Patents
Literature
222 results about "Haemophilia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
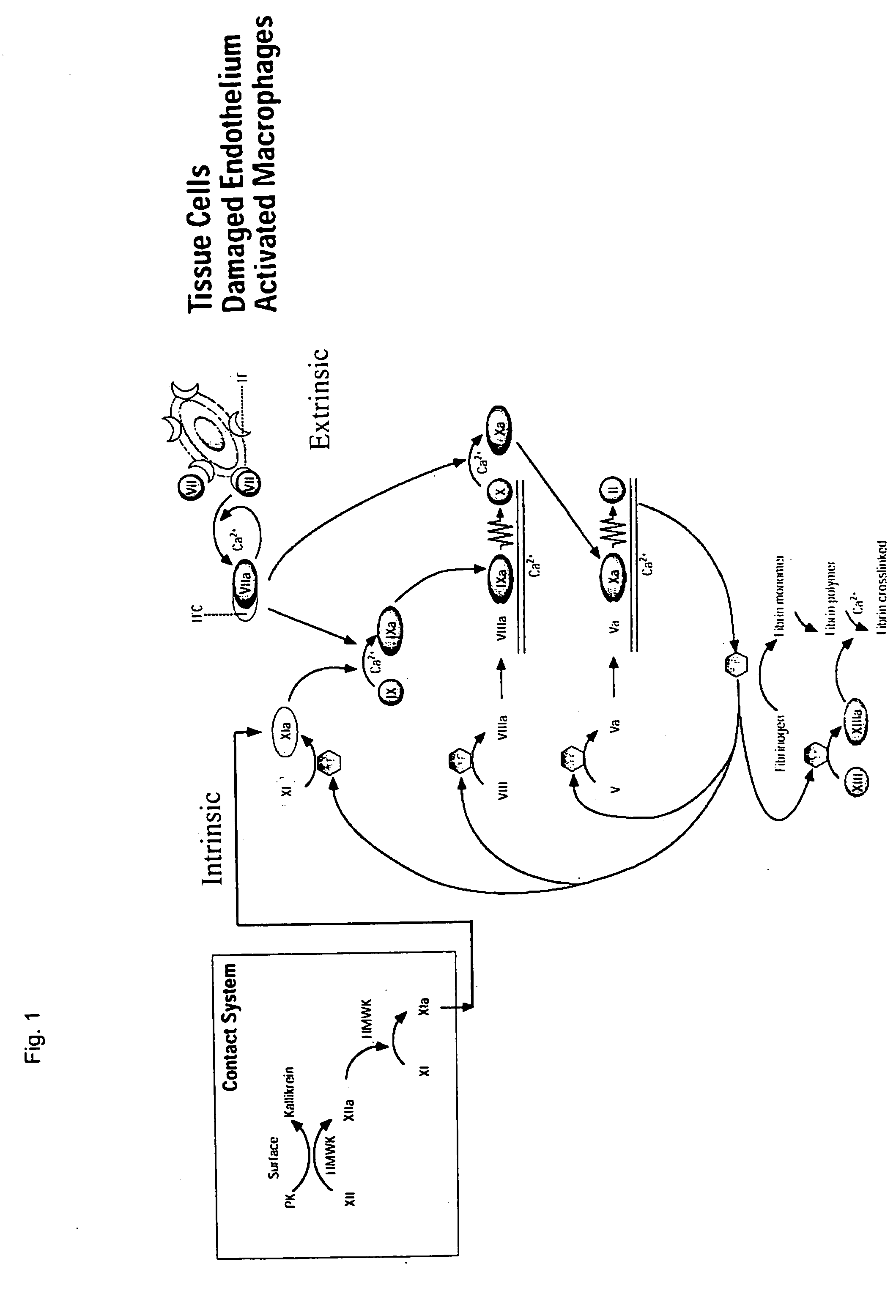
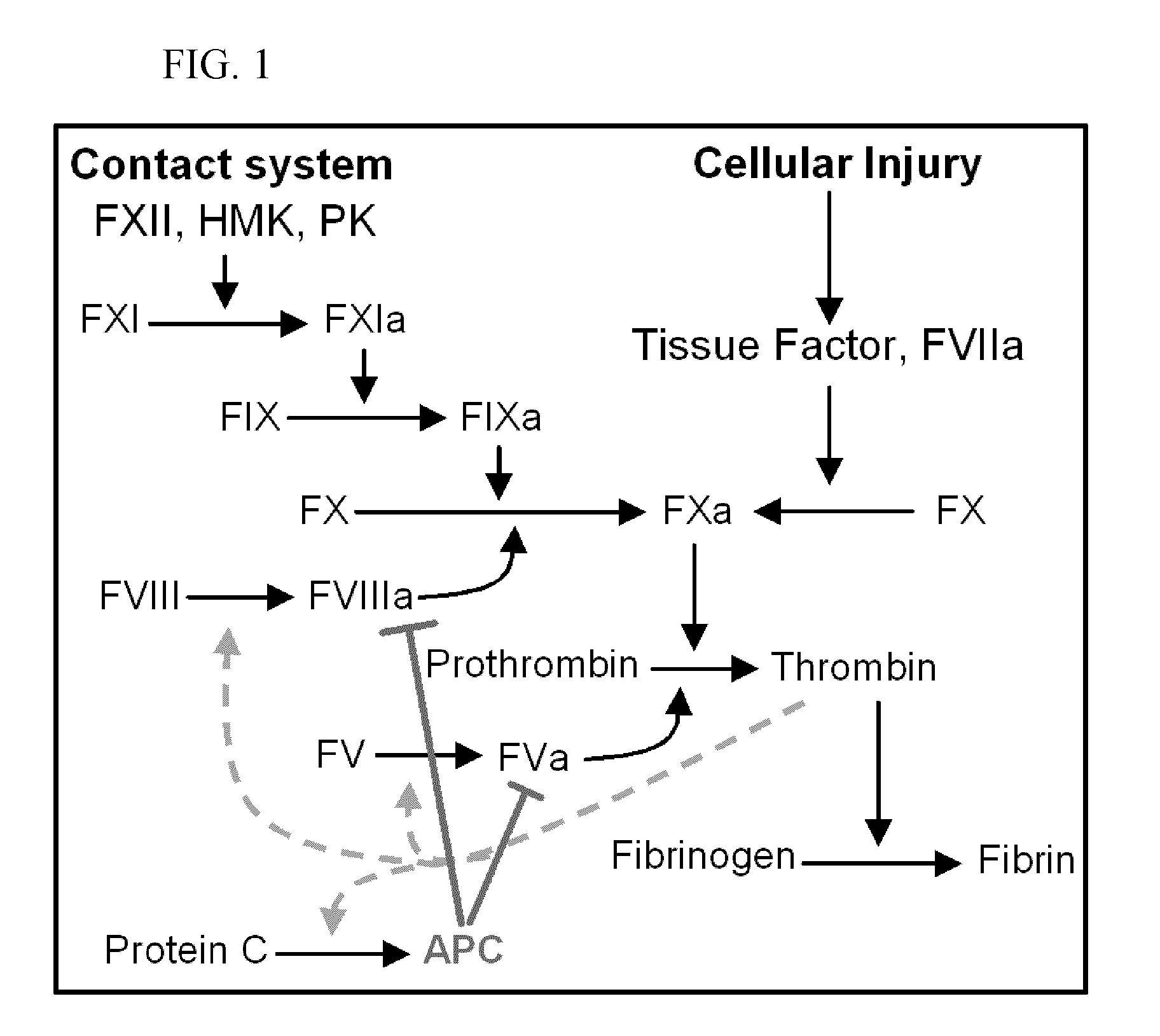
An inherited disorder in which the blood does not clot due to insufficient clotting factors.
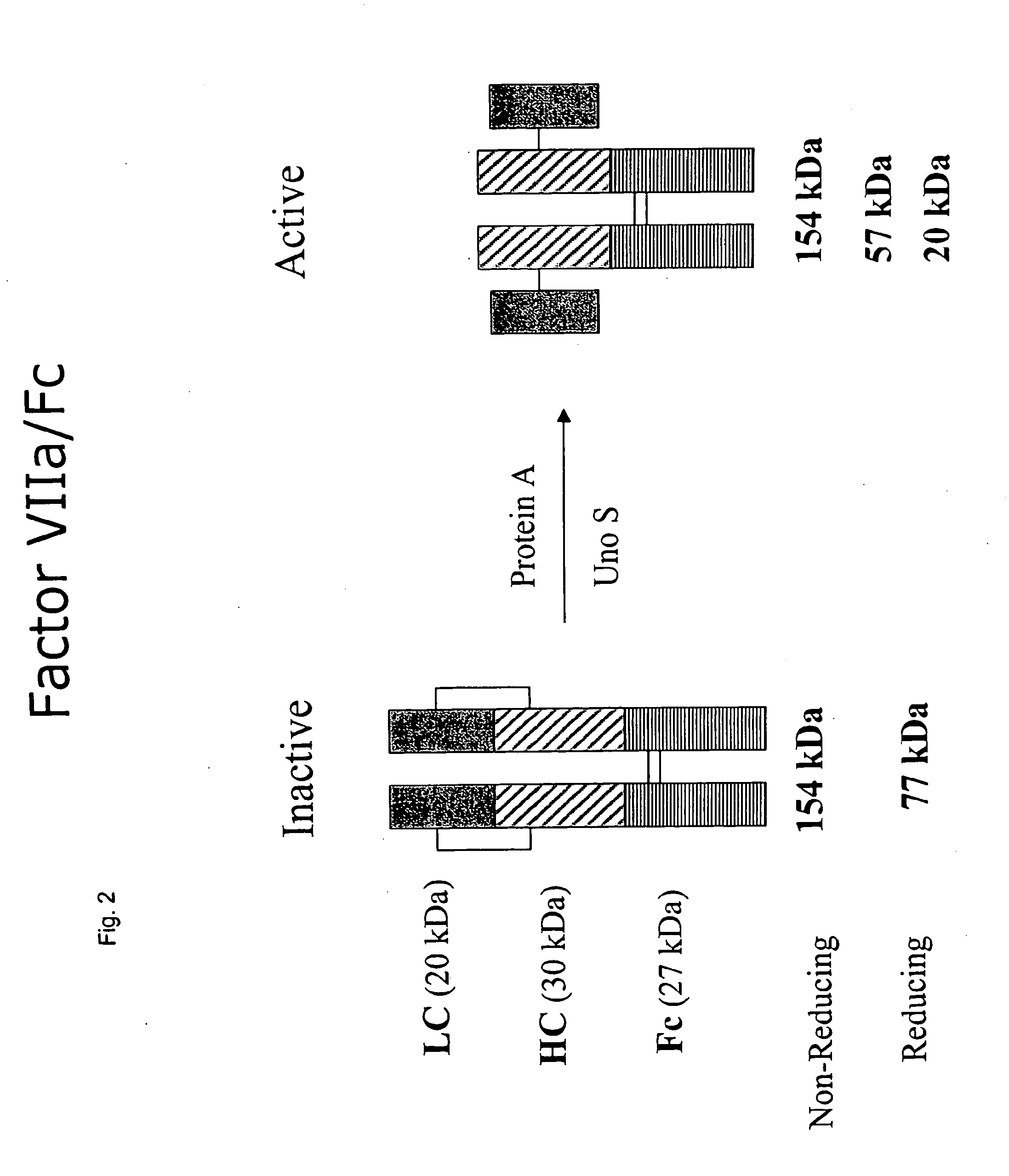
Clotting factor-Fc chimeric proteins to treat hemophilia
InactiveUS20050147618A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHemostatic DisordersChimera Protein
The invention relates to a chimeric protein comprising at least one clotting factor and at least a portion of an immunoglobulin constant region. The invention relates to a method of treating a hemostatic disorder comprising administering a therapeutically effective amount of a chimeric protein wherein the chimeric protein comprises at least one clotting factor and at least a portion of an immunoglobulin constant region.
Owner:BIOVERATIV THERAPEUTICS INC
Factor VIII compositions and methods
InactiveUS20050100990A1Reduce gapExtended half-lifeFactor VIIPeptide/protein ingredientsHalf-lifeNucleotide
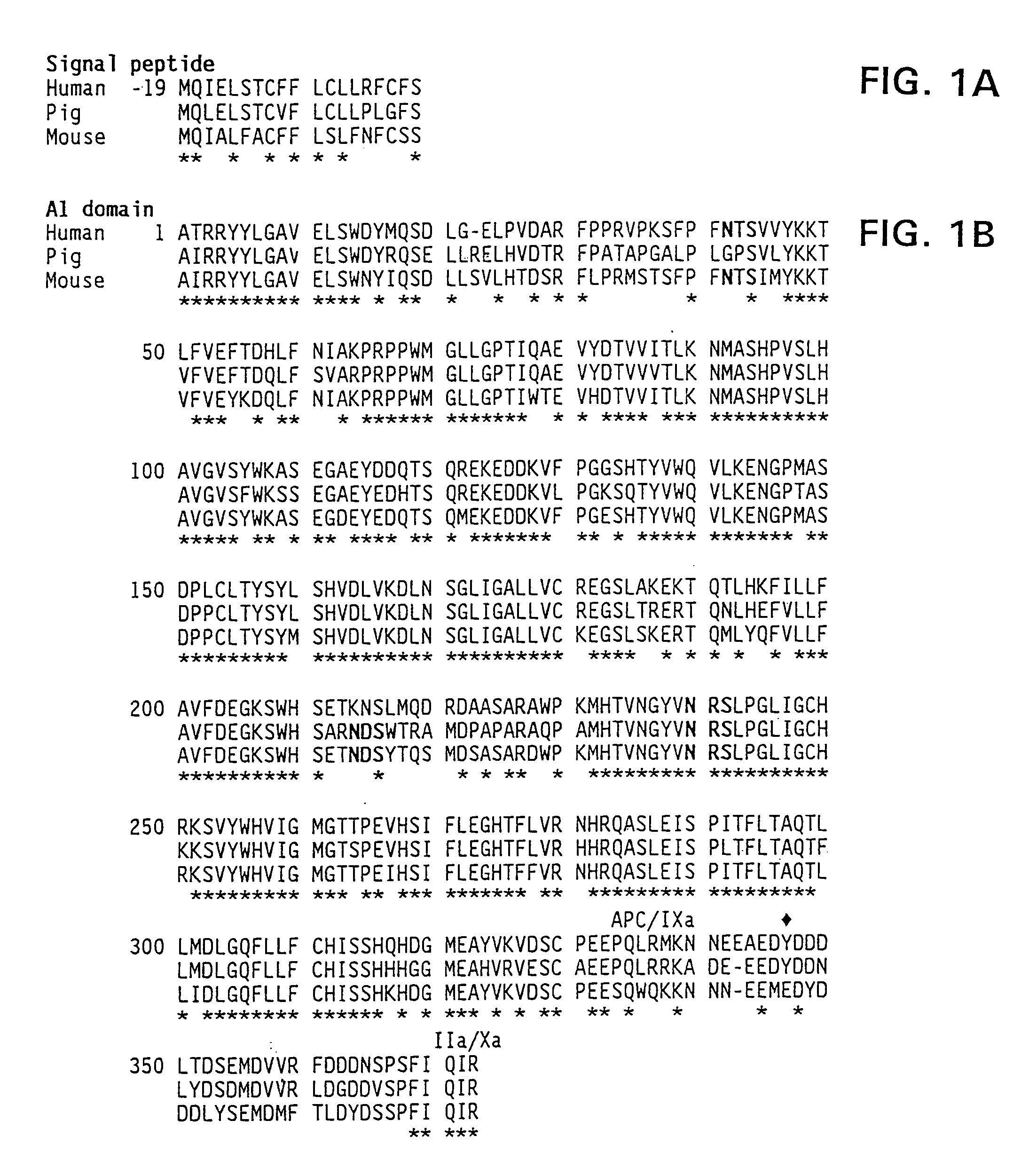
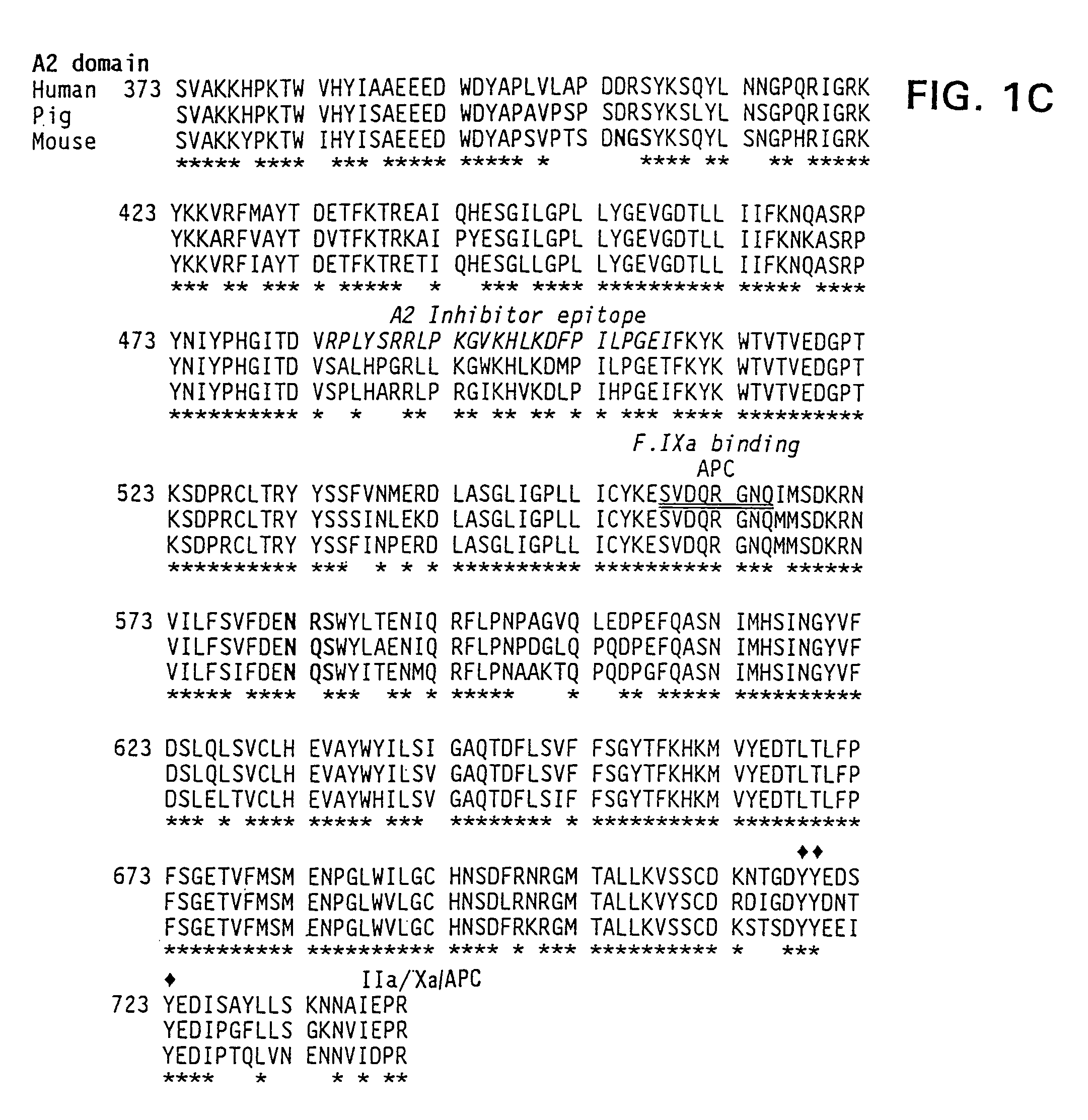
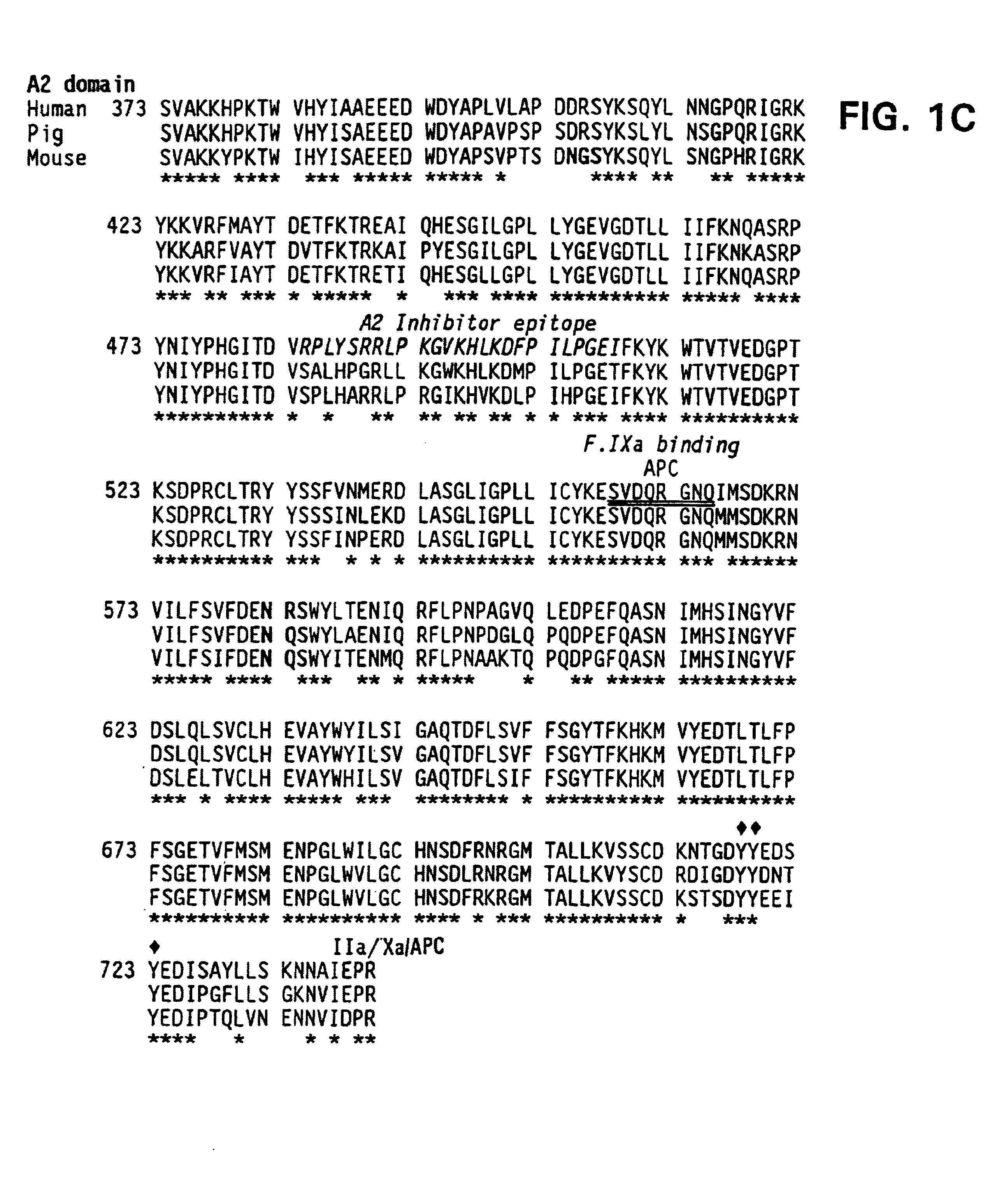
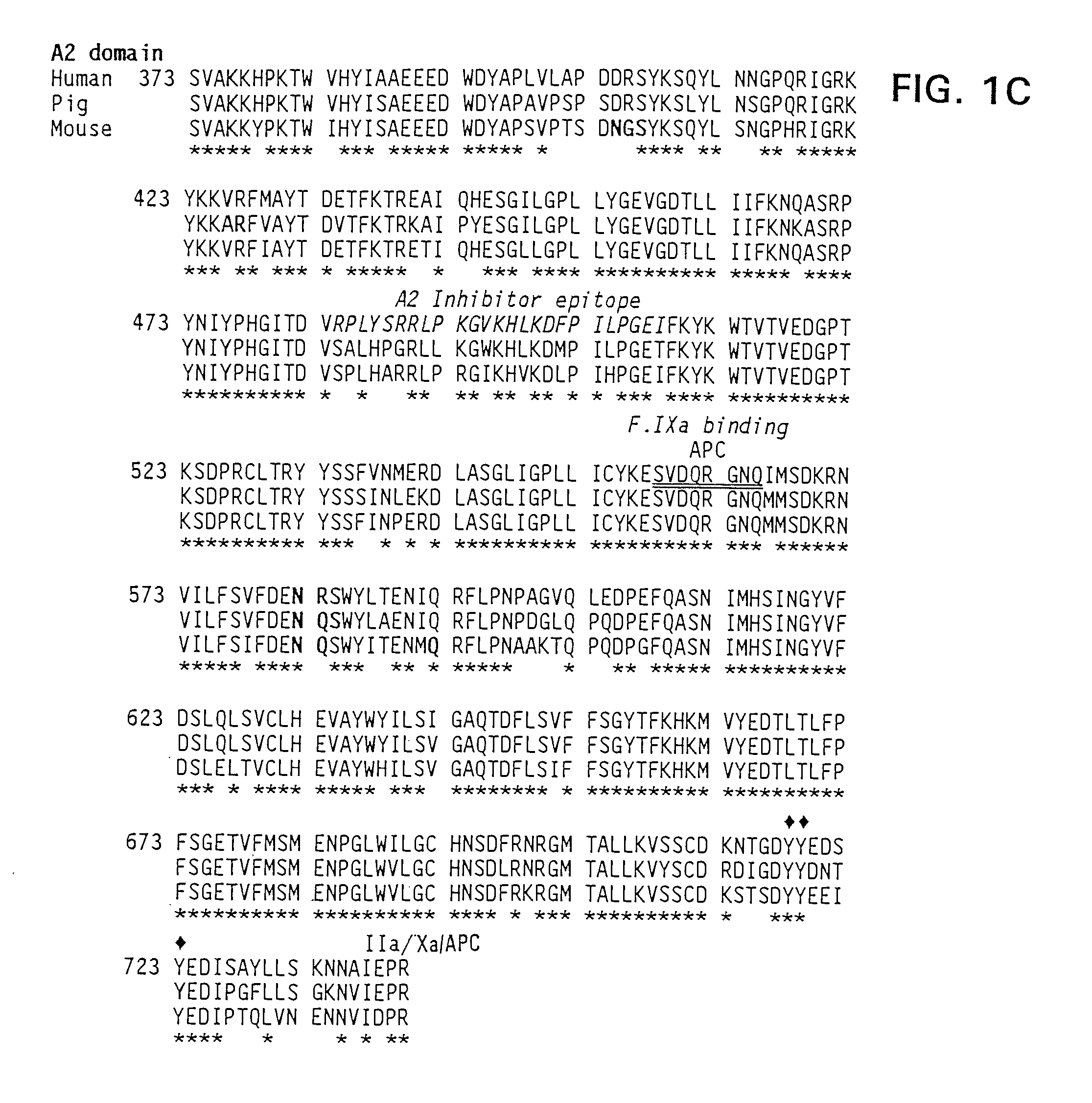
The present invention provides methods of increasing the half-life and / or specific activity of factor VIII. More specifically, the invention provides methods of increasing the half-life and / or specific activity of factor VIII by substituting one or more amino acids in the A2 domain. It further provides methods for producing such factor VIII mutants. The invention also provides polynucleotides encoding the mutant factor VIII, and methods of treating hemophilia using the polypeptides and polynucleotides of the invention.
Owner:UNIV OF MARYLAND
Modified fVIII having reduced immunogenicity through mutagenesis of A2 and C2 epitopes
InactiveUS20050123997A1Low immunogenicityImmunoglobulins against blood coagulation factorsFactor VIIEpitopeVaccine Immunogenicity
Specific amino acid loci of human fVIII interact with inhibitory antibodies of hemophilia patients after being treated with fVIII. Modified fVIII is disclosed in which the amino acid sequence is changed by multiple substitutions in human fVIII A2 and C2 domains. The modified fVIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory antibodies.
Owner:EMORY UNIVERSITY
Hepatocellular chimeraplasty
InactiveUS6524613B1Decrease in levelReduce riskOrganic active ingredientsBiocideGenetic ChangeFhit gene
The present invention concerns compositions and methods for the introduction of specific genetic changes in endogenous genes of the cells of an animal. The genetic changes are effected by oligonucleotides or oligonucleotide derivatives and analogs, which are generally less than about 100 nucleotides in length. The invention provides for macromolecular carriers, optionally incorporating ligands for clathrin coated pit receptors. In one embodiment the ligand is a lactose or galactose and the genetic changes are made in hepatocytes. By means of the invention up to 40% of the copies of a target gene have been changed in vitro. Repair of mutant genes having a Crigler-Najjar like phenotype and Hemophilia B phenotype were observed.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV +1
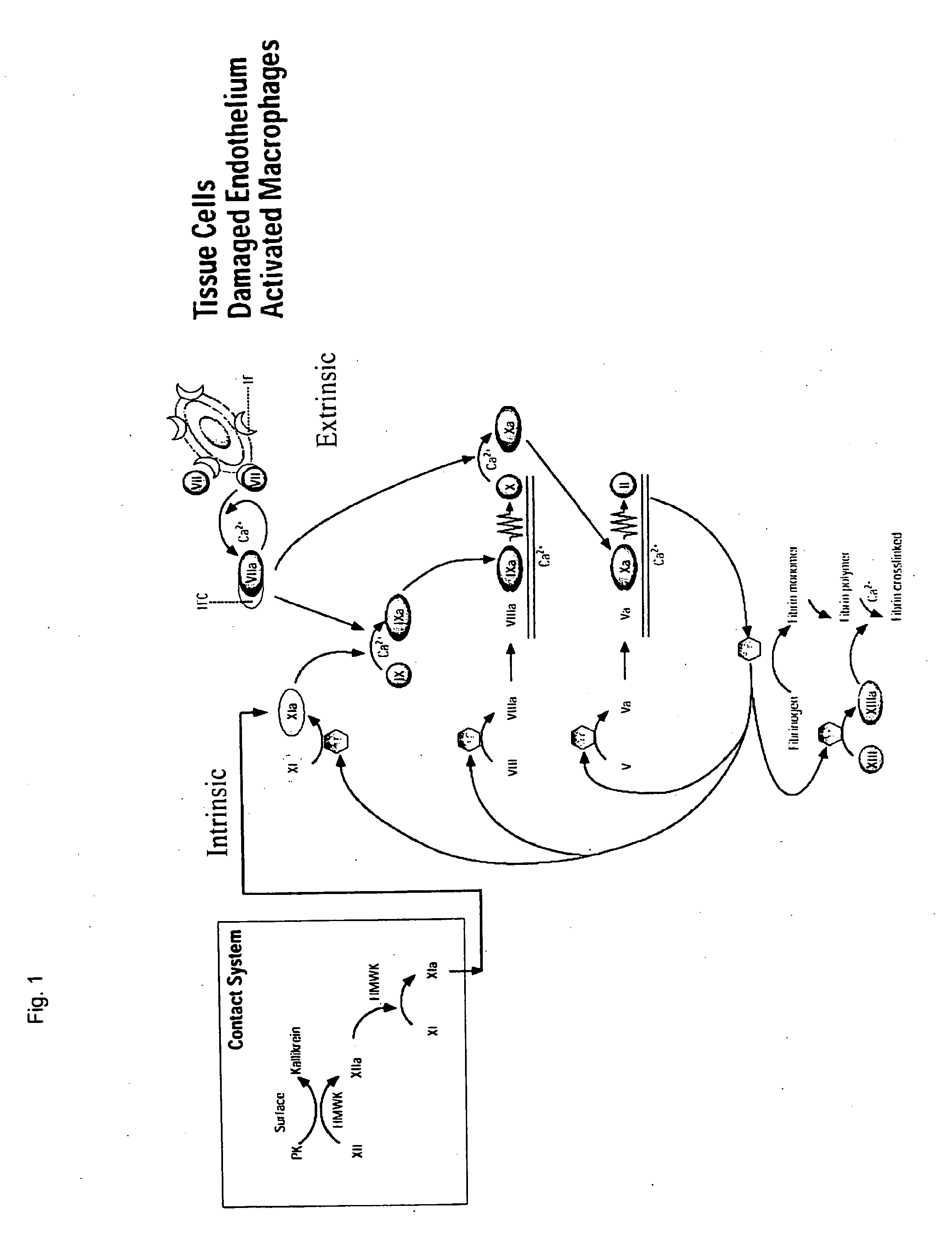
Method of providing hemostasis in Anti-coagulated blood
InactiveUS20080254146A1Promote formationBiocideAnimal repellantsBULK ACTIVE INGREDIENTActive ingredient
A method of clotting blood includes the step of administering a therapeutically effective amount of a composition comprising zeolite as the active ingredient to a wound from which the blood emanates. A method of arresting blood flowing from a wound includes the steps of providing a patient being inflicted with a bleeding wound and administering a therapeutically effective amount of a composition comprising zeolite as the active ingredient to the bleeding wound. A method of facilitating the formation of blood clots includes the step of contacting blood with a negatively charged surface wherein upon contacting the blood with the negatively charged surface a clotting mechanism is initiated. In any of the foregoing methods, the blood has a compromised ability to form clots. The blood may be from a person diagnosed with hemophilia or von Willebrand disease.
Owner:TELEFLEX LIFE SCI LTD
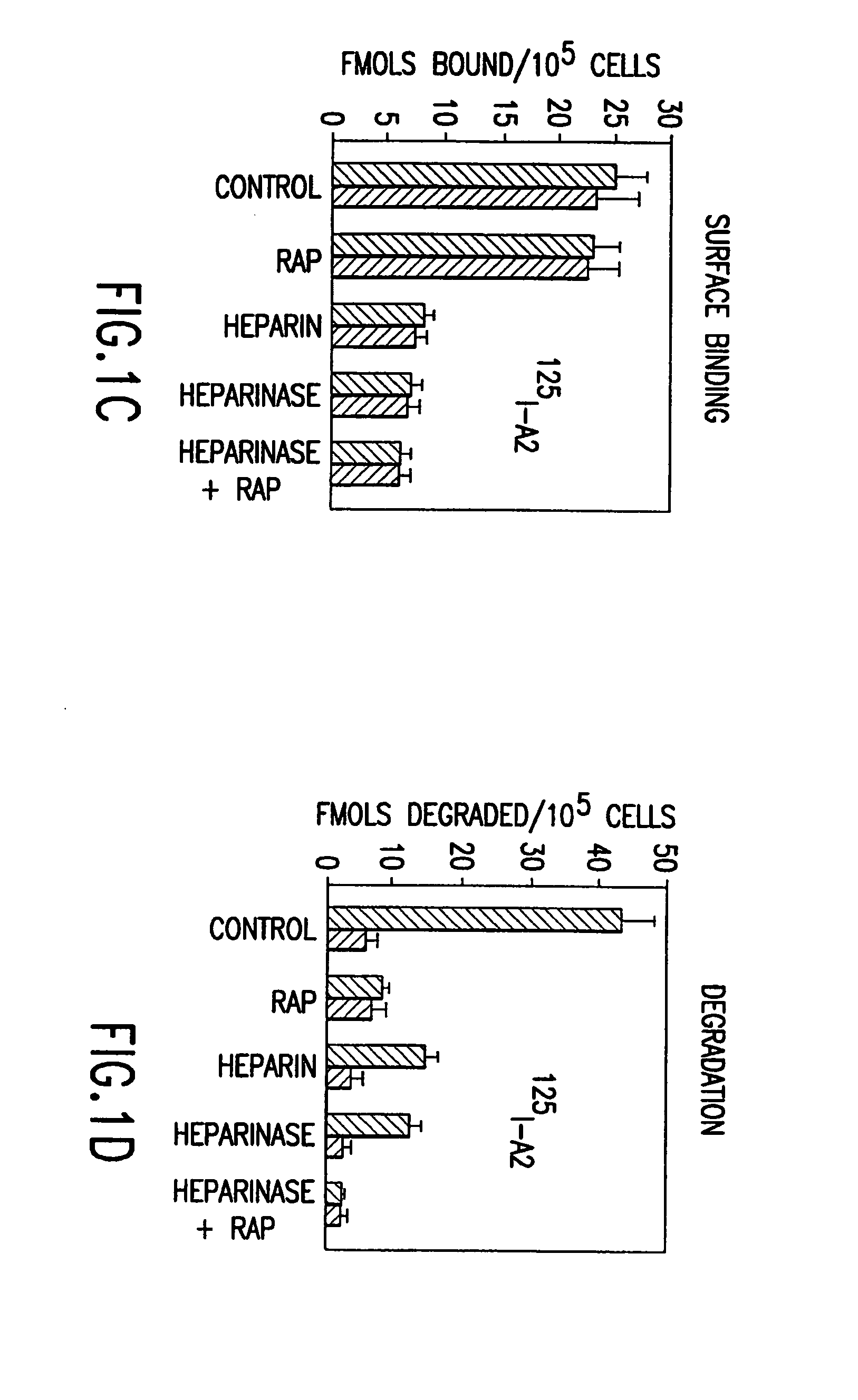
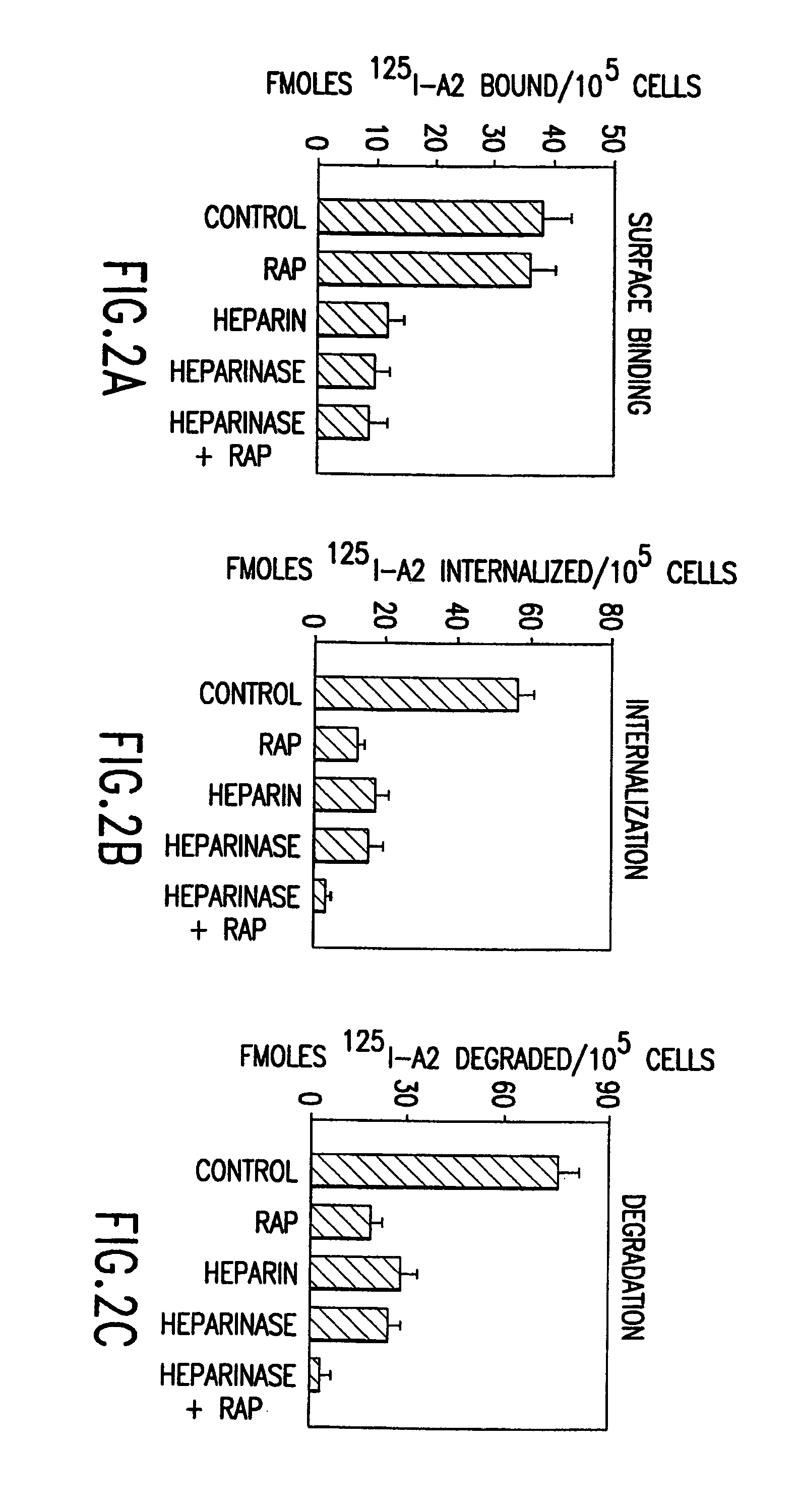
Mutant human factor IX with an increased resistance to inhibition by heparin
InactiveUS7125841B2Improve the immunityShorten clotting timePeptide/protein ingredientsMammal material medical ingredientsFactor iiBiology
The present invention is related to a novel composition of matter and methods of using the same. More particularly, the invention describes mutant human factor IX which has an increased resistance to inhibition by heparin. Methods of making and using this composition for the therapeutic intervention of hemophilia are disclosed.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
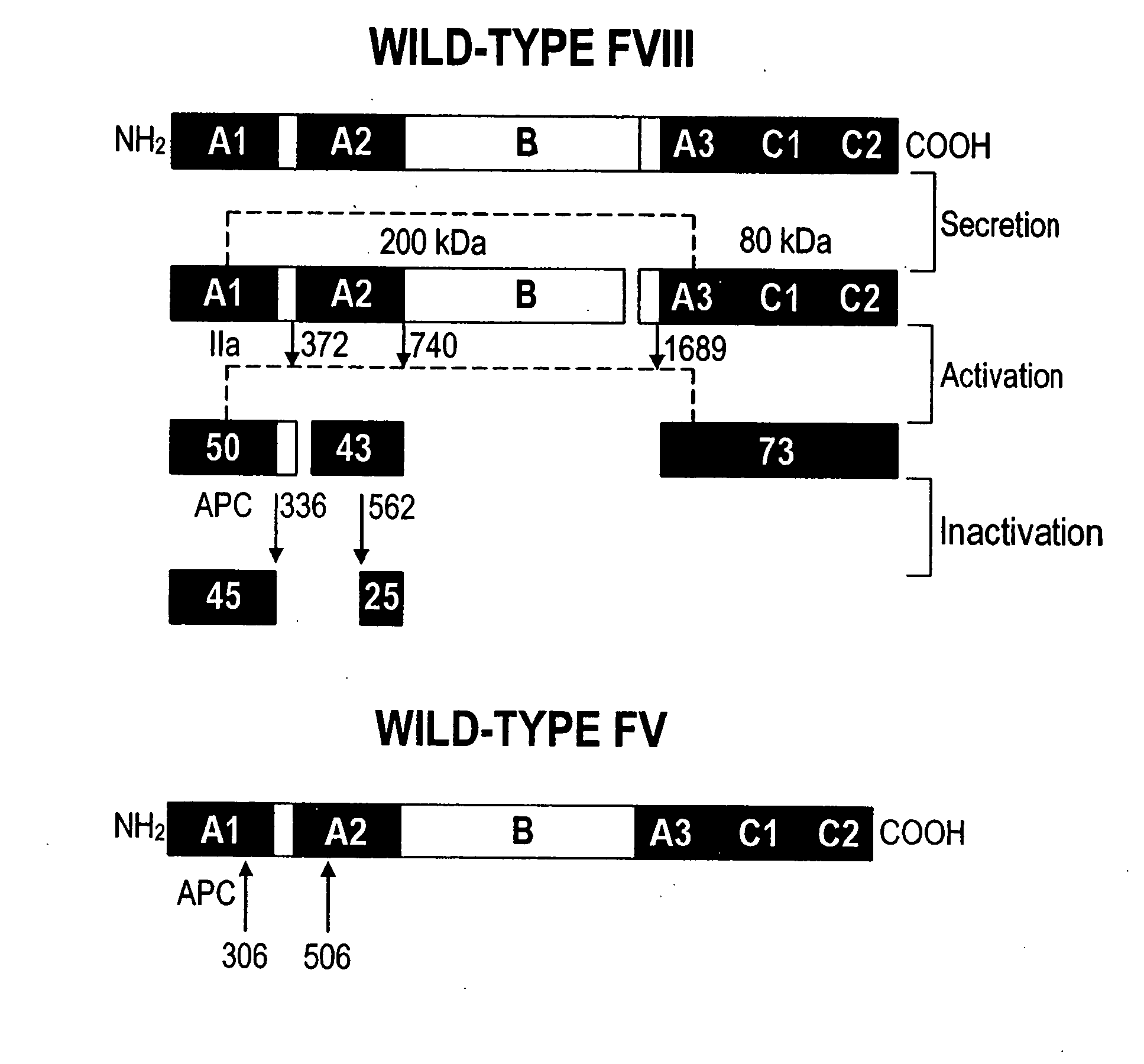
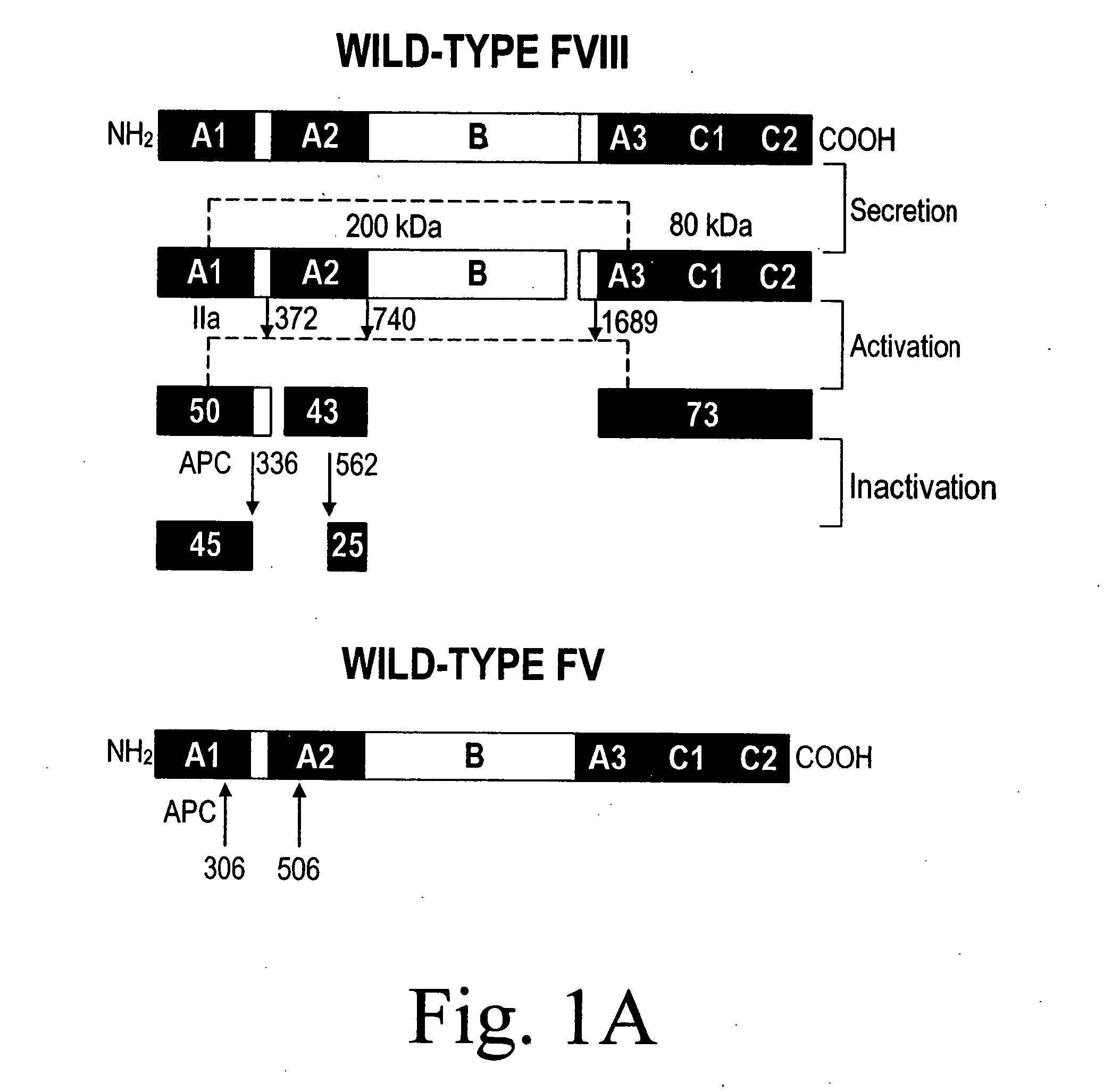
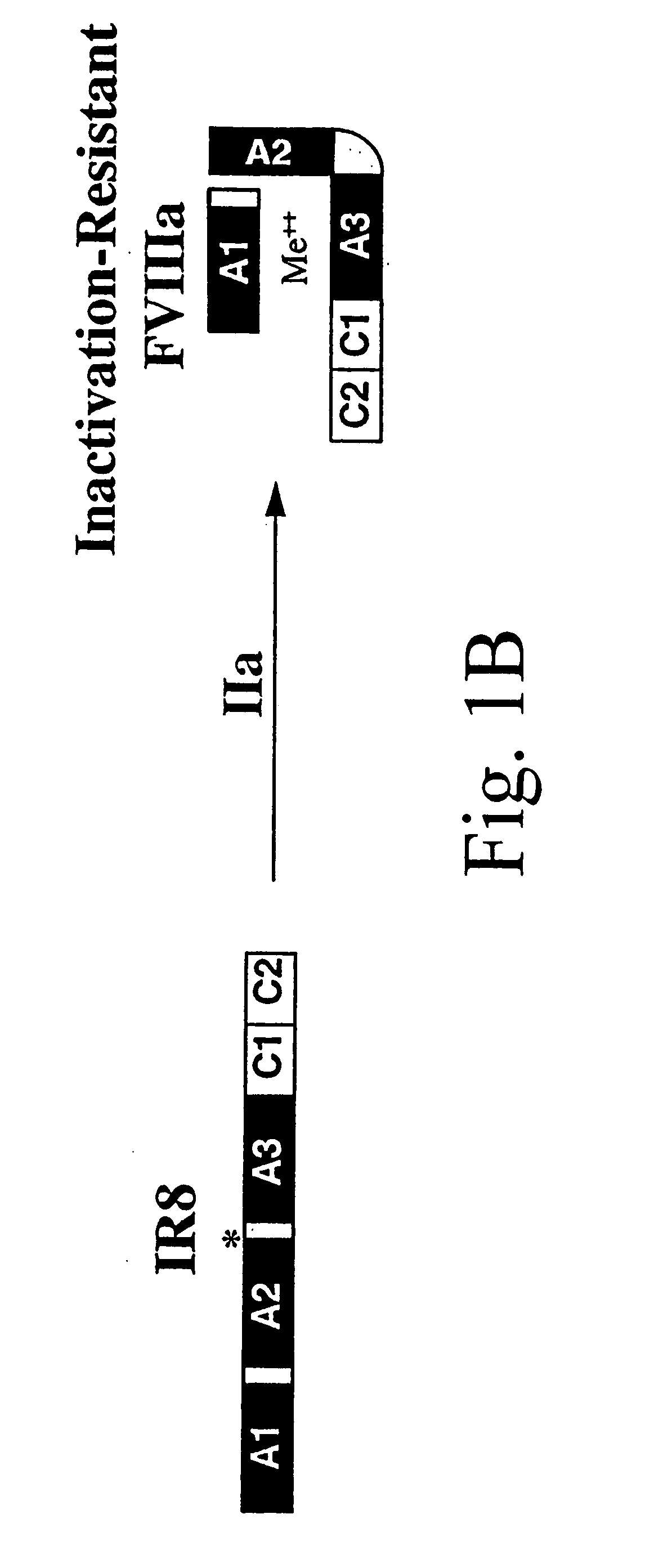
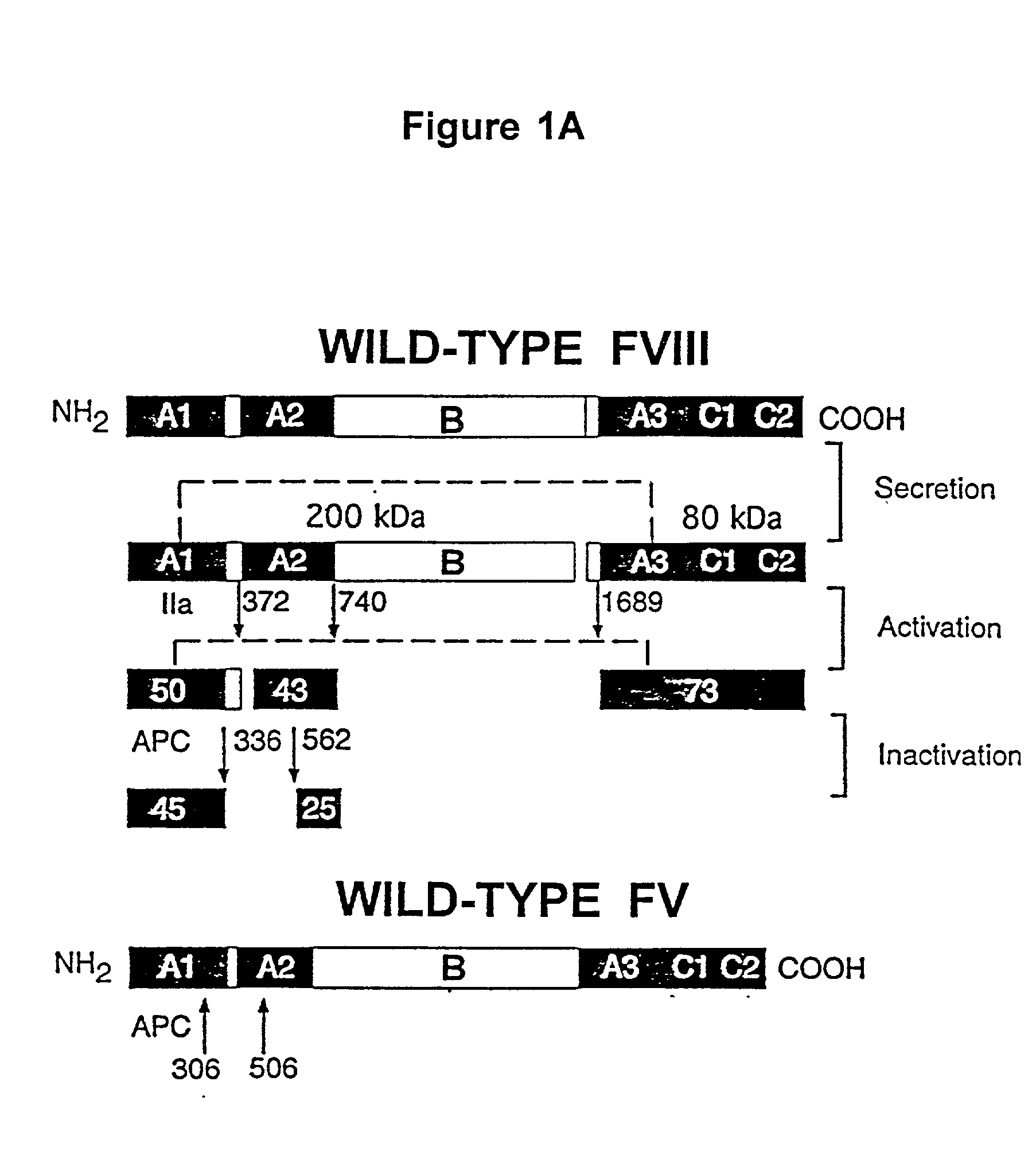
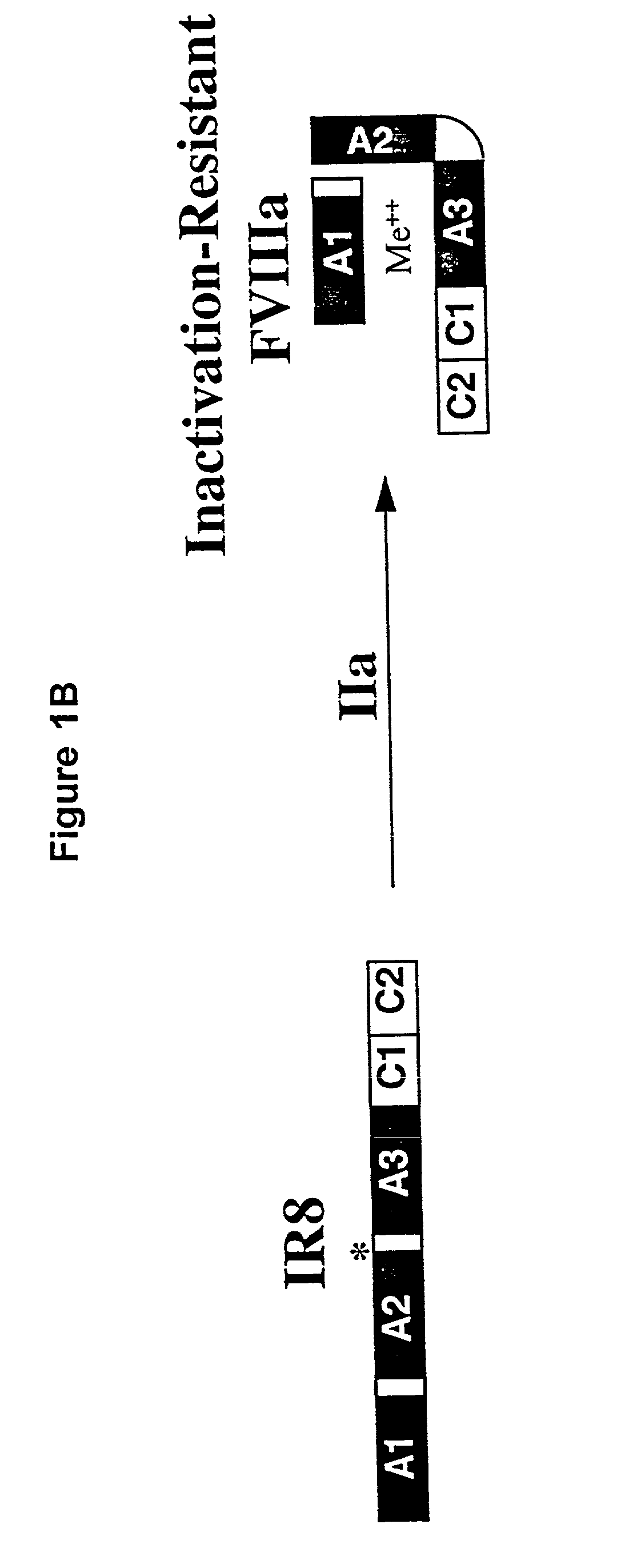
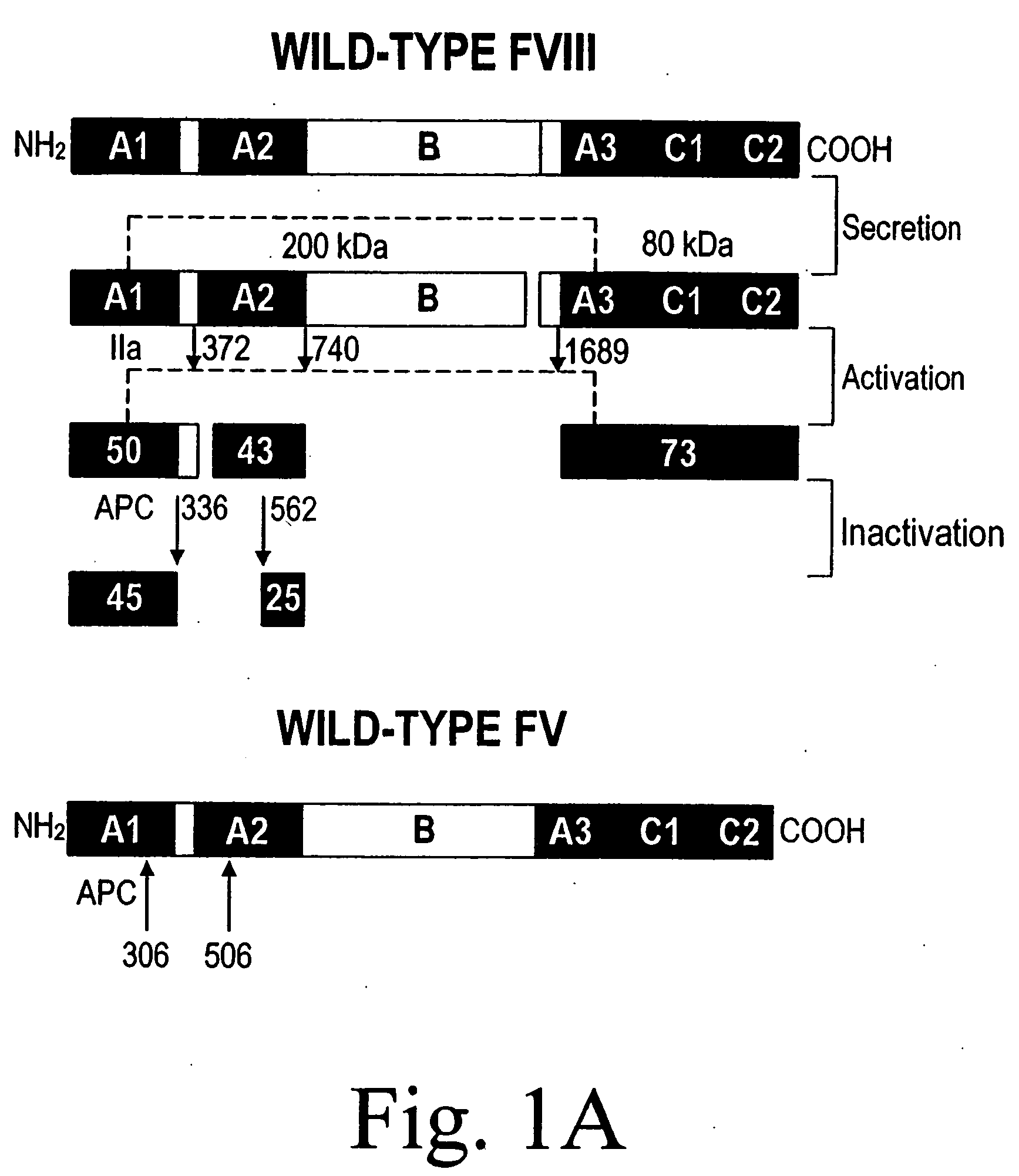
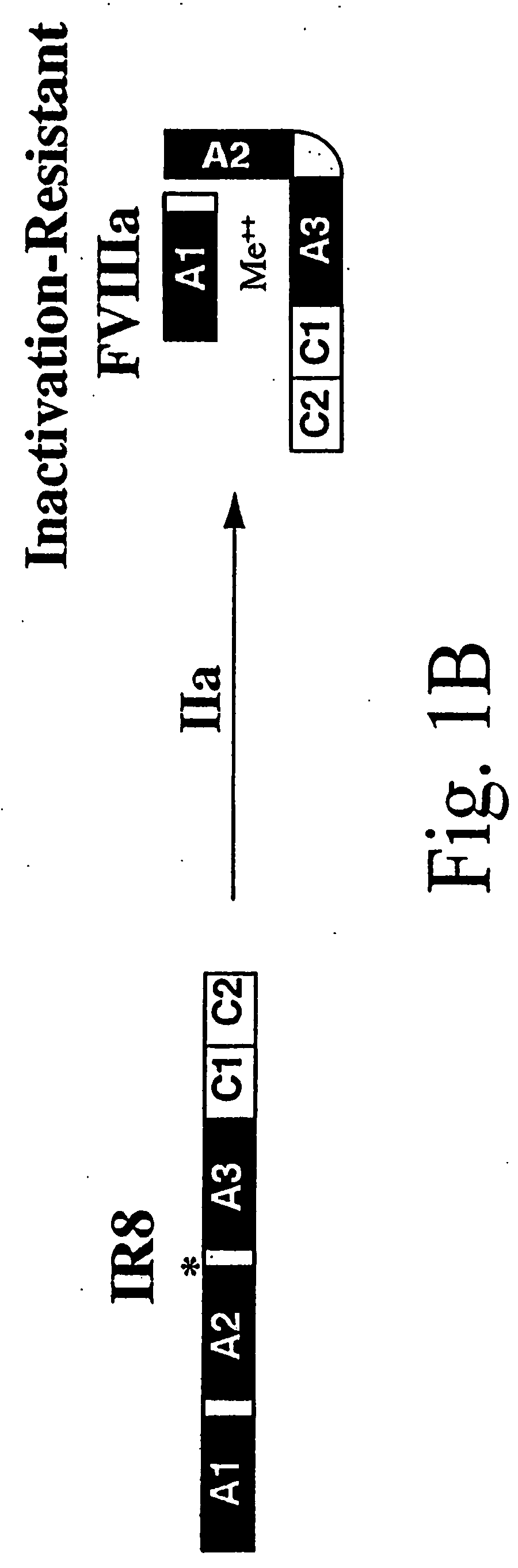
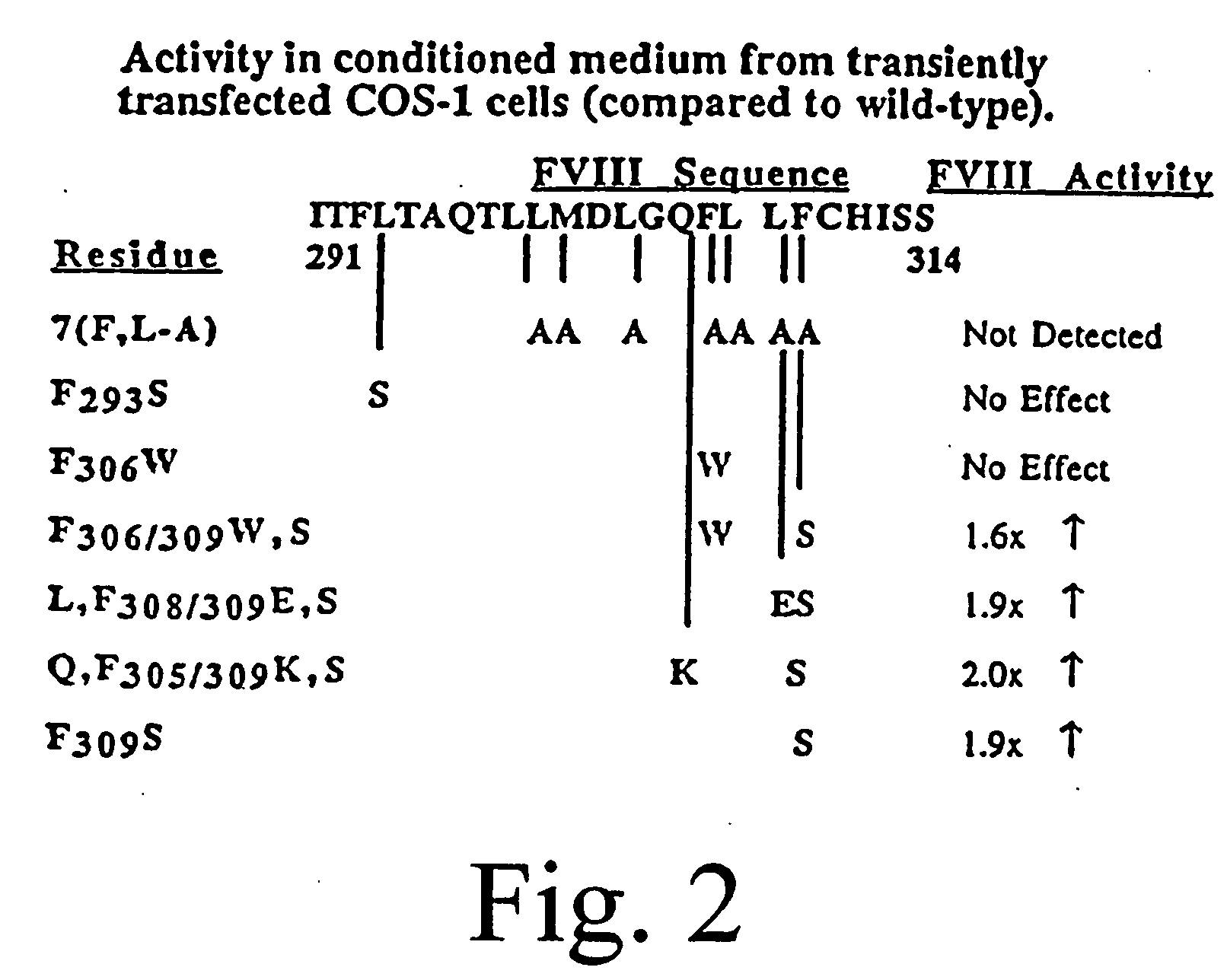
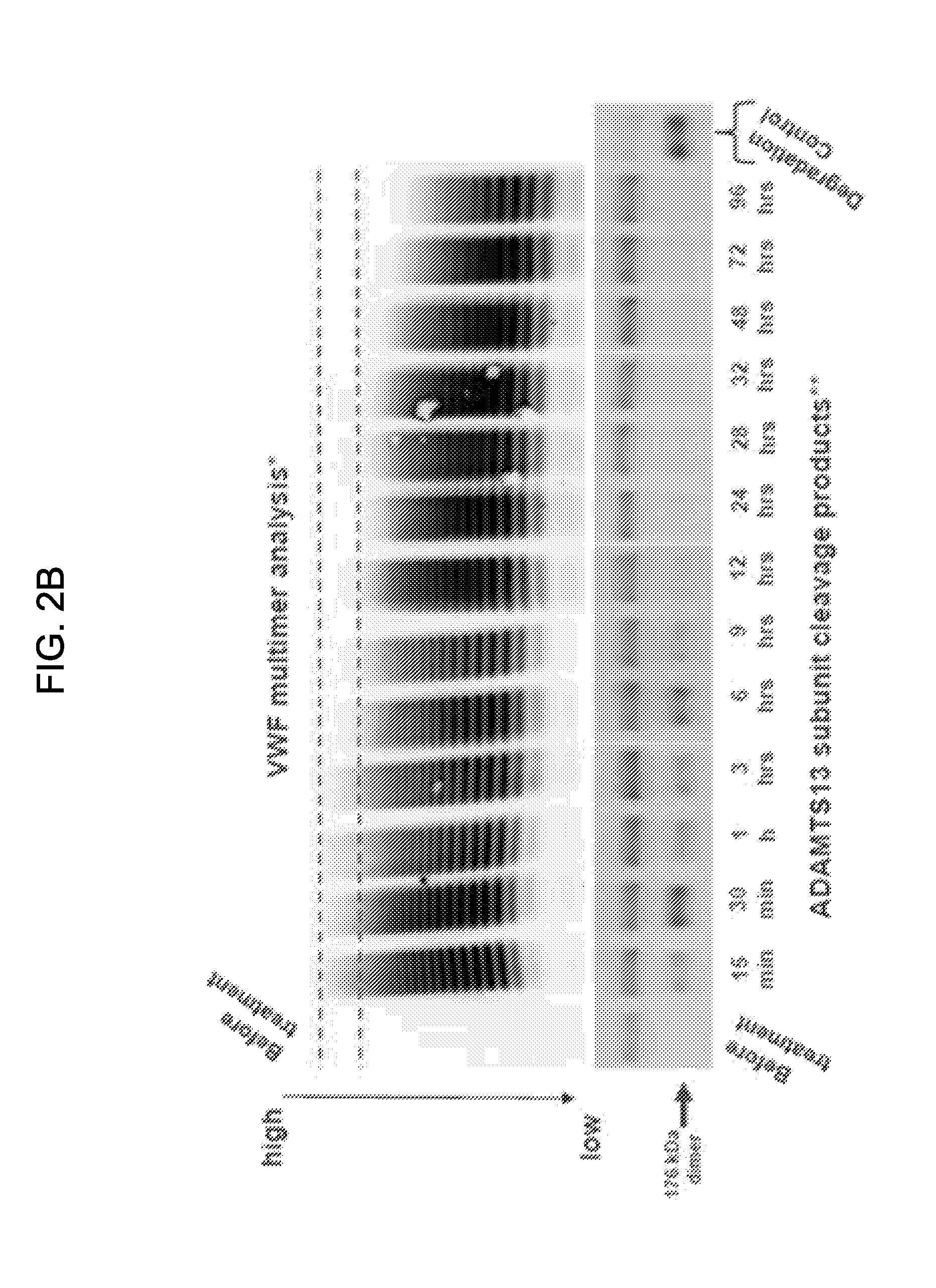
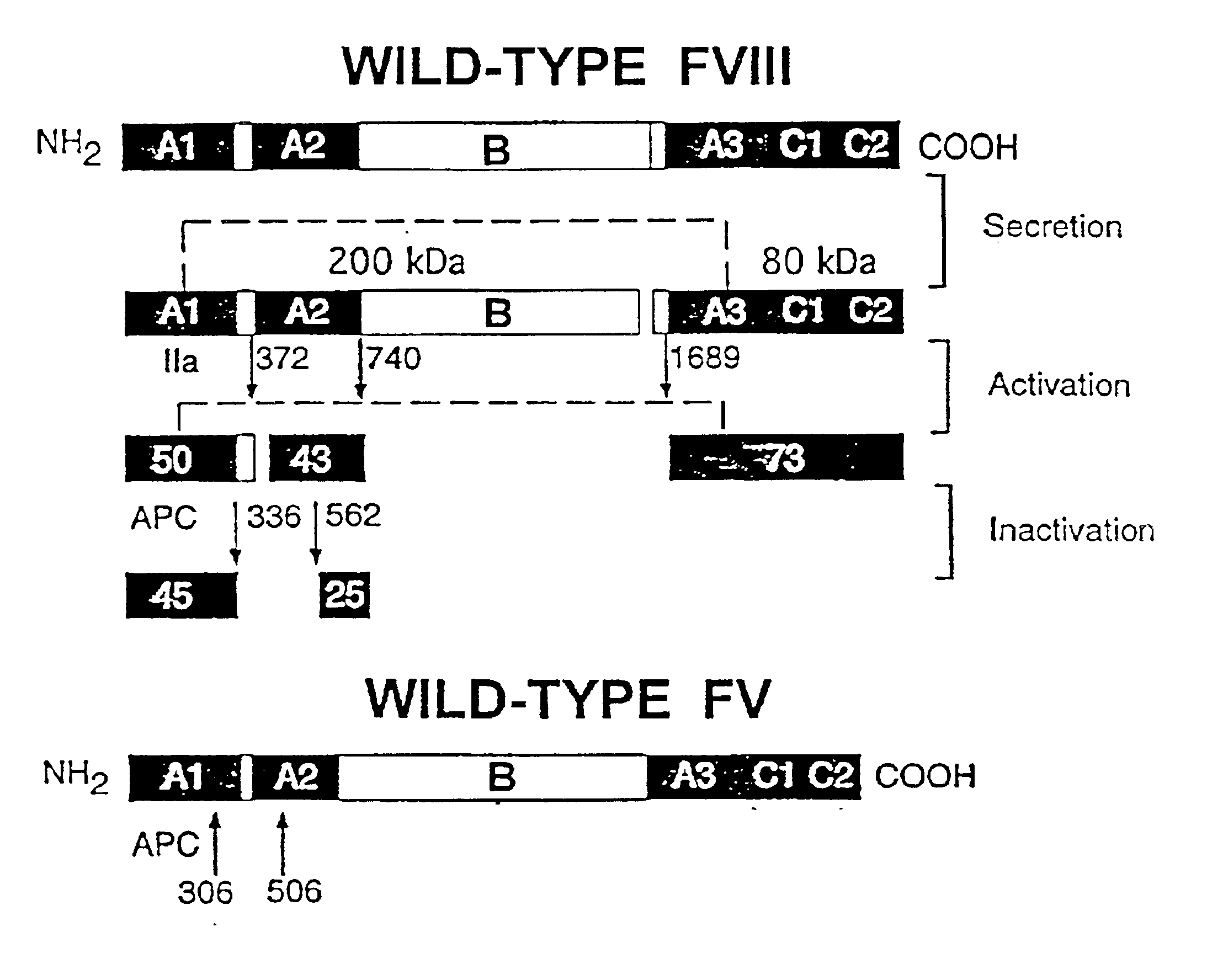
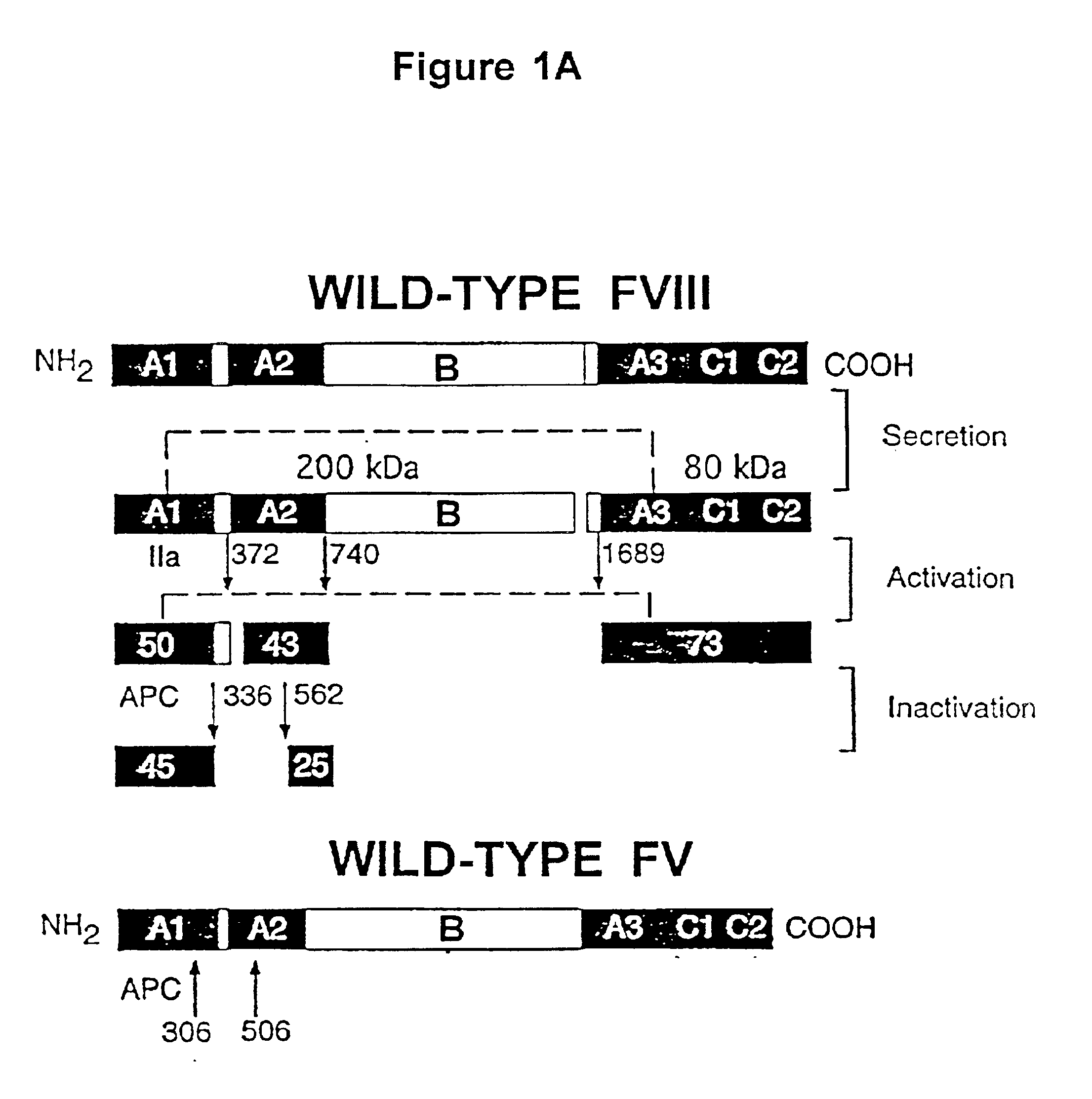
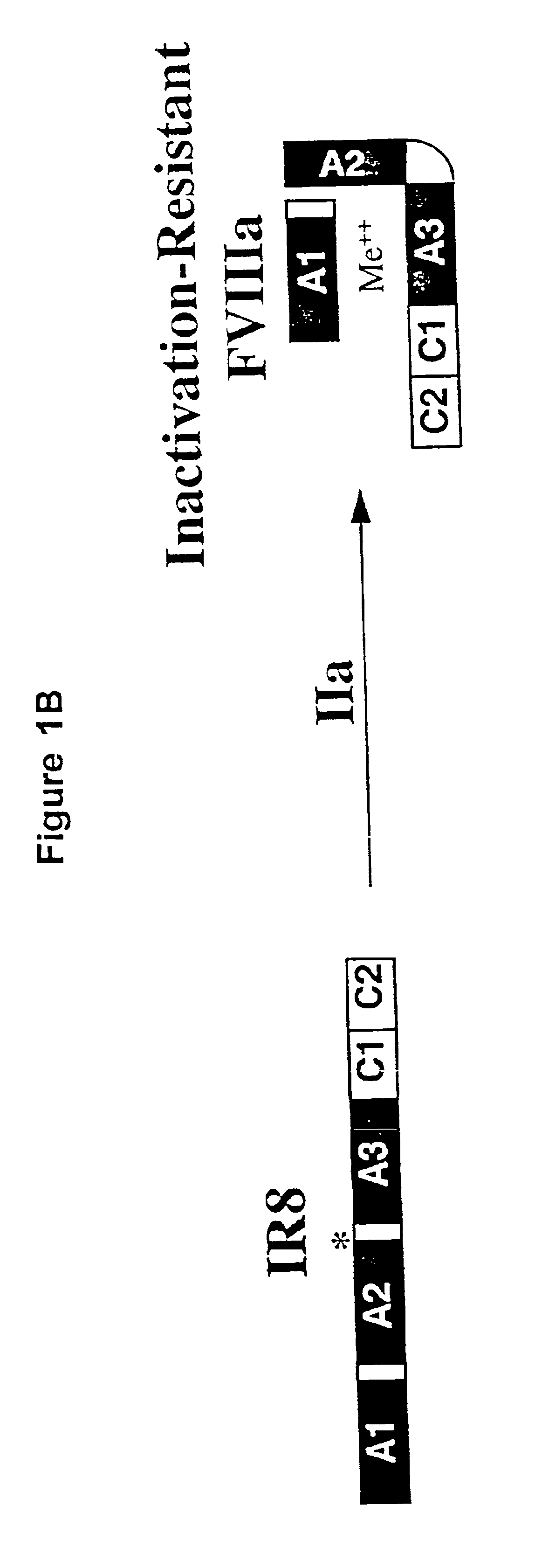
Inactivation resistant factor VIII
InactiveUS20040092442A1Increase secretionIncrease FVIII expressionFactor VIIBacteriaNucleotideBinding site
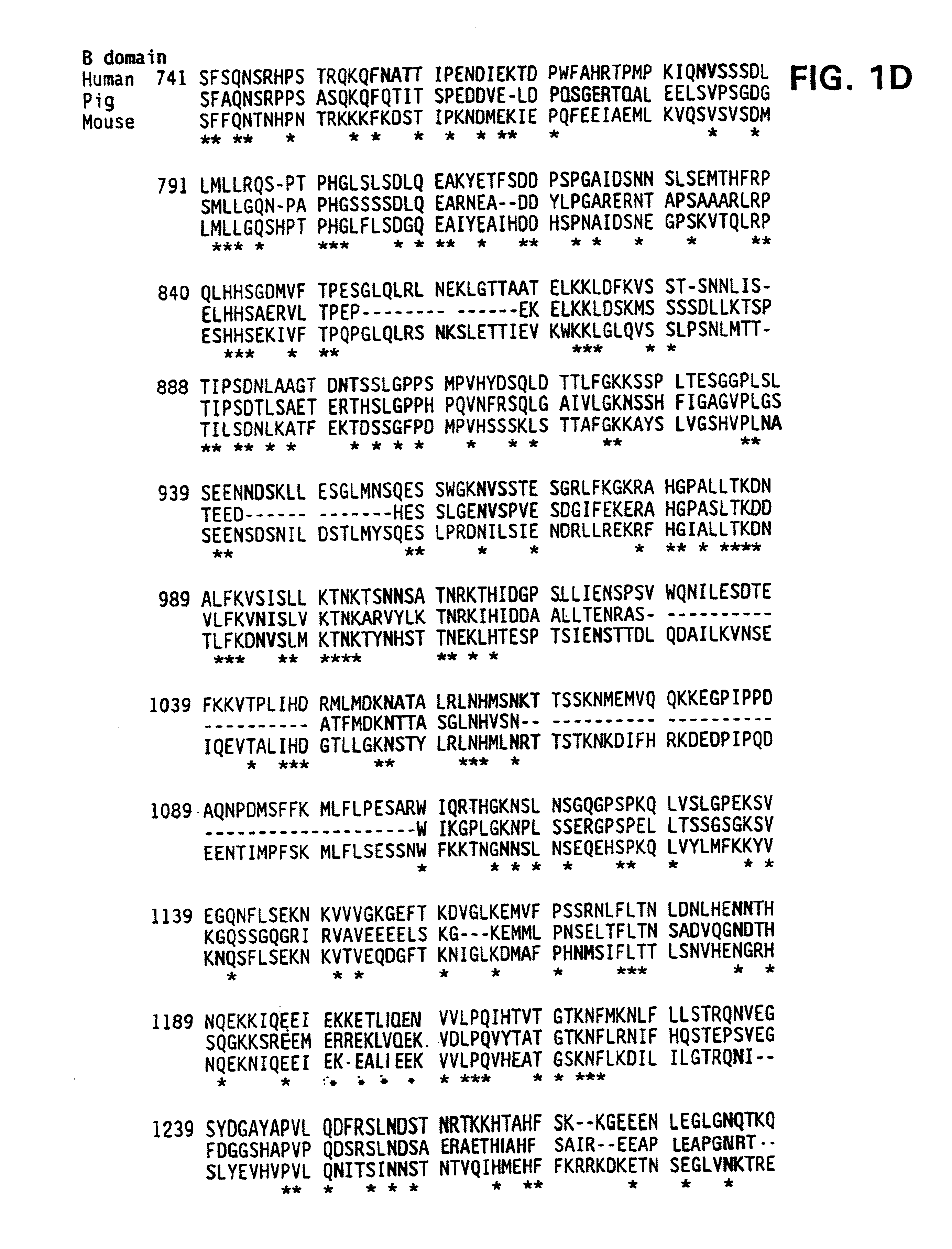
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe3O9 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated, an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:UNIV OF MICHIGAN THE
CLOTTING FACTOR-Fc CHIMERIC PROTEINS TO TREAT HEMOPHILIA
ActiveUS20110182896A1Reduce riskEffective treatmentPeptide/protein ingredientsHydrolasesHemostatic DisordersImmunoglobulin IgE
Owner:BIOVERATIV THERAPEUTICS INC
Modified factor VIII
Owner:EMORY UNIVERSITY
Factor VIII compositions and methods
The present invention provides methods of increasing the half-life and / or specific activity of factor VIII. More specifically, the invention provides methods of increasing the half-life and / or specific activity of factor VIII by substituting one or more amino acids in the A2 domain. It further provides methods for producing such factor VIII mutants. The invention also provides polynucleotides encoding the mutant factor VIII, and methods of treating hemophilia using the polypeptides and polynucleotides of the invention.
Owner:UNIV OF MARYLAND BALTIMORE
Modified factor VIII
Modified porcine factor VIII is disclosed in which most of the B domain has been removed through genetic engineering. This modified factor VIII is particularly useful for treatment of hemophiliacs, especially those undergoing bleeding episodes.
Owner:EMORY UNIVERSITY
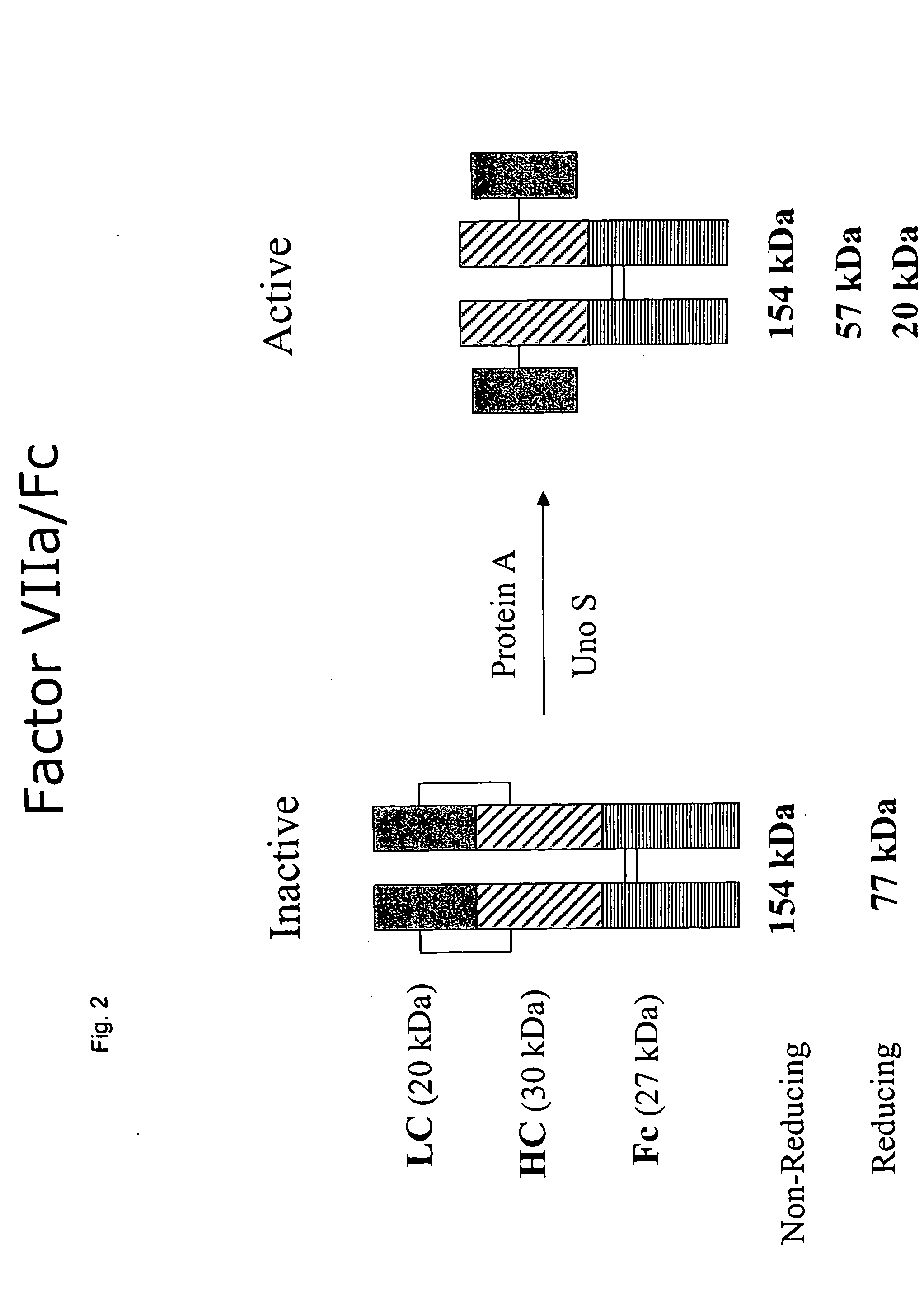
Factor VII polypeptides for preventing formation of inhibitors in subjects with haemophilia
InactiveUS20050032690A1Inhibition formationImprove stabilityOrganic active ingredientsFactor VIIFactor VIIaBlood coagulation factor VIII
The invention provides a method for preventing formation of inhibitors to blood coagulation factor VIII or factor IX in a subject having haemophilia, the method comprising administering (via intravenous, subcutaneous, intradermal, or intramuscular routes) to a previously untreated subject an effective dosage of factor VIIa or a factor VII-related polypeptide.
Owner:NOVO NORDISK AS
Inactivation resistant factor VIII related applications
InactiveUS20020132306A1Reduce the possibilityLow costOrganic active ingredientsBiocideBinding siteENCODE
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe3O9 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated and an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:RGT UNIV OF MICHIGAN
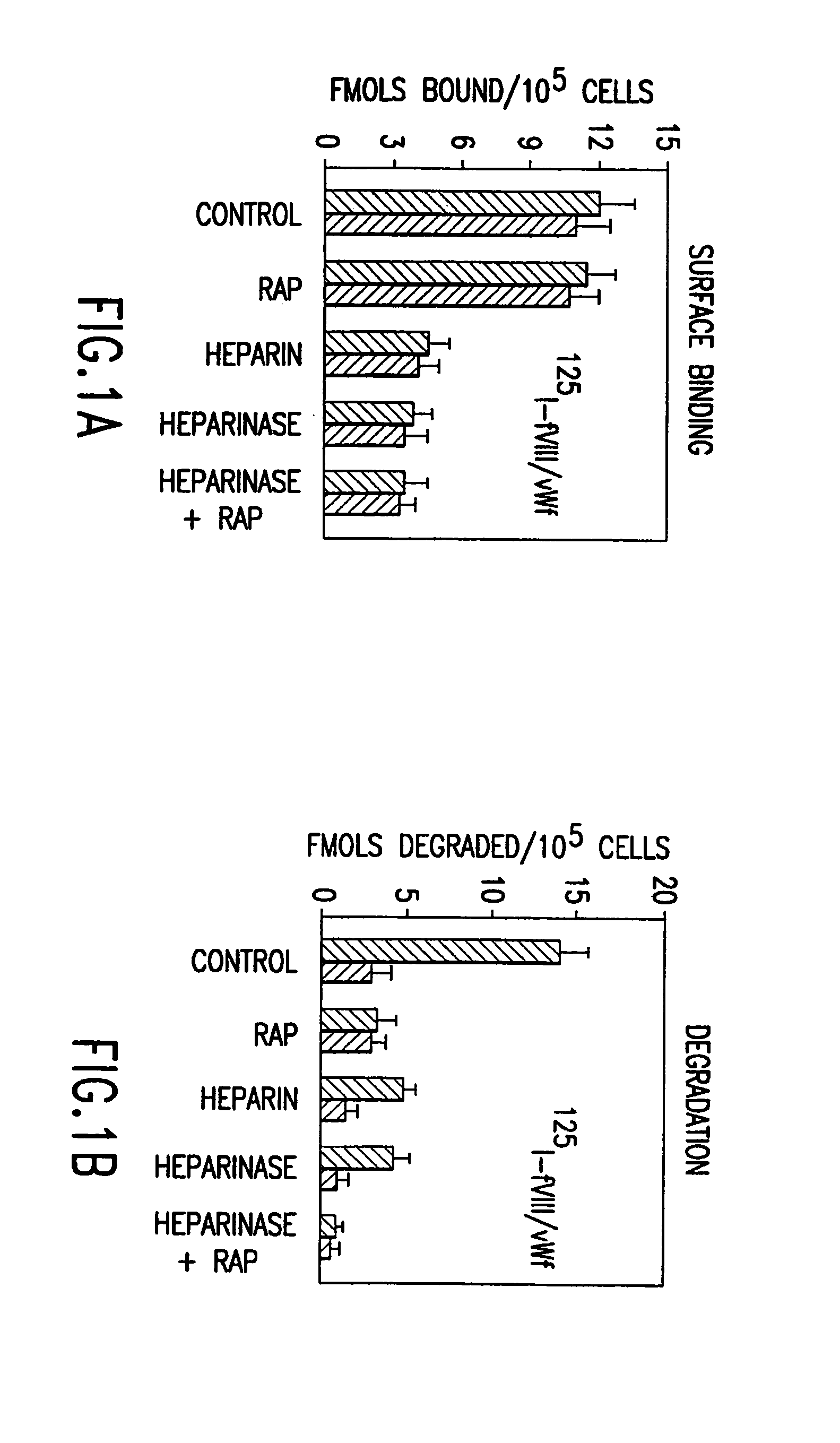
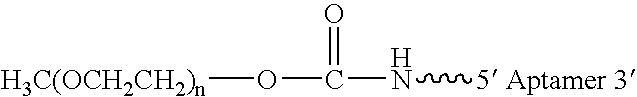
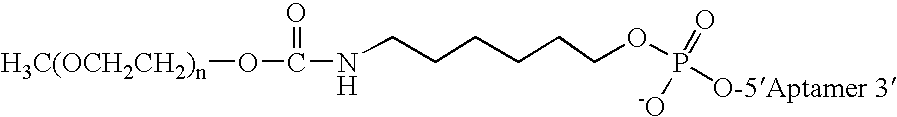
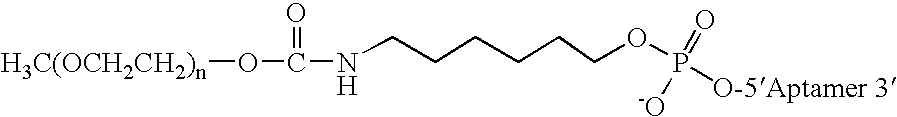
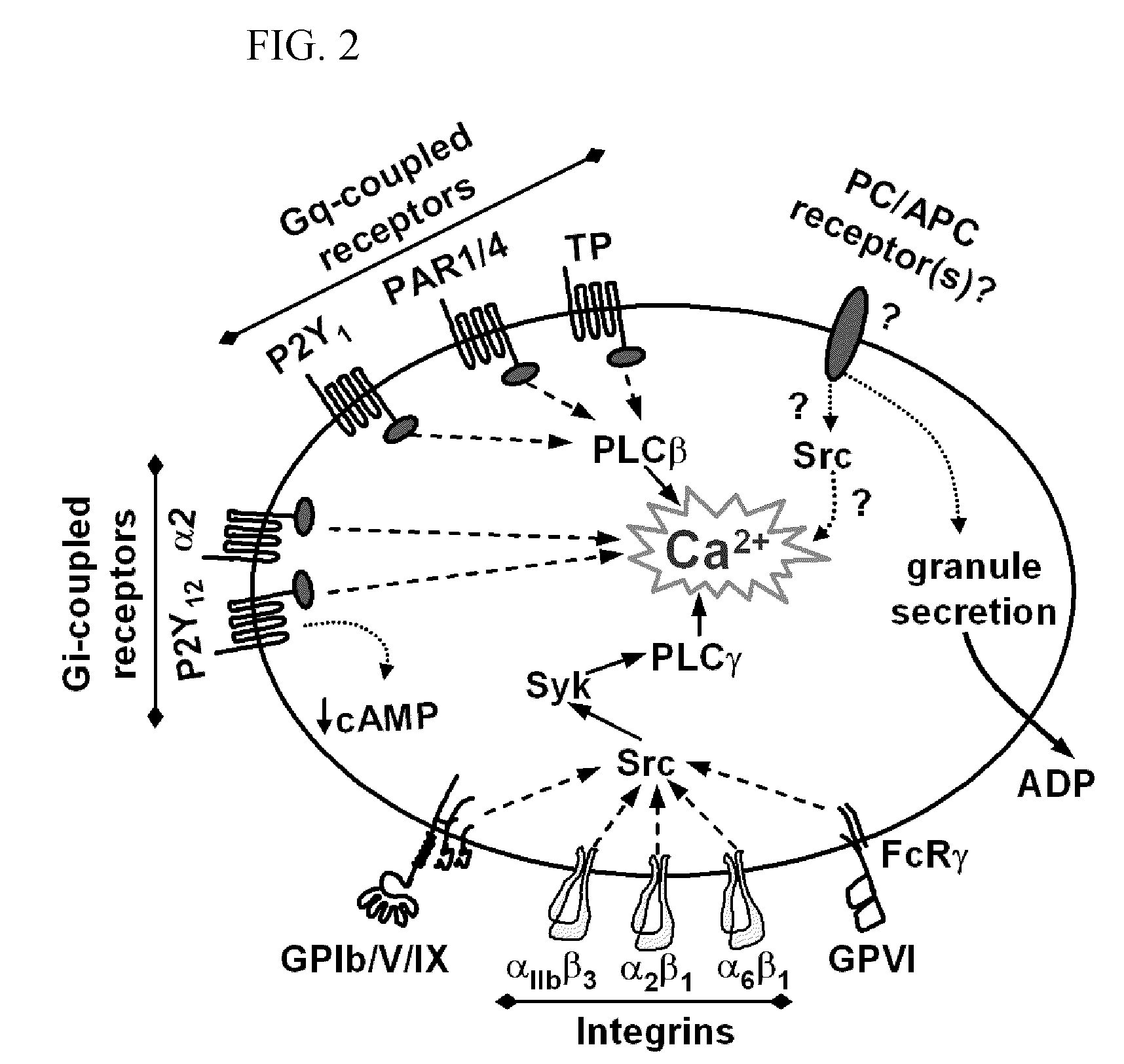
Aptamers to von Willebrand Factor and their use as thrombotic disease therapeutics
InactiveUS20060264369A1Shorten bleeding timeInhibits platelet aggregationPeptide/protein ingredientsGenetic material ingredientsAptamerDisease
The invention relates generally to the field of nucleic acids and more particularly to aptamers capable of binding to von Willebrand Factor useful as therapeutics in and diagnostics of thrombotic diseases and / or other diseases or disorders in which von Willebrand Factor mediated platelet aggregation has been implicated. The invention further relates to materials and methods for the administration of aptamers capable of binding to von Willebrand Factor.
Owner:ARCHEMIX CORP
Inactivation resistant factor VIII
InactiveUS20060293238A1Increase secretionHigh expressionFactor VIIPeptide/protein ingredientsBinding siteENCODE
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe309 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated, an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:RGT UNIV OF MICHIGAN
Medical management system
InactiveUS20080046295A1Drug and medicationsComputer-assisted treatment prescription/deliveryTreatment periodMedical treatment
A medical management system. The burdensome task of medication logging is greatly reduced for a patient when compared to conventional methods to perform these tasks. Moreover, the invention allows for pharmaceutical supplier / providers and healthcare providers the ability better to manage, track, and trend the medication and products that must be provided to patients. This integrated ability allows for better management of the inventory of highly perishable and life-saving medications for chronically ill patients. The invention also allows for highly accurate communication between patients and other parties including his / her medical doctor, a pharmaceutical supplier / provider who provides medications and products for the patient, as well as the insurance provider providing medical insurance coverage for the patient. The system integrates embedded functionality that ensures that in the accidental event that a patient is prescribed tainted medication, the patient is warned of the taint in due time before self-administering the medication to avoid potentially contracting a disease or other ailment from the tainted medication. Patient medication is tracked and trending over the lifetime of the medication and the treatment period for the patient. Exceptions that outlay the trending of the patient's medication are identified and properly communicated, sometimes automatically, to a healthcare provider or medical doctor who treats the patient. The system is adaptable for use generally or to treat specific diseases, such as chronic diseases as hemophilia, diabetes, asthma, HIV, and other diseases requiring long term treatment and ongoing administration of medications and products.
Owner:BAYER HEALTHCARE LLC
Recombinant factor viii having reduced inactivation by activated protein c
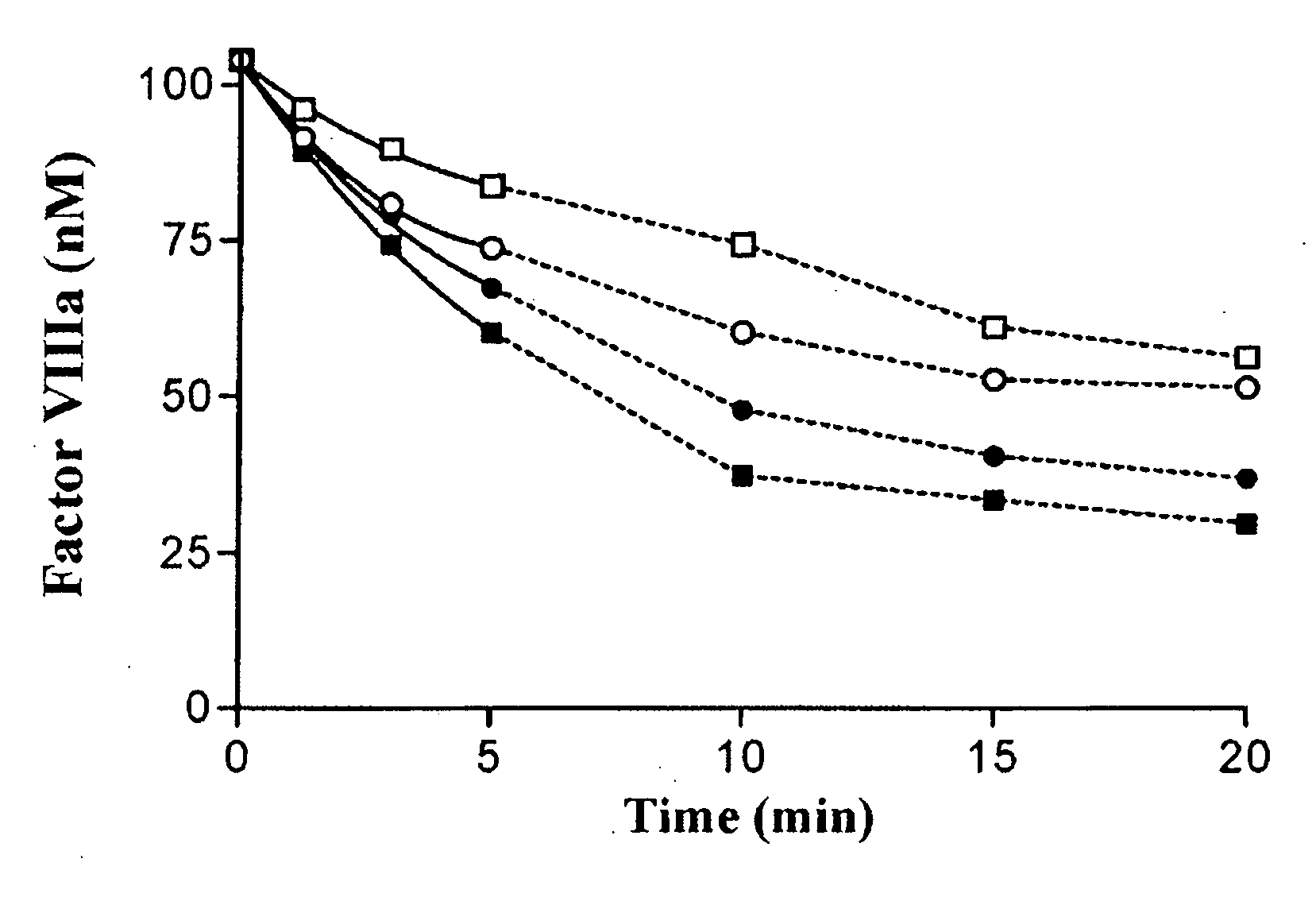
ActiveUS20090118185A1High catalytic efficiencyPromote localizationFactor VIIFungiProtein activationFactor ii
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
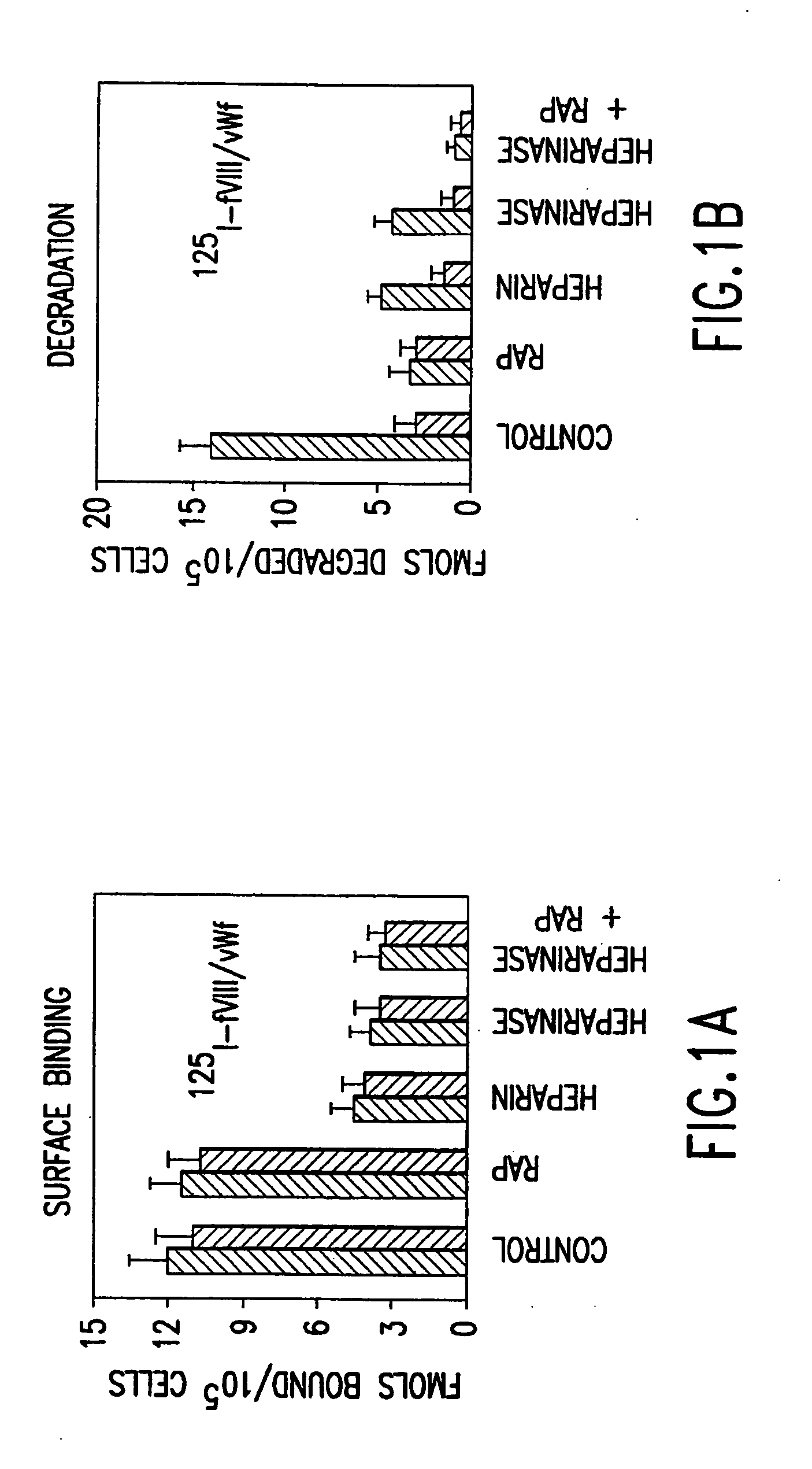
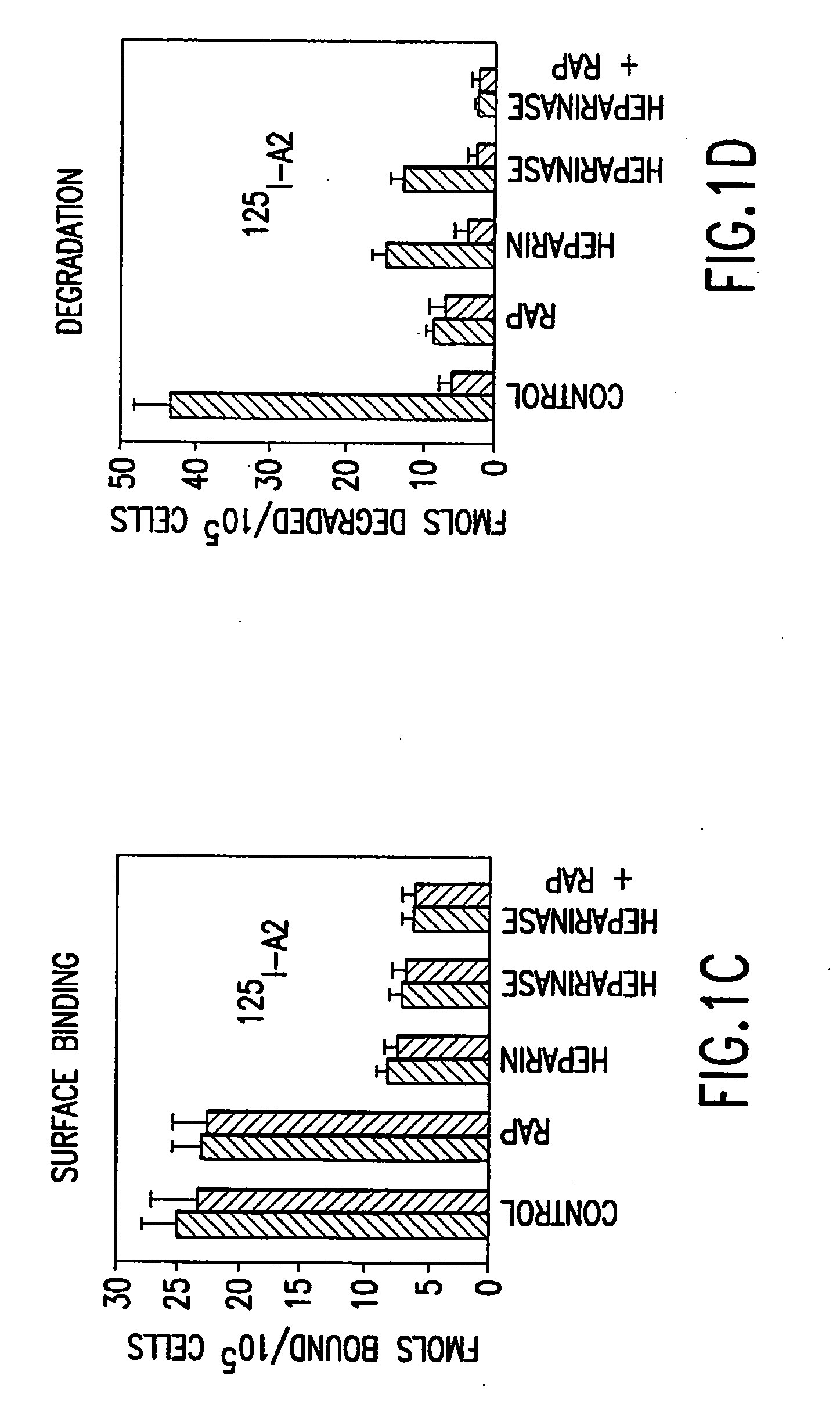
Recombinant expression vector system for variants of coagulation factor VIII and von Willebrand factor
ActiveUS20100183556A1Good coagulationReduce the possibilityOrganic active ingredientsBiocideFactor iiExon
Disclosed is an expression vector system for variants of coagulation factor VIII (FVIII) and von Willebrand factor (vWF). In detail, mutant vWF the size of which is significantly reduced by deleting exons but which has remarkably increased FVIII stabilizing and activating efficiency, and an expression vector system useful for the treatment of hemophilia which is capable of expressing the same along with FVIII are disclosed. Use of the mutant vWF with a reduced size enables effective expression of FVIII in a viral vector and significantly enhanced FVIII activity. Further, the viral vector may be effectively used to treat hemophilia through gene therapy.
Owner:KOREA UNIV IND & ACADEMIC CALLABORATION FOUND
Minimal adenoviral vector
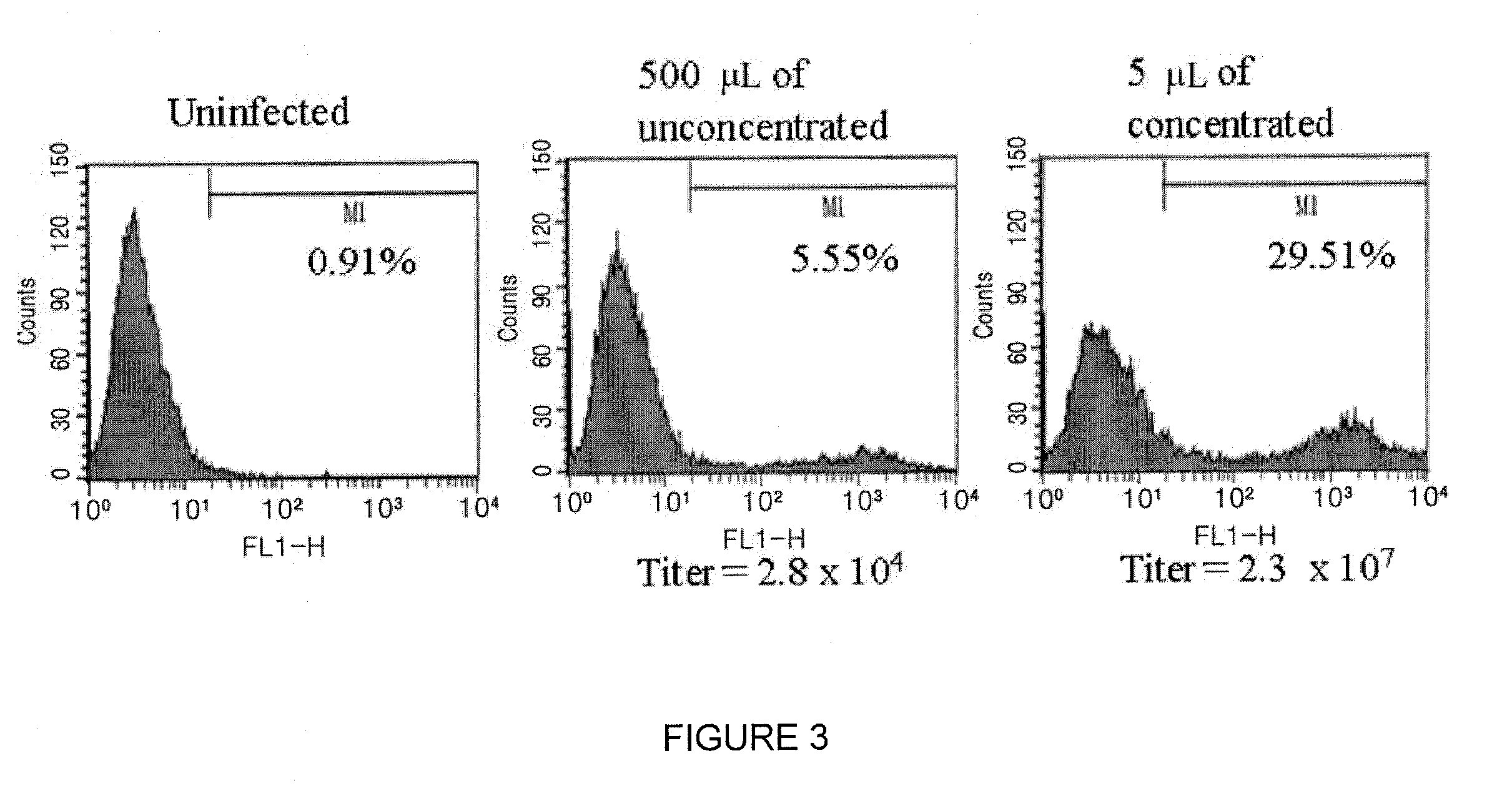
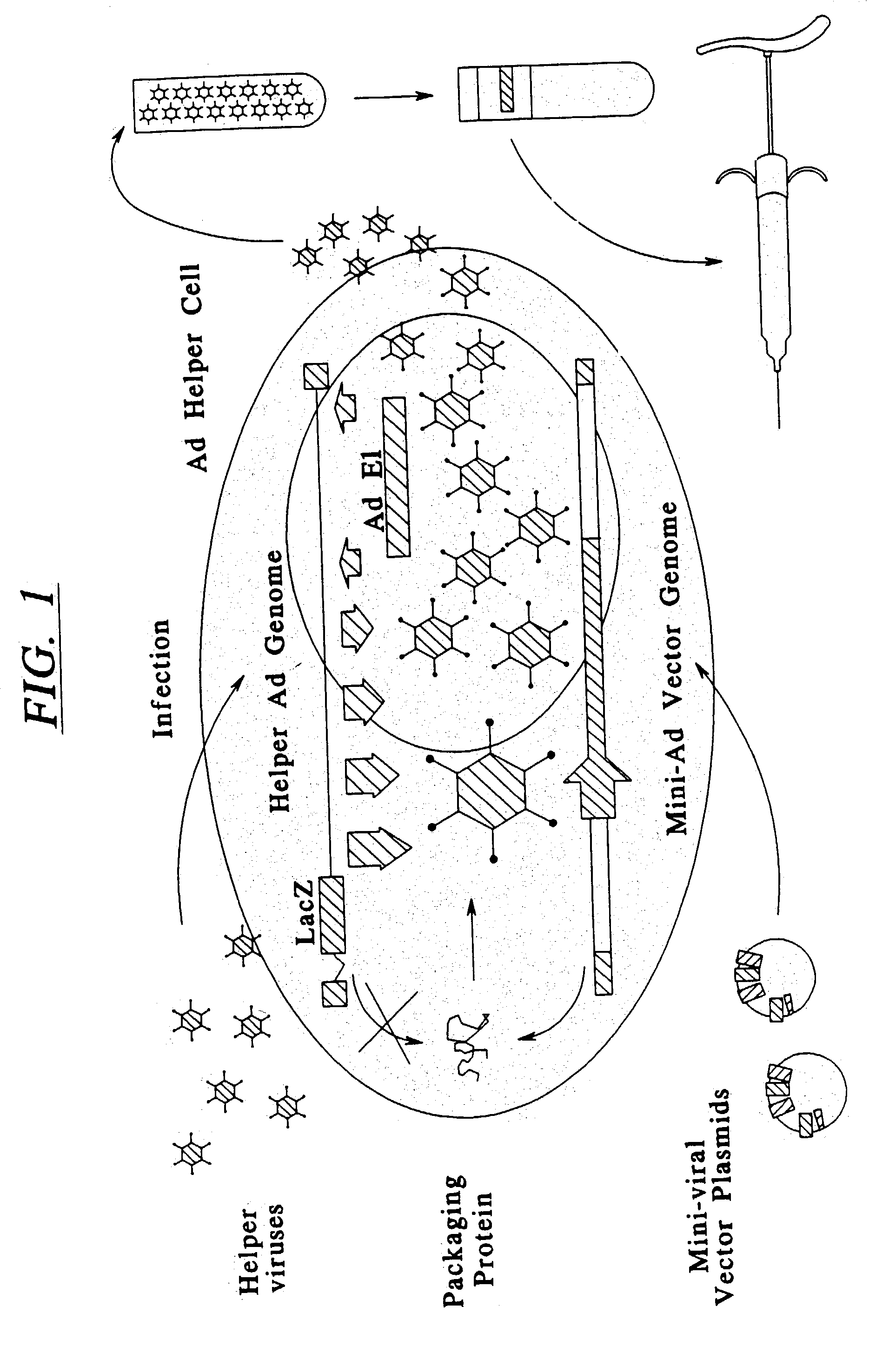
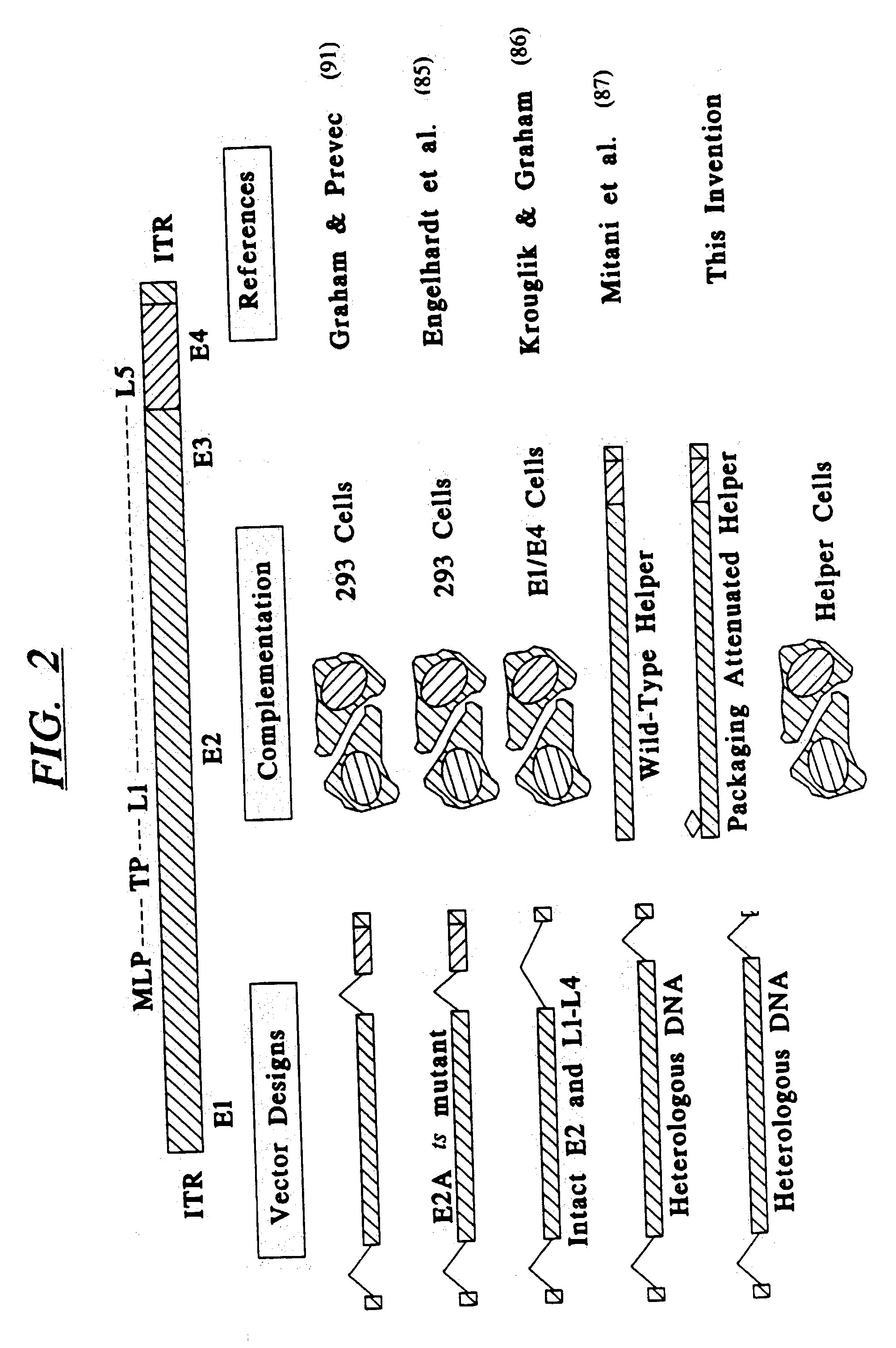
InactiveUS20030192066A1Accurate assessmentComplicate studies of long-term delivery of FVIIIBiocideFactor VIIVaccinationGene transfer
This invention is related to adenoviral (Ad) vectors and their applications in the field of genetic medicine, including gene transfer, gene therapy, and gene vaccination. More specifically, this invention is related to the Ad vectors that carry the minimal cis-element of the Ad genome (mini-Ad vector) and are capable of delivering transgenes and / or heterologous DNA up to 36 kb. The generation and propagation of the mini-Ad vectors require trans-complementation of a packaging-attenuated and replication-defective helper Ad (helper) in an Ad helper cell line. This invention further comprises a methodology for generating a mini-adenoviral (mini-Ad) vector for use in gene therapy of hemophilia and animal test systems for in vivo evaluation of the Ad vectors. More specifically, this invention describes factor VIII (FVIII) Ad vectors that only contain minimal cis-elements of the Ad genome (so called mini-Ad) and comprise a human FVIII cDNA with other supporting DNA elements up to 36 kb. The FVIII mini-Ad can be generated and preferentially amplified through the assistance of a packaging-attenuated helper Ad and a helper cell line. This invention also reports designs and methods for producing transgenic mouse models that can be used for in vivo testing the mini-Ad.
Owner:GENSTAR THERAPEUTICS
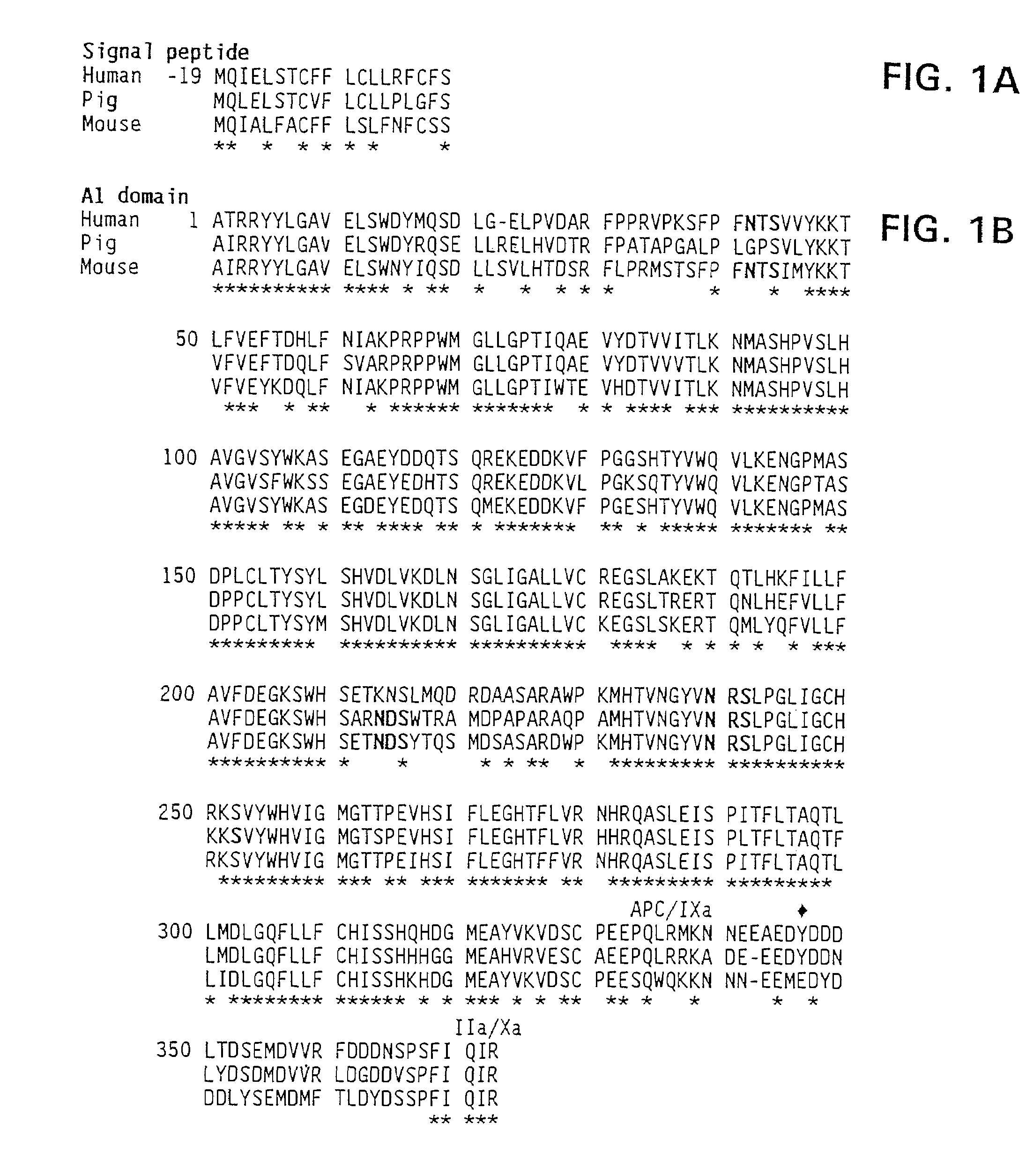
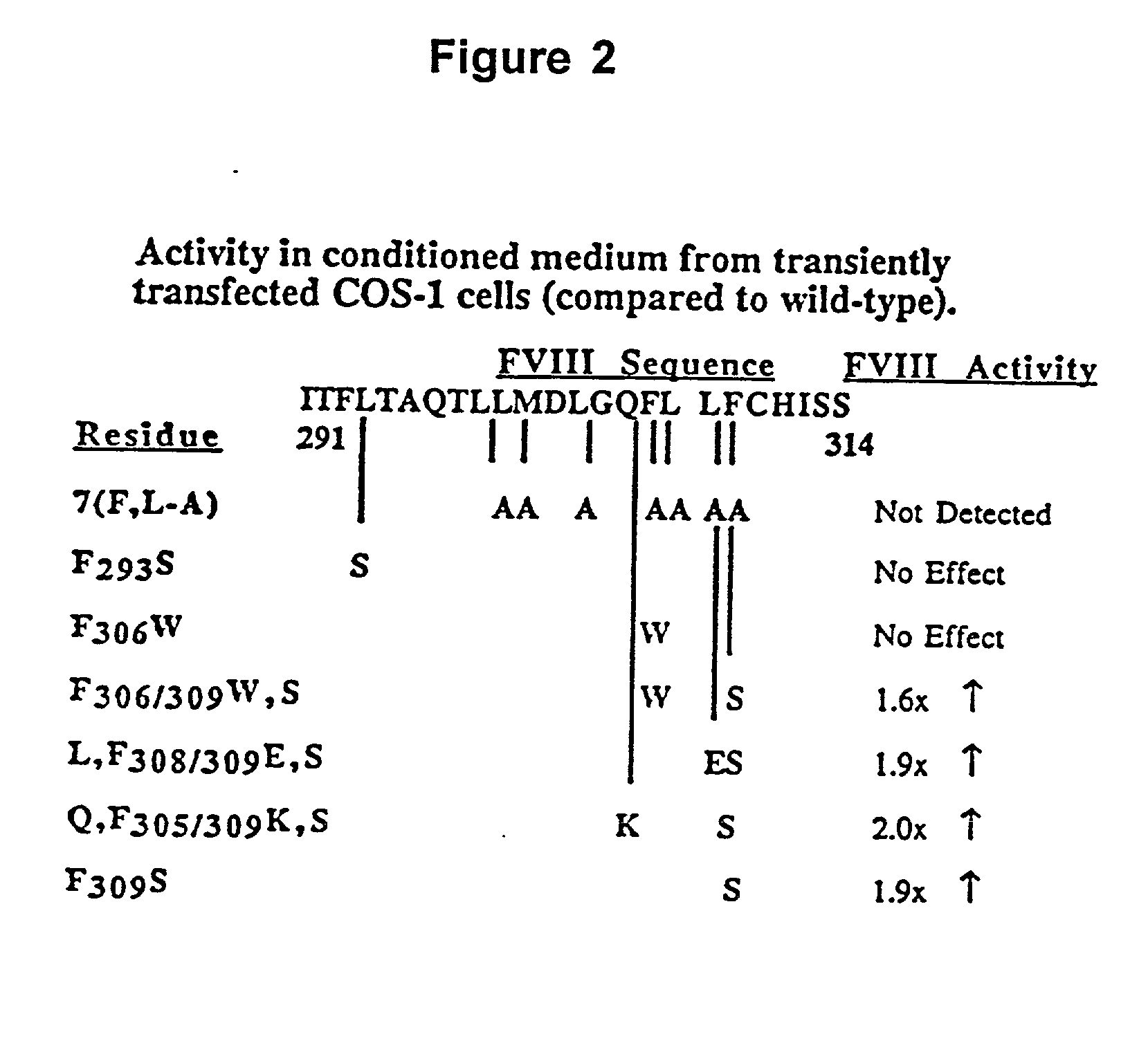
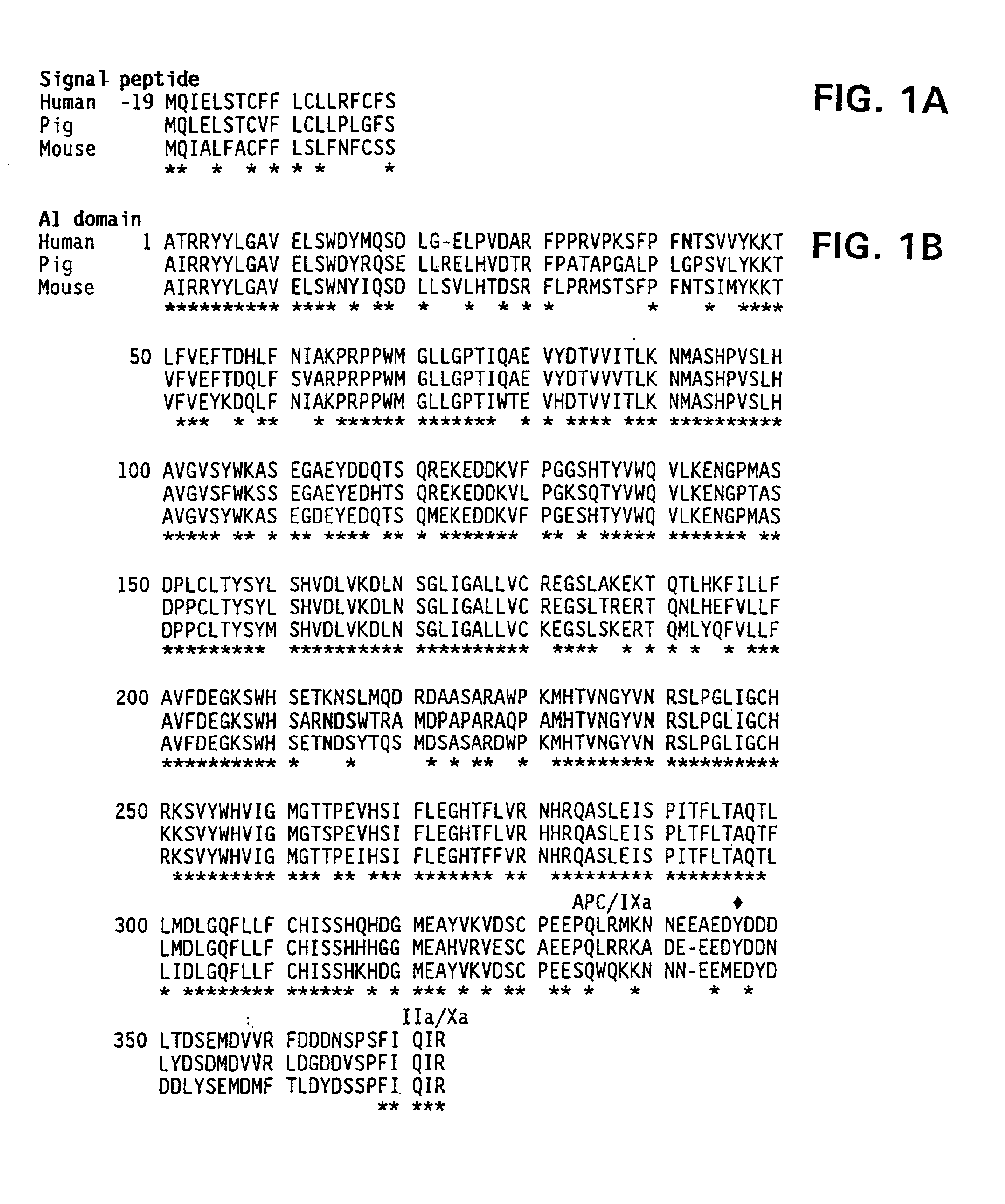
Nucleic acid molecules encoding modified factor VIII proteins, expression products, and methods of making the same
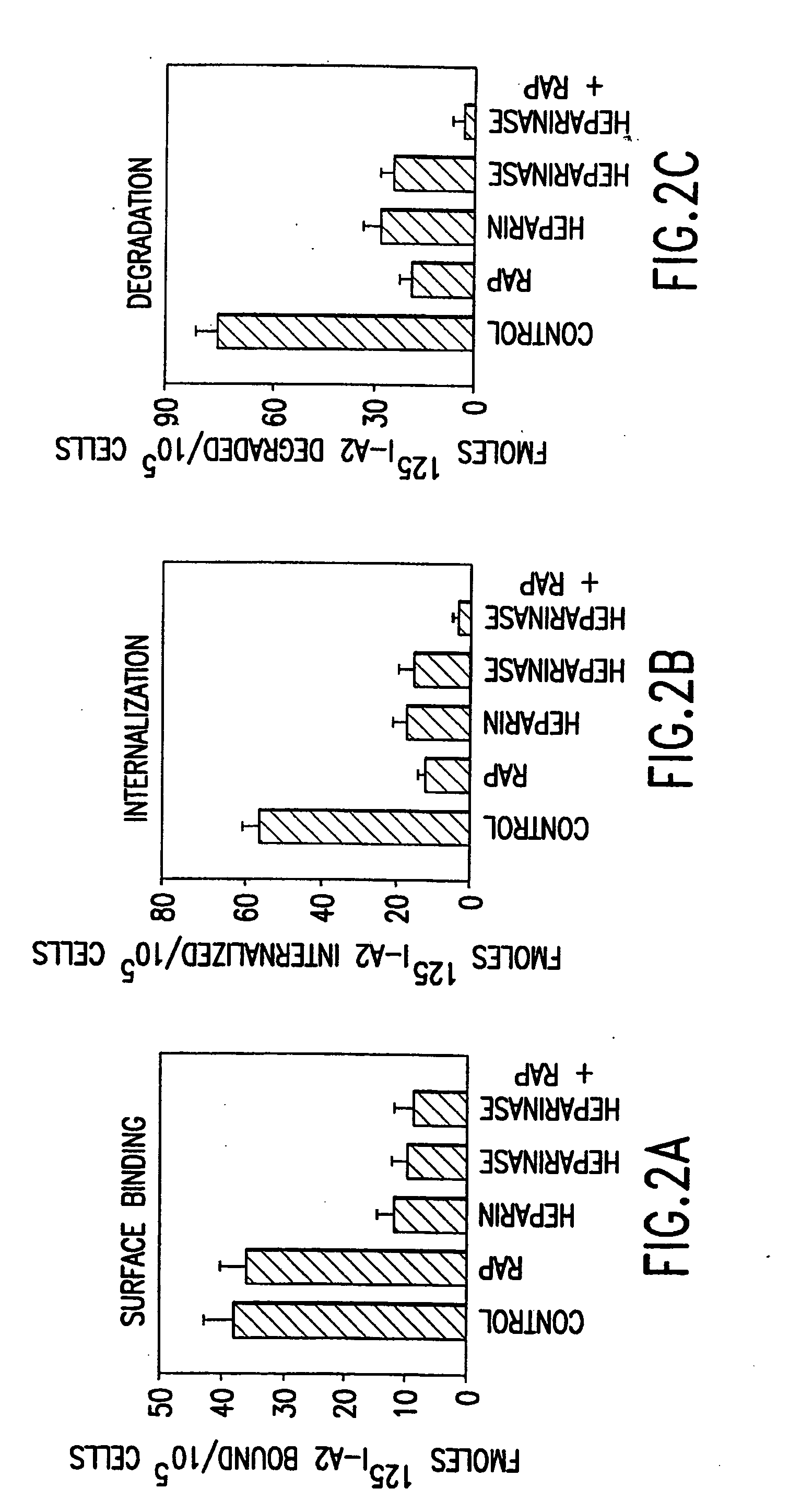
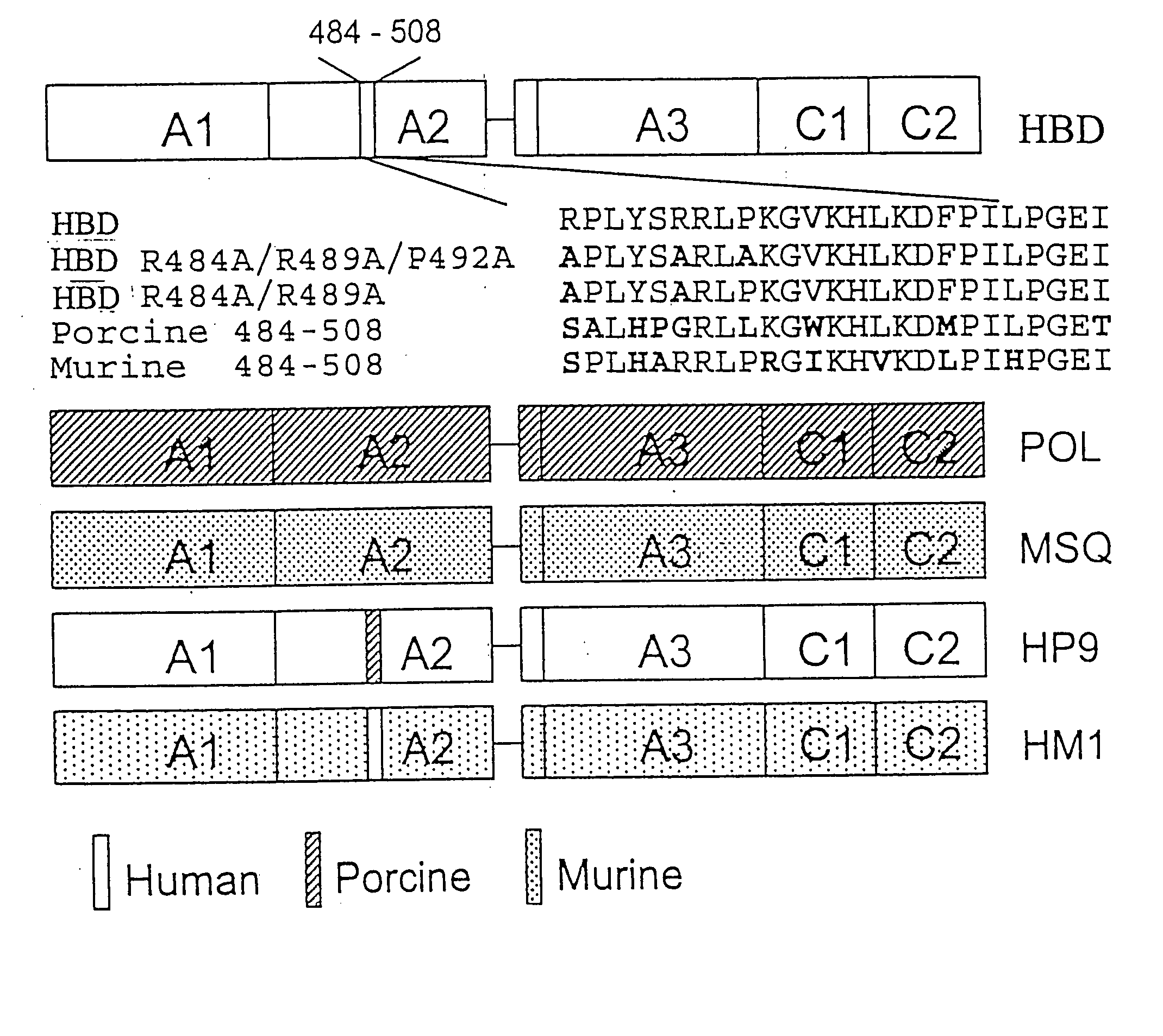
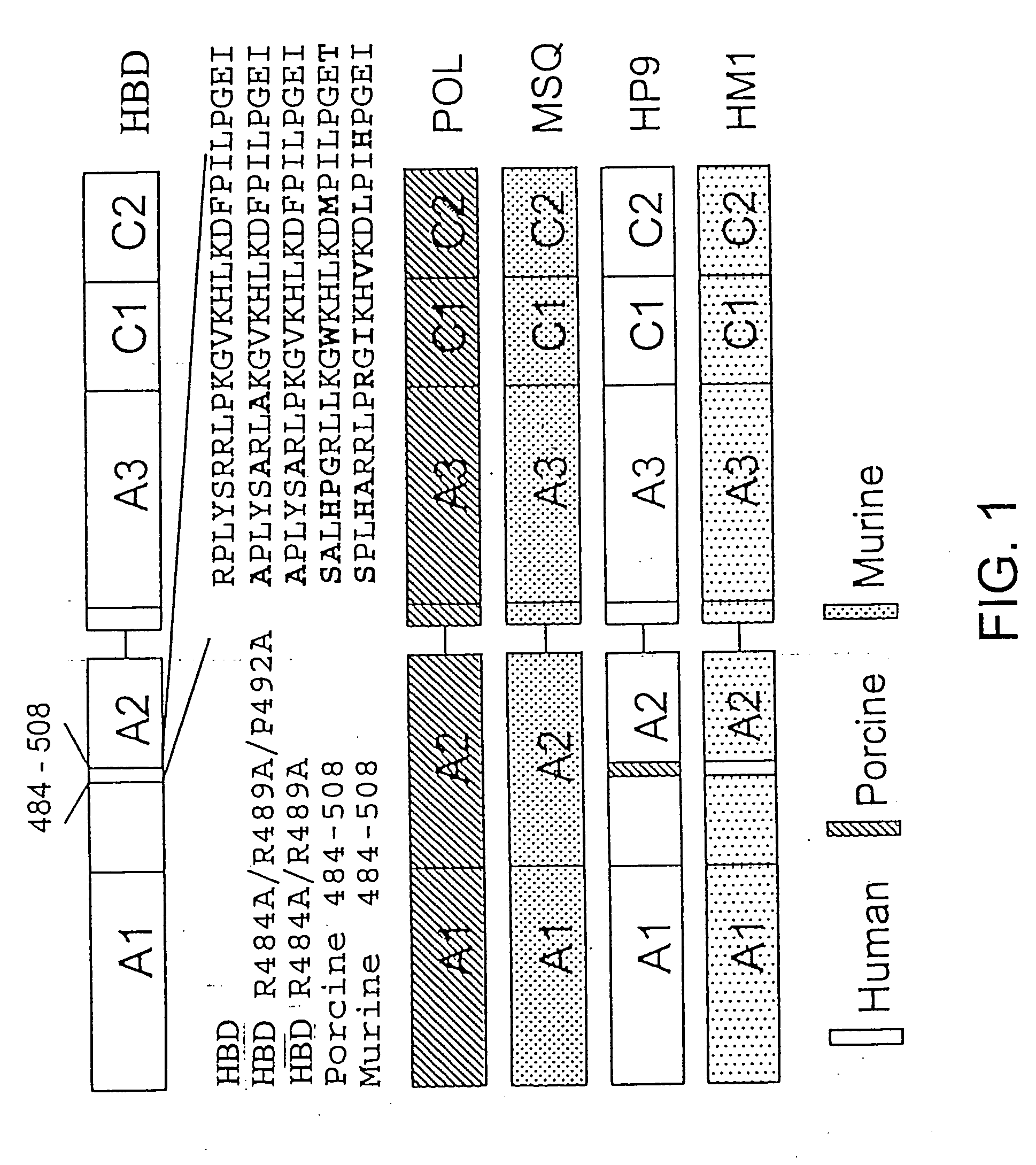
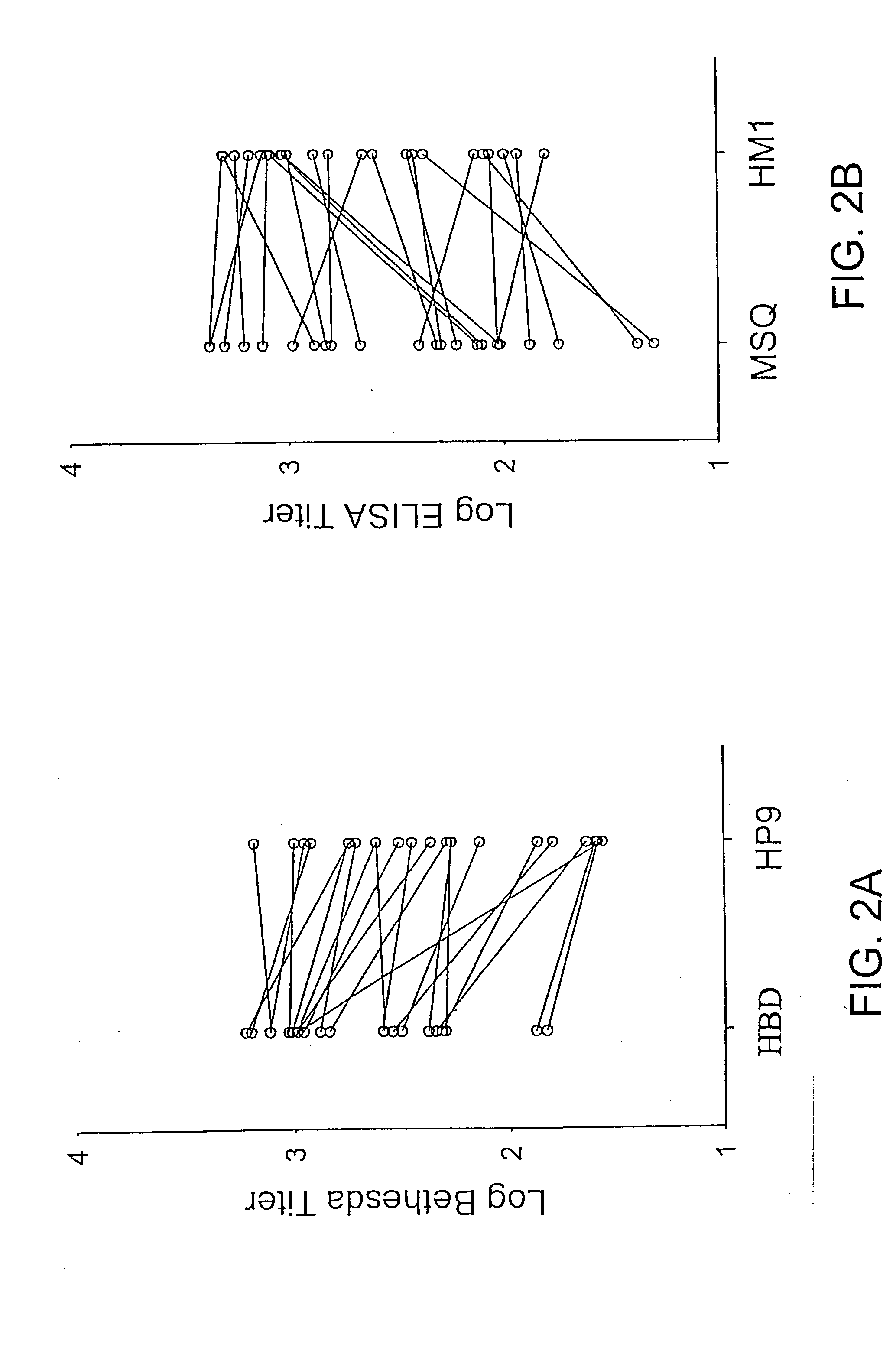
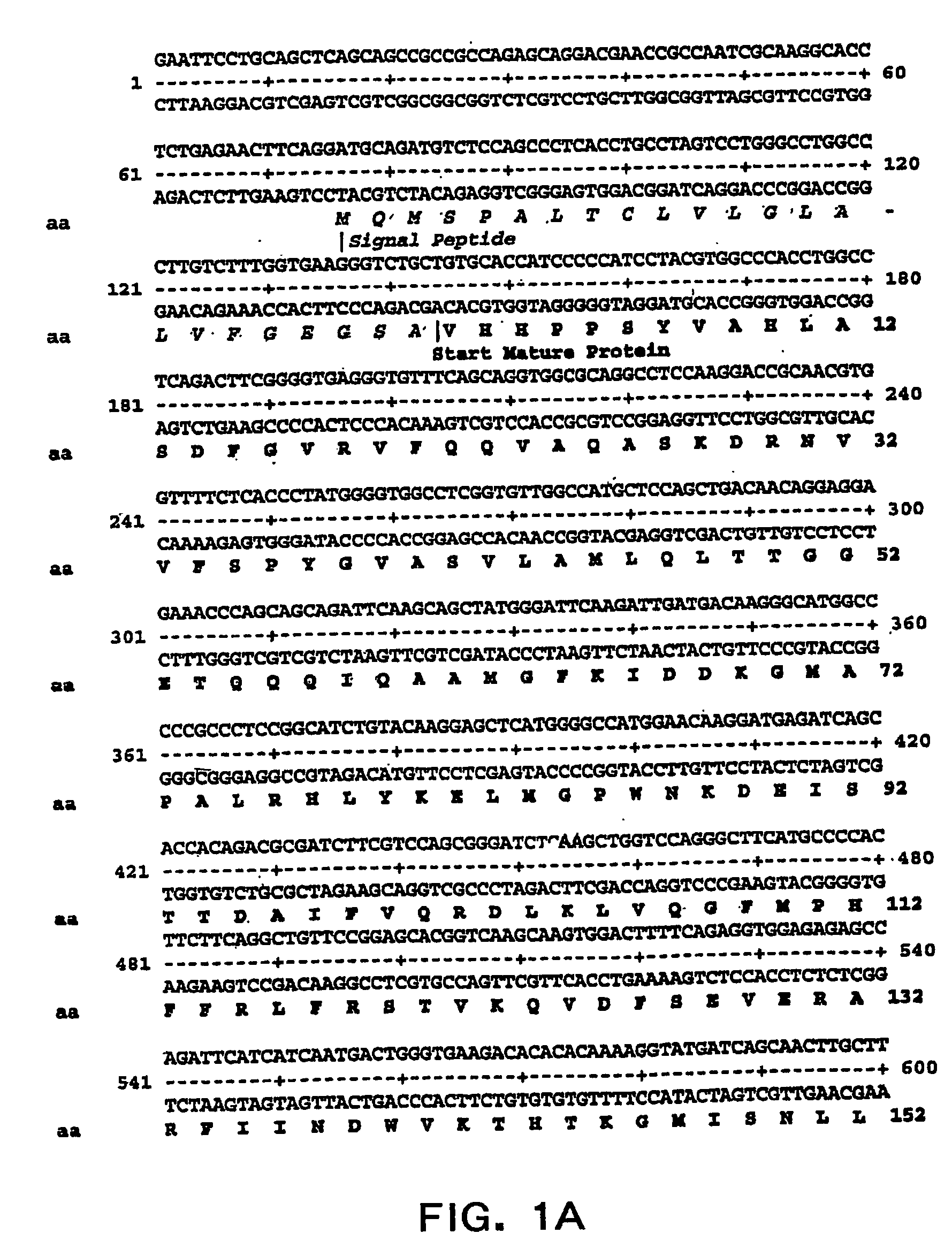
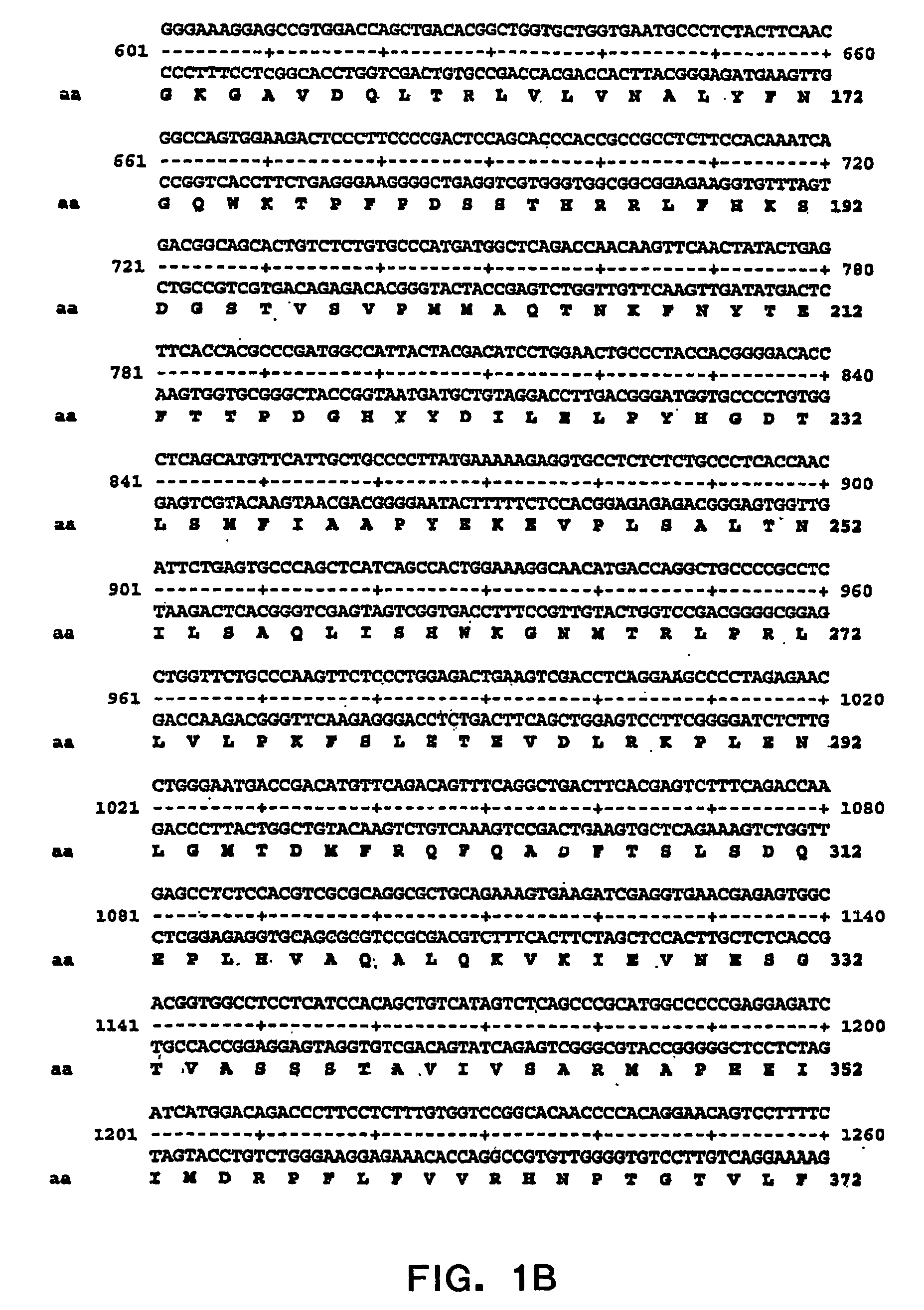
Specific amino acid loci of human factor VIII interact with inhibitory antibodies of hemophilia patients who have developed such antibodies after being treated with factor VIII. Modified factor VIII is disclosed in which the amino acid sequence is changed by a substitution at one or more amino acids of positions 484-508 of the A2 domain. The modified factor VIII is useful as a clotting factor supplement for hemophiliacs.
Owner:GENERAL ELECTRIC CO +1
Compositions and methods for less immunogenic protein formulations
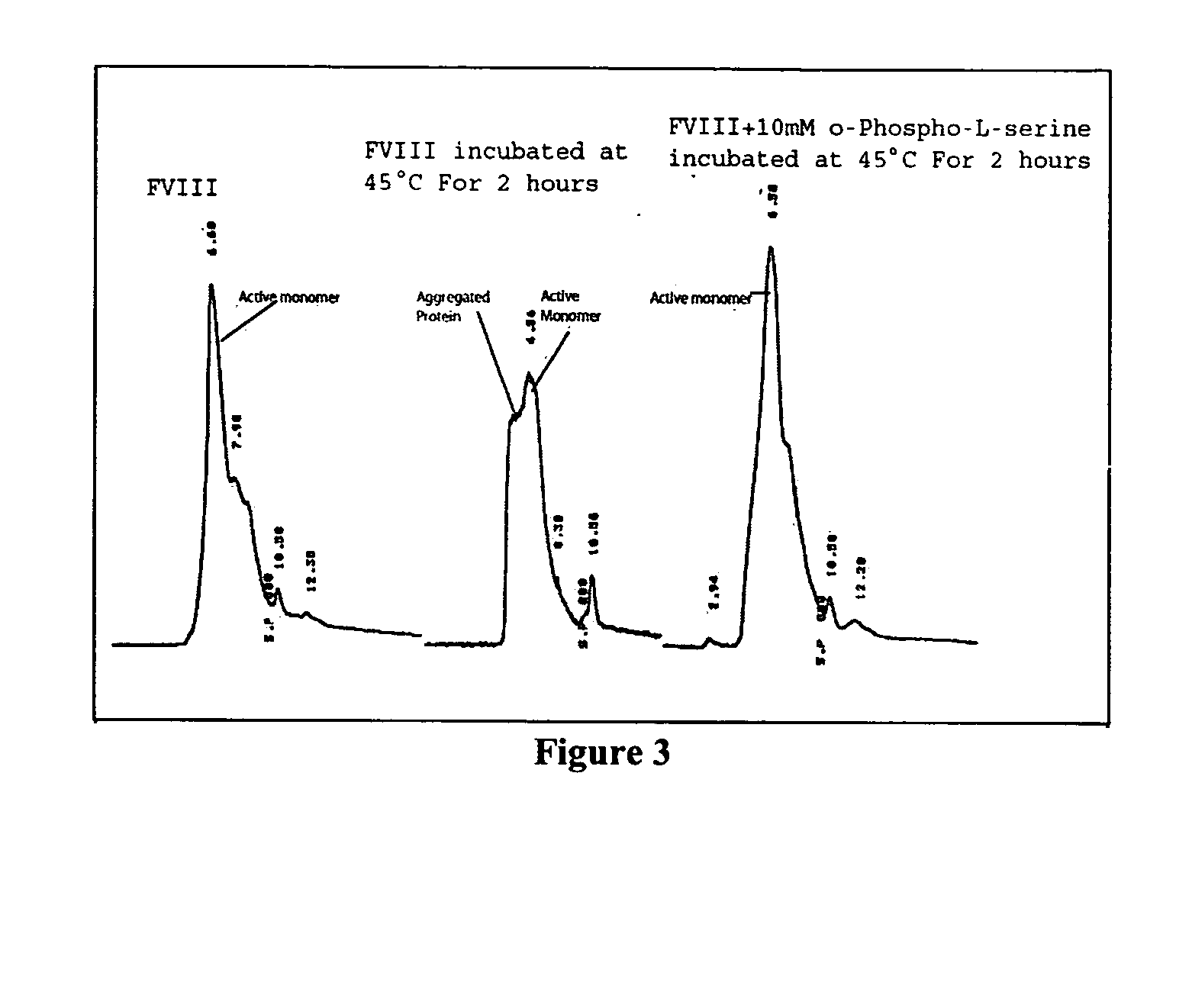
Composition and methods are described for low immunogenic protein formulations. An example of such a protein is antihemophilic factor (FVIII). The composition generally includes the protein, polypeptide or peptide, one or more agents that bind to epitope domains of the proteins to form a complex. Buffers containing salts may be used to stabilize this interaction. For example, Factor VIII and serine containing phospholipids in buffer salts containing Ca<2+> and Na<+> can be used to prepare protein-lipid structures. These complexes are useful for treatment of diseases such as Hemophilia. A method for the formation of novel non-liposomal structures is also disclosed.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Factor VIII Polypeptide Formulations
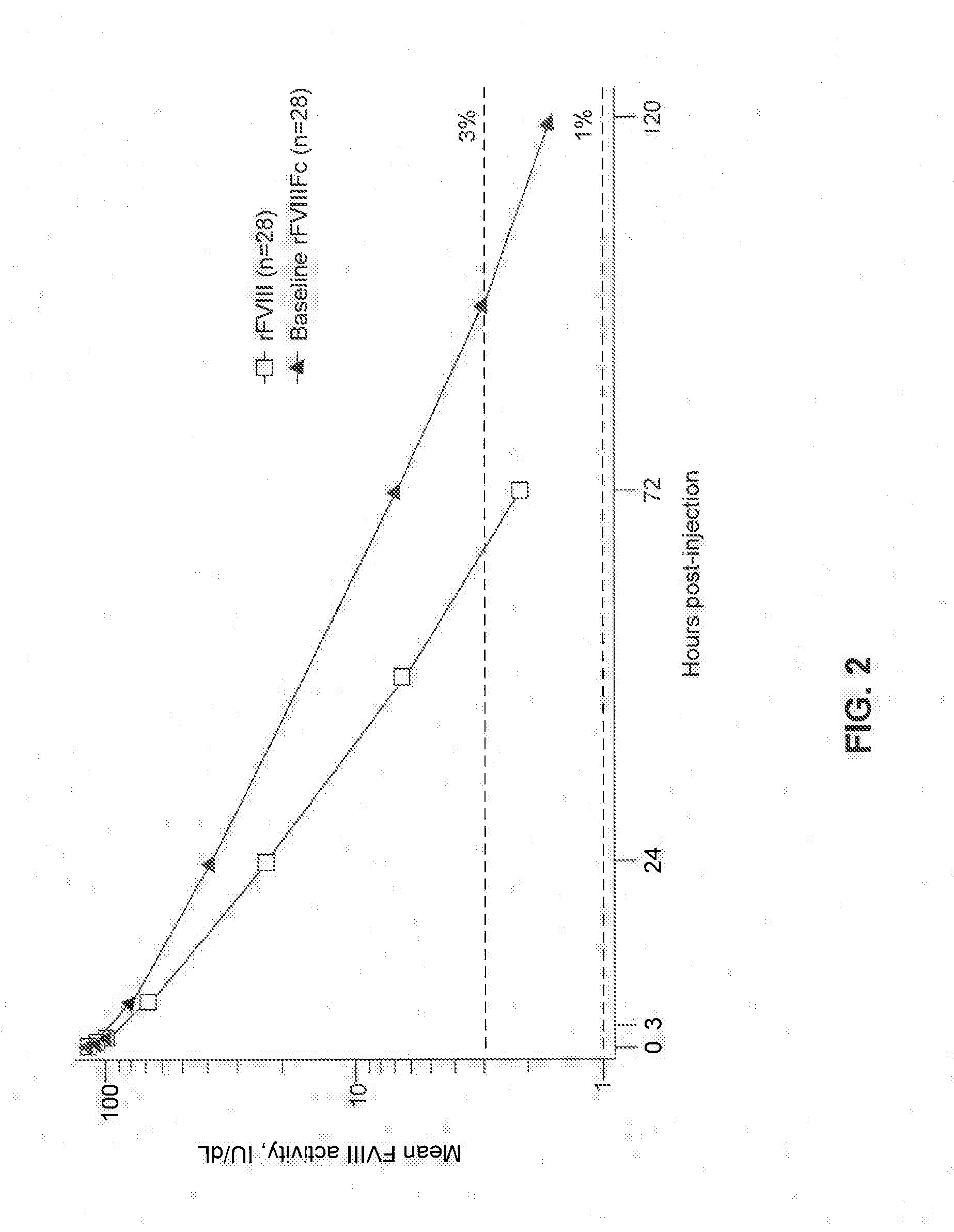
The present invention provides a formulation of a Factor VIII polypeptide, e.g., FVIII-Fc, and methods of using the same. The FVIII polypeptide can be a recombinant FVIII protein, a short-acting FVIII protein, or a long-acting FVIII protein. The pharmaceutical formulation comprising a FVIII polypeptide can be used for individual prophylaxis, weekly prophylaxis, episodic (on-demand) treatment, or perioperative management of hemophilia.
Owner:BIOVERATIV THERAPEUTICS INC
Modified plasminogen inhibitor type-1 and methods based thereon
InactiveUS20050158295A1Reduced enzymatic activityExtended half-lifePeptide/protein ingredientsTissue cultureAbnormal tissue growthFiber
The present invention is based upon the discovery that modified plasminogen activator inhibitor type-I (PAI-1) in which two or more amino acid residues that do not contain a sulfliydryl group have been replaced with amino acid residues that contain a sulfhydryl group and, therefore, forms intramolecular disulfide bonds, have increased in vivo half-life. Also disclosed are the modified PAI-1 proteins, derivatives and analogs thereof, specific antibodies, nucleic acid molecules and host cells. Methods for producing modified PAI-1, derivatives and analogs are also provided. The invention further relates to Therapeutics, pharmaceutical compositions and method of using the composition for treatment. The invention may be used to inhibit angiogenesis in a subject, thereby treating diseases or conditions associated with undesired angiogenesis and cell proliferation. Such conditions include psoriasis, chronic inflammation, tumor invasion and metastasis invention are useful for the treatment, prophylaxis, management and amelioration of cardiovascular diseases such as, but not limited to those that are related to hyerfibrinolysis, hemophilia, and vessel leakage syndrome.
Owner:UNIVERSITY OF TOLEDO
Treatment of coagulation disease by administration of recombinant vwf
ActiveUS20120316116A1Extended half-lifeFactor VIIPeptide/protein ingredientsFactor VIII vWFVon willebrand
The present invention provides methods of treating coagulation disease, including hemophilia and von Willebrand disease by administering recombinant von Willebrand Factor alone or in combination with Factor VIII.
Owner:TAKEDA PHARMA CO LTD
Inactivation resistant factor VIII
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe3O9 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated and an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:RGT UNIV OF MICHIGAN
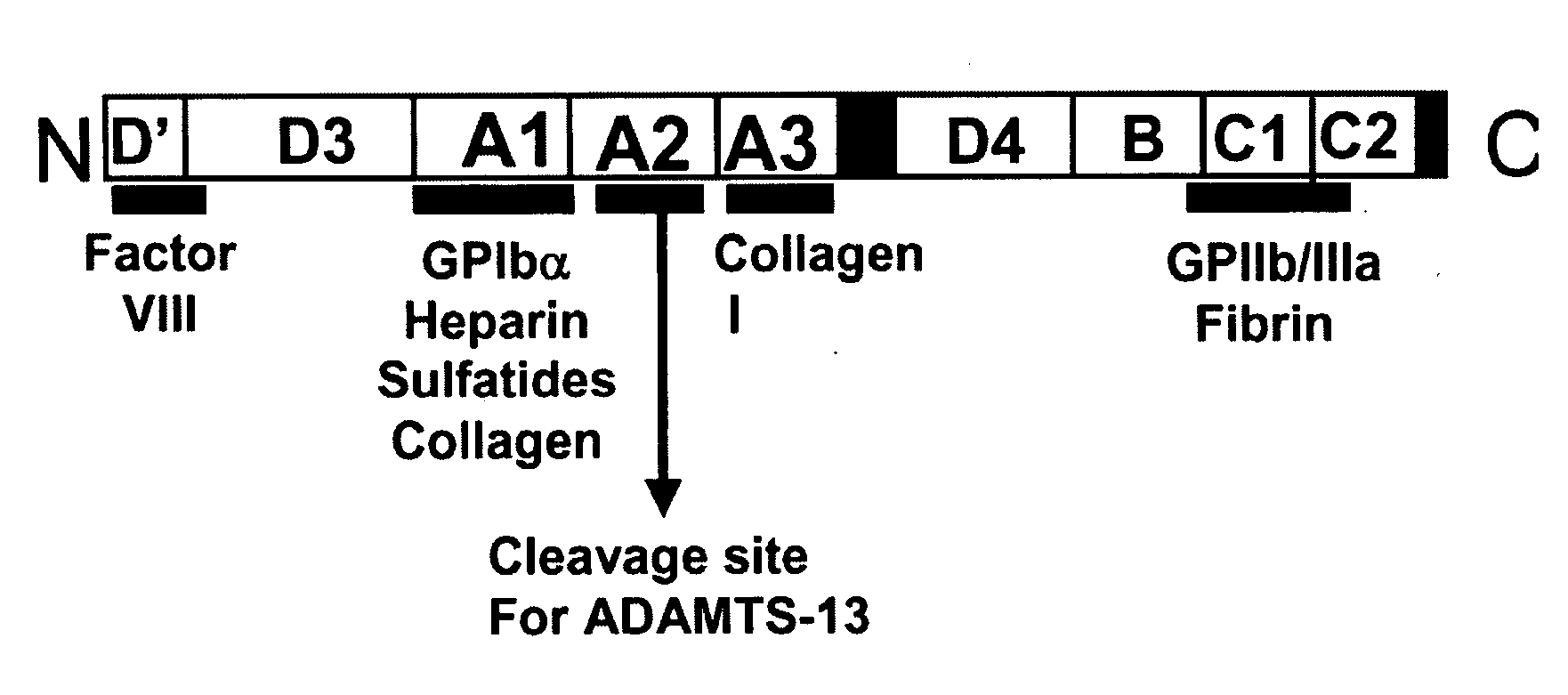
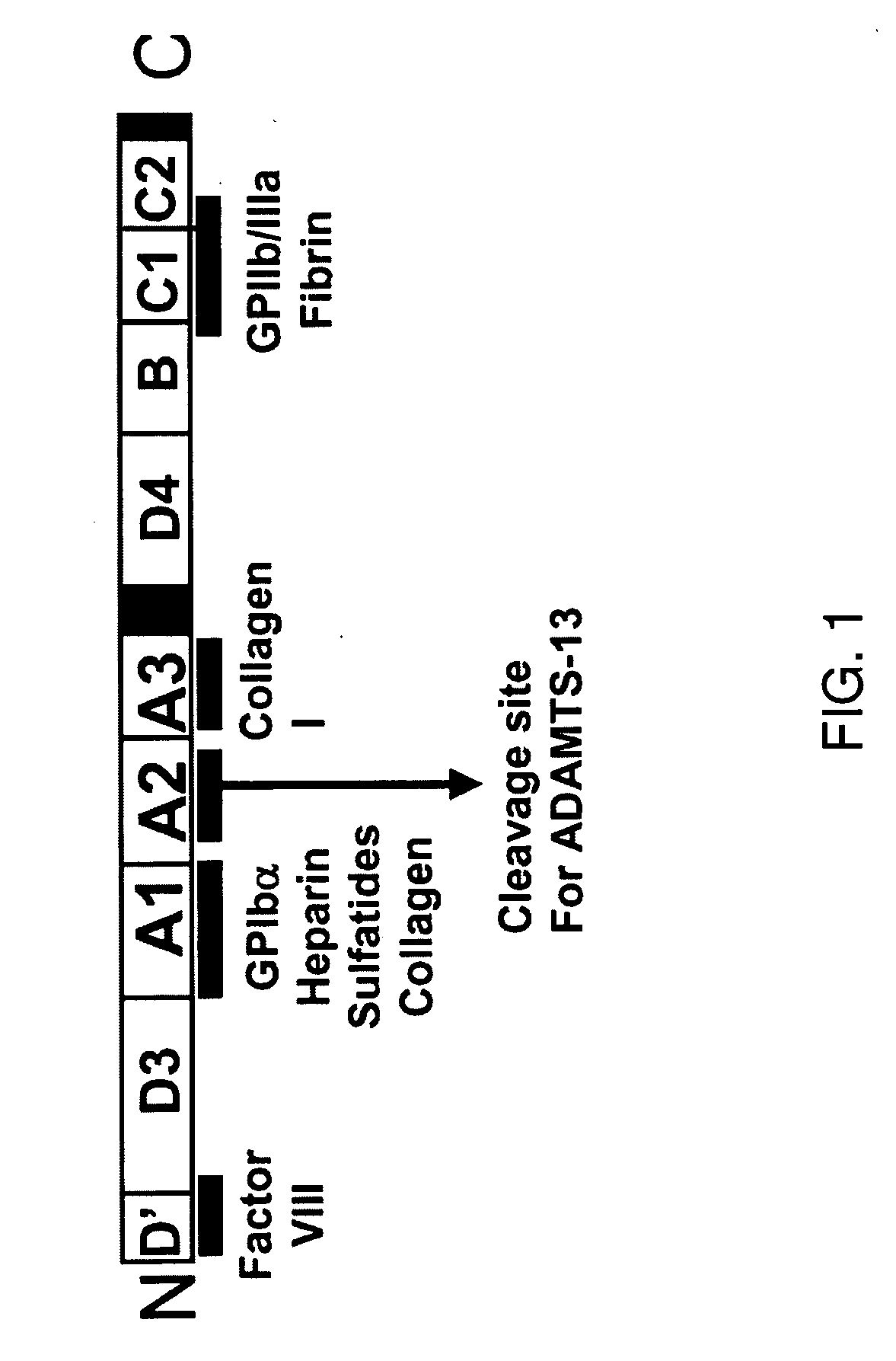
Treatment of medical condition with a2 domain of von willebrand factor
ActiveUS20090118161A1Reduce complicationsAvoid deathPeptide/protein ingredientsMicrobiological testing/measurementFactor VIII vWFFactor ii
The present invention is directed to methods for the prevention, treatment and / or diagnosis of a medical condition, such as sepsis, systemic inflammatory reaction syndrome, and / or thrombosis, for example. In particular, the method employs part or all of the A2 domain of von Willebrand factor. In certain cases, a recombinant A2 domain is utilized for the treatment of sepsis, systemic inflammatory reaction syndrome, and / or thrombosis, for example.
Owner:BAYLOR COLLEGE OF MEDICINE
Modified Factor VIII
InactiveUS20070135342A1Superior coagulant activityFactor VIIHydrolasesCombinatorial chemistryBleeding episodes
Modified porcine factor VIII is disclosed in which most of the B domain has been removed through genetic engineering. This modified factor VIII is particularly useful for treatment of hemophiliacs, especially those undergoing bleeding episodes.
Owner:EMORY UNIVERSITY
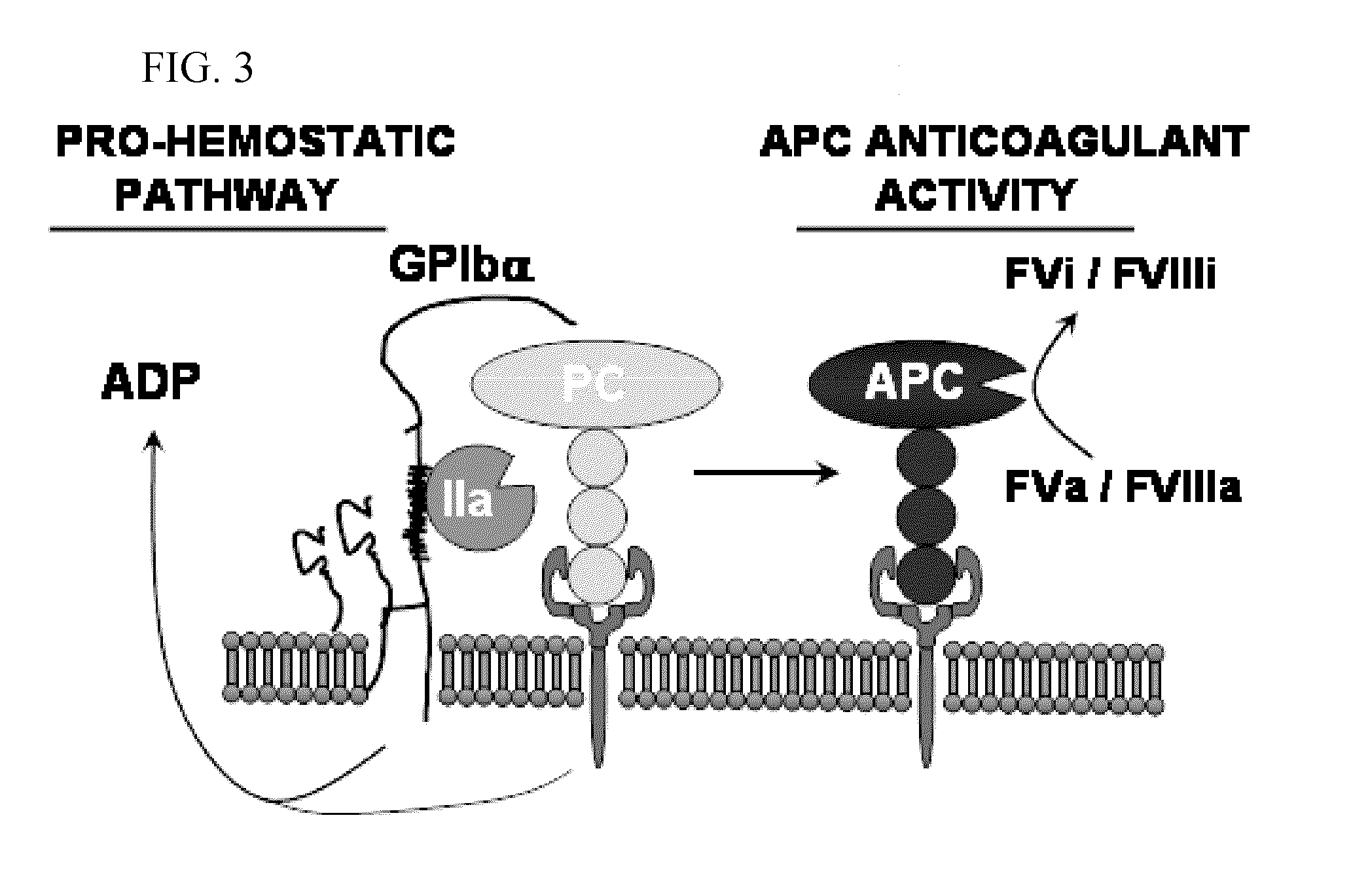
Protein c for use in maintaining hemostasis
InactiveUS20100184672A1Effective hemostasisGood hemostasisPeptide/protein ingredientsGenetic material ingredientsPlatelet disorderInjury cause
It is disclosed herein that protein C functions as a hemostatic agent. Thus, provided is a method of preventing, treating or ameliorating abnormal bleeding in a subject, comprising administering to the subject a protein C polypeptide or polynucleotide. Abnormal bleeding can result from a bleeding disorder, such as hemophilia or a platelet disorder, or from a bleeding episode, such as from a traumatic injury.
Owner:OREGON HEALTH & SCI UNIV +1
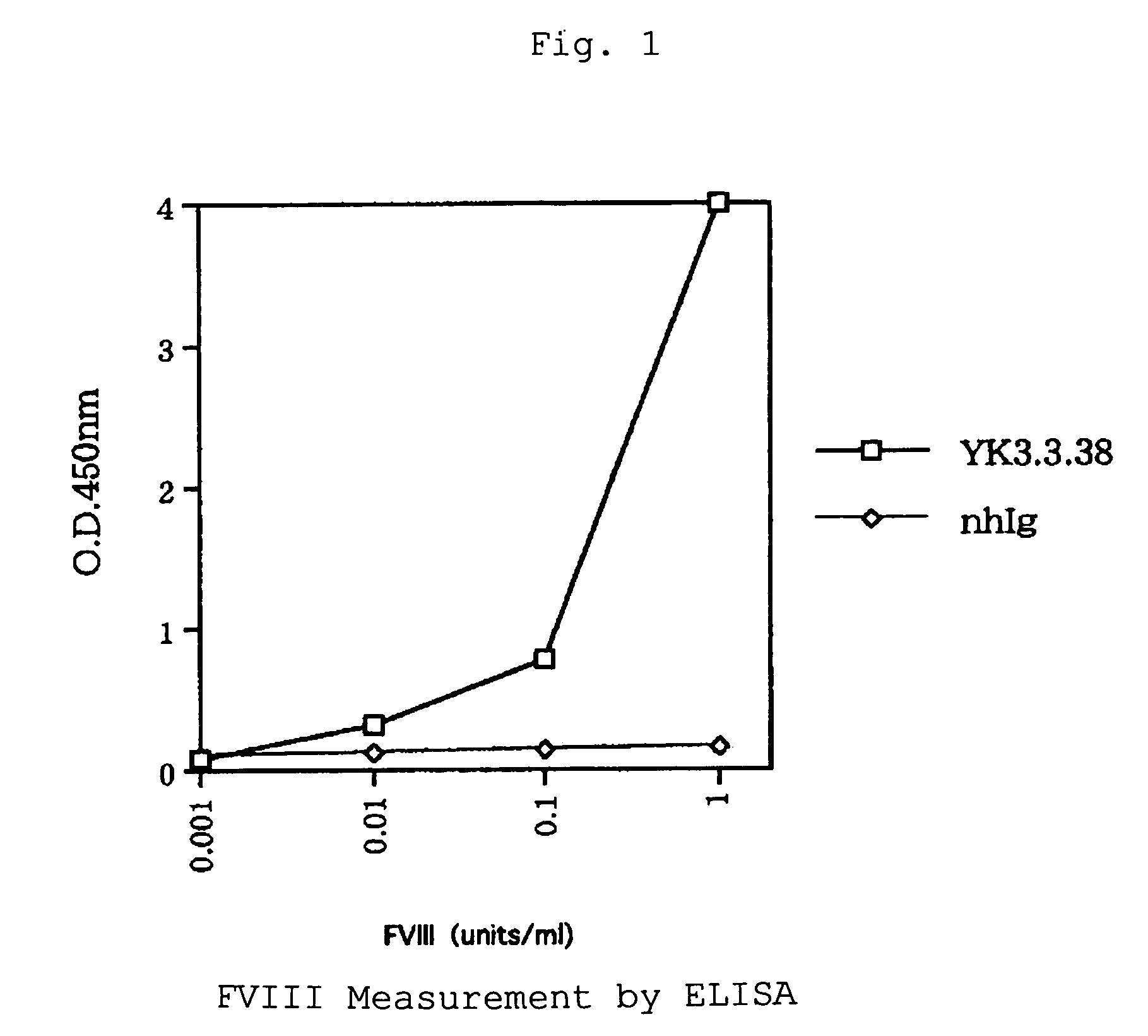
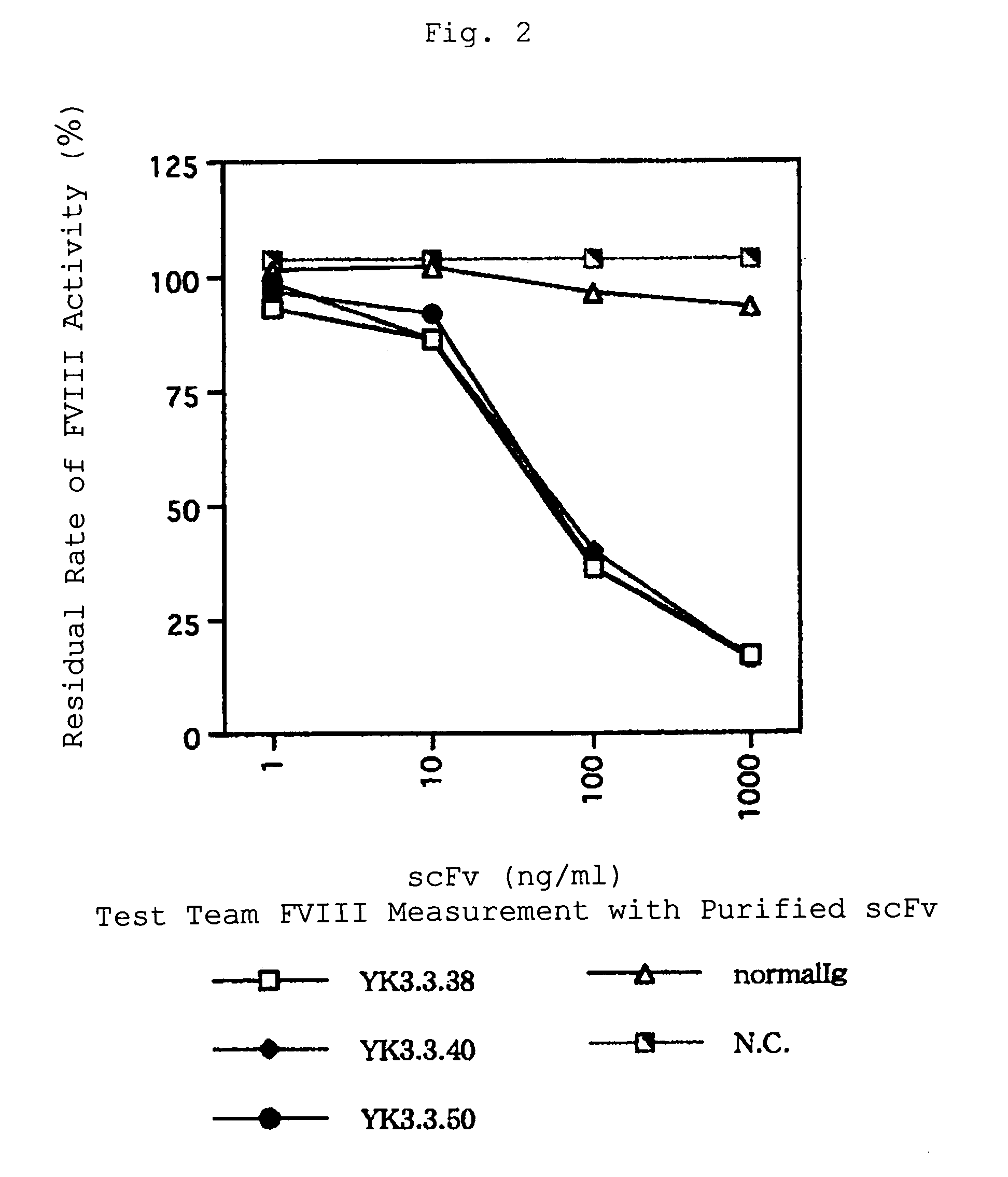
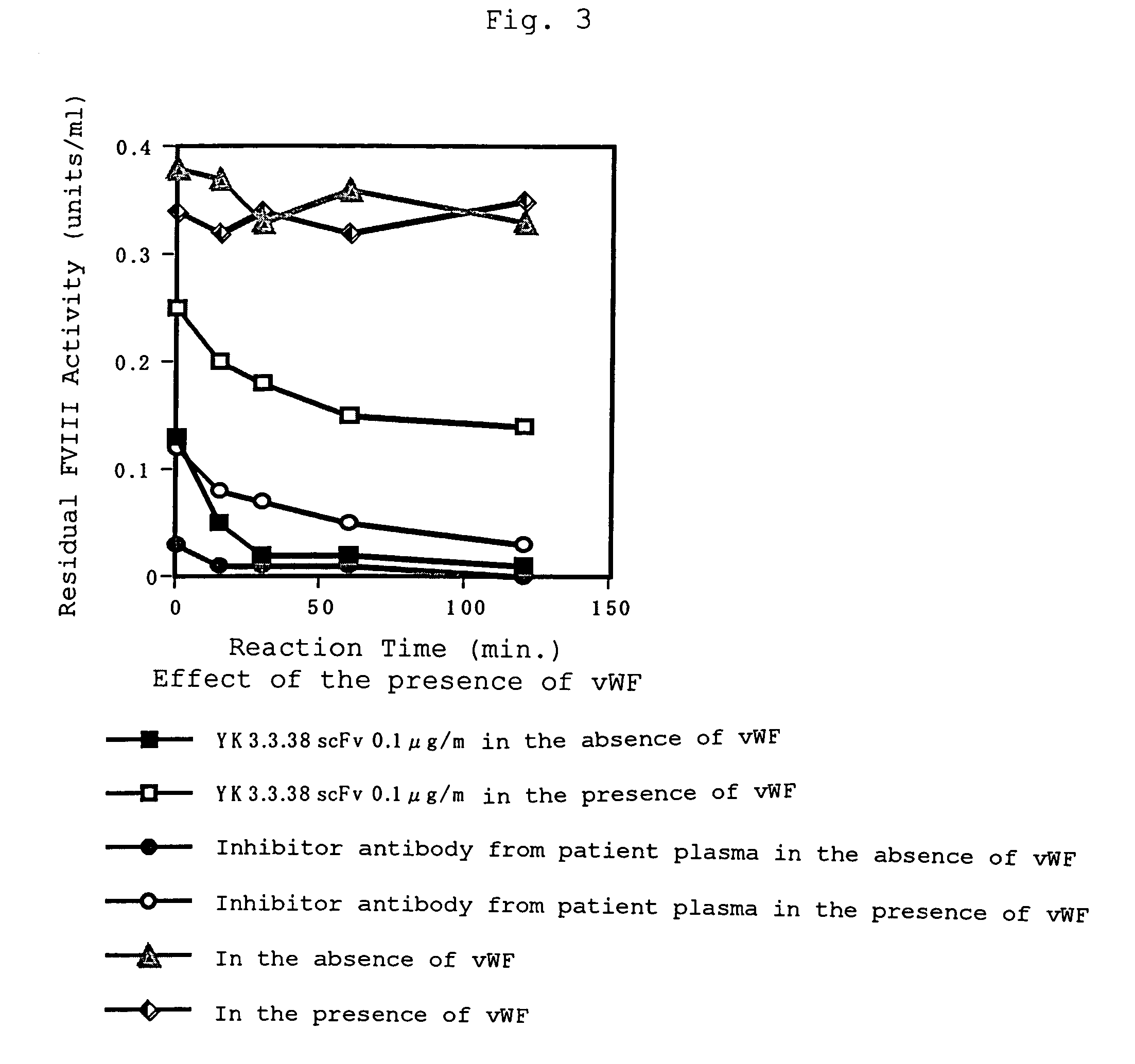
Human-type anti-blood coagulation factor VIII antibody
InactiveUS7214785B2Immunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIRandom combination
The present invention provides a human antibody against blood coagulation factor VIII (hereinafter also referred to as “FVIII”) and an antibody fragment that binds to human FVIII and specifically inhibits the coagulation activity of human FVIII. ScFv display phage libraries, prepared by using scFv DNAs constructed by random combinations of immunoglobulin VH chain genes and VL chain genes from lymphocytes from hemophilia A patients, is reacted with FVIII immobilized to a solid phase via anti-FVIII monoclonal antibody, and scFv clones capable of binding to FVIII are cloned to reveal VH and VL chains of FVIII-specific antibody.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Genetic factors associated with inhibitor development in hemophilia a
The present invention provides methods for predicting the risk of an individual developing antibodies to factor VIII by identifying a single nucleotide polymorphism of an immune response or immune modifier gene. The invention further provides oligonucleotides, diagnostic kits, microarrays, and isolated nucleic acids comprising single nucleotide polymorphisms of immune response or immune modifier genes.
Owner:ASTERMARK JAN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com