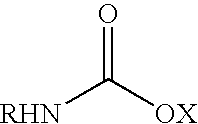
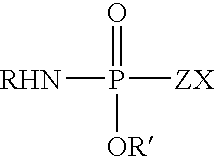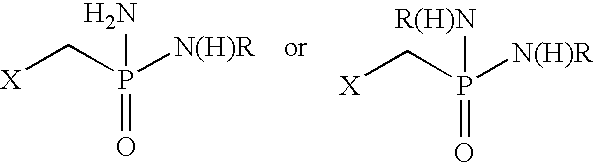Fatty amine drug conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Examples of Paclitaxel Conjugates
[0173]The length of the fatty moiety chain is governed by the synthetic procedure. For example, preparation of the fatty amine from the corresponding fatty acid (with an even number of carbons) generally results in a carbon chain with an odd number of carbons. Alternatively, preparation of the fatty amine from the corresponding fatty alcohol (with an even number of carbons) generally results in a carbon chain with an even number of carbons. The following paclitaxel-fatty amine conjugates with an even number and odd number of carbons in the fatty moiety, respectively, are prepared in accordance with the methods of the invention:
example 2
Preparation of N-Methyl Fatty Amine Conjugates
[0174]The conjugates may be prepared with N-methyl group at the amino moiety of the fatty amine as shown below:
to prevent internal self-immolative destruction. N-methylated fatty amines may be prepared from fatty acids using the procedures described in the Examples and described generally (in Yamada, F., et al. Heterocycles 1986, 24, 1223 and Somei, M., et al. Heterocycles 1987, 26, 895, both hereby incorporated by reference).
example 3
Synthesis of a Fatty Amine-Adefovir Conjugate Via a Urea Linkage
[0175]Adefovir (PMEA) was conjugated to a fatty amine using the following procedures:
wherein Y is methyl or ethyl, (PhO)2P(O)N3 is diphenylphosphoryl azide, and TMSBr is bromotrimethylsilane. One skilled in the art will appreciate that the number of carbons in the fatty amine moiety of the conjugate is governed by both the starting material and synthetic method chosen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell proliferation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com