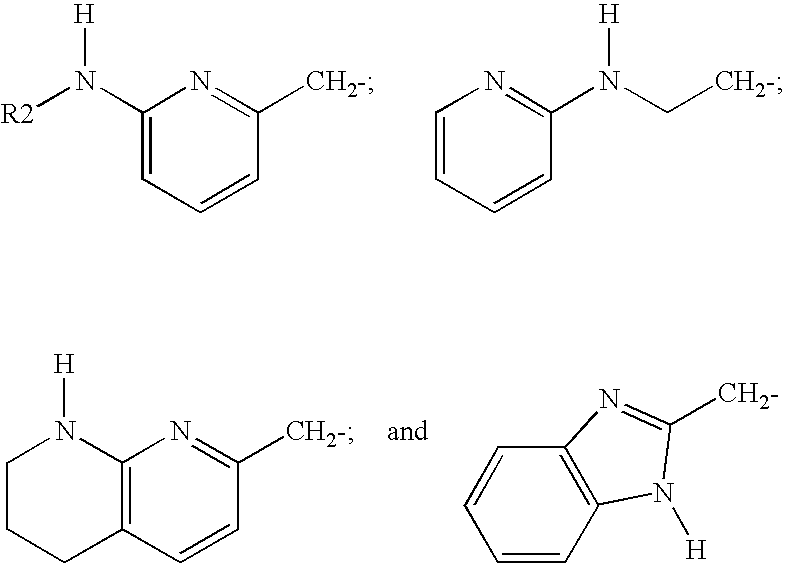
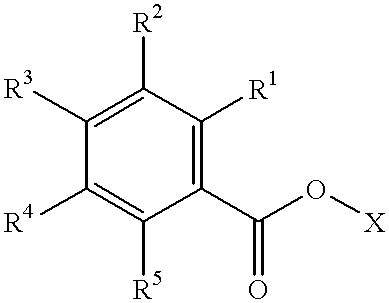
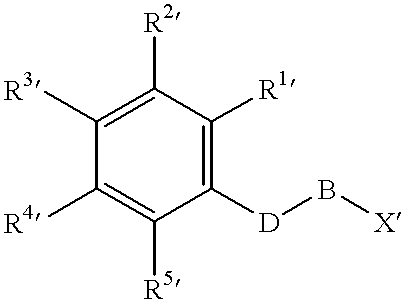
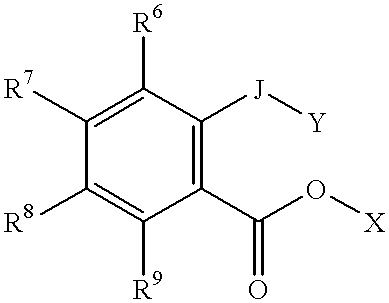
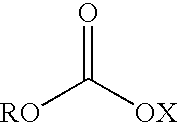
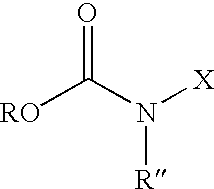
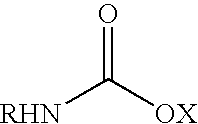
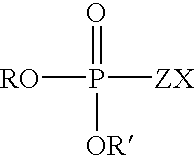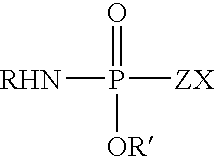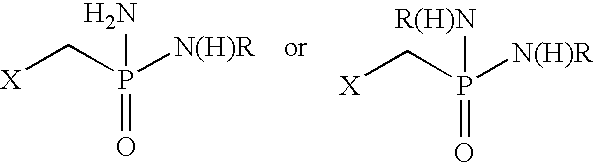Patents
Literature
109results about How to "Reduce drug toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
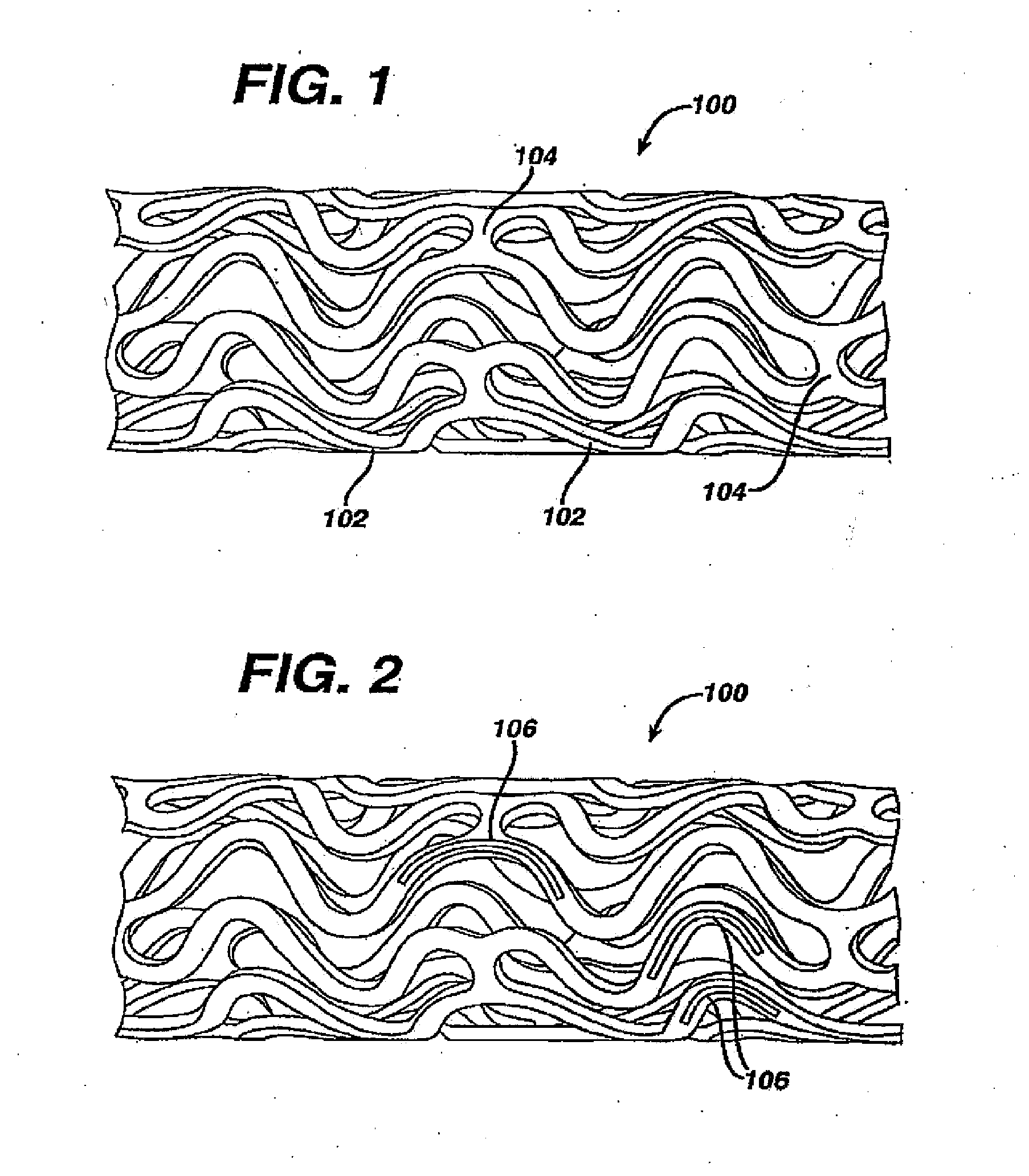
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
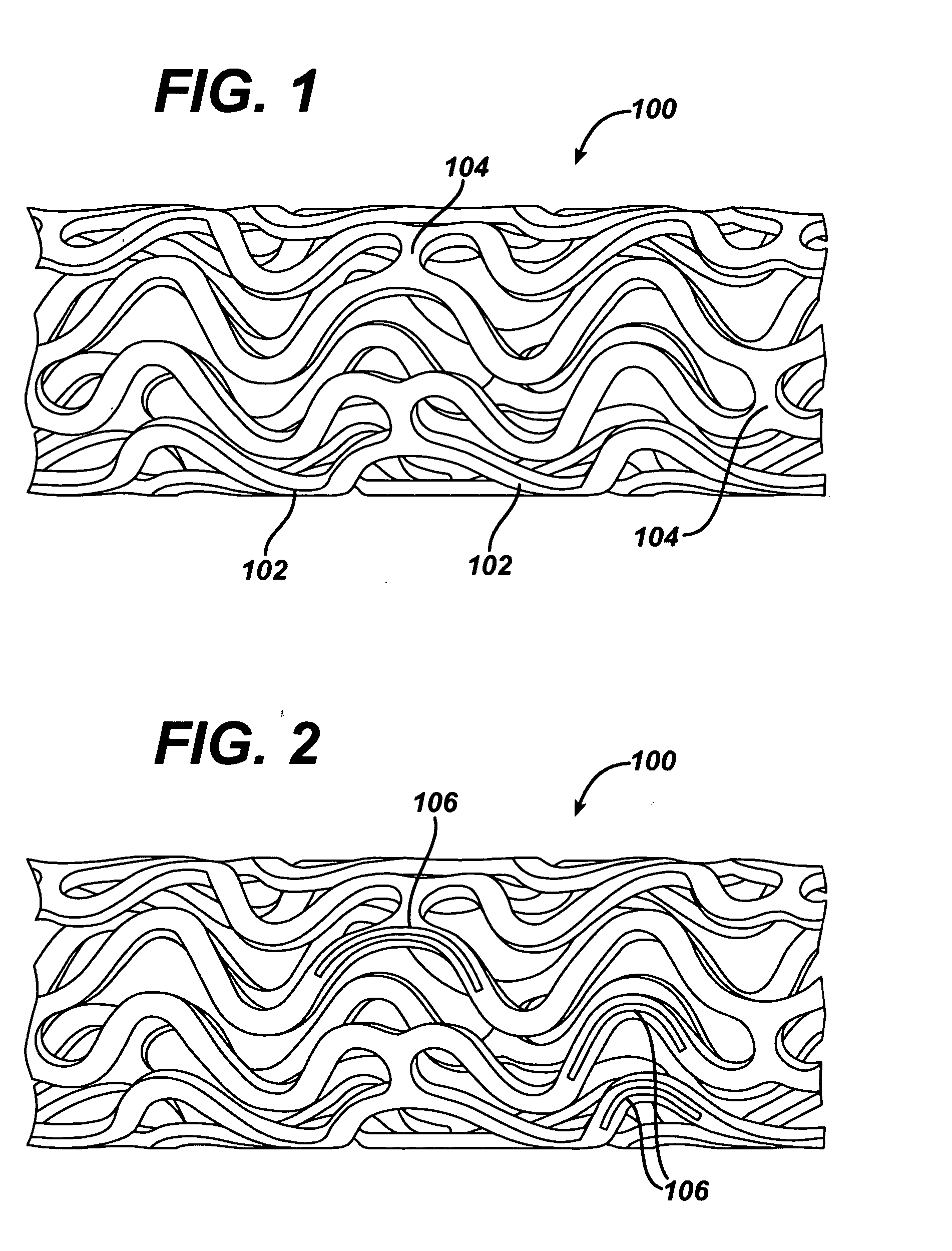
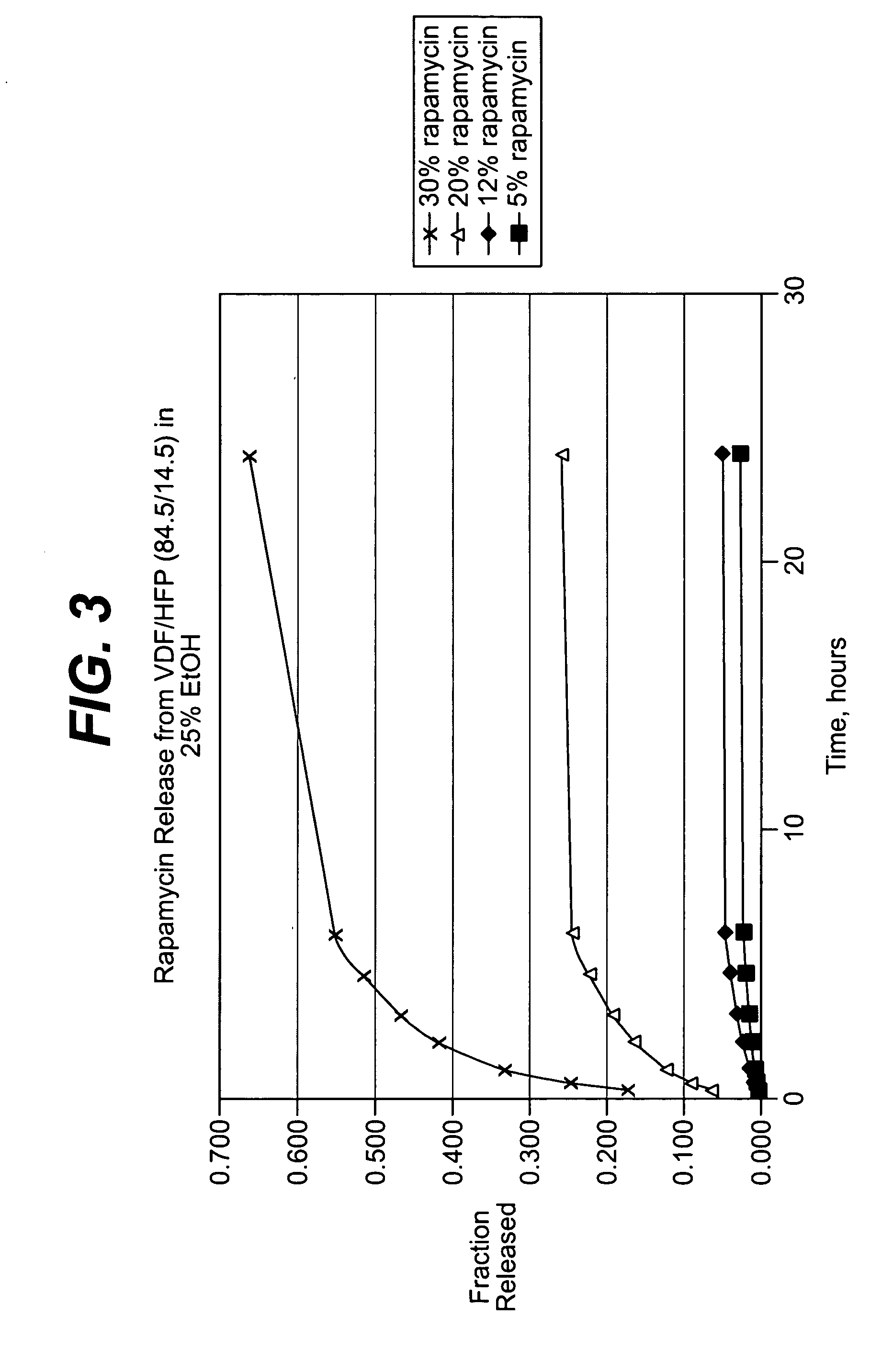
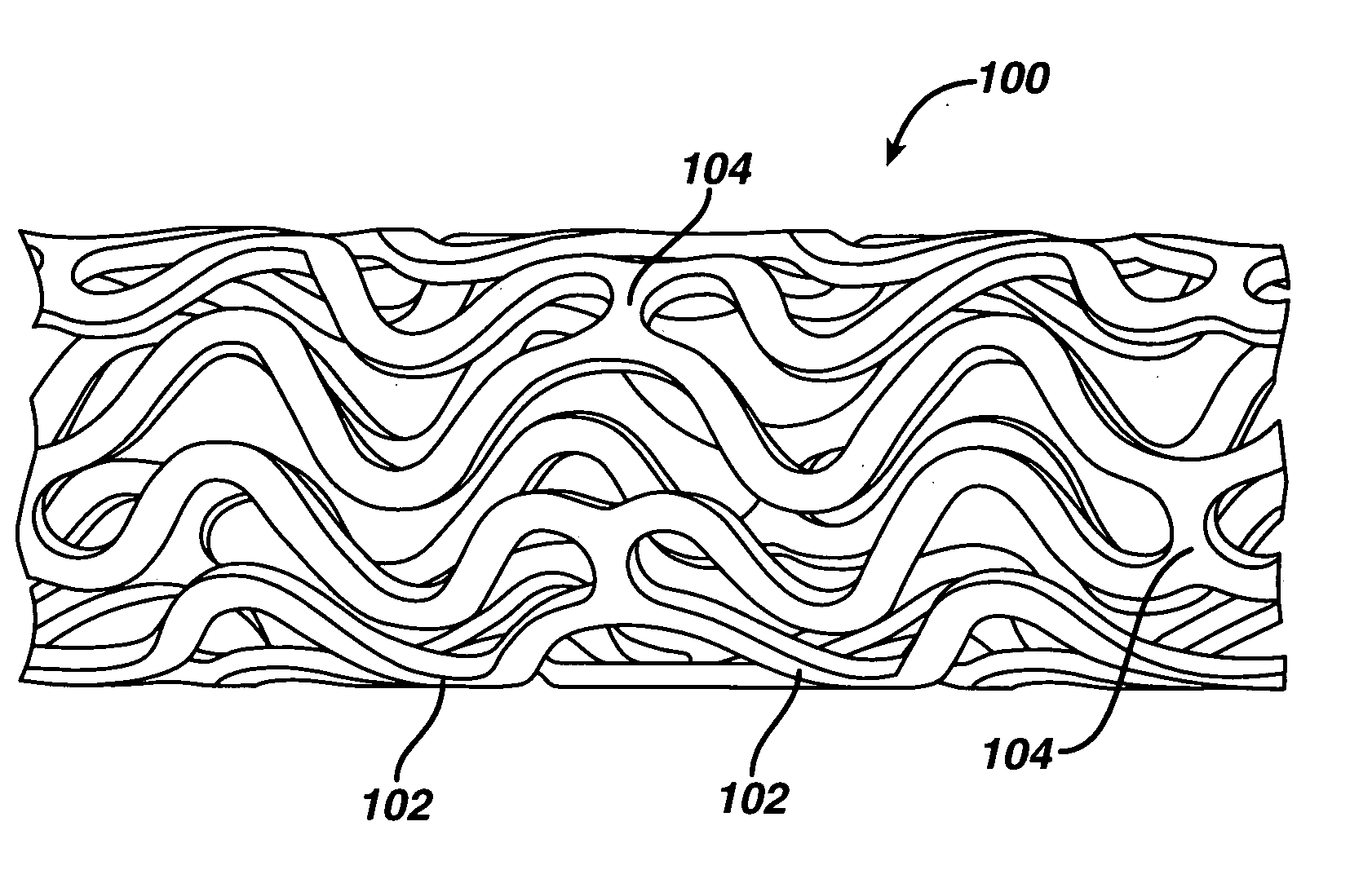
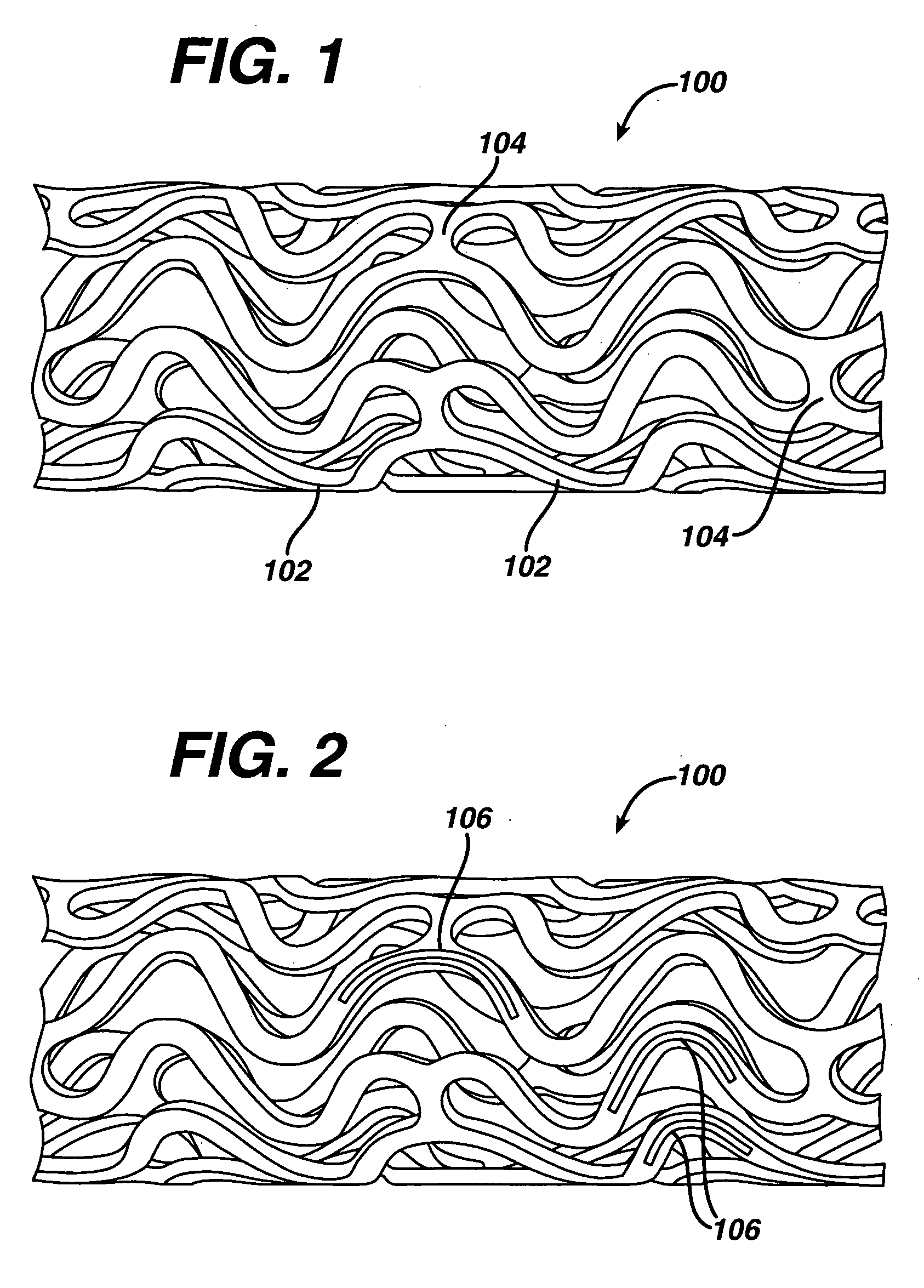
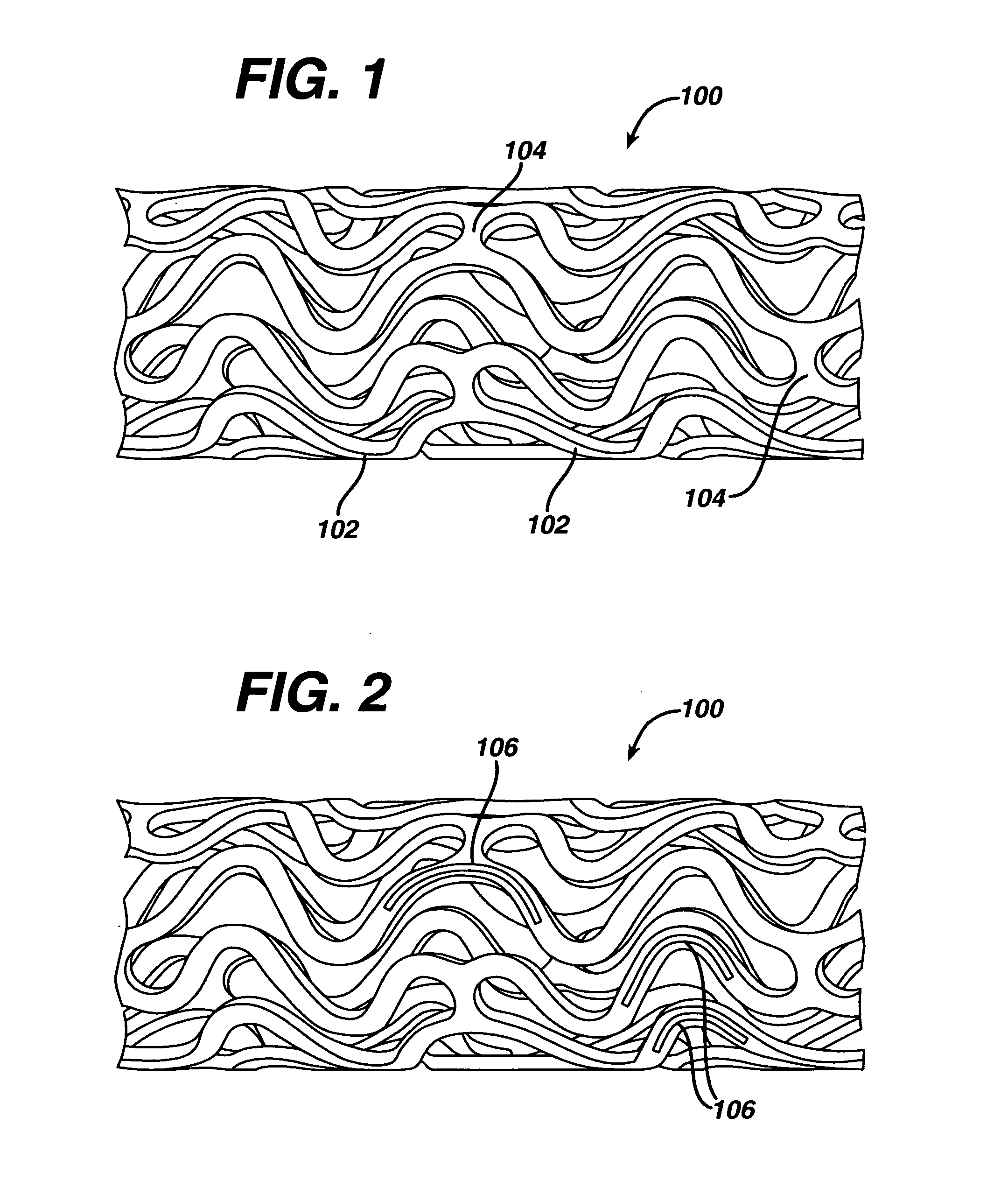
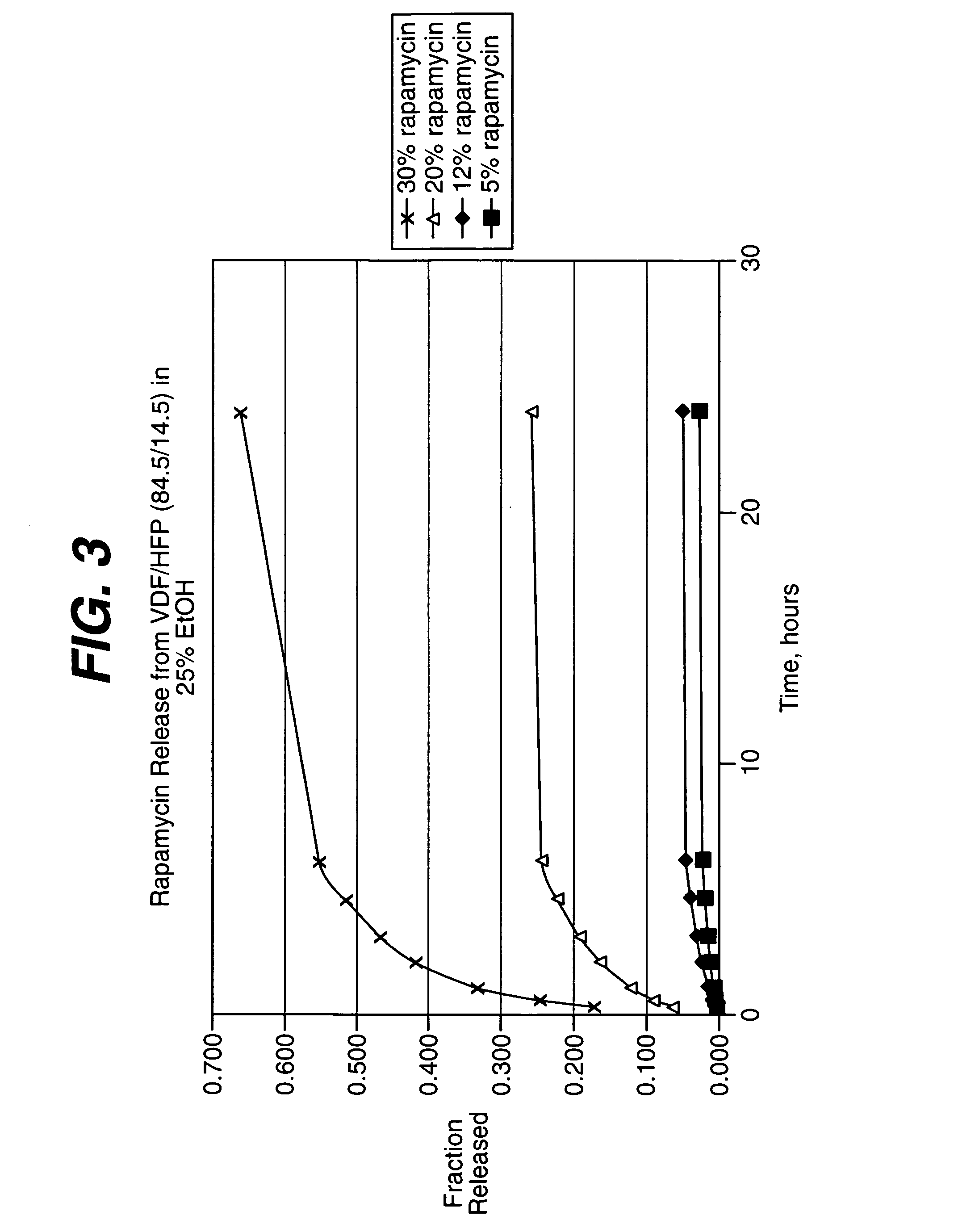
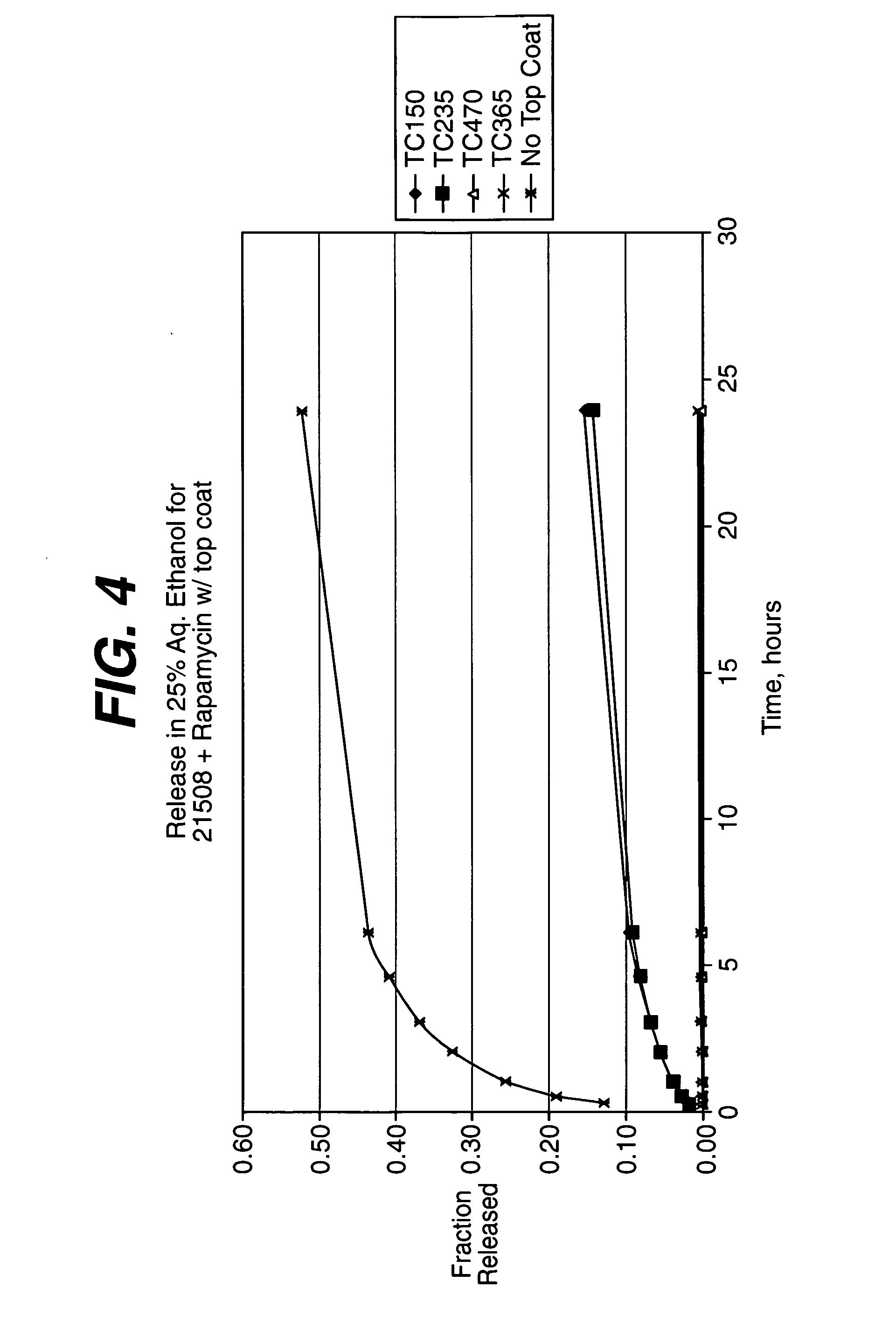
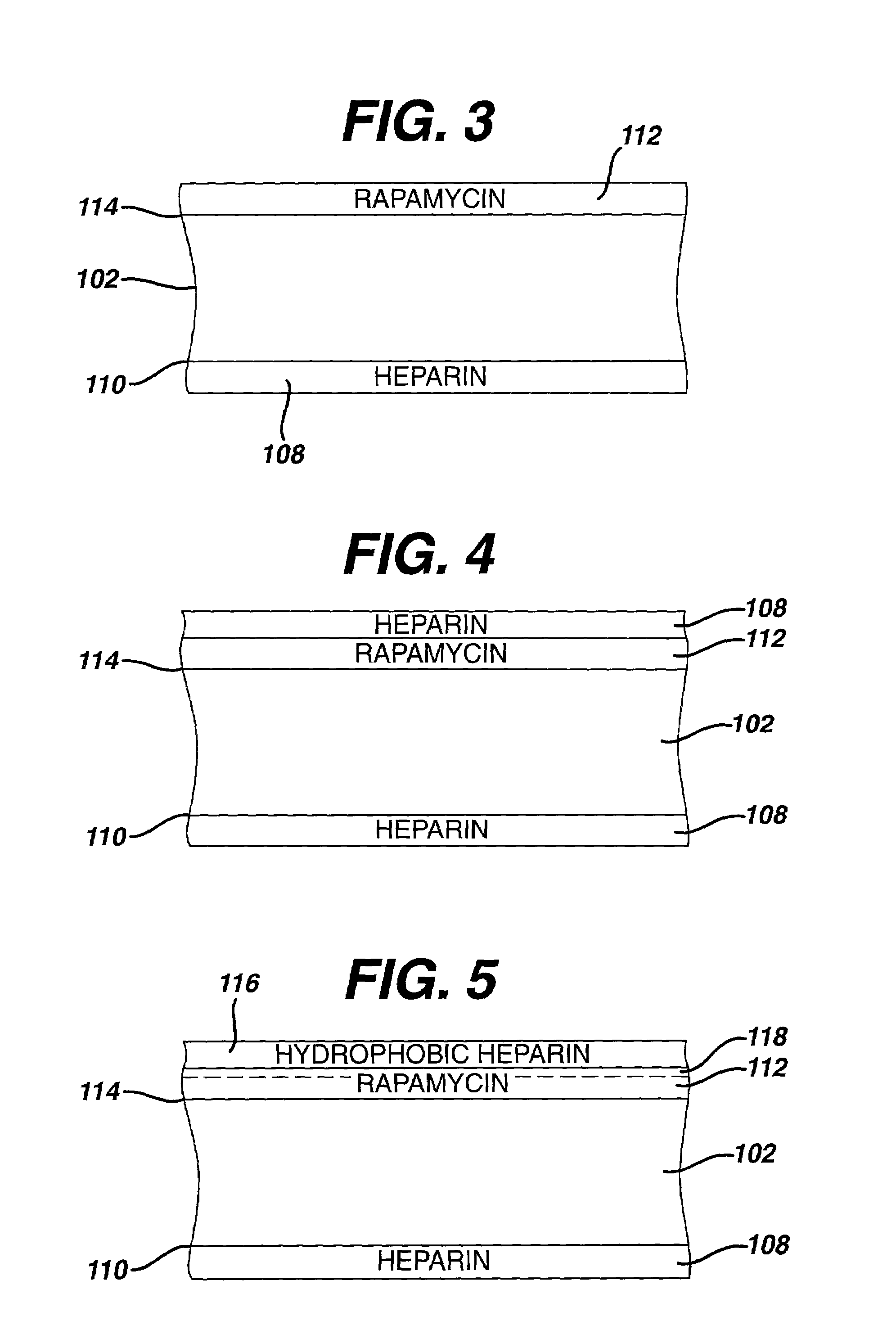
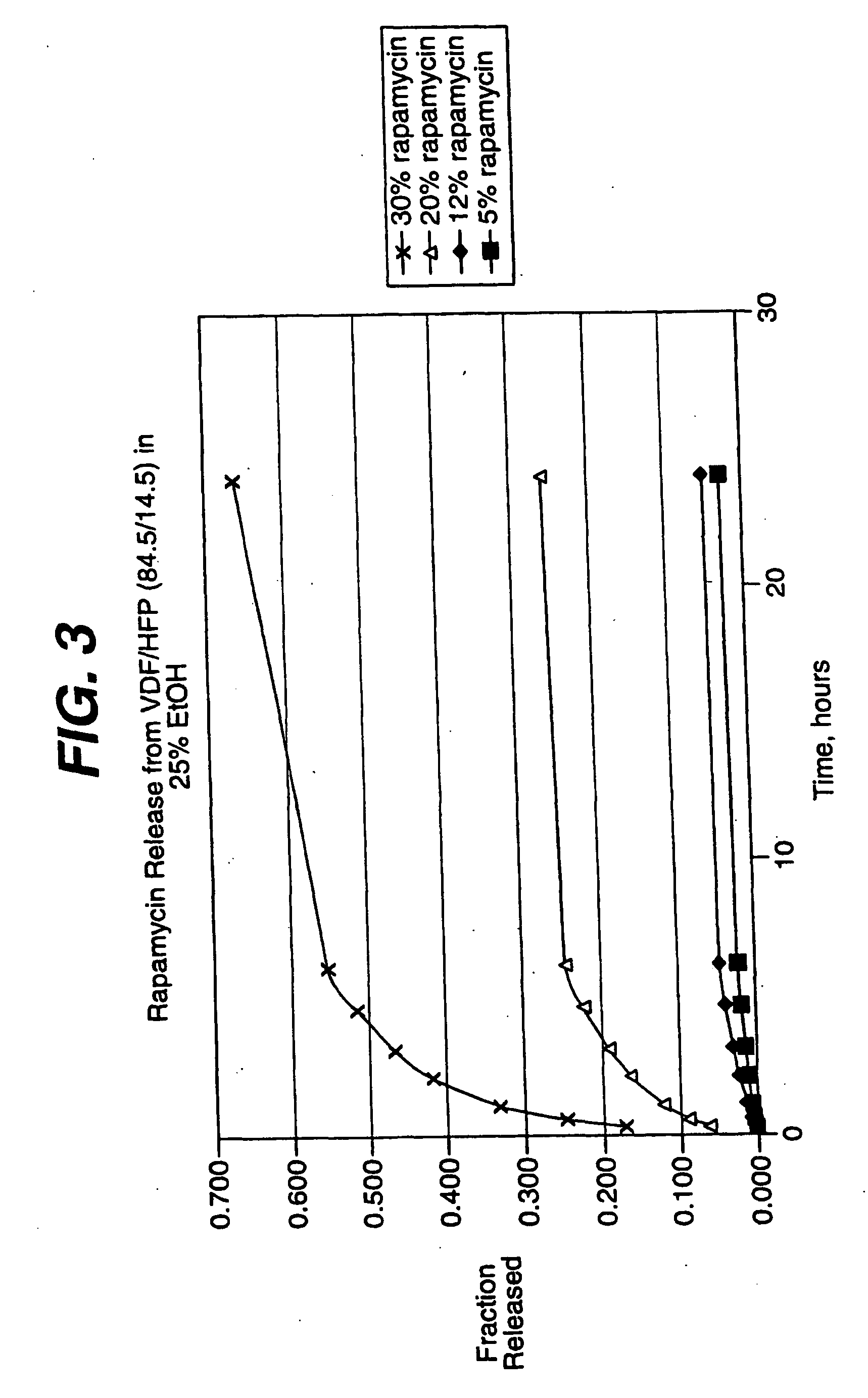
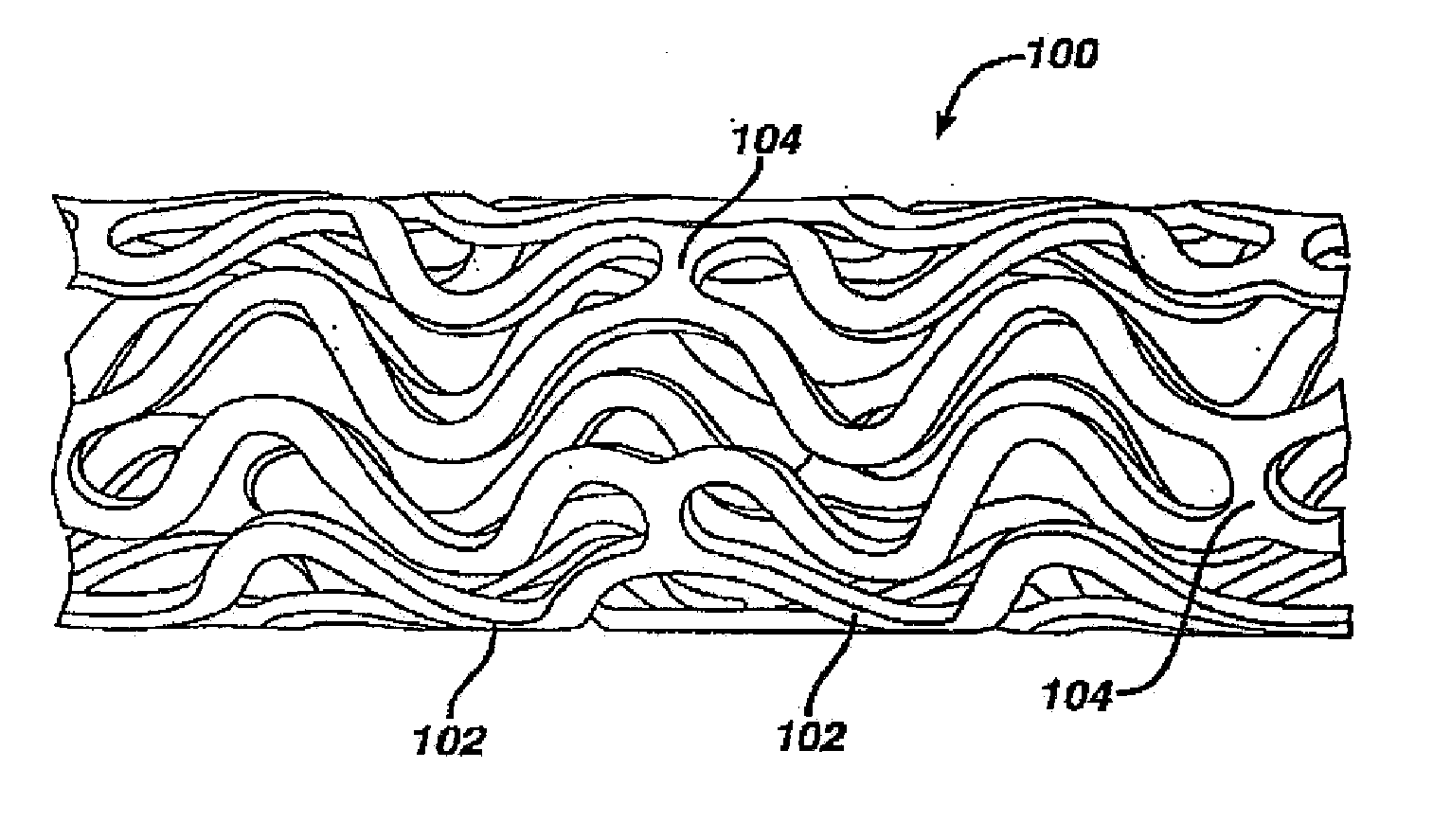
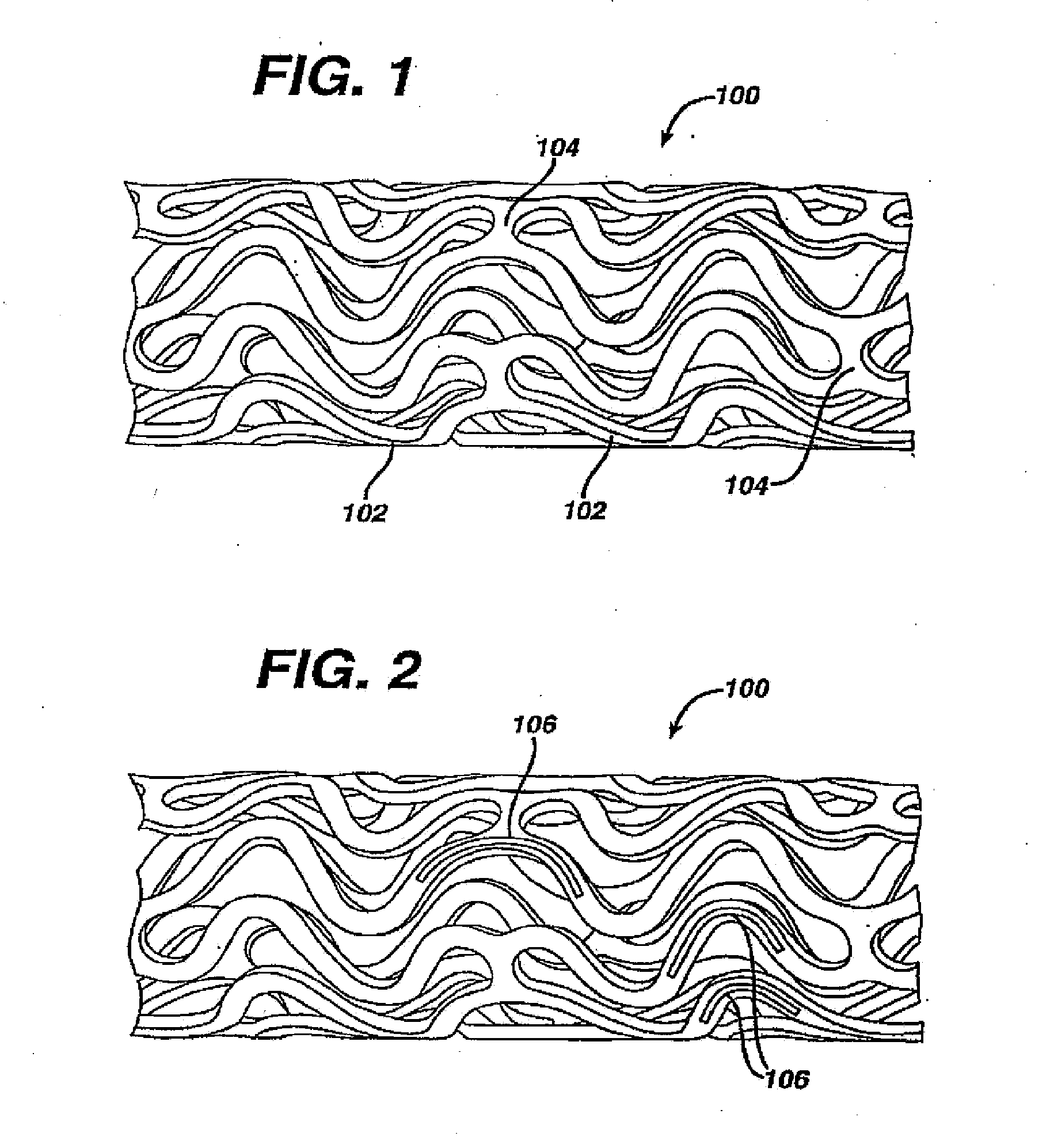
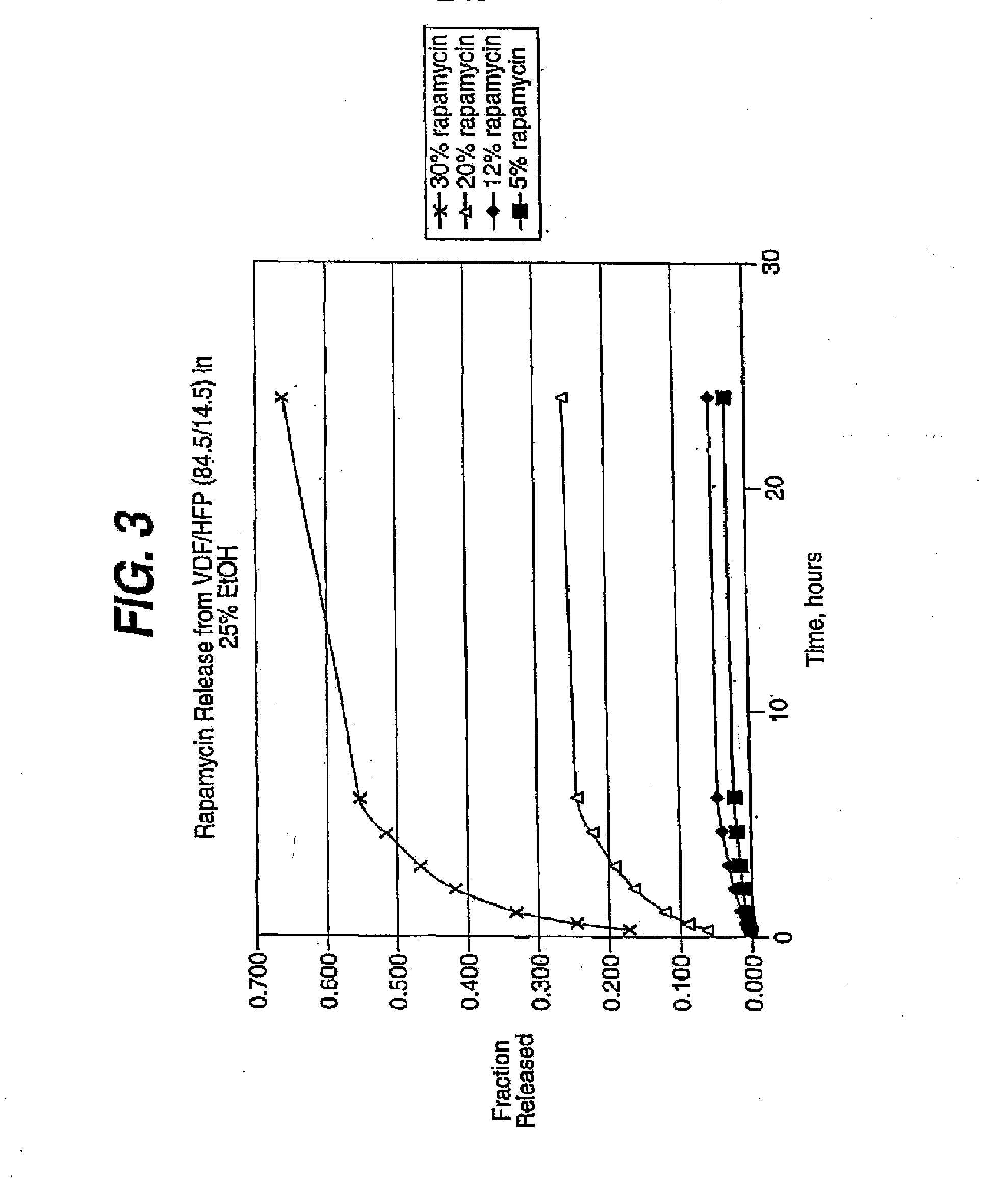
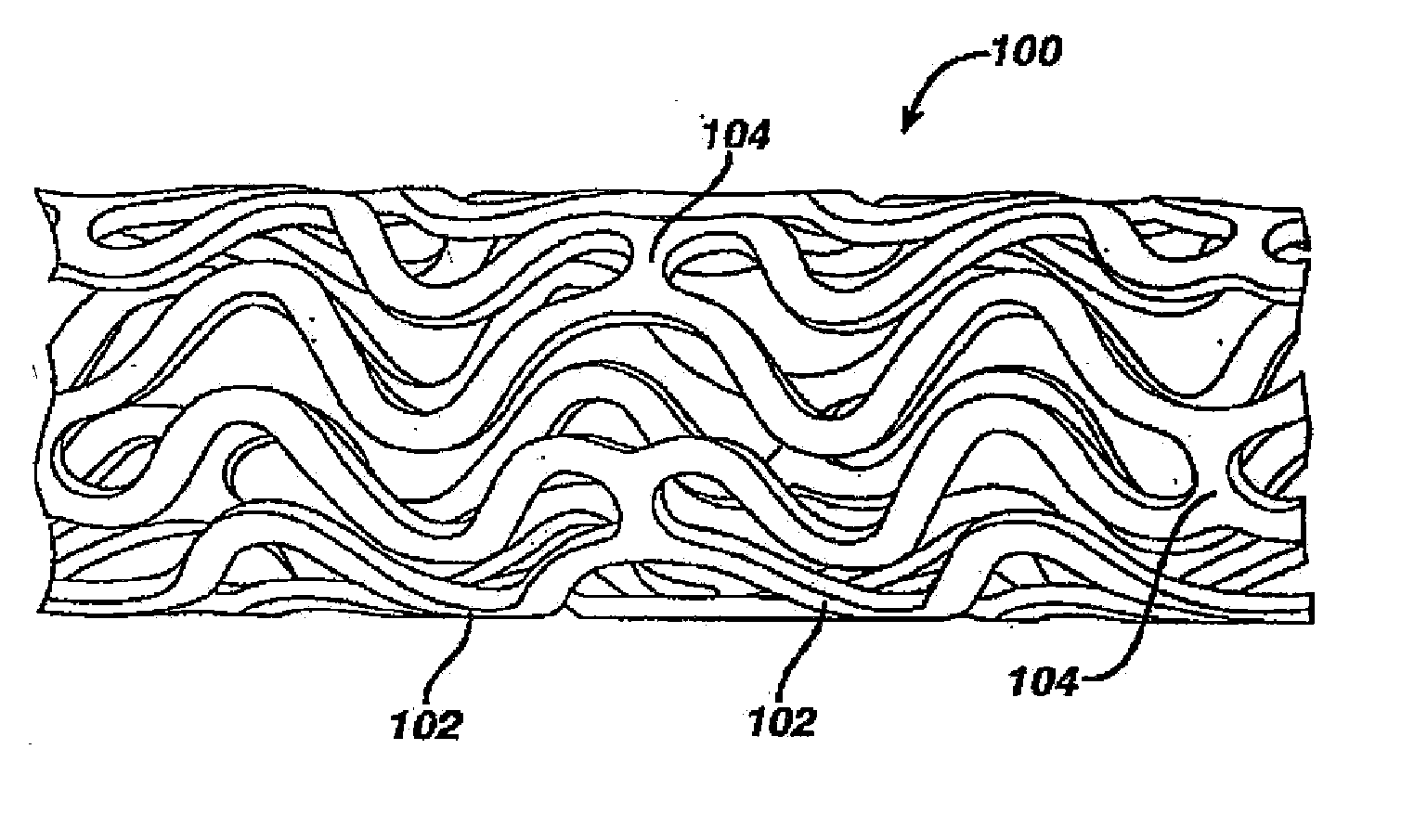
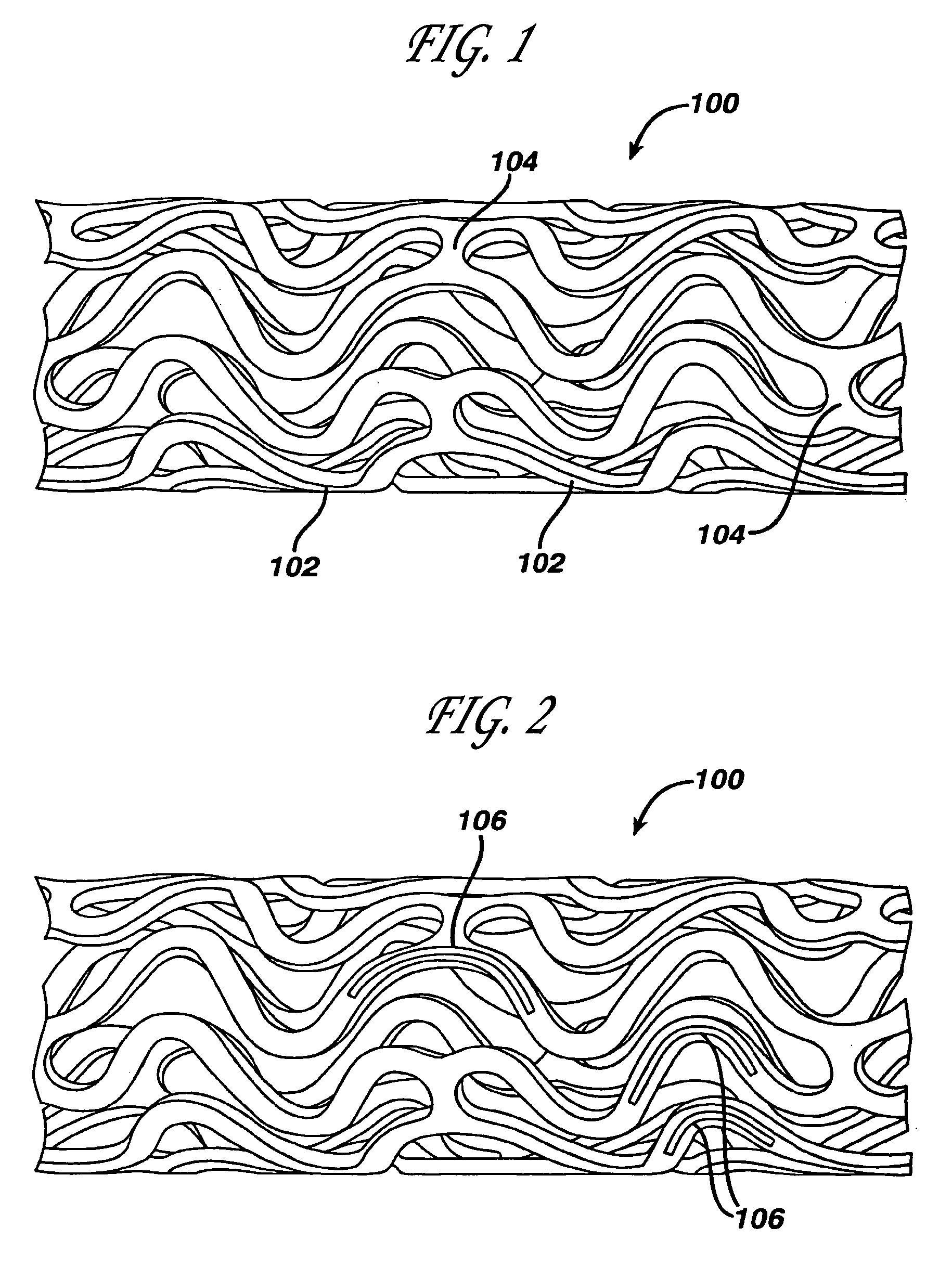
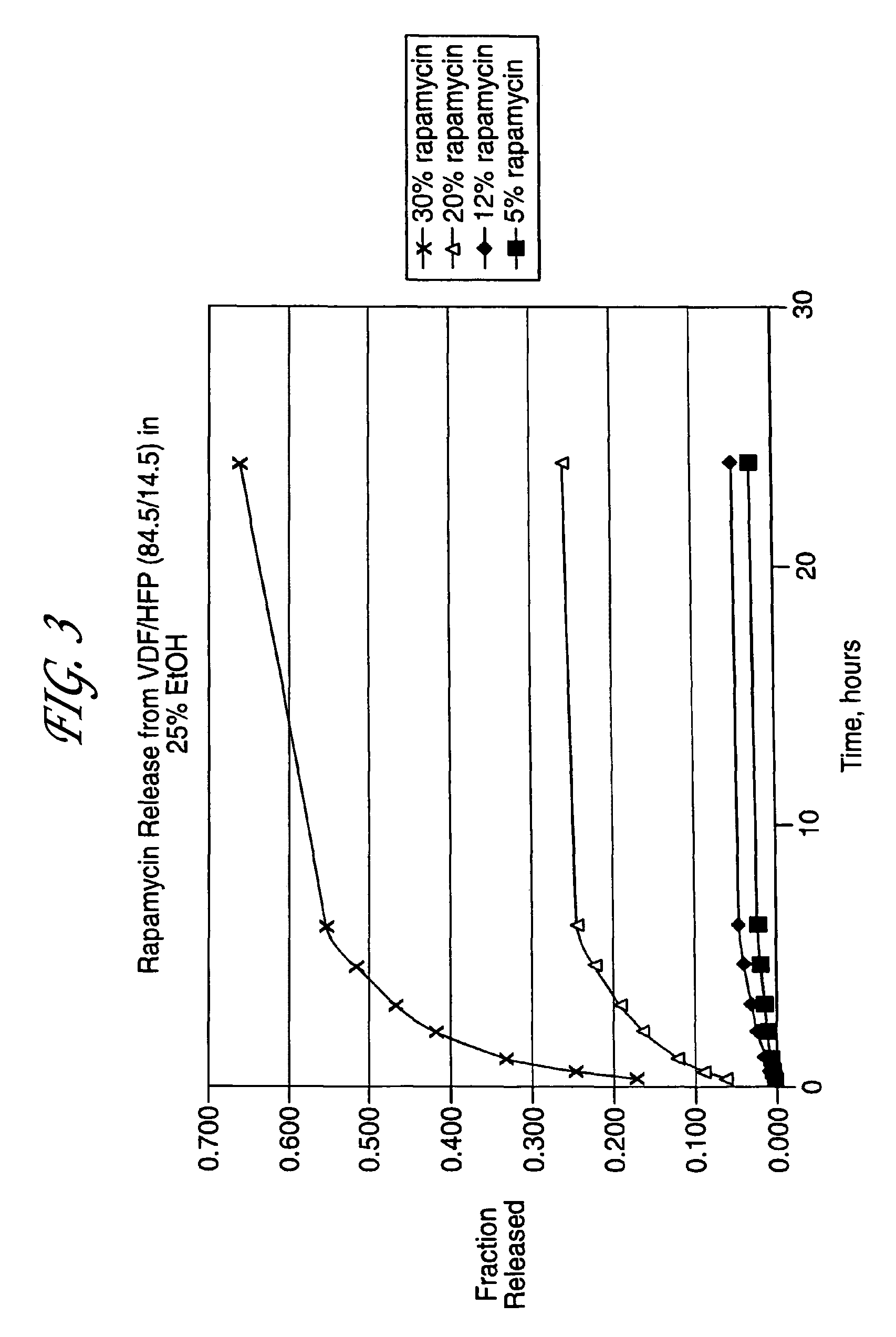
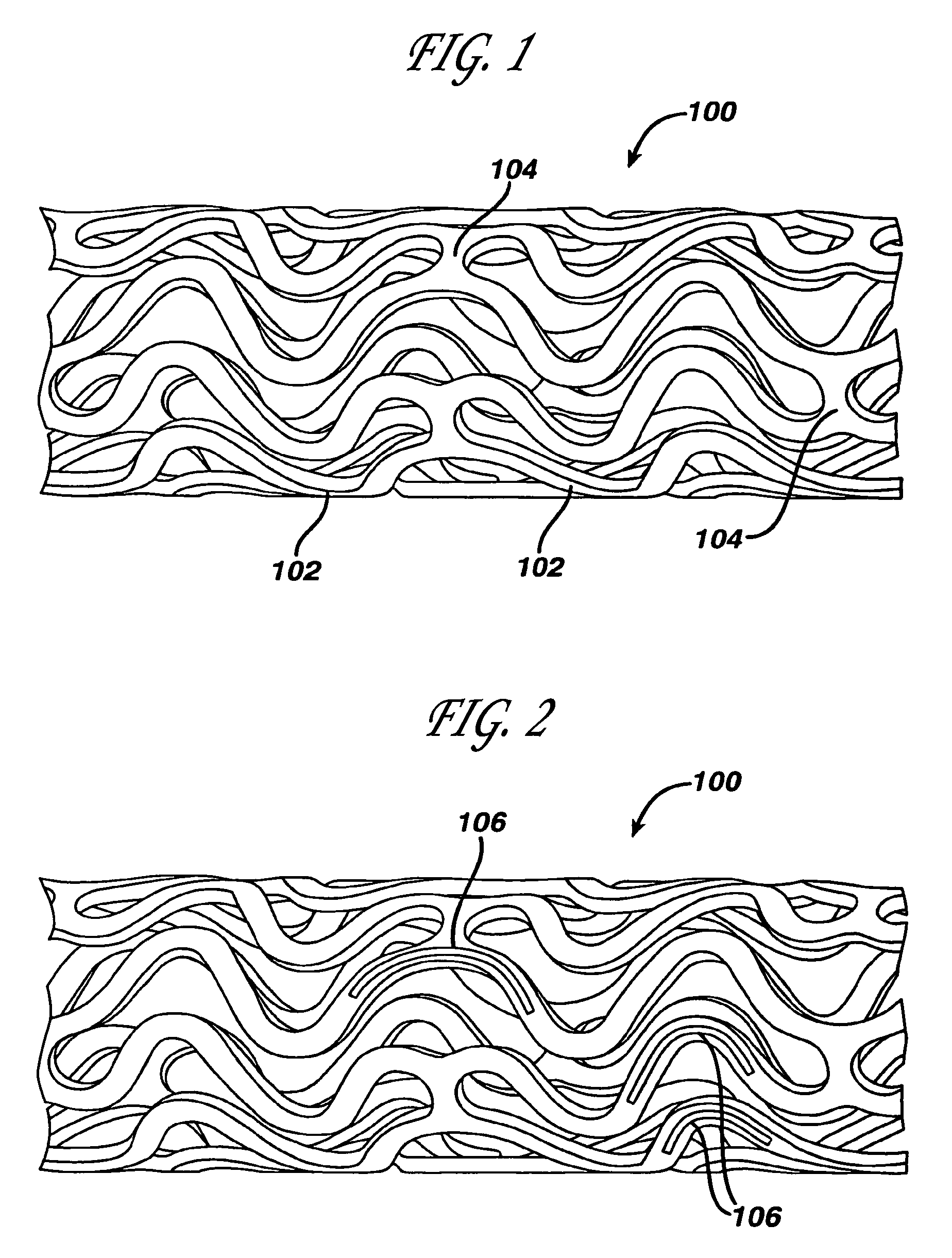
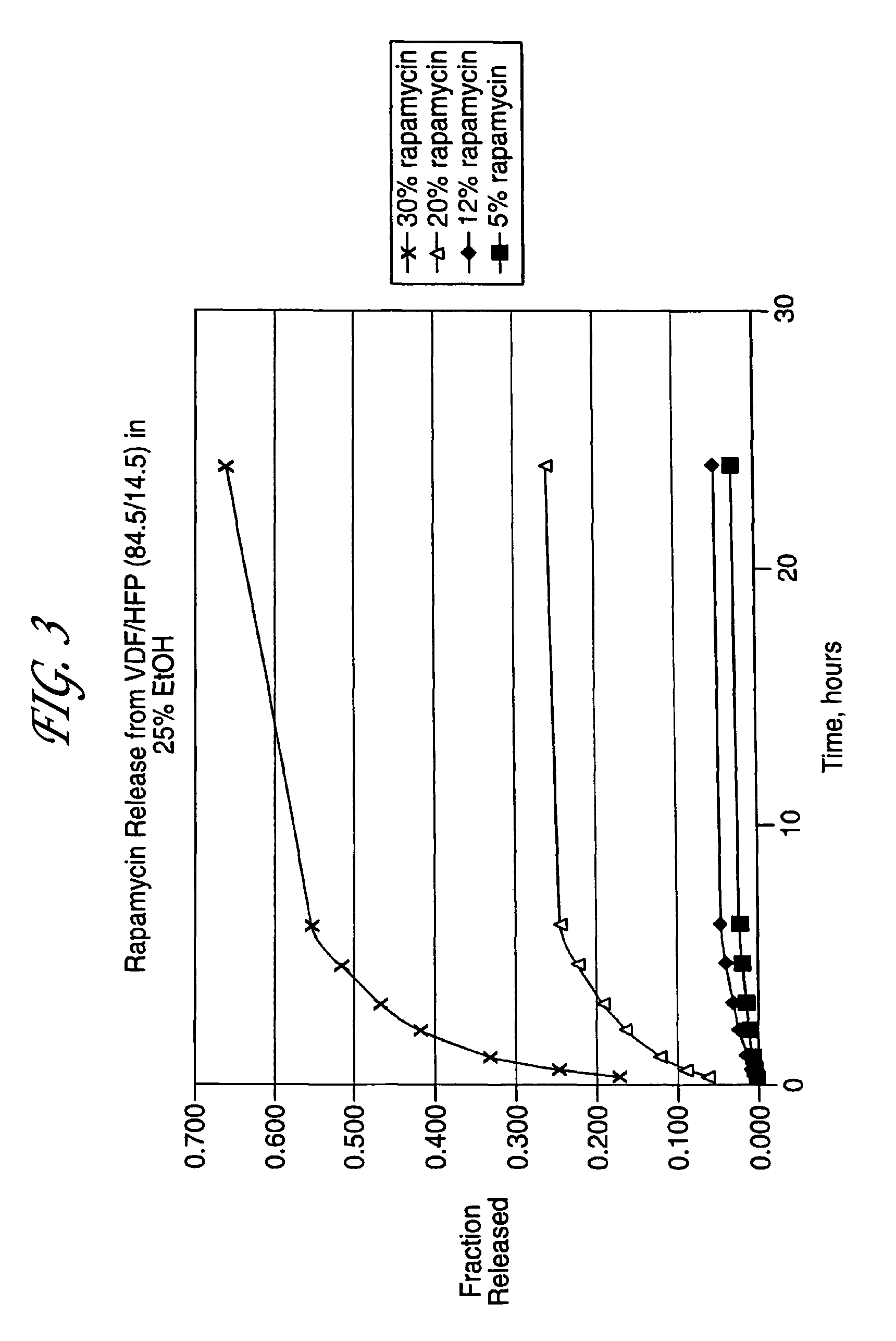
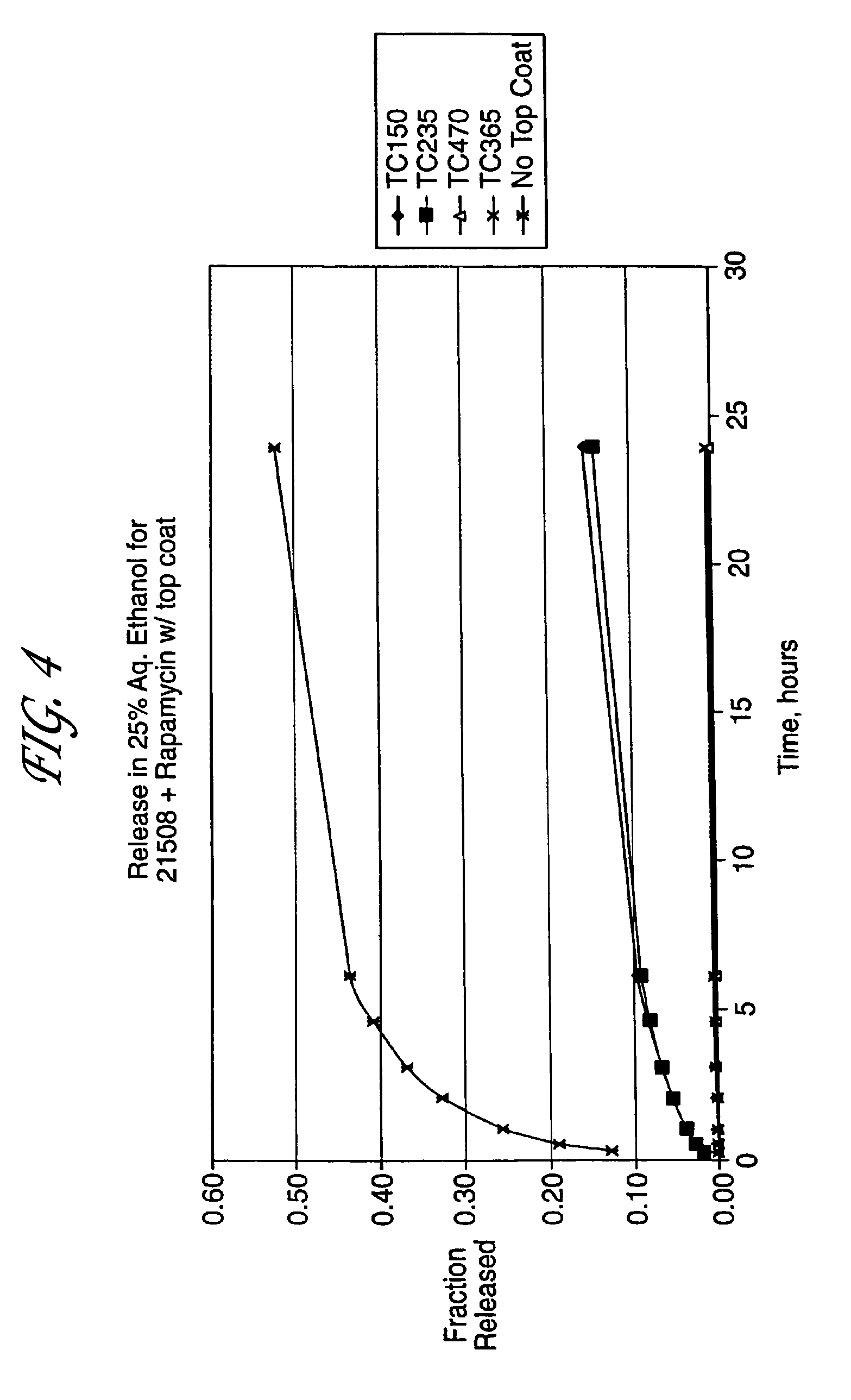
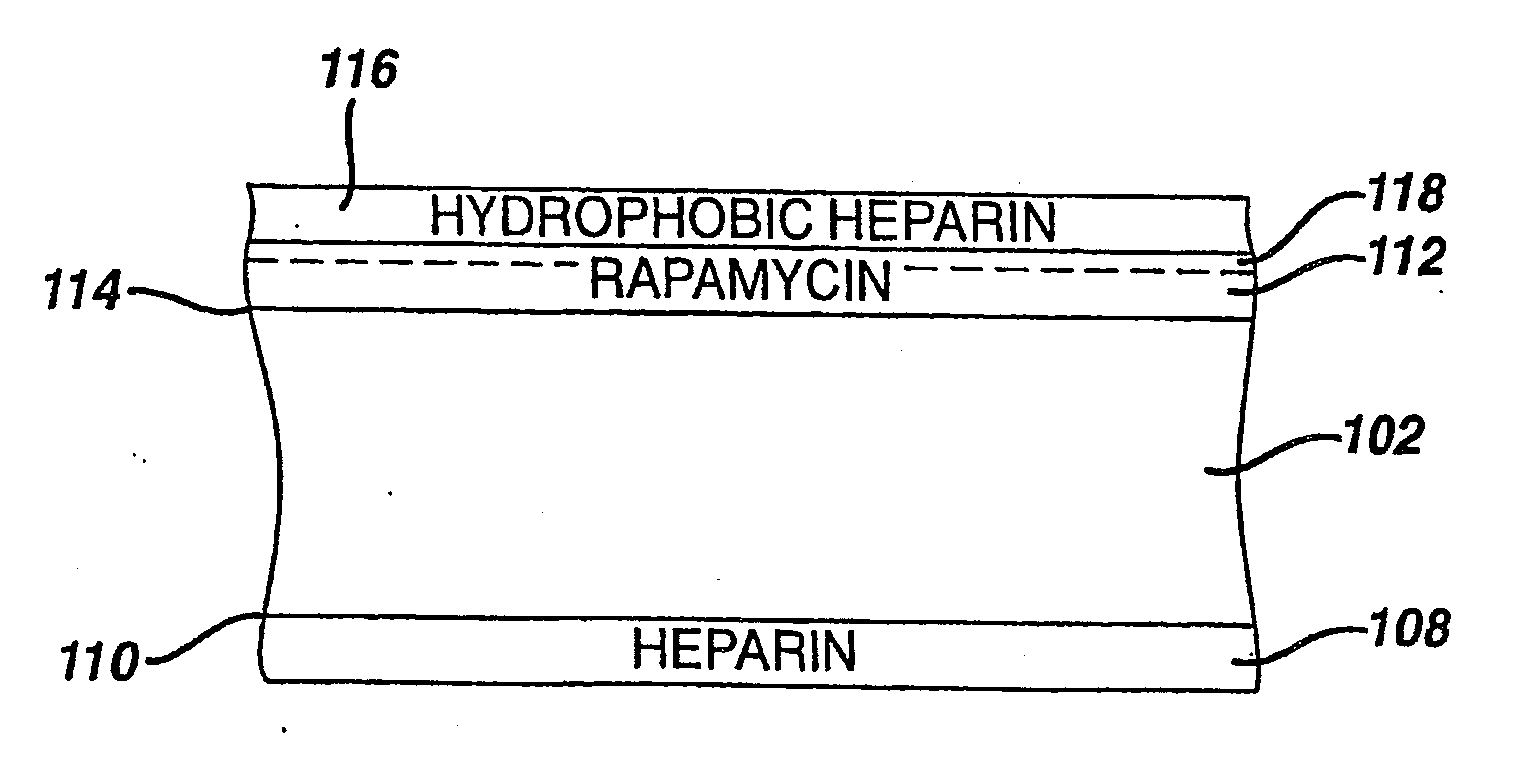
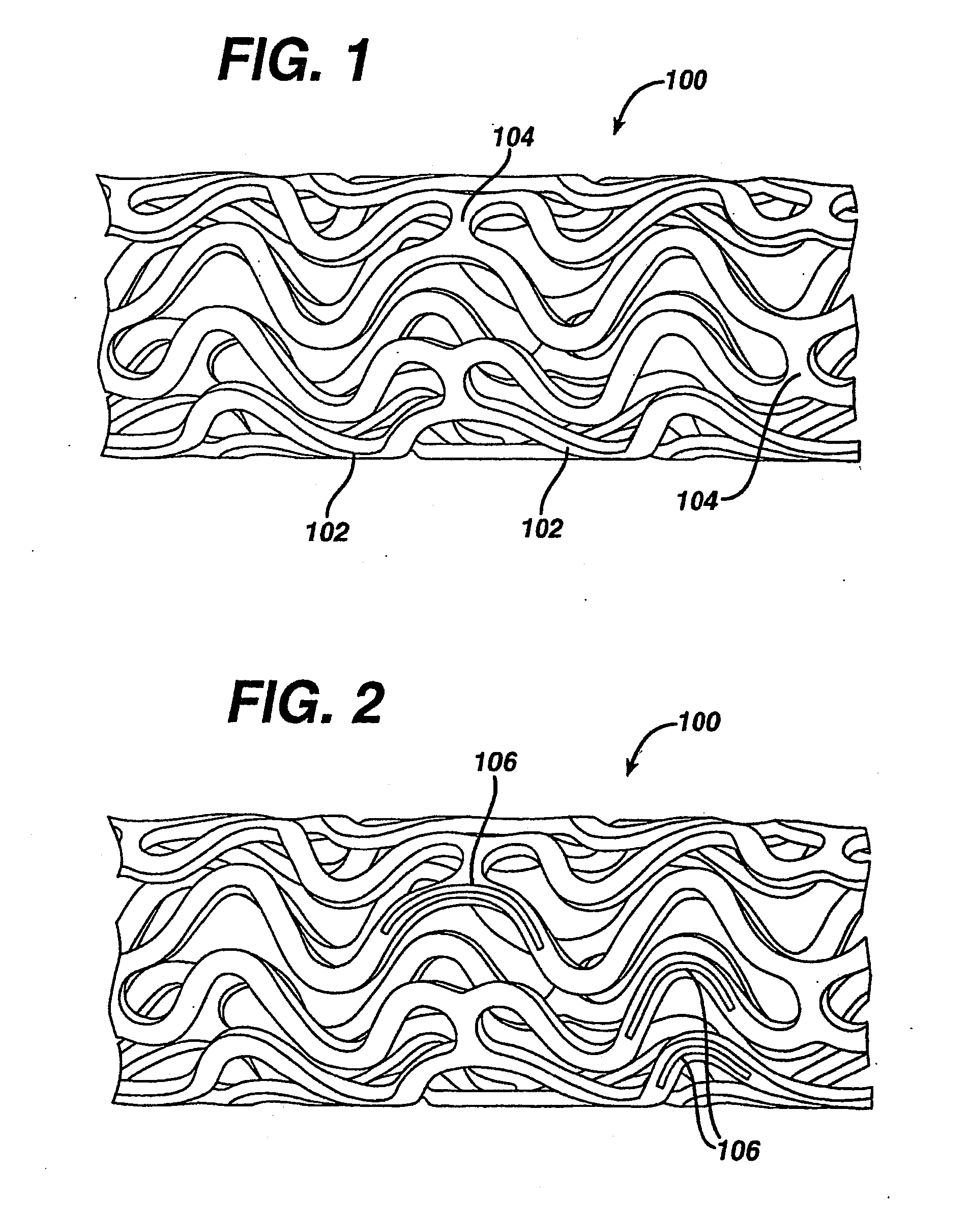
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Endovascular graft with differentiable porosity along its length
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device such as a stent-graft. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. A stent-graft fabricated from a thin-walled, high strength material provides for a more durable and lower profile endoprosthesis. The stent-graft comprises one or more stent segments covered with a fabric formed by the weaving, knitting or braiding of a biocompatible, high tensile strength, abrasion resistant, highly durable yarn such as ultra high molecular weight polyethylene. The one or more stent segments may be balloon expandable or self-expanding. The fabric may be attached to the stent segments utilizing any number of known materials and techniques. In addition, the pore size of the graft material may be varied.
Owner:CORDIS CORP
Intraluminal device and therapeutic agent combination for treating aneurysmal disease
InactiveUS20070083258A1Avoid dilationReduce drug toxicityStentsSurgeryCompound (substance)Biocompatible material
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Medical devices, drug coatings and methods for maintaining the drug coatings thereon
InactiveUS7056550B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyMedical device
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC +1
Coated aneurysmal repair device
InactiveUS20050249776A1Reduce drug toxicityGood curative effectBiocideStentsBlood vesselDisease cause
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Polymer conjugated protein micelles
ActiveUS8697098B2Promote absorptionConvenient treatmentMaterial nanotechnologyOrganic active ingredientsSolubilityConjugated protein
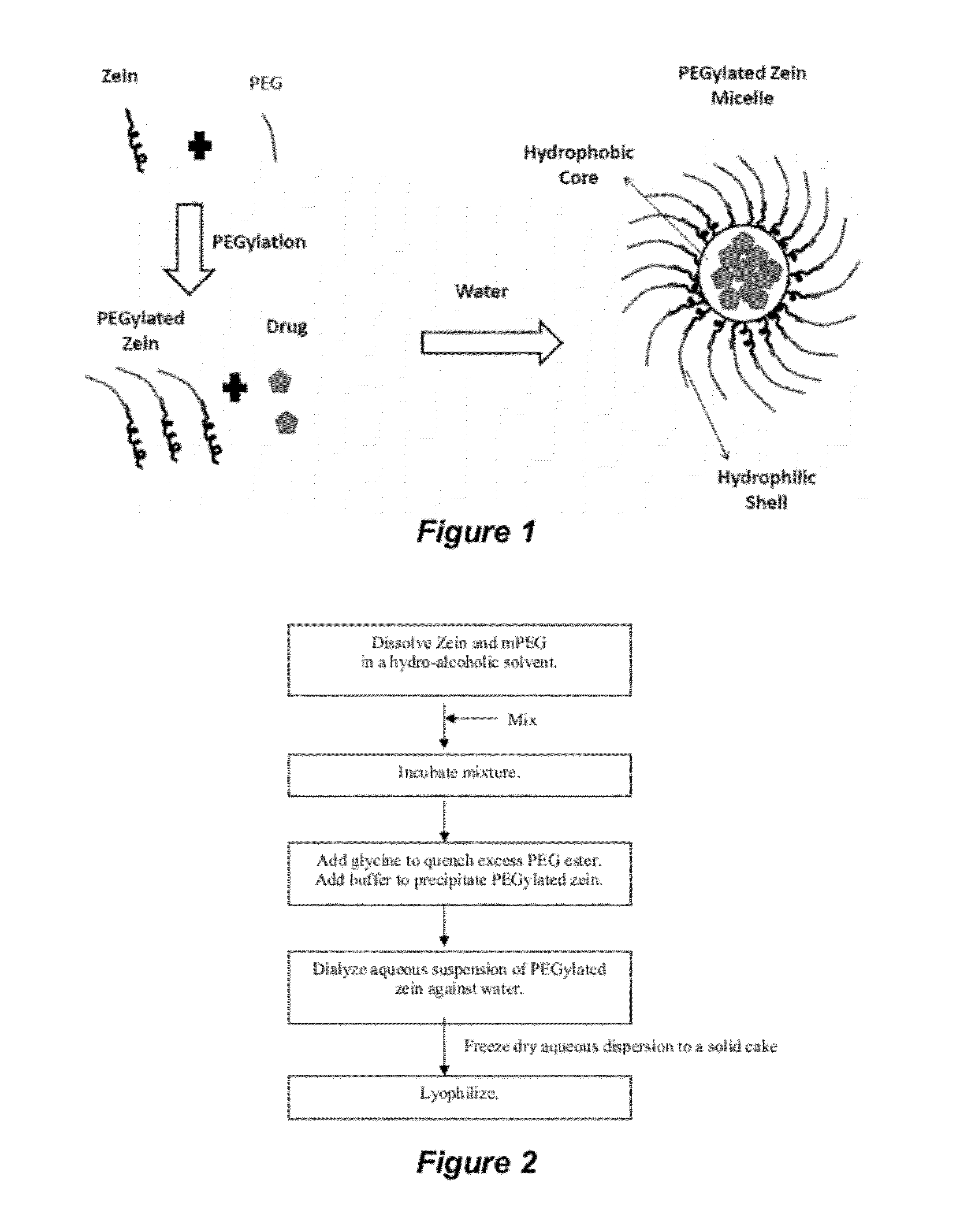
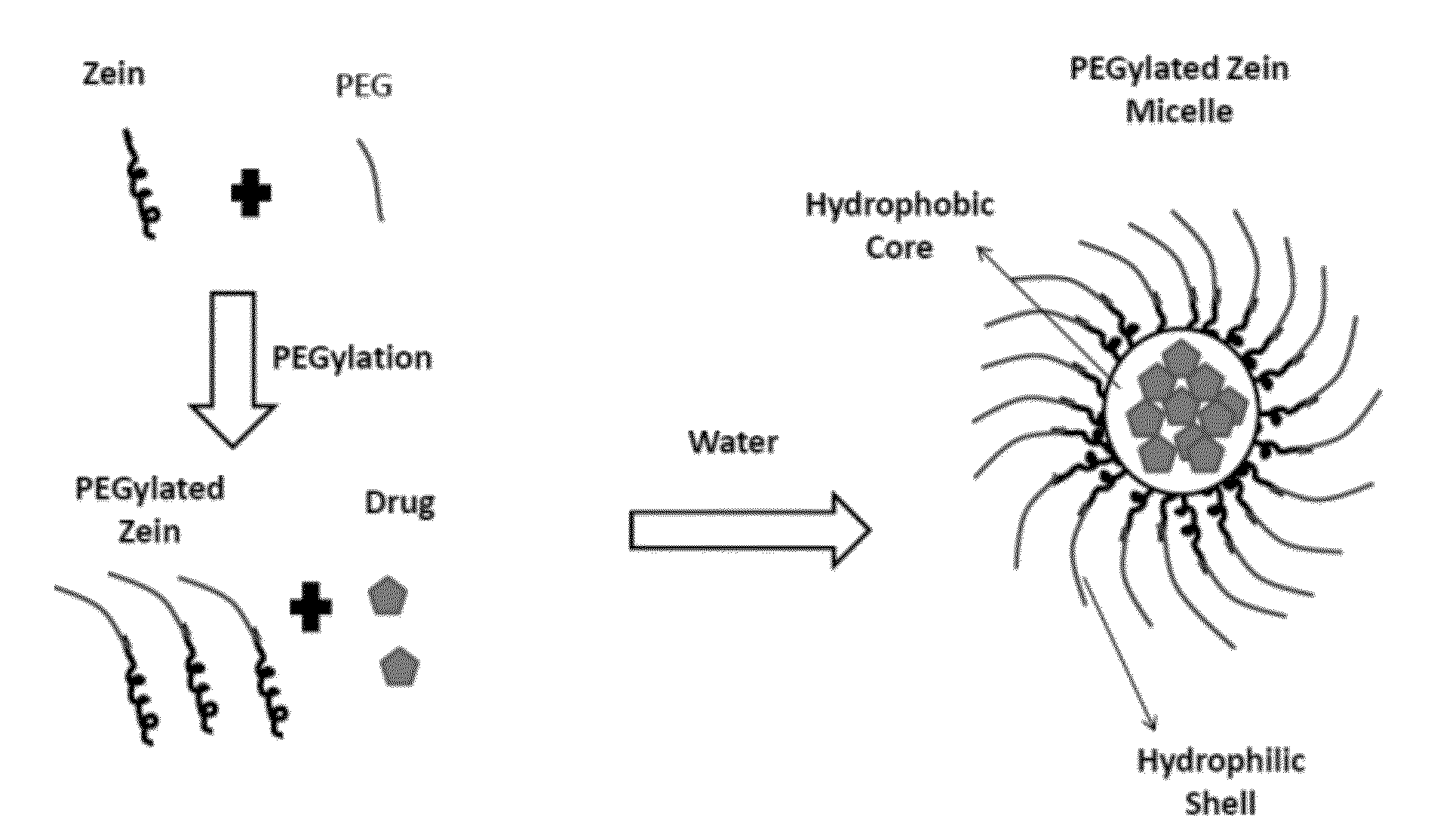
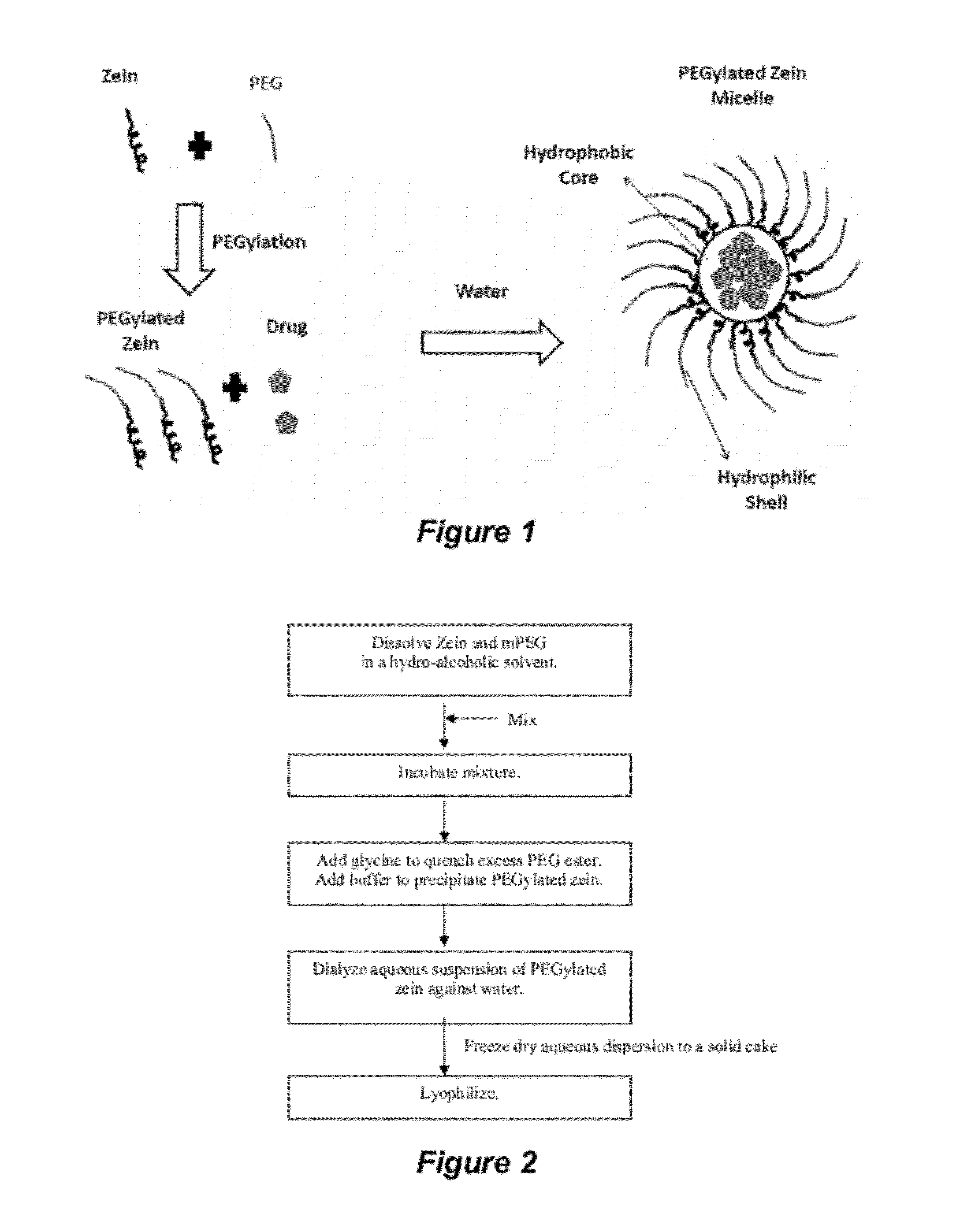
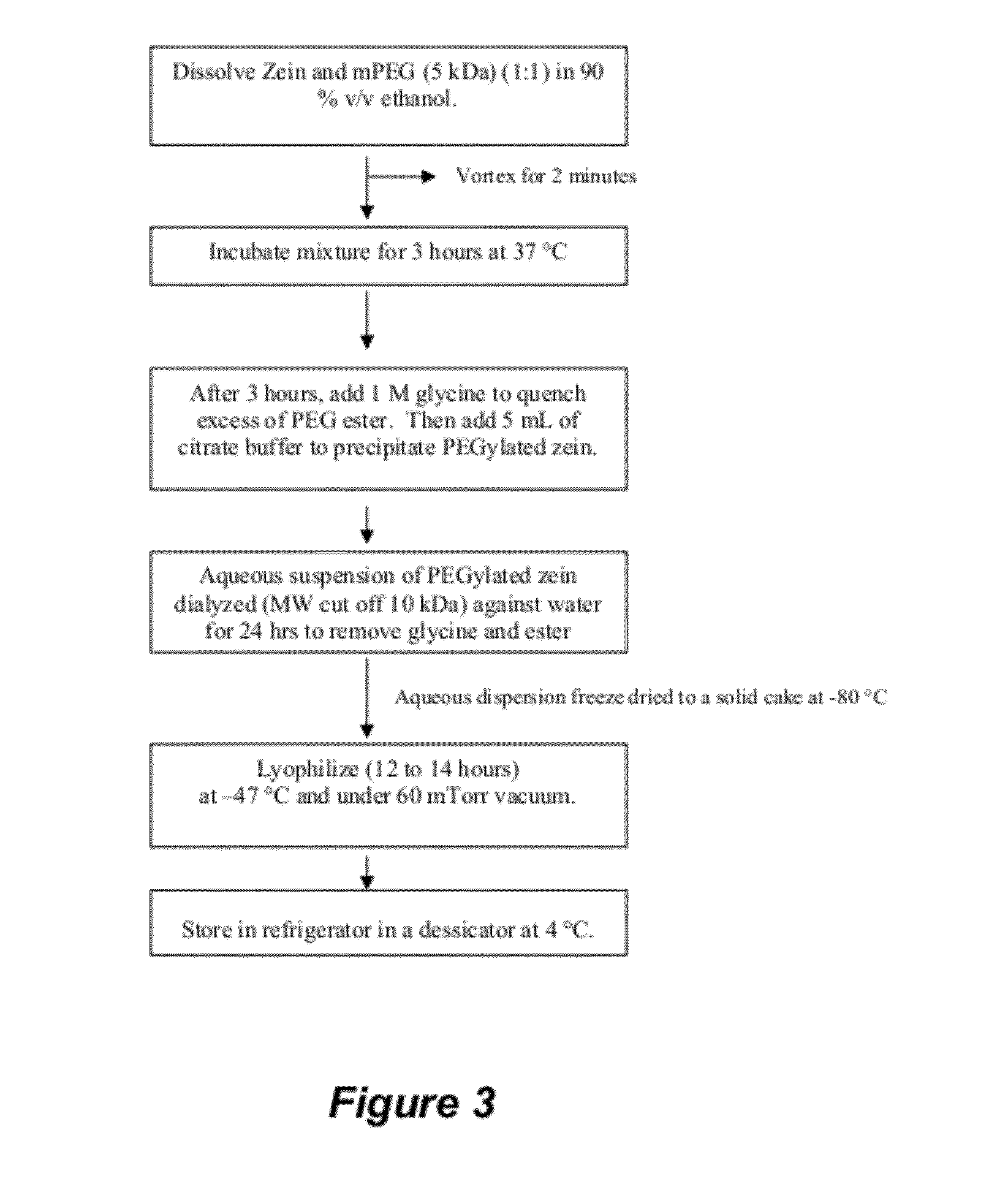
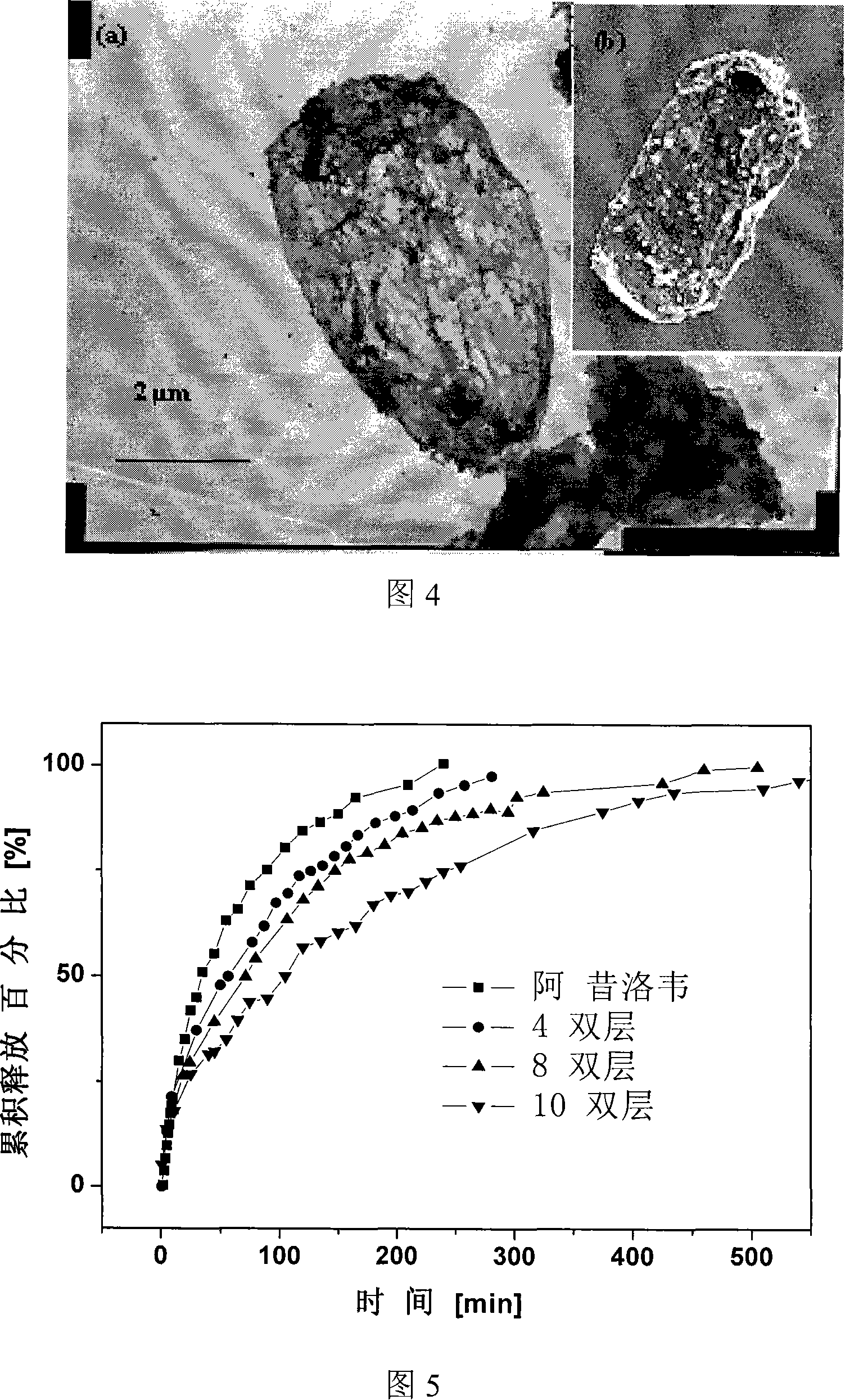
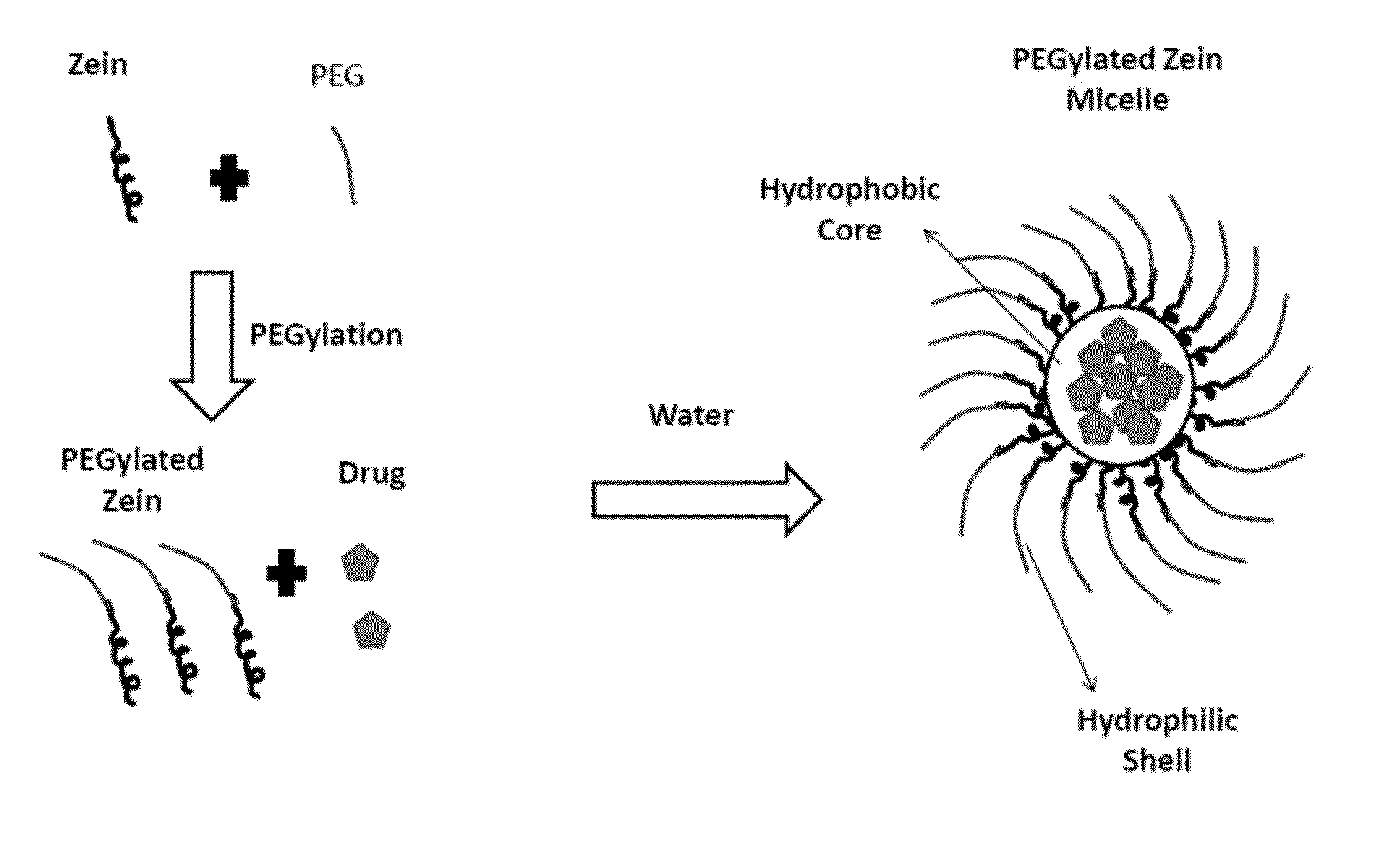
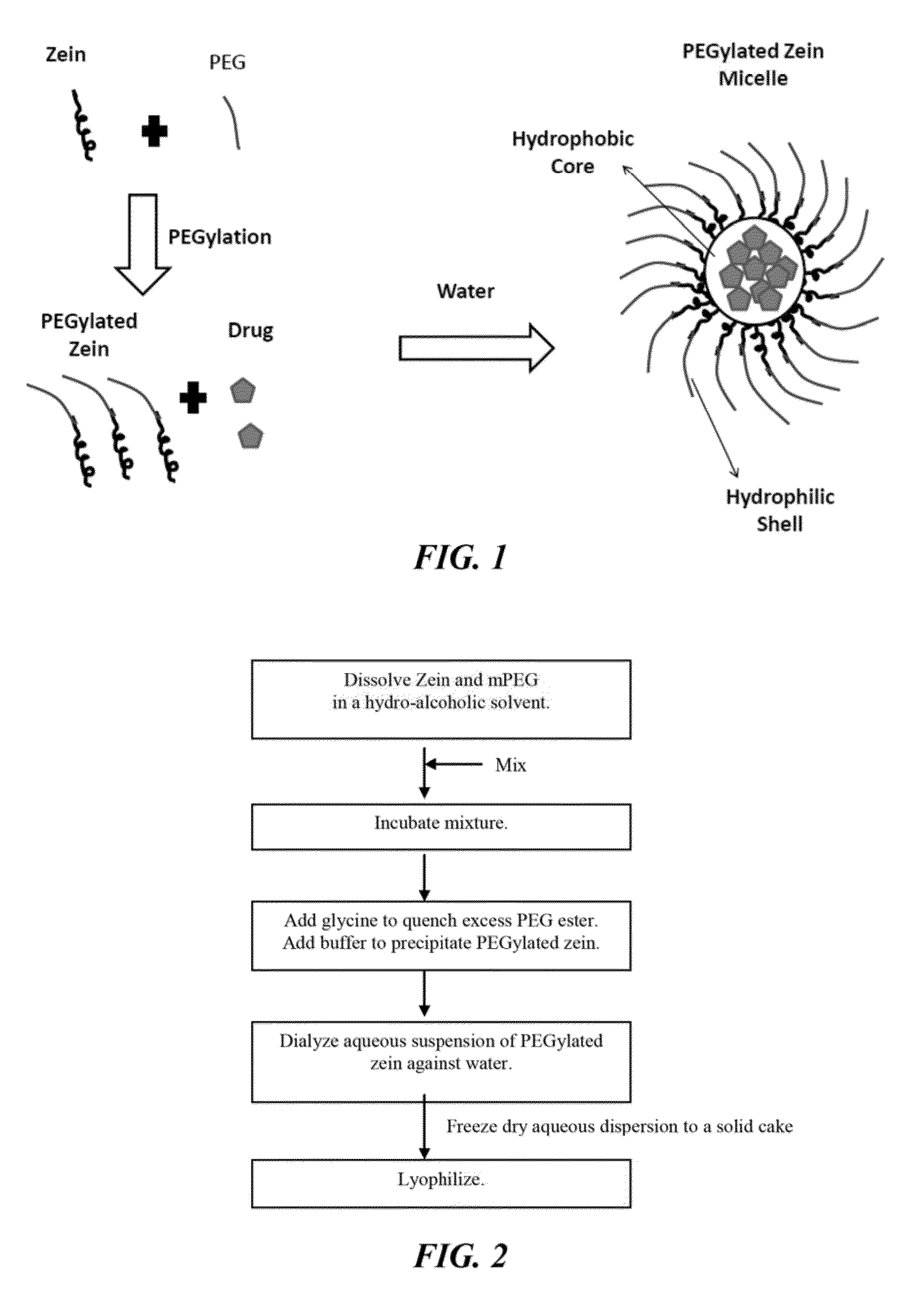
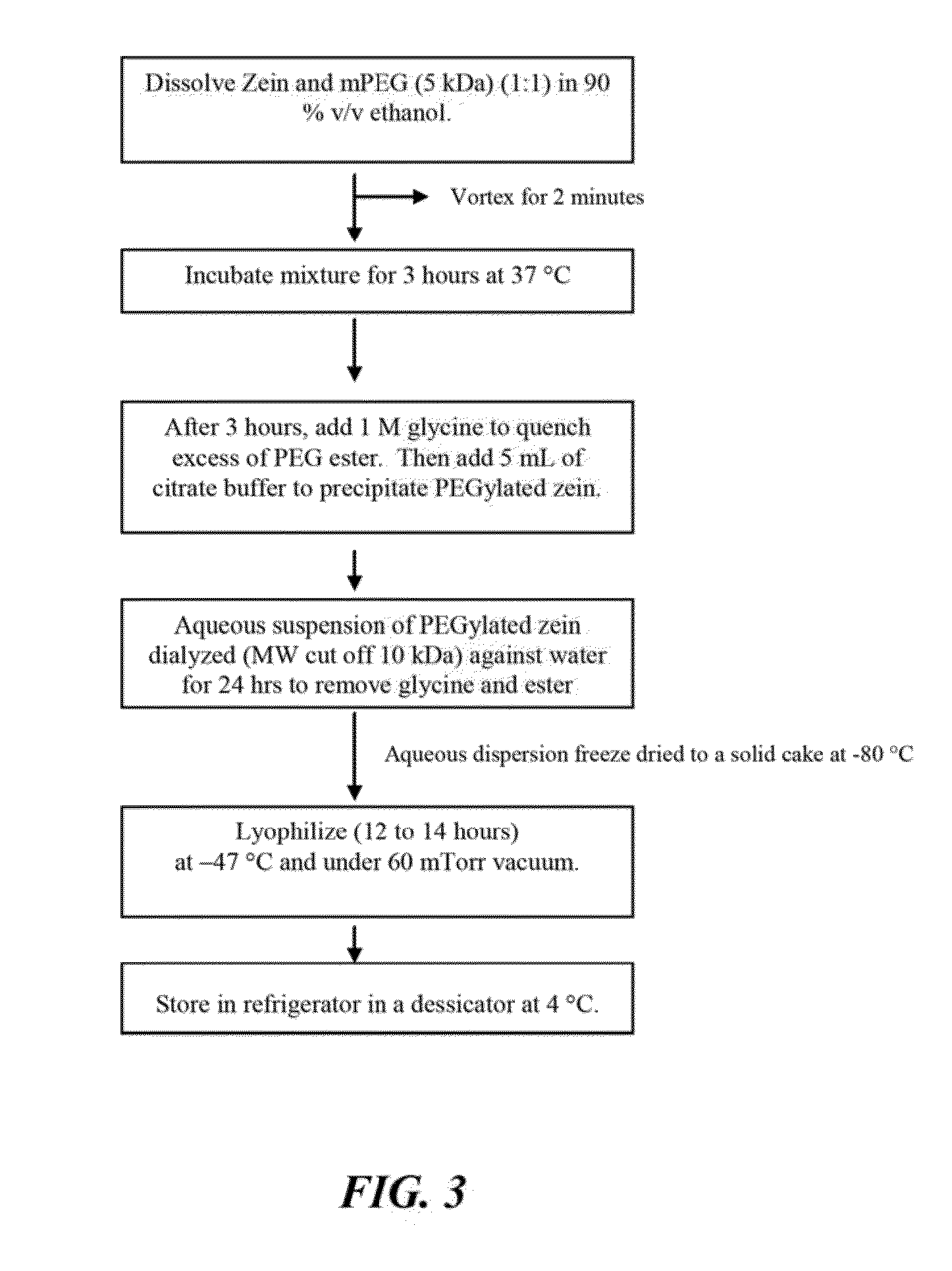
The invention encompasses micelle assemblies, compositions having micelle assemblies, and methods for preparing micelle assemblies and compositions thereof. The invention also encompasses a prolamine protein conjugated to a polymer, such as a polyethylene glycol (PEG) chain, which conjugates can be used to prepare micelle assemblies. The invention further encompasses methods of encapsulating molecules using the conjugates of the invention. The micelle assemblies can be used for a variety of applications, such as treating cancer, targeting tumors, reducing the toxicity of a drug in vivo, increasing the efficacy of an encapsulated agent in vivo, protecting an encapsulated agent against degradation, and enhancing the water solubility of a drug or other agent.
Owner:SOUTH DAKOTA STATE UNIVERSITY
Local drug delivery devices and methods for maintaining the drug coatings thereon
InactiveUS7261735B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsMedical deviceDrug coating
Local drug delivery medical devices are utilized to deliver therapeutic dosages of drugs, agents or compounds directly to the site where needed. The local drug delivery medical devices utilize various materials and coating methodologies to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
Medical devices, drug coatings and methods of maintaining the drug coatings thereon
InactiveUS20060222756A1Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
System for treating aneurysmal disease
InactiveUS20070026042A1Reduce drug toxicityGood curative effectSuture equipmentsBiocideCompound (substance)Biocompatible material
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051885A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Products and drug delivery vehicles
InactiveUS20040208844A1Ameliorate and prevent disease stateGood water solubilityAntipyreticAnalgesicsMedicineDrug delivery
Owner:SMITHKLINE BECKMAN CORP
Prodrugs activated by targeted catalytic proteins
InactiveUS6258360B1Minimal systemic exposureIncrease concentrationOrganic active ingredientsAntibody mimetics/scaffoldsCatalytic antibodyProtein C
Prodrugs that are activated by and conjugated to a catalytic antibody conjugated to a moiety that binds to a tumor cell population are provided.
Owner:WELLSTAT BIOCATALYSIS
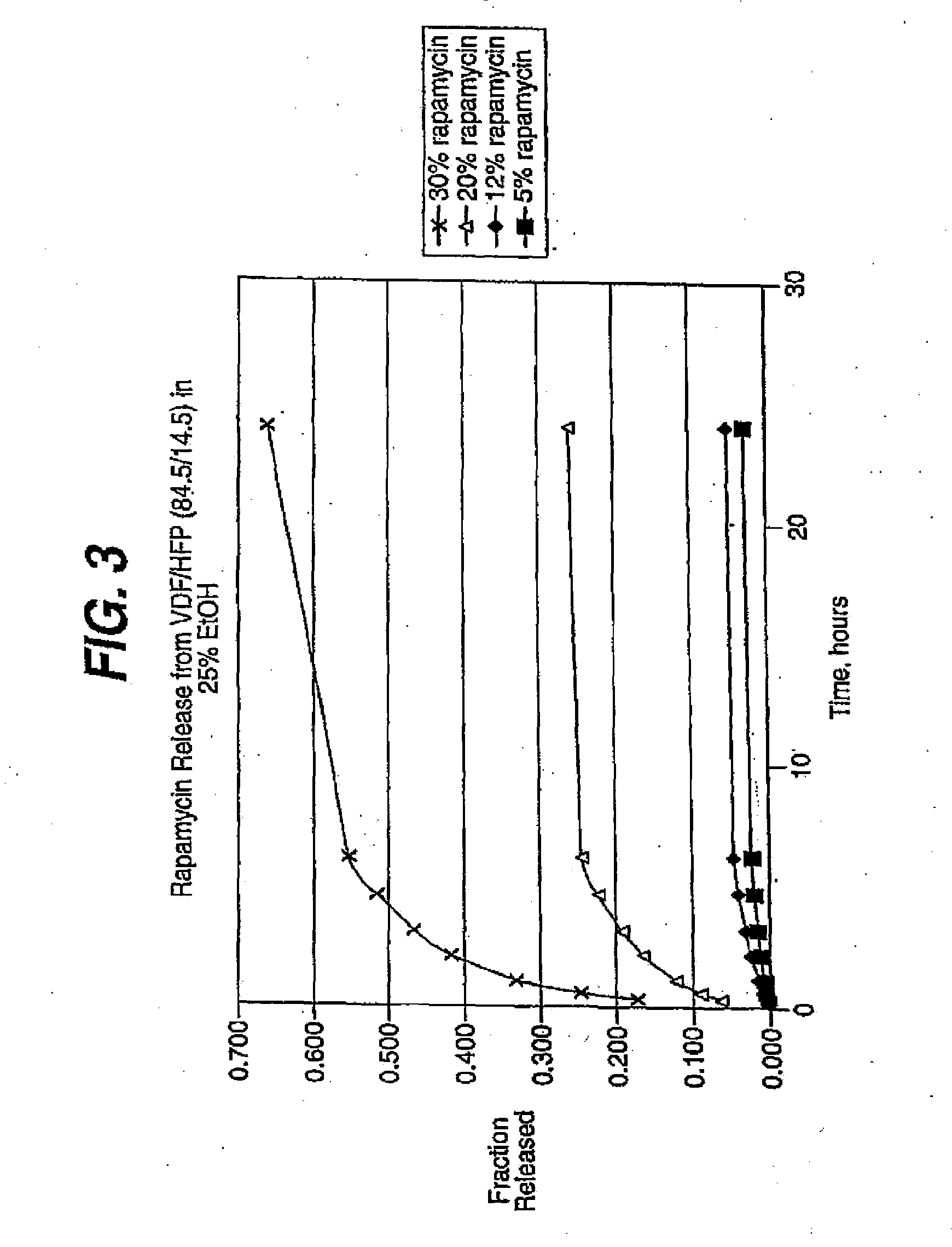
Deuterated rapamycin compounds, method and uses thereof
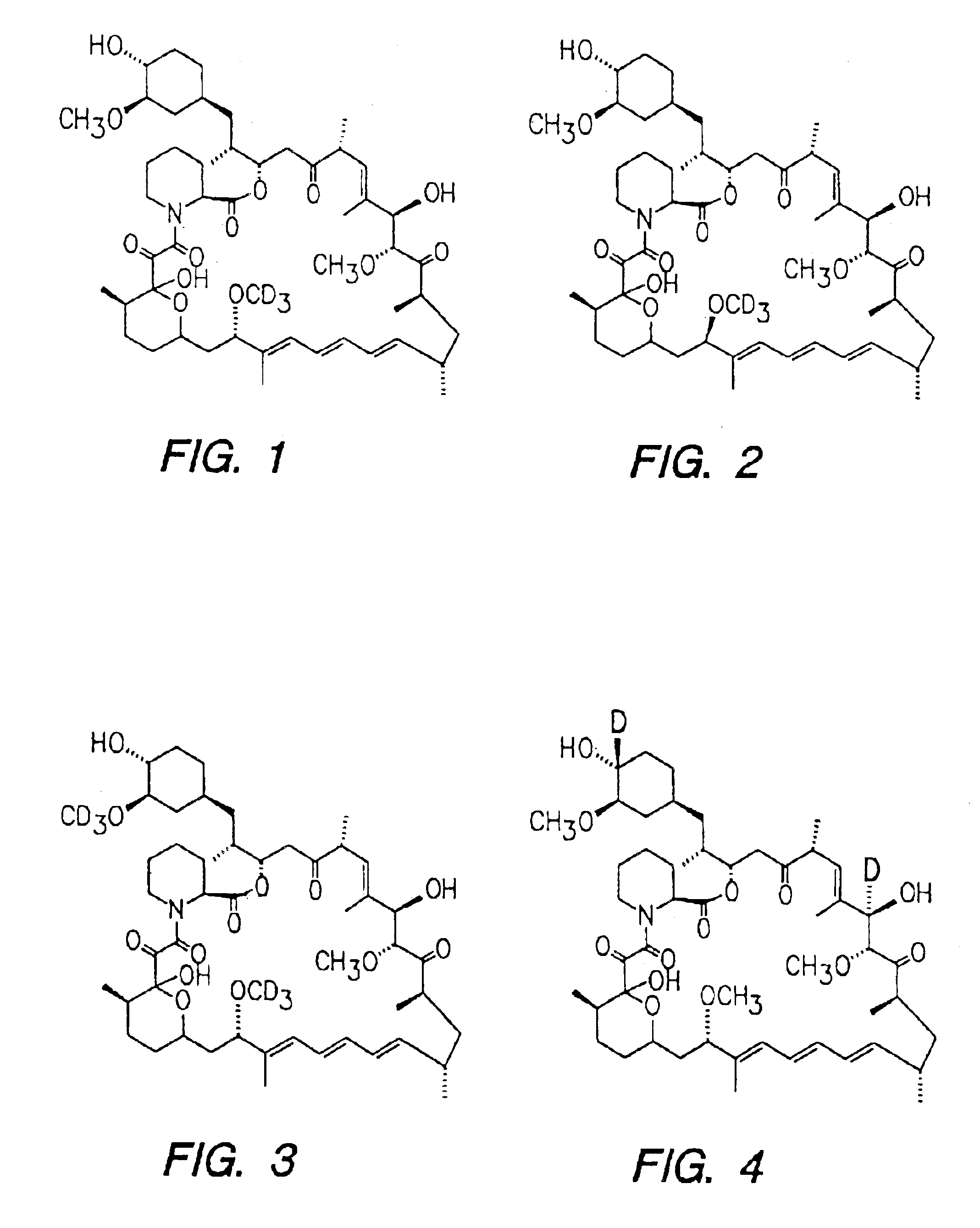
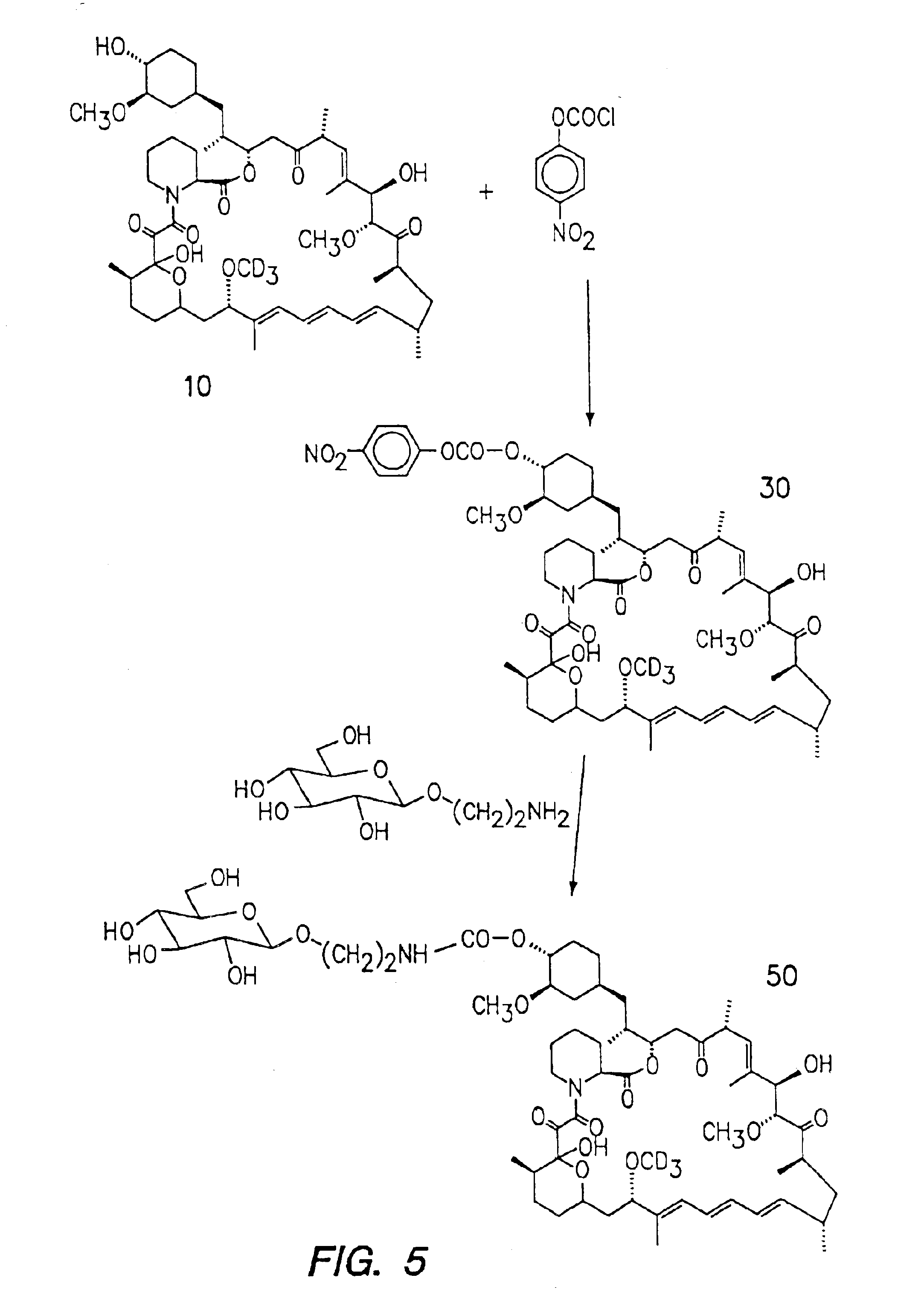
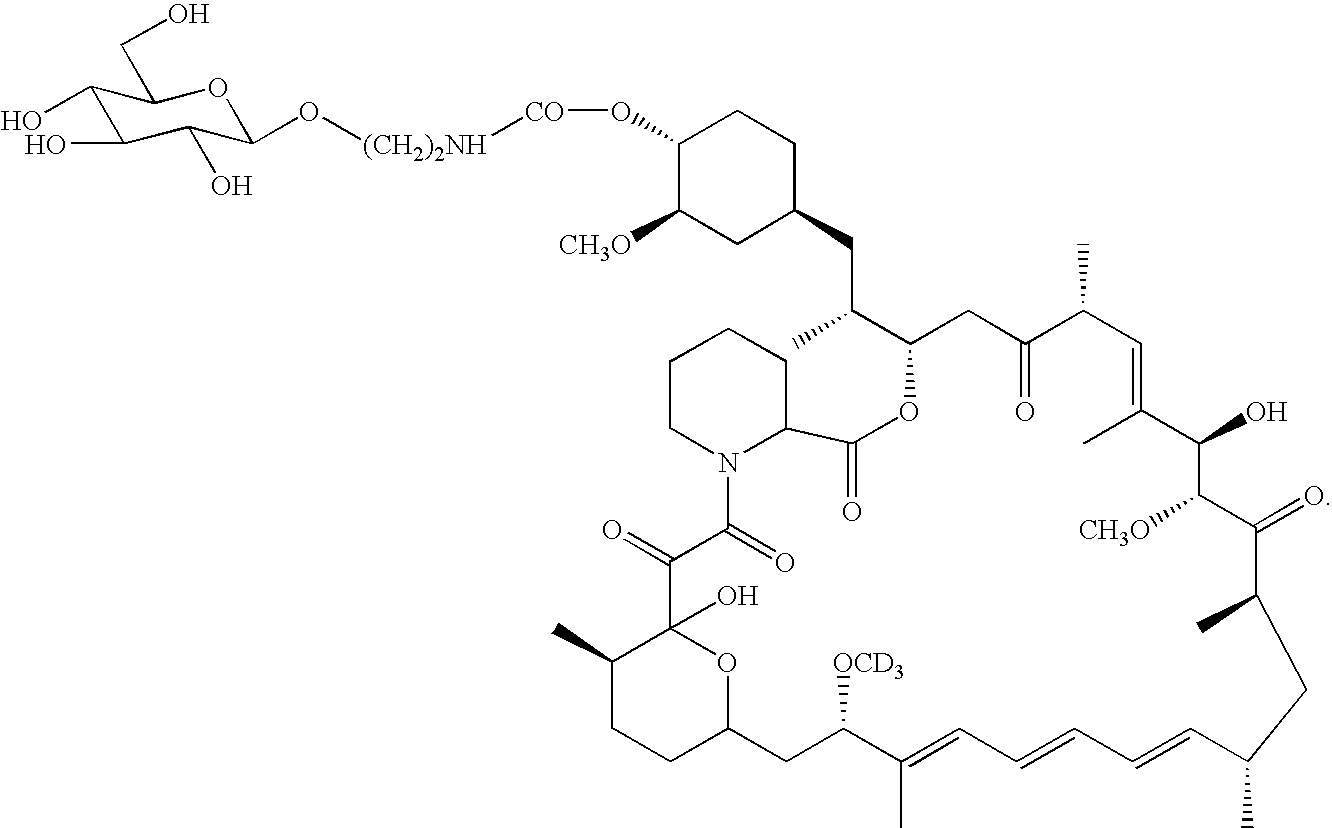
InactiveUS6939878B2Reduce oxidation rateReduce formationBiocideOrganic chemistryAutoimmune diseaseHost disease
The synthesis of deuterated analogues of rapamycin is disclosed together with a method for use for inducing immunosupression and in the treatment of transplantation rejection, graft vs host disease, autoimmune diseases, diseases of inflammation leukemia / lymphoma, solid tumors, fungal infections, hyperproliferative vascular disorders. Also described is a method for the synthesis of water soluble deuteratred rapamycin compounds and their use as described above.
Owner:ISOTECHNIKA INC
Medical gel and preparation method for utilizing medical gel to prepare gel wet-patch
InactiveCN105816907AIntrinsically antimicrobialImprove stabilityAbsorbent padsBandagesAlcoholGlycerol
The invention discloses a medical gel, which is composed of the following components and their weight ratio: 10%-60% magnesium sulfate, 0.01%-5% plant extract, 0.5%-15% glycerin, 0.1%-5% ethanol , 0.5%-20% hydrophilic polymer material, 0.5%-15% PEG40-hydrogenated castor oil, 0.001%-5% antibacterial agent, and the balance is purified water; Antibacterial, anti-inflammatory and analgesic plant extracts. The invention also discloses a gel wet compress prepared by using the medical gel and a preparation method thereof. The medical gel of the invention has the effects of removing edema, reducing inflammation, relieving pain, inhibiting bacteria, protecting skin, etc., is non-irritating to human body, is safe to use, has quick onset and good curative effect.
Owner:上海昌颌医药科技有限公司 +1
Fatty alcohol drug conjugates
InactiveUS7816398B2Reduce drug toxicityIncreasing amount of can tolerateBiocideNervous disorderFatty alcoholVirus
The invention provides conjugates of fatty alcohols and pharmaceutical agents useful in treating cancer, viruses, psychiatric disorders. Compositions, pharmaceutical preparations, and methods of preparation of the fatty alcohols-pharmaceutical agent conjugates are provided.
Owner:LUITPOLD PHARMA INC
Combined fruit and vegetable fresh-keeping pad and preparation method and application thereof
ActiveCN104985892AQuick releaseReduce releaseFruit and vegetables preservationSynthetic resin layered productsPhytotoxicitySulfite
The invention provides a combined fruit and vegetable fresh-keeping pad and a preparation method and application thereof. The combined fruit and vegetable fresh-keeping pad comprises a non-woven cloth layer, a 1-methylcyclopropene release layer, a first thin film layer, a bi-component gas release layer and a second thin film layer in sequence. The 1-methylcyclopropene release layer is formed by fixing 1-methylcyclopropene powder on the first thin film layer. The bi-component gas release layer is formed by fixing a sulfite, chlorate and slow-release agent mixture to the second thin film layer. The air permeation volume of the first thin film layer is 1500-2000 cm<3> / m<2>.d.pa, and the air permeation volume of the second thin film layer is 200-500 cm<3> / m<2>.d.pa. The combined fruit and vegetable fresh-keeping pad can prevent quality deterioration caused by senescence or rottenness of stems or fruits, prolong the shelf life of picked grapes and reduce the phytotoxicity of SO2 gas. The preparation method of the combined fruit and vegetable fresh-keeping pad is simple, easy to implement and low in cost.
Owner:乌鲁木齐市格瑞德保鲜科技有限公司 +1
Methods for reducing toxicity of a chemotherapeutic drug
ActiveUS20190099498A1Low toxicityImprove securityPowder deliveryOrganic active ingredientsChemotherapeutic drugsChemotherapy related toxicity
This disclosure relates to methods for improving the therapeutic index of a chemotherapeutic drug in the treatment of patients afflicted with cancer, by reducing chemotherapy-related toxicity to a level that allows the chemotherapeutic drug to be used in humans.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20070179594A1Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:ETHICON INC
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20070276473A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
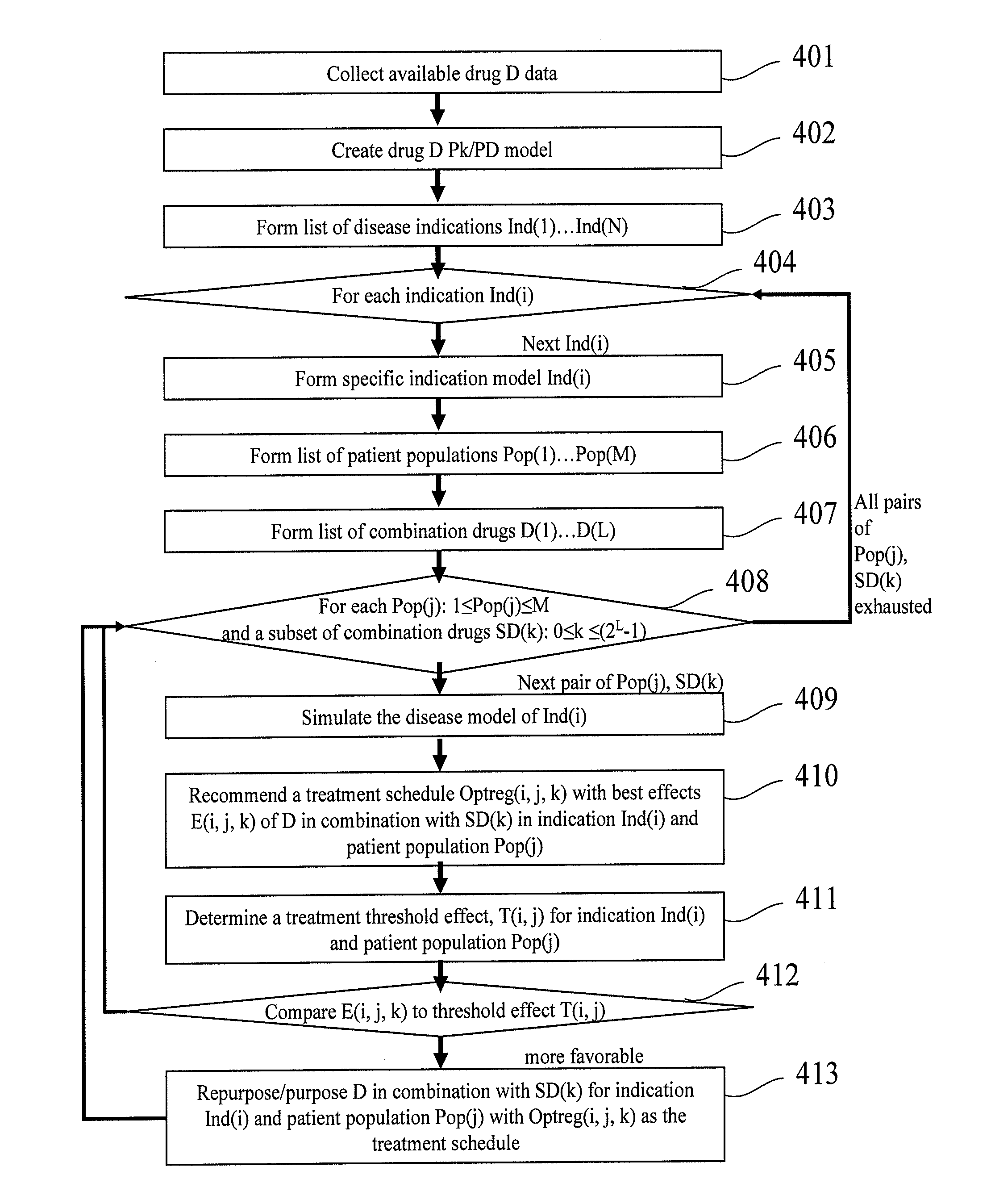
Techniques for Purposing a New Compound and for Re-Purposing a Drug
InactiveUS20100161301A1Reduce drug toxicityImprove efficacyChemical property predictionAnalogue computers for chemical processesTrial drugMathematical model
A method for repurposing a pharmaceutical compound. The method includes identifying a pharmaceutical compound, the pharmaceutical compound corresponding to a drug that has failed in clinical development or an approved drug. A mathematical model describing the physiological processes related to at least one disease and the effects of the pharmaceutical compound on the disease is created. The model is adjusted based upon information from preclinical or clinical trials. A new treatment protocol is suggested to salvage the failed drug or a new way to use an approved drug. The suggested treatment protocol is displayed. Systems and computer program products encompassing the above techniques are also disclosed.
Owner:OPTIMATA
Polymer conjugated protein micelles
ActiveUS20120219600A1Enhanced chemical stabilityImprove systemic circulationMaterial nanotechnologyOrganic active ingredientsProtein proteinDrug
The invention encompasses micelle assemblies, compositions having micelle assemblies, and methods for preparing micelle assemblies and compositions thereof. The invention also encompasses a prolamine protein conjugated to a polymer, such as a polyethylene glycol (PEG) chain, which conjugates can be used to prepare micelle assemblies. The invention further encompasses methods of encapsulating molecules using the conjugates of the invention. The micelle assemblies can be used for a variety of applications, such as treating cancer, targeting tumors, reducing the toxicity of a drug in vivo, increasing the efficacy of an encapsulated agent in vivo, protecting an encapsulated agent against degradation, and enhancing the water solubility of a drug or other agent.
Owner:SOUTH DAKOTA STATE UNIVERSITY
Notiginseng total saponin liposome and its preparation
InactiveCN1491658ALow toxicityNo immunosuppressive effectOrganic active ingredientsSenses disorderMedicineCerebral embolism
The present invention discloses total notoginseng saponin liposome and its preparation. The total notoginseng saponin liposome contains total notoginseng saponin 5-30 wt%, filming material 50-80 wt%, additive 1-10 wt% and some other supplementary material. It may be prepared into transdermal preparation, aerosol, oral liquid, eye preparation, tablet or capsule; is used in treating apoplexy, hemiplegia, atherosclerotic thrombotic cerebral infarction, cerebral embolism, etc.; and has excellent medicinal application foreground.
Owner:YUNNAN PHYTOPHARML
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051884A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Fatty amine drug conjugates
The invention provides conjugates of fatty amines and pharmaceutical agents useful in treating cancer, viruses, psychiatric disorders. Compositions, pharmaceutical preparations, and methods of preparations of the fatty amine-pharmaceutical agent conjugates are provided.
Owner:LUITPOLD PHARMA INC
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051883A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Method of producing hepatic targeting drug microcapsule
InactiveCN101129342AHigh selectivityLess by-productsPharmaceutical non-active ingredientsMicroballoon preparationDrug capsuleVinyl ester
The invention discloses a method for preparing a medicine with liver target taxis for slow-releasing the nanometer microcapsule. The method comprises the following steps: proceeding with enzymatic reaction of liver target taxis gene with sugar monomer of cerebrose residue and ethylated carboxylate; copolymerizing vinyl ester with glycosyl and unsaturated cationic or anion; getting liver target taxis polyelectrolyte; getting the medicinal microcapsule with liver target taxis by multilayer packaging polyelectrolyte on the surface of medicine by electrostatic action, wherein the deactivation speed of microcapsule medicine can be controlled. The polymerization method is simple, high effective and non-toxicity. The coating method has the high efficient, the simple operation and the mild technology, which can cycle several times. The preparing medicine can save for a long time, which can release step by step, can accumulate in the liver, improves the medicinal effect of disease portion, reduces the toxic effect of the other healthy organ, and has the good application prospect.
Owner:ZHEJIANG UNIV
Polymer conjugated protein micelles
ActiveUS20140370110A1Good chemical stabilitySustained releaseCosmetic preparationsBiocideDrugToxicity
The invention encompasses micelle assemblies, compositions having micelle assemblies, and methods for preparing micelle assemblies and compositions thereof. The invention also encompasses a prolamine protein conjugated to a polymer, such as a polyethylene glycol (PEG) chain, which conjugates can be used to prepare micelle assemblies. The invention further encompasses methods of encapsulating molecules using the conjugates of the invention. The micelle assemblies can be used for a variety of applications, such as treating cancer, targeting tumors, reducing the toxicity of a drug in vivo, increasing the efficacy of an encapsulated agent in vivo, protecting an encapsulated agent against degradation, and enhancing the water solubility of a drug or other agent.
Owner:SOUTH DAKOTA STATE UNIVERSITY
Medical devices, drug coatings and methods for maintaining the drug coatings thereon
InactiveUS7591844B2Reduce drug toxicityGood curative effectStentsSynthetic resin layered productsBiological bodyMedical device
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
Medical Devices, Drug Coatings And Methods for Maintaining the Drug Coatings Thereon
InactiveUS20070179596A1Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com