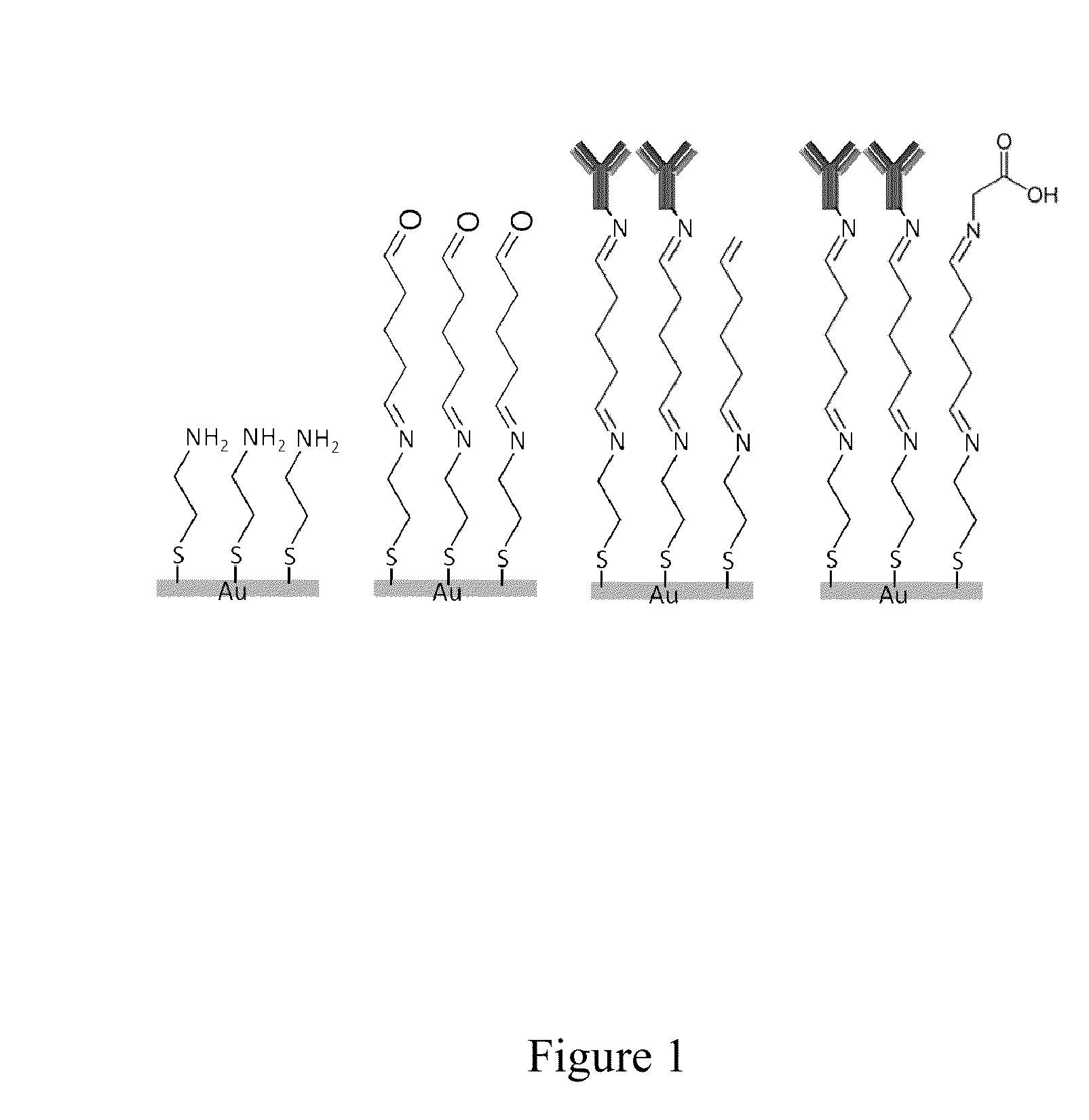
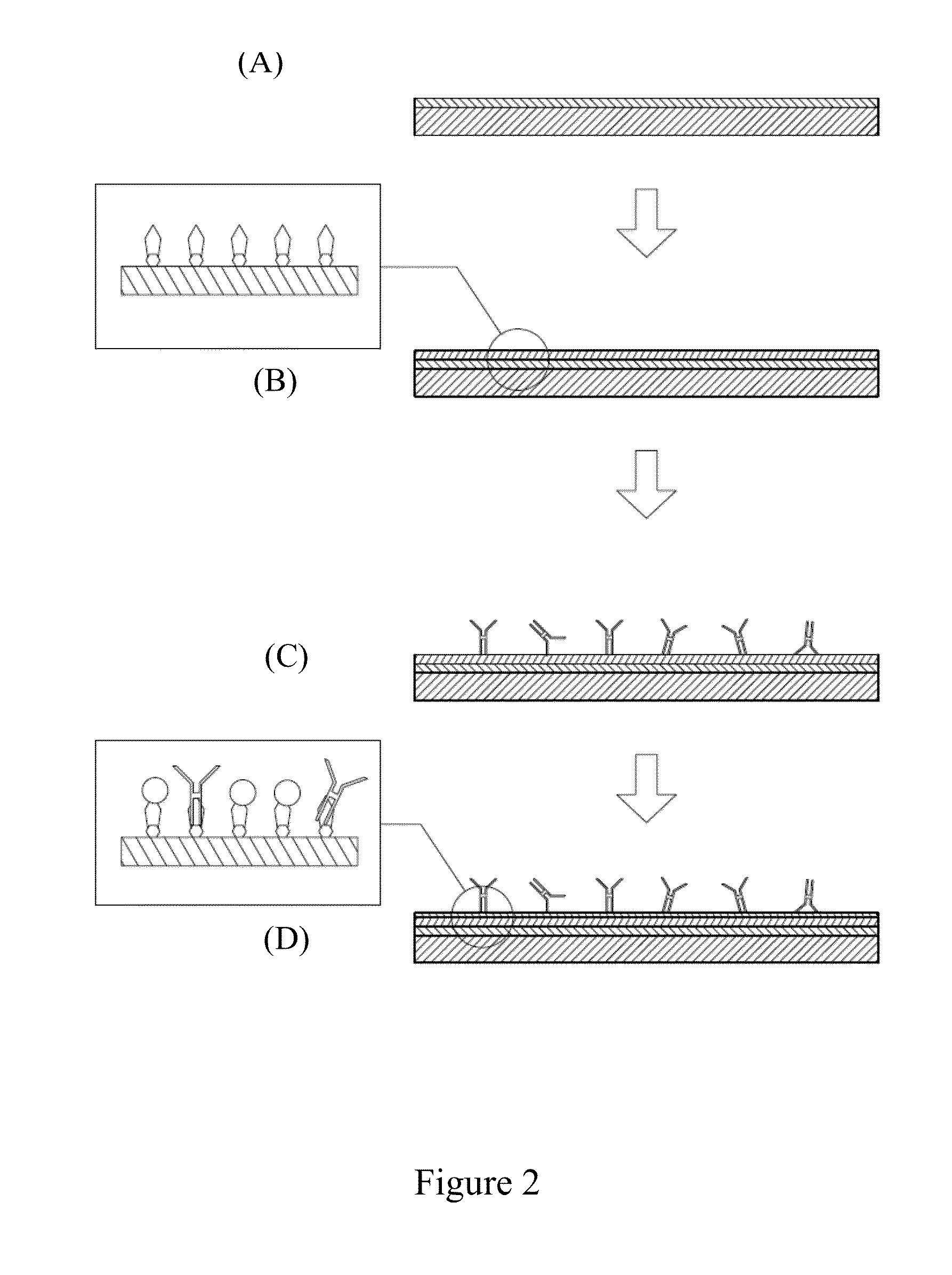
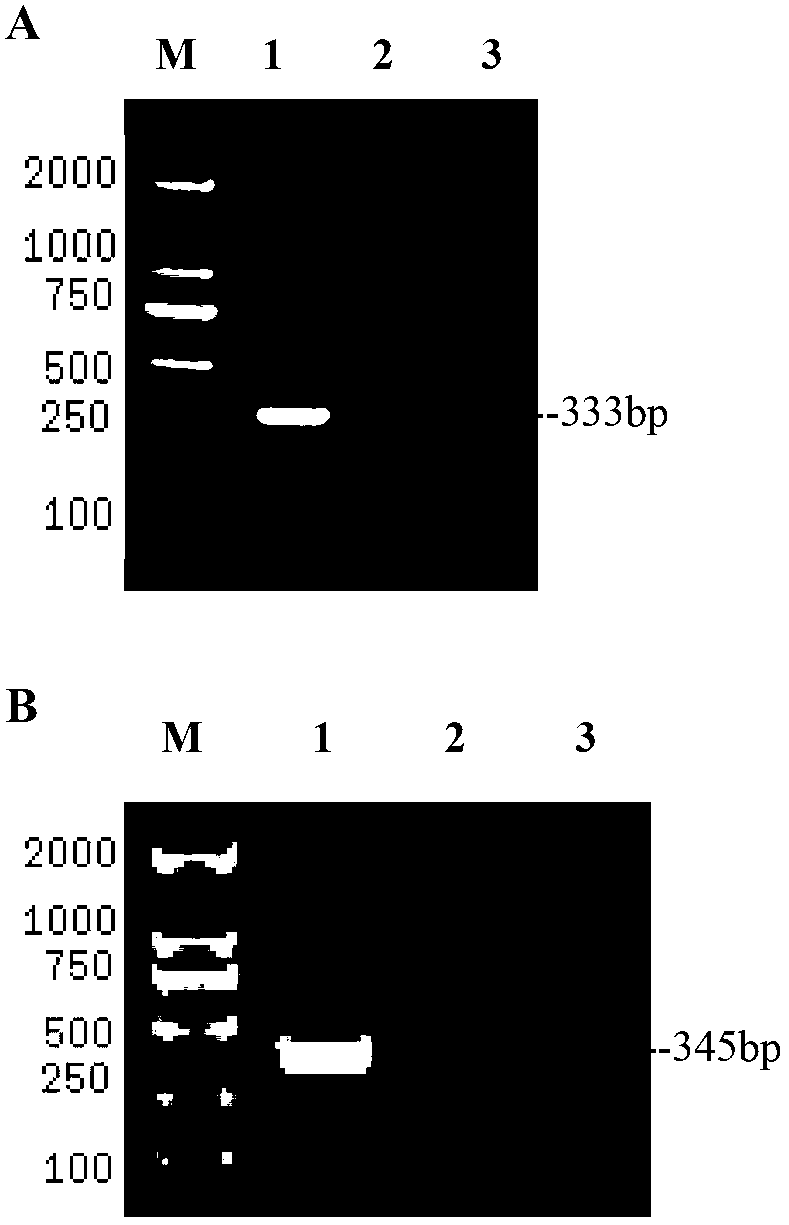
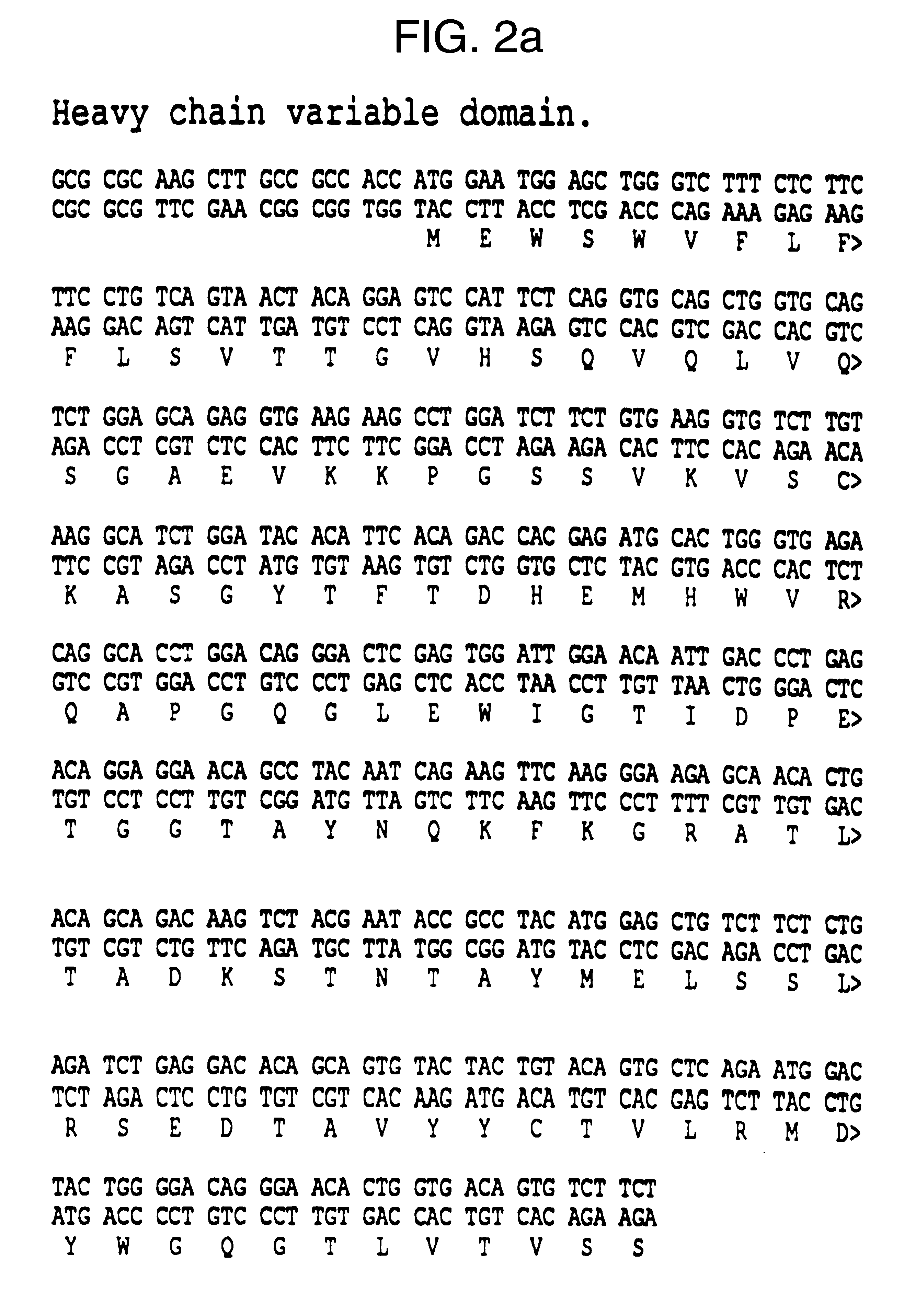Patents
Literature
96 results about "Natural antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Natural antibodies may refer to antibodies that: are produced without any previous infection, vaccination, other foreign antigen exposure or passive immunization, contrasted to regular and irregular antibodies.
Antibody
InactiveUS20070274985A1Generate efficientlyEffective isolationAntibody mimetics/scaffoldsImmunoglobulins against animals/humansNatural antibodySingle-Chain Antibodies
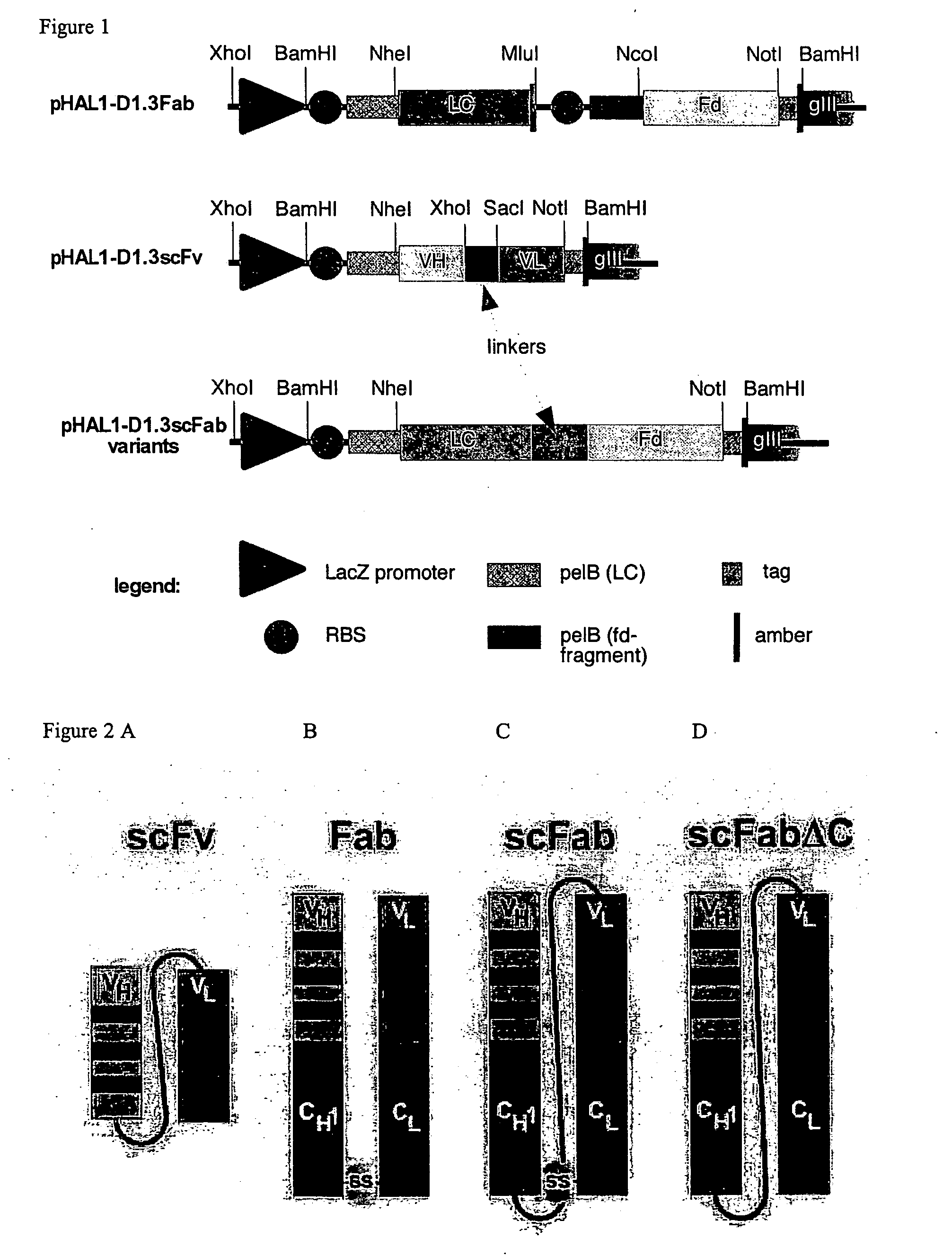
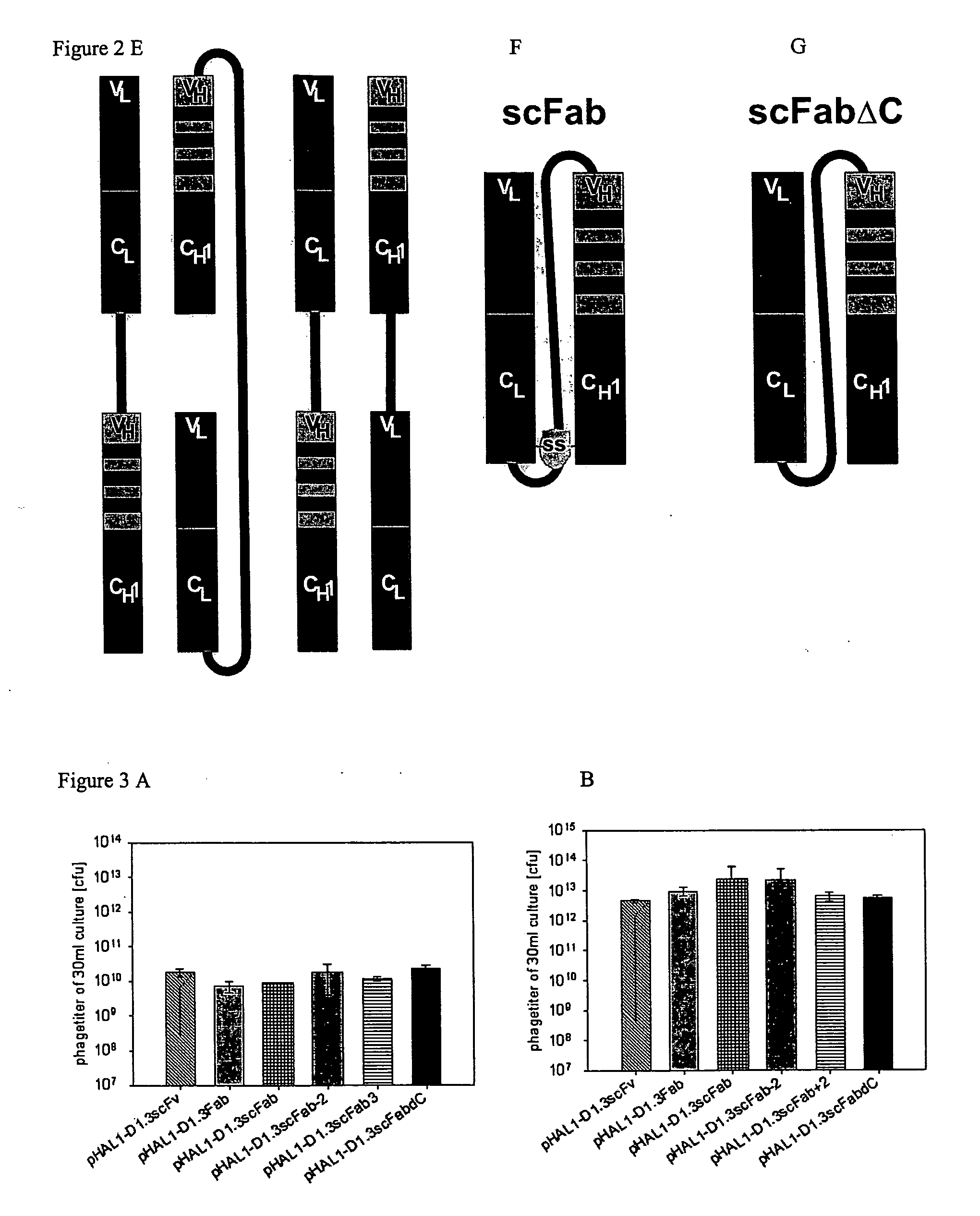
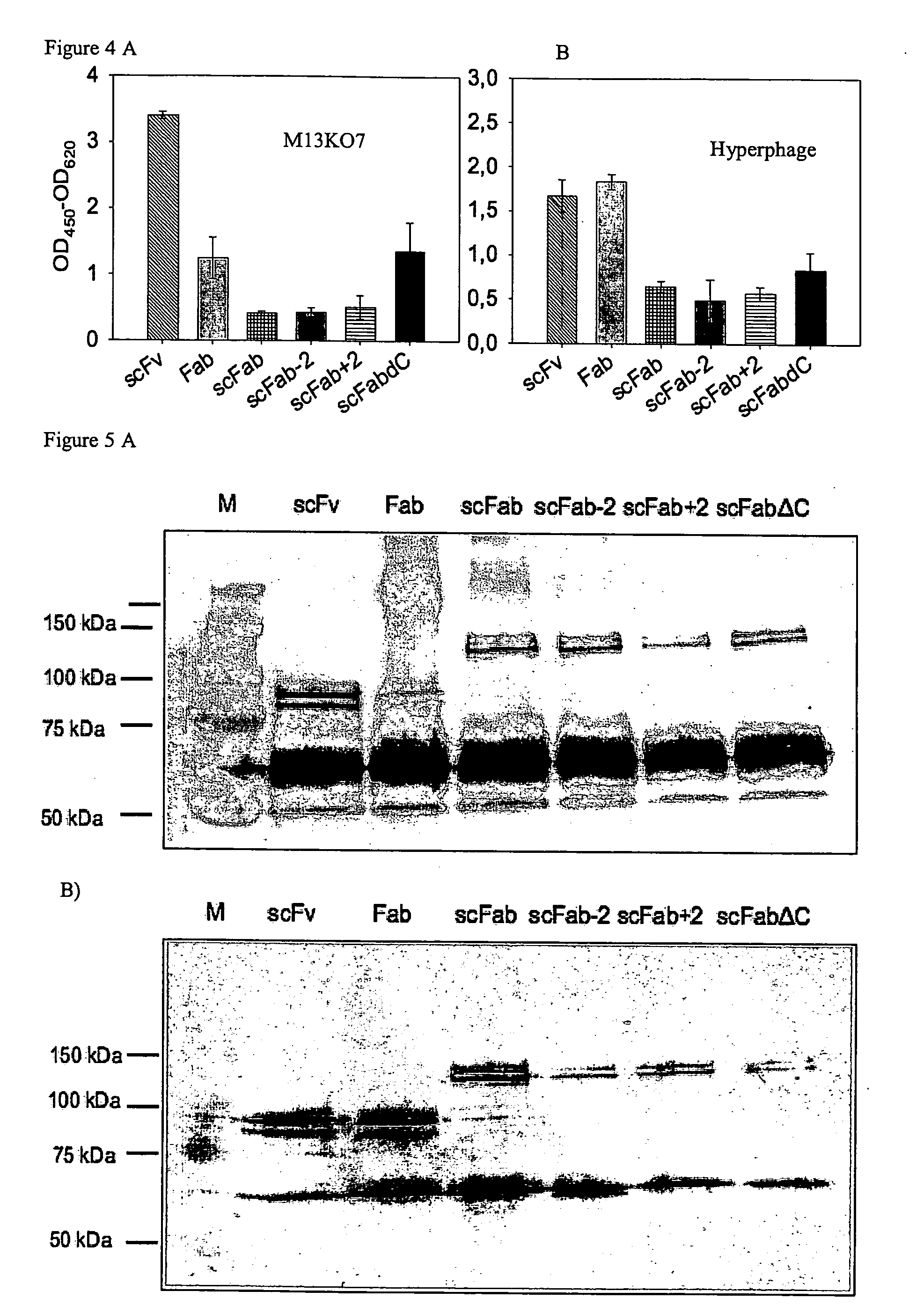
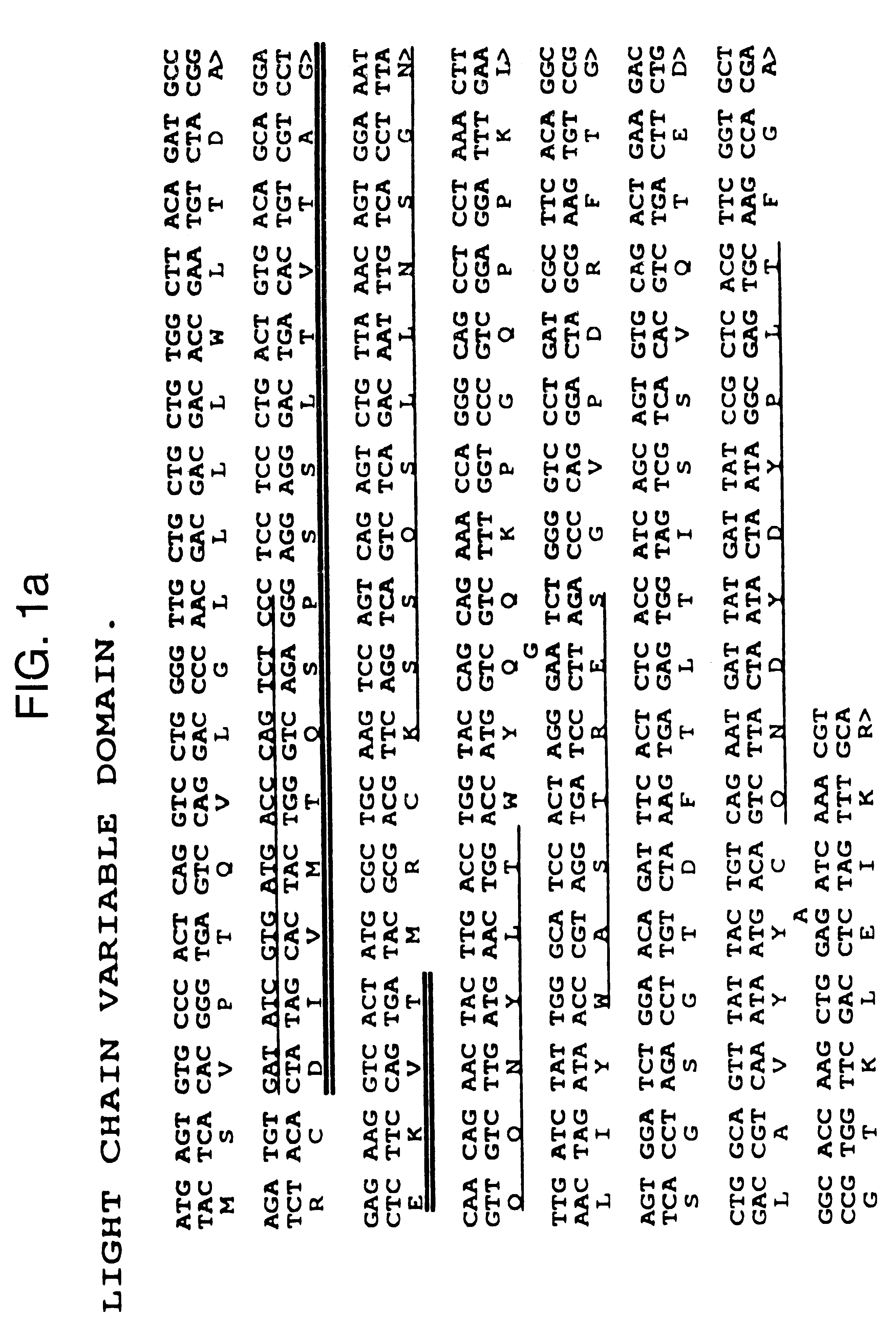
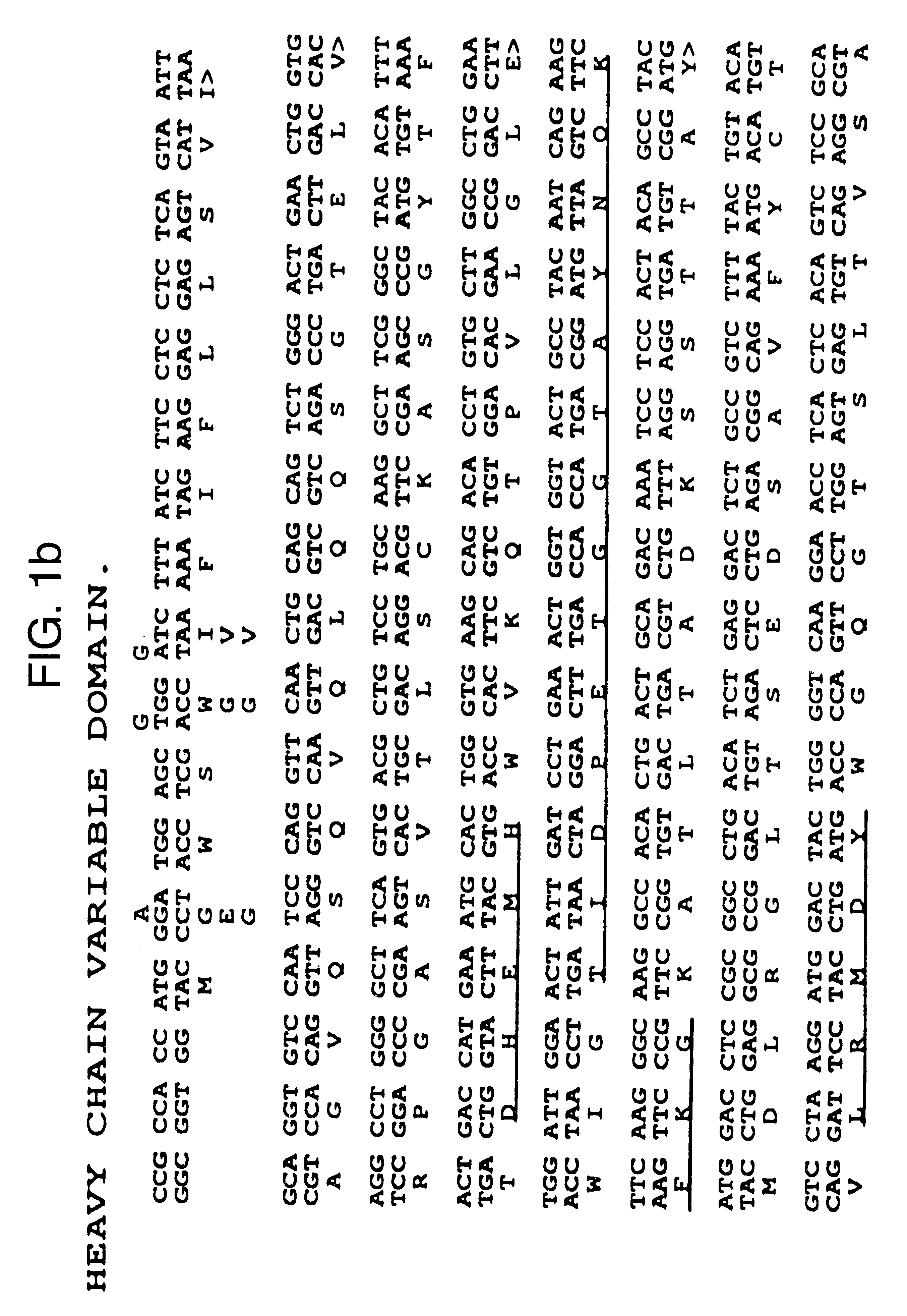
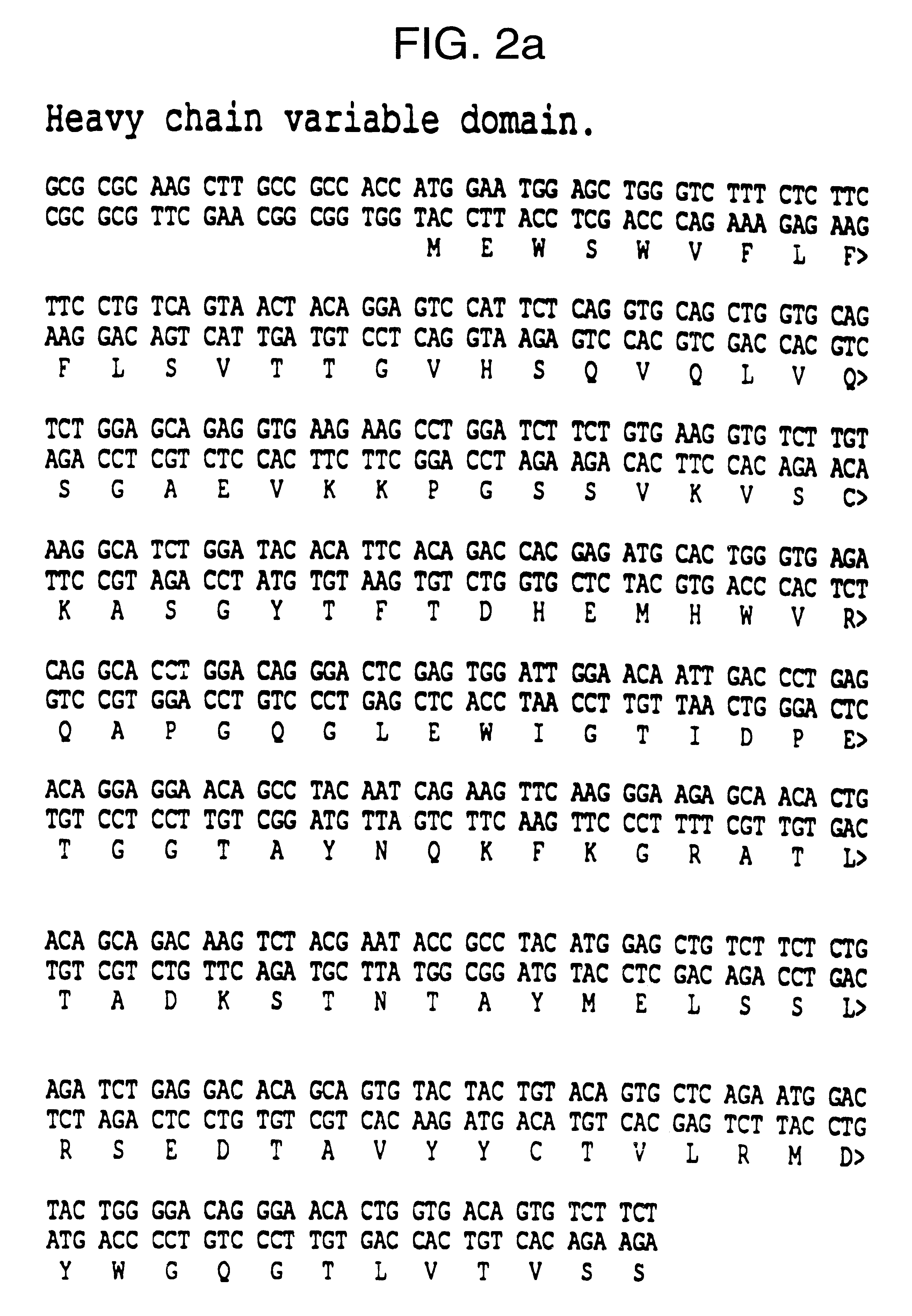
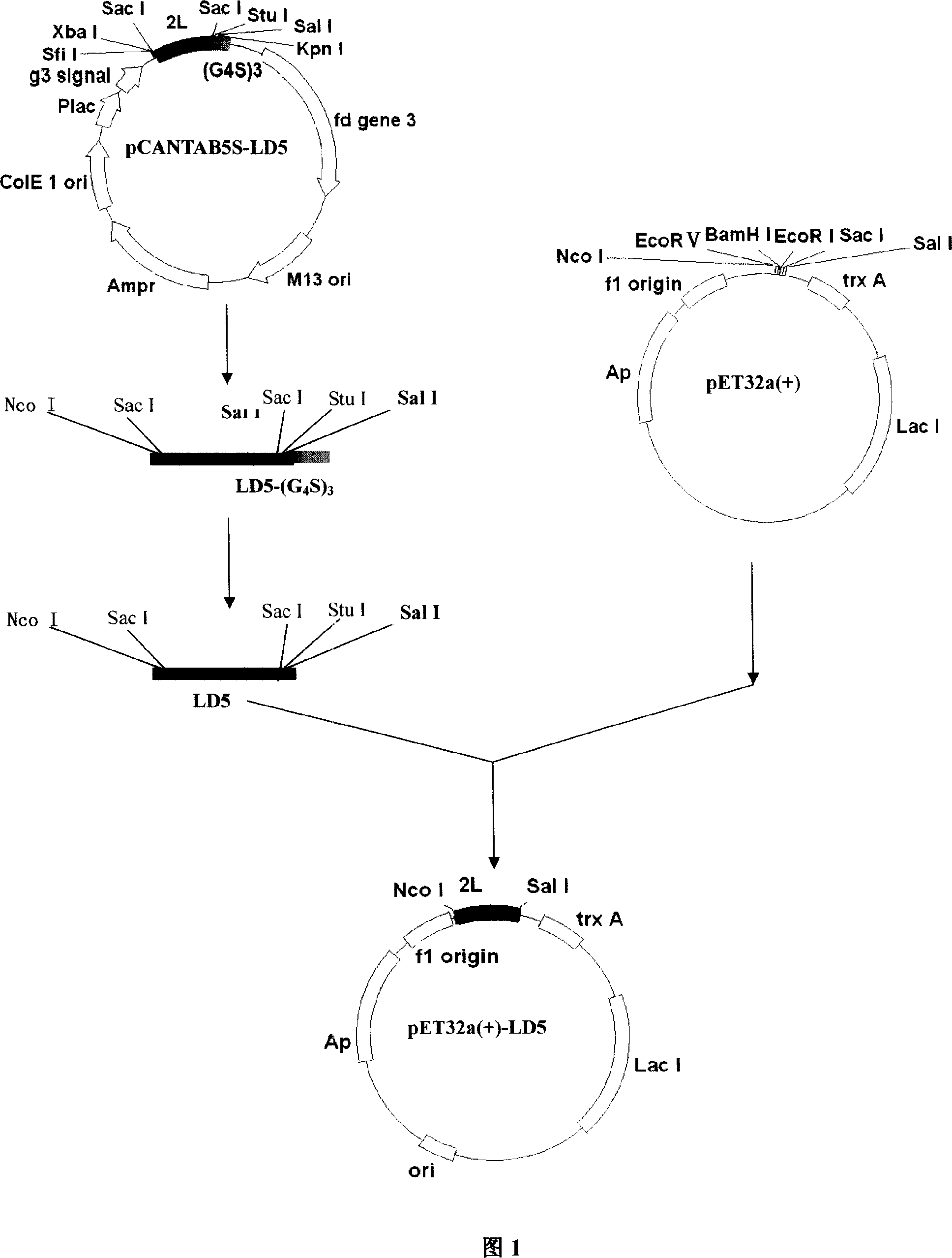
The present invention refers to synthetic antibody molecules which comprise domains from naturally occuring antibodies, e.g. domains derivable from IgG, preferably of human origin, in a novel arrangement. Single chain molecules are provided which are suitable for expression in micro-organisms in their active conformation, which single chain molecules generally comprise a VL domain, a CL domain, and a VH domain, a CH1 domain, linked by a linker arranged between VUCL and VH / CH1. Accordingly, these antibody molecules can be termed single chain Fabs (scFabs). These antibody molecules are single chain proteins, which can also be associated to dimers, including heteromeric antibodies, wherein at least two single chain antibody molecules are associated.
Owner:TECH UNIV BRAUNSCHWEIG
DESIGN AND GENERATION OF HUMAN DE NOVO pIX PHAGE DISPLAY LIBRARIES
InactiveUS20100021477A1Easy to understandIncrease diversityBacteriaLibrary screeningNatural antibodyStructure function
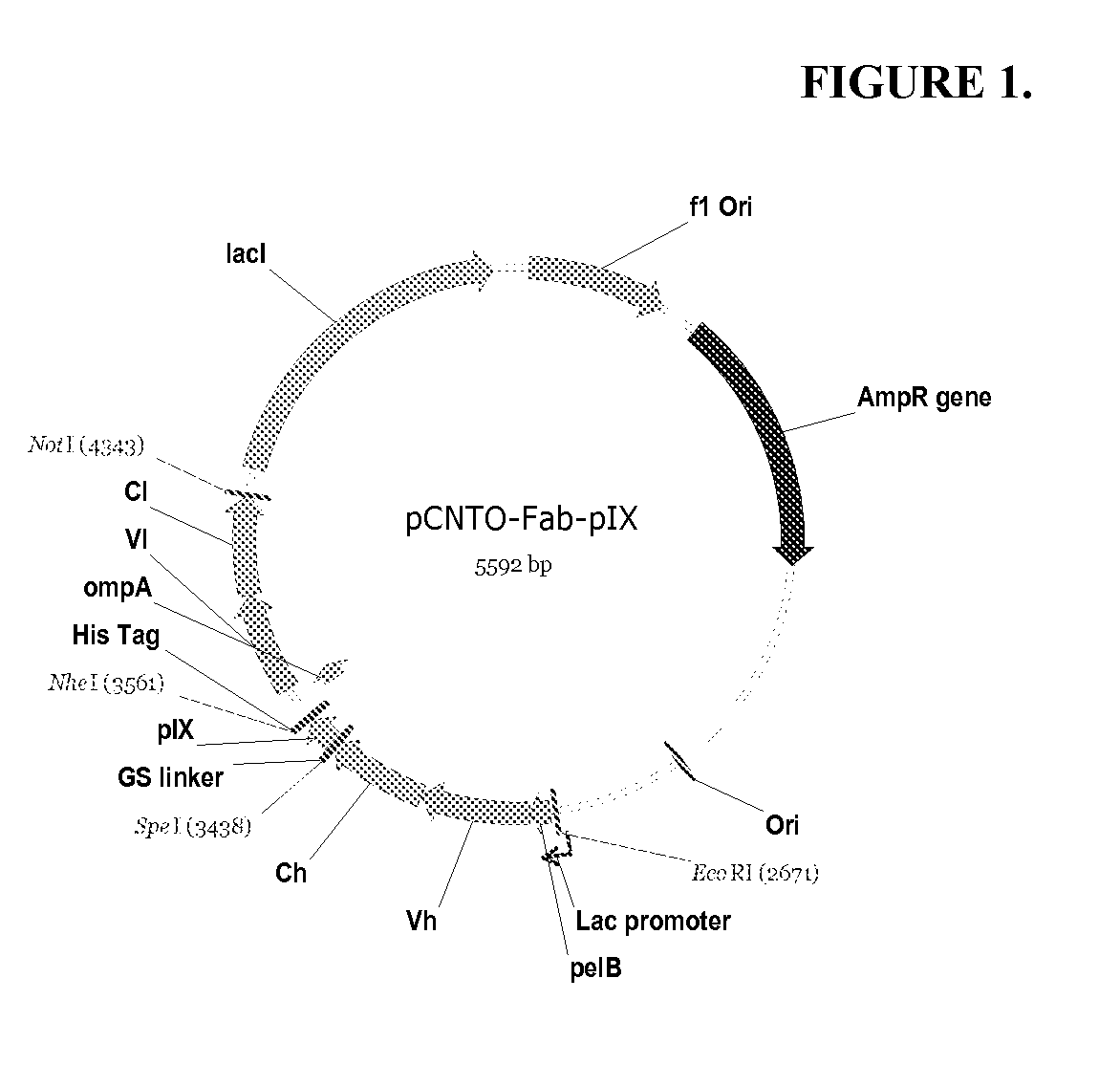
Described and claimed herein are combinatorial synthetic Fab libraries displayed on a phage pIX protein. The libraries were built on scaffolds representing the most frequently used genes in human antibodies, which were diversified to mirror the variability of natural antibodies. After selection using a diverse panel of proteins, numerous specific and high-affinity Fabs were isolated. By a process called in-line maturation the affinity of some antibodies was improved up to one hundred-fold yielding low pM binders suitable for in vivo use. This work thus demonstrates the feasibility of displaying complex Fab libraries as pIX-fusion proteins for antibody discovery and lays the foundations for studies on the structure-function relationship of antibodies.
Owner:JANSSEN BIOTECH INC
Medicament for treating prostate diseases
InactiveUS7582294B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsNatural antibodyHeifer calf
A medicament based on antibodies contains an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to the prostate-specific antigen, the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to homeopathic technology. In order to obtain the antibodies, the prostate-specific antigen isolated from the prostatic tissues of cattle or prepared synthetically is employed; a mixture of various, mostly centesimal, homeopathic dilutions is used. The method of treating diseases of the urogenital sphere consists in using activated forms of ultra-low doses of antibodies to prostate-specific antigen prepared by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Curing method for pathologic syndrome and medicinal preparation
A method of treating a pathological syndrome includes administration of an activated form of ultra-low doses of antibodies to an antigen, wherein said activated form is obtained by repeated consecutive dilution combined with external impact, and the antigen is a substance or a pharmaceutical agent exerting influence upon the mechanisms of formation of this particular pathological syndrome. Pharmaceutical agent for treating a pathological syndrome contains activated form of ultra-low doses of monoclonal, polyclonal or natural antibodies to an antigen, wherein said activated form is prepared by means of repeated consecutive dilution and external treatment, predominantly based on homeopathic technology, and said antigen is a substance or a drug acting as a direct cause of the pathological syndrome or involved in regulation of mechanisms of its formation. At that, activated forms of ultra-low doses of antibodies are raised against antigens of exogenous or endogenous origin, against autologous antigens, fetal antigens; anti-idiotypic antibodies are used too.
Owner:EPSHTEIN OLEG I
Media and method for treating pathological syndrome
InactiveUS7572441B2Energy modified materialsImmunoglobulins against cytokines/lymphokines/interferonsNatural antibodyUltra low dose
A medicament based on antibodies contains an activated form of monoclonal, polyclonal, or natural antibodies to interferon in low or ultra-low doses prepared by multiple consecutive dilutions and exposure to external factors, preferably in accordance with homeopathic technology. In order to obtain antibodies, human or heterologous interferon alpha, beta, or gamma, including recombinant interferon, is used; a mixture of various, mostly centimal, homeopathic dilutions being employed. A method of treating a pathologic syndrome, whose formation is affected by interferon, consists in the use of activated forms of antibodies to interferon alpha, beta, or gamma in low or ultra-low doses obtained by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Human plasma hyaluronidase
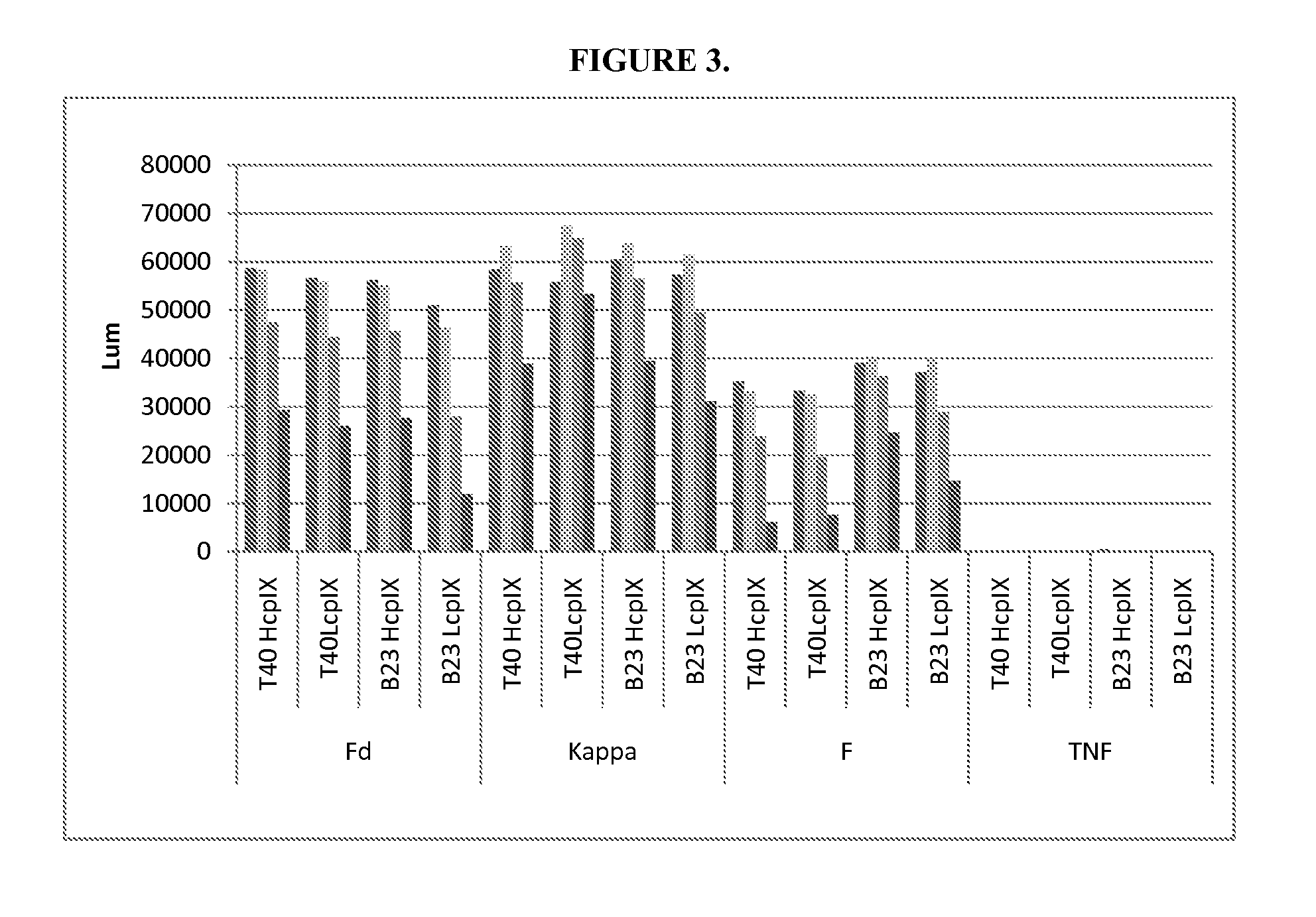
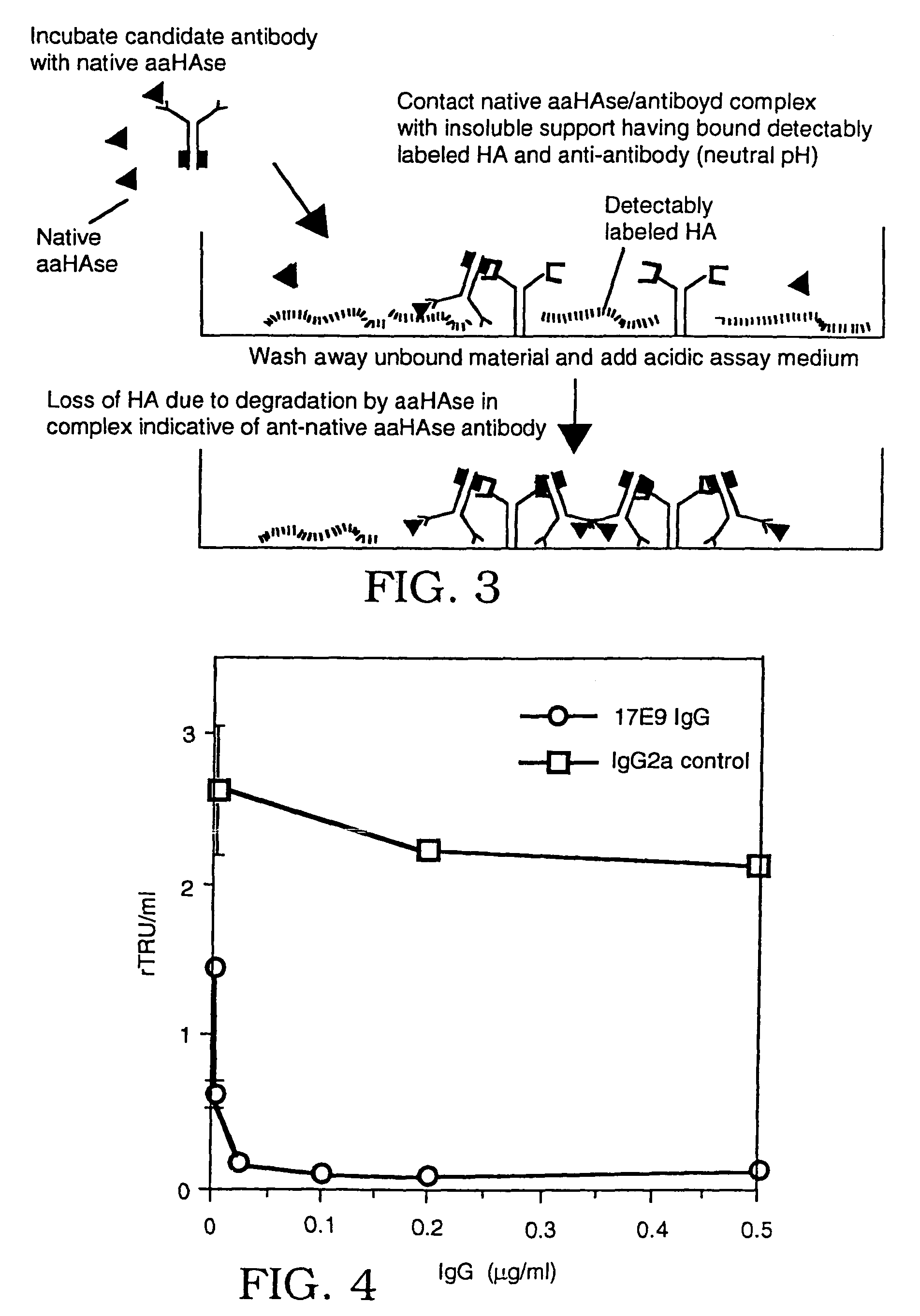
InactiveUS7105330B2Less likely to induceControl of level of activityPeptide/protein ingredientsMicroorganism based processesPurification methodsScreening method
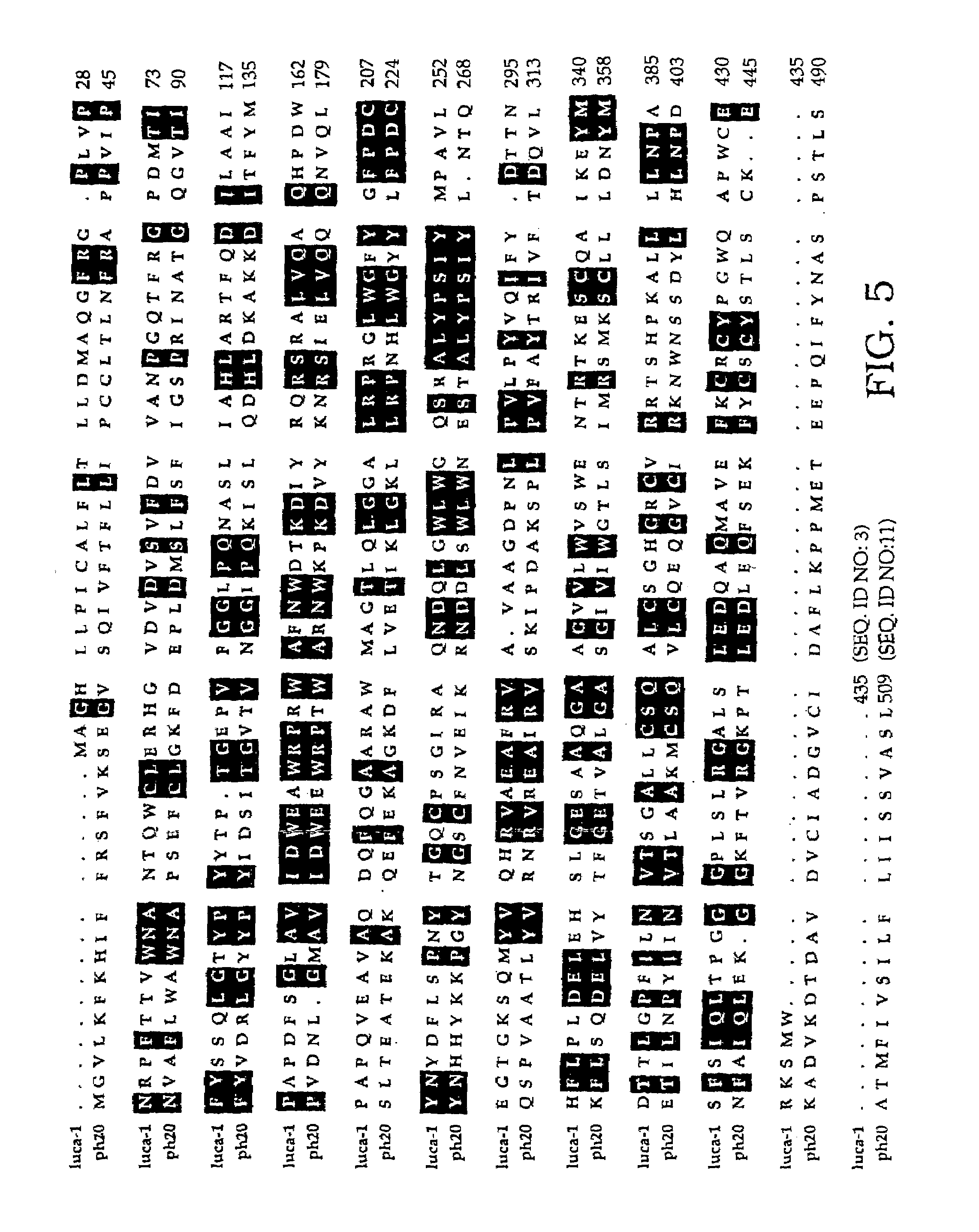
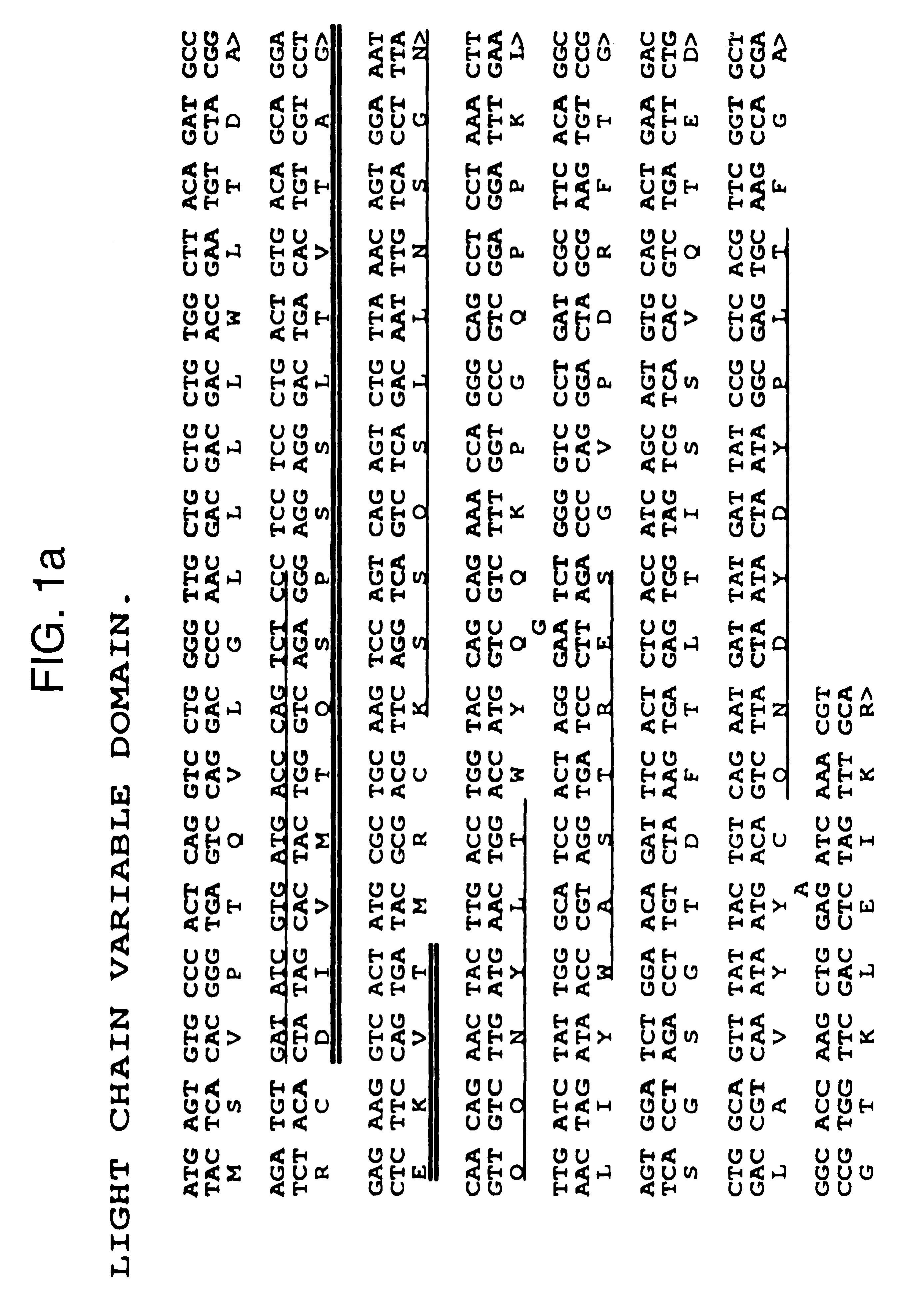
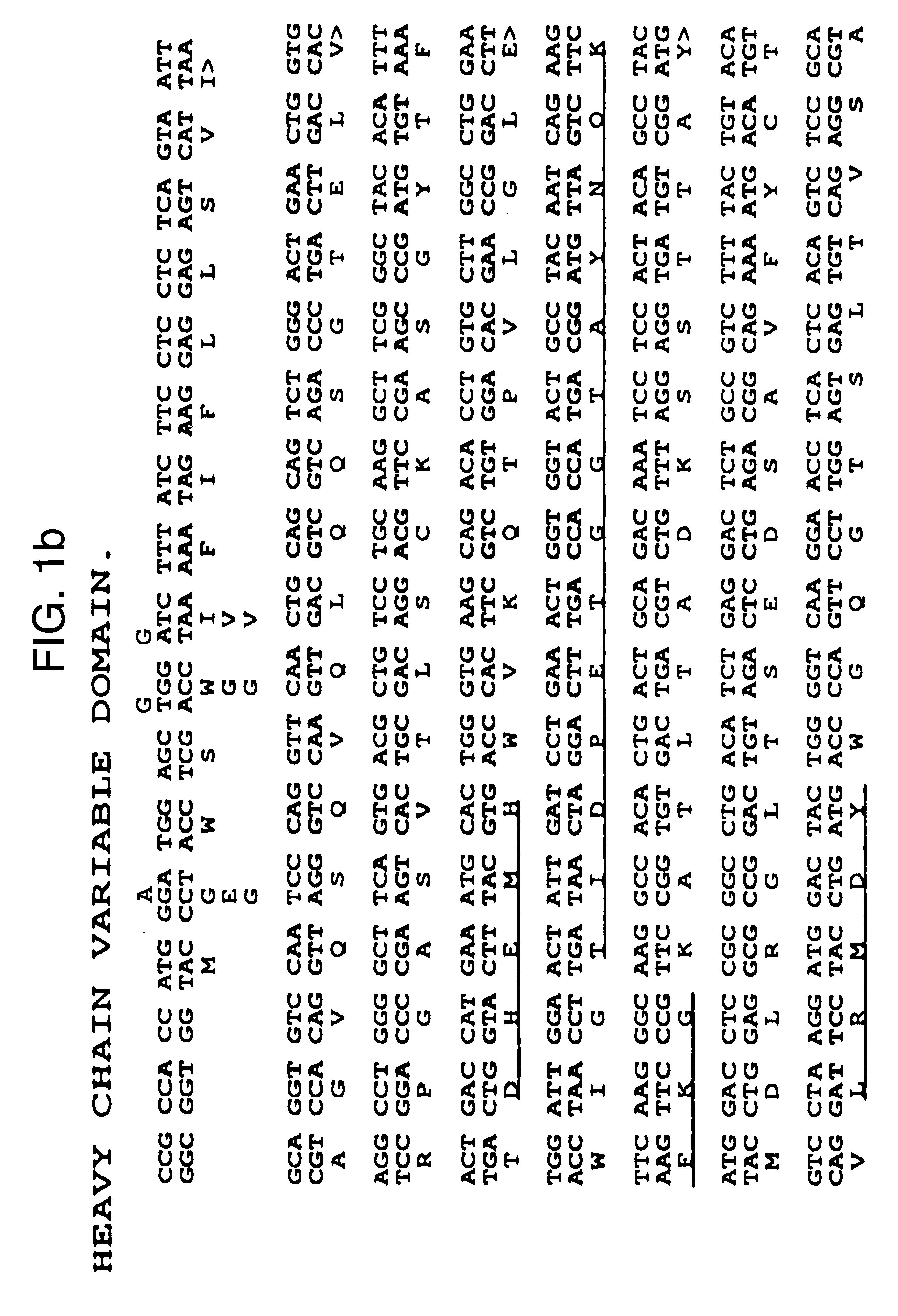
The invention is based on the discovery of methods for purification of an acid active hyaluronidase found in human plasma (hpHAse), including both biochemical and immunoaffinity purification methods. The method of immunoaffinity purification of the invention is based on the discovery of a method for identifying antibodies that specifically bind native hpHAse (anti-native hpHAse antibodies), and anti-native hpHAse antibodies identified by this screening method. The invention also features an assay for sensitive detection of HAse activity using biotinylated hyaluronic acid (bHA). Purification and characterization of hpHAse lead to the inventors' additional discovery that hpHAse is encoded by the LuCa-1 gene, which gene is present in the human chromosome at 3p21.3, a region associated with tumor suppression. The invention additionally features methods of treating tumor-bearing patients by administration of hpHAse and / or transformation of cells with hpHAse-encoding DNA.
Owner:RGT UNIV OF CALIFORNIA
Medicinal agent for treating erectile dysfunction
A medicament based on antibodies contains an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to endothelial nitric oxide synthase (NO synthase), the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to the homeopathic technology. A method of treating erectile dysfunctions and vegetative disturbances of male climax by regulating the level of cyclic guanosine monophosphate (cGMP) in the cavernous bodies on sexual stimulation, the method being characterized by the use of activated forms of ultra-low doses of antibodies to the entire molecule of the endothelial NO synthase or to its polypeptide fragments, activated forms being prepared by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Antibodies against E-selectin
InactiveUS6204007B1Avoid consumptionAlteration of antibody side chain interactionPeptide/protein ingredientsAntipyreticNatural antibodyFc(alpha) receptor
This invention relates to whole antibodies of neutral isotype having specificity for E-selectin, process for their preparation (using vectors), pharmaceutical compositions containing them, and their use in therapy and diagnosis. Said antibodies are variants of natural antibodies altered in the Fc region, especially in the CH2 domain, so that the interactions with antibodies Fc receptors and complement are very low.
Owner:CELLTECH R & D LTD
Method of correcting immunopathological reactions and a medicament
Owner:EPSHTEIN OLEG I
Novel method for the selection of specific affinity binders by homogeneous noncompetitive assay
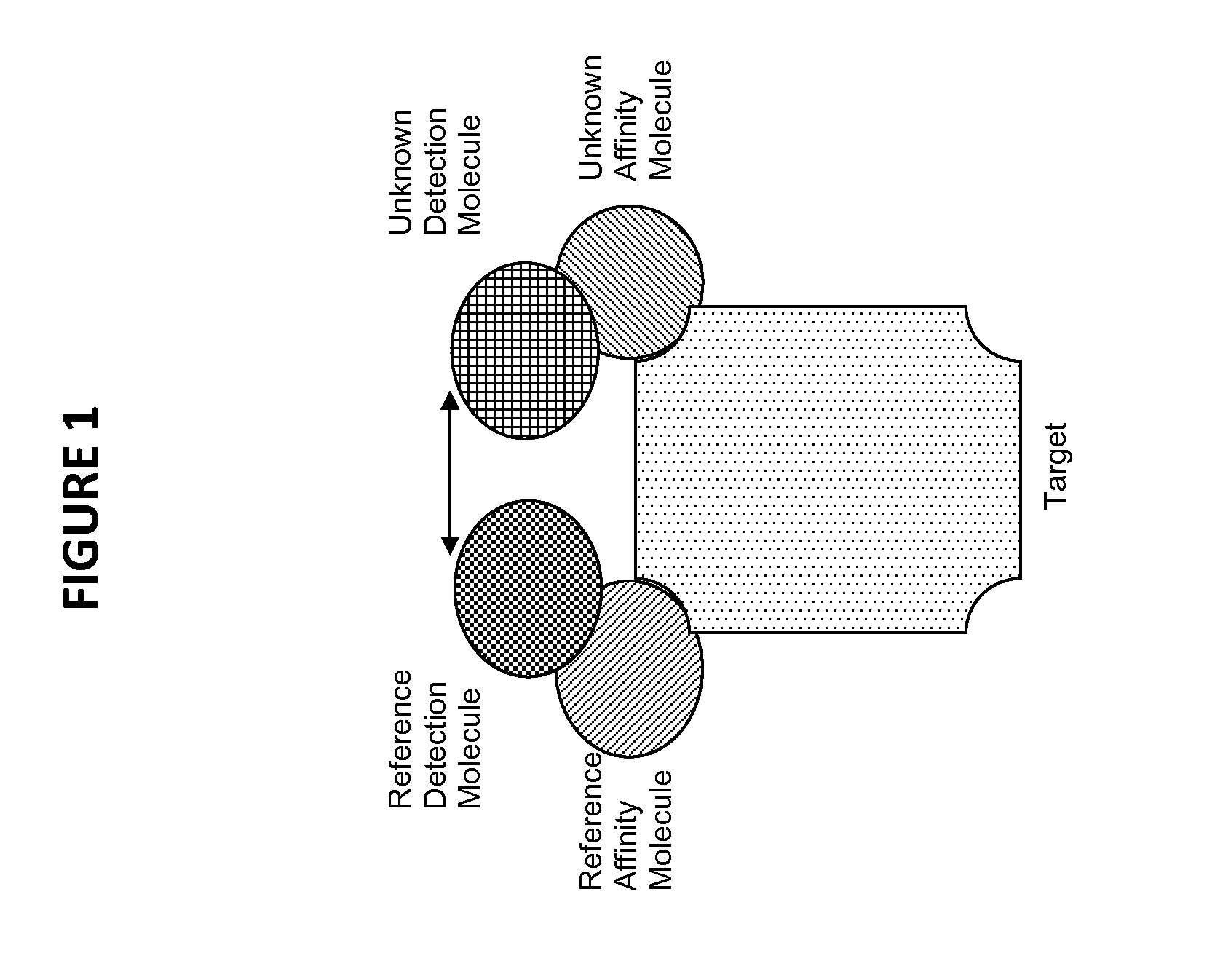
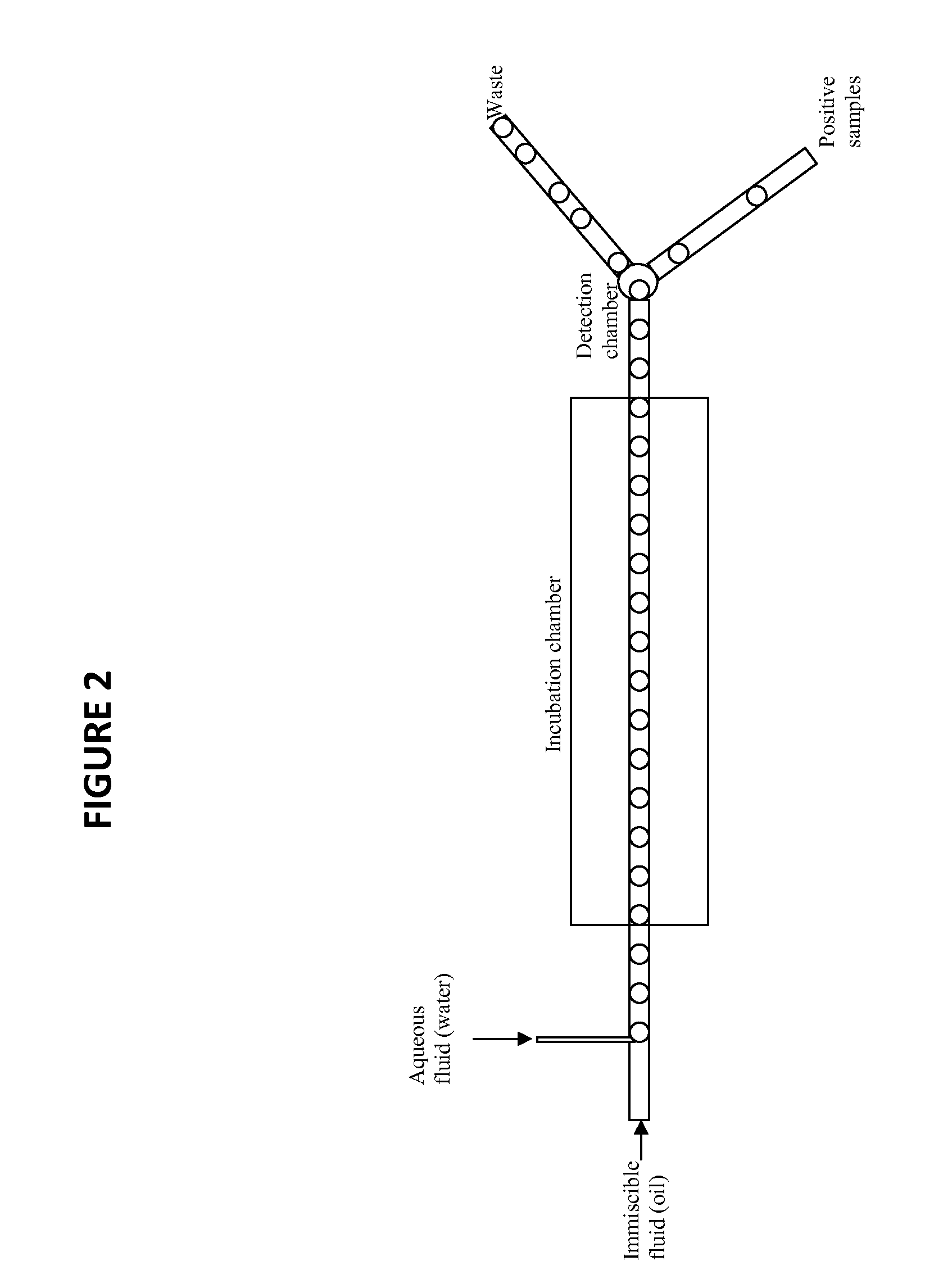
The invention generally relates to the field of immunochemistry including antibody therapy, diagnostics, and basic research and specifically relates to the area of selecting affinity molecules such as natural antibodies, including artificial antibodies, antibody mimics, and aptamers. The invention relates particularly to a method of selecting affinity molecules using a homogeneous noncompetitive assay in a high throughput process.
Owner:DYNAMIC AFFINITY REAGENTS
High-Yield Method For The Production Of Human Antibodies Blocking The Biological Activity Of A Human Cytokine
The invention concerns a pharmaceutical composition comprising, as the active ingredient, human natural antibodies of the IgG isotype, that neutralize the activity of a human cytokine selected from VEGF, IFNα, IL-4, TNFα and TGFβ, the said neutralizing antibodies inhibiting at least 50% of the maximum biological activity induced by an amount ranging from 0.006 ng to 0.05 ng of the said cytokine in vitro.
Owner:NEOVACS SA
Prosuction of antibodies or (functionalized) fragments thereof derived from heavy chain immunoglobulins of camelidae
InactiveUS7794981B2High catalytic activityImprove efficiencyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsNatural antibodyComplementarity determining region
A process is provided for the production of an antibody or a fragment or functionalized fragment thereof using a transformed lower eukaryotic host containing an example DNA sequence encoding the antibody or (functionalized) fragment thereof, wherein the antibody or (functionalized) fragment thereof is derived from a heavy chain immunoglobulin of Camelidae and is devoid of light chains, and wherein the lower eukaryotic host is a mould, preferably belonging to the genera Aspergillus or Trichoderma, or a yeast, preferably belonging to the yeast genera Saccharomyces, Kluyveromyces, Hansenula, or Pichia. The heavy chain fragment can contain at least the whole variable domain. A complementary determining region (CDR) different from the CDR belonging to the natural antibody ex Camelidae can be grafted on the framework of the variable domain of the heavy chain immunoglobulin. The catalytic antibodies can be raised in Camelidae against transition state molecules. The functionalized antibody or fragment thereof can comprise a fusion protein of both a heavy chain immunoglobulin from Camelidae or a fragment thereof and another polypeptide, e.g., an enzyme, preferably an oxido-reductase. Also provided are new products obtainable by a process as described, and compositions containing a product produced by a process as described, which composition may contain a new product as provided.
Owner:BAC IP
Medicinal agent and method for curing erectile dysfunction
A medicament based on antibodies contains an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to endothelial nitric oxide synthase (NO synthase), the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to the homeopathic technology. A method of treating erectile dysfunctions and vegetative disturbances of male climax by regulating the level of cyclic guanosine monophosphate (cGMP) in the cavernous bodies on sexual stimulation, the method being characterized by the use of activated forms of ultra-low doses of antibodies to the entire molecule of the endothelial NO synthase or to its polypeptide fragments, activated forms being prepared by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
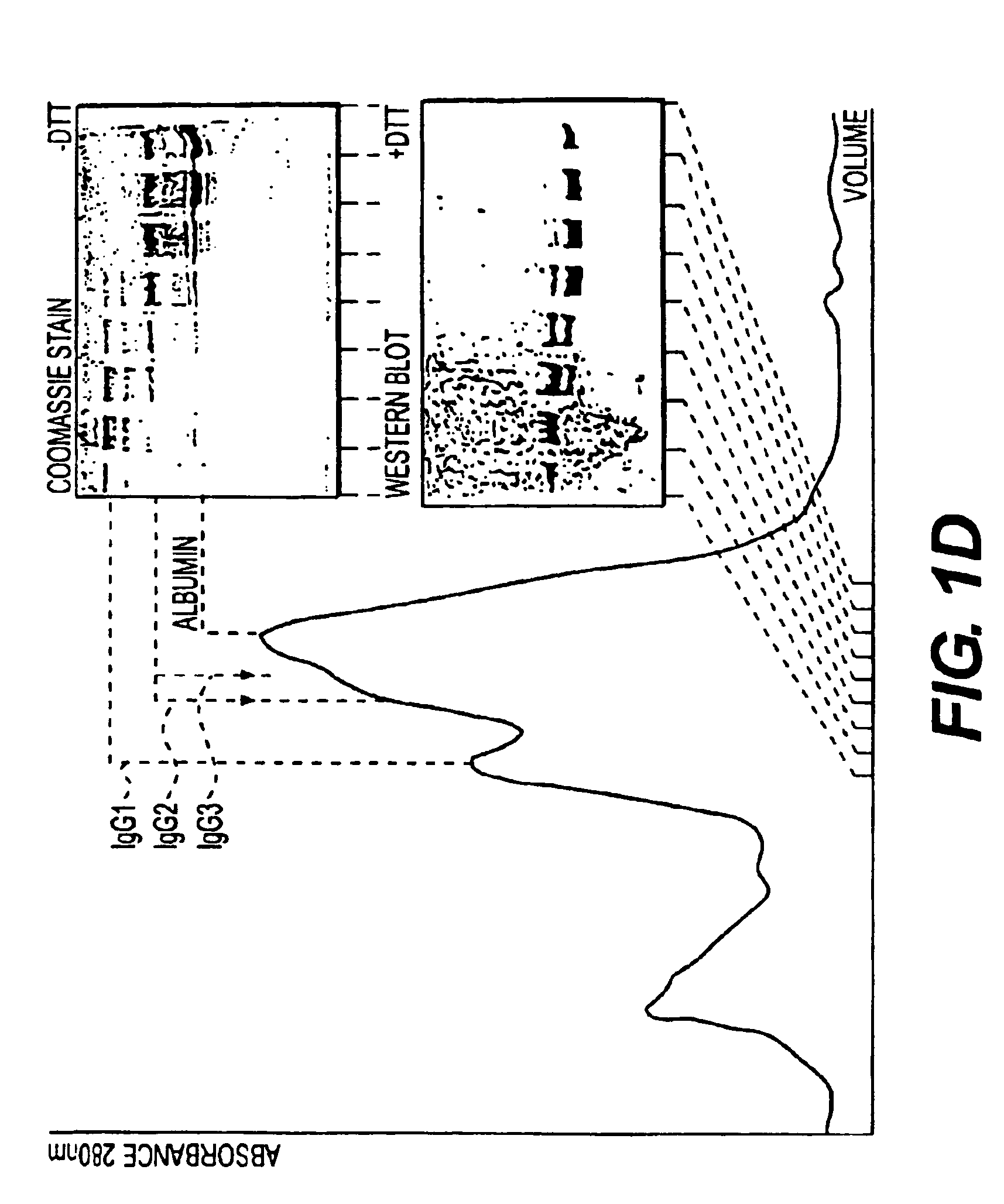
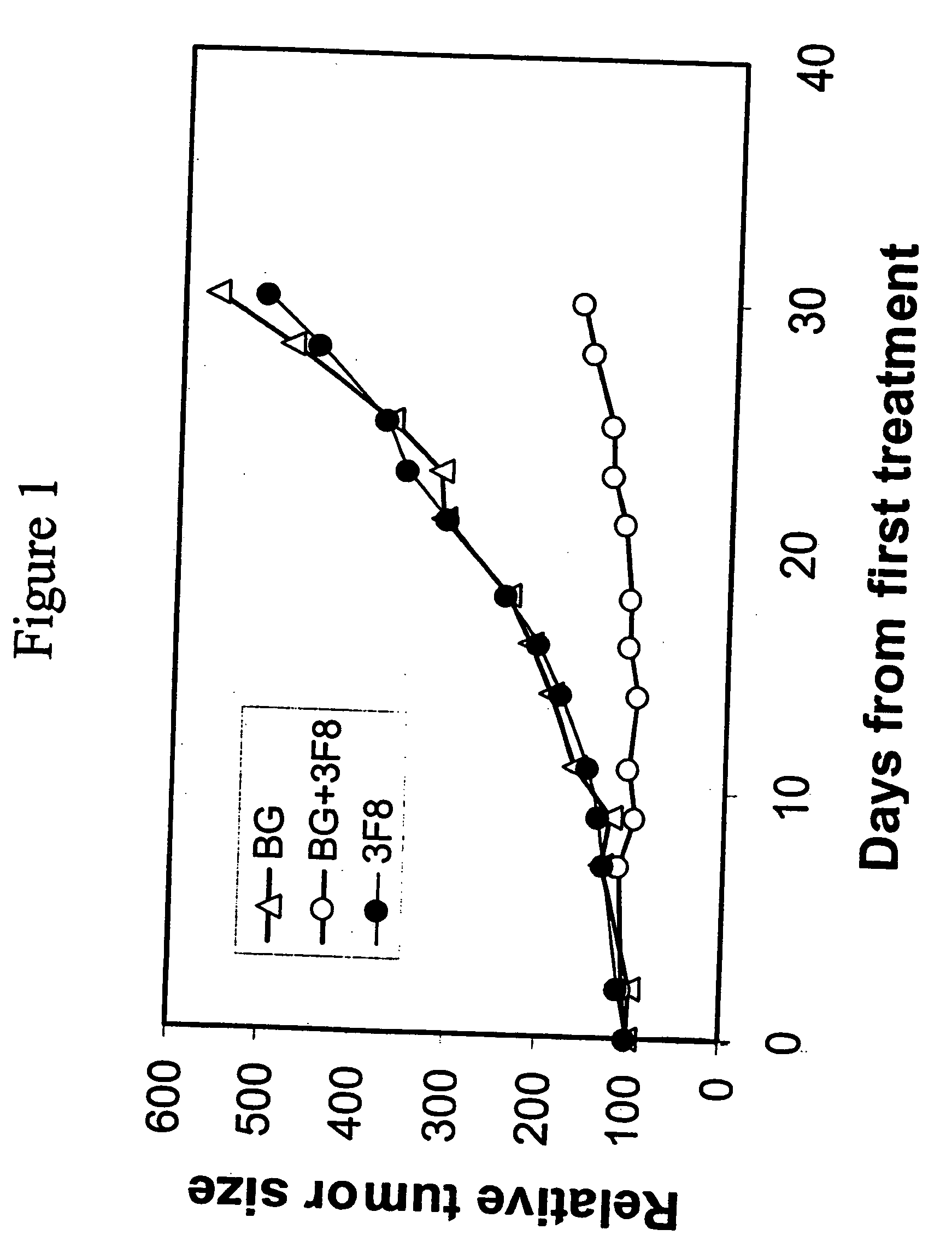
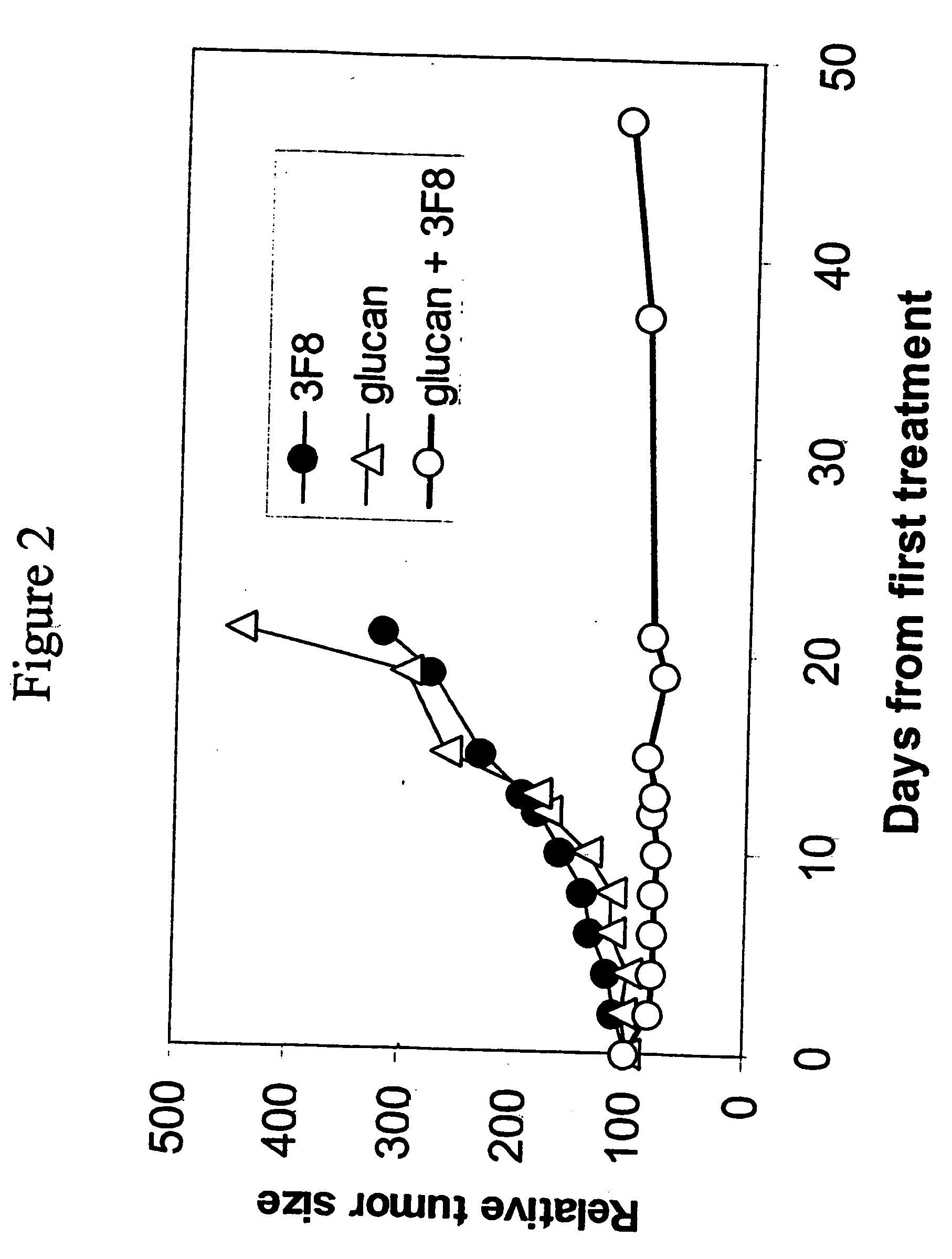
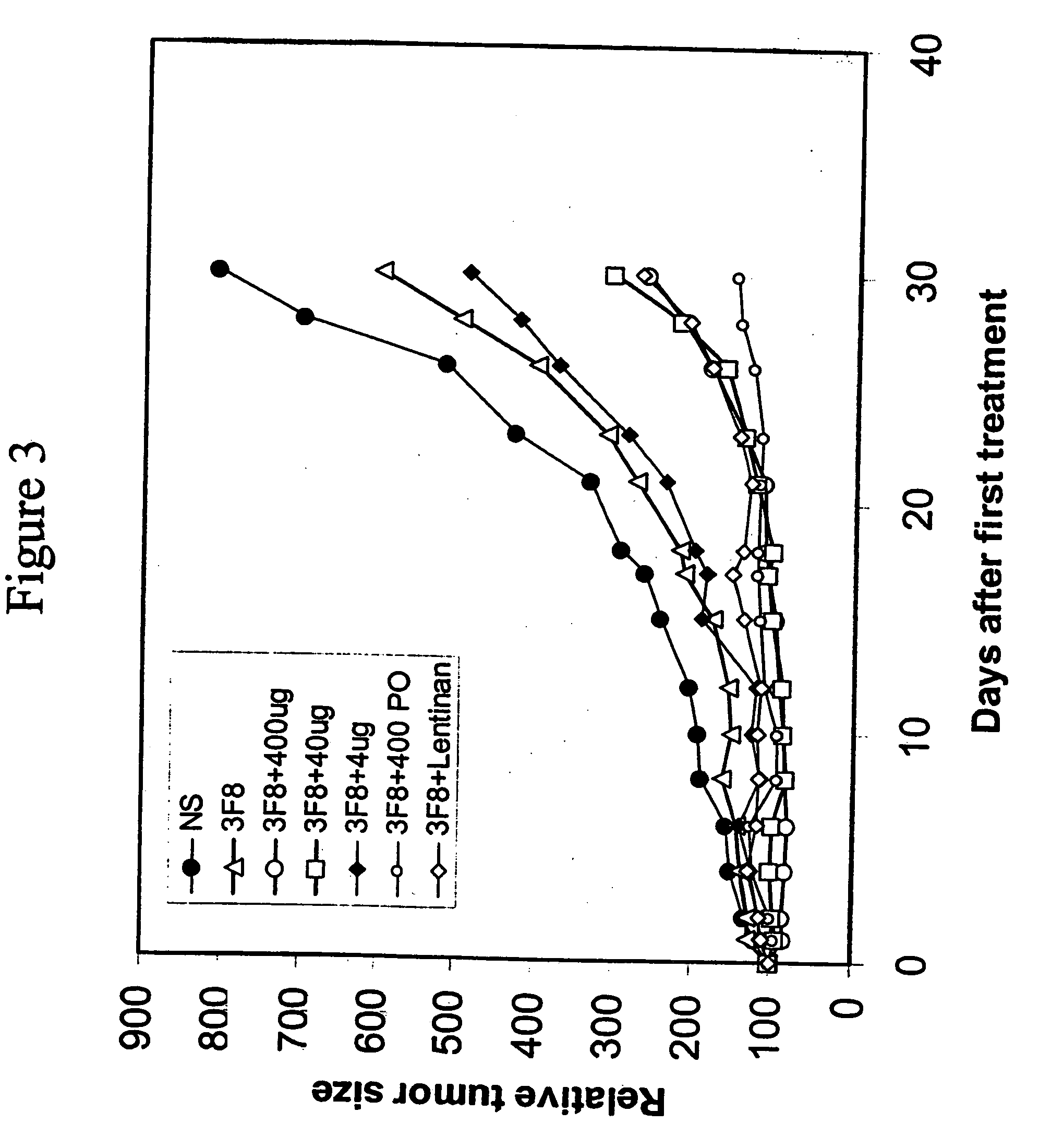
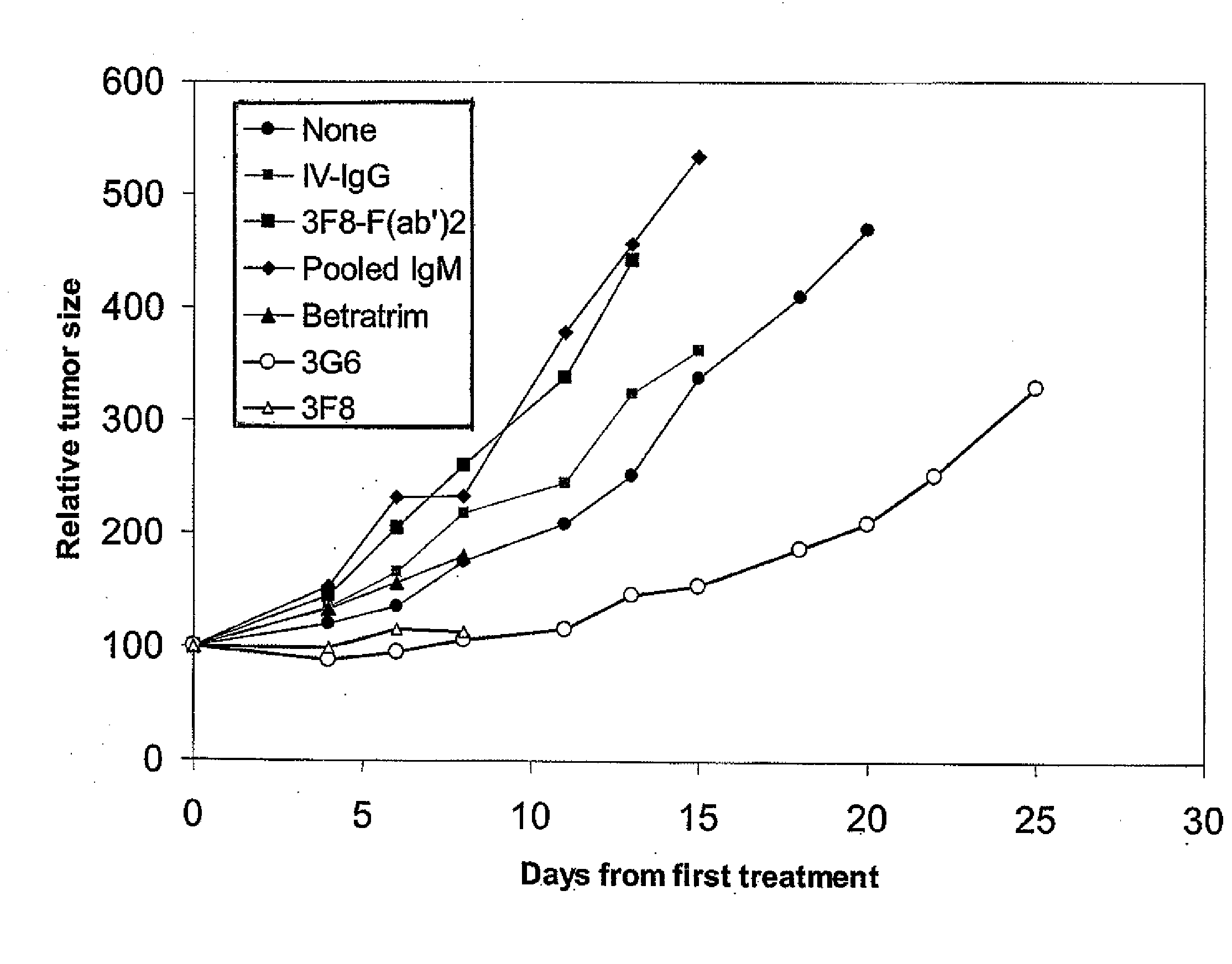
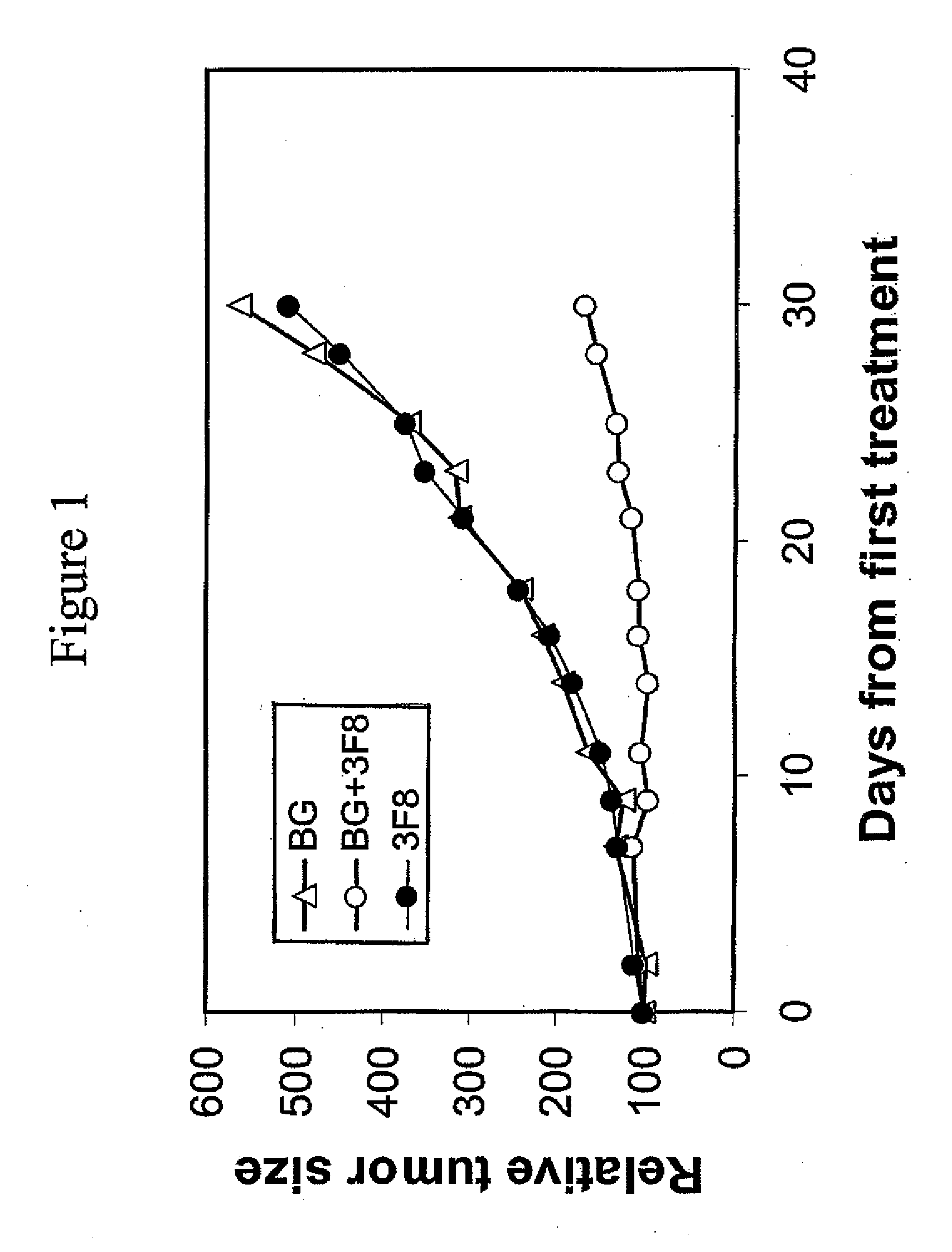
Therapy-enhancing glucan
This invention provides a composition comprising an effective amount of glucan capable of enhancing efficacy of antibodies. This invention further provides the above compositions and a pharmaceutically acceptable carrier. This invention also provides a method for treating a subject with cancer comprising administrating the above-described composition to the subject. This invention provides a composition comprising effective amount of glucan capable of enhancing efficacy of vaccines. This invention also provides a method of treating a subject comprising administrating the above pharmaceutical composition to the subject. This invention provides a composition comprising effective amount of glucan capable of enhancing efficacy of natural antibodies. This invention provides a composition comprising effective amount of glucan capable of enhancing host immunity. This invention also provides a composition comprising effective amount of glucan capable of enhancing the action of an agent in preventing tissue rejection.
Owner:SLOAN KETTERING INST FOR CANCER RES
Magnetic molecularly imprinted polymer-fluorescence analysis method
ActiveCN103558203AAchieve "class-specific" adsorptionMagnetic features are obviousPreparing sample for investigationFluorescence/phosphorescenceNatural antibodyMagnetite Nanoparticles
The invention discloses a magnetic molecularly imprinted polymer-fluorescence analysis method, relates to a rapid detection of pesticides, and aims to solve the problem of difficulty in preparation of small molecular natural antibody in the conventional quick detection method. The method comprises the following steps: 1, preparing polyethylene glycol and oleic acid-coated magnetic nanoparticles; 2, preparing a magnetic molecularly imprinted polymer of triazine pesticides; 3, determining a fluorescence signal by using a fluorescence spectrophotometer. The common natural antibody which is difficult to prepare in the normal quick detection method is substituted by using a magnetic molecularly imprinted biomimetric material; quick detection of a non-immune method of the triazinemicromoleuclar pesticides is technically realized through competitive combination of the pesticide molecules and the fluorescence probe on the magnetic imprinting. The method is used for quickly detecting the residue of the triazine pesticides.
Owner:BEIJING PURKINJE GENERAL INSTR
Method for correcting immune respones and medical agent
The principle of the present invention is the use of an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to cytokines implicated in the course of inflammation, including autoimmune inflammation, the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to the homeopathic technology. It is possible to use a mixture of activated forms of antibodies to various cytokines involved in the course of the inflammatory process. In addition, employed as a medicament for correcting pathologic immune reactions based on antibodies to the tumor necrosis factor alpha (TNF-α) can be an activated form of antibodies to TNF-α obtained by multiple consecutive dilutions and exposure to external factors; activation preferably being performed in accordance with the homeopathic technology, and the forms preferably being applied in a mixture of various, predominantly centesimal, homeopathic dilutions.
Owner:EPSHTEIN OLEG I
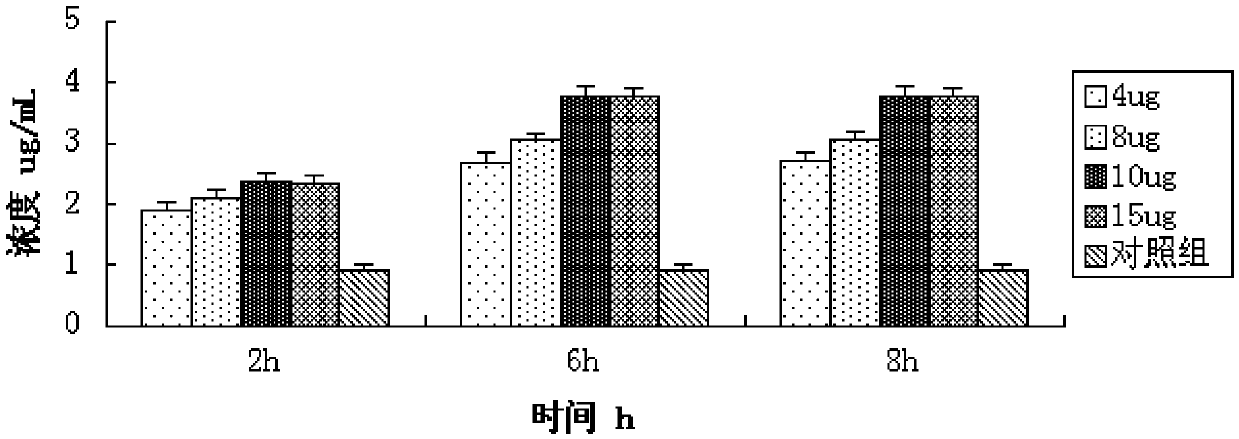
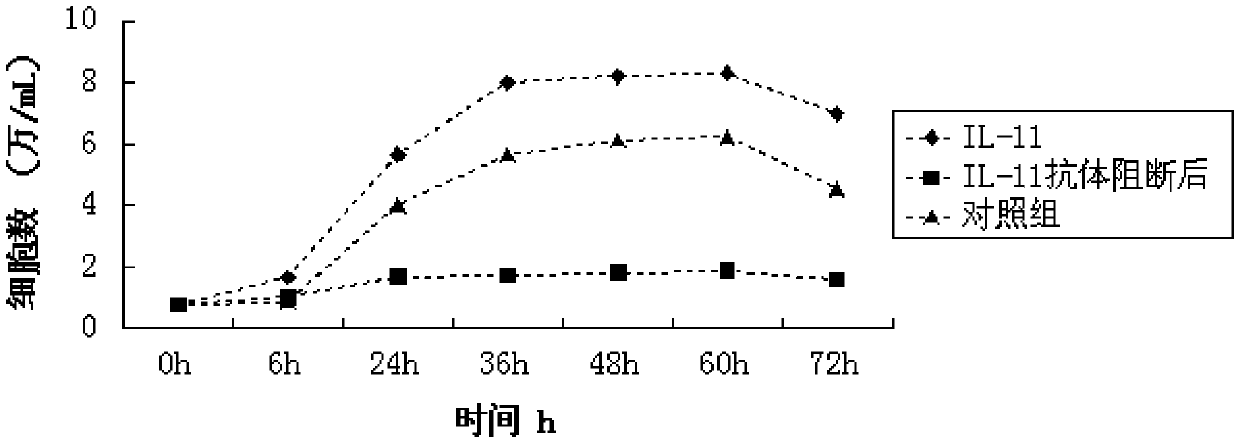
Application of IL-11 monoclonal antibody in inhabiting PI3K/Akt signal channel
ActiveCN105497893AInhibit expressionSmall toxicityAntibody ingredientsImmunoglobulinsNatural antibodySide effect
The invention discloses the application of an IL-11 monoclonal antibody in inhabiting a PI3K / Akt signal channel. The concentration of the IL-11 monoclonal antibody is 8 mug / mL, and the dosage of the IL-11 monoclonal antibody is 10 mug. The safe and efficient PI3K / Akt signal channel inhibitor, namely the IL-11 monoclonal antibody is found, and can be specifically combined with IL-11 secreted by cells to induct PTEN transcription, up-regulate PTEN expression and inhibit the expression of AKT on the downstream portion of the PI3K / Akt signal channel, and then the whole signal channel is inhibited. The inhibitor is a natural antibody, so that toxic and side effects are reduced and safety and efficiency are high.
Owner:HEILONGJIANG HEIKE TECH CO LTD
Prosuction of antibodies or (functionalized) fragments thereof derived from heavy chain immounogobulins of camelidae
InactiveUS20050130266A1Improve physical stabilityImprove efficiencyPolypeptide with localisation/targeting motifFungiNatural antibodyComplementarity determining region
A process is provided for the production of an antibody or a fragment or functionalized fragment thereof using a transformed lower eukaryotic host containing an example DNA sequence encoding the antibody or (functionalized) fragment thereof, wherein the antibody or (functionalized) fragment thereof is derived from a heavy chain immunoglobulin of Camelidae and is devoid of light chains, and wherein the lower eukaryotic host is a mould, preferably belonging to the genera Aspergillus or Trichoderma, or a yeast, preferably belonging to the yeast genera Saccharomyces, Kluyveromyces, Hansenula, or Pichia. The heavy chain fragment can contain at least the whole variable domain. A complementary determining region (CDR) different from the CDR belonging to the natural antibody ex Camelidae can be grafted on the framework of the variable domain of the heavy chain immunoglobulin. The catalytic antibodies can be raised in Camelidae against transition state molecules. The functionalized antibody or fragment thereof can comprise a fusion protein of both a heavy chain immunoglobulin from Camelidae or a fragment thereof and another polypeptide, e.g., an enzyme, preferably an oxido-reductase. Also provided are new products obtainable by a process as described, and compositions containing a product produced by a process as described, which composition may contain a new product as provided.
Owner:BAC IP
Method of treating a pathological syndrome and a pharmaceutical agent
A method of treating a pathological syndrome includes administration of an activated form of ultra-low doses of antibodies to an antigen, wherein said activated form is obtained by repeated consecutive dilution combined with external impact, and the antigen is a substance or a pharmaceutical agent exerting influence upon the mechanisms of formation of this particular pathological syndrome. Pharmaceutical agent for treating a pathological syndrome contains activated form of ultra-low doses of monoclonal, polyclonal or natural antibodies to an antigen, wherein said activated form is prepared by means of repeated consecutive dilution and external treatment, predominantly based on homeopathic technology, and said antigen is a substance or a drug acting as a direct cause of the pathological syndrome or involved in regulation of mechanisms of its formation. At that, activated forms of ultra-low doses of antibodies are raised against antigens of exogenous or endogenous origin, against autologous antigens, fetal antigens; anti-idiotypic antibodies are used too.
Owner:EPSHTEIN OLEG I
Natural IgM antibodies and inhibitors thereof
ActiveUS20050276811A1Improve bioavailabilityProlong half-life in vivoPeptide/protein ingredientsAntipyreticNatural antibodyDisease
The invention provides natural IgM antibody inhibitors that may be used to treat various inflammatory diseases or disorders.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +2
C-reactive protein imprinted polymer film and microchip system utilizing the same
ActiveUS20130156645A1Well formedImproves imprinting substrateComponent separationLayered productsNatural antibodyCapacitance
The present invention is a C-reactive protein imprinted polymer film. The C-reactive protein antibody imprinted polymer film comprises a plurality of imprinted nanocavities with unified orientation and distribution formed by removing a plurality of C-reactive proteins from a polymer film. Its ability to capture the target proteins can achieve 99% compared with the natural antibodies. The present invention further provides a C-reactive protein microchip system formed by the dynamic capacitance sensing method with the above imprinted polymer film. The C-reactive protein microchip system comprises a body having a first chamber and a second chamber, and a detector.
Owner:MACKAY MEMORIAL HOSPITAL +1
Staphylococcal enterotoxin micromolecule antibody and its preparation method and use
InactiveCN103224561AImmunoglobulins against bacteriaVector-based foreign material introductionDisulfide bondingNatural antibody
The invention discloses a staphylococcal enterotoxin micromolecule antibody and its preparation method and use. The staphylococcal enterotoxin micromolecule antibody has an amino acid sequence shown in the formula of SEQ ID No.1 in the sequence table. The preparation method comprises the following steps of designing and constructing eukaryotic mono-promoter co-expression vectors of staphylococcal enterotoxin monoclonal antibody light chain and heavy chain variable region genes, introducing cysteine residues to C- ends of the light chain and the heavy chain to form interchain disulfide bonds by an intracellular peptide fragment self-assembling principle, and simulating antigen binding domain spatial conformation of natural antibodies to obtain high-efficiency expression stable-secretion mammal cell line and the high-singularity strong-affinity micromolecule antibody. The staphylococcal enterotoxin micromolecule antibody can be used for staphylococcal enterotoxin detection, cell indirect immunofluorescence detection and flow cytometry detection.
Owner:TIANJIN UNIV
Humanized anti-IL-1beta antibodies
ActiveUS7541033B2Improve stabilityAvoid destructionNervous disorderAntipyreticNatural antibodyDisease
Owner:ELI LILLY & CO
Anti-pd-l1/Anti-pd-1 natural antibody structure-like heterodimeric bispecific antibody and preparation thereof
ActiveUS20200299412A1Highly effective and specific killing effectLow side effectsHybrid immunoglobulinsPeptide/protein ingredientsNatural antibodyHeterologous
Provided are an anti-PD-L1 / anti-PD-1 natural antibody structure-like heterodimeric bispecific antibody and a preparation thereof. In particular, provided are a highly stable heterodimeric anti-PD-L1 / anti-PD-1 bispecific antibody with characteristics of a natural IgG and without mismatches heavy chain-light chain, and a preparation thereof. The bispecific antibody can bind to both target molecules and is more effective in treating a complex disease.
Owner:BEIJING HANMI PHARMA CO LTD
Antibodies against E-selectin
InactiveUS6407214B1Avoid consumptionAlteration of antibody side chain interactionPeptide/protein ingredientsAntipyreticFc(alpha) receptorNatural antibody
This invention relates to whole antibodies of neutral isotype having specificity for E-selection, process for their preparation (using vectors), pharmaceutical compositions containing them, and their use in therapy (e.g. for inflammatory disorders) and diagnosis. Said antibodies are variants of natural antibodies altered in the Fc region, especially in the CH2 domain, so that the interactions with antibodies Fc receptors and complement are absent or very low.
Owner:CELLTECH R & D LTD
Medicinal agent and method for curing prostate diseases
InactiveUS20050266007A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsNatural antibodyHeifer calf
A medicament based on antibodies contains an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to the prostate-specific antigen, the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to homeopathic technology. In order to obtain the antibodies, the prostate-specific antigen isolated from the prostatic tissues of cattle or prepared synthetically is employed; a mixture of various, mostly centesimal, homeopathic dilutions is used. The method of treating diseases of the urogenital sphere consists in using activated forms of ultra-low doses of antibodies to prostate-specific antigen prepared by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Evolved immunoglobulin binding molecule, and its preparation method and uses
InactiveCN1966526ACombined broad spectrumImprove bindingHybrid immunoglobulinsBacteriaNatural antibodyGenetic engineering
The invention relataes to novel evolutionary immunoglobulin binding molecules, as well as their preparation methods and applications. The invention discloses separated evolutionary immunoglobulin binding molecules, which are proteins with amino acid sequences as shown by SEQ ID NO : 1, or conservative variant proteins with immunoglobulin binding activity. The invention also discloses the gene encoding, genetic engineering preparation methods and applications of immunoglobulin binding molecules. The disclosed immunoglobulin molecule broad-spectrum combined with various immuneglobulin shows high immunoglobulin whole molecule binding activity, and can be used in large-scale purification of genetic engineering antibodies, purification of natural antibodies and monoclonal antibodies, enzyme-linked immunosorbent assay, and immuno-chromatography and immunohistochemical methods for immune antibody detection and diagnosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
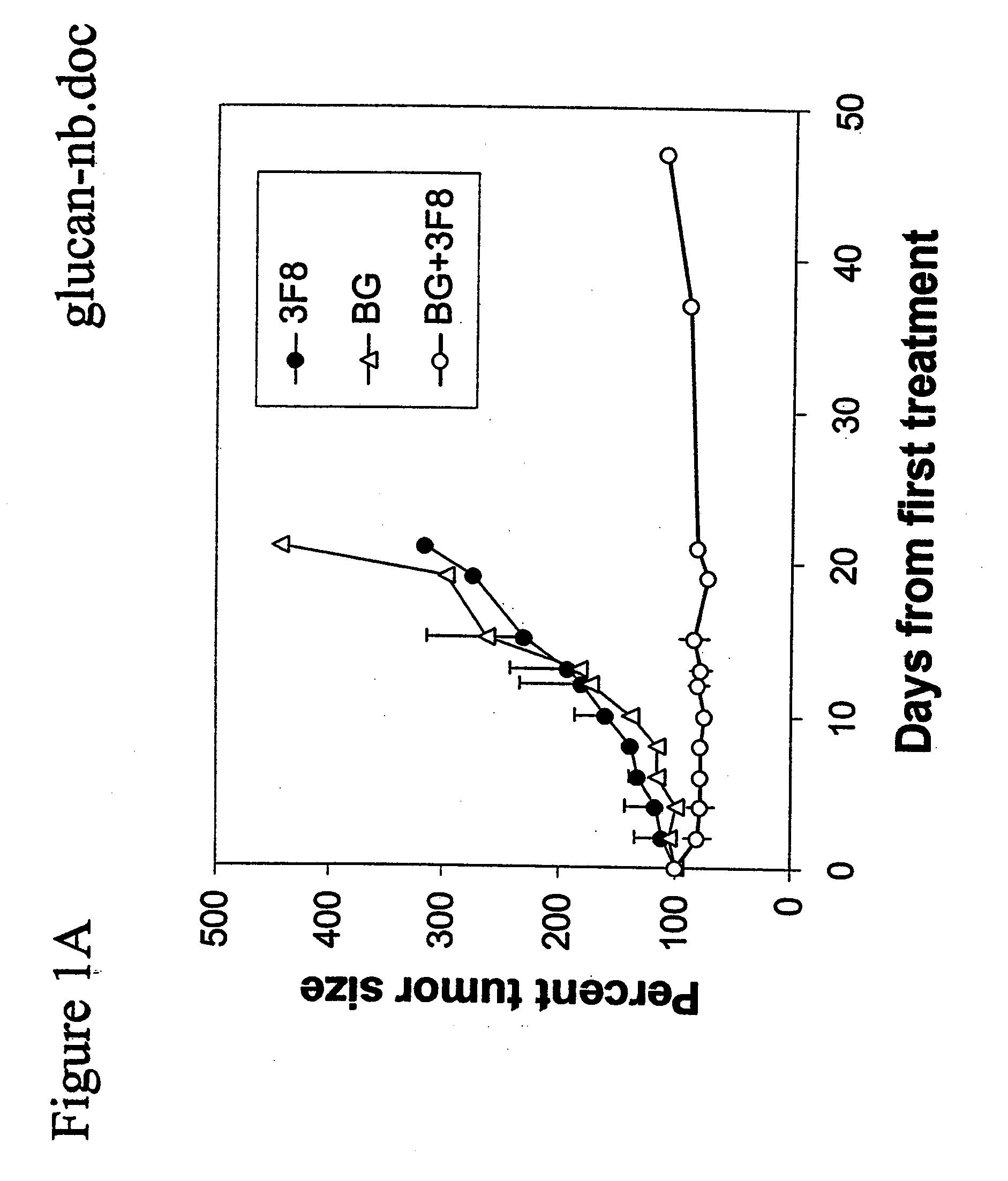
Therapy-enhancing glucan
This invention provides a composition comprising an effective amount of glucan capable of enhancing efficacy of antibodies. This invention further provides the above compositions and a pharmaceutically acceptable carrier. This invention also provides a method for treating a subject with cancer comprising administrating the above-described composition to the subject. This invention provides a composition comprising effective amount of glucan capable of enhancing efficacy of vaccines. This invention also provides a method of treating a subject comprising administrating the above pharmaceutical composition to the subject. This invention provides a composition comprising effective amount of glucan capable of enhancing efficacy of natural antibodies. This invention provides a composition comprising effective amount of glucan capable of enhancing host immunity. This invention also provides a composition comprising effective amount of glucan capable of enhancing the action of an agent in preventing tissue rejection.
Owner:SLOAN KETTERING INST FOR CANCER RES
Rhesus monkey erythrocyte adsorber
ActiveCN106110421ASave bloodEasy to store regularlyOther blood circulation devicesMedical devicesNatural antibodyRed blood cell
The invention relates to a rhesus monkey erythrocyte adsorber, belonging to the field of medicine science. According to the rhesus monkey erythrocyte adsorber, rhesus monkey erythrocytes replace human Rh positive O erythrocytes, the rhesus monkey erythrocytes are washed by 4 DEG C normal saline and 37 DEG C normal saline, so that immune antibodies or natural antibody attaching to the surfaces of the erythrocytes can be removed, after antigenicity detection, cell sediment is added into a cylindrical adsorber made of a material with high biocompatibility till 4 / 5 of the volume is occupied, then 3.5% of mannitol erythrocyte preserving fluid is added, so that the concentration of the erythrocyte achieves 80%, shaking is carried out gently for uniform mixing, the opening is sealed, and the product is stored at 4 DEG C for regular use, wherein a screen is arranged at the opening of a purifier, so that a line of defense for preventing cell debris from being filtered out is formed, after the plasma is filtered, Rh antibodies are combined with Rh positive rhesus monkey erythrocytes to form a fixed compound, the broken erythrocyte debris and the macromolecular compound combined with the broken erythrocyte debris are blocked by the screen of the purifier, and the plasma with morbid substance removed is filtered by the purifier and then conveyed back, so that the purpose of treating blood-group-incompatible haemolytic diseases is achieved by eliminating Rh antibodies of the plasma.
Owner:ATTACHED OBSTETRICS & GYNECOLOGY OSPITAL MEDICALCOLLEGE ZHEJIANG UNIV +1
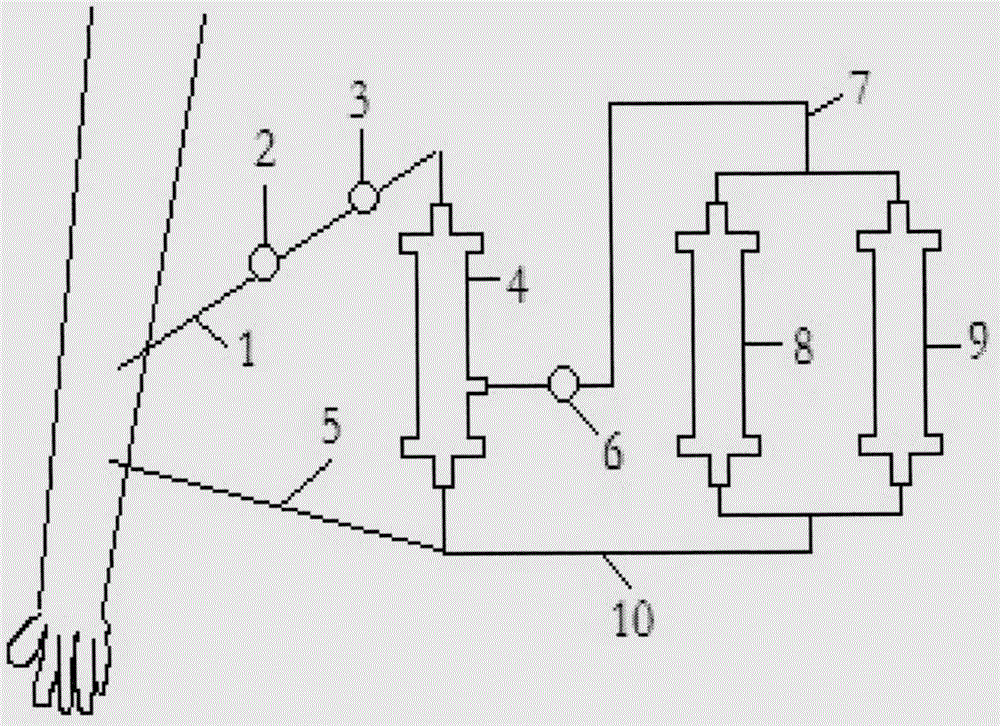
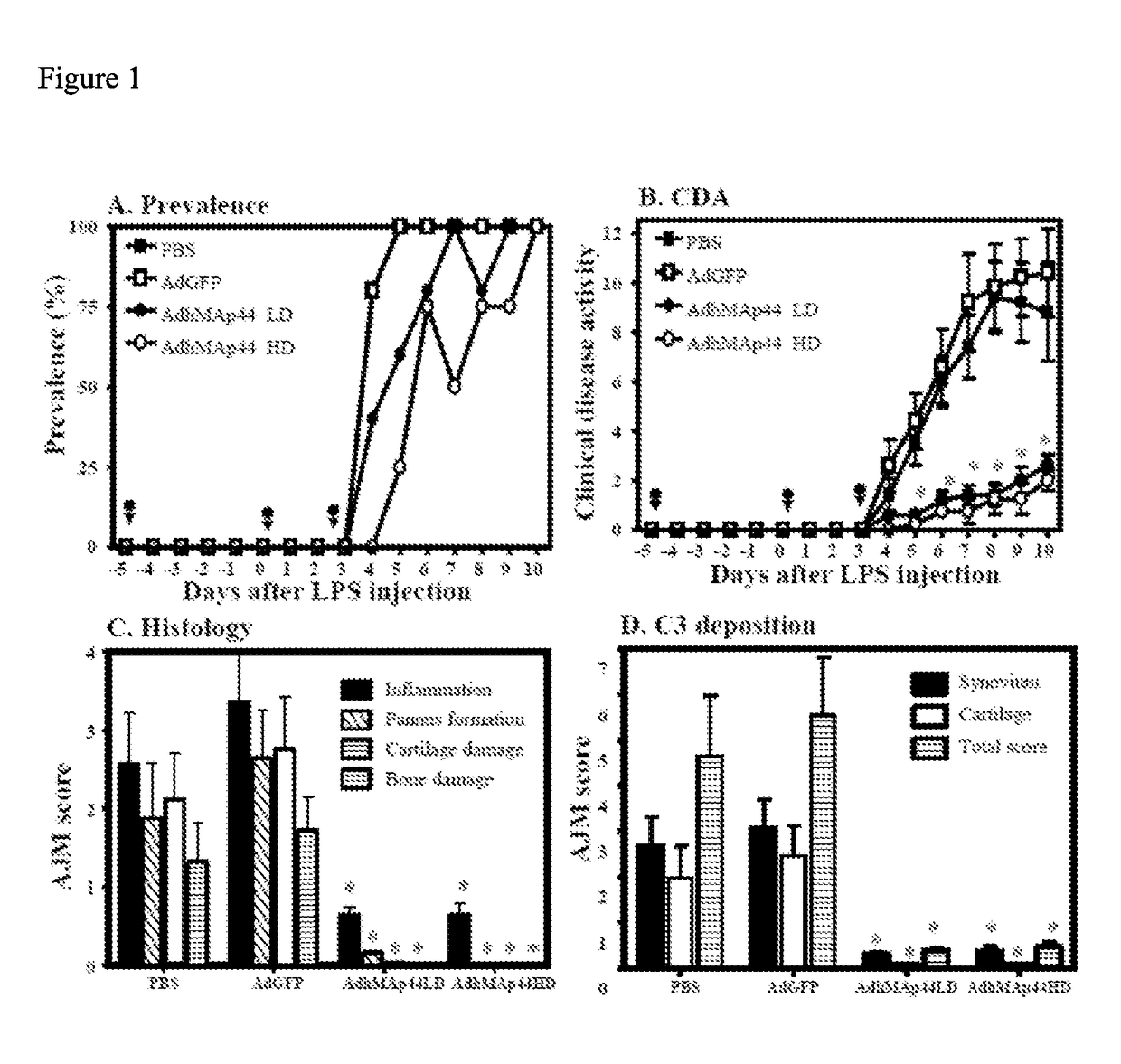
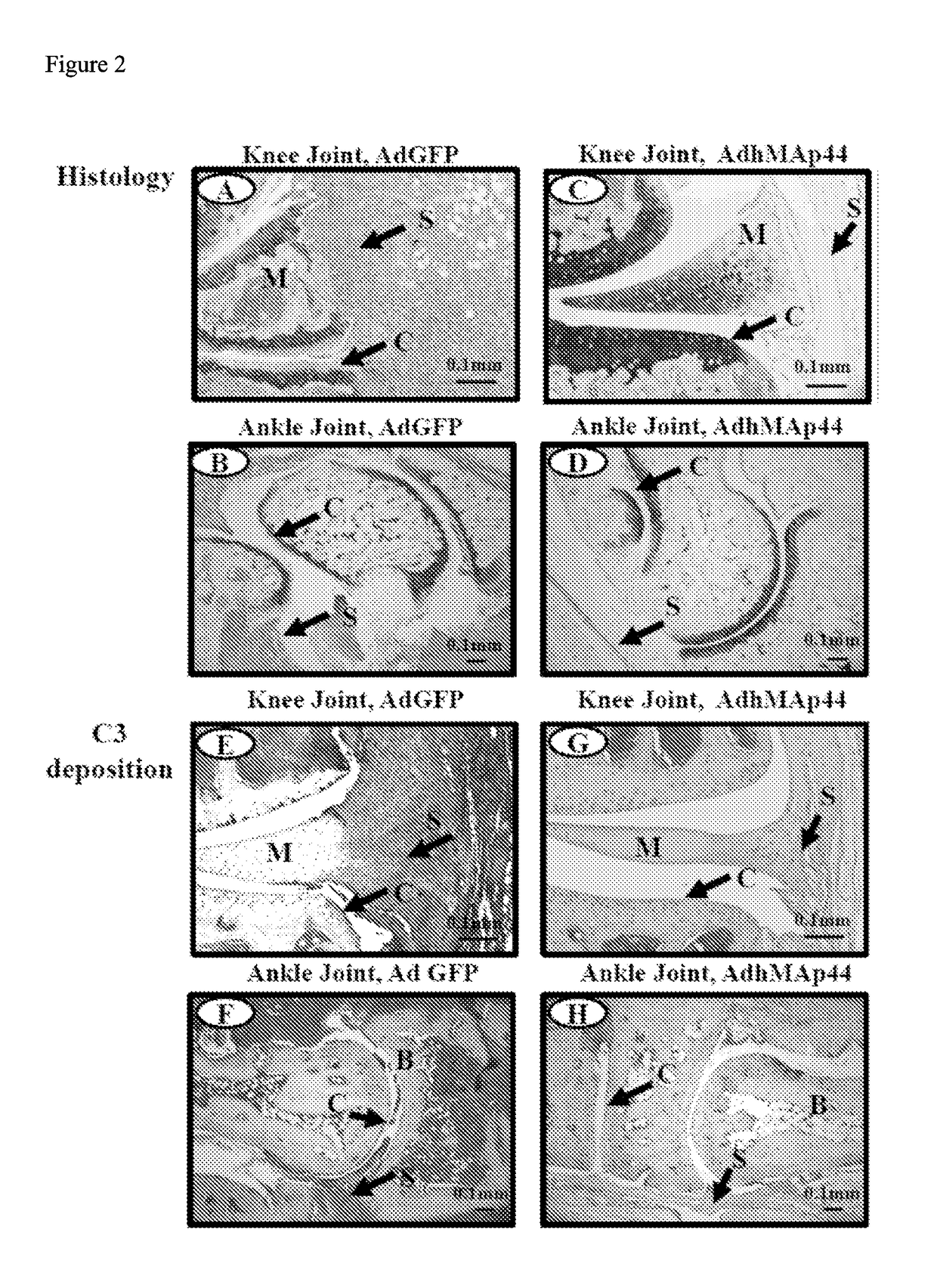
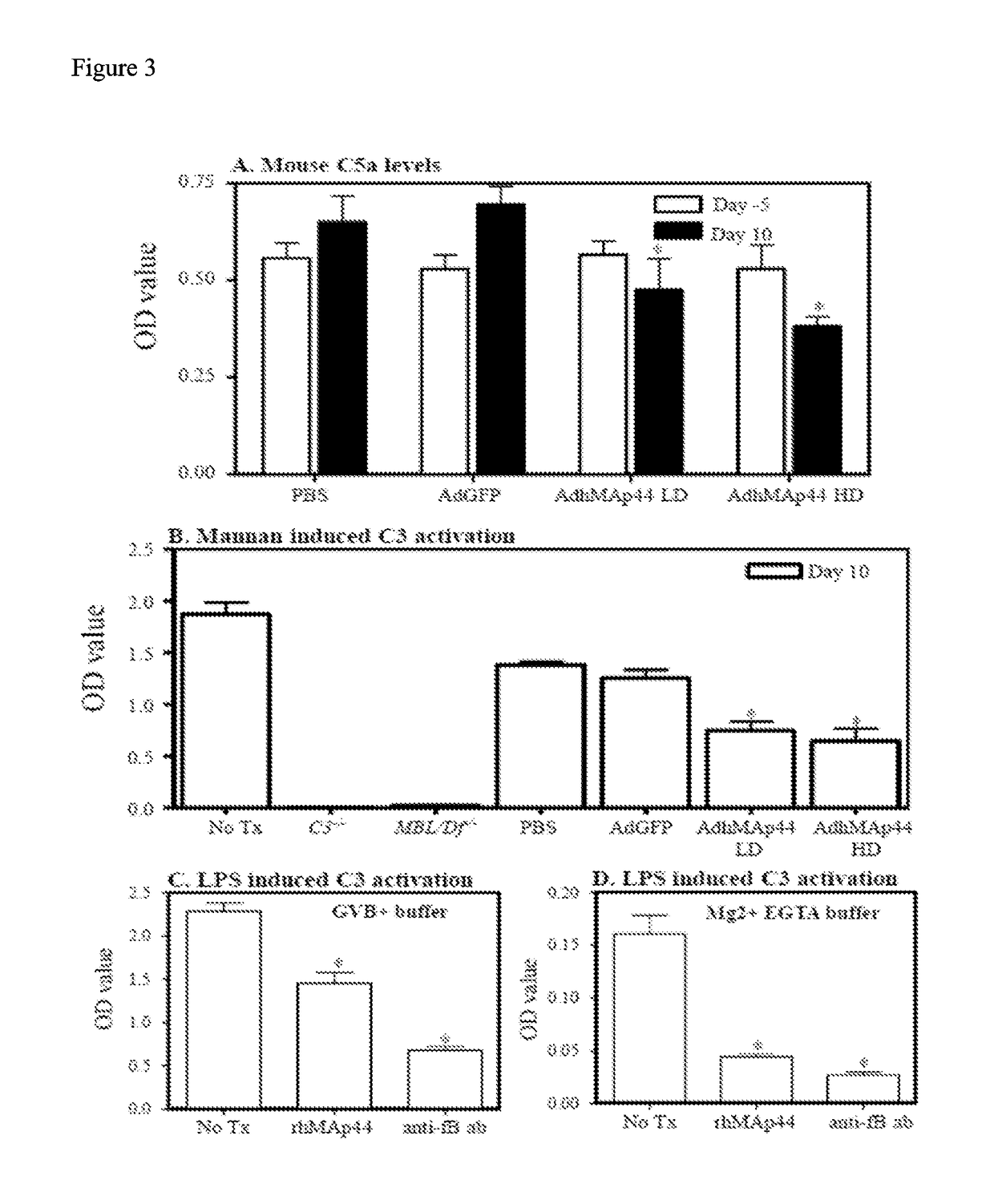
Map44 polypeptides and constructs based on natural antibodies and uses thereof
The present invention provides delivery methods and constructs for treating inflammatory diseases in an individual. The targeted delivery approach utilizes an antibody that recognizes an epitope found to be present at sites of inflammation. The antibody is used to deliver a MAp44 polypeptide or fragment thereof to sites of inflammation, where it inhibits the lectin pathway of complement activation.
Owner:UNIV OF COLORADO THE REGENTS OF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com