Sirolimos sustained and controlled release preparation and preparation method thereof
A technology of sirolimus and controlled-release preparations, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem that solutions are not easy to transport and store, and reduce the incidence of adverse reactions , Sirolimus instability and other problems, to achieve the effect of improving the safety of clinical medication, reducing the incidence of adverse reactions, and the preparation process is simple and feasible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
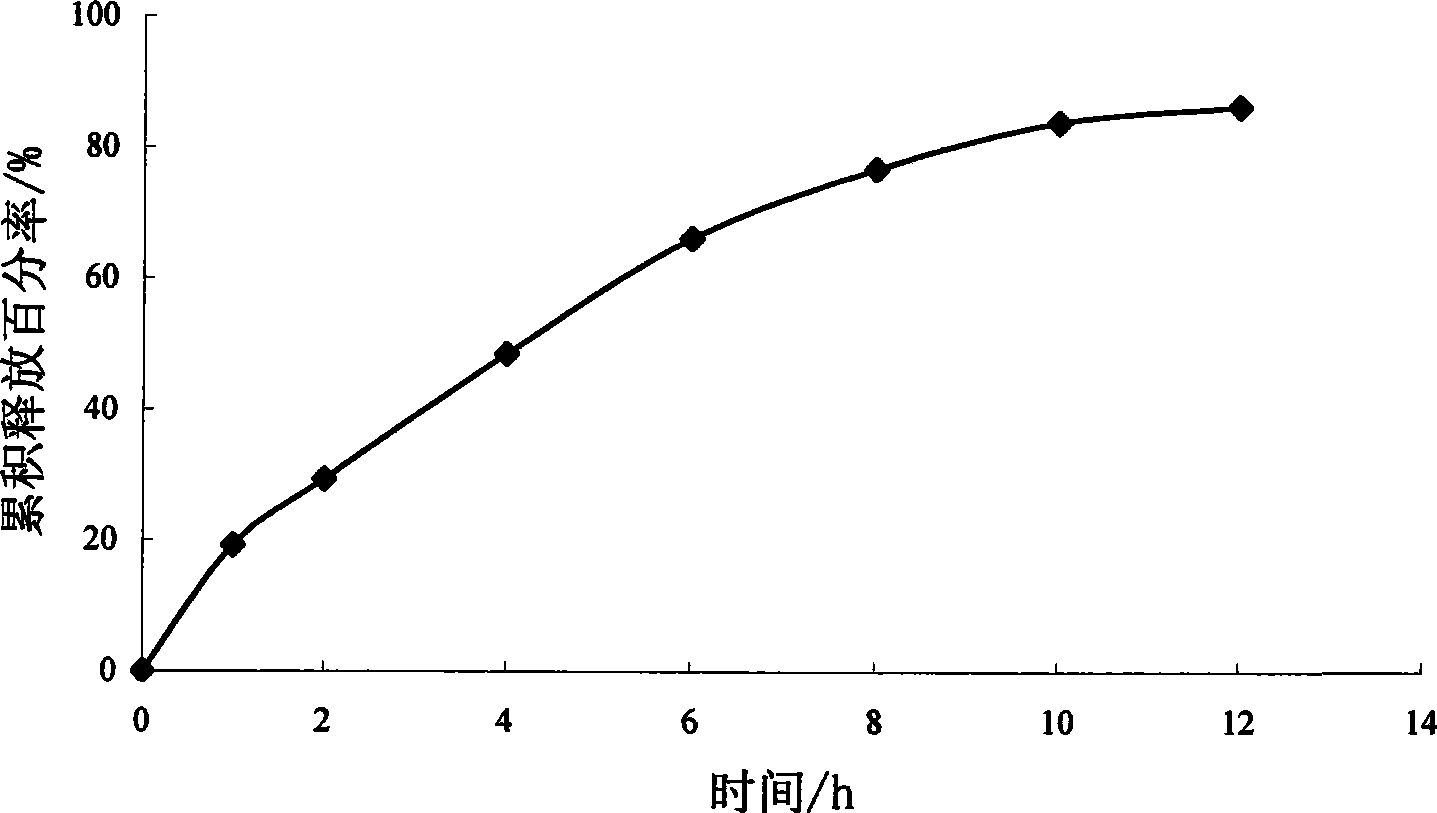
[0074] Example 1 Preparation of sirolimus hydrogel matrix tablet
[0075] Sirolimus 1g Polyoxyethylene-40-stearate 10g Micropowder silica gel 0.7g Hydroxypropyl Methyl Cellulose K4M 42g microcrystalline cellulose 84g lactose 2.8g Magnesium stearate 0.7g A total of 1000 pieces
[0076] Preparation Process:
[0077] Dissolve sirolimus in an appropriate amount of ethanol solution, heat polyoxyethylene-40-stearate in a water bath at 60-70°C until it melts, mix well, stir vigorously and pour the molten material onto a stainless steel plate Form a thin layer, let it cool quickly into a solid, then place the solid in a vacuum oven at 25-40°C for 24 hours, take it out, add a small amount of micropowder silica gel to grind (micropowder silica gel as a lubricant, can reduce the solid dispersion during the grinding process) loss), after passing through an 80-mesh sieve, mix it with hydroxypropyl methylcellulose K4M, microcrystall...
Embodiment 2
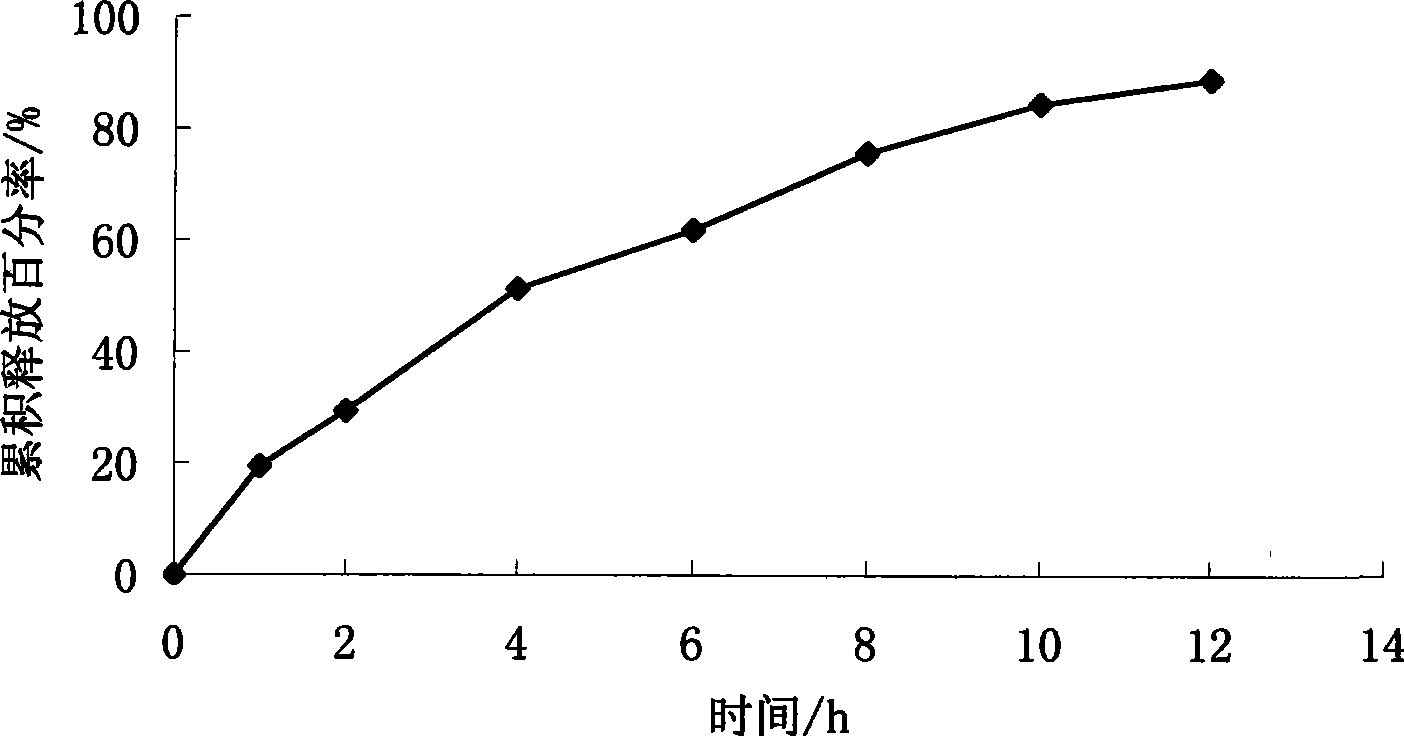
[0080] Example 2 Preparation of sirolimus membrane-controlled sustained-release tablets
[0081] Chip Sirolimus 1g Poloxamer 188 15g Micropowder silica gel 0.7g lactose 130g Magnesium stearate 0.7g 1% aqueous solution of polyoxyethylene pyrrolidone Appropriate amount
[0082] Coating material Ethyl cellulose 15g polyethylene glycol 400 1.5g Diethyl phthalate 1.5g acetone Appropriate amount A total of 1000 pieces
[0083] Preparation Process:
[0084] Dissolve sirolimus in an appropriate amount of ethanol solution, heat Poloxamer 188 in a water bath at 60-70°C to melt, mix well, stir vigorously, and pour the melt on a stainless steel plate to form a thin layer. Cool it quickly into a solid, then place the solid in a vacuum oven at 25-40°C for 24 hours, take it out, add a small amount of micropowder silica gel to grind (micropowder silica gel can be used as a lubricant to reduce...
Embodiment 3
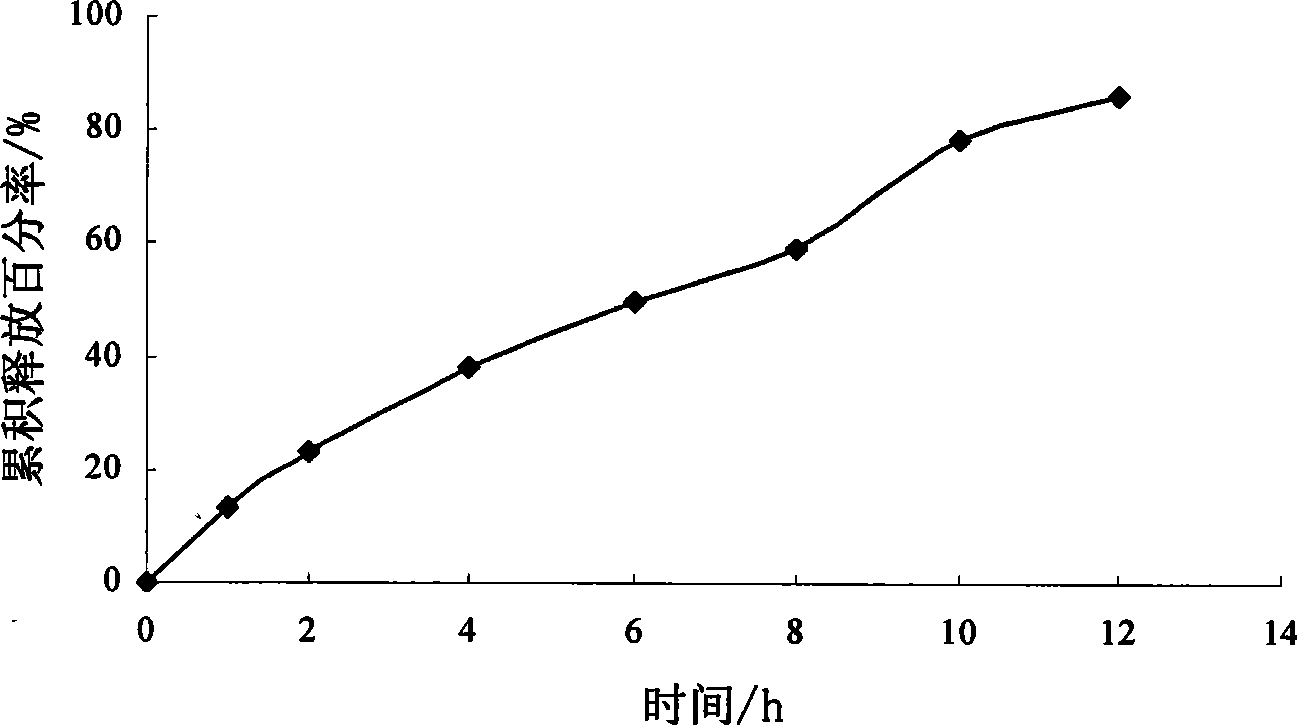
[0086] Example 3 Preparation of Sirolimus Osmotic Pump Tablets
[0087] Chip Sirolimus 1g Hydroxypropyl-β-cyclodextrin 20g polyoxyethylene 30g Sodium chloride 70g Magnesium stearate 0.7g Coating material Cellulose acetate 12g polyethylene glycol 400 1.5g Acetone: water (100:1, v:v) Appropriate amount A total of 1000 pieces
[0088] Preparation Process:
[0089] Dissolve sirolimus and hydroxypropyl-β-cyclodextrin with an appropriate amount of absolute ethanol, sonicate in an ultrasonic instrument for 20 minutes, then transfer it to a rotary evaporator to evaporate the solvent to dryness at a temperature of 30-40°C, and precipitate Put the product in a vacuum drying oven at 25-40°C for 24 hours, take it out, grind it, pass through an 80-mesh sieve, mix it with polyoxyethylene, sodium chloride, and magnesium stearate, and press it into tablets. Coating with the above-mentioned coating materials, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com