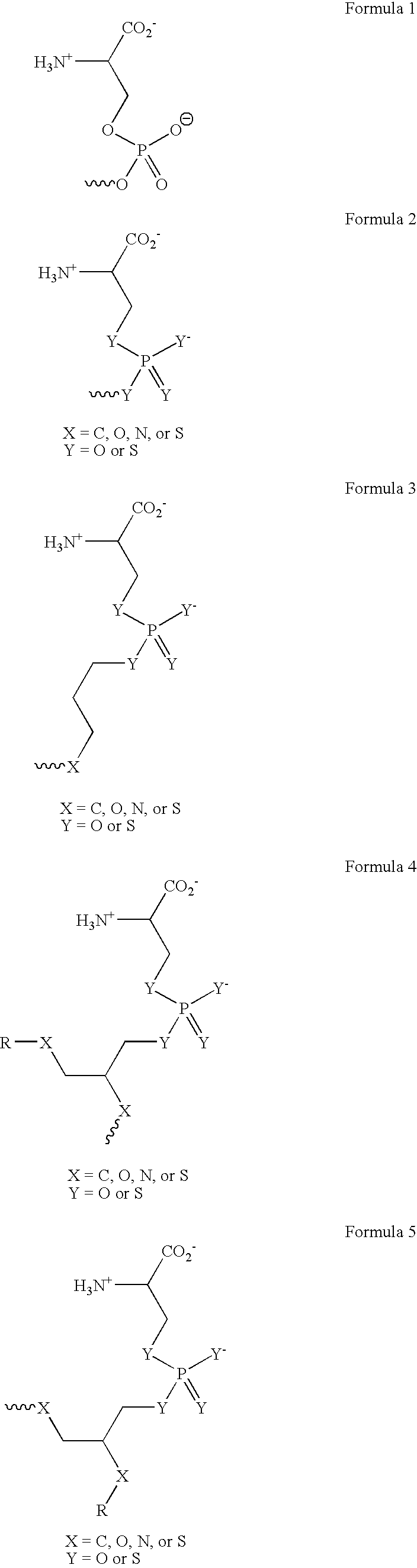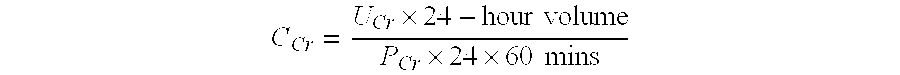Patents
Literature
53 results about "Thrombospondin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thrombospondins are a family of secreted glycoproteins with antiangiogenic functions. Due to their dynamic role within the extracellular matrix they are considered matricellular proteins. The first member of the family, thrombospondin 1 (THBS1), was discovered in 1971 by Nancy L. Baenziger.
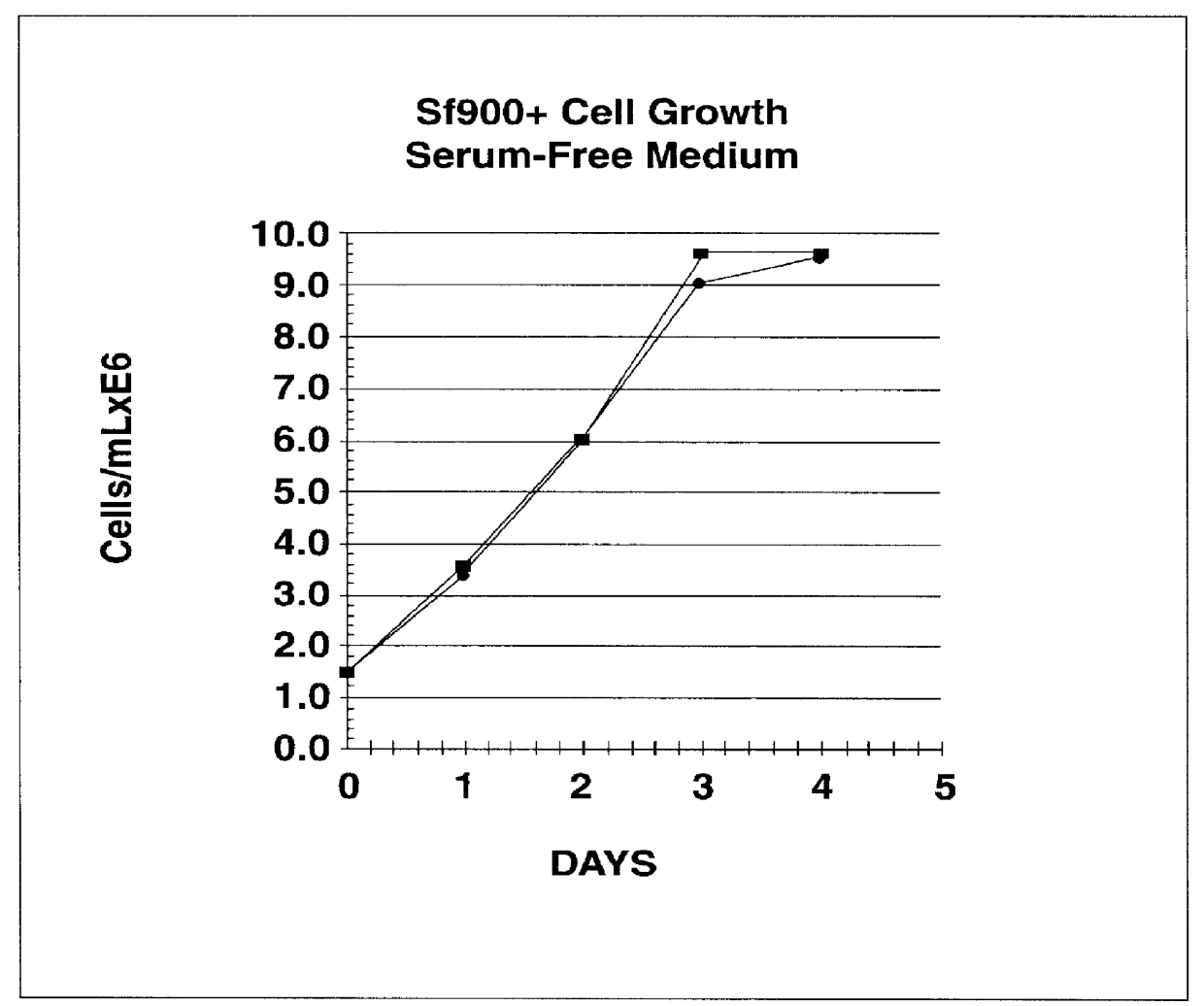
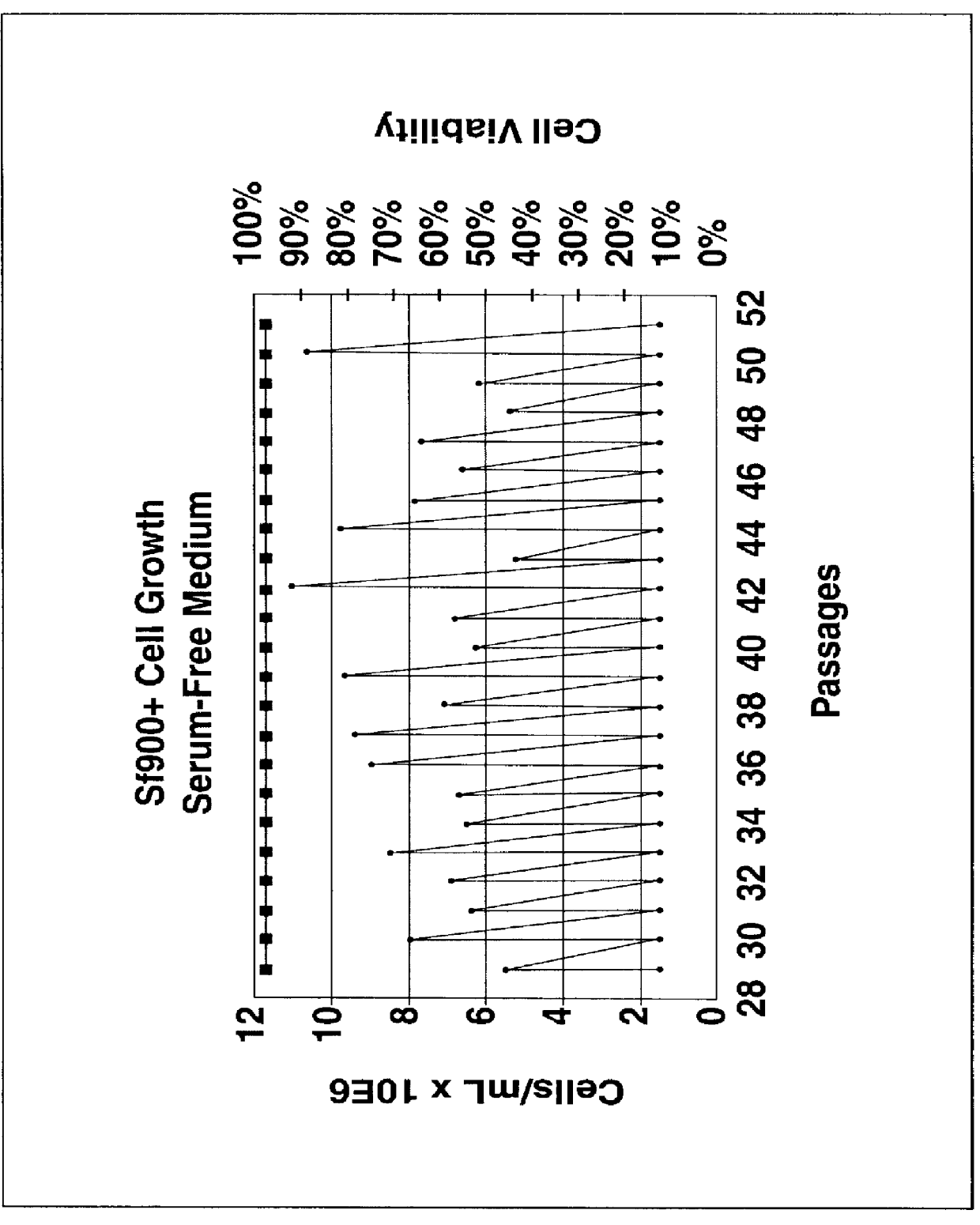
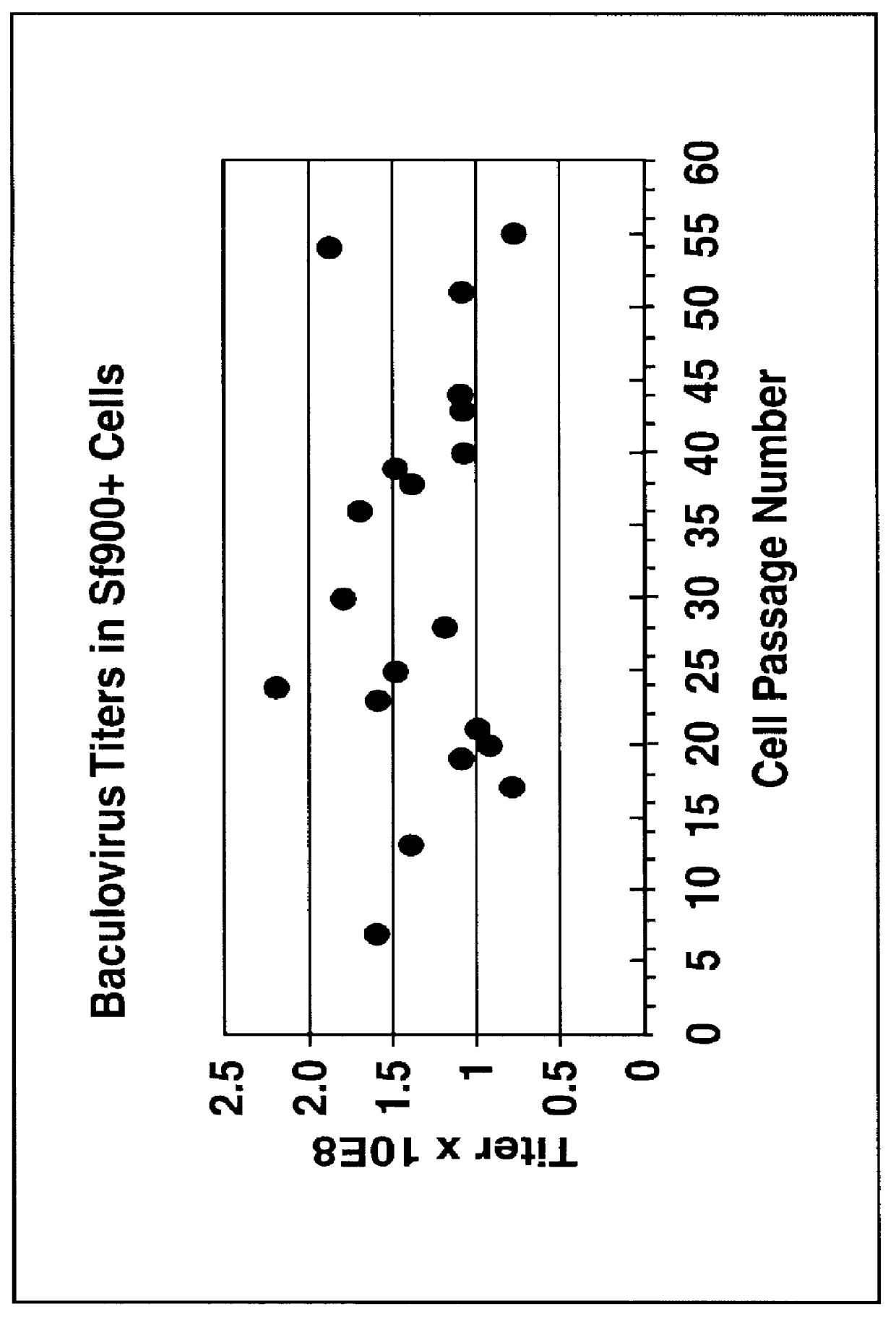
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
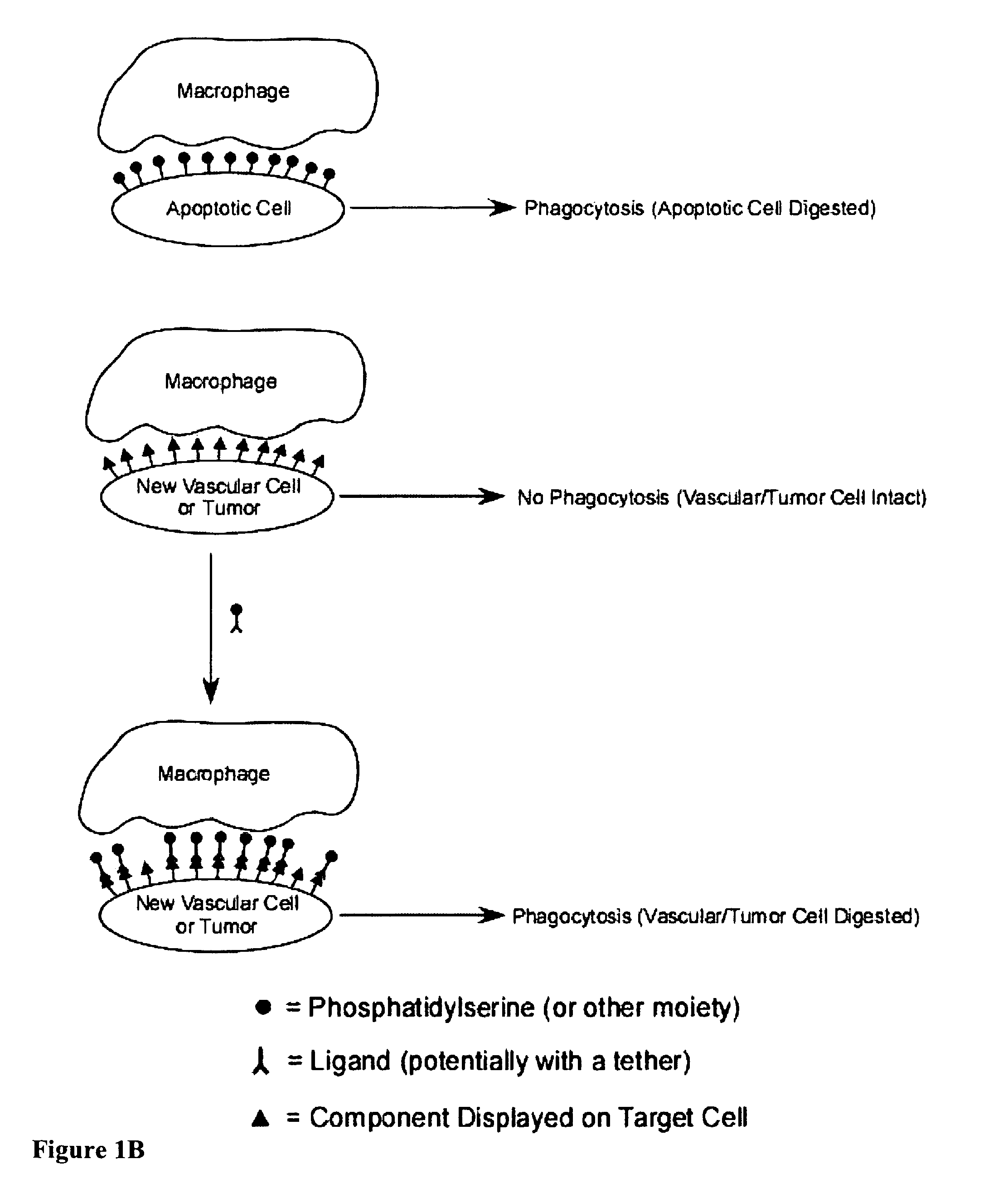
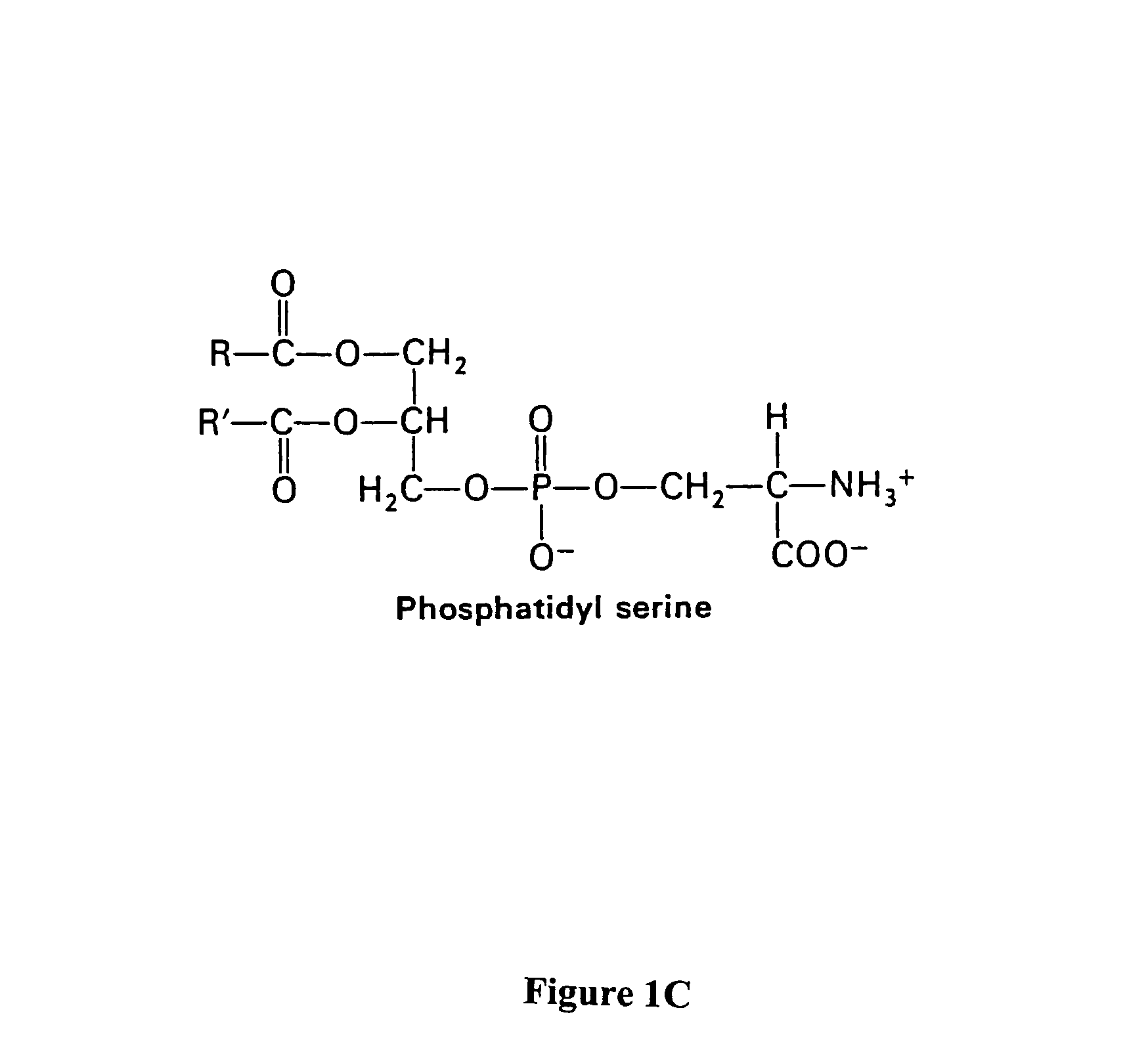
Compositions and methods for enhancing phagocytosis or phagocyte activity
ActiveUS20050113297A1Enhances and incites phagocytosisBetter targets for phagocytosisSenses disorderPeptide/protein ingredientsWhite blood cellCell type specific
The present invention provides a system for enhancing clearance or destruction of undesirable cells or noncellular molecular entities by tagging such cells or noncellular molecular entities with a marker that targets the cells or noncellular molecular entities for phagocytosis (phagocytic marker). The target cells can be, for example, endothelial cells, tumor cells, leukocytes, or virus-infected cells. In certain embodiments of the invention the tagging is accomplished by administering a composition comprising an antibody or ligand linked to the phagcytotic marker, wherein the antibody or ligand binds to a cell type specific marker present on or in the cell surface of a target cell. In preferred embodiments of the invention, the phagocytic marker comprises phosphatidylserine or a group derived from phosphatidylserine, thrombospondin-1, annexin I, or a derivative of any of these.
Owner:APELLIS PHARMA
Methods of Purifying Recombinant Adamts13 and Other Proteins and Compositions Thereof
ActiveUS20110081700A1Reduce formationExtension of timeInactivation/attenuationBiomass after-treatmentHydroxylapatiteAnion-exchange chromatography
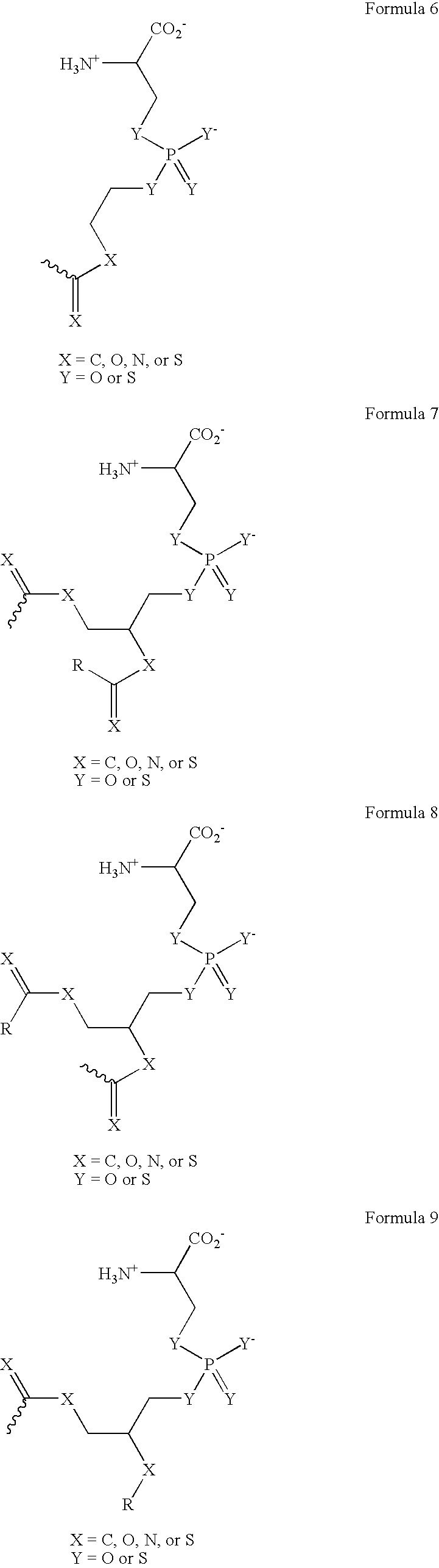
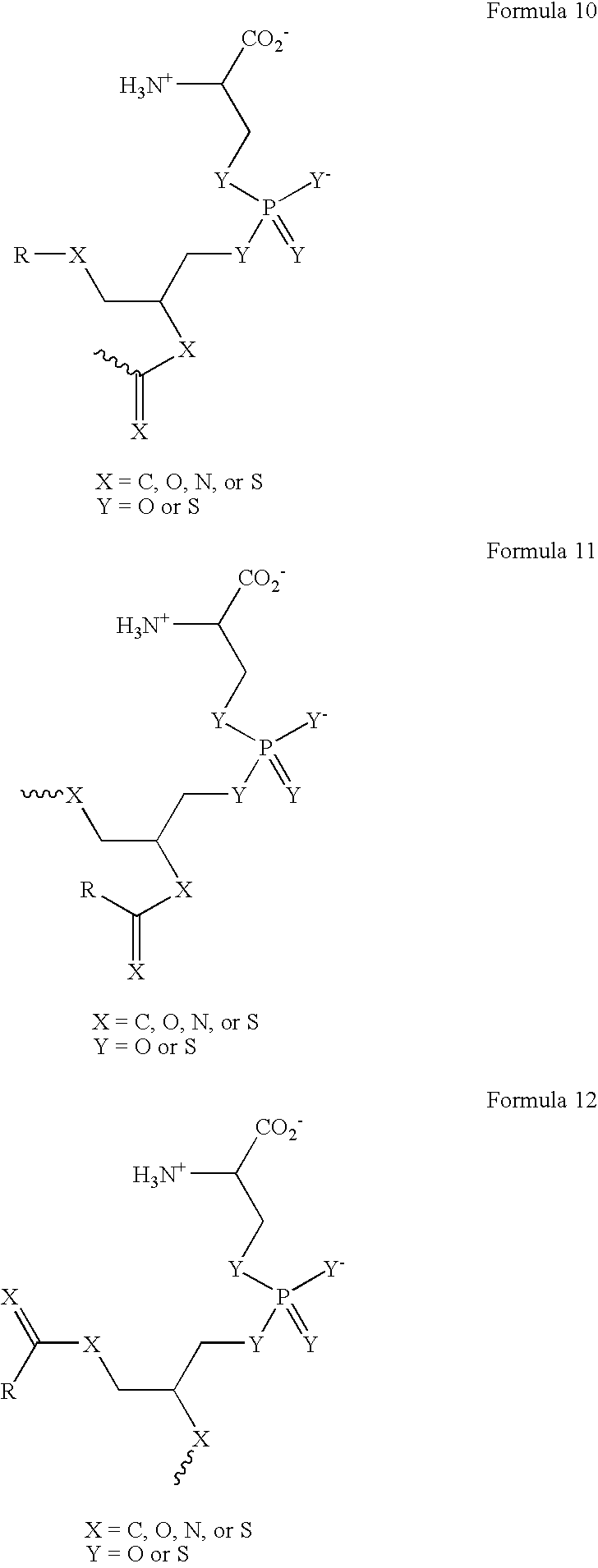
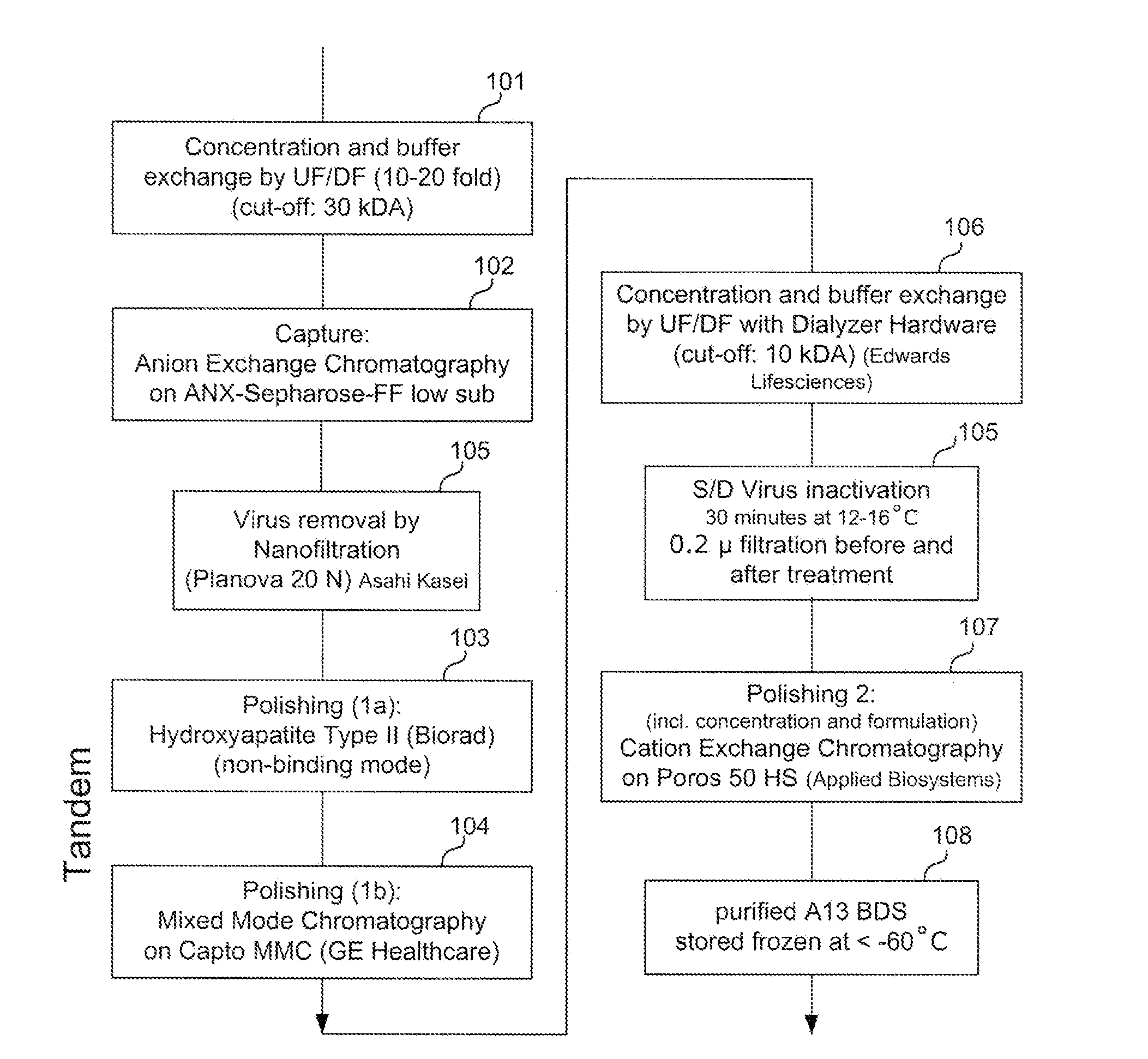
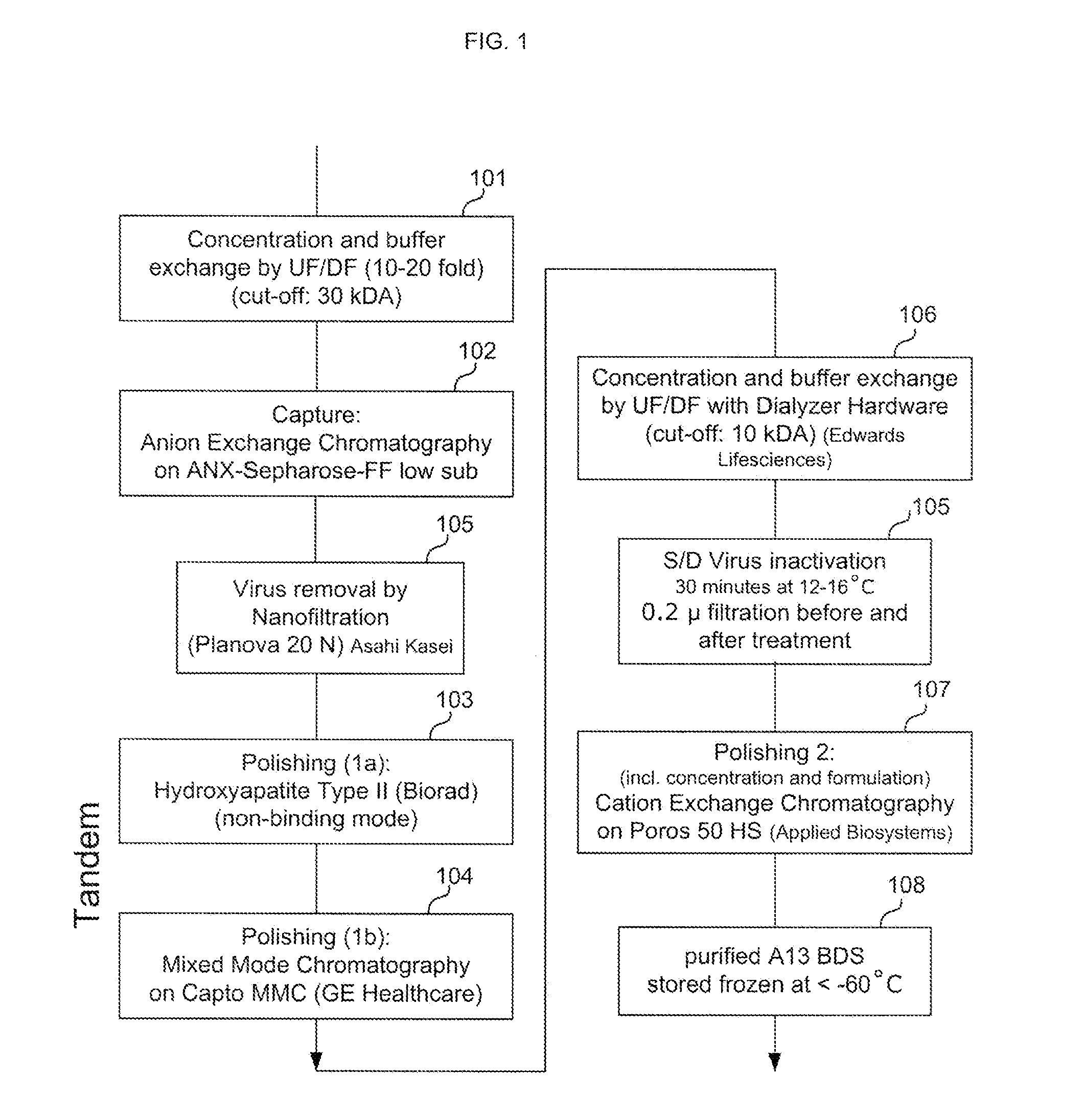
Provided herein are methods for purifying recombinant A Disintegrin-like and Metallopeptidase with Thrombospondin Type 1 Motif 13 (ADAMTS13) protein from a sample. The method comprises enriching for ADAMTS13 protein by chromatographically contacting the sample with hydroxyapatite under conditions that allow ADAMTS13 protein to appear in the eluate or supernatant from the hydroxylapatite. The methods may further comprise tandem chromatography with a mixed mode cation exchange / hydrophobic interaction resin that binds ADAMTS13 protein. Additional optional steps involve ultrafiltration / diafiltration, anion exchange chromatography, cation exchange chromatography, and viral inactivation. Also provided herein are methods for inactivating virus contaminants in protein samples, where the protein is immobilized on a support. Also provided herein are compositions of ADAMTS13 prepared according to said methods.
Owner:TAKEDA PHARMA CO LTD
Methods for inhibiting vascular permeability
The present invention relates to methods for decreasing or inhibiting disorders associated with vascular hyperpermeability and to methods of screening for compounds that affect permeability, angiogenesis and stabilize tight junctions. In one aspect of the present invention there is provided a method of decreasing or inhibiting vascular hyperpermeability in an individual in need of such treatment. The method includes administering to the individual an effective amount of an antiangiogenic compound selected from the group consisting of endostatin, thrombospondin, angiostatin, tumstatin, arrestin, recombinant EPO and polymer conjugated TNP-470. Other antiangiogenic compounds are disclosed herein.
Owner:CHILDRENS MEDICAL CENT CORP
Compositions and methods for producing bioactive fusion proteins
Disclosed is a composition of matter involving a recombinant fusion protein comprising a a pharmacologically active protein partner, and a small pharmacologically inactive protein domain partner of human origin, such as but not limited to, a 10th fibronectin III domain, a SH3 domain, a SH2 domain, a CH2 domain of IgG1, a PDZ domain, a thrombospondin repeat domain, an ubiquitin domain, a leucine-rich repeat domain, a villin headpiece HP35 domain, a villin headpiece HP76 domain, or a fragment or modification of any of these. Also disclosed are nucleic acids (e.g., DNA constructs) encoding the fusion protein, expression vectors and recombinant host cells for expression of the fusion protein, and pharmaceutical compositions containing the recombinant fusion protein and a pharmaceutically acceptable carrier, and method of producing a pharmacologically active recombinant fusion protein.
Owner:AMGEN INC
Compositions and methods for enhancing phagocytosis or phagocyte activity
ActiveUS8198020B2Better targets for phagocytosisEnhances and incites phagocytosisSenses disorderPeptide/protein ingredientsWhite blood cellCell type specific
The present invention provides a system for enhancing clearance or destruction of undesirable cells or noncellular molecular entities by tagging such cells or noncellular molecular entities with a marker that targets the cells or noncellular molecular entities for phagocytosis (phagocytic marker). The target cells can be, for example, endothelial cells, tumor cells, leukocytes, or virus-infected cells. In certain embodiments of the invention the tagging is accomplished by administering a composition comprising an antibody or ligand linked to the phagcytotic marker, wherein the antibody or ligand binds to a cell type specific marker present on or in the cell surface of a target cell. In preferred embodiments of the invention, the phagocytic marker comprises phosphatidylserine or a group derived from phosphatidylserine, thrombospondin-1, annexin I, or a derivative of any of these.
Owner:APELLIS PHARMA
Compositions and methods for producing bioactive fusion proteins
InactiveUS20090118181A1Low immunogenic riskReduce riskBacteriaPeptide/protein ingredientsDNA constructActive protein
Disclosed is a composition of matter involving a recombinant fusion protein comprising a a pharmacologically active protein partner, and a small pharmacologically inactive protein domain partner of human origin, such as but not limited to, a 10th fibronectin III domain, a SH3 domain, a SH2 domain, a CH2 domain of IgG1, a PDZ domain, a thrombospondin repeat domain, an ubiquitin domain, a leucine-rich repeat domain, a villin headpiece HP35 domain, a villin headpiece HP76 domain, or a fragment or modification of any of these. Also disclosed are nucleic acids (e.g., DNA constructs) encoding the fusion protein, expression vectors and recombinant host cells for expression of the fusion protein, and pharmaceutical compositions containing the recombinant fusion protein and a pharmaceutically acceptable carrier, and method of producing a pharmacologically active recombinant fusion protein.
Owner:AMGEN INC
Modulation of synaptogenesis
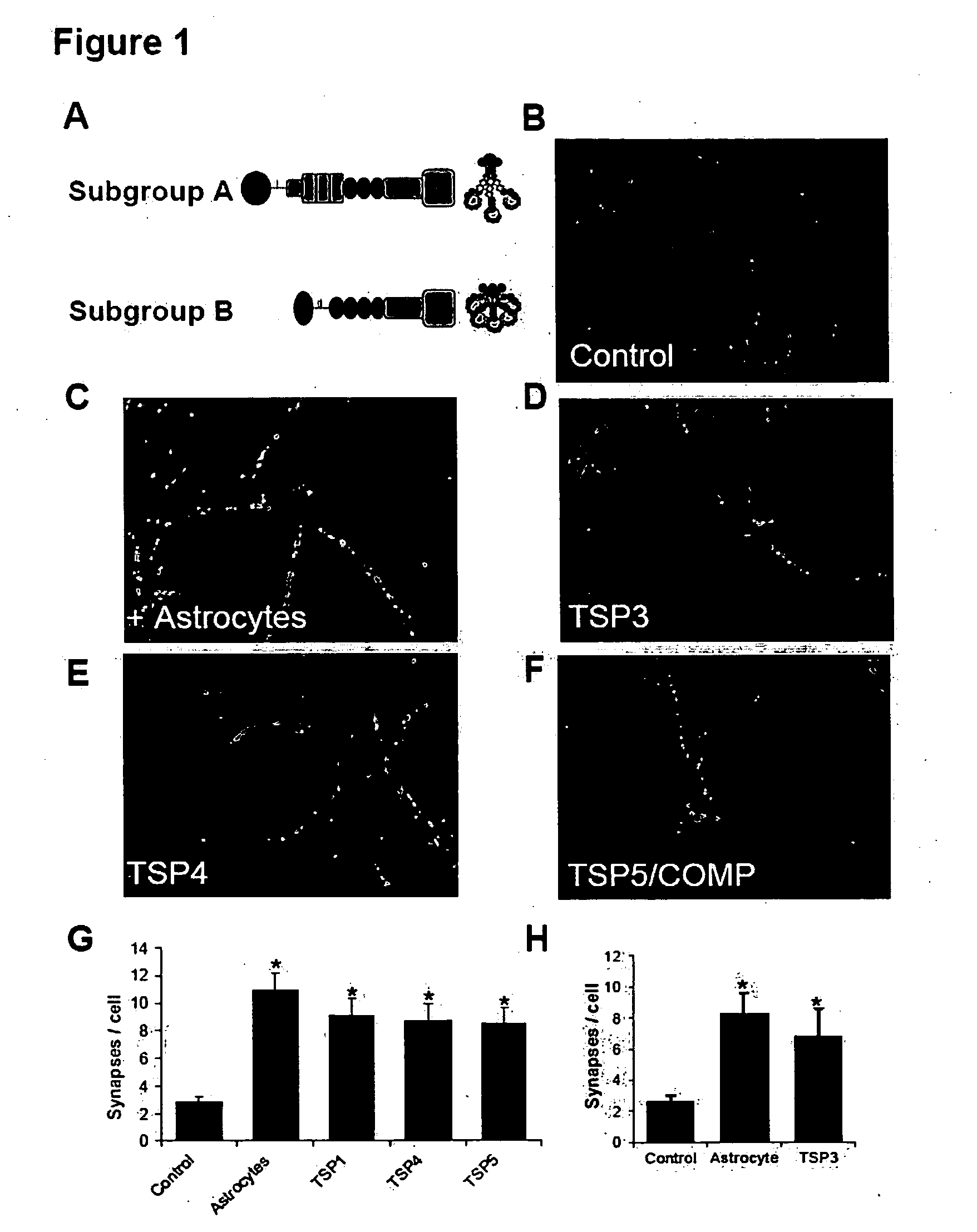
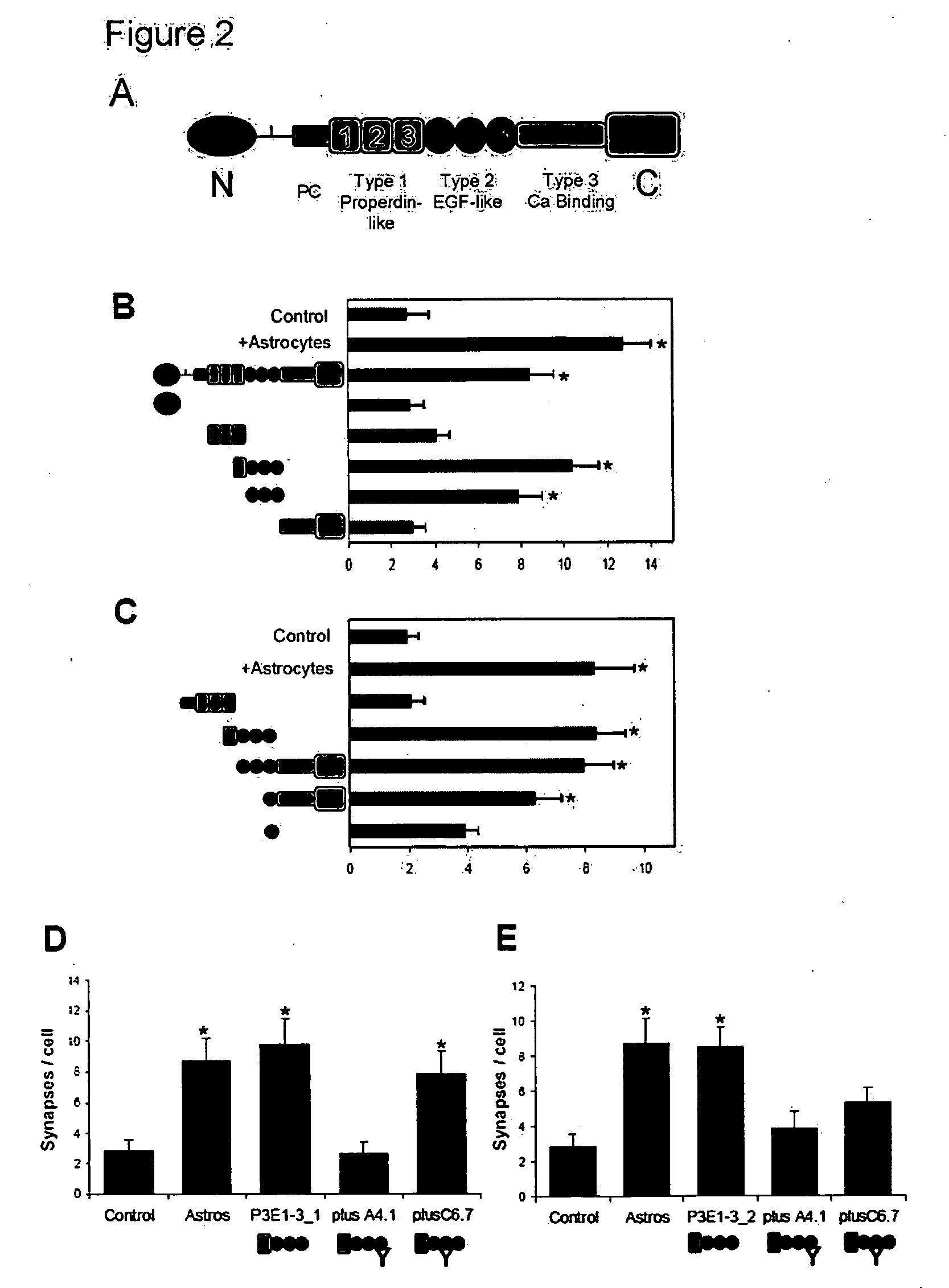
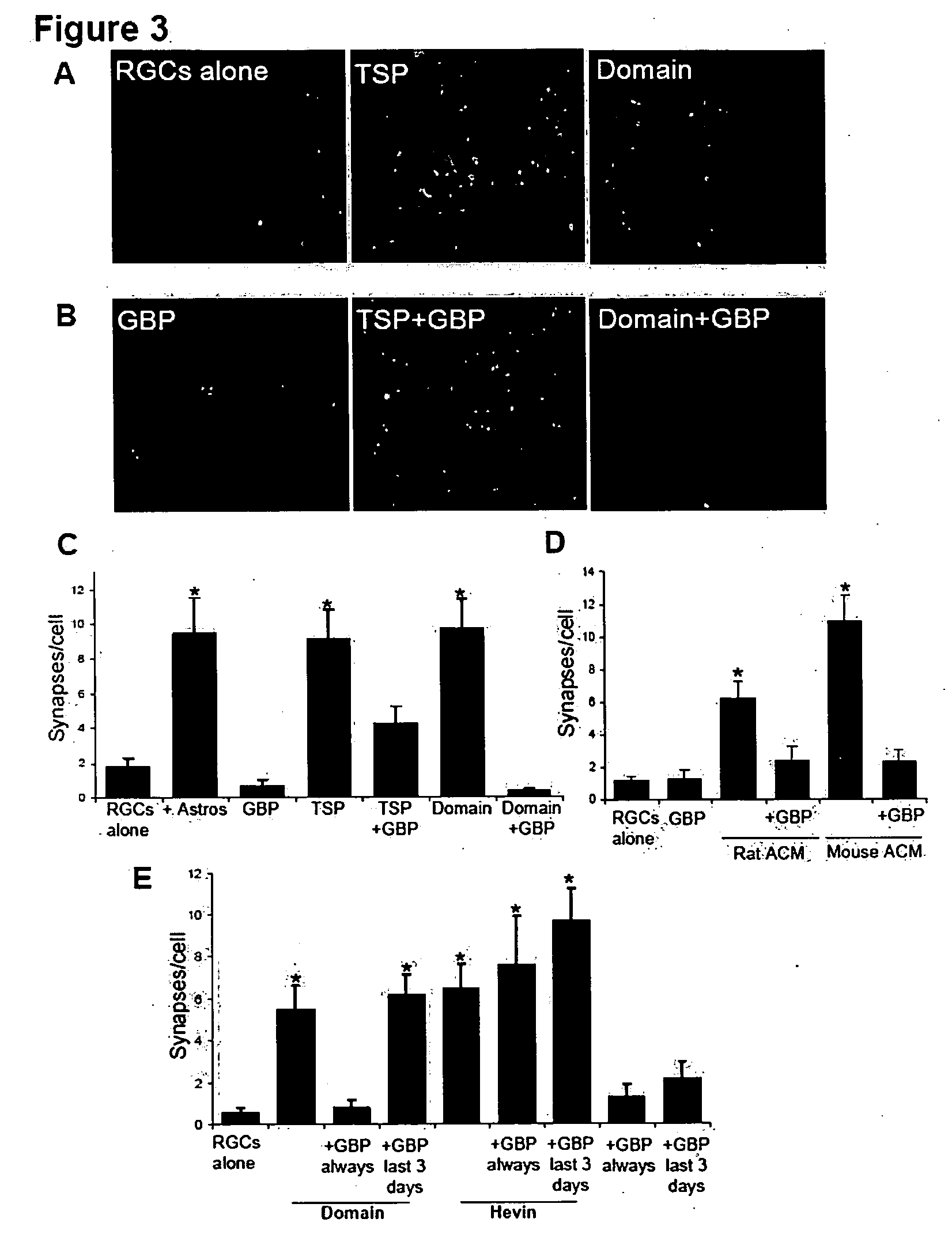
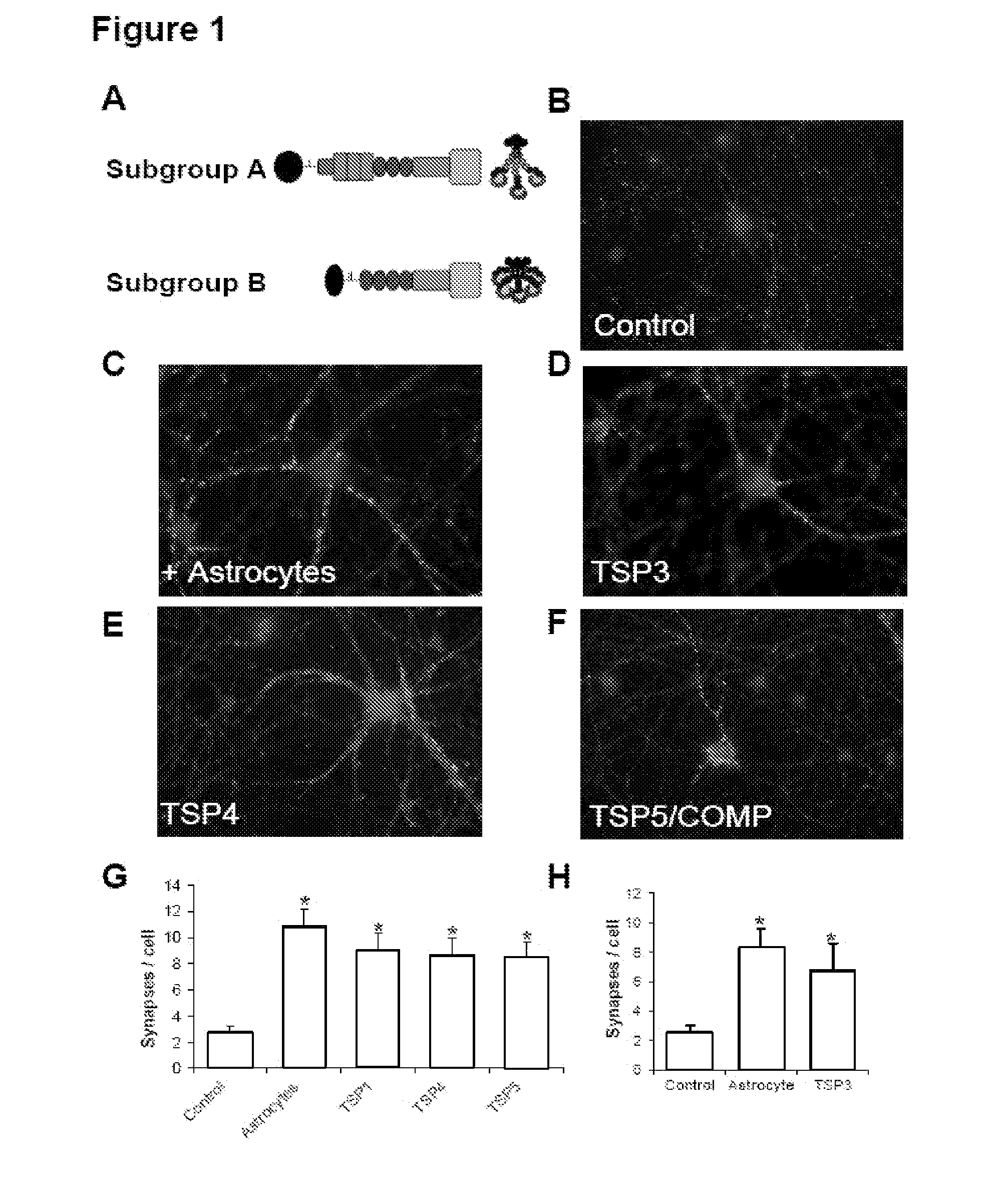
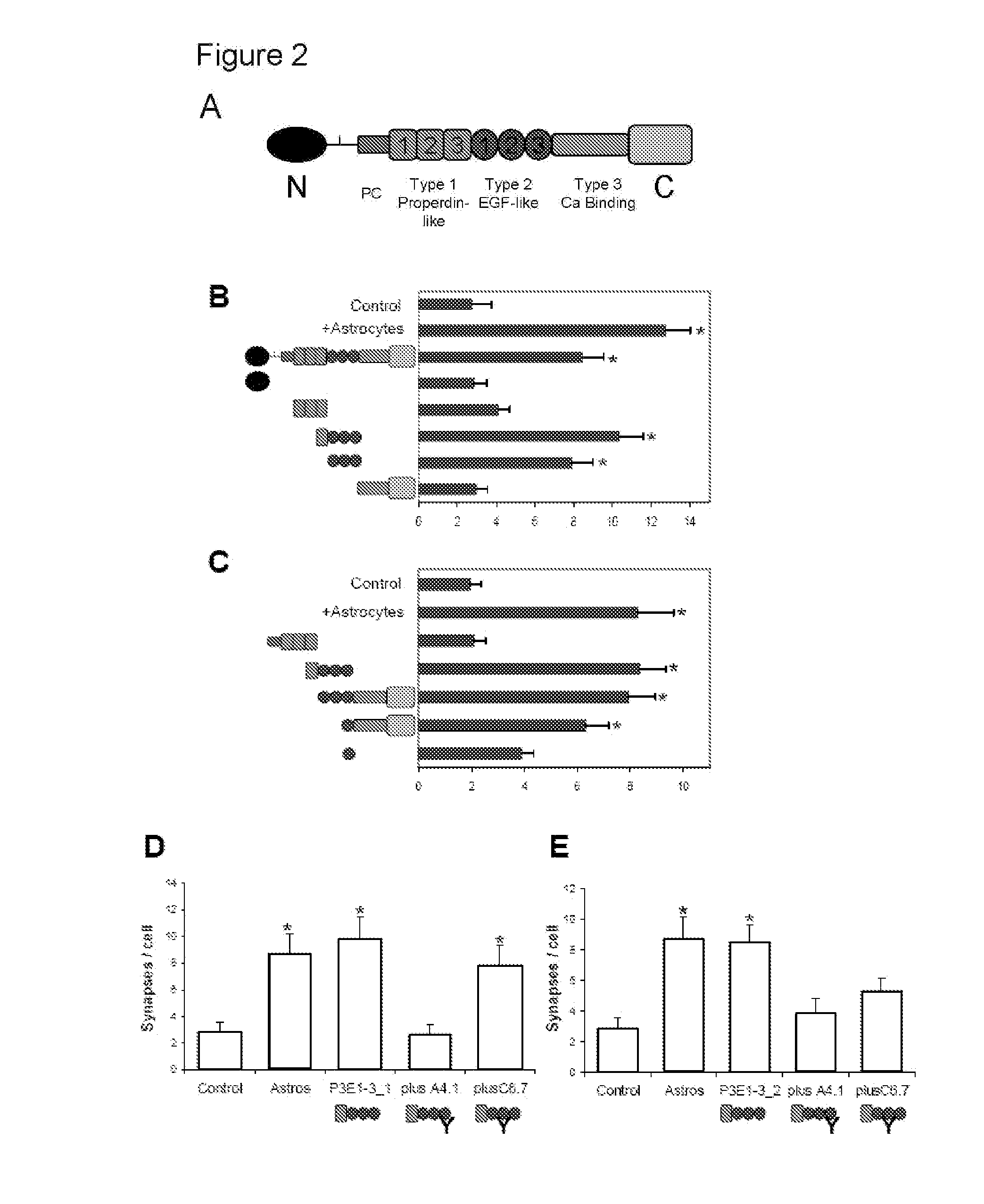
InactiveUS20090053232A1Promoting synaptogenesisSynapse formation is increasedOrganic active ingredientsSenses disorderThrombospondinSynaptogenesis
The present invention describes methods and compositions for modulating synaptogenesis and axon and / or dendritic growth. The methods include the use of agents that modulate a thrombospondin and / or an α2δ subunit of a calcium channel.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods for inhibiting vascular permeability
InactiveUS20050112063A1Inhibition formationPrevent leakagePeptide/protein ingredientsUrinary disorderDiseaseAngiostatins
The present invention relates to methods for decreasing or inhibiting disorders associated with vascular hyperpermeability and to methods of screening for compounds that affect permeability, angiogenesis and stabilize tight junctions. In one aspect of the present invention there is provided a method of decreasing or inhibiting vascular hyperpermeability in an individual in need of such treatment. The method includes administering to the individual an effective amount of an antiangiogenic compound selected from the group consisting of endostatin, thrombospondin, angiostatin, tumstatin, arrestin, recombinant EPO and polymer conjugated TNP-470. Other antiangiogenic compounds are disclosed herein.
Owner:DANA FARBER CANCER INST INC +1
Angiogenesis inhibitor comprising meteorin as an active ingredient
ActiveUS20100048471A1Induce functional maturity of the blood-brain barrierInhibit angiogenesisSenses disorderNervous disorderVascular diseaseVascular problem
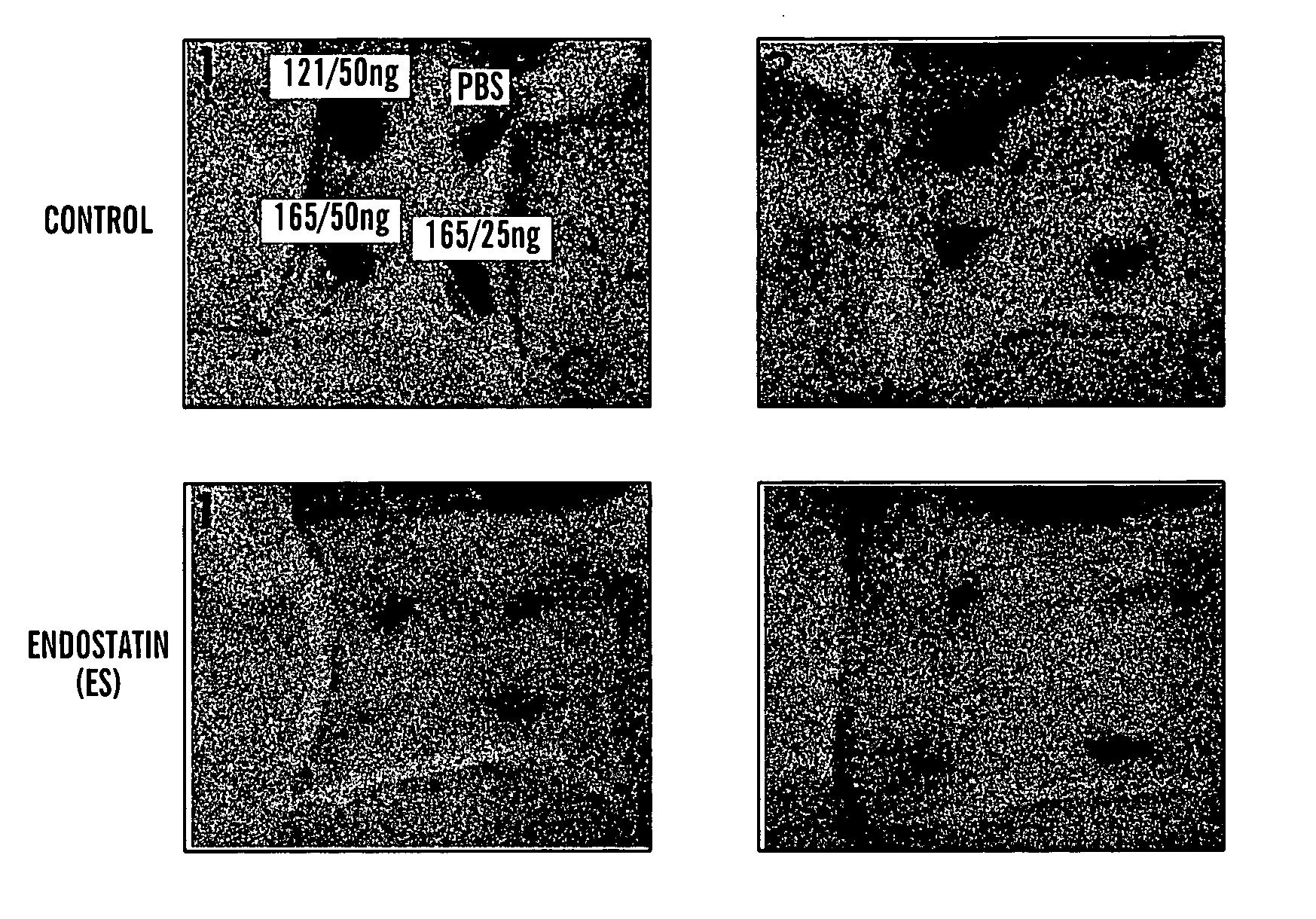
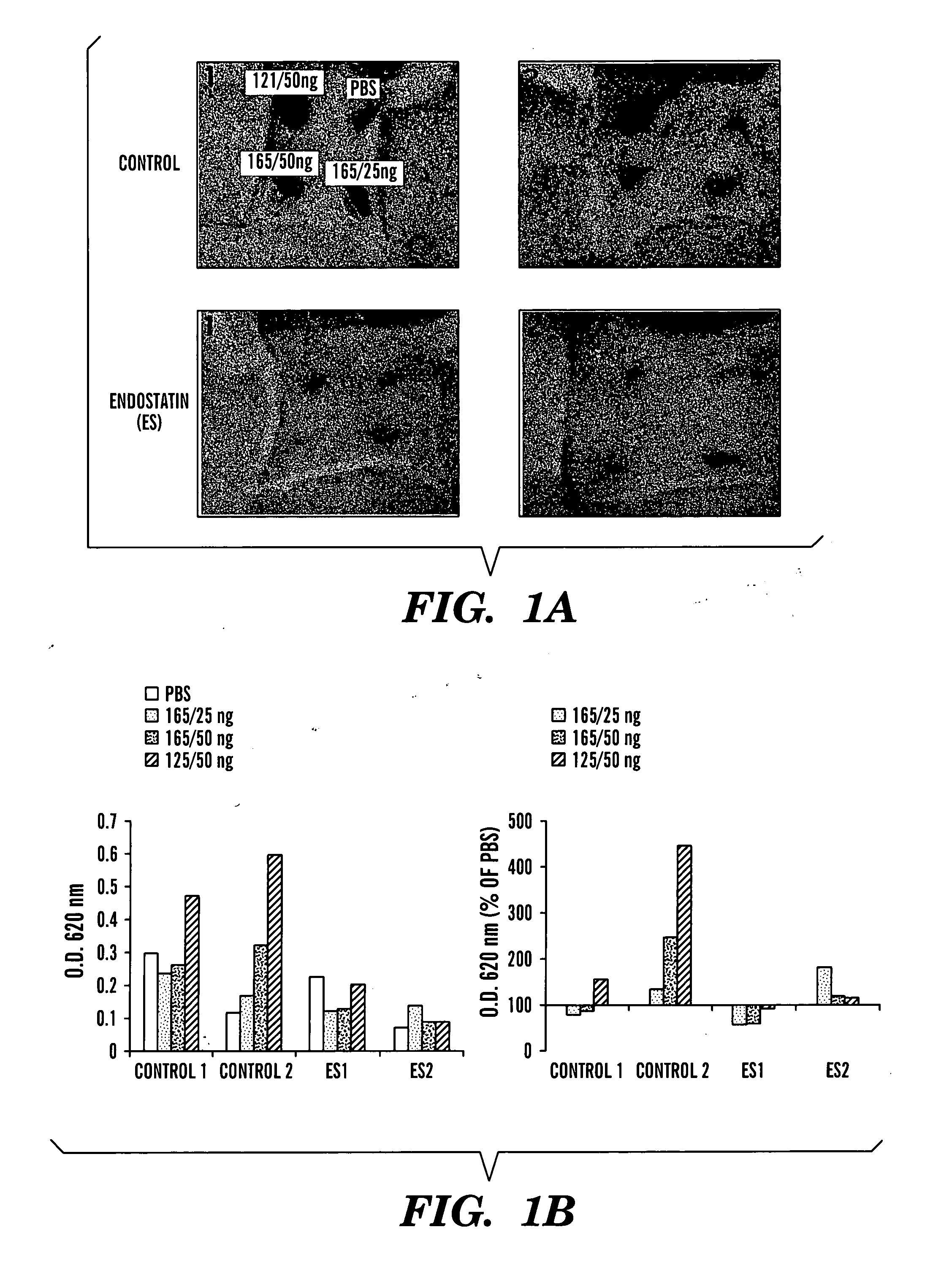
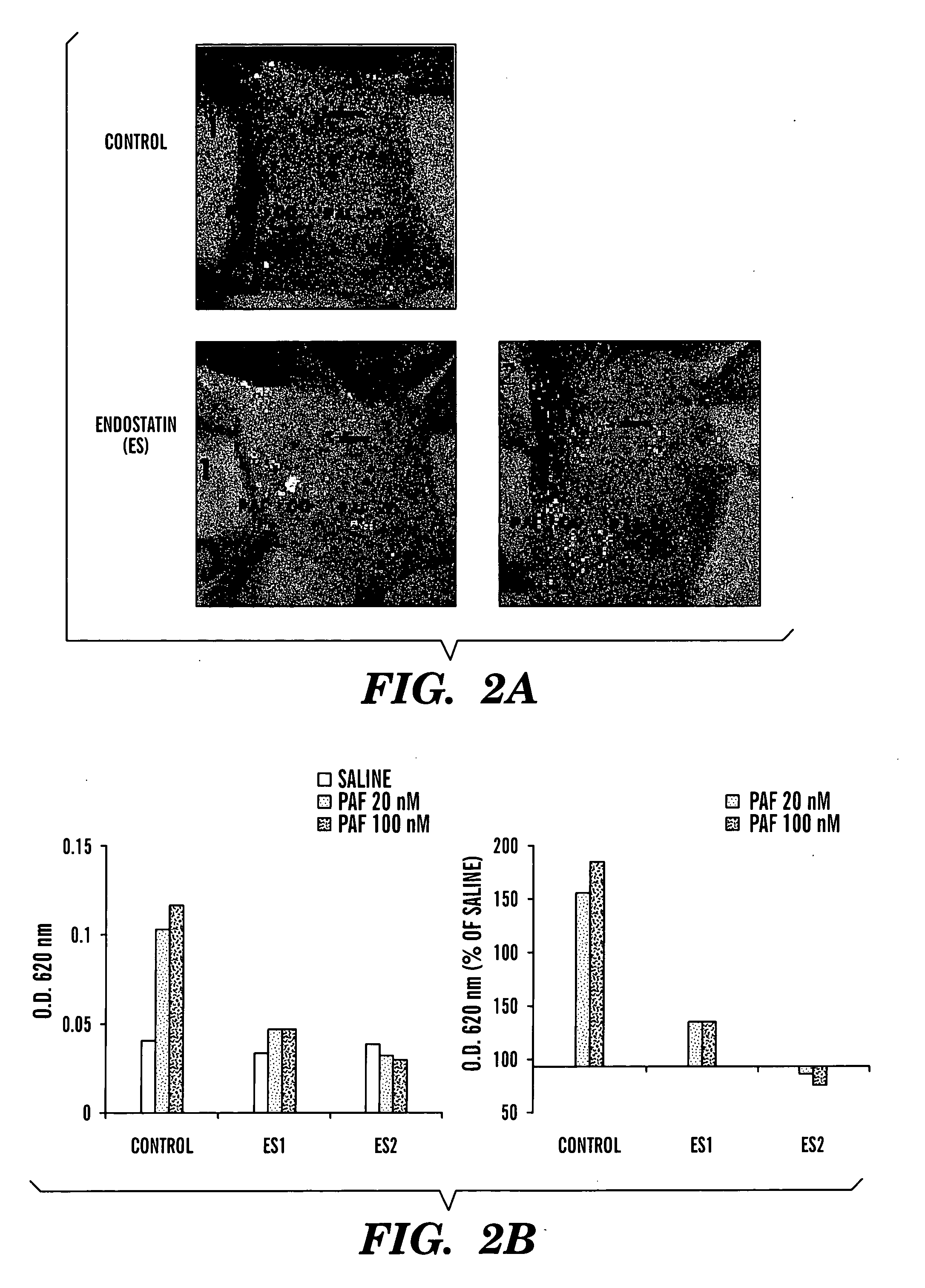
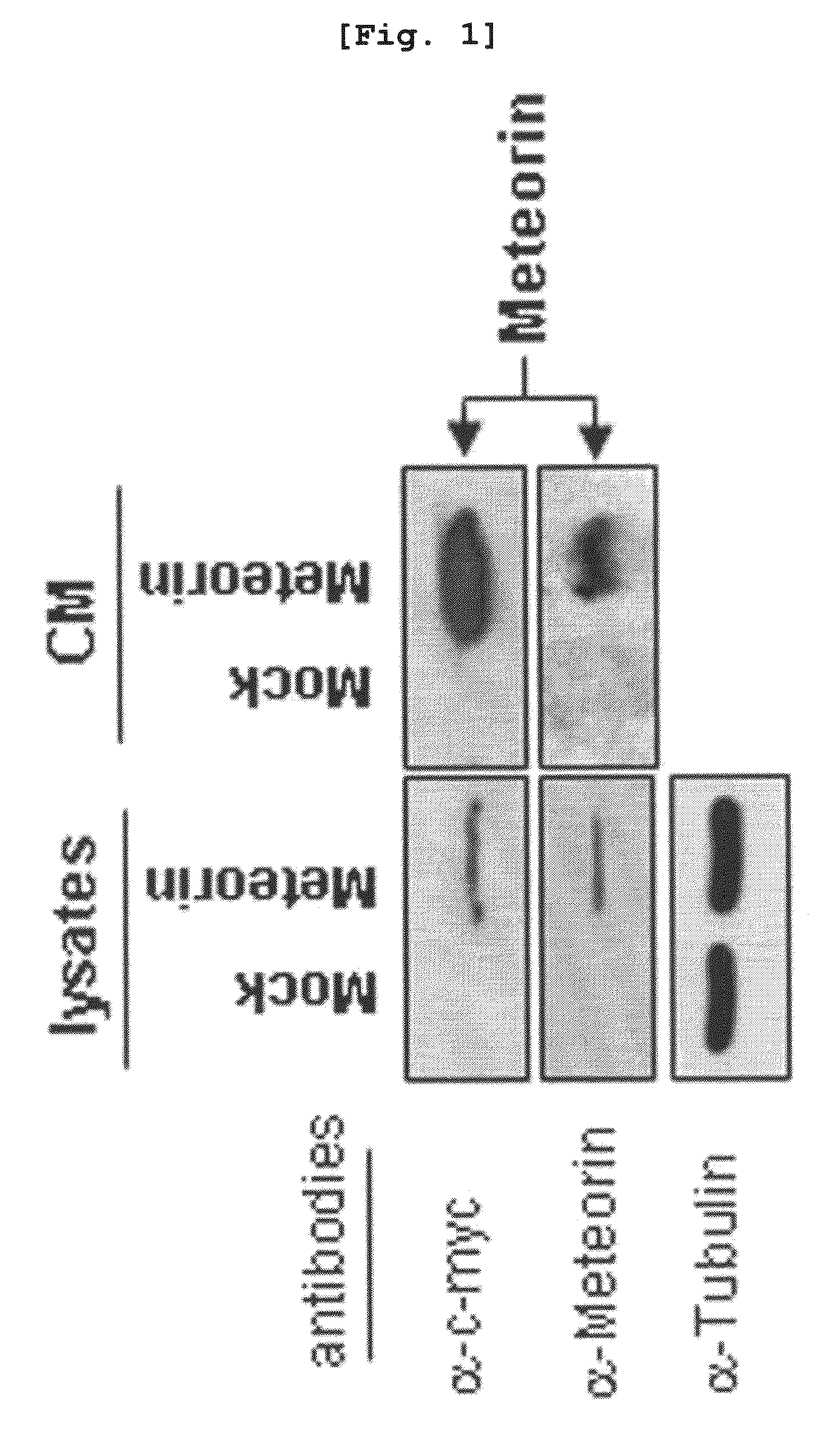
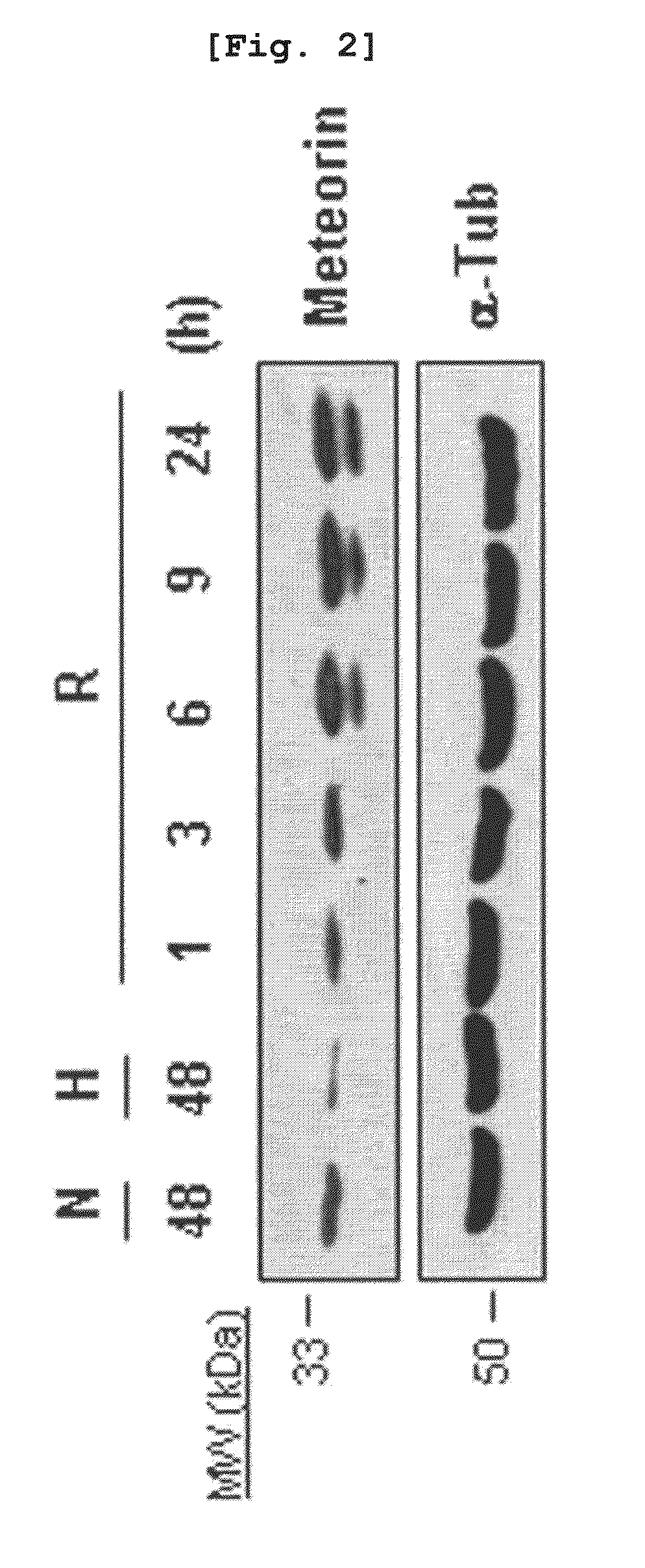
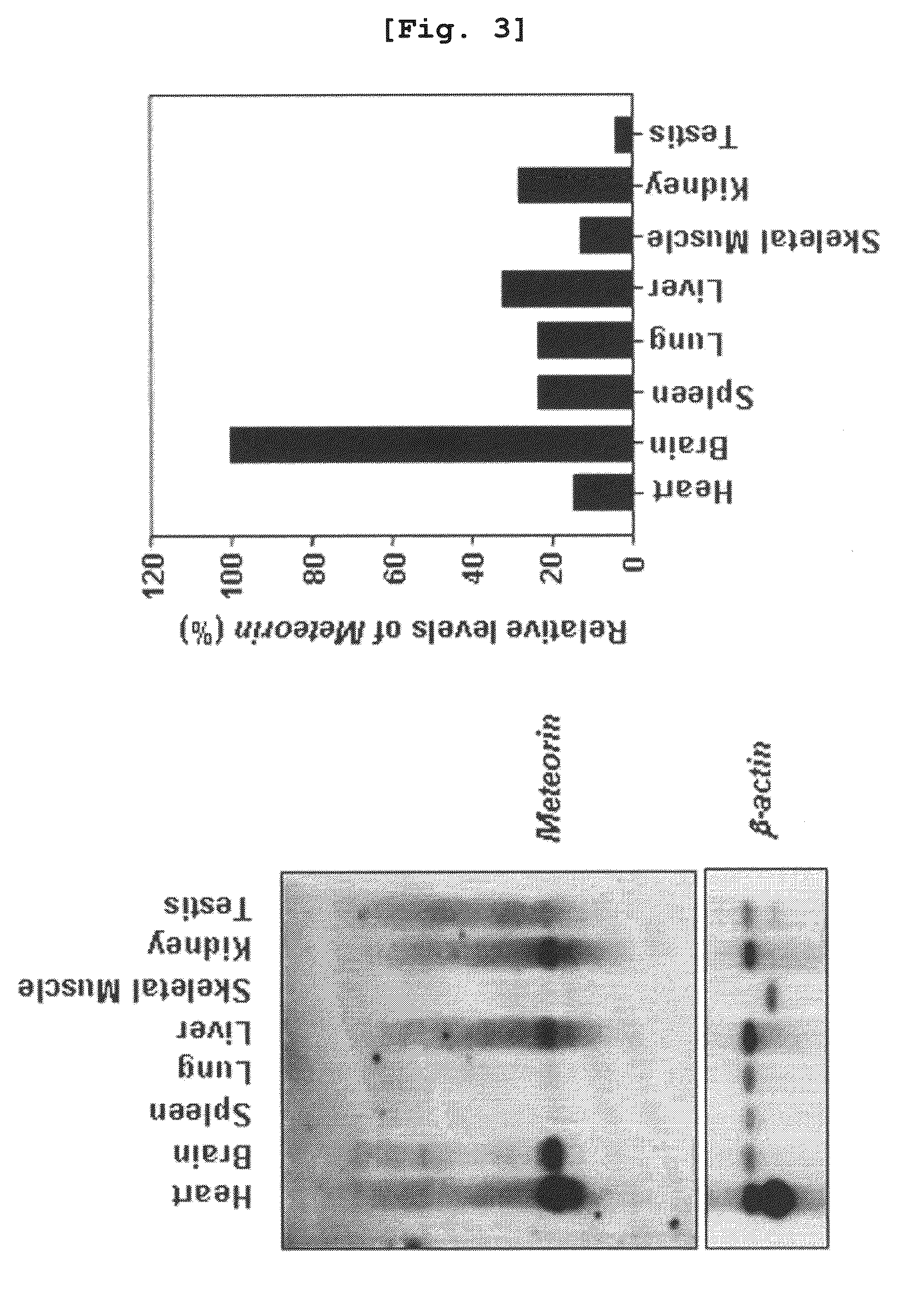
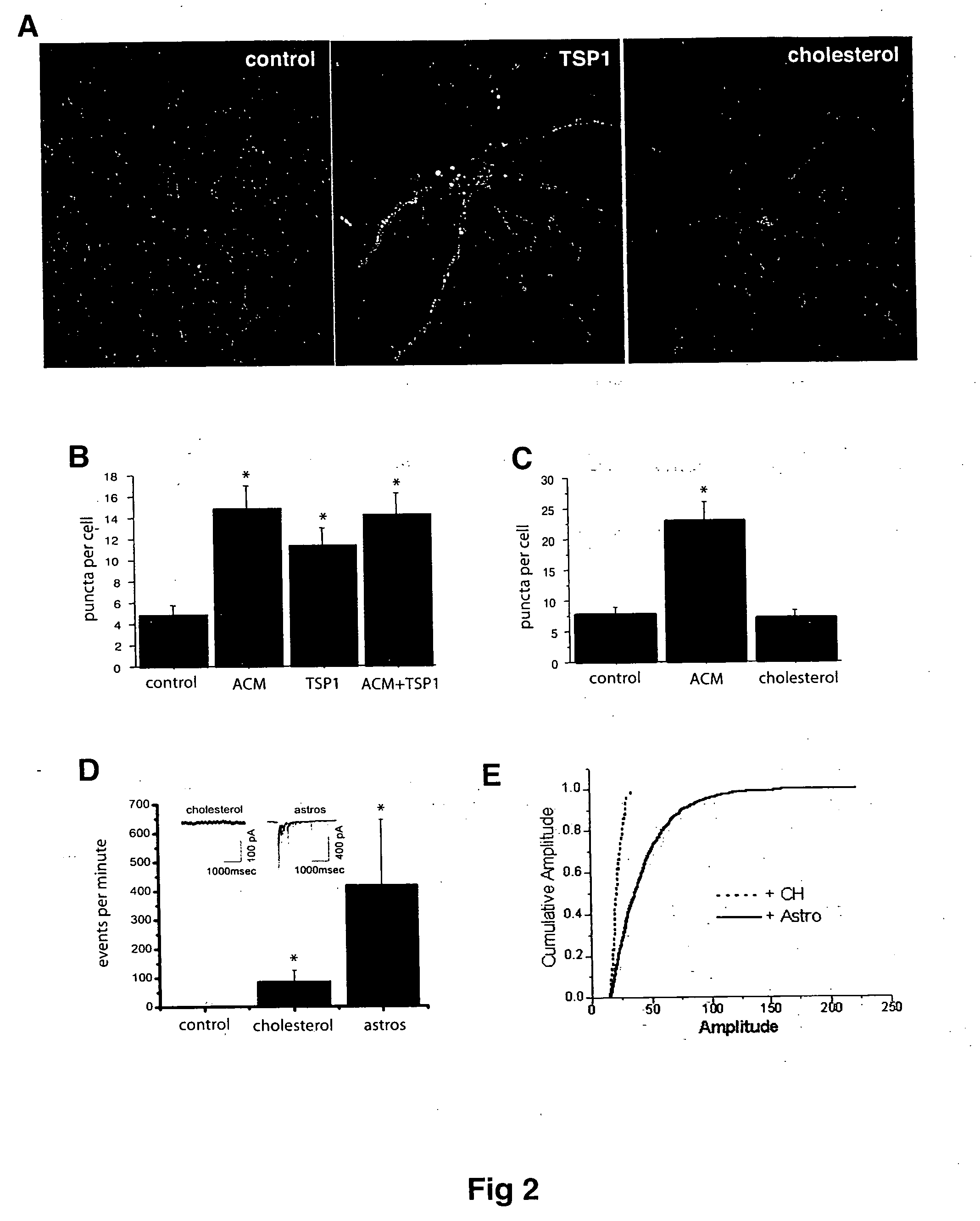
The present invention relates to an angiogenesis inhibitor comprising meteorin as an active ingredient that is highly expressed in astrocytes of the brain and retina in the late embryonic stage and after the birth of a mouse. It is in particular highly detected in astrocyte endfeet surrounding blood vessels and promotes the expression of thrombospondin-1 / -2 (TSP-1 / -2) via autocrine pathway and thus inhibits angiogenesis. The meteorin of the present invention can be effectively used for pharmaceutical compositions and health foods that prevent vascular diseases by inhibiting angiogenesis.
Owner:SEOUL NAT UNIV R&DB FOUND
Modulation of synaptogenesis
InactiveUS20060019880A1Synaptogenesis is enhancedNervous disorderSaccharide peptide ingredientsSynaptogenesisEmbryonic brain
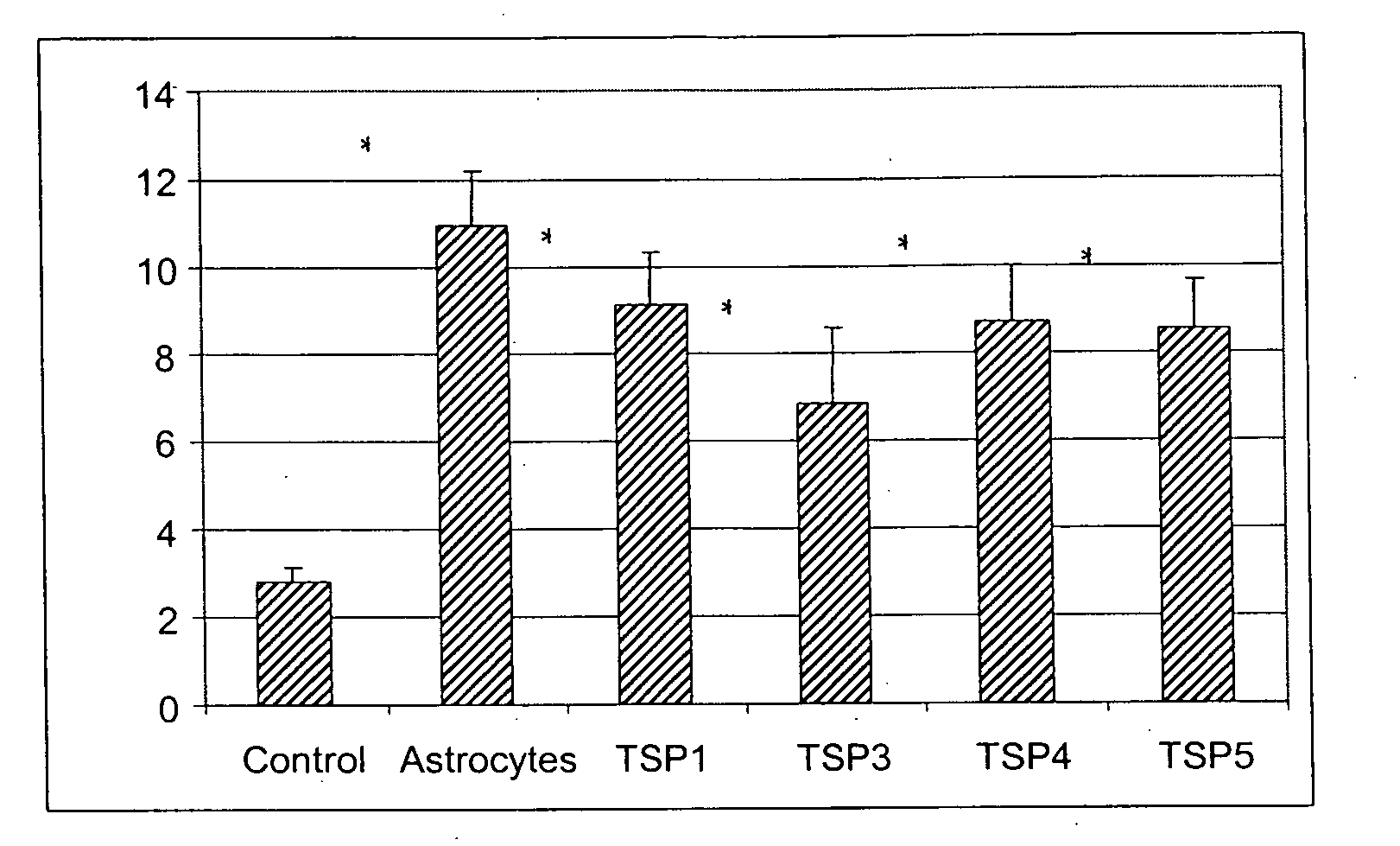
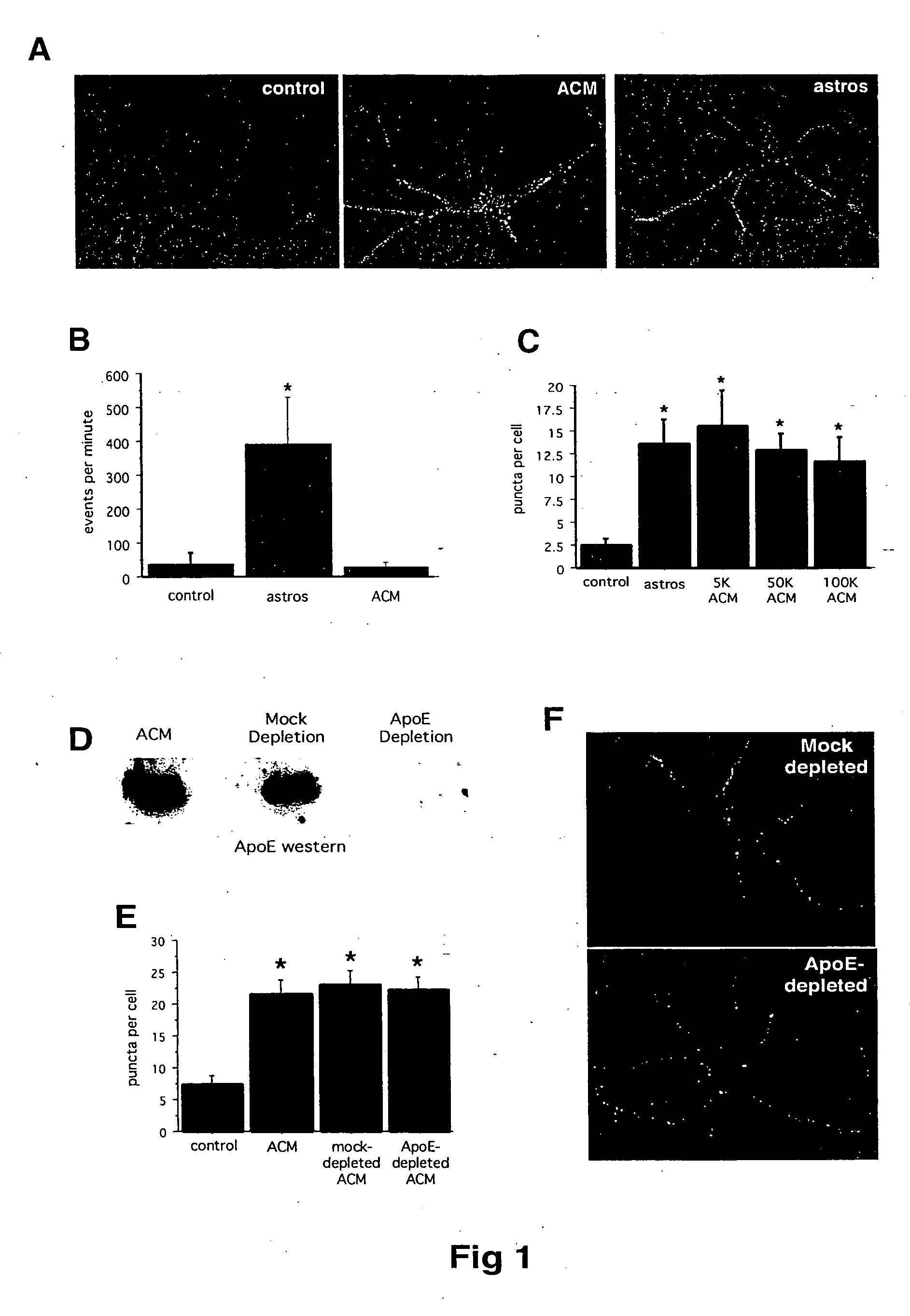
Soluble proteins, e.g. thrombospondins, can trigger synapse formation. Such proteins are synthesized in vitro and in vivo by astrocytes, which therefore have a role in synaptogenesis. These thrombospondins are only expressed in the normal brain exactly during the period of developmental synaptogenesis, being off in embryonic brain and adult brain but on at high levels in postnatal brain. Methods are provided for protecting or treating an individual suffering from adverse effects of deficits in synaptogenesis, or from undesirably active synaptogenesis. These findings have broad implications for a variety of clinical conditions, including traumatic brain injury, epilepsy, and other conditions where synapses fail to form or form inappropriately. Synaptogenesis is enhanced by contacting neurons with agents that are specific agonists or antagonists of thrombospondins. Conversely, synaptogenesis is inhibited by contacting neurons with inhibitors or antagonists of thrombospondins.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
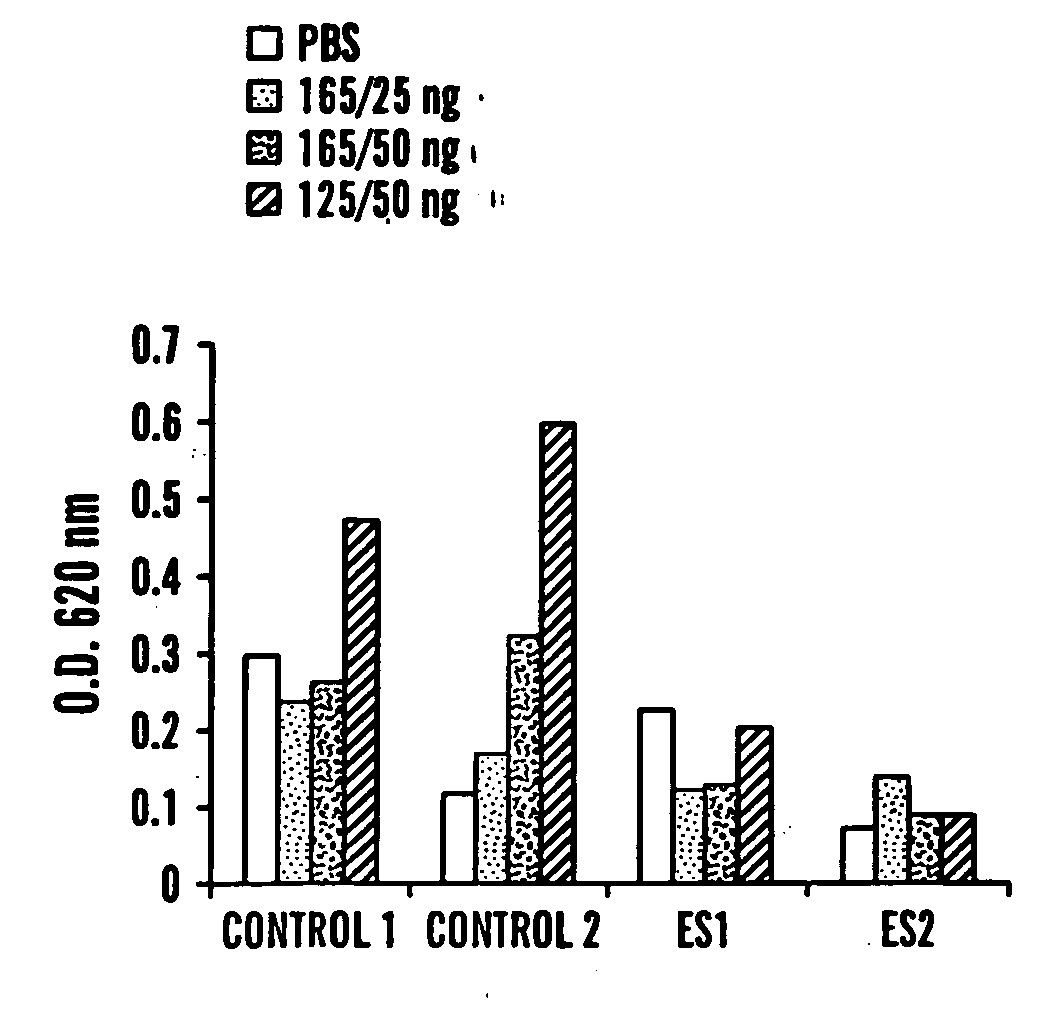
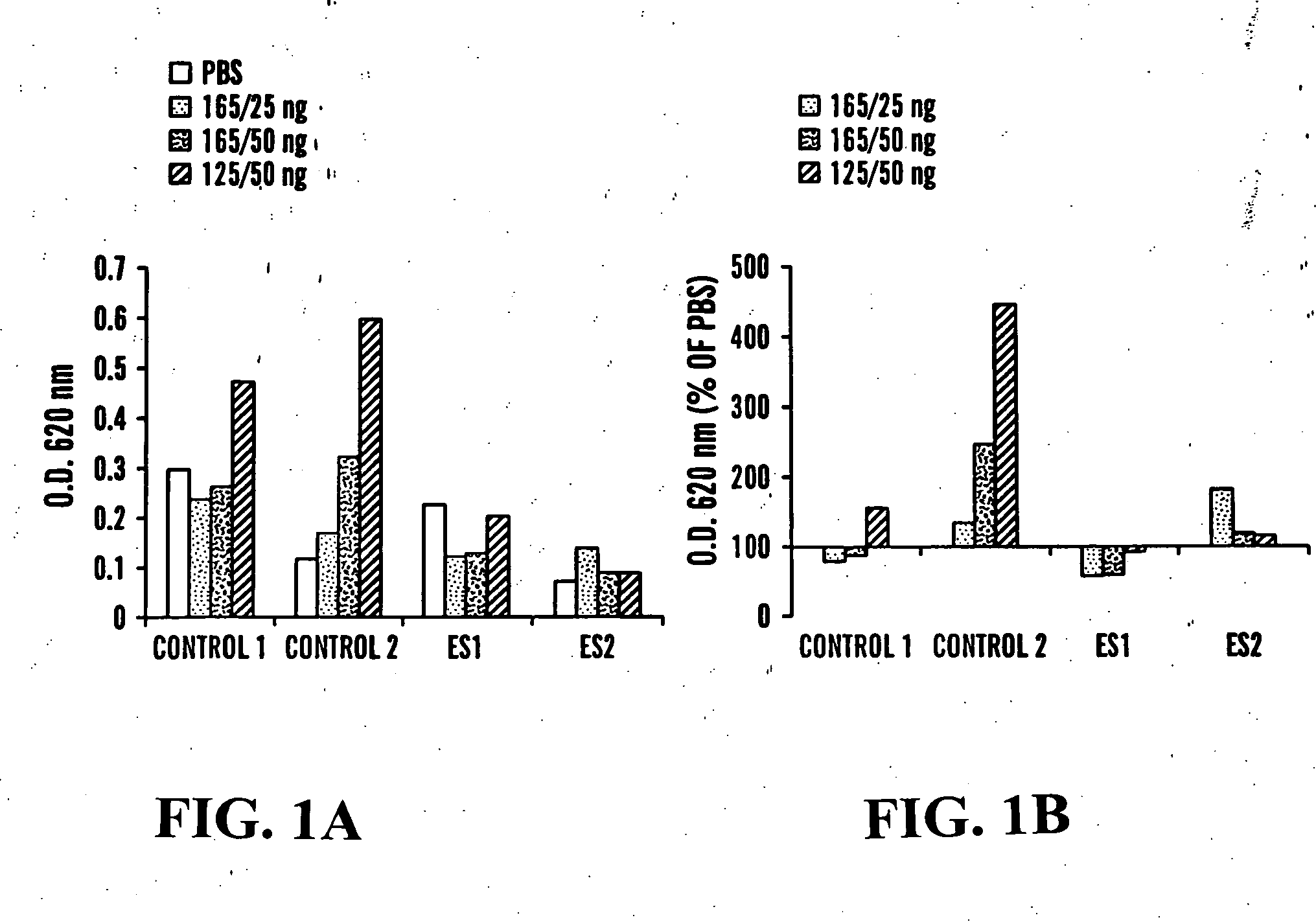
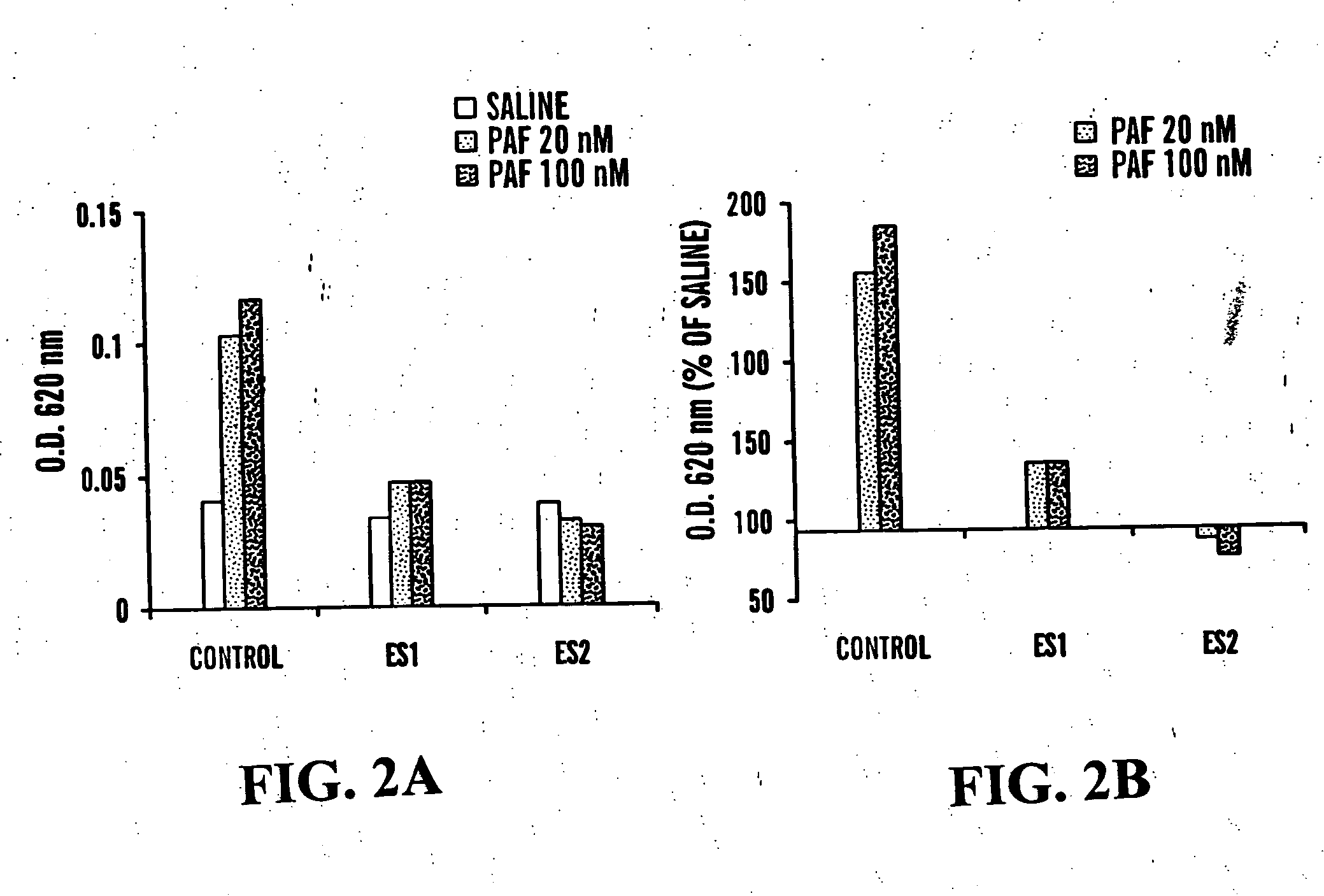
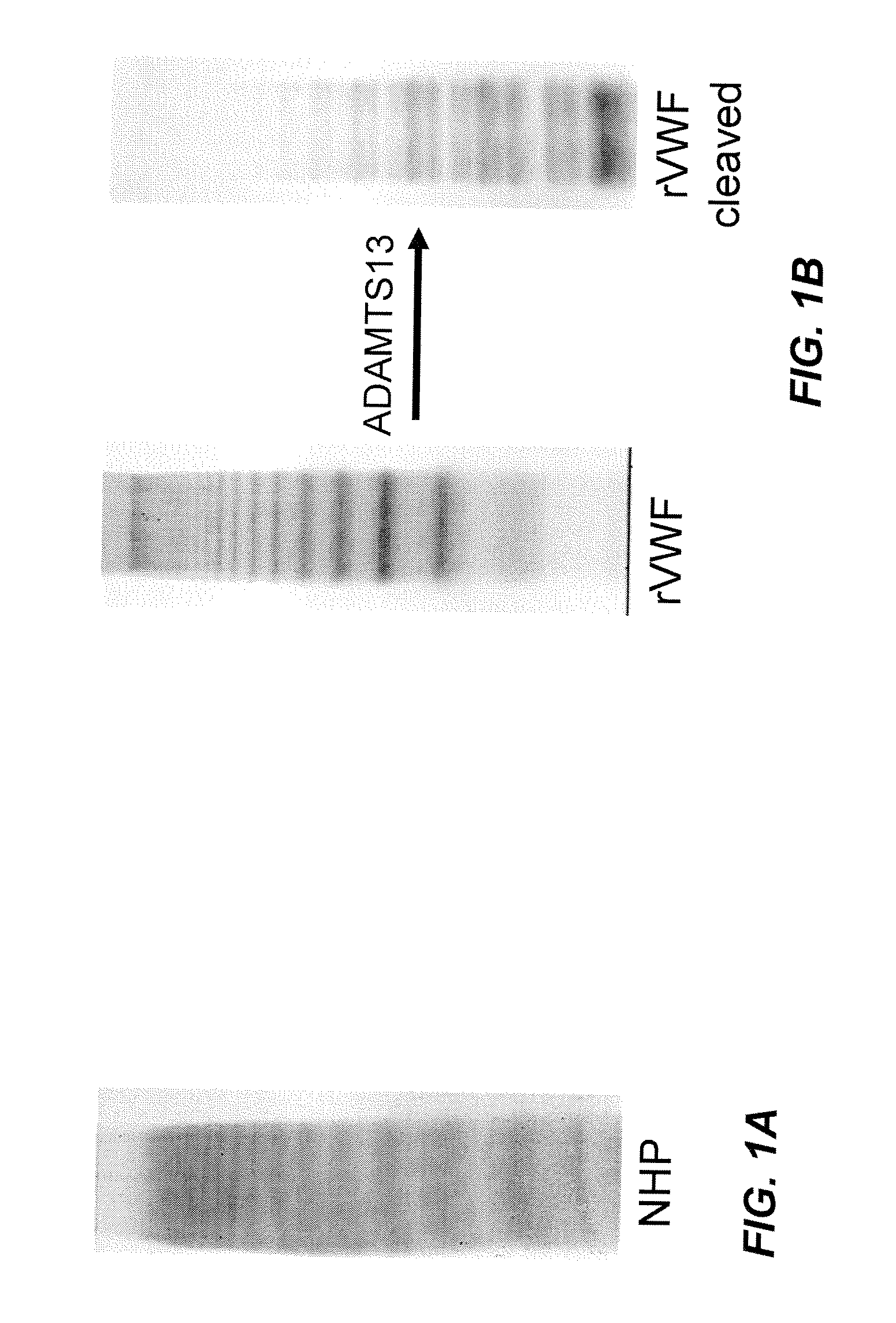
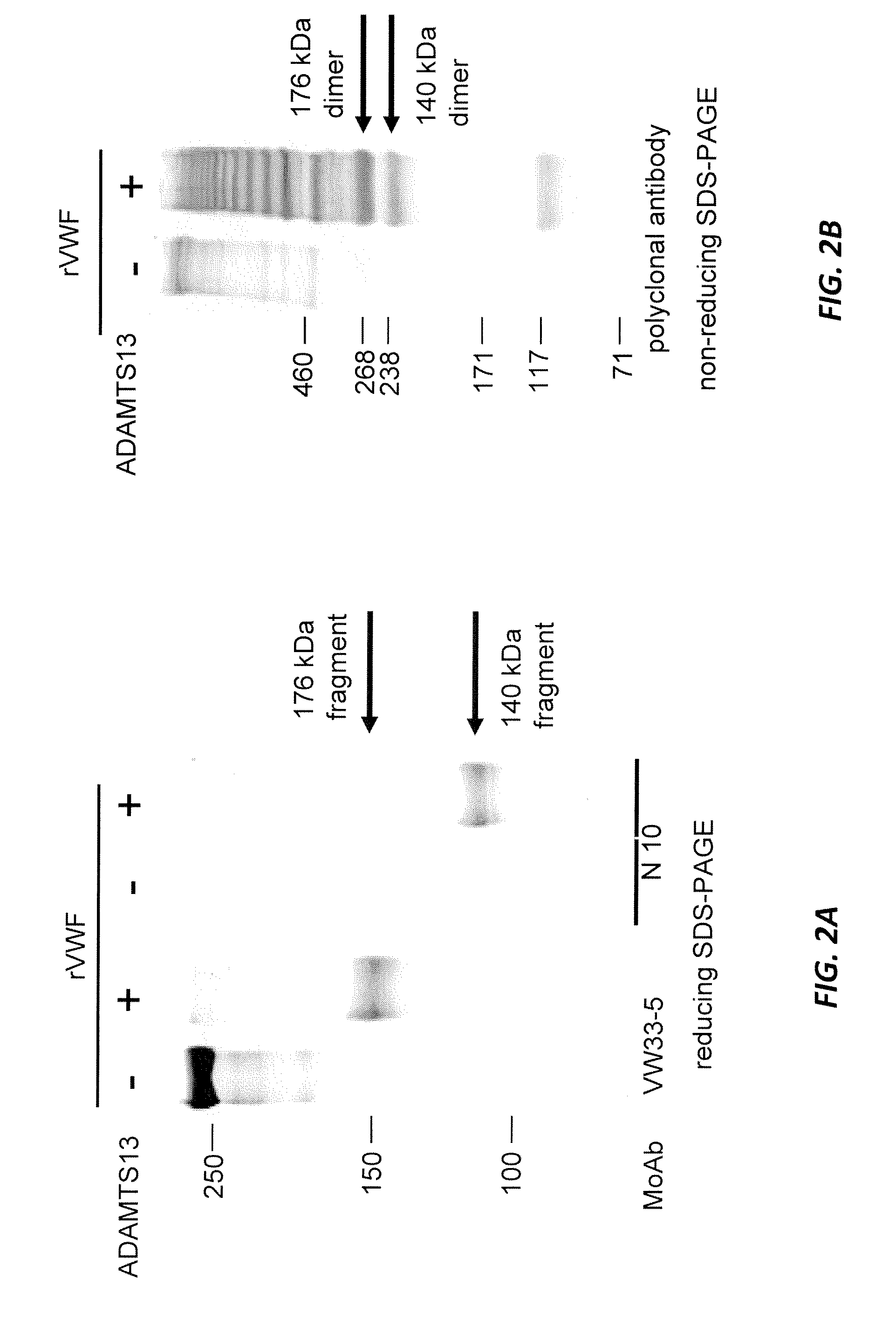
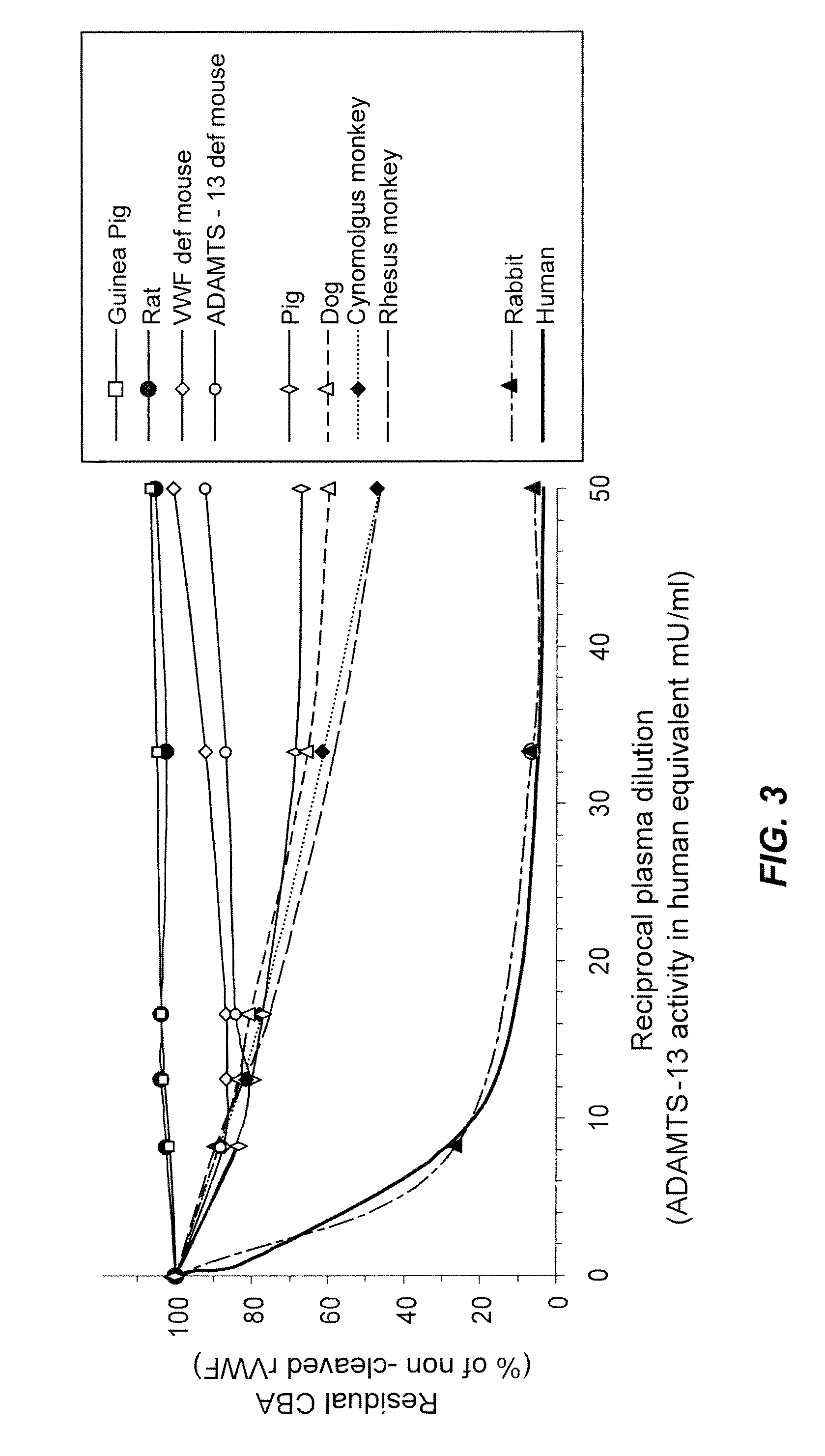
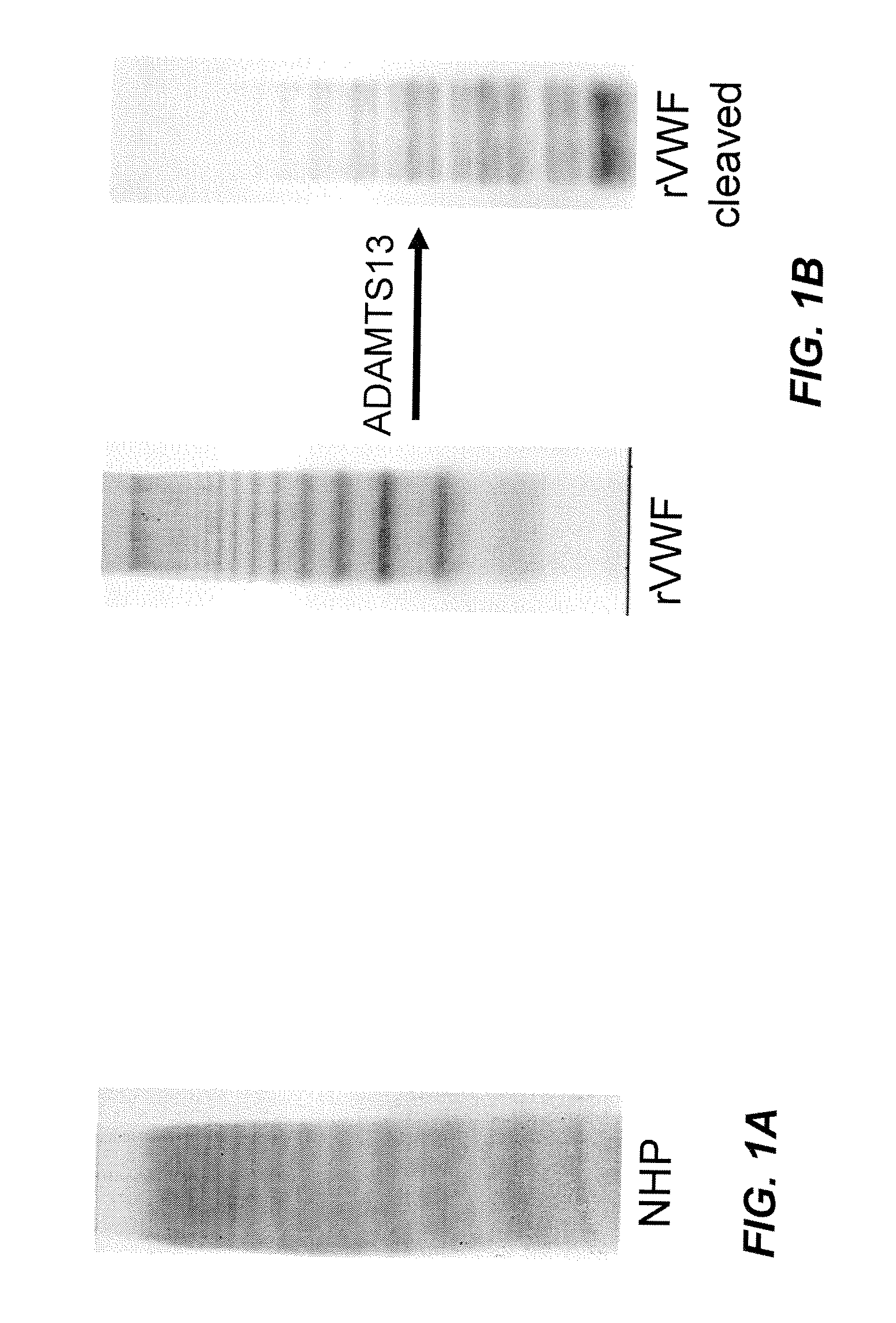
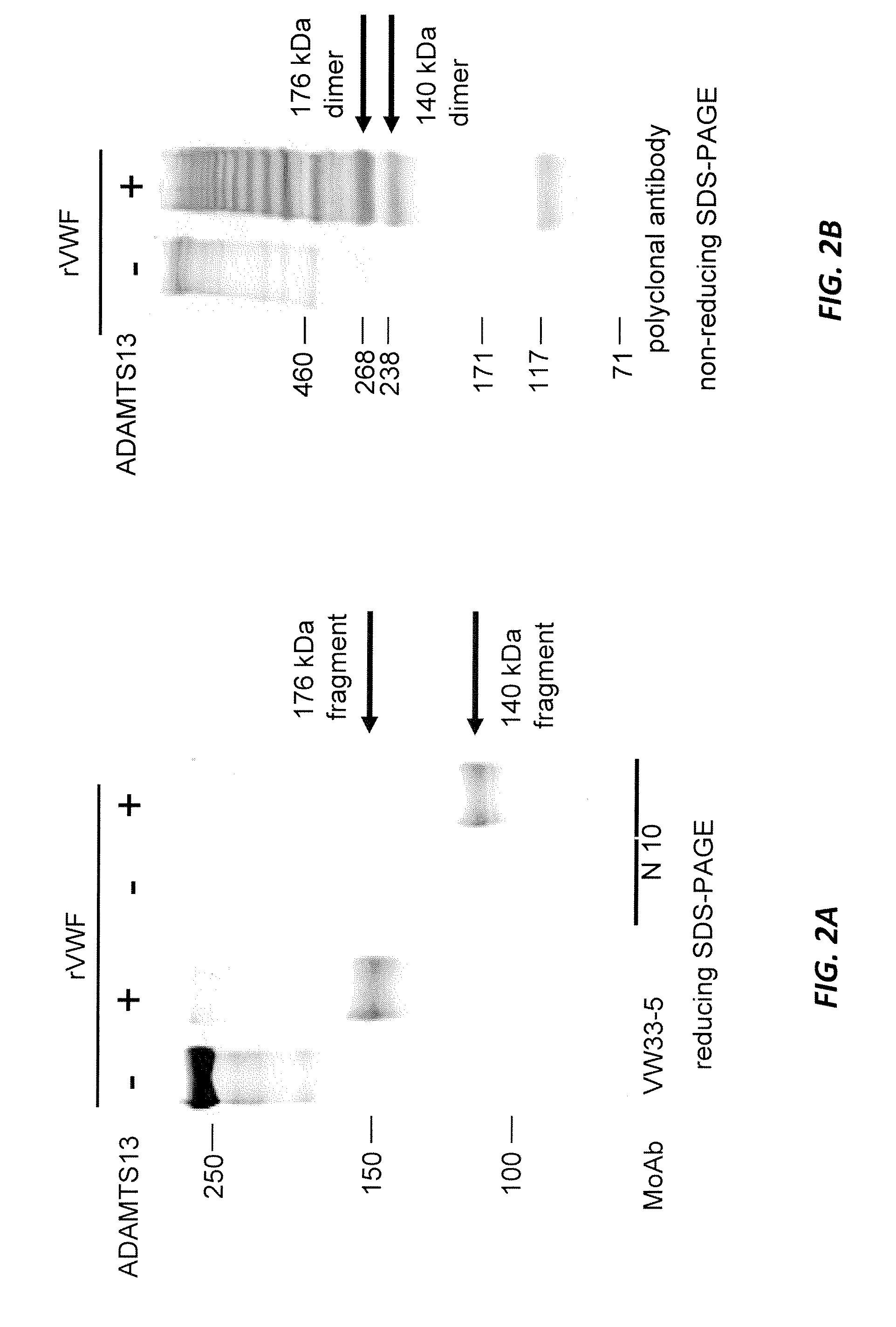
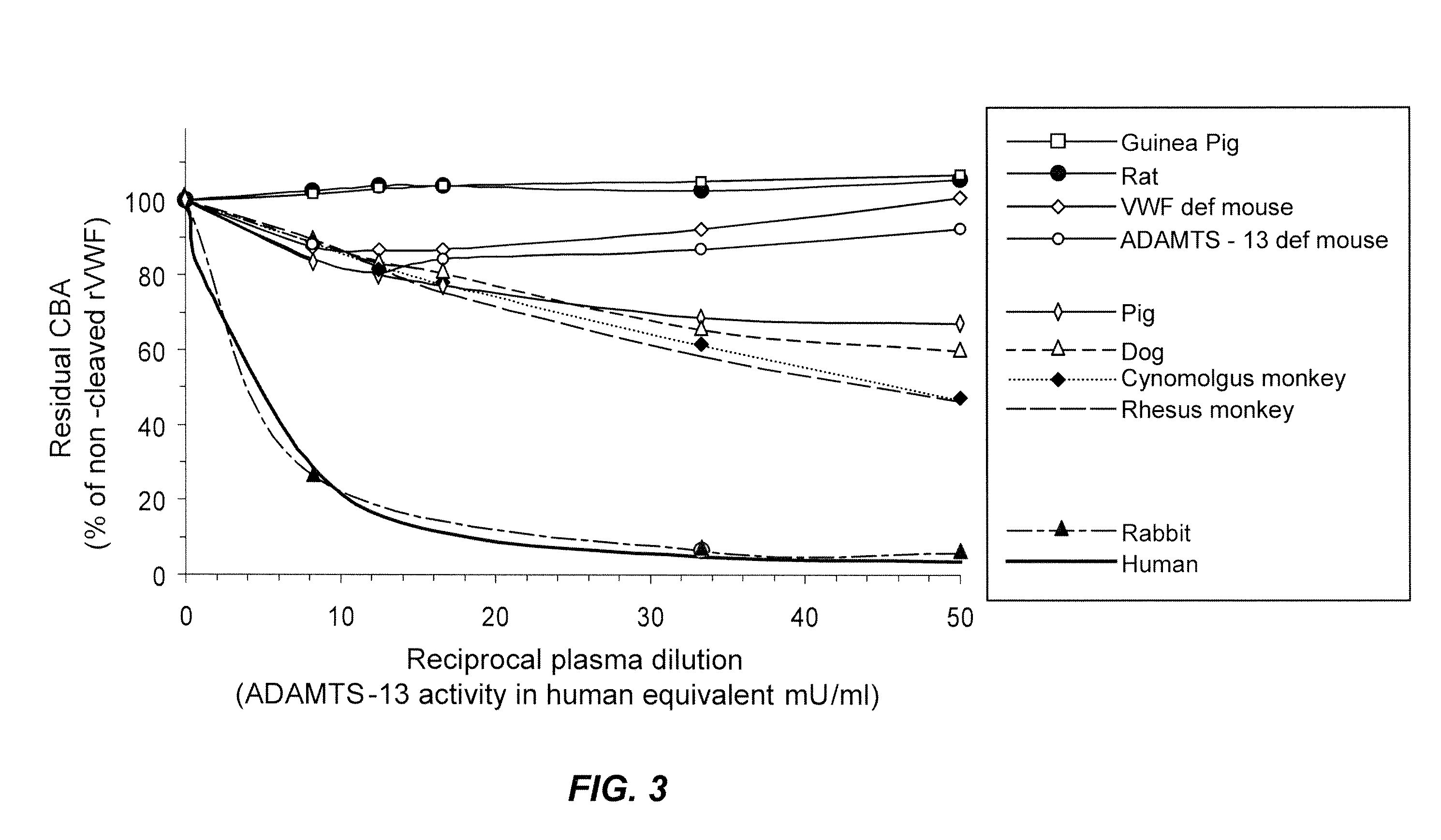
Methods of measuring ADAMTS13-mediated in vivo cleavage of von Willebrand factor and uses thereof
ActiveUS8415114B2Microbiological testing/measurementDisease diagnosisAdamts13 activityFactor VIII vWF
The invention generally relates to methods of measuring cleaved von Willebrand factor (VWF) fragments. More specifically, the invention relates to methods of measuring the ability of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) to cleave VWF in vivo. The invention also relates to methods of using various animal models which demonstrate ADAMTS13 activity similar to that of a human. The invention further relates to methods of measuring the cleavage products of rVWF in mammals, particularly in humans and in human plasma.
Owner:TAKEDA PHARMA CO LTD
Method for preparing cornea lamina material
ActiveCN102552979AStrong ability to pass onReduce the risk of contaminationProsthesisBiocompatibility TestingStem cell culture
The invention discloses a method for preparing a cornea lamina material. The method comprises the following steps of: carrying out accellular processing on a cornea-sclera complex of a mammal eyeball, culturing an amnion epithelial stem cell, inducing and secreting TSP (Thrombospondin)-1 and co-culturing accellular cornea stroma and the amnion epithelial stem cell, sizing, packaging and sterilizing, and the like. According to an obtained cornea lamina transplanting material, the promotion and the balance among the biocompatibility, the stability, the transparency, the mechanical strength and the bioactivity are realized; the integrity of cornea collagen molecules and a lamina structure can be kept to the largest extent, and compared with that prepared with the traditional method, the accellular cornea stroma prepared according to the method disclosed by the invention is larger in tensile strength and has the moisture content and the transparency close to those of normal cornea; and various cell growth factors and the TSP-1 are gathered, and the cornea lamina material has special effects of inflammatory resistance and vascularization resistance, has the capability of greatly enhancing the bioactivity of the cornea lamina material and can be widely used for repairing complicated ocular surface coloboma such as inflammation and corneal ulcer grown in a vascularization way.
Owner:SHAANXI RUISHENG BIOTECH
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20140147864A1Eliminate needMicrobiological testing/measurementDisease diagnosisFactor iiRenal Failures
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Proheparin-binding EGF-like growth factor, Tenascin C, Angiopoietin-related protein 4, Fibroblast growth factor 19, Fibroblast growth factor 21, Heparin-binding growth factor 1, Angiopoietin-related protein 6, Proepiregulin, Probetacellulin, Amphiregulin, Angiogenin, Thrombospondin-2, and Collagen alpha-1 (XVIII) chain as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
PLA2R-THSD7A fusion protein as well as application and kit thereof
ActiveCN106432508AImmunoreactiveImprove the detection rateCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsEpitopeThrombospondin
The invention relates to the medical field and particularly relates to a PLA2R-THSD7A fusion protein as well as application and kit thereof. The fusion protein comprises a first region and a second region, wherein the first region has at least 85% of sequence homology with a phospholipase A2 receptor (PLA2R), and the second region has at least 85% of sequence homology with 1-type thrombospondin 7A domain (THSD7A). The fusion protein prepared by virtue of the preparation method integrates epitopes of two antibodies including PLA2R and THSD7A and simultaneously has the immunoreactivities of the two antibodies, and the detection ratio of spontaneity MN-relevant autoantibodies is increased and can reach 78.1%.
Owner:SHENZHEN BLOT BIOTECH
Modulation of synaptogenesis
The present invention describes methods and compositions for modulating synaptogenesis and axon and / or dendritic growth. The methods include the use of agents that modulate a thrombospondin and / or an α2δ subunit of a calcium channel.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
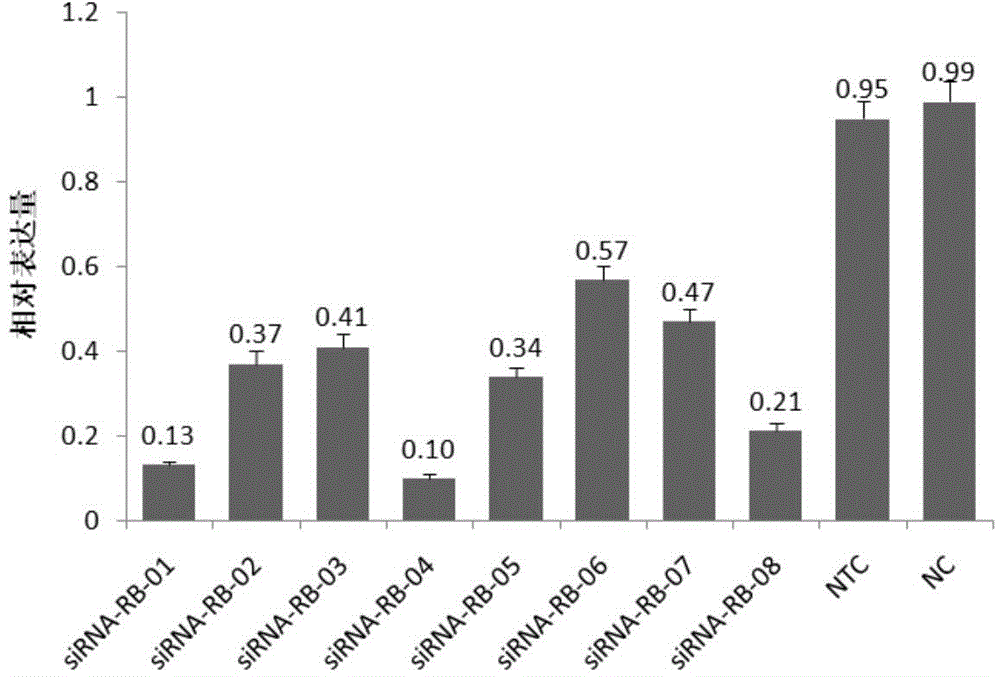
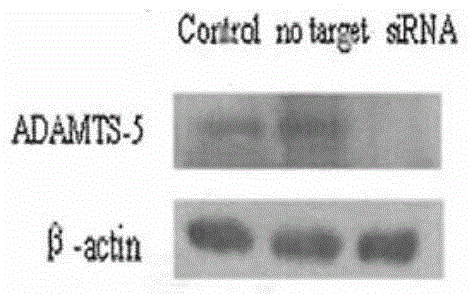
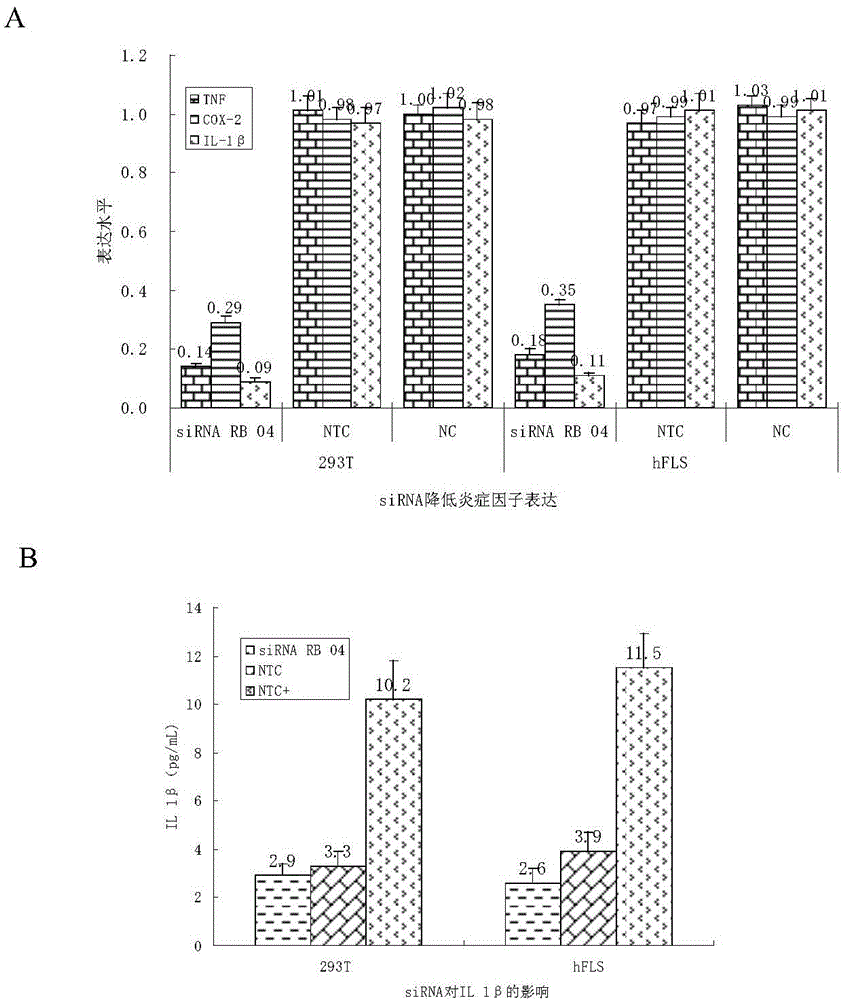
SiRNA (small interfering ribonucleic acid) composition inhibiting ADAMTS-5 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs-5) and ADAM17 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs 17) genes and application of siRNA composition
ActiveCN104560997AAchieve healingAntipyreticGenetic material ingredientsInflammatory factorsMolecular composition
The invention discloses an siRNA (small interfering ribonucleic acid) composition inhibiting an ADAMTS-5 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs-5) gene and an ADAM17 (a disintegrin-like and metalloproteinase with thrombospondin type 1motifs 17) gene and an application of the siRNA composition. The invention discloses the chemically modified double-chain siRNA molecular composition which comprises at least one of double-chain siRNA molecules as shown as (1) and at least one double-chain siRNA molecules as shown as (2). The siRNA composition can serve as a treatment medicine of arthritis and relevant inflammation, and the siRNA composition and a preparation of the siRNA composition can be injected locally through a bone articular cavity to inhibit inflammatory factor expression to achieve treatment of the joint inflammation.
Owner:ARGORNA PHARM CO LTD
Methods of measuring adamts13-mediated in vivo cleavage of von willebrand factor and uses thereof
ActiveUS20100143957A1Microbiological testing/measurementDisease diagnosisFactor VIII vWFAdamts13 activity
The invention generally relates to methods of measuring cleaved von Willebrand factor (VWF) fragments. More specifically, the invention relates to methods of measuring the ability of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) to cleave VWF in vivo. The invention also relates to methods of using various animal models which demonstrate ADAMTS13 activity similar to that of a human. The invention further relates to methods of measuring the cleavage products of rVWF in mammals, particularly in humans and in human plasma.
Owner:TAKEDA PHARMA CO LTD
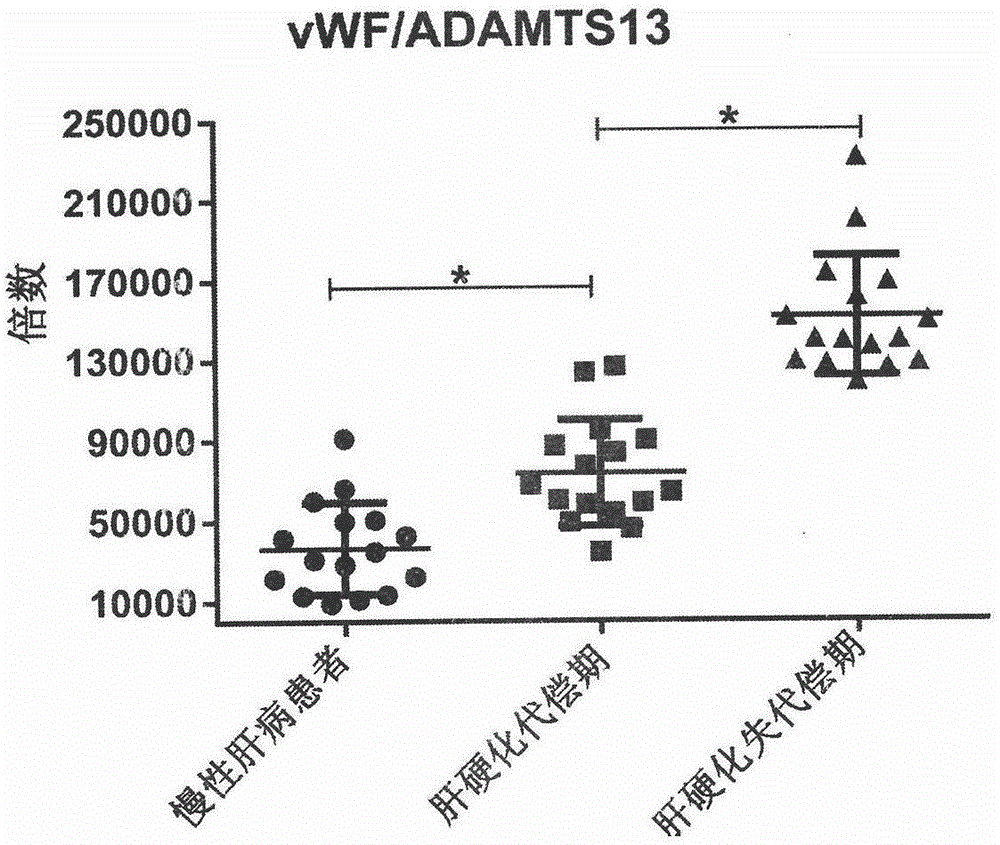
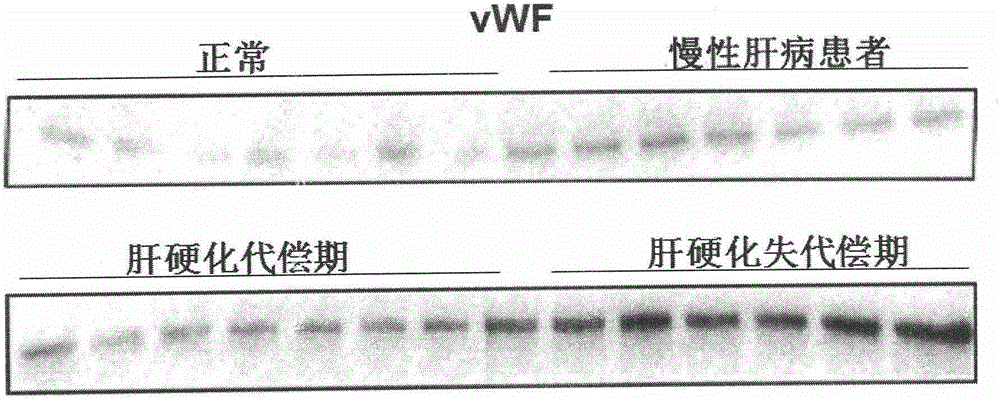
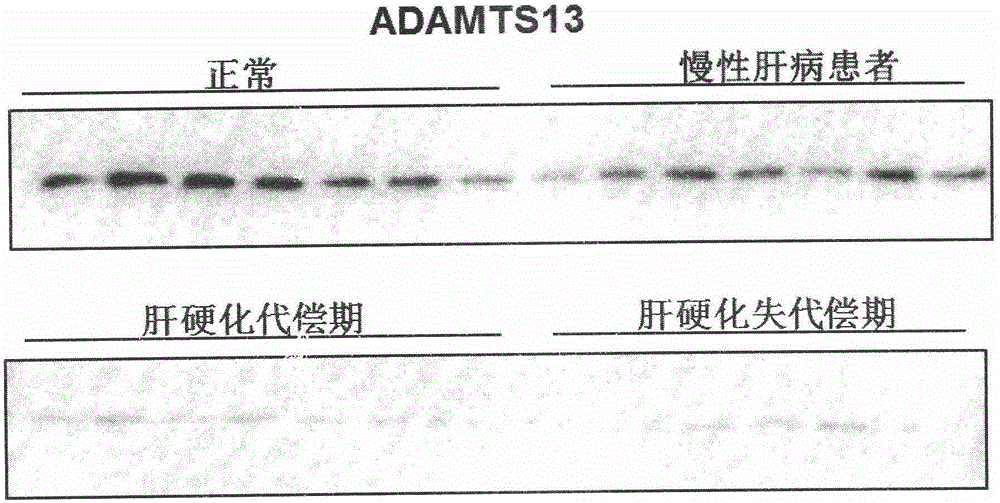
Biomarkers VWF and ADAMTS13 and use thereof in liver cirrhosis diagnostic reagents
ActiveCN106066401AEasy to operateEasy screening and selectionDisease diagnosisBiological testingProtein compositionAmino acid
Owner:CHONGQING ZAOQITIAN BIOTECHNOLOGY CO LTD
Immune Disease Medicament Comprising a Modulator of the Binding Between a Heparin Bindin Domain of Thrombospondin-1 and a Beta1 Integrin
An article of manufacture comprising packaging material and a pharmaceutical composition is disclosed, the article of manufacture being identified in print in or on the packaging material for treatment of an immunity-related disease in a subject in need thereof. The pharmaceutical composition comprises a pharmaceutically acceptable carrier and, as an active ingredient, a compound being capable of modulating an interaction between a heparin-binding domain of a thrombospondin and a receptor of the heparin-binding domain.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
In vitro method for identifying thoracic aortic aneurysms (TAA) in a subject
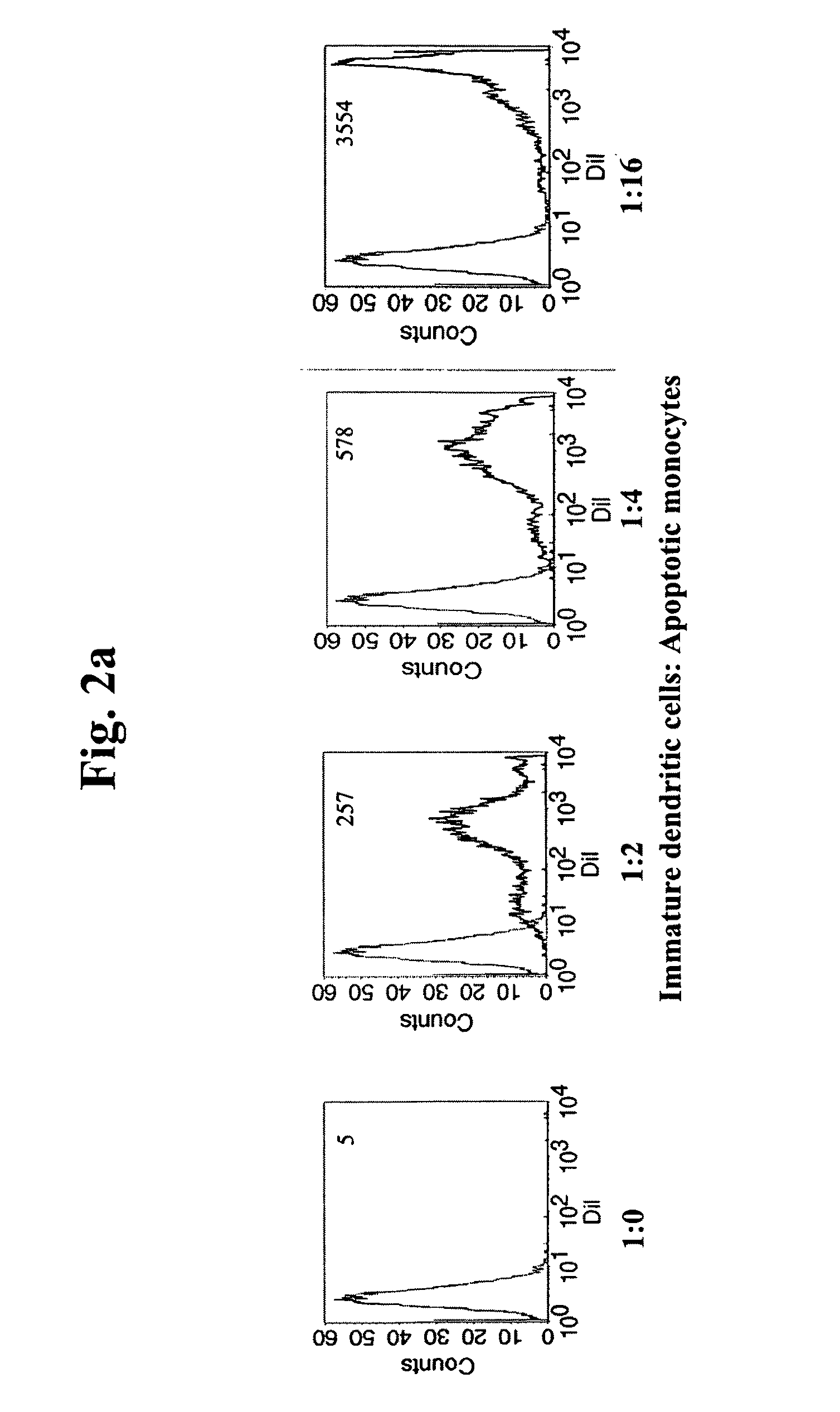
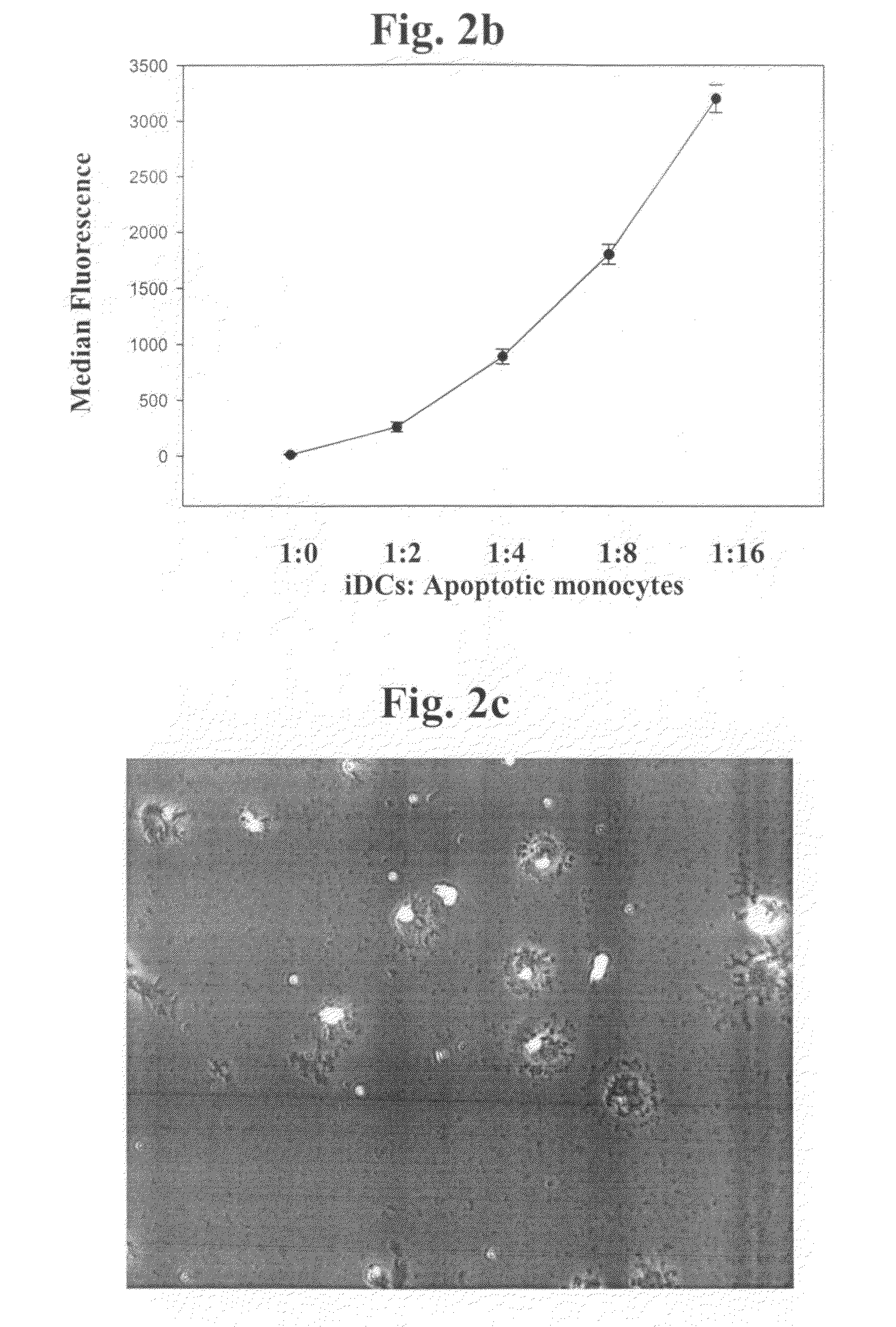
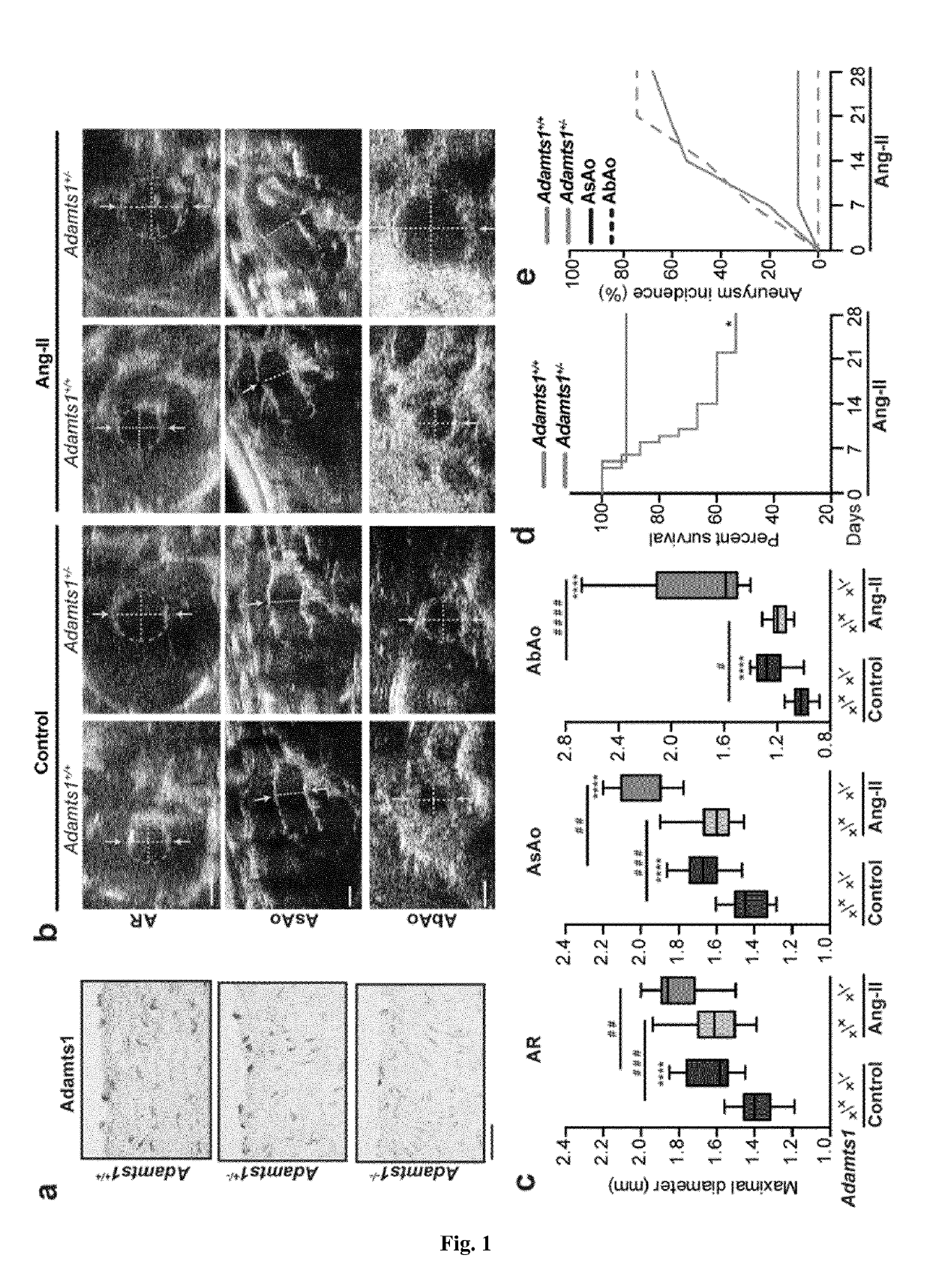
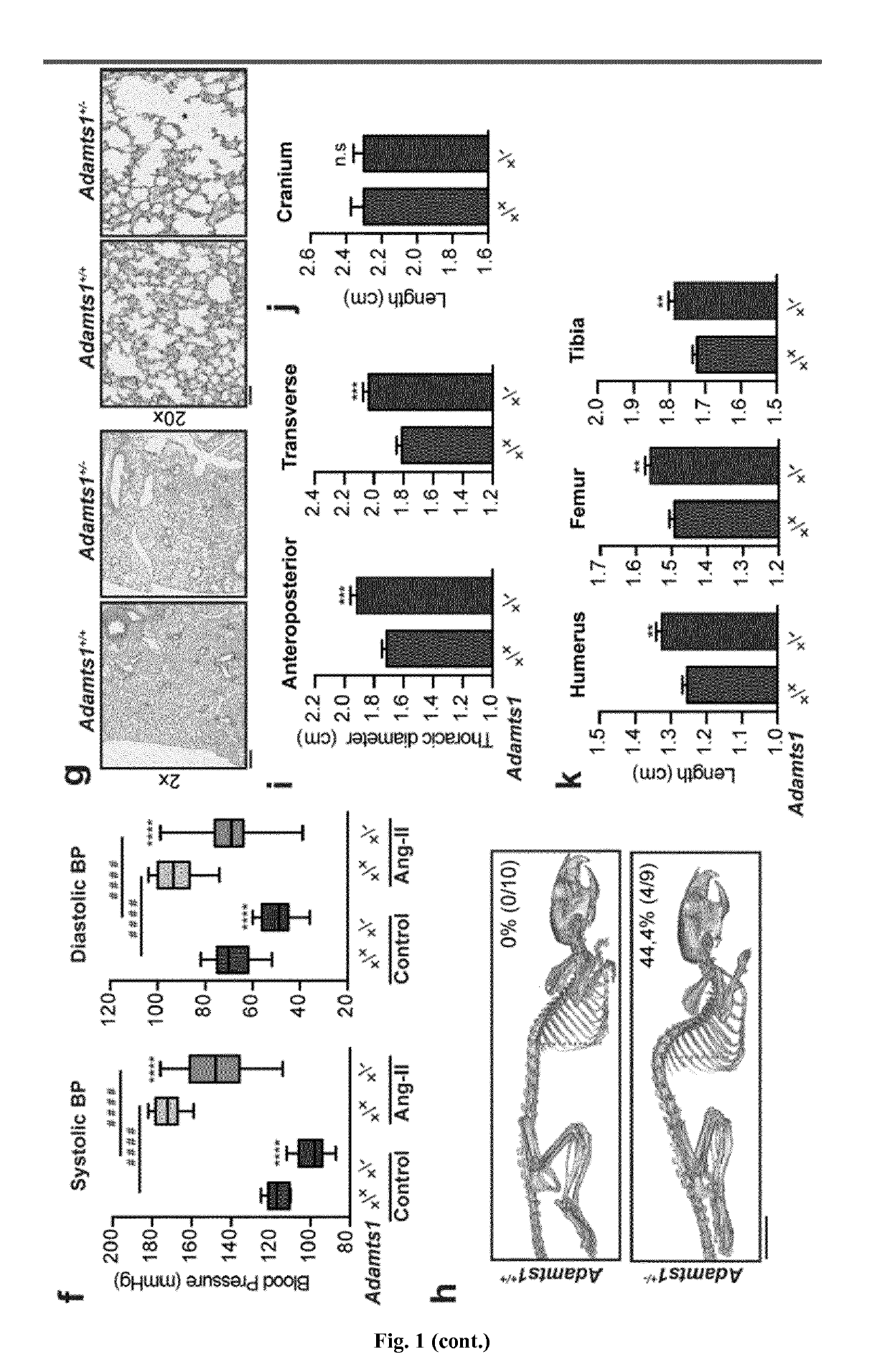
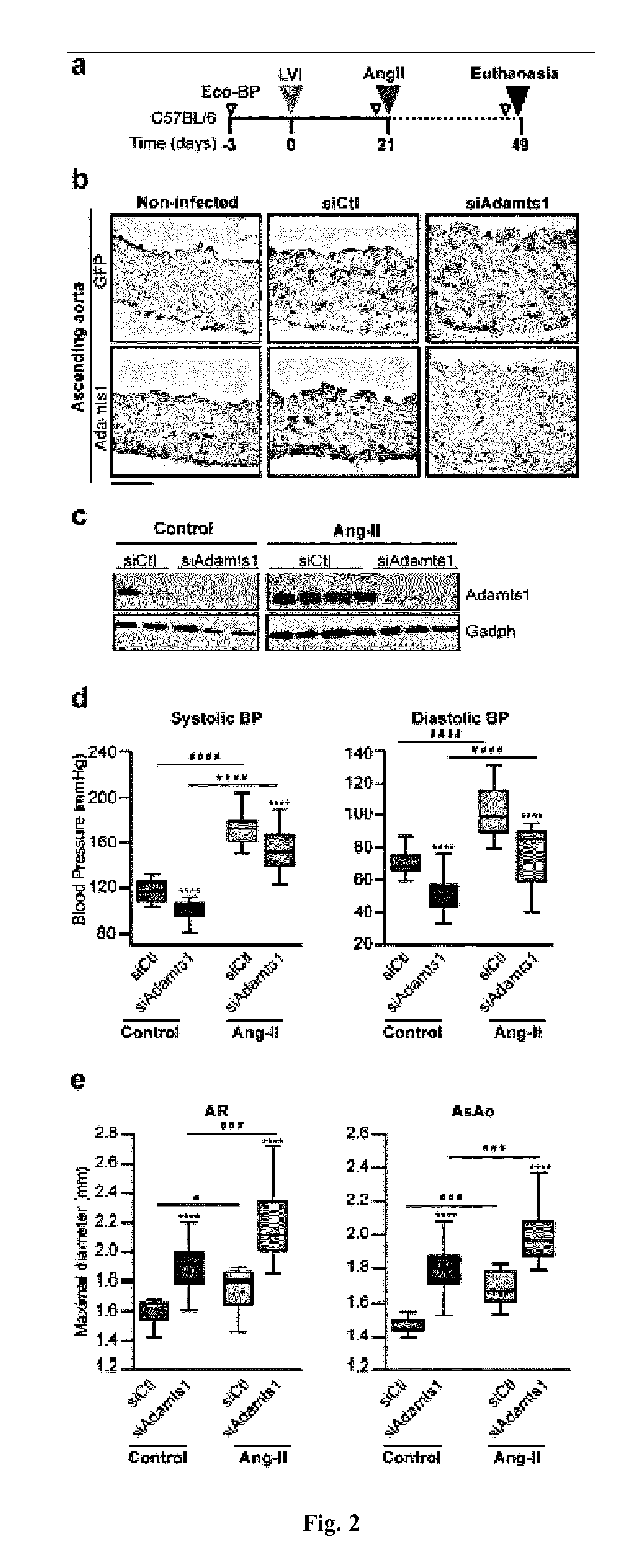
The present invention refers to an In vitro method for screening for subjects at risk of developing thoracic aortic aneurysm (TAA) or a disease causing TAA comprising: (a) measuring the expression pattern or level of at least A Disintegrin And Metalloproteinase with Thrombospondin Motifs 1 (ADAMTS1) obtained from an isolated biological sample of the subjects to be screened; and (b) comparing said expression pattern or level of at least ADAMTS1 of the subjects to be screened with an already established expression pattern or level, wherein reduced expression of at least ADAMTS1 is indicative of a thoracic aortic aneurysm (TAA).
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +2
Early diagnosis reagent for of kawasaki disease and application thereof
PendingCN111007258ASimple and fast operationLow costDisease diagnosisBiological testingDiagnosis earlyThrombospondin
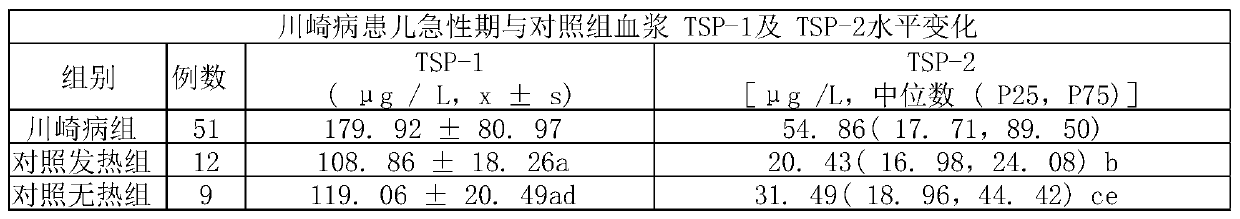
The invention provides an early diagnosis reagent for the children Kawasaki disease and application thereof. The reagent is a thrombospondin detection reagent and can be used for early diagnosis of Kawasaki disease. The invention also provides application of the reagent in preparation of a kit for early diagnosis of Kawasaki disease. The kawasaki disease is diagnosed in an assisted mode by detecting the content of thrombospondin in blood plasma of a suspected child patient and thus the suspected child patient can be diagnosed and treated in the early stage, so that cardiovascular diseases andother pathological changes are prevented.
Owner:AFFILIATED CHILDRENS HOSPITAL OF CAPITAL INST OF PEDIATRICS
Thrombospondin-1 polypeptides and methods of using same
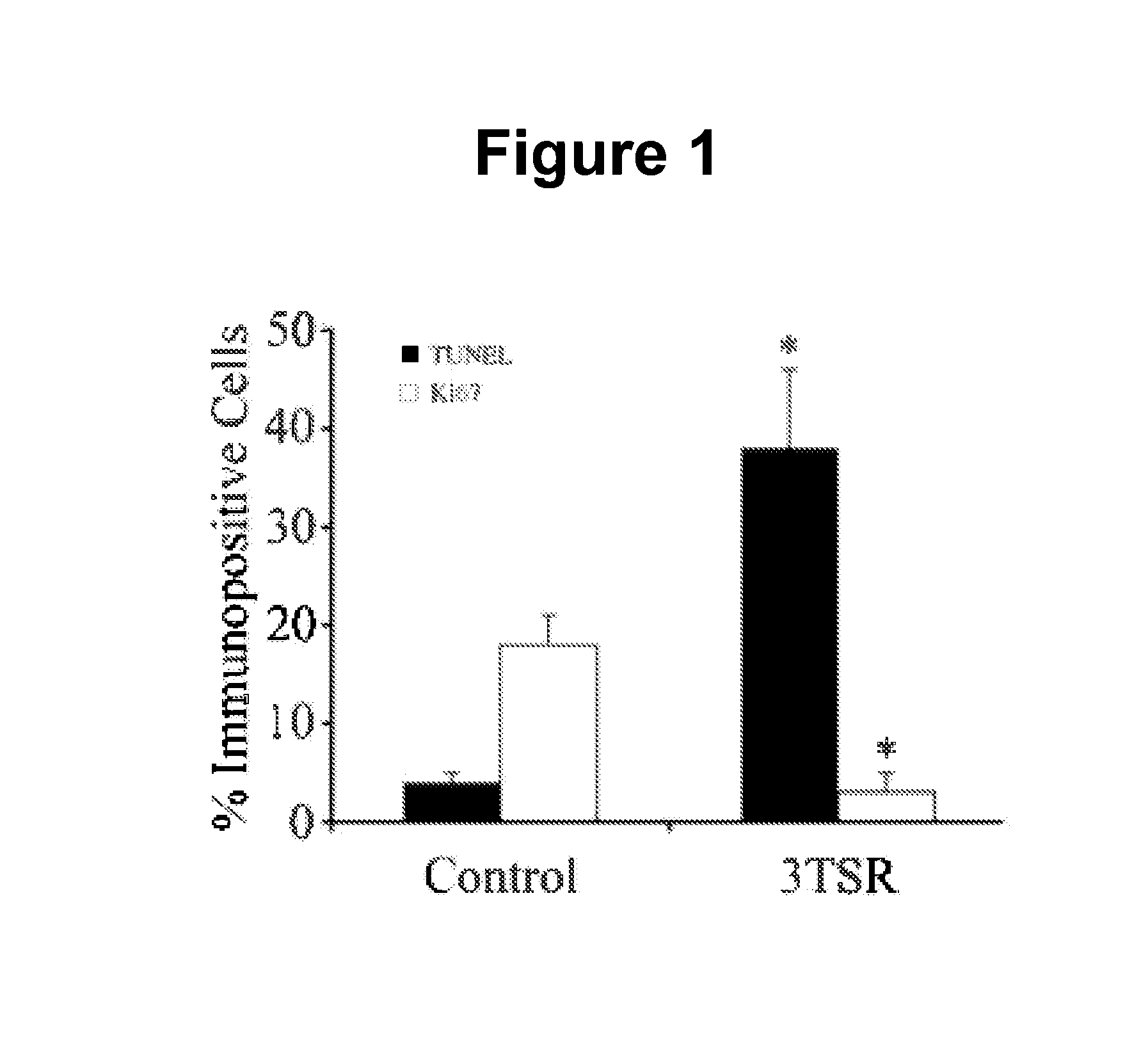
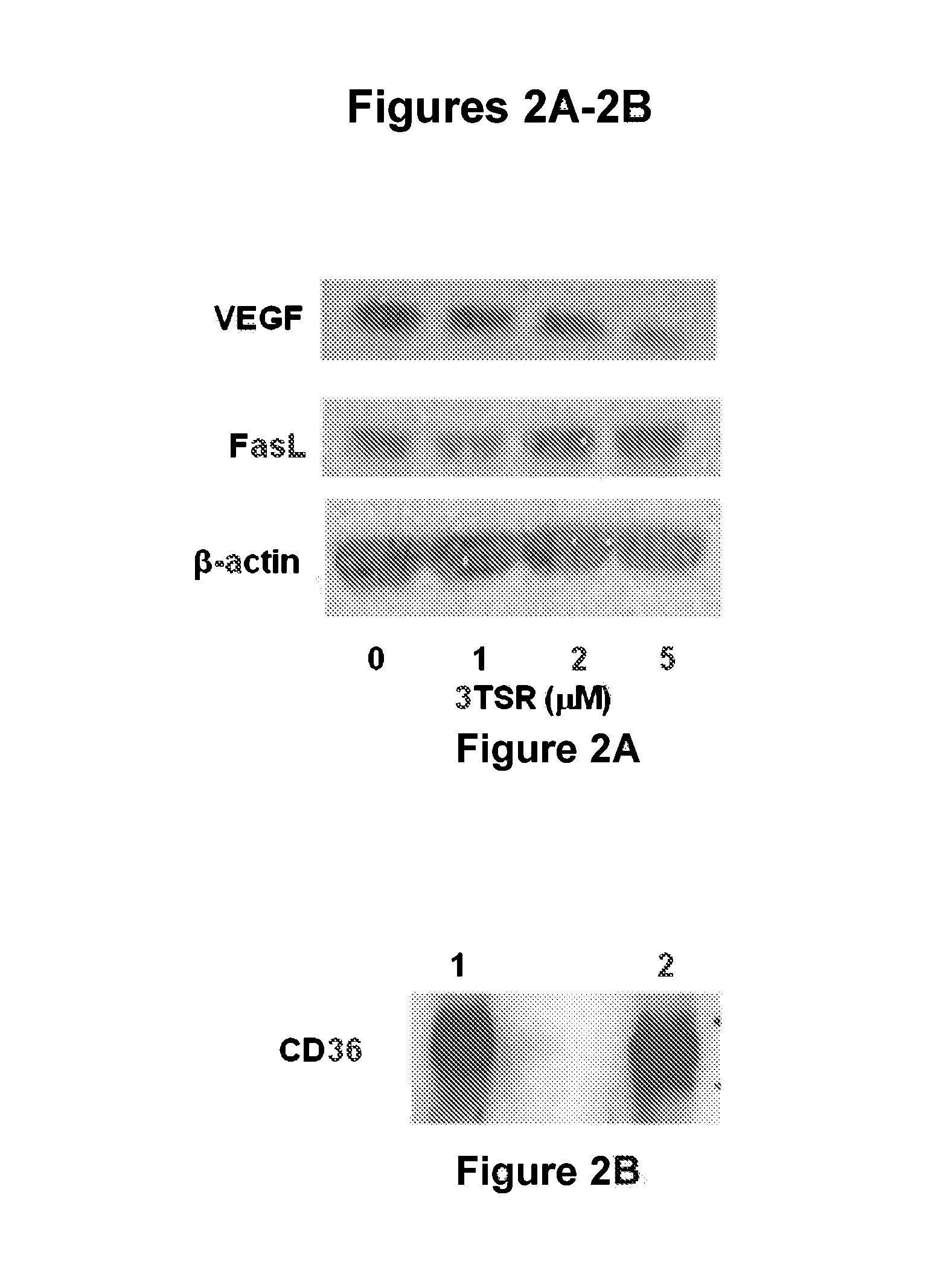
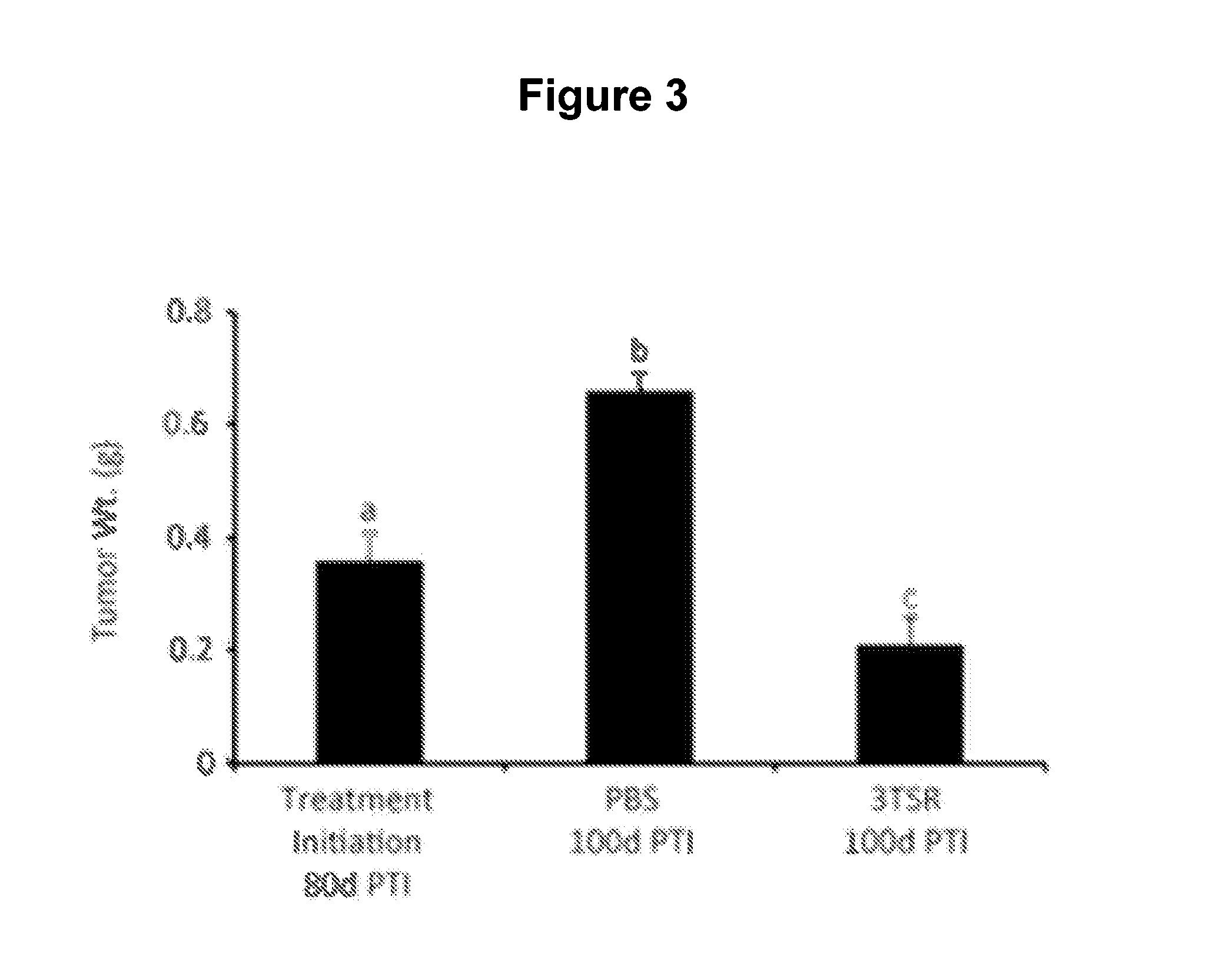
The invention features thrombospondin-1 (TSP-1) polypeptides (e.g., 3TSR-Fc fusion proteins), nucleic acid molecules encoding the TSP-1 polypeptides, and compositions thereof. The invention also features methods of making and using the TSP-1 polypeptides of the invention (e.g., using 3TSR-Fc fusion proteins to treat a subject having a disorder associated with pathological angiogenesis, e.g., cancer, e.g., epithelial ovarian cancer (EOC)).
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Method for detecting multiple types of ovine babesiasis and detection kit thereof
ActiveCN107164506ASpecific differential testRapid differential testMicrobiological testing/measurementDNA/RNA fragmentationDiseaseCurrent technology
The invention discloses a method for detecting ovine babesiasis indeterminate species, Babesia motasi LZ subspecies and Babesia motasi NB subspecies as well as a detection kit thereof. The detection method takes ovine babesiasis thrombospondin-related anonymous protein TRAP gene as a detection identifier. The kit comprises six primers shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3, SEQ ID No.4, SEQ ID No.5, and SEQ ID No.6, The method takes TRAP as the detection identifier, the difference between species and subspecies is large, the detection sensitivity is better than that of the current technologies. The kit can simultaneously amplify several target DNA molecules in a PCR reaction, and realizes a purpose of simultaneously discriminating and detecting a plurality of microbes in a same sample. Compared with the other molecule detection technologies, the method has the advantages of simpleness, rapidity, high efficiency and saved cost, is suitable for rapid detection of cause of disease, and can be a hotspot technology for scientific research and clinical diagnosis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Autoantibody immunochromatographic strip and test card for simultaneously detecting M-type phospholipase A2 receptor and type 1 thrombospondin 7A domain
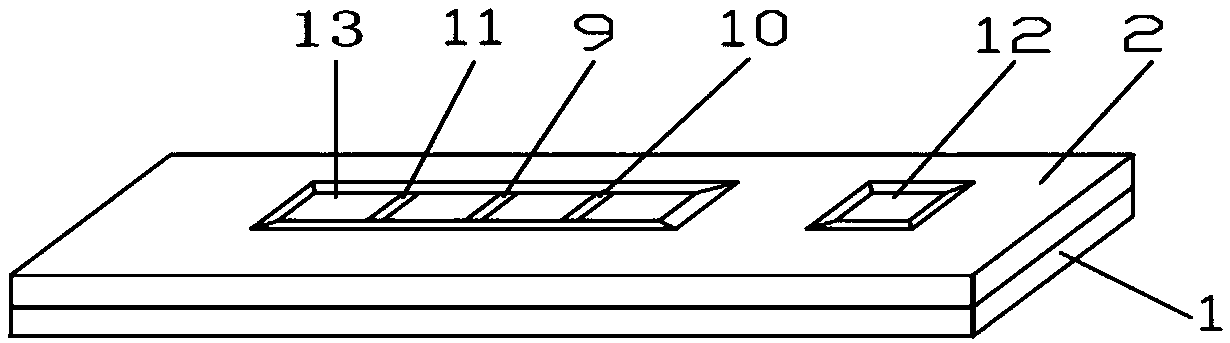
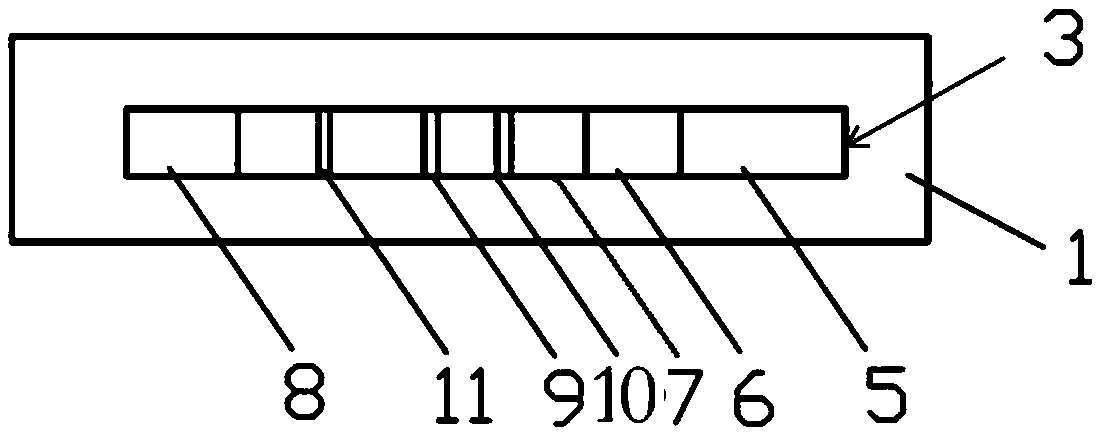
The invention relates to the field of clinical immunology detection, in particular to an autoantibody immunochromatographic strip and a test card for simultaneously detecting an M-type phospholipase A2 receptor and a type 1 thrombospondin 7A domain. The autoantibody immunochromatographic strip and the test card for simultaneously detecting the M-type phospholipase A2 receptor and the type 1 thrombospondin 7A domain comprises a bottom plate, an absorbent paper, a nitrocellulose coating film, an antibody binding pad, a sample pad, and a shell. The absorbent paper, the nitrocellulose coating film, the antibody binding pad and the sample pad are sequentially adhered to the bottom plate along the length direction of the bottom plate. The shell encloses the immunochromatographic strip. The shellfurther comprises a base and a clamping cover. The clamping cover is provided with an observing opening and a sample adding opening. The opening of the sample adding opening is located at the upper part of the sample pad. The opening of the observing opening is located at the upper side of the coating film.
Owner:ZHEJIANG SCI-TECH UNIV
Compositions and methods for grafts modified with a non-thrombogenic and pro-migratory cell-derived extracellular matrix
PendingUS20220152273A1Reducing and eliminating riskGood biocompatibilitySurgeryPharmaceutical containersCell-Extracellular MatrixThrombogenicity
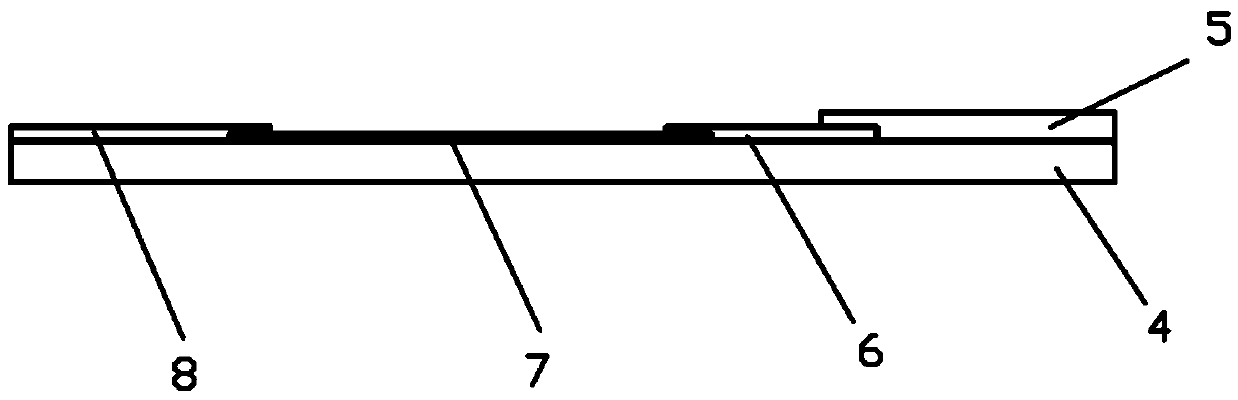
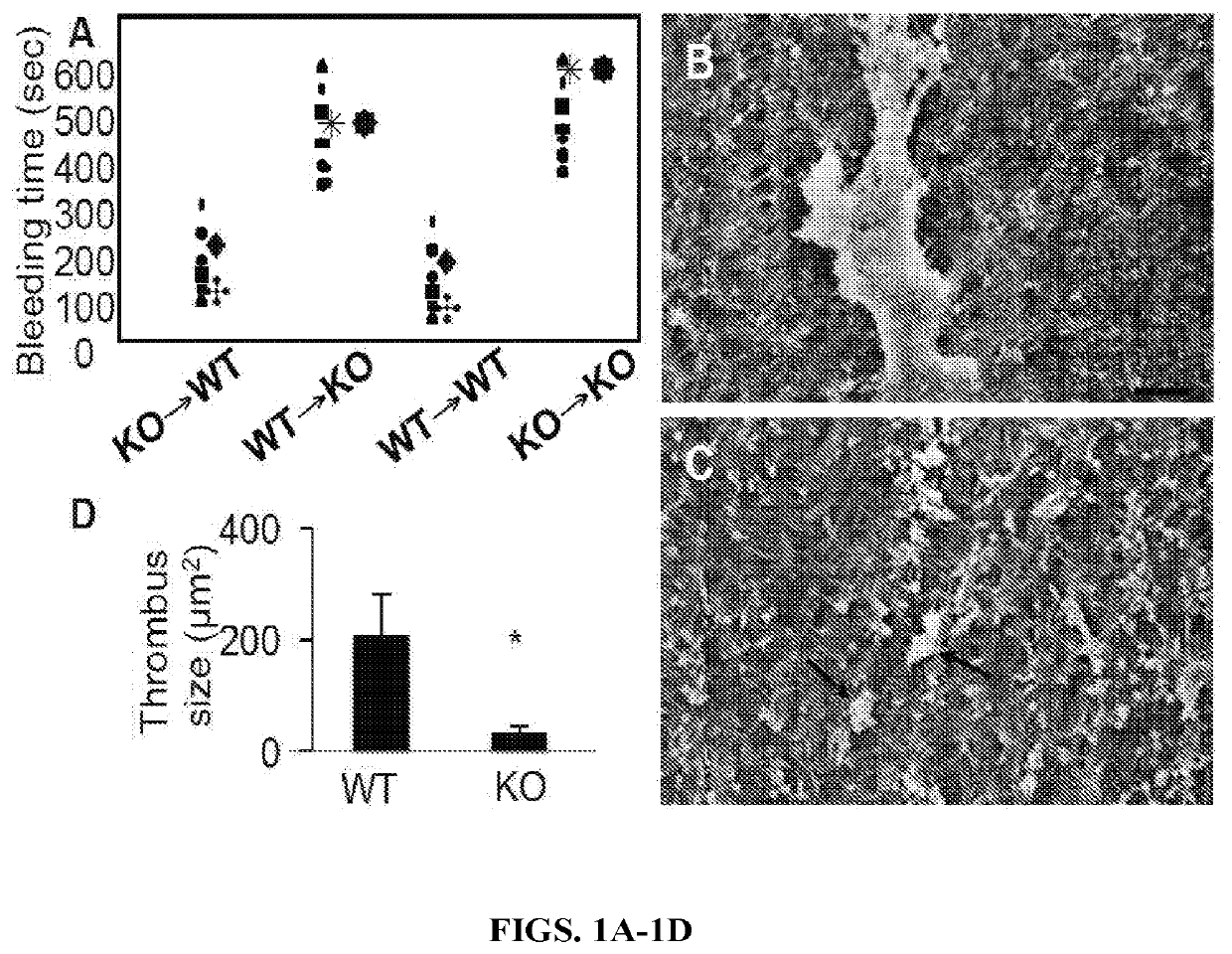
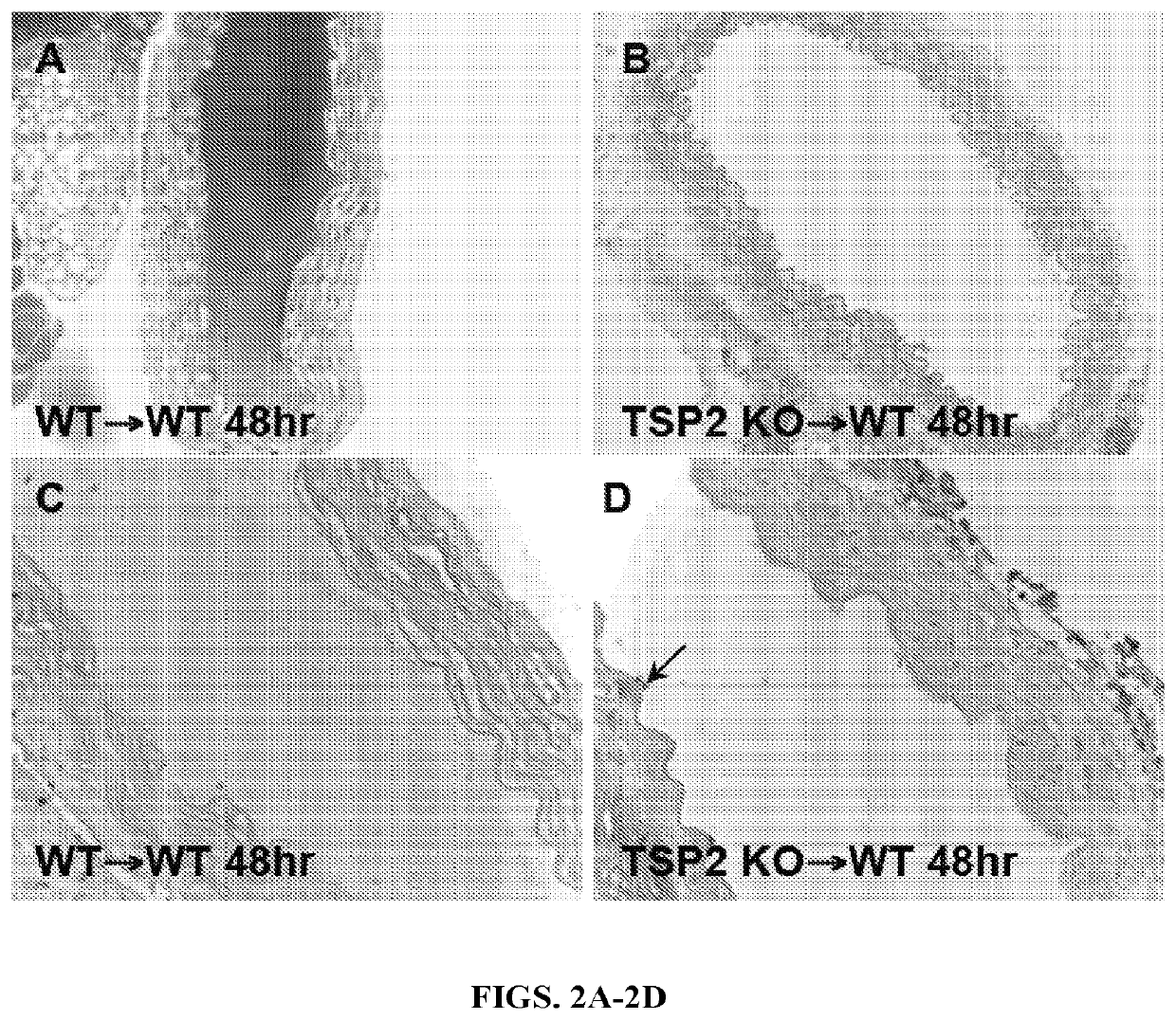
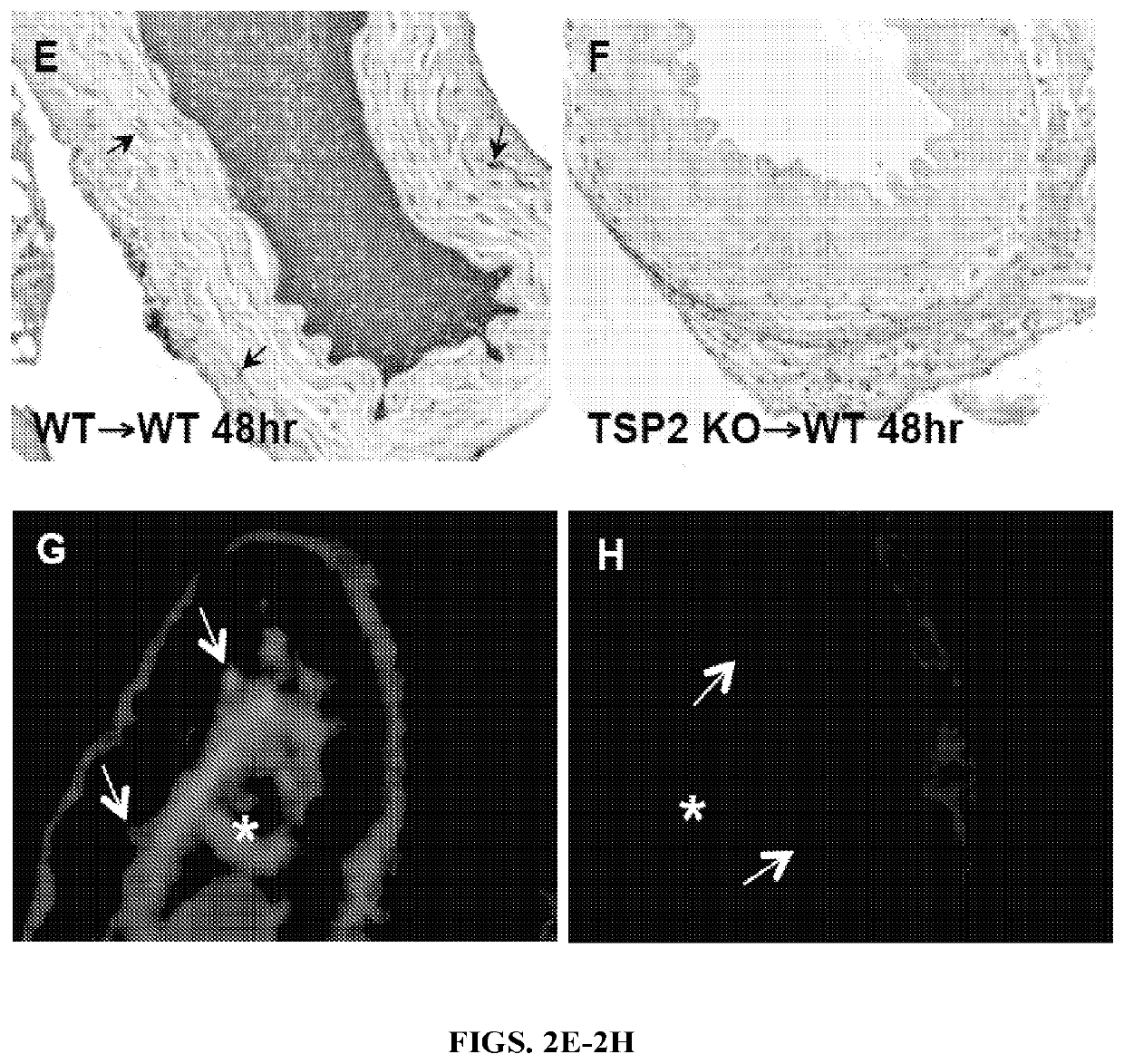
The present invention relates to novel compositions and methods for reducing or eliminating the thrombogenicity of a graft by modifying the graft with a cell-derived extracellular matrix lacking thrombospondin-2 (TSP2-null ECM) to render it non-thrombogenic when transplanted to a subject in need thereof. The invention also provides a method for improving the biocompatibility of a medical device or an implant by modifying the medical device or implant with a cell-derived TSP2-null ECM, whereby the medical device or implant is rendered non-thrombogenic and pro-migratory.
Owner:YALE UNIV
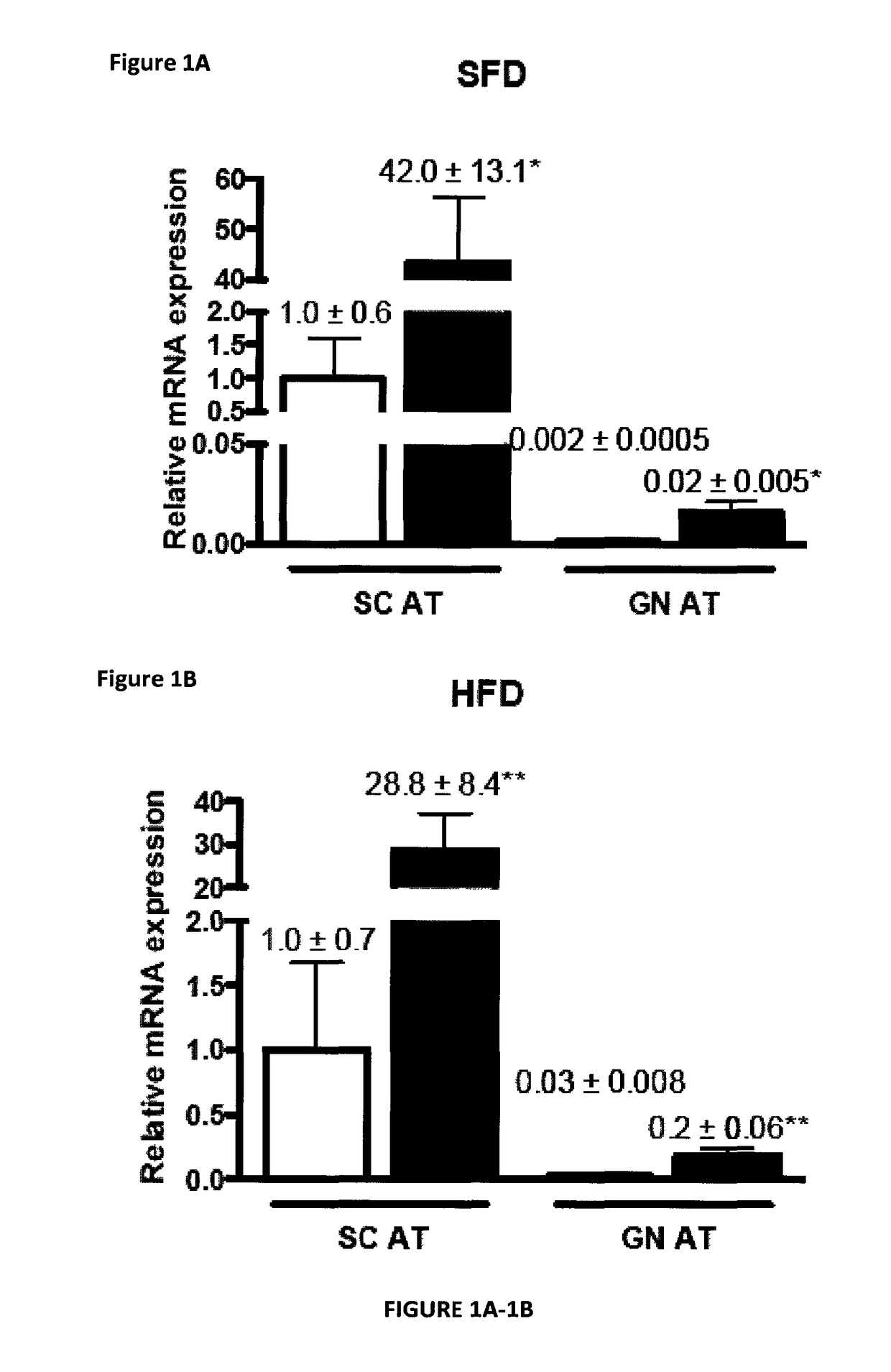
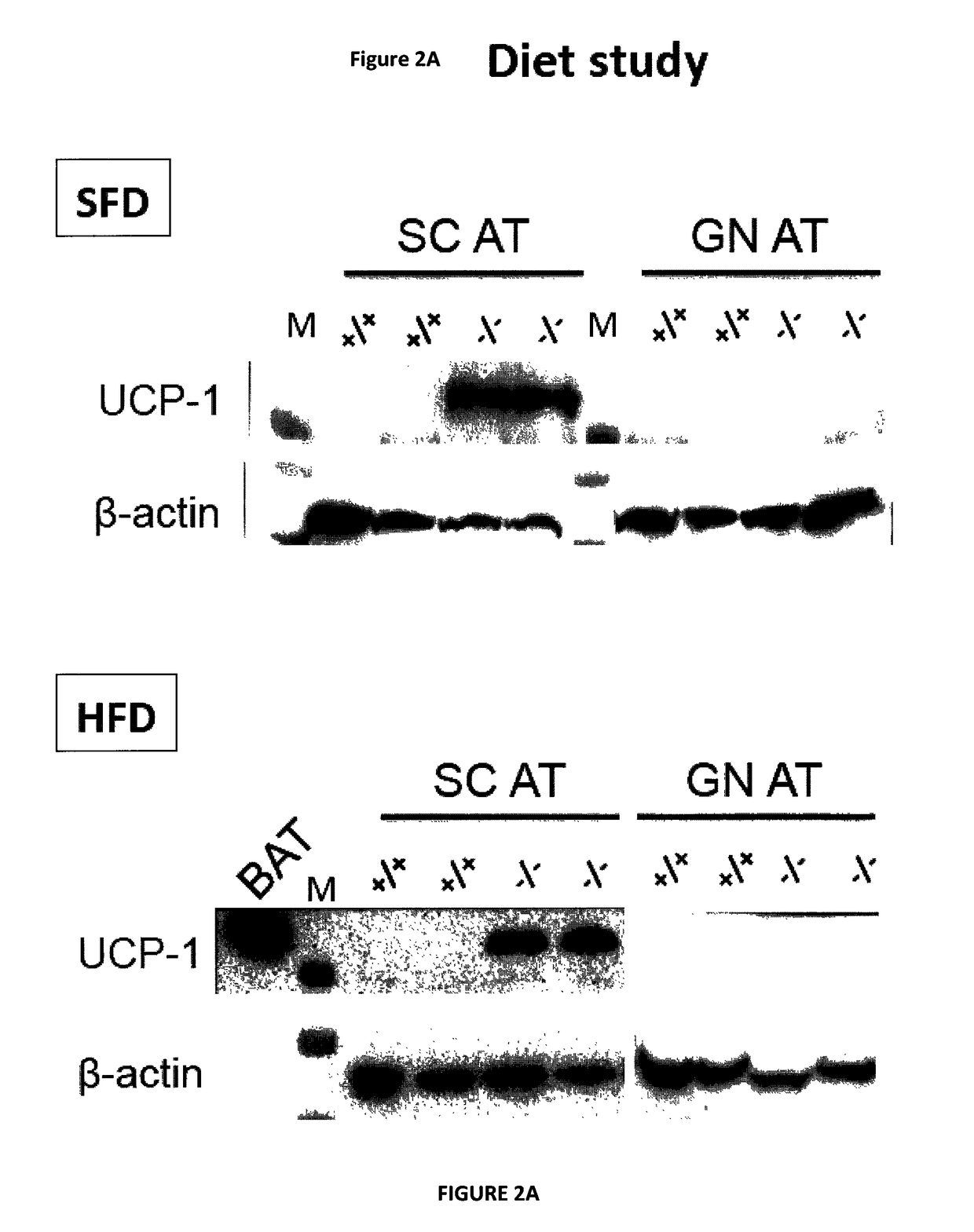
Modulating adipose tissue and adipogenesis
InactiveUS20180009903A1InducePrevent reduce developmentMicrobiological testing/measurementDisease diagnosisDiseaseAntigen
The invention relates to the field of obesity and related metabolic diseases. More specifically, the invention relates to methods of reducing aggrecanase activity or antigen in mammals in order to enhance brown adipose tissue (BAT) development, to promote conversion of white adipose tissue (WAT) into BAT in vivo, and to limit triglyceride accumulation and steatosis in the liver. The invention also relates to a strategy of neutralization or depletion of ADAMTS5 as a strategy to inhibit adipogenesis and more specifically, the invention relates to a method of reducing aggracanase-2 (ADAMTS5, A Disintegrin and Metalloproteinase with Thrombospondin motif 1; member 5) antigen and / or activity in mammals in order to impair differentiation of precursor cells into mature adipocytes (i.e., adipogenesis).
Owner:KATHOLIEKE UNIV LEUVEN KU LEUVEN RES & DEV
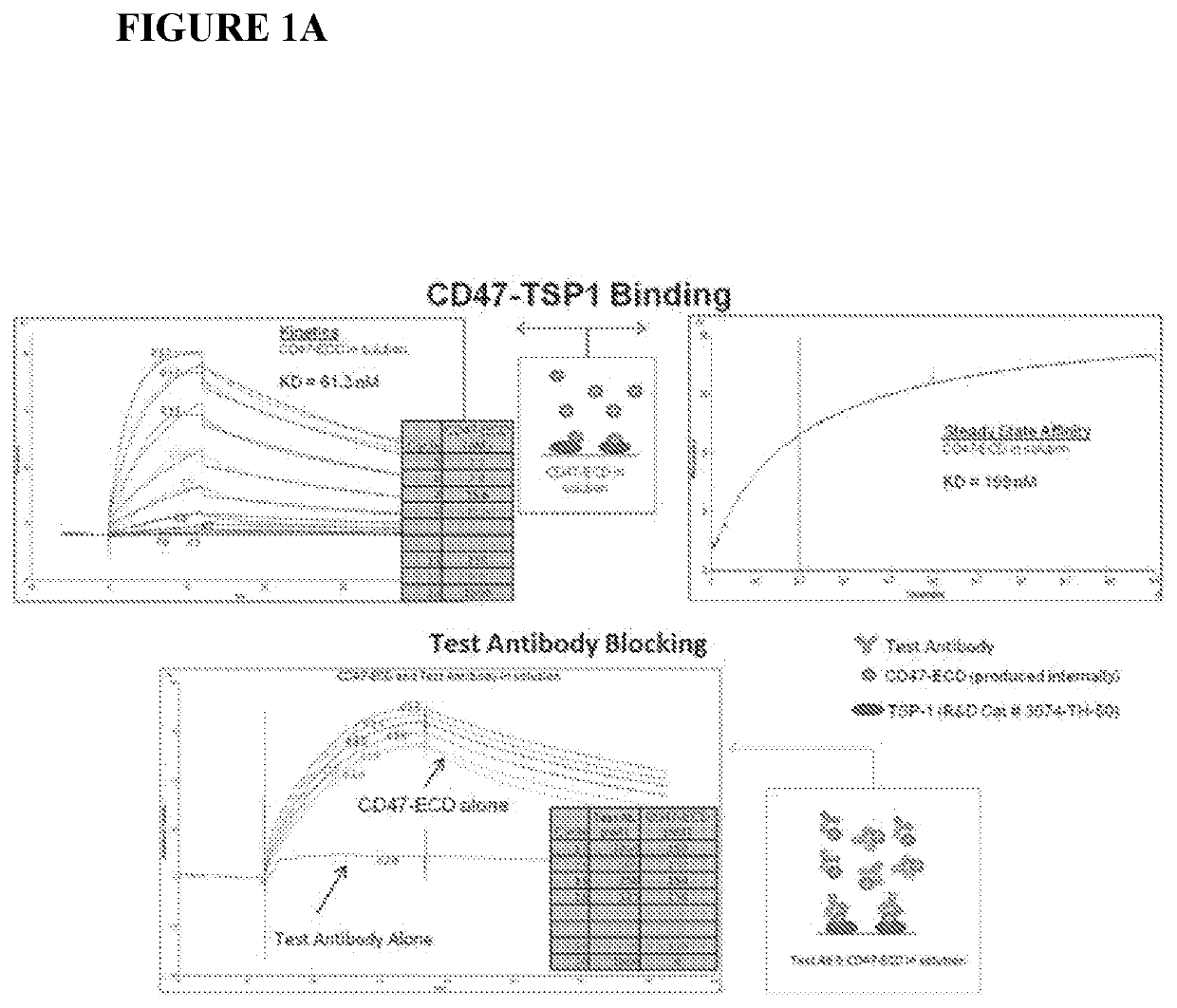
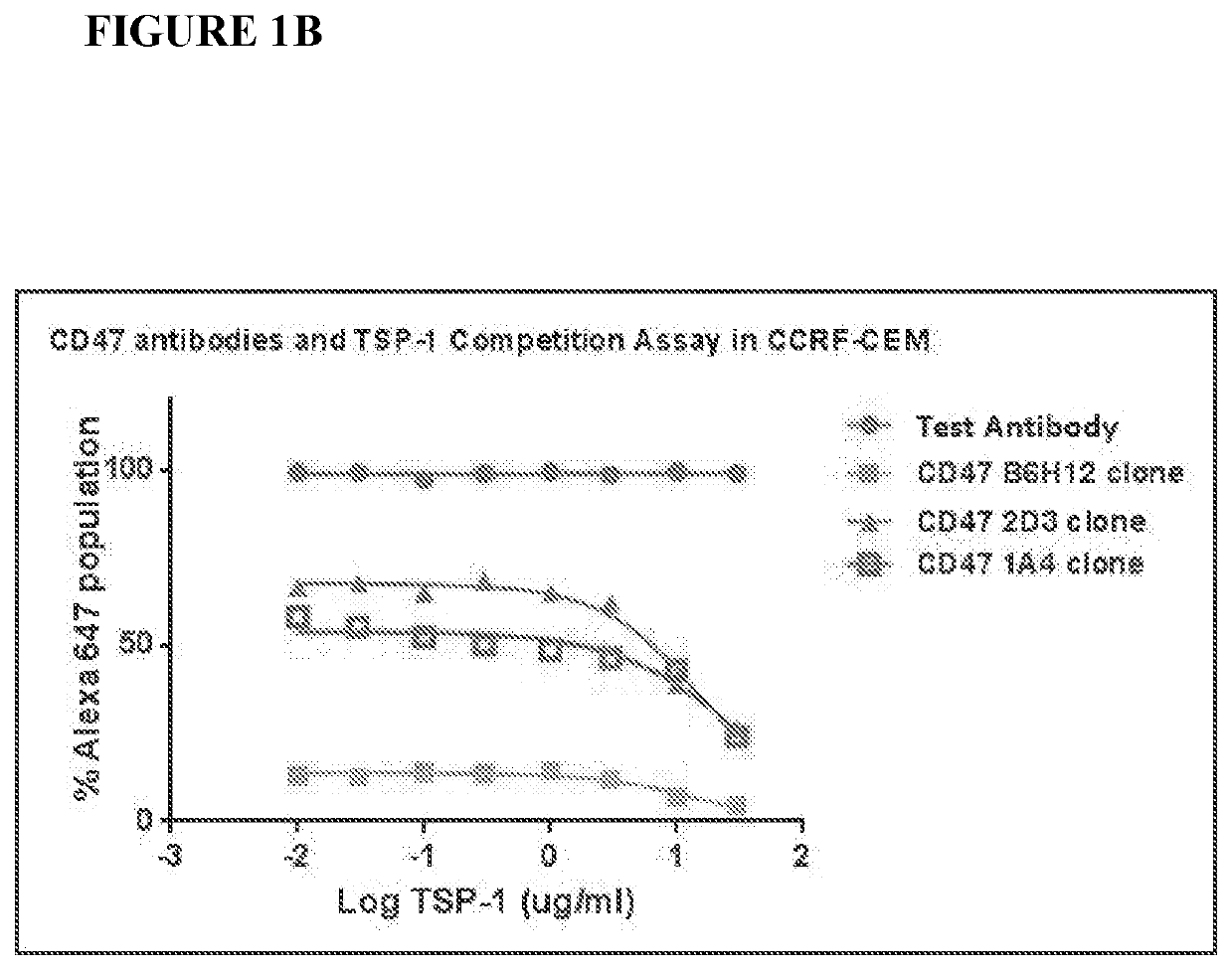
Cd47 antibodies and methods of use thereof
InactiveUS20200255515A1Good antitumor activityLarge levelImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSignal-regulatory protein alphaAntiendomysial antibodies
Integrin-associated protein (IAP or CD47) antibodies that specifically inhibit the interaction between CD47 and the CD47-signal regulatory protein alpha (SIRPa) but not the interaction between CD47 and thrombospondin-1 (TSP-1), and methods of using these monoclonal antibodies as therapeutics are provided. Further disclosed are sequences of variable heavy chains and variable light chains of the antibodies provided.
Owner:CELGENE CORP
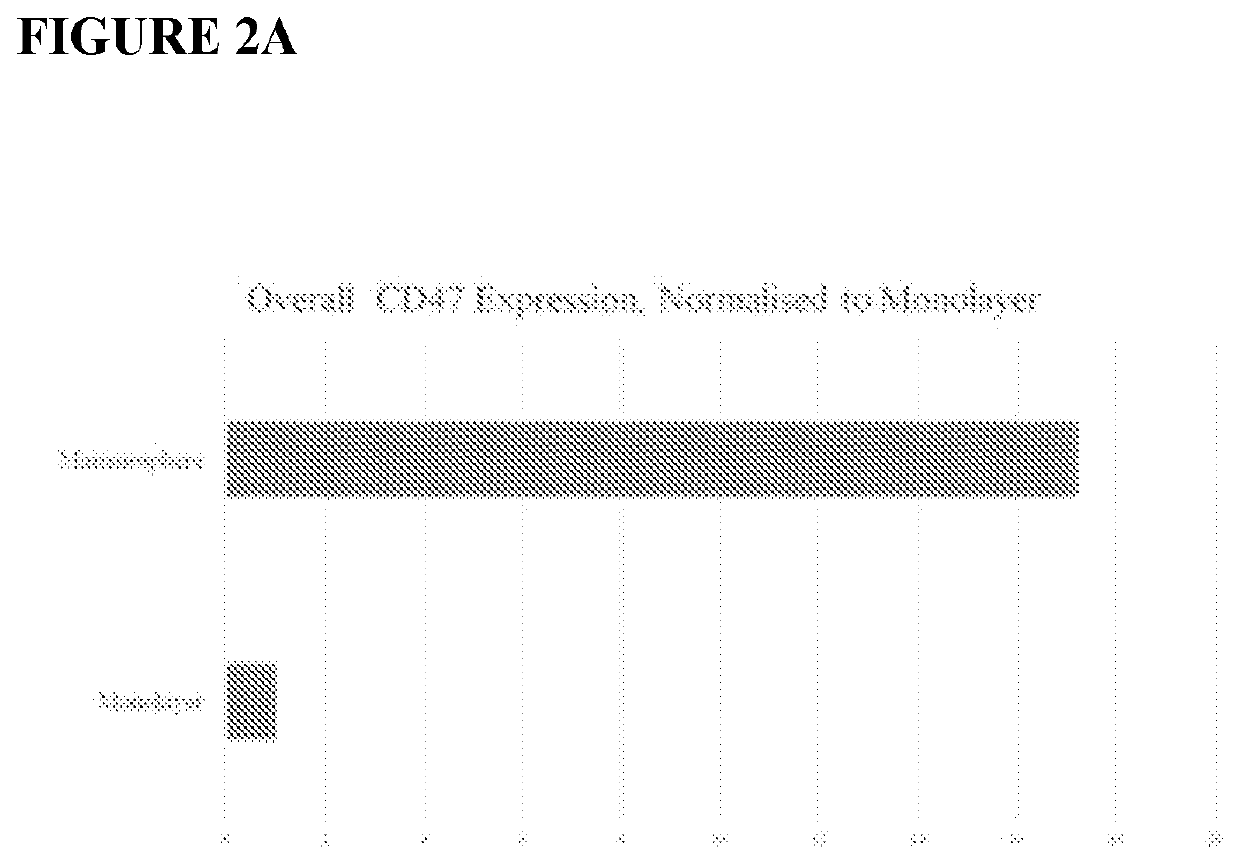
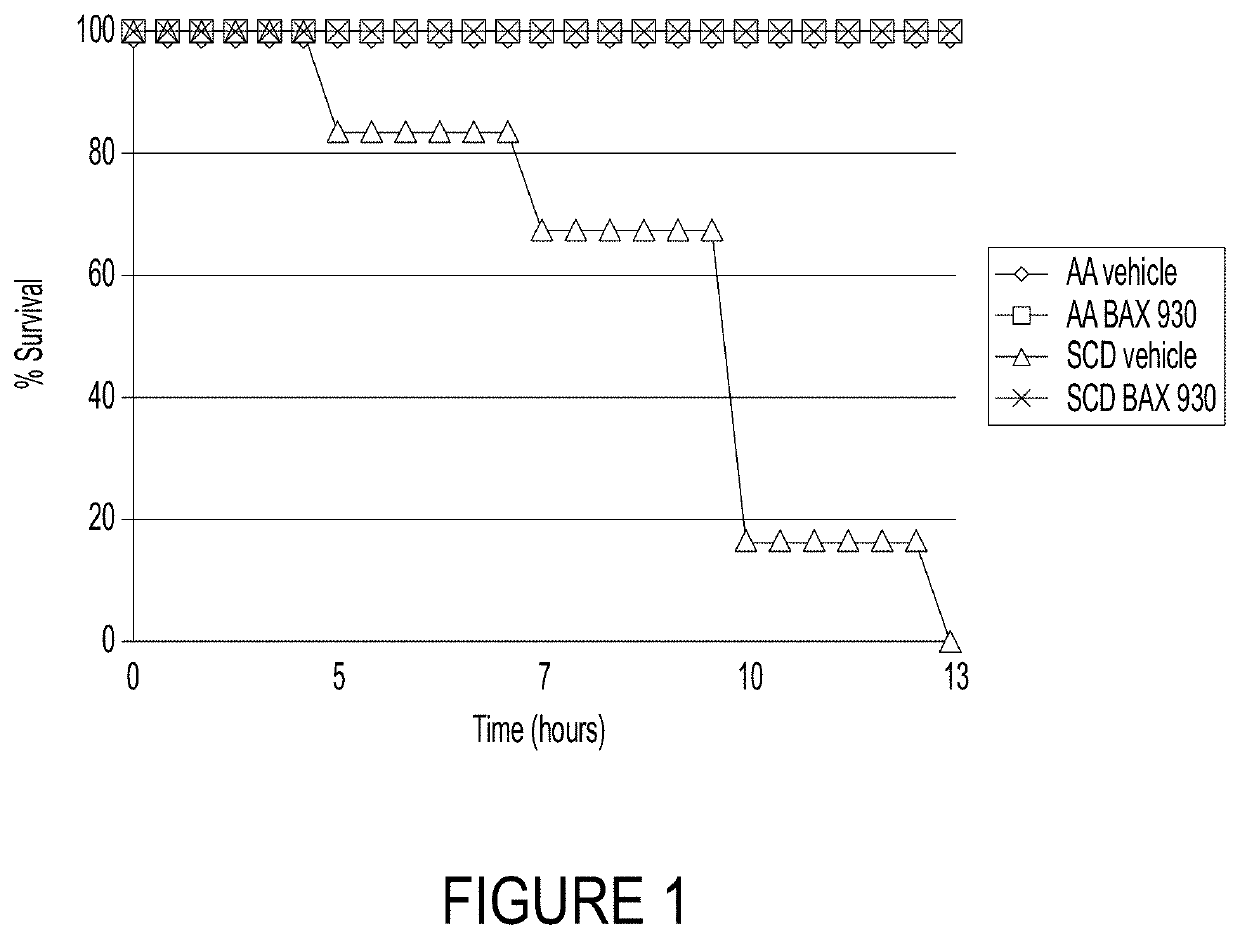
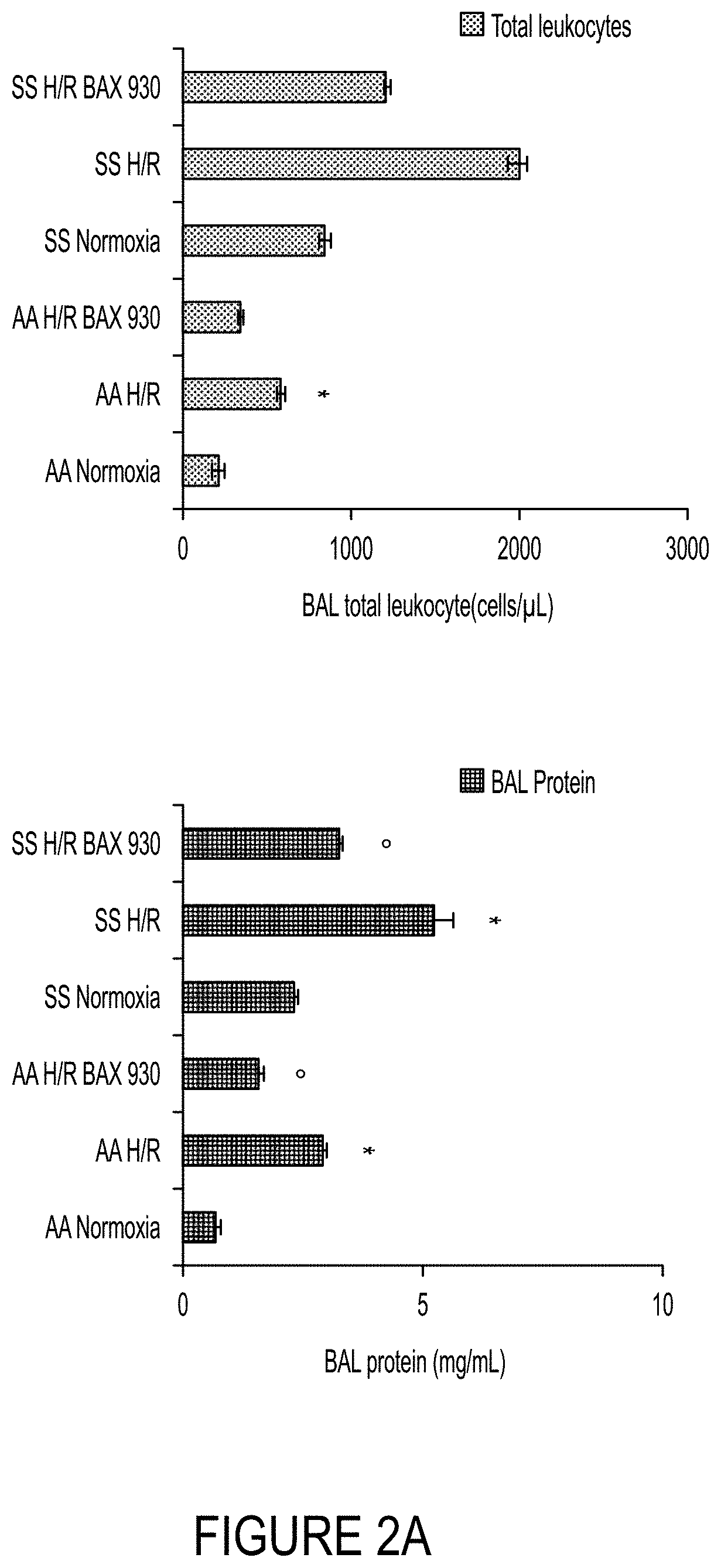
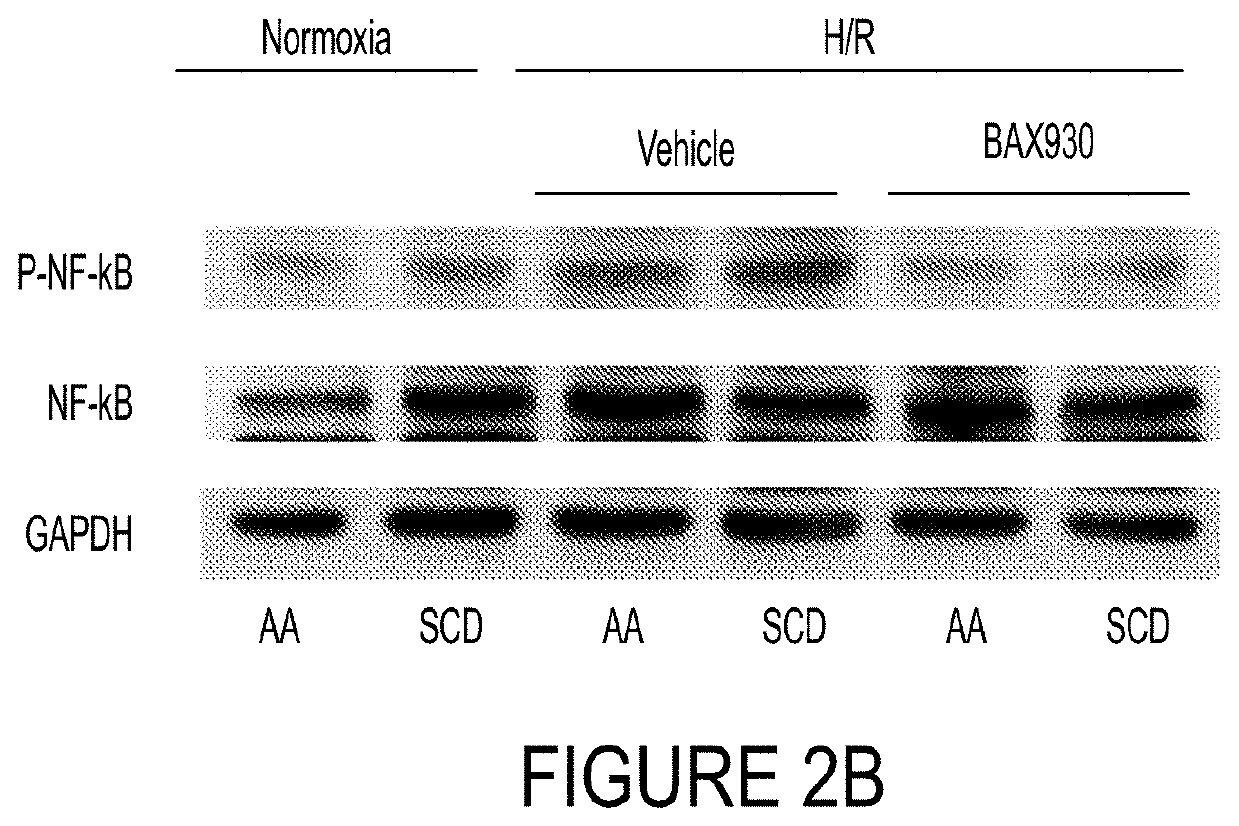
Use of ADAMTS13 for treating, ameliorating and/or preventing vaso-occlusive crisis in sickle cell disease, acute lung injury and/or acute respiratory distress syndrome
ActiveUS11191818B2Treating and ameliorating and preventing VOCReduce expressionNervous disorderPeptide/protein ingredientsPulmonary InjuryALI - Acute lung injury
Owner:TAKEDA PHARMA CO LTD
Thrombospondin-1 derived peptides and treatment methods
InactiveUS8546323B2Stabilize atherosclerotic-plaqueReduction tendencyPeptide/protein ingredientsAntipyreticLimb ischemiaWhole body
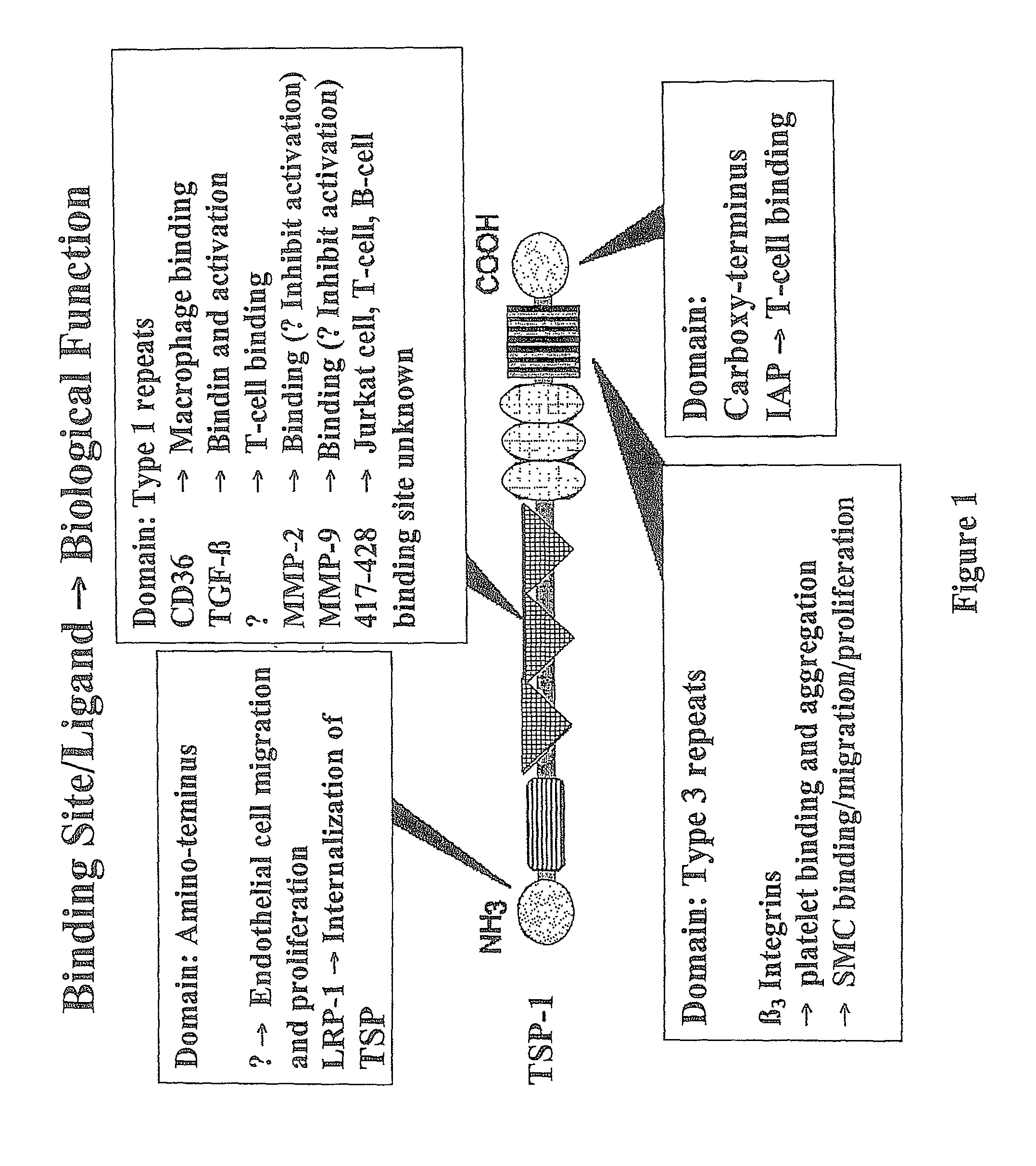
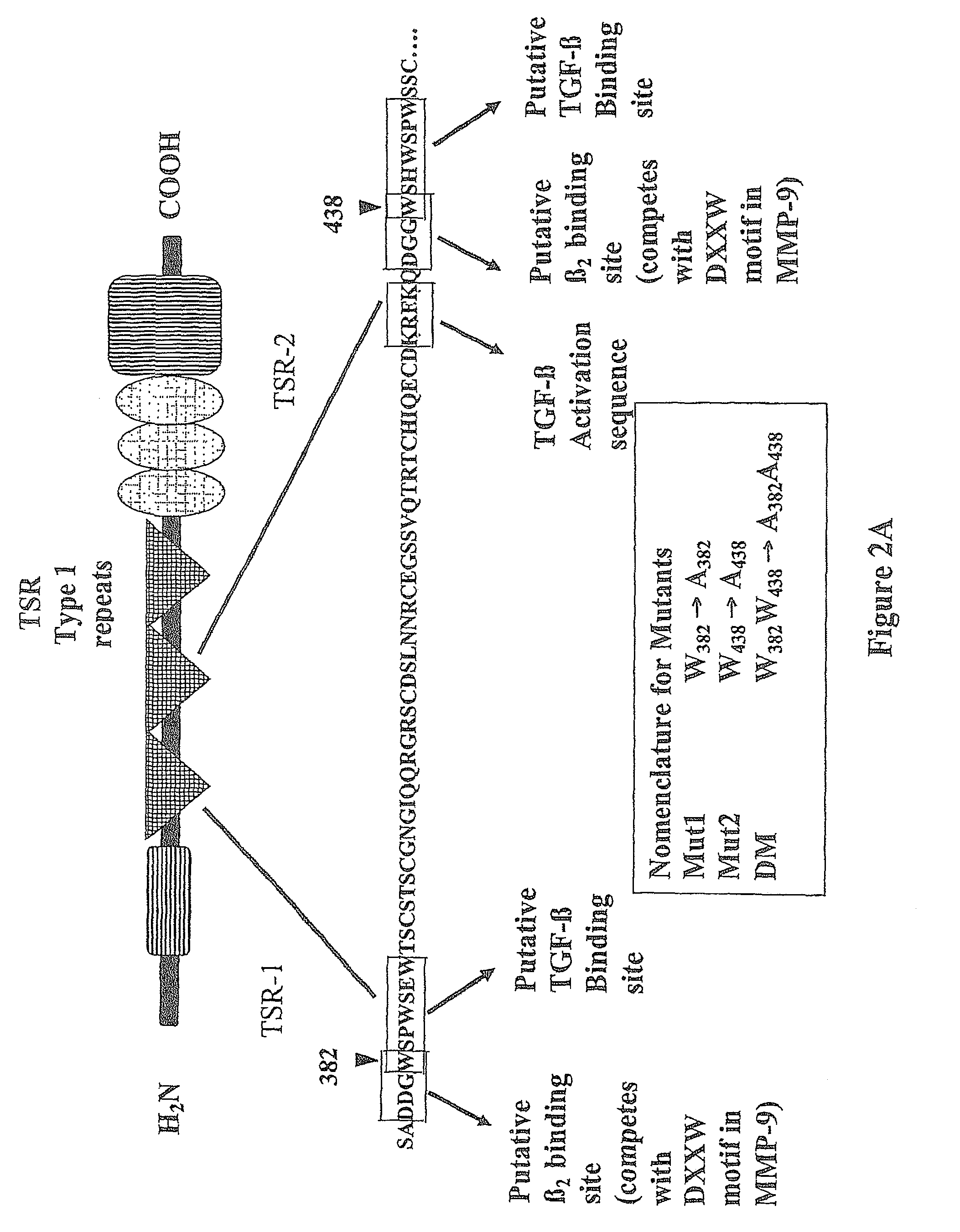
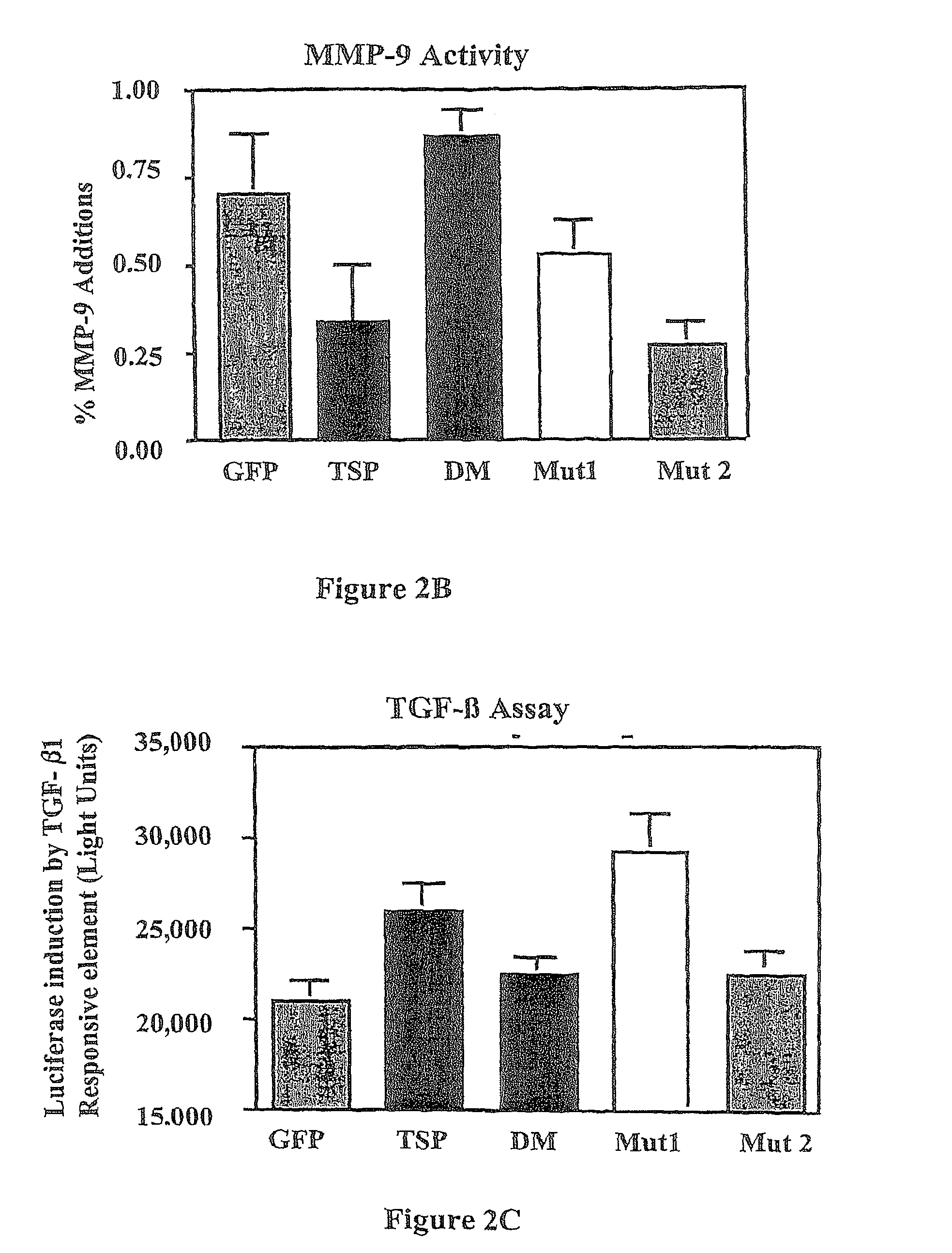
Treatments employing the matricellular protein thrombospondin-1 (TSP-I) and related compositions are disclosed for stabilizing atherosclerotic plaque and decreasing occurrence of plaque rupture events leading to, for example, myocardial infarction, stroke, and acute limb ischemia. Various peptides, including certain synthetic peptides, related to TSP-I are also disclosed. Such peptides have utility in stabilizing plaque in various contexts, including the disease states mentioned above. Some of these peptides include one or more sequences related to active sites of TSP-I for regulating, e.g., TGF-ss1 and MMP-9 activity. Experimental data show that a representative peptide provides a beneficial effect with systemic injection of the peptide.
Owner:AGAH RAMTIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com