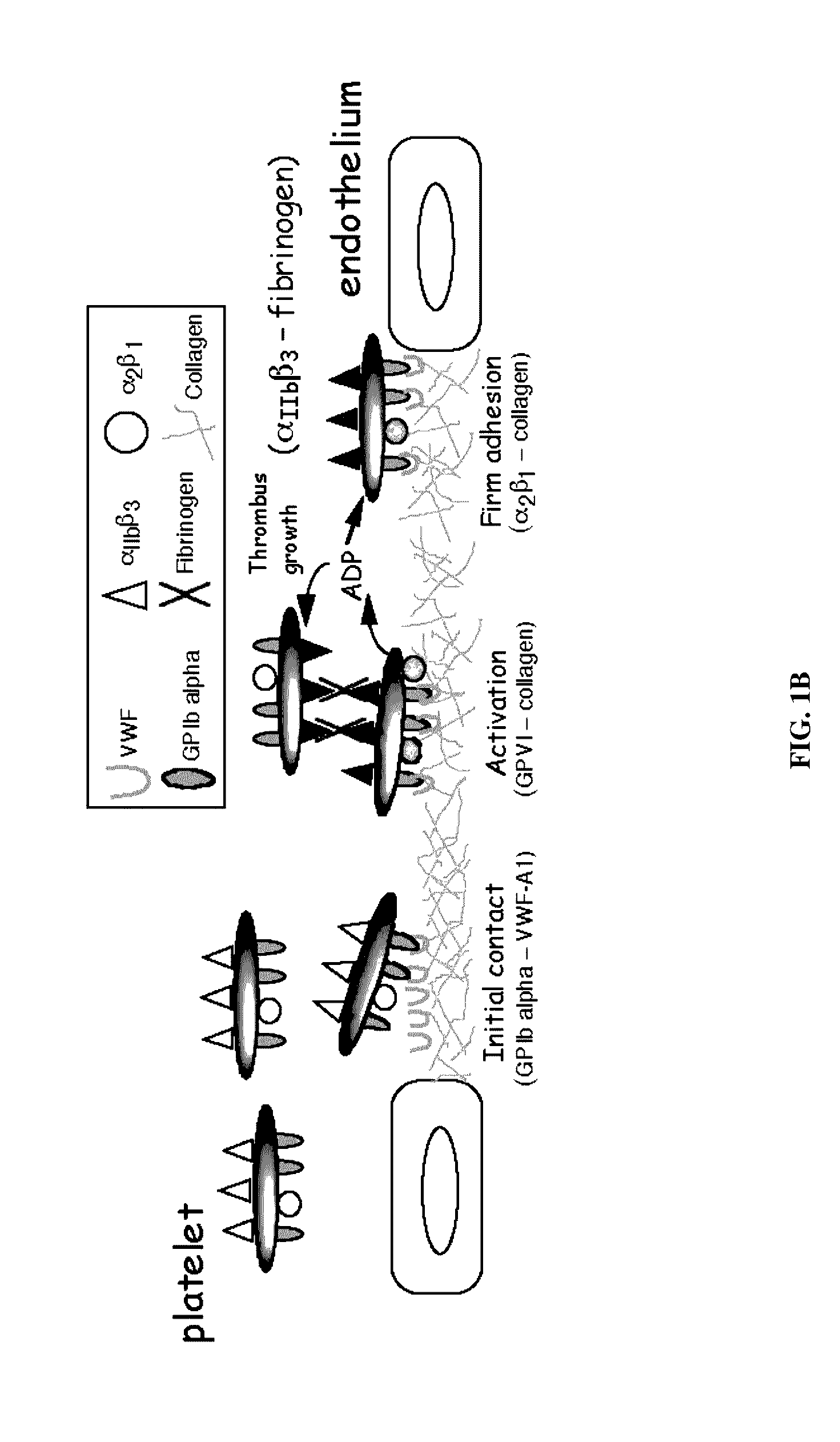
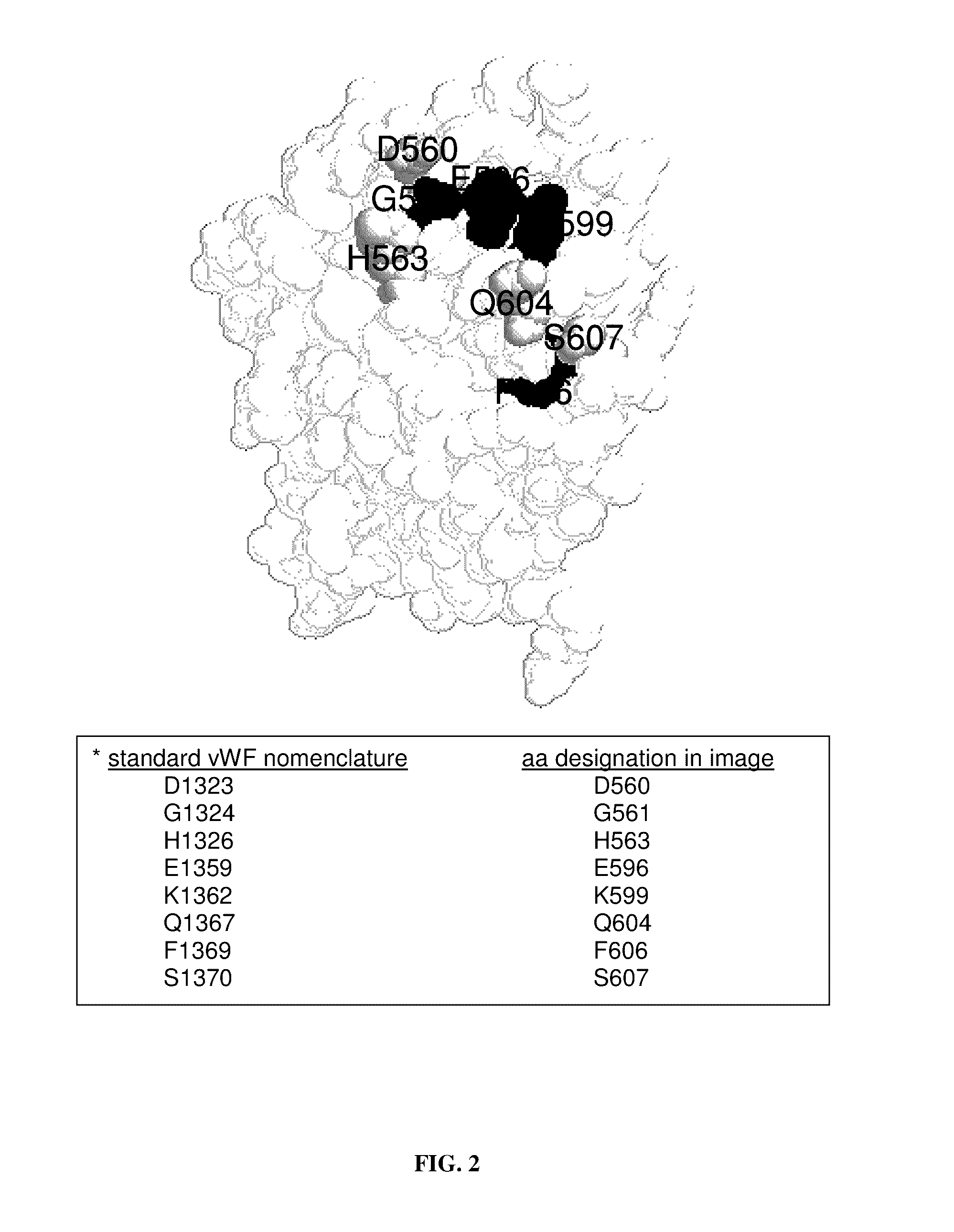
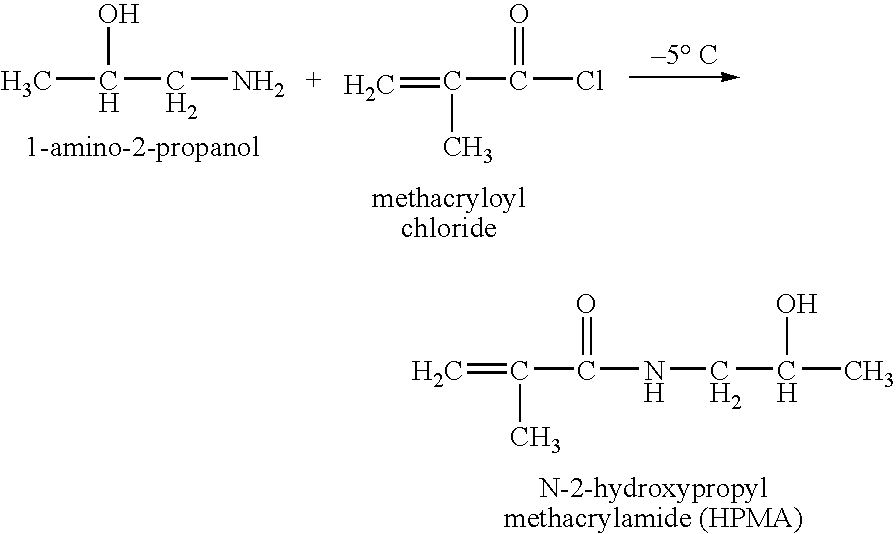Patents
Literature
47 results about "Von willebrand" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
InactiveUS20060063714A1Good storage stabilityPeptide/protein ingredientsInorganic non-active ingredientsFactor VIIaClotting factor deficiency
The invention relates to a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (e.g. human Factor VIIa) and a buffering agent; wherein the molar ratio of non-complexed calcium ions (Ca2+) to the Factor VII polypeptide is lower than 0.5. The composition may further comprise a stabilizing agent (e.g. copper or magnesium ions, benzamidine, or guanidine), a non-ionic surfactant, a tonicity modifying agent, an antioxidant and a preservative. The composition is useful for treating a Factor VII-responsive syndrome, such as bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor XI deficiency, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
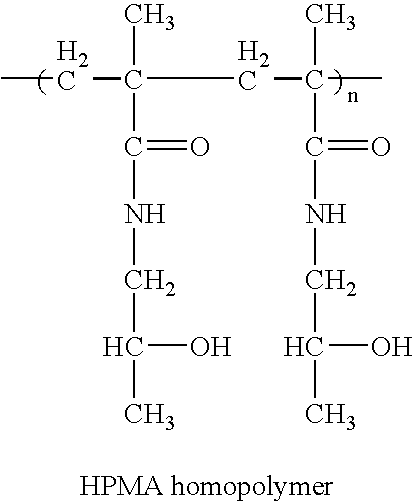
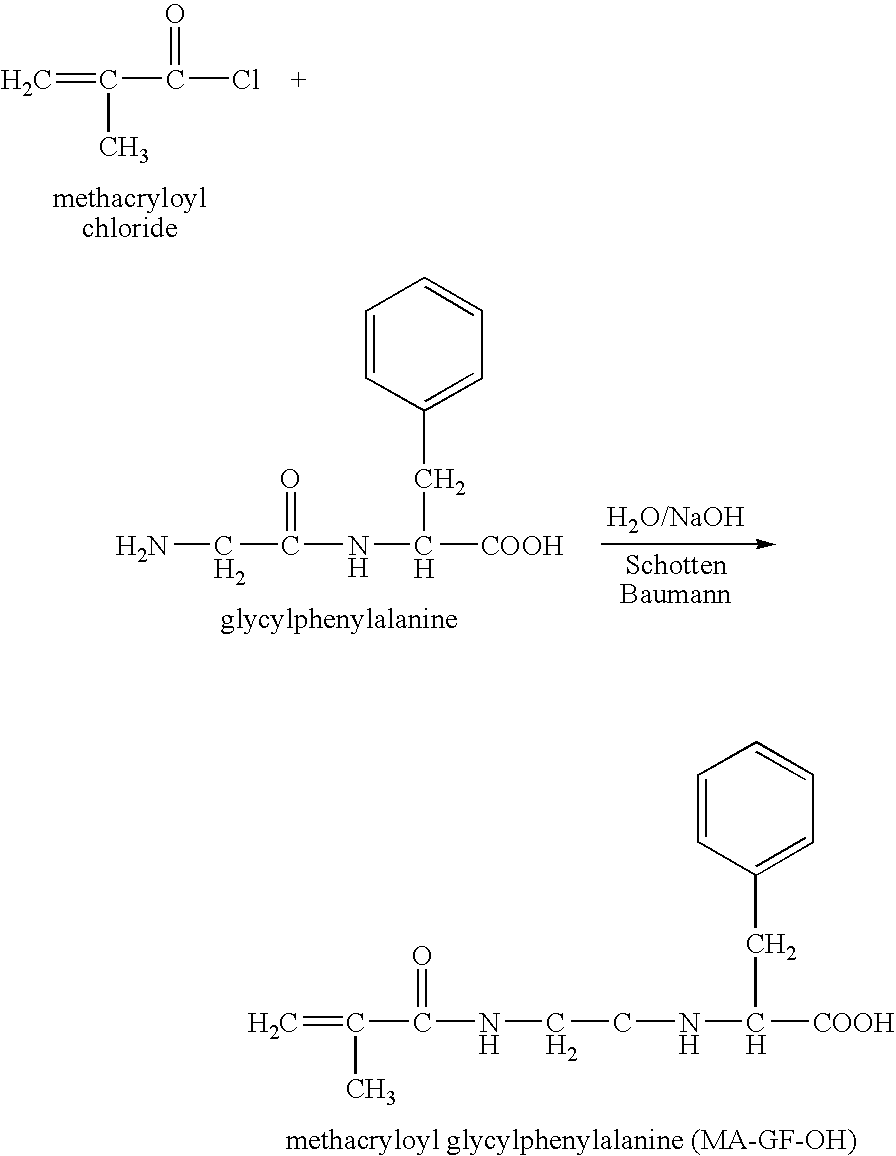
Hpma-polyamine conjugates and uses therefore
InactiveUS20060014695A1Prevent proliferationStimulate immune responsePeptide/protein ingredientsGenetic material ingredientsSomatostatin analogTreatment effect
The inventions provide compositions and methods for nucleic acid delivery comprising IIPMA conjugated to a polyamine. These compositions have the benefit of the steric hindrance of HPMA and the nucleic acid binding capability of a polyamine. Useful polyamines for this purpose include spermine, spermidine and their analogues, and DFMO. These polyamines have the ability not only to bind nucleic acids, but also have anti-cancer effects themselves. The compounds provided can also include ligand binding domains, such as vascular endothelial growth factors, somatostatin and somatostatin analogs, transferring, melanotropin, ApoE and ApoE peptides, von Willebrand's factor and von Willebrand's factor peptides, adenoviral fiber protein and adenoviral fiber protein peptides, PD 1 and PD 1 peptides, EGF and EGF peptides, RGD peptides, CCK peptides, antibody and antibody fragments, folate, pyridoxyl and sialyl-LewisX and chemical analogs. Methods for using these compositions to achieve a therapeutic effect, including for vaccination, are also provided.
Owner:UNIV OF MARYLAND
Kunitz domain polypeptide zkun10
Proteinase inhibitors comprising a Kunitz domain are disclosed. The Kunitz domain comprises a motif of amino acid residues as shown in SEQ ID NO:4, and the sequence of the Kunitz domain is shown in residues 57 through 107 of SEQ ID NO:2. The polypeptide also includes an N-terminal collagen domain in which a von Willebrand domain resides, and is shown in SEQ ID NO: 5. Also disclosed are methods for making the proteinase inhibitors, and expression vectors and cultured cells that are useful within the methods. The proteinase inhibitors may be used as components of cell culture media, in protein purification, and as inhibitors of protease degradation of plasma proteins.
Owner:ZYMOGENETICS INC
Antibodies that bind to a portion of the VWC domain of connective tissue growth factor
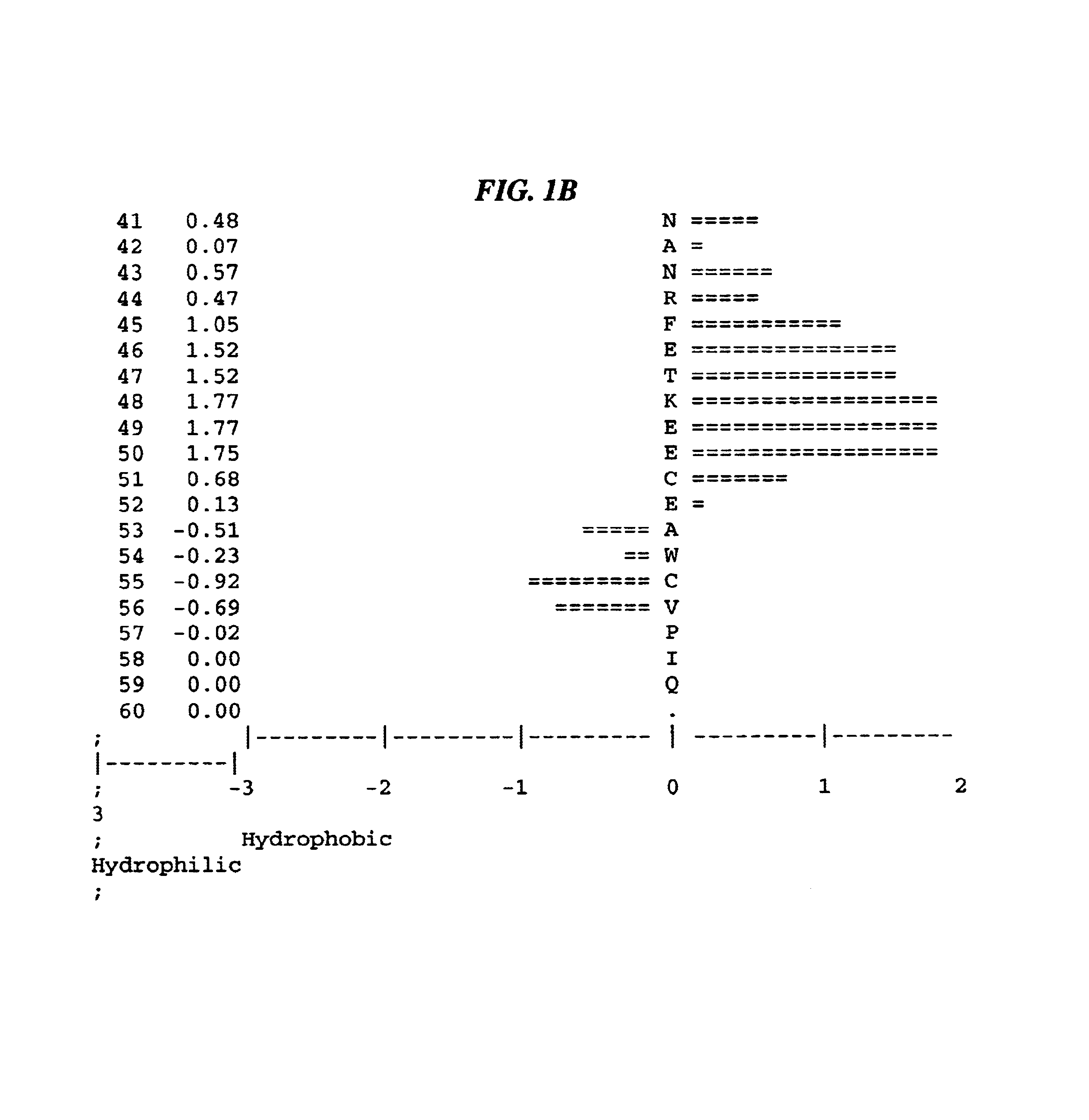
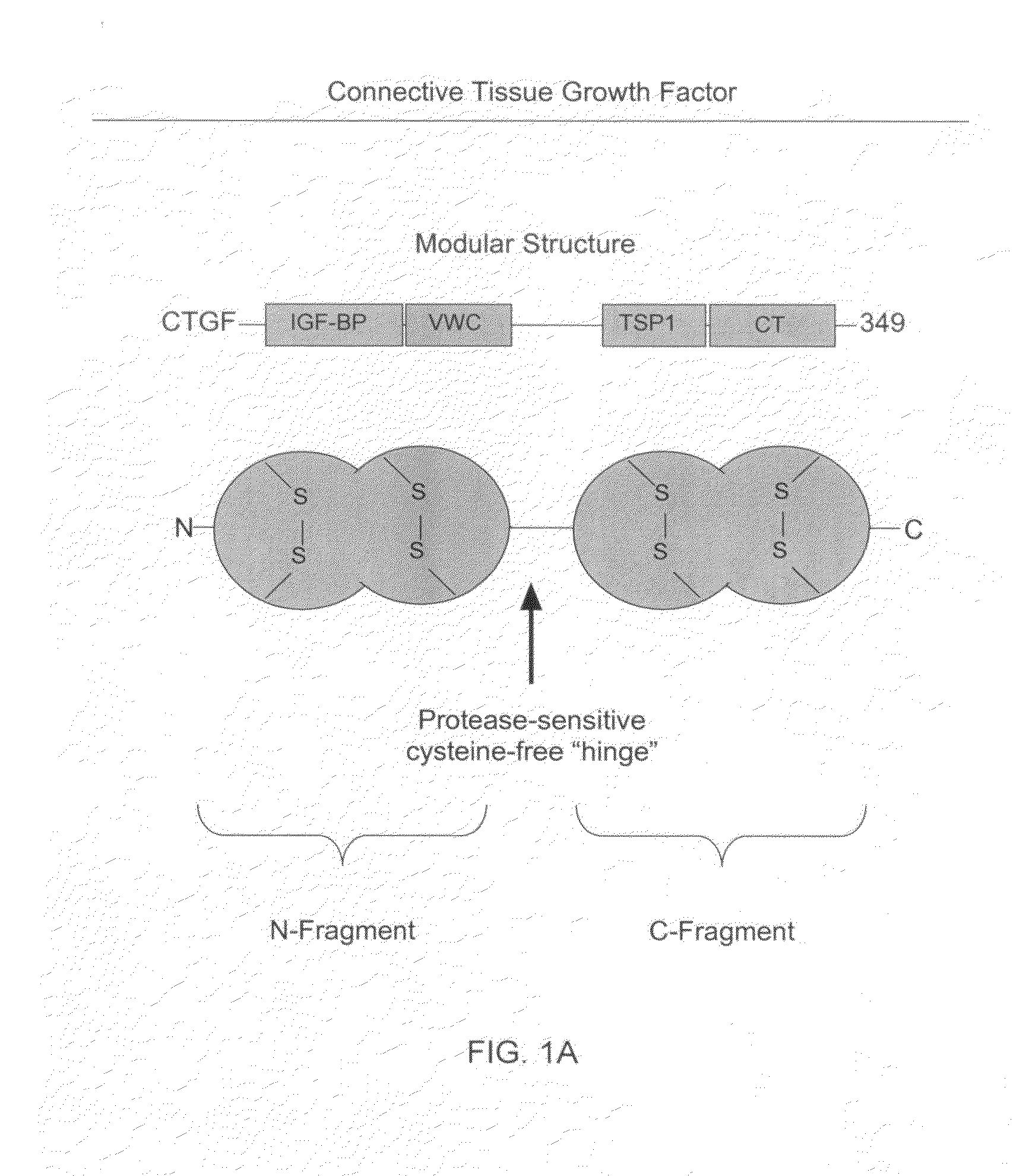
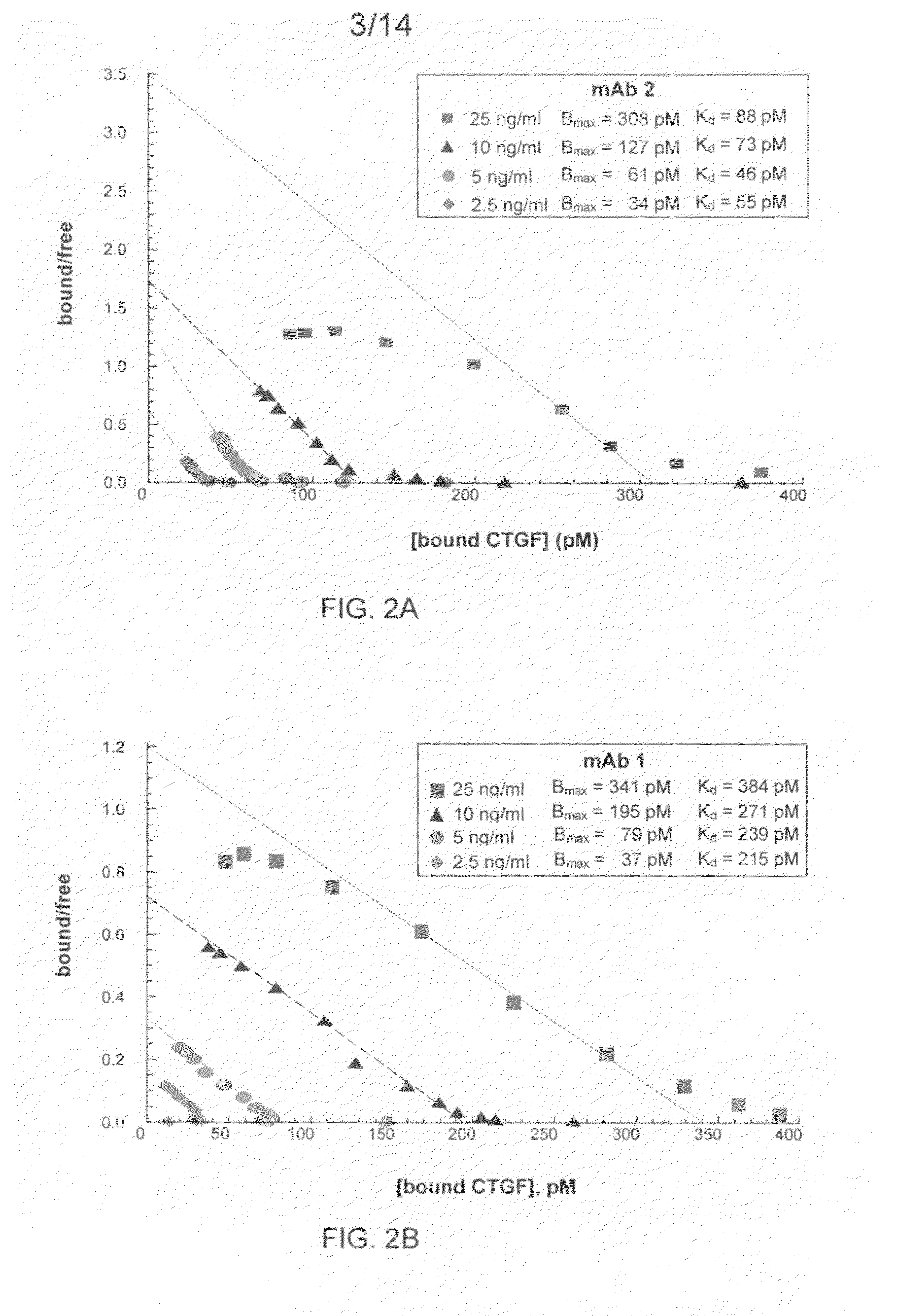
The present invention relates to antibodies that bind to a portion of the Von Willebrand's factor (VWC) domain of connective tissue growth factor (CTGF). The antibodies are particularly directed to regions of CTGF involved in biological activities associated with fibrosis. The invention also relates to methods of using the antibodies to treat disorders associated with CTGF including localized and systemic fibrotic disorders including those of the lung, liver, heart, skin, and kidney.
Owner:FIBROGEN INC
Treatment of coagulation disease by administration of recombinant vwf
ActiveUS20120316116A1Extended half-lifeFactor VIIPeptide/protein ingredientsFactor VIII vWFVon willebrand
The present invention provides methods of treating coagulation disease, including hemophilia and von Willebrand disease by administering recombinant von Willebrand Factor alone or in combination with Factor VIII.
Owner:TAKEDA PHARMA CO LTD
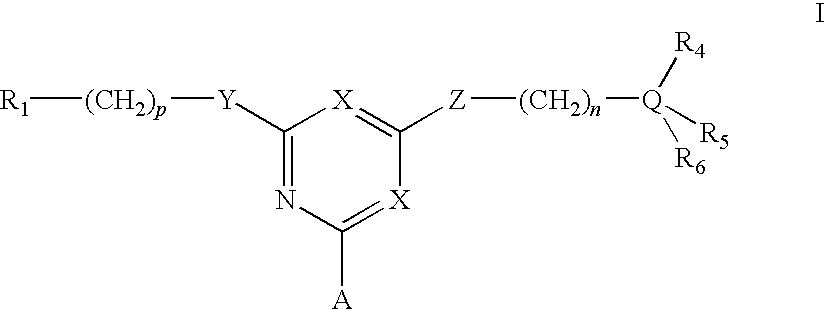
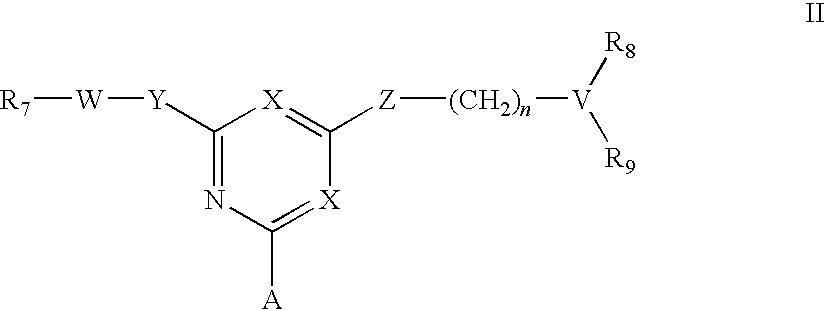
Affinity adsorbents for Factor VIII and von Willebrand's Factor
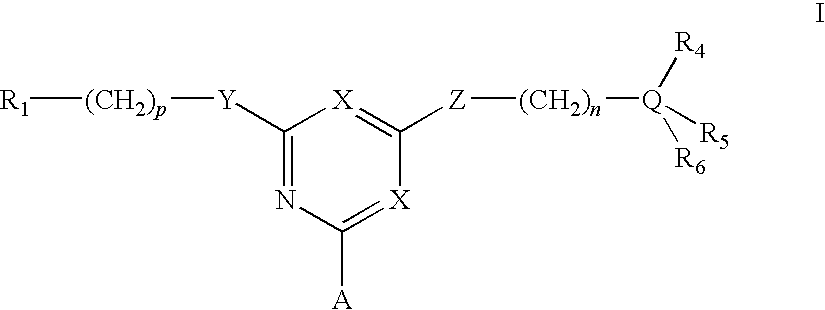
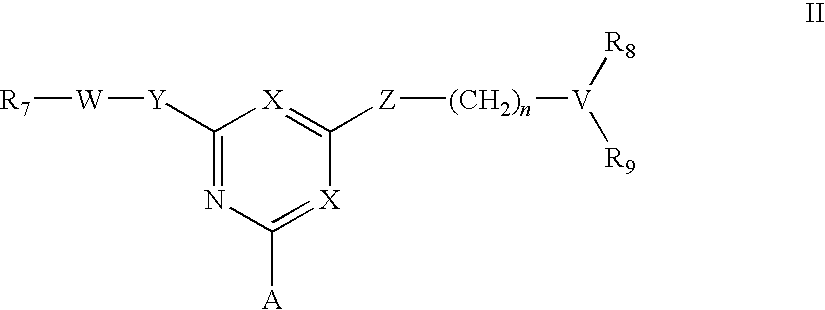
For the separation, removal, isolation, purification, characterization, identification or quantification of Factor VIII, von Willebrand's Factor or a protein that is a analogue of either, an affinity adsorbent is used that is a compound of formula (II) wherein one X is N and the other is N, C—Cl or C—CN; A is a support matrix, optionally linked to the triazine ring by a spacer; Y is O, S or NR2; Z is O, S or N—R3; R2 and R3 are each H, C1-6 alkyl, C1-6 hydroxyalkyl, benzyl or &bgr;-phenylethyl; B and W are each an optionally substituted hydrocarbon linkage containing from 1 to 10 carbon atoms; D is H, OH or a primary amino, secondary amino, tertiary amino, quaternary ammonium, imidazole, guanidino or amidino group; or B-D is —CHCOOH—(CH2)3-4—NH2; and R7 is a group bearing a positive charge at neutral pH.
Owner:PROMETIC BIOSCIENCES LTD
Treatment of medical condition with a2 domain of von willebrand factor
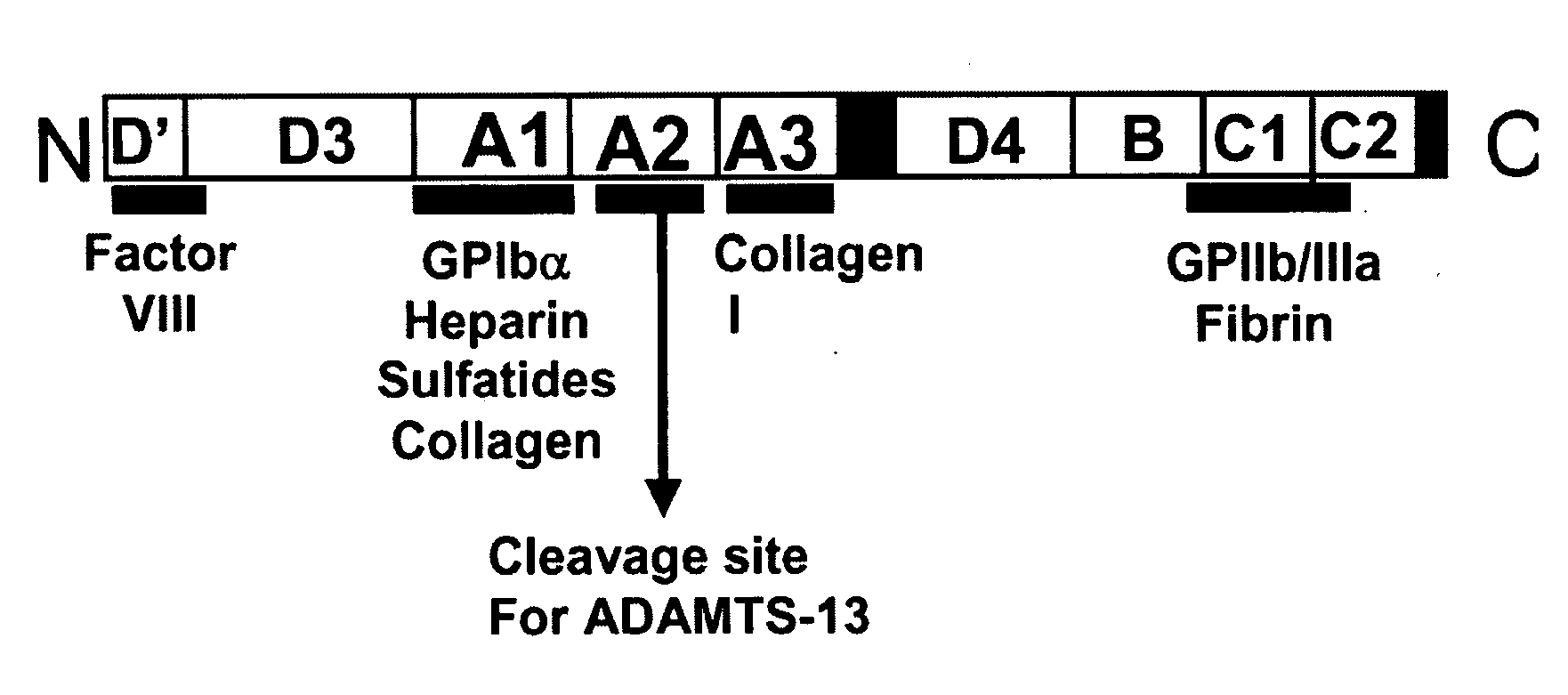
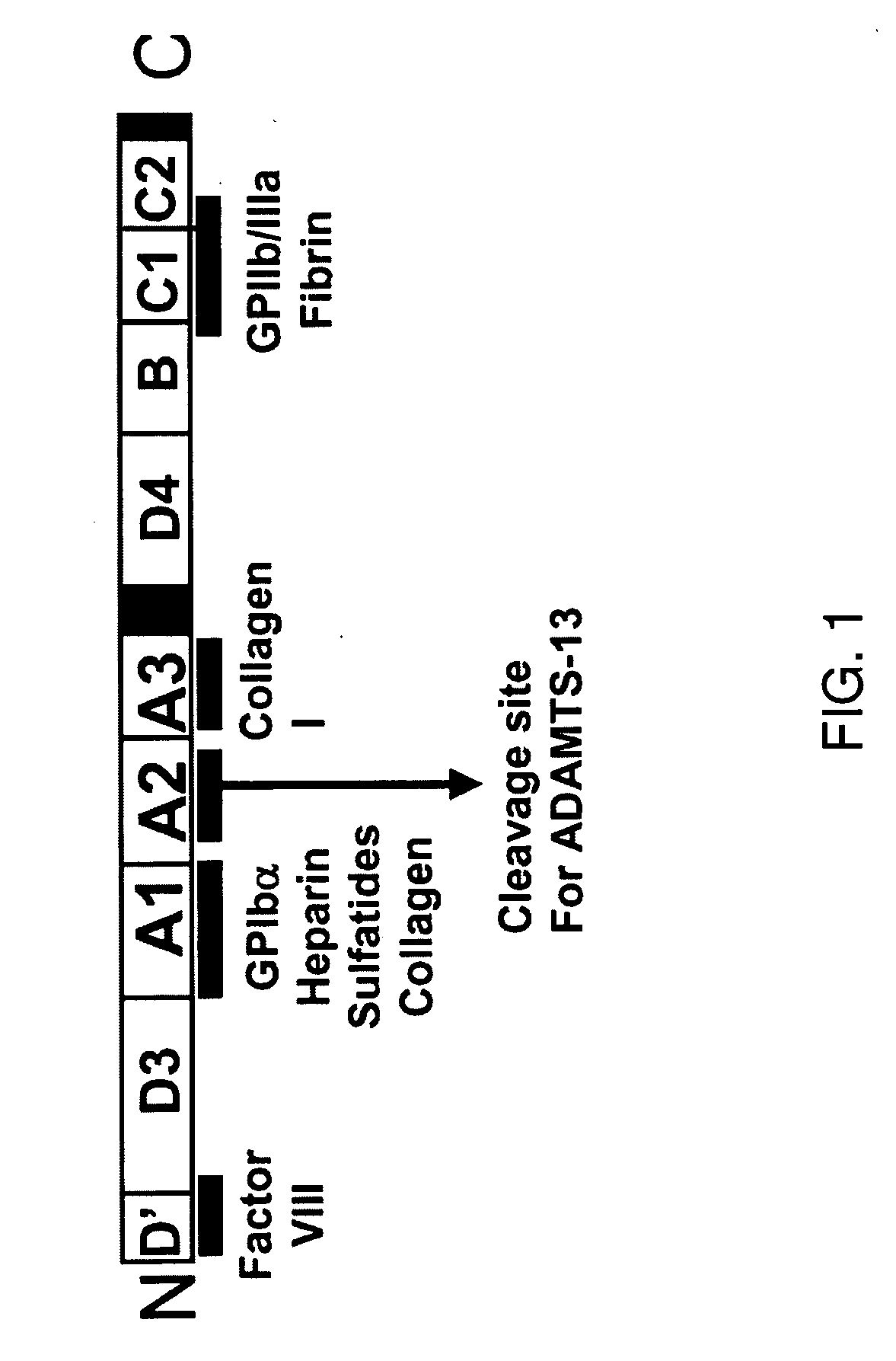
ActiveUS20090118161A1Reduce complicationsAvoid deathPeptide/protein ingredientsMicrobiological testing/measurementFactor VIII vWFFactor ii
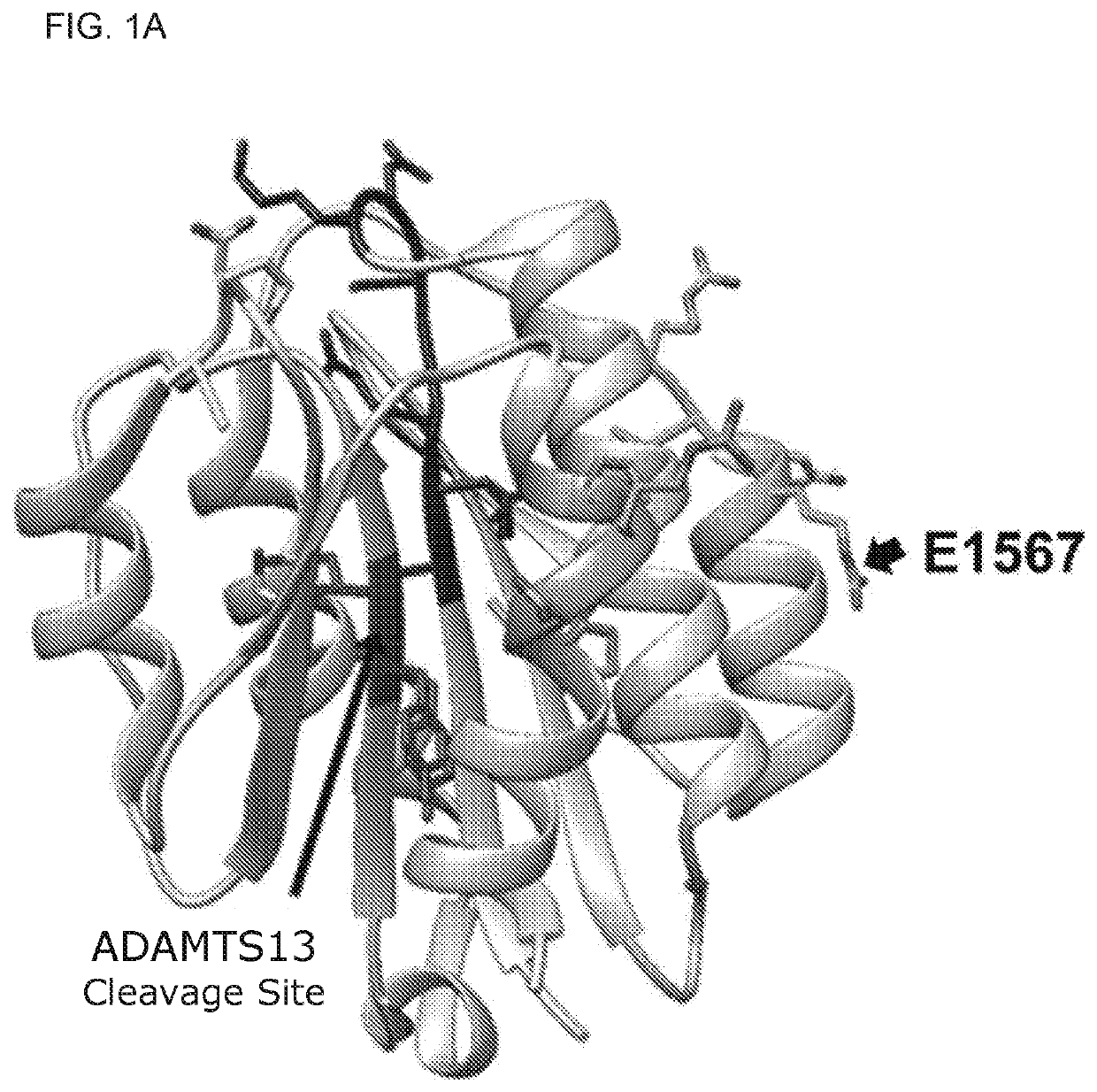
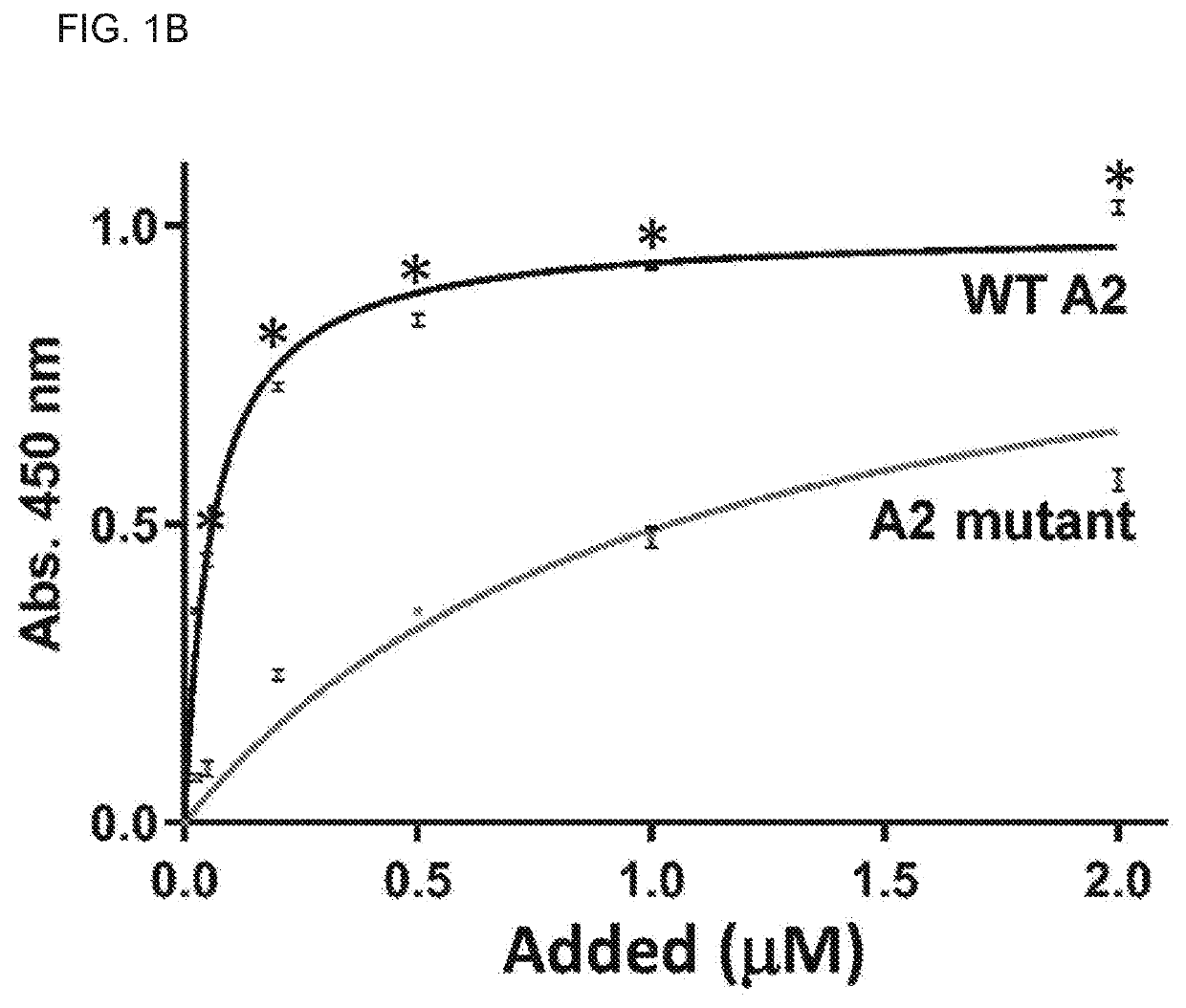
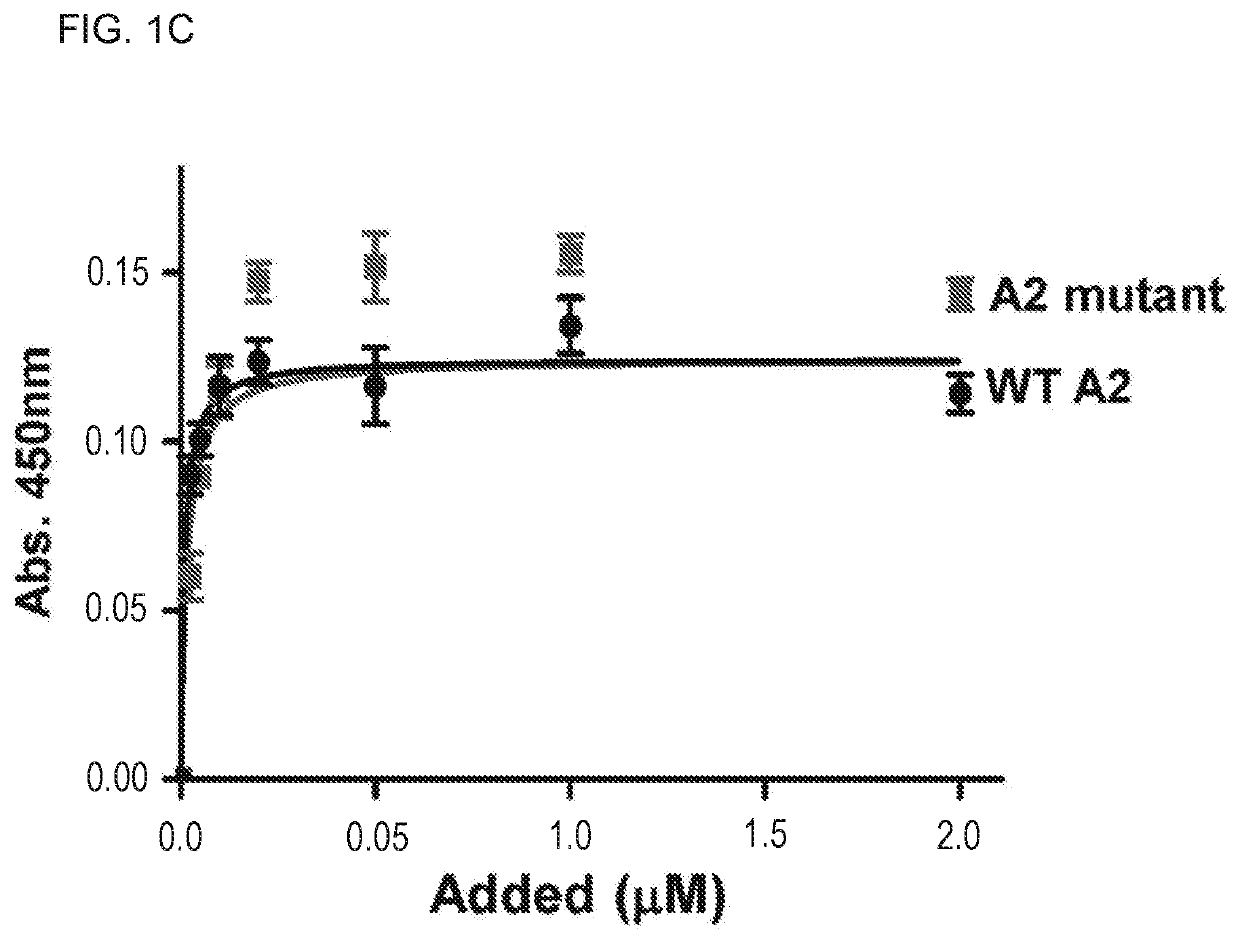
The present invention is directed to methods for the prevention, treatment and / or diagnosis of a medical condition, such as sepsis, systemic inflammatory reaction syndrome, and / or thrombosis, for example. In particular, the method employs part or all of the A2 domain of von Willebrand factor. In certain cases, a recombinant A2 domain is utilized for the treatment of sepsis, systemic inflammatory reaction syndrome, and / or thrombosis, for example.
Owner:BAYLOR COLLEGE OF MEDICINE
Von willebrand factor specific binders and methods of use therefor
InactiveUS20110158996A1Organic active ingredientsPeptide/protein ingredientsDosing regimenVon willebrand
The invention provides new uses for specific binders to the Al domain of the von Willebrand Factor (vWF), in particular the use in patients with stable angina undergoing elective percutaneous coronary intervention. Furthermore, dosing schedules and use of suitable assays such as RIPA and RICO in the particular disease settings are provided.
Owner:ABLYNX NV
Liquid, aqueous pharmaceutical composition of Factor VII polypeptides
InactiveUS20060166882A1Improve stabilityHeavy metal active ingredientsBiocideClotting factor deficiencyOxidation state
Owner:NOVO NORDISK HEALTH CARE AG
Use of plasma proteins concentrates containing VWF with a high proportion of high molecular weight multimers
ActiveUS7335634B2Prevents a bleeding diathesisReduces pre-, peri- and postoperative blood lossBiocidePeptide/protein ingredientsExtracorporeal circulationPostoperative Blood Loss
Use of plasma proteins concentrates containing VWF with a high proportion of high molecular weight multimers prevents a bleeding diathesis and reduces pre-, peri- and postoperative blood loss in acquired Von Willebrand syndromes such as in cardiovascular diseases requiring surgical procedures, especially those requiring extracorporeal circulation.
Owner:CSL BEHRING GMBH
Compounds suitable for treatment of haemophilia
The present invention relates to Von Willebrand (VWF) compounds as well as compositions suitable for treatment of blood clotting diseases. The present invention also relates to pharmaceutical compositions, freeze-dried or liquid, comprising (i) a Factor VIII molecule and (ii) a VWF compound.
Owner:NOVO NORDISK AS
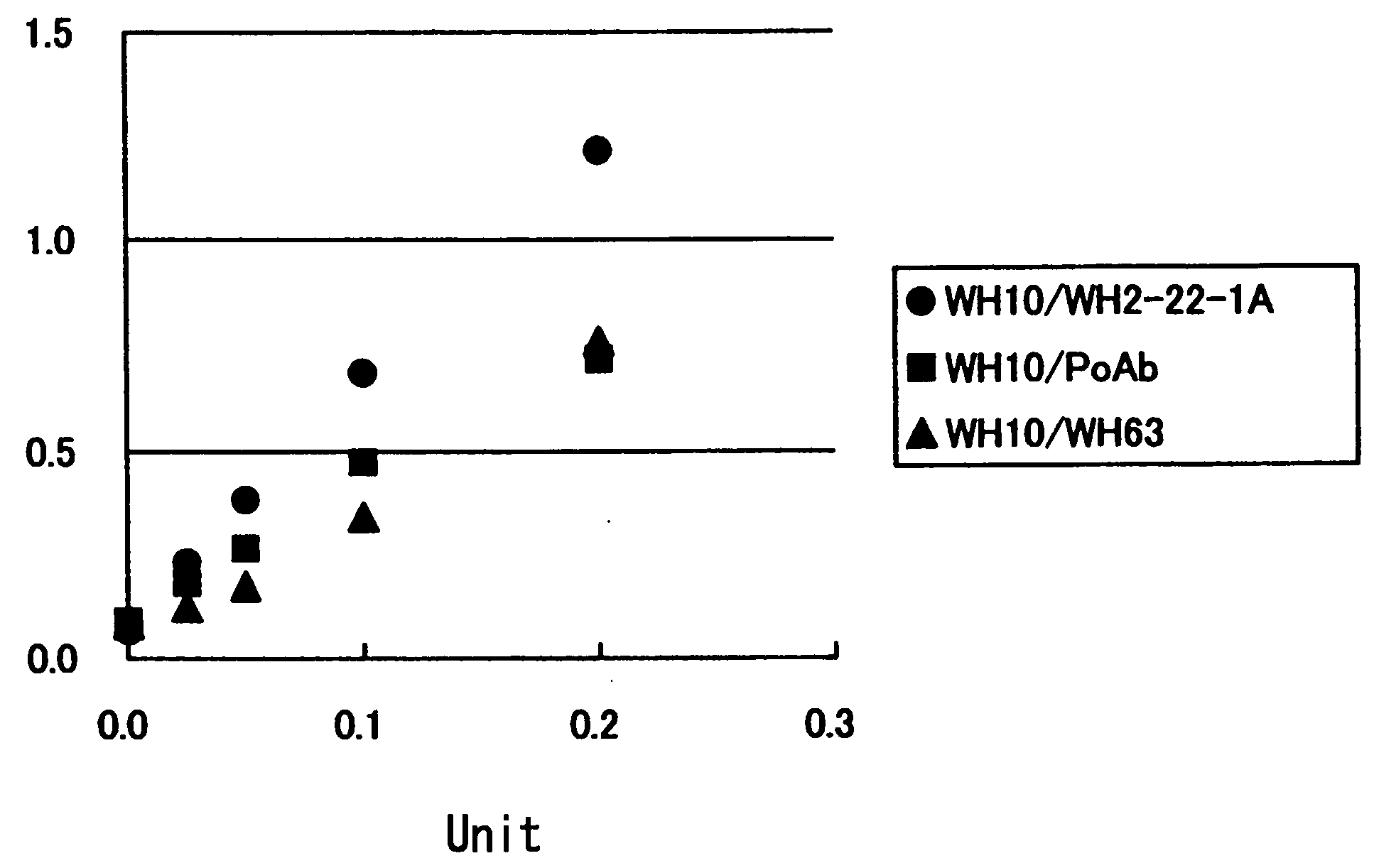
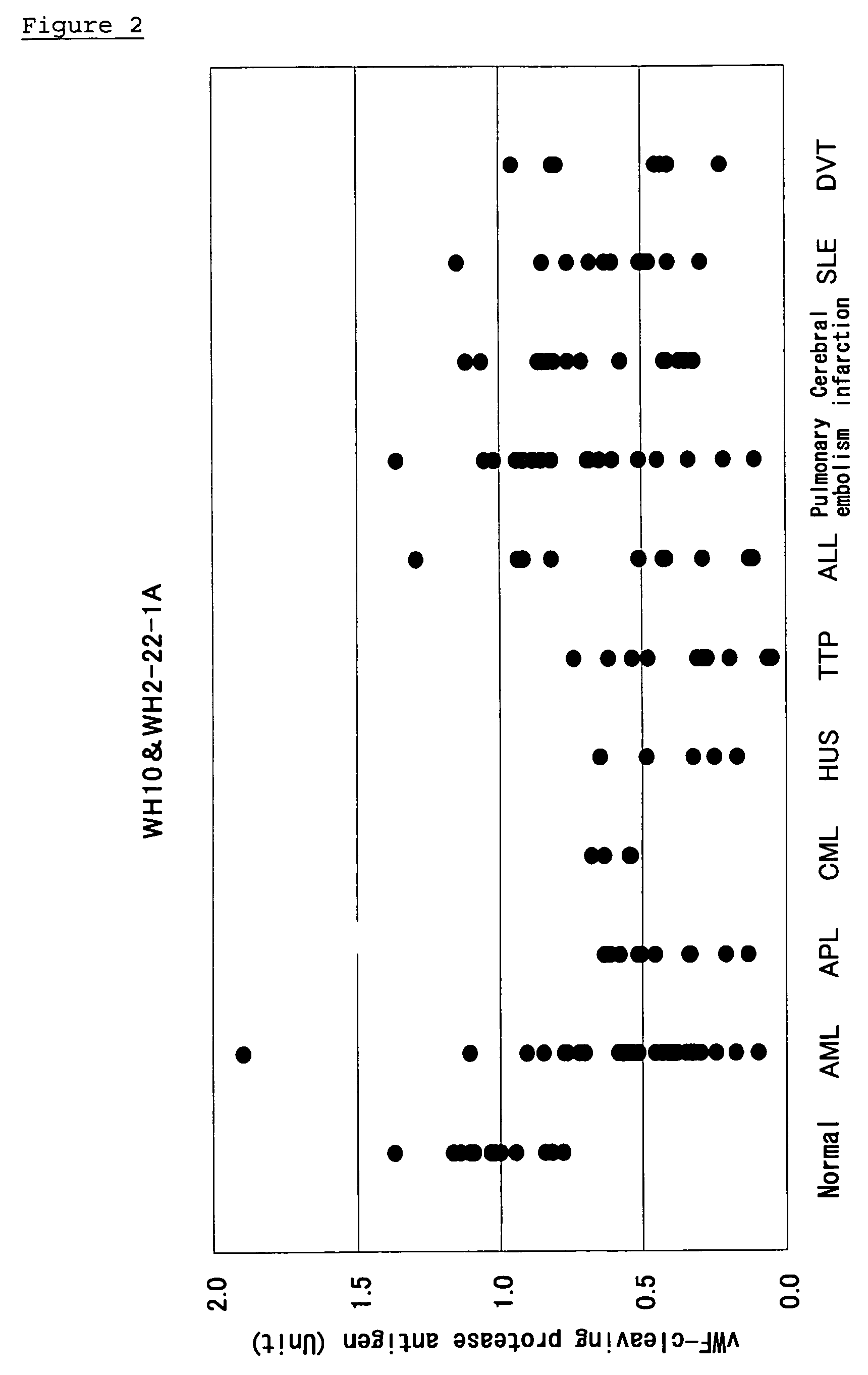
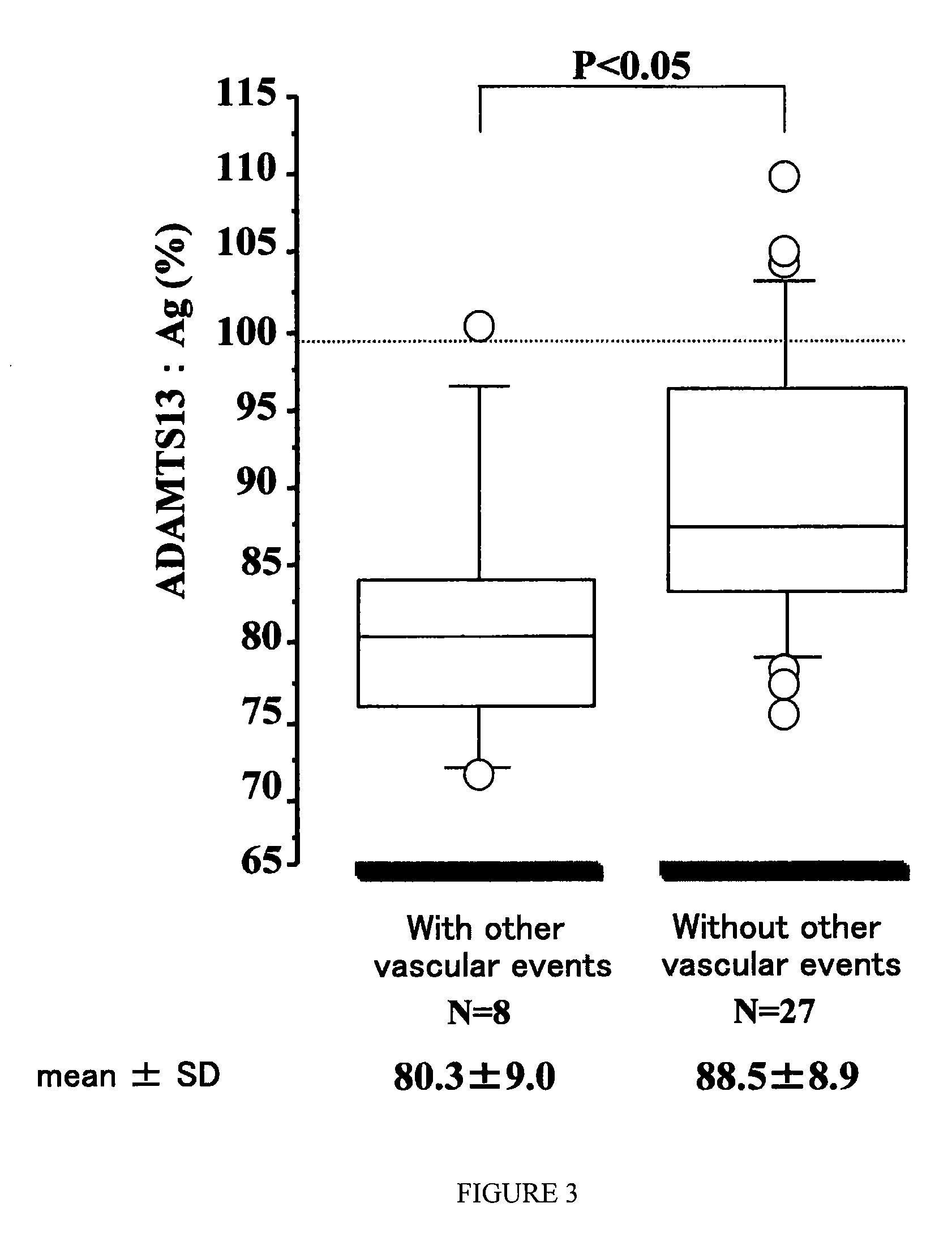
Method of Detecting Thrombosis by Measuring Von Willenbrand Factor-Cleaving Protease
ActiveUS20070275414A1Microbiological testing/measurementDisease diagnosisProteinase activityVon willebrand
A method of detecting thrombosis or the degree of thrombophilia by measuring a von Willebrand factor cleaving protease, and a kit for detecting thrombosis or the degree of thrombophilia, comprising an antibody or a fragment thereof specifically binding to a von Willebrand factor-cleaving protease, are disclosed. The detection method and the detection kit have an excellent convenience, rapidity, and specificity.
Owner:KM BIOLOGICS CO LTD
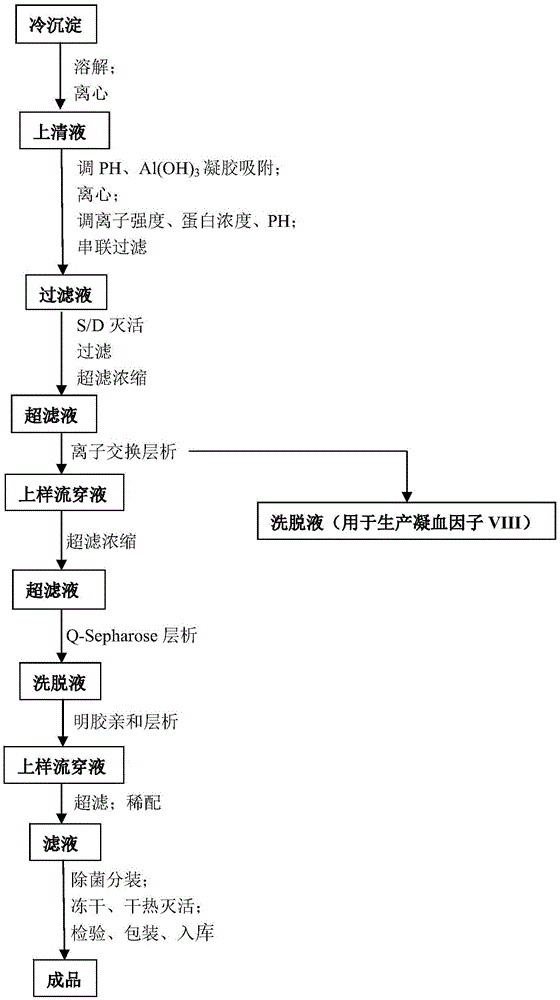
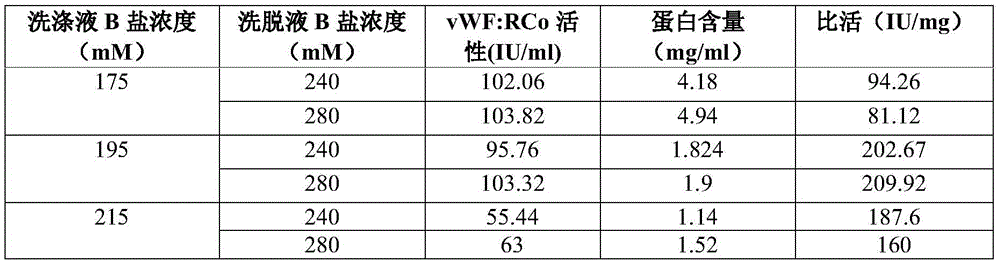
Process for the preparation of a von Willebrand (FvW) factor concentrate by chromatography and a FvW concentrate thus obtainable
InactiveUS7888476B2Increase ionic strengthQuality improvementFactor VIIApolipeptidesIonic strengthFactor VIII vWF
This invention relates to a process for the preparation of a very high purity von Willebrand factor concentrate from a biological fraction containing von Willebrand factor, including a separation by anion exchange chromatography using a vinyl polymer support of weak base type, the separation comprising the steps of loading of the chromatographic support with the fraction containing von Willebrand factor, previously equilibrated with a suitable buffer, with a predetermined flowrate allowing the retention of the von Willebrand factor, washing of the support with an acidic buffer with a flowrate higher than the flowrate of the step a) until the not-retained proteins and the contaminants are removed, flushing and equilibrating of the chromatographic support with the buffer and using the flowrate of the step a), and elution of the von Willebrand factor by increasing of the ionic strength of the step c). The invention also relates to a von Willebrand factor concentrate for therapeutic use likely to be obtained by implementing the process wherein the rate of Factor VIII:C / FvW:RCo is less than 0.06%.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
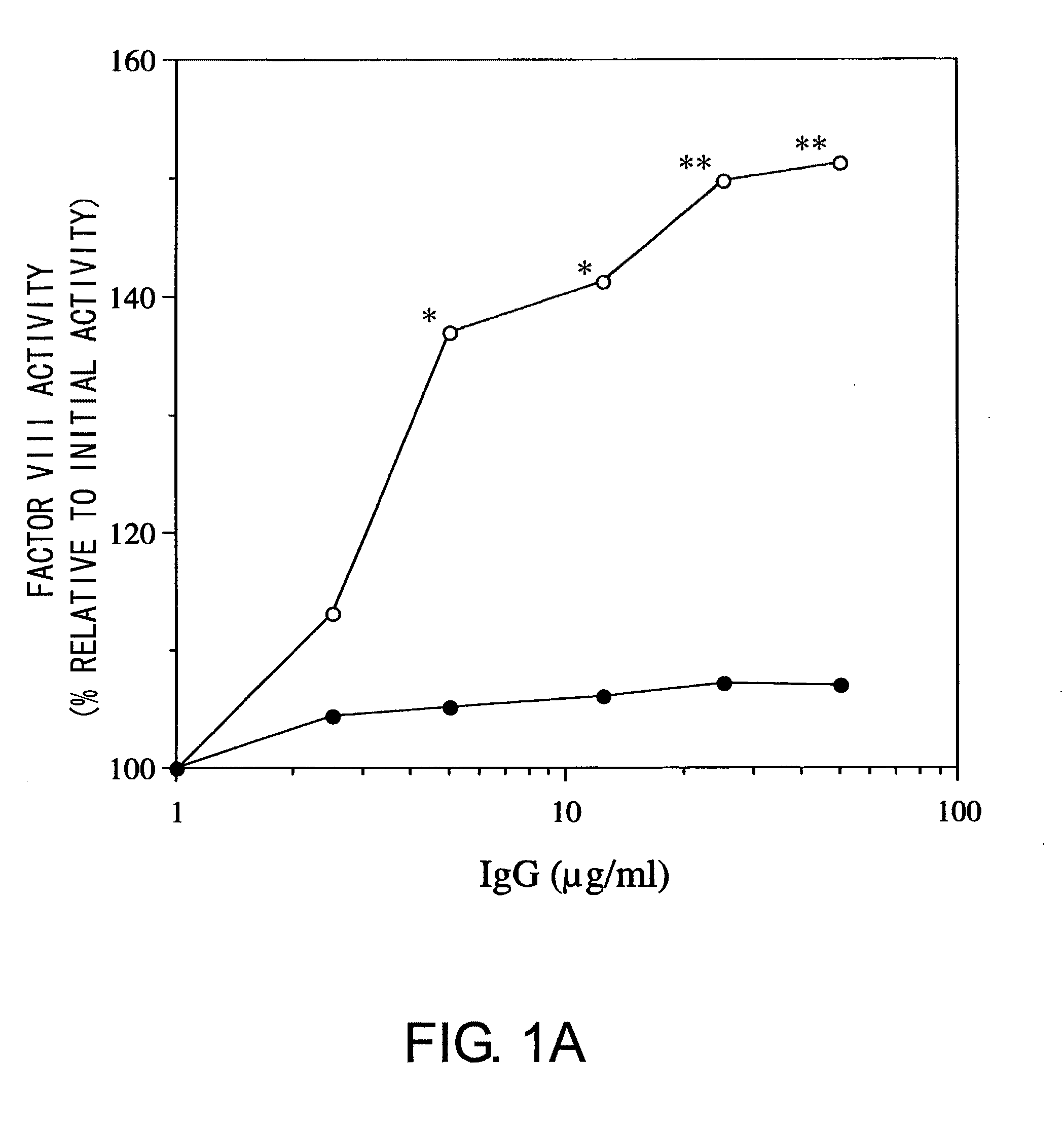
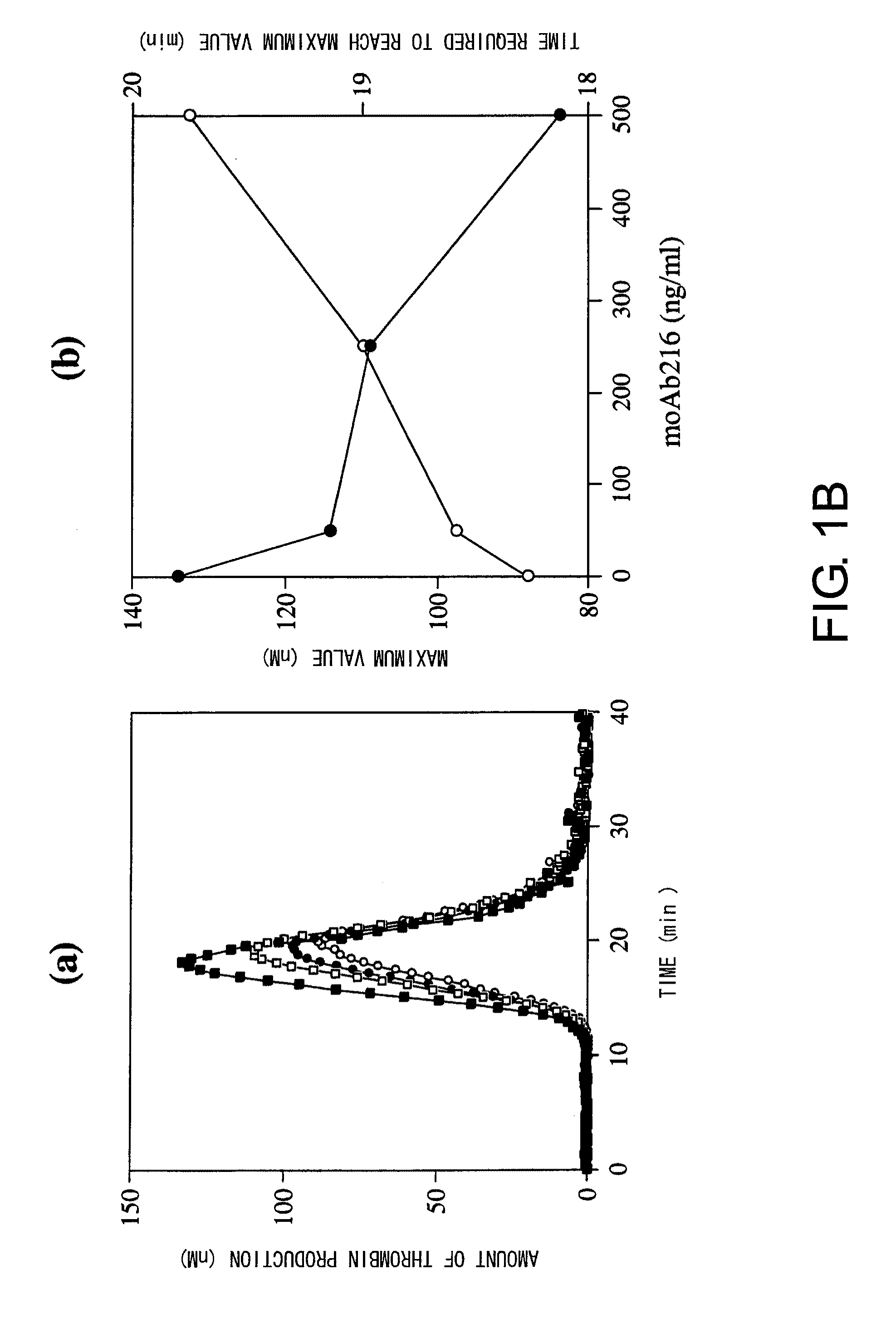
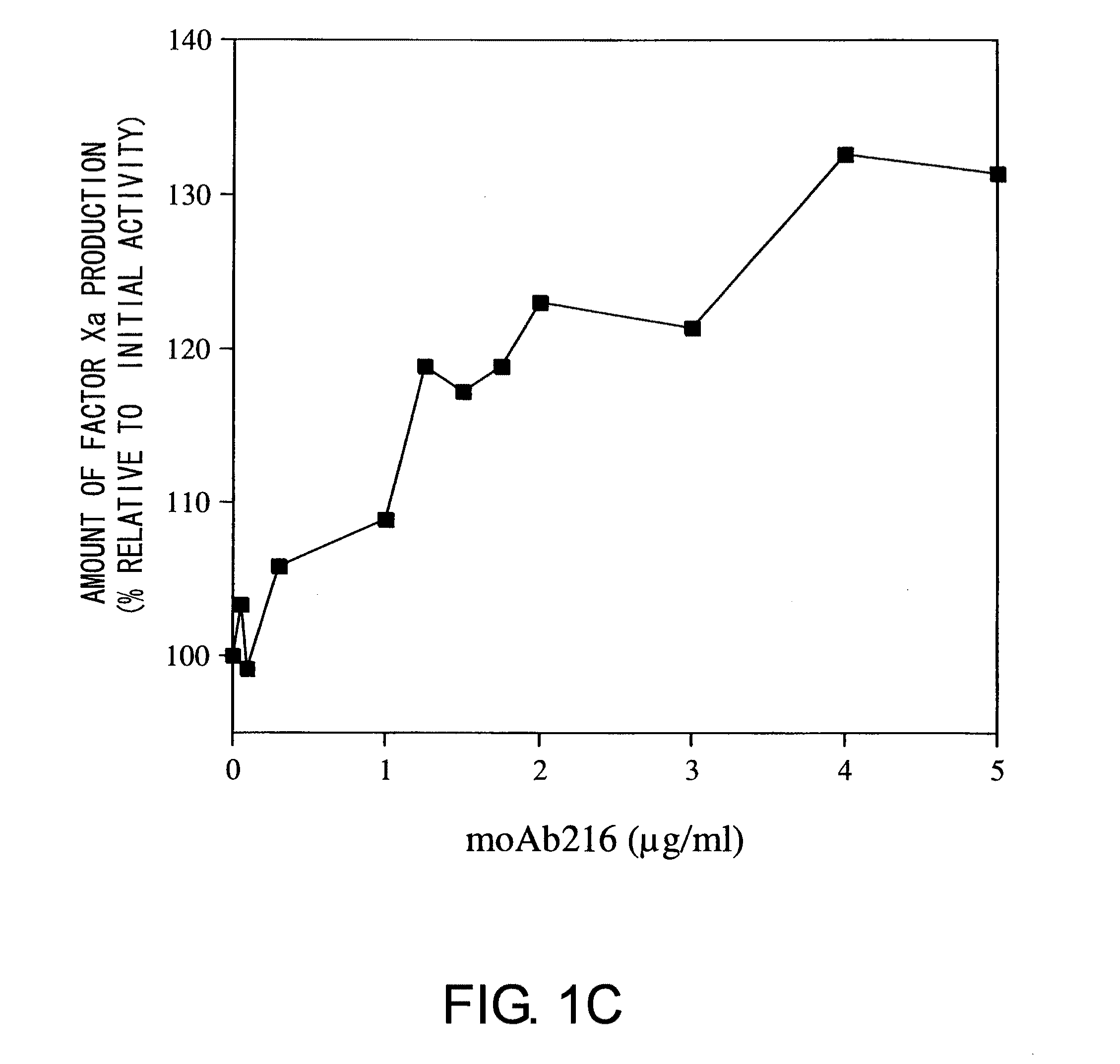
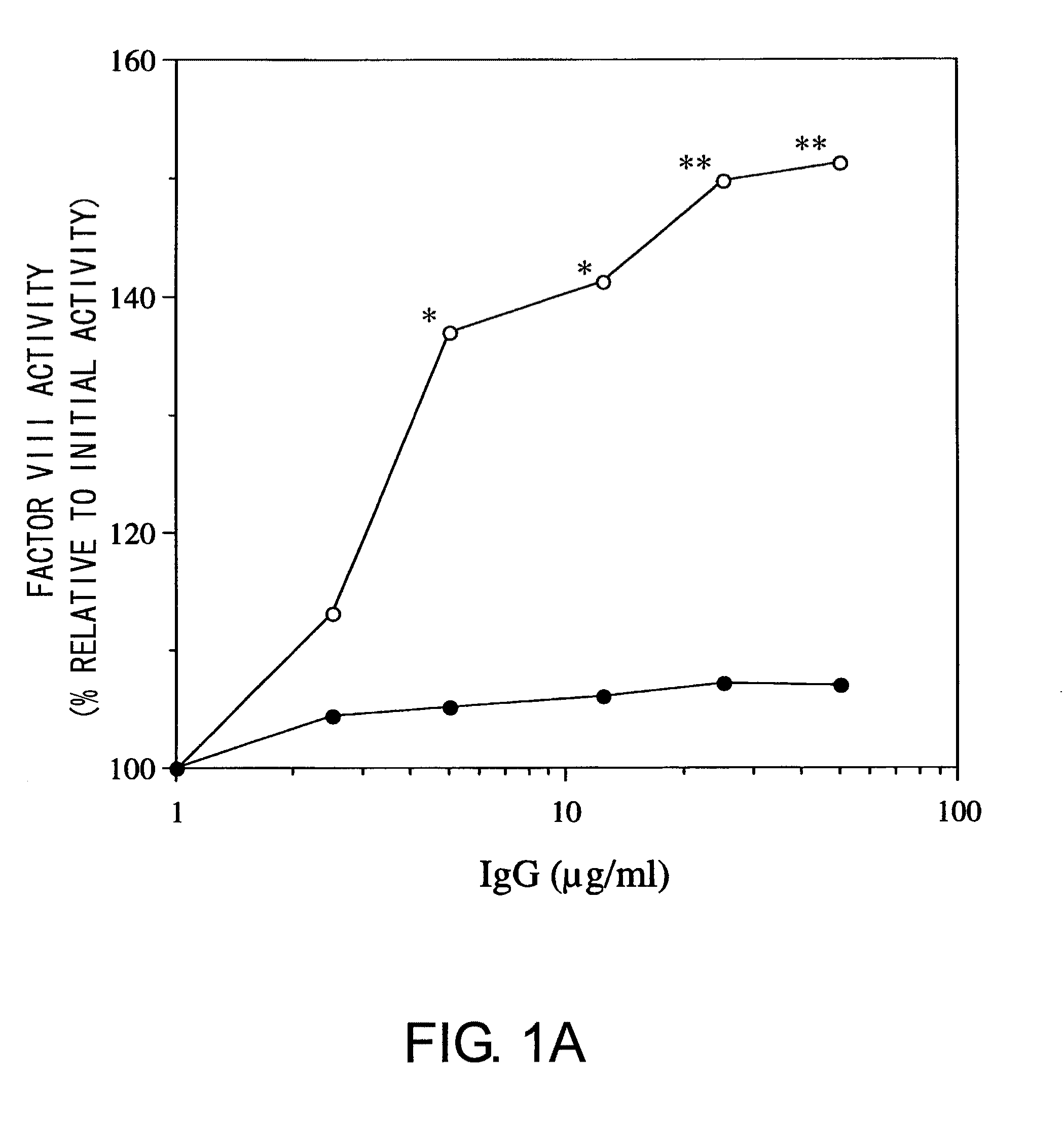
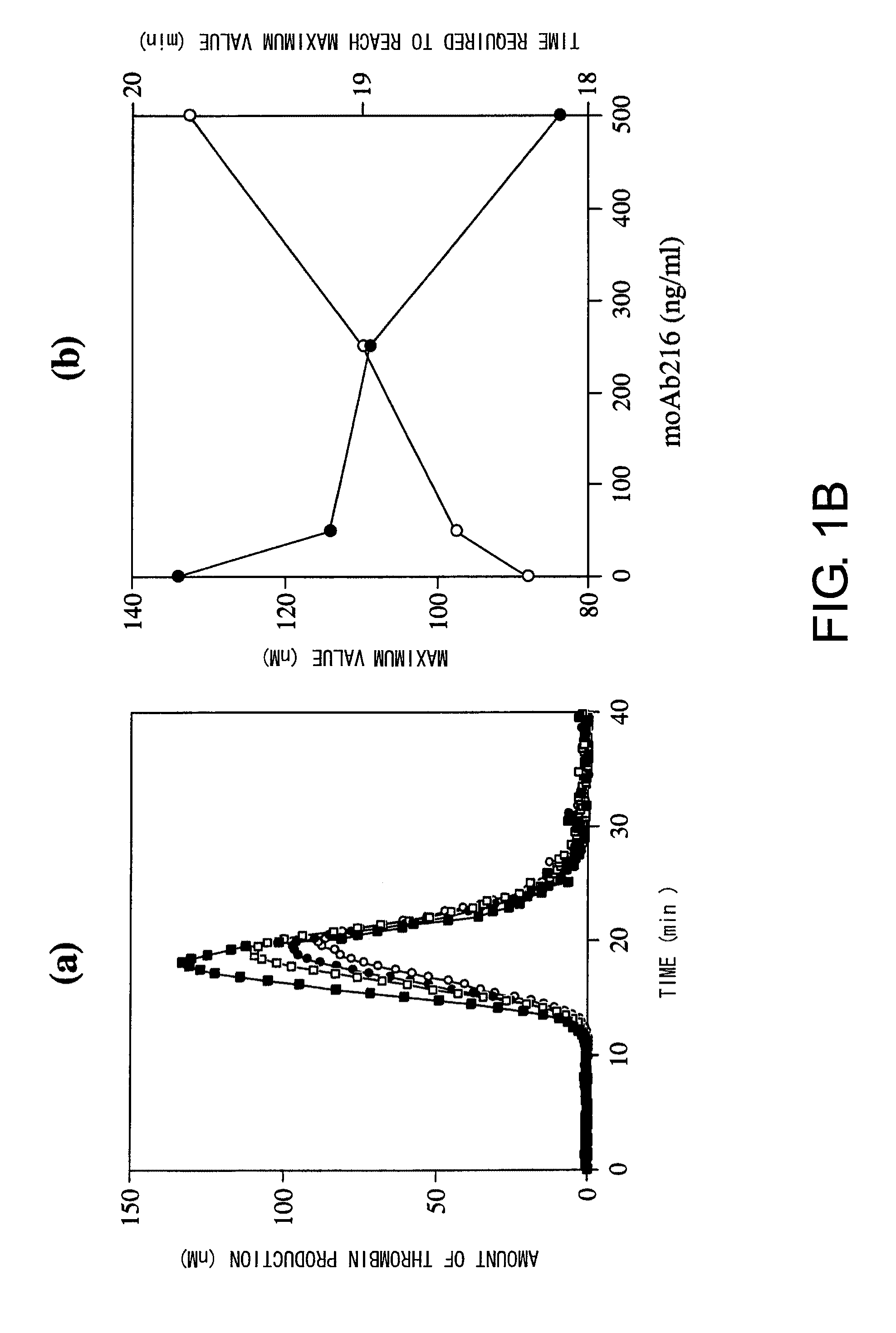
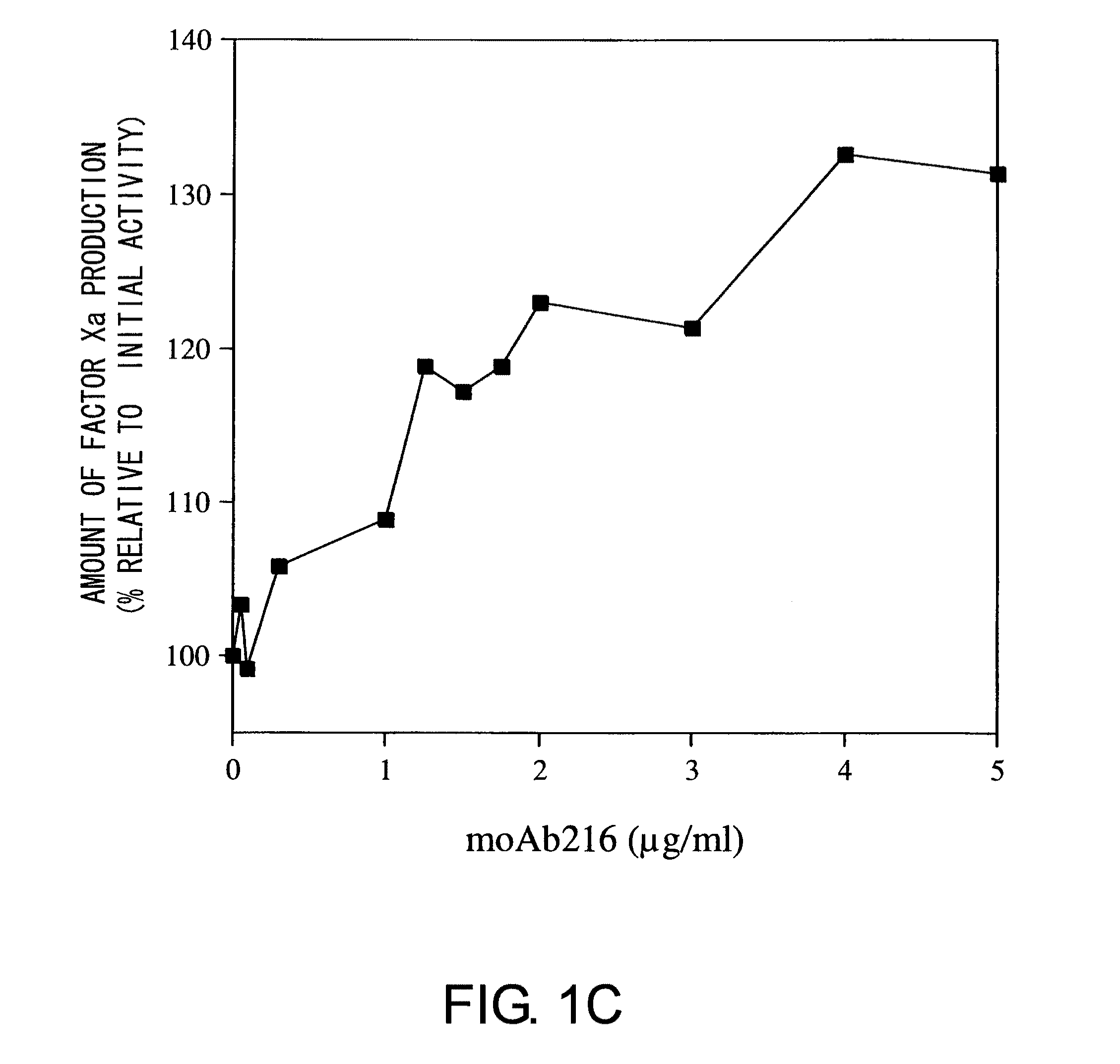
Blood Coagulation Factor VIII Activation-Enhancing Antibodies
ActiveUS20090297503A1Increases coagulation-enhancing activity of Factor VIIIHigh activityImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIVon willebrand
For the first time, the present invention provides antibodies that enhance the generation of activated blood coagulation factor VIII. The antibodies enhance the cleavage of blood coagulation factor VIII at the Arg of position 372 and suppress the cleavage at the Arg of position 336 by recognizing and binding to the A2 domain of blood coagulation Factor VIII. Such antibodies are expected to be useful in preventing or treating diseases that develop or progress due to decrease or loss of the blood coagulation factor VIII activity, for example, hemophilia A, acquired hemophilia, and von Willebrand's disease.
Owner:CHUGAI PHARMA CO LTD +1
Affinity Adsorbents for Factor VIII and Von Willebrand's Factor
For the separation, removal, isolation, purification, characterisation, identification or quantification of Factor VIII, von Willebrand's Factor or a protein that is a analogue of either, an affinity adsorbent is used that is a compound of formula (II) wherein one X is N and the other is N, C—Cl or C—CN; A is a support matrix, optionally linked to the triazine ring by a spacer; Y is O, S or NR2; Z is O, S or N—R3; R2 and R3 are each H, C1-6 alkyl, Cu, hydroxyalkyl, benzyl or phenylethyl; B and W are each an optionally substituted hydrocarbon linkage containing from 1 to 10 carbon atoms; D is H, OH or a primary amino, secondary amino, tertiary amino, quaternary ammonium, imidazole, guanidino or amidino group; or B—D is —CHCOOH—(CH2)3-4—NH2; and R7 is a group bearing a positive charge at neutral pH.
Owner:PROMETIC BIOSCIENCES LTD
Blood coagulation factor VIII activation-enhancing antibodies
ActiveUS8252287B2Increases coagulation-enhancing activity of Factor VIIIHigh activityImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIVon willebrand
For the first time, the present invention provides antibodies that enhance the generation of activated blood coagulation factor VIII. The antibodies enhance the cleavage of blood coagulation factor VIII at the Arg of position 372 and suppress the cleavage at the Arg of position 336 by recognizing and binding to the A2 domain of blood coagulation Factor VIII. Such antibodies are expected to be useful in preventing or treating diseases that develop or progress due to decrease or loss of the blood coagulation factor VIII activity, for example, hemophilia A, acquired hemophilia, and von Willebrand's disease.
Owner:CHUGAI PHARMA CO LTD +1
Process for the preparation of a von Willebrand (FvW) factor concentrate by chromatography and a FvW concentrate thus obtainable
InactiveUS20060036081A1Quality improvementHigh specific activityFactor VIIApolipeptidesChromatographic separationVon willebrand
This invention relates to a process for the preparation of a very high purity von Willebrand factor concentrate from a biological fraction containing von Willebrand factor, including a separation by anion exchange chromatography using a vinyl polymer support of weak base type, the said separation comprising the steps of loading of the chromatographic support with the fraction containing von Willebrand factor, previously equilibrated with a suitable buffer, with a predetermined flowrate allowing the retention of the von Willebrand factor, washing of the support with an acidic buffer with a flowrate higher than the flowrate of the step a) until the not-retained proteins and the contaminants are removed, flushing and equilibrating of the chromatographic support with the buffer and using the flowrate of the step a), and elution of the von Willebrand factor by increasing of the ionic strength of the step c). The invention also relates to a von Willebrand factor concentrate for therapeutic use likely to be obtained by implementing of the process wherein the rate of Factor VIII:C / FvW:RCo is less than 0.06%.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
Transgenic Non-Human Animals Expressing Human Blood Clotting Factors and Uses Thereof
Owner:BAXALTA GMBH
Method and kit for detecting condition in patient with disturbance of consciousness
ActiveUS20090220990A1Inhibit progressHigh clinical valueMicrobiological testing/measurementDisease diagnosisVascular diseaseFactor VIII vWF
A method for detecting a condition in a patient with disturbance of consciousness, by analyzing an amount and / or activity of a von Willebrand factor-cleaving protease, and a kit for detecting a condition in a patient with disturbance of consciousness, comprising an antibody or a fragment thereof which specifically binds to a von Willebrand factor-cleaving protease, or a von Willebrand factor or a fragment thereof, are disclosed. Examples of the detection of a condition include a detection of cerebrovascular disease, a detection of arteriosclerotic vascular disease, and a detection or prediction of severity.
Owner:MITSUBISHI CHEM MEDIENCE
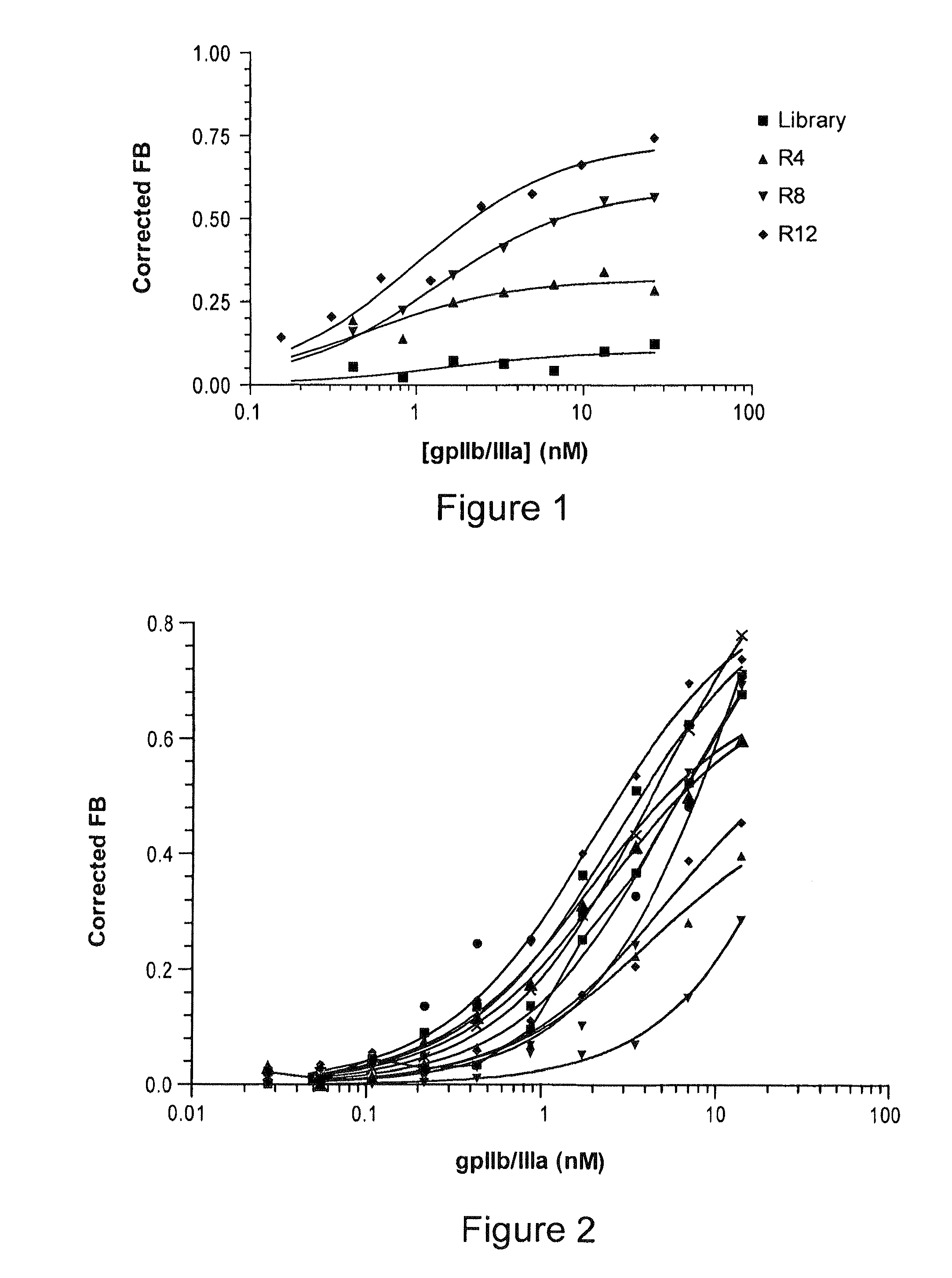
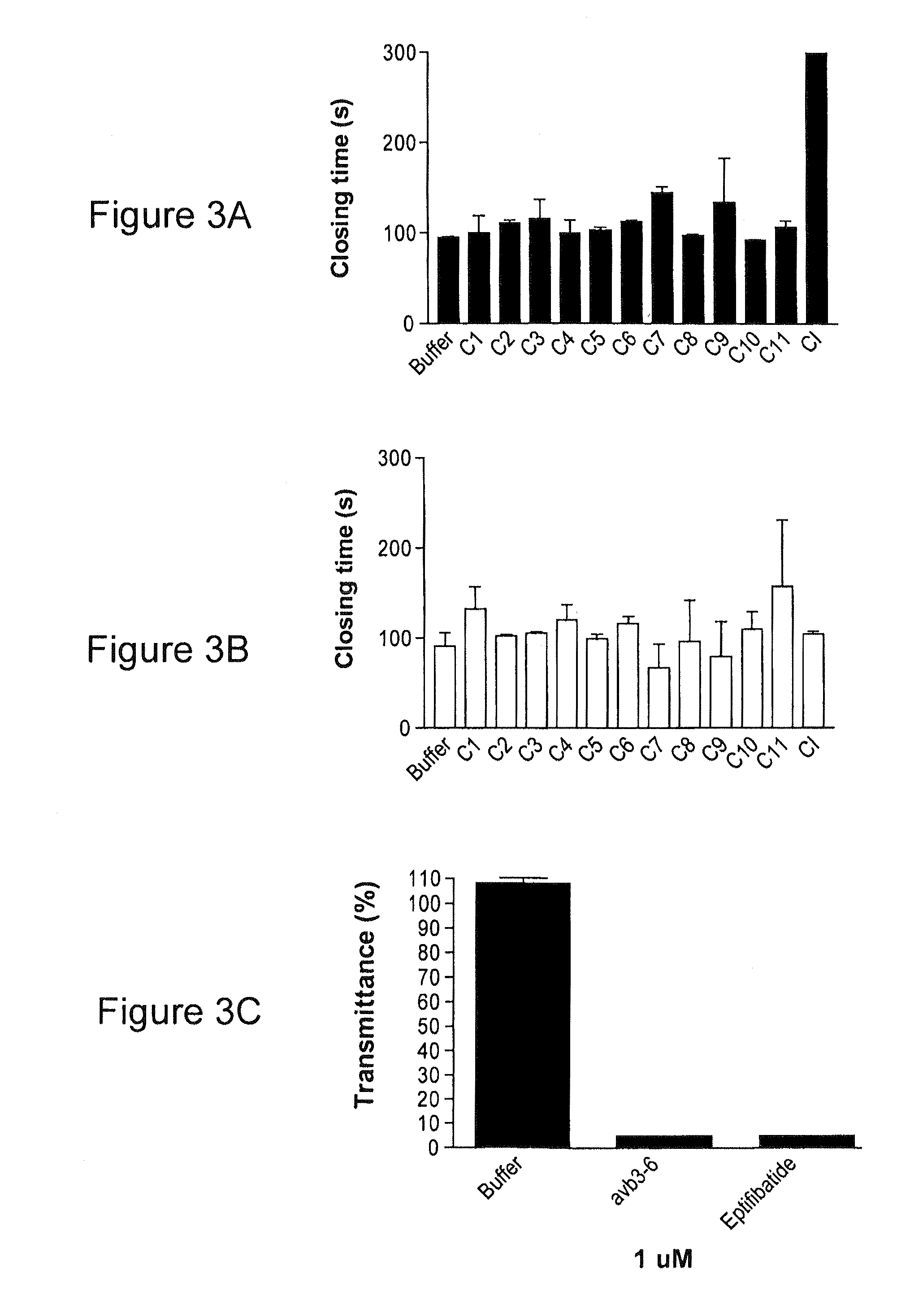
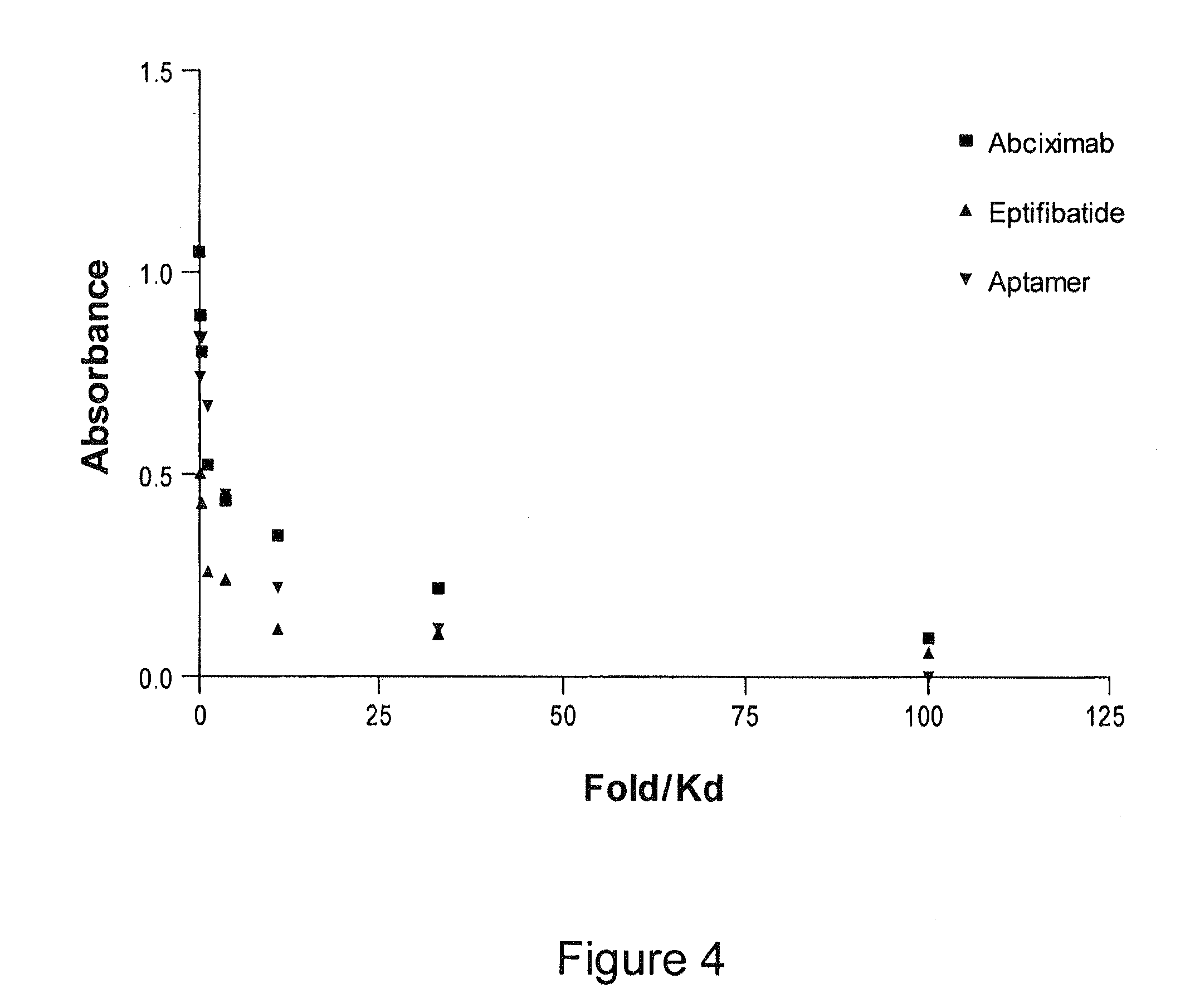
Reversible platelet inhibition
The present invention relates, in general, to receptors and to platelet aggregation and, in particular, to a method of inhibiting platelet aggregation using an aptamer that binds to and inhibits the activity of a receptor, such as glycoprotein IIb / IIIa (gpIIb / IIIa), and to aptamers suitable for use in such a method. The invention also relates to antidotes to antiplatelet agents and to methods of using such antidotes to reverse aptamer-induced platelet inhibition. The invention further relates to von Willebrand Factor (VWF) inhibitors, and antidotes therefore, and to methods of using same.
Owner:DUKE UNIV
vWF (von willebrand factor) activity protection fluid
InactiveCN105622747AIncrease profitGuaranteed purityFactor VIIPeptide preparation methodsChromatographic separationBlood coagulation factor VIII
The invention discloses a vWF (von Willebrand factor) activity protection fluid. The vWF activity protection fluid is used in preparation for extracting von Willebrand factors from cryoprecipitated blood coagulation factor VIII waste. In a preparation technology of the von Willebrand factors, glycine is added into a chromatographic buffer solution, and lysine, glycine and albumin are added into protein fluid before freeze drying after chromatography to protect vWF activity. The vWF activity protection fluid has the advantages that by means of adding the glycine into the chromatographic buffer solution, vWF activity loss can be reduced in a chromatographic separation and purification process; the albumin, the lysine and the glycine are added into protein dialysate to serve as freeze-drying protectants, so that von Willebrand factor molecules can be stabilized; the albumin serving as an excellent protein stabilizer is capable of effectively adsorbing protein surfaces; the lysine and the glycine, serving as micromolecular amino acids, are capable of protecting a protein structure, increasing collapse temperature of a finished product and stopping protein damage caused by collapse during freeze-drying, so that biological activity is kept.
Owner:华润博雅生物制药集团股份有限公司
Synthetic platelets
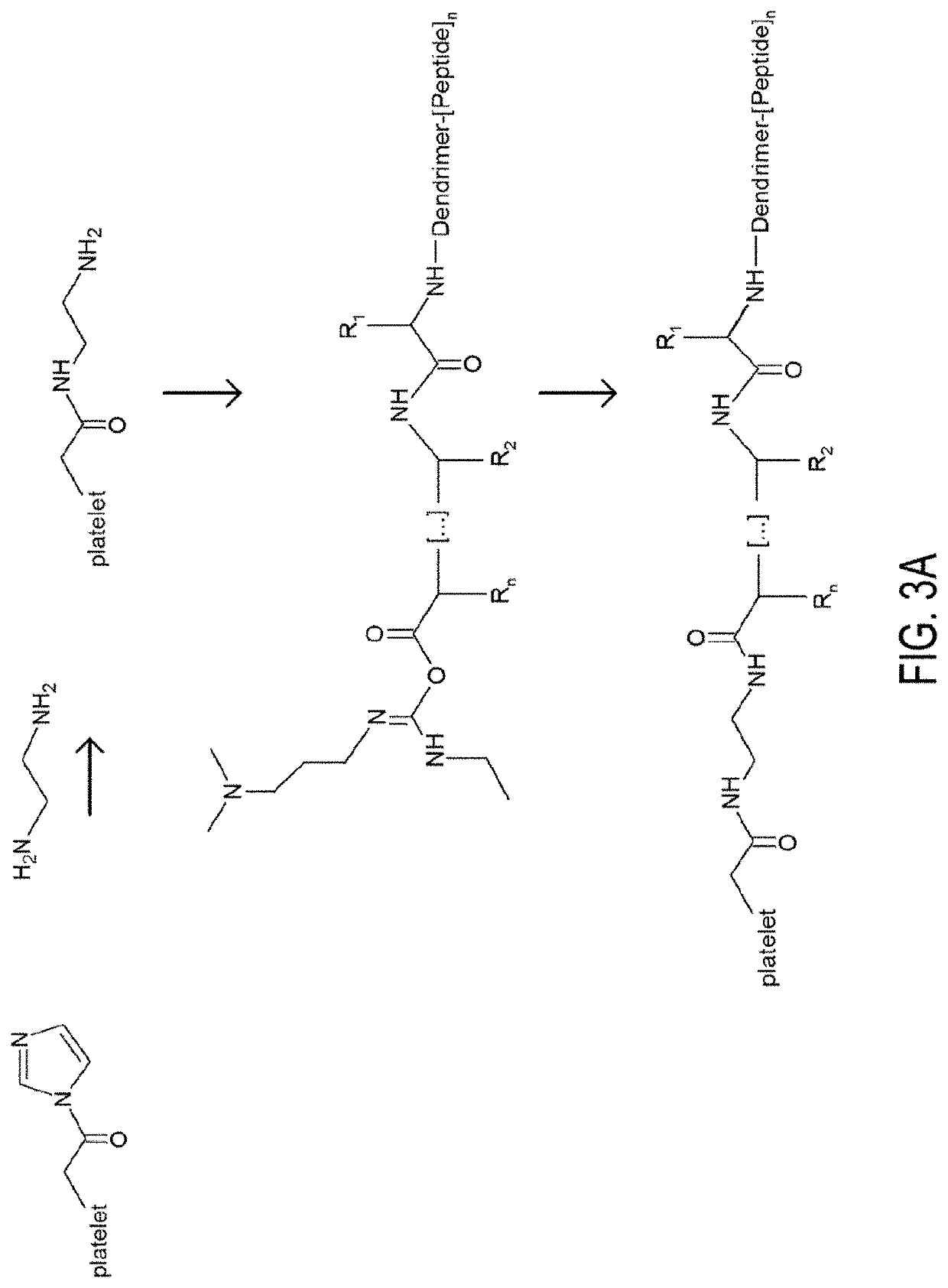
Provided herein are particles comprising a polymer substrate comprising one or more hyaluronic acid chains; and two or more peptide moieties bound directly to each hyaluronic acid chain. In some embodiments, the two or more peptide moieties comprising collagen-binding peptide (CBP) and von Willebrand binding peptide (VBP). The particles can be utilized in, e.g., methods of hemostatic treatment.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
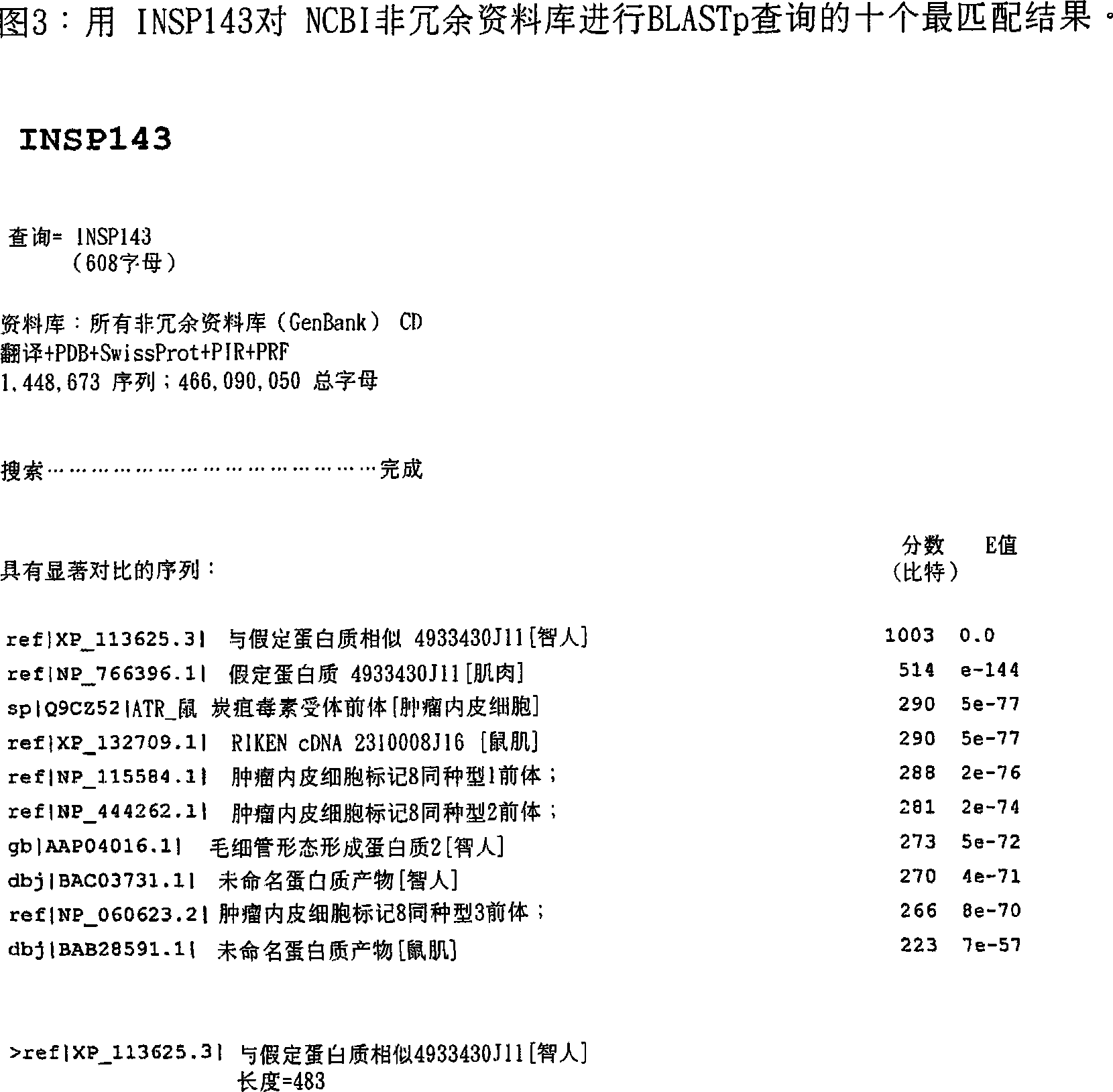
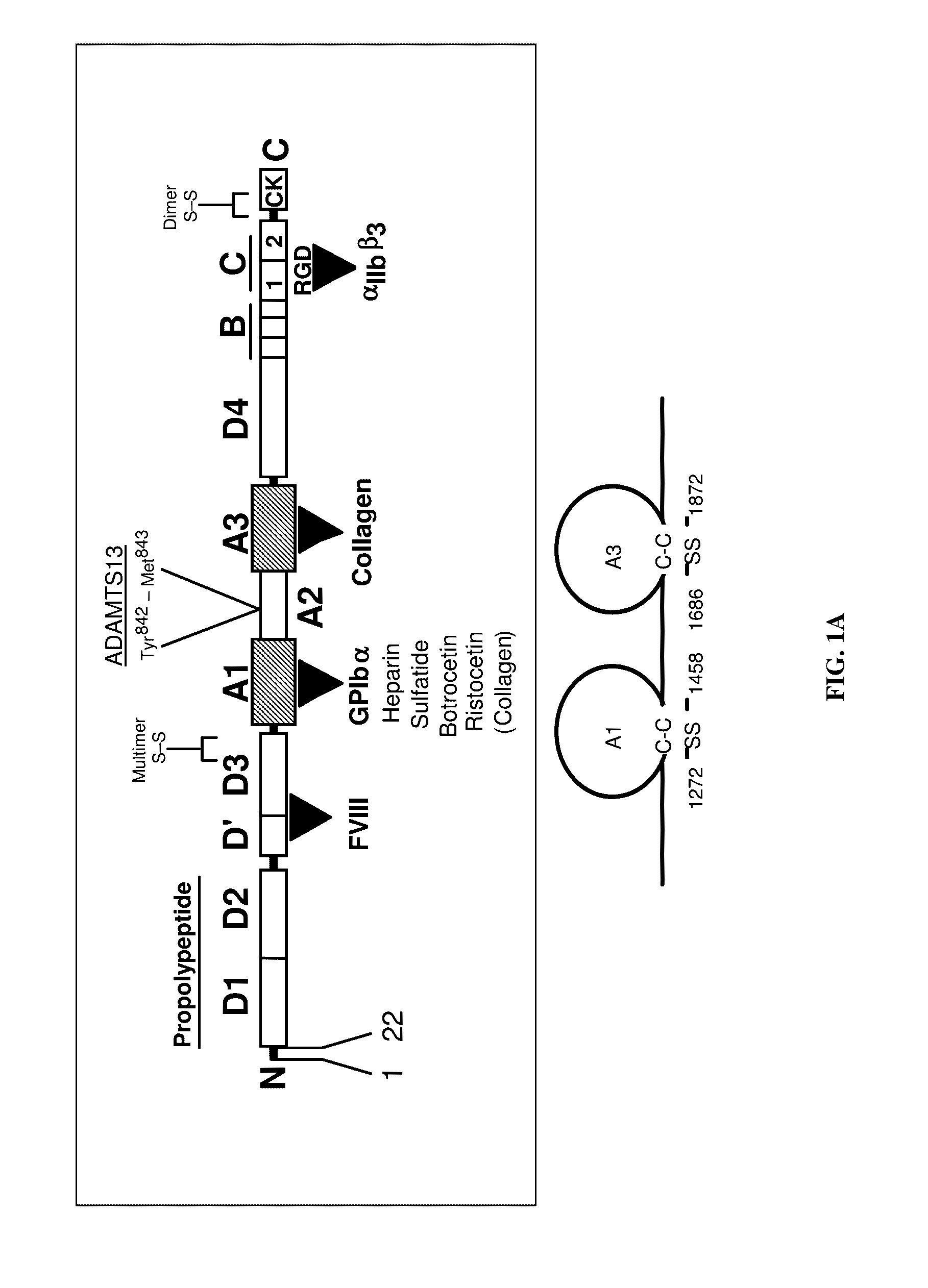
vWFA and/or ANTú›IG domain containing proteins
This invention relates to novel proteins (herein termed INSP141, INSP142, INSP143, and INSP144), herein identified as anthrax receptor-like proteins containing von Willebrand factor A (vWFA) and Anthrax receptor extracellular (ANT_IG) domains and to the use of these proteins and nucleic acid sequences from the encoding genes in the diagnosis, prevention and treatment of disease.
Owner:ARES TRADING SA
Preparation comprising factor viii and von willebrand factor peptides
ActiveUS20180185455A1Reduces inhibitor formationImprove stabilityFactor VIIPeptide/protein ingredientsFactor VIII vWFVon willebrand
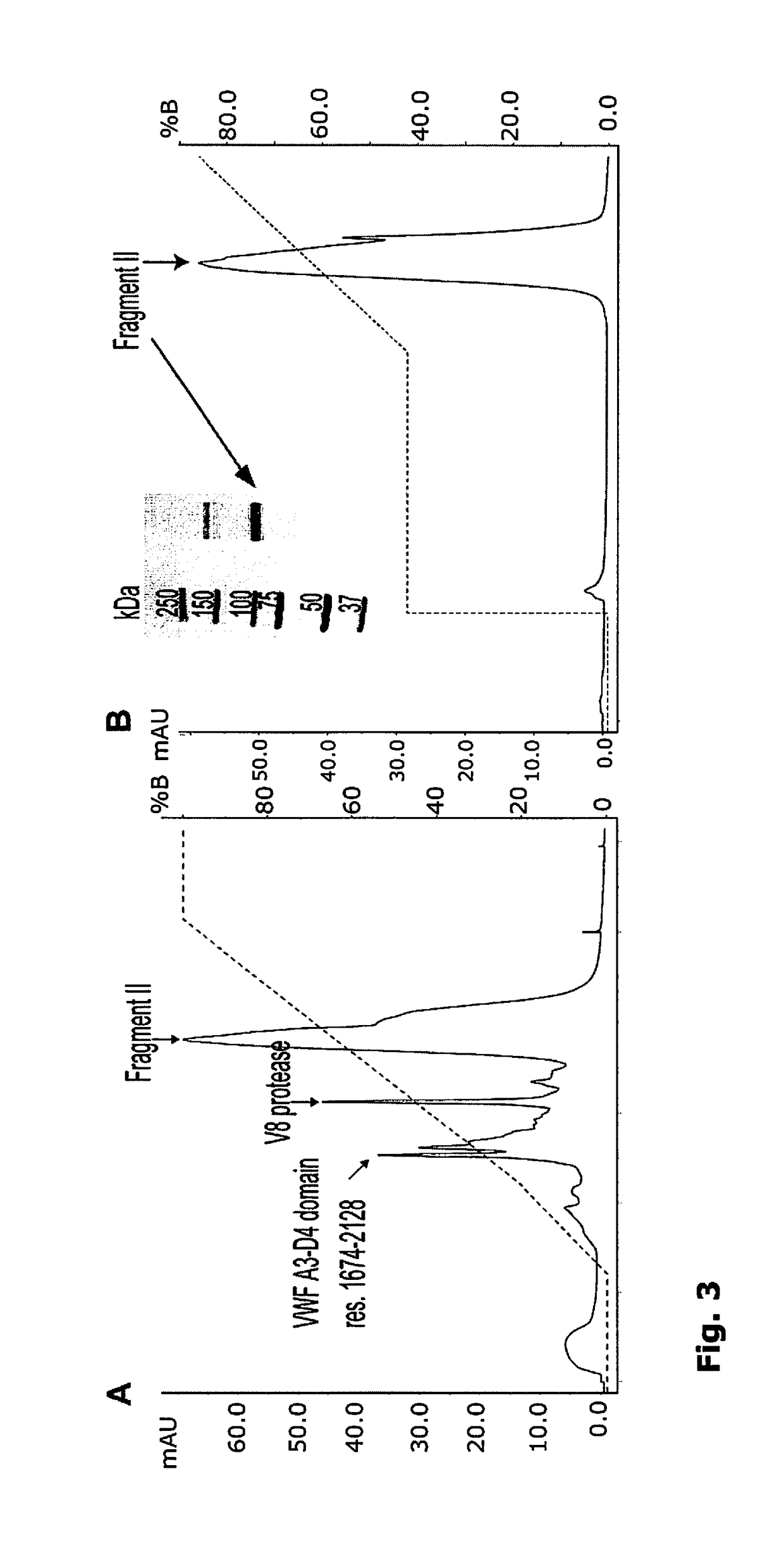
A composition comprising a complex of Factor VIII and one or more Von Willebrand Factor peptides, wherein the Von Willebrand Factor peptides comprise at least the amino acids 764 to 1035 and 1691 to 1905 of SEQ ID No. 1 but not amino acids 2255 to 2645 of SEQ ID No. 1.
Owner:OCTAPHARMA
Uses of a2 domain of von willebrand factor
PendingUS20220193204A1Improve survivalImprove bleedingPeptide/protein ingredientsAntiviralsFactor VIII vWFVon willebrand
Embodiments of the disclosure encompass methods and compositions for maintaining a healthy fibrin network in an individual. The disclosure includes methods of targeting fibrin in an individual for the purpose of restoring fibrin that is subject to a level of fibrinolysis that is deleterious, such as excessive or reduced with respect to the general population. Such modifications of fibrin in an individual may include direct targeting of fibrin with the A2 domain of von Willebrand factor or a functional derivative or fragment thereof. In specific embodiments, the methods restore to a normal level any imbalance between coagulation and inflammation.
Owner:BAYLOR COLLEGE OF MEDICINE
Substance with antithrombotic activity and method for detecting glycokallidin
InactiveUS7608695B2Simple methodEasy to measureFactor VIICell receptors/surface-antigens/surface-determinantsGlycoprotein IbFactor VIII vWF
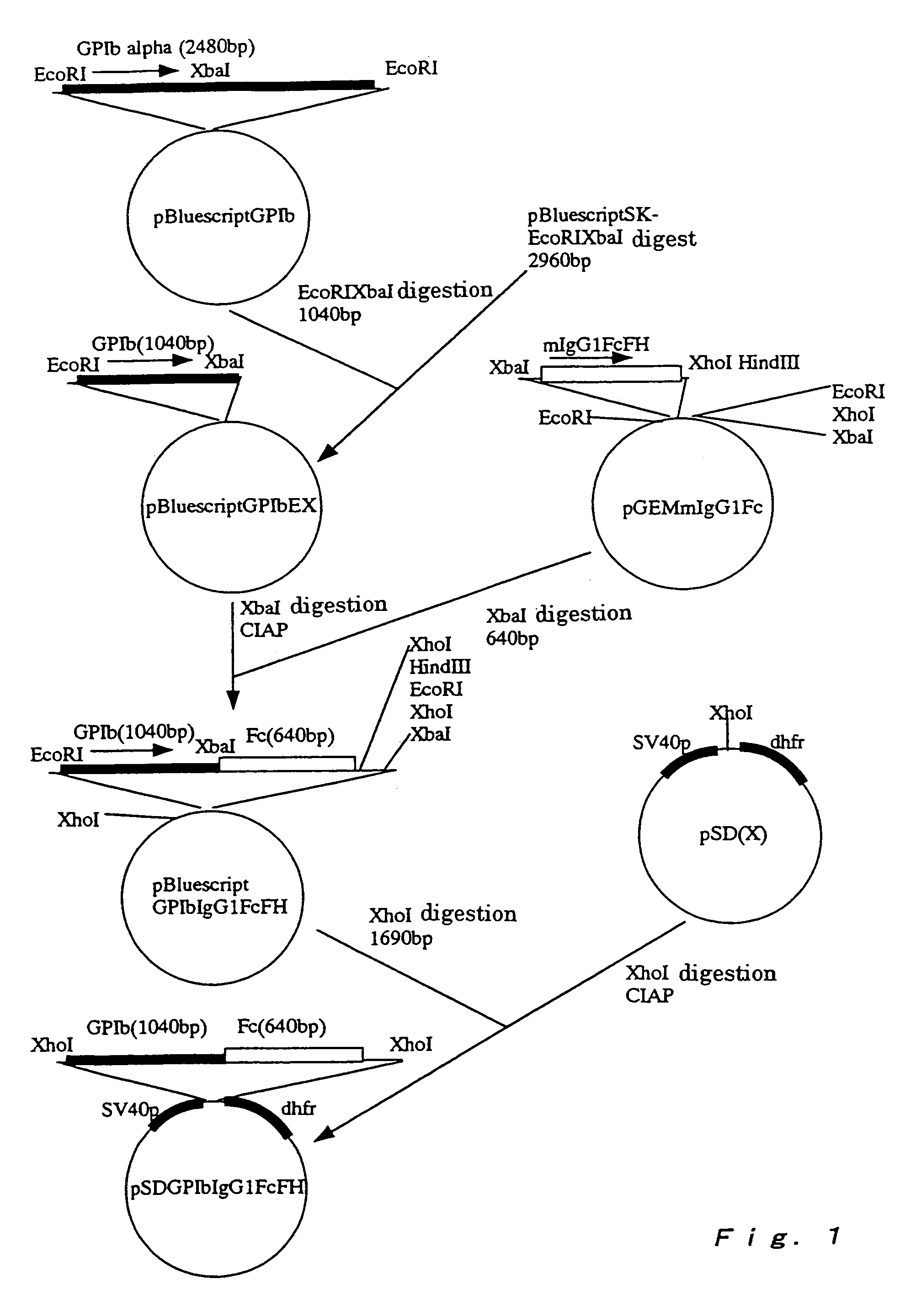
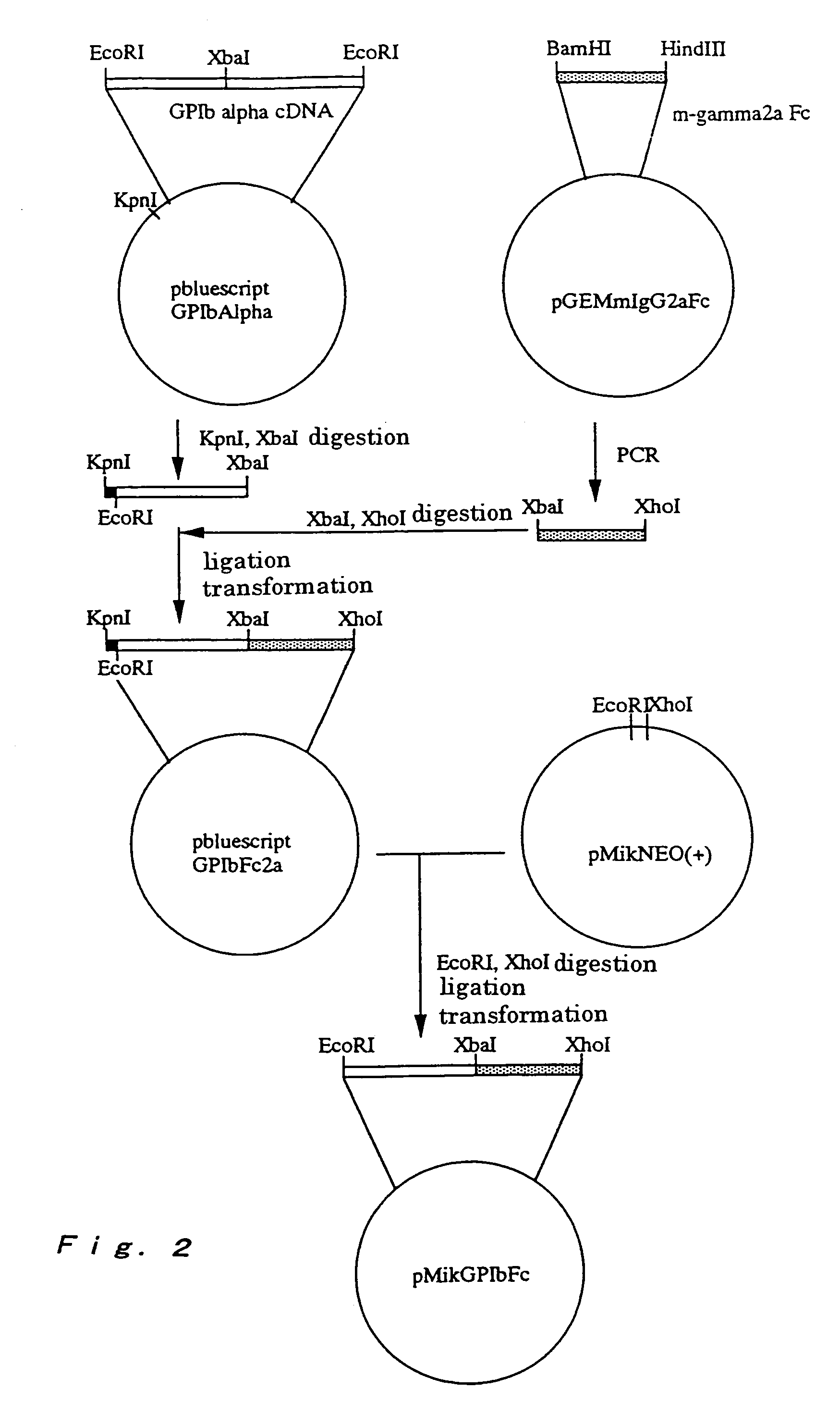
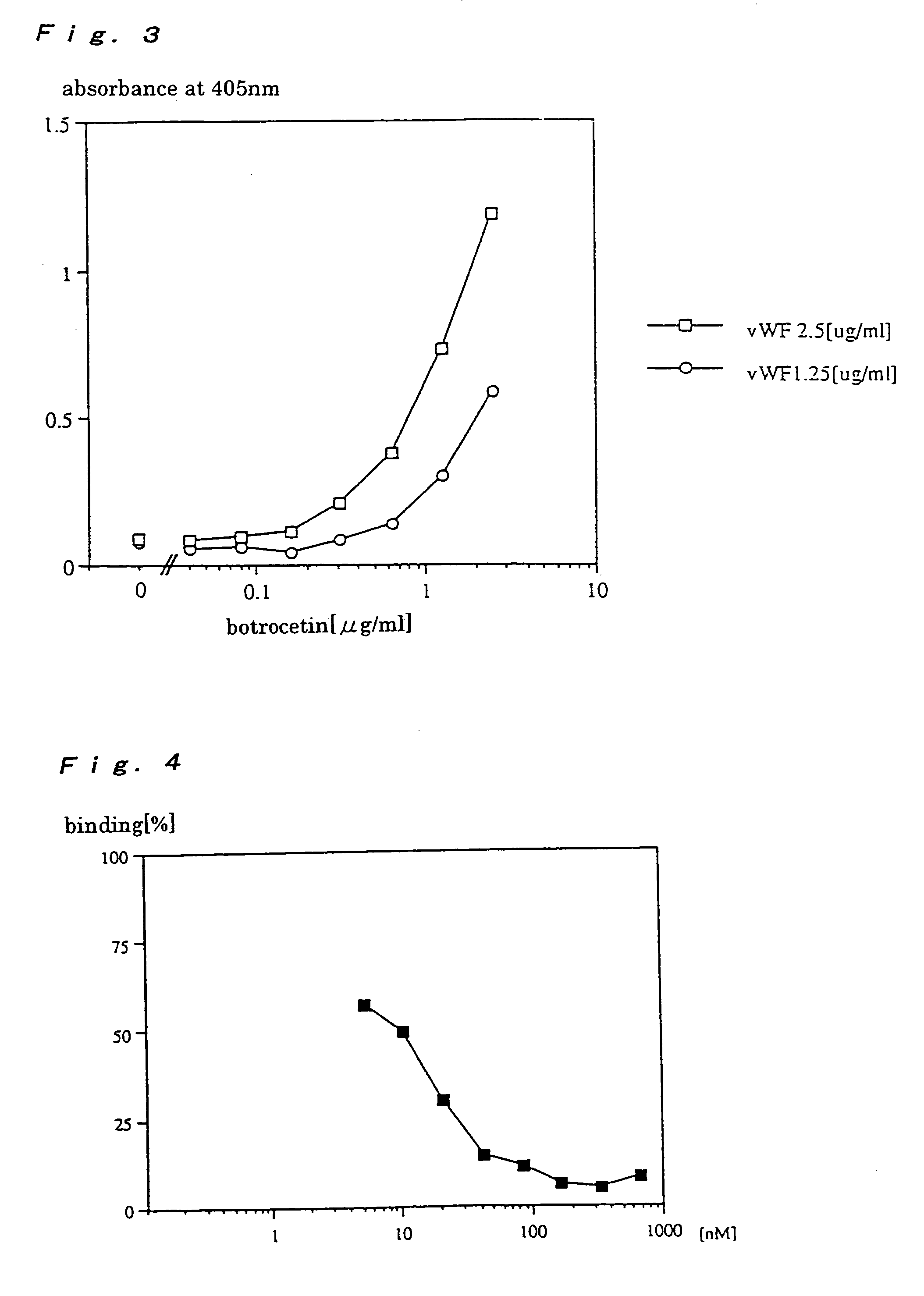
A method for conveniently detecting binding between the von Willebrand factor and glycoprotein Ib and a means to be used therein. The von Willebrand factor fixed in a reactor immobilized in a reaction vessel in the presence of bottrocetin is bound to a chimeric protein constructed by fusing the carboxyl terminal of a partial protein containing the von Willebrand factor-binding site of glycoprotein Ib with the amino terminal of the Fc region of an immunoglobulin molecule. Then the Fc region of the above immunoglobulin molecule is detected to thereby detect the binding between the von Willebrand factor and the glycoprotein Ib or inhibition of this binding.
Owner:AJINOMOTO CO INC
Method for treating von willebrand's disease
Use of factor XIII for treating von Willebrand's disease. A patient having von Willebrand's disease is treated by administering factor XIII generally in conjunction with factor VIII concentrate, 1-desamino-8-D-arginine vasopressin (DDAVP) or desmopressin.
Owner:ZYMOGENETICS INC
Methods for Testing Anti-Thrombotic Agents
ActiveUS20160345550A1Altering the kinetics of the interactionImprove the connection rateCompound screeningFactor VIIFactor VIII vWFVon willebrand
The invention provides a transgenic non-human animal expressing von Willebrand Factor A1 protein containing at least one mutation selected from the group consisting of: 1263P>S, 1269N>D, 1274K>R, 1287M>R, 1302G>D, 1308H>R, 1313R>W, 1314I>V, 1326R>H, 1329L>I, 1330E>G, 1333A>D, 1344T>A, 1347I>V, 1350T>A, 1370G>S, 1379H>R, 1381T>A, 1385T>M 1391P>Q, 1394A>S, 1397L>F, 1421S>N, 1439L>V, 1442G>S, 1449R>Q, 1466A>P, 1469Q>L, 1472Q>H, 1473V>M, 1475H>Q, 1479S>G, and any combination thereof.
Owner:DIACOVO THOMAS DR +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com