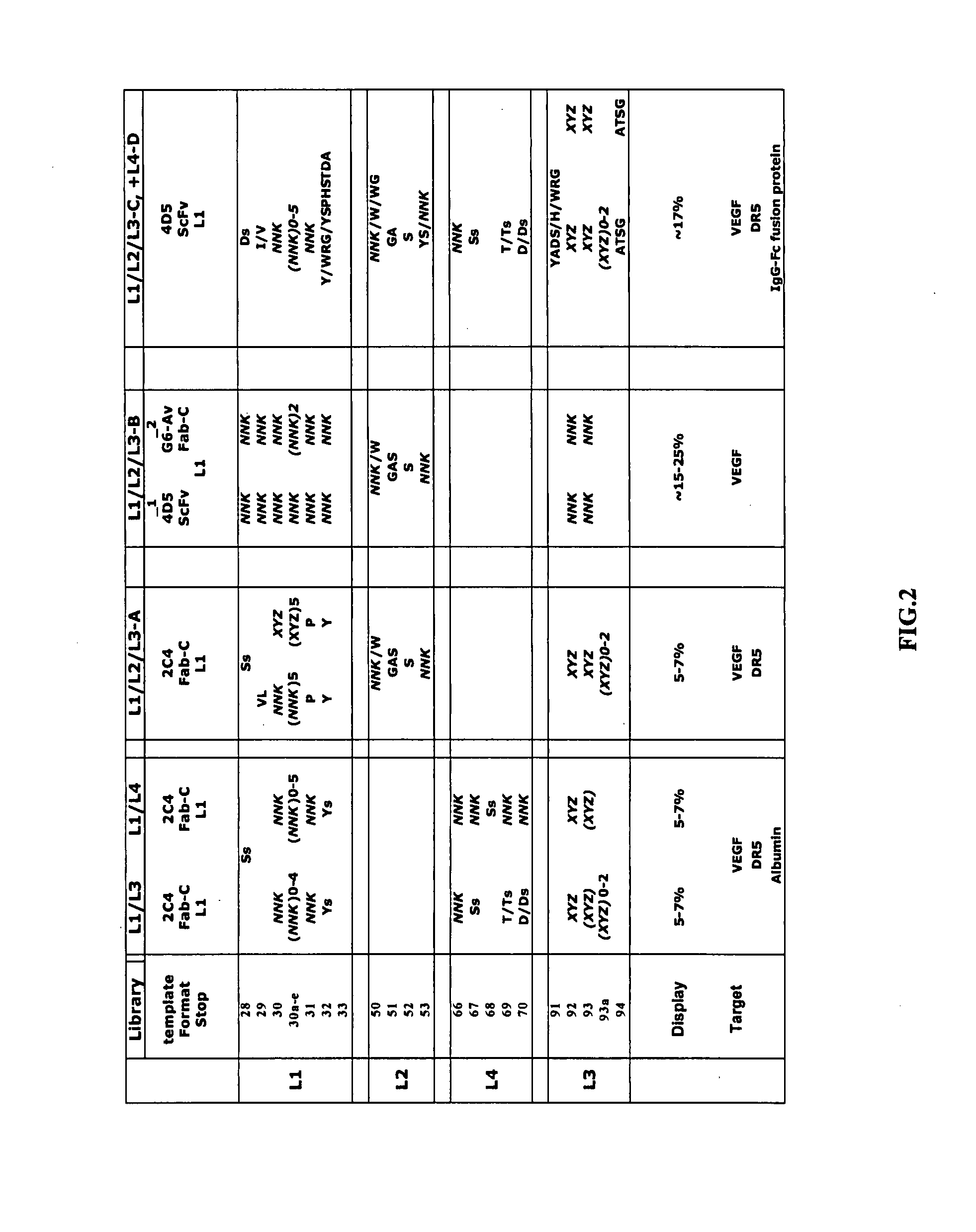
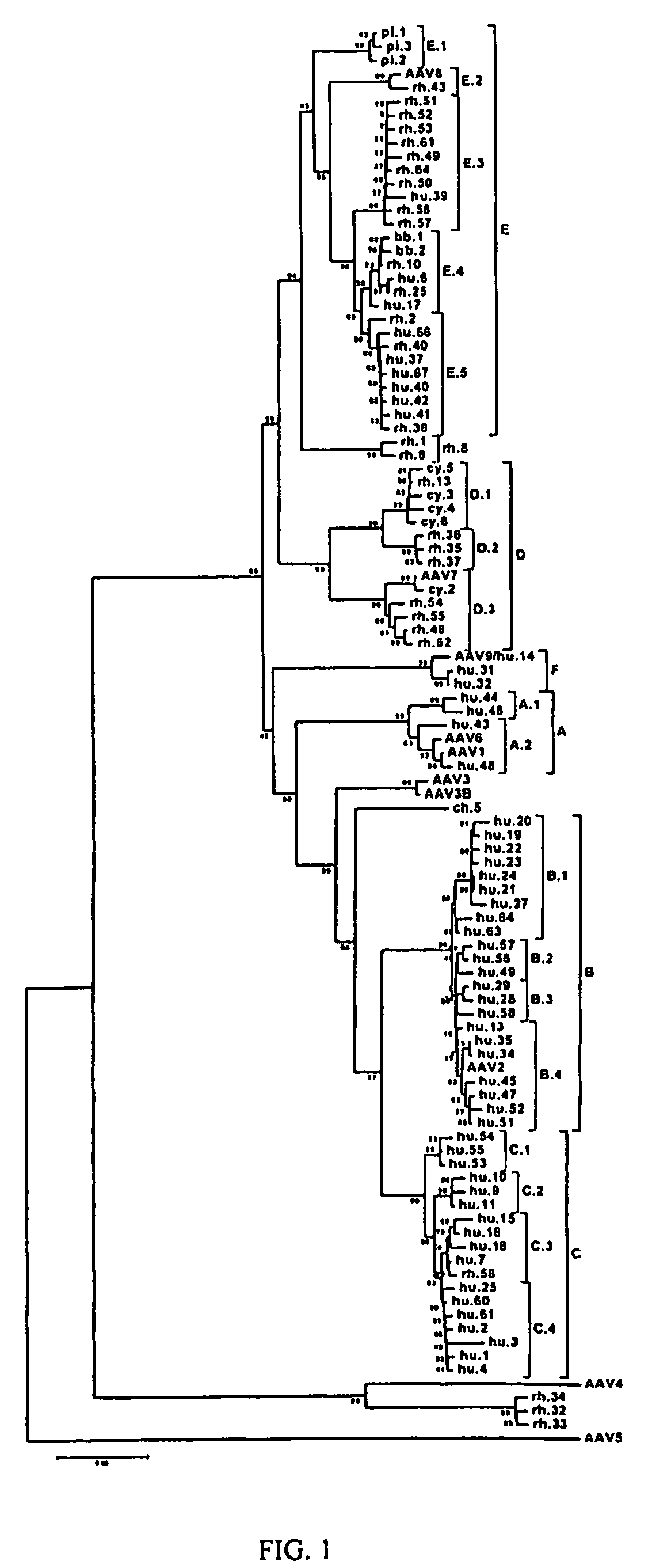
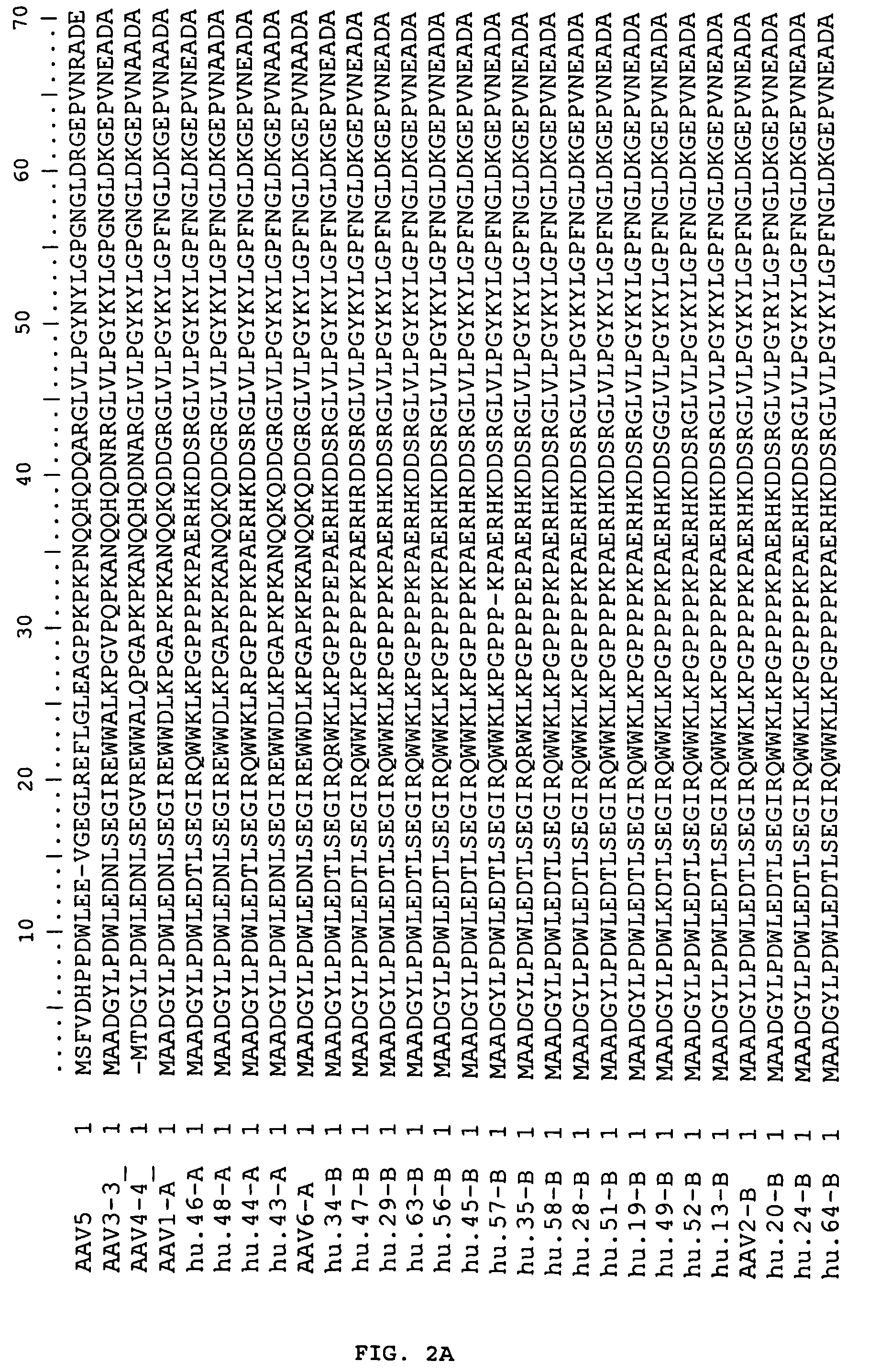
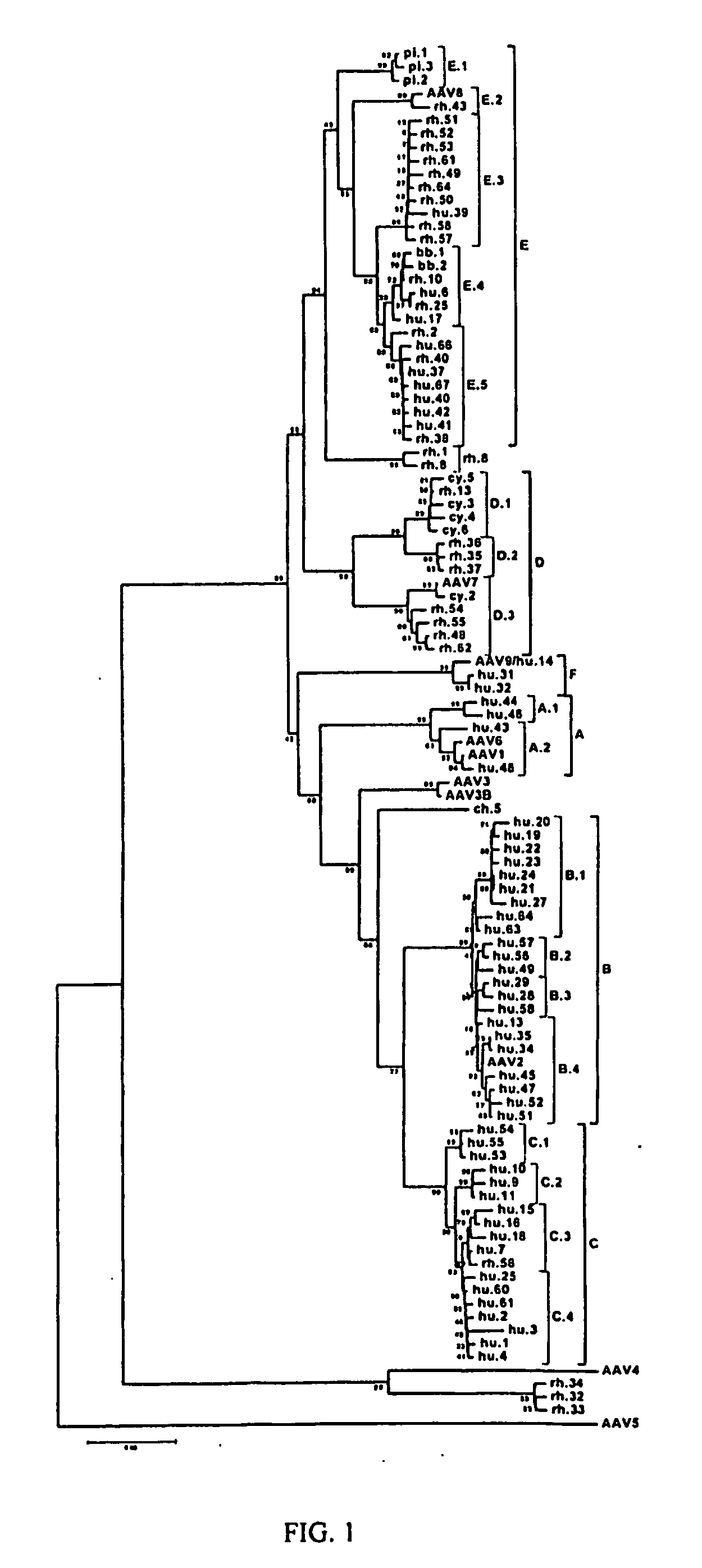
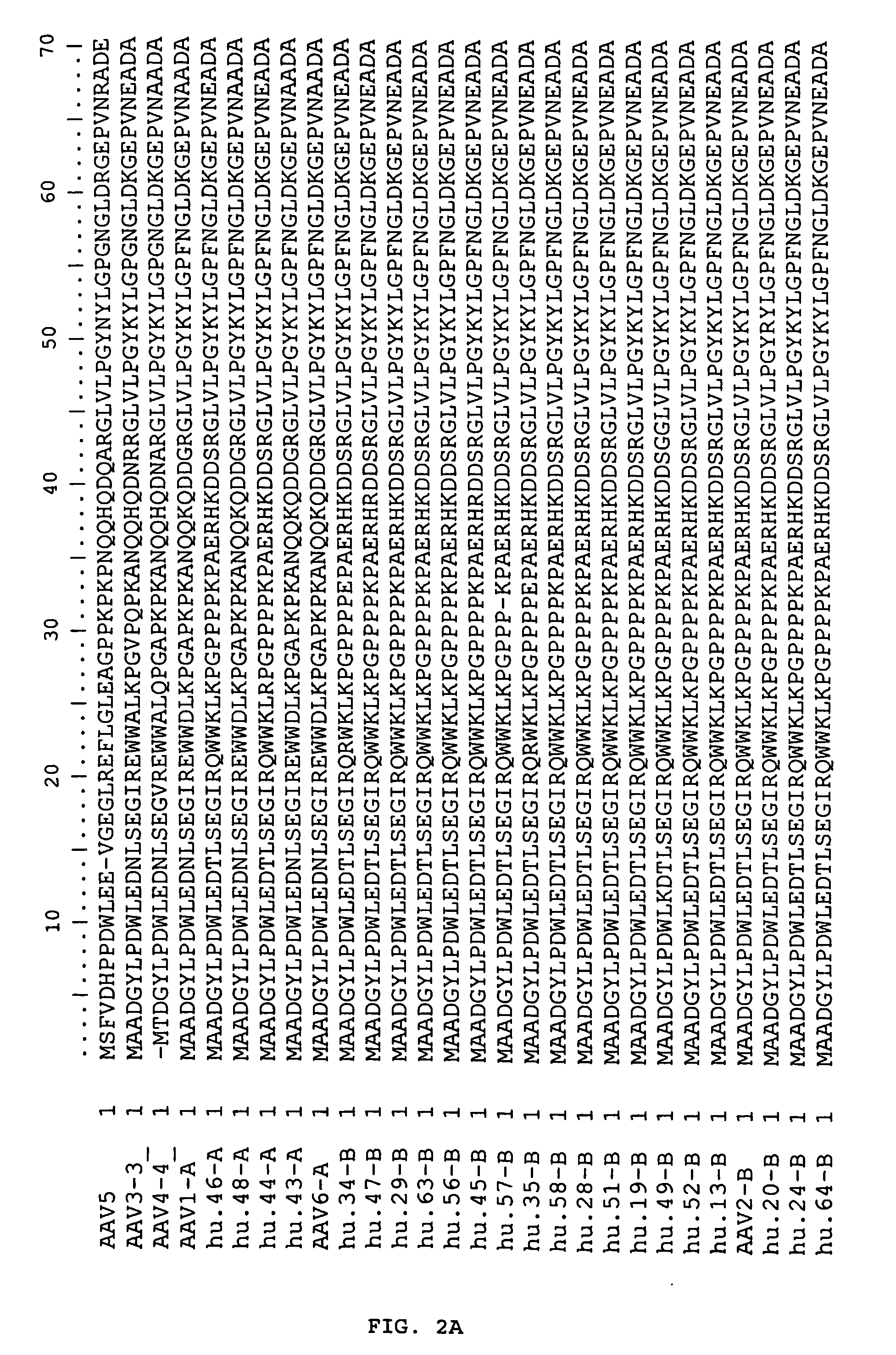
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
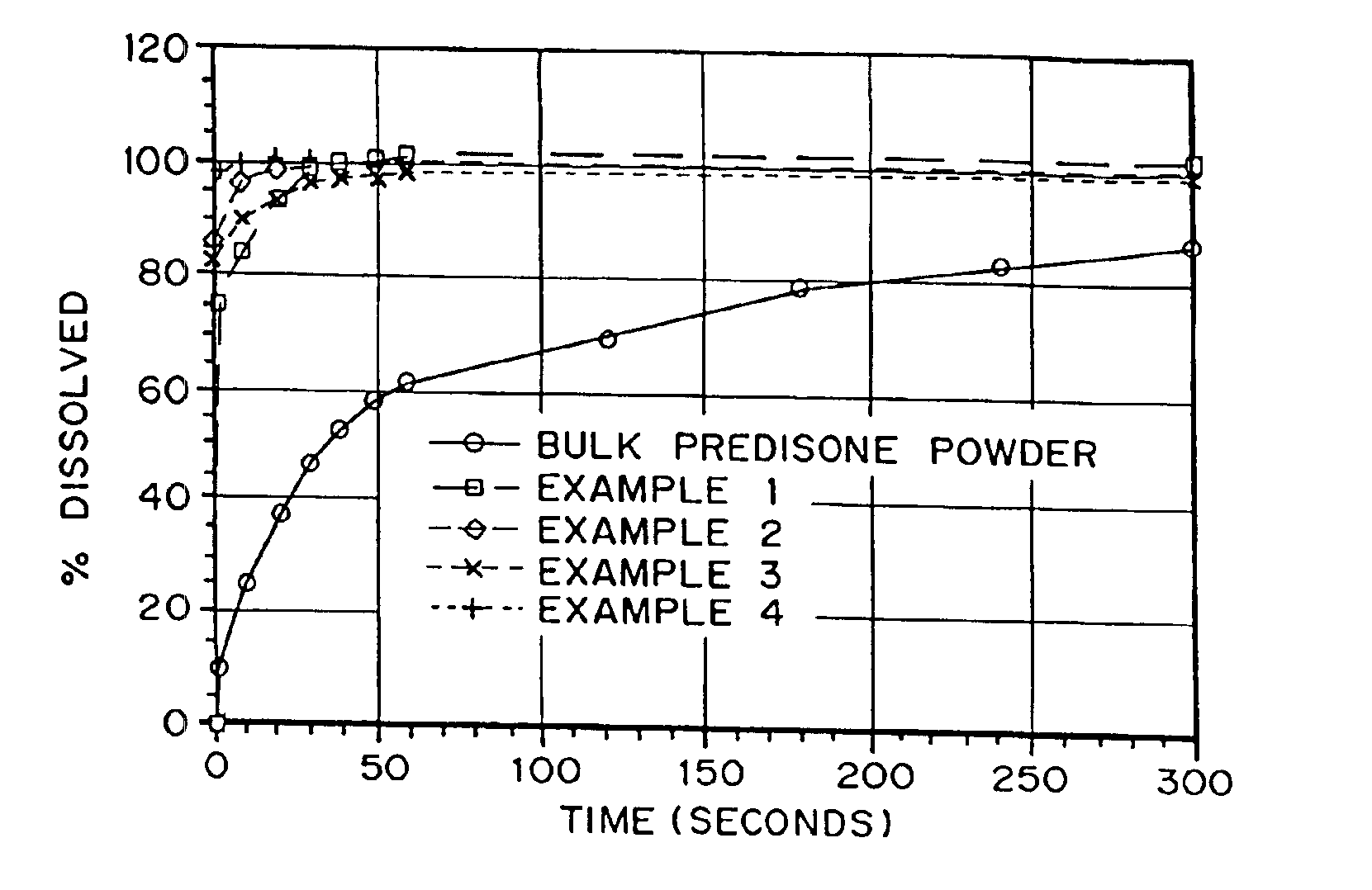
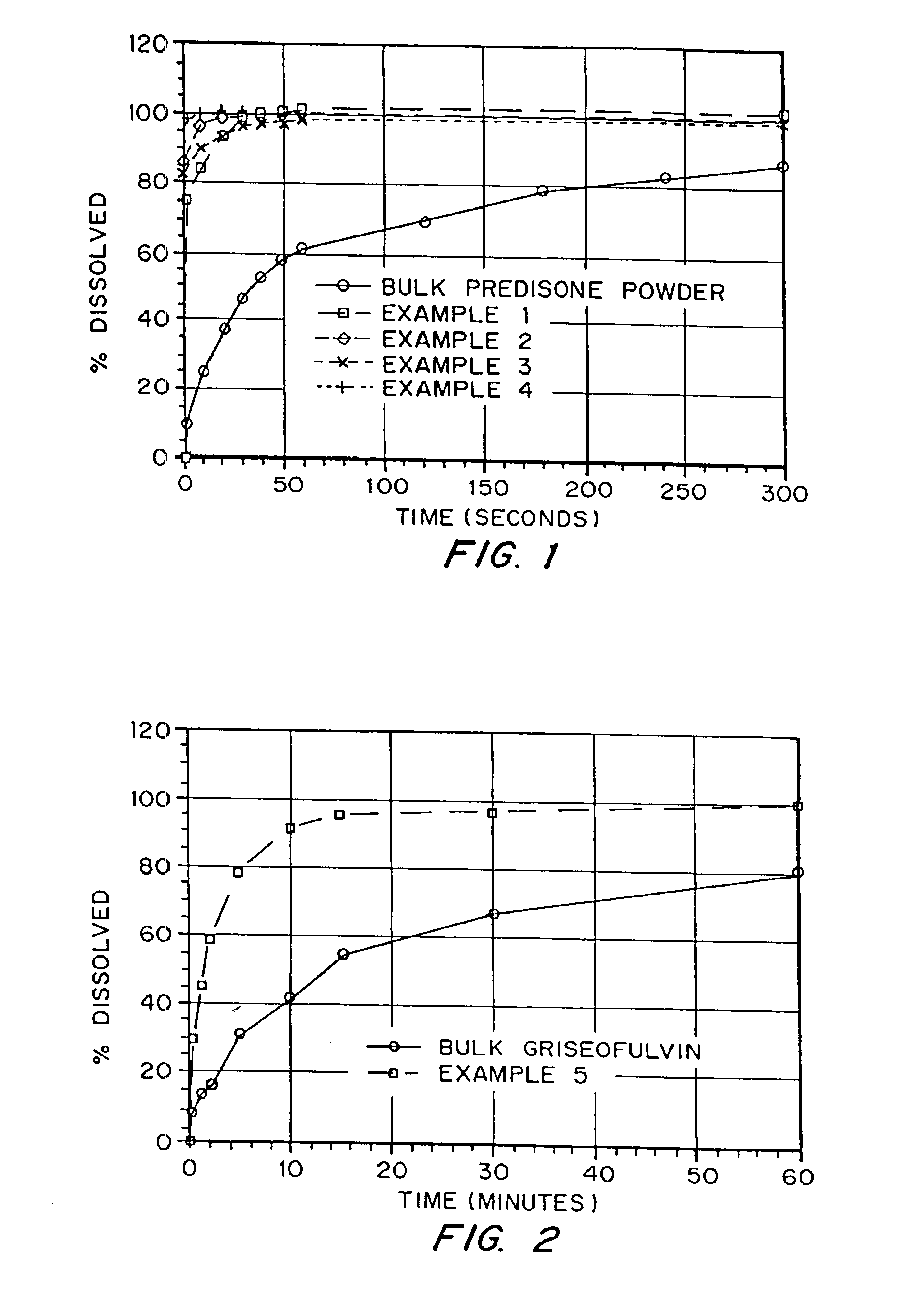
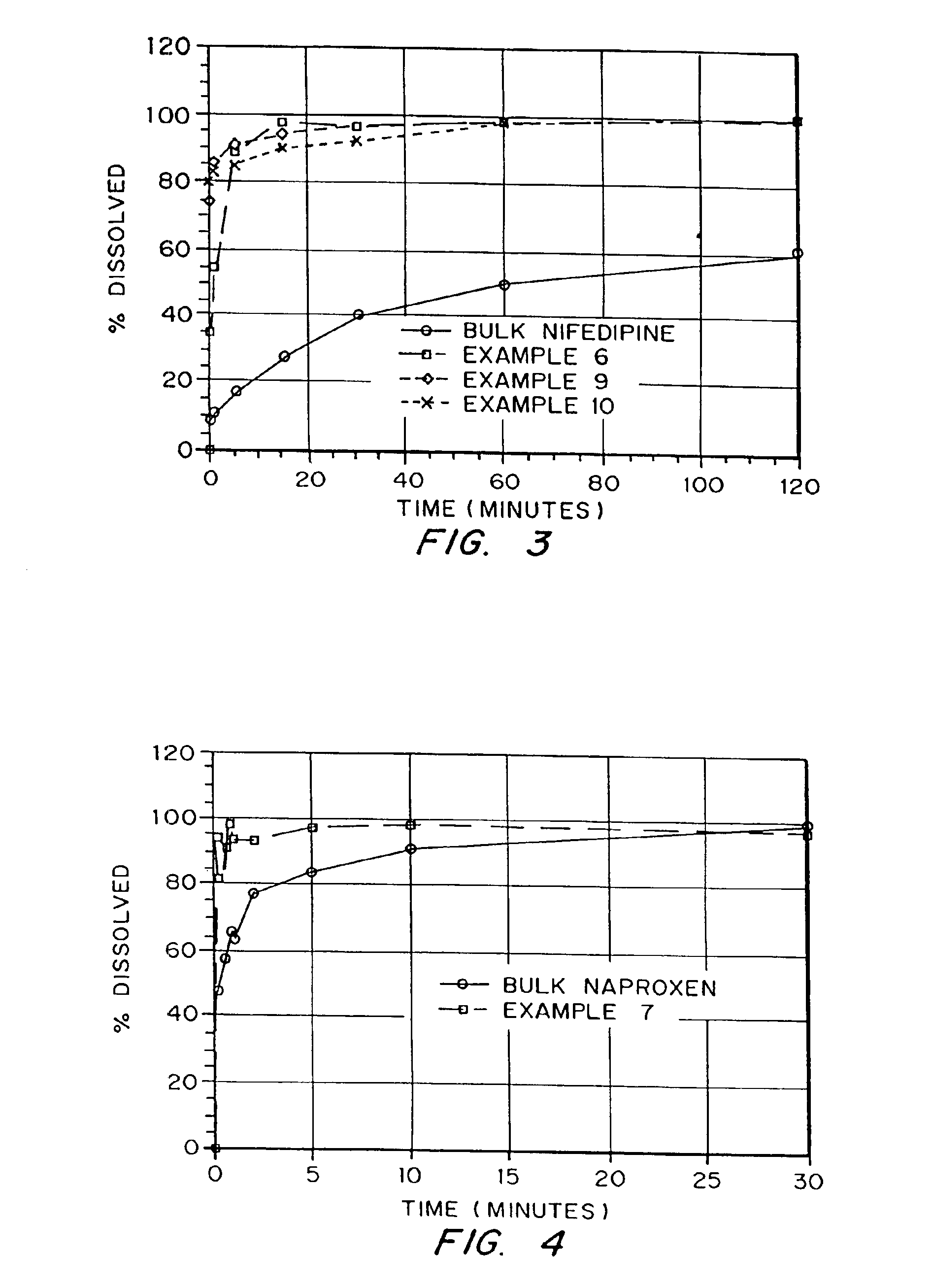
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Receptor specific transepithelial transport of therapeutics
InactiveUS6485726B1Effective strategyImprove abilitiesBacterial antigen ingredientsPeptide/protein ingredientsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
Nucleic Acid-Lipopolymer Compositions
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
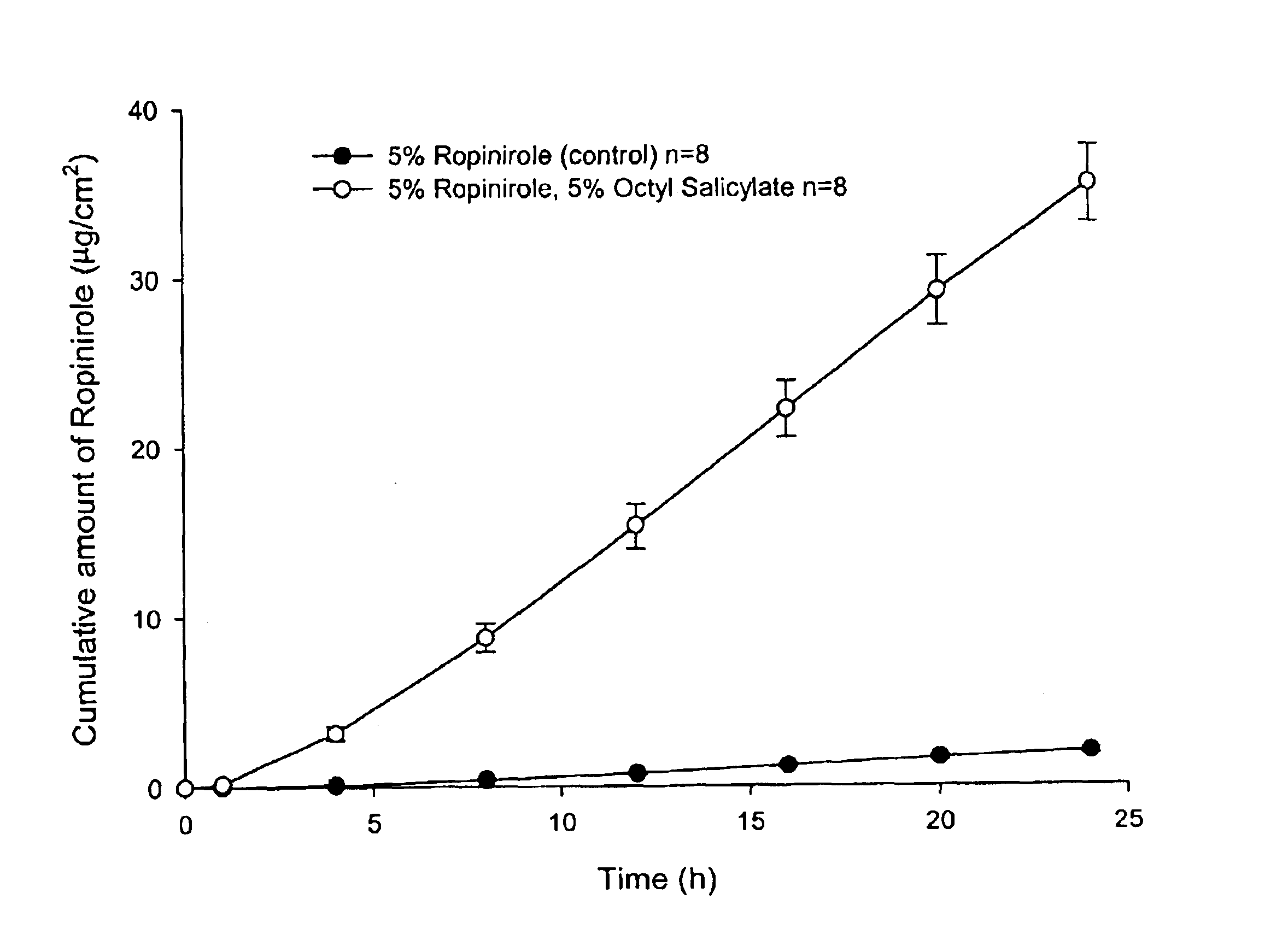
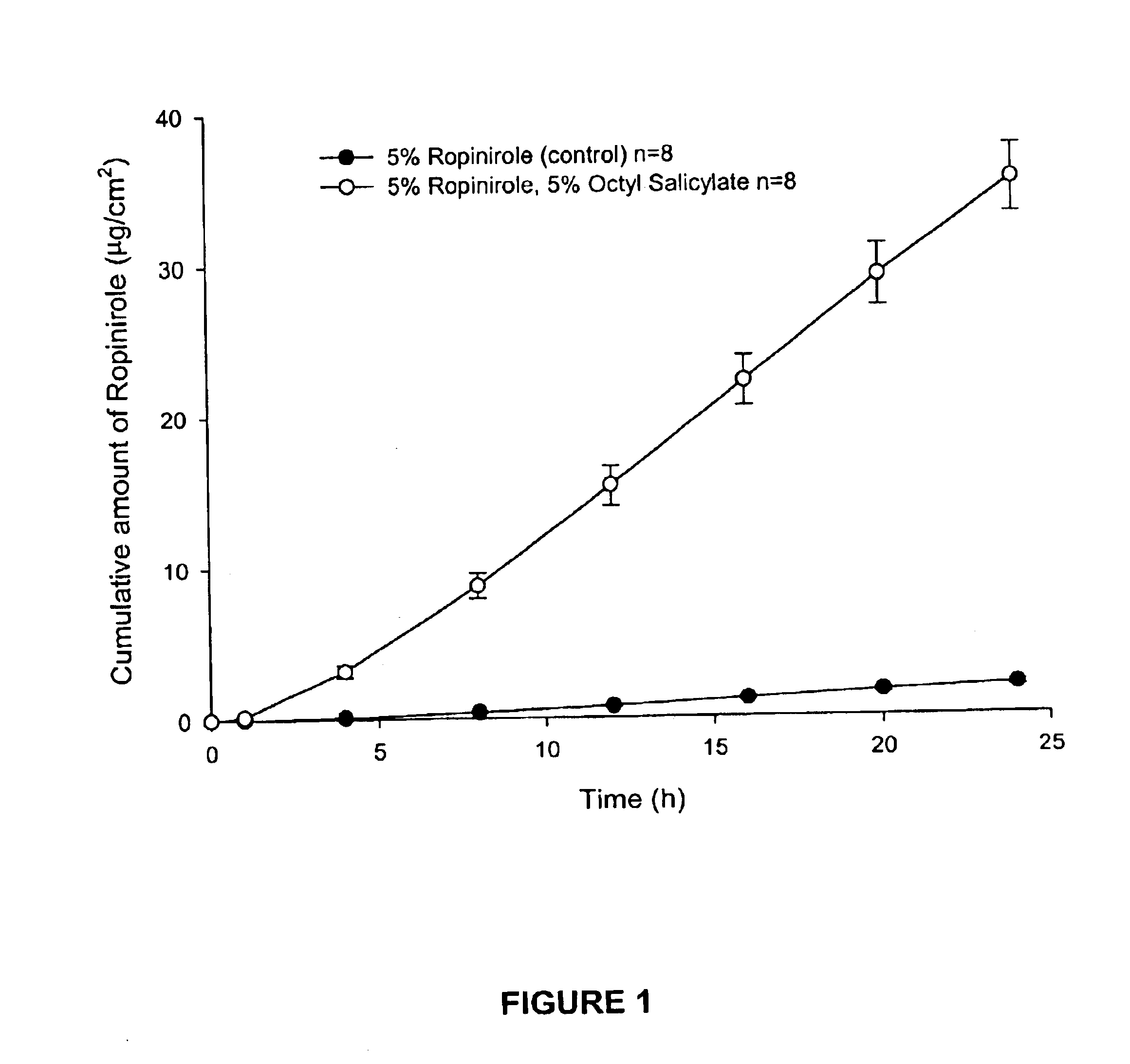
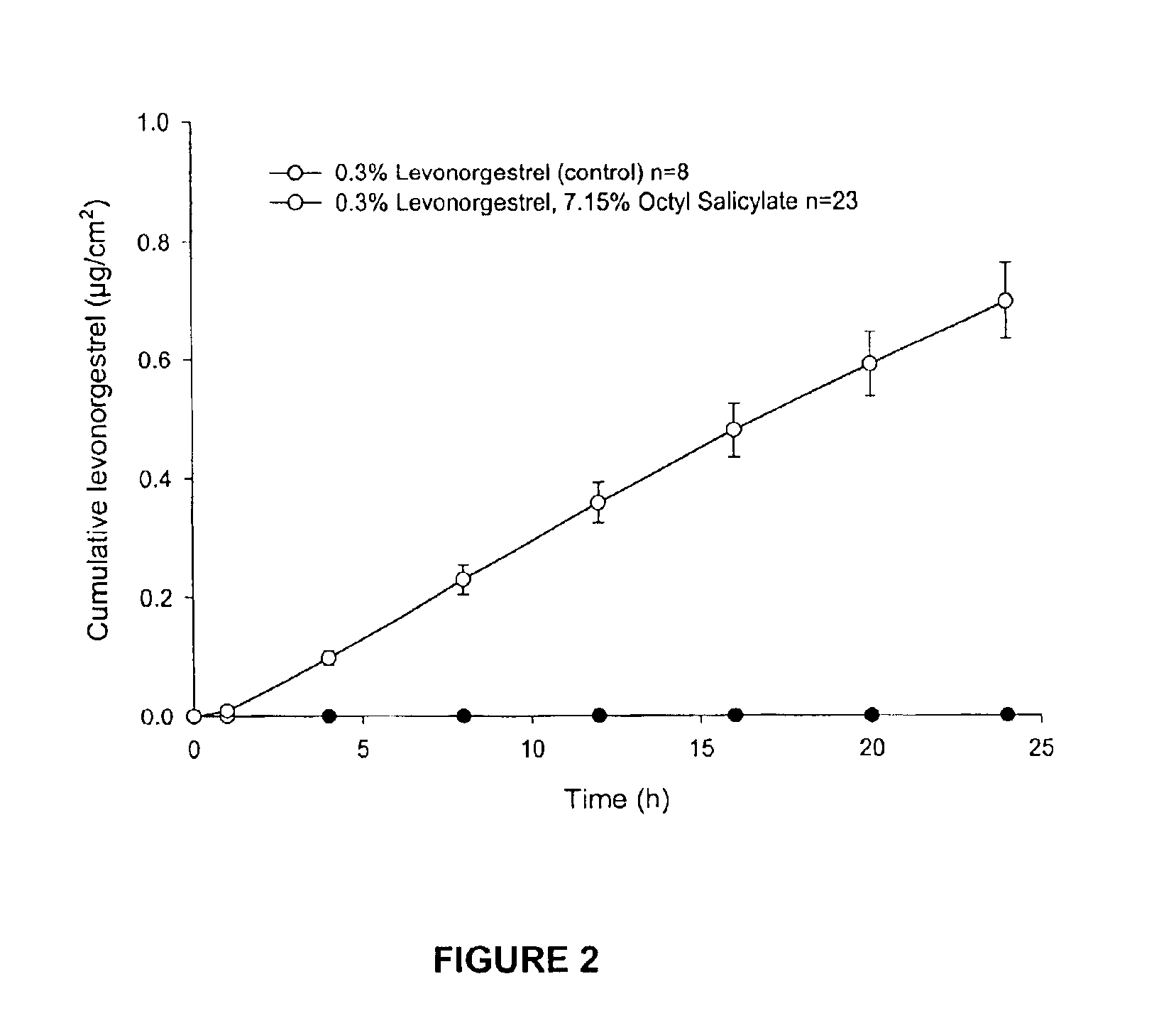
Transdermal delivery of antiparkinson agents
InactiveUS6929801B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyMedicine
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of an antiParkinson agent; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting antiParkinson agent to an animal which comprises applying an effective amount of the antiParkinson agent in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS
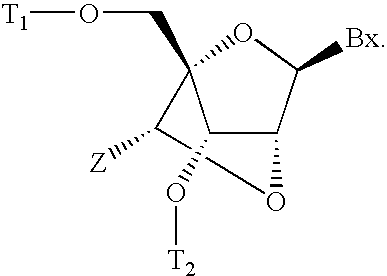
6-disubstituted or unsaturated bicyclic nucleic acid analogs
The present disclosure describes 6-disubstituted bicyclic nucleosides, oligomeric compounds prepared therefrom and methods of using the oligomeric compounds. More particularly, the 6-disubstituted bicyclic nucleosides each comprise a 2′-O—C(Ri)(R2)-4′ or 2′-O—C=(R3)(R.4)-4′ bridge wherein each R is, independently a substituent group and Ri and R2 include H. The 6-disubstituted bicyclic nucleosides are useful for enhancing properties of oligomeric compounds including nuclease resistance. In certain embodiments, the oligomeric compounds provided herein hybridize to a portion of a target RNA resulting in loss of normal function of the target RNA.
Owner:IONIS PHARMA INC
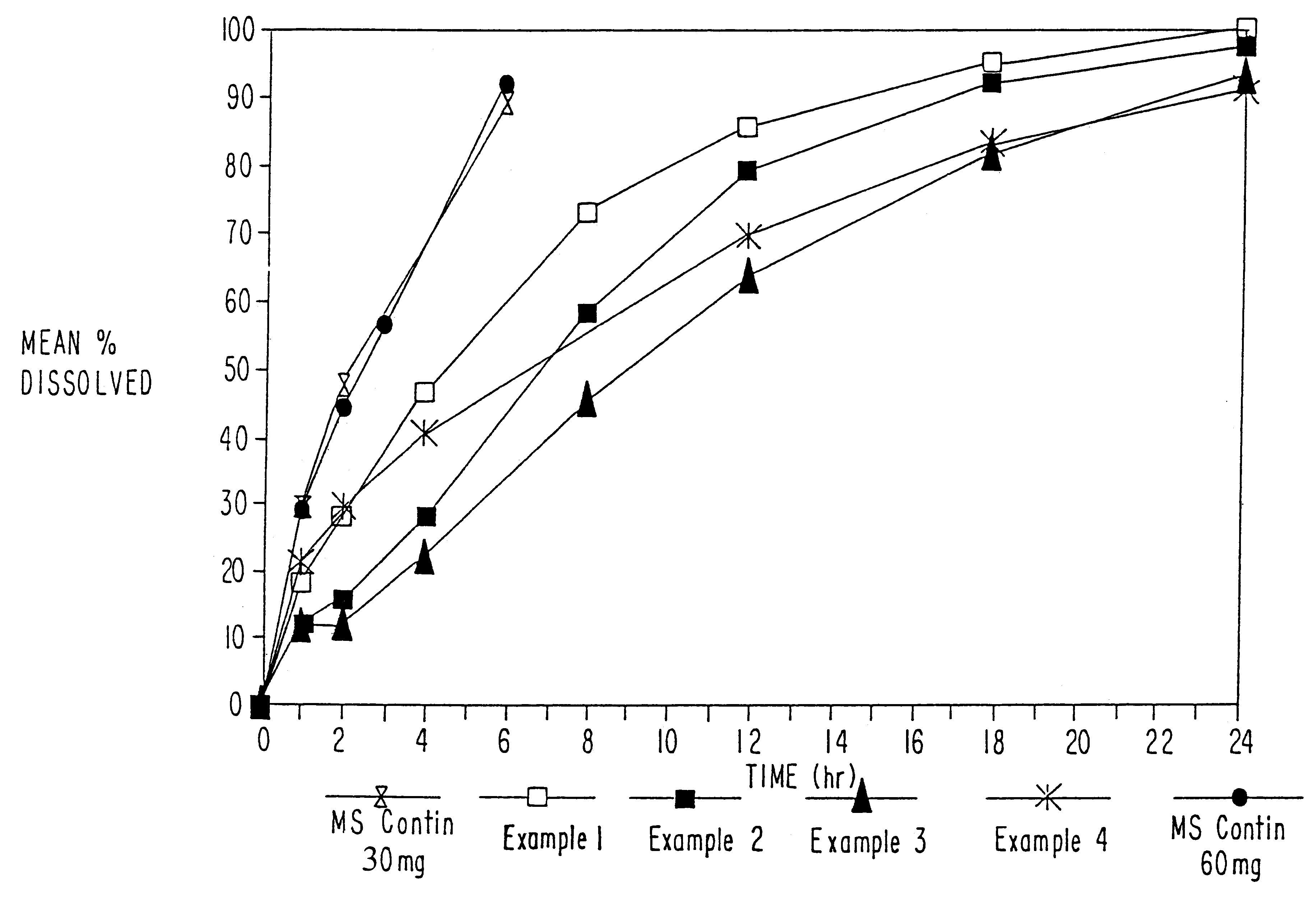
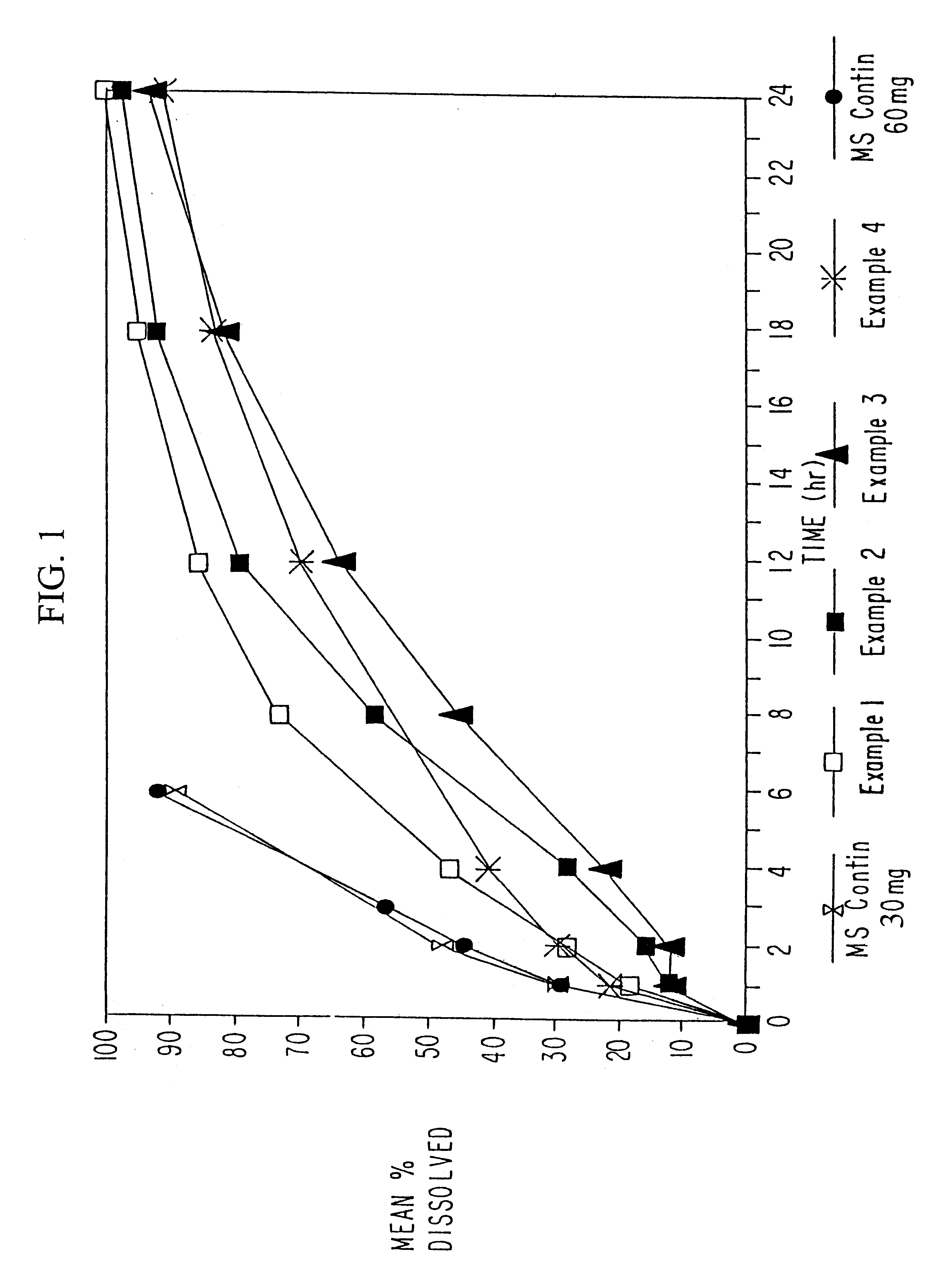
Orally administrable opioid formulations having extended duration of effect
InactiveUS6294195B1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Sustained release oral solid dosage forms of opioid analgesics are provided as multiparticulate systems which are bioavailable and which provide effective blood levels of the opioid analgesic for at least about 24 hours. A unit dose of the opioid analgesic contains a plurality of substrates including the opioid analgesic in sustained release form. The substrates have a diameter from about 0.1 mm to about 3 mm.
Owner:PURDUE PHARMA LP
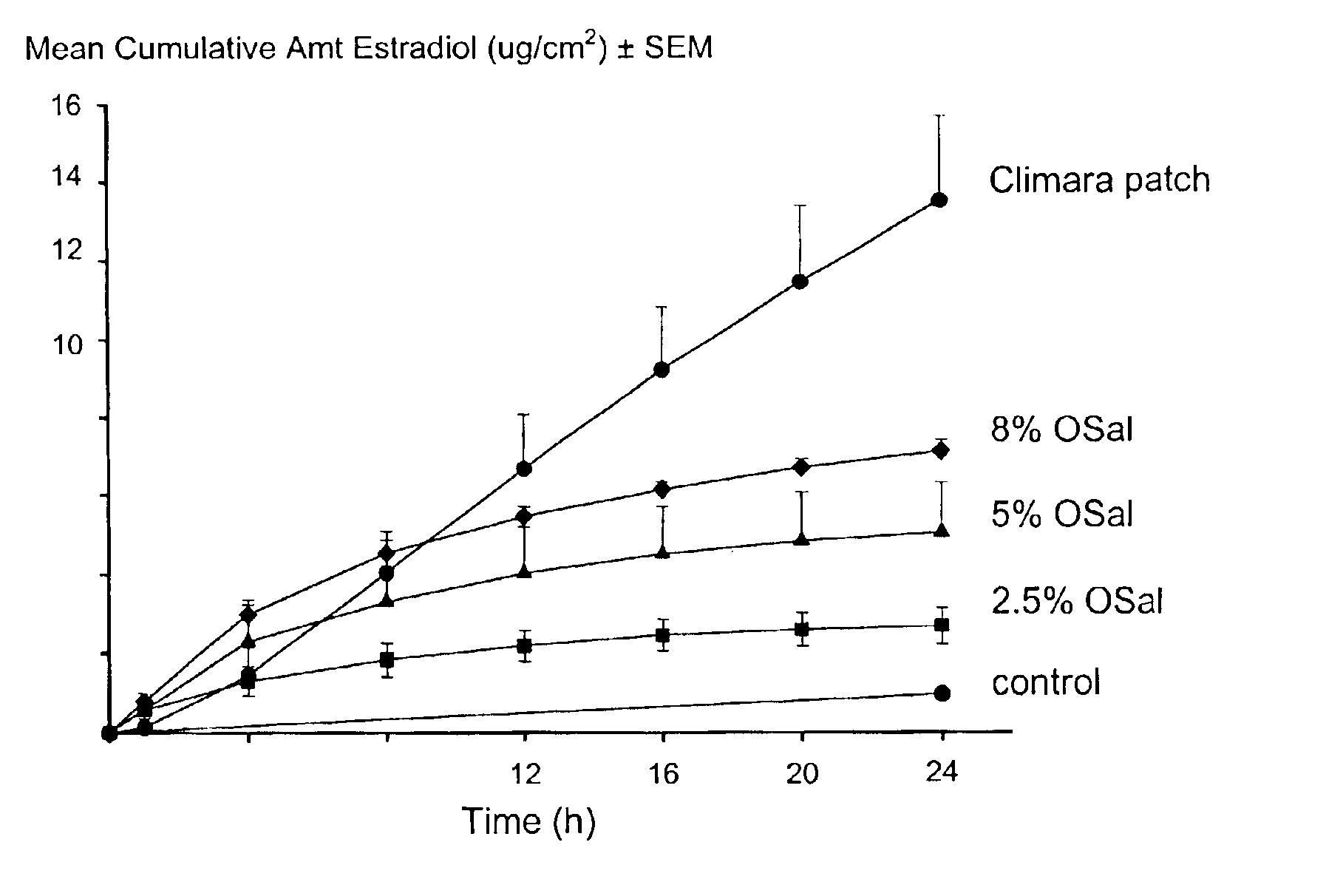
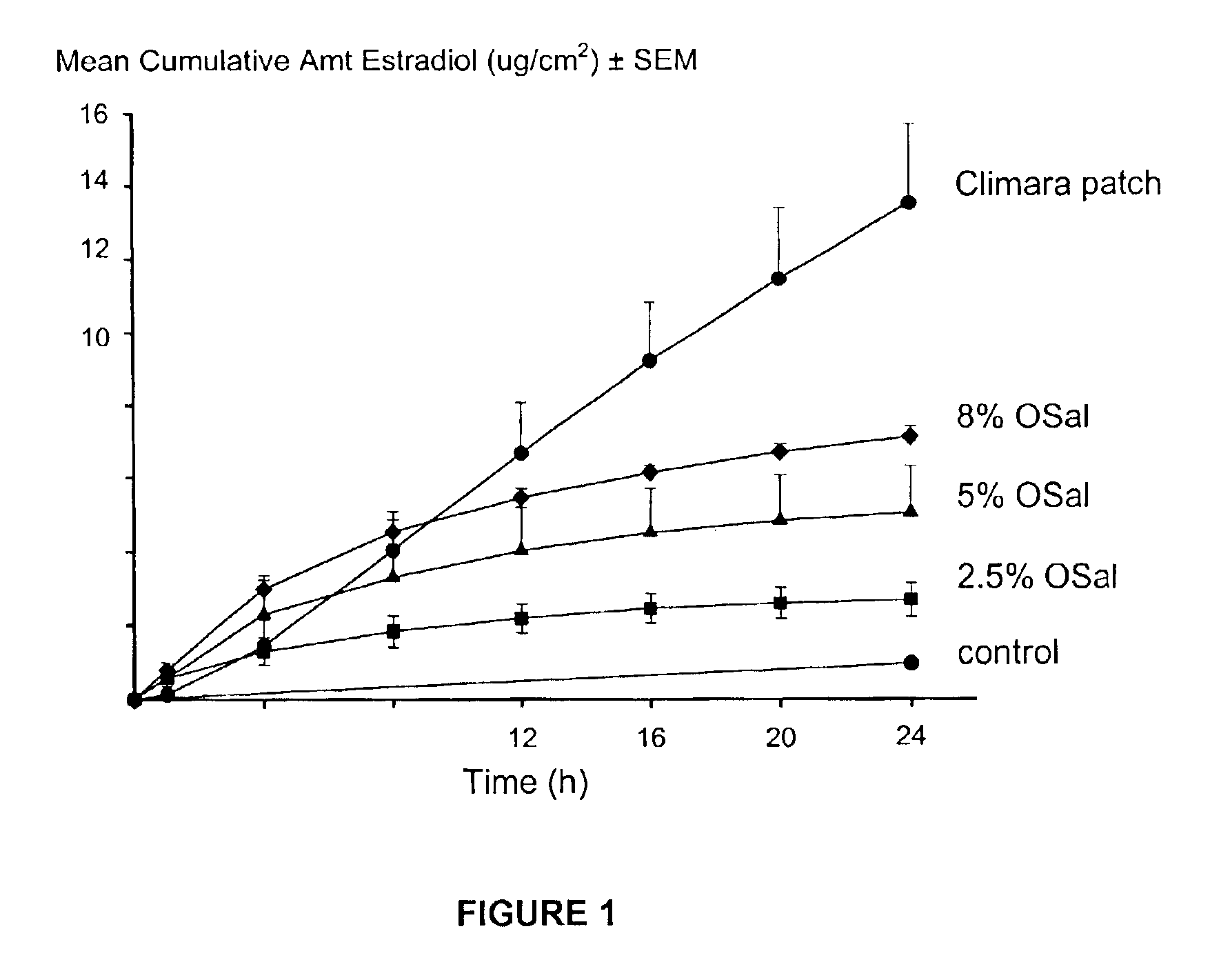
Transdermal delivery of hormones
InactiveUS6923983B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyHormones regulation
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of a hormone; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting hormone to an animal which comprises applying an effective amount of the hormone in the form of the drug delivery system of the present invention
Owner:ACRUX DDS
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Transdermal delivery system
The present invention relates to the discovery of a transdermal delivery system that can deliver high molecular weight pharmaceuticals and cosmetic agents to skin cells. A novel transdermal delivery system with therapeutic and cosmetic application and methods of use of the foregoing is disclosed.
Owner:ORIX +1
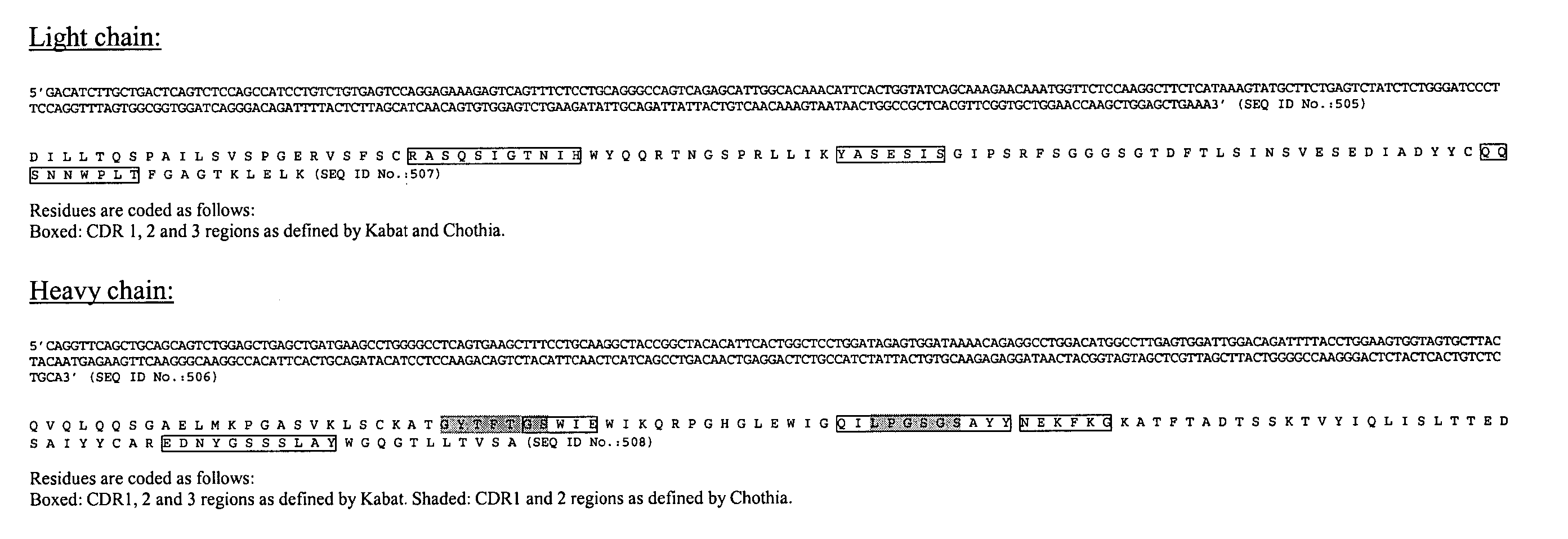
Antibody specificity transfer using minimal essential binding determinants
The present invention provides methods of making antibodies having the binding specificity of a reference antibody. Antibodies generated by the methods of the inventions have at least one minimal essential binding specificity determinant from a heavy chain or light chain CDR3 from the reference antibody. The method can be used, e.g., in humanization procedures. The invention also provides libraries and antibodies made in accordance with the methods.
Owner:HUMANIGEN INC
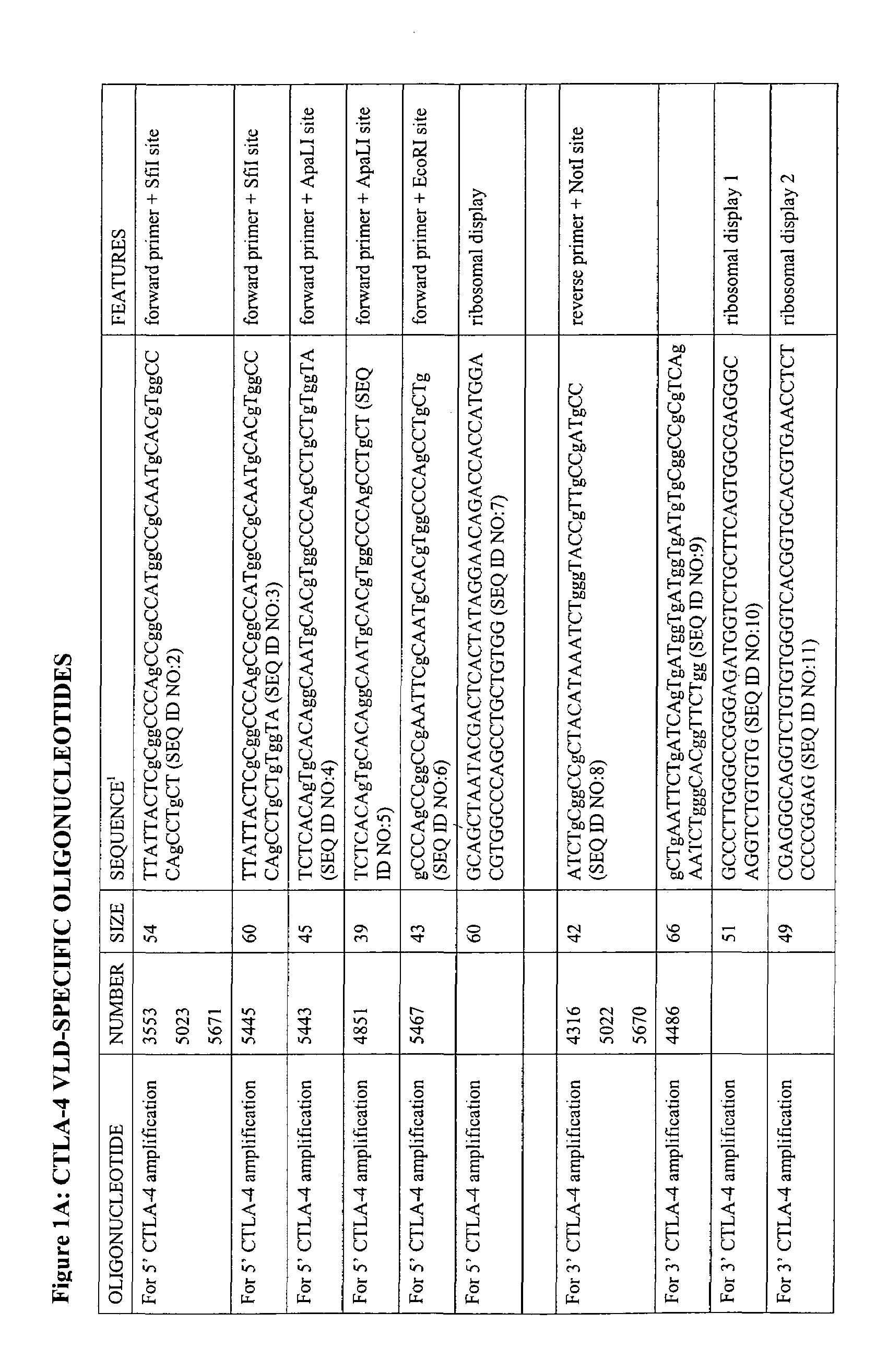
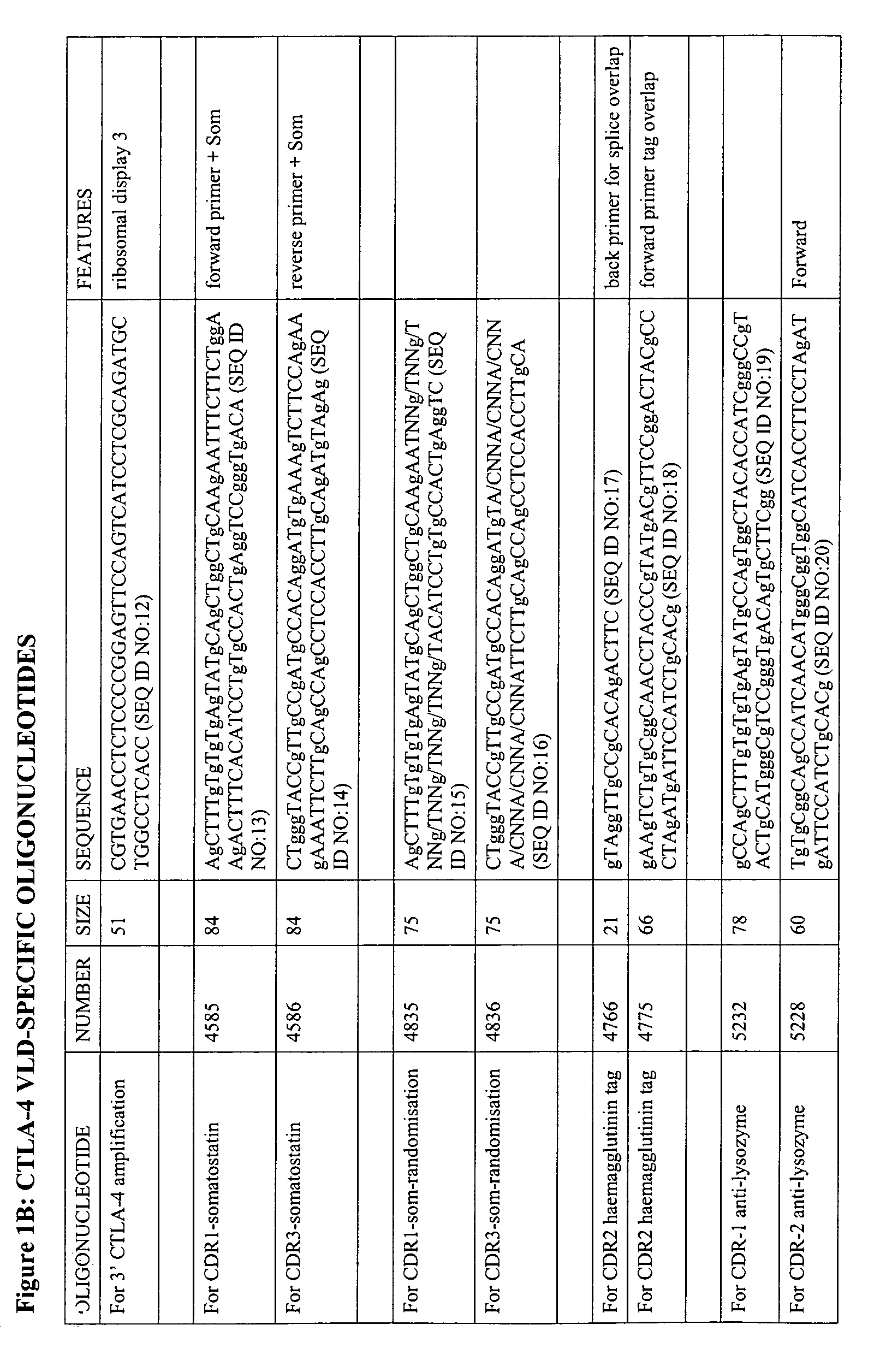
V-like domain binding molecules
The present invention relates to binding moieties comprising at least one monomeric V-like domain (VLD) derived from a non-antibody ligand, the at least one monomeric V-like domain being characterized in that at least one CDR loop structure or part thereof is modified or replaced such that the solubility of the modified VLD is improved when compared with the unmodified VLD.
Owner:ARANA THERAPEUTICS VIC
Serum albumin binding peptides for tumor targeting
InactiveUS20050287153A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthPeptide ligand
Peptide ligands having affinity for serum albumin are useful for tumor targeting. Conjugate molecules comprising a serum albumin binding peptide fused to a biologically active molecule demonstrate modified pharmacokinetic properties as compared with the biologically active molecule alone, including tissue (e.g., tumor) uptake, infiltration, and diffusion.
Owner:GENENTECH INC
Method for altering the lifespan of eukaryotic organisms
A method for altering the lifespan of a eukaryotic organism. The method comprises the steps of providing a lifespan altering compound, and administering an effective amount of the compound to a eukaryotic organism, such that the lifespan of the organism is altered. In one embodiment, the compound is identified using the DeaD assay.
Owner:UNIVERSITY OF ROCHESTER
Broad-spectrum delta -endotoxins
InactiveUS6110464AImprove insecticidal effectBroad-range specificityNanotechBacteriaAureobasidium sp.Toxin
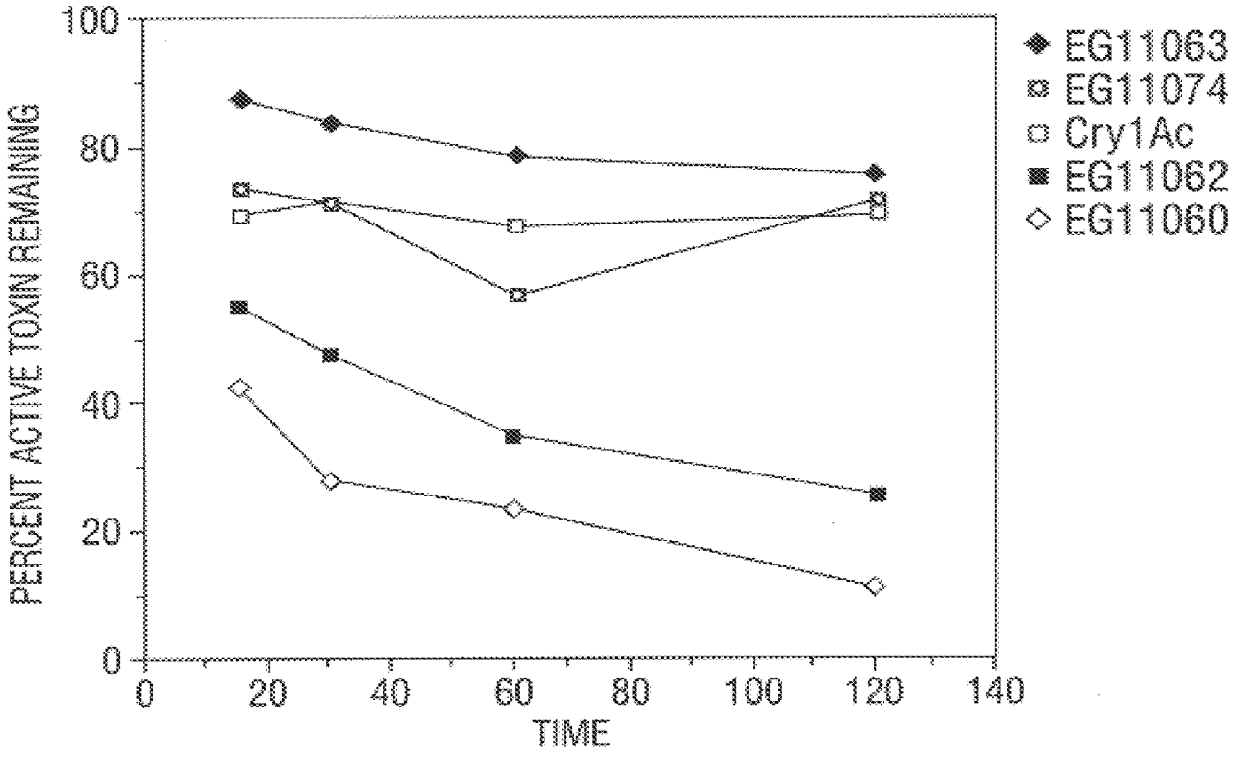
Disclosed are novel synthetically-modified B. thuringiensis chimeric crystal proteins having improved insecticidal activity against coleopteran, dipteran and lepidopteran insects. Also disclosed are the nucleic acid segments encoding these novel peptides. Methods of making and using these genes and proteins are disclosed as well as methods for the recombinant expression, and transformation of suitable host cells. Transformed host cells and transgenic plants expressing the modified endotoxin are also aspects of the invention.
Owner:MONSANTO TECH LLC
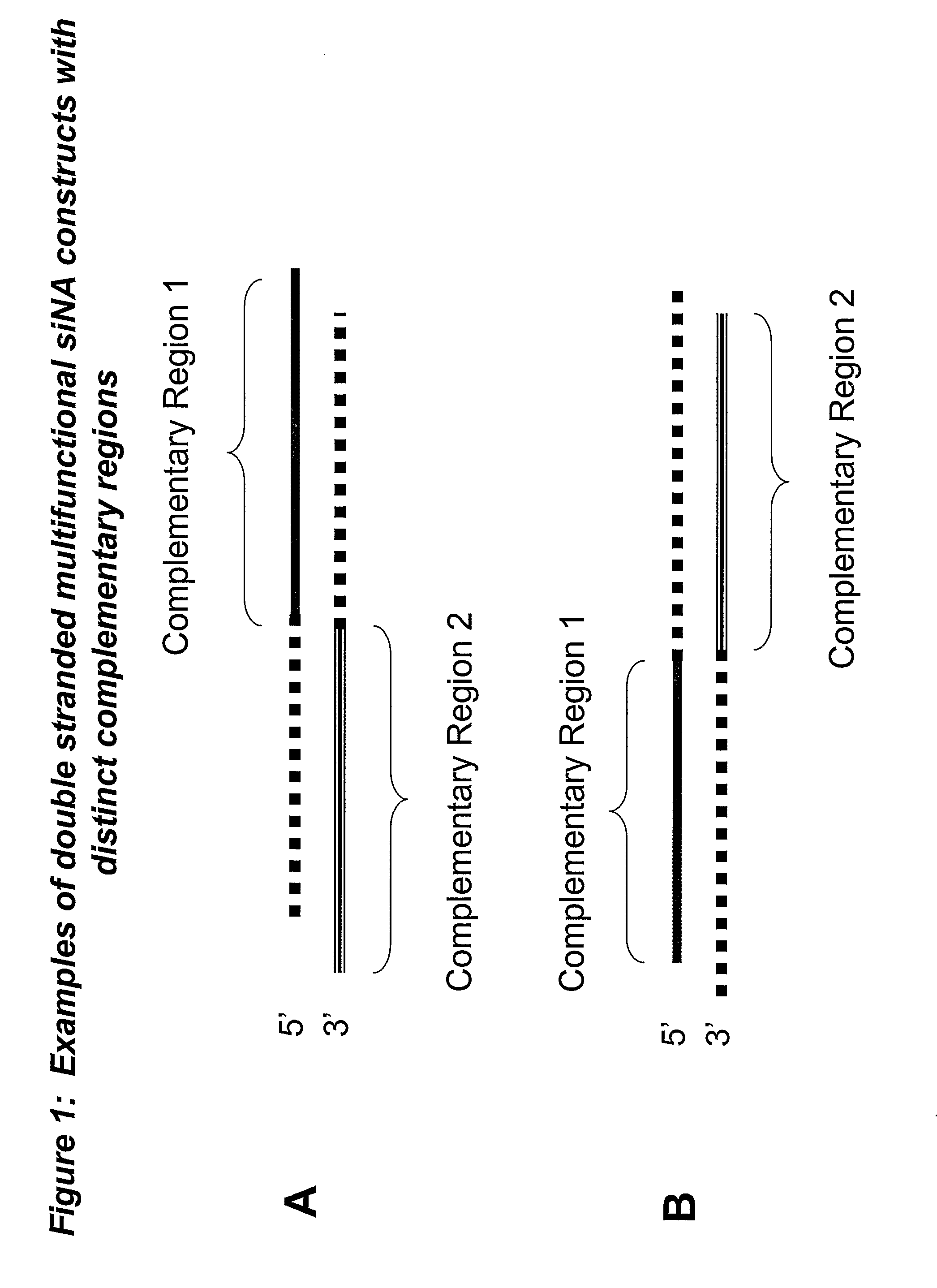
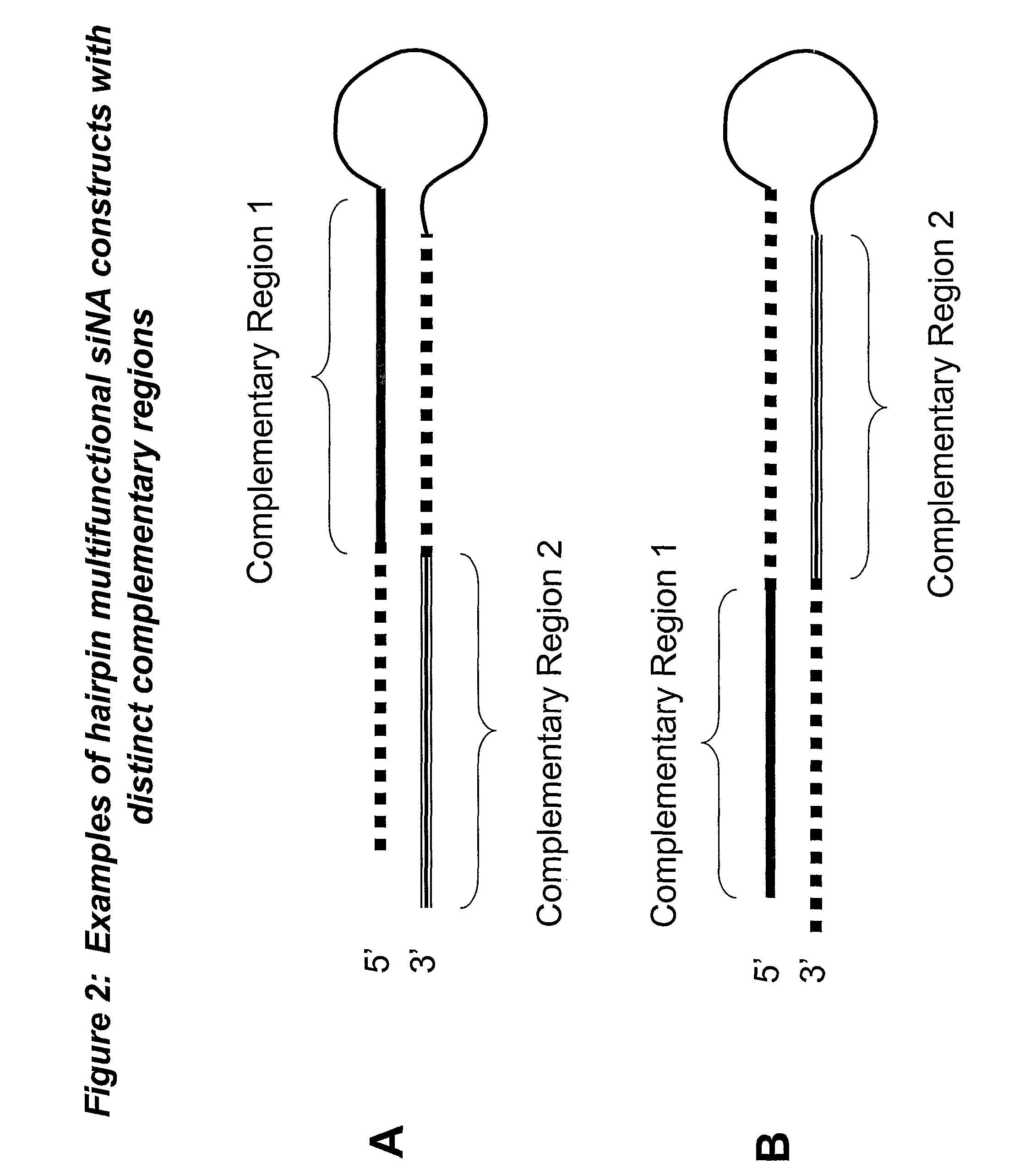
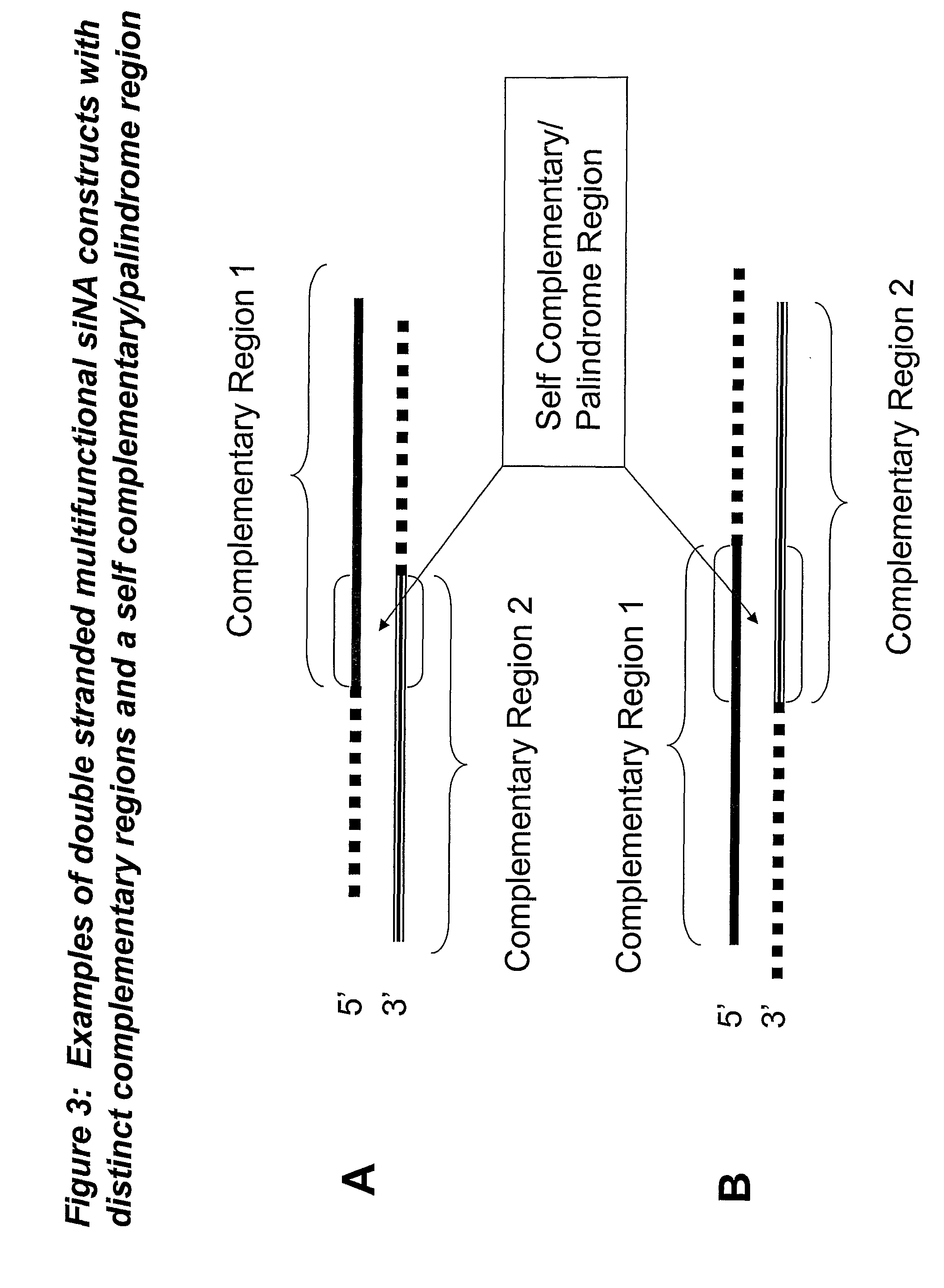
RNA interference mediated inhibition of gene expression using multifunctional short interfering nucleic acid (multifunctional siNA)
ActiveUS7858769B2Down regulating or inhibiting the expression of one or more target nucleicInhibit and reduce expressionSugar derivativesPeptide/protein ingredientsDiseaseBiological body
The present invention concerns methods and nucleic acid based reagents useful in modulating gene expression in a variety of applications, including use in therapeutic, veterinary, agricultural, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to multifunctional short interfering nucleic acid (multifunctional siNA) molecules that modulate the expression of one or more genes in a biologic system, such as a cell, tissue, or organism via RNA interference (RNAi). The bifunctional short interfering nucleic acid (multifunctional siNA) molecules of the invention can target more than one regions of nucleic acid sequence in a single target nucleic acid molecule or can target regions of nucleic acid sequence in differing target nucleic acid molecules. The self multifunctional siNA molecules are useful in the treatment of any disease or condition that responds to modulation of gene expression or activity in a cell, tissue, or organism.
Owner:SIRNA THERAPEUTICS INC
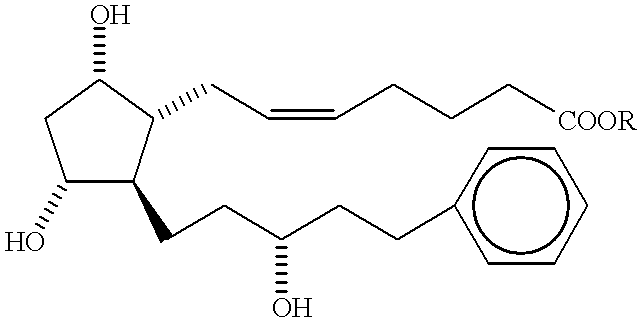
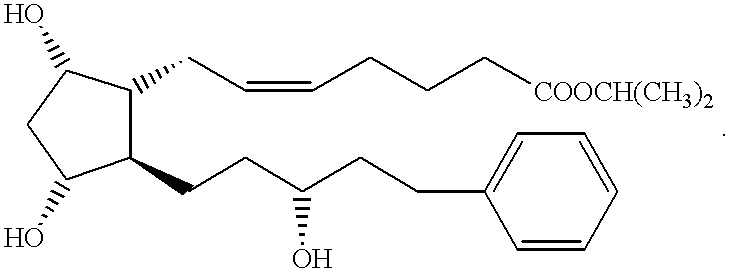
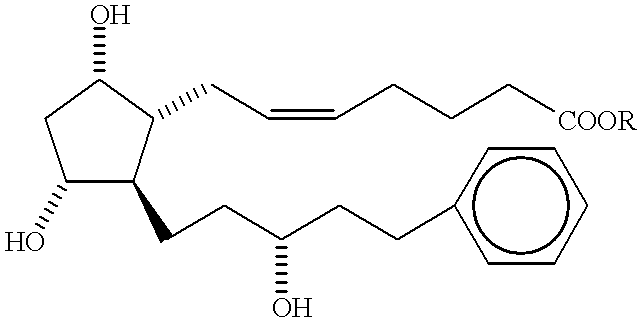
Method of treating macular degeneration with a prostaglandin derivative
InactiveUS6225348B1Reduce elevated intraocular pressureReduce morbidityBiocideElcosanoid active ingredientsProstaglandin F2alphaProstaglandin PGE
A method of treating age-related macular degeneration in an eye is described, comprising contacting the eye with a therapeutic amount of a prostaglandin F2alpha derivative. The preferred prostaglandin F2alpha derivative is latanoprost, and the preferred dosage is between about 1.5 mug and about 4.5 mug per day per eye.
Owner:PAULSEN ALFRED W
Generation of modified molecules with increased serum half-lives
InactiveUS20020142374A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsSerum igeHalf-life
In accordance with the present invention, there are provided methods for the extension of serum half-lives of proteinaceous molecules, particularly antibody molecules, and compositions of molecules modified in accordance with the methods of the invention. In accordance with a first aspect of the present invention, there is provided a method of modifying the half-life of an antibody through providing an antibody containing an FcRn binding domain or the genes encoding such antibody and physically linking the antibody or the antibody as encoded to a second FcRn binding domain. In accordance with a second aspect of the present invention, there is provided a molecule that contains at least two distinct FcRn binding moieties.
Owner:ABQENIX INC
Gene silencing
InactiveUS6753139B1Reduce non-sequence specific bindingShorten the lengthBiocideElectrolysis componentsBiological bodyTranscriptional gene silencing
Owner:PLANT BIOSCI LTD
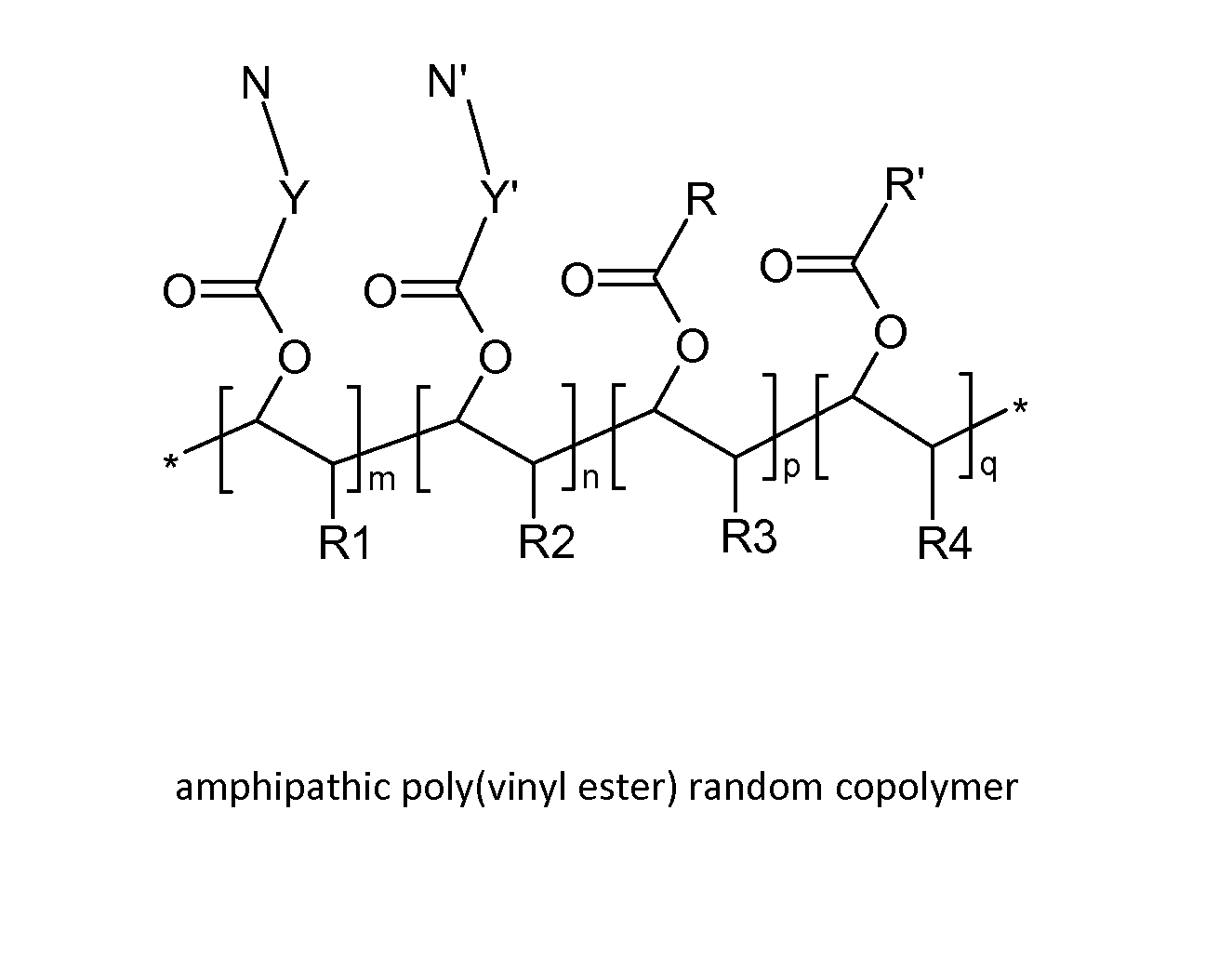
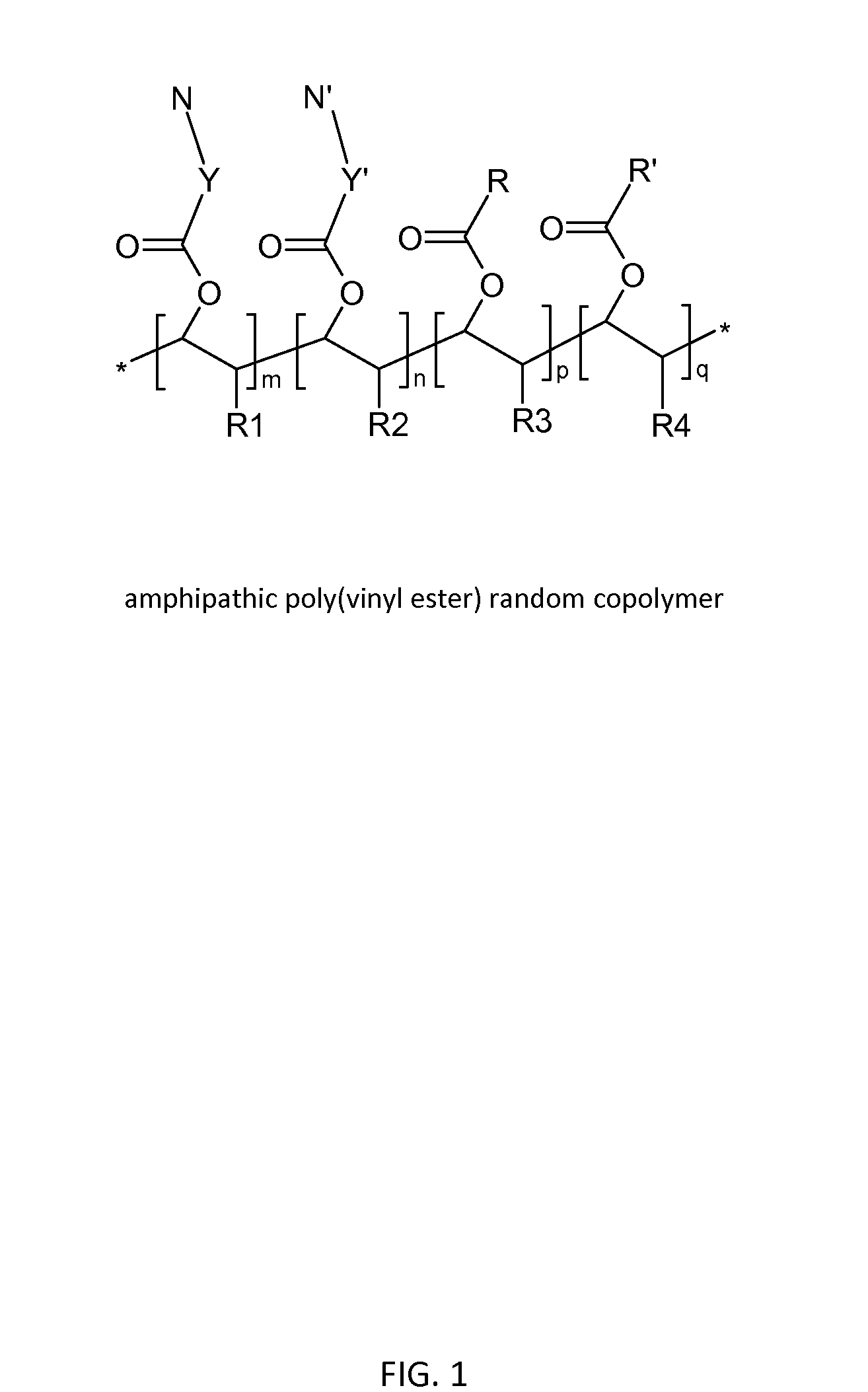
Poly(vinyl ester) Polymers for In Vivo Nucleic Acid Delivery
ActiveUS20130121954A1Suppression problemBeneficial level of expressionPharmaceutical non-active ingredientsDrug compositionsMembrane activityVinyl ester
The present invention is directed membrane active poly(vinyl ester) polymers and compositions for targeted delivery of RNA interference (RNAi) polynucleotides to cells in vivo. RNAi polynucleotides are conjugated to the poly(vinyl ester) polymers and the polymers are reversibly modified to enable in vivo targeted delivery. Membrane activity of the poly(vinyl ester) provides for movement of the RNAi polynucleotides from outside the cell to inside the cell. Reversible modification provides physiological responsiveness.
Owner:ARROWHEAD MADISON
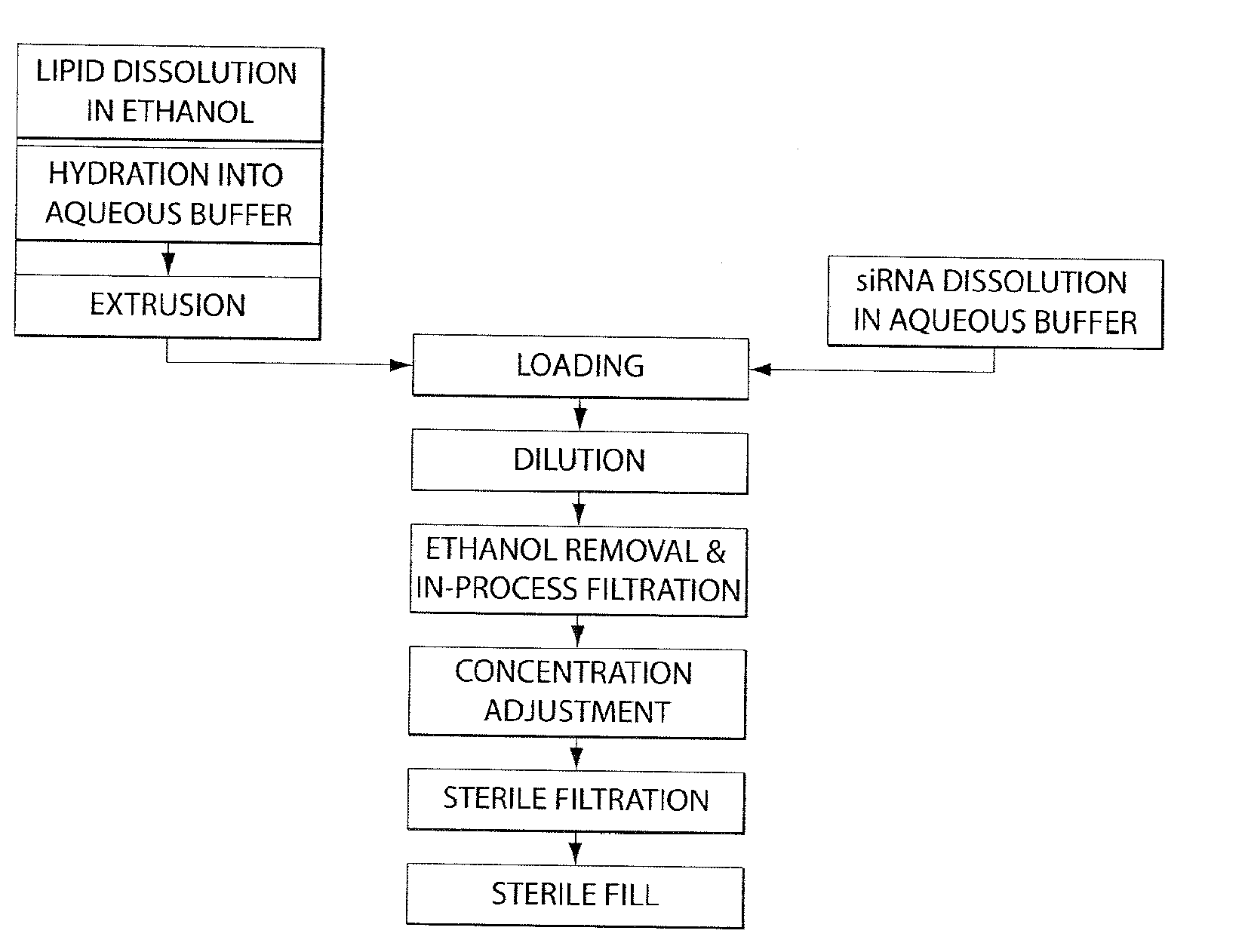
Lipid formulation
The invention features an improved lipid formulation comprising a cationic lipid of formula (A), a neutral lipid, a sterol and a PEG or PEG-modified lipid, where R1 and R2 are independently alkyl, alkenyl or alkynyl, each can be optionally substituted, and R3 and R4 are independently lower alkyl or R3 and R4 can be taken together to form an optionally substituted heterocyclic ring. In one embodiment, R1 and R2 are independently selected from oleoyl, pamitoyl, steroyl, linoleyl and R3 and R4 are methyl. Also disclosed are targeting lipids, and specific lipid formulations comprising such targeting lipids.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
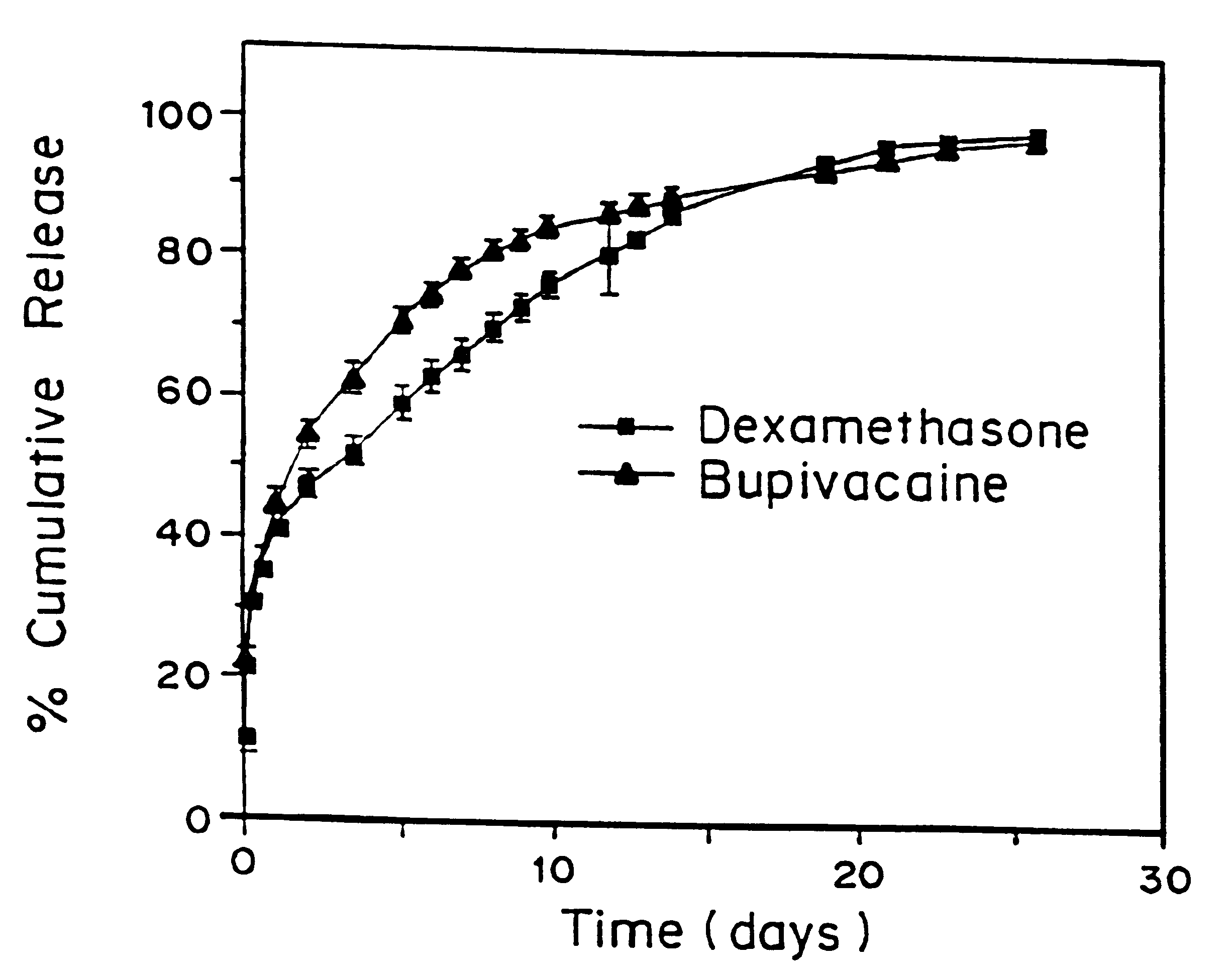
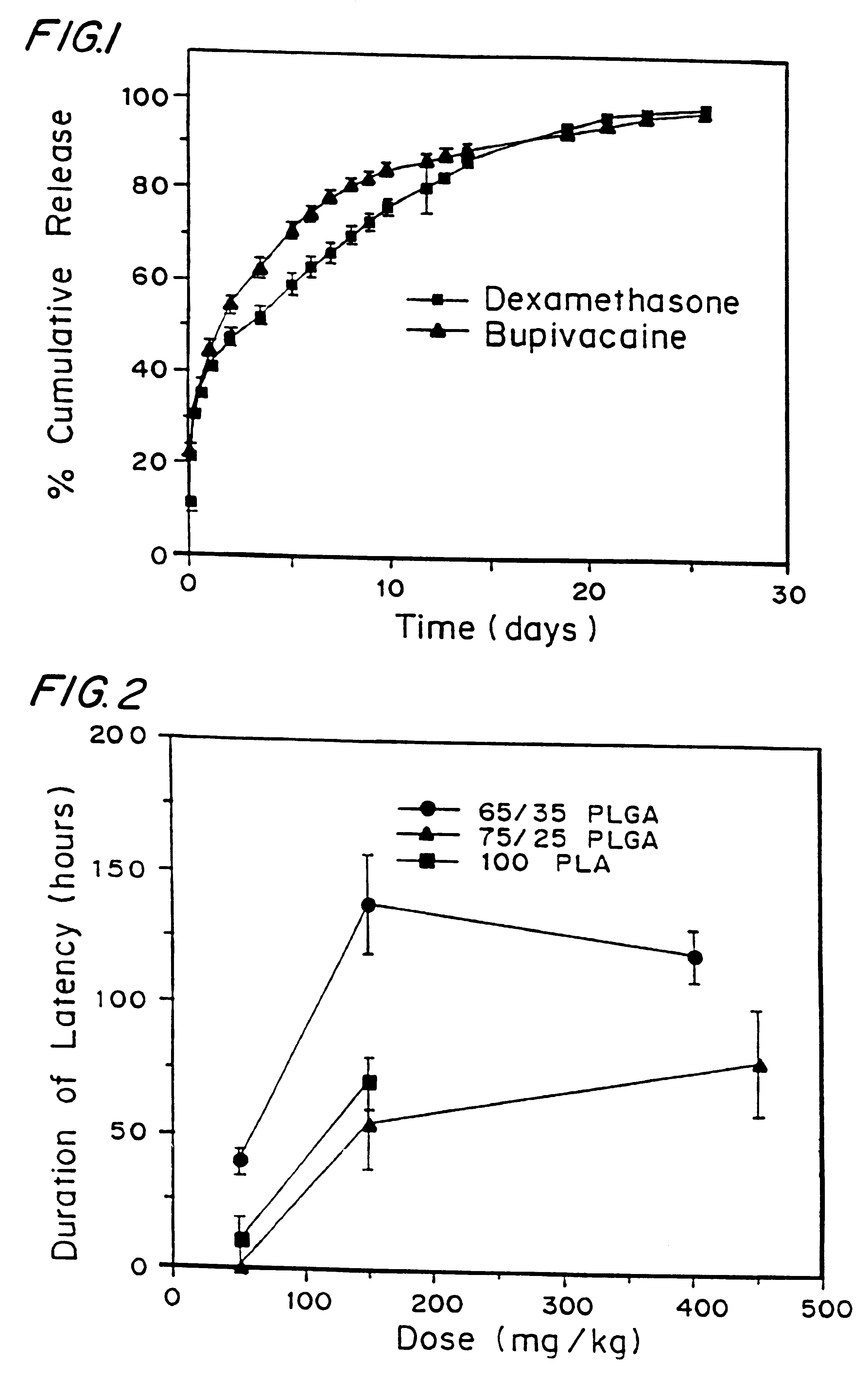
High load formulations and methods for providing prolonged local anesthesia
A formulation for inducing sustained local anesthesia in a patient comprising a substrate comprising a high load of local anesthetic by weight and an effective amount of a biocompatible, controlled release material to obtain a. reversible nerve blockade or anesthesia effect when implanted or injected in a patient, and a non-toxic glucocorticosteroid agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the glucocorticosteroid agent.
Owner:CHILDRENS MEDICAL CENT CORP
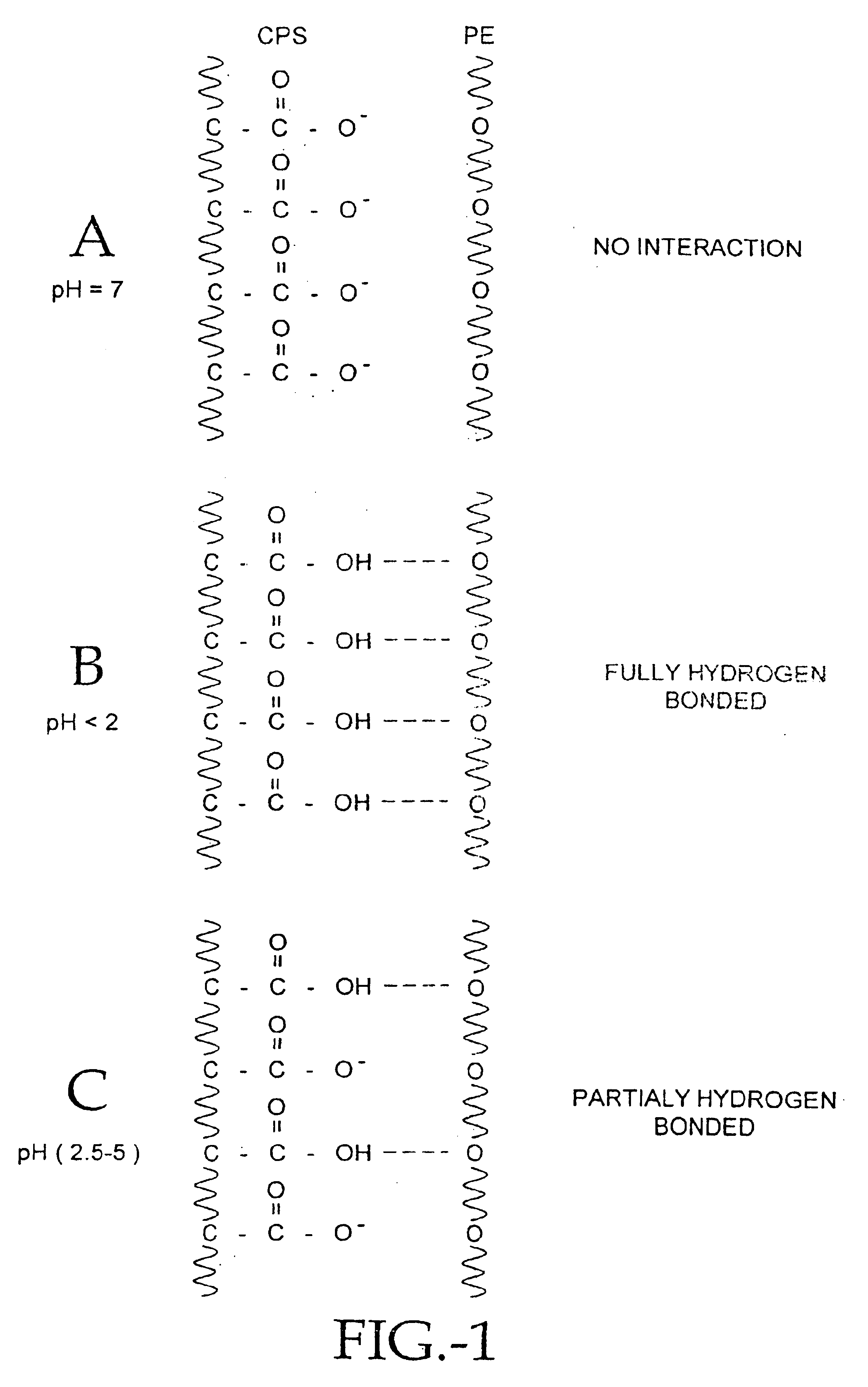
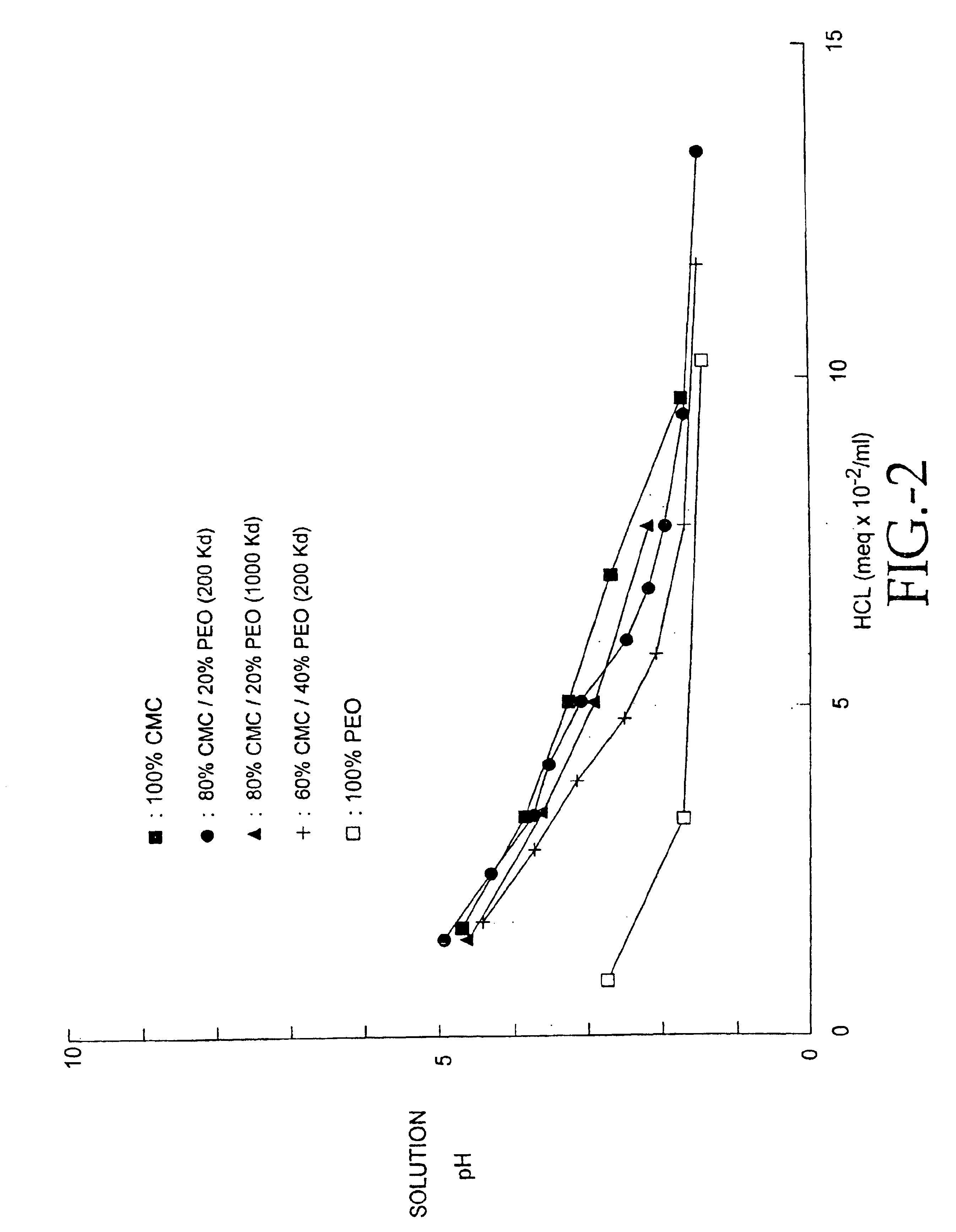
Compositions of polyacids and polyethers and methods for their use in reducing adhesions
InactiveUS6869938B1Improve anti-adhesion performancePrevent reformationBiocideSurgeryMicrosphereThrombogenicity
The present invention relates to improved methods for making and using bioadhesive, bioresorbable, anti-adhesion compositions made of intermacromolecular complexes of carboxyl-containing polysaccharides, polyethers, polyacids, polyalkylene oxides, multivalent cations and / or polycations. The polymers are associated with each other, and are then either dried into membranes or sponges, or are used as fluids or microspheres. Bioresorbable, bioadhesive, anti-adhesion compositions are useful in surgery to prevent the formation and reformation of post-surgical adhesions. The compositions are designed to breakdown in-vivo, and thus be removed from the body. Membranes are inserted during surgery either dry or optionally after conditioning in aqueous solutions. The anti-adhesion, bioadhesive, bioresorptive, antithrombogenic and physical properties of such membranes and gels can be varied as needed by carefully adjusting the pH and / or cation content of the polymer casting solutions, polyacid composition, the polyalkylene oxide composition, or by conditioning the membranes prior to surgical use. Multi-layered membranes can be made and used to provide further control over the physical and biological properties of antiadhesion membranes. Membranes and gels can be used concurrently. Antiadhesion compositions may also be used to lubricate tissues and / or medical instruments, and / or deliver drugs to the surgical site and release them locally.
Owner:FZIOMED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com