Ester compound, preparation method and application thereof
A compound and drug technology, applied in the field of ester compounds, its preparation and application, can solve the side effects of rhabdomyolysis syndrome and other problems, achieve the effect of low toxicity, lower cholesterol and low-density lipoprotein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
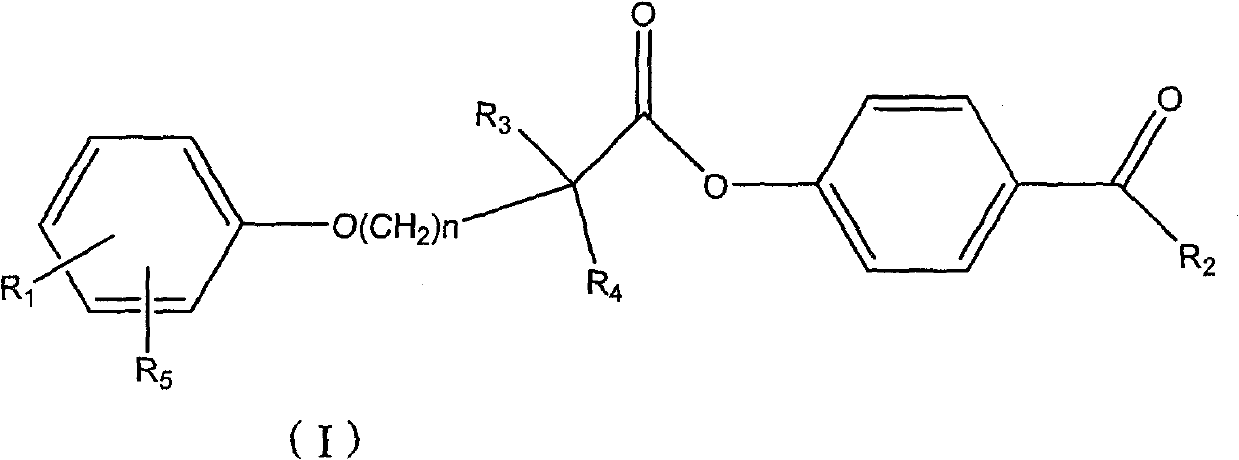
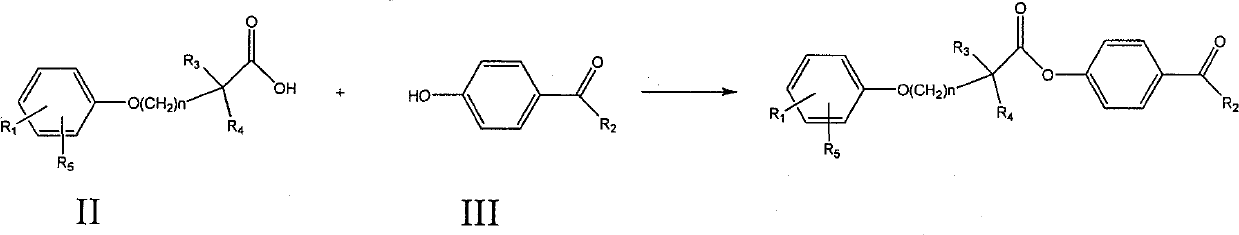
[0085] Preparation of 4-acetylphenyl 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy]-2-methylpropanoate
[0086] 2-[4-[2-(4-Chlorobenzamido)ethyl]phenoxy]-2-methylpropionic acid 1g, dicyclohexylcarbodiimide 0.57g, p-hydroxyacetophenone 0.38g , 60ml of dichloromethane was placed in a 100ml single-necked bottle, reacted at room temperature for 5 hours, evaporated to dryness, and recrystallized from ethanol to obtain 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy] - 4-acetylphenyl 2-methylpropionate 800mg. MS (ESI): 480 (M+H + ). 1 H NMR (400MHz, CDCl 3 )δ(ppm): 1.774(6H), 2.607(3H), 2.898-2.934(2H), 2.687-3.736(2H), 6.019(1H), 6.945-6.967(2H), 7.127-7.182(4H), 7.367 -7.389 (2H), 7.610-7.632 (2H), 7.981-8.003 (2H).
Embodiment 2
[0088] Preparation of 4-propionylphenyl 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy]-2-methylpropanoate
[0089] 2-[4-[2-(4-Chlorobenzamido)ethyl]phenoxy]-2-methylpropionic acid 1g, dicyclohexylcarbodiimide 0.57g, p-hydroxypropiophenone 0.40g, 60ml of dichloromethane was placed in a 100ml single-necked bottle, reacted at room temperature for 5 hours, evaporated to dryness, and recrystallized from ethanol to obtain 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy]- 4-propionylphenyl 2-methylpropionate 700mg. MS (ESI): 494 (M+H + ).
Embodiment 3
[0091] Preparation of 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy]-2-methylpropanoic acid 4-butyrylacetylphenyl ester
[0092] 2-[4-[2-(4-Chlorobenzamido)ethyl]phenoxy]-2-methylpropionic acid 1g, dicyclohexylcarbodiimide 0.57g, p-hydroxybutyrophenone 0.38 g, 60ml of dichloromethane was placed in a 100ml single-necked bottle, reacted at room temperature for 5 hours, evaporated to dryness, and recrystallized from ethanol to obtain 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy ]-4-butyrylphenyl 2-methylpropionate 600 mg. MS (ESI): 508 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com