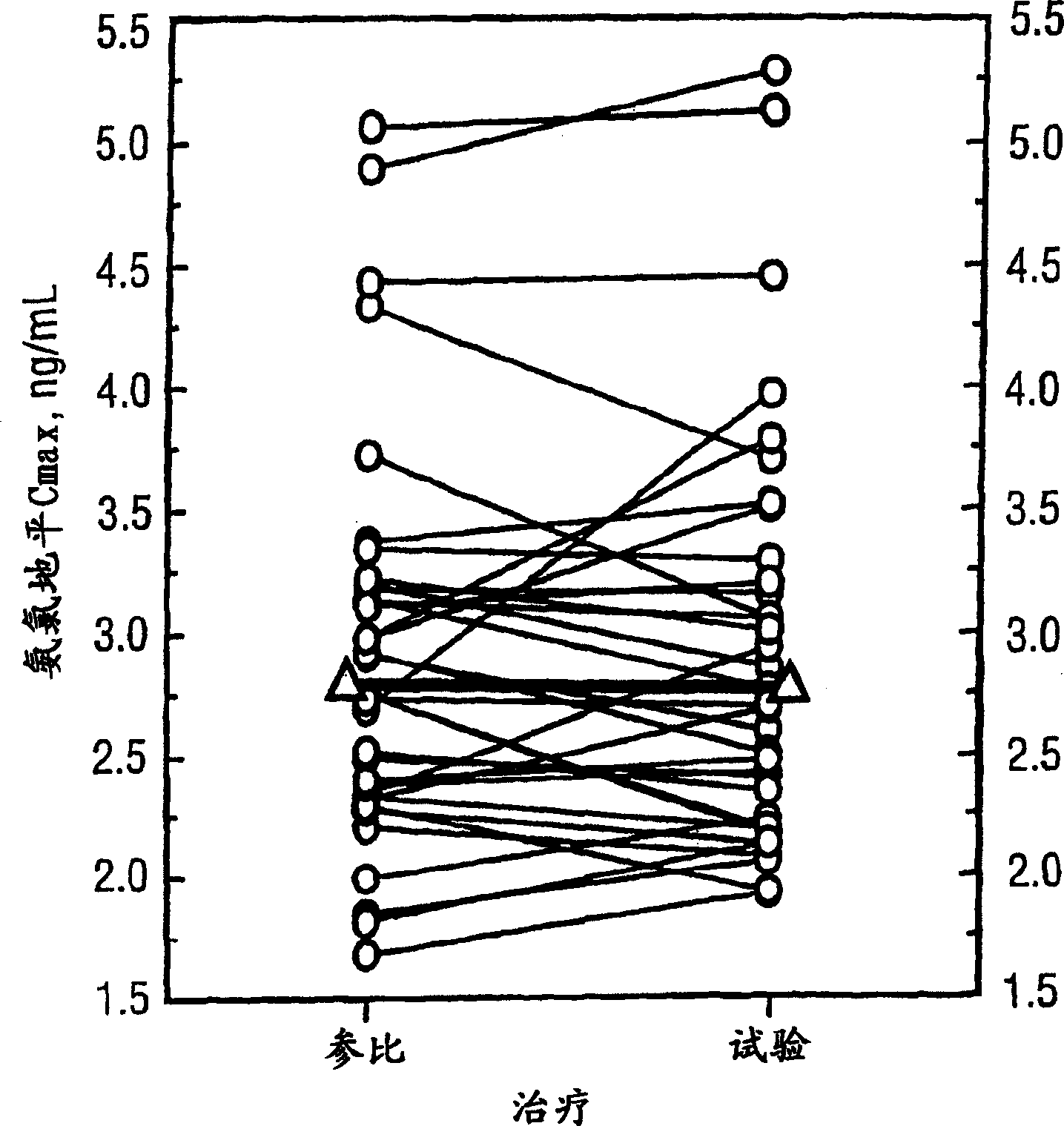
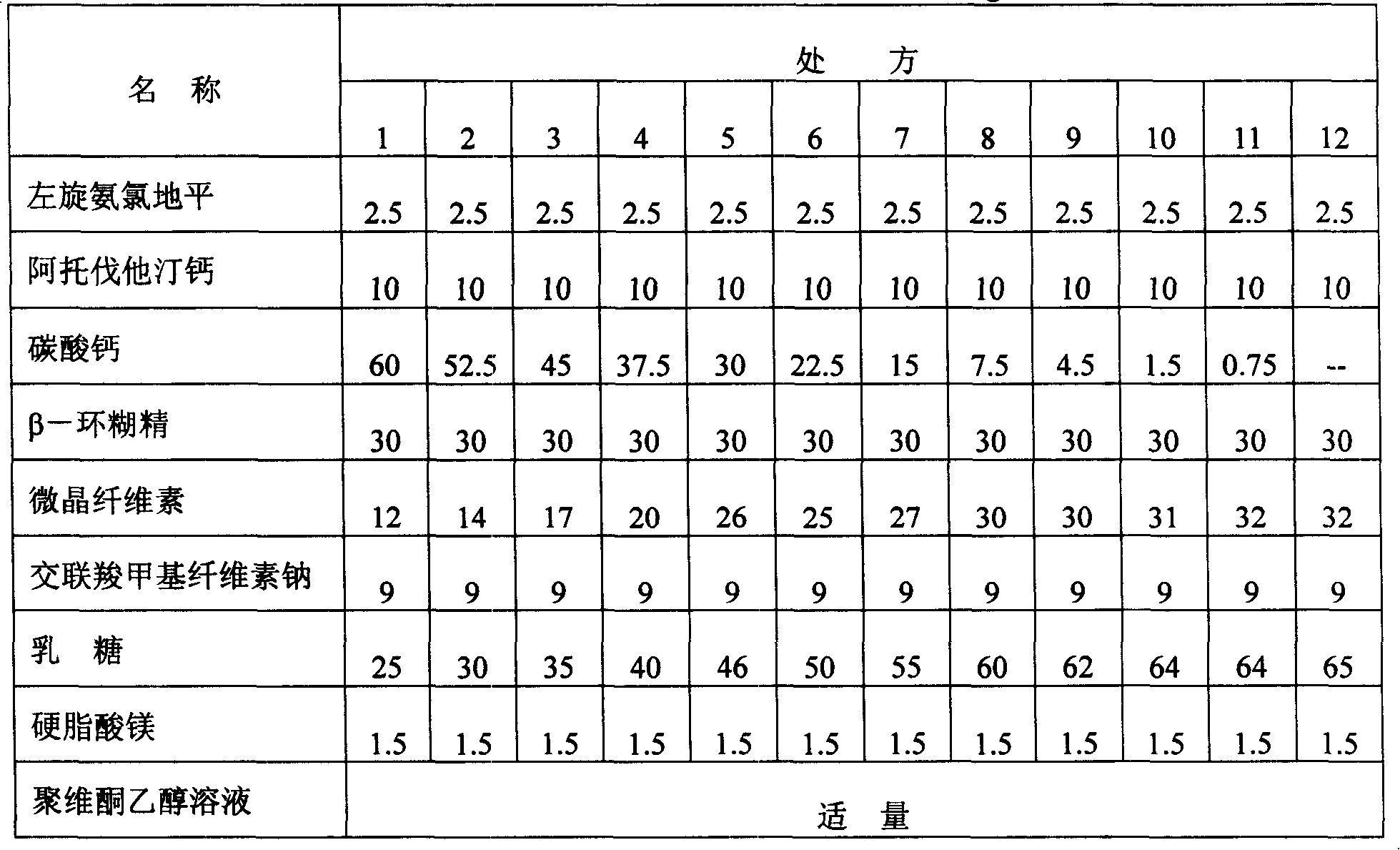
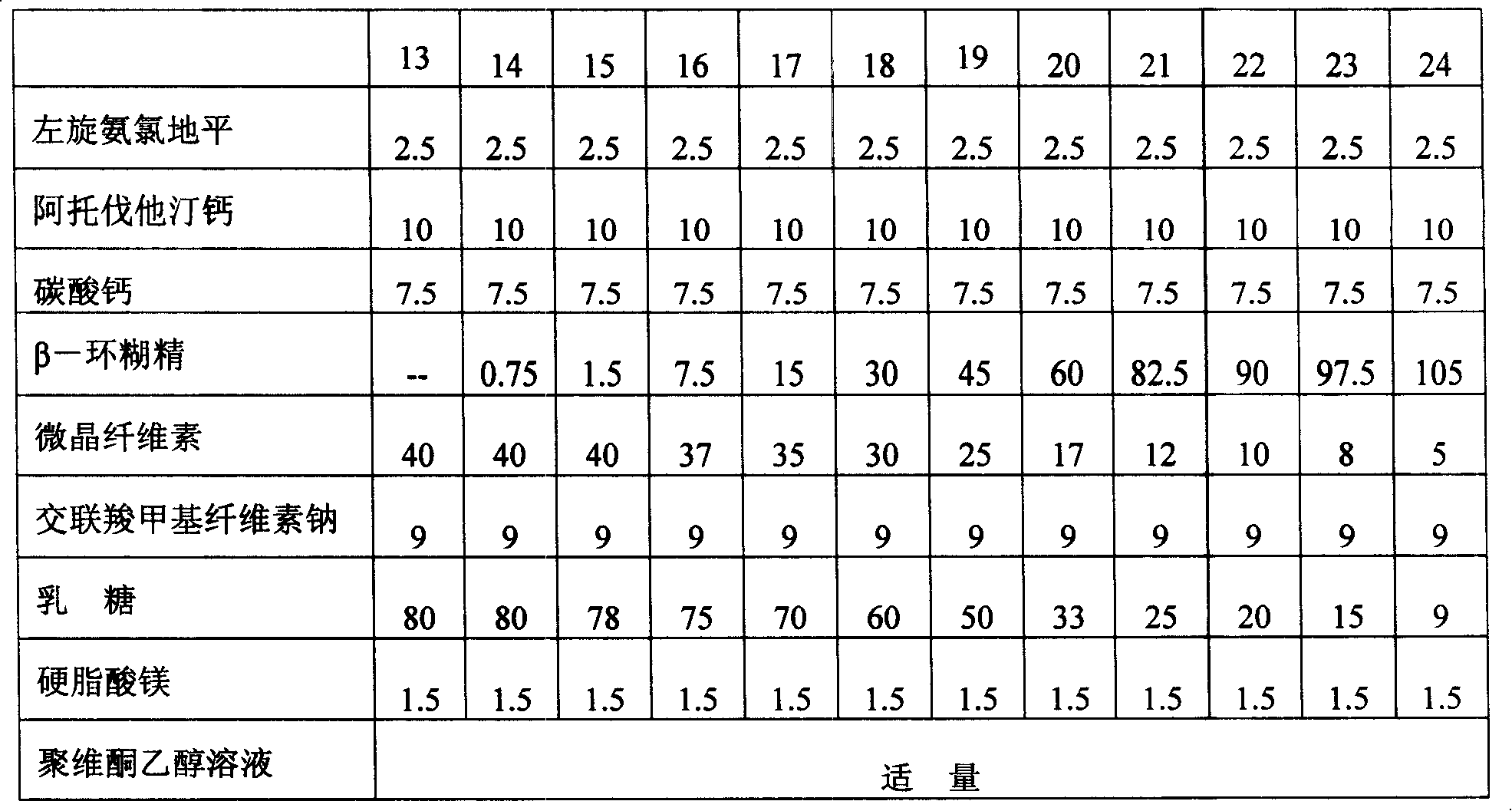
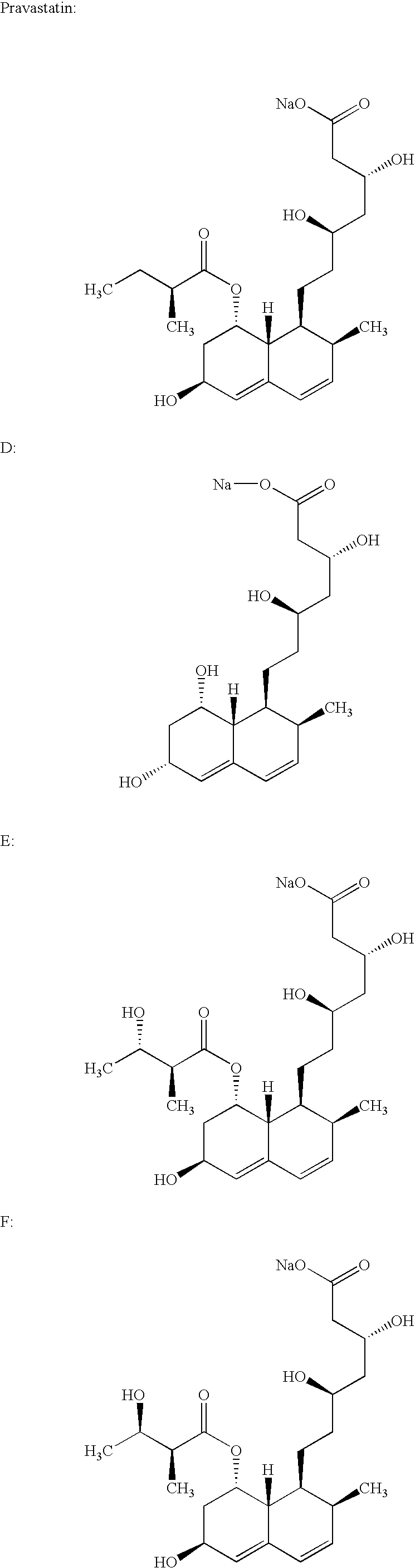
Patents
Literature
271 results about "Atorvastatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Atorvastatin is used along with a proper diet to help lower "bad" cholesterol and fats (such as LDL, triglycerides) and raise "good" cholesterol (HDL) in the blood.
Atorvastatin formulation
InactiveUS20090311322A1Reduce impactReduces food effectBiocidePowder deliveryBioavailabilityAtorvastatin
Provided are atorvastatin compositions which reduce the effect of food on the bioavailability of atorvastatin and methods for making such compositions. Also provided are methods of reducing low density lipoprotein by administering the compositions of the invention.
Owner:TEVA PHARMA IND LTD
Treatment of arteriosclerosis and xanthoma
A combination of one or more HMG-CoA reductase inhibitors (for example pravastatin, lovastatin, simvastatin, fluvastatin, rivastatin or atorvastatin) with one or more insulin sensitizers (for example troglitazone, pioglitazone, englitazone, BRL-49653, 5-(4-{2-[1-(4-2'-pyridylphenyl)ethylideneaminooxy]ethoxy}benzyl)thiazolidine-2,4-dione, 5-{4-(5-methoxy-3-methylimidazo[5,4-b]pyridin-2-ylmethoxy)benzyl}thiazolidine-2,4-dione or its hydrochloride, 5-[4-(6-methoxy-1-methylbenzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione, 5-[4-(1-methylbenzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione and 5-[4-(5-hydroxy-1,4,6,7-tetramethylbenzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione) exhibits a synergistic effect and is significantly better at preventing and / or treating arteriosclerosis and / or xanthoma than is either of the components of the combination alone.
Owner:DAIICHI SANKYO CO LTD
Pharmaceutical compositions of atorvastatin
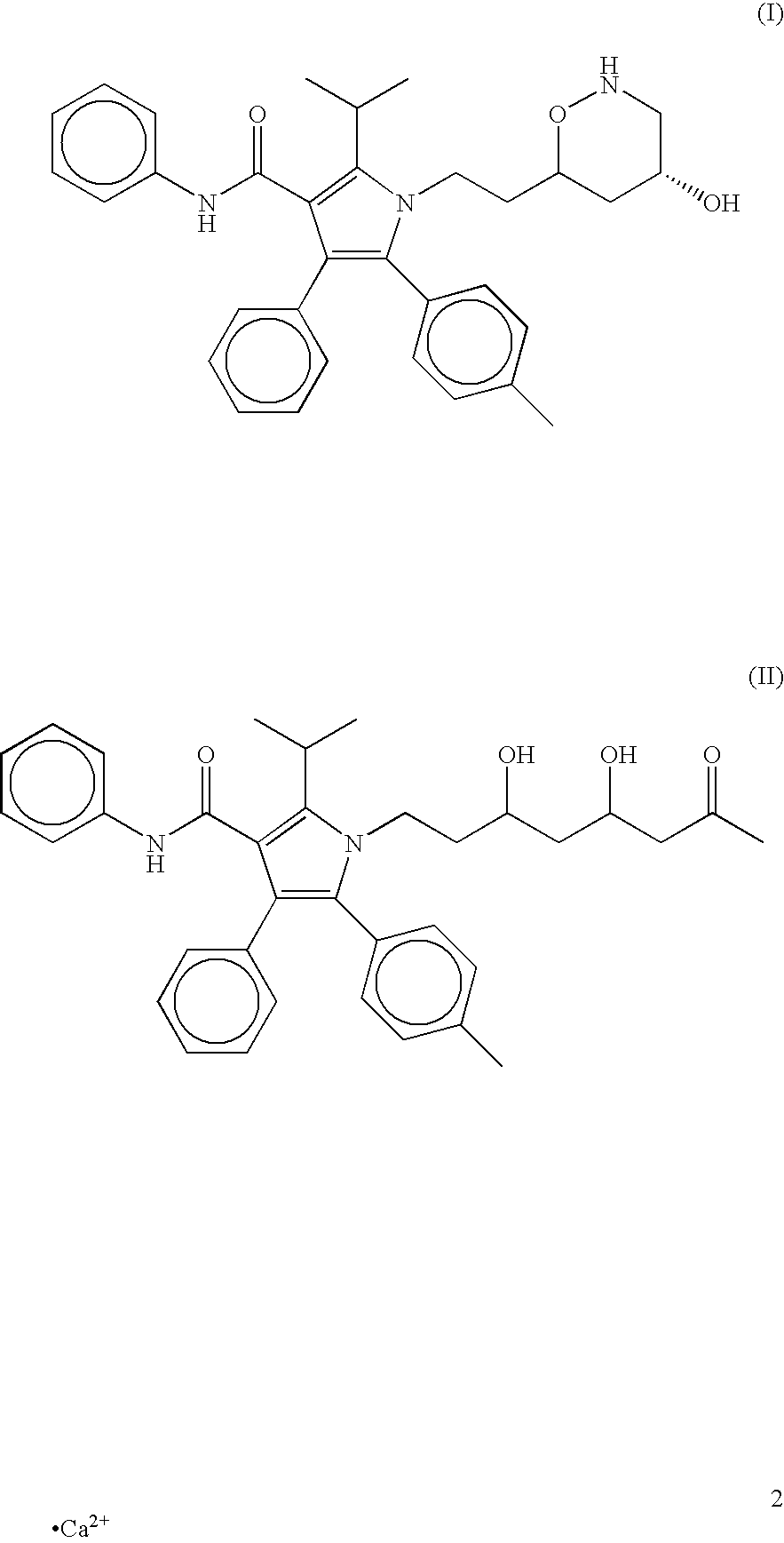
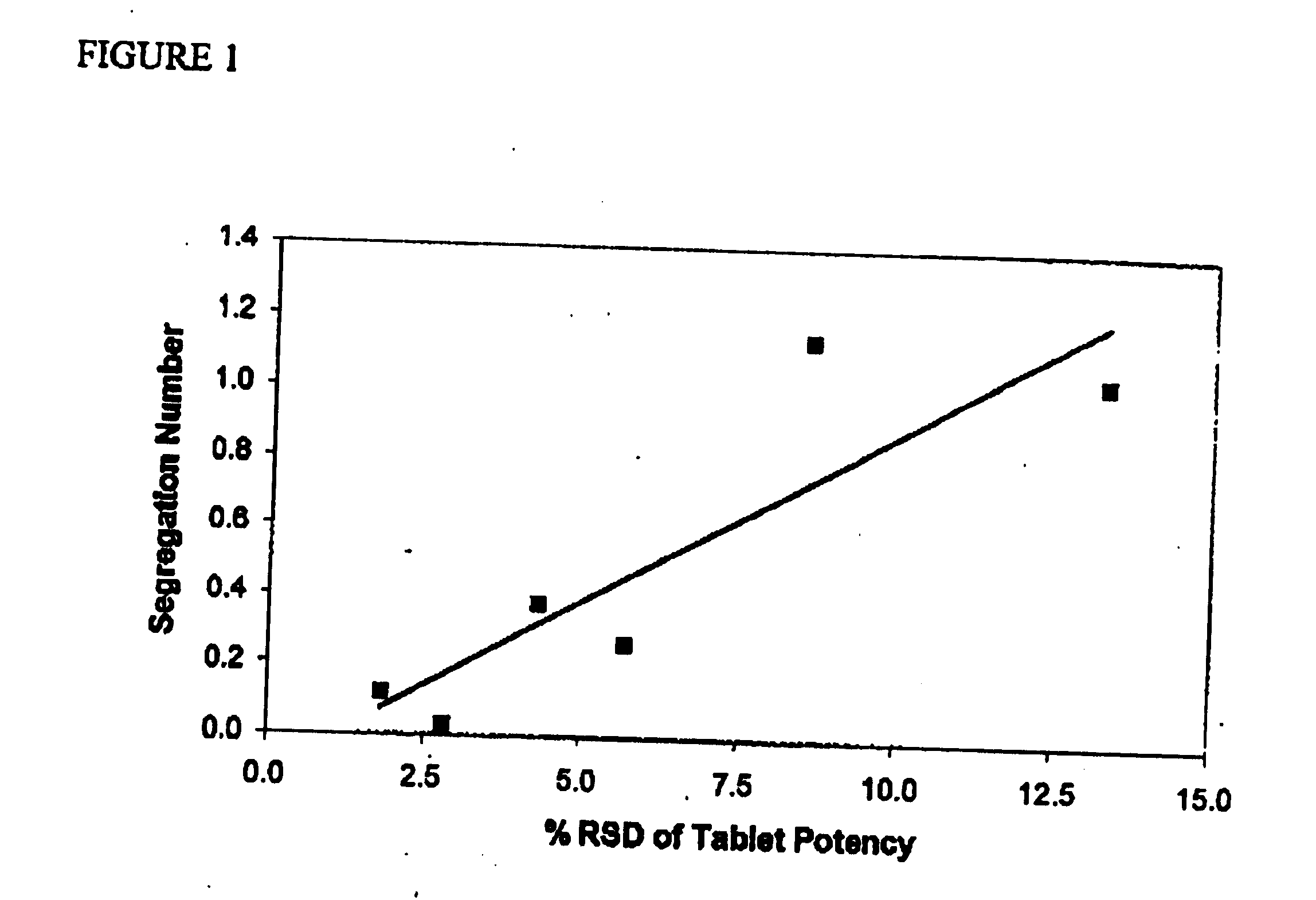
A unit dosage form comprising atorvastatin or a pharmaceutically acceptable salt thereof, prepared without a granulation step, wherein the measured atorvastatin potency of said dosage form shows a relative standard deviation (RSD) for atorvastatin potency per unit dosage form of not more than about 7.8%, when said unit dosage form is prepared at a rate that greater than 10,000 unit dosage forms per hour per single unit dosage form per machine, as well as the unit dsoage form in combination with at least one active drug, methods for preparing unit dosage form, kits for containing such compositions and a method of treating hypercholesterolemia and / or hyperlipidemia, osteoporosis, benign prostatic hyperplasia (BPH), and Alzheimer's Disease using a therapeutically effective amount of the unit dosage form.
Owner:BERCHIELLI ALFRED +2
Compositions comprising fenofibrate and rosuvastatin
InactiveUS20050096391A1Improve bioavailability in vivoSubstance may accumulateBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
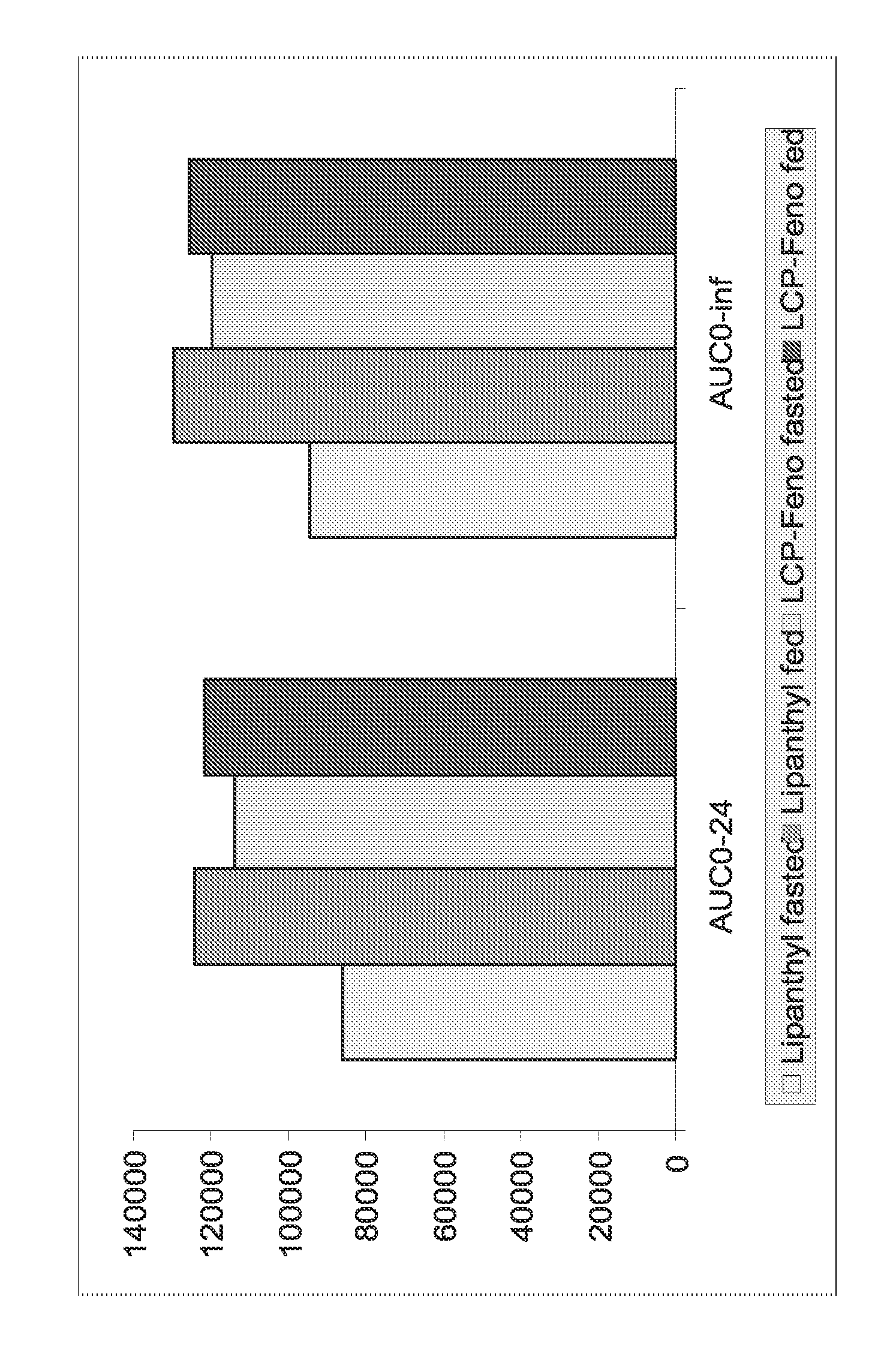
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor rosuvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCrosuvastatin) of between about 150 and about 12,000. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and rosuvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Process for the synthesis of atorvastatin and phenylboronates as intermediate compounds
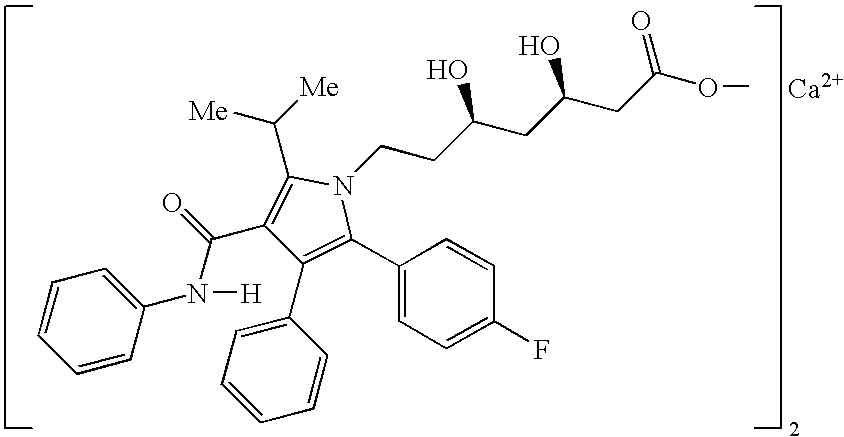
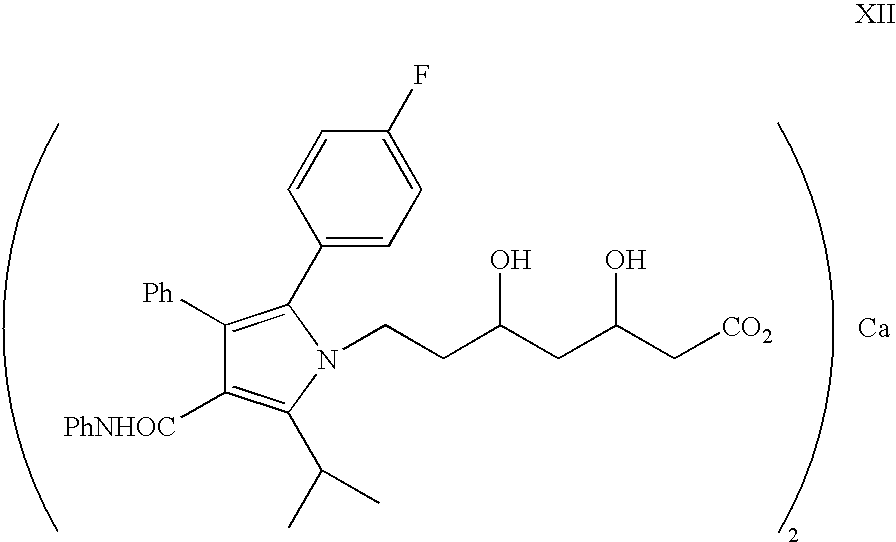
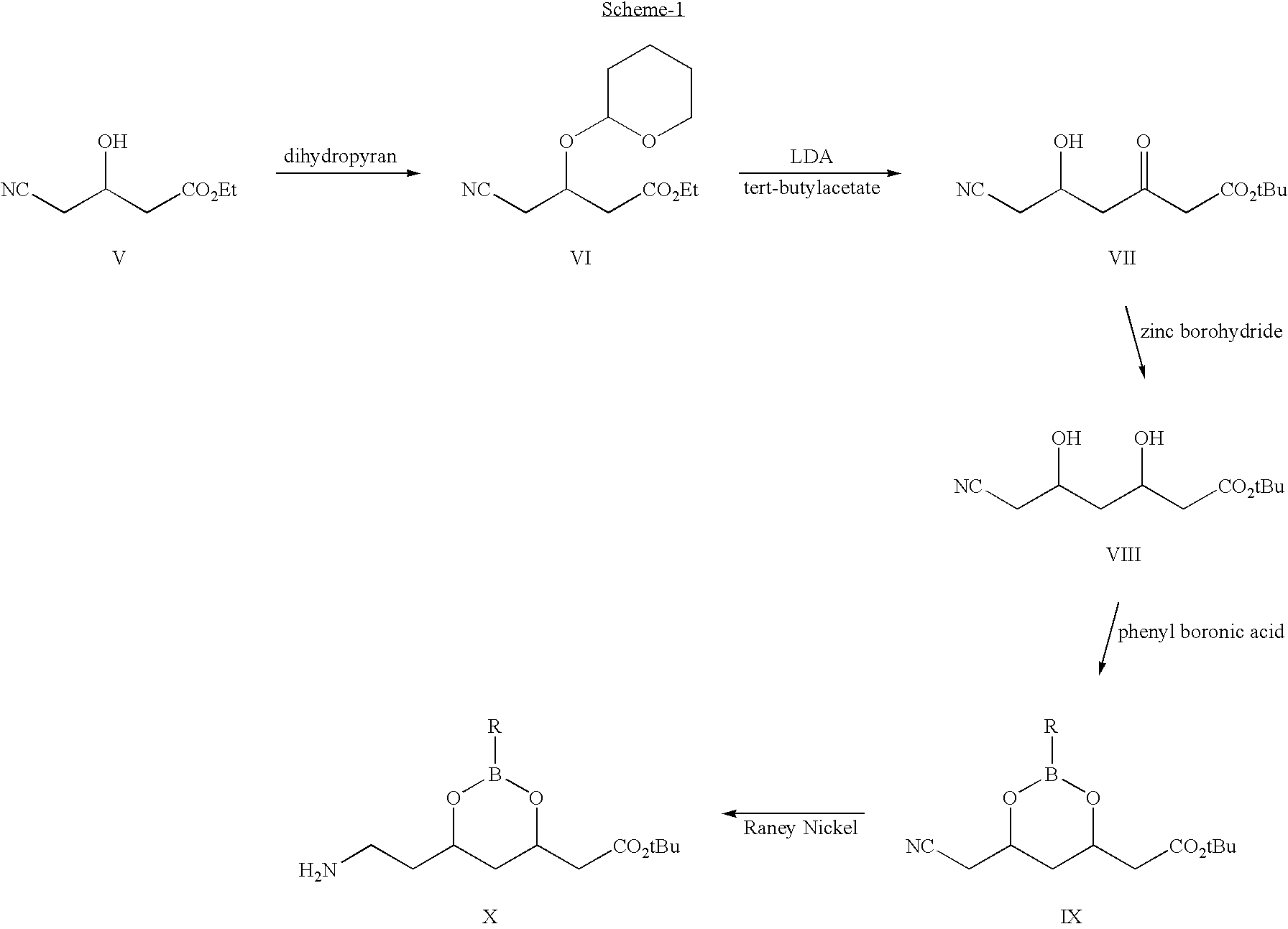
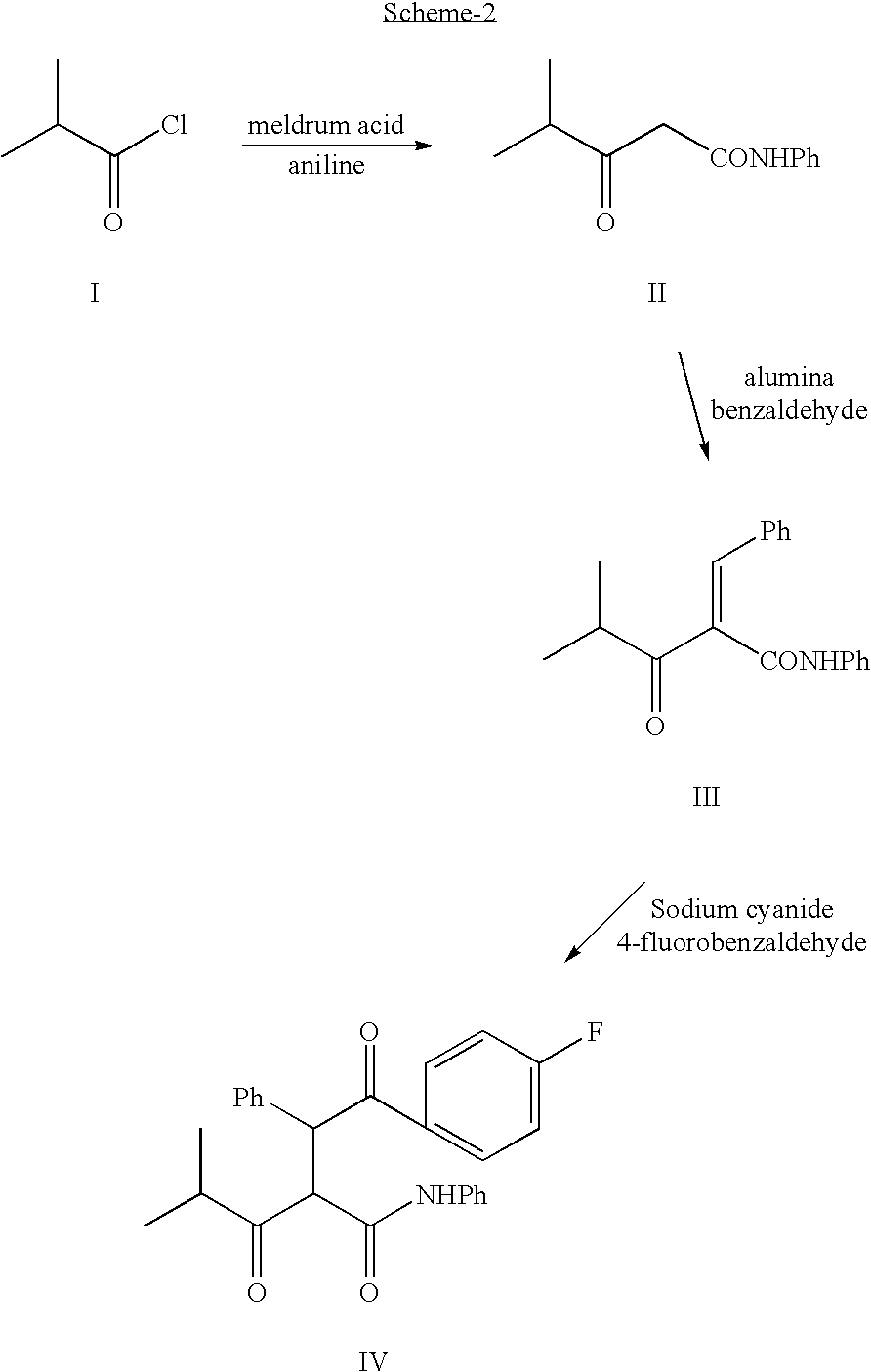
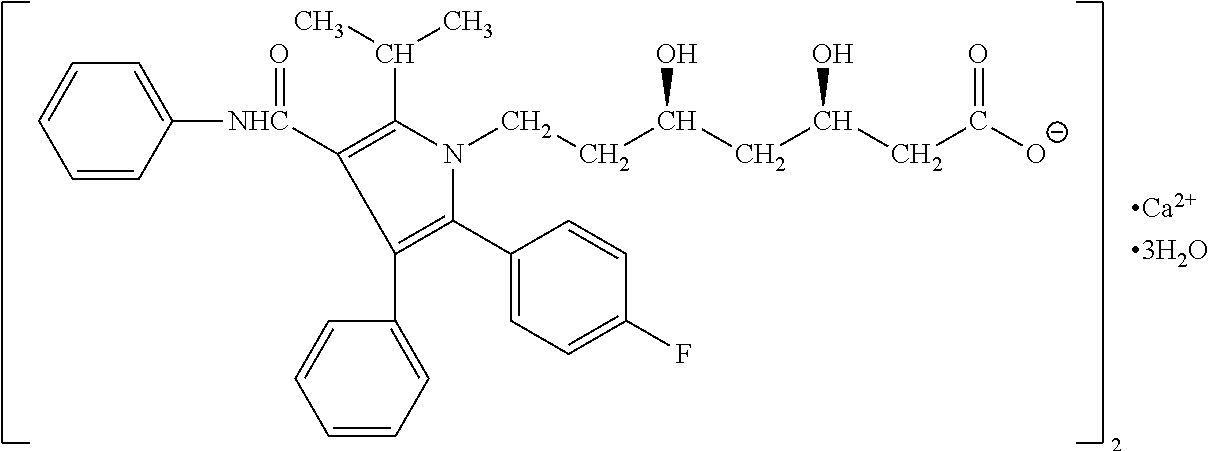
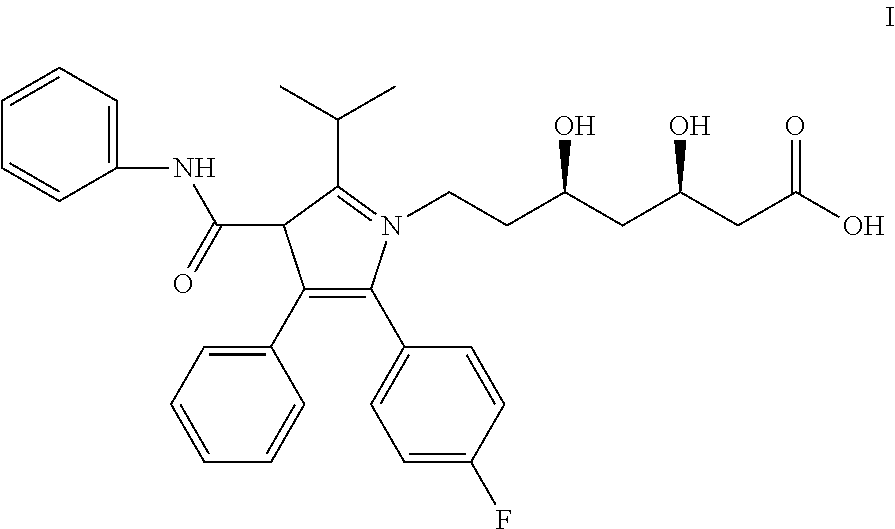
The present invention discusses a novel process for the synthesis of [R-(R*,R*)]-2-(4-fluorophenyl)-B,D-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid hemi calcium, atorvastatin. The compound so prepared is useful as inhibitor of the HMG-CoA reductase and may thus be used as hypolipidemic and hypocholesterolemic agent.
Owner:BIOCON LTD
Pharmaceutical compositions comprising fenofibrate and atorvastatin
InactiveUS20070014846A1Improve bioavailabilityReducing inter-individual variationBiocidePill deliveryParticulatesHMG-CoA reductase
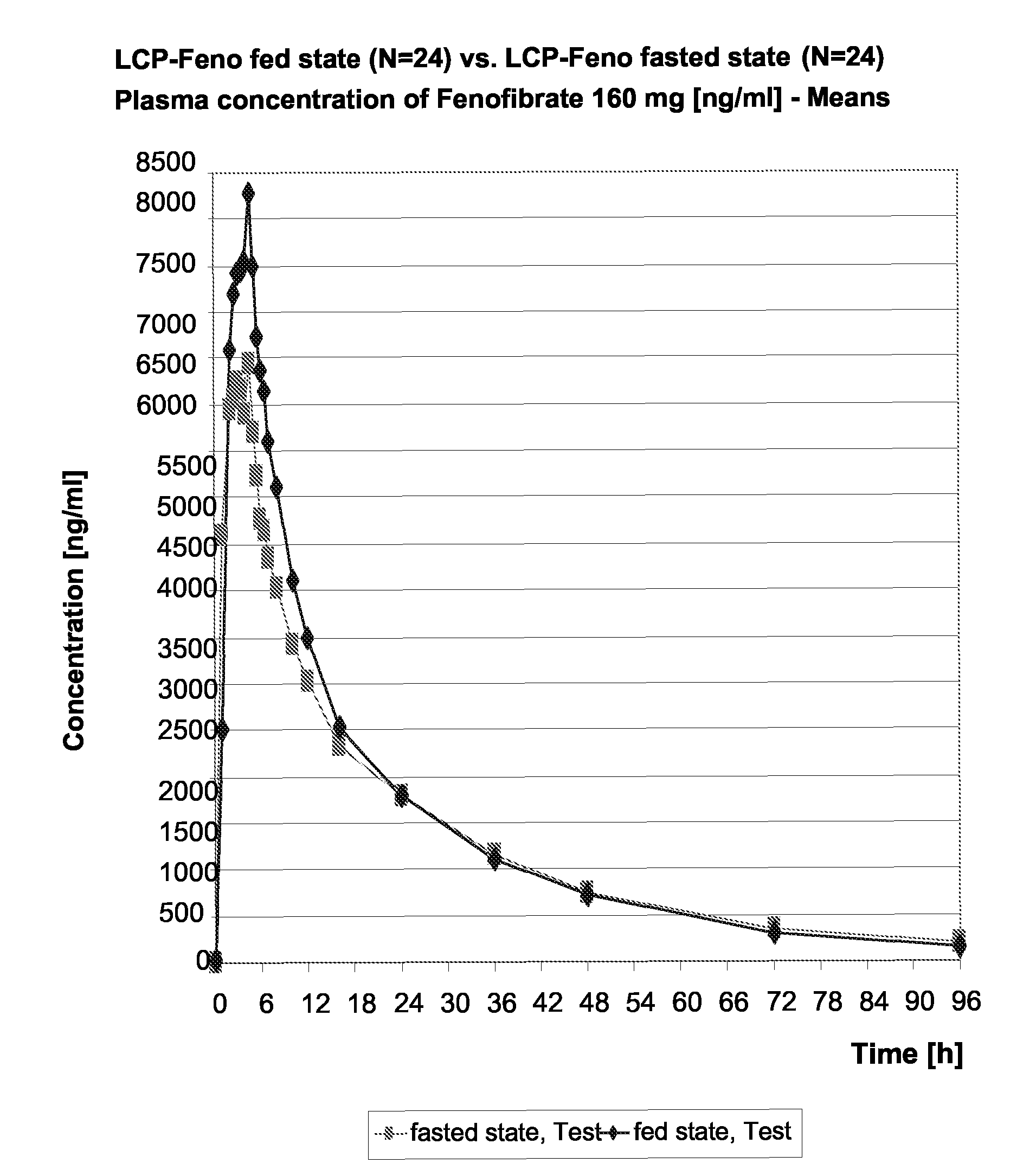
Pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor atorvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCatorvastatin) of between about 250 and about 10,000. The solid compositions are manufactured without any need of addition of water or aqueous medium. Atorvastatin is optionally provided as a controlled release or a delayed release formulation resulting in a maintained LDL-lowering effect at a reduced dosage, and fenofibrate is provided in a formulation having increasing bioavailability and reduced food effect.
Owner:VELOXIS PHARMA
Synergistic effects of amlodipine and atorvastatin metabolite as a basis for combination therapy
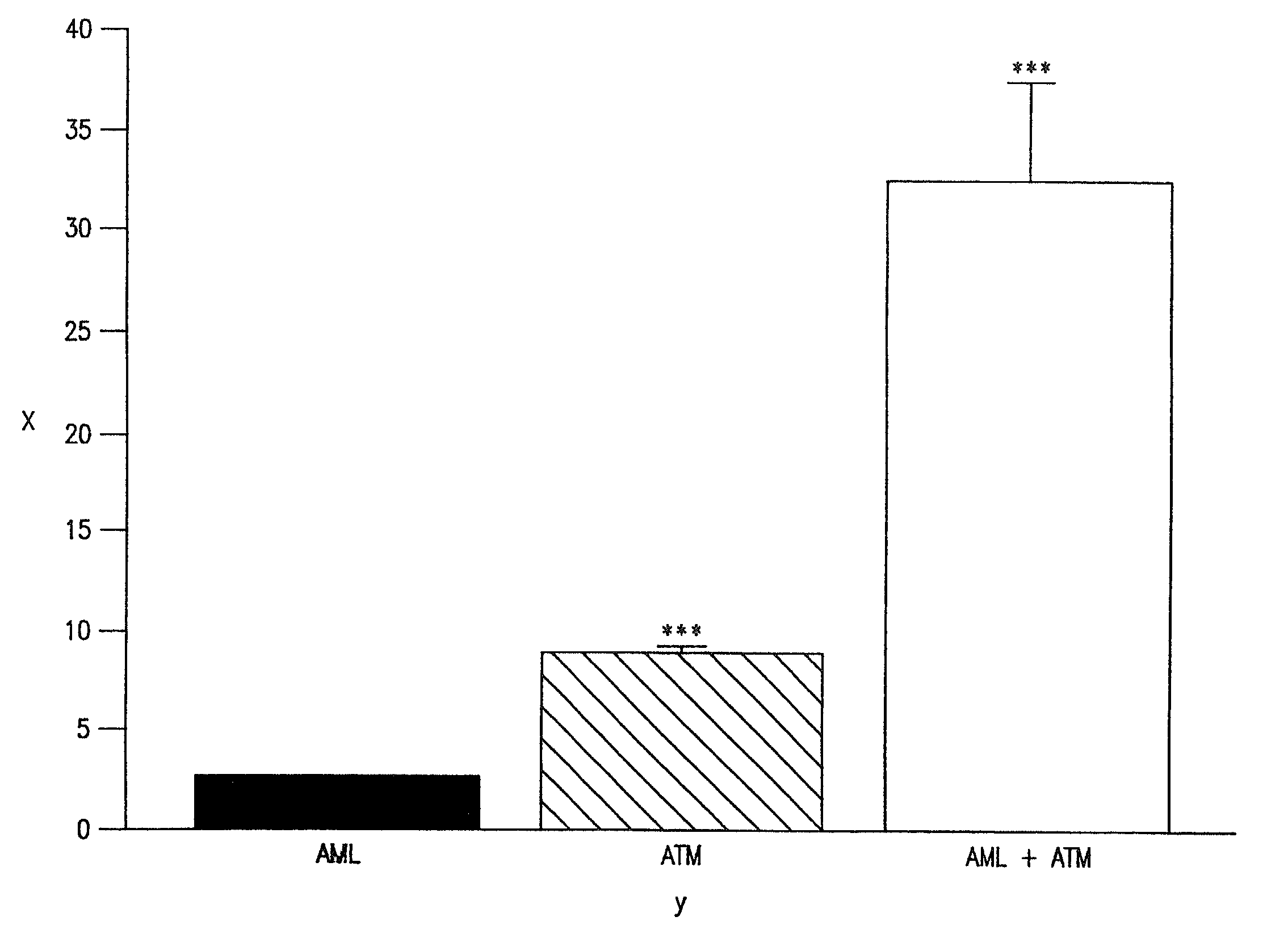
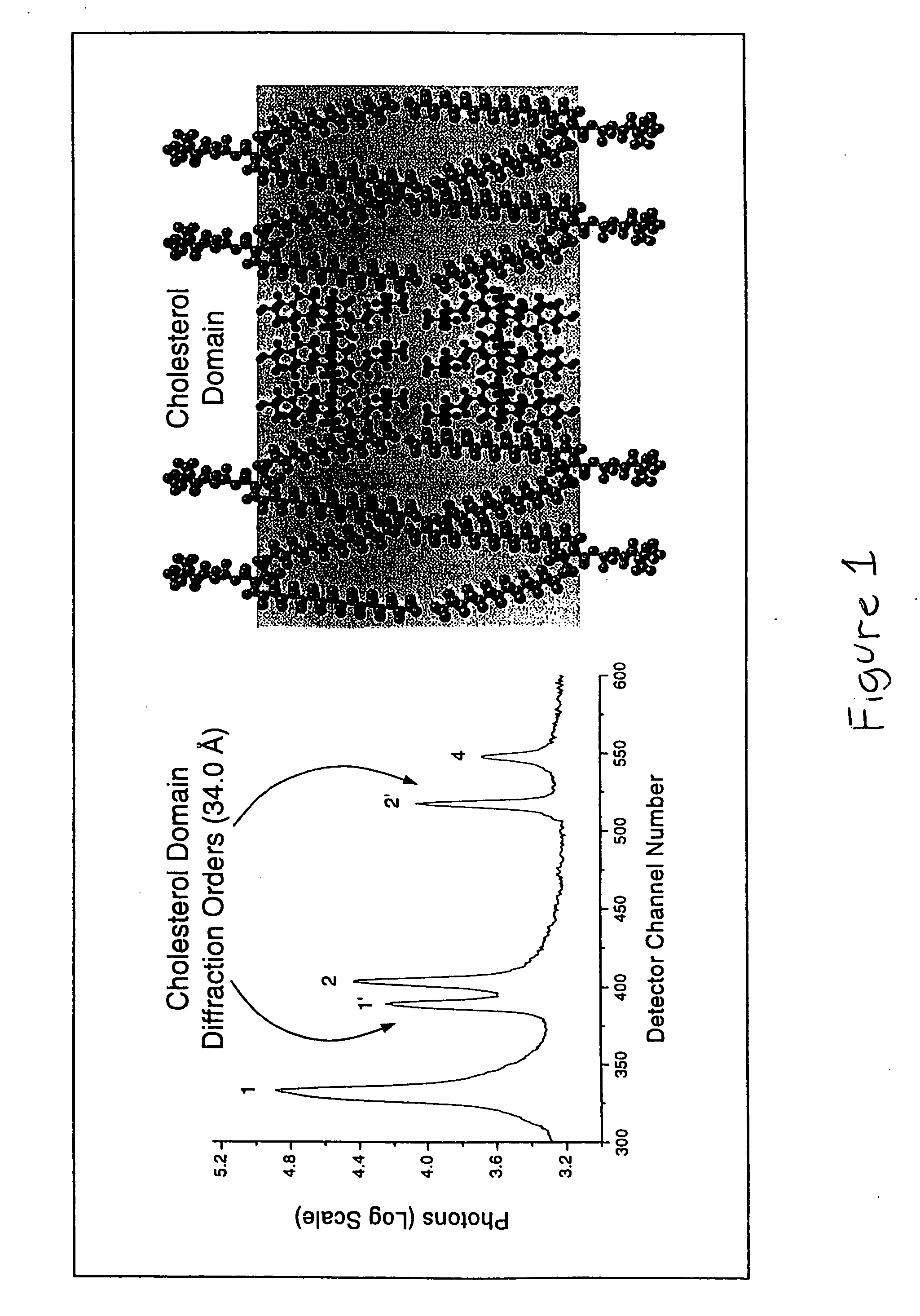
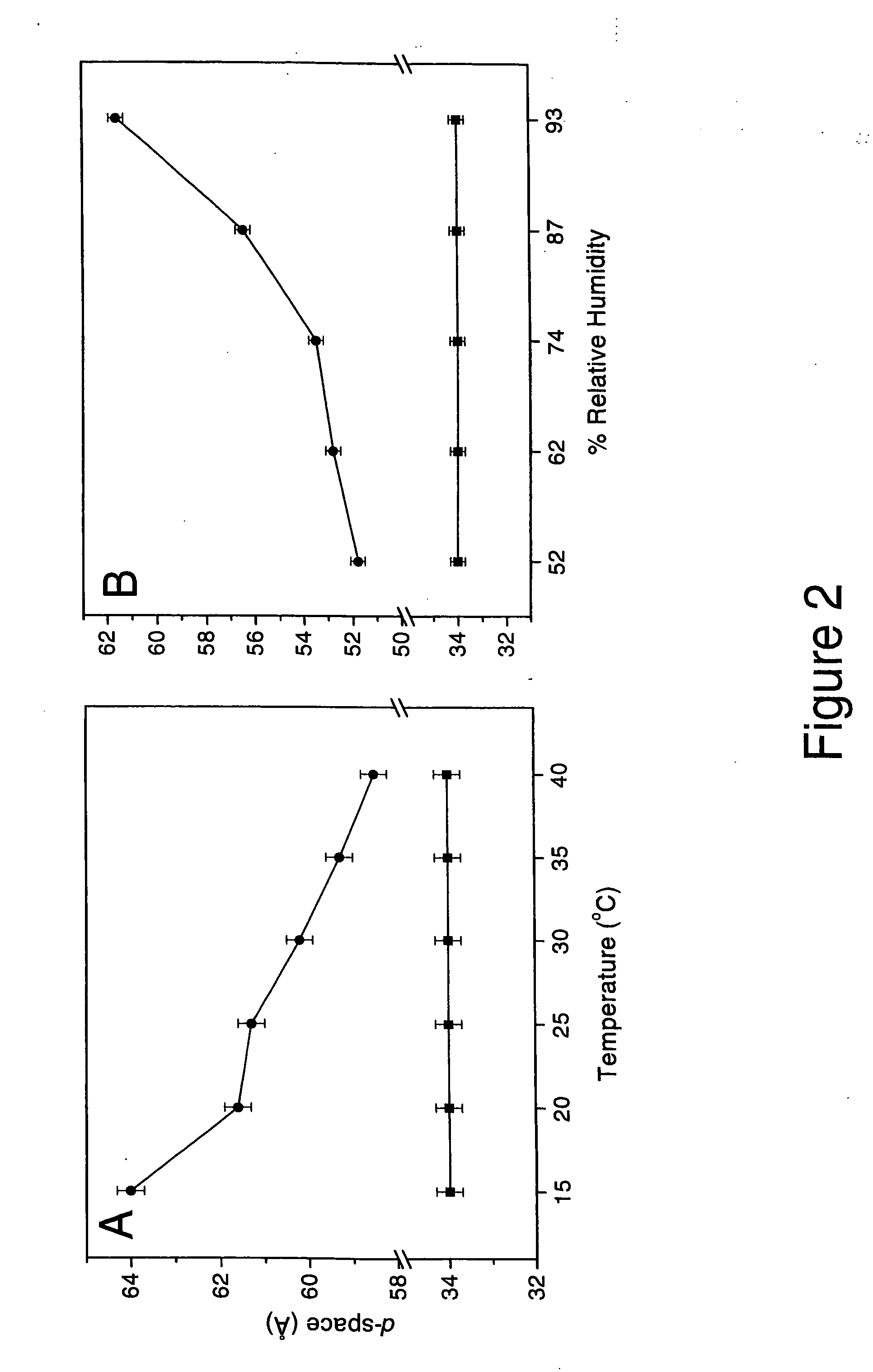
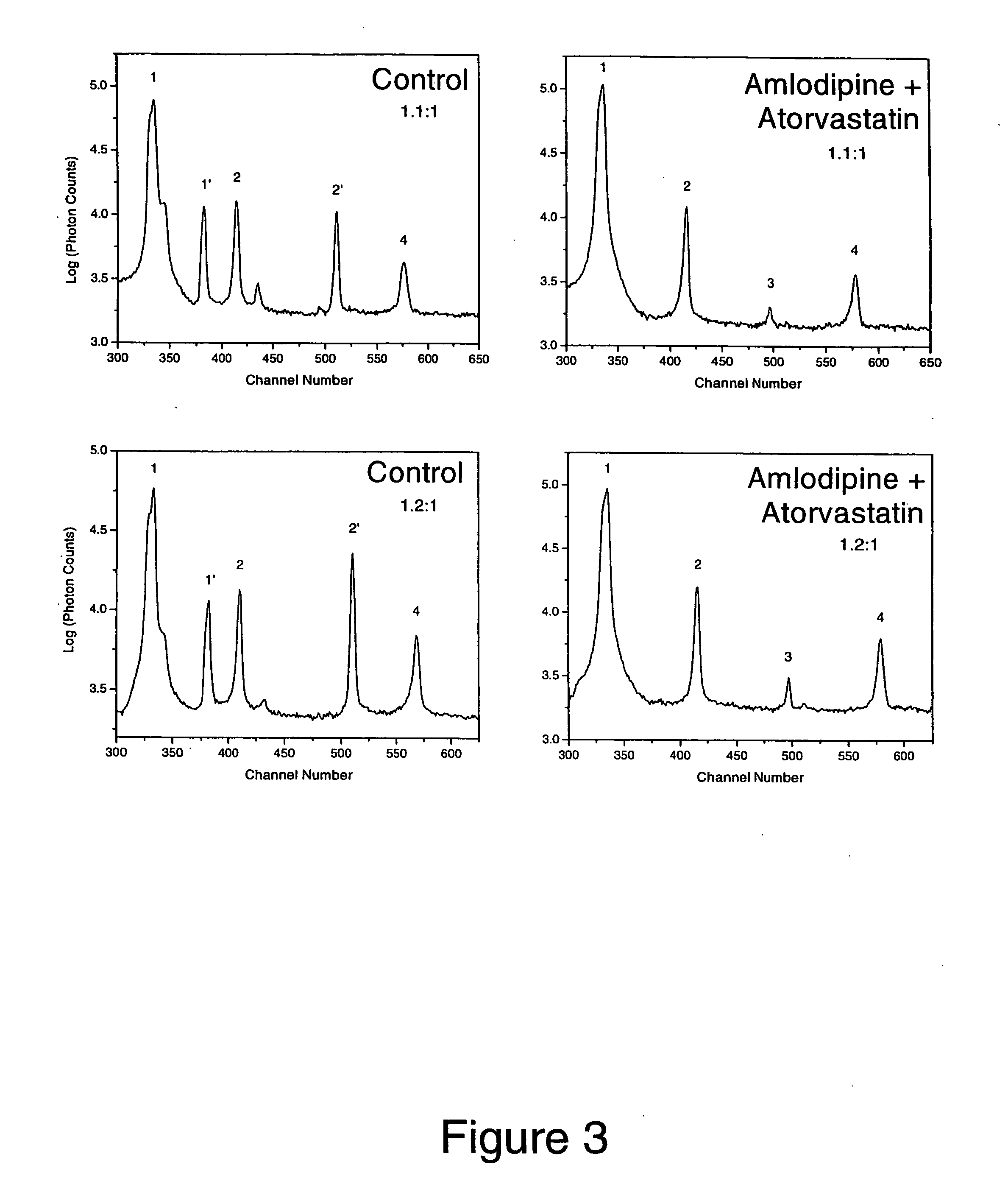
The combination of amlodipine with atorvastatin metabolite shows a synergistic antioxidant effect on lipid peroxidation in human low-density lipoproteins and membrane vesicles enriched with polyunsaturated fatty acids. Inhibition of oxy-radical damage by this drug combination was observed at therapeutic levels in a manner that could not be reproduced by the combination of amlodipine with other statins or the natural antioxidant, vitamin E. The basis for this potent activity is attributed to the chemical structures of these compounds and their molecular interactions with phospholipid molecules, as determined by x-ray diffraction analyses. This combination therapy can be used to treat cardiovascular disorders, especially coronary artery disease, by increasing the resistance of low-density lipoproteins and vascular cell membranes against oxidative modification.
Owner:MASON R PRESTON
Methods and compositions for inhibiting inflammation associated with pulmonary disease
The present invention provides an aerosol formulation of a 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitor. The HMG-CoA reductase inhibitor can be, for example, a statin such as lovastatin, pravastatin, simvastatin, cerivastatin, fluvastatin, atorvastatin or mevastatin. The invention also provides a method of treating a pulmonary disease with an aerosol formulation of a HMG-CoA reductase inhibitor.
Owner:UNIV OF WASHINGTON
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Compositions comprising fenofibrate and pravastatin
InactiveUS20050096390A1Substance may accumulateImprove bioavailability in vivoBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor pravastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCpravastatin) of between about 90 and about 6300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and pravastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
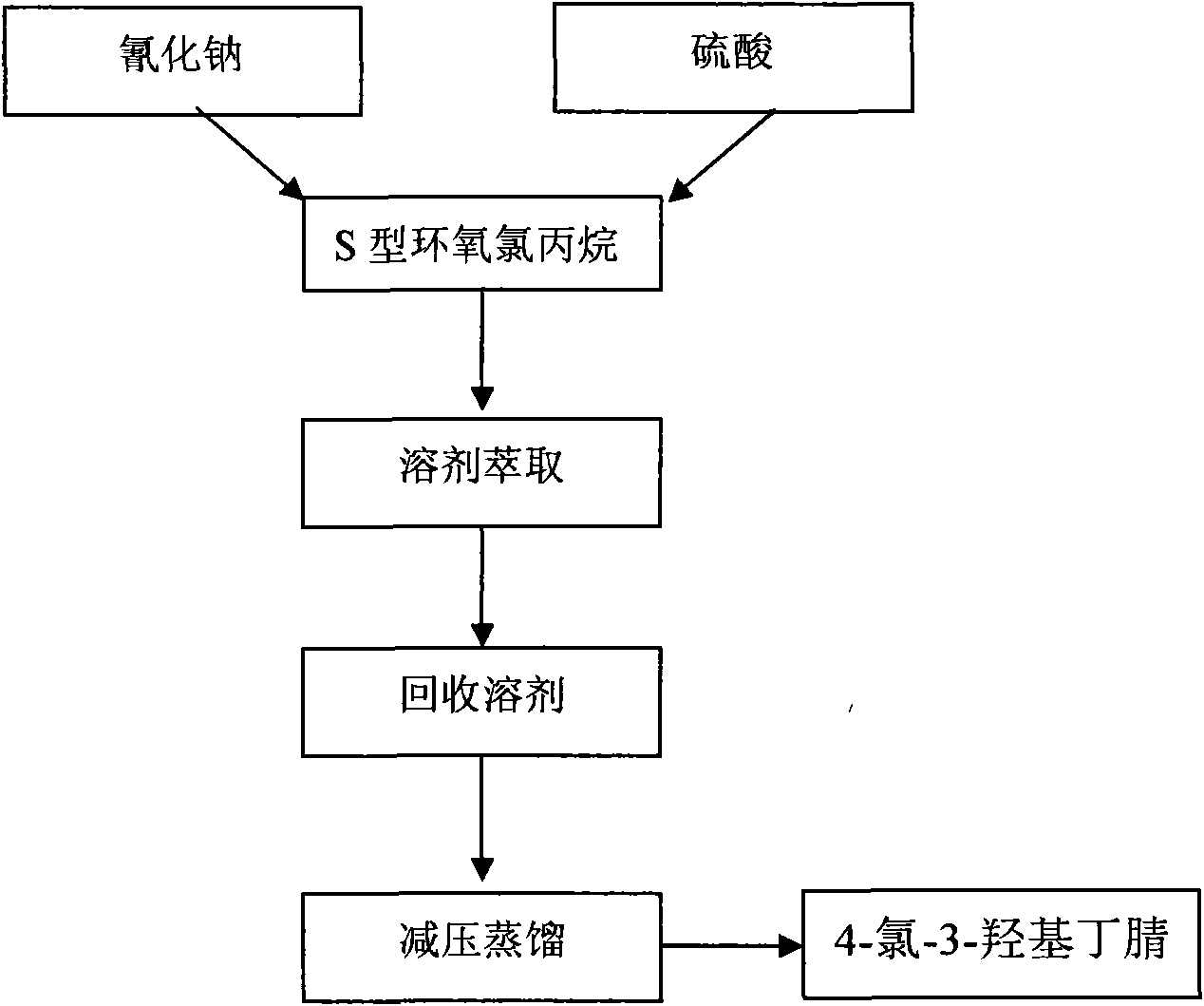
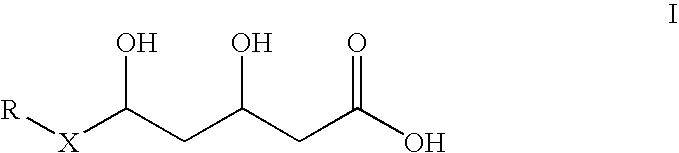
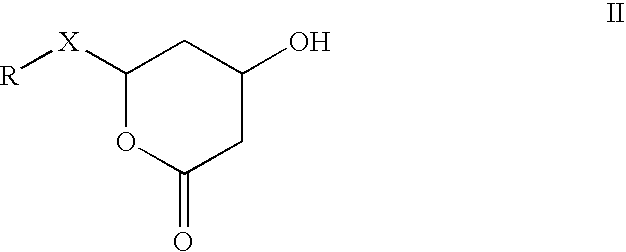
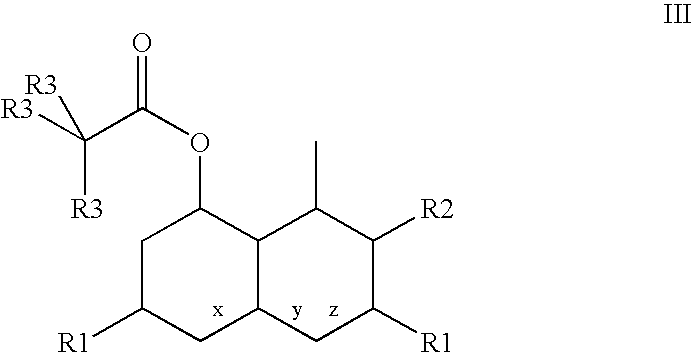
Manufacturing method of atorvastatin intermediate (R)-(-)-4-nitrile-3-hydroxybutyrate
InactiveCN101838221AIncrease profitEmission reductionPreparation by cyanide reactionState of artEnantiomer
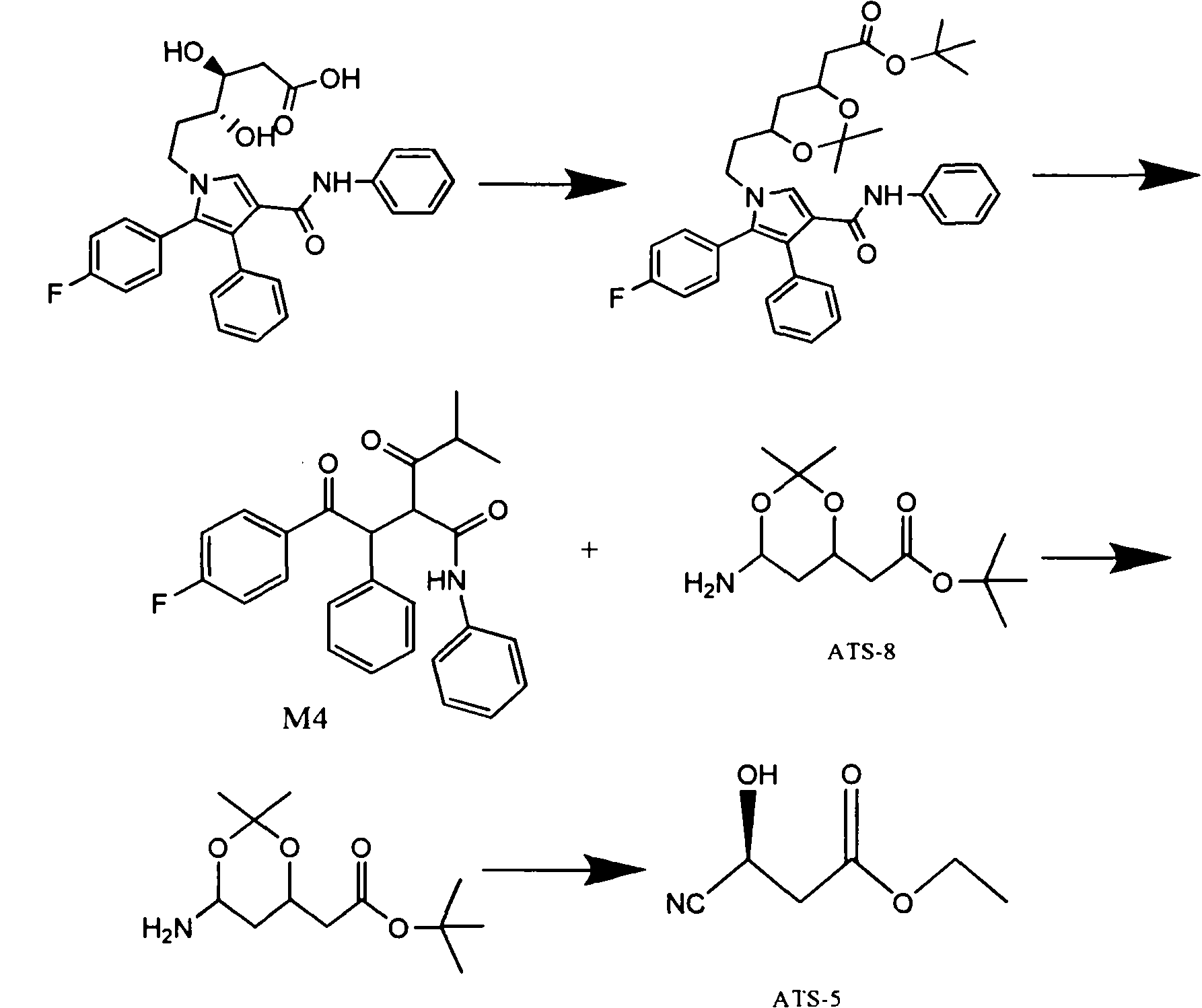
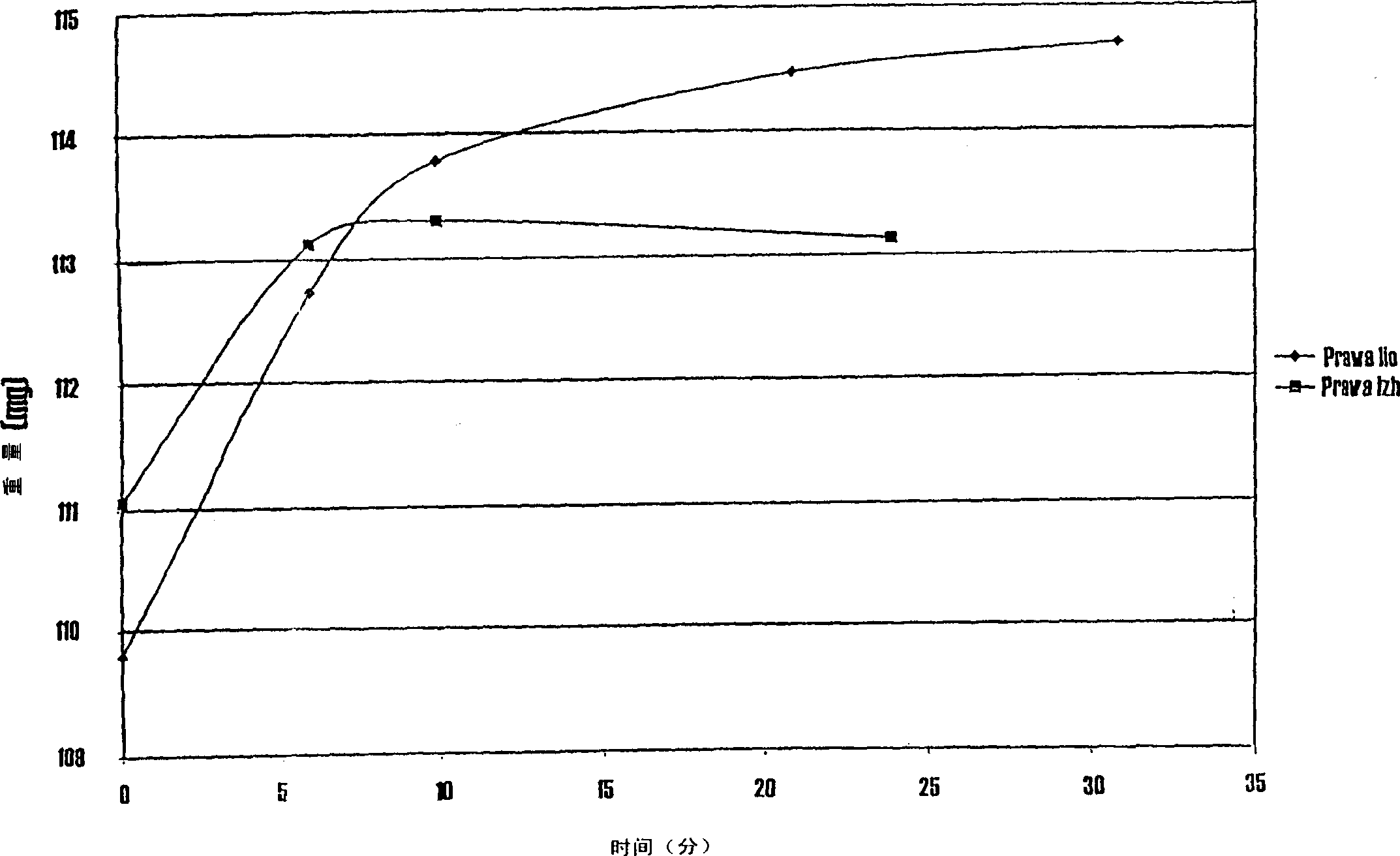
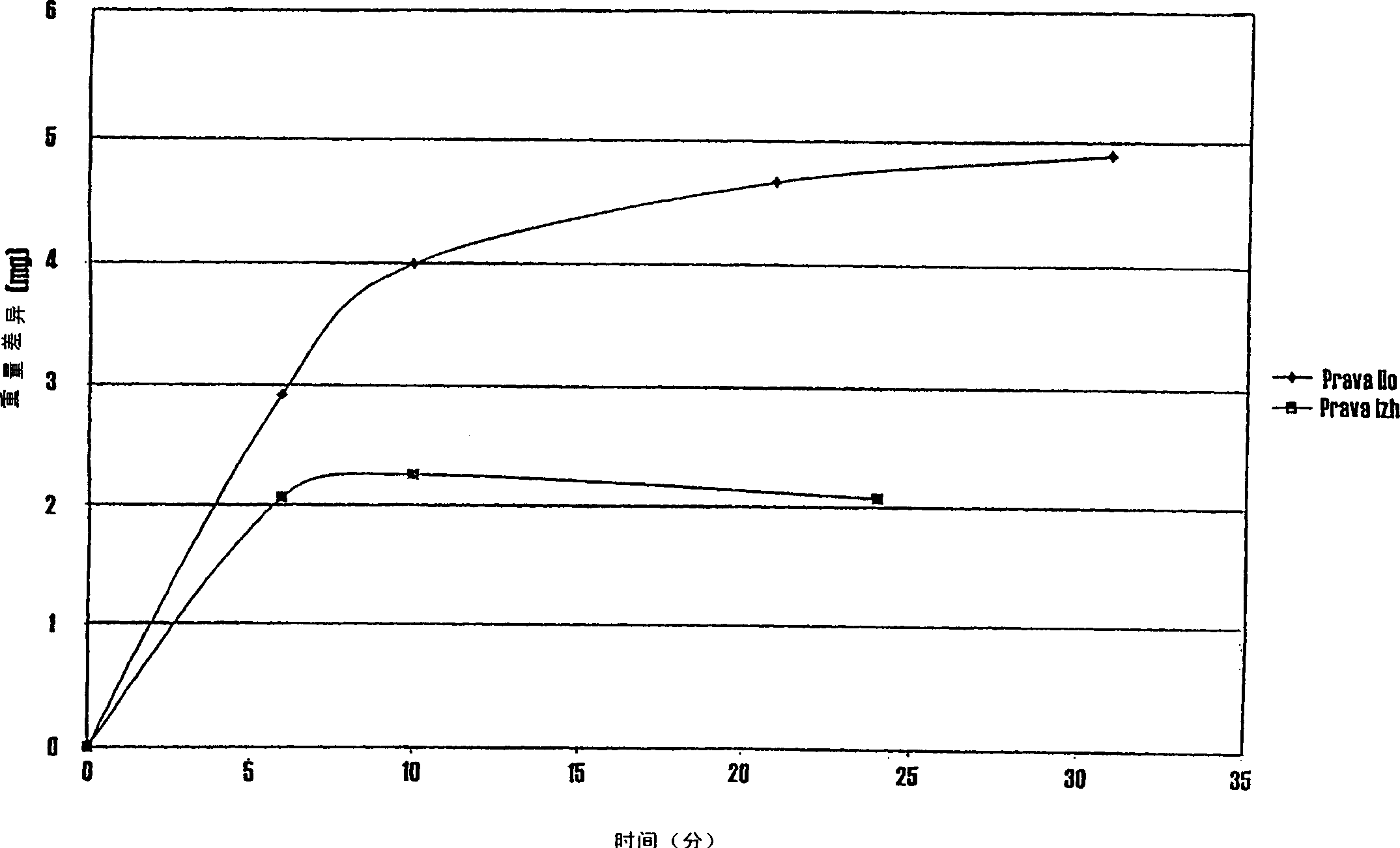
The invention relates to a manufacturing method of atorvastatin intermediate (R)-(-)-4-nitrile-3-hydroxybutyrate (ATS-5), which solves the problems of high cost, expensive raw material price and the like of the prior art. The manufacturing method is characterized in that the atorvastatin intermediate is prepared from epichlorohydrin as a raw material through ring-opening addition, glycolysis esterification, cyaniding substitution synthesis and refining. The invention has simple synthesis route, abundant raw material resources, low equipment requirements and no high-temperature high-pressure reaction; the content of produced ATS-5 is more than 98 percent, the content of water is lower than 0.2 percent, and the content of enantiomer is less than 1.0 percent. The manufacturing method is a mature production process for manufacturing the intermediate of medicine atorvastatin and is applicable to medium-sized and small enterprises.
Owner:HUANGGANG HUAYANG PHARMA
Phosphates of secondary alcohols
InactiveUS20060281715A1High metabolismReduced bioavailabilityBiocideNervous disorderAlcoholVenlafaxine
According to the invention, there is provided a phosphate derivative of a compound having a secondary hydroxy group. The compound having a secondary hydroxyl group may, for example, be chosen from pravastatin, atorvastatin venlafaxine, their derivatives and mixtures thereof.
Owner:VITAL HEALTH SCIENCES PTY LTD
Pharmaceutical formulations for carrier-mediated transport statins and uses thereof
InactiveUS20050119331A1Improve bioavailabilityMechanism is preventedBiocideOrganic chemistryLipid formationCholesterol
The present invention relates to formulations comprising therapeutically effective amounts of at least one acid-stable, carrier-mediated transport statin, at least one poorly water-soluble, carrier-mediated transport statin, or at least one large molecular weight, carrier-mediated transport statin, such as atorvastatin and rosuvastatin, or a pharmaceutically acceptable salt thereof, and methods of their use. The present formulations and methods are designed to exhibit a controlled-release of a therapeutic amount of the statin in the small intestine, thereby limiting systemic exposure of the statin and maximizing liver-specific absorption of the drug. The formulations and methods of the present invention are particularly useful for treating and / or preventing conditions that are benefited by decreasing levels of lipids and / or cholesterol in the body.
Owner:CIRC PHARM RES & DEV LTD
Stable pharmaceutical formulation comprising HMC-CoA reductase inhibitor
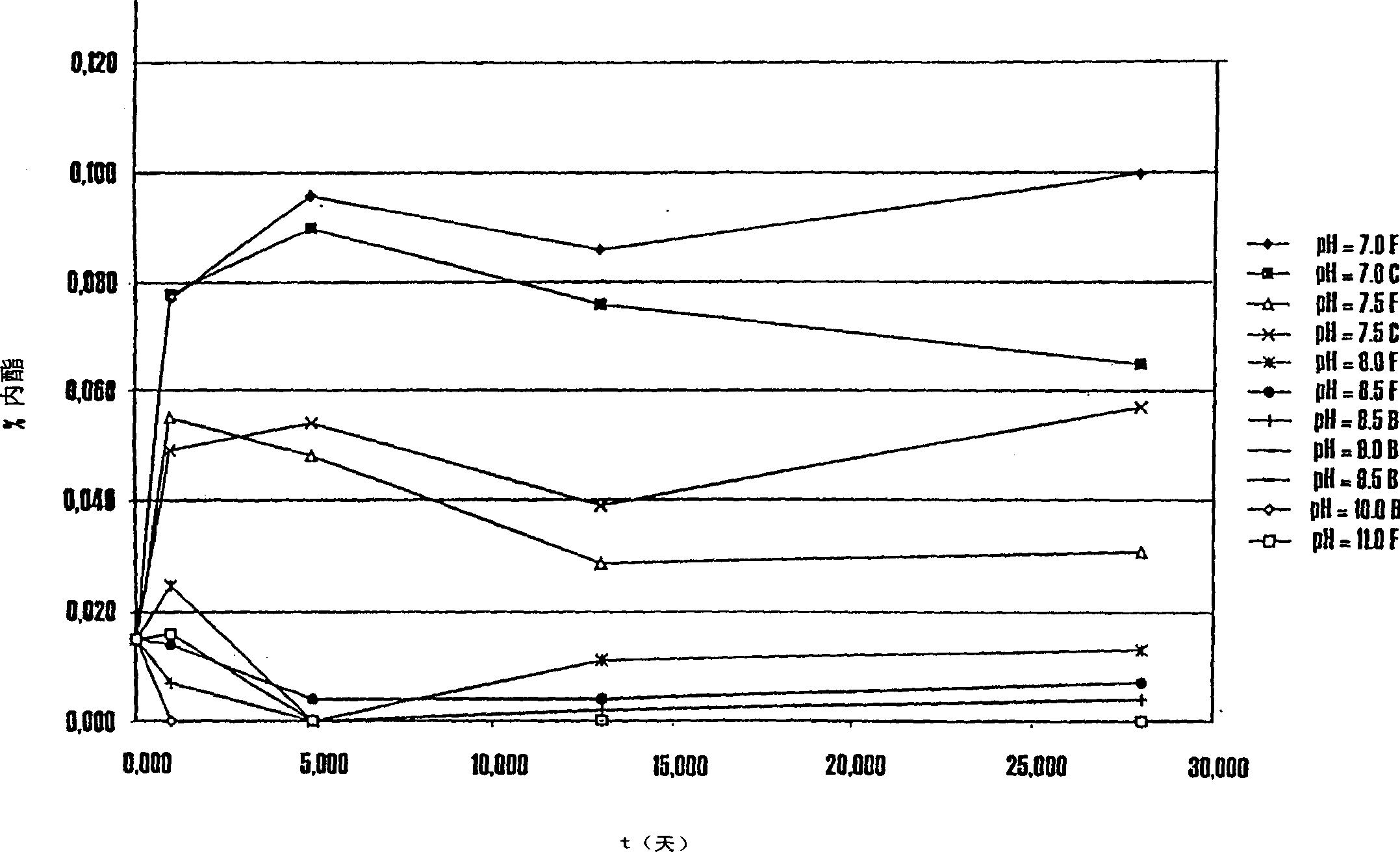
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, and some are obtained by treating the fermentation products using the methods of chemical synthesis or they are the products of total chemical synthesis. The aforementioned active substances may be destabilised by the environmental factors, their degradation may also be accelerated by interactions with other pharmaceutical ingredients, such as fillers, binders, lubricants, glidants and disintegrating agents, therefore the pharmaceutical ingredients and the process for preparation of the pharmaceutical formulation should be meticulously chosen to avoid the aforementioned undesired interactions and reactions. The present invention relates to a stable solid pharmaceutical formulation for the treatment of hypercholesterolemia and hyperlipidemia. More precisely, the present invention relates to the new stable solid pharmaceutical formulation containing as an active ingredient a HMG-CoA reductase inhibitor, such as atorvastatin, pravastatin, fluvastatin and cerivastatin or pharmaceutically acceptable salts thereof.
Owner:LEK PHARMA & CHEM
Methods for treating disorders associated with hyperlipidemia in a mammal
The invention is directed to methods for treating hyperlipidemia in a mammal. The methods involve combination therapies using a microsomal triglyceride transfer protein (MTP) inhibitor (for example, BMS-201038 and implitapide) and a HMG-CoA reductase inhibitor (for example simvastatin or atorvastatin). Co-administration of the MTP inhibitor with the HMG-CoA reductase inhibitor produces a therapeutic benefit, for example, a reduction in the concentration of cholesterol and / or triglycerides in the blood stream, but with fewer or reduced side effects than when higher dosages of the MTP inhibitor are used during monotherapy to provide the same or similar therapeutic benefit.
Owner:AEGERION PHARM INC
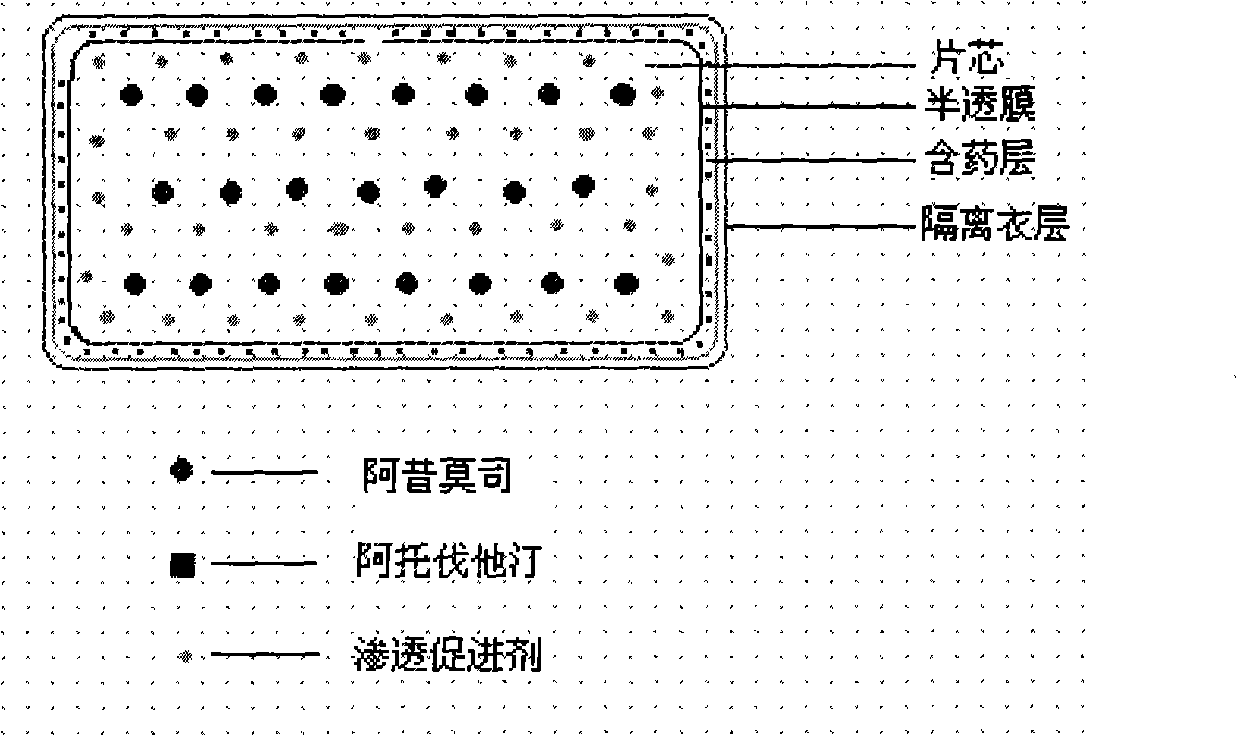
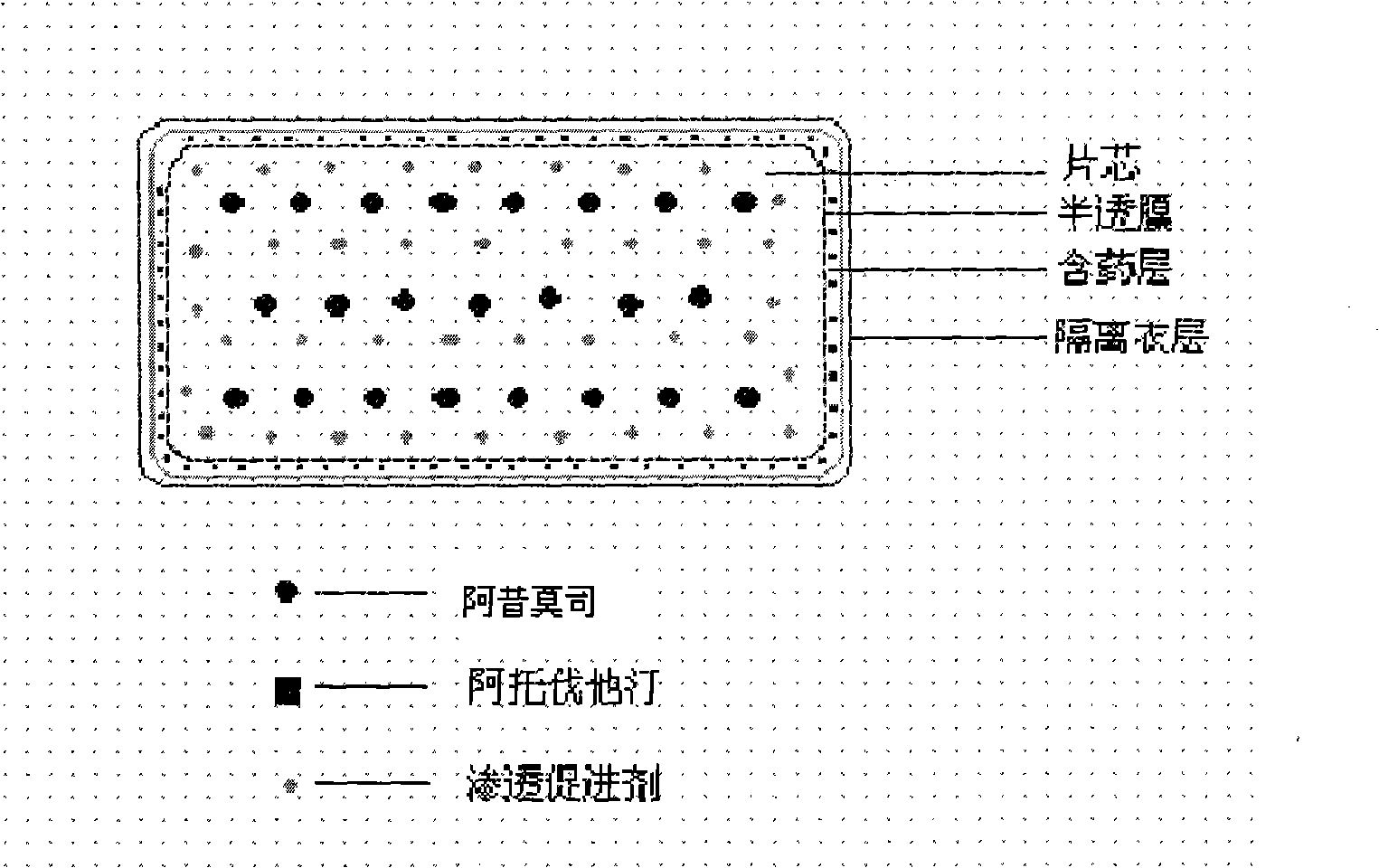
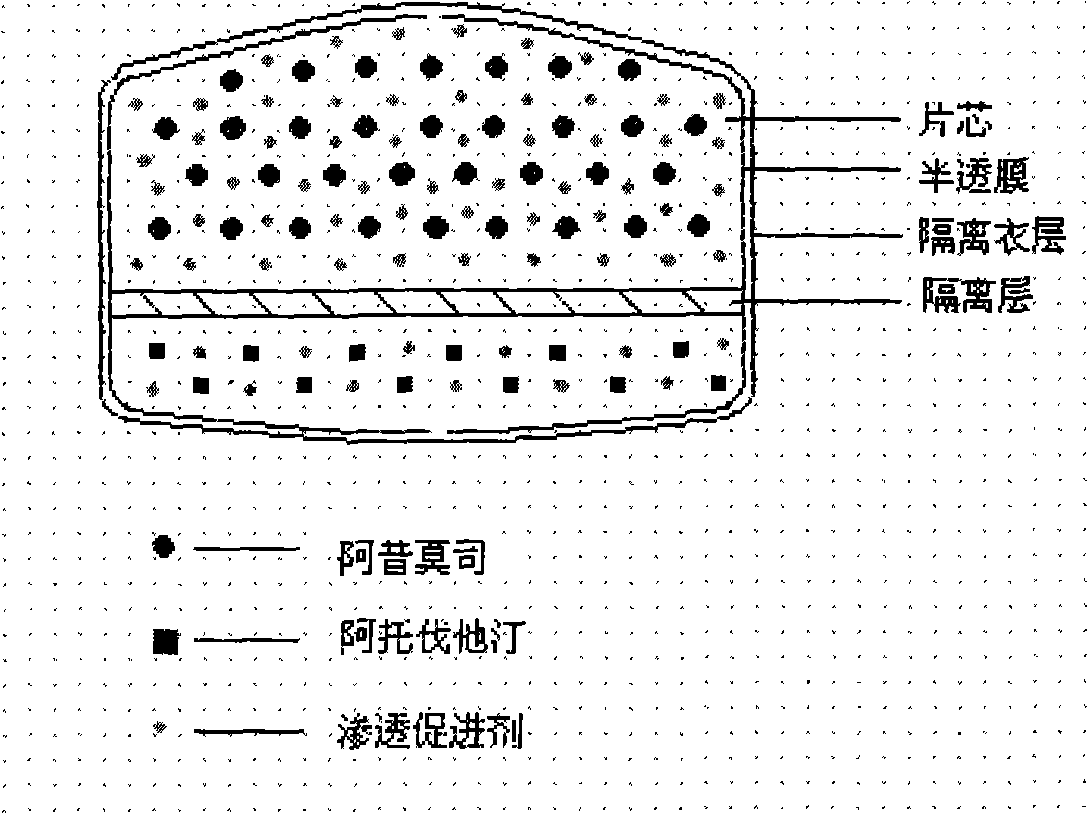
Osmotic pump controlled release preparation composition and preparation thereof
ActiveCN101352426AFully functionalSmall toxicityMetabolism disorderPharmaceutical delivery mechanismSide effectBULK ACTIVE INGREDIENT
The invention relates to an osmotic pump controlled release pharmaceutical composition which contains two medicine active ingredients and is used for treating hyperlipoidemia and a preparation method thereof, the pharmaceutical composition has a nicotinic acids medicine such as acipimox and the other one estatina medicine such as atorvastatin; wherein, the nicotinic acids medicine such as acipimox is a controlled release part, and the estatina medicine such as atorvastatin is a quick release part; or both the acipimox and the atorvastatin are the controlled release part. The compound osmotic pump preparation of the invention has the advantages of comprehensive action, low toxicity and side effects and convenient use.
Owner:LUNAN PHARMA GROUP CORPORATION
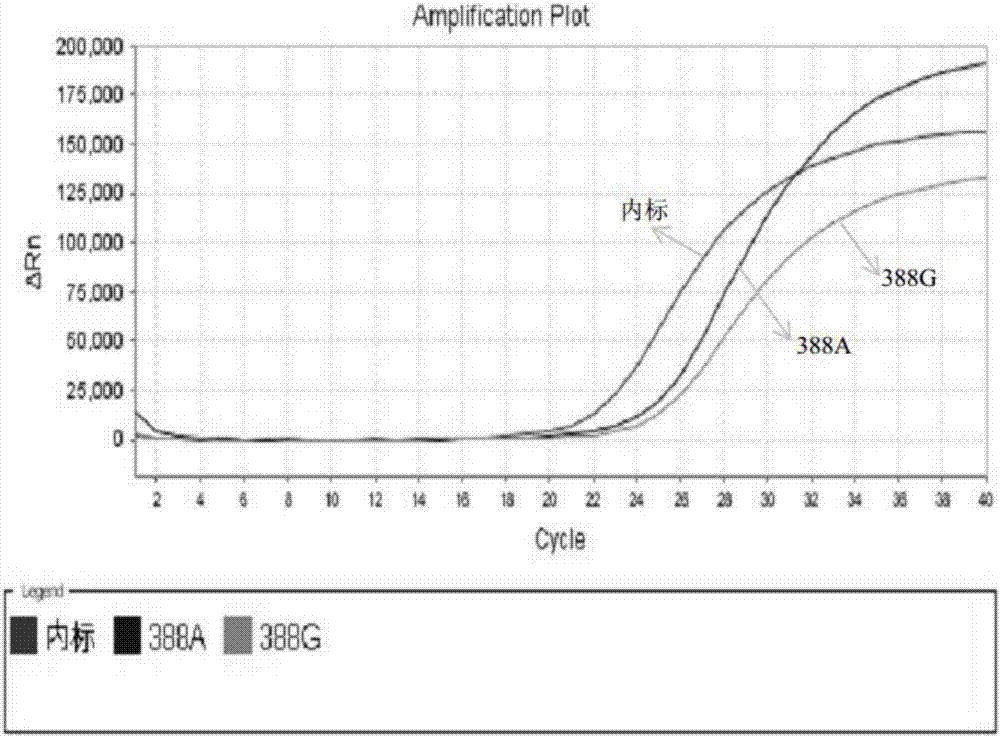
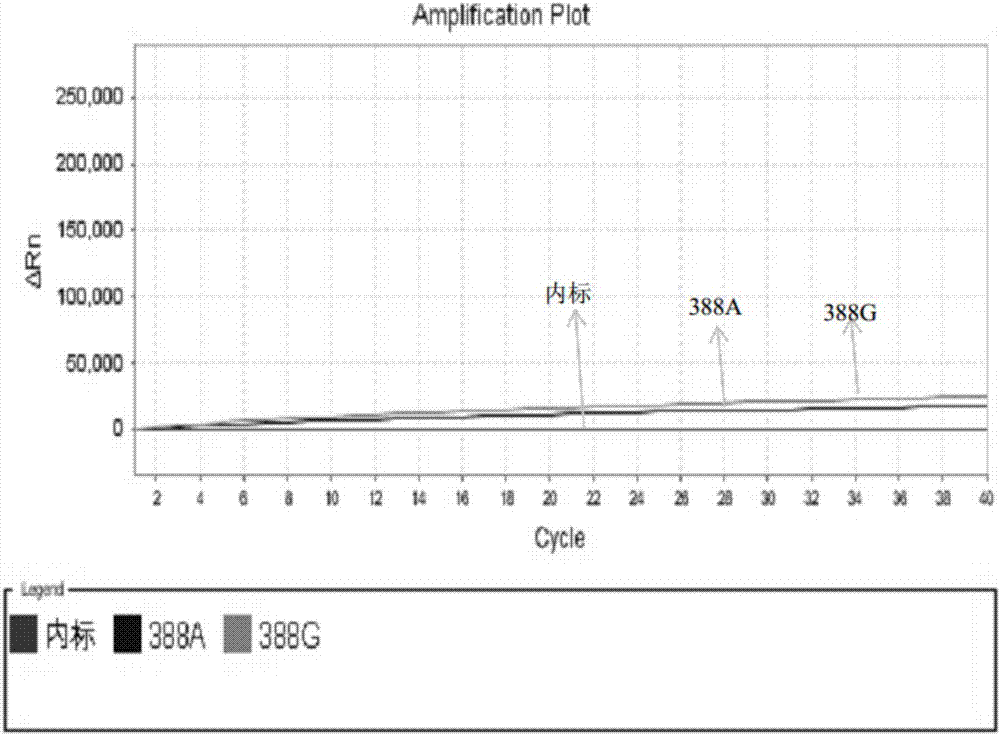
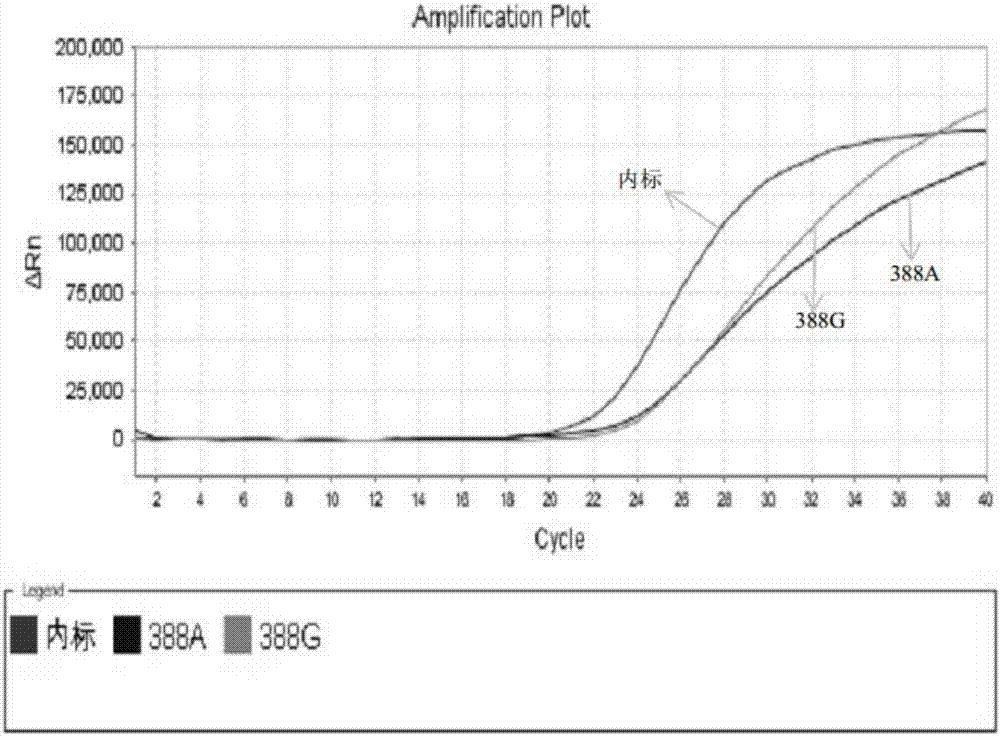
Kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation
The invention belongs to the technical field of gene mutation detection, and concretely discloses a kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation. Through meticulous design, multi-time verification, screening and optimization, specific primers and probes based on a Taqman allelic gene resolution analysis method are obtained; eight functional variation of the SLCO1B1, APOE and LDLR genes can be detected; the time from DNA (Deoxyribonucleic Acid) extraction to fluorescent PCR (Polymerase Chain Reaction) to result obtaining is less than four hours; and the manual operation time is less than two hours. The kit comprising the primer pairs and the probe pairs has the advantages that the time is saved; convenience is realized; the sensitivity is high; and both the positive conformity rate and the negative conformity rate of a sample are higher than 99 percent, and the like. A detection method provided by the invention is mainly used for the personalized medication auxiliary diagnosis of statins such as simvastatin, atorvastatin, fluvastatin and rosuvastatin.
Owner:钟诗龙
Compositions containing HMG Co-A reductase inhibitors and policosanol
InactiveUS6890941B1Lowering of total serum cholesterol levelInhibiting cholesterol synthesisBiocideHydroxy compound active ingredientsLipid storageElevated serum cholesterol
The present invention provides pharmaceutical compositions, methods, combinations, and kits for treating a disorder related to elevated serum cholesterol concentration, for example, hypercholesterolemia, atherosclerosis, elevated LDL plasma levels, low HDL plasma levels, hypertriglyceridemia, hyperlipidemia, hypertension, cholesterol gallstones, and lipid storage diseases. The compositions, methods, combinations, and kits of the present invention are pharmaceutical compositions comprising atherapeutically effective amount of a lipid regulating agent, such as a HMG-CoA reductase inhibitor, and compound that inhibits cholesterol synthesis at a point between the formation of acetate and mevalonate. A typical pharmaceutical composition of the invention contains and effective amount of atorvastatin and an effective amount of policosanol.
Owner:PROCAPS
A kit for simultaneously detecting statin metabolizing gene multisite mutations
The invention relates to a kit for simultaneously detecting statin metabolizing gene multisite mutations. The kit includes primer pairs and probe pairs for detecting APOE, SLCO1B1, CETP, ABCB1 and MTHFR gene sites. MGB (minor groove binder) probes and a real-time fluorescent PCR technique are applied in the kit. The kit has advantages of capability of being time saving and convenient, high sensitivity, capability of allowing positive and negative coincidence rates of a sample to be 99.5% or above, and the like. The kit is mainly used for personalized medication assisted diagnosis of statins such as simvastatin, atorvastatin and pravastatin.
Owner:NINGBO MEIJING MEDICAL TECH
Process for forming amorphous atorvastatin
ActiveUS20050032880A1Improve solubilityImprove processing efficiencyBiocideNervous disorderSolventAtorvastatin
Forming amorphous atorvastatin comprises the steps of dissolving atorvastatin in a hydroxylic solvent, followed by rapidly evaporating the solvent. In another aspect, a composition comprises particles of amorphous atorvastatin and a core.
Owner:UPJOHN US 1 LLC
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
Synergistic effect of amlodipine and atorvastatin on aortic endothelial cell nitric oxide release
The combination of amlodipine and atorvastatin act to synergistically synthesize NO production. Moreover, the addition of a tertiary compound complements this combination of amlodipine and atorvastatin in NO production.
Owner:MASON R PRESTON
Combinations of statins and anti-obesity agent and glitazones
Co-therapy of an anti-obesity agent, a statin, and a glitazone is disclosed along with fixed combinations thereof. Atorvastatin, rosiglitazone, and orlistat are preferred as the various components. Non-glitazone antidiabetic agents may be optionally added to the therapy and / or to the fixed combination product.
Owner:PALEPU NAGESWARA R
Pharmaceutical compositions of atorvastatin
The present invention provides stable pharmaceutical compositions comprised of atorvastatin and sodium bicarbonate or L-arginine. The compositions are prepared as bulk drug compositions and also as oral dosage units, such as tablets or capsules. The compositions are useful for preparation of monolithic and bi-layer tablets containing atorvastatin as the only active agent or combined with one or more additional active agents. The compositions are useful for treating hypercholesterolemia and related conditions.
Owner:MERCK SHARP & DOHME CORP
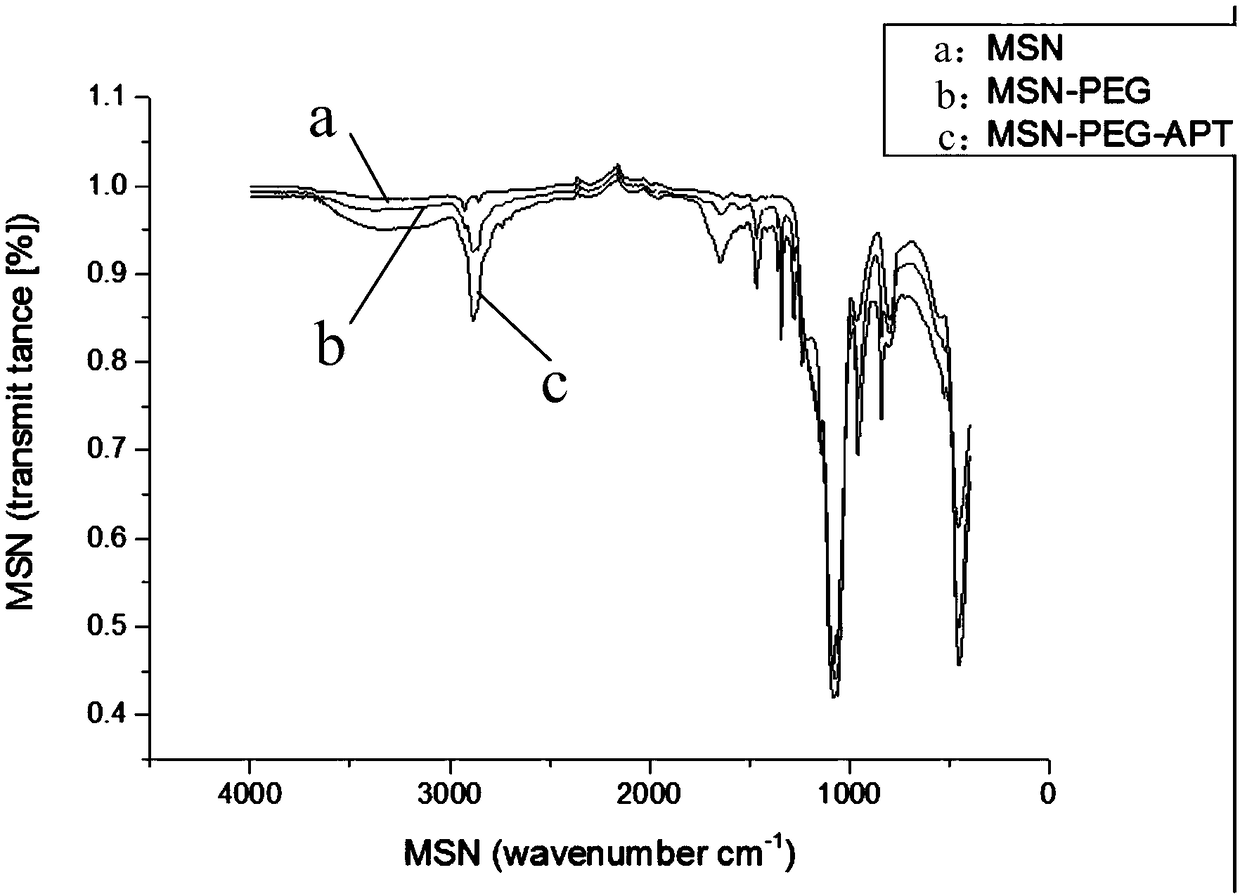
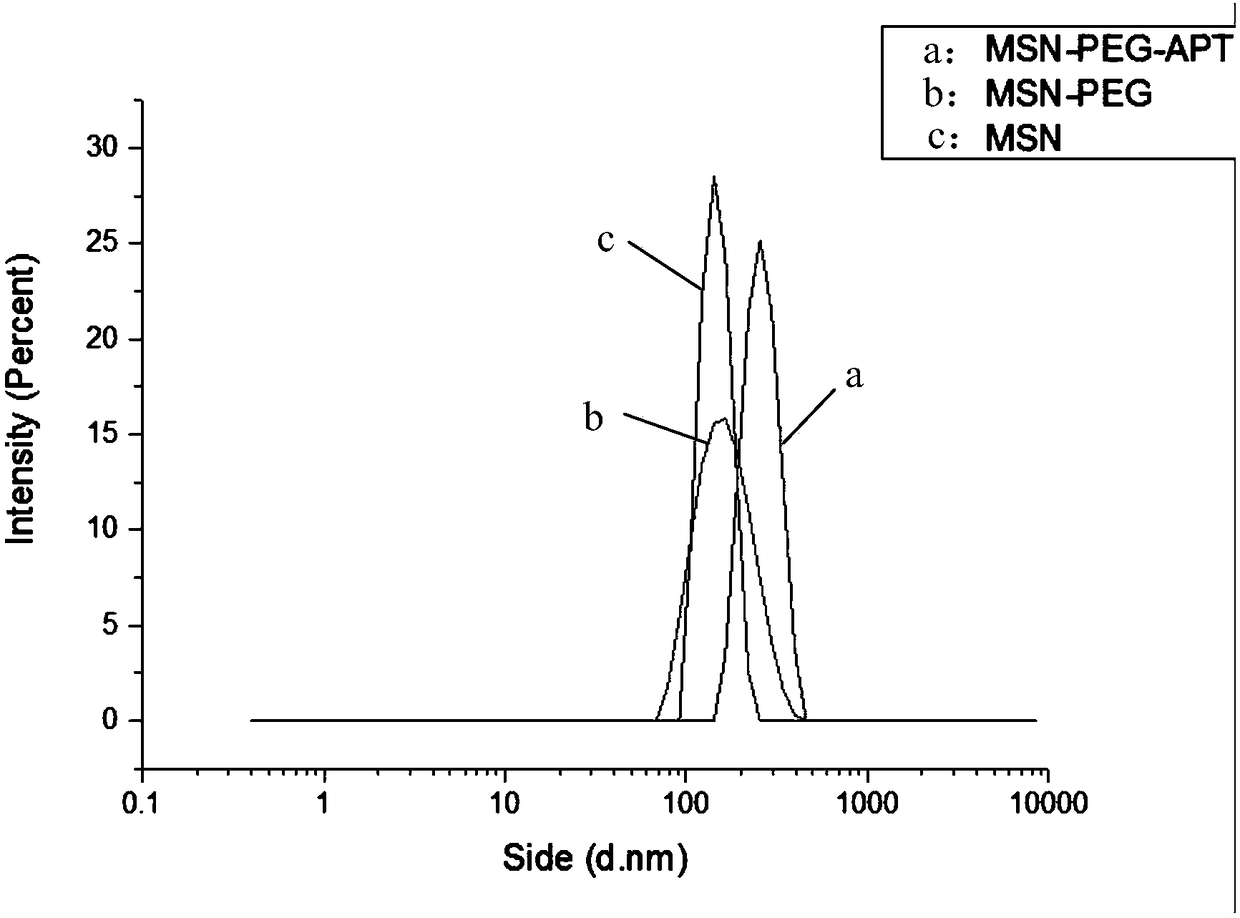
Mesoporous silica nanoparticles modified by nucleic acid aptamer targeting polyethylene glycol and preparation method thereof
InactiveCN109044993AEasy to retouchImproving immunogenicityPharmaceutical non-active ingredientsMicrocapsulesLipid formationLipid lowering drug
The invention discloses mesoporous silica nanoparticles modified by nucleic acid aptamer targeting polyethylene glycol and a preparation method thereof. The method comprises the following steps: synthesizing mesoporous silica through TEOS(tetraethyl orthosilicate), CTAB(cetyltrimethyl ammonium bromide), alkali and water; modifying amino on the surface of the mesoporous silica through APTES (aminopropyltriethoxysilane) in an ethanol solution; and connecting the primary amine end group of the mesoporous silica to the carboxyl group of polyethylene glycol by reacting, connecting the terminal carboxyl group of a nucleic acid aptamer to the amine group of the polyethylene glycol on the mesoporous silica by reacting, and carrying, by the mesoporous silica nanoparticles, a lipid-lowering drug such as atorvastatin. The mesoporous silica nanoparticles and the preparation method thereof have the characteristics that the nucleic acid aptamer is bound with the biological target with high affinityand high specificity, the polyethylene glycol can improve the water dispersion and the sustained release capability of the carrier, and the mesoporous silica can carry hydrophilic and hydrophobic drugs of different molecular weights due to the characteristics of unique mesoporous structure, huge specific surface area, easy surface modification and the like.
Owner:SOUTH CHINA UNIV OF TECH
Combination therapy comprising atorvastatin and an antihypertensive agent
InactiveCN1268053APeptide/protein ingredientsMetabolism disorderDrug compoundSecondary hyperlipidemia
The present invention relates to a pharmaceutical compound of atorvastatin or its pharmaceutically acceptable salt and an antihypertensive drug, a kit containing the compound and the use of the compound for treating patients with angina pectoris, atherosclerosis, mixed hypertension and hyperlipidemia Methods of treatment of individuals with cardiac disease and individuals with cardiac risk symptoms, including humans. The present invention also relates to a combination of atorvastatin or a pharmaceutically acceptable salt thereof and an antihypertensive agent with additive and synergistic effects, which can be used for treating angina pectoris, atherosclerosis, Individuals with mixed hypertension and hyperlipidemia and individuals with cardiac risk symptoms, including humans, are treated.
Owner:PFIZER INC
Pharmaceutical compositions of amlodipine and atorvastatin
InactiveCN1617717AMetabolism disorderInorganic non-active ingredientsPharmaceutical drugPharmaceutical medicine
The present invention describes a pharmaceutical composition comprising two components: (a) a component comprising granules of atorvastatin or a pharmaceutically acceptable salt thereof and a carrier, including an alkalizing agent to form a pH greater than 5; and (b) Two components comprising amlodipine or a pharmaceutically acceptable salt thereof and a carrier, excluding an alkalizing agent for forming a pH greater than 5, wherein the two components are mixed to form a final composition in solid dosage form, and a method of preparing the composition, A kit containing such a composition, and the use of a therapeutically effective amount of the pharmaceutical composition to treat angina pectoris, atherosclerosis, hypertension and hyperlipidemia complications and / or hypercholesterolemia and to treat heart disease risk signs.
Owner:WARNER LAMBERT CO LLC
Compound of atorvastatin and levorotatory amlodipine and preparing method thereof
ActiveCN101224205AExcellent indicatorsHigh dissolution rateMetabolism disorderPill deliveryCyclodextrinLevamlodipine
The invention relates to a combination of atorvastatin and levamlodipine, mainly consists of levamlodipine or officinal salt thereof, atorvastatin or officinal salt thereof, alkali metal salt, and cyclodextrin and derivatives thereof and guarantees the stability and the bioavailability of atorvastatin and levamlodipine.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Process for obtaining HMG-CoA reductase inhibitors of high purity
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, Streptomyces, Actinomadura, Micromonospora, some are obtained by treating the fermentation products using the method of chemical synthesis or they are the products of total chemical synthesis. The purity of the active ingredient is an important factor for manufacturing the safe and effective pharmaceutical, especially if the pharmaceutical product must be taken on a longer term basis in the treatment or prevention of high plasma cholesterol. The accumulation of the impurities from the pharmaceuticals of lower purity may cause many side effects during the medical treatment. The present invention relates to a new industrial process for the isolation of HMG-CoA reductase inhibitors using so-called displacement chromatography. Use of the invention enables one to obtain HMG-CoA reductase inhibitors of high purity, with high yields, lower production costs and suitable ecological balance.
Owner:LEK PHARMA D D
Stabilized atorvastatin
InactiveUS20070190138A1Amount being removedReduce the amount requiredBiocideGranular deliveryOral medicationMETHYLPROPANEDIOL
A stable pharmaceutical composition for oral administration comprising atorvastatin and an amount of a pharmaceutically acceptable organic alkalizing compound capable of establishing a microenvironment for atorvastatin having a pH of at least about 5, for example 2-amino-2-(hydroxymethyl)-1,3-propanediol (trometamol).
Owner:VELOXIS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com