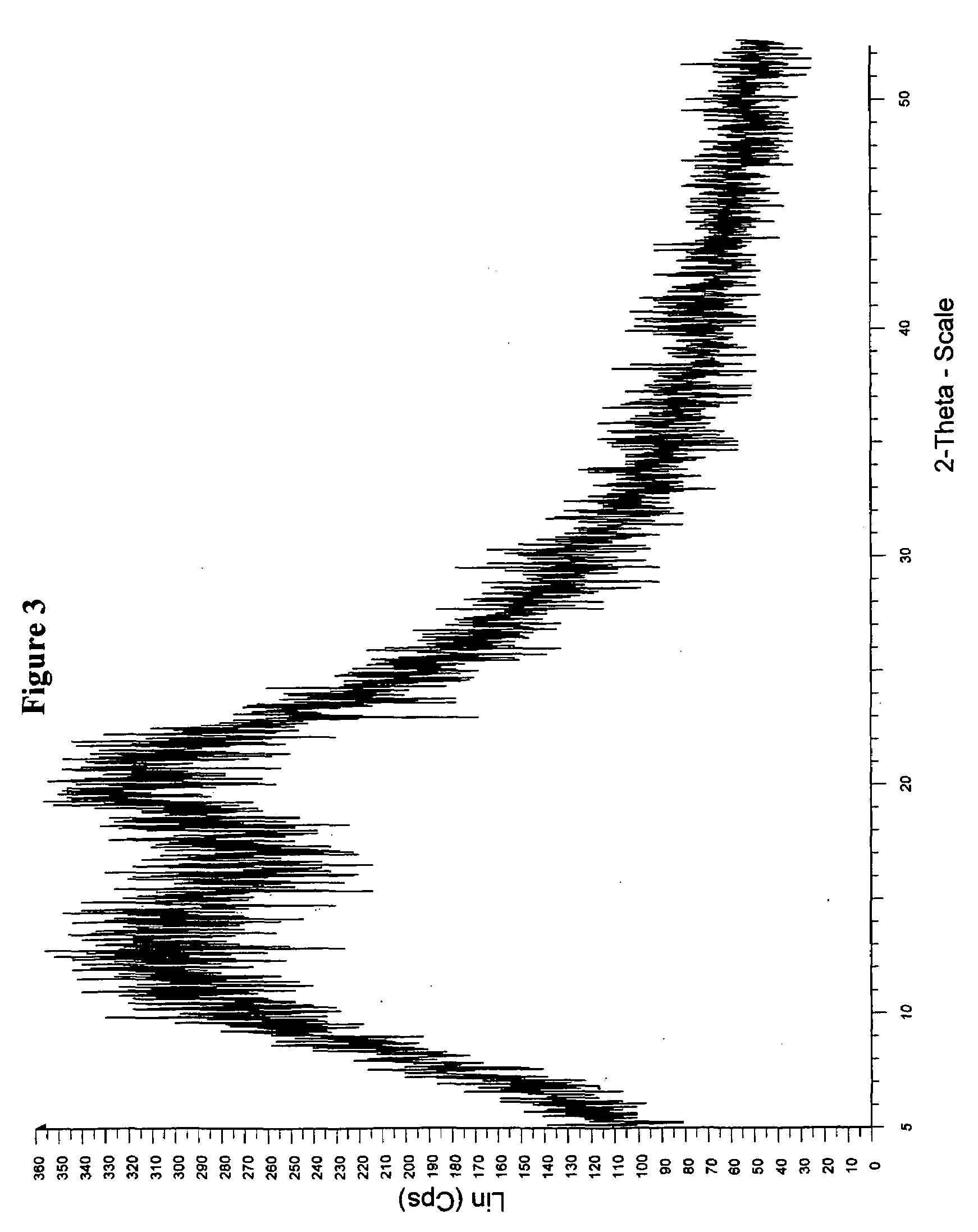
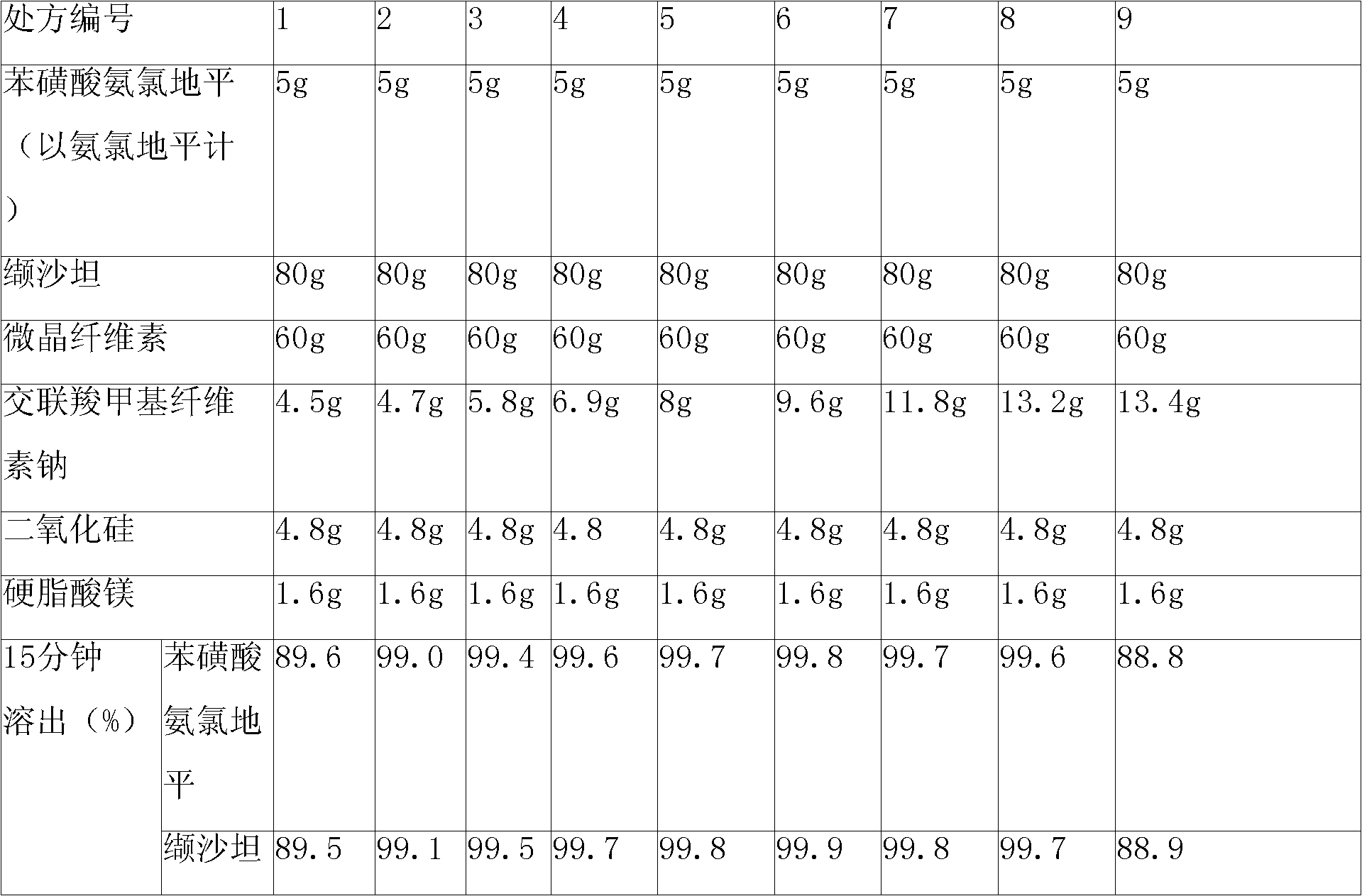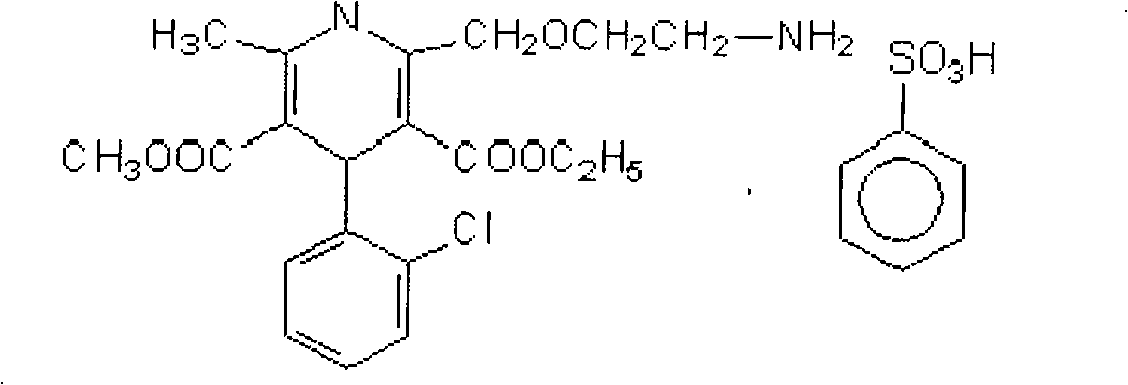Patents
Literature
396 results about "Valsartan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
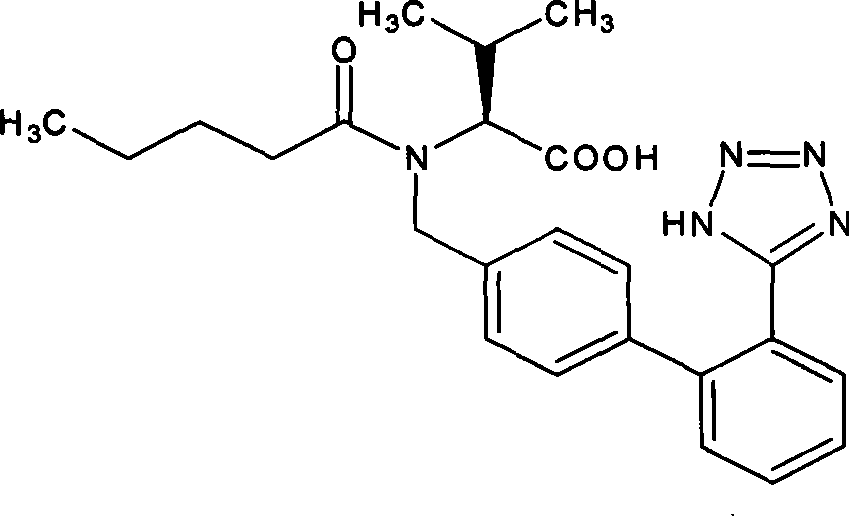
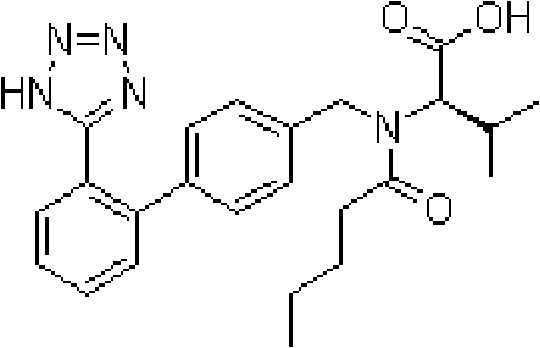
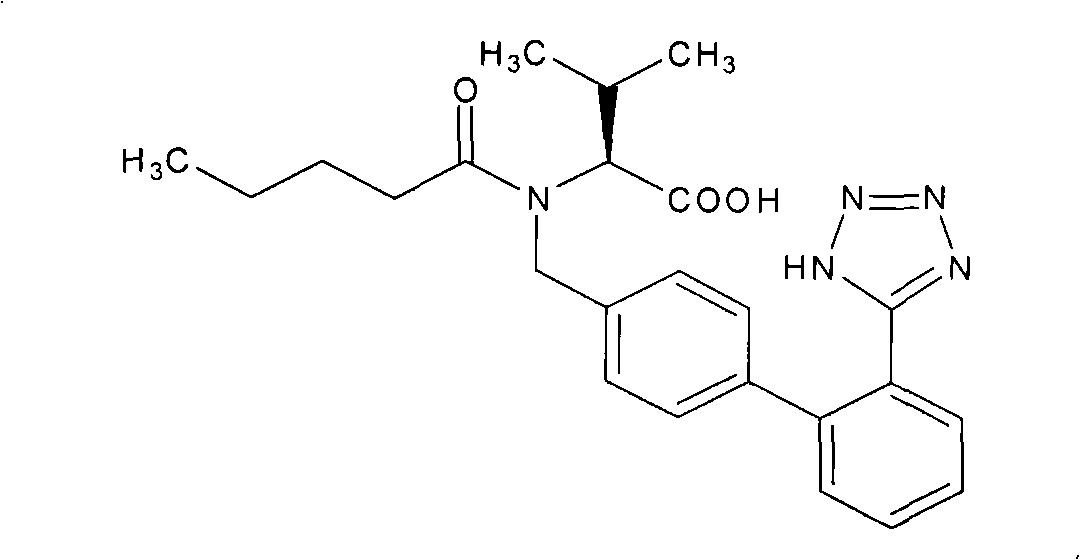
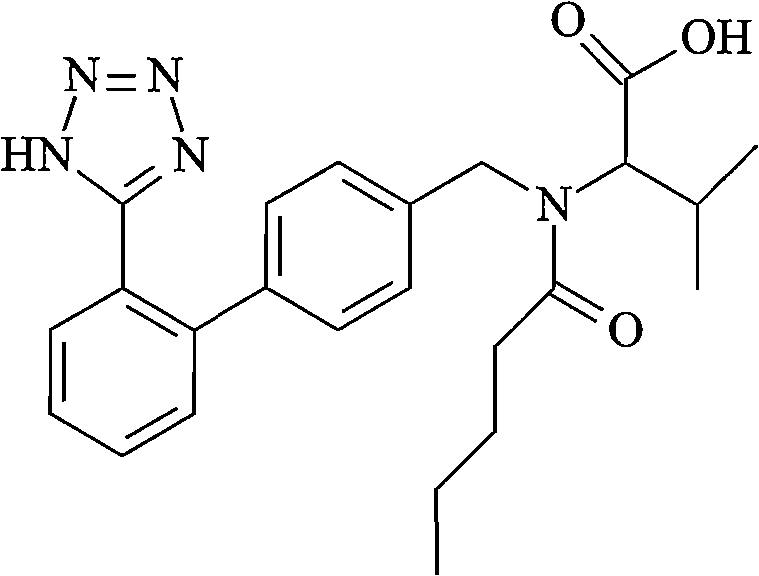
Valsartan is used to treat high blood pressure and heart failure. It is also used to improve the chance of living longer after a heart attack.
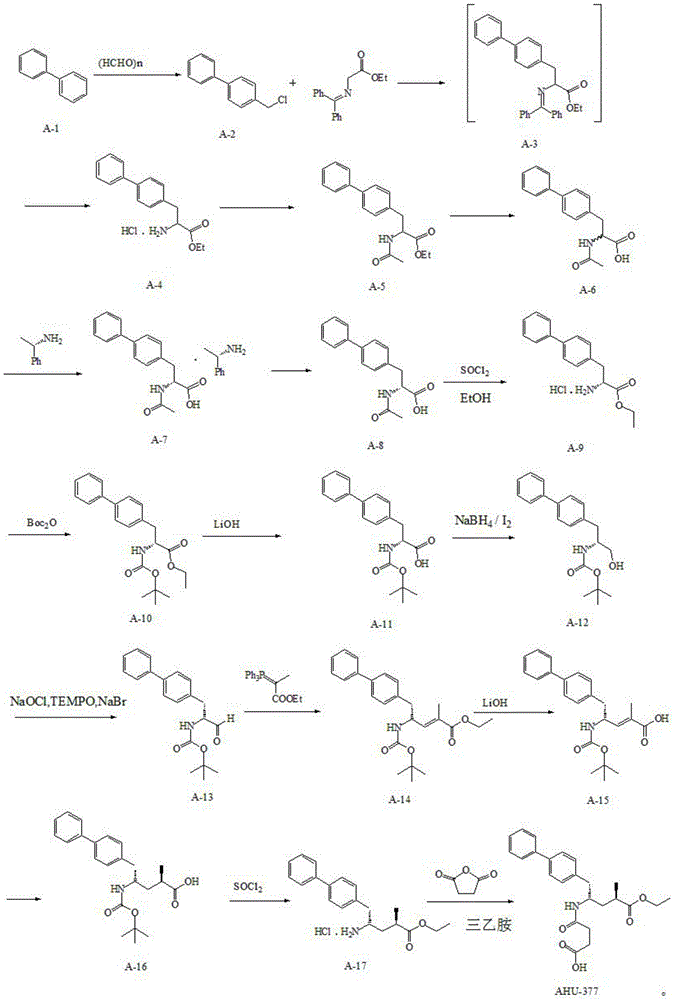
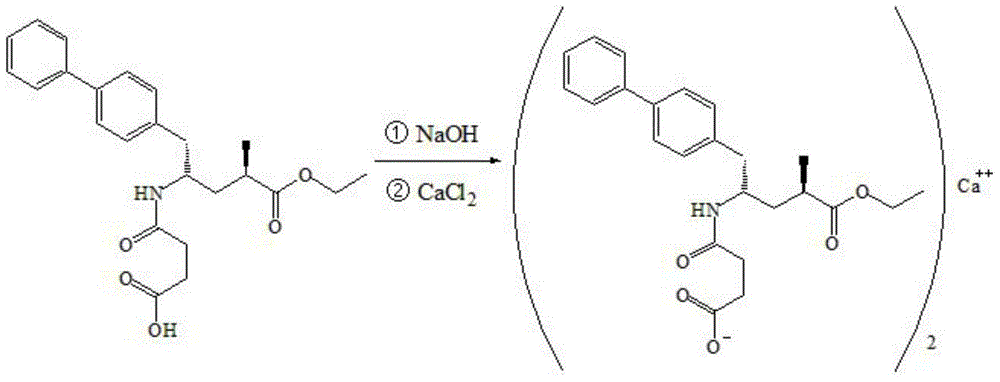
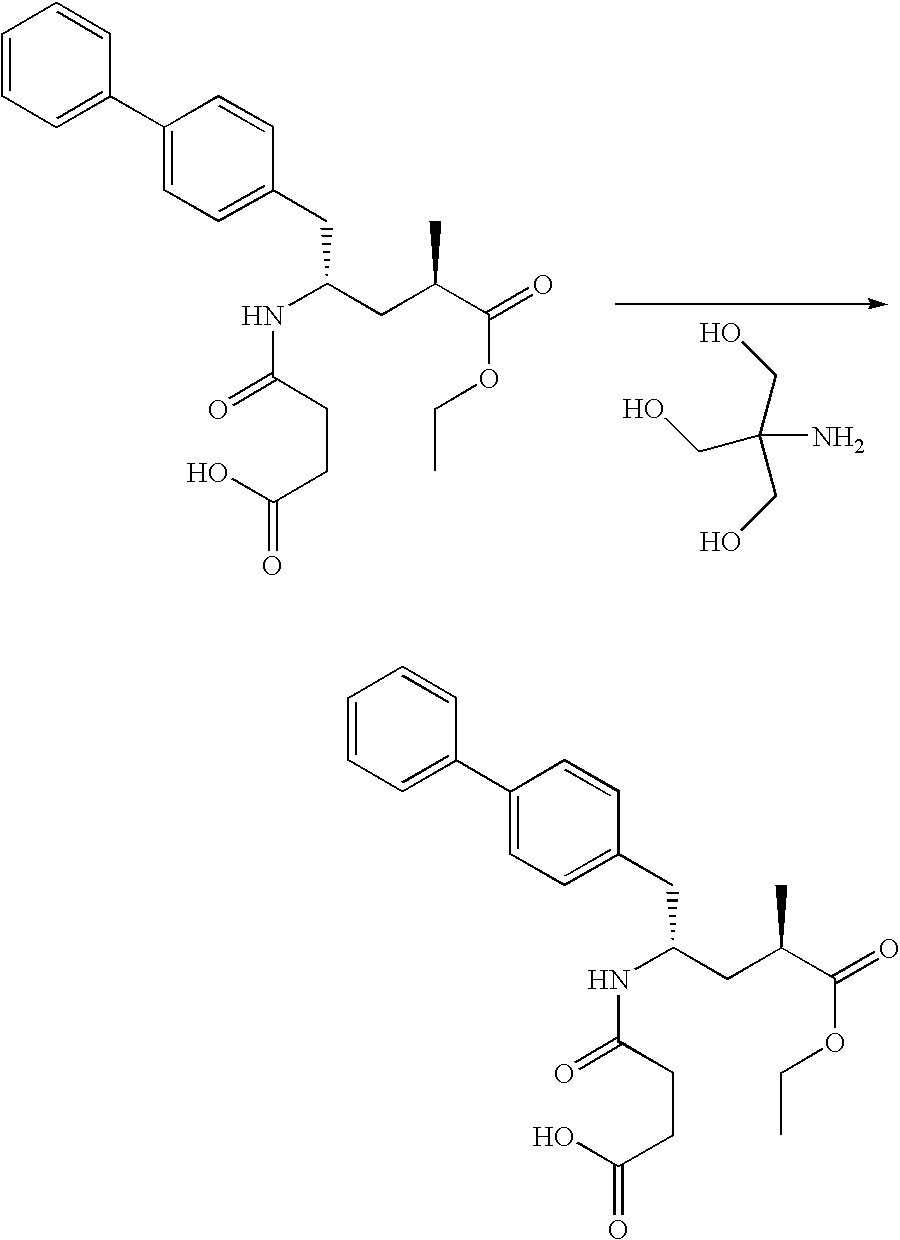
Preparation method for dual inhibitor LCZ696 of angiotensin II receptor and neprilysin
InactiveCN105168205AQuality improvementHigh purityOrganic compound preparationCarboxylic acid amides preparationValsartanHigh volume manufacturing
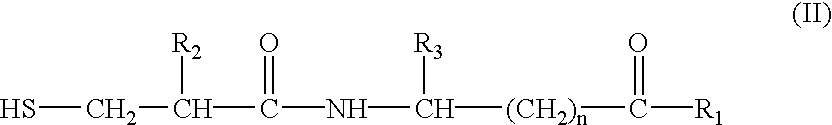
The invention specifically relates to a preparation method for a dual inhibitor LCZ696 of angiotensin II receptor and neprilysin, which belongs to the technical field of drug synthesis. The preparation method comprises the following steps: preparing valsartan and AHU-377 or AHU-377 calcium salt at first; and then mixing valsartan with AHU-377 or AHU-377 calcium salt under stirring so as to prepare LCZ696. The LCZ696 prepared in the invention has good quality and high purity; and the preparation method has the advantages of simplicity, low energy consumption, low cost and suitability for large batch production.
Owner:TAILITE MEDICINE HUBEI
Pharmaceutical Formulation of Valsartan
InactiveUS20100222334A1Maintain good propertiesLow variabilityBiocideNervous disorderValsartanOral medication
The present invention relates to a pharmaceutical composition in a form of suspension for oral administration comprising valsartan or its pharmaceutically acceptable salts and at least one or two or more of the components selected from glycerol or syrup or the mixture thereof, a preservative, a buffer system and a suspending / stabilizing agent. The present invention further relates to the therapeutic uses of the pharmaceutical composition.
Owner:NOVARTIS PHARM CORP
Method for synthesizing diovan
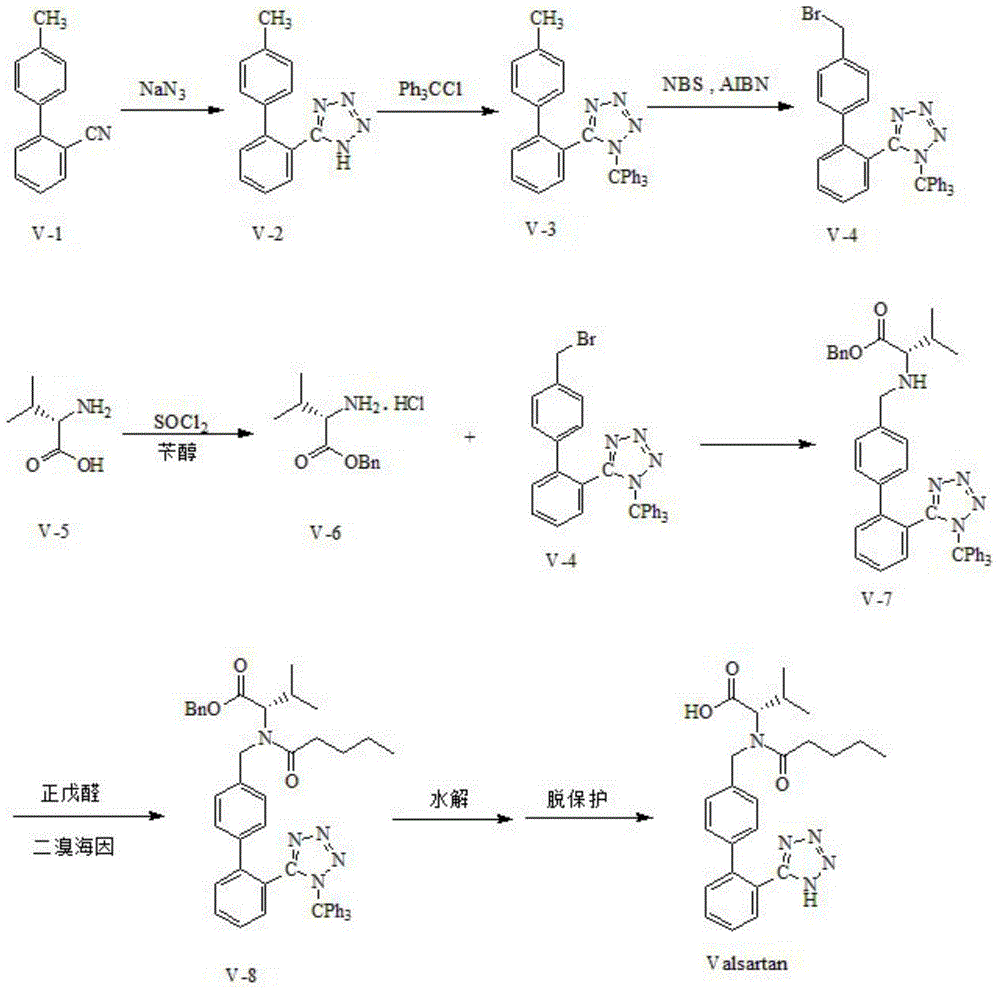
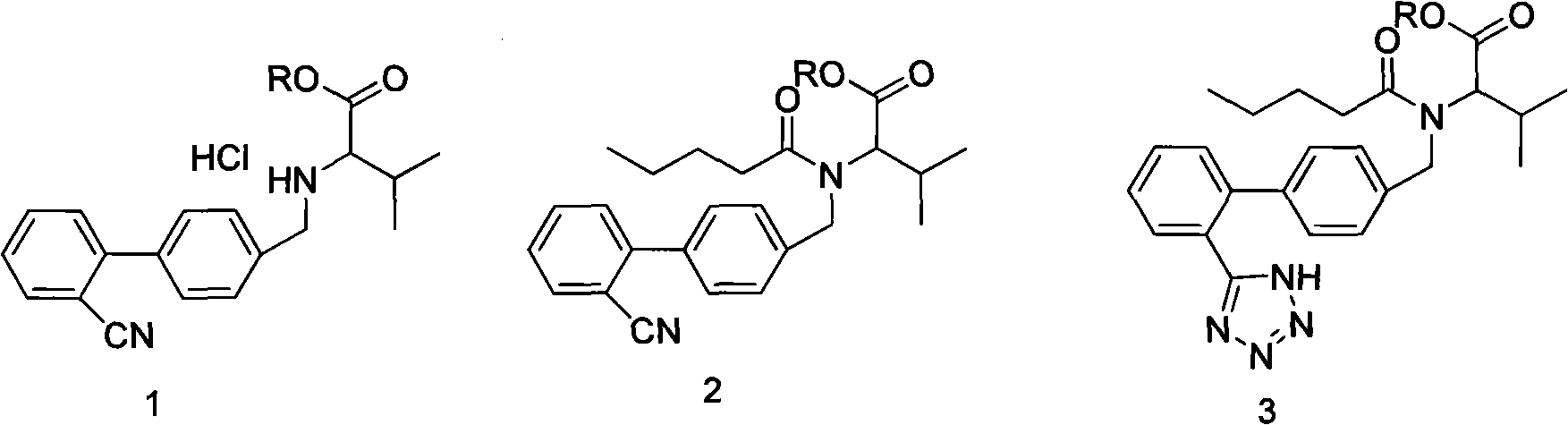
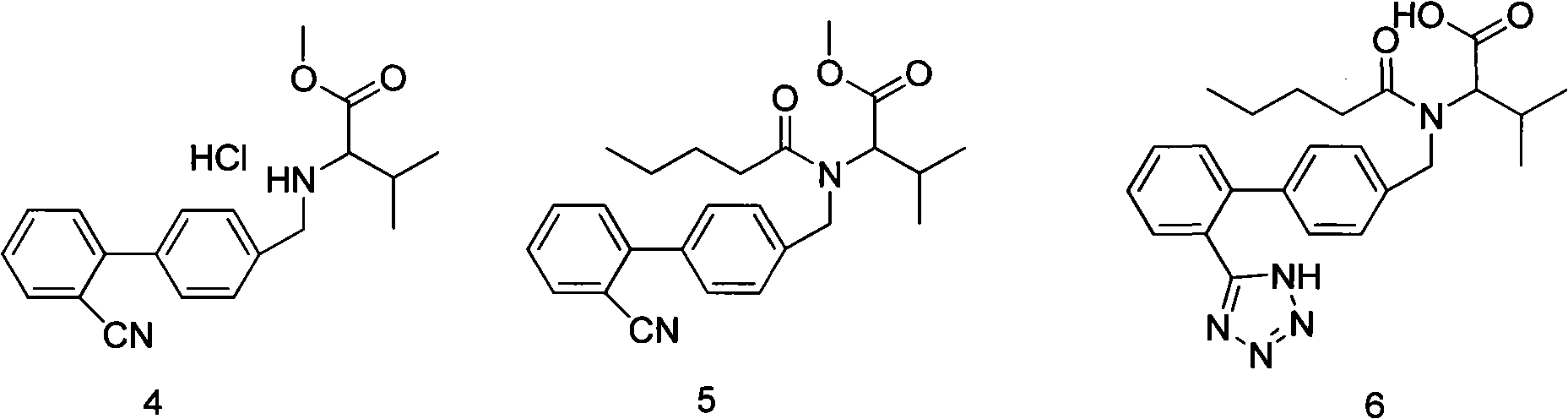
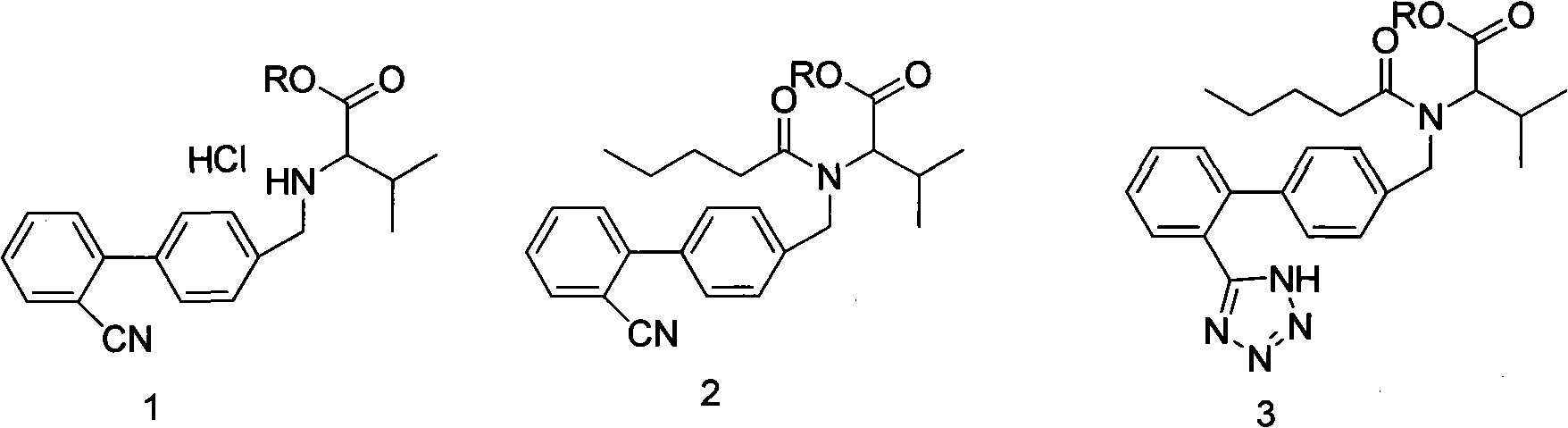
The present invention provides an improved method used for synthesizing valsartan. No tin compounds are required in the reaction. In the method, the condensate of N-[(2'-cyano-1, 1'- biphenyl-4-group)-alkyl]-L-valine ester is used as raw material. And the method comprises valerylation and synthesis of the valsartan. The method has the advantages of easily available raw materials, simple operation, no environmental pollution, high yield, low cost, and suitability for large-scale industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Process for preparing valsartan
Owner:DR REDDYS LAB LTD +1
Diovan compound preparation and preparation method thereof
ActiveCN101485657AImprove liquidityNormal production processPharmaceutical product form changeCapsule deliveryValsartanBULK ACTIVE INGREDIENT
The invention discloses a valsartan compound preparation and a method for preparing the same. In the method, the valsartan or pharmacologically accepted salts of the valsartan and amlodipine or pharmacologically accepted salts of the amlodipine are used as active ingredients, and the active ingredients are pressed to prepare a compact by a rolling method; the compact is screened to prepare granules; and the granules are mixed with pharmaceutic adjuvants to prepare a tablet or a capsule. The method pretreats the active ingredients, so that materials have good fluidity; and the valsartan compound preparation and the method have the characteristics of simple process, low cost and suitability for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
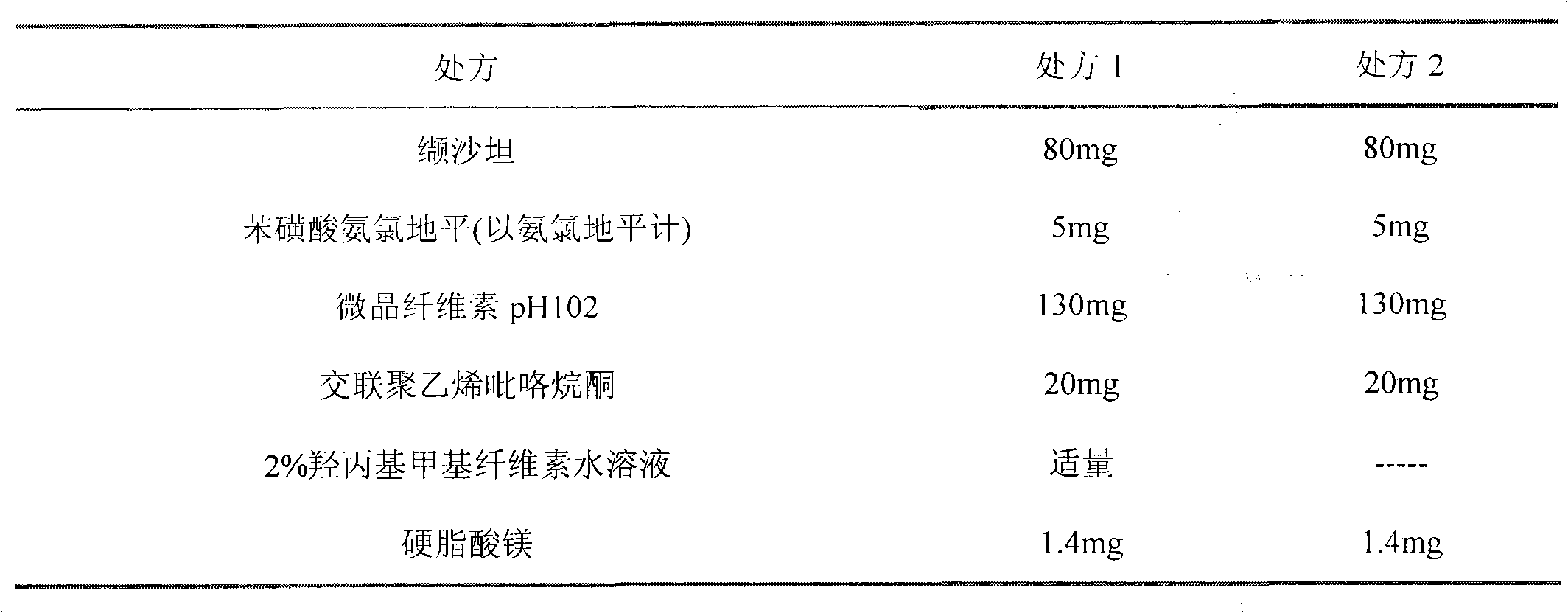
Pharmaceutical composition containing Amlodipine besilate and valsartan and preparation method thereof
InactiveCN101647797ALess investmentSimple production processPharmaceutical product form changeDrageesValsartanMedicine
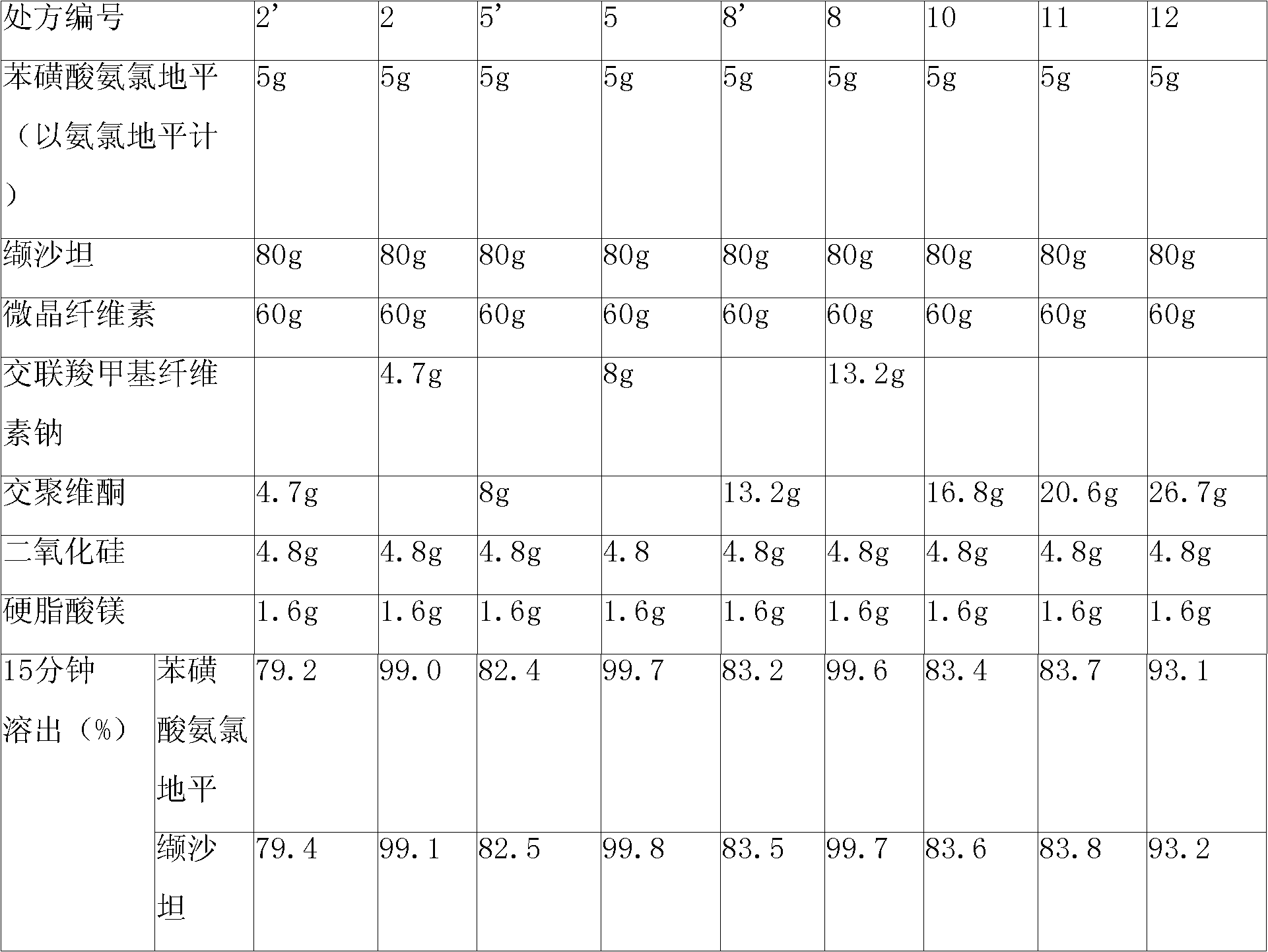
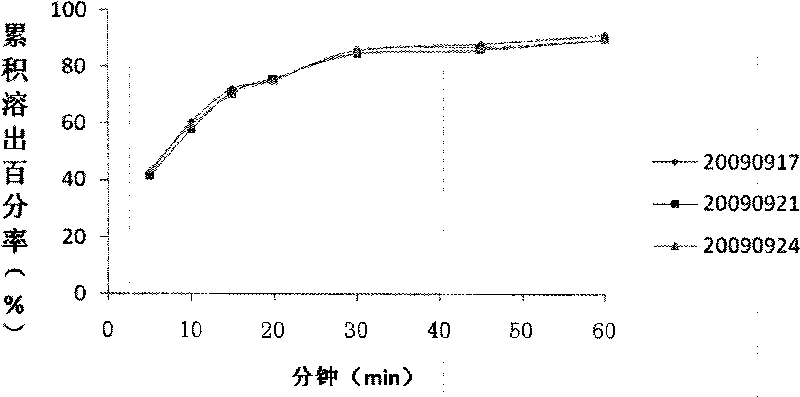
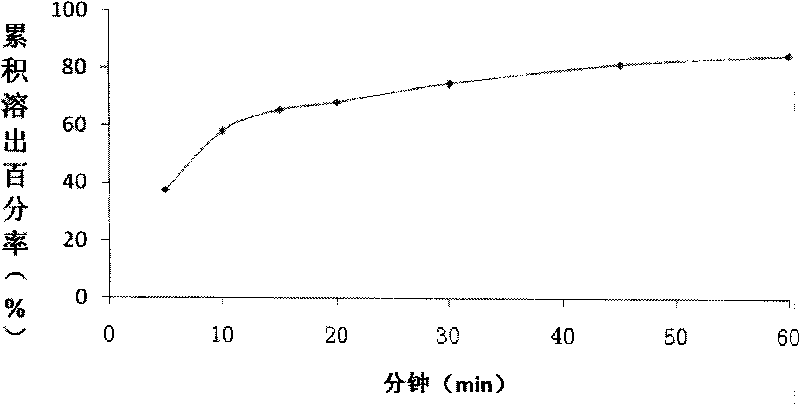
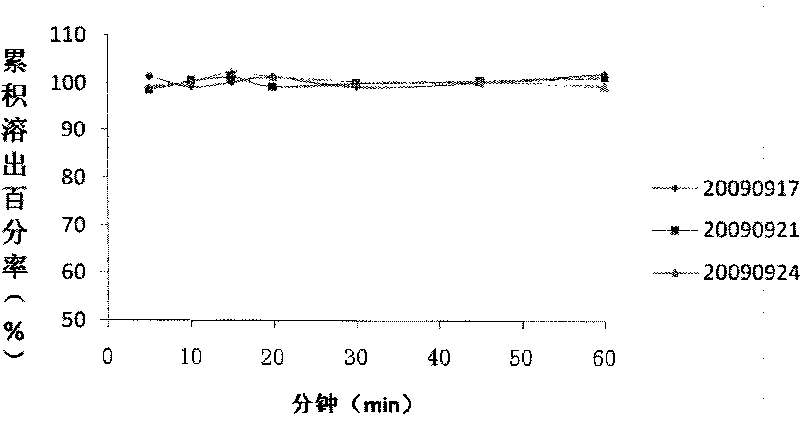
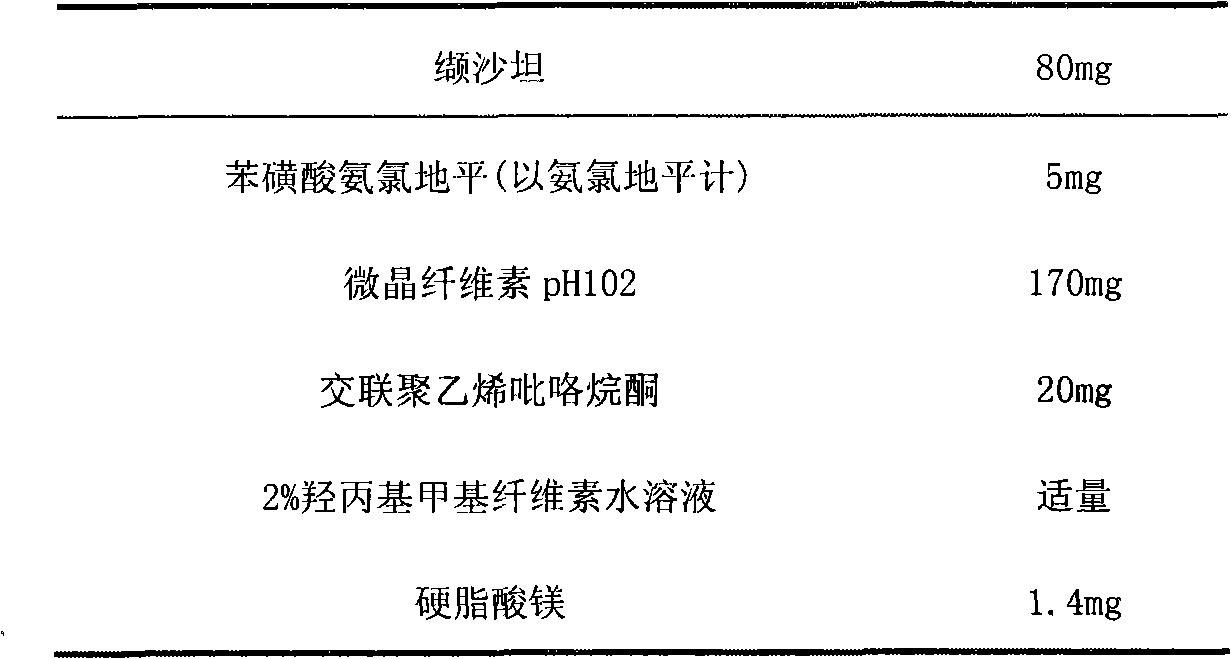
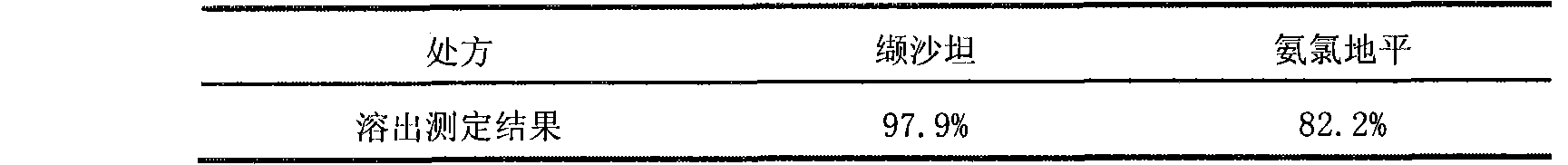
The invention relates to a pharmaceutical composition containing Amlodipine besilate and valsartan and a preparation method thereof. The pharmaceutical composition is prepared from the following ingredients in parts by weight: 5 parts of Amlodipine besilate, 80 parts of valsartan, 60 parts of microcrystalline cellulose, 4.7-13.2 parts of croscarmellose sodium, 4.8 parts of silicon dioxide and 1.6parts of magnesium stearate. The pharmaceutical composition is prepared by using a direct powder compression technique. The invention can reach the dissolution rate of more than 90% by using less disintegrating agent, and has advantages of good stability and faster disintegrating. The preparation method of the invention has simpler production process, reduces the investment of corresponding equipment and plants and saves the production cost; the tablet produced by using direct powder compression technique has faster disintegrating and is helpful to improve the dissolving of pharmaceutical; through detection, the tablet prepared by using the method is dissolved out by more than 90% within 15 min.
Owner:HAINAN JINRUI PHARMA CO LTD
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Preparation of solid coprecipitates of amorphous valsartan
A novel coprecipitate of amorphous valsartan with a pharmaceutically acceptable carrier, e.g. polyvinylpyrolidone (PVP), crosslinked-polyvinylpyrolidone, polyvinylpyrolidone vinyl acetate copolymer (PVP-VA64), a process for the preparation of said novel co-precipitate and the use of said novel coprecipitate in the treatment and / or prophylaxis of hypertension, cardiovascular diseases and conditions associated with thereof and certain complications thereof, are disclosed. A novel solid solution of amorphous valsartan with a pharmaceutically acceptable carrier, preferably with polyethyelene glycol PEG from 4000 to 20,000 of average mol. wt., a process for the preparation thereof and use are disclosed. The said novel coprecipitate of amorphous valsartan and the said novel solid solution of valsartan are stable and may be particularly suitable for pharmaceutical dosage forms.
Owner:MAI DE
Process for refining valsartan
The invention discloses a process for refining valsartan, which includes the following steps: dissolving crude products of the valsartan with an alcoholic solvent or an ester solvent; adding inorganic base to form salt, so that salt of valsartan is obtained through centrifugation; adding the obtained salt of the valsartan into the ester solvent; adding an aqueous solution of an inorganic acid for acidification; delaminating; recovering a drying solvent after the ester solvent layer is washed with water; adding the ester solvent to dissolve materials; and obtaining the valsartan through stirring, cooling crystallization, centrifugation and drying after dissolution. The process is simple in operation, high in yield, low in cost and good in quality of the obtained valsartan, all the reagents are common, cheap and easy to obtain, and simultaneously, the process is beneficial to environmental protection and suitable for industrial production. The quality of the obtained valsartan conforms to a standard of Chinese Pharmacopoeia 2010, and the refining mass yield exceeds 75%.
Owner:浙江新赛科药业有限公司
Process for the preparation of valsartan and its intermediates
The present invention relates to an improved process for the preparation of valsartan and its intermediates in substantially pure enantiomeric form. In particular, the present invention provides a process for preparing benzyl valsartan intermediate substantially free of organotin impurities. The valsartan produced from such benzyl valsartan intermediate requires significantly lower catalyst loading and has superior purity.
Owner:IPCA LAB LTD
Dispersible tablets containing valsartan and amlodipine besylate and preparation method thereof
InactiveCN101843615AAvoid stabilityAvoid the disadvantages of inconvenient storage and transportationPill deliveryHeterocyclic compound active ingredientsValsartanActive agent
The invention discloses to dispersible tablets containing valsartan and amlodipine besylate and a preparation method thereof, and relates to medicament dispersible tablets and the preparation method thereof, solving the problem of poor stability, slow disintegration and dissolution, low oral bioavailability and high cost existing in the traditional medicine containing valsartan and amlodipine besylate. The dispersible tablets are prepared from valsartan, amlodipine besylate, disintegrant, diluent, adhesive, lubricant, fluidizer, surfactant and flavoring agent. The method comprises the following steps of: weighting and screening materials; mixing the valsartan, disintegrant and diluent to obtain mixed powder A; mixing amlodipine besylate and the mixed powder A to obtain mixed powder B; mixing the adhesive, surfactant and the mixed powder B to prepare soft material; screening, granulating and drying the soft material; mixing and screening and the dried granules with the fluidizer, lubricant, disintegrant and flavoring agent; and finally carrying out size stabilization and tabletting. The medicine prepared by the method has good stability, fast disintegration and dissolution, high oral bioavailability and low cost.
Owner:包丽昕
Valsartan-containing solid preparation and preparation method thereof
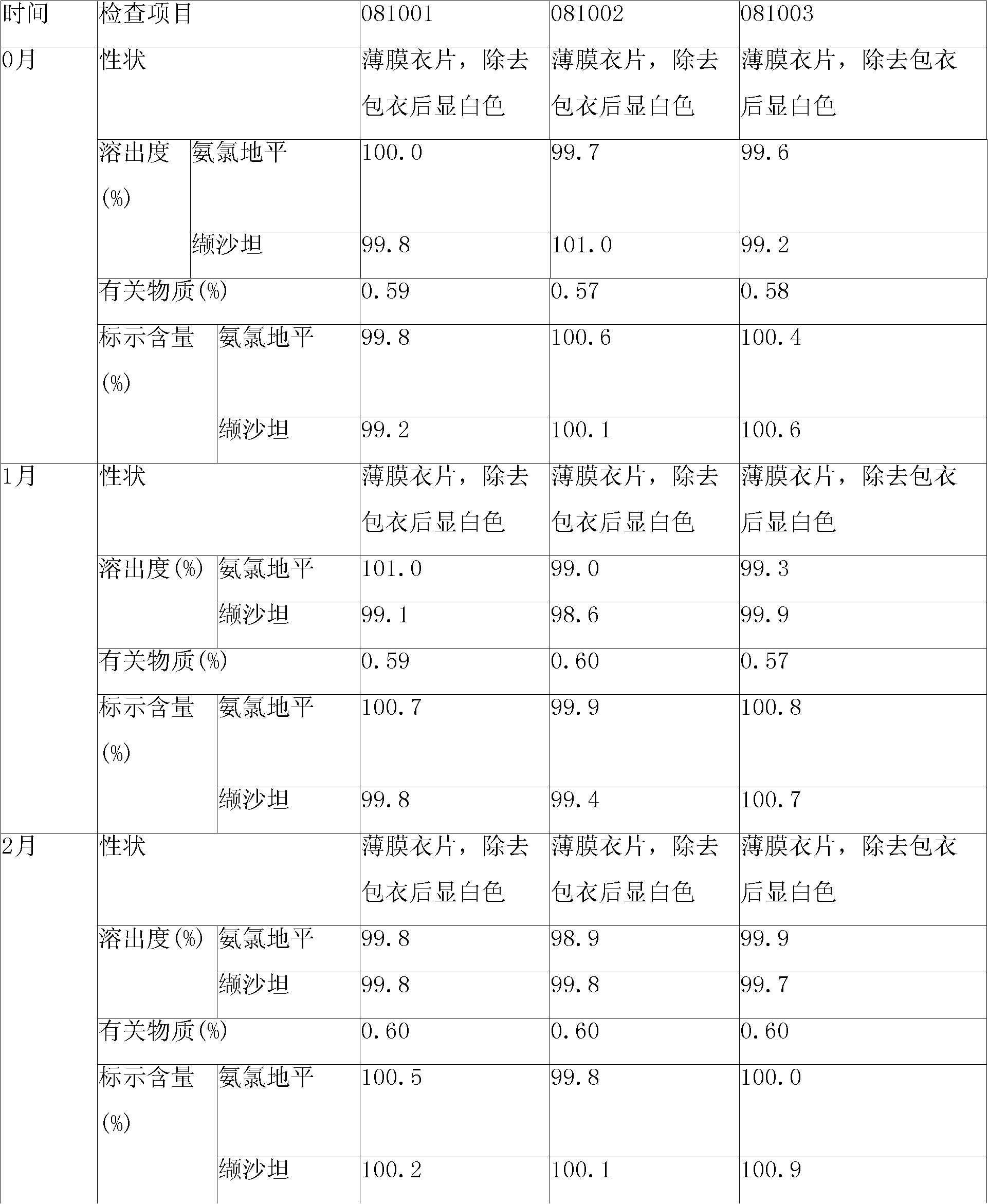
ActiveCN101829111AIncrease humidityImprove stabilityPharmaceutical non-active ingredientsCapsule deliveryValsartanMedicine
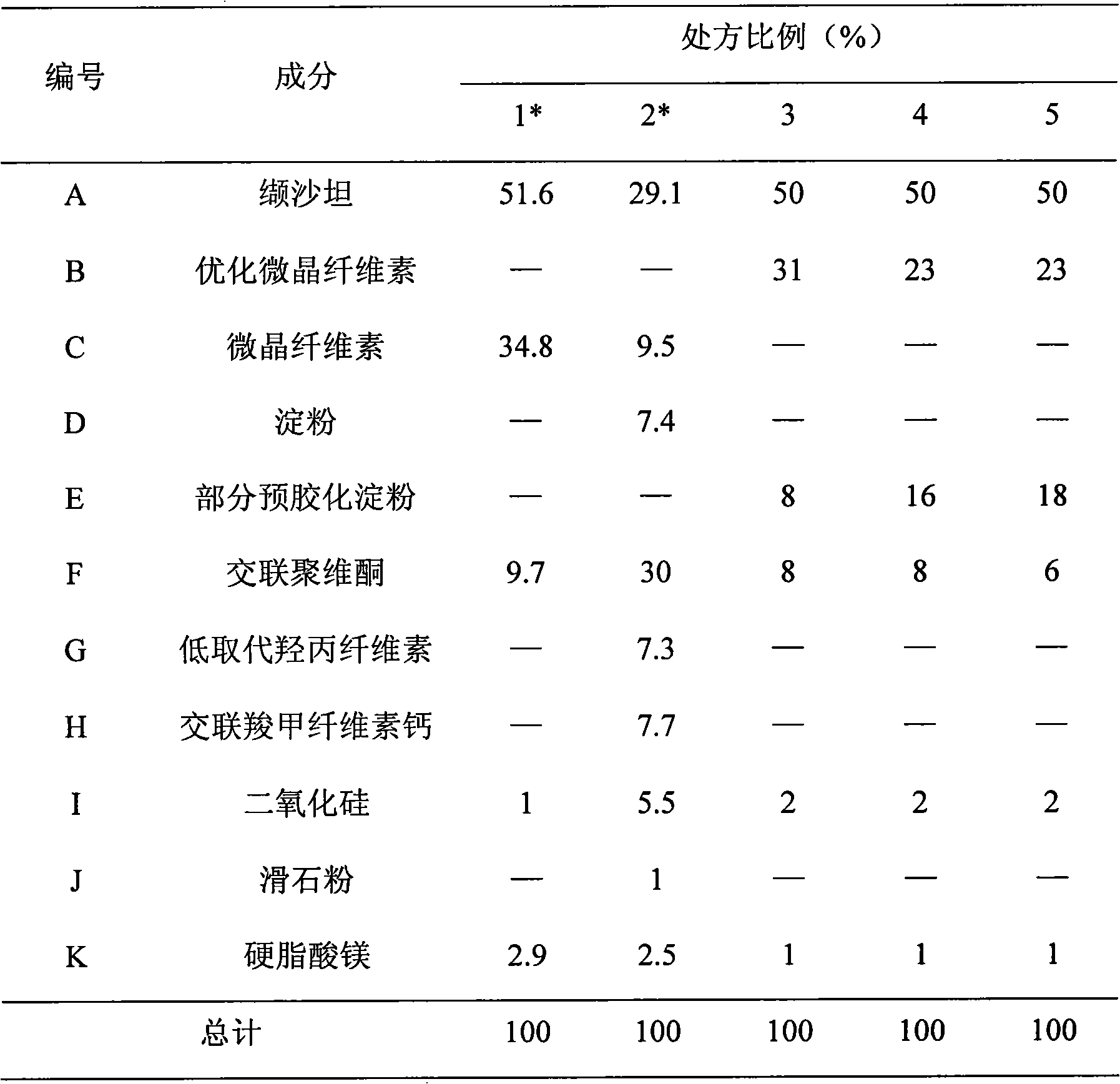
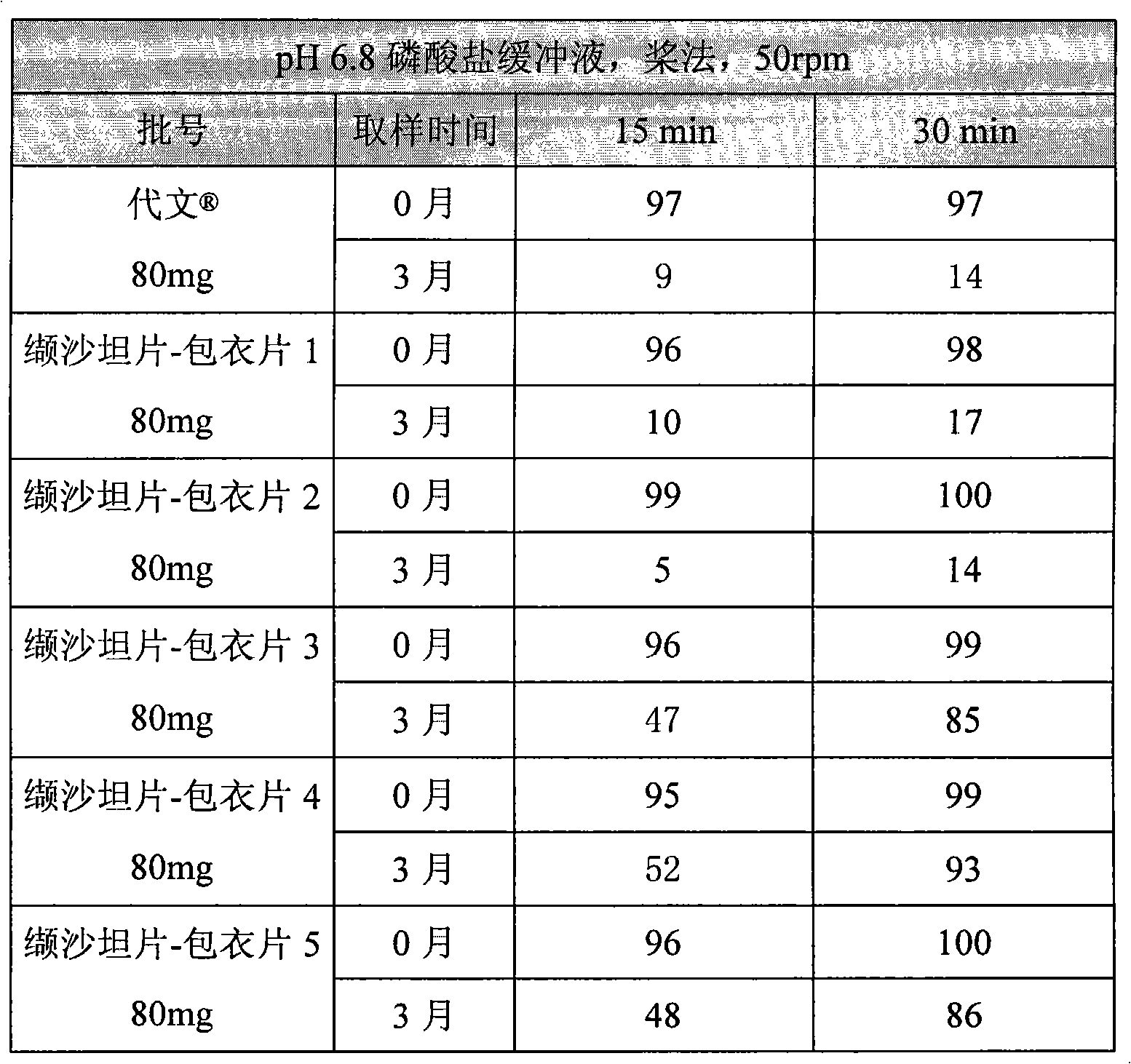
The invention relates to a valsartan-containing medicine preparation which at least comprises valsartan or pharmaceutically acceptable salt thereof that is taken as active ingredient, and at least two disintegrating agents, wherein the proportion of the two disintegrating agents is within the range of 1:2-2:1. The invention also relates to a preparation method of the valsartan-containing medicine preparation, which comprises the steps of: first, pelletizing the valsartan or the pharmaceutically acceptable salt thereof by a roller press way, and then mixing with the at least two disintegrating agents and other pharmaceutic adjuvant; and finally, tabletting or filling into capsules. Compared with the prior art, the valsartan-containing medicine preparation has better stability. The method has simple technique and high production efficiency, and is suitable for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Preparation method of high purity valsartan
The invention discloses a preparation method of high purity valsartan, which comprises the following steps: drying after a valsartan crude product is recrystallized through esters solvent, dissolving in inorganic base aqueous solution, then adjusting the inorganic base aqueous solution to be acidic by using hydrochloric acid, washing materials by using acid water solution after centrifugation, drying and crushing, and producing the high purity valsartan through centrifugation and drying. The method is simple to operate, the prepared valsartan is high in purity and has the quality that relevant impurities are not detected, chiral isomers are not detected, individual solution remains is smaller than 100 ppm, frequently-used reagents are used, the cost is low, the access is easy, the cost is lower, and meanwhile, the preparation method is beneficial to environmental protection and suitable for industrialized production.
Owner:浙江新赛科药业有限公司
Compound dispersible tablet for treating hypertension
The invention relates to a medicinal oral preparation containing Valsartan and Hydrochlorothiazide, more specifically the dispersible tablets for enhancing the beneficial effects of Valsartan and Hydrochlorothiazide.
Owner:江苏万高药业股份有限公司
Solid preparation of angiotensin receptor inhibitor and amlodipine and new preparation method thereof
InactiveCN101507715AQuality improvementImprove stabilityPill deliveryPharmaceutical non-active ingredientsValsartanAngiotensin receptor
The invention relates to an angiotensin receptor inhibitor and a solid preparation of amlodipine and a novel method for preparing the same. The method comprises the following steps that: 1, valsartan and amlodipine are screened respectively until the grain size of the valsartan and amlodipine is qualified; 2, the valsartan and amlodipine are added with proper amount of excipient respectively and the mixture is evenly mixed; and 3, the evenly mixed powder are added with proper amount of bonding agent or wetting agent respectively to obtain grain in proper grain size, and the grain is dried.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Improved method for synthesizing valsartan
The invention provides an improved method for synthesizing valsartan. The valsartan synthesized by the method can be further purified to give high-purity valsartan. In the method, tin compounds are not used, N-[(2'-cyano-1,1'-biphenyl-4-yl)alkyl]-ester L-valine monohydrochloride is used as a raw material, pentaacylation, diazotization and saponification are performed to obtain the valsartan, a crystallization process is controlled and thus the high-purity valsartan is obtained. The method has the advantages that: the operation is simple; the yield is high; the product purity is high; and the industrial production is easy.
Owner:ZHEJIANG MENOVO PHARMA
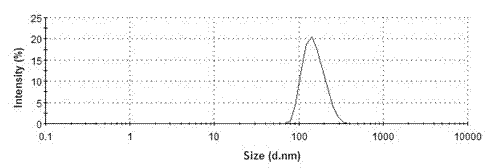
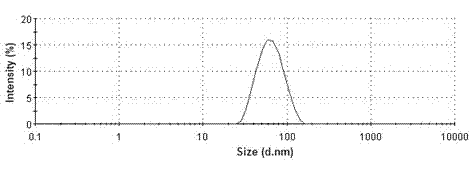
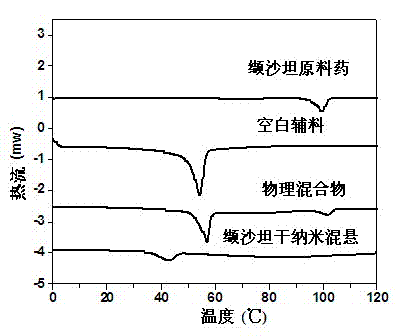
Valsartan spray-dried nanosuspension and preparation method of valsartan spray-dried nanosuspension
InactiveCN102920654AUniform particle sizeLarge particle sizePowder deliverySolution deliveryValsartanAnti solvent
The invention discloses a valsartan nanosuspension, a spray-dried power, and a preparation method of the valsartan nanosuspension and the spray-dried power. A valsartan spray-dried nanosuspension and a preparation method of the valsartan spray-dried nanosuspension belong to the field of nano-drug preparations. The valsartan spray-dried nanosuspension consists of 1 part of valsartan, 0.1-1 part of a stabilizing agent and 10-100 parts of a spray-drying protecting agent by mass; the valsartan spray-dried nanosuspension is prepared by an anti-solvent precipitation method and / or a high-pressure homogenizing method and / or an acid-alkali neutralization method in combination with a spray-drying technology. The drug particles in the spray-dried powder are reduced to a nano-grade, and the hydrophilicity, the in vitro dissolution rate and the bioavailability of the valsartan are greatly improved. The powder can be directly processed or be processed together an excipient into tablets, capsules and the like. The valsartan spray-dried nanosuspension has the advantages of simple preparation process, stable product quality and easy implementation of productization.
Owner:SHENYANG PHARMA UNIVERSITY
Method for refining Valsartan containing more than 10% of isomer
The invention discloses a method for refining Valsartan containing higher isomer impurity, namely Valsartan containing more than 10% (HPLC detection peak area method) of N isomer is refined by adopting butanone or the mixture solvent of butanone and esters and ethers, the N isomer can be remarkably reduced to about 1.0%, and the yield can be as high as more than 50%.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
A kind of valsartan capsule and preparation method thereof
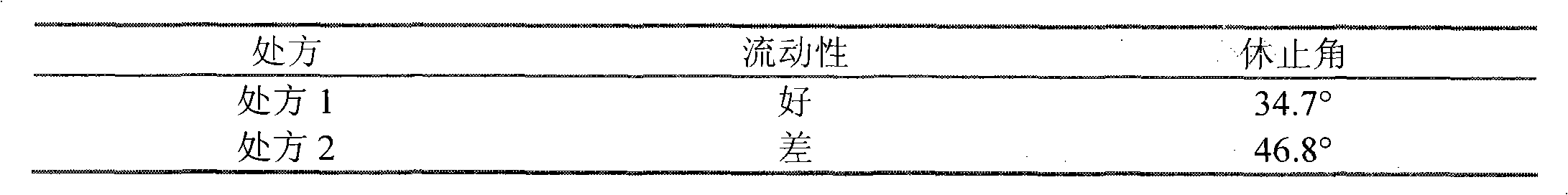
The invention relates to valsartan capsules and a preparation method thereof. The valsartan capsules comprise the following components in parts by weight: valsartan 50-200 parts, microcrystalline cellulose 30-160 parts, crosslinked povidone 1-10 parts, sodium dodecyl sulfate 0.8-4 parts, povidone K30 1-9 parts, and magnesium stearate 1-3 parts. The valsartan capsules provided by the invention canbe used for treating mild and moderate primary hypertension. The valsartan capsules have the advantages of reasonable formula, feasible process, stable and reliable quality, and good stability, dissolution and bioavailability. The valsartan capsules are prepared by using a wet granulation process, the addition method of solubilizer sodium dodecyl sulfate is proper, the concentration of the bonding agent is proper, and the granules have angle of repose smaller than 40 degrees, belong to powders with good fluidity and can meet the need of capsule filling. The valsartan capsules have short production process and low production costs, and are easy for industrial production.
Owner:HAINAN JINRUI PHARMA
Extended release gastro-retentive oral drug delivery system for valsartan
An extended release gastro-retentive drug delivery system of Valsartan. The drug delivery system contains a release portion containing the Valsartan, a gastro-retentive portion for retaining the drug delivery system in the stomach and an optional secondary portion for delivering a secondary pulse of Valsartan. In another embodiment, there is provided a swellable unfolding membrane comprising Valsartan for sustained administration of Valsartan to the upper GI tract of a patient.
Owner:NOVARTIS PHARM CORP
Valsartan and amlodipine compound preparation and preparation method thereof
InactiveCN102091069AEffective growth controlAvoid direct contactPill deliveryCapsule deliveryValsartanBULK ACTIVE INGREDIENT
The invention discloses valsartan and amlodipine compound preparation and a preparation method thereof. In the invention, valsartan or pharmaceutically acceptable salts thereof and amlodipine or pharmaceutically acceptable salts thereof are used as active ingredients, the valsartan and a pharmaceutical auxiliary material are mixed and rolled by a rolling process to form a pressed material; the pressed material is sieved to obtain a granular material; and the granular material is mixed with amlodipine to form tablets or capsules. In the invention, the valsartan and the auxiliary material are granulated first and then mixed with the amlodipine equally, so the liquidity of the material is improved; and the prepared product is very stable, and the process is simple and suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Dispersing tablet of valsartan and preparation method thereof
ActiveCN101732270AImprove bioavailabilitySmall inter-individual differencesPill deliveryPharmaceutical non-active ingredientsValsartanAdhesive
The invention belongs to the field of medicine preparations, which in particular relates to a dispersing tablet of valsartan and a preparation method thereof. Because the valsartan has lower bioavailability when being orally taken and absorbed and larger difference between application individuals, the invention provides the dispersing tablet of the valsartan, which contains the valsartan, hydroxy propyl-beta-schardinger dextrin, a filling agent, a disintegrating agent, an adhesive and a lubricant. After the valsartan is included by the hydroxy propyl-beta-schardinger dextrin (HP-beta-CD), the valsartan is very easy to dissolve in water, enhances the stability of the medicine, promotes the in-vivo release of the medicine, increases the absorption, enhances the bioavailability and lowers the difference between the application individuals.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for crystallizing valsartan
ActiveCN103086993ASimple and fast operationSuitable for industrial productionOrganic chemistryAlkaneValsartan
The invention discloses a method for crystallizing valsartan. The method comprises the following steps of: dissolving a valsartan crude product by using an ester solvent; cleaning the ester solvent layer by using water to recover the dry solvent; adding an ester solvent for dissolving the material; adding an alkane solvent after dissolving; stirring and cooling to a first stage temperature of 15 to 20 DEG C, and preserving the heat; then cooling to a second stage temperature of -3 to 3 DEG C, and preserving the heat; centrifuging; and drying to obtain the valsartan. The method is simple to operate, high in yield and low in cost, the mass yield is over 85 percent, the obtained valsartan is high in quality, and the used reagents are common reagents which are cheap and easily obtained. Meanwhile, the method is an industrial crystallization method which is very suitable for industrial production and provides a technical support for large-scale industrial production.
Owner:浙江新赛科药业有限公司
Methods of treatment and pharmaceutical composition
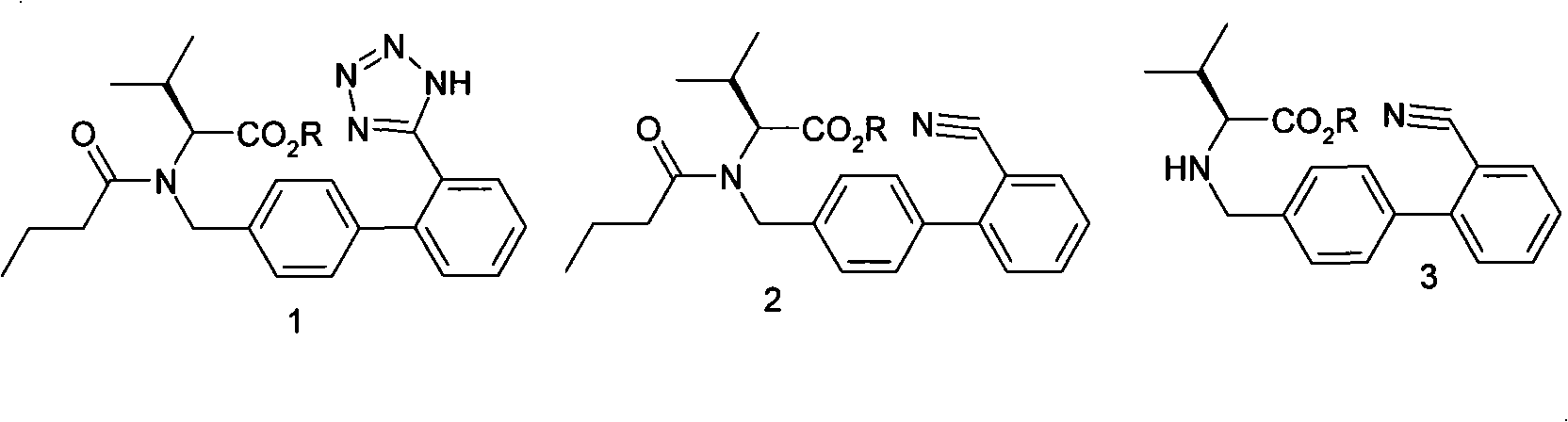
The invention relates a pharmaceutical composition comprising a combination of:(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof; and(ii) a NEP inhibitor or a pharmaceutically acceptable salt thereof and optionally a pharmaceutically acceptable carrier and to a method for the treatment or prevention of a condition or diseaseselected from the group consisting of hypertension, heart failure, such as (acute and chronic) congestive heart failure, left ventricular dysfunction and hypertrophic cardiomyopathy, diabetic cardiac myopathy, supraventricular and ventricular arrhythmias, atrial fibrillation, atrial flutter, detrimental vascular remodeling, myocardial infarction and its sequelae, atherosclerosis, angina (whether unstable or stable), renal insufficiency (diabetic and non-diabetic), heart failure, angina pectoris, diabetes, secondary aldosteronism, primary and secondary pulmonary hypertension, renal failure conditions, such as diabetic nephropathy, glomerulonephritis, scleroderma, glomerular sclerosis, proteinuria of primary renal disease, and also renal vascular hypertension, diabetic retinopathy, the management of other vascular disorders, such as migraine, peripheral vascular disease, Raynaud's disease, luminal hyperplasia, cognitive dysfunction, such as Alzheimer's, glaucoma and stroke, comprising administering a therapeutically effective amount of the pharmaceutical composition to a mammal in need thereof.
Owner:NOVARTIS PHARM CORP
Valsartan dispersible tablet and preparation method thereof
ActiveCN101167723AGood curative effectLittle side effectsPill deliveryMacromolecular non-active ingredientsValsartanMedicine
The invention provides valsartan dispersible tablets and a preparation method thereof, which contain effective doses of valsartan and pharmaceutical adjuvants, which include disintegrants, diluents, binders, lubricants, glidants and surface Active agent, based on 100 parts of valsartan, the dosage of disintegrating agent is 2-50 parts, the dosage of diluent is 10-150 parts, the dosage of binder is 2-25 parts, and the dosage of lubricant is 0.5-20 parts. The dosage of the liquid agent is 0.2-10 parts, and the surfactant is 0.1-2.5 parts; the present invention also provides the preparation method of the valsartan dispersible tablet. Compared with other dosage forms, the valsartan dispersible tablet has a good dispersion state , Short disintegration time, rapid drug dissolution, convenient administration, low production cost, no need for special equipment, convenient and stable carrying and transportation, etc.
Owner:HAINAN HUALON PHARM
Compound amlodipine and valsartan solid preparation and preparation method thereof
InactiveCN101744813AEasy to operateGood value for industrial productionPharmaceutical product form changeCapsule deliveryValsartanMedicine
The invention discloses a compound amlodipine and valsartan solid preparation and a preparation method thereof. The preparation can be a tablet or a capsule and contains 2.5-10g of amlodipine or pharmaceutically acceptable salt thereof (calculated according to the amlodipine) and 40-640g of valsartan or pharmaceutically acceptable salt thereof (calculated according to the valsartan). The preparation method of the compound amlodipine and valsartan solid preparation comprises the steps of mixing the two medicines and the corresponding medicinal auxiliary materials according to the proportion, mixing with the corresponding lubricant or glidant after granulating in a wet method, and pressing into a tablet or filling into a capsule so as to obtain the compound amlodipine and valsartan solid preparation. In the invention, the tablet or the capsule is prepared after the two active substances with unequal doses are granulated in the wet method, and the method is simple and convenient, is easy to control the quality and is suitable for the industrialized production. More than 85% of the amlodipine in the preparation is dissolved out within 30 minutes, and the invention has favorable market popularization prospect.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Valsartan amlodipine capsule medicine compound and preparation method thereof
The invention relates to a valsartan amlodipine capsule medicine compound and a preparation method thereof. The compound uses valsartan and amlodipine as raw materials, containing one or more medicinal excipient. The preparation method thereof comprises the step of respectively mixing the valsartan and amlodipine with selective thinner, adhesive, disintegrating agent, antitack agent and lubricant, adding a proper wetting agent to make into softwood, sieving for making into wet grain, drying the wet grain, sieving, mixing uniformly and making into orally taken preparation which has obvious effect for treating hypertension.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Valsartan compound and new manufacturing method thereof
InactiveCN102093302AEasy to refineEffectively refinedOrganic chemistryCardiovascular disorderValsartanSide effect
The invention provides a valsartan compound and a new manufacturing method thereof. In the invention, through specially designed methods for acid and alkali conversion and absorption by macroporous absorption resin, the aim of refining and purifying can be fulfilled; and thus, the high-purity valsartan compound is obtained, the product quality of the preparation is improved, the toxic and side effects are reduced, and the clinic medicine safety of the valsartan in the preparation of antihypertensive medicines is guaranteed.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
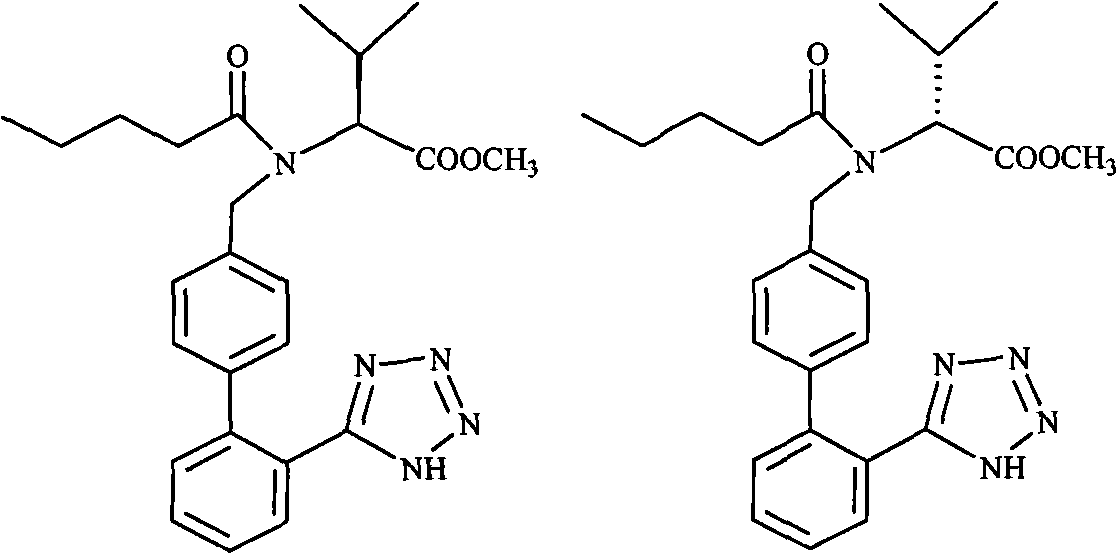
Process for preparing Valsartan
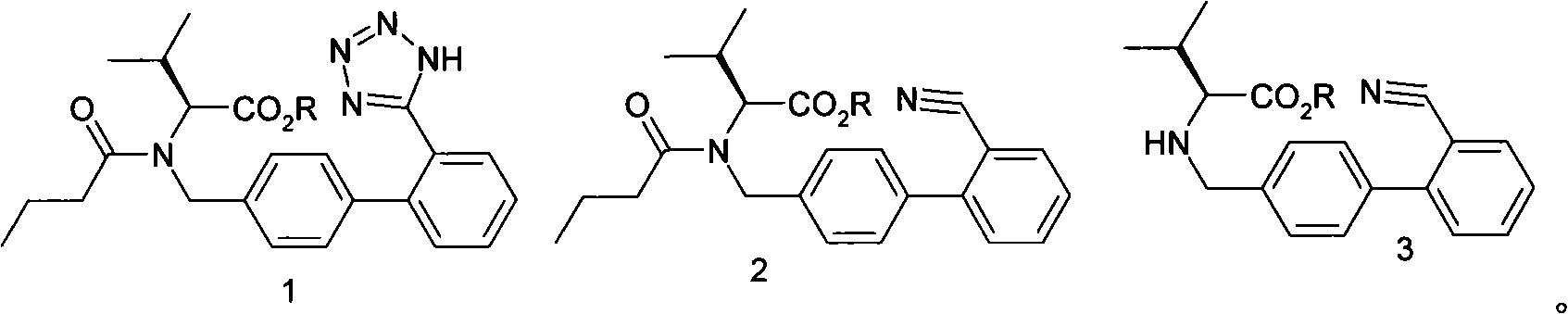
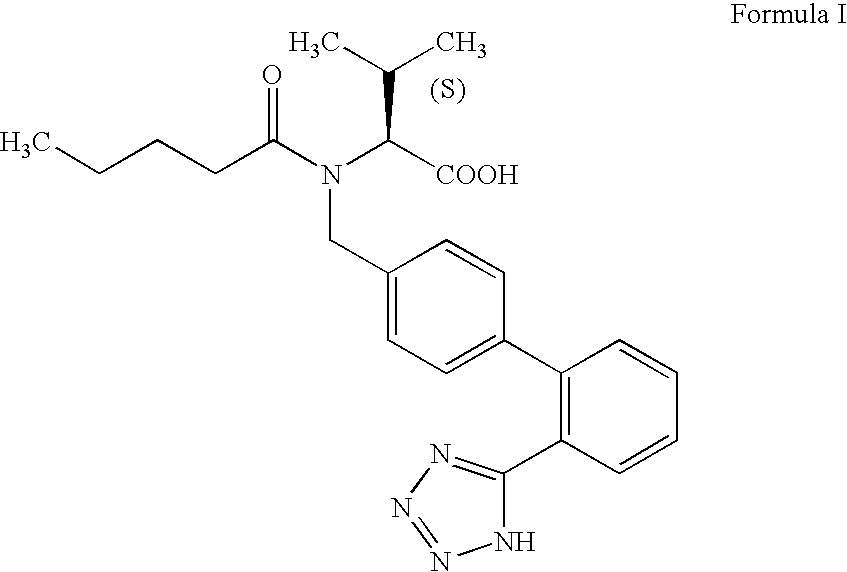
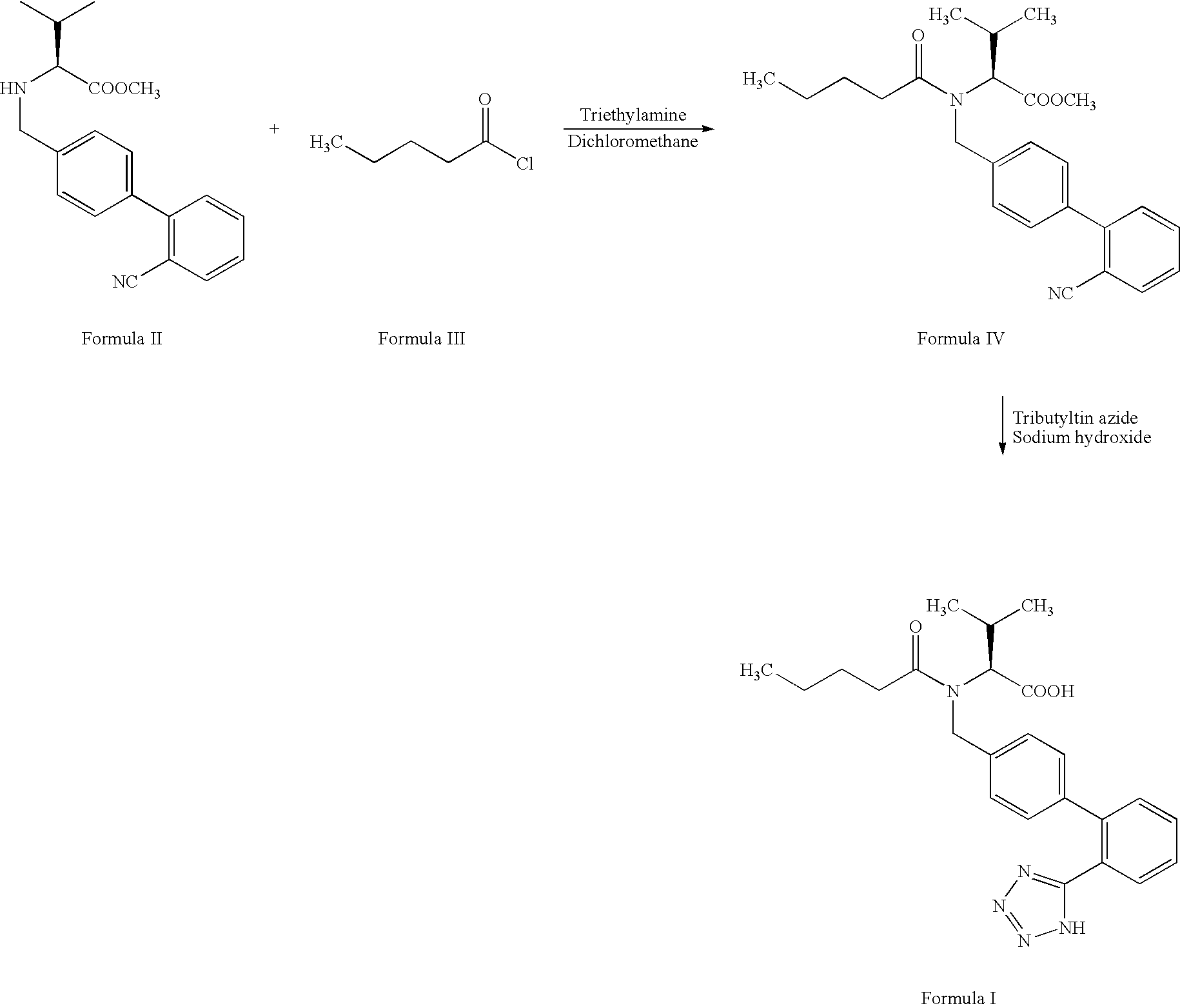
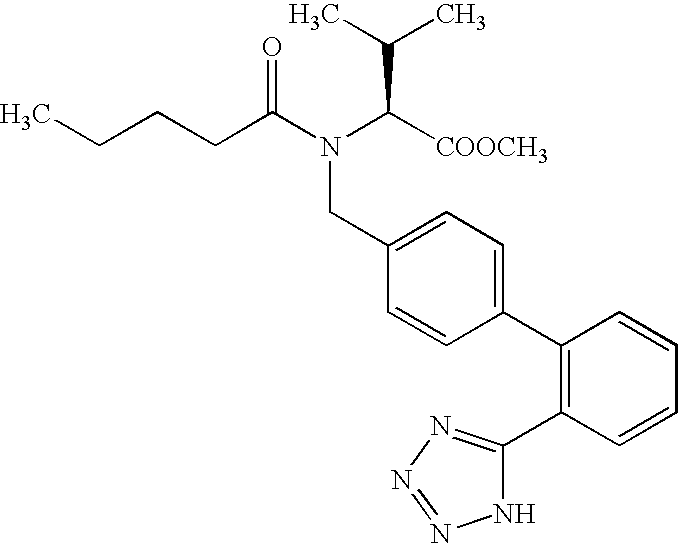
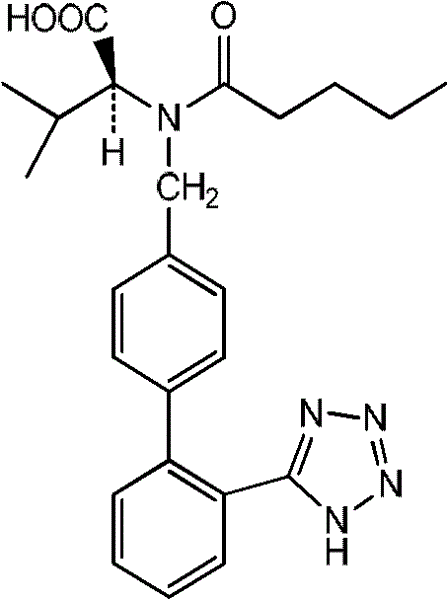
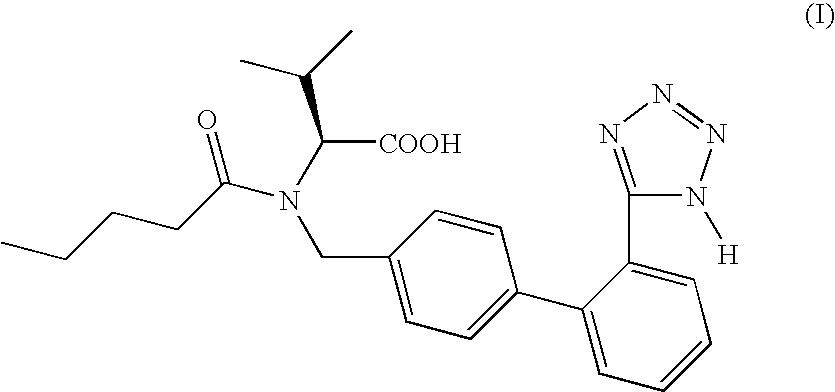
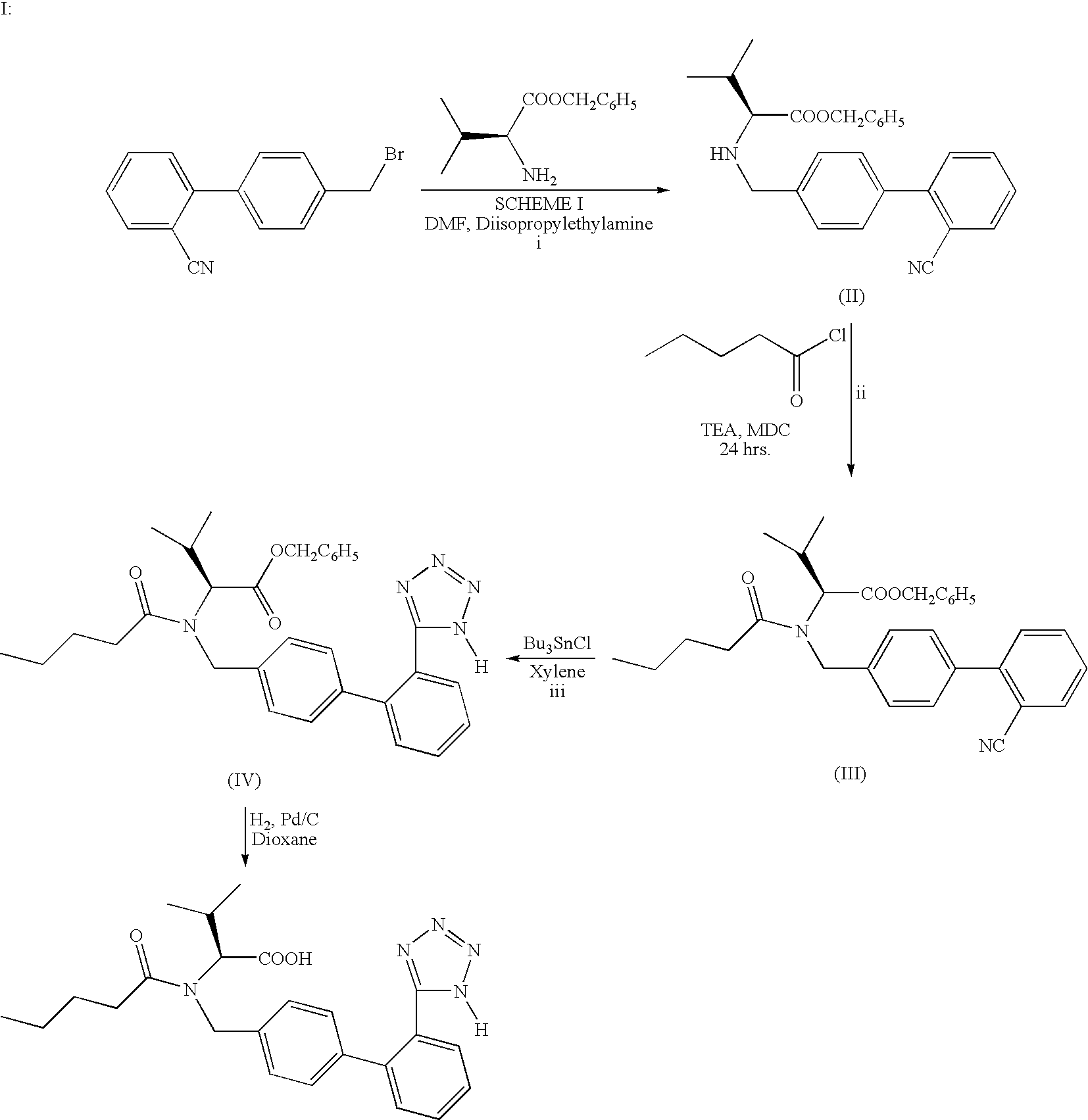
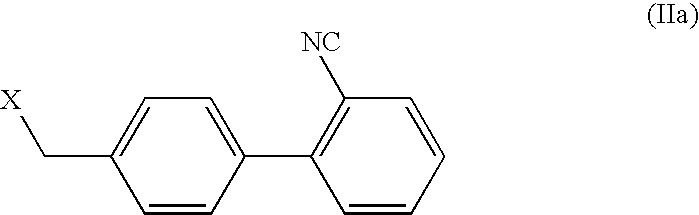
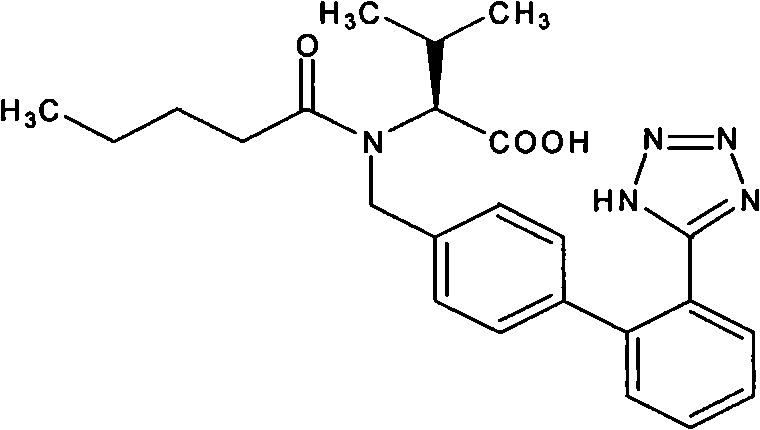
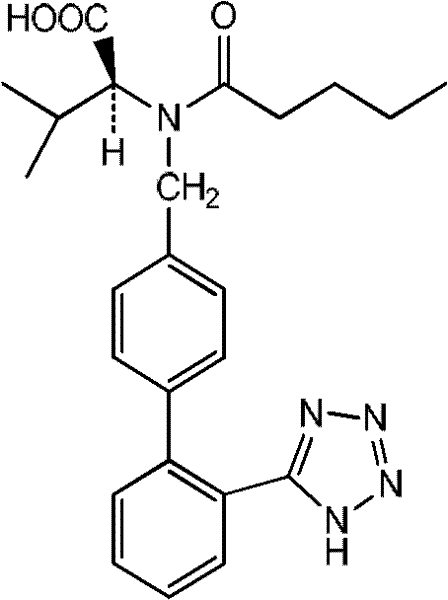
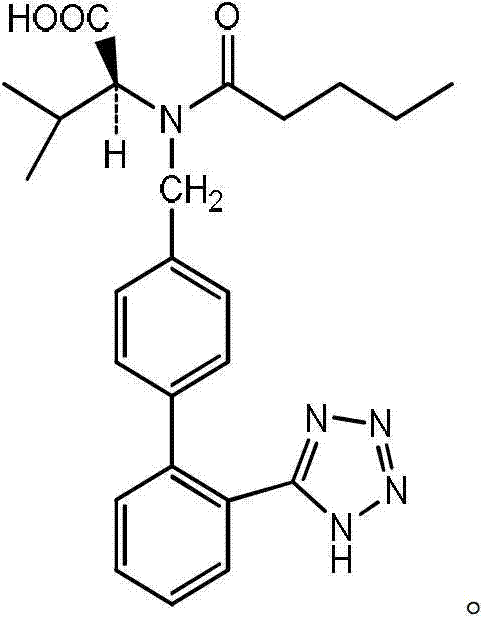
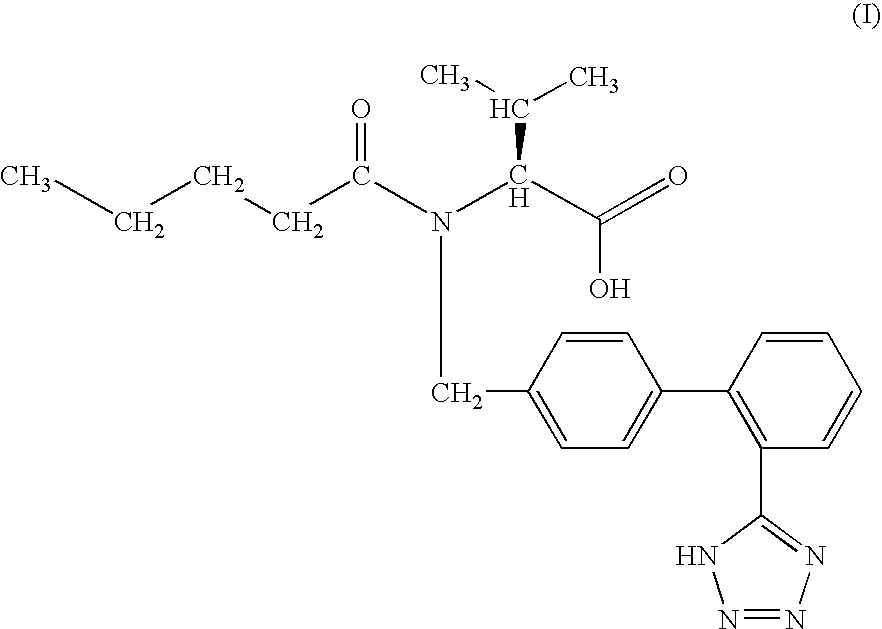
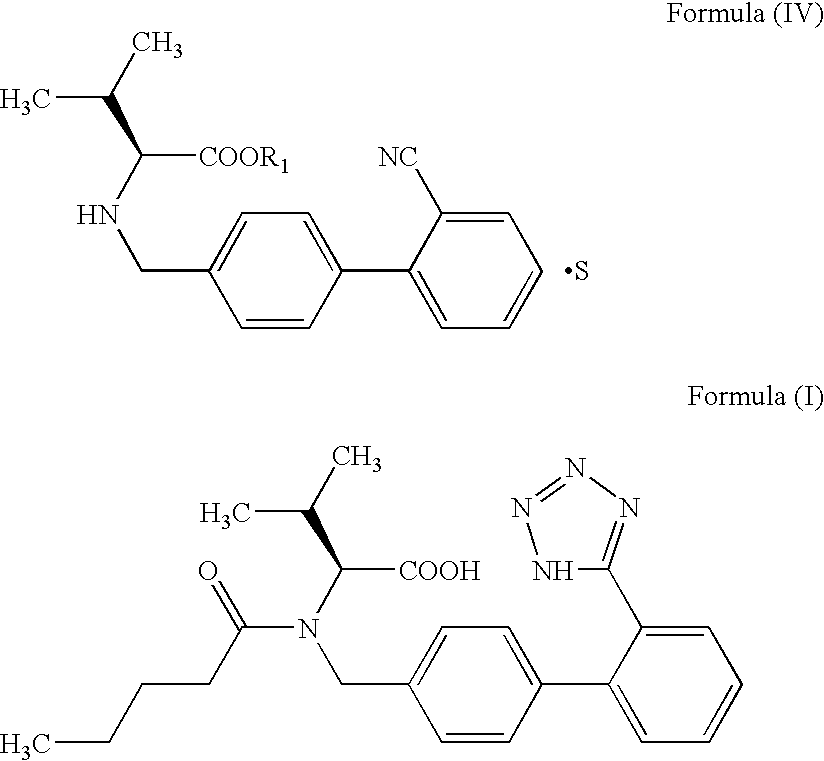
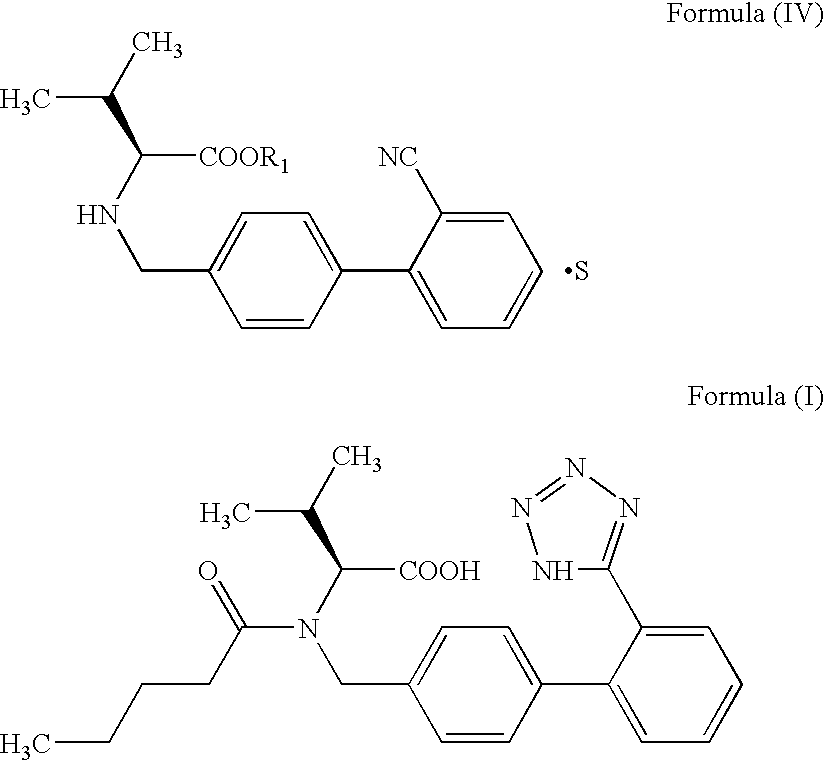
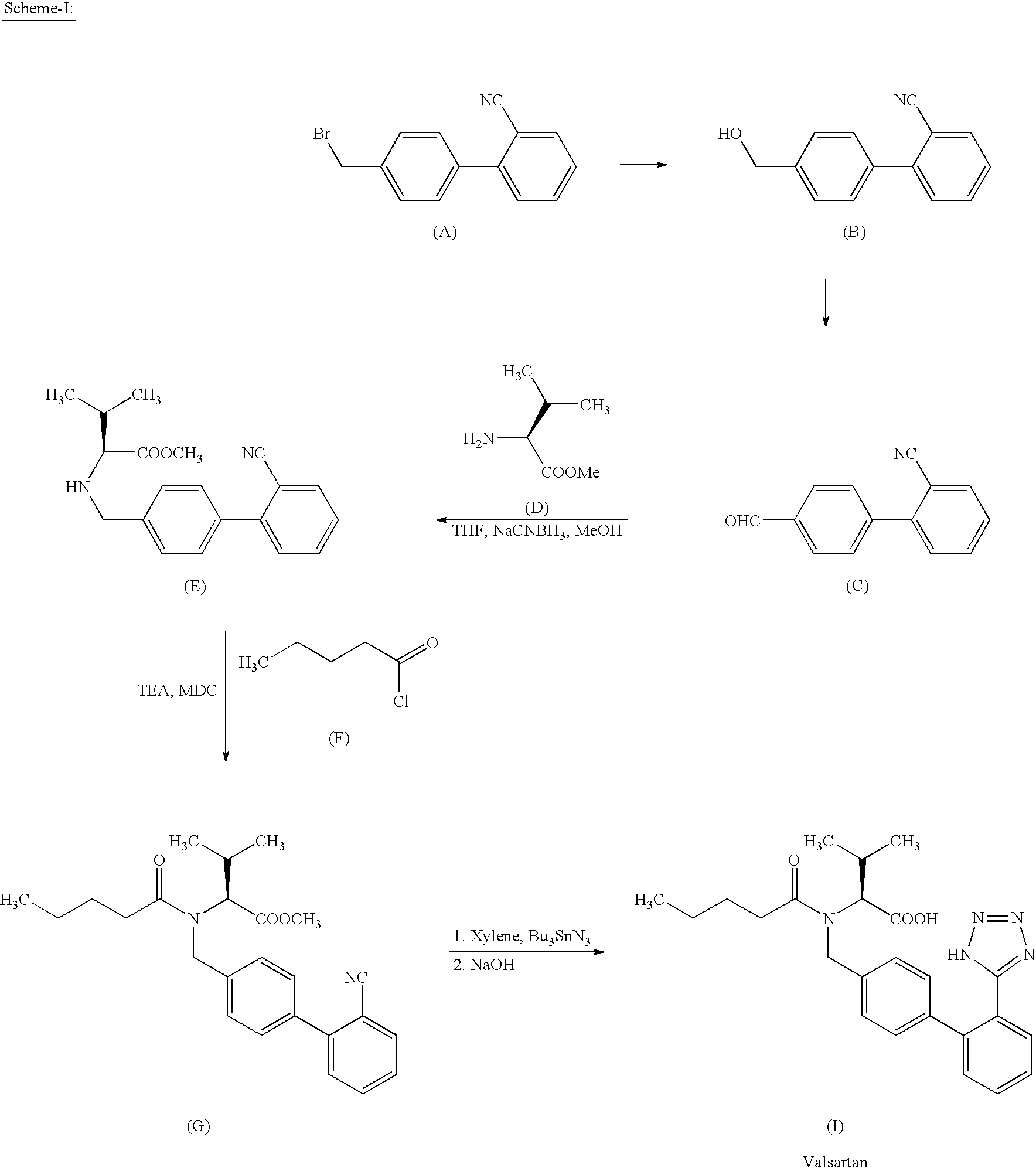
The invention relates to novel compound of formula (IV), which is an organic acid salt of N-[(2′-cyanobiphenyl-4-yl)methyl]-(L)-valine ester. This compound is an useful intermediate for process of preparation of Valsartan of formula (I), chemically known as (S)—N-(1-Carboxy-2-methylprop-1-yl)-N-pentanoyl-N-[2′-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl]amine. This invention also relates to a process for preparing Valsartan using novel intermediate of formula (IV).
Owner:ALEMBIC LTD
Compound valsartan benzenesulfonic acid amlodipine medicament composition and new preparation method thereof
ActiveCN101836981APrescription stableReasonable prescriptionCapsule deliveryOil/fats/waxes non-active ingredientsValsartanPharmaceutical Substances
The invention relates to a compound valsartan benzenesulfonic acid amlodipine medicament composition and a new preparation method thereof. The compound valsartan benzenesulfonic acid amlodipine medicament composition is in a capsule type and is prepared by adopting the preparation method of a solid dispersion technique so that the dissolution of a medicament and the adsorption of the medicament in gastrointestinal tracts can be obviously promoted. The preparation method overcomes the defects of poor dissolution outside benzenesulfonic acid amlodipine and low bioavailability, greatly improve the quality of a preparation product and can realize industrial production.
Owner:天津汉嘉医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com