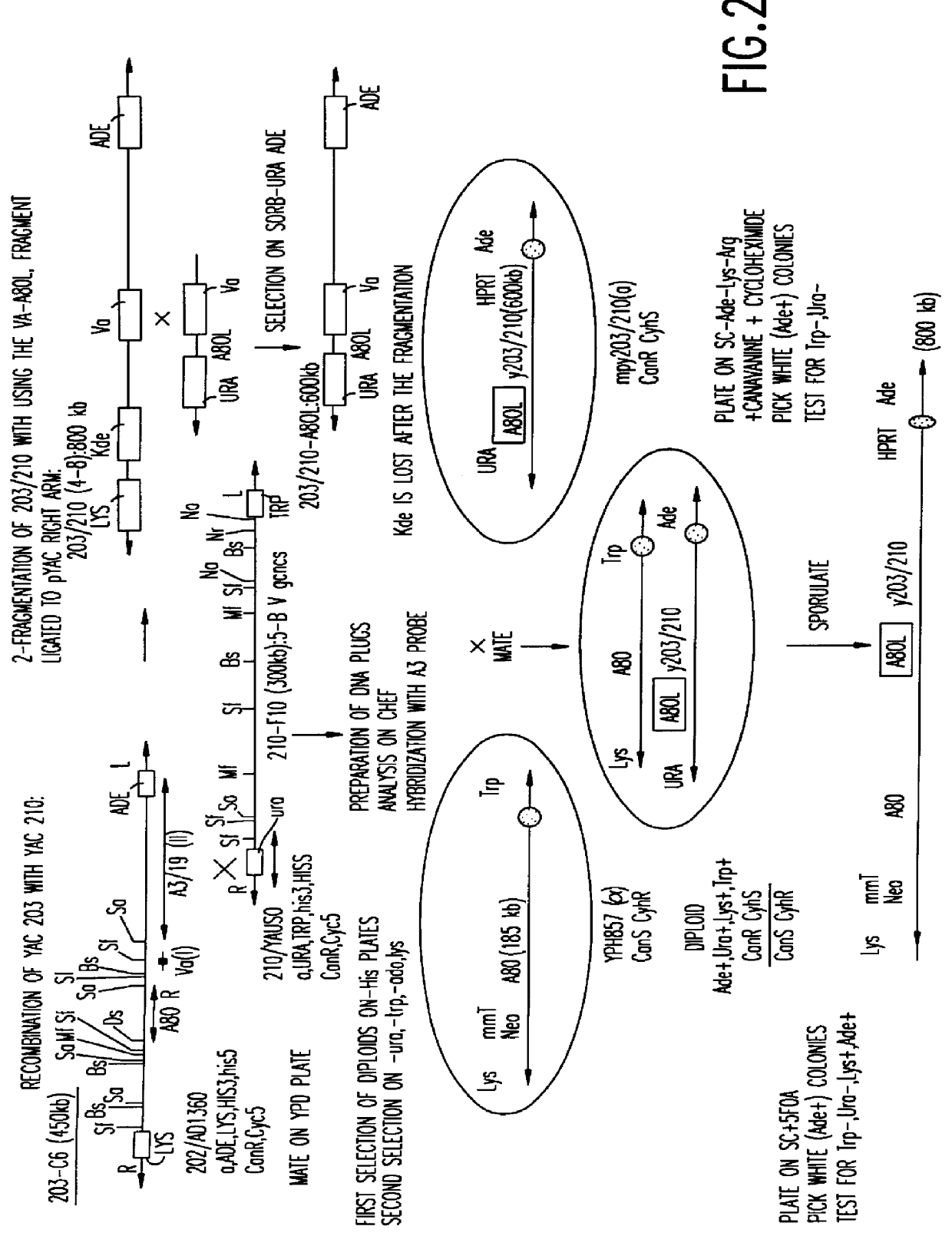
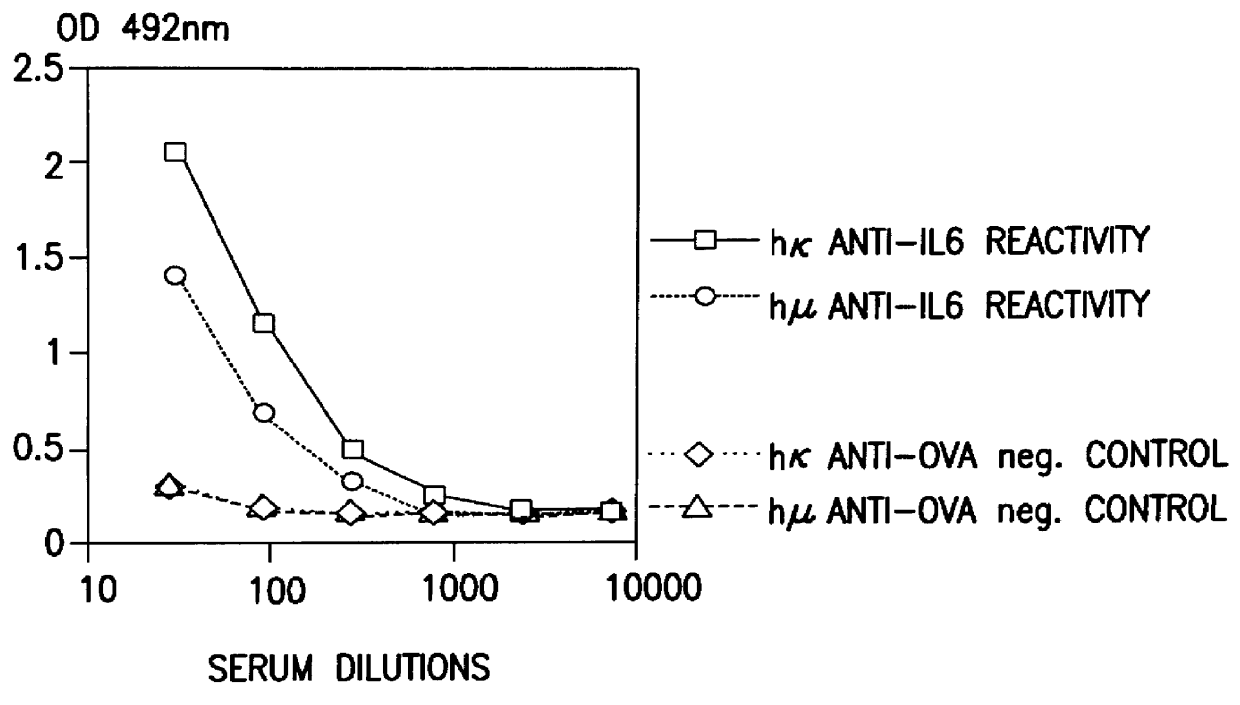
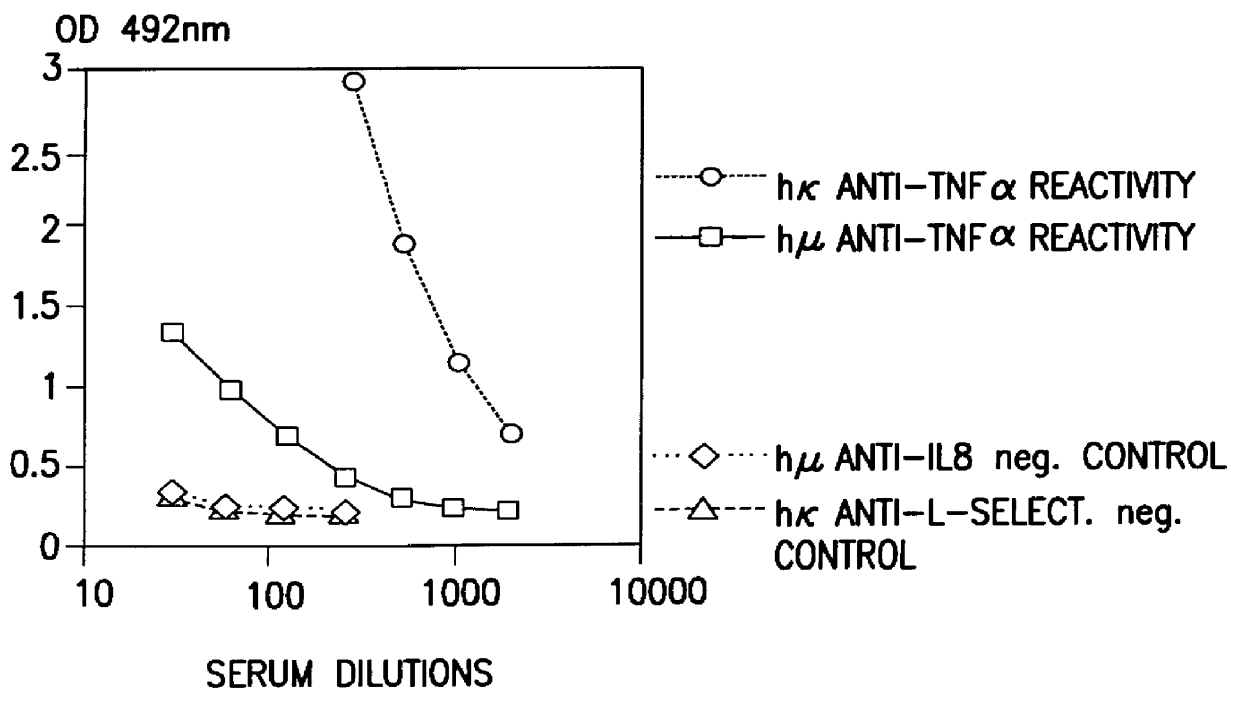
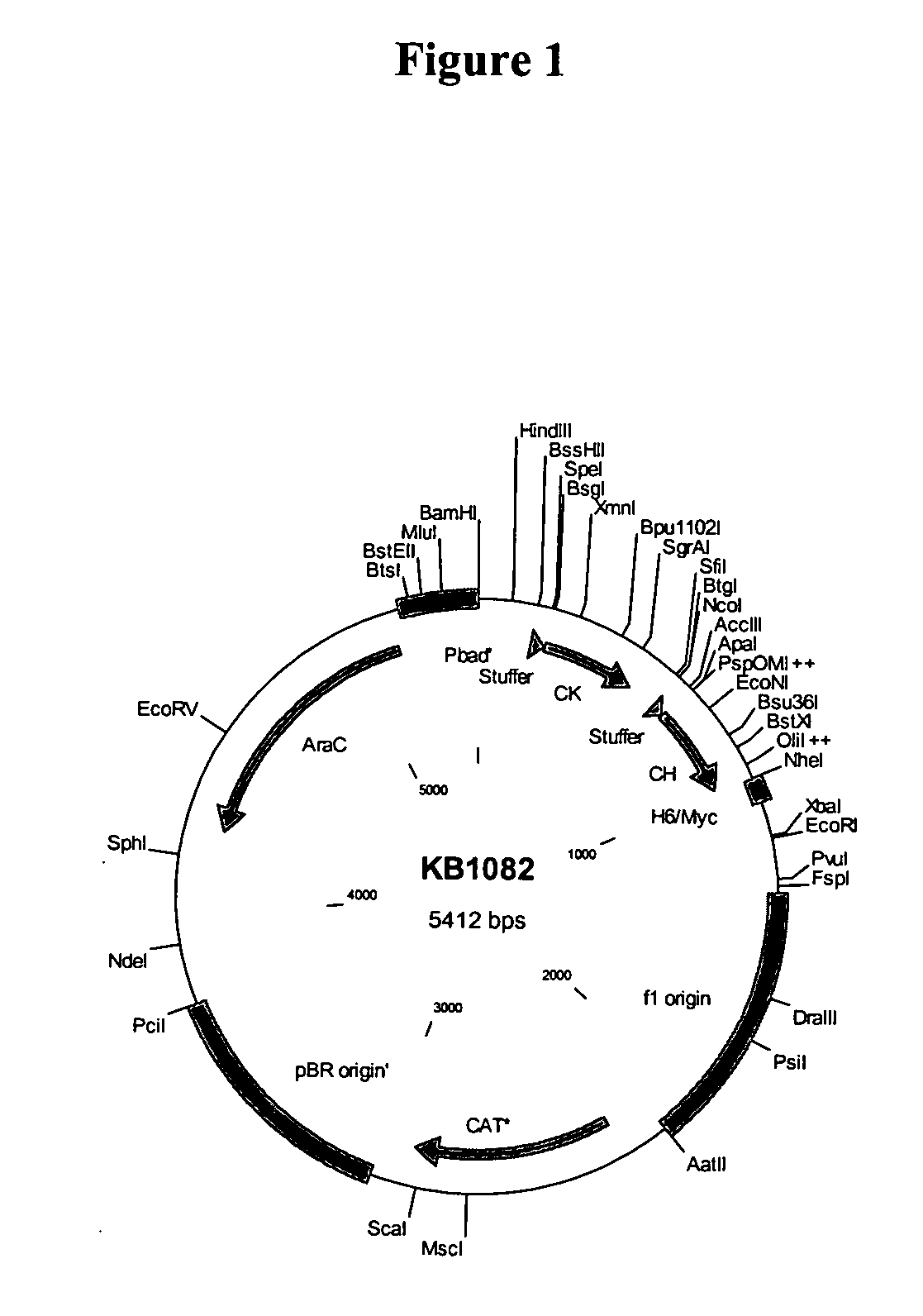
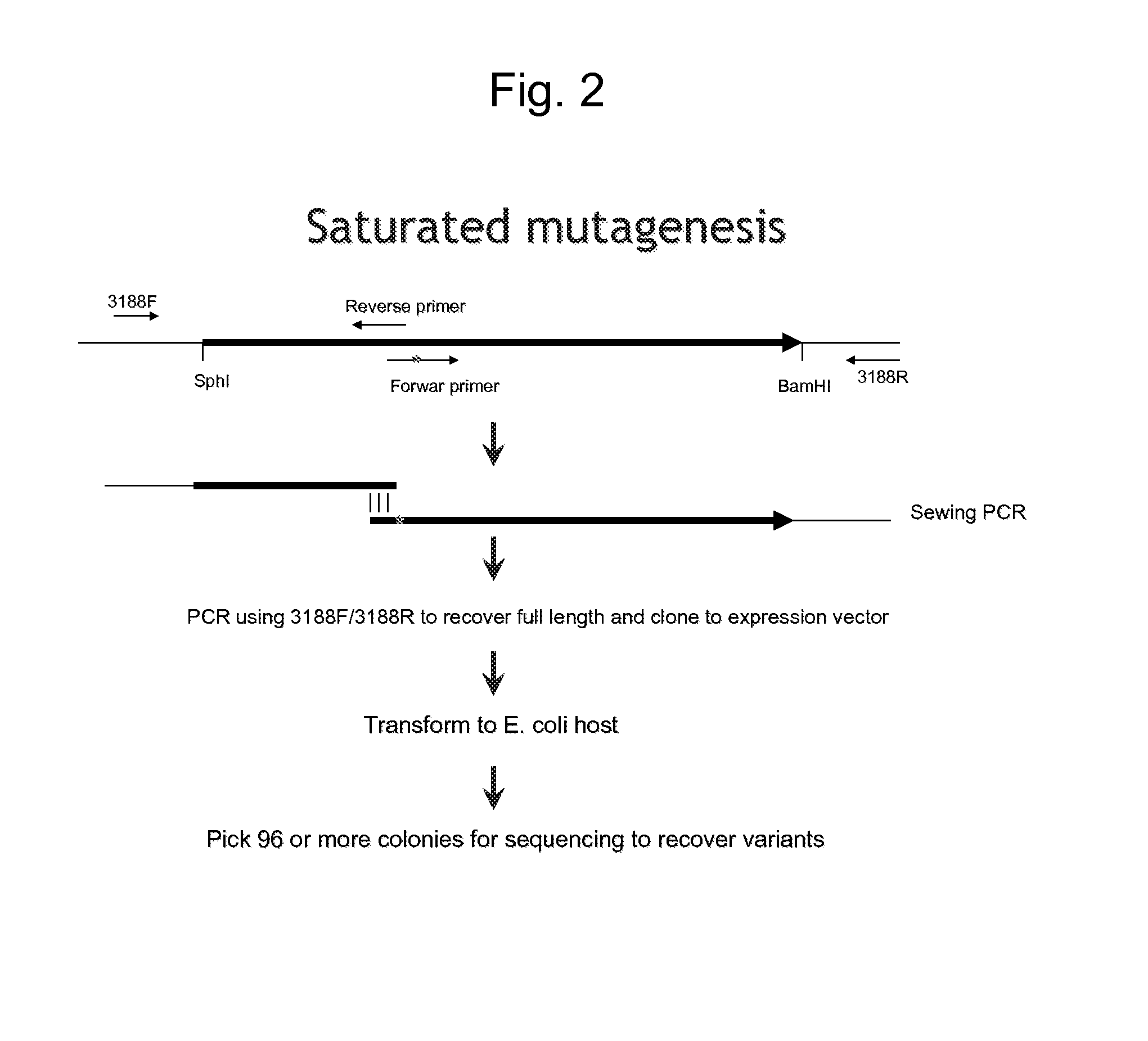
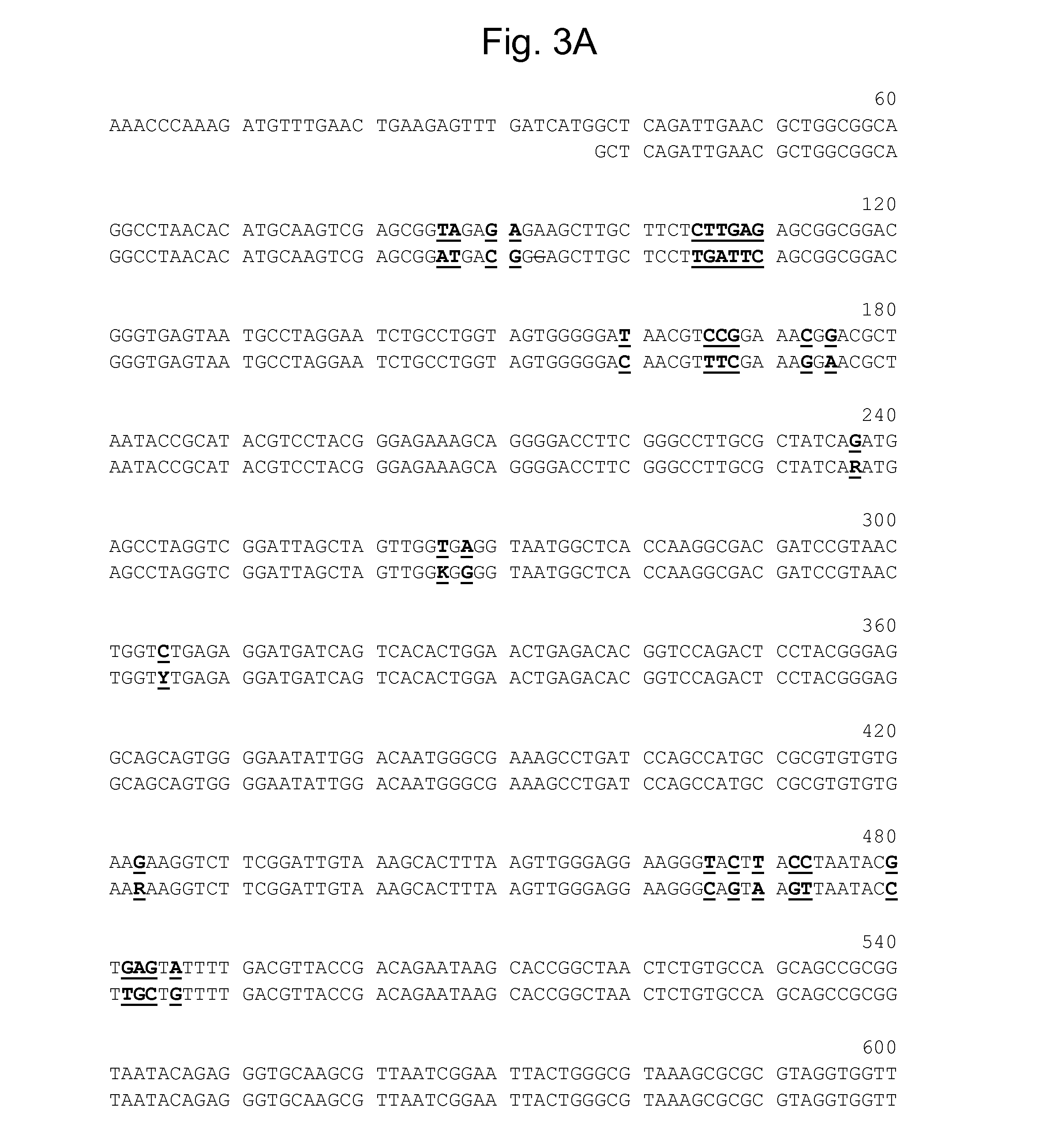
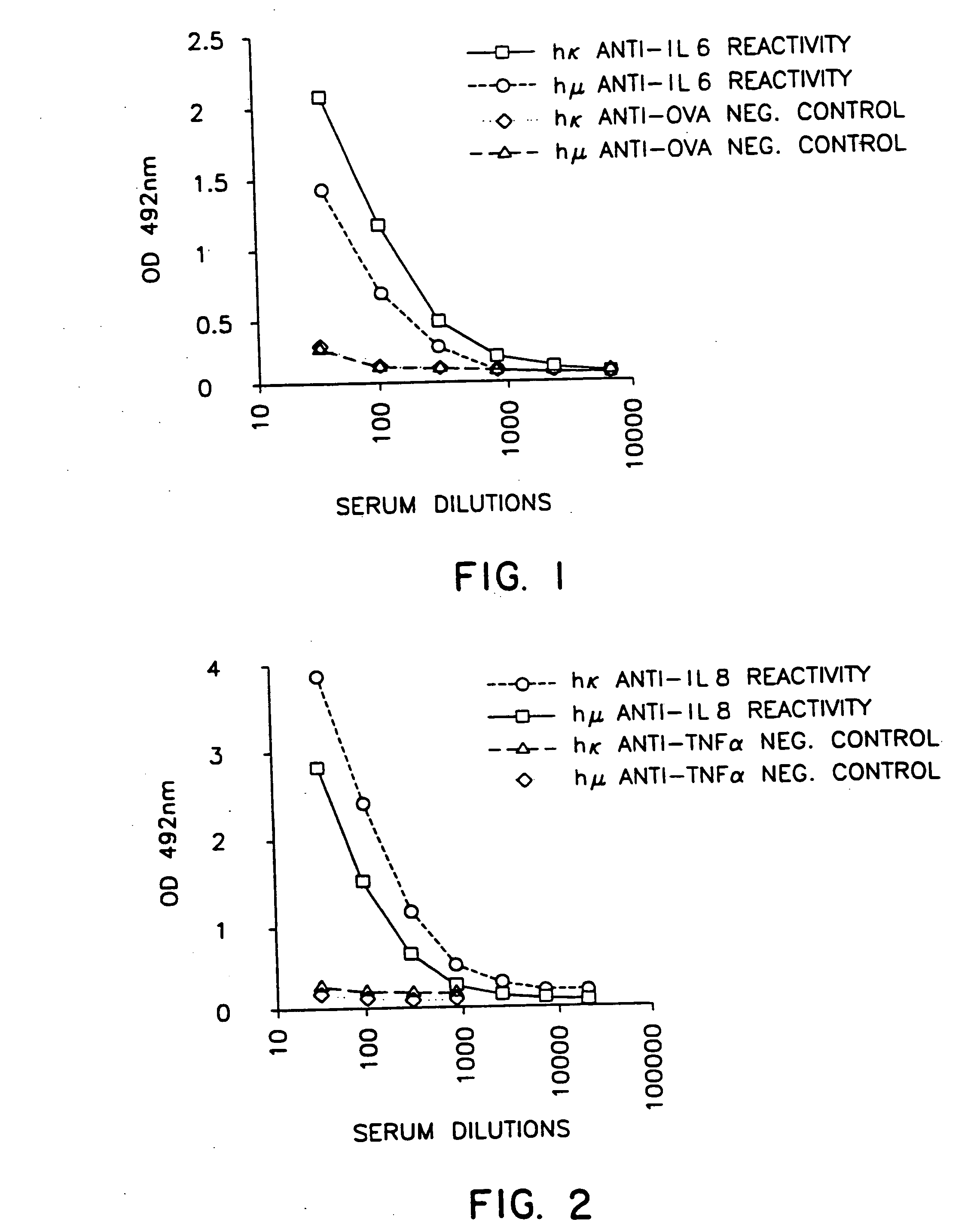
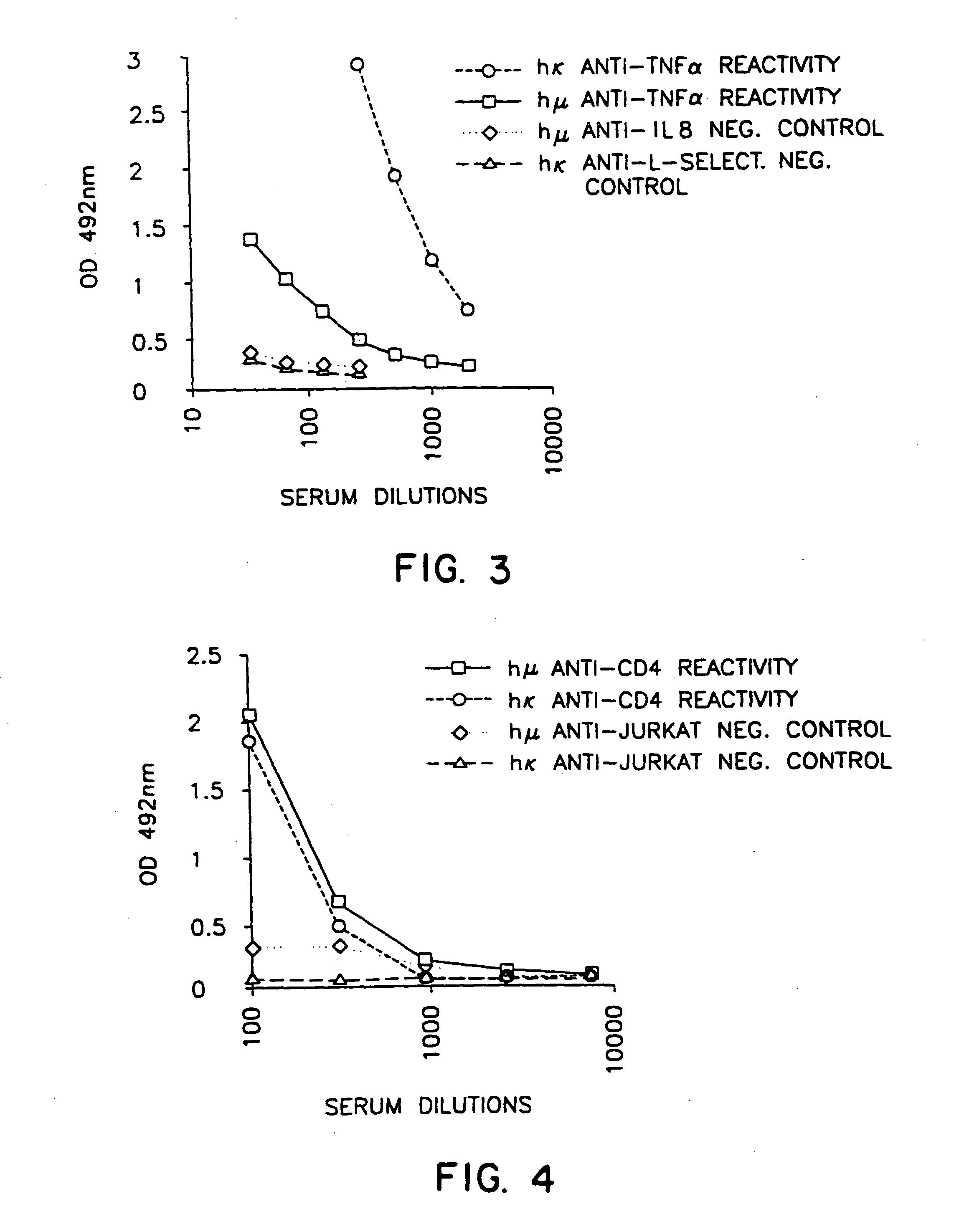
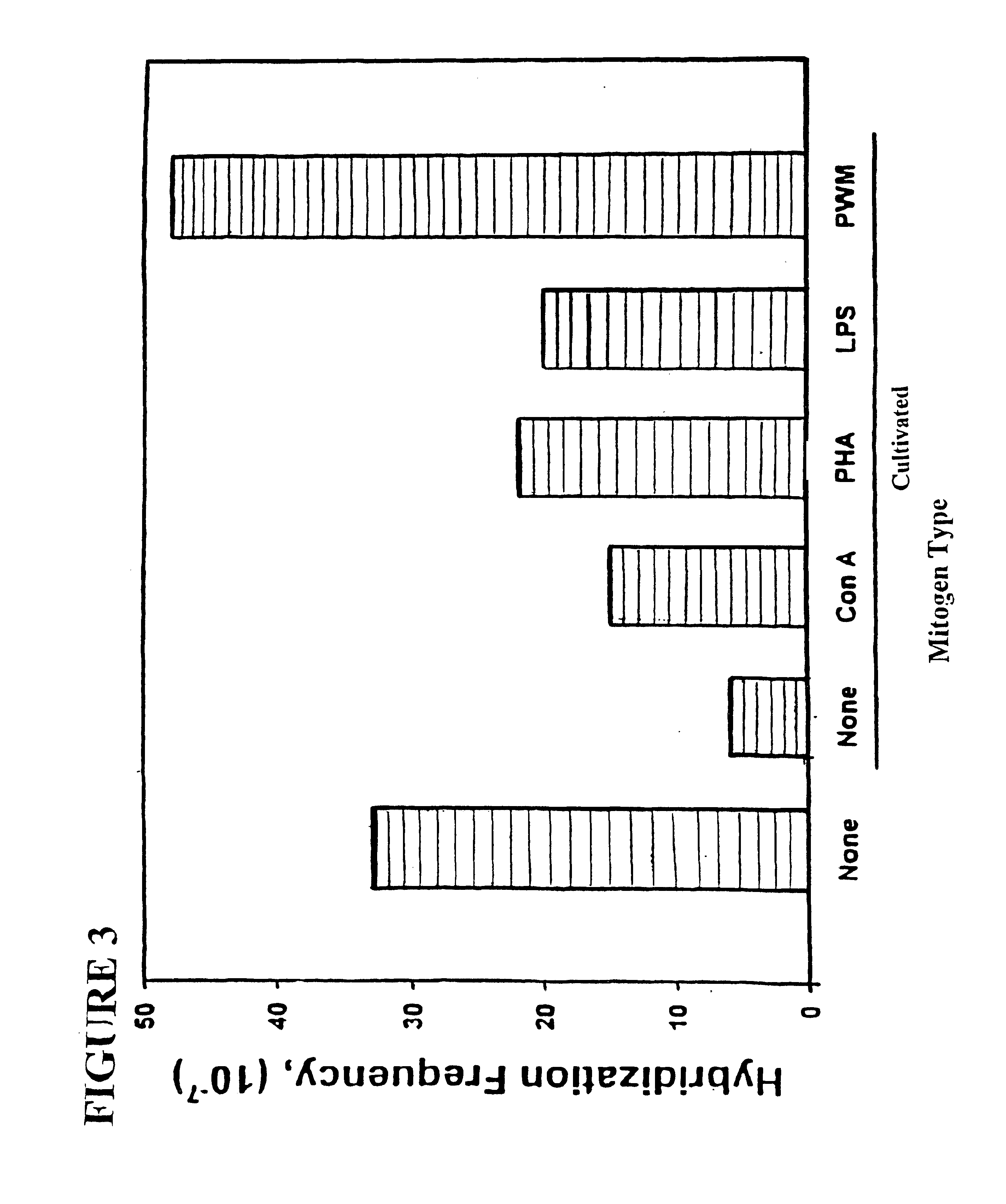
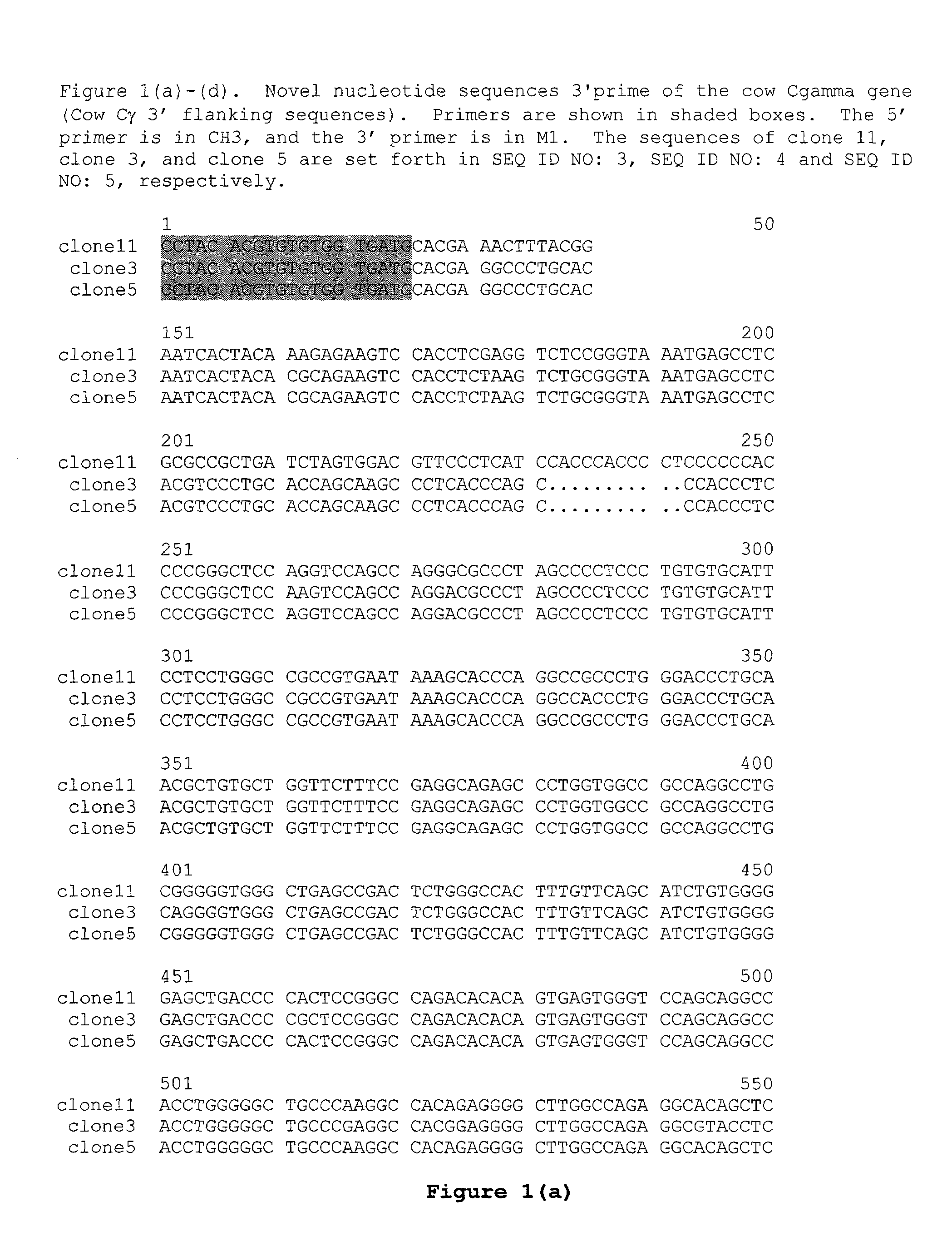
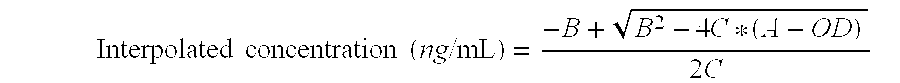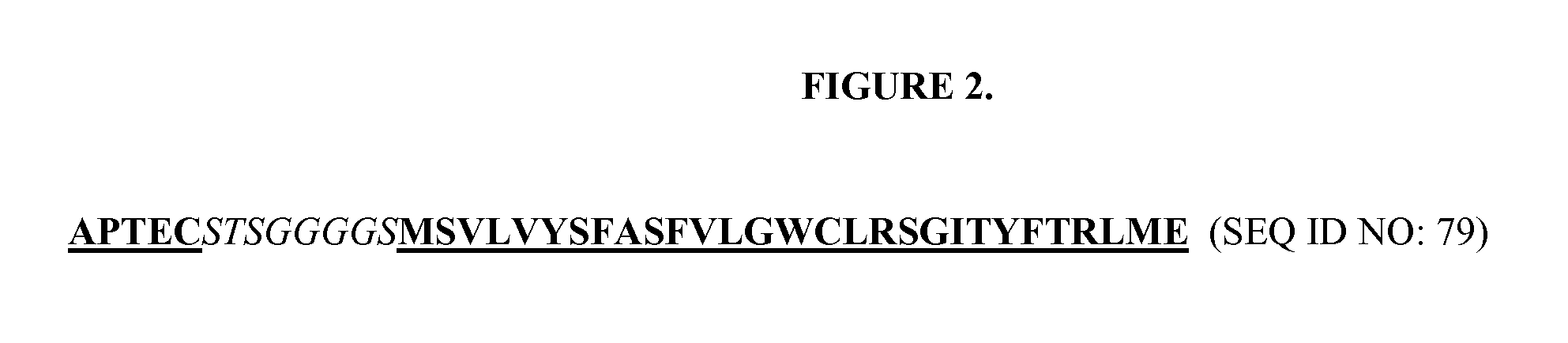Patents
Literature
2417results about "Immunoglobulins against bacteria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human antibodies derived from immunized xenomice
Fully human antibodies against a specific antigen can be prepared by administering the antigen to a transgenic animal which has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled. Various subsequent manipulations can be performed to obtain either antibodies per se or analogs thereof.
Owner:AMGEN FREMONT INC
Toxin genes and methods for their use
Compositions and methods for conferring pesticidal activity to bacteria, plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a delta-endotoxin polypeptide are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants and bacteria. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated delta-endotoxin nucleic acid molecules are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed, and antibodies specifically binding to those amino acid sequences. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:61-121 and 133-141, or the nucleotide sequence set forth in SEQ ID NO:1-60, 124-132, and 142-283, as well as variants and fragments thereof.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
Axmi-031, axmi-039, axmi-040 and axmi-049, a family of novel delta-endotoxin genes and methods for their use
Compositions and methods for conferring pesticidal activity to bacteria, plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a delta-endotoxin polypeptide are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants and bacteria. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated delta-endotoxin nucleic acid molecules are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed, and antibodies specifically binding to those amino acid sequences. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:2, 4, 6, 8, 10, 12, 15, 17, 19, 21, 23, 25, 27, 29, 31, 33, 35, or 38, or the nucleotide sequence set forth in SEQ ID NO:1, 3, 5, 7, 9, 11, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, or 37, as well as variants and fragments thereof.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
Antibody specificity transfer using minimal essential binding determinants
The present invention provides methods of making antibodies having the binding specificity of a reference antibody. Antibodies generated by the methods of the inventions have at least one minimal essential binding specificity determinant from a heavy chain or light chain CDR3 from the reference antibody. The method can be used, e.g., in humanization procedures. The invention also provides libraries and antibodies made in accordance with the methods.
Owner:HUMANIGEN INC
Monoclonal antibodies and detection methods for phosphinothricin-N-acetyl-transferase enzyme
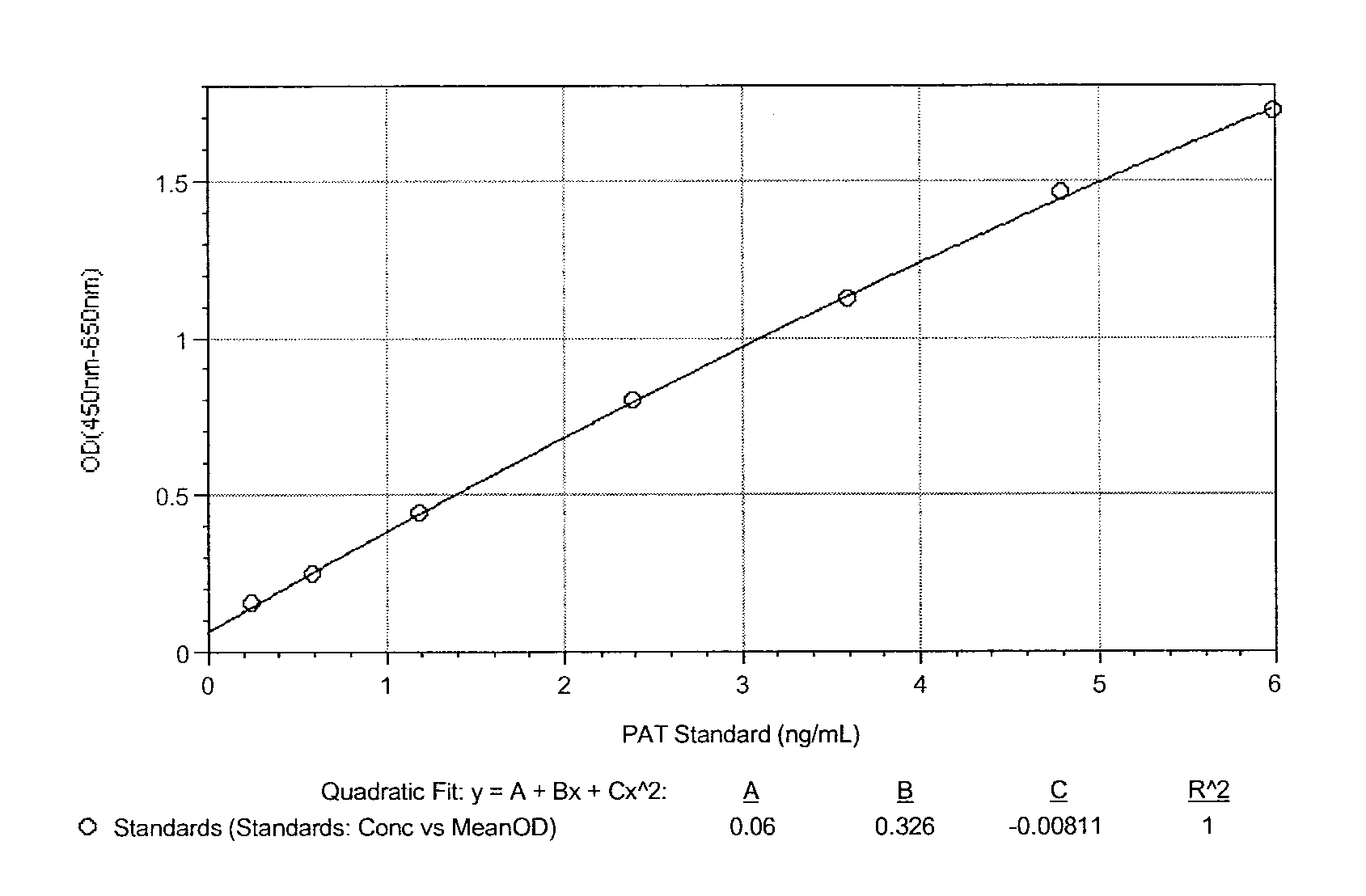
ActiveUS9371394B2Immunoglobulins against bacteriaBiological material analysisN-acetyltransferaseAcetyltransferase
Described herein are monoclonal antibodies and methods useful for determining and quantitating the presence of a phosphinothricin-N-acetyl-transferase enzyme. The claimed antibodies and methods are particularly useful for identifying and quantitating the presence of phosphinothricin-N-acetyl-transferase expressed in trangenic plants.
Owner:CORTEVA AGRISCIENCE LLC
CRY1C polypeptides having improved toxicity to lepidopteran insects
Disclosed are novel synthetically-modified B. thuringiensis nucleic acid segments encoding delta -endotoxins having insecticidal activity against lepidopteran insects. Also disclosed are synthetic crystal proteins encoded by these novel nucleic acid sequences. Methods of making and using these genes and proteins are disclosed as well as methods for the recombinant expression, and transformation of suitable host cells. Transformed host cells and transgenic plants expressing the modified endotoxin are also aspects of the invention. Also disclosed are methods for modifying, altering, and mutagenizing specific loop regions between the alpha helices in domain 1 of these crystal proteins, including Cry1C, to produce genetically-engineered recombinant cry* genes, and the proteins they encode which have improved insecticidal activity. In preferred embodiments, novel Cry1C* amino acid segments and the modified cry1C* nucleic acid sequences which encode them are disclosed.
Owner:MONSANTO CO (MONSANTO CY)
Broad-spectrum insect resistant transgenic plants
InactiveUS6281016B1Improve insecticidal effectBroad-range specificityBiocideNanotechAureobasidium sp.Toxin
Disclosed are novel synthetically-modified B. thuringiensis chimeric crystal proteins having improved insecticidal activity against coleopteran, dipteran and lepidopteran insects. Also disclosed are the nucleic acid segments encoding these novel peptides. Methods of making and using these genes and proteins are disclosed as well as methods for the recombinant expression, and transformation of suitable host cells. Transformed host cells and tansgenic plants expressing the modified endotoxin are also aspects of the invention.
Owner:MONSANTO CO (MONSANTO CY)
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Novel Insecticidal Proteins and Methods for Their Use
Compositions and methods for controlling pests are provided. The methods involve transforming organisms with a nucleic acid sequence encoding an insecticidal protein. In particular, the nucleic acid sequences are useful for preparing plants and microorganisms that possess insecticidal activity. Thus, transformed bacteria, plants, plant cells, plant tissues and seeds are provided. Compositions are insecticidal nucleic acids and proteins of bacterial species. The sequences find use in the construction of expression vectors for subsequent transformation into organisms of interest, as probes for the isolation of other homologous (or partially homologous) genes. The insecticidal proteins find use in controlling, inhibiting growth or killing lepidopteran, coleopteran, dipteran, fungal, hemipteran, and nematode pest populations and for producing compositions with insecticidal activity.
Owner:PIONEER HI BRED INT INC
Human antibodies derived from immunized xenomice
Antibodies with fully human variable regions against a specific antigen can be prepared by administering the antigen to a transgenic animal which has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled. Various subsequent manipulations can be performed to obtain either antibodies per se or analogs thereof.
Owner:KUCHERLAPATI RAJU +4
Collagen binding protein compositions and methods of use
InactiveUS6288214B1Prevent and lessen adhesionReduce adhesionAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:TEXAS A&M UNIVERSITY
Human monoclonal antibodies against bacillus anthracis protective antigen
ActiveUS20050287149A1Neutralizing activityLess immunogenicAntibacterial agentsBacteriaV(D)J recombinationBispecific antibody
Isolated human monoclonal antibodies which bind to Anthrax protective antigen are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:MEDAREX LLC
Tumor-associated marker
This invention provides monoclonal antibody-producing hybridomas designated 27.F7 and 27.B1. The invention also provides methods for detecting TIP-2 antigen-bearing cancer cells in a sample, detecting the presence of TIP-2 antigen, optionally on the surface of cancer cells, immunohistochemical screening of a tissue section for the presence of TIP-2 antigen bearing cancer cells, diagnosing cancer in a subject, monitoring progression of cancer wherein the cancer cells are TIP-2 antigen-bearing cells, delivering exogenous material to TIP-2 antigen-bearing cancer cells of a human subject, and treating cancer in a human subject. This invention further provides a kit for detecting the presence of TIP-2 antigen-bearing cancer cells. This invention also provides isolated peptides having the amino acid sequences Lys Leu Leu Gly Gly Gln Ile Gly Leu (SEQ ID No:3) and Ser Leu Leu Gly Cys Arg His Tyr Glu Val (SEQ ID NO:4).
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Production of humanized antibodies in transgenic animals
InactiveUS7129084B2Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Therapeutic using a bispecific antibody
Multivalent, multispecific molecules having at least one specificity for a pathogen and at least one specificity for the HLA class II invariant chain (Ii) are administered to induce clearance of the pathogen. In addition to pathogens, clearance of therapeutic or diagnostic agents, autoantibodies, anti-graft antibodies, and other undesirable compounds may be induced using the multivalent, multispecific molecules.
Owner:IMMUNOMEDICS INC
Method for linking sequences of interest
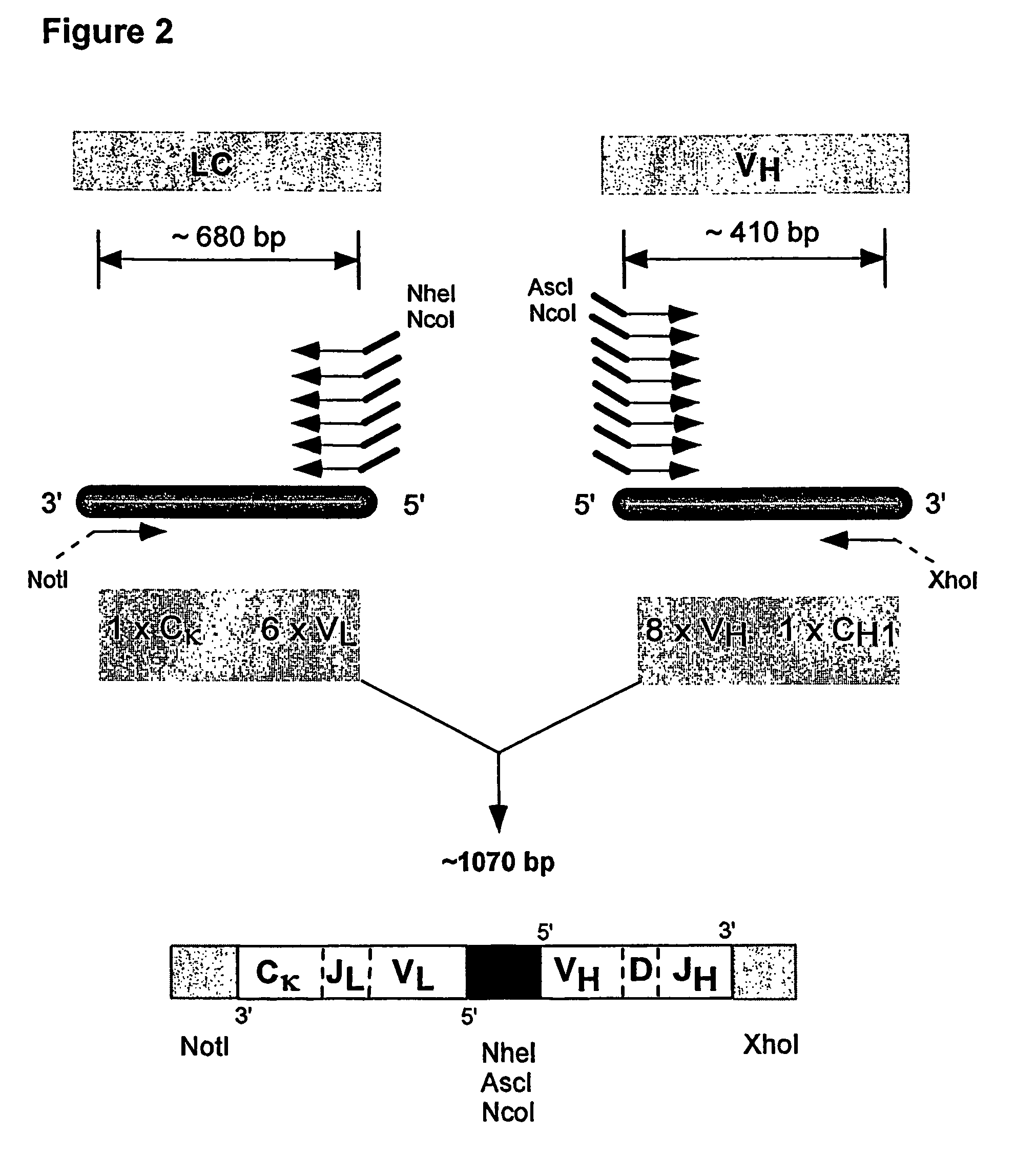
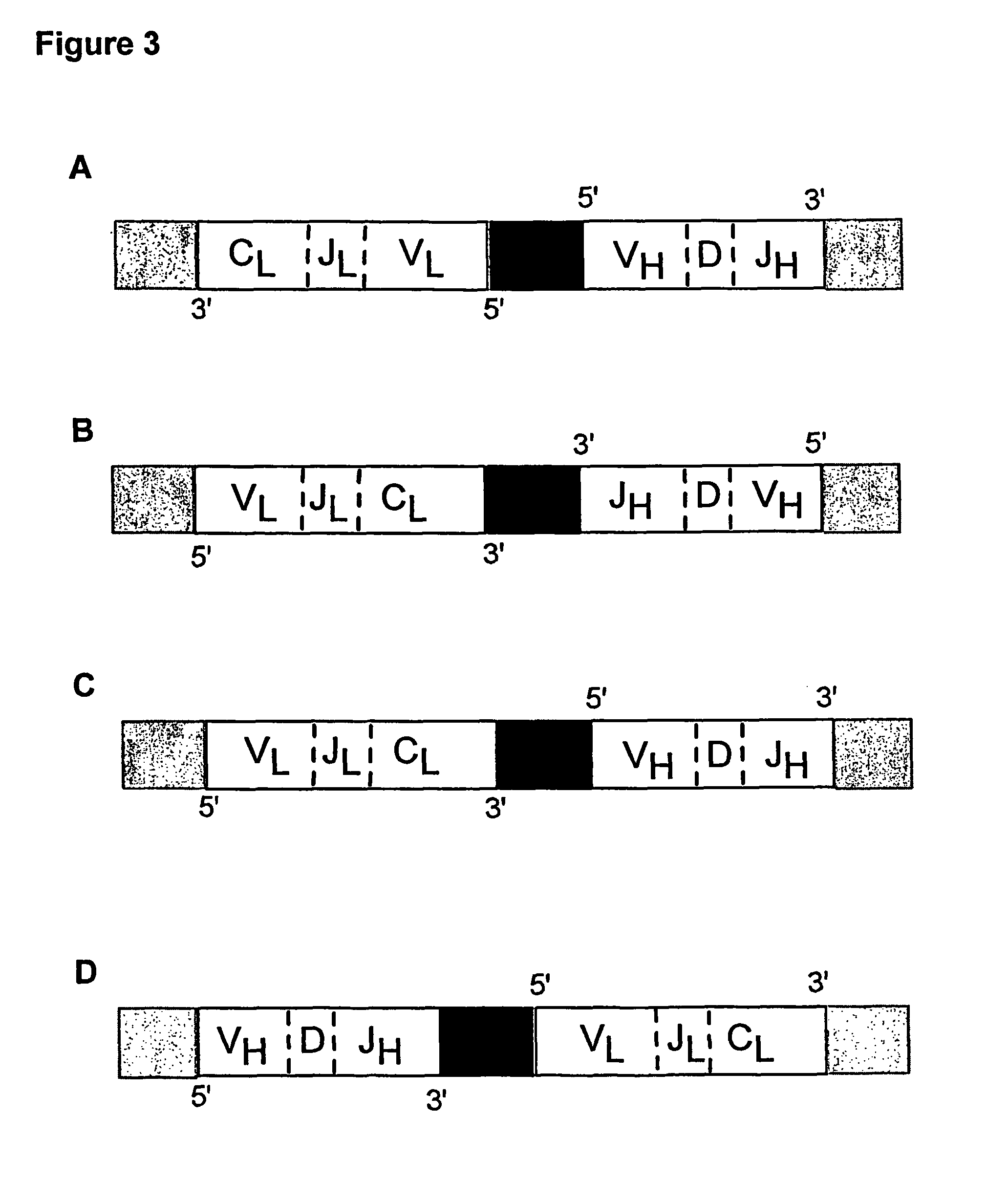
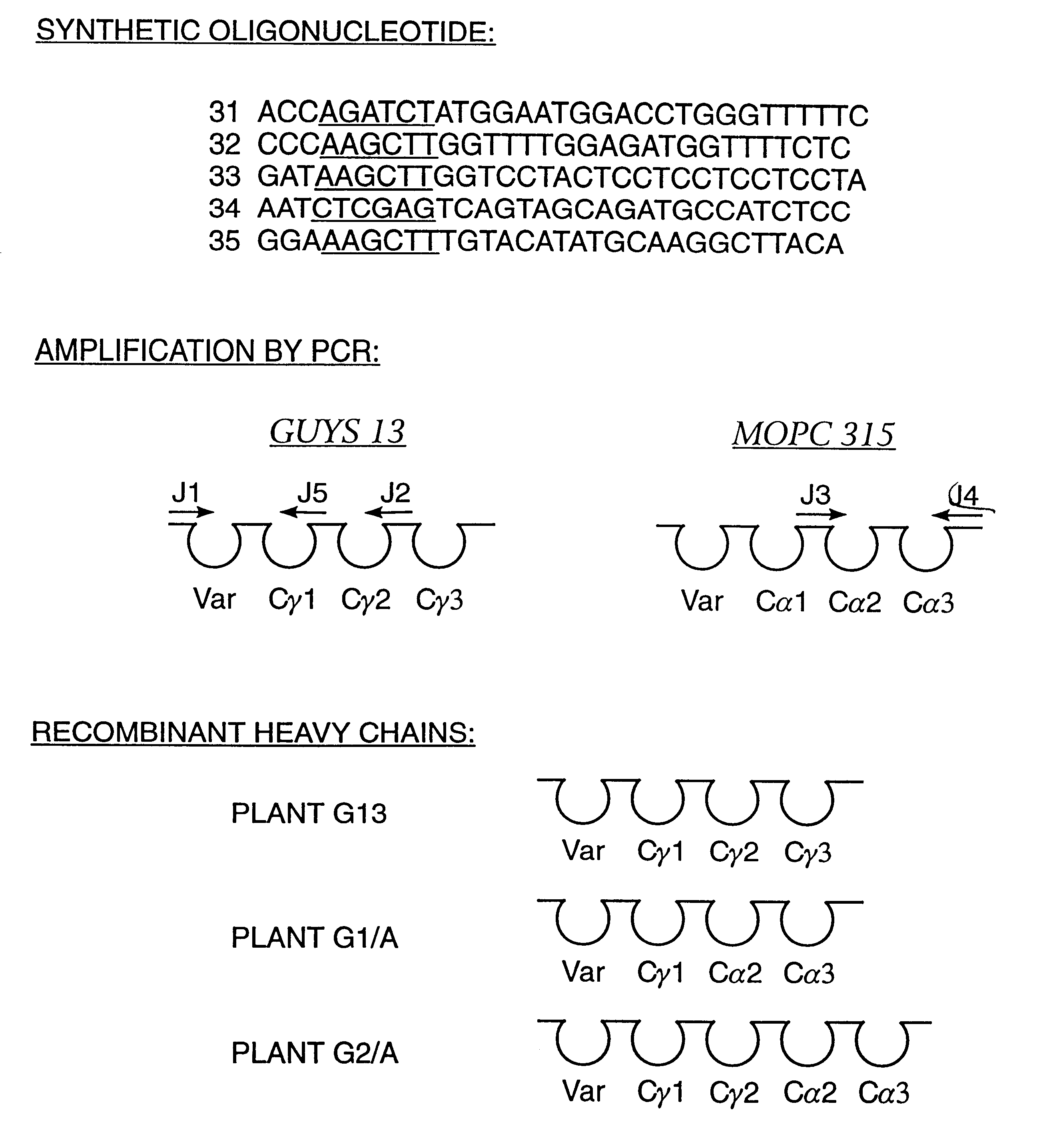
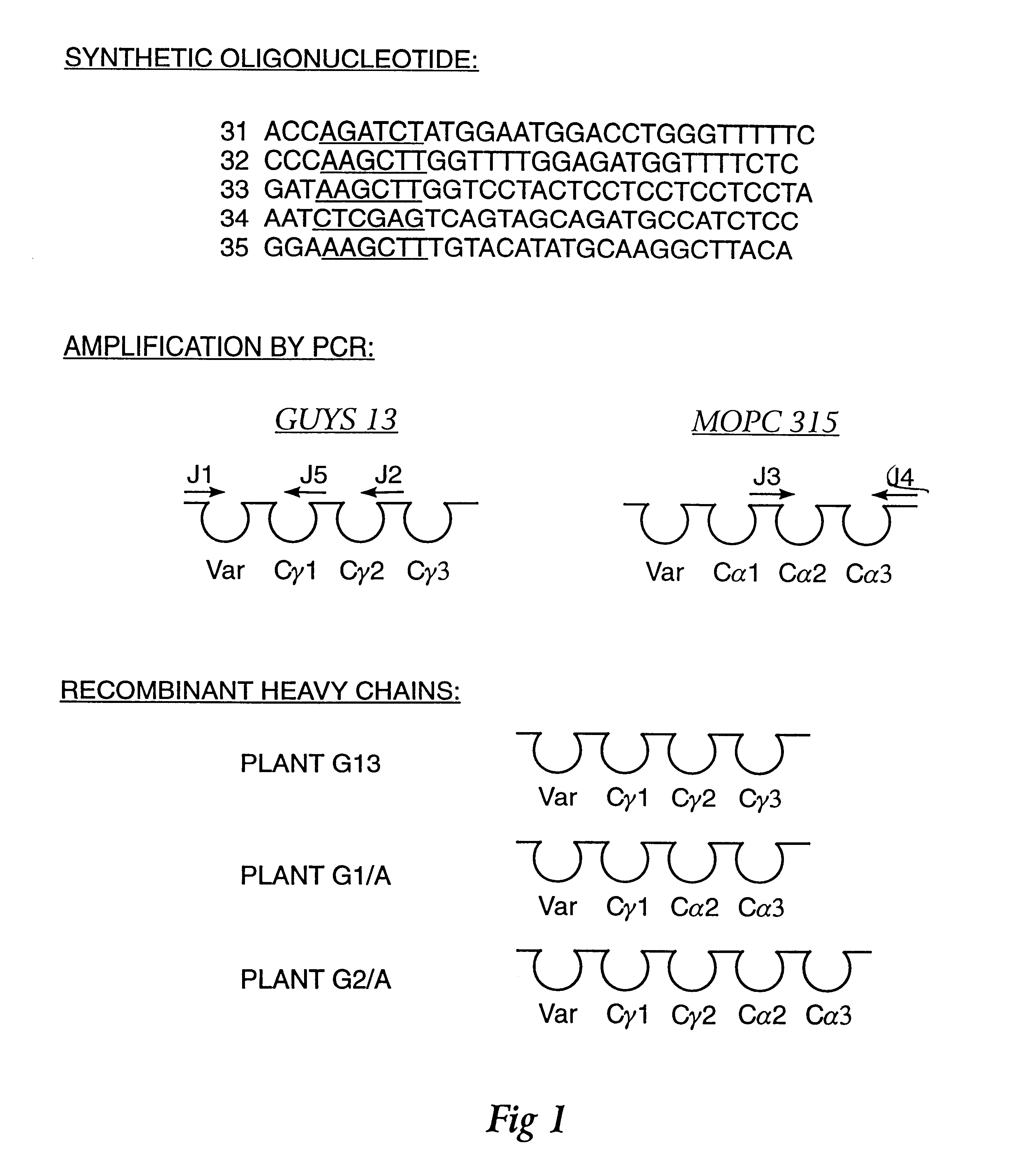
ActiveUS7749697B2Efficient methodAntibacterial agentsSugar derivativesNucleotideNucleotide sequencing
Multiplex overlap-extension RT-PCR provides an efficient method of linking two or more nucleotide sequences encoding for domains or subunits of a heteromeric protein, in a single reaction. Especially, the linkage of variable region encoding sequences from e.g. immunoglobulins, T cell receptors or B cell receptors is eased with the method of the present invention. This allows for a more efficient way of generating libraries of variable region encoding sequences. The capability to perform the multiplex overlap-extension RT-PCR using template derived from an isolated single cell enables the generation of cognate pair libraries in a high-throughput format.
Owner:LES LAB SERVIER
P. aeruginosa mucoid exopolysaccharide specific binding peptides
InactiveUS6962813B2Enhance opsonization and phagocytosisEnhance cytocidal effectAnimal cellsImmunoglobulins against bacteriaDiseaseMonoclonal antibody
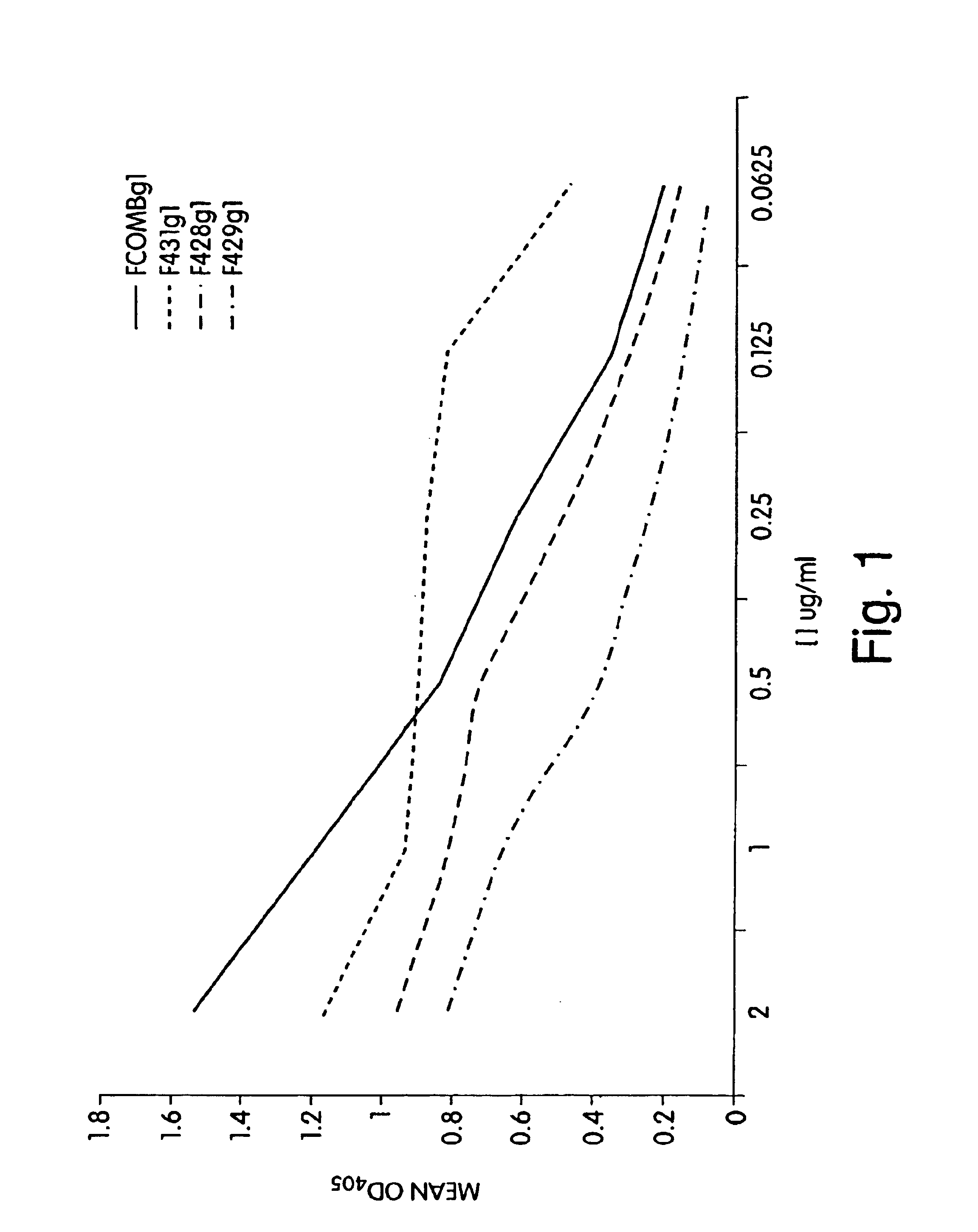
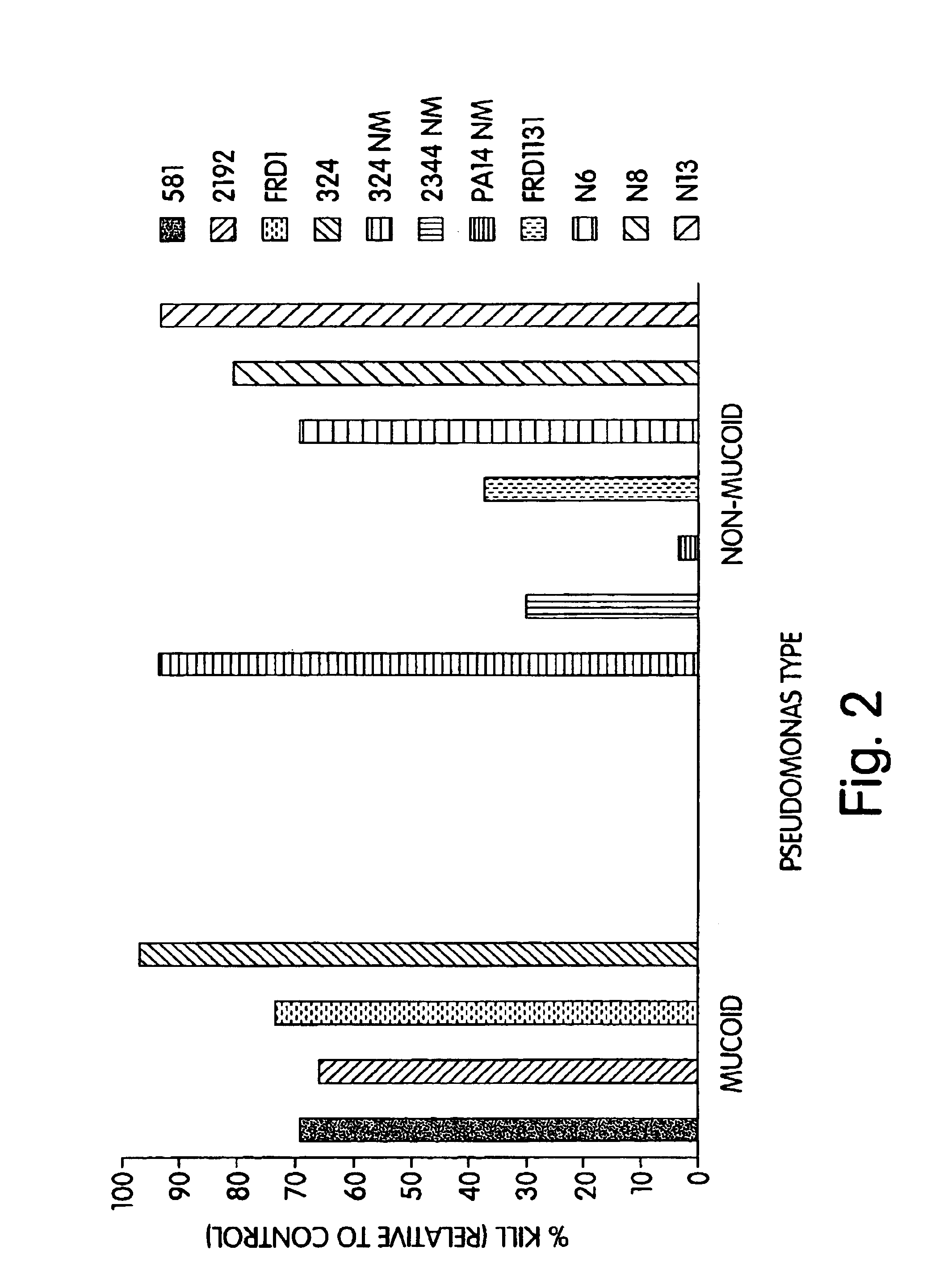
The present invention relates to peptides, particularly human monoclonal antibodies, that bind specifically to P. aeruginosa mucoid exopolysaccharide. The invention further provides methods for using these peptides in the diagnosis, prophylaxis and therapy of P. aeruginosa infection and related disorders (e.g., cystic fibrosis). Some antibodies of the invention enhance opsonophagocytic killing of multiple mucoid strains of P. aeruginosa. Compositions of these peptides, including pharmaceutical compositions, are also provided, as are functionally equivalent variants of such peptides.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
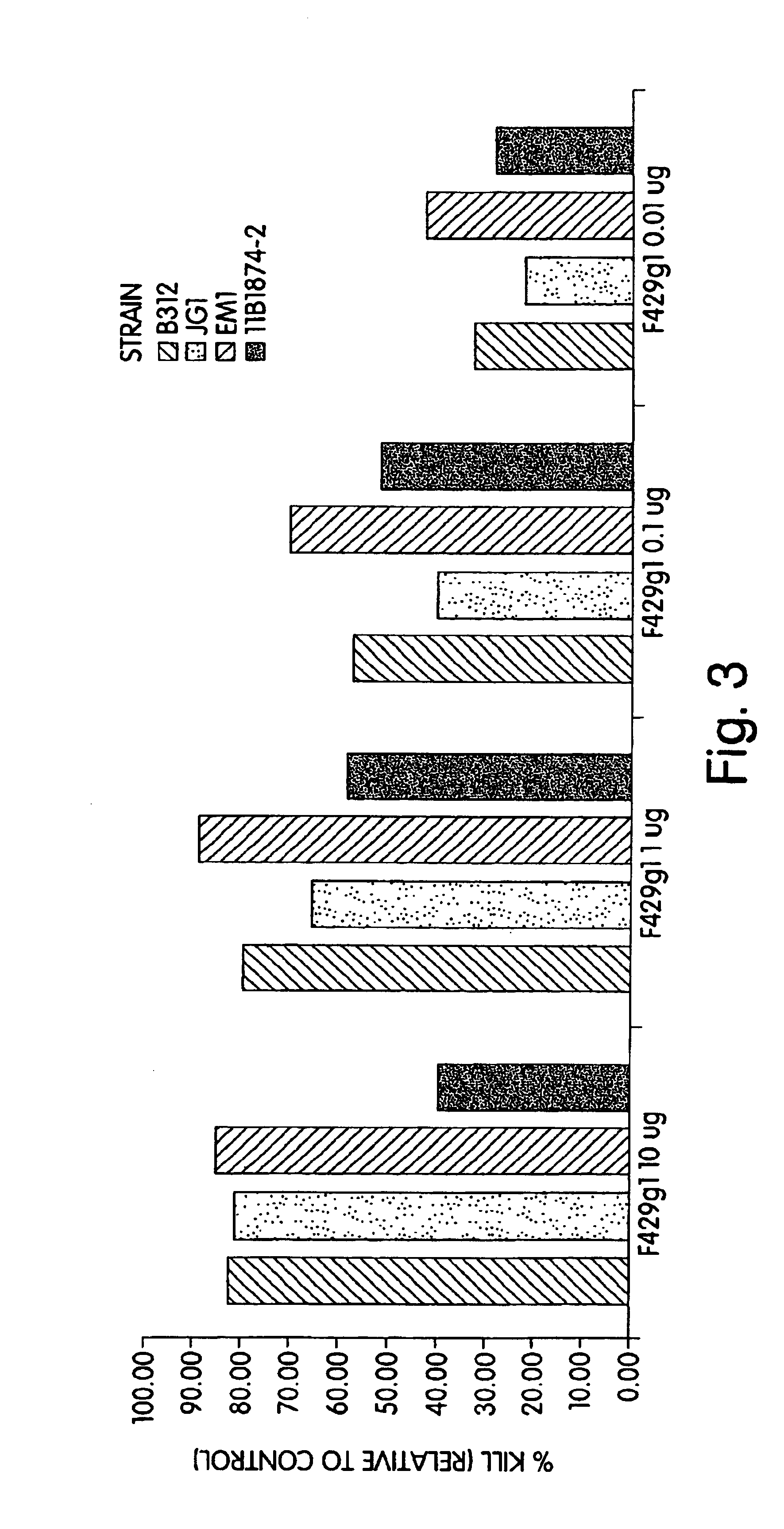
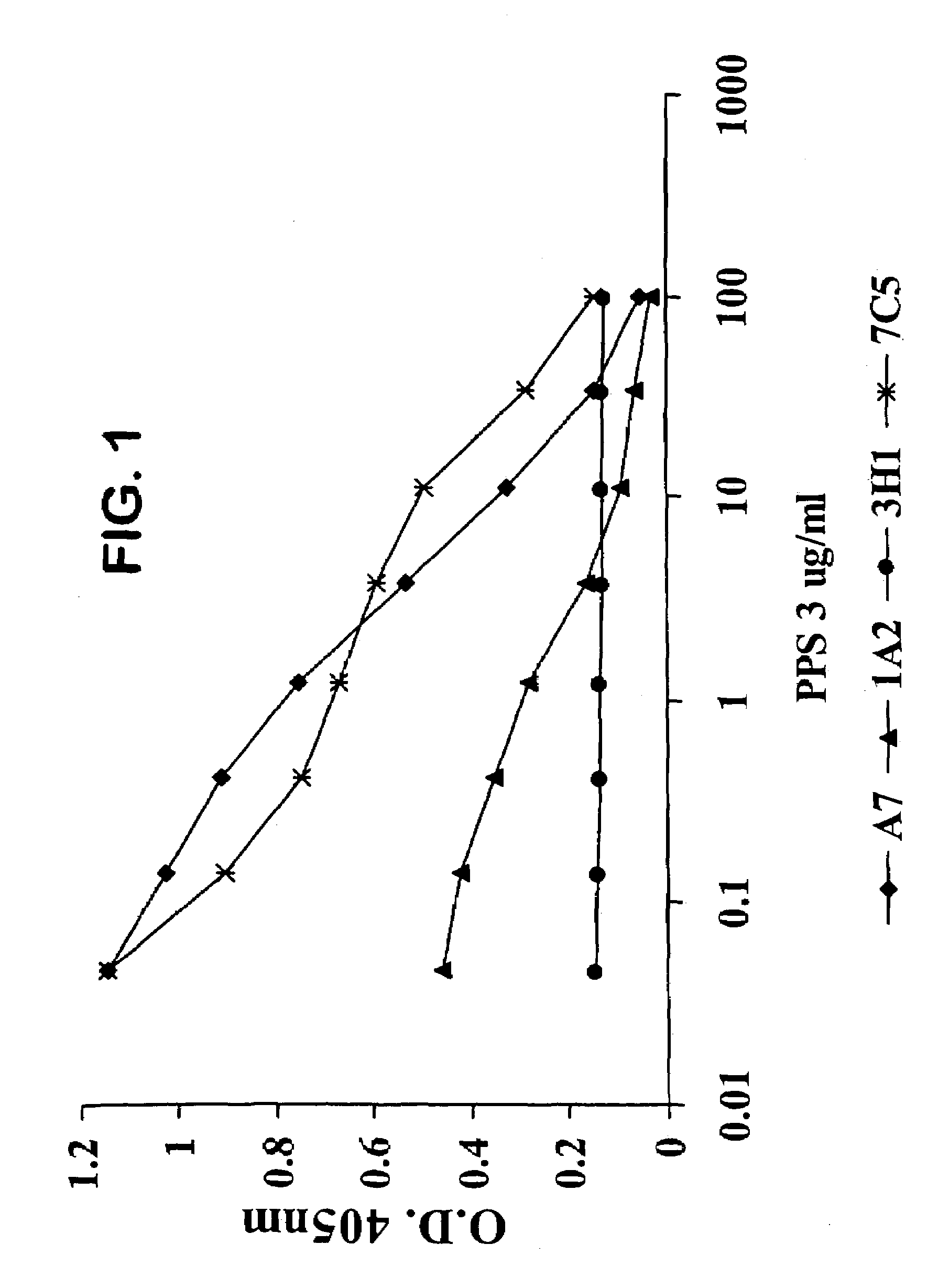
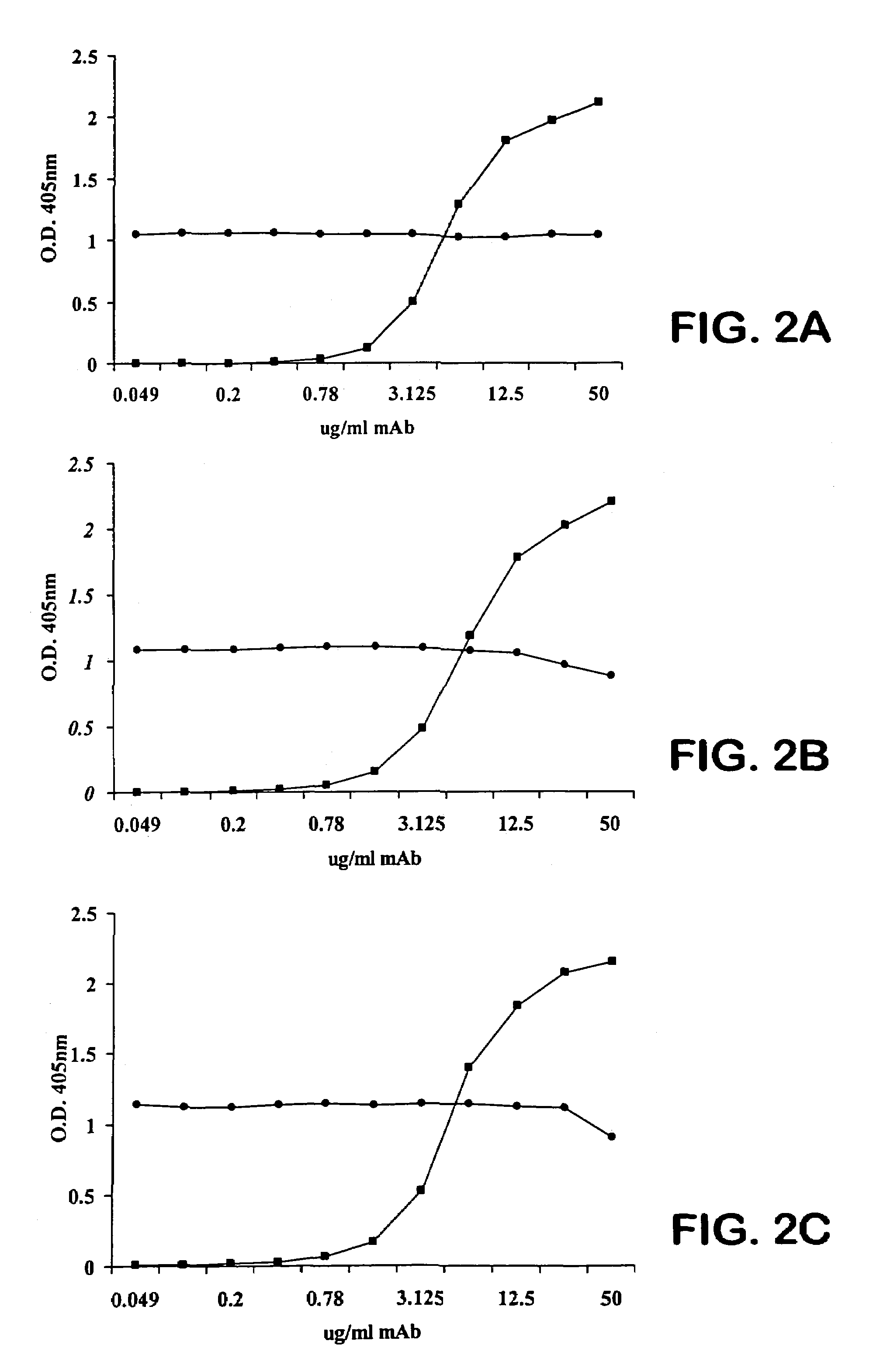
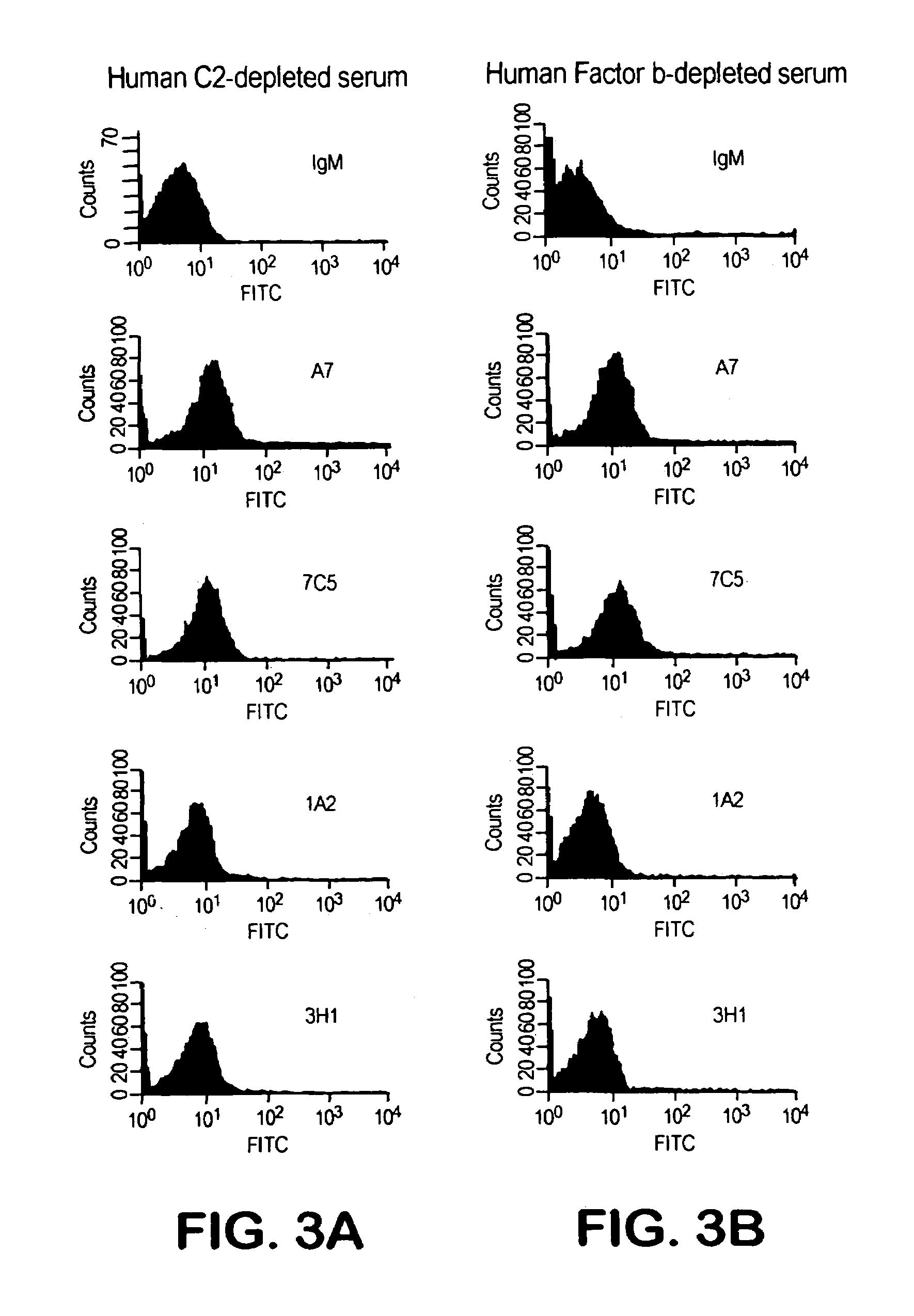
Human antipneumococcal antibodies from non-human animals
The invention described herein provides human antibodies produced in non-human animals that specifically bind to Streptococcus pneumoniae capsular polysaccharide (PPS-3). The invention further provides methods for making the antibodies in a non-human animal and for expressing the antibodies in cells including hybridomas and recombinant host cell systems. Kits and pharmaceutical compositions comprising the antibodies are also provided in addition to methods of treating, inhibitng or preventing S. pneumoniae infection or conditions or disorders caused by such infection by administering to a patient the pharmaceutical compositions described herein.
Owner:ABQENIX INC
IBD-associated microbial antigens and methods of using same
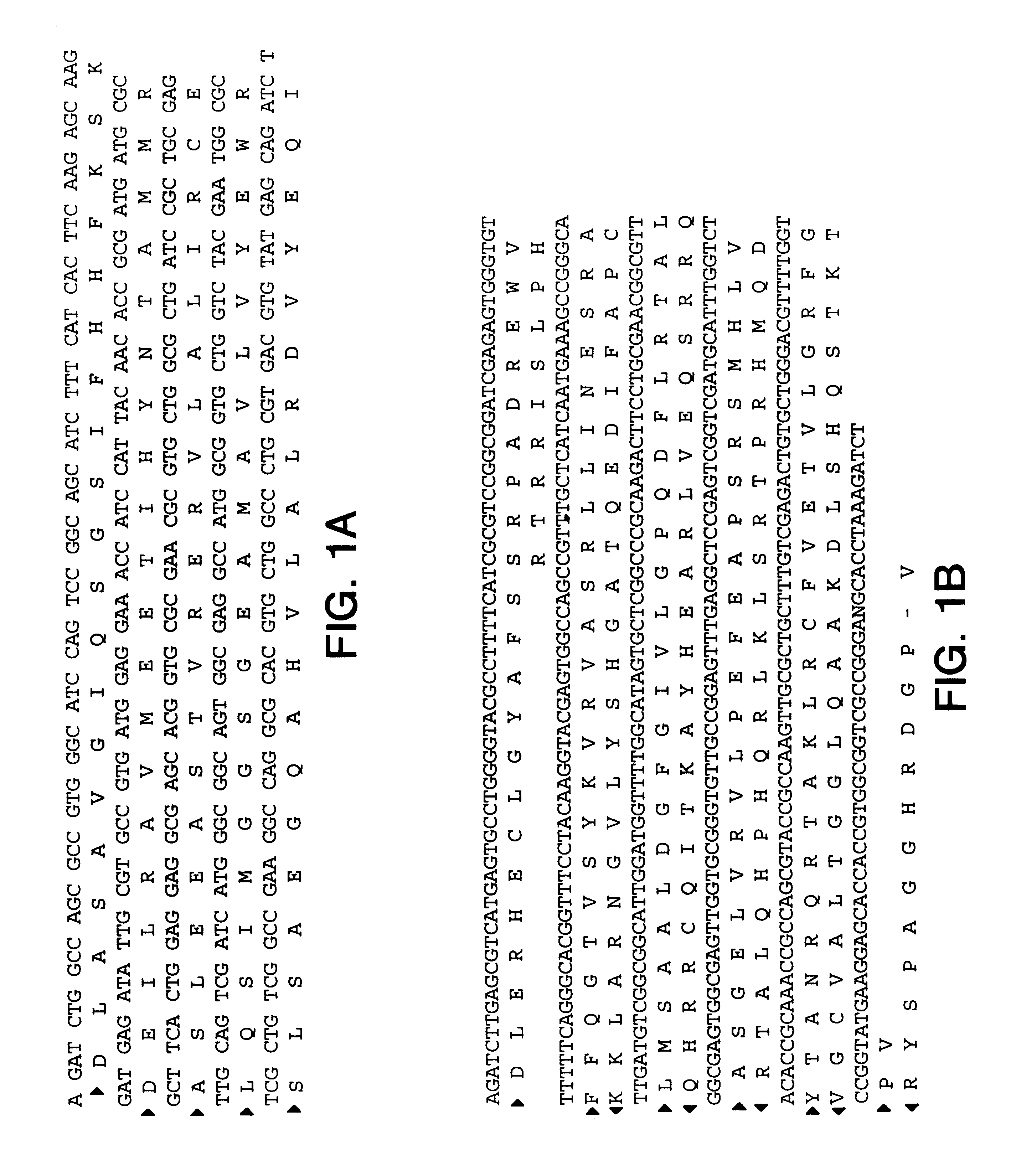
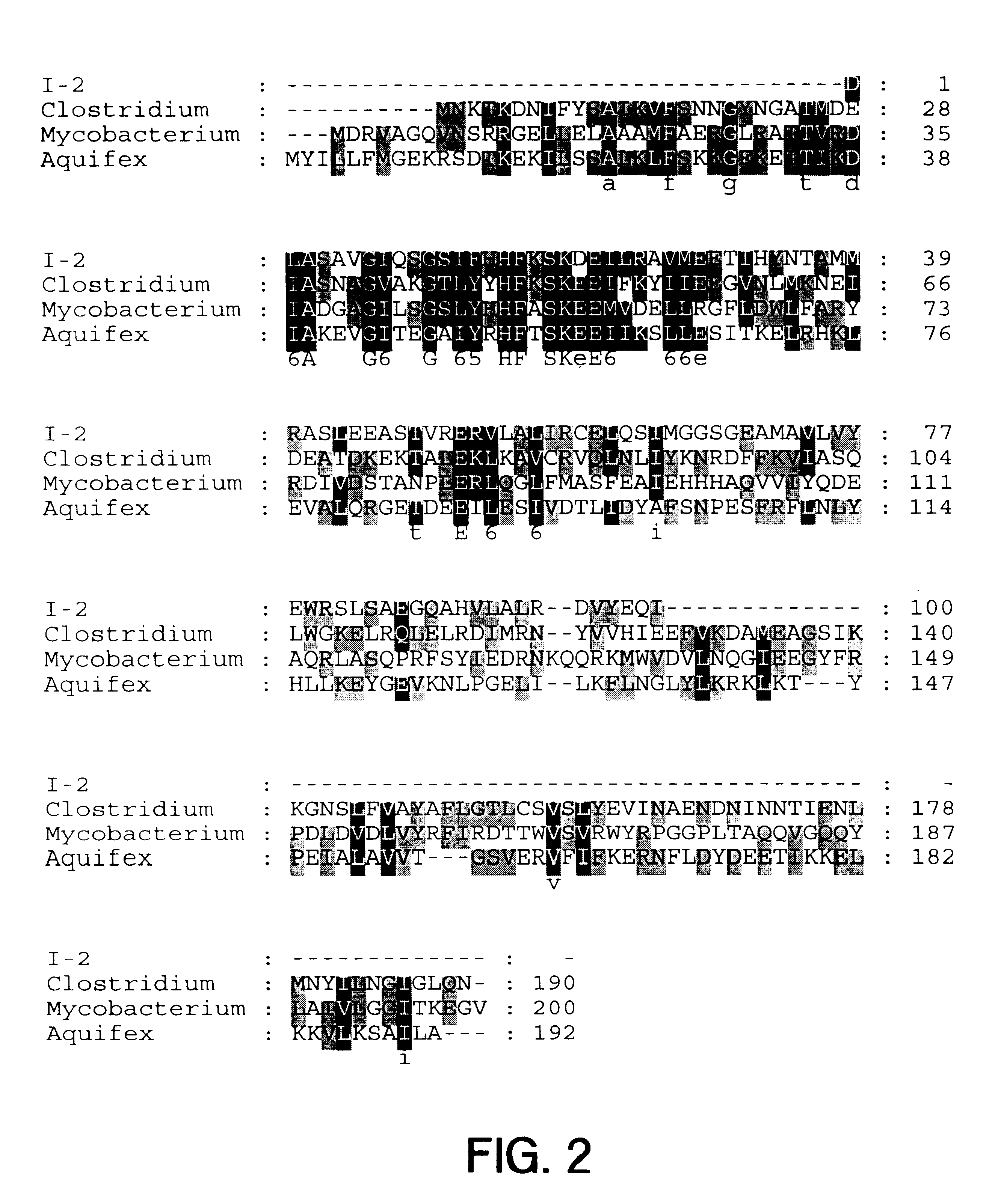
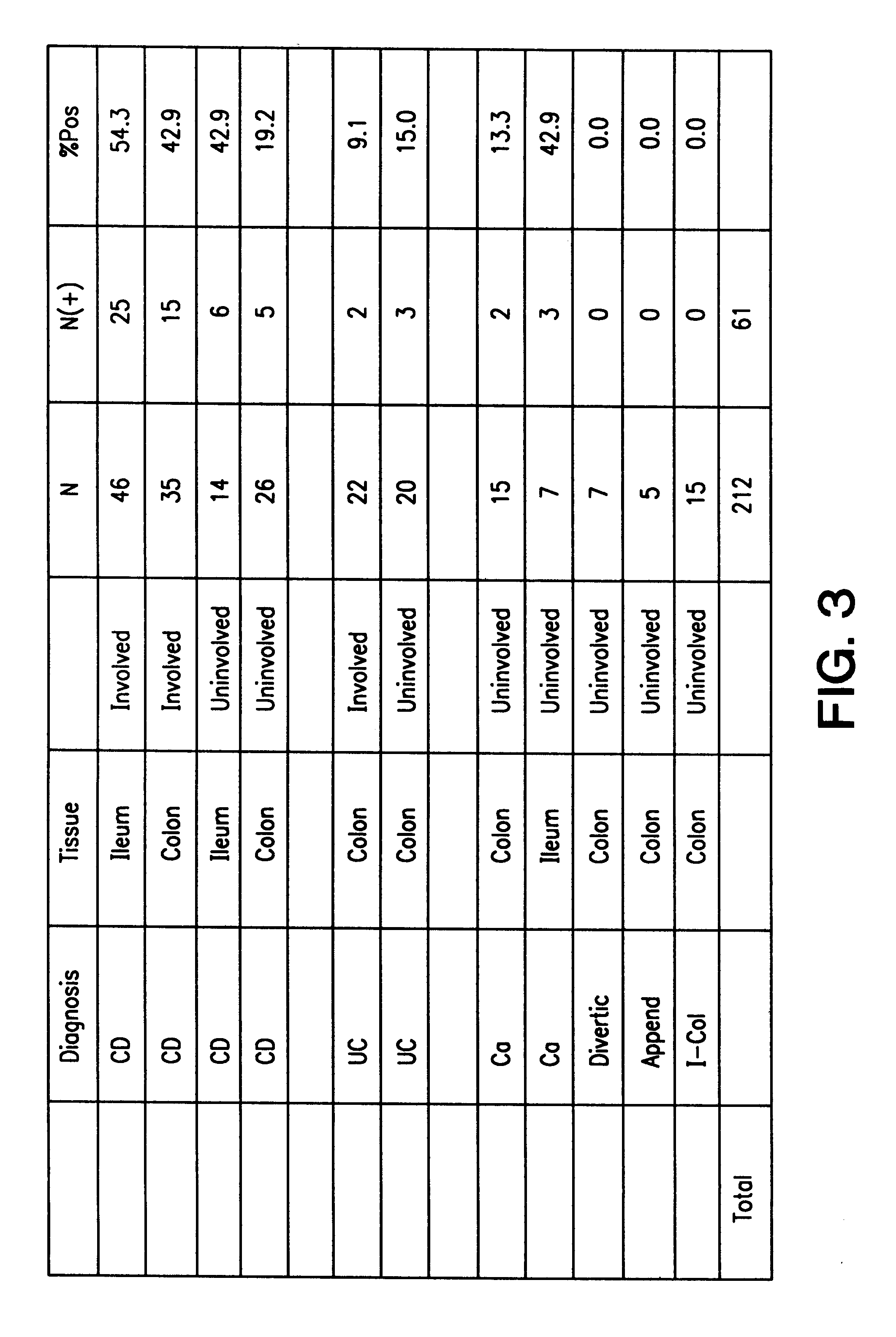
The present invention provides nucleic acid and amino acid sequence of the novel I-1 and I-2 polypeptides, which are associated with human inflammatory bowel disease (IBD). Methods of diagnosing and treating inflammatory bowel disease using the IBD-associated I-1 and I-2 antigens also are provided.
Owner:RGT UNIV OF CALIFORNIA
Streptococcus pneumoniae 37-kDa surface adhesin a protein
The invention provides a nucleic acid encoding the 37-kDa protein from Streptococcus pneumoniae. Also provided are isolated nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. The invention also provides purified polypeptides encoded by the nucleic acid encoding the 37-kDa protein from and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are antibodies which selectively binds the polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are vaccines comprising immunogenic polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Further provided is a method of detecting the presence of Streptococcus pneumoniae in a sample comprising the steps of contacting a sample suspected of containing Streptococcus pneumoniae with nucleic acid primers capable of hybridizing to a nucleic acid comprising a portion of the nucleic acid encoding the 37-kDa protein, amplifying the nucleic acid and detecting the presence of an amplification product, the presence of the amplification product indicating the presence of Streptococcus pneumoniae in the sample. Further provided are methods of detecting the presence of Streptococcus pneumoniae in a sample using antibodies or antigens, methods of preventing and treating Streptococcus pneumoniae infection in a subject.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Pesticidal genes from brevibacillus and methods for their use
Compositions and methods for conferring insecticidal activity to bacteria, plants, plant cells, tissues and seeds are provided. Compositions including a coding sequence for a Brevibacillus-derived delta-endotoxin polypeptide are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants and bacteria. Compositions also include transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated delta-endotoxin nucleic acid molecules are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed, and antibodies specifically binding to those amino acid sequences. In particular, the present invention provides for isolated nucleic acid molecules having nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:2, 4, 7, or 10, or the nucleotide sequence set forth in SEQ ID NO:1, 3, 5, 6, 8, 9, 11, 12, 13, 14, or 15, as well as variants and fragments thereof.
Owner:ATHENIX
Method for the production of porous carbon-based molded bodies, and use thereof as cell culture carrier systems and culture systems
The present invention relates to methods for producing carbon-based molded bodies. In particular, the present invention relates to methods for producing porous carbon-based molded bodies by carbonizing organic polymer materials mixed with non-polymeric fillers and subsequently dissolving the fillers out from the carbonized molded bodies. The present invention further relates to methods for producing porous carbon-based molded bodies by carbonizing organic polymer materials mixed with non-polymeric fillers which are substantially completely decomposed during the carbonization. The present invention also relates to a method for producing porous carbon-based molded bodies by carbonizing organic polymer materials, the carbon-based molded bodies being partially oxidized following carbonization so as to produce pores. In addition, the present invention relates to porous molded bodies produced according to one of said methods and the use thereof, especially as cell culture carriers and / or culture systems.
Owner:CINVENTION AG
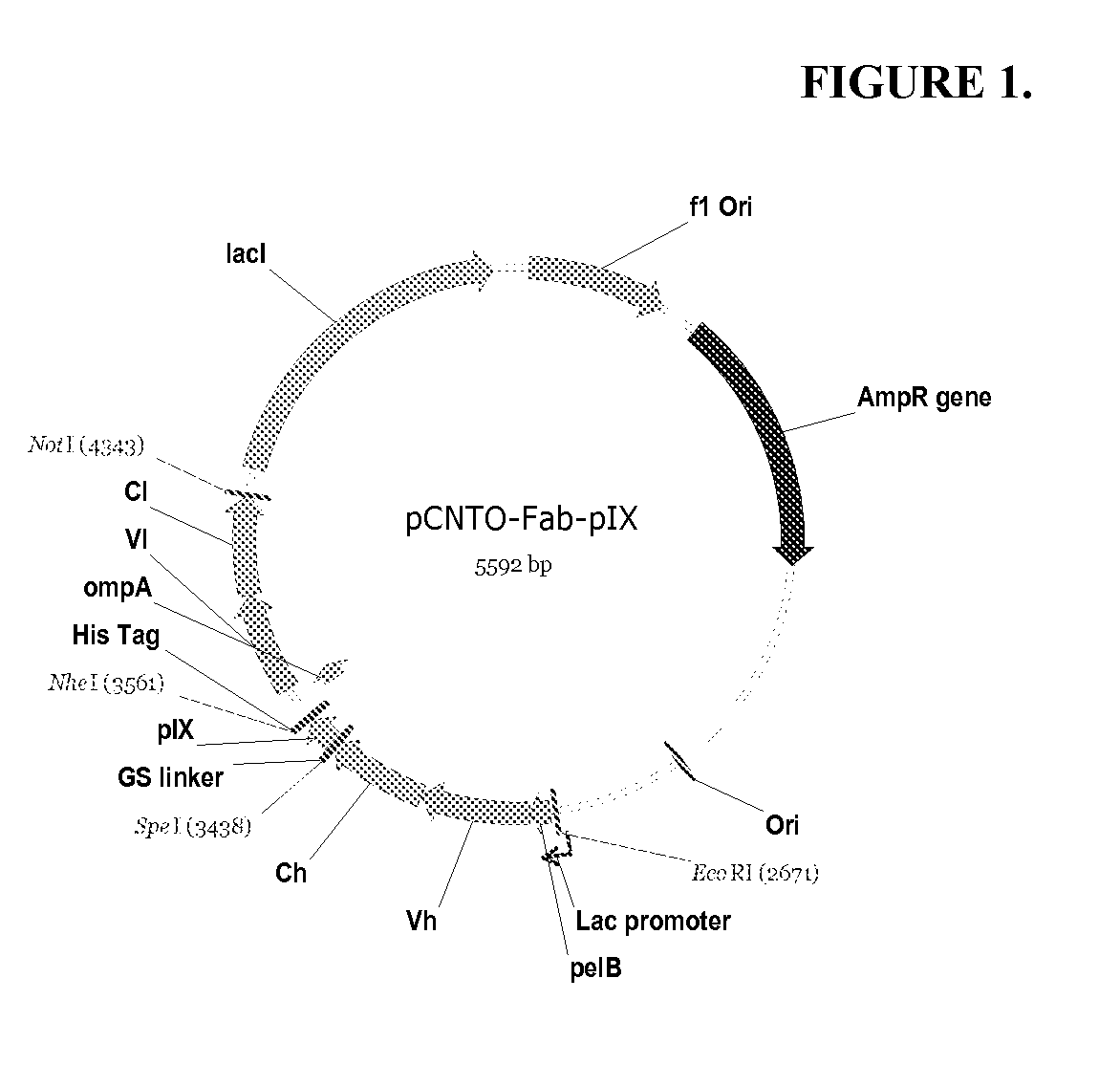
DESIGN AND GENERATION OF HUMAN DE NOVO pIX PHAGE DISPLAY LIBRARIES
InactiveUS20100021477A1Easy to understandIncrease diversityBacteriaLibrary screeningNatural antibodyStructure function
Described and claimed herein are combinatorial synthetic Fab libraries displayed on a phage pIX protein. The libraries were built on scaffolds representing the most frequently used genes in human antibodies, which were diversified to mirror the variability of natural antibodies. After selection using a diverse panel of proteins, numerous specific and high-affinity Fabs were isolated. By a process called in-line maturation the affinity of some antibodies was improved up to one hundred-fold yielding low pM binders suitable for in vivo use. This work thus demonstrates the feasibility of displaying complex Fab libraries as pIX-fusion proteins for antibody discovery and lays the foundations for studies on the structure-function relationship of antibodies.
Owner:JANSSEN BIOTECH INC
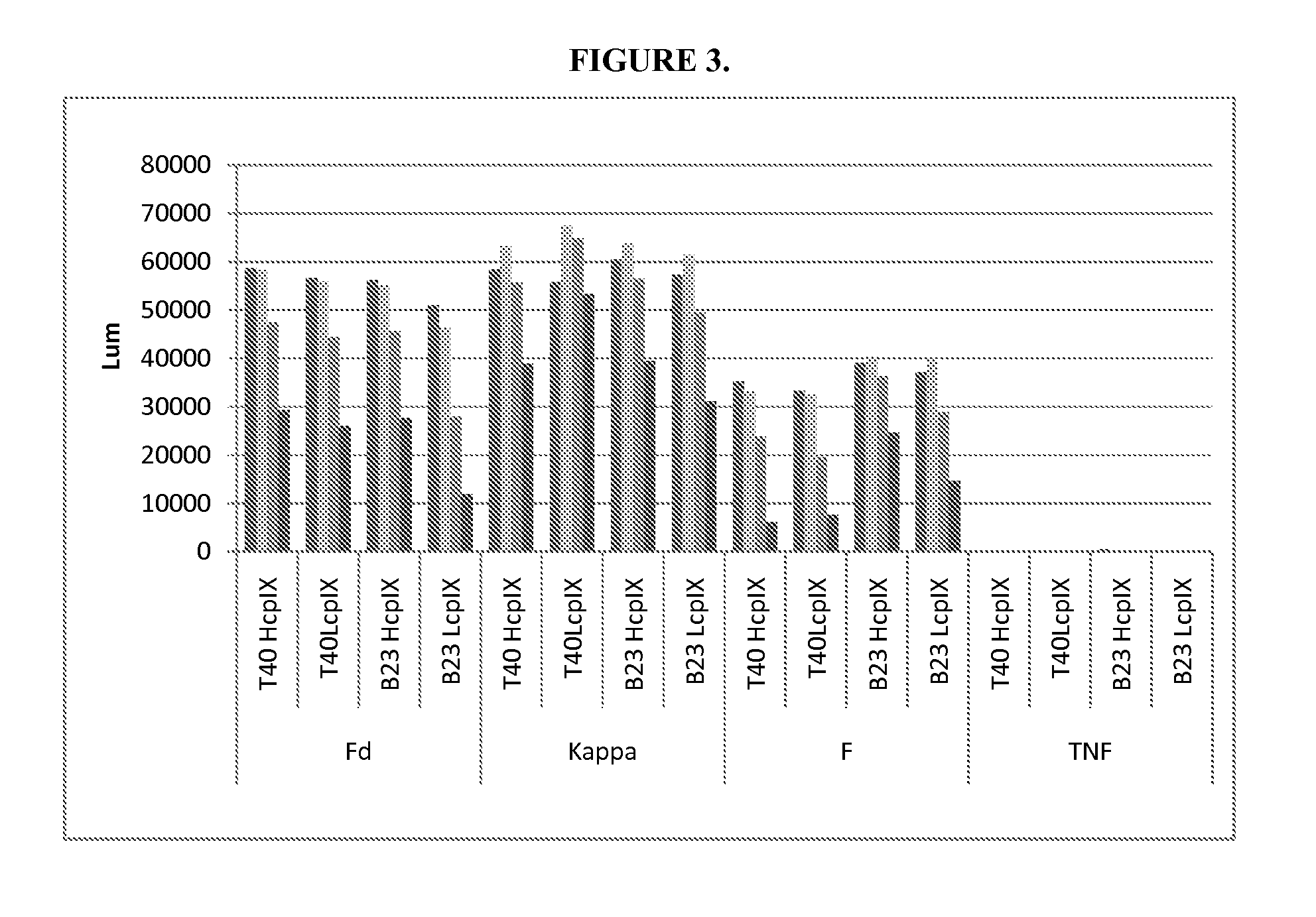
Method for producing immunoglobulins containing protection proteins in plants and their use
The immunoglobulins of the present invention are useful therapeutic immunoglobulins against mucosal pathogens such as S. mutans. The immunoglobulins contain a protection protein that protects the immunoglobulins in the mucosal environment.The invention also includes the greatly improved method of producing immunoglobulins in plants by producing the protection protein in the same cell as the other components of the immunoglobulins. The components of the immunoglobulin are assembled at a much improved efficiency. The method of the invention allows the assembly and high efficiency production of such complex molecules.The invention also contemplates the production of immunoglobulins containing protection proteins in a variety of cells, including plant cells, that can be selected for useful additional properties. The use of immunoglobulins containing protection proteins as therapeutic antibodies against mucosal and other pathogens is also contemplated.
Owner:UNTD MED & DENT SCHLS OF GUYS & ST THOMASS HOSP
Mutated immunoglobulin-binding protein
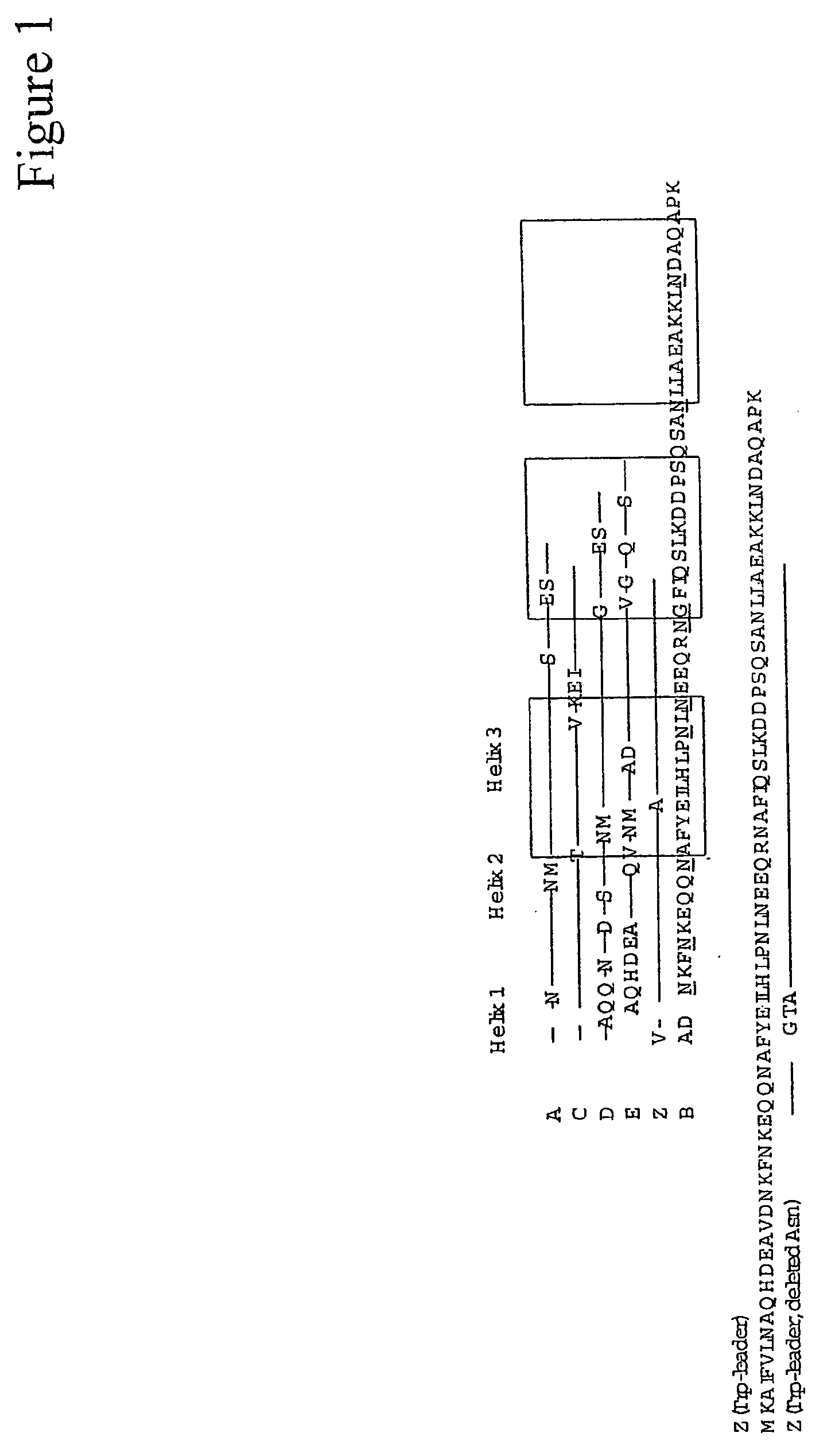
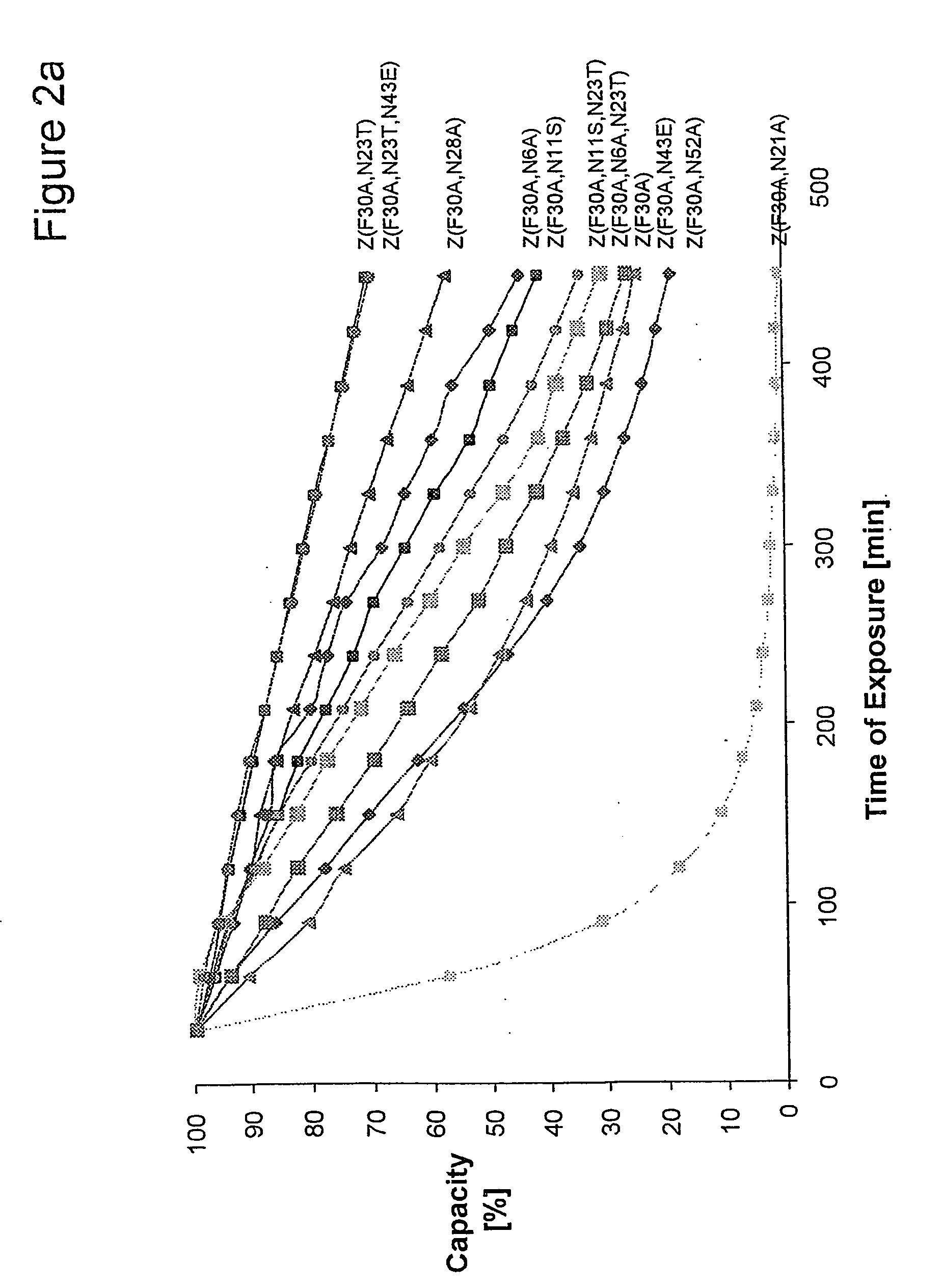
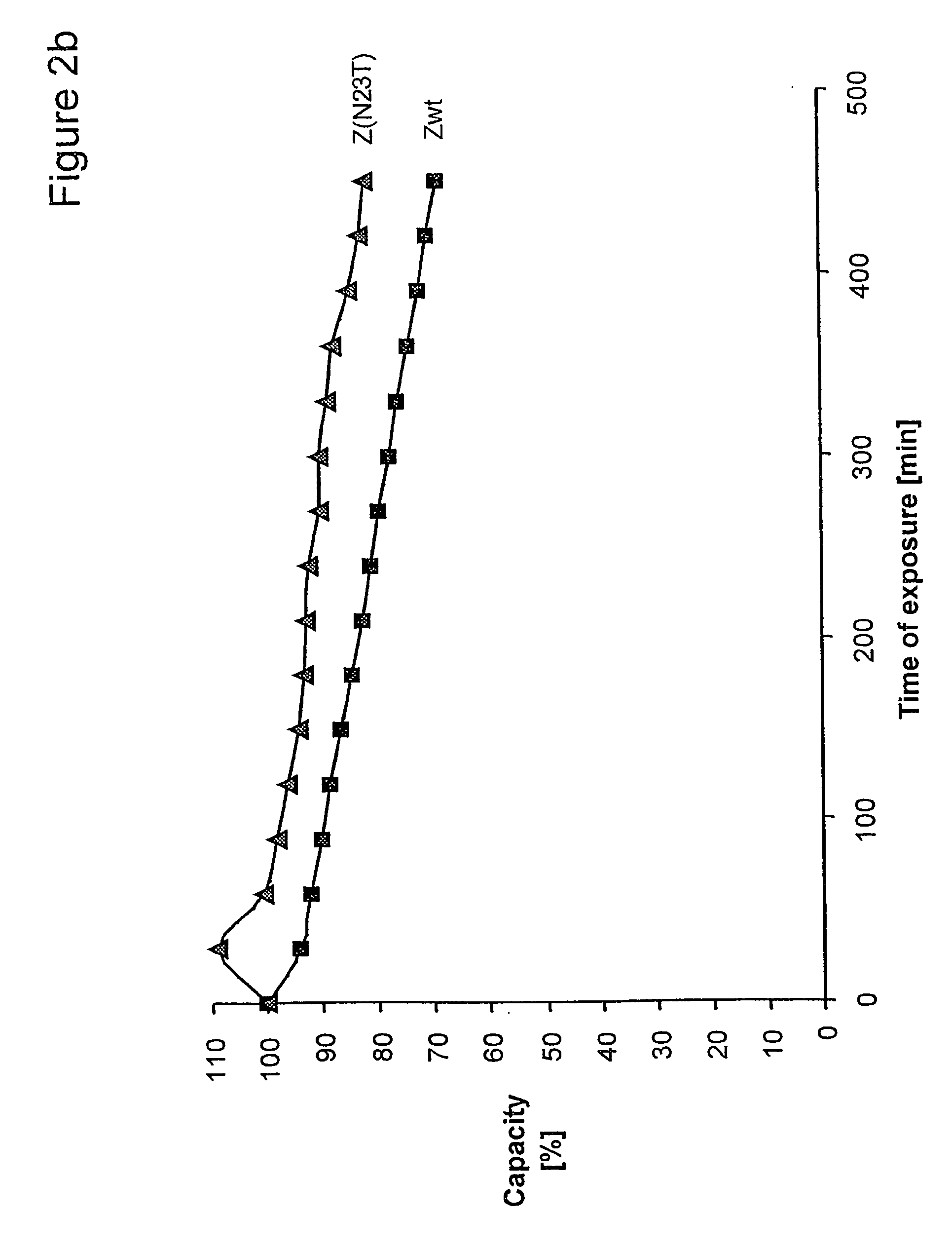
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Meningococcal antigens
InactiveUS6709660B1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsCoccidiaNucleotide
The invention provides proteins from Neisseria meningitidis (strains A & B), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics.
Owner:NOVARTIS AG
Compounds and methods for diagnosis of tuberculosis
Compounds and methods for diagnosing tuberculosis are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of one or more M. tuberculosis proteins, and DNA sequences encoding such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of M. tuberculosis infection in patients and biological samples. Antibodies directed against such polypeptides are also provided.
Owner:CORIXA CORP
Method for linking sequences of interest
Multiplex overlap-extension RT-PCR provides an efficient method of linking two or more nucleotide sequences encoding for domains or subunits of a heteromeric protein, in a single reaction. Especially, the linkage of variable region encoding sequences from e.g. immunoglobulins, T cell receptors or B cell receptors is eased with the method of the present invention. This allows for a more efficient way of generating libraries of variable region encoding sequences. The capability to perform the multiplex overlap-extension RT-PCR using template derived from an isolated single cell enables the generation of cognate pair libraries in a high-throughput format.
Owner:LES LAB SERVIER
Methods of promoting immunopotentiation and preparing antibodies with anti-CD3 antibodies
Disclosed are immunopotentiating agents, and vaccines thereof, which enhance and / or otherwise modify immune responses, and method for their preparation and use in vivo. Immunopotentiating agents can be single agents that act directly, adjuvants added concurrently with the agents, or heteroconjugates wherein the immunopotentiating agent is chemically coupled to the compound against which an immune response is desired. Examples of immunopotentiating agents include monoclonal antibodies, such as anti-CD3, anti-CD2) and anti-CD5 antibodies, and proteins derived from microorganisms (e.g., enterotoxins) which activate T cells. The compounds against which an immune response can be generated, which may be the second component in a heteroconjugate, include compound from abnormal or diseased tissues such as tumors, or infectious agents, such as viruses, bacteria, fungi, protozoal or metozoal parasites, and can be obtained by natural or recombinant means. Methods of using the invention to prepare monoclonal antibodies are particularly disclosed.
Owner:MACROGENICS INC
Mutants of anti-cd40 antibody
ActiveUS20070148163A1High therapeutic effectImmunoglobulins against bacteriaImmunoglobulins against virusesHinge regionMutant
A mutant of a potentially therapeutic anti-CD40 antibody is provided which mutant has reduced ADCC and CDC activities designed to be optimized as a pharmaceutical agent. A mutant of an agonistic anti-CD40 antibody, comprising mutation and / or substitution of at least one amino acid in the constant region to reduce the ADCC and / or CDC activities therein, and a mutant of an antagonistic anti-CD40 antibody, comprising at least one mutation or substitution in the constant region to reduce the ADCC and / or CDC activities therein, both mutants having at least a hinge region derived from a human IgG2.
Owner:KYOWA HAKKO KIRIN CO LTD
Popular searches
Immunoglobulins against cytokines/lymphokines/interferons Immunoglobulins against cell receptors/antigens/surface-determinants Animal repellants Genetic engineering Fermentation Gene therapy Plant genotype modification Foreign genetic material cells Plant growth regulators Vector-based foreign material introduction
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com