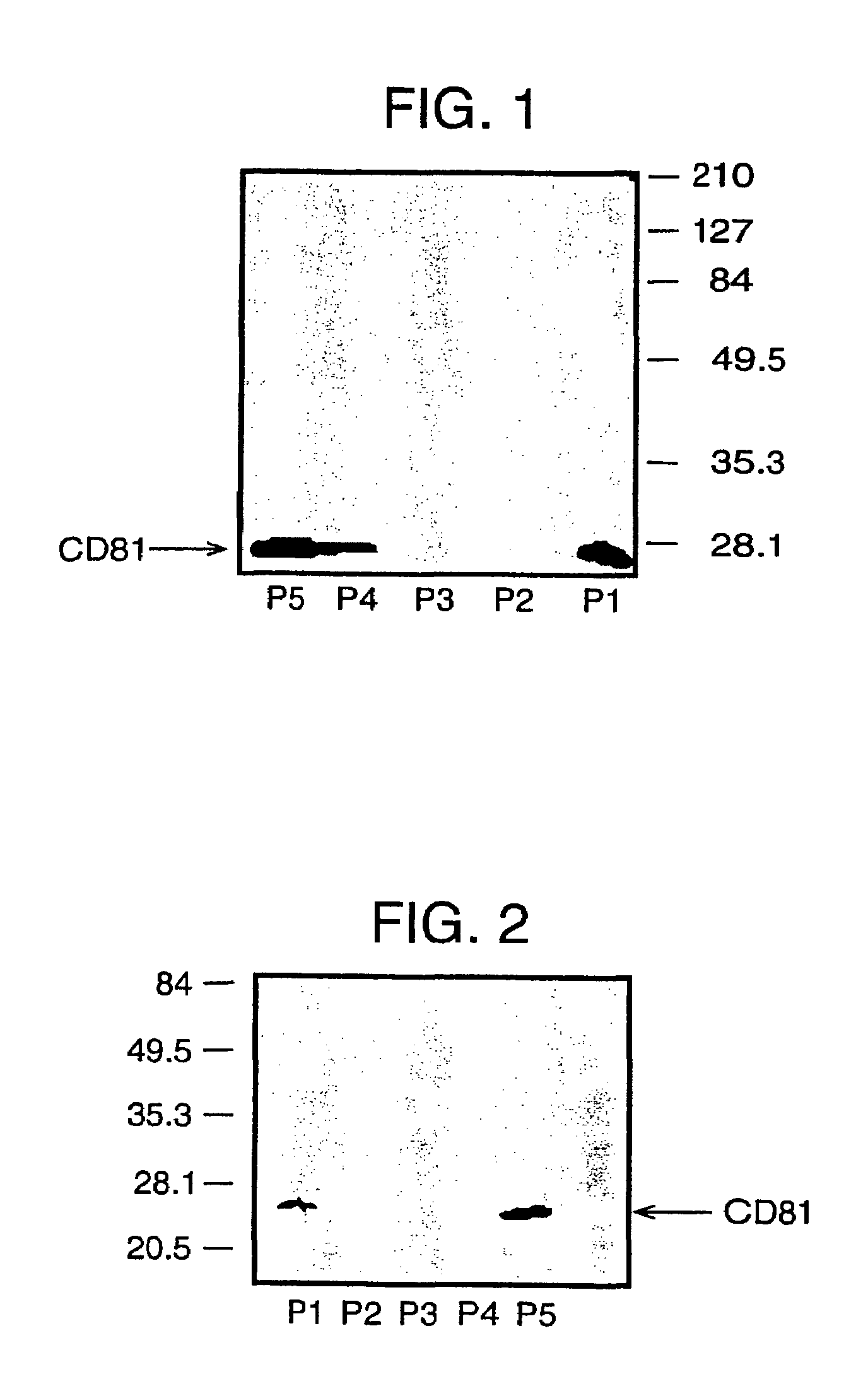
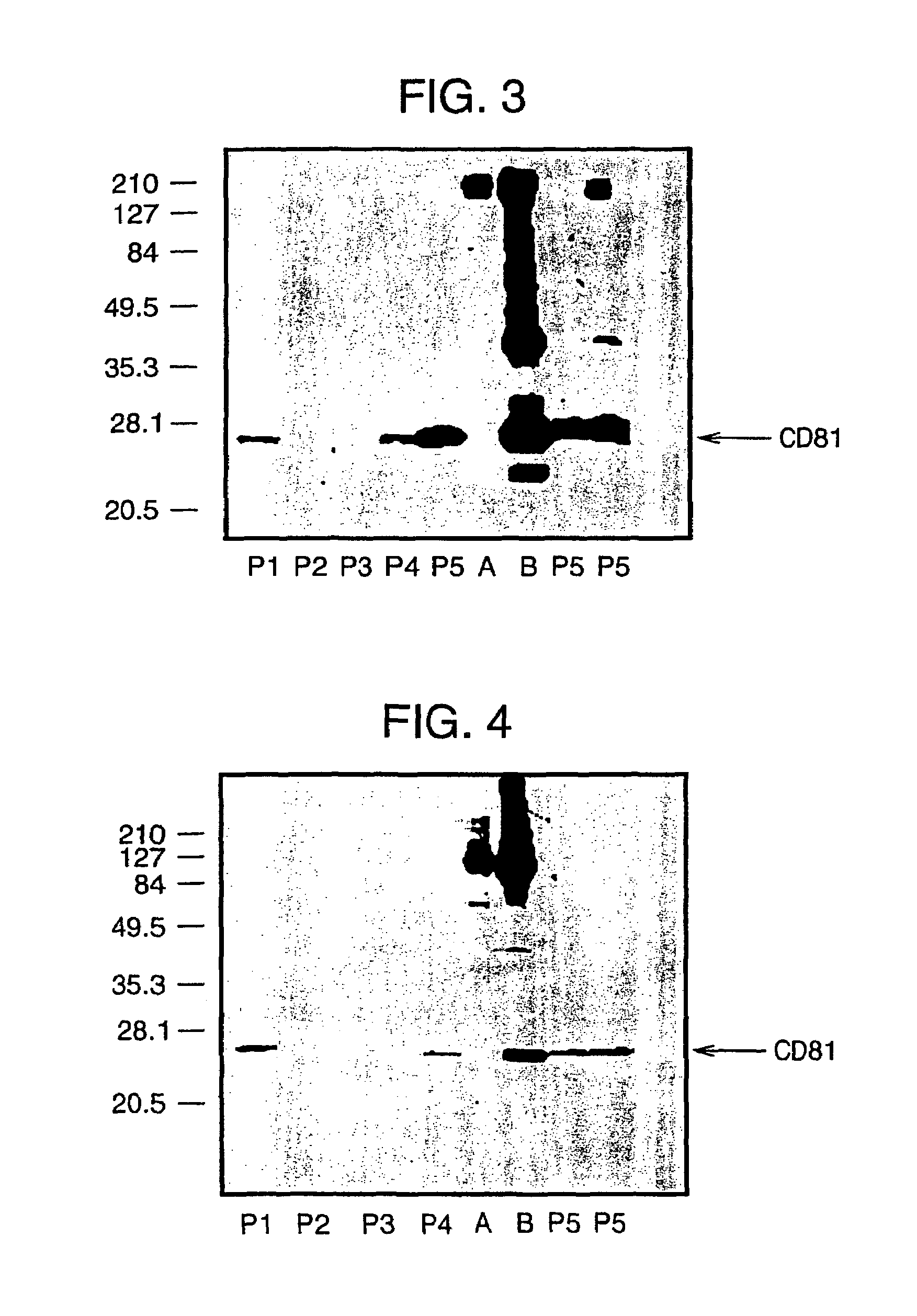
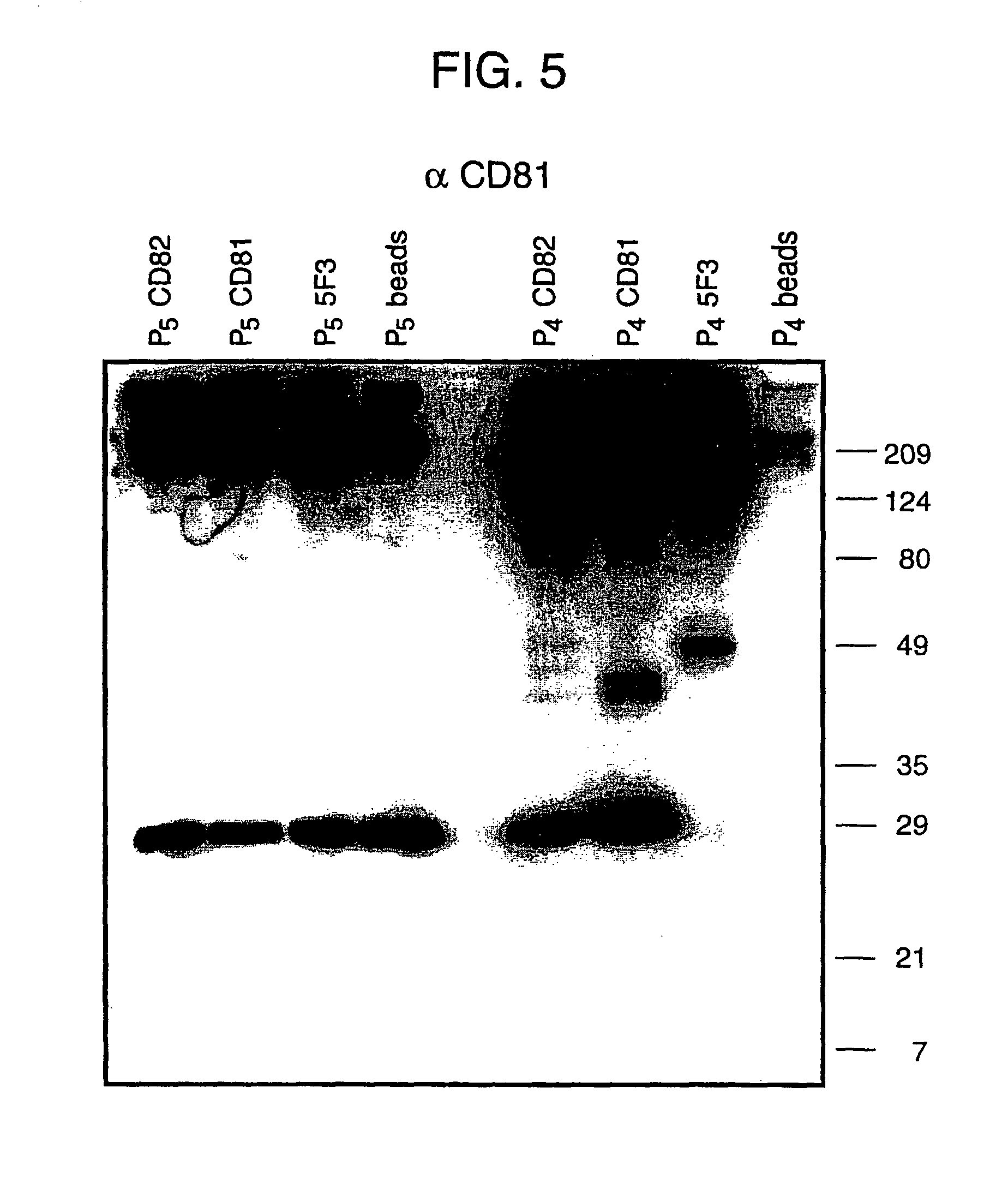
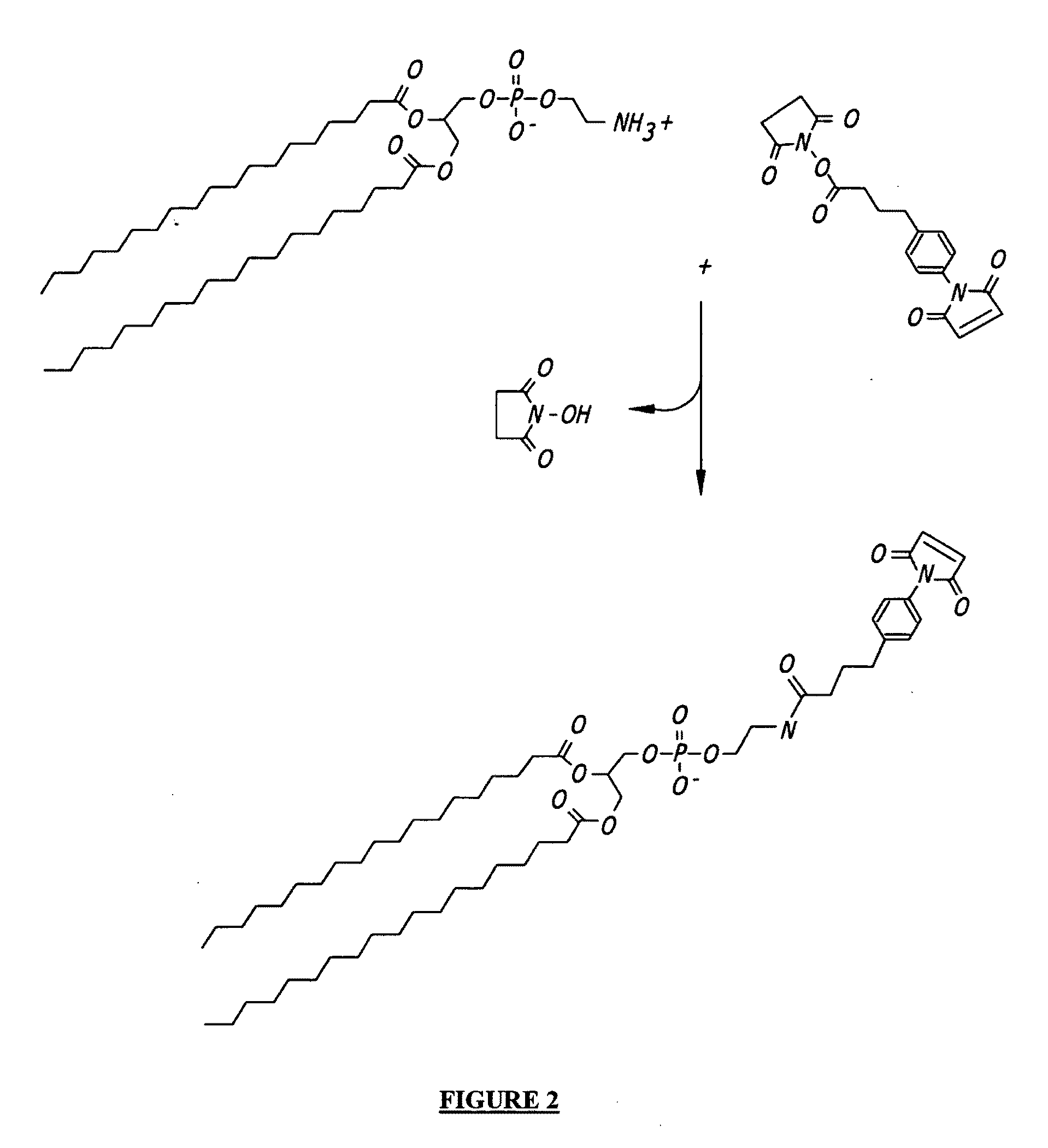
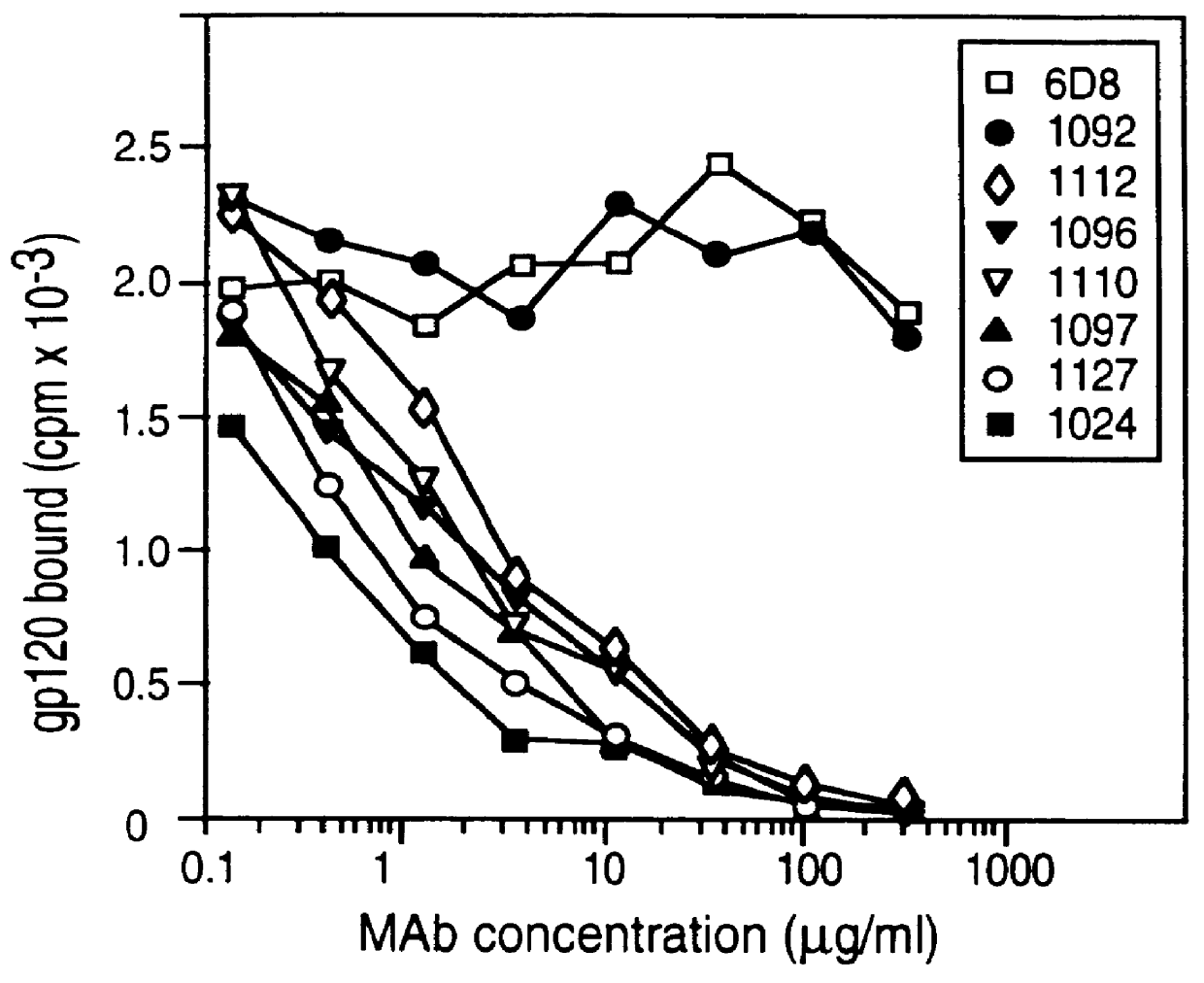
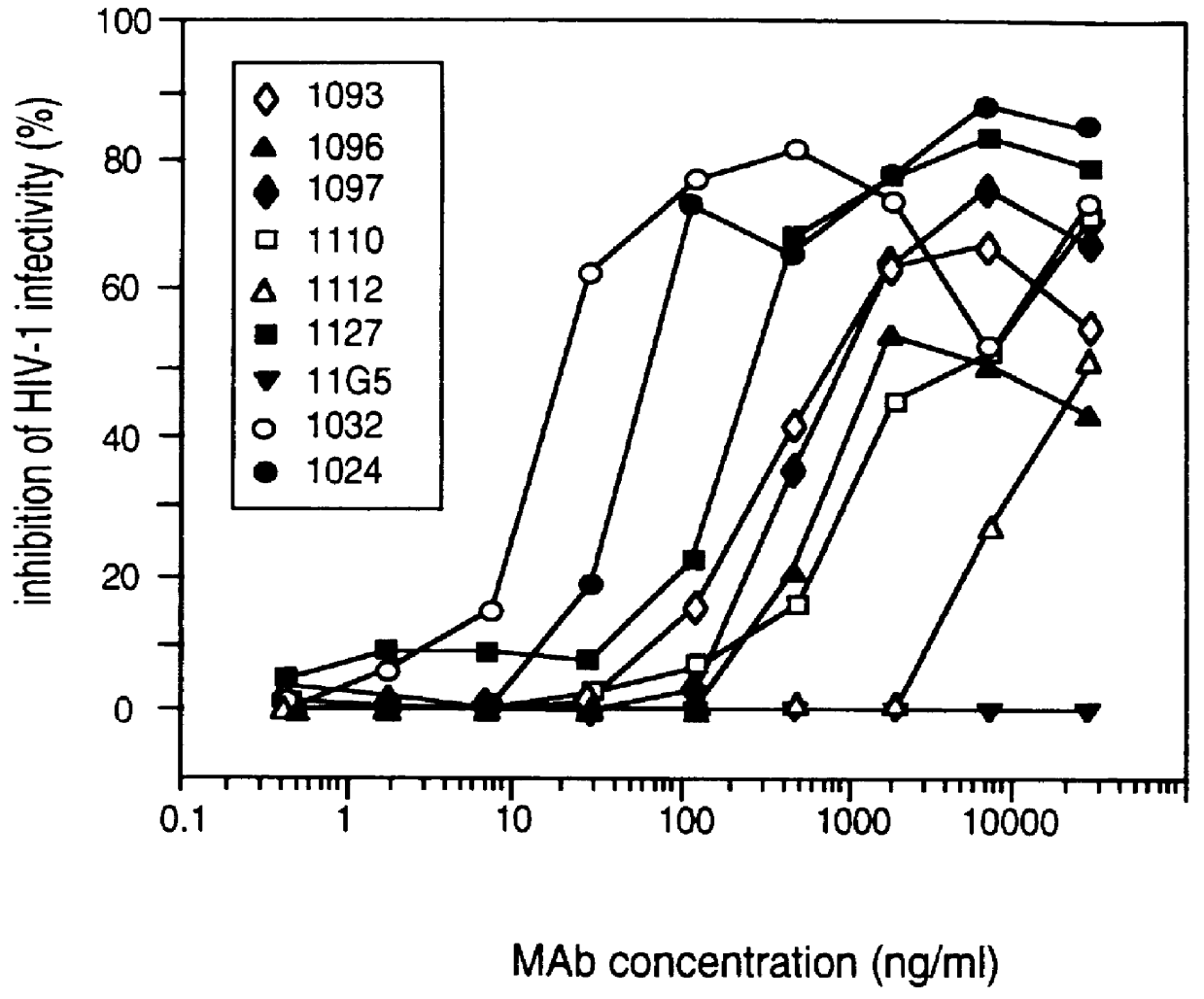
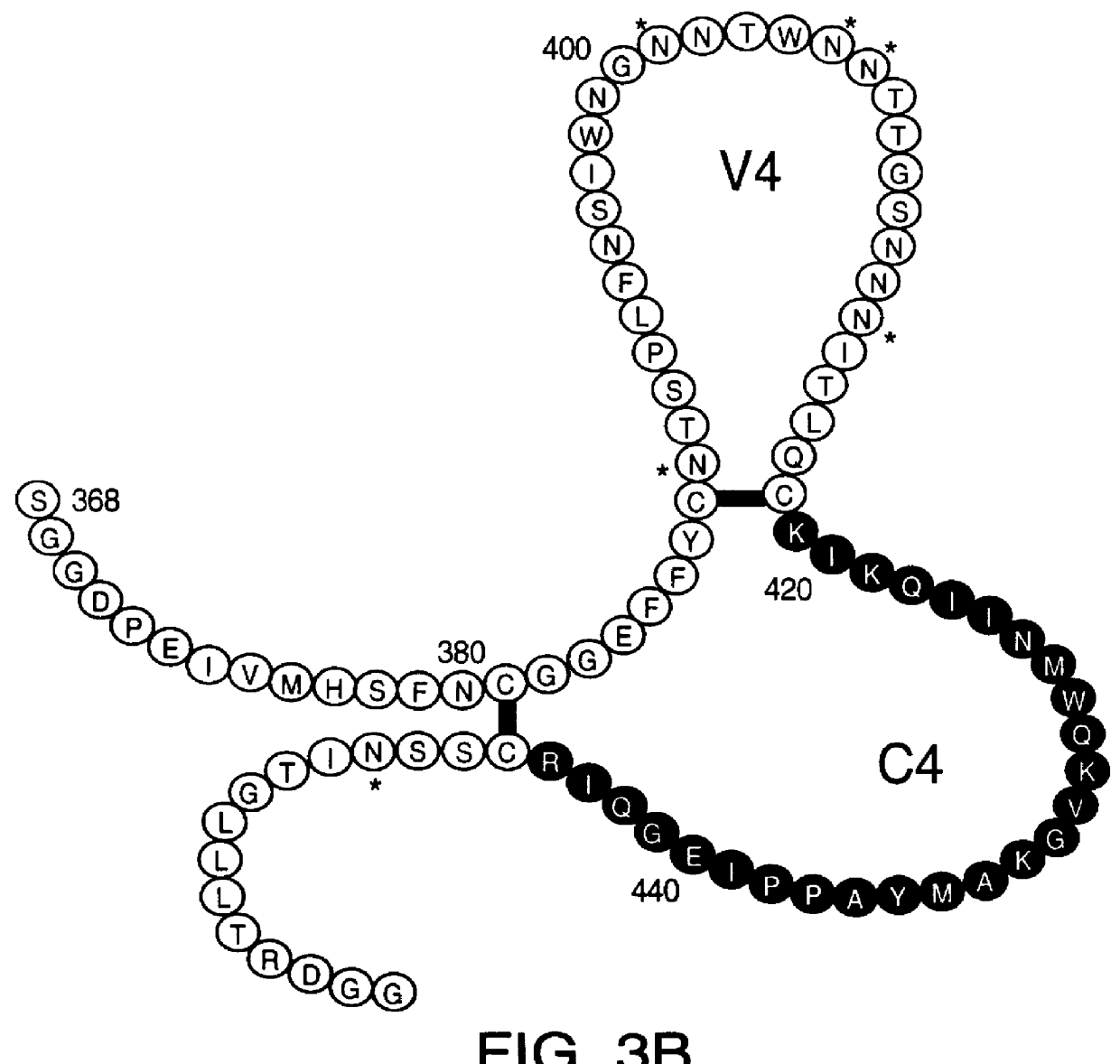
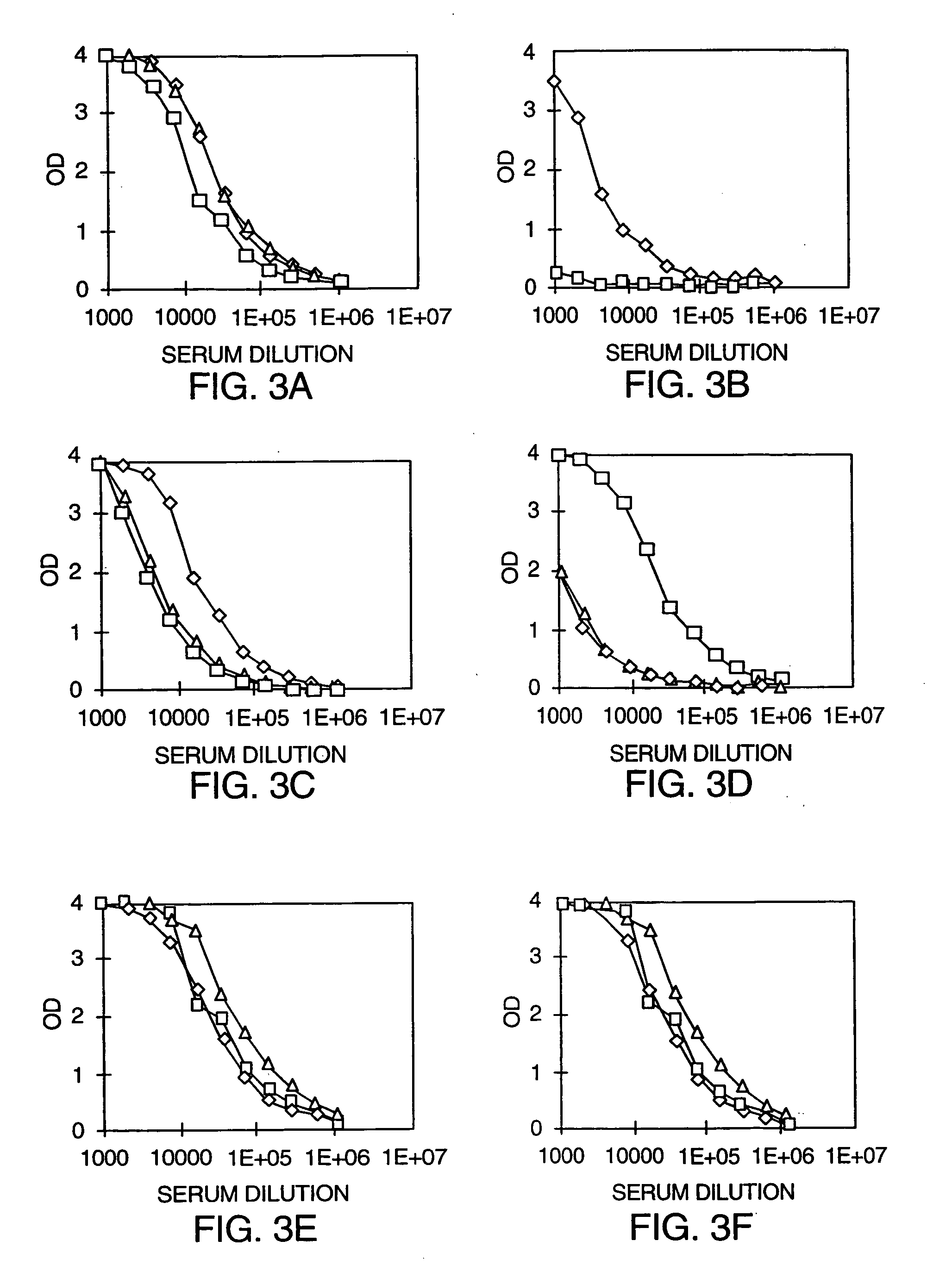
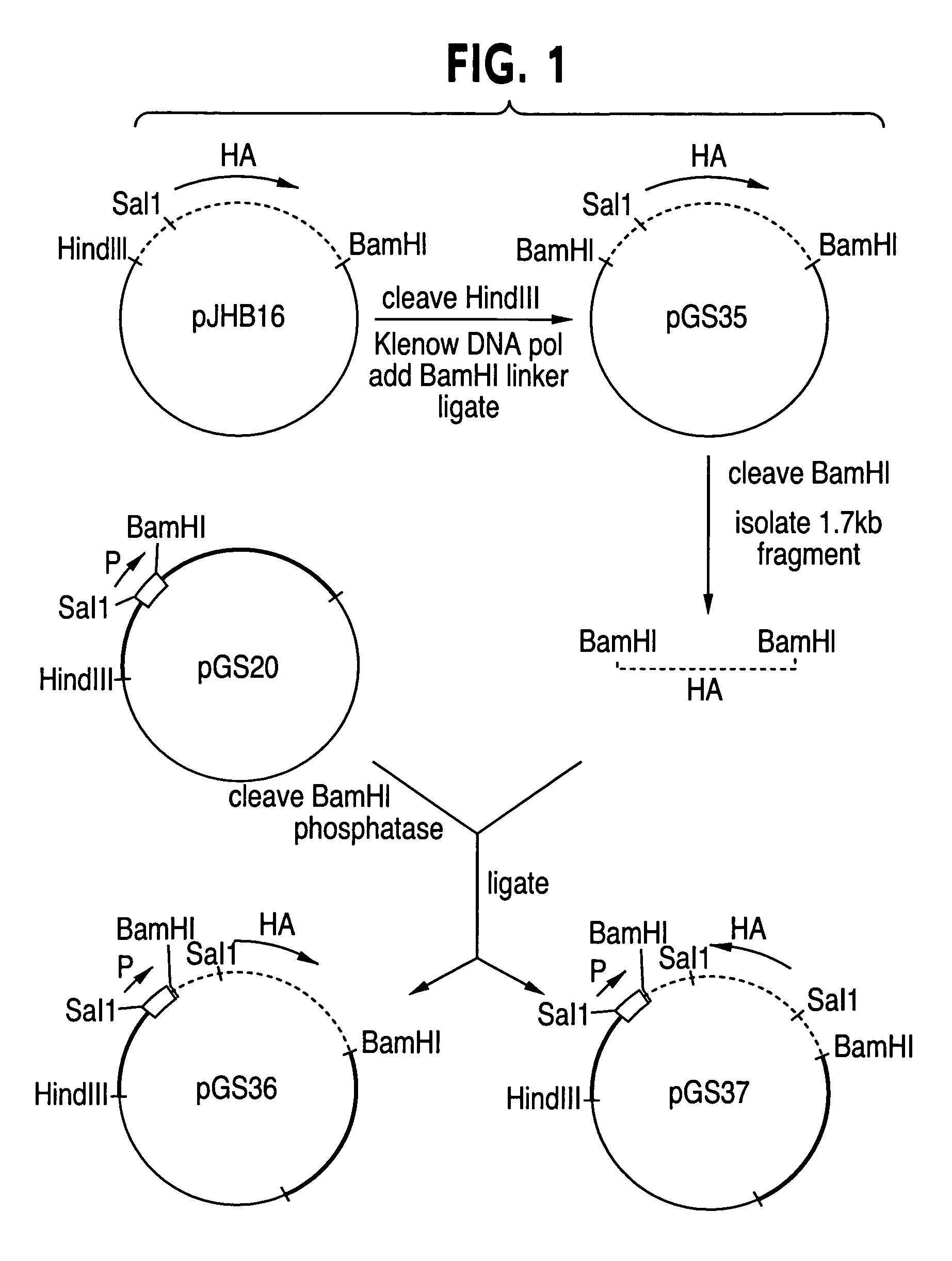
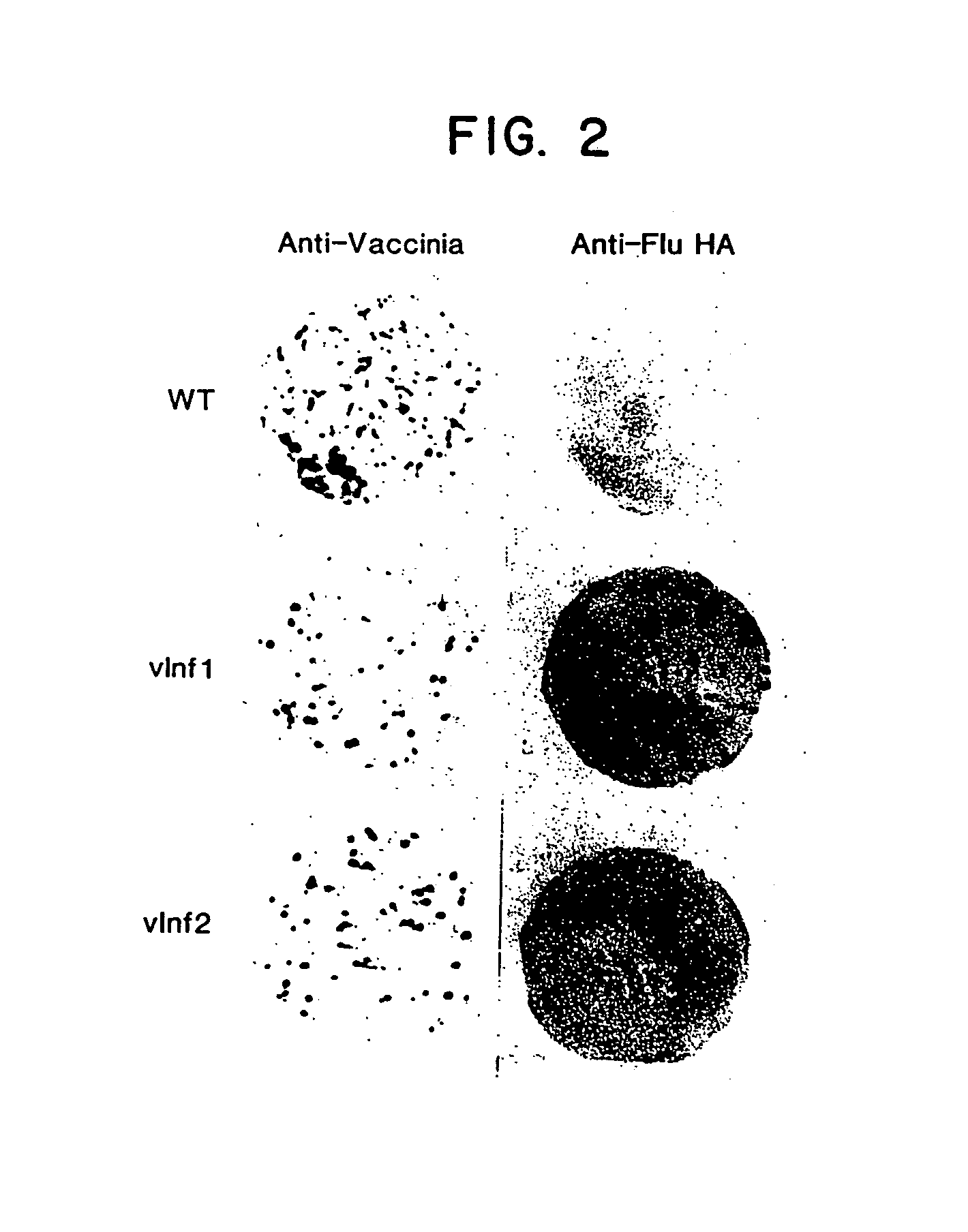
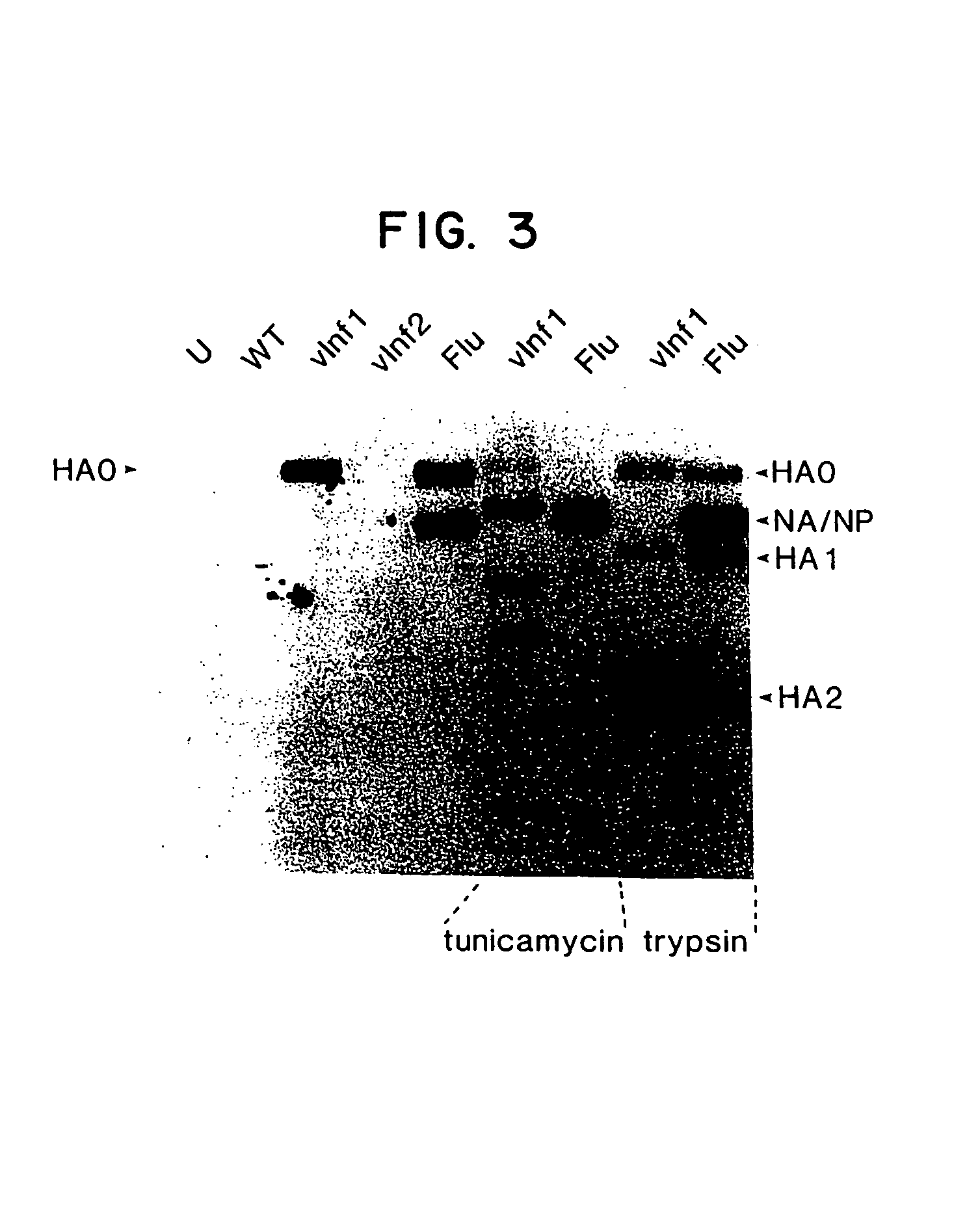
Patents
Literature
10653results about "Virus peptides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
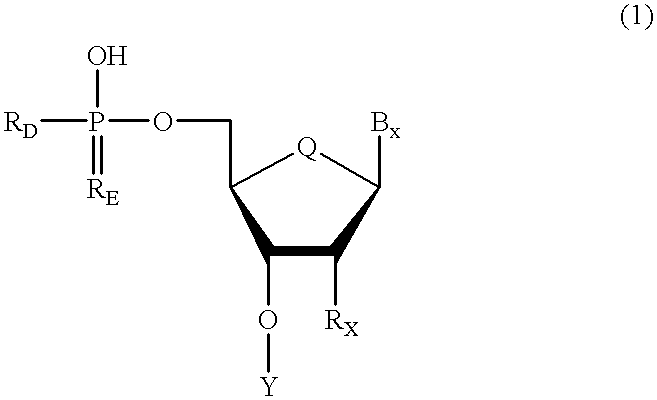
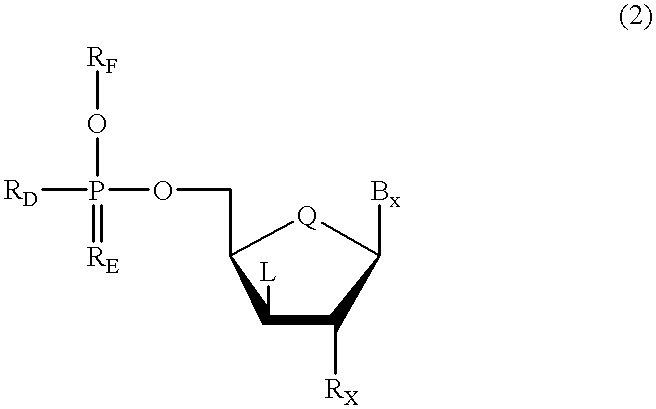
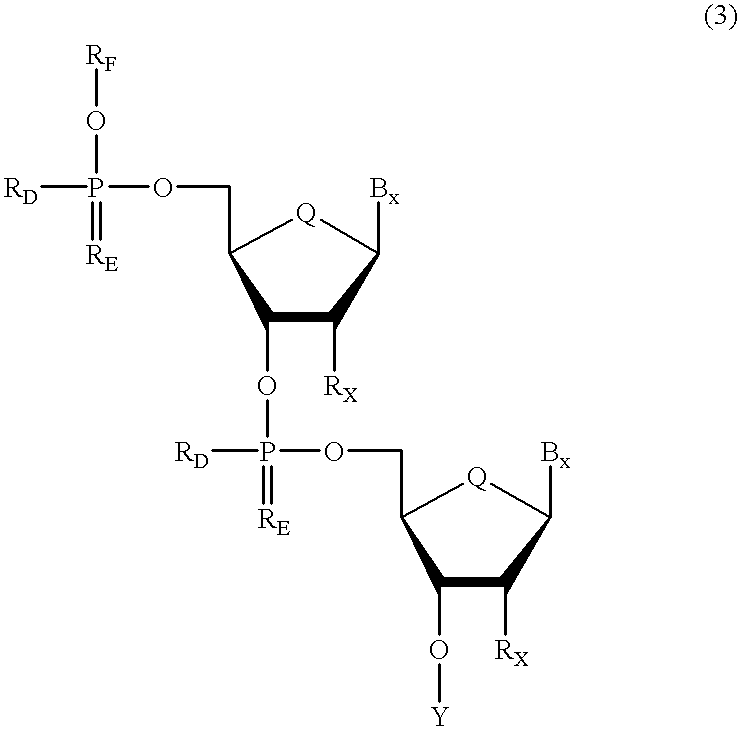
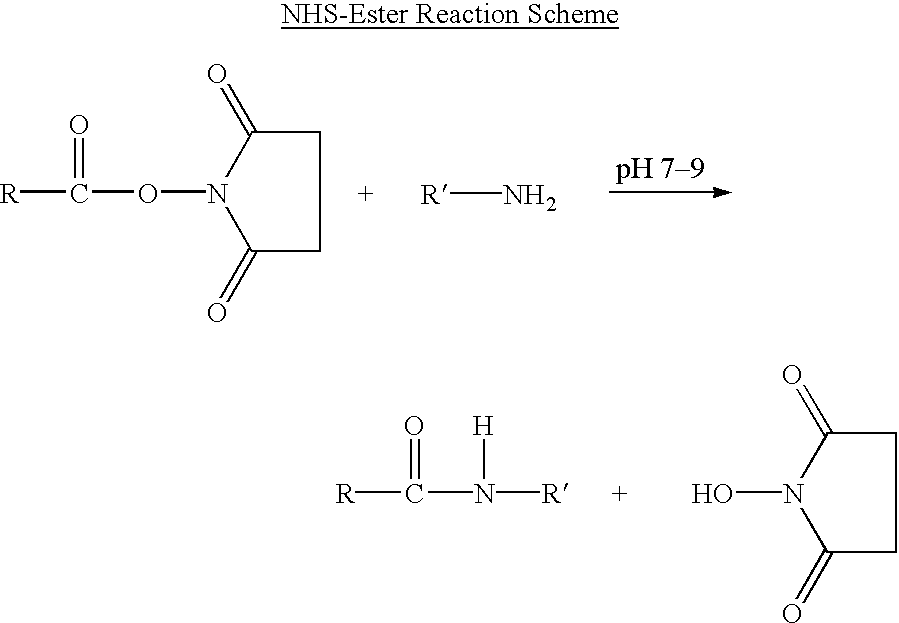
Oligonucleotides having chiral phosphorus linkages
InactiveUS6239265B1Improved pharmacokinetic propertiesImprove propertiesPeptide/protein ingredientsGenetic material ingredientsSugar moietyPhosphoramidate
Sequence-specific oligonucleotides are provided having substantially pure chiral Sp phosphorothioate, chiral Rp phosphorothioate, chiral Sp alkylphosphonate, chiral Rp alkylphosphonate, chiral Sp phosphoamidate, chiral Rp phosphoamidate, chiral Sp phosphotriester, and chiral Rp phosphotriester linkages. The novel oligonucleotides are prepared via a stereospecific SN2 nucleophilic attack of a phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate anion on the 3' position of a xylonucleotide. The reaction proceeds via inversion at the 3' position of the xylo reactant species, resulting in the incorporation of phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate linked ribofuranosyl sugar moieties into the oligonucleotide.
Owner:IONIS PHARMA INC
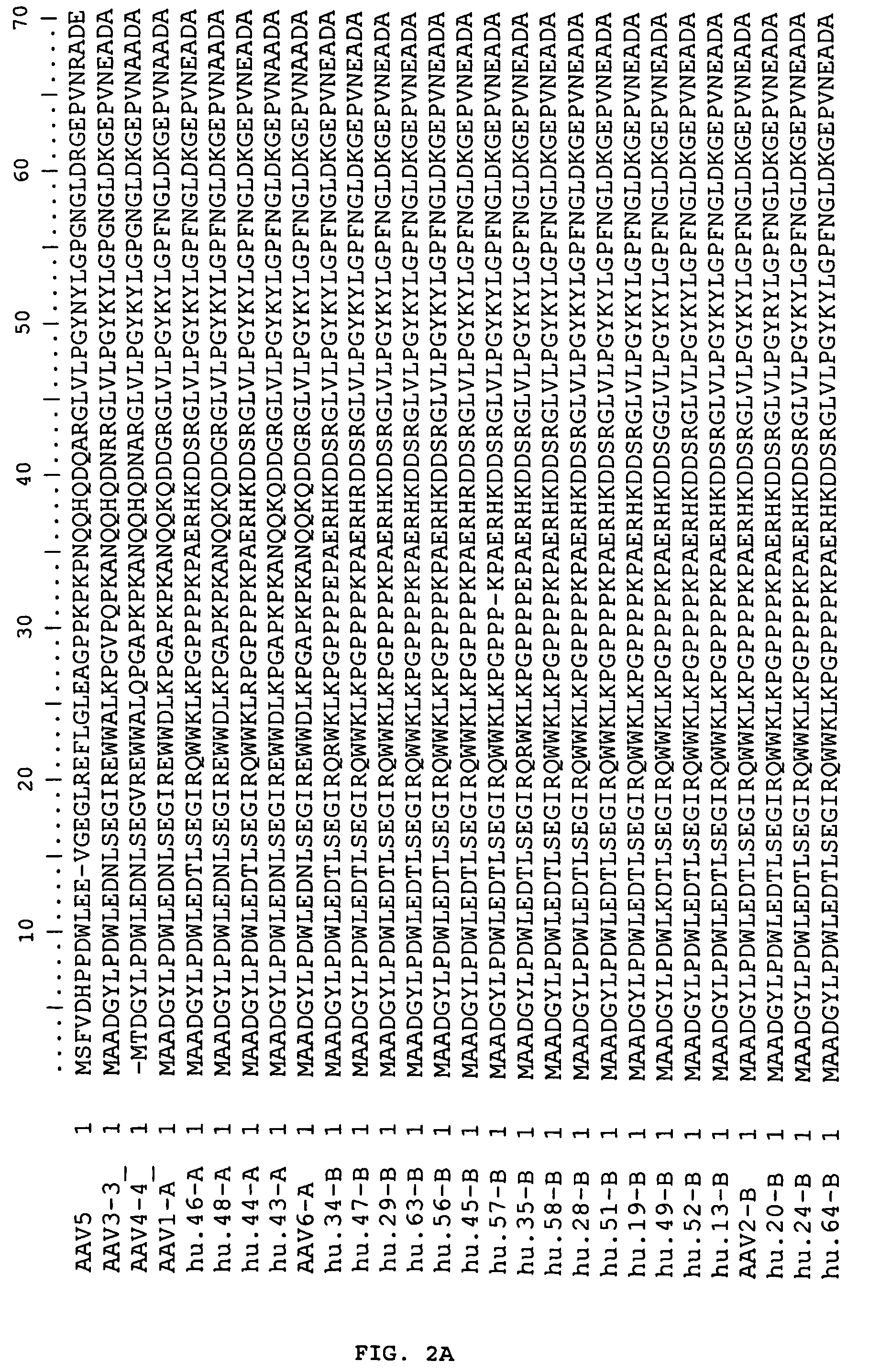
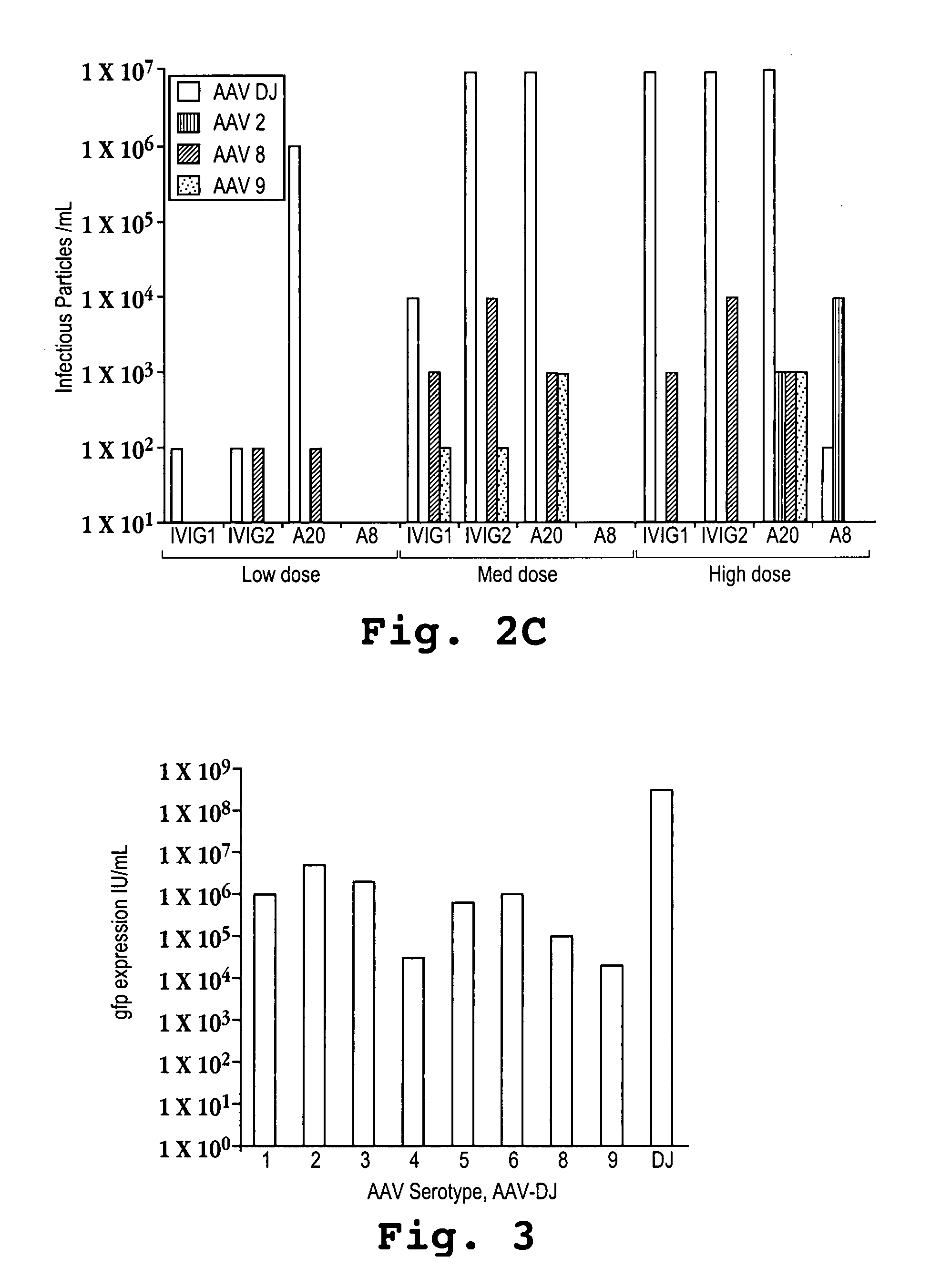
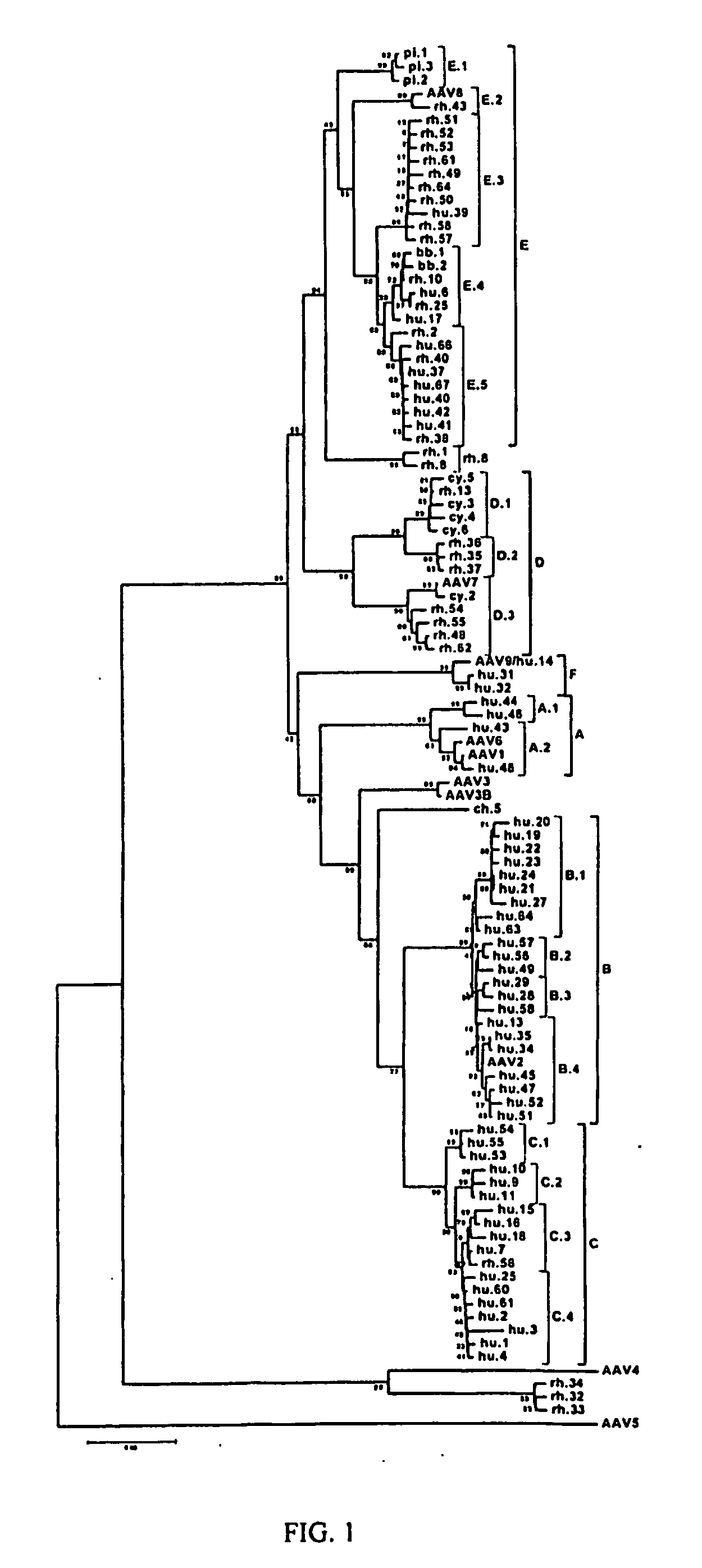
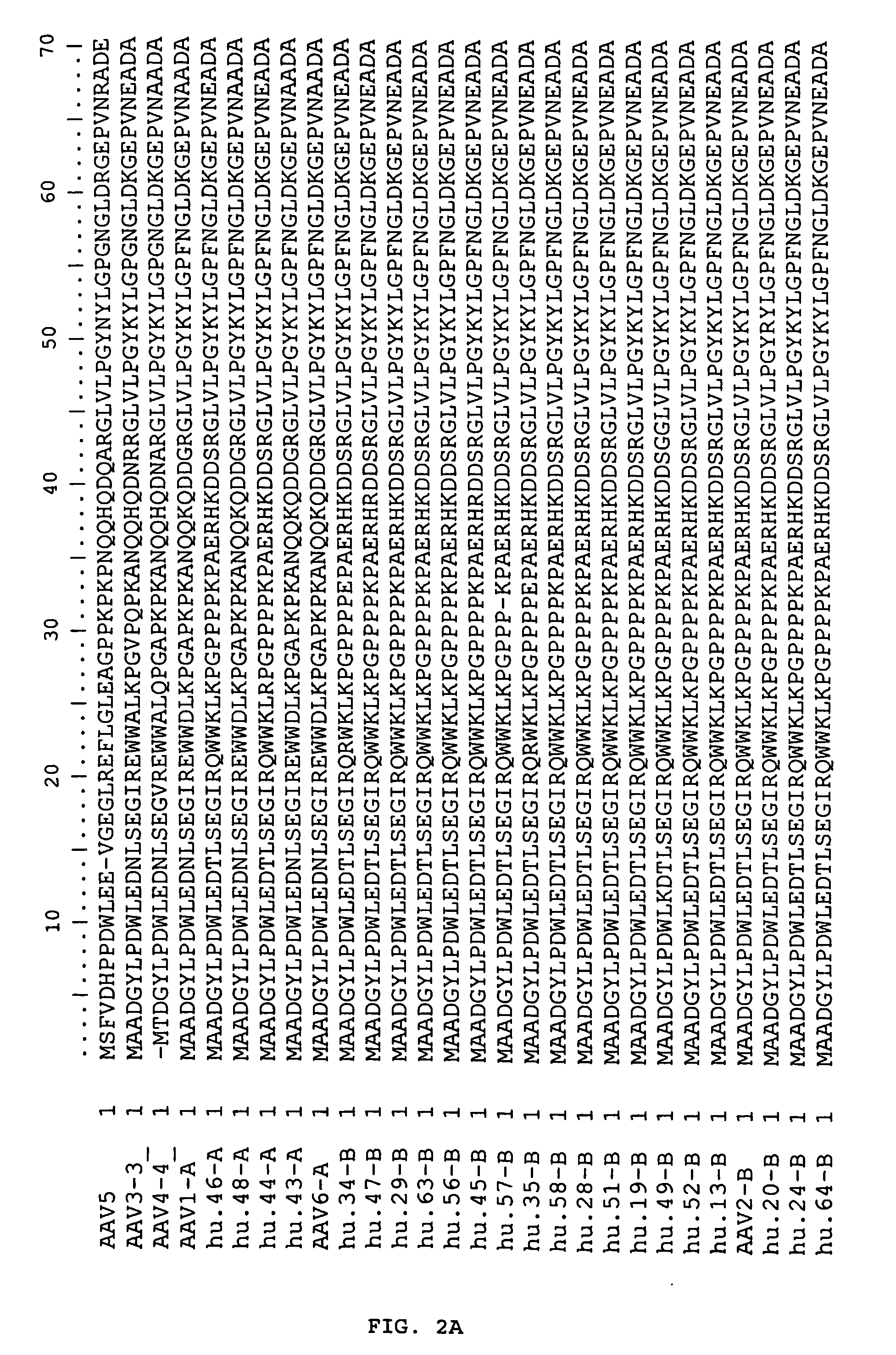
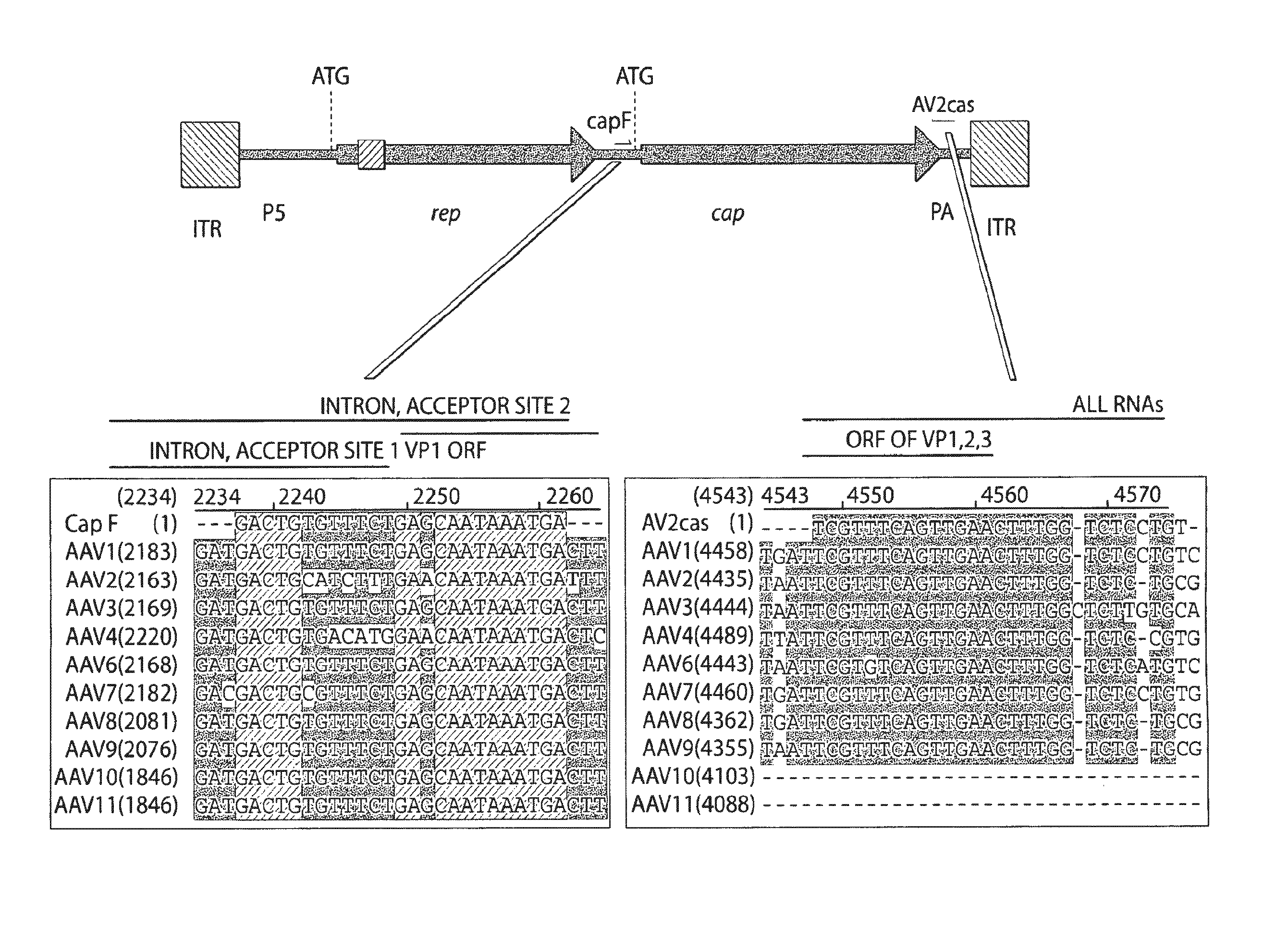
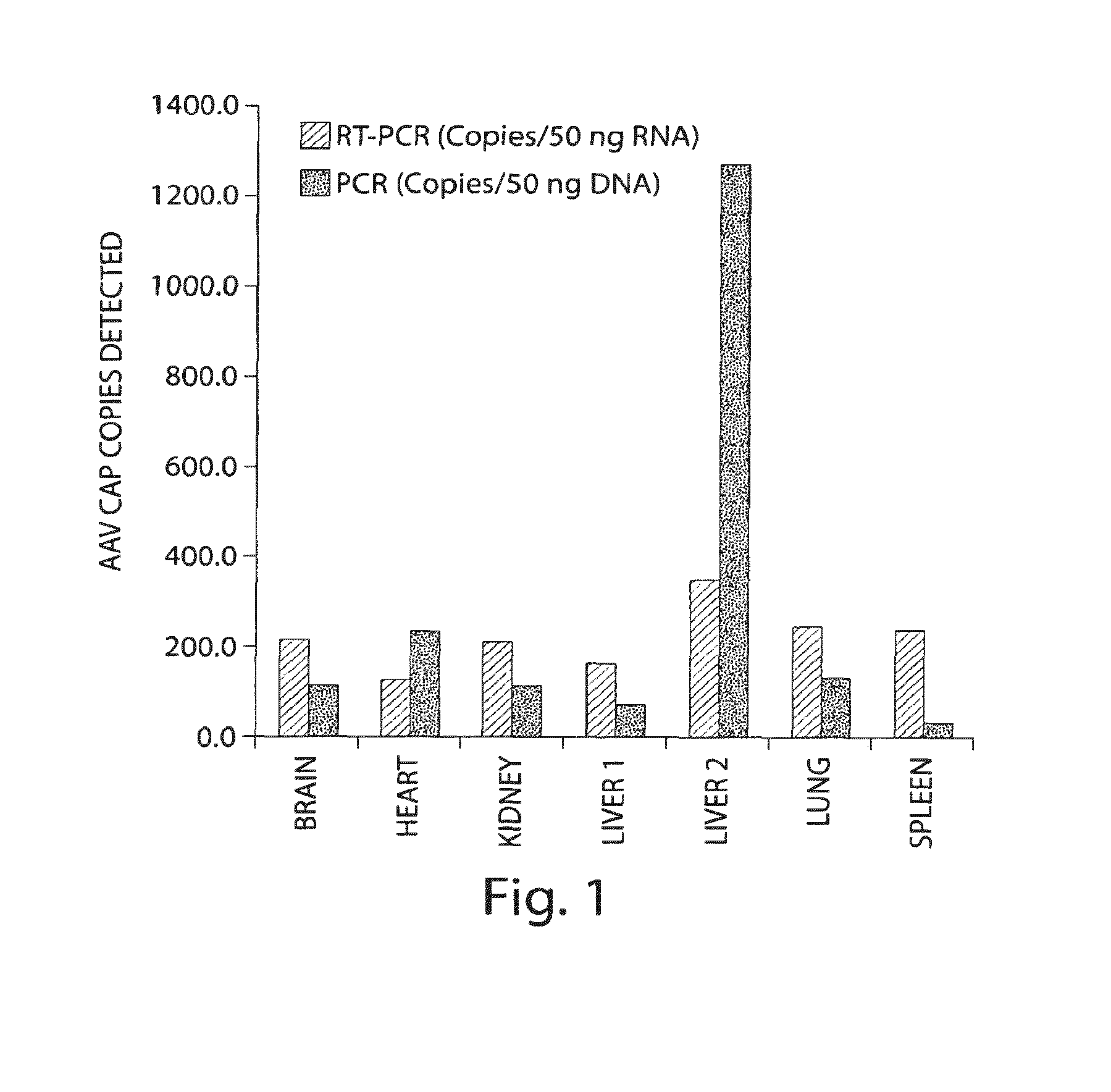
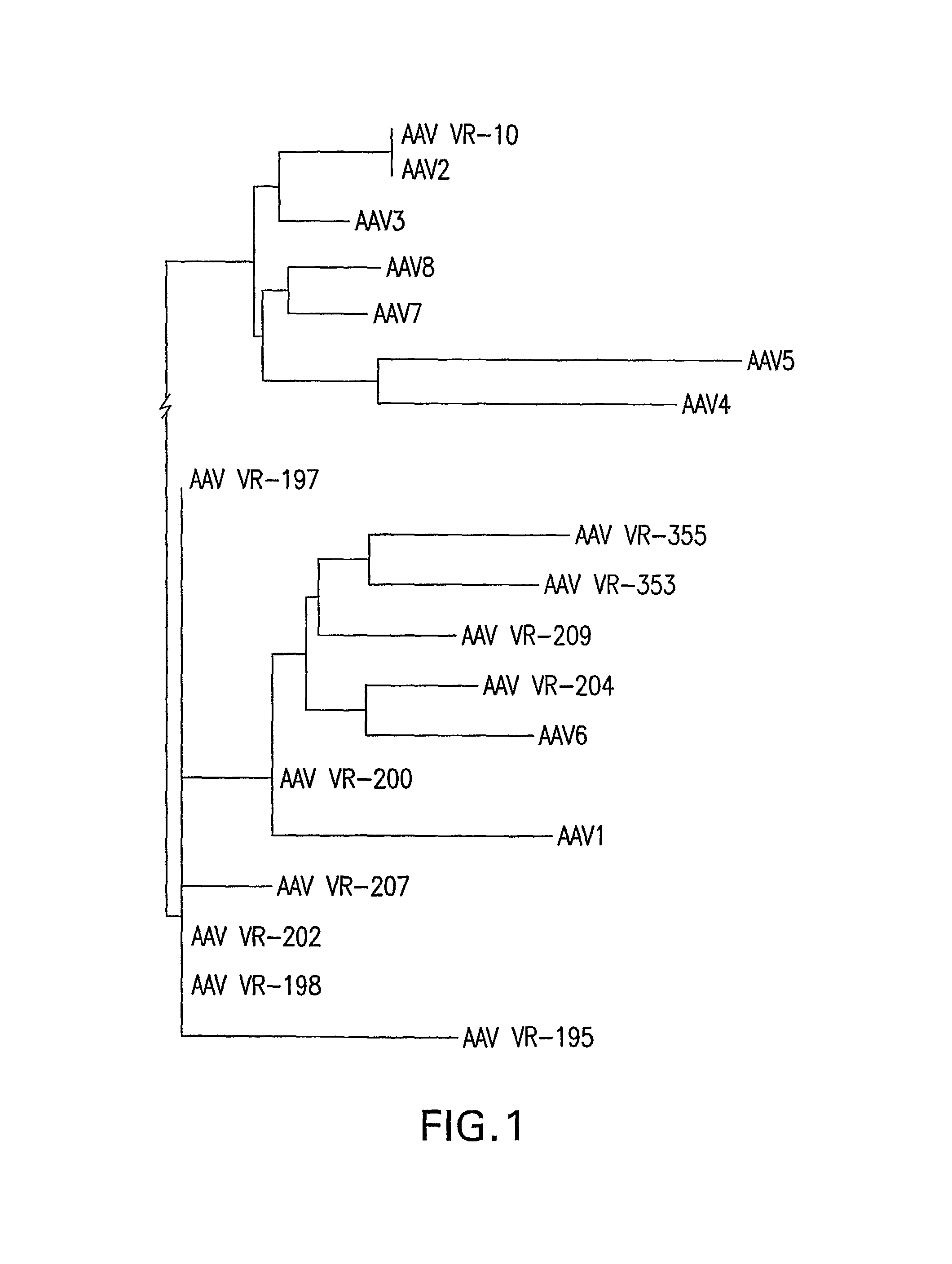
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
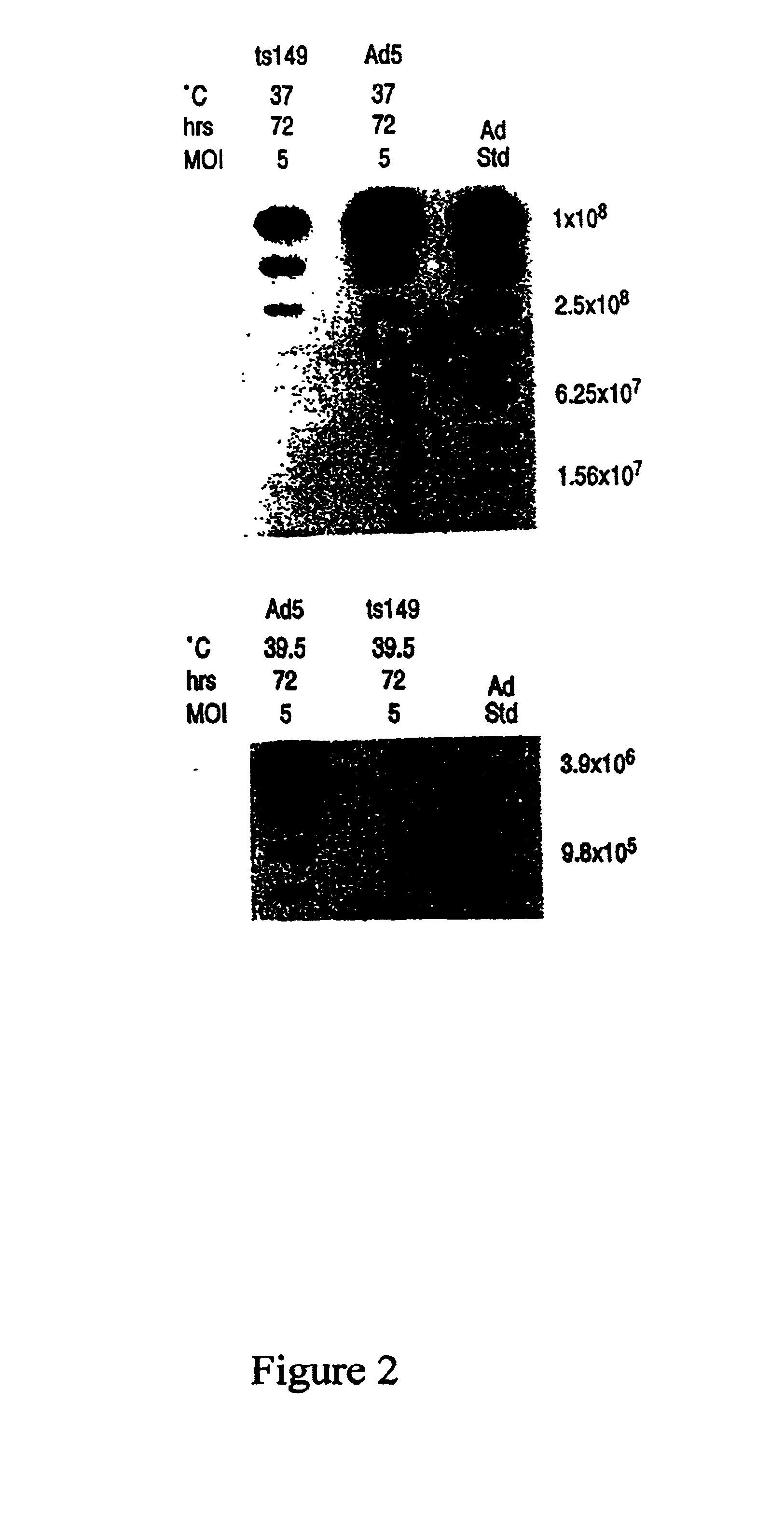
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
AAV capsid library and AAV capsid proteins
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
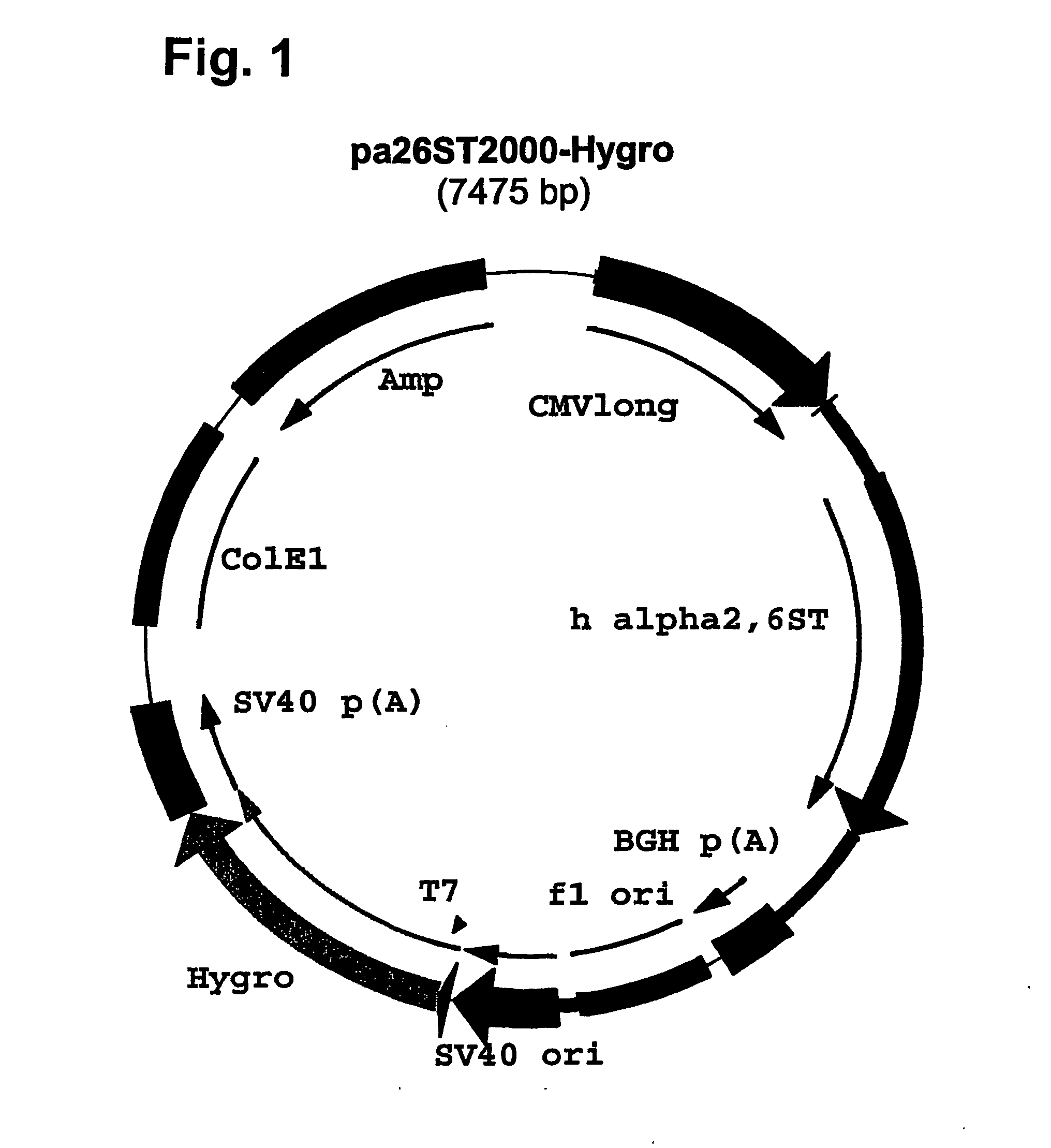
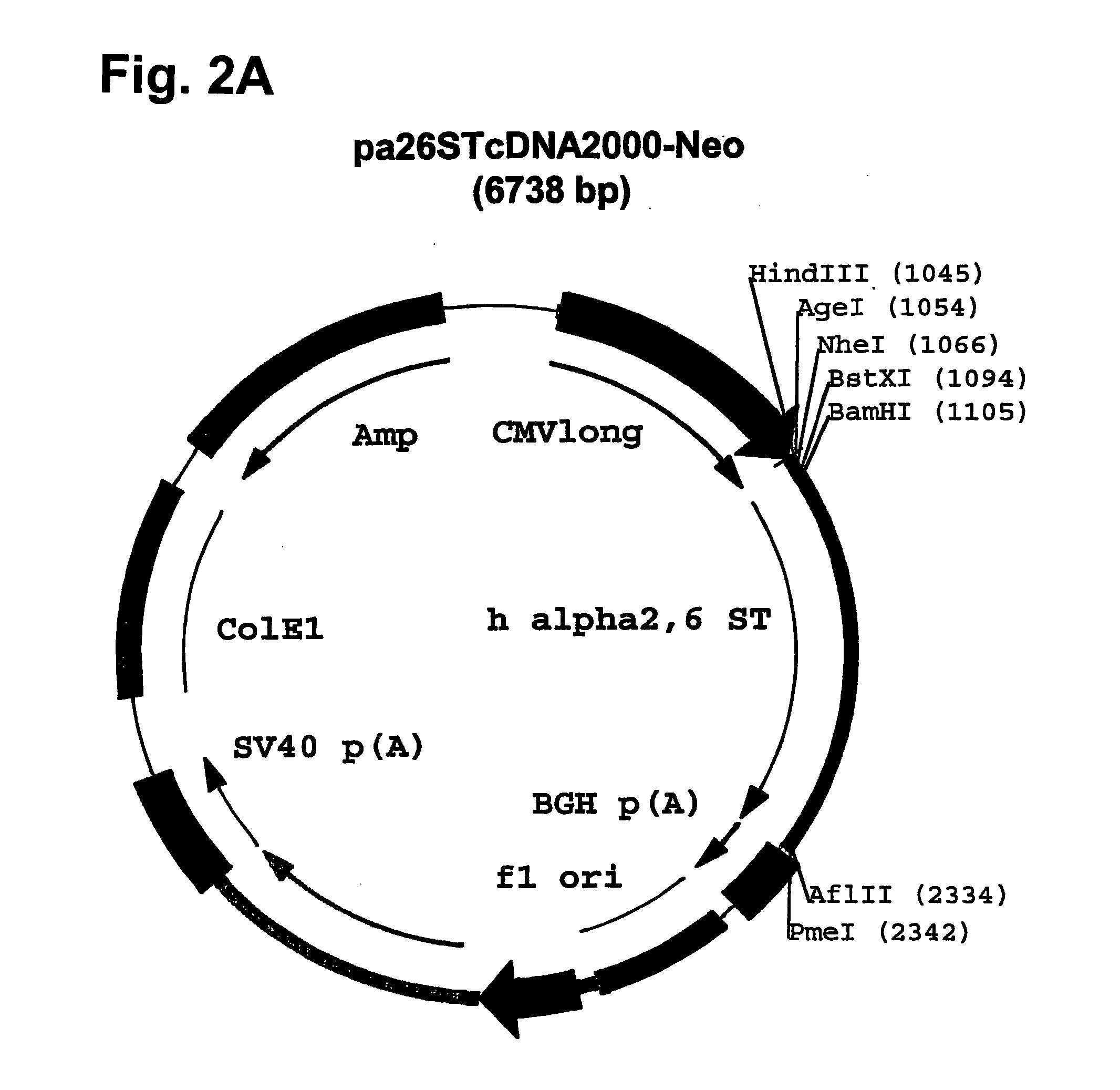
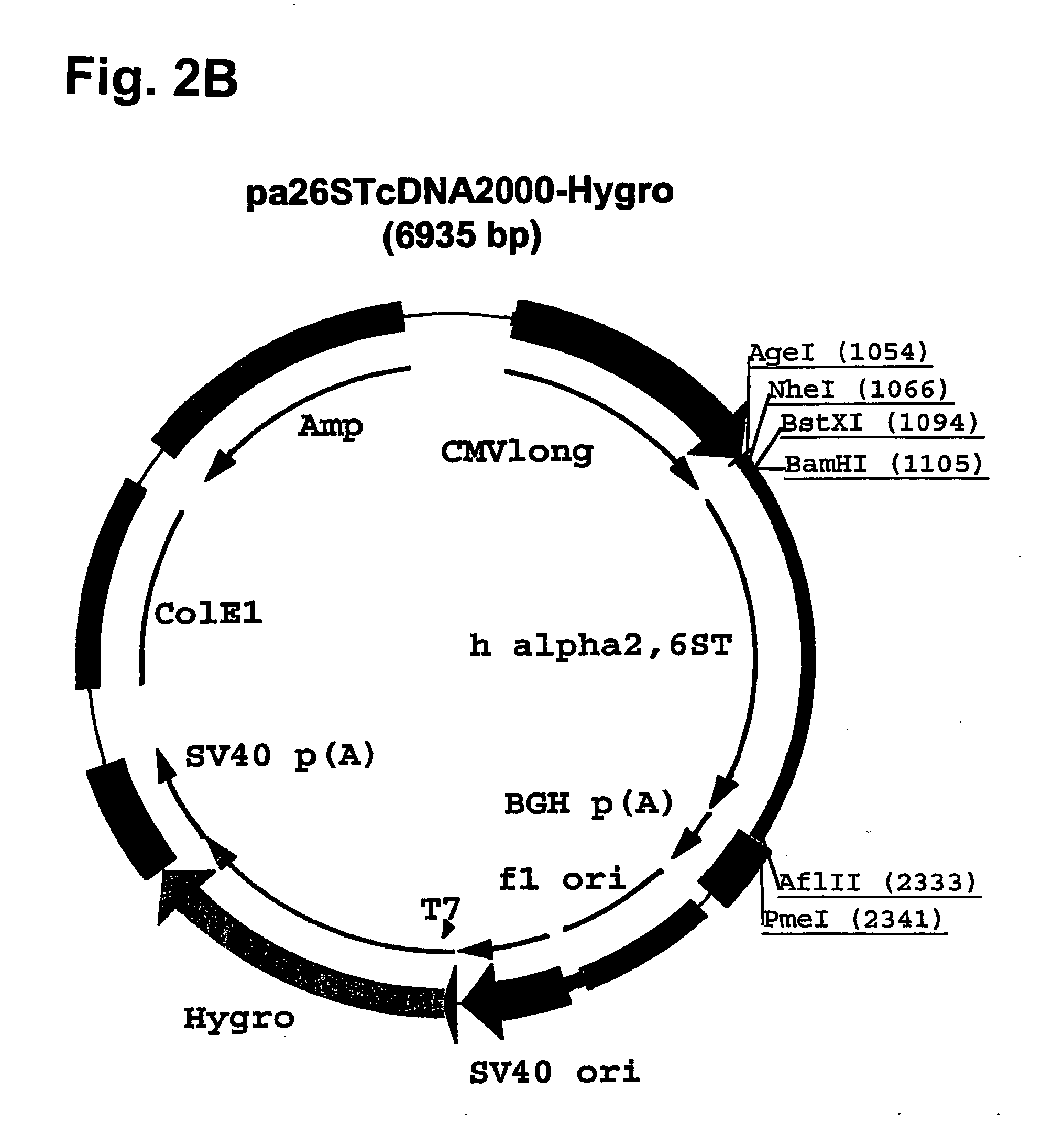
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
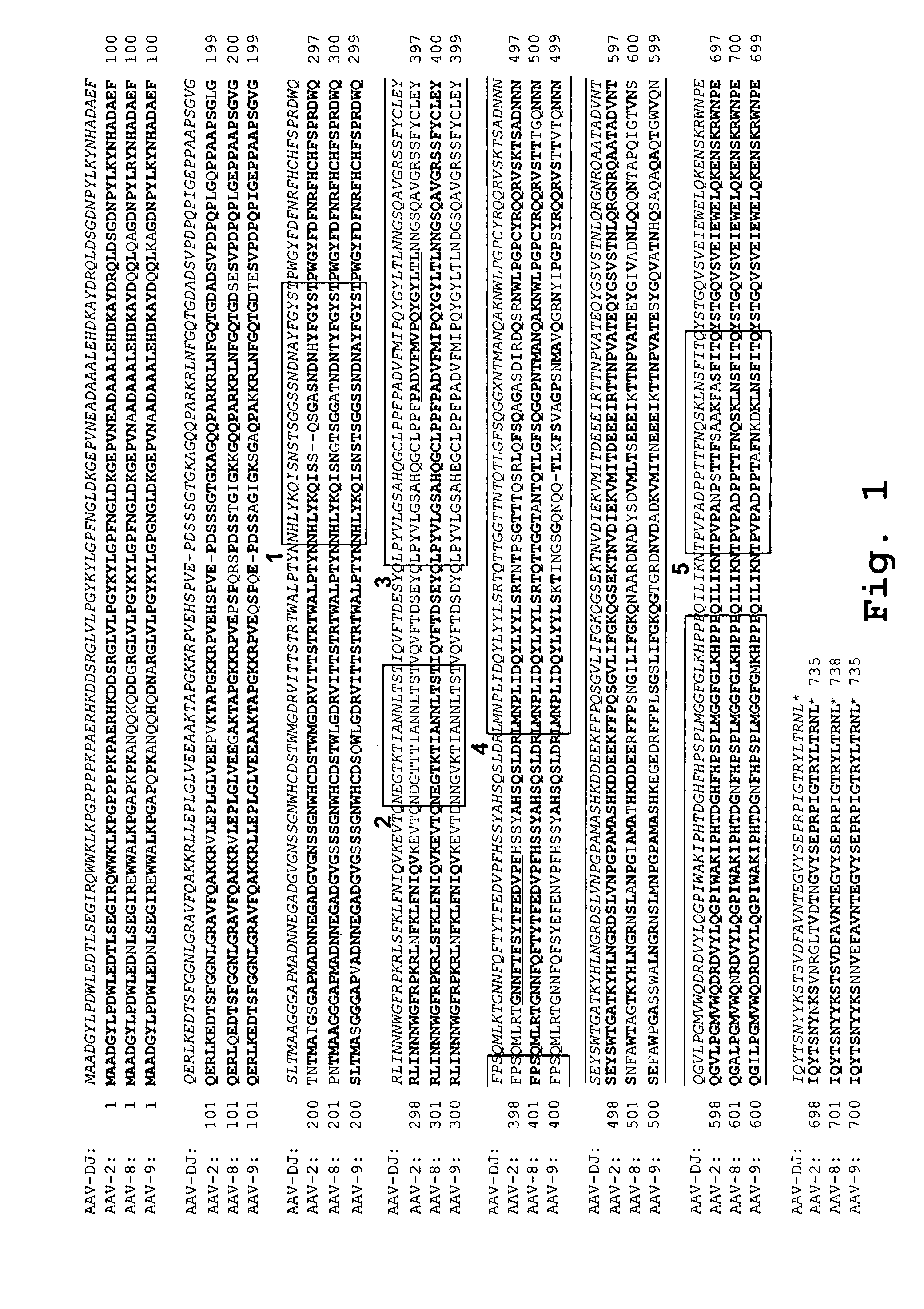
AAV's and uses thereof
The invention in some aspects relates to recombinant adeno-associated viruses having distinct tissue targeting capabilities. In some aspects, the invention relates to gene transfer methods using the recombinant adeno-associate viruses. In some aspects, the invention relates to isolated AAV capsid proteins and isolated nucleic acids encoding the same.
Owner:UNIV OF MASSACHUSETTS
Method of eliminating inhibitory/instability regions from mRNA
InactiveUS6174666B1Microbiological testing/measurementVirus peptidesImmunodeficiency virusInstability
A method of locating an inhibitory / instability sequence or sequences within the coding region of an mRNA and modifying the gene encoding that mRNA to remove these inhibitory / instability sequences by making clustered nucleotide substitutions without altering the coding capacity of the gene is disclosed. Constructs containing these mutated genes and host cells containing these constructs are also disclosed. The method and constructs are exemplified by the mutation of a Human Immunodeficiency Virus-1 Rev-dependent gag gene to a Rev-independent gag gene. Constructs useful in locating inhibitory / instability sequences within either the coding region or the 3' untranslated region of an mRNA are also disclosed.
Owner:UNITED STATES OF AMERICA
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
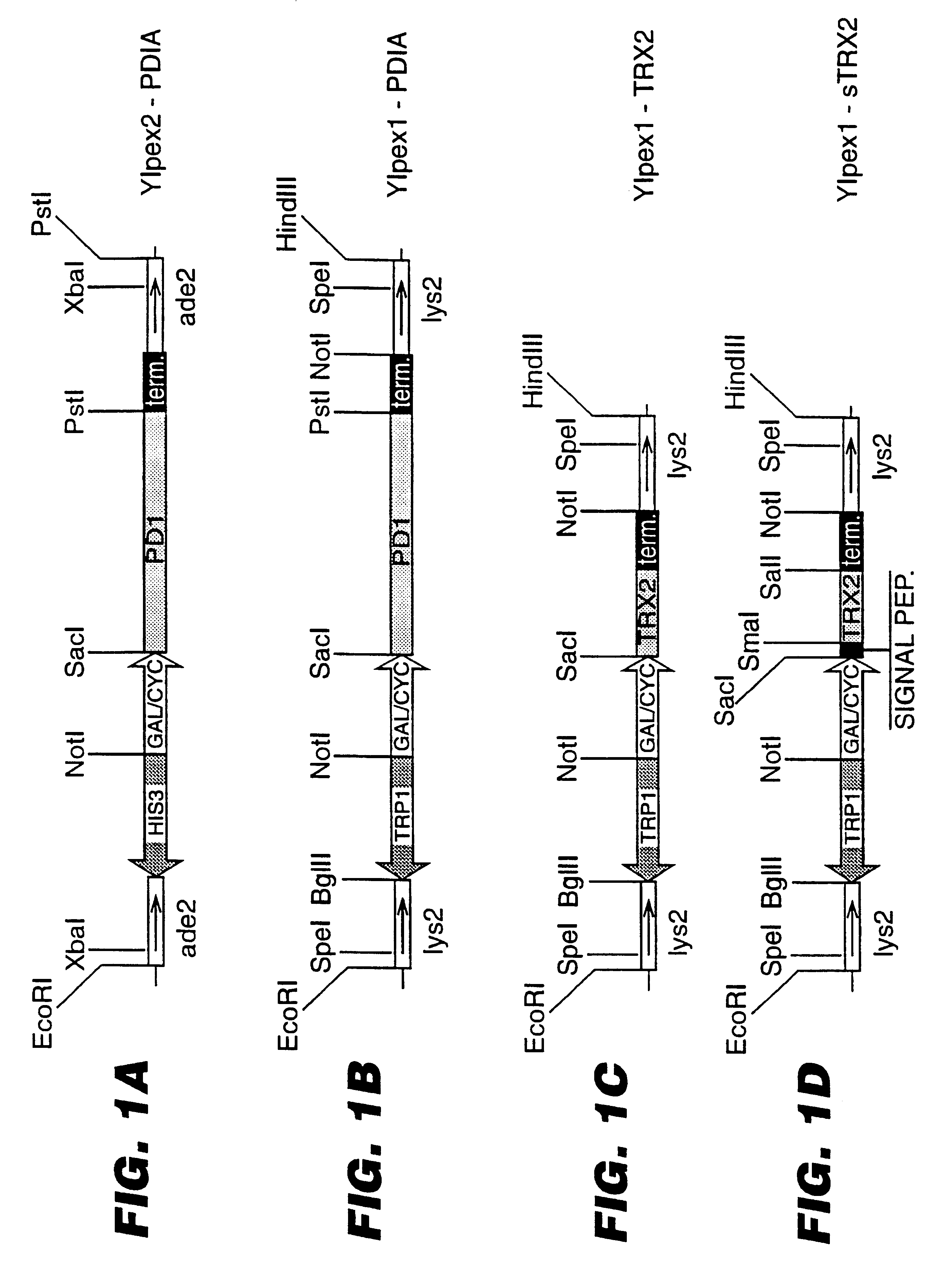
Expression of heterologous proteins
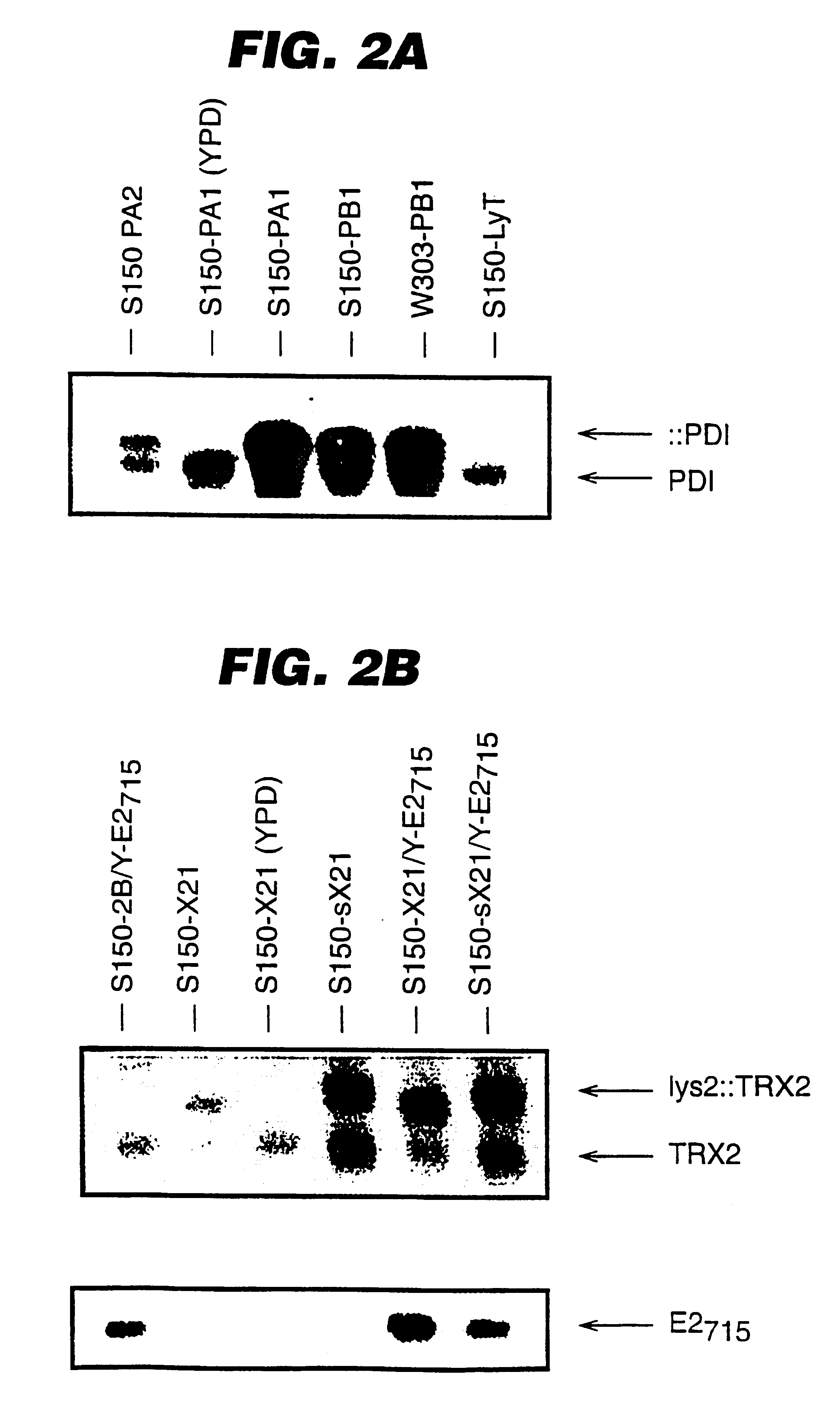
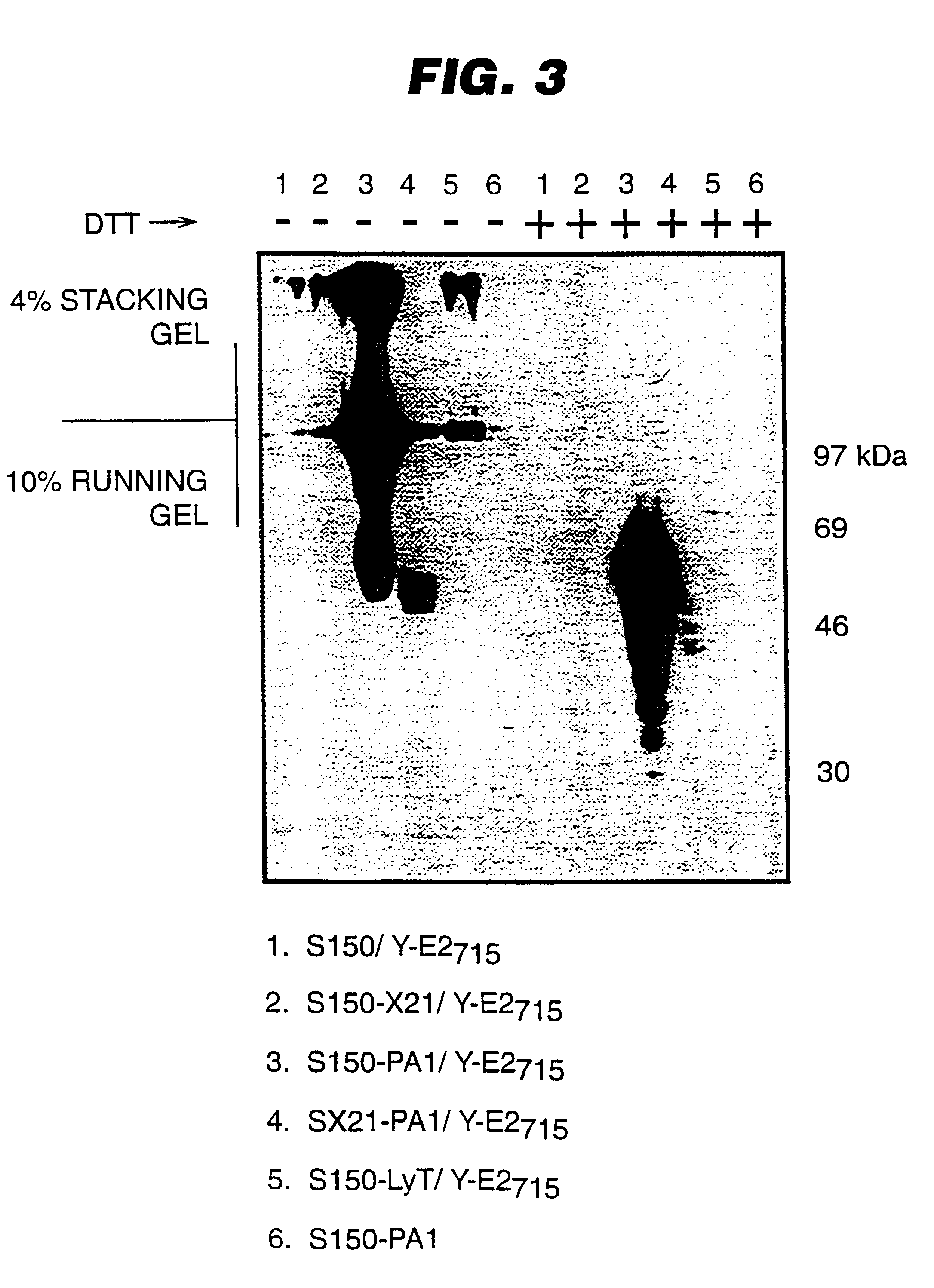
An expression system which provides heterologous proteins expressed by a non-native host organism but which have native-protein-like biological activity and / or structure. Disclosed are vectors, expression hosts and methods for expressing the heterologous proteins. The expression system involves co-expression of protein factor(s) which is / are capable of catalyzing disulphide bond formation and desired heterologous protein(s). The expression system is presented using yeast cells as the preferred host, protein disulphide isomerase (PDI) and thioredoxin (TRX) as the preferred examples of the protein factors and HCV-E2715 envelope glycoprotein and human FIGF as the preferred examples of the heterologous proteins.
Owner:NOVARTIS AG
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
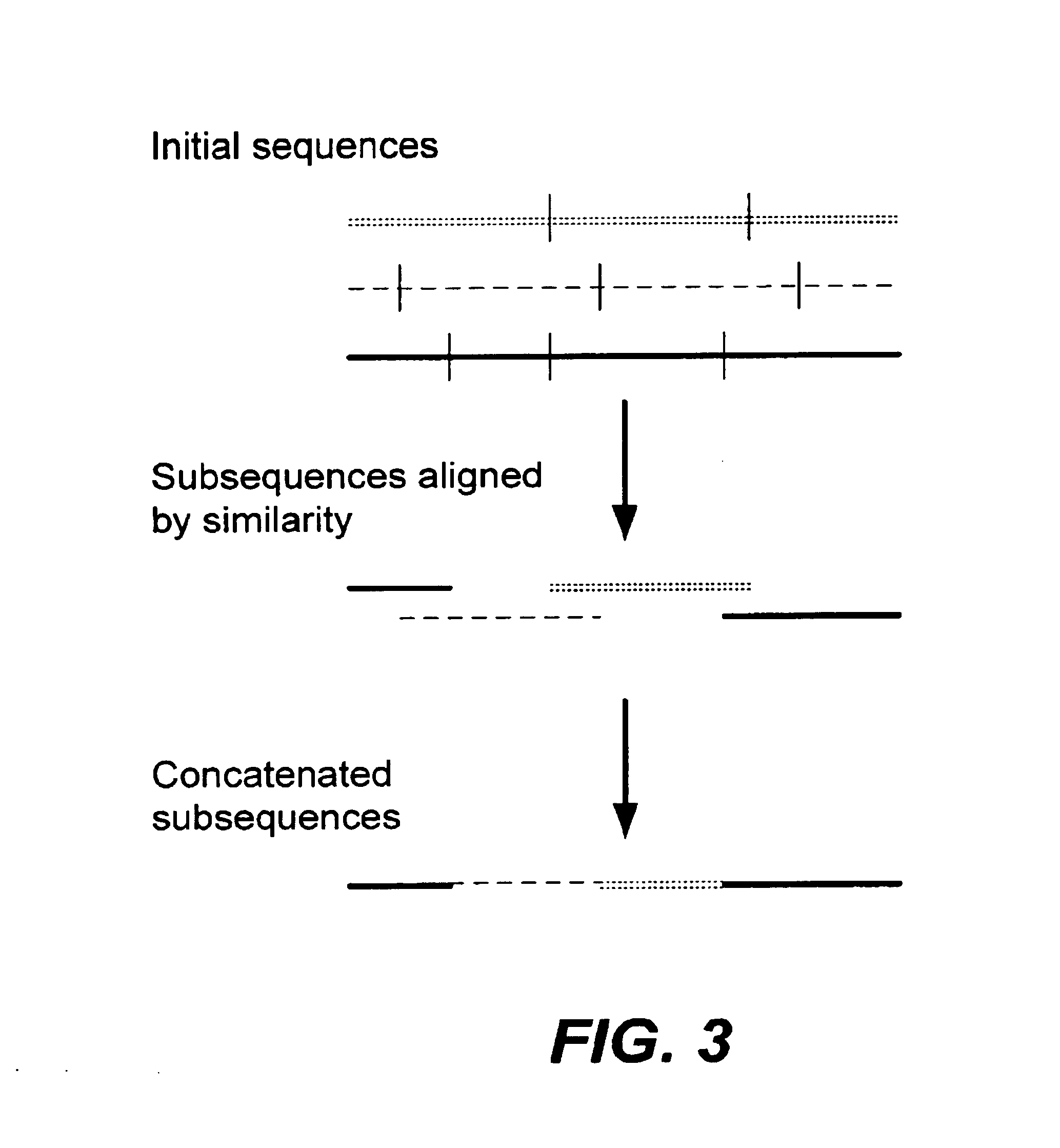
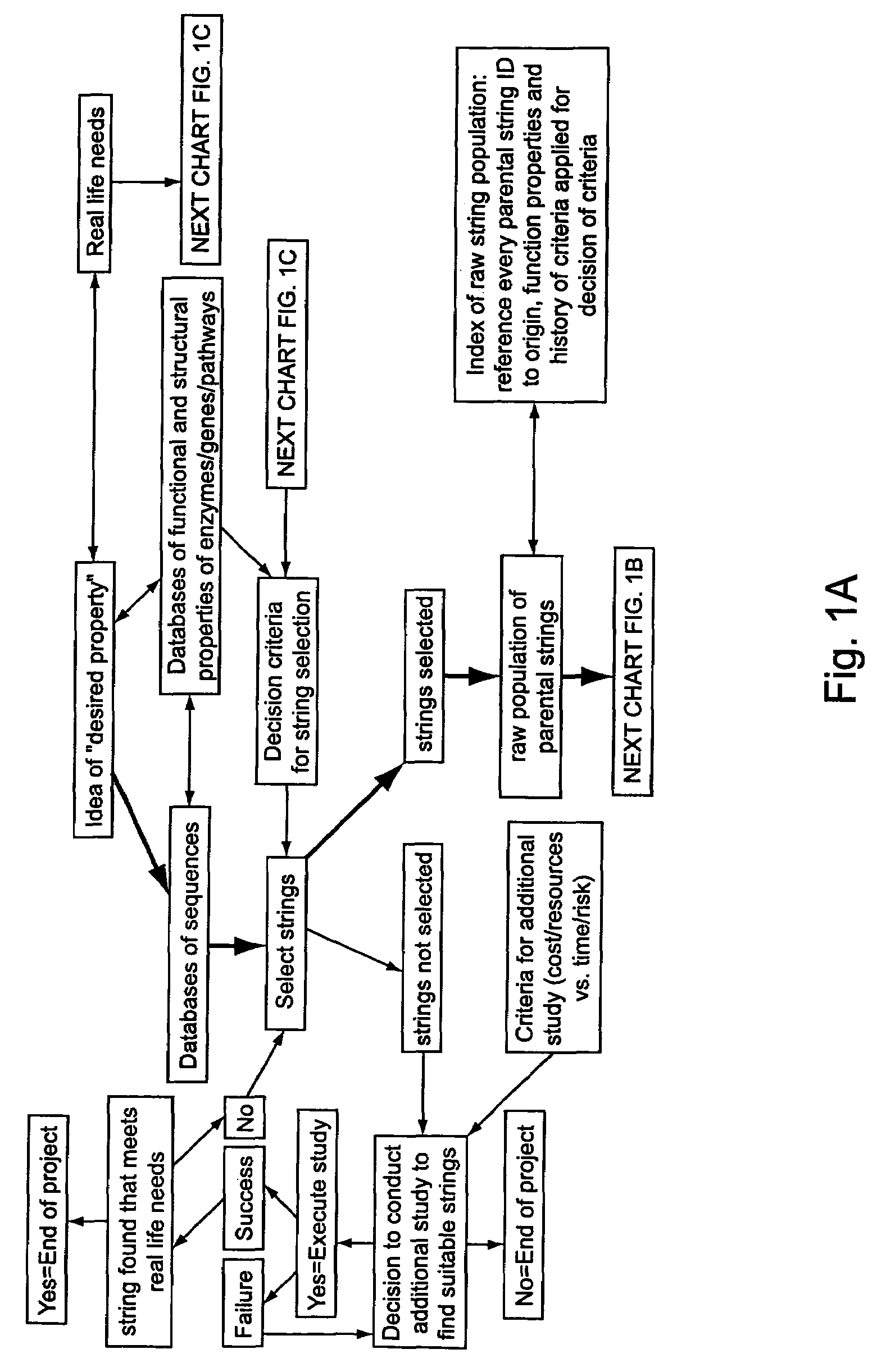
Methods of populating data structures for use in evolutionary simulations
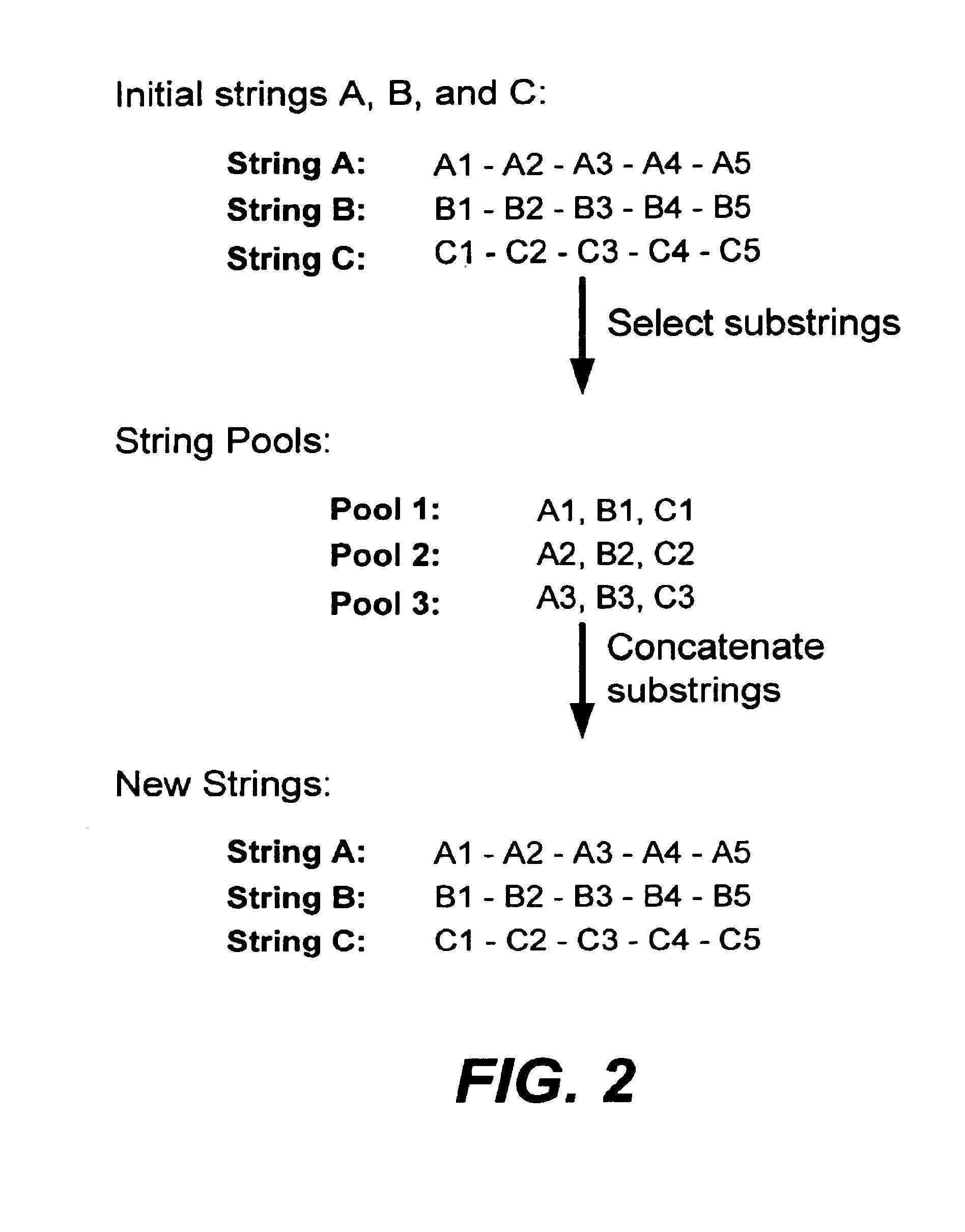
In particular, this invention provides novel methods of populating data structures for use in evolutionary modeling. In particular, this invention provides methods of populating a data structure with a plurality of character strings. The methods involve encoding two or more a biological molecules into character strings to provide a collection of two or more different initial character strings; selecting at least two substrings from the pool of character strings; concatenating the substrings to form one or more product strings about the same length as one or more of the initial character strings; adding the product strings to a collection of strings; and optionally repeating this process using one or more of the product strings as an initial string in the collection of initial character strings.
Owner:CODEXIS MAYFLOWER HLDG LLC
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
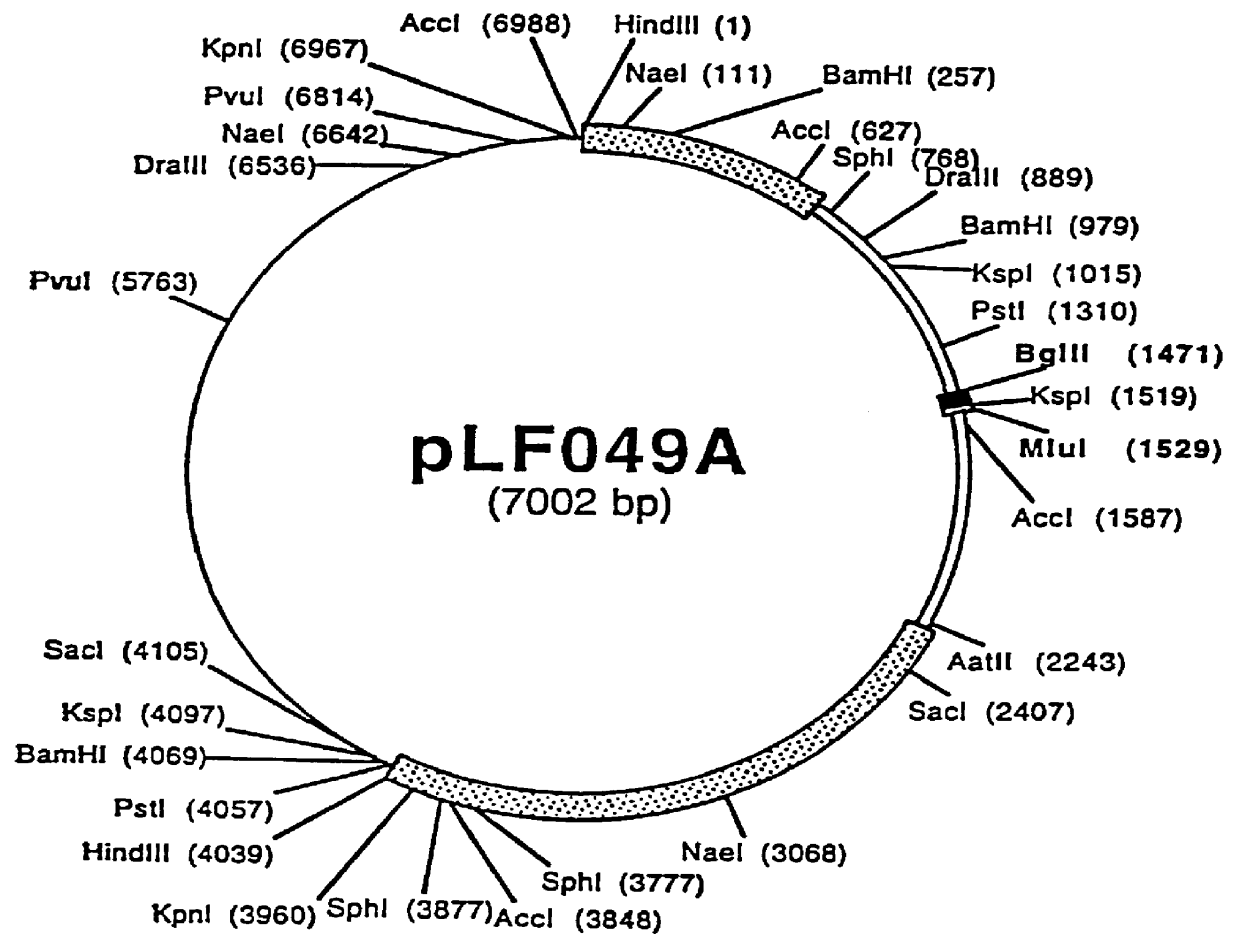
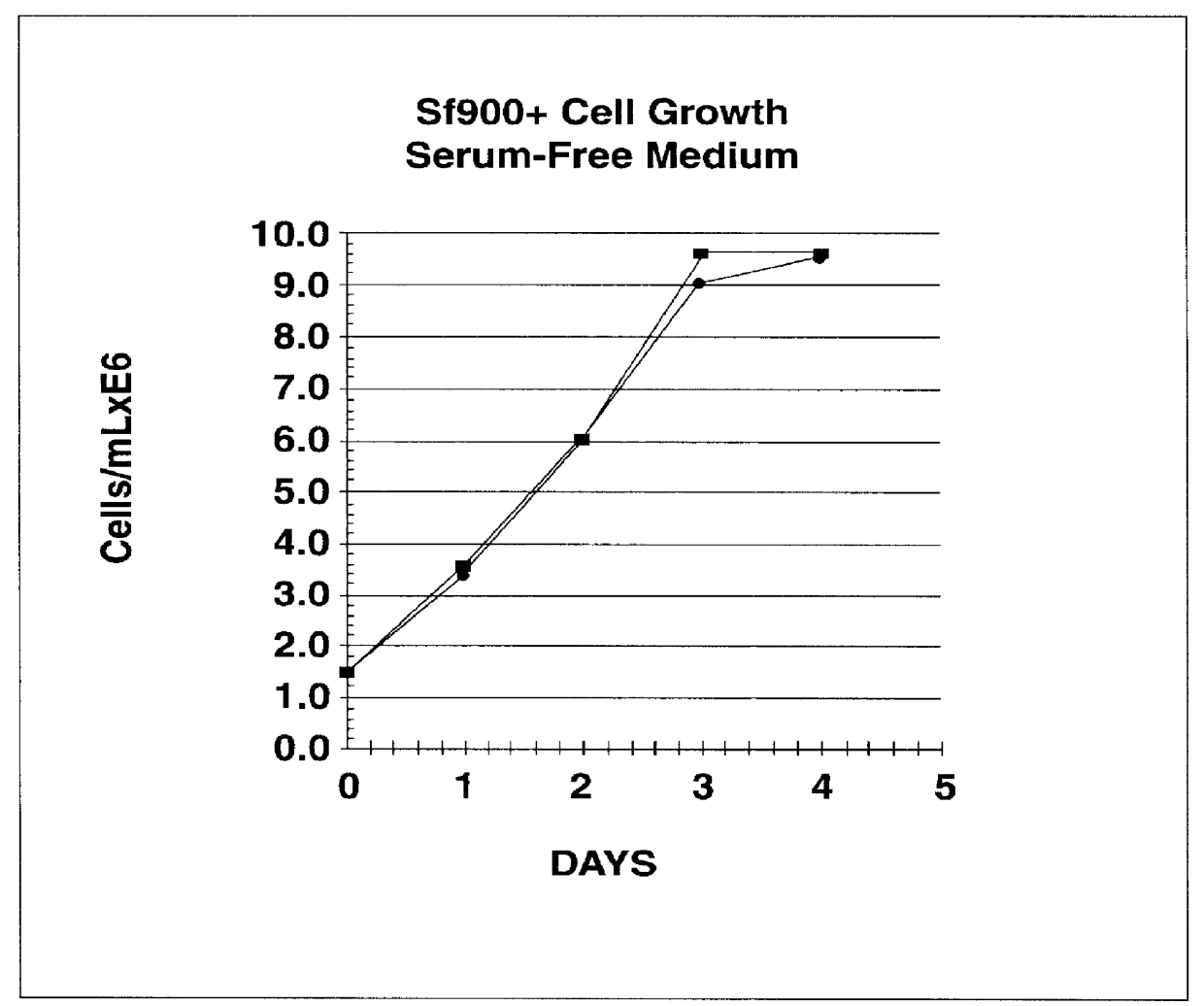
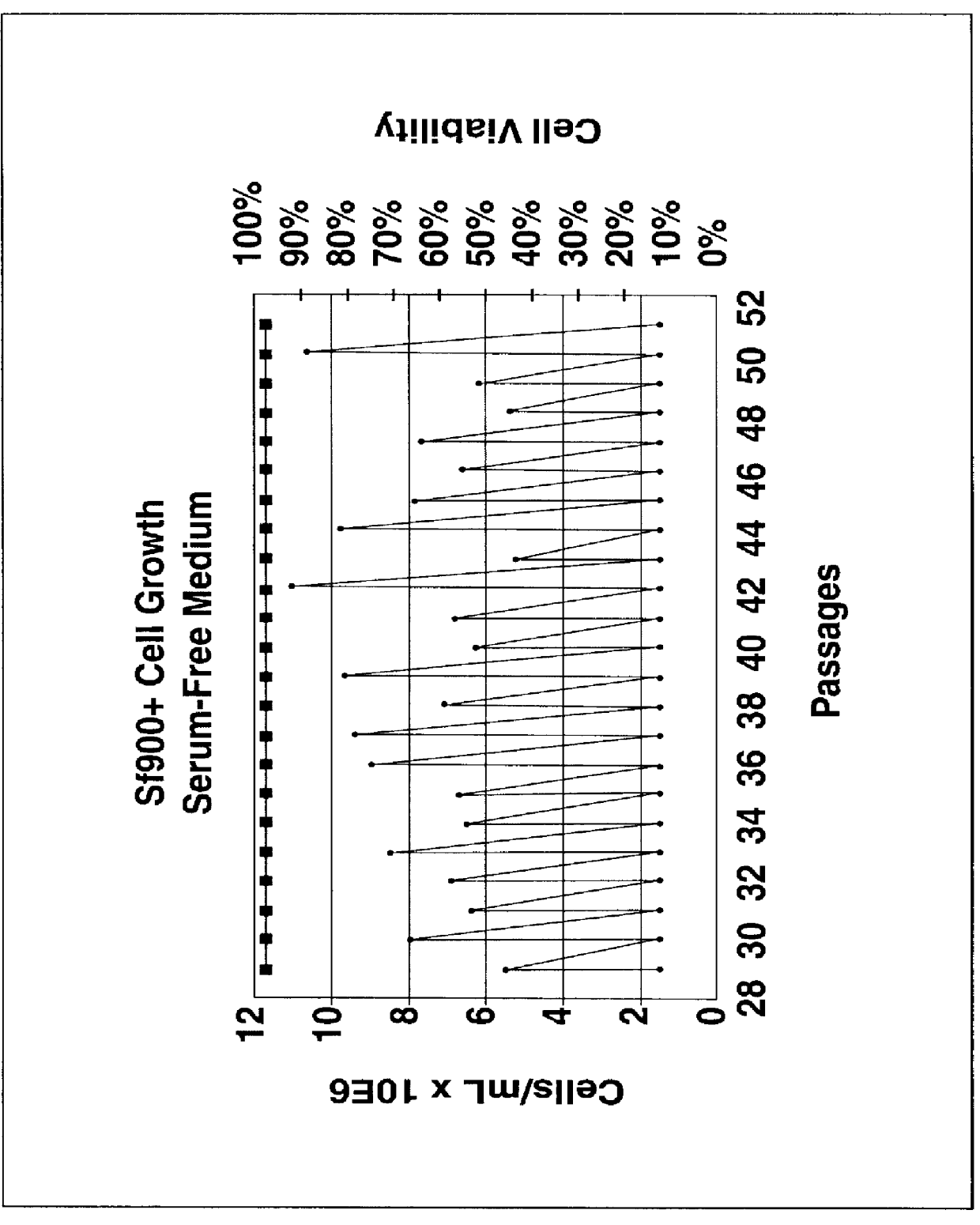
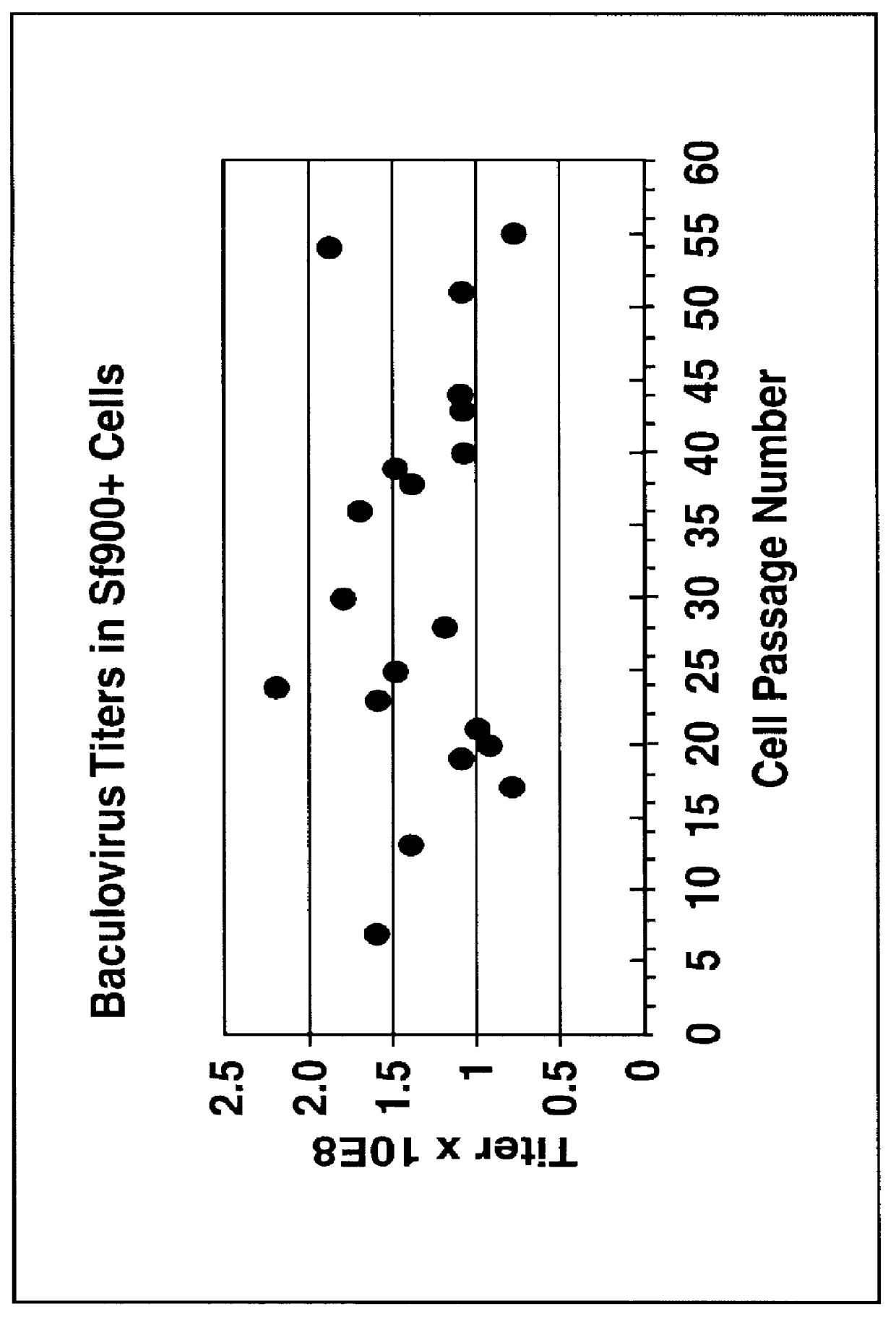
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Method for the preparation of purified HCV RNA by exosome separation
InactiveUS7198923B1Simple preparation processPromote progressSsRNA viruses positive-senseMicrobiological testing/measurementExosomeBlood plasma
Owner:GRIFOLS WORLDWIDE OPERATIONS +1
Identifying oligonucleotides for in vitro recombination
“In silico” nucleic acid recombination methods, related integrated systems utilizing genetic operators and libraries made by in silico shuffling methods are provided.
Owner:CODEXIS INC
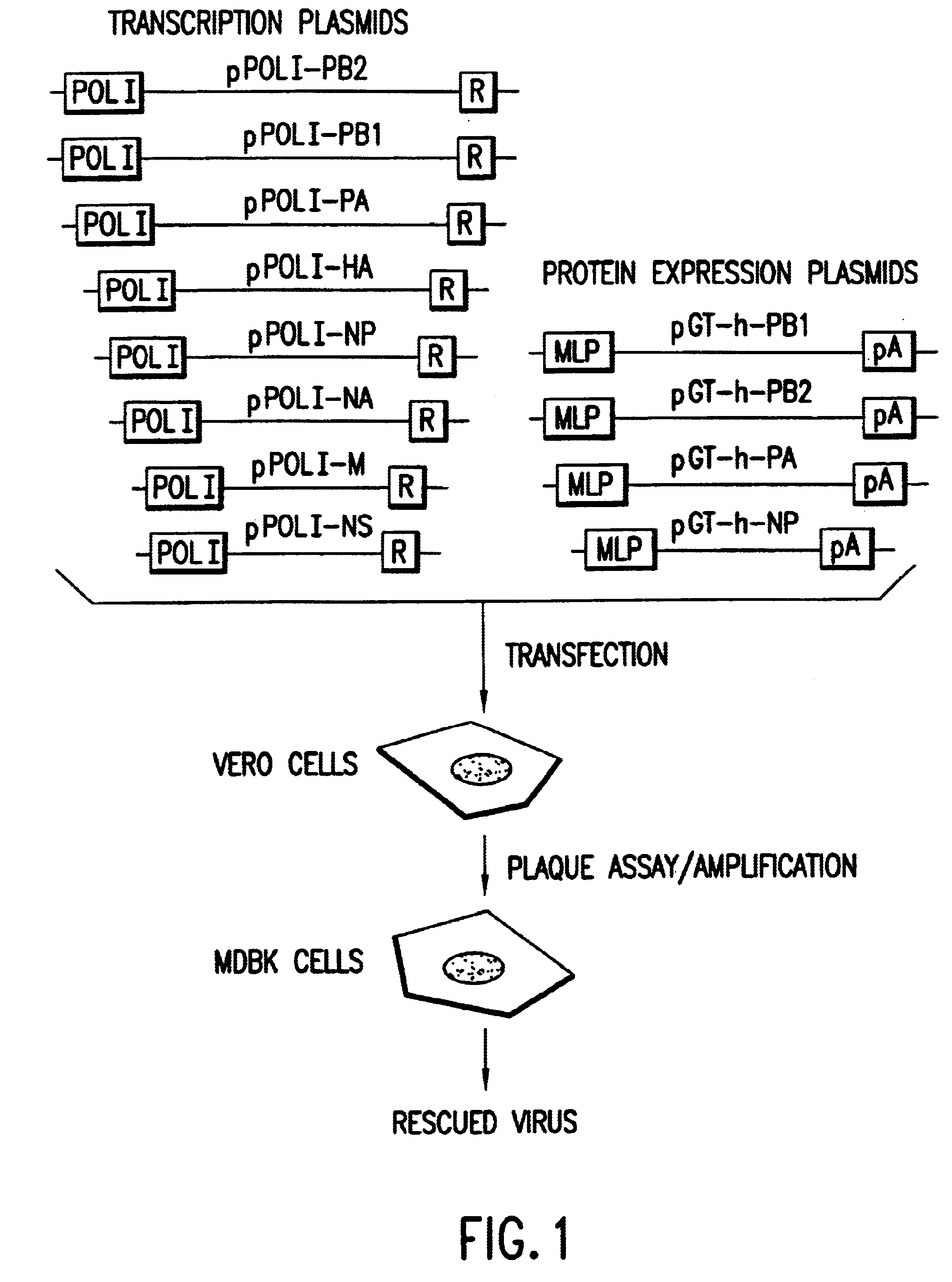
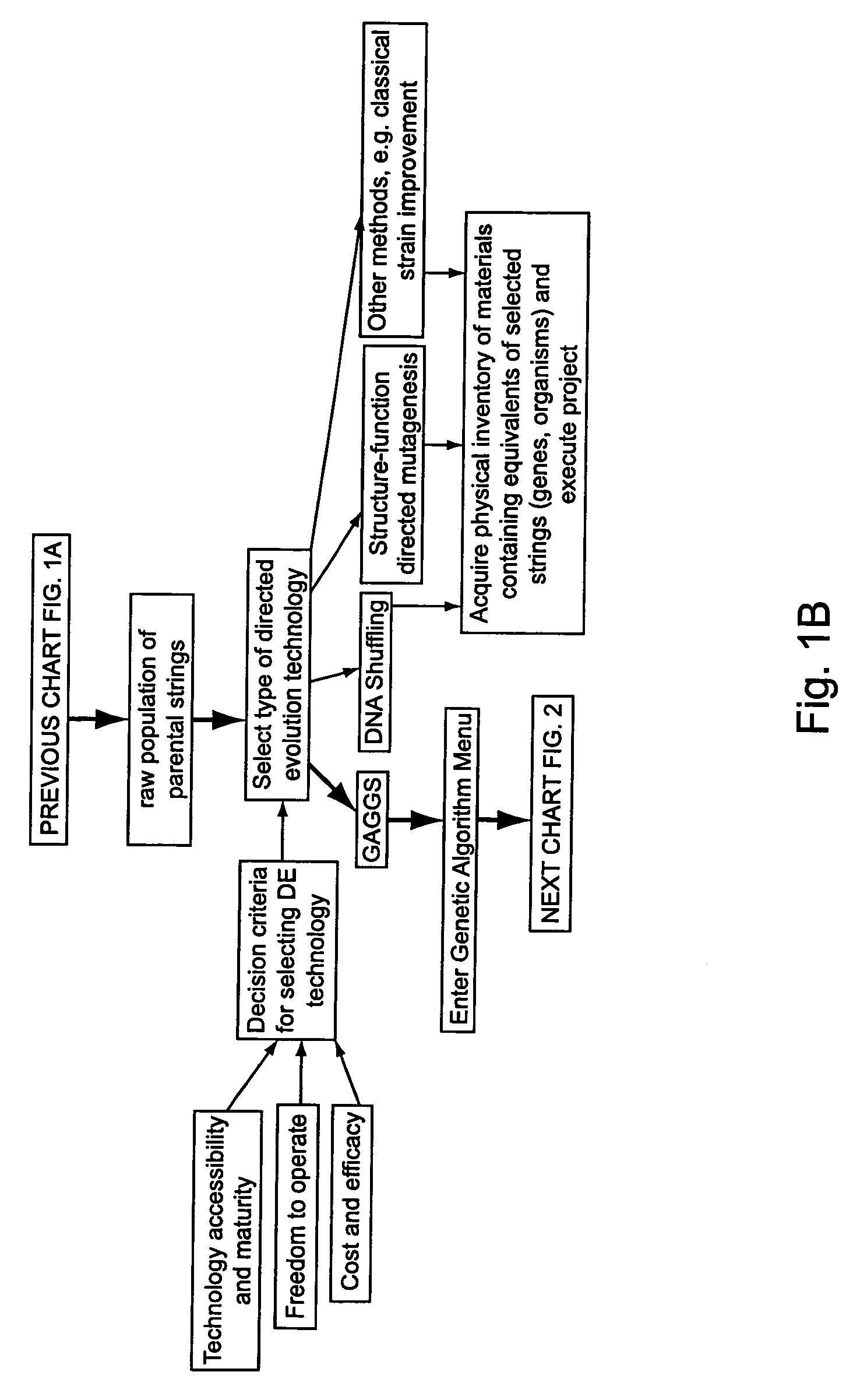
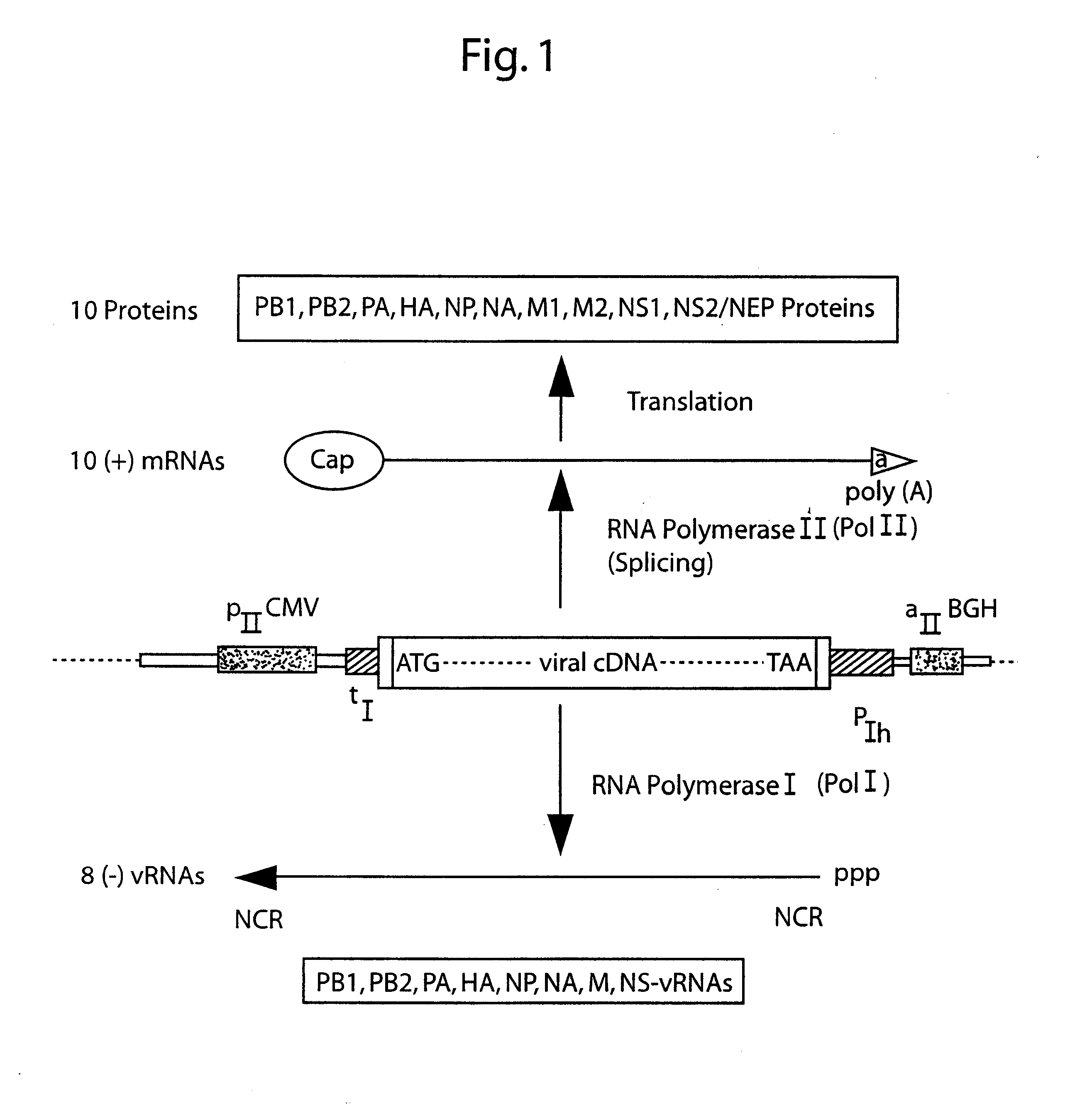
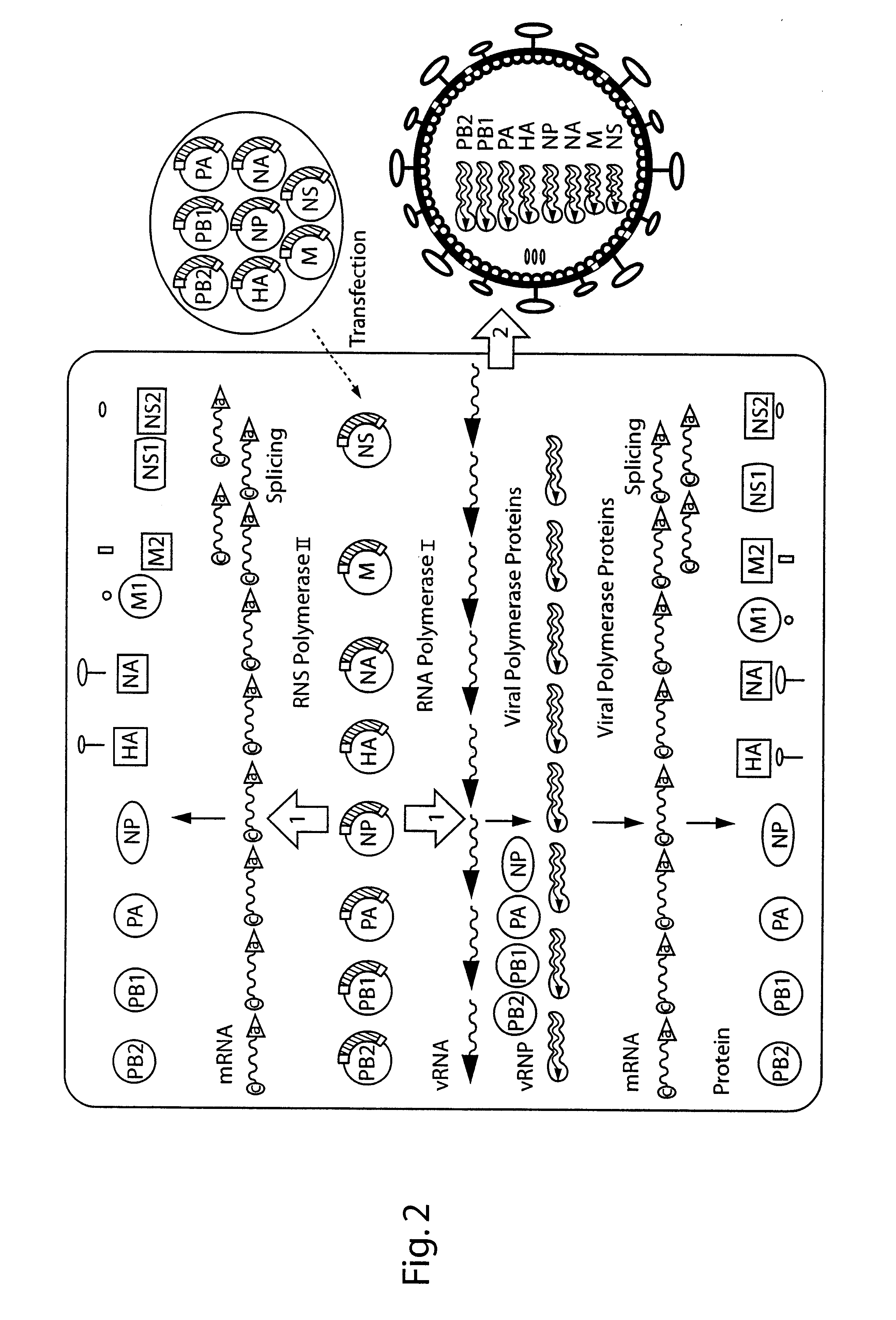
DNA transfection system for the generation of infectious influenza virus
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
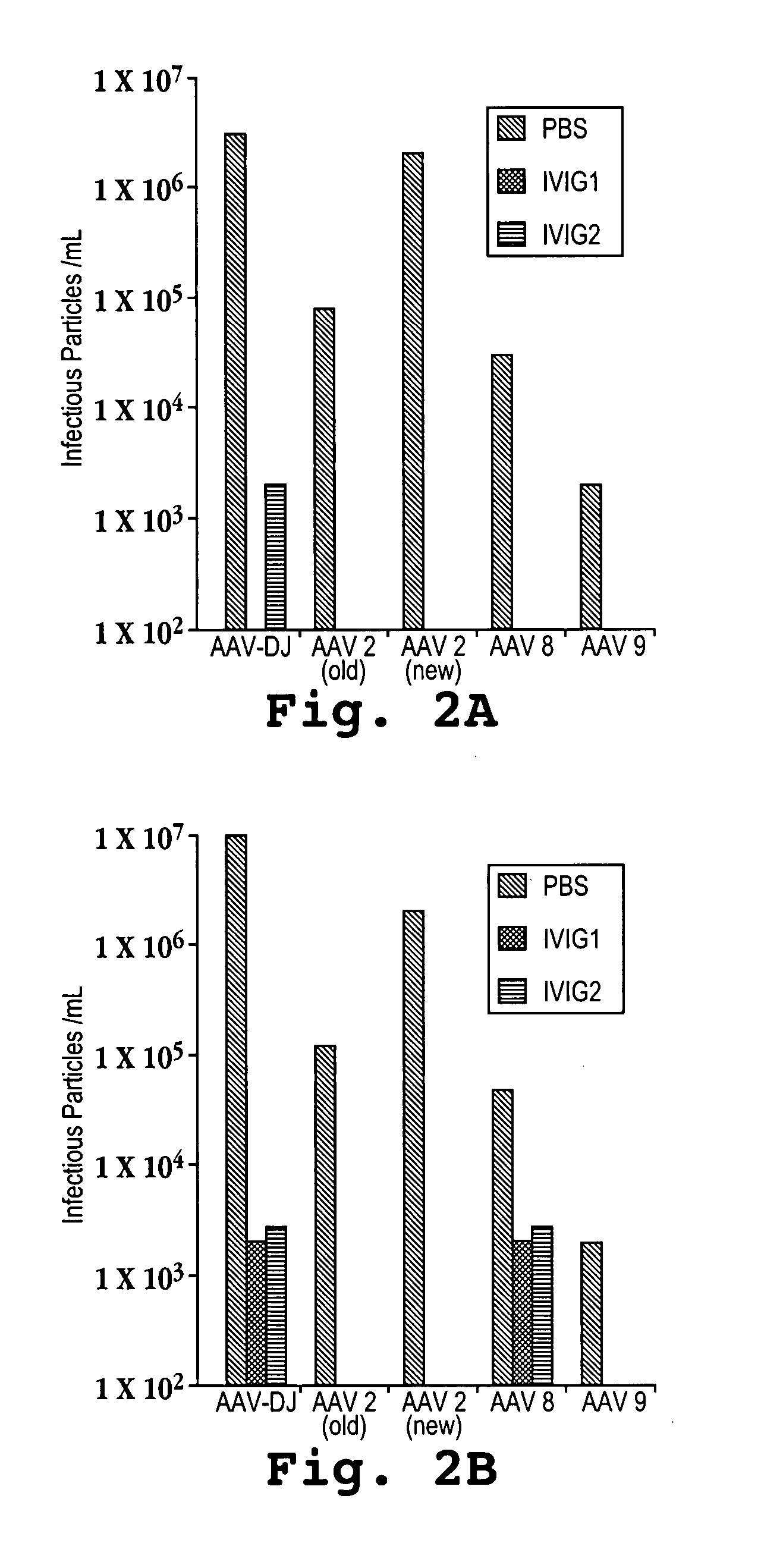
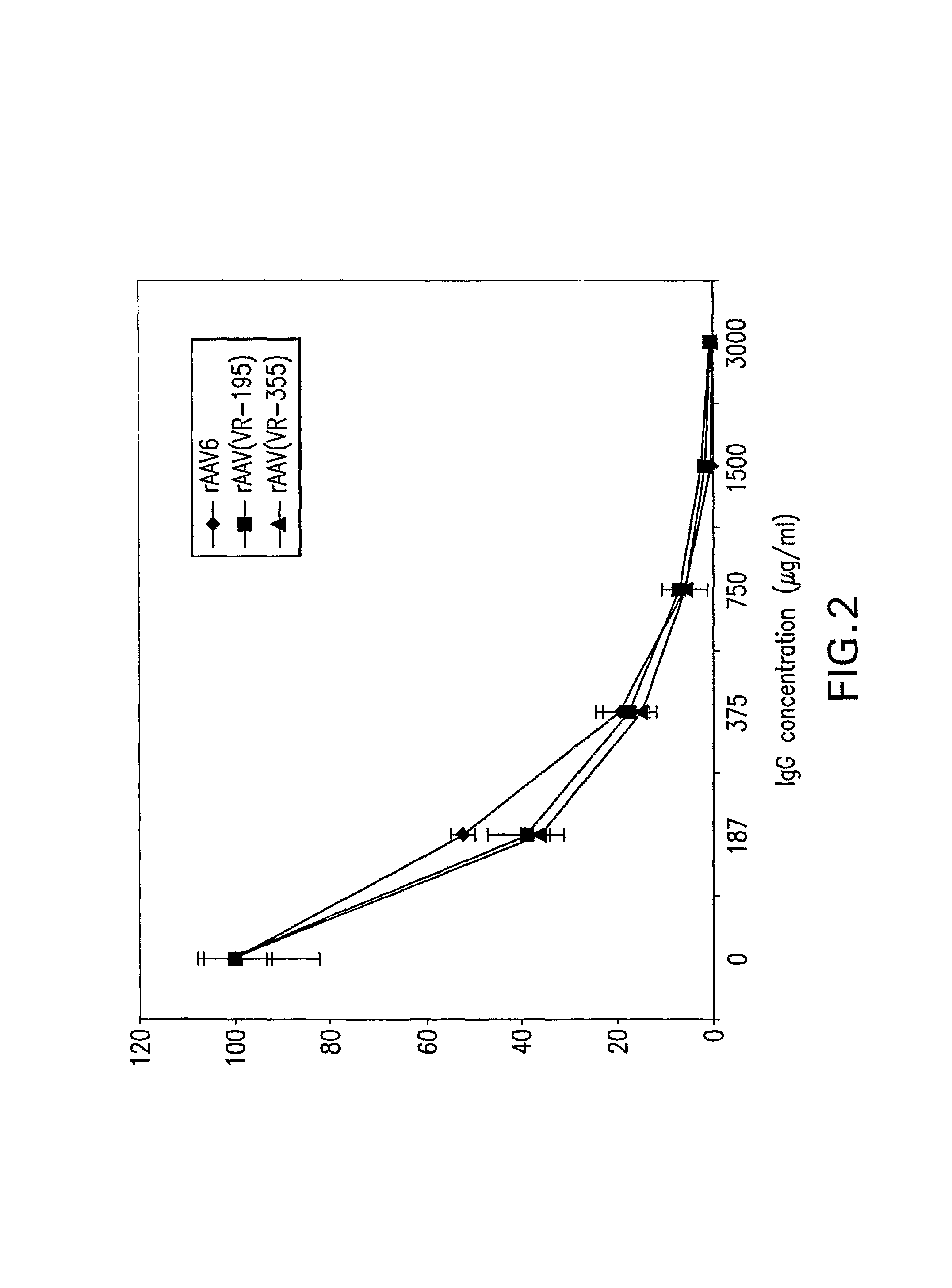
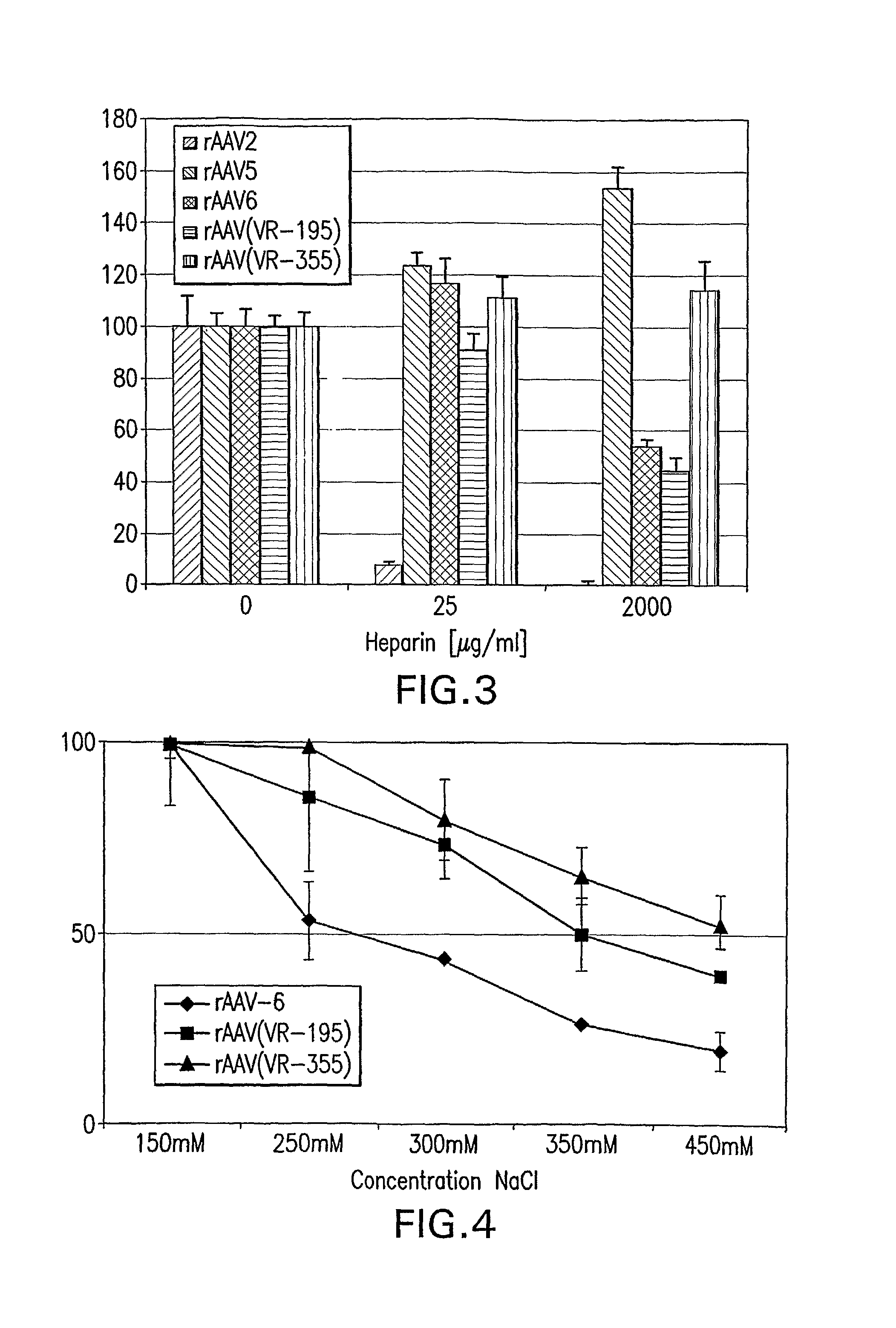
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20090202490A1Reduce the binding forceAltered infectivityOrganic active ingredientsBiocideCell type specificNeutralizing antibody
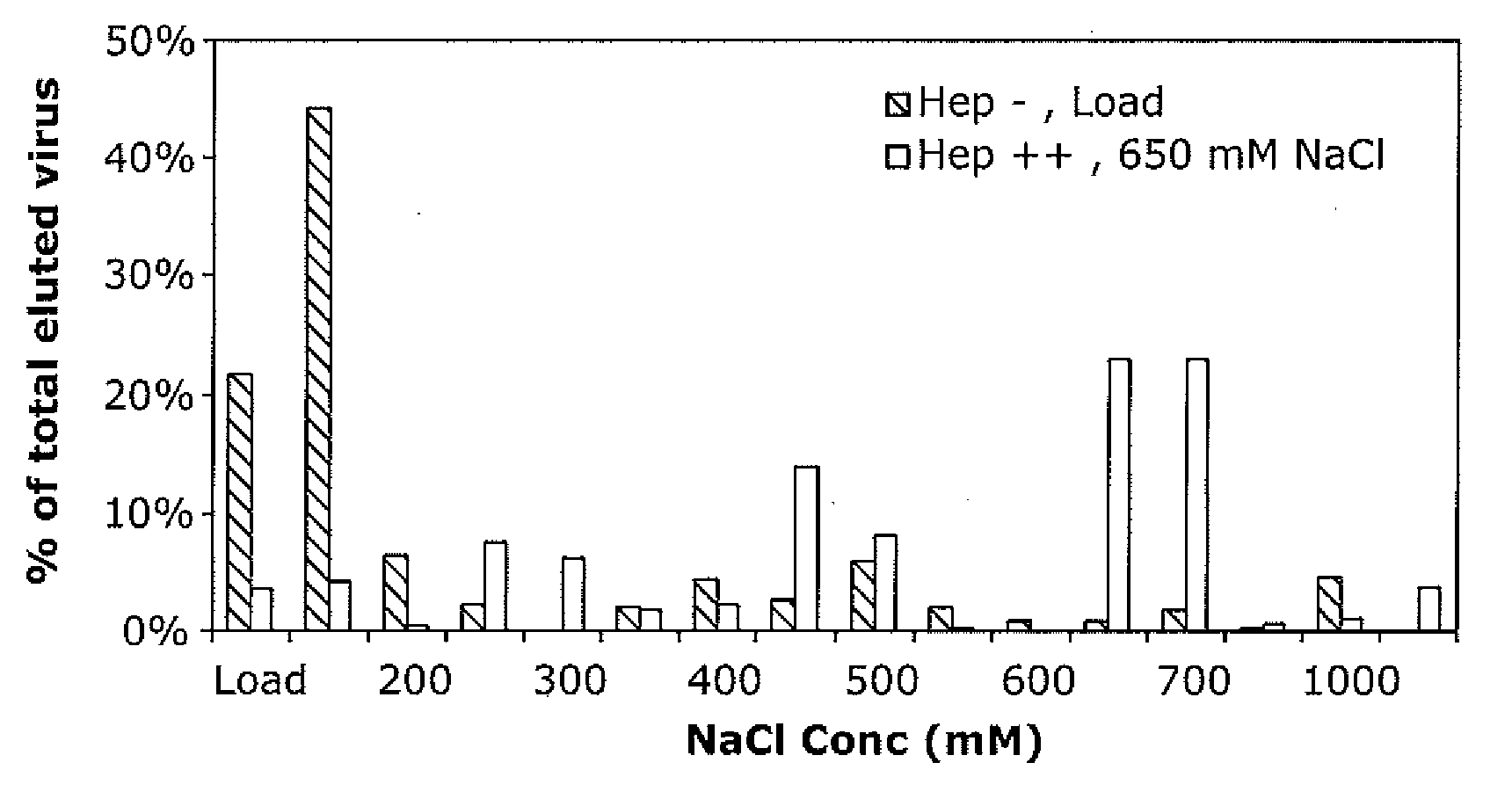
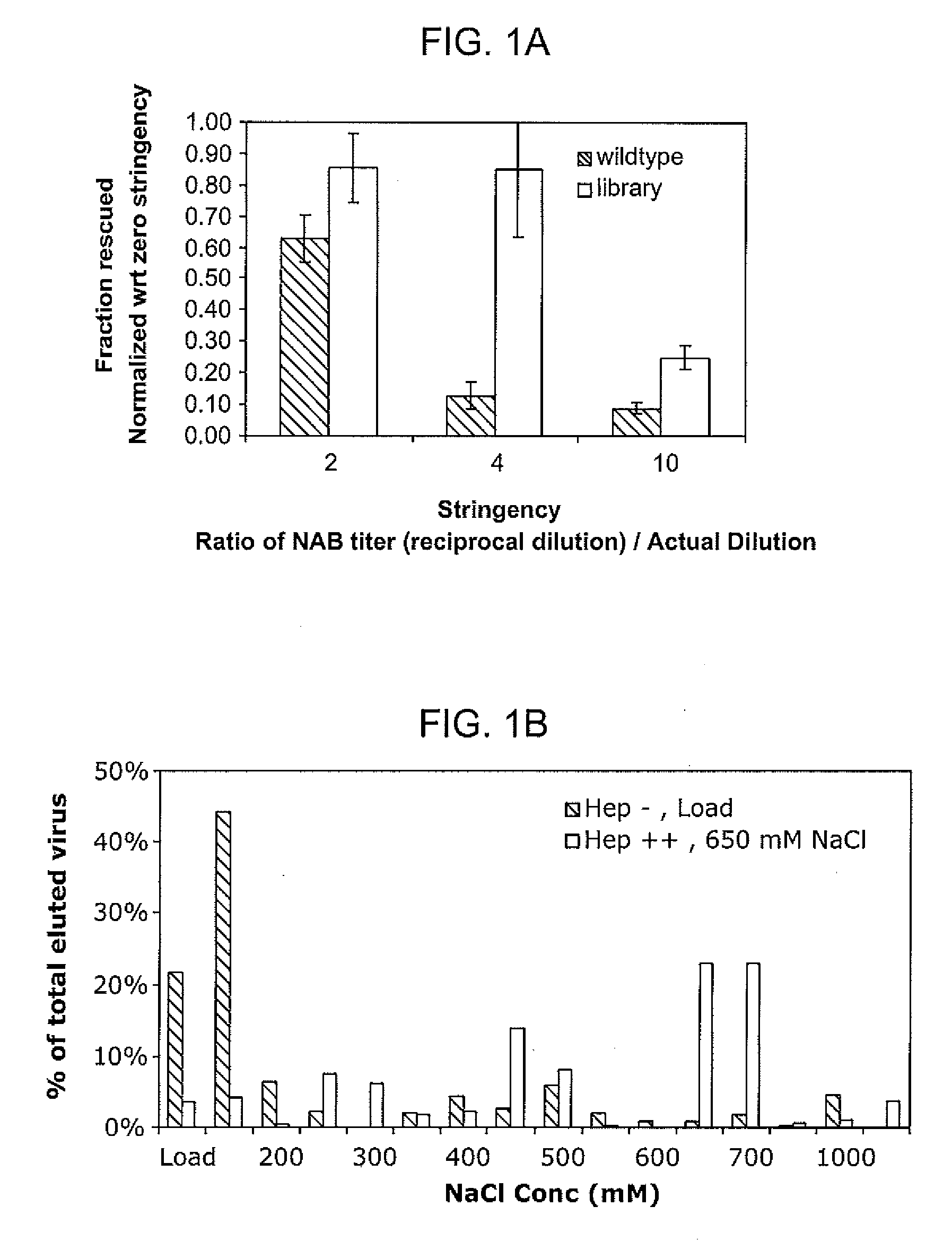
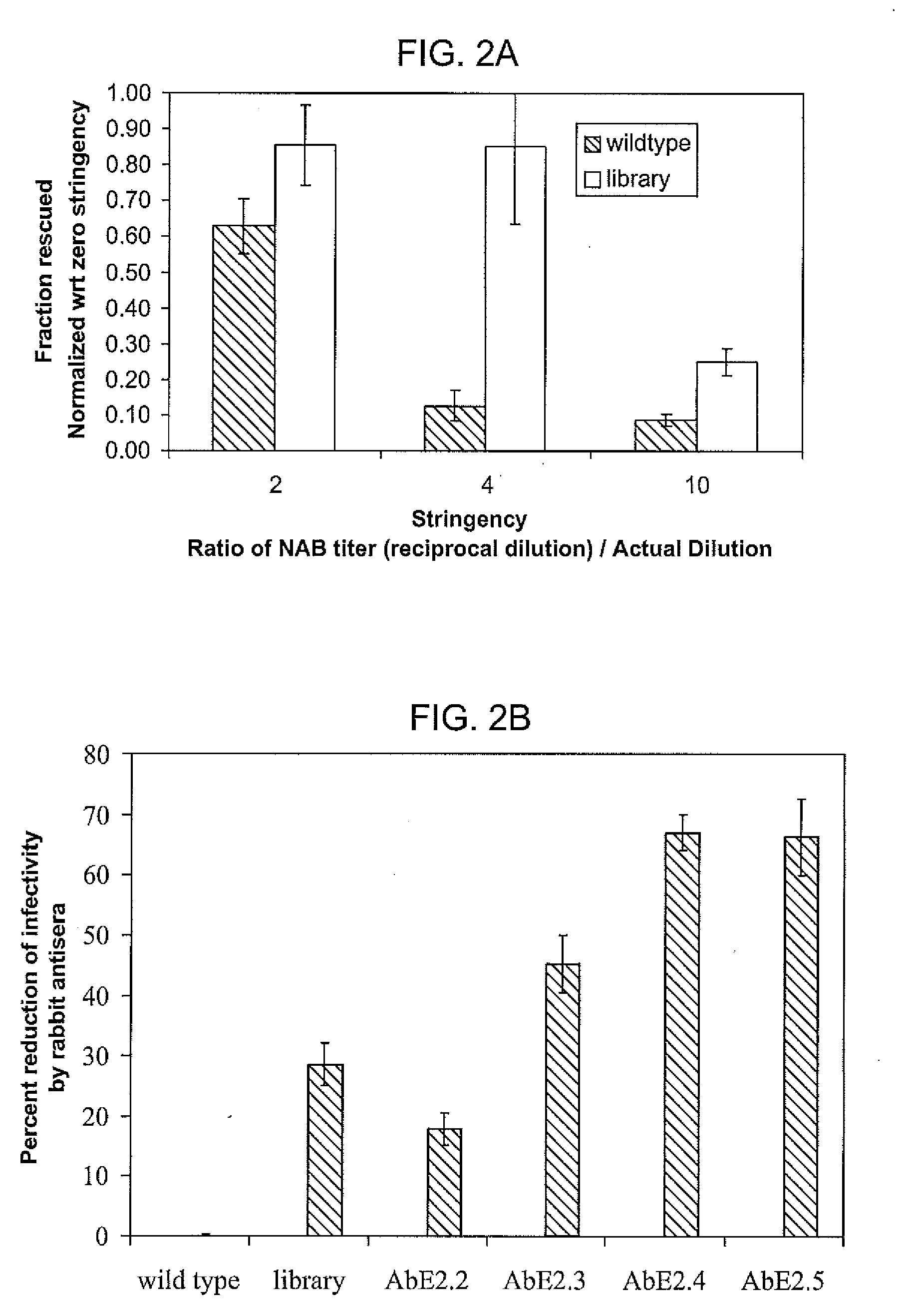
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:RGT UNIV OF CALIFORNIA +1
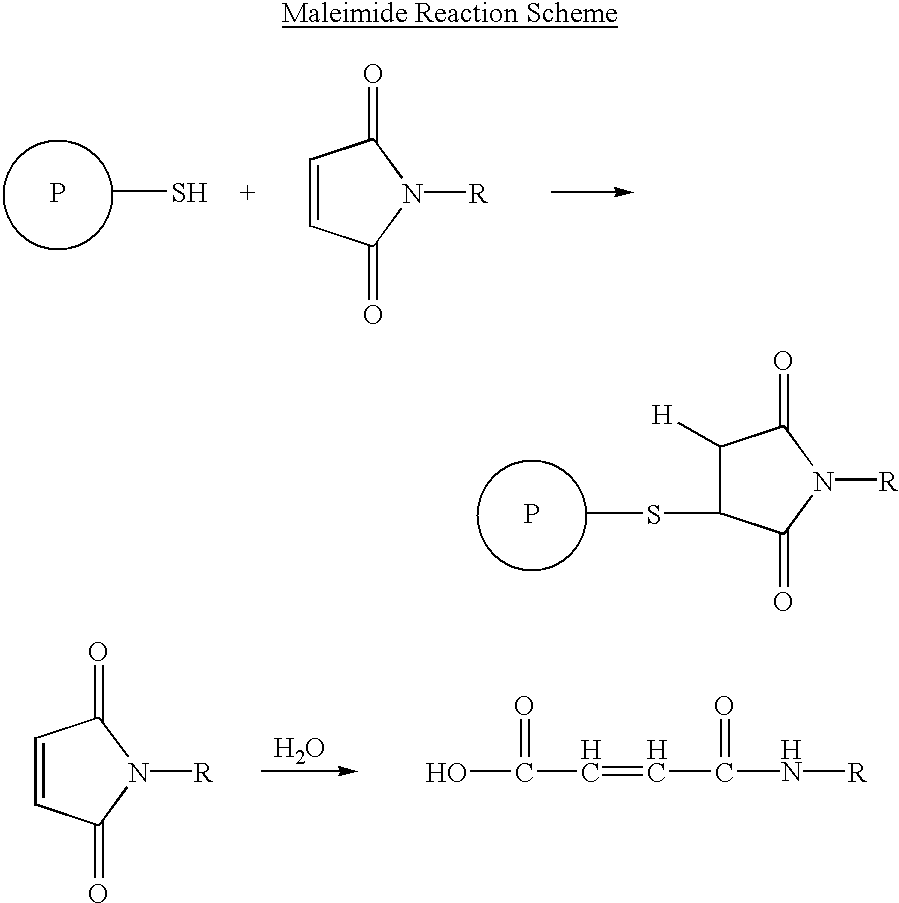
Self-assembling nanoparticle drug delivery system
InactiveUS20090226525A1High binding affinityHigh affinityPowder deliveryMicroencapsulation basedLipid formationMedicine
A self-assembling nanoparticle drug delivery system for the delivery of various bioactive agents including peptides, proteins, nucleic acids or synthetic chemical drugs is provided. The self-assembling nanoparticle drug delivery system described herein includes viral capsid proteins, such as Hepatitis B Virus core protein, encapsulating the bioactive agent, a lipid layer or lipid / cholesterol layer coat and targeting or facilitating molecules anchored in the lipid layer. A method for construction of the self-assembling nanoparticle drug delivery system is also provided.
Owner:CHIMEROS
HIV envelope polypeptides
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Isolation, cloning and characterization of new adeno-associated virus (AAV) serotypes
The present invention provides new adeno-associated virus (AAV) viruses and vectors, and particles derived therefrom. In addition, the present invention provides methods of delivering a nucleic acid to a cell using the AAV vectors and particles.
Owner:THE GOVERNMENT OF THE US SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER
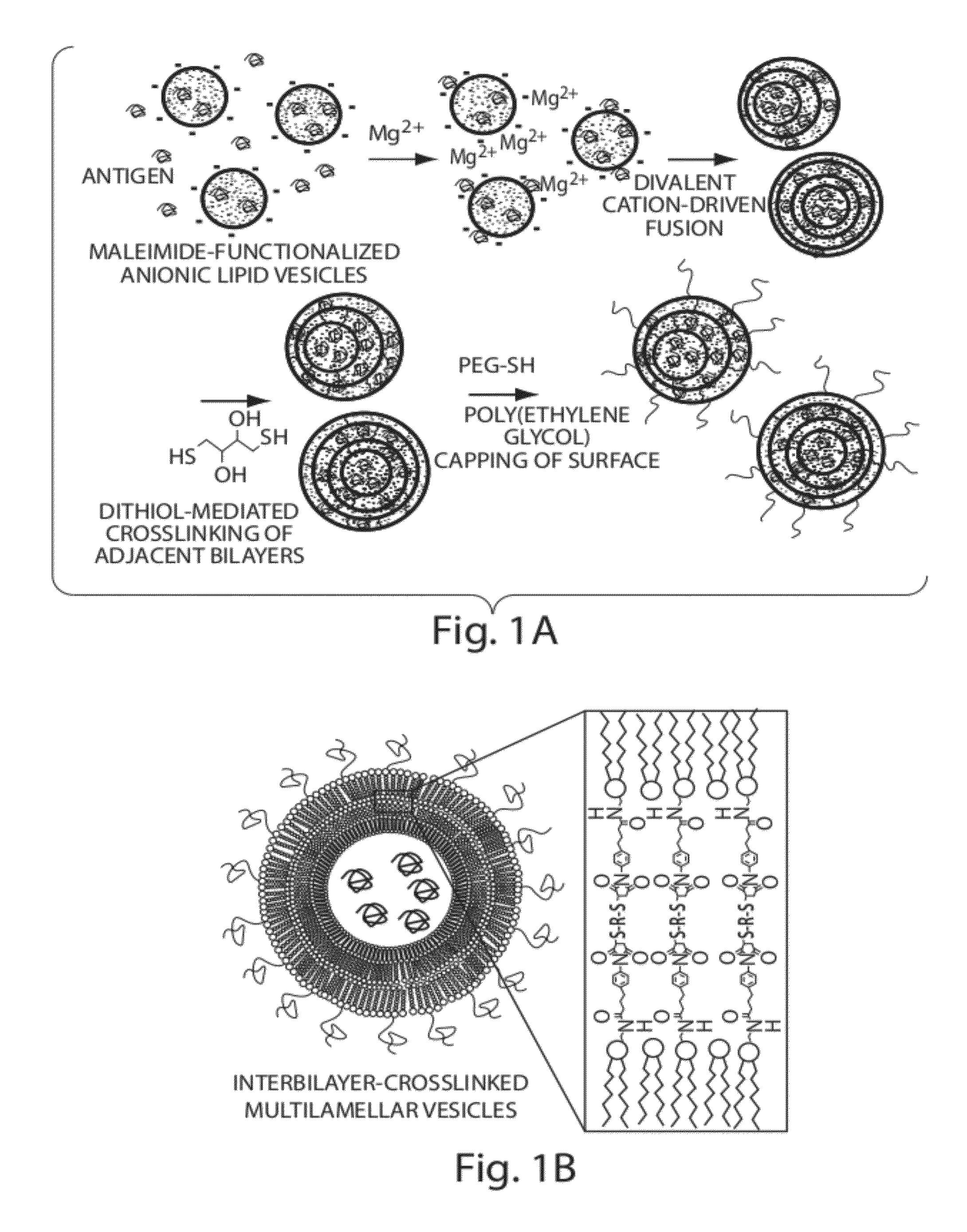
Lipid vesicle compositions and methods of use
ActiveUS20120177724A1Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
Mammalian viral vectors and their uses
InactiveUS6255071B1Stable episomal maintenanceMaintain relatively stableSugar derivativesMicrobiological testing/measurementRetroviral provirusMammal
The present invention relates to methods and compositions for the elucidation of mammalian gene function. Specifically, the present invention relates to methods and compositions for improved mammalian complementation screening, functional inactivation of specific essential or non-essential mammalian genes, and identification of mammalian genes which are modulated in response to specific stimuli.In particular, the compositions of the present invention include, but are not limited to, replication-deficient retroviral vectors, libraries comprising such vectors, retroviral particles produced by such vectors in conjunction with retroviral packaging cell lines, integrated provirus sequences derived from the retroviral particles of the invention and circularized provirus sequences which have been excised from the integrated provirus sequences of the invention. The compositions of the present invention further include novel retroviral packaging cell lines.
Owner:COLD SPRING HARBOR LAB INC
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS20050261229A1Stimulate productionEnhanceVirusesPeptide/protein ingredientsFc receptorAdjuvant
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com