Patents
Literature
990results about "Medical insufflators" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
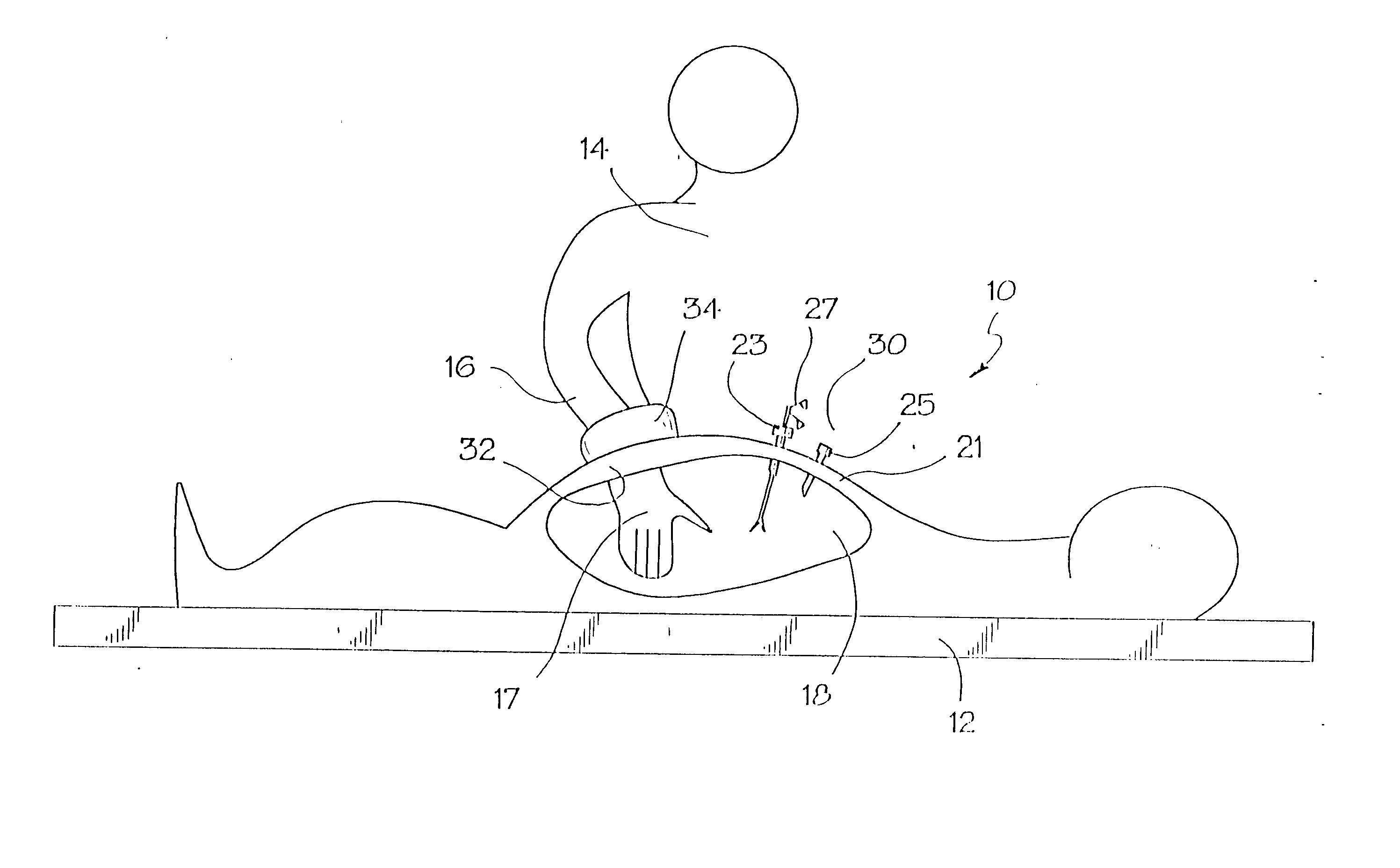
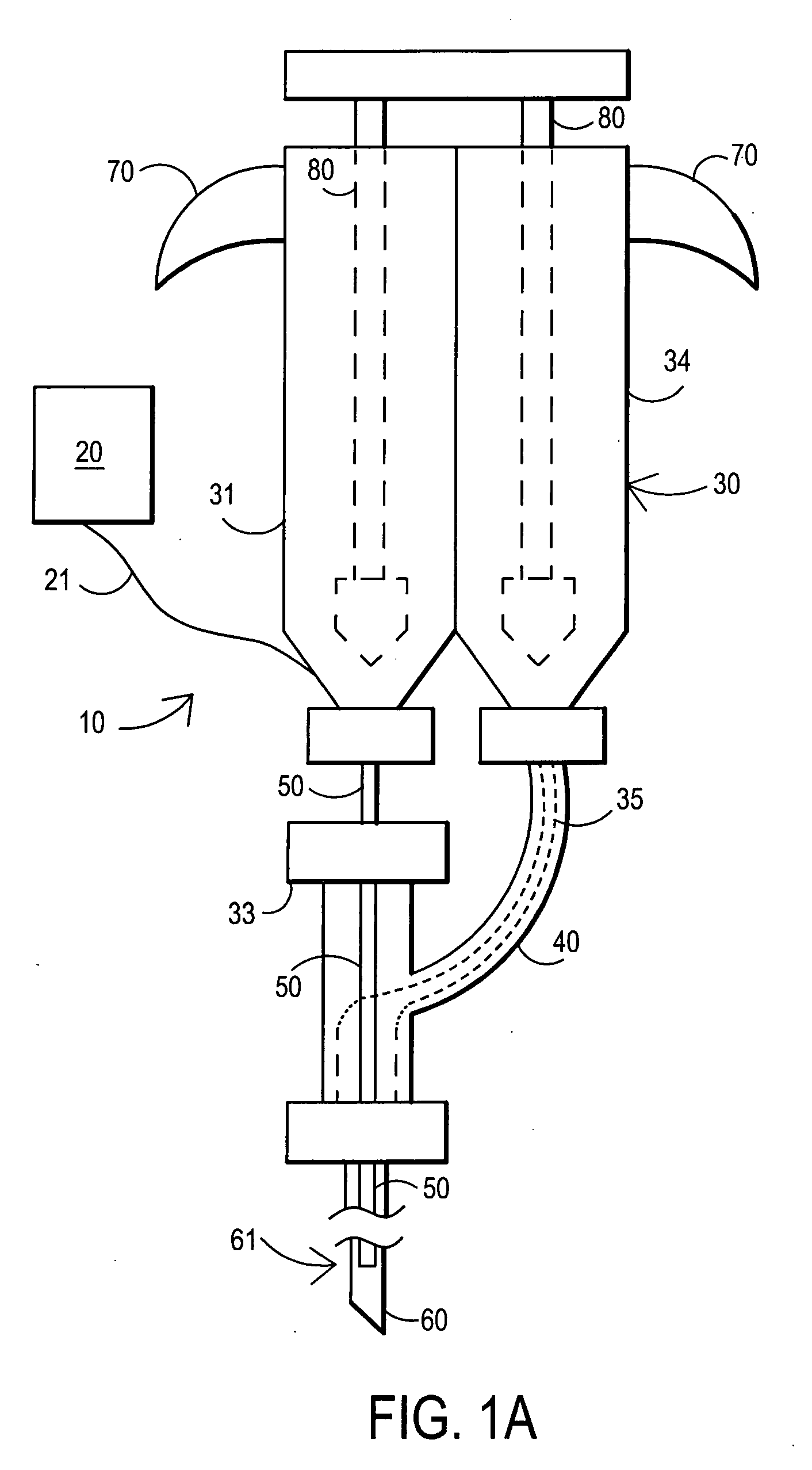
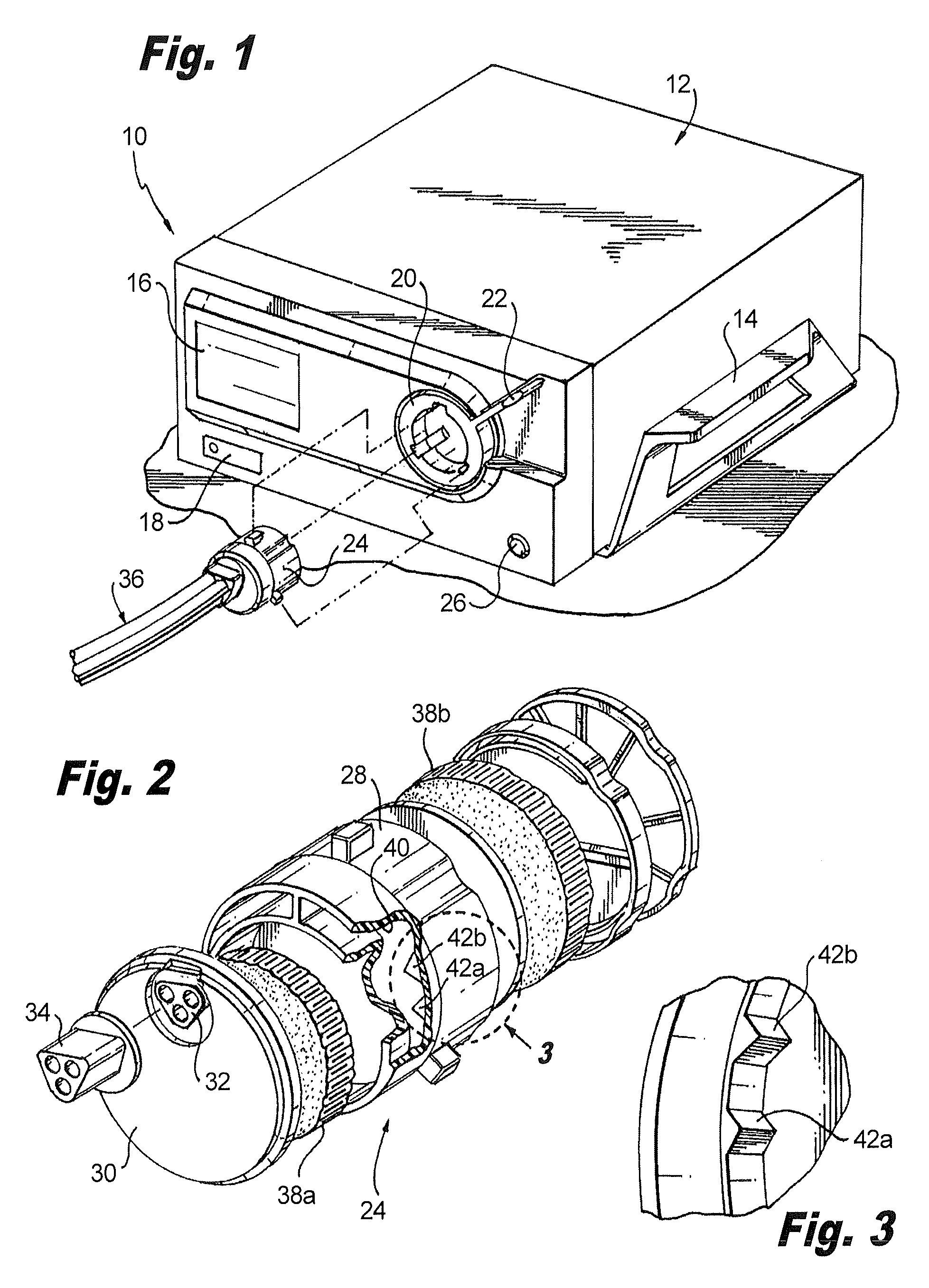
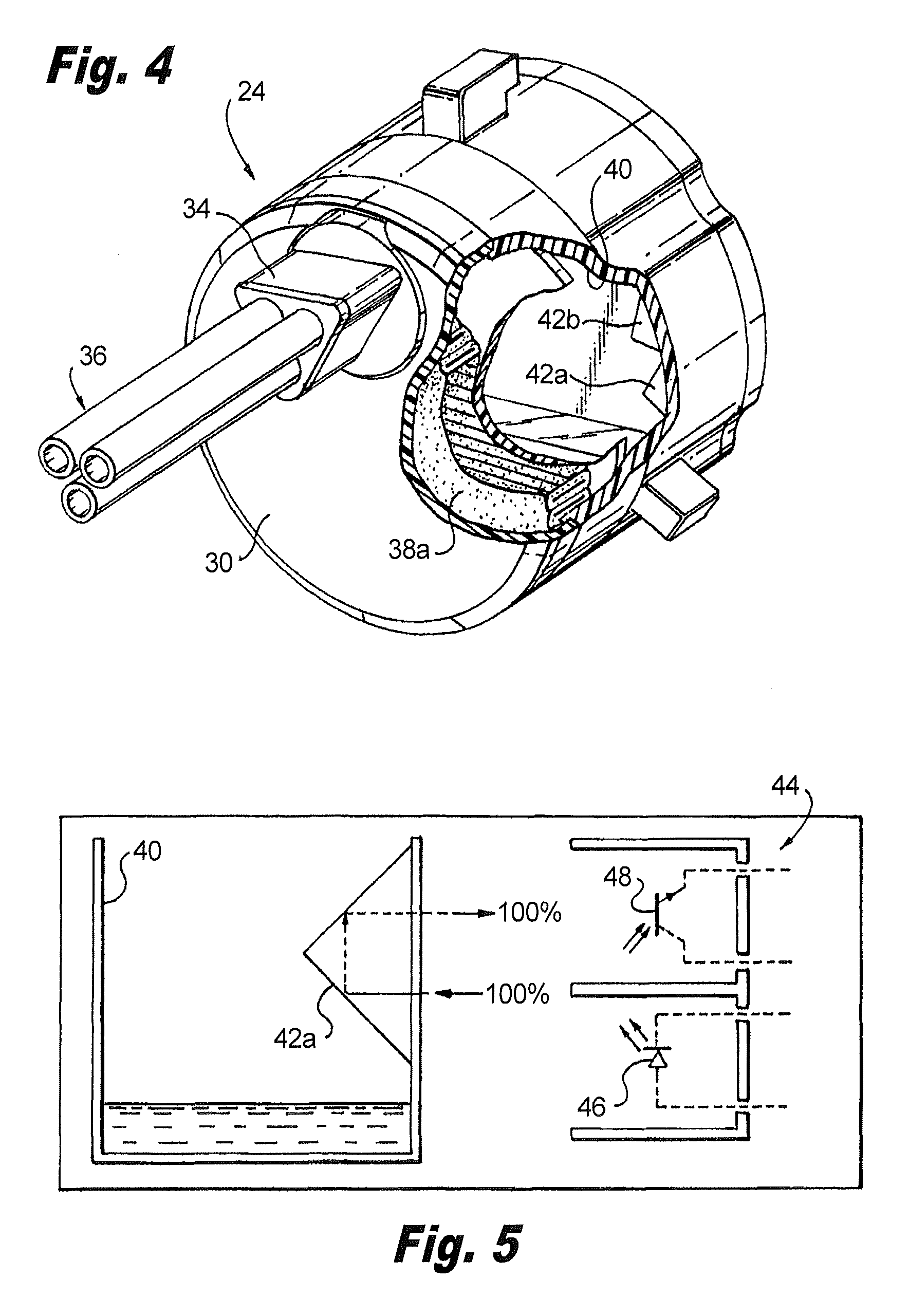
Methods and apparatus for performing transluminal and other procedures
InactiveUS20070135803A1Prevent overflowPrevent leakageSuture equipmentsEar treatmentSurgeryInstrumentation
Owner:INTUITIVE SURGICAL OPERATIONS INC
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
Devices for reduction of post operative ileus
InactiveUS20080086078A1Reducing post-operative ileus and/or gastric stasisReducing post-operative ileusMedical devicesMedical insufflatorsButtressSurgical department
An apparatus and method for reducing post-operative ileus and / or gastric stasis is described. The method can include applying to the intestine a therapeutically effective amount of a composition comprising a drug that is effective in reducing post-operative ileus and / or gastric stasis, such as by introducing the composition through a surgical access device, such as a trocar or endoscope. The apparatus can include a surgical fastener and a buttress comprising the composition.
Owner:ETHICON ENDO SURGERY INC
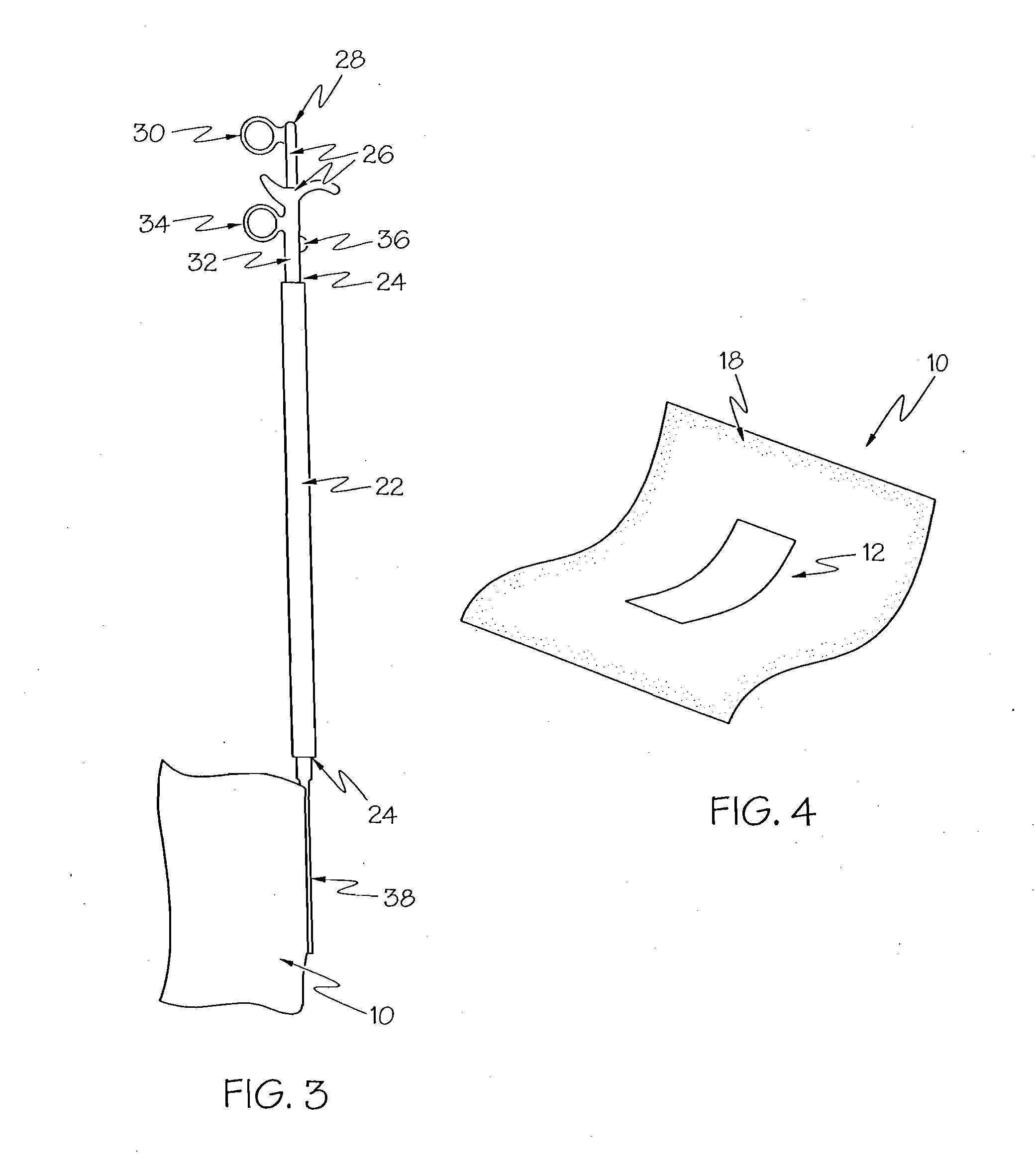
Insertion auxiliary implement
An insertion auxiliary implement of the present invention includes: a tubular part into which a flexible endoscope insertion part which is insertable into a body cavity, and one of a treatment tool and a channel into which the treatment tool is insertable, are insertable; and a sealing member which has through holes for supporting the endoscope insertion part and one of the treatment tool and the channel in the tubular part, and which airtightly and movably contacts each of a periphery of the endoscope insertion part, a periphery of the treatment tool or a periphery of the channel, and an inner surface of the tubular part, and thereby maintains airtightness between a distal end and a proximal end inside the tubular part.
Owner:OLYMPUS CORP
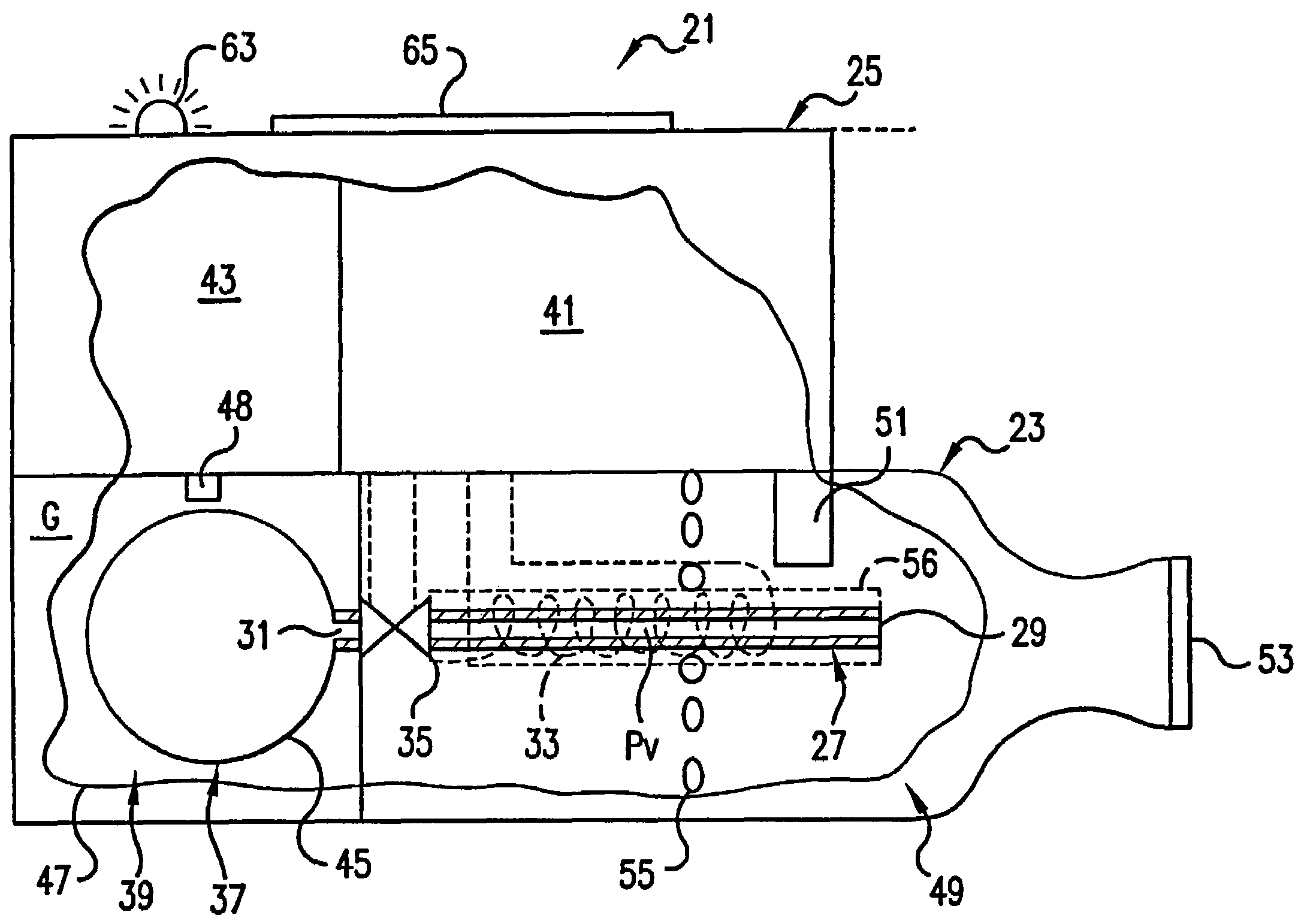
Internally pressurized medical devices
There are provided pressurized surgical instruments for use in pressurized surgical environments. The pressurized surgical instruments include pressurizing systems for maintaining a neutral or positive pressure flow within the surgical instrument during use. The pressurizing systems include a primary sensor for detecting the pressure of the surgical environment and a pressurizing mechanism for balancing the pressure of the surgical instrument with the surgical environment. The pressurizing mechanism includes a controller to receive and compare the signal sent from the primary sensor to the ambient or internal instrument pressure and a pressure delivery system to provide positive pressure to the interior of the surgical instrument in response to a signal received from the controller. The sources of pressure for the pressure delivery systems may be external or self contained within the surgical instrument.
Owner:TYCO HEALTHCARE GRP LP
Aerosol generator and methods of making and using an aerosol generator
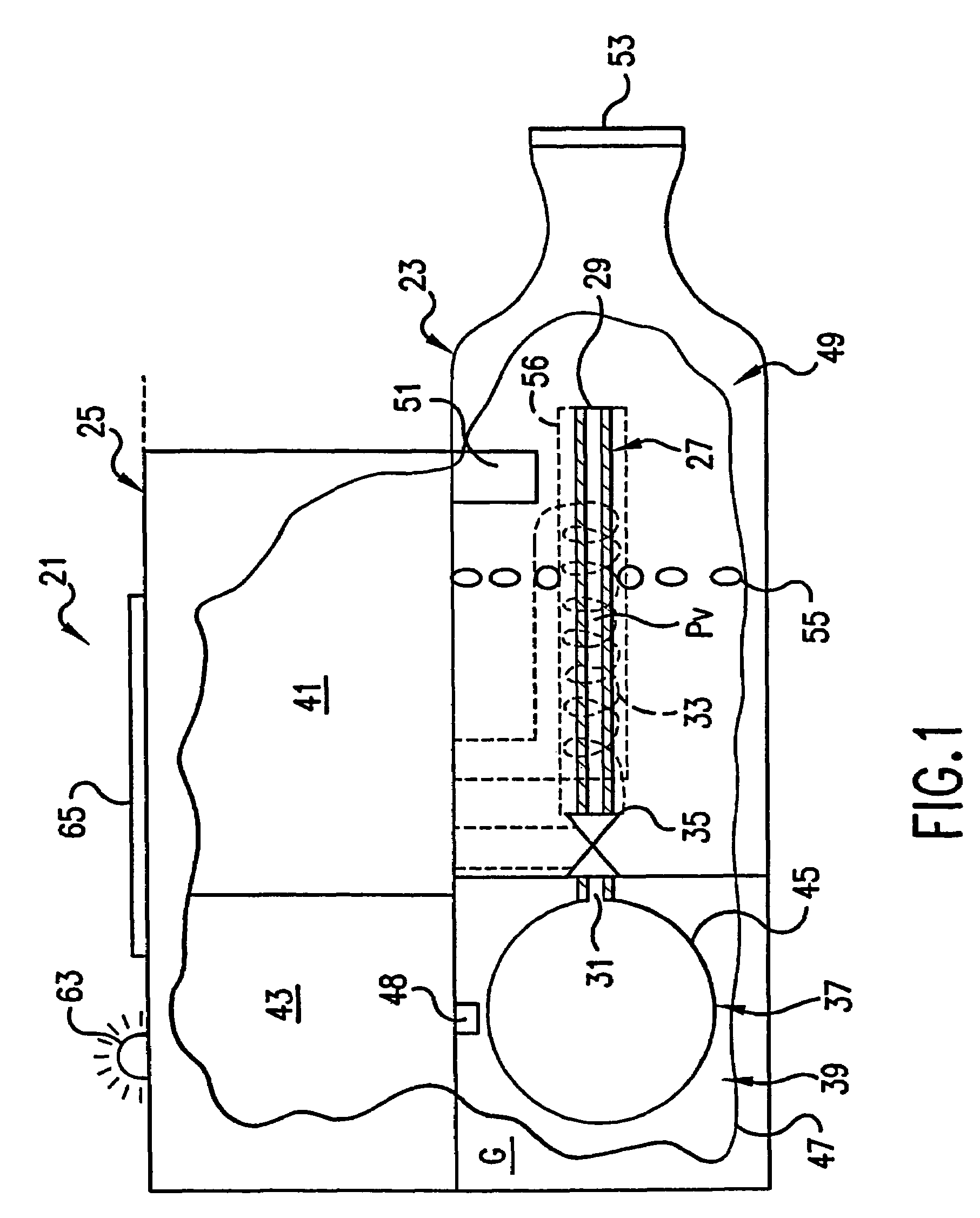
InactiveUS7117867B2Easy to useEasy to moveRespiratorsControlling ratio of multiple fluid flowsRotary valveEngineering
An aerosol generator includes a flow passage having an inlet and an outlet, a heater arranged relative to the flow passage for heating the flow passage, a source of material to be volatilized in communication with the inlet of the flow passage, a valve to open and close communication between the source of material and the inlet of the flow passage, and a pressurization arrangement for causing material in the source of material to be introduced into the flow passage when the valve is in an open position. The aerosol generator further includes a source of power for operating the heater and the valve, and a control device for controlling supply of power from the source of power to the heater and the valve. A metering device in an inhaler includes a pressurized source of medicated fluid and a metering chamber configured to deliver a predetermined volume of fluid to a heated flow passage in the inhaler. The metering chamber can be part of a rotary valve having a bore and a displacement member moveable within the bore from a first position where the fluid is loaded into the bore to a second position where the predetermined volume is ejected out of the bore. Another metering chamber has an elastic portion of a delivery passage in fluid communication with the pressurized source of liquid and the elastic portion of the delivery passage is deformed to eject the predetermined volume.
Owner:PHILIP MORRIS USA INC
Surgical access apparatus and method
A surgical access device includes a single valve that forms a seal with the body wall and provides an access charnel into a body cavity. The valve has properties for creating a zero seal in the absence of an instrument as well as an instrument seal with instruments having a full range of instrument diameter. The valve can include a gel and preferably an ultragel comprised of an elastomer and an oil providing elongation greater than 1000 percent and durometer less than 5 Shore A. The shore valve can be used as a hand port where the instrument comprises the arm of a surgeon, thereby providing hand access into the cavity. A method for making the surgical access device includes the combining or a gelling agent with an oil, preferably in a molding process. A method for using the device includes steps for creating an opening with the instrument. In a particular process, an organ can be removed from the body cavity through the single valve to create an organ seal while the organ is addressed externally of the body cavity. The valve and method are particularly adapted for laparoscopic surgery wherein the abdominal cavity is insufflated with a gas thereby requiring the zero seal, the instrument seal, and the organ seal in various procedures.
Owner:APPL MEDICAL RESOURCES CORP
Instrument access device
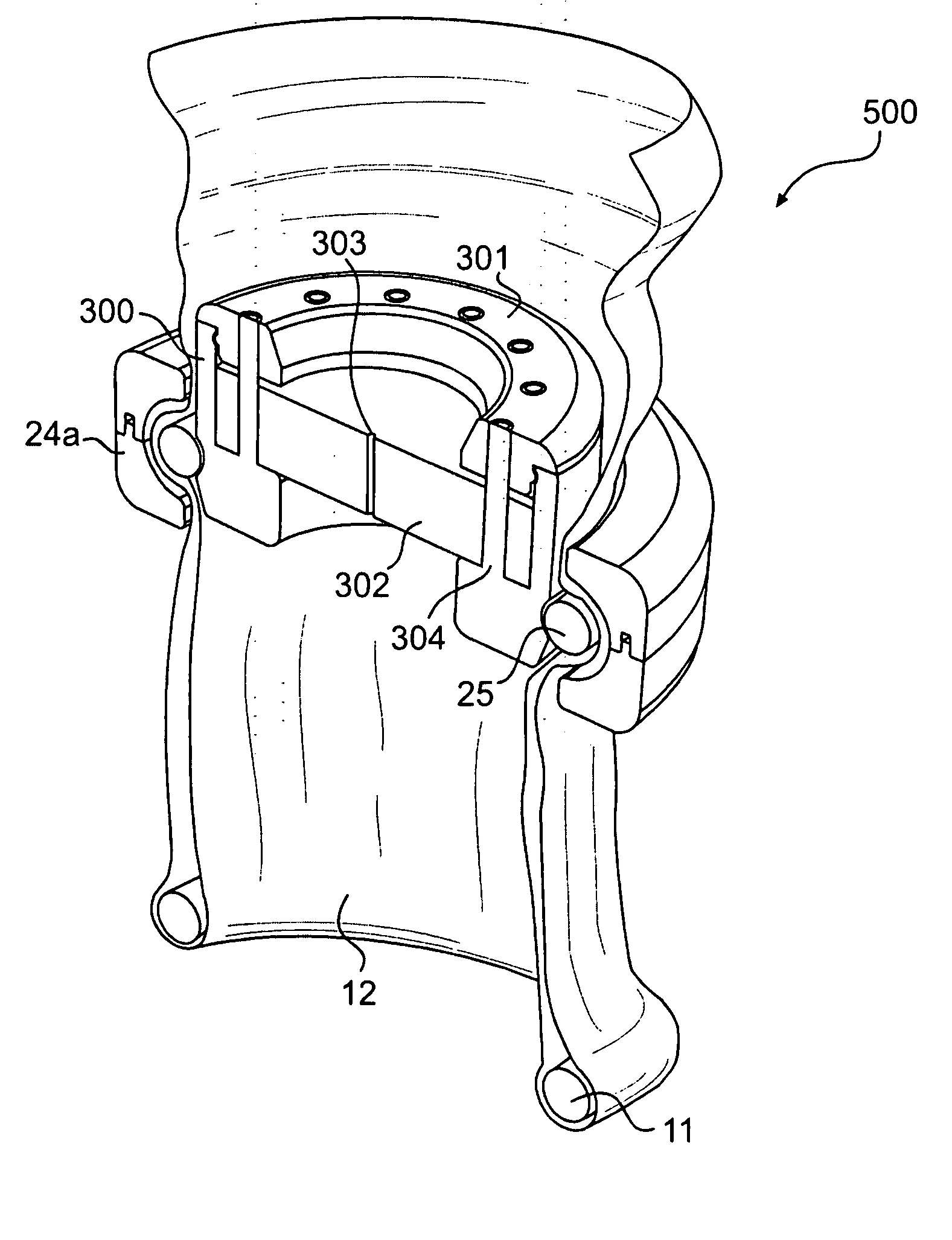
An instrument access device (500) comprises a distal O-ring (11) for insertion into a wound interior, a proximal member for location externally of a wound opening and a sleeve (12) extending in two layers between the distal O-ring (11) and the proximal member. The proximal member comprises an inner proximal ring member (25) and an outer proximal ring member (24) between which the sleeve (12) is led. A seal housing (300) is mounted to the inner proximal ring member (25). A gelatinous elastomeric seal (302) with a pinhole opening (303) therethrough is received in the housing (300). An instrument may be extended through the seal (302) to access the wound interior through the retracted wound opening in a sealed manner.
Owner:ATROPOS LTD
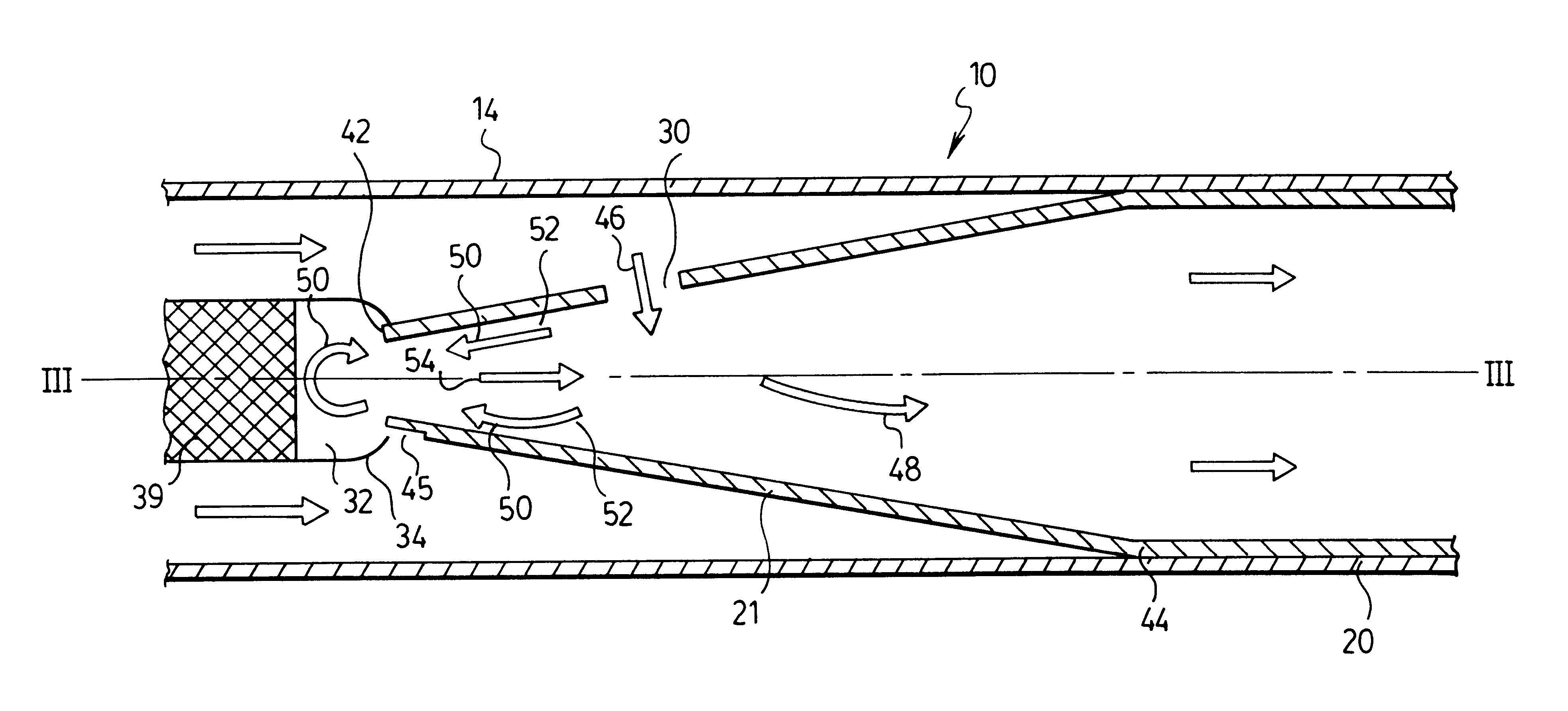
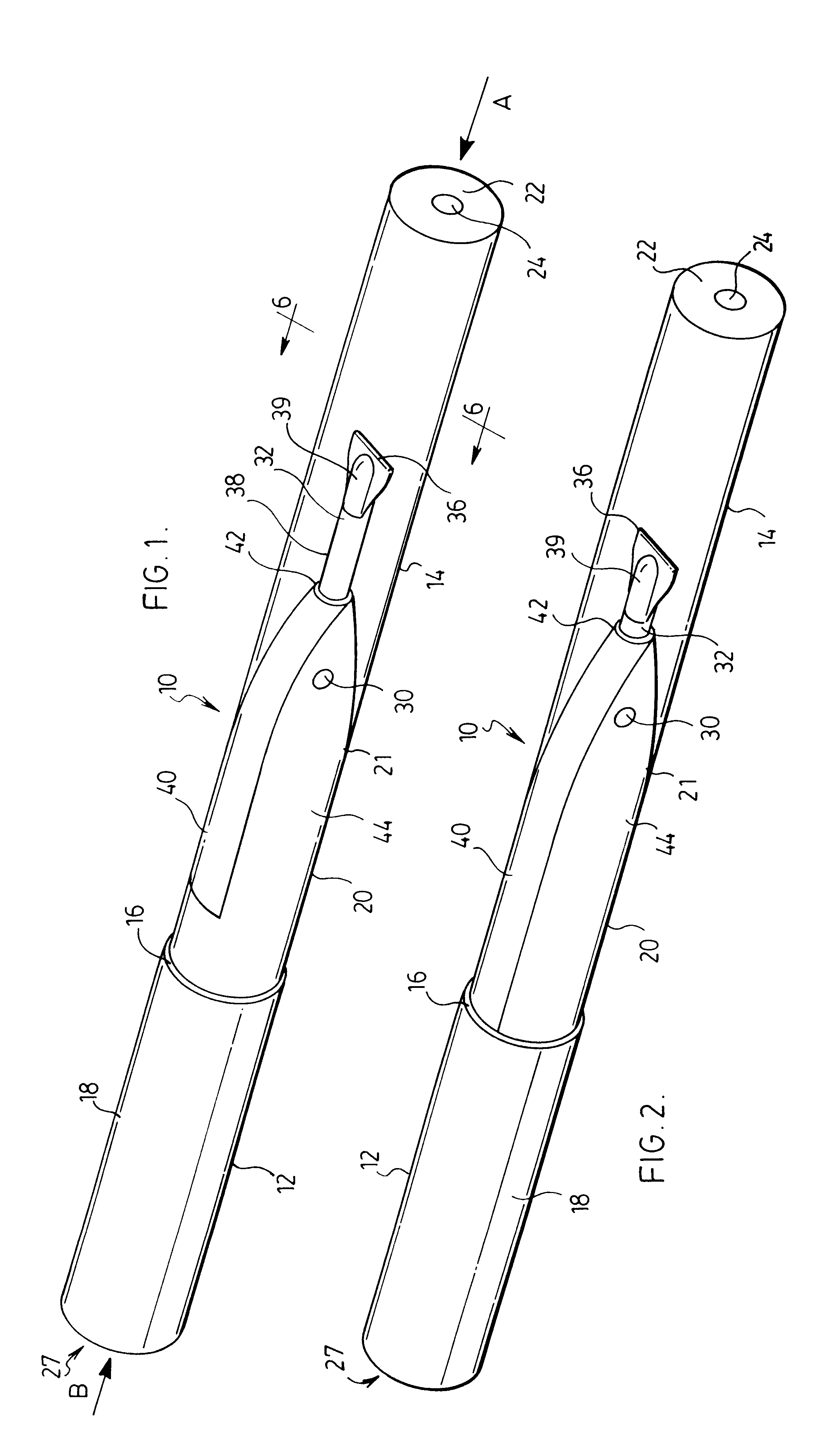
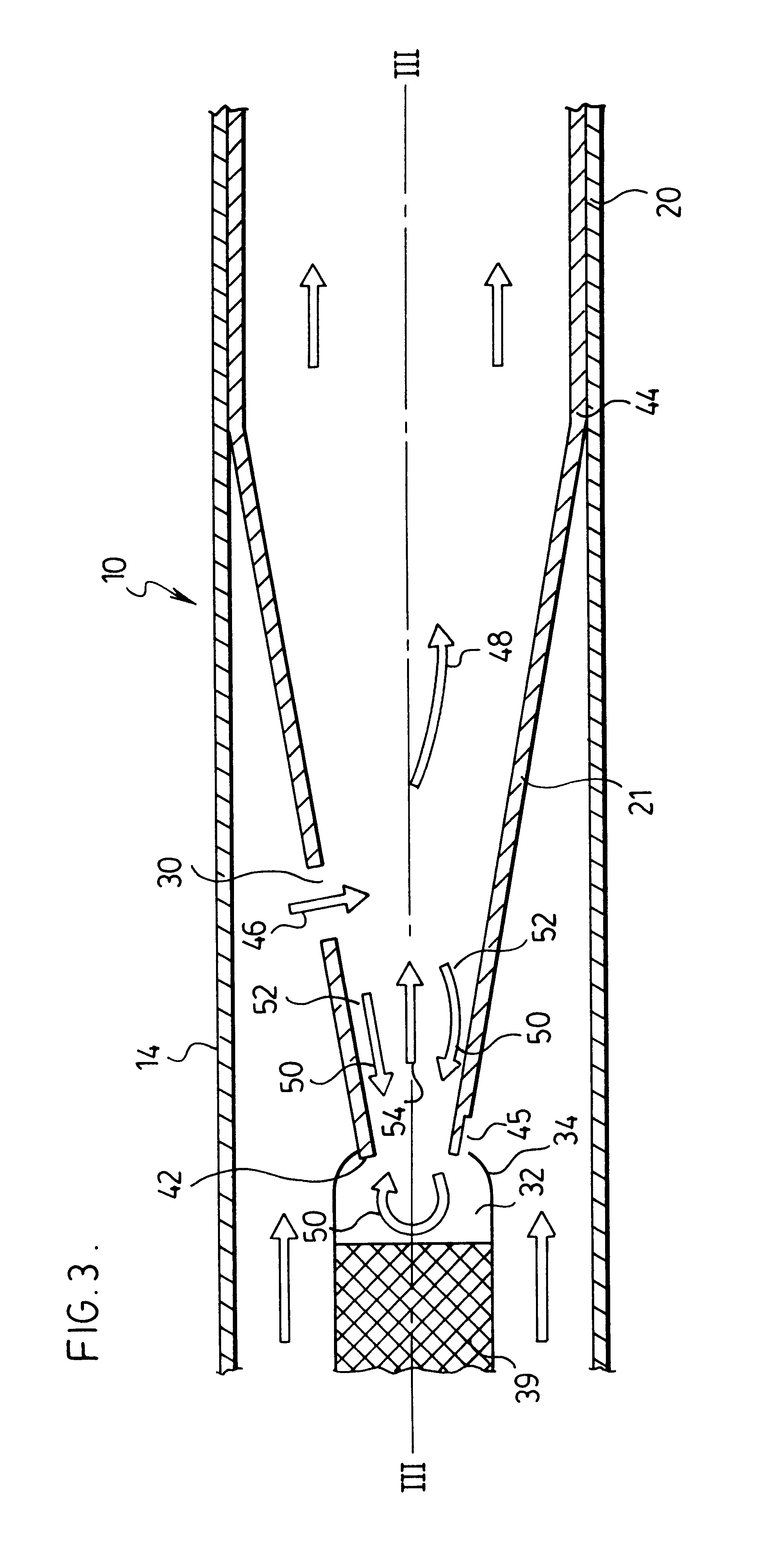
Trocar assembly with pneumatic sealing
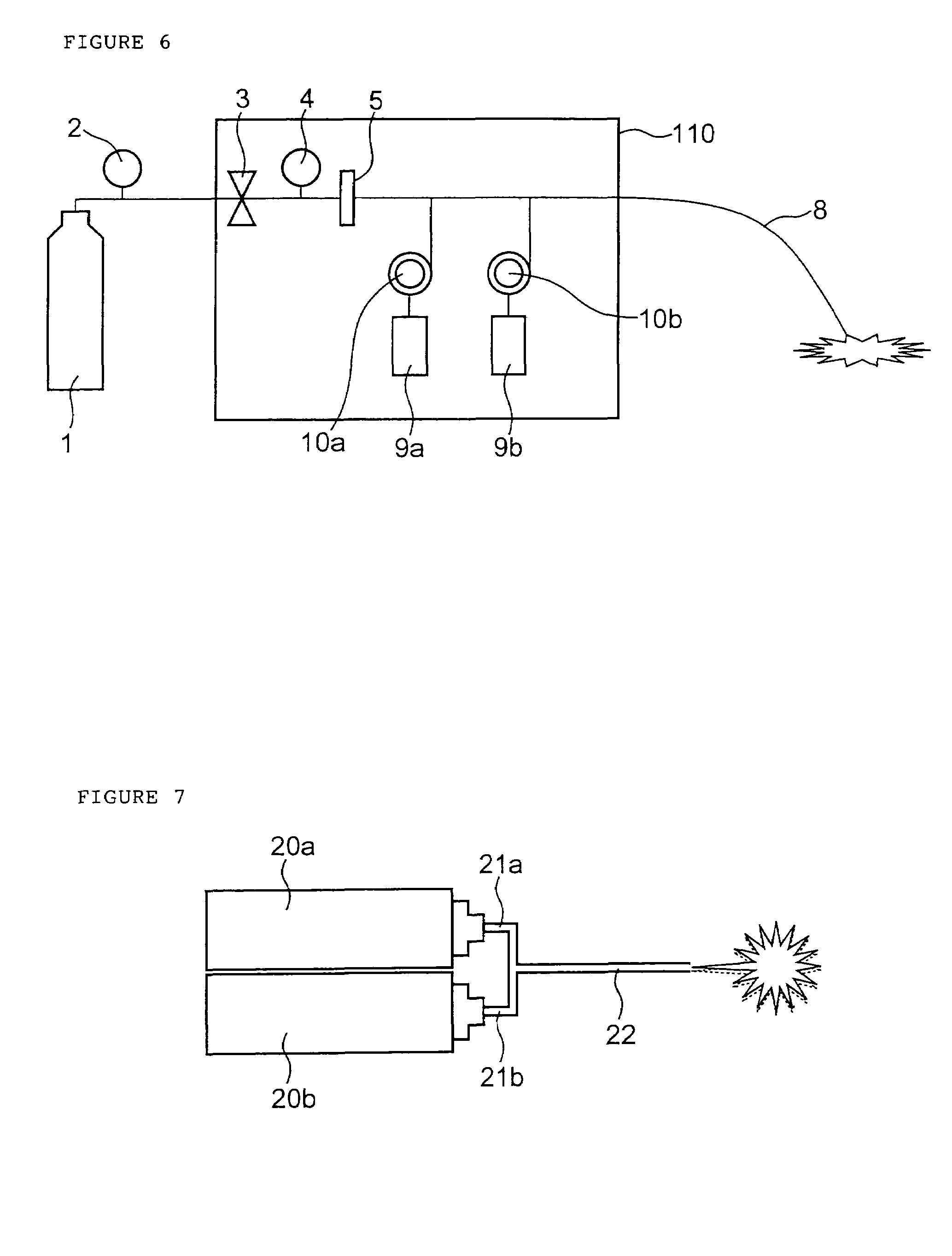
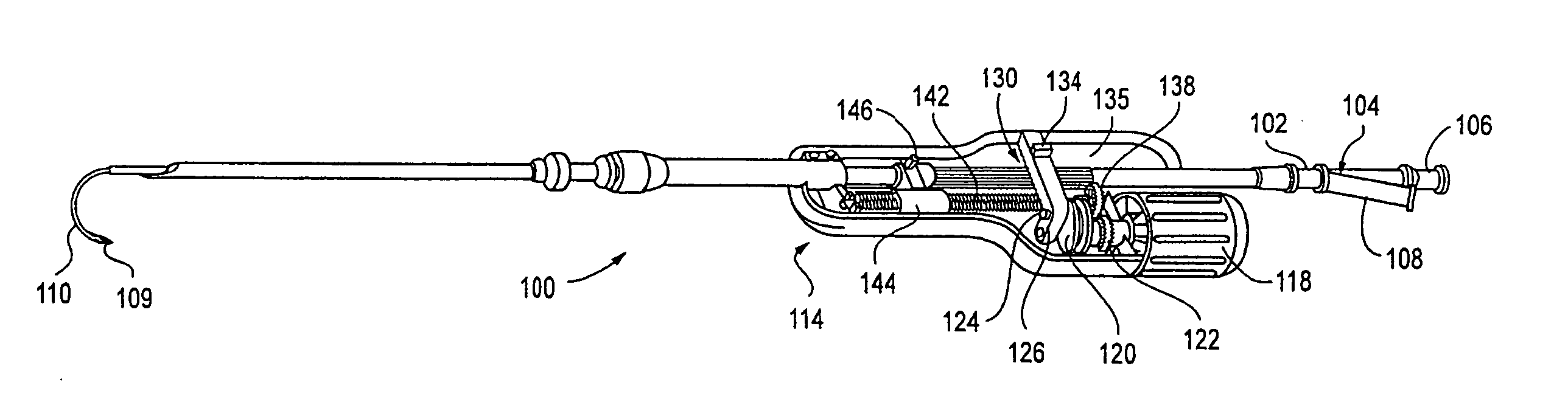
InactiveUS20070088275A1Sufficient massInhibiting proximal egressCannulasDiagnosticsEngineeringSurgical procedures
A trocar for use in a minimally invasive surgical procedure includes an elongated body, nozzle means and means for delivering a pressurized flow of fluid to the nozzle means. The elongated body has a generally tubular configuration with coaxially arranged inner and outer walls and longitudinally opposed proximal and distal end portions, with the inner wall defining a lumen to accommodate passage of an instrument therethrough. The nozzle means is operatively associated with the inner wall of the body for directing pressurized fluid into the lumen to develop a pressure differential in an area within a region extending from a location adjacent a distal end portion of the lumen to a location adjacent a proximal end portion of the lumen, to form a fluid seal around an instrument passing therethrough.
Owner:SURGIQUEST
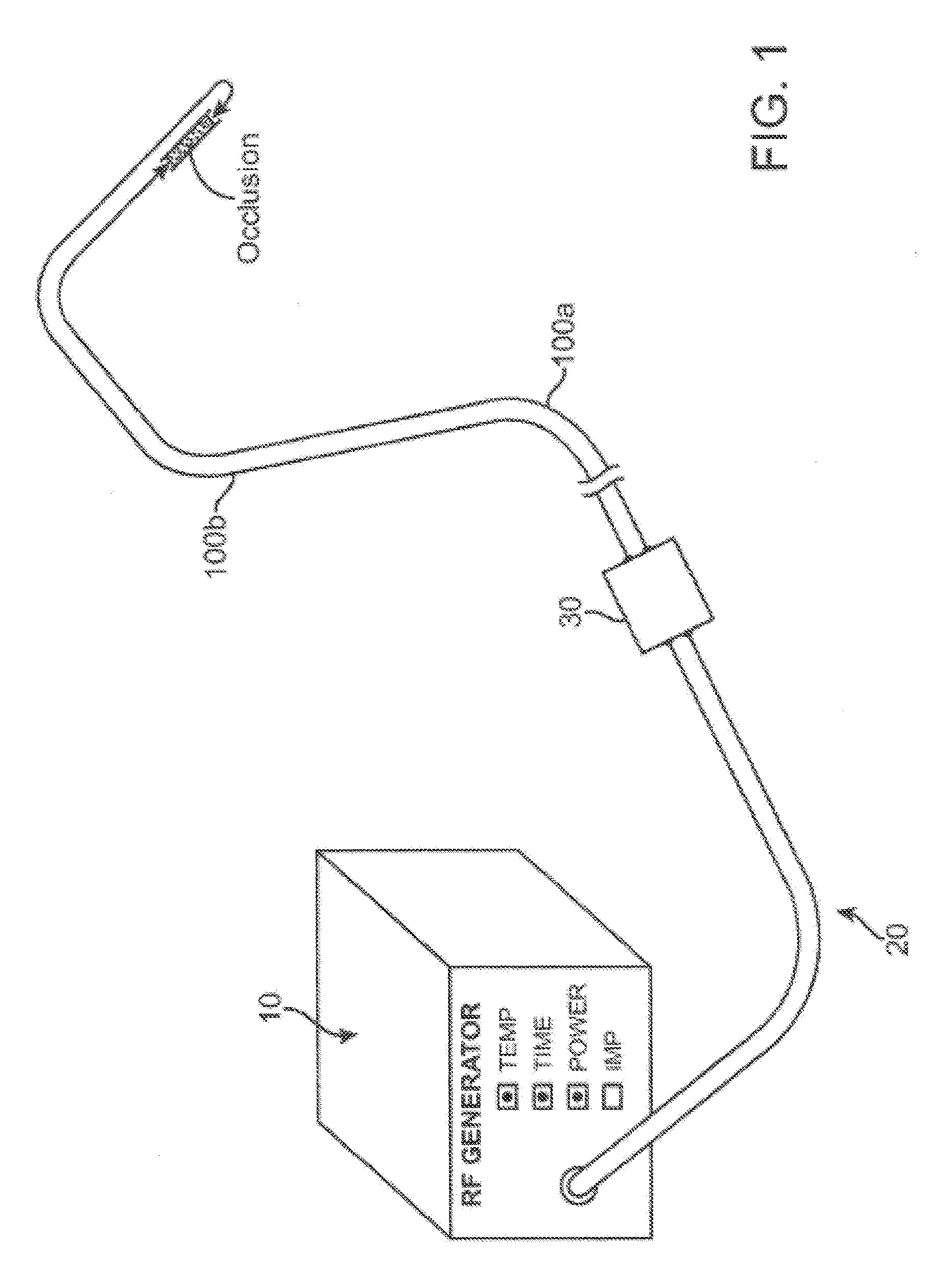
Recanalizing occluded vessels using radiofrequency energy
A method and systems for treating chronic total occlusions, particularly those that are difficult to treat, is disclosed. In this approach, recanalizing the CTO is achieved using a combined antegrade and retrograde approach. The proximal end of the occlusion is penetrated using an antegrade wire, using a traditional approach. Using collateral vessels, the distal end of the occlusion is crossed in a retrograde fashion. By appropriately maneuvering each member and applying radiofrequency energy between the proximal and distal ends of the occlusion, a continuous channel is created.
Owner:ASAHI MEDICAL TECH INC
Delivery of oral drugs
InactiveUS20010020147A1Comfortable and convenient motionComfortable and convenient feelPowder deliveryLiquid surface applicatorsMean diameterHuman patient
Disclosed is a system for delivery of a drug comprising a multiple unit dosing device comprising a housing and an actuator, said device containing multiple doses of multiparticulates comprising drug particles, said device upon actuation delivering a unit dose of said multiparticulates, said drug particles having a mean diameter of greater than 10 mum to about 1 mm such that an effective dose of said drug cannot be delivered into the lower lung of a human patient. Also disclosed are novel methods, devices and dosage forms for delivering a drug.
Owner:PHARMAKODEX LTD
Automatic smoke evacuator and insufflation system for surgical procedures
InactiveUS20070249990A1Easy maintenanceSurgical needlesMedical devicesSurgical siteSurgical department
An automatic smoke evacuation and insufflation system for surgical procedures having a vacuum for removing gas, smoke, and debris from a surgical site and an insufflator for supplying gas to the body cavity of a patient.
Owner:IC MEDICAL INC
Programmable multi-dose intranasal drug delivery device
InactiveUS6948492B2Avoid diversionAvoid abuseRespiratorsLiquid surface applicatorsNasal sprayBiological activation
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
Filter cartridge with internal gaseous seal for multimodal surgical gas delivery system having a smoke evacuation mode
A system is disclosed for delivering gas during a laparoscopic surgical procedure performed within a patient's abdominal cavity requiring smoke evacuation which includes a gas delivery device having a housing with a port for receiving pressurized insufflating gas from a gas source, a pump assembly for circulating gas throughout the system, and a disposable gas conditioning unit or filter cartridge configured for operative association with the gas delivery device.
Owner:SURGIQUEST
Surgical access apparatus and method
Owner:APPL MEDICAL RESOURCES CORP
Inhaler
InactiveUS6234169B1Reduce negative impactHigh simulationRespiratorsLiquid surface applicatorsParticulatesInhalation
An inhaler for use by an individual to inhale a particulate medicament from a reservoir comprises a chamber having a first end connectable to the reservoir to be in air flow communication therewith, a second end for delivering the medicament to the individual upon inhalation and a conduit defining an air flow path extending between the first end and the second end; and, an orifice in the chamber between the first end and the second end, the orifice utilizing the Coanda Effect when the reservoir is in air flow communication with the chamber and upon inhalation by the individual to draw medicament from the reservoir into the air flow path.
Owner:SANSA BARBADOS
Metered dose inhaler agitator
A metered dose inhaler including a mechanism for agitating the medicament formulation prior to its separation in a measured dose, for administration to a mammal, including a human. The separated dose is a homogeneous mixture of prescribed medicine in a fluid carrier.
Owner:KOS LIFE SCI
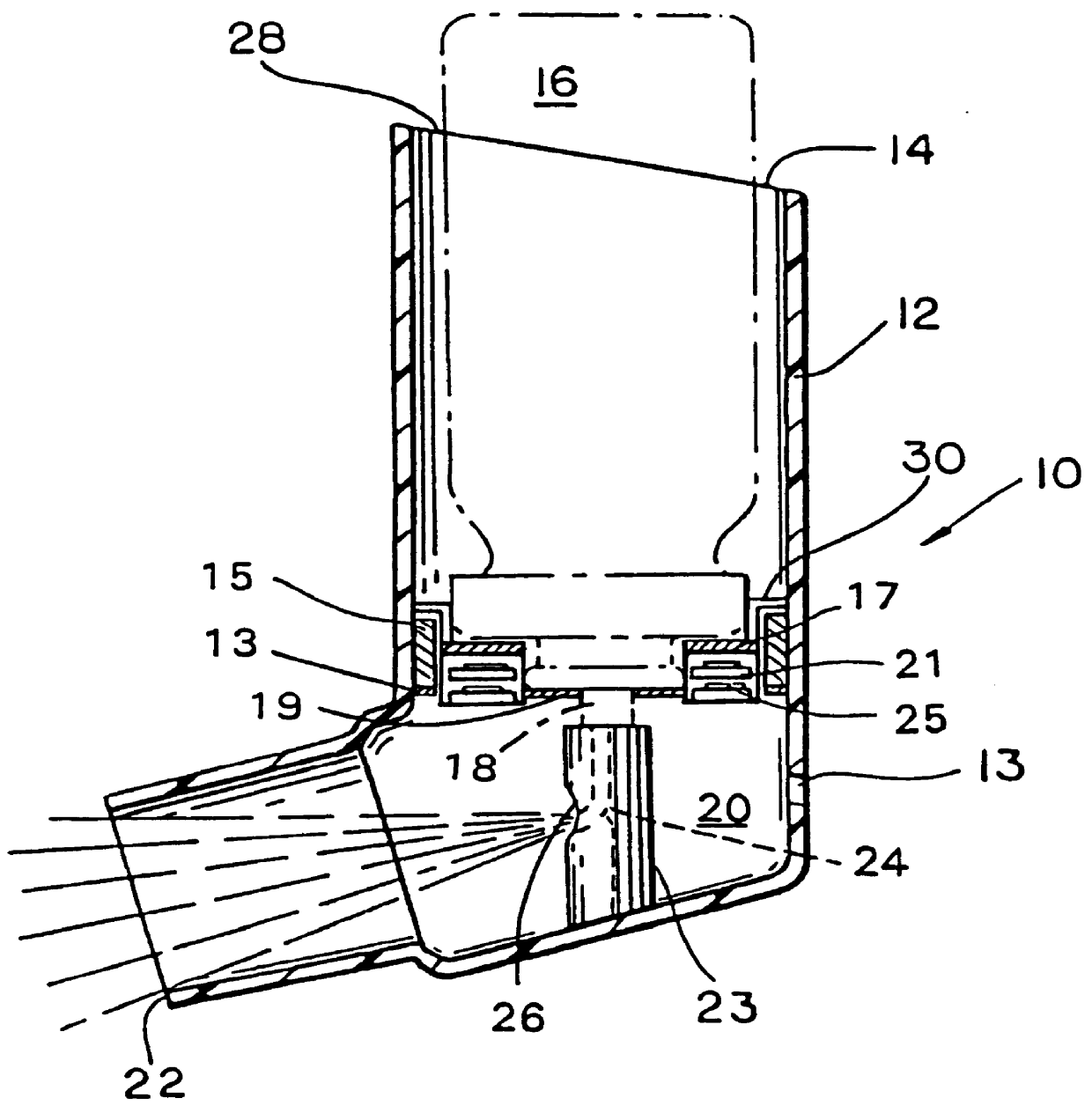
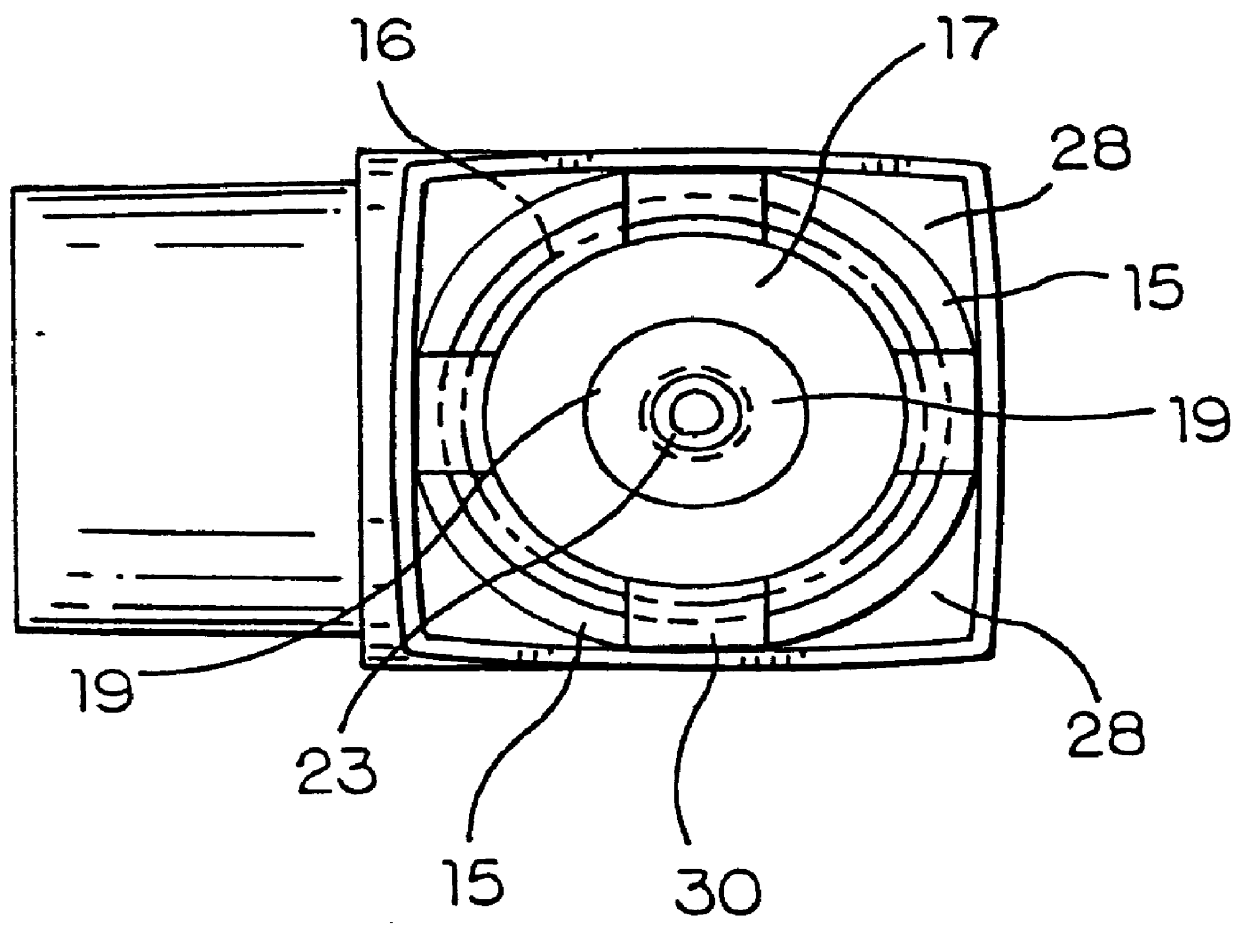
Powder formulation disintegrating system and method for dry powder inhalers
A disperser for dry powders which can be used with different dose systems, dose weights ranging from 2 to 25 mg and different types of powder formulation. In one embodiment, the disperser acts both as a de-agglomeration (disintegration; aerosolization) means and as an air classifier for especially adhesive mixtures. Only fine drug particles are emitted whereas the larger agglomerates and carrier crystals are retained by the disperser. Another embodiment enables time controlled release of carrier crystals in these mixtures. Yet another embodiment has optimized performance with spherical pellets, containing no carrier crystals. Other possible embodiments of the invention make it possible to control the total inhaler resistance and the powder deposition in the upper respiratory tract by means of the addition of a so-called sheath flow of clean air. Modifications also enable carrier retainment in the mouthpiece and elimination of the tangential flow component of the discharge cloud.
Owner:ASTRAZENECA AB
Method for repairing intervertebral discs
ActiveUS20080103564A1Increase pressureHalt leakageDiagnosticsPharmaceutical delivery mechanismIntervertebral discBiomedical engineering
A method of repairing a defect in an annulus fibrosus of an intervertebral disc, without excising the entire nucleus pulposus of the disc. The method includes inserting an introducer needle through the annulus fibrosus by puncturing the annulus fibrosus with the introducer needle, injecting an in situ curable, bio-compatible polymerizable or polymeric material composition into the disc through the introducer needle directly or indirectly so that the in situ curable composition contacts a defect in the annulus fibrosus; and curing said material in situ.
Owner:PAUZA KEVIN
Trocar assembly with pneumatic sealing
A trocar for use in a minimally invasive surgical procedure includes an elongated body, nozzle means and means for delivering a pressurized flow of fluid to the nozzle means. The elongated body has a generally tubular configuration with coaxially arranged inner and outer walls and longitudinally opposed proximal and distal end portions, with the inner wall defining a lumen to accommodate passage of an instrument therethrough. The nozzle means is operatively associated with the inner wall of the body for directing pressurized fluid into the lumen to develop a pressure differential in an area within a region extending from a location adjacent a distal end portion of the lumen to a location adjacent a proximal end portion of the lumen, to form a fluid seal around an instrument passing therethrough.
Owner:SURGIQUEST
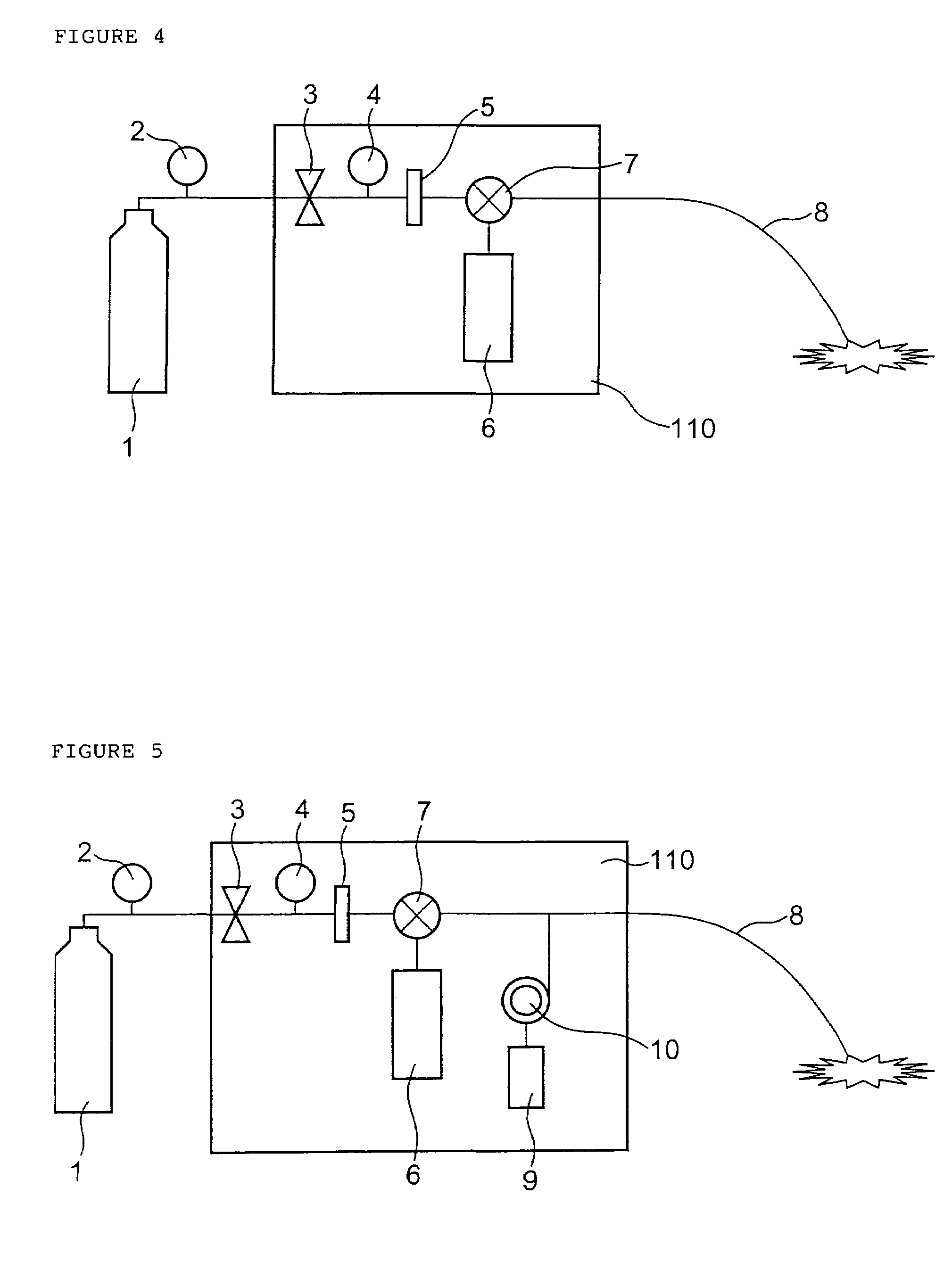
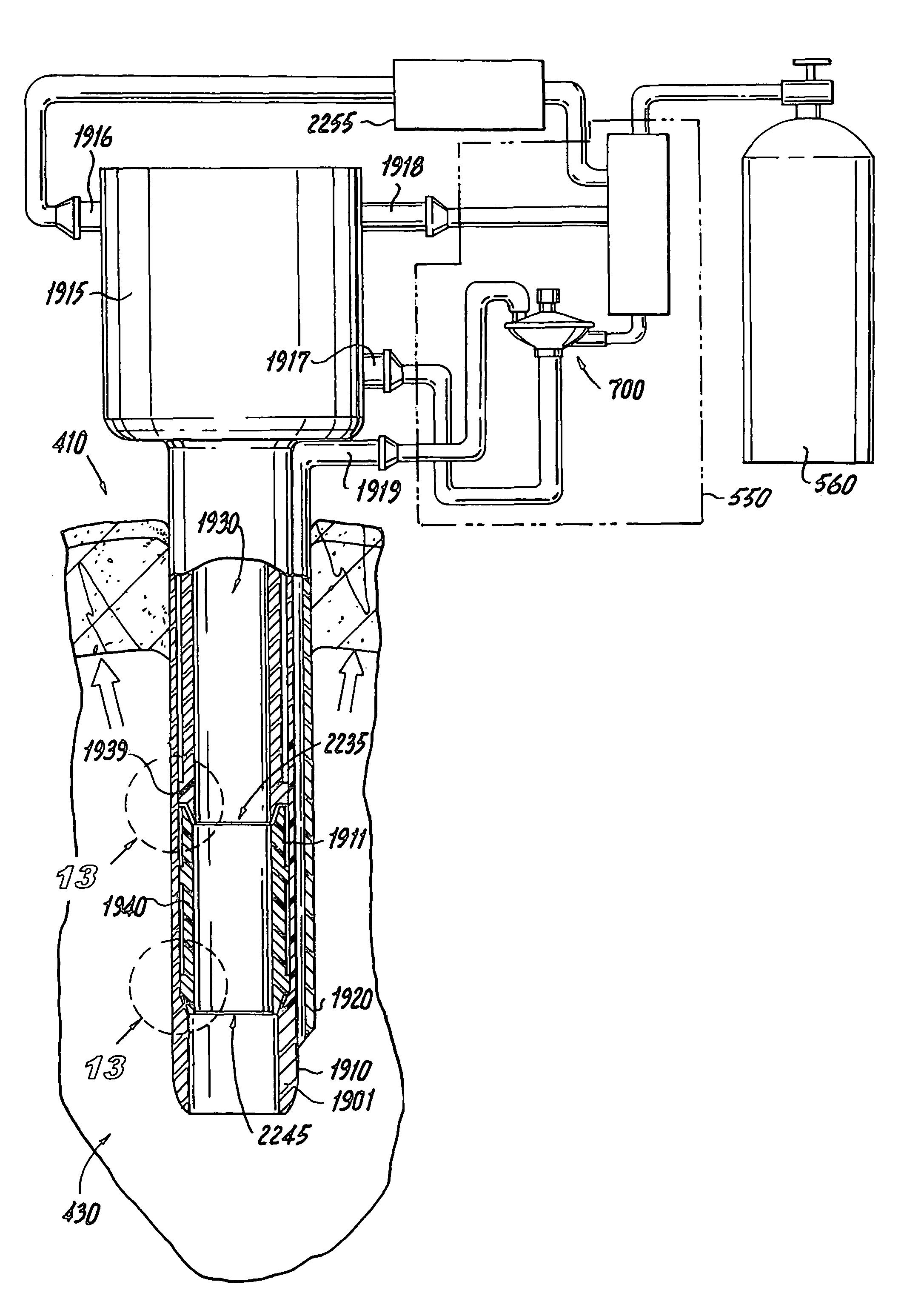
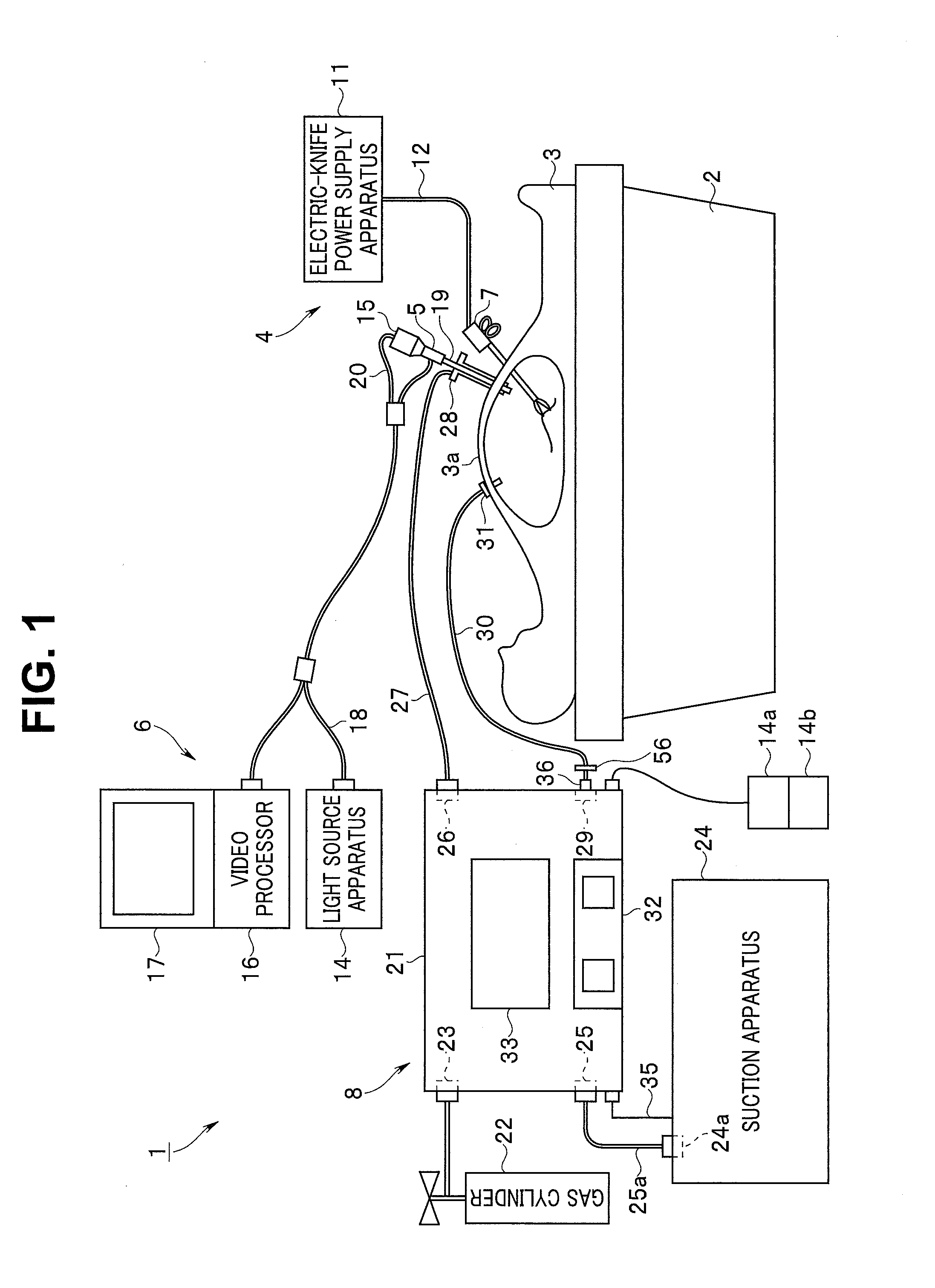
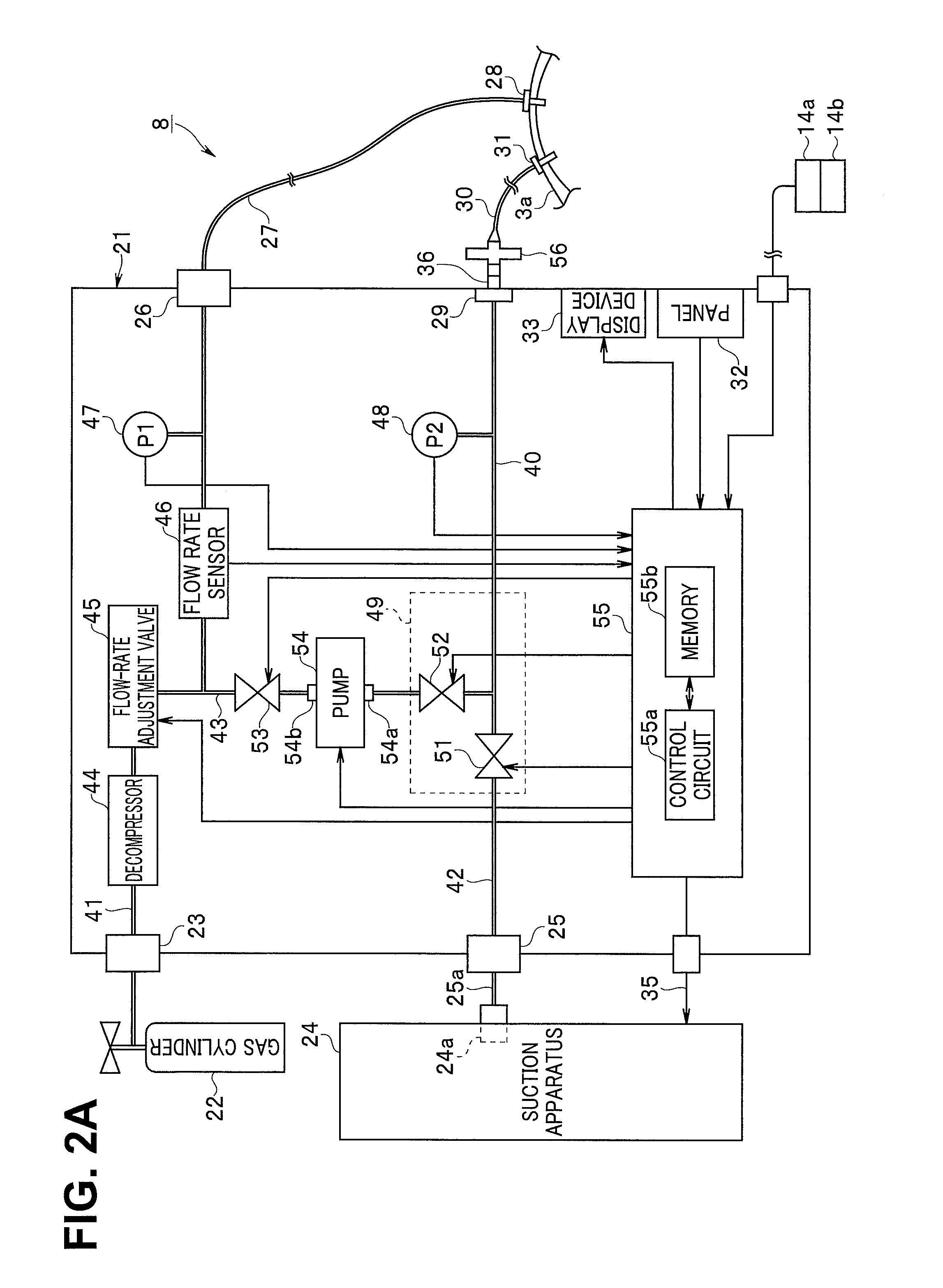
Insufflation apparatus
An insufflation apparatus includes a gas-feeding-conduit connecting section for connecting a conduit that feeds a predetermined gas to a subject, a first conduit that connects a gas-feeding-source connecting section and the gas-feeding-conduit connecting section, a suction-conduit connecting section for connecting a conduit that sucks the predetermined gas from an inside of the subject, a pump for circulating the predetermined gas to the subject, a conduit for circulation that connects the suction-conduit connecting section and the pump, a second conduit that connects a suction-source connecting section and the conduit for circulation, and a control section that controls a switching unit to operate in a circulation operation mode for circulating the predetermined gas to the subject via the pump and a suction operation mode for sucking the predetermined gas to the suction source side via the second conduit.
Owner:OLYMPUS CORP
Smoke evacuation system for continuously removing gas from a body cavity
ActiveUS20180221598A1Prevent over-pressurizationEasy to adjustCannulasSurgical needlesProduct gasContinuous flow
An evacuation system for continuously removing gas from a body cavity of a patient during an endoscopic surgical procedure is disclosed, which includes an inlet flow path leading to a first trocar communicating with the body cavity through which an essentially continuous flow of gas is delivered to the body cavity, an outlet flow path leading from a second trocar communicating with the body cavity though which an essentially continuous flow of gas is removed from the body cavity, a pump communicating at least with the outlet flow path for removing an essentially continuous flow of gas from the surgical cavity, and a processor operatively associated with the pump for controlling at least the essentially continuous flow of gas from the body cavity.
Owner:CONMED CORP
System and method for manipulating a catheter for delivering a substance to a body cavity
ActiveUS20050125002A1Make up for deficienciesGuide needlesEar treatmentINTRODUCTION deviceAerosolization
A system and method for adjusting a catheter to create a medicated atmosphere in an organ, or body cavity is disclosed. The system comprises a catheter, such as an aerosolization catheter, that can be manipulated during use and an introduction device for the introduction and manipulation, by rotational and / or axial positioning, of the aerosolization catheter. The method includes inserting the catheter into a body cavity via an introducer apparatus and adjusting an angle or orientation of the exit end of the catheter so that a substance provided to the catheter will be controllably applied to the body cavity at desired locations.
Owner:TRUDELL MEDICAL INT INC
Dry powder inhalers, related blister devices, and associated methods of dispensing dry powder substances and fabricating blister packages
InactiveUS6889690B2Easy to optimizeLimit amount of resistanceSmall article dispensingLiquid surface applicatorsPowder InhalerInhalation
The present invention includes dry powder inhalers and associated multi-dose dry powder packages for holding inhalant formulated dry powder substances and associated fabrication and dispensing methods. The multi-dose package can include a platform body comprising at least one thin piezoelectric polymer material layer defining at least a portion of a plurality of spatially separated discrete elongate dry powder channels having an associated length, width and height; and a metallic material attached to selected portions of the piezoelectric polymer material including each of the regions corresponding to the elongate dry powder channels to, in operation, define active energy releasing vibratory channels. In operation, the elongate channels can be selectively individually activated to vibrate upon exposure to an electrical input.The dry powder inhaler includes an elongate body having opposing first and second outer primary surfaces with a cavity therebetween and having opposing top and bottom end portions and a multi-dose sealed blister package holding a plurality of discrete meted doses of a dry powder inhalable product located in the cavity of the elongate body. The inhaler also includes an inhalation port formed in the bottom end portion of the elongate body, the inhalation port configured to be in fluid communication with at least one of the discrete meted doses during use and a cover member that is pivotably attached to the elongate body so that it remains attached to the body during normal operational periods of use and moves to a first closed position to overlie the inhalation port at the bottom end portion of the body during periods of non-use and moves to a second open position away from the inhalation port during periods of use to allow a user to access the inhalation port.
Owner:ORIEL THERAPEUTICS INC
Apparatus and methods for directly displacing the partition between the middle ear and inner ear at an infrasonic frequency
ActiveUS7179238B2Alleviate vertigo, tinnitus, fullness of the ear and/or hearing lossUltrasound therapyElectrotherapyMiddle ear functionInner Ear Diseases
An apparatus for displacing a partition between a middle ear and an inner ear to treat the symptoms of Meniere's disease or endolymphatic hydrops comprises a treatment member for being disposed in the middle ear adjacent the partition and a driver for driving the treatment member to move against the partition to thereby displace the partition at an infrasonic frequency to influence fluid distribution in the inner ear. A method for treating an ear comprises the steps of disposing a treatment device within the middle ear and moving at least a portion of the treatment device against the partition at an infrasonic frequency to displace the partition to influence fluid in the inner ear.
Owner:MEDTRONIC XOMED INC
Flexible access assembly with multiple ports
An access assembly configured to receive one or more surgical instruments is provided. The access assembly includes a flexible housing having a proximal end and a distal end, the housing defining a longitudinal passageway extending from the proximal end to the distal end and a seal assembly received within the longitudinal passageway of the housing. The seal assembly defining a plurality of ports each configured to receive an instrument inserted therethrough in a sealing manner.
Owner:TYCO HEALTHCARE GRP LP
System for surgical insufflation and gas recirculation
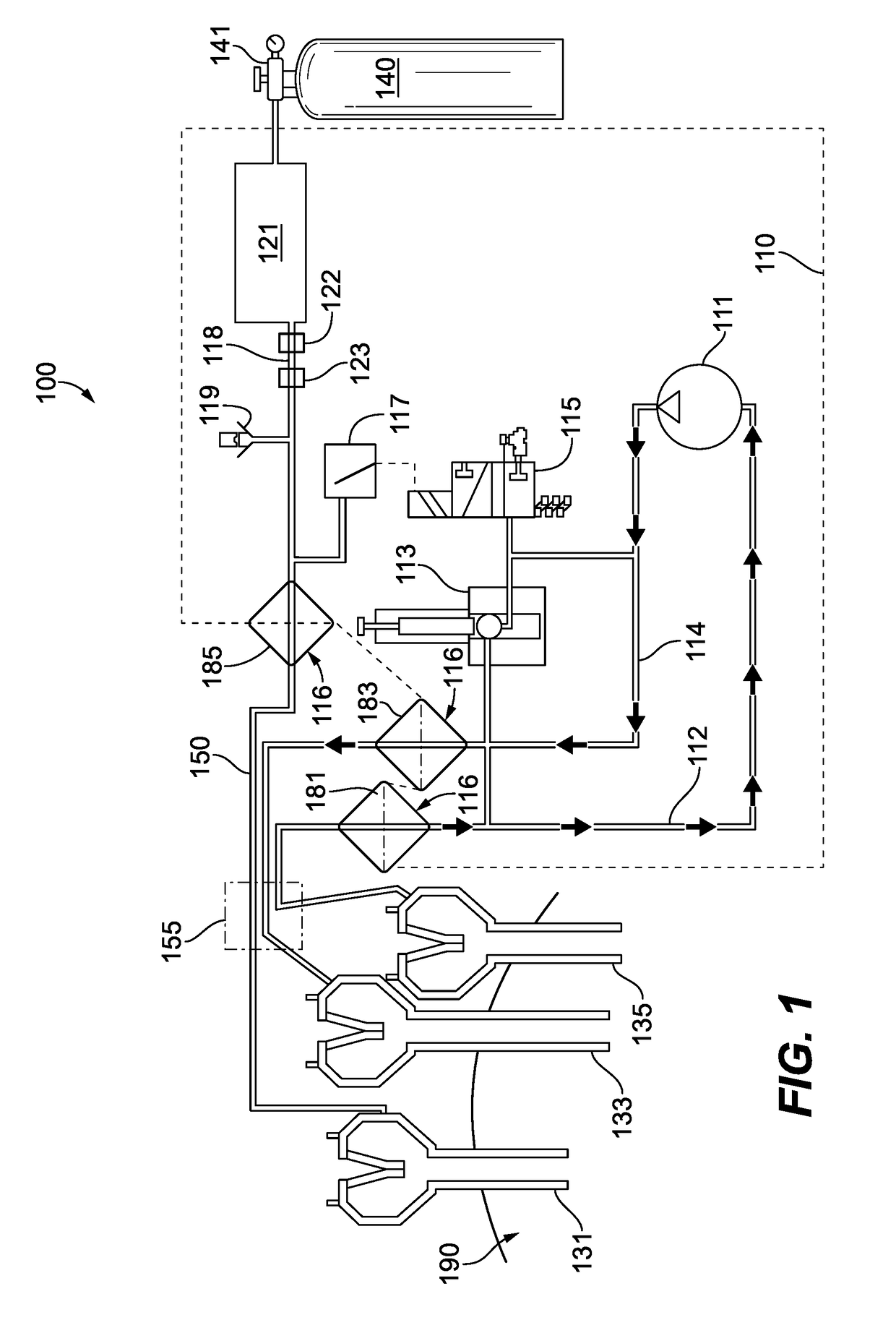
ActiveUS20090137943A1Avoid pressure lossAvoid lostCannulasInfusion syringesControl signalPressure controlled ventilation
A system for insufflation and recirculation of insufflation fluid in a surgical procedure. The system includes a control unit having a fluid pump, a supply conduit, a return fluid conduit and a pressure-controlled valve. The fluid pump is adapted and configured to circulate insufflation fluid through the system. The supply conduit is in fluid communication with an output of the fluid pump and configured and adapted for delivering pressurized insufflation fluid to an output port of the control unit. The return conduit is in fluid communication with an input of the fluid pump for delivering insufflation fluid to the fluid pump and is configured and adapted for returning insufflation fluid from an input port of the control unit. The pressure-controlled is in fluid communication with the supply conduit and the return conduit, and is adapted and configured to receive a control signal and respond to the control signal by opening, thereby fluidly connecting the supply conduit and the return conduit with one another.
Owner:SURGIQUEST
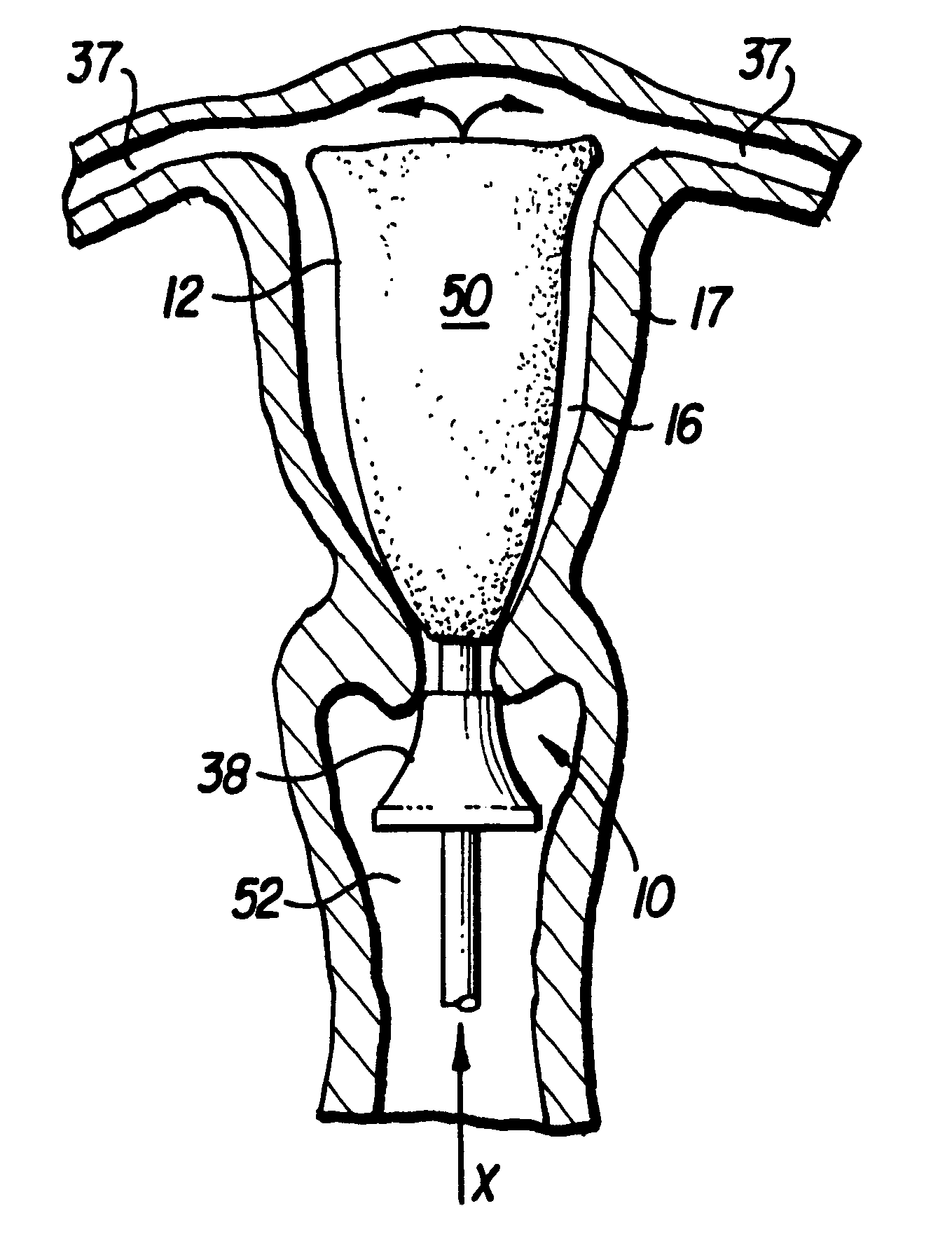
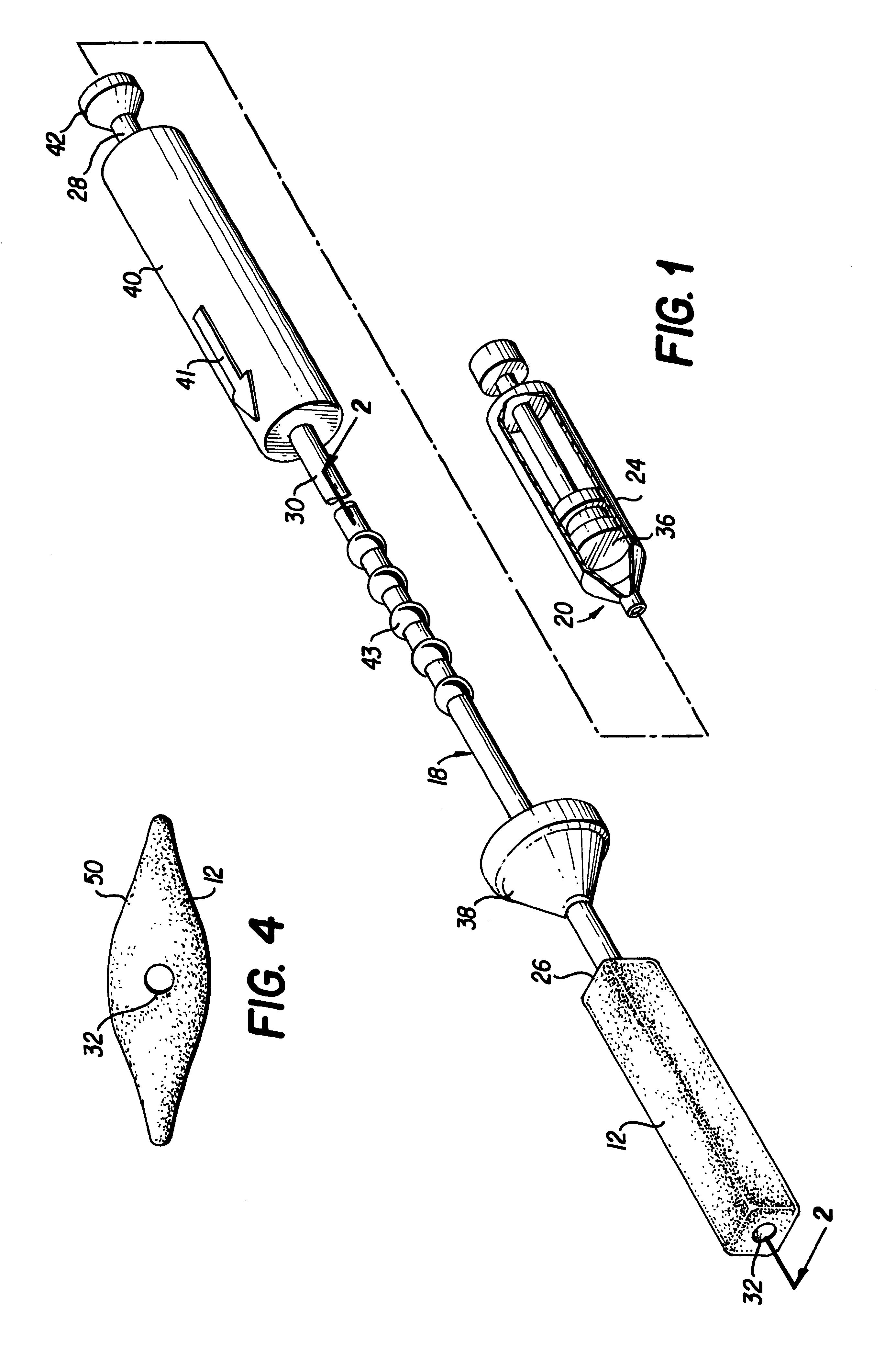
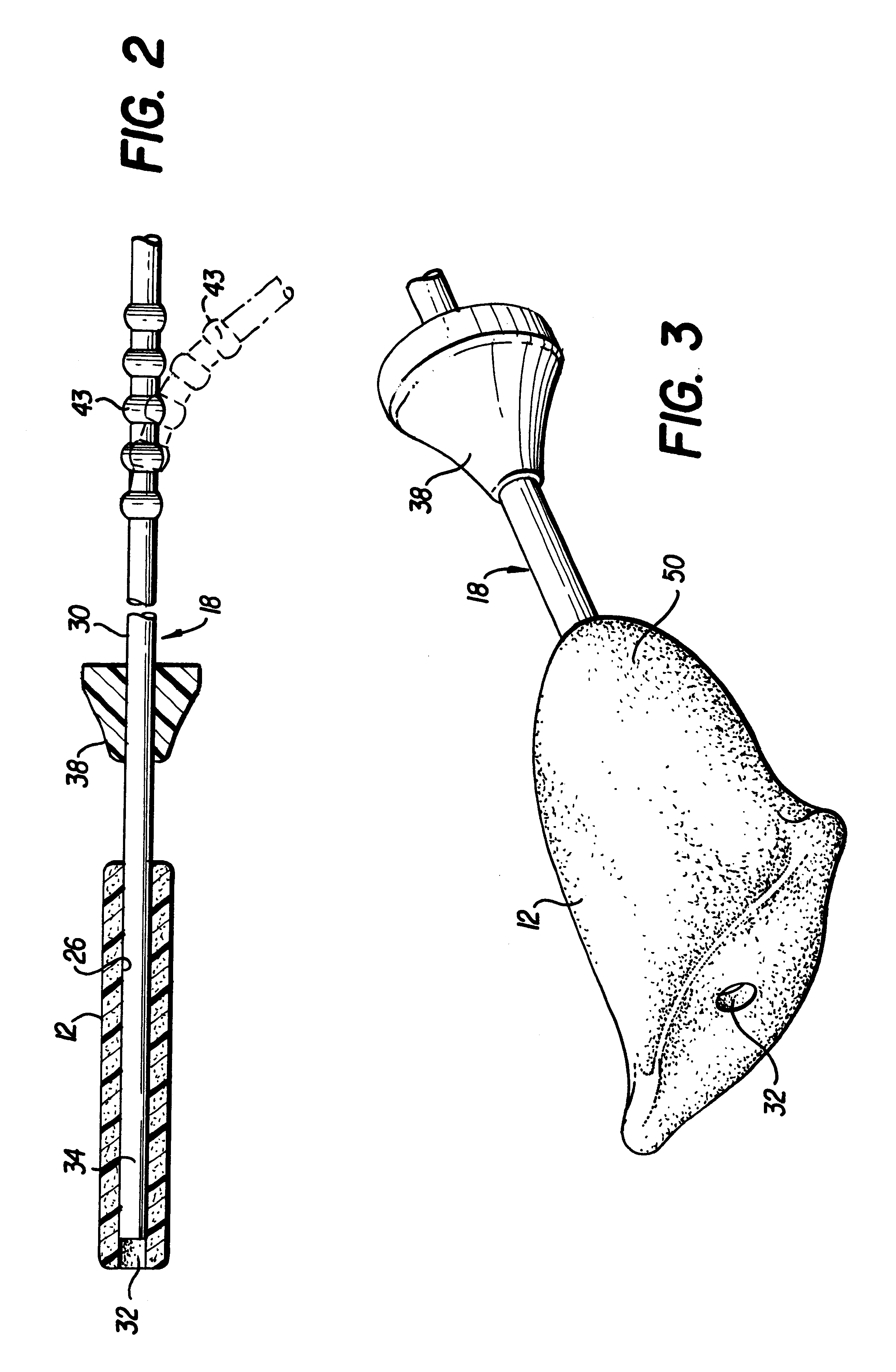
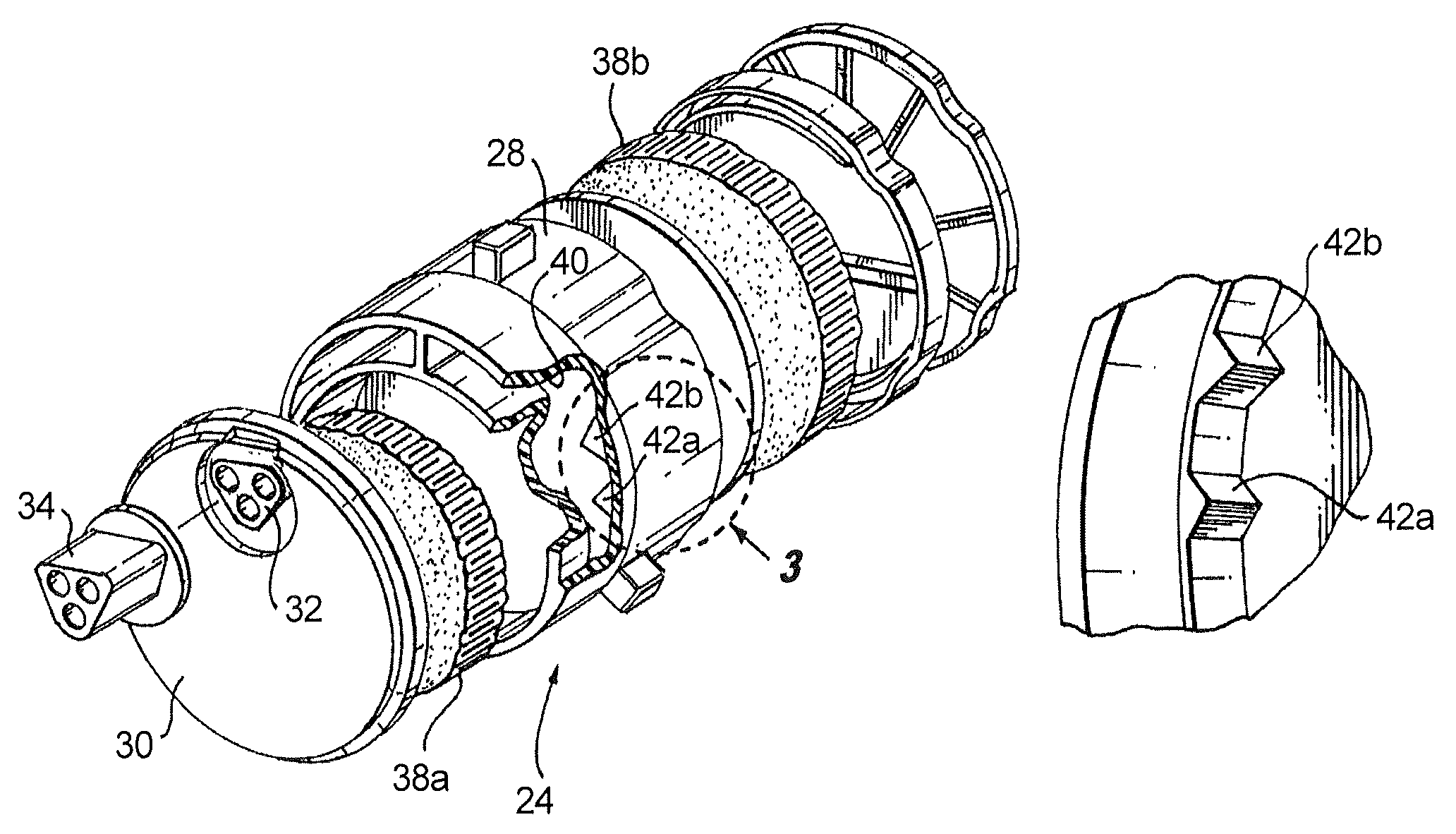
Apparatus and method for delivering and deploying an expandable body member in a uterine cavity
InactiveUS6395012B1Maximize contactSufficient pressureStentsBalloon catheterEndometrial layerGynecology
An apparatus and method delivers an expandable body member into a uterine cavity of a uterus through a cervical opening and deploys the expandable body member in the uterine cavity. The expandable body member in a compressed state is inserted into the uterine cavity. A fluid is provided to the uterine cavity at a pressure sufficient to inflate the uterine cavity. The expandable body member expands from the compressed state to the expanded state. After expansion of the expandable body member and inflation of the uterine cavity, the fluid pressure is relieved thereby collapsing the uterine cavity about the expandable body member in the expanded state such that an endometrial layer of the uterus and a surface of the expandable body member substantially contact each other in a facially opposing relationship maximizing contact therebetween. The apparatus which performs the method includes an elongated tubular member having the expandable body member connected to a distal portion of the elongated tubular member and a fluid-providing device connected to a proximal portion of the elongated tubular member.
Owner:YOON INBAE +1
Filter interface for multimodal surgical gas delivery system
Owner:SURGIQUEST
Disposable aerosol generator system and methods for administering the aerosol
InactiveUS20020078951A1Avoid contamination of fluidNegates needRespiratorsMedical devicesBiomedical engineeringAerosol generator
A disposable aerosol generator for use with an inhaler device which includes a heater adapted to volatilize fluid stored in the disposable aerosol generator and method of using the inhaler. The disposable body includes a sealed chamber and an outlet, the chamber being located between first and second layers of material. The chamber holds a predetermined volume of a fluid which is expelled through the outlet when the fluid in the chamber is volatilized by the heater. The disposable body can include a series of spaced apart aerosol generators, each of which can be advanced to a release position at which the heater can heat one of the fluid containing chambers. Prior to heating the fluid, the outlet can be formed by severing the first and / or second layer with a piercing element and the volatilized fluid can be expelled from the outlet into a passage of a dispensing member.
Owner:PHILIP MORRIS USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com