Patents
Literature
97 results about "Pravastatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
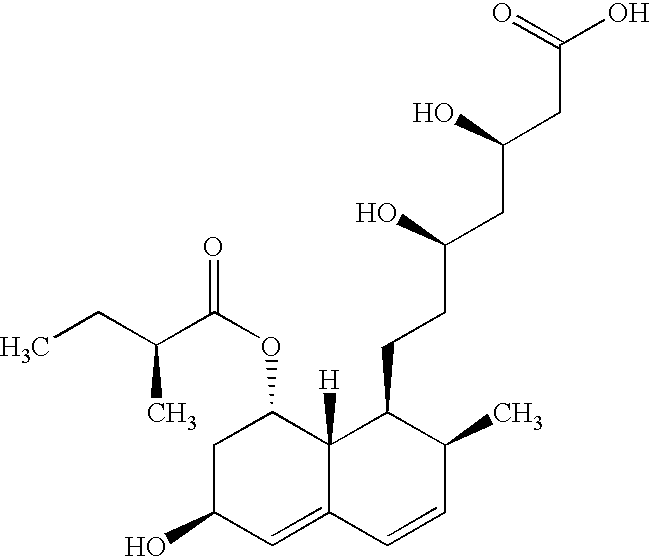
Pravastatin is used along with a proper diet to help lower "bad" cholesterol and fats (such as LDL, triglycerides) and raise "good" cholesterol (HDL) in the blood.
Treatment of arteriosclerosis and xanthoma
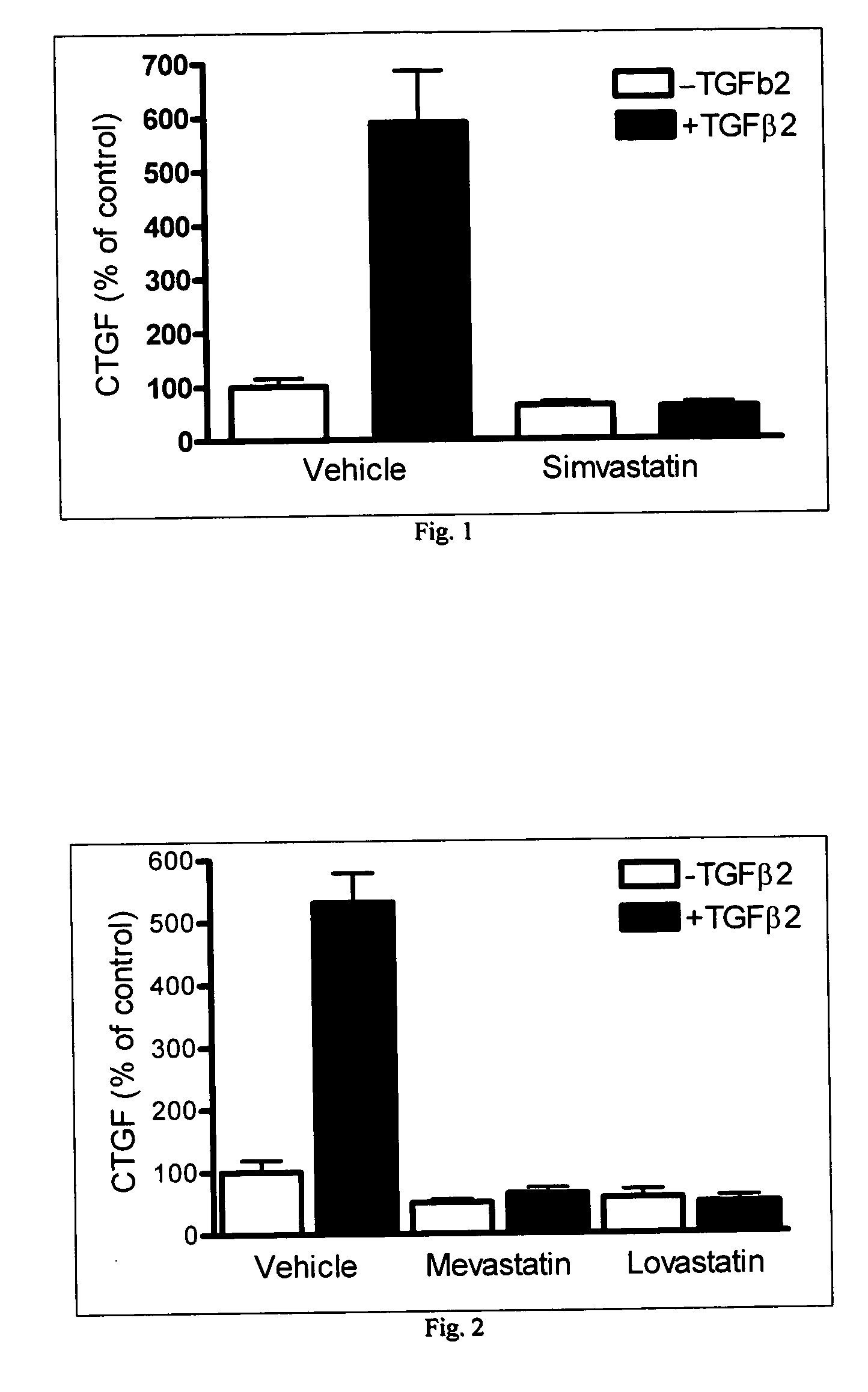
A combination of one or more HMG-CoA reductase inhibitors (for example pravastatin, lovastatin, simvastatin, fluvastatin, rivastatin or atorvastatin) with one or more insulin sensitizers (for example troglitazone, pioglitazone, englitazone, BRL-49653, 5-(4-{2-[1-(4-2'-pyridylphenyl)ethylideneaminooxy]ethoxy}benzyl)thiazolidine-2,4-dione, 5-{4-(5-methoxy-3-methylimidazo[5,4-b]pyridin-2-ylmethoxy)benzyl}thiazolidine-2,4-dione or its hydrochloride, 5-[4-(6-methoxy-1-methylbenzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione, 5-[4-(1-methylbenzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione and 5-[4-(5-hydroxy-1,4,6,7-tetramethylbenzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione) exhibits a synergistic effect and is significantly better at preventing and / or treating arteriosclerosis and / or xanthoma than is either of the components of the combination alone.
Owner:DAIICHI SANKYO CO LTD
Controlled release drug delivery system of pravastatin
InactiveUS20050089572A1Minimizes instabilityGood formulation stabilityBiocideAnimal repellantsControlled releaseDelivery system
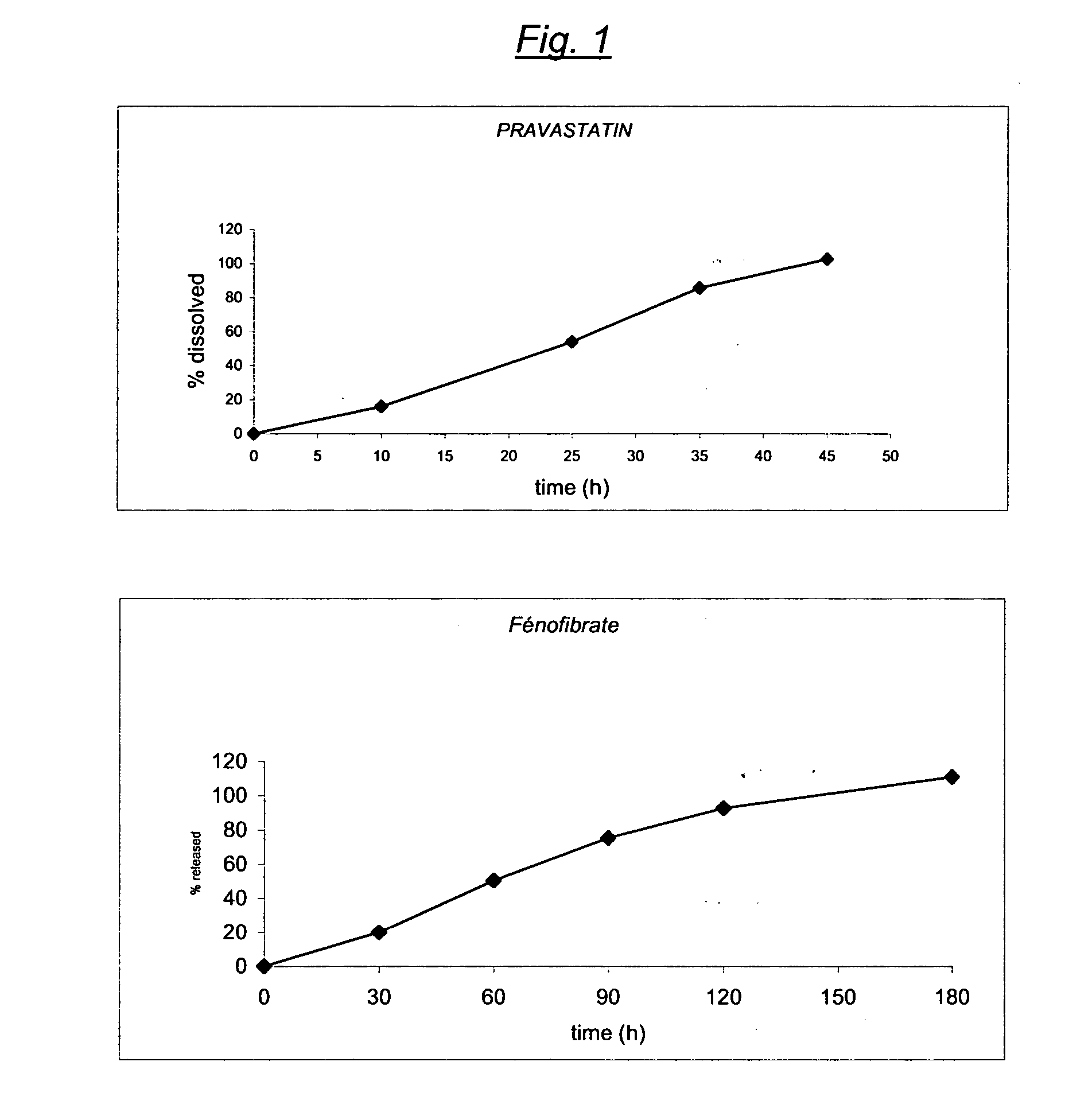
The present invention relates to an oral drug delivery system comprising pravastatin or its pharmaceutically acceptable salts such that the system provides enhanced stability in the acidic environment of the stomach and exhibits controlled release of the drug.
Owner:RANBAXY LAB LTD
Compositions comprising fenofibrate and pravastatin
InactiveUS20050096390A1Substance may accumulateImprove bioavailability in vivoBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
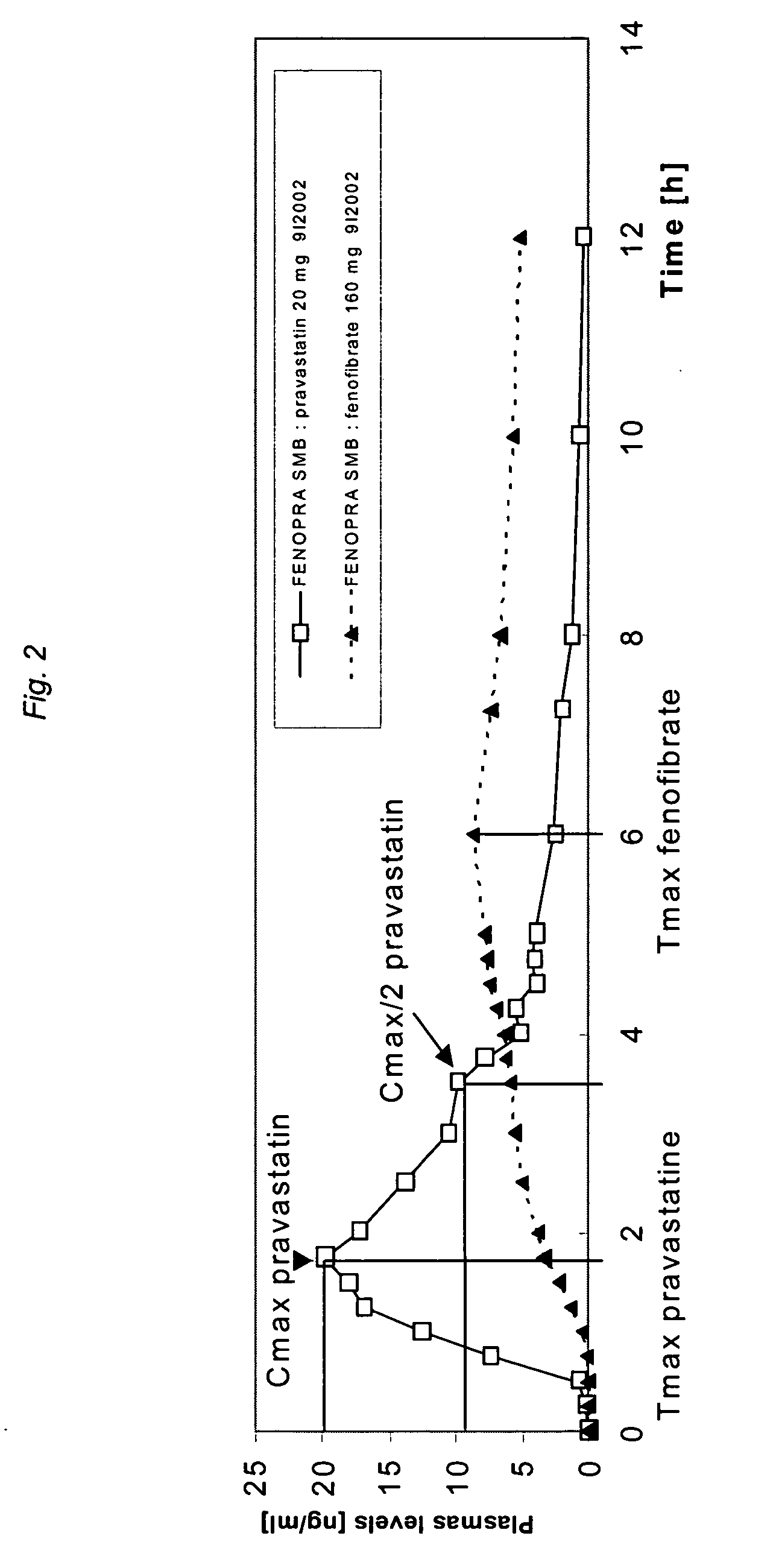
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor pravastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCpravastatin) of between about 90 and about 6300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and pravastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Method for preparing high optical purity pitavastatin calcium raw material drug
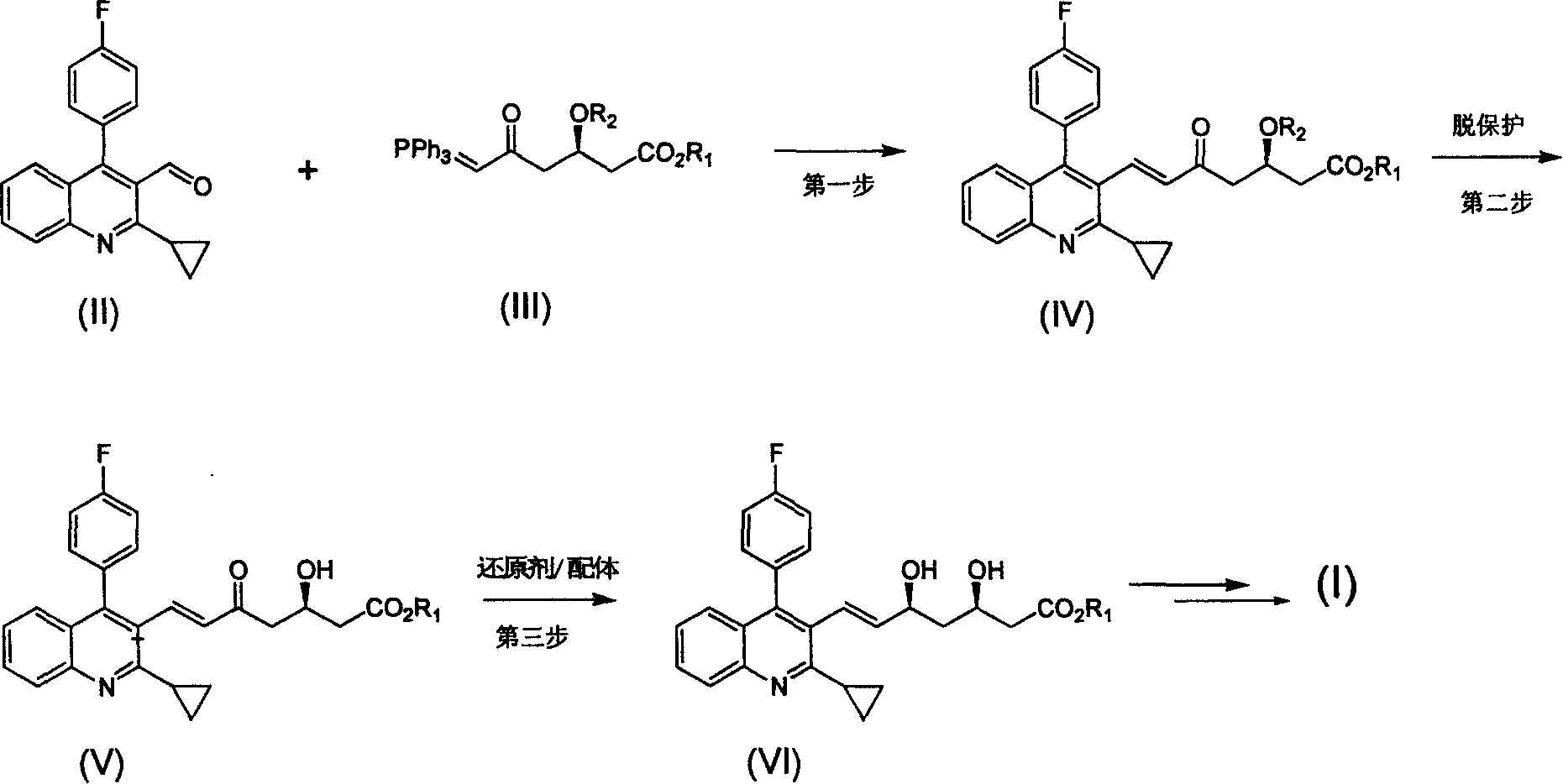
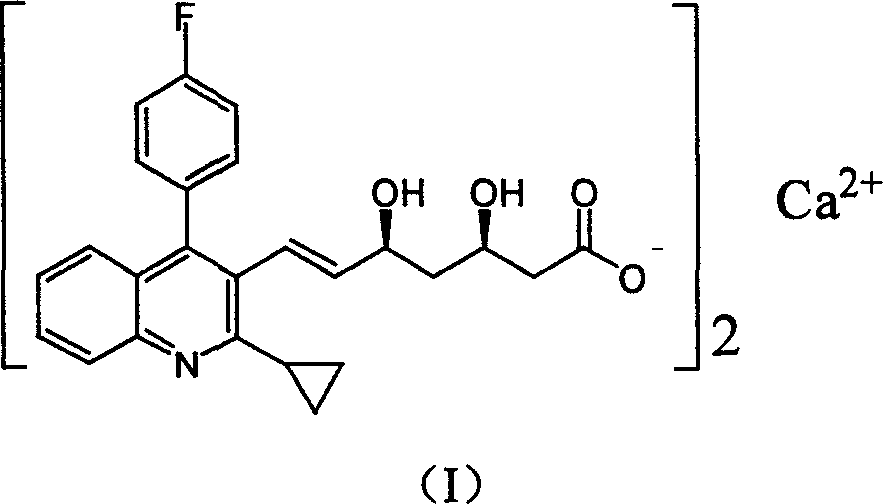
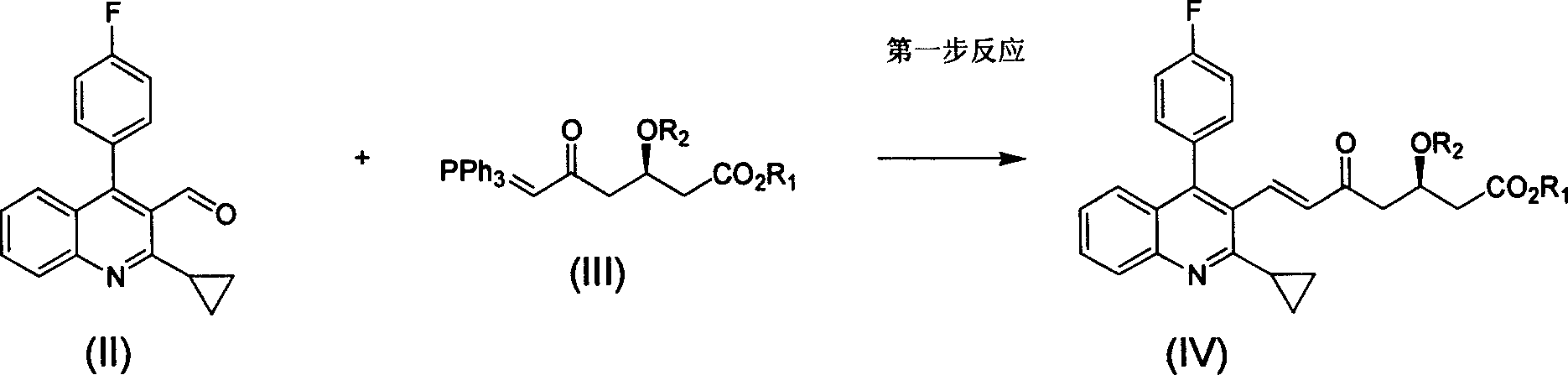
The invention relates the method of preparing the raw material of high optical purity pravastatin calcium. The method comprises the following steps: adding the 2- cyclopropyl-4-(4- fluorophenyl)-3-quinoline aldehyde II and (3R)-3- alkoxy silane-5- carbonyl-6- triphenyl phosphor heptene acid ester III in dissolvent, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)-3- alkoxy silane-6- triphenyl phosphor heptene acid ester IV, removing the protection of IV, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)- hydroxyl -6- triphenyl phosphor heptene acid ester V, deacidizing it in the mixture dissolvent of alcohol and ether with NaBH4 or KBH4 at -100-0Deg.C, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-(3R, 5S)- dihydroxy -6- triphenyl phosphor heptene acid ester VI, hydrolyzing it with alkali, and getting pravastatin calcium. The material is used to prepare HMG-CoA reductase inhibiting agent.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Phosphates of secondary alcohols
InactiveUS20060281715A1High metabolismReduced bioavailabilityBiocideNervous disorderAlcoholVenlafaxine
According to the invention, there is provided a phosphate derivative of a compound having a secondary hydroxy group. The compound having a secondary hydroxyl group may, for example, be chosen from pravastatin, atorvastatin venlafaxine, their derivatives and mixtures thereof.
Owner:VITAL HEALTH SCIENCES PTY LTD
Polysaccharide MA from Mytilus coruscus with hypolipidemic activity and preparation method thereof
The invention relates to the technical field of a medicine, provides a mussel polysaccharide MA separated from common mussel and a preparation method thereof. Proven through blood fat lowering test in animal bodies, the mussel polysaccharide MA is significant in treating white rat hyperlipemia induced by high-fat feedstuff, and can reduce the level of total cholesterol, triglyceride and low density lipoprotein in white rates. The action of the mussel polysaccharide MA is equal to the action of pravastatin as a positive medicine or Xuezhikang. Accordingly, the mussel polysaccharide MA can be applied for preparing medicines or food for lowering blood fat, which is of far reaching importance for making the most of living marine resources.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
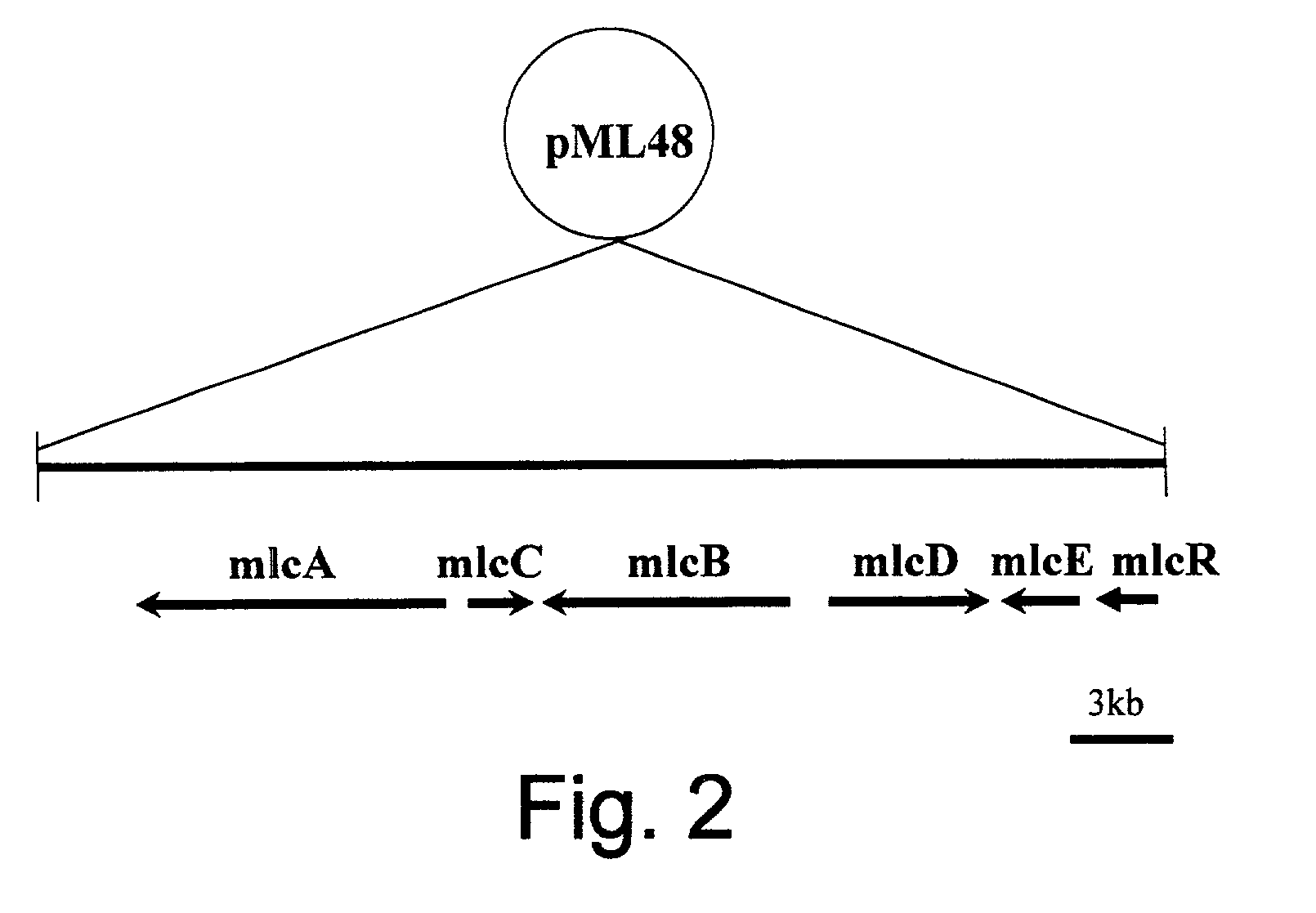
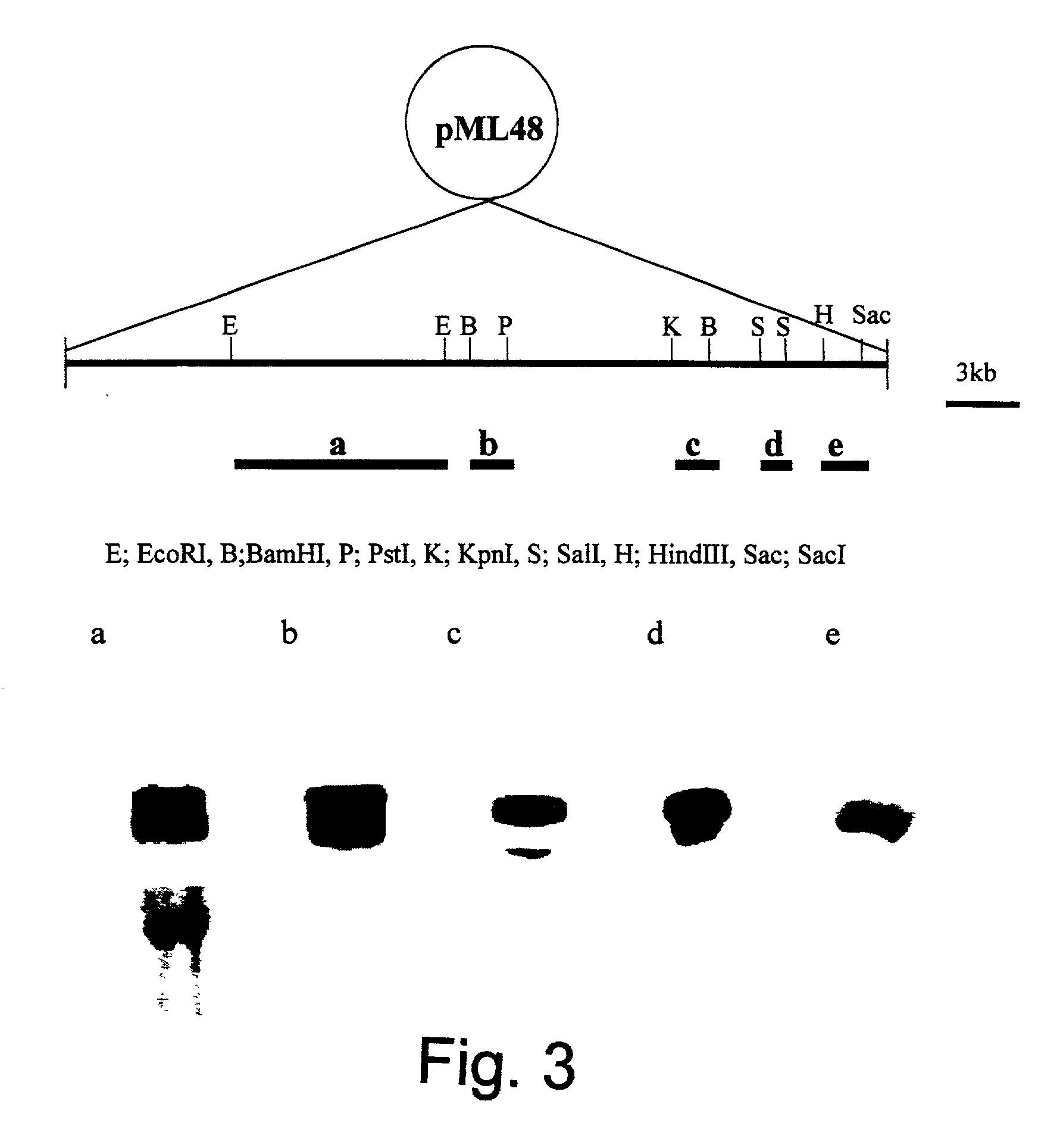
Fermentation of pravastatin
InactiveUS20100048938A1Increase synthesisReduce consumptionFungiOrganic chemistryMicroorganismHydroxylase gene
The present invention provides a microorganism containing a compactin biosynthesis gene and a gene for conversion of compactin into pravastatin. In a preferred example, said compactin biosynthesis gene is mIcA and / or mIcB and / or mIcC and / or mIcD and / or mIcE and / or mIcF and / or mIcG and / or mIcH and / or mIcR and said gene for conversion of compactin into pravastatin is a hydroxylase gene. Furthermore, the present invention provides a method for producing a compound of interest such as a statin. In a preferred example said statin is pravastatin.
Owner:DSM IP ASSETS BV
A kit for simultaneously detecting statin metabolizing gene multisite mutations
The invention relates to a kit for simultaneously detecting statin metabolizing gene multisite mutations. The kit includes primer pairs and probe pairs for detecting APOE, SLCO1B1, CETP, ABCB1 and MTHFR gene sites. MGB (minor groove binder) probes and a real-time fluorescent PCR technique are applied in the kit. The kit has advantages of capability of being time saving and convenient, high sensitivity, capability of allowing positive and negative coincidence rates of a sample to be 99.5% or above, and the like. The kit is mainly used for personalized medication assisted diagnosis of statins such as simvastatin, atorvastatin and pravastatin.
Owner:NINGBO MEIJING MEDICAL TECH
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
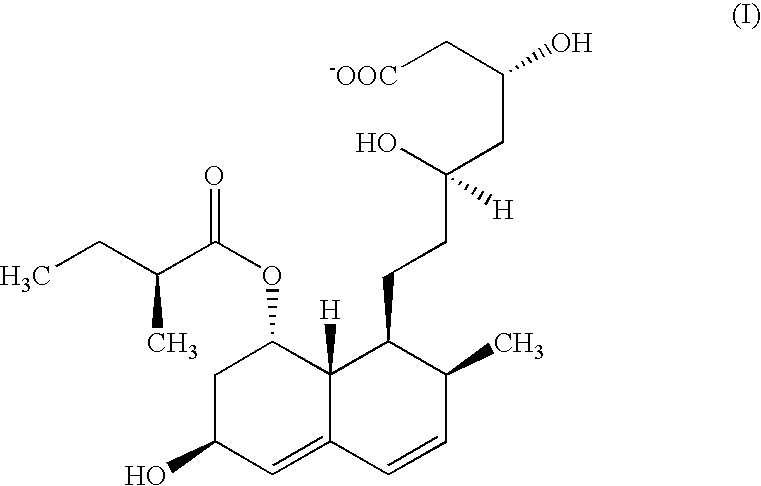
Microbial process for preparing pravastatin
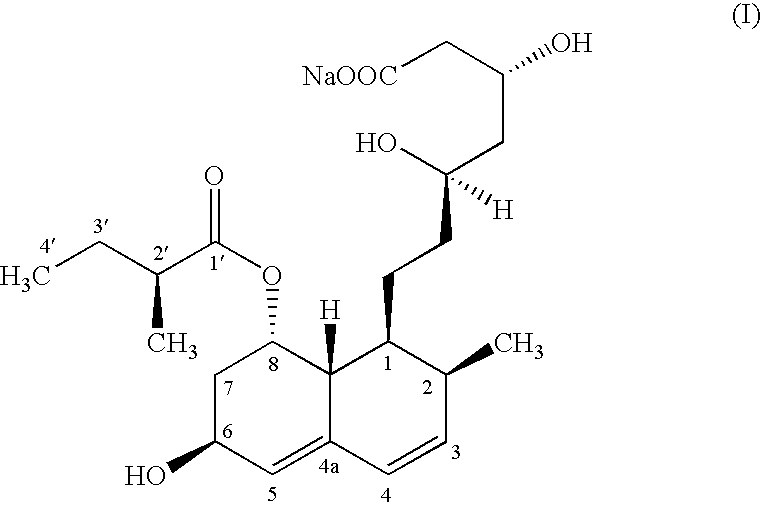
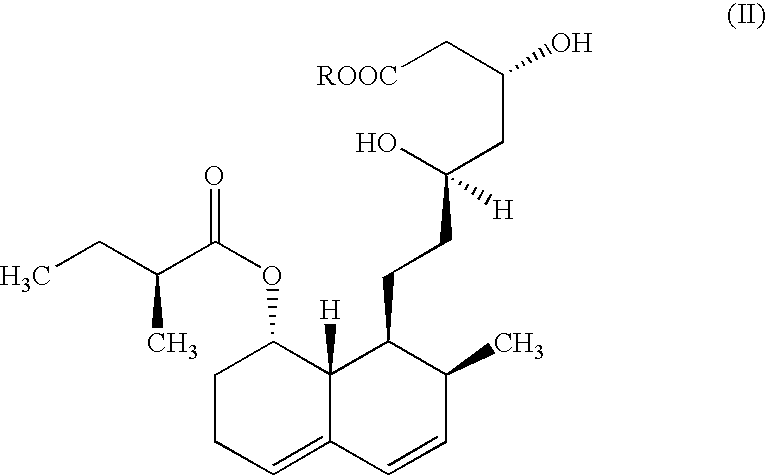
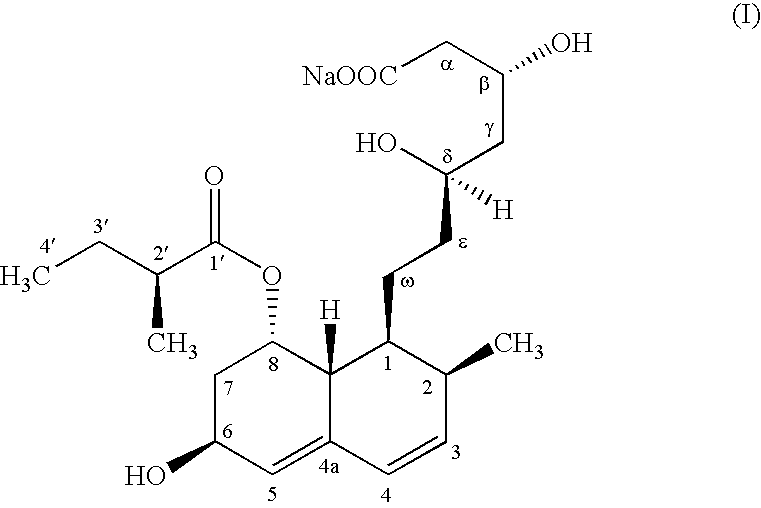
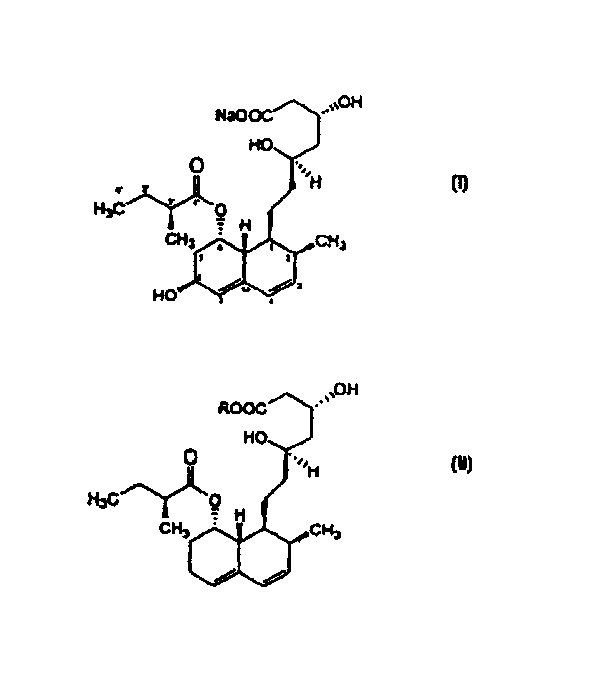
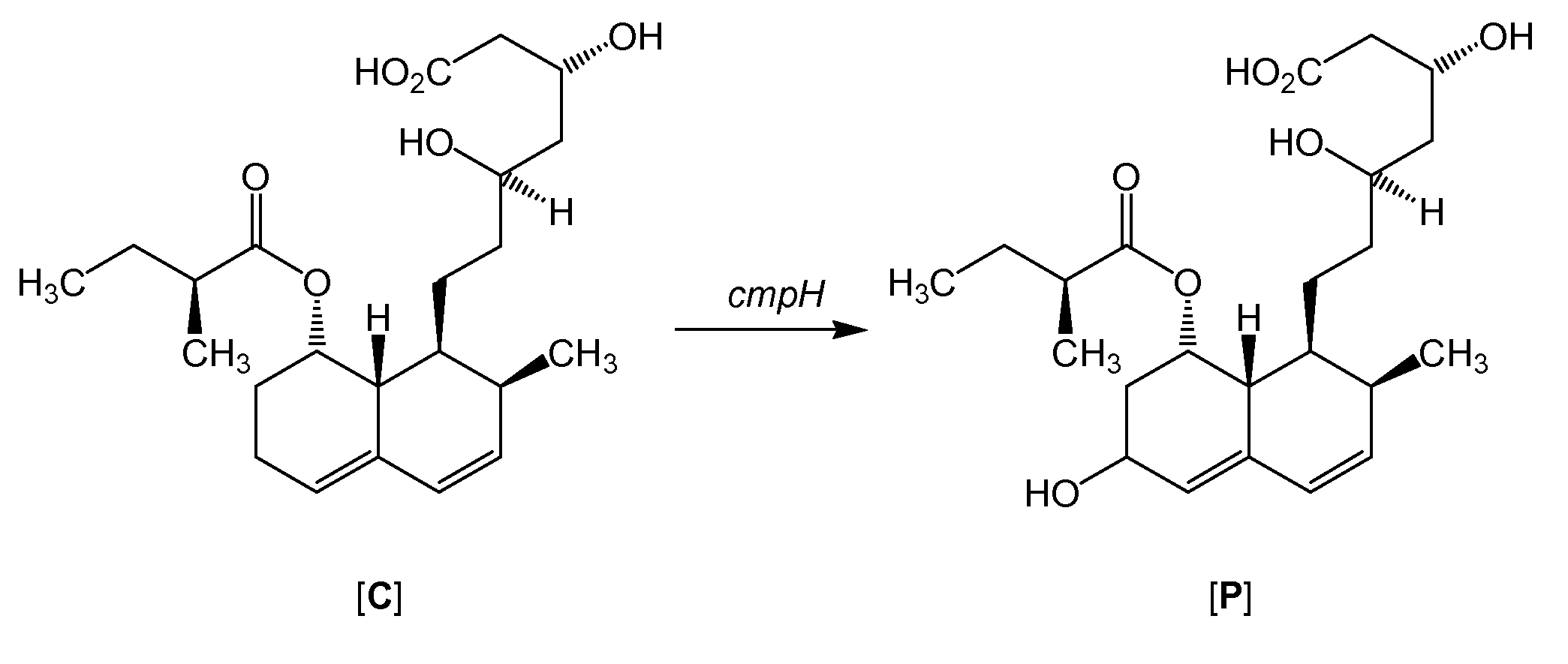
The present invention relates to a new microbial process for the preparation of the compound formula (I)from a compound of general formula (II)wherein R stands for an alkali metal or ammonium ion, by the submerged cultivation of a mold strain able to 6beta-hydroxylate a compound of the Formula (II) in aerobic fermentation and by the separation and purification of the product of Formula (I) formed in the course of the bioconversion. The process comprises cultivating a strain of Mortierella maculata filamentous mold species that is able to 6beta-hydroxylate a compound of the general Formula (II), on a nutrient medium containing assimilable carbon and nitrogen sources and mineral salts and separating the product formed from the fermentation broth, then isolating the compound of formula (I) and purifying the same. Novel strains of Mortierella maculata are also disclosed.
Owner:INST FOR DRUG RES
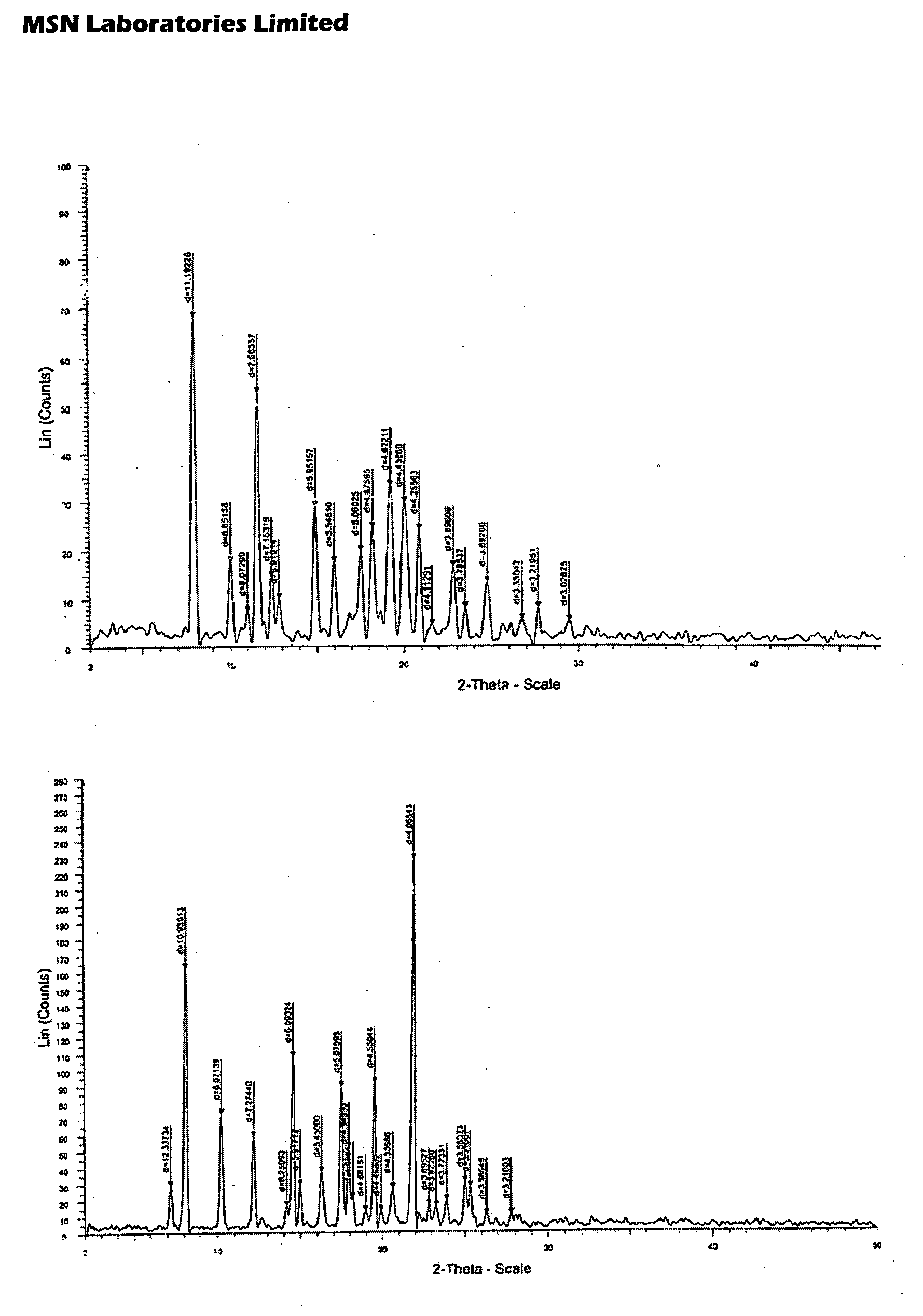
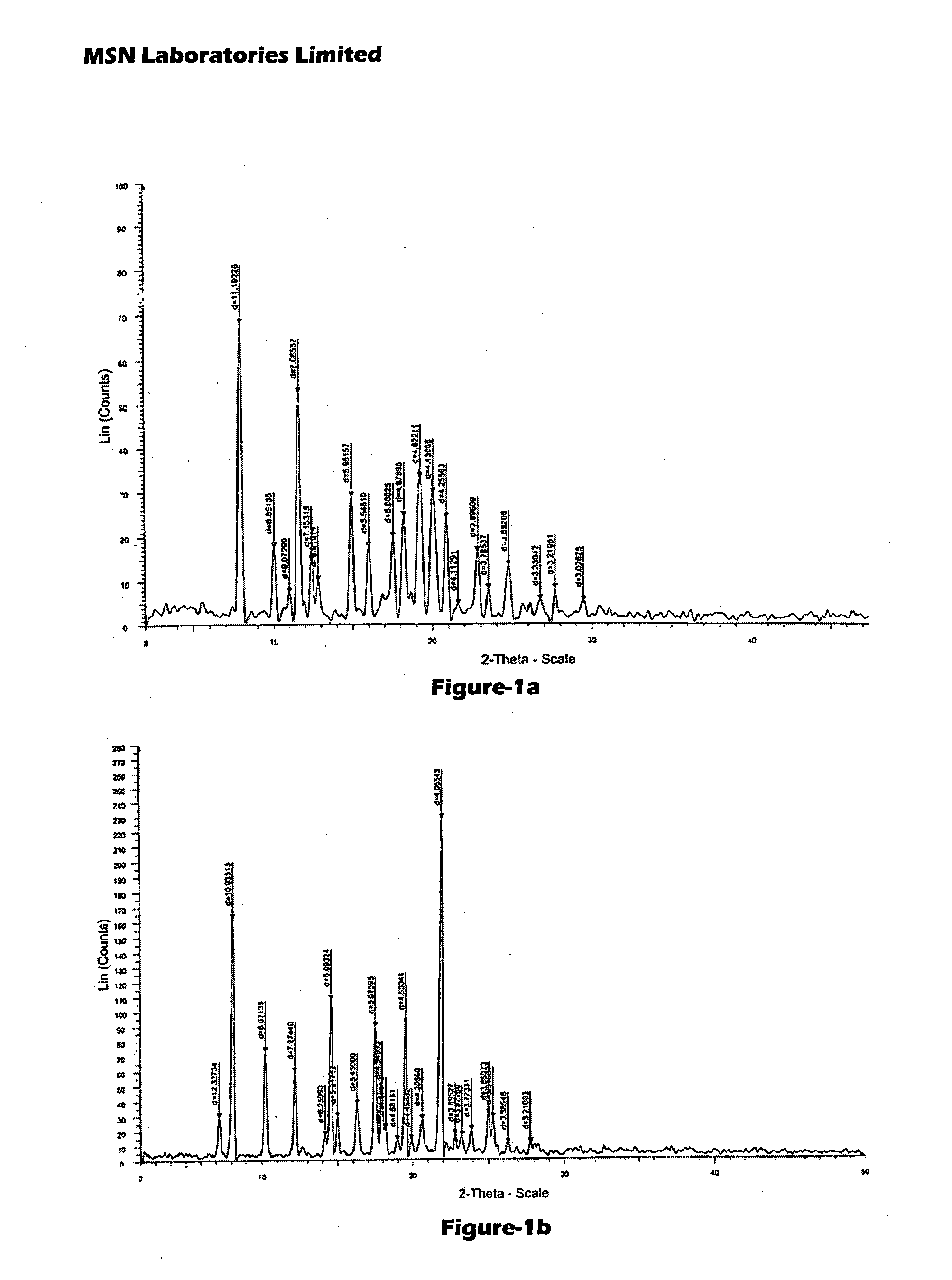
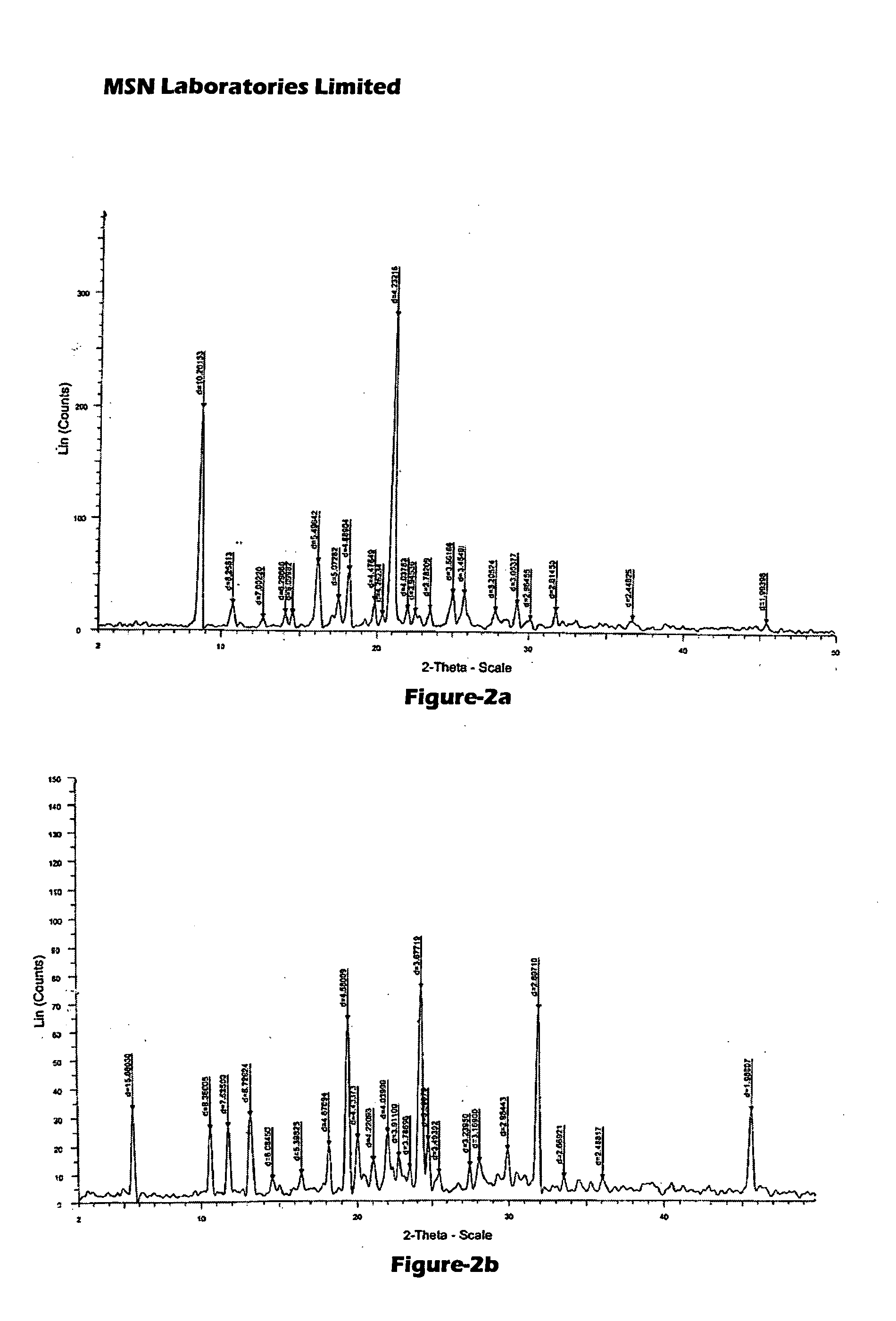
Process for Preparing Pitavastatin, Intermediates and Pharmaceuctically Acceptable Salts Thereof
Owner:MSN LAB PTE LTD
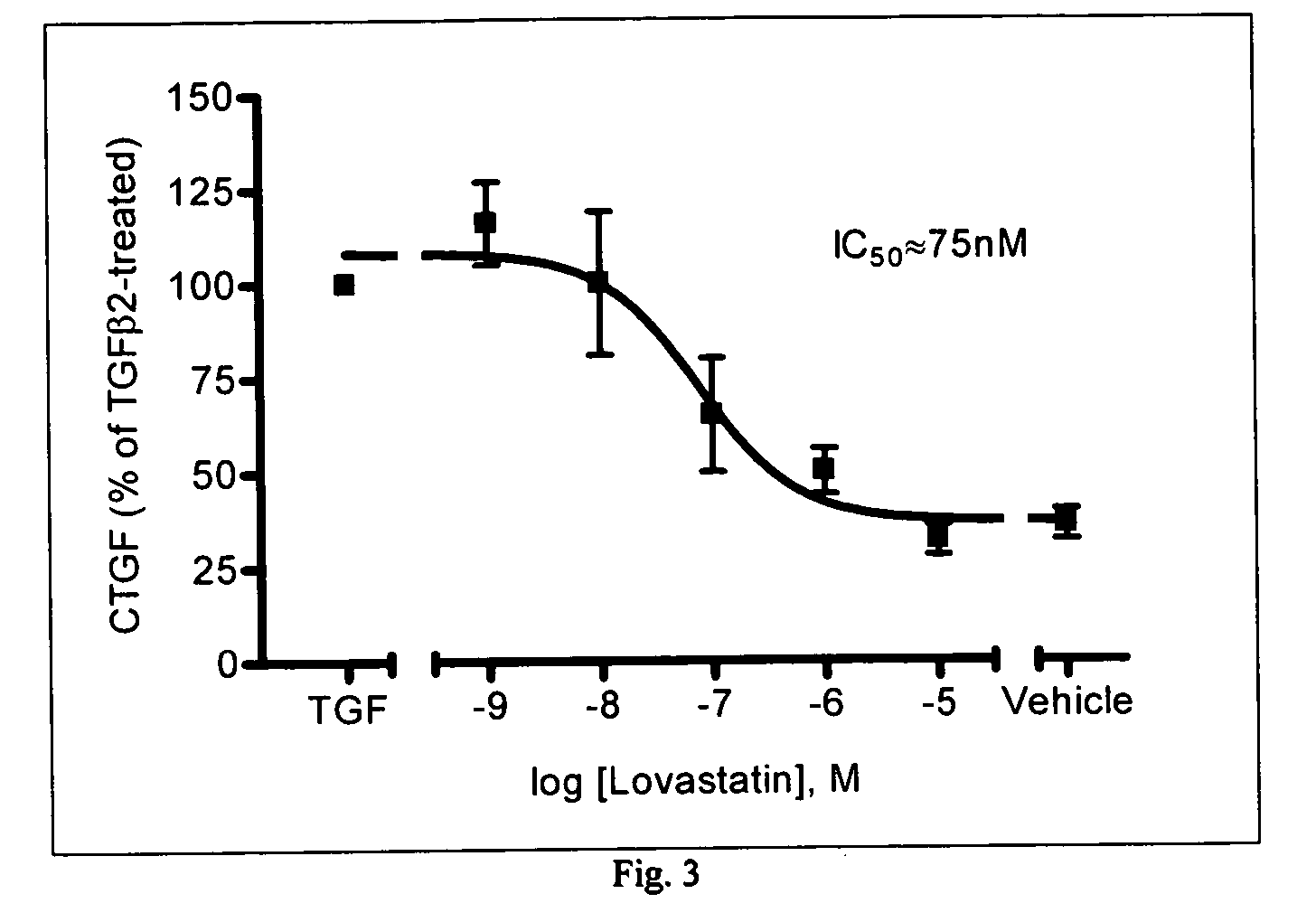
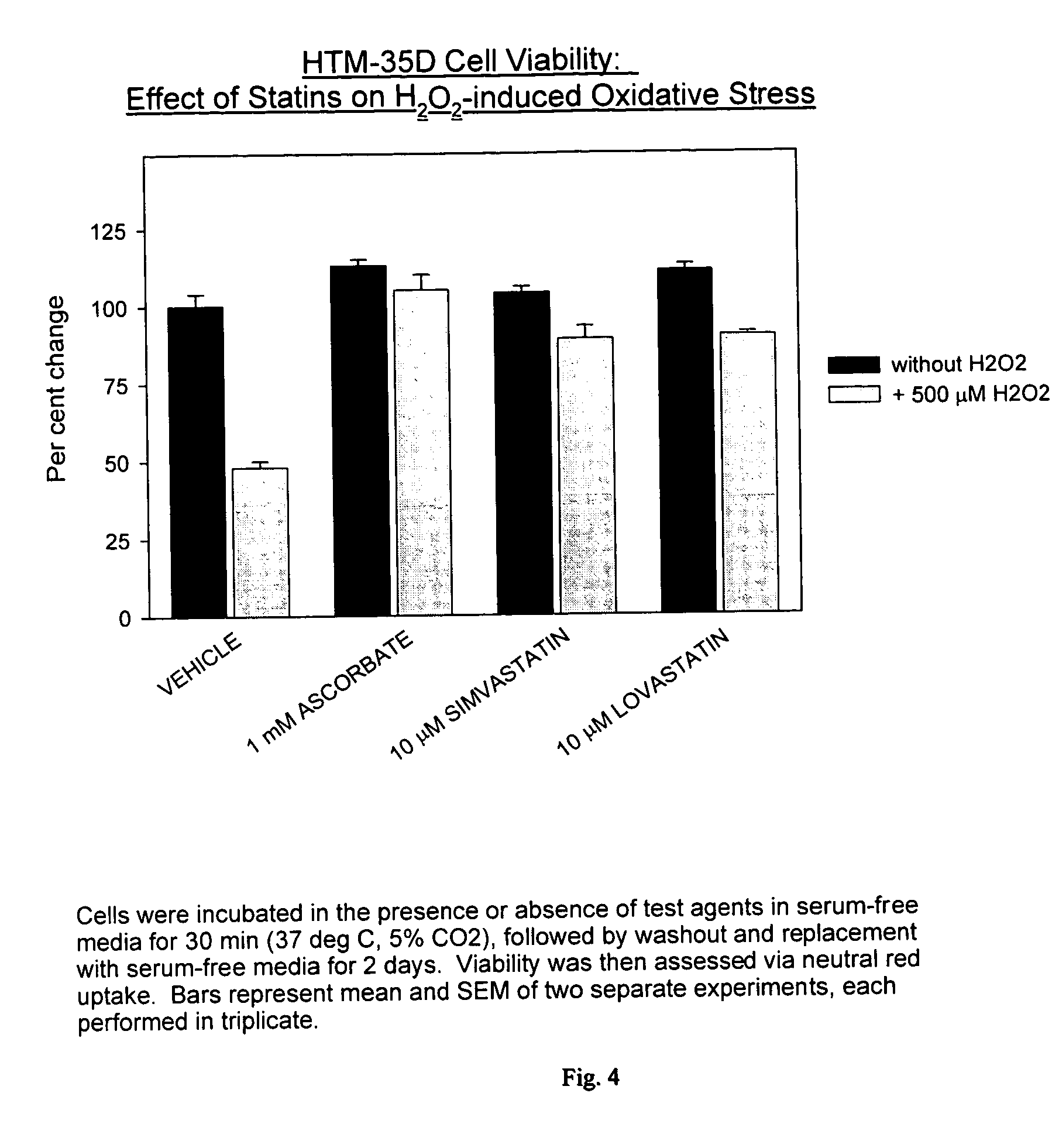
Statins for the treatment of ocular hypertension and glaucoma
InactiveUS20050239871A1Good chemical stabilityLowering and controlling normal or elevated intraocular pressureBiocideSenses disorderPravastatinStatine
The use of HMG-CoA reductase inhibitors (e.g., statins) to treat glaucoma, control intraocular pressure, preserve the trabecular meshwork, protect against ocular neurodegeneration and / or protect against glaucomatous retinopathy is described. The preferred HMG-CoA reductase inhibitors, which are statins having an RI value of 0.2 to 0.7 (e.g., pravastatin), are administered via topical application to the affected eye(s) of the patient.
Owner:ALCON INC
Fermentation process for producing pravastatin by transforming compactin by using actinomadura yumaense
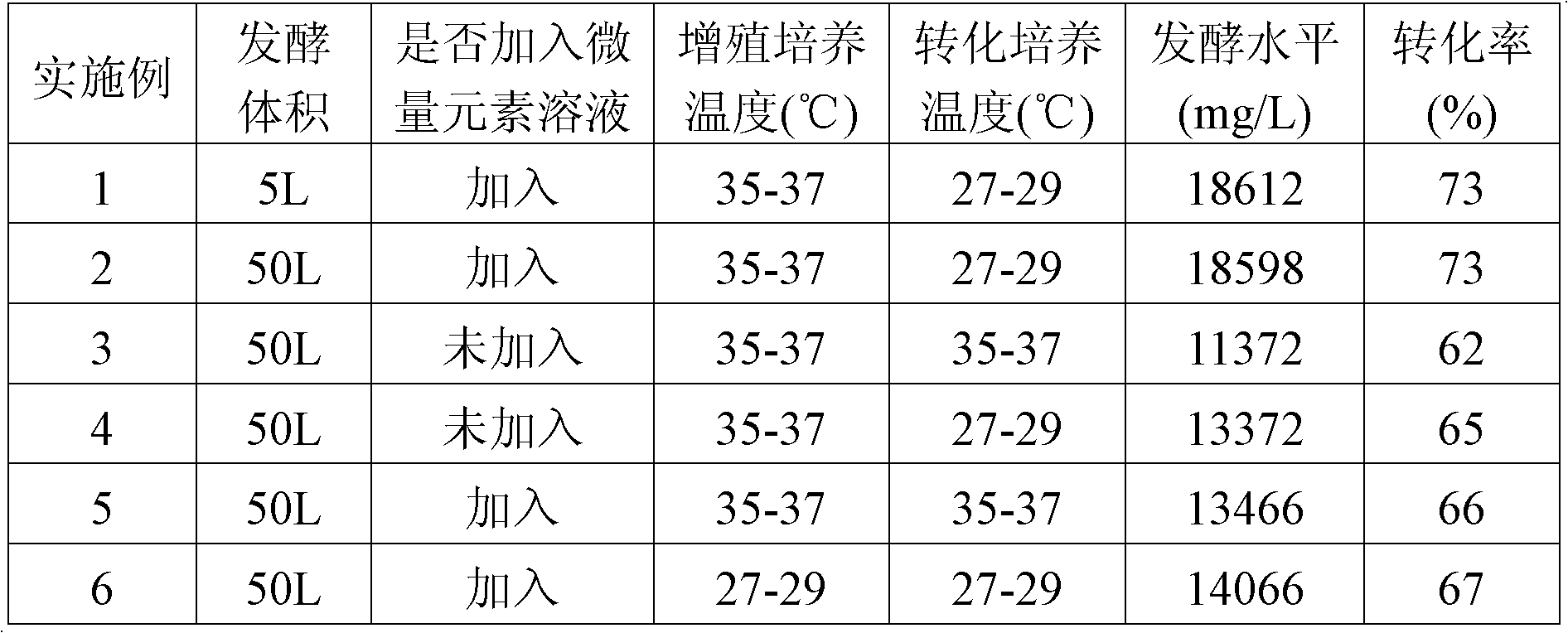
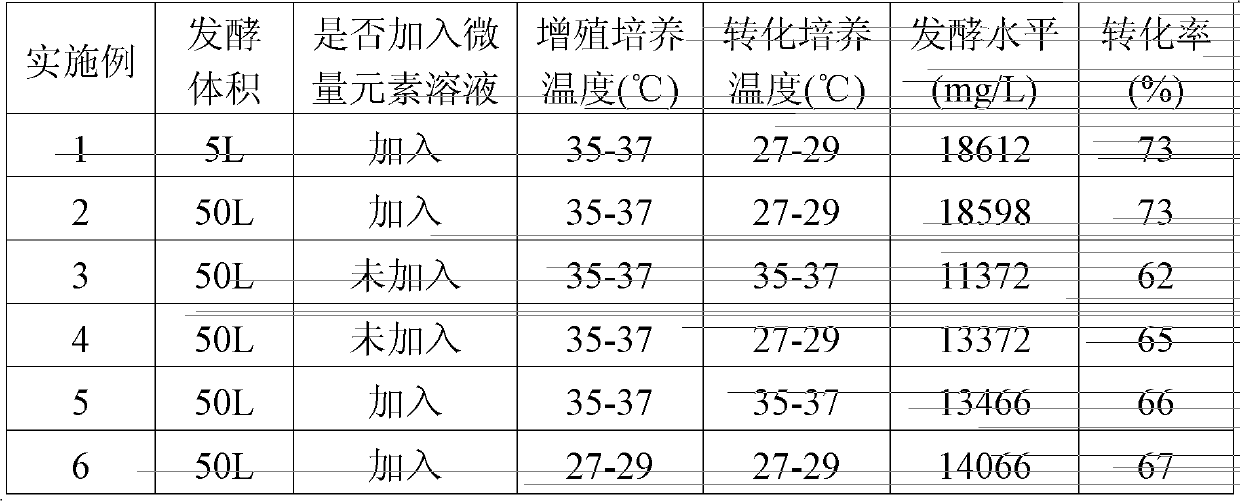
ActiveCN102757986AImprove conversion abilityRaise the level of fermentationMicroorganism based processesFermentationMicroorganismTrace element
The invention discloses a fermentation process for producing pravastatin by transforming compactin by using actinomadura yumaense. According to the fermentation process based on the conventional fermentation process, the transformation ability of the microbial substrate and the fermentation level of the pravastatin are improved by adding trace element solution into a fermentation culture medium and utilizing the effect of the trace elements completely; the transformation ability of the microbial transformation bacteria-actinomadura yumaense on the compactin and the fermentation level of the pravastatin are effectively improved by controlling the culture temperature of the actinomadura yumaense and the transformation temperature of the compactin by using actinomadura yumaense; and the fermentation level is far higher than that of the prior art. The fermentation process is simple and convenient to operate, low in cost and suitable for large-scale production.
Owner:GUANGDONG BLUE TREASURE PHARMA
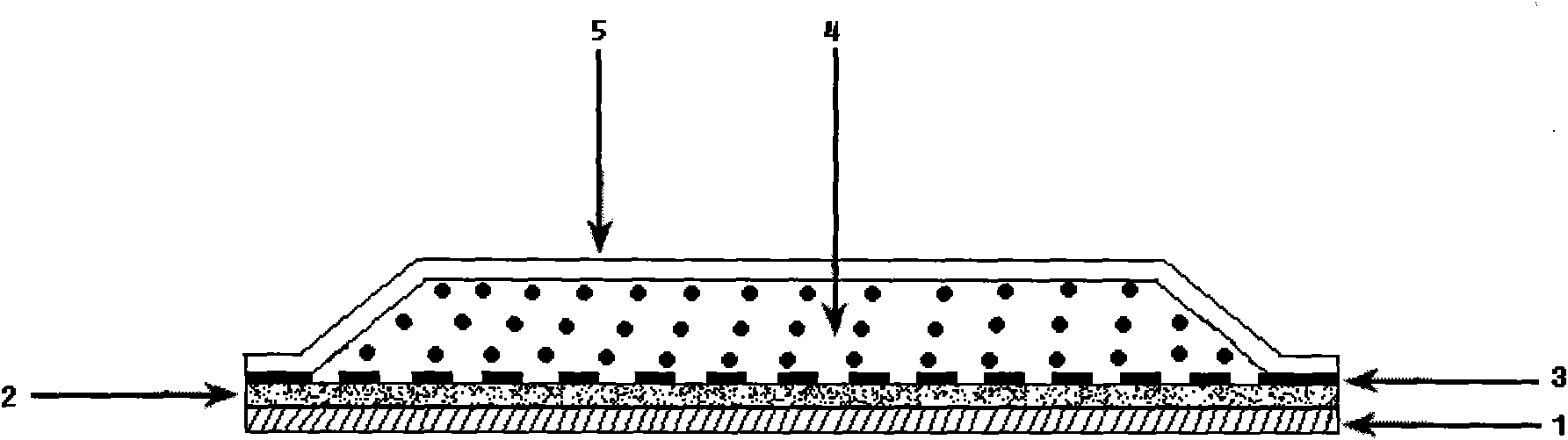
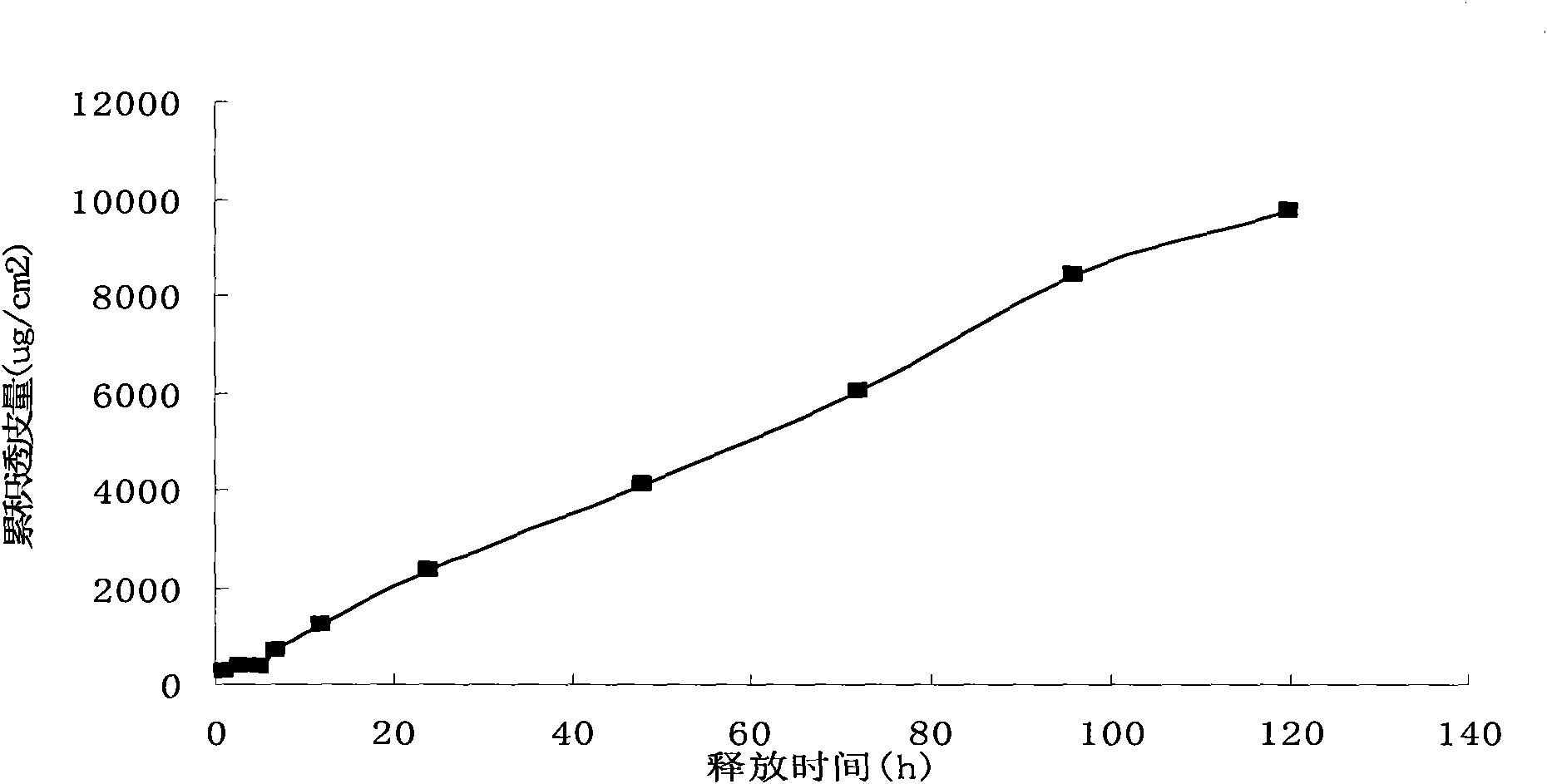
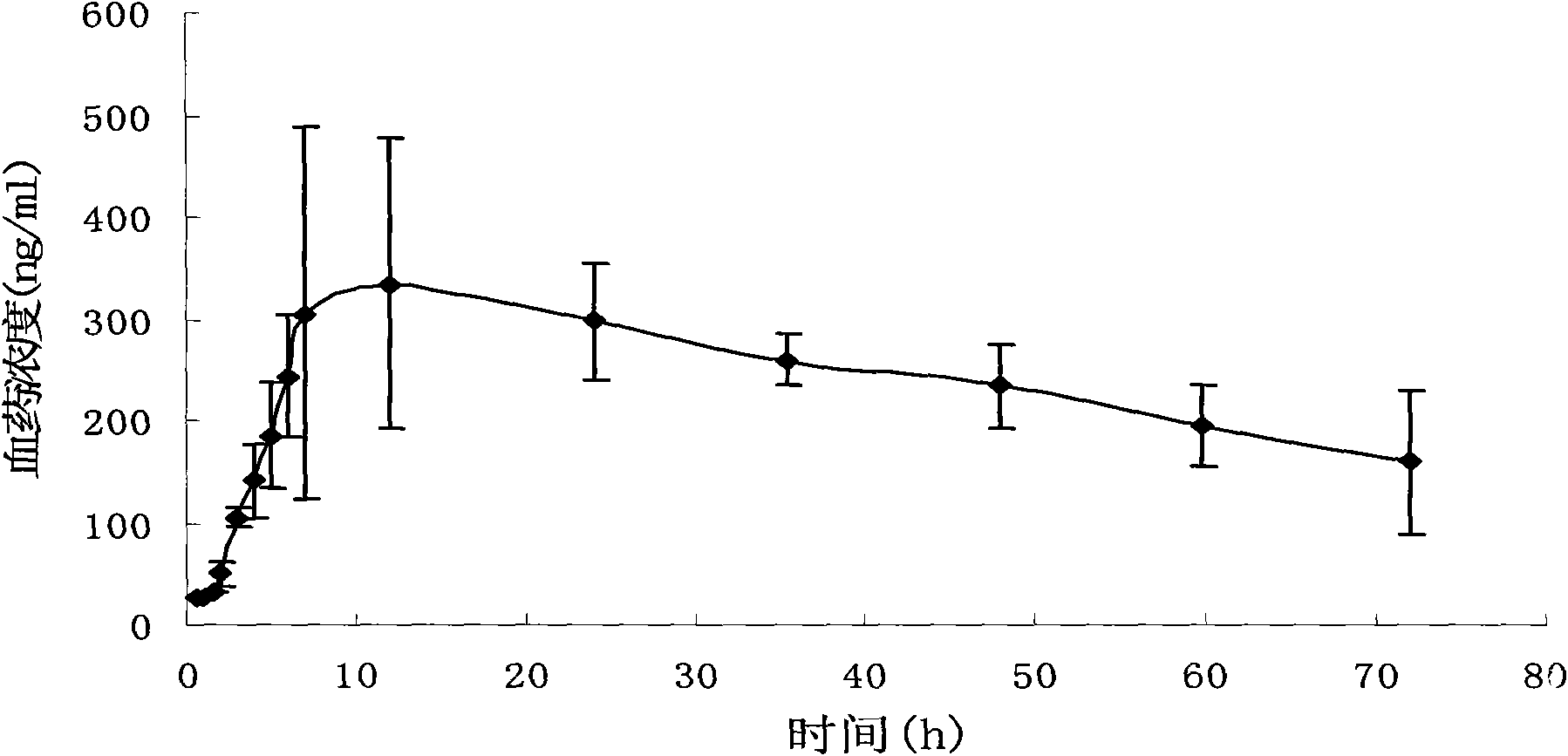
Pravastatin transdermal administration preparation and preparation method thereof
ActiveCN101856342ALittle side effectsLower total cholesterolMetabolism disorderEster active ingredientsIrritationRelease time
The invention relates to a pravastatin transdermal administration preparation and a preparation method thereof. In the invention, pravastatin or a pharmaceutically acceptable salt of the pravastatin serves as a medicament active ingredient, and the medicament active ingredient is a filling closed transdermal preparation which consists of a protective layer, an adhesive layer, a controlled-release membrane layer, a medicament reservoir and a backing layer; the medicament reservoir comprises 1 to 20 percent of pravastatin, and the balance of reservoir substrate; the adhesive layer comprises 0 to 10 percent of pravastatin, and the balance of pressure-sensitive adhesive; the reservoir substrate comprises 5 to 50 percent of transdermal enhancer, while the adhesive layer comprises 0 to 20 percent of transdermal enhancer; and the medicament release area of the preparation is 1 to 100 cm<2>. The pravastatin transdermal administration preparation is convenient to use and carry; the first pass effect of a liver and a gastrointestinal tract is avoided; and an in-vivo experiment of an animal proves that the preparation has no irritation or sensitization to skin. The pravastatin transdermal administration preparation can keep stable and persistent blood concentration, the continuous medicament releasing time is 1 to 7 days, so that the preparation provides the more convenient and safer treatment method for a patient.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Improved statin production
The present invention provides a method for the fermentative production of compactin, lovastatin, pravastatin or simvastatin comprising culturing a host, preferably a filamentous fungus, comprising the polynucleotide of the lovE transcription regulator gene from Aspergillus terreus. Furthermore, the invention provides a host for the production of above mentioned statines comprising the polynucleotide of the lovE transcription regulator gene from Aspergillus terreus.
Owner:DSM IP ASSETS BV
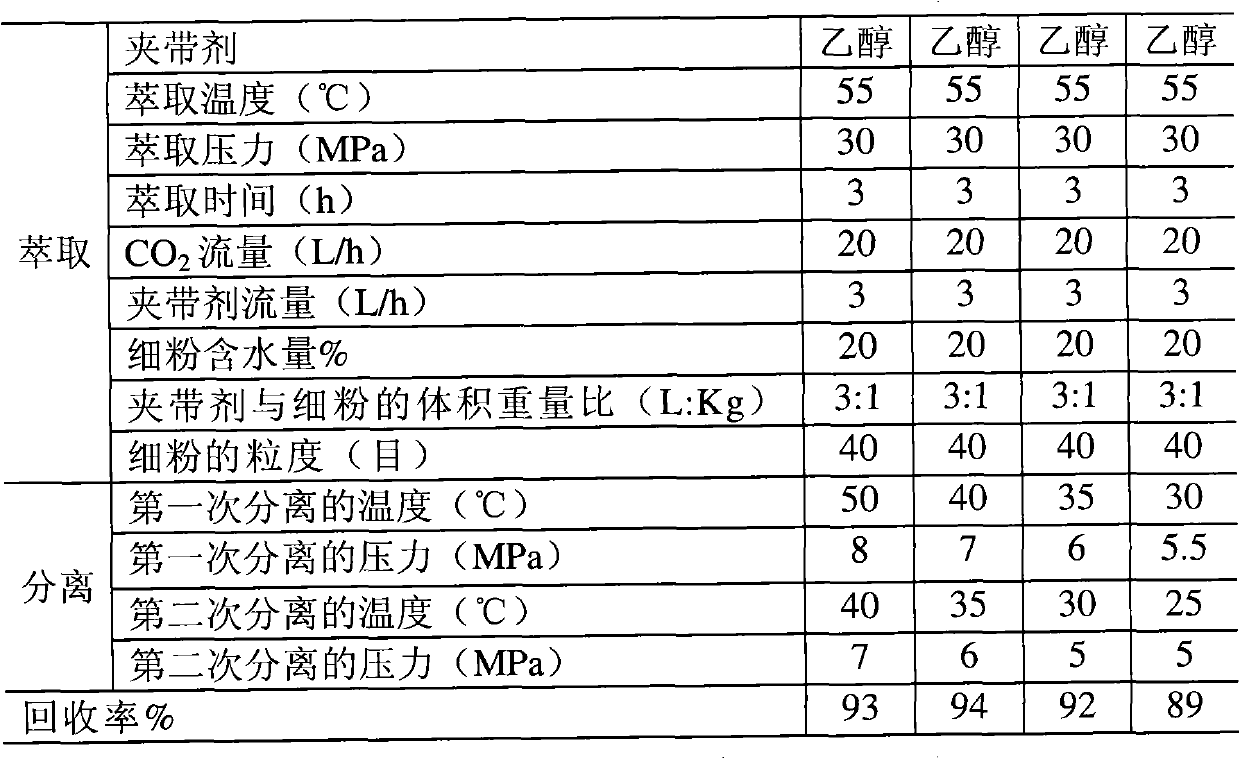
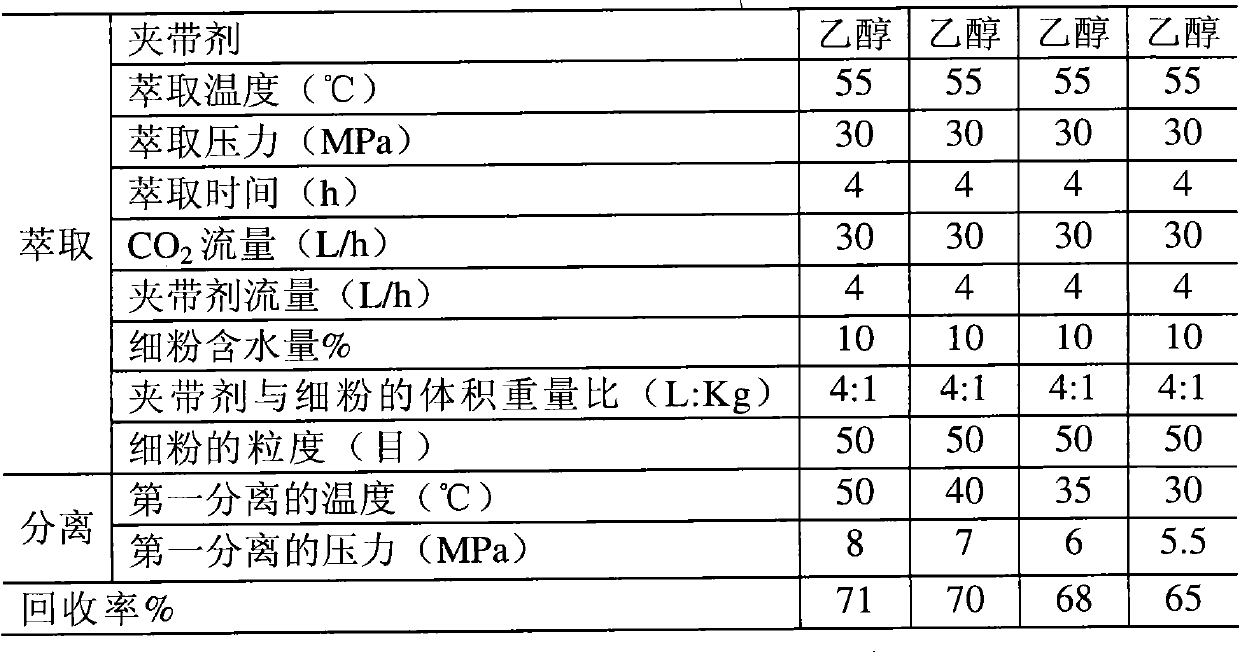
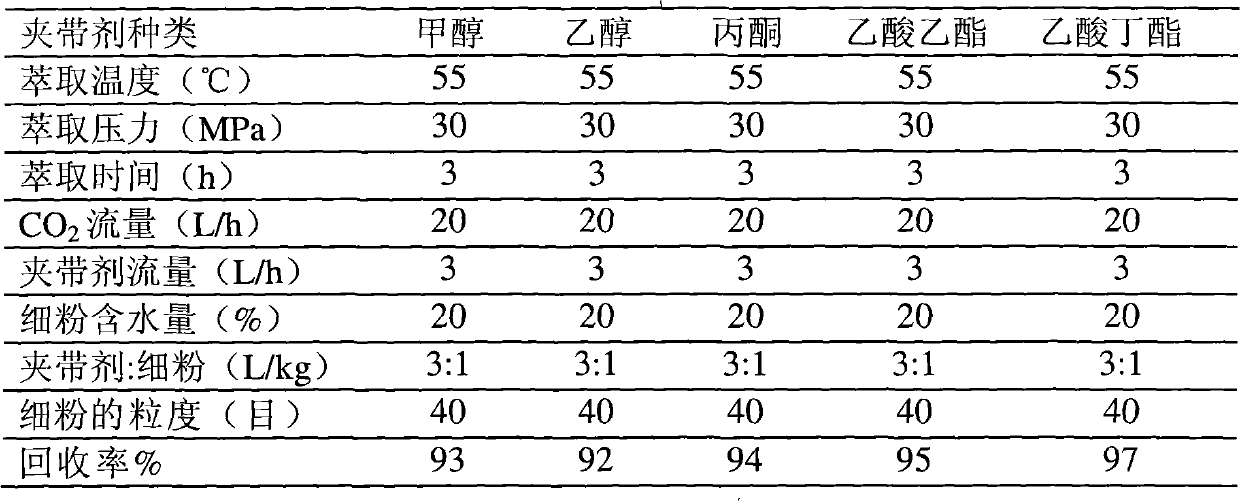
Method for extracting and separating statins substance
ActiveCN102086183APrevent oxidationPrevent escapeOrganic chemistryBulk chemical productionAcetic acidButyl acetate
The invention relates to a method for extracting and separating statins substances by utilizing a supercritical CO2 extraction process. The method comprises the steps of extracting and separating, wherein in the extracting step, an entrainer is one or more of ethanol, acetone, ethyl acetate and butyl acetate. The method provided by the invention is suitable for extracting and separating the statins substances, such as mevastatin, pravastatin or lovastatin and the like. By adopting the method, the usage of organic solvents can be greatly reduced, the production cost is lowered, the environmental pollution is reduced, and the quality and the recovery rate of a finished product are improved, therefore, the statins substances are prepared economically and cleanly.
Owner:NEW FOUNDER HLDG DEV LLC +2
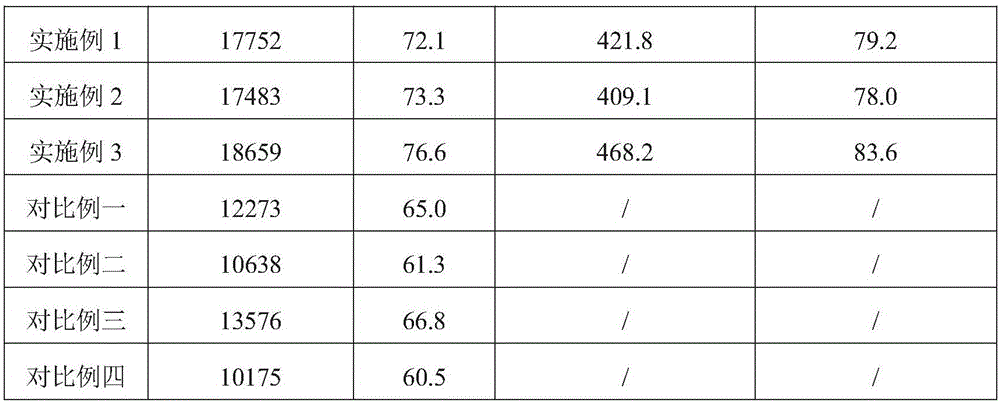
Process for producing pravastatin on large scale
ActiveCN105821086ARealize large-scale productionRaise the level of fermentationBacteriaOrganic compound preparationMicroorganismOrganic solvent
The invention discloses a process for producing pravastatin on a large scale. The process comprises the steps of fermenting, extracting and refining, wherein a plant extracting solution composed of an exocarpium benincasae extracting solution and a water melon peel extracting solution is used in the fermentation process, drug resistance and the conversion capacity of microorganisms to the substrate compactin and the fermentation level of pravastatin are effectively improved, the yield of pravastatin is increased, and in the steps of extracting and refining, the pH value of the solution, types and dosage of organic solvent, extracting time and temperature, cooling operation and crystallization time are controlled strictly to ensure the yield and purity of pravastatin. The process is stable, cost is low, and large-scale industrial production of pravastatin is achieved.
Owner:GUANGDONG BLUE TREASURE PHARMA
Fermentation process for producing pravastatin by transforming compactin by using actinomadura yumaense
ActiveCN102757986BImprove conversion abilityRaise the level of fermentationMicroorganism based processesFermentationMicroorganismTrace element
The invention discloses a fermentation process for producing pravastatin by transforming compactin by using actinomadura yumaense. According to the fermentation process based on the conventional fermentation process, the transformation ability of the microbial substrate and the fermentation level of the pravastatin are improved by adding trace element solution into a fermentation culture medium and utilizing the effect of the trace elements completely; the transformation ability of the microbial transformation bacteria-actinomadura yumaense on the compactin and the fermentation level of the pravastatin are effectively improved by controlling the culture temperature of the actinomadura yumaense and the transformation temperature of the compactin by using actinomadura yumaense; and the fermentation level is far higher than that of the prior art. The fermentation process is simple and convenient to operate, low in cost and suitable for large-scale production.
Owner:GUANGDONG BLUE TREASURE PHARMA
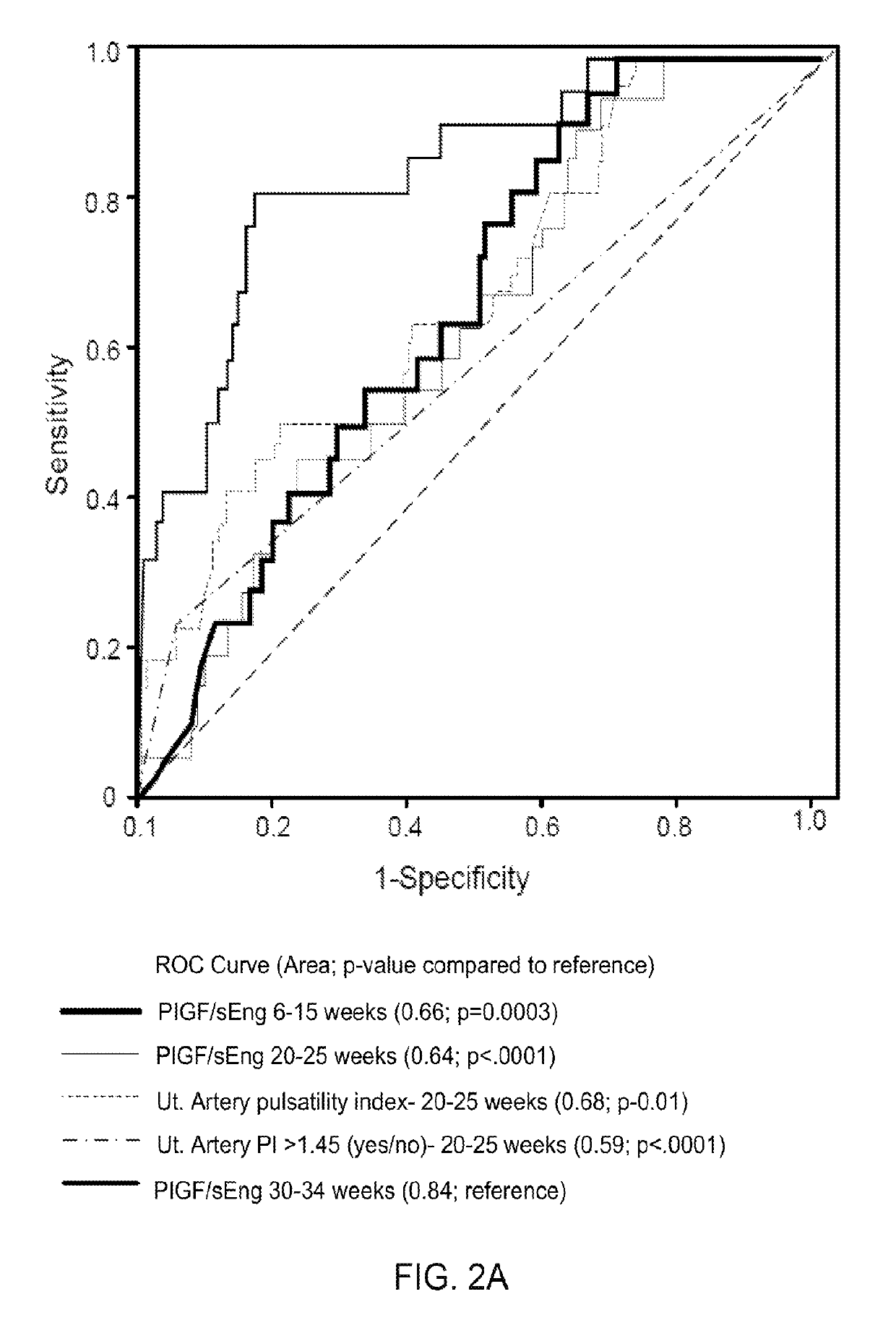
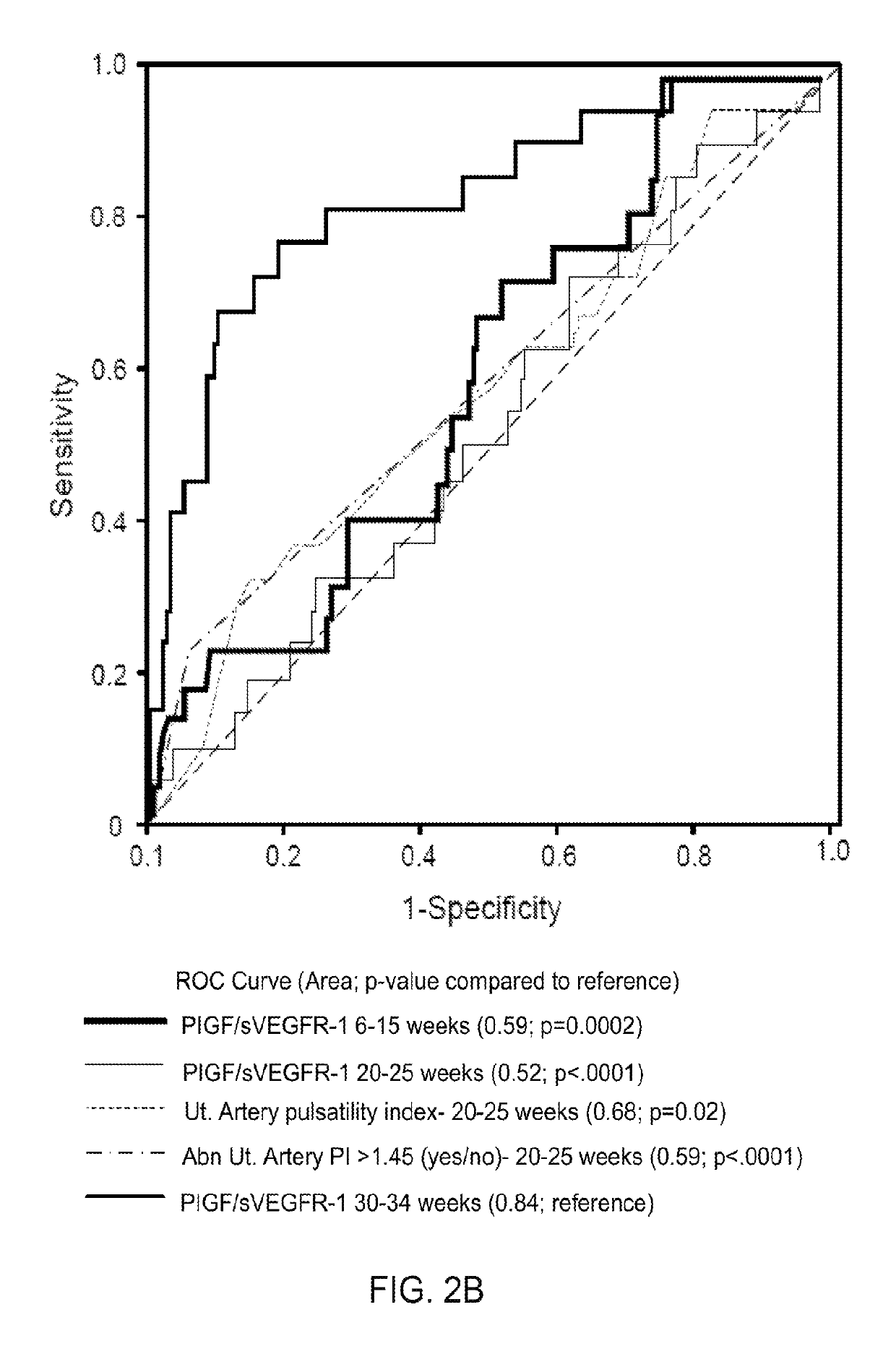
Systems and methods to identify and treat subjects at risk for obstetrical complications
ActiveUS10281475B2Health-index calculationMicrobiological testing/measurementObstetricsWater soluble
Provided are systems and methods for assessing the presence or risk of obstetrical complications, particularly those related to an angiogenic and anti-angiogenic imbalance. Also provided are methods of treating an angiogenic and anti-angiogenic imbalance with water-soluble statins, such as pravastatin.
Owner:WAYNE STATE UNIV +1
Method for purification of pravastatin or a pharmacologically acceptable salt thereof
InactiveUS20030204105A1High purityOrganic compound preparationCarboxylic acid esters preparationInorganic saltsMicroorganism
The present invention provides methods for purification of pravastatin or a pharmacologically acceptable salt thereof using a salting-out technique. Inorganic salts are added to an aqueous solution of pravastatin of a pharmacologically acceptable salt thereof which was obtained from culturing of microorganisms, to selectively precipitate the pravastatin or pharmacologically acceptable salt.
Owner:SANKYO CO LTD
Lipid peroxide-lowering compositions
InactiveUS6998422B2Decrease lipid peroxide levelBiocidePeptide/protein ingredientsPravastatinPantethine
Owner:SANKYO CO LTD
Stable pravastatin pharmaceutical compositions
The present invention provides oral pravastatin formulations comprising a physical mixture of pravastatin and at least one pharmaceutically-acceptable excipient, wherein the composition for at least 6 months after its preparation is stable and has a pH of greater than about 7 to less than 9, as well as methods for the preparation and use of these stable formulations.
Owner:ACTAVIS ELIZABETH
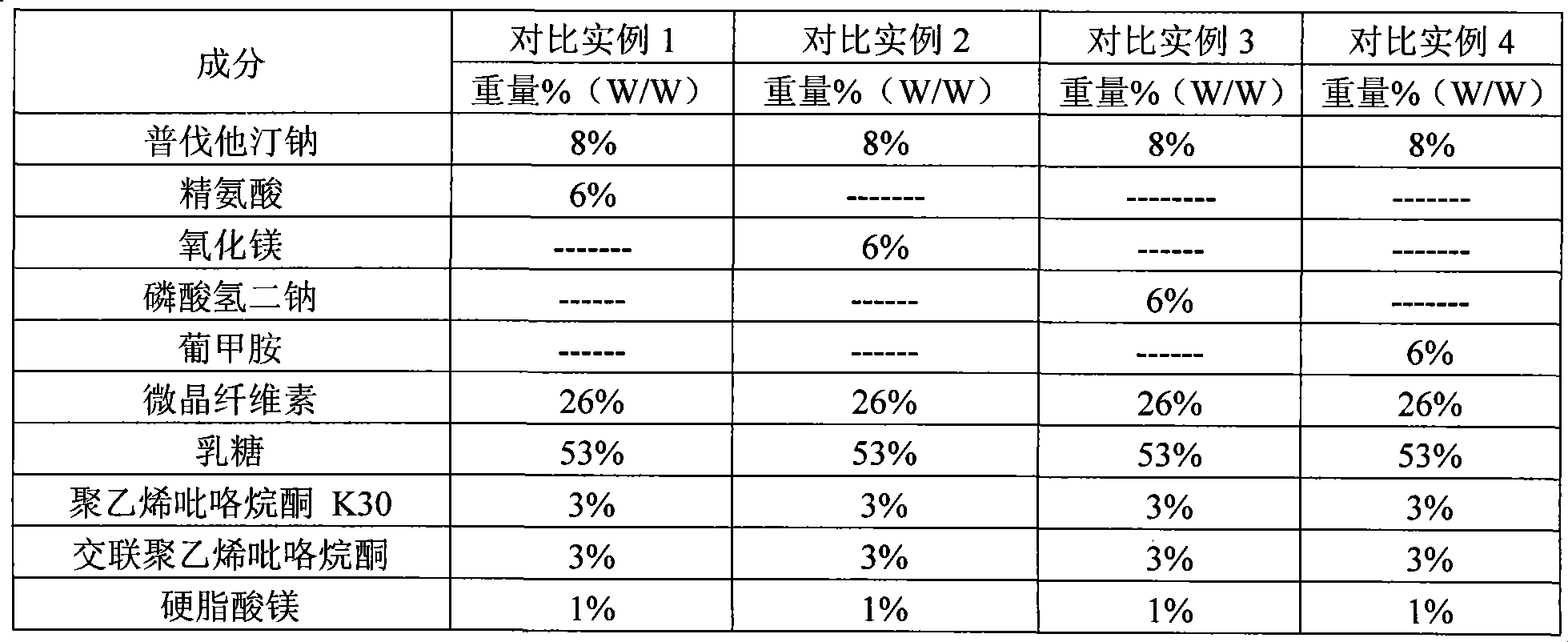
Stable pravastatin medicament composition and preparation method thereof
ActiveCN101461799APharmaceutical non-active ingredientsEster active ingredientsTraditional medicineIothalamate Meglumine
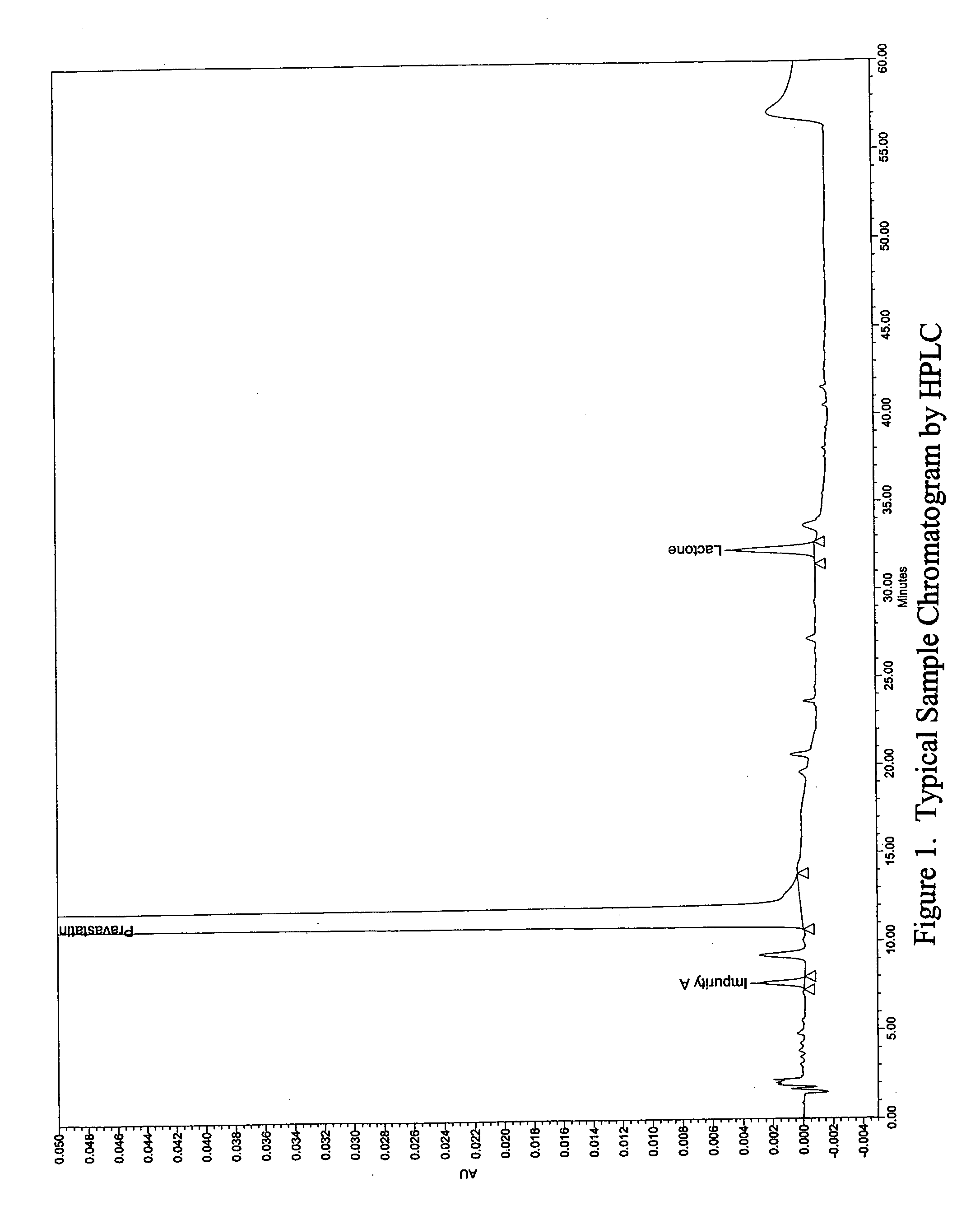
The invention relates to the field of pharmaceutical preparation, in particular to a steady pravastatin medicine composition and a preparation method thereof. The method is characterized in that the stability of the pravastatin medicine composition is remarkably improved by adding a certain amount of meglumine. A high-destructive experiment and long-term storage show that the pravastatin medicine composition has a tiny lactone degradation product.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Use of statin and aspirin in preparation of medicine for treating blood high viscosity syndrome
InactiveCN1965843AImprove treatment efficiencyLower specific viscosityOrganic active ingredientsMetabolism disorderAspirinLovastatin
Owner:BEIJING HUAANFO BIOMEDICAL RES CENT
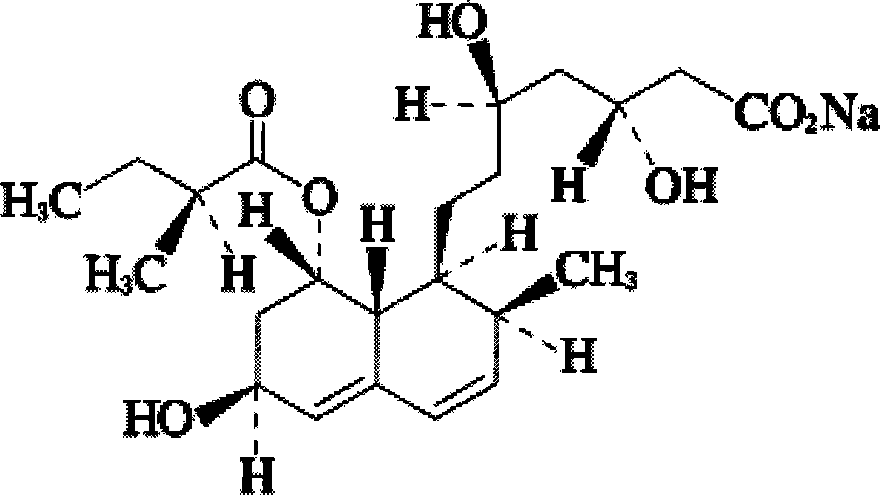
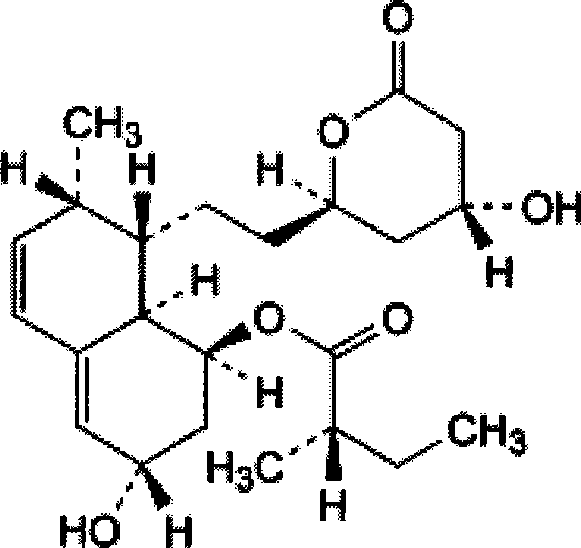
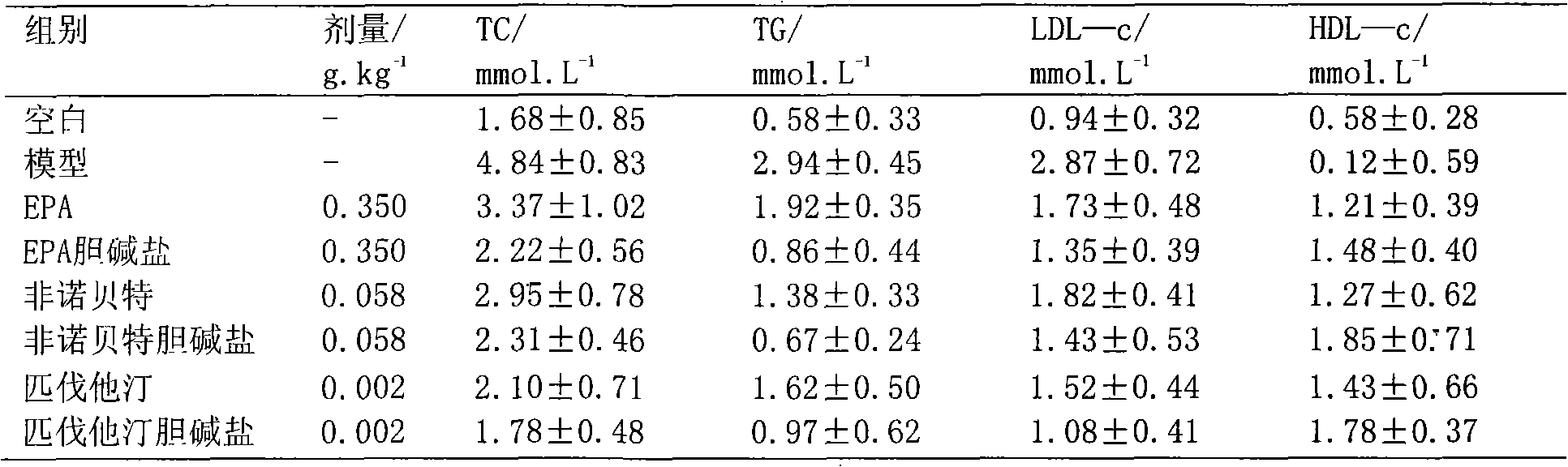
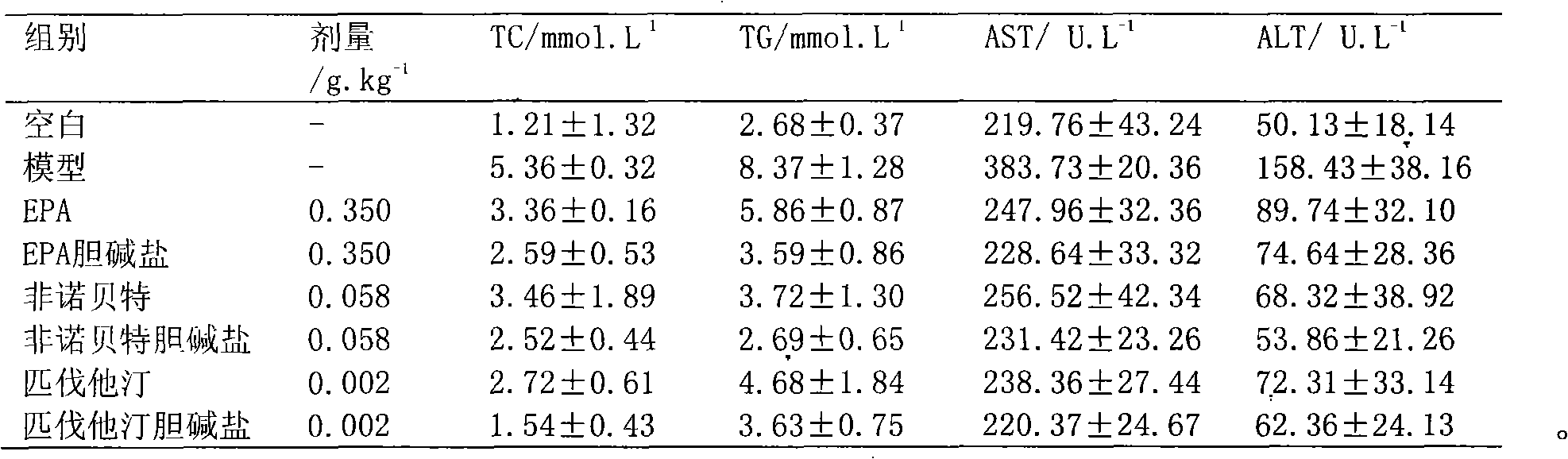
Choline salt of hypolipidemic drug and preparation method and pharmaceutical use thereof
InactiveCN101863780APromote absorptionEfficient use ofOrganic chemistryElcosanoid active ingredientsDocosahexaenoic acidPitavastatin
The invention relates to a choline salt of a hypolipidemic drug and a preparation method and a pharmaceutical use thereof. The invention provides a choline salt of a class of hypolipidemic drugs, and the hypolipidemic drugs include but not limited to clofibrate, libet, fenofibrate, ciprofibrate, gemfibrozil, acipimox, niacin, lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, pitavastatin, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and other unsaturated fatty acids. The choline salt of the hypolipidemic drug can be used for treating hyperlipidemia and other cardiovascular diseases. The invention also provides a preparation method of the choline salt of the hypolipidemic drug.
Owner:北京利乐生制药科技有限公司
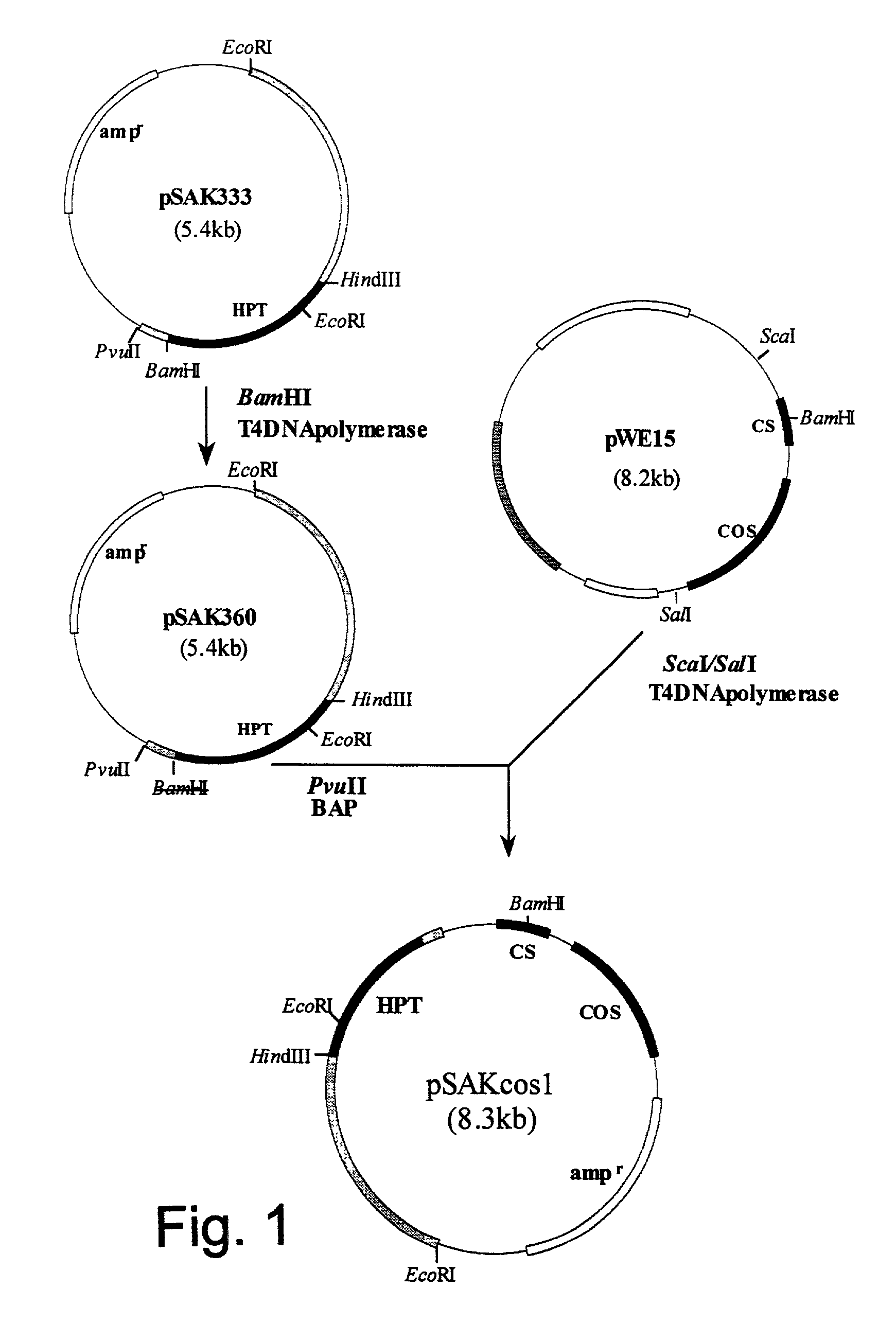
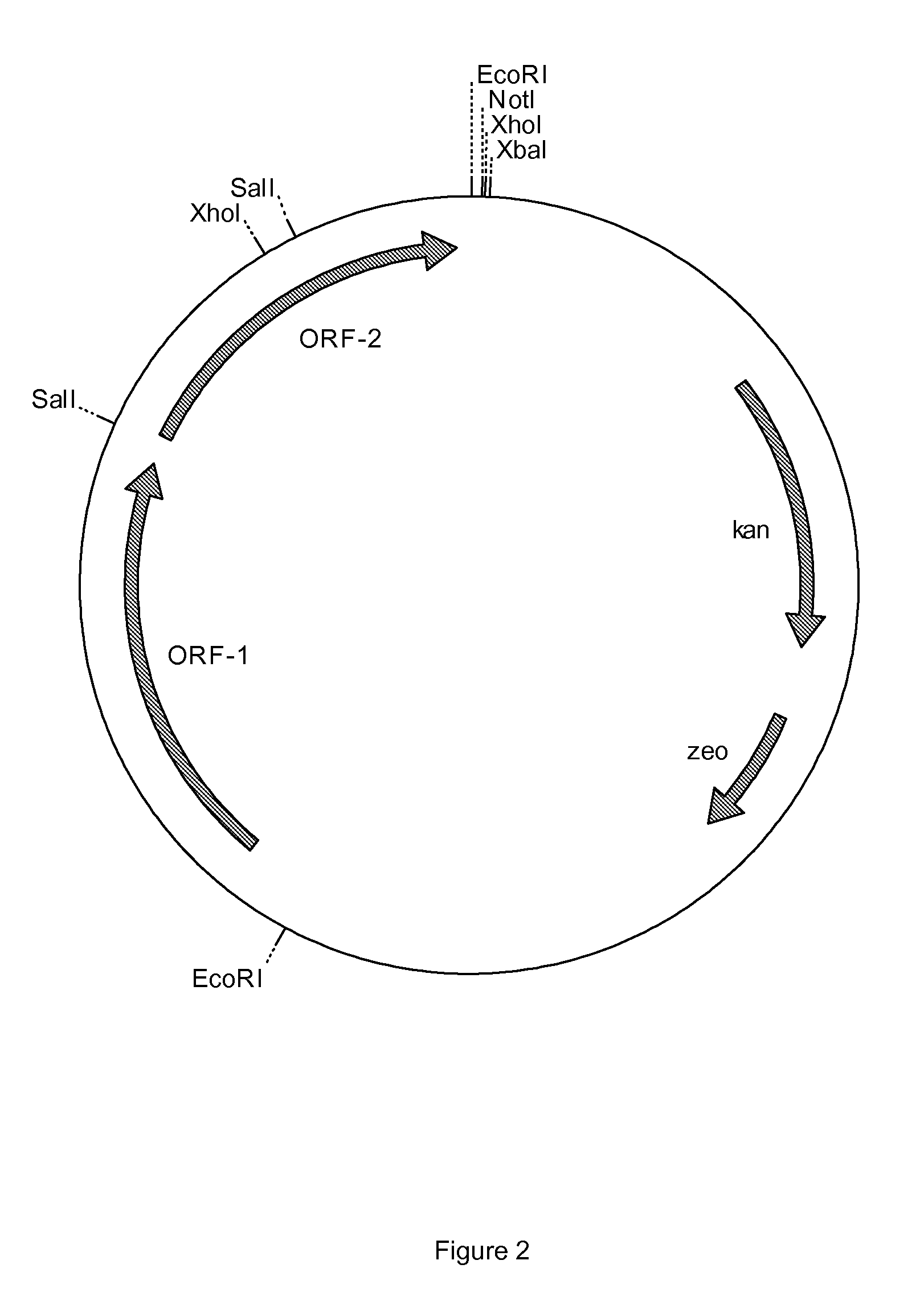
Genes from a gene cluster
ML-236B is an inhibitor of HMG-CoA reductase and useful in preparing another such inhibitor. pravastatin. The preparation of ML-236B using an ML-236B-producing microorganism is enhanced using a polynucleotide encoding a gene related to the polyketide synthase cluster occuring in such a microorganism.
Owner:SANKYO CO LTD
Microbial process for preparing pravastatin
The present invention relates to a new microbial process for the preparation of compound formula (I) from a compound of general formula (II) wherein R stands for an alkali metal or ammonium ion, by the submerged cultivation of a mold strain able to 6 beta -hydroxylate a compound of Formula (II) in aerobic fermentation and by the separation and purification of the product of Formula (I) formed in the course of the bioconversion. The process comprises cultivating a strain of Mortierella maculata filamentous mold species that is able to 6 beta -hydroxylate a compound of general Formula (II), on a nutrient medium containing assimilable carbon and nitrogen sources and mineral salts and separating the product formed from the fermentation broth, then isolating the compound of formula (I) and purifying the same. Novel strains of Mortierella maculata are also disclosed.
Owner:INSTITUTE FOR DRUG RESEARCH LTD (HU)
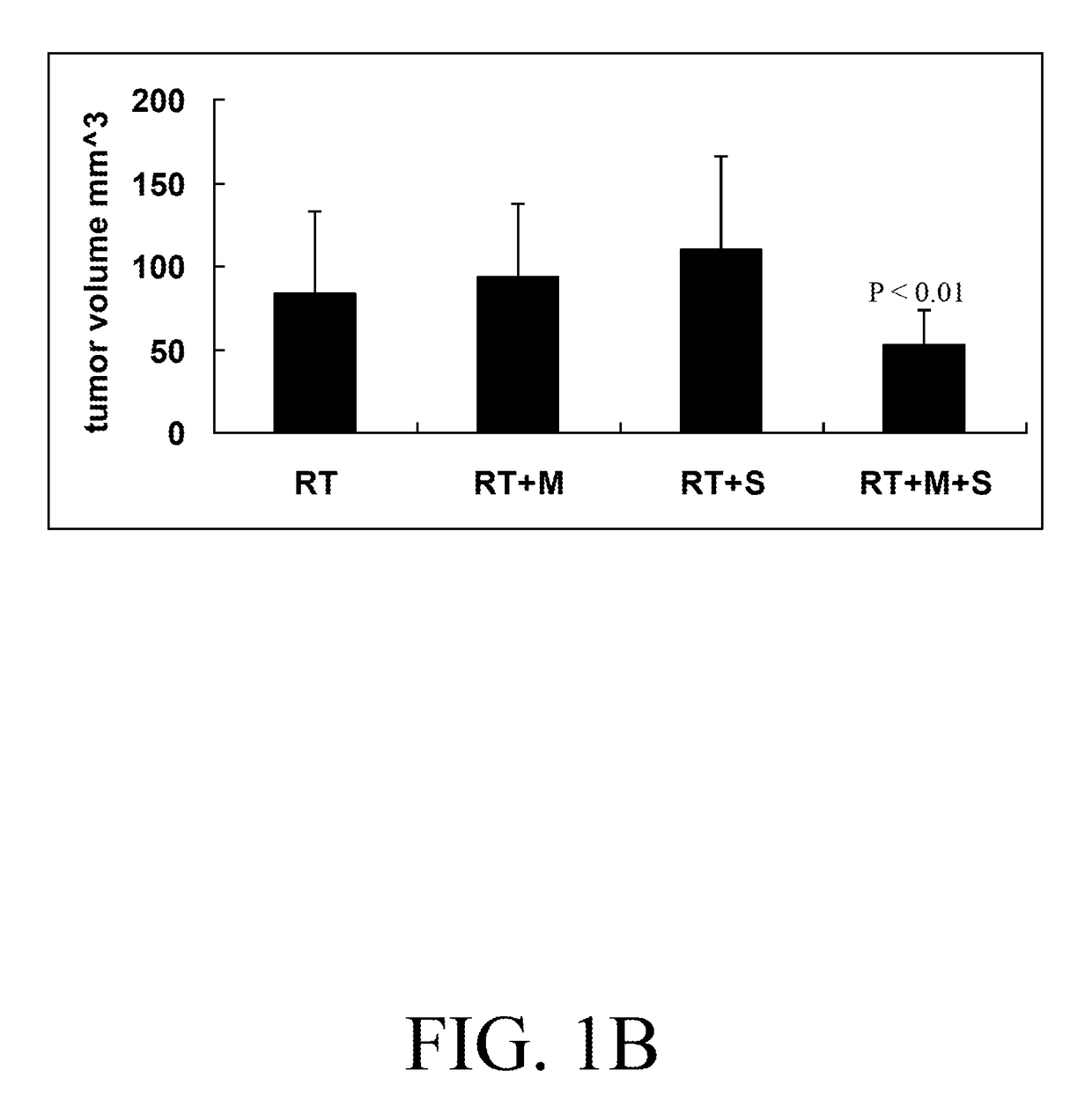
Pharmaceutical combination for cancer treatment and the therapeutic use thereof
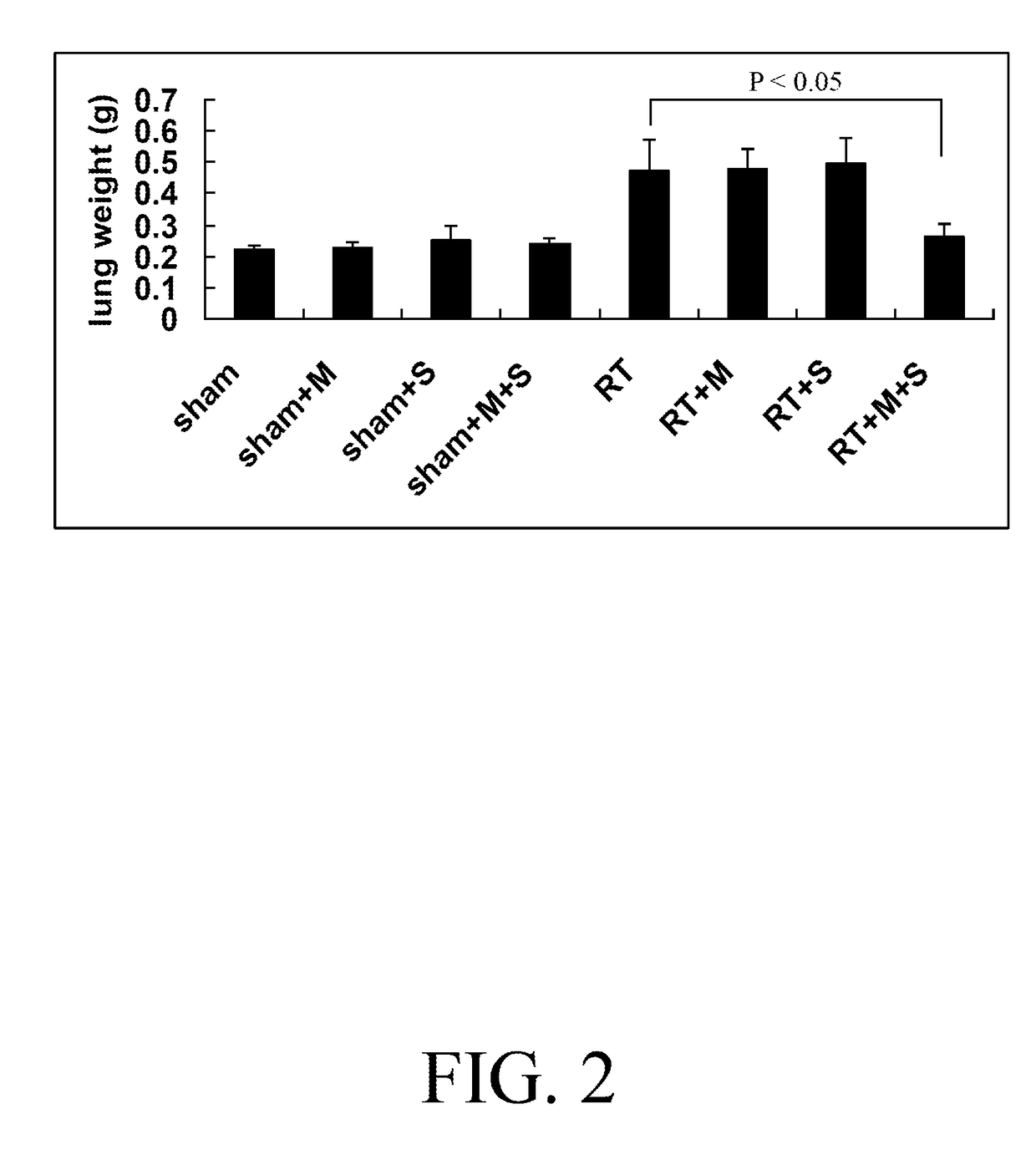
A pharmaceutical combination for sensitizing a subject to a cancer treatment comprises an amount of metformin and an amount of statin. The amount of metformin and the amount of statin together constitute an effective amount of the pharmaceutical combination. The statin is selected from a group consisting of atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin. The cancer treatment is preferably a radiotherapy. The pharmaceutical combination may be included in a pharmaceutical composition. A method of treating cancer is also provided. The method comprises administering the pharmaceutical combination to a subject and delivering an effective dose of radiation to the subject.
Owner:SHINER PHARM CORP
Stable controlled release pharmaceutical compositions containing fenofibrate and pravastatin
InactiveUS20070092567A1Eliminate side effectsReduced bioavailabilityBiocideMetabolism disorderControlled releaseFENOFIBRIC ACID
A controlled Release Pharmaceutical composition comprising an effective amount of Pravastatin and Fenofibrate, characterised in that the difference, in absolute value, between the times of maximal concentration (Tmax) of Pravastatin and Fenofibric acid is not less than 1.5 hours upon administration with food to humans.
Owner:GALEPHAR PHARMA RES
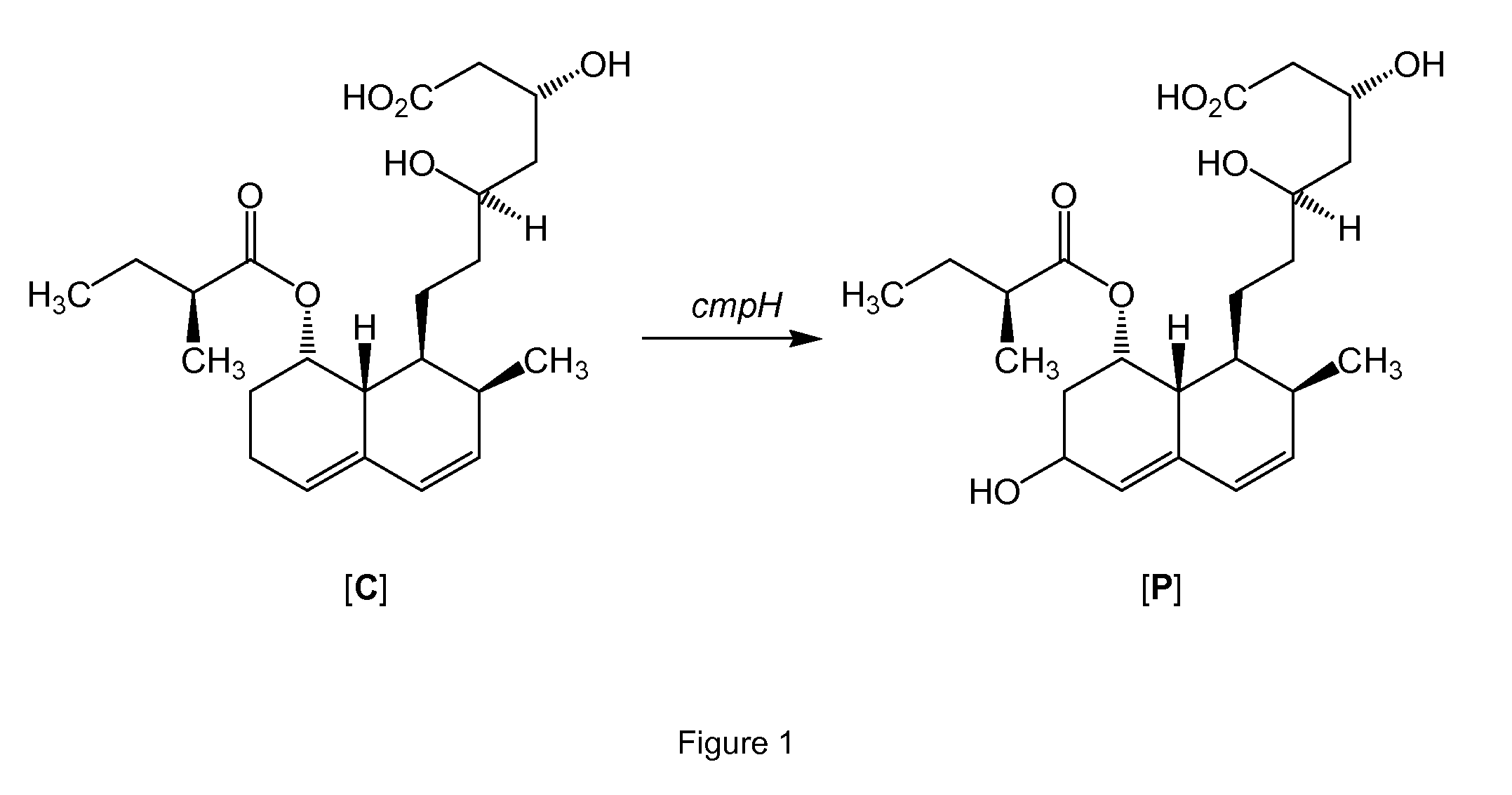
Process for preparing pravastatin
InactiveUS20100217032A1Improve the level ofEfficient executionSugar derivativesMetabolism disorderNucleotideA-DNA
The present invention provides a polypeptide having an amino acid sequence according to SEQ ID NO 3, SEQ ID NO 6 or SEQ ID NO 43-59. The present invention also provides a polynucleotide comprising a DNA sequence encoding these polypeptides and a method for isolating polynucleotides encoding polypeptides capable of improving the compactin into pravastatin conversion. Furthermore, the present invention provides a method for producing pravastatin and a pharmaceutical composition comprising pravastatin.
Owner:DSM SINOCHEM PHARMA NETHERLANDS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com