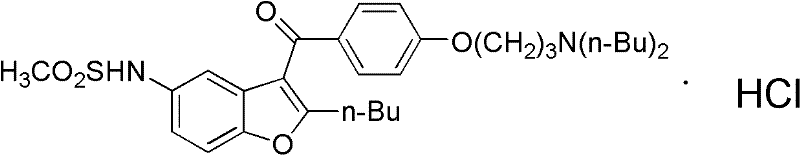
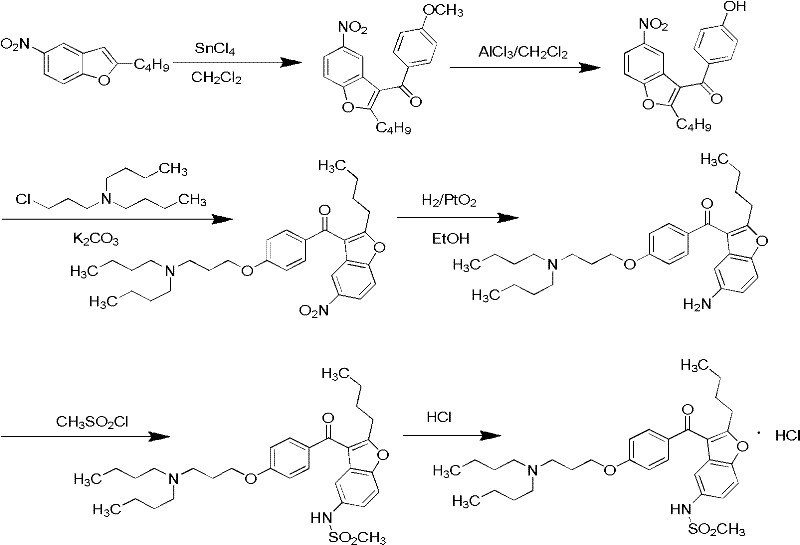
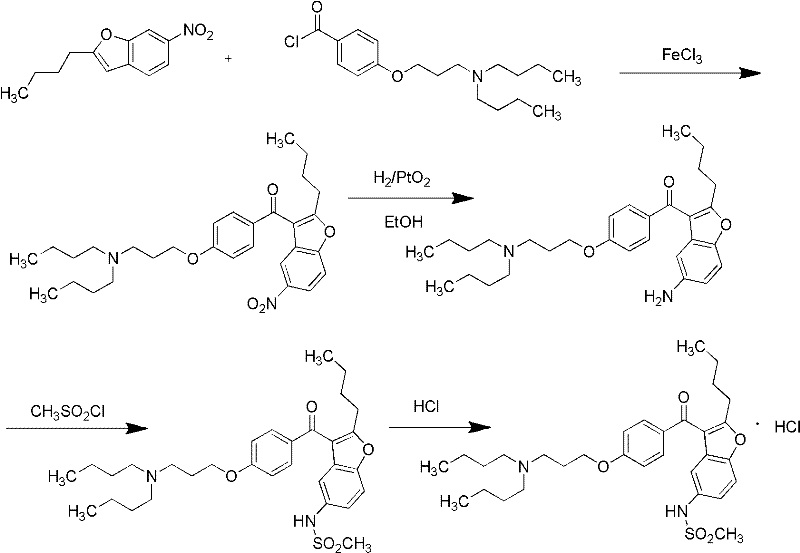
Method for preparing dronedarone hydrochloride
A technology for dronedarone hydrochloride and hydrochloric acid solution, which is applied in the field of preparation of dronedarone hydrochloride, can solve the problems of being unsuitable for industrial production, high preparation cost, and high price, and achieves the elimination of high-pressure hydrogenation reaction, simple operation, Less toxic and polluting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1). Preparation of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran
[0034] 72g of 1-bromo-3-chloropropane was added to 300ml of acetone, and 21g of anhydrous potassium carbonate was added. Heating to reflux at 56°C, while slowly adding a mixed solution of 51g of 2-butyl-3-(4-hydroxybenzoyl)-5-nitrobenzofuran and 200ml of acetone dropwise. After the dropwise addition, the reaction was refluxed for 4h. After the reaction is complete, cool to 25°C, filter with suction, wash the filter cake with acetone, combine the filtrates, evaporate the solvent acetone and excess 1-bromo-3-chloropropane to obtain 2-n-butyl-3-[4-(3 -Chloropropoxy)benzoyl]-5-nitrobenzofuran 59g, yield 94.4%. HPLC purity 98.1%.
[0035] (2). Preparation of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-aminobenzofuran
[0036] Get 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran 65g obtained in (1), ammonium chloride 17g, reduced iron powder 44g and 75% (volume fraction) ethanol was m...
Embodiment 2
[0044] (1). Preparation of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran
[0045] 236g of 1-bromo-3-chloropropane was added to 600ml of toluene, and 25.5g of sodium bicarbonate was added. Heat at 110°C to reflux, and slowly add dropwise a mixed solution of 51g of 2-butyl-3-(4-hydroxybenzoyl)-5-nitrobenzofuran and 300ml of toluene. After the dropwise addition was completed, the reaction was refluxed for 2.5 hours. After the reaction is complete, cool to 25°C, filter with suction, wash the filter cake with toluene, combine the filtrates, evaporate the solvent toluene and excess 1-bromo-3-chloropropane to obtain 2-n-butyl-3-[4-(3 -Chloropropoxy)benzoyl]-5-nitrobenzofuran 58g, yield 92.8%. HPLC purity 98.0%.
[0046] (2). Preparation of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-aminobenzofuran
[0047] Get 91g of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran obtained in (1), 150ml of 6mol / L hydrochloric acid solution, reduce zinc powder 43g and 1200...
Embodiment 3
[0055] (1). Preparation of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran
[0056] 29g of 1-bromo-3-chloropropane was added to 400ml of ethyl acetate, and 24g of anhydrous sodium carbonate was added. Heat at 77°C to reflux, and slowly add a mixed solution of 77g of 2-butyl-3-(4-hydroxybenzoyl)-5-nitrobenzofuran and 300ml of ethyl acetate dropwise. After the dropwise addition was completed, the reaction was refluxed for 6 hours. After the reaction is complete, cool to 25°C, filter with suction, wash the filter cake with ethyl acetate, combine the filtrates, evaporate the solvent ethyl acetate to obtain 2-n-butyl-3-[4-(3-chloropropoxy)benzene Formyl]-5-nitrobenzofuran 78g, yield 83.2%. HPLC purity 97.5%.
[0057] (2). Preparation of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-aminobenzofuran
[0058] Get 130g of 2-n-butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran obtained in (1), 90ml of glacial acetic acid, 175g of reduced iron powder and 50 % (volume fra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com