Patents
Literature
185902results about "Organic active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
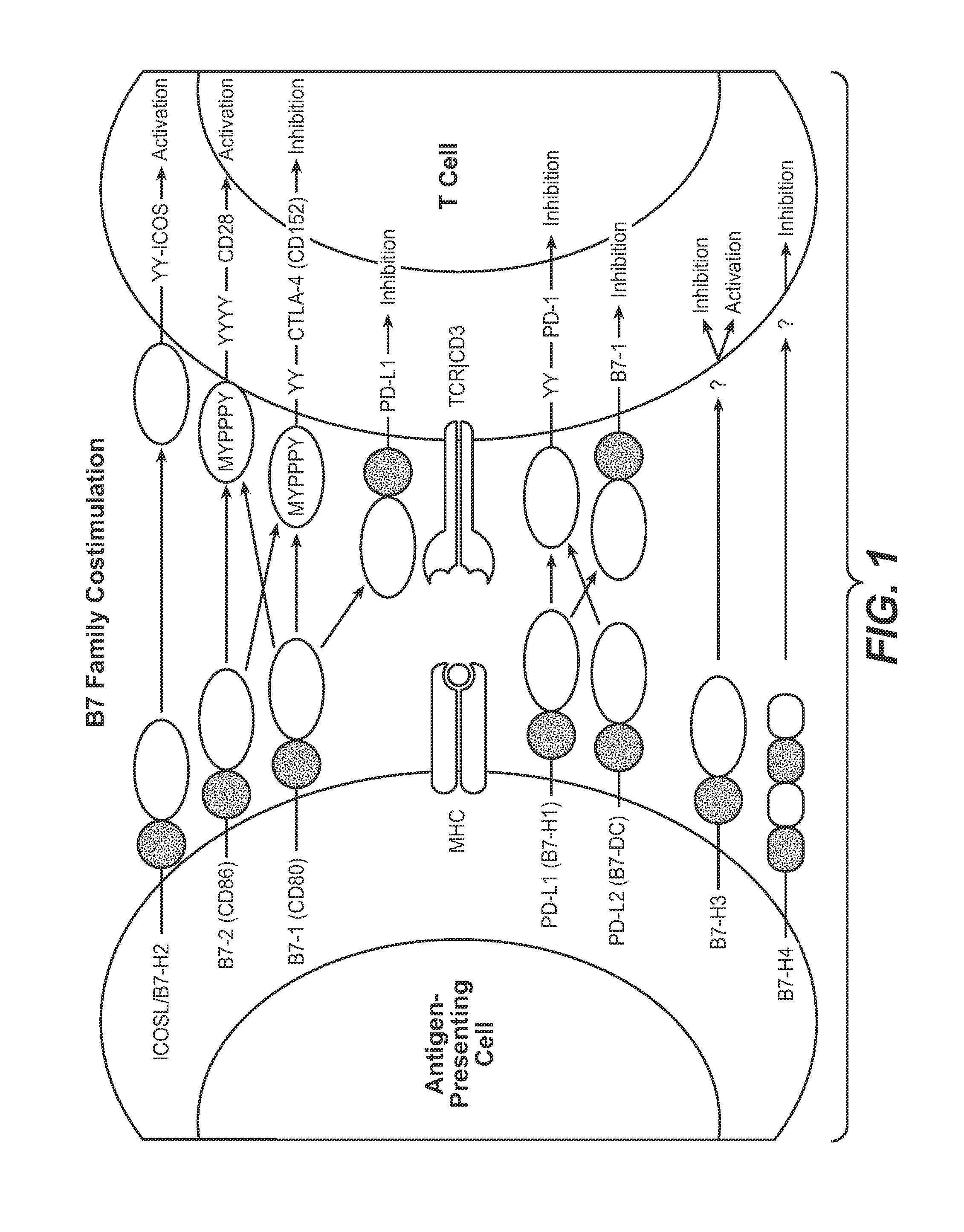
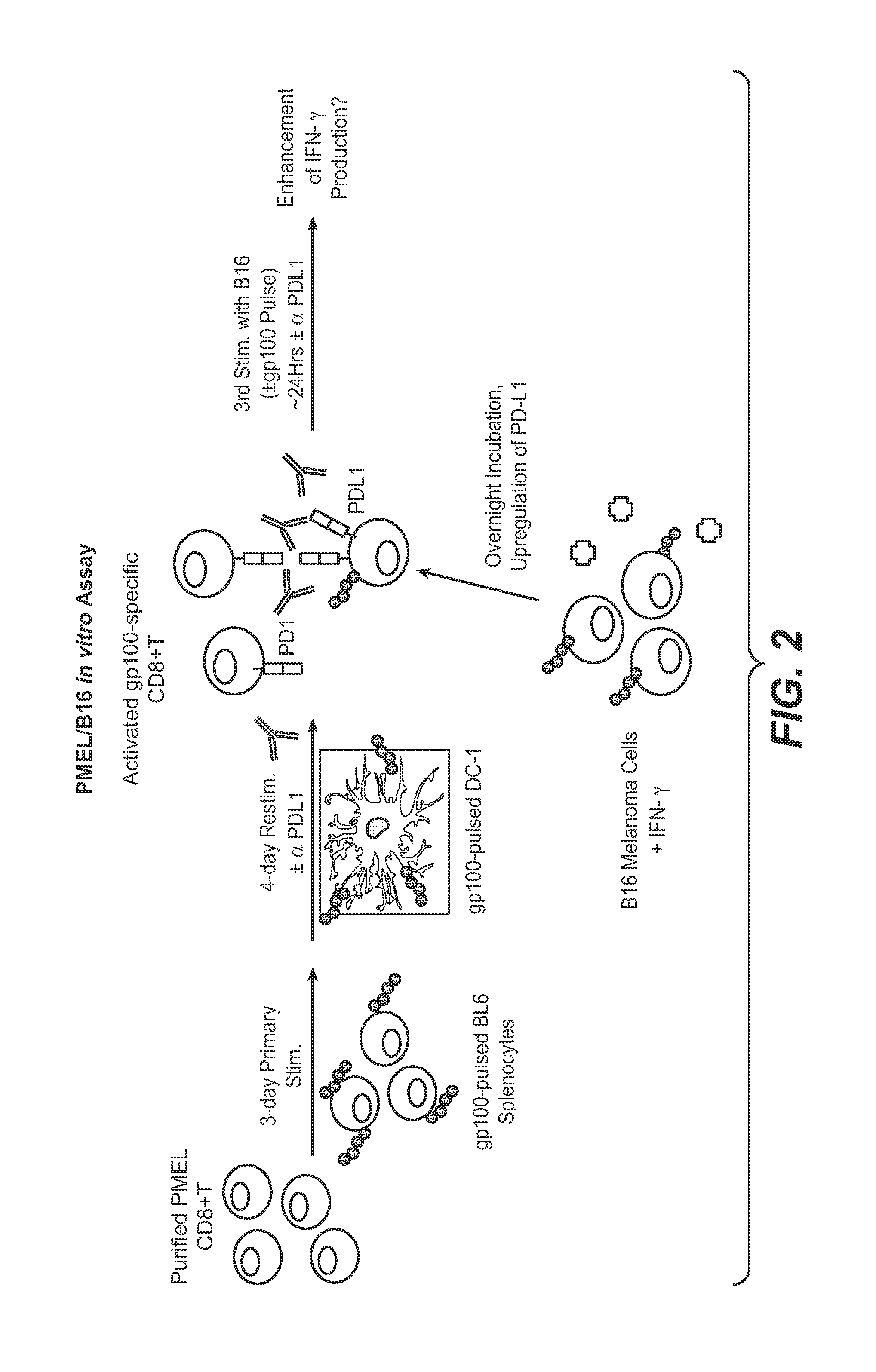
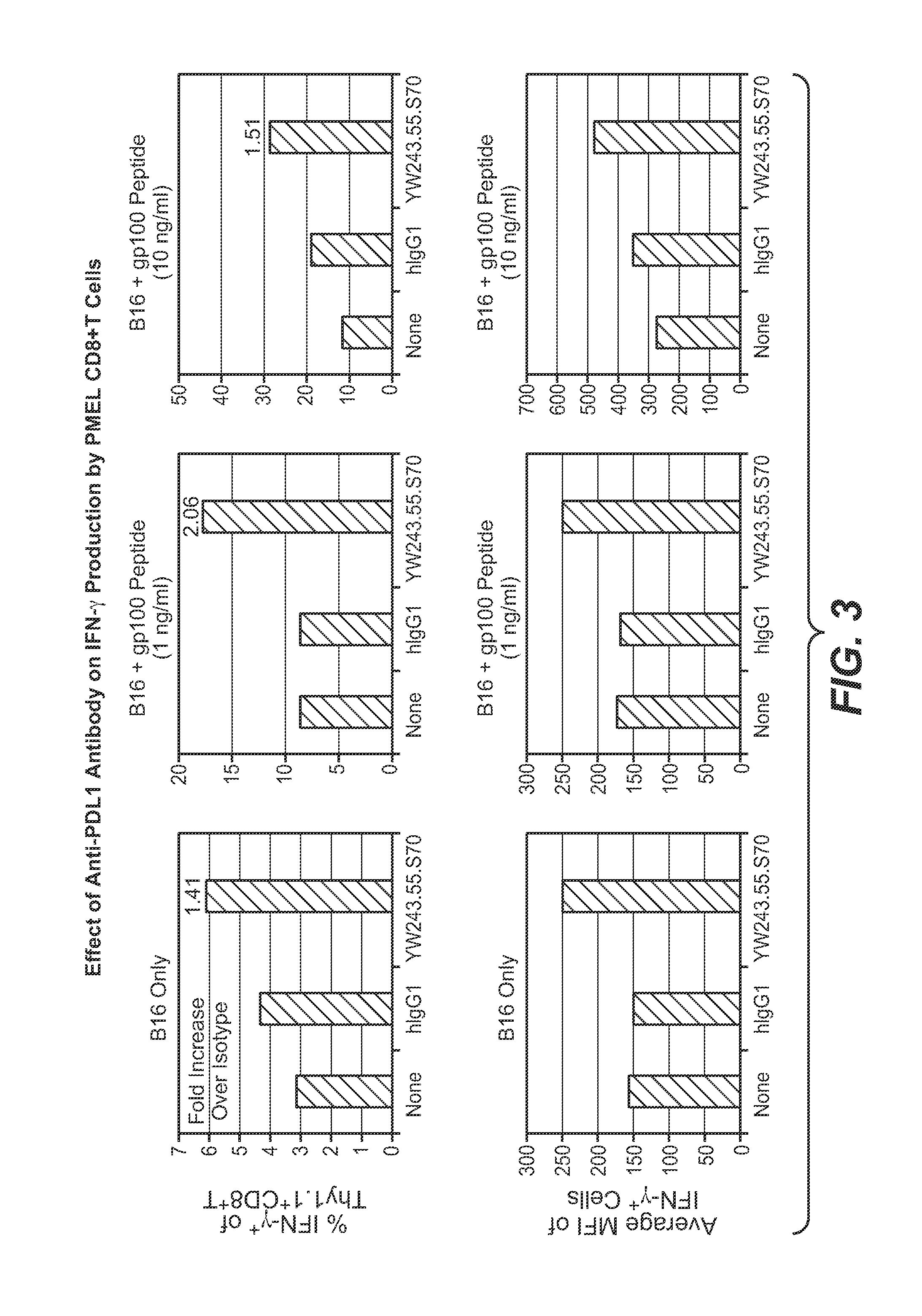
Anti-PD-L1 antibodies, compositions and articles of manufacture
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
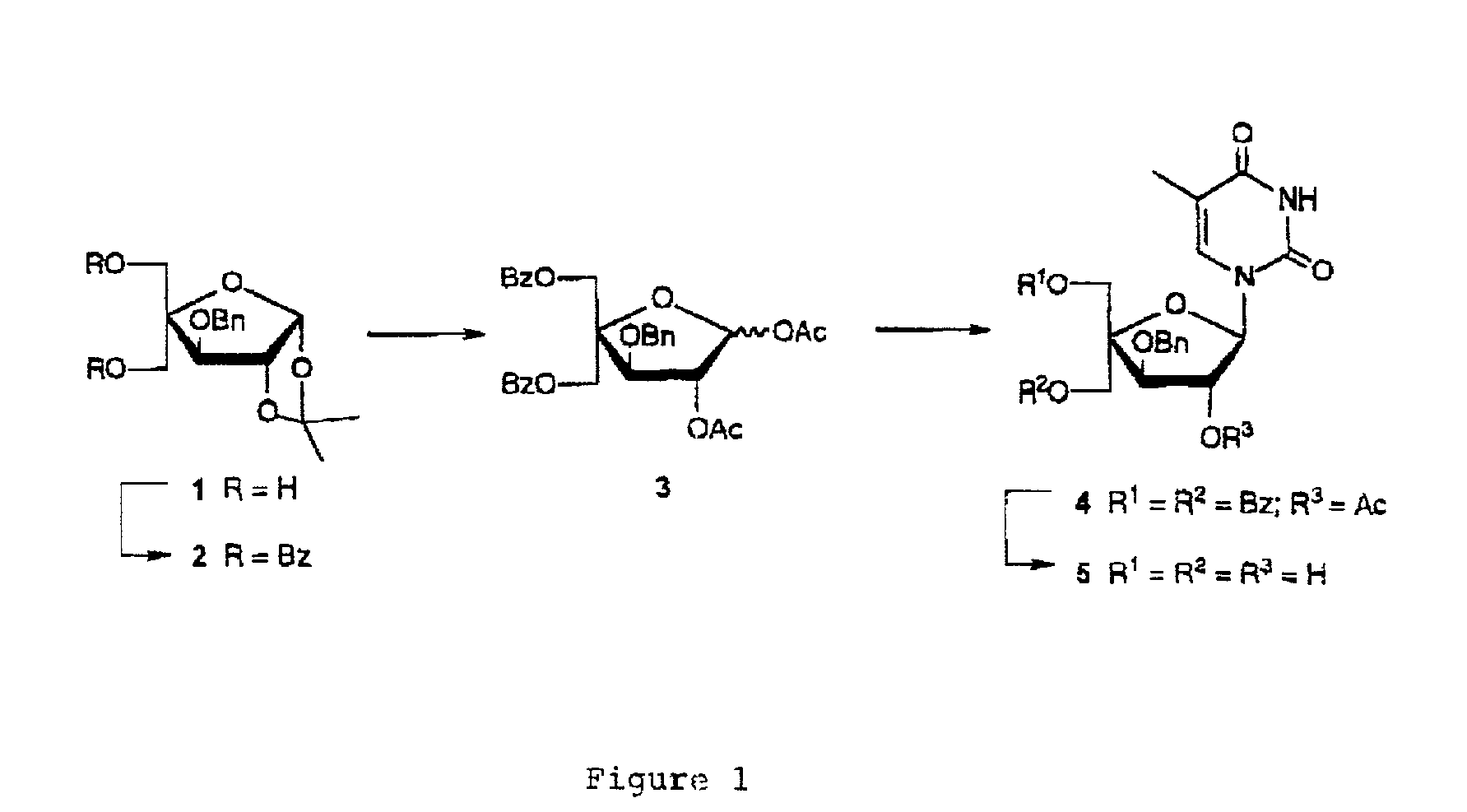
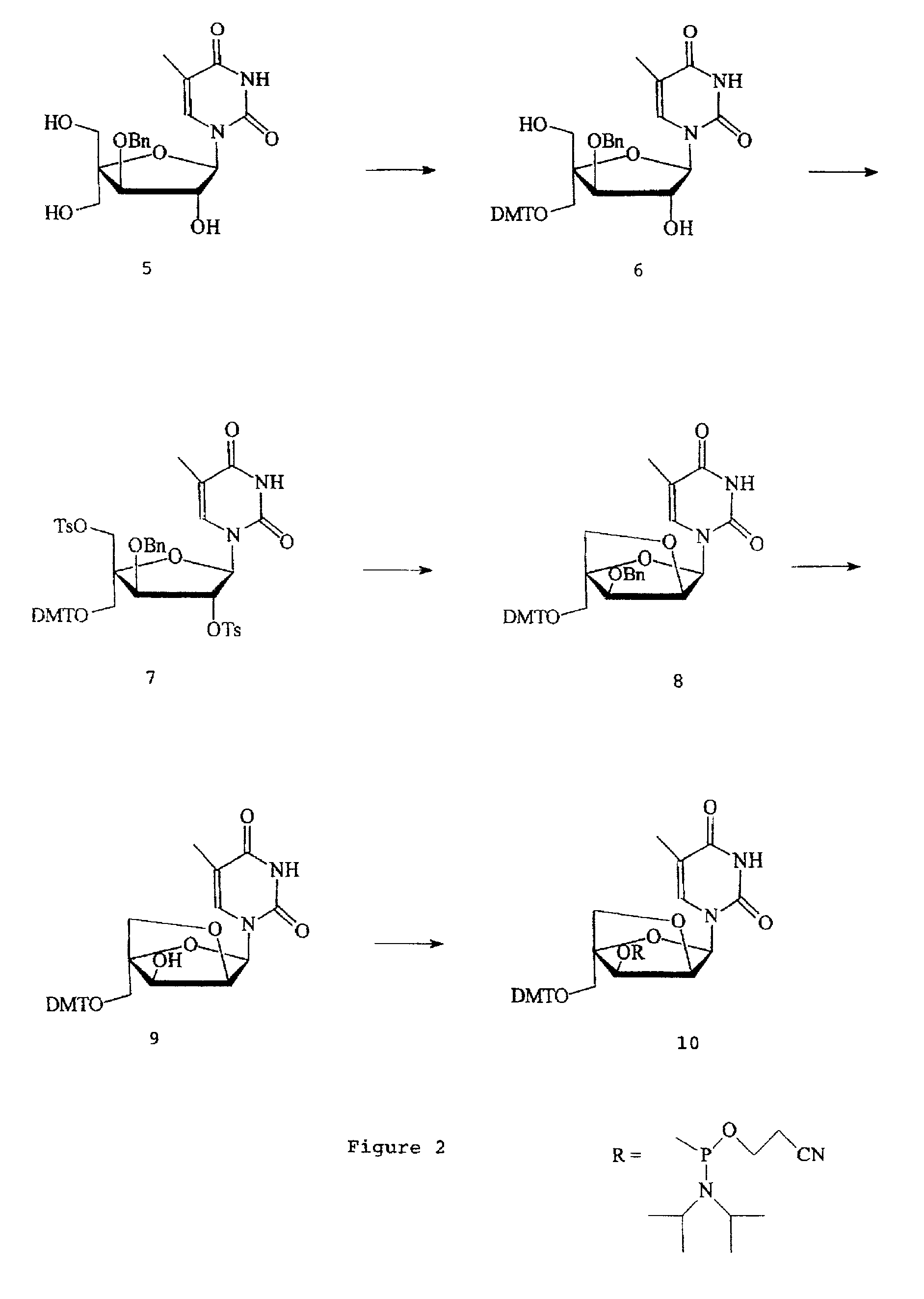
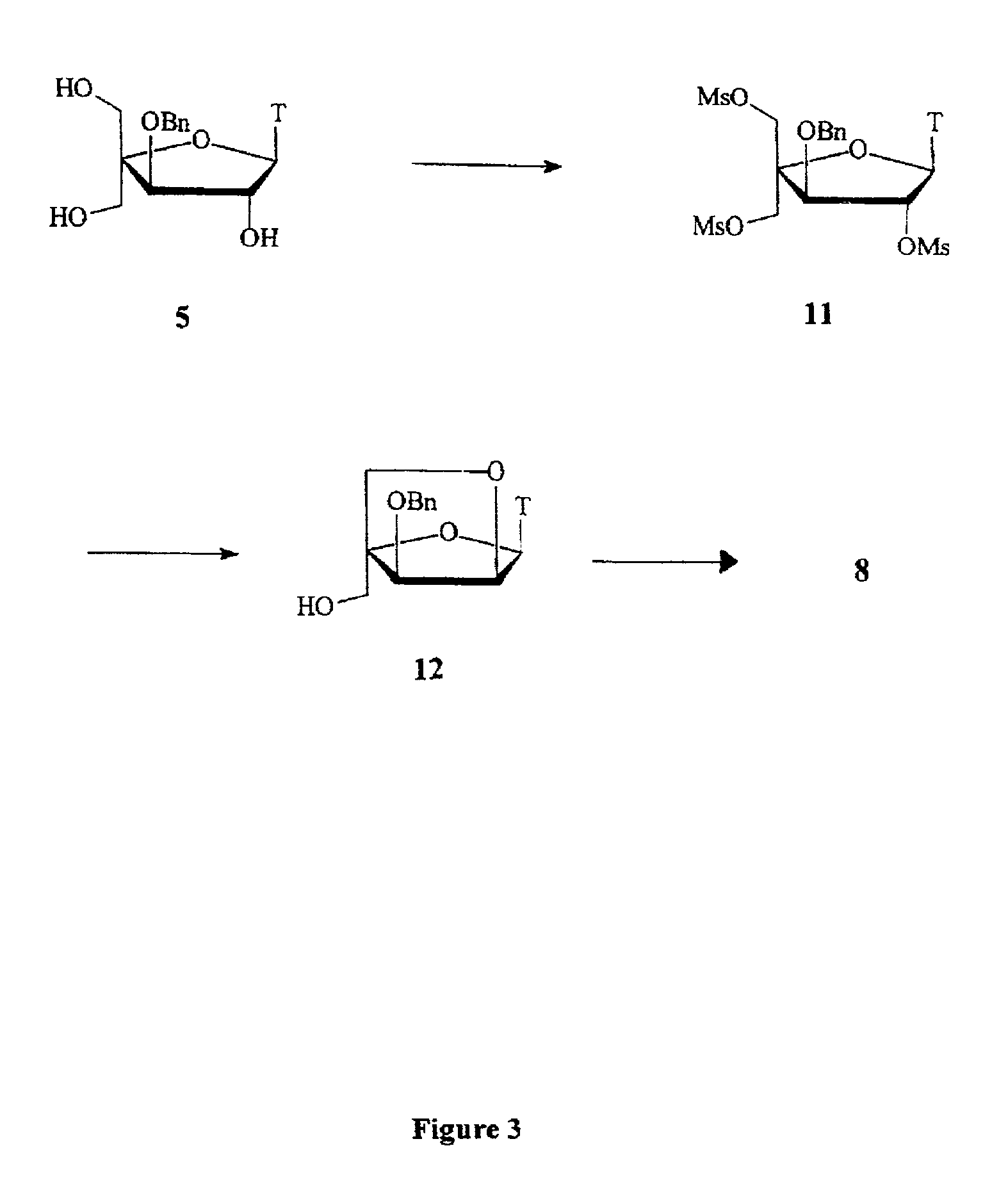
L-ribo-LNA analogues
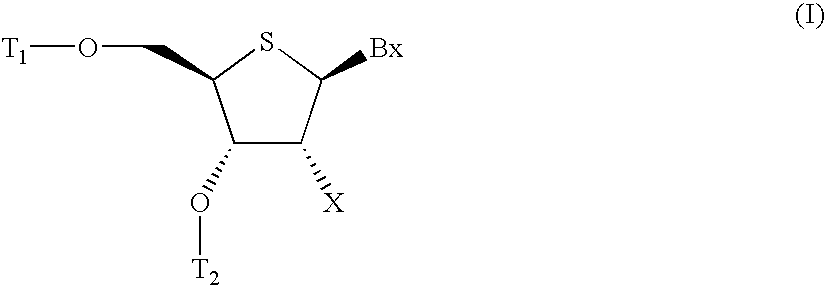
Provided are L-ribo bicyclic nucleotide compounds as well as syntheses of such compounds. The nucleoside compounds of the invention are useful in forming oligonucleotides that can produce nucleobase specific duplexes with complementary single stranded and double stranded nucleic acids.
Owner:SANTARIS PHARMA AS
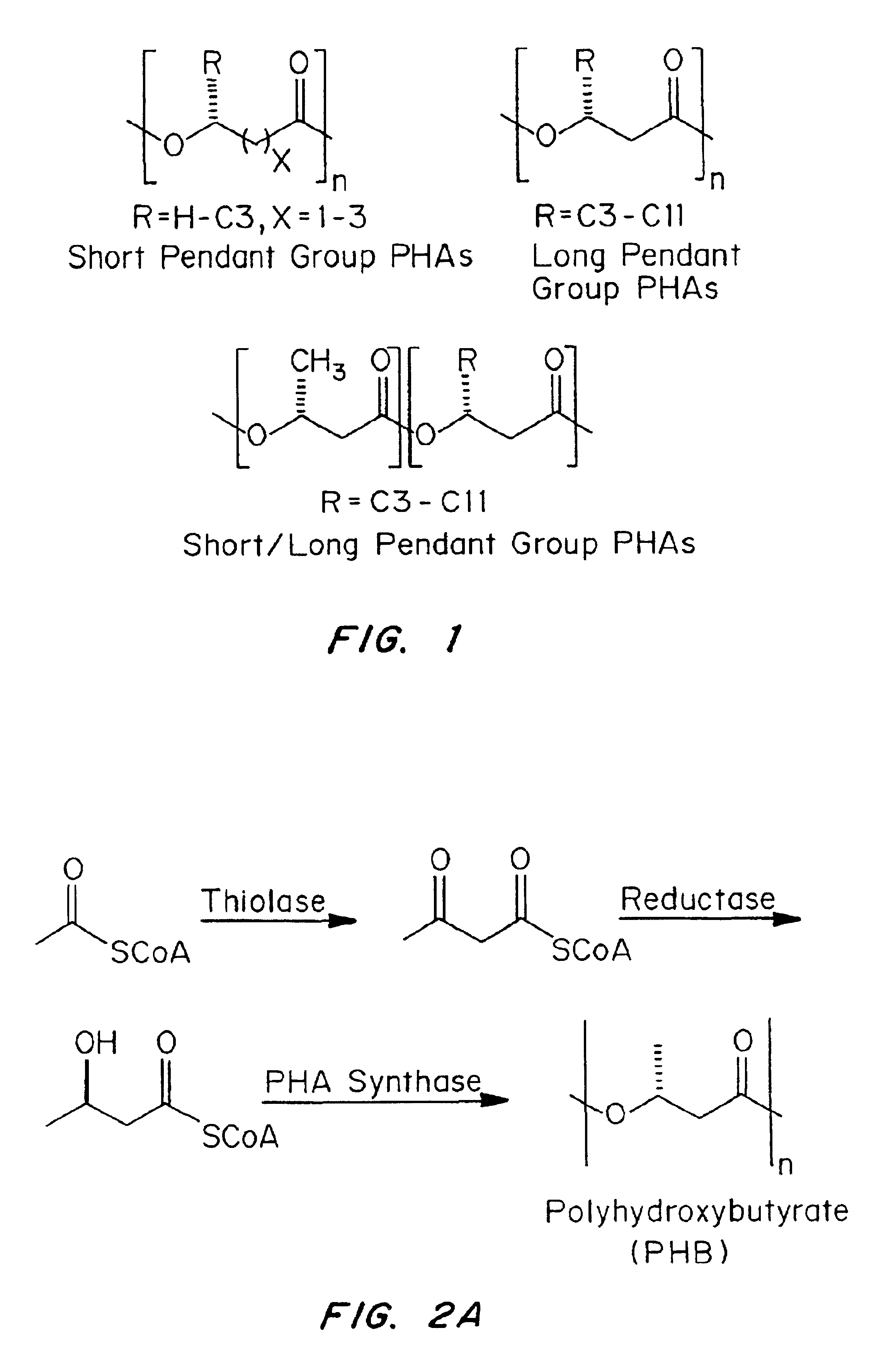
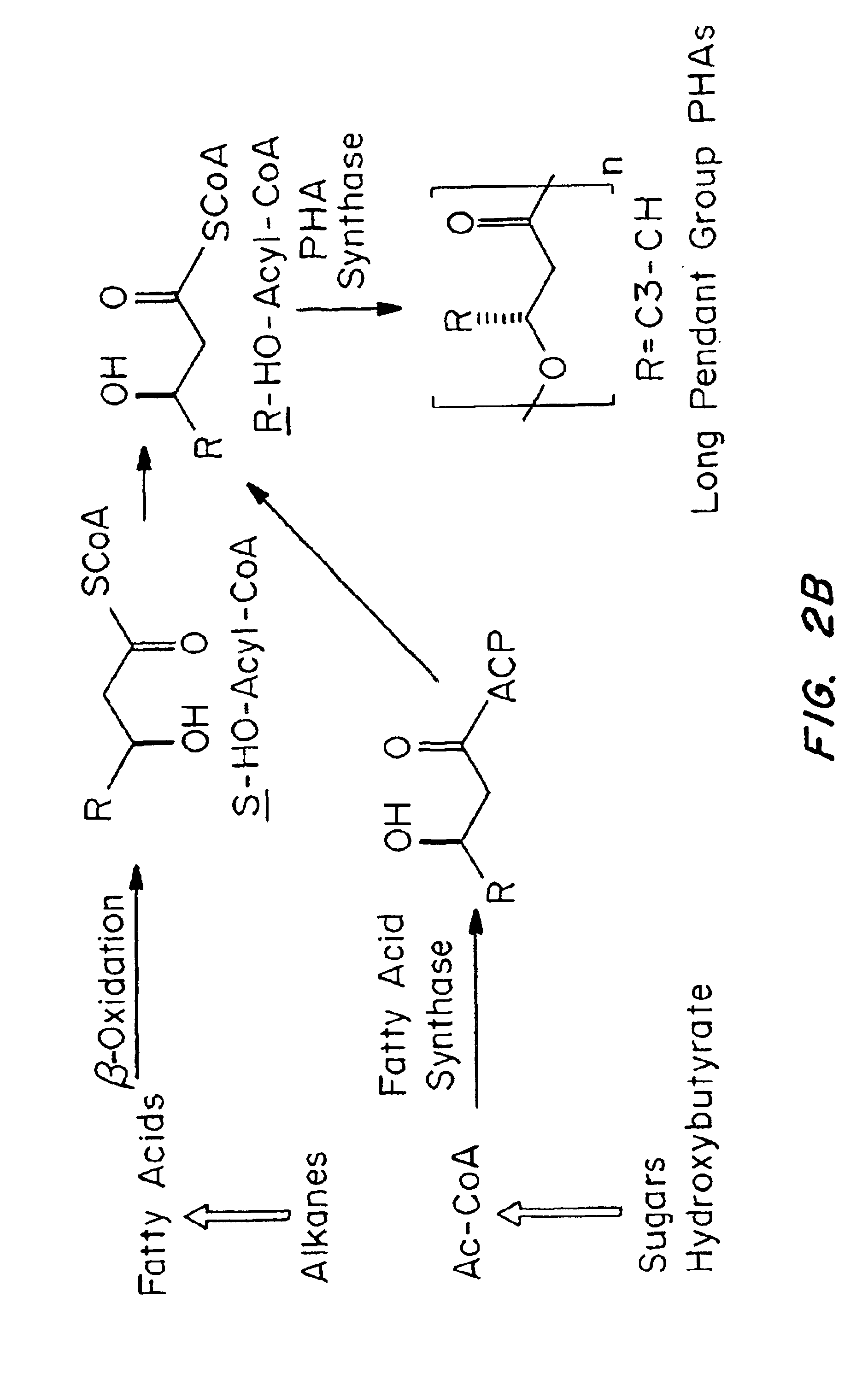
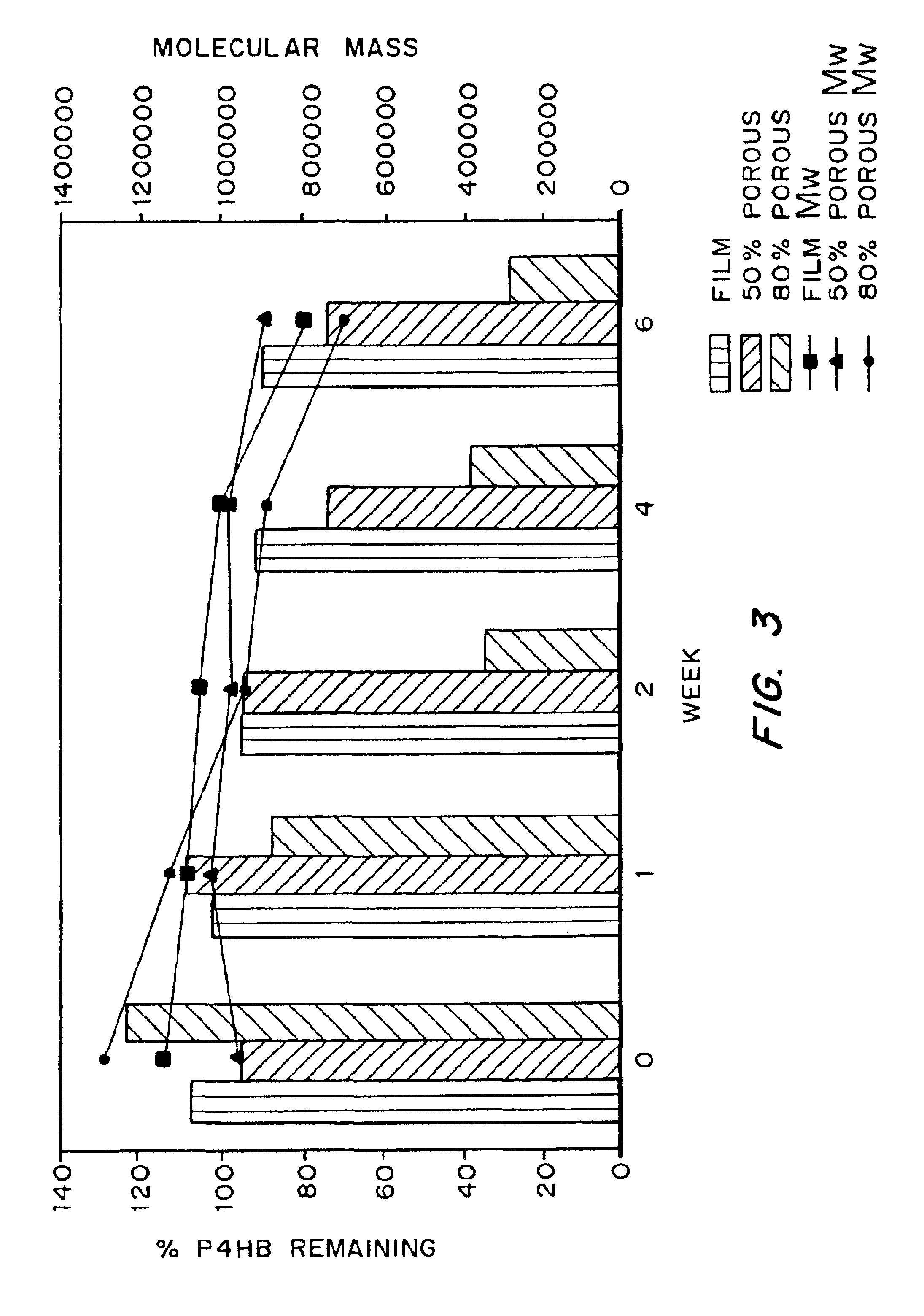
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Oligonucleotide analogues
Novel oligomers, and synthesis thereof, comprising one or more bi-, tri, or polycyclic nucleoside analogues are disclosed herein. The nucleoside analogues have a “locked” structure, termed Locked Nucleoside Analogues (LNA). LNA's exhibit highly desirable and useful properties. LNA's are capable of forming nucleobase specific duplexes and triplexes with single and double stranded nucleic acids. These complexes exhibit higher thermostability than the corresponding complexes formed with normal nucleic acids. The properties of LNA's allow for a wide range of uses such as diagnostic agents and therapeutic agents in a mammal suffering from or susceptible to, various diseases.
Owner:EXIQON AS
Reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
InactiveUS6852330B2Sufficient structural integritySufficient propertyPowder deliveryOrganic active ingredientsTissue repairSoft tissue repair
A biocompatible tissue repair stimulating implant or “scaffold” device, and methods for making and using such a device, are provided. The implant includes one or more layers of a bioabsorbable polymeric foam having pores with an open cell pore structure. A reinforcement component is also present within the implant to contribute enhanced mechanical and handling properties. The implant houses a biological component that may be released to tissue adjacent the location in which the implant is implanted to faciliate and / or expedite the healing of tissue. This biological component resides primarily within the foam component of the implant, being incorporated within pores formed within the foam.
Owner:DEPUY SYNTHES PROD INC
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Avenanthramide-containing compositions
Methods and compositions for treating or preventing a skin condition, an inflammation, an irritation or an allergy associated with an ectoparasitic infection or infestation on an animal. The methods involve applying to the skin of the animal a pharmaceutical composition that contains a therapeutically effective amount of one or more than one avenanthramide, an optional ecto and / or endo-parasiticidal agent, and a pharmaceutically acceptable diluent or carrier
Owner:CEAPRO
Energetically-controlled delivery of biologically active material from an implanted medical device
InactiveUS7101394B2Facilitated releaseReduce deliveryOrganic active ingredientsElectrotherapyMedical deviceBiomedical engineering
A medical device and system capable of providing on-demand delivery of biologically active material to a body lumen patient, and a method of making such medical device. A first coating layer comprising a biologically active material and optionally a polymeric material is disposed on the surface of the medical device. A second coating layer comprising magnetic particles and a polymeric material is disposed on the first coating layer. The second coating layer, which is substantially free of a biologically active material, protects the biologically active material prior to delivery. The system includes the medical device and a source of energy, such as an electromagnetic or mechanical vibrational energy. When the patient is exposed to the energy source, the magnetic particles move out of the second coating layer and create channels therein through which the biologically active material can be released.
Owner:BOSTON SCI SCIMED INC
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Methods and compositions for selecting siRNA of improved functionality
InactiveUS20050255487A1Improve efficiencyGood curative effectOrganic active ingredientsGenetic material ingredientsGene silencingSilencing gene
Efficient sequence specific gene silencing is possible through the use of siRNA technology. By selecting particular siRNAs by rational design, one can maximize the generation of an effective gene silencing reagent, as well as methods for silencing genes. Methods, compositions, and kits generated through rational design of siRNAs are disclosed.
Owner:THERMO FISHER SCIENTIFIC INC
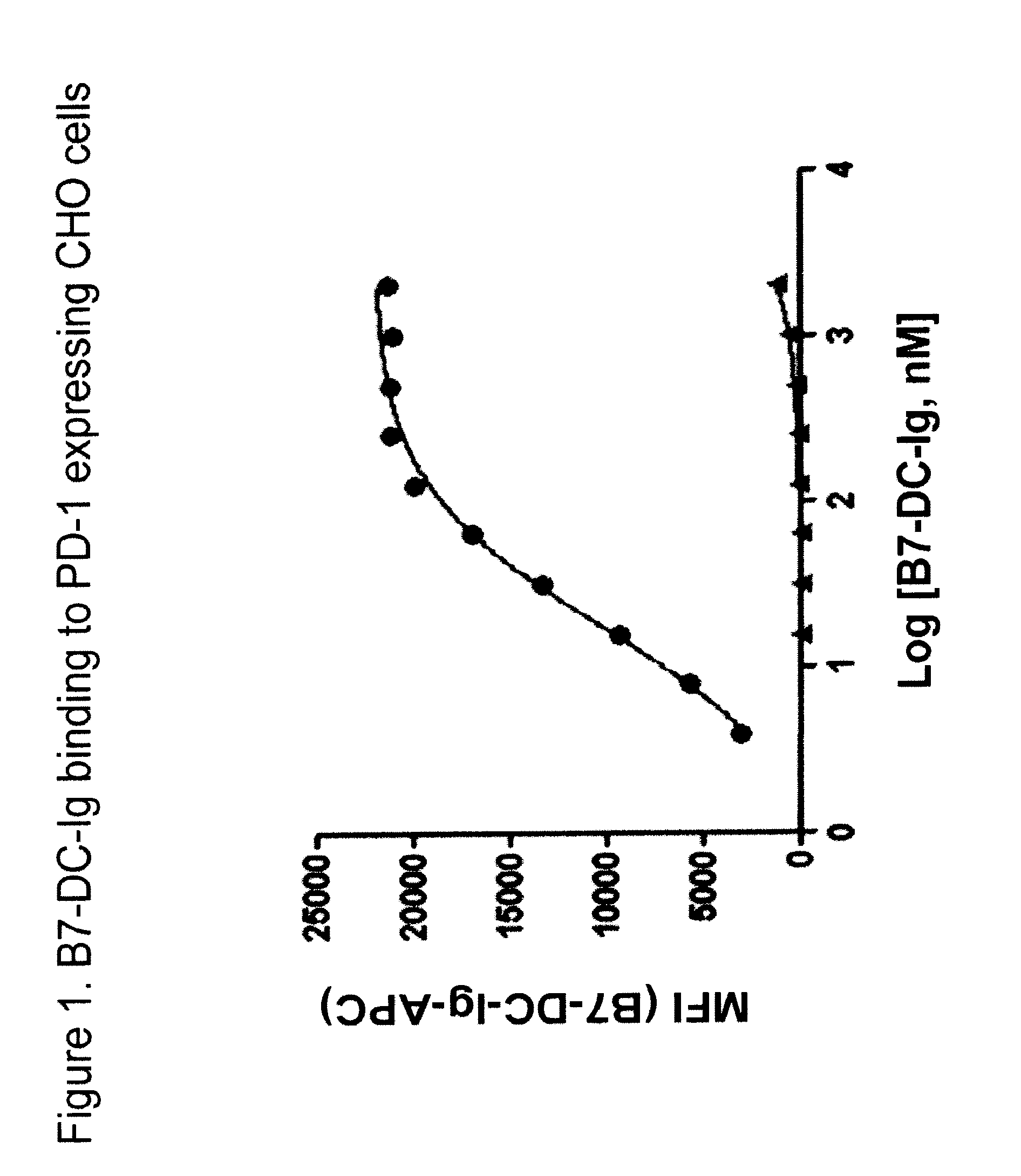
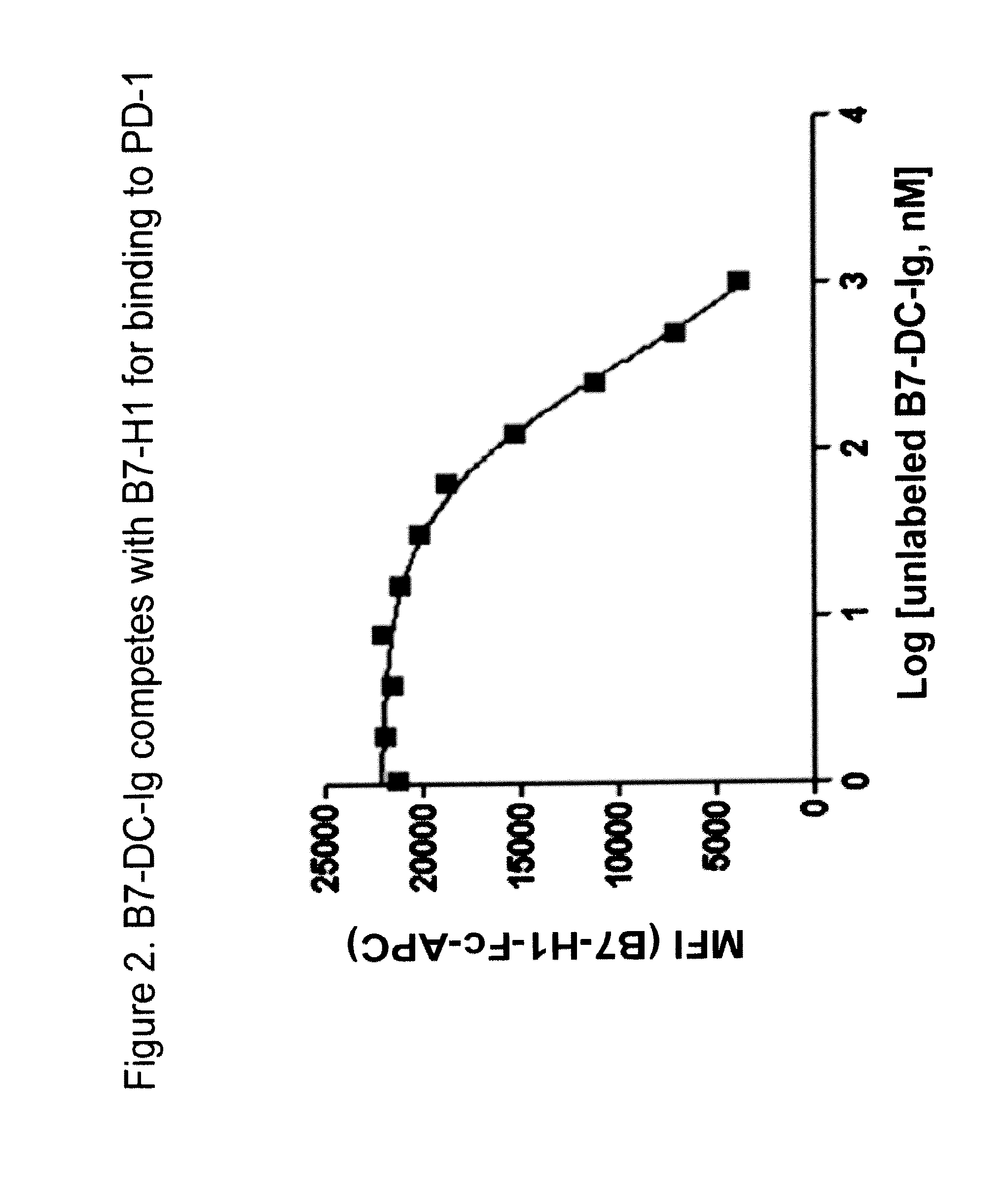
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114649A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsT cellInfective disorder
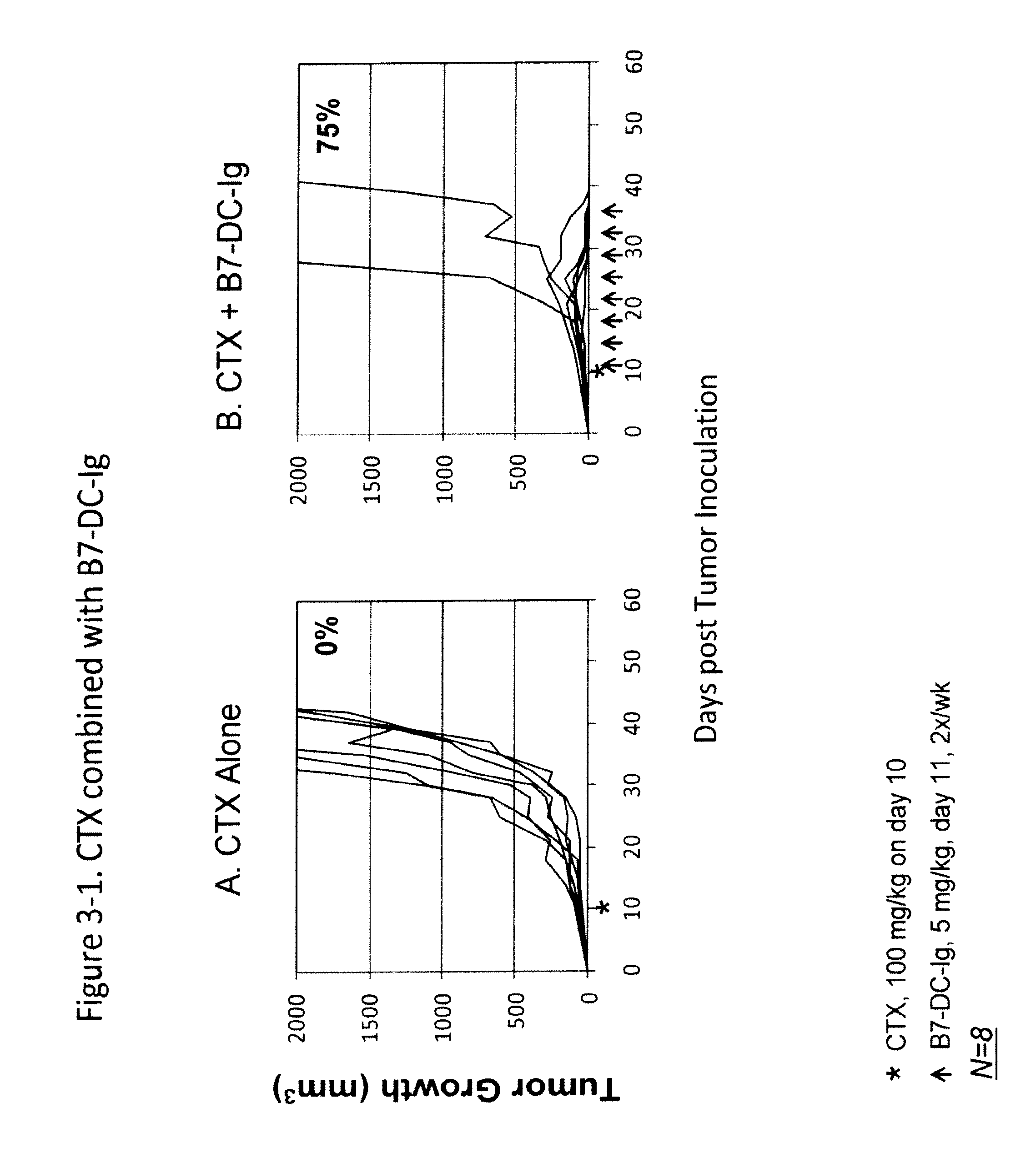
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
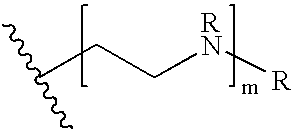
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
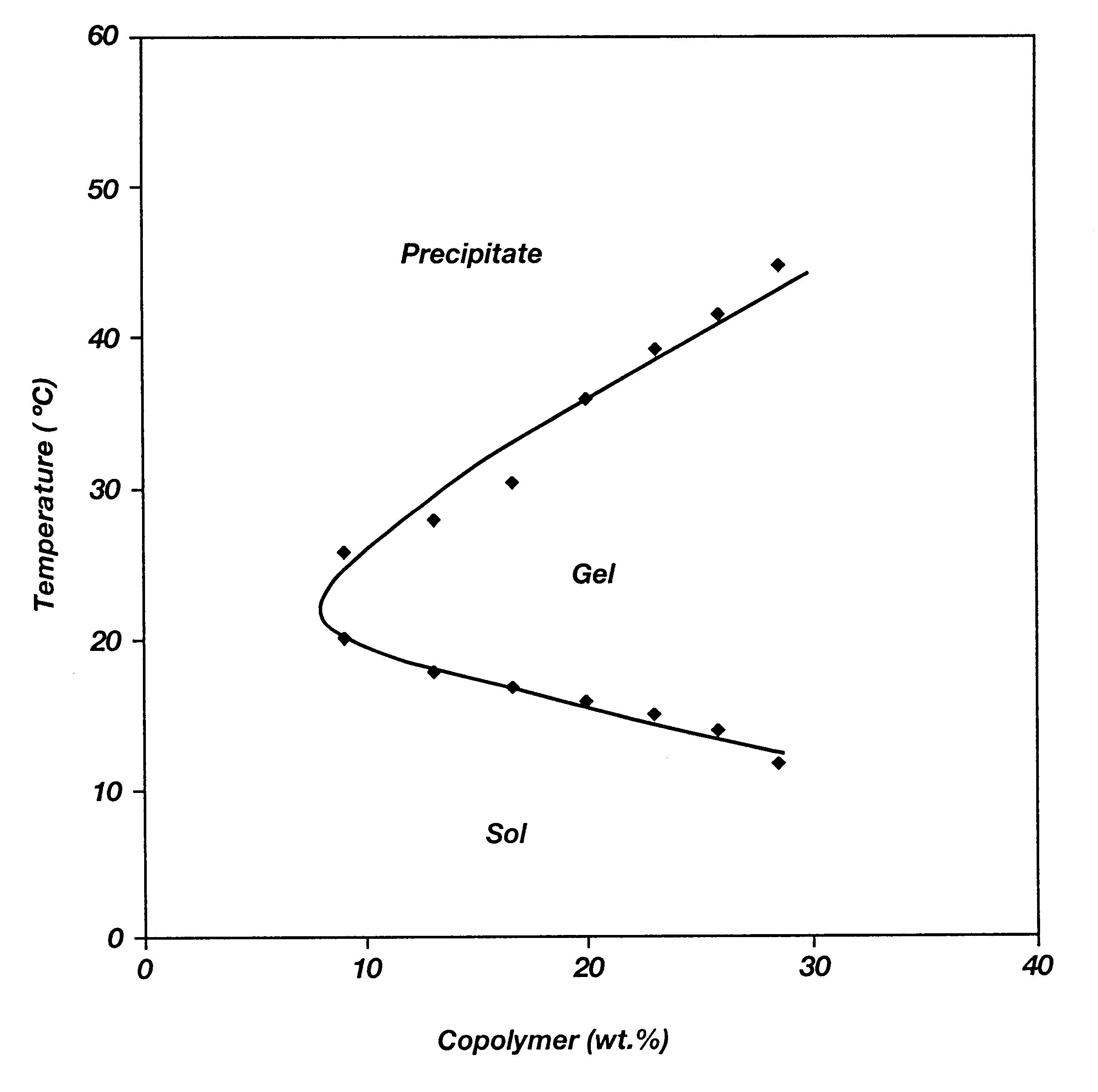
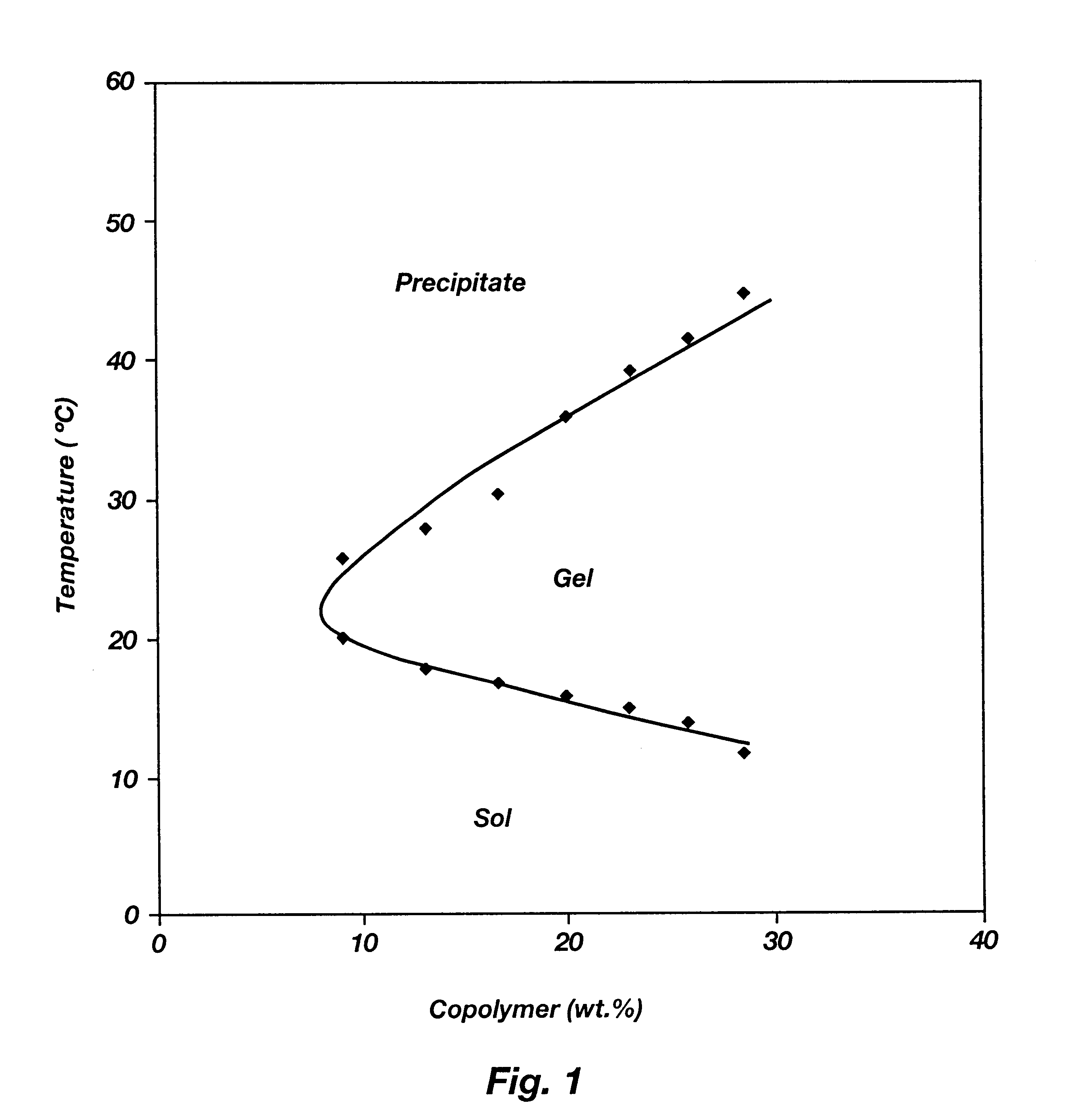
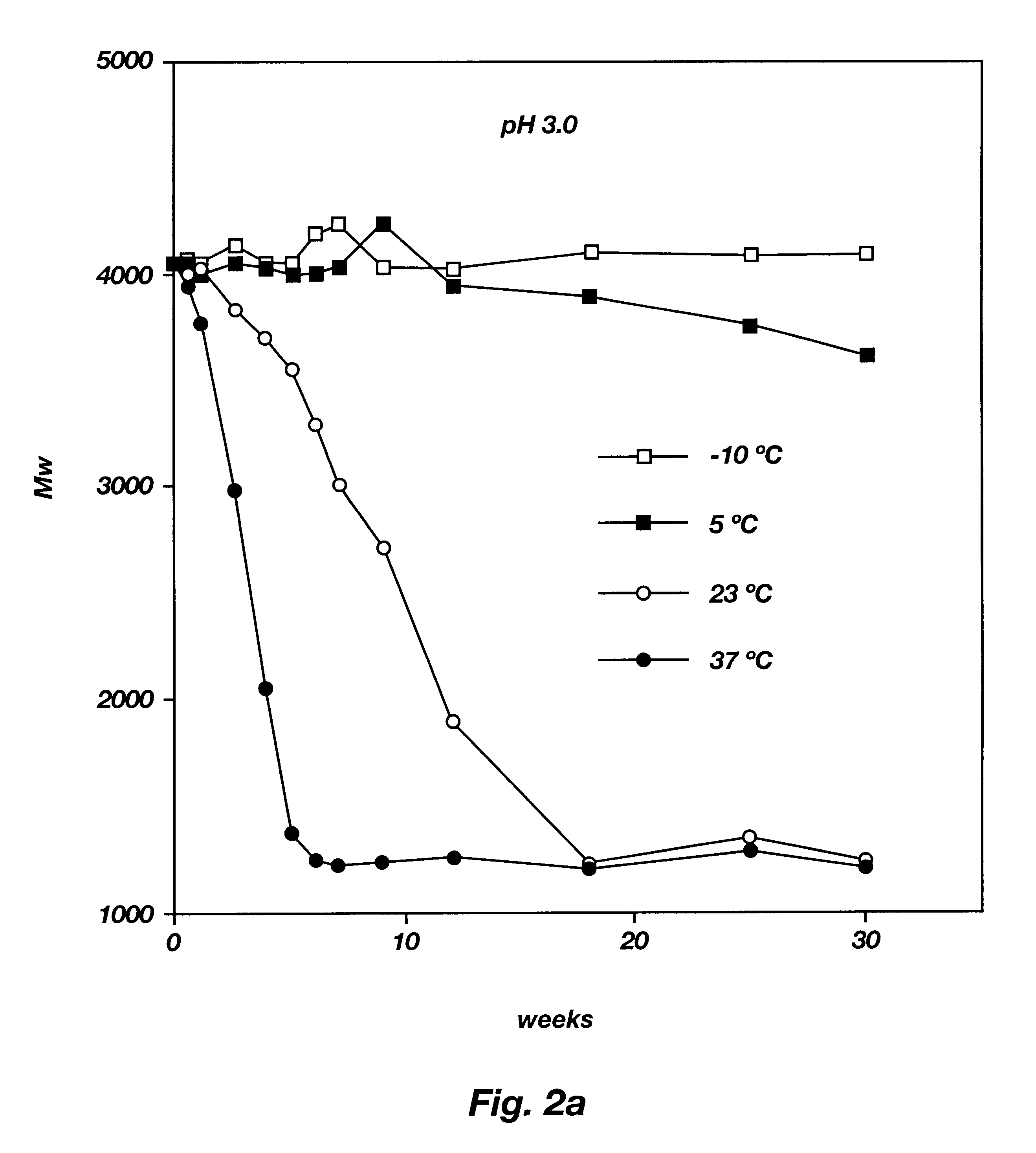
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Tissue electroperforation for enhanced drug delivery
The present invention relates to a method and a device for transporting a molecule through a mammalian barrier membrane of at least one layer of cells comprising the steps of: ablating the membrane with an electric current from a treatment electrode; and utilizing a driving force to move the molecule through the perforated membrane.
Owner:LIFESCAN INC
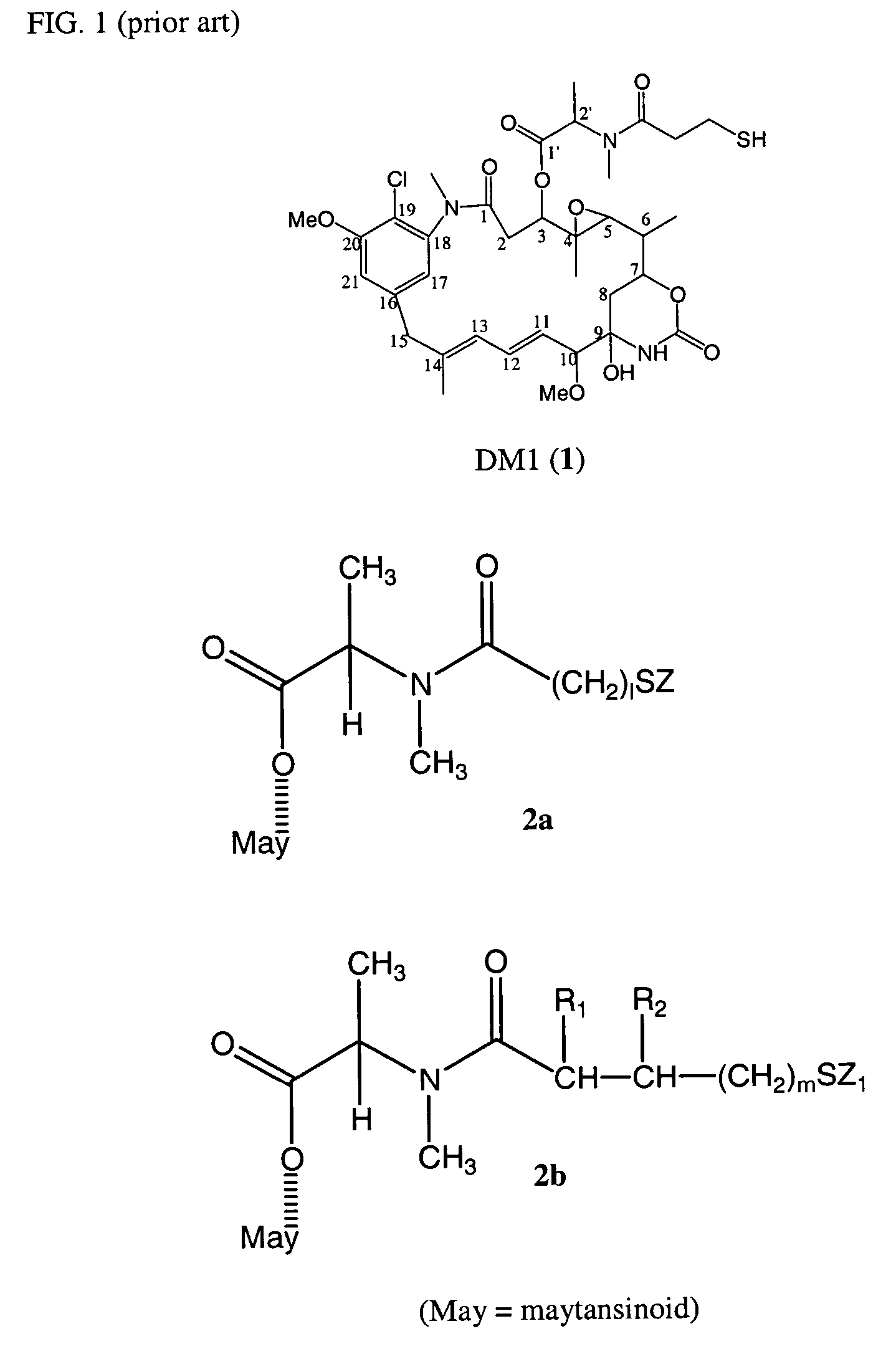
Cytotoxic agents comprising new maytansinoids
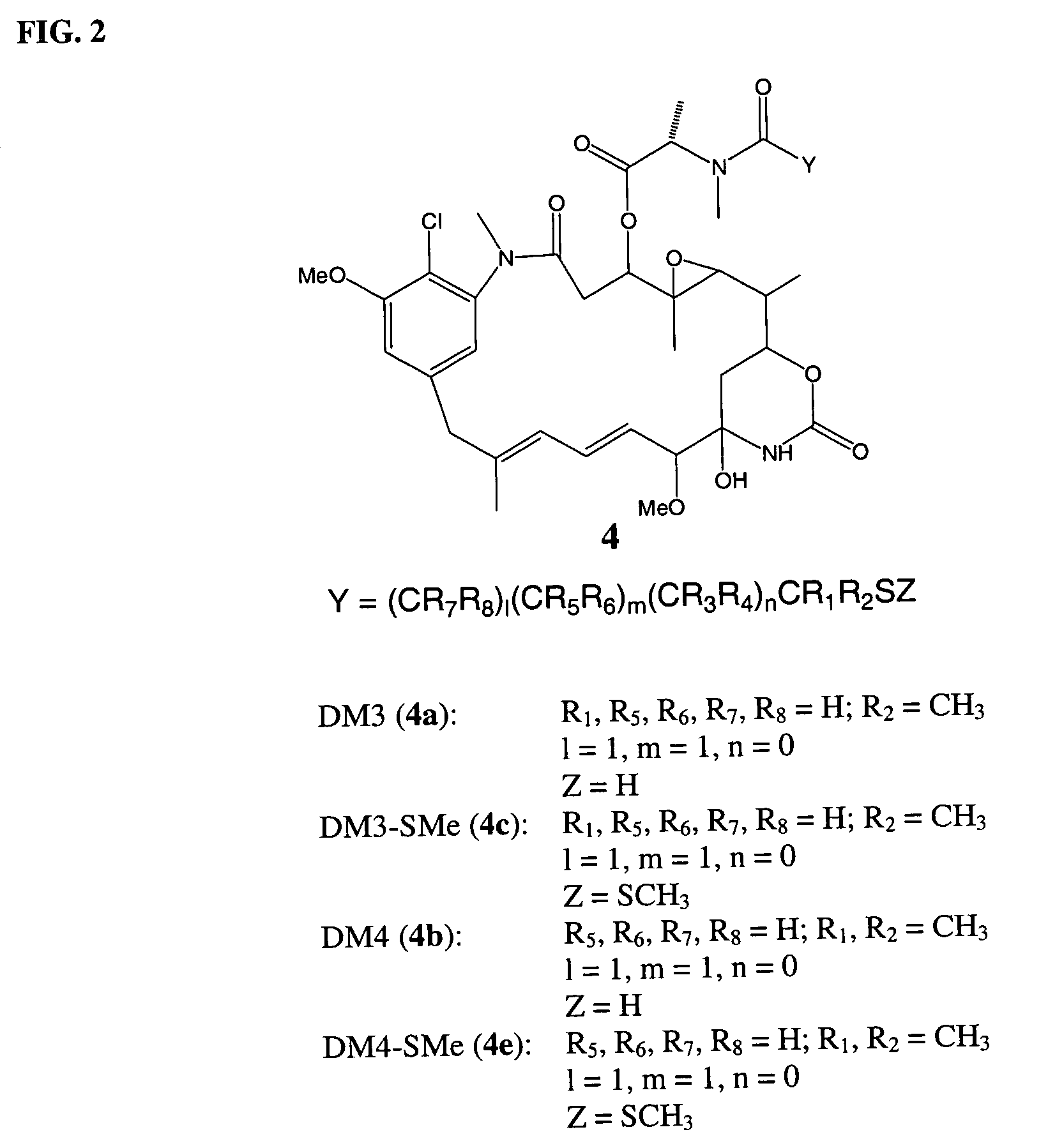
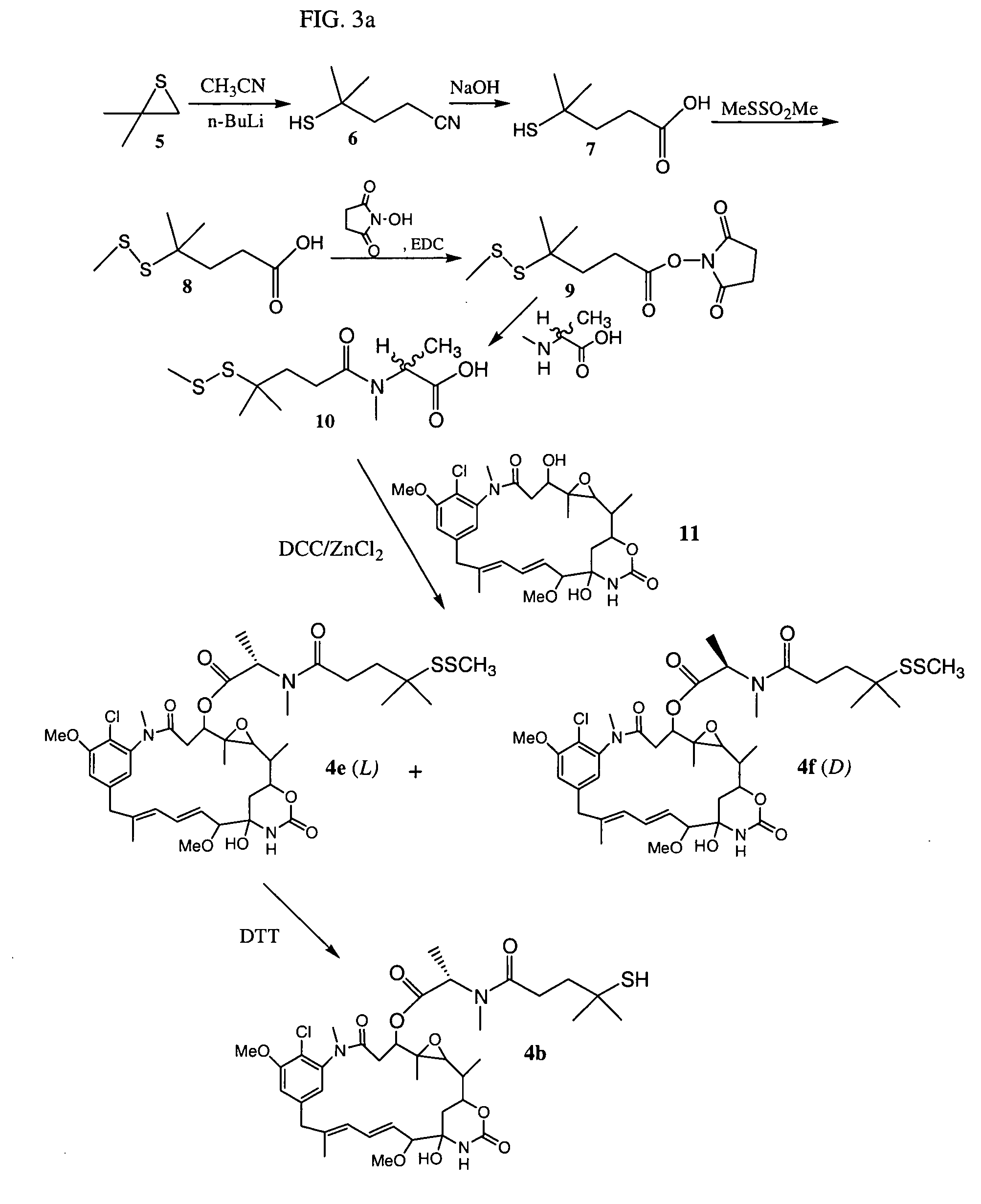
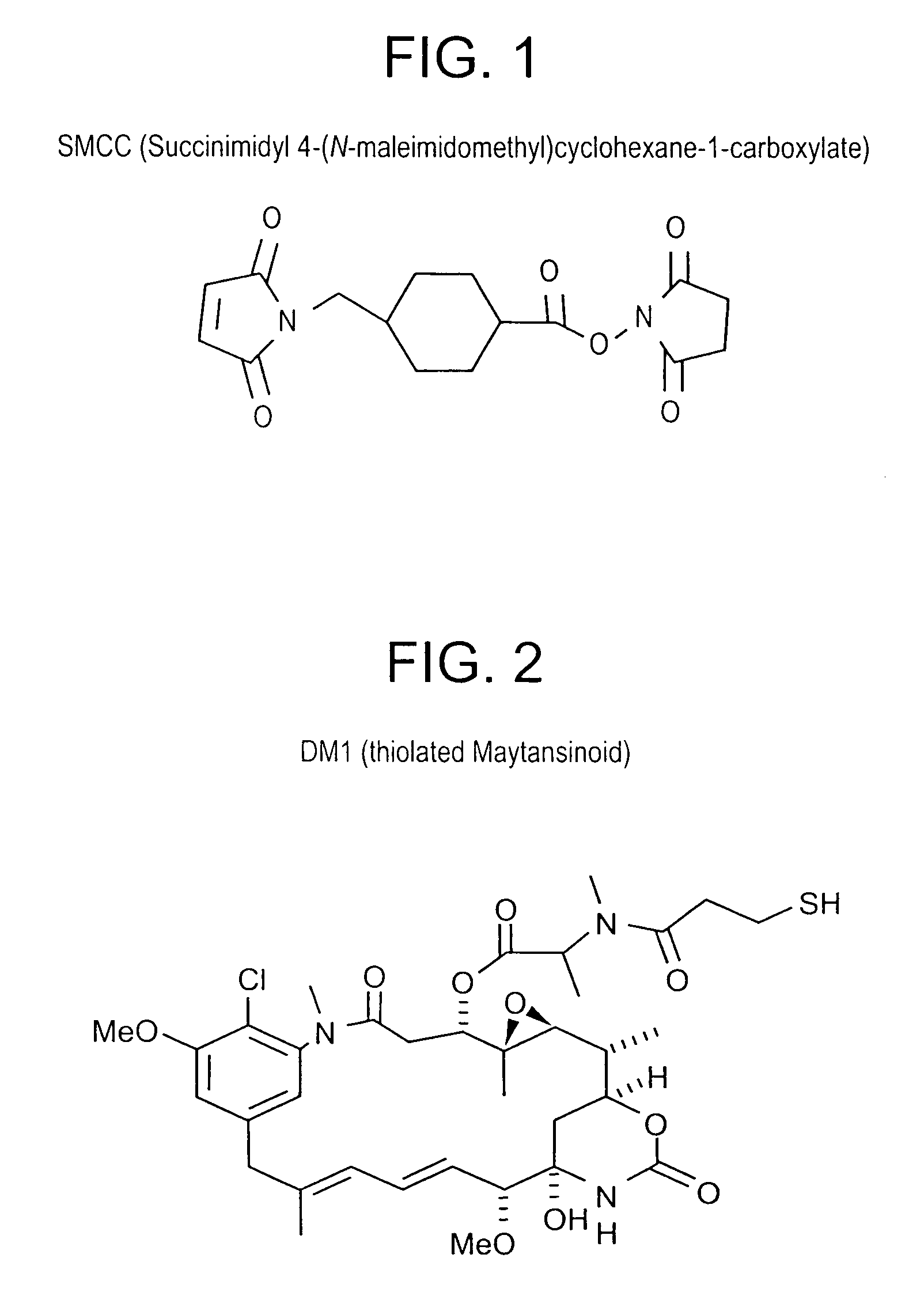
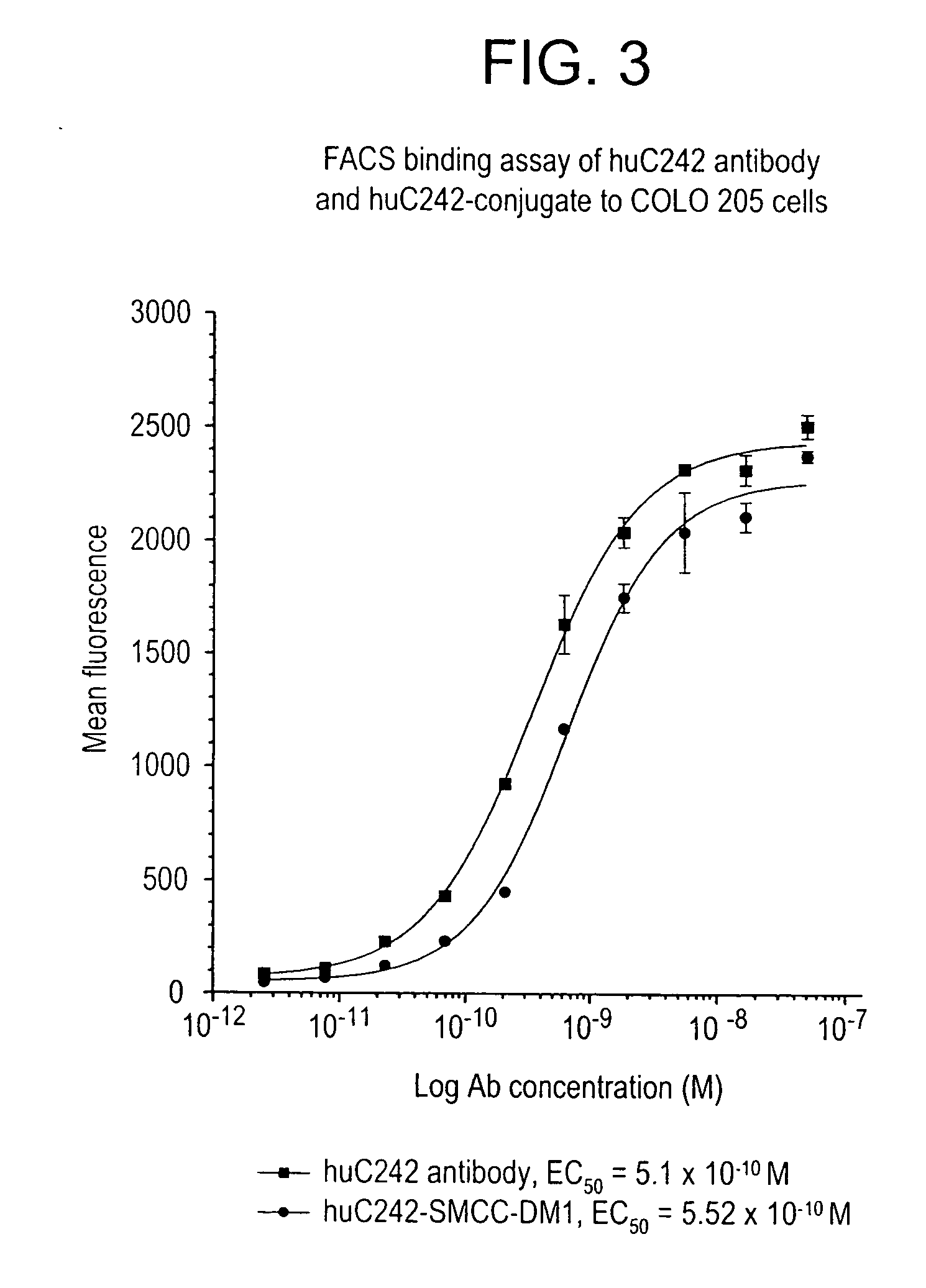
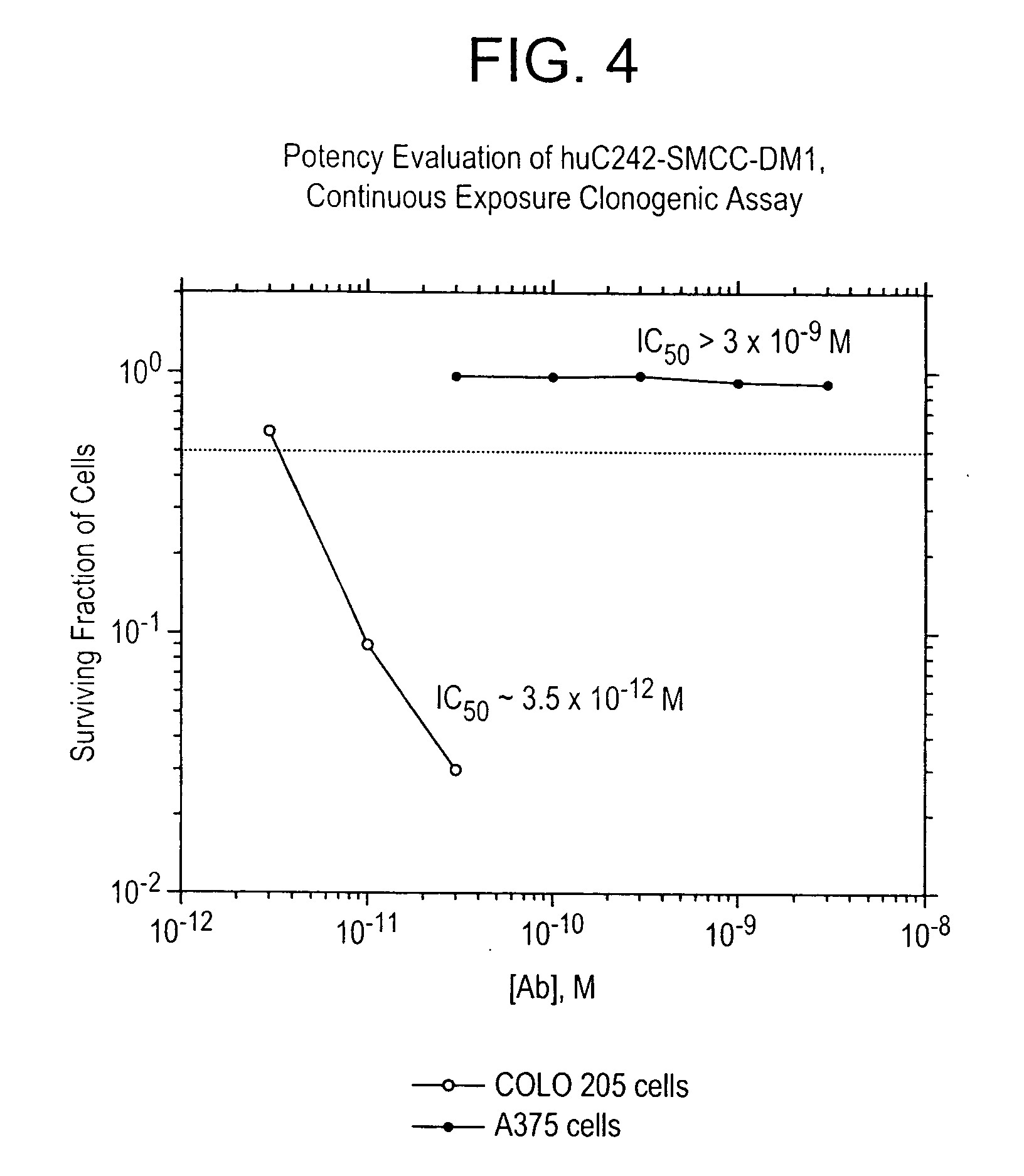
ActiveUS7276497B2Improve anti-tumor activityImprove biological activityOrganic active ingredientsOrganic chemistryAnimal tumorEfficacy
New thiol and disulfide-containing maytansinoids bearing a mono or di-alkyl substitution on the α-carbon atom bearing the sulfur atom are disclosed. Also disclosed are methods for the synthesis of these new maytansinoids and methods for the linkage of these new maytansinoids to cell-binding agents. The maytansinoid-cell-binding agent conjugates are useful as therapeutic agents, which are delivered specifically to target cells and are cytotoxic. These conjugates display vastly improved therapeutic efficacy in animal tumor models compared to the previously described agents.
Owner:IMMUNOGEN INC
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Antimicrobial polymer compositions and the use thereof
An antimicrobial composition comprising: a complex of an anionic polyester with an antimicrobial cationic surfactant, wherein the anionic polyester has at least one carboxylic group. A medical device having an antimicrobial composition comprising: a complex of an anionic polyester with an antimicrobial cationic surfactant wherein the anionic polyester has at least one carboxylic group.
Owner:ETHICON INC
Multiparticulate modified release composition
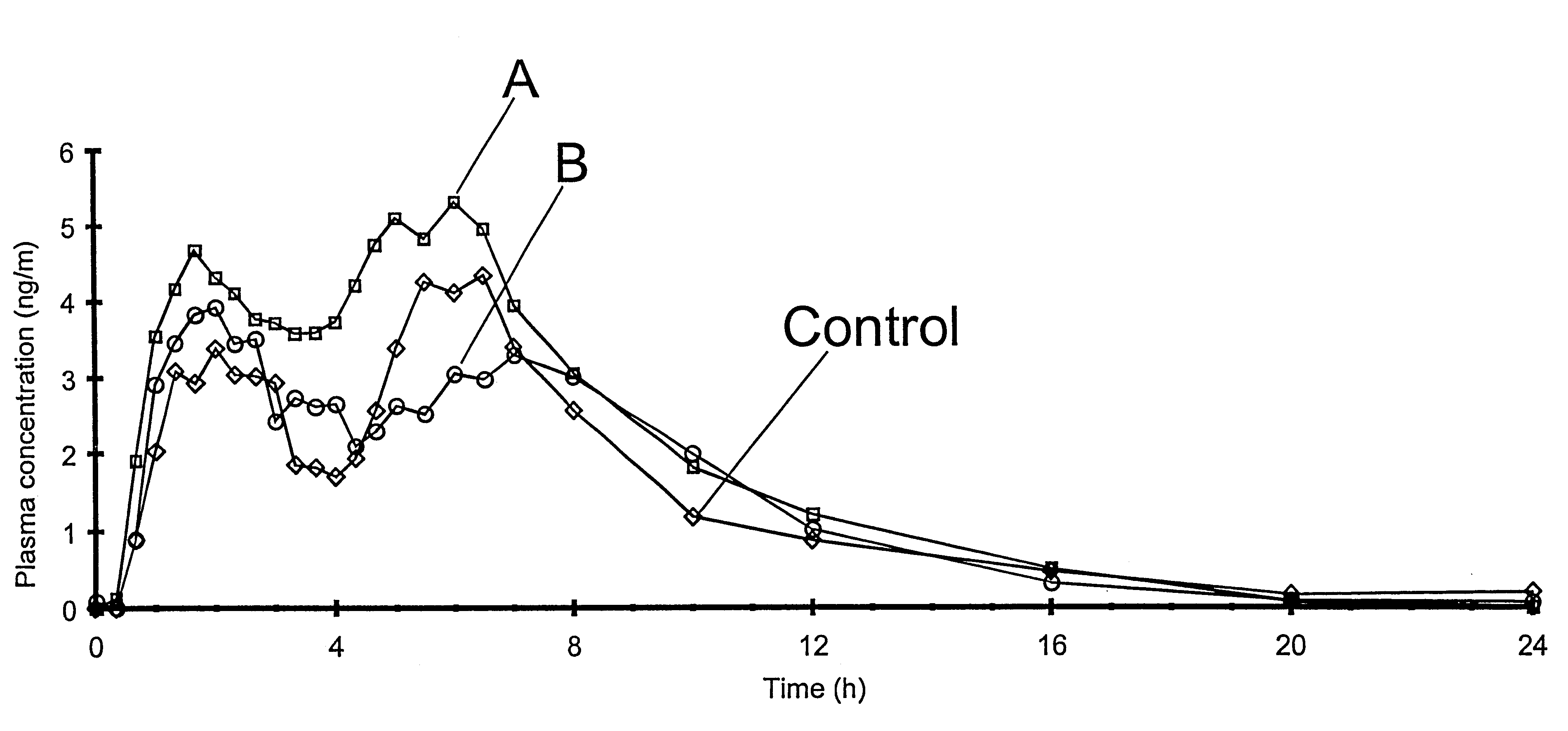
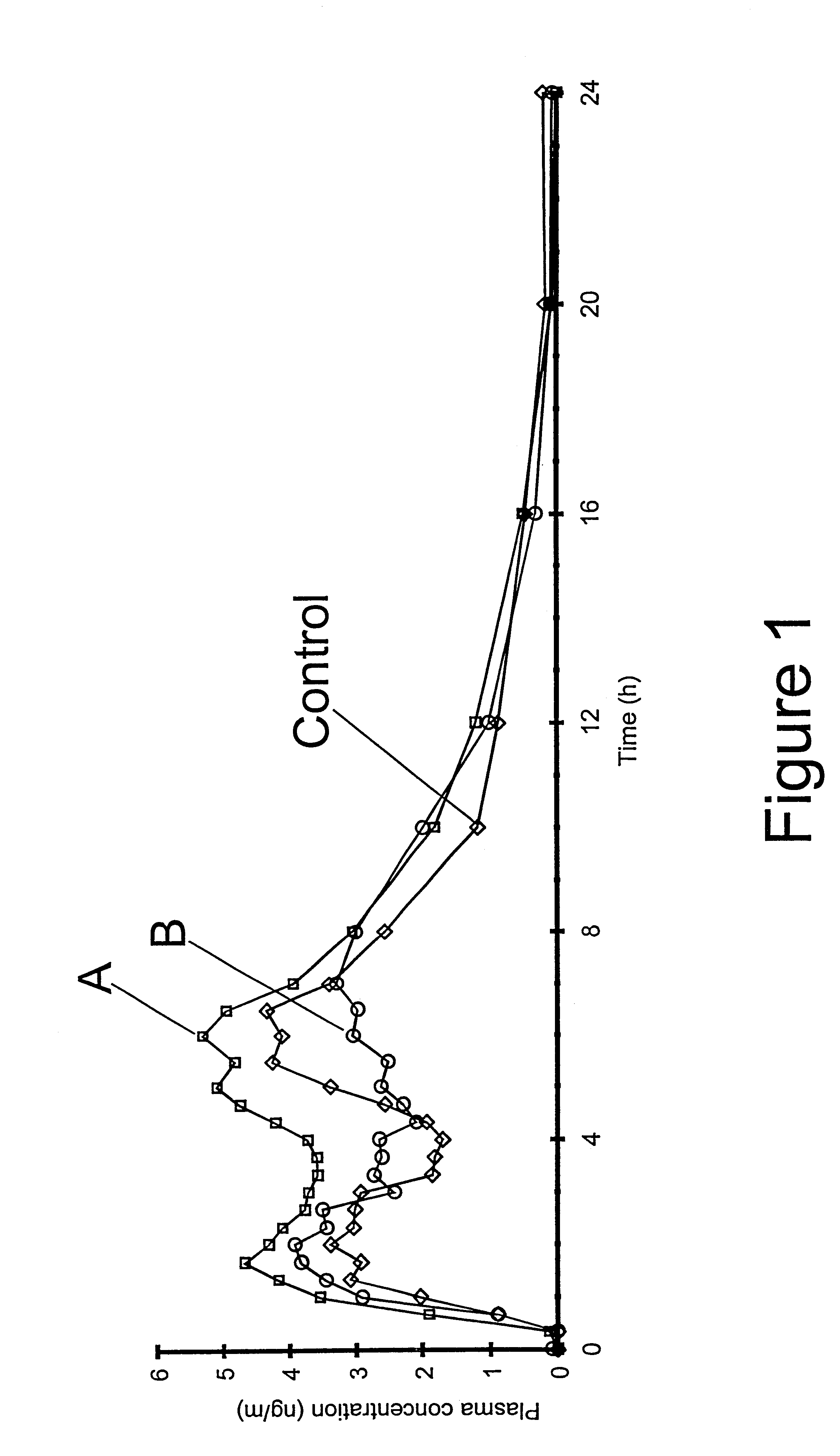
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
Methods and compositions for therapeutic use of RNA interference
InactiveUS20030157030A1Effective amountImprove angiogenesisOrganic active ingredientsPowder deliveryGene expressionSmall interfering RNA
Abstract of Disclosure The present invention provides methods and compositions for attenuating expression of a target gene in vivo. In general, the method includes administering RNAi constructs (such as small-interfering RNAs (i.e., siRNAs) that are targeted to particular mRNA sequences, or nucleic acid material that can produce siRNAs in a cell), in an amount sufficient to attenuate expression of a target gene by an RNA interference mechanism, e.g., in a sequence-dependent, PKR-independent manner. In particular, the subject method can be used to alter the growth, survival or differentiation of cells for therapeutic and cosmetic purposes.
Owner:INSERT THERAPEUTICS INC
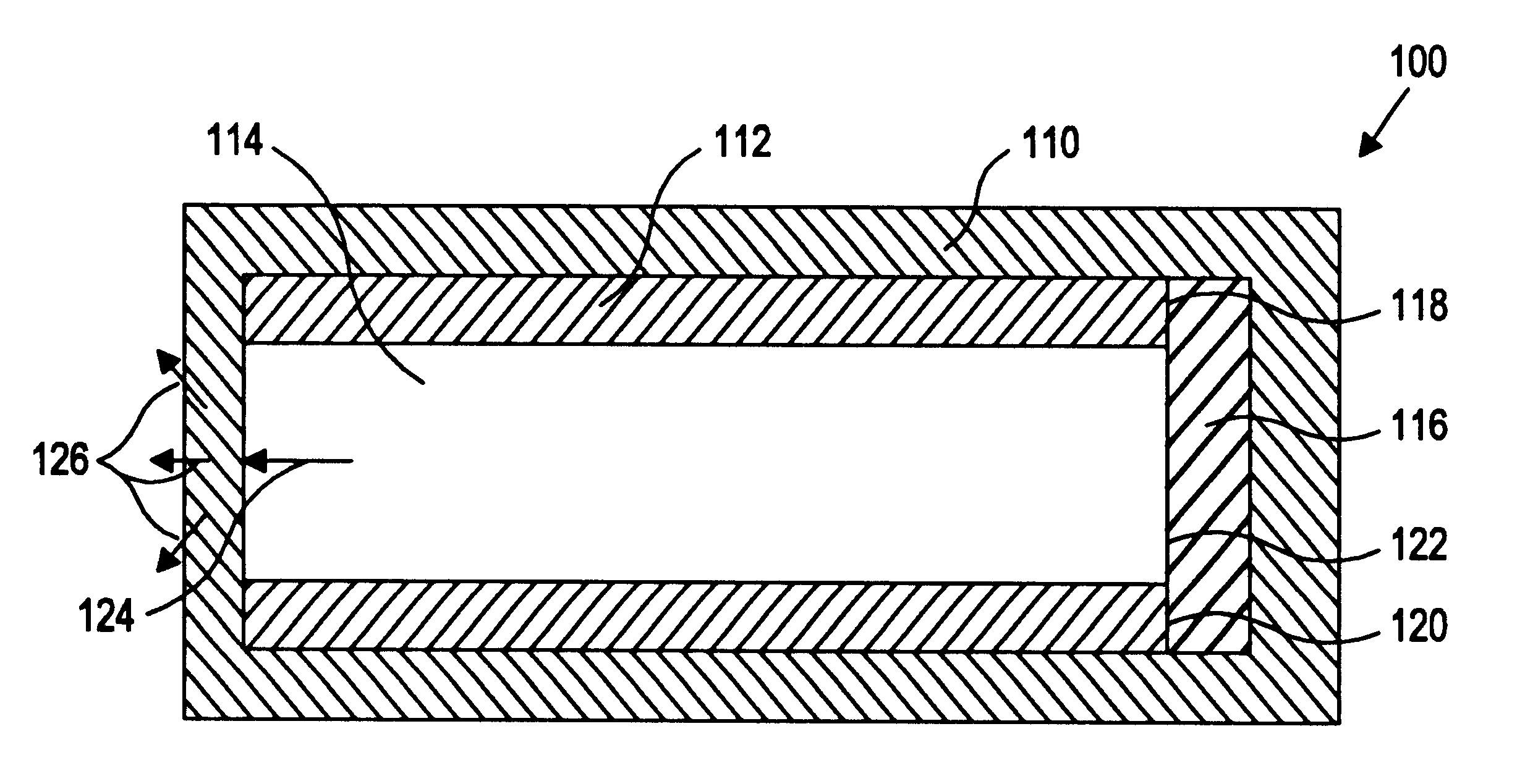
Sustained release drug delivery devices, methods of use, and methods of manufacturing thereof
InactiveUS6375972B1Without of effectWithout riskOrganic active ingredientsSenses disorderBiological bodySustained release drug
A method and device for treating a mammalian organism to obtain a desired local or systemic physiological or pharmacological effect is provided. The method includes administering a sustained release drug delivery system to a mammalian organism in need of such treatment at an area wherein release of an effective agent is desired and allowing the effective agent to pass through the device in a controlled manner. The device includes an inner core or reservoir including the effective agent, an impermeable tube which encloses portions of the reservoir, and a permeable member at an end of the tube.
Owner:EYEPOINT PHARMA INC
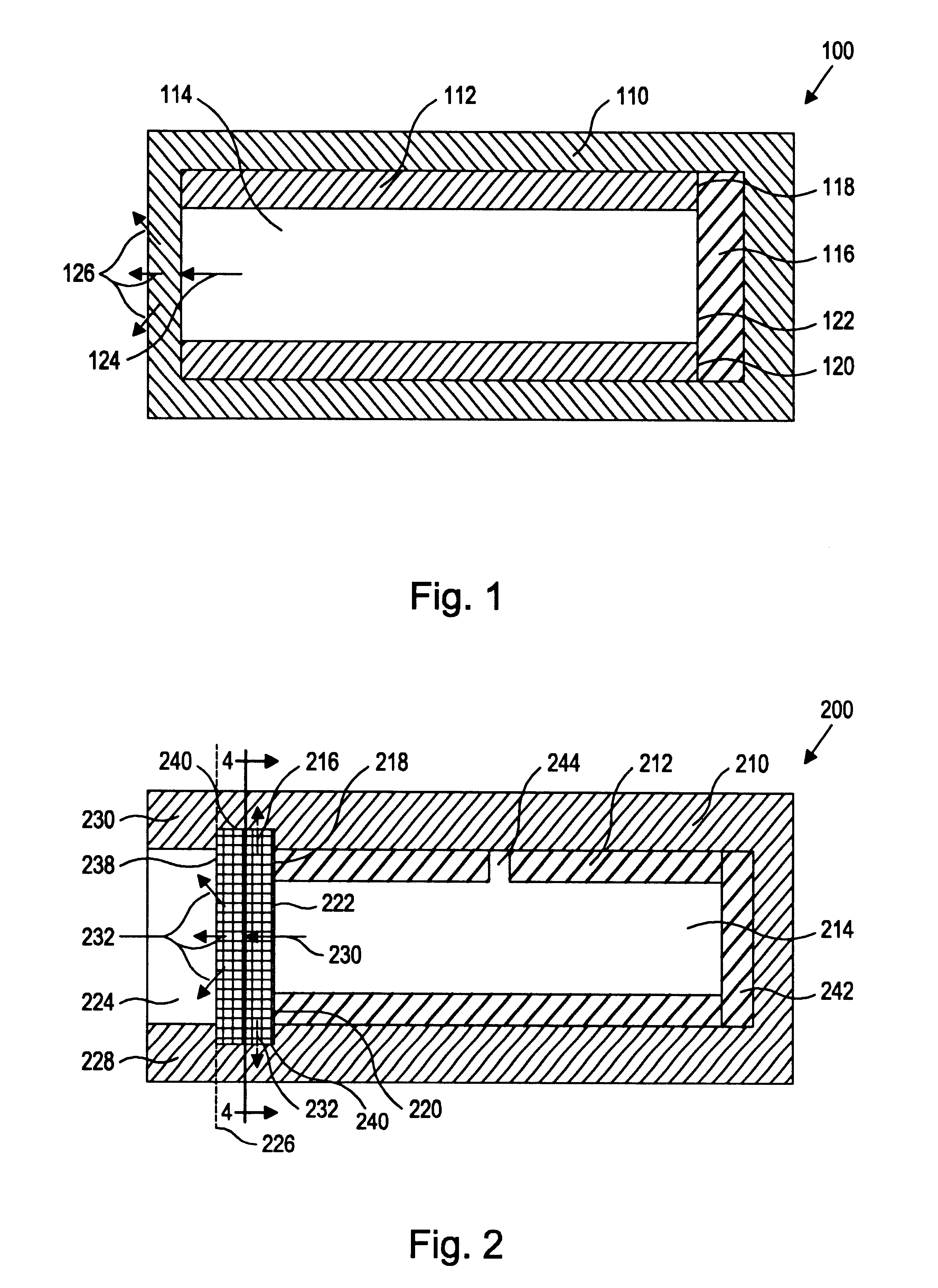
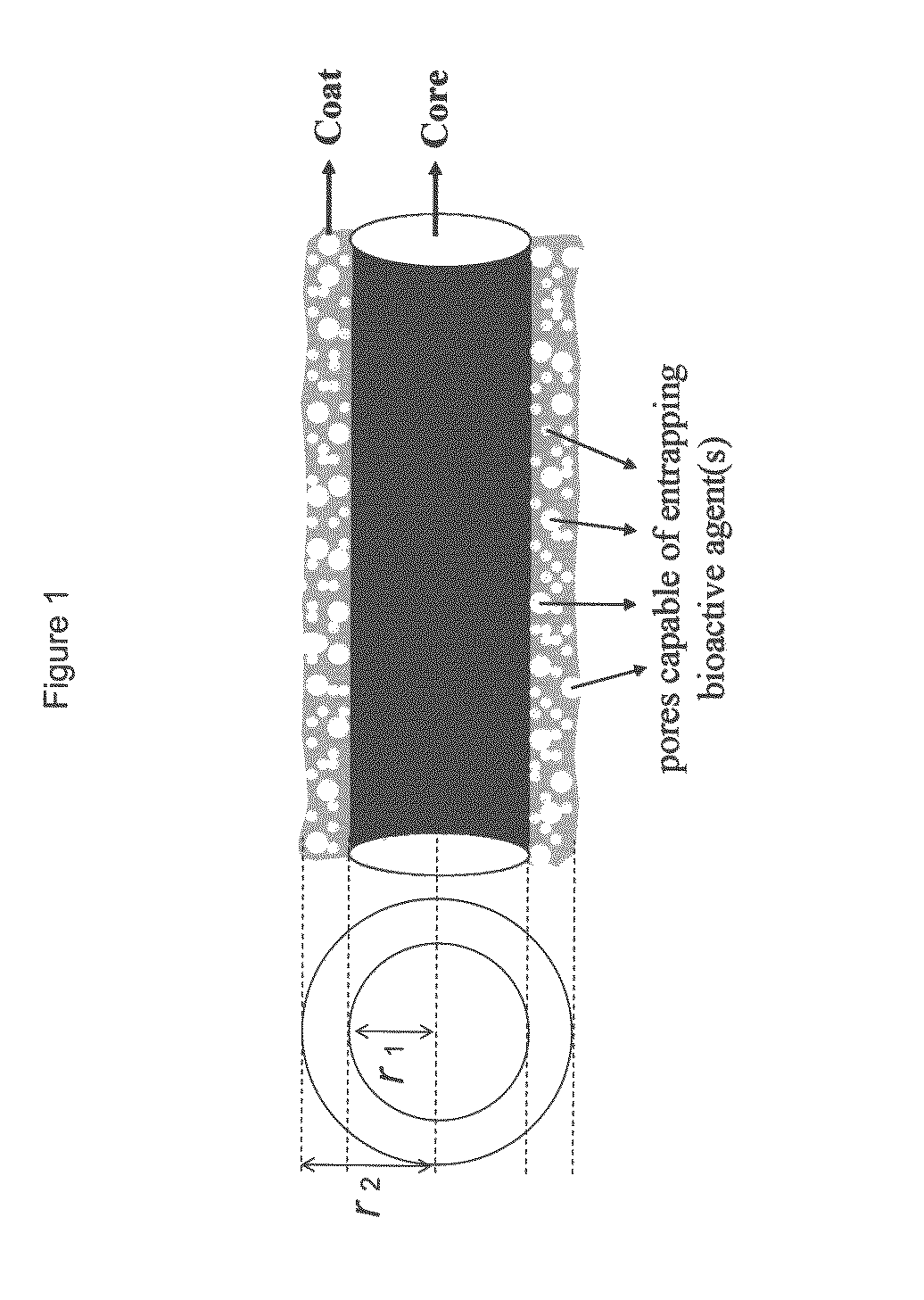
Drug-delivering composite structures
Composite structures composed of a fibril core and a polymeric coat and designed capable of encapsulating both hydrophobic and hydrophilic bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures, and medical devices and disposable articles made therefrom.
Owner:ZILBERMAN MEITAL
Combination therapies employing GITR binding molecules
ActiveUS8591886B2Small sizeImprove securityOrganic active ingredientsNervous disorderInternal medicine
Owner:GITR
Treatment of conditions through modulation of the autonomic nervous system
InactiveUS20050153885A1Effective treatmentOrganic active ingredientsNervous disorderNervous systemMedicine
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one aldosterone antagonist in a manner that is effective to treat the subject for the condition. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Extended release dosage form
InactiveUS6245357B1Free to changeOrganic active ingredientsNervous disorderExtended Release Dosage Form
A dosage form comprising a composition comprising a drug surrounded by an interior and an exterior wall with an exit for administering the drug to a patient; and a method of using the dosage form are disclosed for an indicated therapy.
Owner:ALZA CORP
Lipid formulations for nucleic acid delivery
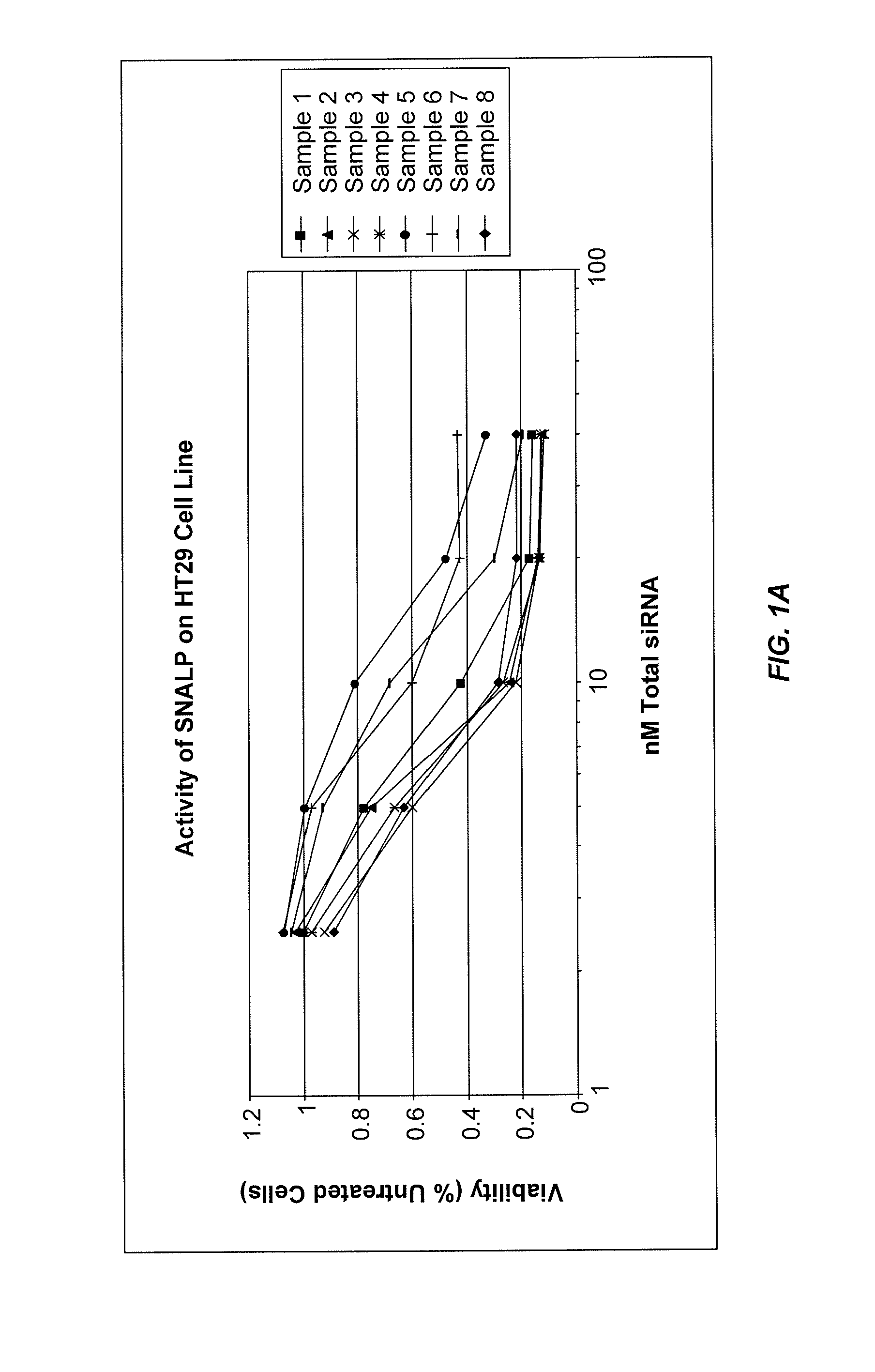
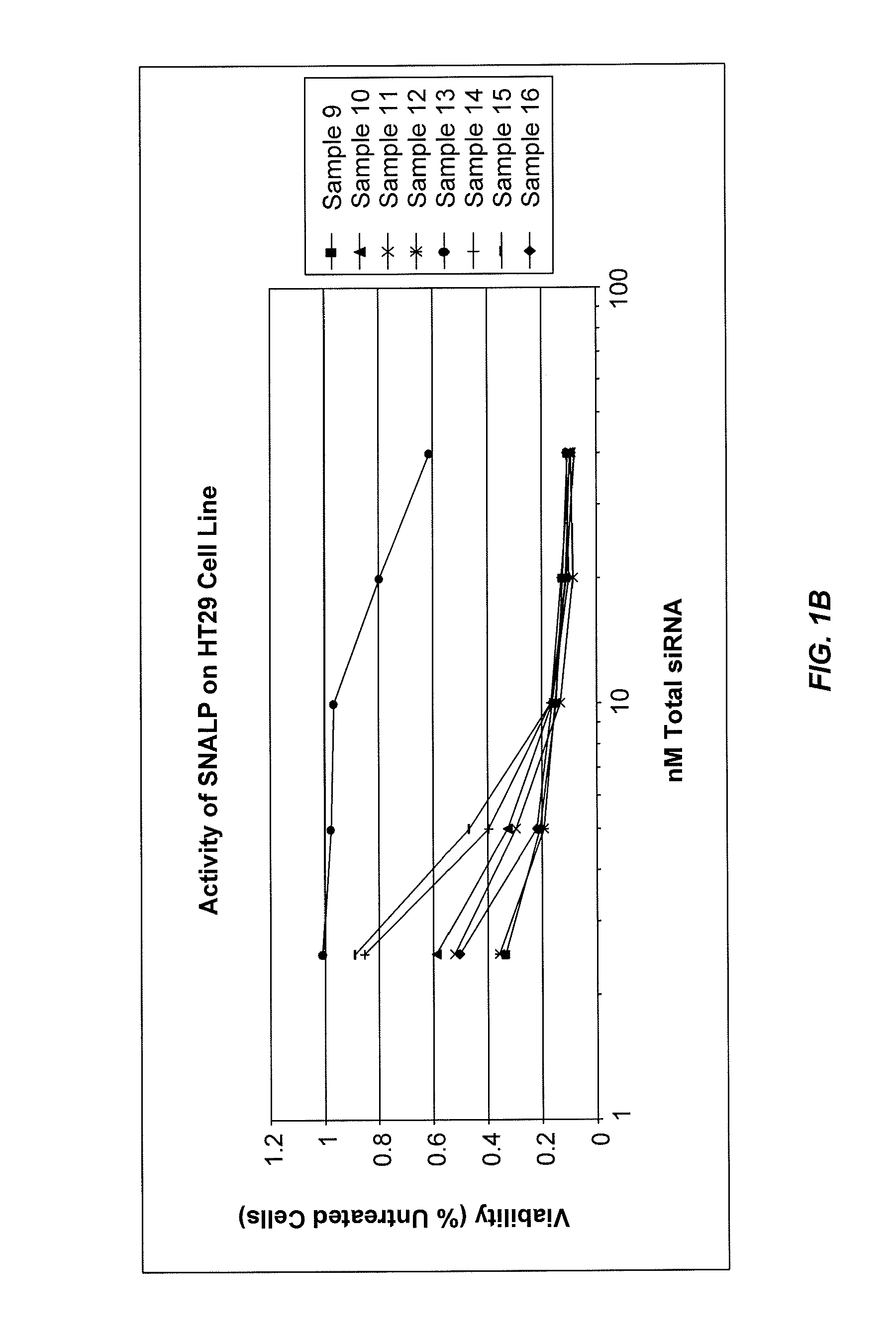
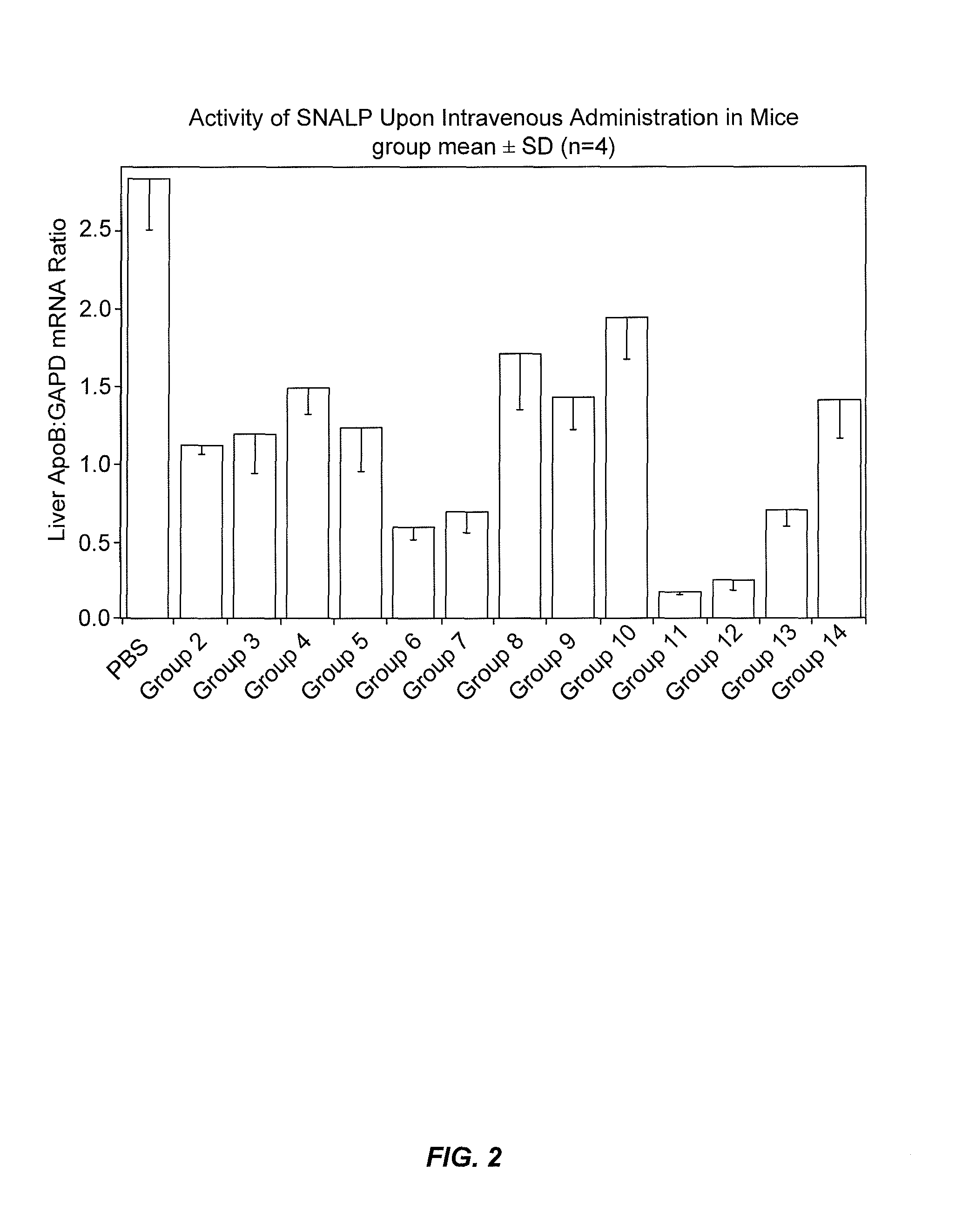
The present invention provides novel, stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (such as one or more interfering RNA), methods of making the SNALP, and methods of delivering and / or administering the SNALP.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
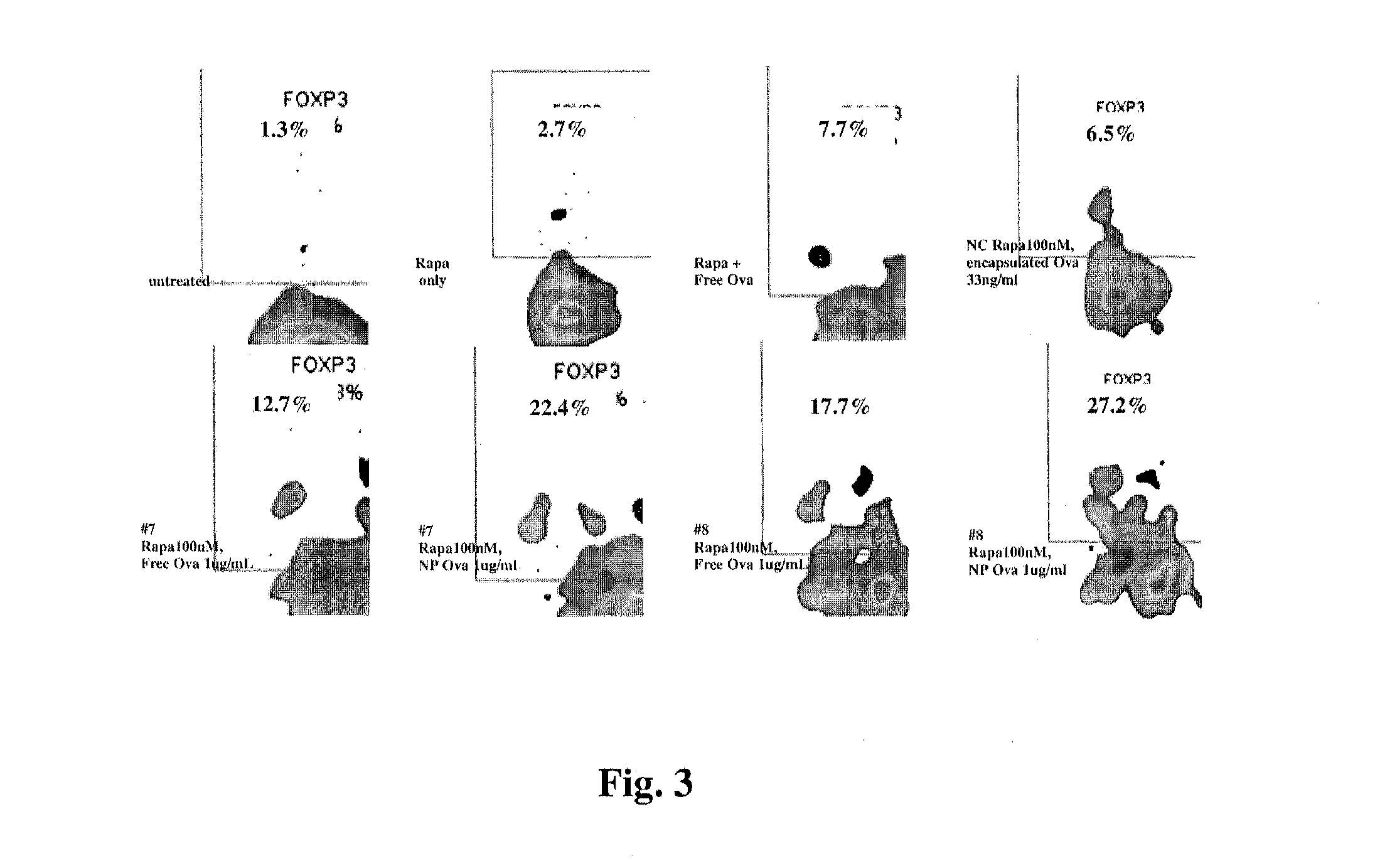
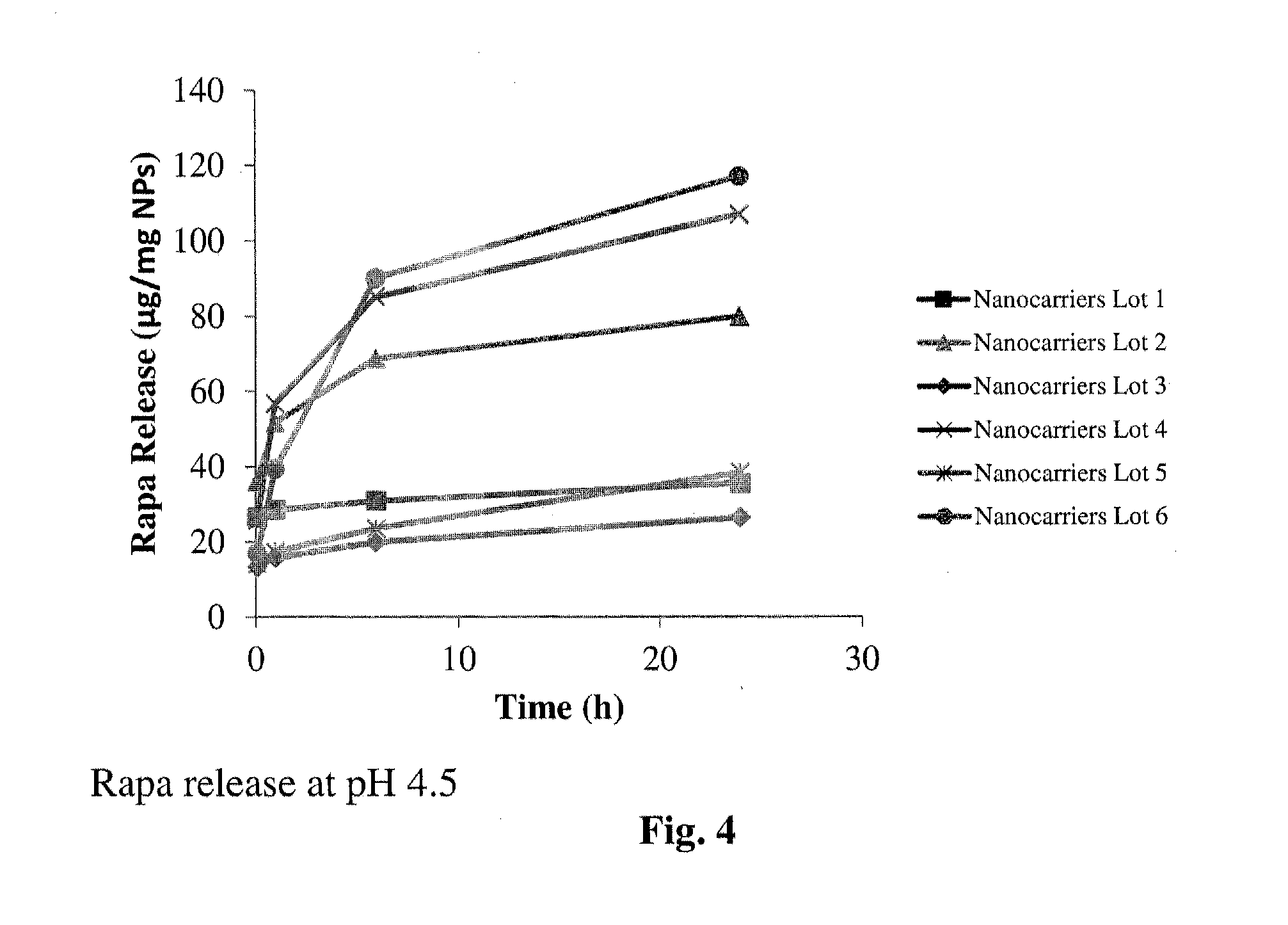
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
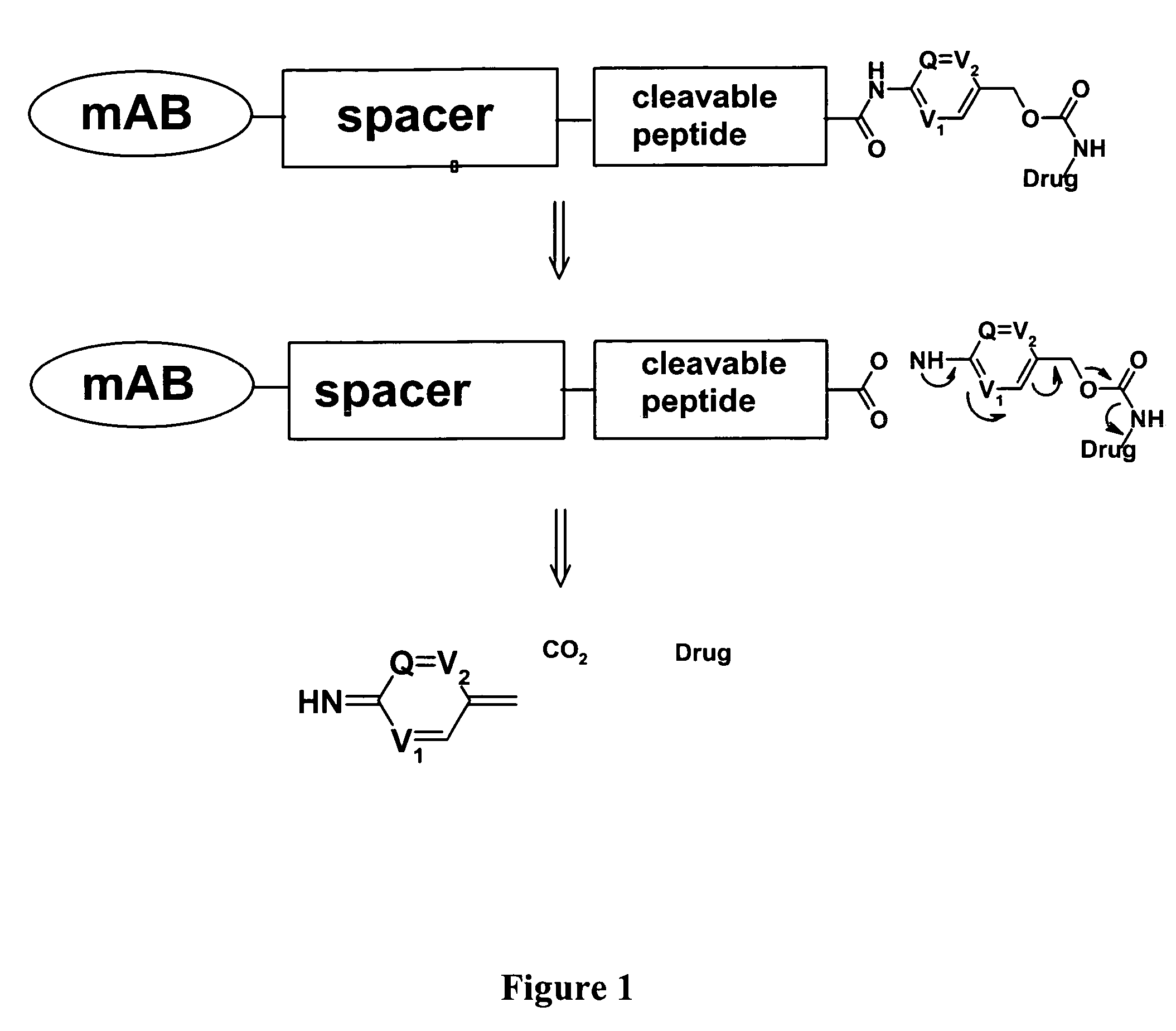
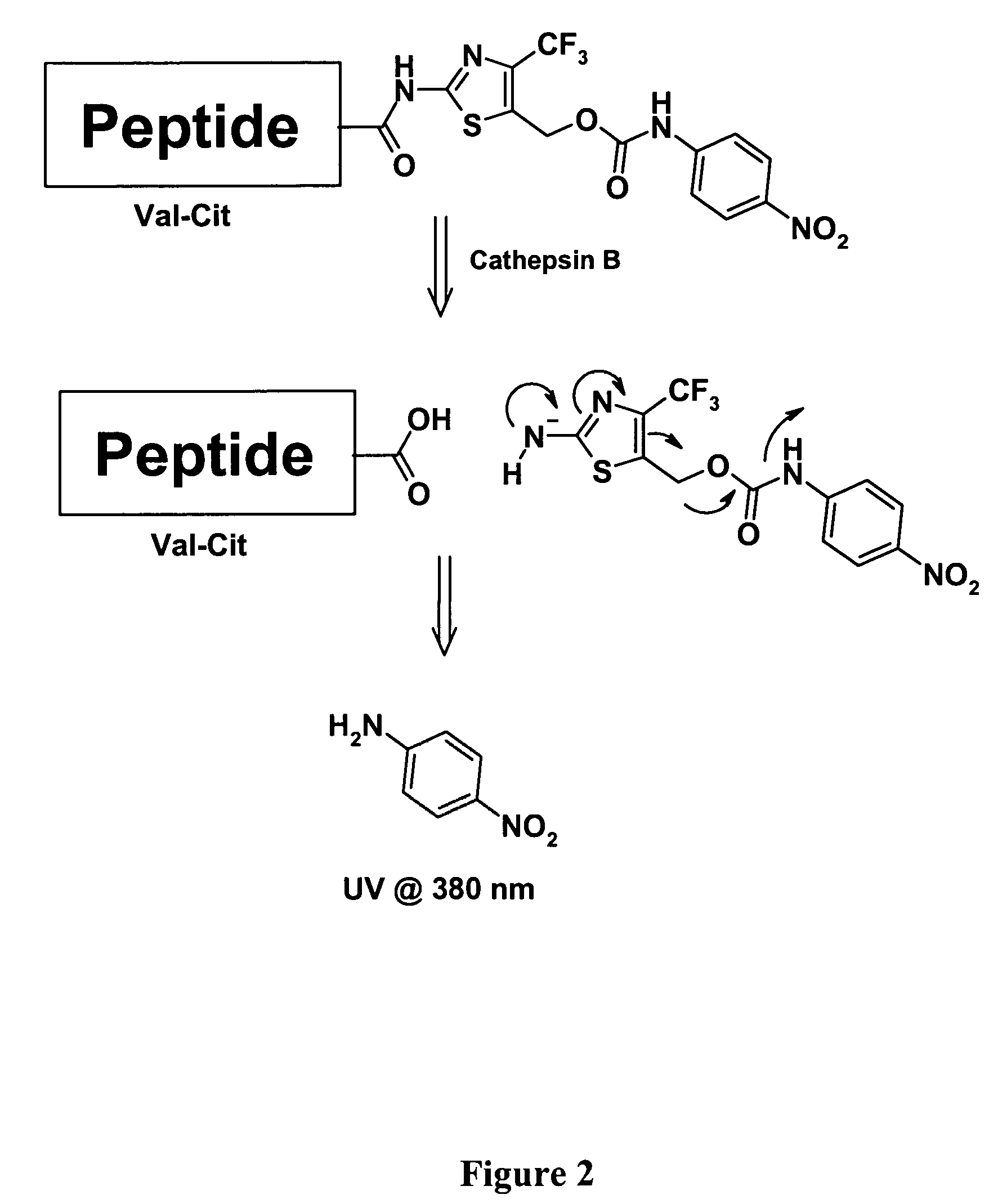
Heterocyclic self-immolative linkers and conjugates
The present invention provides heterocyclic linker compounds useful for linking drug moieties to ligands. The compounds also include drug-ligand conjugates comprising a ligand capable of targeting a selected cell population, and a drug connected to the ligand by a heterocyclic linker moiety. The linker moiety comprises a peptide sequence that is a substrate for an intracellular enzyme, for example a cathepsin, that cleaves the peptide at an amide bond. The peptide further contains a self-immolating moiety which connects the drug and the protein peptide sequence. Upon cleavage of the peptide sequence by an intracellular enzyme the self-immolating moiety cleaves itself from the drug moiety such that the drug moiety is in an underivatized and active form.
Owner:SEAGEN INC
Smokeless Tobacco Product
InactiveUS20080196730A1Create sensationOrganic active ingredientsTobacco treatmentSmokeless tobaccoAutomotive engineering
The present invention relates generally to a smokeless tobacco product in the form of a completely dissolvable vehicle (1; 11) comprising concentrated tobacco extract contained in a dissolvable matrix. In one embodiment, the vehicle (11) is a multilayer three-dimensional vehicle whose several layers (12, 13, 14) dissolve layer by layer in the mouth of a user.
Owner:NOVUS SCI
N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV
The invention discloses certain N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing said compounds as an active ingredient thereof, and the use of said compounds in inhibiting dipeptidyl peptidase-IV.
Owner:NOVARTIS AG
Porous polymeric matrices made of natural polymers and synthetic polymers and optionally at least one cation and methods of making
A porous polymeric matrix containing at least one natural polymer and at least one synthetic polymer and optionally at least one cation. Furthermore, a method of making a porous polymeric matrix involving mixing at least one natural polymer and inorganic salts with a solution comprising at least one solvent and at least one synthetic polymer to form a slurry, casting the slurry in a mold and removing the solvent to form solid matrices, immersing the solid matrices in deionized water to allow natural polymer cross-linking and pore creation to occur simultaneously, and drying the matrices to create a porous polymeric matrix; wherein the matrix contains a cation. Also, a method of making a porous polymeric matrix, involving mixing at least one natural polymer in an aqueous solvent and mixing at least one synthetic polymer in an organic solvent, combining the mixtures and casting in a mold, and separately removing said aqueous solvent and said organic solvent to form a porous polymeric matrix; wherein the porous polymeric matrix does not contain a cation.
Owner:US SEC AGRI
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com