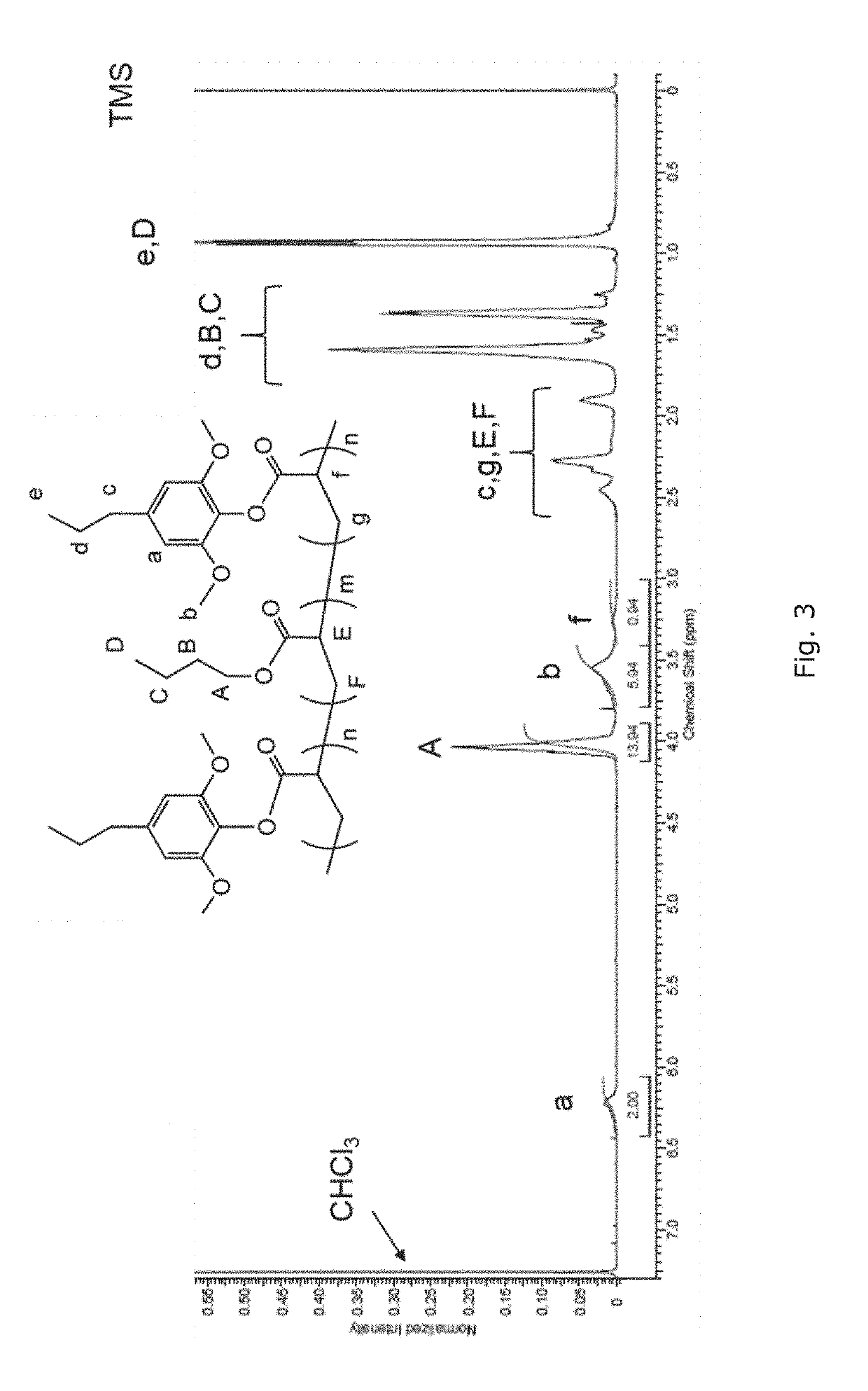
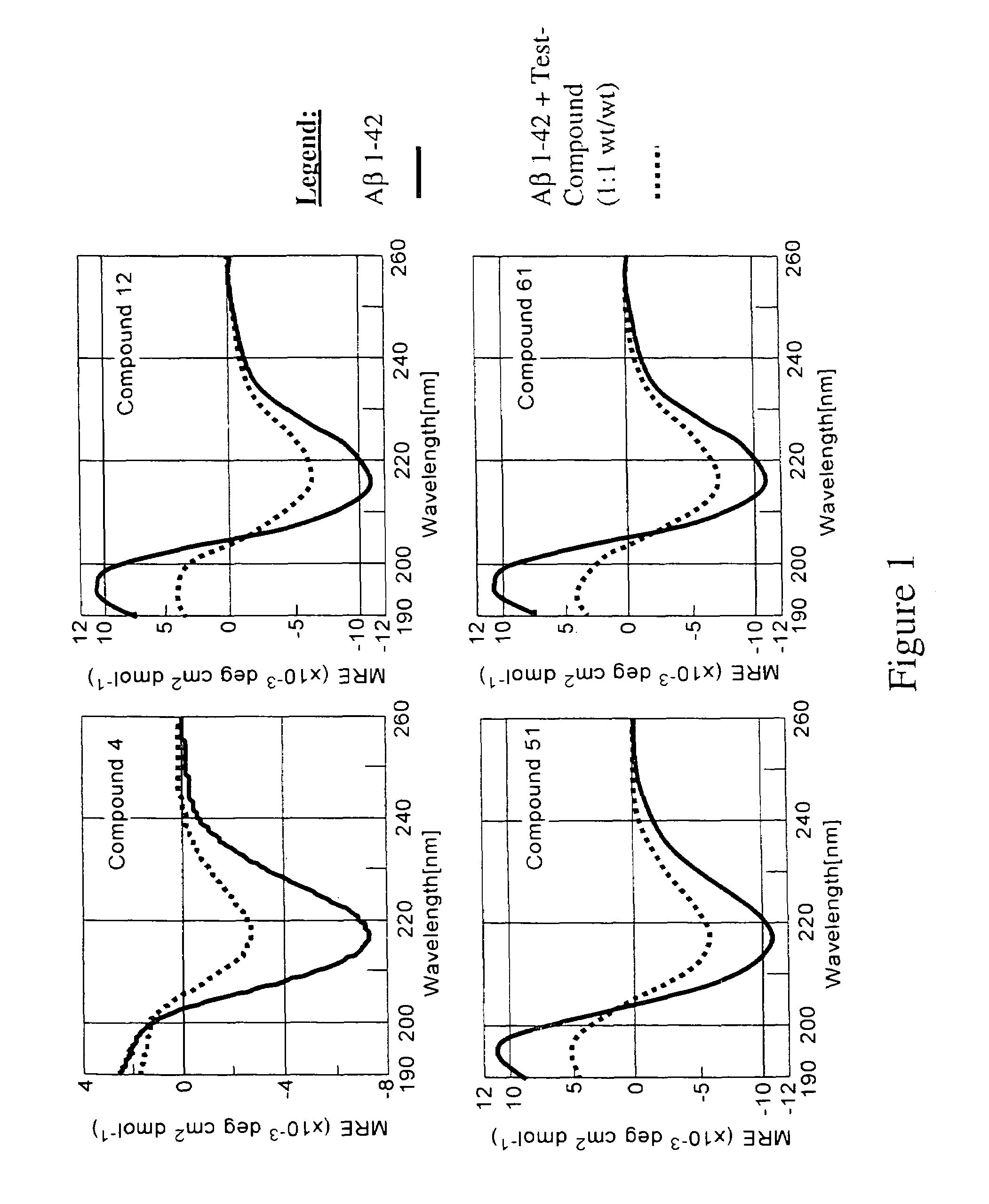
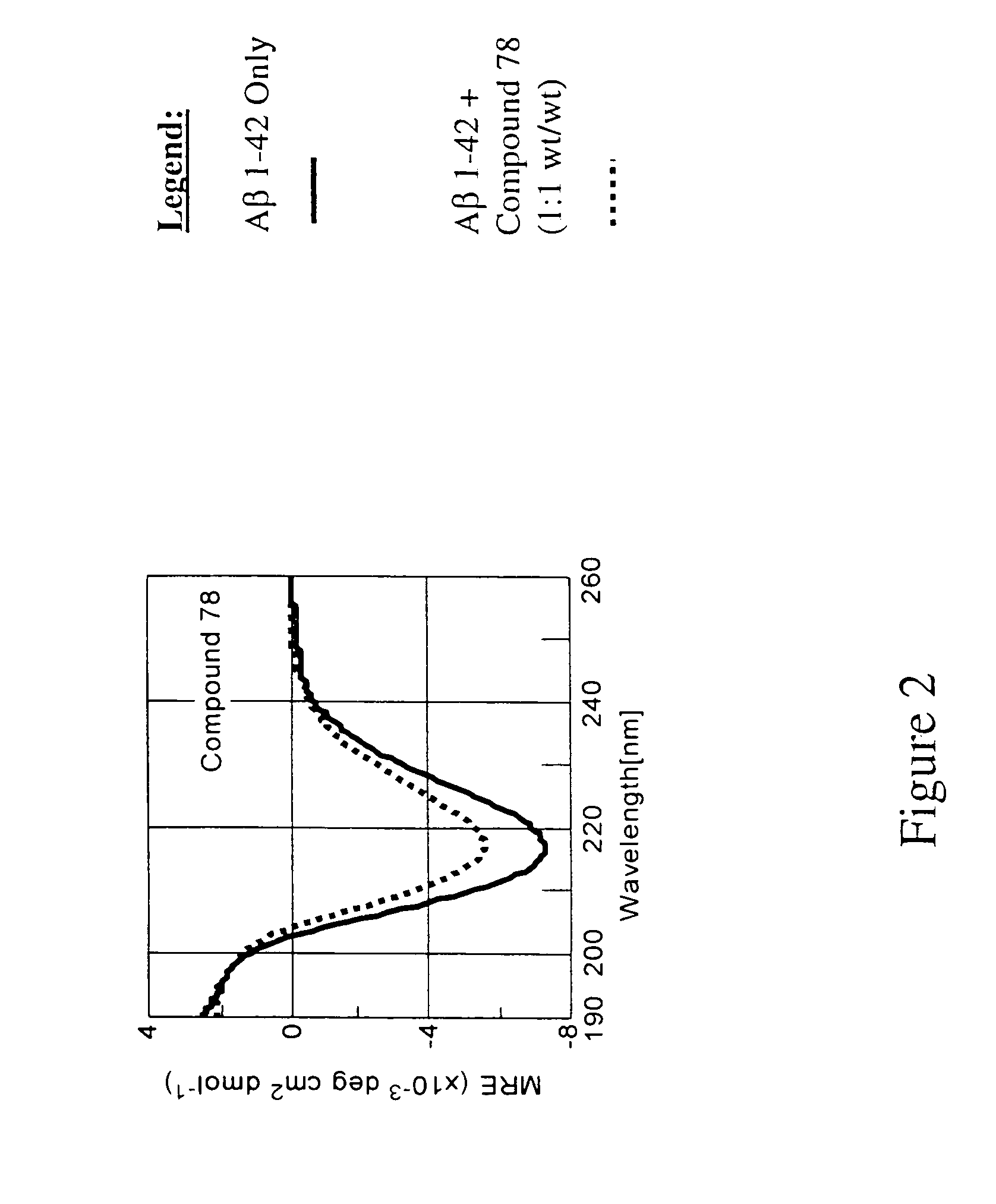
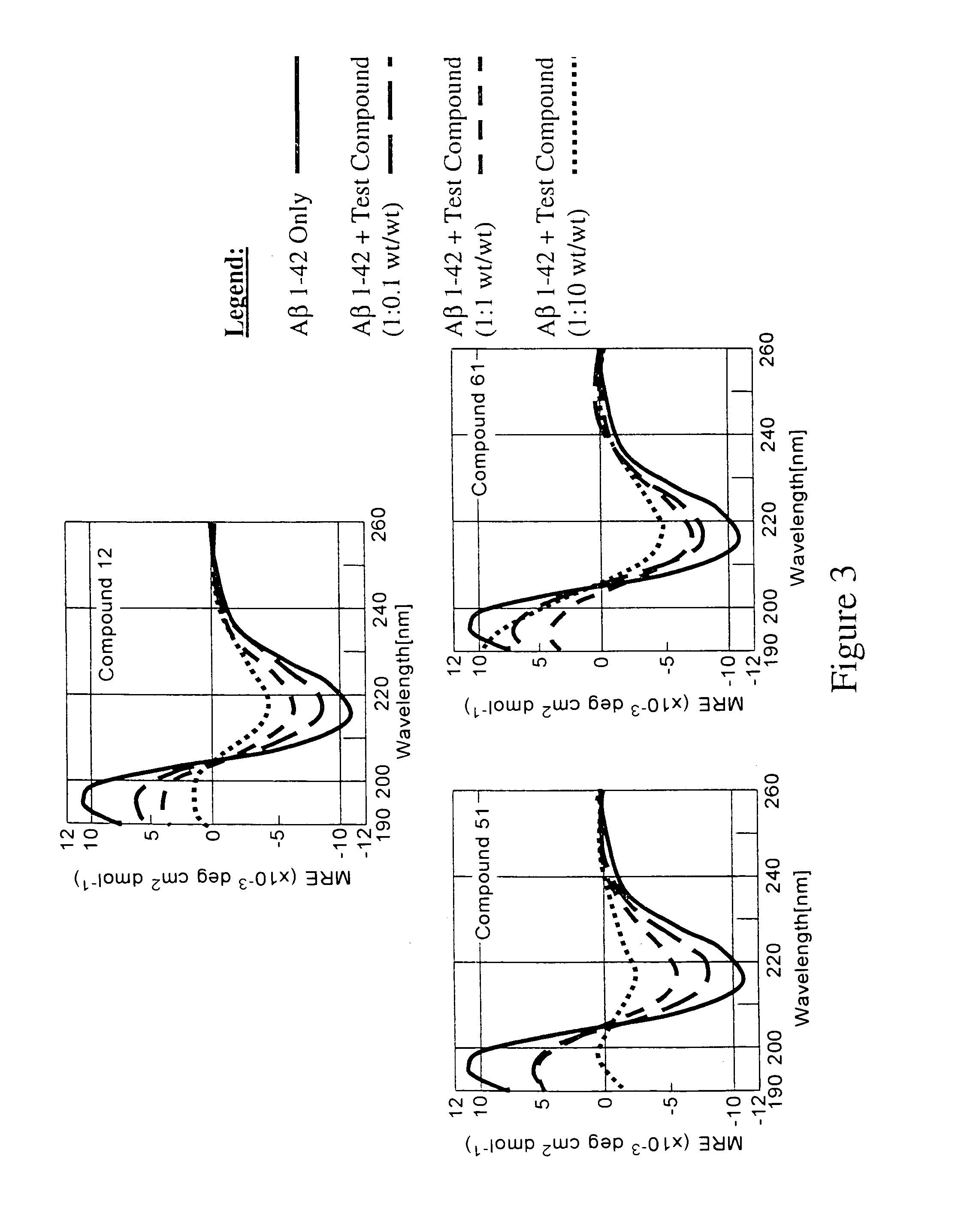
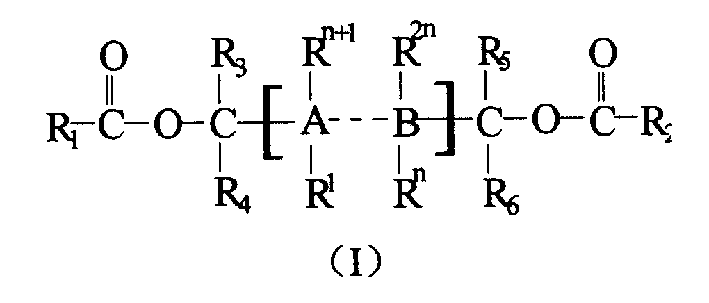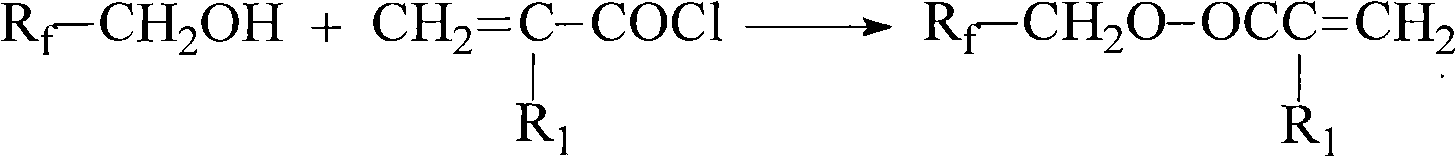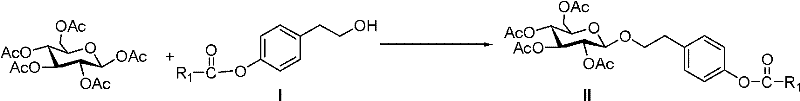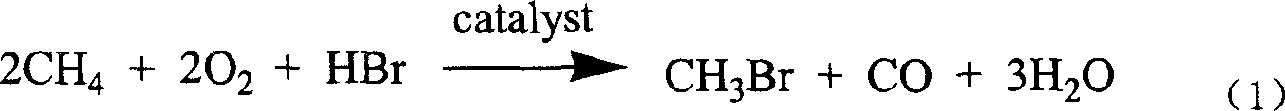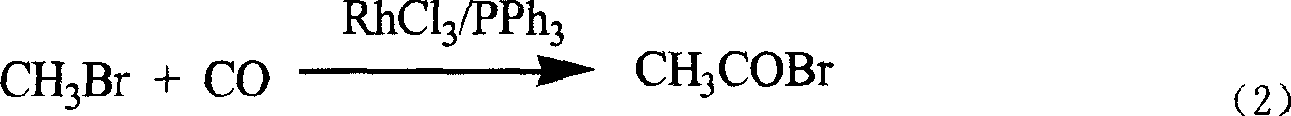Patents
Literature
1070results about "Preparation from carboxylic acid halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyester compound for preparing olefine polymerizing catalyst
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthesis method of D,L-danshensu isopropyl ester
ActiveCN103980120ARaw materials are cheap and easy to getShort reaction stepsPreparation from carboxylic acid halidesOrganic compound preparationHydrogenSynthesis methods
The invention relates to a synthesis method of D,L-danshensu isopropyl ester. The method comprises taking protocatechuic aldehyde as an initial raw material, protecting phenolic hydroxyl by benzyl, carrying out Darzens epoxidation, followed by carrying out catalytic reduction with palladium catalyst / hydrogen or Raney nickel / hydrogen, and thus obtaining the D,L-danshensu isopropyl ester. The purity of the product synthesized by the method can reach 98%, and the yield can reach 55%. Moreover, the synthesis method has the advantages of simple and easily obtained raw materials, short routes and high yield, and is suitable for large-scale industrialized production.
Owner:NORTHWEST UNIV
Synthesis method of racemic bornyl beta-(3,4-dihydroxyphenyl)-alpha-hydroxypropionate
ActiveCN104030923ASynthetic Method AdvantagesRaw materials are cheap and easy to getPreparation from carboxylic acid halidesOrganic compound preparationHydrogenSynthesis methods
The invention relates to a synthesis method of racemic bornyl beta-(3,4-dihydroxyphenyl)-alpha-hydroxypropionate. The method comprises the following steps: carrying out Darzens epoxidation on an initial raw material benzyl protected protocatechualdehyde, and carrying out palladium catalyst / hydrogen or Raney nickel / hydrogen catalytic reduction to obtain racemic bornyl beta-(3,4-dihydroxyphenyl)-alpha-hydroxypropionate. The purity and the yield of a product synthesized by adopting the method reach 98% and 48.6% respectively. The synthesis method has the advantages of simple and easily available raw material, short route, high yield, and suitableness for large scale industrialized production.
Owner:NORTHWEST UNIV +1
Production of terephthalic acid di-esters
InactiveUS7799942B2Speed up reactionSpeed up the conversion processPreparation from carboxylic acid halidesOrganic compound preparationAlcoholTerephthalic acid
Disclosed is a process for the preparation of a terephthalic acid di-ester by the esterification of terephthalic acid with an alcohol at elevated and normal temperature and pressure while the water of the reaction is removed from the reaction mixture via an inert gas or a column.
Owner:EASTMAN CHEM CO
Production of di-(2-ethylhexyl) terephthalate
ActiveUS7276621B2Speed up the conversion processSpeed up reactionPreparation from carboxylic acid halidesOrganic compound preparation2-EthylhexanolETHYLHEXYL ACETATE
Disclosed is a process for the preparation of di-(2-ethylhexyl) terephthalate by the esterification of terephthalic acid with 2-ethylhexanol at elevated temperature and pressure while the water of reaction is removed from the reaction mixture.
Owner:EASTMAN CHEM CO
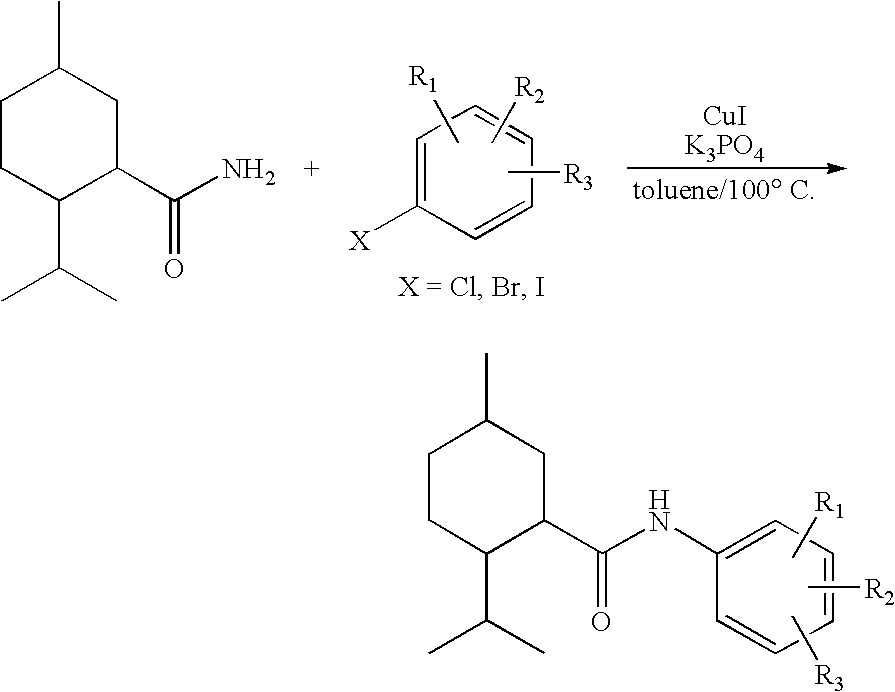
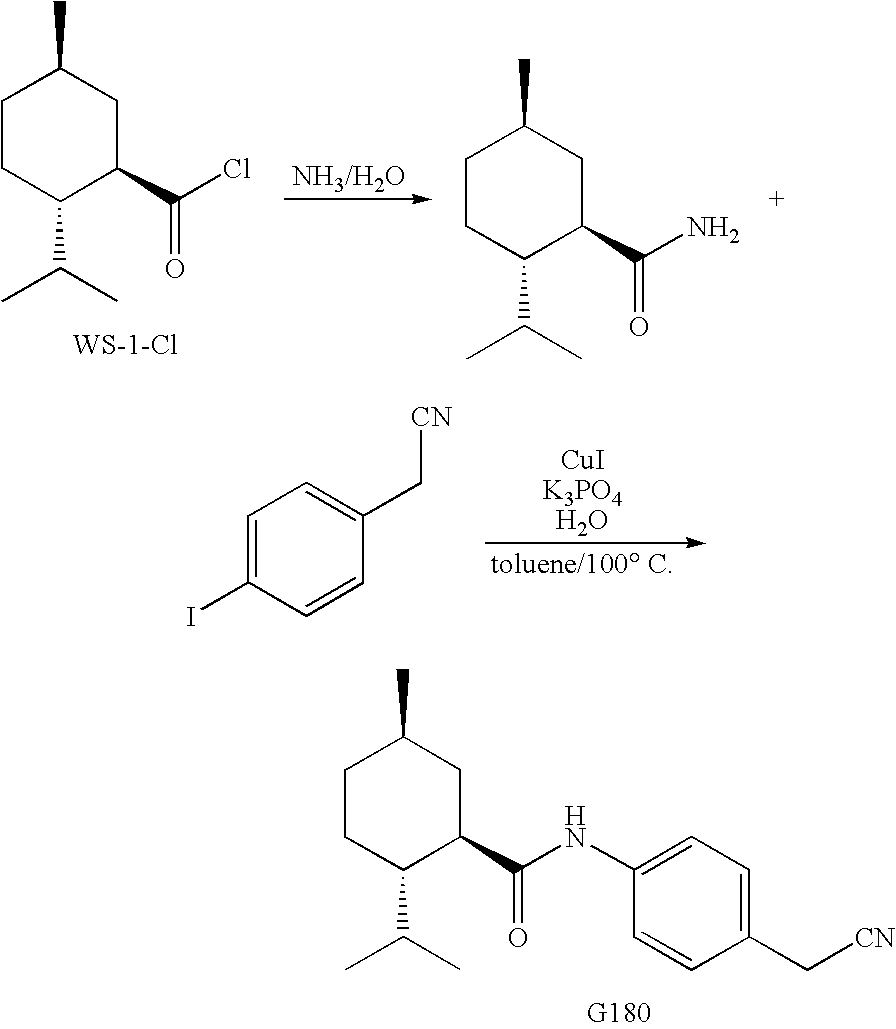
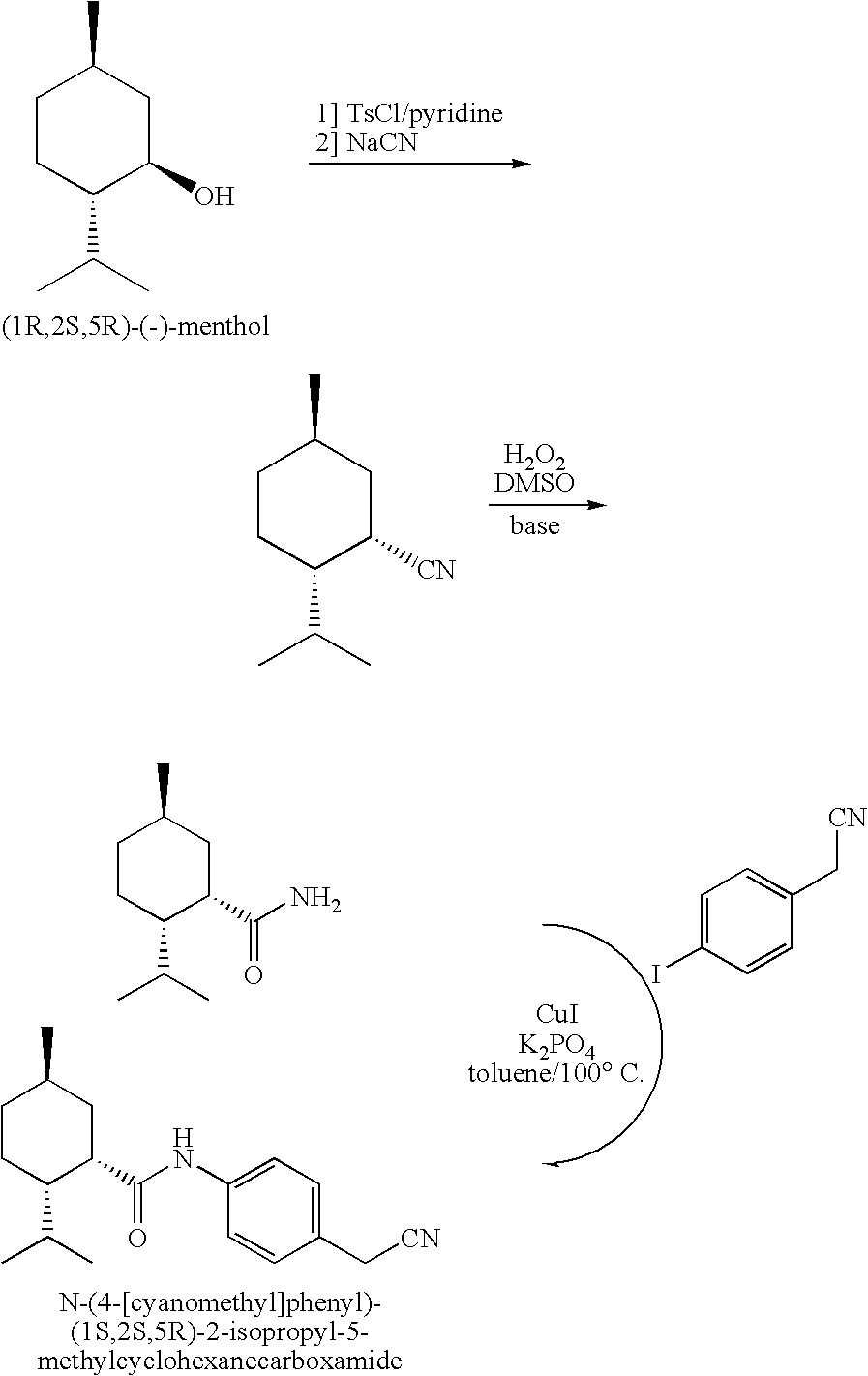
Synthesis of Cyclohexane Derivatives Useful as Sensates in Consumer Products
ActiveUS20100076080A1Improve cooling effectPotent and long lasting cooling effectBiocideCosmetic preparationsCooling effectL menthol
The present invention provides synthetic routes for preparing various isomers of cyclohexane-based coolants, such as menthyl esters and menthanecarboxamide derivatives, in particular those substituted at the amide nitrogen, for example with an aromatic ring or aryl moiety. Such structures have high cooling potency and long lasting sensory effect, which make them useful in a wide variety of consumer products. One synthetic route involves a copper catalyzed coupling of a primary menthanecarboxamide with an aryl halide, such reaction working best in the presence of potassium phosphate and water. Using this synthetic route, specific isomers can be prepared including the menthanecarboxamide isomer having the same configuration as l-menthol and new isomers such as a neoisomer having opposite stereochemistry at the carboxamide (C-1) position. The neoisomer unexpectedly has potent and long lasting cooling effect. Preparation schemes for neoisomers of other menthyl derivatives which are useful as coolants, including esters, ethers, carboxy esters and other N-substituted carboxamides are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
Synthesis of polyanhydrides
InactiveUS7411031B2Reduce yieldHigh yieldPreparation from carboxylic acid halidesOrganic compound preparationPolymer sciencePolyanhydrides
The present invention provides a method for forming compounds of FormulaHO—C(═O)R1—X—R2—X—R1—C(═O)—O—Hwherein compound (I) can be polymerized to provide a polymer that contains therapeutically active compounds. In the compounds of the invention, each R1 is group that will provide the therapeutically active compound upon hydrolysis of the polymer; each X is independently an ester linkage or an amide linkage; and R2 is a linking group.
Owner:RUTGERS THE STATE UNIV +1
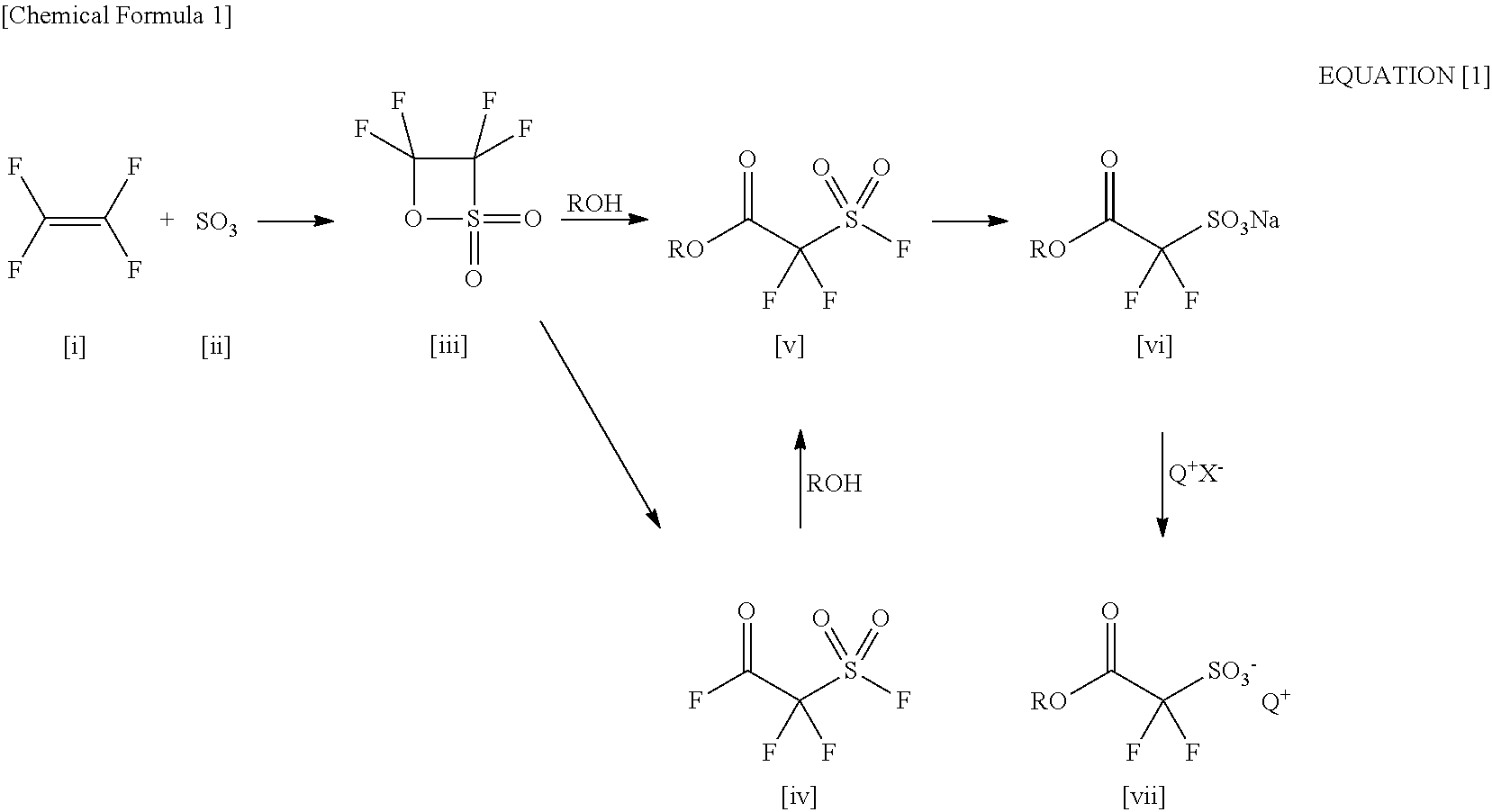
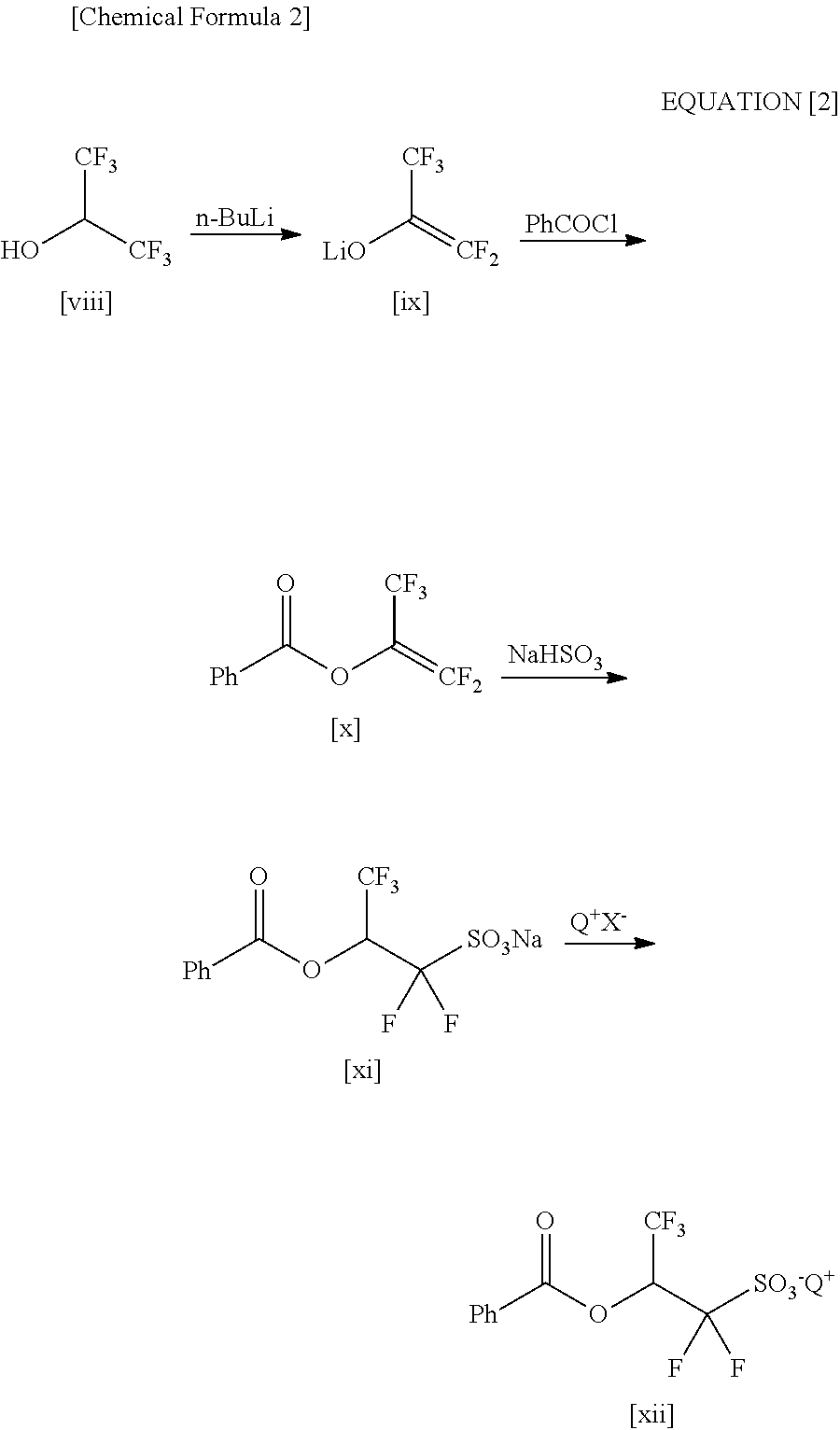
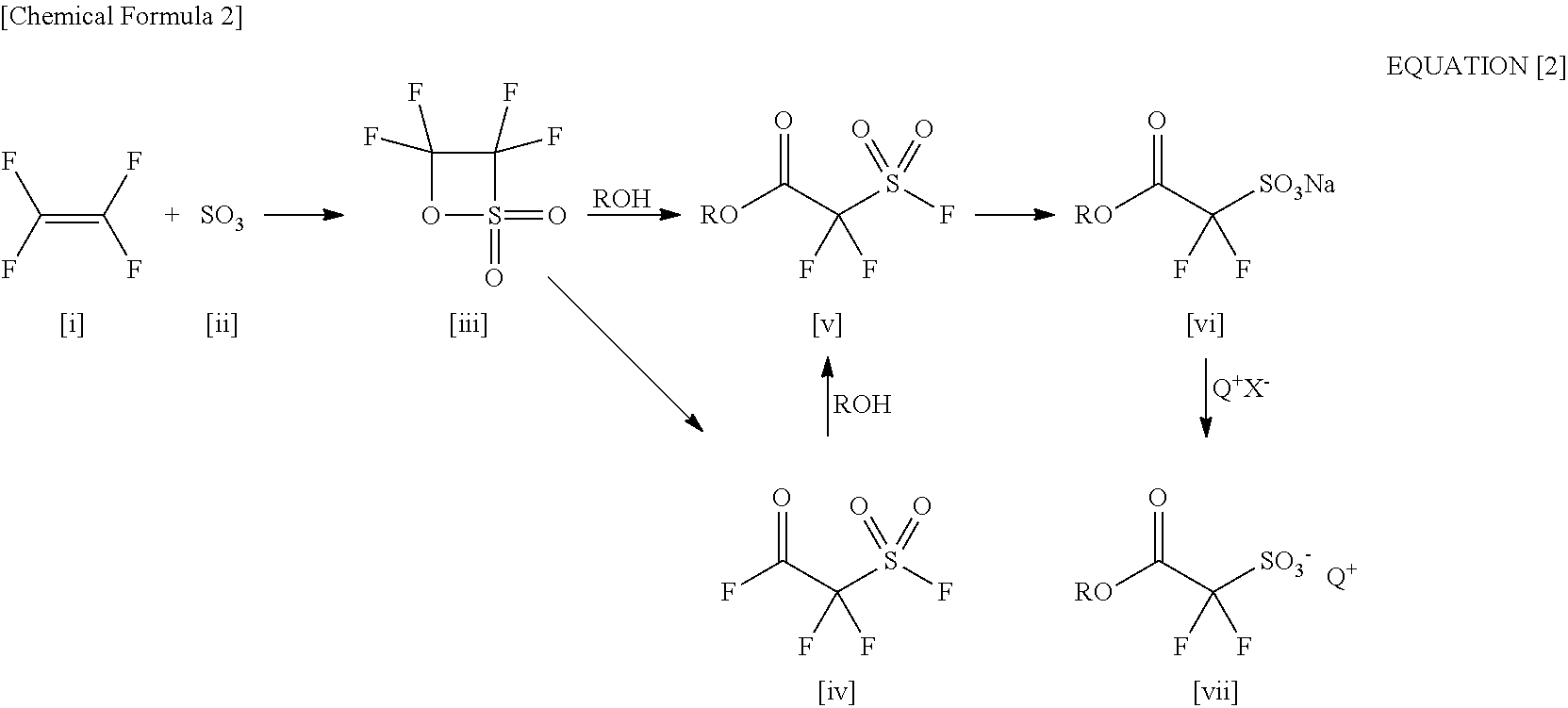
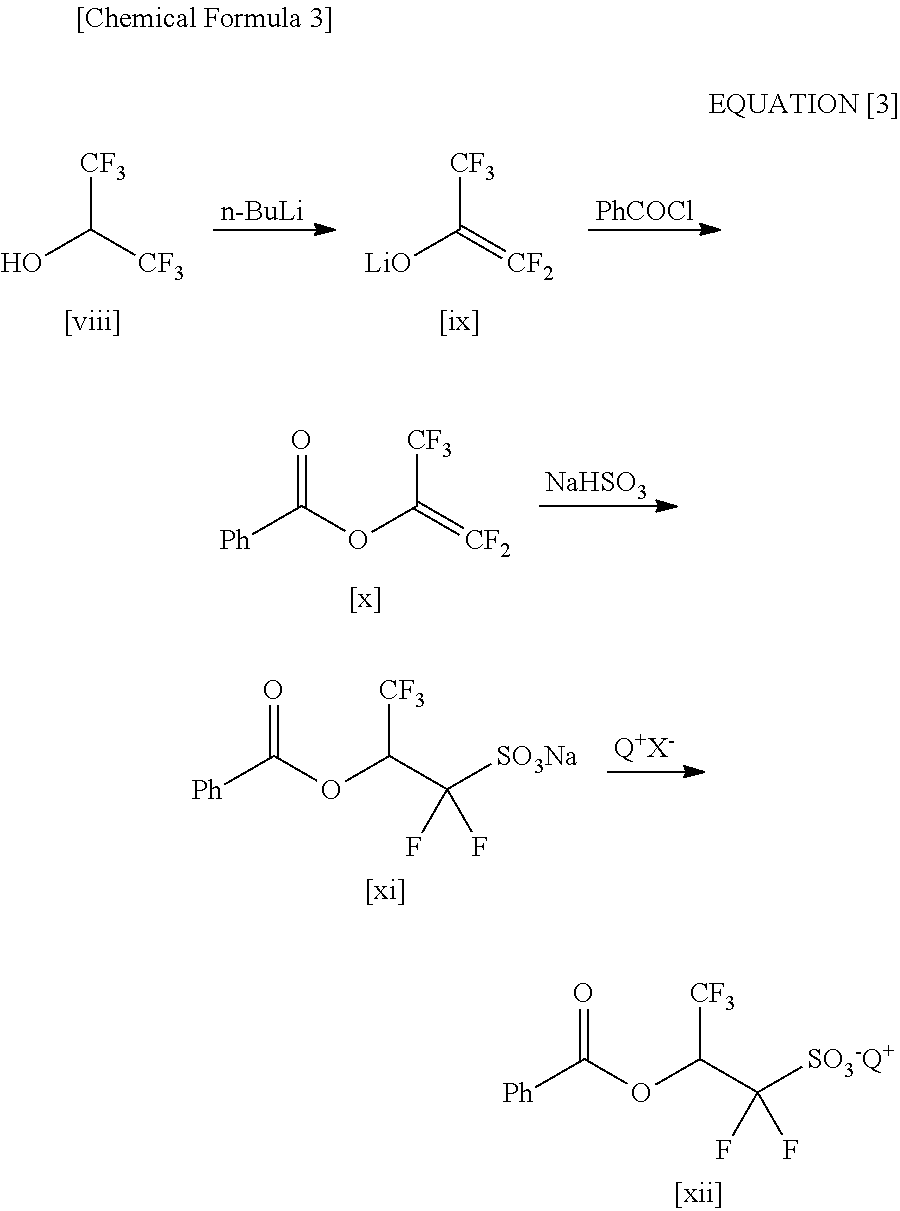
2-(Alkylcarbonyloxy)-1, 1-Difluoroethanesulfonic Acid Salt and Method for Producing the Same
ActiveUS20110034721A1Economically and readily producingPreparation from carboxylic acid halidesOrganic compound preparationSulfonateEthanesulfonic acid
By using an organic base when a carboxylic acid bromodifluoroethyl ester is sulfinated by using a sulfinating agent, there is obtained 2-(alkylcarbonyloxy)-1,1-difluoroethanesulfinic acid ammonium salt. By oxidizing the 2-(alkylcarbonyloxy)-1,1-difluoroethanesulfinic acid ammonium salt, there is obtained 2-(alkylcarbonyloxy)-1,1-difluoroethanesulfonic acid ammonium salt. By using the 2-(alkylcarbonyloxy)-1,1-difluoroethanesulfonic acid ammonium salt as a raw material and exchanging it into an onium salt directly or through saponification / esterification, there can be obtained a 2-alkylcarbonyloxy-1,1-difluoroethanesulfonic acid onium salt.
Owner:CENT GLASS CO LTD
Sirtuin activating compounds and methods for making the same
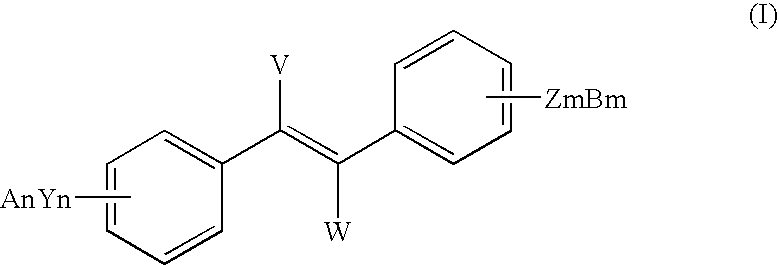
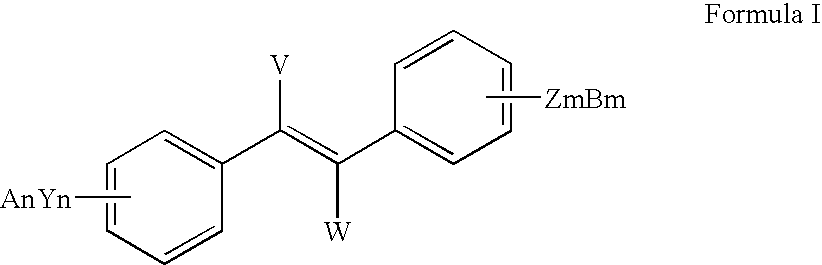
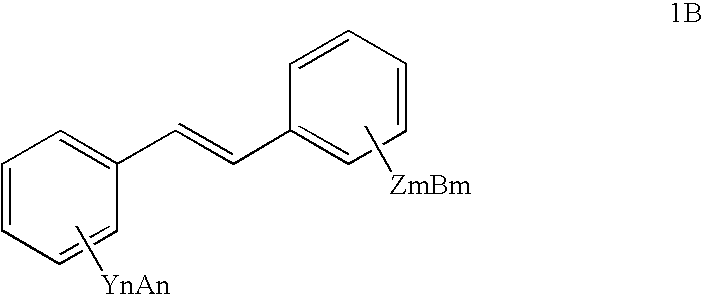
InactiveUS7714161B2Preparation from carboxylic acid halidesOrganic compound preparationHalideCarbon atom
The present invention includes methods for preparing resveratrol, resveratrol esters and substituted and unsubstituted stilbenes of the formula given below; where each Y is —O or halogen, each Z is —O or halogen, each n and each m is independently the value of 0, 1, 2, 3, 4 or 5, each A and each B is independently selected from Pn, R or absent, each V and each W is independently selected from Pn, straight or branched alkyl of from (2) to (6) carbon atoms and cycloalkyl of from (3) to (8) carbon atoms, alkoxy, phenyl, benzyl or halogen, R is independently selected from the group comprising alkyl with at least one carbon atom, aryl and aralkyl, Pn is an alcohol protecting group and diastereoisomers of the foregoing. The compounds are made from a multi-step process including a N-heterocyclic carbene-type ligand coupling in the presence of a base with benzyol halide and styrene coupling partners. These compounds show increased stability for use in the food, cosmetic and pharmaceutical industries.
Owner:BRIGHAM YOUNG UNIV
Method for preparing fluorine-containing acrylate
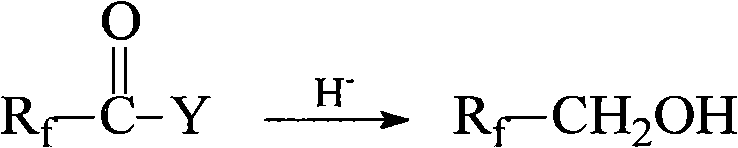
ActiveCN102010334ANo destructionExcellent water and oil repellencyPreparation from carboxylic acid halidesVegetal fibresHuman healthBioaccumulation
The invention belongs to the technical field of textile fabric finishing agents, and relates to a method for preparing an intermediate fluorine-containing acrylate monomer of a water-proof and oil-proof organic fluorine fabric finishing agent. The method is characterized by comprising the following steps of: (1) in the presence of a reducing agent, preparing polyfluoroalcohol by using a perfluoroalkylacyl compound as a raw material; and (2) slowly dripping (methyl) acryloyl chloride to prepare fluorine-containing acrylate in the presence of a catalyst by using the polyfluoroalcohol prepared in the step (1) and using phenothiazine as a polymerization inhibitor. The number of long-chain perfluoroalkyl carbon atoms in the prepared fluorine-containing acrylate is less than 8, and the fluorine-containing acrylate can be degraded and has no biological accumulation. The fluorine-containing acrylate has no hidden dangers of long-chain perfluoroalkyl to destroy the environment and harm human health. Fluorine-containing acrylate copolymer emulsion prepared by using the prepared fluorine-containing acrylate monomer is used as a water-proof and oil-proof finishing agent for textiles, and the finished textiles have excellent water-proof and oil-proof performance.
Owner:江苏梅兰化工有限公司
Processes for Production of 2-Bromo-2,2-Difluoroethanol and 2-(Alkylcarbonyloxy)-1,1-Difluoroethanesulfonic Acid Salt
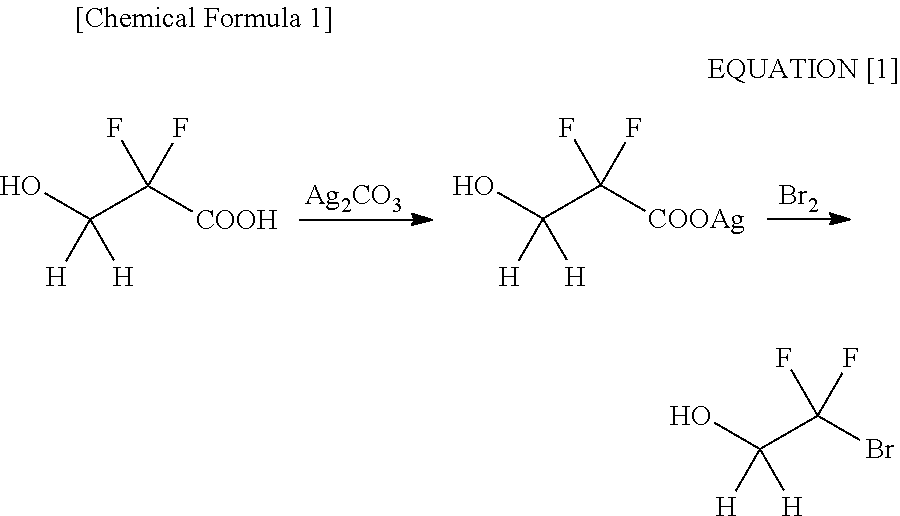
ActiveUS20110015431A1Preparation from carboxylic acid halidesOrganic compound preparationHalogenAlkoxy group
Disclosed is a process for producing 2-bromo-2,2-difluoroethanol, which comprises reducing a bromodifluoroacetic acid derivative represented by the formula [1] by using an ate hydride complex as a reducing agent. 2-Bromo-2,2-difluoroethanol thus produced can be used as the starting material to carry out the esterification step, the sulfination step and the oxidation step in this order, thereby producing a 2-alkylcarbonyloxy-1,1-difluoroethanesulfonic acid salt, wherein A represents a substituted or unsubstituted linear, branched or cyclic alkoxy group having 1 to 20 carbon atoms, a substituted or unsubstituted aryloxy group having 6 to 15 carbon atoms, a heteroaryloxy group having 4 to 15 carbon atoms, or a halogen atom.
Owner:CENT GLASS CO LTD
Preparation method of 2-(4-haloethyl) phenyl-2-methyl propionic ester and synthesis method of bilastine
InactiveCN102675101AAvoid expensive reagentsRaw materials are cheap and easy to getPreparation from carboxylic acid halidesPropanoic acidPtru catalyst
The invention discloses a preparation method of 2-(4-haloethyl) phenyl-2-methyl propionic ester and a synthesis method of bilastine, which comprises the following steps of: carrying out acylation reaction on 2, 2-dimethyl phenylacetate and halogen acetyl halides under the action of catalyst, to generate 2-(4-halogen acetyl) phenyl-2-methyl propionic ester; carrying out kishner-wolff-huang reduction reaction on the 2-(4-halogen acetyl) phenyl-2-methyl propionic ester, to reduce the carbonyl so as to generate the 2-(4-haloethyl) phenyl-2-methyl propionic ester; having condensation reaction with 1-ethoxy ethyl-2-pyridine-4-group benzimidazole by taking the 2-(4-haloethyl) phenyl-2-methyl propionic ester as a midbody to obtain esterified bilastine; and hydrolyzing, to generate the bilastine. The novel synthesis method of the bilastine provided by the invention can easily obtain raw materials, and is simple to operate, lower in cost, environment-friendly, and completely suitable for the industrial production.
Owner:王蕾
Polyorganosiloxane compound, method for preparing the same, and copolycarbonate resin comprising the same
ActiveUS20150315380A1Improve mechanical propertiesMaintain transparencyPreparation from carboxylic acid halidesPlastic/resin/waxes insulatorsOrganic chemistryMechanical property
A polyorganosiloxane compound, a method of preparing the same, and a copolycarbonate resin comprising the same are disclosed. Particularly, a copolycarbonate resin, which may be applied to a variety of applications, and in particular, comprises a polyorganosiloxane compound used as an impact modifier, a modifier, or a comonomer of a copolycarbonate resin and has improved mechanical properties such as low-temperature impact strength, is disclosed.
Owner:LG CHEM LTD
Production of terephthalic acid di-esters
InactiveUS20070161815A1Speed up reactionSpeed up the conversion processPreparation from carboxylic acid halidesOrganic compound preparationAlcoholTerephthalic acid
Disclosed is a process for the preparation of a terephthalic acid di-ester by the esterification of terephthalic acid with an alcohol at elevated and normal temperature and pressure while the water of the reaction is removed from the reaction mixture via an inert gas or a column.
Owner:EASTMAN CHEM CO
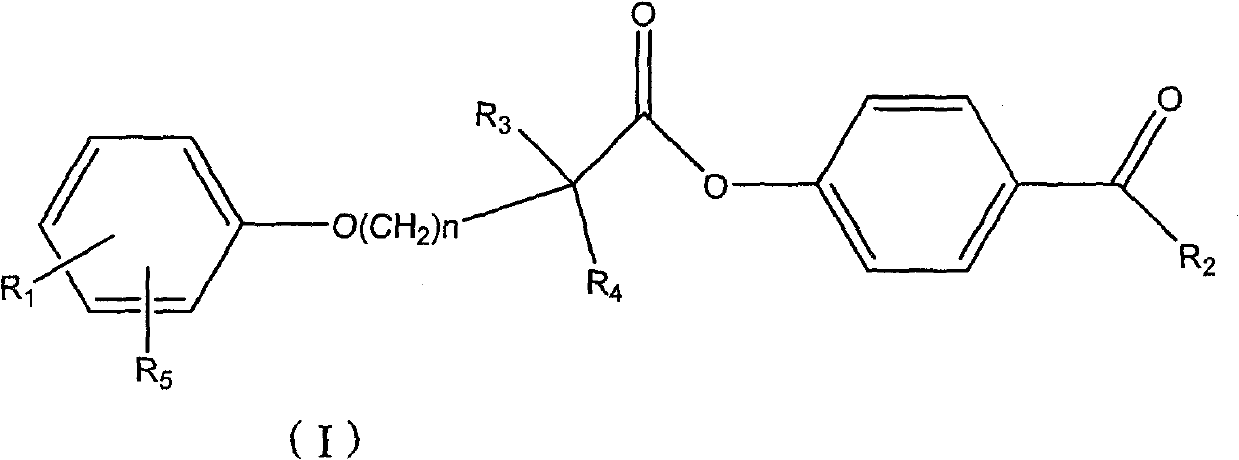
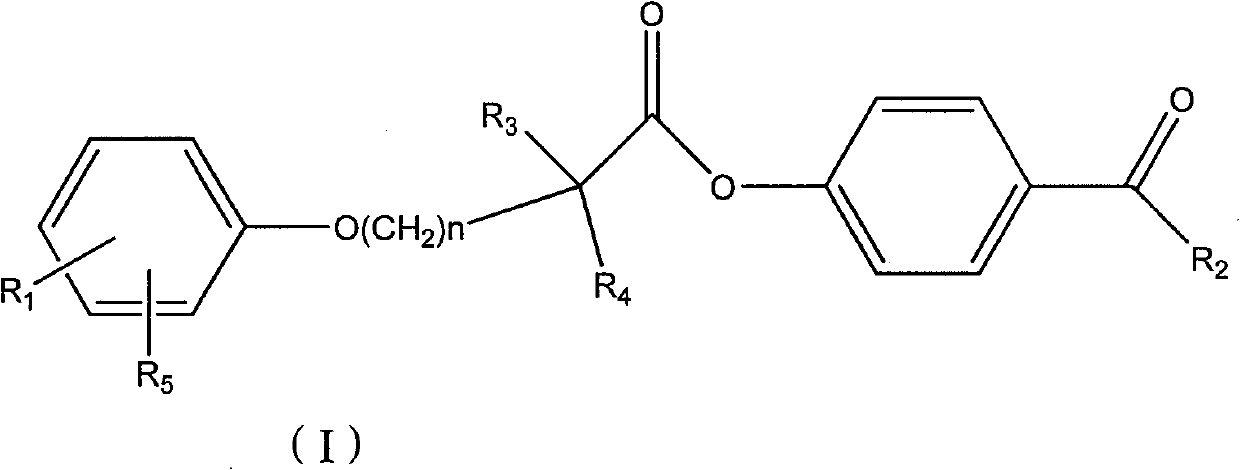
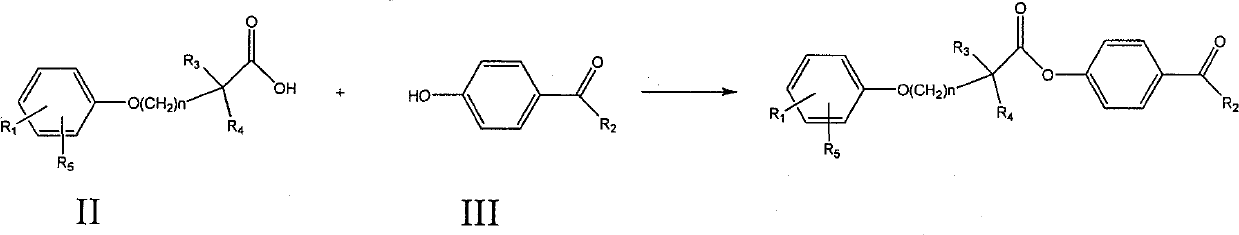
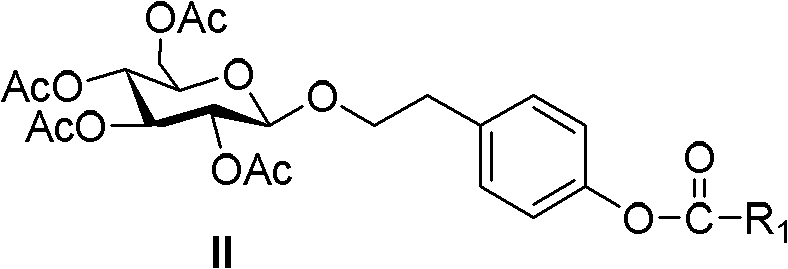
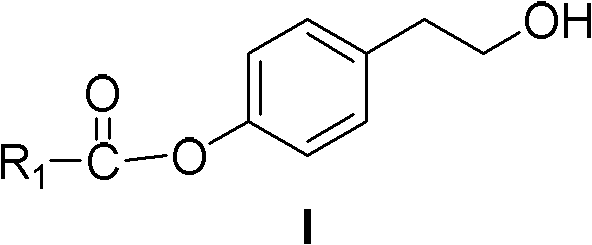
Ester compound, preparation method and application thereof
ActiveCN102093246ALower levelLow toxicityOrganic active ingredientsPreparation from carboxylic acid halidesSolubilityOrganic solvent
The invention discloses a compound shown as a formula I or pharmaceutically acceptable salt thereof. In the formula I, R1, R2, R3, R4, R5 and n are defined as the specification. The invention also discloses a preparation method for the compound of the formula I, a composition containing the compound and application of the compound. The compound of the formula I is stable in vitro, has good dissolubility in a pharmaceutically applied organic solvent, and has good bioavailability in the body of an animal. Meanwhile, the invention discloses the preparation method for the compound of the formula I and the application of the compound of the formula I in preparation of medicaments for regulating blood fat and preventing and treating biliary calculus.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Salidroside chemical synthesis method for industrialization
ActiveCN102304157APossibility of industrializationReduce pollutionPreparation from carboxylic acid halidesSugar derivativesTyrosolChemical synthesis
The invention discloses a method for chemically synthesizing natural product salidroside. In the method, lewis acid serving as a catalyst, tyrosol of acyl protected phenolic hydroxyl serving as a glycosylation receptor, and beta-D-5 acetyl glucose serving as glycosylation donor perform a glycosylation reaction to obtain 1-[2-(4-acyloxy phenyl) ethyl]-2,3,4,6-O-tetraacetyl-beta-D-glucopyranoside; and acyl protecting group is removed in an alkaline condition to obtain salidroside. Compared with the traditional method, the method has easily available raw materials, the glycosylation receptor, the glycosylation product and the final product can be prepared through recrystallization, column chromatography and other purification processes are eliminated, the reaction step is short, the preparation process is simple, the glycosylation reaction catalyst has low cost and is easily available, so that the preparation cost is remarkably reduced; and a heavy metal salt catalyst is not used in the reaction, so that the synthesis product is more suitable to be used as a raw material of medicaments and health-care products. The method is suitable for industrial production of salidroside.
Owner:PEKING UNIV
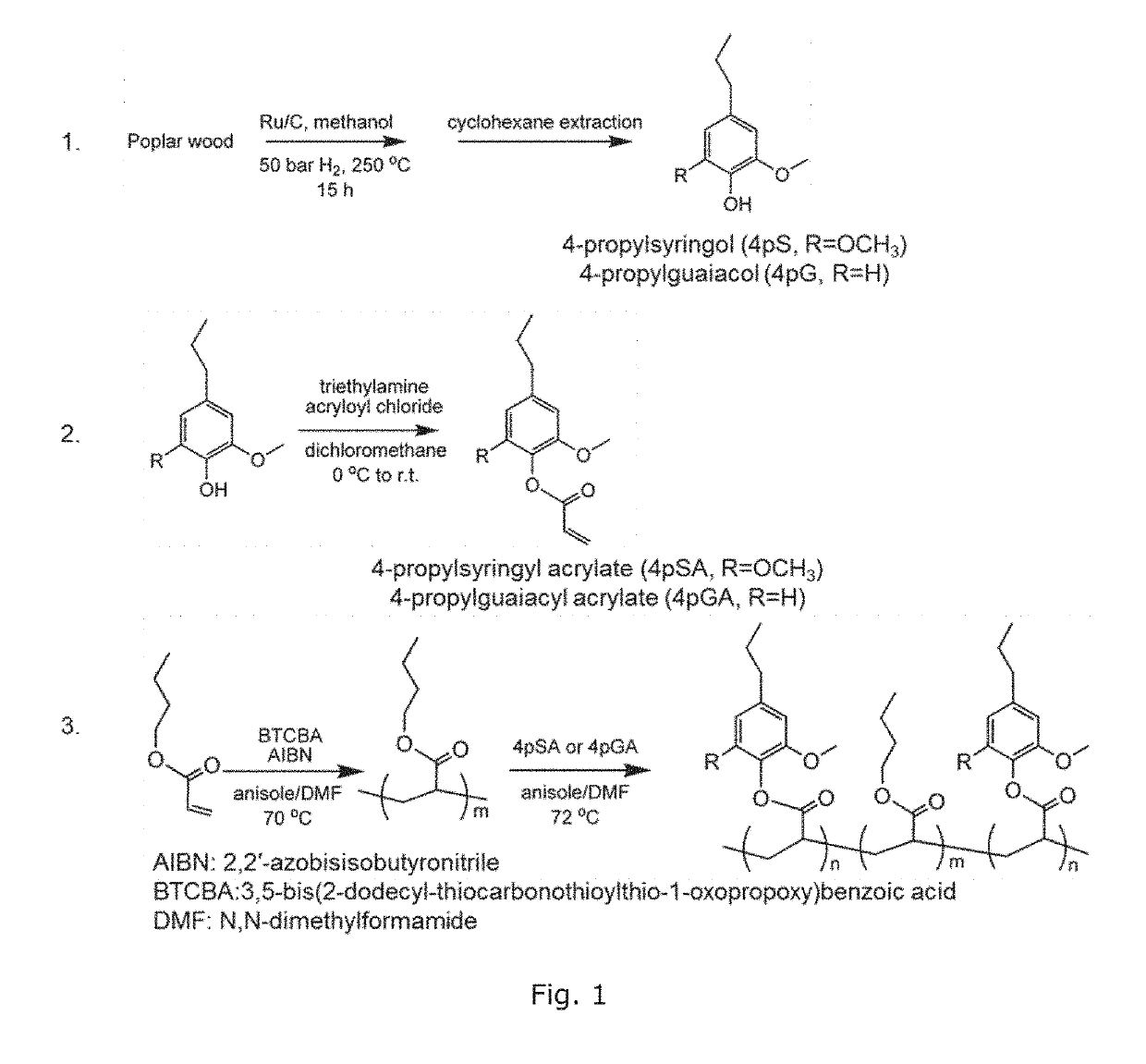
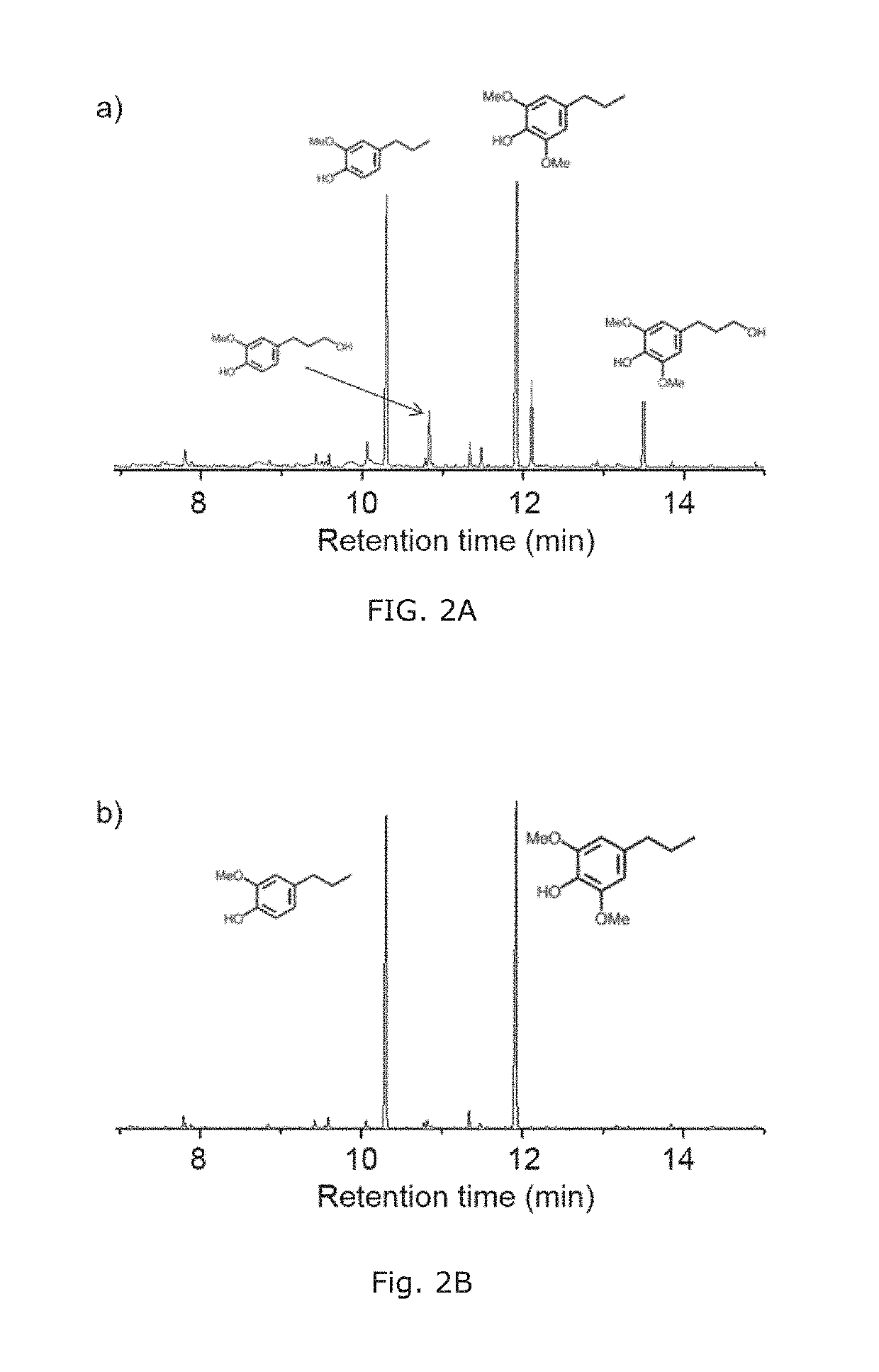
Bio-based polymers from raw lignocellulosic biomass
ActiveUS20190144590A1High yieldSolid electrolytesPreparation from carboxylic acid halidesCellulosePolymer electrolytes
Disclosed herein is a method of making polymerizable bio-based monomers containing one phenolic hydroxyl group which has been derivatized to provide at least one polymerizable functional group which is an ethylenically unsaturated functional group (such as a [meth]acrylate group), where the precursors of the polymerizable bio-based monomers are derived from raw lignin-containing biomass. Also disclosed herein are bio-based copolymers prepared from such bio-based monomers and a co-monomer, and methods of making and using such bio-based copolymers. In particular, the bio-based copolymers can be used as pressure sensitive adhesives, binders, and polymer electrolytes.
Owner:UNIVERSITY OF DELAWARE
Compounds, compositions and methods for the treatment of amyloid diseases and synucleinopathies such as alzheimer's disease, type 2 diabetes, and parkinson's disease
Bis- and tris-dihydroxyaryl compounds and their methylenedioxy analogs and pharmaceutically acceptable esters, their synthesis, pharmaceutical compositions containing them, and their use in the treatment of amyloid diseases, especially Aβ amyloidosis, such as observed in Alzheimer's disease, IAPP amyloidosis, such as observed in type 2 diabetes, and synucleinopathies, such as observed in Parkinson's disease, and the manufacture of medicaments for such treatment.
Owner:PROTAMED
Synthesis of polyanhydrides
InactiveUS20050131199A1Reduce yieldHigh yieldPreparation from carboxylic acid halidesOrganic compound preparationPolyanhydridesHydrolysis
The present invention provides a method for forming compounds of Formula HO—C(═O)R1—X—R2—X—R1—C(═O)—O—H wherein compound (I) can be polymerized to provide a polymer that contains therapeutically active compounds. In the compounds of the invention, each R1 is group that will provide the therapeutically active compound upon hydrolysis of the polymer; each X is independently an ester linkage or an amide linkage; and R2 is a linking group.
Owner:RUTGERS THE STATE UNIV +1
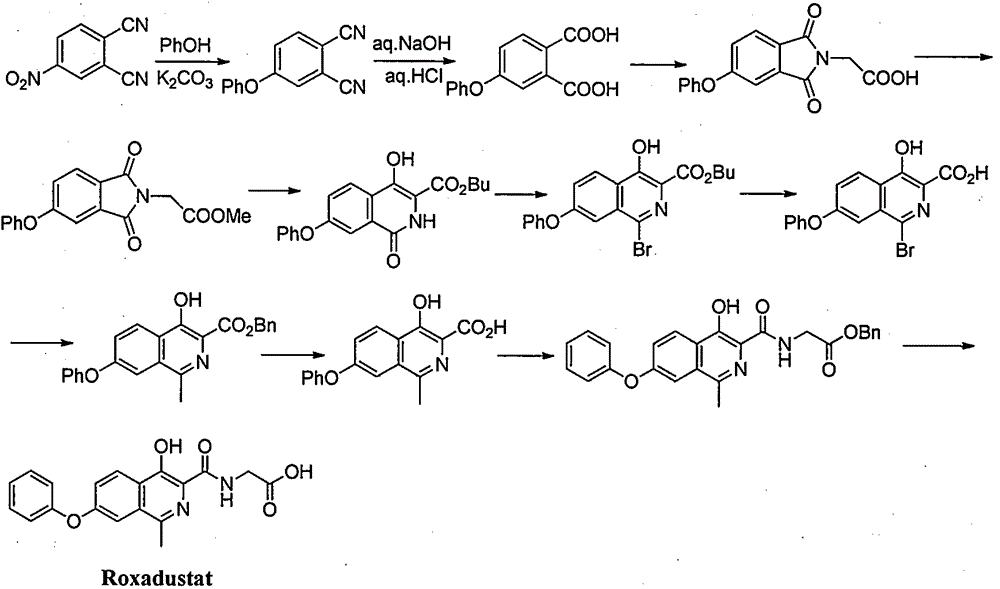
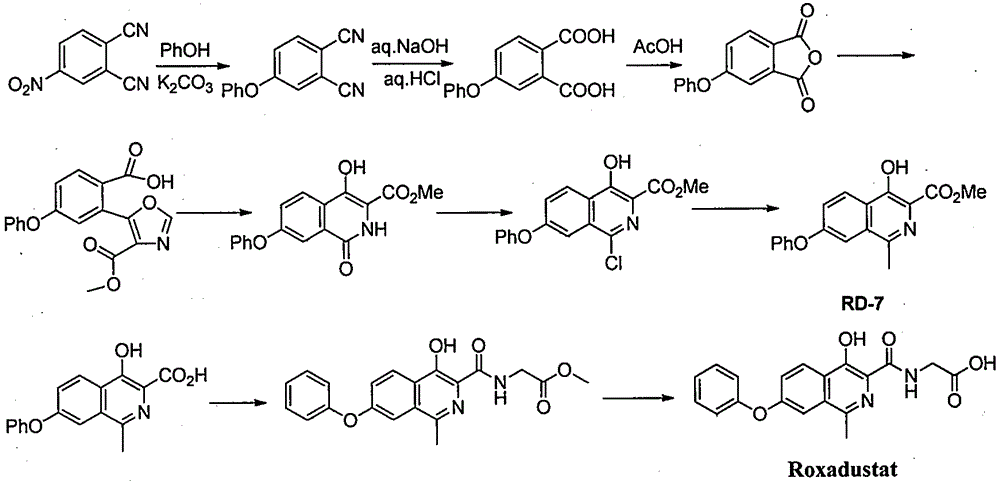
Preparation method for intermediate of Roxadustat
InactiveCN106478503ARaw materials are easy to getSimple processPreparation from carboxylic acid halidesSulfonic acid amide preparationAromatizationPhenol
The invention provides a preparation method for an intermediate VI of Roxadustat. The preparation method comprises the following steps: reacting 3-methyl-5-bromoisobenzofuran-1(3H)-one (I), which serves as a starting raw material, with phenol so as to prepare an intermediate II, then, subjecting the II to a ring-opening substitution reaction so as to prepare a compound III, and subjecting the compound III to a substitution reaction so as to prepare a key intermediate V; and subjecting the V to alkali condensation, deprotection and aromatization, thereby preparing the target intermediate VI. The preparation method for the intermediate VI of the Roxadustat, provided by the invention, has the advantages that the raw materials are readily available, the process is simple, the operation is convenient, the reaction yield is high, the atom utilization ratio is high, and the industrial production is facilitated. The reaction general formula is represented by the formulae shown in the description.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
Rosin or rosin derivative long chain flexible monomer preparation method
InactiveCN103232349AImprove flexibilityPreparation from carboxylic acid halidesSteroidsMethacryloyl chlorideRosin
The present invention relates to a rosin or rosin derivative long chain flexible monomer preparation method, which comprises the following steps: 1) placing rosin or a rosin derivative in dichloromethane or tetrahydrofuran, adding an acyl chlorination reagent, carrying out a reaction for 1-12 h at a temperature of 0-85 DEG C to synthesize rosin acyl chloride or rosin derivative acyl chloride; 2) mixing the rosin acyl chloride or the rosin derivative acyl chloride and a long chain aliphatic diol according to a certain molar ratio, dissolving in tetrahydrofuran, adding a catalyst, carrying out a reaction for 5-24 h at a temperature of 50-80 DEG C to synthesize rosin long chain aliphatic diol monoester or rosin derivative long chain aliphatic diol monoester, and carrying out precipitation purification in a specific solvent; and (3) mixing the purified rosin long chain aliphatic diol monoester or the purified rosin derivative long chain aliphatic diol monoester and acryloyl chloride or methacryloyl chloride according to a certain molar ratio, adding a catalyst and a polymerization inhibitor hydroquinone, and carrying out a reaction for 4-24 h at a temperature of -10-10 DEG C to synthesize a rosin long chain flexible monomer or a rosin derivative long chain flexible monomer, wherein the synthesized rosin long chain flexible monomer or the synthesized rosin derivative long chain flexible monomer can be subjected to a free radical polymerization reaction.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
New technological process of synthesizing acetyl bromide, acetic acid, acetate from methane
ActiveCN1724503AEasily hydrolyzedReduces aggravated sedimentationPreparation from carboxylic acid halidesPreparation from carboxylic acid halideSolventOxygen
The invention discloses a novel process for preparing acetyl bromide, acetic acid and acetic ester from methane, which consists of reacting methane, oxygen and aqueous solution of HBr to obtain CH3Br and CO through catalytic reaction, then subjecting CH3Br and CO to carbonation reaction so as to prepare acetyl bromide in organic medium containing catalyst, then directly hydrolyzing the acetyl bromide and a certain amount of water into acetic acid, and reacting acetyl bromide and alcohols to obtain the acetic esters of the corresponding alcohols. The HBr can be regenerated as circular reaction medium and used again for the reaction for preparing CH3Br and CO from methane. The organic medium of the carbonylation reaction contains catalyst such as rhodium (Rh) compound, hydriodate and / or organo-phosphorus ligand, and a certain proportion of solvents.
Owner:MICROVAST POWER SYST CO LTD
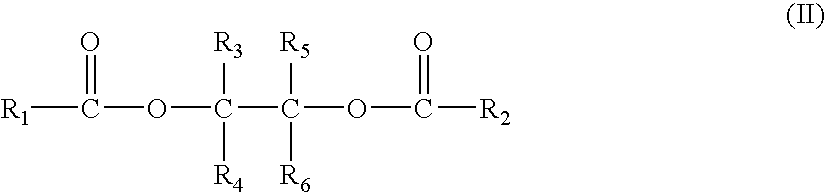
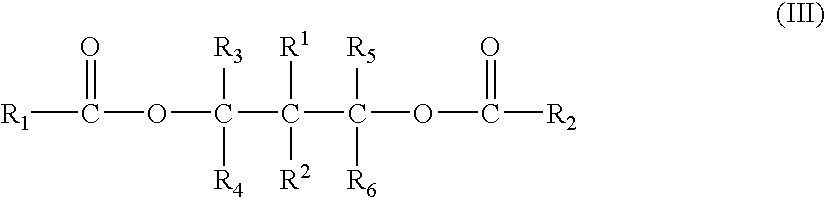
Polyol ester compounds useful in preparation of a catalyst for olefins polymerization, process for preparing the same and use thereof
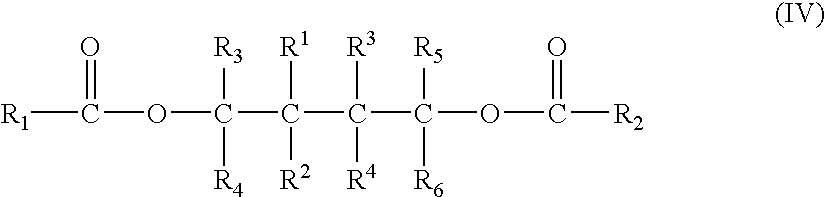
The present application relates to polyol ester compounds, having general formula (I): R1CO—O—CR3R4-A-CR5R6—O—CO—R2 (I) wherein, R, and R2 groups, which may be identical or different, can be substituted or unsubstituted hydrocarbyl having 1 to 20 carbon atoms, R3-R6 groups, which may be identical or different, can be selected from the group consisting of hydrogen, halogen or substituted or unsubstituted hydrocarbyl having 1 to 20 carbon atoms, R1-R6 groups optionally contain one or more hetero-atoms replacing carbon, hydrogen atom or the both, said hetero-atom is selected from the group consisting of nitrogen, oxygen, sulfur, silicon, phosphorus and halogen atom, two or more of R3-R6 groups can be linked to form saturated or unsaturated monocyclic or polycyclic ring; A is a single bond or bivalent linking group with chain length between two free radicals being 1-10 atoms, wherein said bivalent linking group is selected from the group consisting of aliphatic, alicyclic and aromatic bivalent radicals, and can carry C1-C20linear or branched substituents; one or more of carbon atom and / or hydrogen atom on the substituents can be replaced by a hetero-atom selected from the group consisting of nitrogen, oxygen, sulfur, silicon, phosphorus, and halogen atom, and two or more said substituents on the linking group as well as above-mentioned R3-R6 groups can be linked to form saturated or unsaturated monocyclic or polycyclic ring. The compounds of formula (I) find use in preparing a catalyst for olefin polymerization.
Owner:CHINA PETROCHEMICAL CORP +1
Crystal forms of a hcv protease inhibitor
Owner:MERCK SHARP & DOHME LLC +1
Ester glycosyl phase selective oleophylic gelator as well as preparation method and application thereof in oil gelatinization
InactiveCN104803850AEasy to separateTo achieve the effect of gellingFatty/oily/floating substances removal devicesPreparation from carboxylic acid halidesOil phaseRaw material
The invention discloses an ester glycosyl phase selective oleophylic gelator as well as a preparation method and application thereof in oil gelatinization. Mannitol and medium / long alkyl chain acyl chlorides are taken as raw materials, and the selective oleophylic gelator is prepared through O-esterification reaction. Due to the / long alkyl chain, the ester glycosyl phase selective oleophylic gelator disclosed by the invention can selectively enter an oil phase in an oil-water mixture, self-assembling can be achieved in the oil phase through the weak interaction of intermolecular hydrogen bonds, a physically crosslinked supramolecular structure can be formed, furthermore the oil phase is immobilized through interfacial tension and capillary action, then the oil phase has no mobility, and the effect of gelatinization can be achieved. The problems that a conventional similar gelator is complex in preparation process, high in catalyst cost, and low in gelatinization efficiency are solved, and the ester glycosyl phase selective oleophylic gelator has a relatively good application prospect in the fields of offshore overflow oil recycling and rapid industrial oil-containing wastewater separation.
Owner:TIANJIN UNIV
Synthesis method of rhein and diacerein
InactiveCN1789229AAvoid pollutionConvenient sourcePreparation from carboxylic acid halidesOrganic compound preparationM-CresolAcetylation
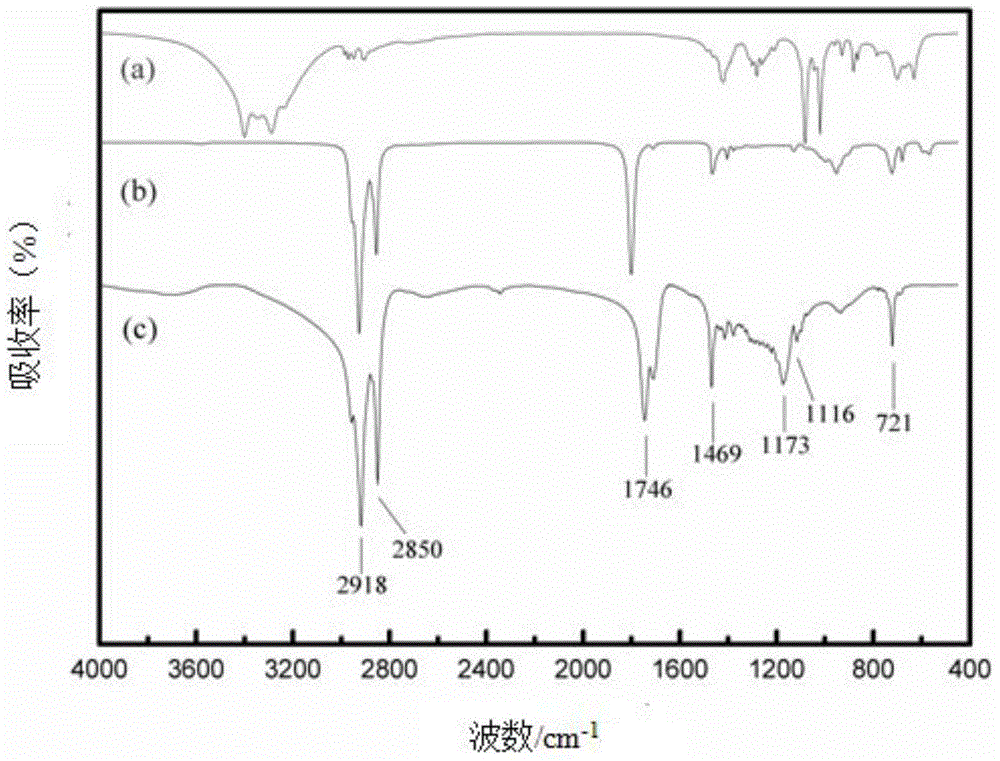
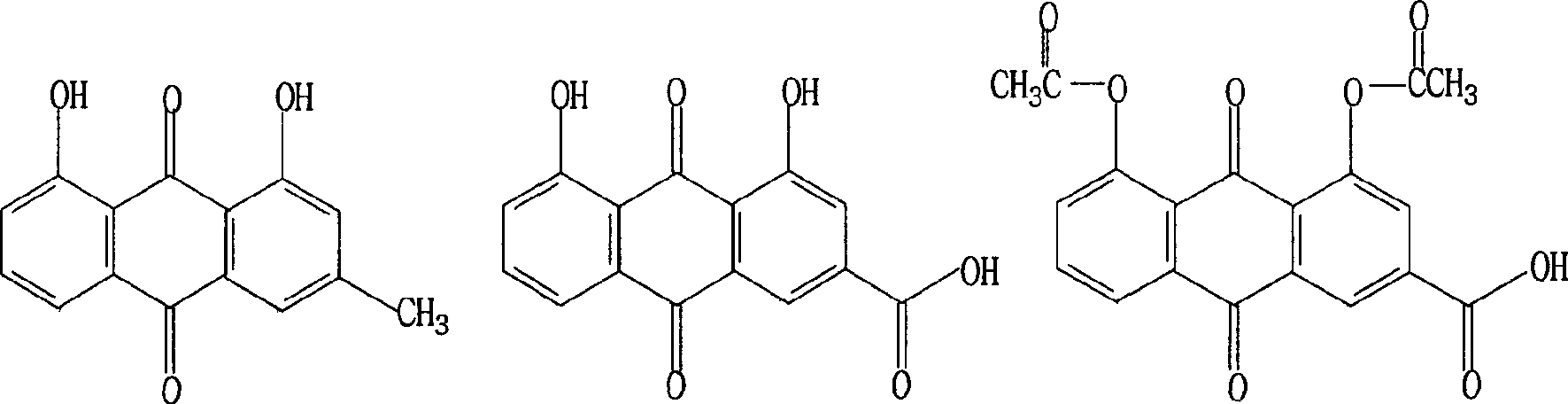
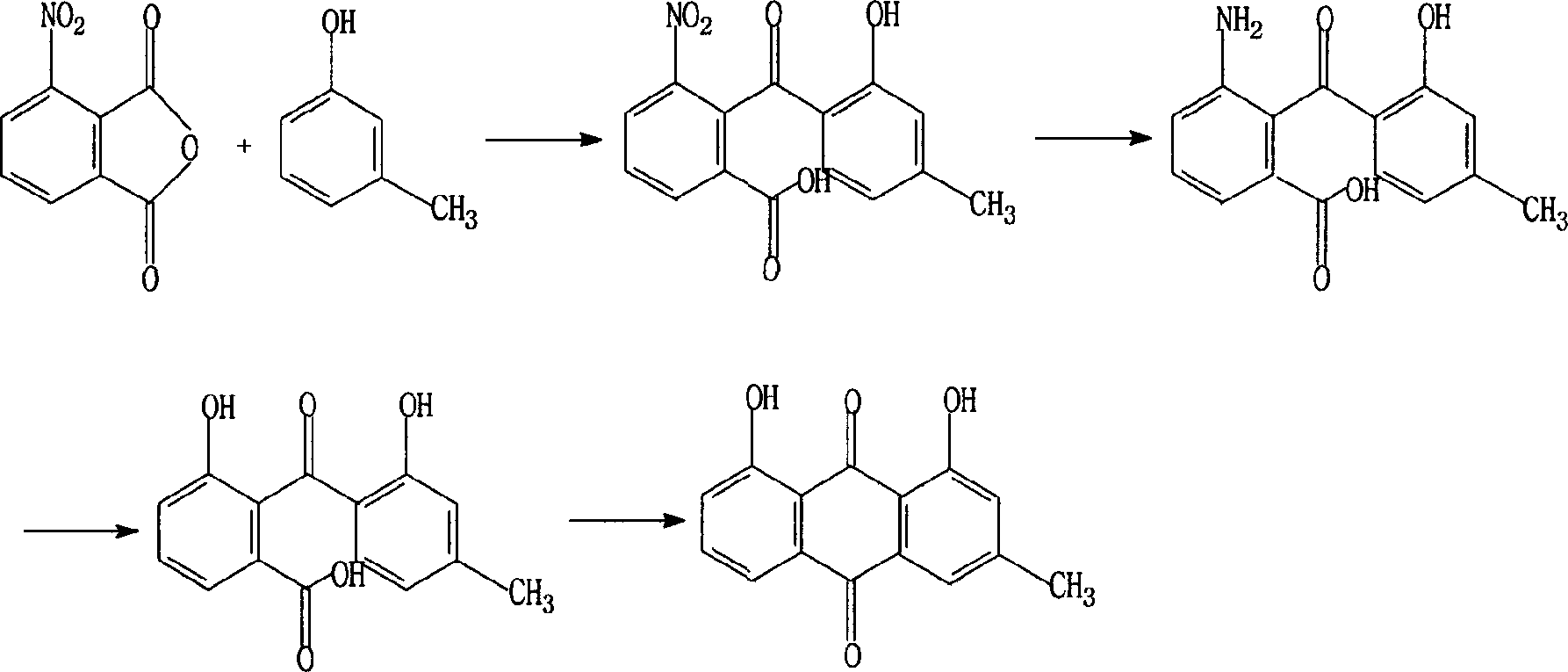
The invention relates to a method for synthesizing the emodic acid which is the essential component of the traditional Chinese medicine rhubarb horsetails and duroruiyin which is antipyretic analgesic and osteoarthritis treating medicine, employing 3-nitro-ortho-phthalic anhydride as start raw material, reacting with m-cresol fuke, through reaction of reduction, diazonium salt, ring-combining to prepare intermediate compound chrysophanol, oxygenating to emodic acid, preparing duroruiyin through acetylation, the total productivity of the six reaction is 31.1%, the process is optimize through pilot-scale experiment a nd suitable for industrial production.
Owner:NAN JING RHINE PHARM TECH
Preparation methods of glyoxylic acid L-menthyl alcohol ester and monohydrate of glyoxylic acid L-menthyl alcohol ester
ActiveCN102516078AEasy to operateMild reaction conditionsPreparation from carboxylic acid halidesFenchyl alcoholL menthol
The invention relates to preparation methods of glyoxylic acid L-menthyl alcohol ester and a monohydrate of the glyoxylic acid L-menthyl alcohol ester, and belongs to the technical field of fine chemical industry. The glyoxylic acid L-menthyl alcohol ester is prepared by the following steps: reacting L-menthol with monohalogen or dihalogen acetyl halide or anhydride to generate monohalogen or dihalogen acetic acid L-menthyl alcohol ester; and reacting in the presence of a pro-oxidant to obtain the glyoxylic acid L-menthyl alcohol ester. The monohydrate of the glyoxylic acid L-menthyl alcohol ester is prepared by the following steps: cooling and diluting a reaction solution using monohalogen acetic acid L-menthyl alcohol ester as a raw material; treating by using a dimethylsulfoxide (DMSO) solution of P2O5 and triethylamine to obtain the glyoxylic acid L-menthyl alcohol ester; washing, extracting and concentrating a reaction solution using dihalogen acetic acid L-menthyl alcohol ester as a raw material to obtain the glyoxylic acid L-menthyl alcohol ester; and treating by using sodium hydrogen sulfite and formaldehyde to obtain the monohydrate of the glyoxylic acid L-menthyl alcohol ester. The total yield of the glyoxylic acid L-menthyl alcohol ester synthesized by the technology can reach over 72 percent; the total yield of the monohydrate of the glyoxylic acid L-menthyl alcohol ester can reach over 80 percent; the purity can reach over 99.5 percent; the technical process is simple and convenient; the raw materials are available; the yield is high; the product purity is high; and the process is suitable for industrial production.
Owner:ZHEJIANG XIANFENG TECH +1
Plasticizers from less branched alcohols
ActiveUS6982295B2Improve compatibilityReduce volatilityPreparation from carboxylic acid halidesPlastic/resin/waxes insulatorsAlcoholPlasticizer
Less branched C13 alcohols are used to provide plasticizer esters particularly suitable for high temperature applications such as wire and cable insulation.
Owner:EXXONMOBIL CHEM PAT INC
Method for continuously synthesizing ethyl 4-chloroacetoacetates
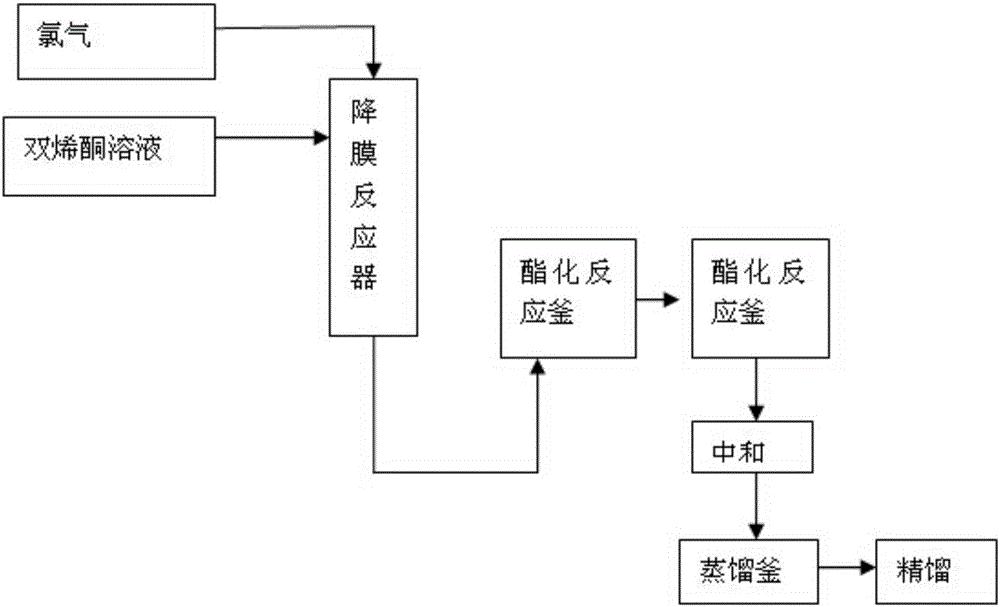
ActiveCN105693509AReduce volumeIncrease the effective cooling areaPreparation from carboxylic acid halidesCarboxylic acid halides preparationSolventEthyl Chloride
The invention relates to a method for continuously synthesizing ethyl 4-chloroacetoacetates. The method comprises the following steps: pumping a cooled diketene solution into a falling film reactor through a metering pump, and enabling the cooled diketene solution to uniformly form a film through a distributor, and flow into the reactor from a tower top; meanwhile, controlling the flow of chlorine gas through a gas flow meter; introducing the chlorine gas into the reactor through a gas distributor; carrying out a reaction on the diketene and the chlorine gas on the surface of the falling film reactor; after the reaction is finished, enabling reaction liquid to flow out from a tower bottom and enter an esterification reaction kettle; pumping in different alcohols and overflowing into a next esterification reaction kettle to continually react; absorbing hydrogen chloride gas, which is generated in a reaction process, with water; after the reaction is finished, washing with water and neutralizing to remove the residual hydrogen chloride; distilling an organic phase to recycle a solvent and excessive alcohol raw materials to obtain a crude product; rectifying the crude product to obtain an ethyl 4-chloroacetoacetates product. With the adoption of the method, reaction time can be shortened, energy consumption is reduced, polychlorides are controlled, reaction selectivity is improved, rectification is relatively easy, and yield and purity of the product are improved.
Owner:山东德澳精细化学品有限公司
Borneol-based macromolecule antibacterial material
ActiveCN103881010AGood biocompatibilityImprove adhesionBiocidePreparation from carboxylic acid halidesBiocompatibility TestingGram-positive bacterium
Owner:北京市晟名宽科技发展有限公司
Popular searches
Carbonyl compound preparation Organic-compounds/hydrides/coordination-complexes catalysts Carboxylic acid nitrile preparation Toilet preparations Ether/acetal active ingredients Carboxylic acid amides preparation Preparation from nitriles Animal repellants Preparation by hydrolysis Amide active ingredients
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com