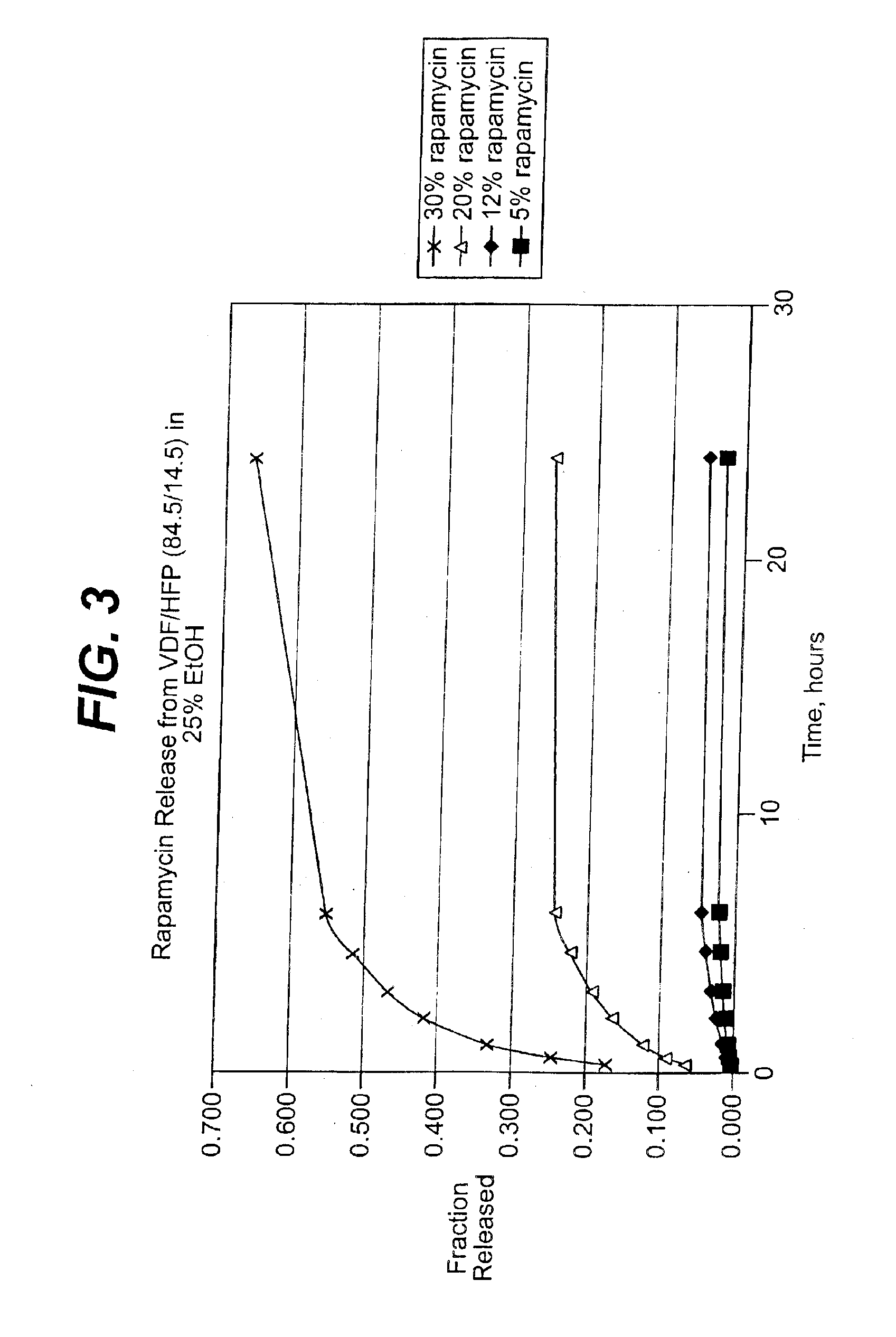
Patents
Literature
18189results about "Stents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
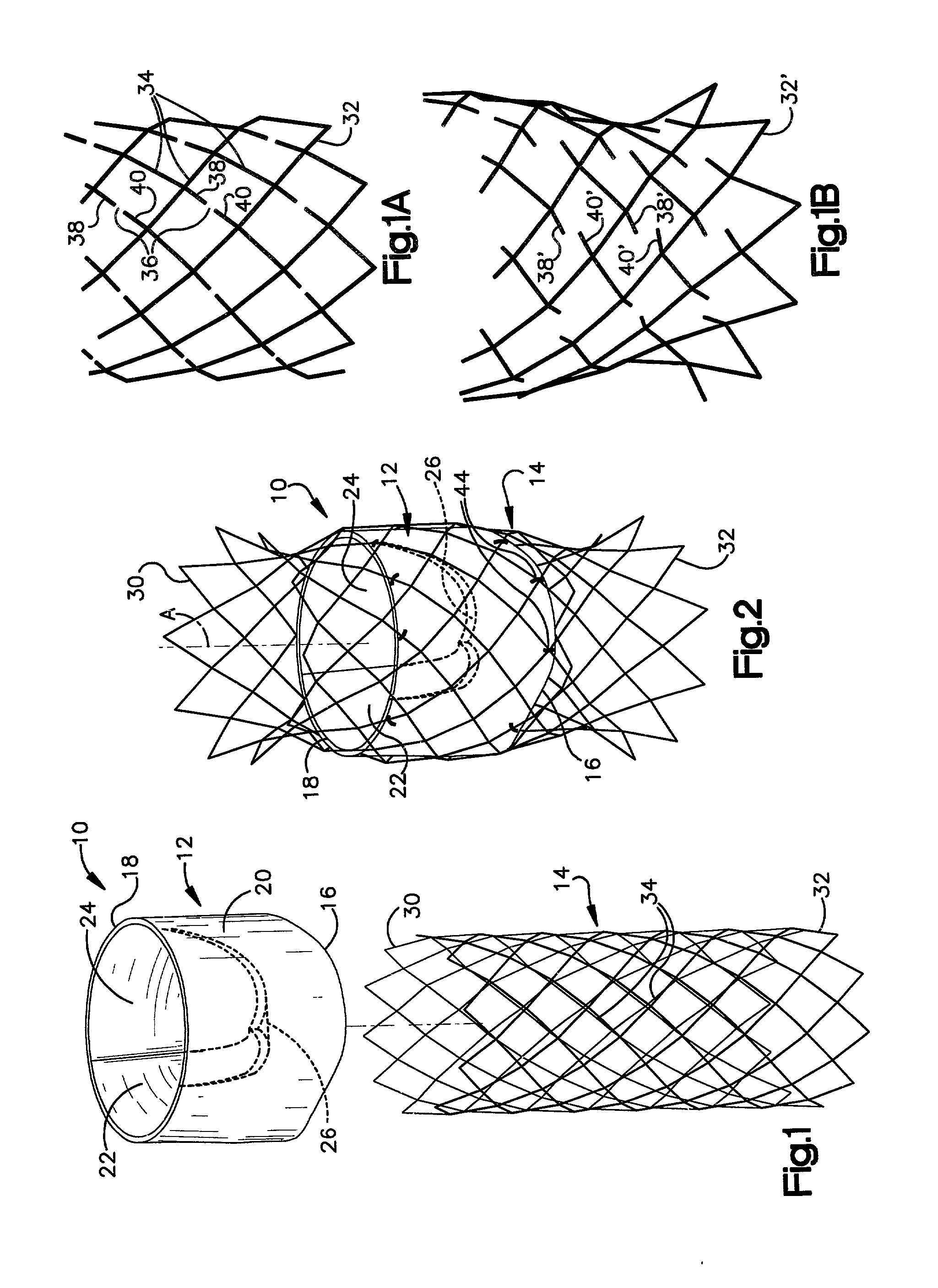
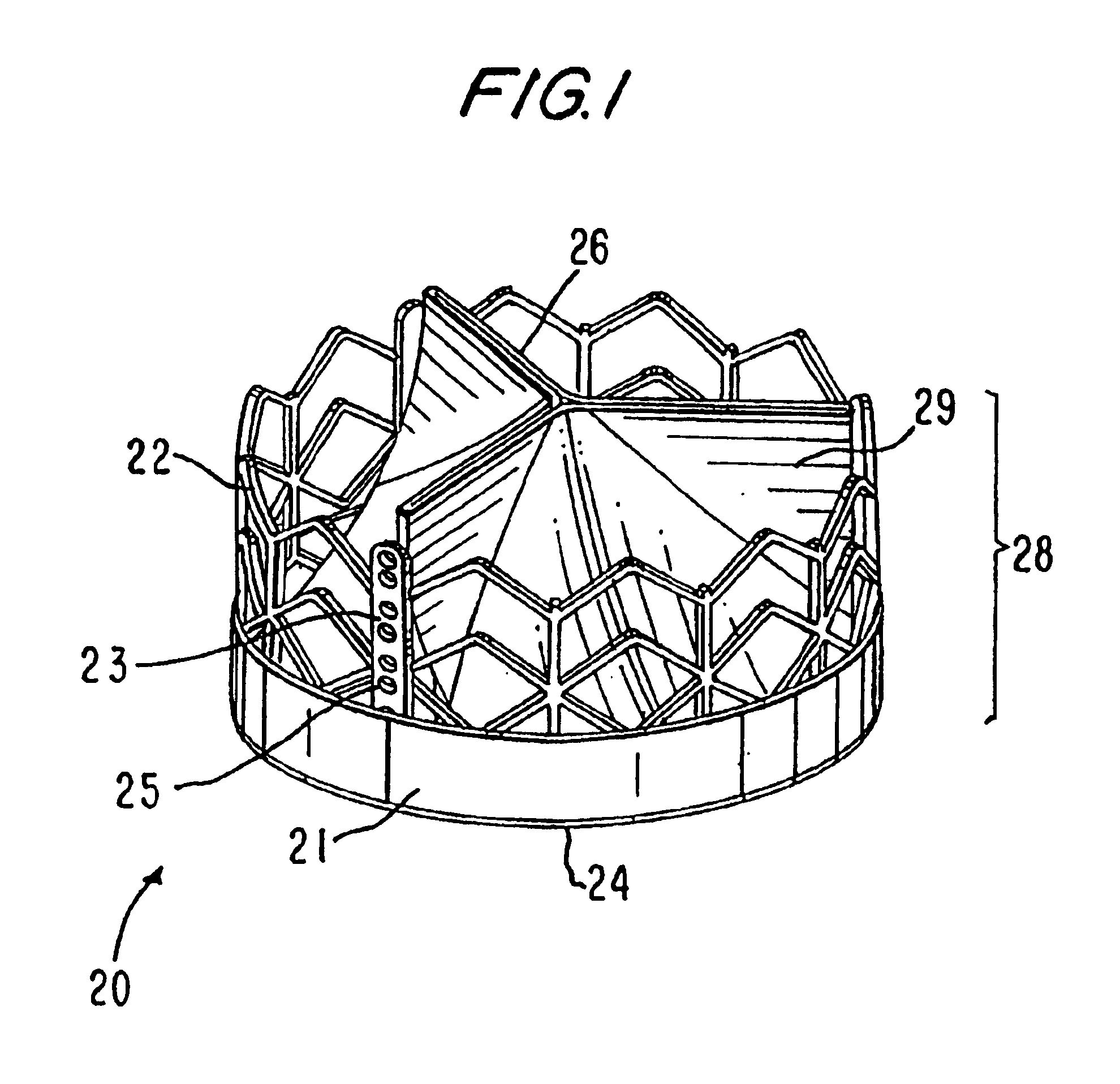
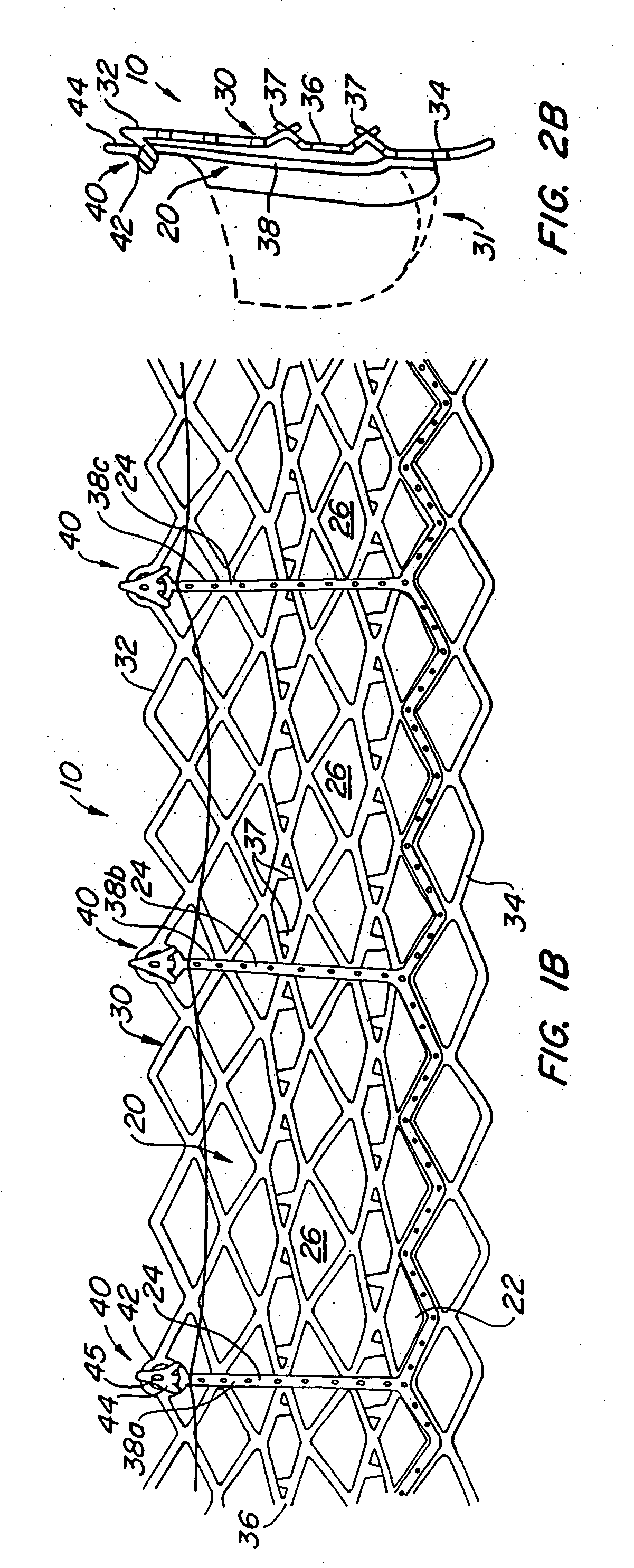
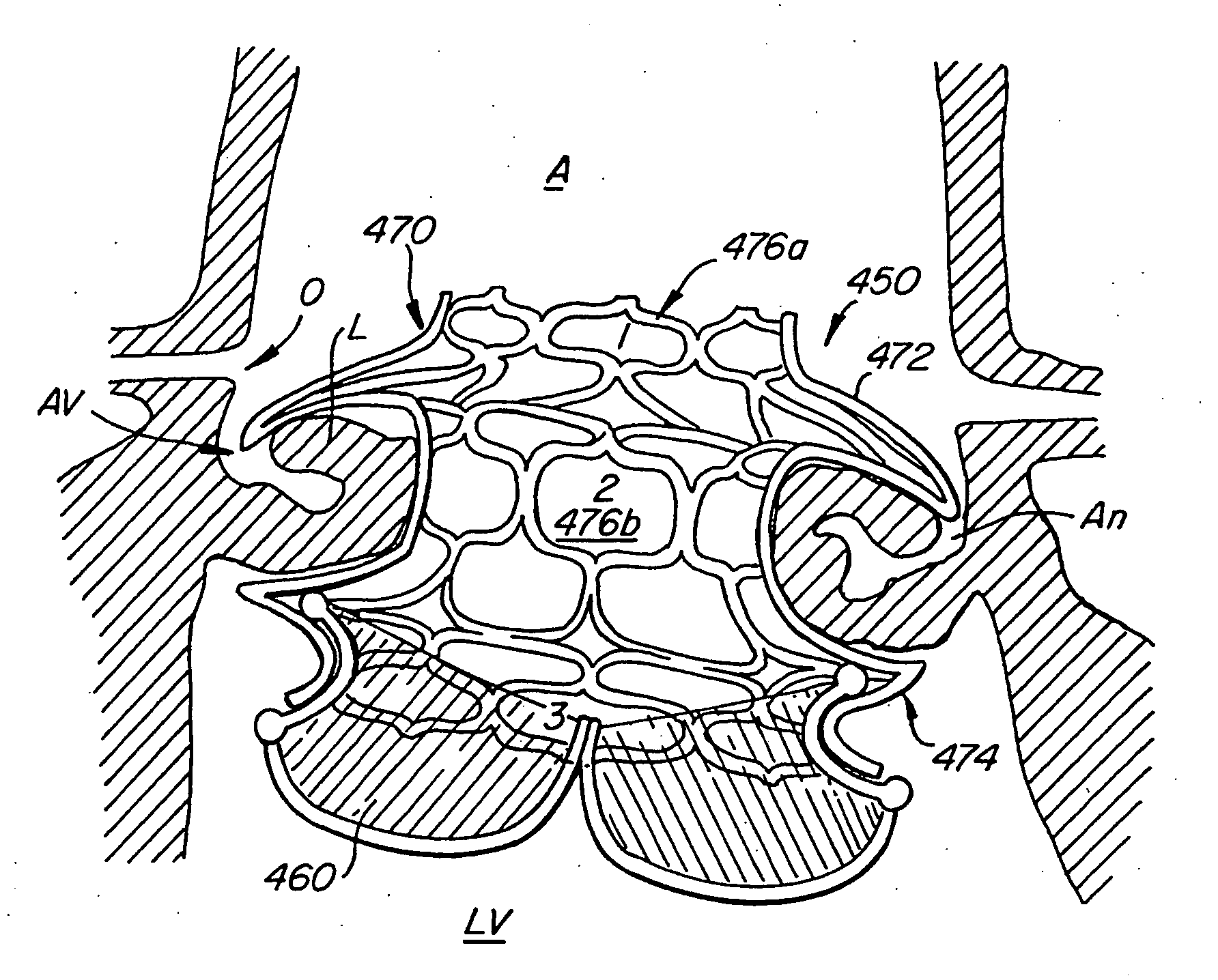
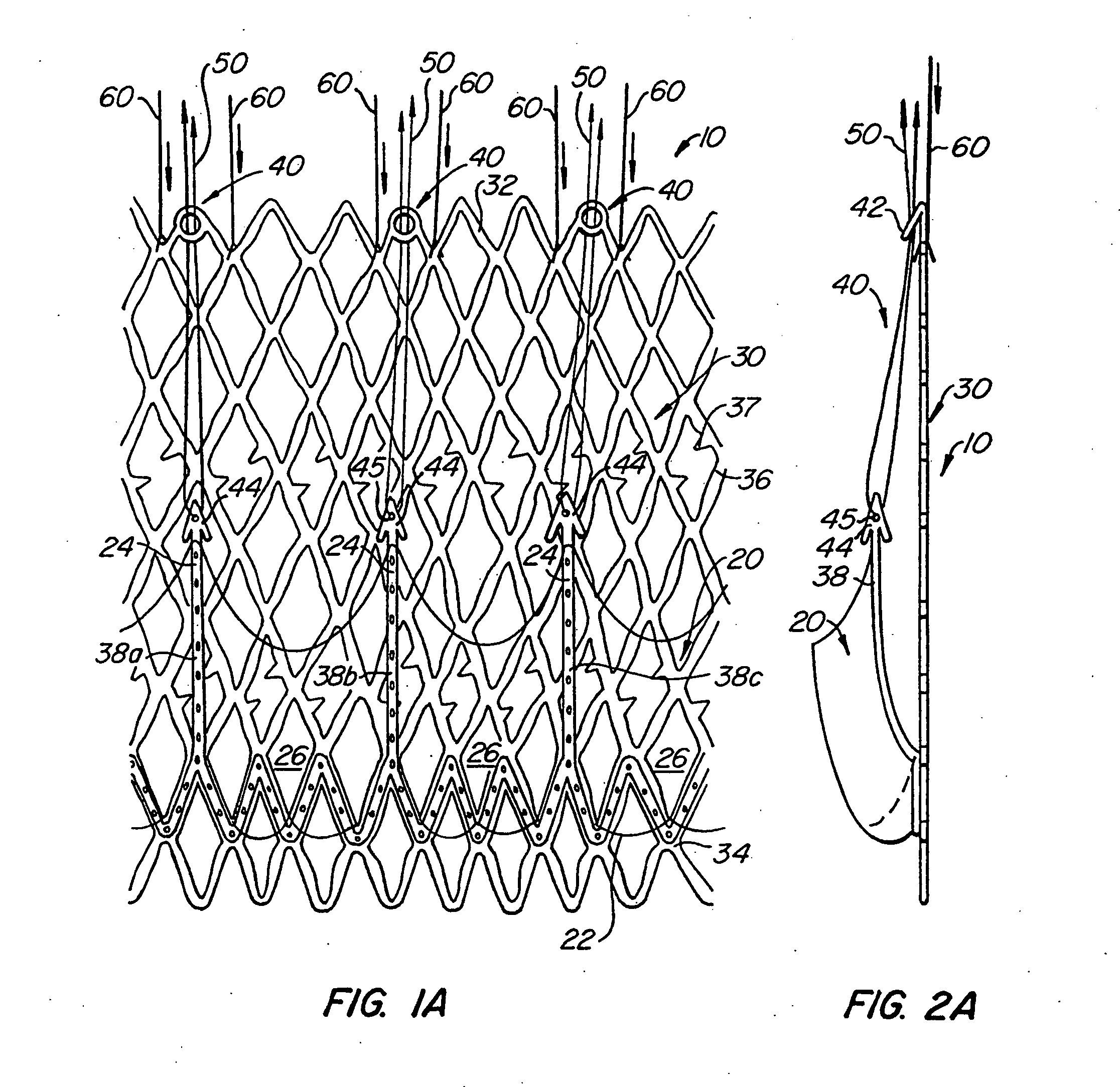
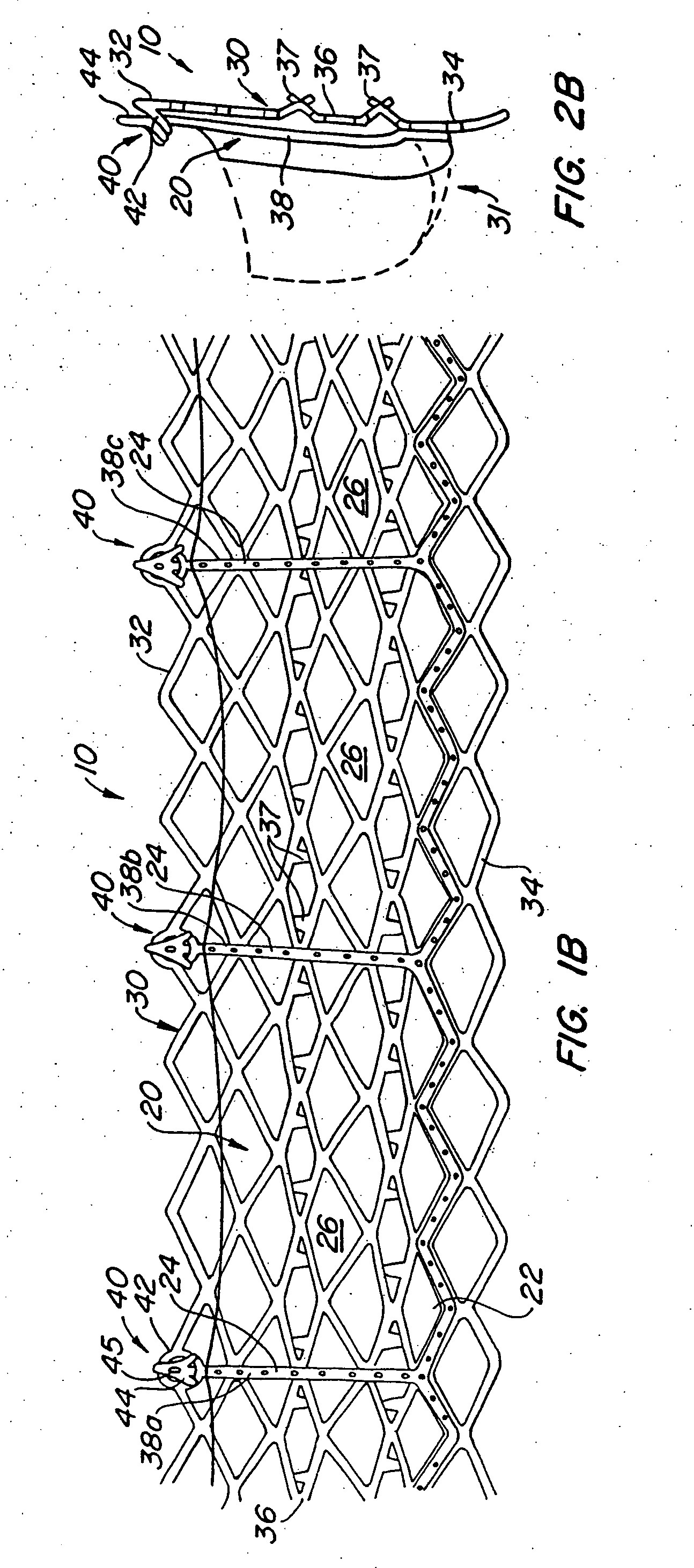
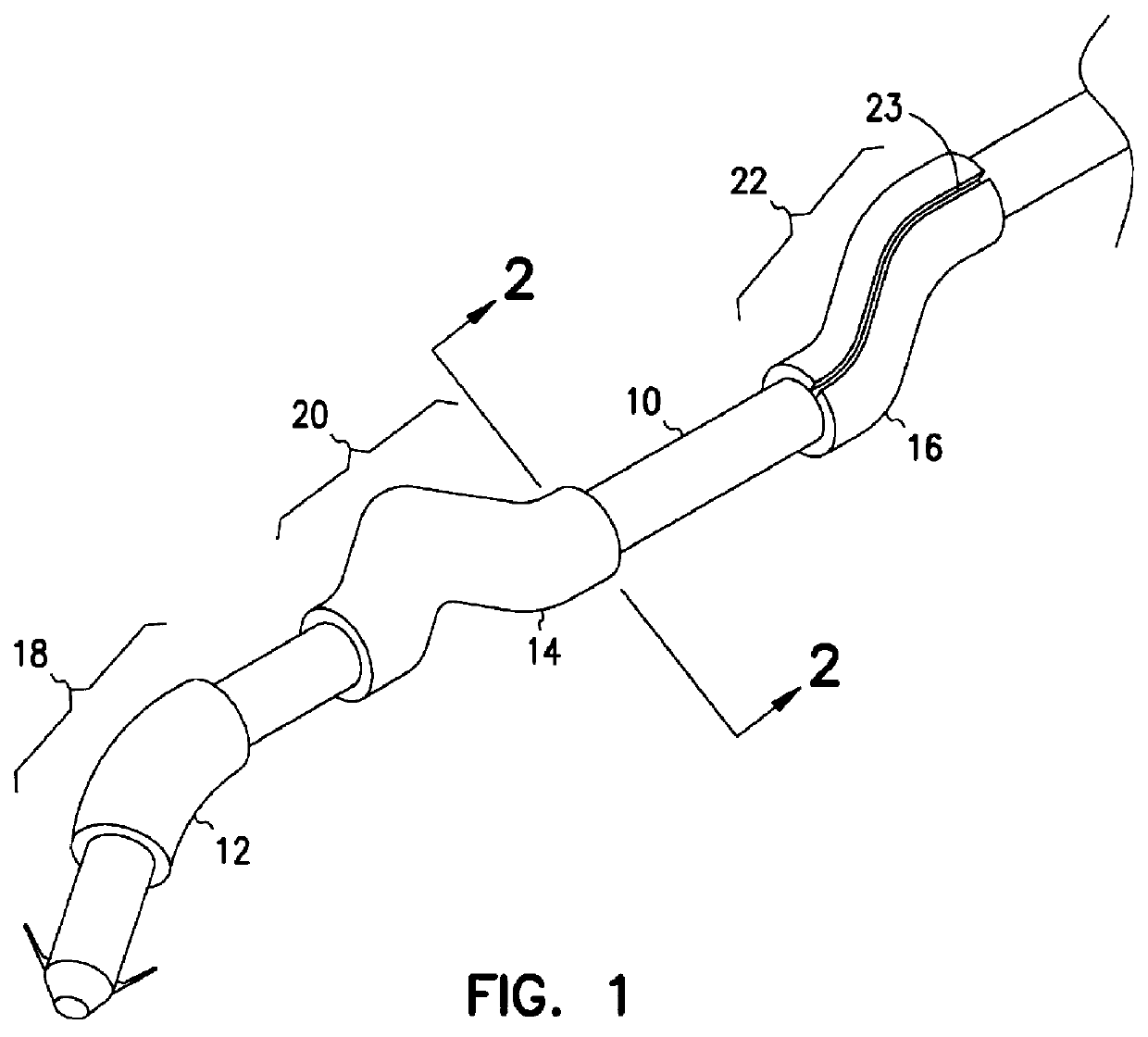
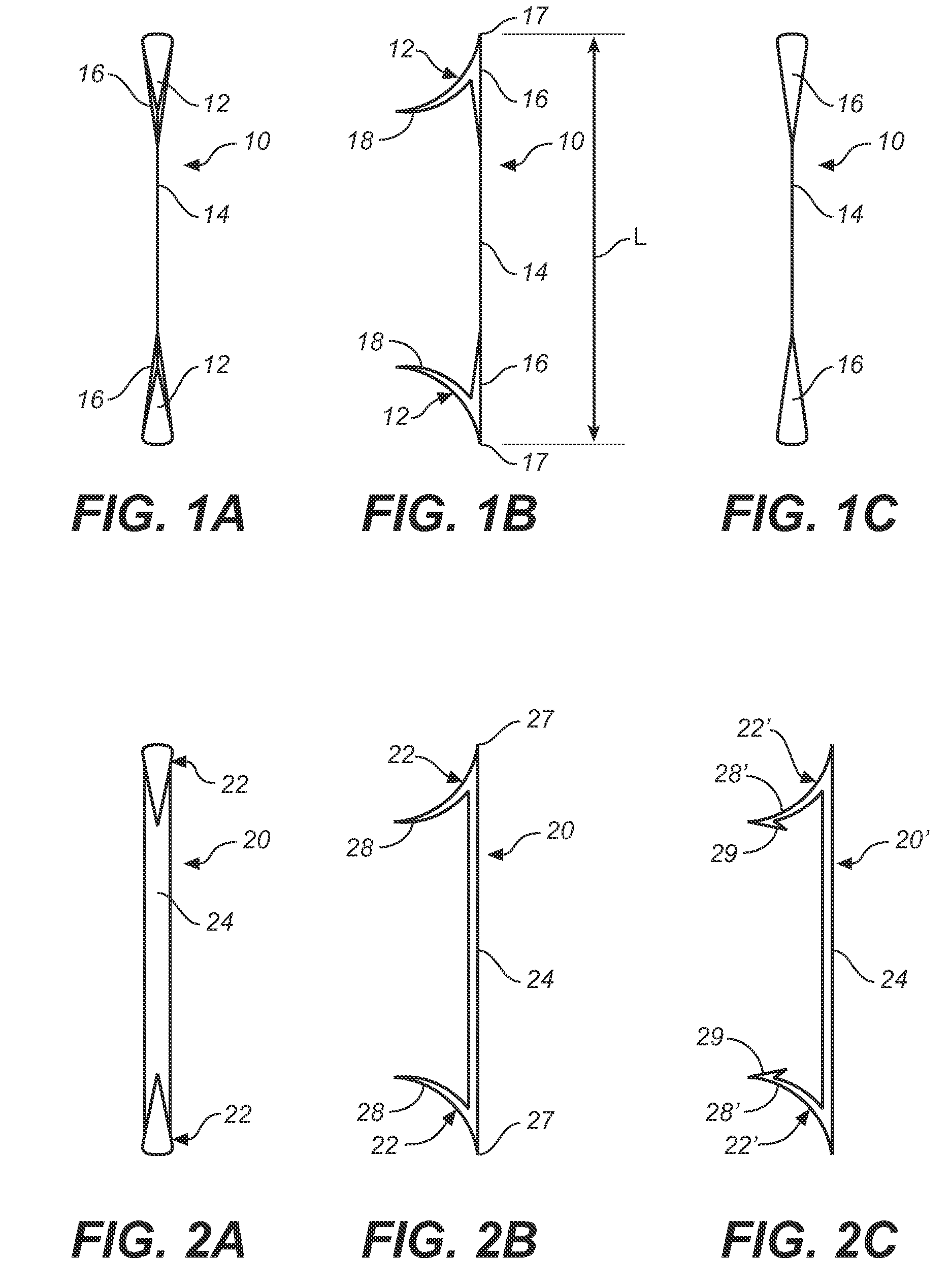
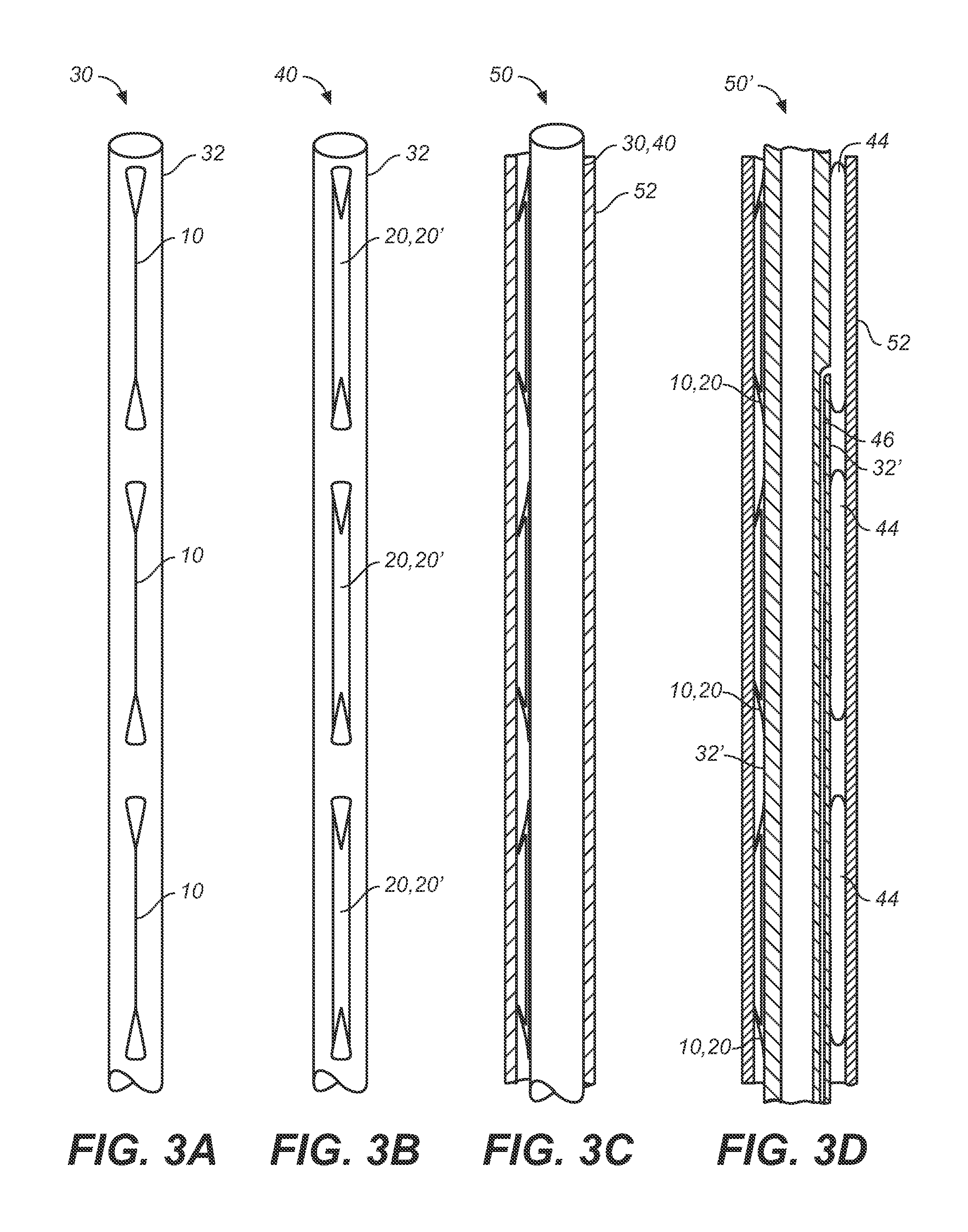
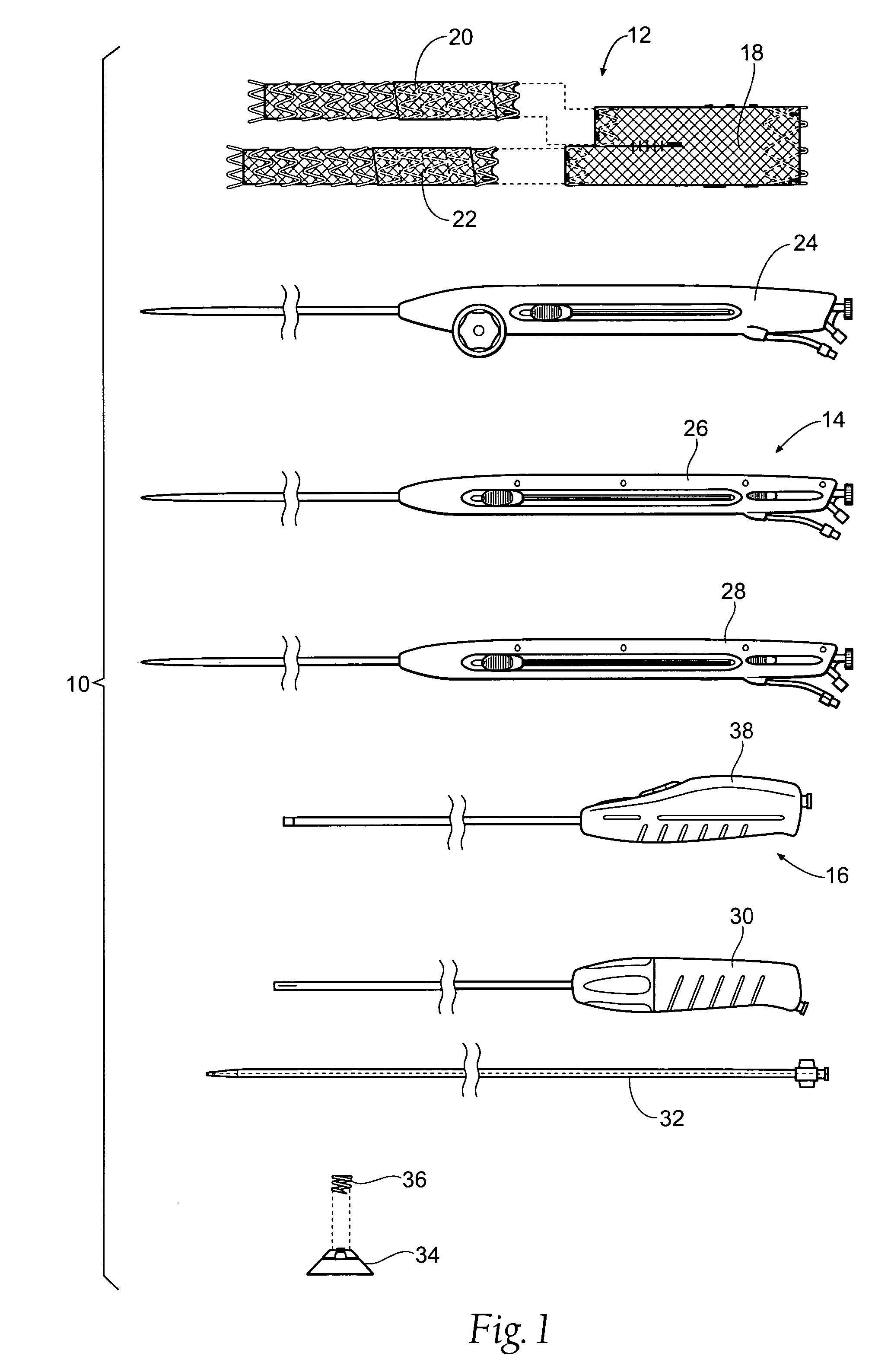
Heart valve prosthesis and sutureless implantation of a heart valve prosthesis
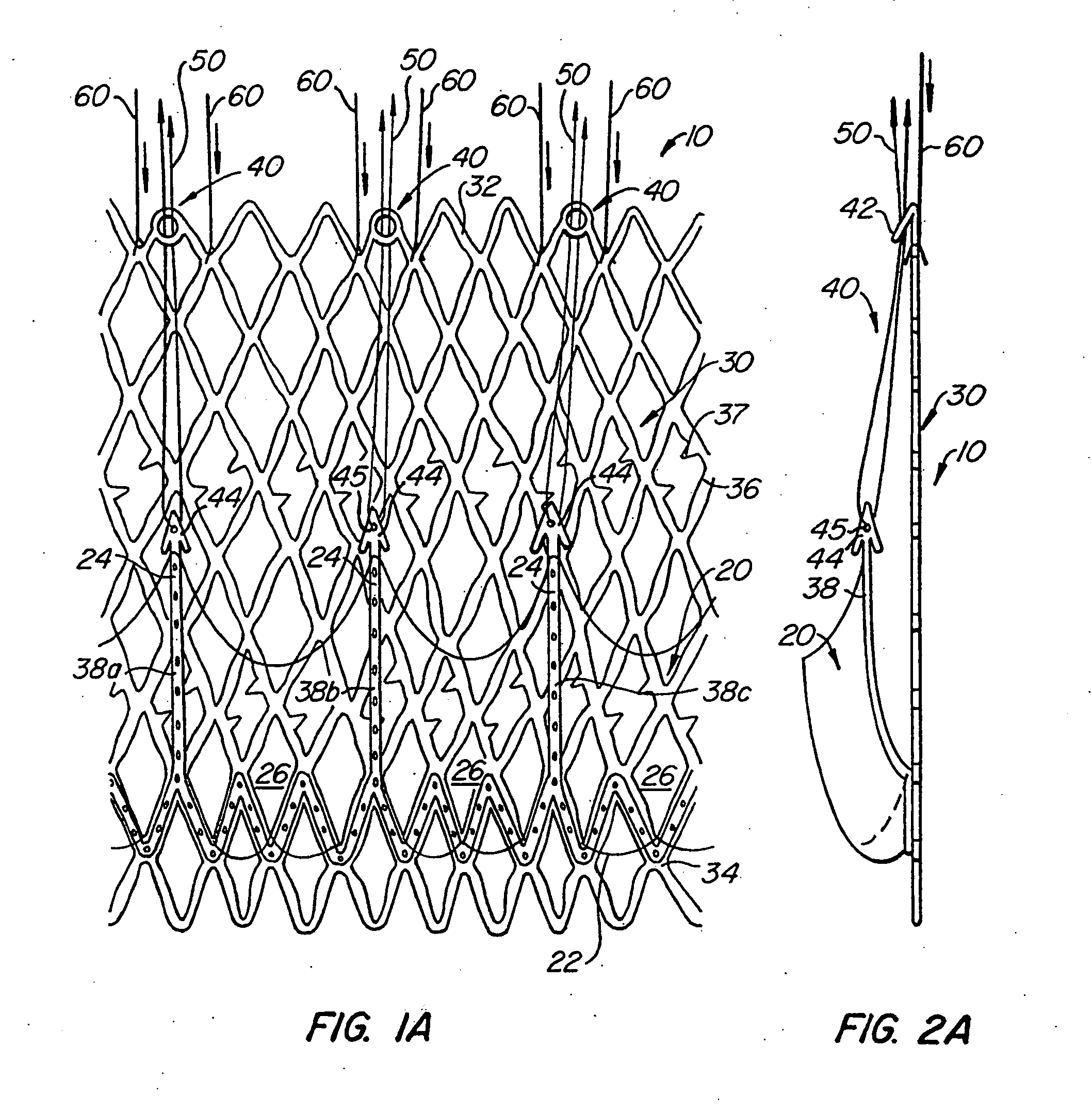
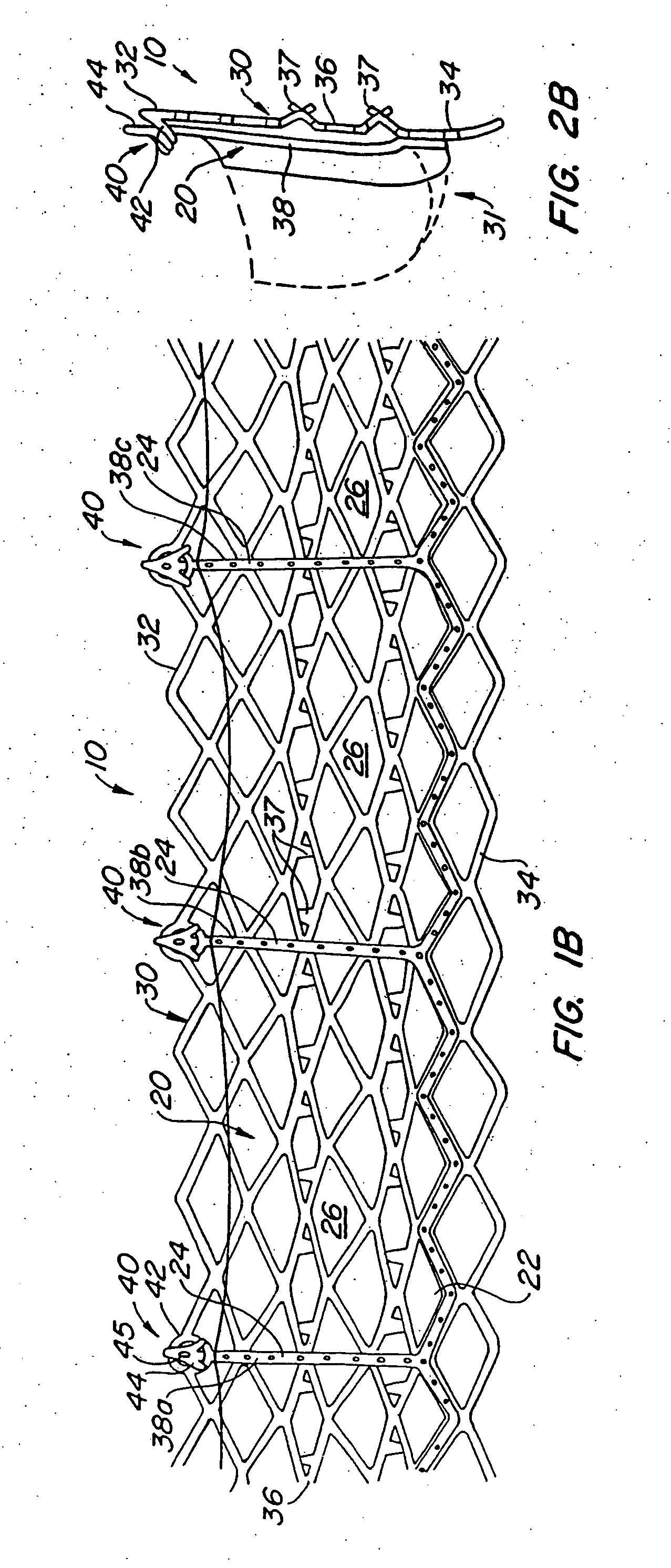
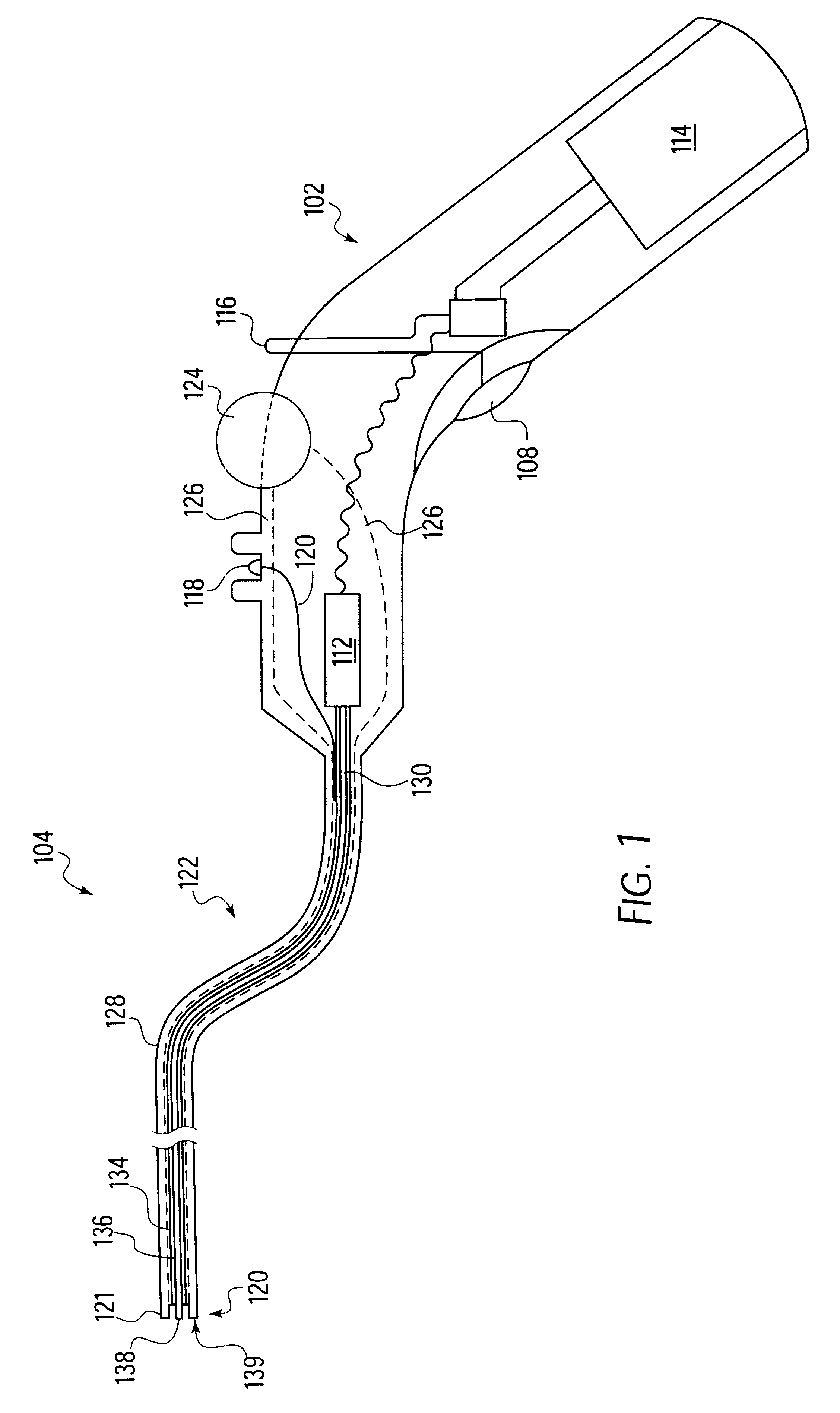
A heart valve prosthesis and method of implanting the prosthesis are disclosed. A valve is mounted within a support apparatus that is deformable between a first condition and a second condition. The prosthesis has a cross-sectional dimension in the second condition that is less than a cross-sectional dimension of the supported valve when in first condition. The prosthesis can be implanted into a patient's heart, such as during a direct vision procedure through a tubular implantation apparatus that maintains the prosthesis in its second condition until discharged from the tubular apparatus.
Owner:EDWARDS LIFESCI CARDIAQ
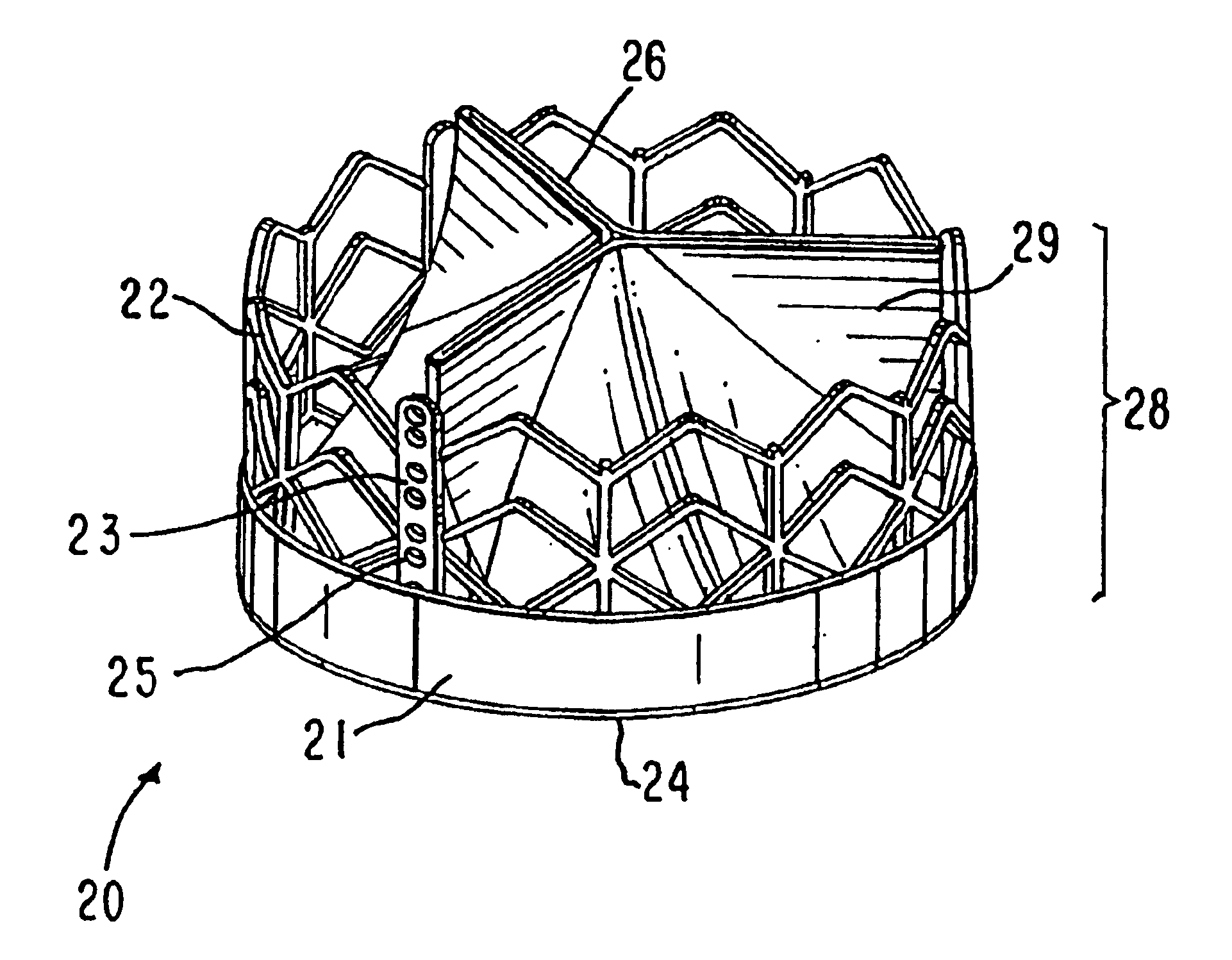
Implantable prosthetic valve
InactiveUS6893460B2Prevent blood flowImprove sealingStentsBalloon catheterGuide tubeBiomedical engineering
A valve prosthesis device is disclosed suitable for implantation in body ducts. The device comprises support stent, comprised of a deployable construction adapted to be initially crimped in a narrow configuration suitable for catheterization through the body duct to a target location and adapted to be deployed by exerting substantially radial forces from within by means of a deployment device to a deployed state in the target location, the support stent provided with a plurality of longitudinally rigid support beams of fixed length; valve assembly comprising a flexible conduit having an inlet end and an outlet, made of pliant material attached to the support beams providing collapsible slack portions of the conduit at the outlet. When flow is allowed to pass through the valve prosthesis device from the inlet to the outlet the valve assembly is kept in an open position, whereas a reverse flow is prevented as the collapsible slack portions of the valve assembly collapse inwardly providing blockage to the reverse flow.
Owner:EDWARDS LIFESCI PVT
Value prosthesis for implantation in body channels
A valve prosthesis which is especially useful in the case of aortic stenosis and capable of resisting the powerful recoil force and to stand the forceful balloon inflation performed to deploy the valve and to embed it in the aortic annulus, comprises a collapsible valvular structure and an expandable frame on which said valvular structure is mounted. The valvular structure is composed of physiologically compatible valvular tissue that is sufficiently supple and resistant to allow the valvular structure to be deformed from a closed state to an opened state. The valvular tissue forms a continuous surface and is provided with strut members that create stiffened zones which induce the valvular structure to follow a patterned movement in its expansion to its opened state and in its turning back to its closed state.
Owner:EDWARDS LIFESCI PVT
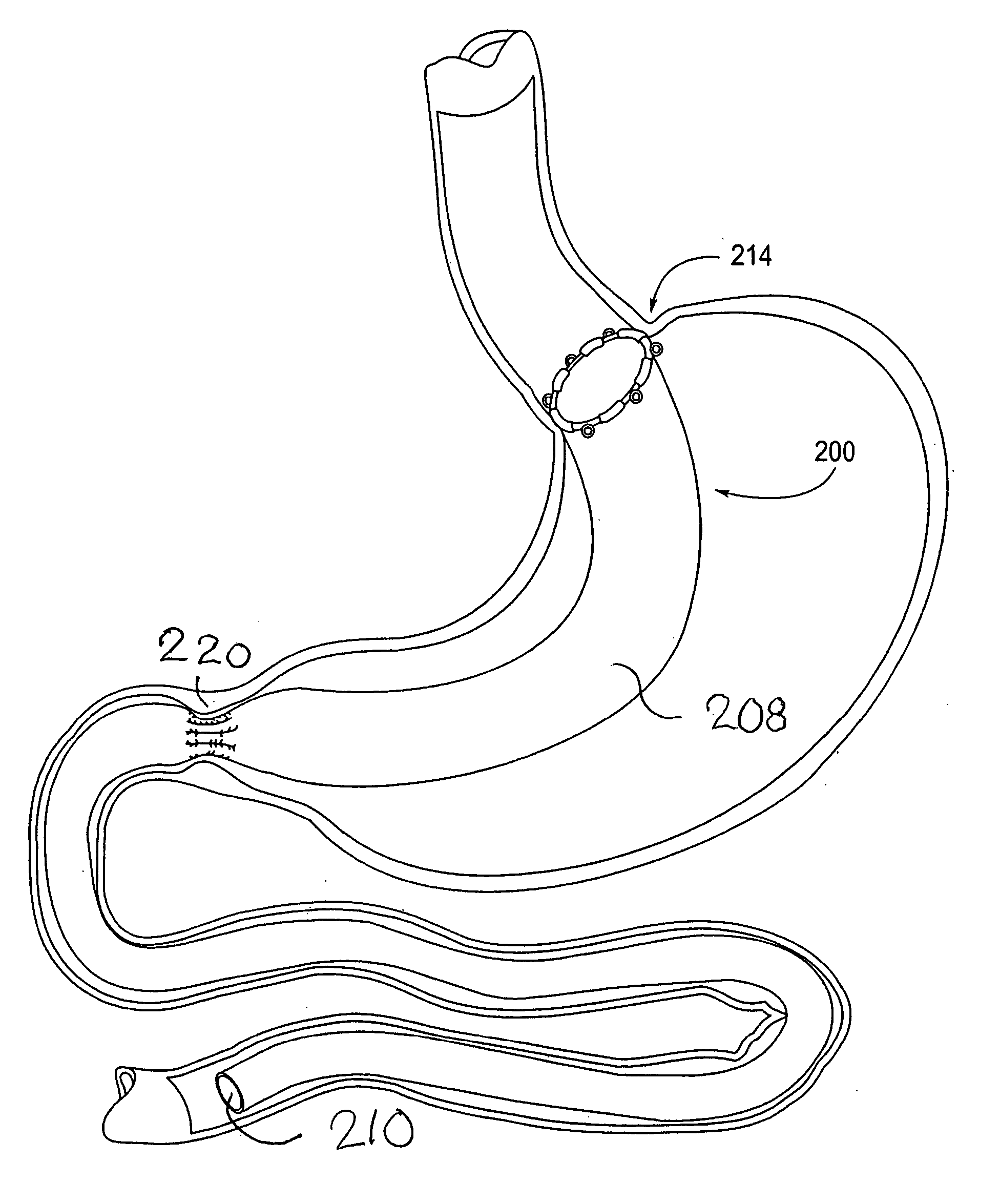
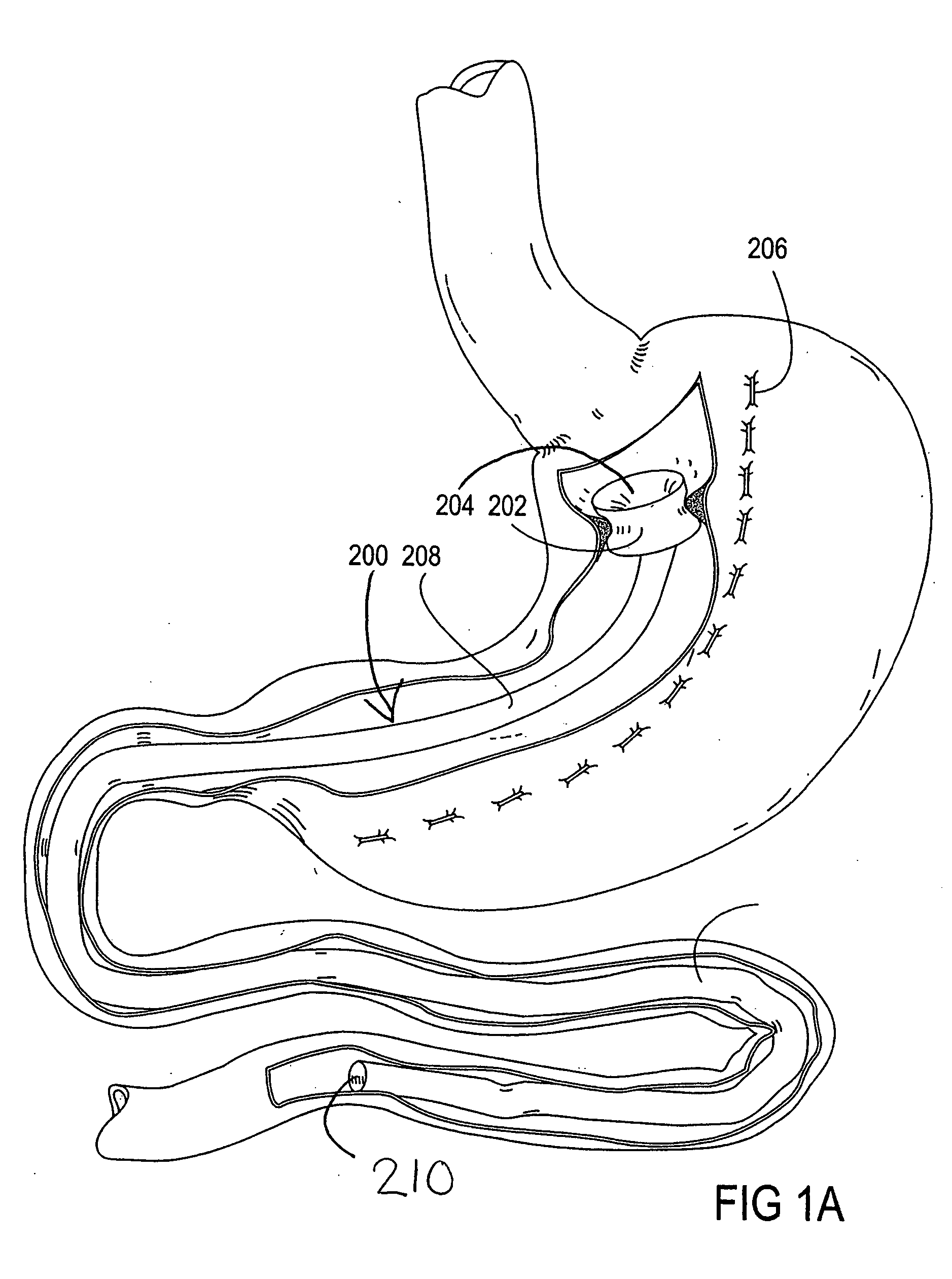
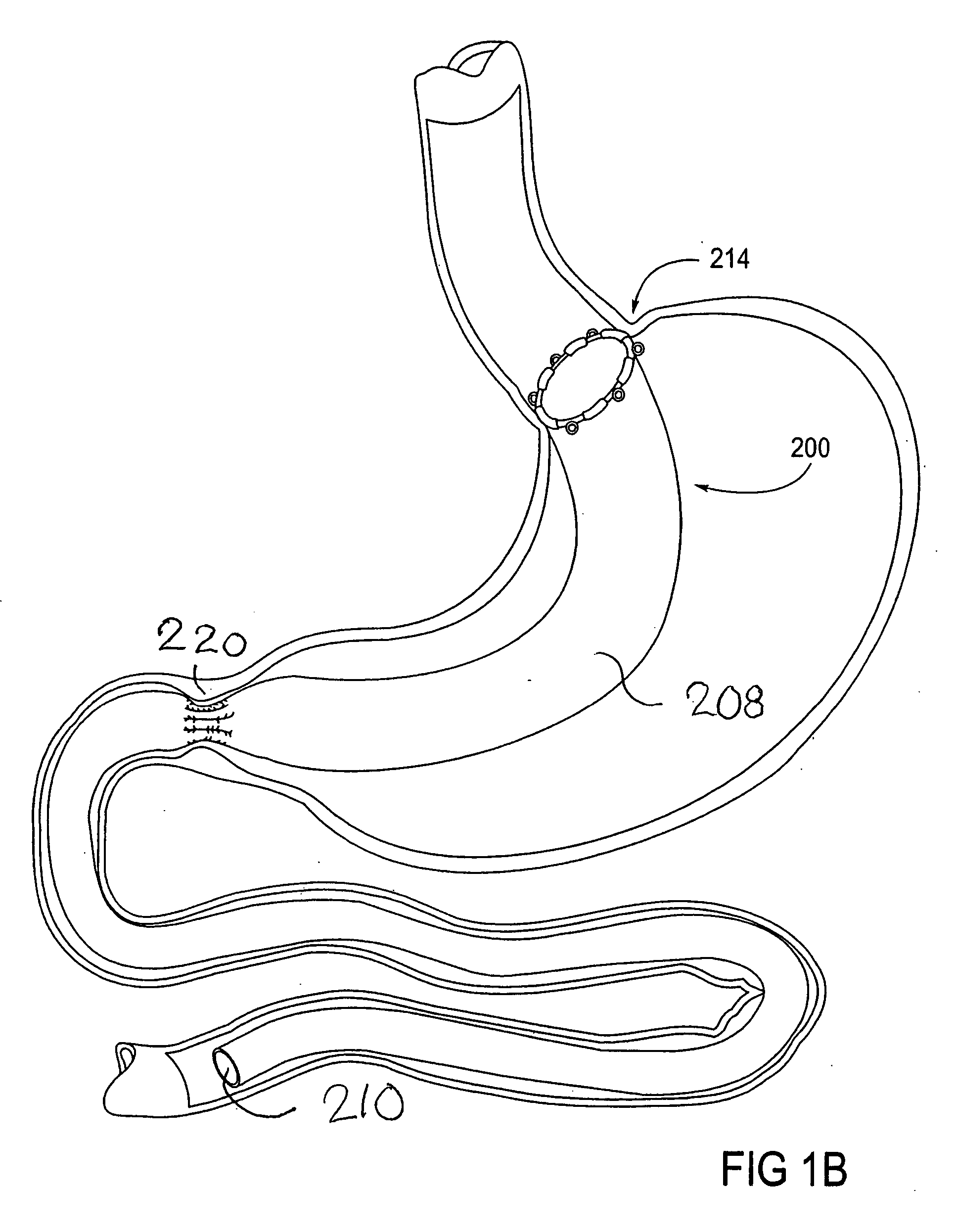
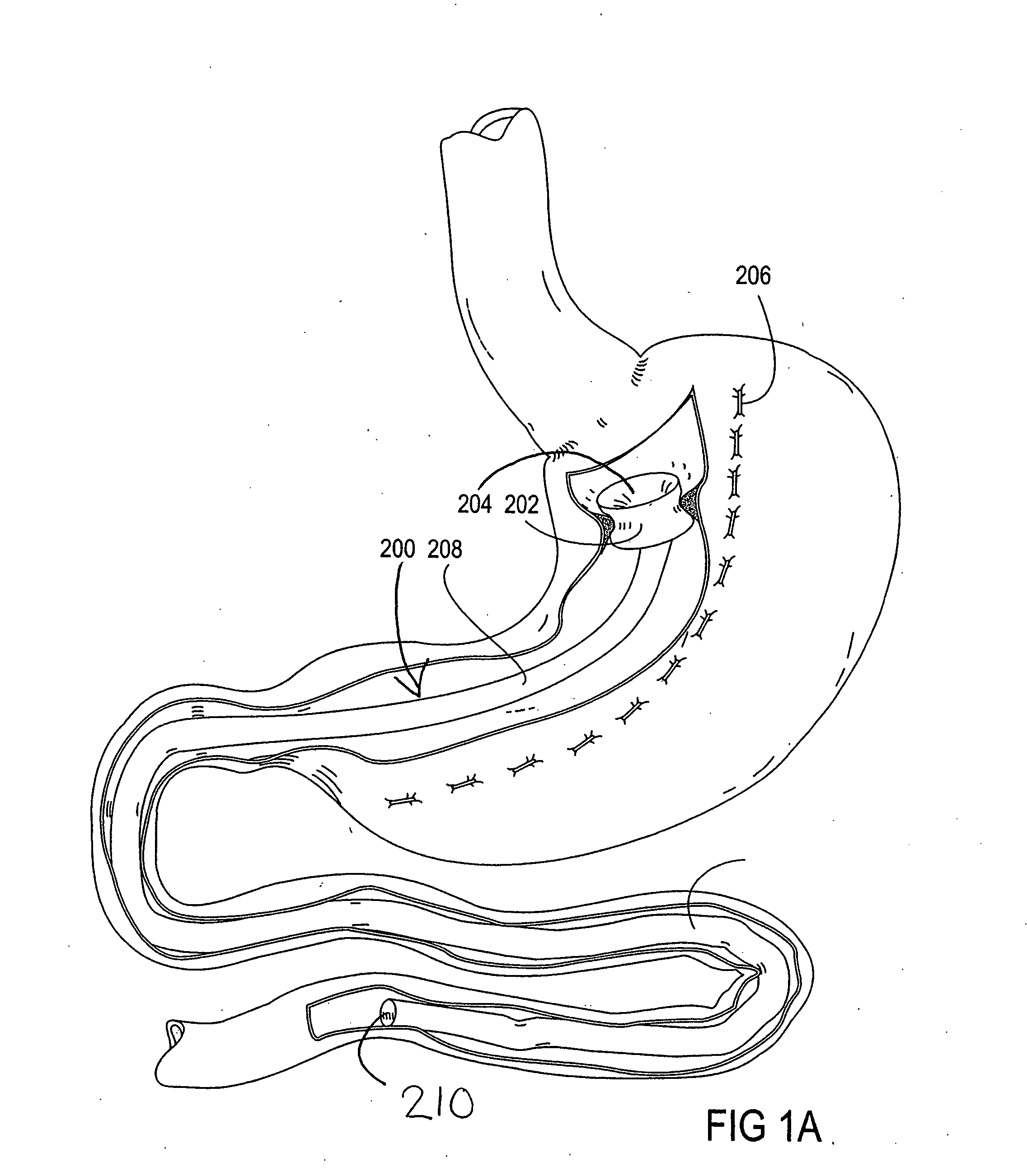
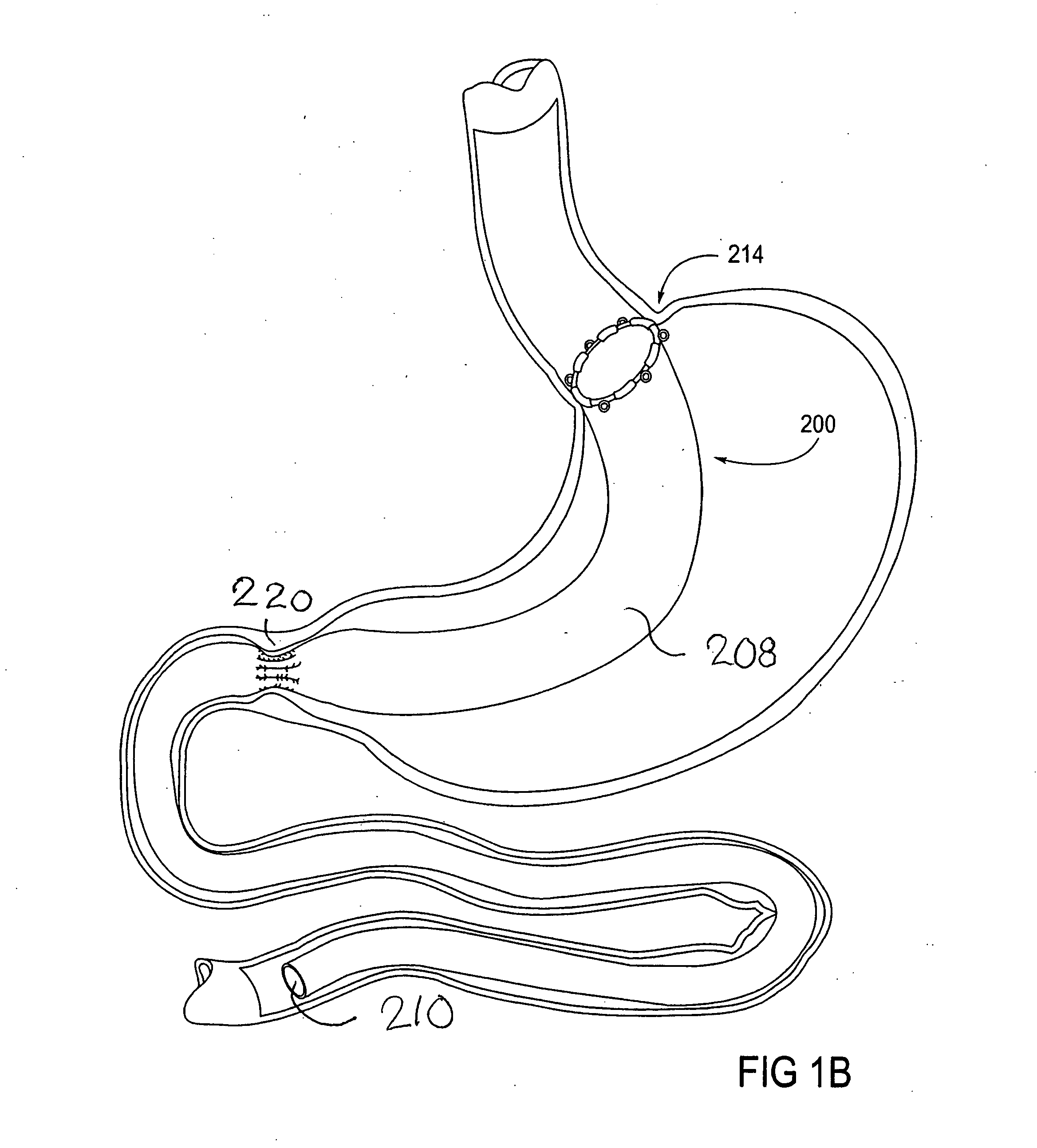
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Repositionable heart valve and method
Owner:BOSTON SCI SCIMED INC
Apparatus and method for fixation of vascular grafts
InactiveUS7351258B2Reduce leakageOvercome disadvantagesStentsBlood vesselsThree vesselsVascular graft
An apparatus for facilitating securement of a vascular graft within a blood vessel, includes a shaft dimensioned for passage within a blood vessel and having an expansion member movable between a contracted condition and an expanded condition and a fastener array comprising at least one fastener disposed about a peripheral portion of the expansion member. The one fastener is deployable into a wall of the blood vessel upon movement of the expansion member to the expanded condition thereof, to thereby engage the vascular graft to secure the vascular graft to a wall of the blood vessel. The fastener array preferably includes a plurality of fasteners. The fasteners may be operatively connected to each other and releasably secured to the peripheral portion of the expansion member.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Tear and abrasion resistant expanded material and reinforcement
InactiveUS20070207186A1Increase flexibilityLess complex manufacturing processStentsSurgeryDiseaseEngineering
The present invention is a more durable expanded material that enables thinner wall thicknesses and a more flexible reinforcement suitable for stenting. The present invention is especially useful in the construction of grafts, stents, and stent-grafts which are used, for example, in repairing or replacing blood vessels that are narrowed or occluded by disease, aneurismal blood vessels, or other medical treatments. The inventive material and configurations allow expansion or contraction in size or adjustment in size in an incremental manner so that the optimum size, shape, and fit with other objects can be obtained. The present invention is also optionally capable of more accurately delivering one or more active ingredients such as drugs over longer periods of time. The present invention optionally includes surface modifications and additives that increase the surface adhesion of active ingredients, coatings, or combinations thereof. Finally, the present invention optionally includes growing cells on the inventive material so that the expanded material, reinforcement, or combinations thereof are useful, for example, in producing lab-grown blood vessels or organs.
Owner:SCANLON JOHN JAMES +1
Externally expandable heart valve anchor and method
InactiveUS20050137686A1Reduce paravalvular leakageReduce regurgitationStentsBalloon catheterBlood vesselVALVE PORT
Owner:BOSTON SCI SCIMED INC
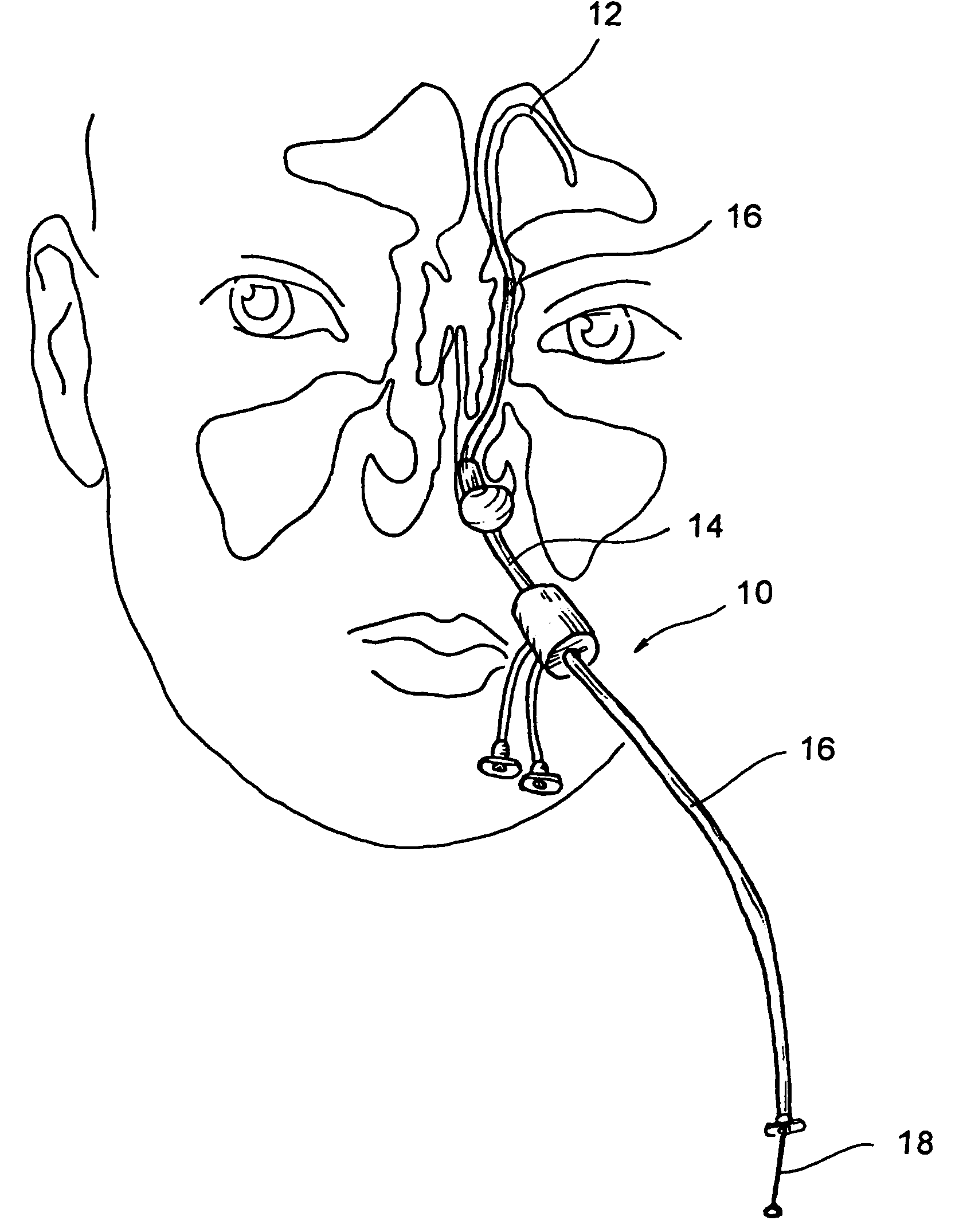
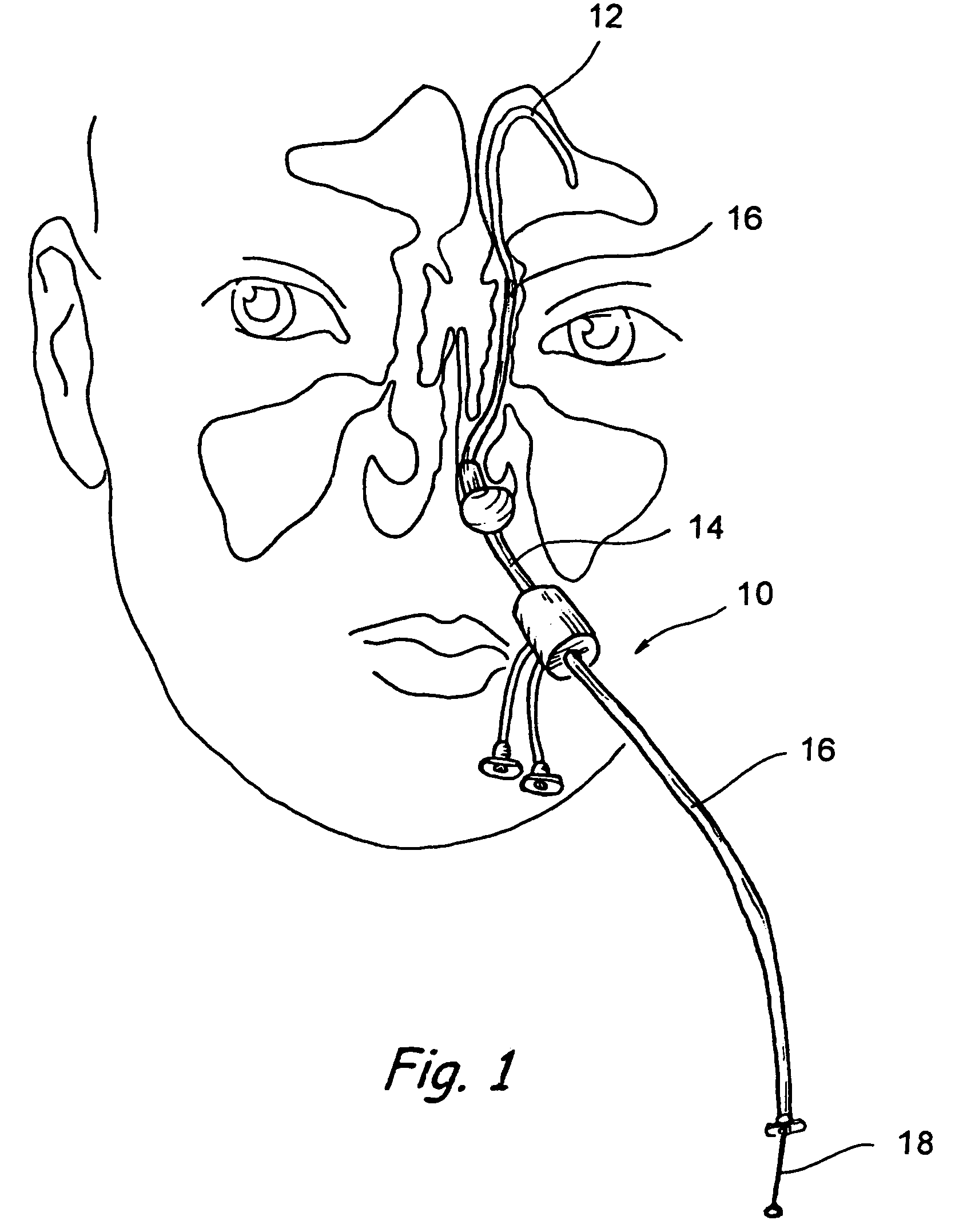
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
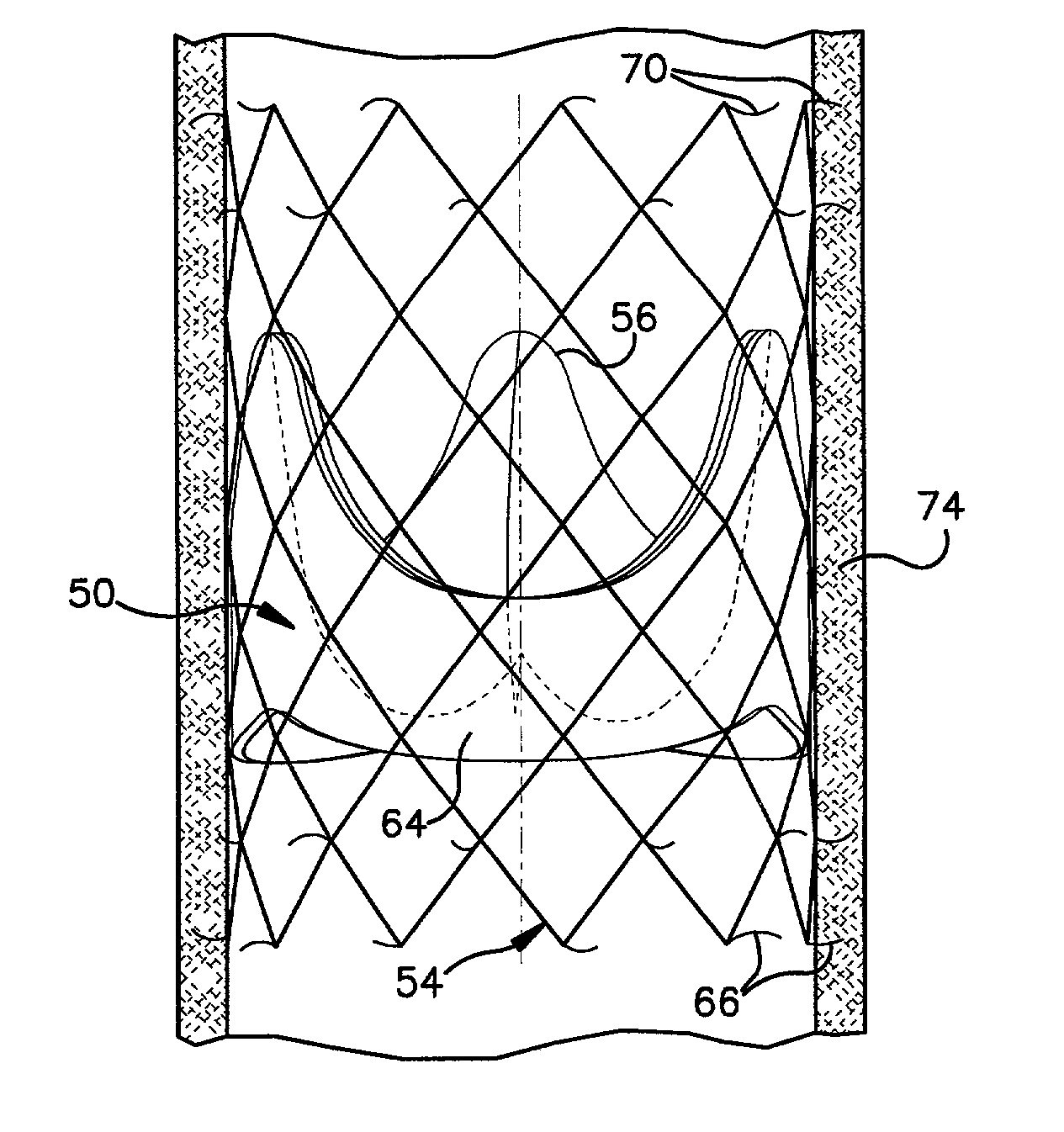
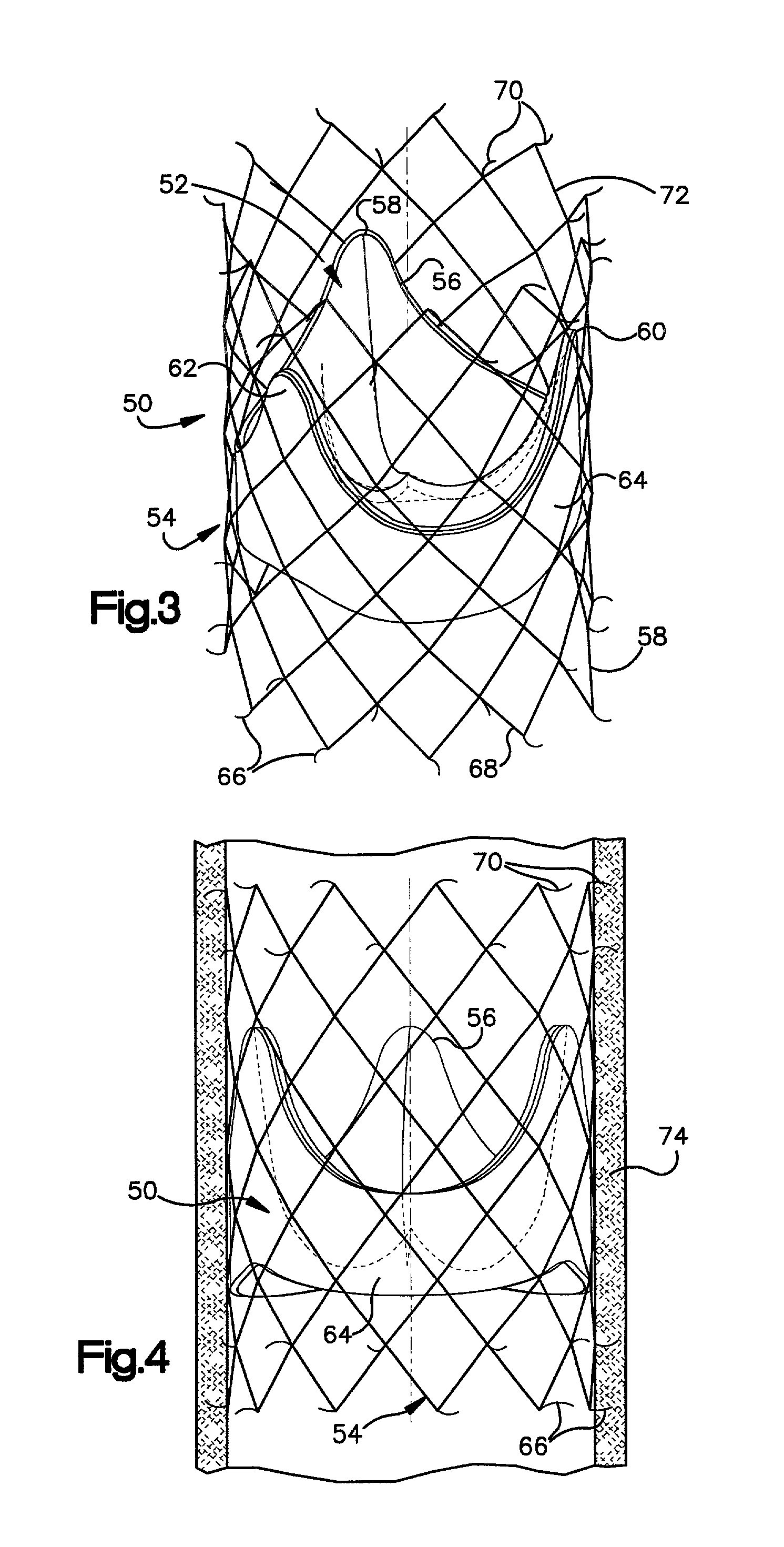
Stent-valves for valve replacement and associated methods and systems for surgery
InactiveUS20070213813A1Simple methodReduce riskStentsBalloon catheterLess invasive surgeryInsertion stent
Stent-valves (e.g., single-stent-valves and double-stent-valves), associated methods and systems for their delivery via minimally-invasive surgery, and guide-wire compatible closure devices for sealing access orifices are provided.
Owner:SYMETIS
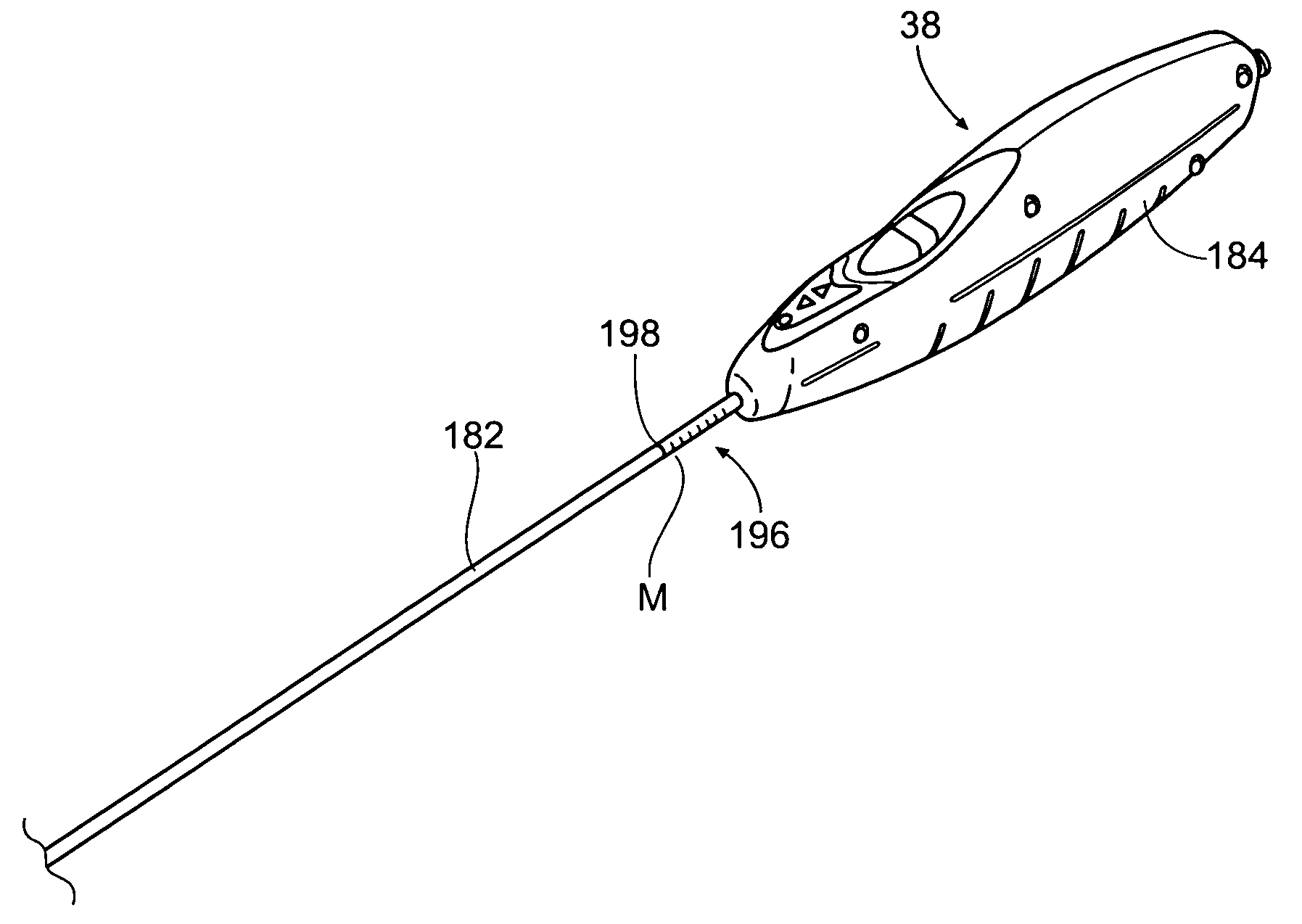
Transapical heart valve delivery system and method
ActiveUS20070112422A1Facilitate positioning of valveHelp positioningStentsBalloon catheterProsthetic heartLeft ventricular apex
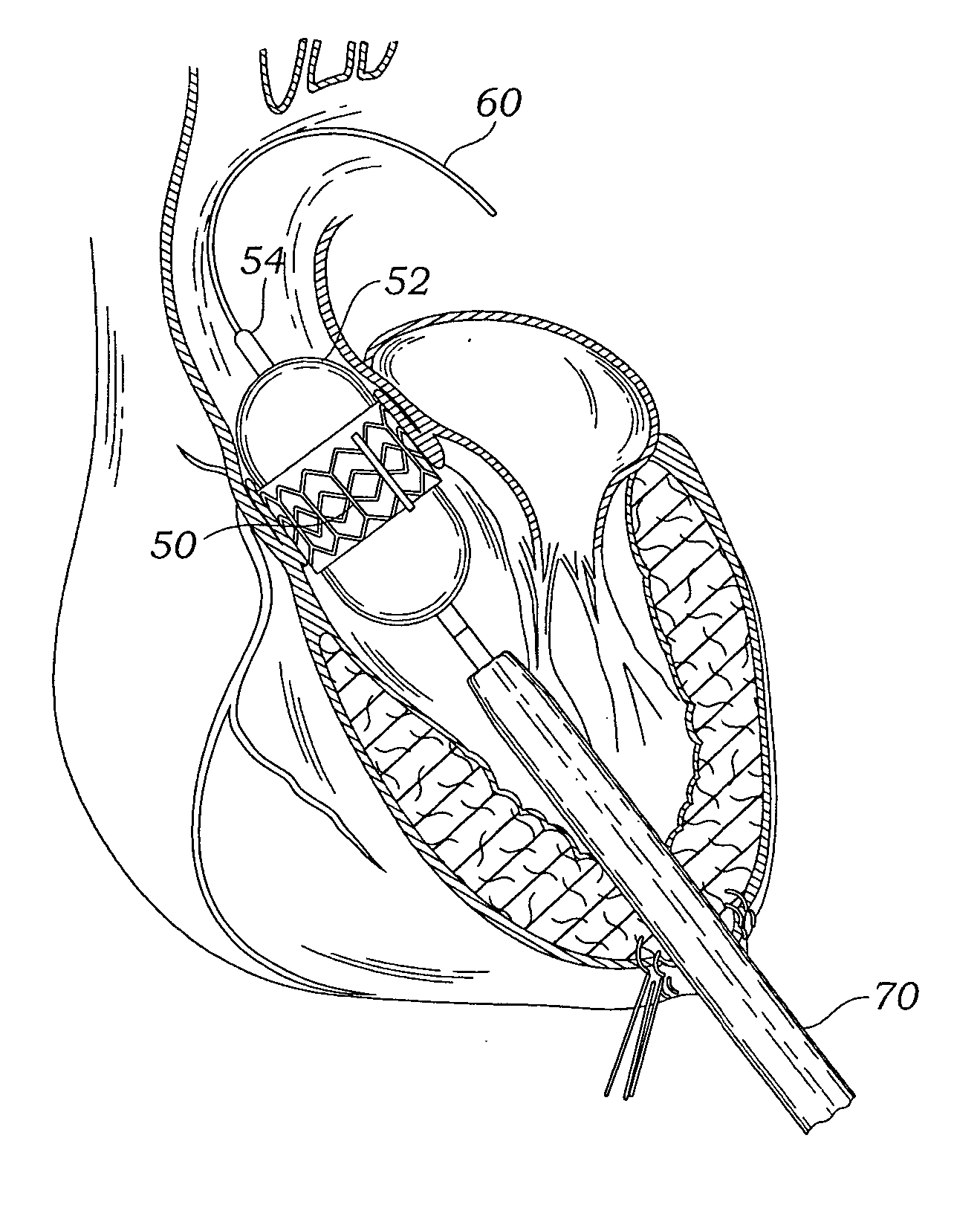
A delivery system and method for delivering a prosthetic heart valve to the aortic valve annulus. The system includes a balloon catheter having a steering mechanism thereon for delivering a balloon-expandable prosthetic heart valve through an introducer in an antegrade fashion to the aortic annulus. The balloon catheter passes through an introducer that accesses the left ventricle through its apex and a small incision in the chest wall. The balloon catheter includes a deflecting segment just proximal to the distal balloon to facilitate positioning of the prosthetic heart valve in the proper orientation within the aortic annulus. A slider in a deflection handle may be coupled to a deflection wire that actuates the deflecting segment. The method includes using two concentric rings of purse-string sutures around the puncture in the left ventricular apex to maintain a good seal around instruments passed therethrough. The prosthetic heart valve may be installed over the existing calcified leaflets, and a pre-dilation valvuloplasty procedure may also be utilized.
Owner:EDWARDS LIFESCIENCES CORP
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
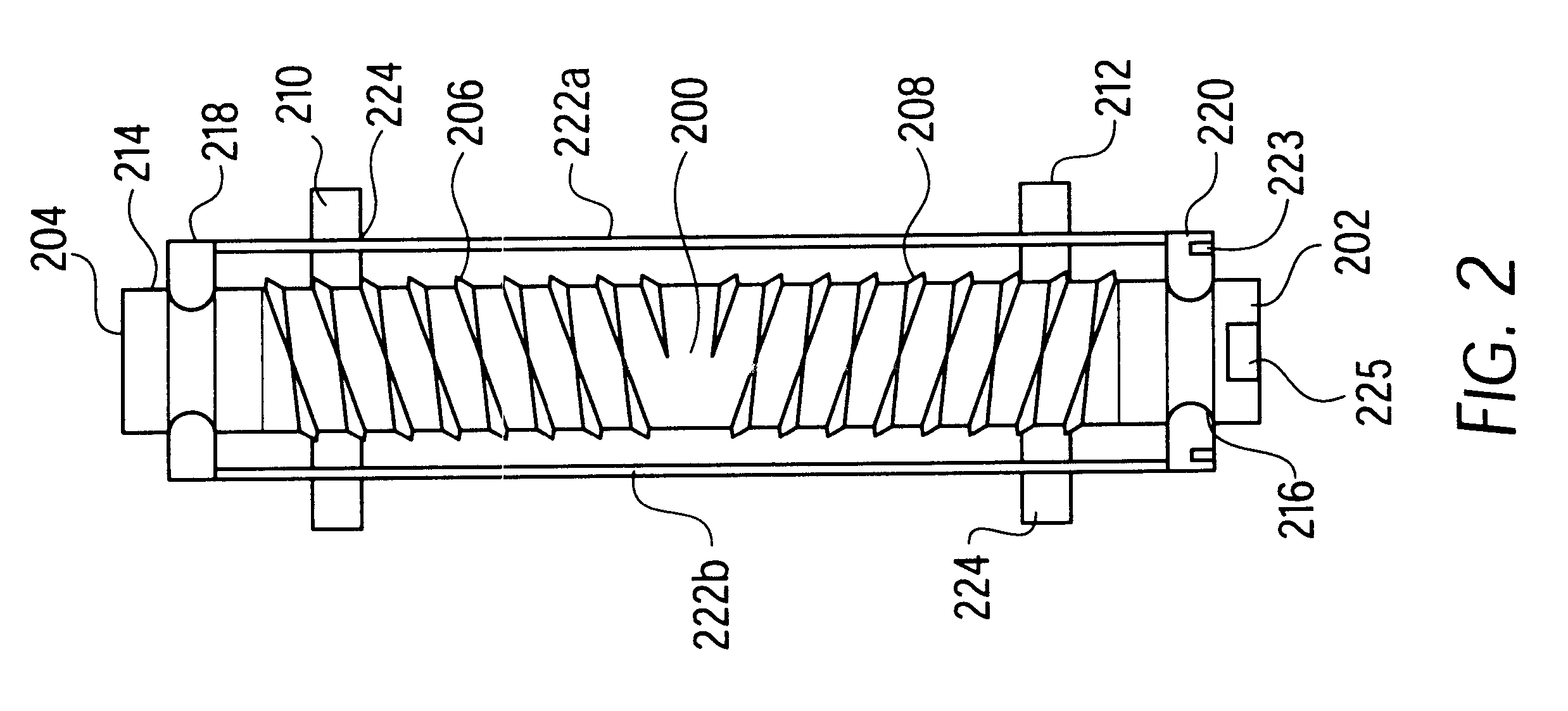
Vessel and lumen expander attachment for use with an electromechanical driver device
A selectively coupleable and remotely actuateable surgical attachment for use with an electromechanical driver device for use in expanding occluded vessels and lumens. The attachment includes an axial rod which is coupleable to the electromechanical driver device, and which is selectively rotateable in accordance with the action of the electromechanical driver device. The rod is also threaded in opposing orientations at either end thereof. A pair of nuts are mounted to the rod, and specifically on the opposing threads. The rod and nuts are disposed within an axial track which permits the rod to turn, but constrains the rotational motion of the nuts, thereby permitting the nuts to move axially along the threadings of the rod when the rod turns. Attached to the nuts are a series of jointed spokes which are coupled to a flexible tubular shroud which surrounds the entire assembly. The jointed spokes are radially extended and retracted in accordance with the motion of the nuts in an umbrella-like fashion in accordance with the actuation of the electromechanical driver device. The radial expansion of the flexible tubular shroud provides the necessary force to expand an occluded vessel or lumen.
Owner:DORROS GERALD M D
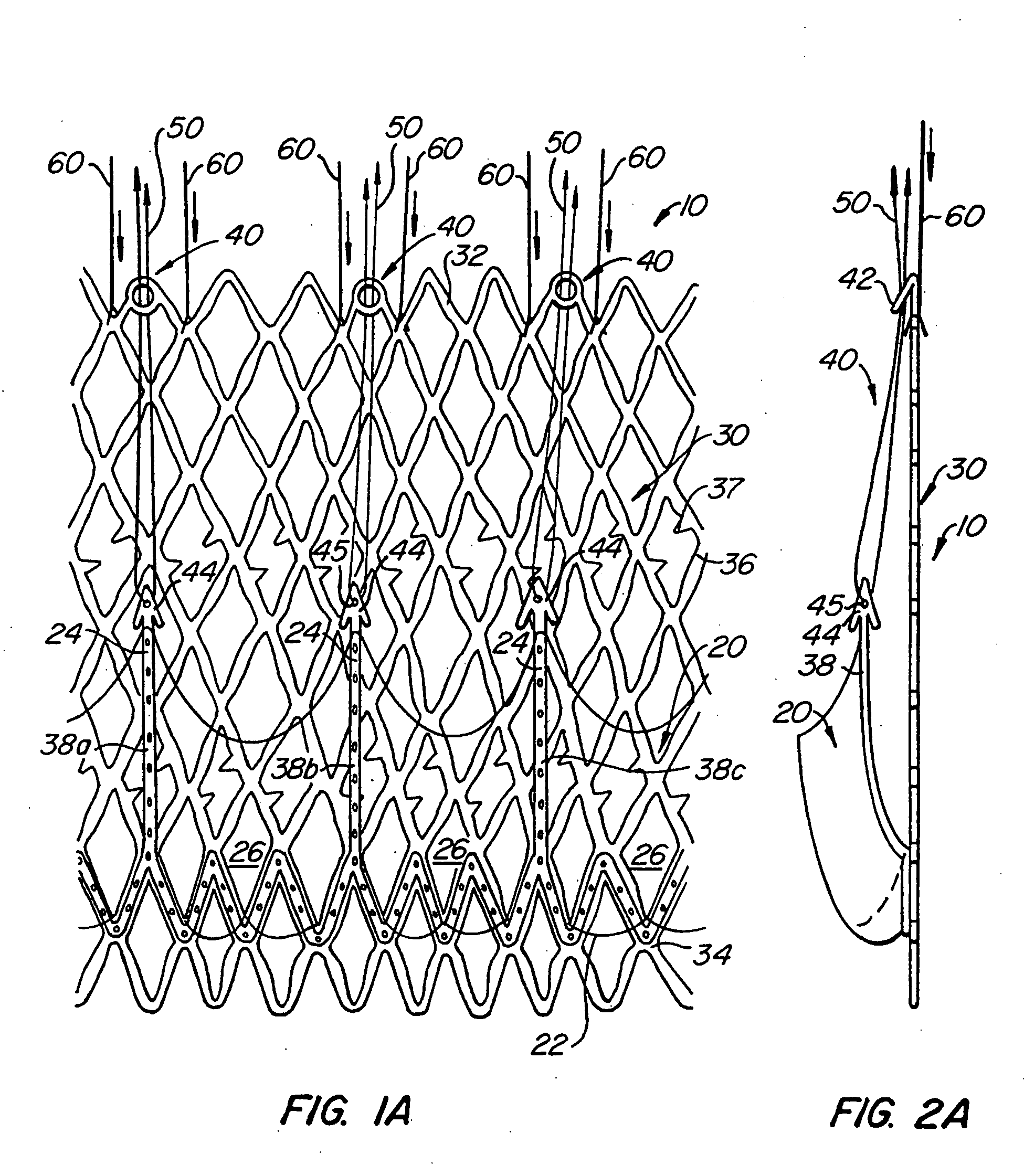
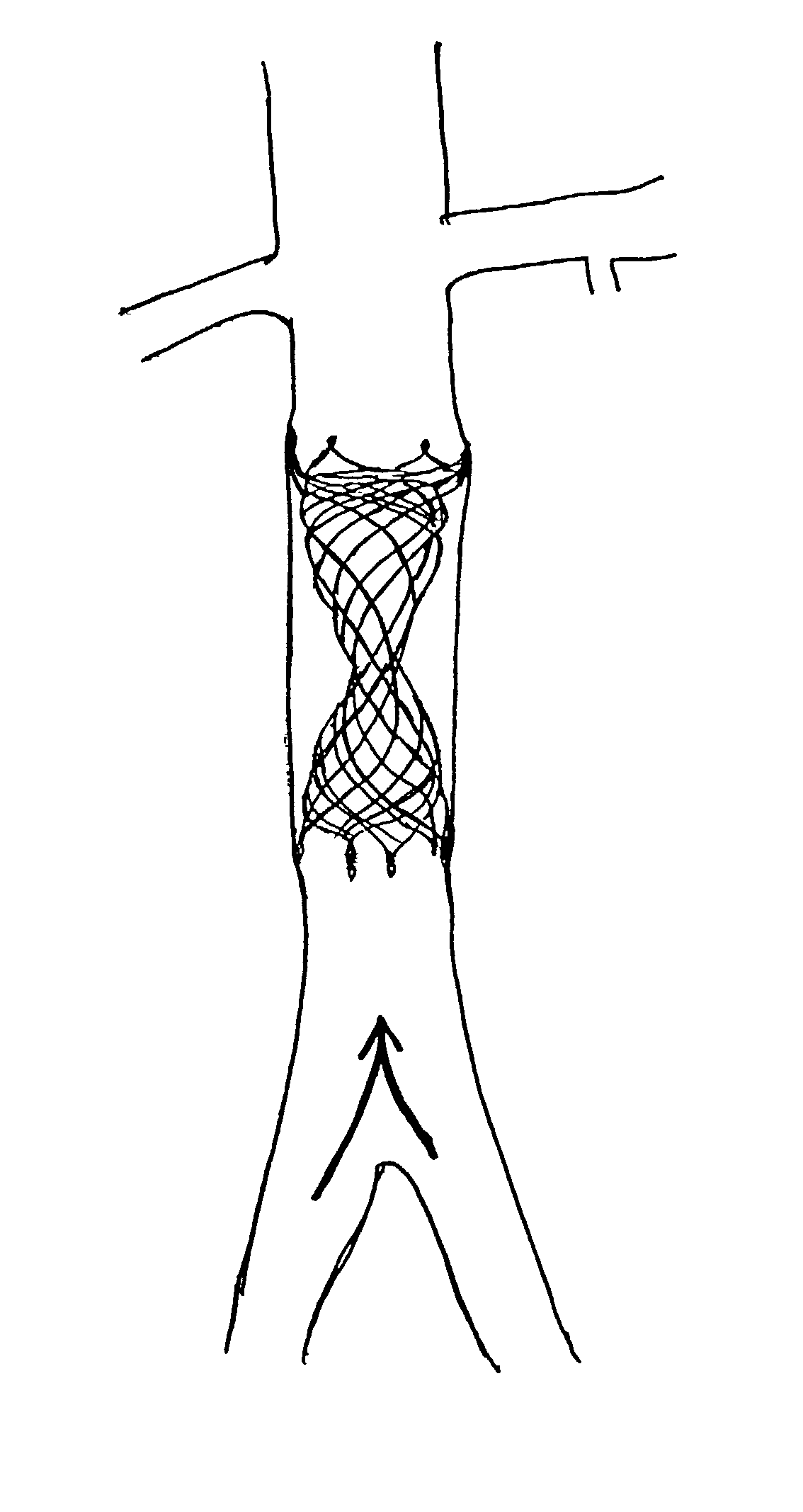
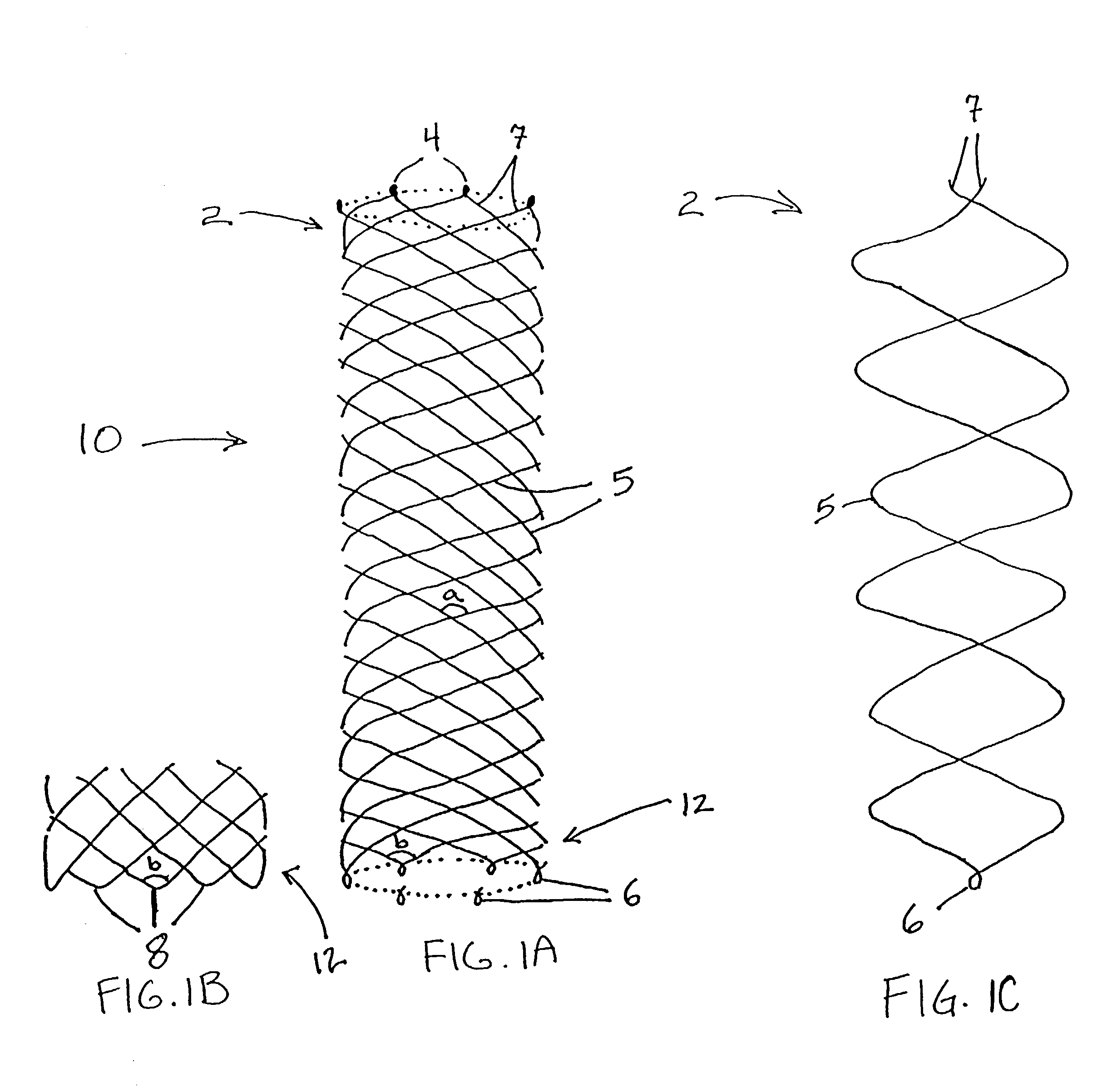
Delivery devices
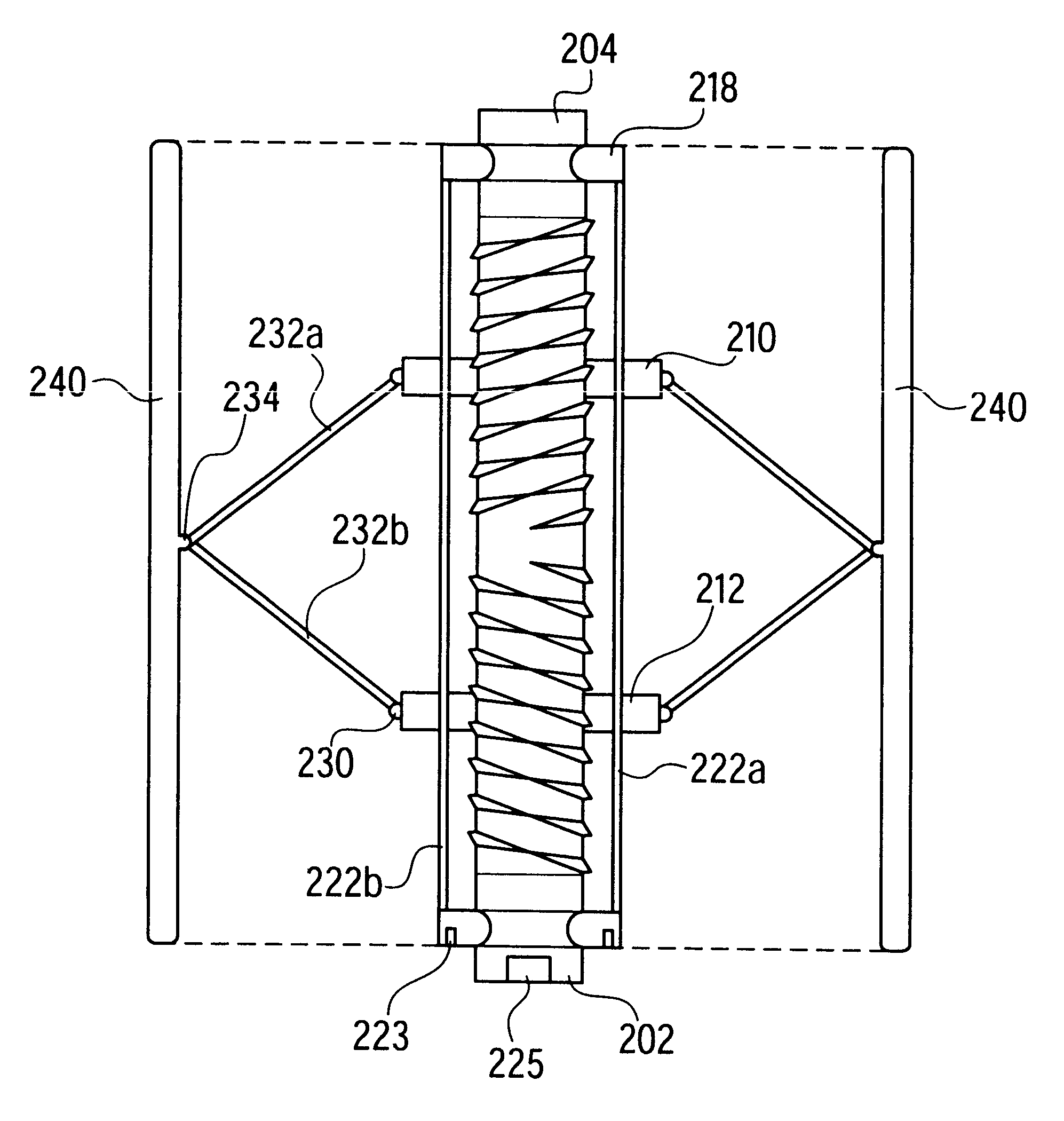
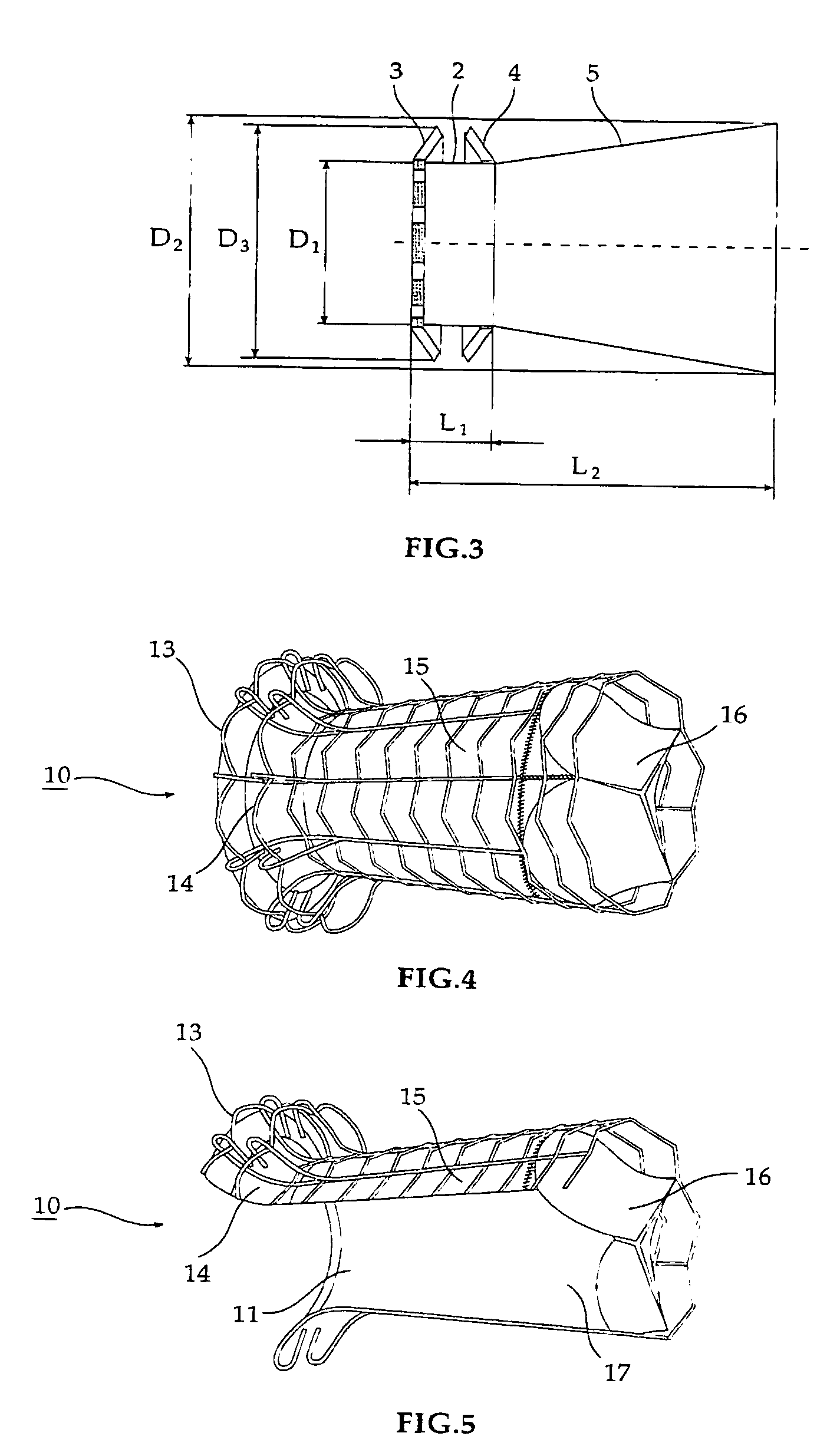
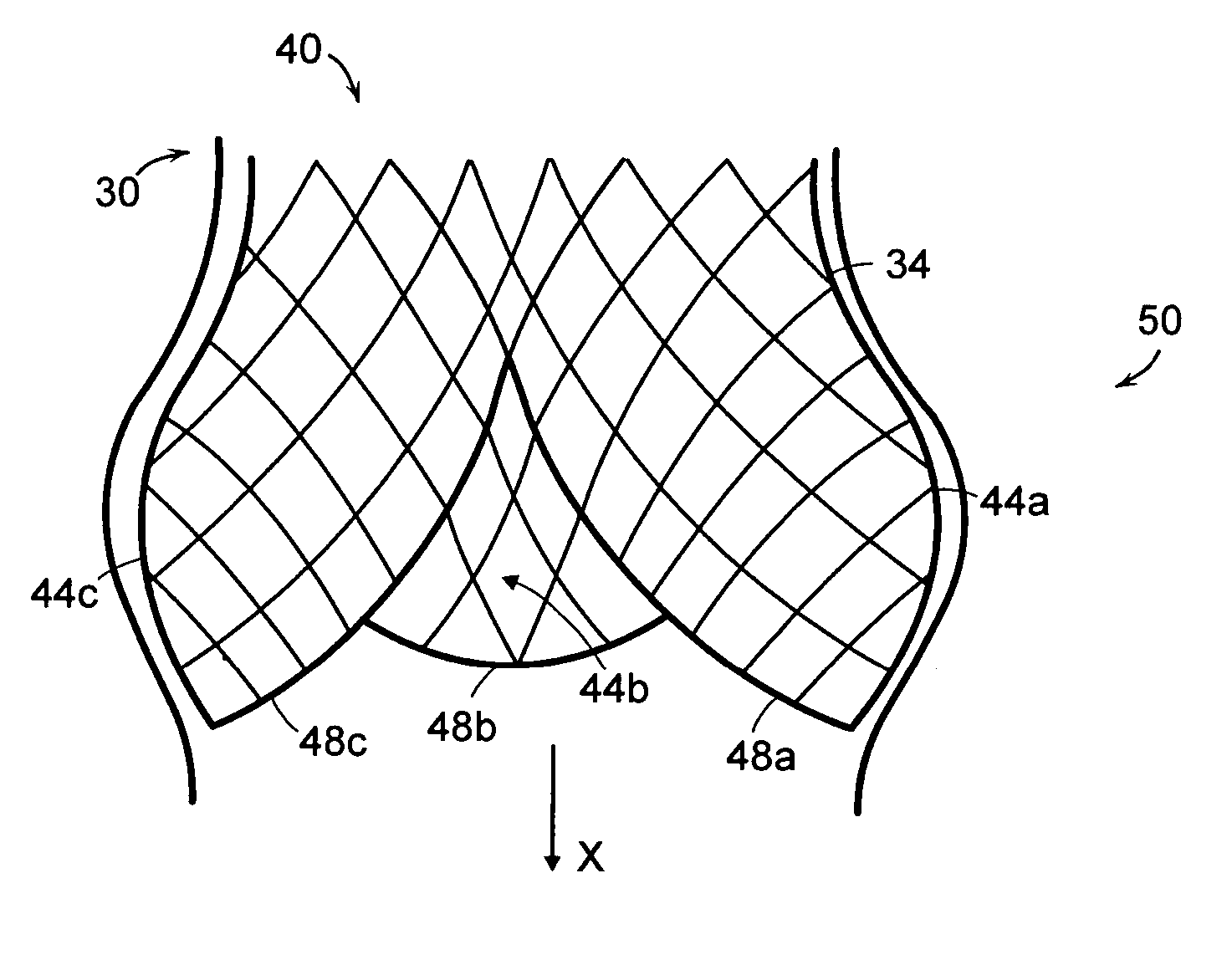
Self-expandable, woven intravascular devices for use as stents (both straight and tapered), filters (both temporary and permanent) and occluders for insertion and implantation into a variety of anatomical structures. The devices may be formed from shape memory metals such as nitinol. The devices may also be formed from biodegradable materials. Delivery systems for the devices include two hollow tubes that operate coaxially. A device is secured to the tubes prior to the implantation and delivery of the device by securing one end of the device to the outside of the inner tube and by securing the other end of the device to the outside of the outer tube. The stents may be partially or completely covered by graft materials, but may also be bare. The devices may be formed from a single wire. The devices may be formed by either hand or machine weaving. The devices may be created by bending shape memory wires around tabs projecting from a template, and weaving the ends of the wires to create the body of the device such that the wires cross each other to form a plurality of angles, at least one of the angles being obtuse. The value of the obtuse angle may be increased by axially compressing the body.
Owner:HYODOH HIDEKI +2
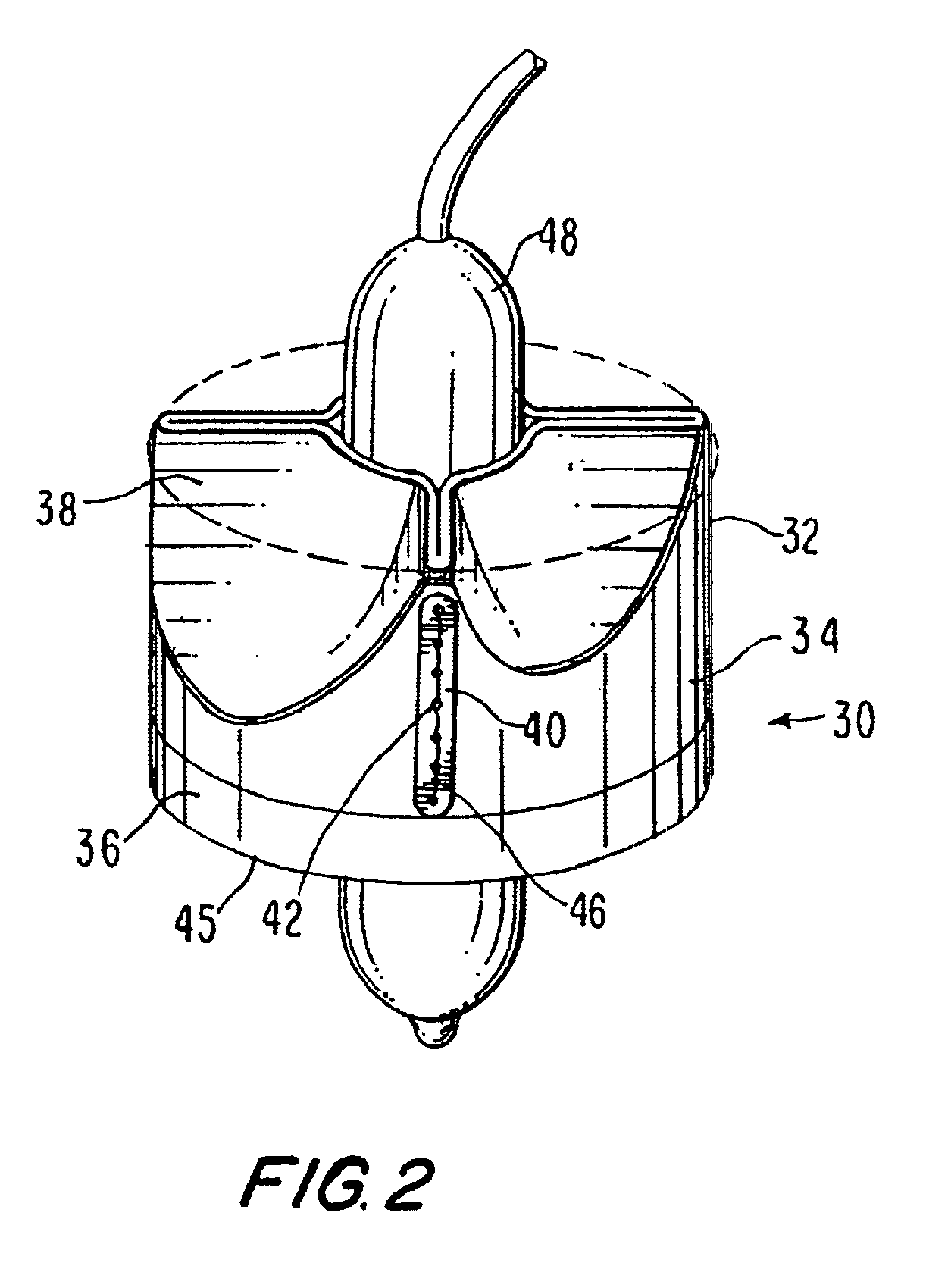
Fluid flow prosthetic device
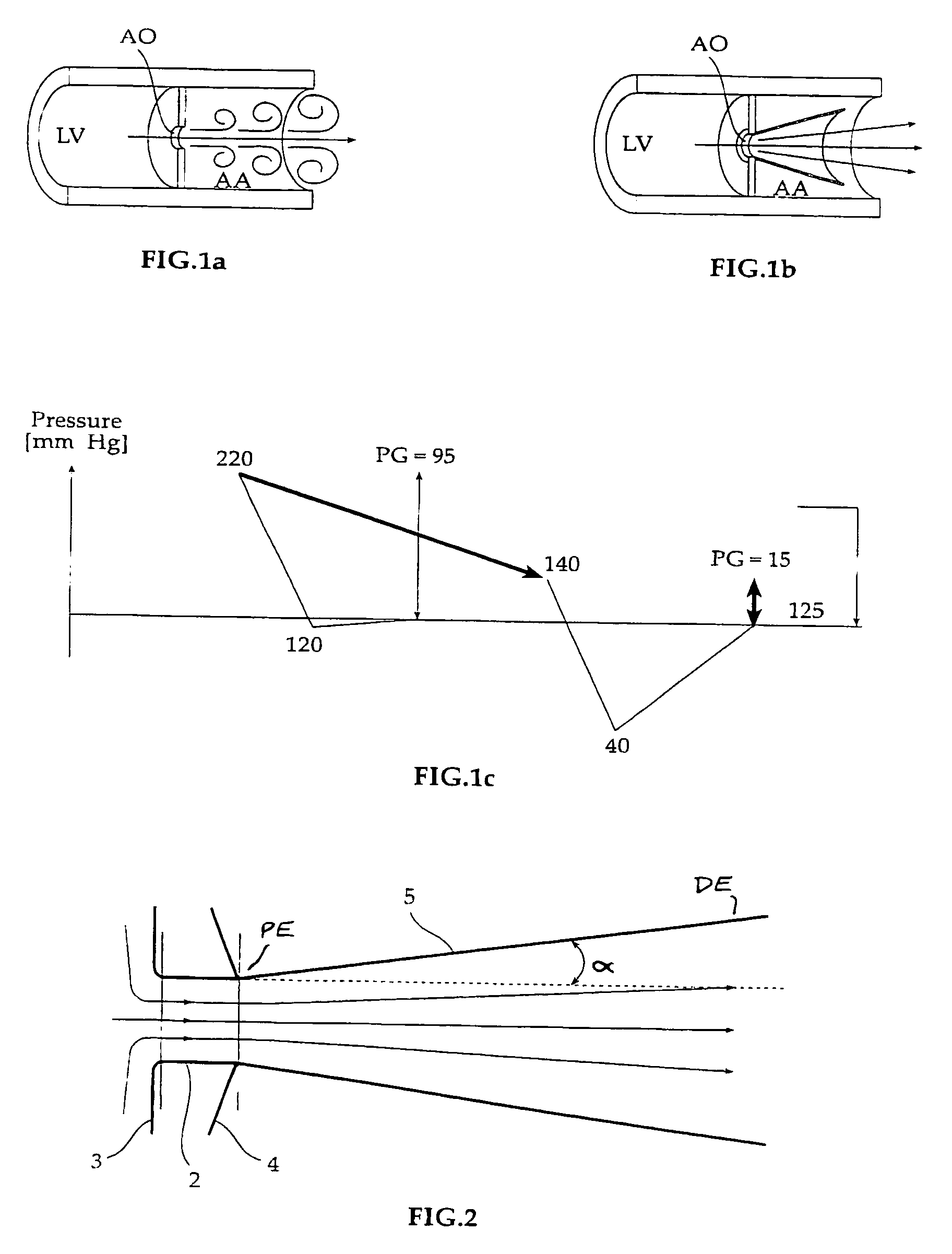
A prosthetic device including a valve-orifice attachment member attachable to a valve in a blood vessel and including a fluid inlet, and a diverging member that extends from the fluid inlet, the diverging member including a proximal end near the fluid inlet and a distal end distanced from the proximal end, wherein a distal portion of the diverging member has a larger cross-sectional area for fluid flow therethrough than a proximal portion thereof. The diverging member may have a diverging taper that causes fluid to flow therethrough with pressure recovery at the distal end thereof.
Owner:MEDTRONIC VENTOR TECH
Means and method of replacing a heart valve in a minimally invasive manner
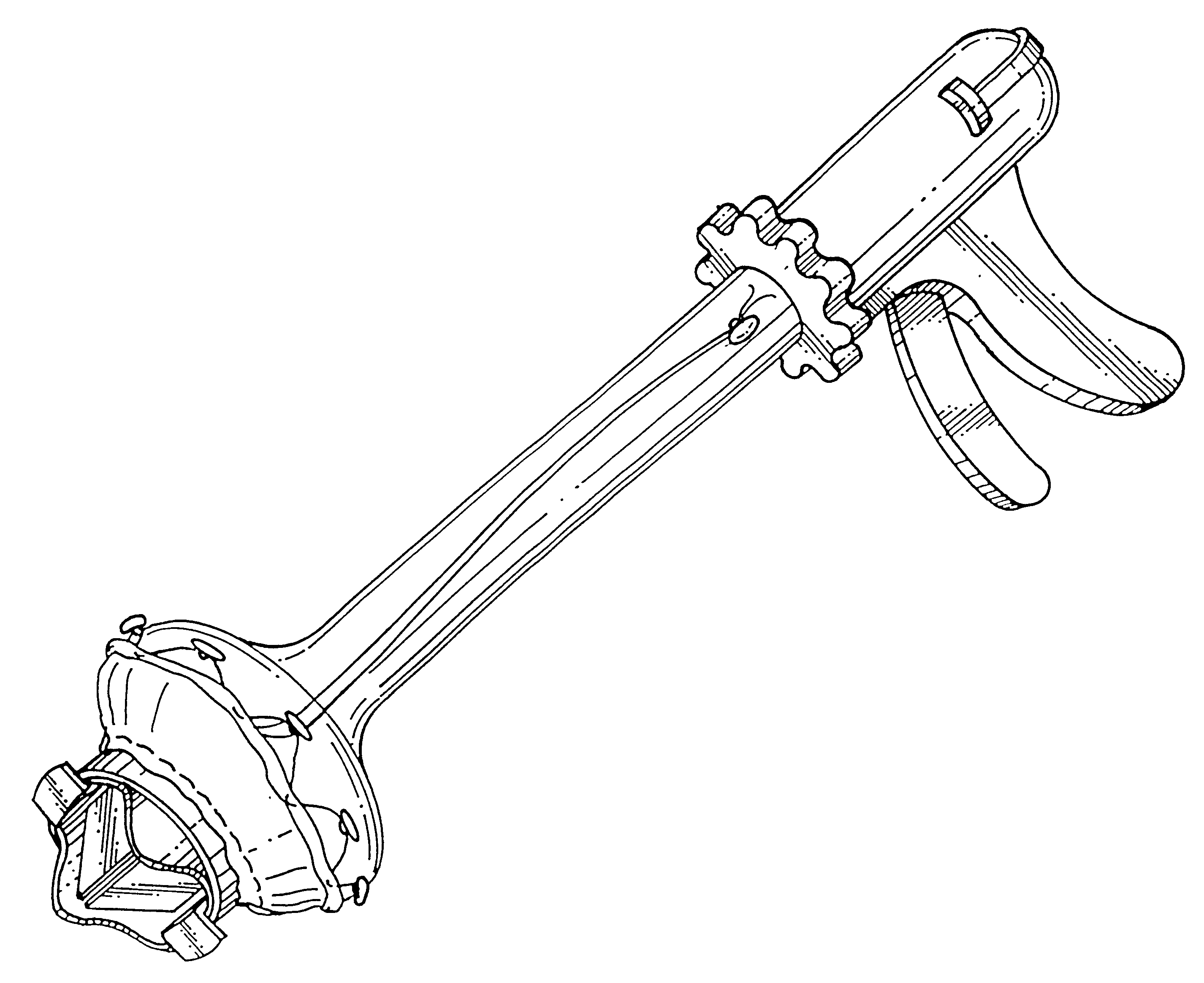
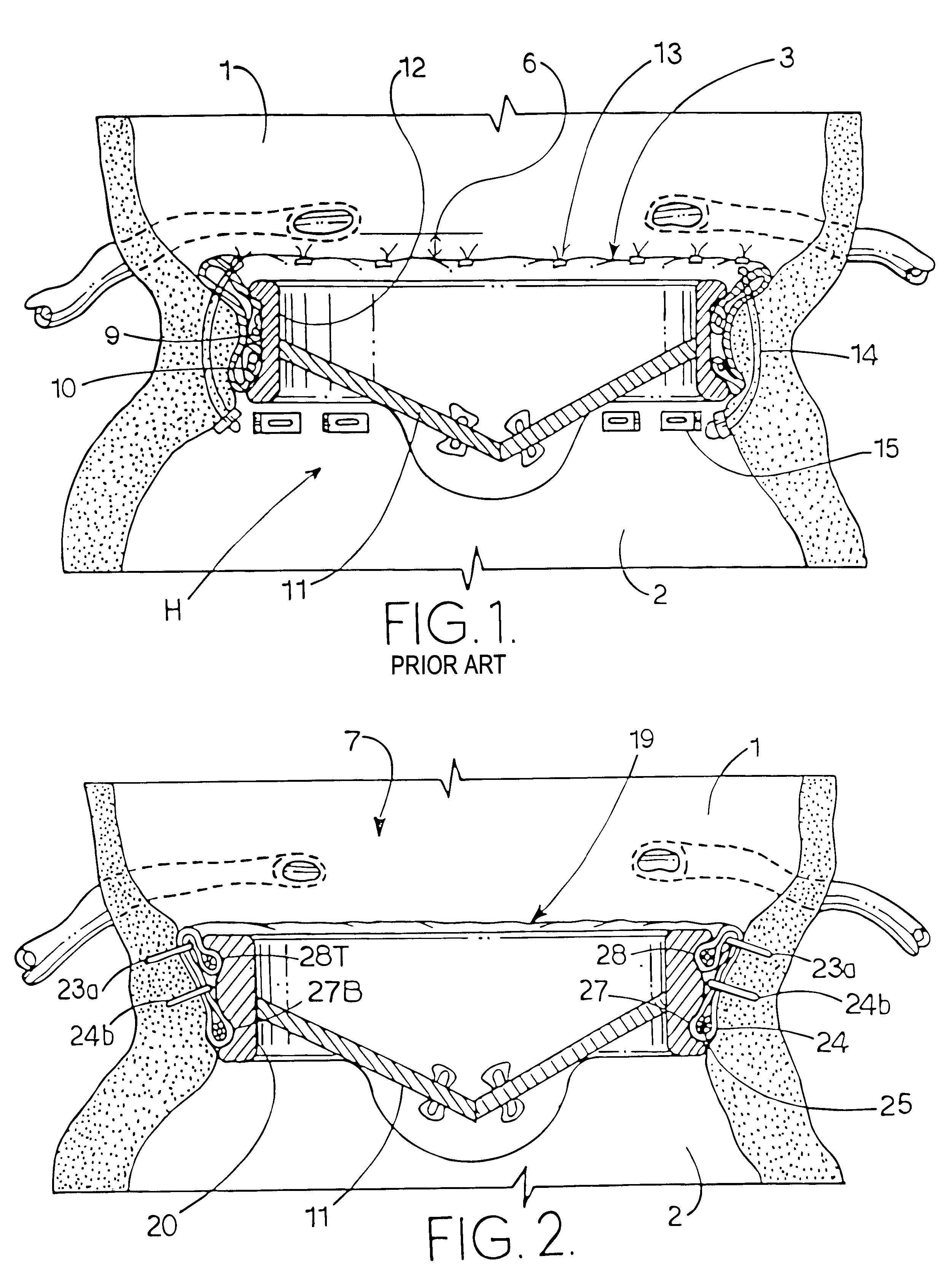
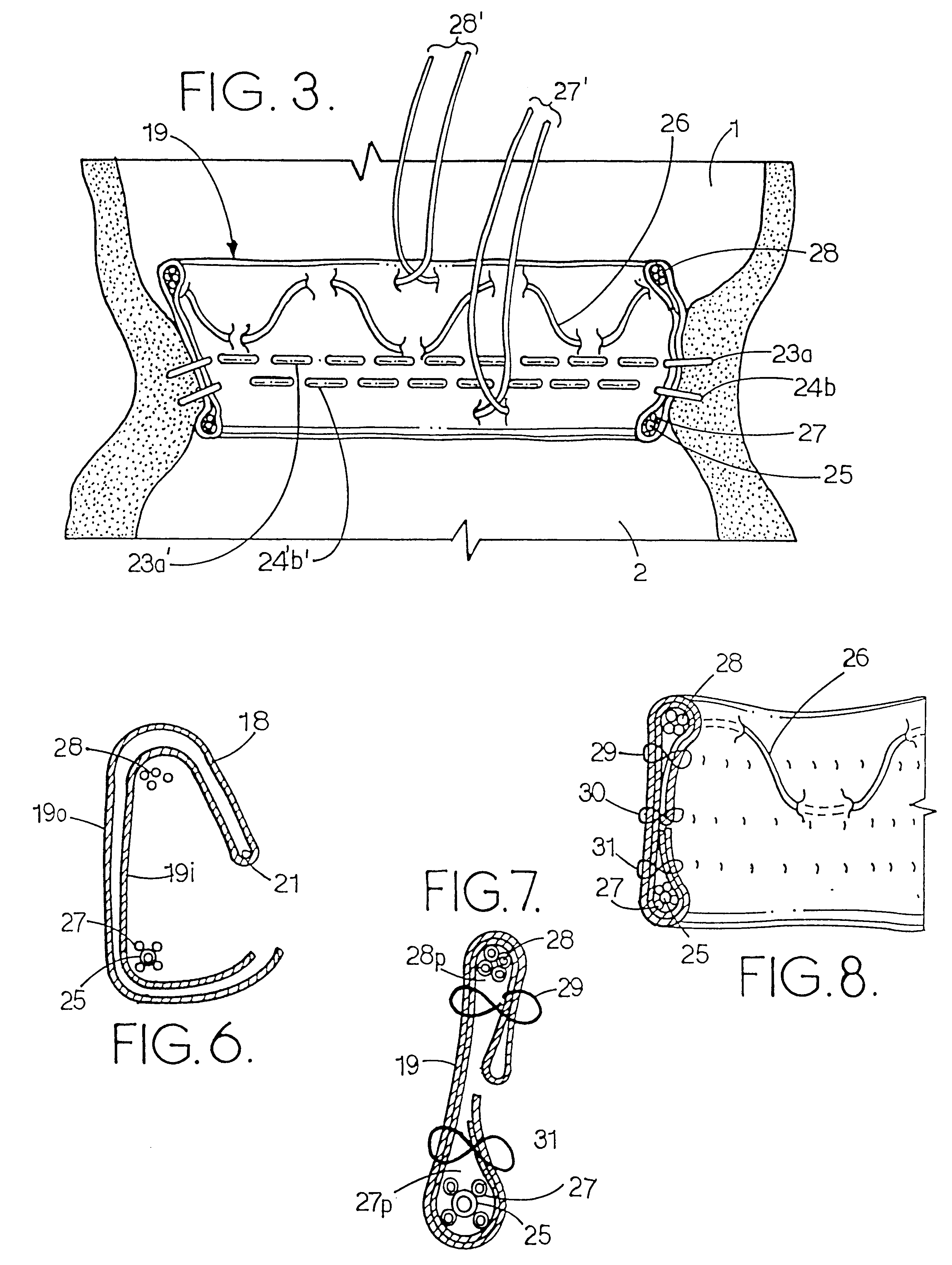
A heart valve can be replaced using minimally invasive methods which include a sutureless sewing cuff that and a fastener delivery tool that holds the cuff against the patient's tissue while delivering fasteners to attach the cuff to the tissue from the inside out. The tool stores a plurality of fasteners and is self-contained whereby a fastener is delivered and placed all from inside a vessel. The fasteners are self-forming whereby they do not need an anvil to be formed. Anchor elements are operated from outside the patient's body to cinch a prosthesis to an anchoring cuff of the valve body. The cuff is releasably mounted on the tool and the tool holds the cuff against tissue and drives the fastener through the cuff and the tissue before folding over the legs of the fastener whereby secure securement between the cuff and the tissue is assured. Fasteners are placed and formed whereby fasteners are located continuously throughout the entire circumference of the cuff. A minimally invasive surgical method is disclosed, and a method and tool are disclosed for repairing abdominal aortic aneurysms in a minimally invasive manner. Fasteners that are permanently deformed during the process of attaching the cuff are disclosed as are fasteners that are not permanently deformed during the attaching process.
Owner:MEDTRONIC INC +1
Transcatheter delivery of a replacement heart valve
A replacement heart valve apparatus. The heart valve apparatus includes a stent and a valve frame having a substantially cylindrical body defining a lumen. The valve frame includes a plurality of curved wire pairs attached to the substantially cylindrical body. Each curved wire pair includes an inner curved wire and an outer curved wire. The wire frame further having a plurality of leaflets. Each leaflet is attached to a respective inner curved wire and extends over a respective outer curved wire, so as to position the body of the leaflet within the lumen of the valve frame.
Owner:CHILDRENS MEDICAL CENT CORP
Endovascular fastener applicator
Endovascular fastener applicator for endoluminally fastening prosthetic grafts to vessels, are provided. The endovascular fastener applicator includes a delivery assembly configured for positioning within a vessel, and a control assembly mounted to a proximal end of the outer sheath for extracorporeal control of the delivery assembly. The delivery assembly includes an expandable portion disposed adjacent a distal end of an outer sheath and being expandable to support a prosthetic in contact with an inner surface of a vessel; a yoke assembly disposed within the expandable portion; an applicator head assembly pivotably mounted to the yoke assembly and movable between a loading position longitudinally aligned with the yoke assembly, and a firing position oriented substantially perpendicular to the yoke assembly; and a fastener assembly connectable to a distal end of the expandable portion, the fastener assembly retaining at least one fastener therein.
Owner:TYCO HEALTHCARE GRP LP
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Low profile heart valve and delivery system
Apparatus for endovascularly replacing a patient's heart valve, including: a delivery catheter having a diameter of 21 french or less; an expandable anchor disposed within the delivery catheter; and a replacement valve disposed within the delivery catheter. The invention also includes a method for endovascularly replacing a heart valve of a patient. In some embodiments the method includes the steps of: inserting a catheter having a diameter no more than 21 french into the patient; endovascularly delivering a replacement valve and an expandable anchor to a vicinity of the heart valve through the catheter; and deploying the anchor and the replacement valve.
Owner:BOSTON SCI SCIMED INC
Apparatus for imparting physician-determined shapes to implantable tubular devices
A tubular sleeve is provided for enabling a physician to impart a preselected shape in an implantable tubular device, such as a cardiac lead, a catheter, or some other tubular structure. The sleeve may be deformed by the surgeon before or at the time of implantation to customize the shape of the tubular device to the particular anatomical structures to be encountered by the device. To retain the deformation imparted by the physician, the sleeve may be composed of a heat-sensitive shape-memory material or an elastomeric material provided with a plastically deformable rib.
Owner:INTERMEDICS
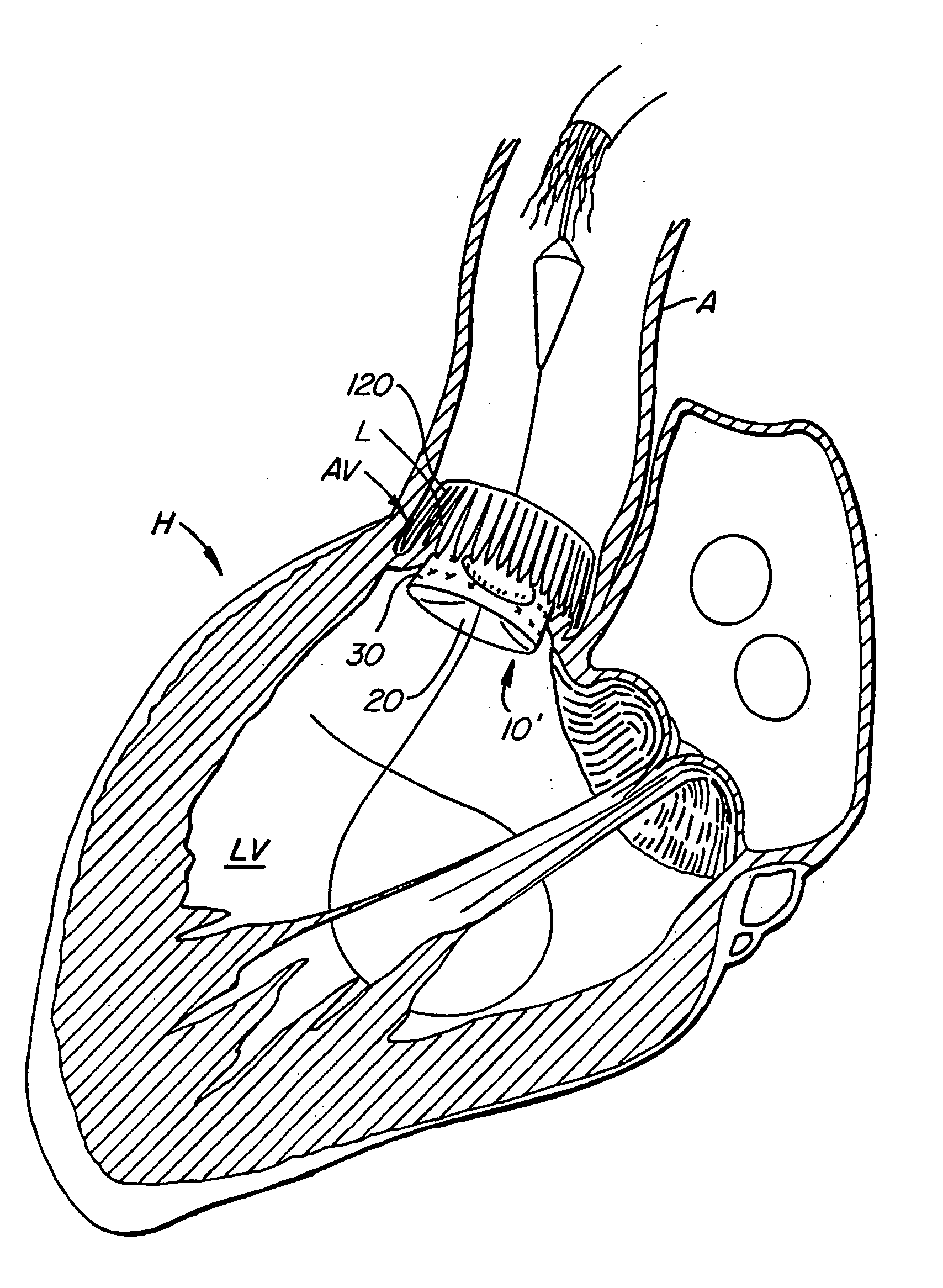
Methods and devices for repair or replacement of heart valves or adjacent tissue without the need for full cardiopulmonary support
ActiveUS20060074484A1Reduce in quantityEliminate needEar treatmentCannulasAortic dissectionVentricular aneurysm
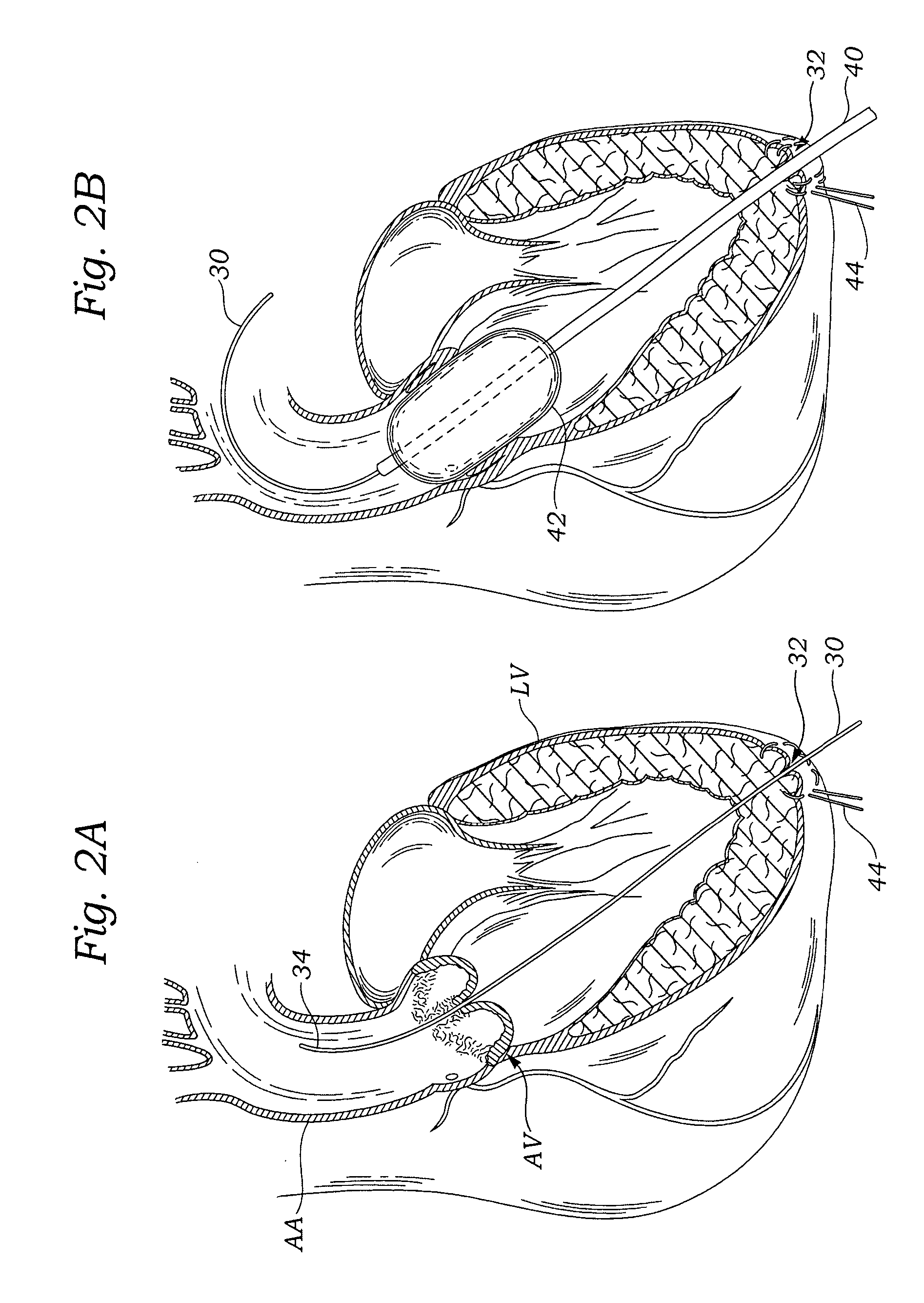
Methods and systems for endovascular, endocardiac, or endoluminal approaches to a patient's heart to perform surgical procedures that may be performed or used without requiring extracorporeal cardiopulmonary bypass. Furthermore, these procedures can be performed through a relatively small number of small incisions. These procedures may illustratively include heart valve implantation, heart valve repair, resection of a diseased heart valve, replacement of a heart valve, repair of a ventricular aneurysm, repair of an arrhythmia, repair of an aortic dissection, etc. Such minimally invasive procedures are preferably performed transapically (i.e., through the heart muscle at its left or right ventricular apex).
Owner:EDWARDS LIFESCI CARDIAQ
Methods and apparatus for endovascular heart valve replacement comprising tissue grasping elements
ActiveUS20060058872A1Accurate placementPrevent blood backflowStentsBalloon catheterHeart valve replacementBlood vessel
The present invention provides an apparatus for endovascularly replacing a patient's heart valve. In some embodiments, the apparatus includes an expandable anchor supporting a replacement valve, the anchor and replacement valve being adapted for percutaneous delivery and deployment to replace the patient's heart valve, the anchor having a braid having atraumatic grasping elements adapted to grasp tissue in a vicinity of the patient's heart valve.
Owner:BOSTON SCI SCIMED INC
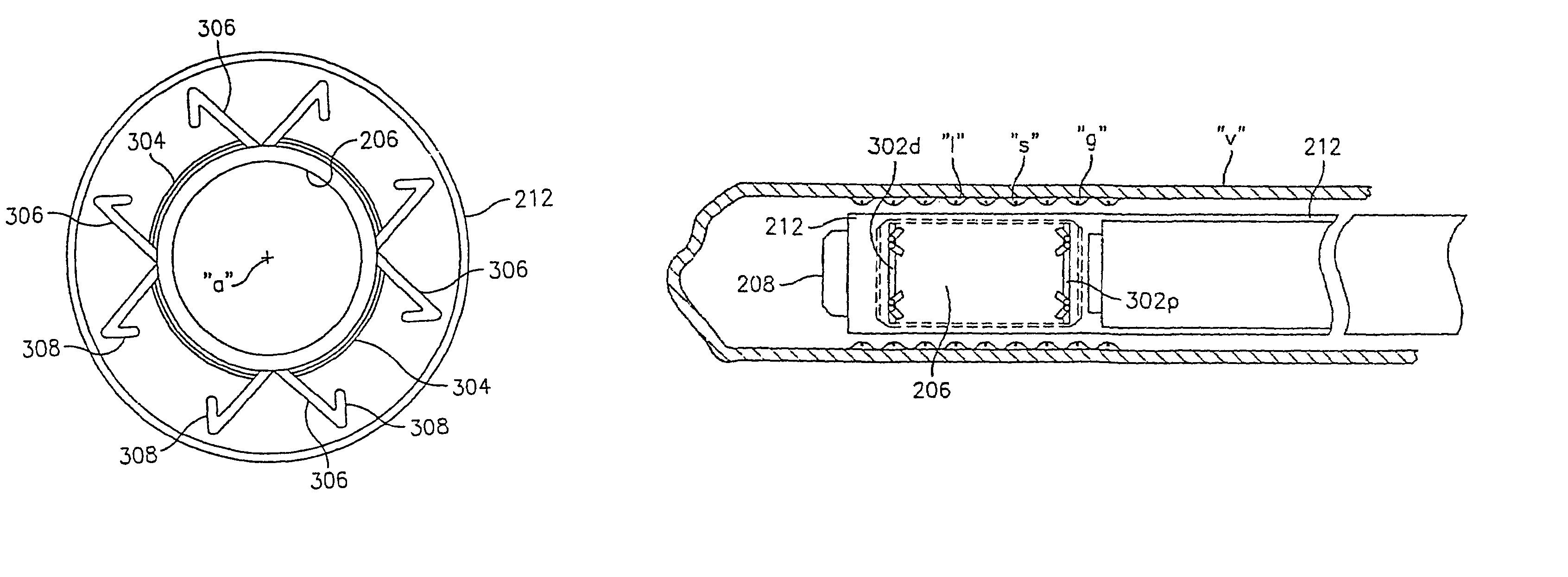
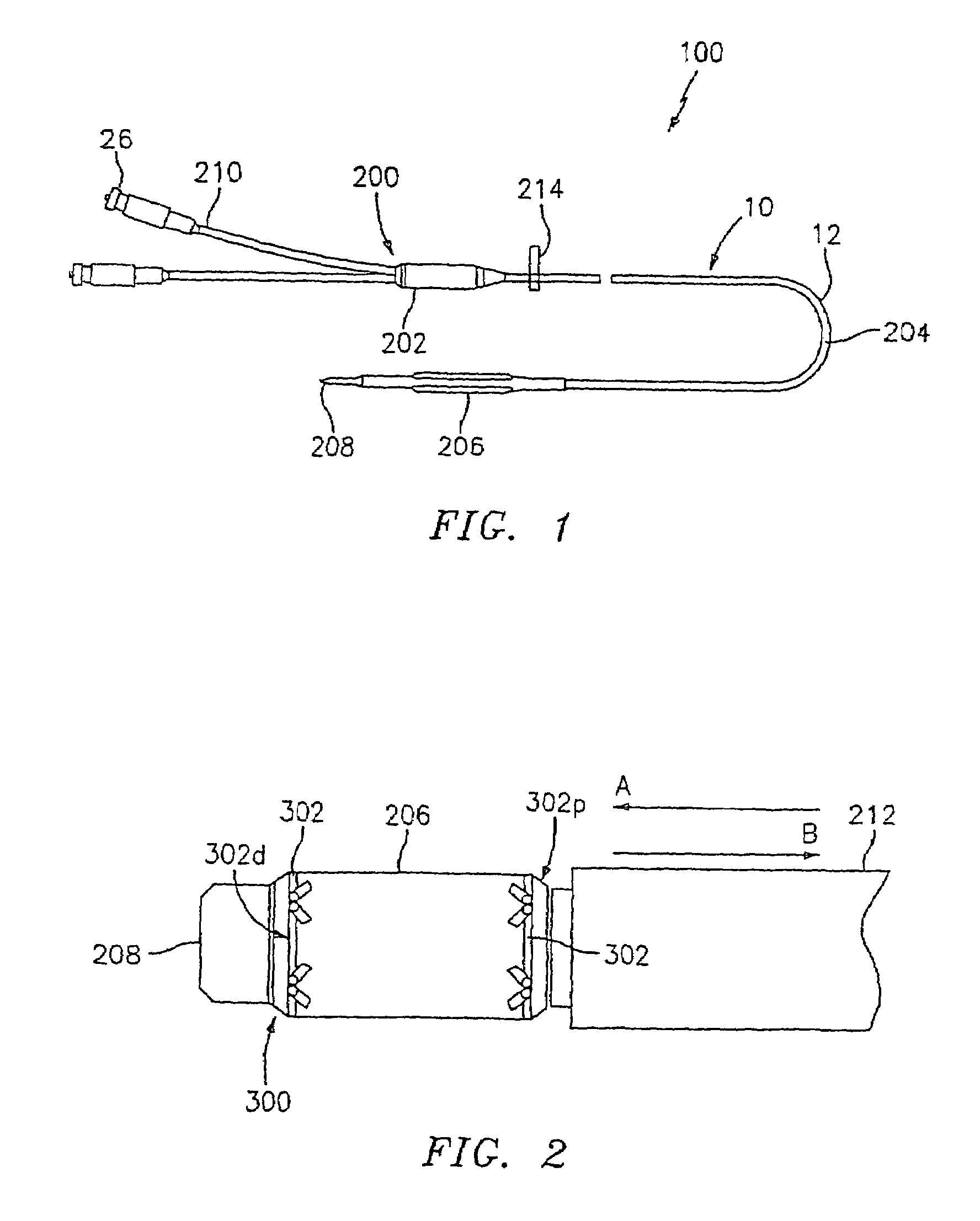
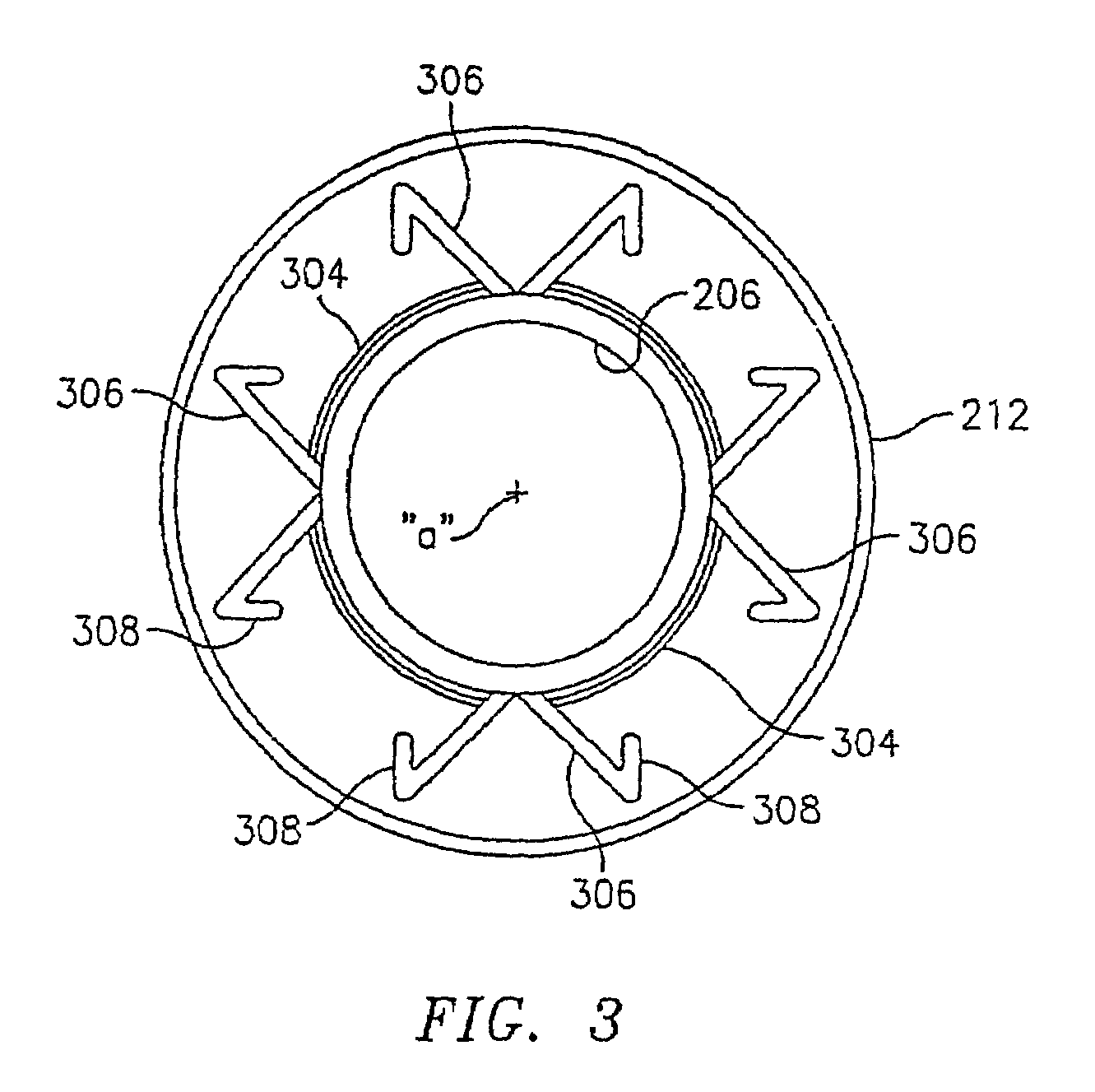
Implantable sensor
InactiveUS6895265B2Thickness minimizationTransport of glucose to the sensor is not altered over timeStentsCatheterAnalyteInsulin pump
A sensor is disclosed, for implantation within a blood vessel to monitor an analyte in blood. In one embodiment, the analyte is glucose. A signal indicative of glucose level is transmitted to an external receiver. The signal may also be used to drive an internal or externally worn insulin pump. Methods are also disclosed.
Owner:SILVER JAMES H
Prosthesis fixation apparatus and methods
A method of securing a prosthesis placed at a desired site in a passageway of a human body comprises delivering a fastener having a proximal piercing end portion and a distal piercing end portion to a site where a prosthesis having a tubular wall has been placed in the passageway, which has a wall, advancing the proximal piercing end portion beyond the prosthesis, penetrating the proximal piercing end portion into the wall of the passageway without passing the proximal piercing end portion through the tubular wall of the prosthesis, and passing the distal piercing end portion through the tubular wall of the prosthesis and into the wall of the passageway. One surgical fastener delivery apparatus for delivering a surgical fastener to a target site comprises a support having a first end, a second end, and a longitudinal axis and being adapted for placement in a passageway in a human body. A surgical fastener having a first piercing end portion, a second piercing end portion and a central portion extending therebetween and having a longitudinal axis is releasably mounted to the support with the central portion longitudinal axis generally parallel to the support longitudinal axis.
Owner:MEDTRONIC VASCULAR INC
Endovascular aneurysm devices, systems, and methods
Devices, systems, and methods for implanting prostheses in the body lumens rely on tacking or anchoring the prostheses with separately introduced fasteners. After initial placement, a fastener applier system is introduced within the expanded prostheses to deploy a plurality of fasteners to at least one prosthesis end. The fasteners are usually helical fasteners which are releasably restrained on the fastener driver, and are delivered by rotation of the fastener driver. The fasteners may be applied singly, typically in circumferentially spaced-apart patterns about the interior of at least one end of the prosthesis. A lumen extension or lumens may be coupled to the prosthesis to extend the reach of the prosthesis within the implantation site. Fasteners may also be applied to the lumen extensions.
Owner:MEDTRONIC VASCULAR INC
Fluid flow prosthetic device
A prosthetic device including a valve-orifice attachment member attachable to a valve in a blood vessel and including a fluid inlet, and a diverging member that extends from the fluid inlet, the diverging member including a proximal end near the fluid inlet and a distal end distanced from the proximal end, wherein a distal portion of the diverging member has a larger cross-sectional area for fluid flow therethrough than a proximal portion thereof. The diverging member may have a diverging taper that causes fluid to flow therethrough with pressure recovery at the distal end thereof.
Owner:MEDTRONIC VENTOR TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com