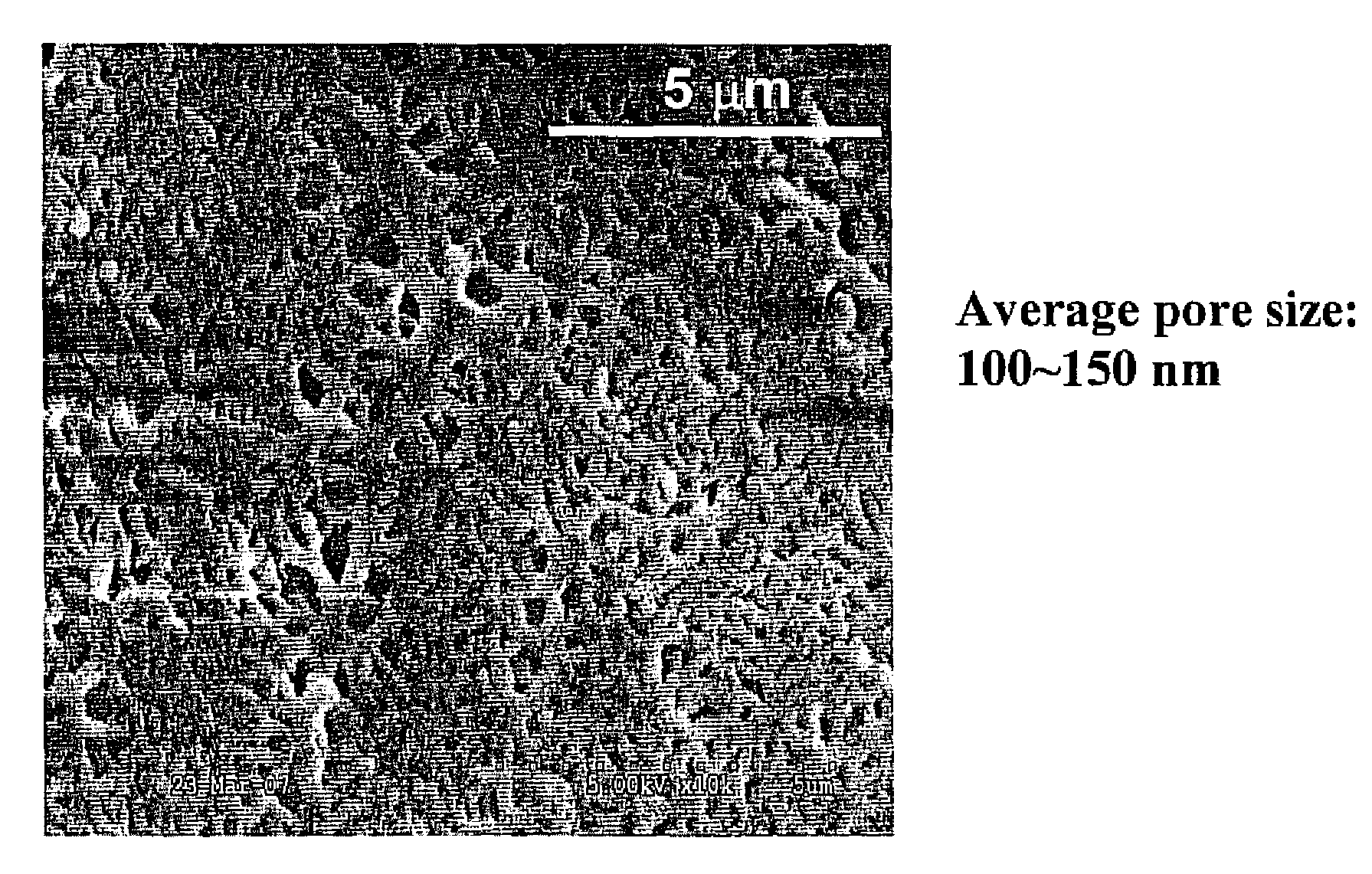Patents
Literature
299 results about "Hydrogel matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
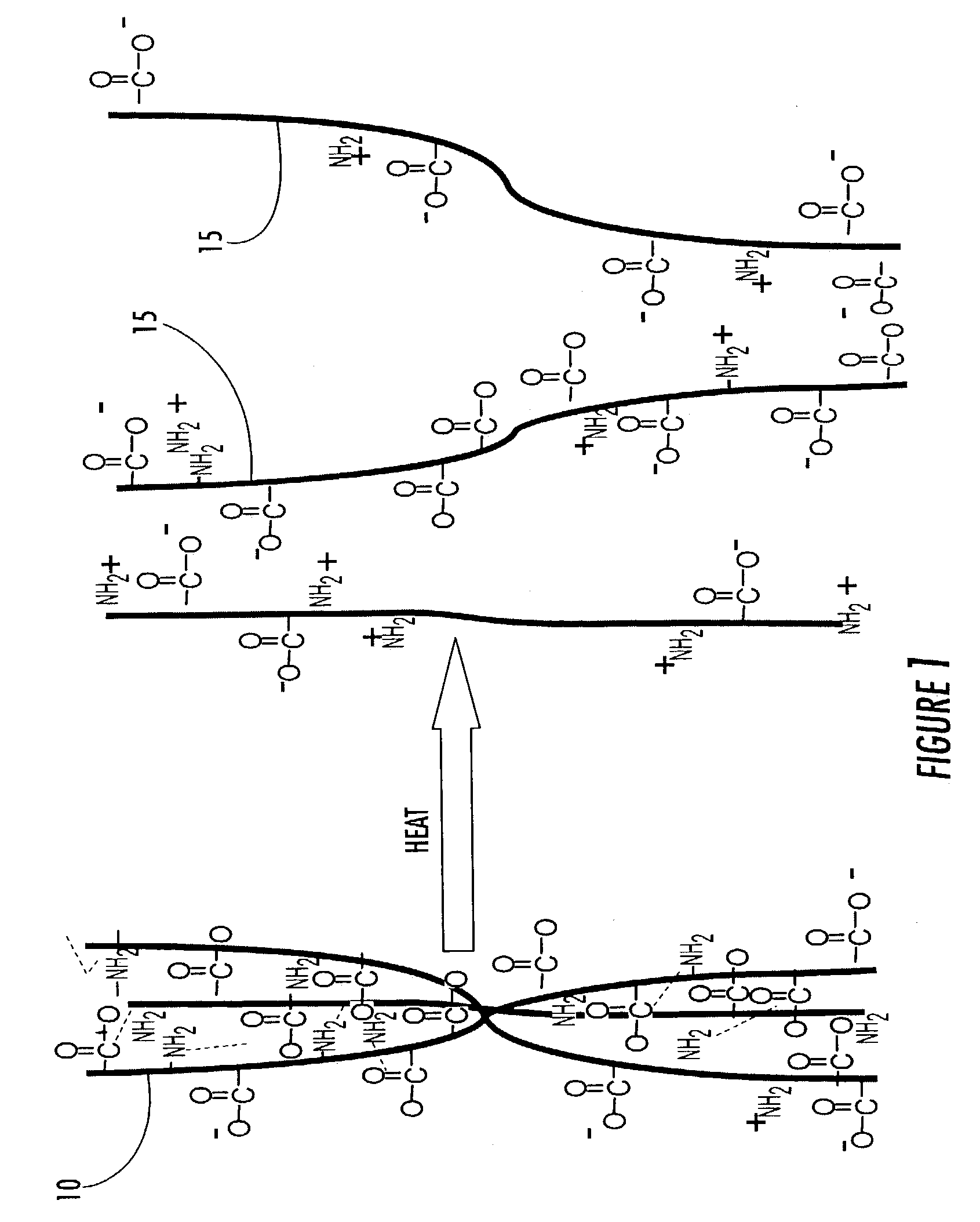
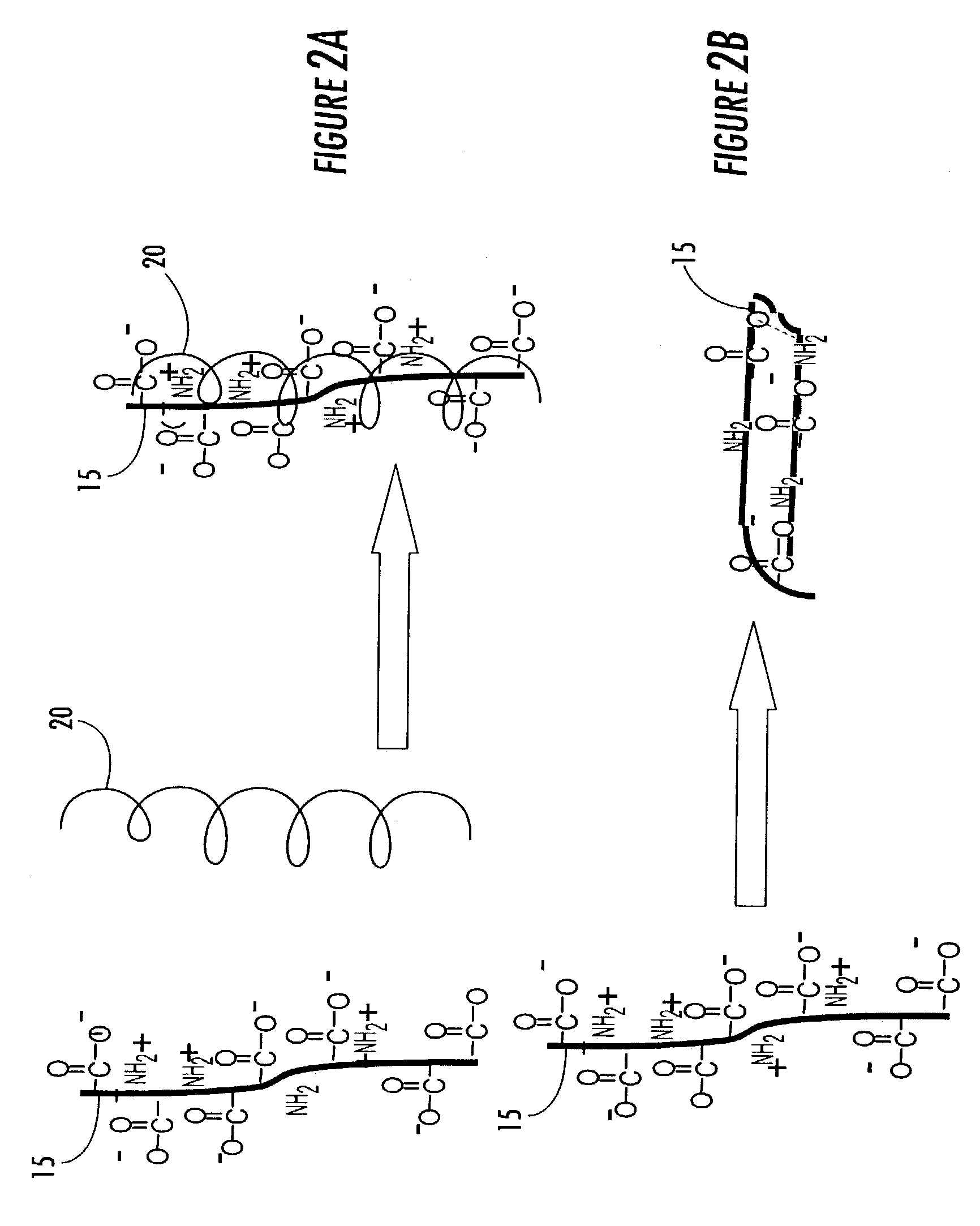
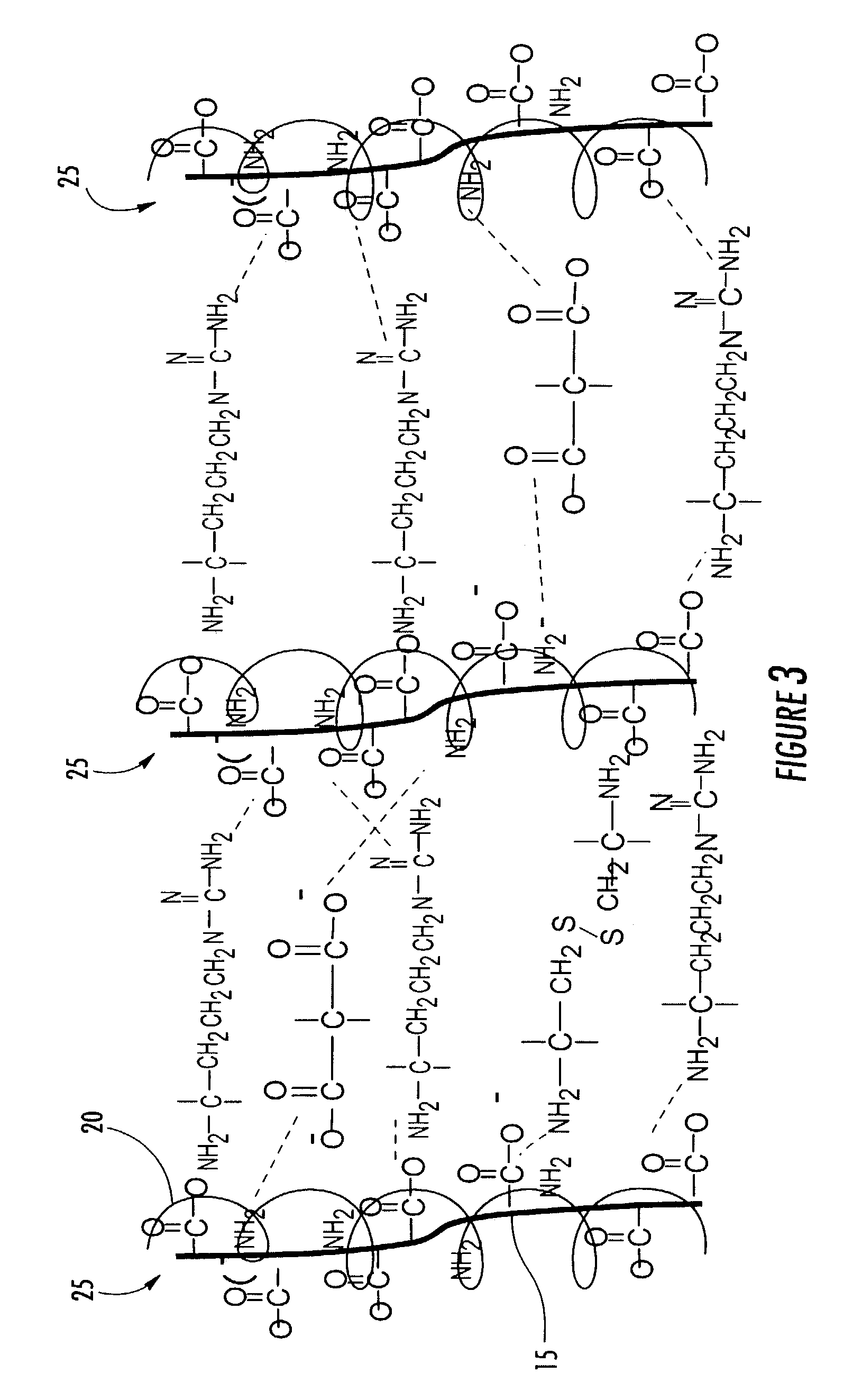
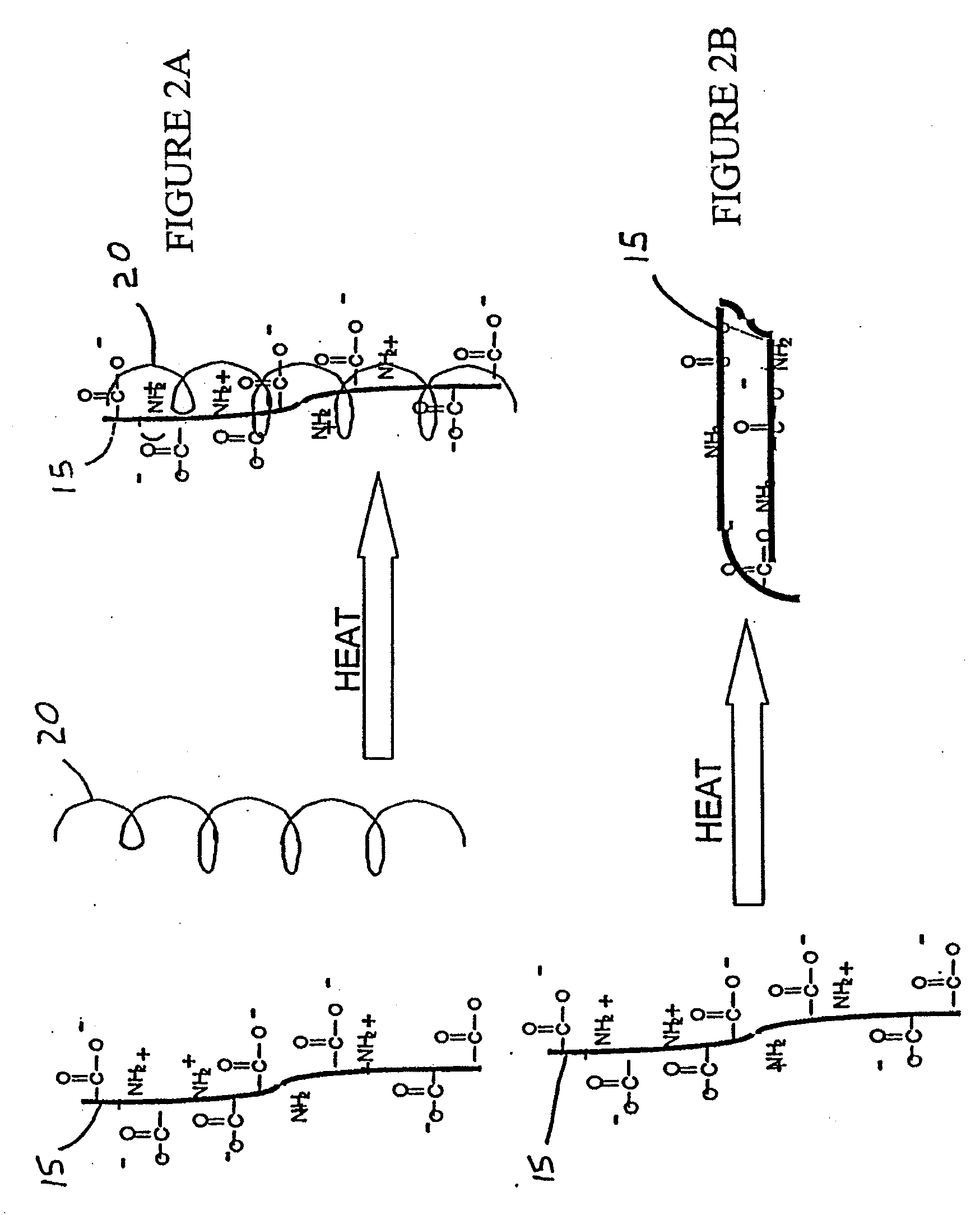
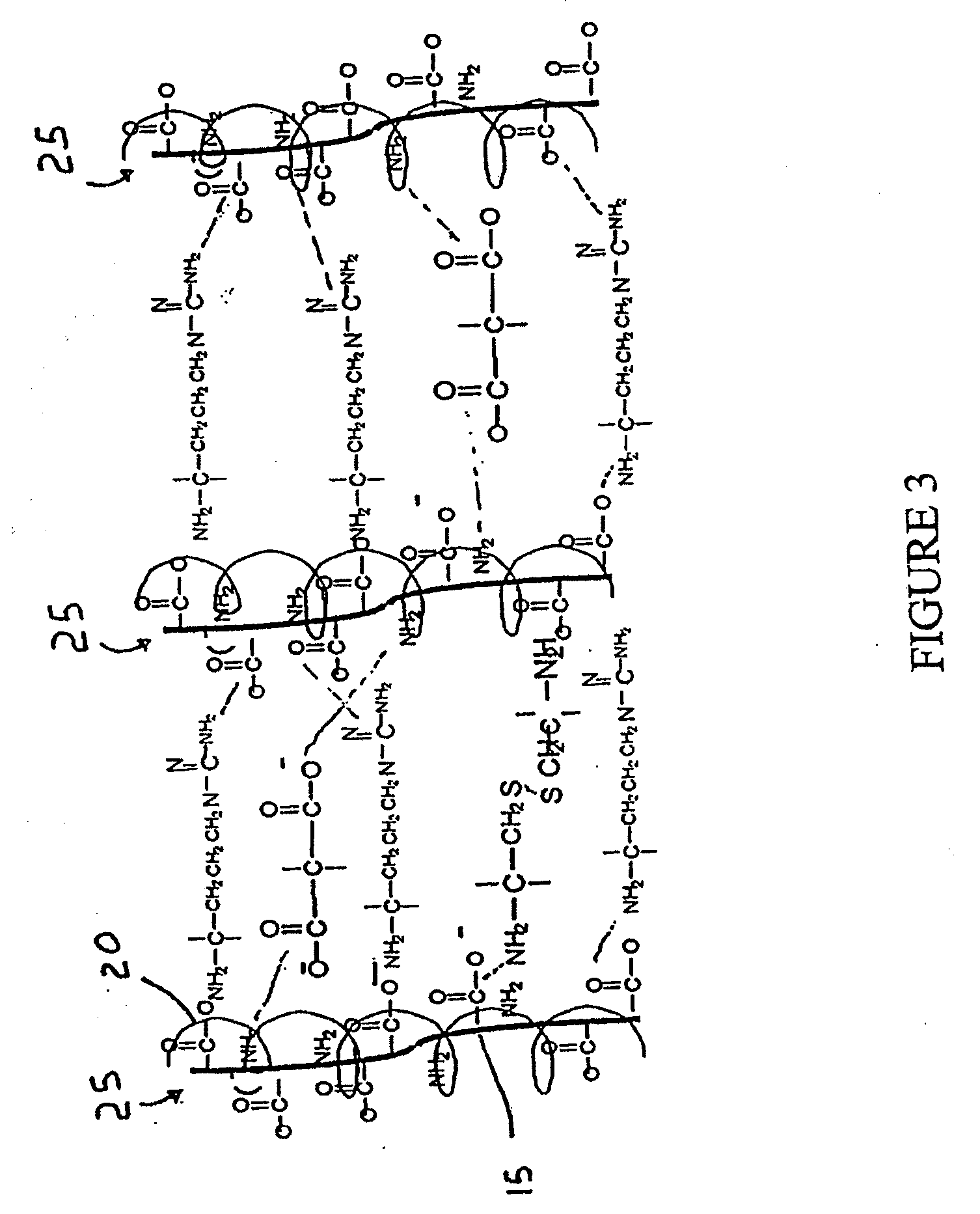
Immobilized bioactive hydrogel matrices as surface coatings
InactiveUS7303814B2Improves tissue integrationImprove intimate contactSurgerySkeletal disorderHydrogel matrixMedical device
The present invention is directed to a stabilized bioactive hydrogel matrix coating for substrates, such as medical devices. The invention provides a coated substrate comprising a substrate having a surface, and a bioactive hydrogel matrix layer overlying the surface of the medical device, the hydrogel matrix comprising a first high molecular weight component and a second high molecular weight component, the first and second high molecular weight components each being selected from the group consisting of polyglycans and polypeptides, wherein at least one of the first and second high molecular weight components is immobilized (e.g., by covalent cross-linking) to the surface of the substrate.
Owner:PIONEER SURGICAL TECH INC
Gel composition for cellular adhesion inhibition
InactiveUS20070031498A1Avoid stickingInhibiting adhesion of cellPowder deliveryBiocideAdhesion processPolyethylene glycol
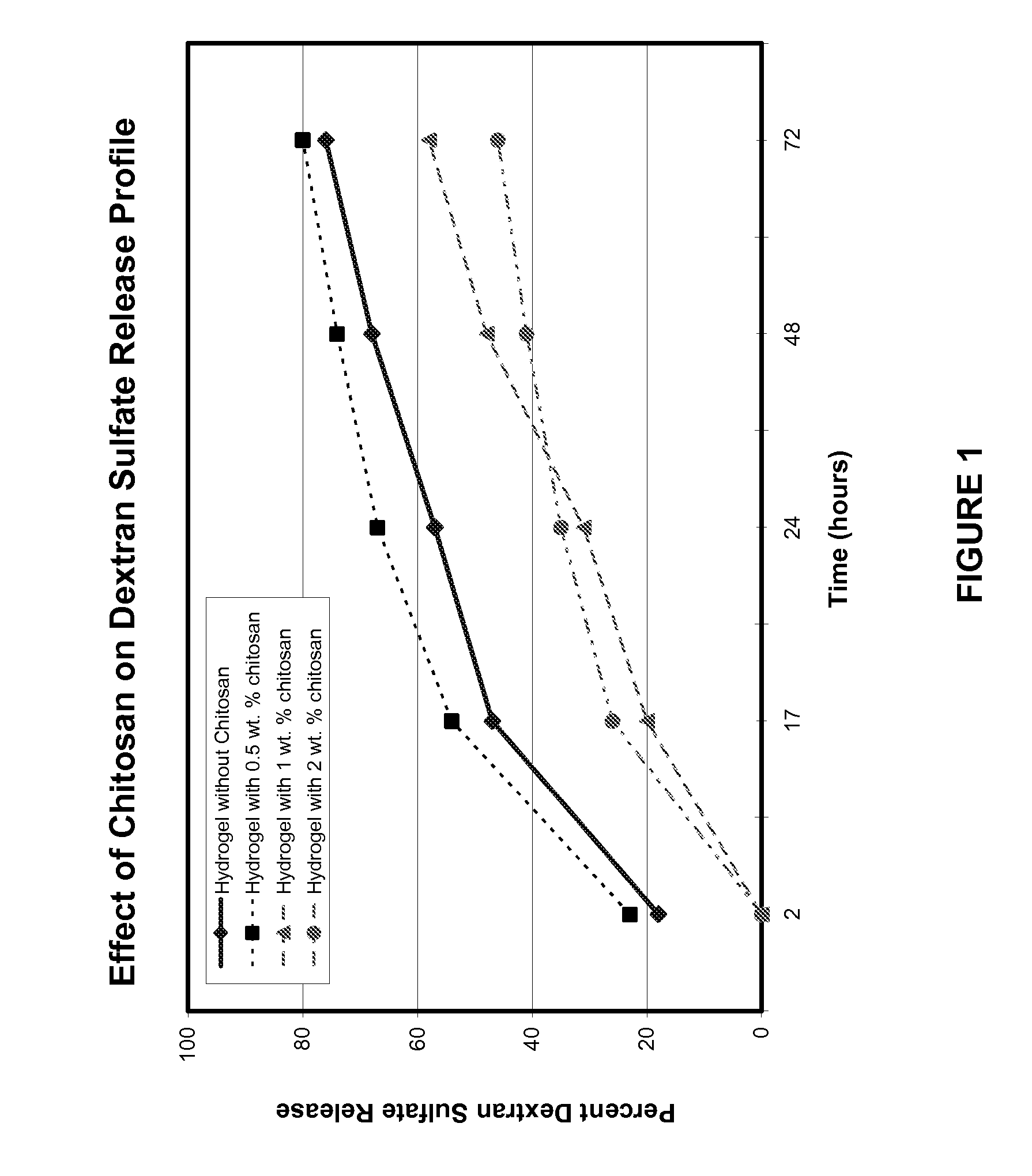
The invention includes compositions for inhibiting cellular adhesion, methods of preparation of such compositions, and methods for preventing cell adhesion at a surgical site comprising application of such compositions. The compositions generally comprise a cellular adhesion inhibitory agent, such as dextran sulfate, and a crosslinked hydrogel matrix, preferentially physically entrapping the adhesion inhibitory agent. The hydrogel matrix can include a first gel component, such as an electrophilically functionalized polyethylene glycol polymer, and at least one additional gel component, preferably nucleophilically functionalized, and preferentially selected from the group consisting of polyethylene glycol polymers, polypeptides, and polysaccharides. The compositions are useful for delivering the cellular adhesion inhibitory agent to a site in need of adhesion inhibition and providing either immediate or metered delivery of the inhibitory agent.
Owner:TRIAD
Tunable sustained release of a sparingly soluble hydrophobic therapeutic agent from a hydrogel matrix
ActiveUS20100291191A1Improve solubilityGood effectAntibacterial agentsBiocideSolubilityMethyl cellulose
The incorporation of polymeric excipients into an injectable hydrogel matrix, for example, methyl cellulose in the case of a hydrogel matrix comprising hyaluronan and methylcellulose (HAMC) has been found to increase the solubility of sparingly soluble hydrophobic drugs and tune their rate of release. The hydrogel matrix may also include other sparingly soluble hydrophobic food or cosmetic agents.
Owner:THE GOVERNINIG COUNCIL OF THE UNIV OF TORANTO
Bioactive wound dressings and implantable devices and methods of use
The present invention provides wound dressings, optionally surgically implantable, containing biodegradable, polymers and hydrogels having allogenic or autologous precursor cells, such as stem cells and progenitor cells dispersed within. Alternatively, the wound dressings can have conditioned medium obtained from the precursor cells dispersed within. The wound dressings promote tissue restoration processes at a site of application or implantation. Additional bioactive agents can also be dispersed within the polymer / hydrogel matrix, which can be formulated to biodegrade at a controlled rate by adjusting the composition. Methods are also provided for using such biodegradable wound dressings as a delivery device or carrier for the precursor cells, conditioned medium and bioactive agents, or as coatings on implantable medical devices, to promote tissue restoration at a lesion site.
Owner:MEDIVAS LLC
Porous non-biodegradable hydrogel admixed with a chemoattractant for tissue replacement
InactiveUS20090017096A1Facilitate in vivo tissue ingrowthEasy to integrateBone implantPolyesterTissue defect
A non-biodegradable hydrogel matrix containing microspheres of a biodegradable polymer for the purpose of treating, repairing, or replacing damaged biological tissue is described. The biodegradable phase can be admixed with a chemoattractant. Examples of degradable polymers include degradable polyesters such as 50:50 PLA:PGA, the degradation profiles of which are well characterized. The matrix is permanently inserted into a tissue defect to provide mechanical support before, during, and after tissue ingrowth.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY +1
Methods and Compositions for Regenerating Connective Tissue
Connective tissue regenerative compositions and methods of repairing and regenerating connective tissue using such compositions are provided. The compositions generally comprise a bioactive hydrogel matrix comprising a polypeptide, such as gelatin, and a long chain carbohydrate, such as dextran. The hydrogel matrix may further include polar amino acids, as well as additional beneficial additives. Advantageously, the compositions include further components, such as osteoinductive or osteoconductive materials, medicaments, stem or progenitor cells, and three-dimensional structural frameworks. The compositions are useful for regenerating connective tissue, and can be administered to an area having injury to, or a loss of, connective tissue, such as bone, cartilage, tendon, and ligament.
Owner:PIONEER SURGICAL TECH INC
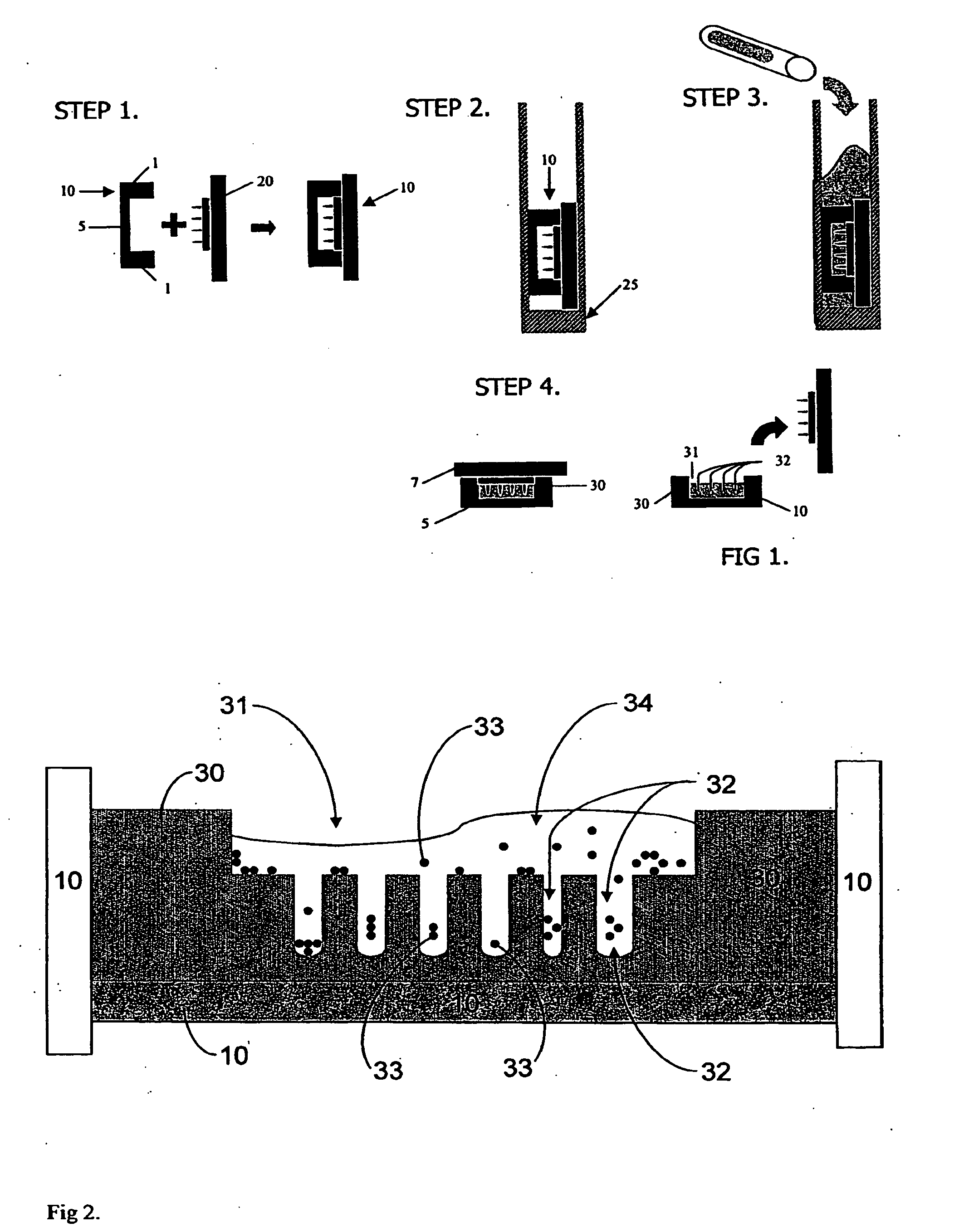
Cell Aggregation and Encapsulation Device and Method
ActiveUS20090018033A1Improve adhesionEnhanced interactionBioreactor/fermenter combinationsBiological substance pretreatmentsCell adhesionAdhesion process
The invention is a cell aggregation device comprising a hydrogel substrate having at least one, preferably a plurality, of cell-repellant compartments recessed into the uppermost surface. Each compartment is composed of an upper cell suspension seeding chamber having an open uppermost portion and a bottom portion, and one, or more than one, lower cell aggregation recess connected to the bottom portion of the upper cell suspension seeding chamber by a port. The diameter of the port may be fully contiguous with the walls of the chambers and walls of the recesses, or the diameter of the port may be more narrow than the walls of the chamber but fully contiguous with the walls of the recesses or more narrow than both the walls of the chamber and the walls of the recesses. The upper cell suspension seeding chambers are formed and positioned to funnel the cells into the lower cell aggregation recesses through gravitational force. The aggregation recesses are formed and positioned to promote cellular aggregation by coalescing cells into a finite region of minimum gravitational energy, increasing intercellular contact and minimizing or preventing cell adherence to the substrate. A device for encapsulating aggregates of live cells is provided. The device comprises (i) a biocompatible, bio-sustainable substrate having a cell-encapsulating face composed of one or more biocompatible, bio-sustainable, spaced-apart, cell-encapsulating compartments extending therefrom and (ii) a coating layer composed of a biocompatible, bio-sustainable polymer that completely surrounds the substrate and the cell-encapsulating compartments. A method for making the device is also provided.
Owner:BROWN UNIVERSITY
Methods and compositions for regenerating connective tissue
InactiveUS20080145404A1Halting progressionAntibacterial agentsPowder deliveryProgenitorLigament structure
Owner:PIONEER SURGICAL TECH INC
Tissue Augmentation With Active Agent For Wound Healing
The invention relates to a bioactive settable hydrogel matrix having a pore creating material, which may also carry a therapeutic agent, and methods of using the same, for example, use in promoting internal wound healing, tissue repair, tissue regeneration.
Owner:WARSAW ORTHOPEDIC INC
Plaster for topical use containing heparin and diclofenac
Plaster for topical use having an analgesic activity and at the same time being able to re-absorb haematomas, comprising:a substrate layer;an adhesive layer in the form of a hydrogel matrix containing a pharmaceutically acceptable diclofenac salt, heparin or a heparinoid;a protective film which can be removed at the moment of use.
Owner:ALTERGON
Method for preparing aquogel matrix for ultraviolet light three-dimensional (3D) printing
InactiveCN104861216AOvercome polymerization inhibitionImprove printing efficiencyCell adhesionPolyvinyl alcohol
The invention relates to a method for preparing an aquogel matrix, in particular to a method for preparing the aquogel matrix for ultraviolet light three-dimensional (3D) printing and belongs to the technical field of preparation of biological materials. An alkenyl group is ingrafted on a chitosan molecular chain, a sulfydryl group is ingrafted on polyvinyl alcohol, and mixing is performed to obtain the aquogel matrix for ultraviolet light 3D printing. According to the aquogel matrix, sulfydryl-alkene 'click' reaction characteristic is fully utilized, gel forming is rapidly achieved under ultraviolet irradiation, inhibition of oxygen in air or a system can be fully overcome, 3D printing efficiency is greatly increased, cytocompatibility of the aquogel matrix is good, enough supporting environment is provided for cell growth, and cell adhesion, growth and proliferation can be facilitated. The preparation method is simple and low in costs, and industrial production is easy.
Owner:WUHAN TEXTILE UNIV
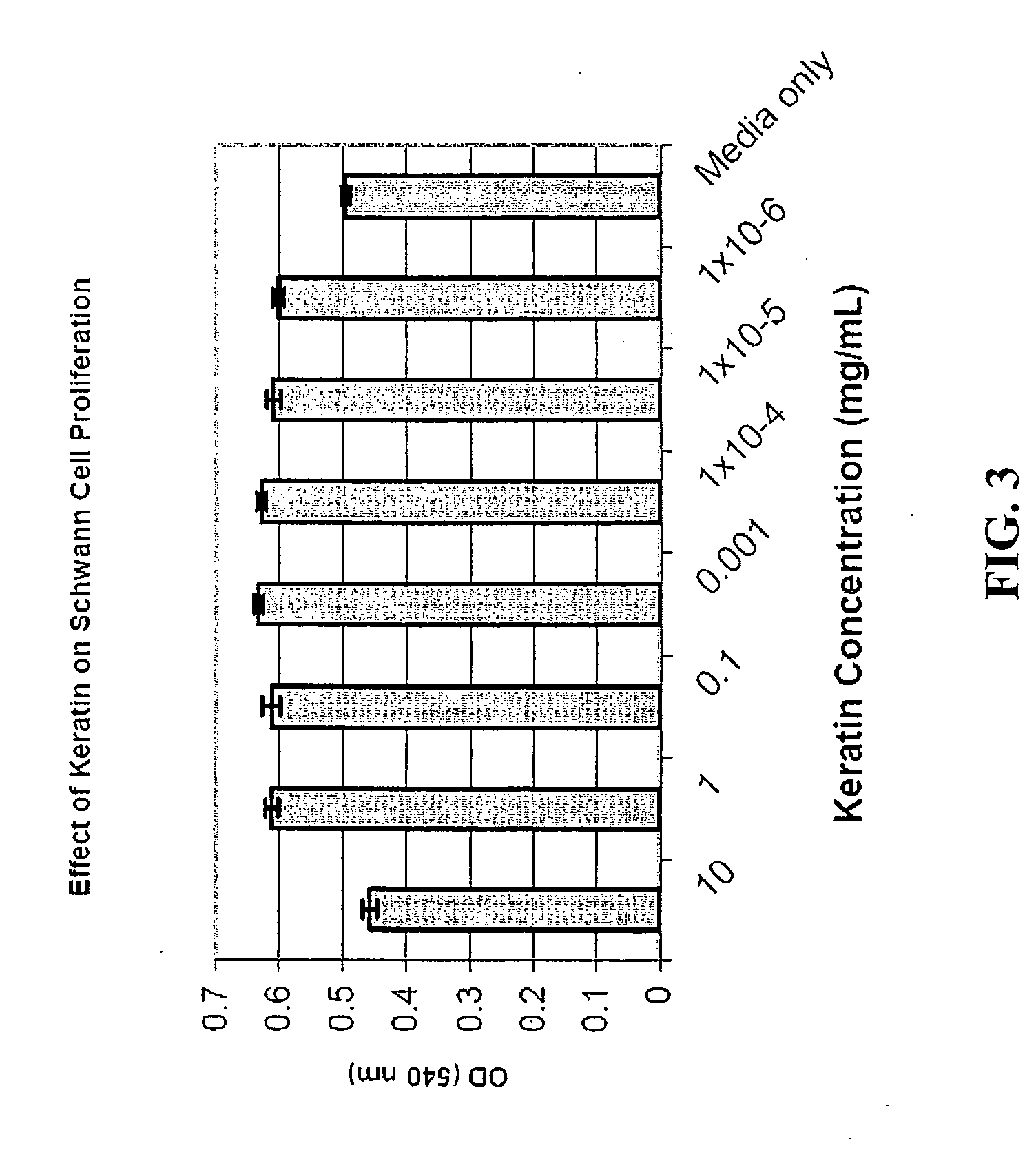
Nerve regeneration employing keratin biomaterials
A keratin hydrogel matrix serves as an effective acellular scaffold for axonal regeneration and facilitates functional nerve recovery.
Owner:WAKE FOREST UNIV HEALTH SCI INC
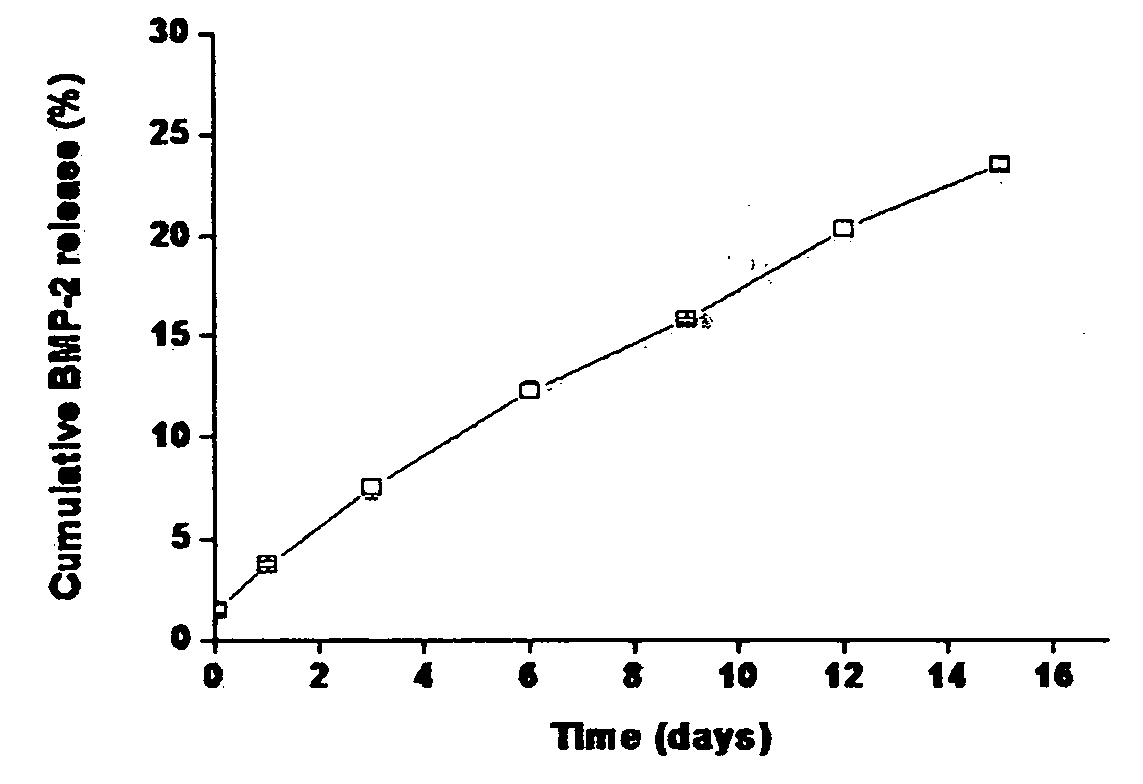
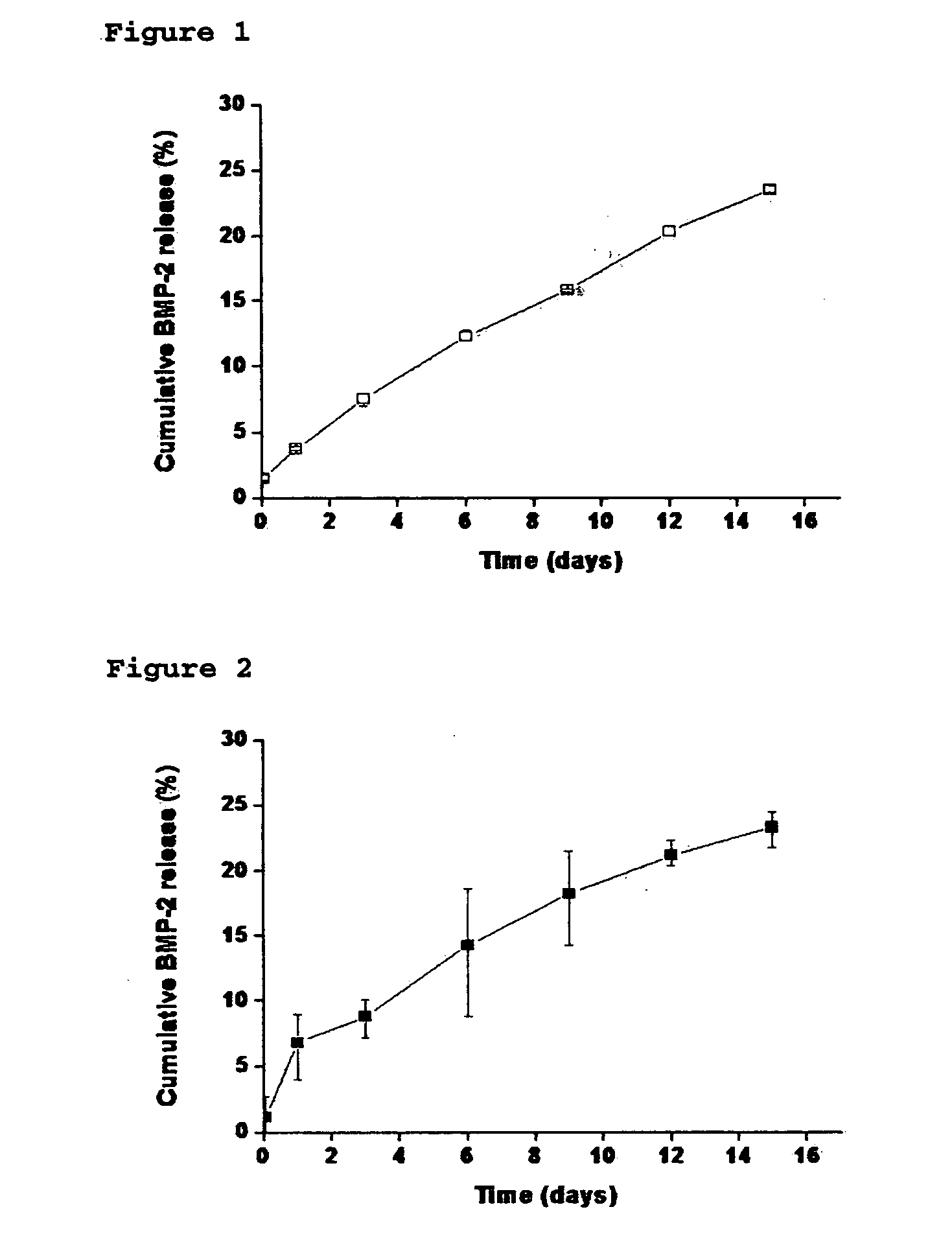
Composite comprising polysaccharide-functionalized nanoparticle and hydrogel matrix, a drug delivery system and a bone defect replacement matrix for sustained release comprising the same, and the preparation method thereof
InactiveUS20070248675A1Powder deliveryPeptide/protein ingredientsFunctionalized nanoparticlesUnit mass
The present invention relates to a nanoparticle-protein-hydrogel composite comprising (1) a polysaccharide-functionalized nanoparticle comprising a core composed of a biodegradable polymer, a hydrogel surface layer composed of a biocompatible polymer emulsifier, and a polysaccharide physically bound to the core and / or the hydrogel layer; (2) a protein forming a specific binding with the polysaccharide; and (3) a hydrogel matrix composed of a biocompatible polymer as a matrix for the nanoparticle. The present also relates to a drug delivery system and a bone defect replacement matrix comprising the composite for sustained release, and the preparation method thereof. Further, the present invention also provides a method for controlling the release rate of a protein drug by changing the content of the polysaccharide in a unit mass of the nanoparticle and / or by changing the content of the nanoparticle in a unit mass of the composite.
Owner:GWANGJU INST OF SCI & TECH
Polyphenol substance hydrogen bond enhanced hydrogel
PendingCN111286045AHigh mechanical strengthReduce the degradation rateAerosol deliveryOintment deliveryTissue repairPharmaceutical drug
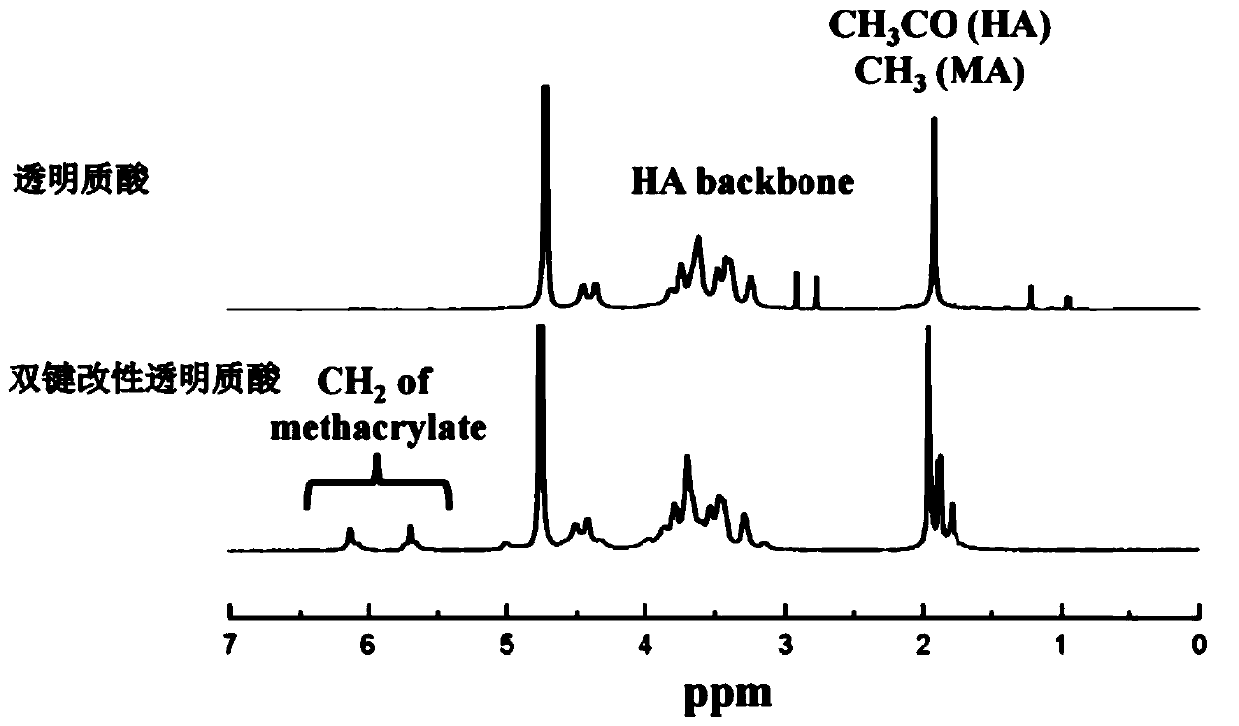
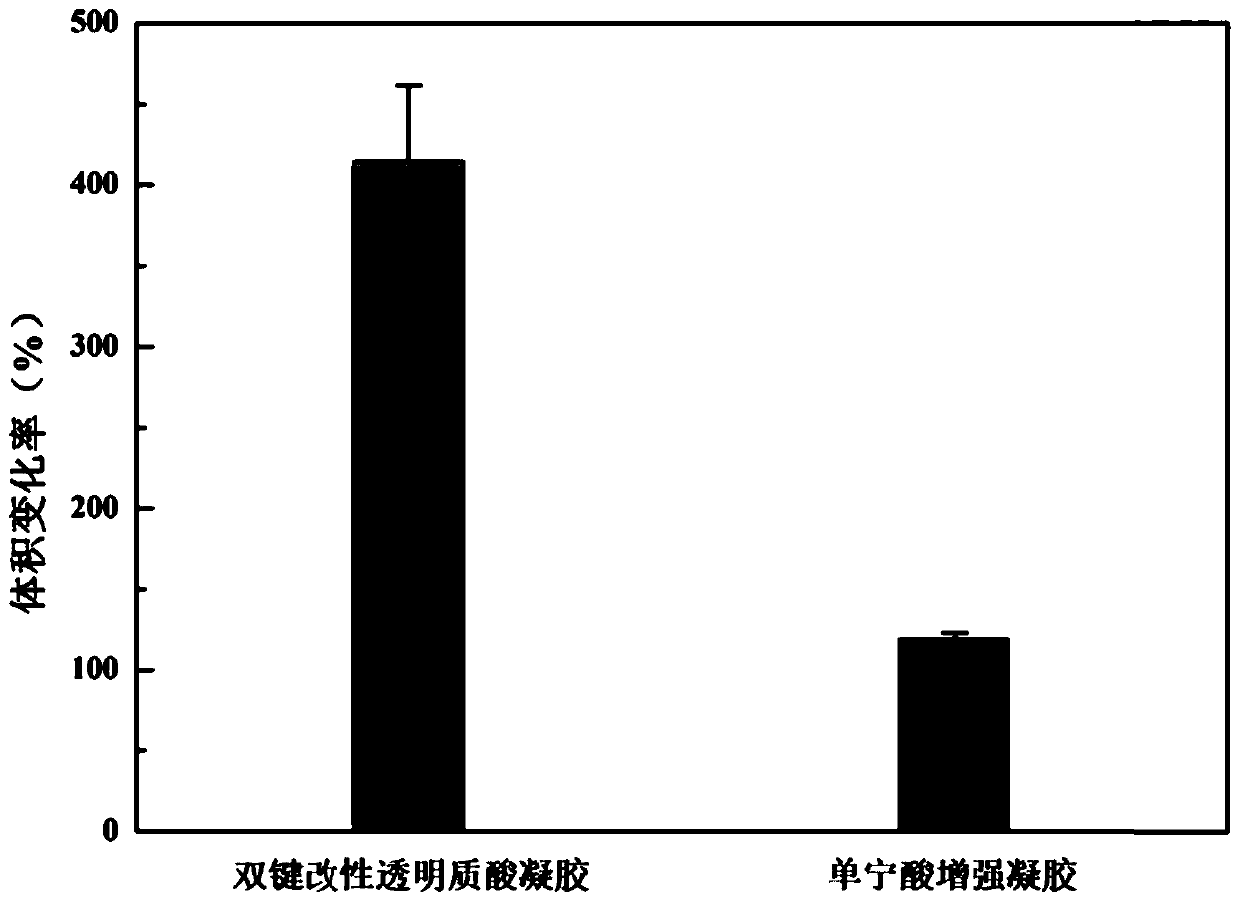
The invention discloses polyphenol substance hydrogen bond enhanced hydrogel. The polyphenol substance hydrogen bond enhanced hydrogel is formed by hydrogen bond crosslinking of polyphenol substancesand hydroxyl-rich hydrogel. A preparation method of the polyphenol substance hydrogen bond enhanced hydrogel comprises the following steps: 1) crosslinking a hydroxyl-rich hydrogel matrix to obtain gel; and 2) soaking the gel in an alkaline solution of polyphenol substances, and carrying out a cross-linking reaction. The polyphenol substance hydrogen bond enhanced hydrogel can be used as a drug carrier or a tissue repair medical material. The mechanical strength of the polyphenol substance hydrogen bond enhanced hydrogel is remarkably improved, the degradation rate is greatly reduced, the comprehensive performance is remarkably improved, and the application prospect is wide.
Owner:GUANGDONG PROV MEDICAL INSTR INST
Method of stimulation hair growth
The invention provides a method of stimulating hair growth, comprising administering a therapeutic amount of a hydrogel matrix to an intradermal or subdermal site where hair growth is desired, the matrix composition comprising gelatin, such as denatured collagen, and a long chain carbohydrate, such as dextran. The matrix may further include polar amino acids, nitric oxide inhibitors and super oxide inhibitors. Injection is a preferred method of administration.
Owner:PIONEER SURGICAL TECH INC
Hybrid target analyte responsive polymer sensor with optical amplification
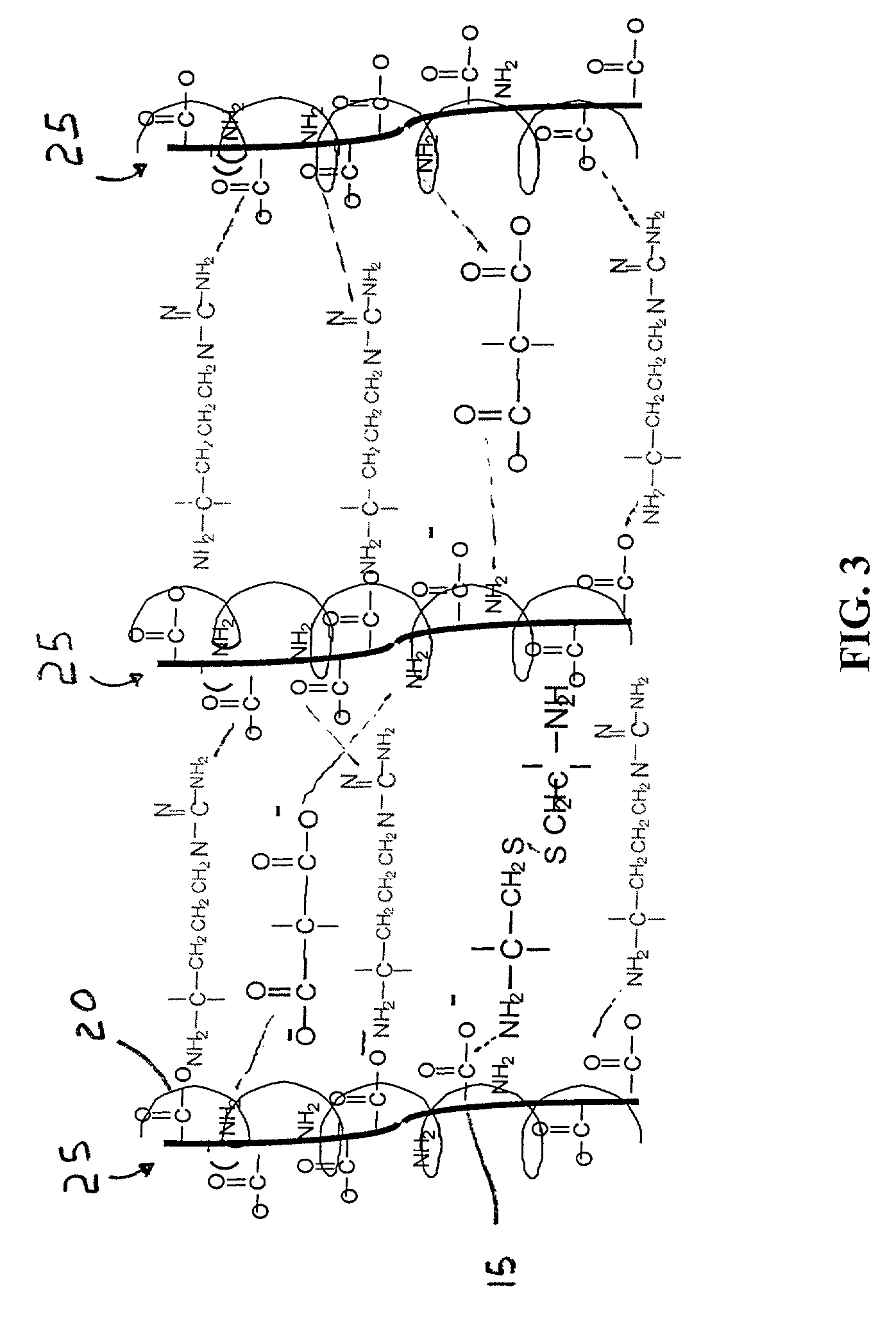
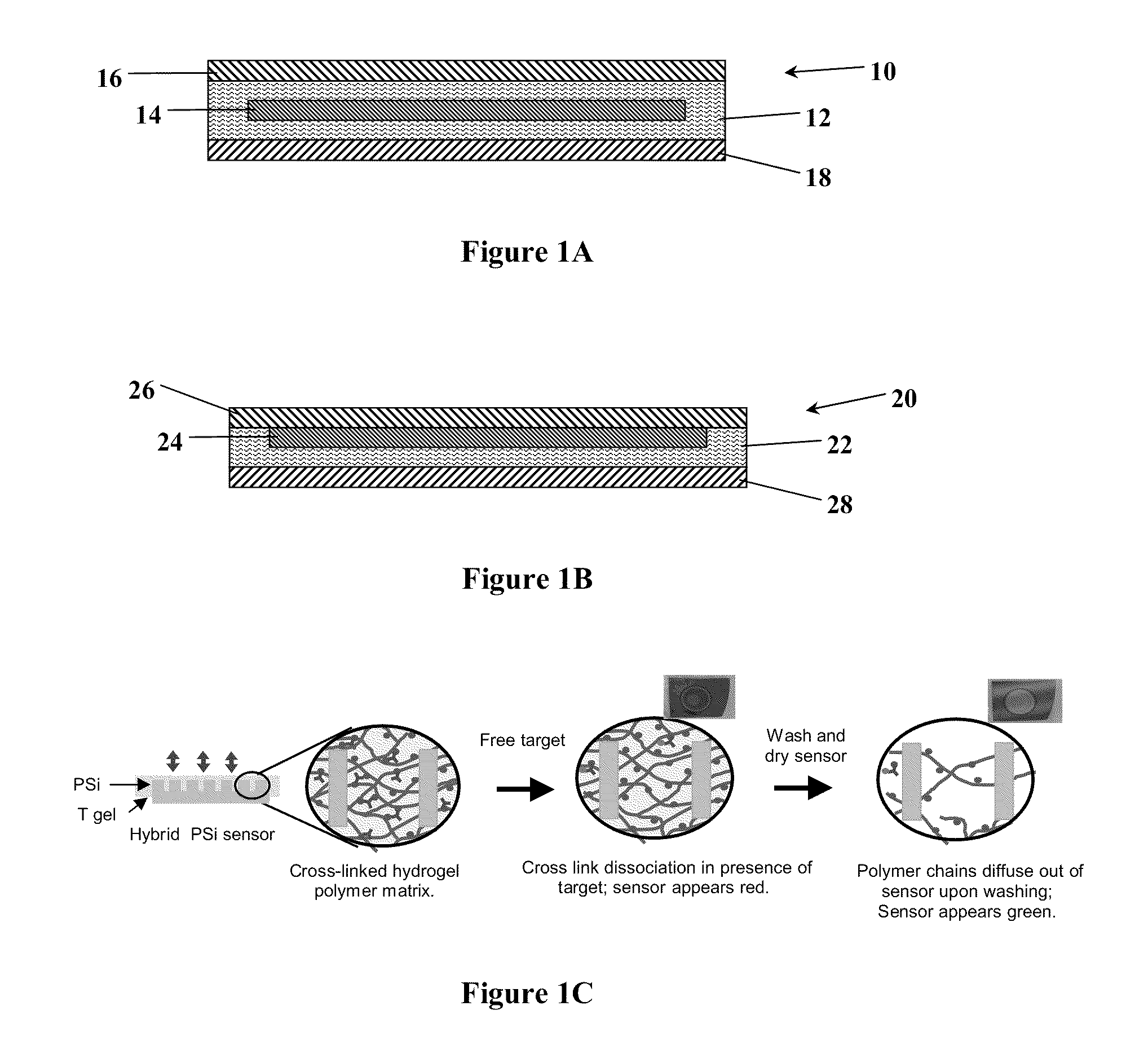
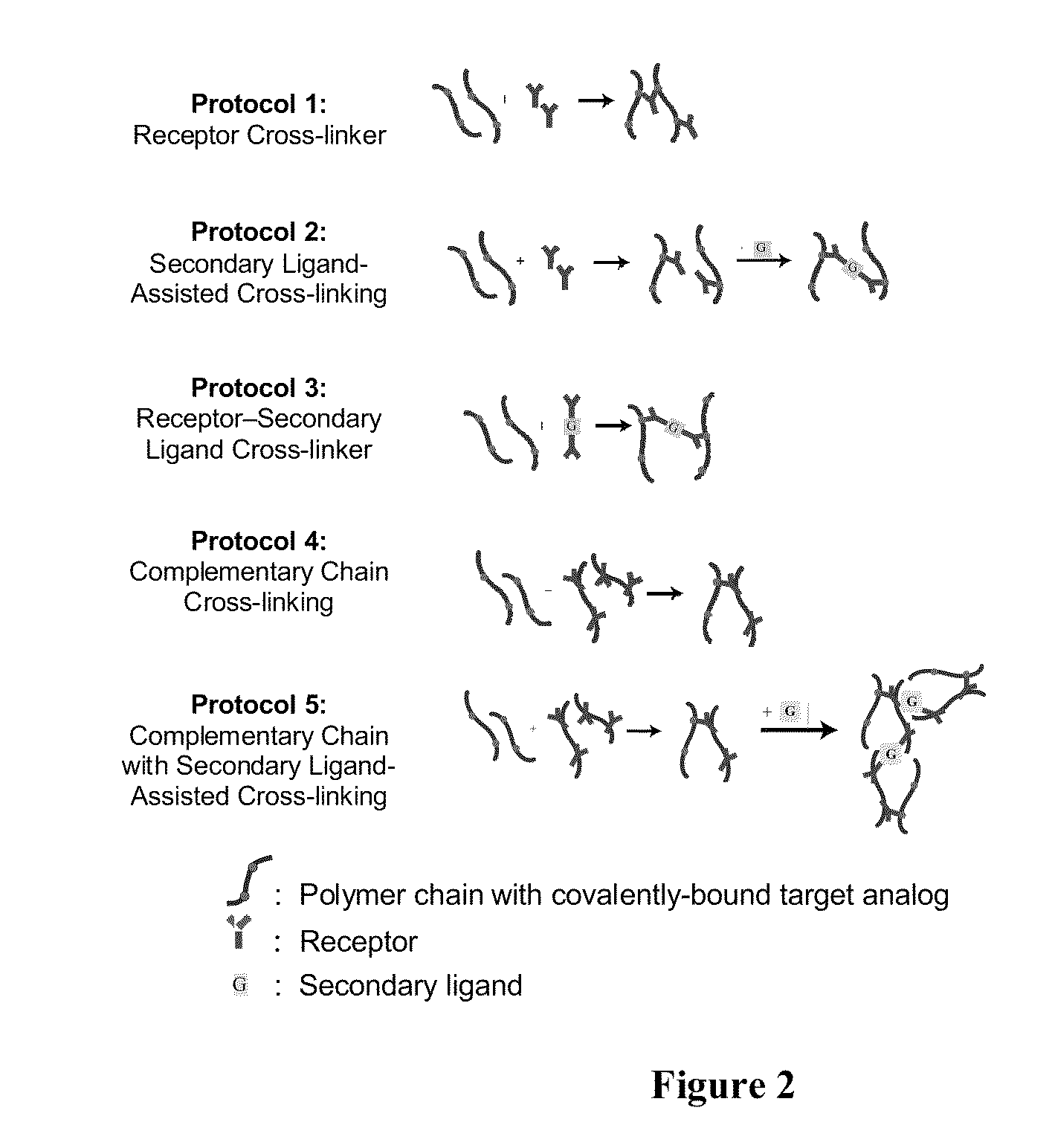
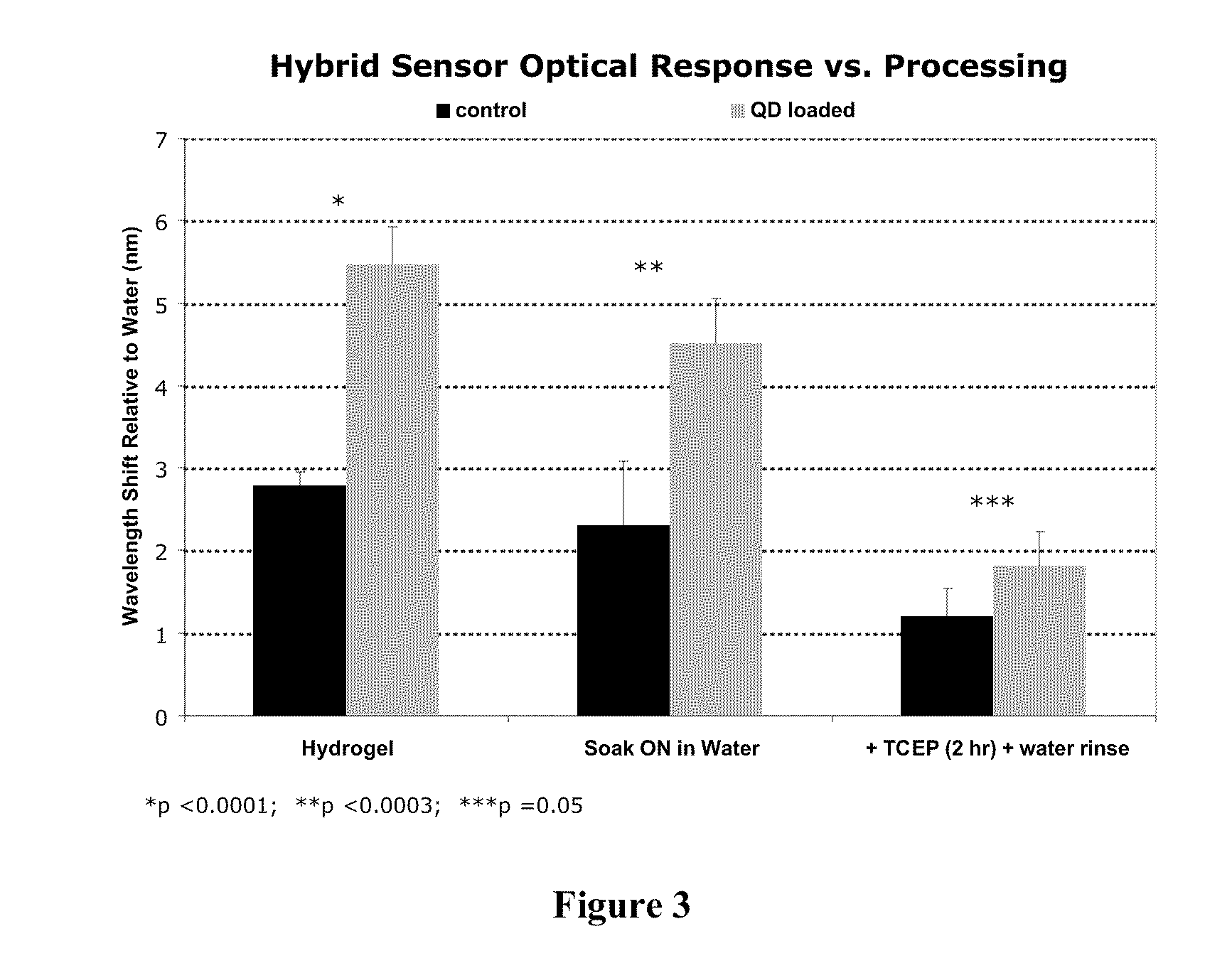
ActiveUS20110212463A1Favorable aqueous environmentIncrease the number ofBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteNanoparticle
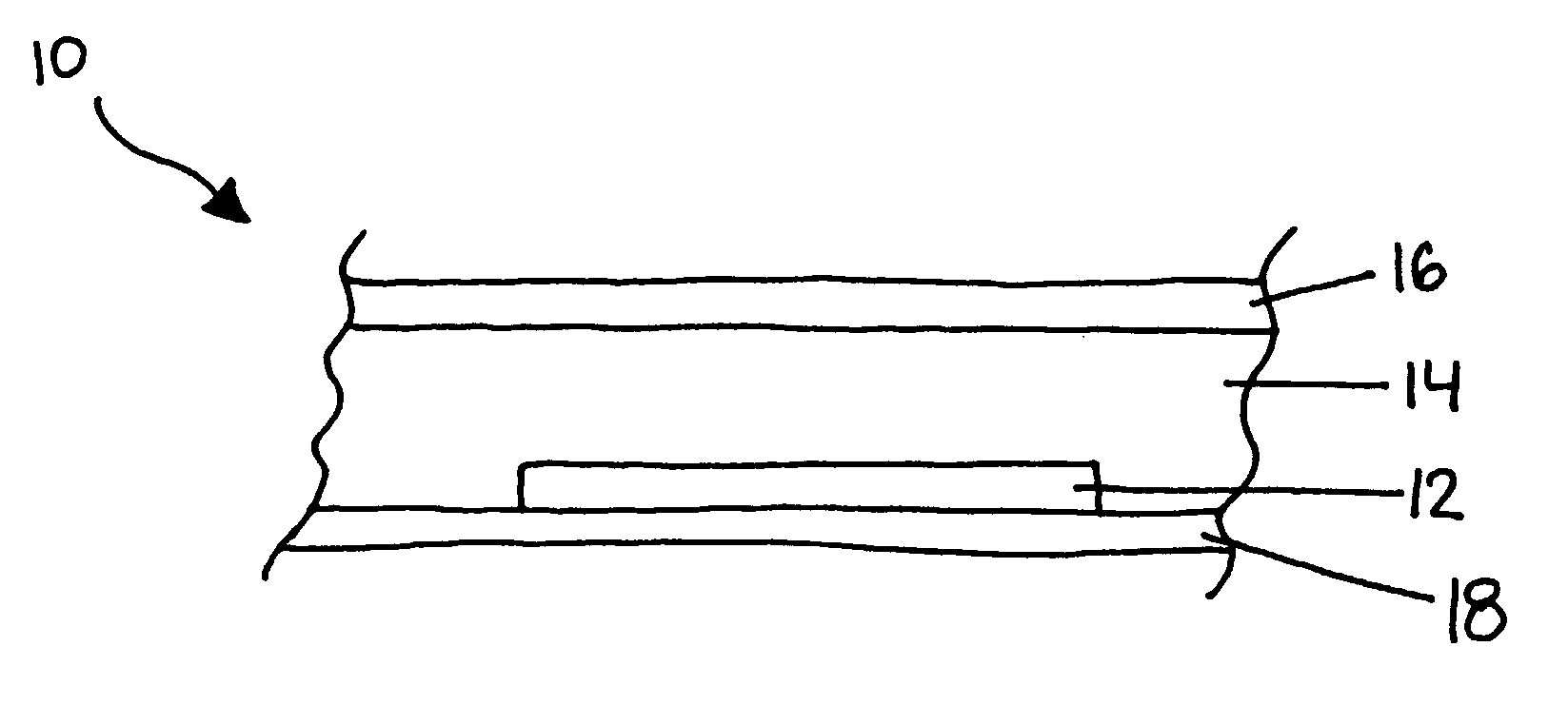
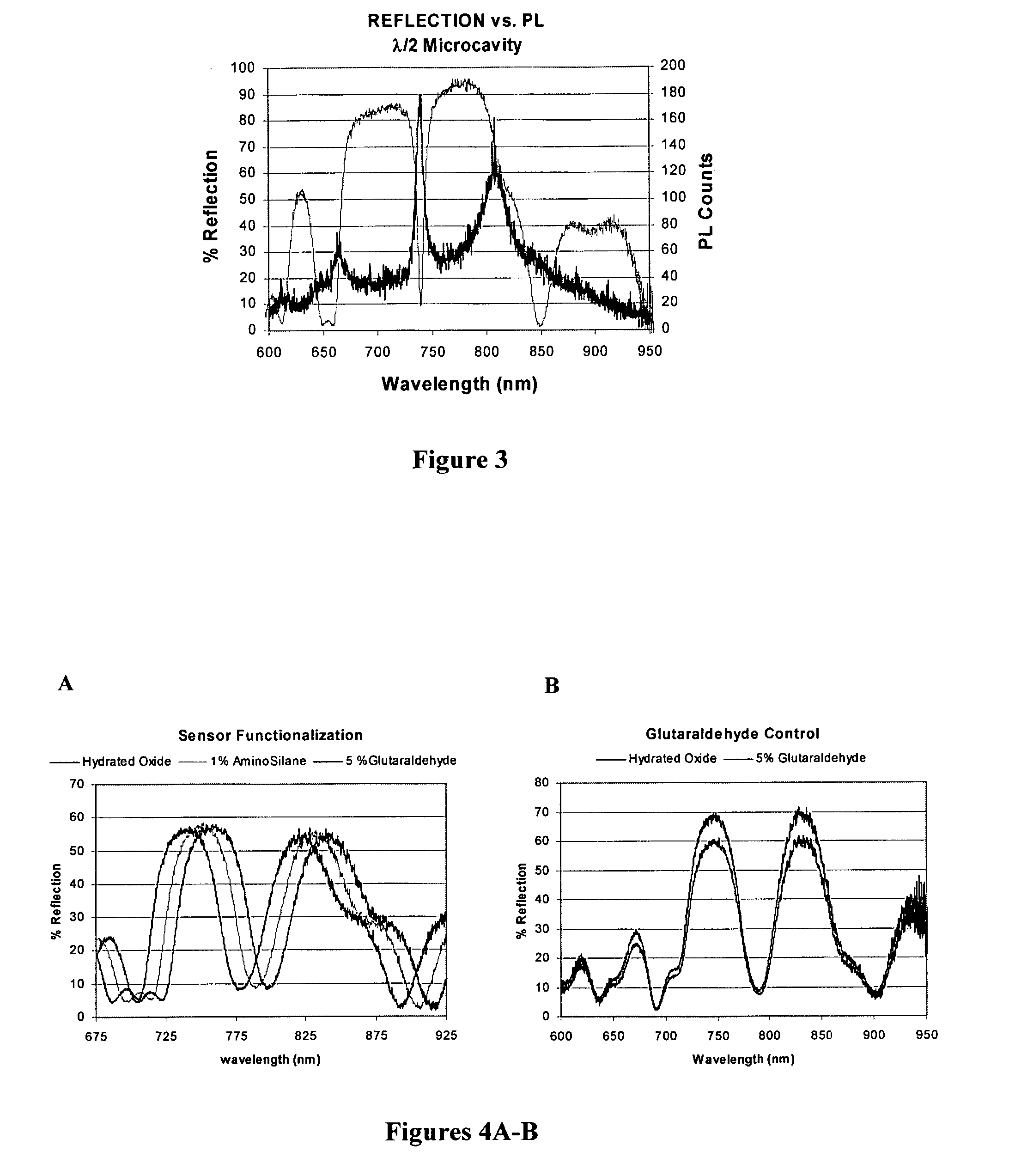
Disclosed is a product that includes an optical sensor; a target-responsive hydrogel matrix on a surface of the optical sensor (where the hydrogel matrix comprises one or more target-specific receptors and one or more target analogs), and one or more high refractive index nanoparticles within the hydrogel matrix, where a detectable change occurs in a refractive index of the hydrogel matrix when contacted with one or more target molecules. Sterile packages and detection devices containing the product, and methods of detecting a target molecule using the product, are also disclosed.
Owner:UNIVERSITY OF ROCHESTER
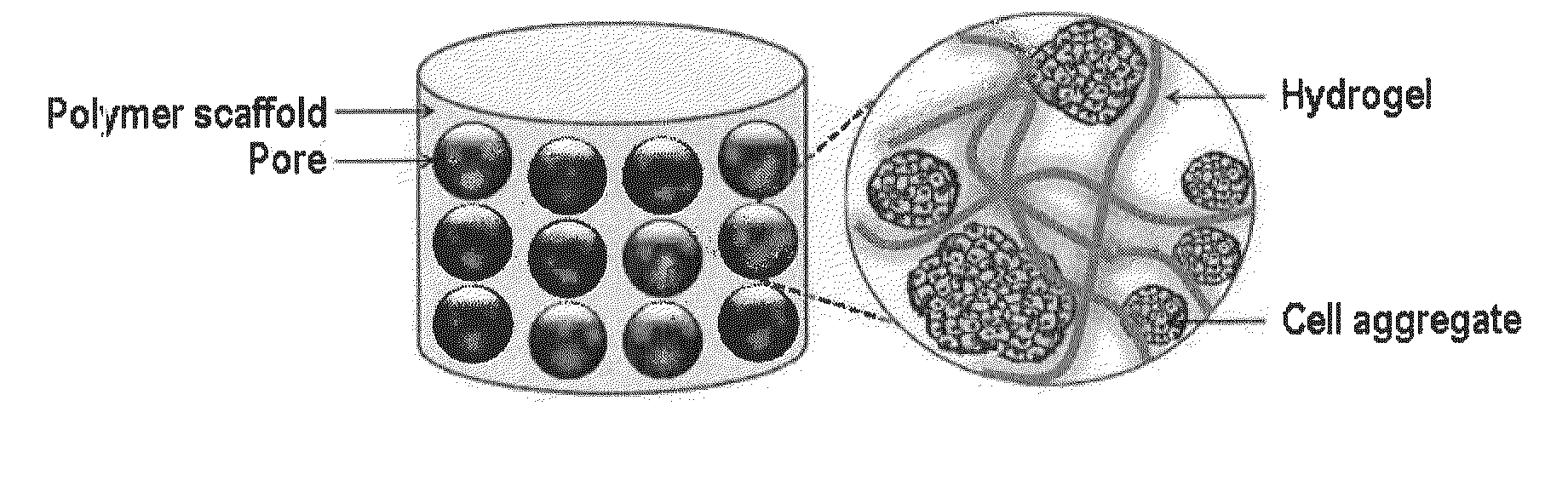
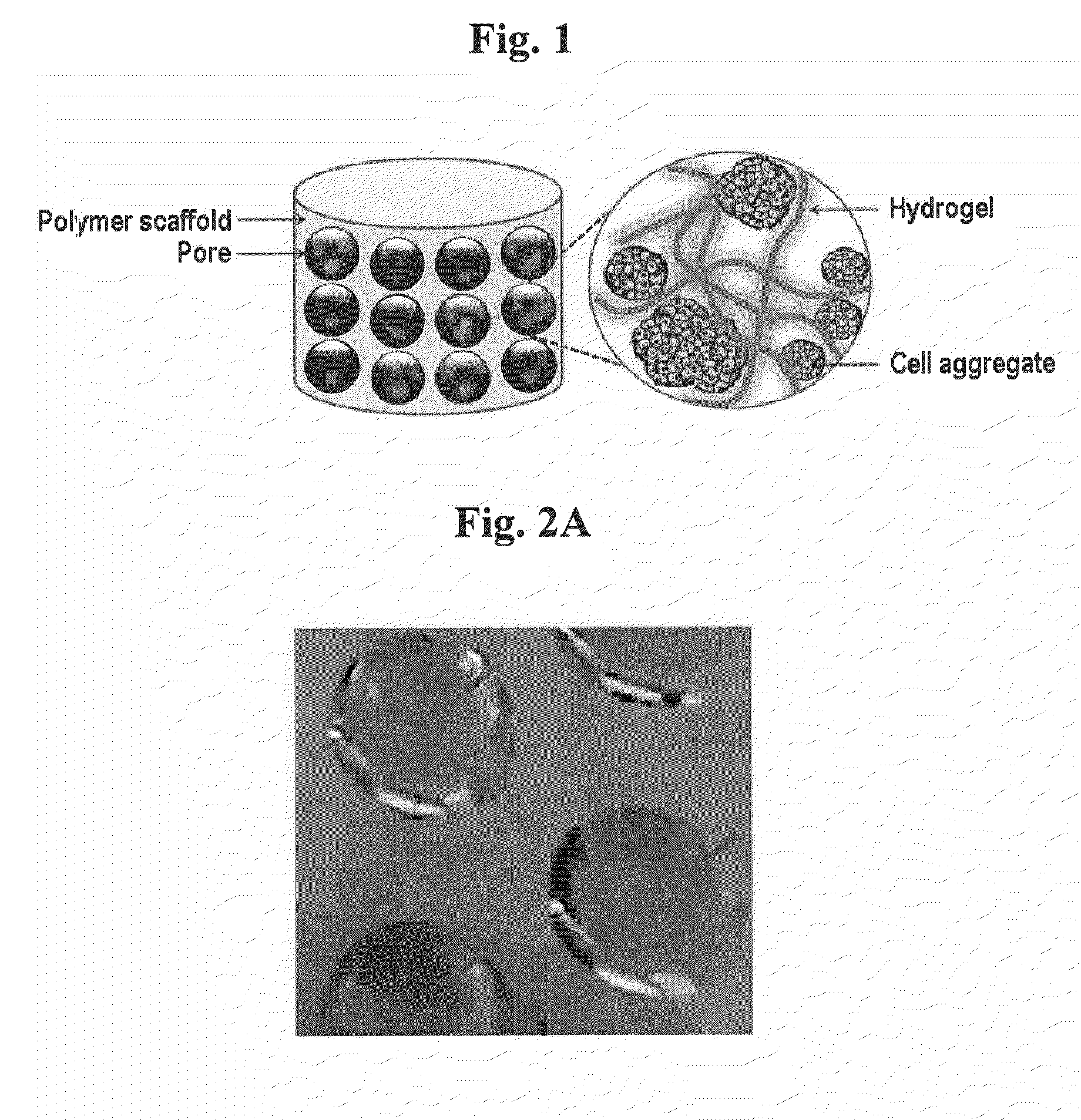
Cell aggregate-hydrogel-polymer scaffold complex for cartilage regeneration, method for the preparation thereof and composition comprising the same
InactiveUS20100120149A1Increase flexibilityHigh mechanical strengthCell differentiationCulture processHydrogel matrixPolymer scaffold
The present invention relates to a cell aggregate-hydrogel-polymer scaffold complex useful for cartilage regeneration which has a structure in which cell aggregates of differentiated chondrocytes are evenly dispersed in a hydrogel matrix, and the resulting hydrogel matrix is immobilized onto the surface of a polymer scaffold while filling up the pores thereof. Since the cell aggregate-hydrogel-polymer scaffold complex according to the present invention can efficiently induce the regeneration of cartilage tissue similar to natural cartilage and retain high mechanical strength, flexibility, and uniform morphology during the cartilage regeneration, it can be effectively used as a cartilage therapeutic agent for the repair of cartilage defects and injuries.
Owner:KOREA INST OF SCI & TECH
Hydrogel patch and preparation method thereof
ActiveCN101926833AMeet the requirements of adhesionHydroxy compound active ingredientsAntipyreticCross-linkPolyvinyl alcohol
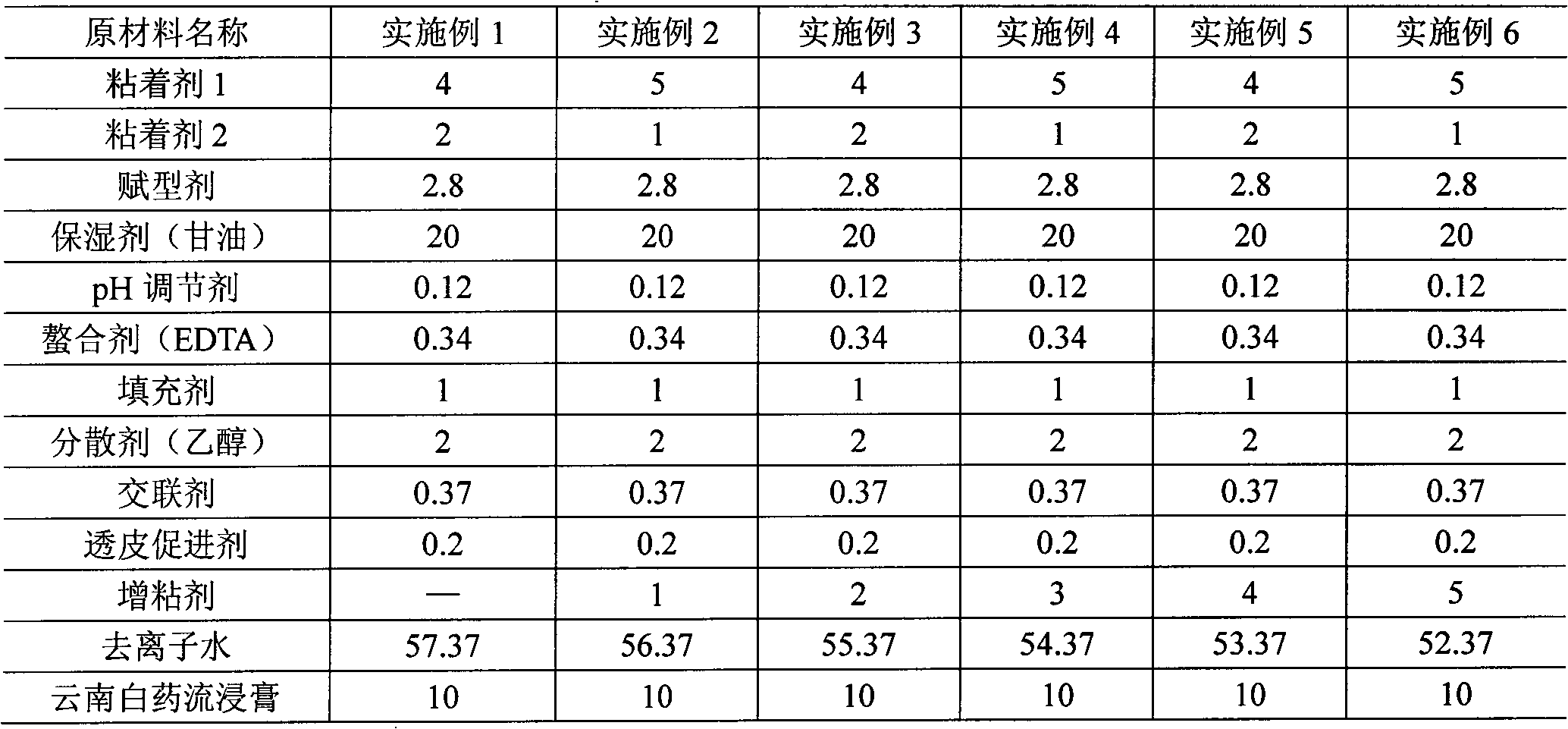
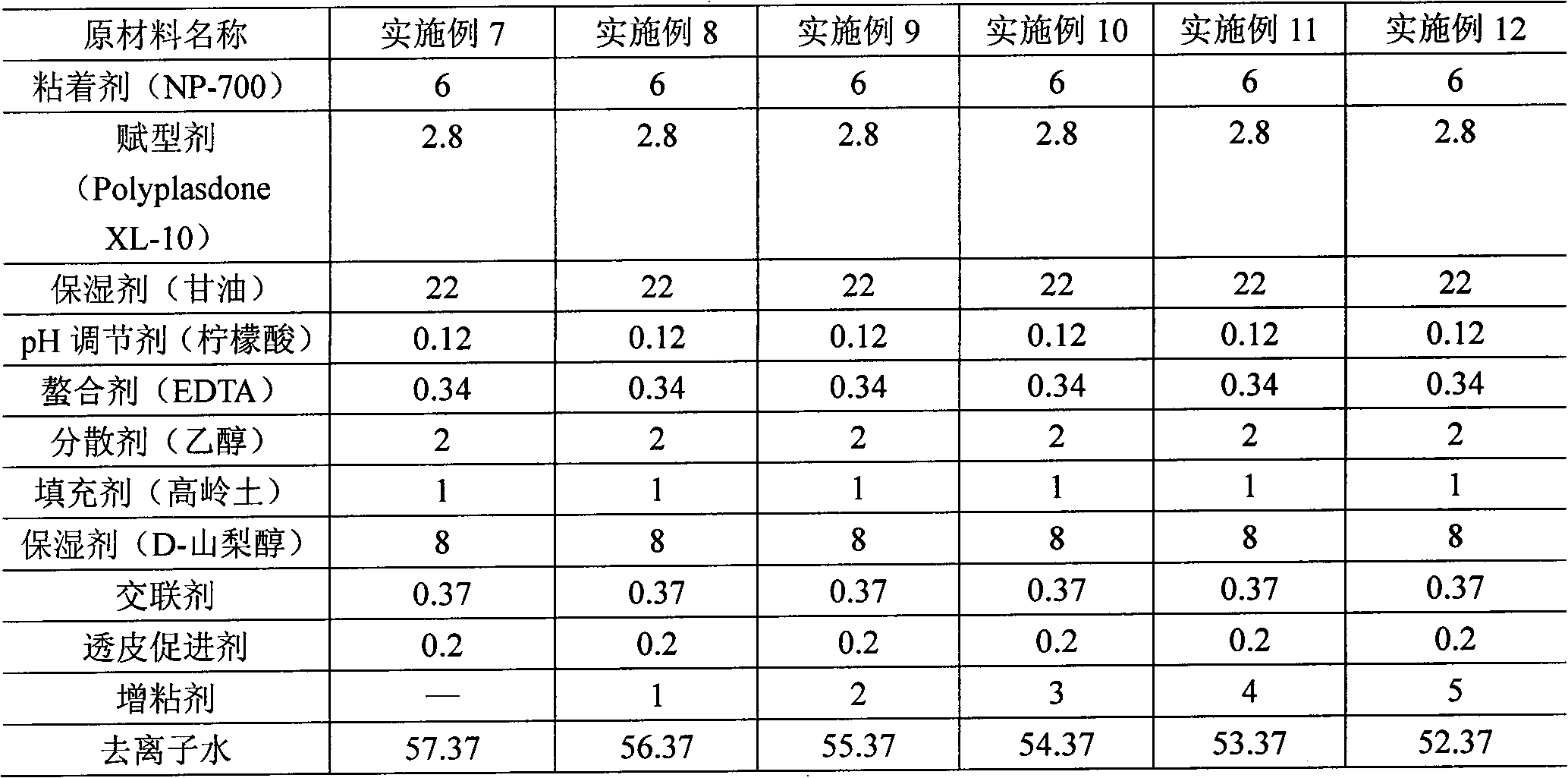
The invention relates to a hydrogel patch and a preparation method thereof. The hydrogel patch consists of three parts: bidirectional flexible non-woven fabric, medicament-containing paste and a cover film layer, wherein the main part, namely the medicament-containing paste is prepared from fluid extract and proper hydrogel matrixes such as an adhesive, a humectant, an excipient, a pH modifying agent, a cross-linking agent, a transdermal enhancer, a filler, a dispersant, a chelating agent, a tackifier and deionized water according to a proportion, wherein the tackifier is at least one of polyvinyl alcohol, povidone, gelatin, carboxymethyl cellulose, collagen, modified starch and plant polysaccharide, or is emulsion formed by polymerizing free radicals of 0-30 percent acrylic acid, 0 to 50 percent vinyl pyrrolidone and 0 to 50 percent 2-acrylamido-2-methylpropanesulfonic acid, and is added in an amount which is 5 to 15 percent based on the total weight. The hydrogel patch solves the common problem of poor viscosity of common hydrogel, and has the characteristics of high adhesion, high compliance with skin, quick response, reusability, moisture retention, permeability and comfortable use.
Owner:云南白药集团无锡药业有限公司
Hydrogel materials and discloses polypyrrole nano composite conductive hydrogel and preparation method thereof
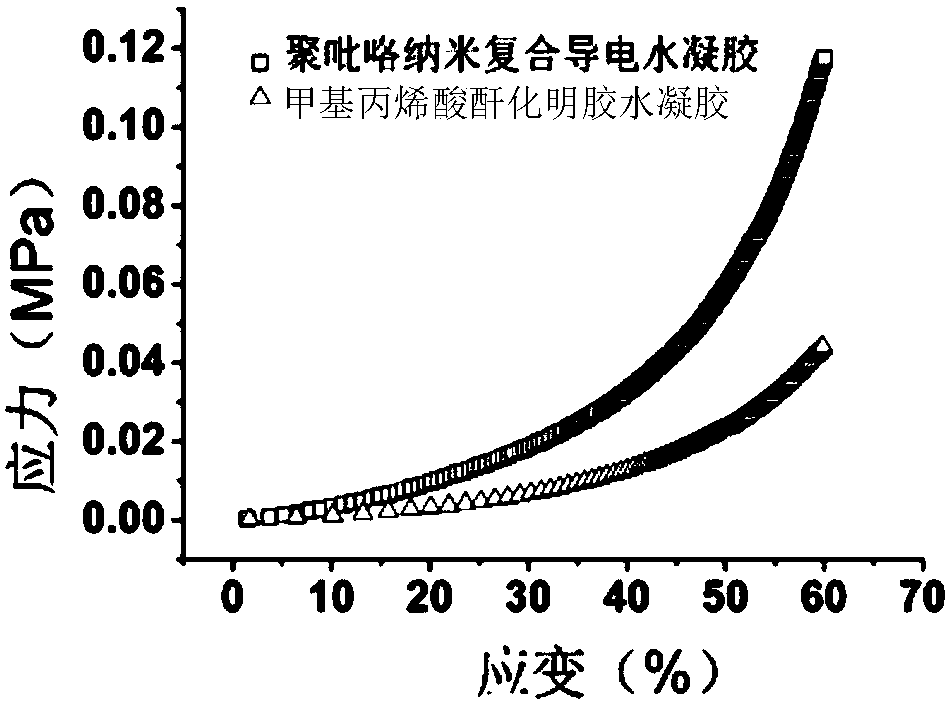
The invention belongs to the technical field of hydrogel materials and discloses polypyrrole nano composite conductive hydrogel and a preparation method thereof. The method comprises the following steps of a, using pyrrole as a monomer and dopamine as a dopant for in-situ chemical oxidative polymerization to prepare a polypyrrole nano material; b, uniformly mixing the polypyrrole nano material, modified gelatin and a photoinitiator in a solvent, and carrying out ultraviolet light initiation polymerization to obtain the polypyrrole nano composite conductive hydrogel. The modified gelatin is obtained by modifying gelatin with methacrylic anhydride. According to the hydrogel material, by modifying polypyrrole with dopamine, the polypyrrole nano material is obtained; the polypyrrole nano material can be well dispersed in a hydrogel matrix material and meanwhile can stably exist under the hydrogen bond action with hydrogel molecules. The prepared conductive hydrogel has excellent conductivity and stable chemical property, and the mechanical property of the hydrogel is improved.
Owner:SOUTH CHINA UNIV OF TECH
Bioactive wound dressings and implantable devices and methods of use
Owner:MEDIVAS LLC
Clay-based hydrogel matrix for three-dimensional printing and preparation method application thereof
InactiveCN106633121AGood biocompatibilityReduce dosageAdditive manufacturing apparatusTissue regenerationAdditive ingredientPolyvinyl alcohol
The invention provides a clay-based hydrogel matrix. The clay-based hydrogel matrix is prepared from, by mass, 10-50% of cross-linking agent, 3-20% of inorganic clay, 0.05-0.1% of ultraviolet light initiator and 30-86% of water, wherein the total mass of the ingredients is 100%, the crosslinking agent is biologically compatible macromolecules with carbon-carbon double bonds, the biologically compatible macromolecules are one or more of polyethylene glycol, polyvinyl alcohol, chitosan, gelatin and hyaluronic acid. The clay-based hydrogel matrix is simple in formula, safe in ingredients and appropriate in viscosity, has a certain pre-formed shape, is suitable for continuously extruded three-dimensional scaffold printing and can achieve that printing is prior to curing for gelling and three-dimensional scaffolds are printed in a large-scale mode, and the three-dimensional scaffold printing efficiency is greatly improved. The invention further provides a preparation method based on the clay-based hydrogel matrix and application.
Owner:SHENZHEN INST OF ADVANCED TECH
Therapeutics dispensing device and methods of making same
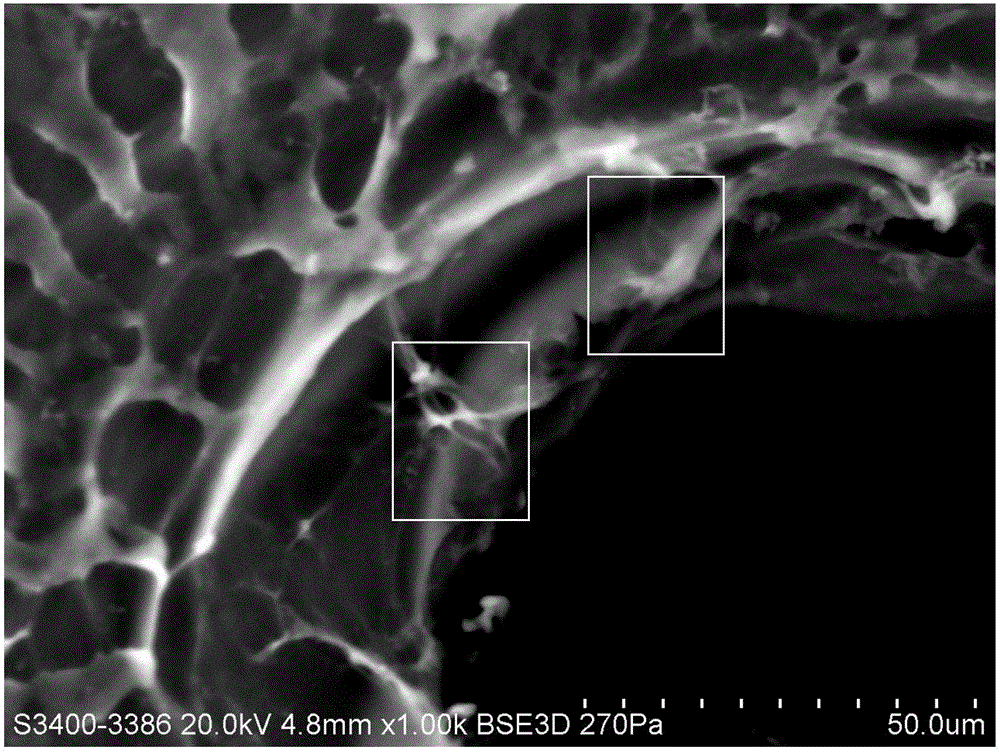
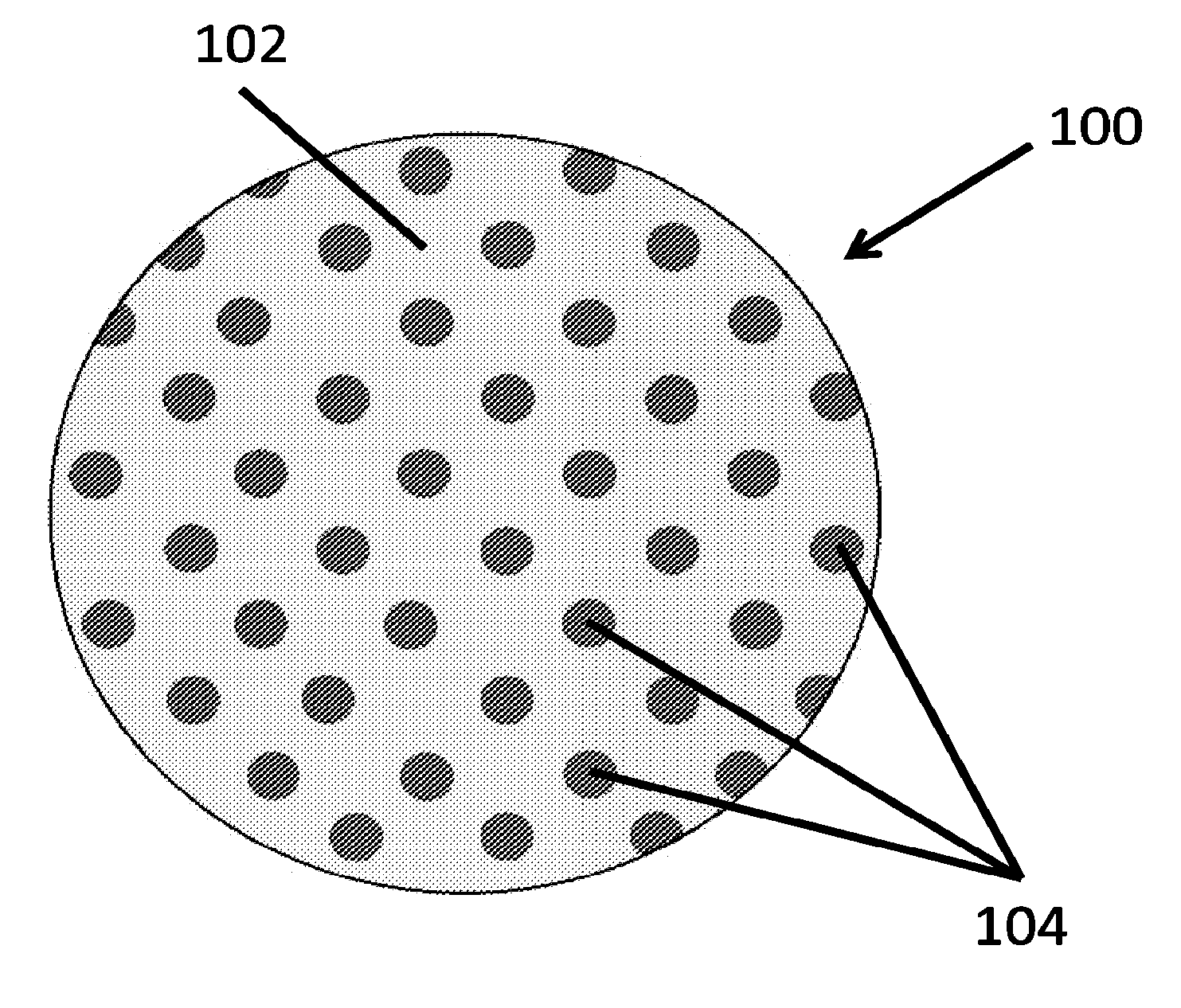
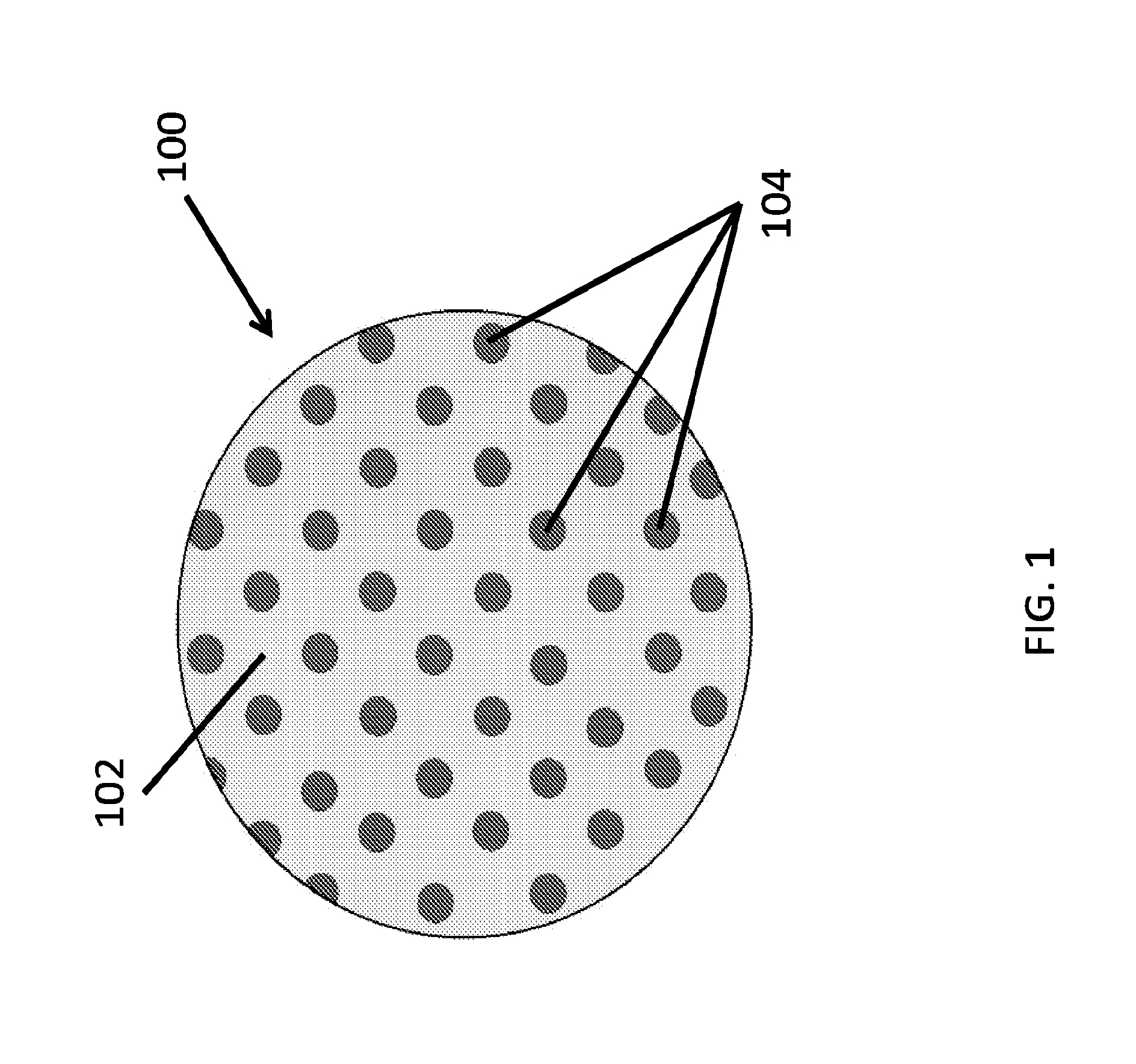
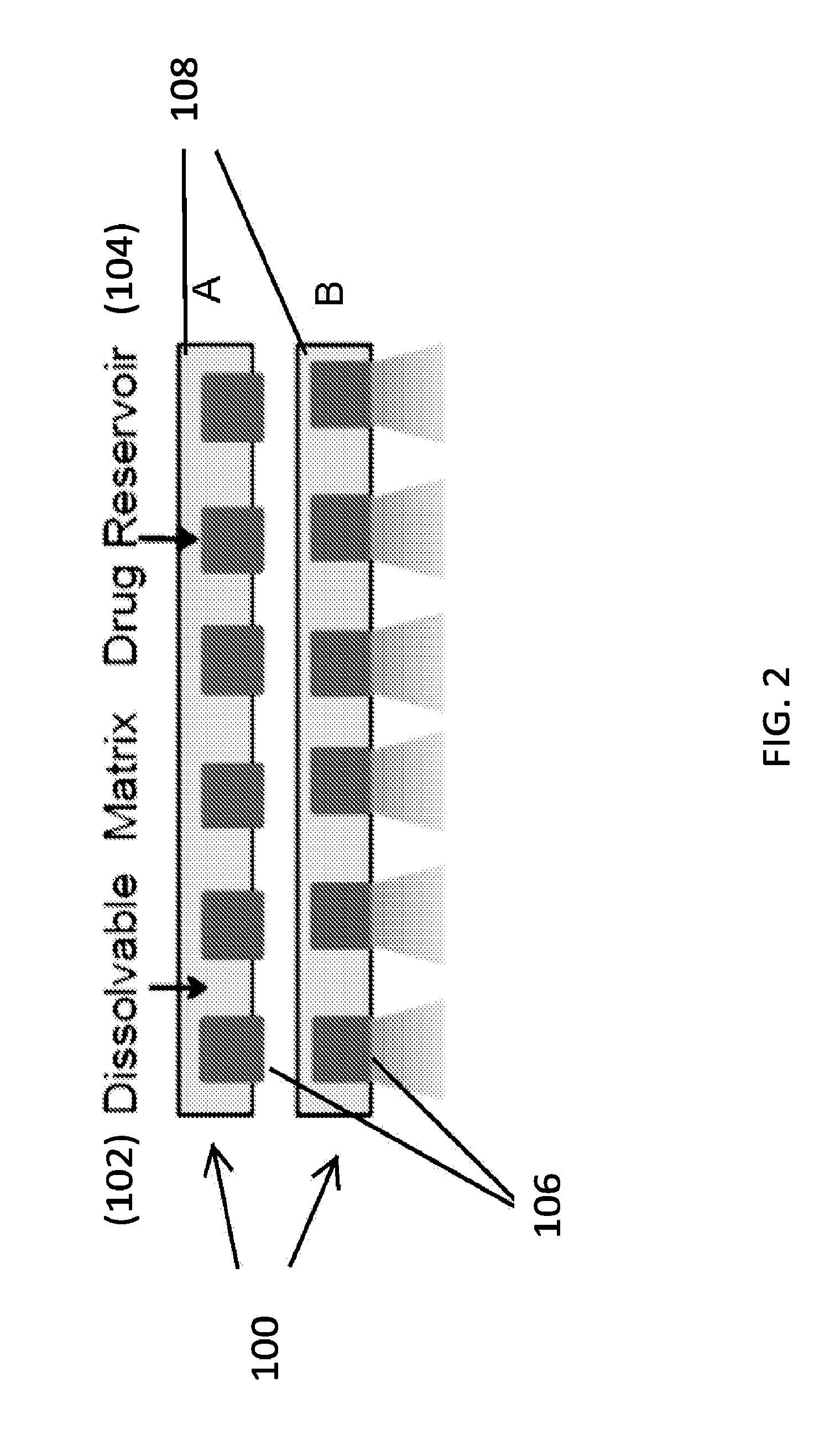
A therapeutics delivery system, and methods of making and using same, are disclosed for environments that rapidly clear any injected therapeutics, such as a patient's eye. The therapeutics delivery system releases the drug in a therapeutically effective concentration for a desired duration of time with a predefined drug kinetics. In one embodiment, the embodiments of the present disclosure release a therapeutically effective concentration for a longer time period than other delivery systems, for instance from a day to a week. Certain embodiments comprise a therapeutics dispensing device comprising a biodissolvable hydrogel matrix for long term drug release that allows the device to be placed directly at the injured site, e.g., onto the surface at or near the injury, and retained there rather than through injection, whether locally or systematically.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Hydrogel-supported porous semiconductor devices
InactiveUS20070184222A1Easy to manageAddress bad outcomesDiagnostics using lightSynthetic resin layered productsHydrogel matrixWound site
A product including a hydrogel matrix and a porous semiconductor material at least partially embedded within the hydrogel matrix. Also disclosed are methods of making the product and a substantially flexible porous semiconductor material, as well as methods of using the product to deliver a therapeutic agent to a subject and detecting the presence of a pathogen and / or infection at a wound site.
Owner:UNIVERSITY OF ROCHESTER
Instant gelling agent for treating allergic rhinitis
InactiveCN101015559AExtended stayOrganic active ingredientsPharmaceutical delivery mechanismNasal cavityGel preparation
The invention belongs to the field of western medicinal preparation, and relates to a preparation with suitable phase variation, detention time prolonging effects and topically administered in nasal cavity, and its preparation method. The method comprises dispersing momestasone furoate or budesonide in hydrophilic gel substrate prepared from acetyl-removed gellan gum and sodium alginate in micro powder form. the gel preparation is prepared and stored in solution from, and administered in solution spraying form; generates phase variation due to the change of environmental ionic strength, pH, or temperature after contacting with tunica mucosa of nasal cavity, and forms gel. The invention has the advantages of simple preparation and accurate administered dose; can prolong the contact time of medicine and schneiderian membrane; and improves therapeutic effect of the medicine to anaphylactic rhinitis.
Owner:FUDAN UNIV
Hydrogel-Filled Drug Delivery Reservoirs
ActiveUS20100121422A1Reduce mesh sizeRate of diffusion be controlHead electrodesOintment deliveryImplantable ElectrodesHydrogel matrix
A cochlear implant electrode includes an implantable electrode carrier having an outer surface with electrode contacts for electrically stimulating nerve tissue of the inner ear of a patient. A drug lumen is within the electrode carrier and adapted to receive a therapeutic fluid. The drug lumen contains delivery openings to the outer surface of the electrode carrier and a hydrogel matrix disposed between the drug lumen and the one or more delivery openings and adapted to swell in volume when exposed to the therapeutic fluid. The hydrogel matrix is adapted to control diffusion of the therapeutic fluid from the drug lumen through the delivery openings to the outer surface of the electrode carrier.
Owner:MED EL ELEKTROMEDIZINISCHE GERAETE GMBH
Preparation method for hydroxylapatite hydrogel used for bone regeneration
The invention relates to a preparation method for a hydroxylapatite hydrogel used for bone regeneration. The preparation method comprises (1) a step of adding hydroxylapatite into an organic solvent, adding n-propylamine and a coupling agent, performing a reaction at 50-80 DEG C for 30-240 min to obtain a hydroxylapatite mixed solution, cooling to 10-30 DEG C after the reaction is finished, washing with washing liquid, and drying to obtain functional hydroxylapatite powder; and (2) a step of homogenously dispersing the functional hydroxylapatite powder, hydrophilic monomers, an initiator and a catalyst into a water solution to obtain hydrogel prepolymerization liquid, and then performing polymerization at 0-40 DEG C for 24-72 h to obtain the hydroxylapatite hydrogel used for bone regeneration. The preparation method is simple in technology and low in cost. The hydroxylapatite is homogenously dispersed in the hydrogel matrix. The hydroxylapatite hydrogel, as a tissue engineering implant material, can induce bone growth and promote bone healing, and has high application value.
Owner:DONGHUA UNIV
Drug delivery vehicle containing vesicles in a hydrogel base
InactiveUS20080260833A1Facilitated releasePowder deliveryOintment deliveryExocytic vesicleActive agent
A drug delivery vehicle having active agent loaded vesicles in a hydrogel matrix; desirably either or both of the vesicles and matrix are made of at least one stimulus responsive polymer so that active agent is released in response to contact with a stimulus.
Owner:BIOCURE
Heat resistant bioactive composition
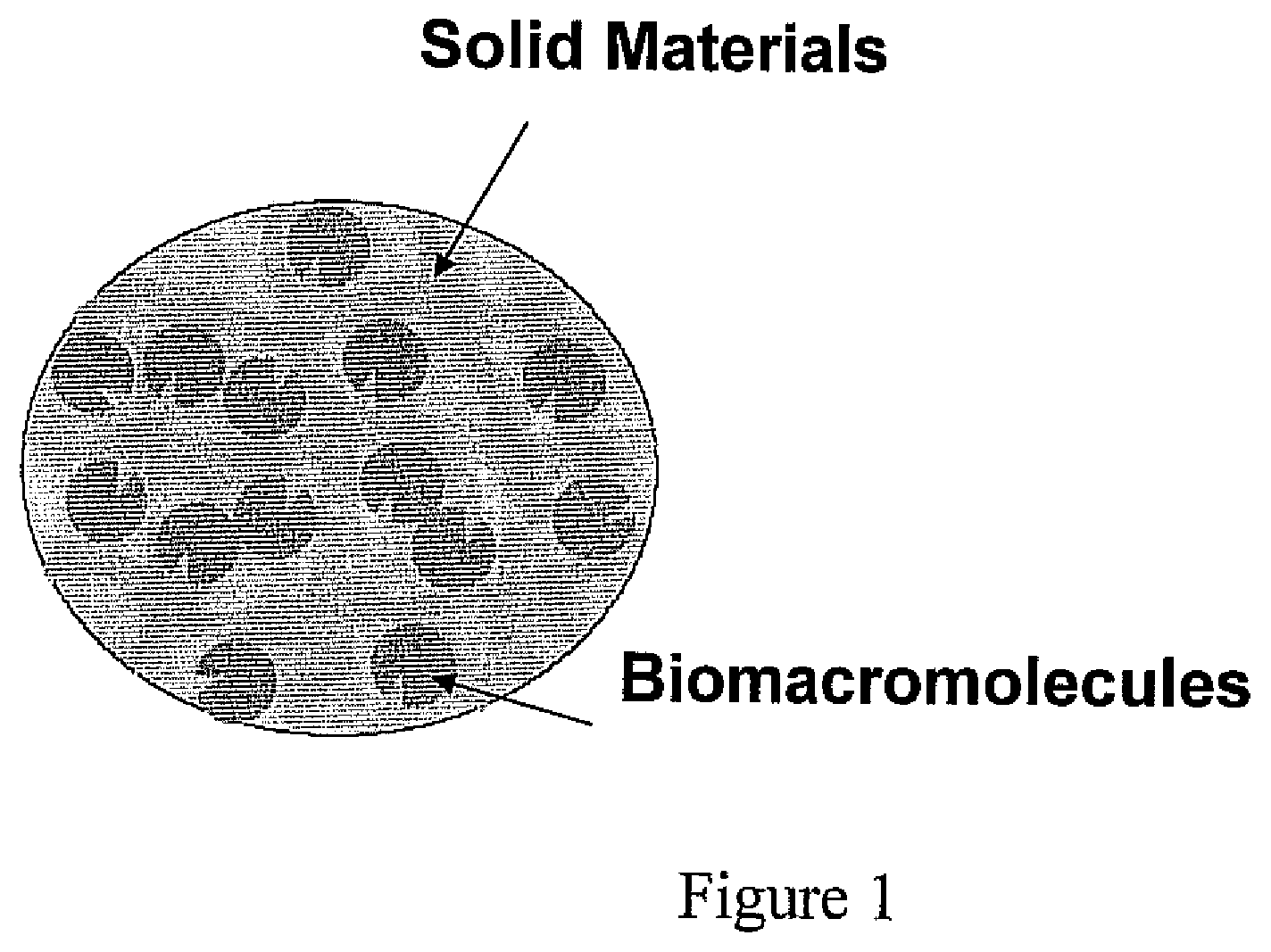
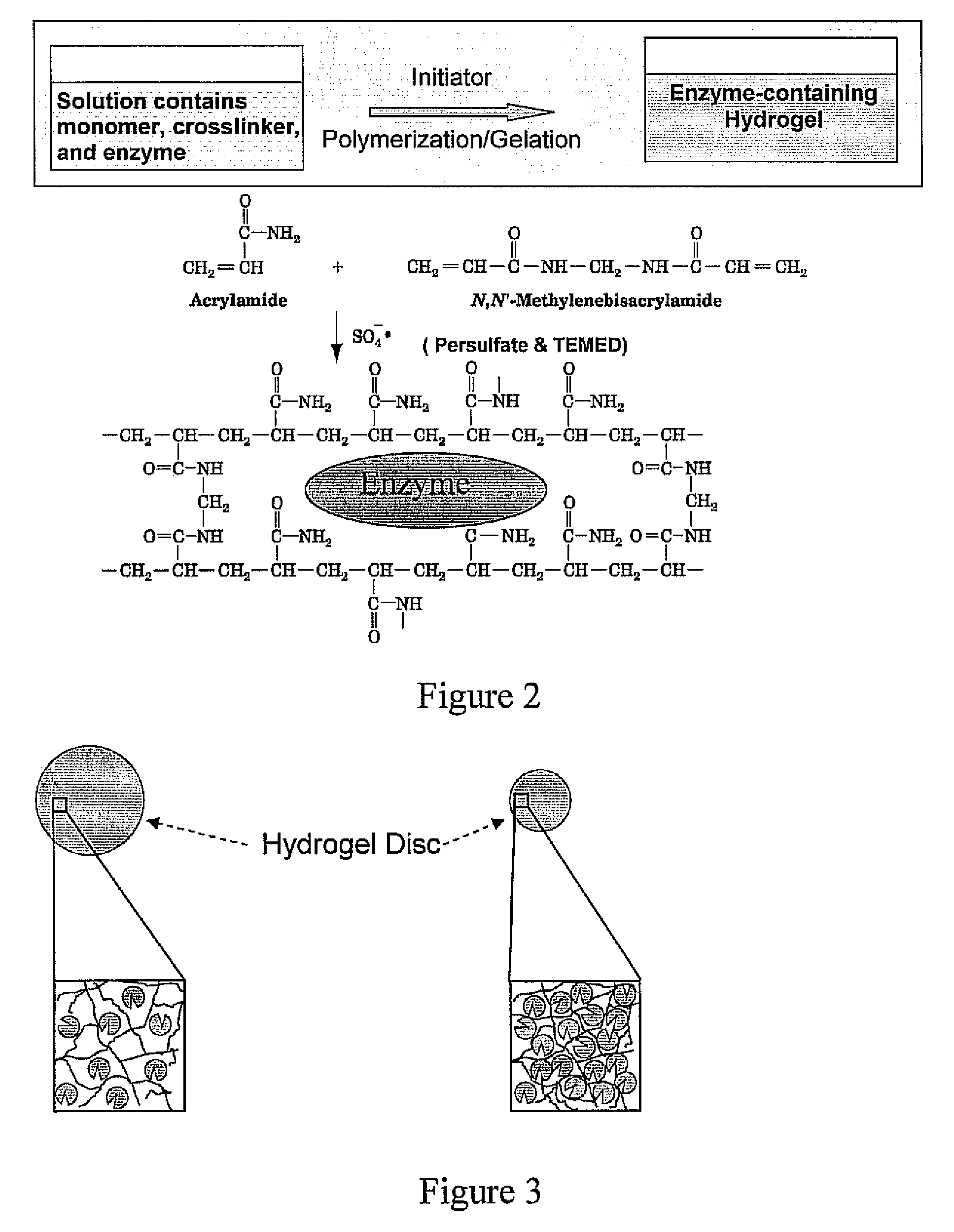
ActiveUS20080293117A1Reduce moisturePeptide/protein ingredientsOintment deliveryHalf-lifeHydrogel matrix
A bioactive composition includes a hydrogel matrix. At least one protein is immobilized in the hydrogel matrix. The digestive protein has a half-life at least 1000 times longer than the half-life of a free digestive protein counterpart.
Owner:TOYOTA MOTOR CO LTD +1
Heat resistant bioactive composition
A bioactive composition includes a hydrogel matrix. At least one protein is immobilized in the hydrogel matrix. The digestive protein has a half-life at least 1000 times longer than the half-life of a free digestive protein counterpart.
Owner:TOYOTA MOTOR CO LTD +1
Medical cold compress plaster and preparation process thereof
InactiveCN105878217ASimple production processEase of mass productionAntipyreticAnalgesicsTriclosanVitamin C
The invention provides a medical cold compress plaster. The medical cold compress plaster is composed of a support layer, a gel layer and an isolating layer, and has the special shape according with the physiological curve of the applied part. An oil phase and a water phase forming the hydrophilic gel layer respectively comprise the following components in percentage by weight: the oil phase: 20-35% of a dispersing agent, 5-7% of macromolecule resin, 0.05-0.15% of dihydroxyaluminium aminoacetate, 0.01-0.15% of ethylene diamine tetraacetic acid, 0.15% of absolute ethyl alcohol, 0.04-0.1% of menthol, 0.1-1% of vitamin E, and 0.05-0.1% of triclosan; the water phase: 0.2-0.8% of tartaric acid, 1.2-2% of carbomer, 0.5-1% of sodium carboxymethylcellulose, 0.01-0.1% of vitamin C, the balance of medical purified water, and the total weight percentage is 100%. A preparation process comprises the following steps: dispersing all the components of the oil phase into the dispersing agent, and uniformly stirring the components; dissolving all the components of the water phase into water, and uniformly stirring the components; mixing the water phase and the oil phase, uniformly stirring the water phase and the oil phase in a vacuum mixing pot, thus obtaining the hydrophilic gel substrate of the medical cold compress plaster, coating the support layer with the hydrophilic gel substrate, meanwhile covering with the isolating layer, and carrying out cutting, solidifying and packaging to obtain the needed medical cold compress plaster product.
Owner:HANGZHOU JIERSI BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com