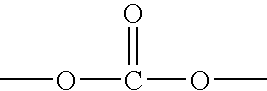
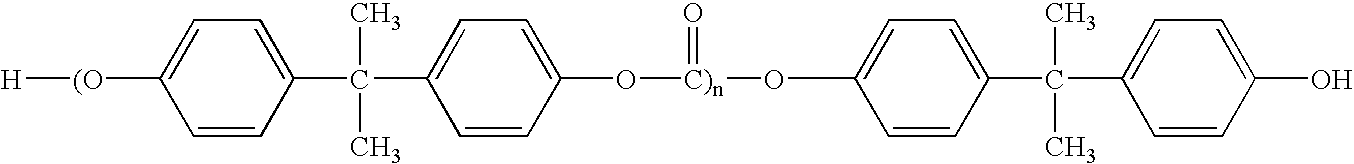
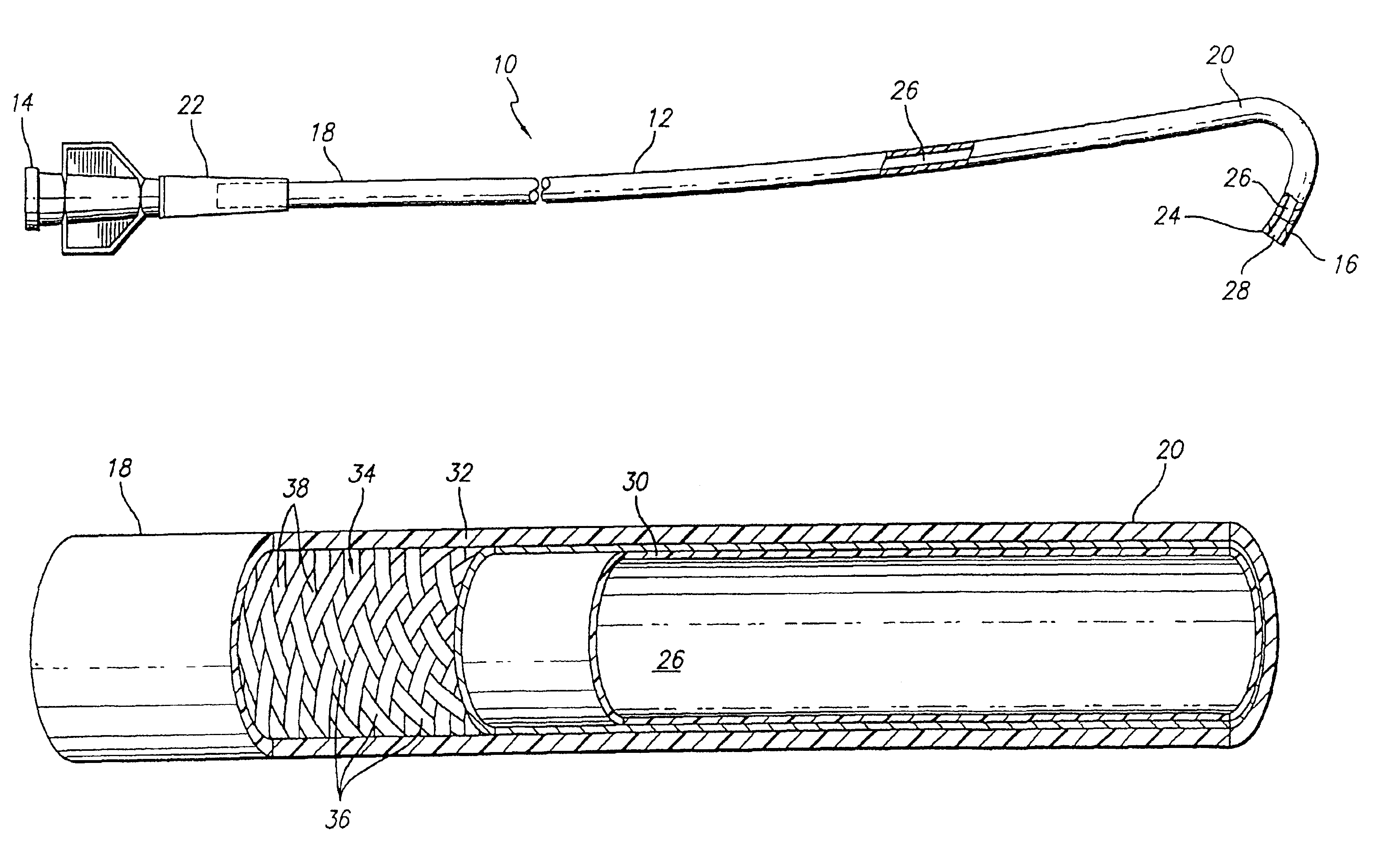
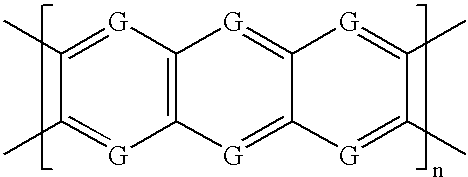
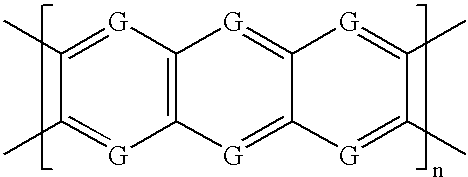
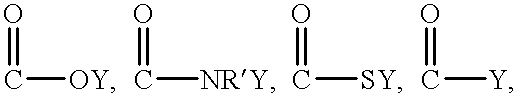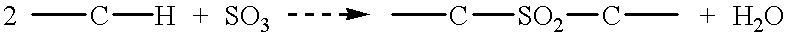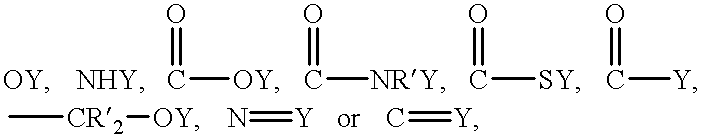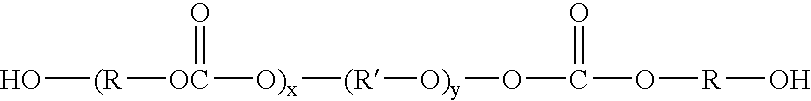Patents
Literature
635 results about "Fibril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
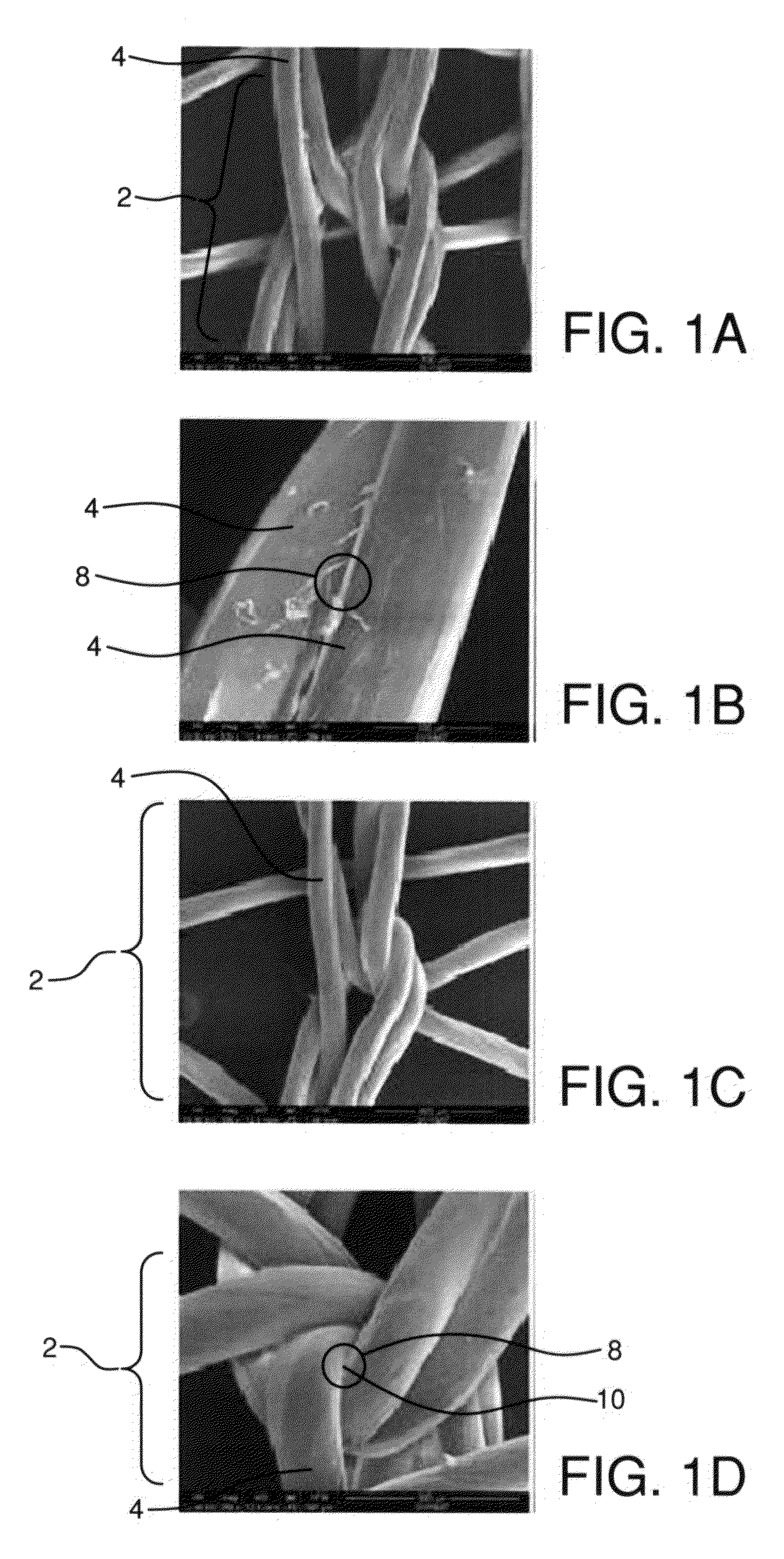
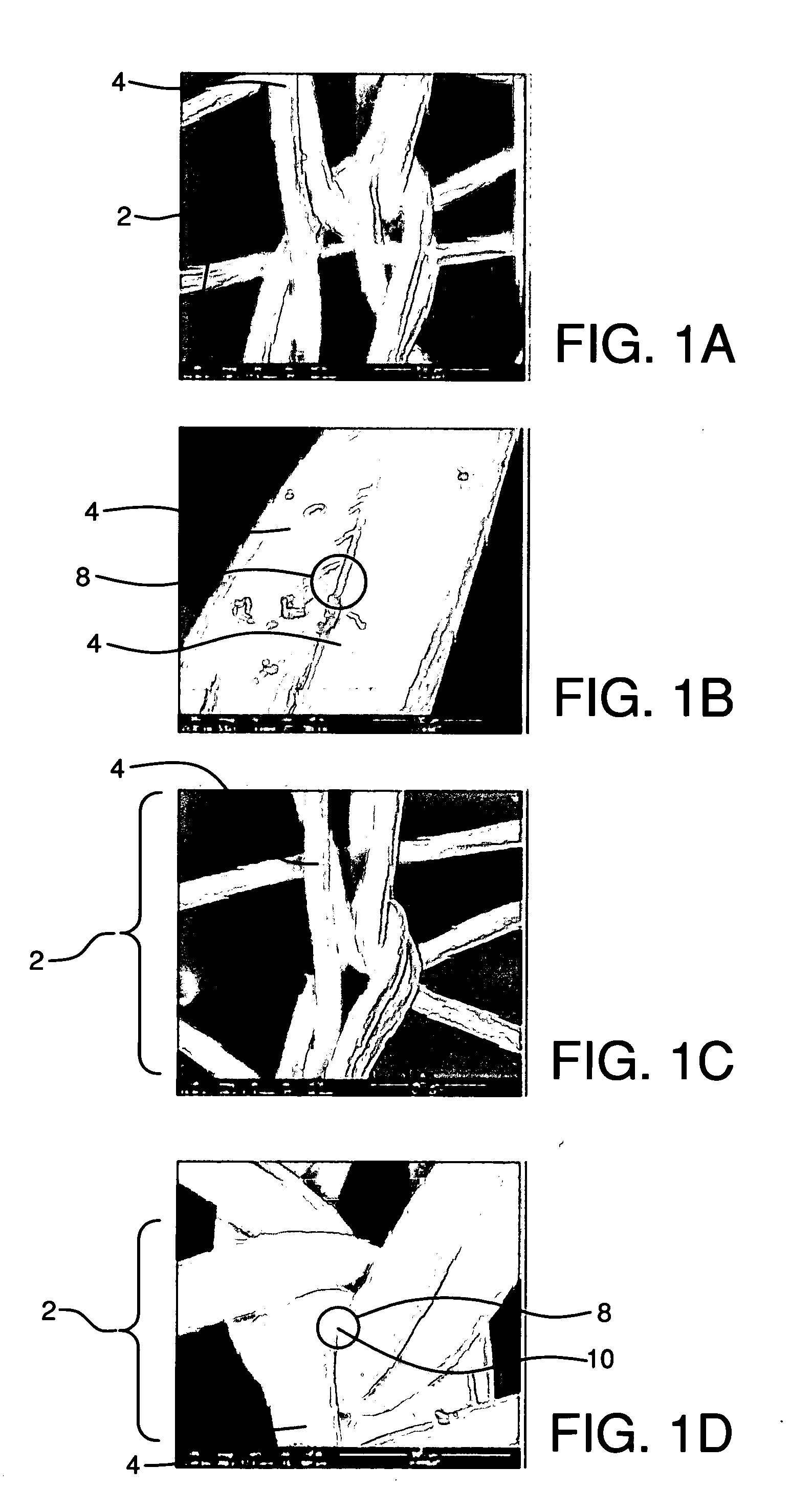
Fibrils (from the Latin fibra) are structural biological materials found in nearly all living organisms. Not to be confused with fibers or filaments, fibrils tend to have diameters ranging from 10-100 nanometers (whereas fibers are micro to milli-scale structures and filaments have diameters approximately 10-50 nanometers in size). Fibrils are not usually found alone but rather are parts of greater hierarchical structures commonly found in biological systems. Due to the prevalence of fibrils in biological systems, their study is of great importance in the fields of microbiology, biomechanics, and materials science.
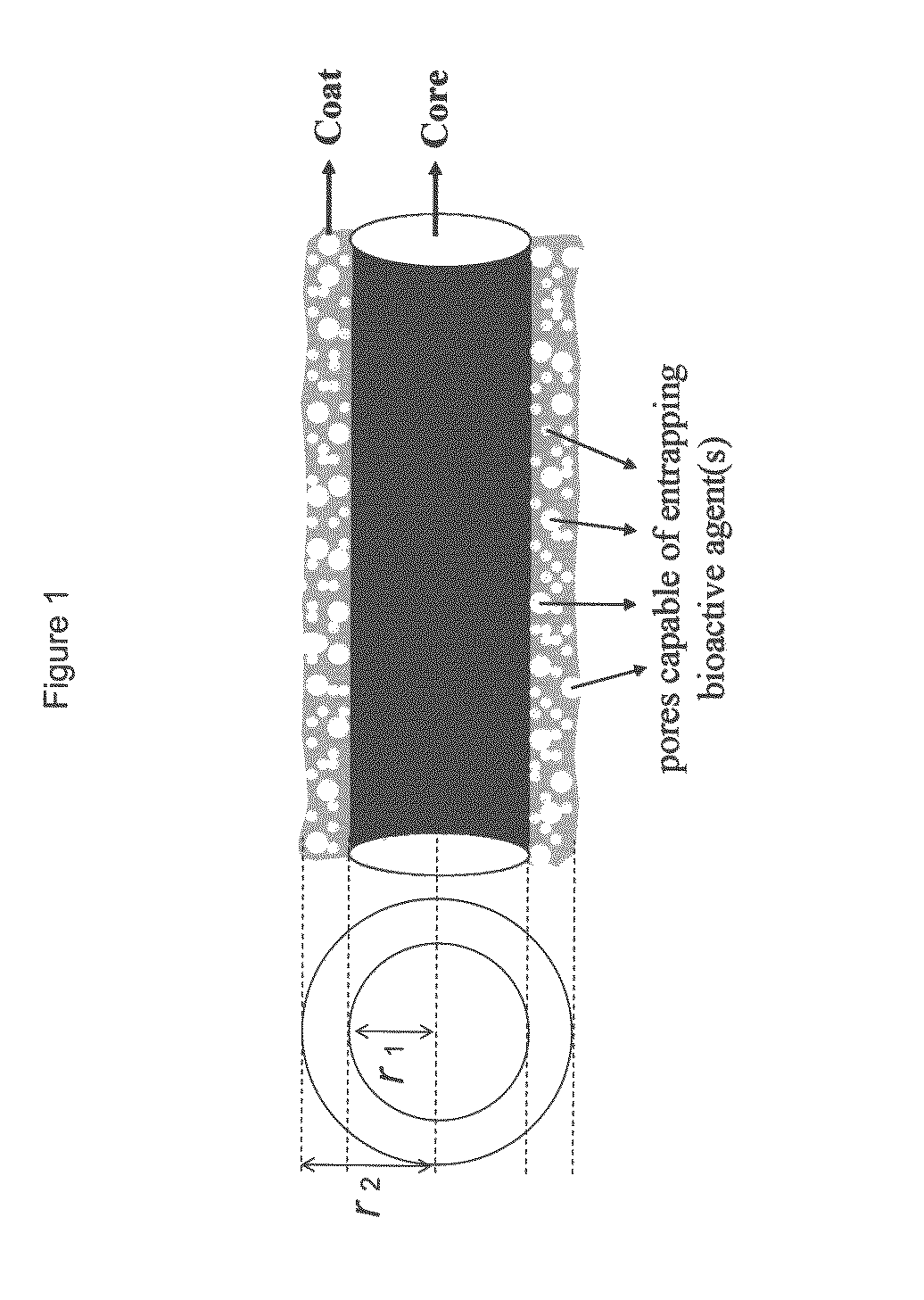
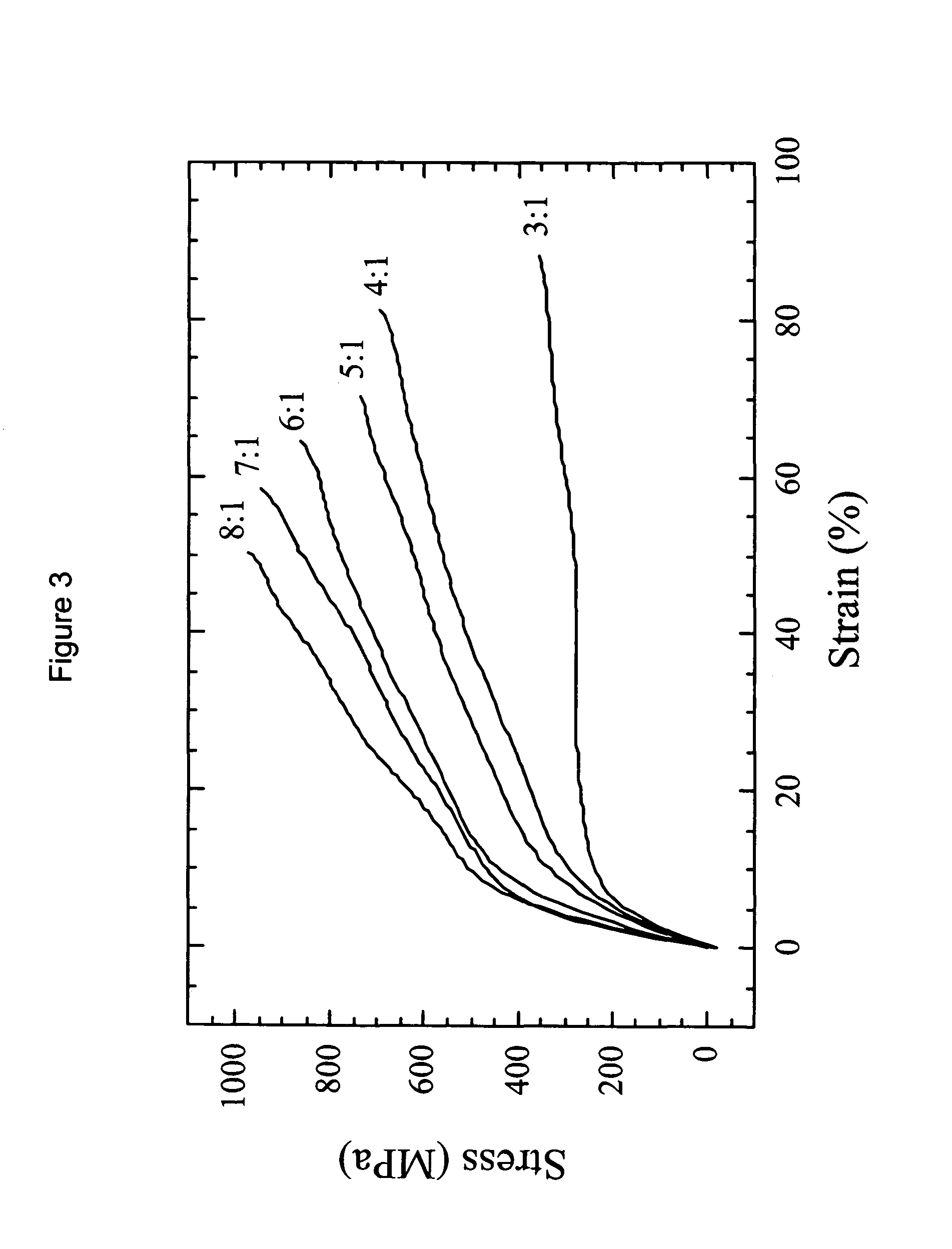
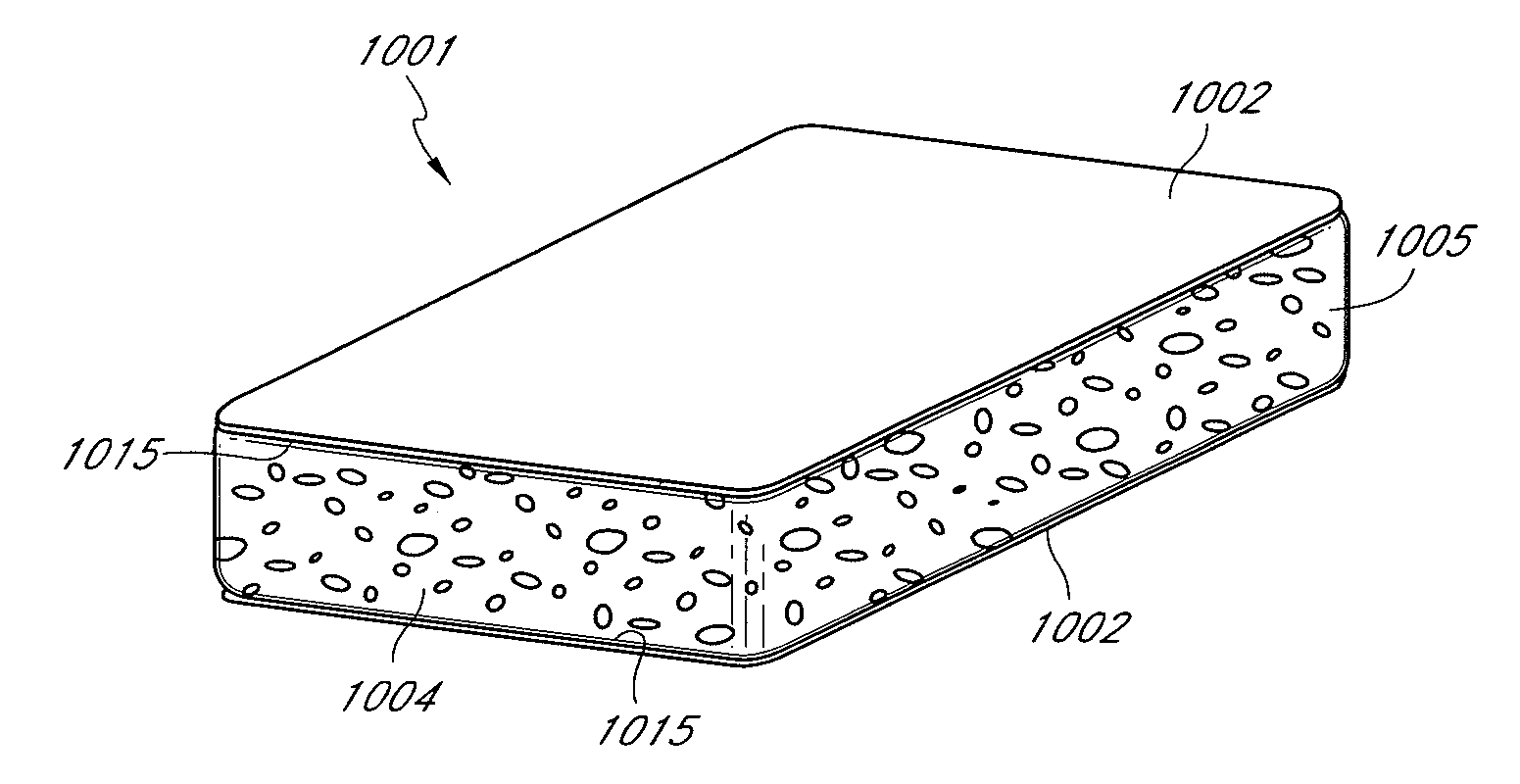
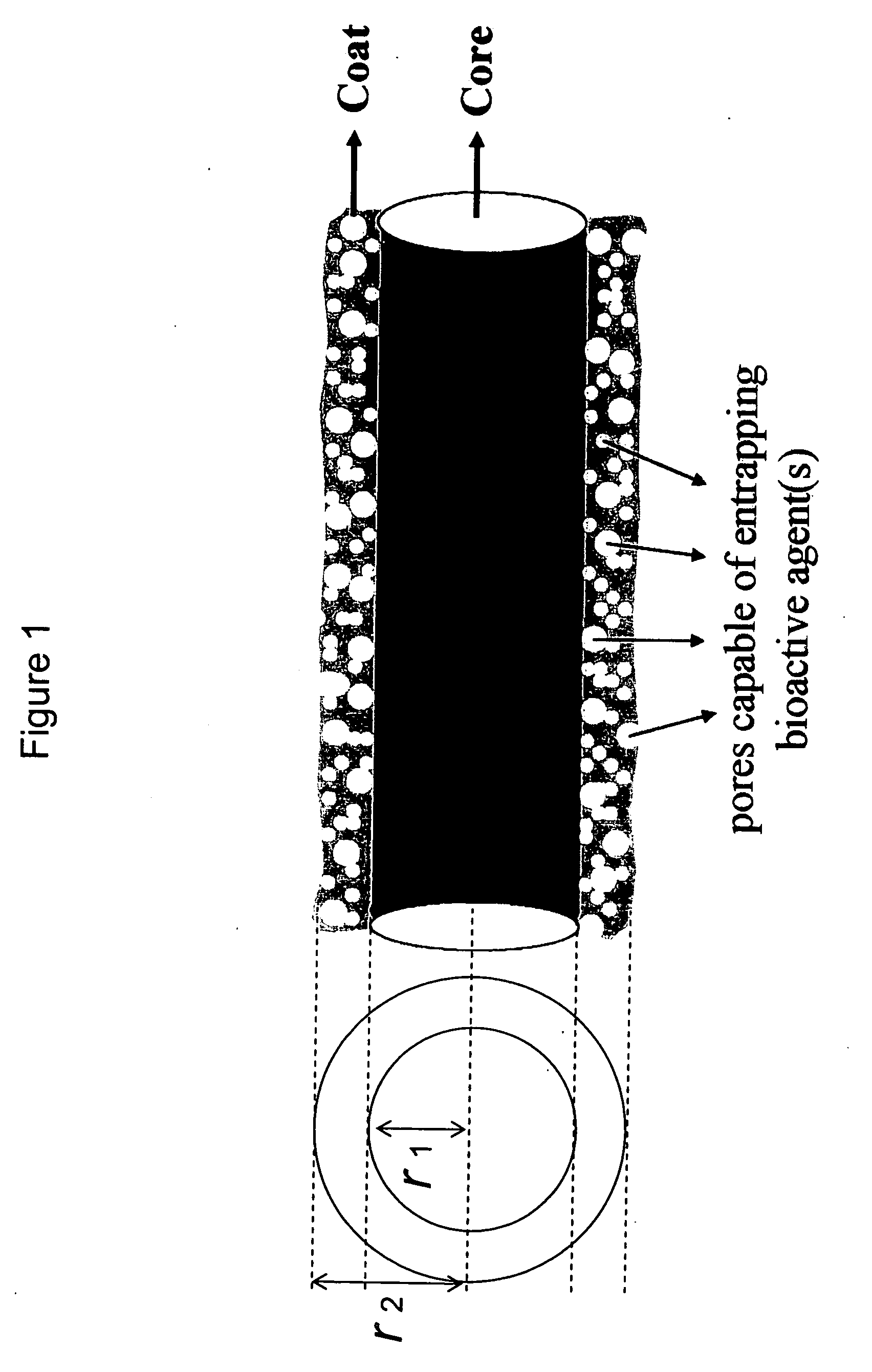
Drug-delivering composite structures
Composite structures composed of a fibril core and a polymeric coat and designed capable of encapsulating both hydrophobic and hydrophilic bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures, and medical devices and disposable articles made therefrom.
Owner:ZILBERMAN MEITAL
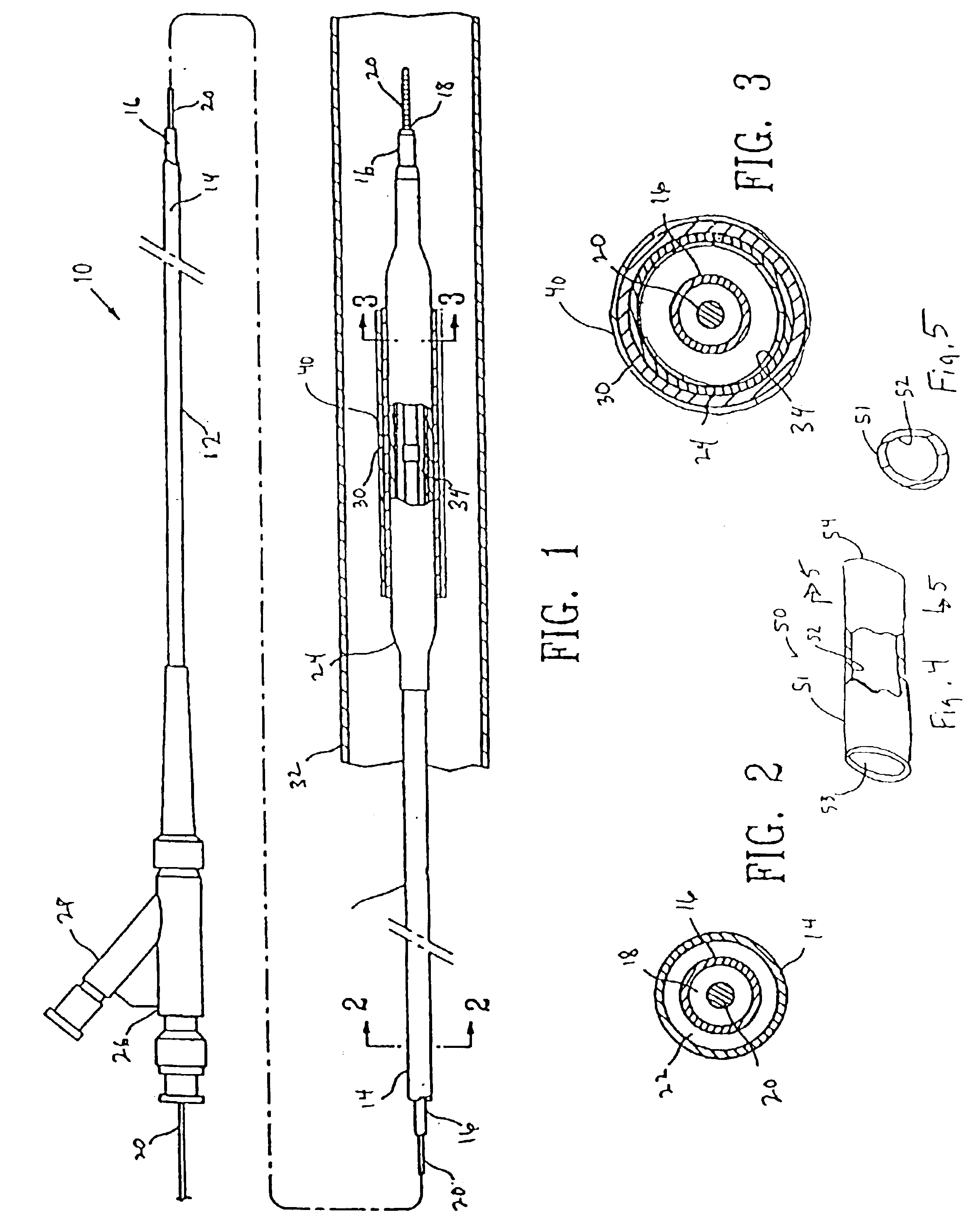
Porous implant with effective extensibility and methods of forming an implant
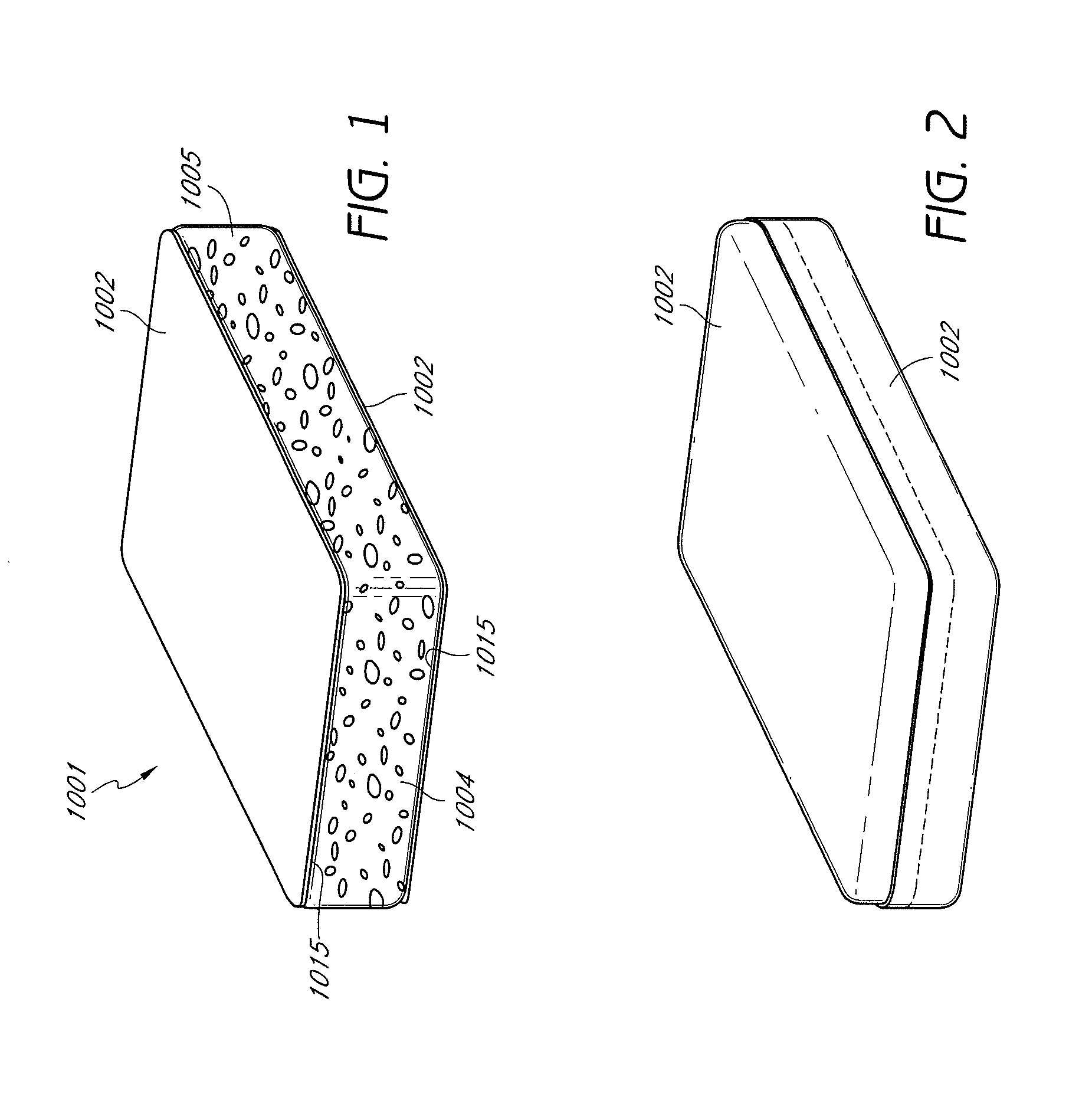
The implant includes an outer layer of ePTFE which exhibits extensibility normally not associated with ePTFE. The ePTFE is reduced in length by deforming the fibrils between the nodes while maintaining the nodes in a substantially flat configuration. Various implant configurations that can include the outer layer described are also disclosed.
Owner:EVERA MEDICAL
Method of making functionalized nanotubes
InactiveUS6203814B1Good dispersionImprove adhesionMaterial nanotechnologyCarbon compoundsChemical MoietyFiber
Owner:HYPERION CATALYSIS INT
System and methods for treatment of alzheimer's and other deposition-related disorders of the brain
InactiveUS20040049134A1Slowing, stopping or avoiding a patient's cognitive lossesMinimal adverse side effectUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseSide effect
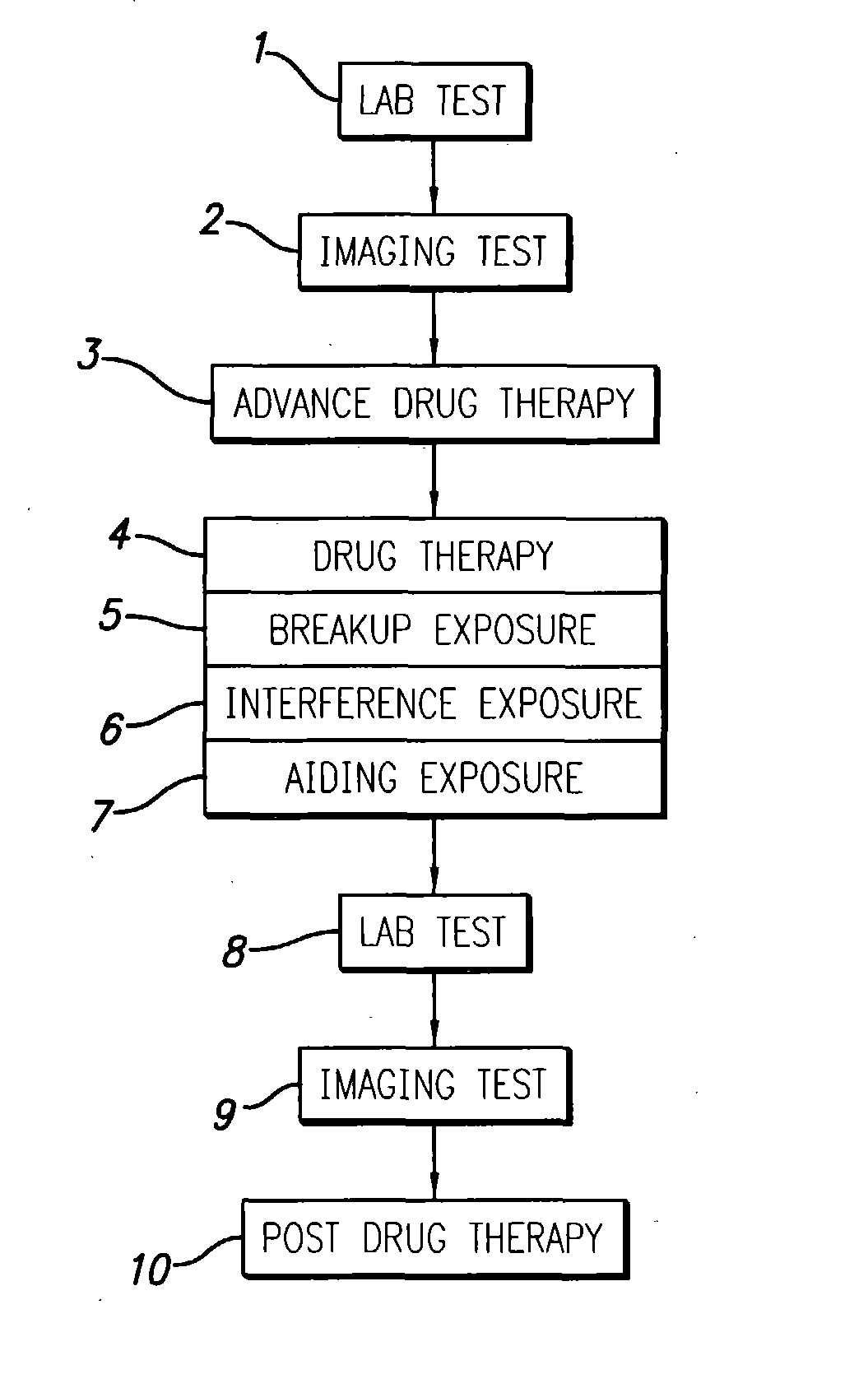
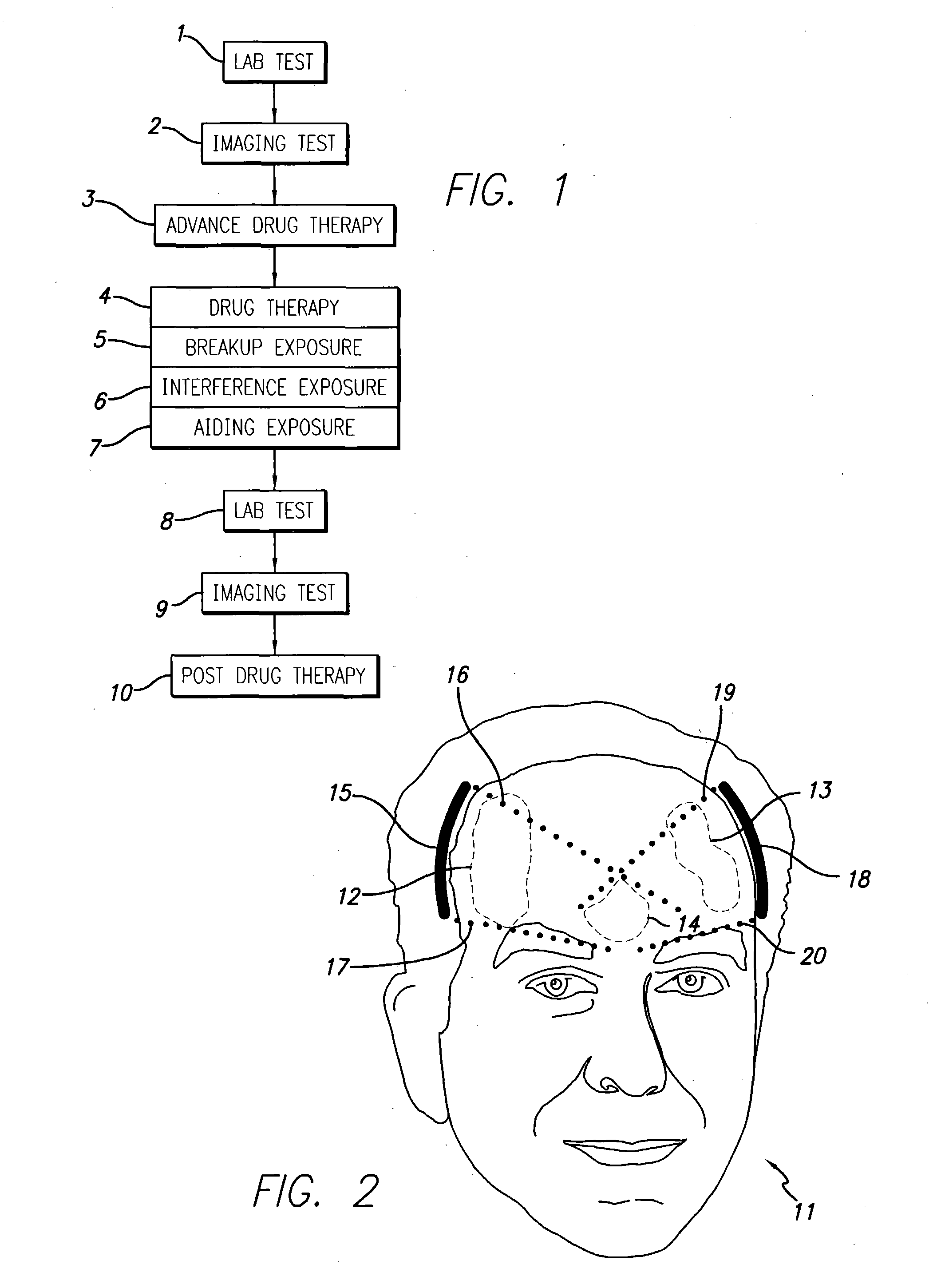
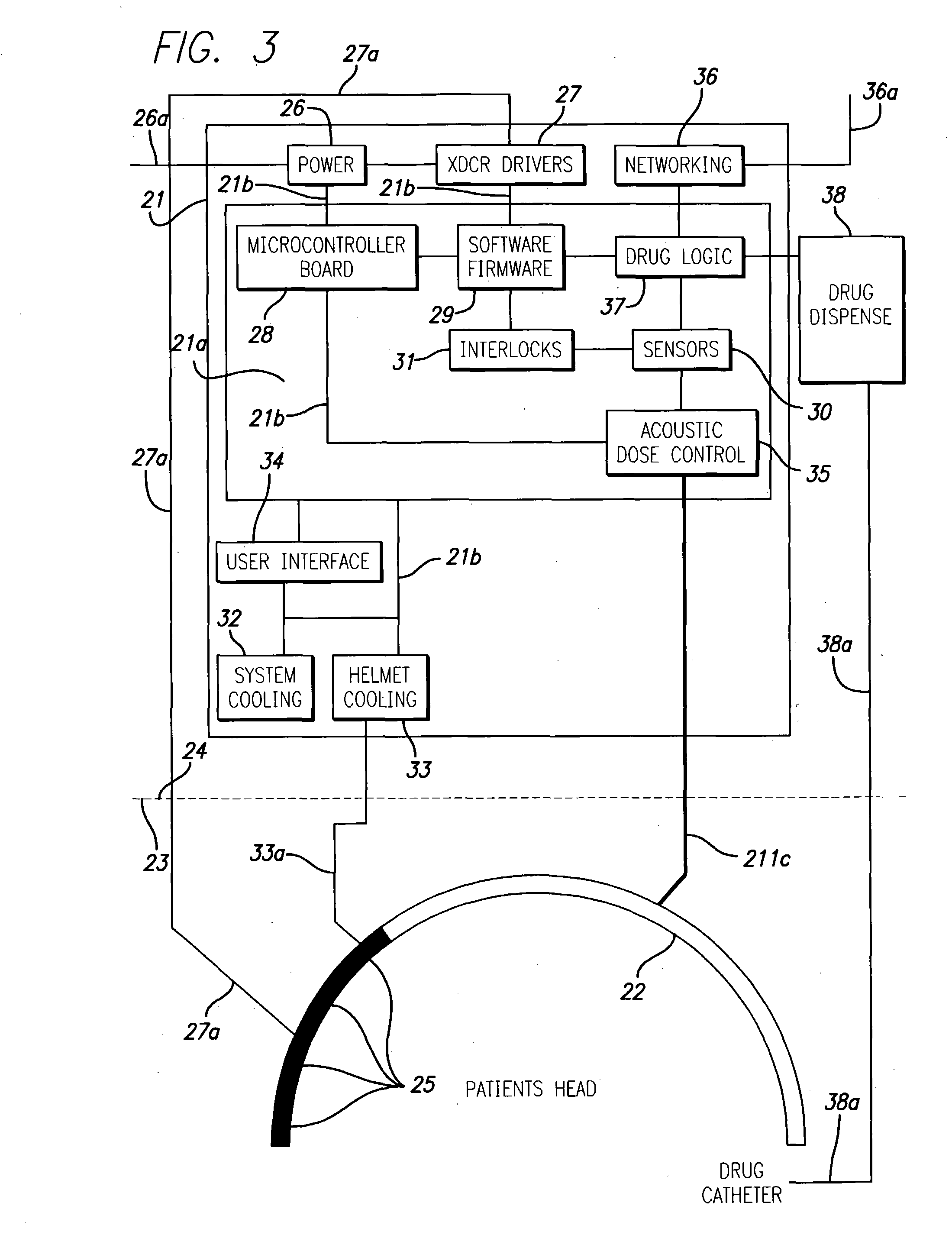
A system and methods are provided for the therapeutic treatment of brain-plaques, fibrils, abnormal-protein related or aggregation-prone protein related deposition-diseases. The system employs acoustic exposure therapy means for delivering therapeutic energy to at least one brain region. The therapy supports at least one of the following processes: (i) physical breakup, erosion, disentanglement, de-aggregation, dissolution, de-agglomeration, de-amalgamation or permeation of the deposits, (ii) interference in at least one deposit formation process, deposition related chemical reaction or biological or genetic pathway contributing to the deposits or deposition-related processes, and (iii) aiding the recovery, growth, regrowth or improved functionality of brain-related cells or functional pathways negatively impacted by, stressed by or disposed to the deposits, deposition-processes or deposition disease state, or supporting the growth of newly transplanted cells anywhere in the brain-related anatomy. The system and methods treat Alzheimer's and other deposition-related disorders of the brain, with minimal adverse side effects to the patient and may be used in cooperation with a drug.
Owner:TOSAYA CAROL A +1
Graphitic nanofibers in electrochemical capacitors
Graphitic nanofibers, which include tubular fullerenes (commonly called "buckytubes"), nanotubes and fibrils, which are functionalized by chemical substitution, are used as electrodes in electrochemical capacitors. The graphitic nanofiber based electrode increases the performance of the electrochemical capacitors. Preferred nanofibers have a surface area greater than about 200 m2 / gm and are substantially free of micropores.
Owner:HYPERION CATALYSIS INT
Dimensionally stable balloons
A medical balloon composed of a micro-composite material which provides for radial expansion of a balloon to a predetermined extent, but which has minimal longitudinal growing during balloon inflation. The micro-composite material includes a fibril component, a matrix component, and optionally, a compatibilizer. The fibril component may preferably be liquid crystal polymer fibers randomly scattered through out the balloon material. The liquid crystal polymers are created by extrusion at high speed. An alternative fibril component may be a PET fibers which are uniformly spaced about the balloon material and extend through out the length of the balloon material tube.
Owner:BOSTON SCI SCIMED INC
Expandable TFE copolymers, method of making, and porous, expended articles thereof
A process for the polymerization of a true tetrafluoroethylene (TFE) copolymer of the fine powder type is provided, wherein the copolymer contains polymerized comonomer units of at least one comonomer other than TFE in concentrations of at least or exceeding 1.0 weight percent, and which can exceed 5.0 weight percent, wherein the copolymer is expandable, that is, the copolymer may be expanded to produce strong, useful, expanded TFE copolymeric articles having a microstructure of nodes interconnected by fibrils.
Owner:WL GORE & ASSOC INC
Graphitic nanotubes in luminescence assays
Graphitic nanotubes, which include tubular fullerenes (commonly called "buckytubes") and fibrils, which are functionalized by chemical substitution, are used as solid supports in electrogenerated chemiluminescence assays. The graphitic nanotubes are chemically modified with functional group biomolecules prior to use in an assay. Association of electrochemiluminescent ruthenium complexes with the functional group biomolecule-modified nanotubes permits detection of molecules including nucleic acids, antigens, enzymes, and enzyme substrates by multiple formats.
Owner:MESO SCALE TECH LLC
Drug-delivering composite structures
Composite structures composed of a fibril core and a polymeric coat and designed capable of encapsulating both hydrophobic and hydrophilic bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures, and medical devices and disposable articles made therefrom.
Owner:ZILBERMAN MEITAL
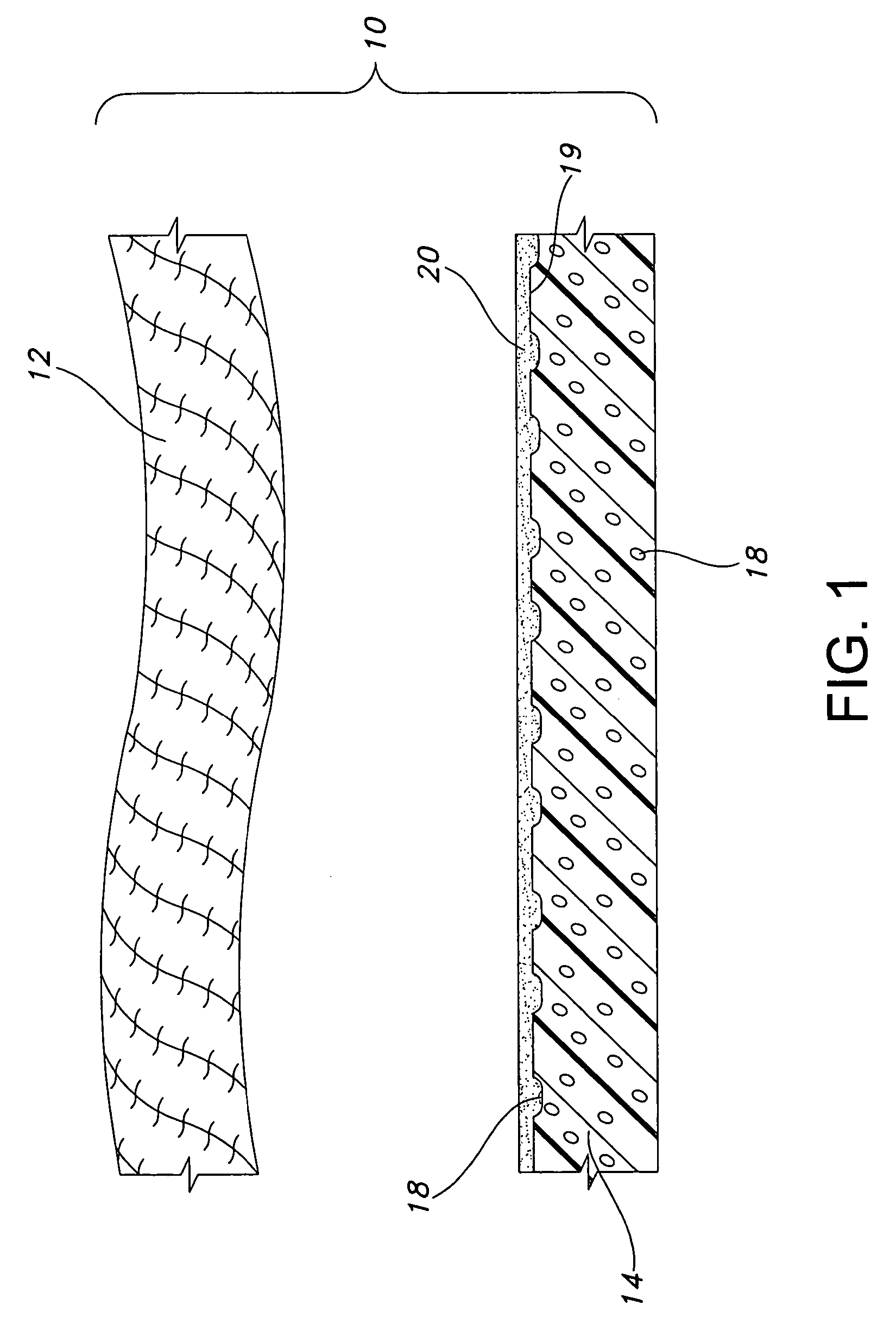
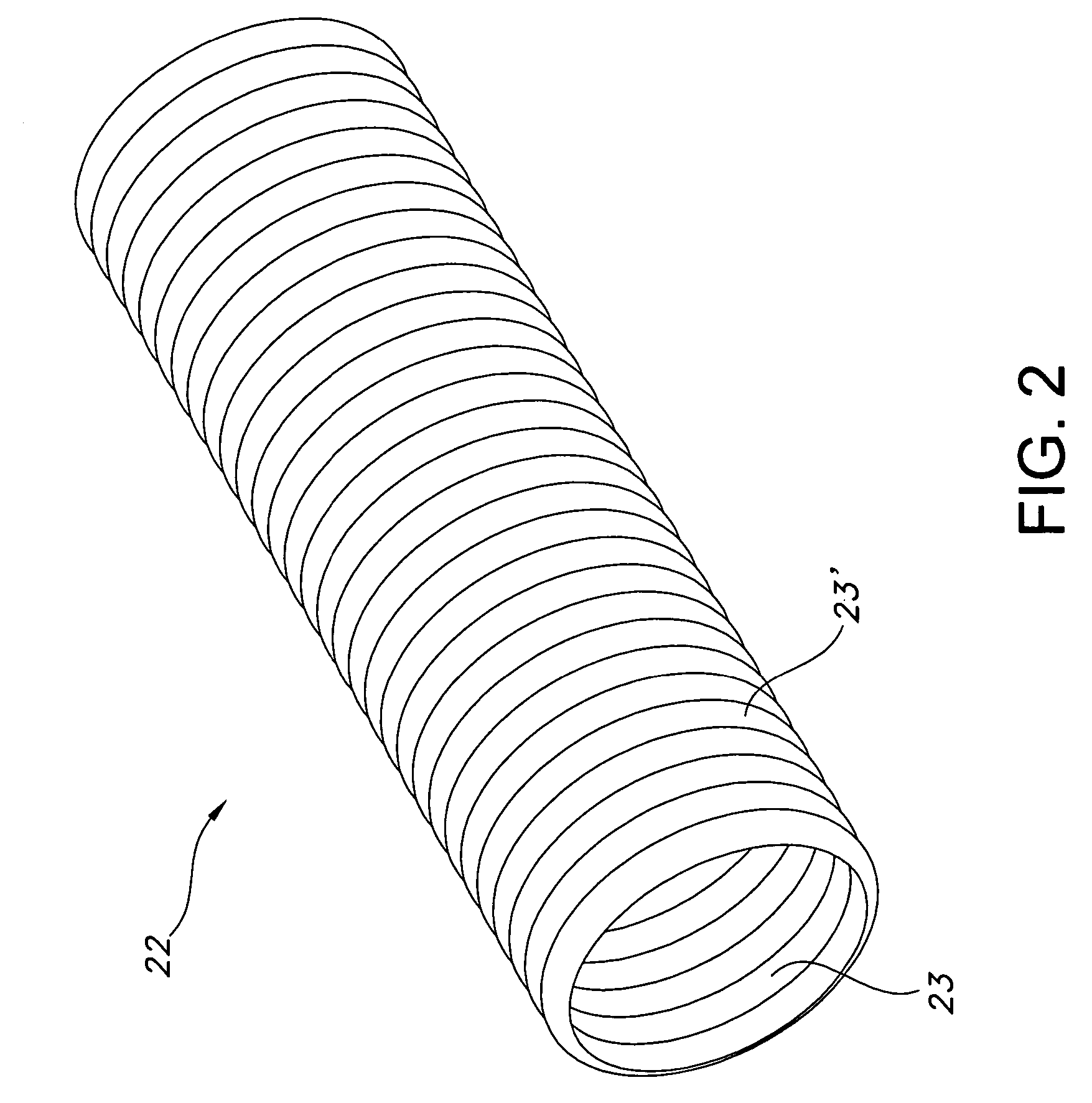
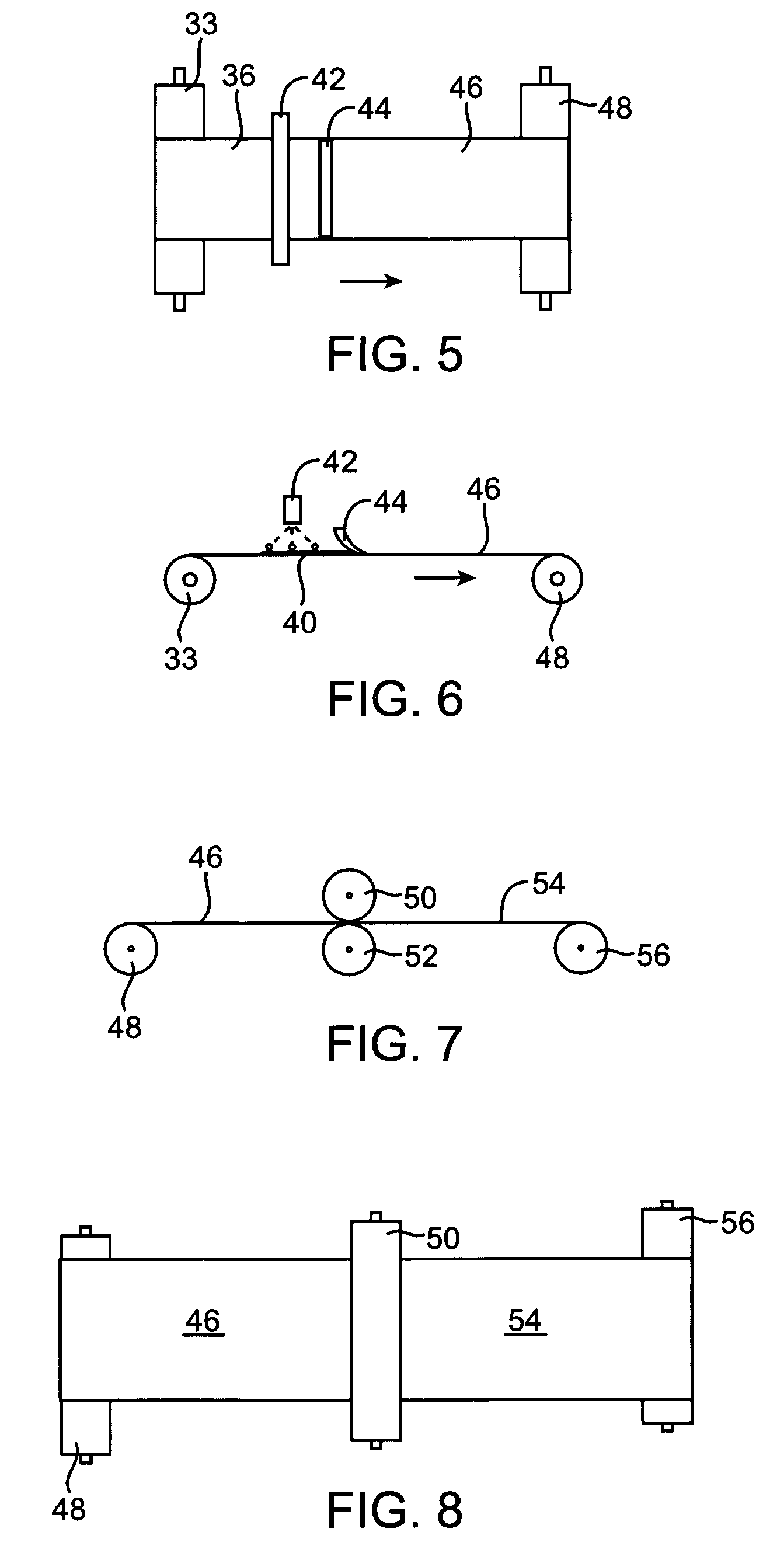
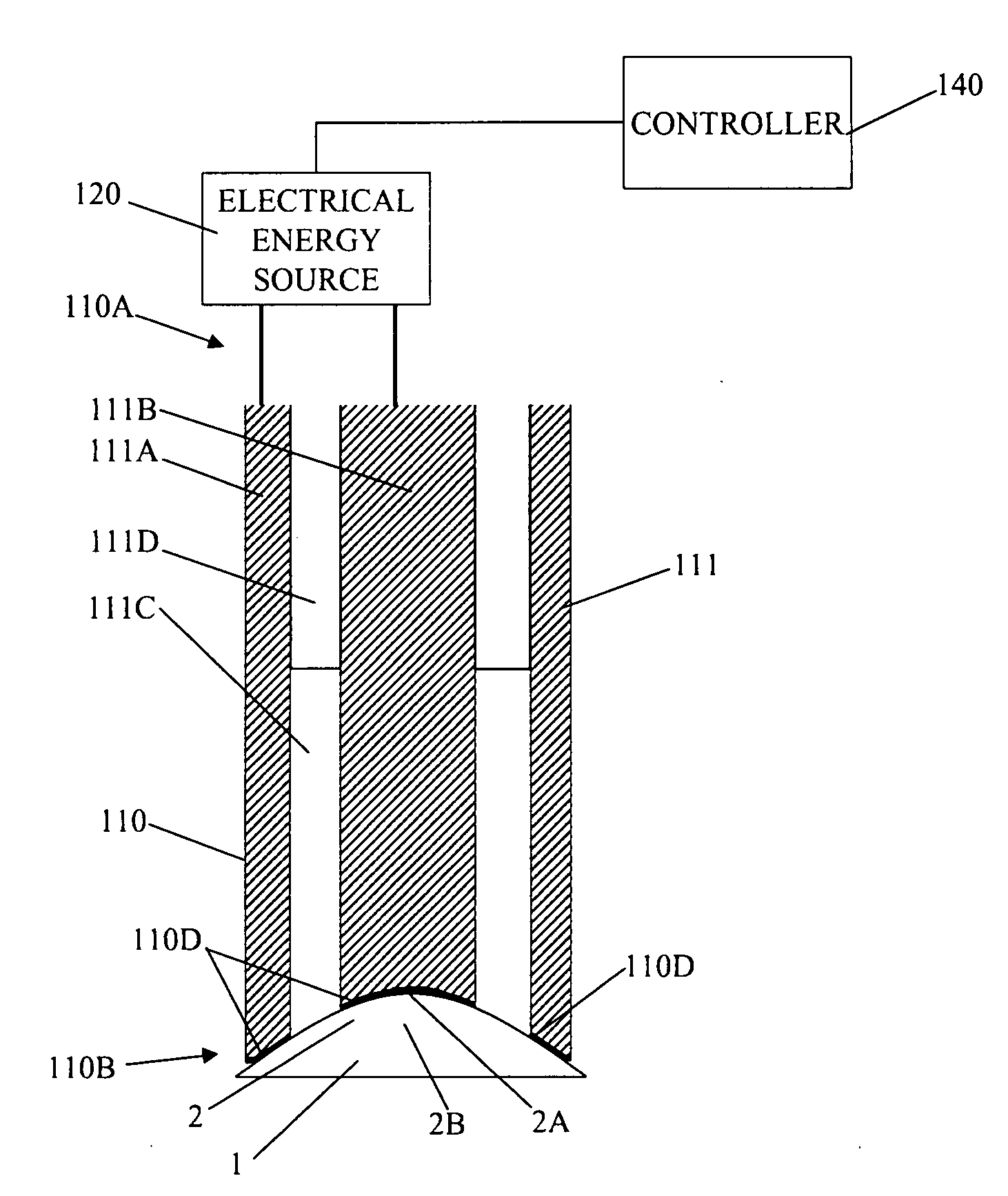
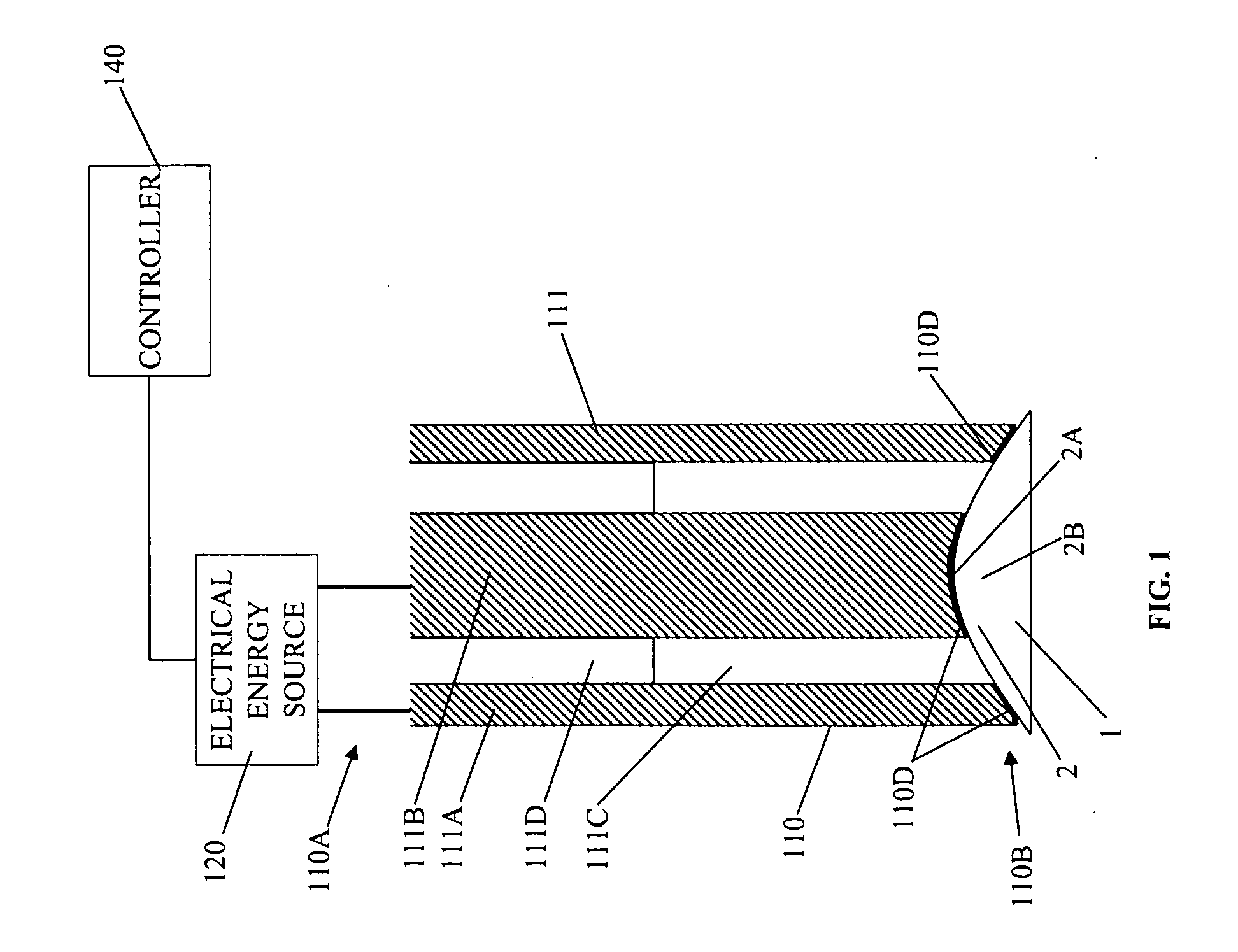
Tissue scaffold having aligned fibrils, apparatus and method for producing the same, and artificial tissue and methods of use thereof
ActiveUS20050009178A1Improve structural strengthMinimal immunological responsePeptide/protein ingredientsHollow filament manufactureFiberRadial position
A tubular tissue scaffold is described which comprises a tube having a wall, wherein the wall includes biopolymer fibrils that are aligned in a helical pattern around the longitudinal axis of the tube where the pitch of the helical pattern changes with the radial position in the tube wall. The scaffold is capable of directing the morphological pattern of attached and growing cells to form a helical pattern around the tube walls. Additionally, an apparatus for producing such a tubular tissue scaffold is disclosed, the apparatus comprising a biopolymer gel dispersion feed pump that is operably connected to a tube-forming device having an exit port, where the tube-forming device is capable of producing a tube from the gel dispersion while providing an angular shear force across the wall of the tube, and a liquid bath located to receive the tubular tissue scaffold from the tube-forming device. A method for producing the tubular tissue scaffolds is also disclosed. Also, artificial tissue comprising living cells attached to a tubular tissue scaffold as described herein is disclosed. Methods for using the artificial tissue are also disclosed.
Owner:UNIVERSITY OF SOUTH CAROLINA
Polymeric web exhibiting a soft and silky tactile impression
A polymeric web exhibiting a soft and silky tactile impression on at least one side thereof is disclosed. The silky feeling side of the web exhibits a pattern of discrete hair-like fibrils, each of the hair-like fibrils being a protruded extension of the web surface and having a side wall defining an open proximal portion and a closed distal portion. The hair-like fibrils exhibit a maximum lateral cross-sectional diameter of between 2 and 5 mils, and an aspect ratio from 1 to 3. Methods and apparatus for making the polymeric web utilize a three-dimensional forming structure having a plurality of protrusions being generally columnar forms having an average aspect ratio of at least about 1.
Owner:THE PROCTER & GAMBLE COMPANY
Elastomerically impregnated ePTFE to enhance stretch and recovery properties for vascular grafts and coverings
This invention relates to an elastomerically recoverable PTFE material that includes a longitudinally compressed fibrils of ePTFE material penetrated by elastomeric material within the pores defining the elastomeric matrix. The elastomeric matrix and the compressed fibrils cooperatively expand and recover without plastic deformation of the ePTFE material. This invention was used for various prosthesis, such as a vascular prosthesis like a patch, a graft and an implantable tubular stents. Furthermore, this invention discloses a method of producing the elastomerically recoverable PTFE material which include the steps of: providing the specified ePTFE, defined by the nodes and fibrils, to meet the desired end use; longitudinally compressing the fibrils of the ePTFE, the pore size sufficiently enough to permit penetration of the elastomeric material; applying the elastomeric material within the pores to provide a structurally integral elastomerically recoverable PTFE material defining an elastomeric matrix. The elastomeric material is applied to the ePTFE by dip, brush or spray coating techniques. The compression step and the application steps are interchangeable to produce the desired properties for the end use material. Finally, the elastomeric material is dried within the pores of the longitudinally compressed ePTFE to solidify the elastomeric matrix.
Owner:LIFESHIELD SCI
Expanded UHMWPE for guiding catheter liners and other lubricious coatings
An intraluminal catheter, such as a guiding catheter, employed for intravascular procedures and having an inner liner formed of expanded Ultra High Molecular Weight Polyethylene (UHMWPE) is disclosed. The expanded UHMWPE is microporous and has an oriented microstructure structure characterized by nodes interconnected by fibrils. The inner liner formed of expanded UHMWPE is very thin to maximize the inner lumen diameter and has excellent mechanical properties.
Owner:ABBOTT CARDIOVASCULAR
Method of making a polymeric web exhibiting a soft and silky tactile impression
A polymeric web exhibiting a soft and silky tactile impression on at least one side thereof is disclosed. The silky feeling side of the web exhibits a pattern of discrete hair-like fibrils, each of the hair-like fibrils being a protruded extension of the web surface and having a side wall defining an open proximal portion and a closed distal portion. The hair-like fibrils exhibit a maximum lateral cross-sectional diameter of between 2 and 5 mils, and an aspect ratio from 1 to 3. Methods and apparatus for making the polymeric web utilize a three-dimensional forming structure having a plurality of protrusions being generally columnar forms having an average aspect ratio of at least about 1.
Owner:THE PROCTER & GAMBLE COMPANY
Elastomerically impregnated ePTFE to enhance stretch and recovery properties for vascular grafts and coverings
An elastomerically recoverable PTFE material is provided including a longitudinally compressed fibrils of ePTFE material penetrated by elastomeric material within the pores defining the elastomeric matrix. The elastomeric matrix and the compressed fibris cooperatively expand and recover without plastic deformation of the ePTFE material. The material may be used for various prosthesis, such as a vascular a prosthesis like a patch, a graft and an implantable tubular stent. Further, a method of producing the elastomerically recoverable PTFE material is provided herein.
Owner:LIFESHIELD SCI
Knit PTFE Articles and Mesh
Disclosed is a knitted article, and a method of producing such an article, having at least one PTFE fiber with oriented fibrils forming multiple fiber cross-over points wherein PTFE fiber is self-bonded in at least one of the cross-over points.
Owner:WL GORE & ASSOC INC
Pressure lamination method for forming composite ePTFE/textile and ePTFE/stent/textile prostheses
A method of forming a composite textile and ePTFE implantable device includes the steps of (a) providing an ePTFE layer having opposed surfaces comprising a microporous structure of nodes interconnected by fibrils; (b) providing a textile layer having opposed surfaces; (c) applying a coating of an elastomeric bonding agent to one of the opposed surfaces of the ePTFE layer or the textile layer; (d) providing a hollow member having an open end and an opposed closed end defining a fluid passageway therebetween and having a wall portion with at least one hole extending therethrough, the hole being in fluid communication with the fluid passageway; (e) concentrically placing the ePTFE layer and the textile layer onto the hollow member and over the at least one hole of the hollow member to provide an interior composite layer and an exterior composite layer, thereby defining a composite assembly, wherein the interior composite layer is one of the ePTFE layer or the textile layer and the exterior composite layer is the other of the ePTFE layer or the textile layer; (f) placing the hollow member with the composite assembly within a pressure chamber; (g) applying a pressure differential so that the pressure within the chamber is greater than a pressure within the fluid passageway of the hollow member; and (h) applying heat to the bonding agent to adhesively bond the textile layer and the ePTFE layer to provide a laminated composite assembly.
Owner:LIFESHIELD SCI
PTFE layers and methods of manufacturing
InactiveUS20060233991A1Reduce porosityHigh degreeStentsSynthetic resin layered productsPorosityFibril
Thin PTFE layers are described having little or no node and fibril microstructure and methods of manufacturing PTFE layers are disclosed that allow for controllable permeability and porosity of the layers. In some embodiments, the PTFE layers may act as a barrier layer in an endovascular graft or other medical device.
Owner:TRIVASCULAR2
Methods and catalysts for the manufacture of carbon fibrils
An improved catalyst for producing carbon fibrils is made by incorporating an effective yield-enhancing amount of a carboxylate into a fibril-forming catalyst. Alternatively, such a catalyst is made by coprecipitating a compound of a metal having fibril-forming catalytic properties and an aluminum and / or magnesium compound, optionally in the presence of carbon particles or carbon fibril aggregates. The catalyst may also be made by incorporating a compound of a fibril-forming metal onto magnesia particles in carbon particles or carbon fibril aggregates. The catalysts, methods of using them to form carbon fibrils and those carbon fibrils are also disclosed.
Owner:HYPERION CATALYSIS INT
Elastomerically impregnated ePTFE to enhance stretch and recovery properties for vascular grafts and coverings
Owner:LIFESHIELD SCI
Knit PTFE Articles and Mesh
Disclosed is a knitted article, and a method of producing such an article, having at least one PTFE fiber with oriented fibrils forming multiple fiber cross-over points wherein PTFE fiber is self-bonded in at least one of the cross-over points.
Owner:WL GORE & ASSOC INC
PTFE layers and methods of manufacturing
Single, continuous PTFE layers having lateral zones of varied characteristics are described. Some of the lateral zone embodiments may include PTFE material having little or no nodal and fibril microstructure. Methods of manufacturing PTFE layers allow for controllable permeability and porosity of the layers, in addition to other characteristics. The characteristics may vary from one lateral zone of a PTFE layer to a second lateral zone of a PTFE layer. In some embodiments, the PTFE layers may act as a barrier layer in an endovascular graft or other medical device.
Owner:TRIVASCULAR2 +1
Eye therapy system
InactiveUS20090149842A1Process stabilityImproving its biomechanical strengthLaser surgeryDiagnosticsCross-linkFibril
Heat is generated in corneal fibrils in a cornea of an eye according to a selected pattern. The heat causes the corneal fibrils corresponding to the selected pattern to transition from a first structure to a second structure. The second structure provides a desired reshaping of the cornea. A cross-linking agent is then activated in the region of corneal fibrils according to the selected pattern. The cross-linking agent prevents the corneal fibrils from changing from the second structure. Thus, embodiments stabilize corneal tissue and improve its biomechanical strength after desired structural changes have been achieved in the corneal tissue. Accordingly, the embodiments help to preserve the desired reshaping of the cornea.
Owner:AVEDRO
Cellulose-based fibrous materials
InactiveUS8012312B2Low densityImprove surface qualityCellulosic pulp after-treatmentCalendersFiberPolymer science
The present invention aims to provide cellulose-based fibrous materials for obtaining papers and sheets having low density, high surface quality, good size stability despite of high strength, and high opacity. Cellulose-based fibrous materials having external fibrils consisting of an assembly of scale-like microfibrils exhibit a higher fiber stiffness, a lower water retention value and a higher specific surface area as compared with fibrous materials having filamentous external fibrils at the same freeness. Papers and sheets having low density, high surface quality, good size stability and high opacity can be obtained by using such fibrous materials.
Owner:NIPPON PAPER IND CO LTD
Medical device formed of ultrahigh molecular weight polyolefin
Medical devices having at least a component, such as a catheter balloon, stent cover and vascular graft, formed of ultrahigh molecular weight polyolefin, such as ultrahigh molecular weight polyethylene. The device component is formed from ultrahigh molecular weight polyethylene that has been processed so that it is microporous and has an oriented node and fibril structure. The device component expands compliantly at low strains and are substantially less compliant at higher strains. The invention also comprises methods for making such medical devices, including the steps of compacting a polyethylene powder and deforming it to impart the oriented structure.
Owner:ABBOTT CARDIOVASCULAR
Flexible Stent-Graft Device Having Patterned Polymeric Coverings
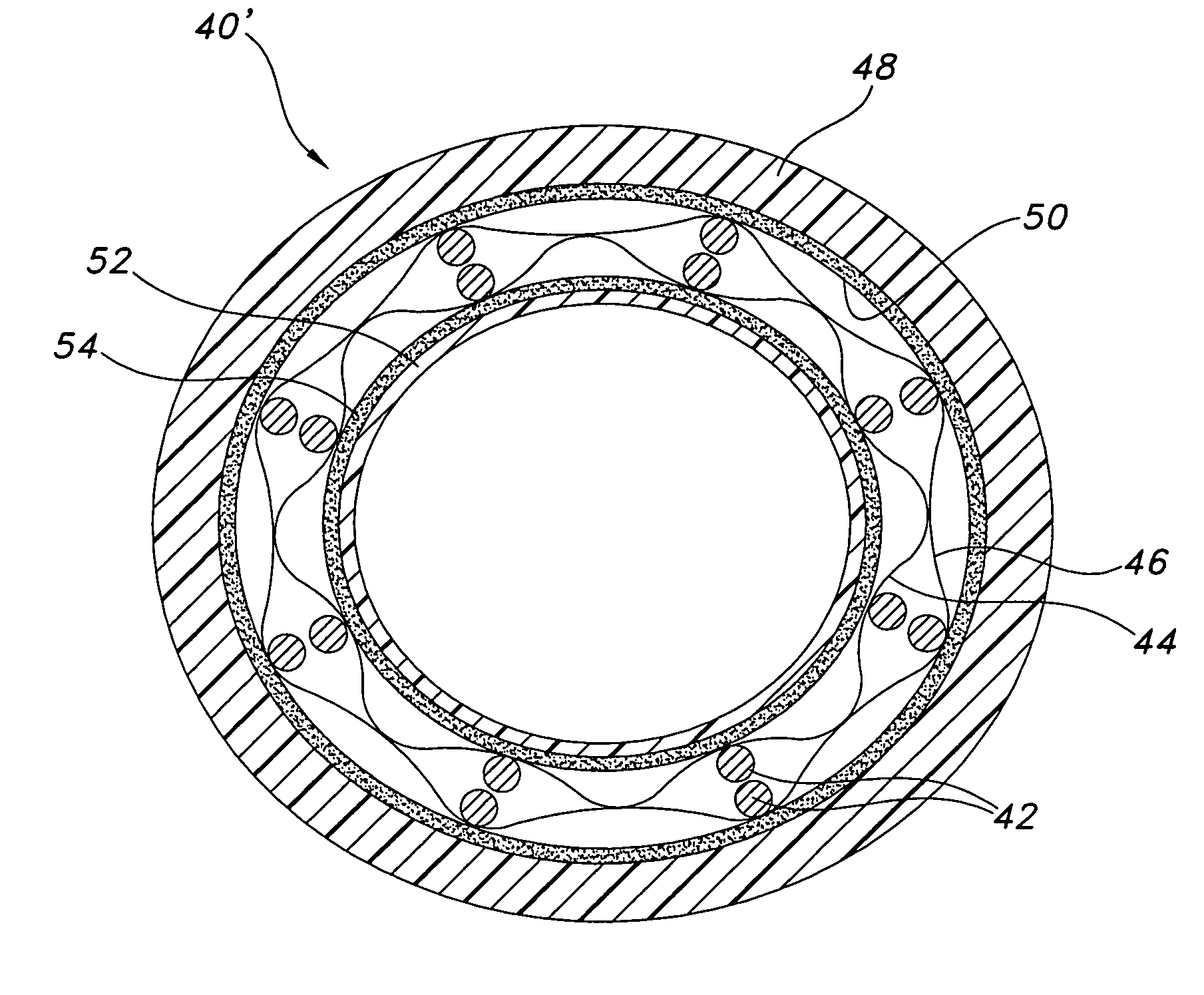
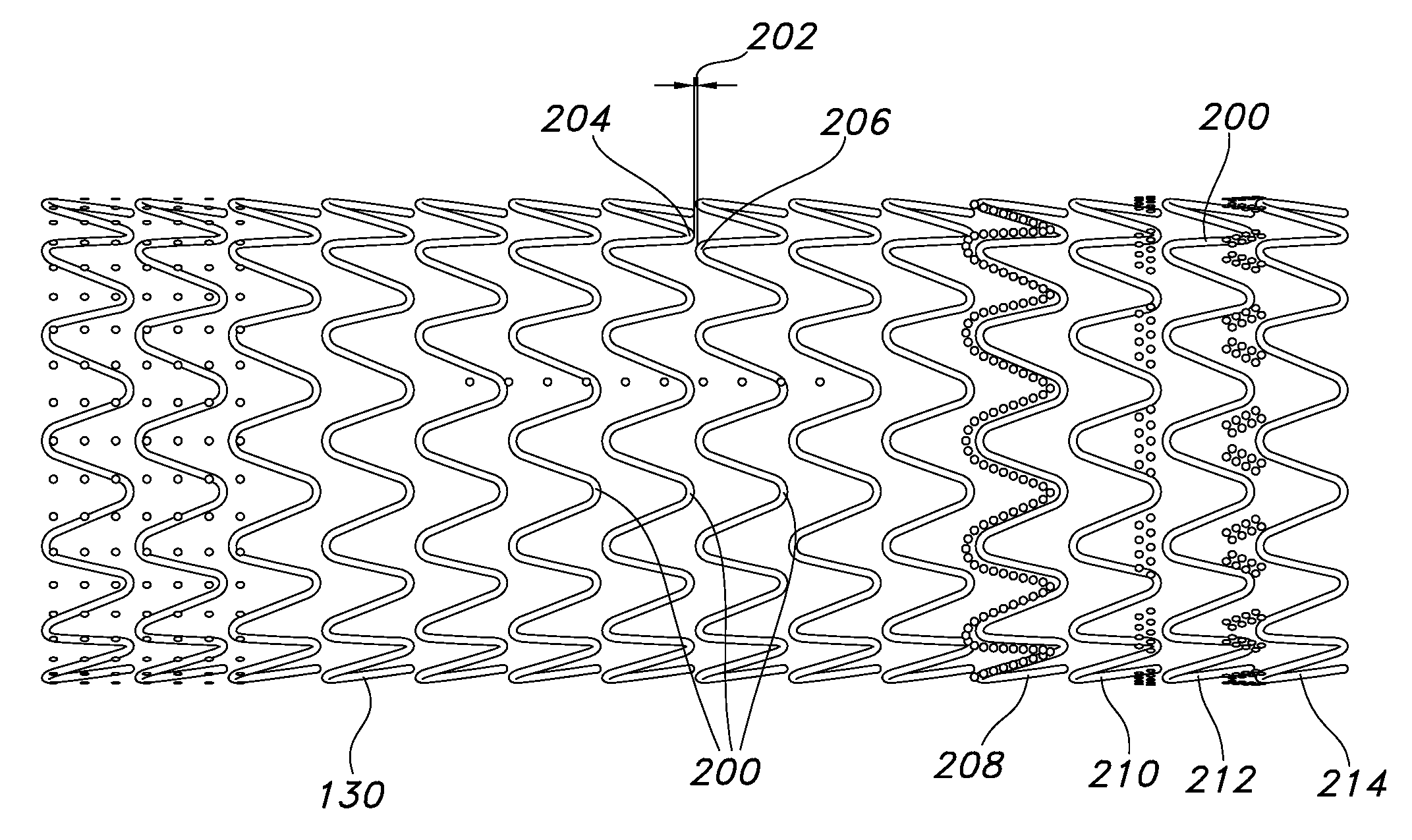
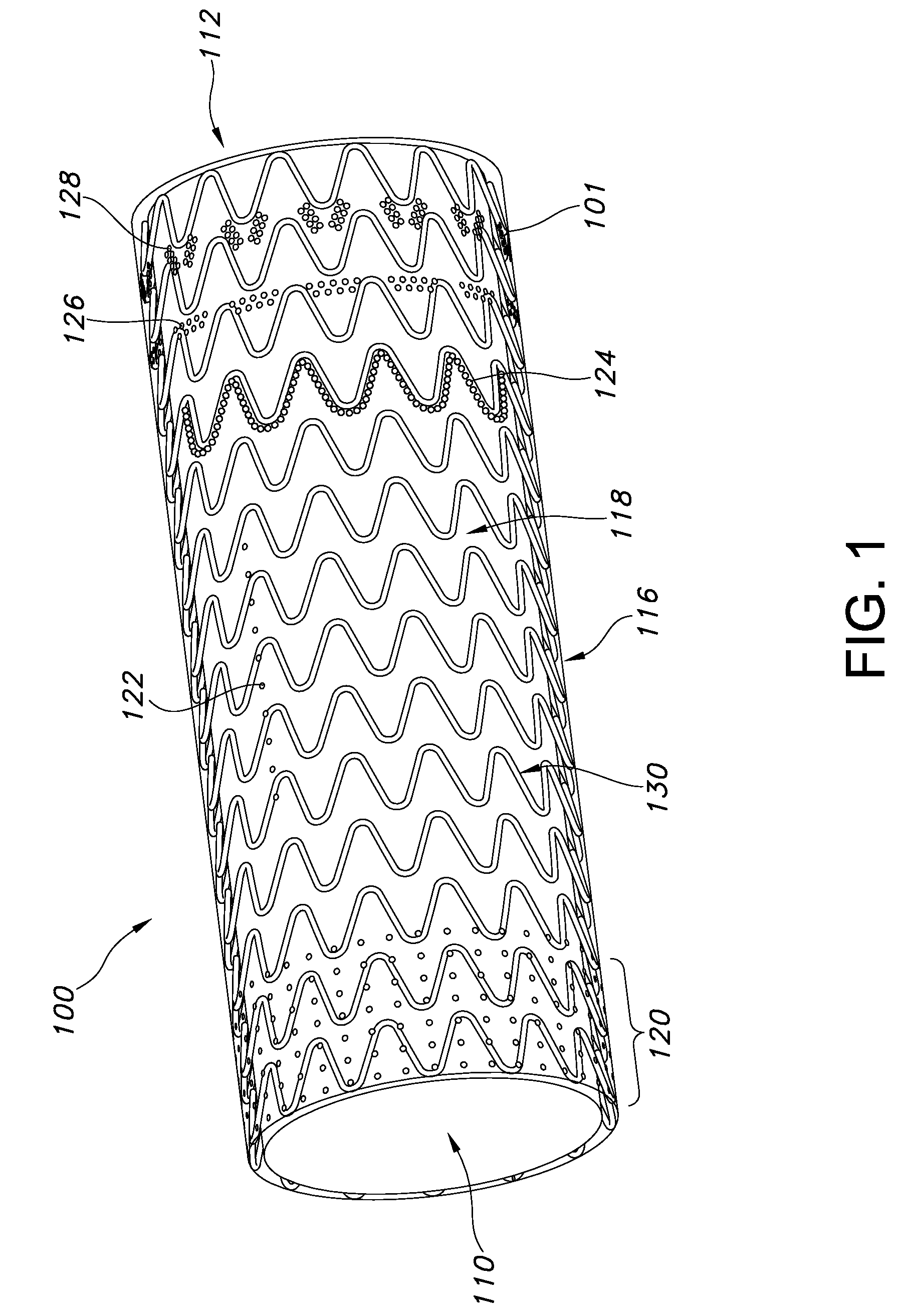
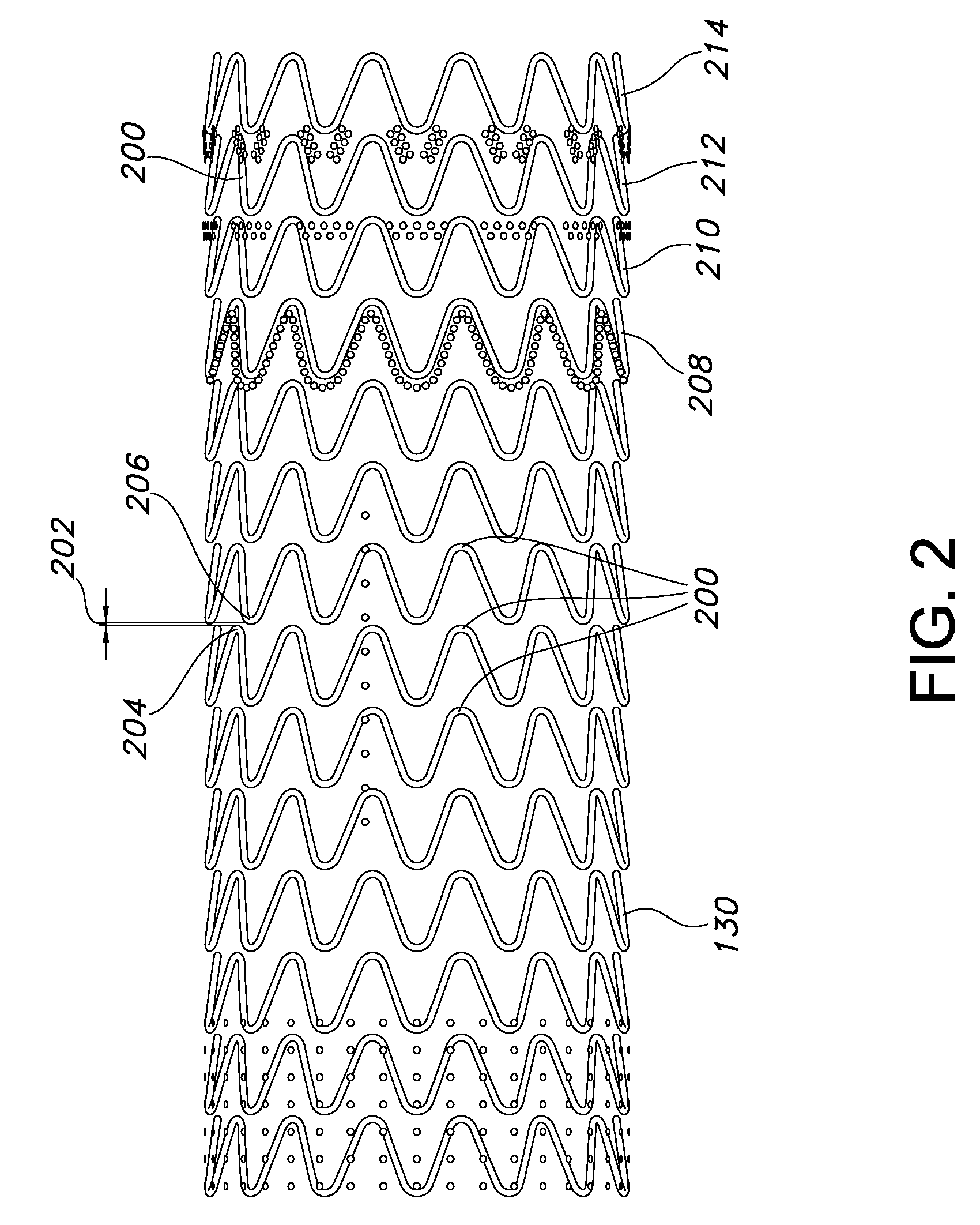
The present invention provides an endoprosthesis comprising a radially distensible, tubular stent comprising opposed open ends and a stent wall structure having opposed exterior and luminal surfaces; and a continuous and seamless ePTFE tubular covering having a node and fibril structure securably disposed to at least one of the stent surfaces, the graft covering comprising at least two laminated layers, wherein the laminated layers include at least one region where the laminated layers are not securely bonded; and the at least one region has a different bending flexibility from the tubular graft covering.
Owner:LIFESHIELD SCI
Graphitic nanofibers in electrochemical capacitors
InactiveUS20030030963A1Improve performanceMaterial nanotechnologyFixed capacitor electrodesFiberFibril
Owner:HYPERION CATALYSIS INT
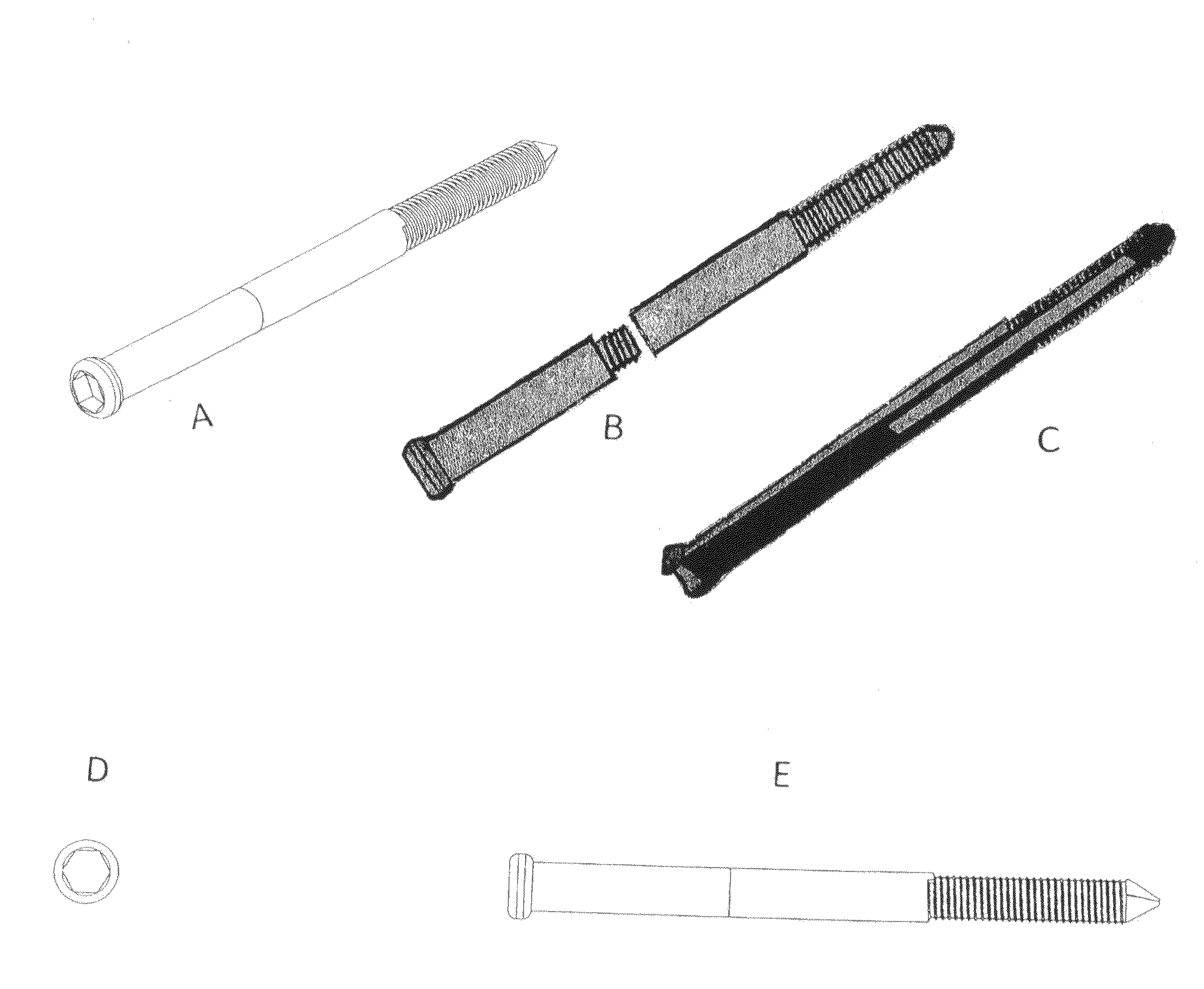
Poly-porous hollow screw for target delivery of growth factors and stem cells:the design and potential clinical application
Present invention depicts a poly-porous (micropore) hollow screws as diffusion chamber filled with core matrix for targeted delivery of growth factors and bone marrow stem cells. The screws comprise at least two parts: the distal part of the screw consists of the tip of the screw made of poly porous material and hollow inside proximally. It has threaded navel attached to the threaded nipple of the distal part of the proximal screw which has the screw head and is made of the solid material of the same kind. The screw head had hexagonal recess targeted for screw driver insertion. Assembly of screw created a chamber in the middle of the screw. The chamber is filled with core matrix consisting of gelatin nano-particles pre-impregnated with BMPs (BMP2 / BMP7 for bone or BMP12 for tendon, ligament) and fibrin sealants or Chitosan dispersed with bone marrow stem cells and / or other growth factors. Bioactive protein core material is prepared during the surgery and filled the chamber of the screw by the surgeon. Fibrin sealants or Chitosan will polymerize to form a gel to hold the growth factors and stem cell in place. The screw can be used as the lag screw or other function to provide mechanical fixation in variety of condition. Once the screw implanted in the human body, the fibrin sealant or Chitosin / gelatin nano-particles are gradually degraded and slowly release growth factors and stem cells via micropores of screw to facilitate the bone healing and regeneration. The gelatin nanoparticles and fibril sealant / or Chitosan matrix also serve as the scaffold and platform for bone in-growth to the screw or alternatively, the stem cell inside of screw can regenerate new bone, providing the biological fixation. At the mean time as the bone regenerate and / or in growth, mechanical strength of the screw increased.
Owner:WU YANGGUAN
Surface modification of ePTFE and implants using the same
A method for modifying an ePTFE surface by plasma immersion ion implantation includes the steps of providing an ePTFE material in a chamber suitable for plasma treatment; providing a continuous low energy plasma discharge onto the sample; and applying negative high voltage pulses of short duration to form a high energy ion flux from the plasma discharge to generate ions which form free radials on the surface of the ePTFE material without changing the molecular and / or physical structure below the surface to define a modified ePTFE surface. The step of applying the high voltage pulses modifies the surface of the ePTFE without destroying the node and fibril structure of the ePTFE, even when the step of applying the high voltage pulses etches and / or carburizes the surface of the ePTFE. The modified surface may have a depth of about 30 nm to about 500 nm. The ions are dosed onto the ePTFE sample at concentrations or doses from about 1013 ions / cm2 to about 1016 ions / cm2.
Owner:LIFESHIELD SCI
Pleated composite ePTFE/textile hybrid covering
InactiveUS7510571B2Enhanced tissue ingrowthHigh suture retention strengthStentsLayered productsFiberFibril
A composite multilayer implantable material having a first inner tubular layer formed of expanded polytetrafluoroethyene having a porous microstructure defined by nodes interconnected by fibrils, wherein said first layer has a plurality of pleated folds, a second tubular layer formed of textile material circumferentially disposed exteriorly to said first layer; and having an elastomeric bonding agent applied to one of said first layer or second layer and disposed within the pores of said microstructure for securing said first layer to said second layer.
Owner:LIFESHIELD SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com