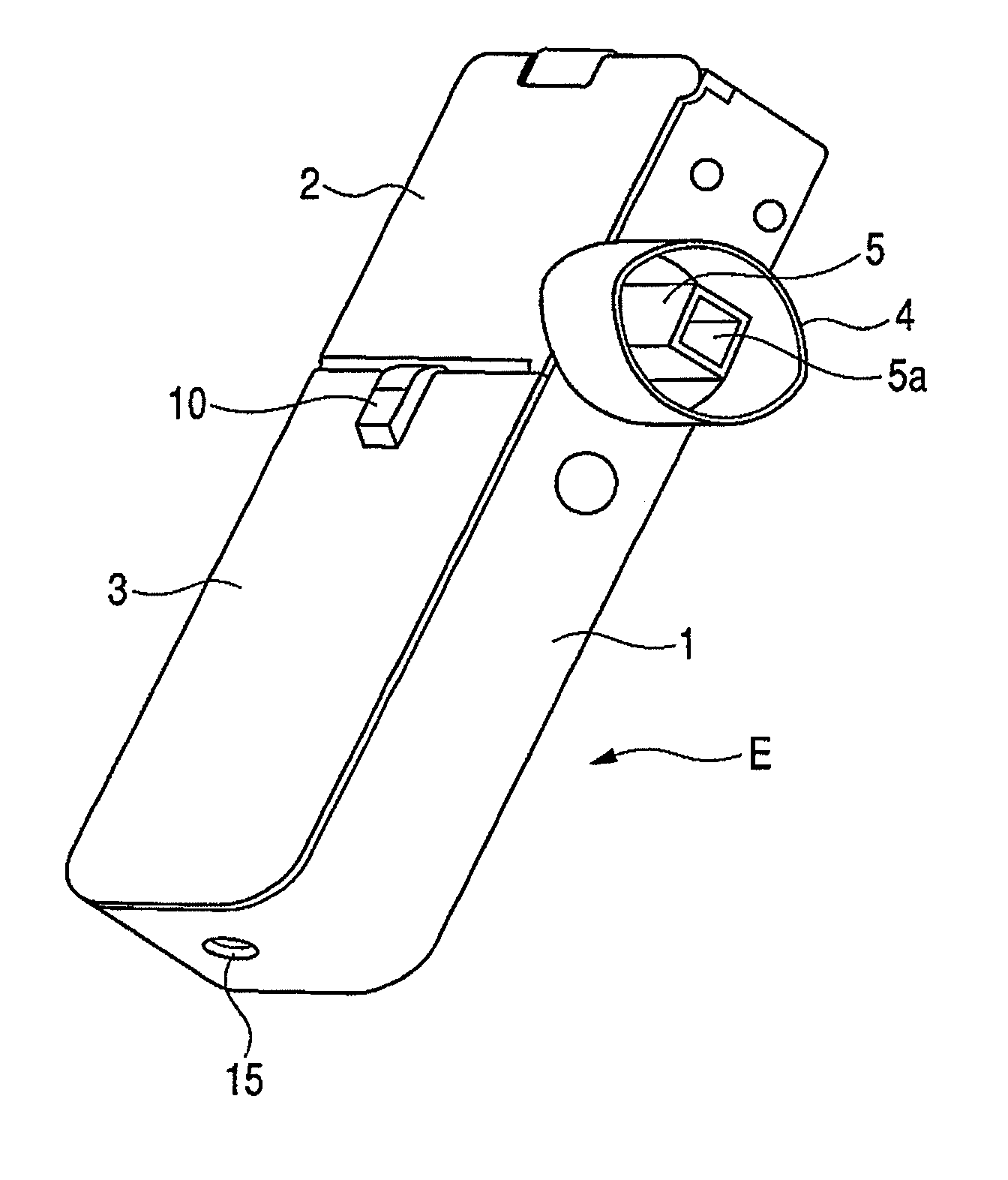
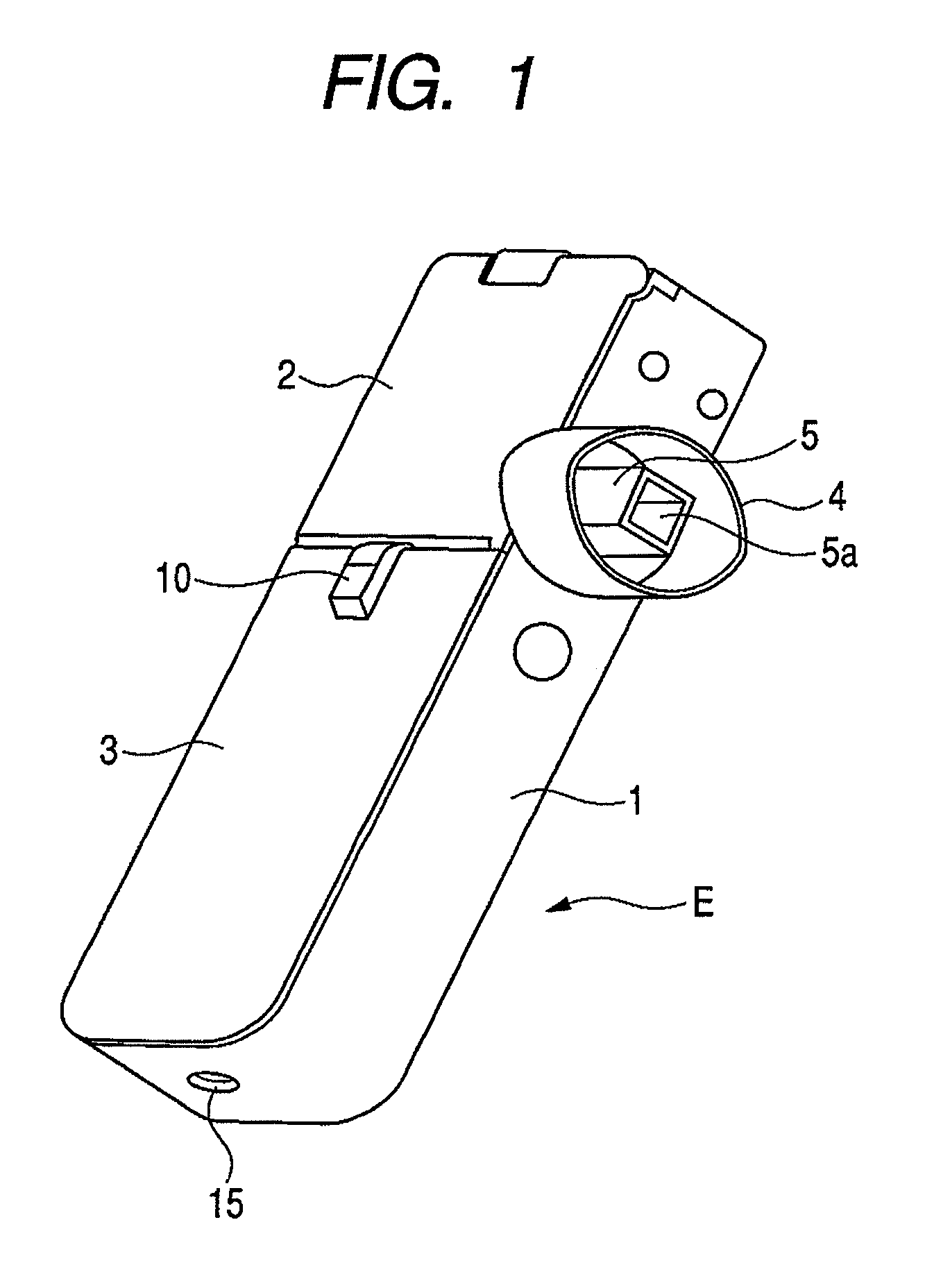
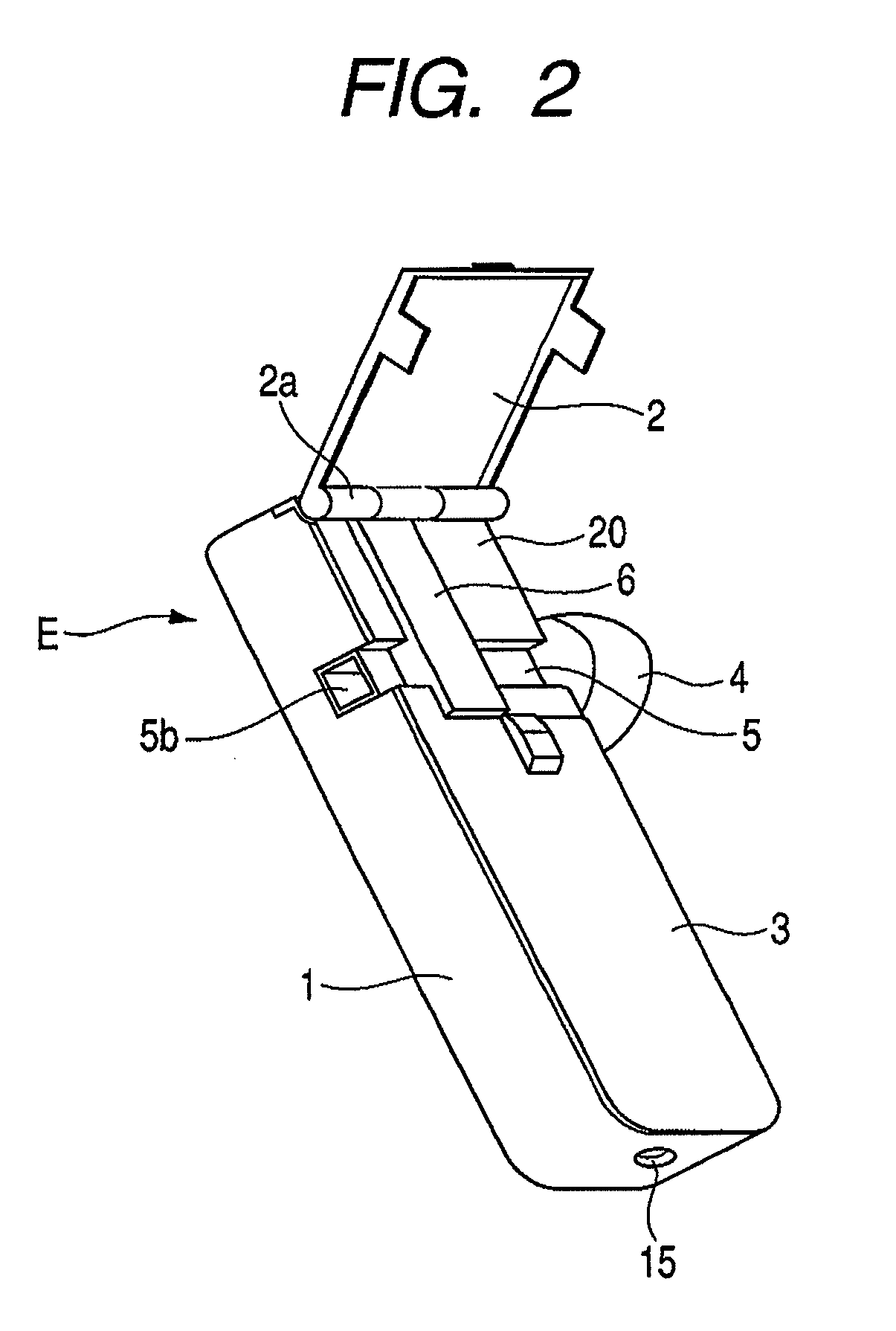
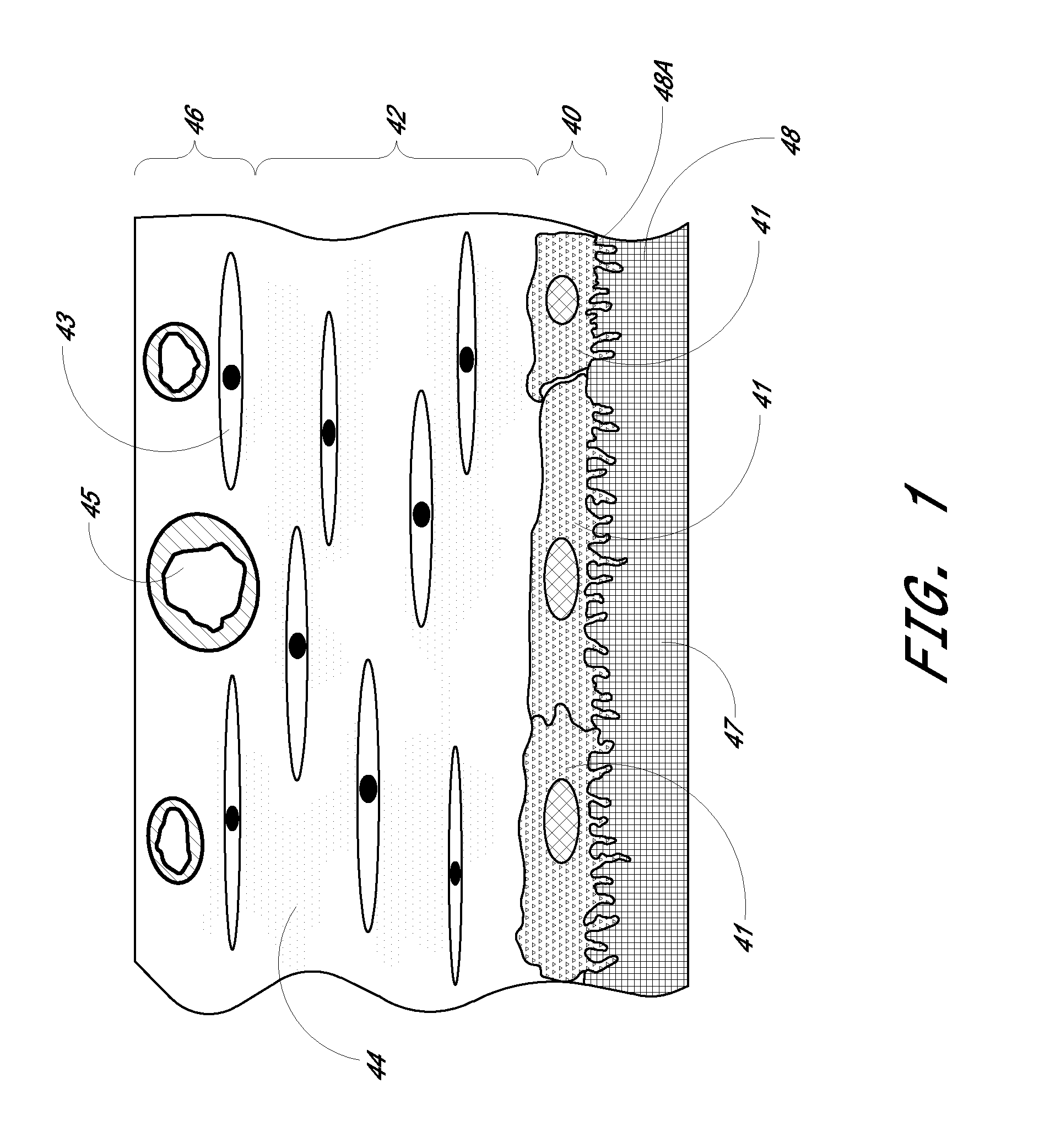
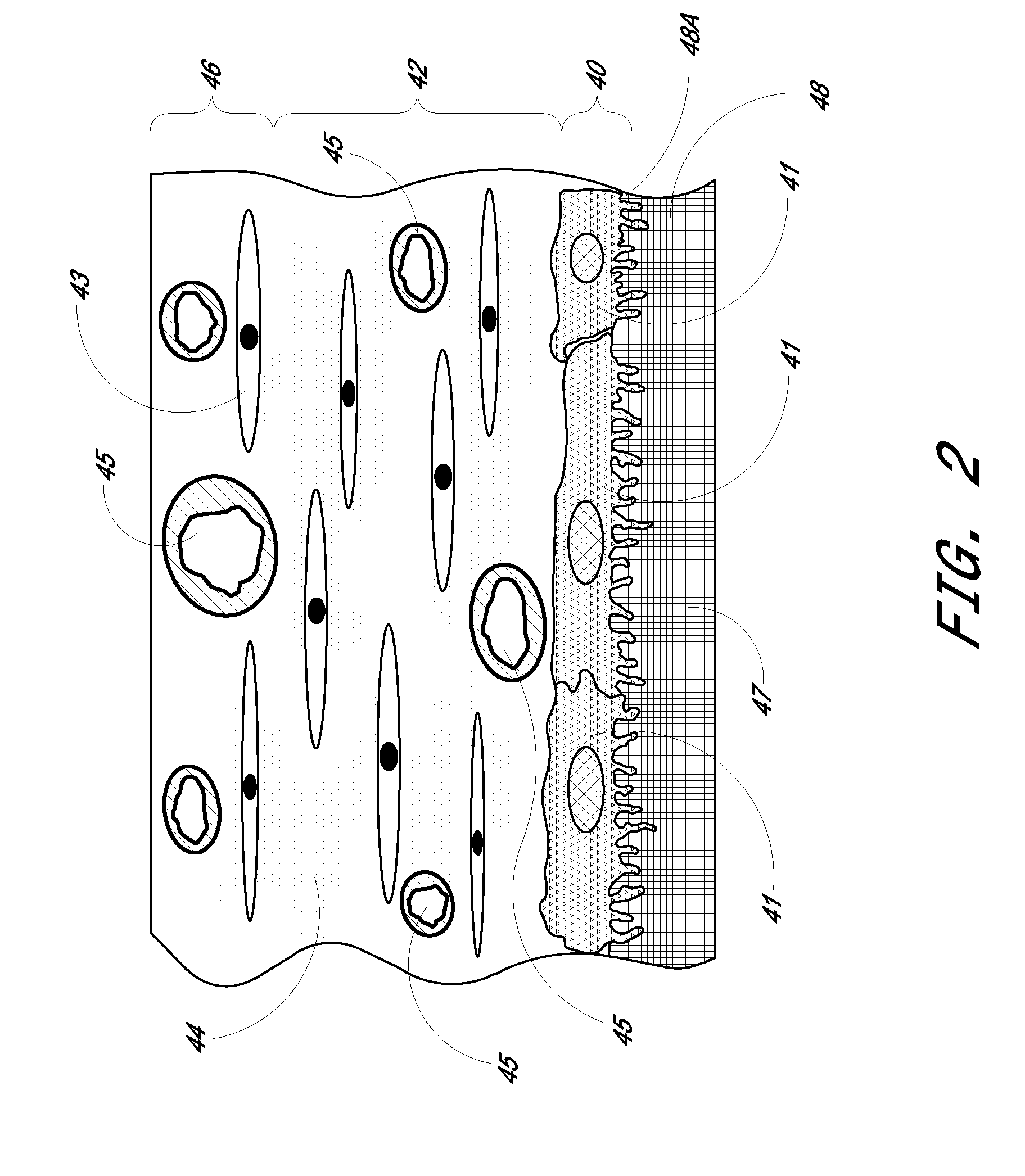
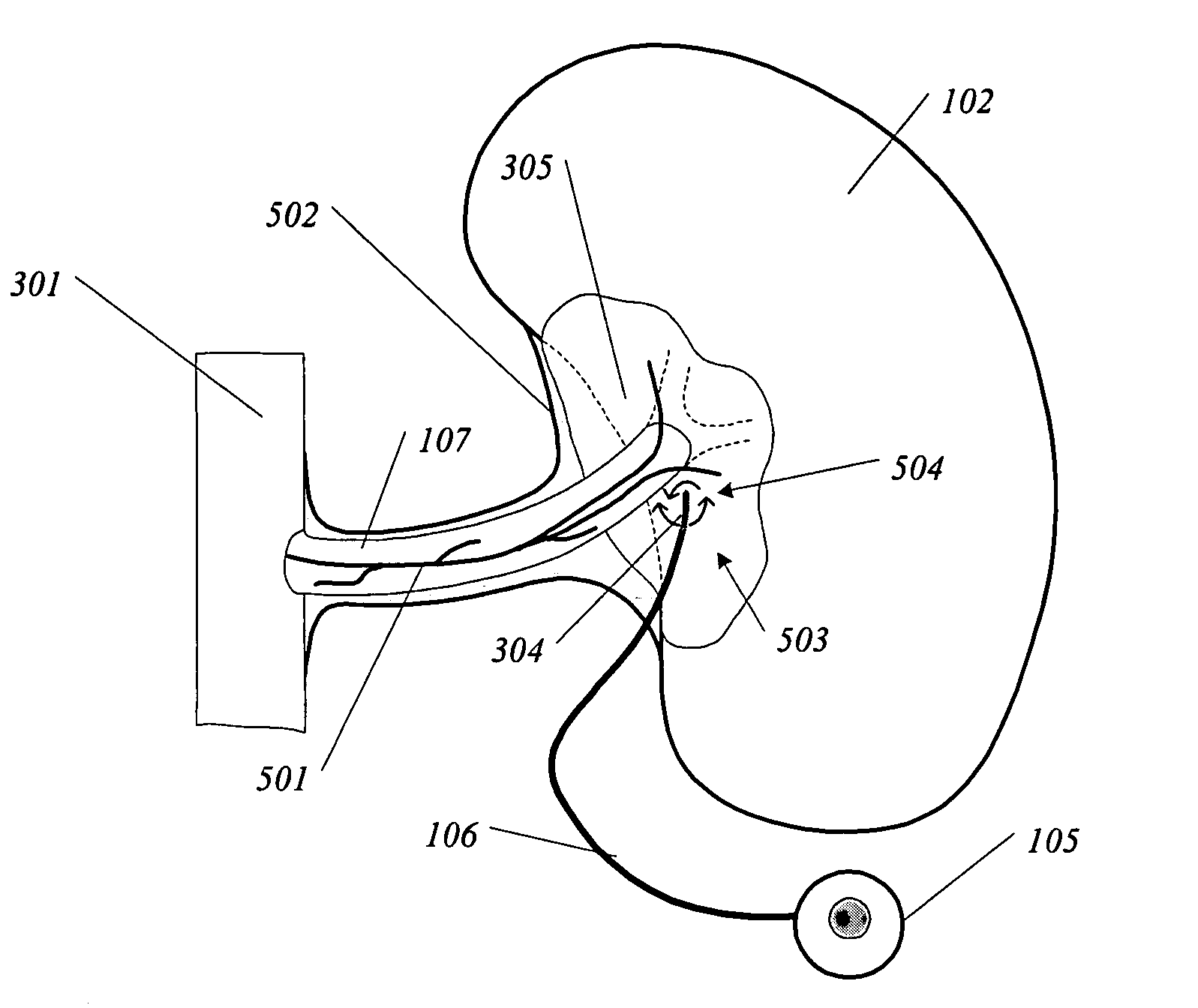
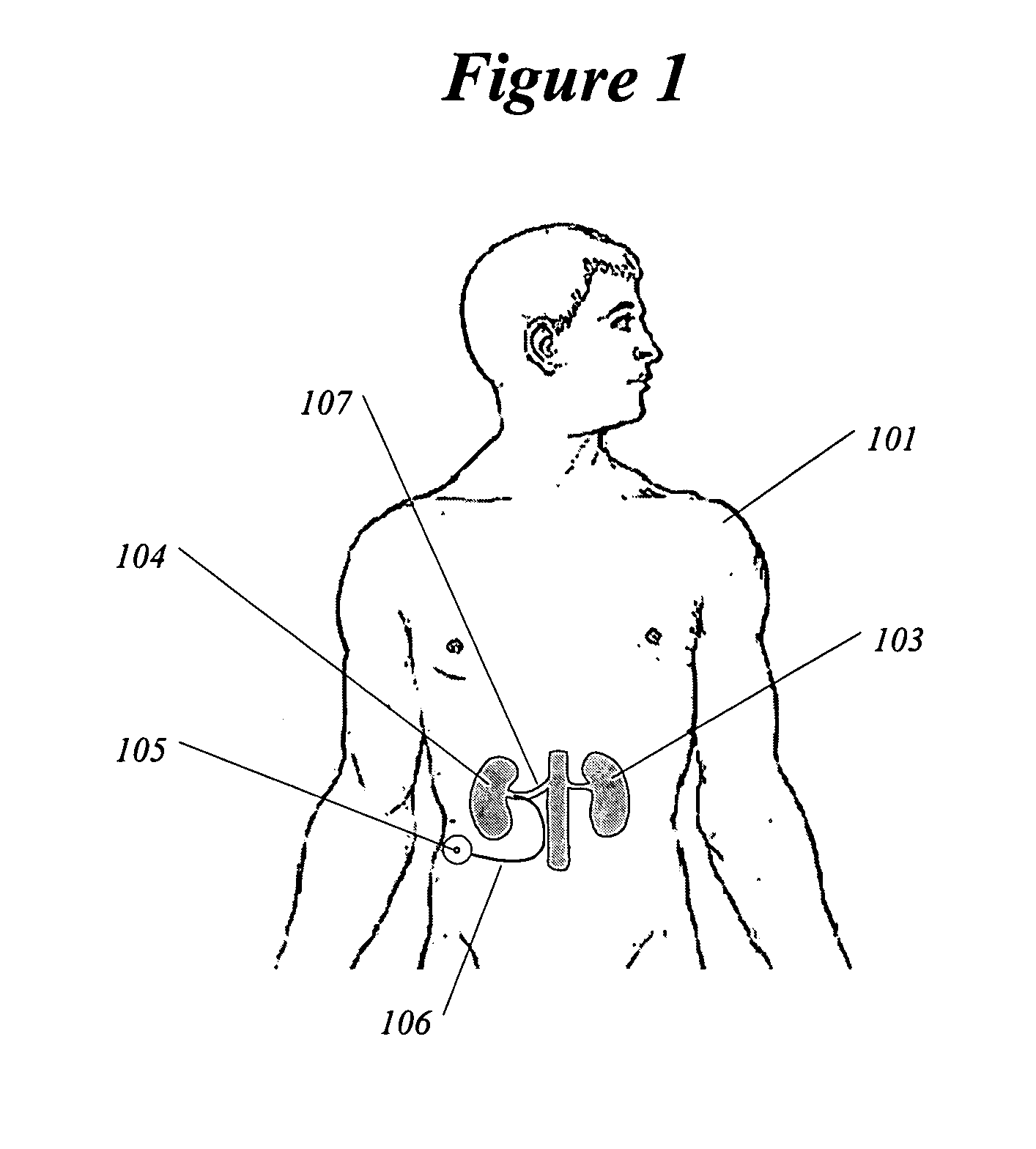
Patents
Literature
134175 results about "Drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A drug is any substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalation, injection, smoking, ingestion, absorption via a patch on the skin, or dissolution under the tongue.
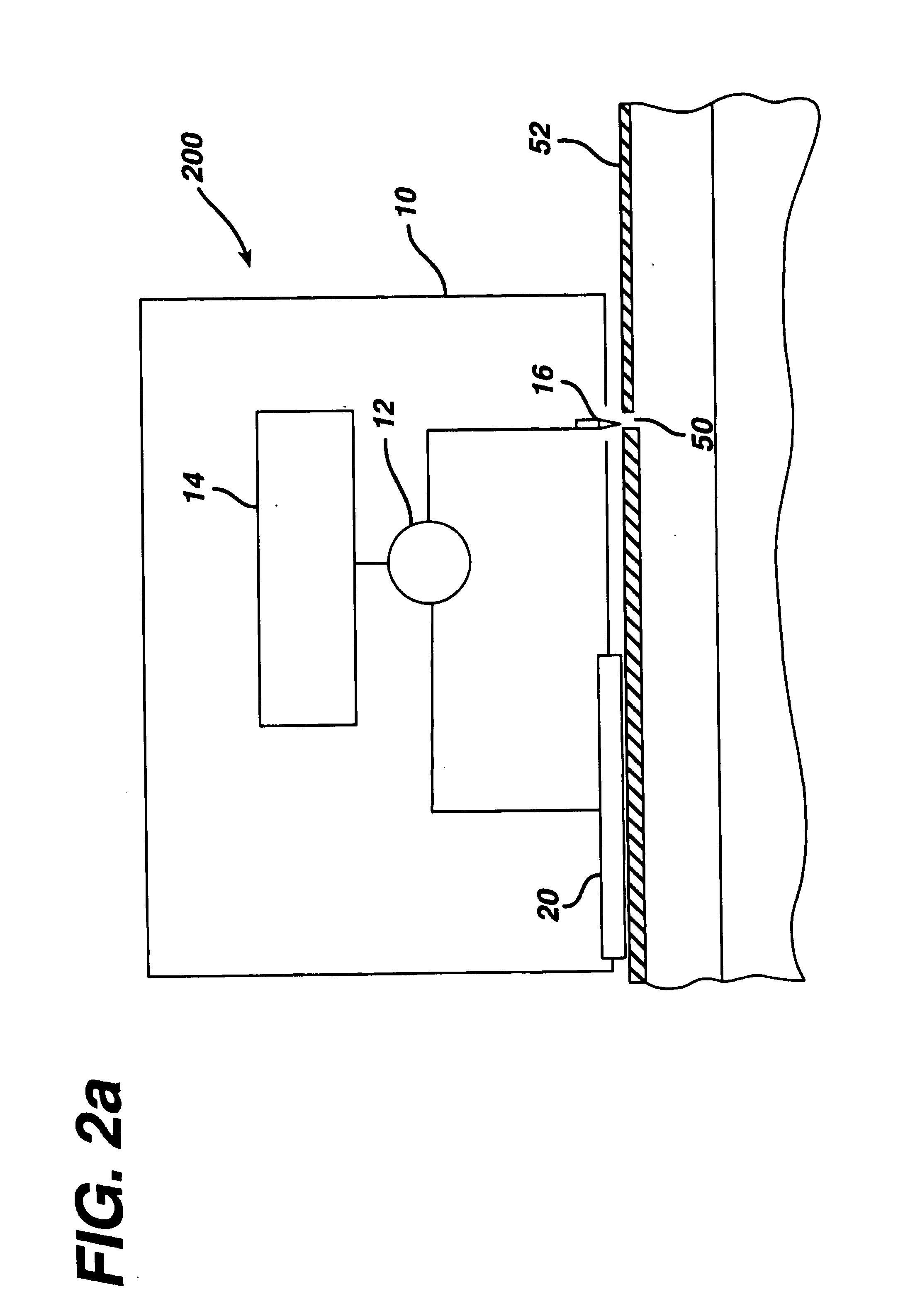
Portable remote patient telemonitoring system
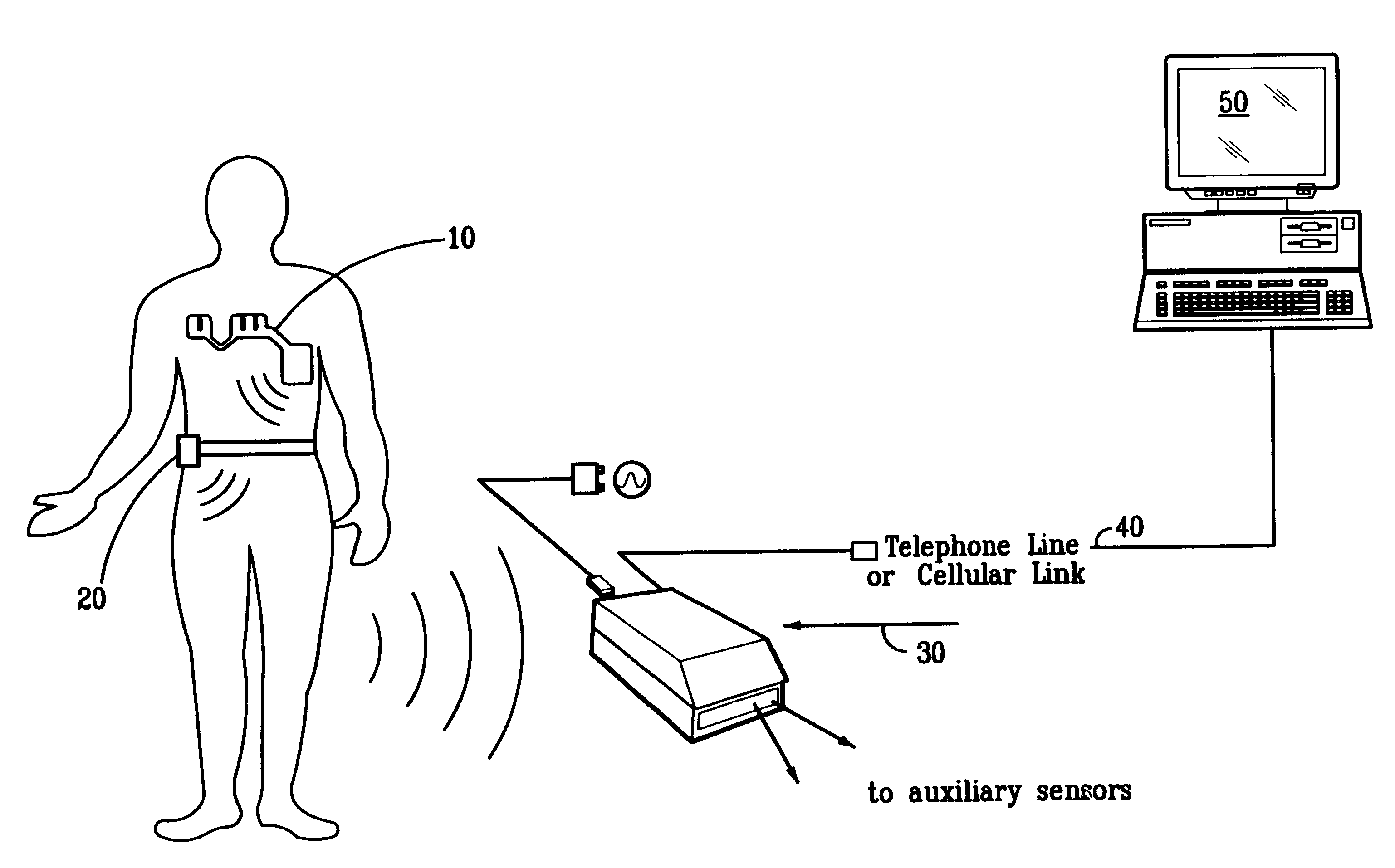
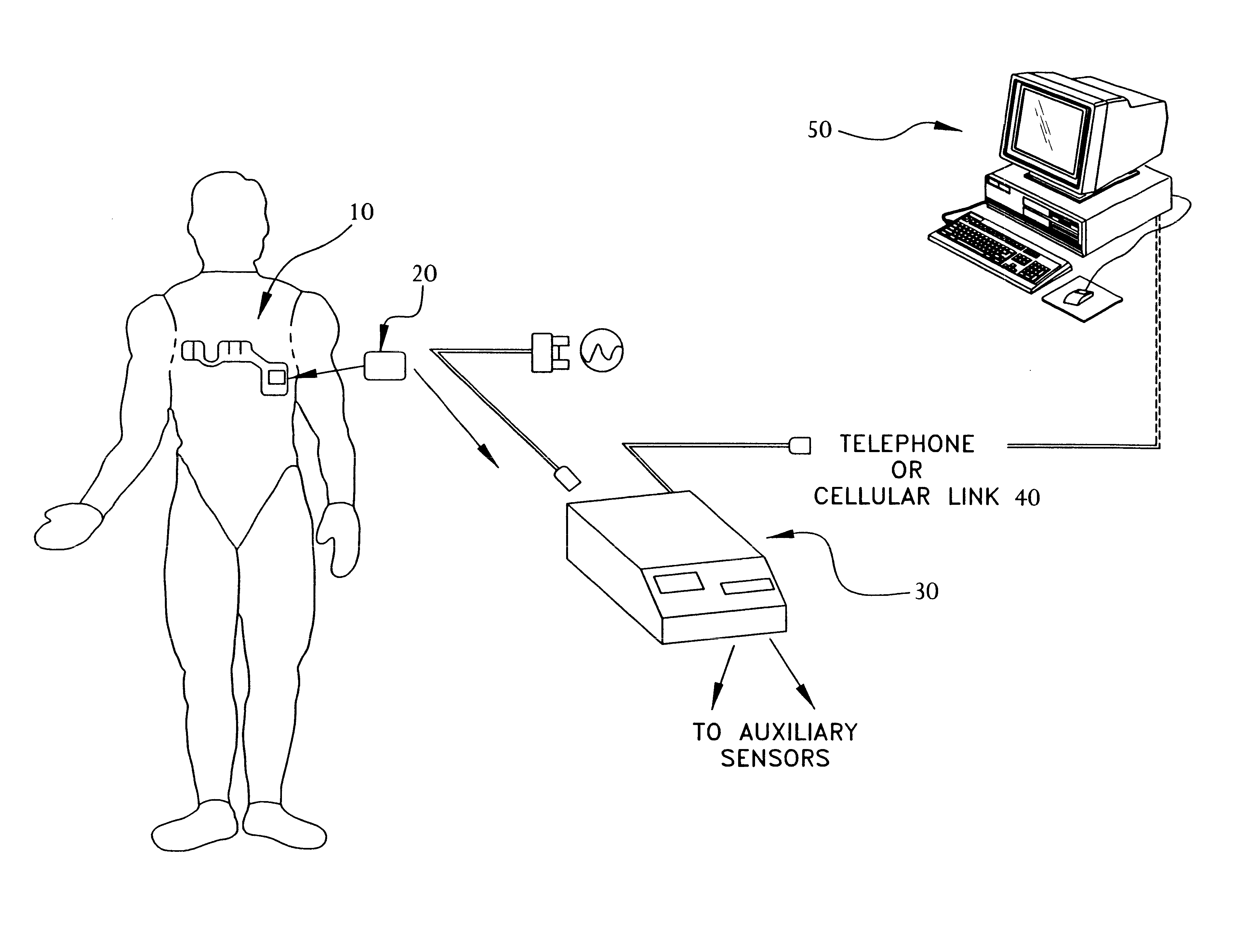
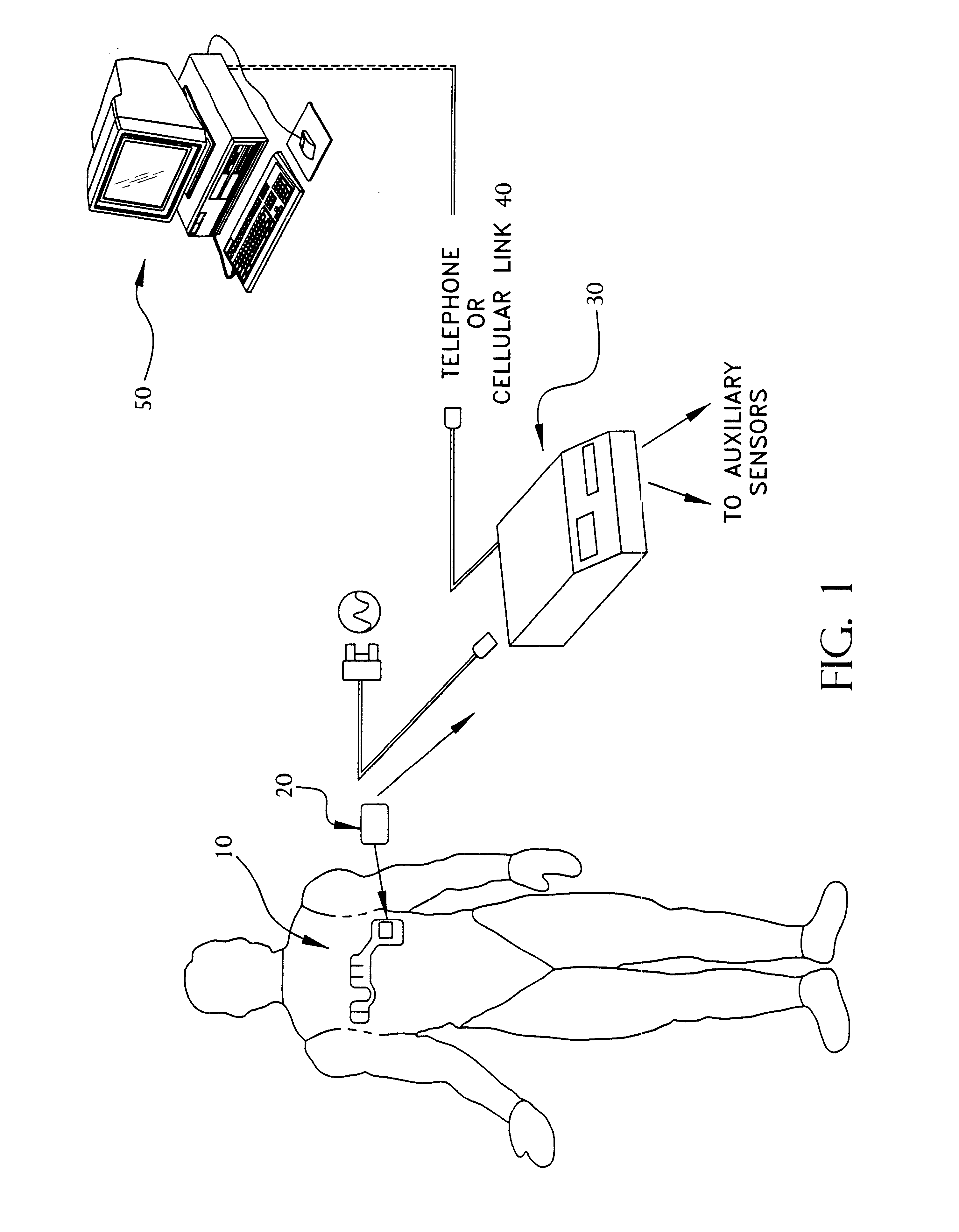
A system and method for monitoring vital signs and capturing data from a patient remotely using radiotelemetry techniques. The system is characterized by a cordless, disposable sensor band with sensors form measuring full waveform ECG, full waveform respiration, skin temperature, and motion, and transmission circuitry for the detection and transmission of vital signs data of the patient. A small signal transfer unit that can either be worn by the patient, e.g., on his or her belt, or positioned nearby receives data from the sensor band, which it then forwards by e.g., radio transmission to a base station that can be located up to 60 meters away. The base station receives data transmissions from the signal transfer unit and is designed to connect to conventional phone lines for transferring the collected data to a remote monitoring station. The base station may also capture additional clinical data, such as blood pressure data, and to perform data checks. Patient safety is enhanced by the ability of the base station to compare clinical data, e.g., ECG, against given profiles and to mark events when appropriate or when the base station is programmed to do so. Such events are indicated to the physician and could be indicated to the patient by reverse transmission to the signal transfer unit. A remote monitoring station allows the presentation and review of data (including events) forwarded by the sensor band. ECG analysis software and a user-friendly graphical user interface are provided to remotely analyze the transmitted data and to permit system maintenance and upkeep. The system of the invention has useful application to the collection of patient clinical data during drug trials and medical testing for regulatory approvals as well as management of patients with chronic diseases.
Owner:CLEARPATH PARTNERS
Medicament dispensing device with a display indicative of the state of an internal medicament reservoir
ActiveUS7331340B2Liquid surface applicatorsPowdered material dispensingDisplay deviceBiomedical engineering
A metered dose inhaler for use with a removable pressurized aerosol canister, or reservoir, having a display for indicating to a user the state of the canister. A memory device on the canister or a housing which houses the canister stores information indicative of doses dispensed from, or remaining in, the canister. That information is processed to provide and display information representative of the state of the canister.
Owner:IVAX CORP
Tear and abrasion resistant expanded material and reinforcement
InactiveUS20070207186A1Increase flexibilityLess complex manufacturing processStentsSurgeryDiseaseEngineering
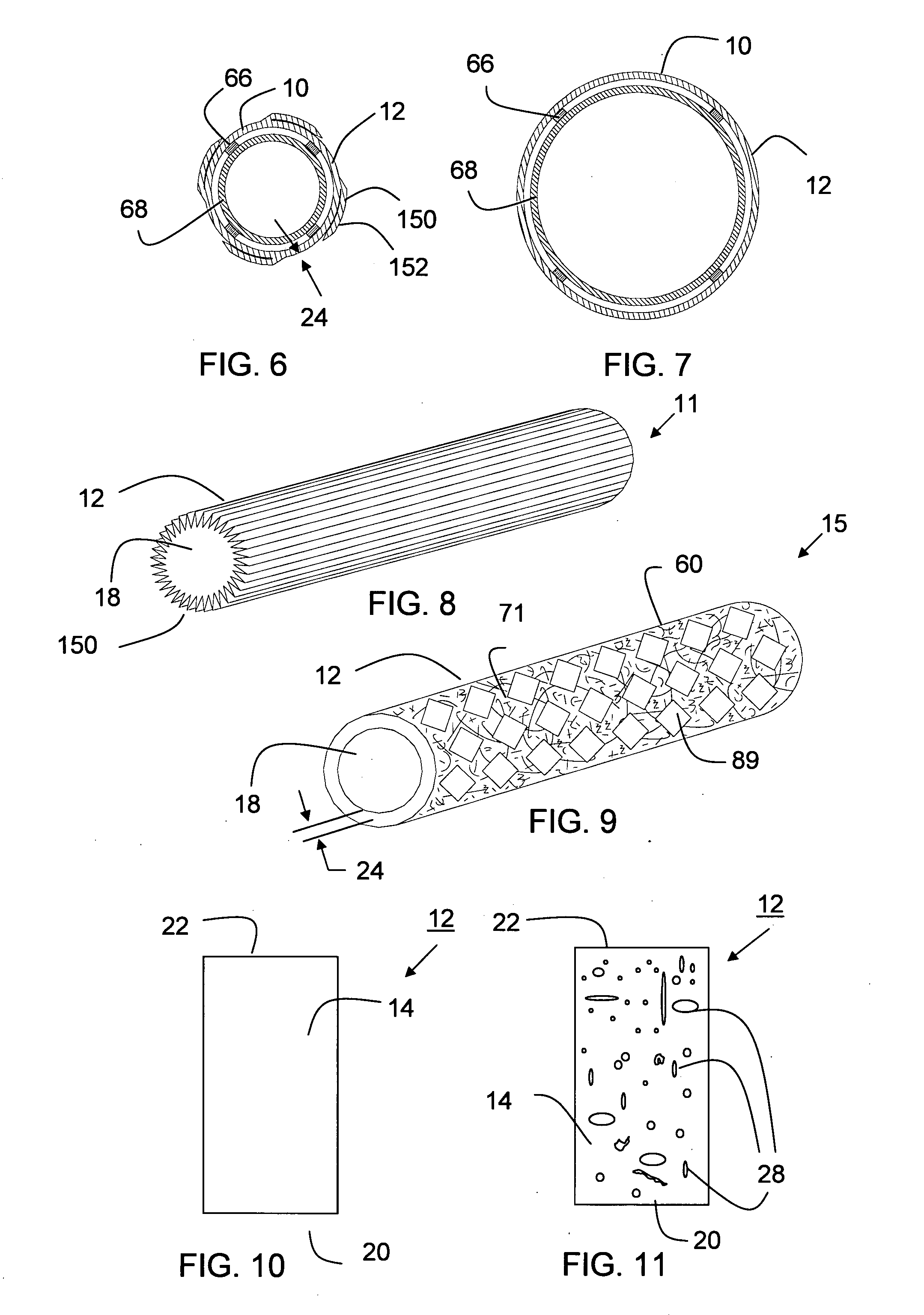
The present invention is a more durable expanded material that enables thinner wall thicknesses and a more flexible reinforcement suitable for stenting. The present invention is especially useful in the construction of grafts, stents, and stent-grafts which are used, for example, in repairing or replacing blood vessels that are narrowed or occluded by disease, aneurismal blood vessels, or other medical treatments. The inventive material and configurations allow expansion or contraction in size or adjustment in size in an incremental manner so that the optimum size, shape, and fit with other objects can be obtained. The present invention is also optionally capable of more accurately delivering one or more active ingredients such as drugs over longer periods of time. The present invention optionally includes surface modifications and additives that increase the surface adhesion of active ingredients, coatings, or combinations thereof. Finally, the present invention optionally includes growing cells on the inventive material so that the expanded material, reinforcement, or combinations thereof are useful, for example, in producing lab-grown blood vessels or organs.
Owner:SCANLON JOHN JAMES +1
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
Extraction of solvents from drug containing polymer reservoirs
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Devices for reduction of post operative ileus
InactiveUS20080086078A1Reducing post-operative ileus and/or gastric stasisReducing post-operative ileusMedical devicesMedical insufflatorsButtressSurgical department
An apparatus and method for reducing post-operative ileus and / or gastric stasis is described. The method can include applying to the intestine a therapeutically effective amount of a composition comprising a drug that is effective in reducing post-operative ileus and / or gastric stasis, such as by introducing the composition through a surgical access device, such as a trocar or endoscope. The apparatus can include a surgical fastener and a buttress comprising the composition.
Owner:ETHICON ENDO SURGERY INC
Methods for reduction of post operative ileus.
InactiveUS20080085296A1Reducing post-operative ileus and/or gastric stasisReducing post-operative ileusBiocideDigestive systemButtressSurgical department
An apparatus and method for reducing post-operative ileus and / or gastric stasis is described. The method can include applying to the intestine a therapeutically effective amount of a composition comprising a drug that is effective in reducing post-operative ileus and / or gastric stasis, such as by introducing the composition through a surgical access device, such as a trocar or endoscope. The apparatus can include a sheet of material, a surgical fastener, or a buttress comprising the composition.
Owner:ETHICON ENDO SURGERY INC
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Intraocular physiological sensor
An implantable intraocular physiological sensor for measuring intraocular pressure, glucose concentration in the aqueous humor, and other physiological characteristics. The implantable intraocular physiological sensor may be at least partially powered by a fuel cell, such as an electrochemical glucose fuel cell. The implantable intraocular physiological sensor may wirelessly transmit measurements to an external device. In addition, the implantable intraocular physiological sensor may incorporate aqueous drainage and / or drug delivery features.
Owner:GLAUKOS CORP
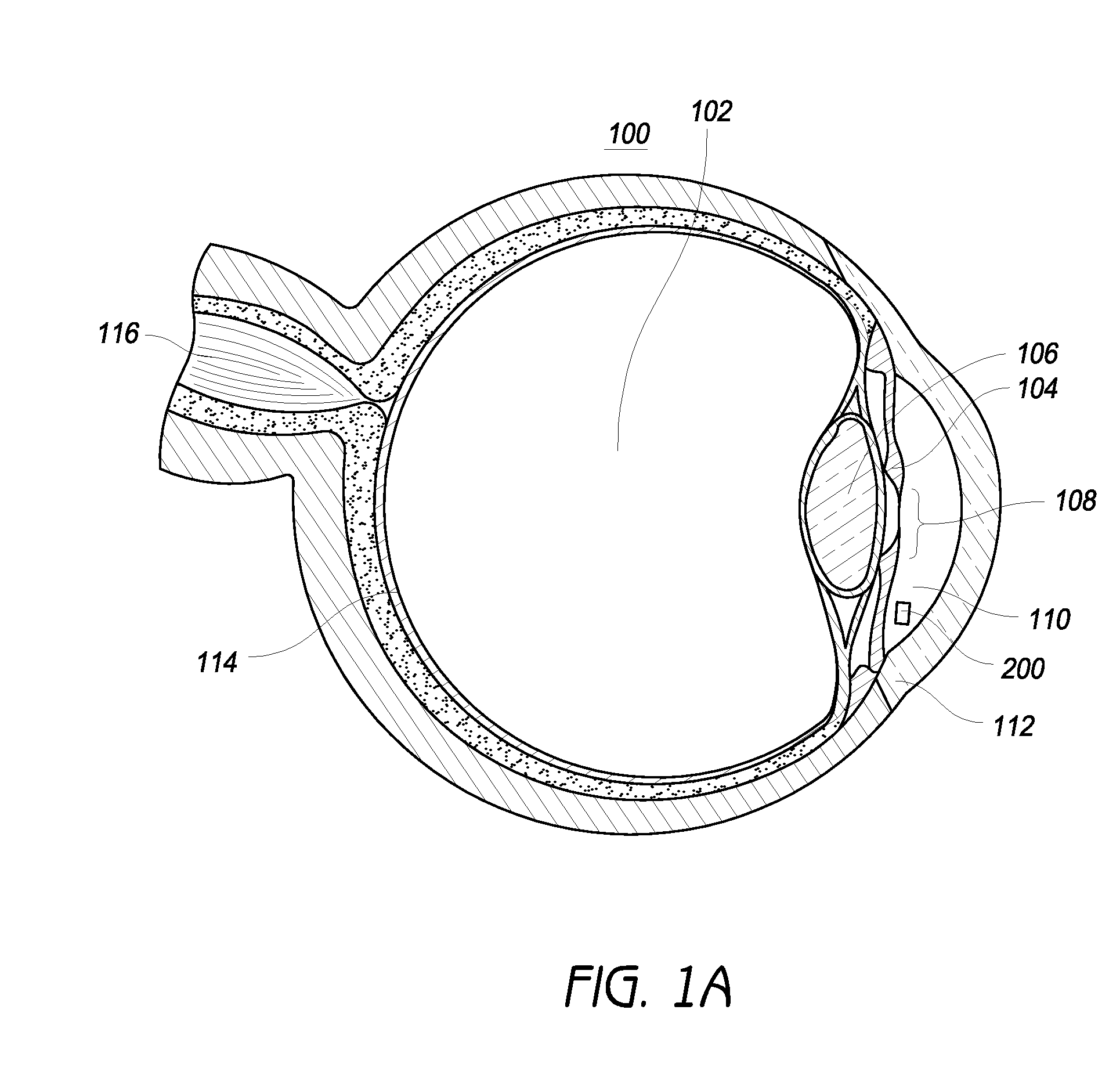
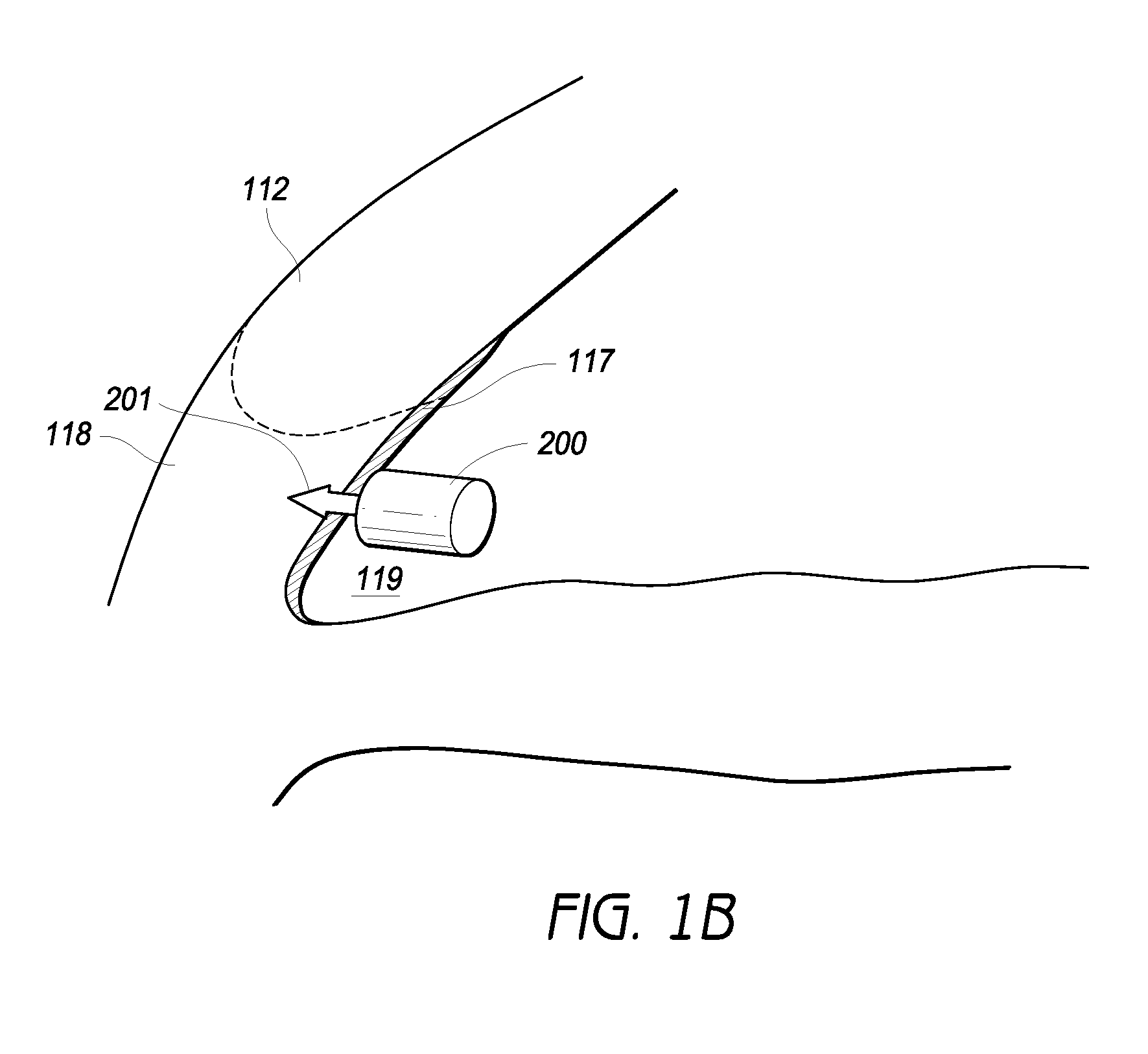
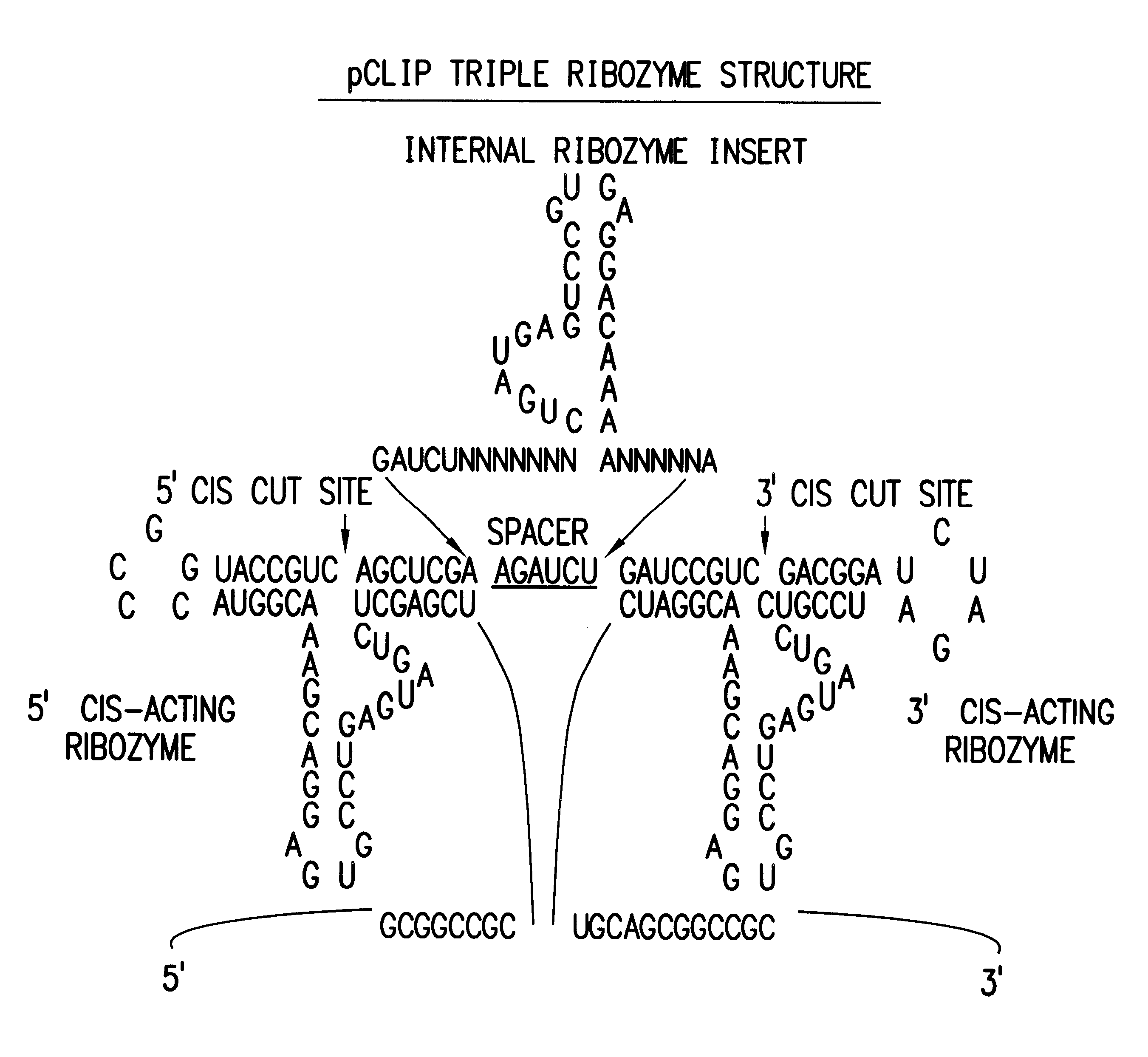
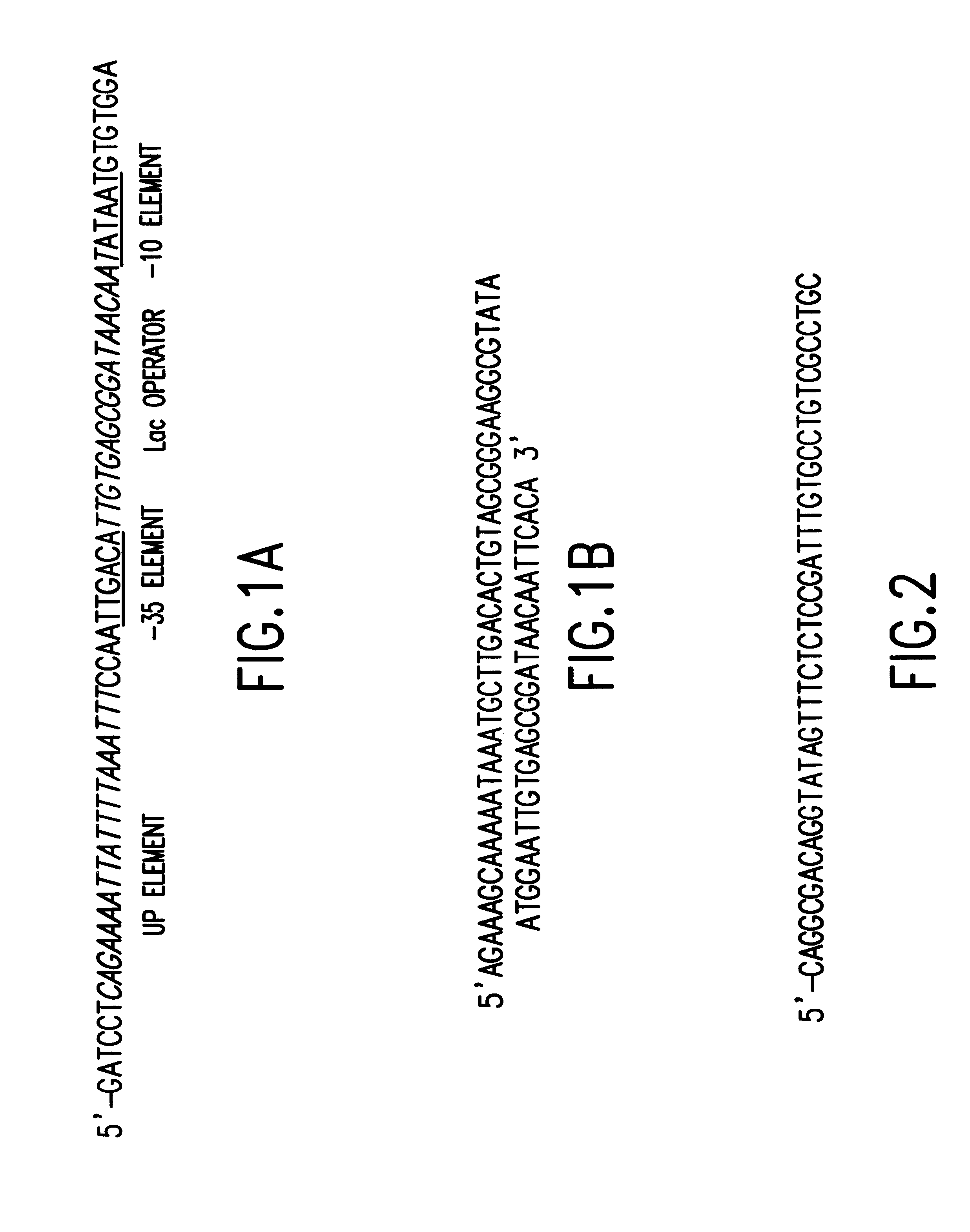
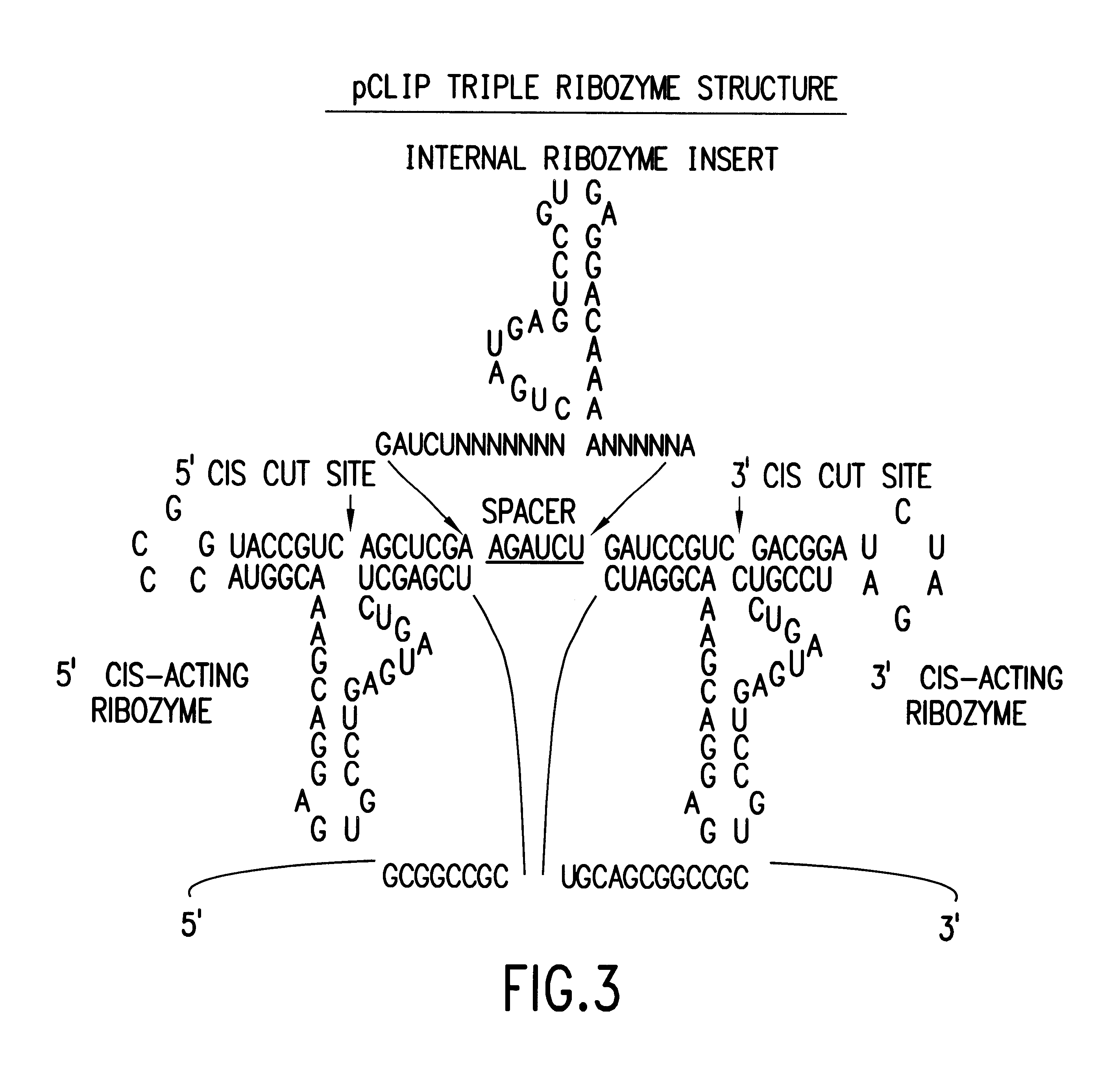
Tissue-specific and pathogen-specific toxic agents and ribozymes
InactiveUS6271359B1Rapidly and effectively expressedImprove stabilityVirusesSugar derivativesCancer cellBiology
The present invention relates to the discovery, identification and characterization of toxic agents which are lethal to pathogens and methods for targeting such toxic agents to a pathogen or pathogen infected cells in order to treat and / or eradicate the infection. In particular, the present invention relates to toxic agents which target bacteria at different stages of the bacterial life cycle, which are delivered alone or in combination to bacteria or bacteria-infected cells. The invention relates to toxic agents which are lethal to diseased cells and methods for targeting such toxic agents to a diseased cell in order to treat and / or eradicate the disease. The present invention relates to promoter elements which are pathogen-specific or tissue-specific and the use of such promoter elements to achieve pathogen-specific or tissue-specific expression of the toxic agent(s) and / or ribozyme(s) of the present invention. Specifically, the invention relates to the delivery of one or more toxic gene products, antisense RNAs, or ribozymes, or combination thereof. The invention provides a novel system by which multiple pathogenic targets may be simultaneously targeted to cause the death of a pathogen, or cell infected with a pathogen. Further, the invention has important implications in the eradication of drug-resistant bacterium and bacterial pathogens. The invention provides a novel system by which multiple targets may be simultaneously targeted to cause the death of a diseased cell. The invention also has important implications in the eradication of drug-resistant pathogens and drug-resistant diseased cells (such as cancer cells).
Owner:MUSC FOUND FOR RES DEV +1
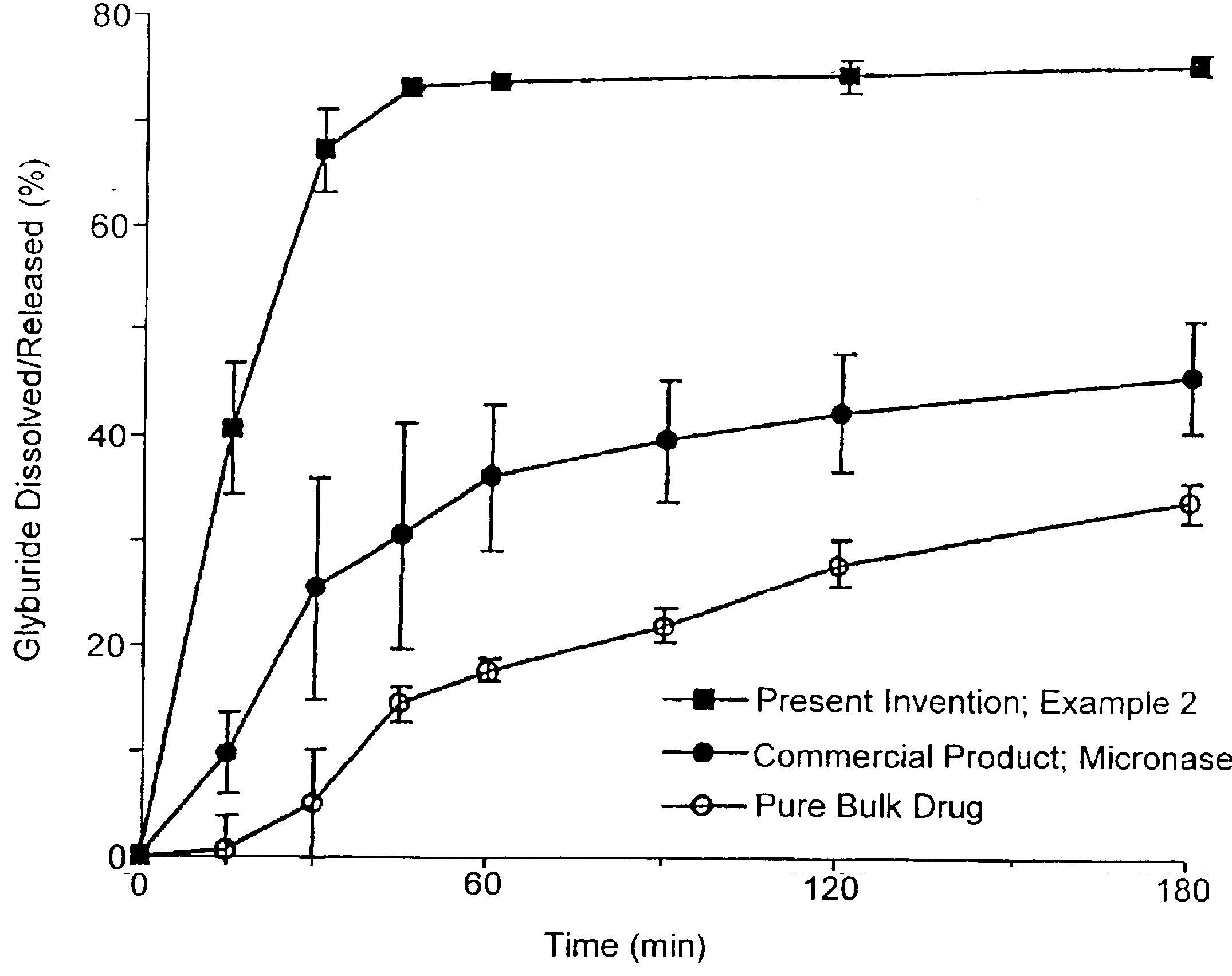
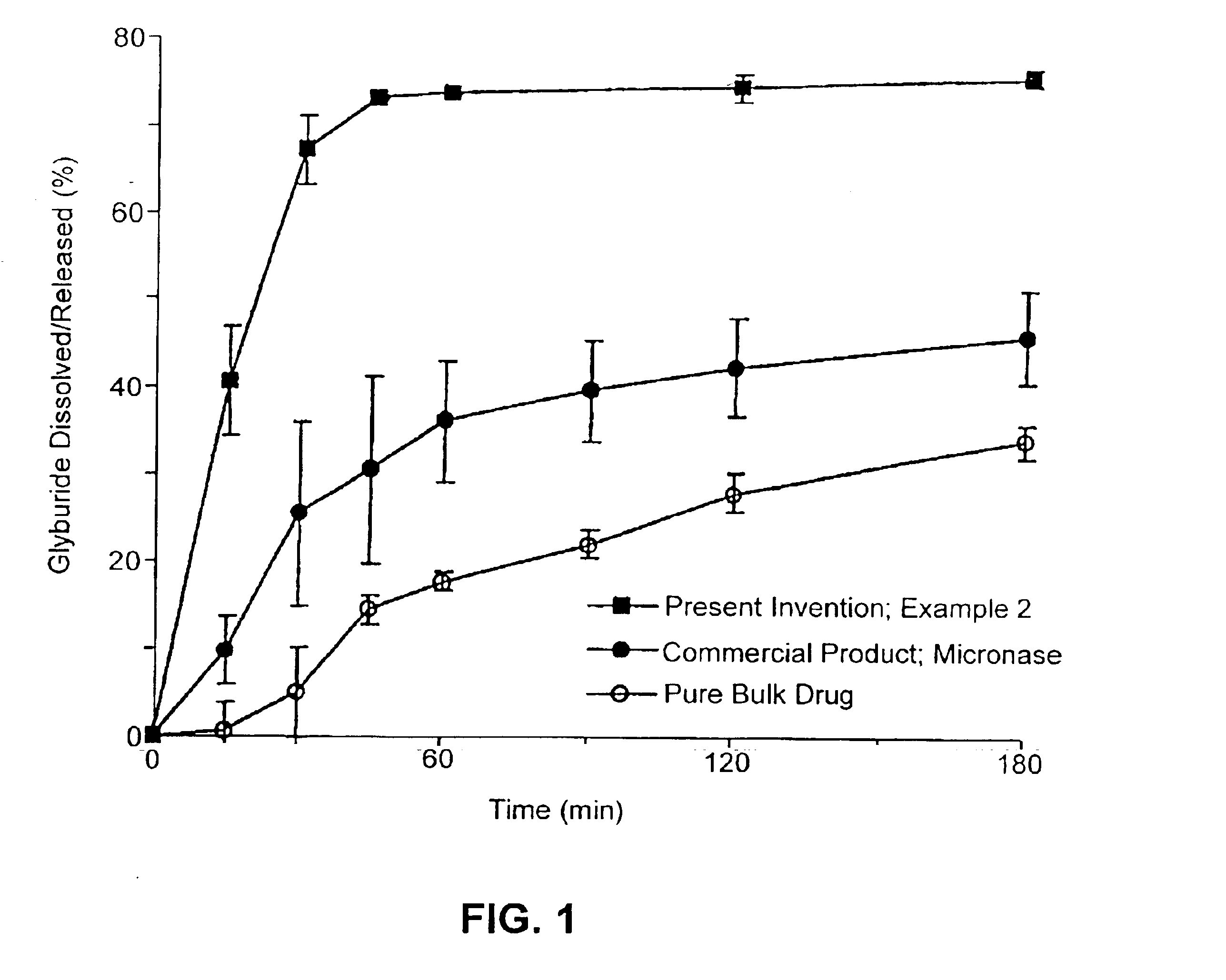
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Systems and methods for modulation of circulatory perfusion by electrical and/or drug stimulation
InactiveUS6845267B2Minimal discomfortAvoid the needElectrotherapyPharmaceutical delivery mechanismElectrical batteryClosed loop
One or more implantable system control units (SCU) apply one or more stimulating drugs and / or electrical pulses to one or more predetermined areas affecting circulatory perfusion. The SCU preferably includes a programmable memory for storing data and / or control parameters, and preferably uses a power source / storage device, such as a rechargeable battery. If necessary, periodic recharging of such a power source / storage device is accomplished, for example, by inductive coupling with an external appliance. The SCU provides a means of stimulating a nerve(s) or other tissue with electrical and / or infusion pulses when desired, without the need for external appliances during the stimulation session. When necessary, external appliances are used for the transmission of data to and / or from the SCU(s) and / or for the transmission of power. In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust electrical and / or drug stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
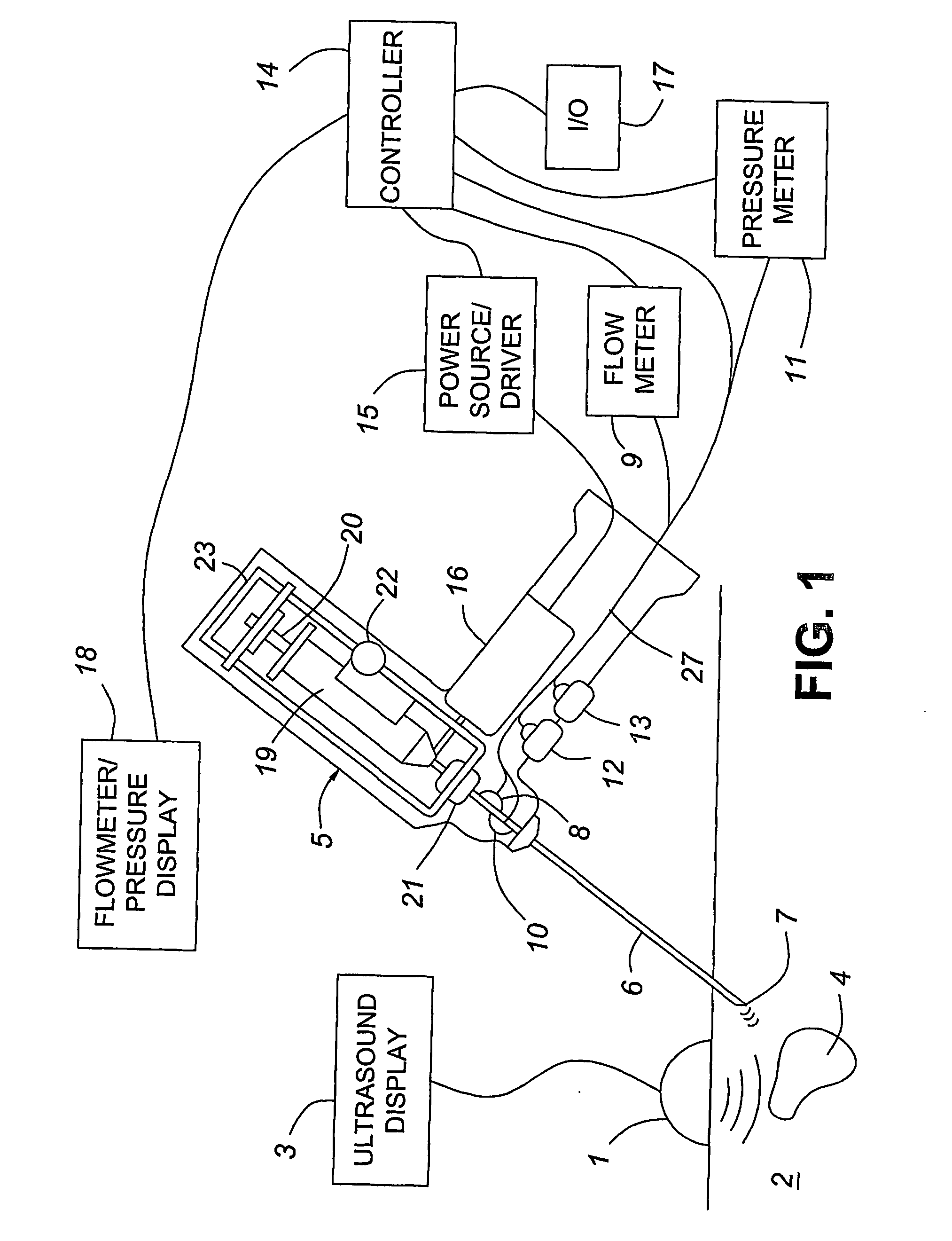
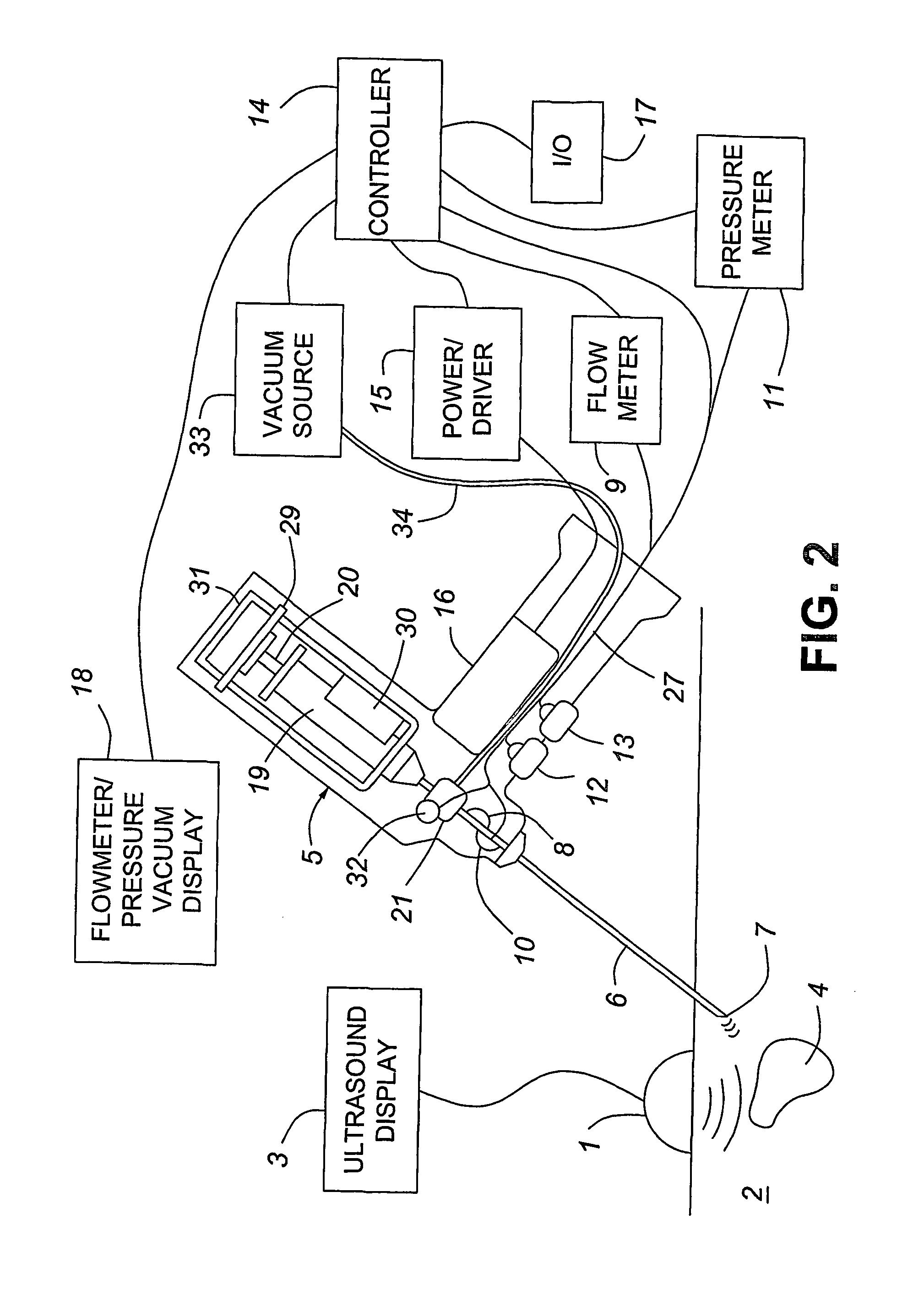
Medical devices with enhanced ultrasonic visibilty
InactiveUS20070197954A1Ultrasonic/sonic/infrasonic diagnosticsElectrotherapyTip positionSolid tissue
A medical device having enhanced ultrasonic visibility is provided. The device permits localized drug delivery, probe positioning, fluid drainage, biopsy, or ultrasound pulse delivery, through the real-time ultrasound monitoring of the needle tip position within a patient. The device permits controlled dispersion of a drug into solid tissue, the lodging of particles into solid tissue, and drug delivery into specific blood vessels. As a needle is inserted, a fluid that contrasts echogenically with the organ environment is injected into the patient. The fluid travels a brief distance before being slowed and stopped by the patient's tissue and this fluid flow will be detectable by ultrasound. The needle position during insertion will be monitored using ultrasound until it is at the desired point of action. A therapeutic drug is then delivered or a probe inserted
Owner:ARTENGA
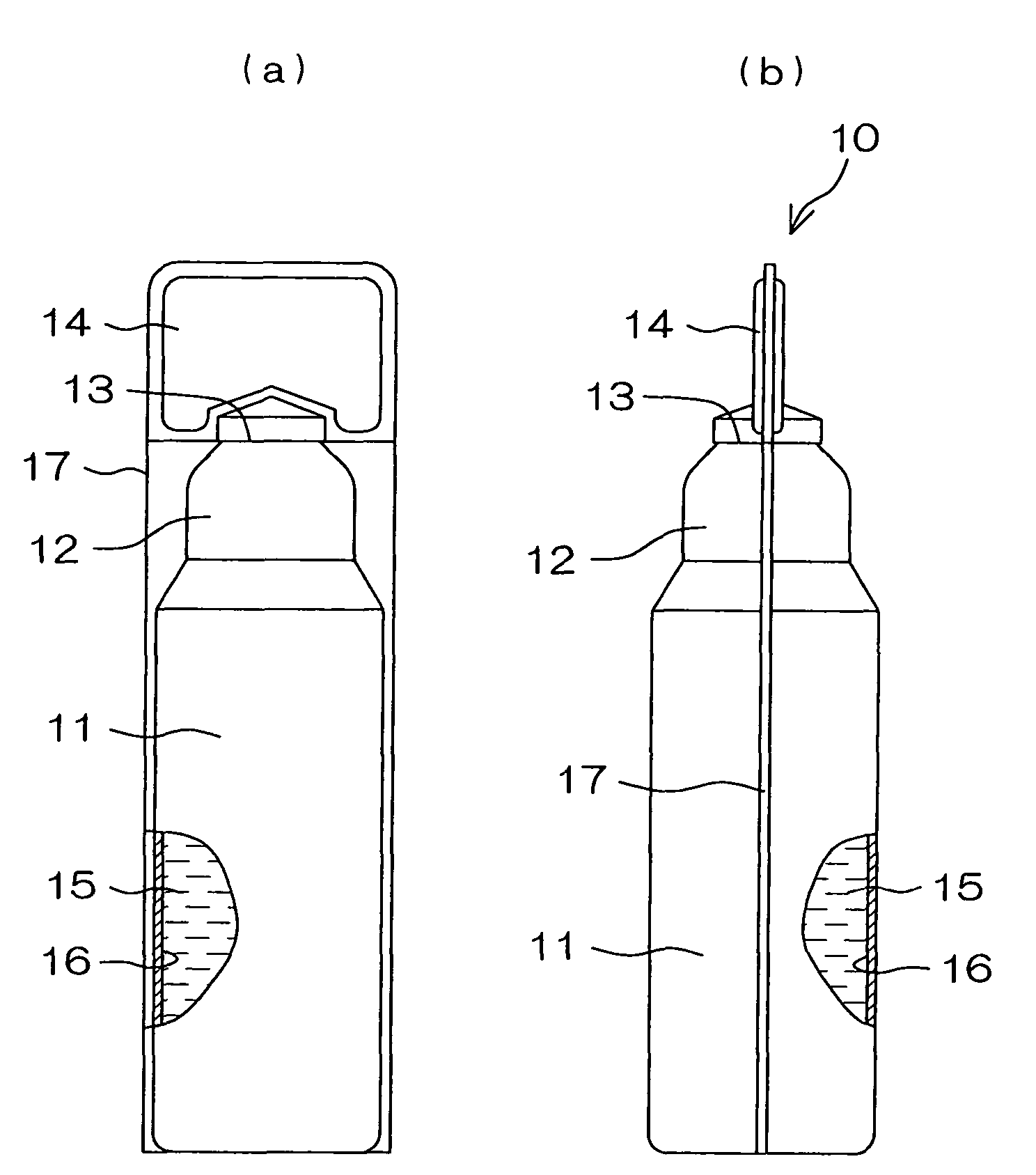
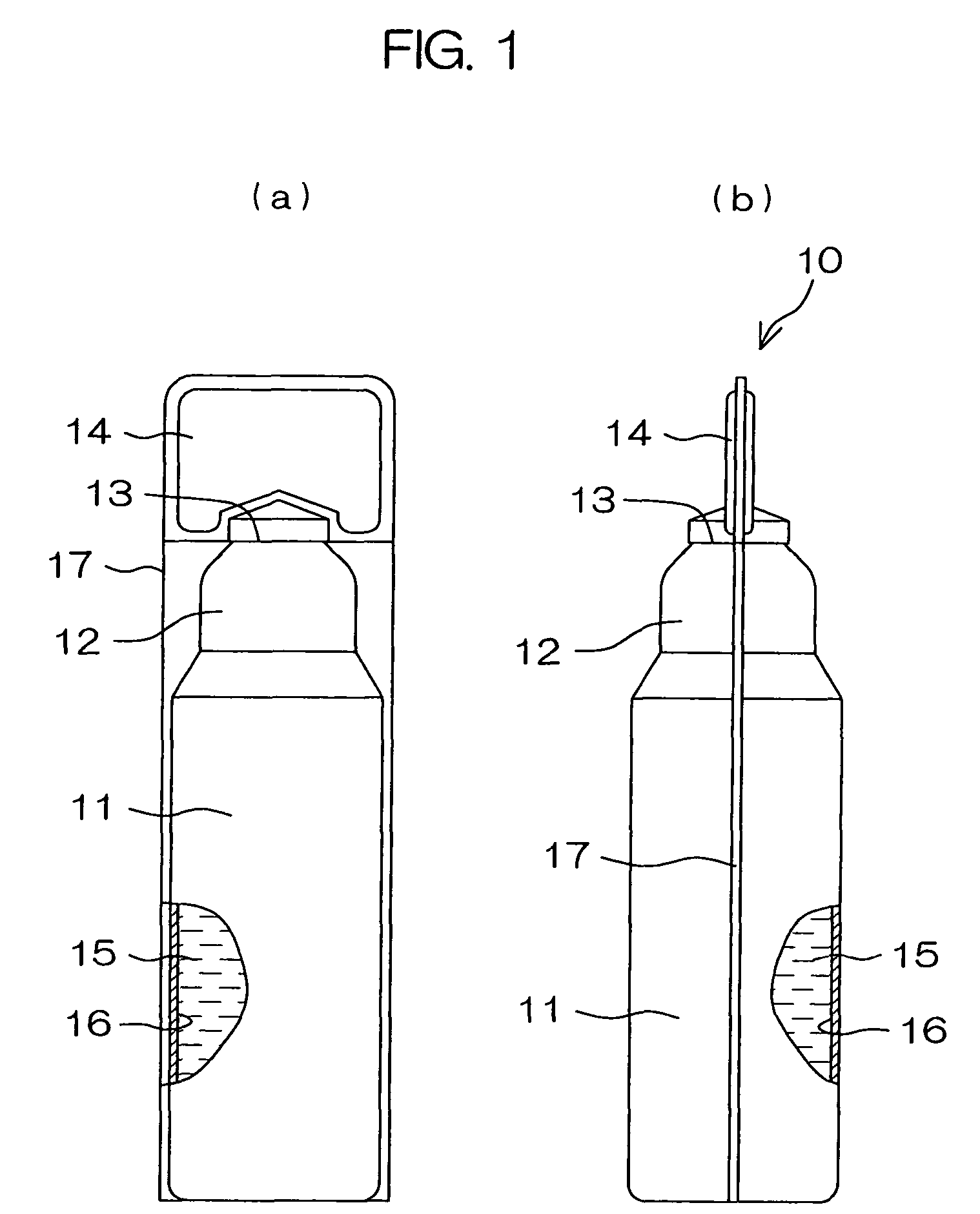
Drug solution filling plastic ampoule and process for producing the same
A drug solution filling plastic ampoule having gas, steam and light ray barrier properties, a drug permeation preventing capability and an absorption / adsorption preventing capability, and a production method for the plastic ampoule. The drug solution filling plastic ampoule includes a container body, a fusion-bonded portion which seals a mouth of the container body, and a wrench-off holder tab connected to the fusion-bonded portion. The ampoule is formed from a parison including two or more layers, at least one of which is a functional layer having at least one characteristic property selected from the group consisting of a gas permeation preventing capability, a steam permeation preventing capability, a light ray permeation preventing capability, a drug permeation preventing capability and a drug absorption / adsorption preventing capability.
Owner:OTSUKA PHARM FAB INC
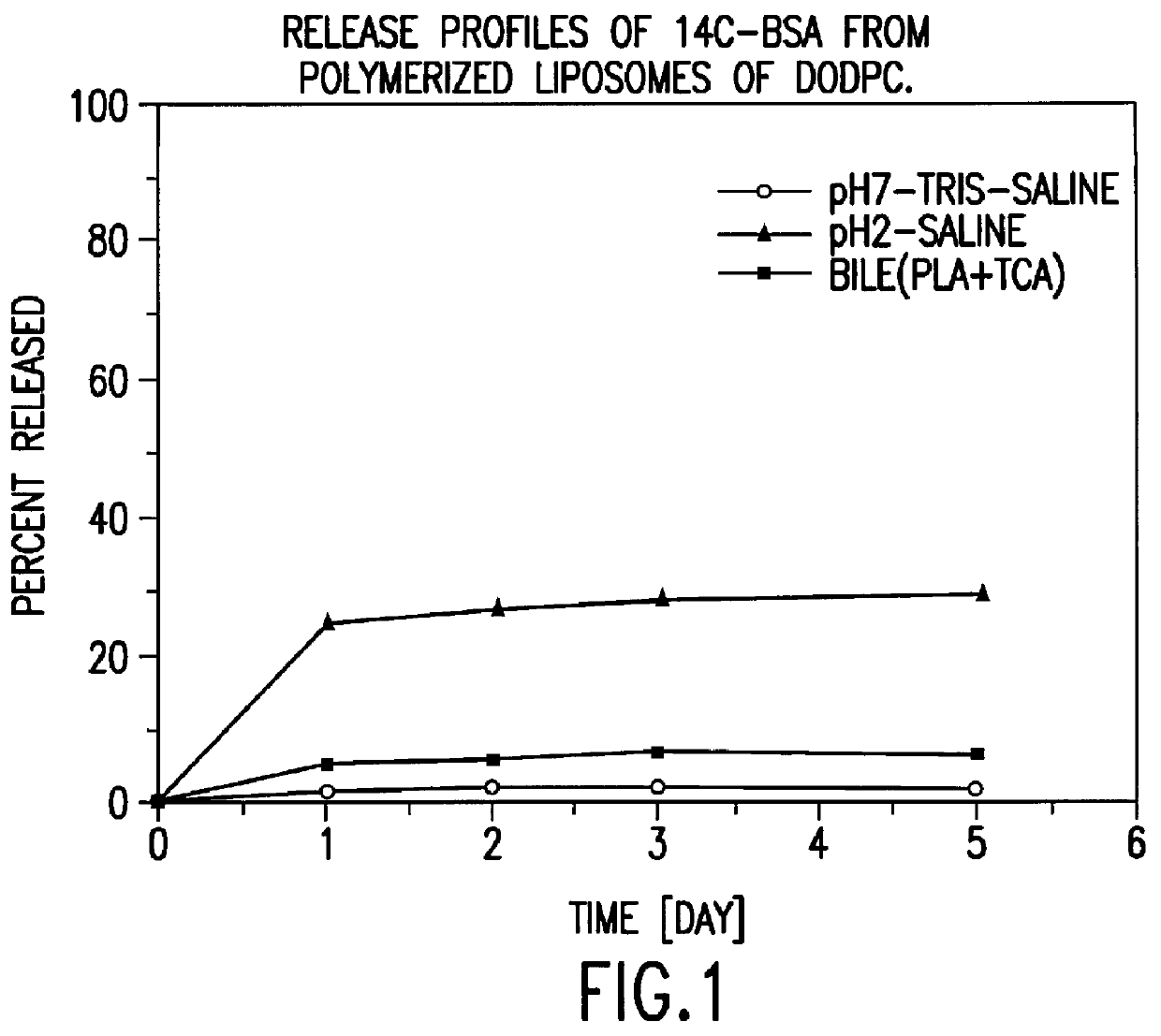
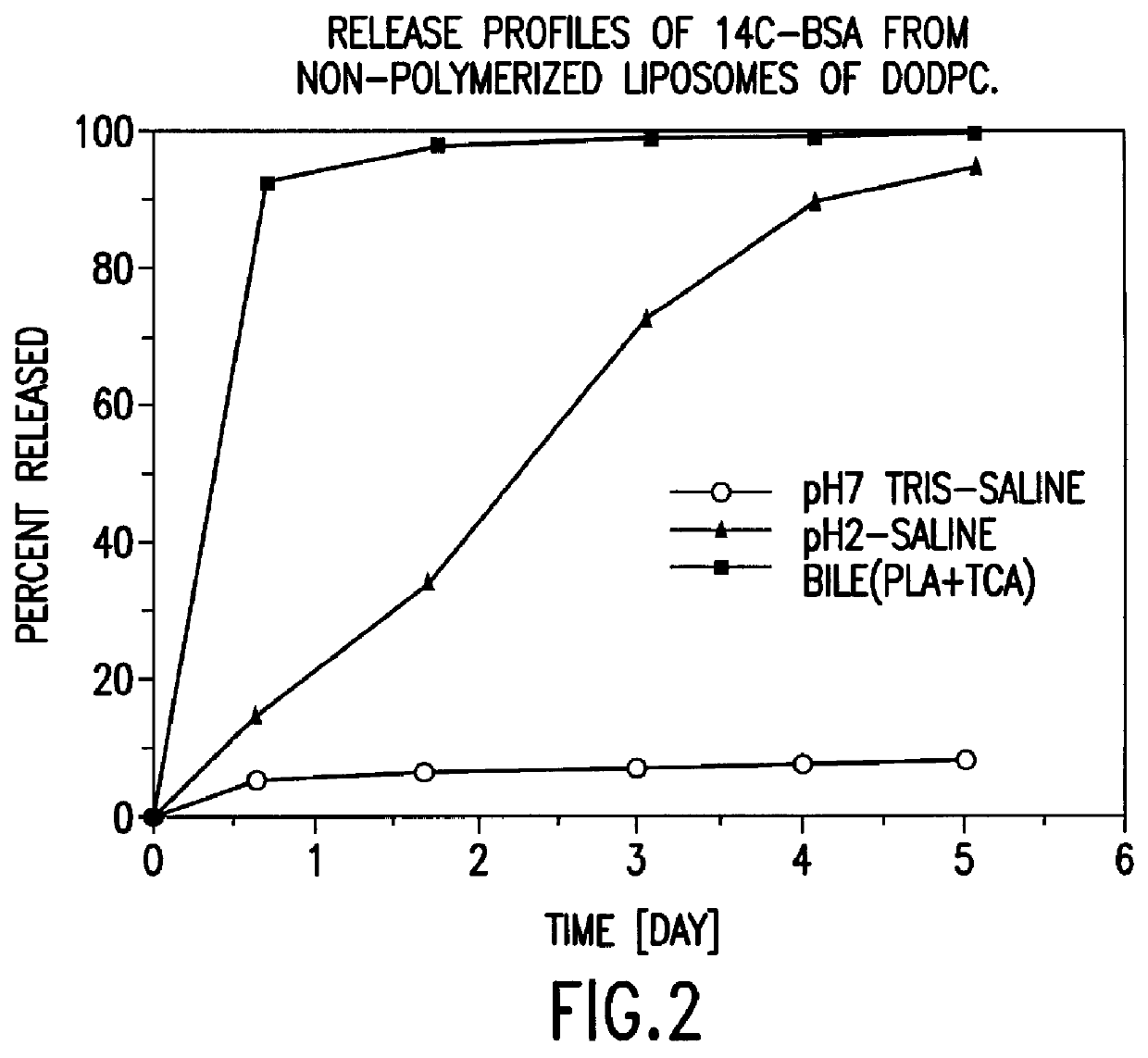
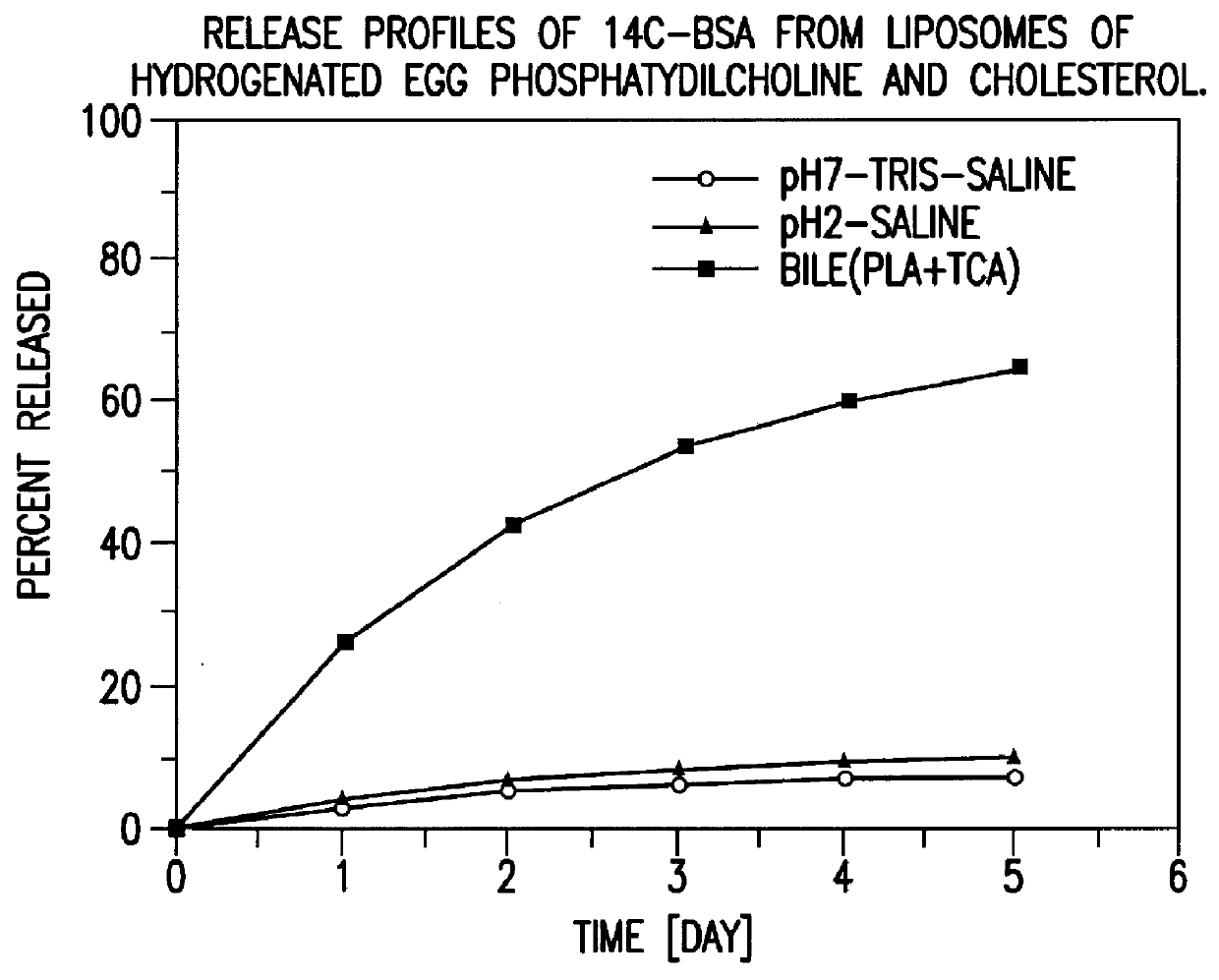
Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery
InactiveUS6060082ALessContainment leakBacterial antigen ingredientsPeptide/protein ingredientsCell typeLiposome
The present invention relates to targeted polymerized liposomes for oral and / or mucosal delivery of vaccines, allergens and therapeutics. In particular, the present invention relates to polymerized liposomes which have been modified on their surface to contain a molecule or ligand which targets the polymerized liposome to a specific site or cell type. More particularly, the invention relates to the use of polymerized liposomes modified to contain a carbohydrate or lectin on their surface.
Owner:MASSACHUSETTS INST OF TECH
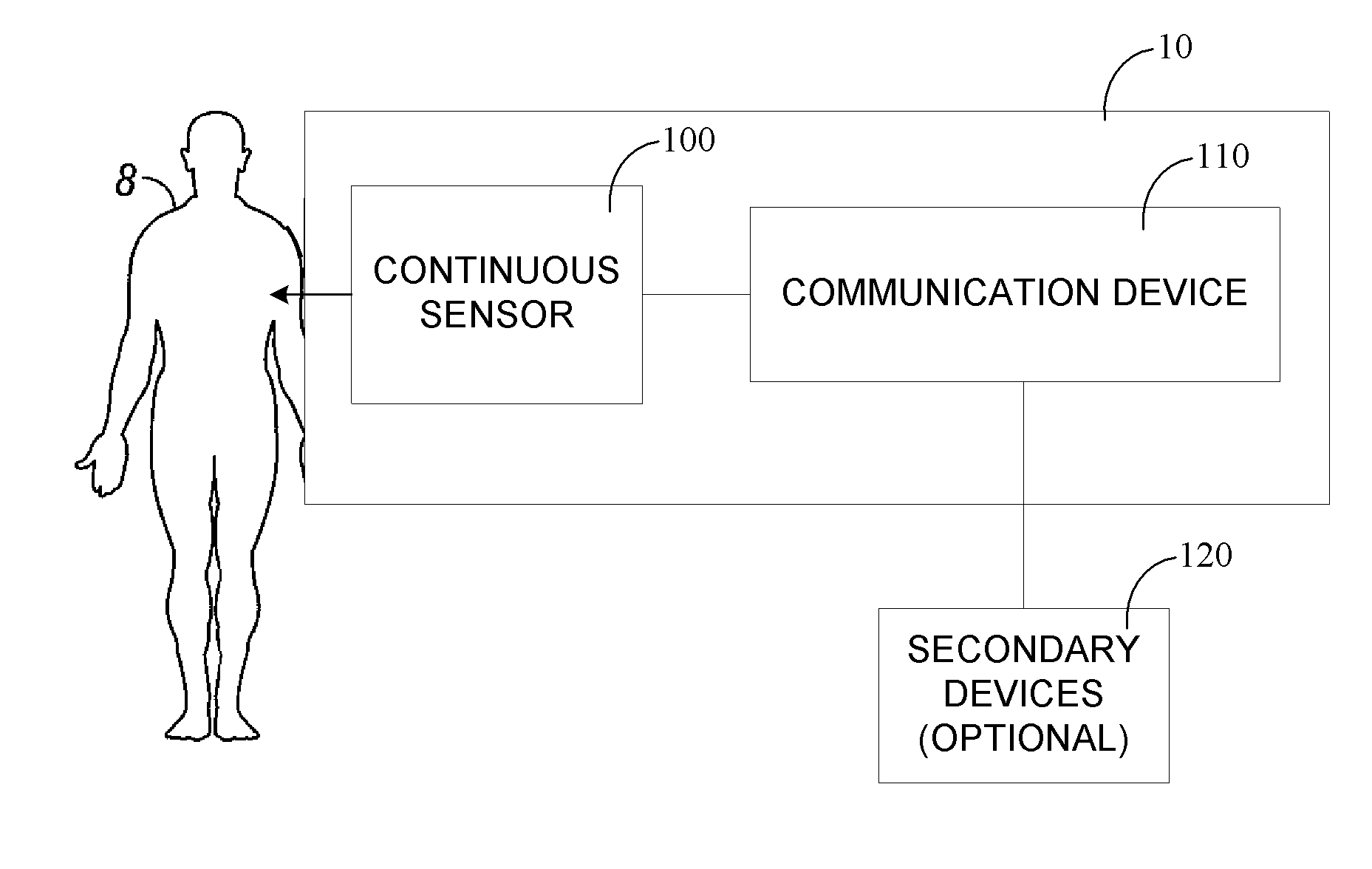
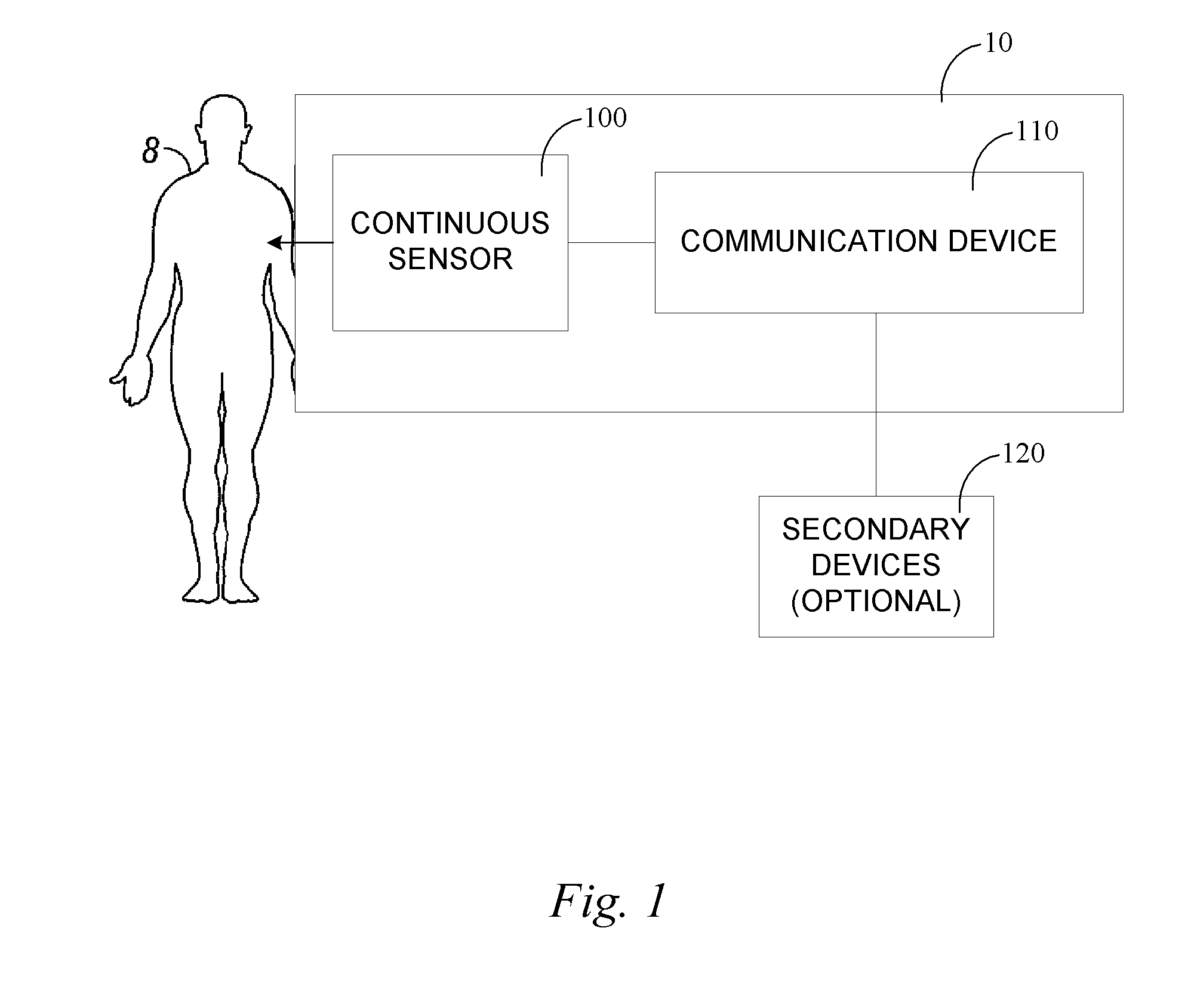
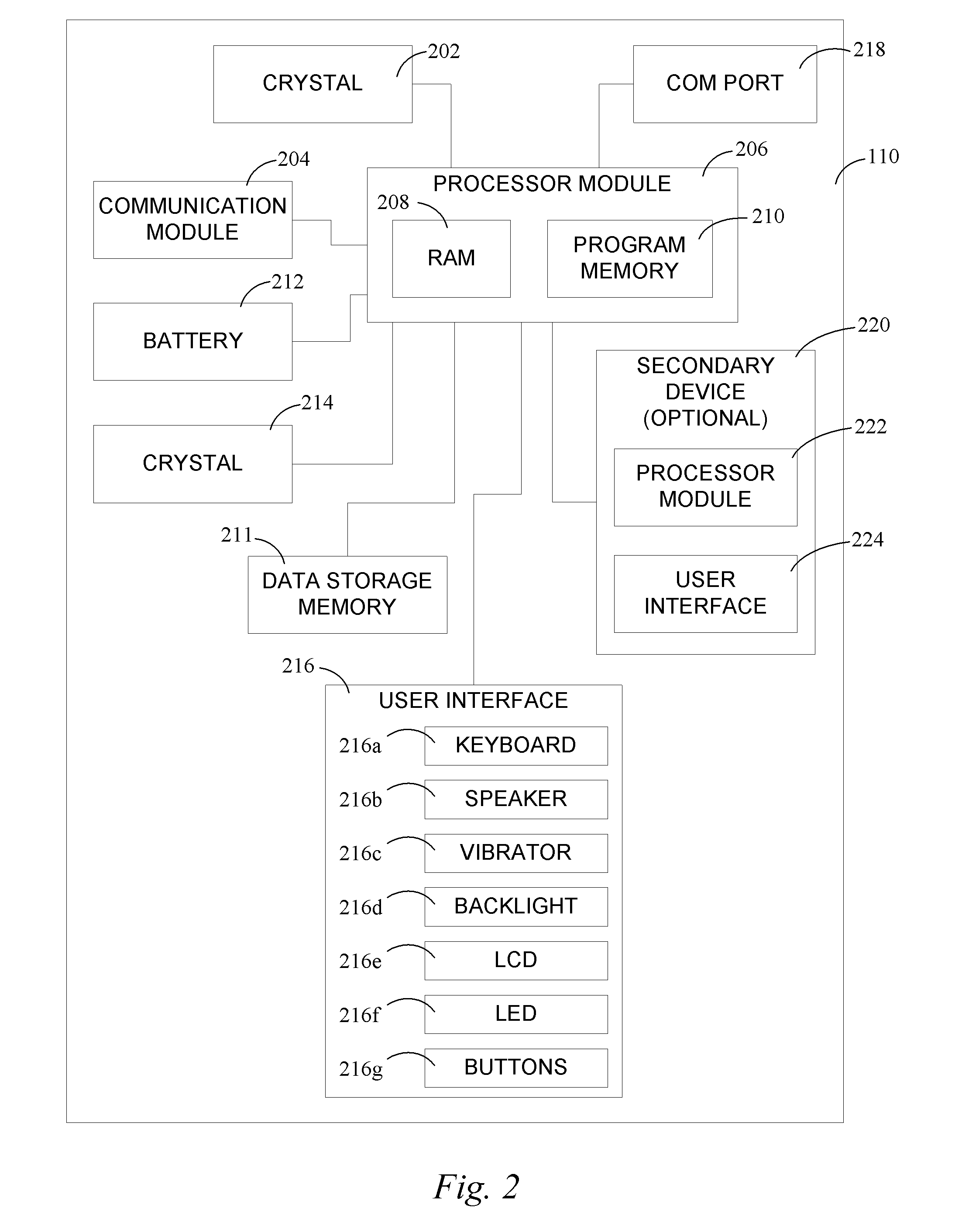
Continuous medicament sensor system for in vivo use
Systems and methods for continuous measurement of a medicament in vivo are provided. In some embodiments, the system is configured to provide information associated with medicament titration and includes a continuous analyte sensor and a communication device. In some embodiments, the system is configured for continuous ambulatory drug testing, including an ambulatory host monitor having a continuous sensor, a location module, a processor module and a transmitter. In some embodiments, the system is configured for continuously monitoring a hormone level and includes a continuous hormone sensor and a communication device configured to output hormone information in real time. Yet another embodiment provides an analyte sensor for continuous monitoring of a host's nutritional status, and is configured for both continuous glucose detection and continuous albumin detection.
Owner:DEXCOM
Portable remote patient telemonitoring system using a memory card or smart card
InactiveUS6454708B1Low costIncrease the number ofSurgeryRespiratory organ evaluationSmart cardFull waveform
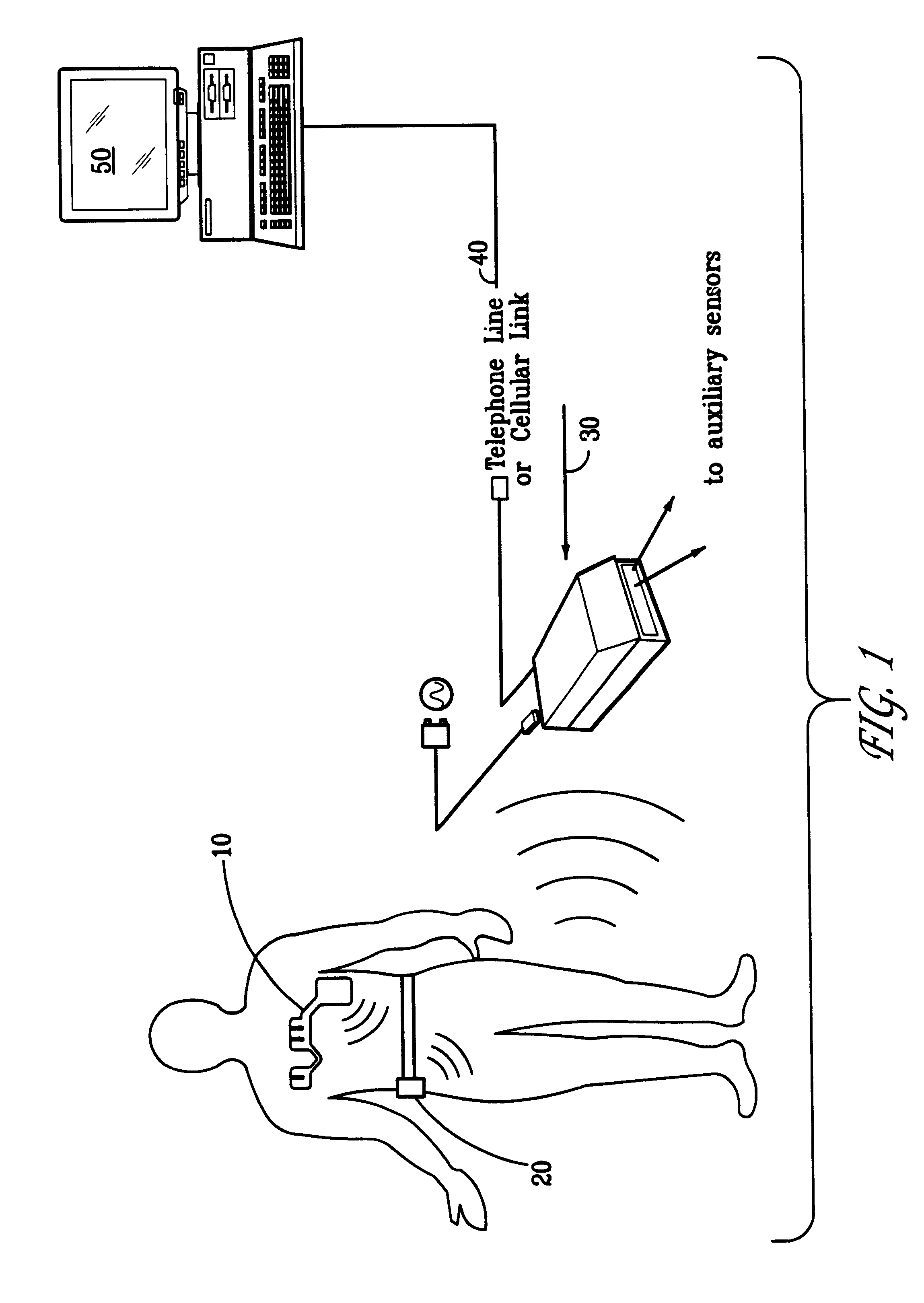
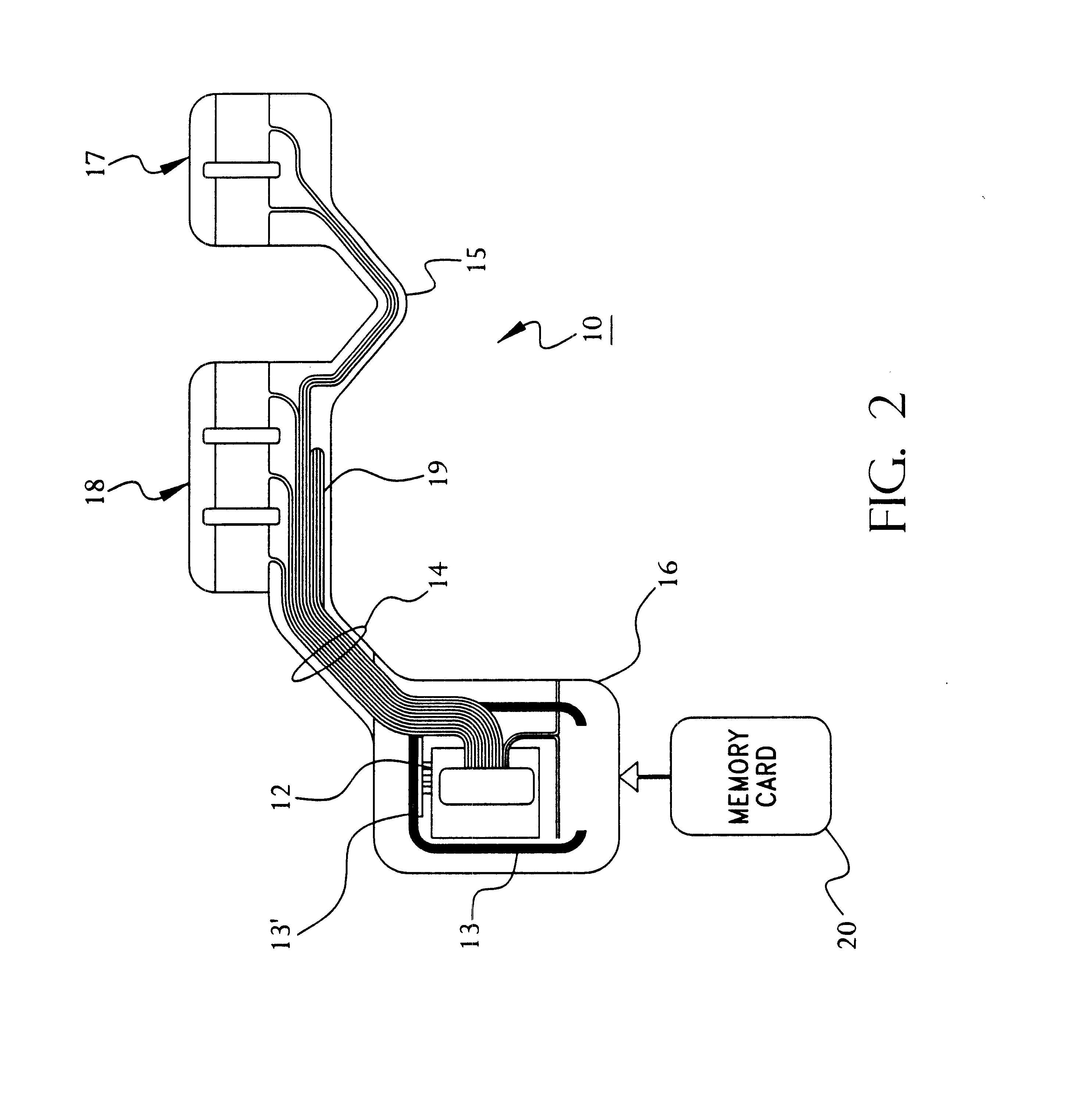
A system and method for monitoring health parameters and capturing data from a subject. The system is characterized by a cordless, disposable sensor band with sensors for measuring full waveform ECG, full waveform respiration, skin temperature, and motion, and a connector which accepts a memory card or a smart card for storage of the measured data. After a predetermined period of time, such as when the sensor band is removed, the memory card or smart card is removed and inserted into a monitoring device which reads the stored health parameter data of the subject. The monitoring device includes a base station that includes a memory / smart card reader and is connected to conventional phone lines for transferring the collected data to a remote monitoring station. The base station may also capture additional clinical data, such as blood pressure data, and to perform data checks. Subject safety is enhanced by the ability of the base station to compare clinical data, e.g. ECG, against given profiles and to mark events when appropriate or when the base station is programmed to do so. The remote monitoring station allows the presentation and review of data (including events) forwarded by the sensor band. ECG analysis software and a user-friendly graphical user interface are provided to remotely analyze the transmitted data and to permit system maintenance and upkeep. In alternative embodiments, a smart card includes the sensor band's electronics and / or signal transmission circuitry in conjunction with a portable data logger so that the electronics may be reused from one disposable sensor band to the next without limiting the patient's range of movement. The system of the invention has useful application to the collection of subject clinical data during drug trials and medical testing for regulatory approvals as well as management of subjects with chronic diseases.
Owner:CLEARPATH PARTNERS
Active suture for the delivery of therapeutic fluids
An active suture that can be used for the delivery of therapeutic fluids to the tissue surrounding a wound is disclosed. The active suture may include a connector designed to join a fluid source, such as a syringe, conventional IV delivery system, or infusion pump to an internal passageway that is embedded within a braided suture. The internal passageway may be comprised of a fine polymeric tube and is capable of conducting and emitting a fluid into at least a portion of the braided suture and surrounding tissue. The invention enables delivery of an efficacious volume of drug bearing solution on the order of milliliters per day, provides a high level of fluid delivery rate control enabling the physician to start or stop drug administration at his / her discretion, and offers a means of providing more than one type of medication that may be selected post-surgically in accord with unexpected patient symptoms that may arise.
Owner:ETHICON INC
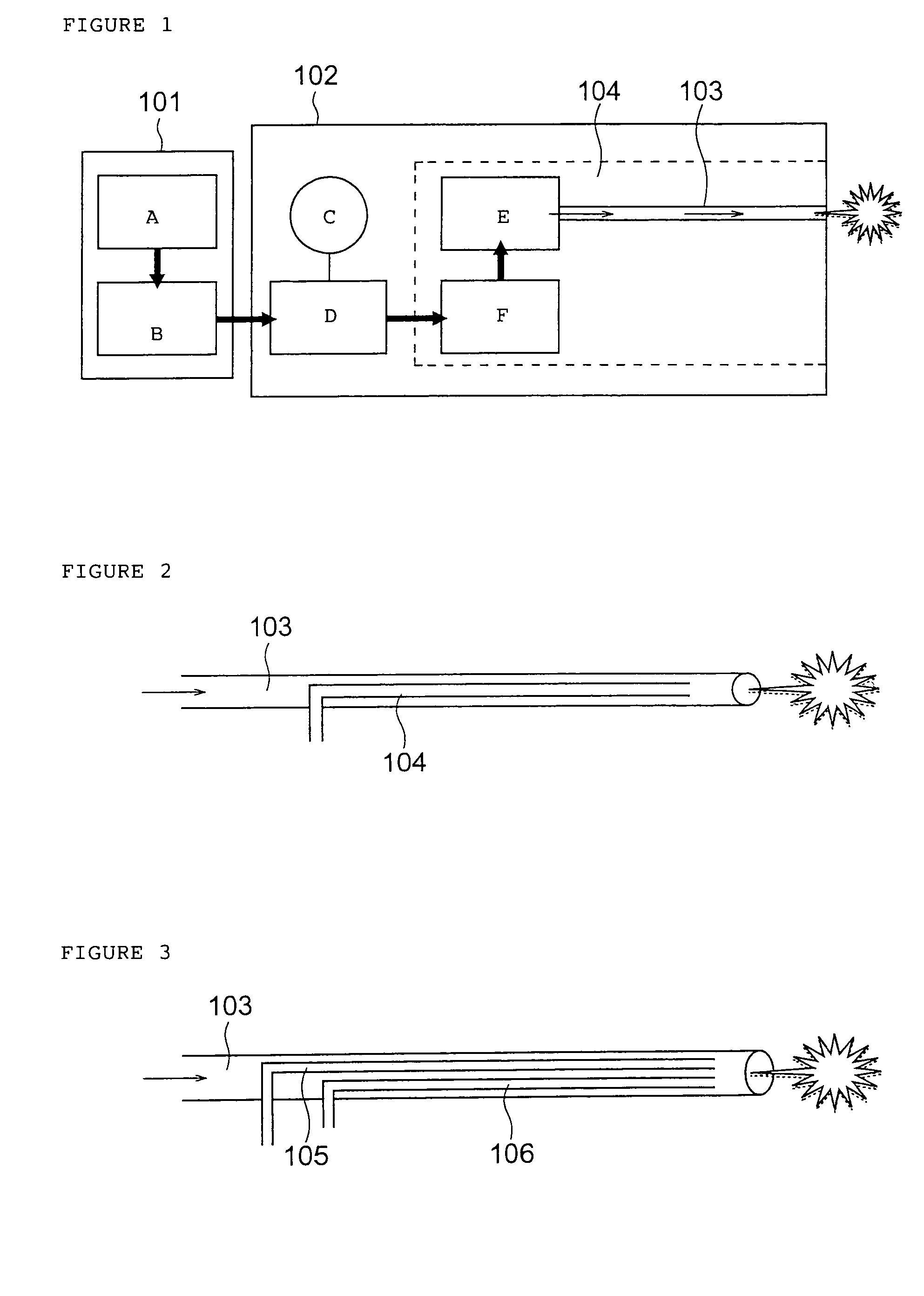
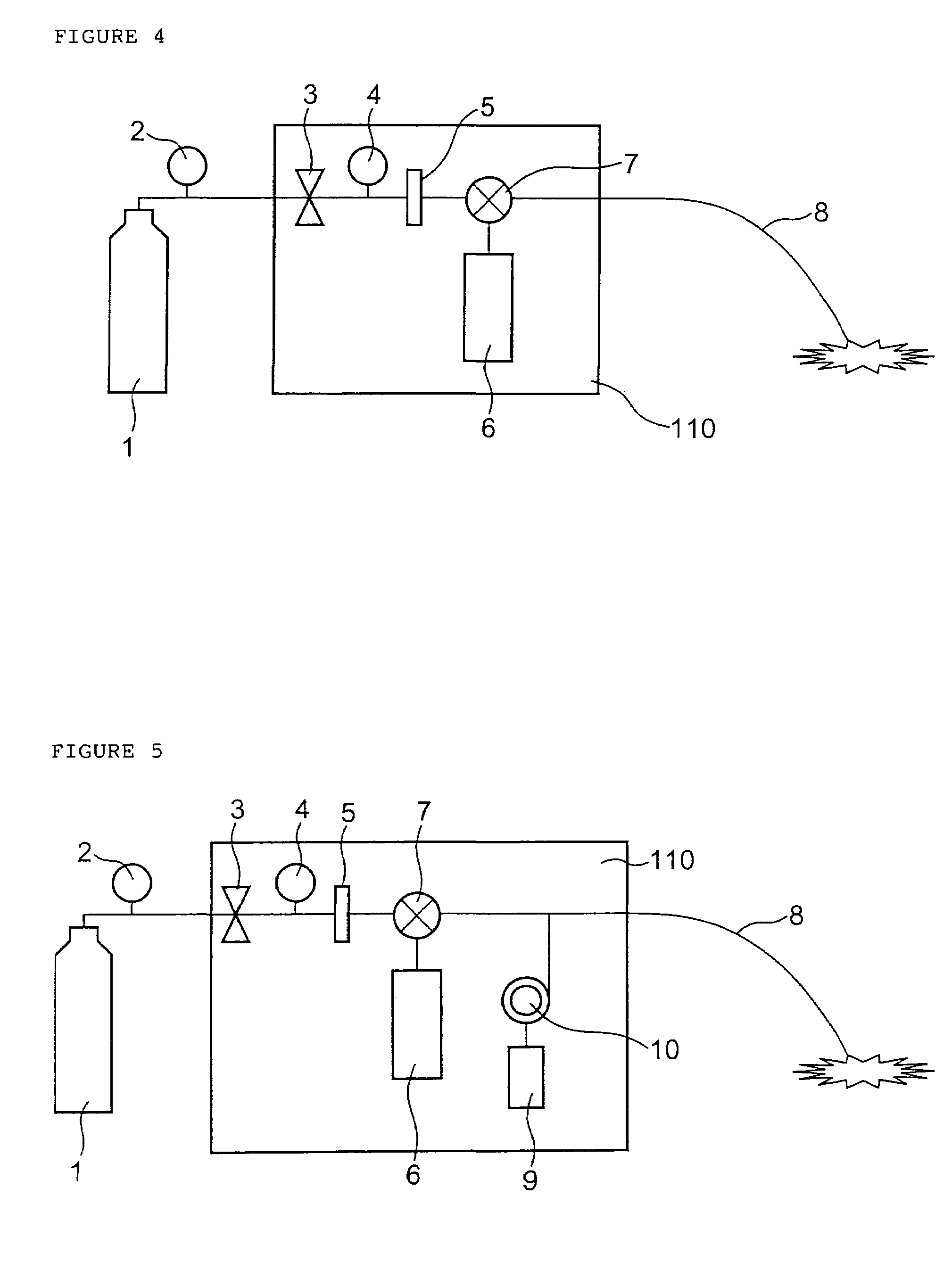
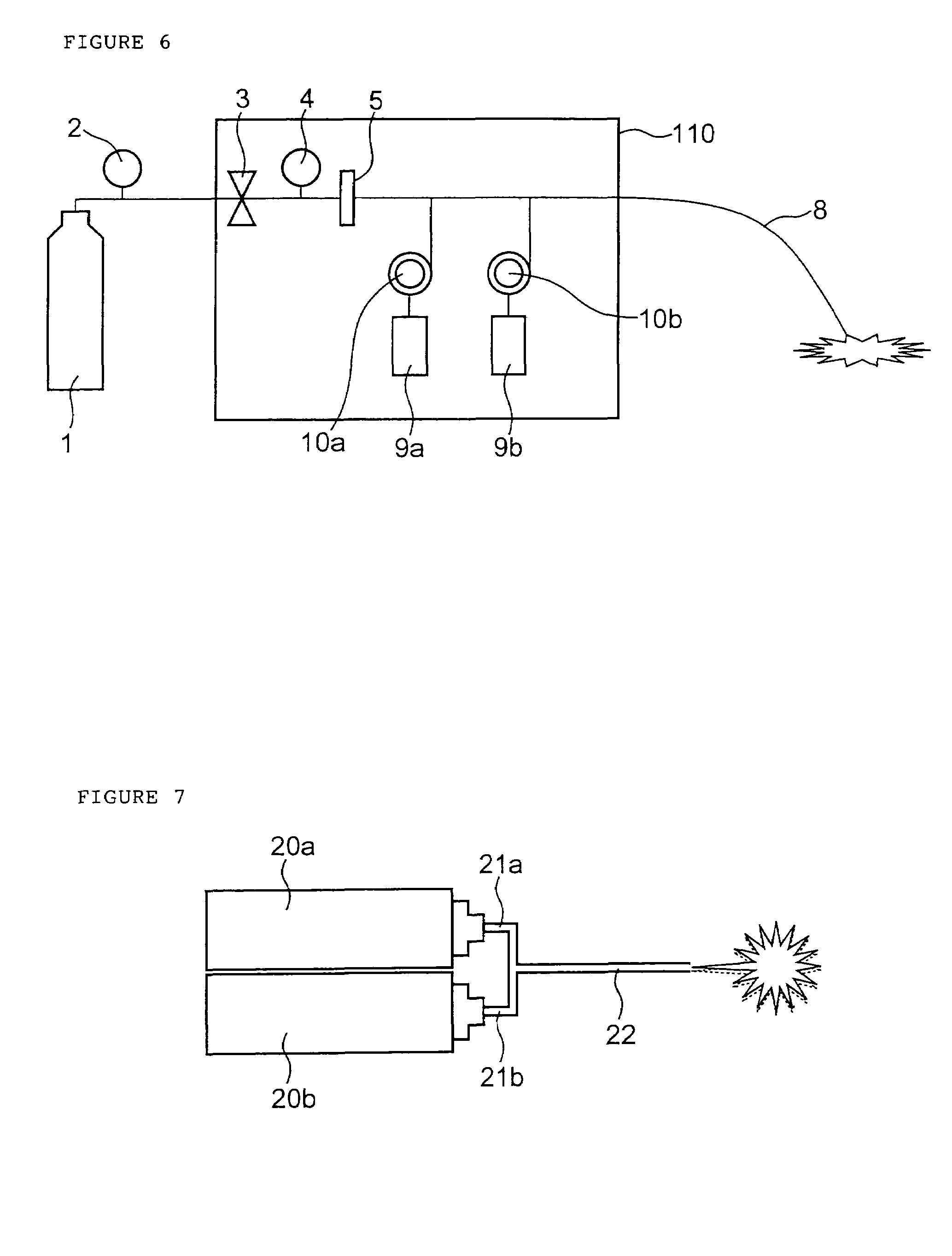
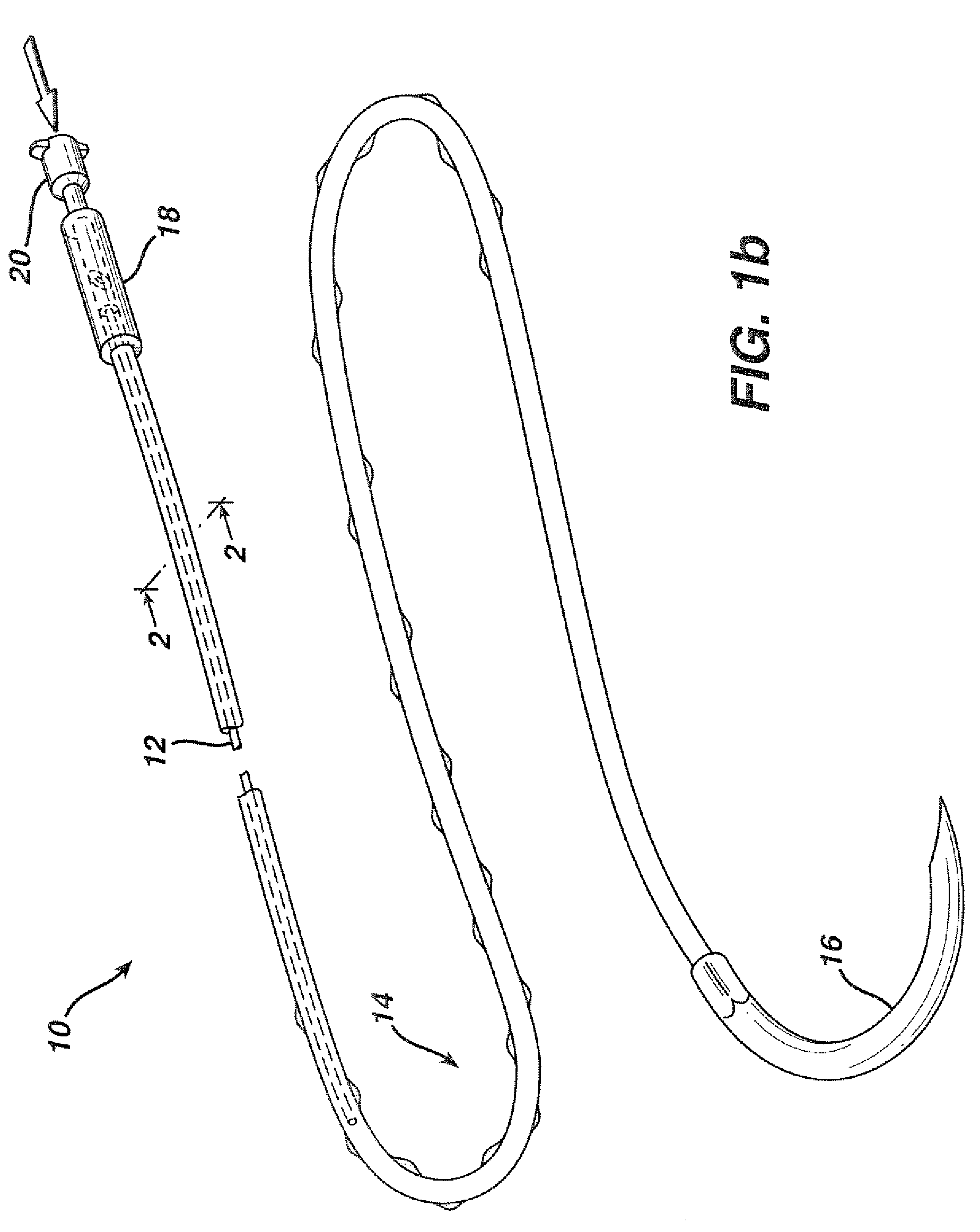
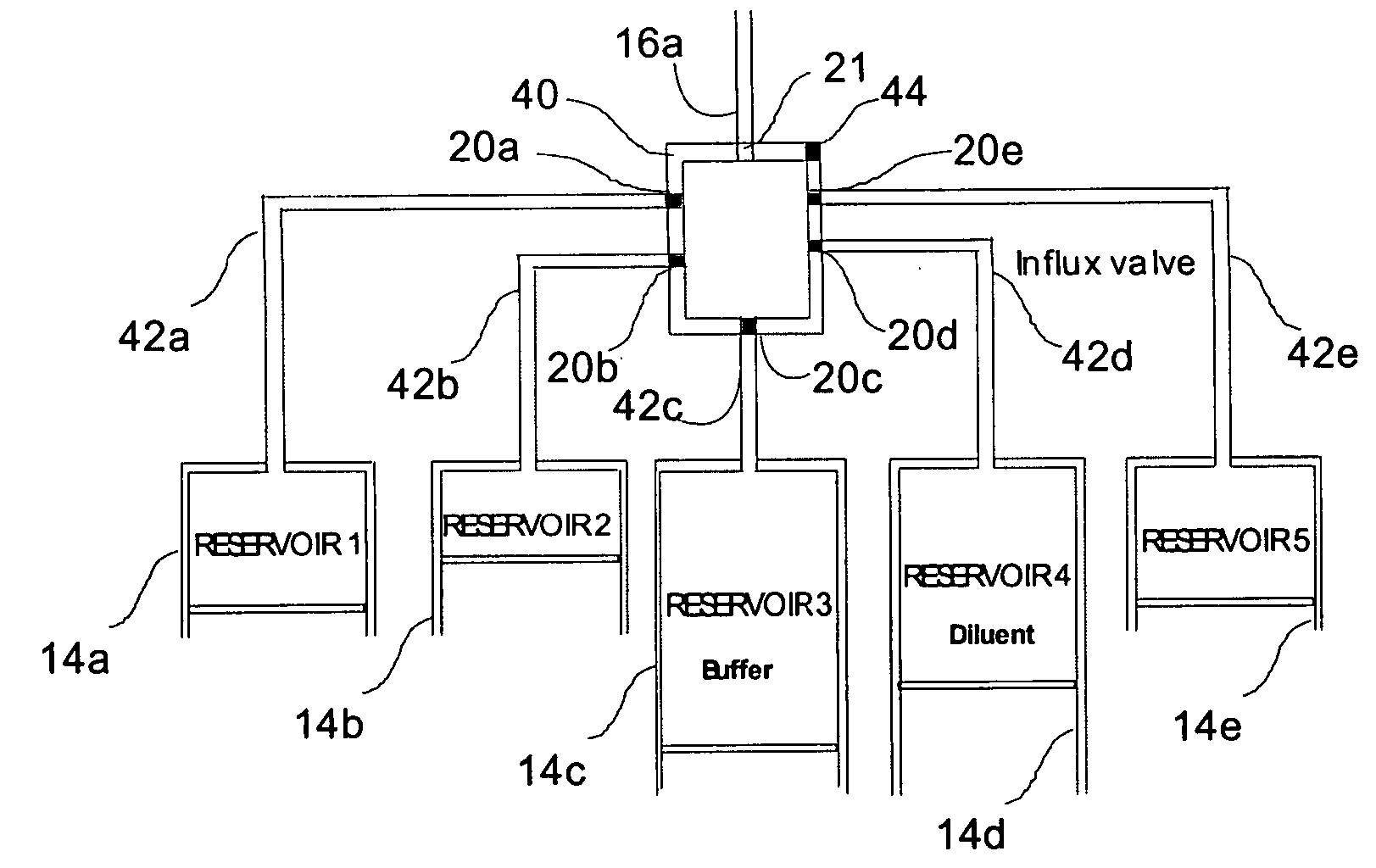
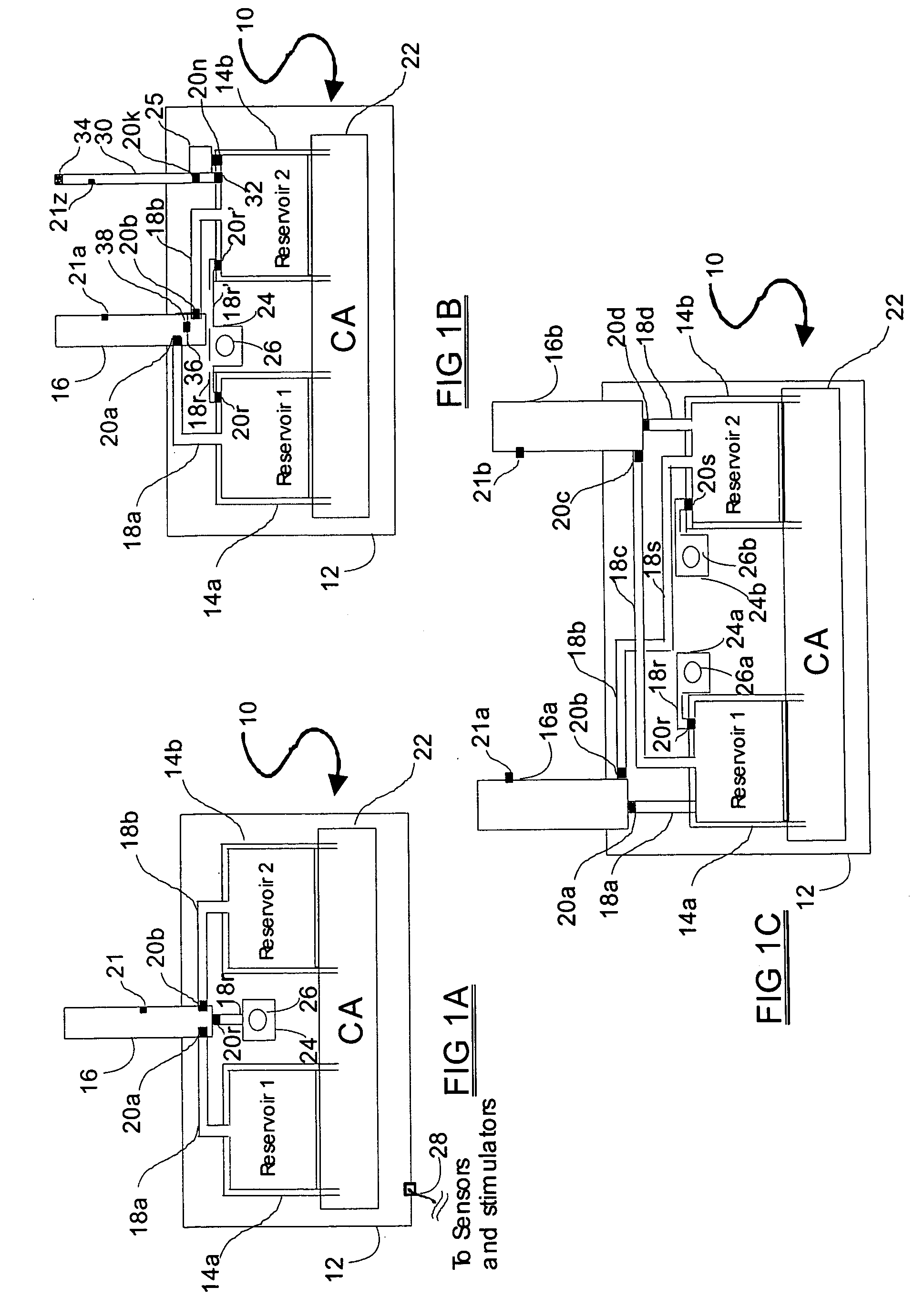
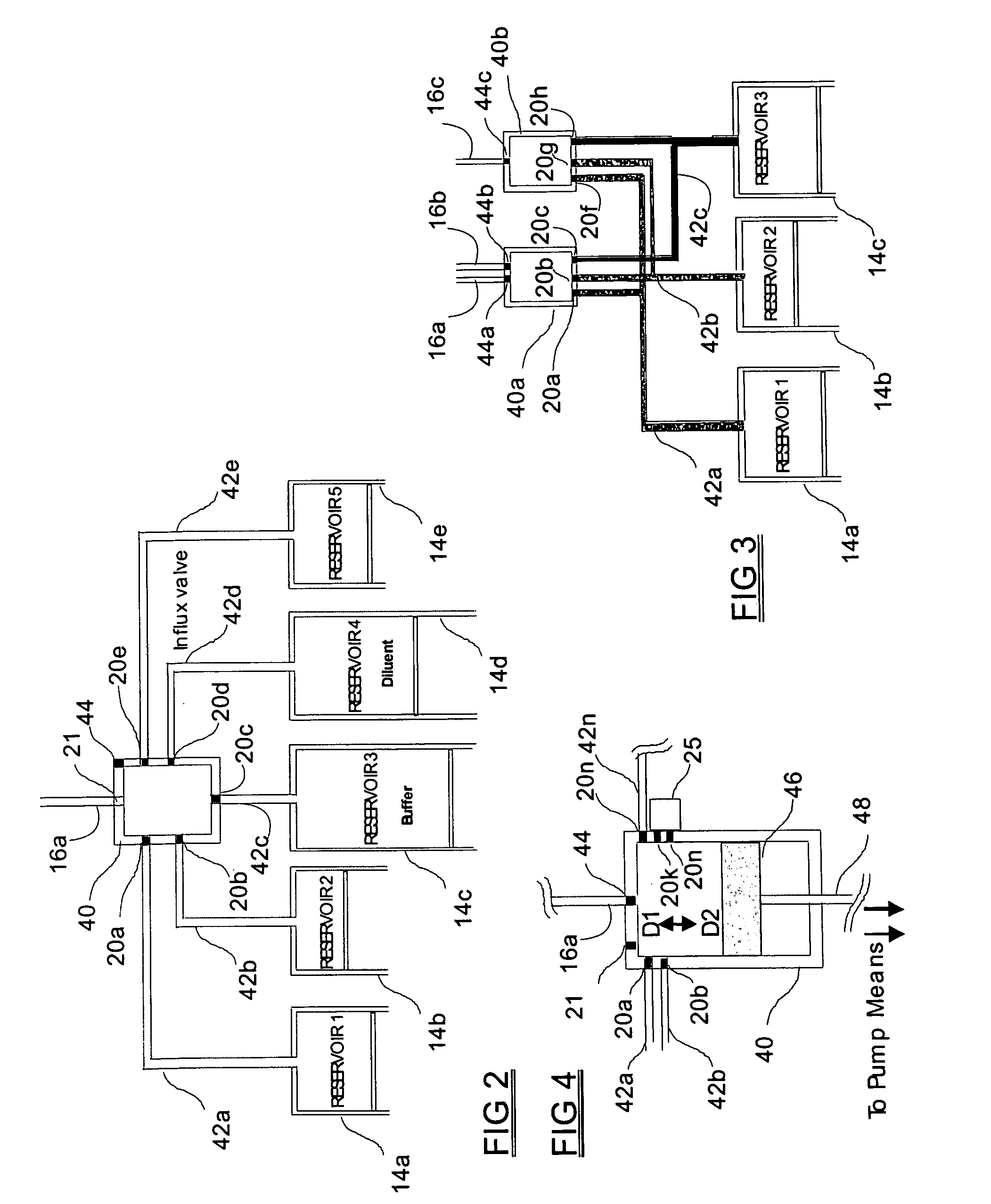
Programmable medical drug delivery systems and methods for delivery of multiple fluids and concentrations
InactiveUS20050277912A1Extension of timeEasy to fillMulti-lumen catheterDrug and medicationsControl mannerDelivery system
A drug delivery system provides for mixing various drugs in an optimally controlled manner, for using flow controllers to guide multiple drugs into a single or into multiple catheters, for enabling a single lumen catheter to treat a specific region with several drugs, for allowing for dilution of a concentrated drug in order to both increase the time between refilling and also for providing any concentration of a drug that might be desired, for using a buffer fluid to deliver exact amounts of several drugs from the same catheter or to separate several drugs within a single catheter, for using external fluid present in the human body either as a diluent or buffer fluid, and for providing for a drug testing / filler apparatus to be used prior to implant to ensure proper function and easy means of filling multiple reservoirs with different fluids, and also after implant for refilling operations. The drug delivery system (DDS) can perform both bolus and continuous delivery of substances, and enable the measured delivery of any one of several drugs to one or more distal locations at independently programmable rates. New types of catheter systems and uses therefore are also described. Catheter hub assemblies allowing for easy replacement of drug delivery systems offer advantages when replacing drug delivery systems. New methods for using the DDS in the promotion of healthy pregnancy and treatment of a developing fetus are also possible.
Owner:JOHN SASHA
Medicine inhaler and medicine ejection method
InactiveUS7896006B2Efficiently inhaledAccurate doseRespiratorsOperating means/releasing devices for valvesOpen air environmentBiomedical engineering
A medicine ejection device is provided allowing a medicine with a desired droplet diameter to be inhaled at a constant rate. The medicine ejection device has a decision part at which the ejection operating conditions of a medicine ejection part for ejecting the medicine are decided in accordance with at least one of an open-air environment or a state of the medicine during use of the device. The device ejects the medicine according to the ejection operating conditions decided by the decision part.
Owner:CANON KK
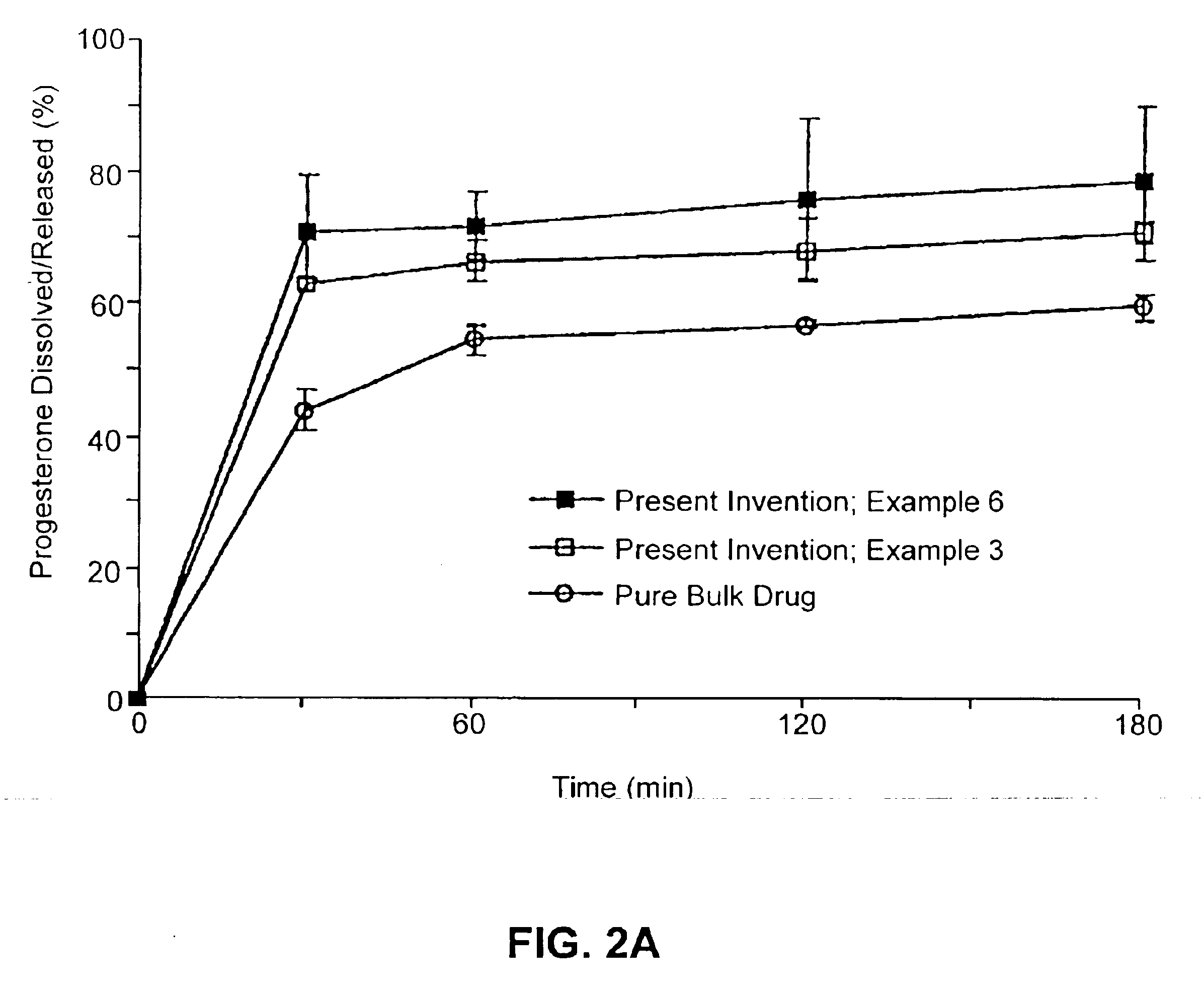
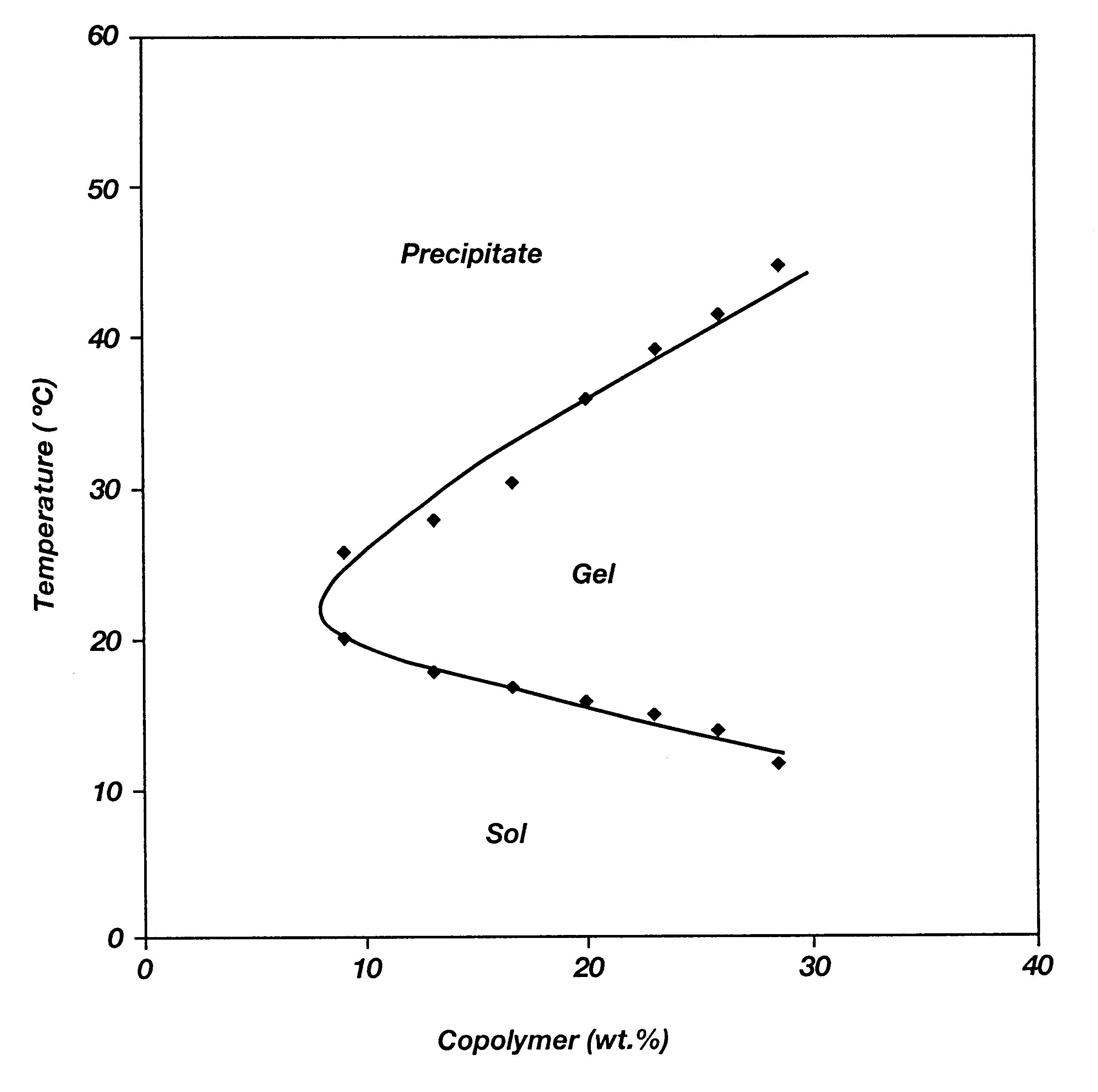
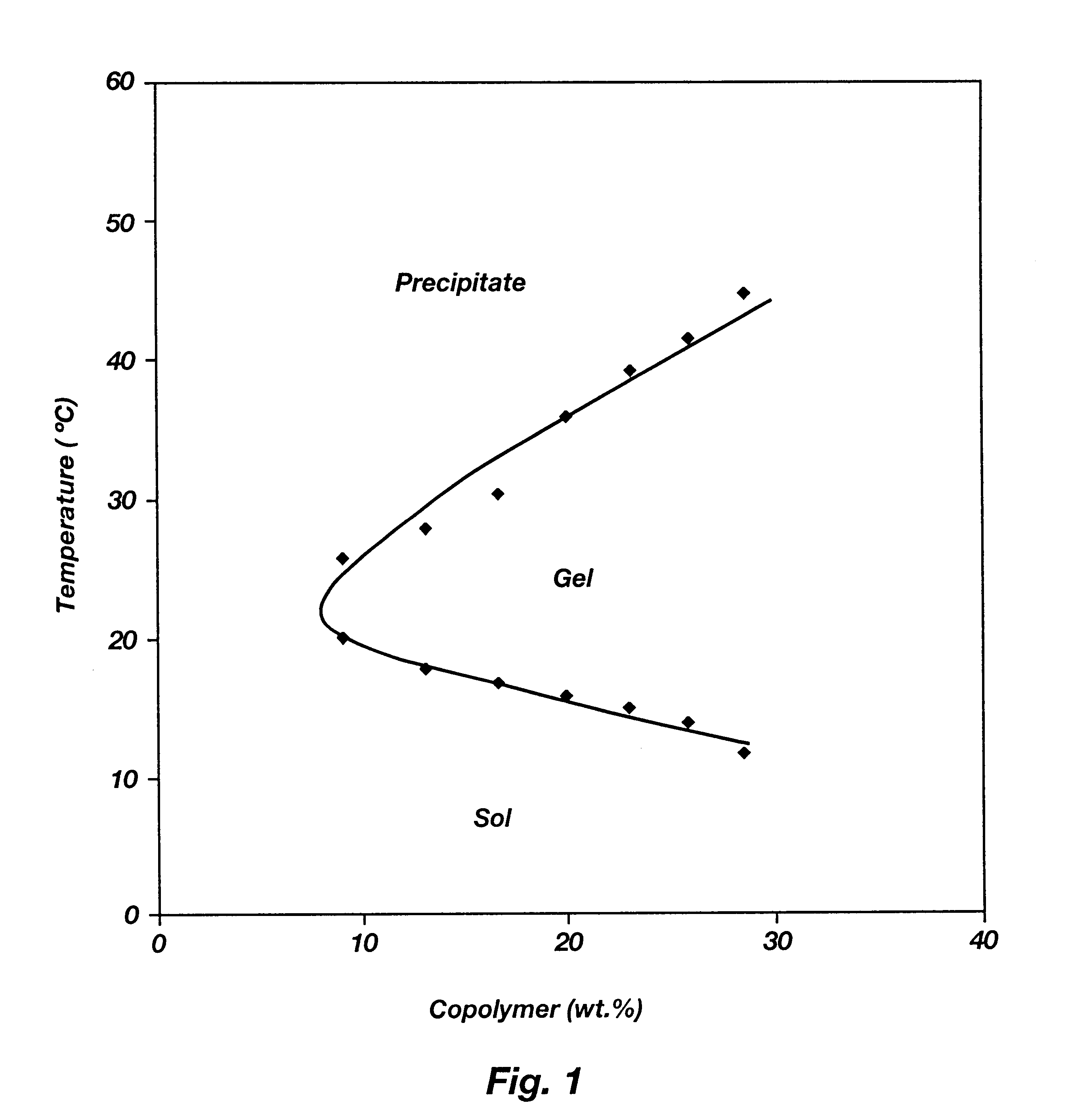
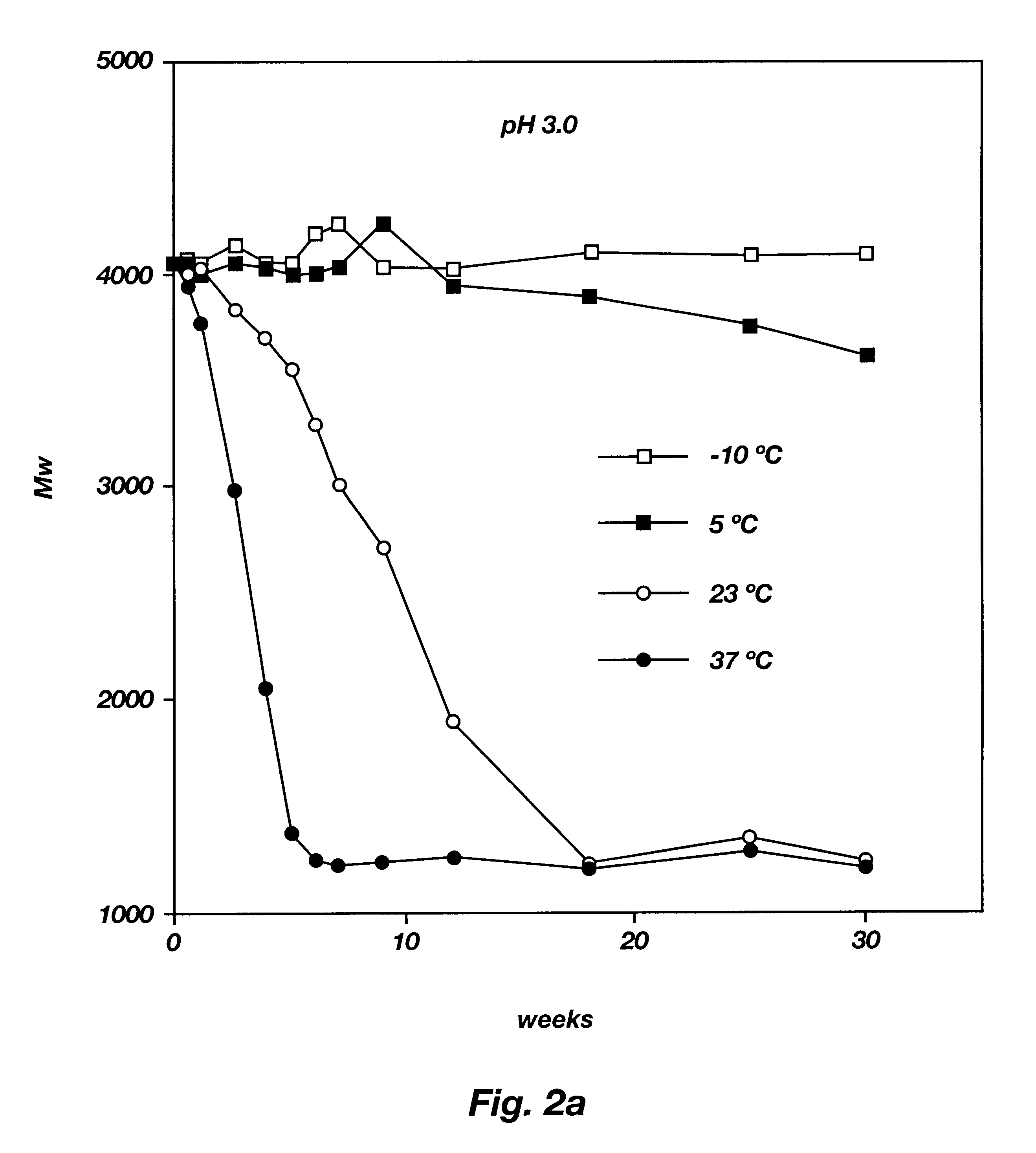
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Tissue electroperforation for enhanced drug delivery
The present invention relates to a method and a device for transporting a molecule through a mammalian barrier membrane of at least one layer of cells comprising the steps of: ablating the membrane with an electric current from a treatment electrode; and utilizing a driving force to move the molecule through the perforated membrane.
Owner:LIFESCAN INC
In vivo biosensor apparatus and method of use
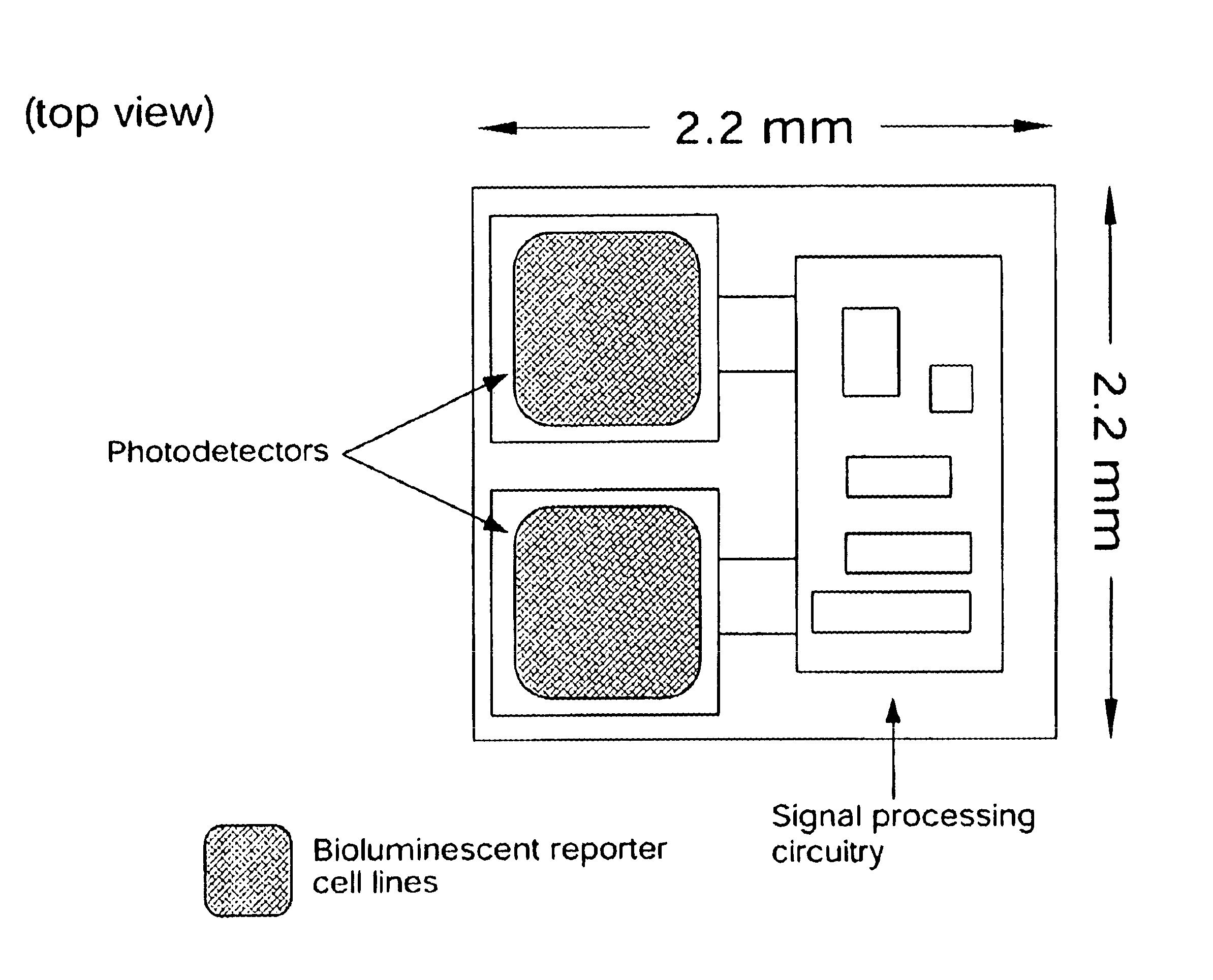
InactiveUS6673596B1Less can be administeredCost-effective administration of drugBioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoGenetically engineered
Disclosed are bioluminescent bioreporter integrated circuit devices that detect selected analytes in fluids when implanted in the body of an animal. The device comprises a bioreporter that has been genetically engineered to contain a nucleic acid segment that comprises a cis-activating response element that is responsive to the selected substance operably linked to a gene encoding a bioluminescent reporter polypeptide. In preferred embodiments, the target analyte is glucose, glucagons, or insulin. Exposure of the bioreporter to the target substance causes the response element to up-regulate the nucleic acid sequence encoding the reporter polypeptide to produce a luminescent response that is detected and quantitated. In illustrative embodiments, the bioreporter device is encapsulated on an integrated circuit that is capable of detecting the emitted light, processing the resultant signal, and then remotely reporting the results. Also disclosed are controlled drug delivery systems capable of being directly or indirectly controlled by the detection device that provide drugs such as insulin to the animal in reponse to the amount of target analyte present in the body fluids.
Owner:UNIV OF TENNESSEE RES FOUND +1
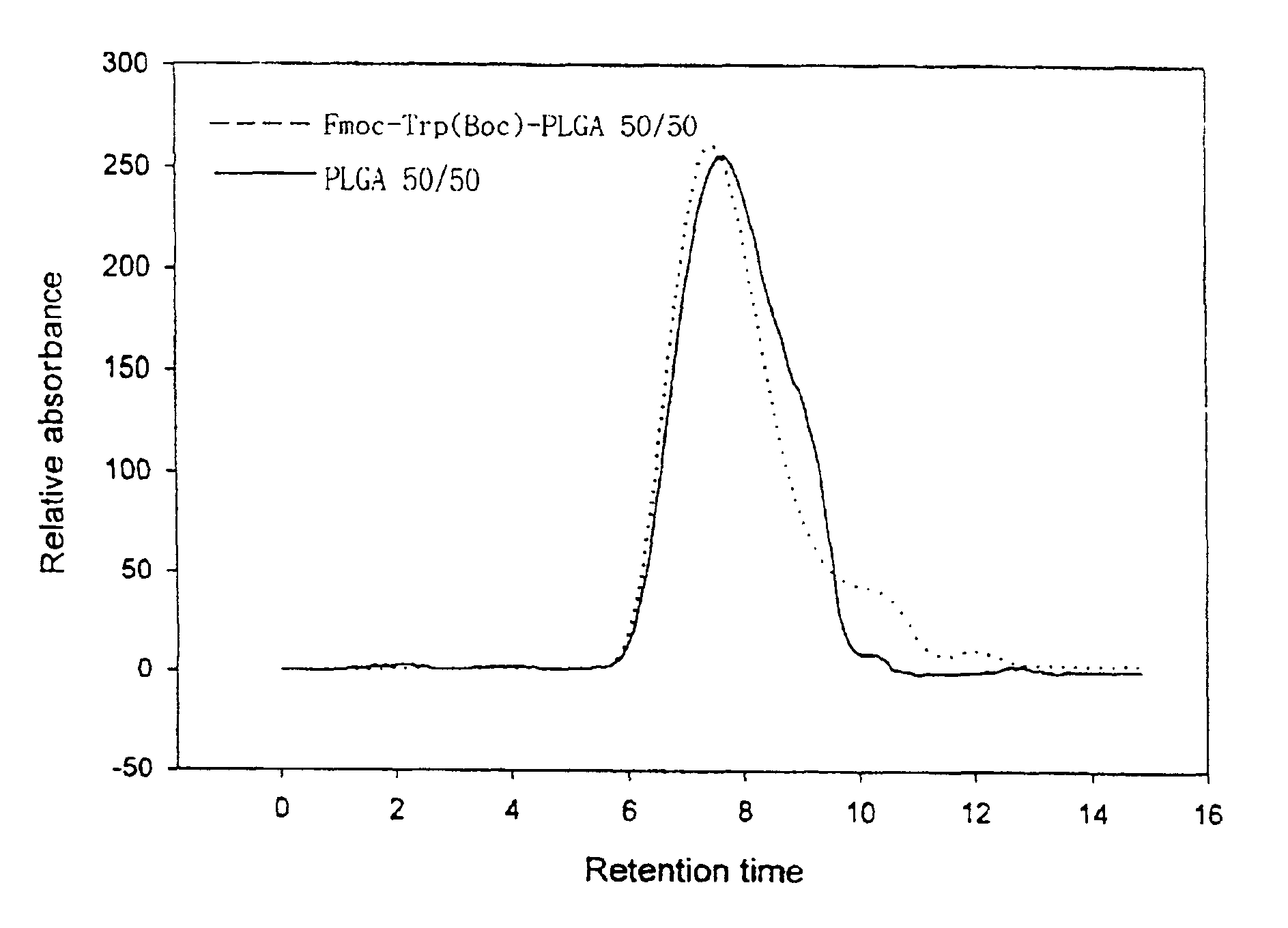
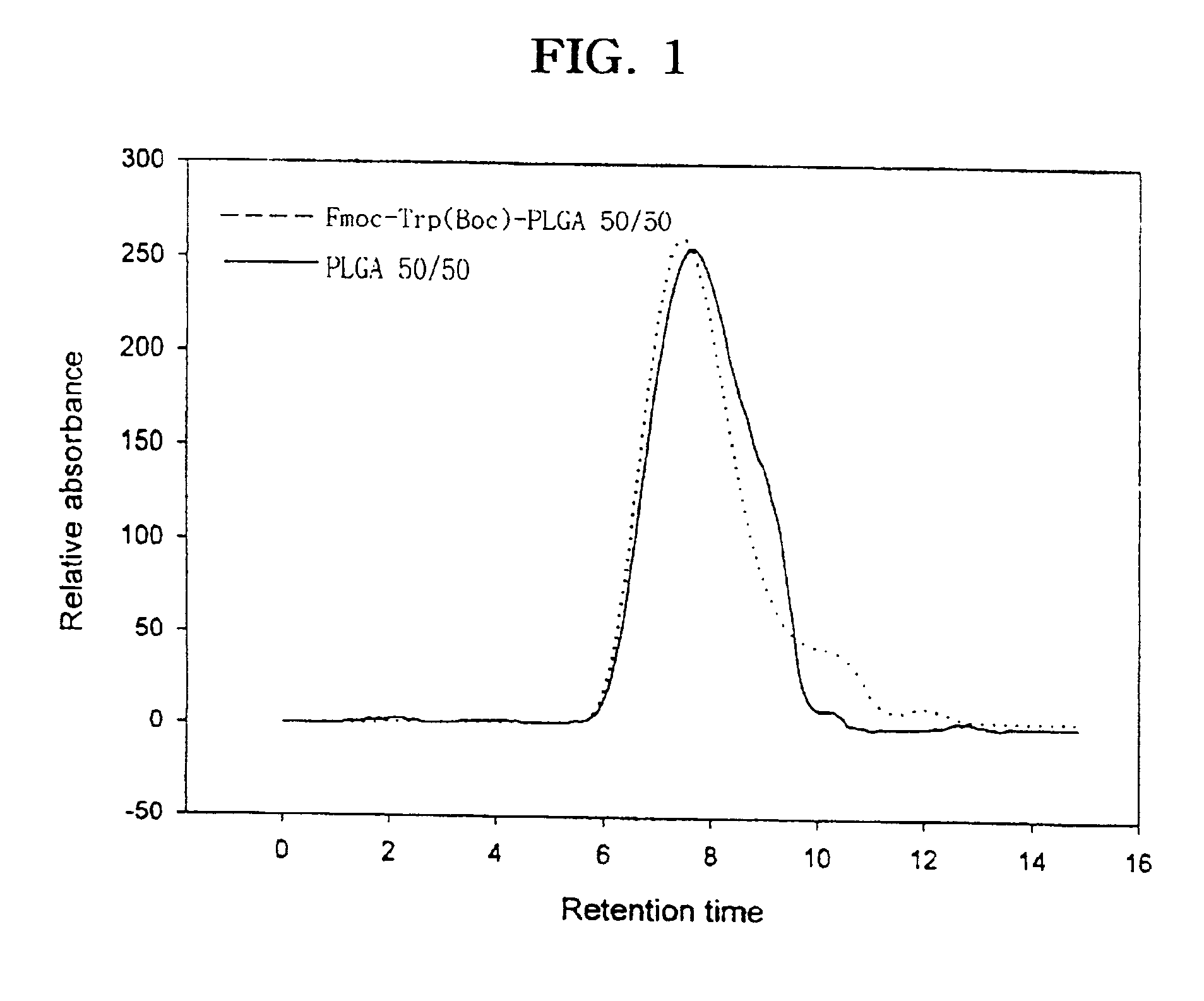
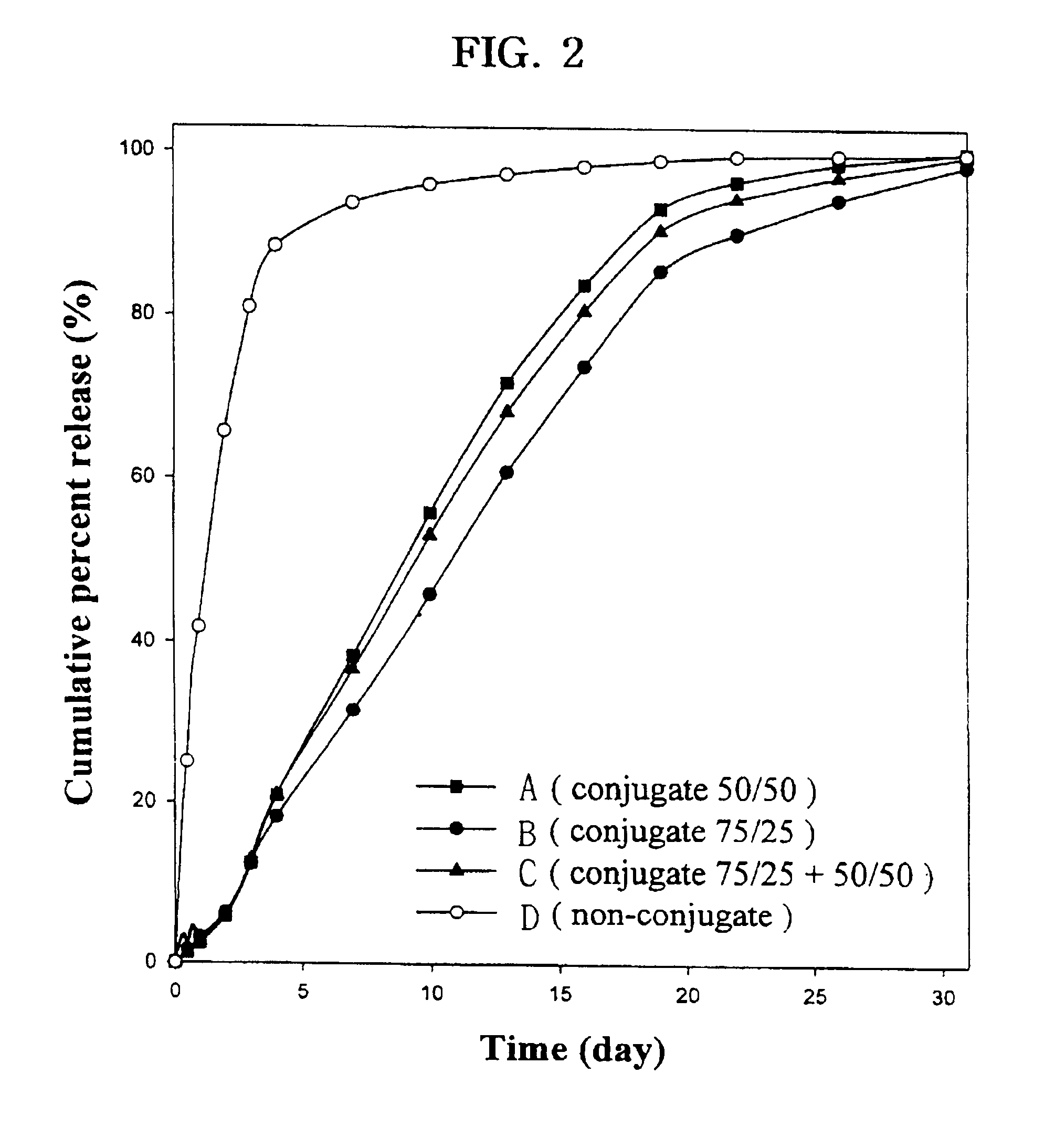
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
Membrane for use with implantable devices
ActiveUS20100087724A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteBiointerface
The present invention provides a biointerface membrane for use with an implantable device that interferes with the formation of a barrier cell layer including; a first domain distal to the implantable device wherein the first domain supports tissue attachment and interferes with barrier cell layer formation and a second domain proximal to the implantable device wherein the second domain is resistant to cellular attachment and is impermeable to cells. In addition, the present invention provides sensors including the biointerface membrane, implantable devices including these sensors or biointerface membranes, and methods of monitoring glucose levels in a host utilizing the analyte detection implantable device of the invention. Other implantable devices which include the biointerface membrane of the present invention, such as devices for cell transplantation, drug delivery devices, and electrical signal delivery or measuring devices are also provided.
Owner:DEXCOM INC
Methods and devices for renal nerve blocking
InactiveUS6978174B2Shorten the progressResolution of overloadPharmaceutical delivery mechanismImplantable neurostimulatorsDiseaseNephropathy
A method and apparatus for treatment of cardiac and renal diseases associated with the elevated sympathetic renal nerve activity by implanting a device to block the renal nerve signals to and from the kidney. The device can be a drug pump eluting implant for targeted delivery of a nerve-blocking agent to the periarterial space of the renal artery.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Method of preventing abuse of opioid dosage forms
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com