Patents
Literature
107 results about "Atrial flutter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An abnormal heart rhythm in the heart's upper chambers (atria).
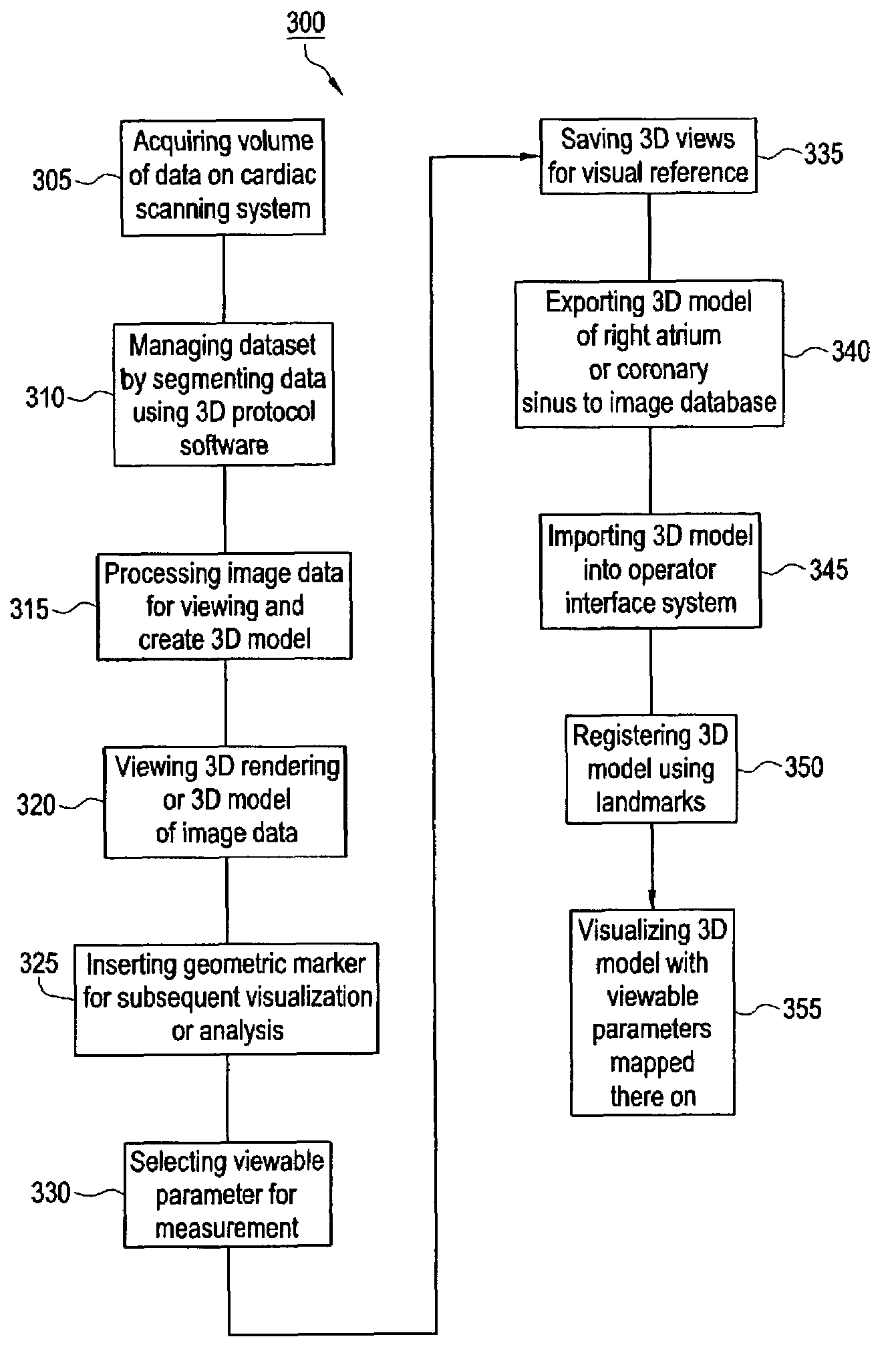
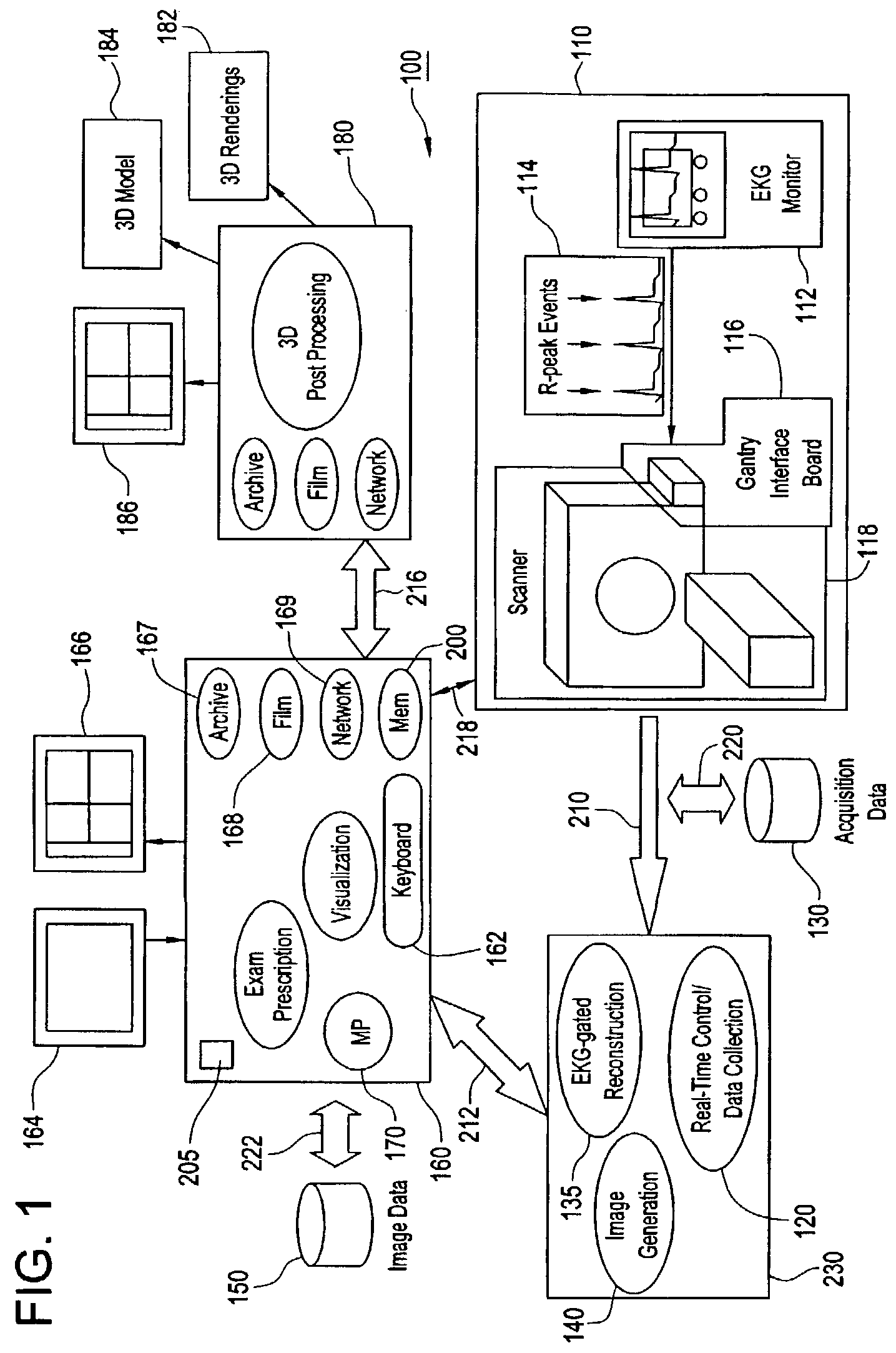
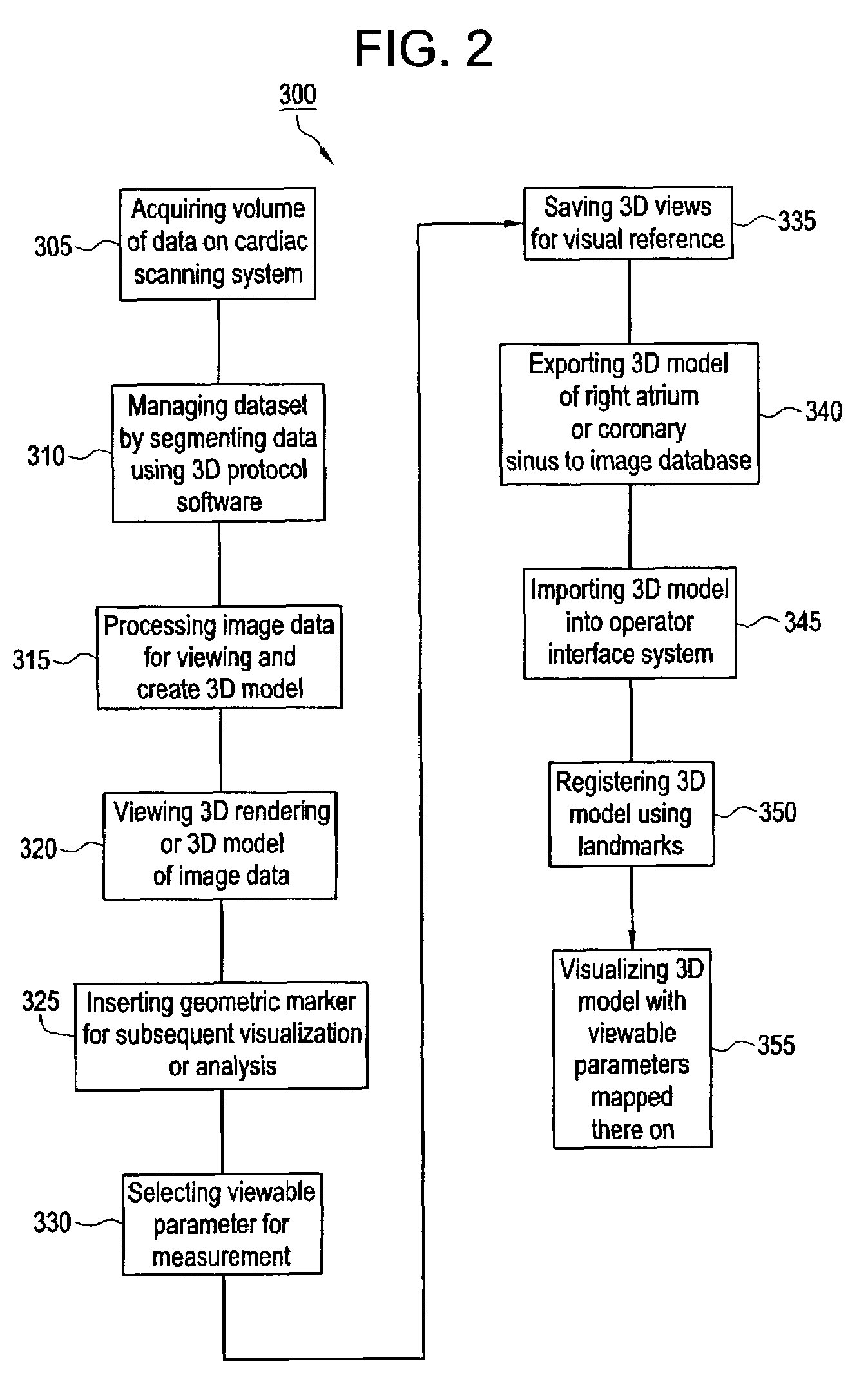
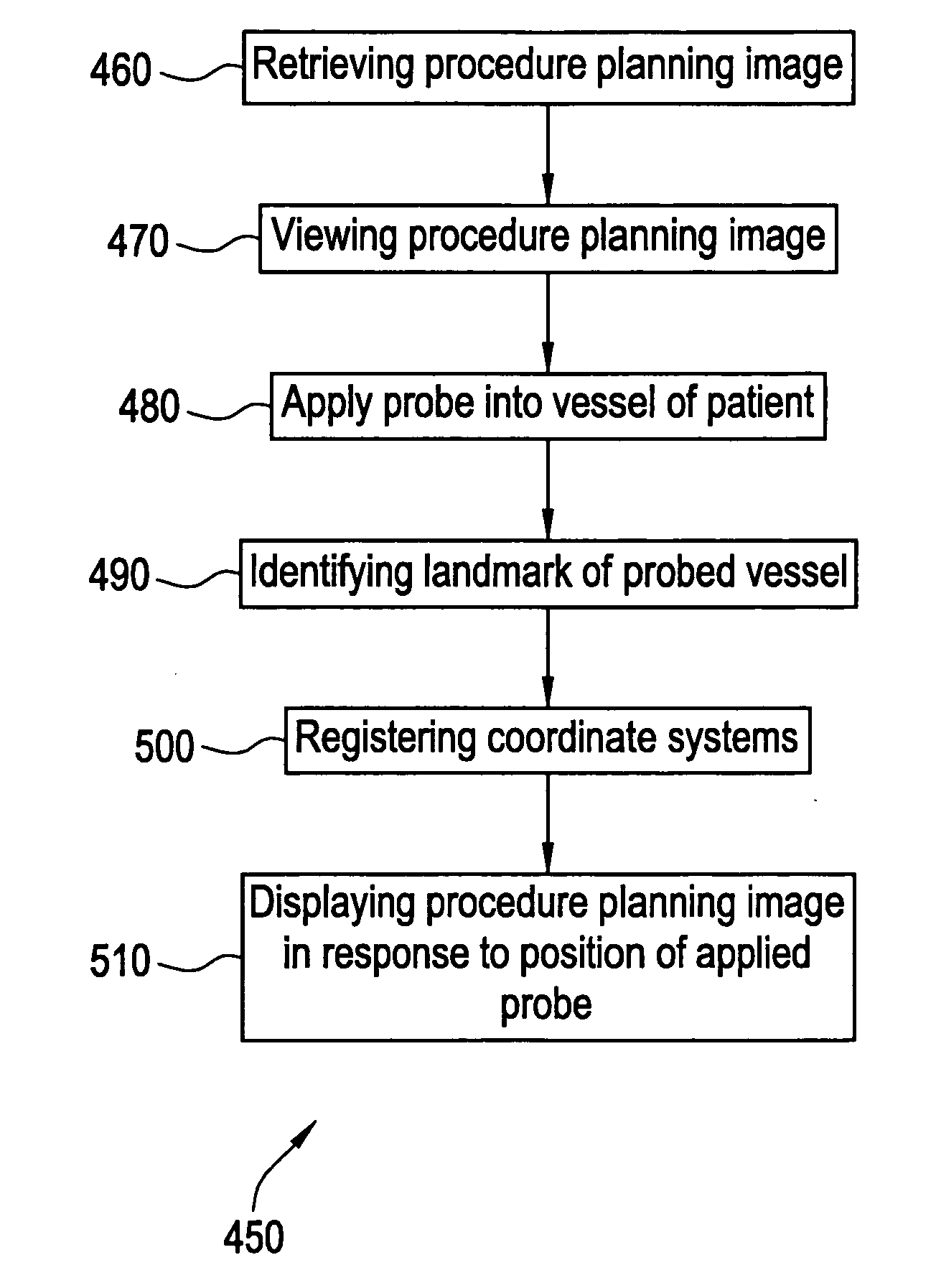
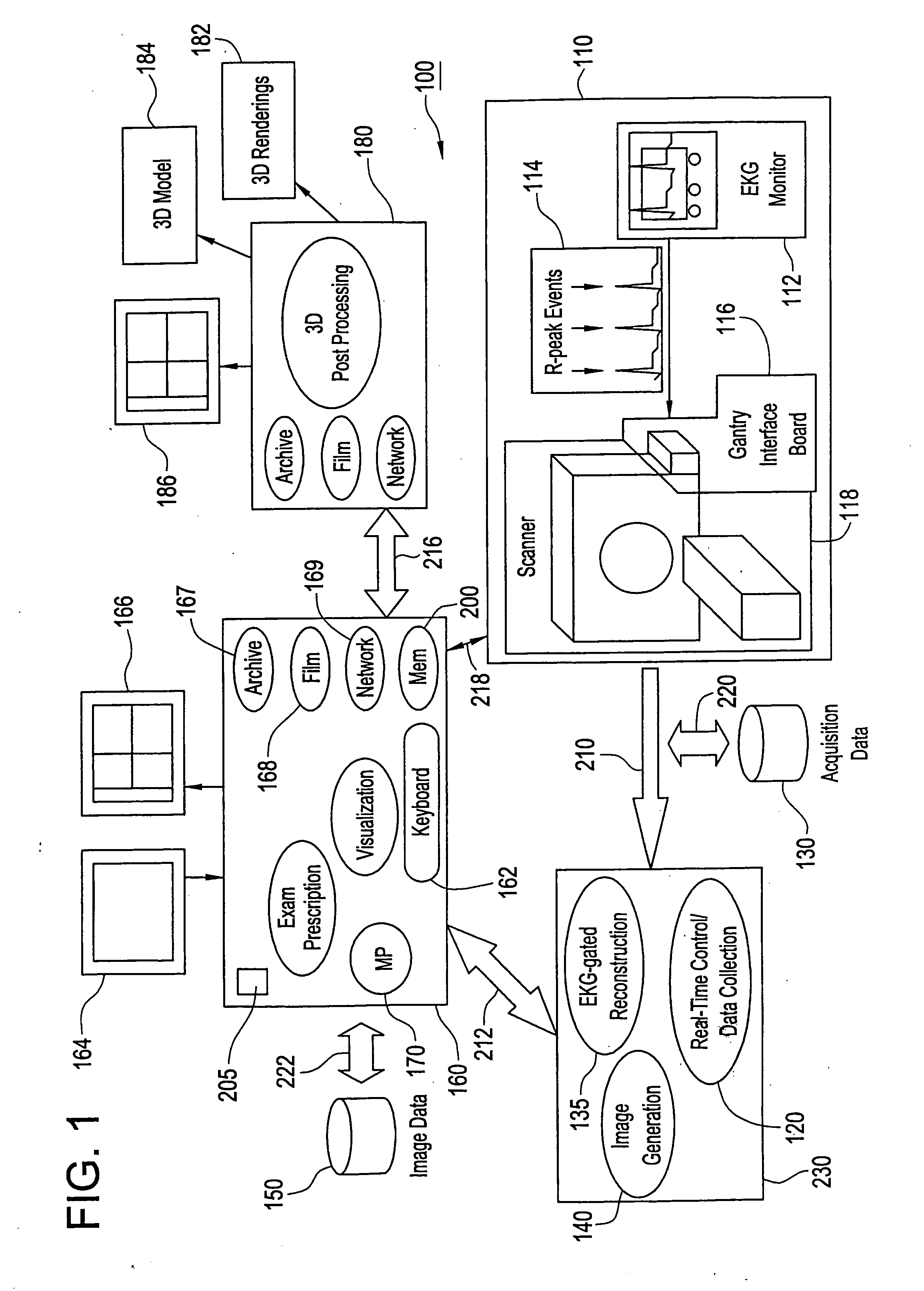
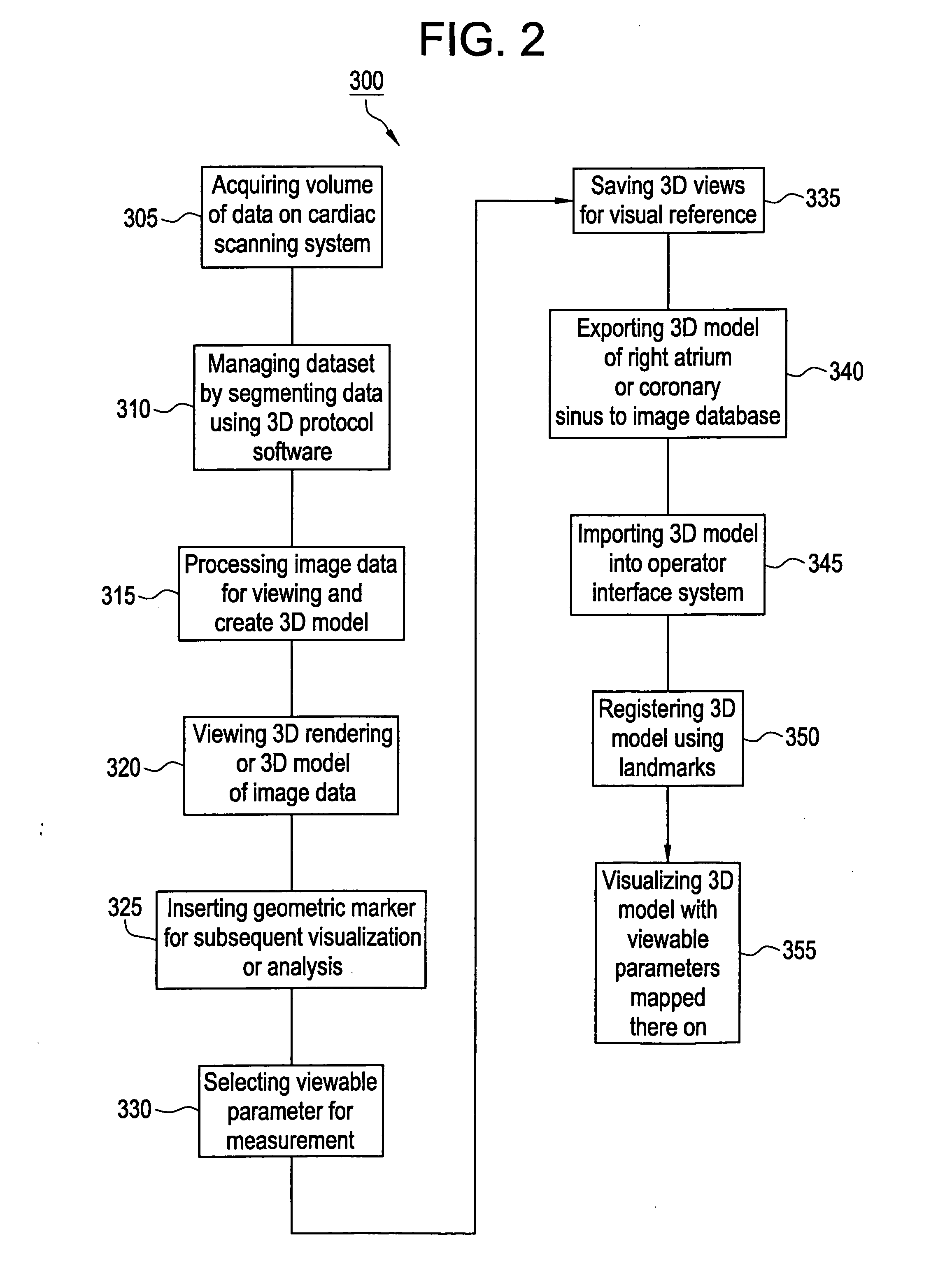
Method and apparatus for medical intervention procedure planning
InactiveUS7346381B2Ultrasonic/sonic/infrasonic diagnosticsMechanical/radiation/invasive therapiesOperator interfaceData acquisition
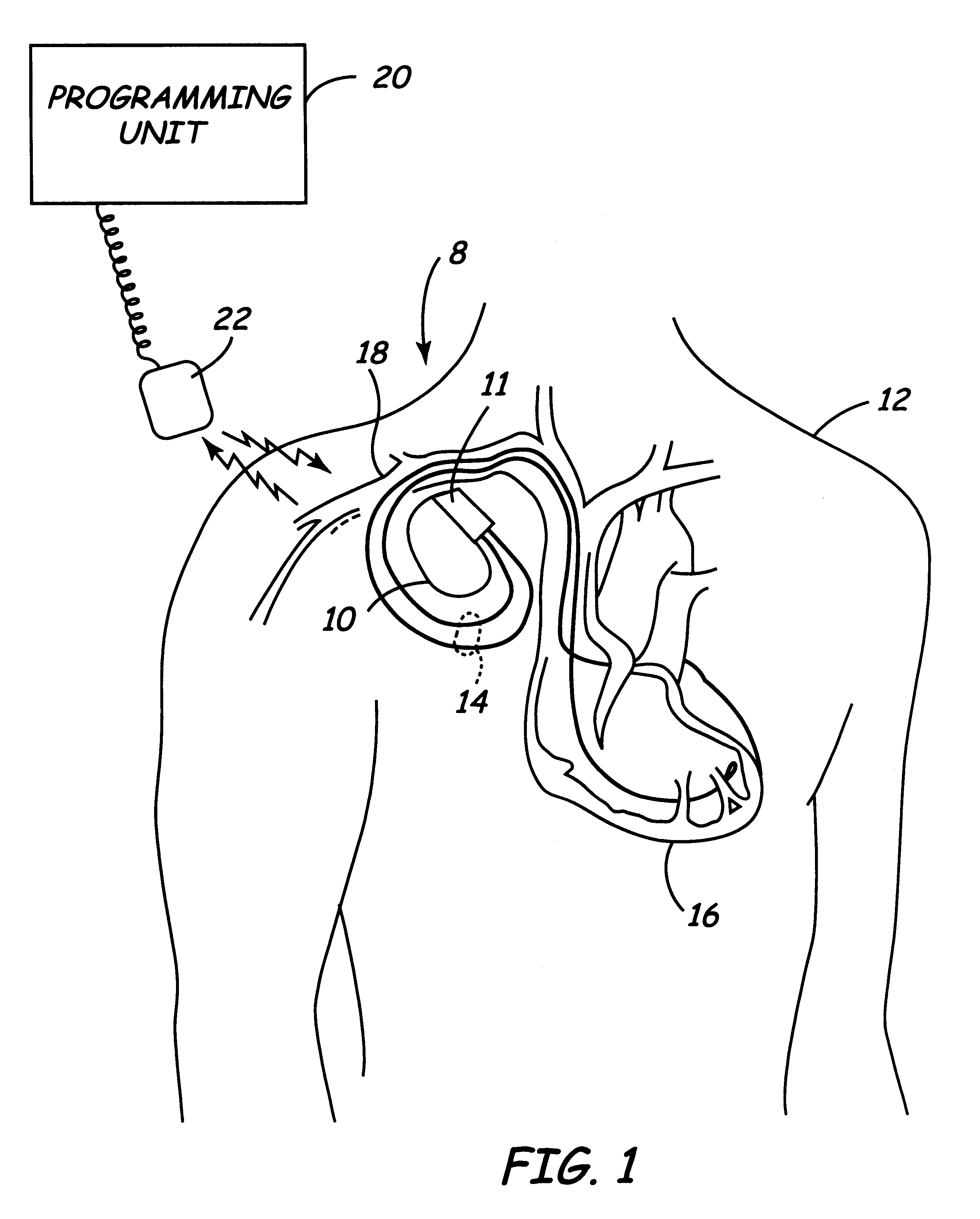
An imaging system for use in medical intervention procedure planning includes a medical scanner system for generating a volume of cardiac image data, a data acquisition system for acquiring the volume of cardiac image data, an image generation system for generating a viewable image from the volume of cardiac image data, a database for storing information from the data acquisition and image generation systems, an operator interface system for managing the medical scanner system, the data acquisition system, the image generation system, and the database, and a post-processing system for analyzing the volume of cardiac image data, displaying the viewable image and being responsive to the operator interface system. The operator interface system includes instructions for using the volume of cardiac image data and the viewable image for bi-ventricular pacing planning, atrial fibrillation procedure planning, or atrial flutter procedure planning.
Owner:GENERAL ELECTRIC CO +1
Vacuum coagulation probes
InactiveUS7063698B2Surgical instruments for heatingSurgical instruments for aspiration of substancesRadio frequencyArticular cartilage
An embodiment of the invention includes a surgical device for coagulating soft tissue such as atrial tissue in the treatment of atrial fibrillation, atrial flutter, and atrial tachycardia; tendon or ligament shrinkage; or articular cartilage removal. The surgical device integrates a suction mechanism with the coagulation mechanism improving the lesion creation capabilities of the device. The surgical device comprises an elongate member having an insulative covering attached about conductive elements capable of coagulating soft tissue when radiofrequency or direct current energy is transmitted to the conductive elements. Openings through the insulative covering expose regions of the conductive elements and are coupled to lumens in the elongate member which are routed to a vacuum source. Suction causes the soft tissue to actively engage the opening thus the integrated, exposed conductive elements to facilitate the coagulation process and ensure the lesions created are consistent, continuous, and transmural. The embodiments of the invention can also incorporate cooling mechanisms associated with the conductive elements and coupled to a fluid source to passively transport fluid along the contacted soft tissue surface to cool thus pushing the maximum temperature deeper into tissue.
Owner:ATRICURE
Vacuum coagulation probe for atrial fibrillation treatment
InactiveUS6893442B2Surgical instruments for heatingSurgical instruments for aspiration of substancesLess invasiveRadio frequency
An embodiment of the invention includes a surgical device for coagulating soft tissue such as atrial tissue in the treatment of atrial fibrillation, atrial flutter, and atrial tachycardia. The surgical device can include at least one elongate member comprising conductive elements adapted to coagulate soft tissue when radiofrequency or direct current energy is transmitted to the conductive elements. Openings through said conductive elements are routed through lumens in the elongate member to a vacuum source to actively engage the soft tissue surface intended to coagulate into intimate contact with the conductive elements to facilitate the coagulation process and ensure the lesions created are consistent, contiguous, and transmural. The embodiments of the invention can also incorporate cooling openings positioned near the conductive elements and coupled with a vacuum source or an injection source to transport fluid through the cooling openings causing the soft tissue surface to cool thus pushing the maximum temperature deeper into tissue. The embodiments of the invention can also incorporate features to tunnel between anatomic structures or dissect around the desired tissue surface to coagulate thereby enabling less invasive positioning of the soft tissue coagulating device and ensuring reliable and consistent heating of the soft tissue.
Owner:ATRICURE
Vacuum coagulation probe for atrial fibrillation treatment
InactiveUS20060009762A1Surgical instruments for heatingSurgical instruments for aspiration of substancesAnatomical structuresLess invasive
An embodiment of the invention includes a surgical device for coagulating soft tissue such as atrial tissue in the treatment of atrial fibrillation, atrial flutter, and atrial tachycardia. The surgical device can include at least one elongate member comprising conductive elements adapted to coagulate soft tissue when radiofrequency or direct current energy is transmitted to the conductive elements. Openings through said conductive elements are routed through lumens in the elongate member to a vacuum source to actively engage the soft tissue surface intended to coagulate into intimate contact with the conductive elements to facilitate the coagulation process and ensure the lesions created are consistent, contiguous, and transmural. The embodiments of the invention can also incorporate cooling openings positioned near the conductive elements and coupled with a vacuum source or an injection source to transport fluid through the cooling openings causing the soft tissue surface to cool thus pushing the maximum temperature deeper into tissue. The embodiments of the invention can also incorporate features to tunnel between anatomic structures or dissect around the desired tissue surface to coagulate thereby enabling less invasive positioning of the soft tissue coagulating device and ensuring reliable and consistent heating of the soft tissue.
Owner:ATRICURE
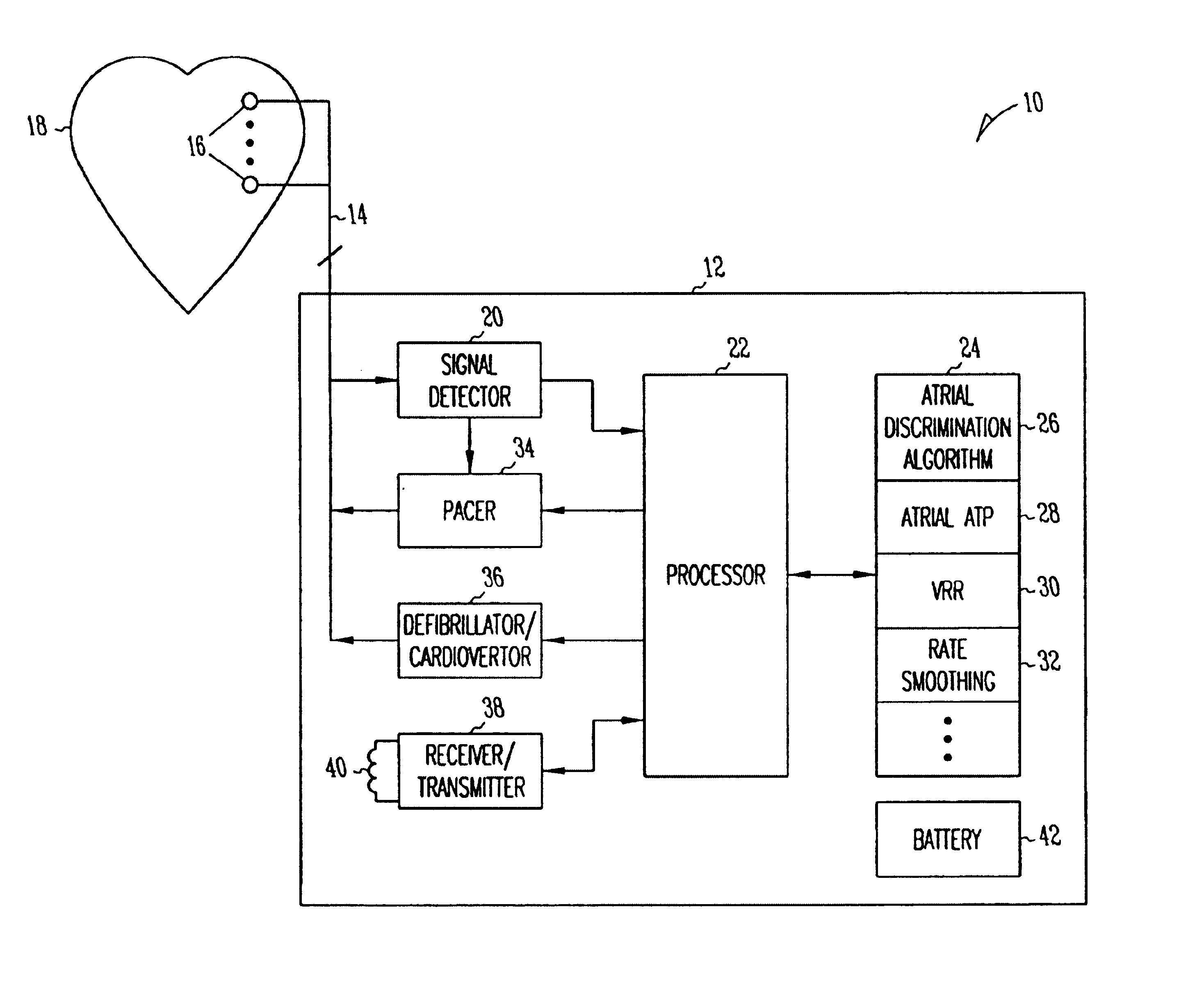
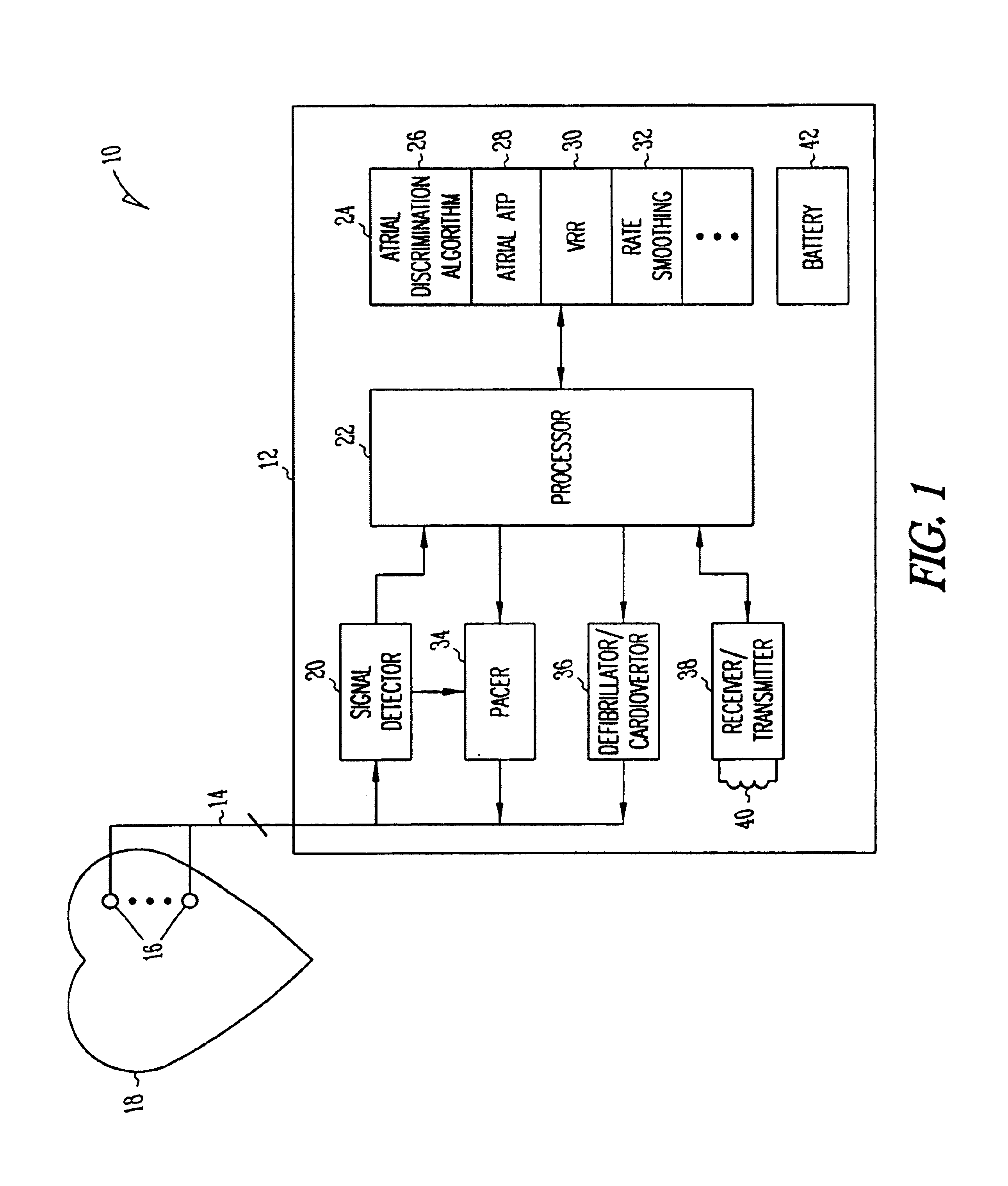
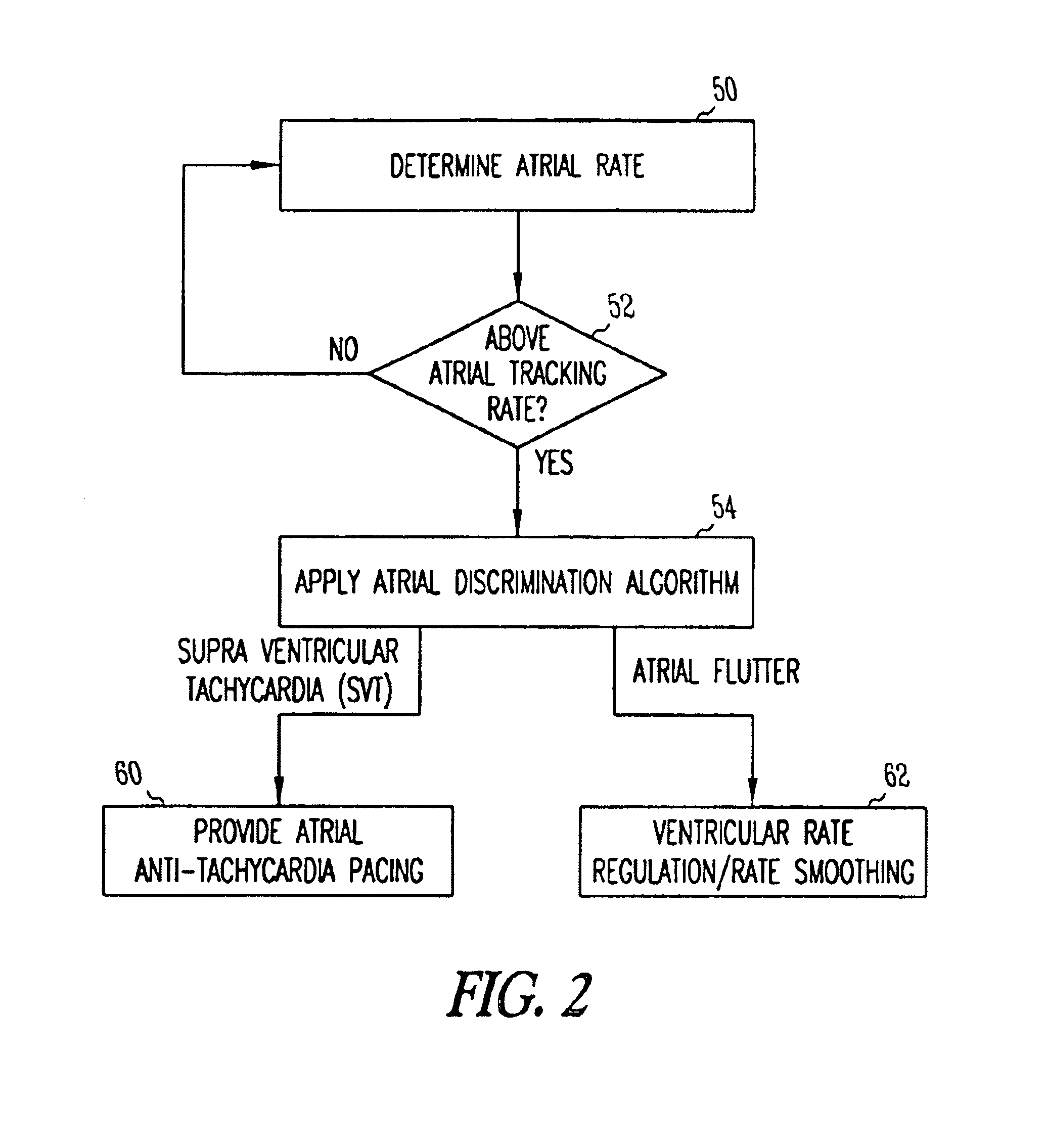
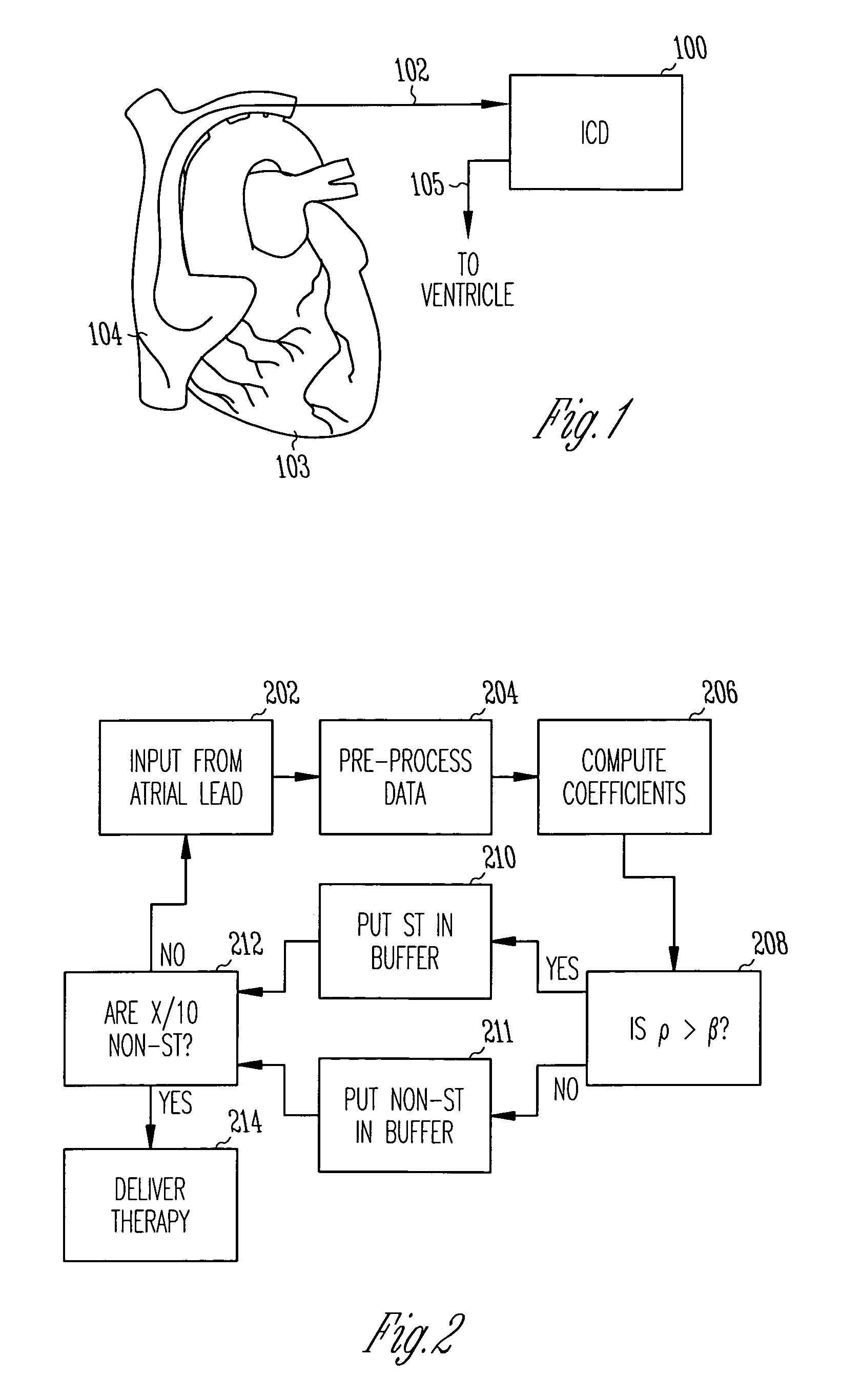
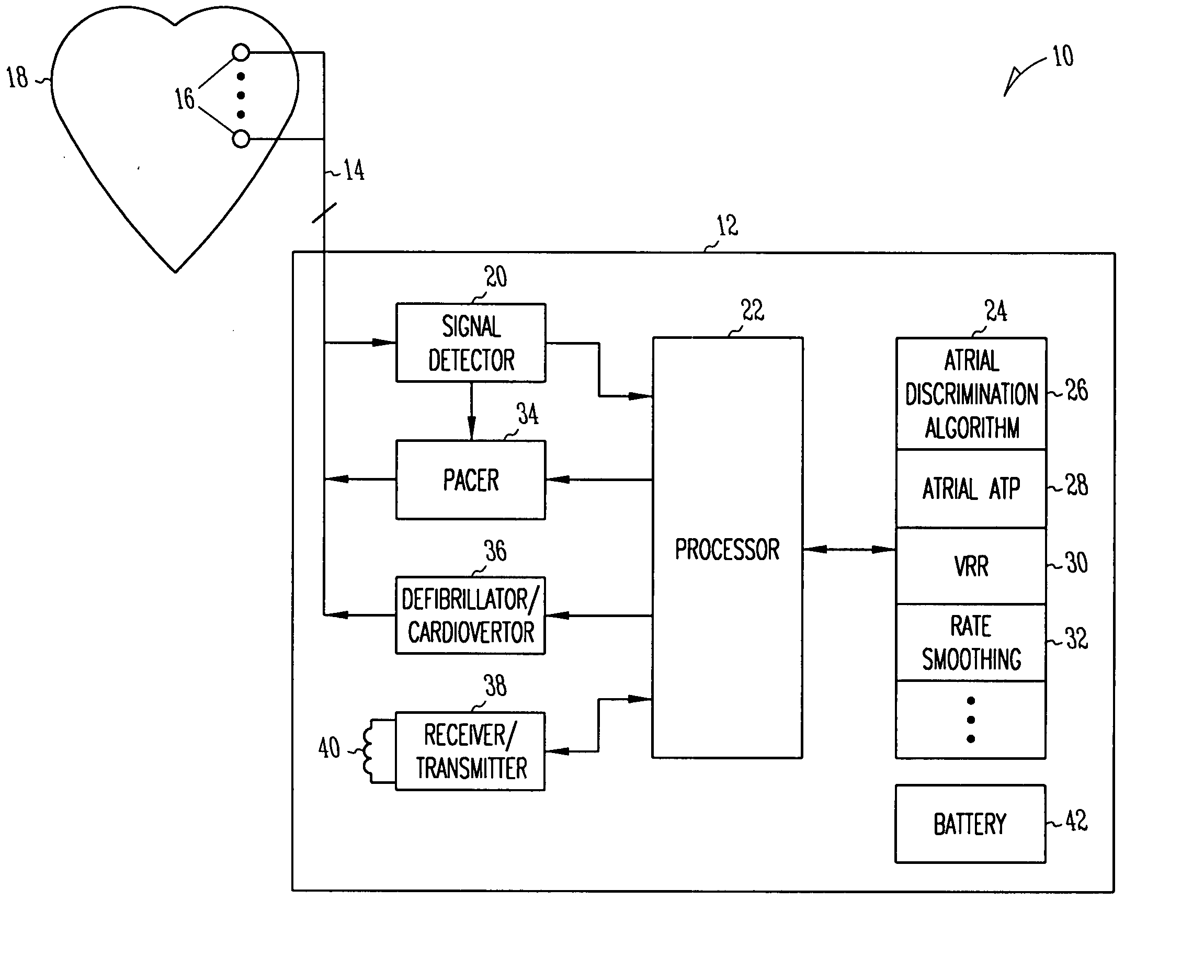
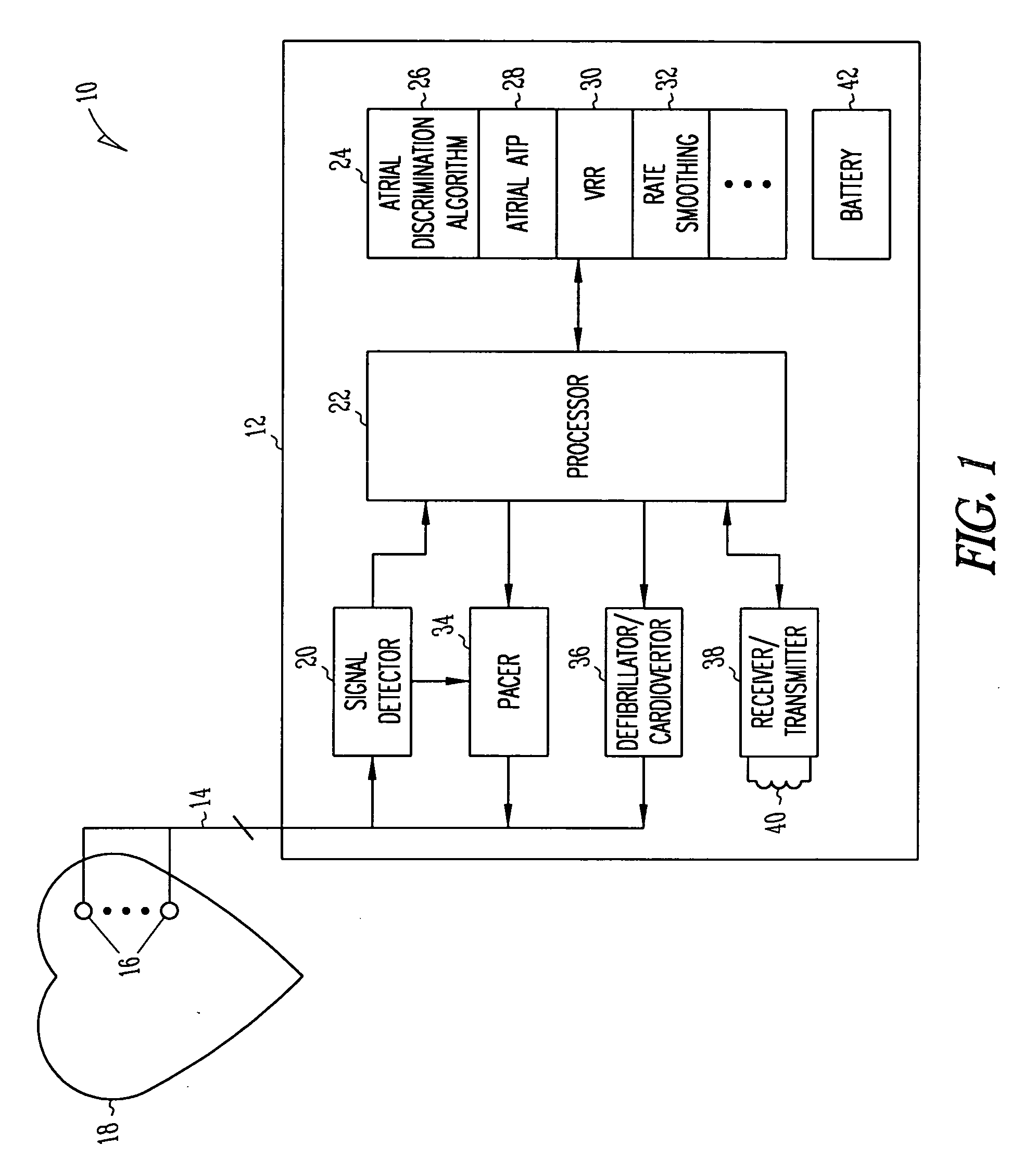
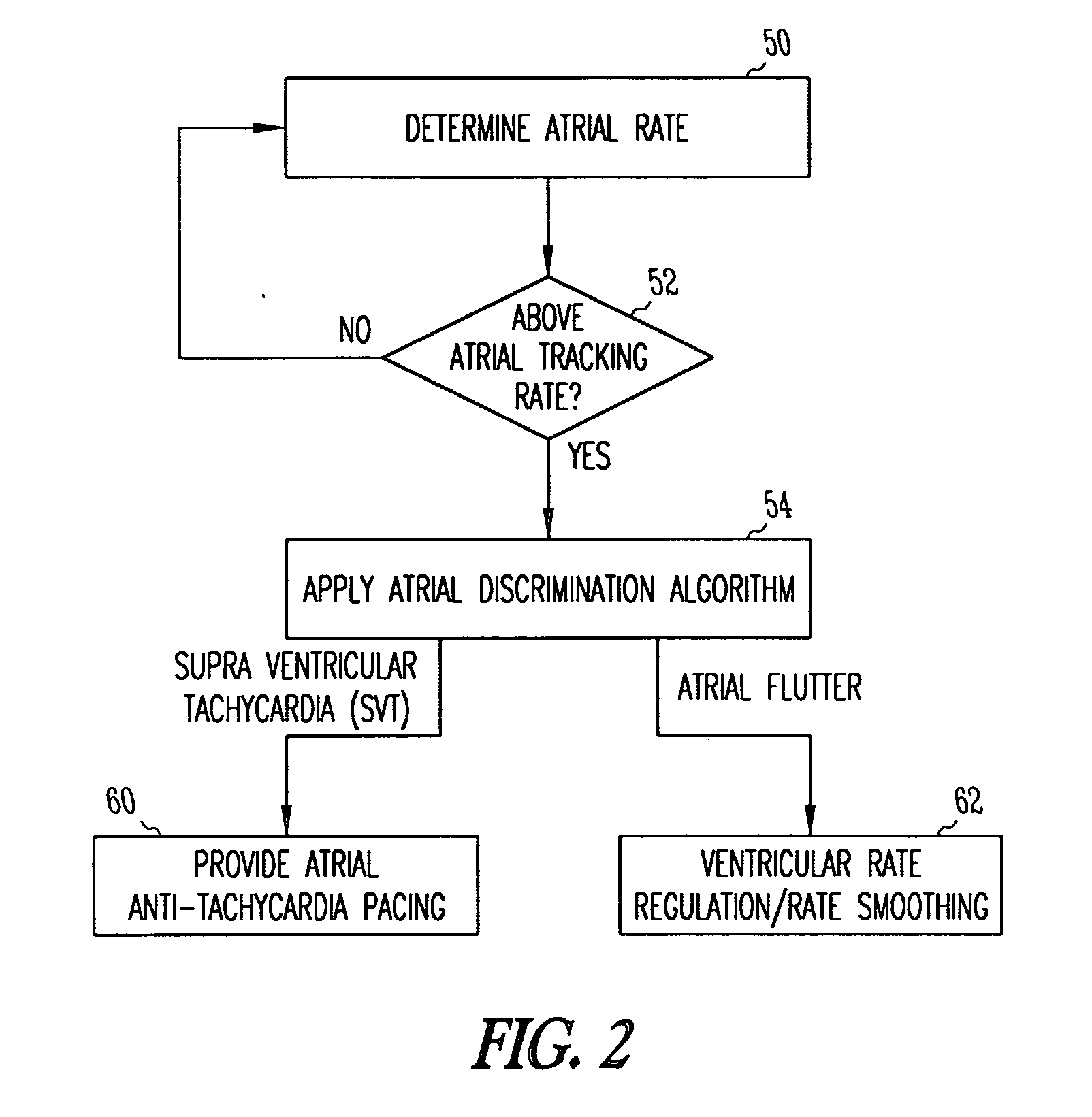
Method and apparatus for using atrial discrimination algorithms to determine optimal pacing therapy and therapy timing
A system and method which employs atrial discrimination algorithms to distinguish between different atrial arrhythmias occurring in a patient for selecting an optimal pacing therapy corresponding to the type of arrhythmia identified. The invention may be implemented in a bradycardia pacemaker or other implantable cardiac device. In response to the detection of an atrial rate above the atrial tracking rate, discrimination criteria are applied to a detected atrial activity signal to distinguish between different types of supraventricular tachycardia, such as fast atrial flutter and other atrial flutter at a relatively slower rate, which may be occurring in the patient. The discrimination criteria may be, for example, rate-based or morphology based. The pacer is controlled to provide pacing therapy to a heart in a manner corresponding to the type of supraventricular tachycardia identified. For example, antitachycardia pacing may be provided to the heart in response to the detection of a relatively lower rate supraventricular tachycardia / other atrial flutter, whereas another pacing control, e.g., ventricular pacing, such as ventricular rate regulation or Rate Smoothing, may be applied if a more rapid rate supraventricular tachycardia / fast atrial flutter is identified. The output of an atrial discrimination algorithm may be tracked and the trend thereof used to improve therapy timing.
Owner:CARDIAC PACEMAKERS INC
Method and apparatus for displaying information retrieved from an implanted medical device
Owner:MEDTRONIC INC
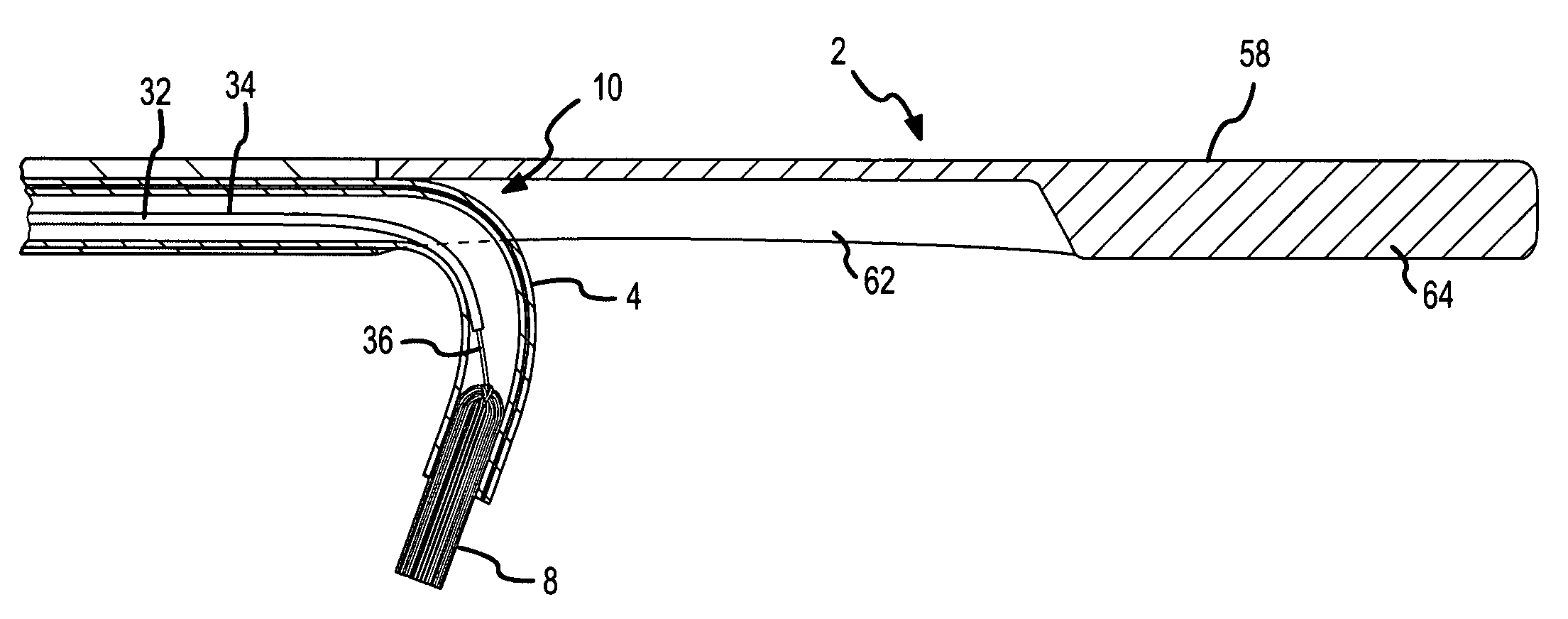
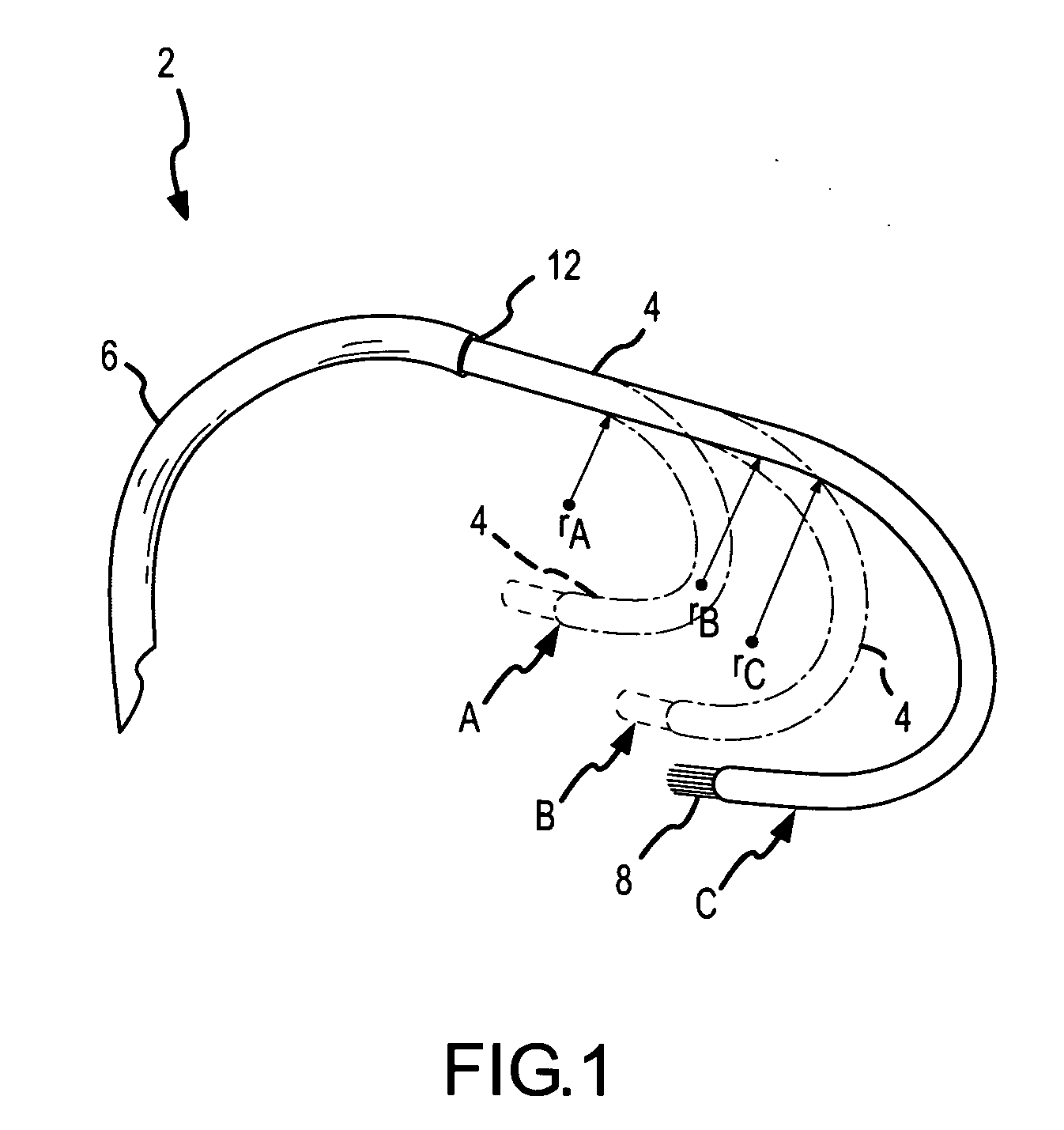
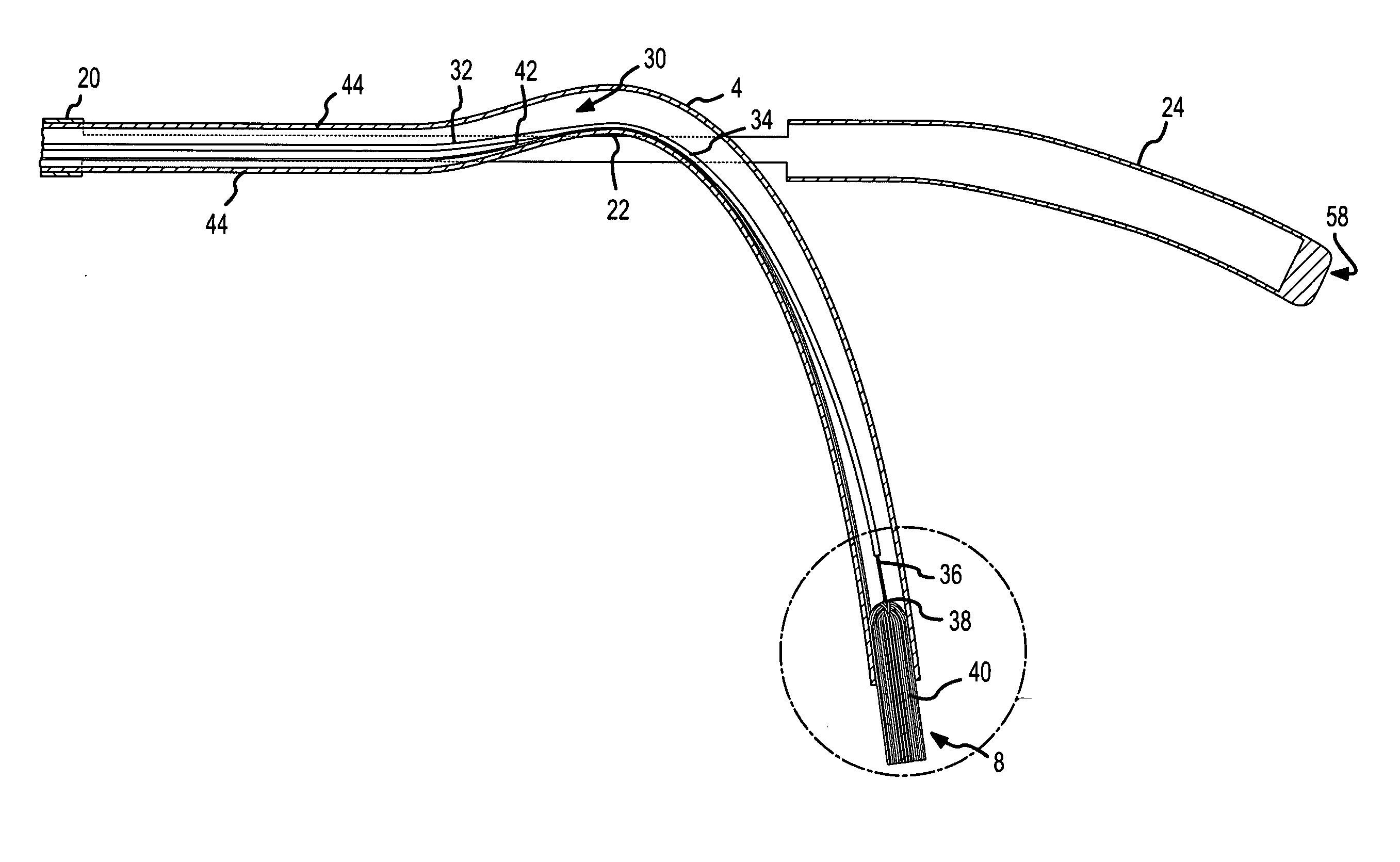
Ablation catheter with suspension system incorporating rigid and flexible components
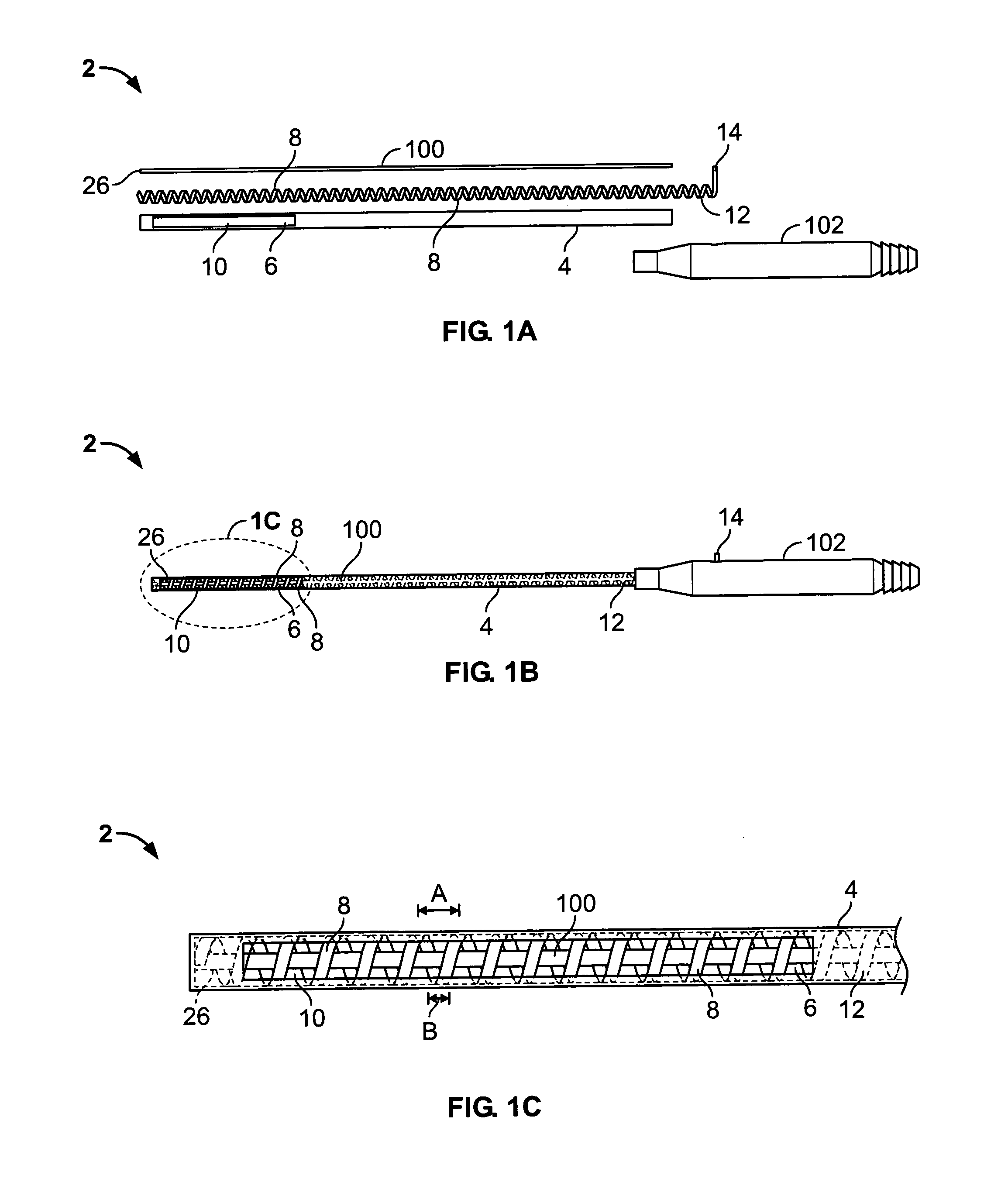
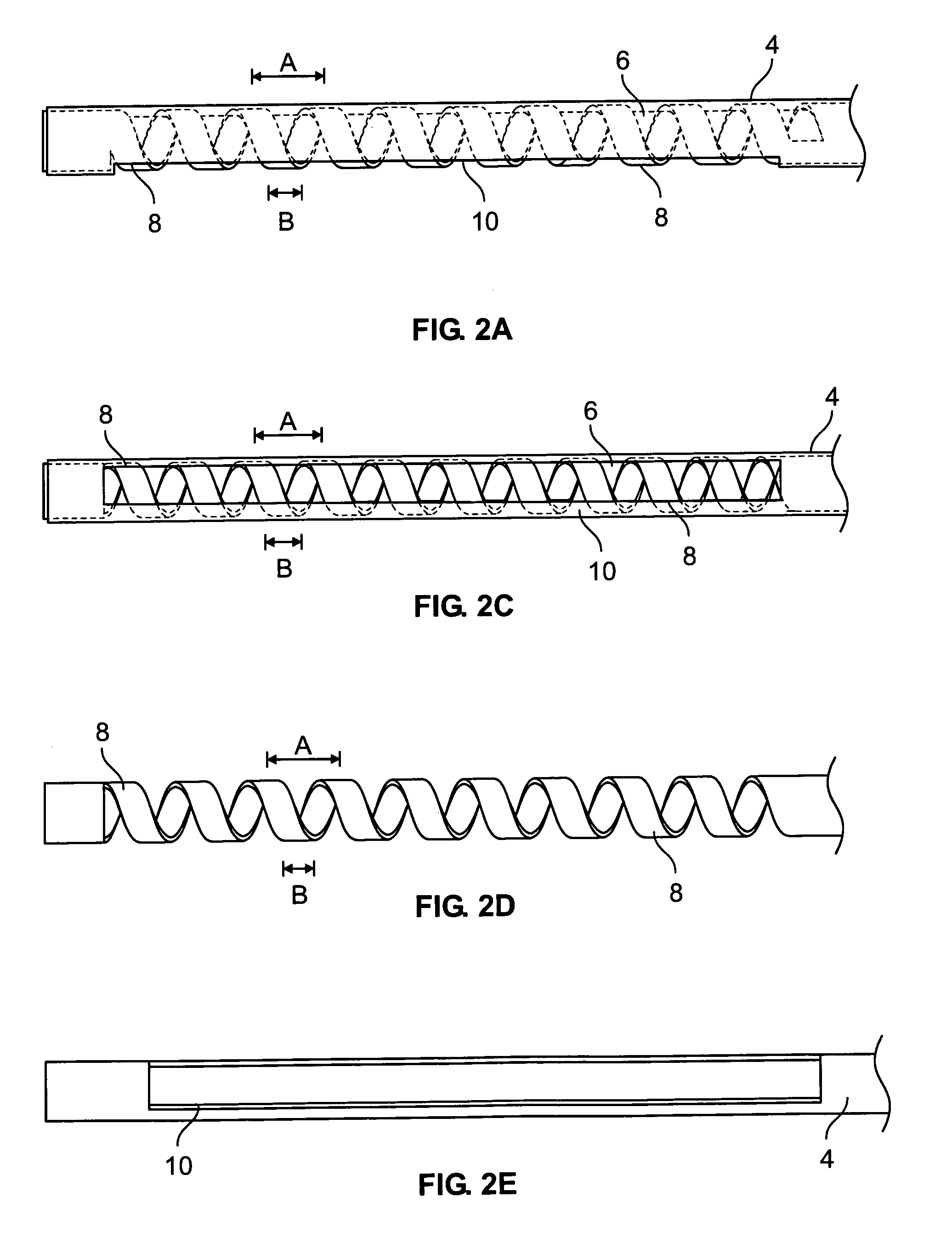
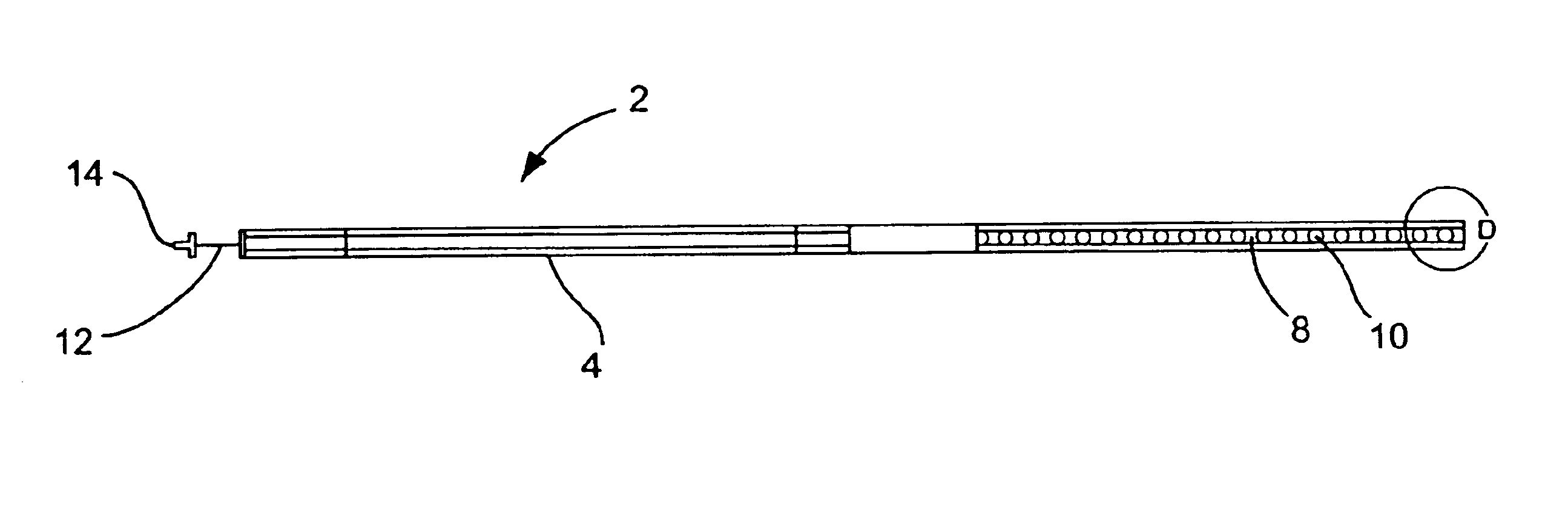
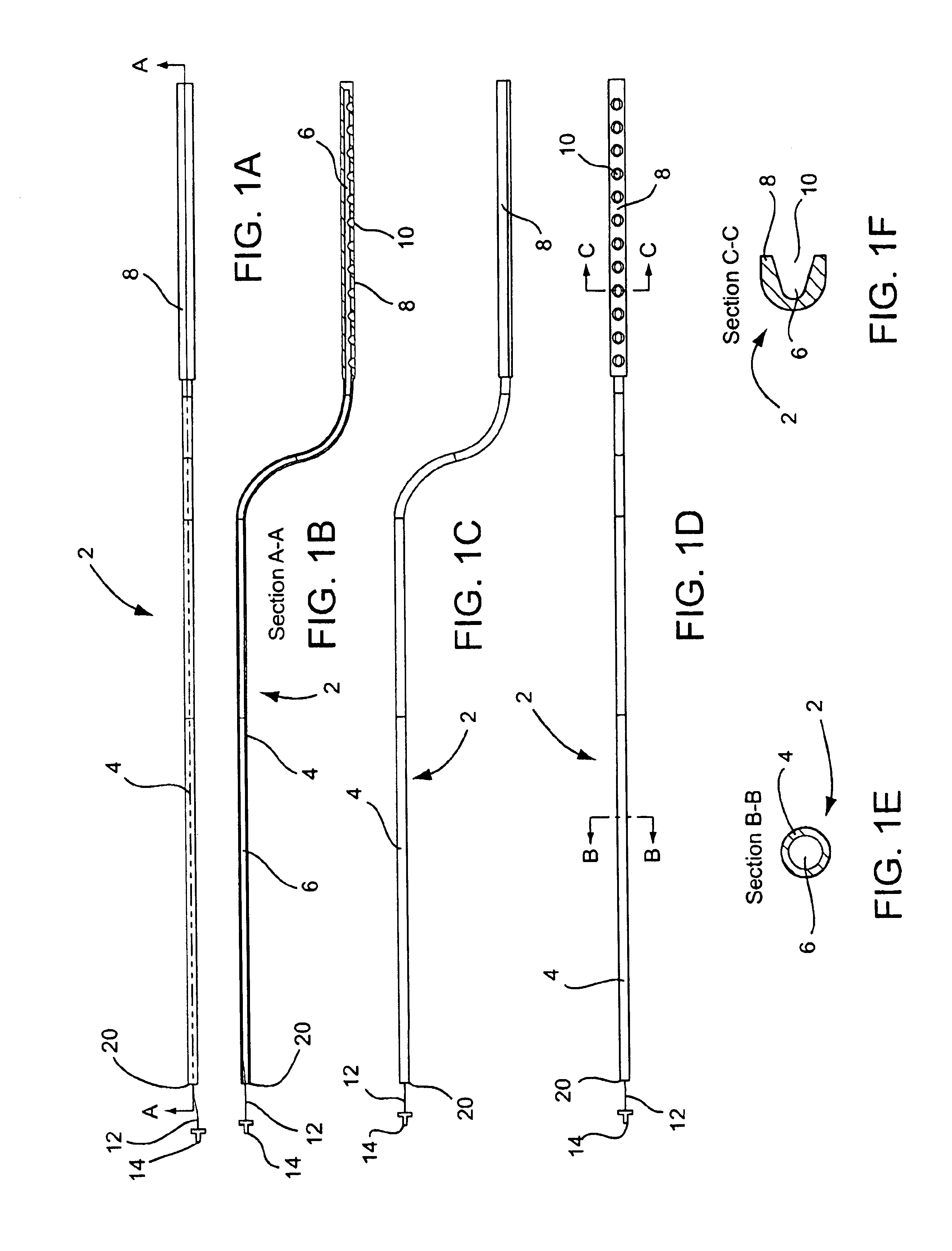
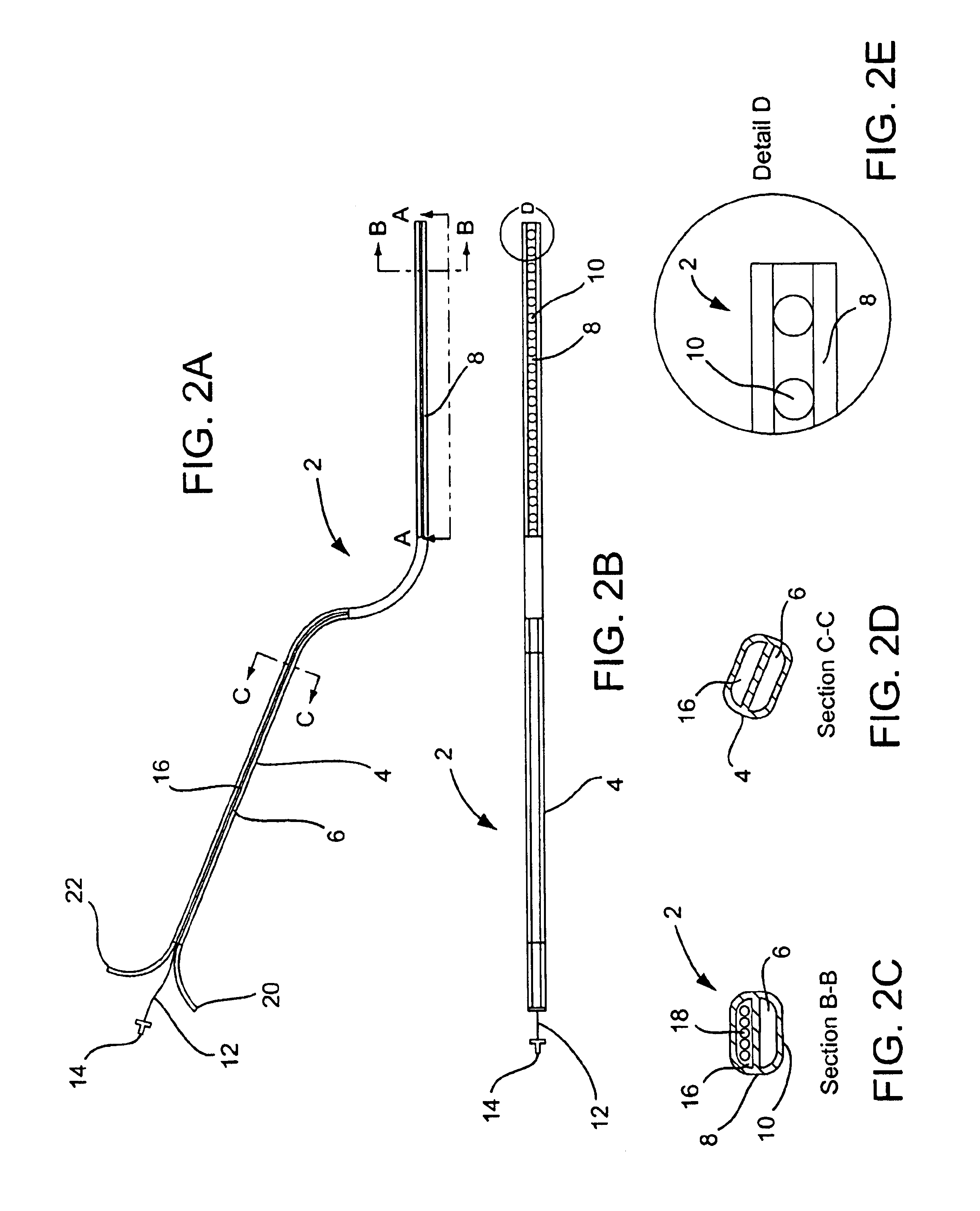
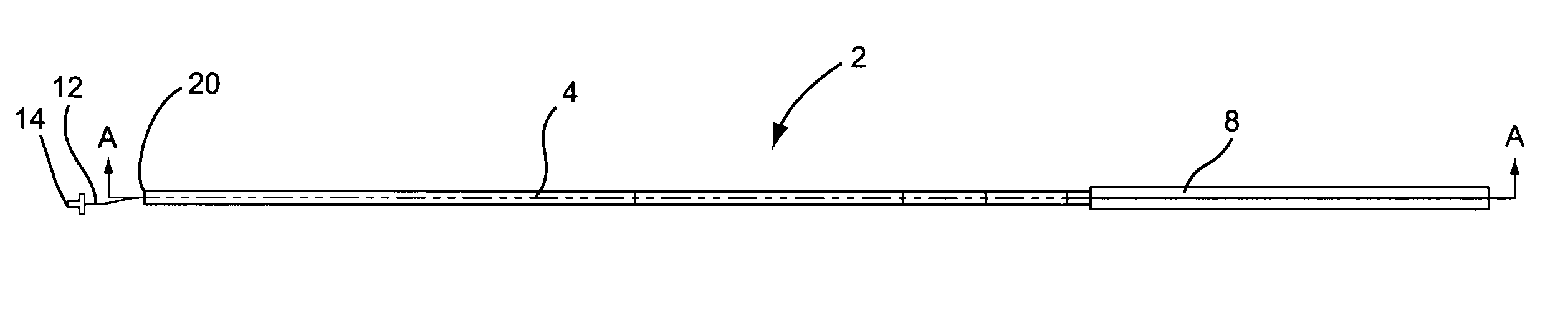
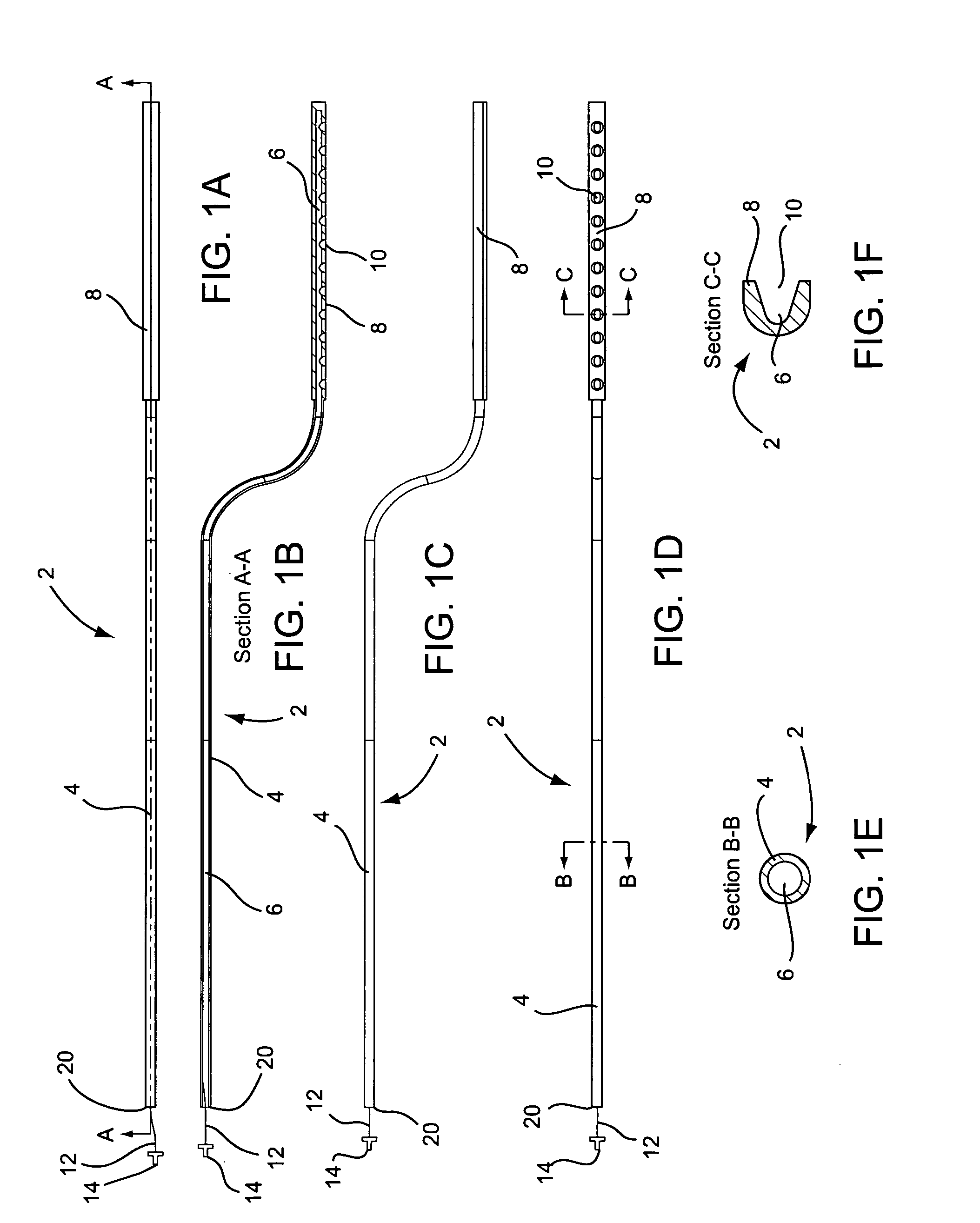
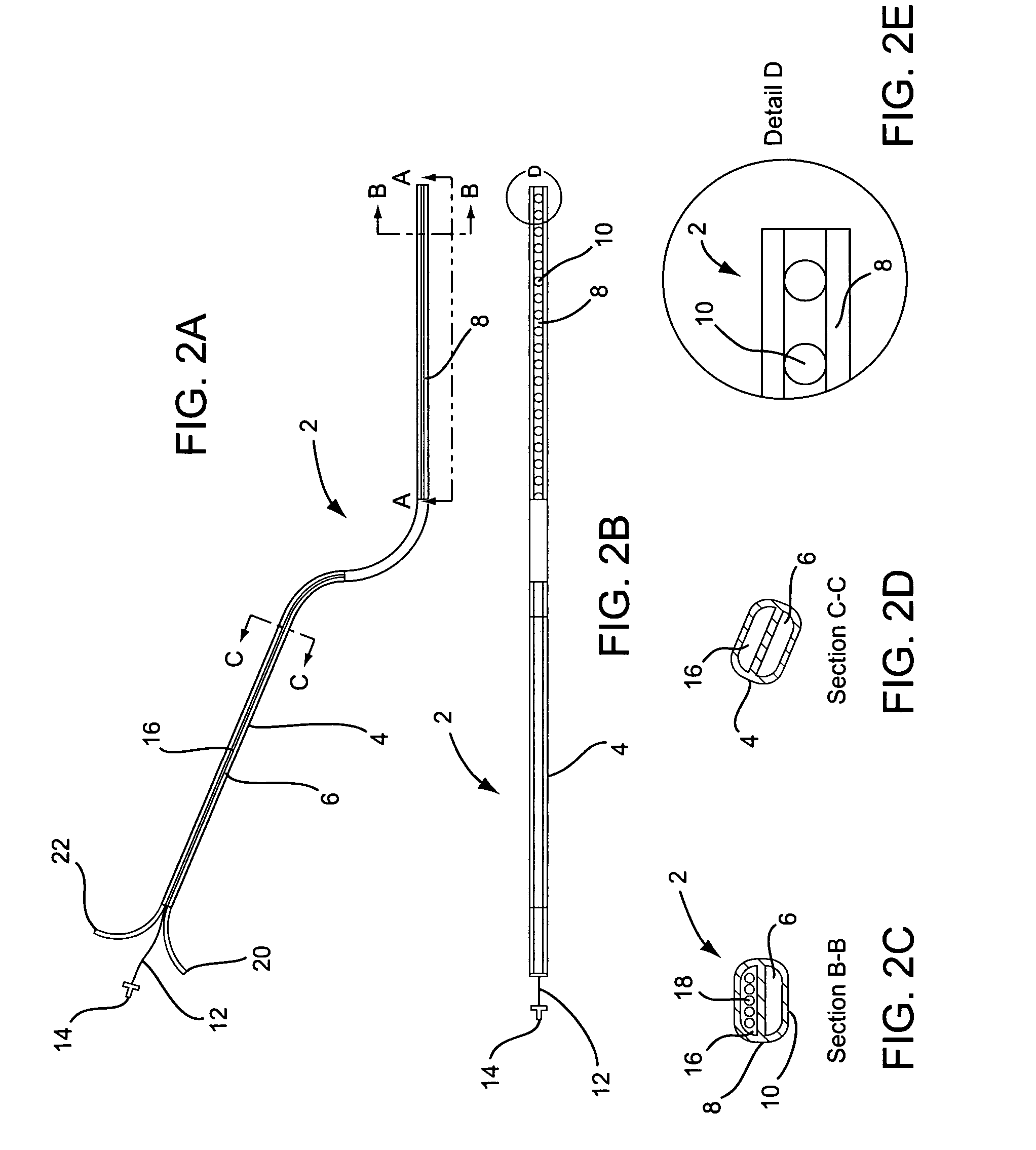
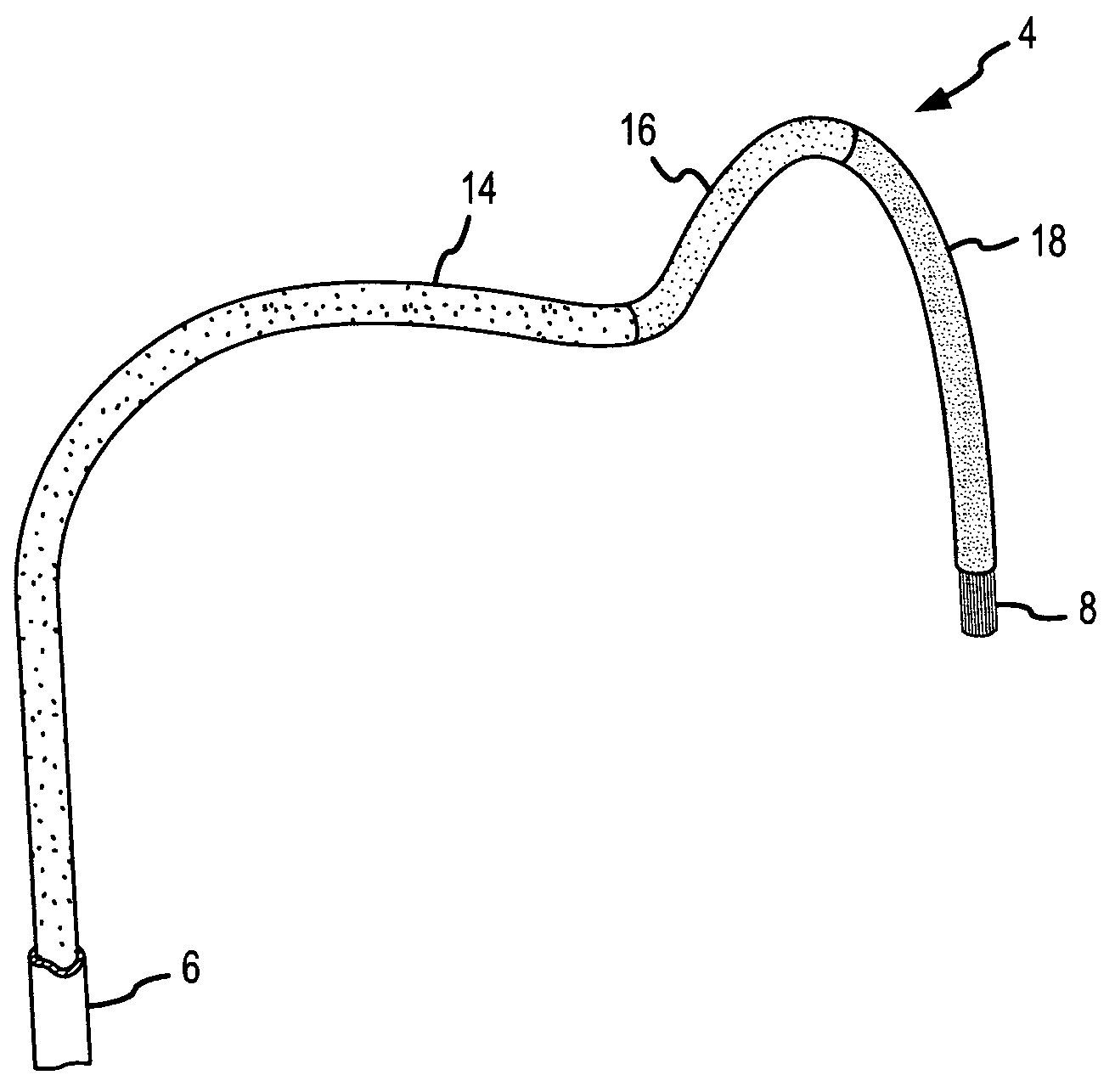
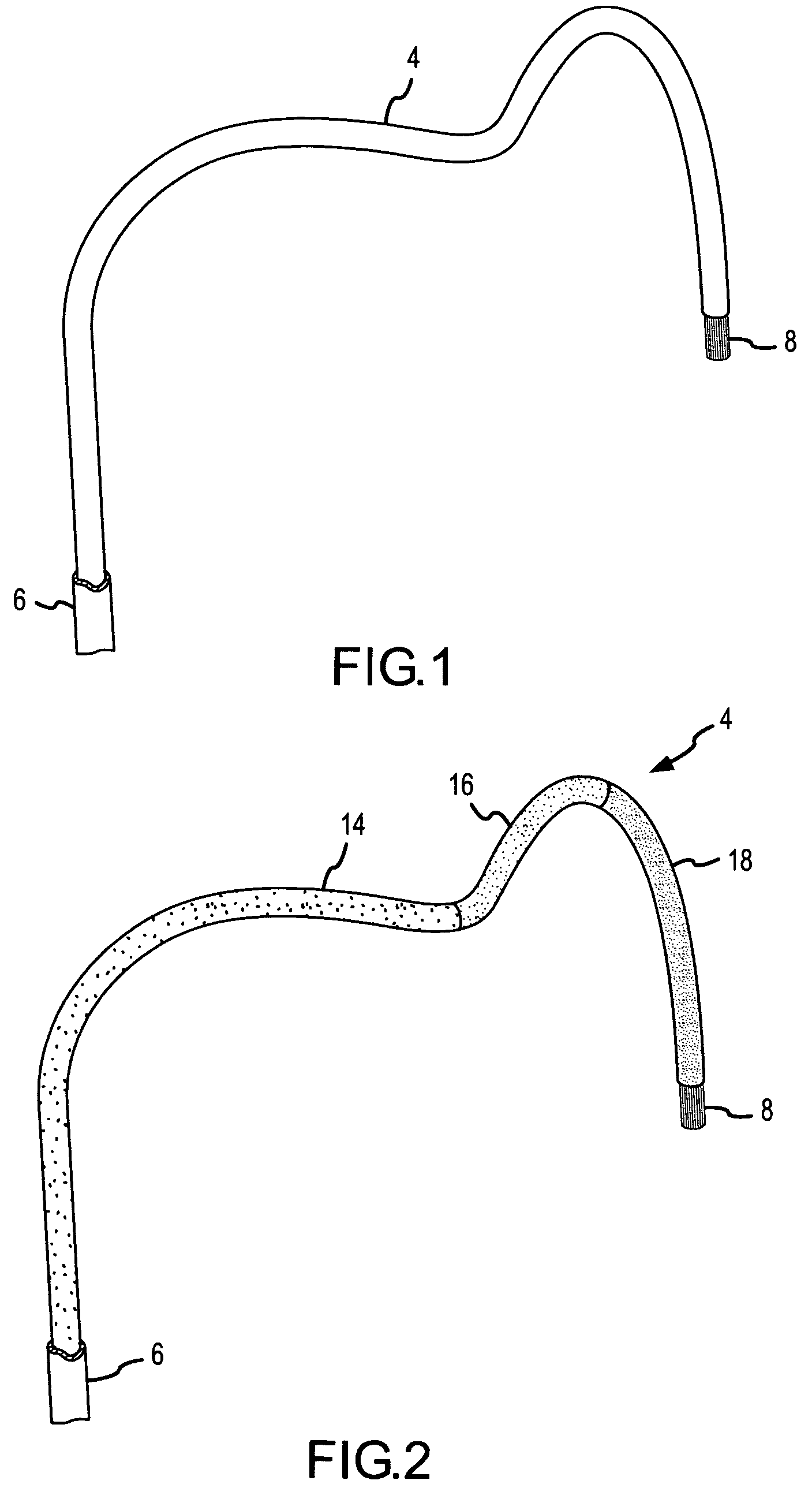
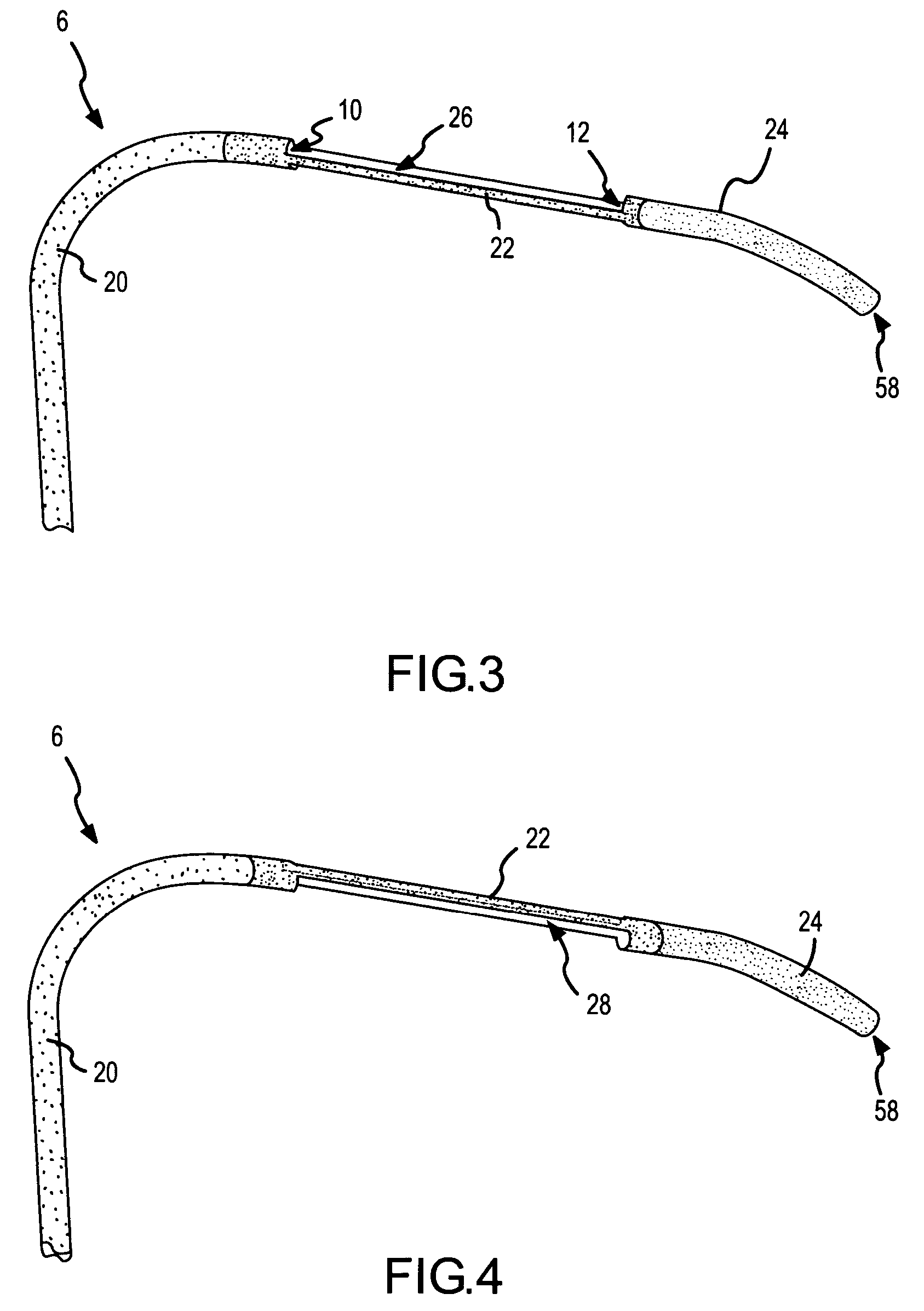
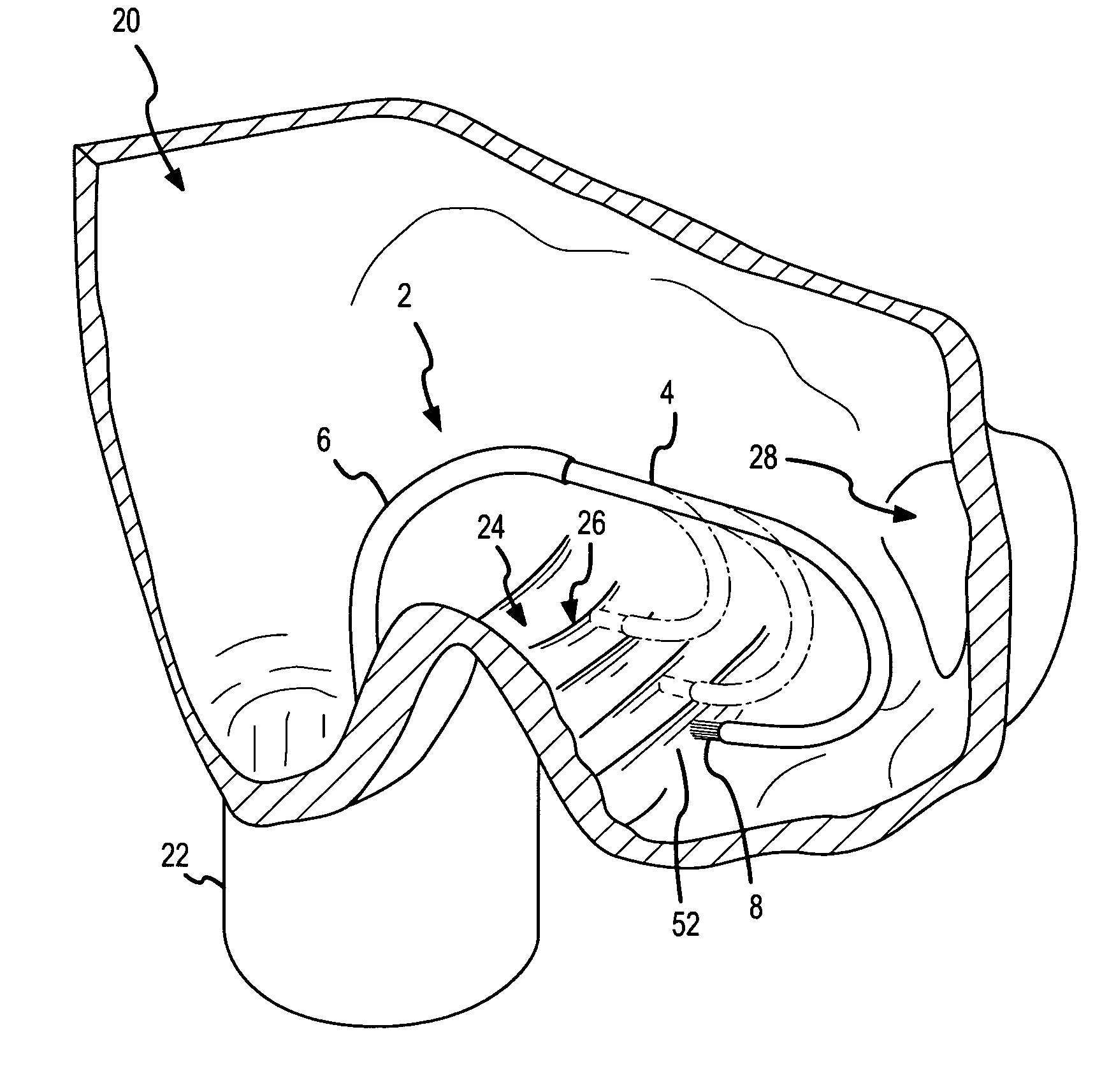
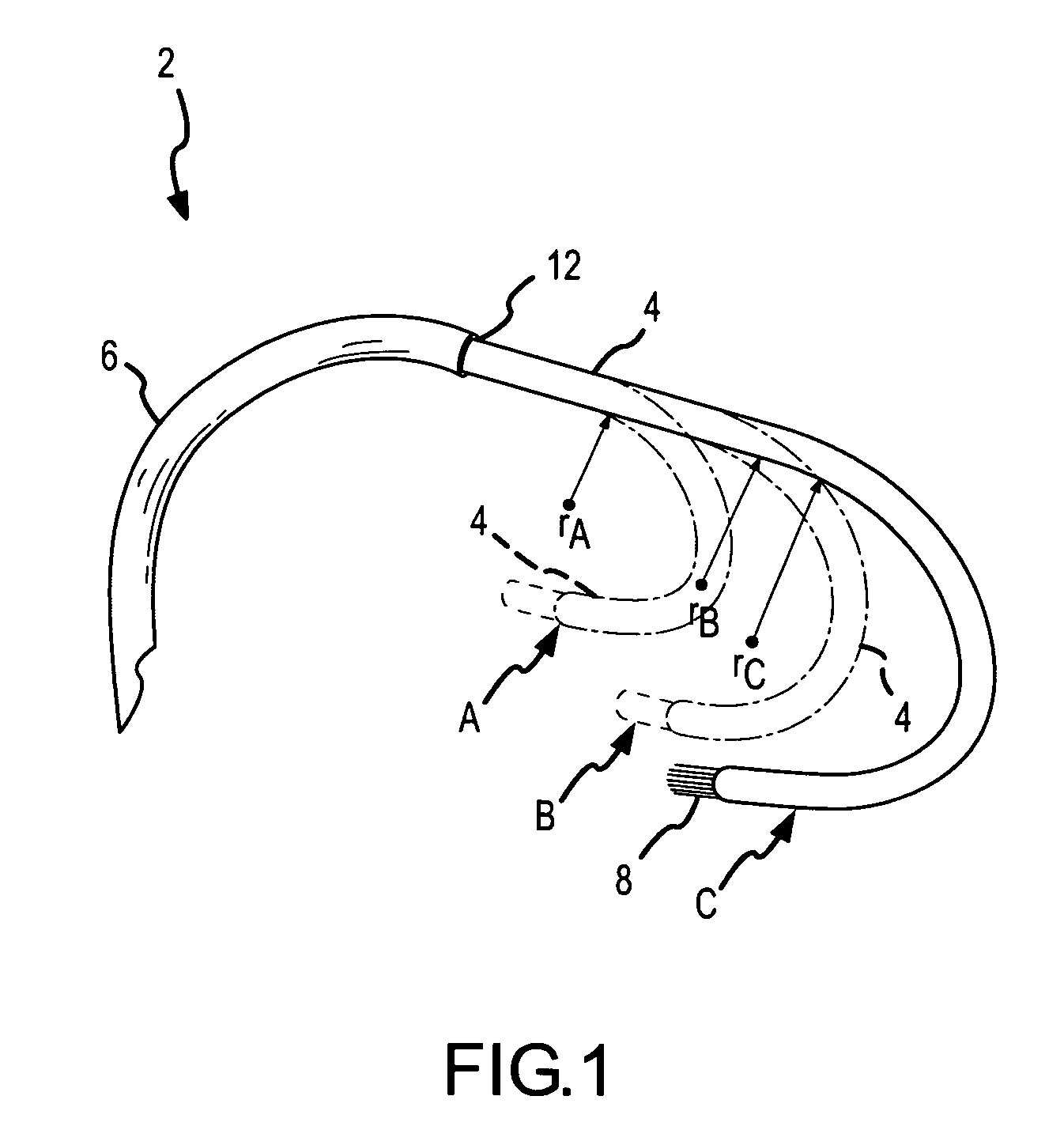
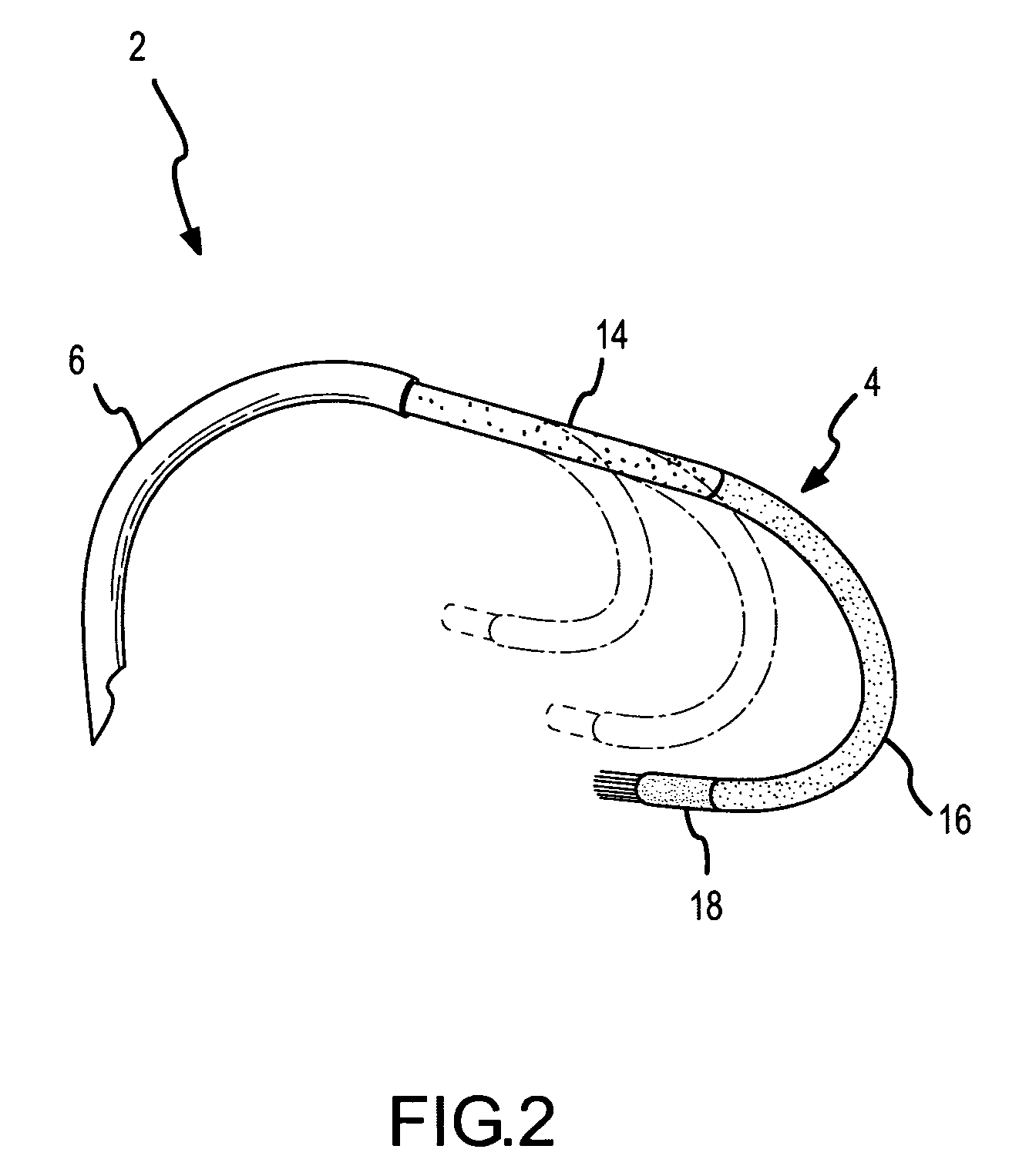
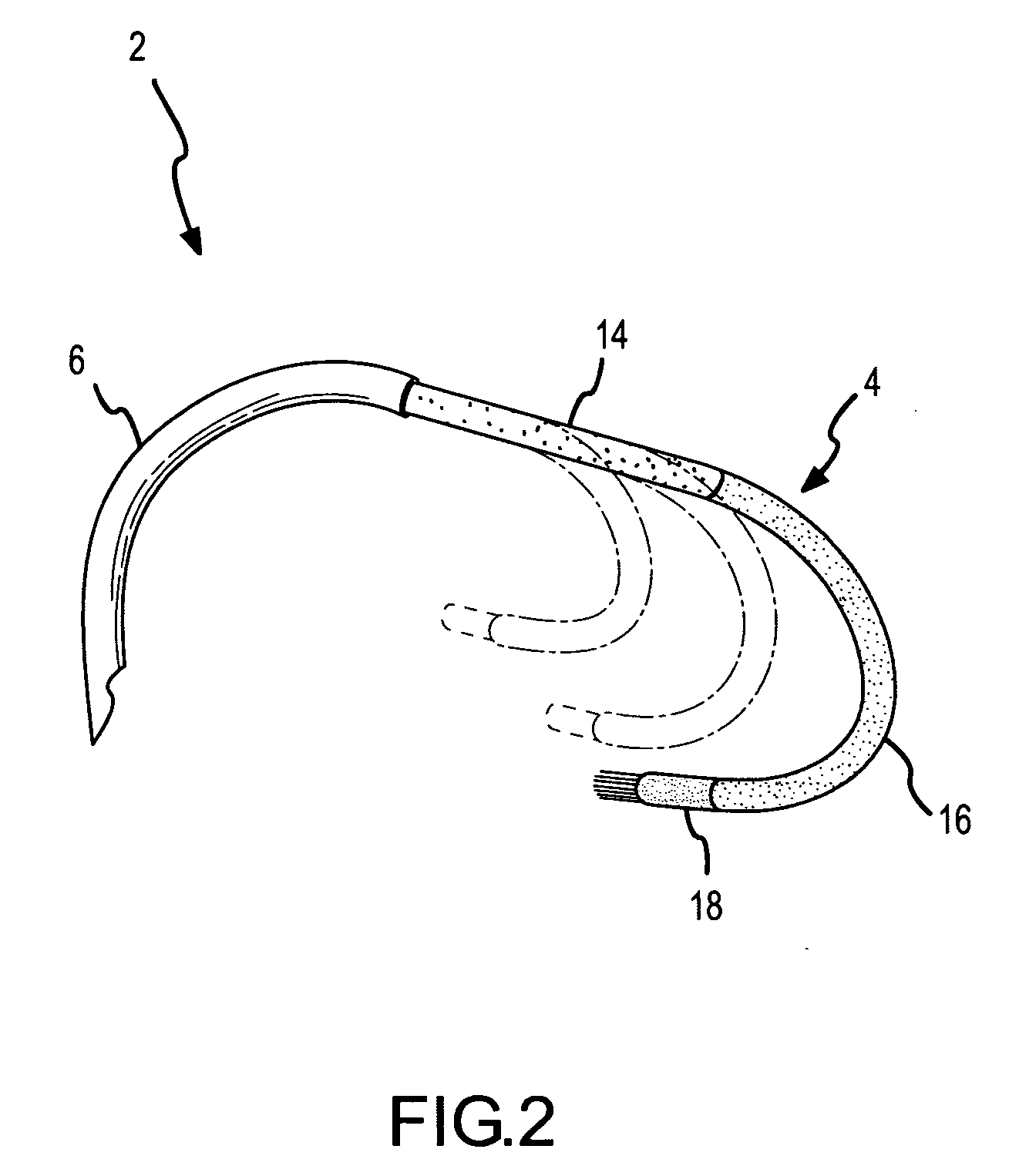
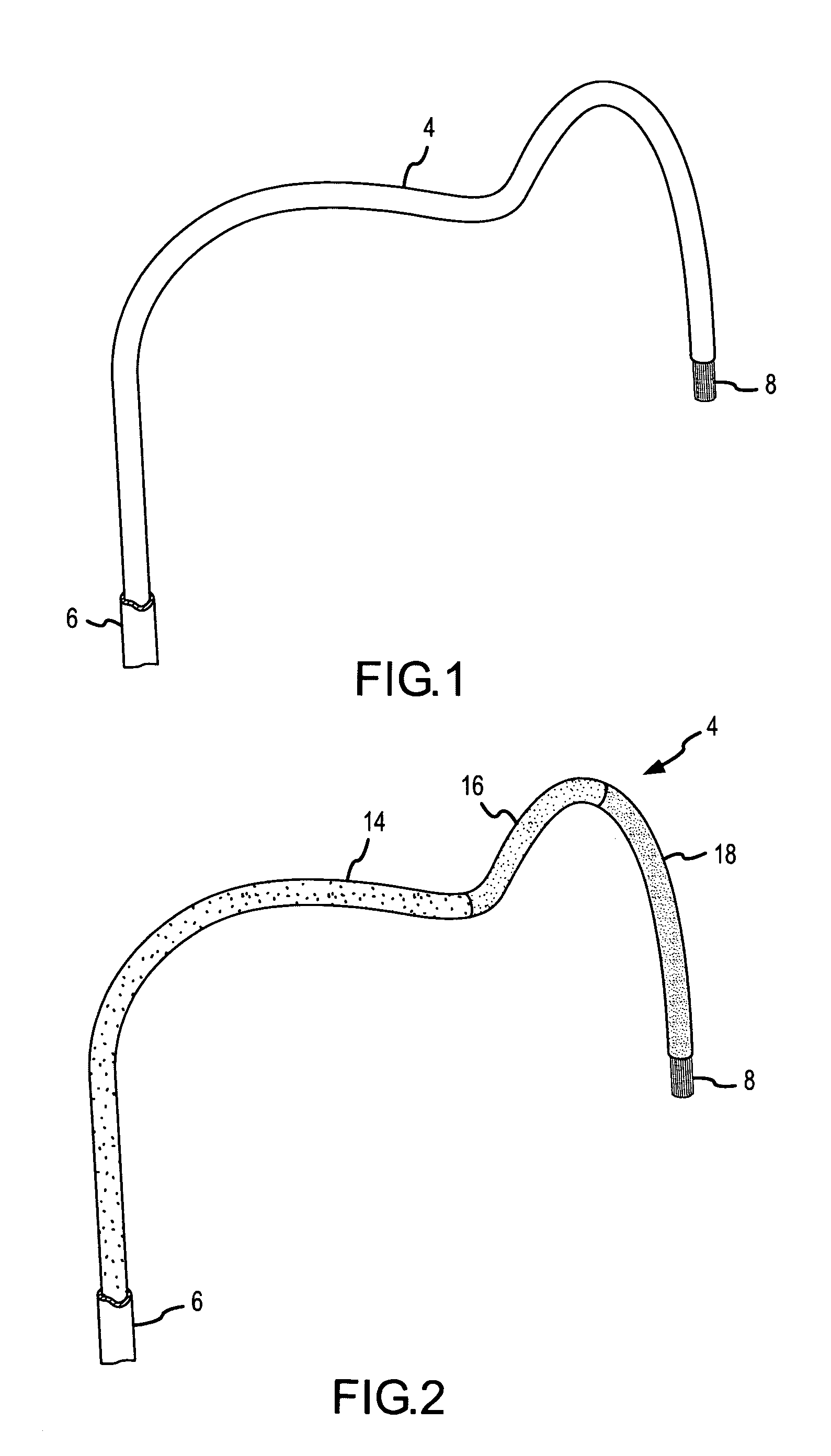
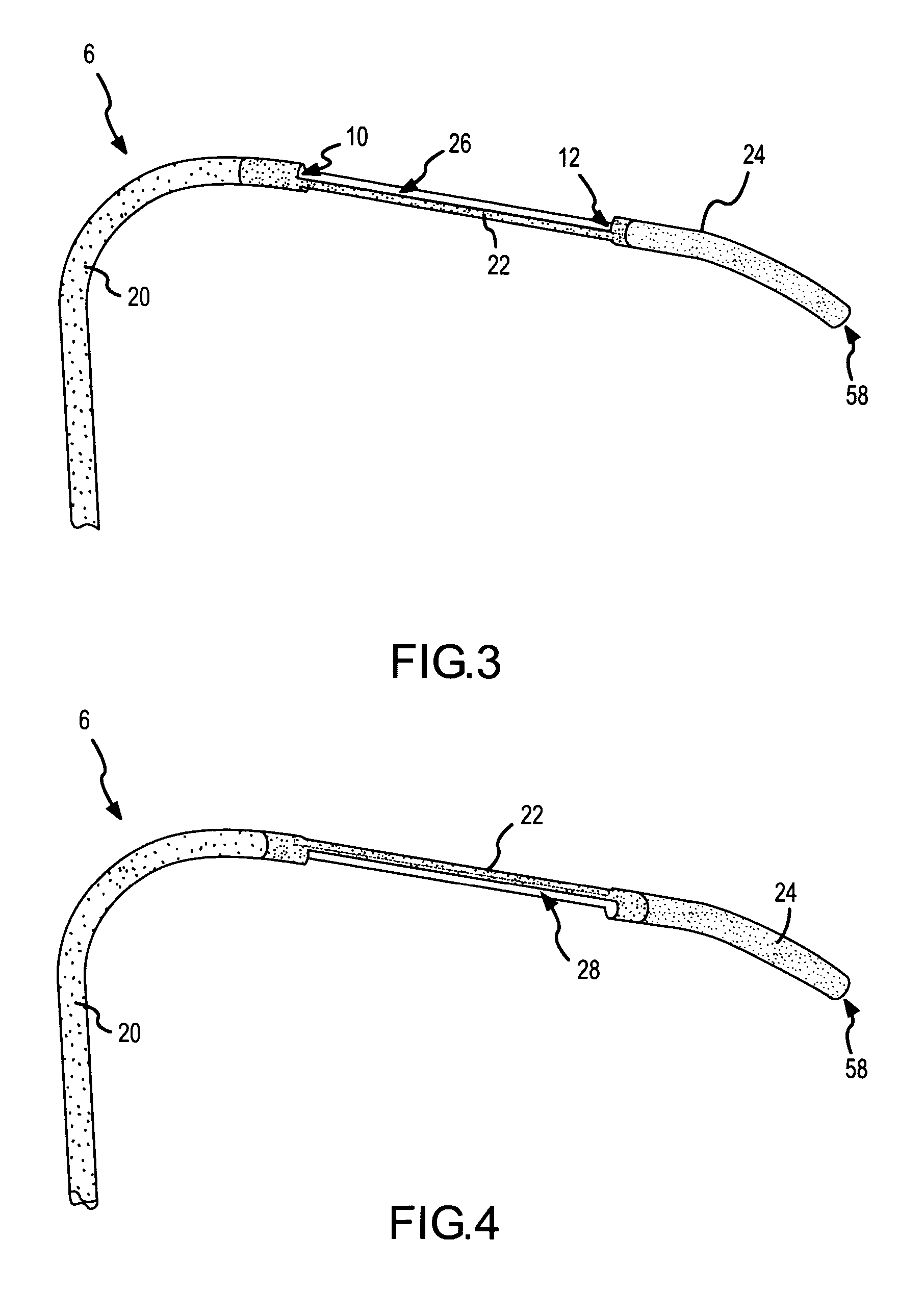
A curved ablation catheter imparts ablative energy to target tissue, for example, along a trabecular slope, e.g., in the right atrium along the isthmus between the ostium of the inferior vena cava and the tricuspid valve. The catheter is formed with a preset curvature that, when deployed, both translates linearly and increases in radius to aid in the formation of spot or continuous linear lesions. A method of treating atrial flutter employs the curved ablation catheter.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Methods of coagulating tissue
InactiveUS20060206113A1Surgical instruments for heatingSurgical instruments for aspiration of substancesRadio frequencyLesion
An embodiment of the invention includes a surgical device for coagulating soft tissue such as atrial tissue in the treatment of atrial fibrillation, atrial flutter, and atrial tachycardia; tendon or ligament shrinkage; or articular cartilage removal. The surgical device integrates a suction mechanism with the coagulation mechanism improving the lesion creation capabilities of the device. The surgical device comprises an elongate member having an insulative covering attached about conductive elements capable of coagulating soft tissue when radiofrequency or direct current energy is transmitted to the conductive elements. Openings through the insulative covering expose regions of the conductive elements and are coupled to lumens in the elongate member which are routed to a vacuum source. Suction causes the soft tissue to actively engage the opening thus the integrated, exposed conductive elements to facilitate the coagulation process and ensure the lesions created are consistent, continuous, and transmural. The embodiments of the invention can also incorporate cooling mechanisms associated with the conductive elements and coupled to a fluid source to passively transport fluid along the contacted soft tissue surface to cool thus pushing the maximum temperature deeper into tissue.
Owner:ATRICURE
Vacuum coagulation probes
InactiveUS20060200124A1Surgical instruments for heatingSurgical instruments for aspiration of substancesArticular cartilageLesion
An embodiment of the invention includes a surgical device for coagulating soft tissue such as atrial tissue in the treatment of atrial fibrillation, atrial flutter, and atrial tachycardia; tendon or ligament shrinkage; or articular cartilage removal. The surgical device integrates a suction mechanism with the coagulation mechanism improving the lesion creation capabilities of the device. The surgical device comprises an elongate member having an insulative covering attached about conductive elements capable of coagulating soft tissue when radiofrequency or direct current energy is transmitted to the conductive elements. Openings through the insulative covering expose regions of the conductive elements and are coupled to lumens in the elongate member which are routed to a vacuum source. Suction causes the soft tissue to actively engage the opening thus the integrated, exposed conductive elements to facilitate the coagulation process and ensure the lesions created are consistent, continuous, and transmural. The embodiments of the invention can also incorporate cooling mechanisms associated with the conductive elements and coupled to a fluid source to passively transport fluid along the contacted soft tissue surface to cool thus pushing the maximum temperature deeper into tissue.
Owner:ATRICURE
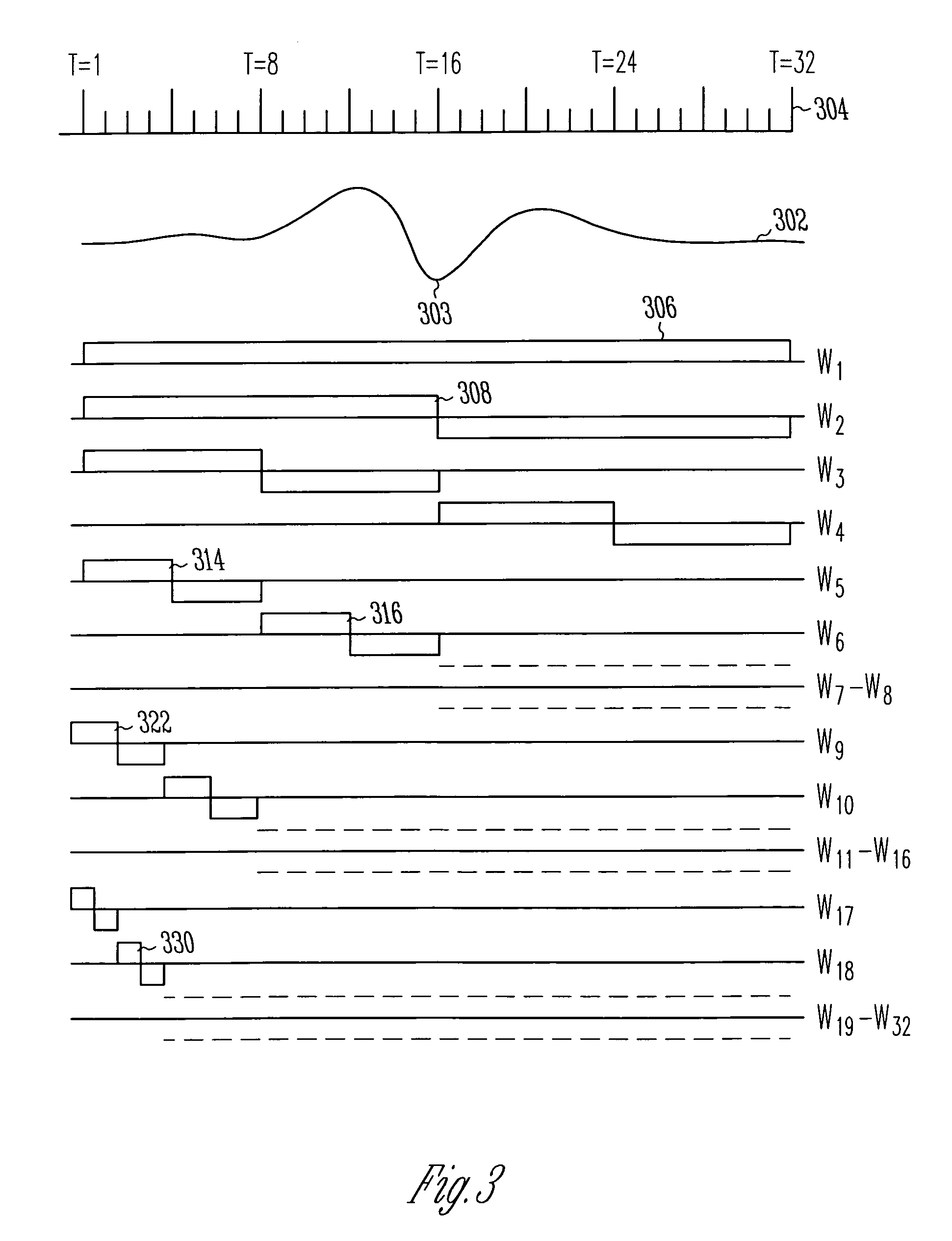
Bayesian discriminator for rapidly detecting arrhythmias
InactiveUS20030216654A1Improve algorithm performanceImprove performanceMedical data miningElectrocardiographyDiscriminatorData set
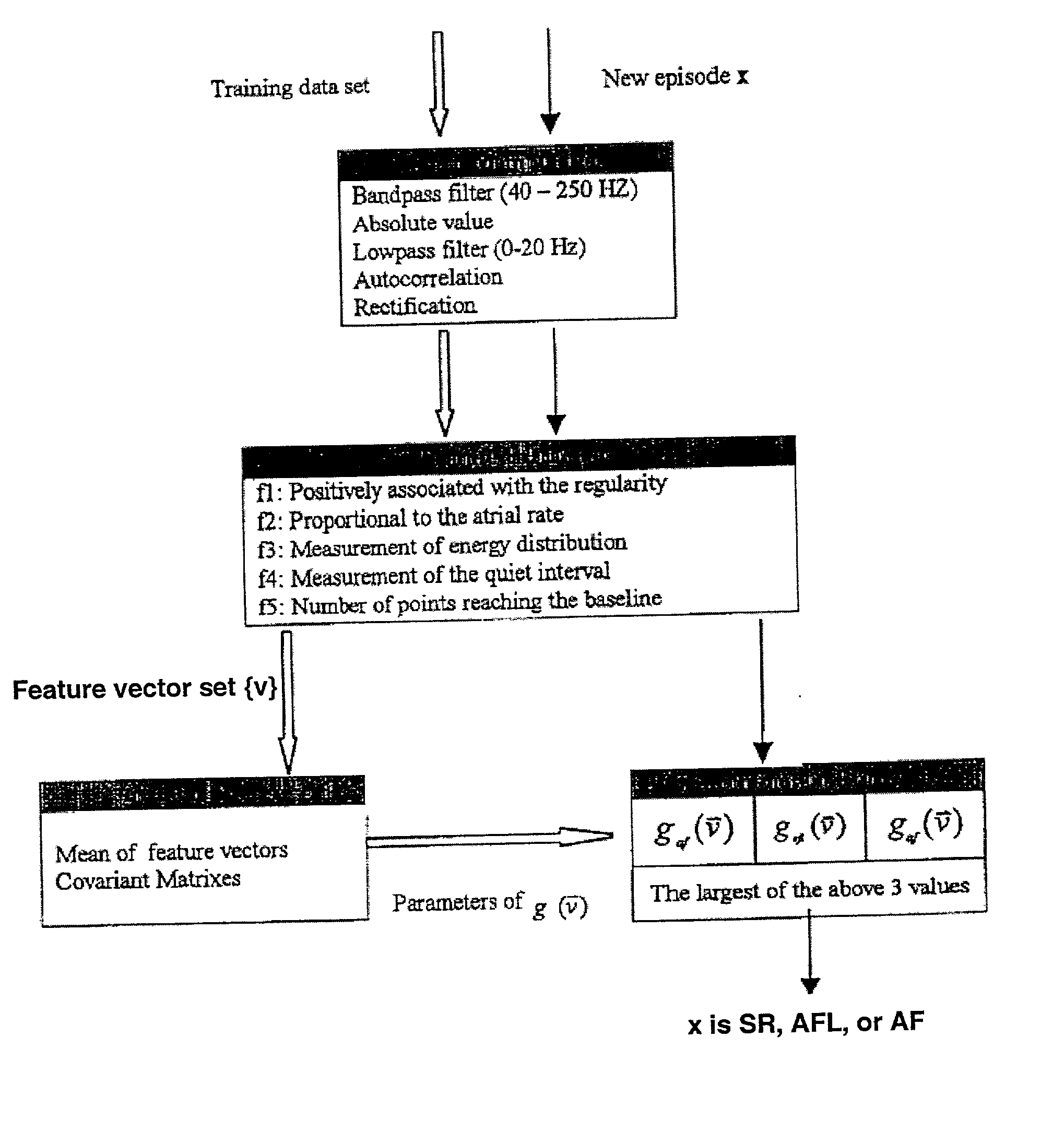
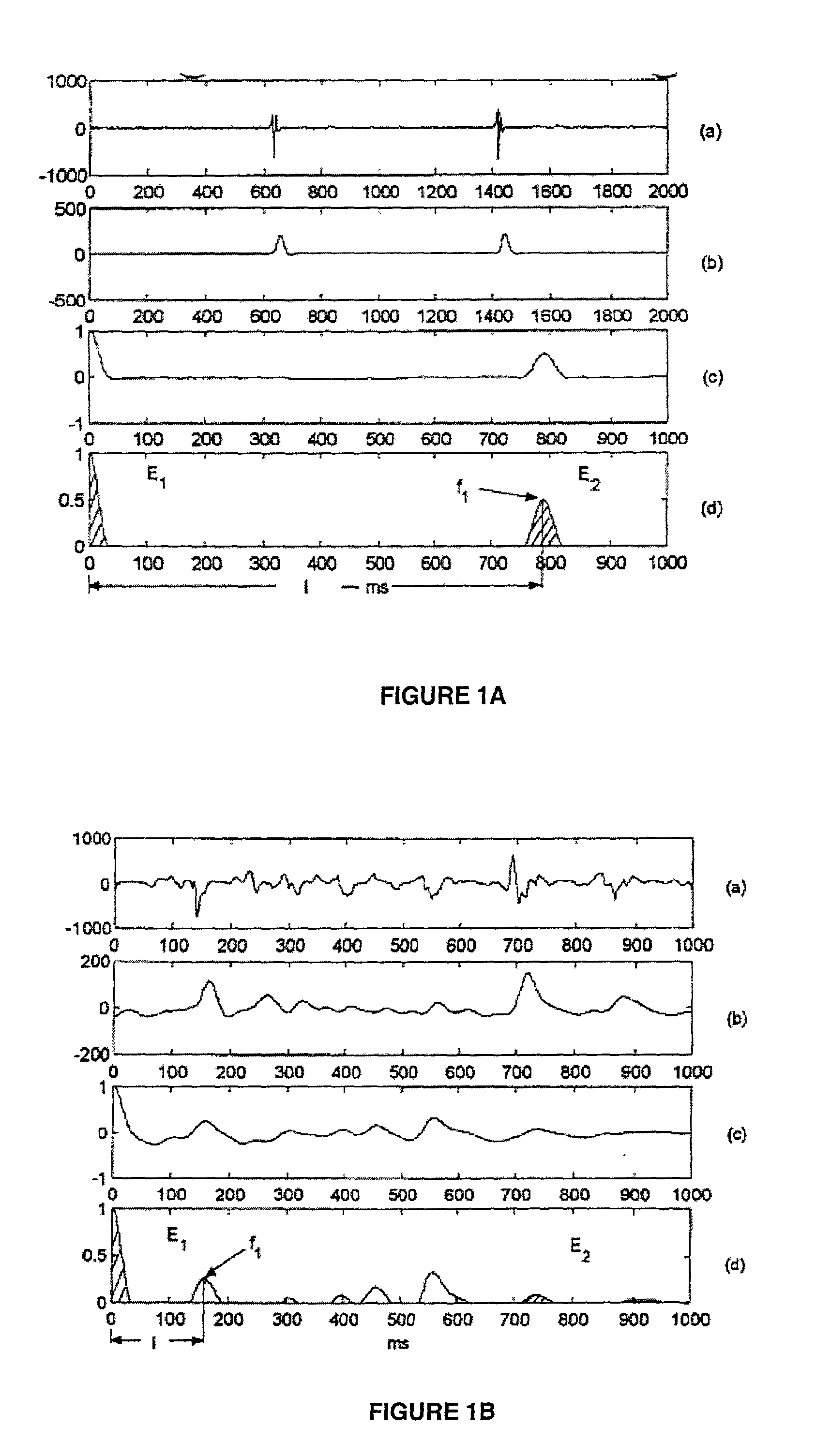
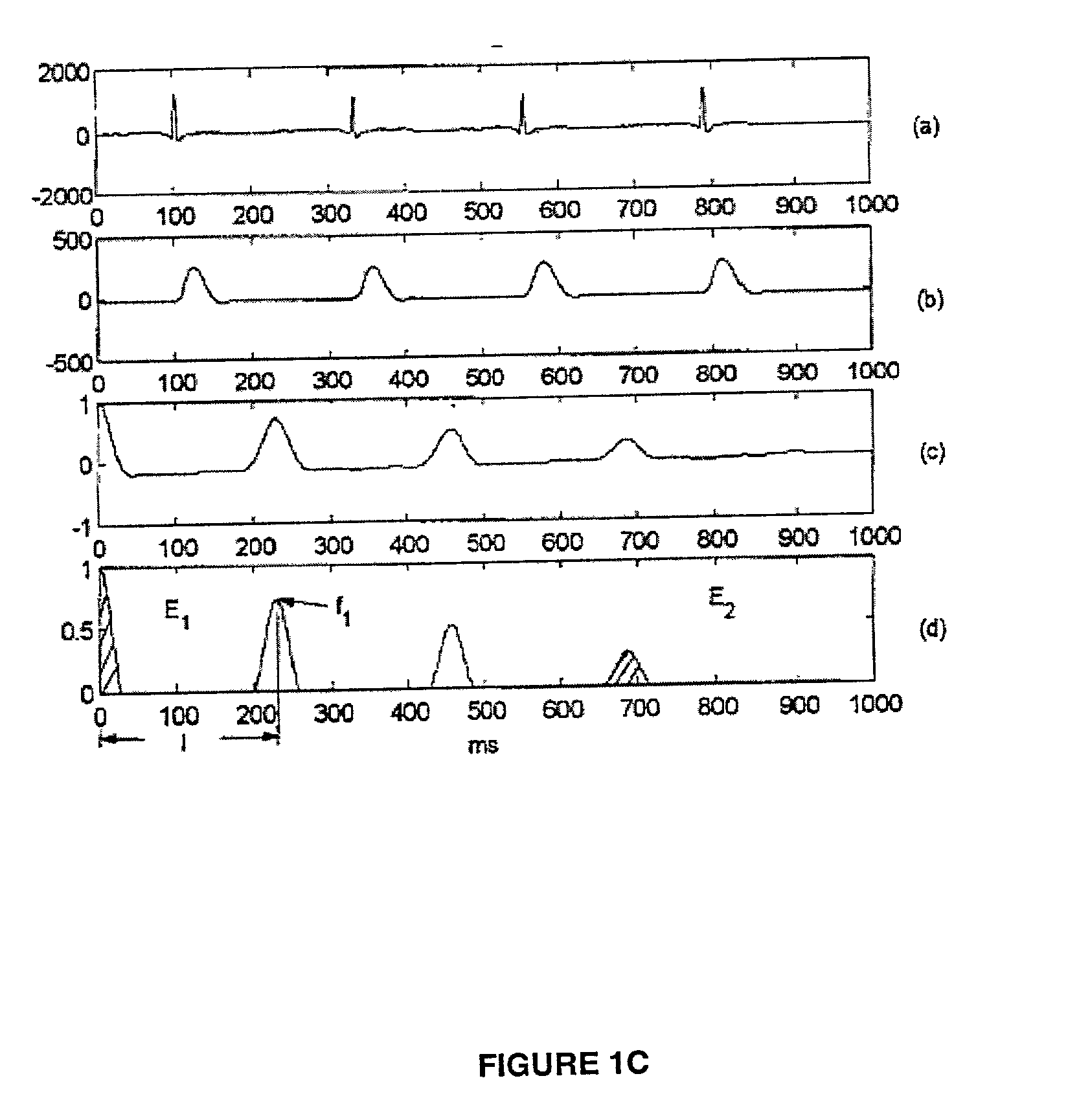
A method for accurate and rapid automated detection of atrial fibrillation (AF), sinus rhythm (SF), and atrial flutter (AFL) is disclosed, which allows distinguishing of these cardiac signals with lowered risk of errors by implanted pacemakers and like devices. The method includes training episodes of intra-cardiac signals (called the closed data set CDS) to evaluate five feature parameters with a discriminator classifying the signal into AF, AFL or sinus rhythm (SR). Comparison with the independent decisions of experienced physicians for each episode reveals specificity, accuracy and sensitivity of greater than 97%. Each episode is a window of intracardiac signal of interval 1-2 seconds with the discriminator providing results in less than 0.25 s. In another aspect, the method is resistant to the presence of noise in the data. In yet another aspect, more feature parameters may be used in alternative implementations including for detecting signals other than AF, AFL & SR.
Owner:HONG KONG THE UNIV OF
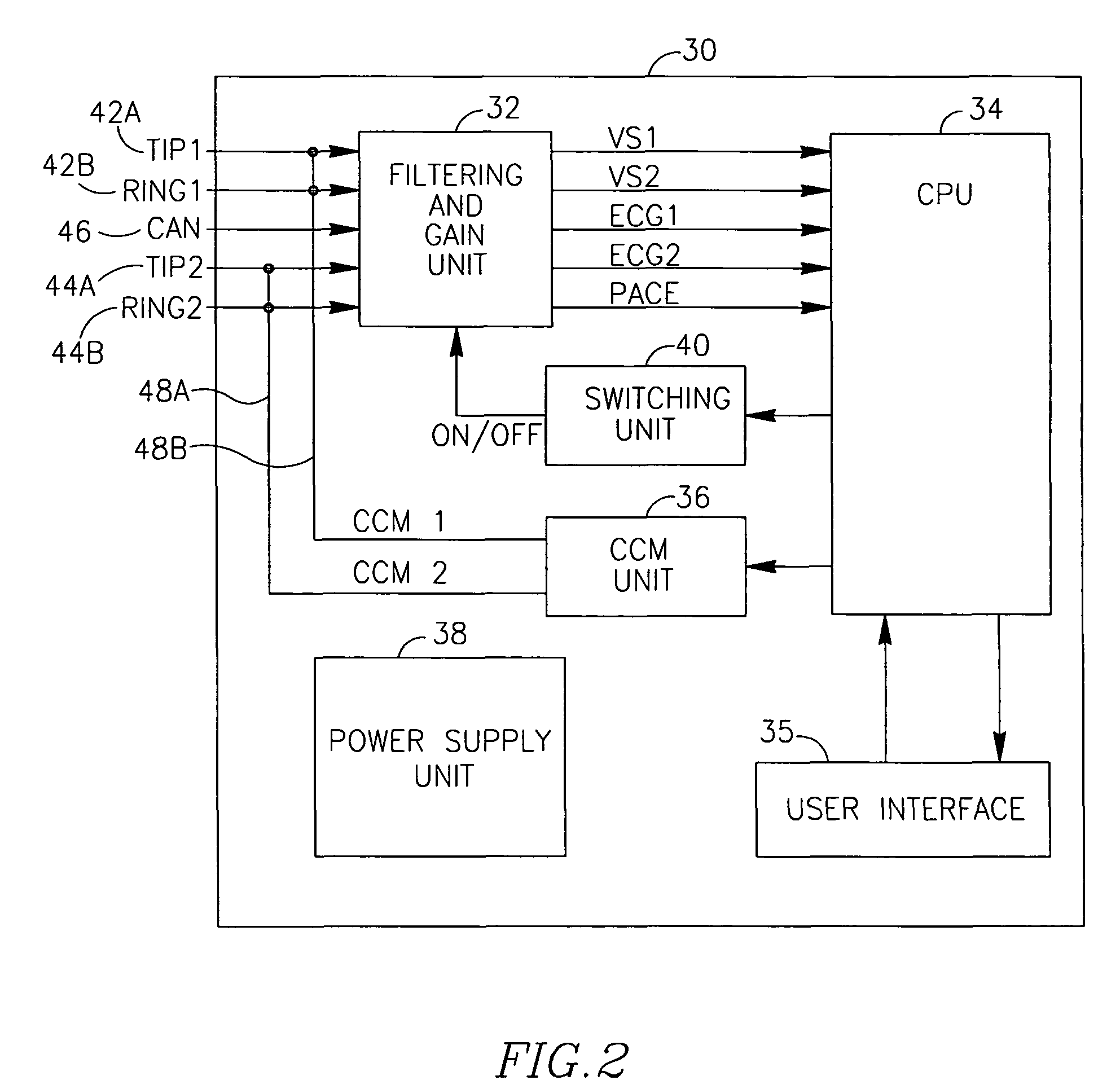
Apparatus and Method for Delivering Electrical Signals to a Heart
ActiveUS20090099618A1Increased effective sensing rangeLong distanceElectrocardiographyHeart stimulatorsEcg signalVentricular dysrhythmia
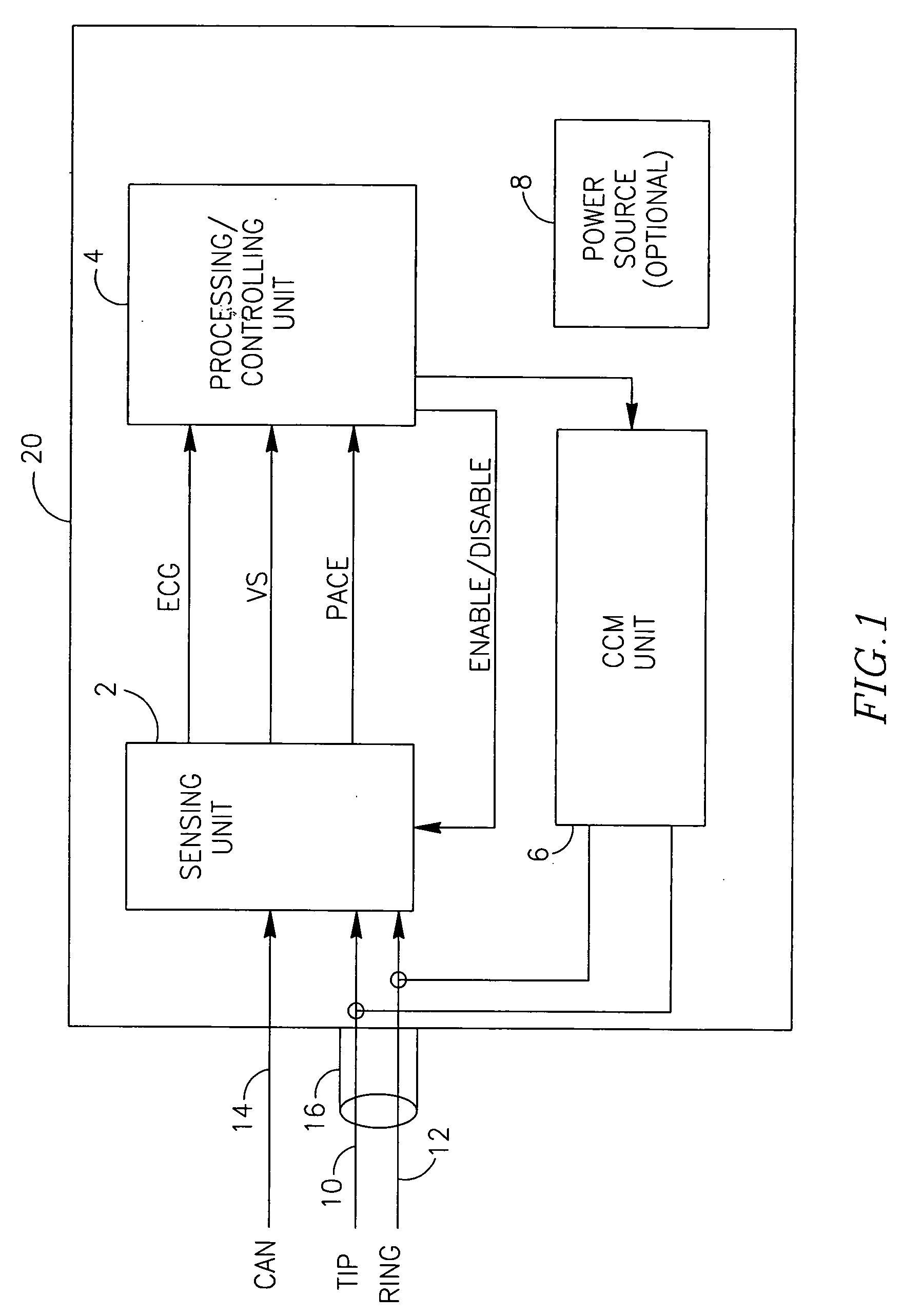
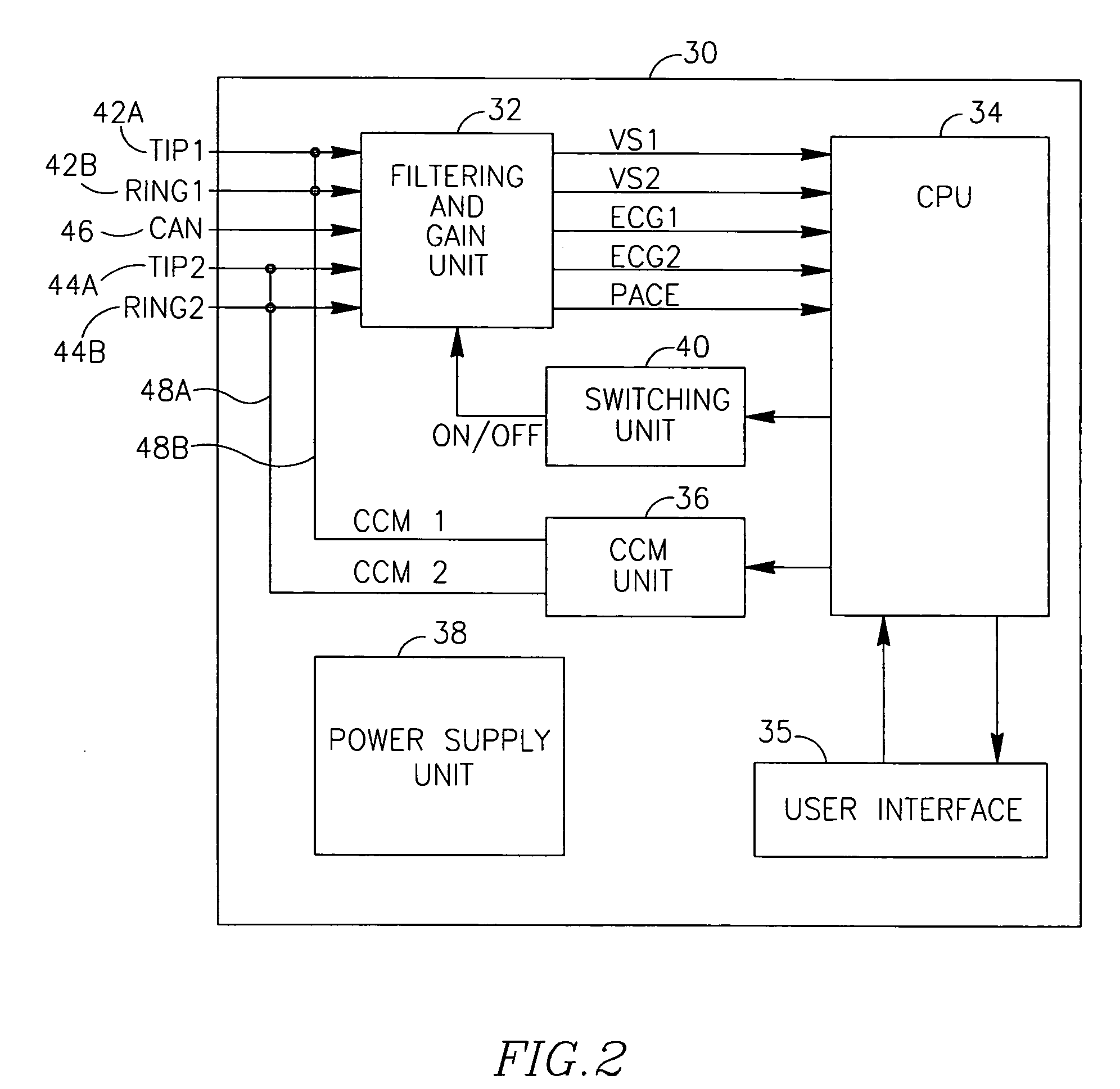
Devices, systems and methods for controlling (inhibiting or enabling) the delivery of electrotherapeutic signals to a heart using sensing of local and / or global ECG signals to detect ventricular arrhythmia or indication of possible ventricular arrhythmia in the heart. The devices, systems and methods process the sensed signals and are capable of delivering electroptherapeutic signals to the heart in the presence of a supra-ventricular arrhythmia such as atrial fibrillation and atrial flutter, while inhibiting the delivering electroptherapeutic signals in the presence of PVCs and / or extopic beats, and / or ventricular arrhythmia. The electrotherapeutic signals may include, among others, pacing signals and cardiac contractility modulating signals.
Owner:IMPULSE DYNAMICS NV
Curved ablation catheter
A curved ablation catheter imparts ablative energy to target tissue, for example, along a trabecular slope, e.g., in the right atrium along the isthmus between the ostium of the inferior vena cava and the tricuspid valve. The catheter is formed with a preset curvature that, when deployed, both translates linearly and increases in radius to aid in the formation of spot or continuous linear lesions. A method of treating atrial flutter employs the curved ablation catheter.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
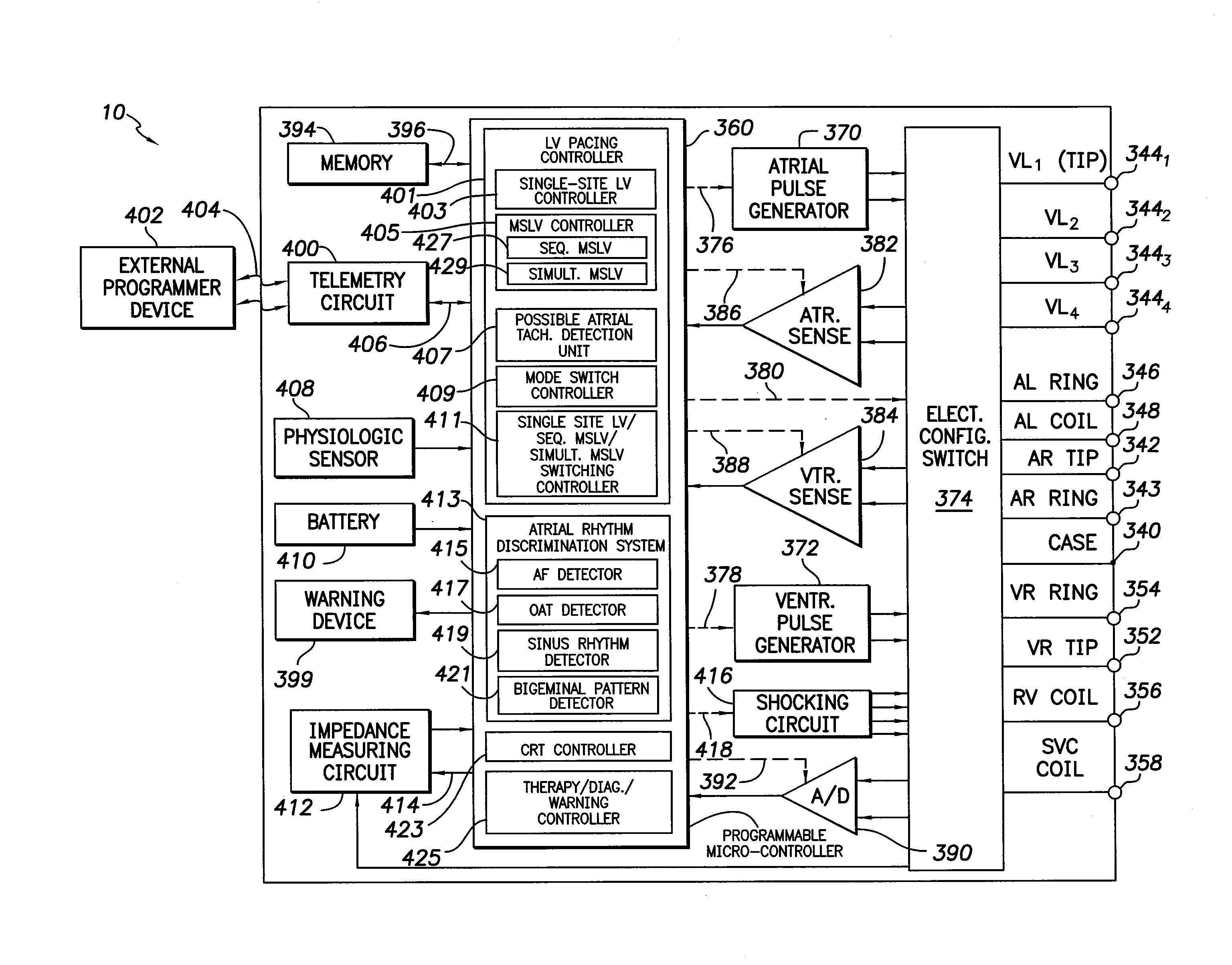
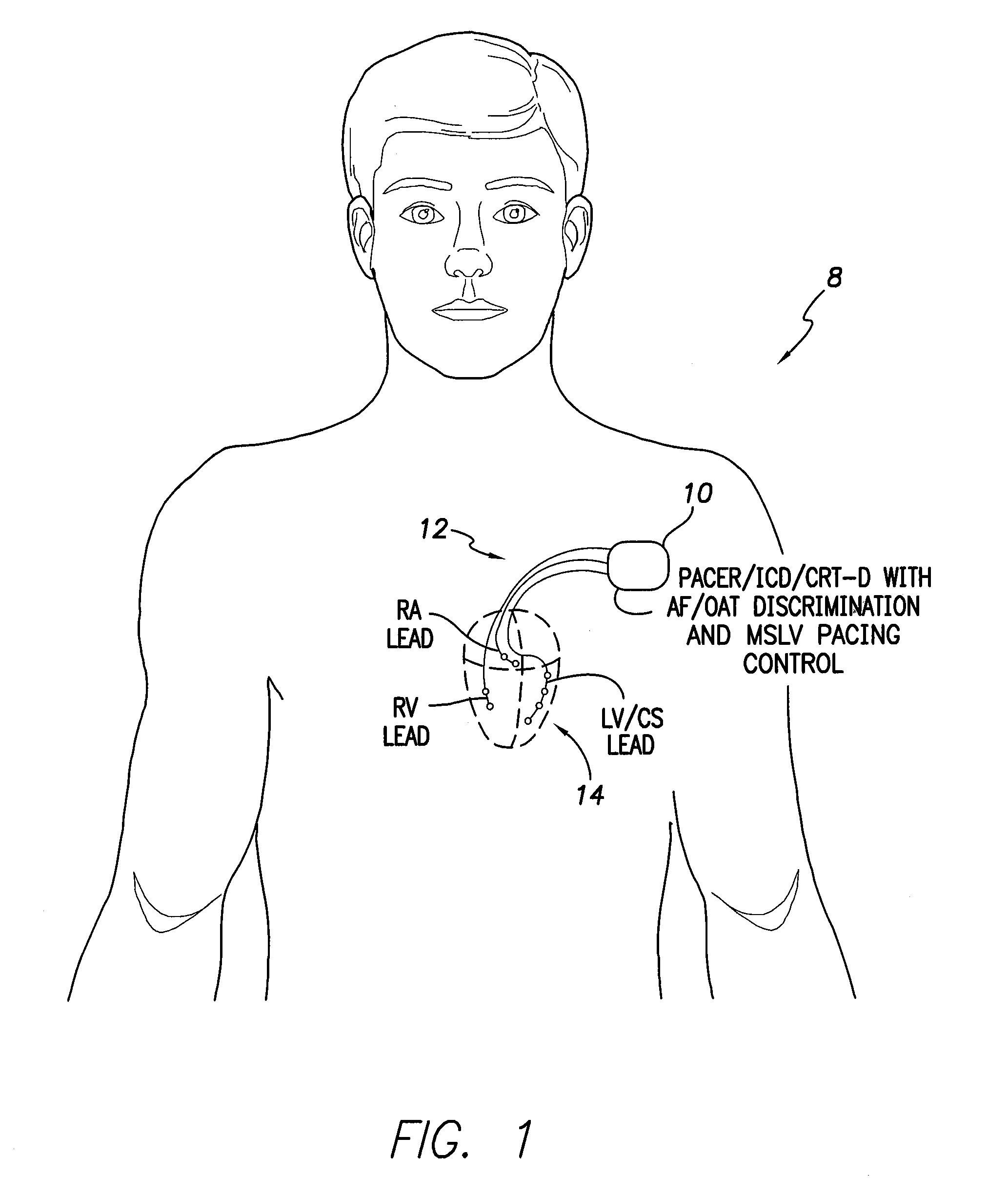
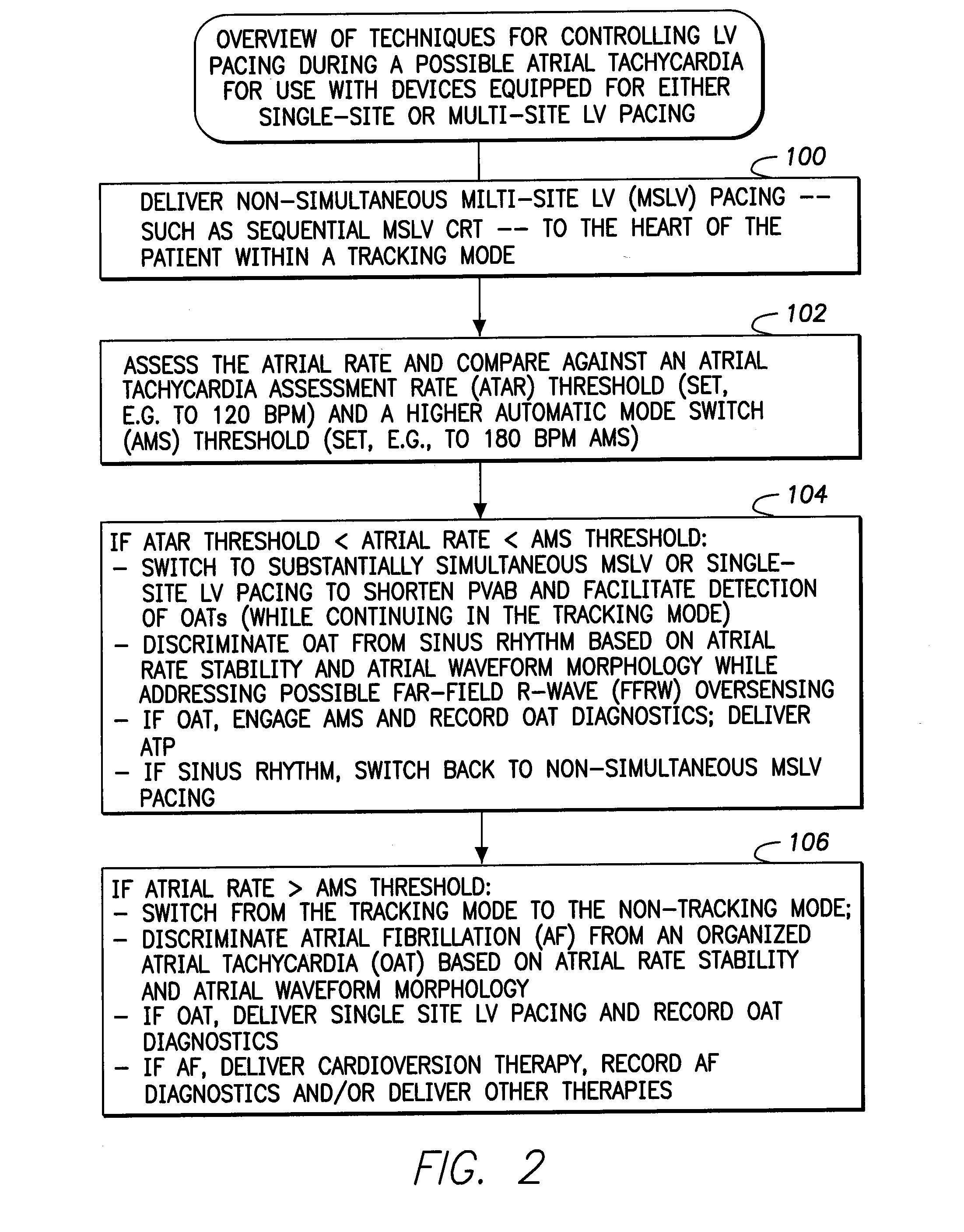
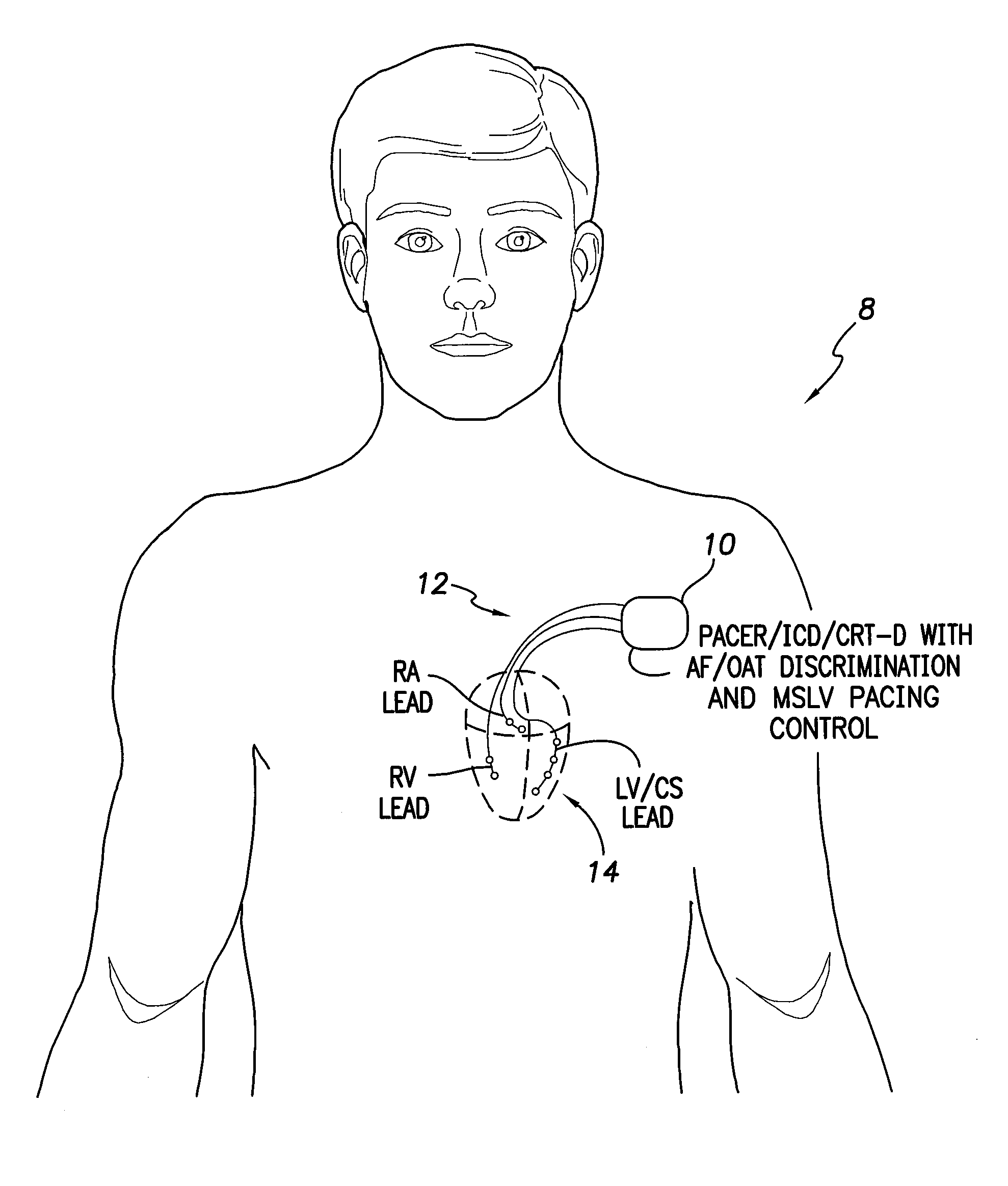
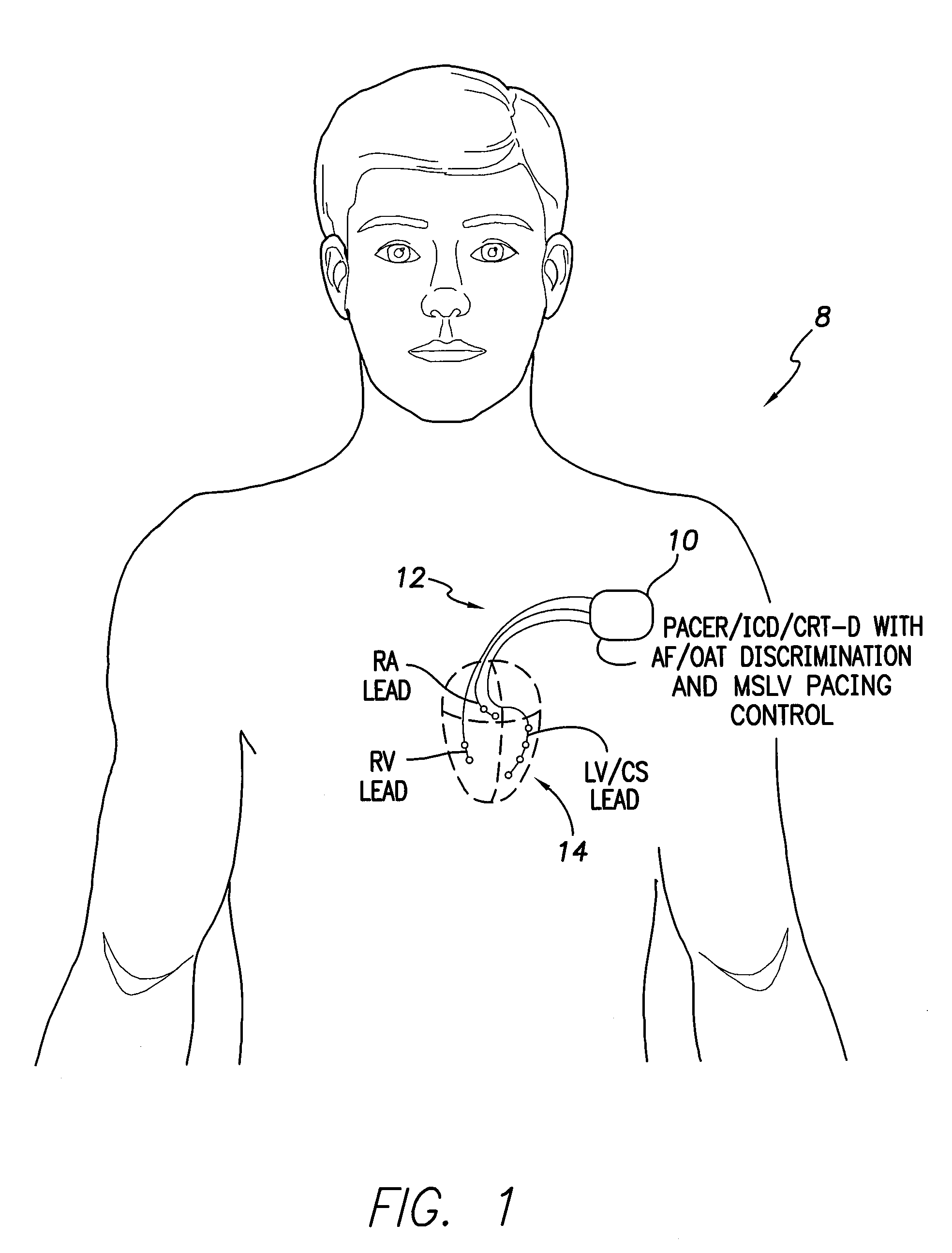
Systems and methods for use by an implantable medical device for controlling multi-site CRT pacing in the presence of atrial tachycardia
Owner:PACESETTER INC
Distinguishing sinus tachycardia from atrial fibrillation and atrial flutter through analysis of atrial channel wavelet transforms
An implantable heart-monitoring device designed to distinguish two differing heart rhythms. The device comprises electrodes, a template, and first, second and third electronic mechanisms. The first electronic mechanism converts electrical representations of heartbeats from the electrodes into digital data and performs calculations including at least a partial discrete wavelet transform upon the digital data to generate a subset of discrete wavelet transform components including components for distinguishing the two differing heart rhythms and also demonstrated to be relatively low in variability from one patient to another. The template contains a corresponding subset of discrete wavelet transform components captured from at least one individual whose heart was beating in accordance with one of the two differing heart rhythms. The second electronic mechanism correlates the subset of transform components against the template components and provides a correlation value. The third electronic mechanism mathematically examines a time series of the correlation values and gives an indication of whether the first or second of the heart rhythms is present.
Owner:CARDIAC PACEMAKERS INC
Curved ablation catheter
ActiveUS20050267459A1Easy to operateImproved linear lesionCatheterDiagnostic recording/measuringVeinRight atrium
A curved ablation catheter imparts ablative energy to target tissue, for example, along a trabecular slope, e.g., in the right atrium along the isthmus between the ostium of the inferior vena cava and the tricuspid valve. The catheter is formed with a preset curvature that, when deployed, both translates linearly and increases in radius to aid in the formation of spot or continuous linear lesions. A method of treating atrial flutter employs the curved ablation catheter.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
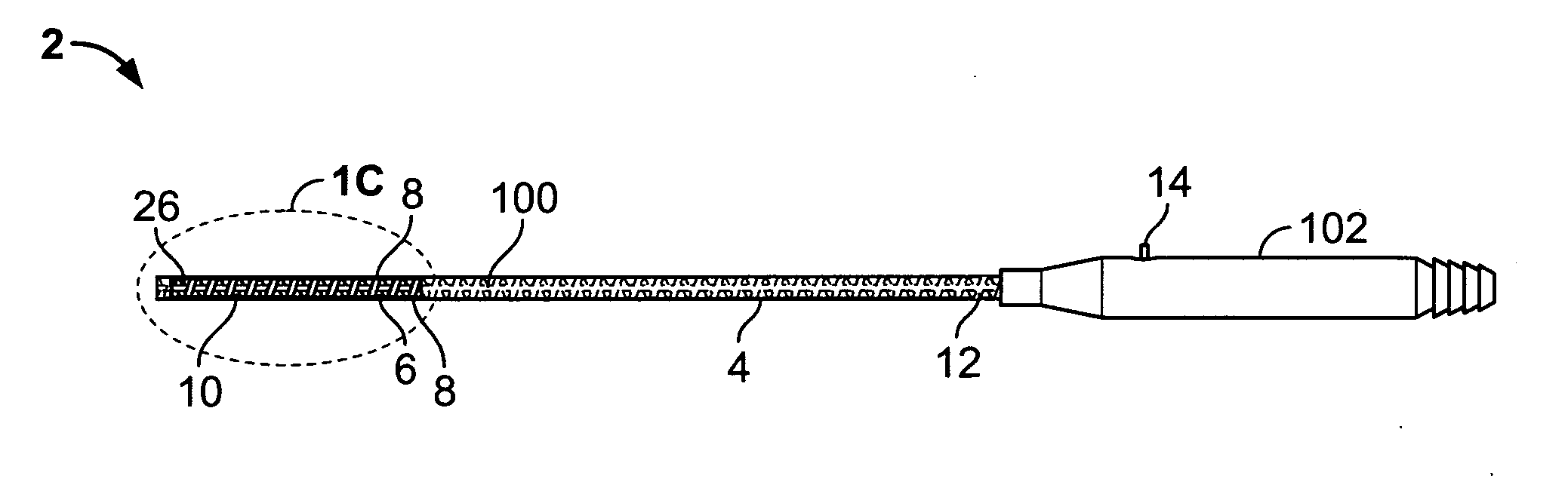
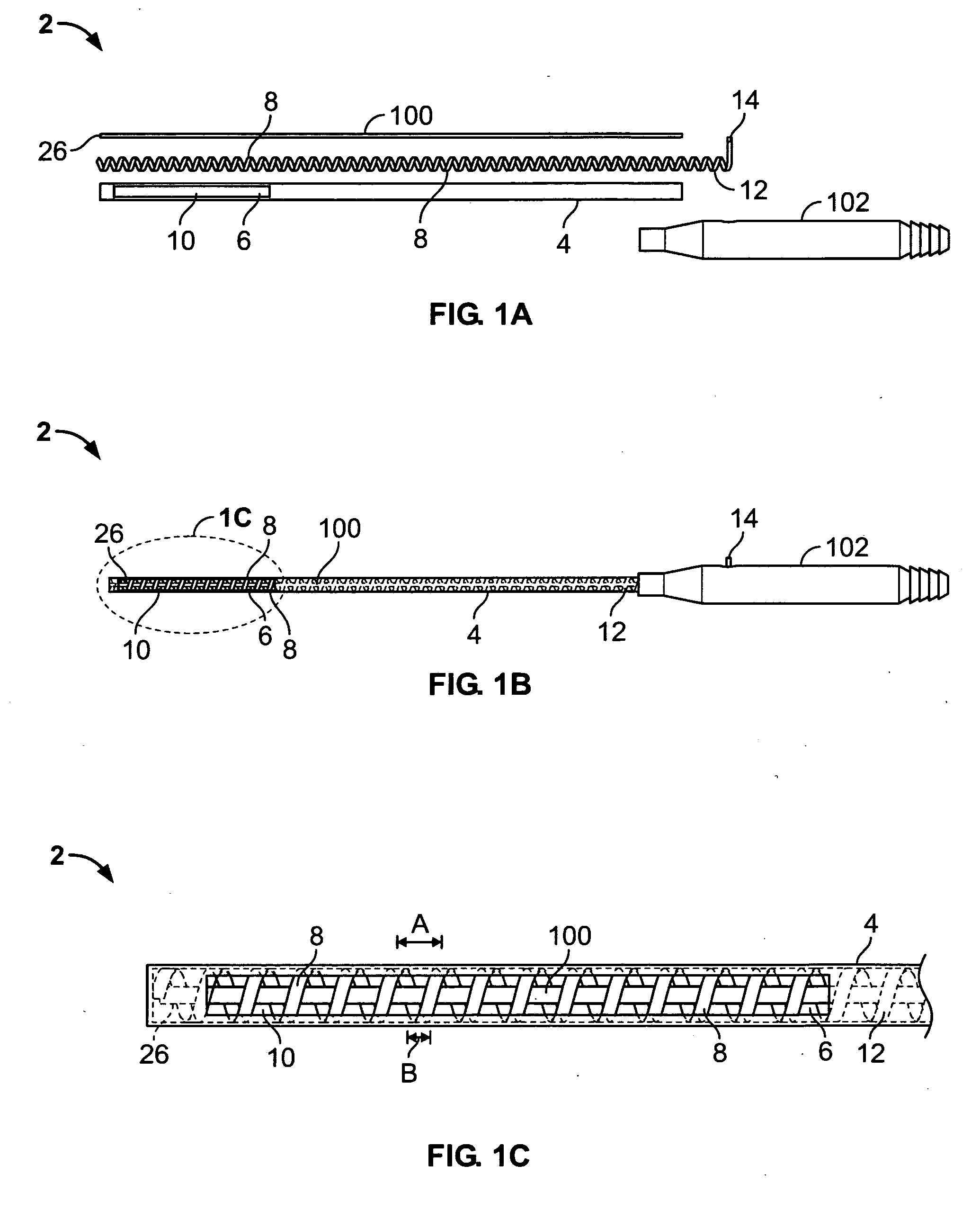
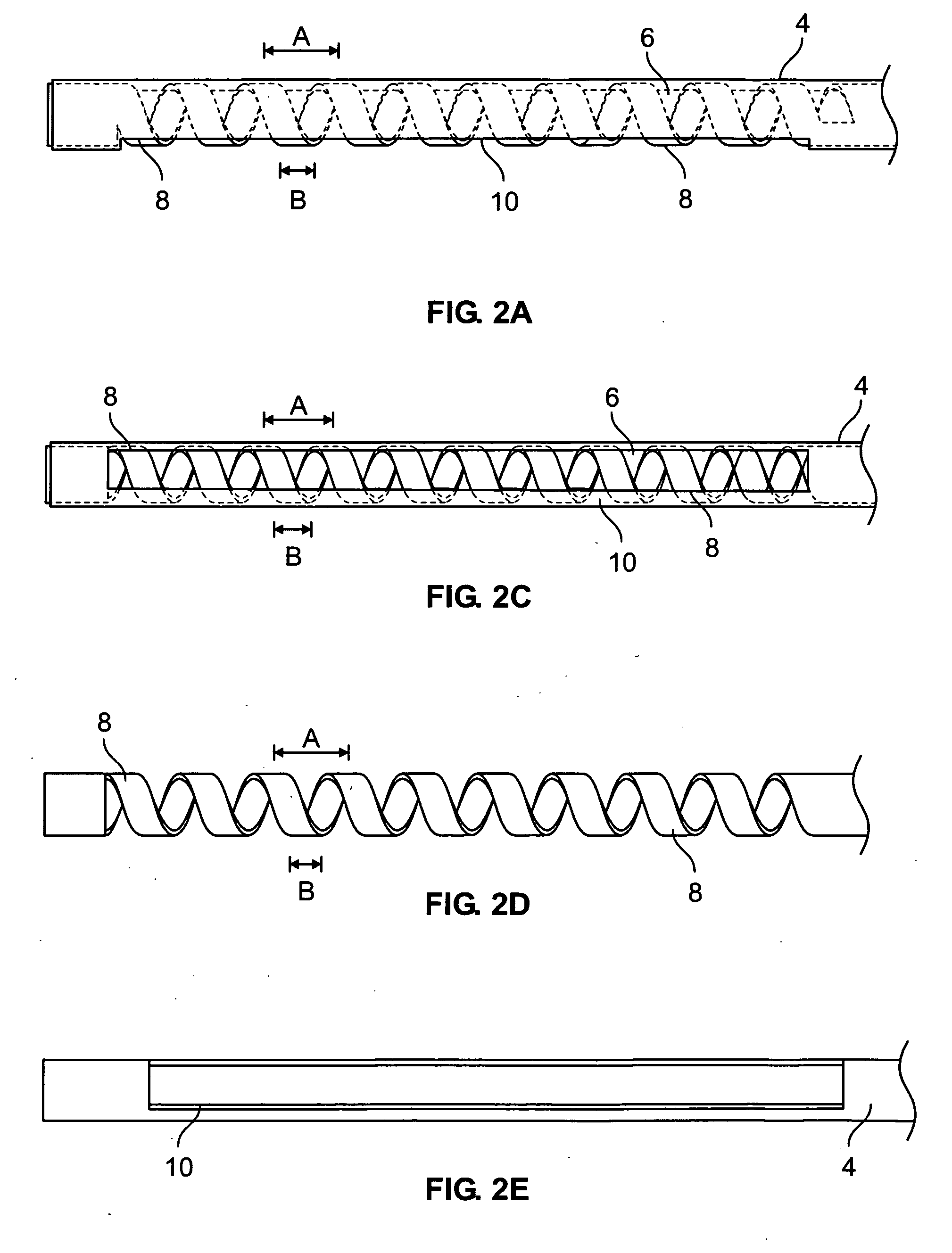
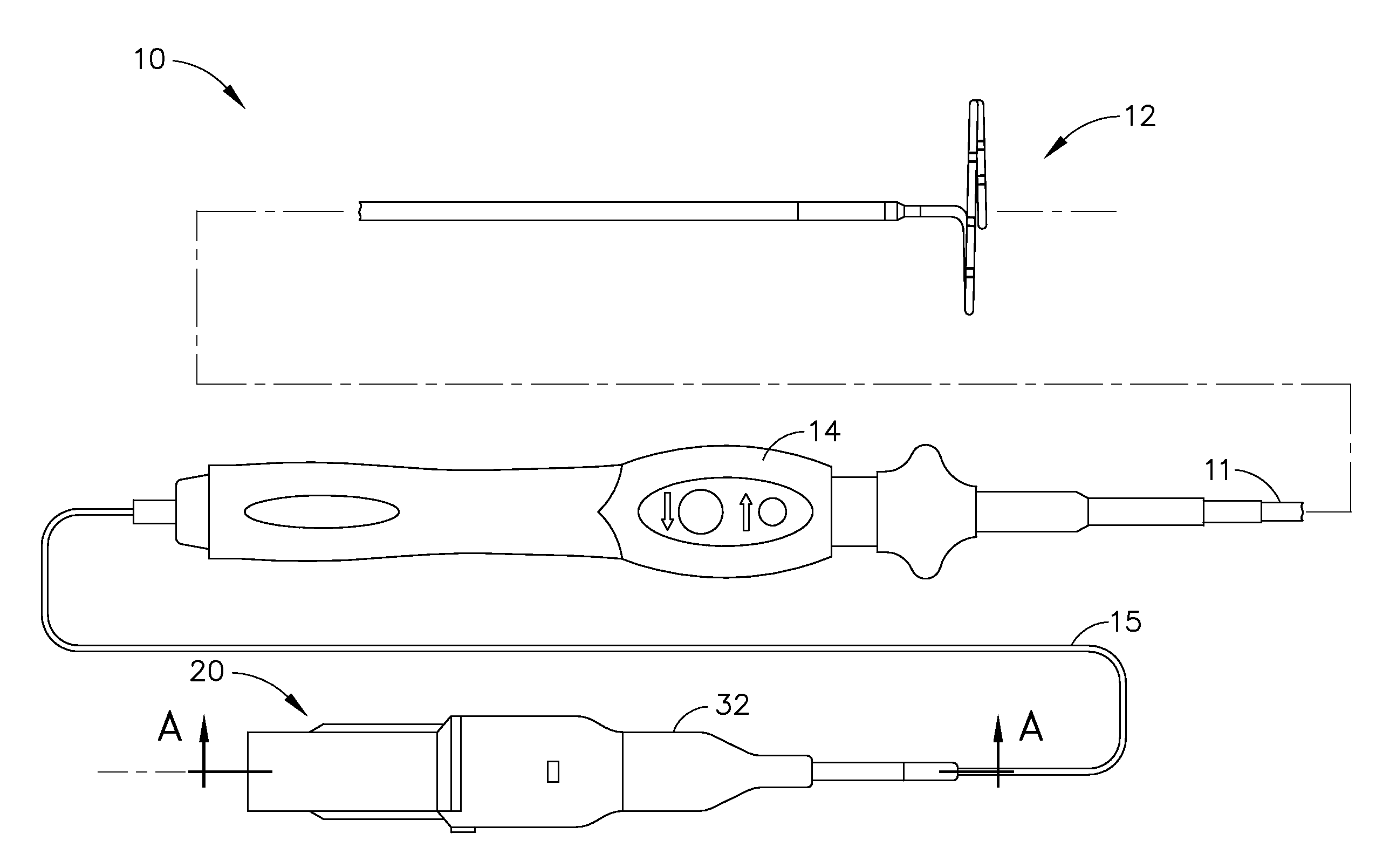
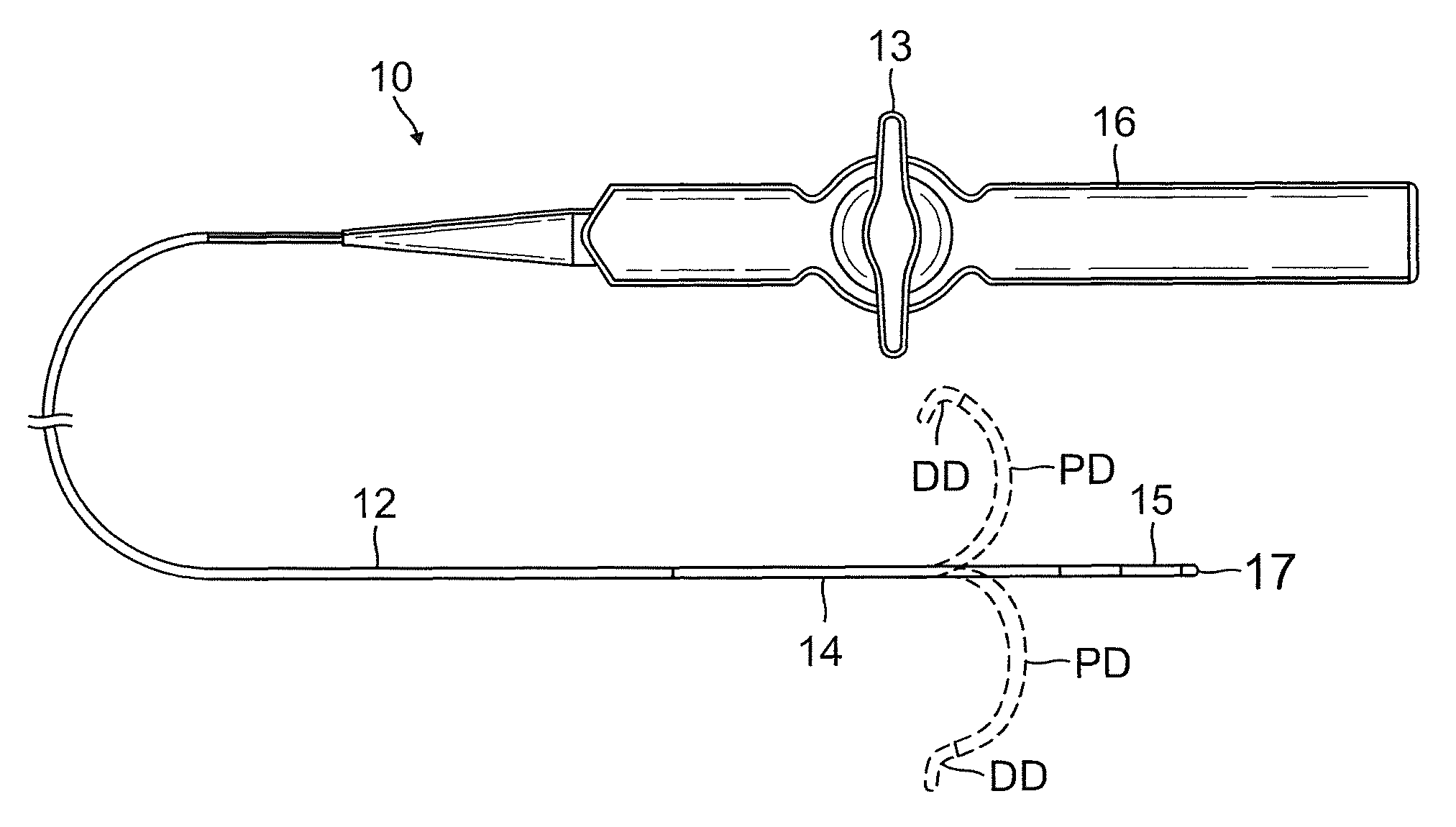
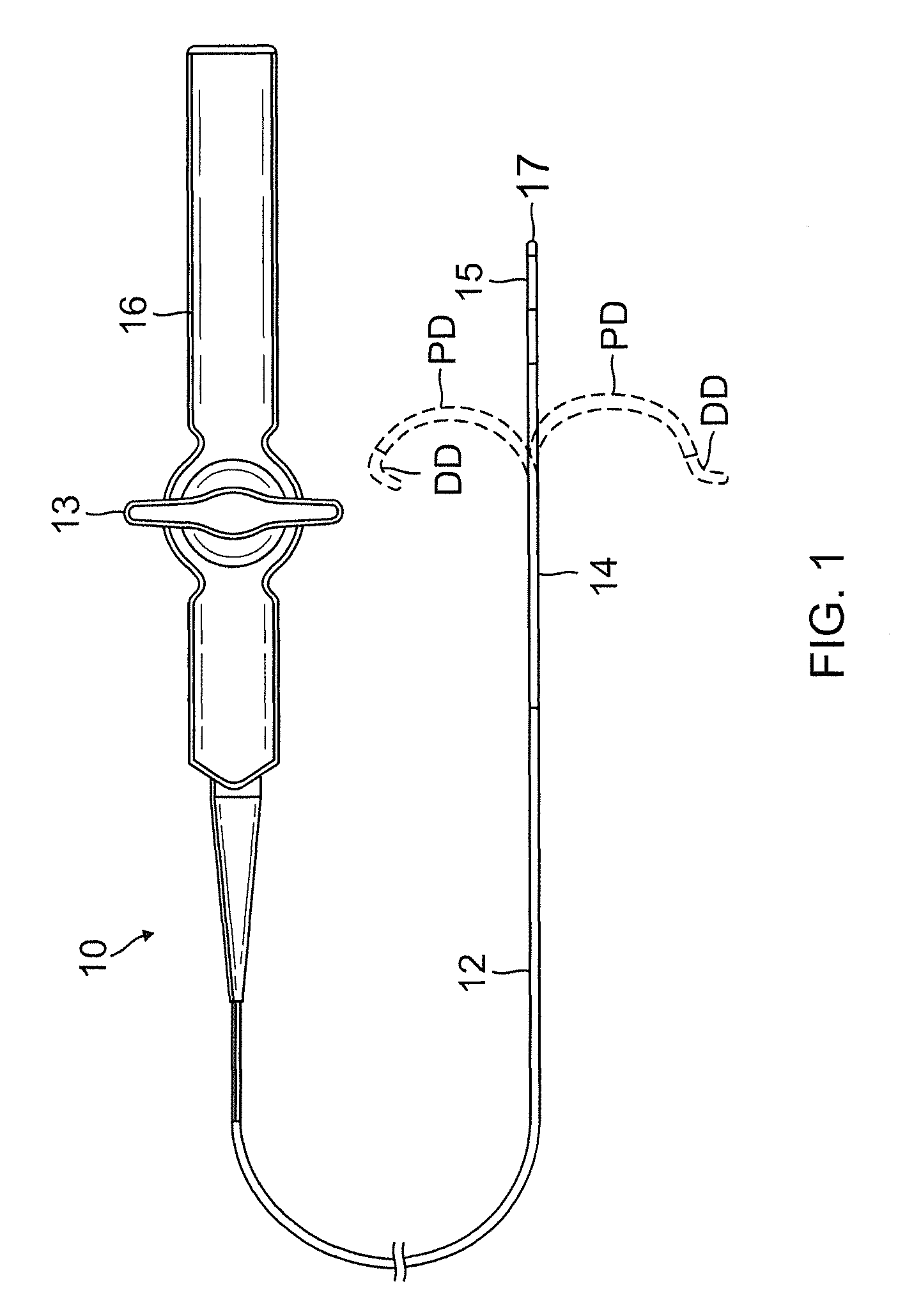
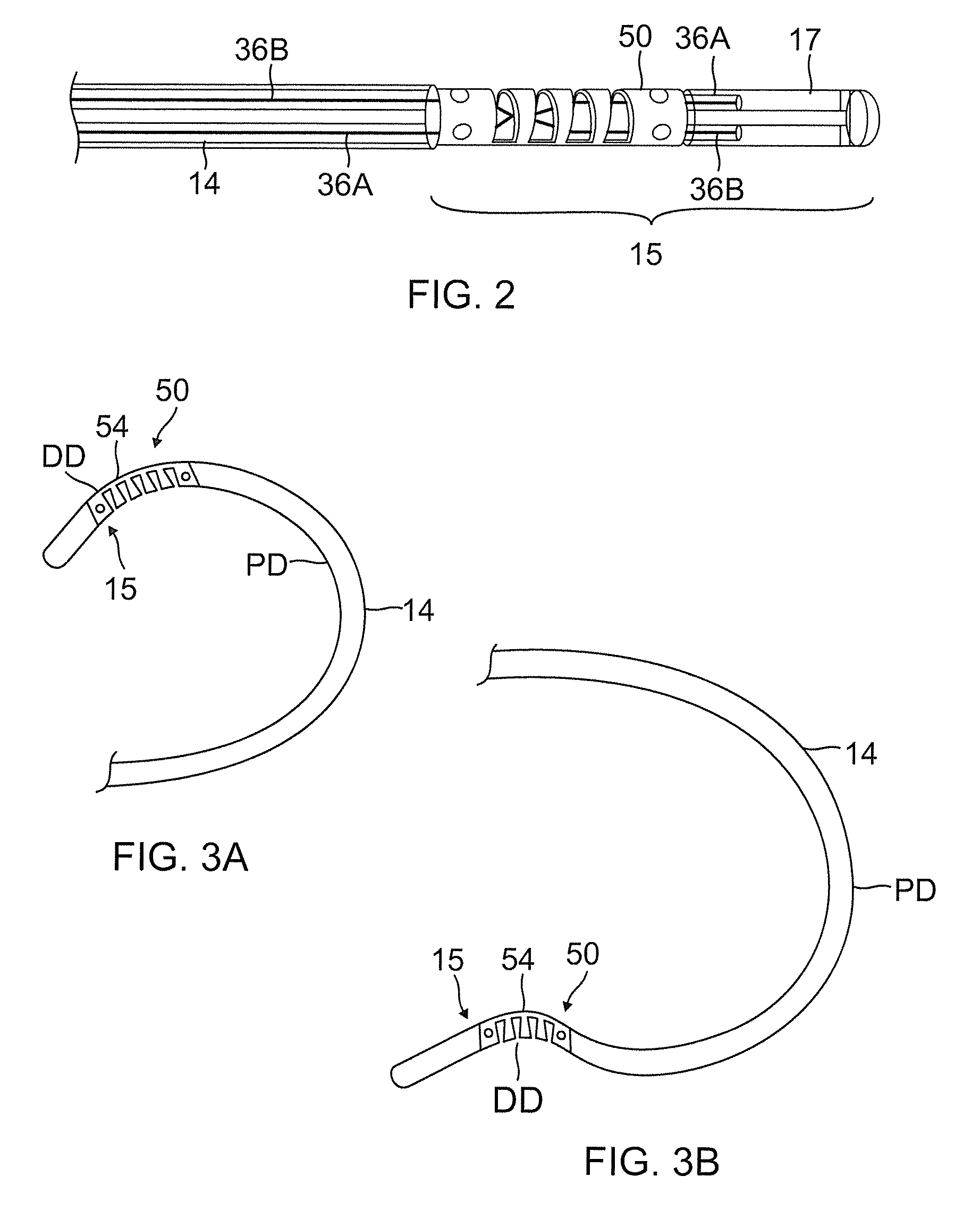
Catheter for treatment of atrial flutter having single action dual deflection mechanism
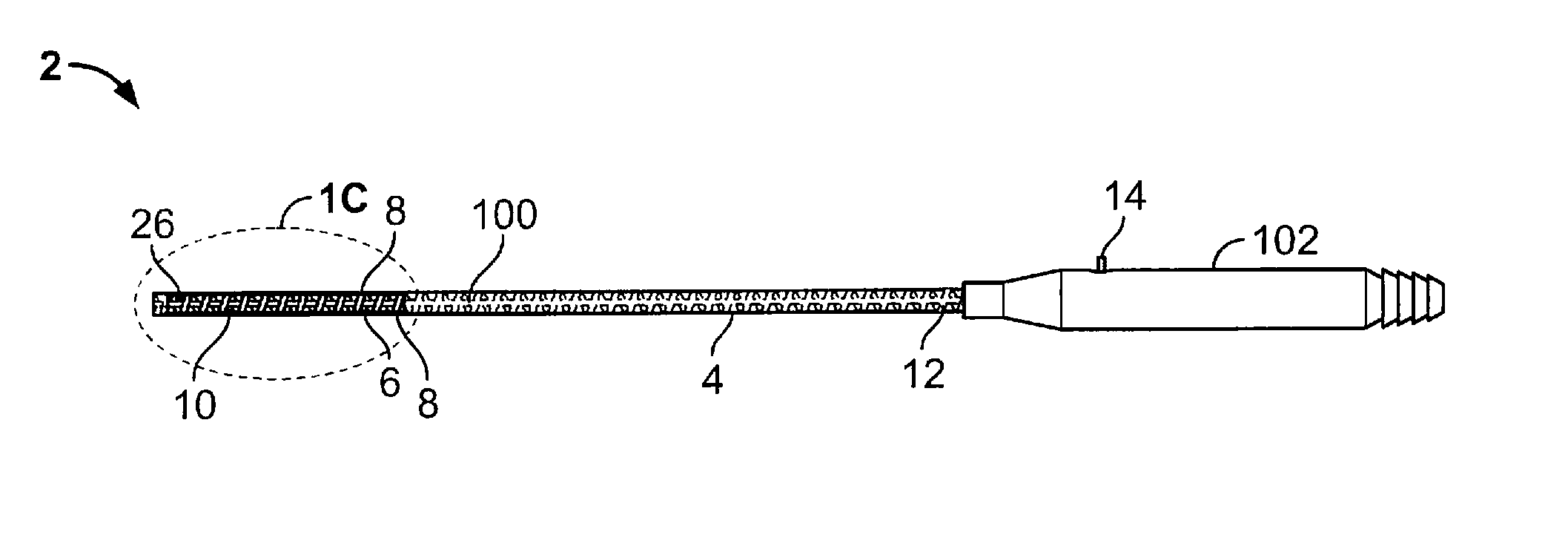
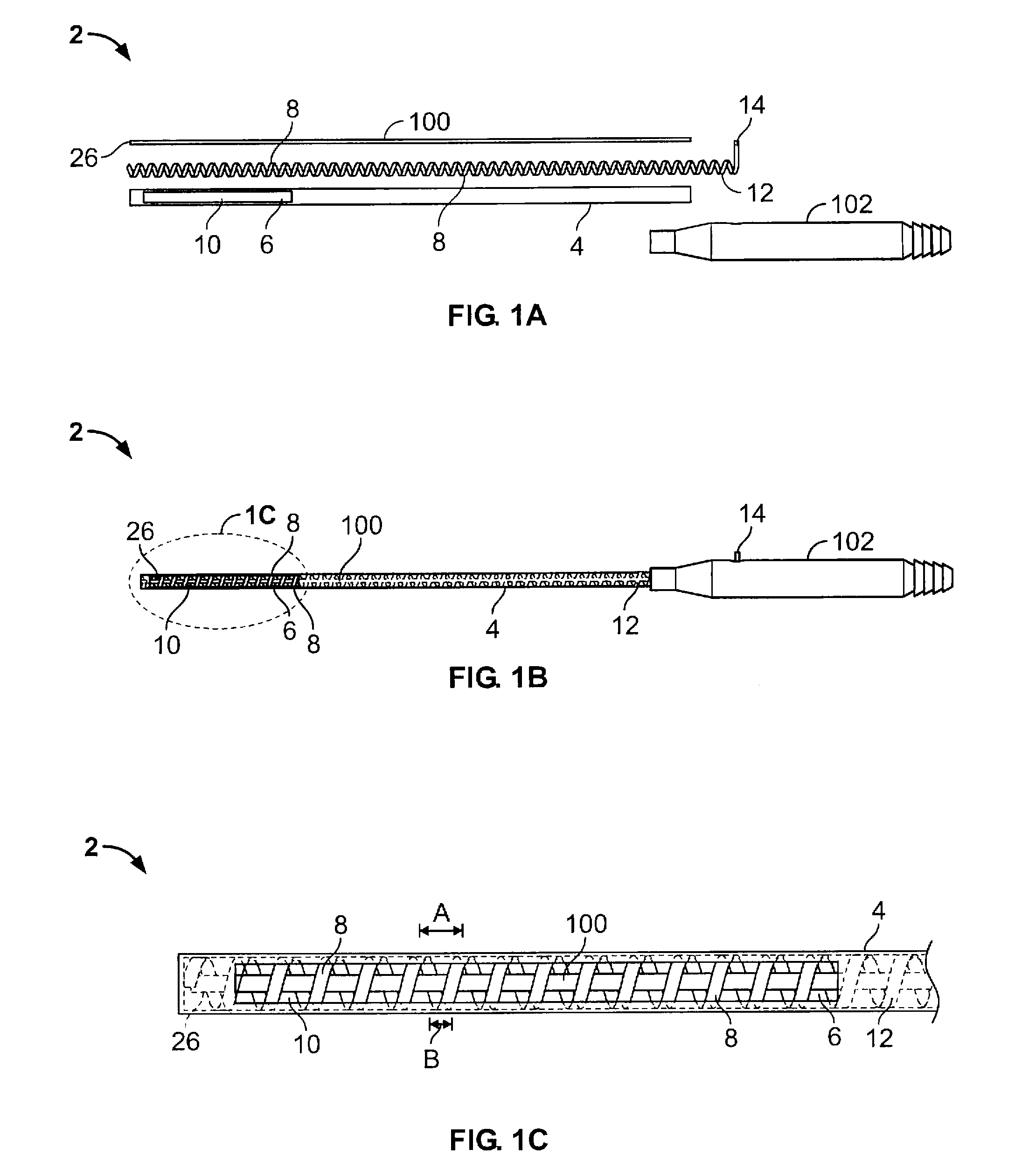
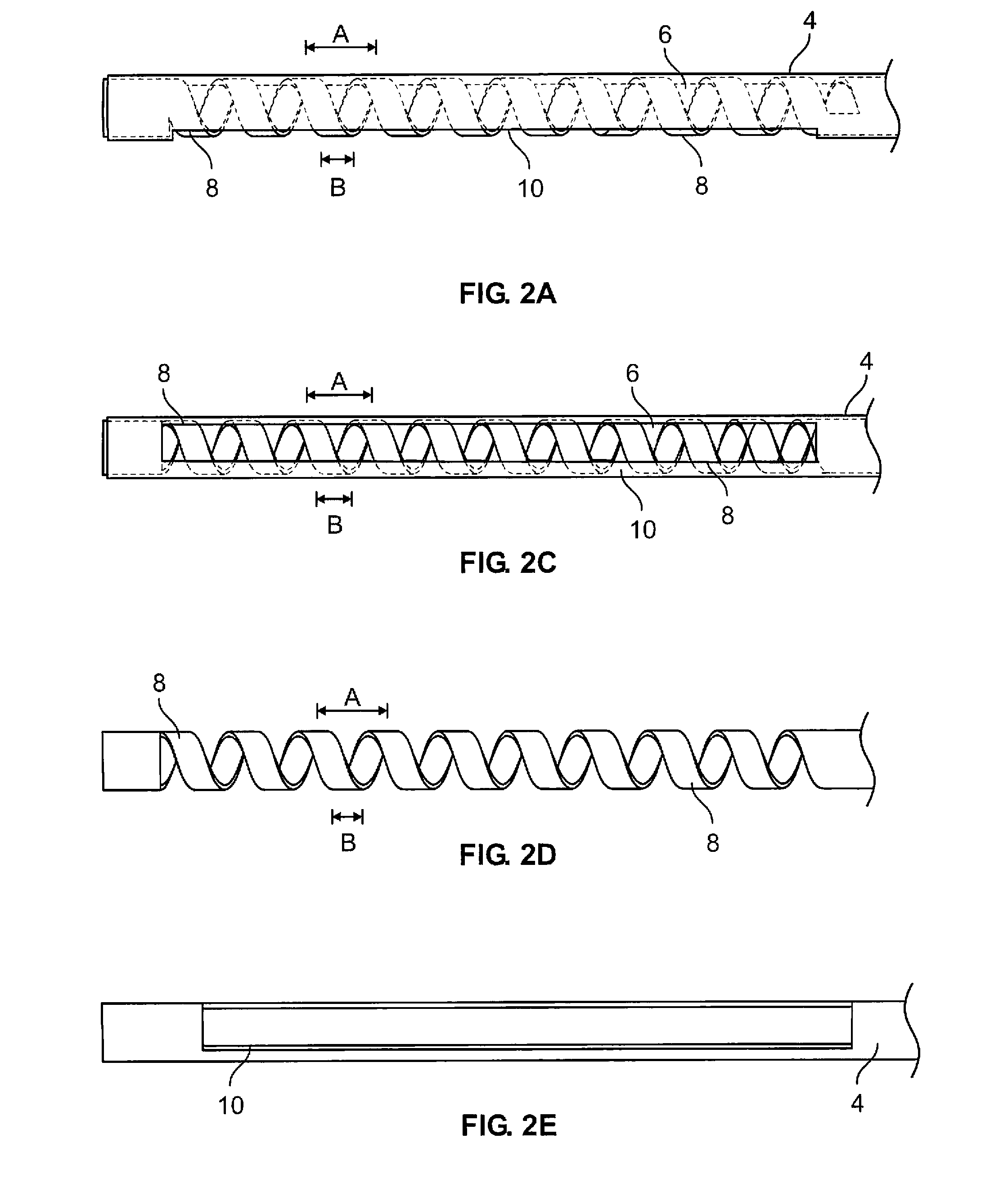
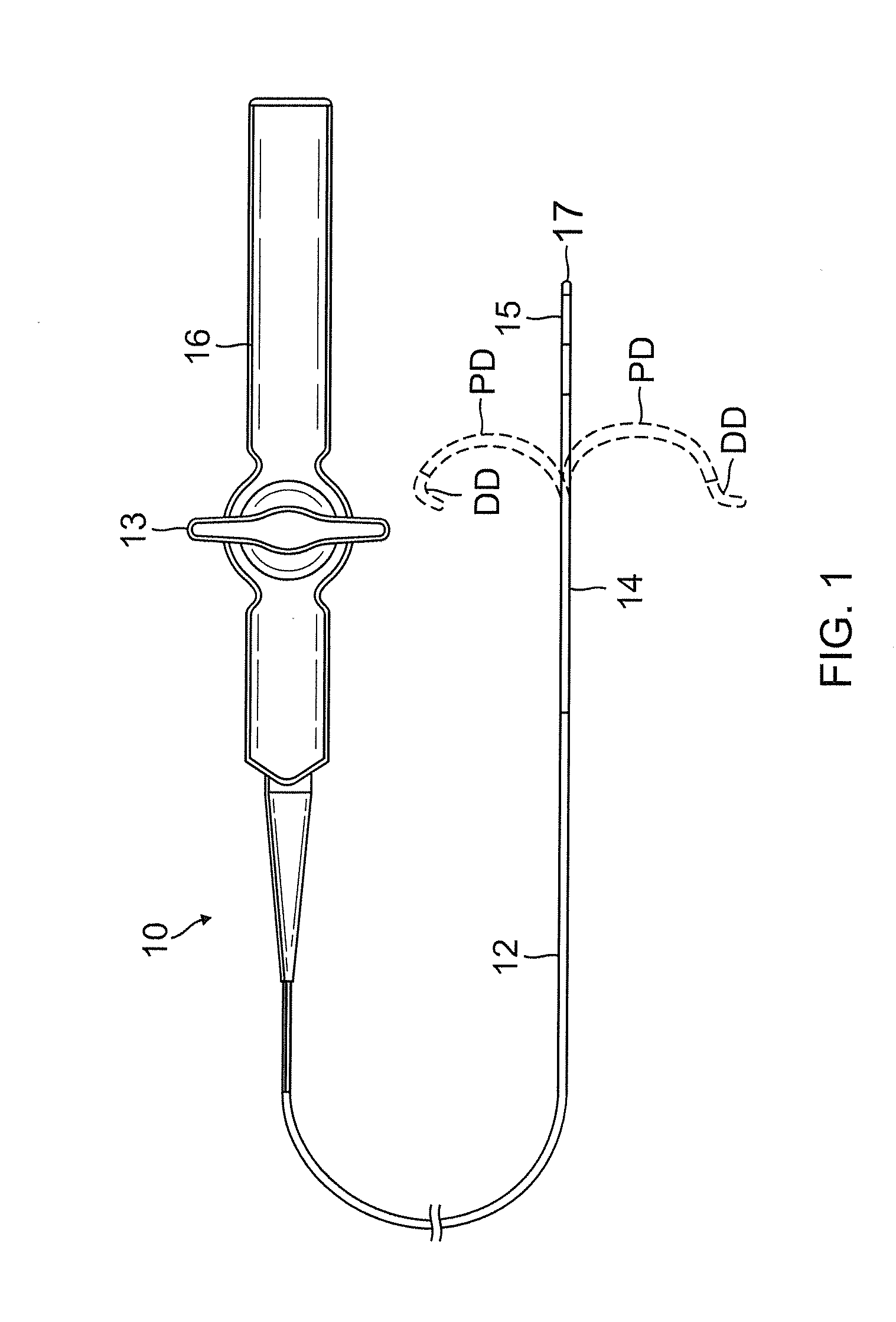
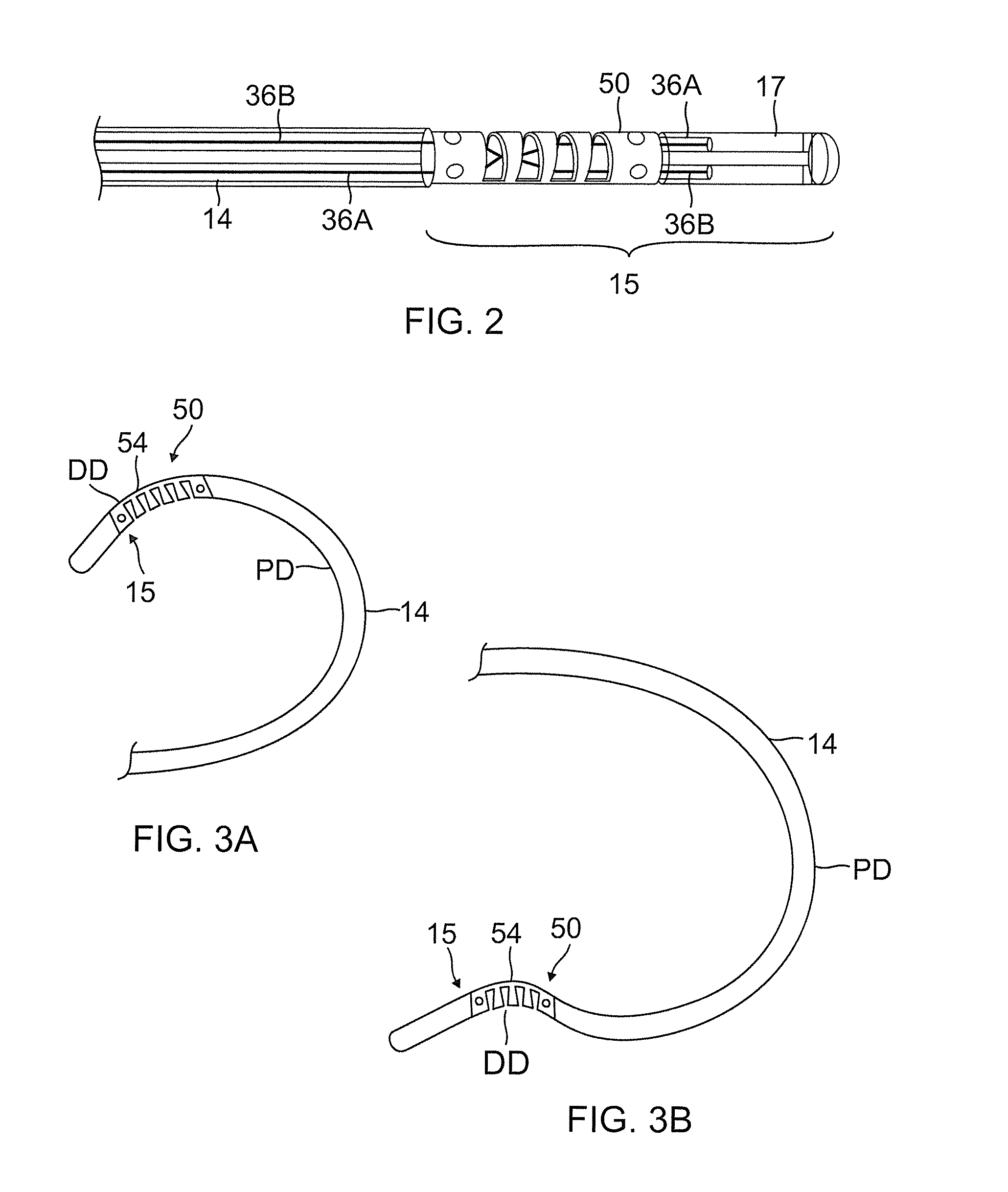
ActiveUS20130231657A1Quantity minimizationEasy constructionMulti-lumen catheterDiagnostic recording/measuringCatheterCardiac arrhythmia
A catheter and method for the treatment of a patient having atrial flutter or other arrhythmia comprises an elongated catheter body having an outer wall, proximal and distal ends, and at least one lumen extending therethrough. Further it has a distal tip section comprising a flexible tubing having a proximal end and a distal end and a plurality of lumens extending therethrough. The proximal end of the tip section is fixedly attached to the distal end of the catheter body. The tip section further comprises a nitinol tube having slots formed therein which causes the distal tip section to deflect using the same puller-wire action used to cause the deflectable catheter to deflect at a point proximal to the distal tip section.
Owner:BIOSENSE WEBSTER (ISRAEL) LTD
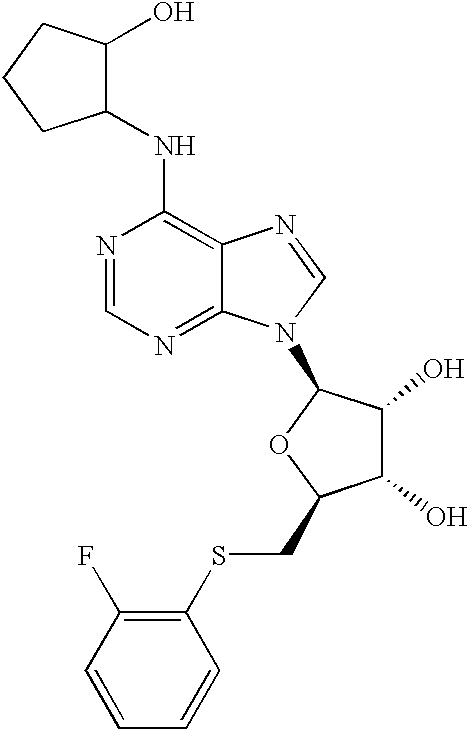
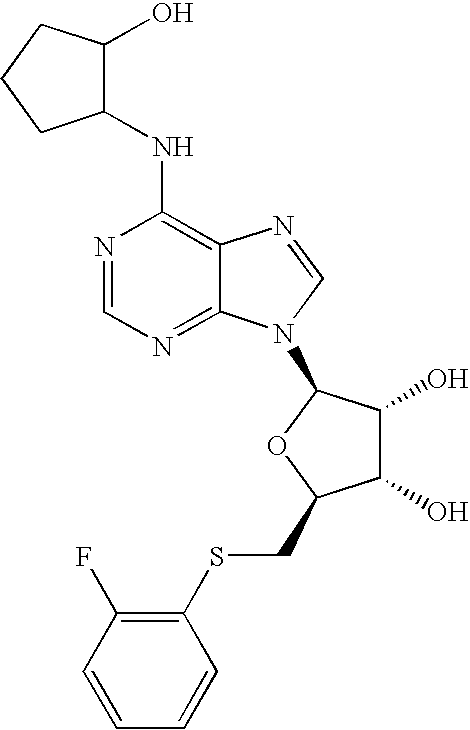
Partial and full agonists of A1 adenosine receptors
InactiveUS6946449B2Avoid actionMaintain liquidityBiocideSugar derivativesAnginaAdenosine A1 receptor
Disclosed are novel compounds that are partial and full A1 adenosine receptor agonists, useful for treating various disease states, in particular tachycardia and atrial flutter, angina, and myocardial infarction.
Owner:GILEAD SCI INC
Catheter having two-piece connector for a split handle assembly
InactiveUS20130296729A1Firmly connectedRelieving strain on wire connectionIncorrect coupling preventionCatheter deviceAtrial flutter
A catheter and method for the treatment of a patient having atrial flutter or other arrhythmia comprises an elongated catheter body having an outer wall, proximal and distal ends, and at least one lumen extending therethrough. Further it has a distal tip section comprising a flexible tubing having a proximal end and a distal end and a plurality of lumens extending therethrough. The proximal end of the tip section is fixedly attached to the distal end of the catheter body. The tip section further comprises a nitinol tube having slots formed therein which causes the distal tip section to deflect using the same puller-wire action used to cause the deflectable catheter to deflect at a point proximal to the distal tip section.
Owner:BIOSENSE WEBSTER (ISRAEL) LTD
Use of autonomic nervous system neurotransmitters inhibition and atrial parasympathetic fibers ablation for the treatment of atrial arrhythmias and to preserve drug effects
InactiveUSRE42961E1Increased occurrence of initiation of atrial flutterShorten the construction periodDiagnosticsHeart defibrillatorsNervous systemRight atrium
Atrial arrhythmias, a major contributor to cardiovascular morbidity, are believed to be influenced by autonomic nervous system tone. The main purpose of this invention was to highlight new findings that have emerged in the study of effects of autonomic nervous system tone on atrial arrhythmias, and its interaction with class III antiarrhythmic drug effects. This invention evaluates the significance of sympathetic and parasympathetic activation by determining the effects of autonomic nervous system using a vagal and stellar ganglions stimulation, and by using autonomic nervous system neurotransmitters infusion (norepinephrine, acetylcholine). This invention evaluates the autonomic nervous system effects on the atrial effective refractory period duration and dispersion, atrial conduction velocity, atrial wavelength duration, excitable gap duration during a stable circuit (such atrial flutter circuit around an anatomical obstacle), and on the susceptibility of occurrence (initiation, maintenance and termination) of atrial re-entrant arrhythmias in canine. This invention also evaluates whether autonomic nervous system activation effects via a local neurotransimitters infusion into the right atria can alter those of class III antiarrhythmic drug, sotalol, during a sustained right atrial flutter. This invention represents an emergent need to set-up and develop a new class of anti-cholinergic drug therapy for the treatment of atrial arrhythmias and to combine this new anti-cholinergic class to antiarrhythmic drugs. Furthermore, this invention also highlights the importance of a local application of parasympathetic neurotransmitters / blockers and a catheter ablation of the area of right atrium with the highest density of parasympathetic fibers innervation. This may significantly reduce the occurrence of atrial arrhythmias and may preserve the antiarrhythmic effects of any drugs used for the treatment of atrial re-entrant arrhythmias.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Apparatus and method for delivering electrical signals to a heart
ActiveUS7966067B2Increased effective sensing rangeLong distanceElectrocardiographyHeart stimulatorsEcg signalVentricular dysrhythmia
Devices, systems and methods for controlling (inhibiting or enabling) the delivery of electrotherapeutic signals to a heart using sensing of local and / or global ECG signals to detect ventricular arrhythmia or indication of possible ventricular arrhythmia in the heart. The devices, systems and methods process the sensed signals and are capable of delivering electroptherapeutic signals to the heart in the presence of a supra-ventricular arrhythmia such as atrial fibrillation and atrial flutter, while inhibiting the delivering electroptherapeutic signals in the presence of PVCs and / or extopic beats, and / or ventricular arrhythmia. The electrotherapeutic signals may include, among others, pacing signals and cardiac contractility modulating signals.
Owner:IMPULSE DYNAMICS NV
Partial and full agonists of A1 adenosine receptors
Disclosed are novel compounds that are partial and full A1 adenosine receptor agonists, useful for treating various disease states, in particular tachycardia and atrial flutter, angina, and myocardial infarction.
Owner:GILEAD SCI INC
Method and apparatus for using atrial discrimination algorithms to determine optimal pacing therapy and therapy timing
A system and method which employs atrial discrimination algorithms to distinguish between different atrial arrhythmias occurring in a patient for selecting an optimal pacing therapy corresponding to the type of arrhythmia identified. In response to the detection of an atrial rate above the atrial tracking rate, discrimination criteria are applied to a detected atrial activity signal to distinguish between different types of supraventricular tachycardia, such as fast atrial flutter and other atrial flutter at a relatively slower rate, which may be occurring in the patient. The pacer is controlled to provide pacing therapy to a heart in a manner corresponding to the type of supraventricular tachycardia identified. The output of an atrial discrimination algorithm may be tracked and the trend thereof used to improve therapy timing. Various embodiments are disclosed herein.
Owner:CARDIAC PACEMAKERS INC
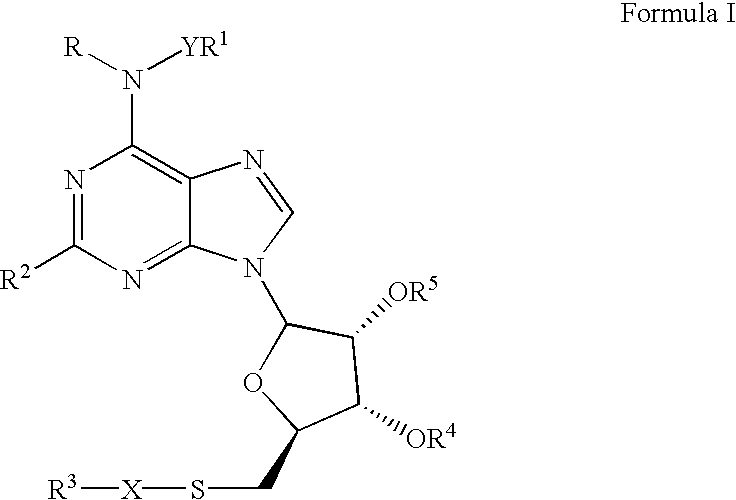
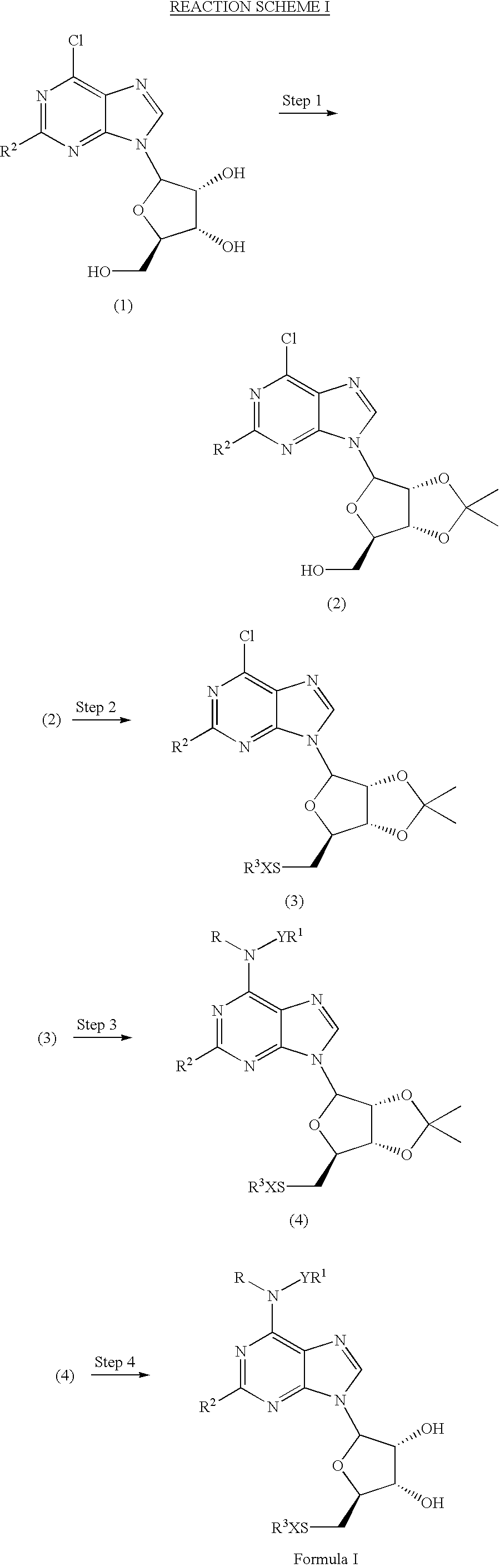
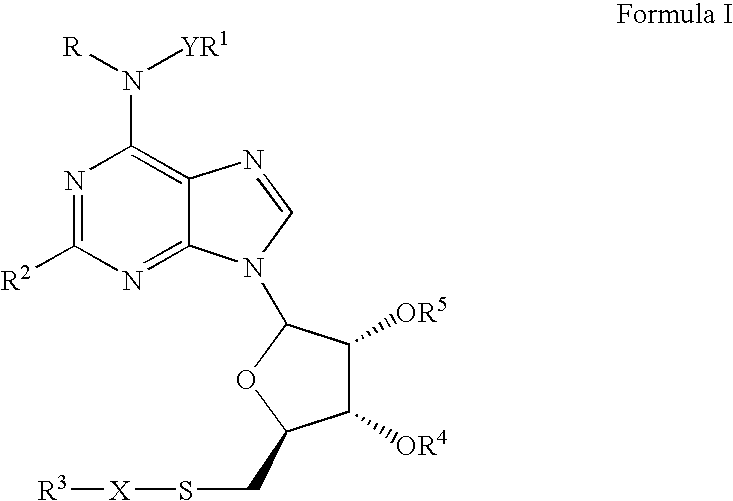
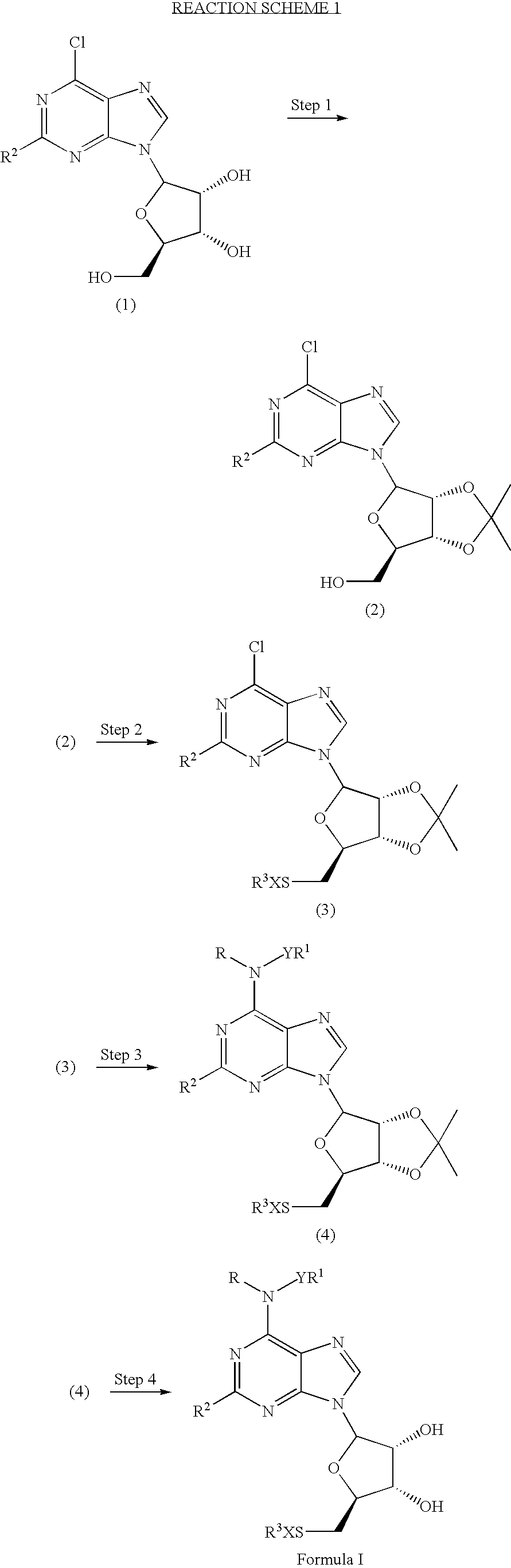
A1 adenosine receptor agonists
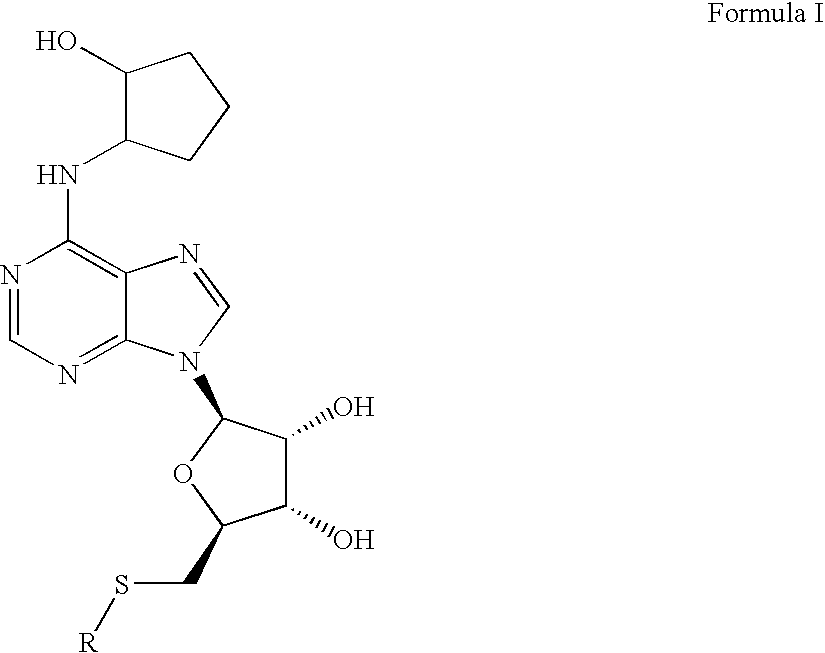
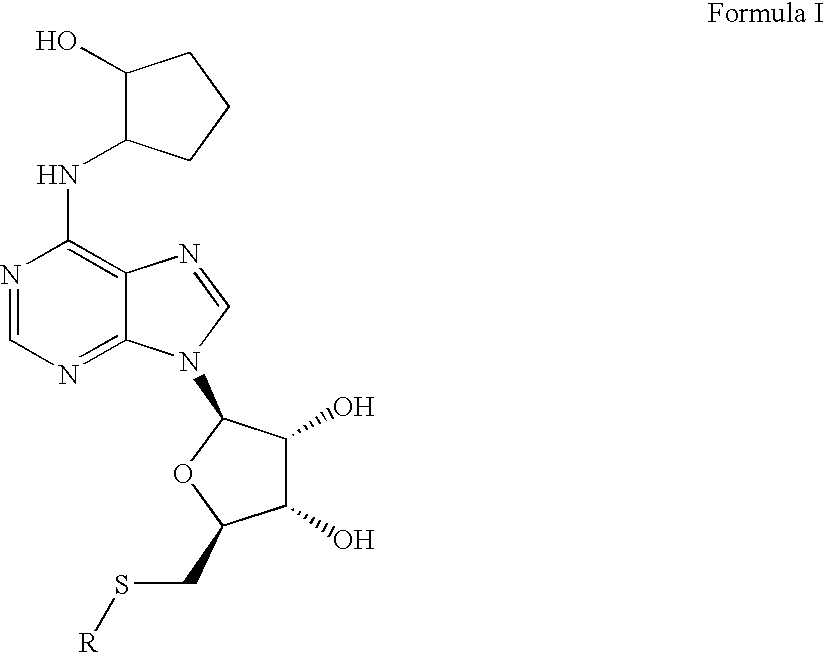
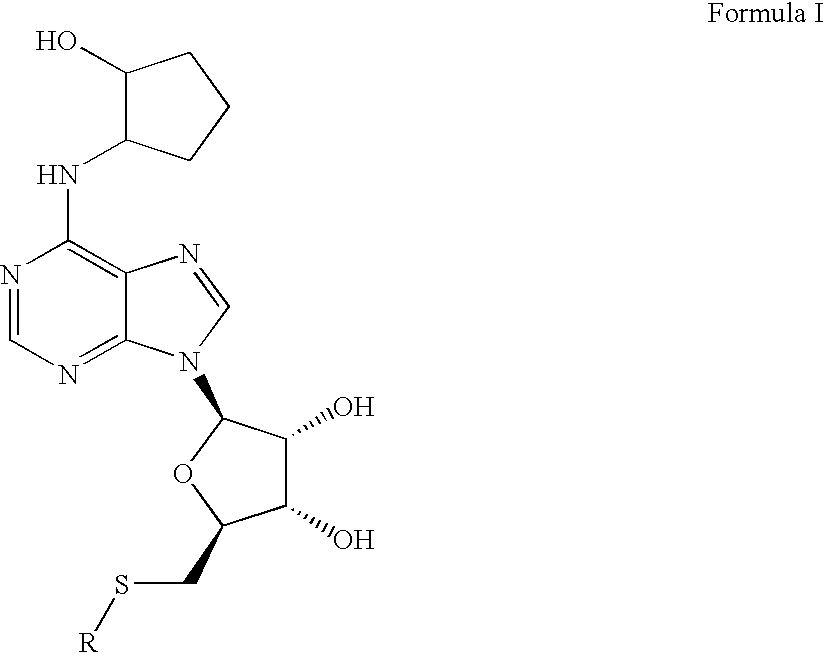
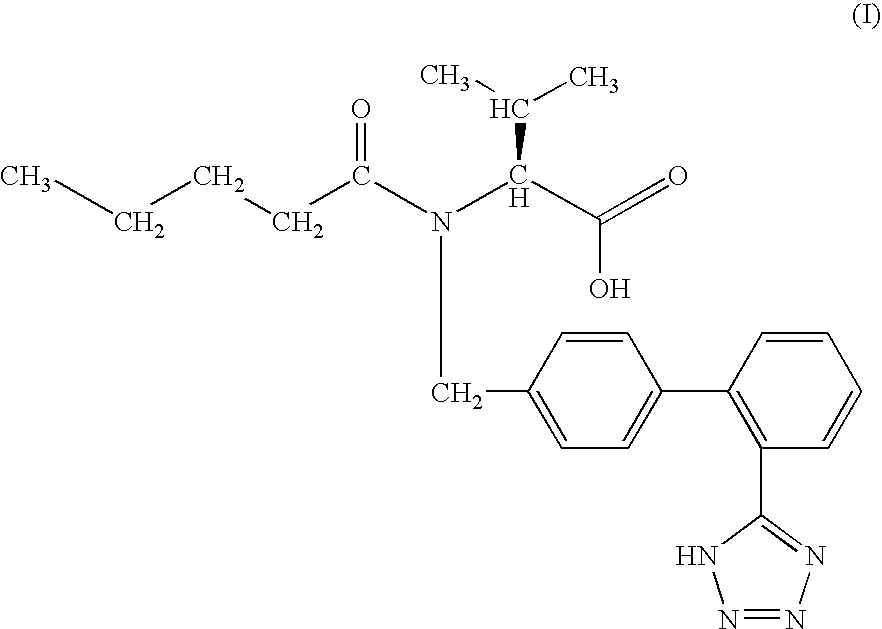
Disclosed is a synthesis suitable for large scale manufacture of novel compounds that are partial and full A1 adenosine receptor agonist having the structure of Formula I: wherein R is optionally substituted phenyl, that are useful for treating various disease states, in particular tachycardia and atrial flutter, angina, and myocardial infarction.
Owner:CV THERAPEUTICS INC
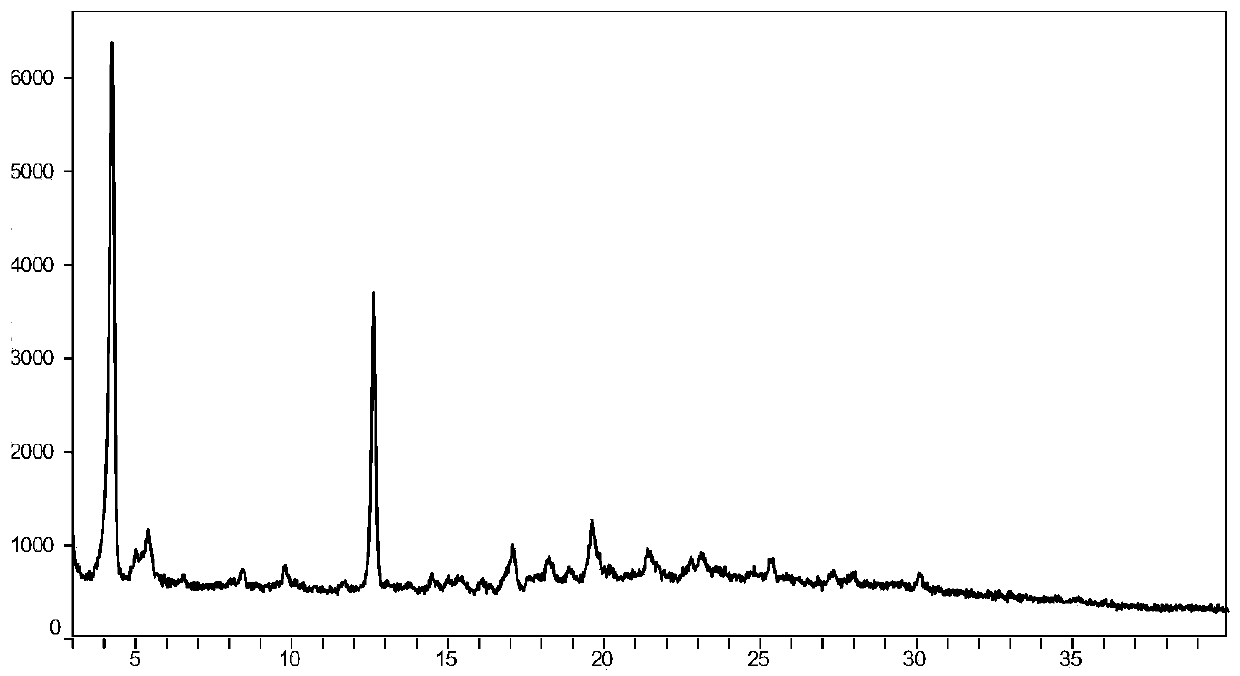
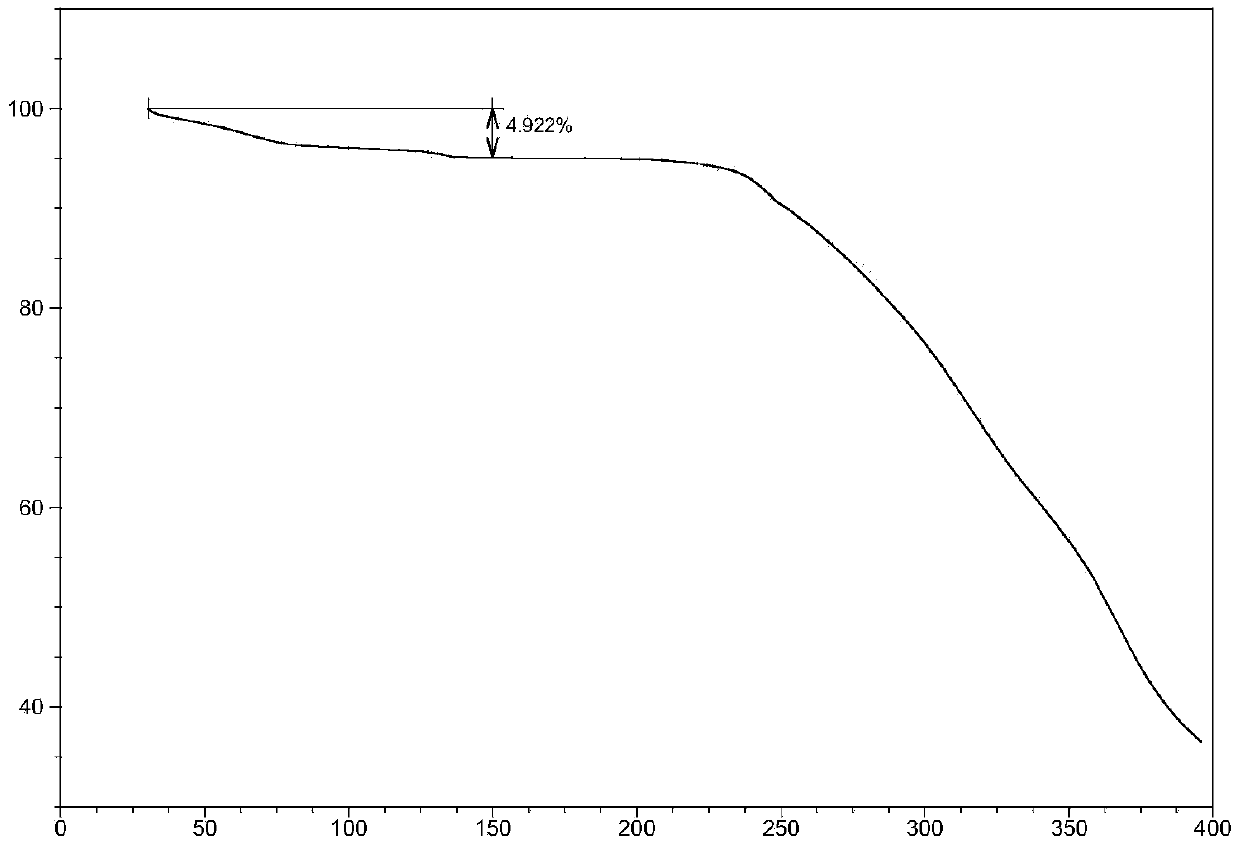
Crystalline ARB-NEPi dicationic compound and preparation method and application thereof
The present invention discloses a crystalline ARB-NEPi dicationic compound and a preparation method and application thereof. In particular, the present invention relates to the crystalline ARB-NEPi dicationic compound having the formula of NEPi.Nax.Ky.ARB.ZH2O. The present invention also relates to a pharmaceutical composition containing an effective amount of the crystalline dicationic compound and application of the pharmaceutical composition in the preparation of drugs for treatment or prevention of neutral-endopeptidase-related diseases, cardiovascular diseases, hypertension, acute and chronic heart failures, congestive heart failure, left ventricular dysfunction, hypertrophic cardiomyopathy, diabetic cardiomyopathy, supraventricular arrhythmia and ventricular arrhythmia, atrial fibrillation, atrial flutter or detrimental vascular remodeling, and the like, and the pharmaceutical composition has broad application prospects.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
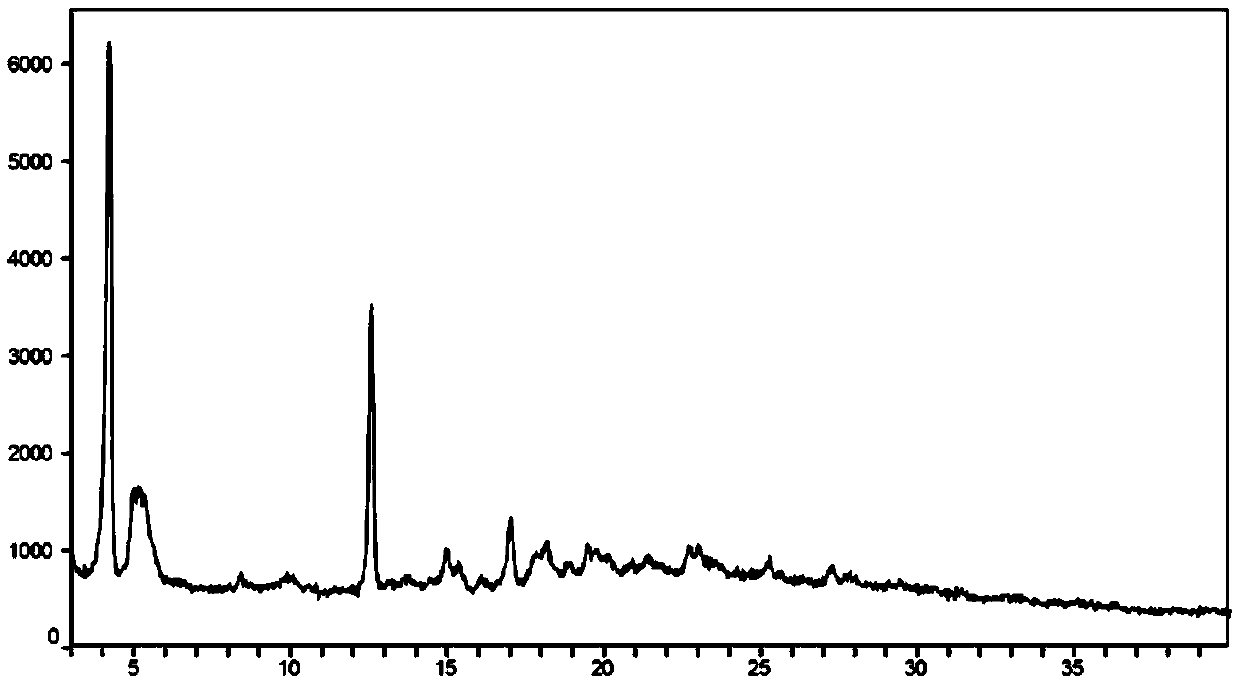
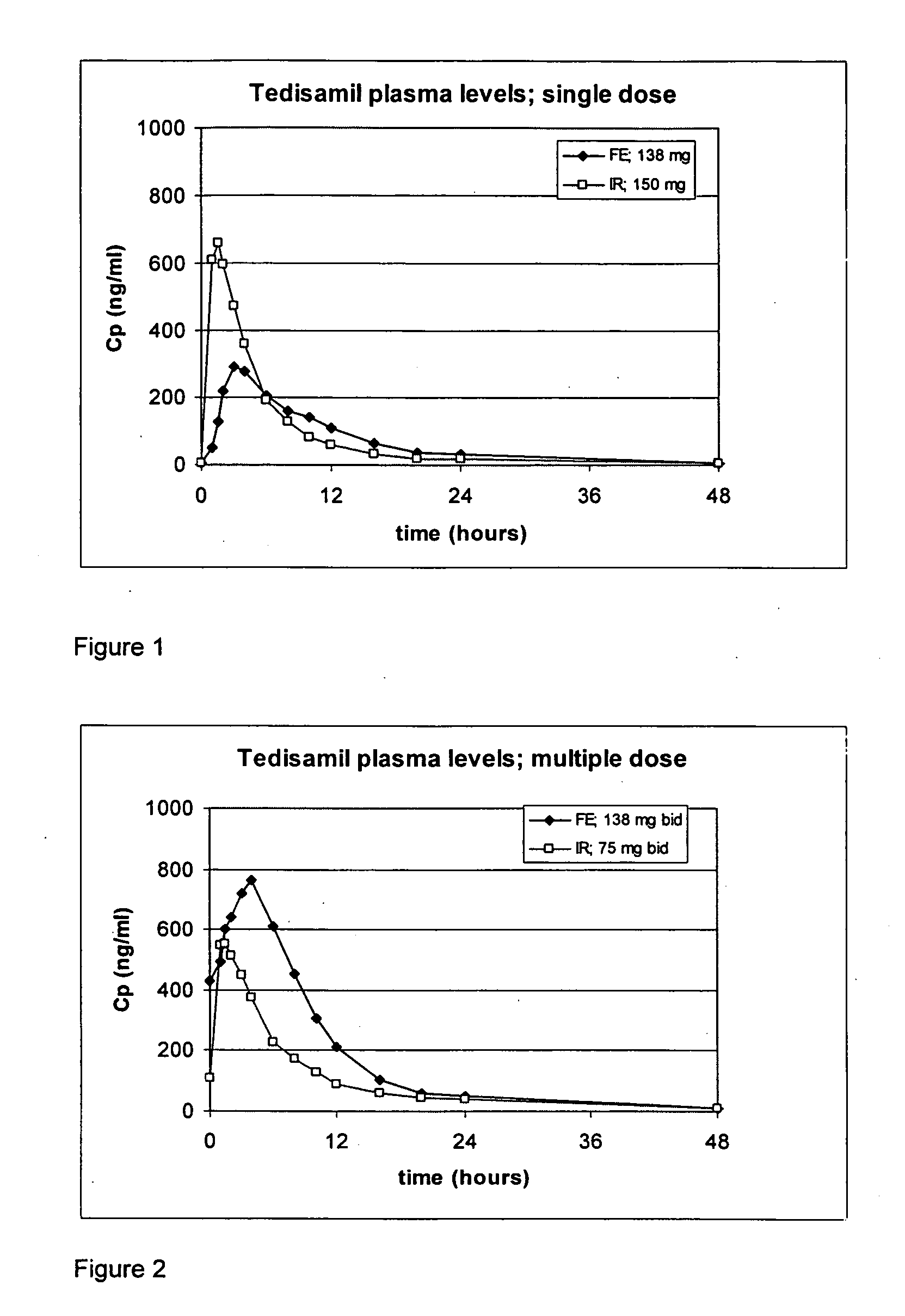
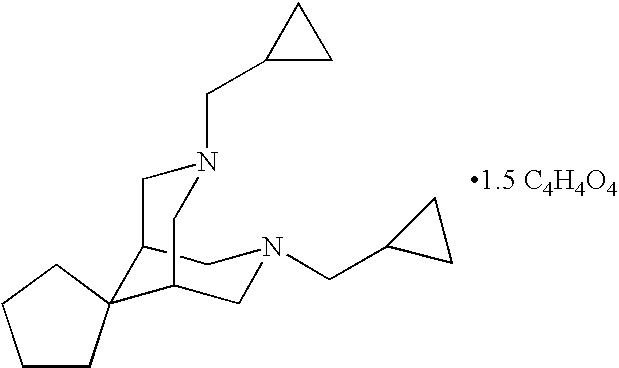
Oral sustained release formulation of tedisamil with gastric retention properties
The present invention relates to a novel sustained release formulation with gastric retention properties comprising tedisamil or a pharmaceutically acceptable salt thereof and the use of this formulation in the prevention and treatment of atrial fibrillation, atrial flutter and cardiac ischemia.
Owner:SOLVAY PHARM BV
Catheter for treatment of atrial flutter having single action dual deflection mechanism
ActiveUS9216056B2Quantity minimizationEasy constructionMulti-lumen catheterMedical devicesCatheter deviceAtrial flutter
A catheter and method for the treatment of a patient having atrial flutter or other arrhythmia comprises an elongated catheter body having an outer wall, proximal and distal ends, and at least one lumen extending therethrough. Further it has a distal tip section comprising a flexible tubing having a proximal end and a distal end and a plurality of lumens extending therethrough. The proximal end of the tip section is fixedly attached to the distal end of the catheter body. The tip section further comprises a nitinol tube having slots formed therein which causes the distal tip section to deflect using the same puller-wire action used to cause the deflectable catheter to deflect at a point proximal to the distal tip section.
Owner:BIOSENSE WEBSTER (ISRAEL) LTD
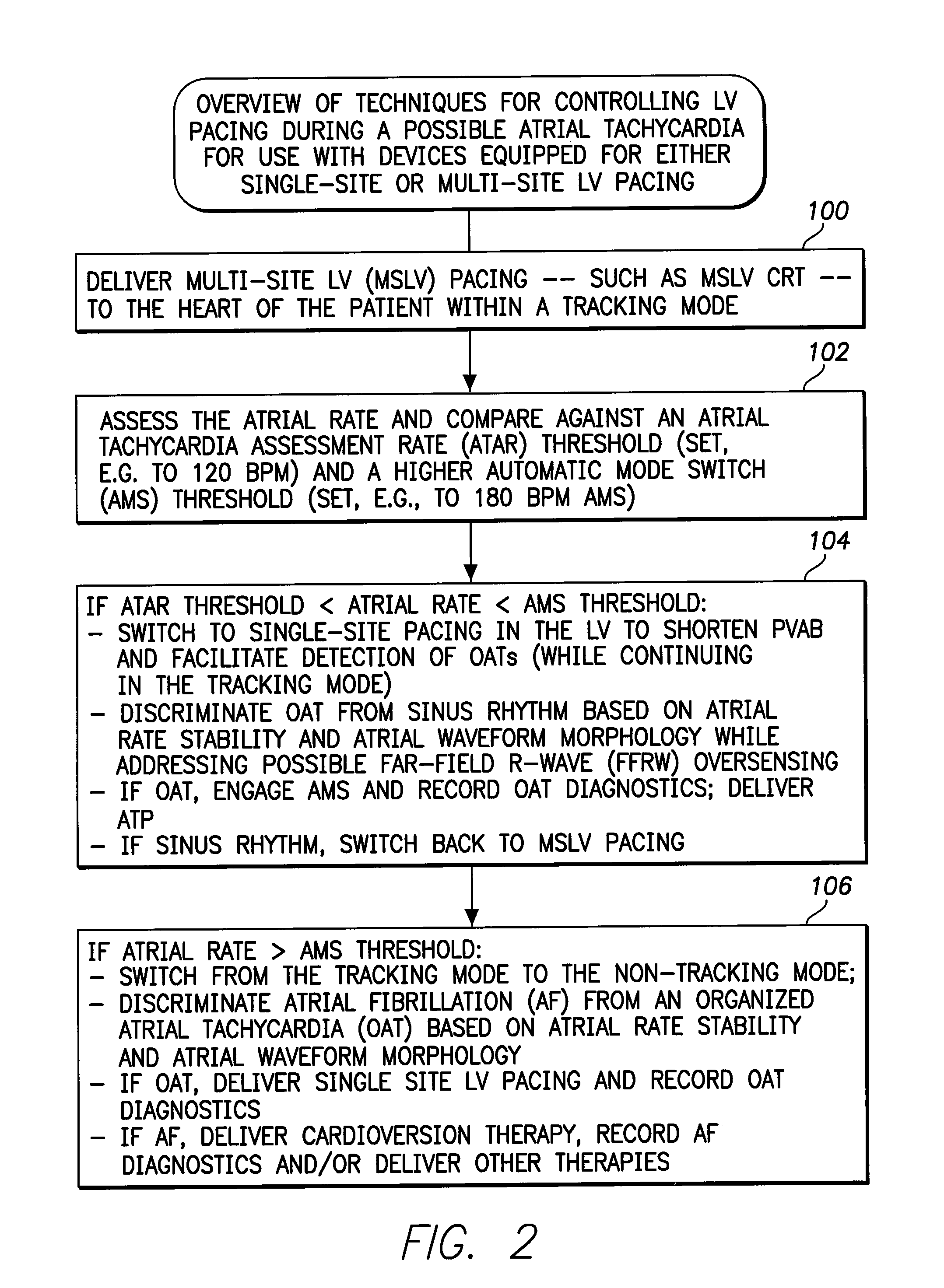
Systems and Methods for Use by an Implantable Medical Device for Controlling Multi-Site CRT Pacing in the Presence of Atrial Tachycardia
Systems and methods are provided for use by implantable medical devices equipped to deliver multi-site left ventricular (MSLV) pacing. MSLV is associated with a relatively long post-ventricular atrial blanking (PVAB) period that might limit the detection of pathologic rapid organized atrial tachycardias (OAT). In one example, MSLV cardiac resynchronization therapy (CRT) pacing is delivered within a tracking mode. A possible atrial tachycardia is detected based on the atrial rate exceeding an atrial tachycardia assessment rate (ATAR) threshold. The device then switches to single-site LV pacing, thereby effectively shortening the PVAB to detect additional atrial events that might otherwise be obscured, and thereby permitting the device to more reliably distinguish organized atrial tachycardias (such as atrial flutter) from sinus tachycardia. The device may also employ an automatic mode switch (AMS) threshold that is set higher than the ATAR threshold for use in switching from tracking modes to nontracking modes.
Owner:PACESETTER INC
Methods of treatment and pharmaceutical composition
The invention relates a pharmaceutical composition comprising a combination of:(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof; and(ii) a NEP inhibitor or a pharmaceutically acceptable salt thereof and optionally a pharmaceutically acceptable carrier and to a method for the treatment or prevention of a condition or diseaseselected from the group consisting of hypertension, heart failure, such as (acute and chronic) congestive heart failure, left ventricular dysfunction and hypertrophic cardiomyopathy, diabetic cardiac myopathy, supraventricular and ventricular arrhythmias, atrial fibrillation, atrial flutter, detrimental vascular remodeling, myocardial infarction and its sequelae, atherosclerosis, angina (whether unstable or stable), renal insufficiency (diabetic and non-diabetic), heart failure, angina pectoris, diabetes, secondary aldosteronism, primary and secondary pulmonary hypertension, renal failure conditions, such as diabetic nephropathy, glomerulonephritis, scleroderma, glomerular sclerosis, proteinuria of primary renal disease, and also renal vascular hypertension, diabetic retinopathy, the management of other vascular disorders, such as migraine, peripheral vascular disease, Raynaud's disease, luminal hyperplasia, cognitive dysfunction, such as Alzheimer's, glaucoma and stroke, comprising administering a therapeutically effective amount of the pharmaceutical composition to a mammal in need thereof.
Owner:NOVARTIS PHARM CORP
Method and apparatus for medical intervention procedure planning
InactiveUS20080146916A1Ultrasonic/sonic/infrasonic diagnosticsMechanical/radiation/invasive therapiesOperator interfaceData acquisition
An imaging system for use in medical intervention procedure planning includes a medical scanner system for generating a volume of cardiac image data, a data acquisition system for acquiring the volume of cardiac image data, an image generation system for generating a viewable image from the volume of cardiac image data, a database for storing information from the data acquisition and image generation systems, an operator interface system for managing the medical scanner system, the data acquisition system, the image generation system, and the database, and a post-processing system for analyzing the volume of cardiac image data, displaying the viewable image and being responsive to the operator interface system. The operator interface system includes instructions for using the volume of cardiac image data and the viewable image for bi-ventricular pacing planning, atrial fibrillation procedure planning, or atrial flutter procedure planning.
Owner:OKERLUND DARIN R +2
Ablation catheter with suspension system incorporating rigid and flexible components
InactiveUS20050267460A1Easy to operateImproved linear lesionSurgical instruments for heatingVeinRight atrium
A curved ablation catheter imparts ablative energy to target tissue, for example, along a trabecular slope, e.g., in the right atrium along the isthmus between the ostium of the inferior vena cava and the tricuspid valve. The catheter is formed with a preset curvature that, when deployed, both translates linearly and increases in radius to aid in the formation of spot or continuous linear lesions. A method of treating atrial flutter employs the curved ablation catheter.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com