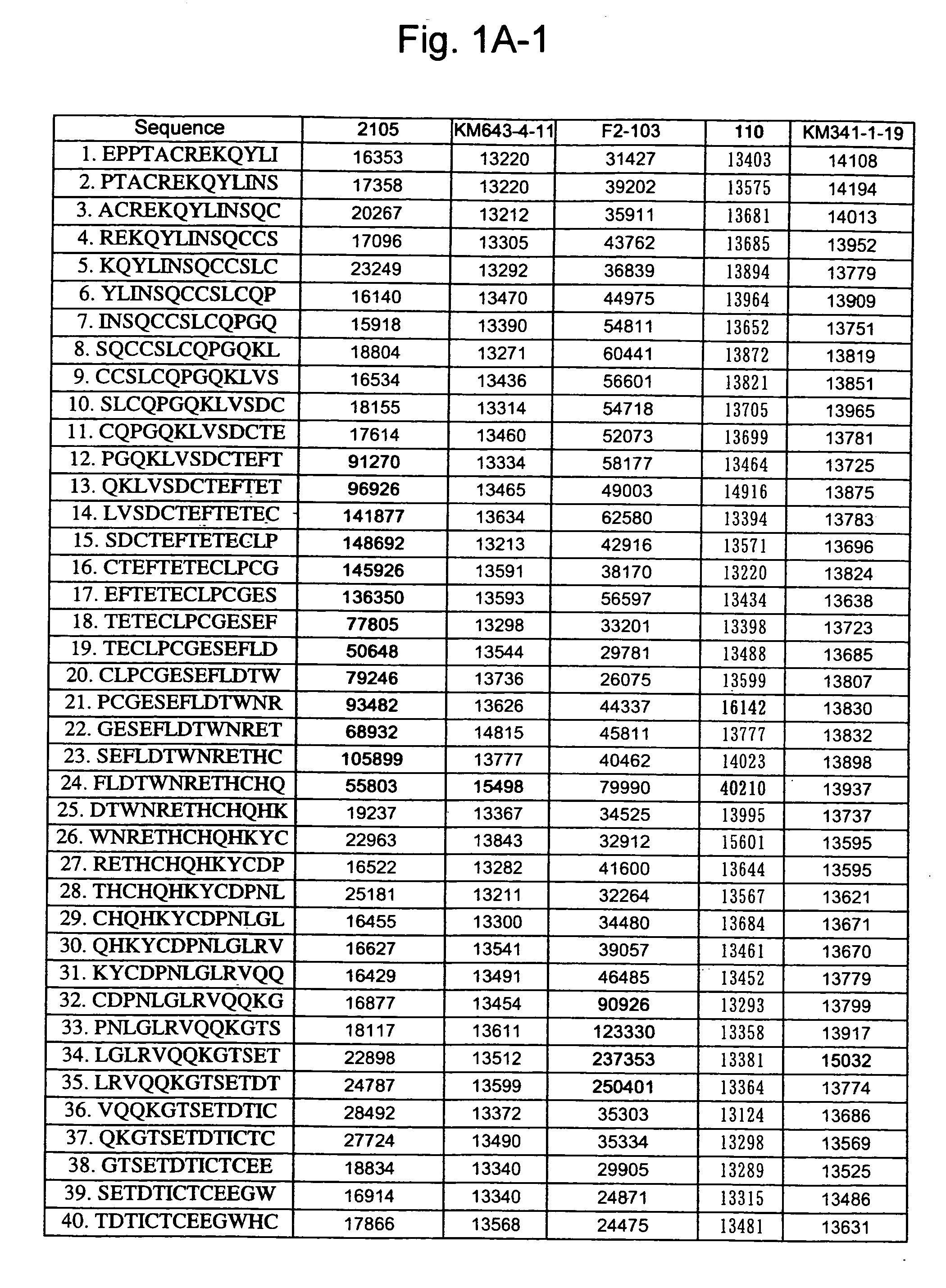
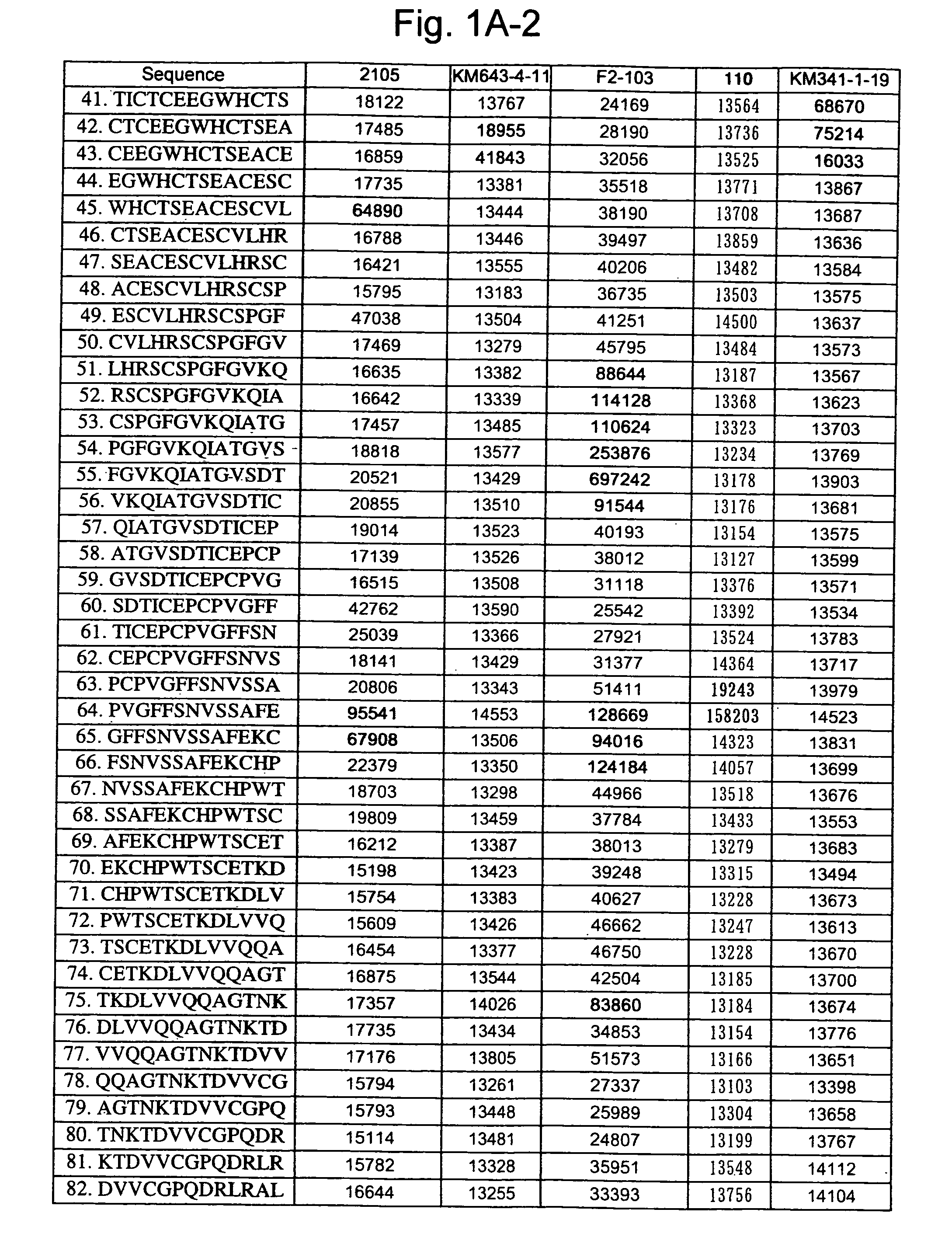
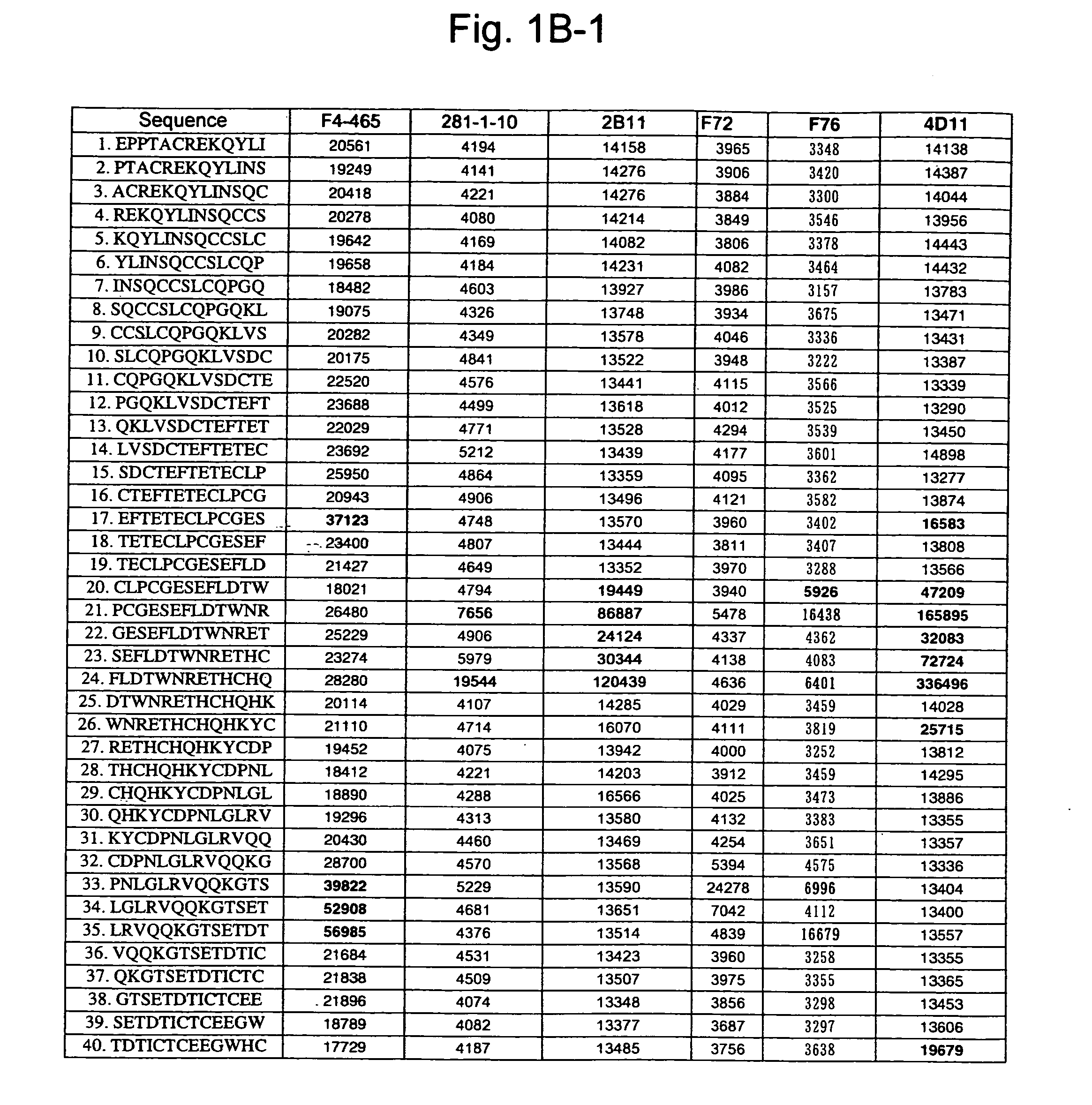
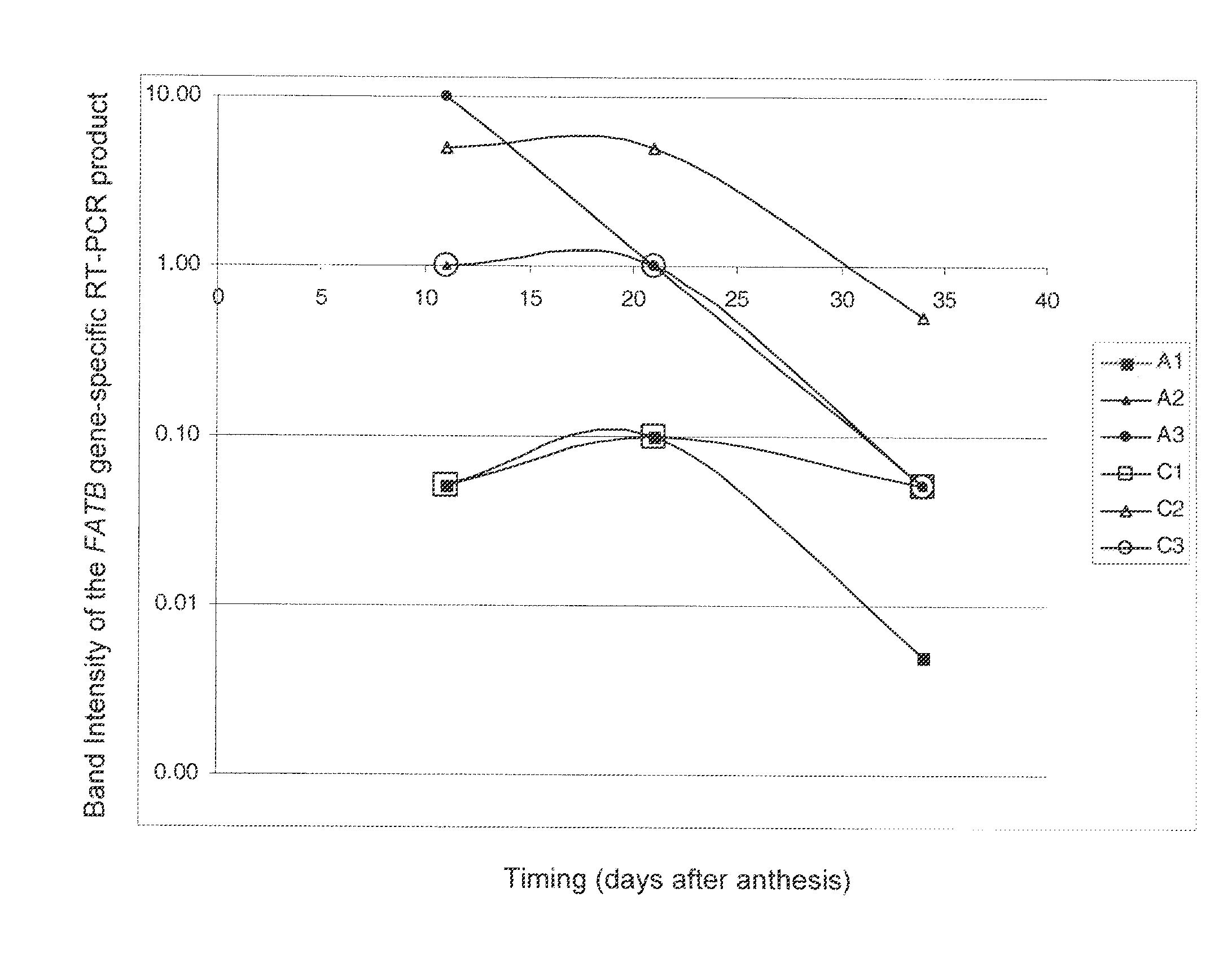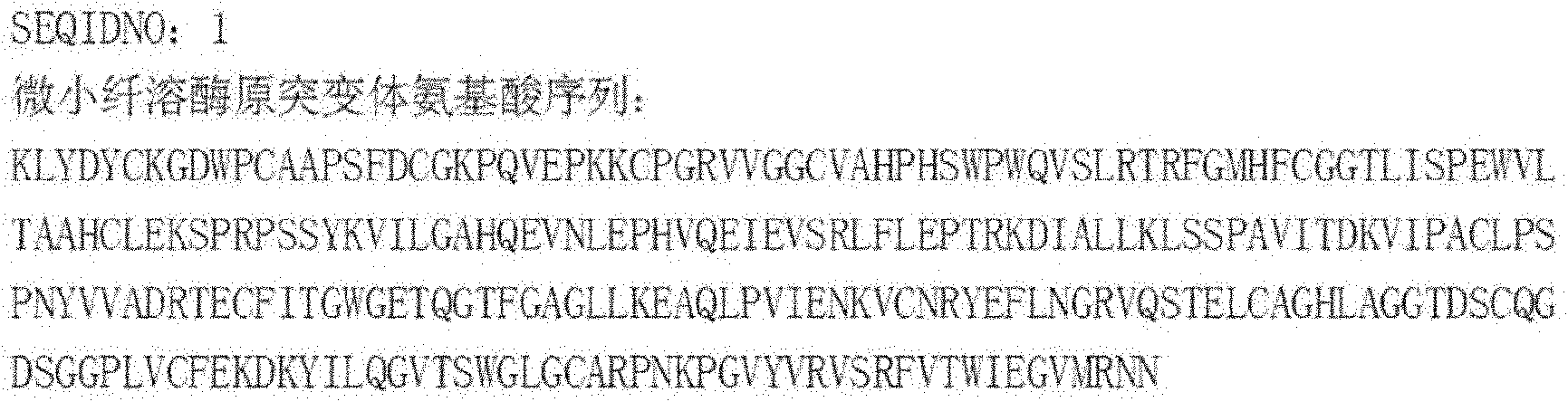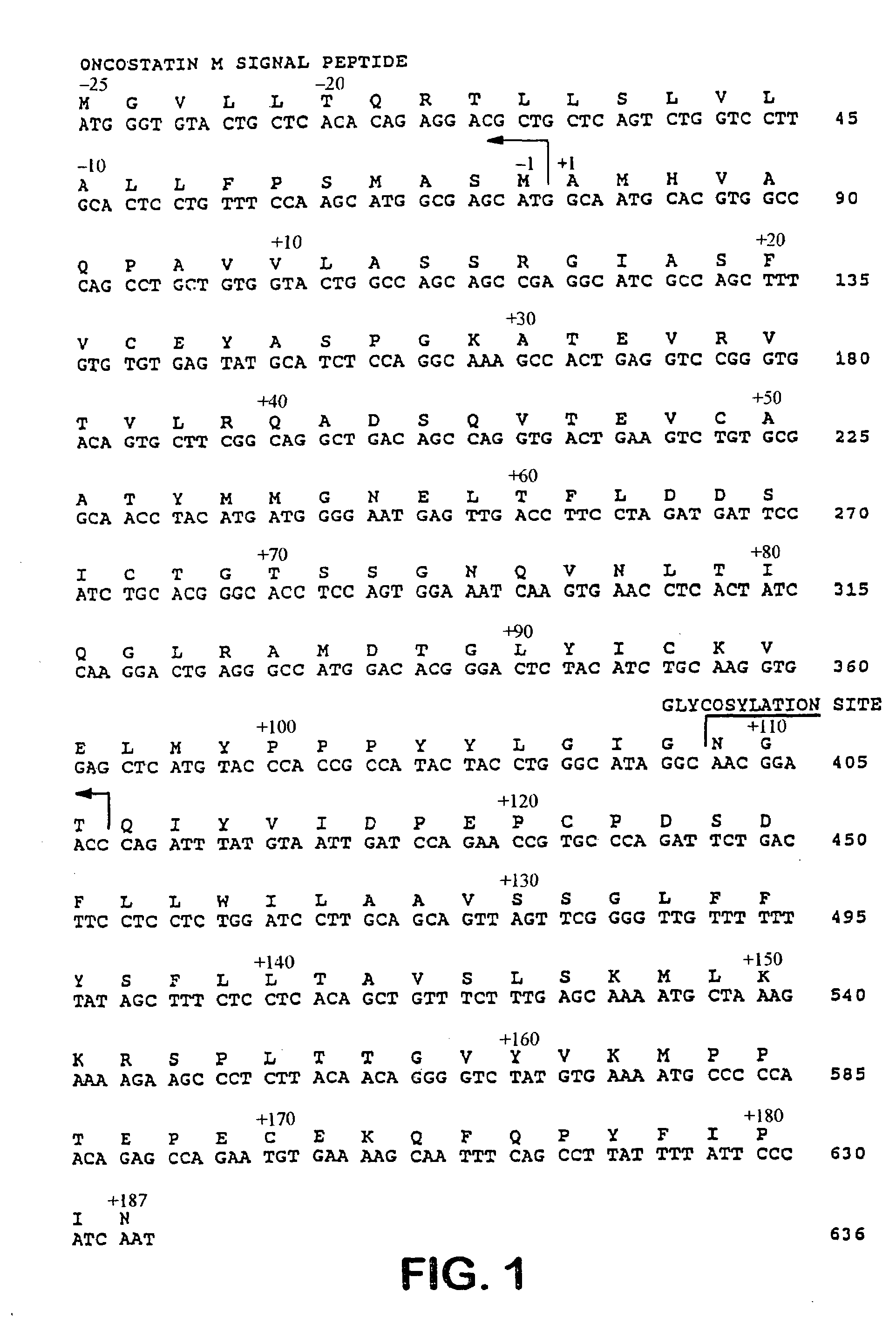Patents
Literature
4407 results about "Mutant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In biology and especially genetics, a mutant is an organism or a new genetic character arising or resulting from an instance of mutation, which is generally an alteration of the DNA sequence of the genome or chromosome of an organism. The term mutant is also applied to a virus with an alteration in its nucleotide sequence whose genome is RNA, rather than DNA. In multicellular eukaryotes, a DNA sequence may be altered in an individual somatic cell that then gives rise to a mutant somatic cell lineage as happens in cancer progression. Also in eukaryotes, alteration of a mitochondrial or plastid DNA sequence may give rise to a mutant lineage that is inherited separately from mutant genotypes in the nuclear genome. The natural occurrence of genetic mutations is integral to the process of evolution. The study of mutants is an integral part of biology; by understanding the effect that a mutation in a gene has, it is possible to establish the normal function of that gene.
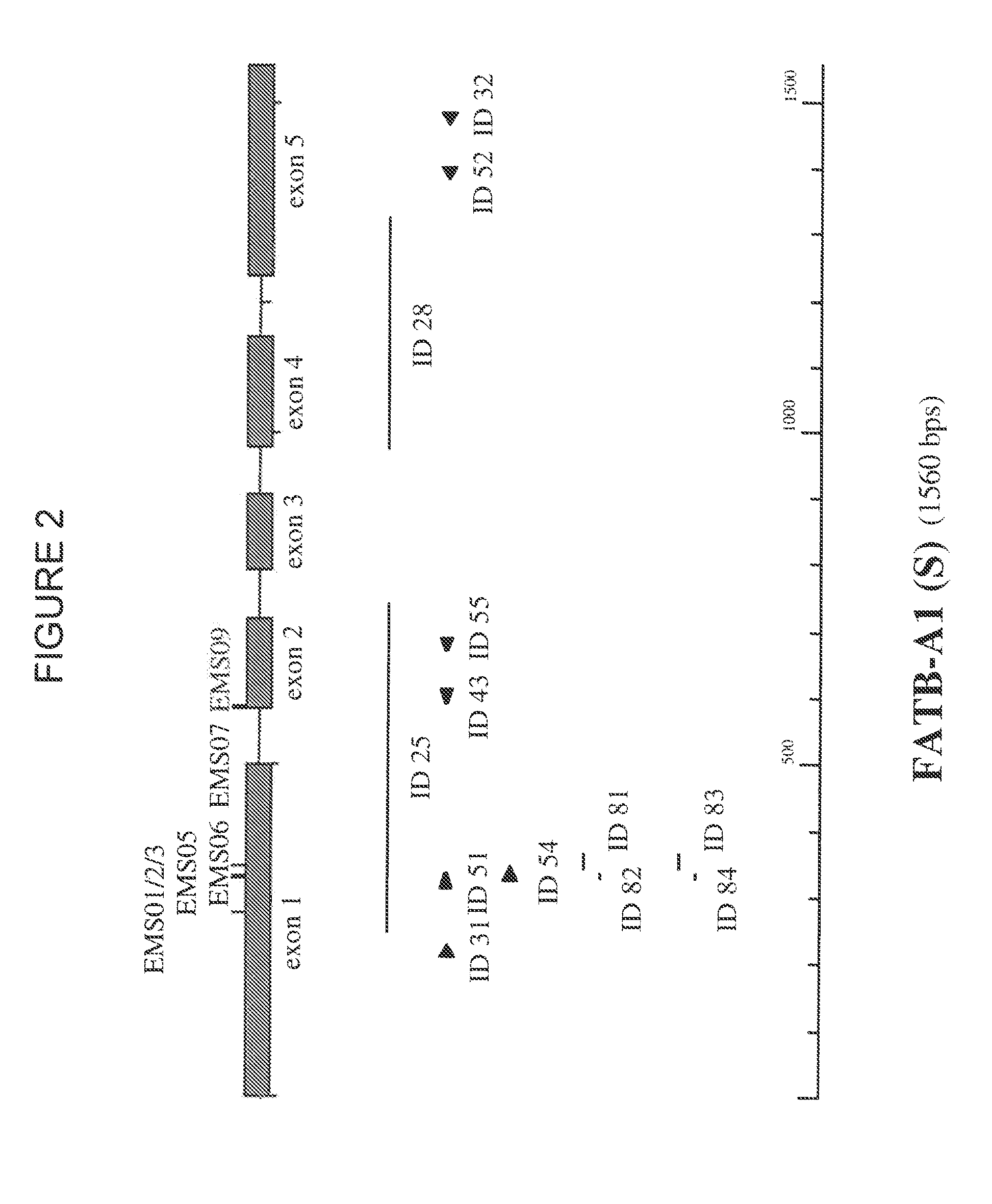
Brassica plant comprising mutant fatty acyl-acp thioesterase alleles
ActiveUS20110145944A1Reduce the amount requiredMinimal level of functional FATB proteinHydrolasesImmunoglobulinsAcyl carrier proteinWild type
The invention relates to crop plants comprising novel seed lipid compositions. Provided are both wild type and mutant nucleic acid molecules encoding Brassica fatty acyl-acyl carrier protein (ACP) thioesterase B proteins (FATB) and the proteins as such. Also provided are Brassica plants, tissue and seeds comprising at least three mutant fatB alleles in their genome, whereby the seed oil fatty acid composition or profile is significantly altered.
Owner:BAYER CROPSCIENCE NV
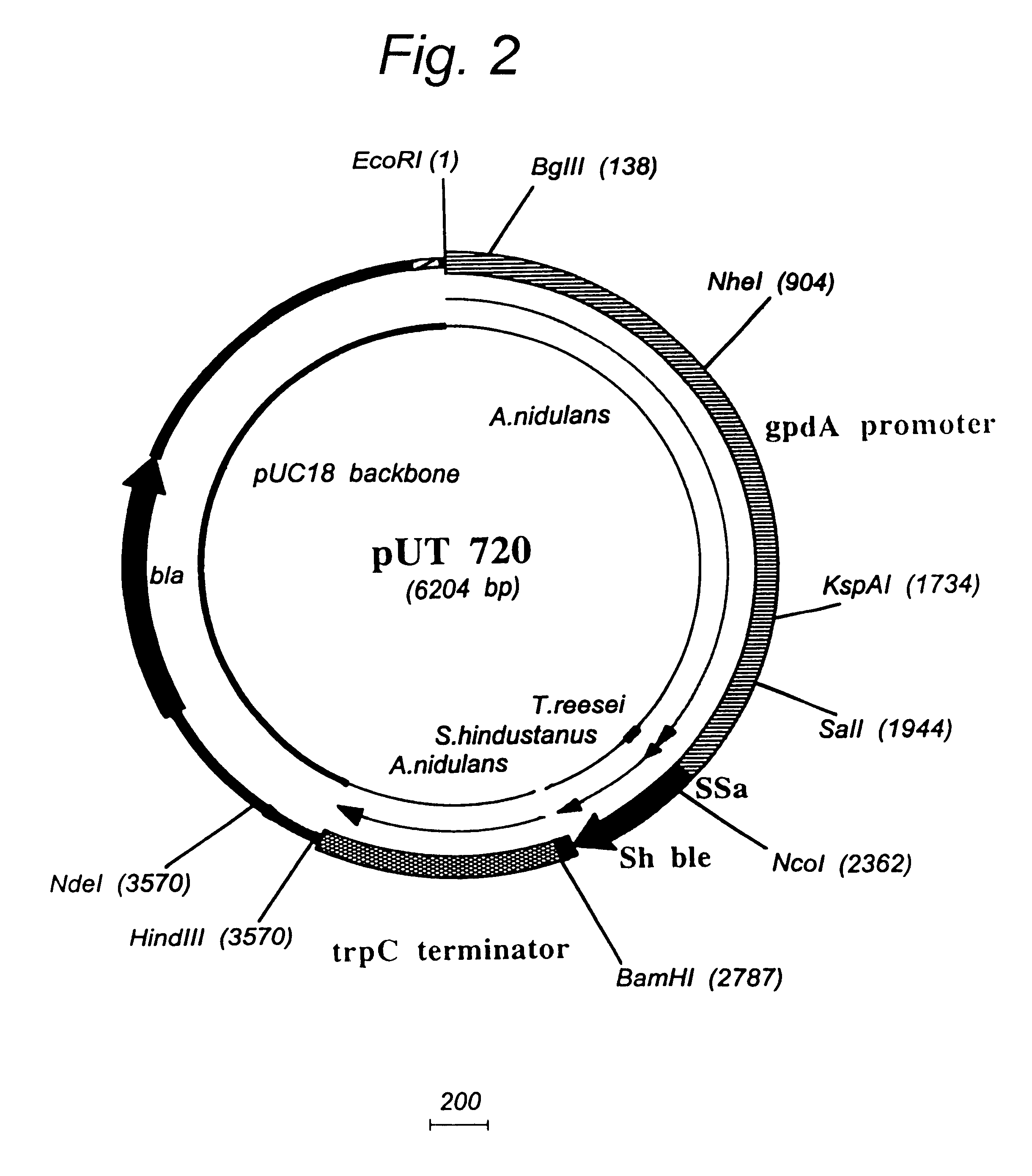
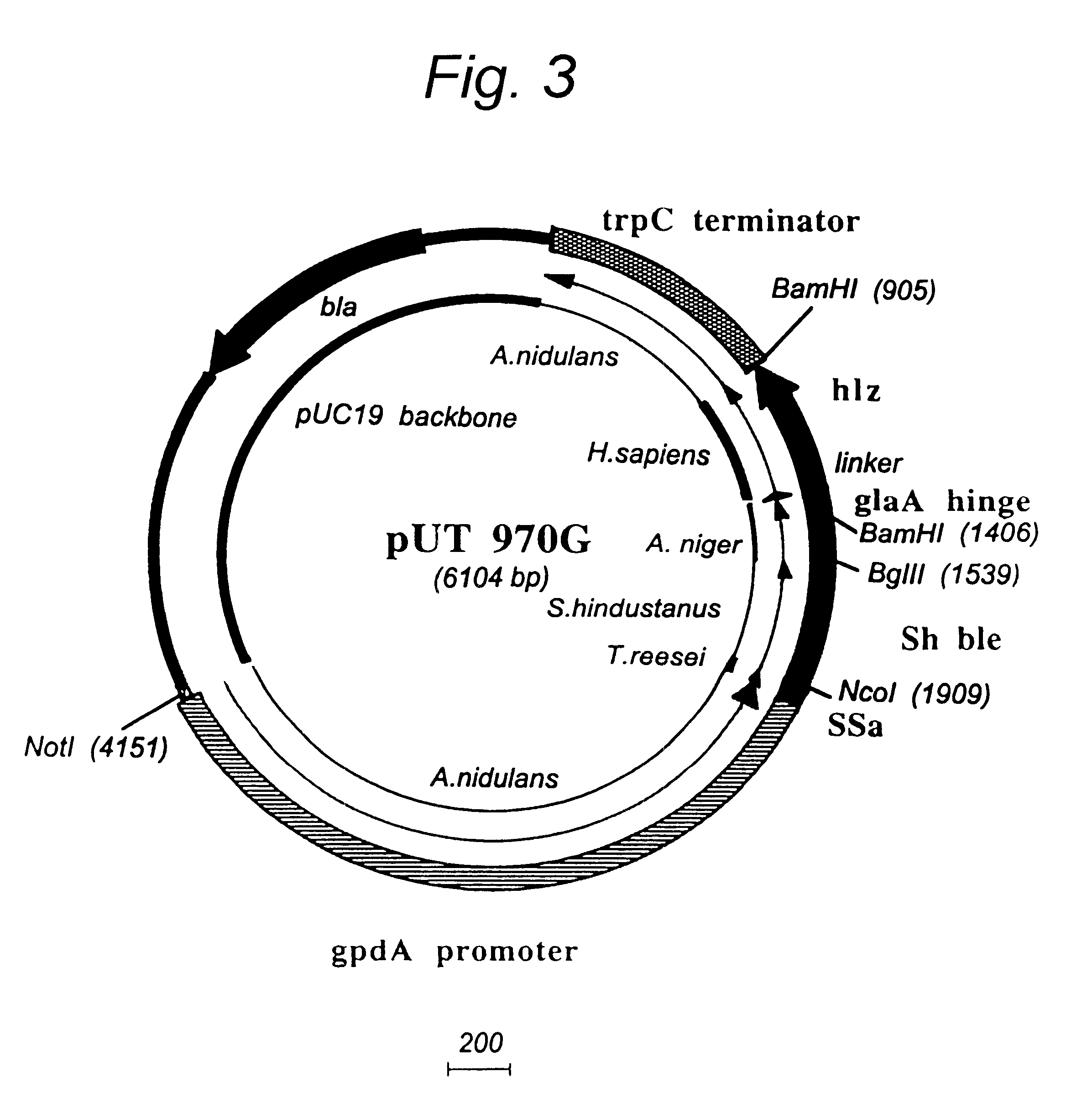
Transformation system in the field of filamentous fungal hosts
A novel transformation system in the field of filamentous fungal hosts for expressing and secreting heterologous proteins or polypeptides is described. The invention also covers a process for producing large amounts of polypeptide or protein in an economical manner. The system comprises a transformed or transfected fungal strain of the genus Chrysosporium, more particularly of Chrysosporium lucknowense and mutants or derivatives thereof. It also covers transformants containing Chrysosporium coding sequences, as well expression-regulating sequences of Chrysosporium genes. Also provided are novel fungal enzymes and their encoding sequences and expression-regulating sequences.
Owner:DANISCO US INC
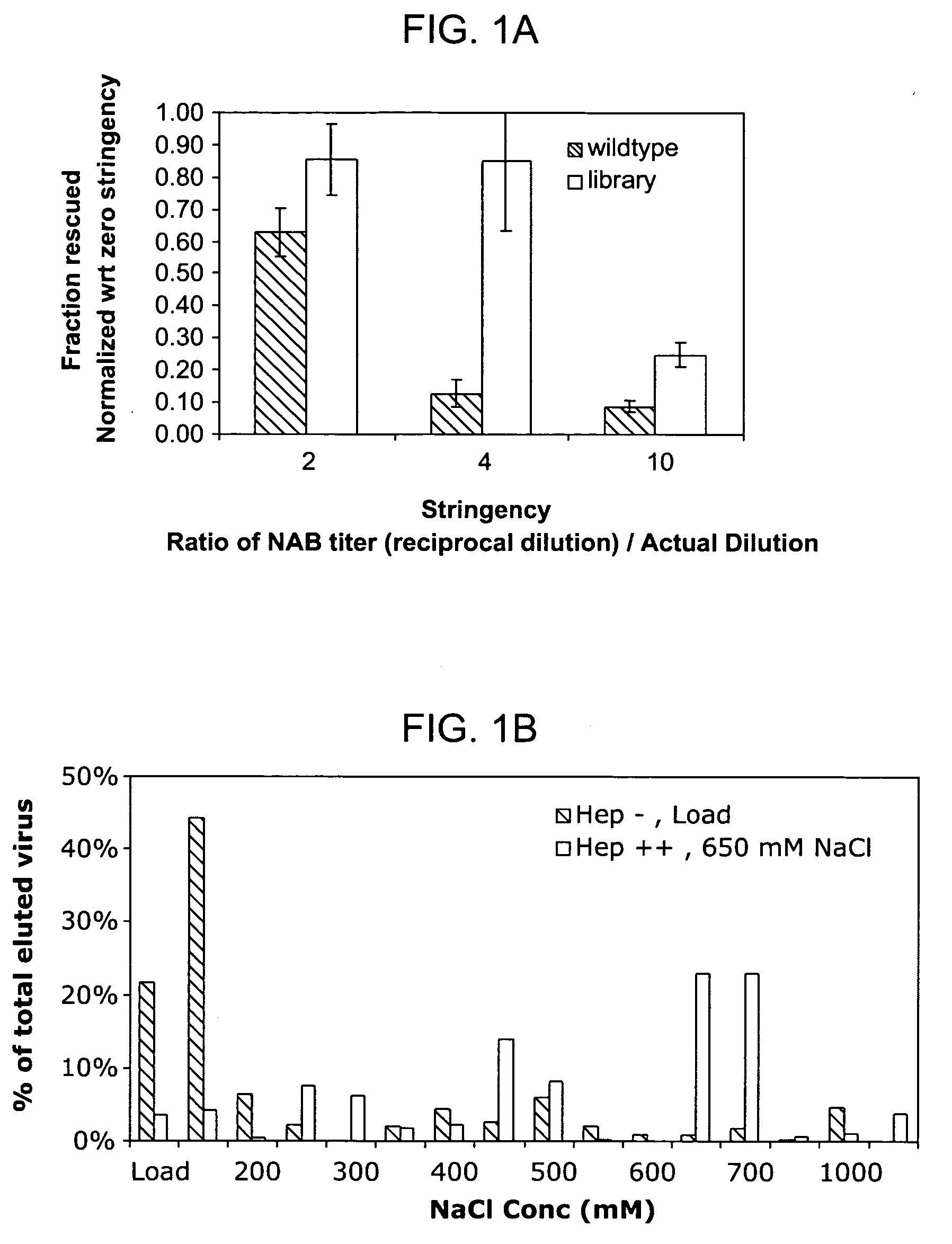
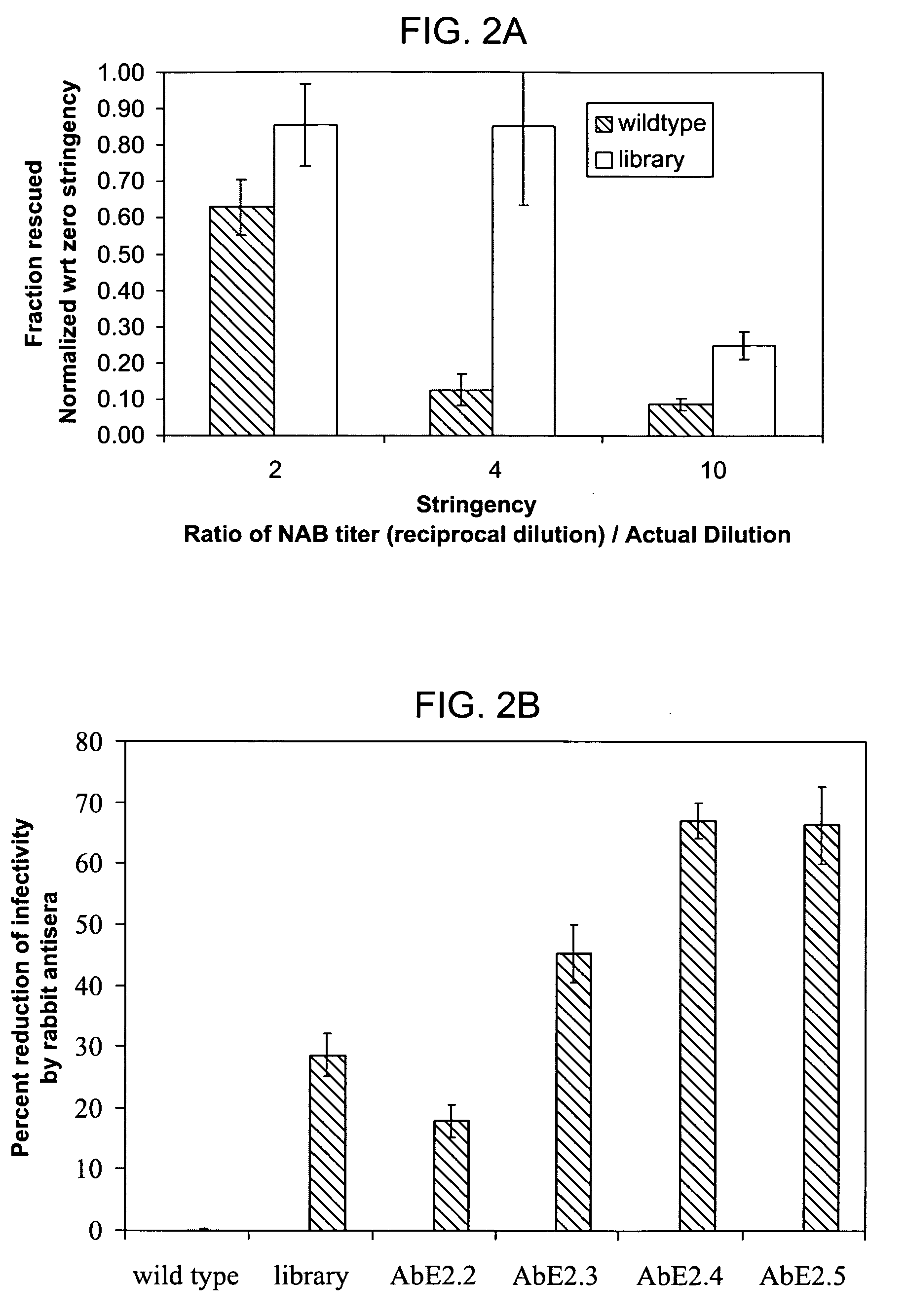
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20050053922A1Reduce the binding forceAltered infectivityAntibacterial agentsVirusesReassortant VirusesNeutralizing antibody
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
Micro plasminogen mutant with function of inhibiting platelet aggregation and preparation method and application thereof
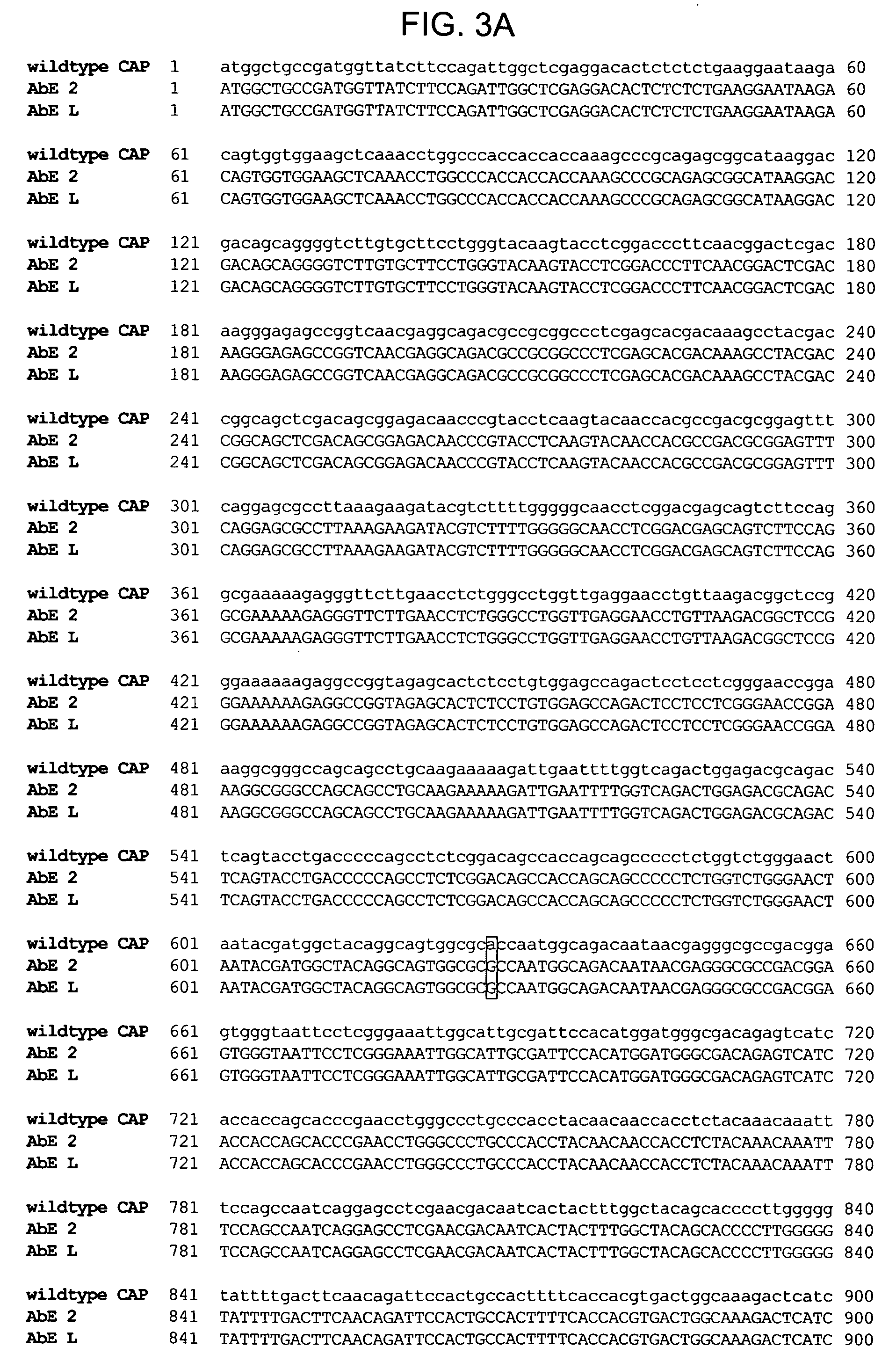
The invention discloses a micro plasminogen mutant with a function of inhibiting platelet aggregation, which has an amino acid sequence of KLYDYCKGDWPCAAPSFDCGKPQVEPKKCPGRVVGGCVAHPHSWPWQVSLRTRFGMHFCGGTLISPEWVLTAAHCLEKSPRPSSYKVILGAHQEVNLEPHVQEIEVSRLFLEPTRKDIALLKLSSPAVITDKVIPACLPSPNYVVADRTECFITGWGETQGTFGAGLLKEAQLPVIENKVCNRYEFLNGRVQSTELCAGHLAGGTDSCQGDSGGPLVCFEKDKYILQGVTSWGLGCARPNKPGVYVRVSRFVTWIEGVMRNN. A preparation method of the micro plasminogen mutant comprises the following steps of: replacing a D-V-P-Q sequence at 7 to 10 positions of a micro plasminogen sequence with a K-G-D-W-P sequence; forming a convex Loop structure on the replaced sequence; and placing a K-G-D sequence at the top end of the Loop structure. Meanwhile, the invention provides application of the micro plasminogen mutant to preparation of a medicament for preventing and treating thrombotic diseases and ophthalmic diseases.
Owner:ZHENGZHOU UNIV
Method for constructing gene site-directed mutation
ActiveCN103388006AEfficient constructionRaise the ratioVector-based foreign material introductionForeign genetic material cellsMulti siteEmbryo
Owner:BIORAY LABORATORIES INC
Soluble CTLA4 mutant molecules
Owner:BRISTOL MYERS SQUIBB CO
Mutants of Aspergillus niger PhyA phytase and Aspergillus fumigatus phytase
ActiveUS7919297B2High nutritional valueImprove bioavailabilityBacteriaSugar derivativesAspergillus fumigatusPhytase
The present invention is directed to an isolated nucleic acid molecule encoding mutant phytases and the isolated mutant phytases themselves. The present invention further relates to methods of using the isolated nucleic acid molecules and the isolated mutant phytases of the present invention.
Owner:CORNELL RES FOUNDATION INC
Methods of producing prenyl alcohols
InactiveUS20070087425A1Efficient cultivationIncrease productivityPolypeptide with localisation/targeting motifFungiBiotechnologyAlcohol
A method of producing a prenyl alcohol, comprising creating a recombinant by transferring into a host a recombinant DNA for expression or a DNA for genomic integration each comprising a prenyl diphosphate synthase gene or a mutant thereof, culturing the resultant recombinant, and recovering the prenyl alcohol from the resultant culture.
Owner:TOYOTA JIDOSHA KK
Methods for treating viral infection using IL-28 and IL-29
ActiveUS7135170B2Reduction in viral infection levelReduce viral infectionBiocidePeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Active variants of FGF with improved specificity
InactiveUS7288406B2Enhanced receptor specificityImprove in vivo activitySugar derivativesPeptide/protein ingredientsDiseaseReceptor subtype
The present invention provides active fibroblast growth factor variants demonstrating enhanced receptor subtype specificity. The preferred novel variants retain binding to FGF Receptor Type 3 (FGFR3) triggering intracellular downstream mechanisms leading to activation of a biological response. Methods of utilizing preferred FGF mutants in preparation of medicaments for the treatment of malignancies and skeletal disorders including osteoporosis and enhancing fracture healing and wound healing processes are provided.
Owner:PROCHON BIOTECH
Factor VIII compositions and methods
InactiveUS20050100990A1Reduce gapExtended half-lifeFactor VIIPeptide/protein ingredientsHalf-lifeNucleotide
The present invention provides methods of increasing the half-life and / or specific activity of factor VIII. More specifically, the invention provides methods of increasing the half-life and / or specific activity of factor VIII by substituting one or more amino acids in the A2 domain. It further provides methods for producing such factor VIII mutants. The invention also provides polynucleotides encoding the mutant factor VIII, and methods of treating hemophilia using the polypeptides and polynucleotides of the invention.
Owner:UNIV OF MARYLAND
Fully active alternansucrases partially deleted in its carboxy-terminal and amino-terminal domains and mutants thereof
Nucleic acid sequences of truncated or mutated alternansucrases, vectors containing these nucleic acids sequences, host cells transformed with the nucleic acid sequences encoding truncated or mutated alternansucrases are provided. Furthermore, a process to recombinantly alternansucrase with a high level of expression, while retaining the enzymatic activity is described.
Owner:CENT NAT DE LA RECHERCHE SCI
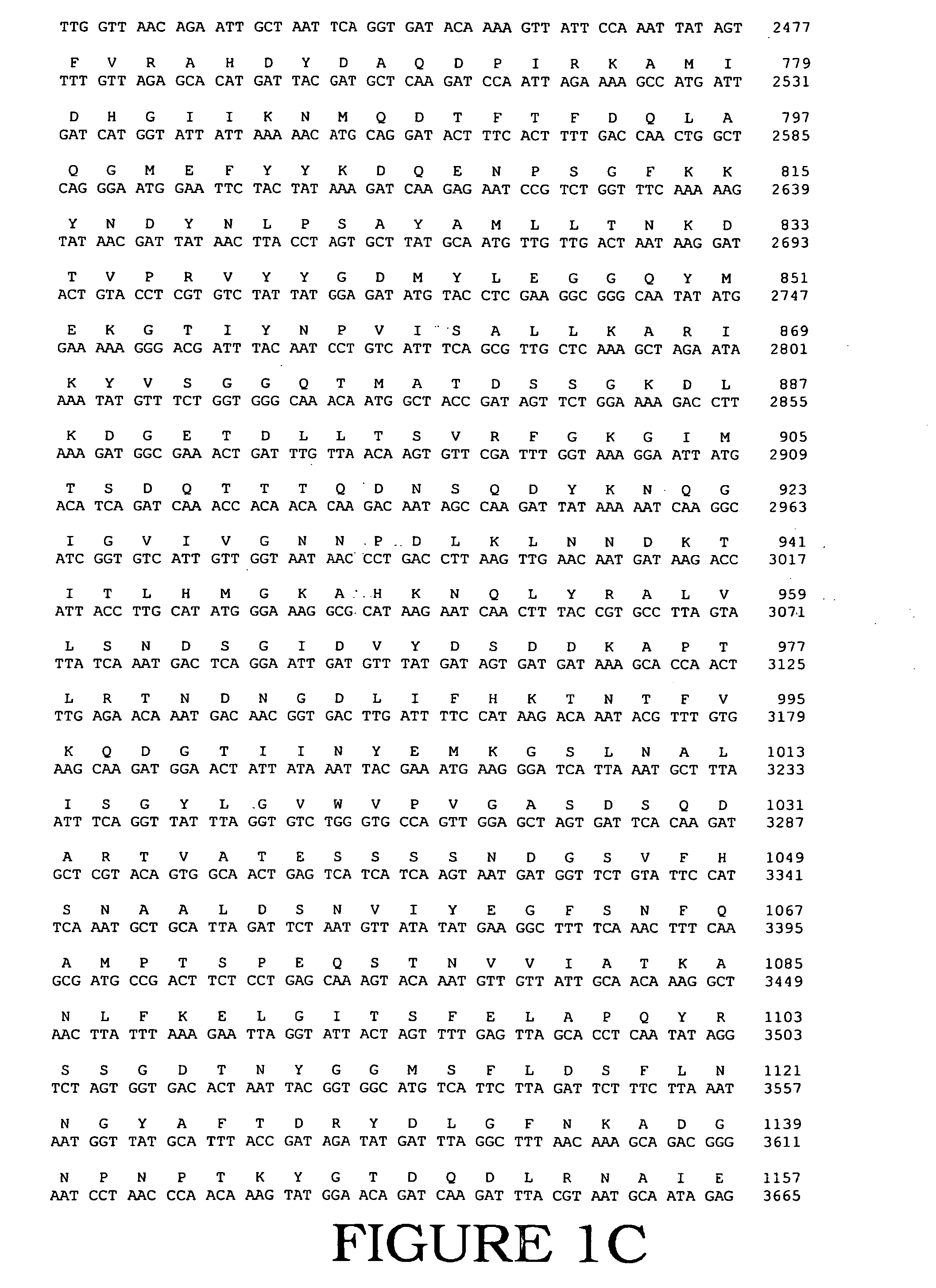
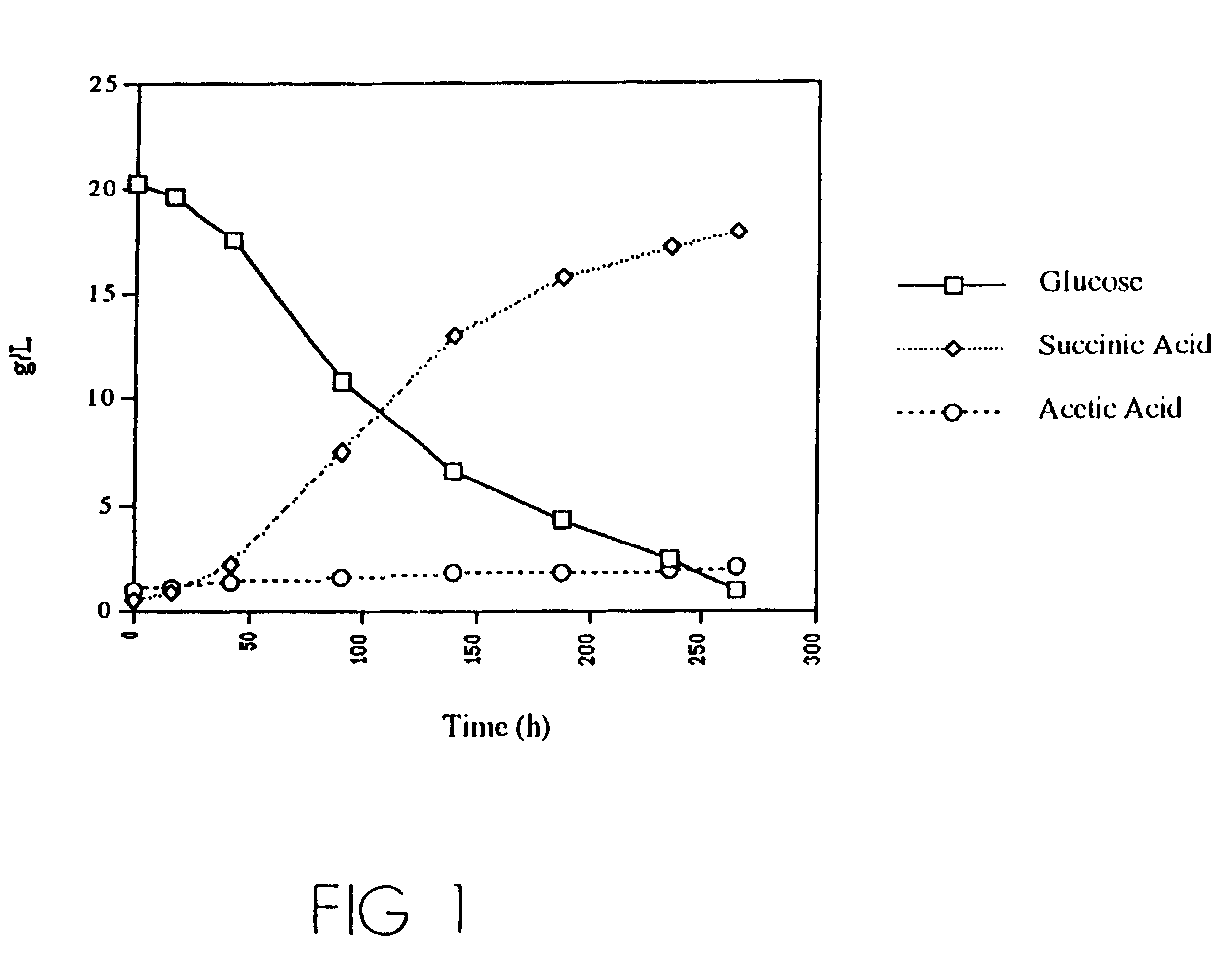
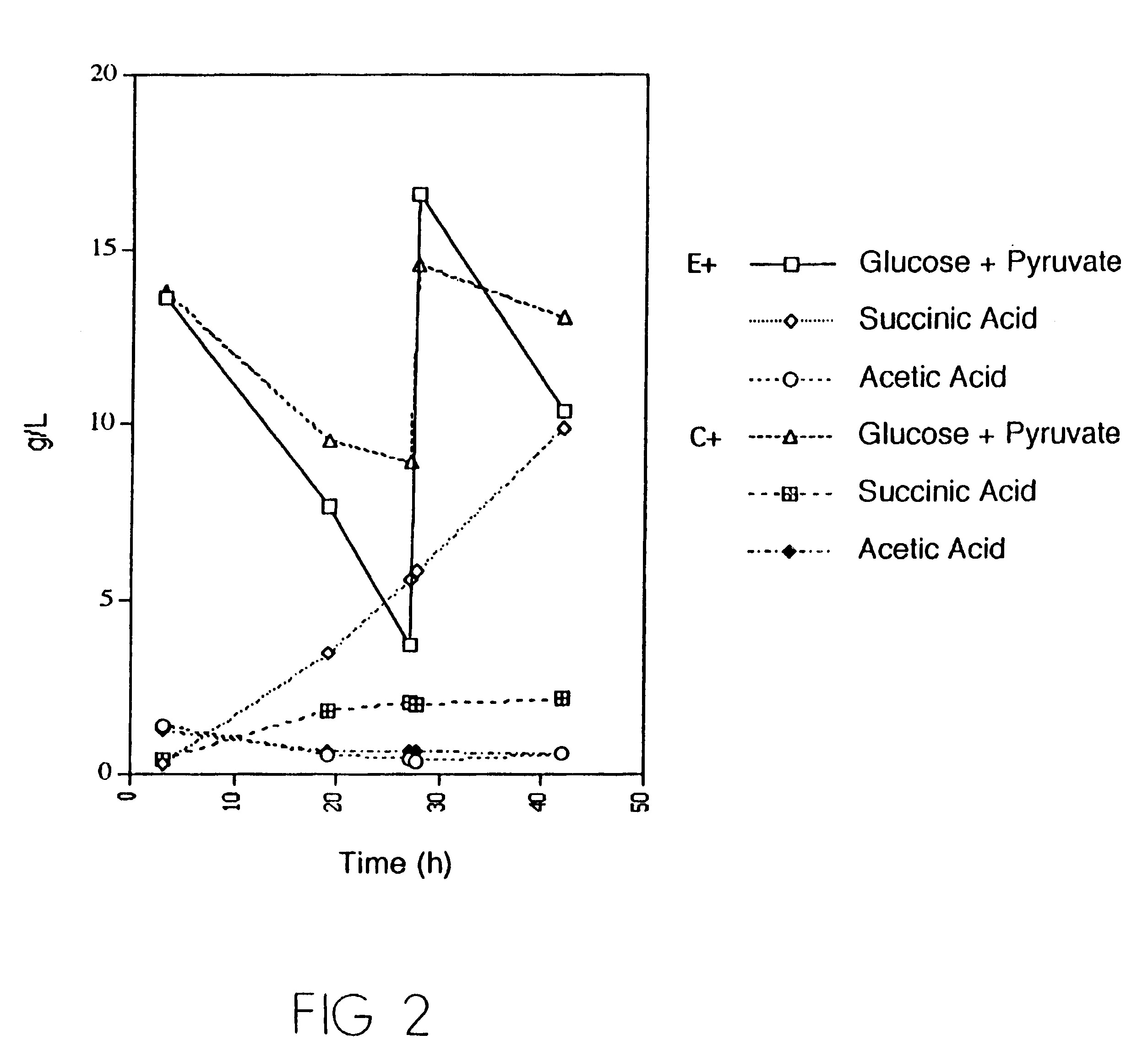
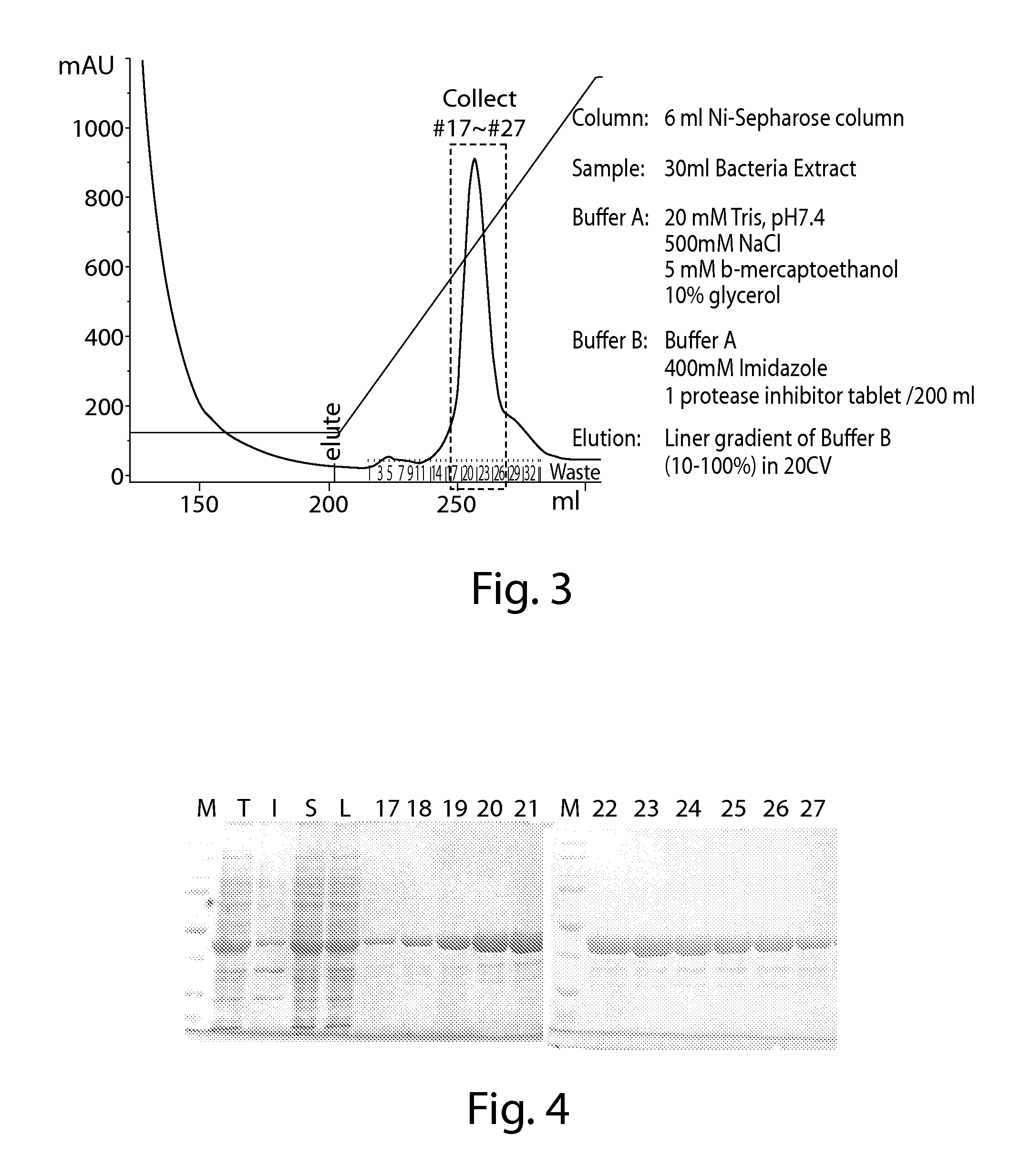
Mutant E. coli strain with increased succinic acid production
InactiveUSRE37393E1Cheap productionIncrease biomassBacteriaUnicellular algaeLactate dehydrogenaseBiological body
A method for isolating succinic acid producing bacteria is provided comprising increasing the biomass of an organism which lacks the ability to catabolize pyruvate, and then subjecting the biomass to glucose-rich medium in an anaerobic environment to enable pyruvate-catabolizing mutants to grow.The invention also provides for a mutant that produces high amounts of succinic acid, which has been derived from a parent which lacked the genes for pyruvate formate lyase and lactate dehydrogenase, and which belongs to the E.coli Group of Bacteria.
Owner:UNIVERSITY OF CHICAGO
Methods and compositions for cell-proliferation-related disorders
InactiveUS20120121515A1Lower Level RequirementsImprove matchDrug and medicationsNMR/MRI constrast preparationsDiseaseMutant
Owner:SERVIER PHARM LLC
Methods for protecting allogeneic islet transplant using soluble CTLA4 mutant molecules
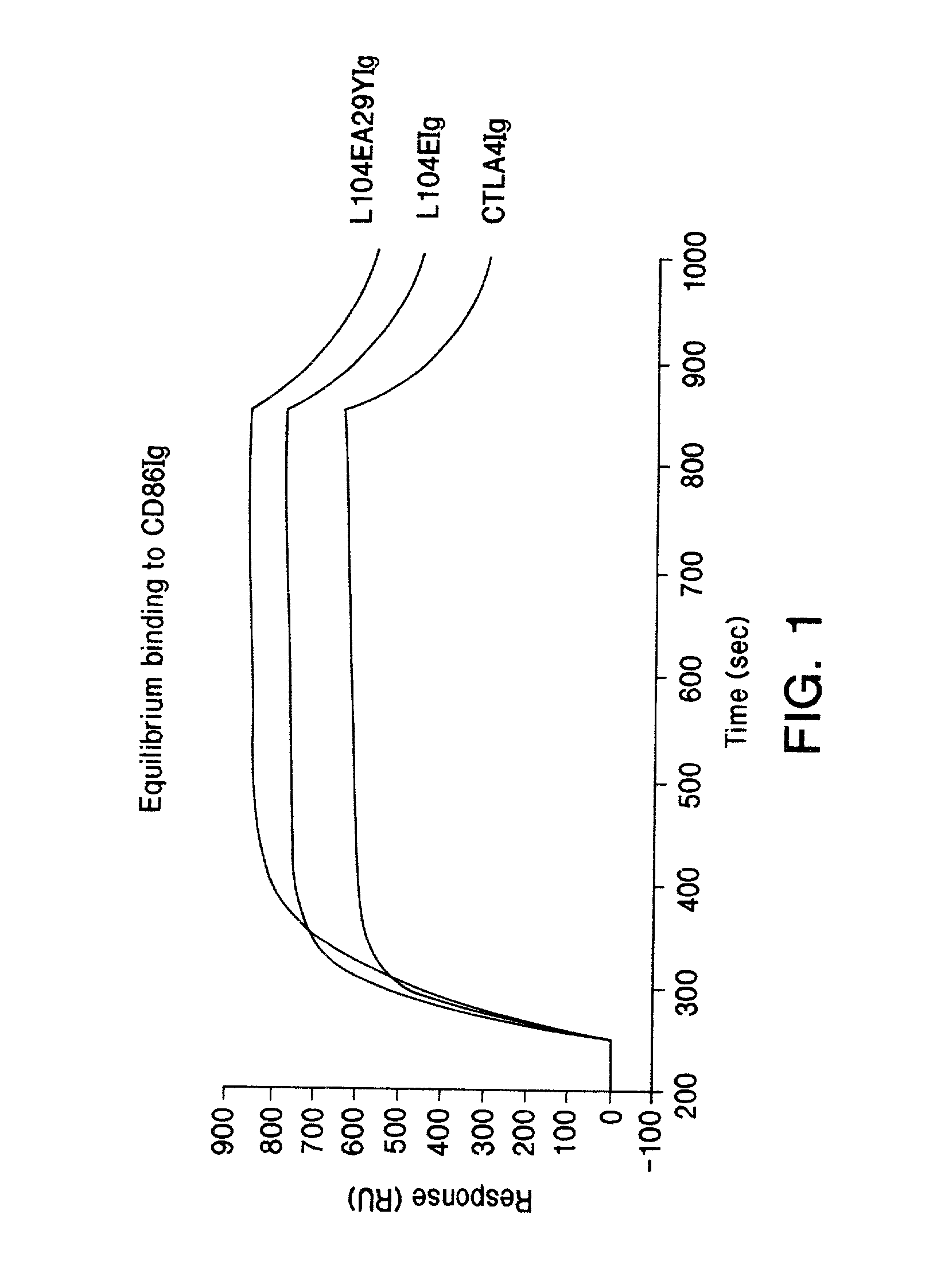
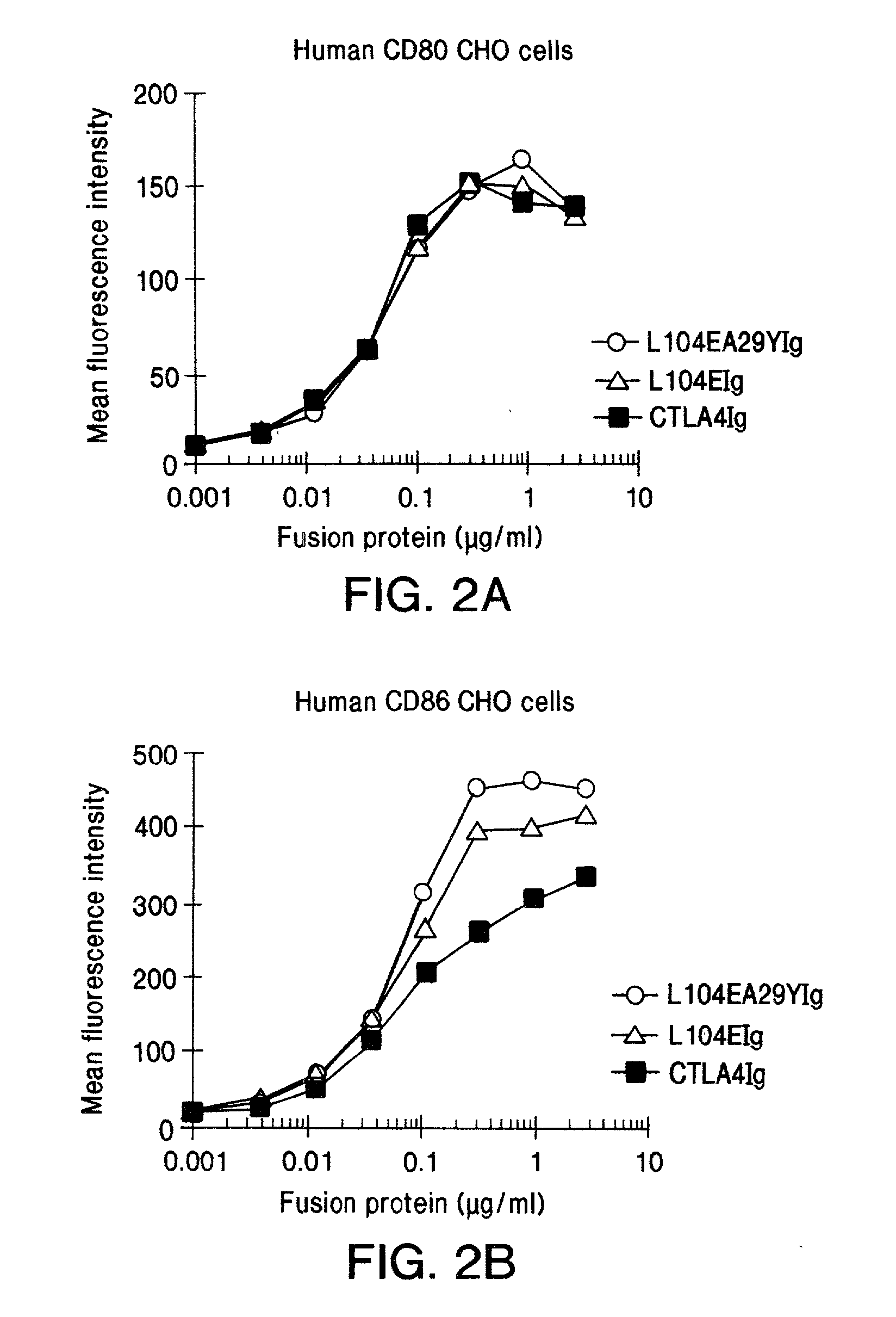
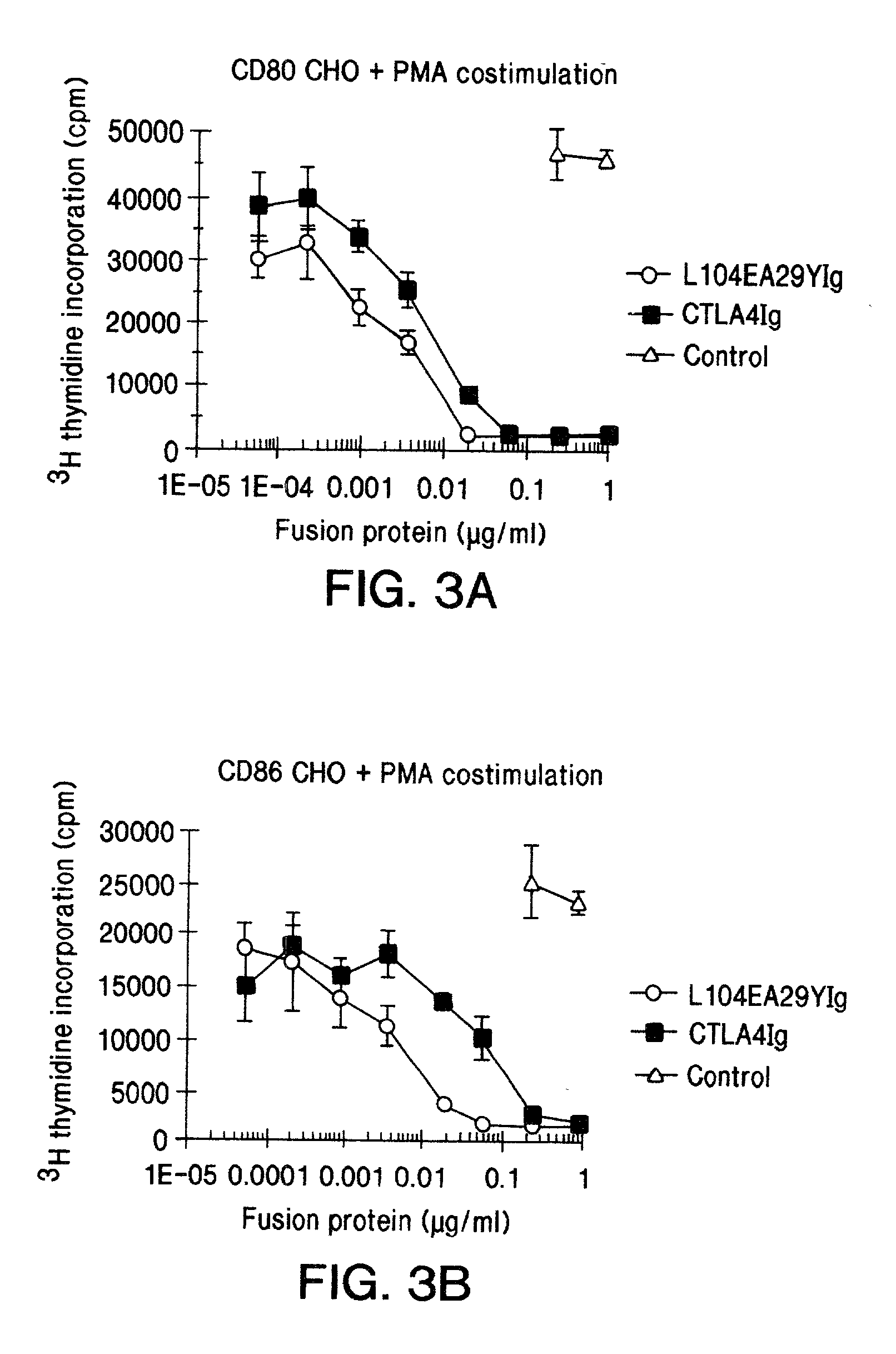
The present invention is a method of inhibiting islet cell transplant rejection, particularly to treat diabetes, such as type-1 and type-2 diabetes, by administering to a subject an effective amount of a soluble CTLA4 mutant molecule. One example of a soluble CTLA4 mutant molecule is L104EA29YIg.
Owner:BRISTOL MYERS SQUIBB CO
Mutants of anti-cd40 antibody
ActiveUS20070148163A1High therapeutic effectImmunoglobulins against bacteriaImmunoglobulins against virusesHinge regionMutant
A mutant of a potentially therapeutic anti-CD40 antibody is provided which mutant has reduced ADCC and CDC activities designed to be optimized as a pharmaceutical agent. A mutant of an agonistic anti-CD40 antibody, comprising mutation and / or substitution of at least one amino acid in the constant region to reduce the ADCC and / or CDC activities therein, and a mutant of an antagonistic anti-CD40 antibody, comprising at least one mutation or substitution in the constant region to reduce the ADCC and / or CDC activities therein, both mutants having at least a hinge region derived from a human IgG2.
Owner:KYOWA HAKKO KIRIN CO LTD
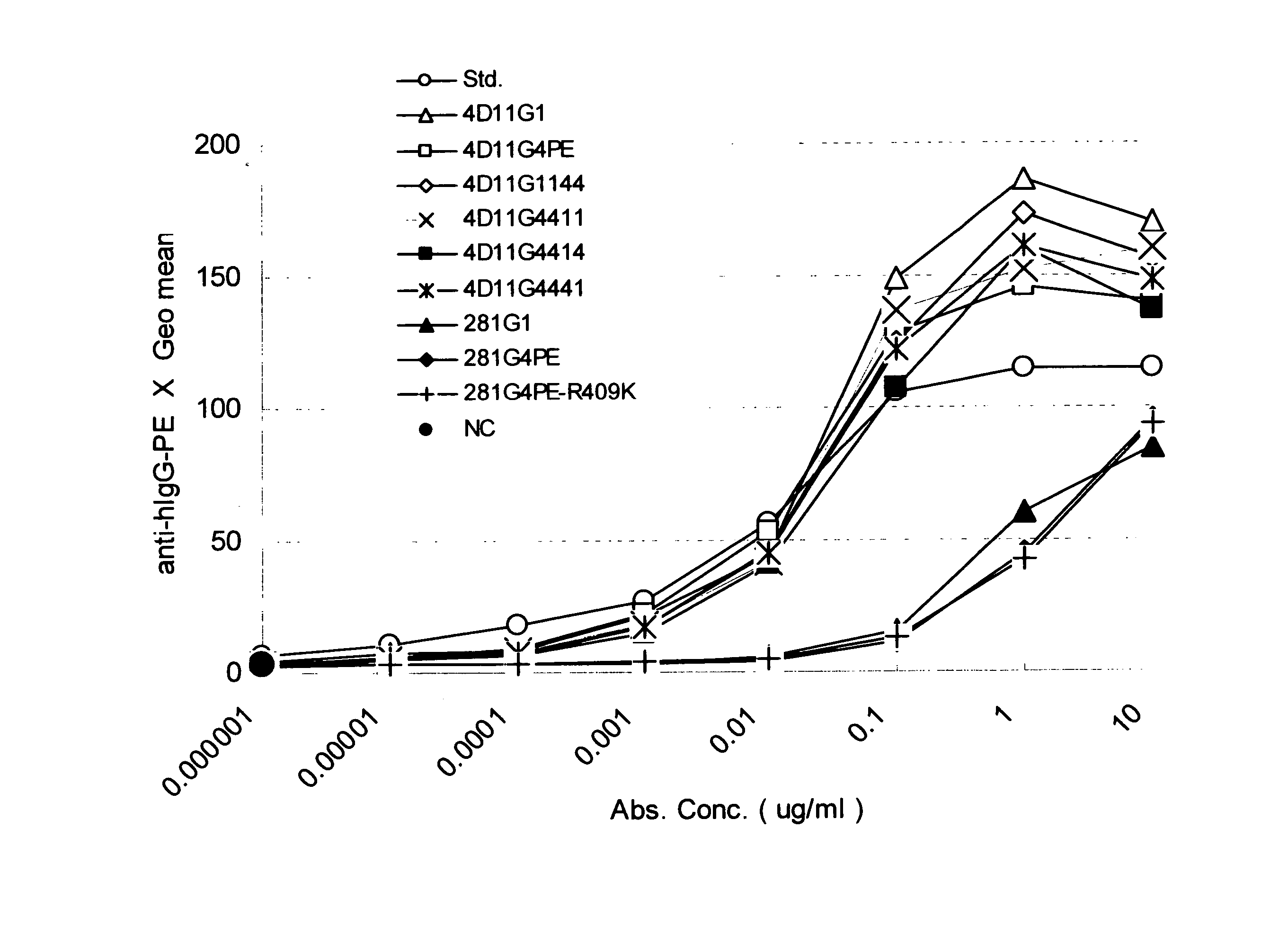
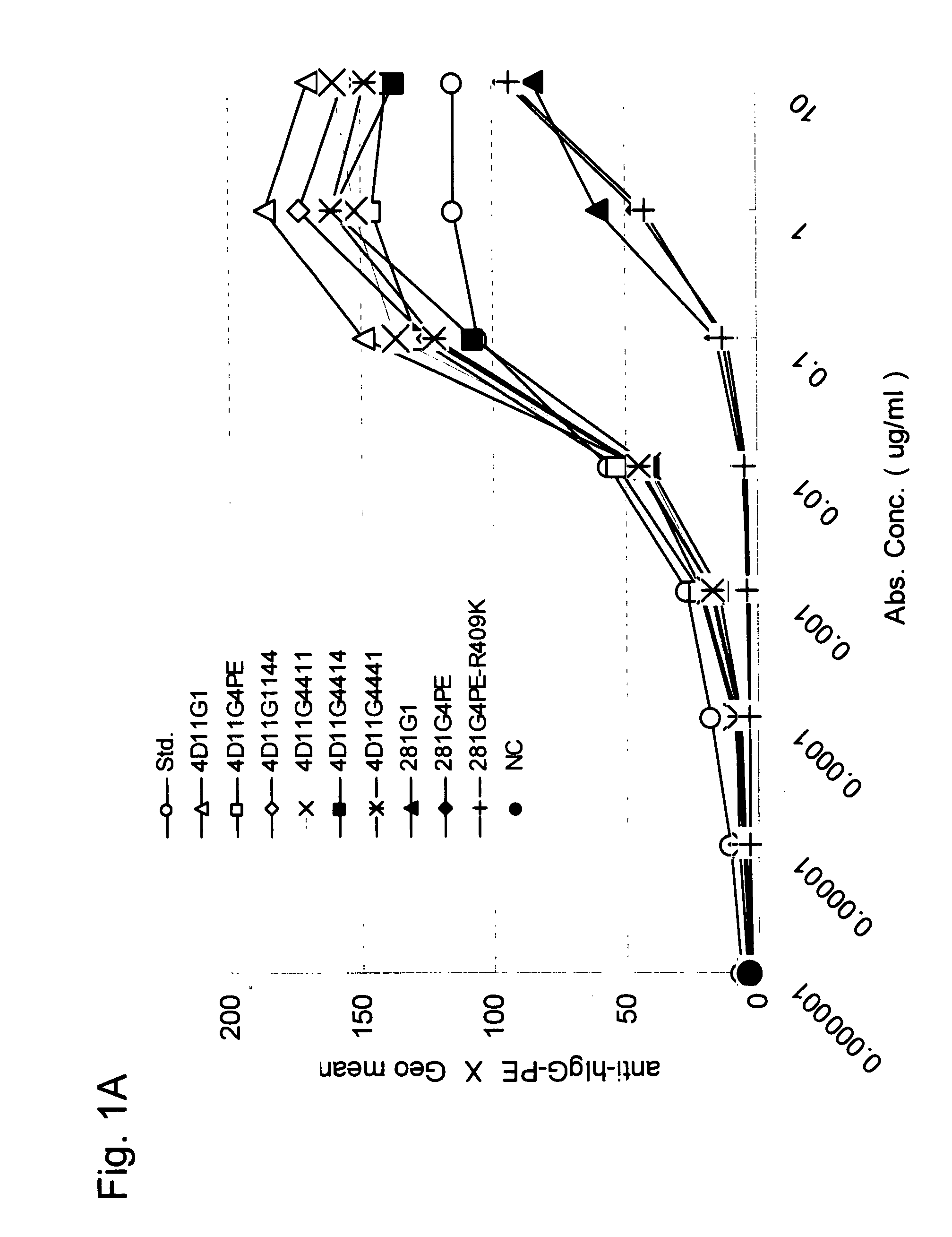
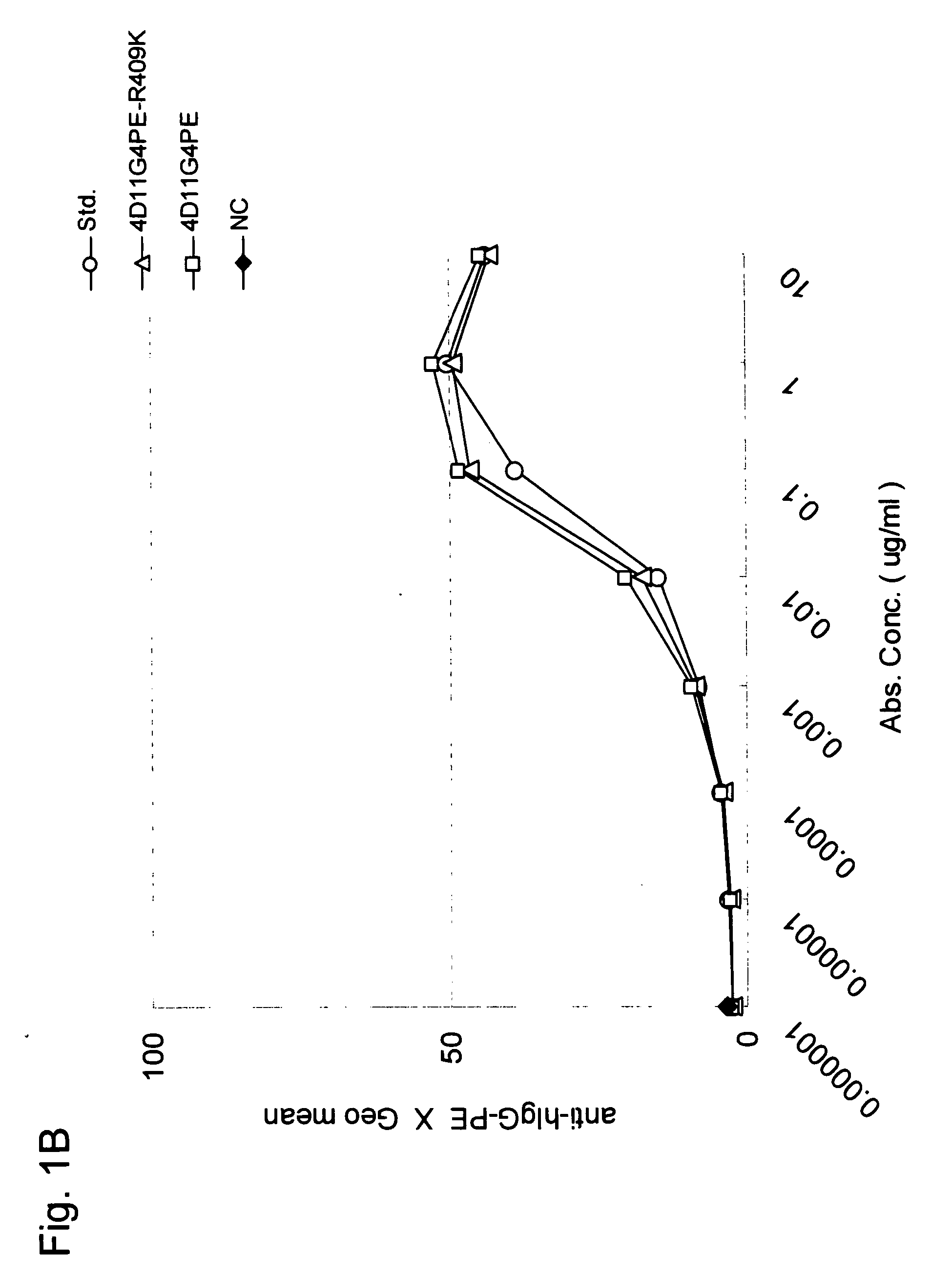
Stabilized Human Igg4 Antibodies
ActiveUS20080063635A1Stable productionReduce adverse effectsNervous disorderAntipyreticArginineMutant
A highly stable mutant of human IgG4 antibody is provided. Such antibody is an antibody in which the CH3 domain of human IgG4 is substituted with the CH3 domain of human IgG1 and which exhibits inhibited aggregate formation, an antibody in which the CH3 and CH2 domains of human IgG4 are substituted with the CH3 and CH2 domains of human IgG1, respectively, or an antibody in which arginine at position 409 indicated in the EU index proposed by Kabat et al. of human IgG4 is substituted with lysine and which exhibits inhibited aggregate formation.
Owner:KYOWA HAKKO KIRIN CO LTD
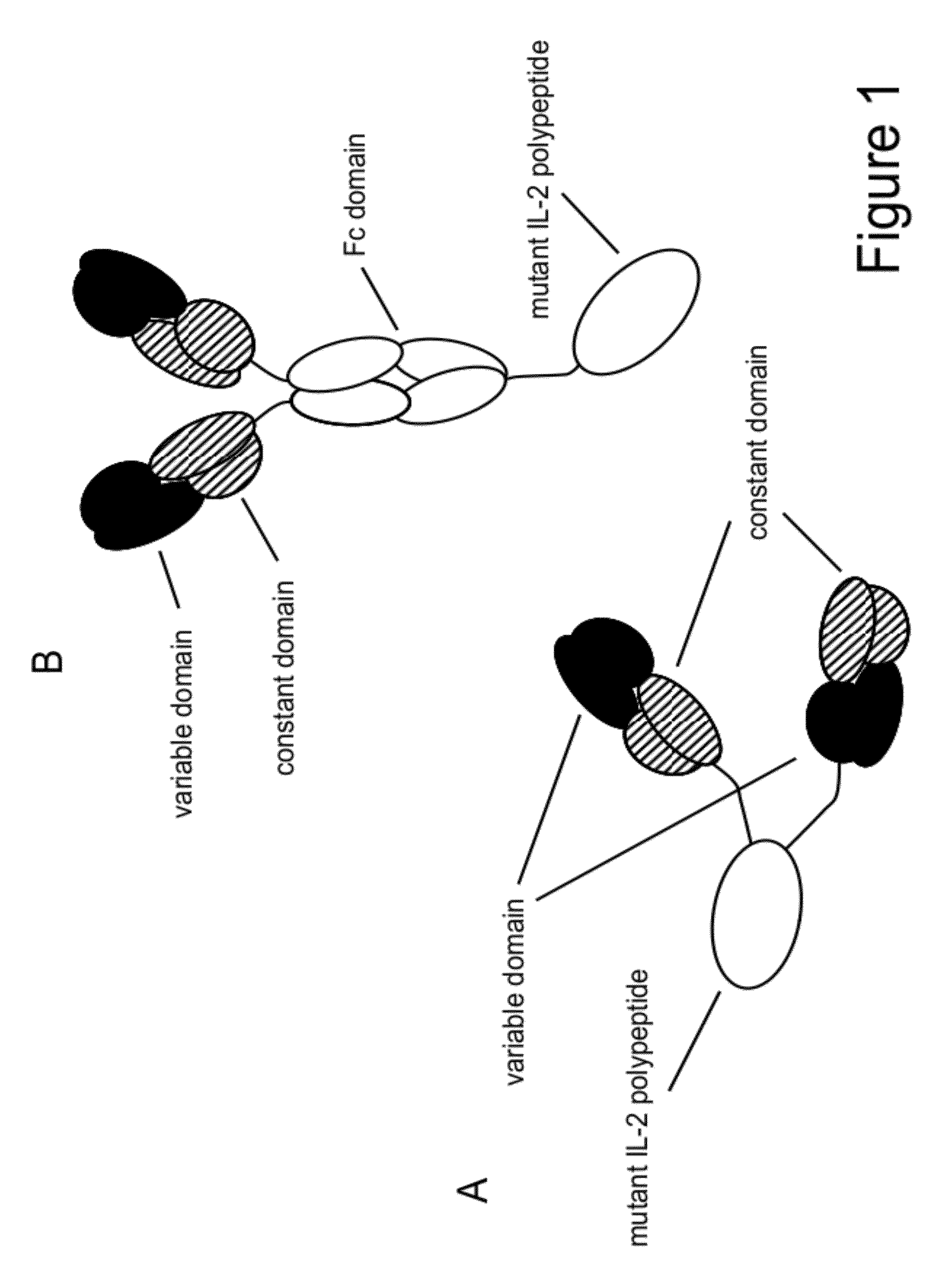
Mutant interleukin-2 polypeptides
ActiveUS9266938B2Eliminates and decrease and delayEliminates and decrease and and and effectPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunotherapeutic agentNucleotide
The present invention generally relates to mutant interleukin-2 polypeptides that exhibit reduced affinity to the α-subunit of the IL-2 receptor, for use as immunotherapeutic agents. In addition, the invention relates to immunoconjugates comprising said mutant IL-2 polypeptides, polynucleotide molecules encoding the mutant IL-2 polypeptides or immunoconjugates, and vectors and host cells comprising such polynucleotide molecules. The invention further relates to methods for producing the mutant IL-2 polypeptides or immunoconjugates, pharmaceutical compositions comprising the same, and uses thereof.
Owner:ROCHE GLYCART AG
Alpha-amylase mutants
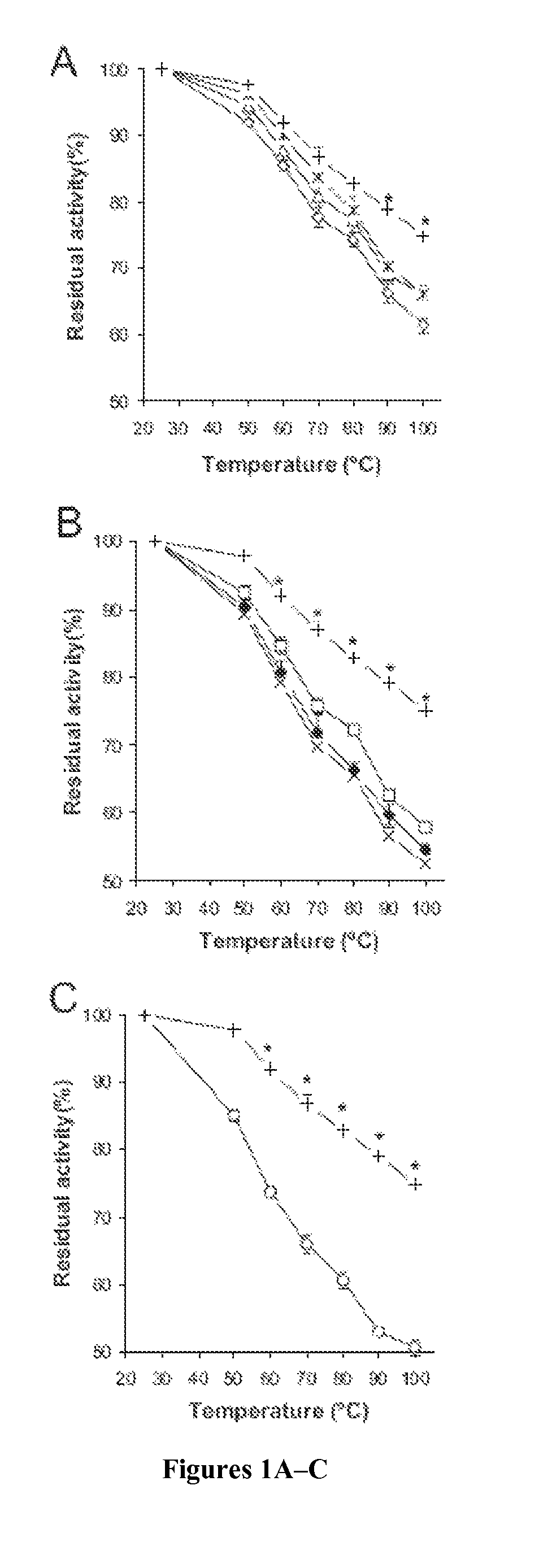
InactiveUS6440716B1Improve stabilitySpecific activityOrganic detergent compounding agentsEnzymesMutantAlpha-amylase activity
The present invention relates to a method of constructing a variant of a parent Termamyl-like alpha-amylase, which variant has alpha-amylase activity and at least one altered property as compared to the parent alpha-amylase, comprisesi) analysing the structure of the parent Termamyl-like alpha-amylase to identify at least one amino acid residue or at least one structural part of the Termamyl-like alpha-amylase structure, which amino acid residue or structural part is believed to be of relevance for altering the property of the parent Termamyl-like alpha-amylase (as evaluated on the basis of structural or functional considerations),ii) constructing a Termamyl-like alpha-amylase variant, which as compared to the parent Termamyl-like alpha-amylase, has been modified in the amino acid residue or structural part identified in i) so as to alter the property, and, optionally,iii) testing the resulting Termamyl-like alpha-amylase variant with respect to the property in question.
Owner:NOVOZYMES AS
BID polypeptides and methods of inducing apoptosis
ActiveUS7247700B2Reduce triggersPeptide/protein ingredientsHydrolasesApoptosisNucleic acid sequencing
Disclosed herein are novel polypeptides and the nucleic acid sequences that encode them. Also disclosed are antibodies that immunospecifically bind to the polypeptide, as well as derivatives, variants, mutants, or fragments of the novel polypeptide, polynucleotide, or antibody specific to the polypeptide. Vectors, host cells, antibodies and recombinant methods for producing the polypeptides and polynucleotides, as well as methods for using same are also included. The invention further discloses therapeutic, diagnostic and research methods for diagnosis, treatment, and prevention of apoptosis associated disorders involving these novel human nucleic acids and proteins.
Owner:DANA FARBER CANCER INST INC
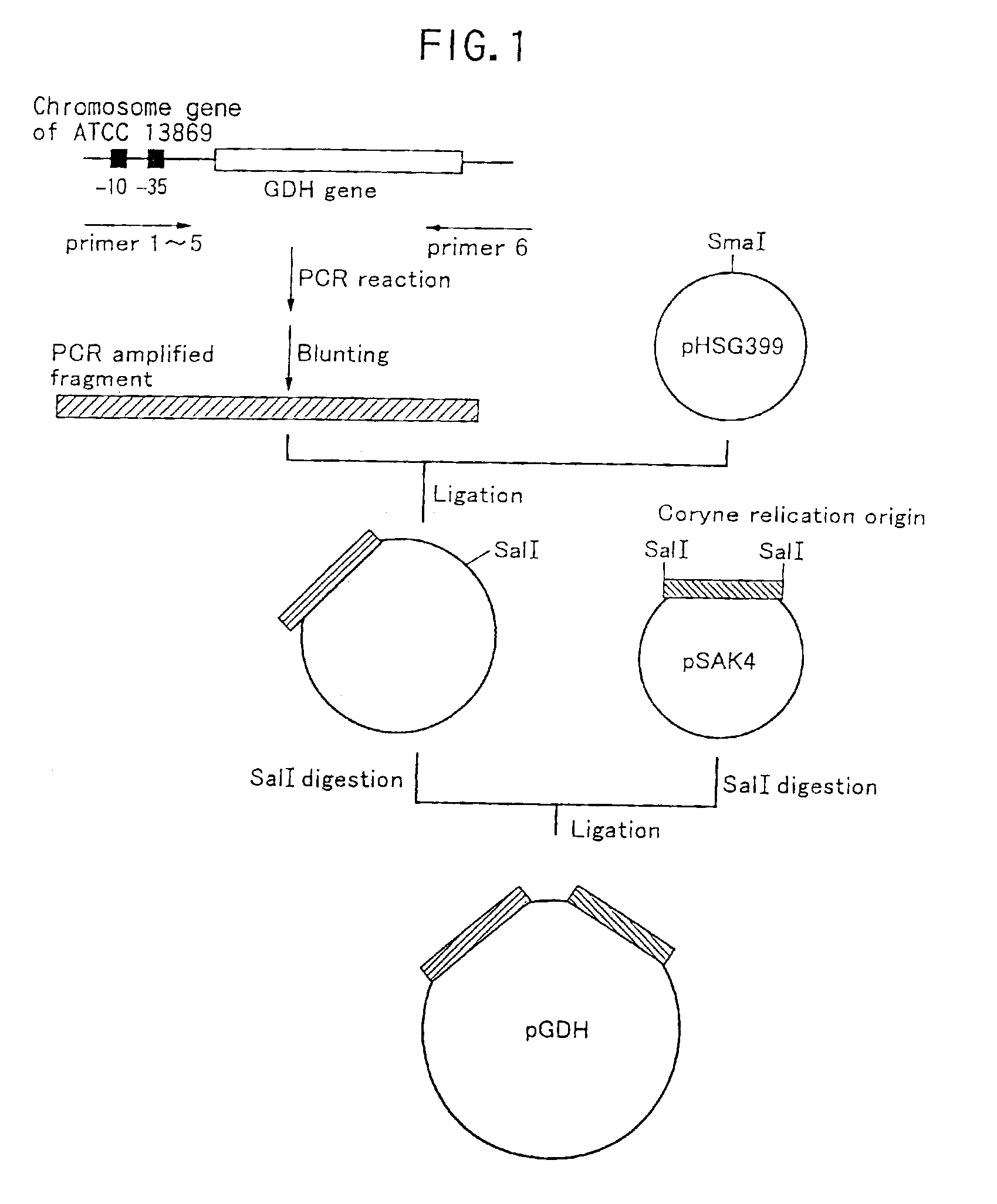
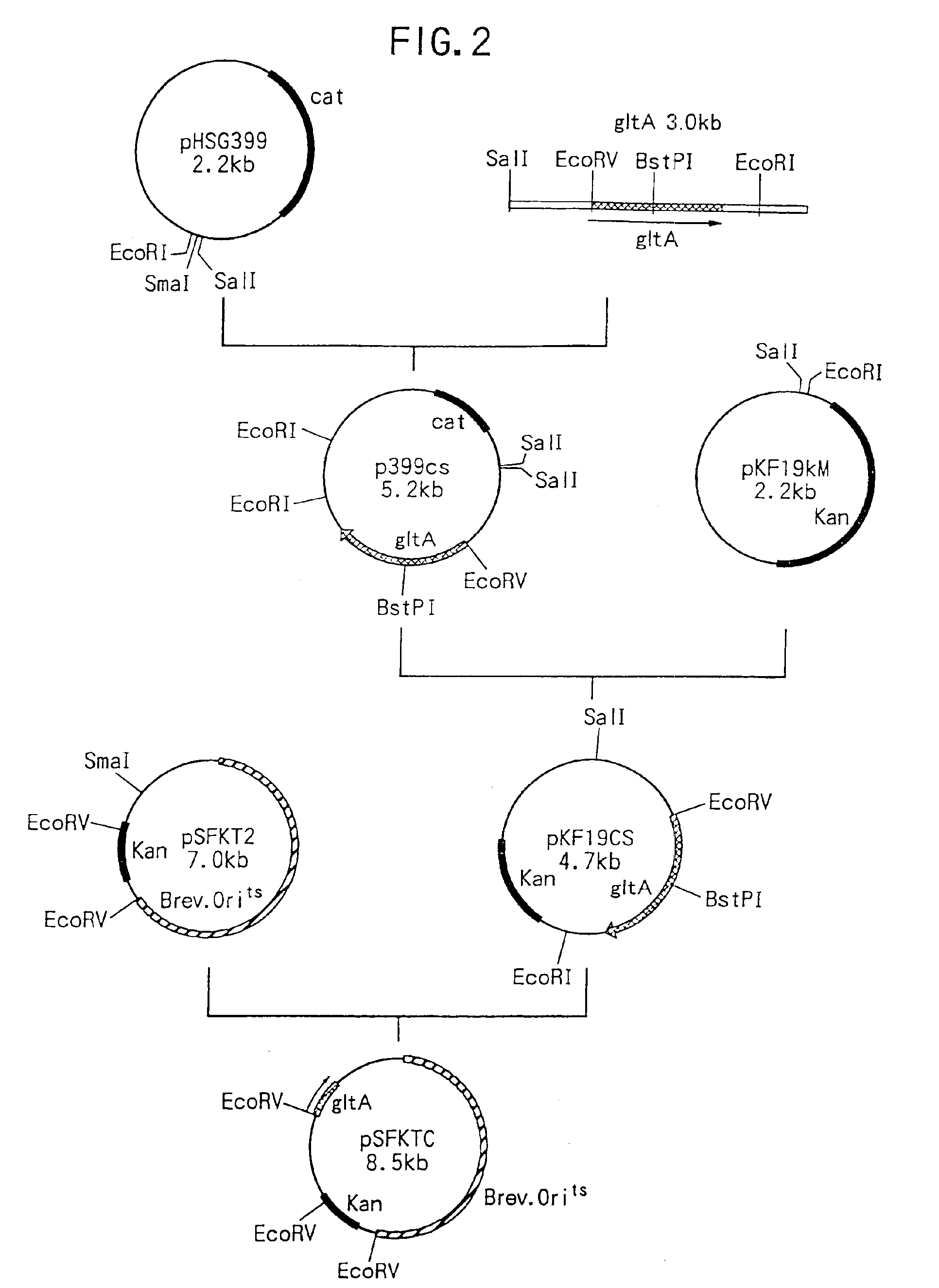
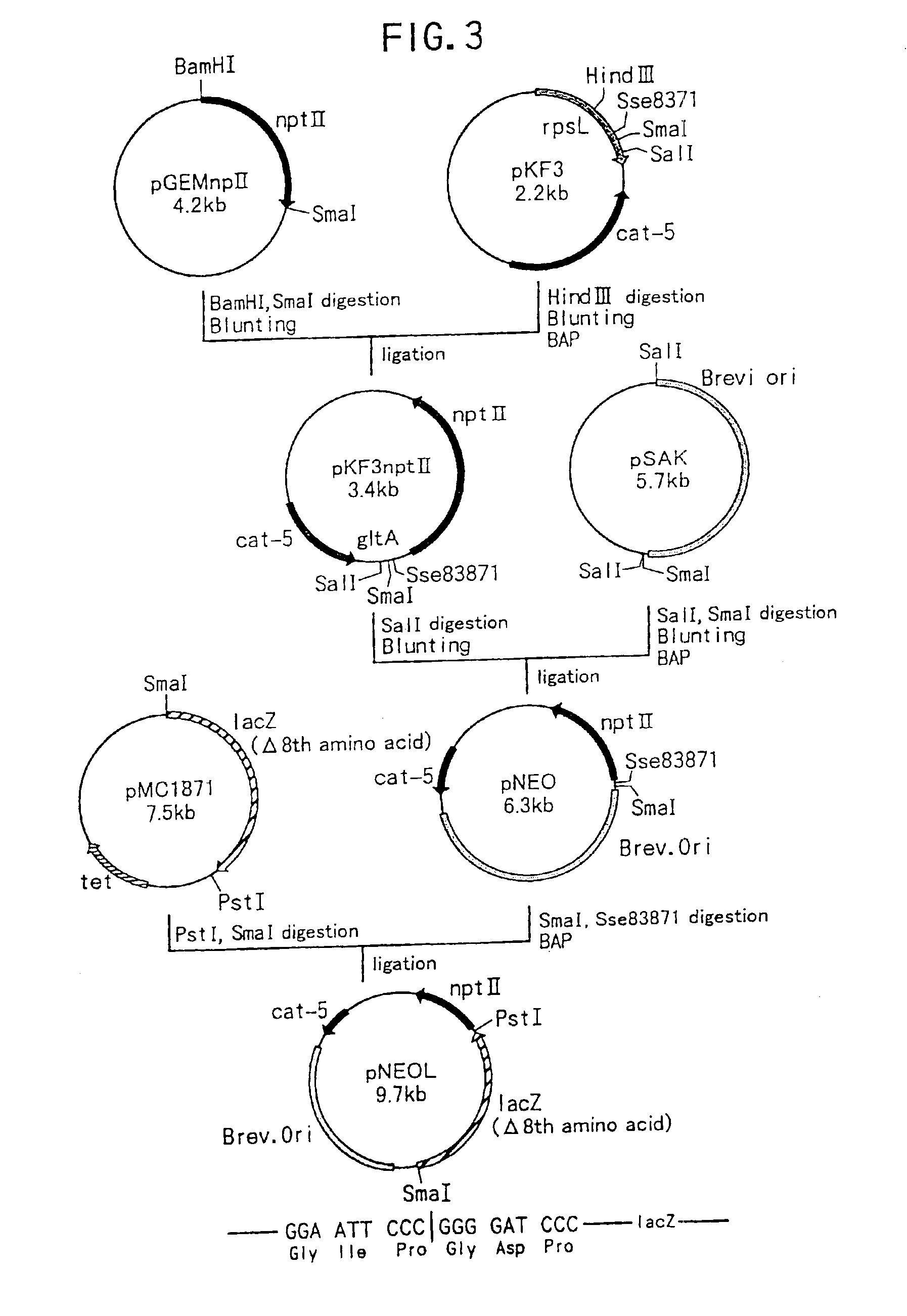
Method of constructing amino acid producing bacterial strains, and method of preparing amino acids by fermentation with the constructed amino acid producing bacterial strains
A method of producing coryneform bacteria having improved amino acid or nucleic acid productivity comprising the steps of introducing a mutation in a promoter sequence of amino acid- or nucleic acid-biosynthesizing genes on a chromosome of a coryneform bacterium to make it close to a consensus sequence, or introducing a change in a promoter sequence of amino acid- or nucleic acid-biosynthesizing genes on a chromosome of a coryneform bacterium by gene recombination to make it close to a consensus sequence, to obtain mutants of the coryneform amino acid- or nucleic acid-producing microorganism, culturing the mutants and selecting a mutant capable of producing the intended amino acid or nucleic acid in a large amount. This method allows the construction of a mutant capable of enriching or controlling the expression of an intended gene without using a plasmid and to promote production of amino acids in a high yield by recombination or mutation.
Owner:AJINOMOTO CO INC
Remodeling and Glycopegylation of Fibroblast Growth Factor (Fgf)
InactiveUS20080176790A1Improve pharmacokineticsCost effectiveOrganic active ingredientsFungiMutantPolynucleotide
The present invention relates to mutants of Fibroblast Growth Factor (FGF), particularly FGF-20 and FGF-21, which contain newly introduced N-linked or O-linked glycosylation site(s). The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:89BIO LTD +1
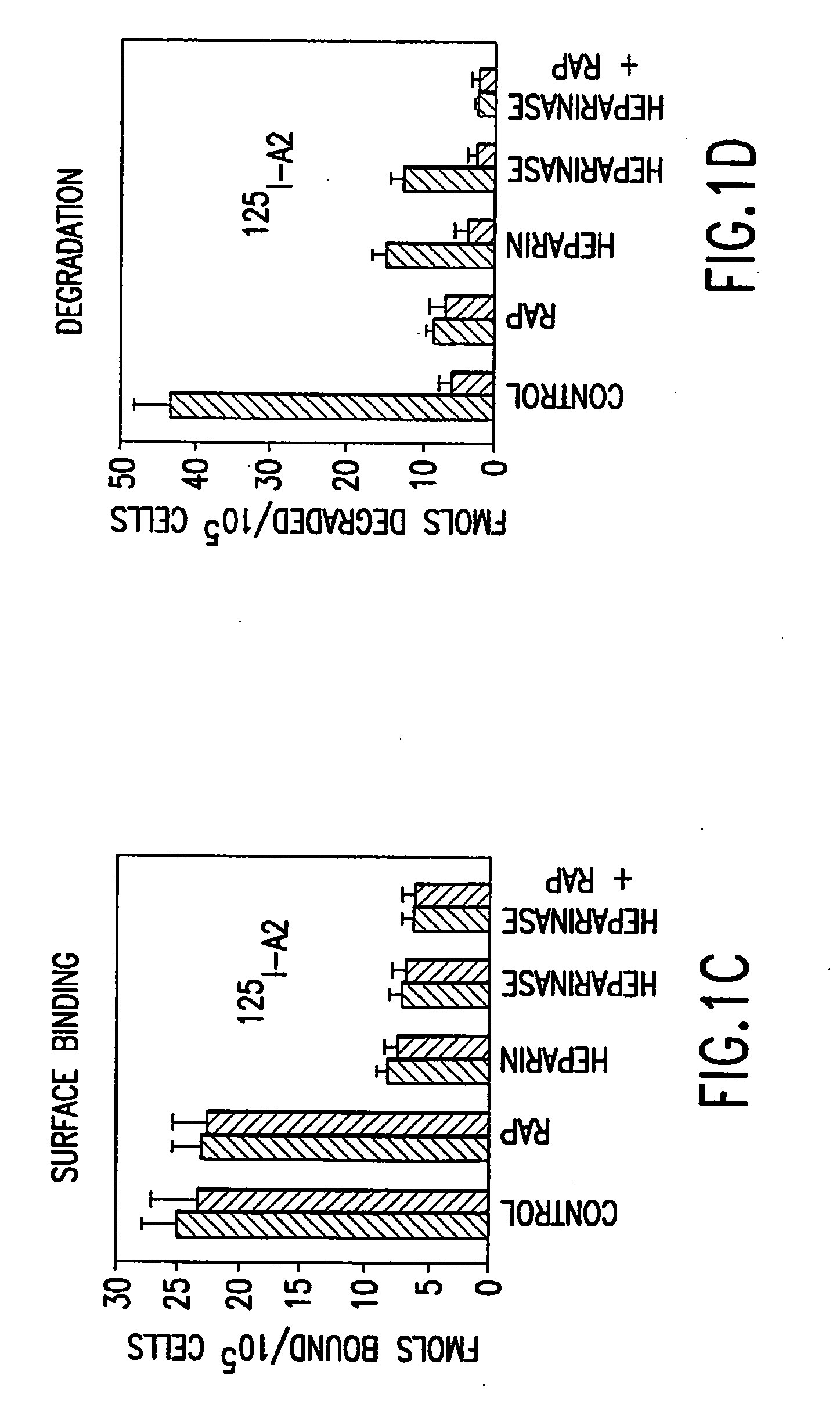
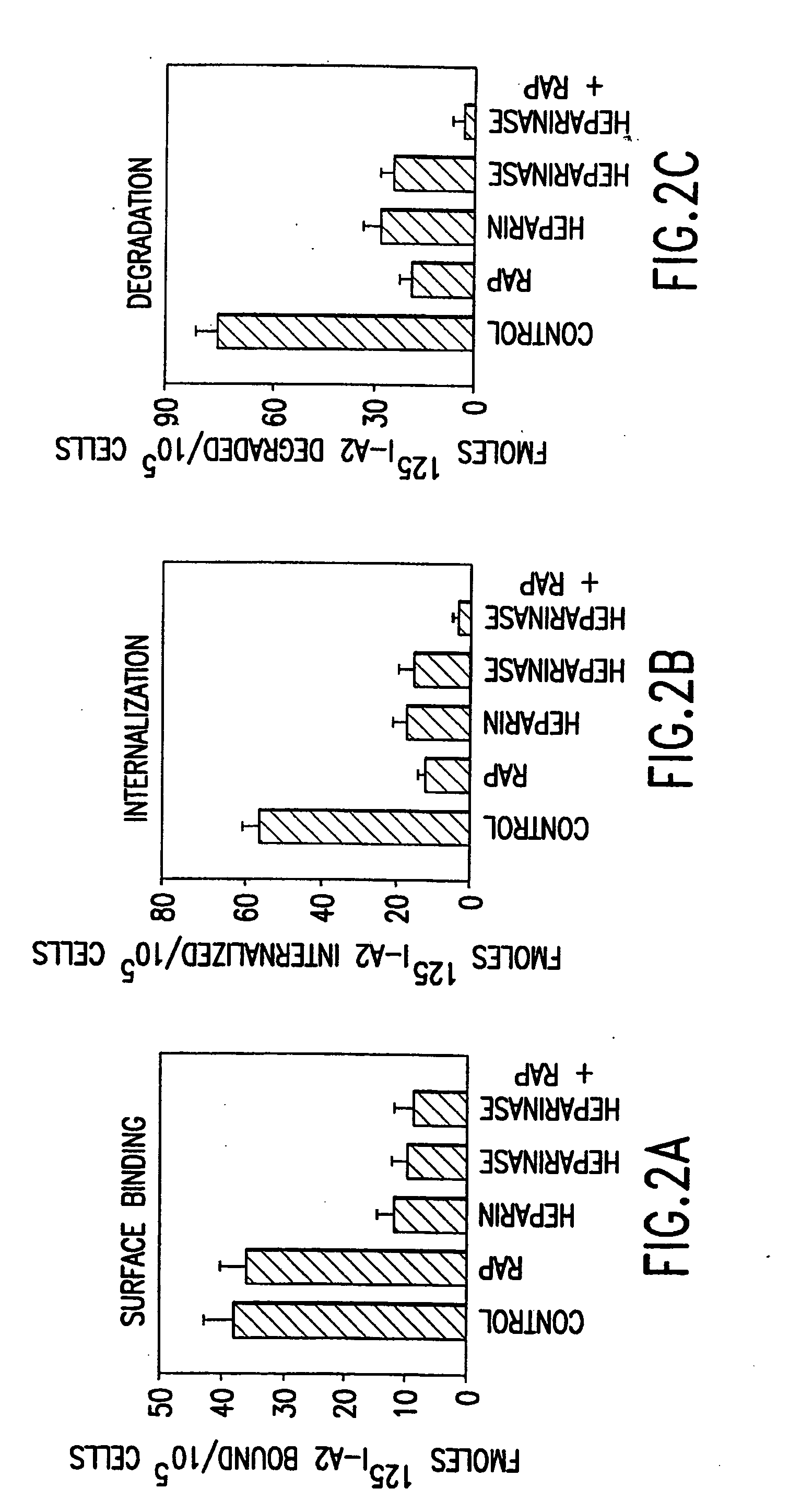
Heparinase III and methods of specifically cleaving therewith
InactiveUS20060067928A1Organic active ingredientsCompound screeningLymphatic SpreadAngiogenesis growth factor
The invention relates to heparinase III and mutants thereof. Modified forms of heparinase III having reduced enzymatic activity which are useful for a variety of purposes, including sequencing of heparin-like glycosaminoglycans (HLGAGs), removing active heparan sulfate from a solution, inhibition of angiogenesis , etc. have been discovered according to the invention. The invention in other aspects relates to methods of treating cancer and inhibiting tumor cell growth and / or metastasis using heparinase III, or products produced by enzymatic cleavage by heparinase III of HLGAGs.
Owner:MASSACHUSETTS INST OF TECH
Heparinase III HLGAG fragments and uses thereof
InactiveUS20050233402A1Accelerate tumor growthInhibit primary tumor growthCompound screeningOrganic active ingredientsLymphatic SpreadSulfate
The invention relates to heparinase III and mutants thereof. Modified forms of heparinase III having reduced enzymatic activity which are useful for a variety of purposes, including sequencing of heparin-like glycosaminoglycans (HLGAGs), removing active heparan sulfate from a solution, inhibition of angiogenesis, etc. have been discovered according to the invention. The invention in other aspects relates to methods of treating cancer and inhibiting tumor cell growth and / or metastasis using heparinase III, or products produced by enzymatic cleavage by heparinase III of HLGAGs.
Owner:MASSACHUSETTS INST OF TECH
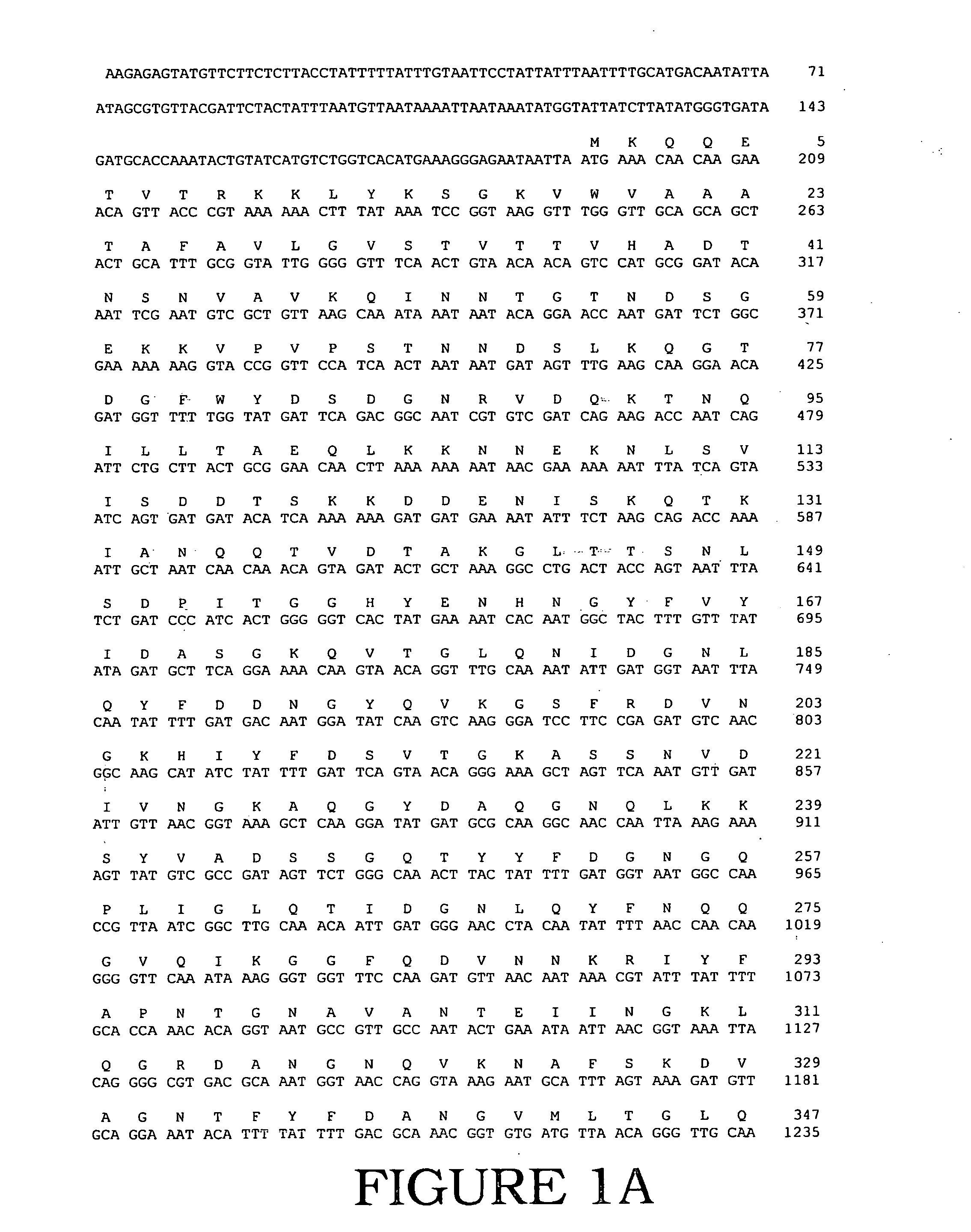
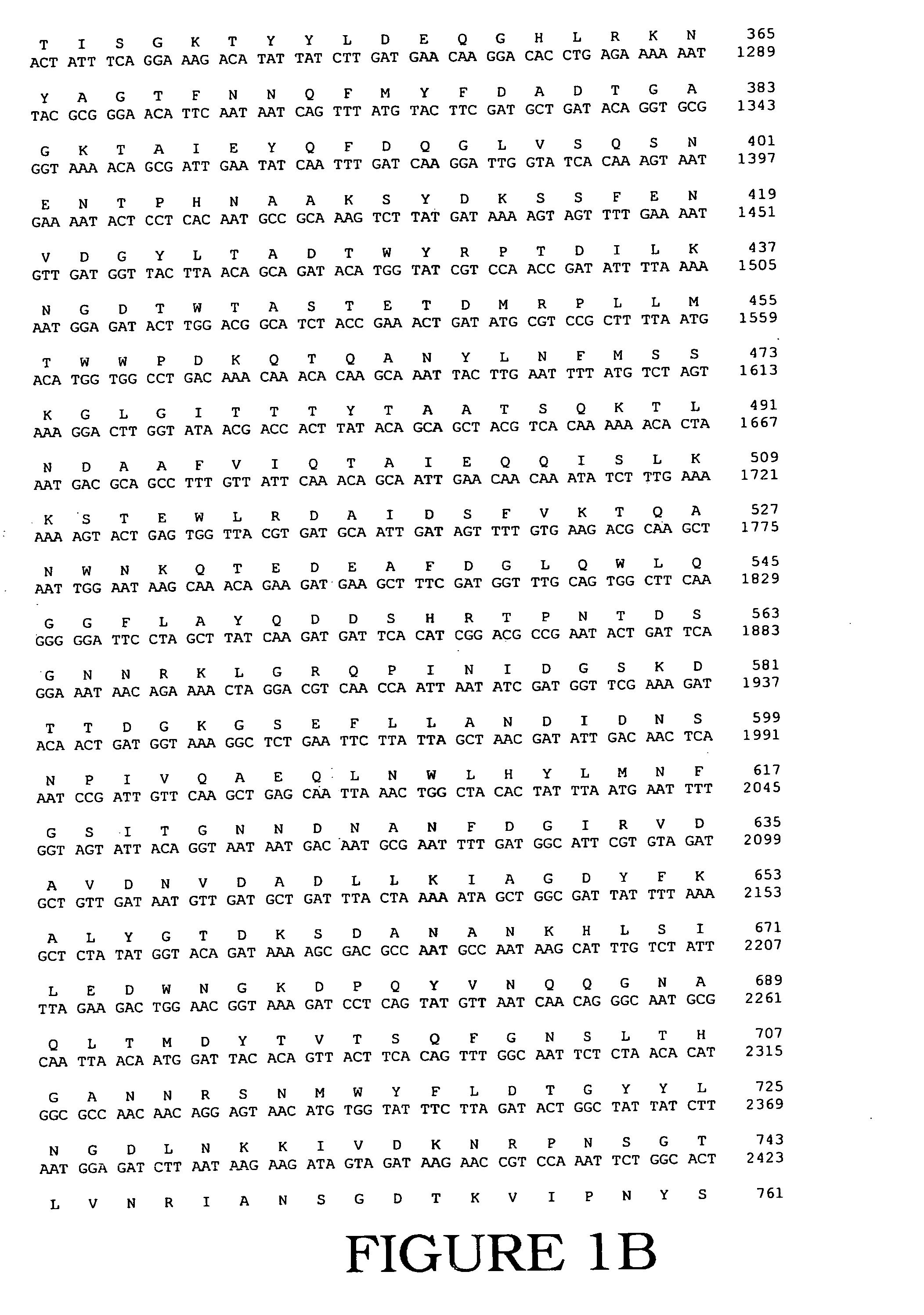
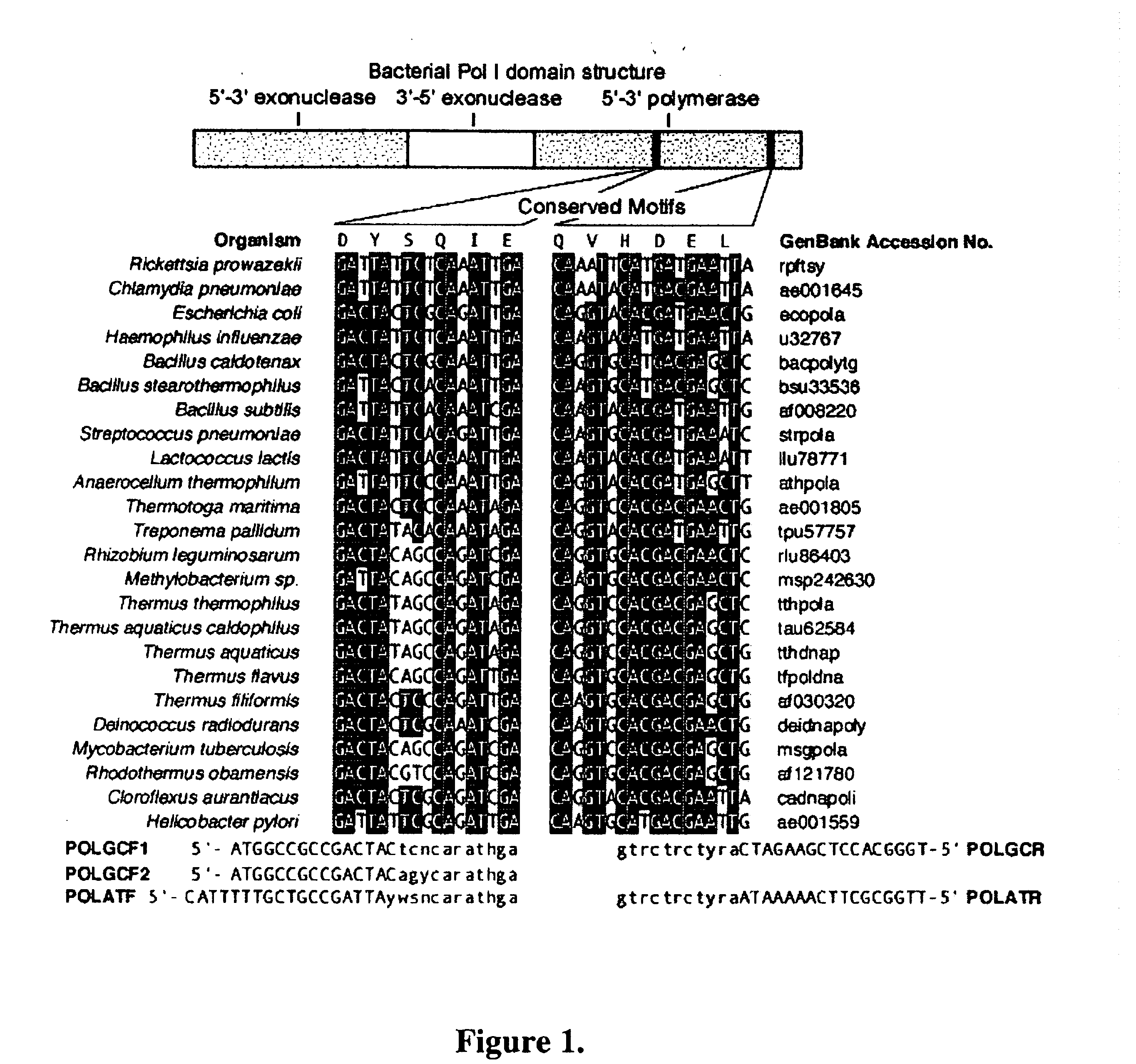
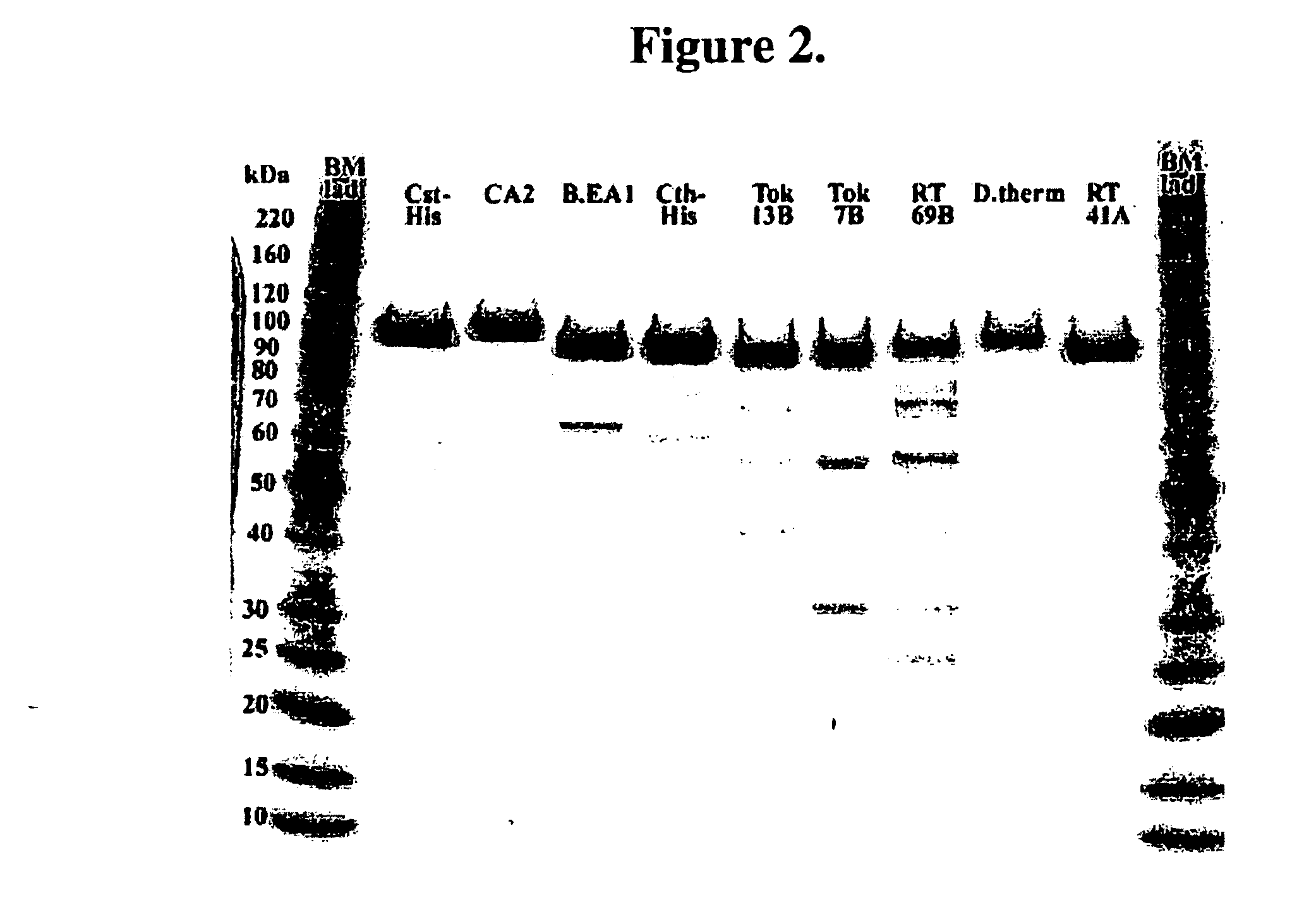
DNA Polymerases and mutants thereof
InactiveUS20070020622A1Enhanced DNA polymerase activityExonuclease activity is relatively decreasedFungiSugar derivativesPolymerase LA-DNA
The present invention provides polypeptides having a nucleotide polymerase activity and method of enhancing polymerase activity. The polypeptides of the present invention may posses both a DNA-dependent DNA polymerase activity and an RNA-dependent DNA polymerase activity, i.e., a reverse transcriptase activity. The polypeptides of the present invention may be used in any application including, but not limited to, DNA sequencing reactions, amplification reactions, cDNA synthesis reactions, and combined cDNA synthesis and amplification reactions, e.g., RT-PCR.
Owner:LIFE TECH CORP
Thraustochytrids, Fatty Acid Compositions, and Methods of Making and Uses Thereof
Owner:DSM IP ASSETS BV
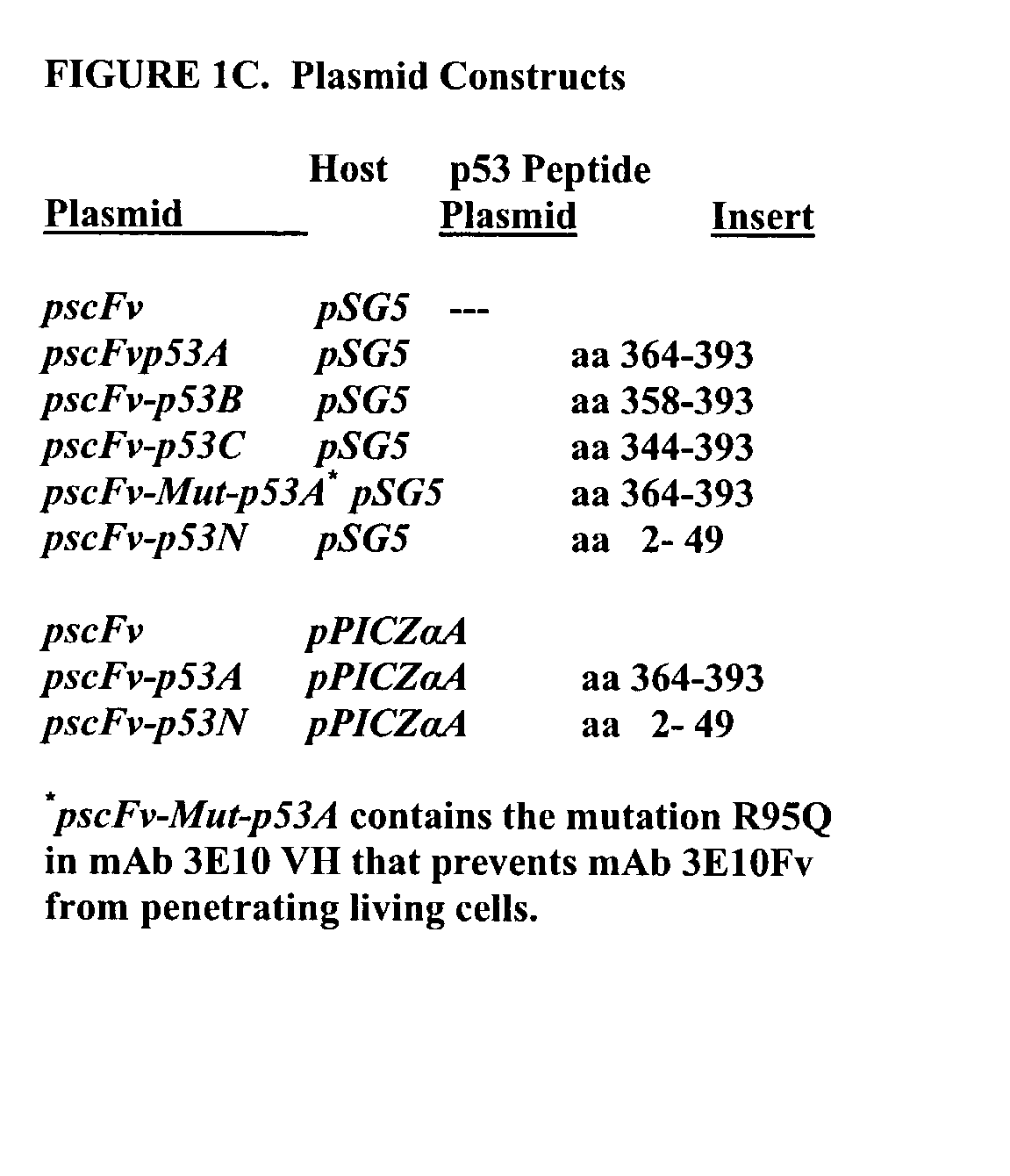
Delivery system using mAb 3E10 and mutants and/or functional fragments thereof
InactiveUS7189396B1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellCytotoxicity
A monoclonal antibody, 3E10, and active fragments thereof that selectively are transported in vivo to the nucleus of mammalian cells without cytotoxic effect are provided. The antibody and other molecules that bind to a variant of myosin IIb heavy chain found in the nucleus of skeletal muscle cells are useful as a non-viral delivery vector to target skeletal muscle in vivo. By contrast, in vitro the monoclonal antibody penetrates and is transported to the nucleus of multiple cell lines derived from different tissue types and can be used in screening tests to identify molecules that modulate growth of cells, such as cancer cells. Non-cytotoxic vectors for delivering a drug, polynucleotide or polypeptide selectively to skeletal muscle cells are also provided.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
DNA polymerases and mutants thereof
InactiveUS20120094332A1High activityImprove thermal stabilityFungiBacteriaPolymerase LReverse transcriptase activity
The present invention provides polypeptides having a nucleotide polymerase activity and method of enhancing polymerase activity. The polypeptides of the present invention may possess both a DNA-dependent DNA polymerase activity and an RNA-dependent DNA polymerase activity, i.e., a reverse transcriptase activity. The polypeptides of the present invention may be used in any application including, but not limited to, DNA sequencing reactions, amplification reactions, cDNA synthesis reactions, and combined cDNA synthesis and amplification reactions, e.g., RT-PCR.
Owner:LIFE TECH CORP
Recombinant mutants of rhabdovirus and methods of use thereof
InactiveUS20050260601A1Decrease in cancer cell viabilityReduce cell viabilityAntibacterial agentsOrganic active ingredientsGlycoproteinMutant
The present invention relates to recombinant Rhabdoviridae, isolated nucleic acids, vectors, cells and compositions comprising same. The recombinant Rhabdoviridae, isolated nucleic acids, vectors, cells and compositions express Rhabdoviral proteins including a mutated matrix protein (M) and / or a mutated glycoprotein (G), in addition to expression of at least one foreign nucleic acid. The present invention also relates to methods of use thereof, including their use in vivo, in anti-cancer applications, such as in the treatment of gliomas. The recombinant Rhabdoviridae of the present invention are also useful in gene therapy and vaccine applications.
Owner:UNIV OF TENNESSEE RES FOUND
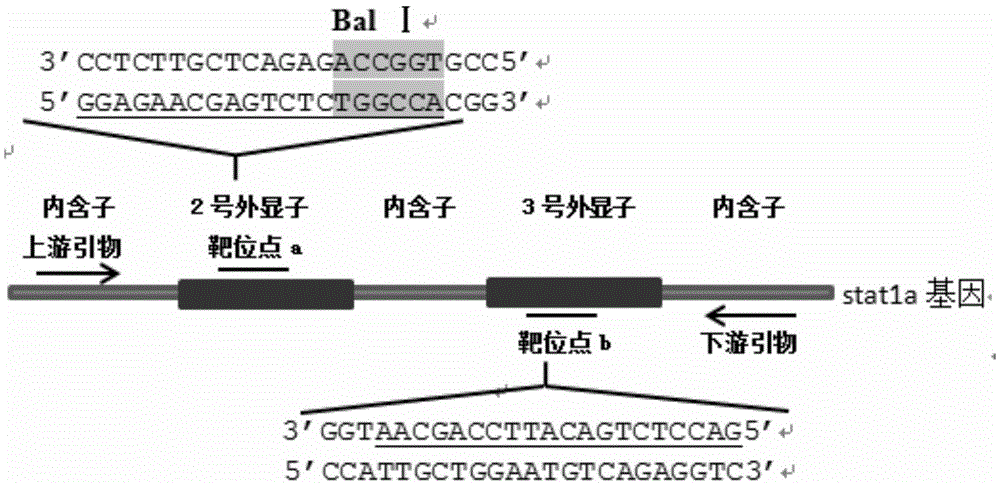
Method for breeding stat1a (signal transducer and activator of transcription 1) gene-deleted zebra fish through gene knockout
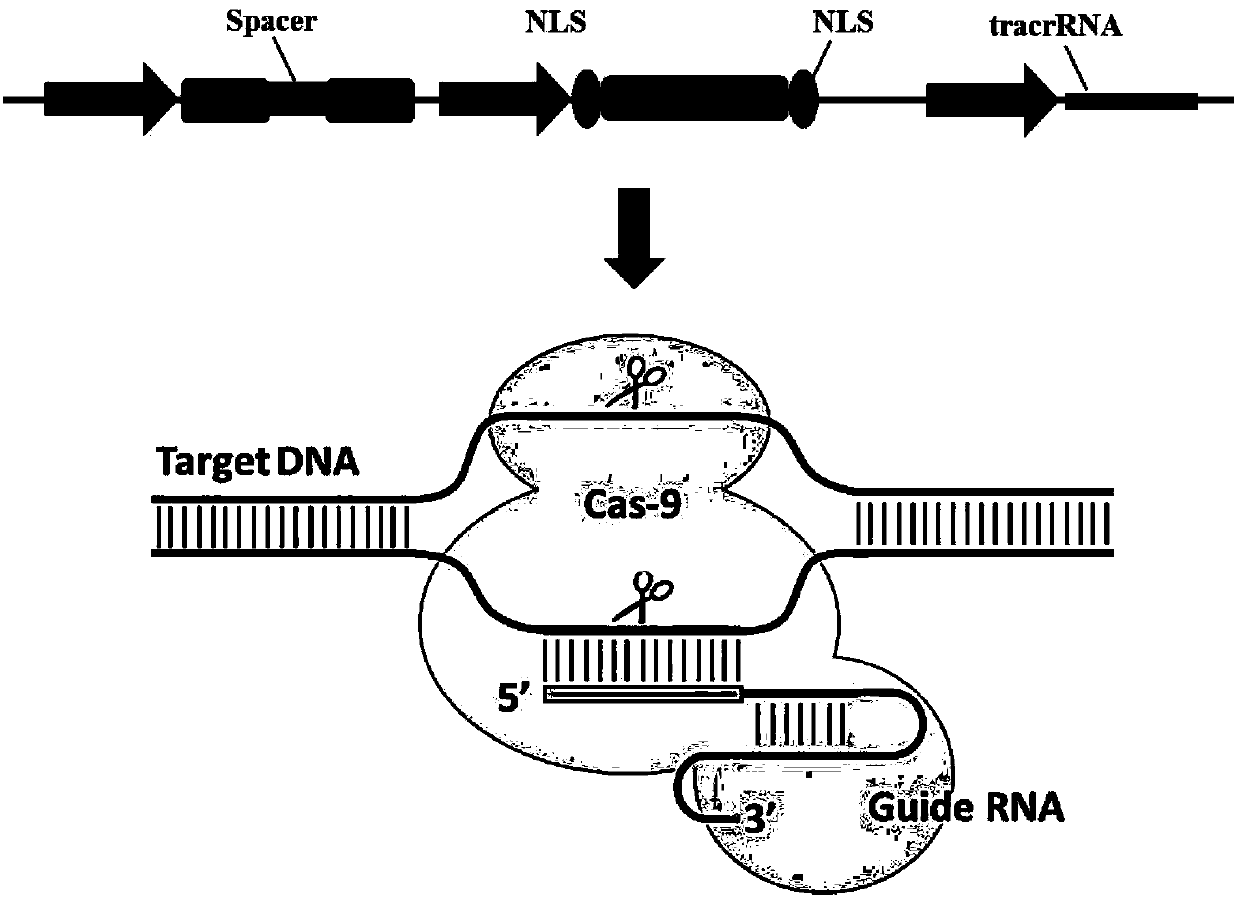
InactiveCN105647969AInefficient shooting techniqueLow costMicrobiological testing/measurementPeptidesFish embryoEmbryo
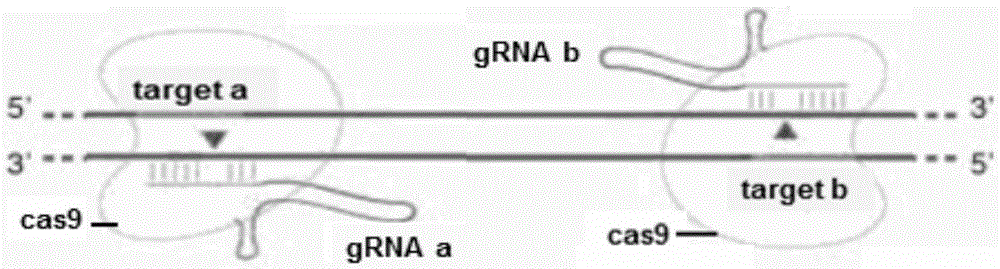
A method for breeding stat1a (signal transducer and activator of transcription 1) gene-deleted zebra fish through gene knockout comprises steps as follows: design of a CRISPR / Cas9 gene knockout target site: a gRNA expression carrier is established and gRNA is synthesized in vitro; micro-injection of a zebra fish embryo; detection of effectiveness of the target site with a T7E1 method through Sanger sequencing; tail cutting identification according to the identification steps after two months of injection; TA cloning of a target sequence; Sanger sequencing of plasmids; obtaining of heritable F1 generation of a zebra fish mutant; obtaining of F2 generation homozygote of the zebra fish mutant, F3 generation pure line inheritance of the gene-deleted zebra fish with the above method, and obtaining of a new zebra fish strain.
Owner:HUNAN NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com