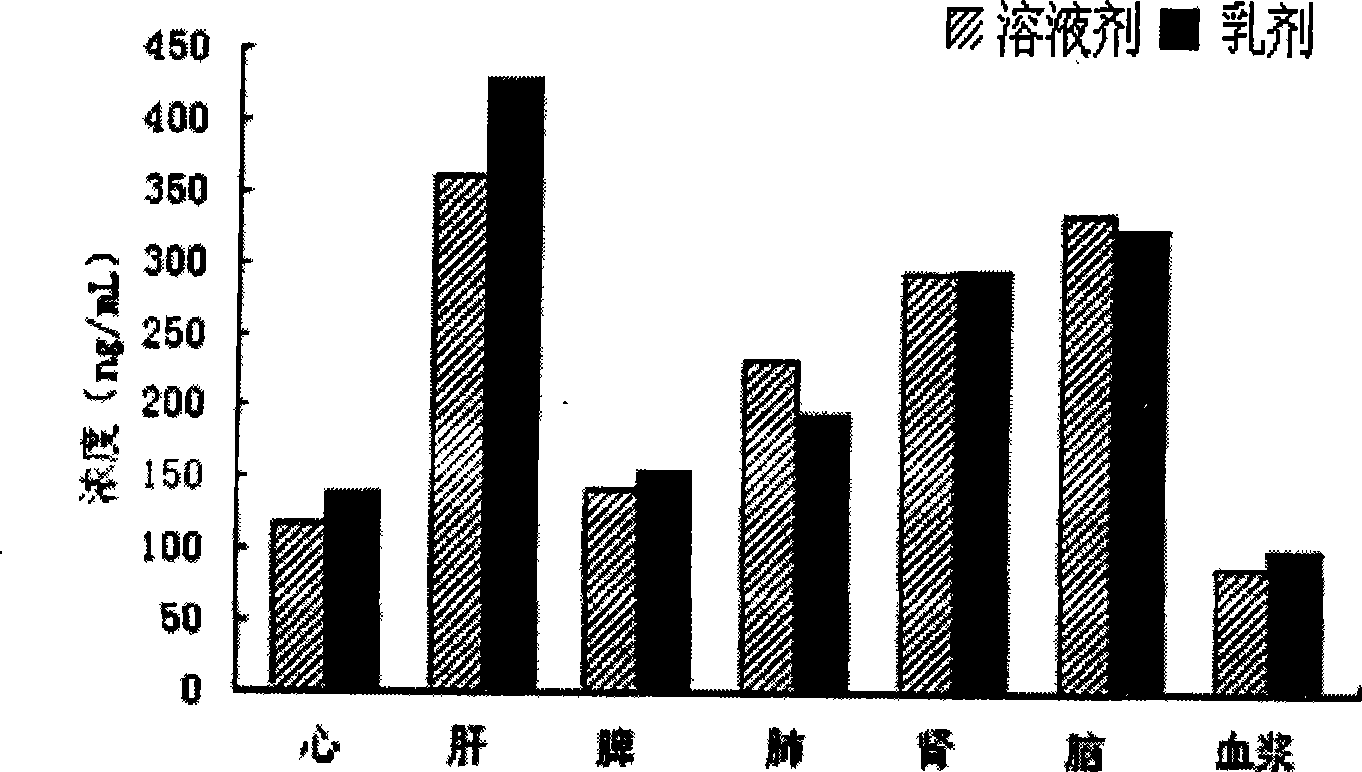
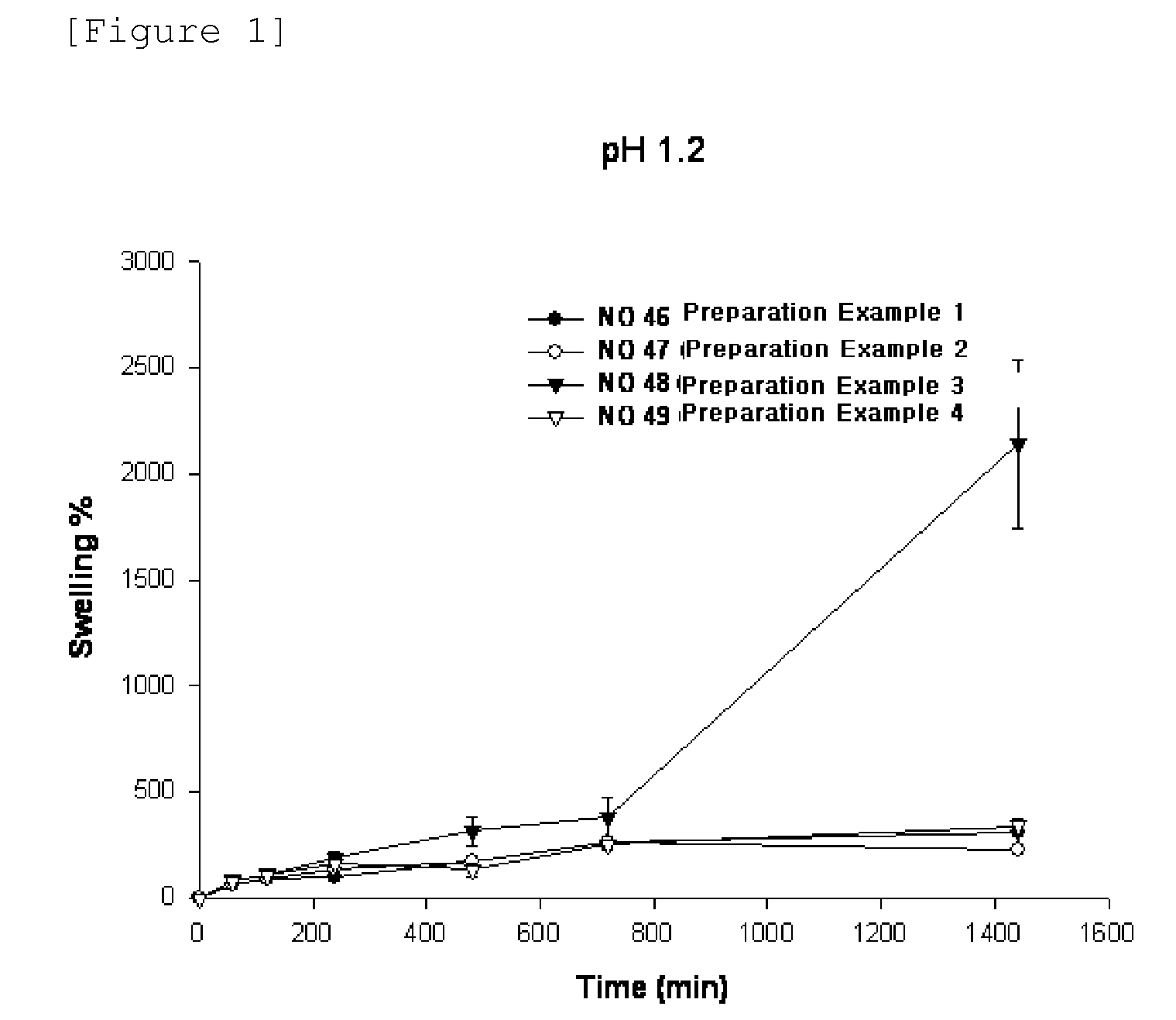
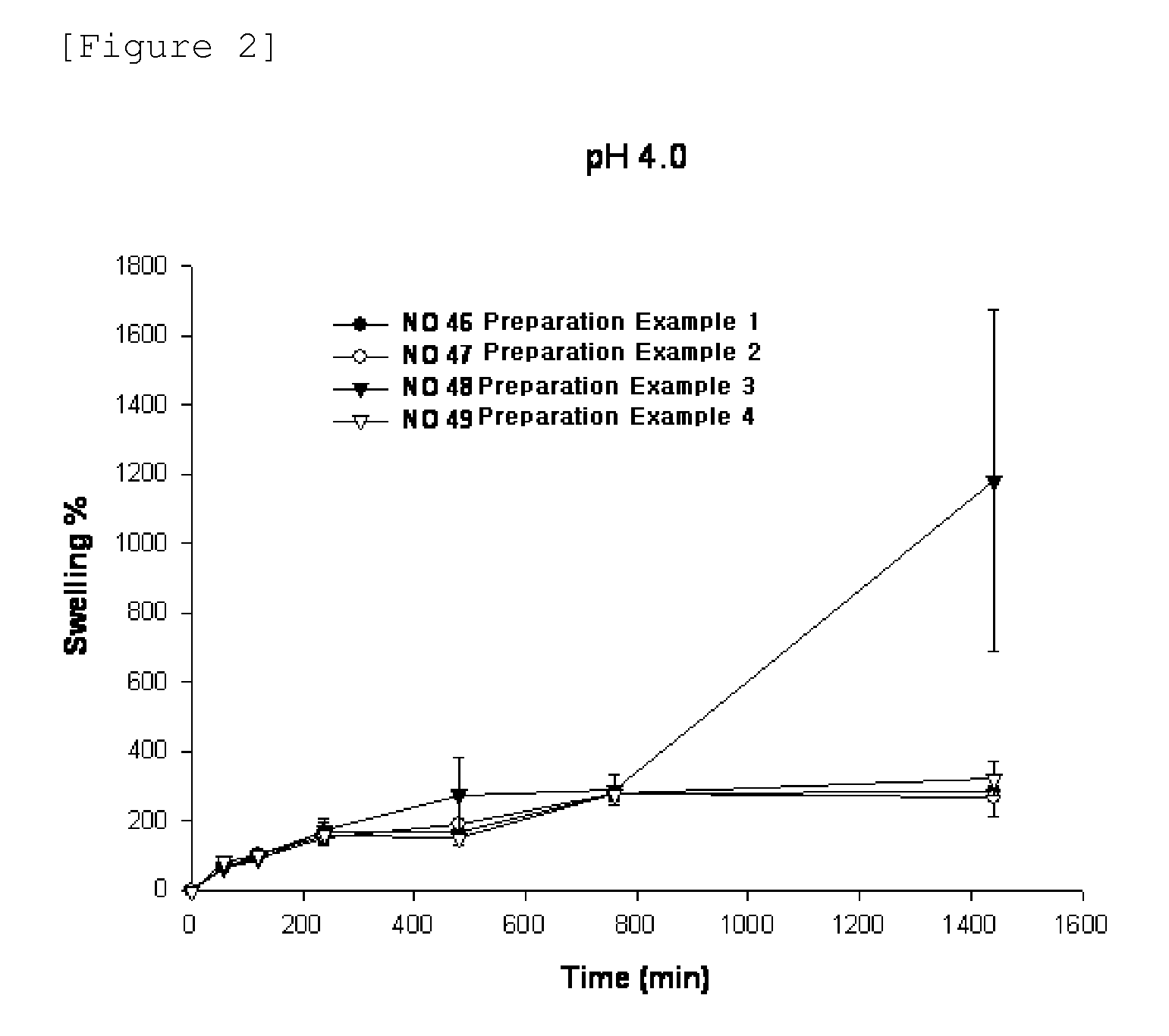
Patents
Literature
1295 results about "Blood concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In medicine, glucose concentration in the blood is more often referred to as the blood sugar level. In a healthy adult, the blood sugar level is usually expected to range from 3.6 mmol/l to 5.8 mmol/l. The glucose concentration in blood does tend to rise after a meal has been ingested, however.
Method of producing a sustained-release preparation
InactiveUS6197350B1Reduce the number of stepsSuitable for industrializationPowder deliveryPeptide/protein ingredientsBlood concentrationOrganic solvent
A method of producing sustained-release microcapsules which comprises dispersing a physiologically active polypeptide into a solution of a biodegradable polymer and zinc oxide in an organic solvent, followed by removing the organic solvent; which provides a sustained-release preparation showing a high entrapment ratio of the physiologically active polypeptide and its constant high blood concentration levels over a long period of time.
Owner:TAKEDA PHARMA CO LTD
Pharmaceutical composition comprising cyclosporin solid-state microemulsion
InactiveUS6306434B1Easy to controlMaintaining blood concentrationPowder deliveryCyclic peptide ingredientsIntestinal fluidBlood concentration
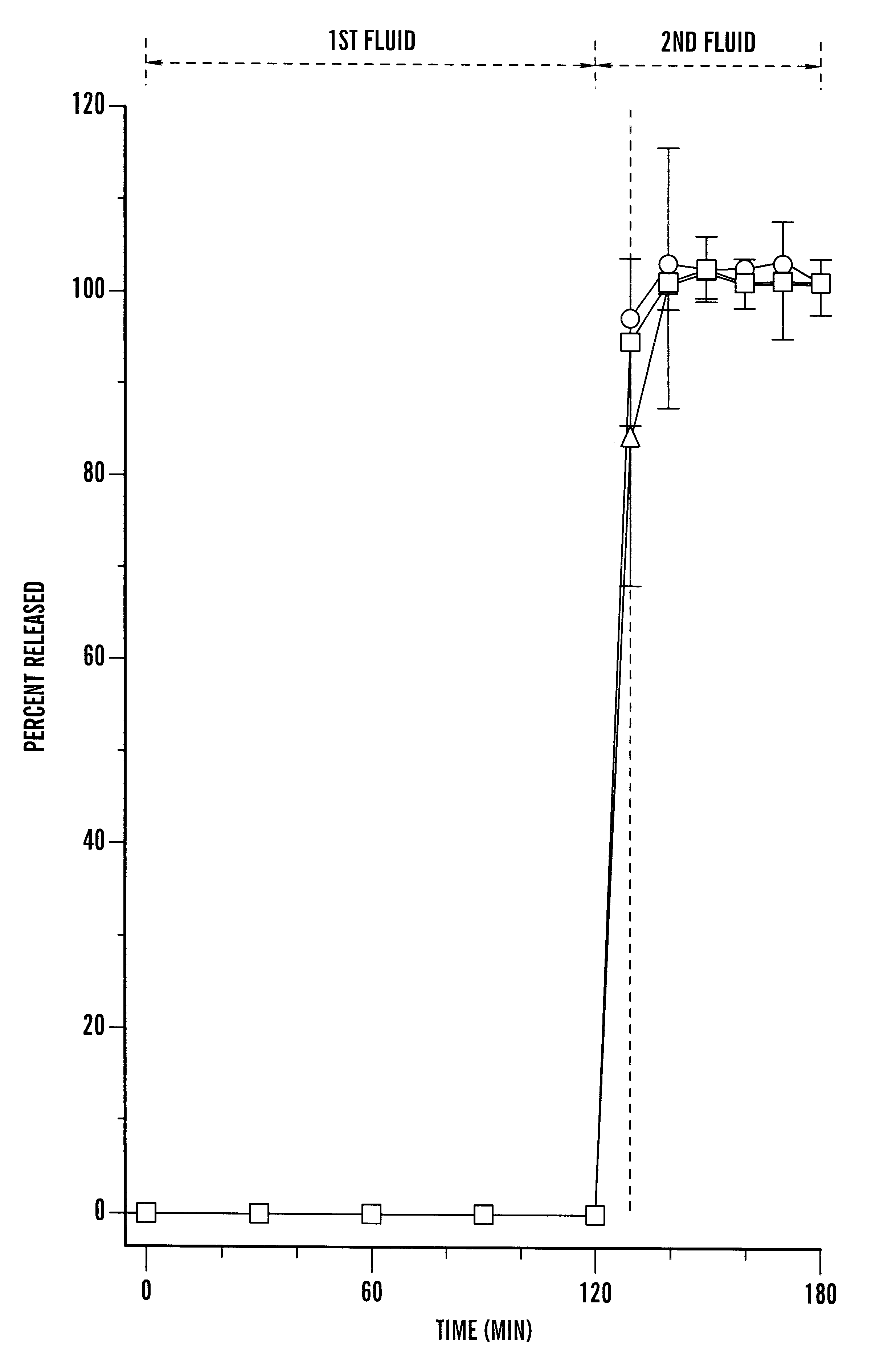
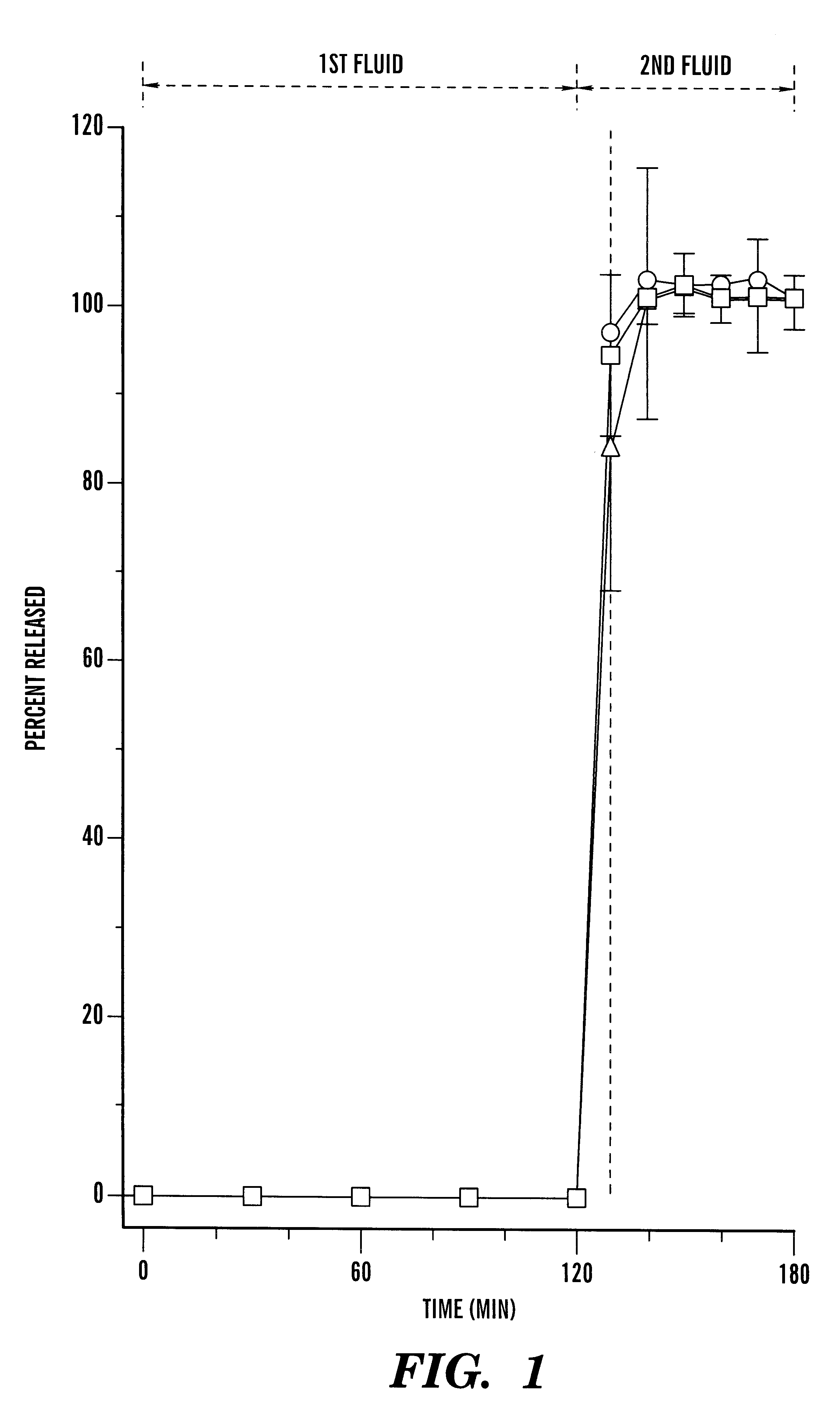
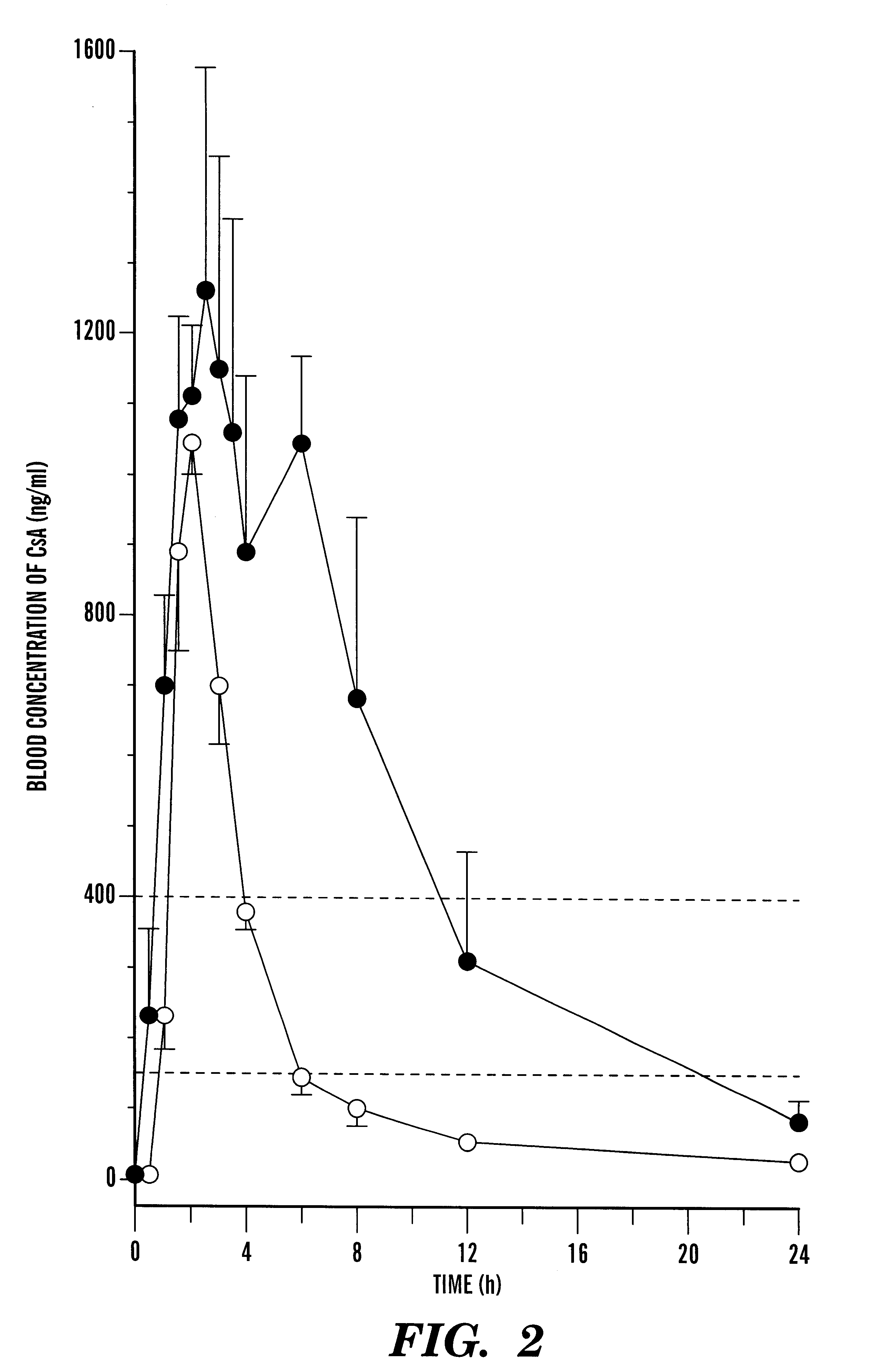
A pharmaceutical composition comprising a cyclosporin solid-state microemulsion is disclosed. In a preferred embodiment, the composition comprises a cyclosporin microemulsion dispersed in an enteric carrier. The composition does not dissolve in external phases such as artificial gastric fluid, but dissolves rapidly in artificial intestinal fluid, whereby it releases the cyclosporin microemulsion, providing rapid delivery of cyclosporin. The composition effectively maintains a therapeutic blood concentration of cyclosporin with once a day dosing, providing for convenience of administration and avoiding adverse effects induced by increasing peak blood cyclosporin concentrations associated with conventional cyclosporin formulations.
Owner:CHONG KUN DANG PHARMA CORP
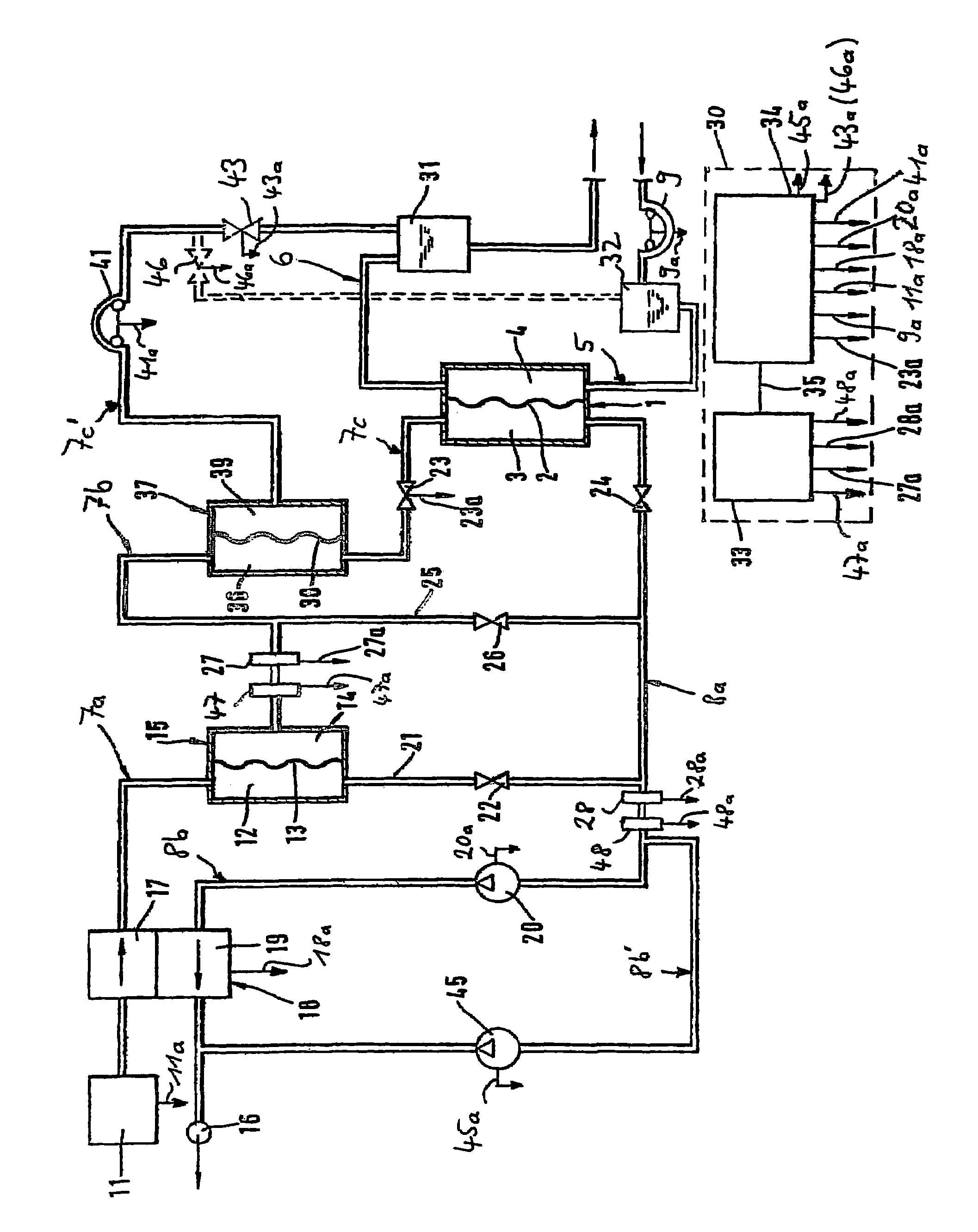
Haemodialysis device
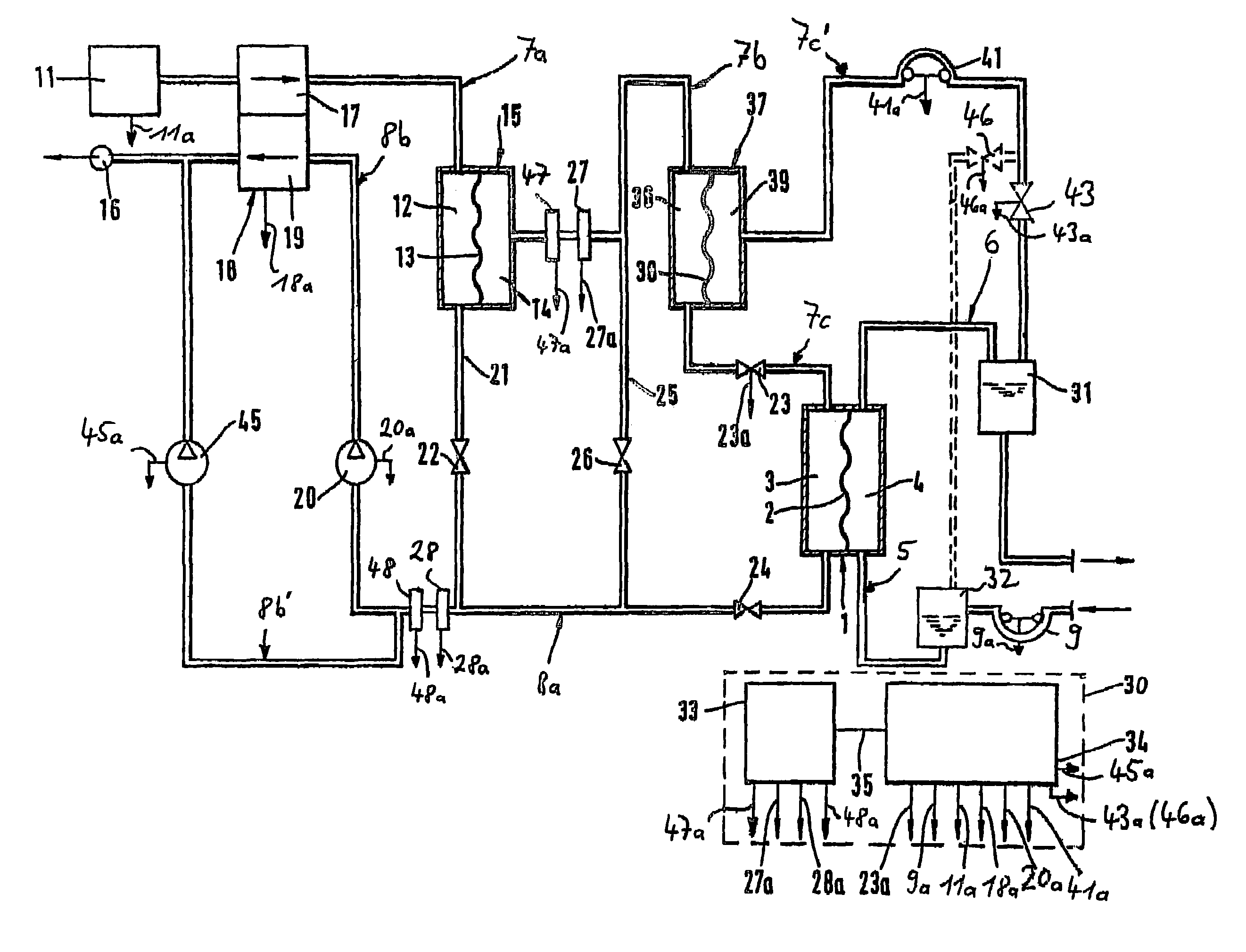
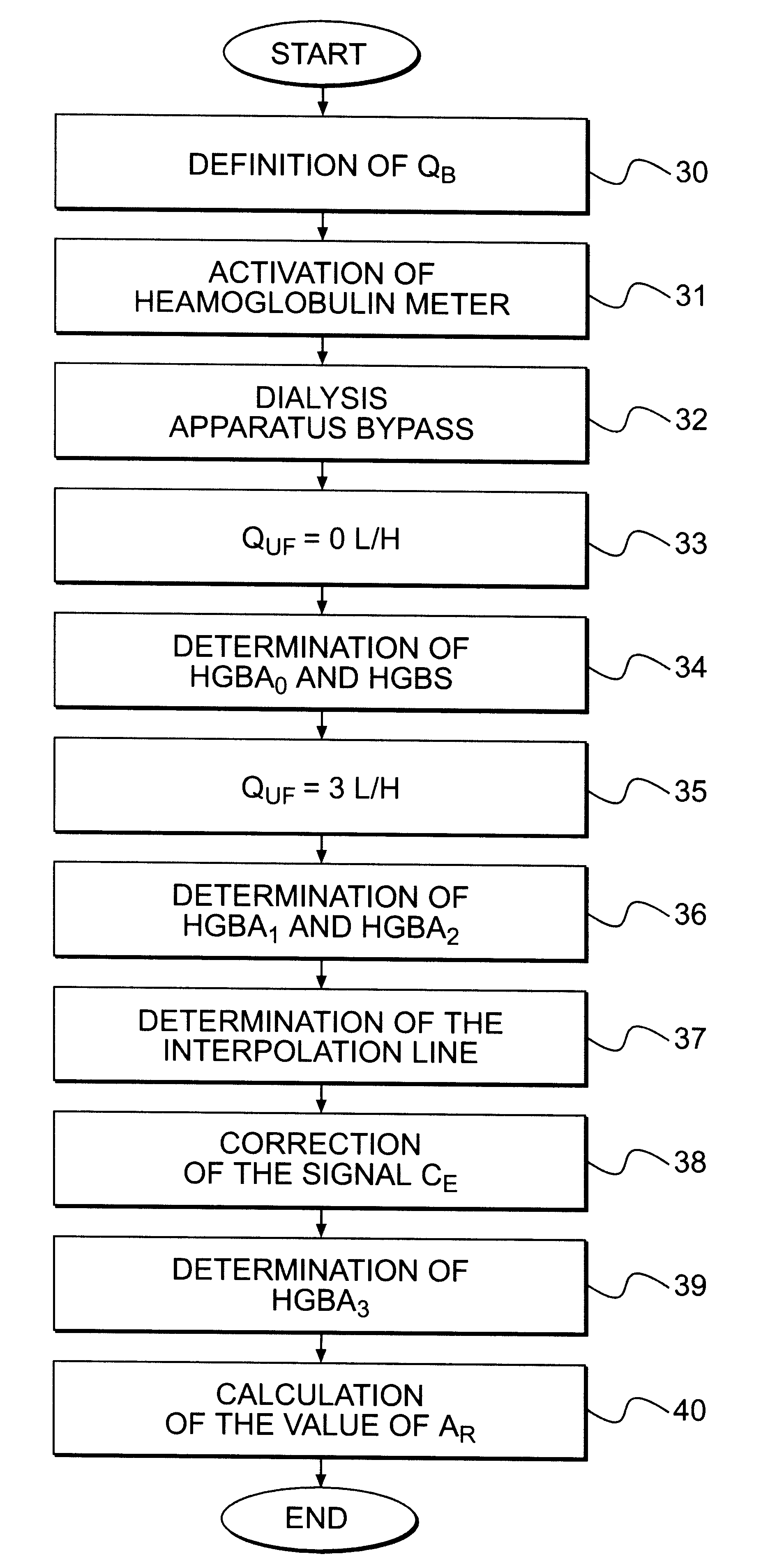
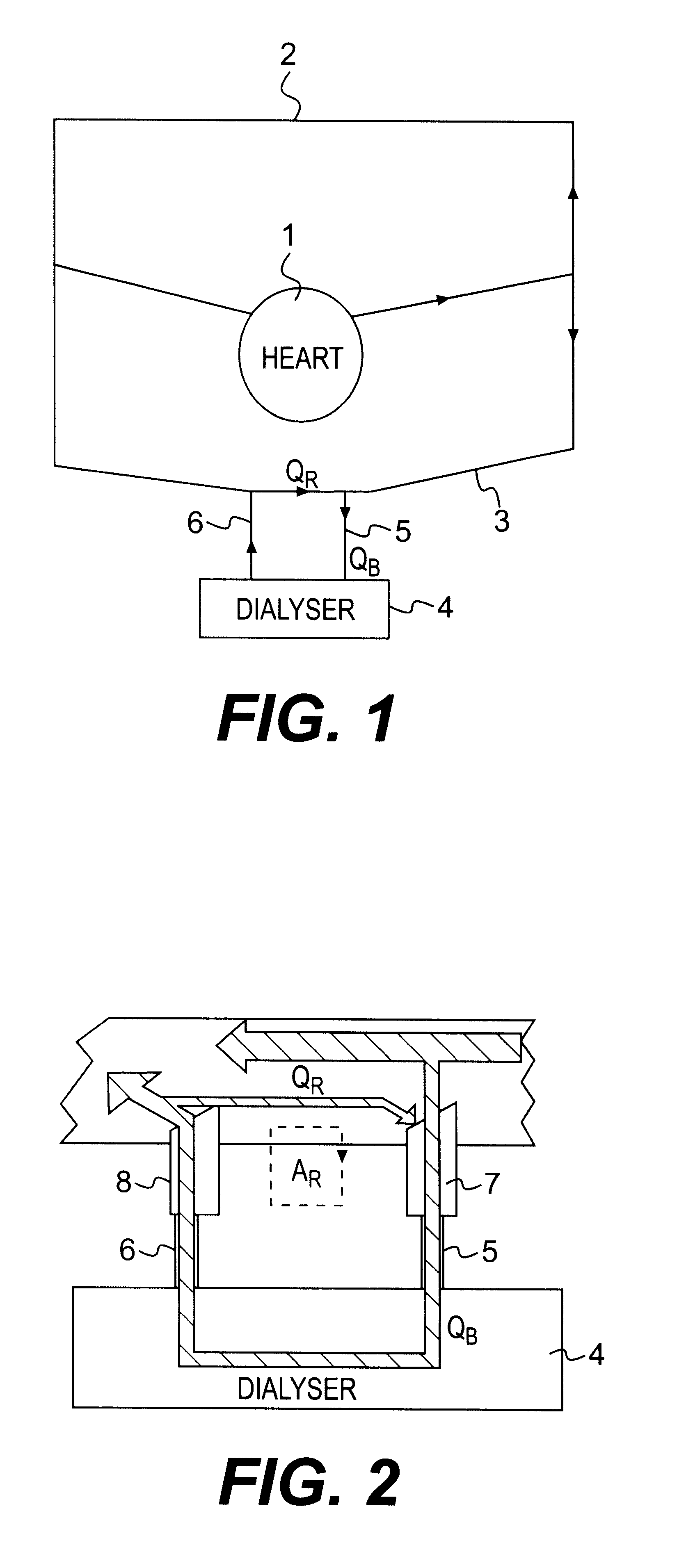
A blood purification device has a blood purification element divided into two chambers by a semipermeable membrane, with a first chamber as part of a dialysis fluid loop and a second chamber as part of an extracorporeal blood loop. By virtue of an analysis unit operatively connected to a sensor in the dialysis fluid loop, the device provides simple and uncomplicated determination of the blood purification performance of the blood purification element for a second material by derivation from a previously established blood purification performance for a first material. In this way, the device also determines the blood concentration of the second material during the blood treatment through measurements in the dialysis fluid without intervention in the treatment sequence.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
Pharmaceutical composition for controlled drug delivery system
The present invention describes a novel controlled release multilayer composition that is capable of delivering a first active agent from one layer immediately followed by continuous controlled delivery of second active agent from matrix forming layer while the dosage form floats and is retained in the fluid of the environment. The floating bilayer system comprises of immediate release layer containing one active agent and a disintegrating agent whereas second floating matrix forming layer comprises a gas generating component, a gelling agent, and a second active agent. The present invention relates more particularly to a controlled release fluoroquinolone compositions, which maintain a therapeutically effective blood concentration of fluoroquinolone for duration with once a day administration.
Owner:J B CHEM & PHARMA
Enabling drug adherence through closed loop monitoring & communication
InactiveUS20070172424A1Low costAddressing slow performanceMedical simulationData processing applicationsBlood concentrationDrug adherence
A method is described for measuring the blood concentration of a medicament through the introduction of a tracer compound. The measurement of the blood concentration of the tracer will yield a result that will enable a prediction of the blood concentration of the medicament. The method further describes ways to utilize the results for monitoring adherence to the medicament and modifying behavior to help patients boost compliance.
Owner:ROSER MARK COSTIN
Levo-oxiracetam slow-release tablet and preparation method thereof
ActiveCN103599083AReduce adverse reactionsImprove securityOrganic active ingredientsNervous disorderInjury brainBlood concentration
The invention relates to a levo-oxiracetam slow-release tablet mainly prepared from levo-oxiracetam, which is prepared from the following raw and auxiliary materials in parts by weight: 0.8-1.2 parts of slow-release framework material, 0.06-0.12 part of flow aid, 0.02-0.05 part of lubricant, 0.02-0.05 part of antisticking agent and 1-1.5 parts of adhesive. The levo-oxiracetam slow-release tablet is used for treating brain injury, and neural afunction and disturbance of memory and intelligence caused by brain injury, and has a smooth surface; the detection proves that the release behavior of the main drug levo-oxiracetam satisfies the requirements for the slow-release tablet; and the main drug levo-oxiracetam is slowly released, so the levo-oxiracetam slow-release tablet can be taken less frequently than the traditional preparation. The main drug levo-oxiracetam in the levo-oxiracetam slow-release tablet is slowly released, and can provide steady and enduring effective blood concentration, thereby avoiding or reducing the phenomena of peaks and troughs of blood concentration, and being beneficial to enhancing the medicine application safety and reducing the untoward reaction of the medicine.
Owner:CHONGQING RUNZE PHARM CO LTD
Blood purification device
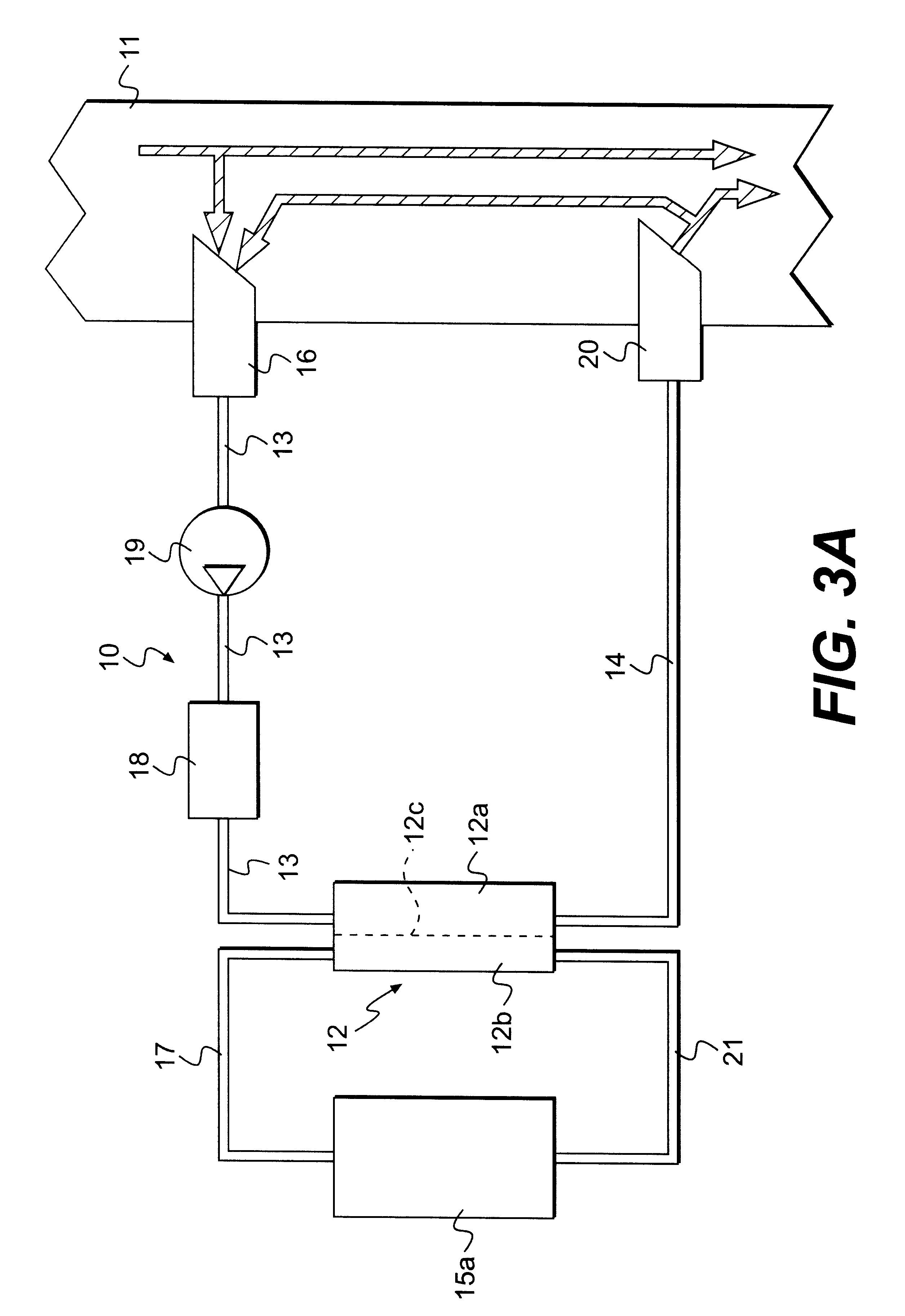
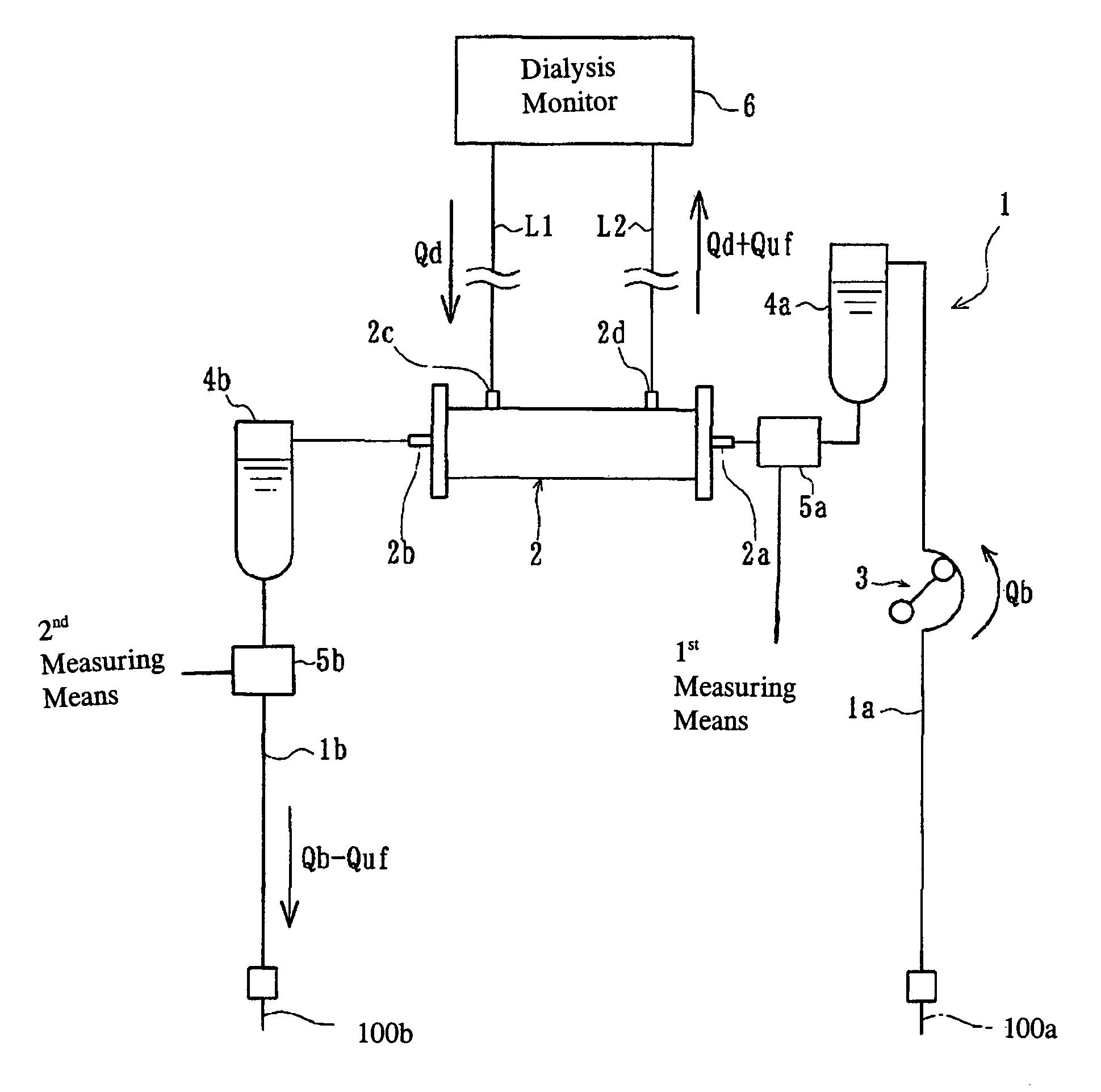
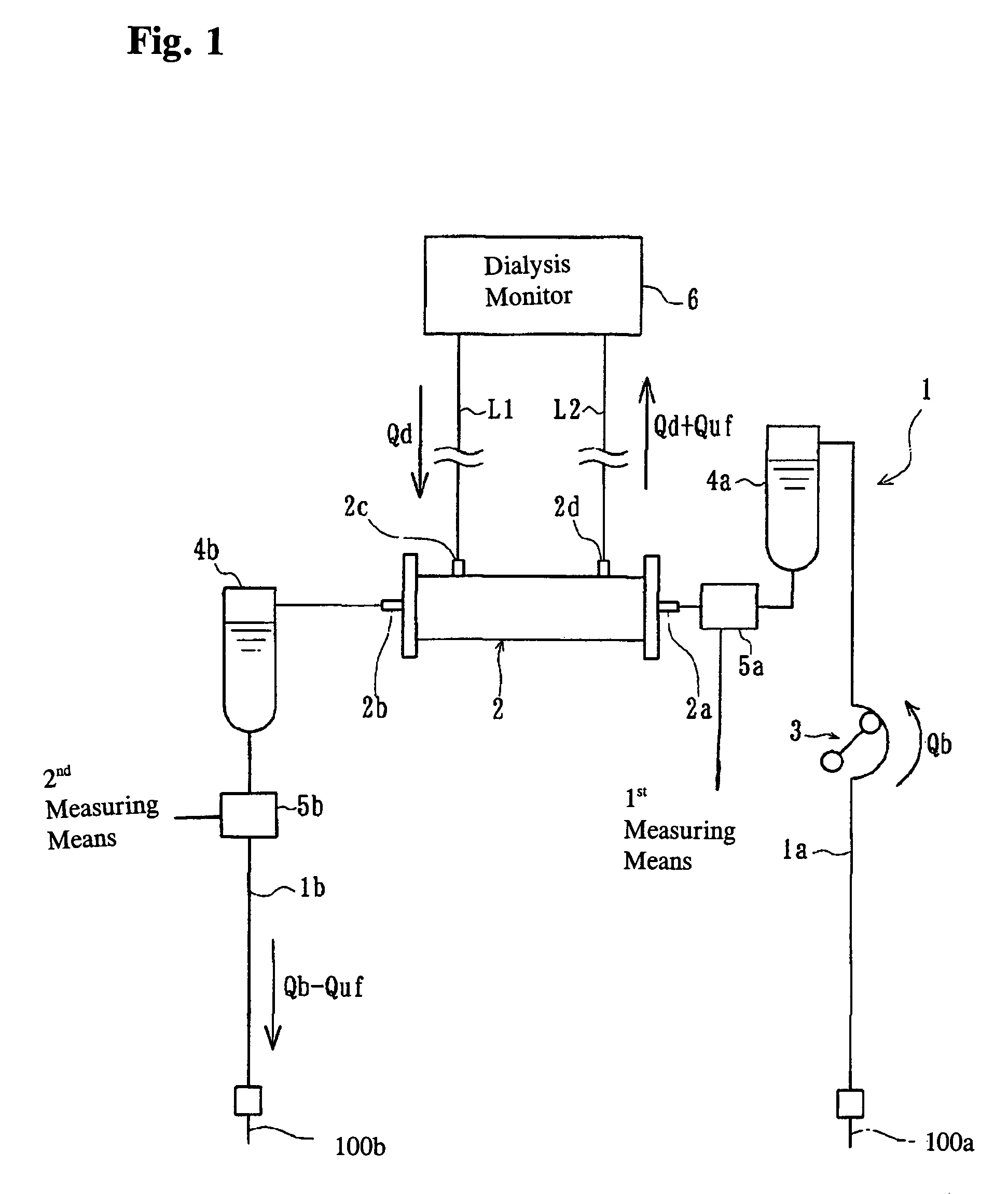
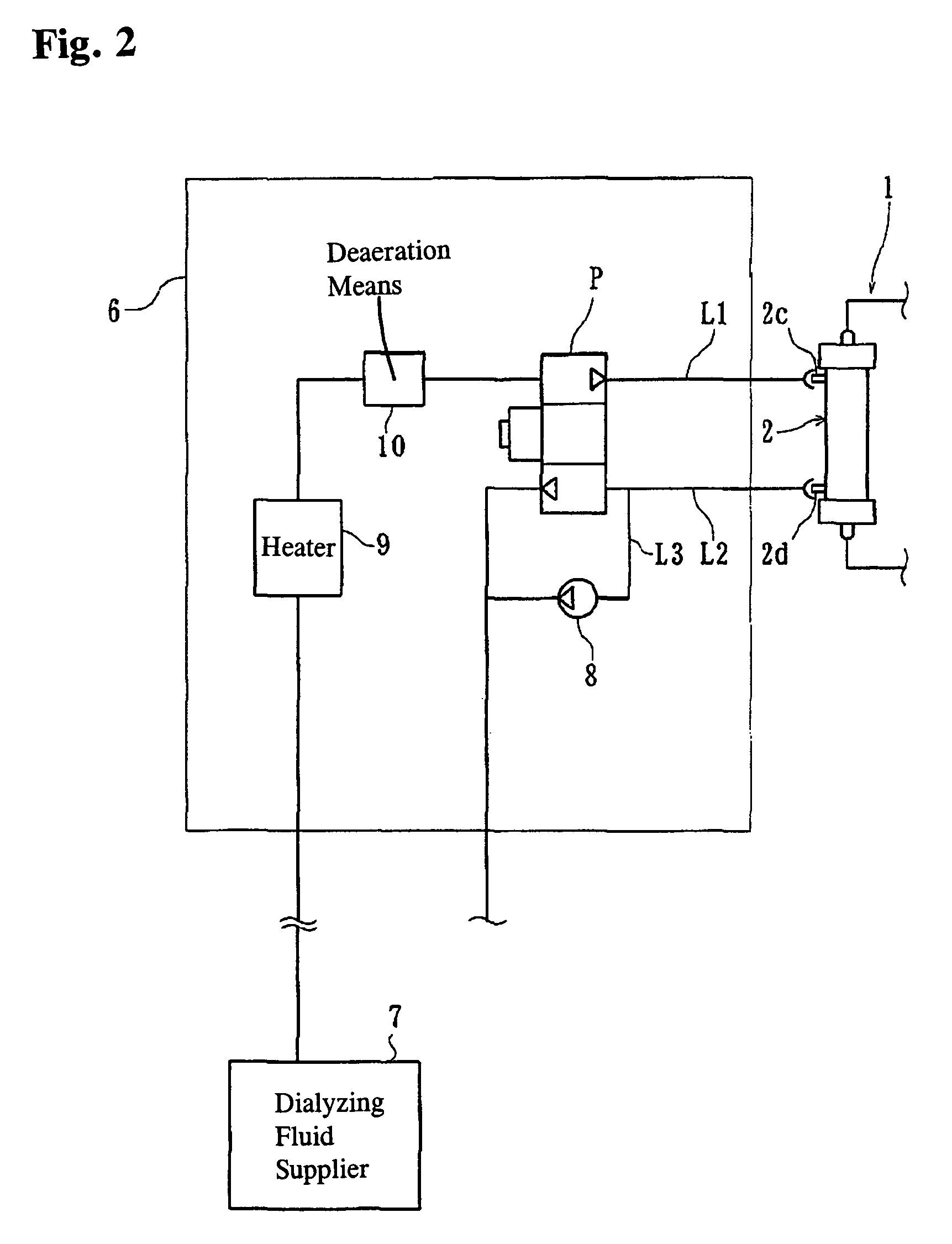
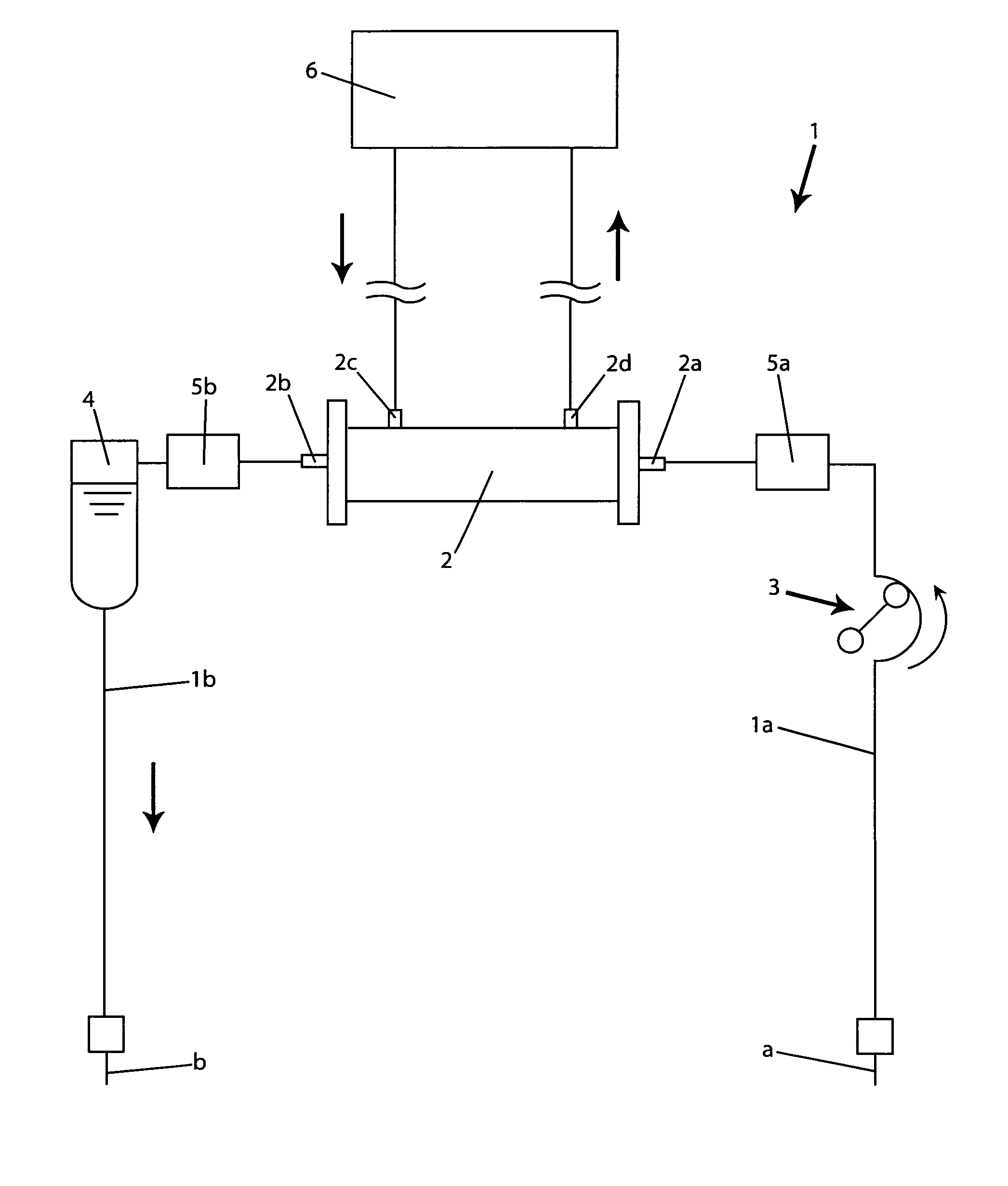
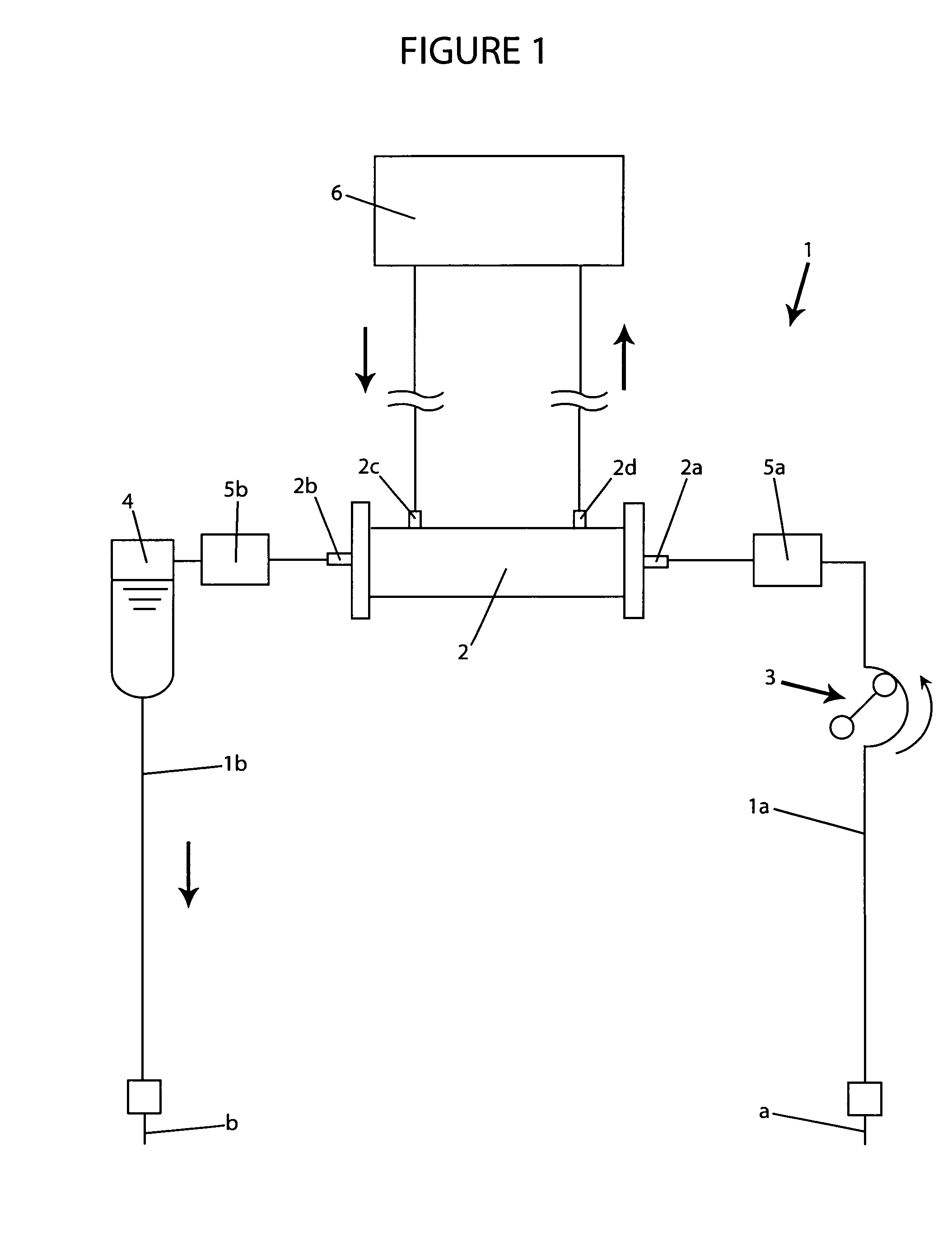
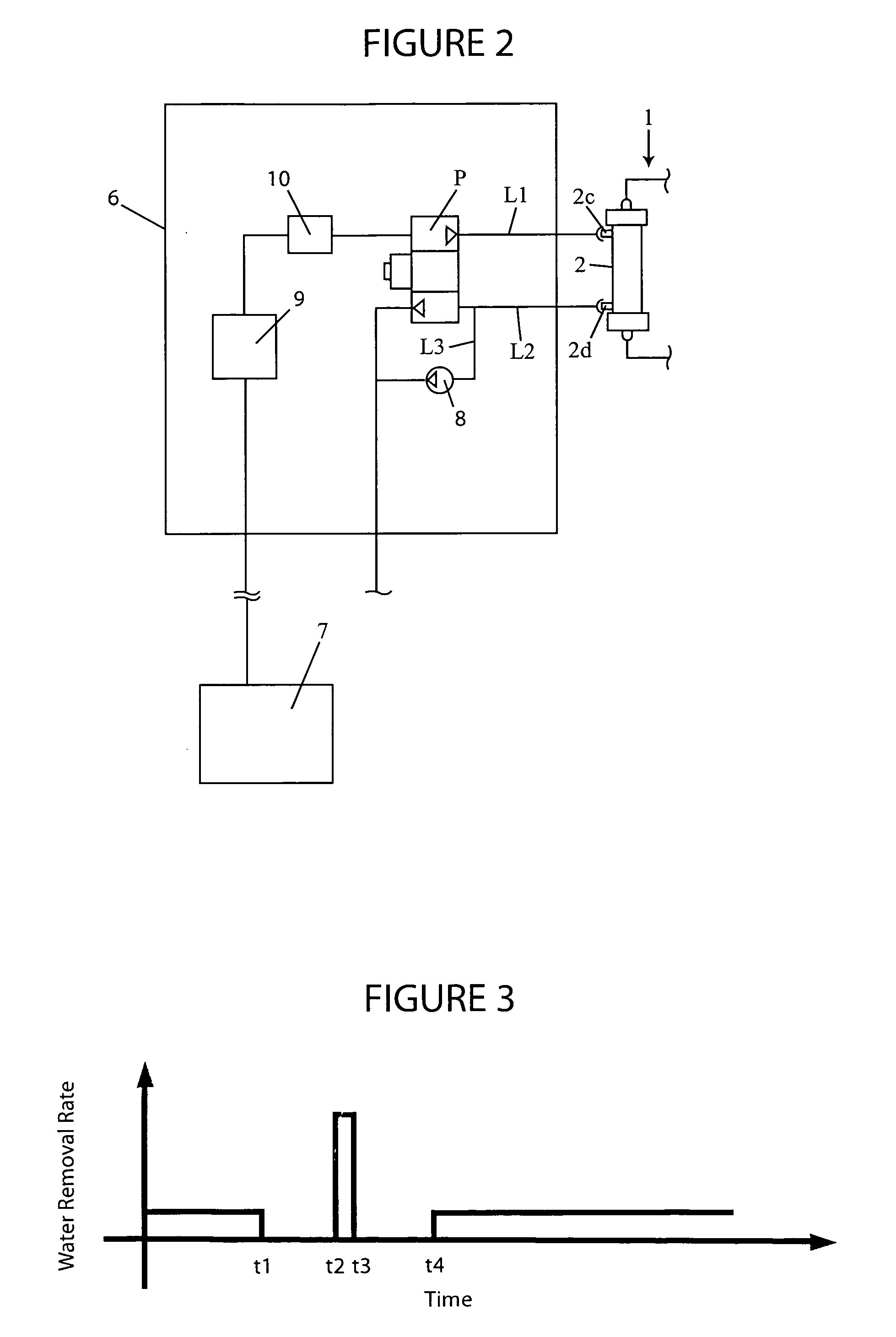
ActiveUS7537688B2Reduce parameterAccurate detectionHaemofiltrationDialysis systemsBlood concentrationUltrafiltration
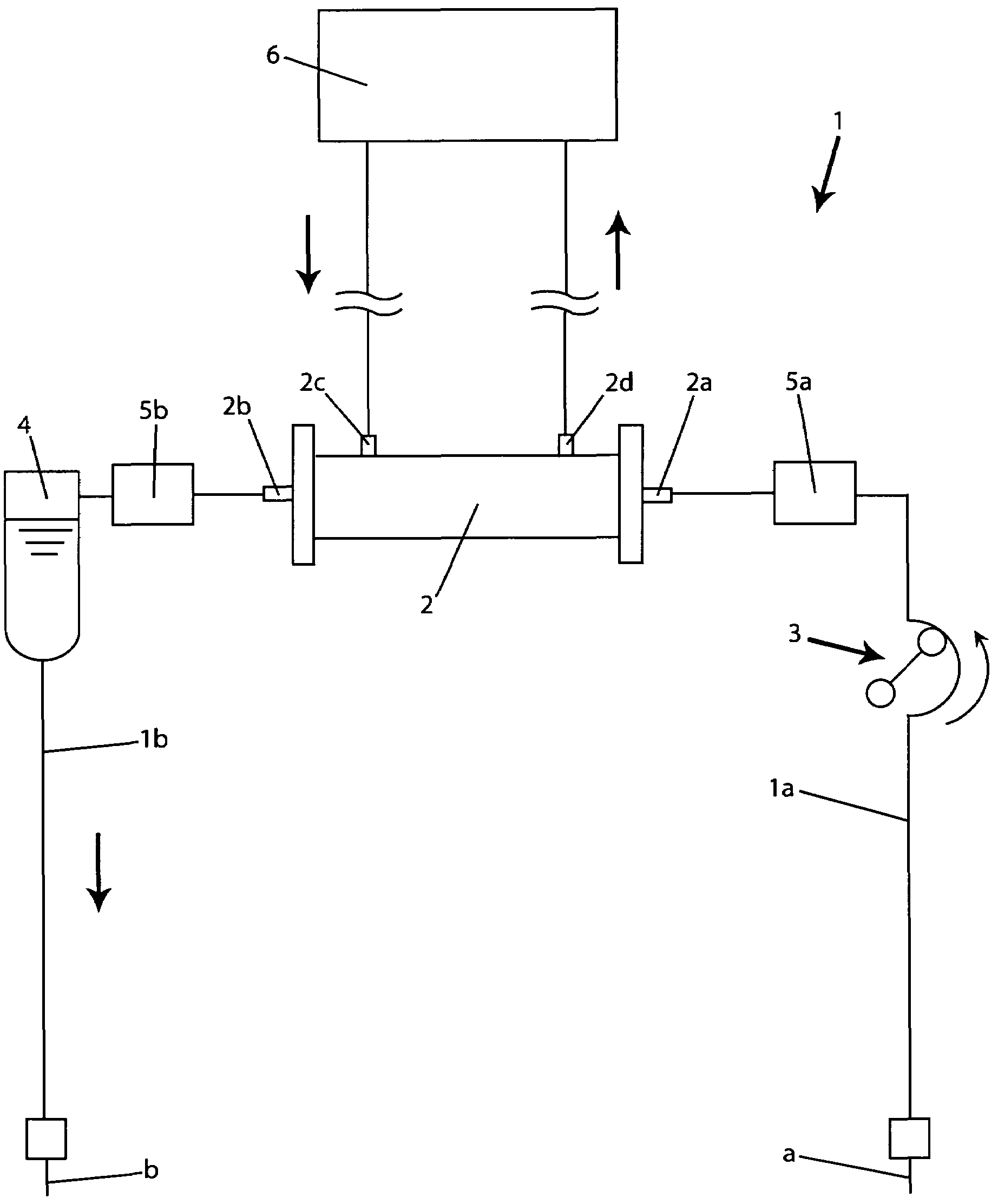
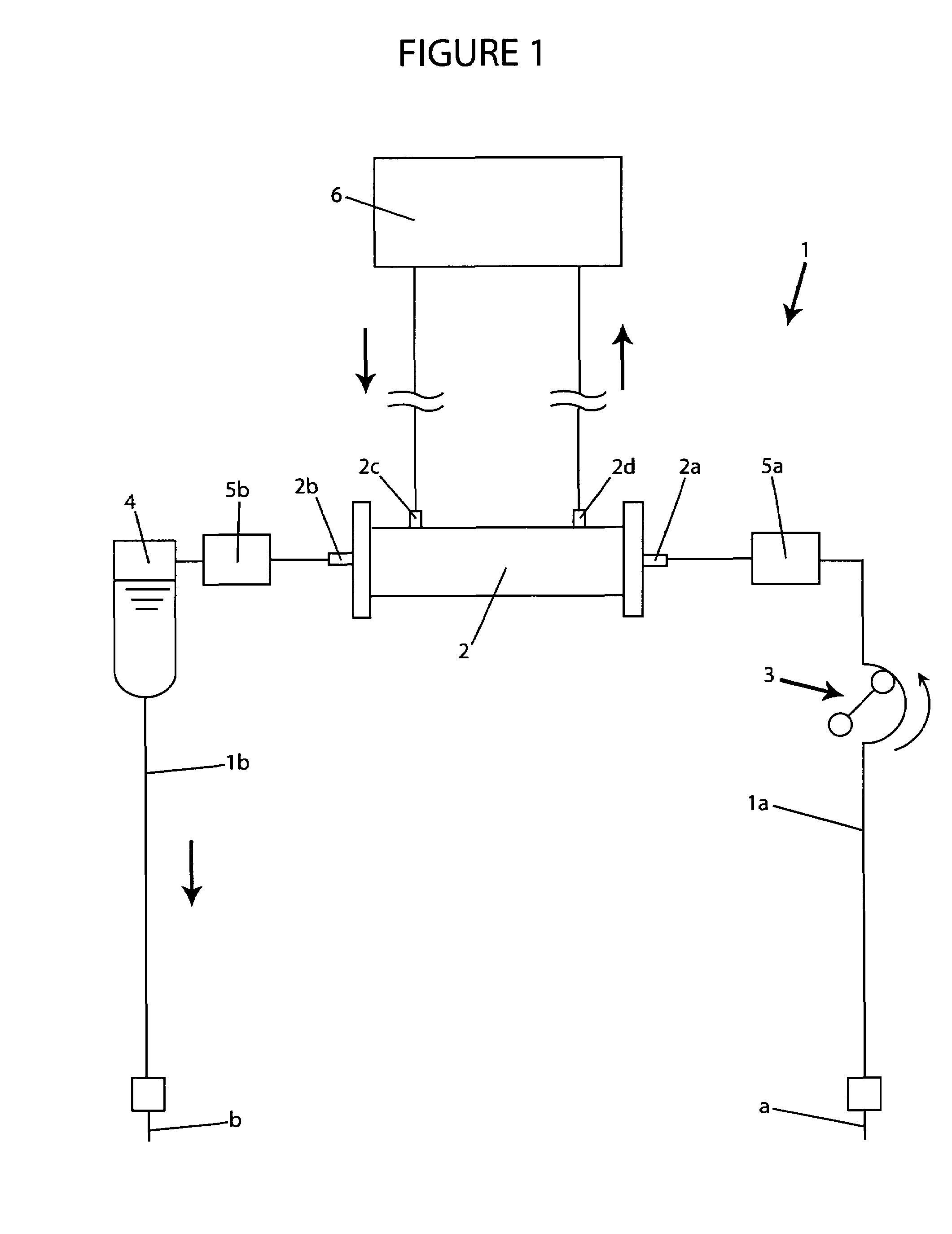
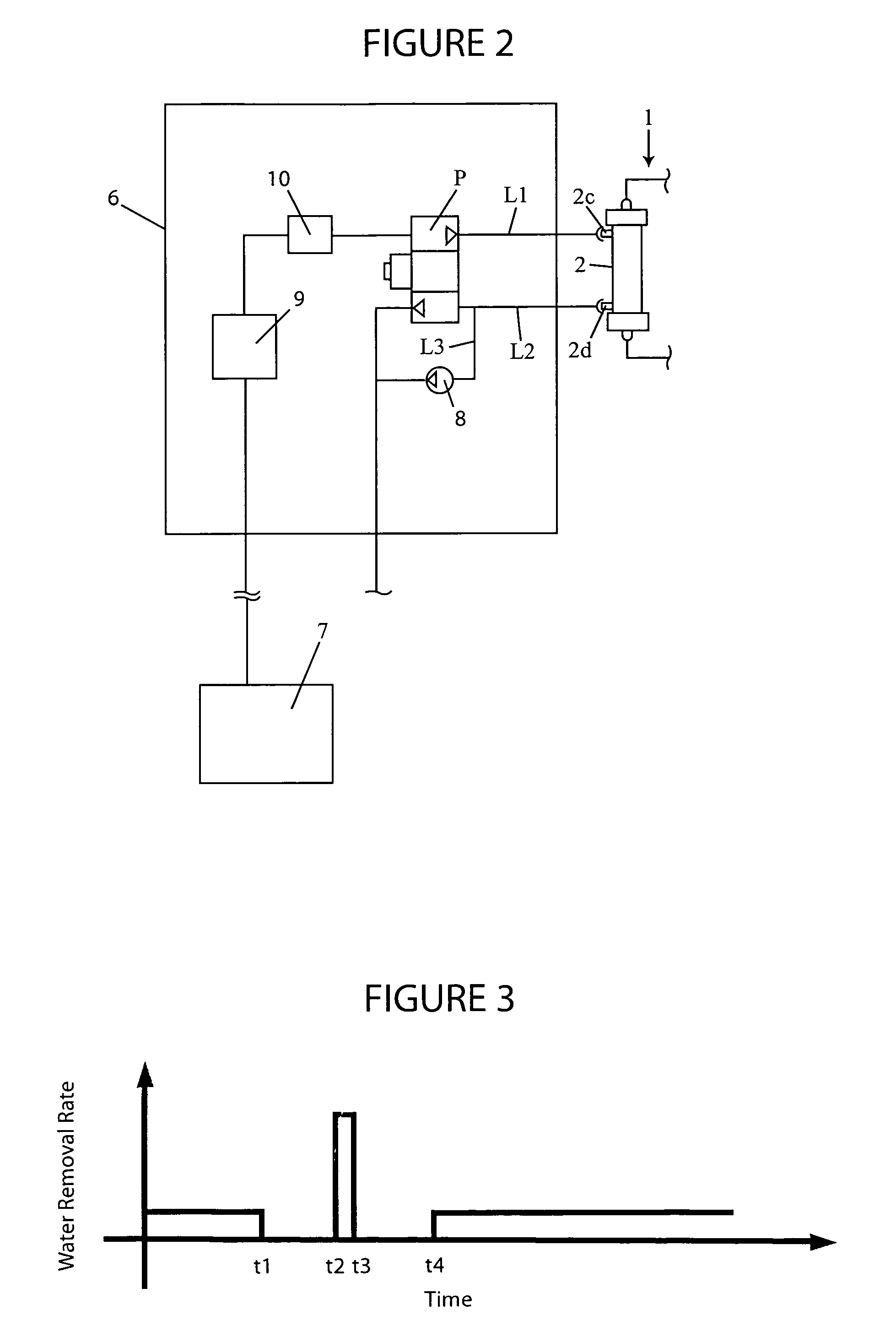
A blood purification device confirms whether a specific peak is provided by e.g. an ultrafiltration pump, to concentrate the blood or not and also accurately measures a blood re-circulation with a minimum of parameters providing a ratio of re-circulating blood. The blood purification device composed of arterial blood circuit route 1a and venous blood circuit route 1b, blood pump 3, dialyzer 2, water removal pump 8 providing the specific peak in the variation of blood concentration by removing water rapidly, and a detector detecting the specific peak, can measure the blood re-circulation thereby. The re-circulating blood flowing is the blood which was introduced again to arterial blood circuit route 1a after it had been returned to a patient from venous blood circuit route 1b. A first detector 5a installed in the arterial blood circuit route 1a and a second detector 5b installed in venous blood circuit route detector.
Owner:NIKKISO COMPANY
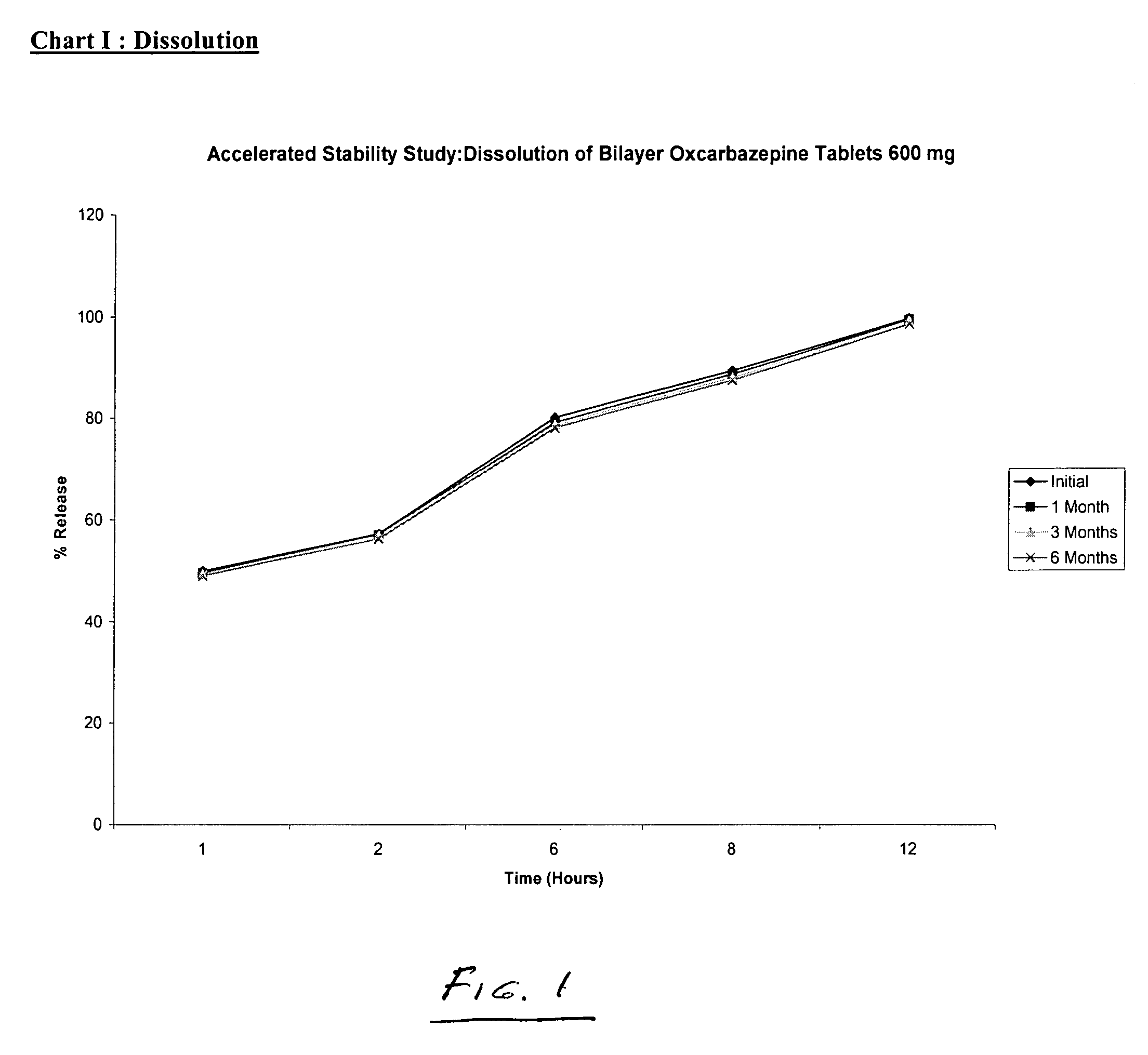
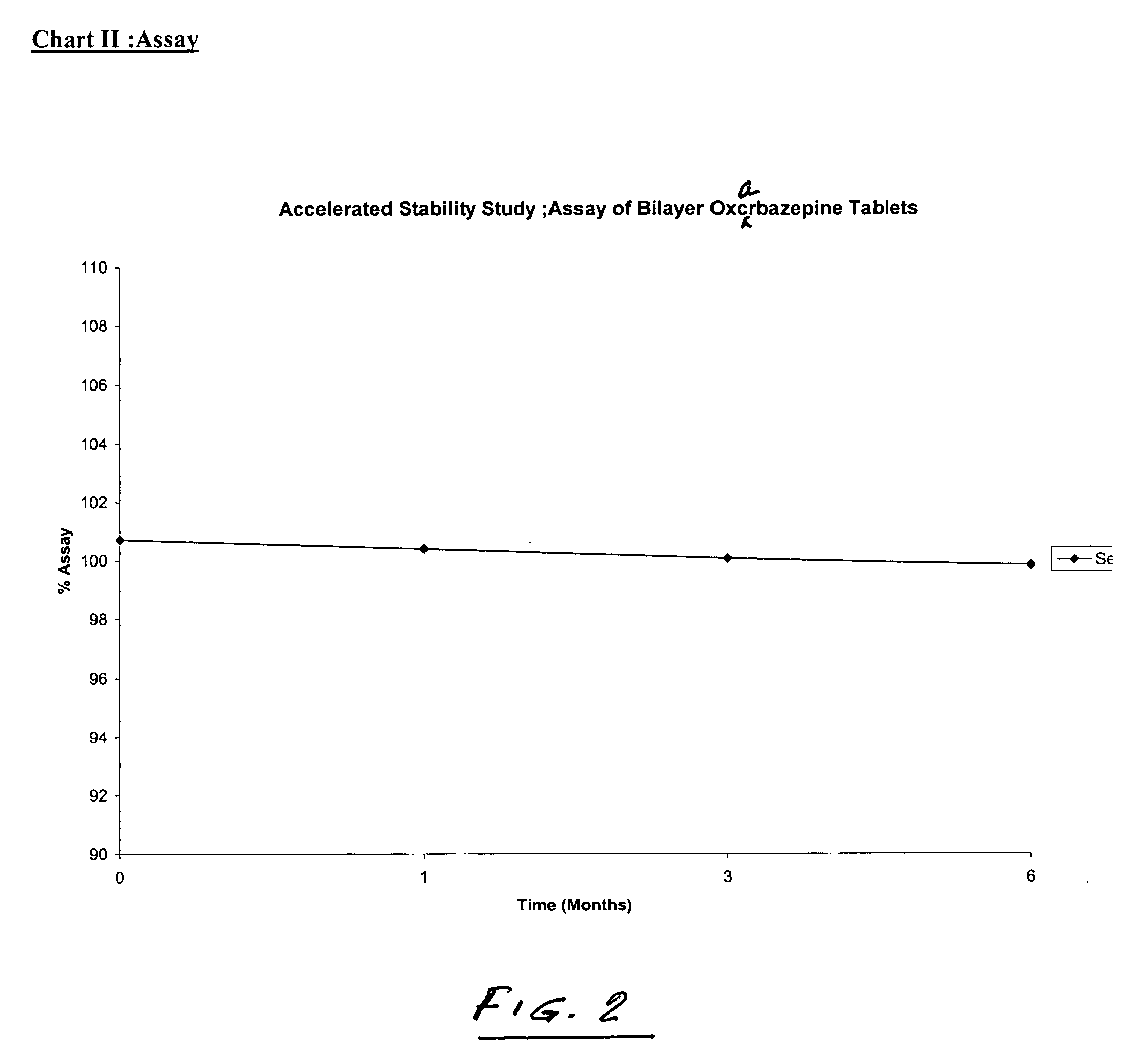
Bilayer tablets of oxcarbazepine for controlled delivery and a process of preparation thereof
InactiveUS20060141037A1Simple manufacturing processBiocidePill deliveryControlled releaseBlood concentration
Bilayer tablet comprising an immediate release first layer comprising an effective amount of oxcarbazepine and at least one pharmaceutically acceptable excipients and a controlled release second layer comprising an effective amount of oxcarbazepine and pharmaceutically acceptable excipients wherein the total amount of oxcarbazepine impurities is less than or equal to about 2% by weight. A process for preparation of controlled release bilayer tablets is capable of delivering oxcarbazepine from one layer immediately followed by a controlled delivery of oxcarbazepine from a matrix forming layer, and a process for preparation of oxcarbazepine bilayer tablets. Bilayer tablets of oxcarbazepine, which maintain a therapeutically effective blood concentration of oxcarbazepine with once a day administration.
Owner:J B CHEM & PHARMA
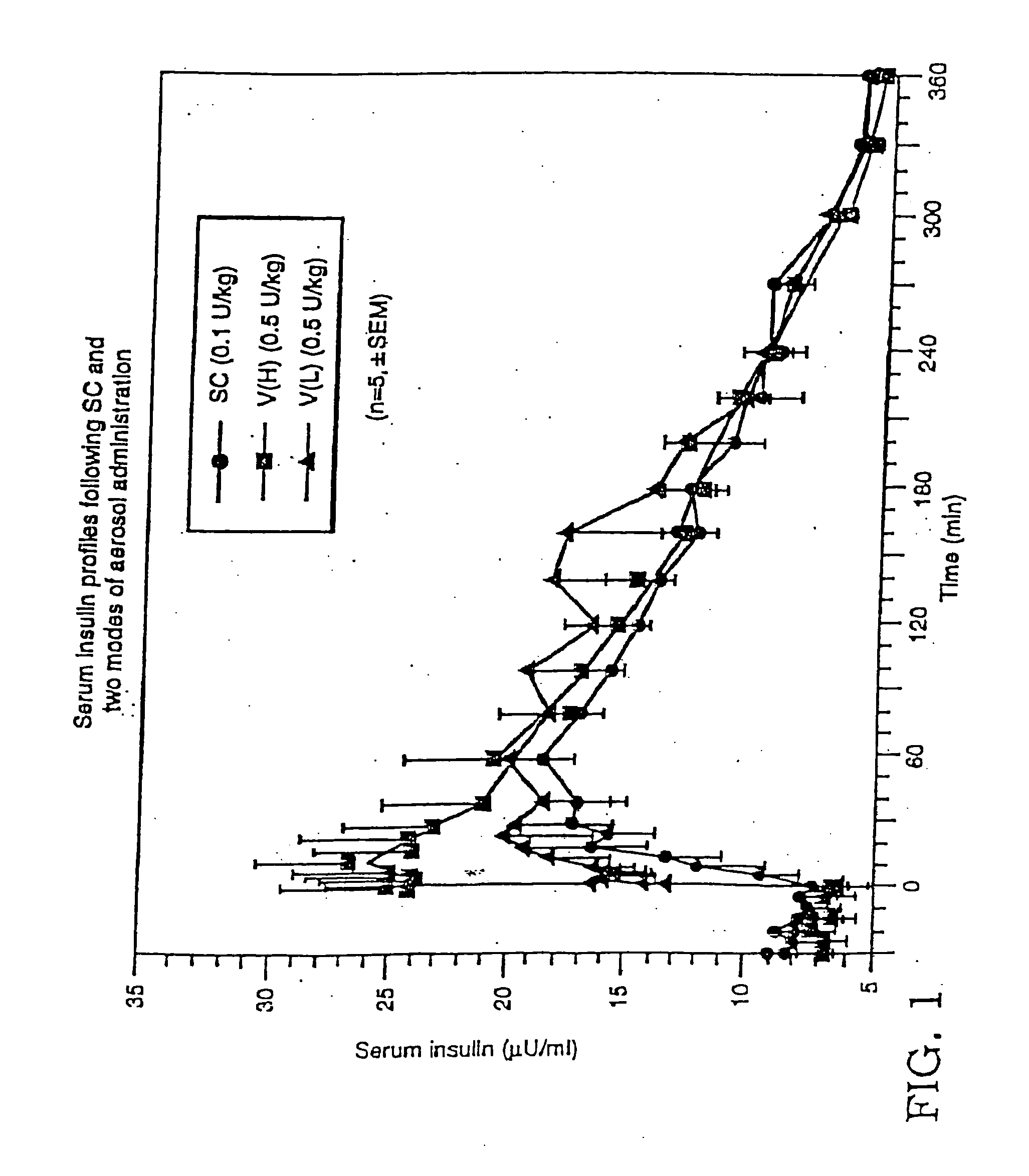
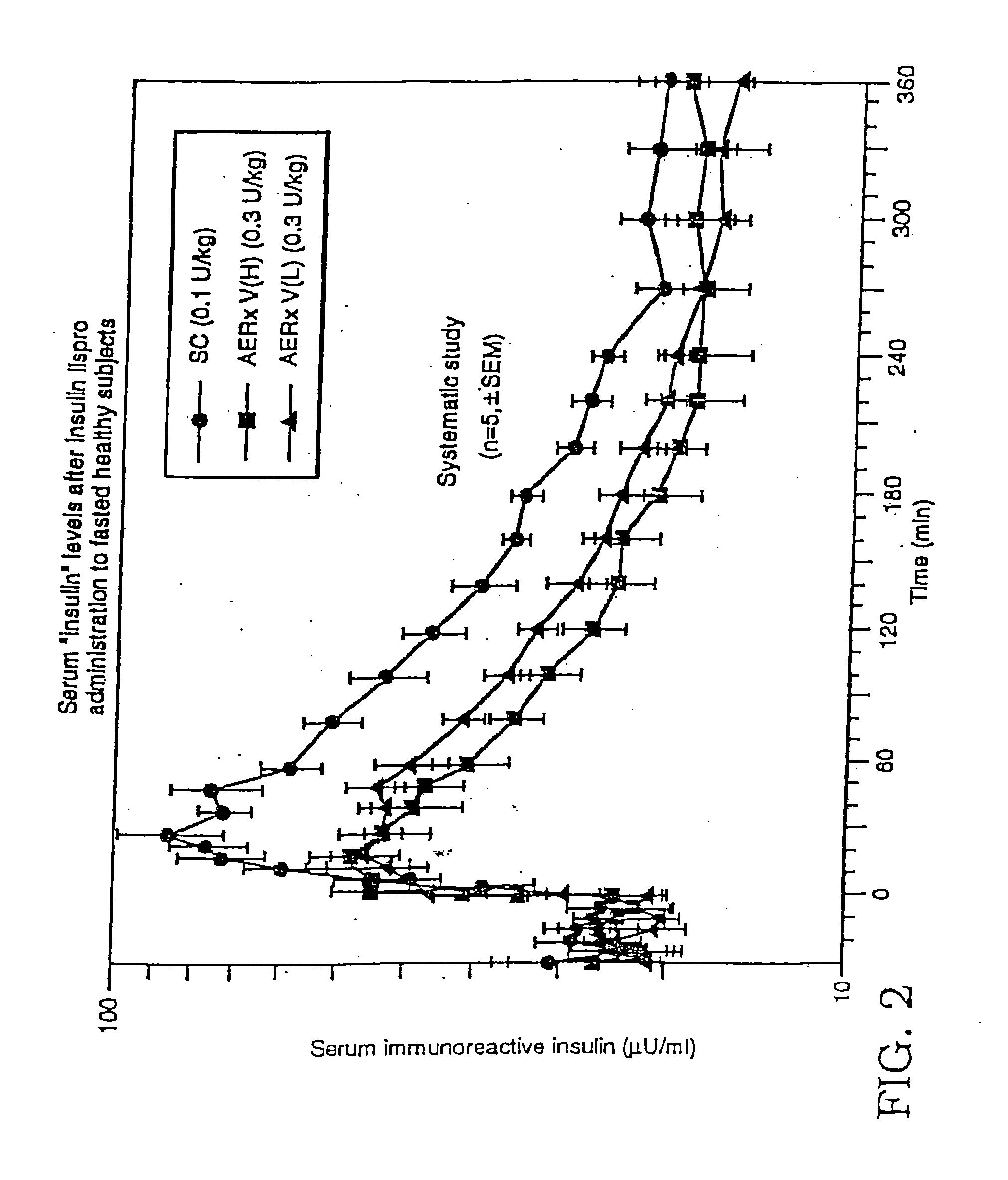
Method of use of monomeric insulin as a means for improving the reproducibility of inhaled insulin
The need for the delivery of insulin by injection can be reduced or eliminated by delivering an aerosolized monomeric insulin formulation. Repeatability of dosing and more particularly the repeatability of the blood concentration versus time profile is improved relative to regular insulin. The blood concentration versus time profile is substantially unaffected by specific aspects of the patient's breathing maneuver at delivery. Further, the rate at which blood glucose is lowered is increased by the use of monomeric insulin. Particles of insulin and in particular monomeric insulin delivered to the surface of lung tissue will be absorbed into the circulatory system. The monomeric insulin may be a dry powder but is preferably in a liquid formulation delivered to the patient from a hand-held, self-contained device which automatically releases an aerosolized burst of formulation. The device includes a sensor which is preferably electronic which measures inspiratory flow and volume which measurement can be used to control the point of drug release.
Owner:ARADIGM
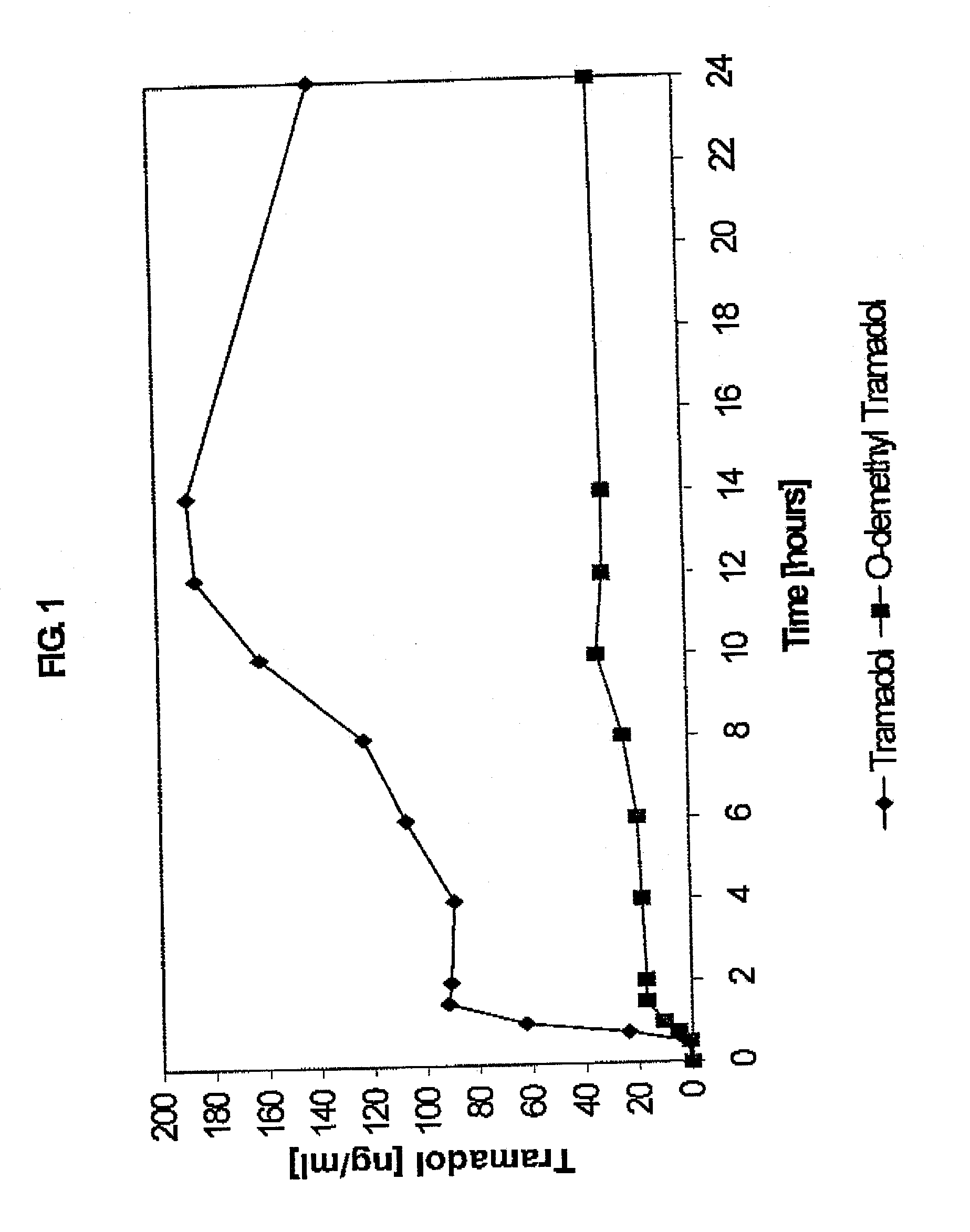
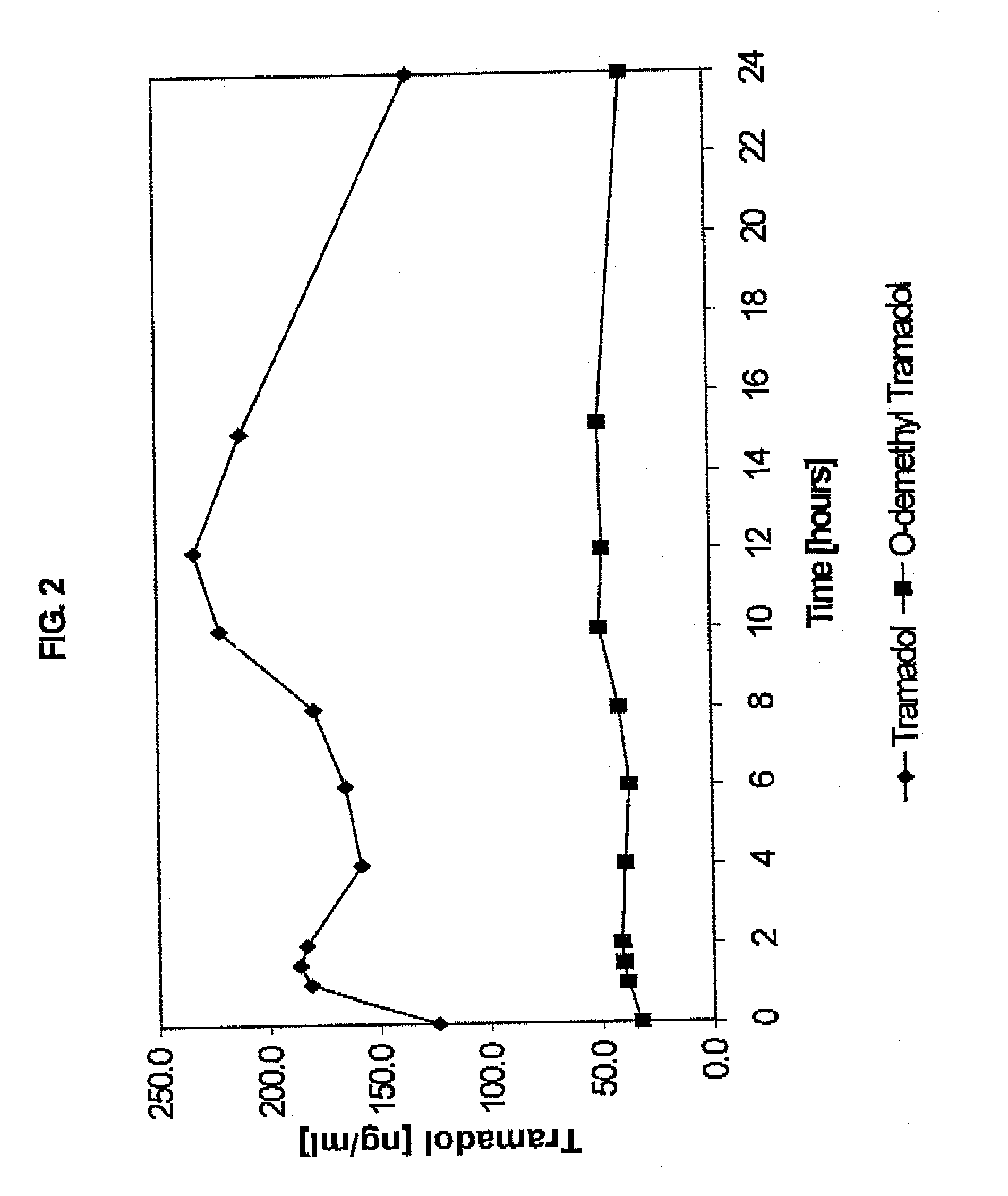
Extended release composition containing Tramadol
InactiveUS20030143270A1Effective controlRelieve painPowder deliveryBiocideBlood concentrationPeak concentration
The present invention relates to a once daily extended release pharmaceutical preparation of Tramadol or its acceptable pharmaceutical salts. The preparation provides, effective blood concentration for a period of about 24 hours with reduced peak concentrations. It is characterized that effective Tramadol levels appear within the first hours after administration, the time to maximal Tramadol content Tmax is at least 10 hours and the peak Tramadol concentration is less than three times the concentration obtained after 24 hours of said administration.
Owner:GALEPHAR PHARMA RES
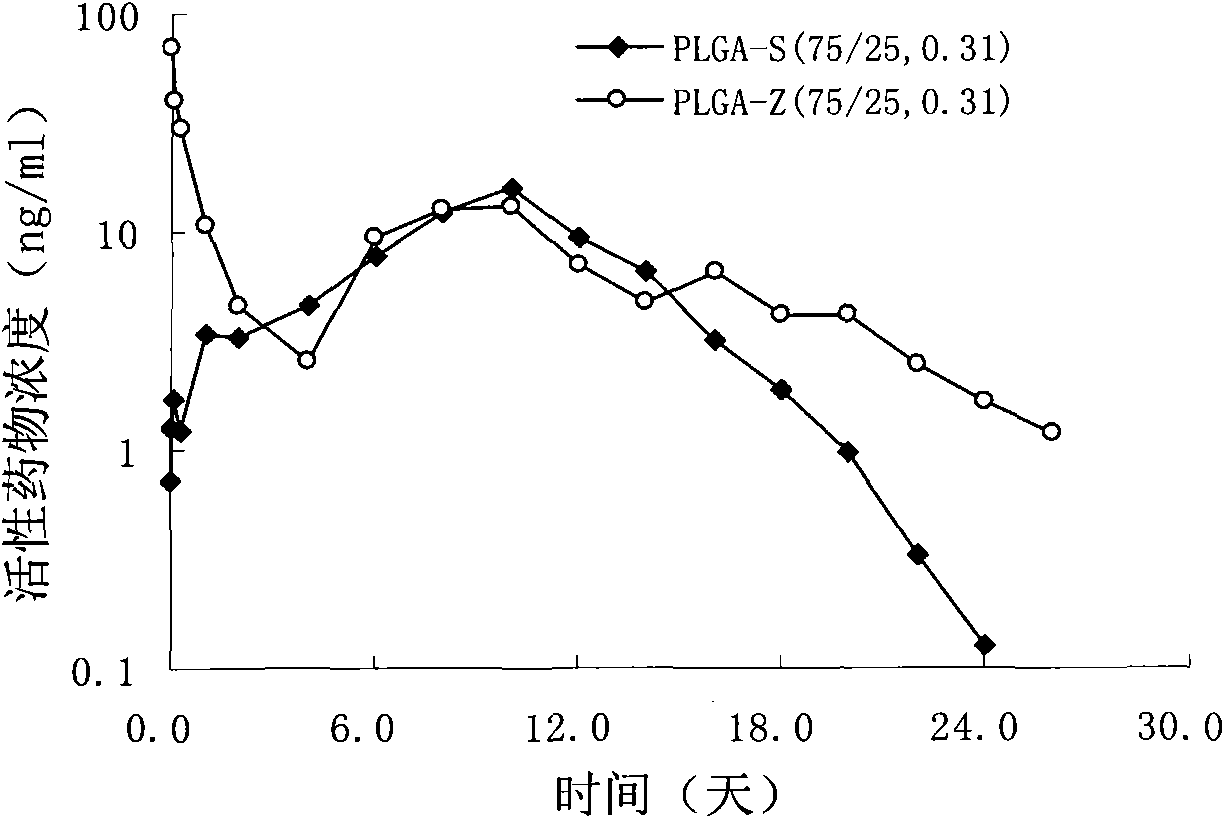
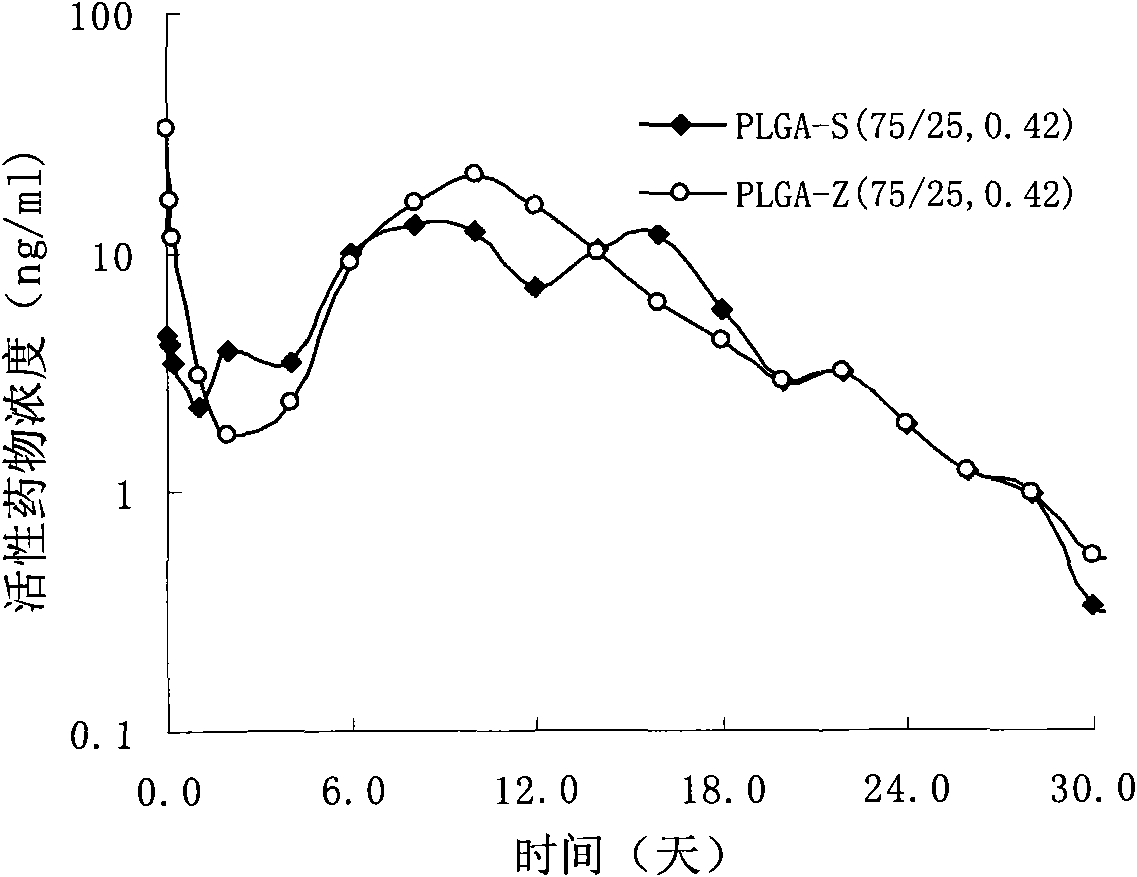
Risperidone slow-release microsphere, preparation method and application thereof
ActiveCN101653422AHigh drug loadingImprove complianceOrganic active ingredientsNervous disorderBlood concentrationMicrosphere
The invention provides a risperidone slow-release microsphere, a preparation method and an application thereof. The microsphere comprises risperidone or 9-hydroxy risperidone or the salt thereof and anon-end-capped lactide-glycollide copolymer. The risperidone slow-release microsphere provided by the invention has higher medicine-carrying quantity, no in-vivo sudden-release phenomenon, stable blood concentration and no medicine release lag period, reduces the administration frequency of a patient greatly, reduces the administration volume of each time, enhances the conformance of the patientand reduces the generation of adverse reactions.
Owner:SHANDONG LUYE PHARMA CO LTD +1
Photoacoustic measurement of analyte concentration in the eye
InactiveUS20070088206A1Quality improvementDiagnostic recording/measuringSensorsAqueous humorSonification
In one aspect, the invention features a method and device for measuring blood concentration of a substance such as glucose in the aqueous humor by illuminating the aqueous humor with a light source at a frequency that is absorbed by the substance to the measured, and then sensing photoacoustically generated sound waves originated within the aqueous humor as a consequence of illumination by the light source. The blood concentration can be estimated from the amplitude of the sound waves received. The method may be combined with other optical techniques for glucose measurement and / or with optical or ultrasonic techniques for topographic mapping of eye structures.
Owner:MINU
Device, system and method for in-vivo detection of bleeding in the gastrointestinal tract
ActiveUS20140296666A1Reduce the impactReduce noiseEndoscopesEndoradiosondesBlood concentrationIn vivo
In-vivo devices, systems and methods for the detection of blood within in-vivo bodily fluids. The methods include irradiating in-vivo fluids passing through a gap in a housing of an in-vivo device introduced to the GI tract of a subject with a plurality of illumination sources positioned on a first side of a gap; detecting with at least one light detector positioned on the opposite side of the gap and facing the illumination sources, light irradiated by the illumination sources; transmitting a plurality of values representing the light detected over time; converting these values to blood concentration values over time, and comparing the blood concentration values to a predetermined threshold value. Based on the comparison, the method includes determining the type of bleeding profile, such that if a plurality of blood concentration values measured consecutively is above the threshold value, the bleeding profile indicates bleeding.
Owner:GIVEN IMAGING LTD
Sustained-release preparations and method for producing the same
The present invention relates to sustained-release preparations prepared by double granulation and methods for producing the same. The sustained-release preparation according to the present invention enables maintenance of effective blood concentration of drug for many hours via sustained release of the drug over 12 hours or more, and further its production is easy owing to convenience of process.
Owner:AMOREPACIFIC CORP
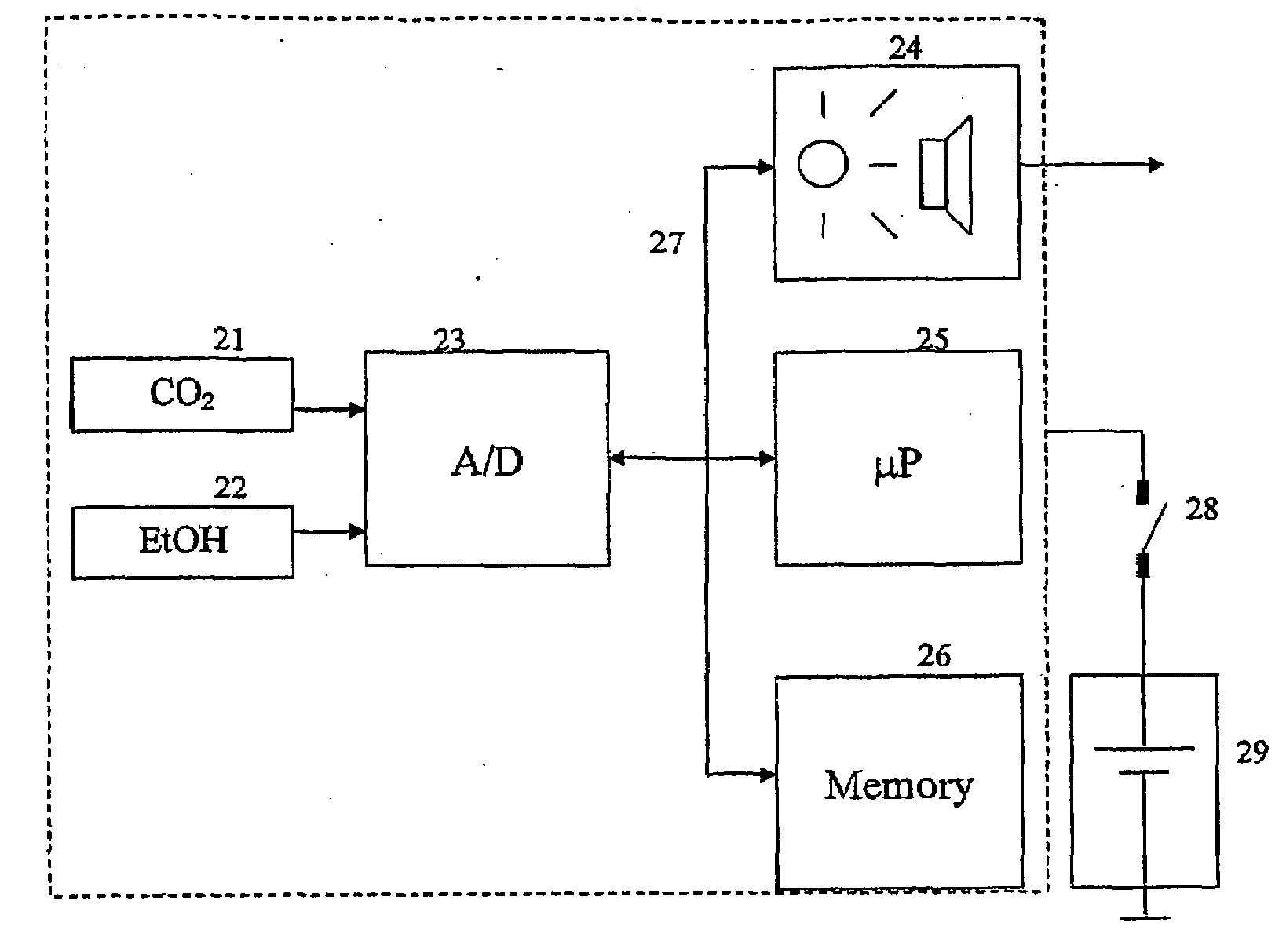
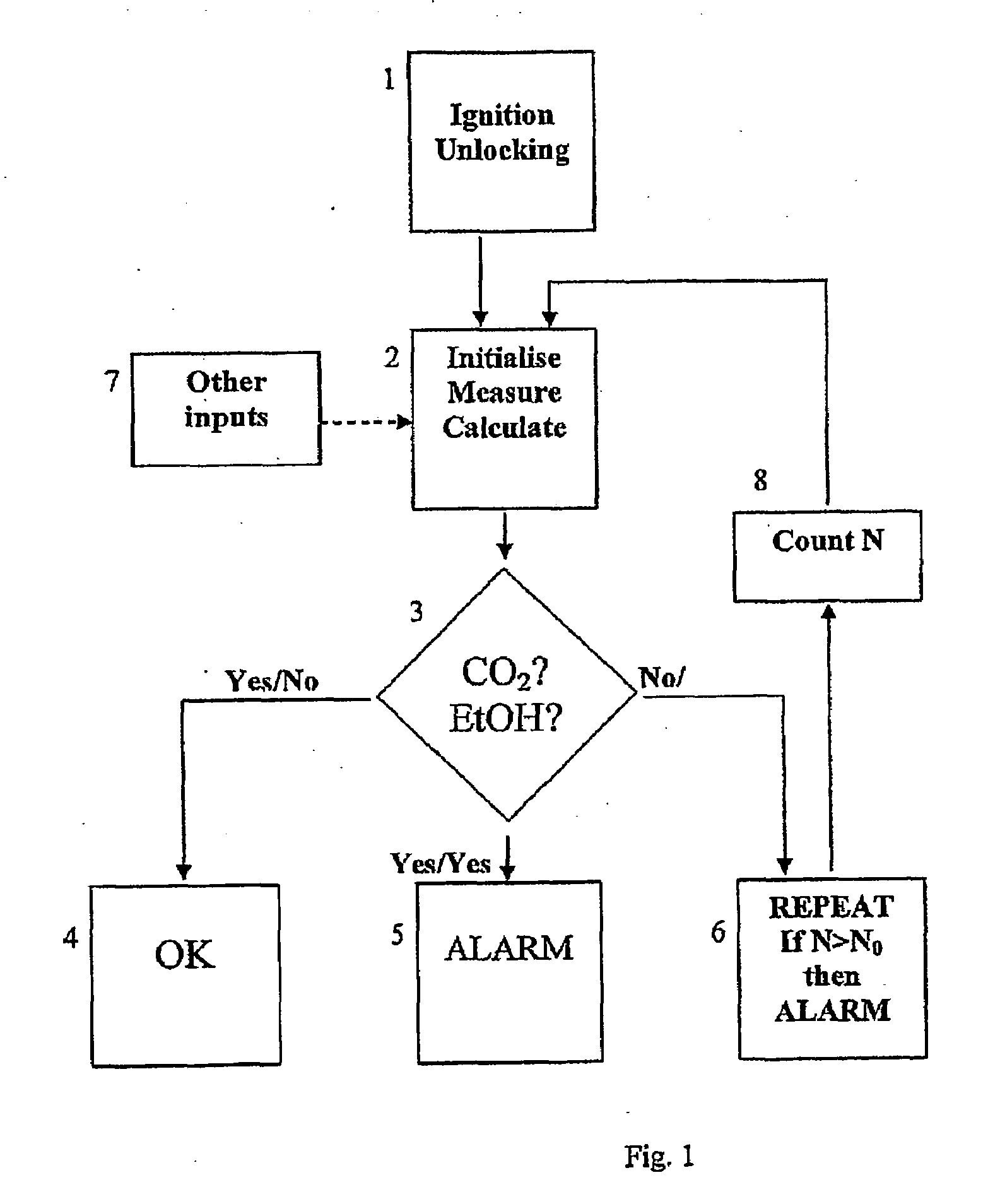
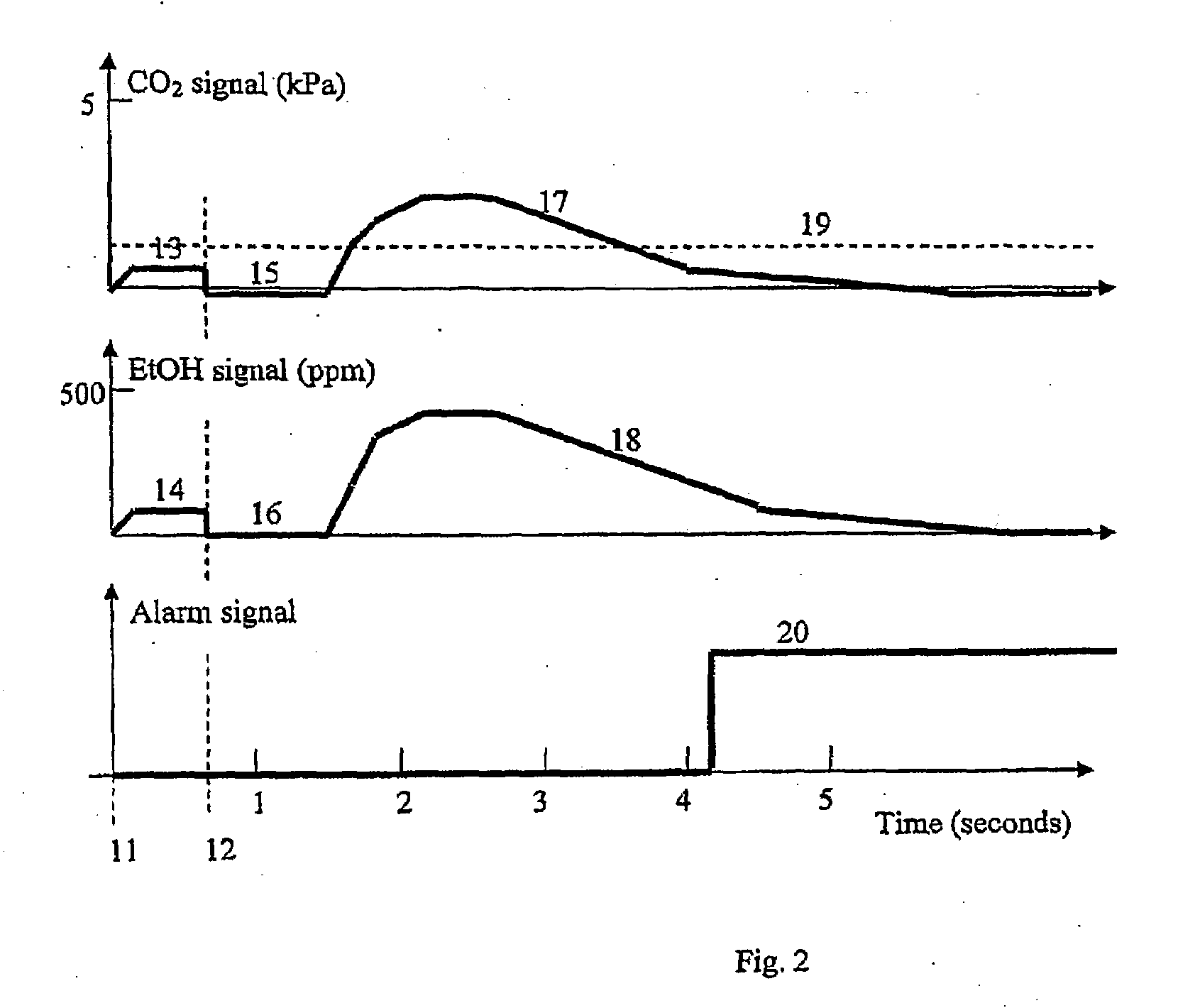
Method and apparatus for assessing blood-concentration of a volatile constituent
InactiveUS20090087920A1Simple methodWithdrawing sample devicesRespiratory organ evaluationExpiratory AirflowBlood concentration
A method and apparatus for of assessing the blood concentration level in a human or animal subject, of a volatile blood constituent (preferably alcohol) is disclosed. The method comprises steps of positioning a sensor (21, 22) within the expiratory gas flow of the subject, wherein the sensor (21, 22) is configured to detect the presence of the constituent and provide a first output signal representative of the concentration of said constituent in air, and also to detect a presence of carbon dioxide and to provide a second output signal representative of the concentration of carbon dioxide in air. The flow of expiratory gases from the subject is sampled to provide a first signal and second signal in respect of the expiratory gases substantially simultaneously. The method also comprises the step of inputting said first and second signals obtained by the sampling step into an algorithm configured to compare the variation of the first signal over time with the variation of the second signal over time and, depending on the result of the comparison, to make said second signal representative of the degree of dilution of said expiratory airflow. The invention further includes an apparatus for carrying out the method.
Owner:AUTOLIV DEV AB
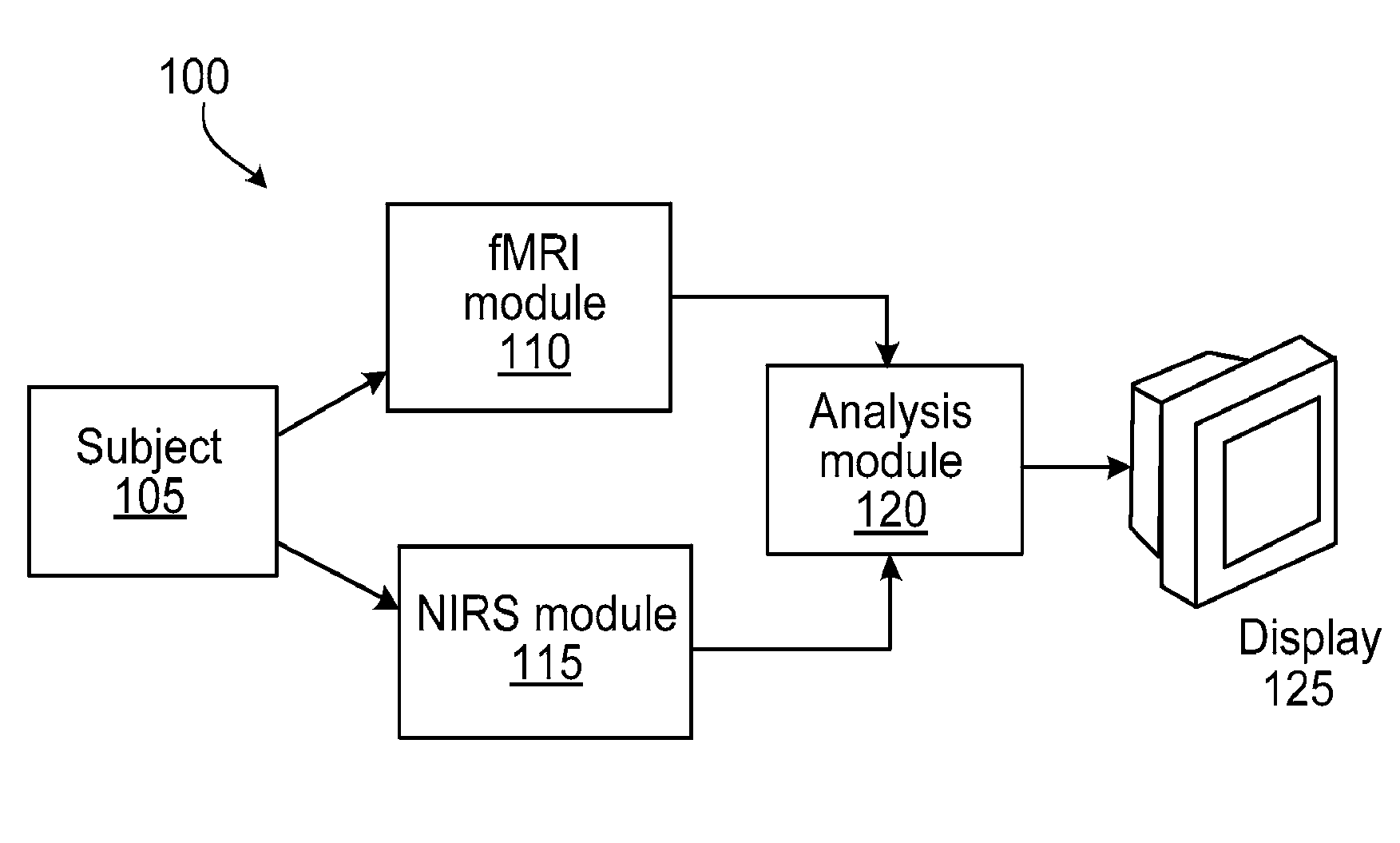
Multi-modal imaging of blood flow
ActiveUS20130144140A1Improved and non-invasive measurement of blood flow and blood volumeLow costDiagnostics using spectroscopyCatheterData setImaging data
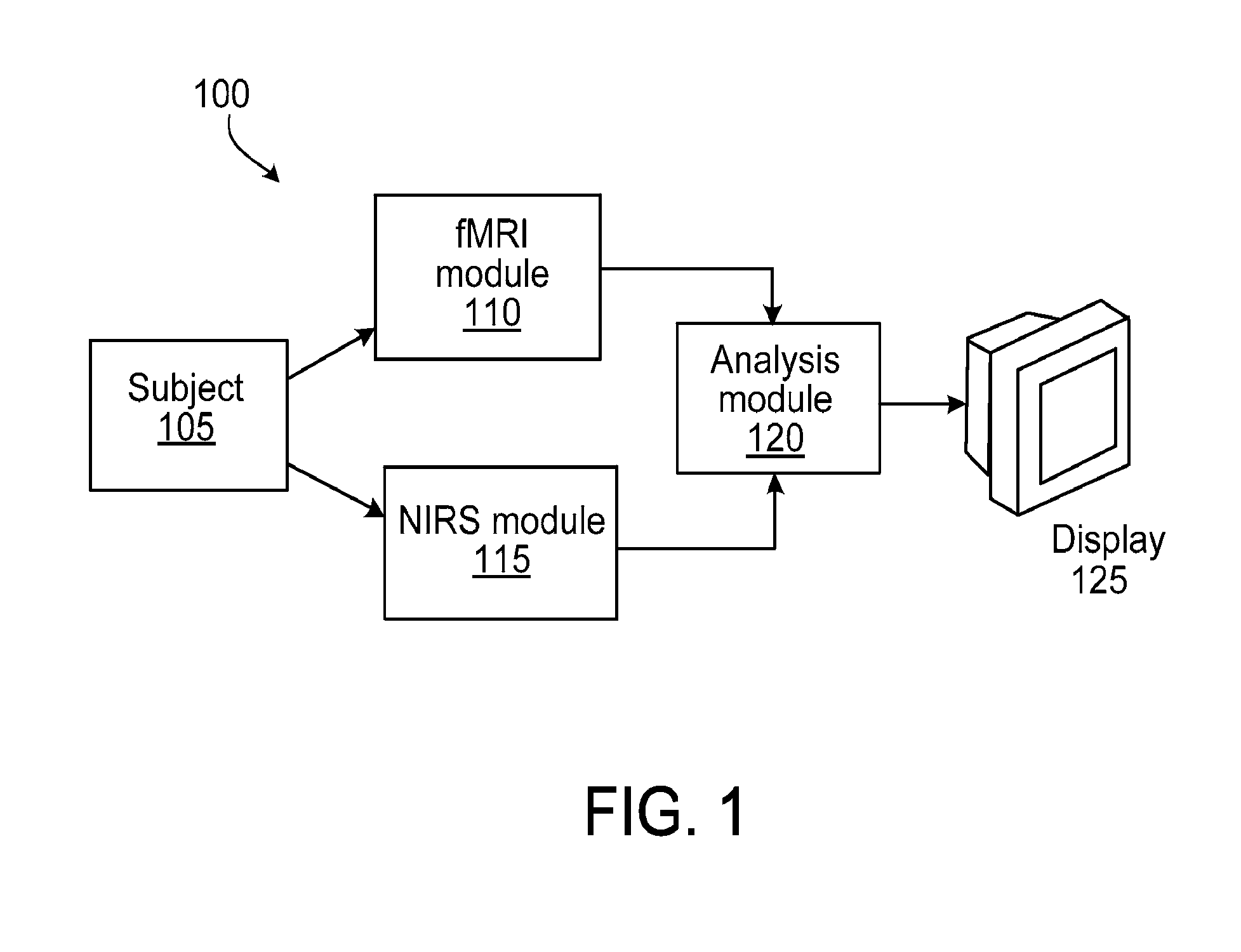
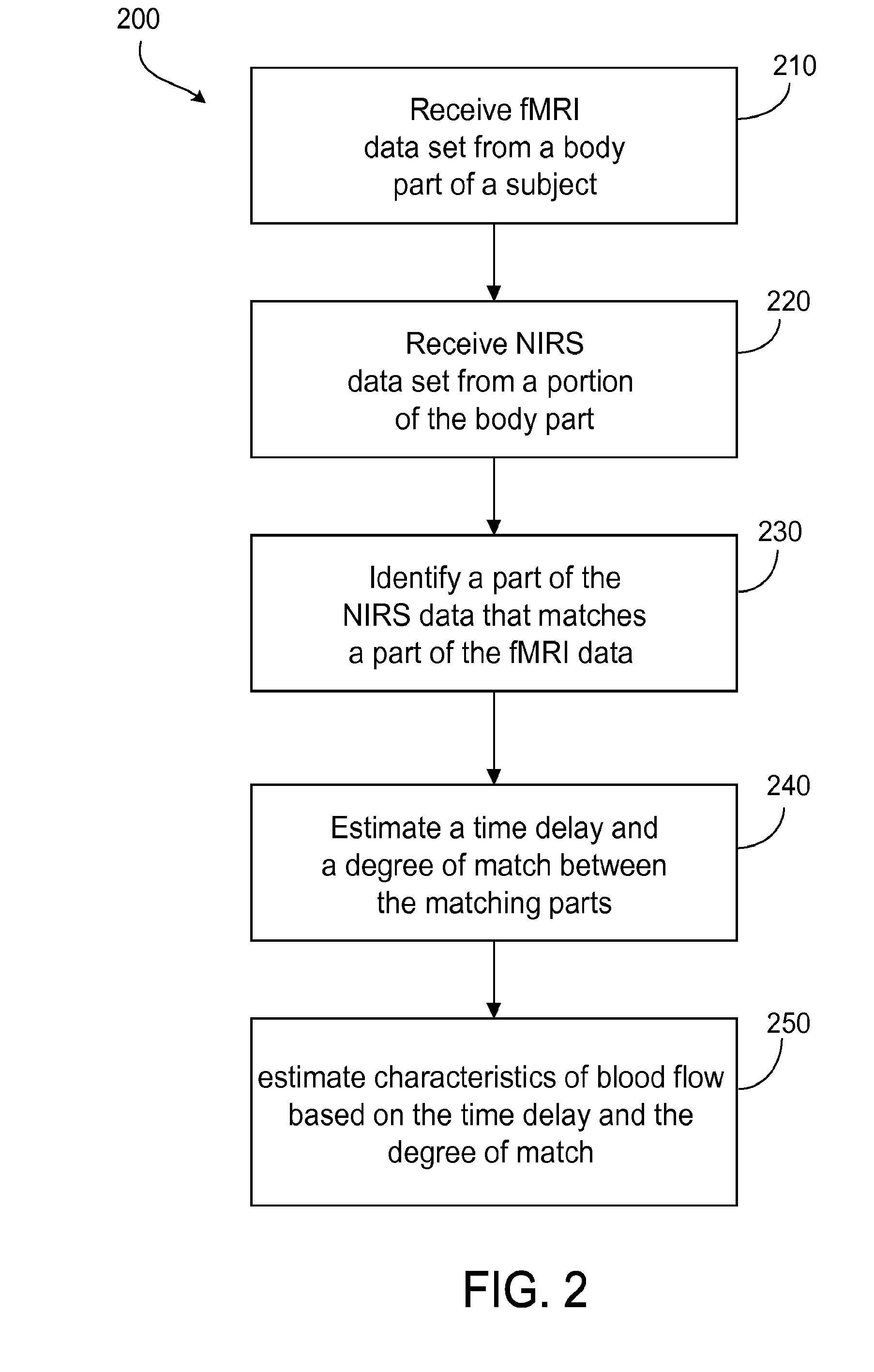
The application features methods, devices, and systems for measuring blood flow in a subject. The computer-implemented methods include receiving functional magnetic resonance imaging (fMRI) data that provides information on at least one of volume or oxygenation of blood at one or more locations in a body over a first predetermined length of time. The methods also include receiving near-infrared spectroscopic (NIRS) imaging or measurement data representing at least one of blood concentration or oxygenation at a first portion of the body over a second predetermined length of time. The methods further include deriving, from the fMRI data corresponding to a second portion of the body, a time varying data set representing changes in blood oxygenation or volume or both blood oxygenation and volume at the second portion over the first predetermined length of time and determining, by a computing device, a time delay and a value of a similarity metric corresponding to a part of the spectroscopic imaging data that most closely matches the time varying data set. The time delay represents a difference between a first time in which blood flows from a third portion in the body to the first portion and a second time in which blood flows to the second portion from the third portion. The value of the similarity metric represents an amount of blood at the second portion. An estimate of a characteristic of at least one of blood flow or blood volume in the second portion at a given time is determined based on the time delay and the value of the similarity metric.
Owner:THE MCLEAN HOSPITAL CORP
Powdery composition for nasal administration
InactiveUS20020012688A1Promote absorptionHigh maximum blood concentrationPowder deliveryMicrocapsulesNasal cavitySolubility
The present invention relates to a powdery composition for nasal administration, which is characterized in that (1) the composition contains (i) a drug, (ii) a water-absorbing and gel-forming base material such as hydroxypropyl cellulose or hydroxypropylmethyl cellulose and (iii) a water-absorbing and water-insoluble base material such as crystalline cellulose or alpha-cellulose, (2) wherein the amount of the water-absorbing and gel-forming base material is about 5-40 wt % based on the total of the water-absorbing and gel-forming base material and the water-absorbing and water-insoluble base material, and (3) wherein the drug is unevenly dispersed more on / in the water-absorbing and water-insoluble base material than on / in the water-absorbing and gel-forming base material. The present invention provides the powdery composition for nasal administration excellent in absorption of the drug from the nasal cavity and having an extremely increased maximum blood concentration comparing a conventional composition for nasal administration even for a drug having a high solubility in water, a drug having a high lipophilicity or a peptide / proteinaceous drug having a large molecular weight.
Owner:TEIJIN LTD
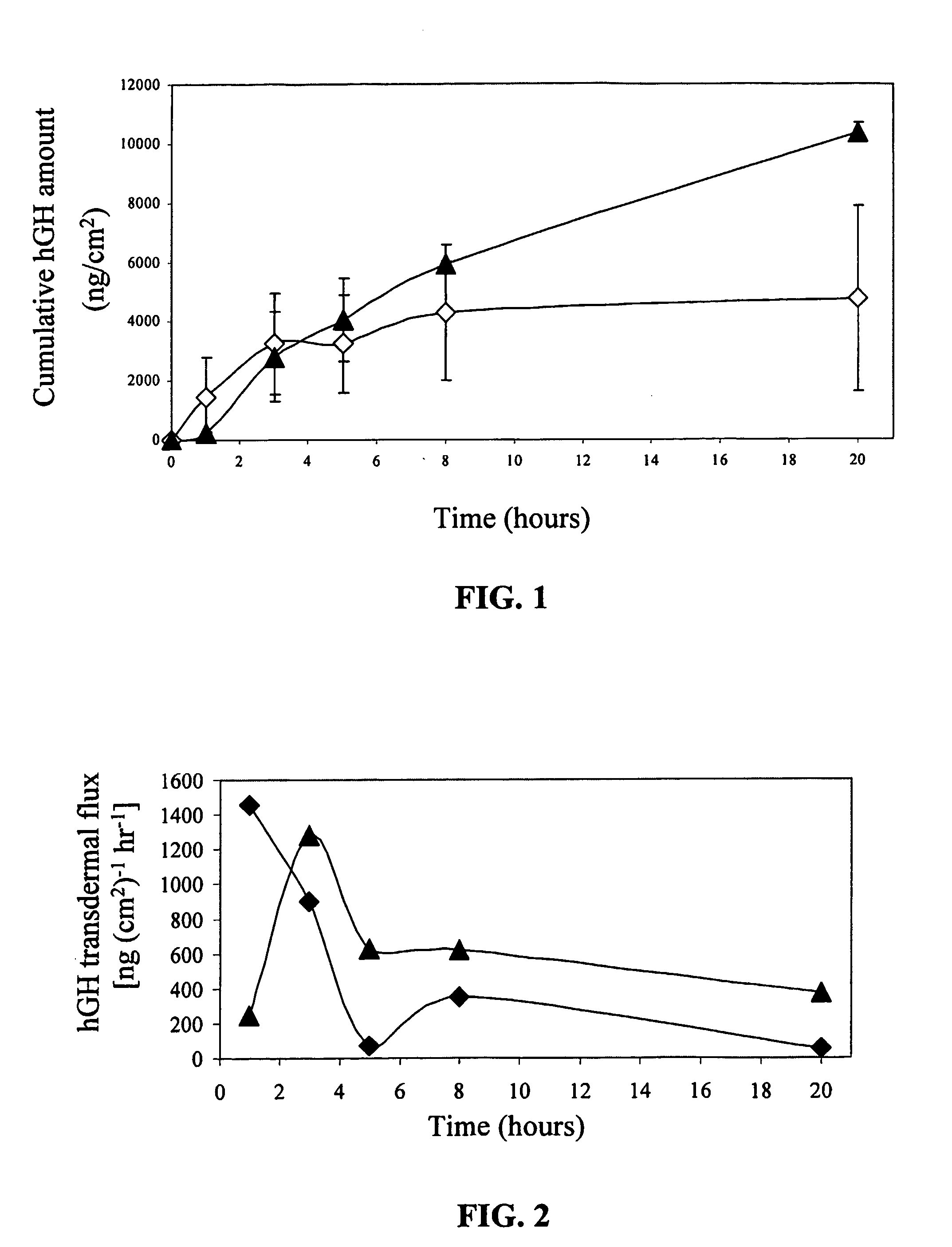
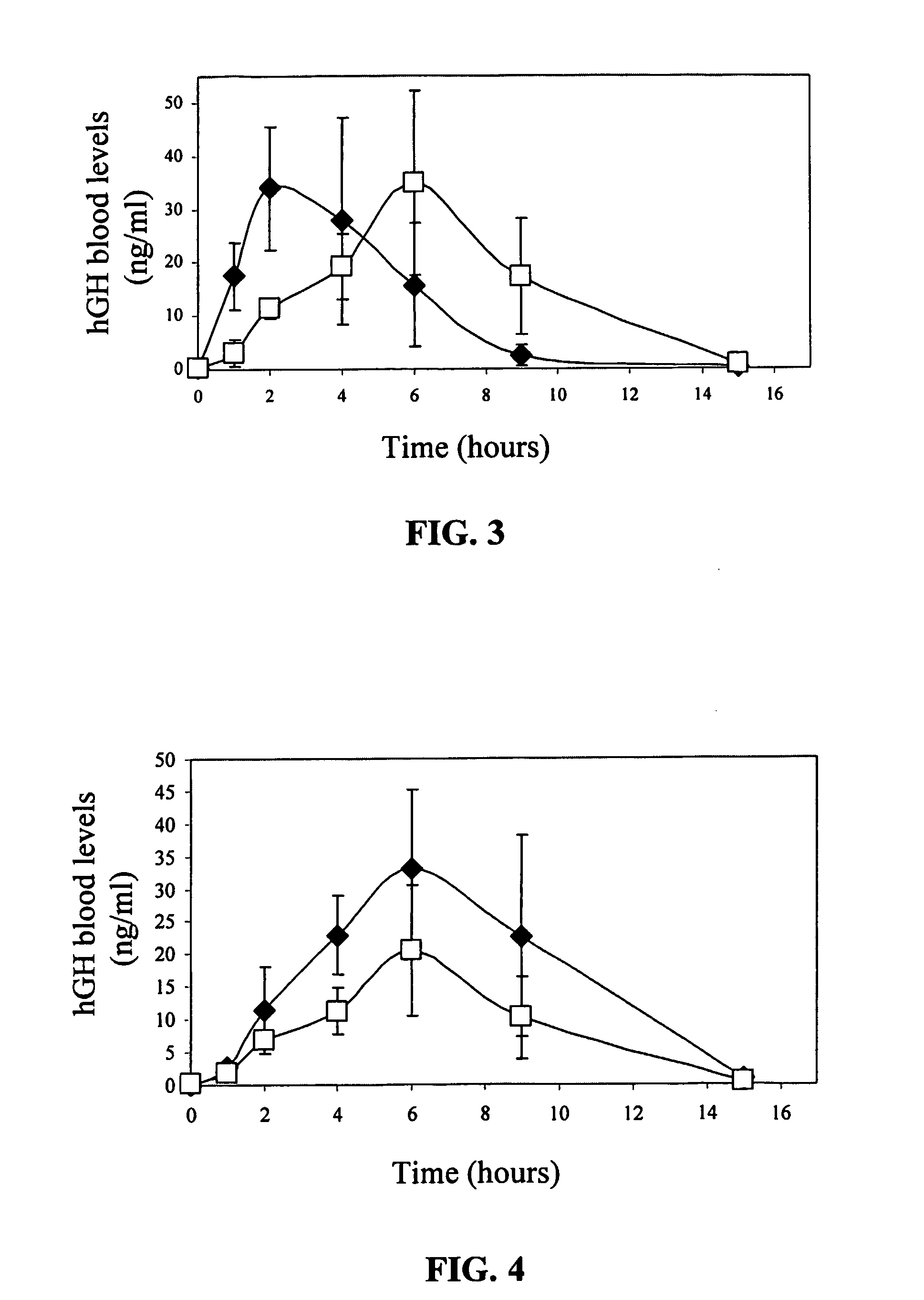
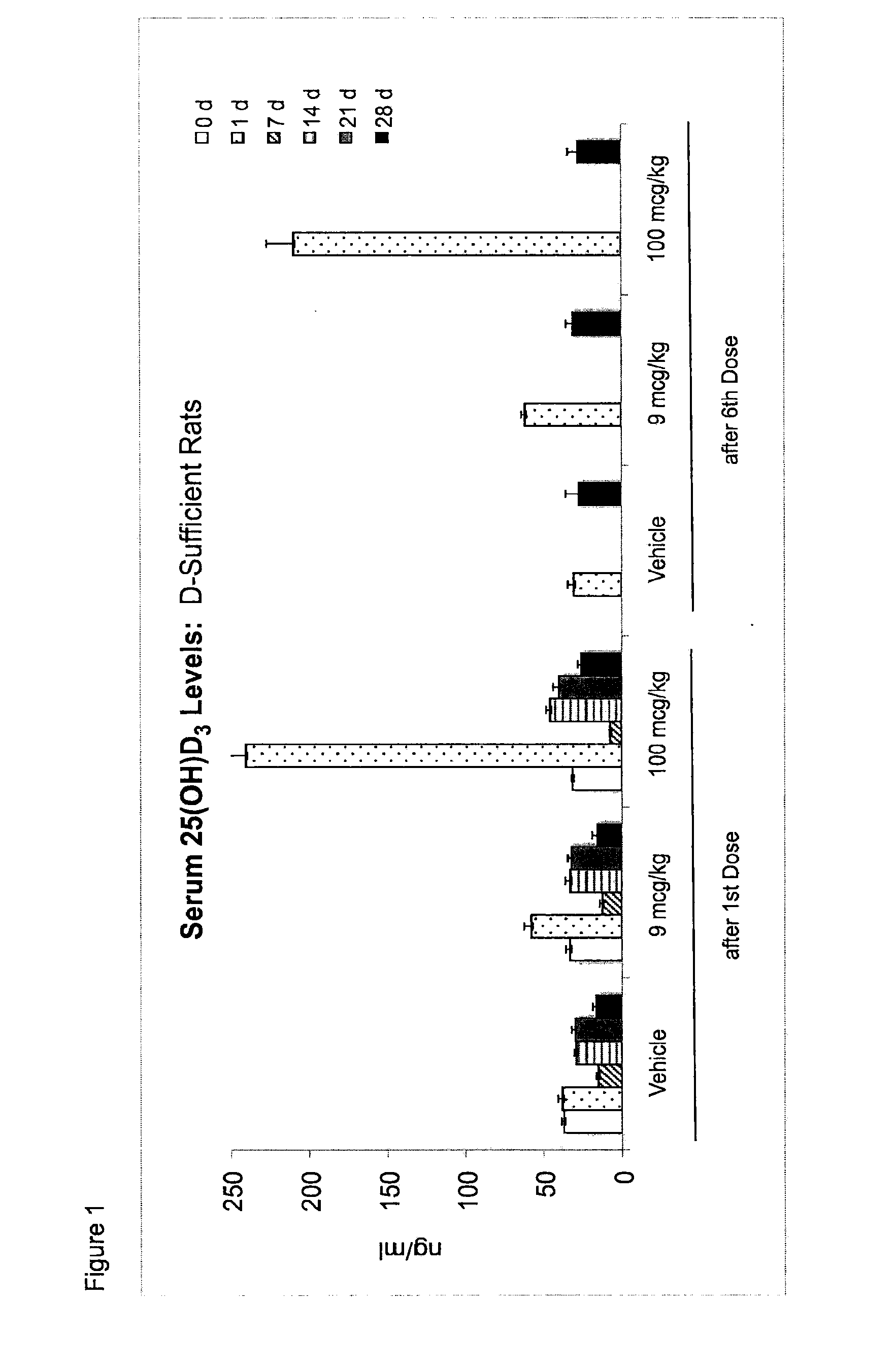
Transdermal System for Sustained Delivery of Polypeptides
InactiveUS20070287949A1Slow and sustained deliveryEffective levelingPowder deliveryElectrotherapyTransdermal patchDrug reservoir
A transdermal system for sustained delivery of high molecular weight hydrophilic drugs, especially peptide-, polypeptide- or protein-drugs, and methods of use thereof, are provided. The system includes an apparatus that generates micro-channels in the skin of a subject in combination with a transdermal patch comprising at least one drug reservoir layer comprising a polymeric matrix and a therapeutic or immunogenic peptide, polypeptide, or protein. The system provides sustained delivery of therapeutic or immunogenic agents, thereby achieving sustained therapeutic blood concentrations of these agents.
Owner:SYNERON MEDICAL LTD
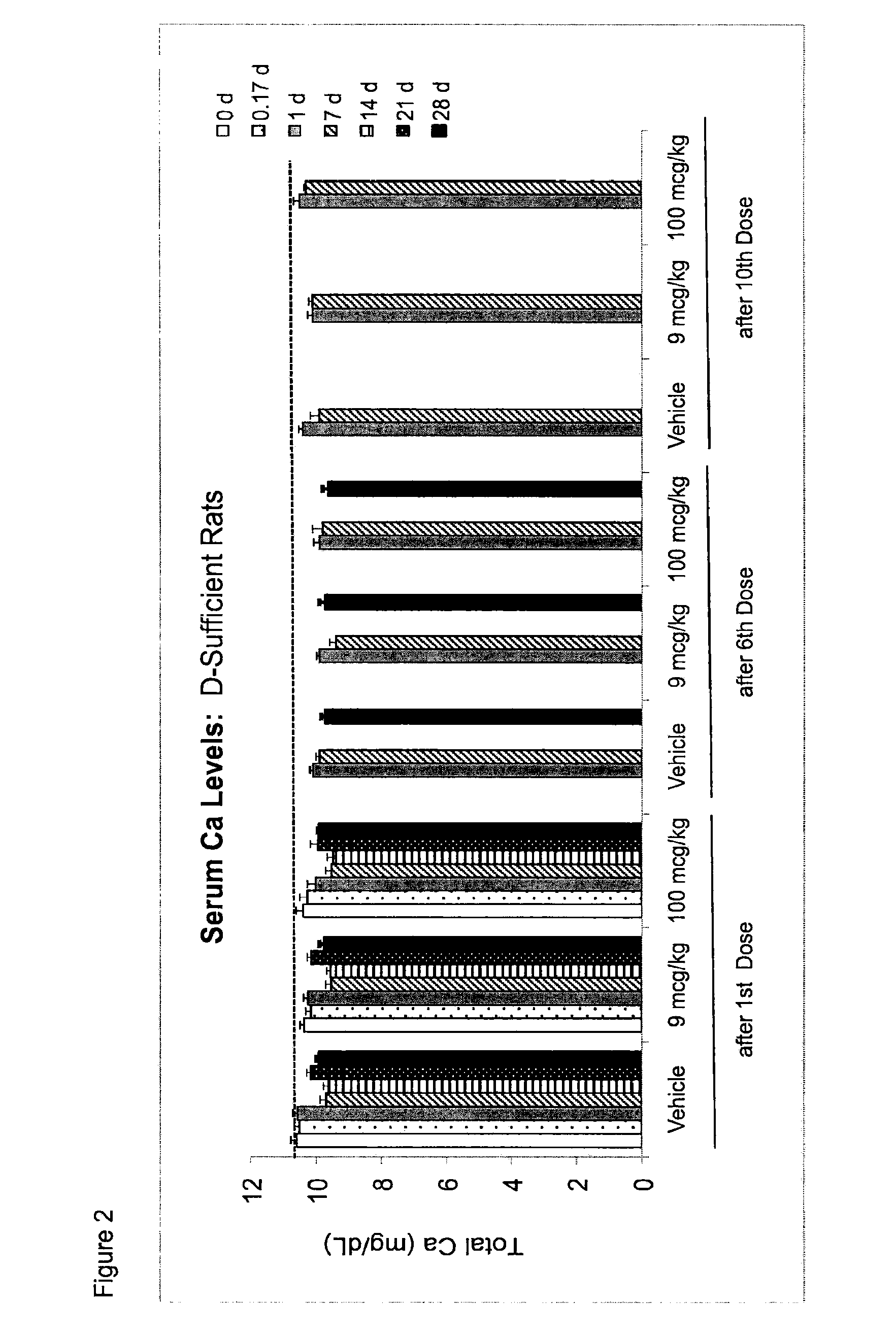
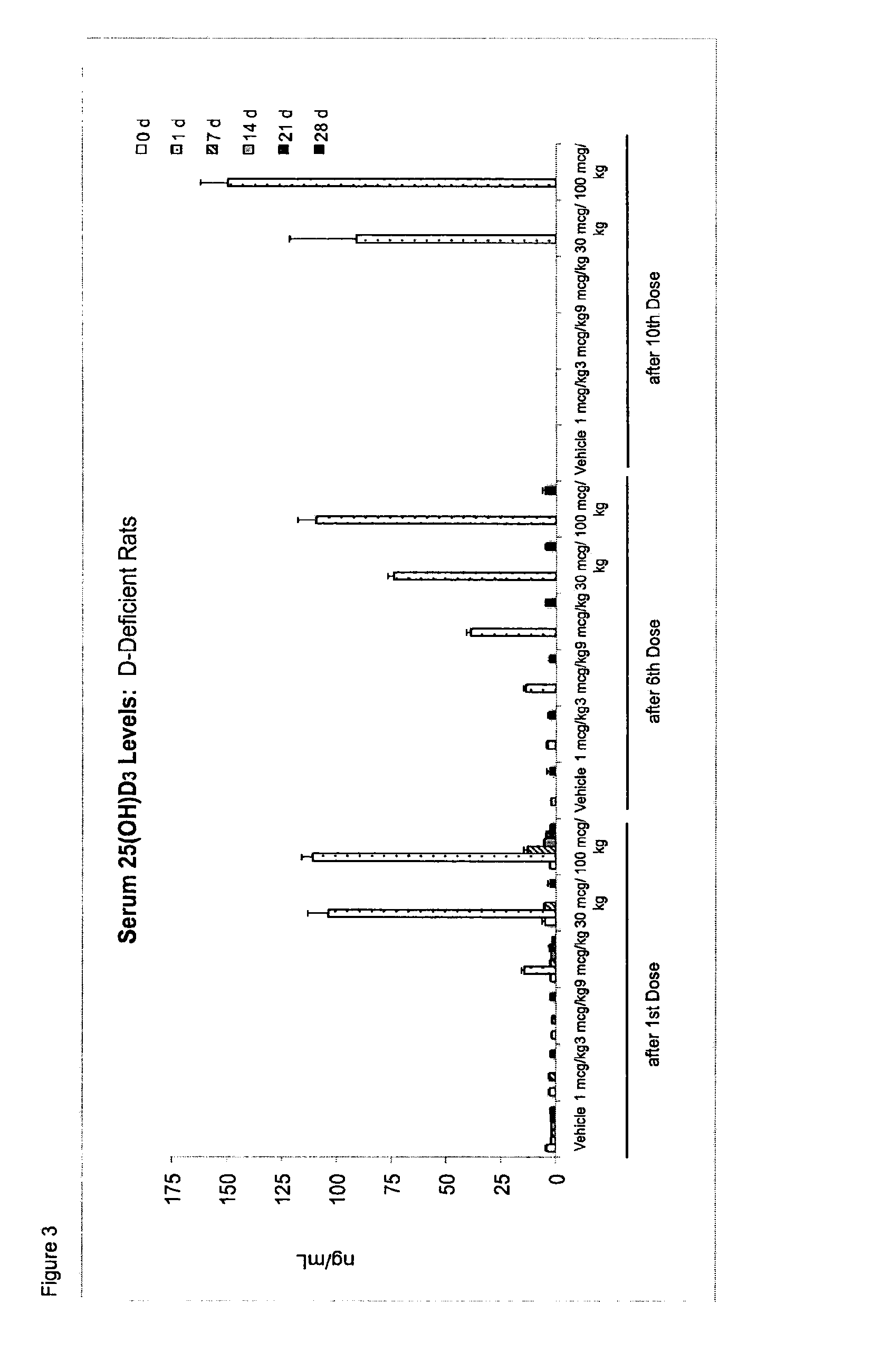
Once-a-week administration of 25-hydroxy vitamin d3 to sustain elevated steady-state pharmacokinetic blood concentration
An oral dosage form comprising a single dose of 25-hydroxy-vitamin D3 sufficient to elevate the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml for at least 7 days and a pharmaceutically suitable oral carrier system, wherein subsequent single doses at least every 7 days are sufficient to sustain the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml at steady-state pharmacokinetics is disclosed. A method of elevating and sustaining the blood level concentration of 25-hydroxy-vitamin D3 in a human in need thereof comprising orally administering or parenterally administering by injection or infusion, at least once every 7 days, a single dose of 25-hydroxy-vitamin D3 sufficient to elevate the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml for at least 7 days, wherein the single doses orally administered at least every 7 days are sufficient to sustain the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml at steady-state pharmacokinetics is disclosed. The human in need thereof may be a human deficient in vitamin D having a serum level concentration of 25-hydroxy-vitamin D3 less than 30 ng / ml.
Owner:WISCONSIN ALUMNI RES FOUND
Sirolimos sustained and controlled release preparation and preparation method thereof
InactiveCN101361703AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityCyclodextrin
The invention provides a Sirolimus slow controlled release preparation and a preparation method thereof. By adopting the solubilizing methods including a solid dispersion technology or cyclodextrin inclusion technology or adding one or a plurality of surface active agents after micronizing drugs and the like, the solubility is dramatically improved and further the bioavailability is improved, therefore, a matrix type slow controlled release preparation is made by adding one or a plurality of framework materials and other accessories, or a diaphragm-controlled or osmotic pump type slow controlled release preparation is made by adopting slow controlled release materials for coating. The Sirolimus slow controlled release preparation has better solubility and dissolution rate, high bioavailability as well as slow controlled release effect, thus being capable of maintaining steady blood concentration, bringing down the concentration of peak, reducing the occurrence of adverse reaction and improving the safety of clinical medicines, in addition, the raw materials can be acquired easily, the preparation process is simple and feasible with high yield rate and low cost, and the large-scale industrial production can be realized and the economic benefit is marked.
Owner:宋洪涛
Method of Safely and Effectively Treating and Preventing Secondary Hyperparathyroidism in Chronic Kidney Disease
InactiveUS20100144684A1Safe and effective usePrevent relapseBiocideOrganic active ingredientsBlood concentrationHormone replacement
A method of treating and preventing secondary hyperparathyroidism in CKD by increasing or maintaining blood concentrations of both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin Din a patient by administering 25-hydroxyvitamin D3 with or without 25-hydroxyvitamin D2 and, as necessary, 1,25-dihydroxyvitamin D2 as a Vitamin D hormone replacement therapy.
Owner:OPKO RENAL LLC
Method for determining the recirculation of blood in a vascular access and a system for implementing same
In an extracorporeal blood treatment system, non-treated blood may be withdrawn from a patient through an arterial line connected to a first compartment of a blood treatment apparatus. The blood treatment apparatus also includes a second compartment separated from the first component by a semi-permeable membrane. Treated blood may be returned to the patient through a venous line connected to the second compartment. In order to determine blood recirculation, i.e., a mixture of treated blood and non-treated blood in the arterial line, the ultrafiltration rate of plasma water through the semi permeable membrane of the treatment apparatus may be temporarily increased or decreased, and the resulting variation of the blood concentration, i.e., of the ratio of the volume of the plasma volume to the blood volume, is determined. The extent of the mixture of the treated blood and the non-treated blood in the arterial line may then be calculated as a function of the blood flow rate in the treatment apparatus, of the amplitude of the variation of the ultrafiltration rate, and of at least two values of the blood concentration, determined before and during the variation of the ultrafiltration flow rate, respectively.
Owner:BAXTER HEALTHCARE SA
Blood purification device
ActiveUS7758532B2Reduce the burden onShorten the time periodOther blood circulation devicesHaemofiltrationMeasurement deviceUltrafiltration
Owner:NIKKISO COMPANY
Lovastatin enteric coated sustained-release pellet capsule and preparation method thereof
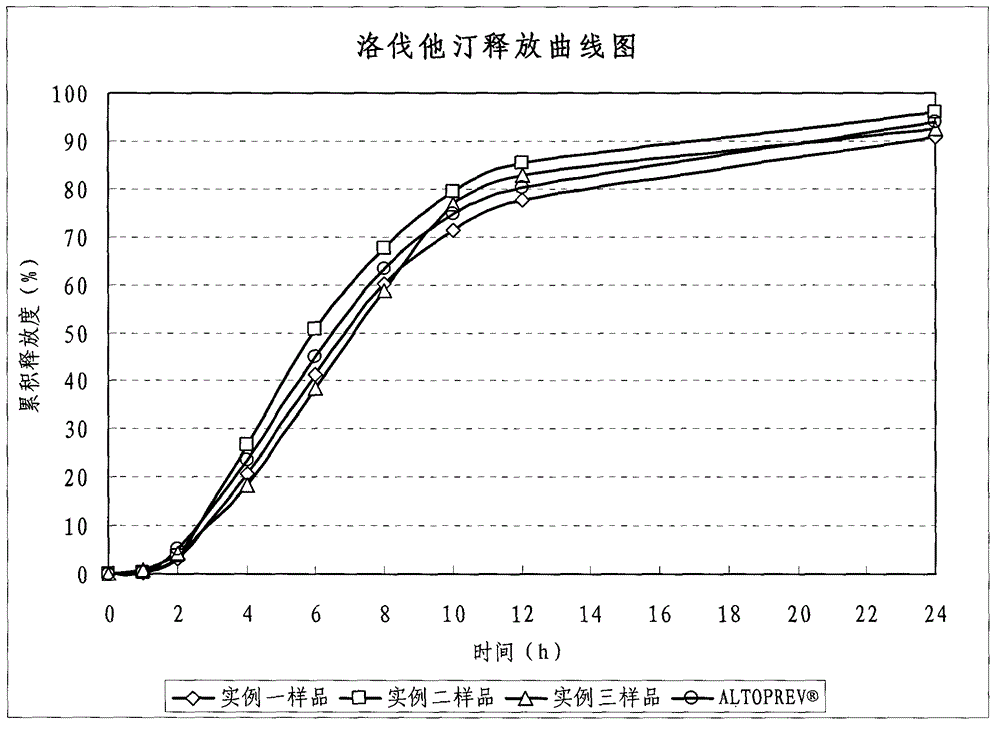
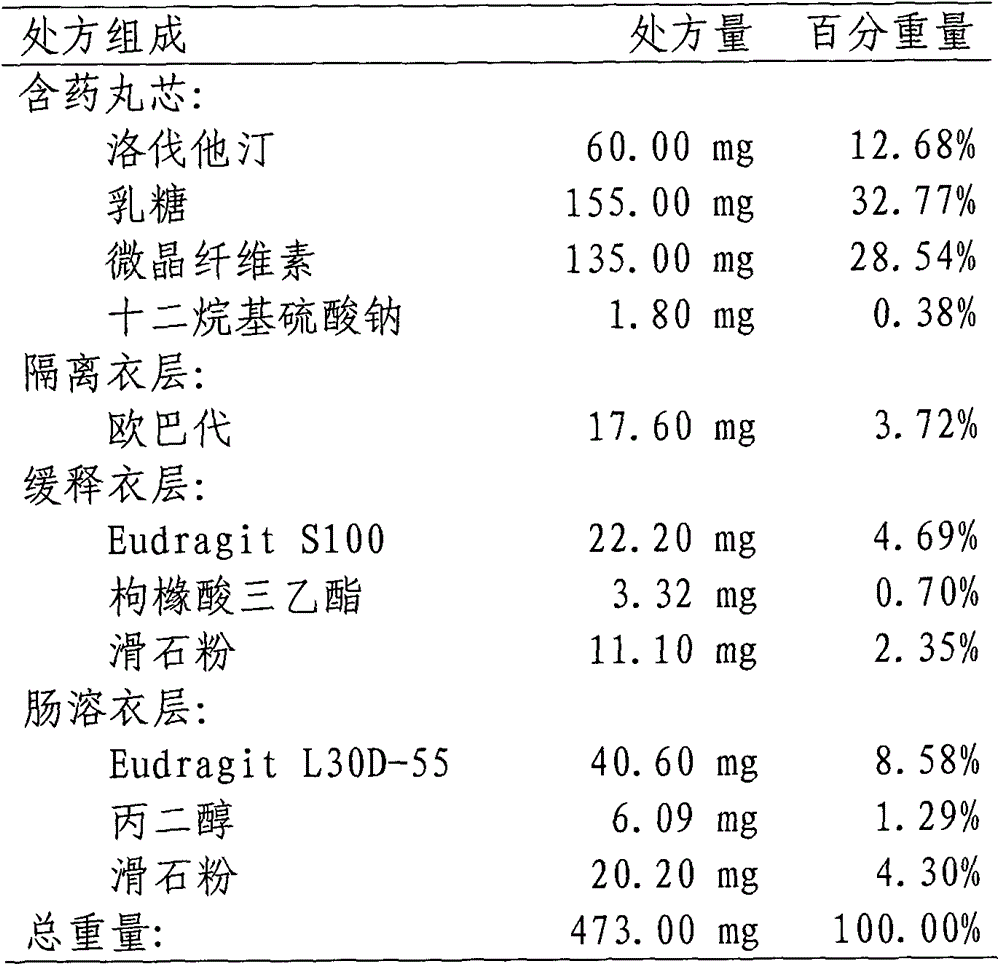
InactiveCN103142552AUniform absorption rateSmall differences in individual bioavailabilityMetabolism disorderGranular deliverySustained release pelletsSide effect
The invention discloses a lovastatin-containing enteric coated sustained-release pellet capsule and a preparation method thereof. The lovastatin-containing enteric coated sustained-release pellet capsule comprises two parts, namely, an enteric coated sustained-release pellet and a hollow capsule, wherein the enteric coated sustained-release pellet comprises 55-86% of medicine-containing pellet core, 2-5% of isolation coating layer, 2-15% of a sustained-release coating layer and 10-25% of enteric-coated coating layer by weight. The prepared lovastatin enteric coated sustained-release pellet capsule is uniform in granule size and stable in medicine release; the medicine is not released in gastric acid but is slowly and constantly released in intestinal tracts and livers, has the characteristic of targeted medicine release, is small in irritation to gastrointestinal tracts, and can reduce the toxic and side effects of the medicine and reduce the number of the medicine administrations, so that the compliance of patients is improved; and meanwhile as the enteric coated sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
Blood purification device
ActiveUS20060043007A1Accurate detectionReduce parameterHaemofiltrationDialysis systemsBlood concentrationUltrafiltration
A blood purification device allows confirming whether a specific peak is provided by a means, such as a ultrafiltration pump, to concentrate the blood or not and also allows measuring certainly and accurately a blood re-circulation with reduction of parameters which can provide a ratio of re-circulating blood. The blood purification device including blood circuit route 1 composed of arterial blood circuit route 1a and venous blood circuit route 1b, blood pump 3, dialyzer 2, water removal pump 8 which can provide the specific peak in the variation of blood concentration by removing water rapidly in a short period of time, and detecting means detecting said specific peak, can measure the blood re-circulation based on the specific peak detected by the detecting means. The re-circulating blood flowing is the blood which was introduced again to arterial blood circuit route 1a after it had been returned to a patient from venous blood circuit route 1b. The detecting means includes first detecting means 5a installed in arterial blood circuit route 1a and second detecting means 5b installed in venous blood circuit route 1b.
Owner:NIKKISO COMPANY
Method of producing a sustained-release preparation
InactiveUS6399103B1Reduce the number of stepsSuitable for industrializationCosmetic preparationsPowder deliveryBlood concentrationOrganic solvent
A method of producing sustained-release microcapsules which comprises dispersing a physiologically active polypeptide into a solution of a biodegradable polymer and zinc oxide in an organic solvent, followed by removing the organic solvent; which provides a sustained-release preparation showing a high entrapment ratio of the physiologically active polypeptide and its constant high blood concentration levels over a long period of time.
Owner:TAKEDA PHARMA CO LTD
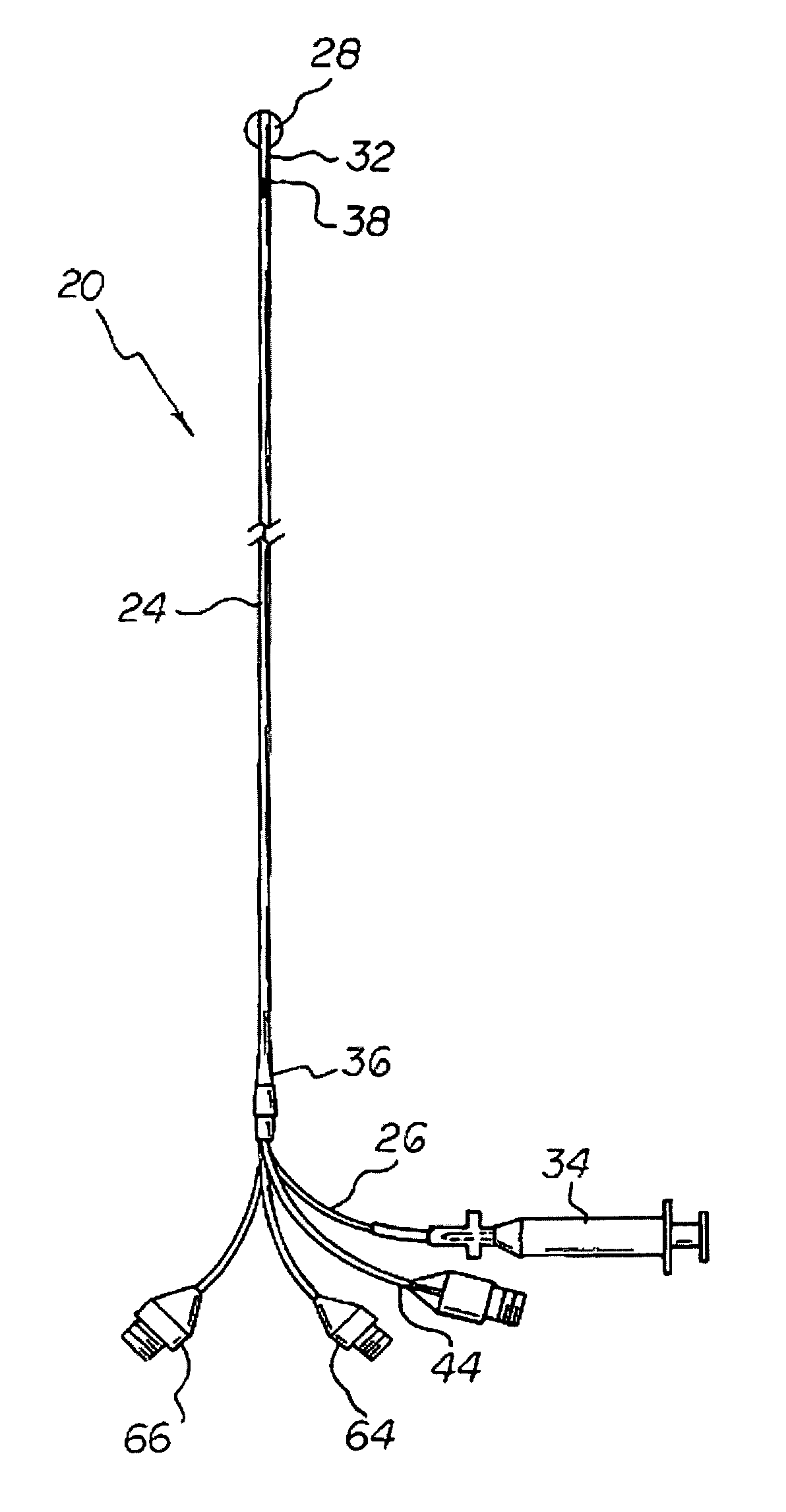
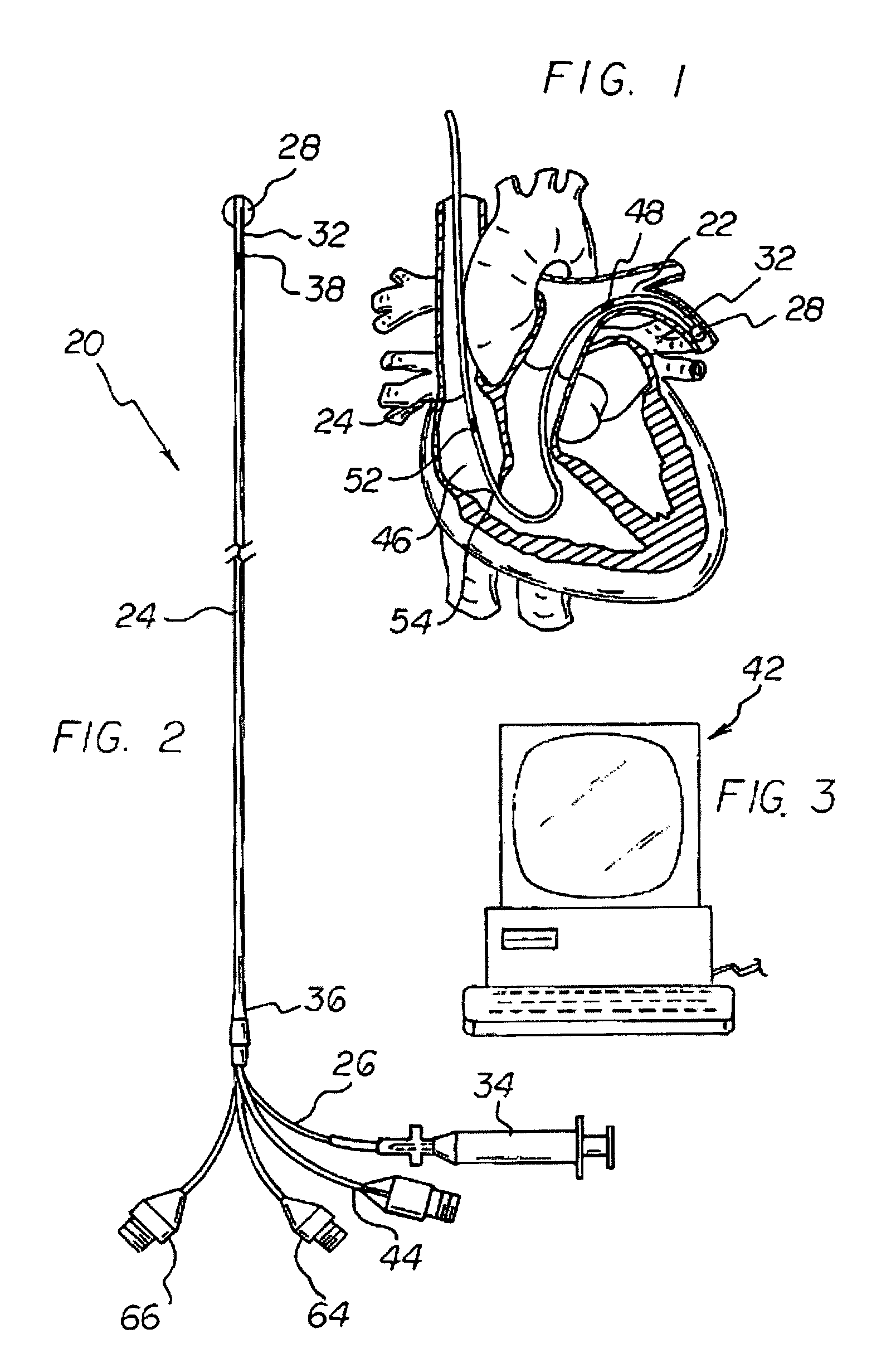
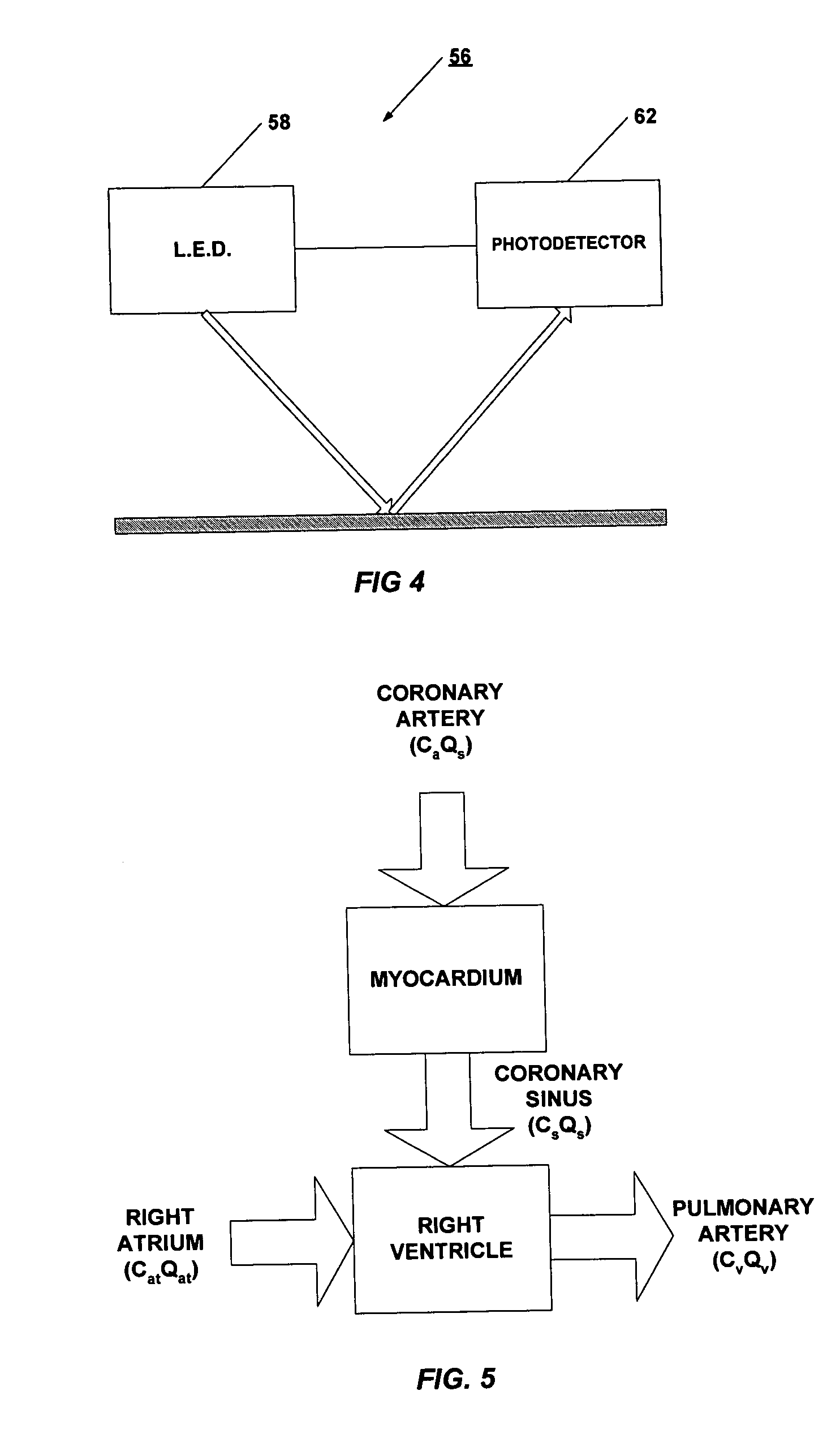
Apparatus and method for measuring myocardial oxygen consumption
Disclosed is a pulmonary artery catheter (“PAC”) that is used in determining myocardial oxygen consumption. Myocardial oxygen consumption is of critical importance because decreased myocardial energy utilization during acute illness may lead to tissue hypoperfusion, multiple organ failure, and eventually death. The inventor has discovered that myocardial oxygen consumption is a function of the difference in oxygen levels in atrial and mixed venous blood. The invention has further discovered that differences in lactate, glucose or any other measurable blood concentration metabolite in atrial or superior vena cava and mixed venous blood can also be used in determining myocardial oxygen consumption.
Owner:GUTIERREZ GUILLERMO
Sinomenine hydrochloride sustained-released injection and preparation method thereof
The invention discloses a sinomenine hydrochloride sustained release injection and a preparation method thereof. The sustained release injection comprises one of synthetic polymer nanometer sinomenine hydrochloride sustained release injection, natural polymer material microsphere sinomenine hydrochloride sustained release injection, lipid sinomenine hydrochloride sustained release injection, multivesicular liposome sinomenine hydrochloride sustained release injection and gelatin sinomenine hydrochloride sustained release injection. Various sinomenine hydrochloride sustained release injections prepared by the invention by different methods can prolong the acting time of drug, reduce drug administration times, keep stable blood concentration in vivo and reduce the toxic and side effects of the drug.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
Nimodipine lipid microsphere injection and preparation method thereof
ActiveCN101485632AImprove solubilityImprove stabilityOrganic active ingredientsNervous disorderSolubilityLipid formation
The invention provides a nimodipine lipid microsphere injection, which is prepared from the following components in percentage by weight: 0.08 percent of nimodipine, 0.5 to 2.3 percent of lecithin for injection, 2 to 8 percent of soybean oil for injection, 2 to 8 percent of medium chain fatty acid for injection, 1 to 3 percent of glycerin, 0.1 to 0.2 percent of tween-80, 0.03 to 0.05 percent of sodium oleic acid, and the balance being water for injection. The preparation method comprises steps of preparation of an oil phase, preparation of water phase, preparation of colostrum, homogenization and canning. In the nimodipine lipid microsphere injection, the soybean oil for injection and the medium chain fatty acid for injection are used to prepare the oil phase, the nimodipine is a fat soluble drug and can be better dissolved in the oil phase, the lipid microsphere in which the soybean oil for injection is the main component has solvent characteristics, is non-toxic, and can guide the fat soluble drugs to be dissolved in emulsion particles and perform the metabolism along with lipid oil drops and slowly release, thereby maintaining the effective blood concentration, lowering toxic and side effects of the drugs, increasing the solubility and stability of the nimodipine drug, improving the drug-loading rate and reducing the hydrolysis of the drugs.
Owner:沈阳信康药物研究有限公司
Novel controlled release-niacin formulation
InactiveUS20090069389A1Easy to controlEffective controlBiocideMetabolism disorderControlled releaseBlood concentration
The present invention relates to a controlled-release niacin formulation. In particular, the present invention relates to a controlled-release niacin formulation, comprising niacin; hydroxypropyl methylcellulose; and a carboxyvinyl polymer, in which the carboxyvinyl polymer and hydroxypropyl methylcellulose are contained in a predetermined weight ratio, and to a preparation method thereof.The controlled-release niacin formulation according to the present invention maintains its matrix shape until completion of release, and maintains its release pattern without fluctuation for a desired time period, unlike a commercial formulation. In particular, since niacin formulations are used for long-term treatment of hyperlipidemia, the controlled-release niacin formulation of the present invention, capable of maintaining effective blood concentration and high stability for a long period of time, is very useful.
Owner:SEOUL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com