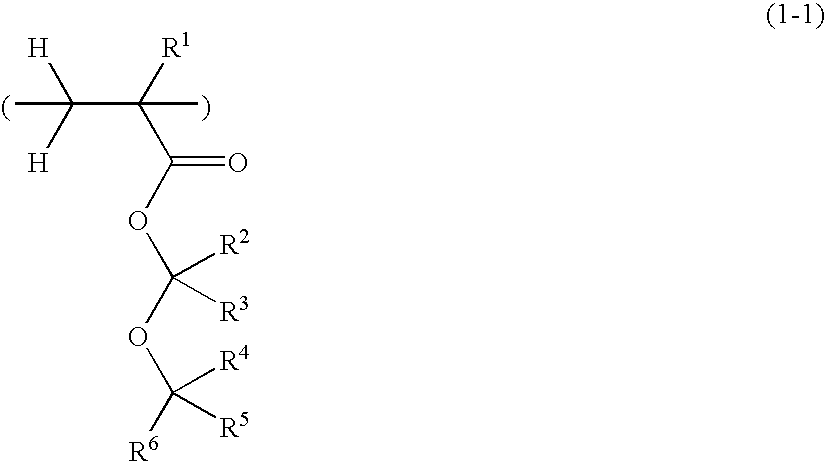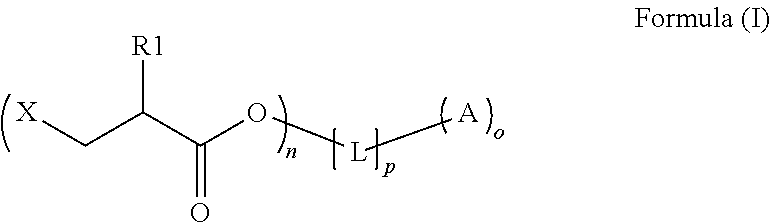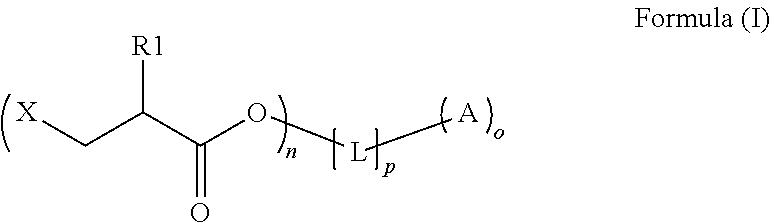Patents
Literature
53 results about "Elimination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In pharmacology the elimination or excretion of a drug is understood to be any one of a number of processes by which a drug is eliminated (that is, cleared and excreted) from an organism either in an unaltered form (unbound molecules) or modified as a metabolite. The kidney is the main excretory organ although others exist such as the liver, the skin, the lungs or glandular structures, such as the salivary glands and the lacrimal glands. These organs or structures use specific routes to expel a drug from the body, these are termed elimination pathways...
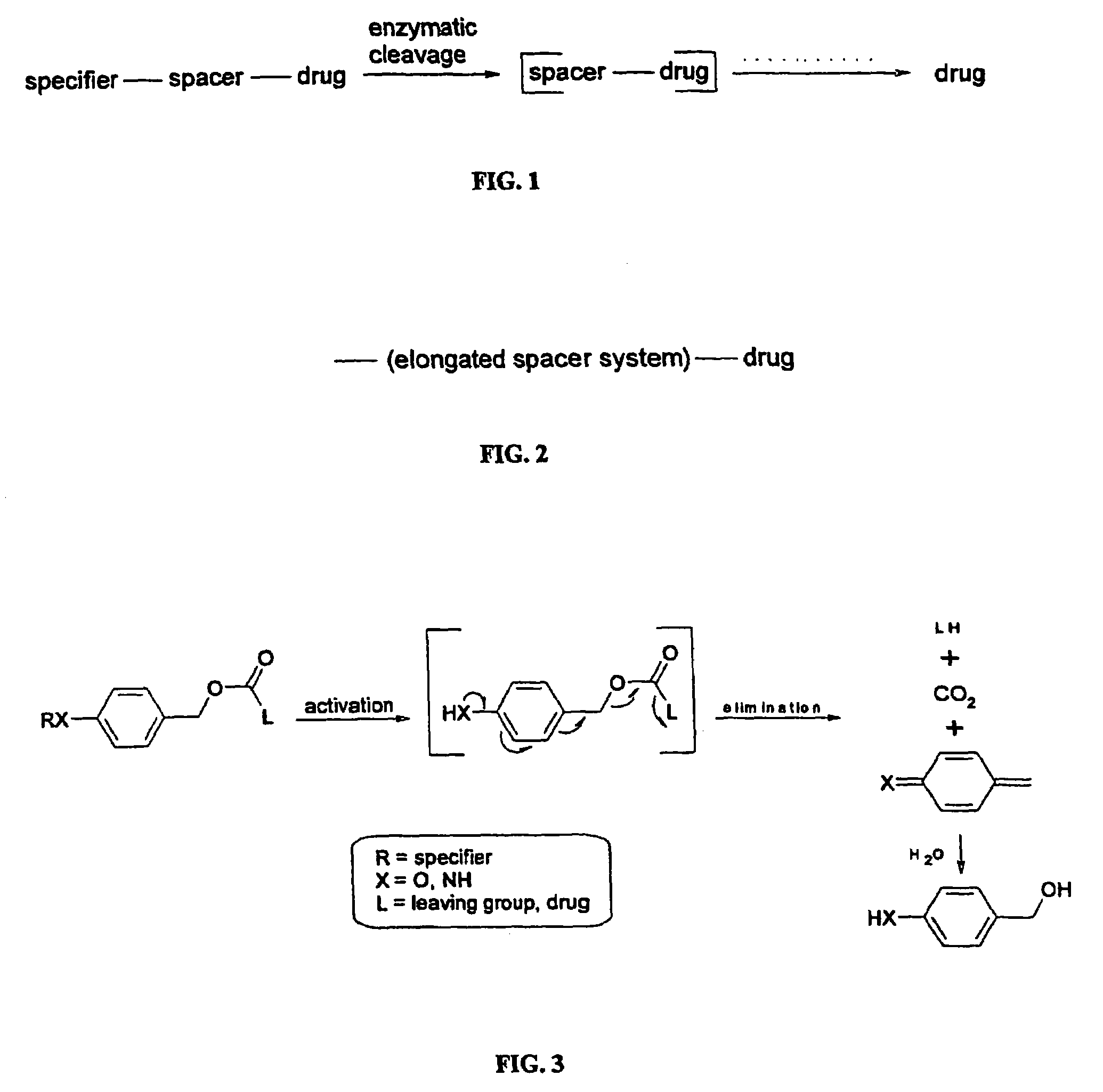
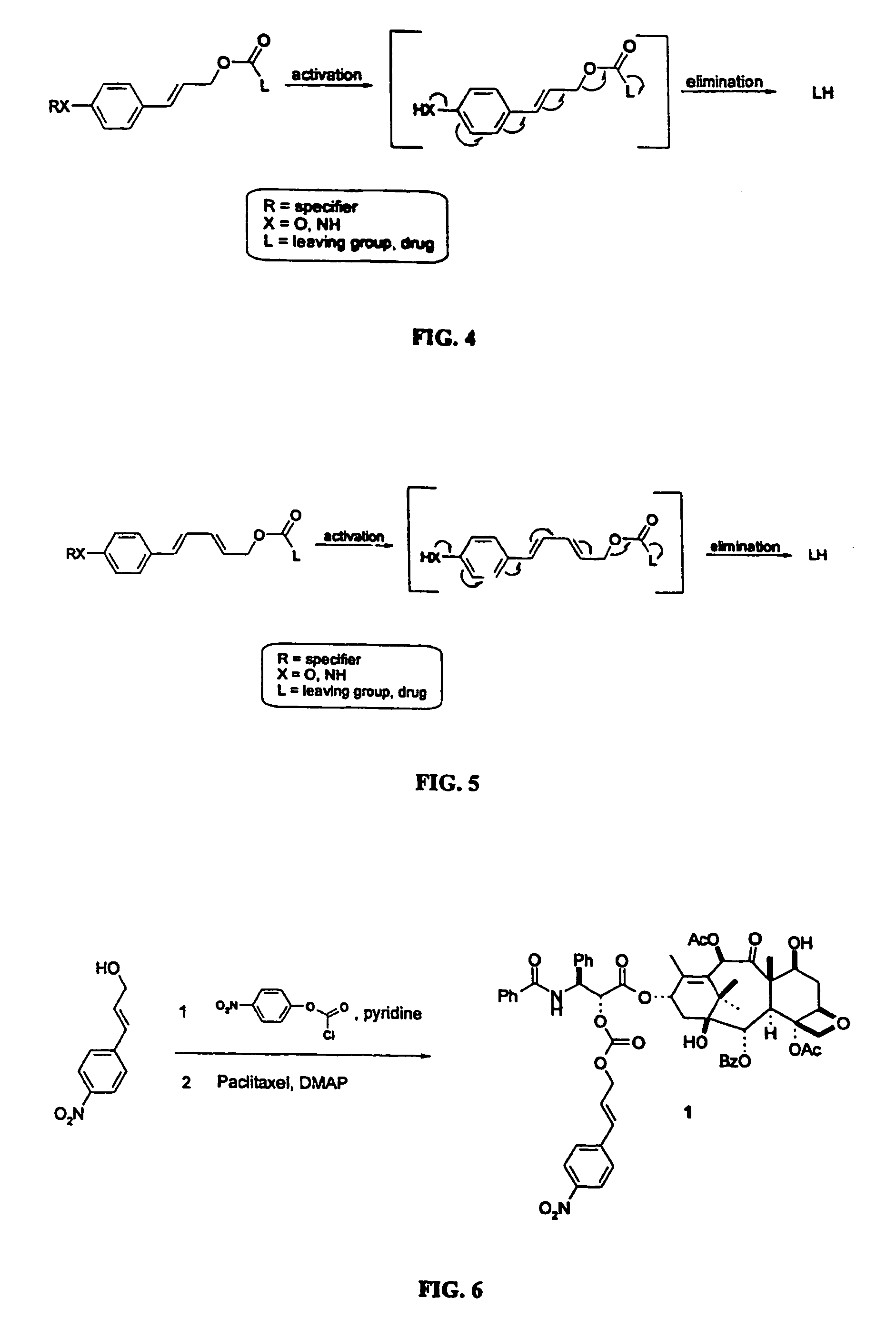
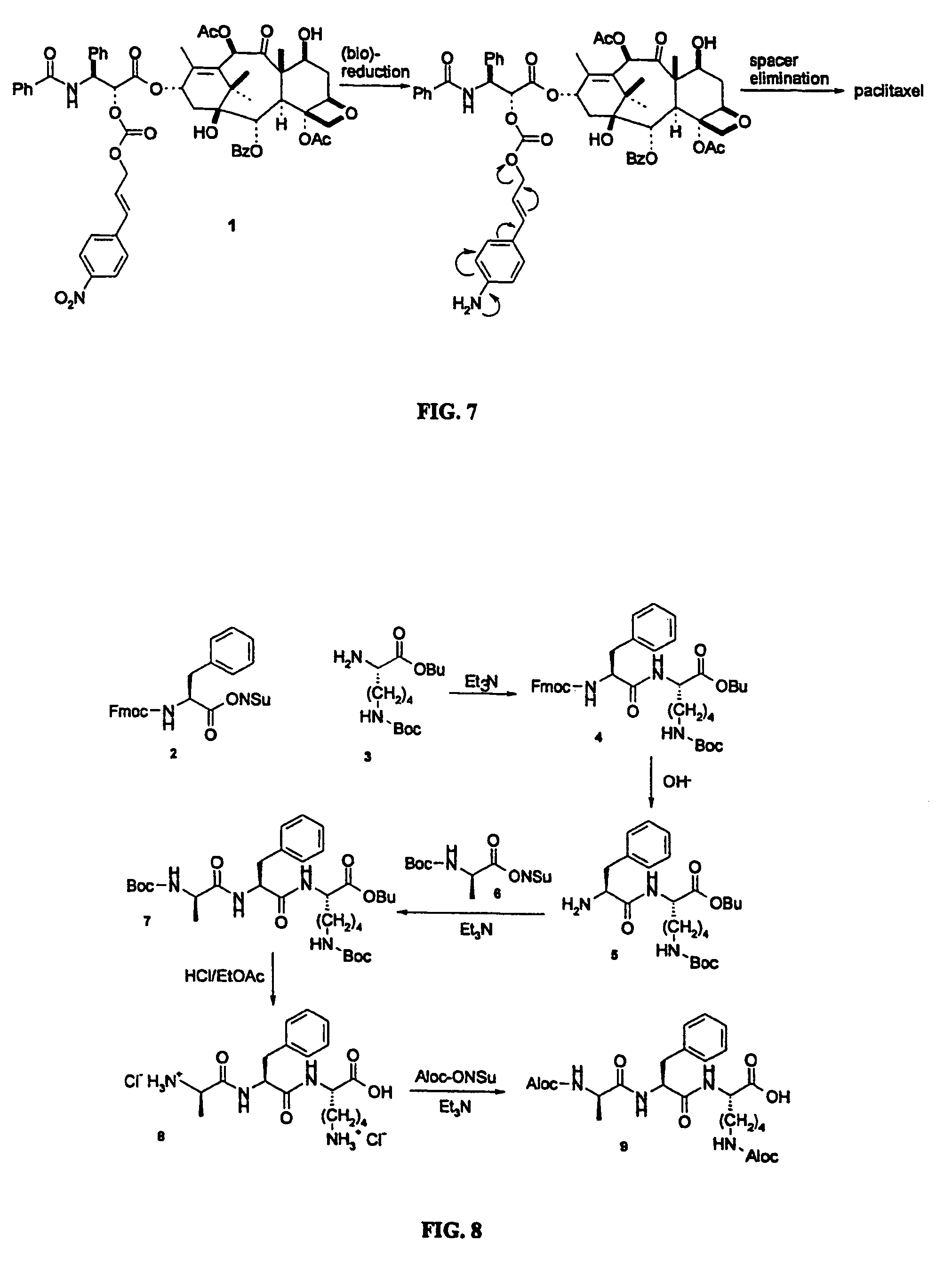
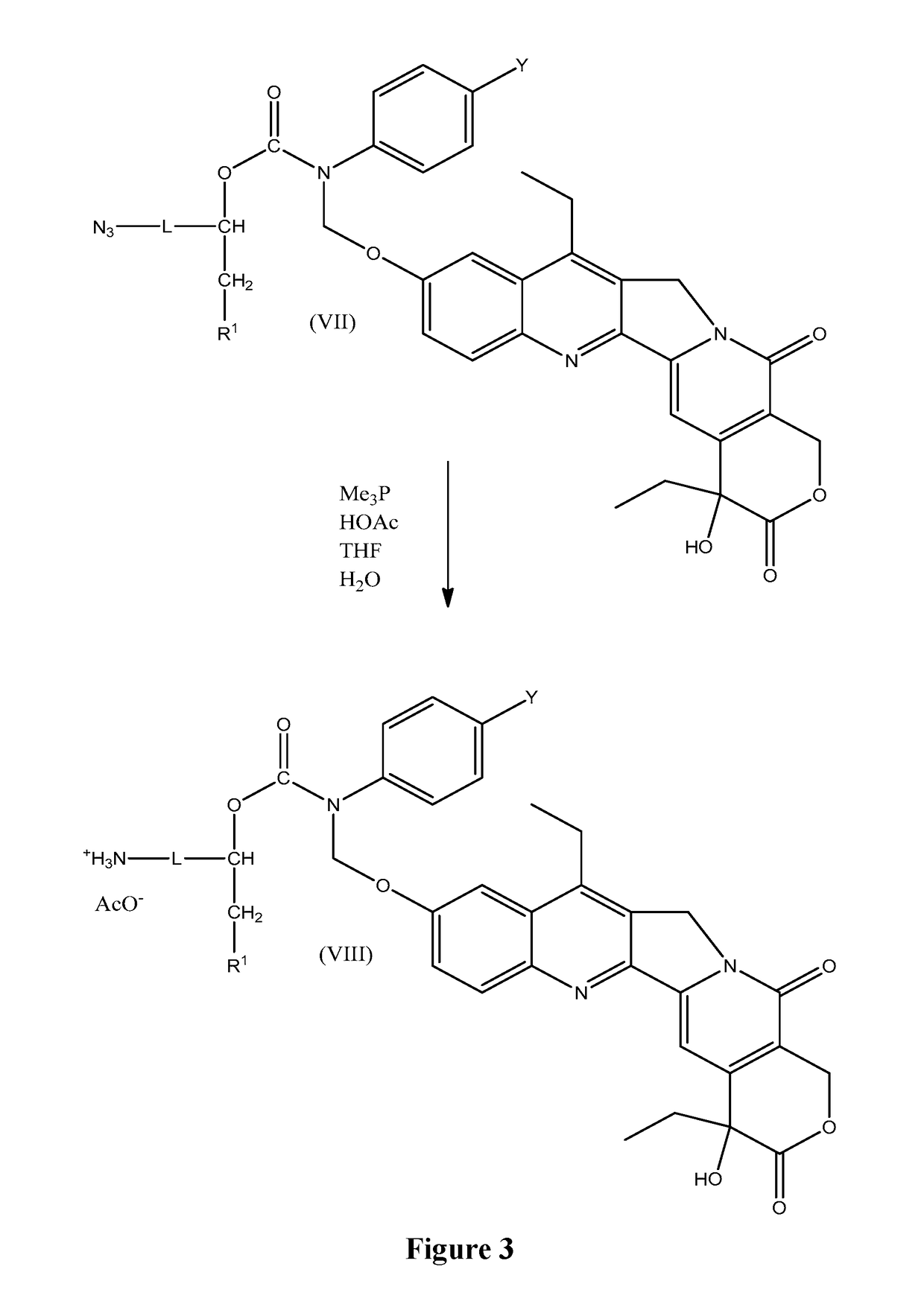
Elongated and multiple spacers in activatible prodrugs
InactiveUS7223837B2Improved kineticsFacilitate enzymatic cleavageAntibacterial agentsOrganic active ingredientsTumor cellsChemistry
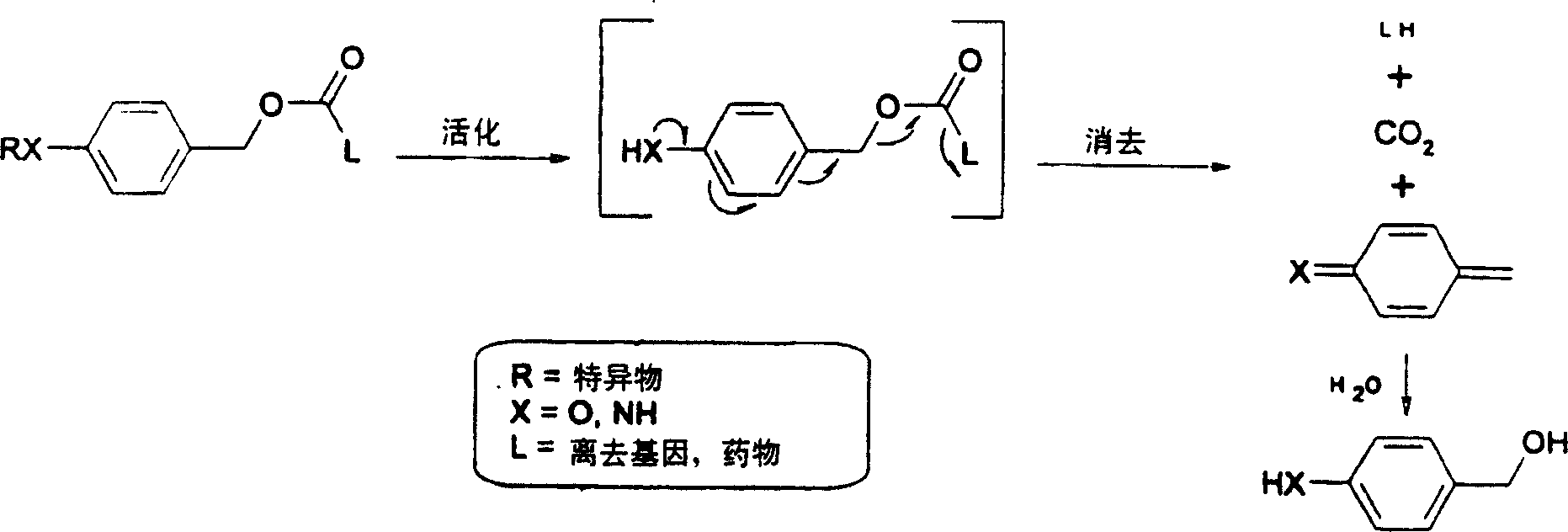
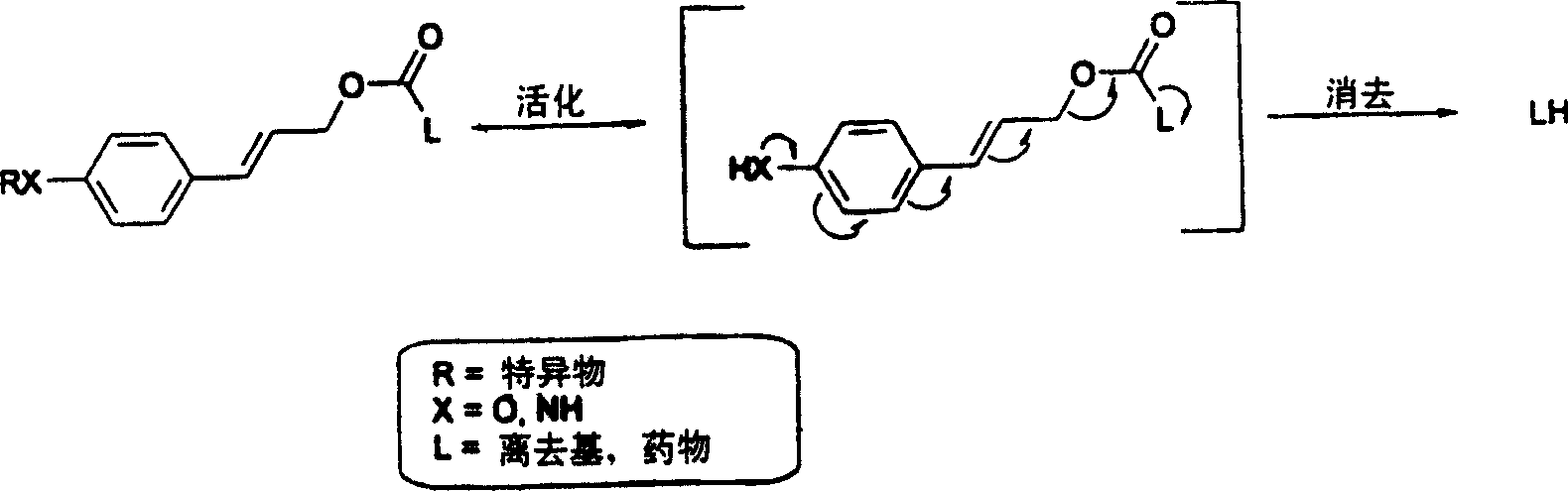
This invention is directed to prodrugs that can be activated at the preferred site of action in order to selectively deliver the corresponding therapeutic parent drugs to target cells or to the target site. This invention will therefore primarily but not exclusively relate to tumor cells as target cells. More specifically the prodrugs are compounds of the formula V—(W)k—(X)l—A—Z, wherein: V is a specifier; (W)k—(X)l—A is an elongated self-elimination spacer system; W and X are each a 1,(4+2n) electronic cascade spacer, being the same or different; A is either a spacer group of formula (Y)m wherein: Y is a 1,(4+2n) electronic cascade spacer, or a group of formula U being a cyclization elimination spacer; Z is a therapeutic drug; k, l and m are integers from 0 (included) to 5 (included); n is an integer of 0 (included) to 10 (included), with the provisos that: —when A is (Y)m: k+l+m≧1, and if k+l+m=1; —when A is U: k+l≧1.
Owner:BYONDIS BV
Parallel Processing Fluidic Method and Apparatus for Automated Rapid Immunohistochemistry
InactiveUS20080213804A1Easy to useShorten time for amountBioreactor/fermenter combinationsBiological substance pretreatmentsSurgical operationGuideline
A sample processing system that may be configured to achieve parallel or coincidental sample processing such as histochemical processing may involve a plurality of samples arranged for coincidental movement perhaps by use of angular microscopic slide movements to cause processing activity that may include repeated elimination and reapplication of a fluidic substance perhaps through the action of capillary motion in order to refresh a microenvironment adjacent to a sample such as a biopsy or other such sample. Snap in antibody and other substances may be included to ease operator actions and to permit location specific substance applications perhaps by including single container multiple chamber multiple fluidic substance magazines, linearly disposed multiple substance source, and primary antibody cartridges. Through refreshing of a microenvironment, depletion of the microenvironment is avoided and the time necessary for slide processing may be dramatically shortened from a more common 60 to 120 minutes to perhaps less than 15 minutes so as to permit use of such a system in an intraoperative or surgical environment such as recommended by the College of American Pathologists intraoperative guidelines or the like. Patients may thus avoid a need to be subjected to an additional surgical procedure when lab results become available to see if tumors or the like were fully removed in a prior procedure.
Owner:CELERUS DIAGNOSTICS
Method And Apparatus For Automated Rapid Immunohistochemistry
InactiveUS20080194034A1Reduce manufacturing costEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsGuidelineMicroscopic scale
A sample processing system that may be configured to achieve parallel or coincidental sample processing such as histochemical processing may involve a plurality of samples arranged for coincidental movement perhaps by use of angular microscopic slide movements to cause processing activity that may include repeated elimination and reapplication of a fluidic substance perhaps through the action of capillary motion in order to refresh a microenvironment adjacent to a sample such as a biopsy or other such sample. Snap in antibody and other substances may be included to ease operator actions and to permit location specific substance applications perhaps by including single container multiple chamber multiple fluidic substance magazines, linearly disposed multiple substance source, and primary antibody cartridges. Through refreshing of a microenvironment, depletion of the microenvironment is avoided and the time necessary for slide processing may be dramatically shortened from a more common 60 to 120 minutes to perhaps less than 15 minutes so as to permit use of such a system in an intraoperative or surgical environment such as recommended by the College of American Pathologists intraoperative guidelines or the like. Patients may thus avoid a need to be subjected to an additional surgical procedure when lab results become available to see if tumors or the like were fully removed in a prior procedure.
Owner:CELERUS DIAGNOSTICS
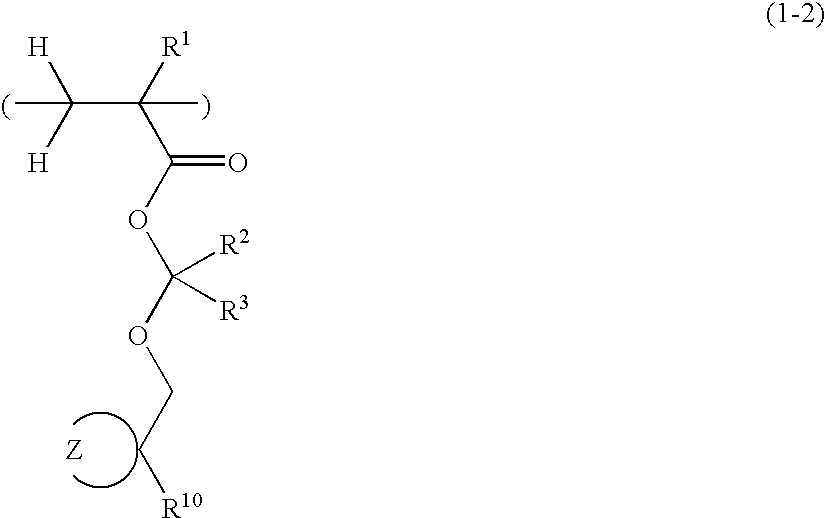
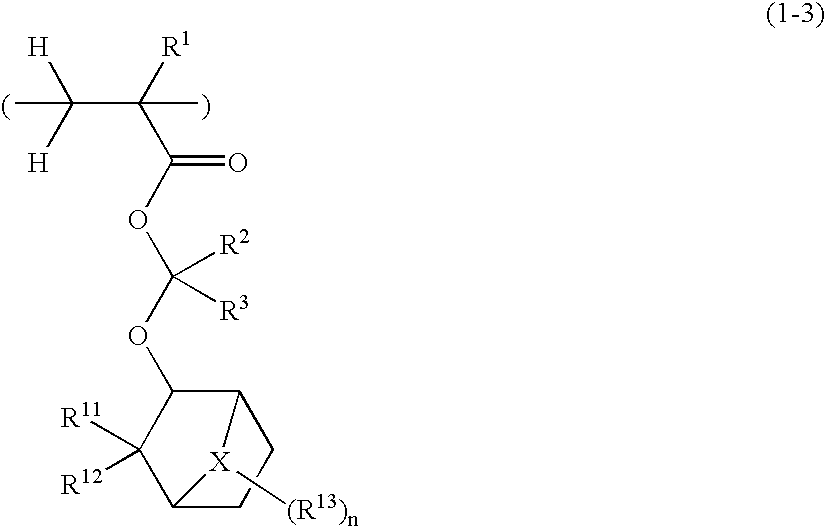
Positive resist compositions and patterning process
A positive resist composition is provided comprising (A) a resin component having a carboxylic acid moiety protected with an acetal protective group which is decomposable under the action of an acid, wherein in the carboxylic acid moiety protected with an acetal protective group, deprotection occurs not by way of β-elimination, and (B) a photoacid generator. The resist composition exhibits a high resolution when processed by ArF lithography.
Owner:SHIN ETSU CHEM IND CO LTD
Positive resist compositions and patterning process
ActiveUS20080008961A1High resolutionUseful in precise microfabricationPhotosensitive materialsRadiation applicationsResistLithographic artist
Owner:SHIN ETSU CHEM IND CO LTD
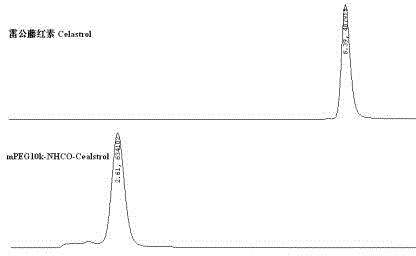
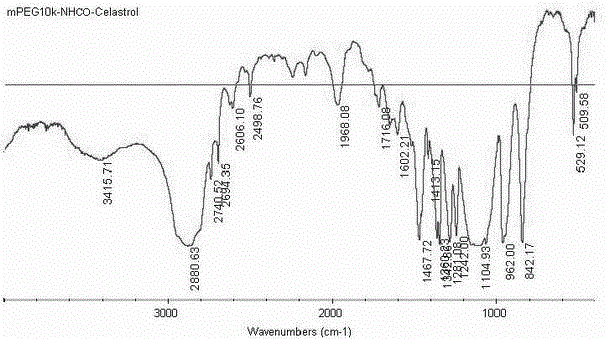
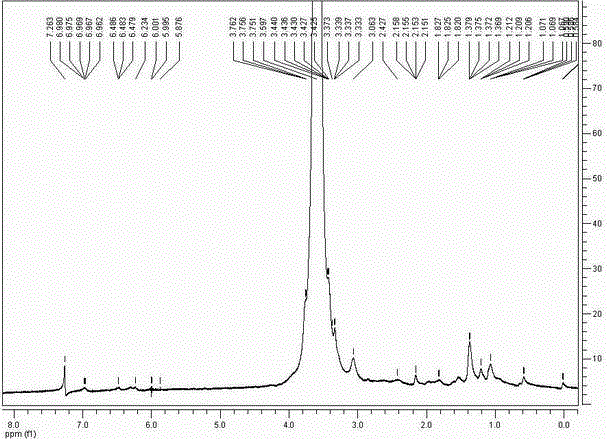
Pegylated celastrol and preparation method and application thereof
InactiveCN102796254ALong elimination half-lifeSmall toxicityOrganic active ingredientsAntineoplastic agentsSide effectElimination
The invention belongs to the technical field of medicine, and relates to pegylated celastrol and a preparation method and application thereof. The pegylated celastrol has a structure shown as a general formula in the specifications. The invention also provides application of the pegylated celastrol and methoxy amino polyethylene glycol 10kDa mono-modified celastrol (mPEG10k-NHCO-celastrol) to preparation of medicines for treating various malignant tumors such as prostatic cancer, lung cancer, liver cancer and cervical cancer. Celastrol is subjected to chemical structural modification by PEG, so that the problem that the celastrol has low solubility can be solved; and an injection can be prepared after the celastrol is modified by the PEG, so that the elimination half life of the celastrol can be prolonged through in-vivo injection, the toxic and side effects of the celastrol are reduced, and the administration frequency of a patient is reduced.
Owner:FUDAN UNIV
Antioxidative peptide derived from yak blood and preparation and application of antioxidative peptide
The invention provides a yak blood antioxidative peptide with intnstive antioxidation activity. The antioxidative peptide has a sequence of TLPDTEKQIKKQ, has reducing power up to 3.24 mg*mL<-1>, has a lipid peroxidation inhibition capability up to 2.43 mg*mL<-1>, an OH elimination capability up to 0.74 mg*mL<-1>, an ABTS<+> elimination capability up to 1.58 mg*mL<-1>, a DPPH elimination capability up to 1.59 mg*mL<-1>, a total antioxidation capability up to 4.27 mg*mL<-1>, has very good use prospects, and can be used for preparing corresponding antioxidants and applied to related antioxidation application fields.
Owner:SICHUAN TOURISM UNIV
Elogated and multiple spacers in activatible prodrugs
InactiveCN1511044AAntibacterial agentsOrganic active ingredientsChemical compoundPharmaceutical drug
This invention is directed to prodrugs that can be activated at the preferred site of action in order to selectively deliver the corresponding therapeutic parent drugs to target cells or to the target site. This invention will therefore primarily but not exclusively relate to tumor cells as target cells. More specifically the prodrugs are compounds of the formula V-(W)k-(X)1-A-Z, wherein: V is a specifier; (W)k-(X)1-A is an elongated self-elimination spacer system; W and X are each a 1,(4+2n) electronic cascade spacer, being the same or different; A is either a spacer group of formula (Y)m wherein: Y is a 1,(4+2n) electronic cascade spacer, or a group of formula U being a cyclisation elimination spacer; Z is a therapeutic drug; k, 1 and m are integers from 0 (included) to 5 (included); n is an integer of 0 (included) to 10 (included), with the provisos that: - when A is (Y)m: k+1+m >= 1, and if k+1+m = 1; - when A is U: k+1 > / = 1.
Owner:SYNTARGA BV
Cubic cyclodextrin framework-RGD composition and preparation method thereof
ActiveCN111440253AImprove securityGood biocompatibilityAntibacterial agentsPeptide/protein ingredientsDiseaseCyclodextrin
The invention provides a cubic cyclodextrin framework-RGD composition (RGD-COF) and a preparation method thereof. Specifically, the cyclodextrin framework-RGD composition of the present invention contains a cyclodextrin framework (COF) having a cubic structure and RGD. The cubic cyclodextrin framework-RGD composition disclosed by the invention can avoid phagocytosis and elimination of macrophages,enhance the mobility and the adhesion to damaged blood vessels and efficiently target and gather at activated blood platelets at the damaged blood vessel parts, and has great application prospects ontargeted diagnosis and treatment of vascular related diseases such as out-of-control hemorrhage, atherosclerosis and cerebral apoplexy. The invention provides a nanoscale cubic cyclodextrin framework-RGD composition which can be used for intravenous injection or a micron-scale cubic cyclodextrin framework-RGD composition which can be used for local external application by utilizing the advantagethat the size of a cyclodextrin-metal organic framework (CD-MOF) is controllable.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Direct sodium removal method, solution and apparatus to reduce fluid overload in heart failure patients
ActiveUS10898631B2Alleviate fluid overloadStable concentrationOrganic active ingredientsWound drainsEliminationKidney Glomerulus
Owner:SEQUANA MEDICAL NV
Pellet used for treating bone fracture
ActiveCN103566278ARelieve painQuick buildHeavy metal active ingredientsAnthropod material medical ingredientsMedicinal herbsAngelica Sinensis Root
The invention discloses a pellet used for treating bone fracture. The pellet is prepared by mixing 46 kinds of medicinal materials, such as flos carthami, semen persicae, radix paeoniae rubra, sanguis draconis, angelica sinensis, ligusticum wallichii, frankincense, myrrh, radix achyranthis bidentatae, radix dipsaci, drynaria rhizome, radix et rhizome rhei, pseudo-ginseng, liquorice, rhizoma corydalis, lumbricus, ground beetle, rhizoma cibotii, ginseng, poria cocos, rhizoma curcumae longae, catechu, amber, pyritum, dried semen strychni, radix rehmanniae praeparata, cucumber seed, dog bone and pearl, at a certain ratio. The pellet can be used for treating soft tissue damages and sequelae caused by bone fracture, and especially curative effect on fracture is excellent. Anti-inflammatory and analgesic effects are excellent, detumescence of affected parts is quick, deep subcutaneous extravasated blood elimination is quick, formation of callus is quick, curative effect is excellent, effect is achieved quickly, and healing phase is short.
Owner:闫书明 +1
Composition for improving body circulation and delaying aging, and application thereof
PendingCN108686010AImprove physical functionAnti agingFood ingredient as antioxidantDispersion deliveryCurative effectPhysical function
The invention specifically relates to a composition for improving body circulation and delaying aging, and application thereof, belonging to the technical field of medicine. The composition has substantial curative effect on adjustment of the qi movement of the internal organs, elimination of toxins in the body and improvement of body circulation, and is capable of improving somatic functions anddelaying aging.
Owner:GUIZHOU PROVINCE JINQIANGUO BIOTECH CO LTD
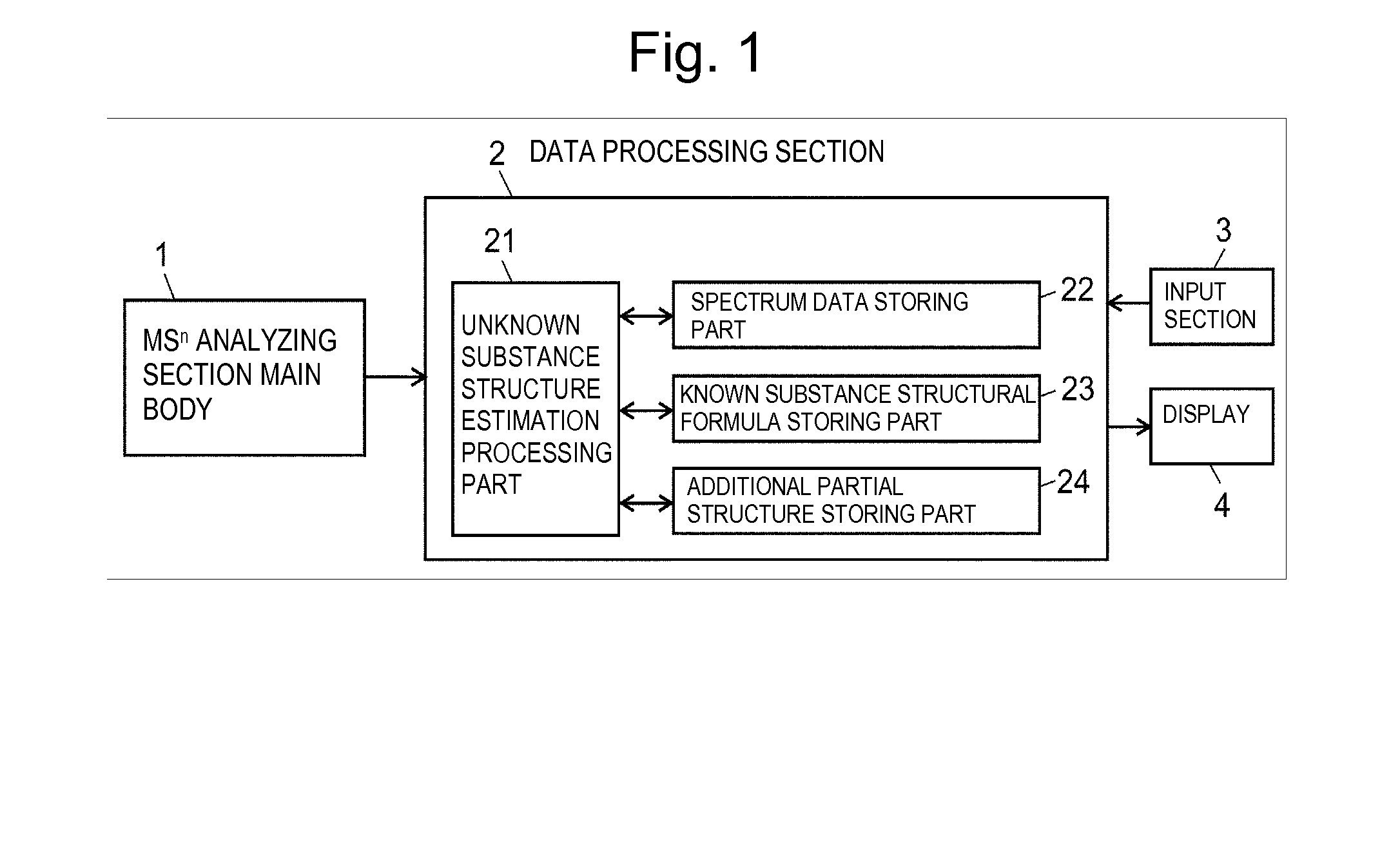
Method and system for mass spectrometry data analysis
ActiveUS20140249766A1Improve reliabilityImprove accuracyParticle separator tubesMolecular entity identificationCrystallographyMass Spectrometry-Mass Spectrometry
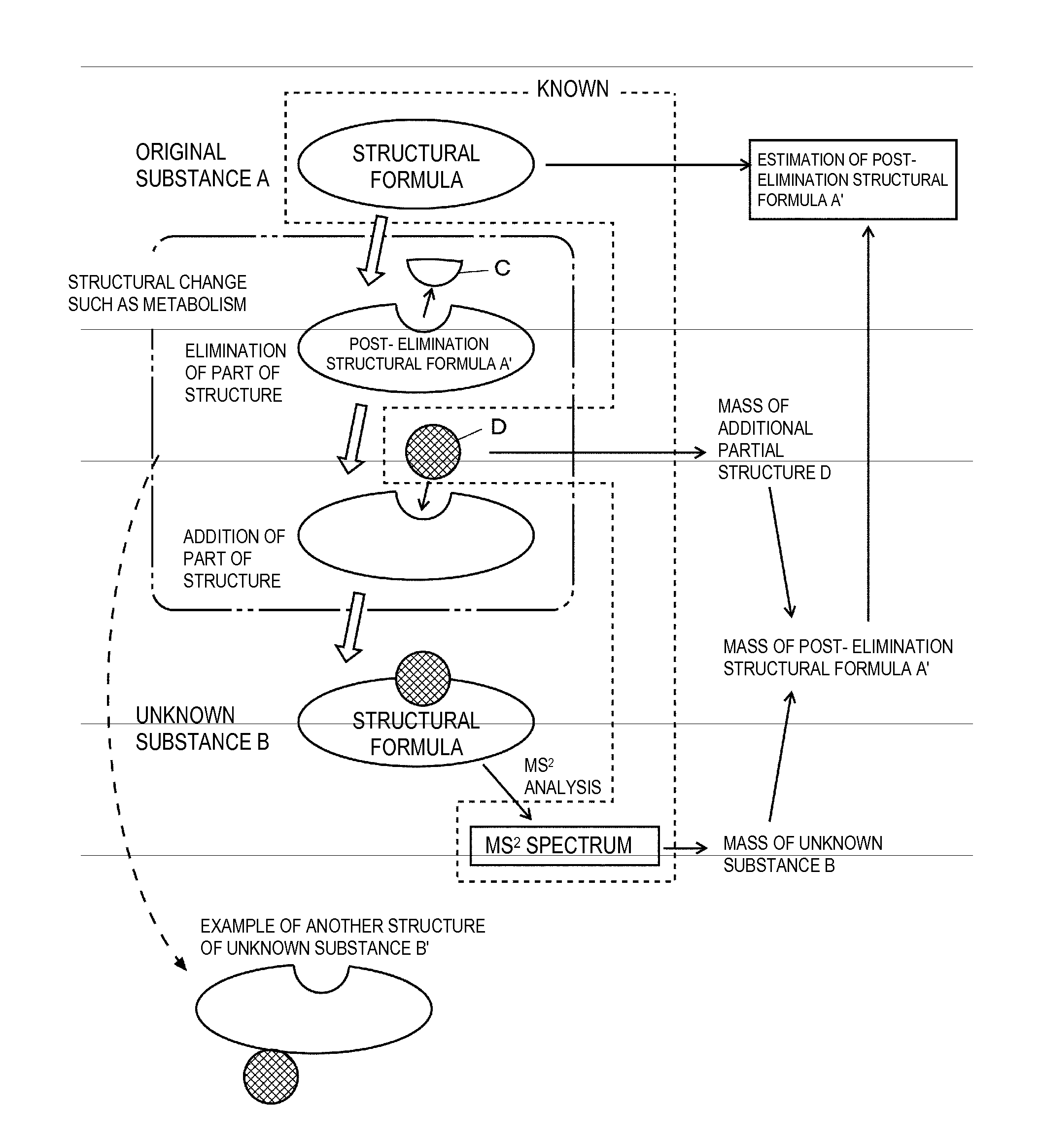
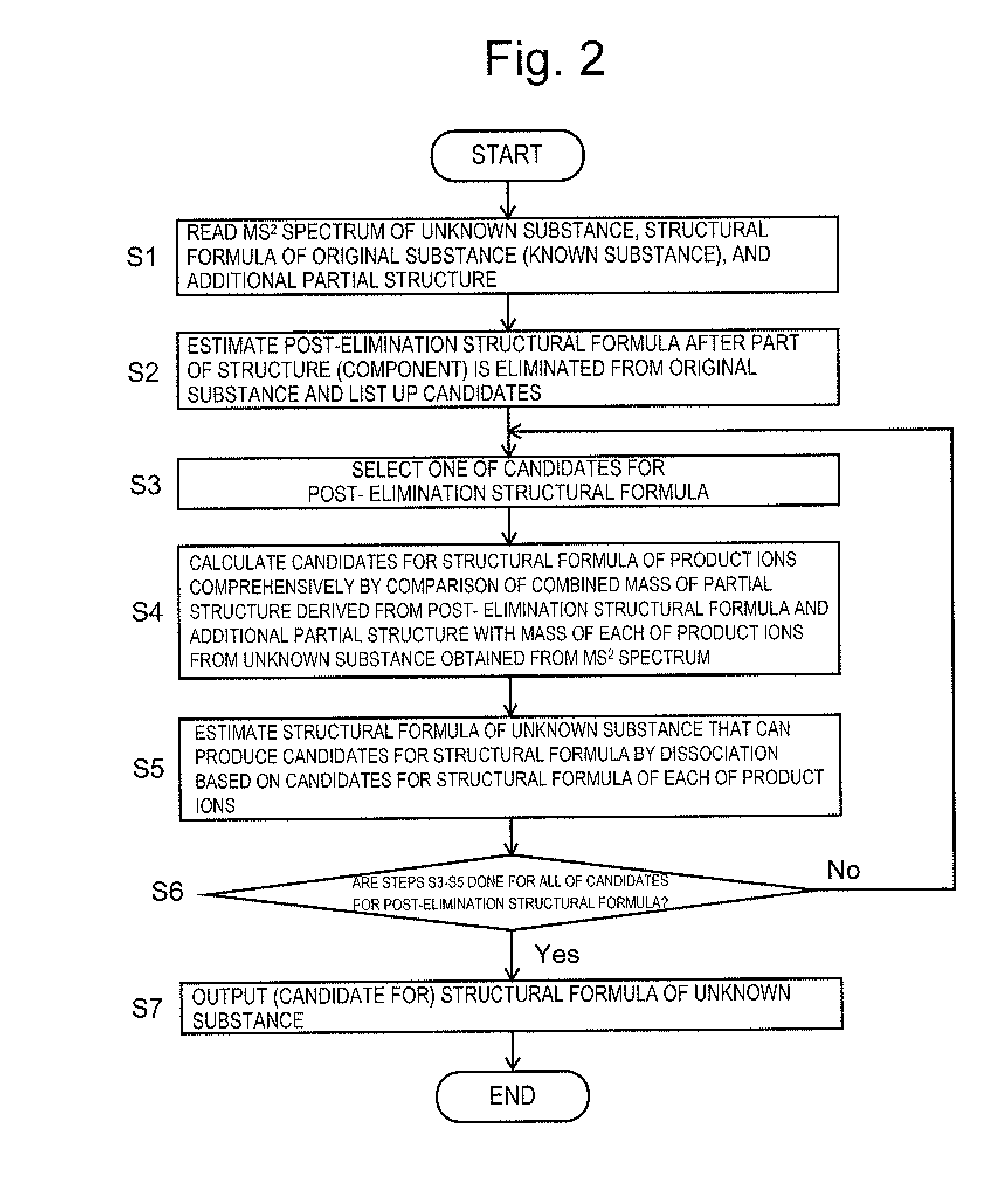
In estimating a structural formula of an unknown substance produced through partial structural change of an original substance having a known structure caused by metabolism or the like, structural change is considered in two stages, the elimination of a partial structure and the addition of another partial structure. First, an additional partial structure is collected as known information in addition to an MSn spectrum of the unknown substance and a structural formula of the original substance. A structural formula at the time when a partial structure is eliminated from the original substance is estimated, and a structural formula of each of product ions is estimated. The structural formula of the unknown substance is determined by estimating a structure that can produce the candidates for structural formulas of the product ions by dissociation.
Owner:SHIMADZU CORP
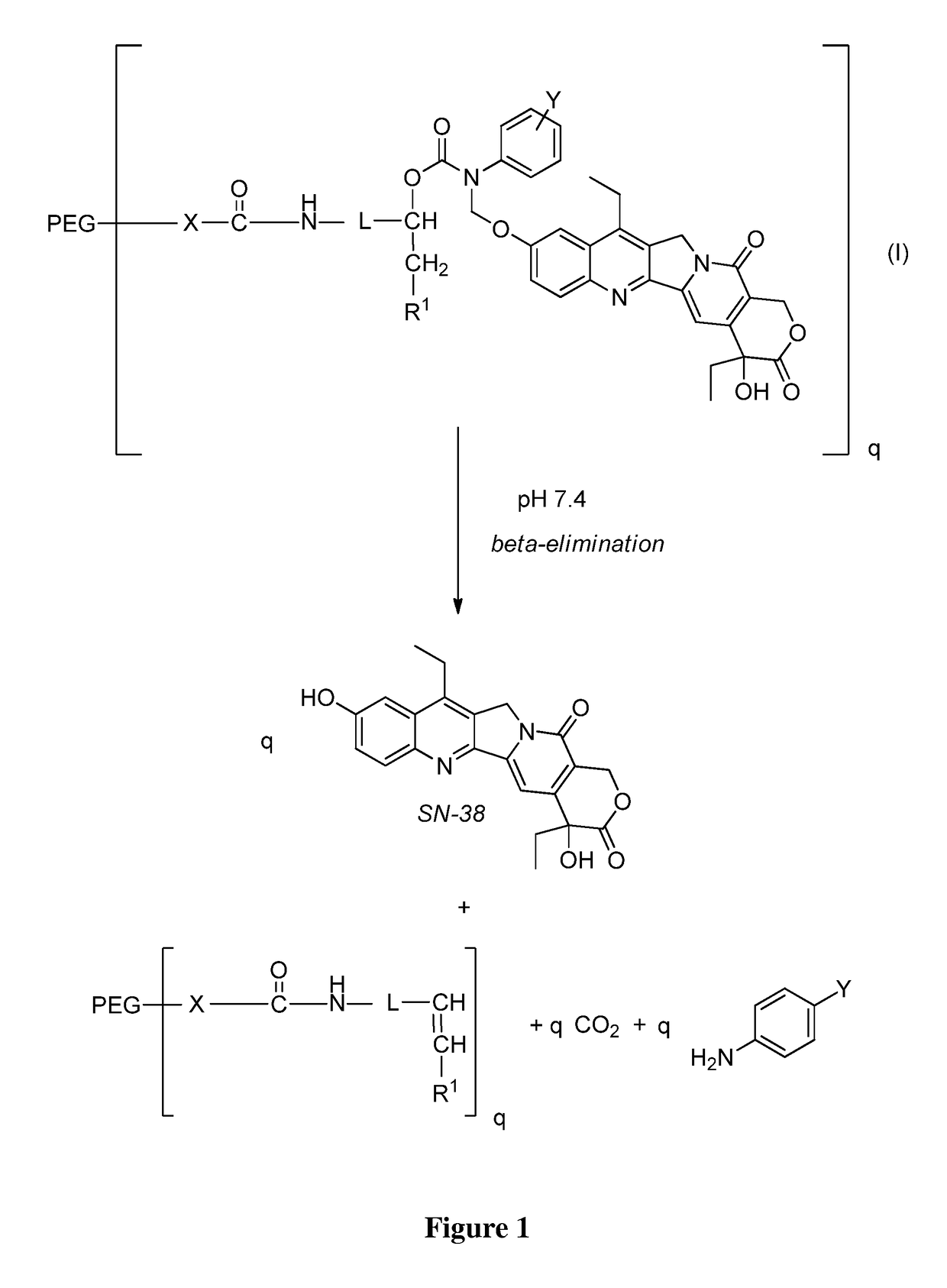
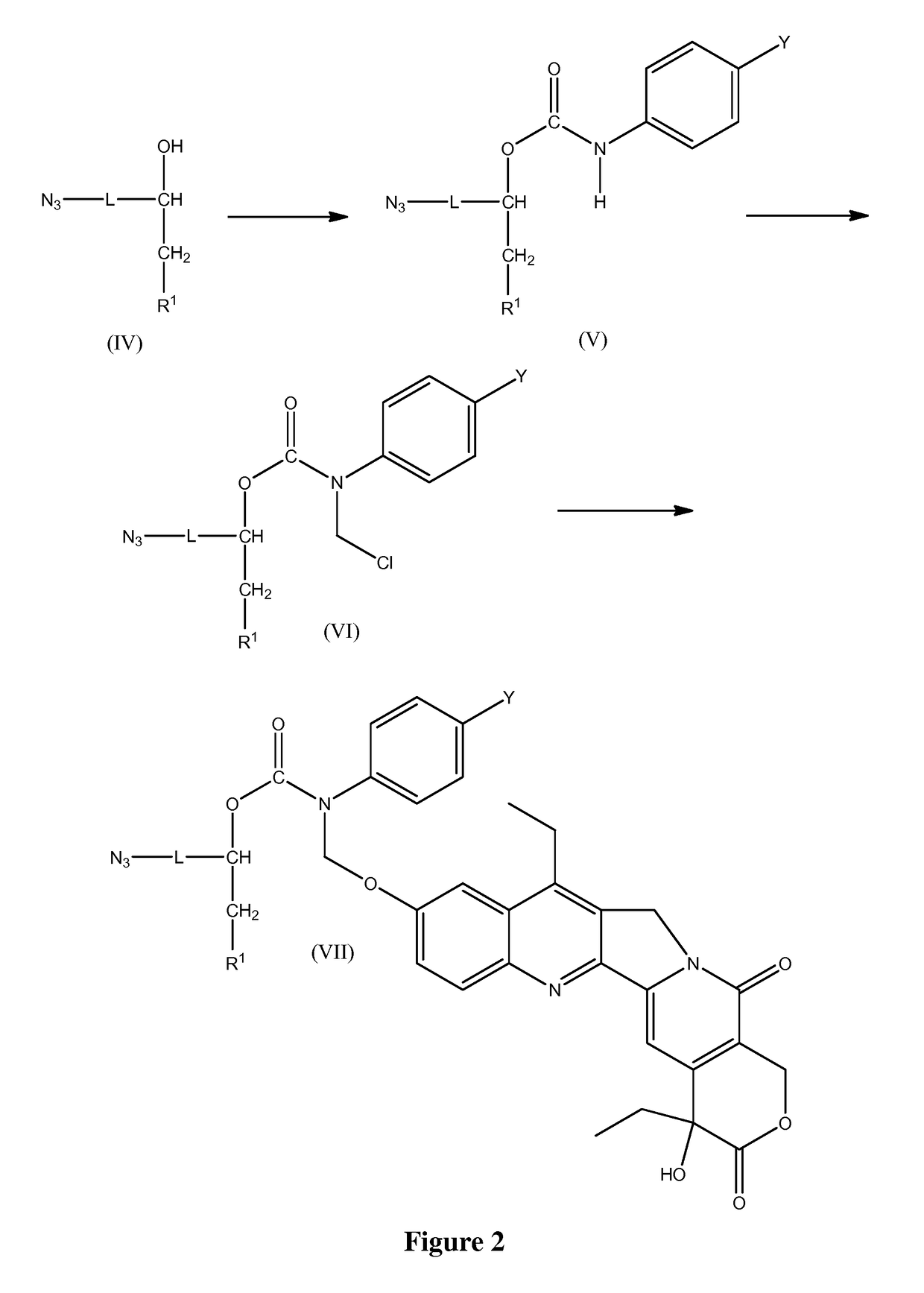
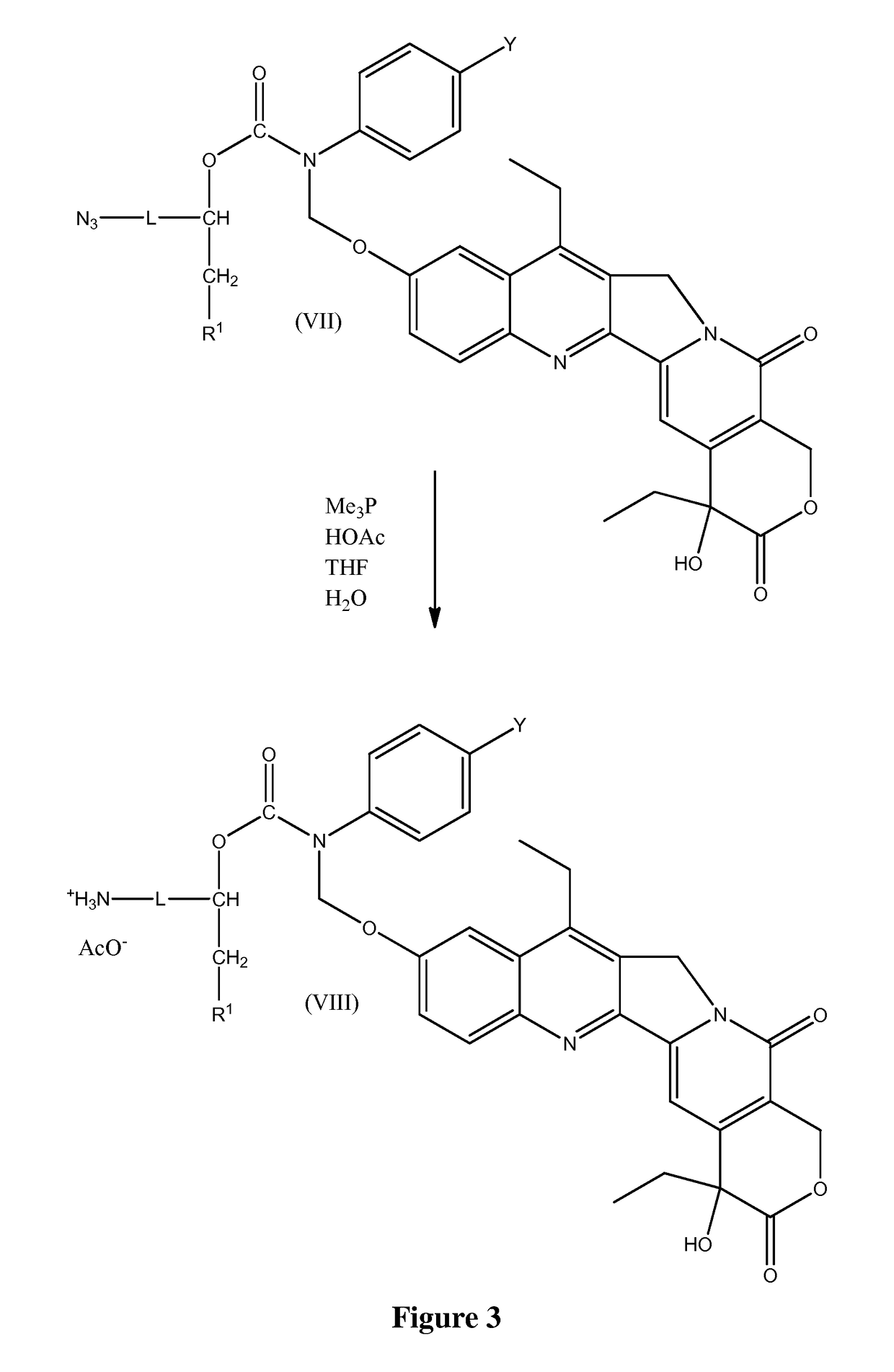
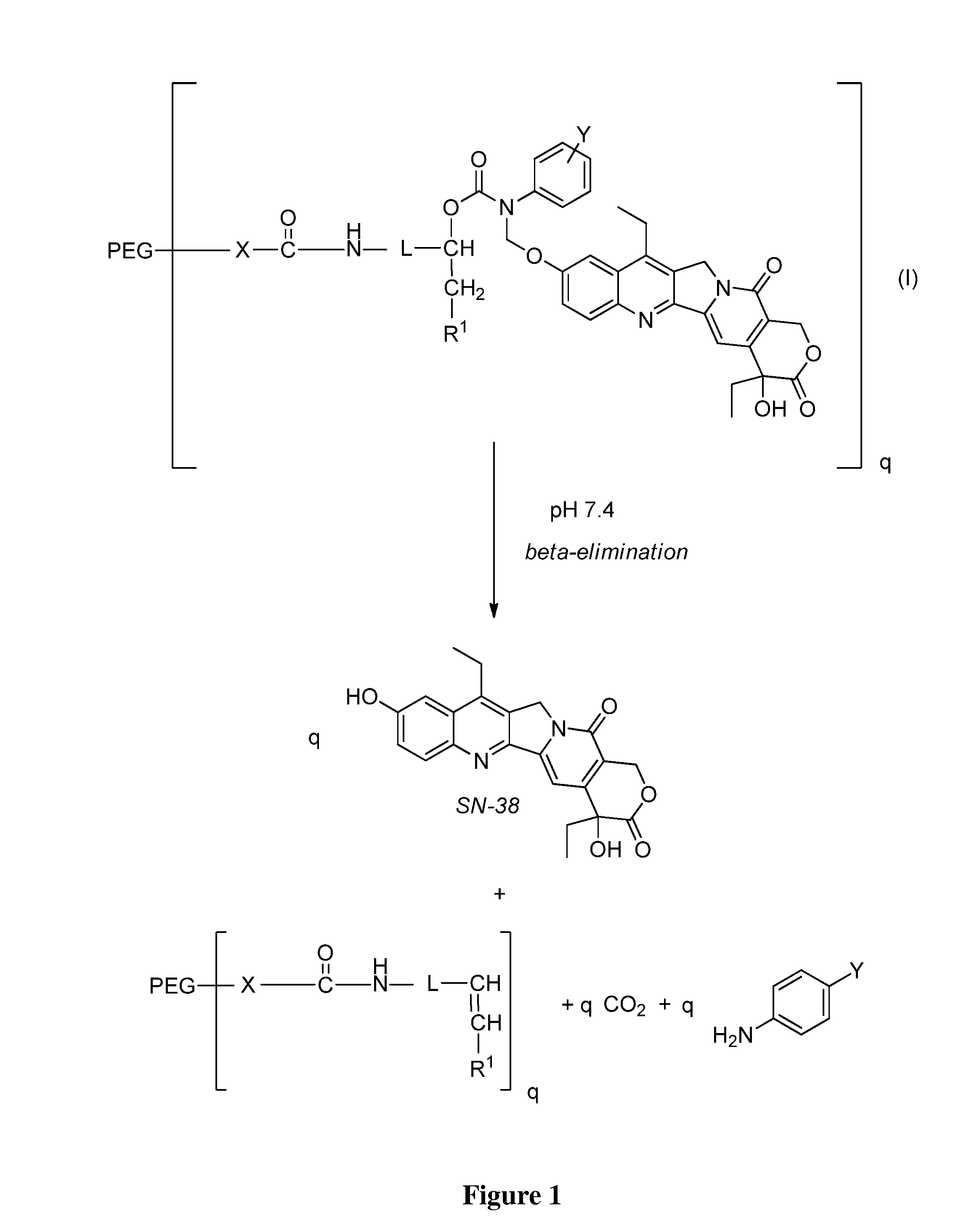
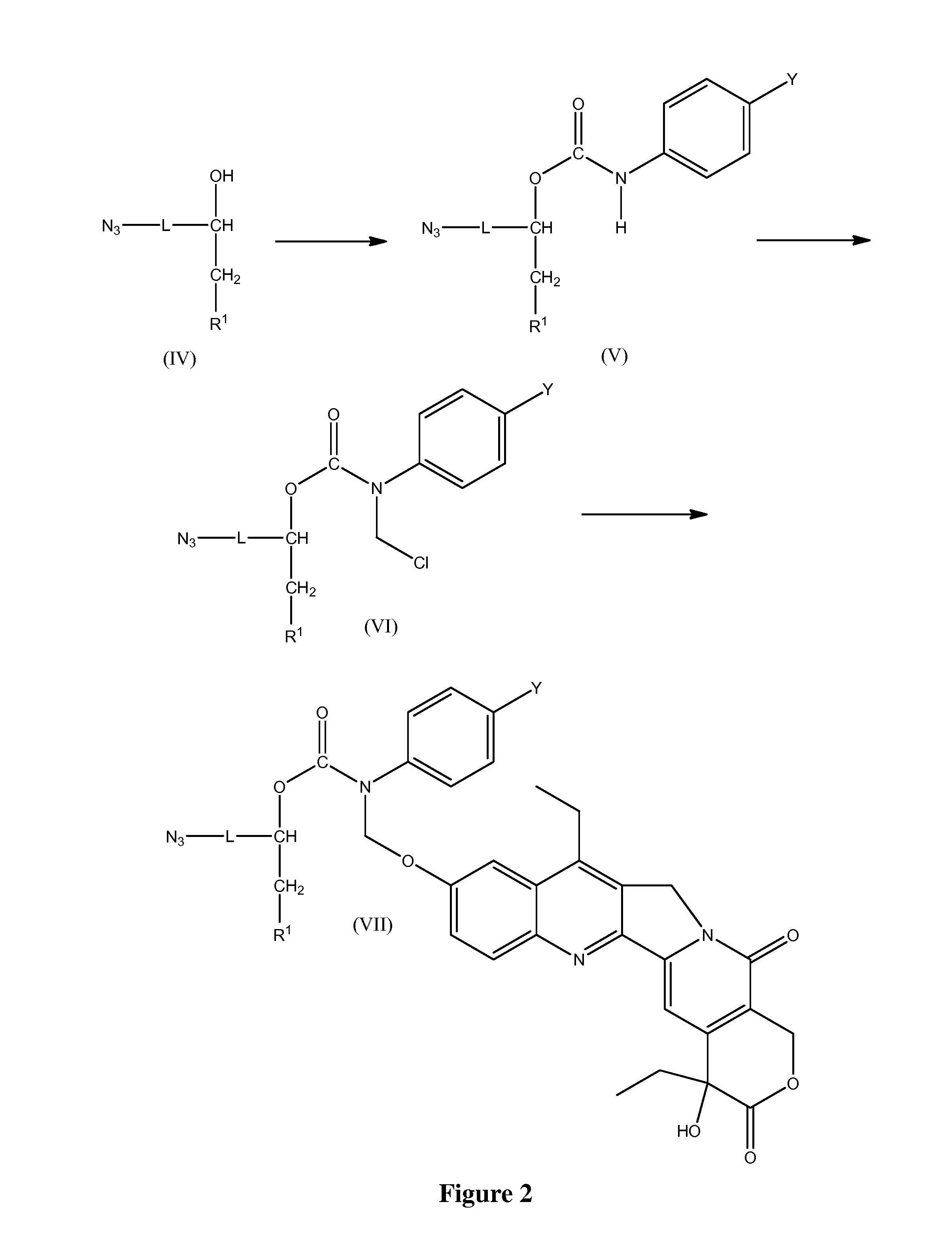
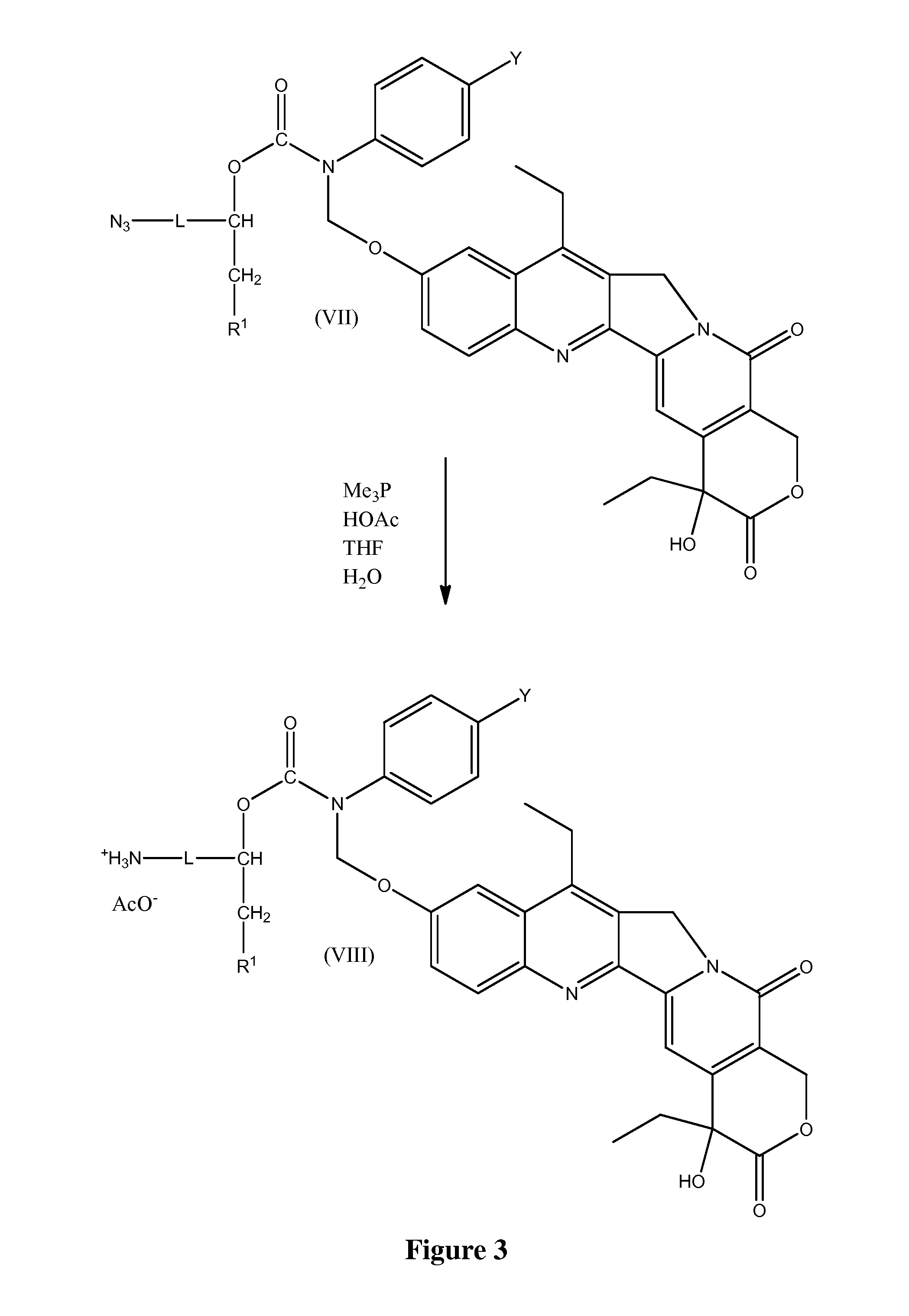
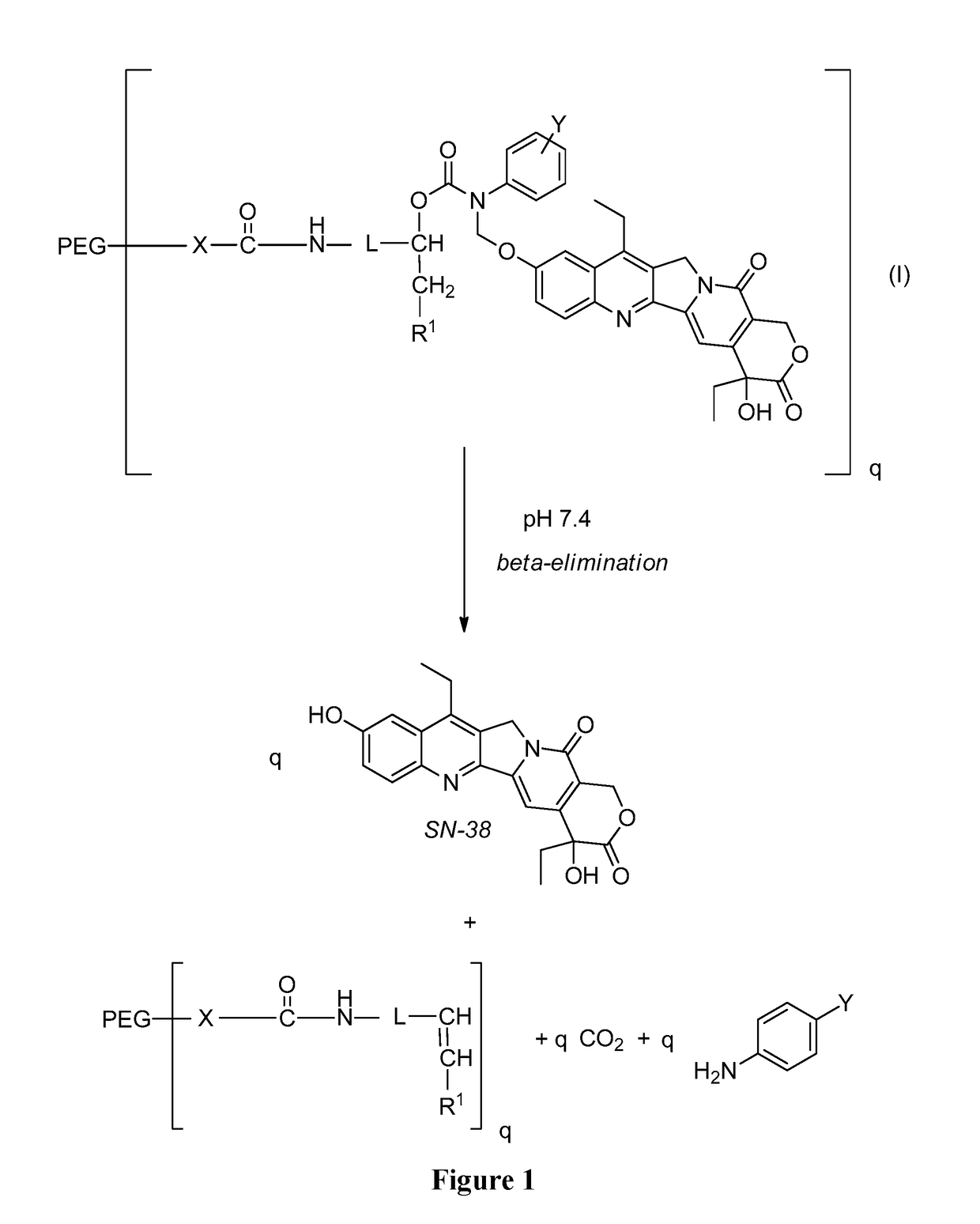
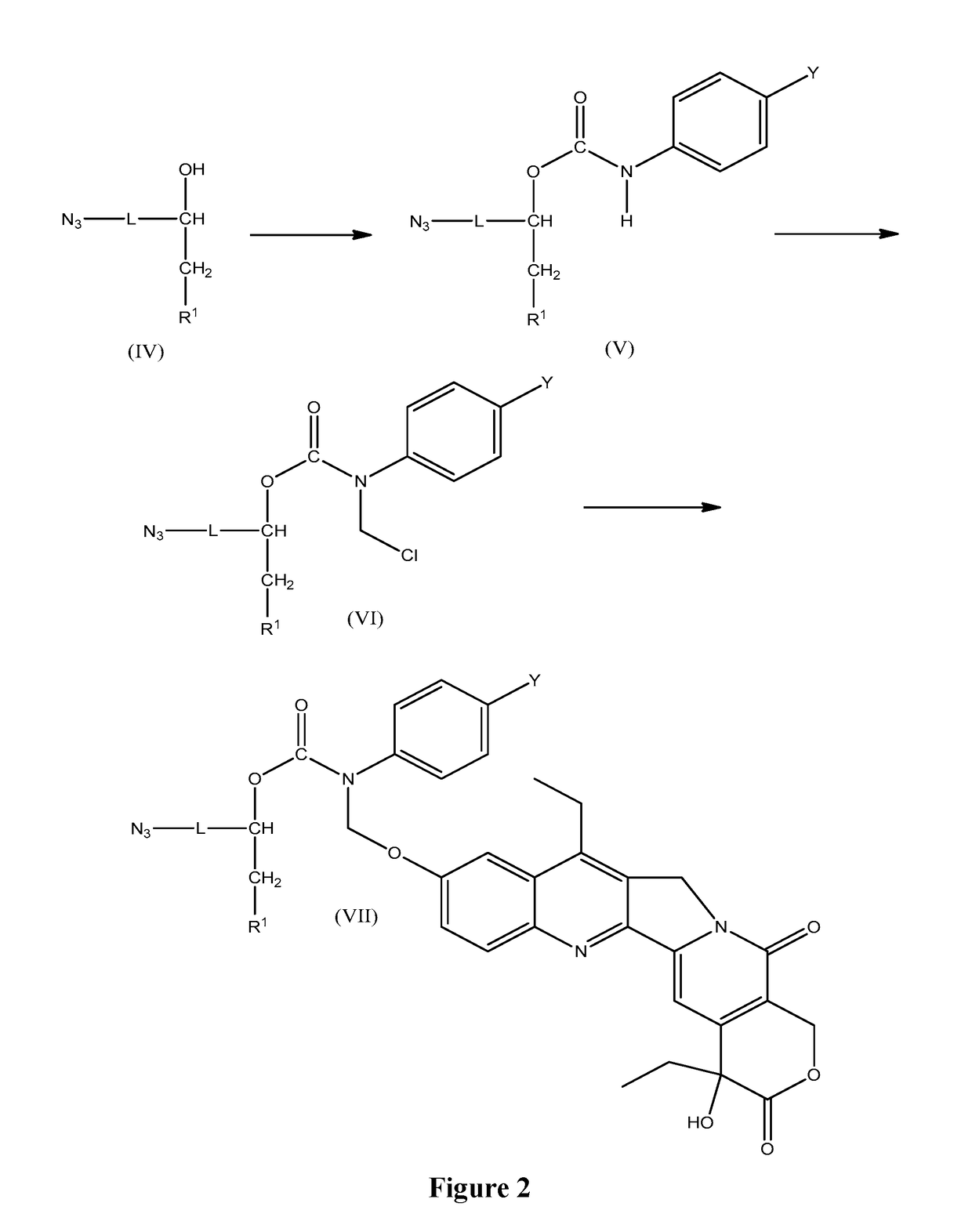
Slow-release conjugates of SN-38
ActiveUS10016411B2Quantity minimizationOrganic active ingredientsOrganic chemistryDrug release rateGlucuronate
Conjugates of SN-38 that provide optimal drug release rates and minimize the formation of the corresponding glucuronate are described. The conjugates release SN-38 from a polyethylene glycol through a β-elimination mechanism.
Owner:PROLYNX LLC
Preparation method of copolymerizable photoinitiators
InactiveUS20100305336A1Simple methodHigh purityPreparation from carboxylic acid halidesOrganic compound preparationArylMethacrylate
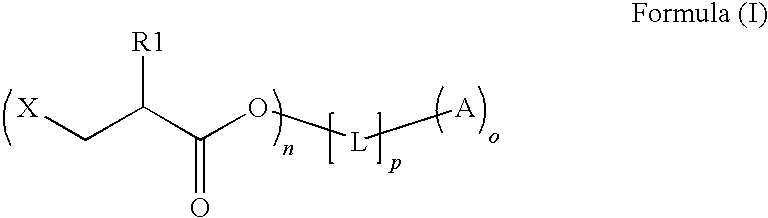
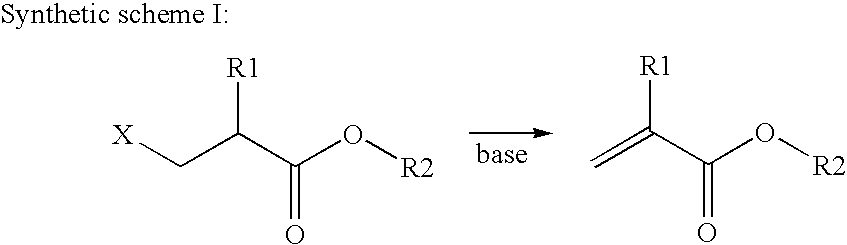
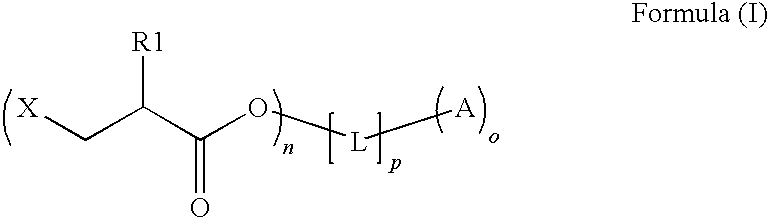
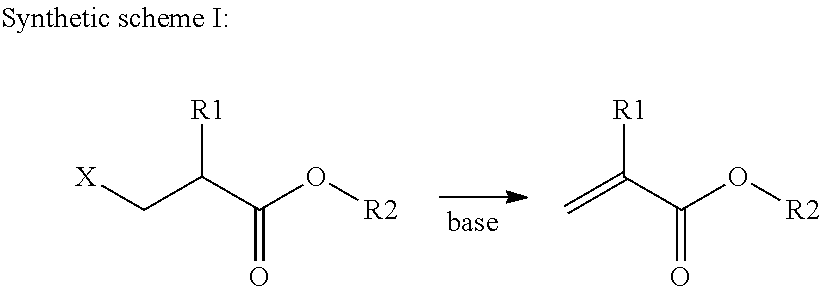
An intermediate for preparing (meth)acrylated photoinitiators according to Formula (I):wherein:R1 is selected from the group consisting of hydrogen and a methyl group;A represents a group including at least one photoinitiating moiety;L represents a n+o-valent linking group including at least one carbon atom;n and o each independently represent an integer from 1 to 4;p is equal to 0 or 1;X represents a group selected from the group consisting of Cl, Br, I, and R2SO3; andR2 represents an optionally substituted group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an alkaryl group-, an aralkyl group, an aryl group and a heteroaryl group. Also, a method for the preparation of (meth)acrylated photoinitiators by β-elimination of HX from the intermediate according to Formula (I).
Owner:AGFA NV
Maca formula capable of strengthening tolerance and explosive force and preparation method thereof
The invention relates to a maca formula capable of strengthening tolerance and explosive force and a preparation method thereof. The maca formula is prepared from, by mass, 50-70 parts of maca extract, 10-15 parts of rhodiola extract, 10-15 parts of eucommia ulmoides leaves, 5-10 parts of calcium pyruvate and 5-10 parts of creatine. According to the maca formula, on one hand, the effect of increasing the explosive force is achieved by increasing energy metabolism, strengthening protein synthesis and promoting muscle growth; on the other hand, the effects of strengthening the tolerance is achieved by regulating substance metabolism, improving antioxidant capacity, strengthening anoxia tolerance capability, accelerating elimination of metabolite and the like; the maca formula is suitable for being processed into dosage forms such as powder, tablets and capsules in pharmacy.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Injection for treating AI (avain in-fluenza)
InactiveCN105193971ANot easy to relapseLower resistancePharmaceutical delivery mechanismAntiviralsTreatment effectSide effect
The invention belongs to the technical field of medicine and medical engineering, and particularly relates to injection for treating AI (avain in-fluenza). The injection comprises, in percentage by mass, 5%-10% of a BS (bursin active peptide solution), 30%-45% of traditional Chinese medicine extracts with radix pseudostellariae as a main material and 50%-60% of water. BS, the traditional Chinese medicine extracts with the radix pseudostellariae as the main material and the water are proportioned in percentage by mass and mixed and filtered for sterilization with double membranes, namely a filter membrane with the specification of 0.45 mu m and a filter membrane with the specification of 0.25 mu m. The efficacy of the injection is remarkably improved, the injection has efficient symptom and root cause treatment effects and has the effects of healthy energy supporting, evil elimination, plague clearing and detoxification, AI is not prone to relapse after being cured, drug resistance is unlikely to be caused, the AI cure rate is high, and the injection can be stored at normal temperature, is environment-friendly, efficient, high-quality and free of residues and toxic and side effects and is a novel specific medicine for treating AI.
Owner:FUJIAN BRADY PHARMA CO LTD
Method for evaluating anti-aging efficacy of cosmetics
ActiveCN112111554AEasy to operateEasy to observeMicrobiological testing/measurementClimate change adaptationBiotechnologyTelomerase
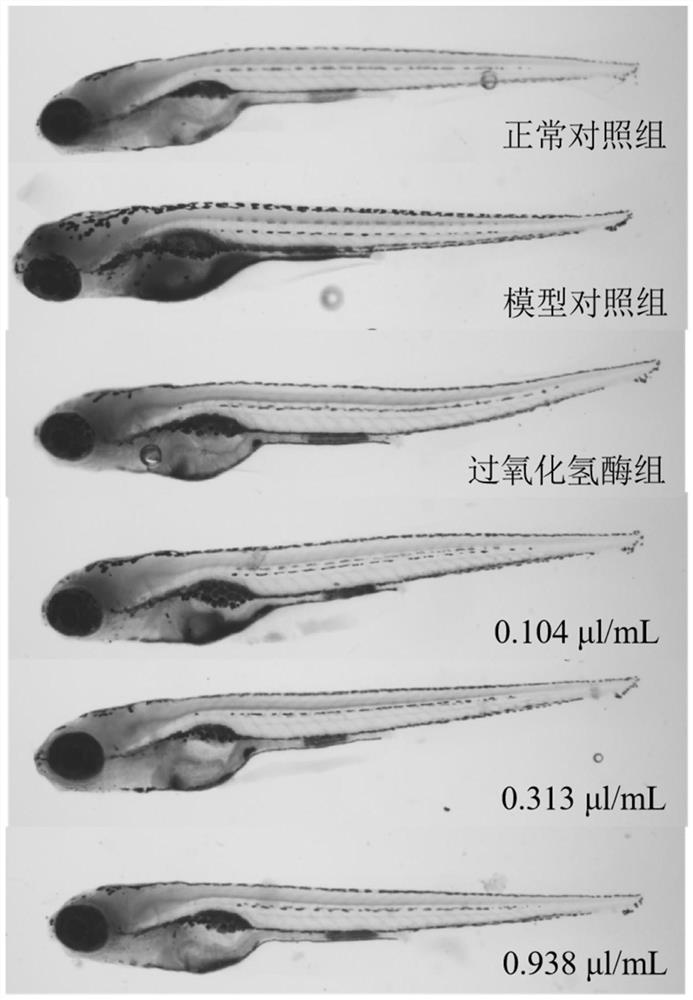
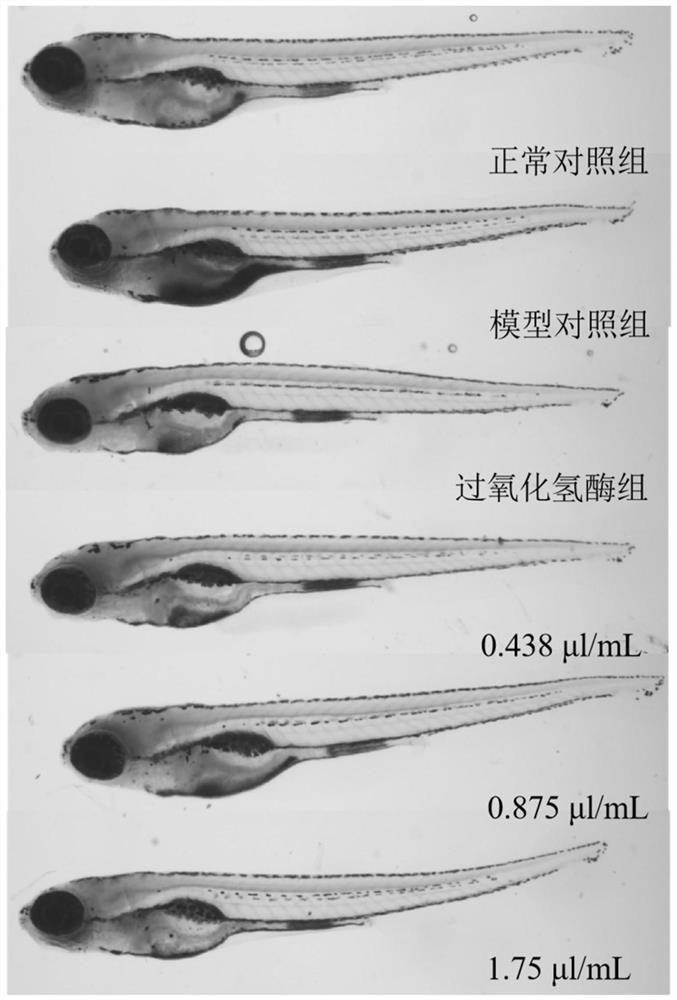
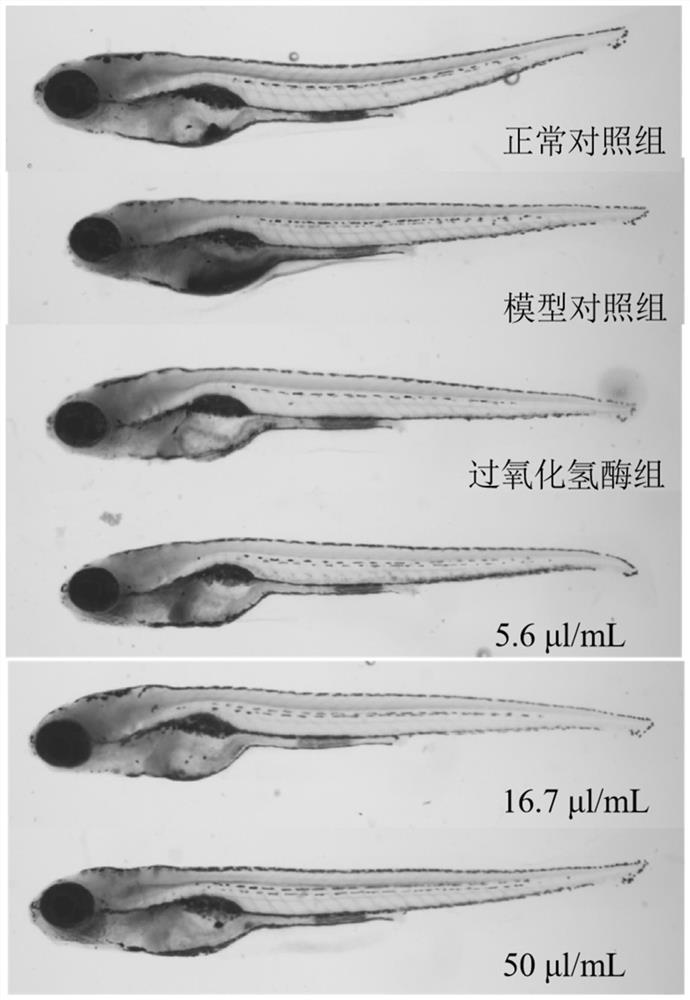
The invention belongs to the technical field of detection. The invention discloses a method for evaluating the anti-aging efficacy of cosmetics. According to the method, indexes such as a beta-galactosidase activity inhibition effect, a telomerase activity enhancement effect, an oxygen free radical elimination effect, a lipid browning content, a survival rate and the like are evaluated. Accordingto the invention, experimental operation and observation are facilitated, and since chemical substances are directly absorbed into a circulating system, and painful and time-consuming injection or invasive surgery is not needed, so that pressure or tissue damage related to a rodent aging model is avoided. Zebra fish is equivalent to mice for 8-10 days in one day, the experiment period is shorter,and the cost is low; The zebra fish, as a complete living body model, can be used for high-throughput screening of drugs, especially screening of chemical drugs. Embryos and juvenile fishes are transparent, various tissue and organ changes can be directly observed under a microscope, and experiment results are visual and easy to understand.
Owner:南京新环检测科技有限公司
Slow-release conjugates of sn-38
ActiveUS20160243106A1Quantity minimizationOrganic active ingredientsOrganic chemistryDrug release ratePolyethylene glycol
Conjugates of SN-38 that provide optimal drug release rates and minimize the formation of the corresponding glucuronate are described. The conjugates release SN-38 from a polyethylene glycol through a β-elimination mechanism.
Owner:PROLYNX LLC
Slow-release conjugates of sn-38
ActiveUS20180289695A1Quantity minimizationOrganic active ingredientsOrganic chemistryDrug release rateGlucuronate
Conjugates of SN-38 that provide optimal drug release rates and minimize the formation of the corresponding glucuronate are described. The conjugates release SN-38 from a polyethylene glycol through a β-elimination mechanism.
Owner:PROLYNX LLC
Application of rotenone in activating activities of ietalurus punetaus nuclear receptor PXR and cytochrome enzyme CYP3A
InactiveCN110028574AAccelerated Residue EliminationResidue reductionOxidoreductasesNuclear receptorEnzyme GeneCvd risk
The present invention studies expression levels of a nuclear receptor PXR and a cytochrome enzyme CYP3A in various tissues of ietalurus punetaus and screens target tissues of gills and foreguts respectively for detecting activities of the PXR and CYP3A, a monomer extracted and purified from natural products is used to conduct mouth-filling on the ietalurus punetaus, the gills and foreguts are collected, the expression levels of the nuclear receptor PXR and cytochrome P450 3A enzyme gene are detected, the natural product monomer of rotenone capable of activating a nuclear receptor PXR pathway and regulating the CYP3A enzyme is screened out, an experiment proves that the rotenone can shorten the residual time of enrofloxacin in muscle with skin (edible tissues) of the ietalurus punetaus from12 d to 7 d, and also significantly reduces the residual amount at the same time point, thus the rotenone can be used to accelerate elimination of quinolone chemical residues in the ietalurus punetaus body, and is of great significance for ensuring quality and safety of aquatic products and reducing risks of intake of the aquatic products containing chemical drug residues by the human body.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Anti-alcoholic dew and preparation method thereof
PendingCN110916036AAvoid absorptionHigh activityPeptide/protein ingredientsDigestive systemBiotechnologyEthanol dehydrogenase
The invention discloses an anti-alcoholic dew and a preparation method thereof. The anti-alcoholic dew is characterized by being prepared from the following raw materials in parts by weight: 30-40 parts of corn peptide, 10-20 parts of vinegar, 20-30 parts of honey, 10-20 parts of fructus lycii, 20-30 parts of mulberries, 10-30 parts of rhizoma polygonati, 30-40 parts of radix astragali, 5-10 partsof radix puerariae and a proper amount of water. Compared with the prior art, the anti-alcoholic dew has homology of medicine and food, takes the corn peptide as a main component, can inhibit the absorption of alcohol by the stomach, increases the activity of ethanol dehydrogenase and acetaldehyde dehydrogenase in vivo, and promotes the metabolism and discharge of alcohol in vivo; alcohol compounds can be quickly decomposed by drinking the anti-alcoholic dew before or after meals, so that the symptoms of patients with severe alcoholic liver can be gradually improved, and the anti-alcoholic dew has good functions of dispelling the effects of alcohol and protecting the liver. Besides, all the components play a synergistic role, so that a good hangover alleviating and liver protecting effectis achieved, and the anti-alcoholic dew is non-toxic and harmless, and can improve the ADH activity of liver tissues, promote ethanol metabolism, accelerate the elimination rate of ethanol metabolites, reduce damage to tissues and cells, play a role in alleviating hangover and promoting awakening, and protect the liver.
Owner:苏州东诚堂生物科技有限公司
Ondansetron composition for injection
InactiveCN103330709AGood treatment effectImprove immunityOrganic active ingredientsPowder deliveryTreatment effectRegimen
The invention provides an andansetron composition for injection, relating to the technical field of drugs and drug preparation. The main drugs of the andansetron composition include andansetron and melatonin, wherein the melatonin comprises a quick-release part and a cyclodextrin encapsulated slow-release part. The andansetron composition for injection, provided by the invention, has the advantages that the treatment effect of the andansetron is improved, the instability of the MT (melatonin), caused by the oral absorption of the MT and the fast distribution and elimination of the MT are avoided, the first-pass effect of the MT is reduced, the dosage of the andansetron is reduced, the quick-release and slow-release combined drug administration design is in line with the physiological secretion characteristic of the MT, the problem of short half-life period of the MT is solved, the biological availability of the product is improved, the melatonin has a synergistic effect on the andansetron for CINV (Chemotherapy Induced Nausea And Vomiting) treatment, after the melatonin and the andansetron are combined, the CINV can be treated, the treatment effect of the andansetron on the CINV are improved, the treatment course is shortened, the usage and the side effect of the andansetron are reduced, and the immunity of human bodies can be improved; and a certain concentration of melatonin maintained in the blood of the human body can be used for effectively reducing the stress reaction of organisms and is facilitated to the treatment of the CINV.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Preparation method of fishskin collagen with certain-range molecular weight
InactiveCN112522356AGood homologyGood compatibilityConnective tissue peptidesPeptide preparation methodsFreeze-dryingEngineering
The invention relates to the technical field of collagen production, in particular to a preparation method of fishskin collagen with certain-range molecular weight. The preparation method specificallycomprises the following steps of: preprocessing fishskin, carrying out enzymolysis membrane cycling separation, carrying out decoloration and fishy smell elimination processing, and carrying out freeze drying. Through specific combined technical steps and parameter conditions, fishskin preprocessing, enzymolysis membrane cycling separation, decoloration and fishy smell elimination processing andfreeze drying are carried out to obtain collagen powder of which the molecular weight is 2-5KD, the triple helix structure of the prepared fishskin collagen is not damaged, and original biological activity is still kept; main ingredients are the 2-5KD collagen powder which has good water solubility and can be diluted by water at any ratio; the fishskin collagen is from the collagen of vertebrates,has good homology and compatibility with the muscle body and the skin of people and has similar molecular weight and structure with the people; and after the fishskin collagen is smeared to the skin,the fishskin collagen can be quickly dissolved into the epidermis, and collagen polypeptide of which the molecular weight is smaller than 5kDa has various physiological activities, including blood pressure reduction and oxidization resistance.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Design, synthesis and application of near-infrared fluorescence imaging agent for targeted tumor VEGFR-3 molecule
ActiveCN108743975AStrong specificityHigh purityIn-vivo testing preparationsNode metastasisPolyethylene glycol
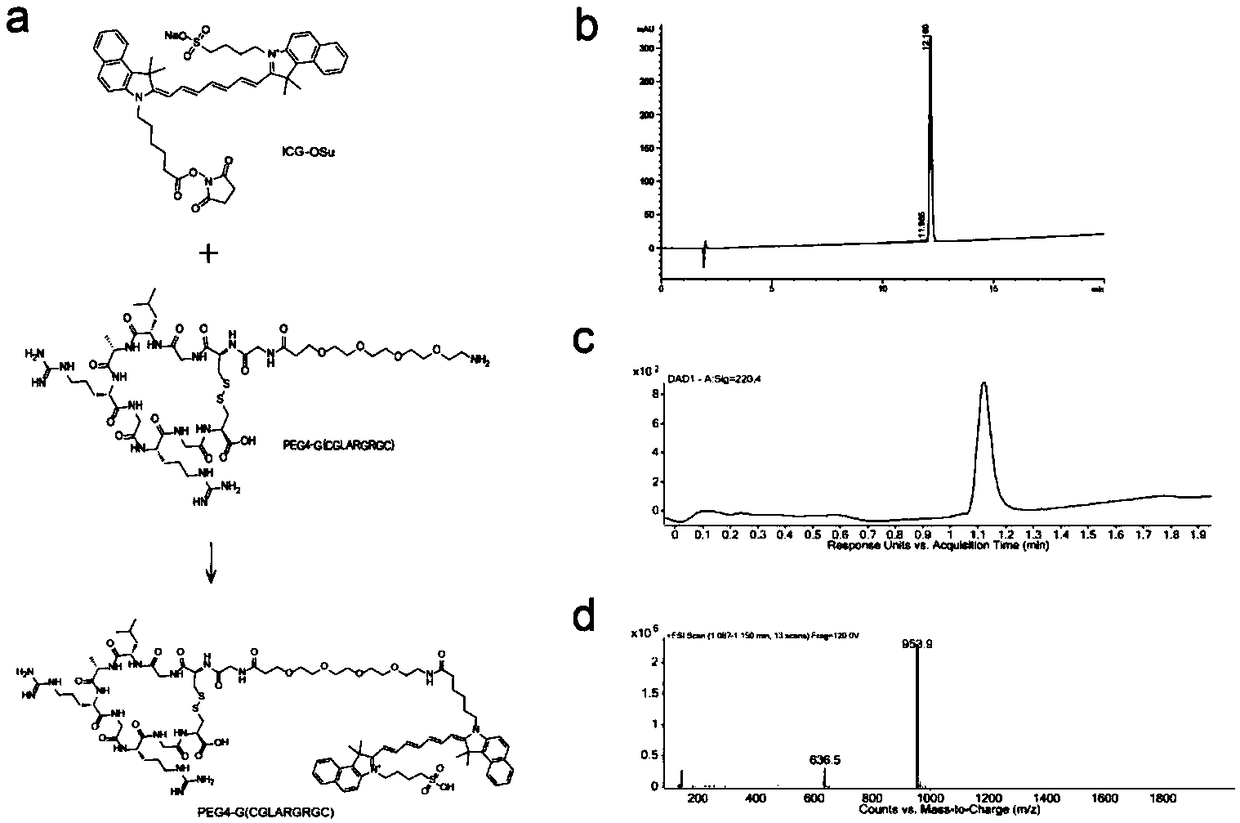
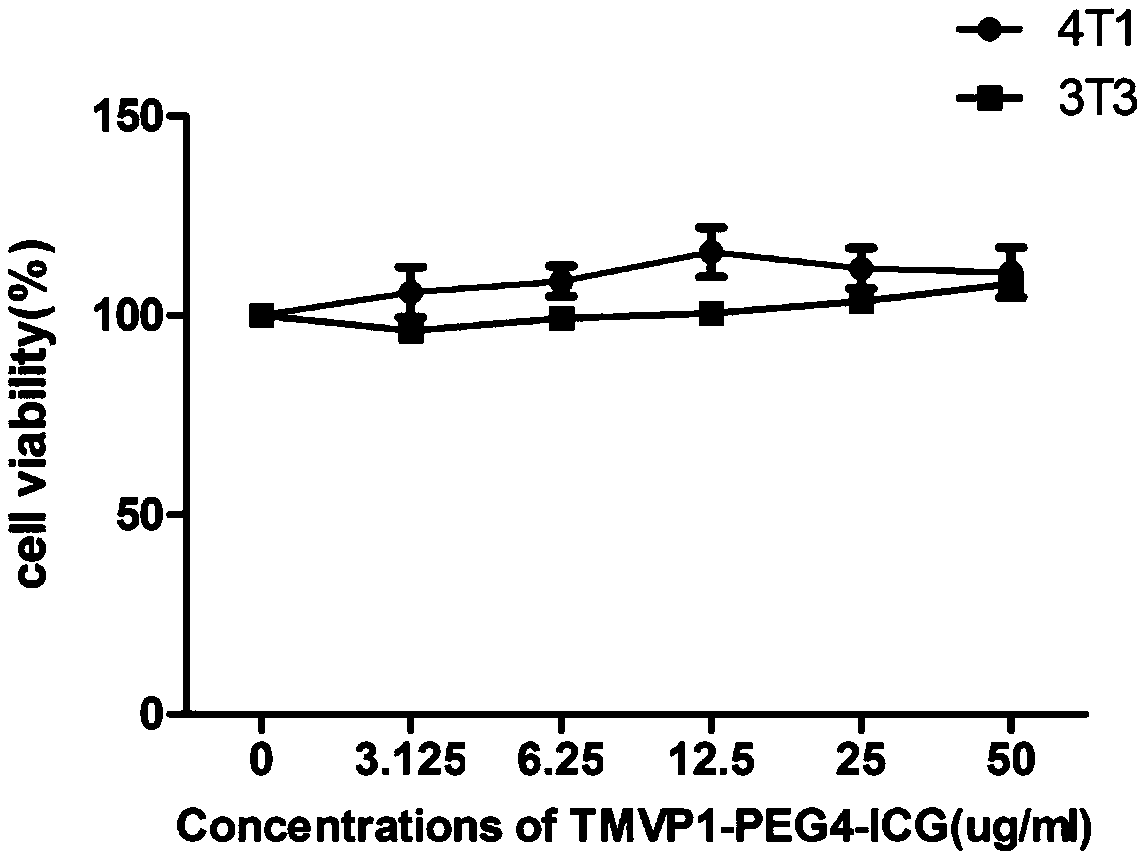
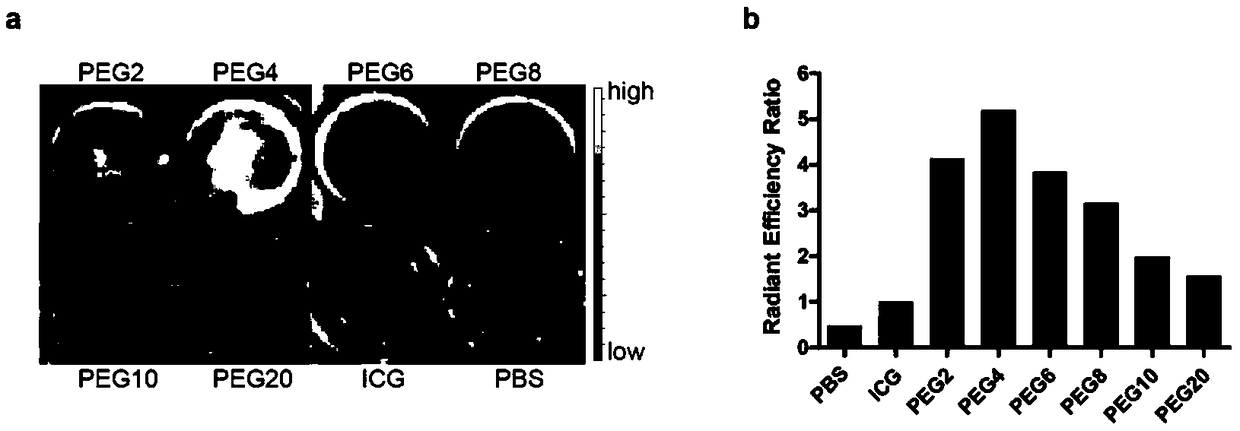
The invention relates to design, synthesis and application of a near-infrared fluorescence imaging agent for a targeted tumor VEGFR-3 molecules, of which the structural formula is ICG-OSu-(PEG)n-G(CGLARGRGC), wherein ICG is near-infrared fluorescence imaging agent indocyanine green, ICG-OSu is sulfonic acid group indocyanine green activated ester, the core LARGR of cyclic polypeptide G(CGLARGRGC)is polypeptide TMVP1 of a targeted VEGFR-3 molecule, which has carboxyl reactivity, and both are bridged through polyethylene glycol (PEG), wherein n is an integer from 2-20. According to the near-infrared molecule imaging agent provided by the invention, the specificity of ICG development cervical cancer, breast cancer focus and lymphatic metastasis focus thereof is greatly improved, and good instructions are provided for later clinical diagnosis of cervical cancer and breast cancer as well as elimination of tumor metastasis lymph node by applying a fluorescent endoscope.
Owner:WUHAN KDWS BIOLOGICAL TECH CO LTD
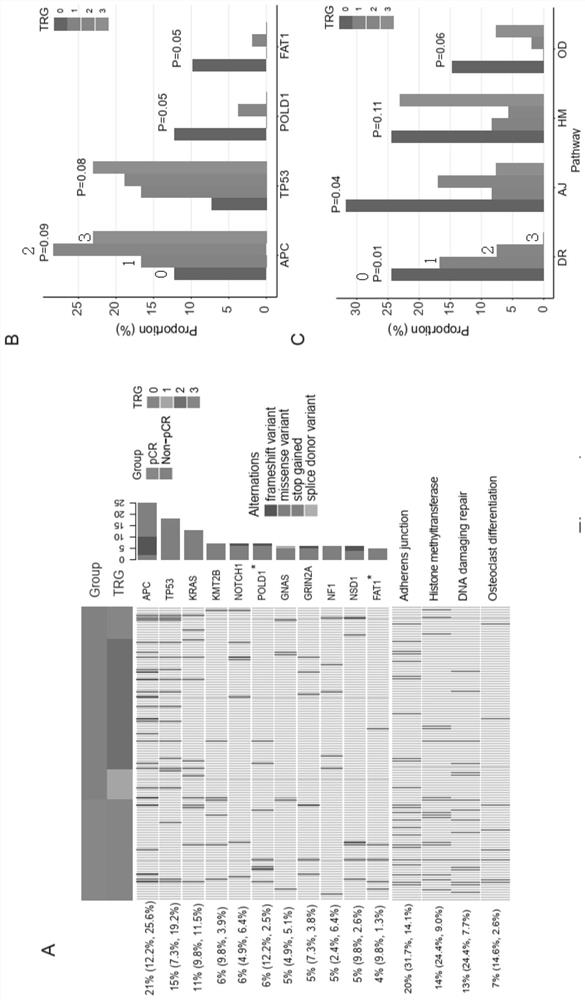
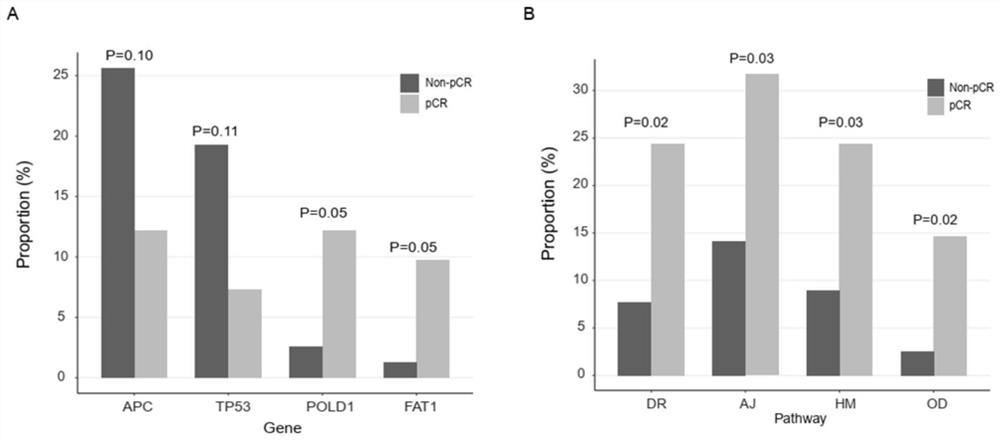
Molecular marker related to rectal cancer and application thereof
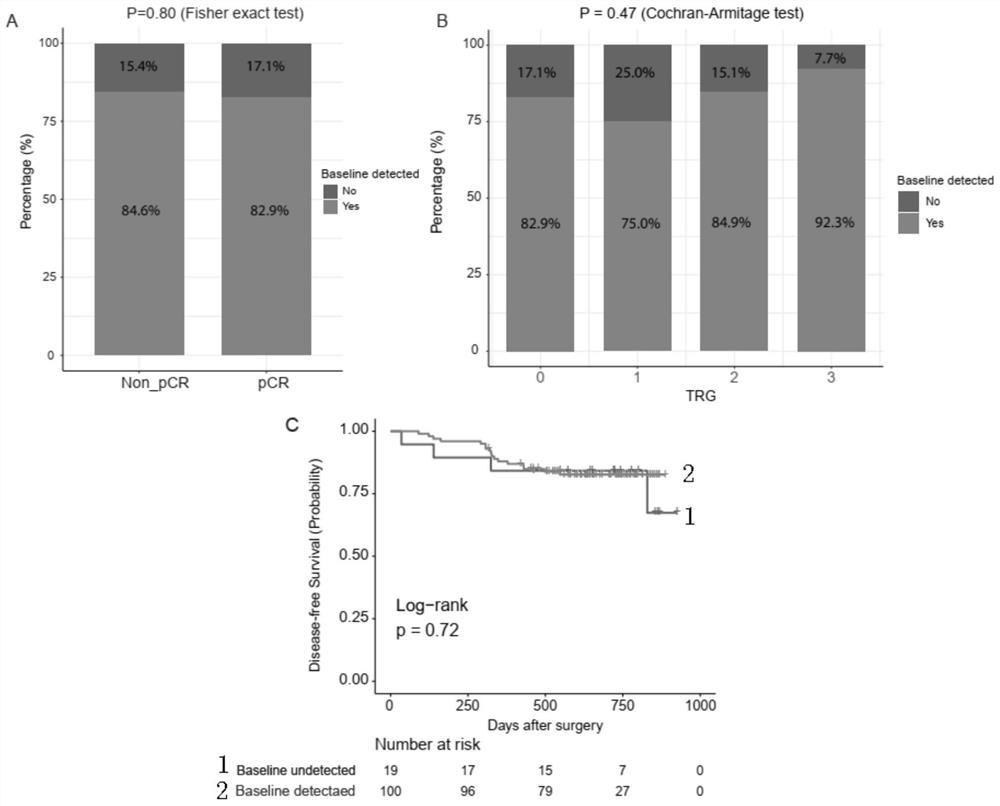
The invention relates to a molecular marker related to the rectal cancer and application thereof, and belongs to the technical field of the medical molecular biology. According to the invention, two key designs are carried out; one design is that ctDNA detection is carried out by applying a whole-exon big panel of 425 cancer-related genes instead of a small panel of a small number of hotspot genes, so that not only is relevance between one single gene mutation and an nCRT treatment effect obtained, but also relevance between genetic mutations of a plurality of signal channels and the nCRT treatment effect is obtained; and the other design is that a plurality of monitoring time points (four time points) are set before an operation, the big panel of the 425 genes is combined, and meanwhile,elimination of the mutations in the ctDNA and dynamic changes of acquired mutations are monitored. Value of ctDNA dynamic monitoring in predication of the nCRT treatment effect is shown, and a new opinion is proposed for a patient adopting a W&W strategy. The invention proves effects of ctDNA detection in the early period of predicting the prognosis of an LARC patient.
Owner:GENESEEQ TECH INC +1
Mini-gastrin analogue, in particular for use in CCK2 receptor positive tumour diagnosis and/or treatment
ActiveUS10953114B2Promote accumulationAccumulation is very lowPeptide/protein ingredientsIsotope introduction to peptides/proteinsDiseaseReceptor
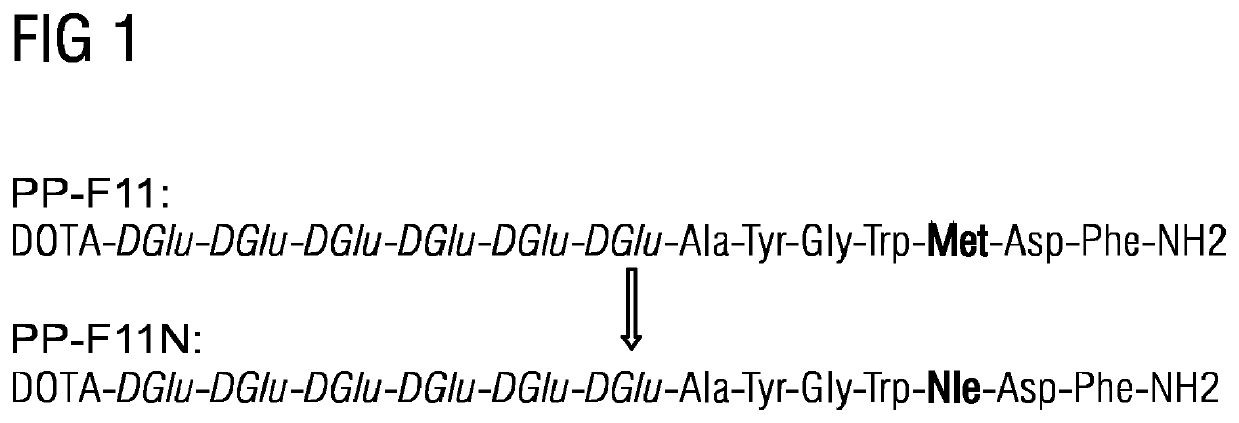
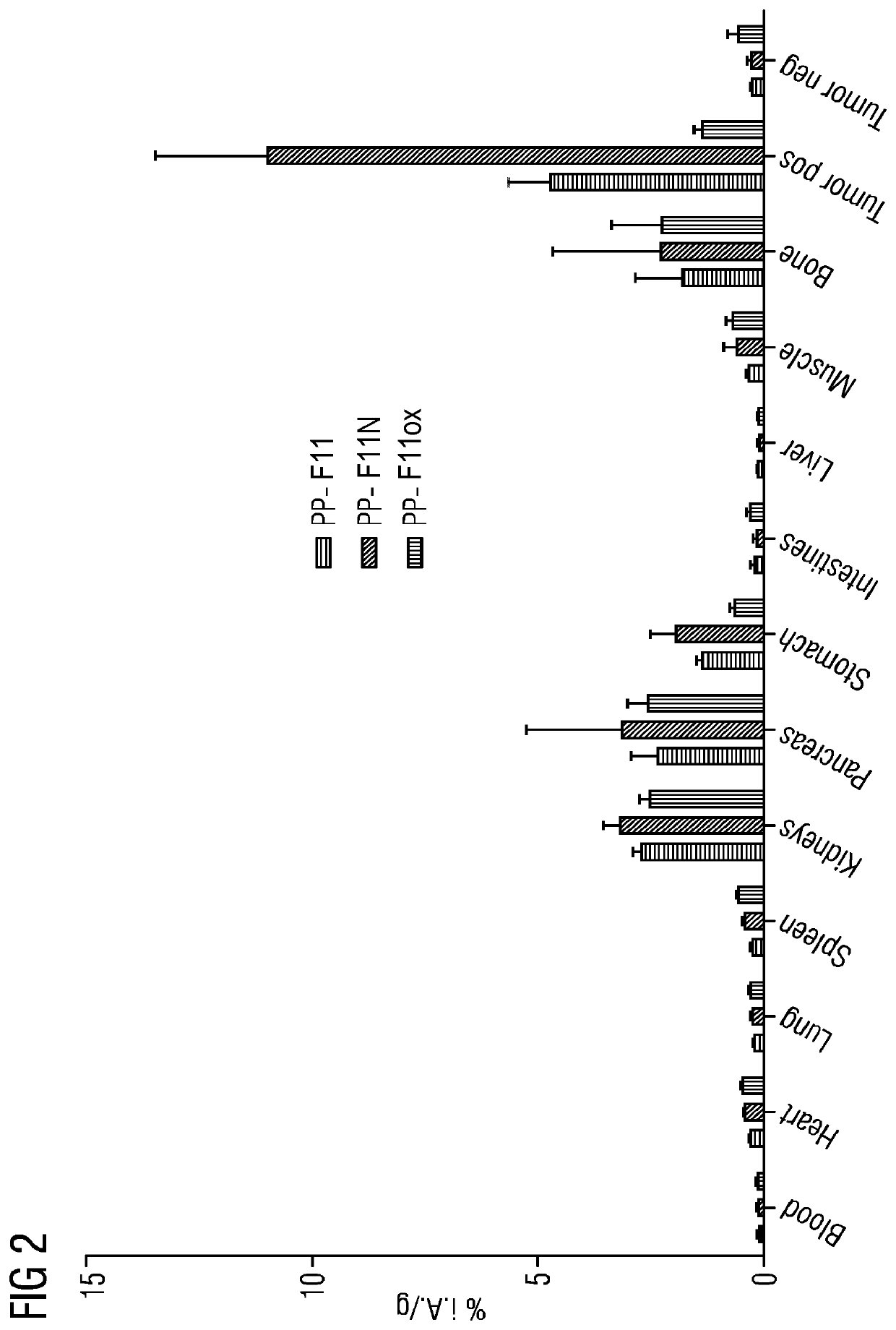
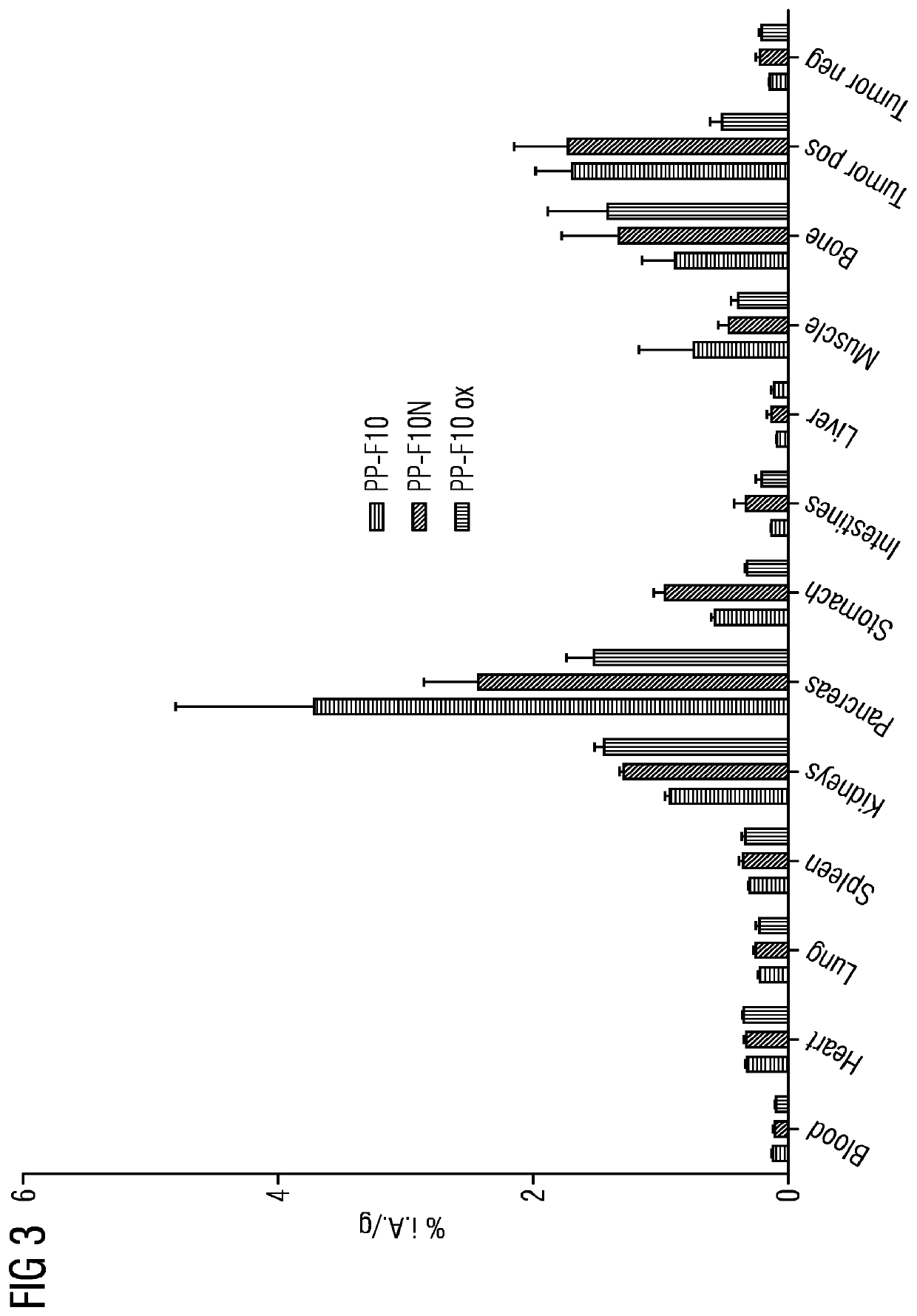
A gastrin analogue shows high uptake in CCK-2 receptor positive tumors and simultaneously a very low accumulation in the kidneys. This is achieved by a mini-gastrin analogue PP-F11 having the formula: PP-F11-X-DGlu-DGlu-DGlu-DGlu-DGlu-DGlu-Ala-Tyr-Gly-Trp-Y-Asp-Phe-NH2, wherein Y is an amino acid replacing methionine and X is a chemical group attached to the peptide for diagnostic and / or therapeutic intervention at CCK-2 receptor relevant diseases. Very suitable compounds with respect to a high tumor to kidney ratio are mini-gastrin analogues with six D-glutamic acids or six glutamines. These compounds still possess a methionine which can be oxidized easily which is a disadvantage for clinical application under GMP due to the forms which may occur. The elimination of the methionine leads to a lower affinity to oxidation which in general favors the tumor-kidney-ratio. Ideally, the methionine is replaced by norleucine. This PP-F11N mini gastrin exhibits currently the best tumor-kidney-ratio and is the most promising candidate.
Owner:PAUL SCHERRER INSTITUT
Method for preparing monoclonal antibody through rapid immunization
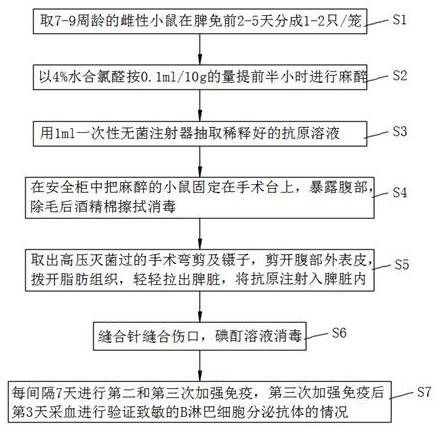
InactiveCN112175070AIncrease activity spaceProduce efficientlySerum immunoglobulinsAntibody secretionSuturing needle
The invention discloses a method for preparing a monoclonal antibody through rapid immunization, which belongs to the technical field of diagnosis, and comprises the following steps: S1, taking animals which are not less than 7 weeks old from cultured animals for cage separation, S2, anesthetizing the animals by using 4% chloral hydrate according to the amount of 0.1 ml / 10g, S3, extracting a diluted antigen solution by using a 1ml disposable sterile syringe, and S4, fixing the narcotized animal on an operating table in a clean environment, exposing the abdomen, and removing hairs from the abdomen; S5, cutting off the outer epidermis of the abdomen by using a pair of surgical curved scissors, pushing aside adipose tissues, slightly pulling out the spleen by using tweezers, and injecting anantigen into the spleen; S6, suturing the wound by using a suture needle; S7, carrying out second and third immunization every 3-14 days. Blood is collected 25 days after the third immunization to verify the antibody secretion condition of the sensitized B lymphocytes; the invention has the advantages of reasonable and practical design, low antigen usage amount, reduced cost, improved antibody titer, and elimination of false positive to some extent. The immune operation is simple, and the situation of unsuccessful immunization caused by immunization to the subcutaneous part does not need to beworried.
Owner:巴德生物科技有限公司
Preparation method of copolymerizable photoinitiators
InactiveUS20140024840A1Simple methodHigh purityPreparation from carboxylic acid halidesOrganic compound preparationArylHydrogen
An intermediate for preparing (meth)acrylated photoinitiators according to Formula (I):wherein:R1 is selected from the group consisting of hydrogen and a methyl group;A represents a group including at least one photoinitiating moiety;L represents a n+o-valent linking group including at least one carbon atom;n and o each independently represent an integer from 1 to 4;p is equal to 0 or 1;X represents a group selected from the group consisting of Cl, Br, I, and R2SO3; andR2 represents an optionally substituted group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an alkaryl group, an aralkyl group, an aryl group and a heteroaryl group. Also, a method for the preparation of (meth)acrylated photoinitiators by β-elimination of HX from the intermediate according to Formula (I).
Owner:AGFA NV
Synthesis of quercetin platinum
InactiveCN101353339AOrganic active ingredientsOrganic chemistryAnticarcinogenic EffectParanasal Sinus Carcinoma
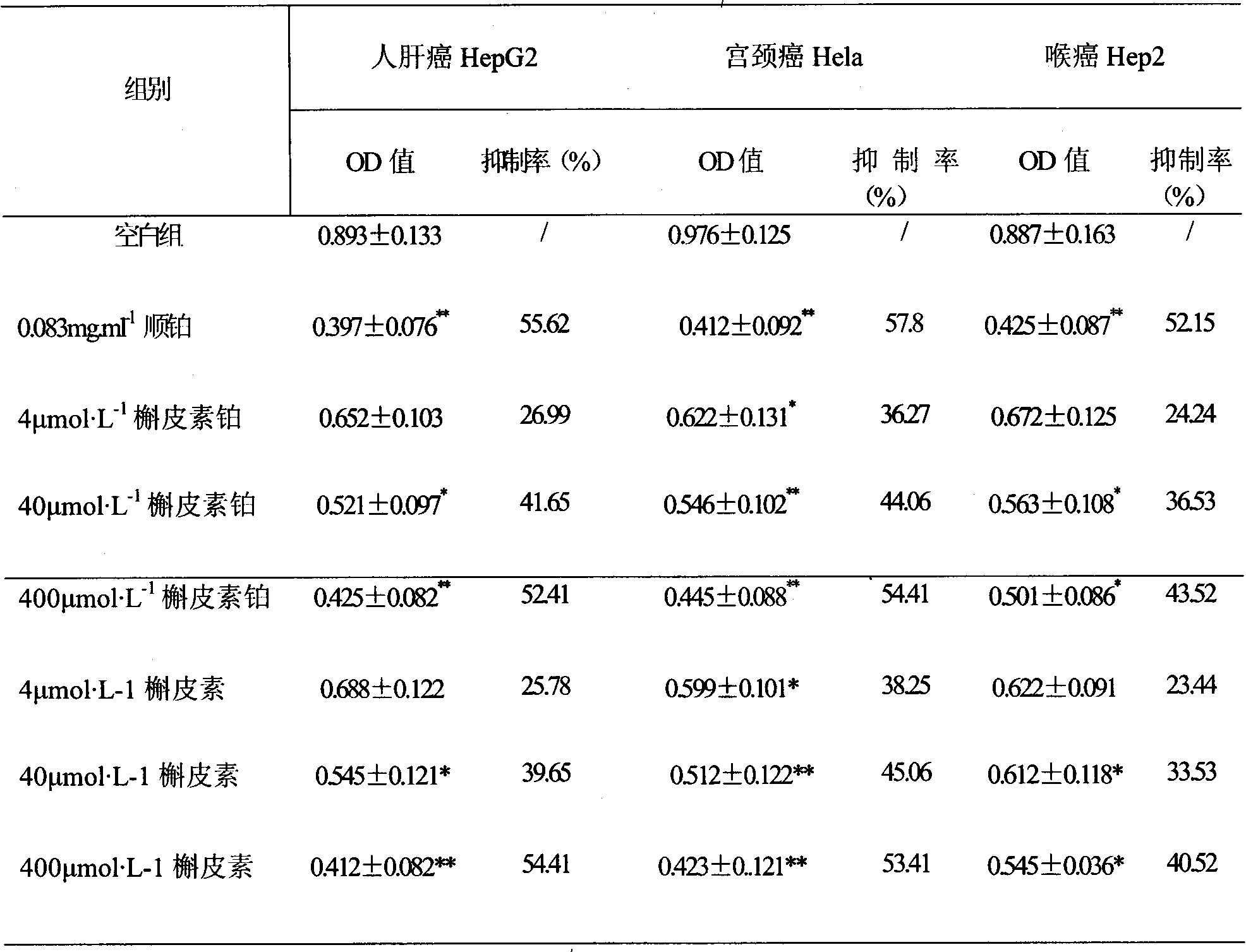
The invention relates to quercetin which has multiple aspects of biological activities, and has effects of phlegm elimination, asthma relief and antiatheroscloresis, etc., and has activities of fighting against the hyperplasia of various tumor cells; platinum complexes have obvious anticancer effect and the combination use of the quercetin and the cisplatin results in synergistic effects. In the invention, the quercetin reacts with a platinum compound under a certain condition for obtaining a quercetin platinum complex by being subjected to reversion, distill, chromatography and separation. The results from the MIT method experiment show that the quercetin platinum significantly inhibits the cell proliferation of tumors such as hepatoma HepG2, cervical carcinoma Hela and laryngocarcinoma Hep2, etc.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com