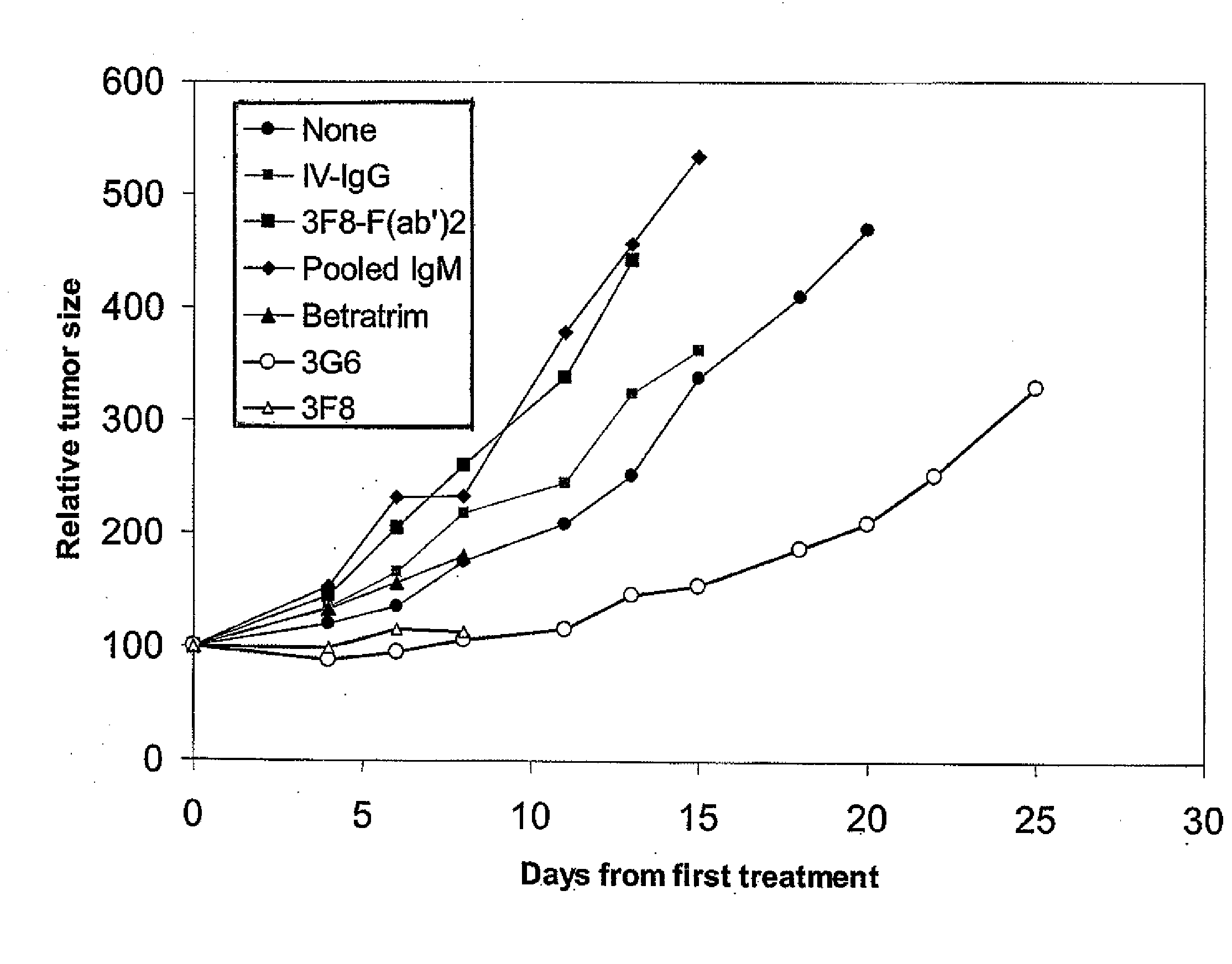
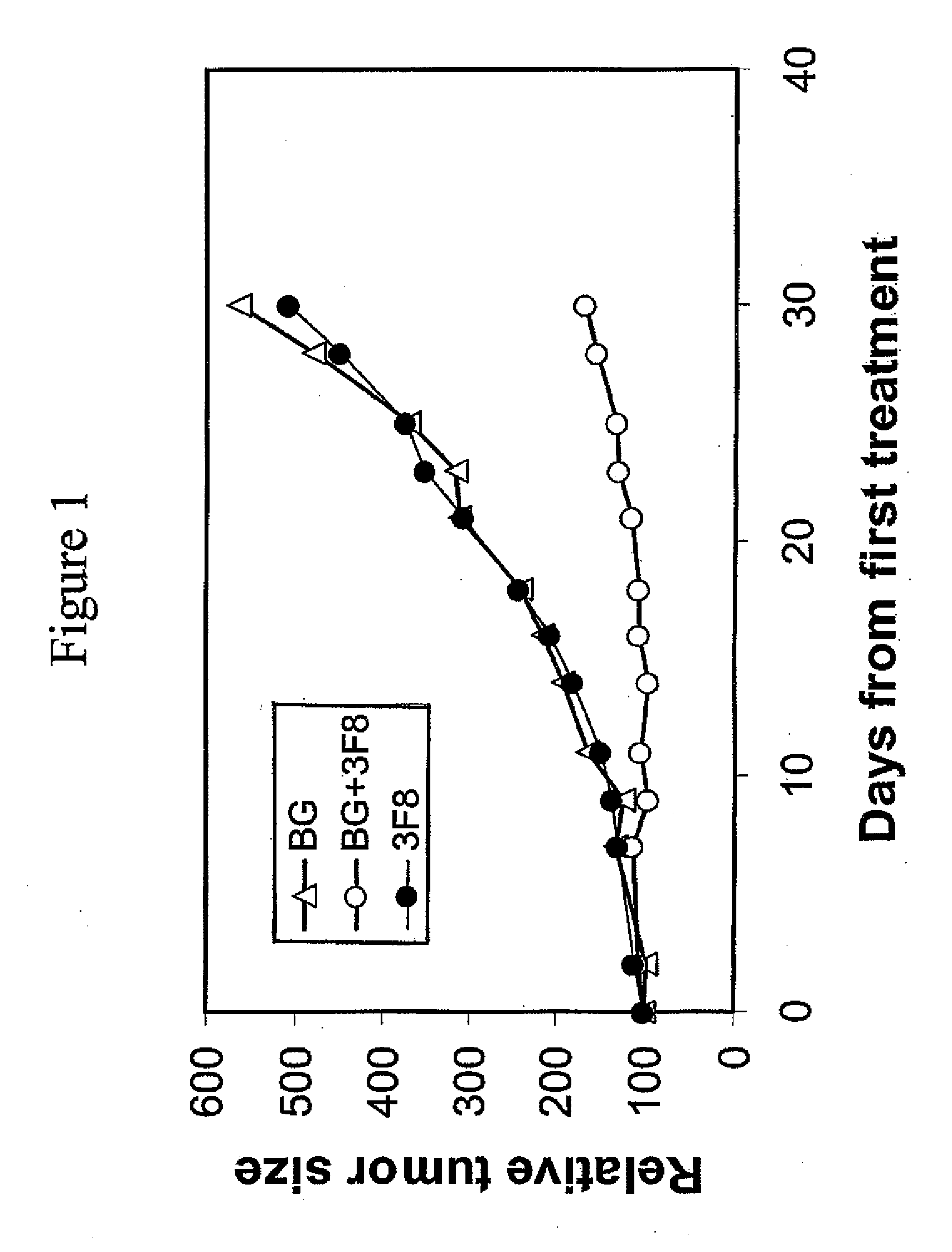
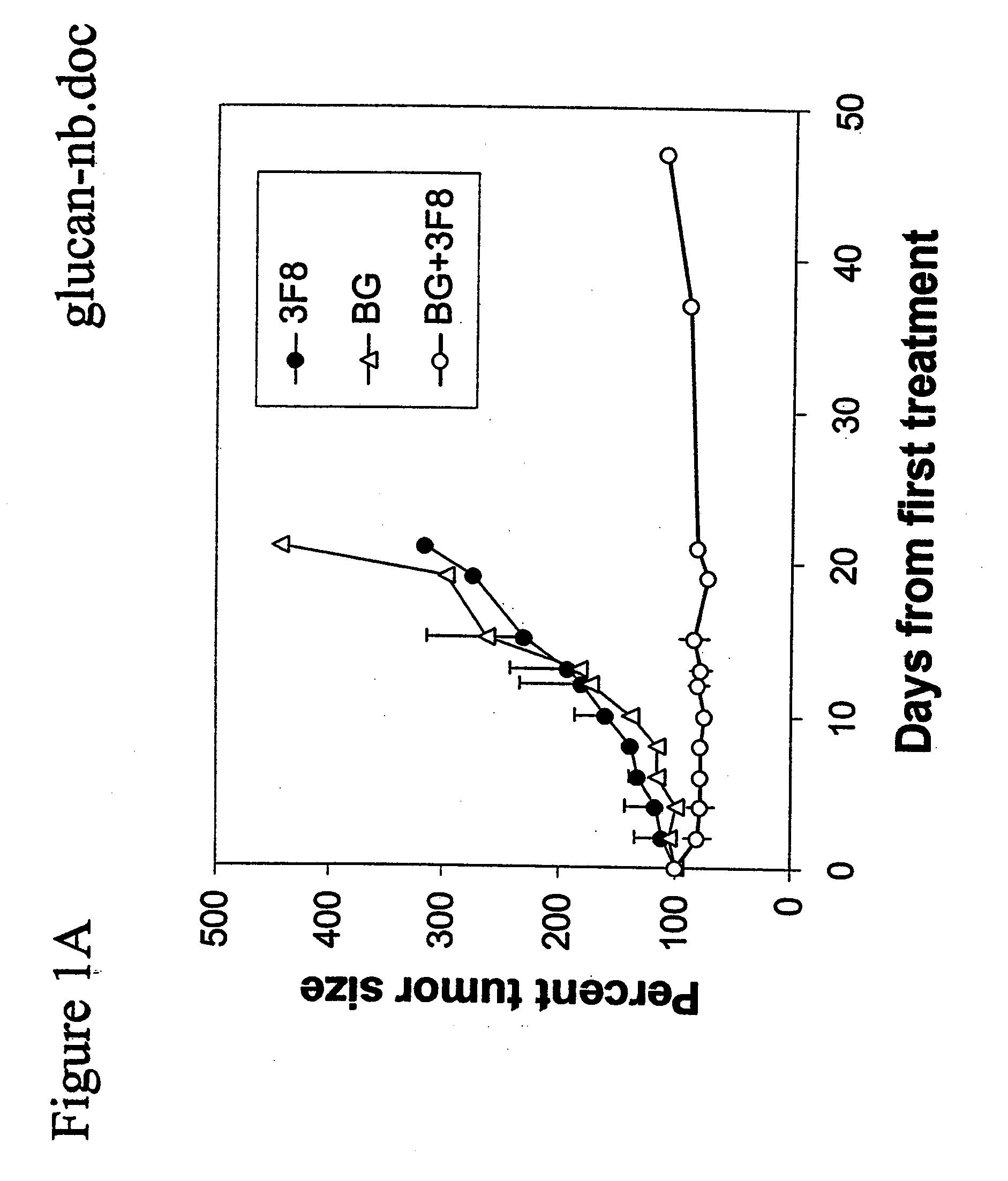
Therapy-enhancing glucan
a technology of glucan and glucan, which is applied in the field of therapy-enhancing glucan, can solve the problems of shrinkage of established tumors and inability to test in vivo the immunomodulatory effect of the tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051]This invention provides a composition comprising an effective amount of glucan capable of enhancing efficacy of antibodies.
[0052]In an embodiment, the antibody is a monoclonal antibody. In a further embodiment, the antibody is an antibody against cancer. In another embodiment, the antibody is a tumor-binding antibody. In a further embodiment, the antibody is capable of activating complement. In a still further embodiment, the antibody is further capable of activating the antibody dependent cell-mediated cytotoxicity.
[0053]In an embodiment, the antibody is directed at the epidermal growth factor receptor. In a further embodiment, the antibody is 528 or C225.
[0054]In another embodiment, the antibody is directed to a ganglioside. In a further embodiment, the ganglioside is GD3. In a still further embodiment, the antibody is R24.
[0055]In a separate embodiment, the ganglioside is GD2. In a further embodiment, the antibody is 3F8.
[0056]In an embodiment, the antigen recognized by the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com