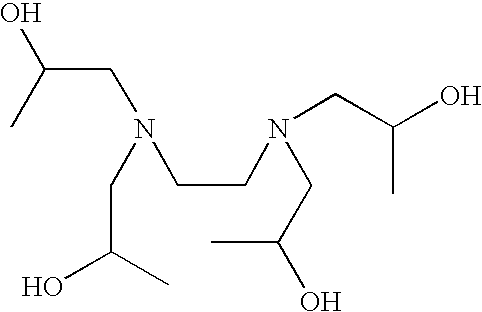
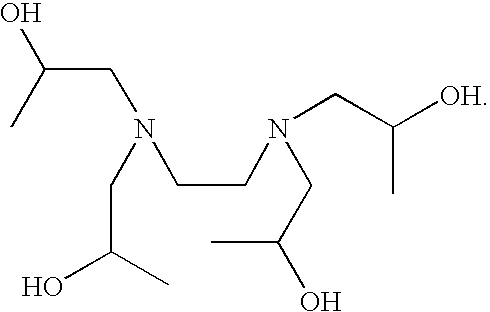
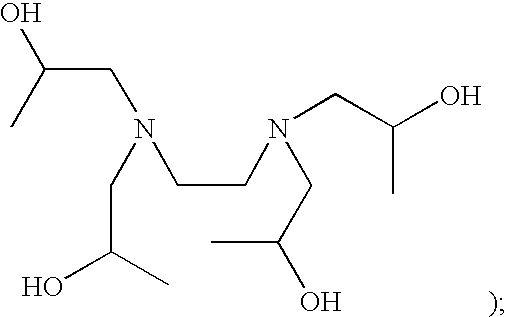
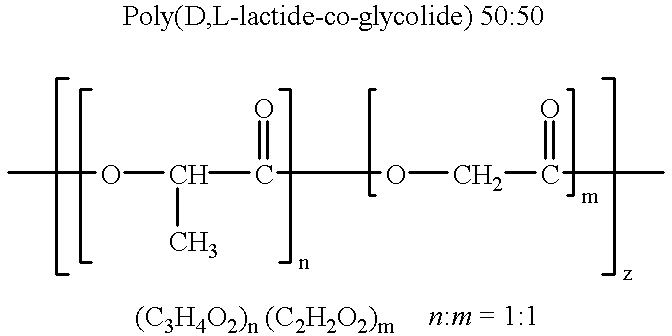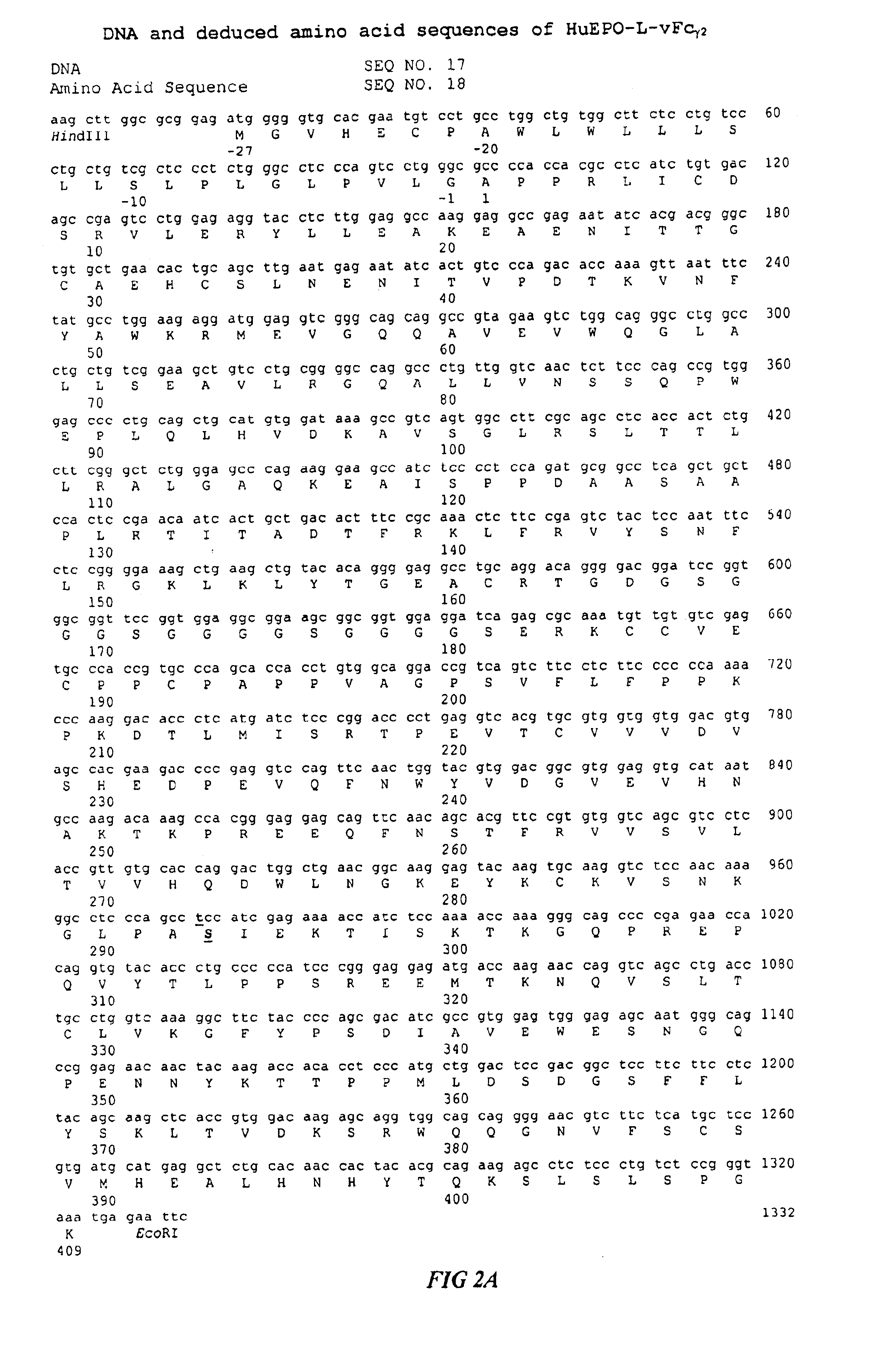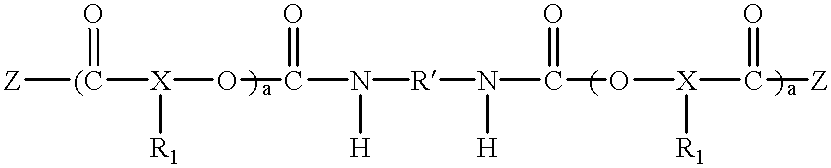Patents
Literature
21161 results about "Biological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
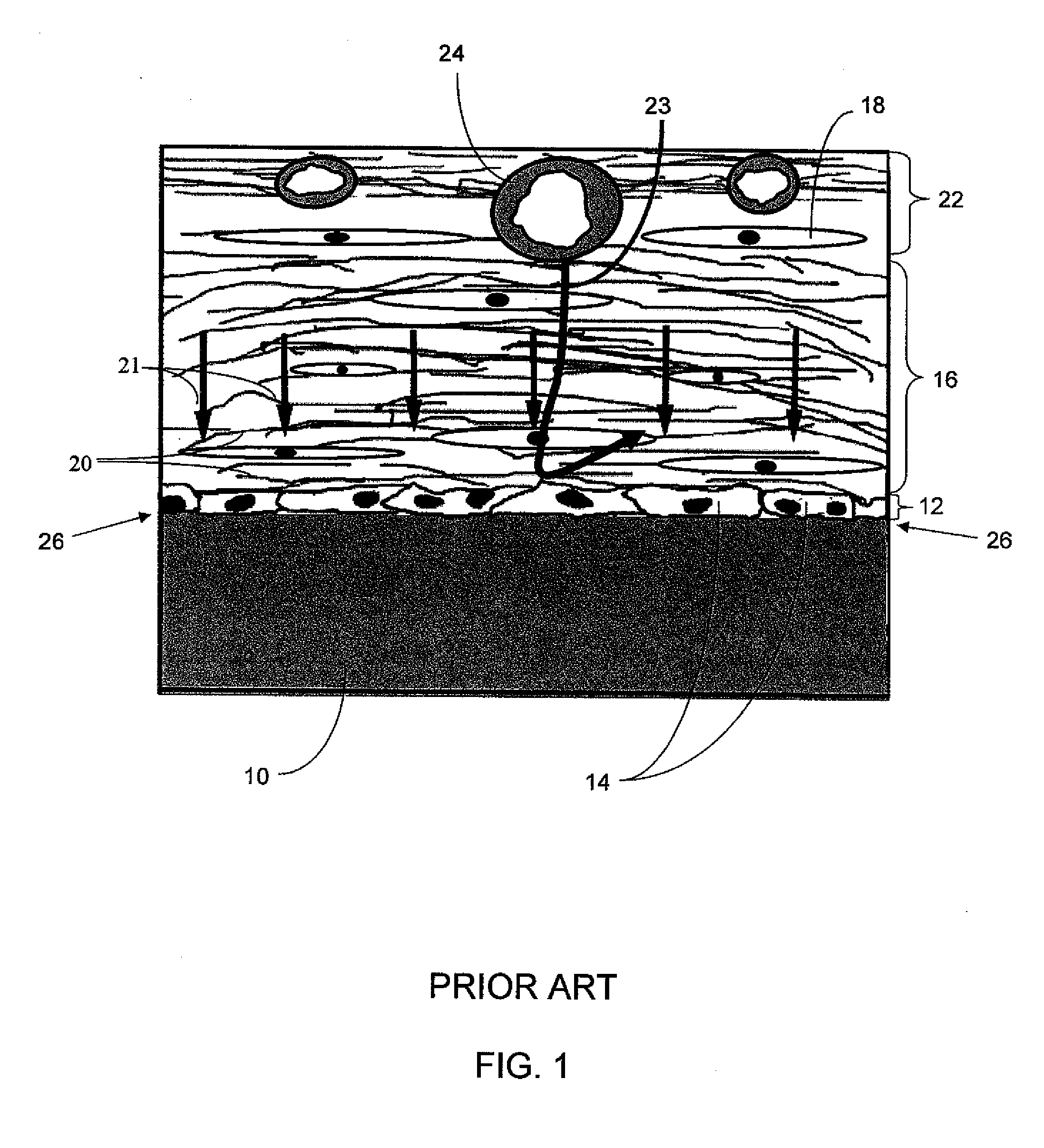
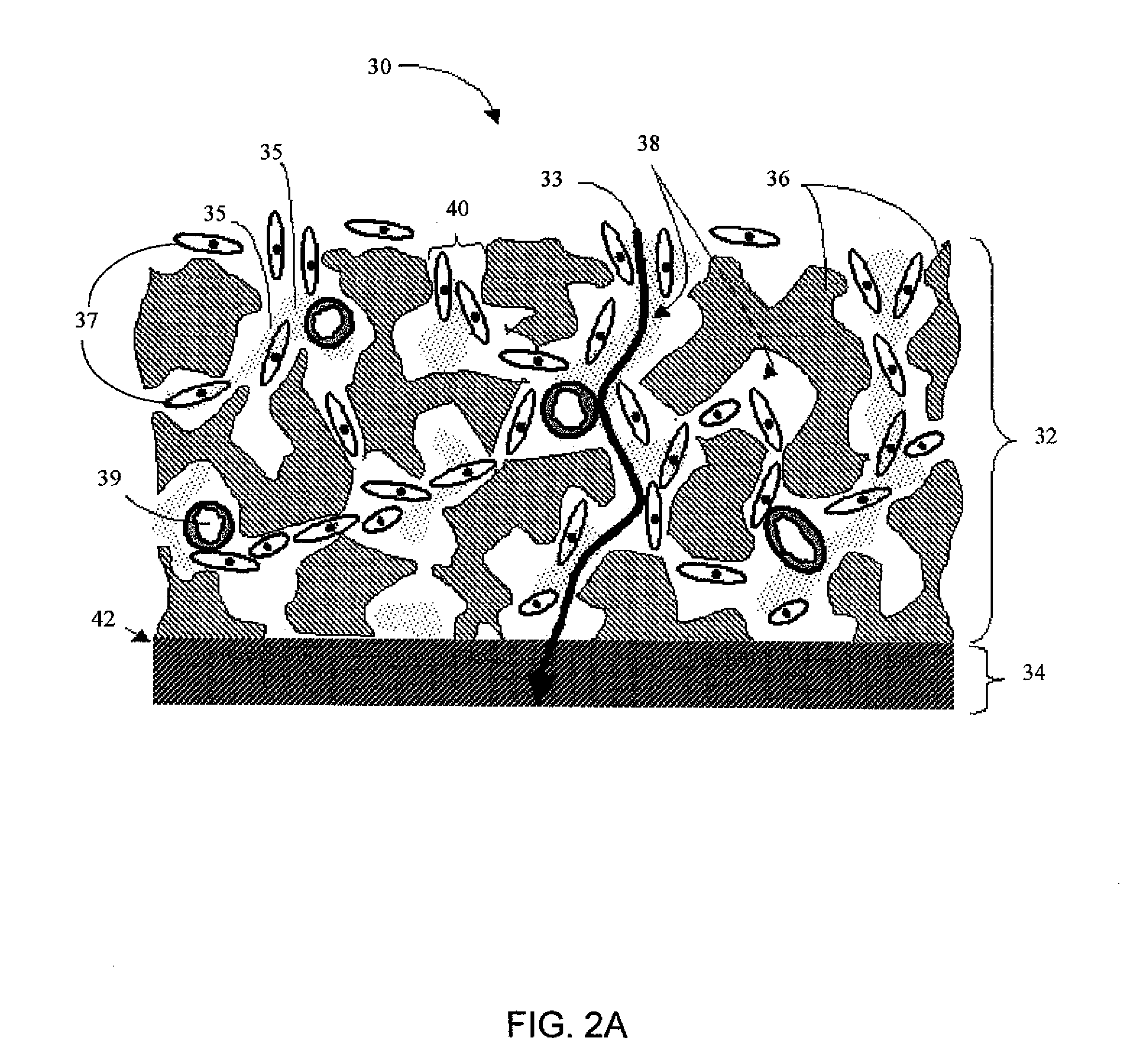
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or pharmacophore but can be modified by the other constituents. Among the various properties of chemical compounds, pharmacological/biological activity plays a crucial role since it suggests uses of the compounds in the medical applications. However, chemical compounds may show some adverse and toxic effects which may prevent their use in medical practice.
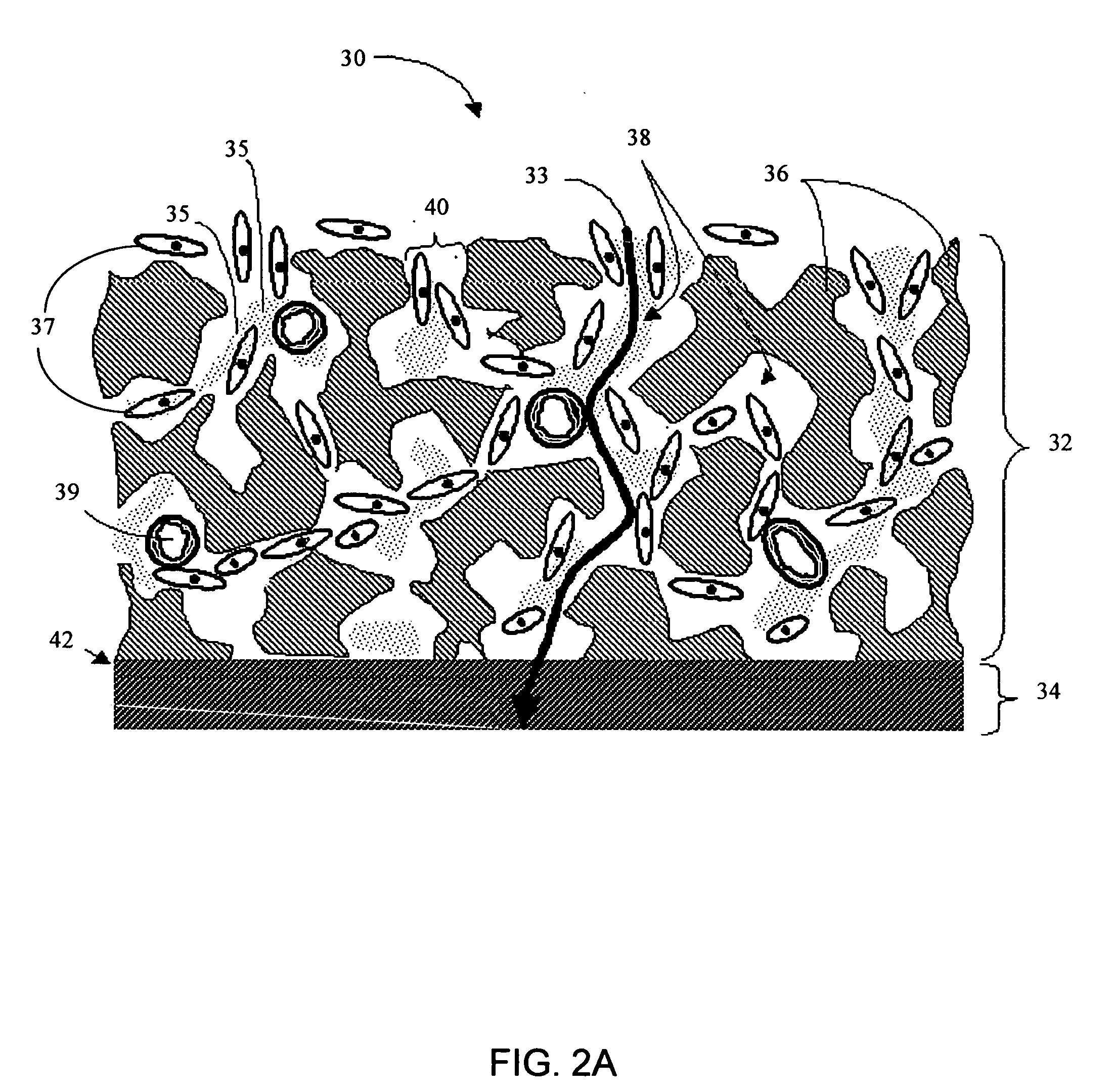
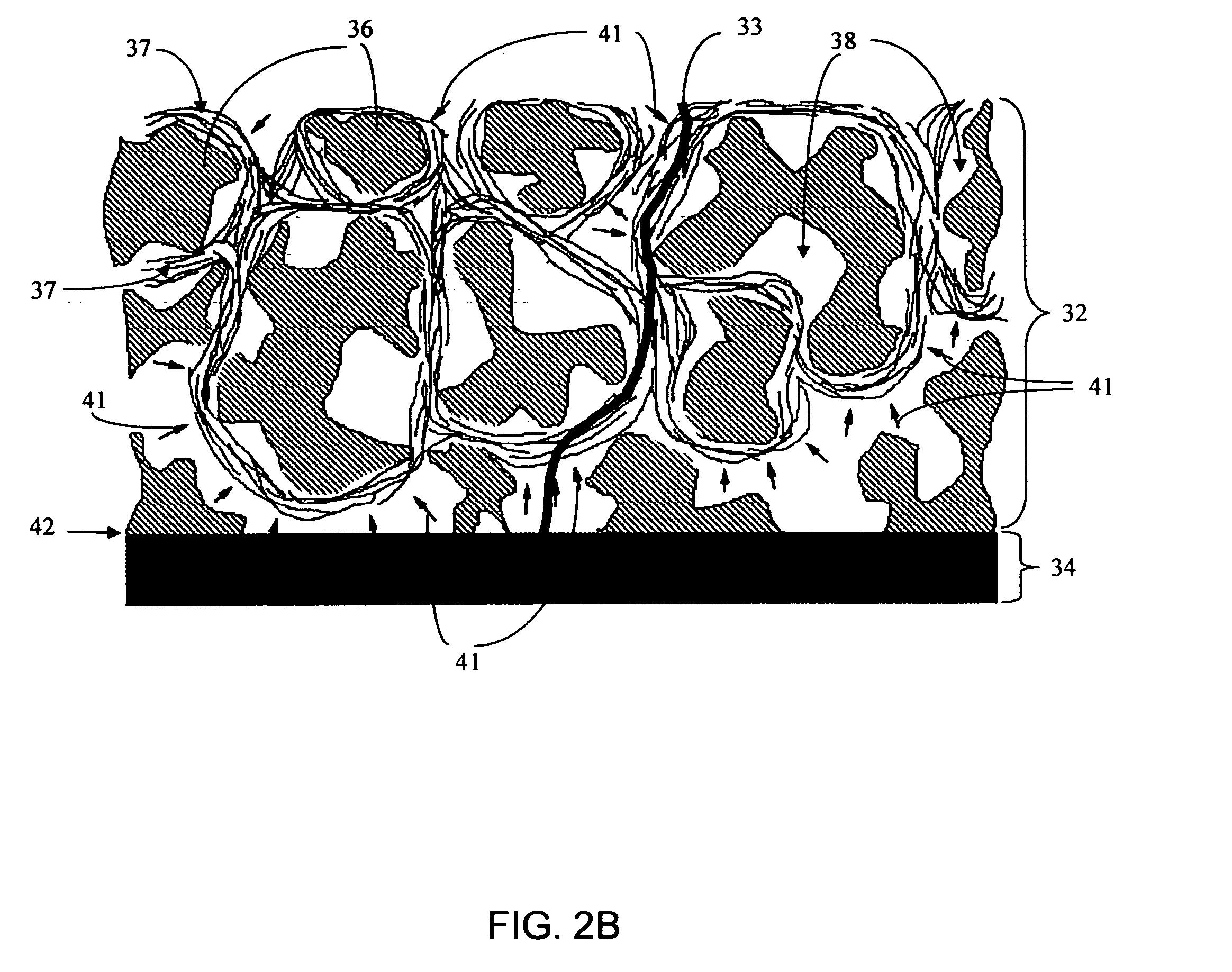
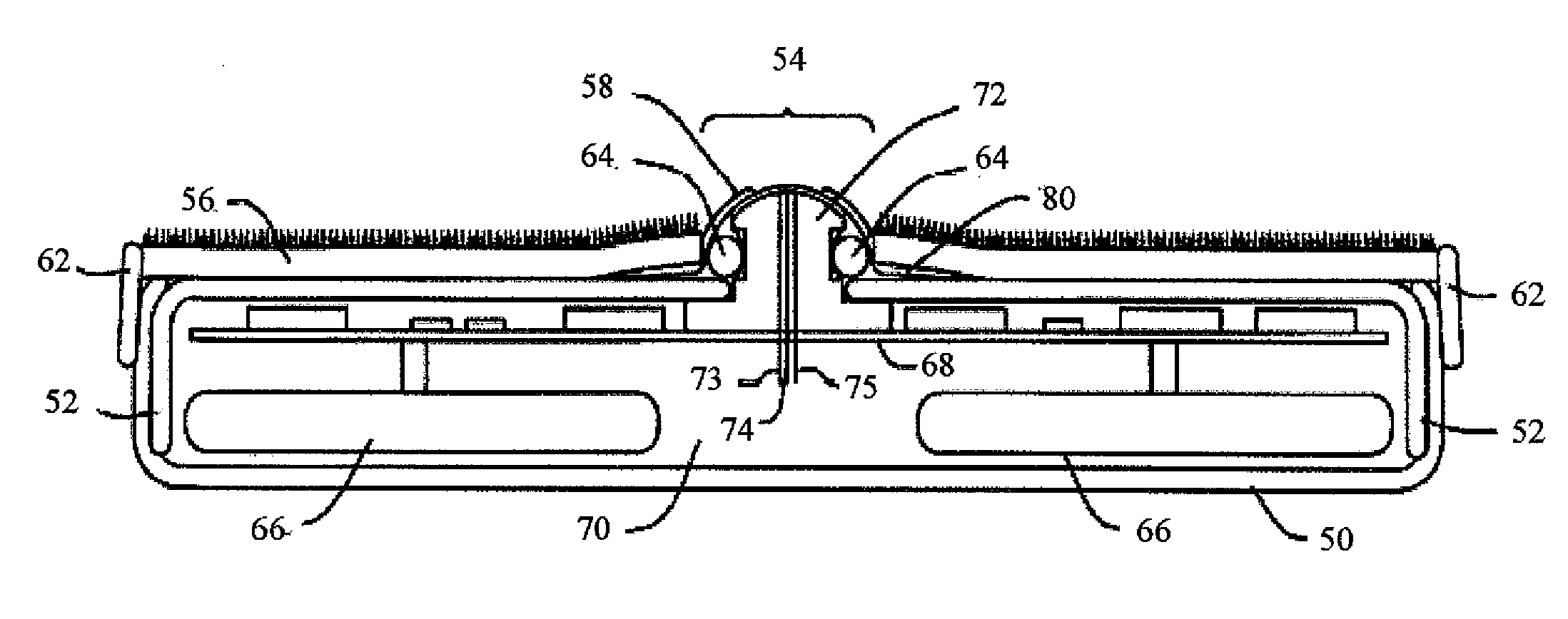
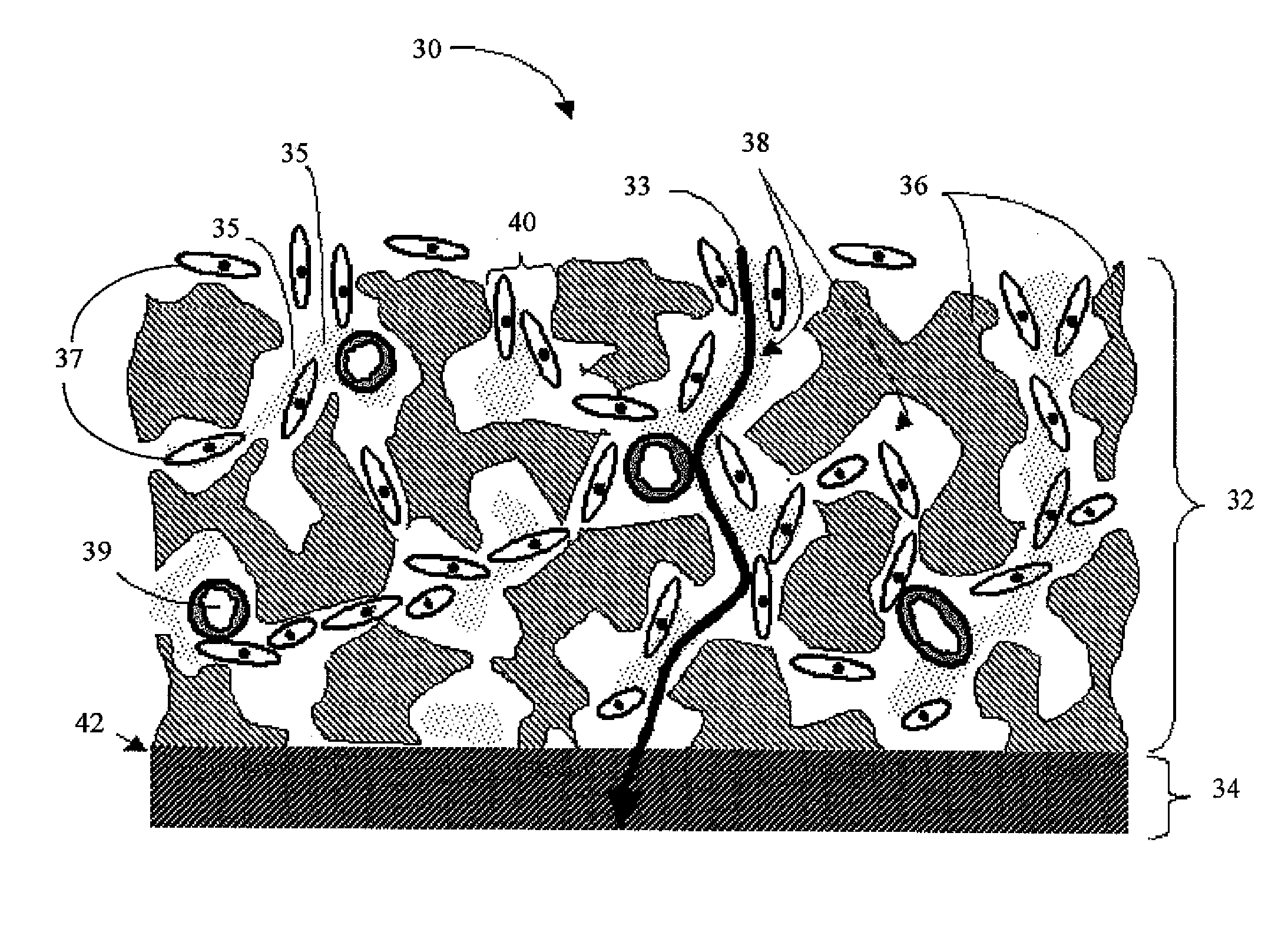
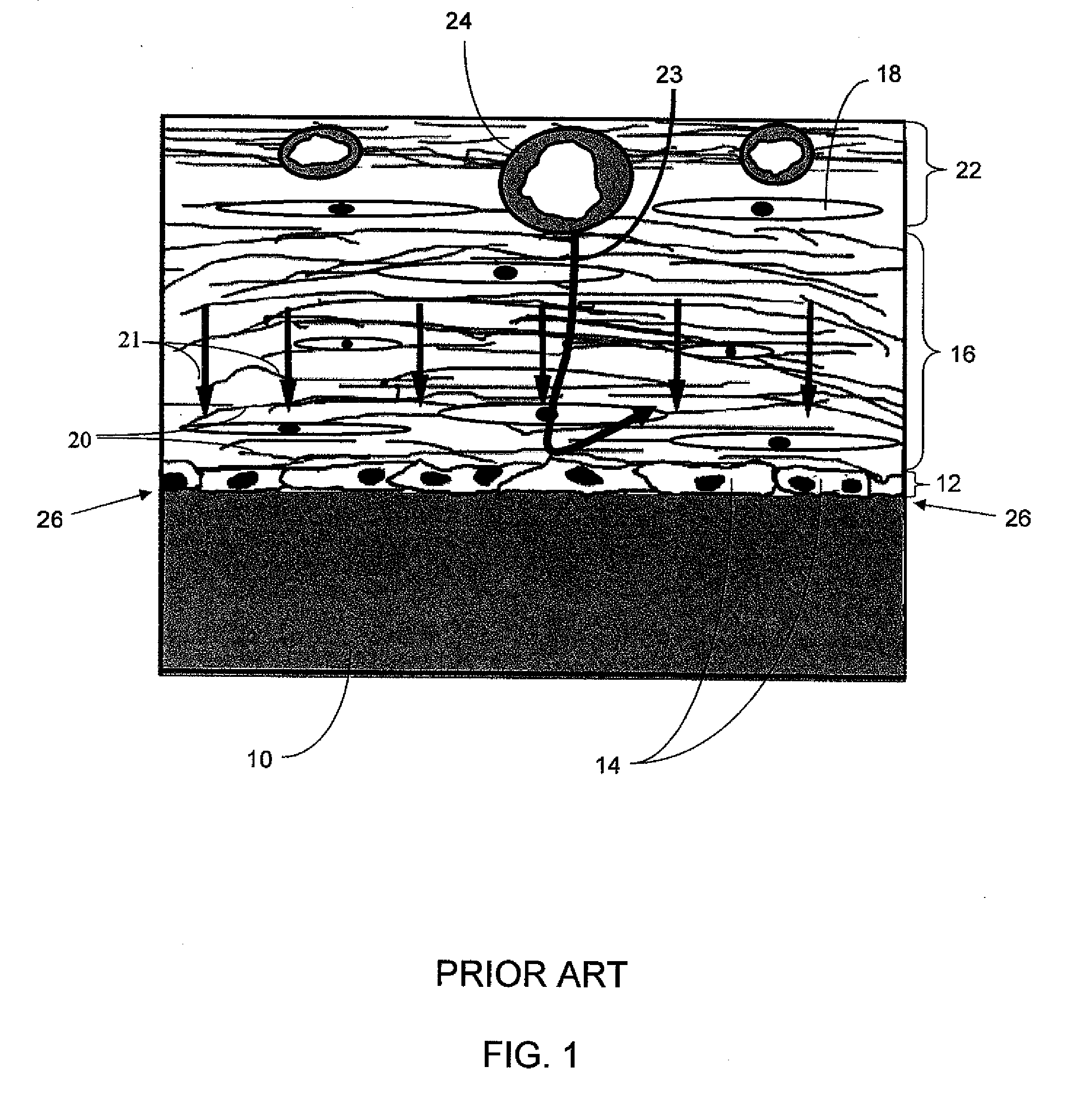
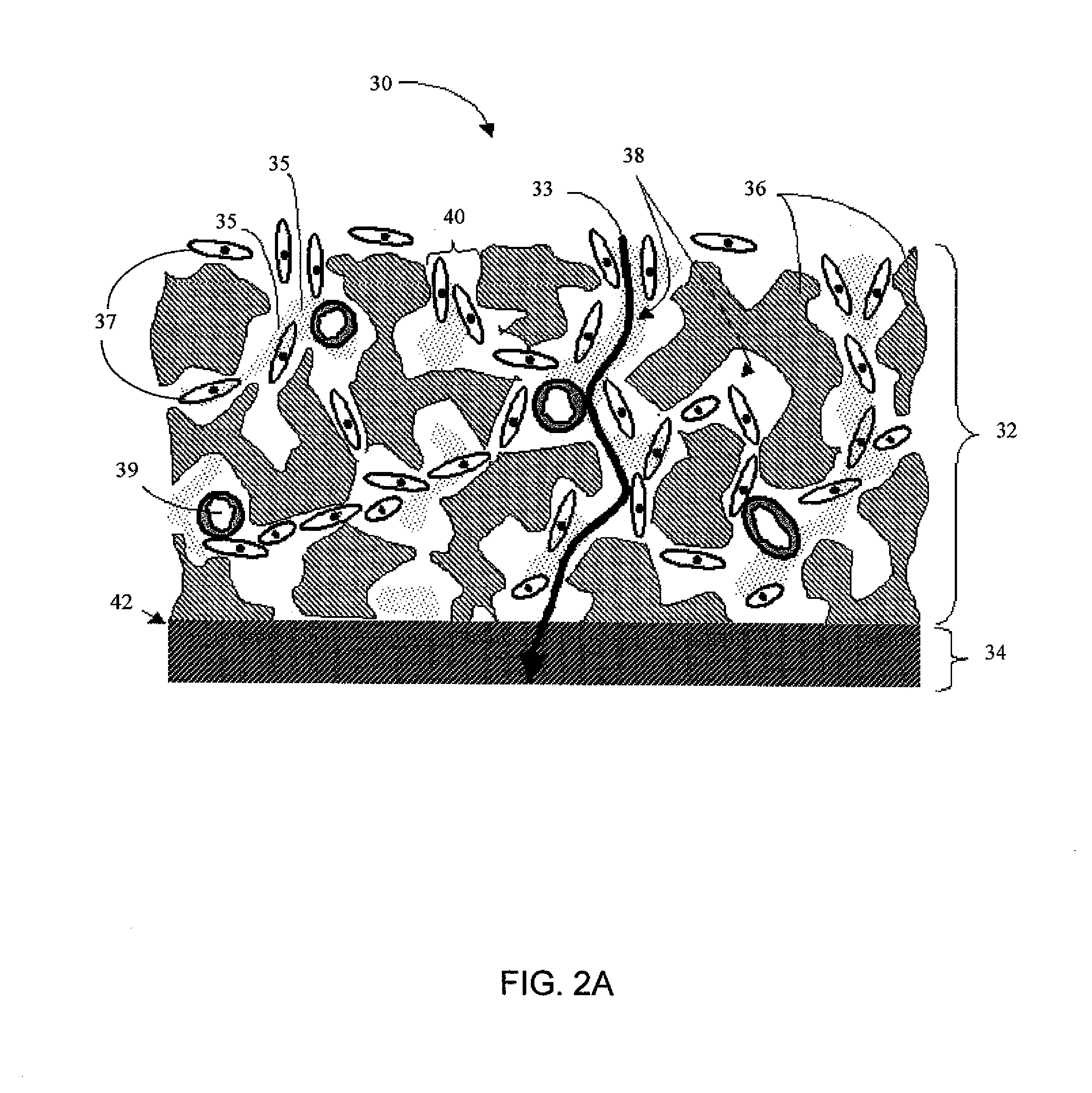
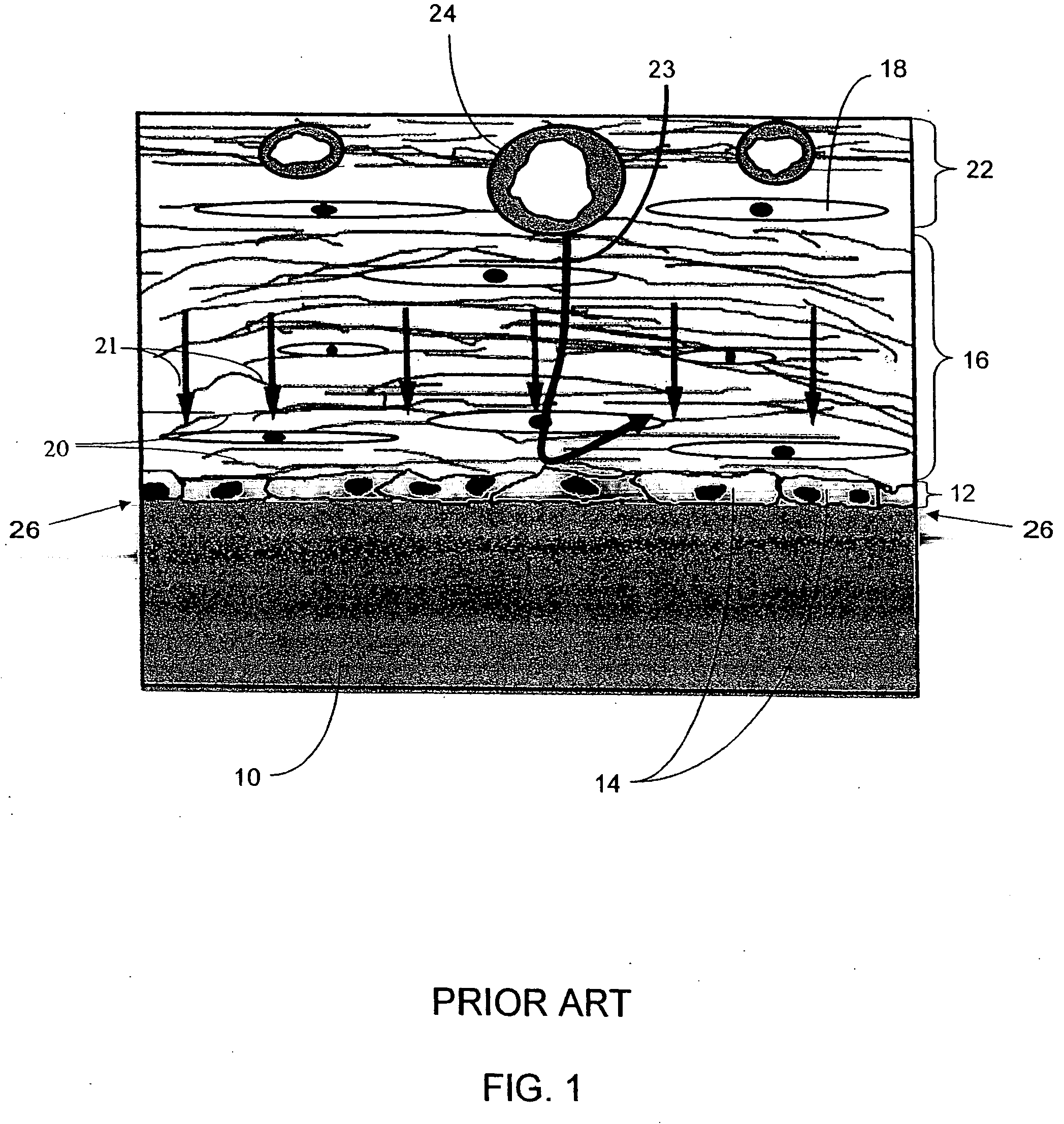
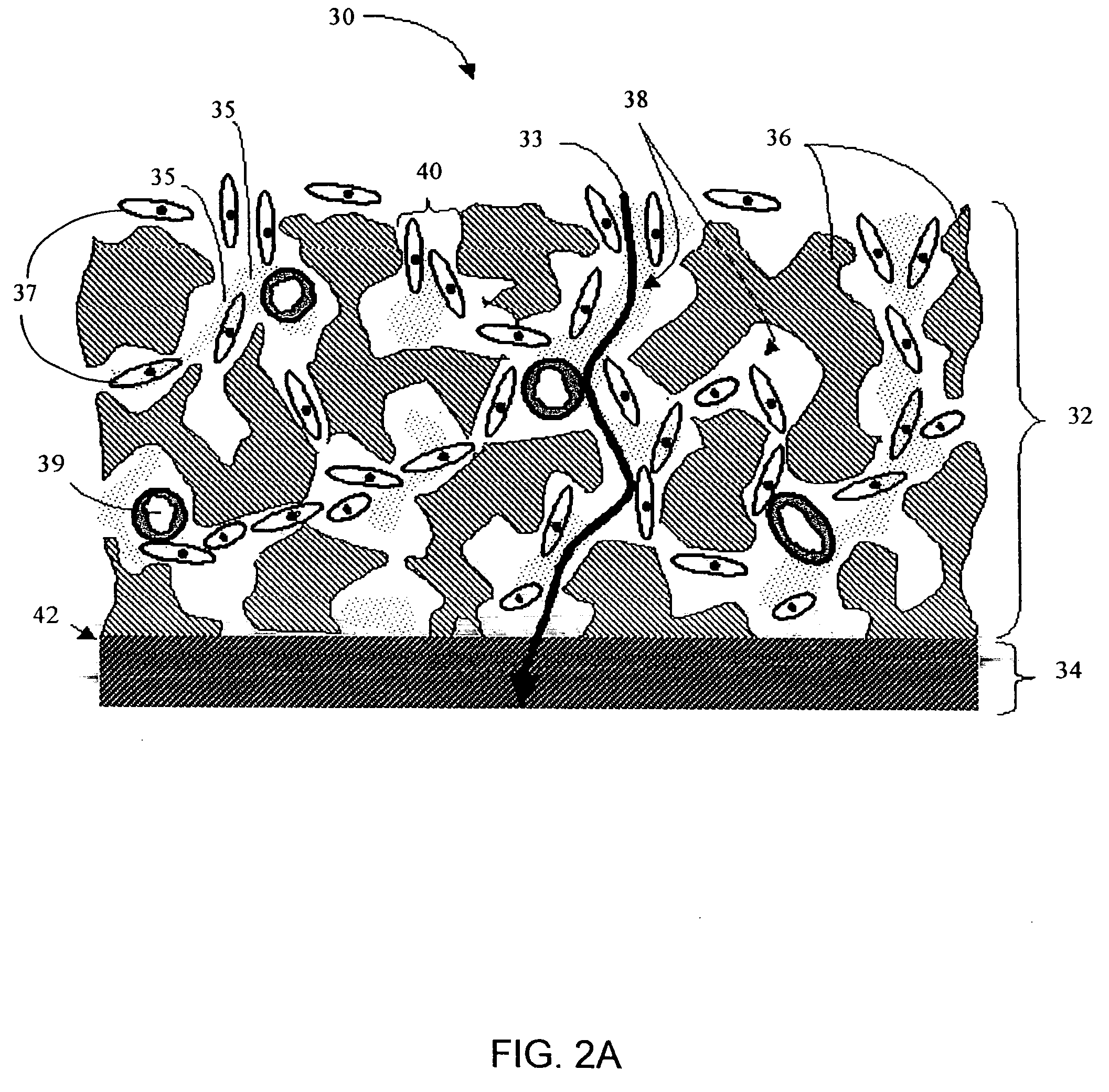
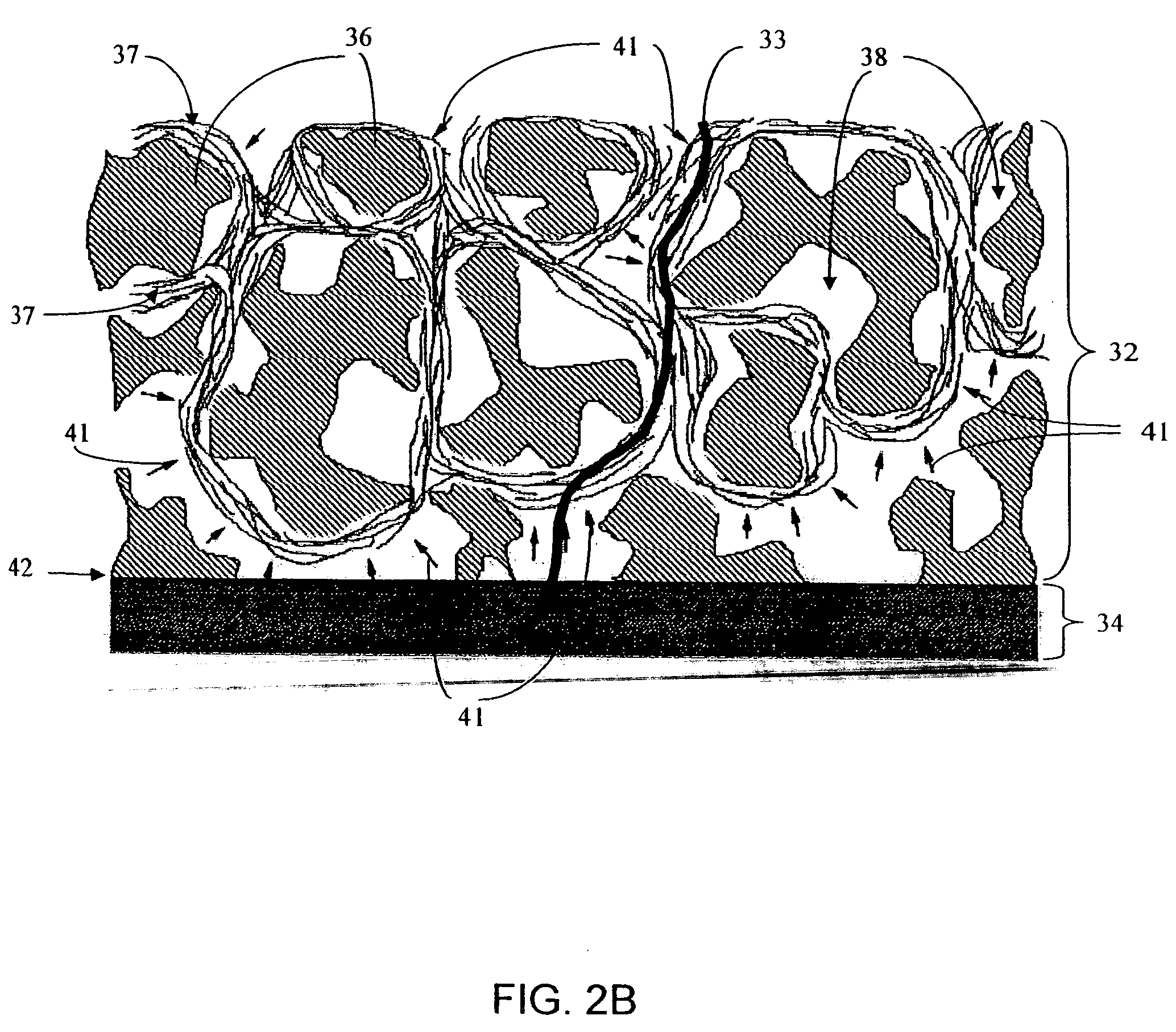
Biointerface membranes incorporating bioactive agents
InactiveUS20050031689A1Improve performancePowder deliveryAdditive manufacturing apparatusBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM
Analyte measuring device
InactiveUS20050033132A1Additive manufacturing apparatusMicrobiological testing/measurementAnalyteCell layer
An implantable analyte-measuring device including a membrane adapted to promote vascularization and / or interfere with barrier cell layer formation. The membrane includes any combination of materials, architecture, and bioactive agents that facilitate analyte transport to provide long-term in vivo performance of the implantable analyte-measuring device.
Owner:DEXCOM
Energetically-controlled delivery of biologically active material from an implanted medical device
InactiveUS7101394B2Facilitated releaseReduce deliveryOrganic active ingredientsElectrotherapyMedical deviceBiomedical engineering
A medical device and system capable of providing on-demand delivery of biologically active material to a body lumen patient, and a method of making such medical device. A first coating layer comprising a biologically active material and optionally a polymeric material is disposed on the surface of the medical device. A second coating layer comprising magnetic particles and a polymeric material is disposed on the first coating layer. The second coating layer, which is substantially free of a biologically active material, protects the biologically active material prior to delivery. The system includes the medical device and a source of energy, such as an electromagnetic or mechanical vibrational energy. When the patient is exposed to the energy source, the magnetic particles move out of the second coating layer and create channels therein through which the biologically active material can be released.
Owner:BOSTON SCI SCIMED INC
Porous medical device and method for its manufacture
ActiveUS7964206B2Thickness of device can be variedControllable porosityBiocideGenetic material ingredientsFiberBioceramic
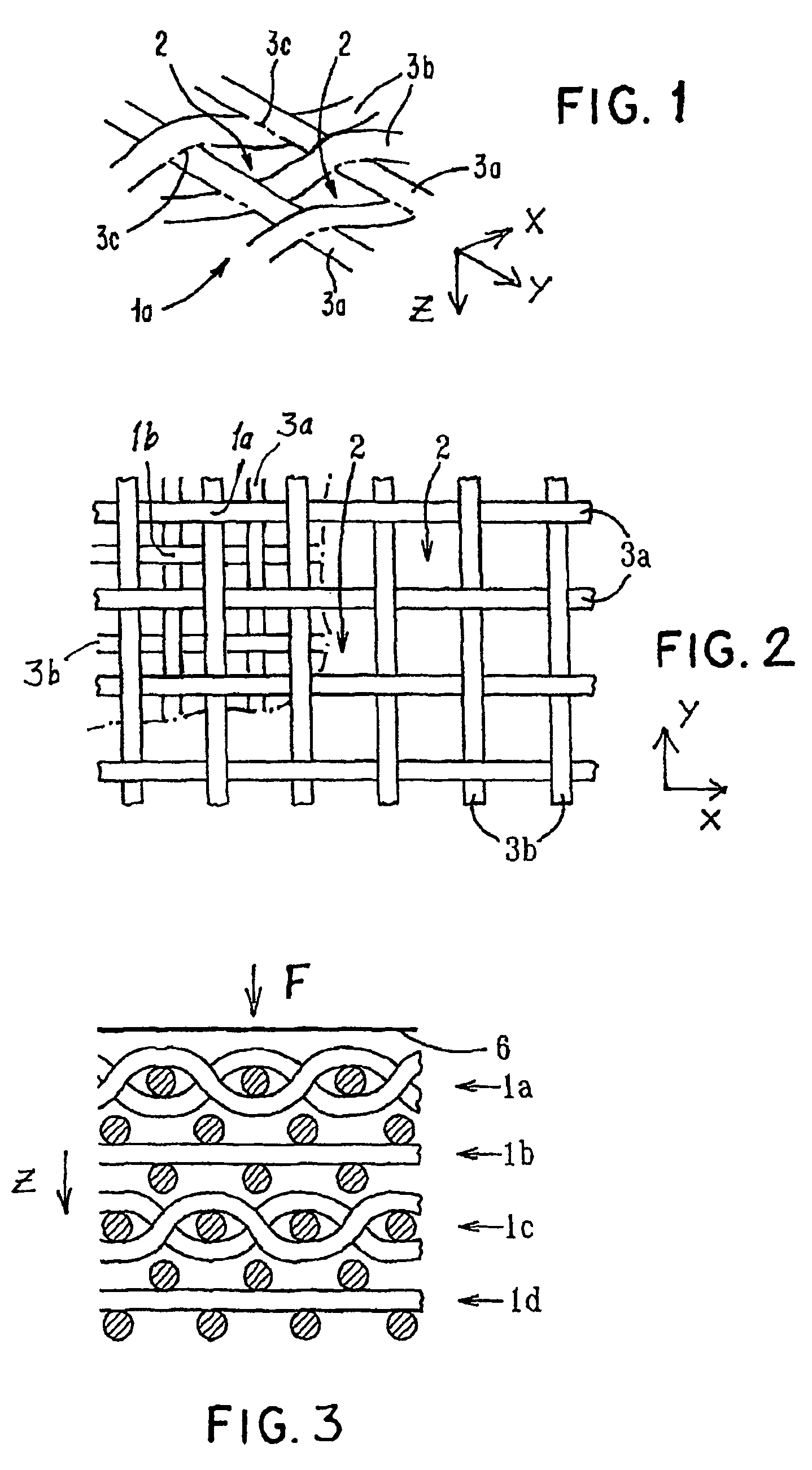
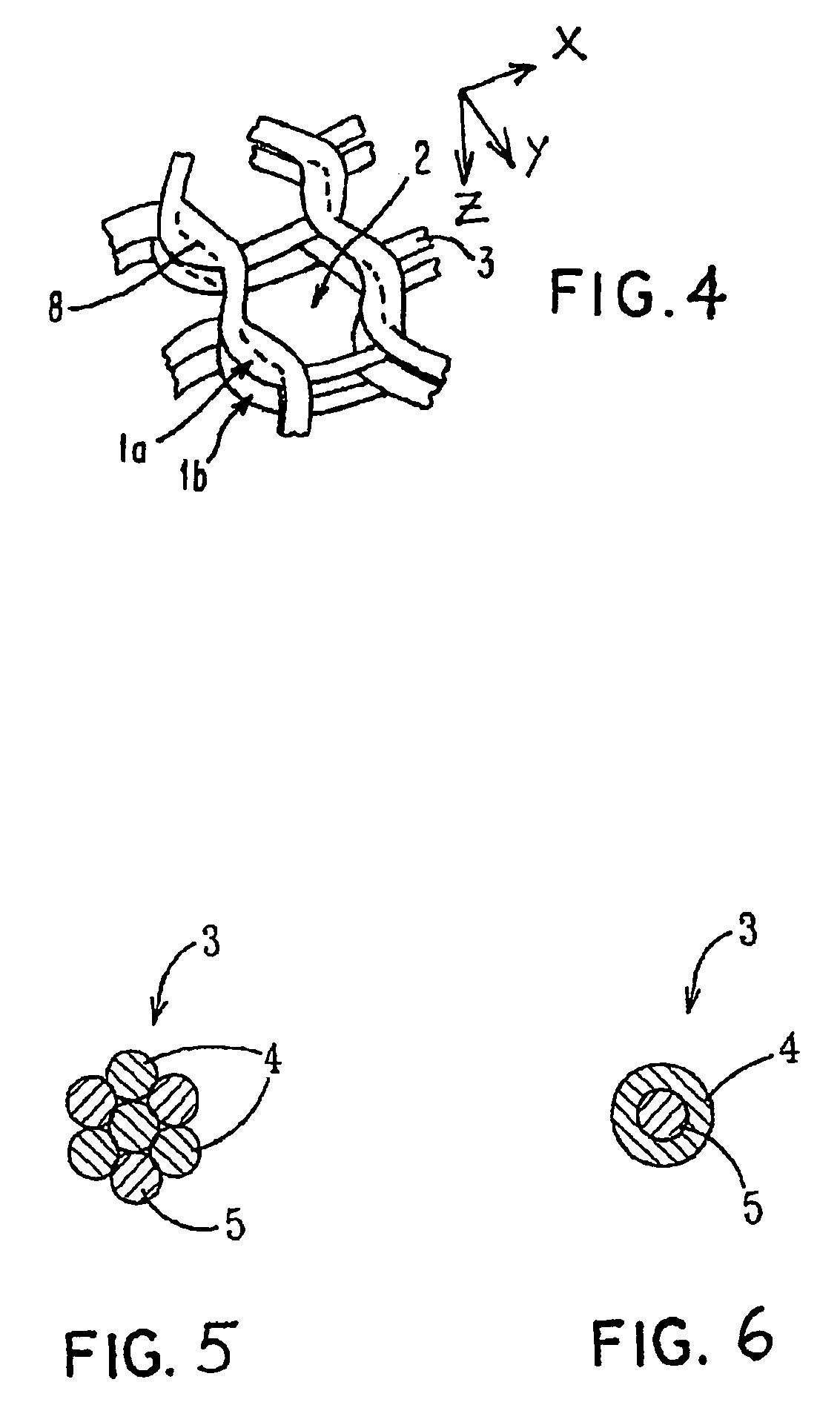
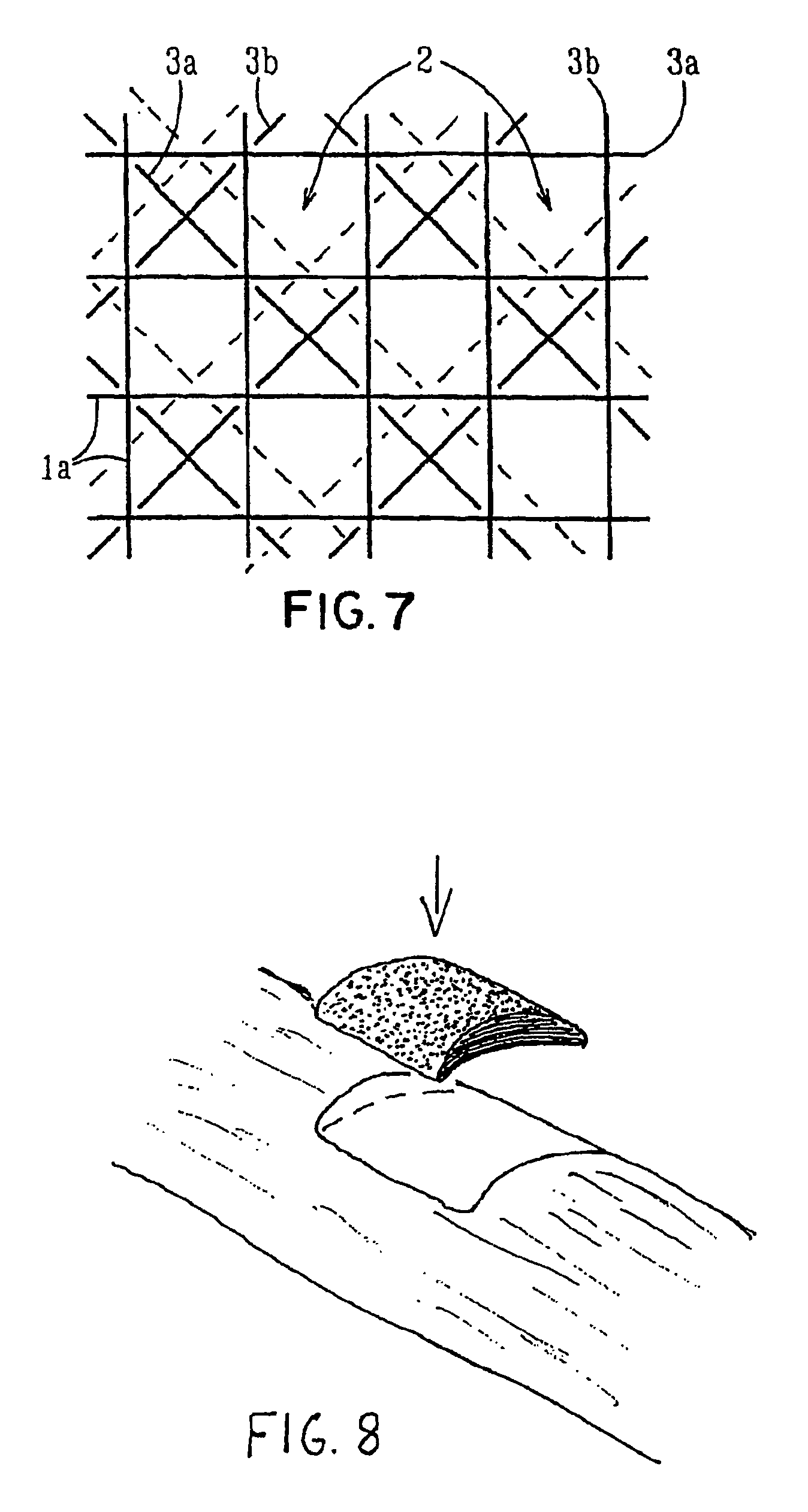
Porous bioabsorbable, bioactive and load-bearing composite medical device structure includes a plurality of regular textile planar layers (1a, 1b . . . ) formed of continuous bioabsorbable polymer matrix and bioceramic fibers acting as reinforcements, both included in continuous fibrous elements (3) forming the textile layers. The layers are placed on top of each other to form a structure having two dimensions (x, y) at right angles to each other according to the two dimensions of the textile layer and a third dimension (z) perpendicular to them and resulting from the piling of the layers. A plurality of passages extend through the layers as a result of the openings (2) defined by portions of the continuous fibrous elements (3) extending substantially in the direction of the plane. The continuous fibrous elements (3) comprise both bioactive ceramic reinforcing fibers which form a reinforcing structure and a bioabsorbable polymer matrix material which forms a matrix which binds the layers together and also binds the portions of continuous fibers defining the openings together, thereby forming the passages and stiffening the structure. This bioactive and bioabsorbable composite structure is suitable to be used as a basic structure in medical devices, especially in osteochondral applications where the load-bearing properties of implant are required.
Owner:BIORETEC
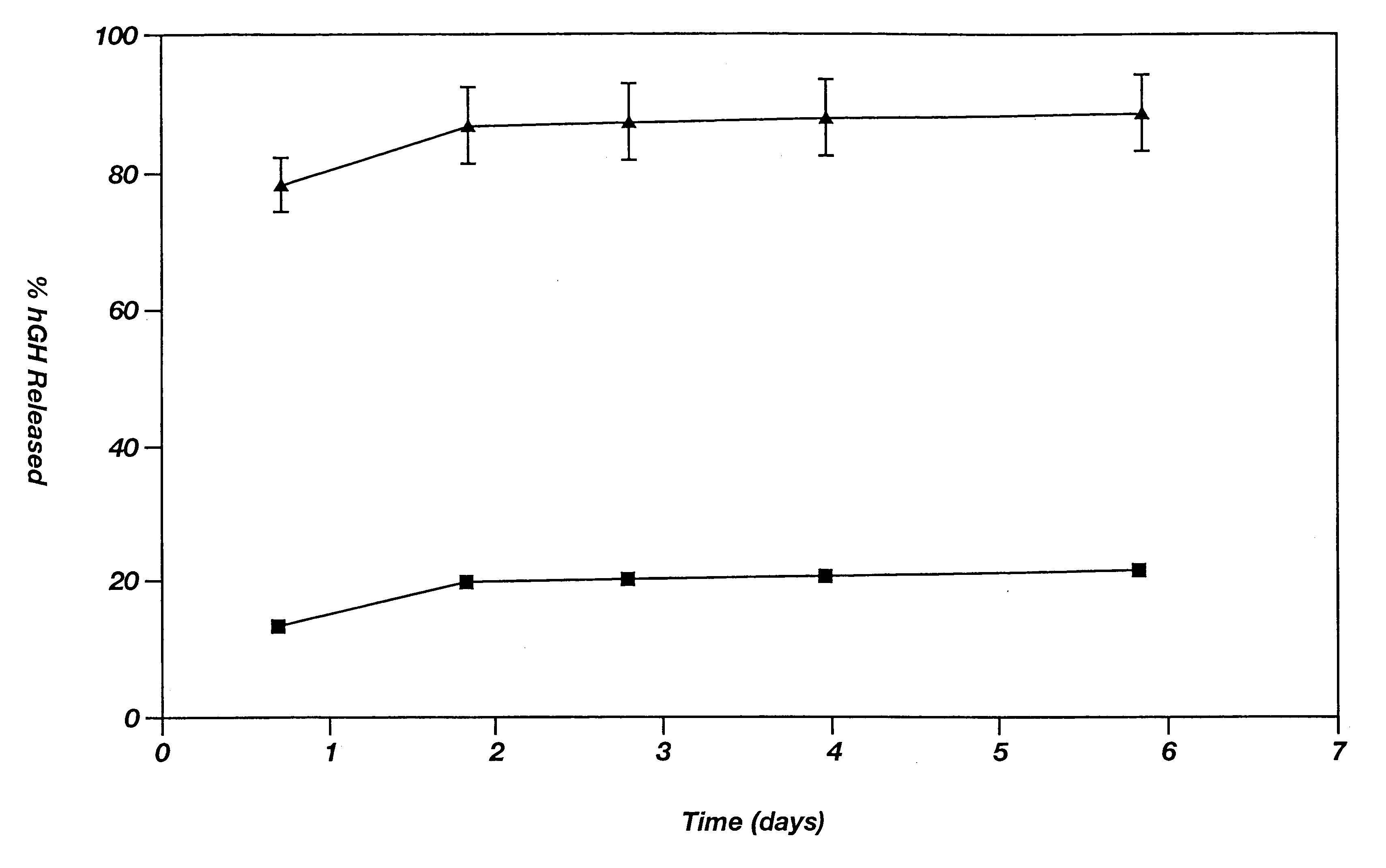
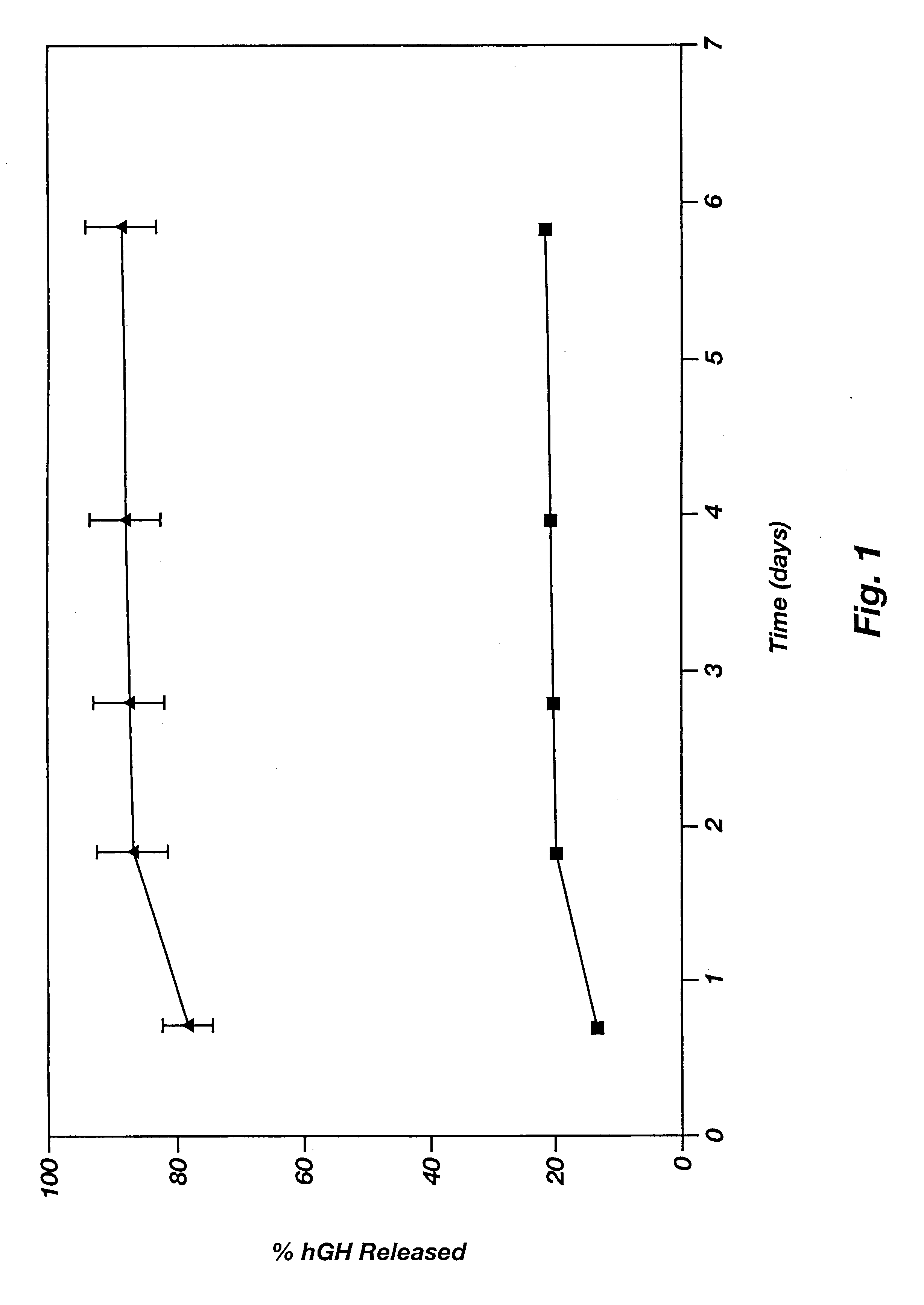
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
Analyte measuring device
InactiveUS20080228054A1Additive manufacturing apparatusMicrobiological testing/measurementAnalyteCell layer
An implantable analyte-measuring device including a membrane adapted to promote vascularization and / or interfere with barrier cell layer formation. The membrane includes any combination of materials, architecture, and bioactive agents that facilitate analyte transport to provide long-term in vivo performance of the implantable analyte-measuring device.
Owner:DEXCOM INC
Implantable devices having swellable grip members
The present disclosure relates to implantable medical devices including swellable tissue gripping elements and methods of forming such devices. The implantable medical device may comprise a biocompatible substrate having a surface comprising at least one swellable grip member. The implantable medical device may take on the form of a surgical mesh, patch, buttress, or pledget and the swellable member may comprise spikes and / or spiked naps. The implantable medical device may also contain a bioactive agent.
Owner:TYCO HEALTHCARE GRP LP
Target analyte sensors utilizing microspheres
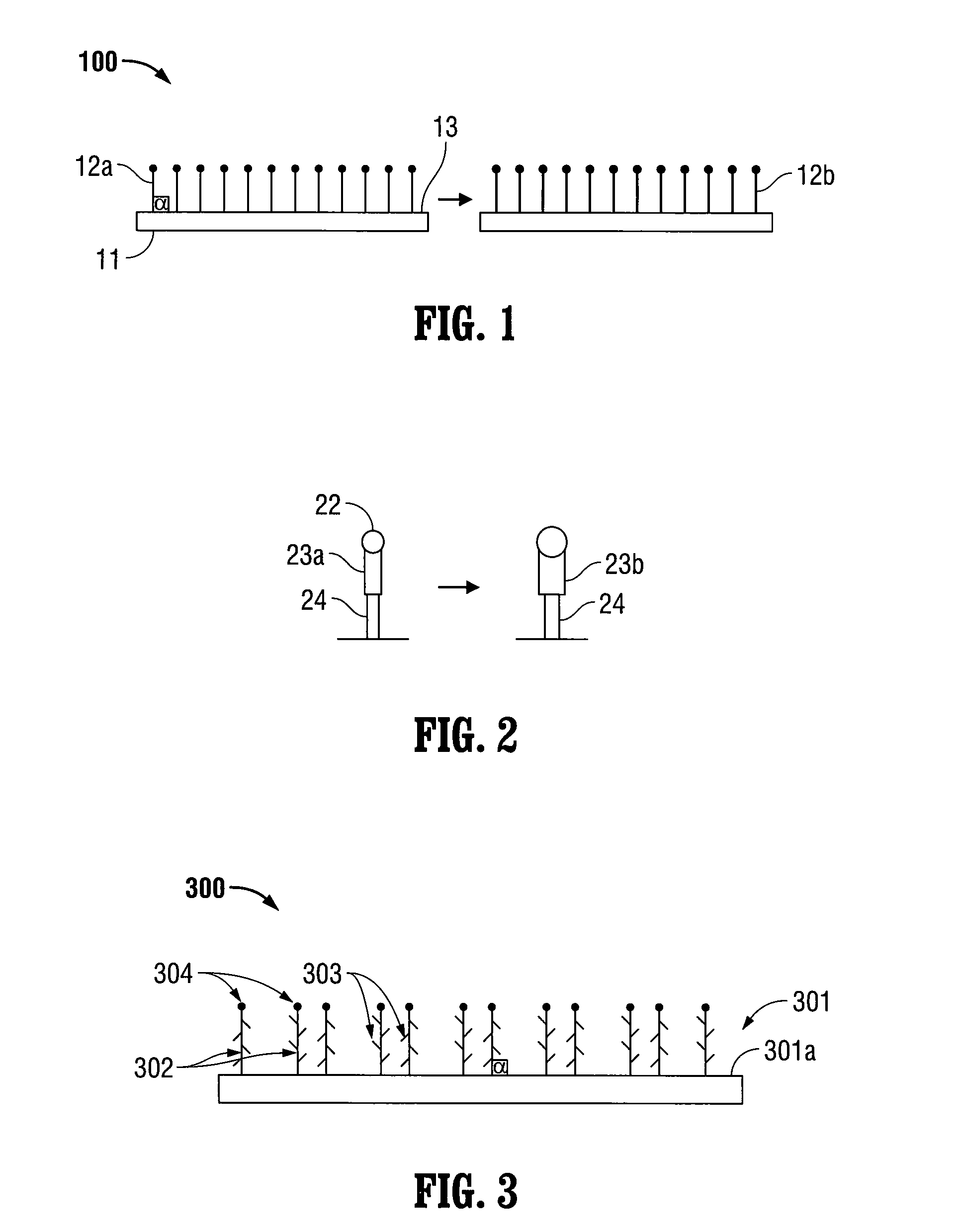
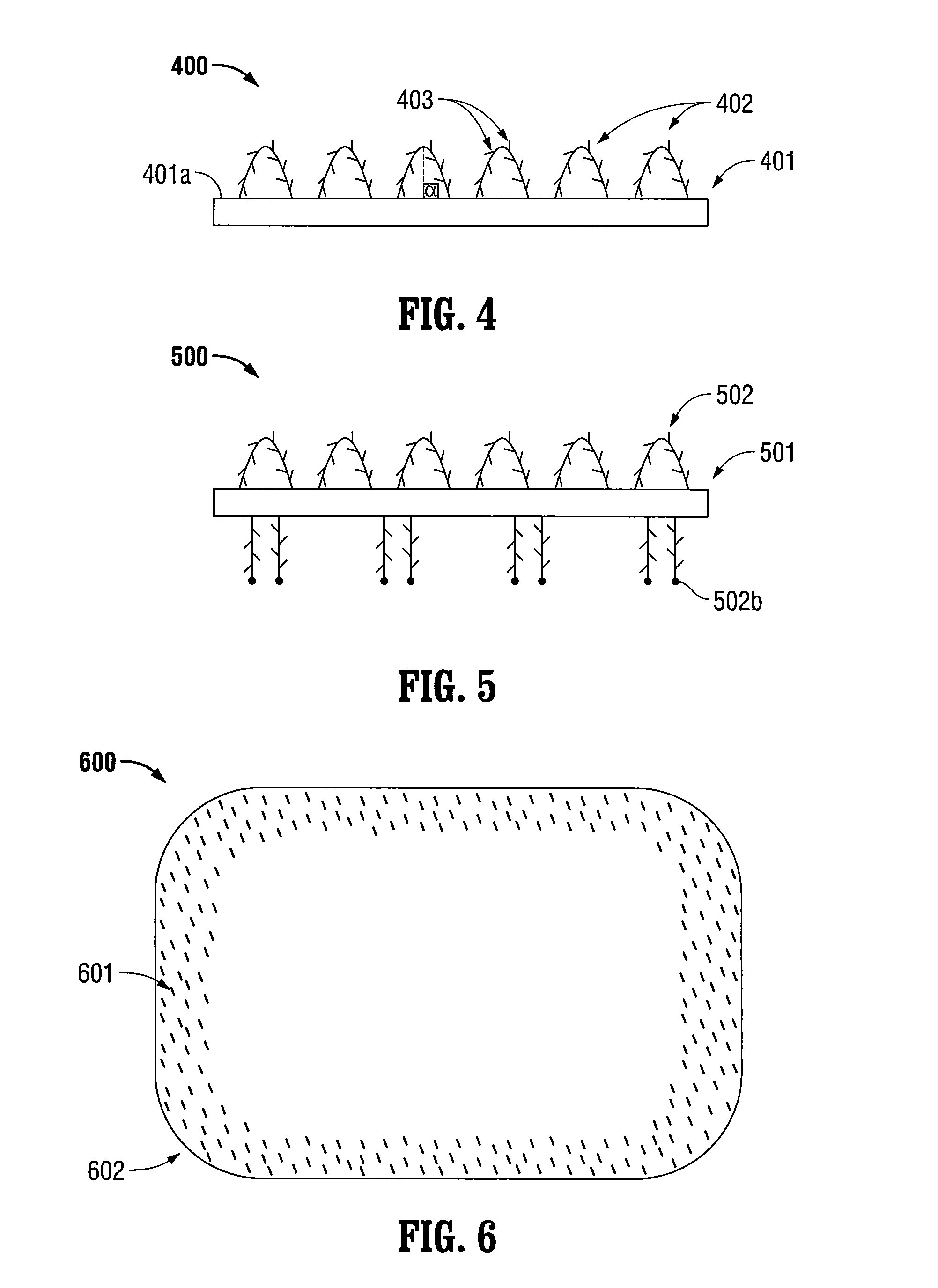
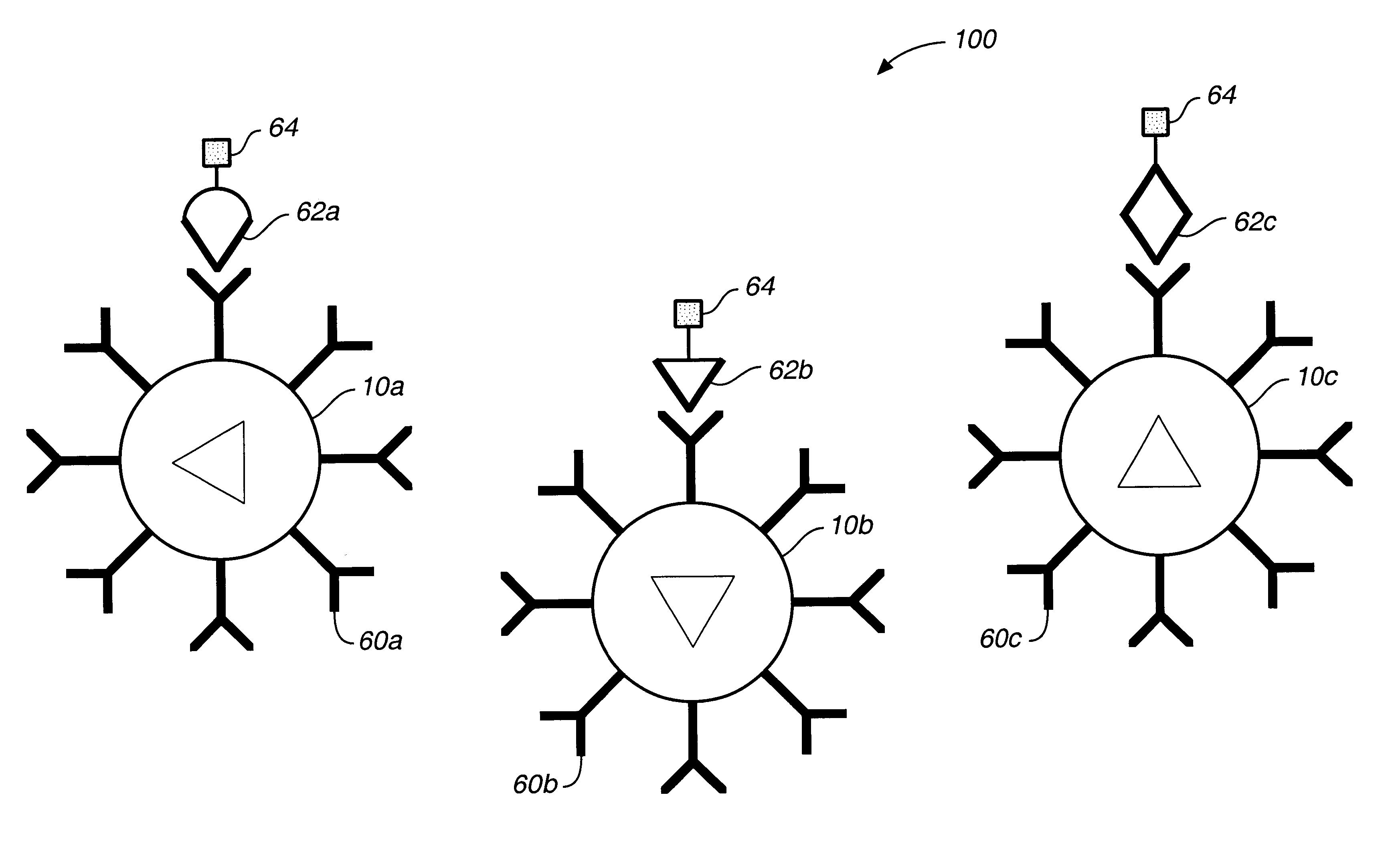
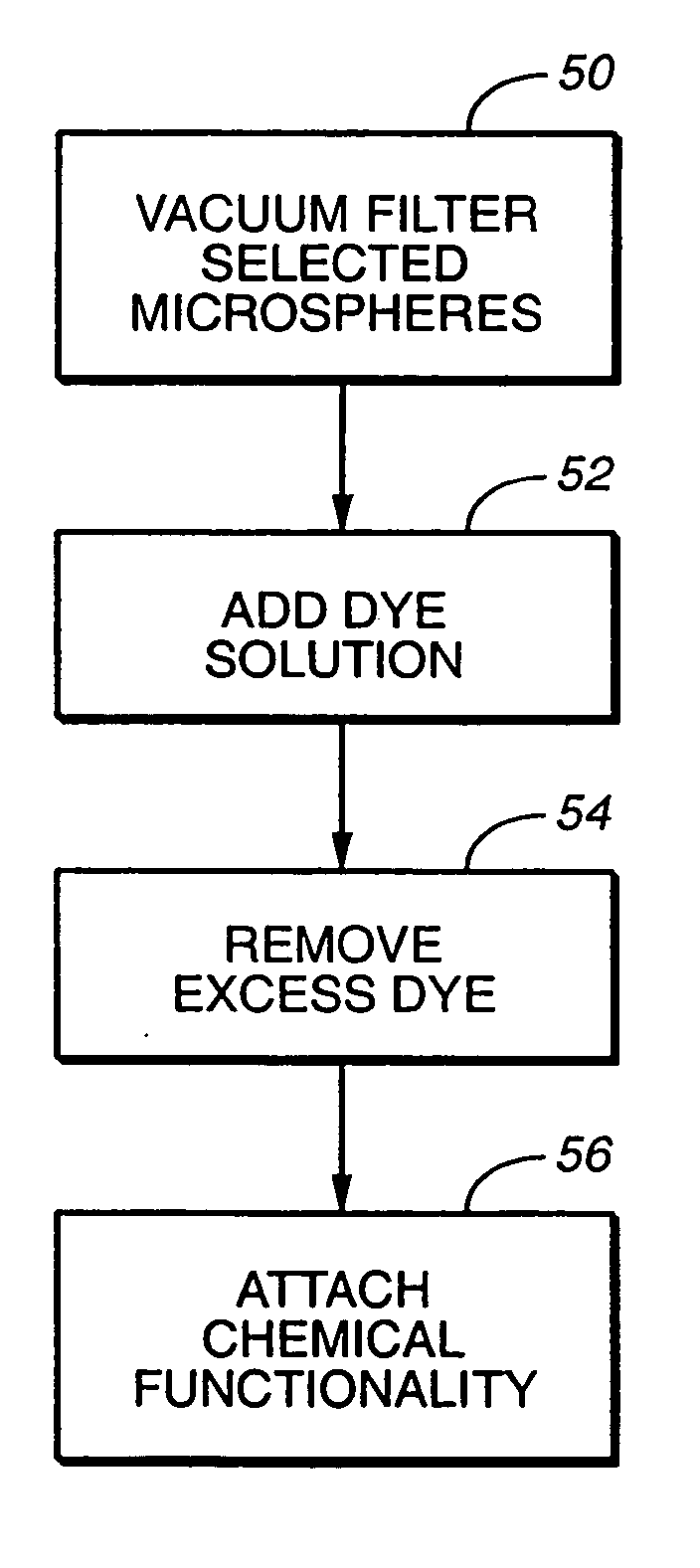
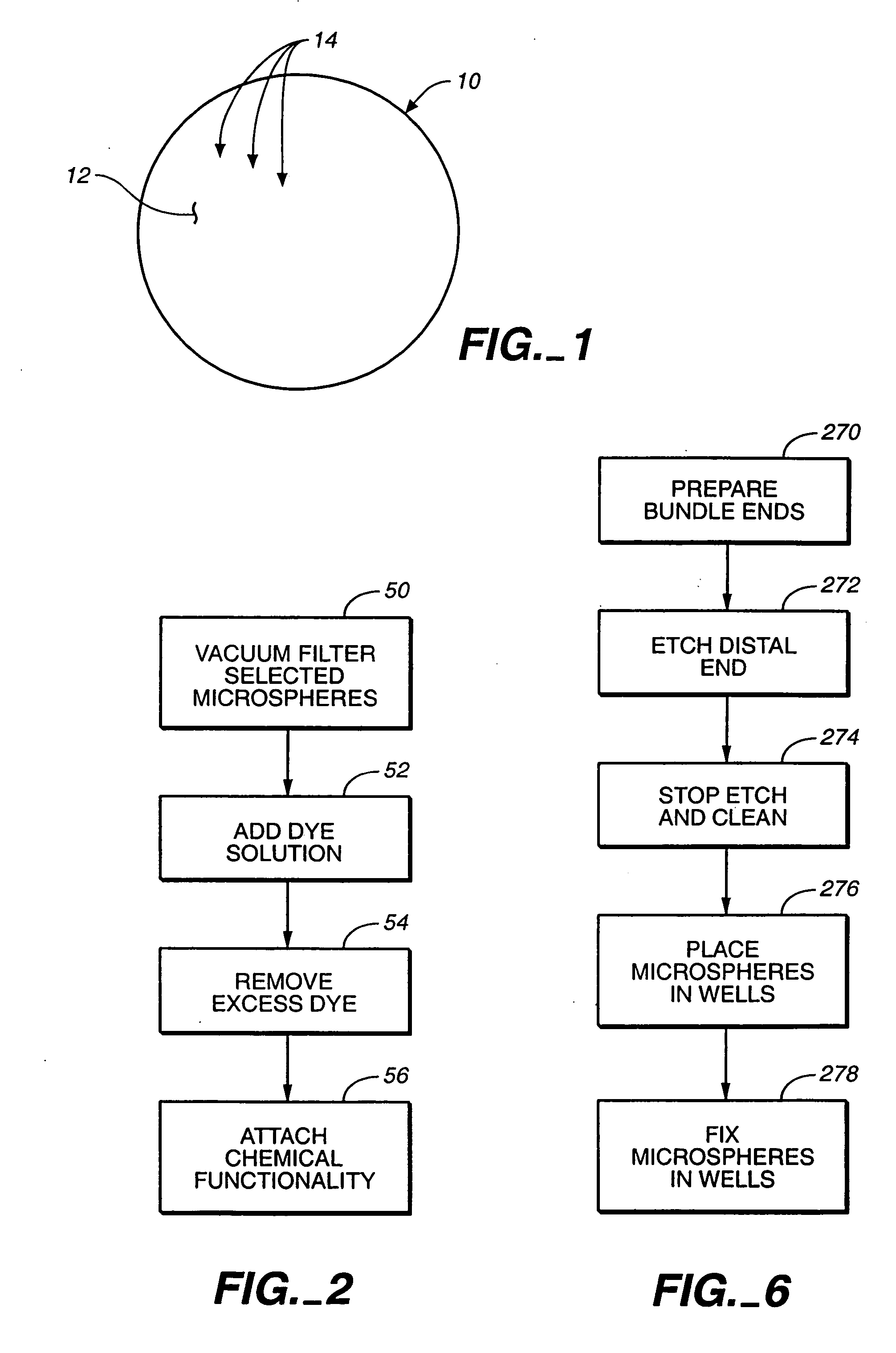
A microsphere-based analytic chemistry system and method for making the same is disclosed in which microspheres or particles carrying bioactive agents may be combined randomly or in ordered fashion and dispersed on a substrate to form an array while maintaining the ability to identify the location of bioactive agents and particles within the array using an optically interrogatable, optical signature encoding scheme. A wide variety of modified substrates may be employed which provide either discrete or non-discrete sites for accommodating the microspheres in either random or patterned distributions. The substrates may be constructed from a variety of materials to form either two-dimensional or three-dimensional configurations. In a preferred embodiment, a modified fiber optic bundle or array is employed as a substrate to produce a high density array. The disclosed system and method have utility for detecting target analytes and screening large libraries of bioactive agents.
Owner:TRUSTEES OF TUFTS COLLEGETHE
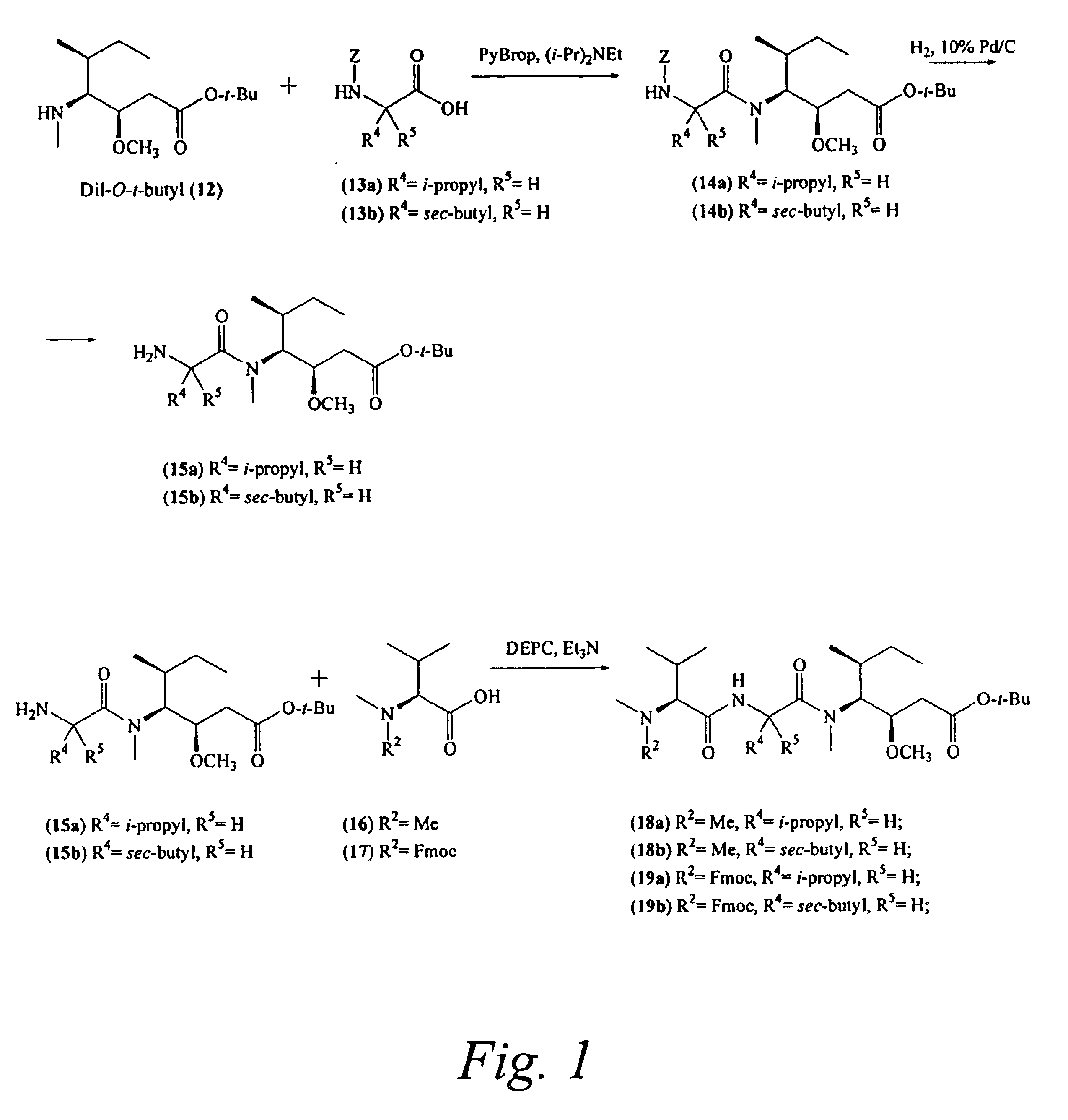
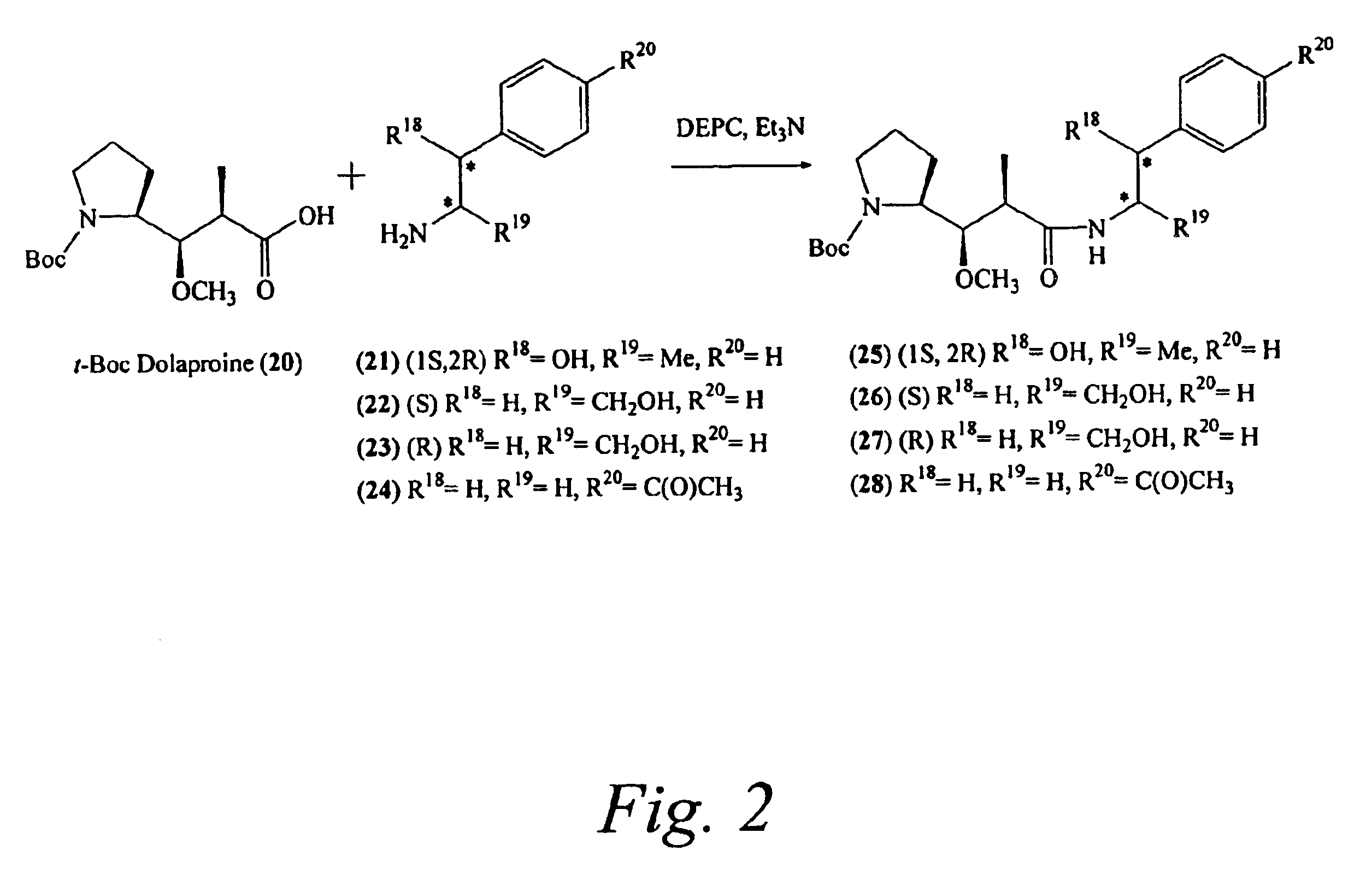
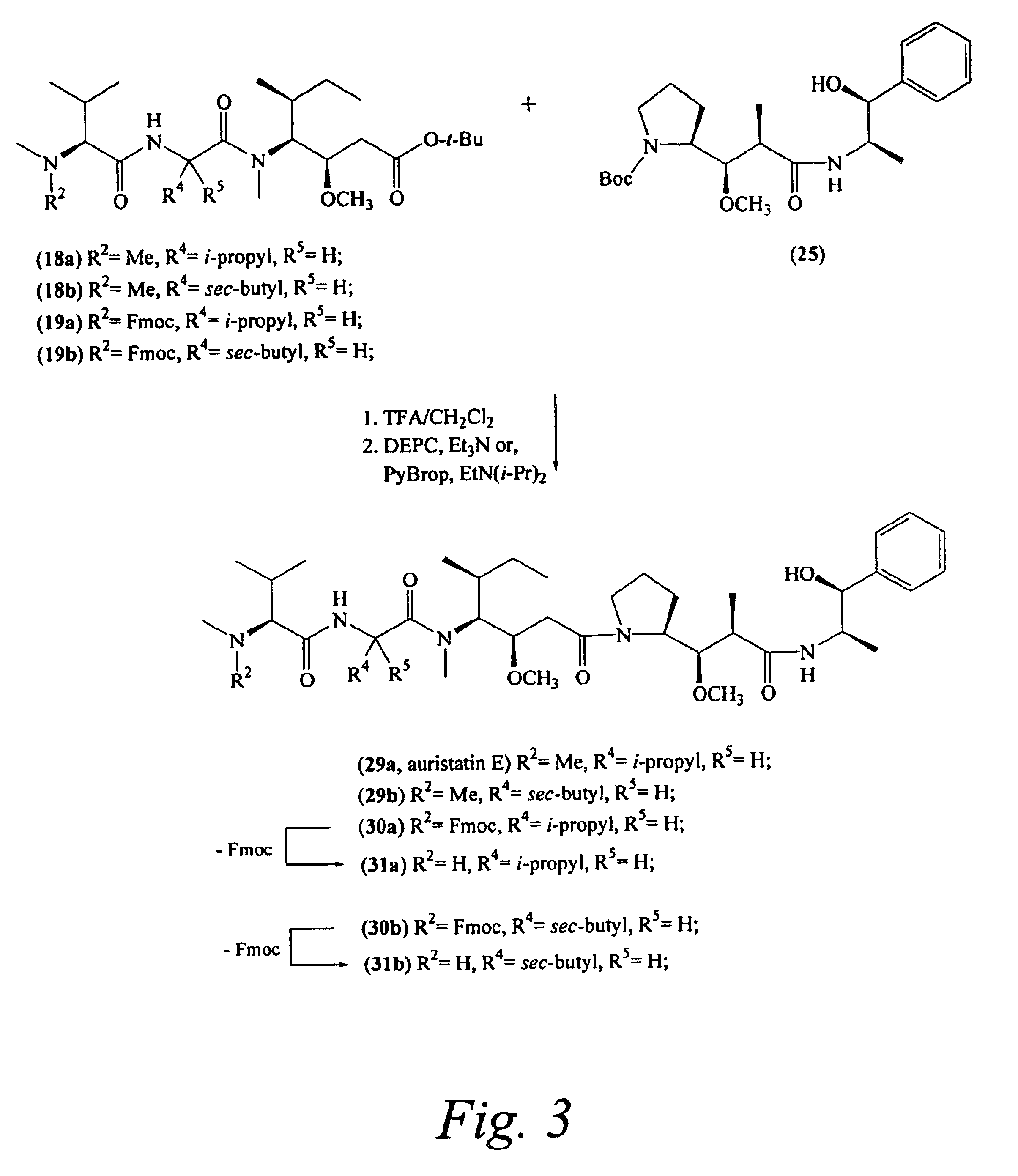
Pentapeptide compounds and uses related thereto
Pentapeptide compounds are disclosed. The compounds have biological activity, e.g., cytotoxicity. Prodrugs having targeting groups and pentapeptide moieities, as well as precursors thereof are also disclosed. For example, precursors having a reactive linker that can serve as a reaction site for joining to a targeting agent, e.g., an antibody, as disclosed.
Owner:SEAGEN INC
Analyte measuring device
InactiveUS20080228051A1Additive manufacturing apparatusMicrobiological testing/measurementAnalyteCell layer
An implantable analyte-measuring device including a membrane adapted to promote vascularization and / or interfere with barrier cell layer formation. The membrane includes any combination of materials, architecture, and bioactive agents that facilitate analyte transport to provide long-term in vivo performance of the implantable analyte-measuring device.
Owner:DEXCOM
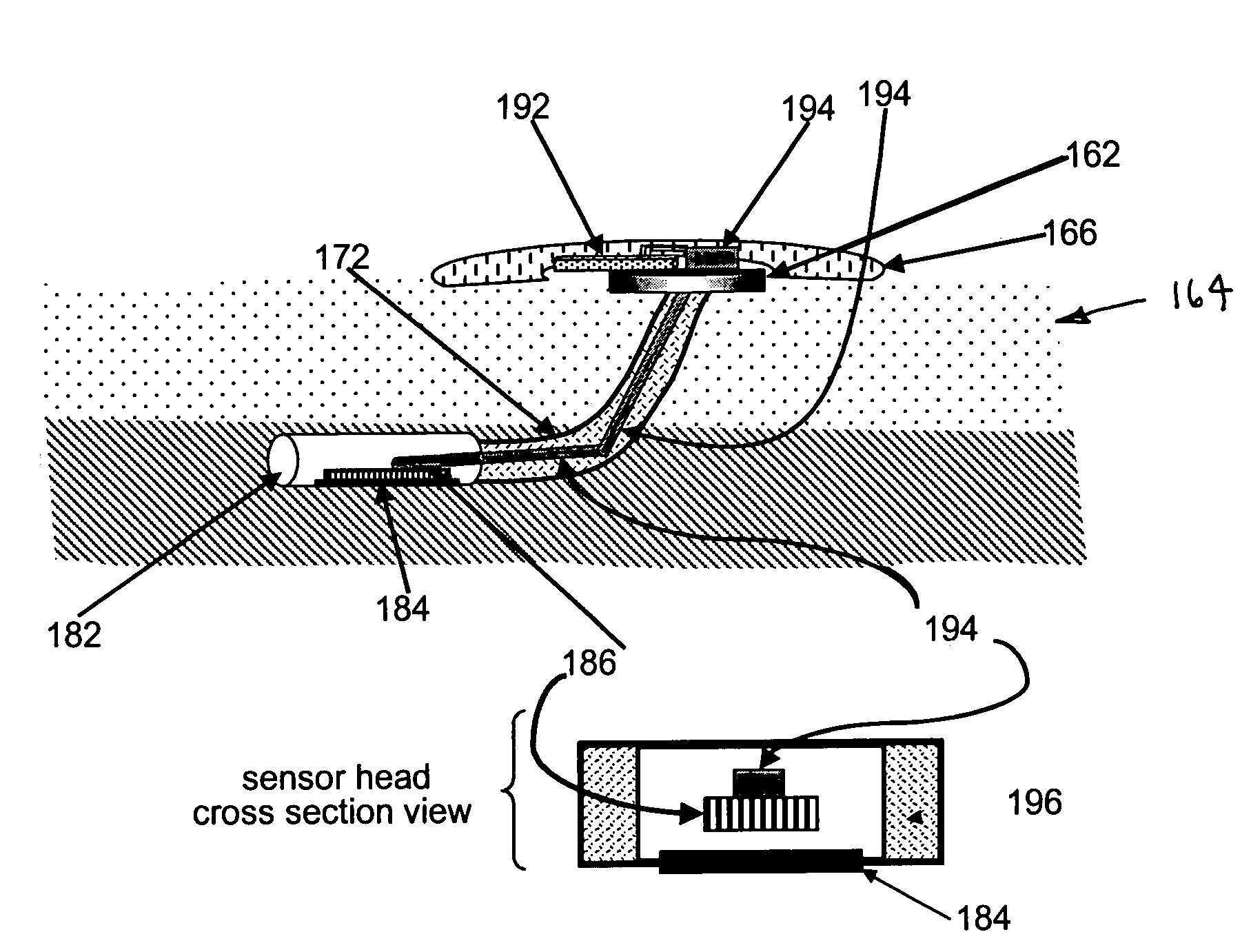
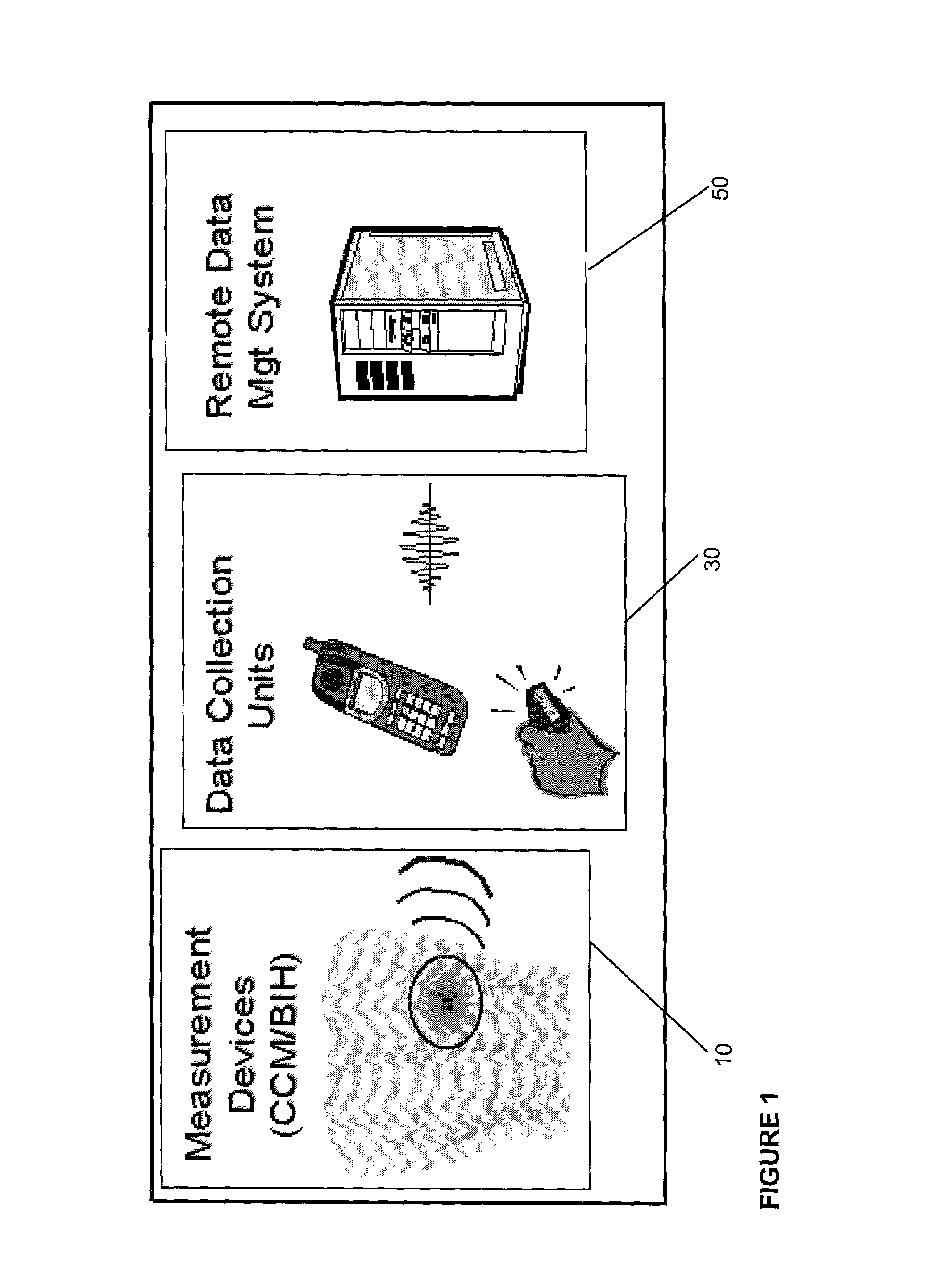
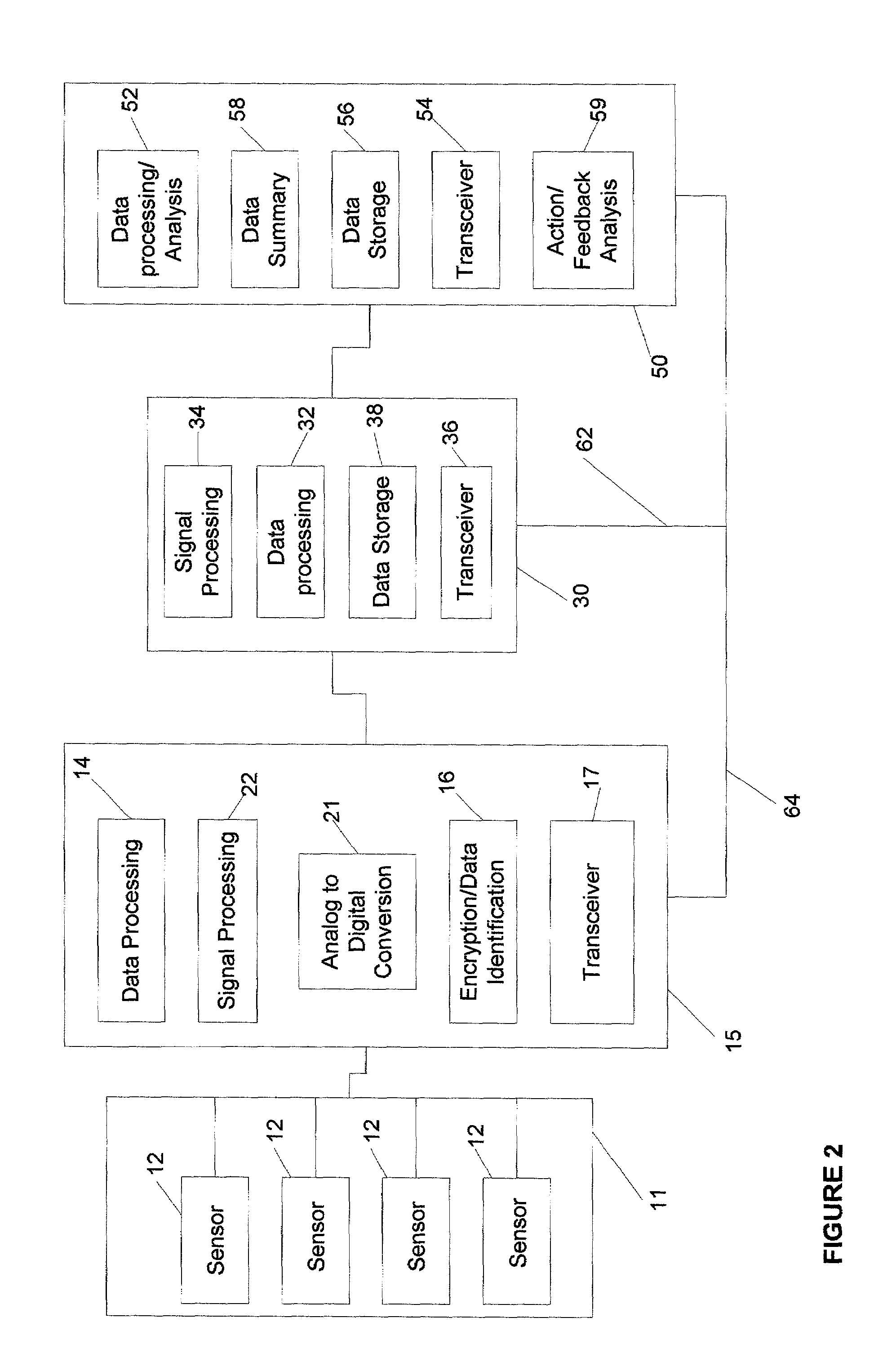
Gateway platform for biological monitoring and delivery of therapeutic compounds
InactiveUS7044911B2Improve data qualityReduce variationBioelectric signal measurementDrug and medicationsEngineeringBiomonitoring
The invention relates to methods and devices for remote or distributed continuous monitoring of physiologically relevant states. The invention provides for methods to automatically detect deviations or other states in physiological parameters and automatically alert a measured subject, user or other authorized party. The device provides for a universal platform for sensors, and further provides for the automatic compensation or distribution of devices or bioactive agents at appropriate levels and / or intervals in response to deviations or other states sensed in various physiological parameters.
Owner:PHILOMETRON
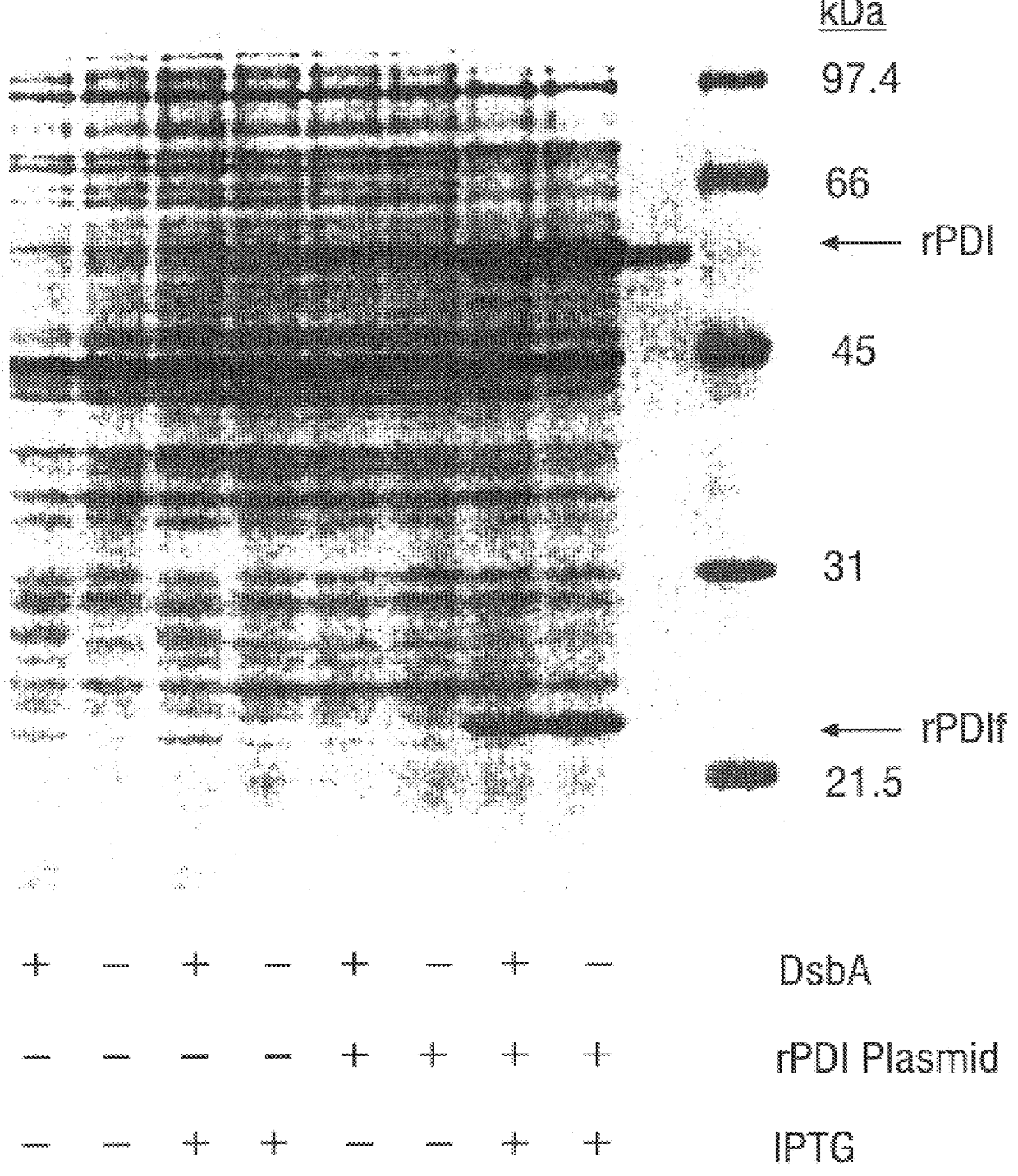
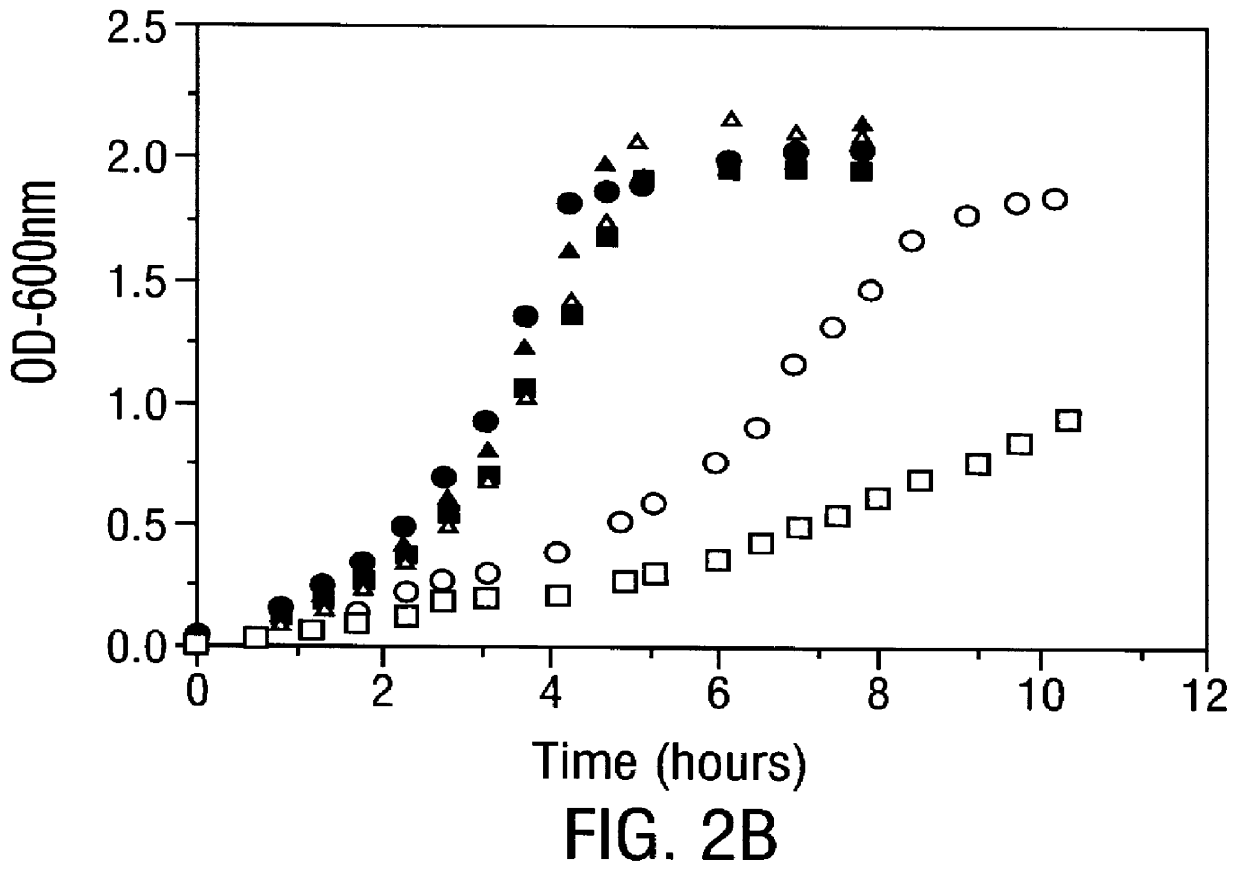
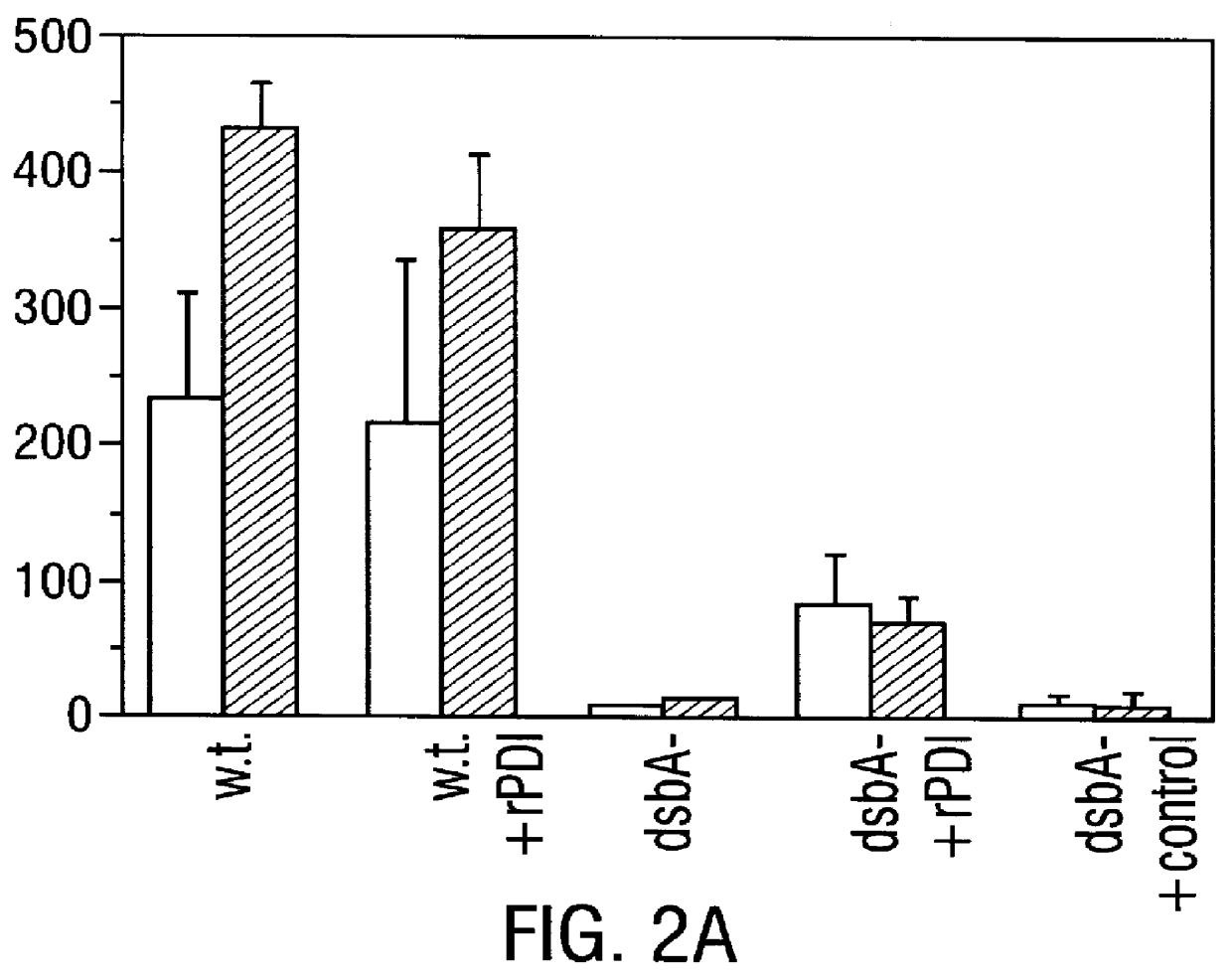
Methods for producing soluble, biologically-active disulfide-bond containing eukaryotic proteins in bacterial cells
InactiveUS6027888AEfficient productionFold preciselyPeptide/protein ingredientsMicroorganismsDisulfide bondingZymogen
Disclosed are methods of producing eukaryotic disulfide bond-containing polypeptides in bacterial hosts, and compositions resulting therefrom. Co-expression of a eukaryotic foldase and a disulfide bond-containing polypeptide in a bacterial host cell is demonstrated. In particular embodiments, the methods have been used to produce mammalian pancreatic trypsin inhibitor and tissue plasminogen activator (tPA) in soluble, biologically-active forms, which are isolatable from the bacterial periplasm. Also disclosed are expression systems, recombinant vectors, and transformed host cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
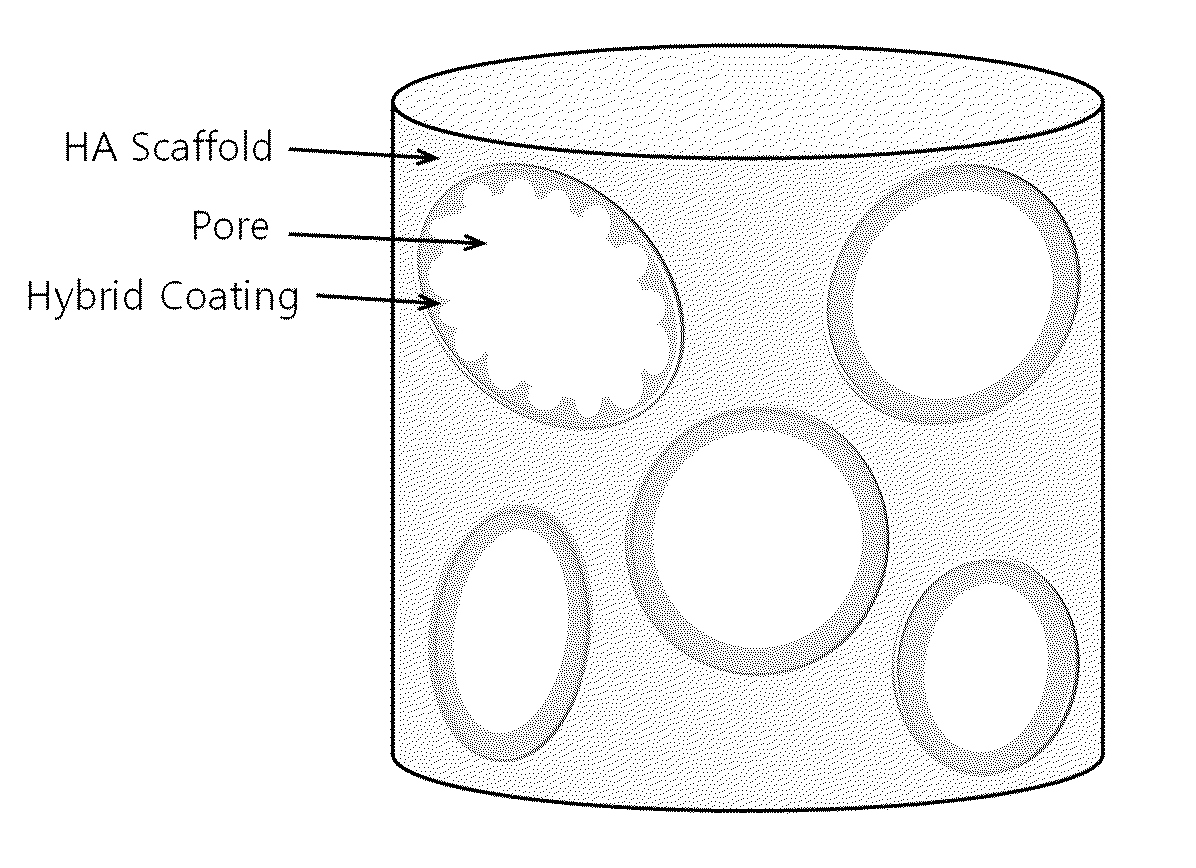
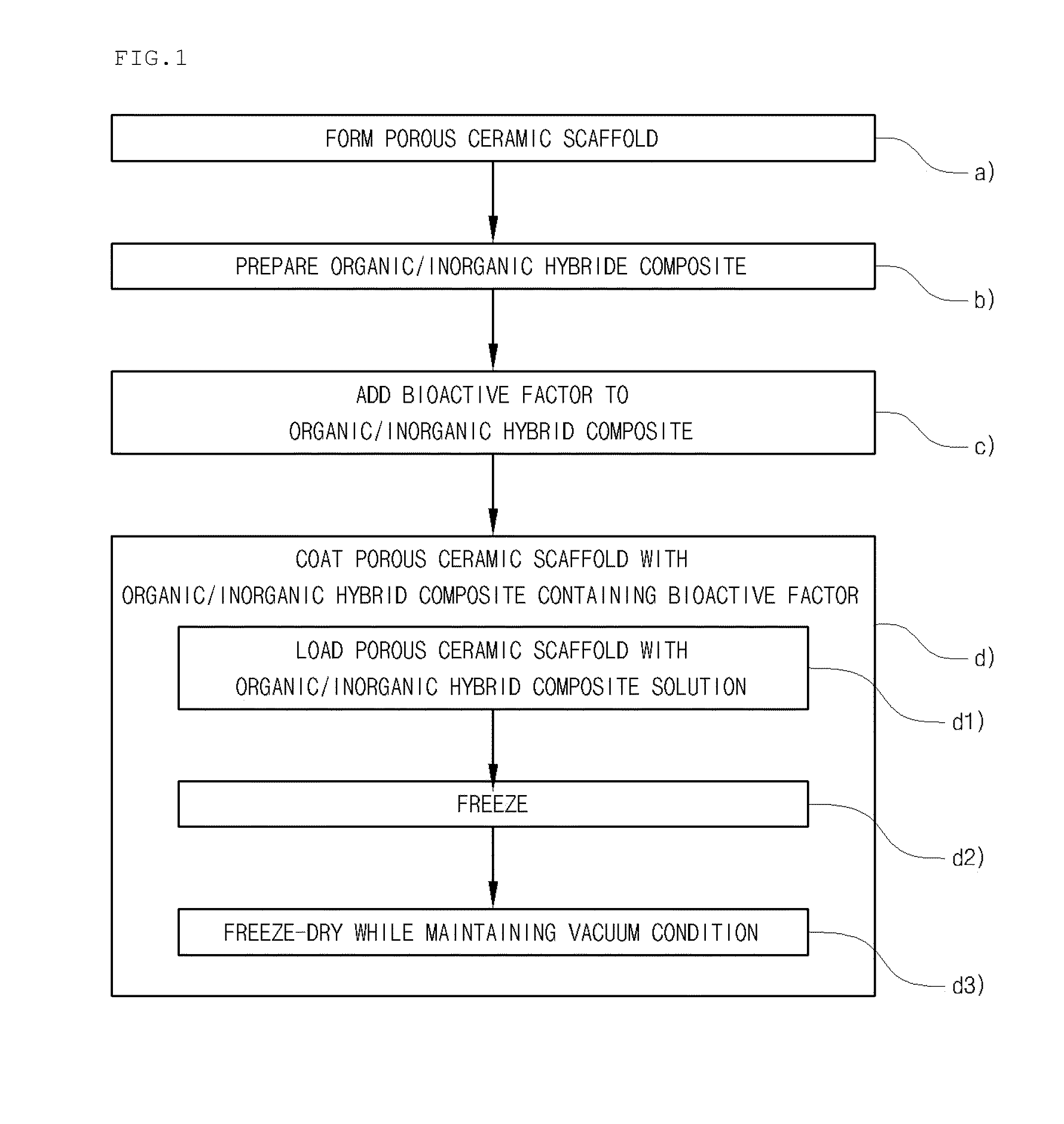
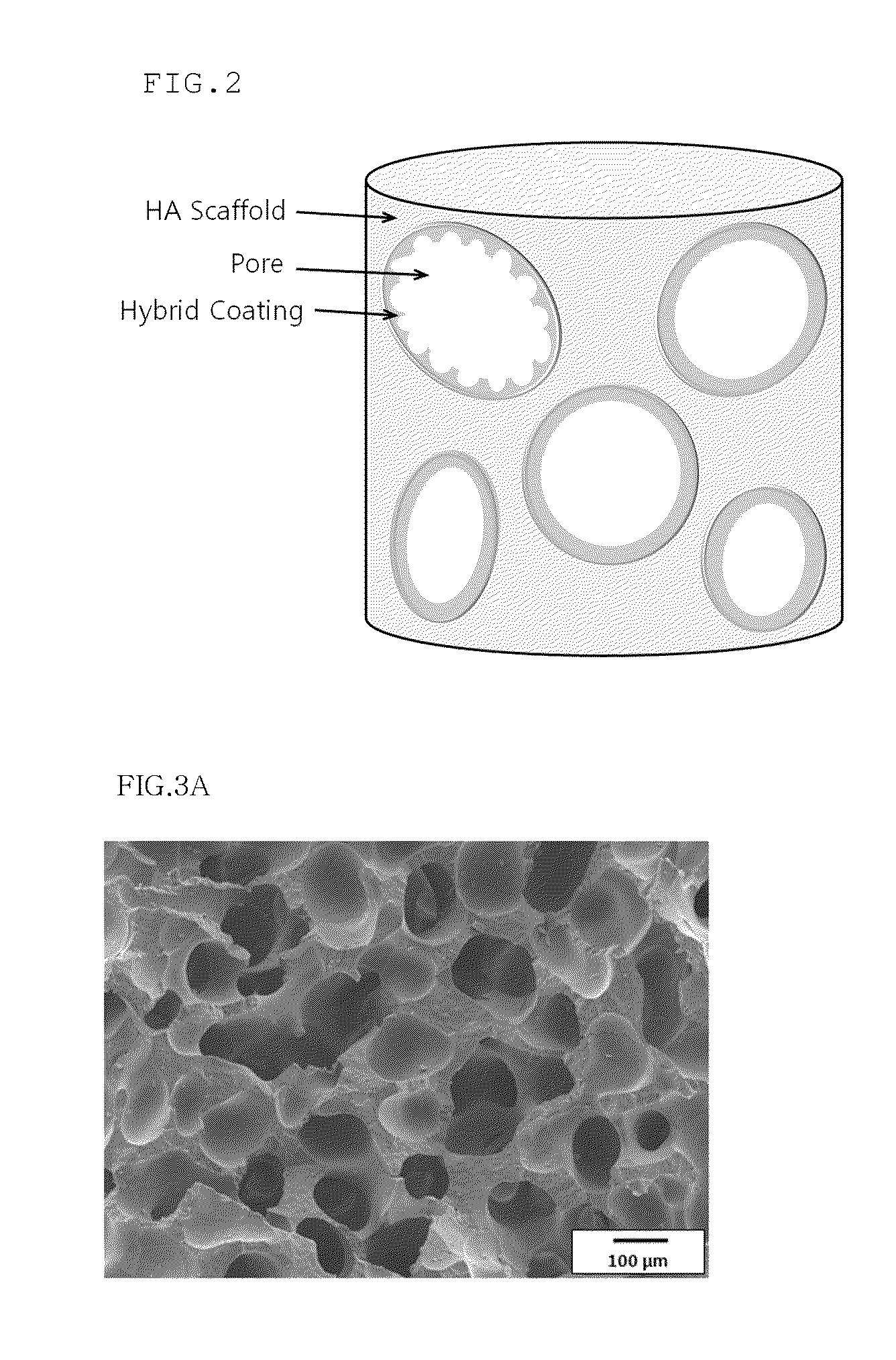
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
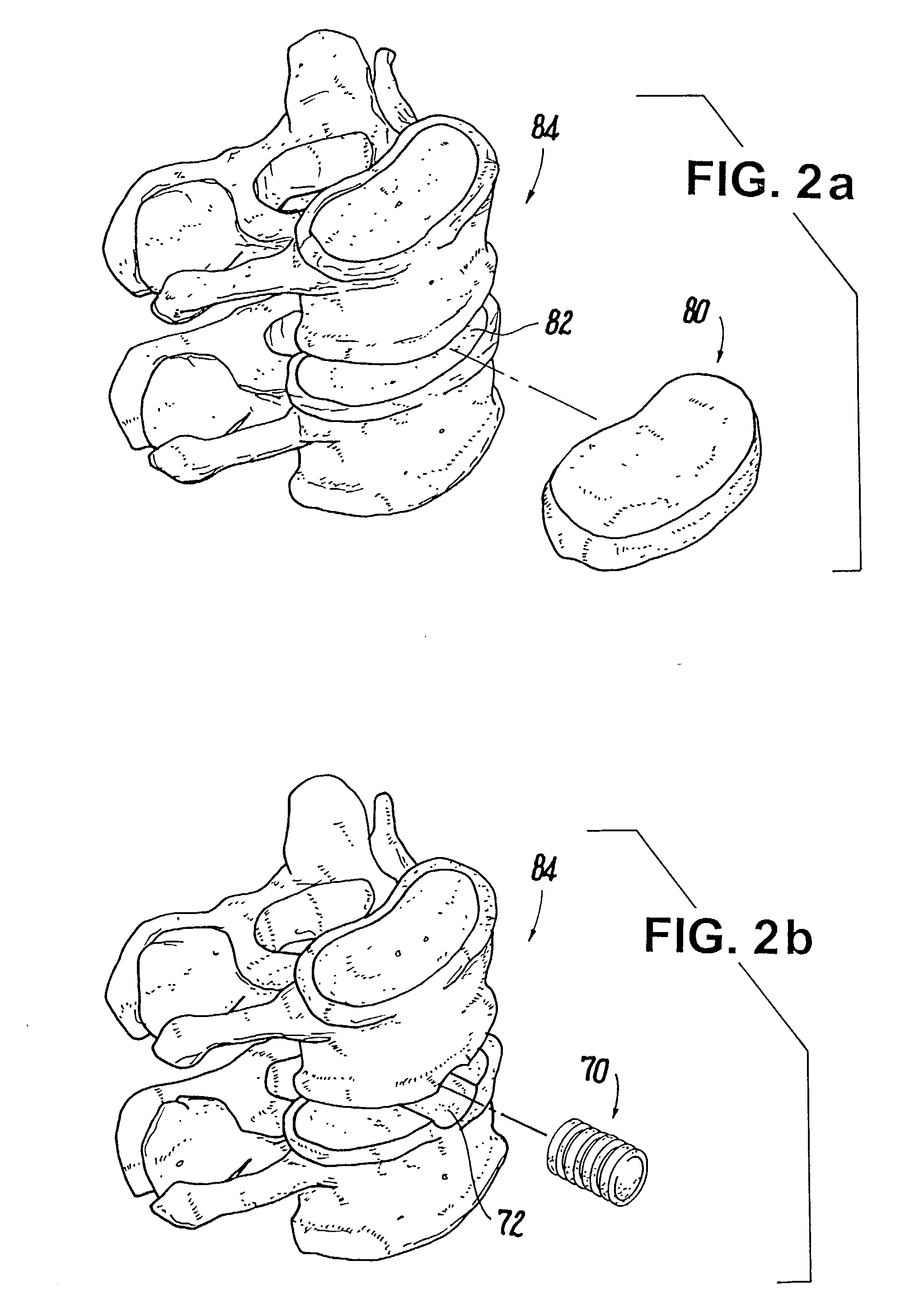
Bioactive spinal implants and method of manufacture thereof
InactiveUS7238203B2Facilitating radiographic assessmentEnhance bone contact and stability and fusionInternal osteosythesisJoint implantsLumbar vertebraeCervical fusions
A bioactive spinal implant used in cervical fusion, Anterior Lumbar Interbody Fusion (ALIF), Posterior Lumbar Interbody Fusion (PLIF), and Transforaminal Interbody Fusion (TLIF), having properties and geometries that enhance bone contact, stability, and fusion between adjacent vertebral bodies.
Owner:VIA SPECIAL PURPOSE CORP +1
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Shaped load-bearing osteoimplant and methods of making same
InactiveUS20030039676A1Promotes new host bone tissue formationPermit of mechanical propertySuture equipmentsDental implantsMedicineHard tissue
A load-bearing osteoimplant, methods of making the osteoimplant and method for repairing hard tissue such as bone and teeth employing the osteoimplant are provided. The osteoimplant comprises a shaped, coherent mass of bone particles which may exhibit osteogenic properties. In addition, the osteoimplant may possess one or more optional components which modify its mechanical and / or bioactive properties, e.g., binders, fillers, reinforcing components, etc.
Owner:WARSAW ORTHOPEDIC INC
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
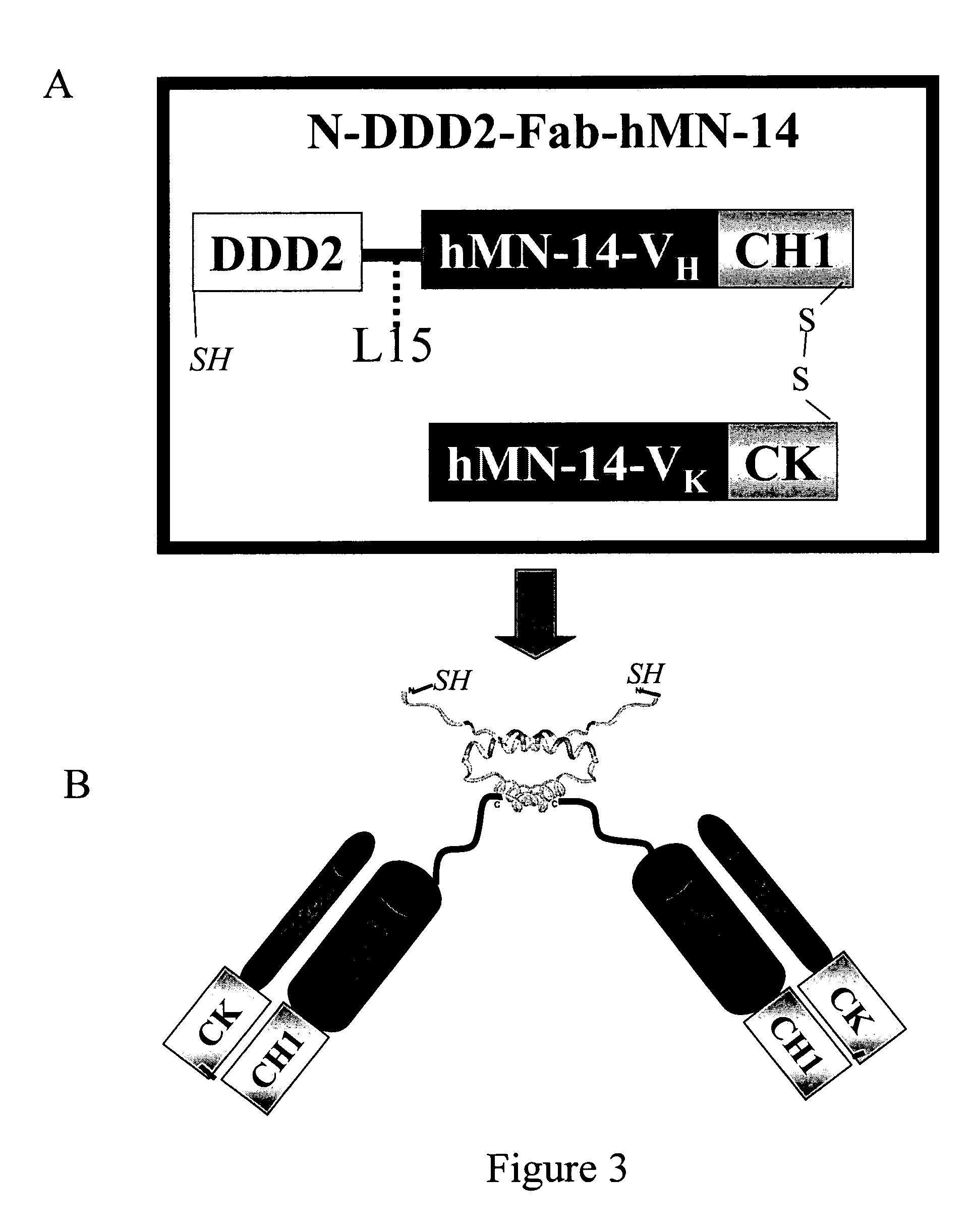
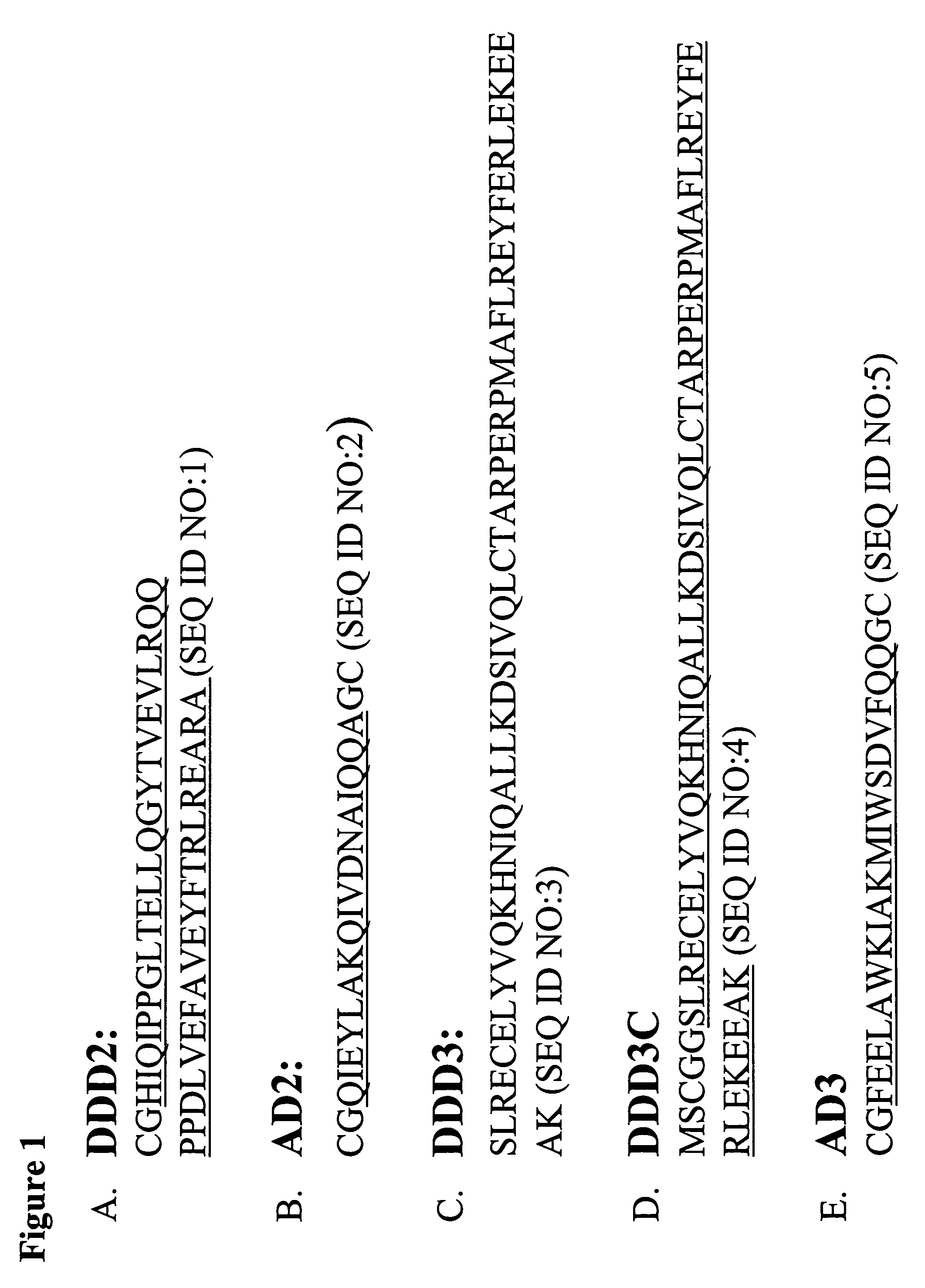
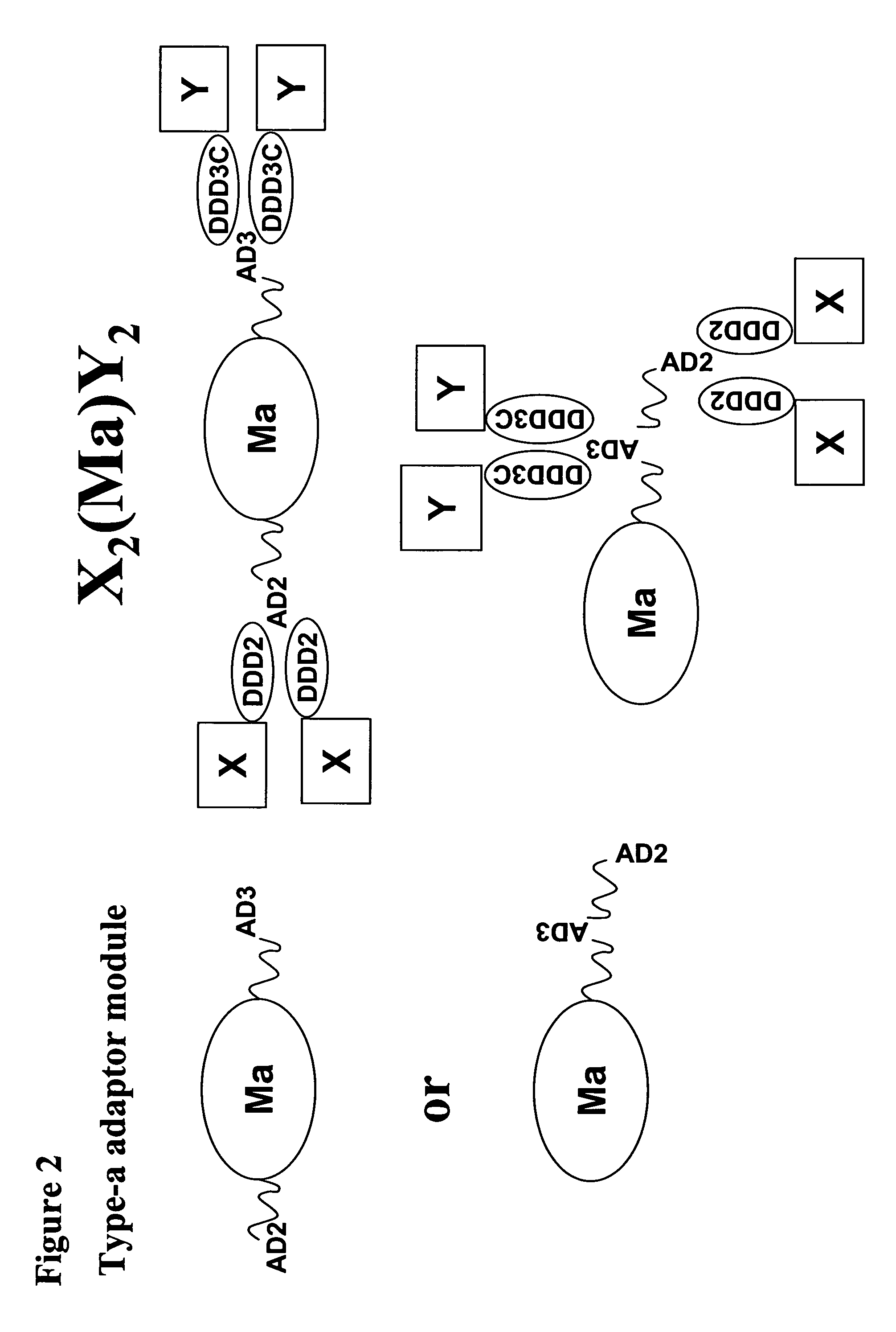
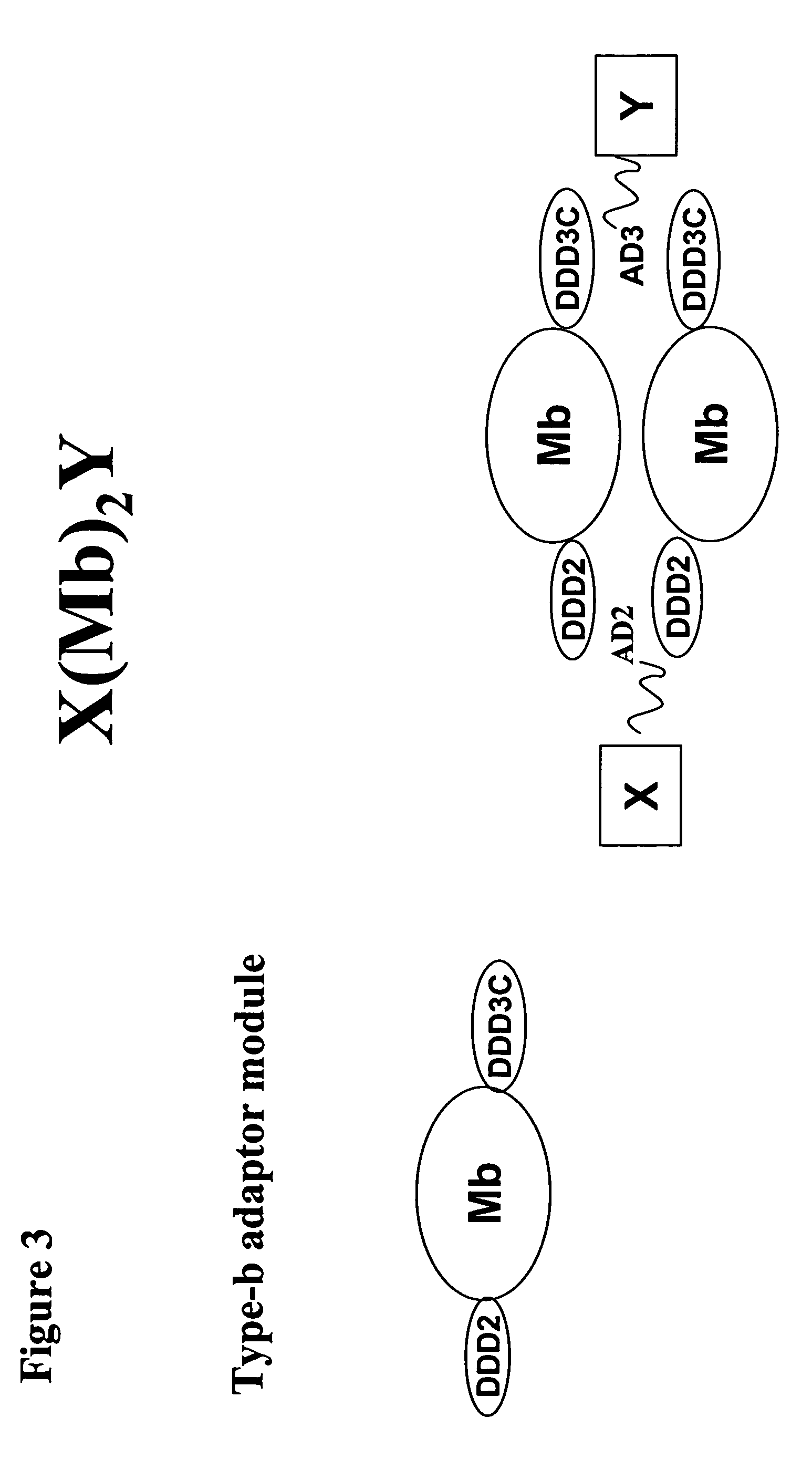
Multivalent immunoglobulin-based bioactive assemblies
ActiveUS7527787B2Efficacious for arrestingInhibition formationPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDiagnostic agent
Owner:IBC PHARMACEUTICALS INC
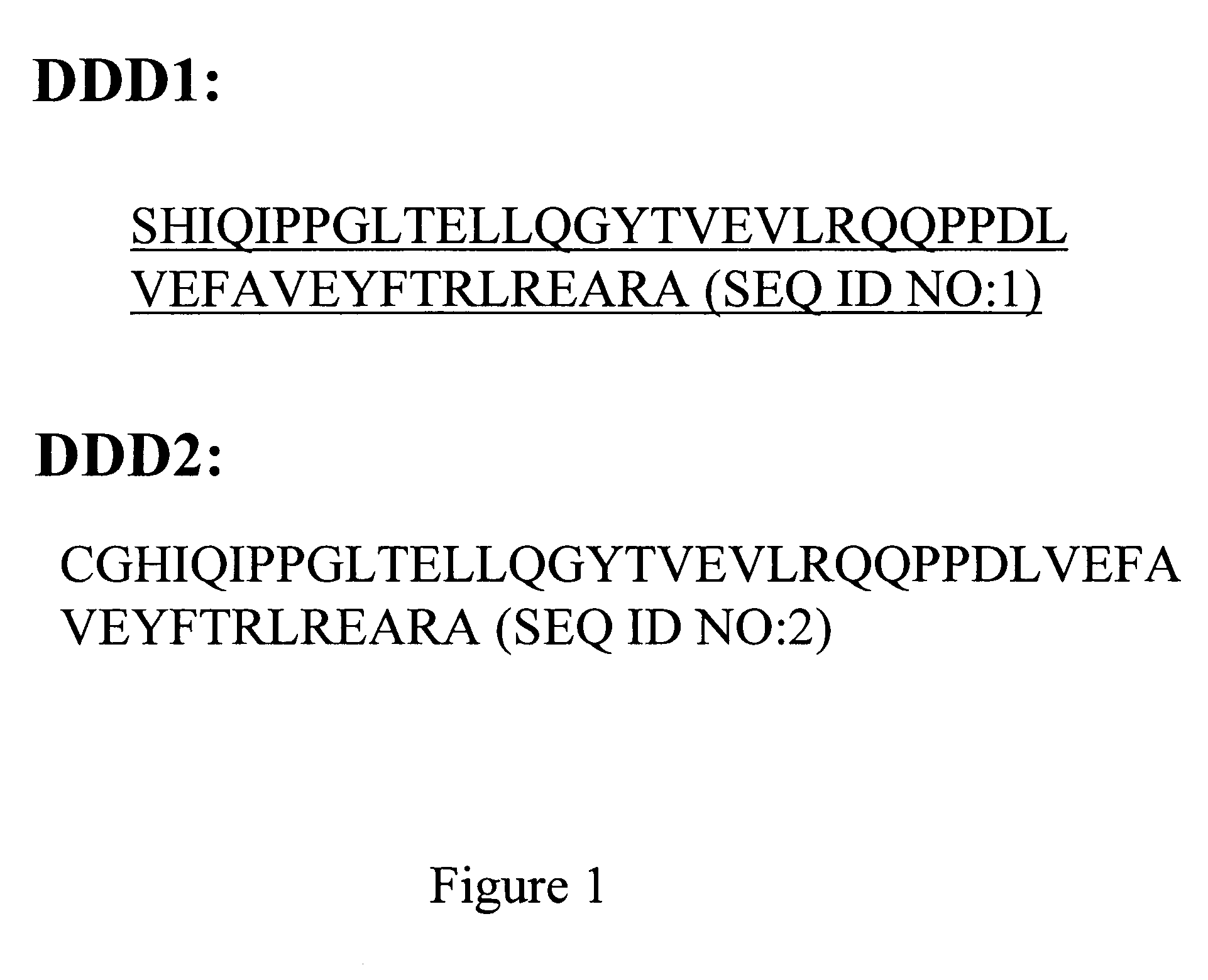
Methods and compositions for generating bioactive assemblies of increased complexity and uses
The present invention concerns methods and compositions for making and using bioactive assemblies of defined compositions, which may have multiple functionalities and / or binding specificities. In particular embodiments, the bioactive assembly is formed using dock-and-lock (DNL) methodology, which takes advantage of the specific binding interaction between dimerization and docking domains (DDD) and anchoring domains (AD) to form the assembly. In various embodiments, one or more effectors may be attached to a DDD or AD sequence. Complementary AD or DDD sequences may be attached to an adaptor module that forms the core of the bioactive assembly, allowing formation of the assembly through the specific DDD / AD binding interactions. Such assemblies may be attached to a wide variety of effector moieties for treatment, detection and / or diagnosis of a disease, pathogen infection or other medical or veterinary condition.
Owner:IBC PHARMACEUTICALS INC
Targeted delivery of active/bioactive and perfuming compositions
Described are controlled, time-release microparticulate active and bioactive compositions (including perfuming compositions) for targeted delivery to surfaces such as skin, hair and fabric and the environment proximate thereto, where the active and bioactive materials have a calculated log10P values of between 1 and 8 (P being the n-octanol-water partition coefficient). Such compositions include the active or bioactive material in single phase, solid solution in a wax or polymer matrix also having coated thereon and / or containing a compatible surfactant. Also described are processes and apparatus for preparing such compositions and processes for using same. Furthermore, certain component(s) of the aforementioned compositions in combination with one another are novel, and other components have novel uses in increasing fragrance substantivity, particularly in hair care preparations such as hair gels and shampoos.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
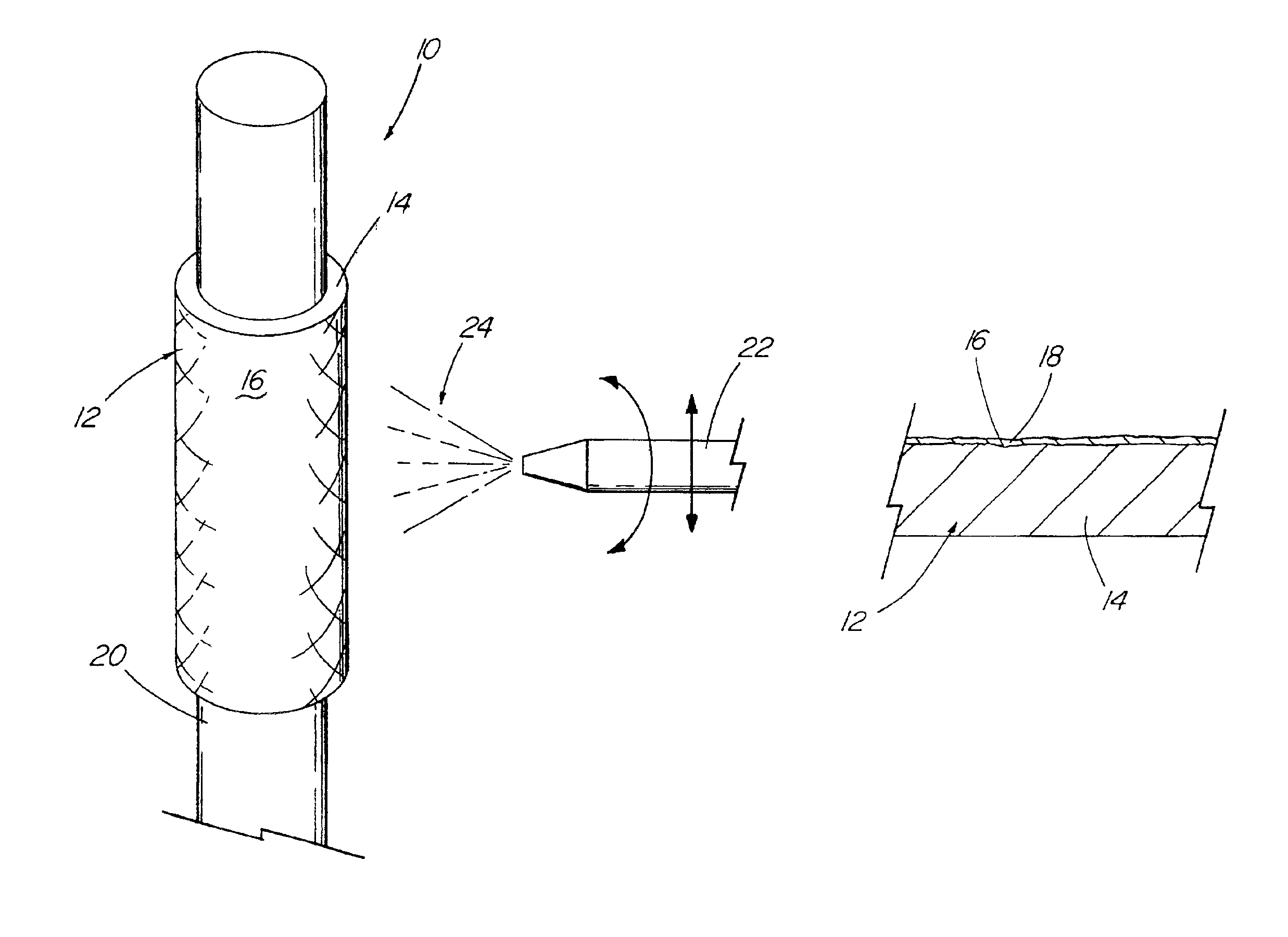
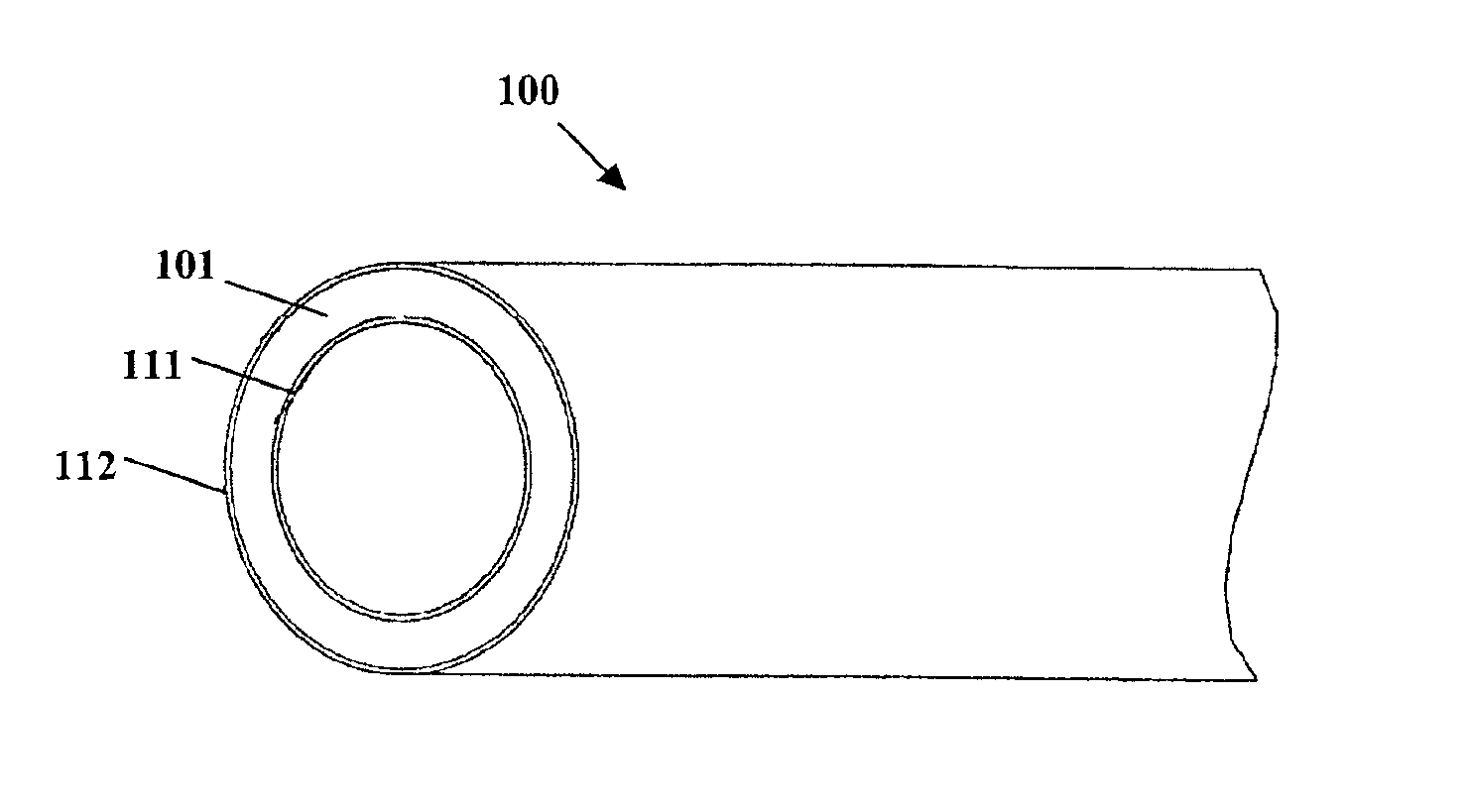
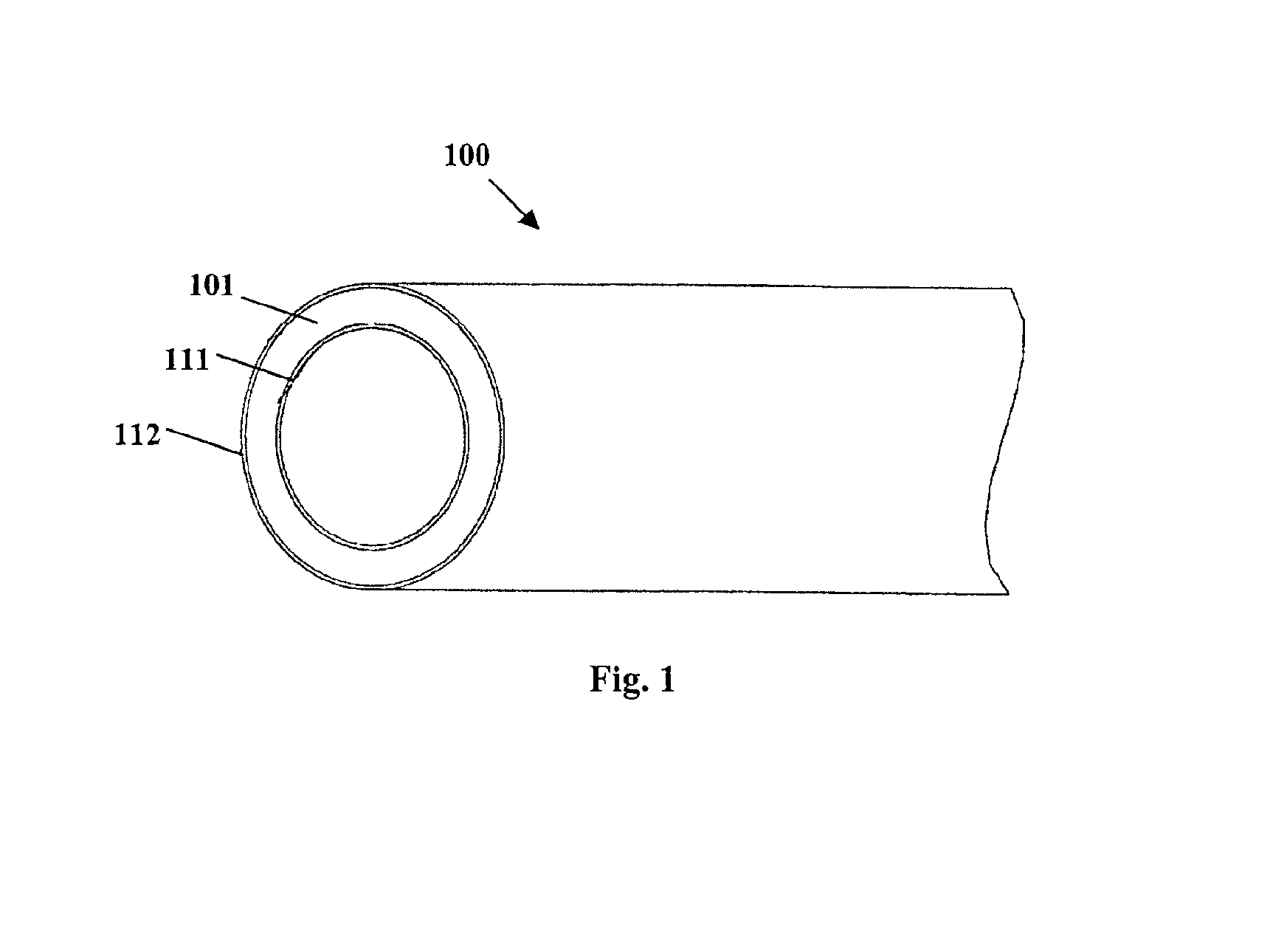
Coated implantable medical device
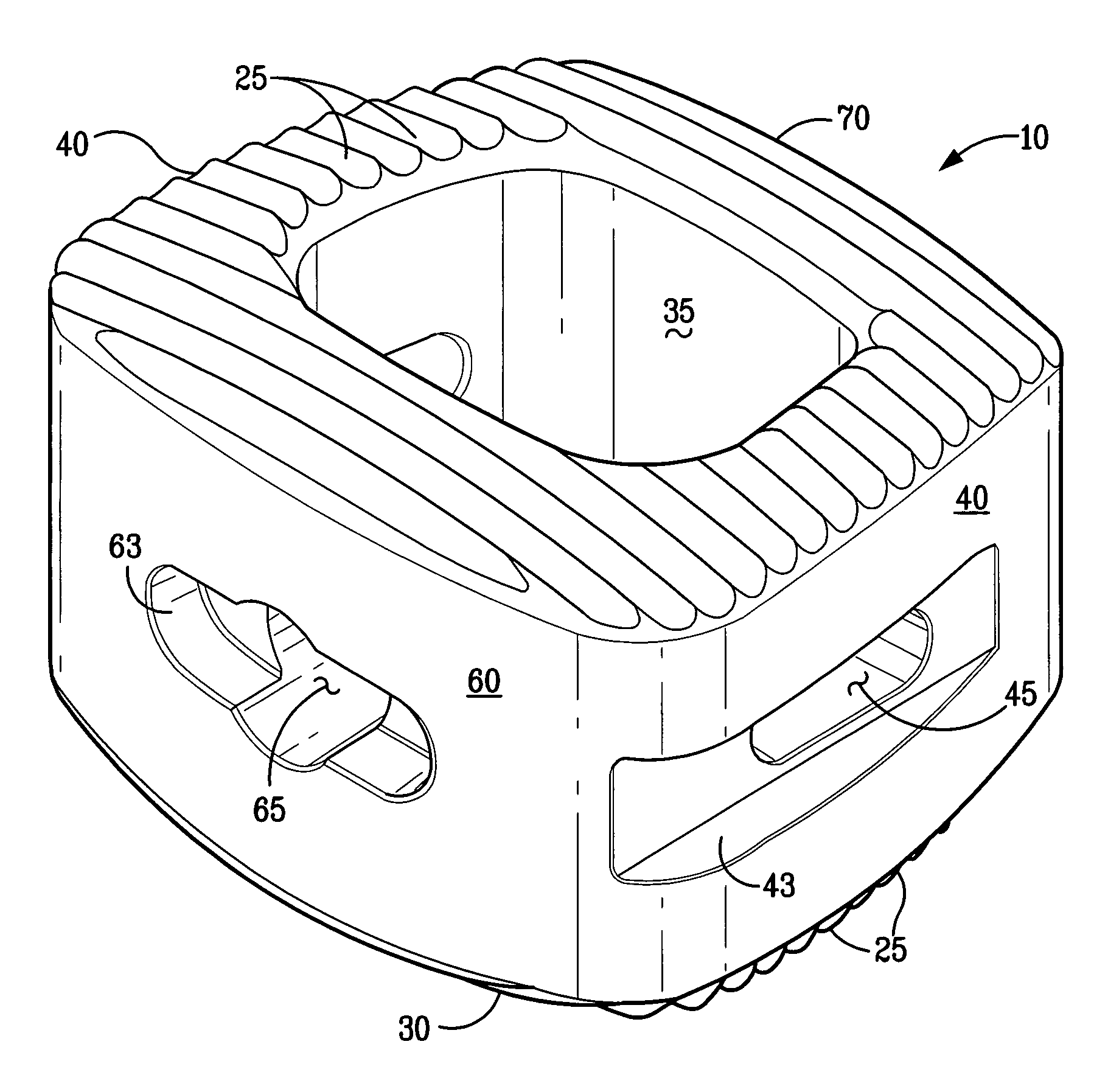
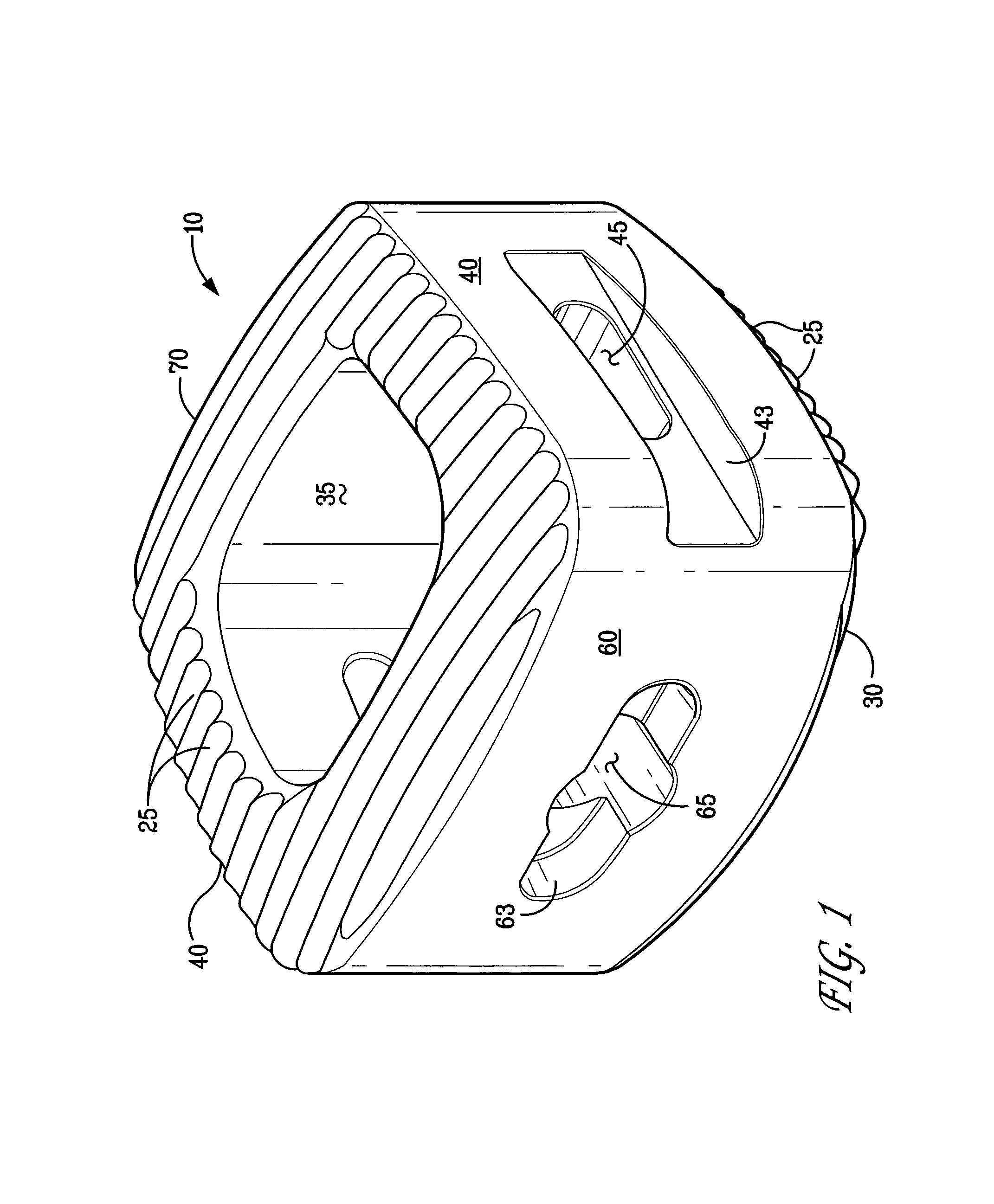
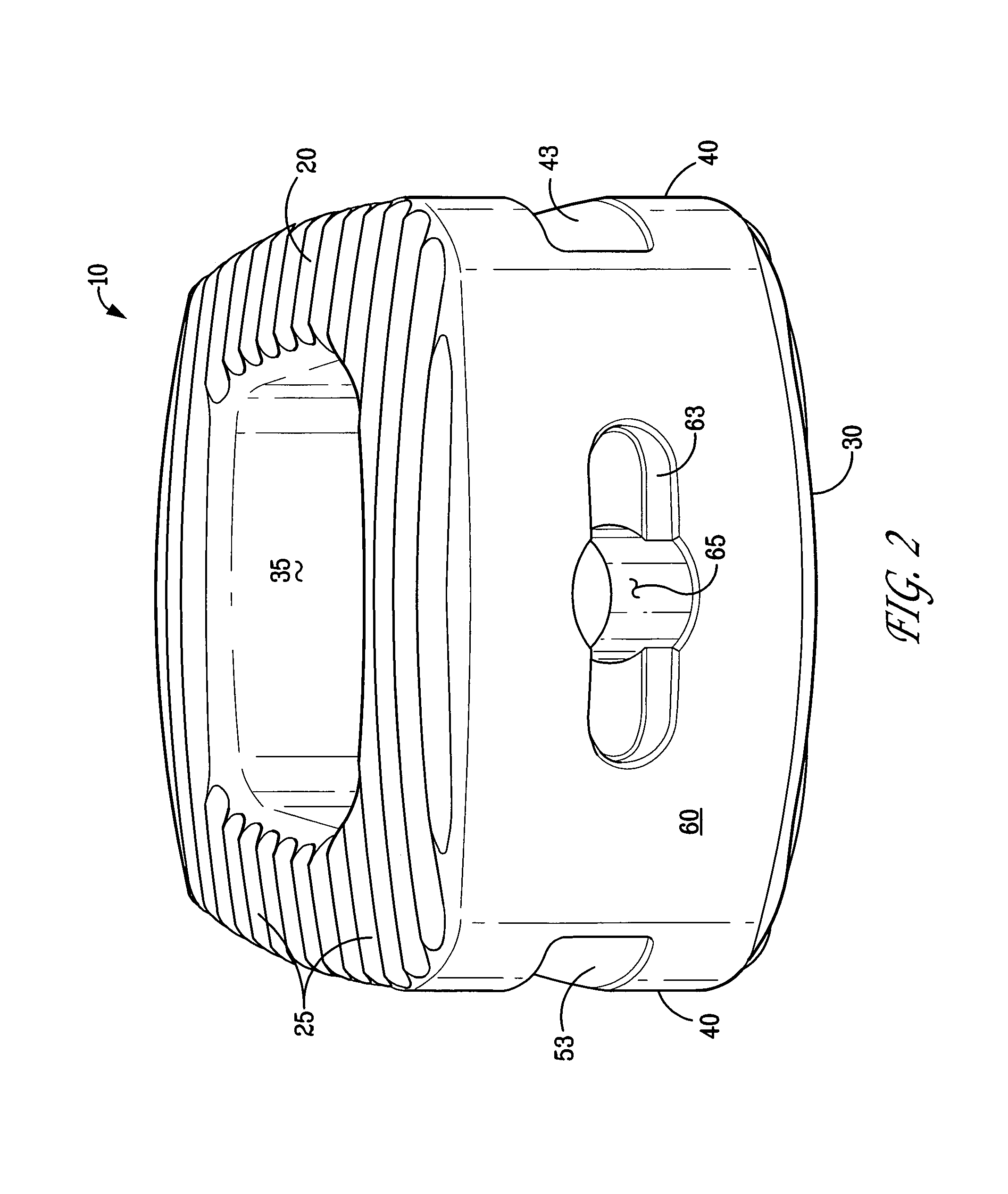
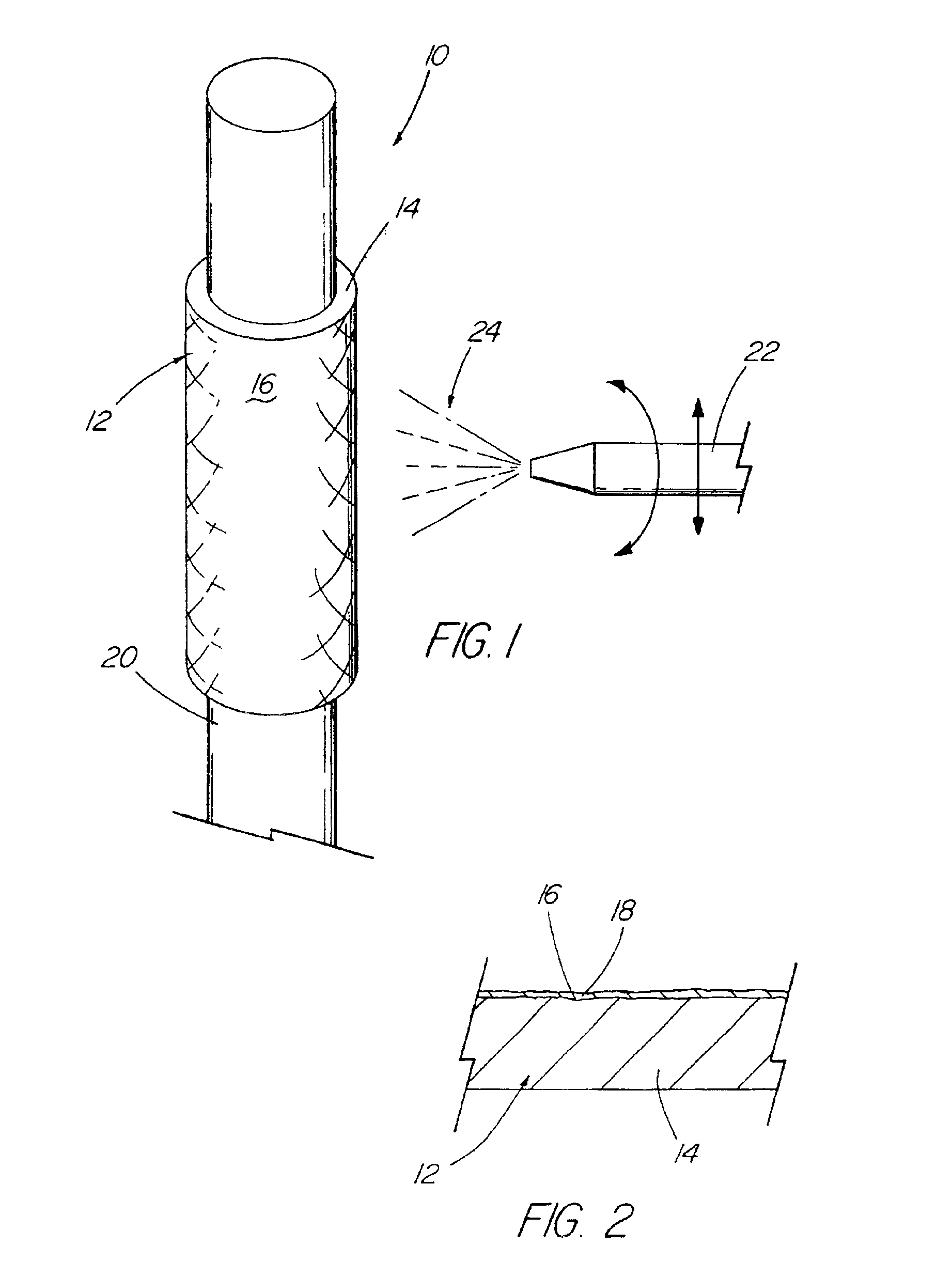
InactiveUS6918927B2Good worker safetyLow costOrganic active ingredientsSurgerySodium bicarbonateMedicine
A medical device (10) includes a structure (12) adapted for introduction into a patient, the structure (12) being formed of a preferably non-porous base material (14) having a roughened or textured surface (16). The structure (12) is conveniently configured as a vascular stent with a base material (14) of stainless steel, nitinol or another suitable material. The medical device (10) also includes a layer (18) of a bioactive material posited directly upon the roughened or textured surface (16) of the base material (14) of the structure (12). The surface (16) of the base material (14) is roughened or textured by etching or by abrasion with sodium bicarbonate or another suitable grit. A preferred roughened or textured surface (16) is thought to have a mean surface roughness of about 10 μin. (about 250 nm) and a surface roughness range between about 1 μin. and about 100 μin. (about 25 nm and about 2.5 μm). The particularly preferred use of sodium bicarbonate as the abrasive to provide roughness or texture to the surface (16) of the base material (14) of the structure (12) is additionally advantageous in the low toxicity of the sodium bicarbonate to production workers, the ease of product and waste cleanup, and the biocompatibility of any residual sodium bicarbonate.
Owner:COOK MEDICAL TECH LLC
Apparatus for preparing a solid phase microparticulate composition
InactiveUS6042792ALow costImproved substantivityCosmetic preparationsComponent separationWaxMicroparticle
Described are controlled, time-release microparticulate active and bioactive compositions (including perfuming compositions) for targeted delivery to services such as skin, hair and fabric and the environment proximate thereto, where the active and bioactive materials have a calculated log10P values of between 1 and 8 (P being the n-octanol-water partition coefficient). Such compositions include the active or bioactive material in single phase, solid solution in a wax or polymer matrix also having coated thereon and / or containing a compatible surfactant. Also described are processes and apparatus for preparing such compositions and processes for using same. Furthermore, certain component(s) of the aforementioned compositions in combination with one another are novel, and other components have novel uses in increasing fragrance substantivity.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Biointerface membranes incorporating bioactive agents
InactiveUS20060198864A1Improve performanceAdditive manufacturing apparatusSurgeryBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM INC
Neonatal Fc receptor (FcRn)-binding polypeptide variants, dimeric Fc binding proteins and methods related thereto
InactiveUS20070148164A1Increase free energyReduced binding affinityAnimal cellsSugar derivativesFc bindingNeonatal Fc receptor
The compositions and methods of the present invention are based, in part, on our discovery that an effector function mediated by an Fc-containing polypeptide can be altered by modifying one or more amino acid residues within the polypeptide (by, for example, electrostatic optimization). The polypeptides that can be generated according to the methods of the invention are highly variable, and they can include antibodies and fusion proteins that contain an Fc region or a biologically active portion thereof.
Owner:BIOGEN MA INC
Methods for detecting target analytes and enzymatic reactions
A microsphere-based analytic chemistry system and method for making the same is disclosed in which microspheres or particles carrying bioactive agents may be combined randomly or in ordered fashion and dispersed on a substrate to form an array while maintaining the ability to identify the location of bioactive agents and particles within the array using an optically interrogatable, optical signature encoding scheme. A wide variety of modified substrates may be employed which provide either discrete or non-discrete sites for accommodating the microspheres in either random or patterned distributions. The substrates may be constructed from a variety of materials to form either two-dimensional or three-dimensional configurations. In a preferred embodiment, a modified fiber optic bundle or array is employed as a substrate to produce a high density array. The disclosed system and method have utility for detecting target analytes and screening large libraries of bioactive agents.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
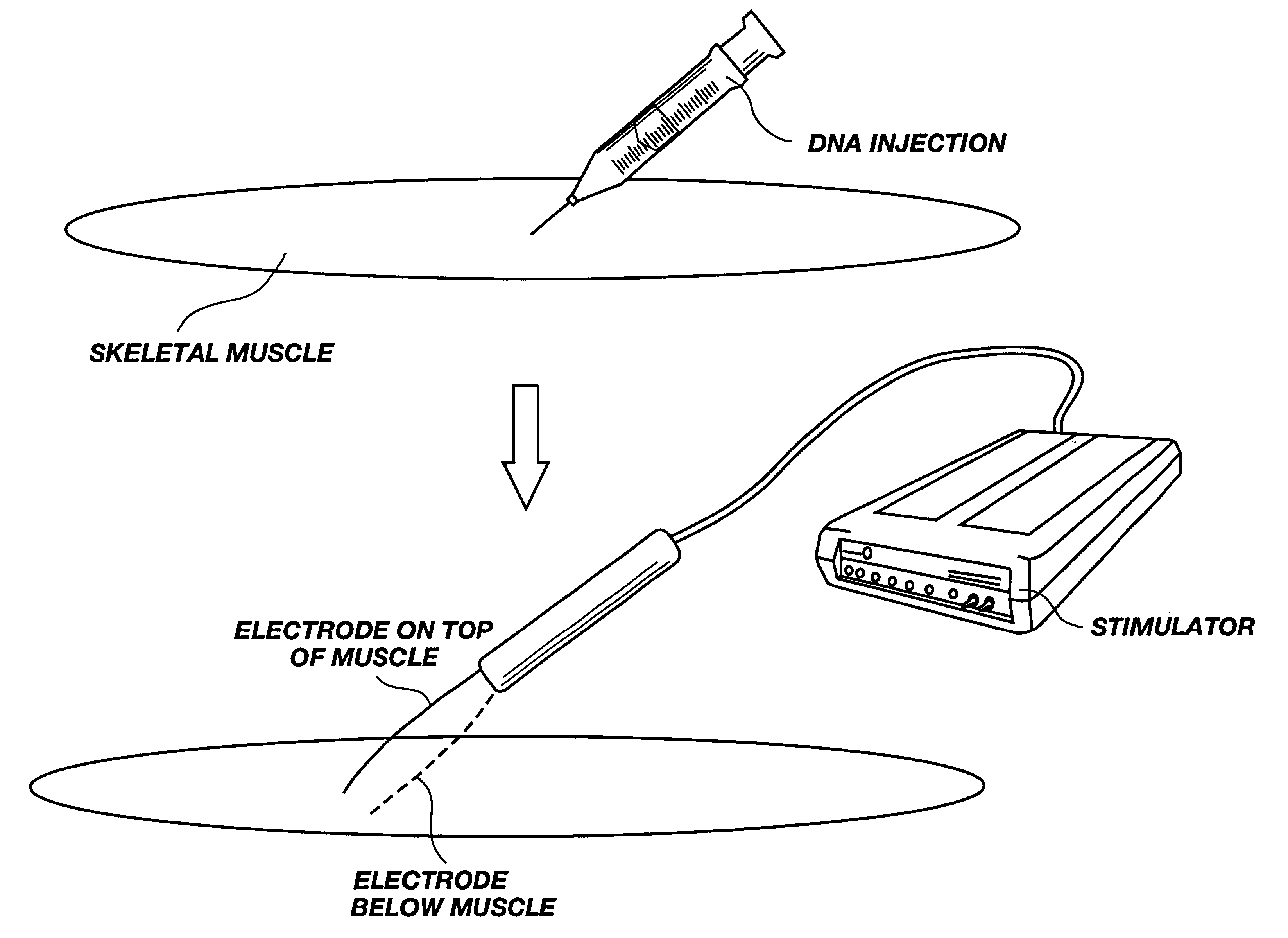
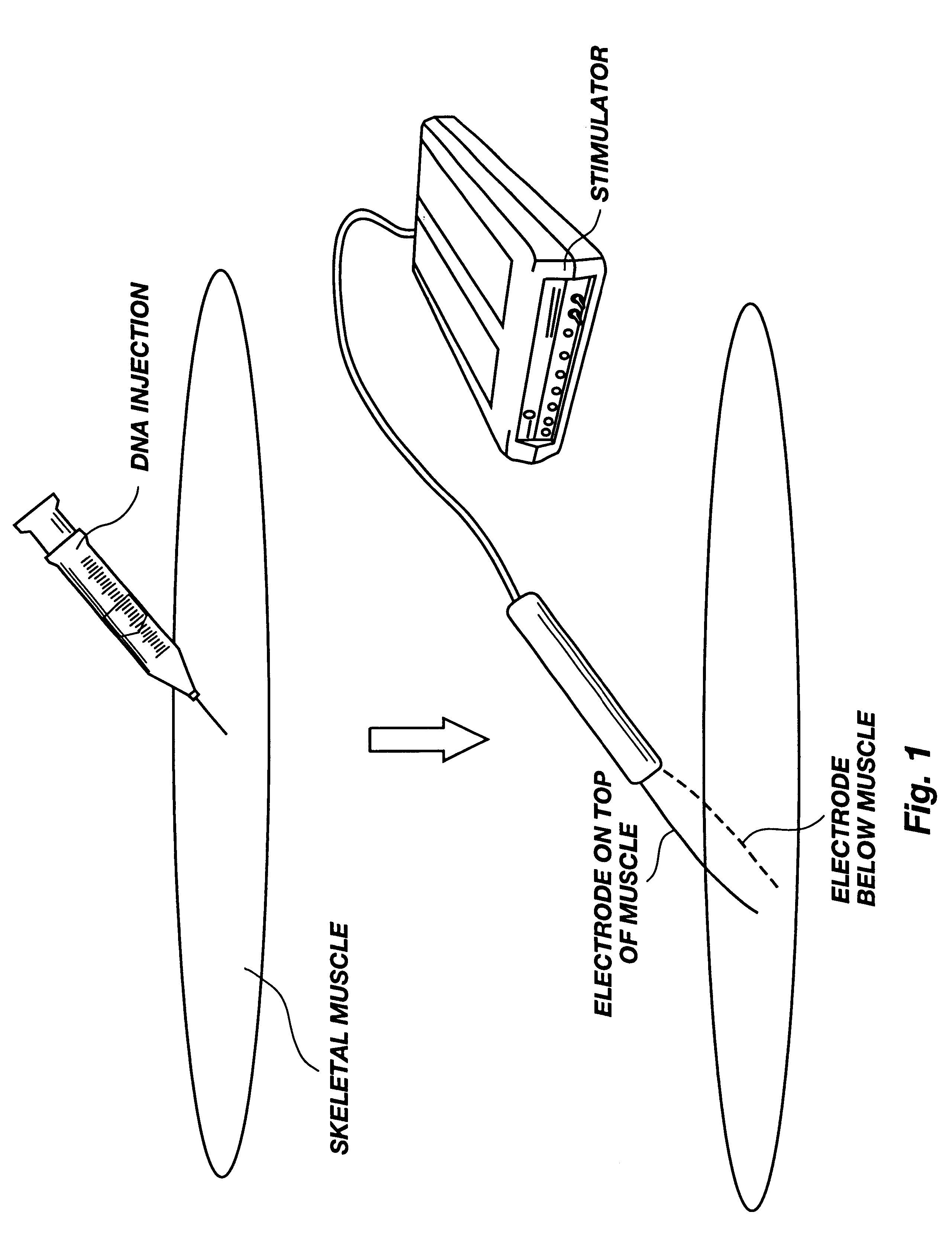
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
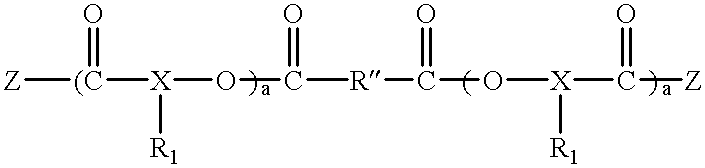
Polyester polyether block copolymers
The present invention relates to novel bioabsorbable polymeric compositions based upon AB polyester polyether or related diblocks and triblocks. Compositions according to the present invention may be used in medical applications, for example, for reducing or preventing adhesion formation subsequent to medical procedures such as surgery, for producing surgical articles including stents and grafts, as coatings, sealants, lubricants, as transient barriers in the body, for materials which control the release of bioactive agents in the body, for wound and bum dressings and producing biodegradable articles, among numerous others.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Implantable or insertable medical device resistant to microbial growth and biofilm formation
InactiveUS6887270B2Prevent preferential partitioningPrevent chemical modificationAntipyreticAnalgesicsActive agentMicrobial adhesion
Disclosed are implantable or insertable medical devices that provide resistance to microbial growth on and in the environment of the device and resistance to microbial adhesion and biofilm formation on the device. In particular, the invention discloses implantable or insertable medical devices that comprise at least one biocompatible matrix polymer region, an antimicrobial agent for providing resistance to microbial growth and a microbial adhesion / biofilm synthesis inhibitor for inhibiting the attachment of microbes and the synthesis and accumulation of biofilm on the surface of the medical device. Also disclosed are methods of manufacturing such devices under conditions that substantially prevent preferential partitioning of any of said bioactive agents to a surface of the biocompatible matrix polymer and substantially prevent chemical modification of said bioactive agents.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com