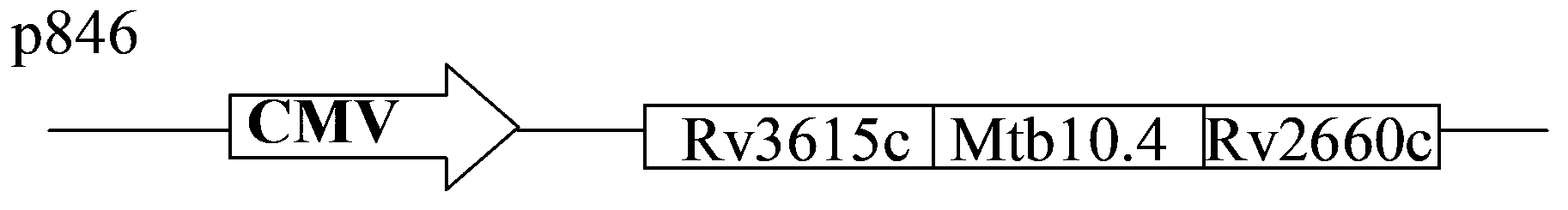Patents
Literature
97 results about "Genetic vaccination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccines at Work. The merits of genetic immunization become most apparent when the actions of traditional vaccines are understood. Traditional preparations consist primarily of a killed or a weakened version of a pathogen (disease-causing agent) or of some piece (subunit) of the agent.
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
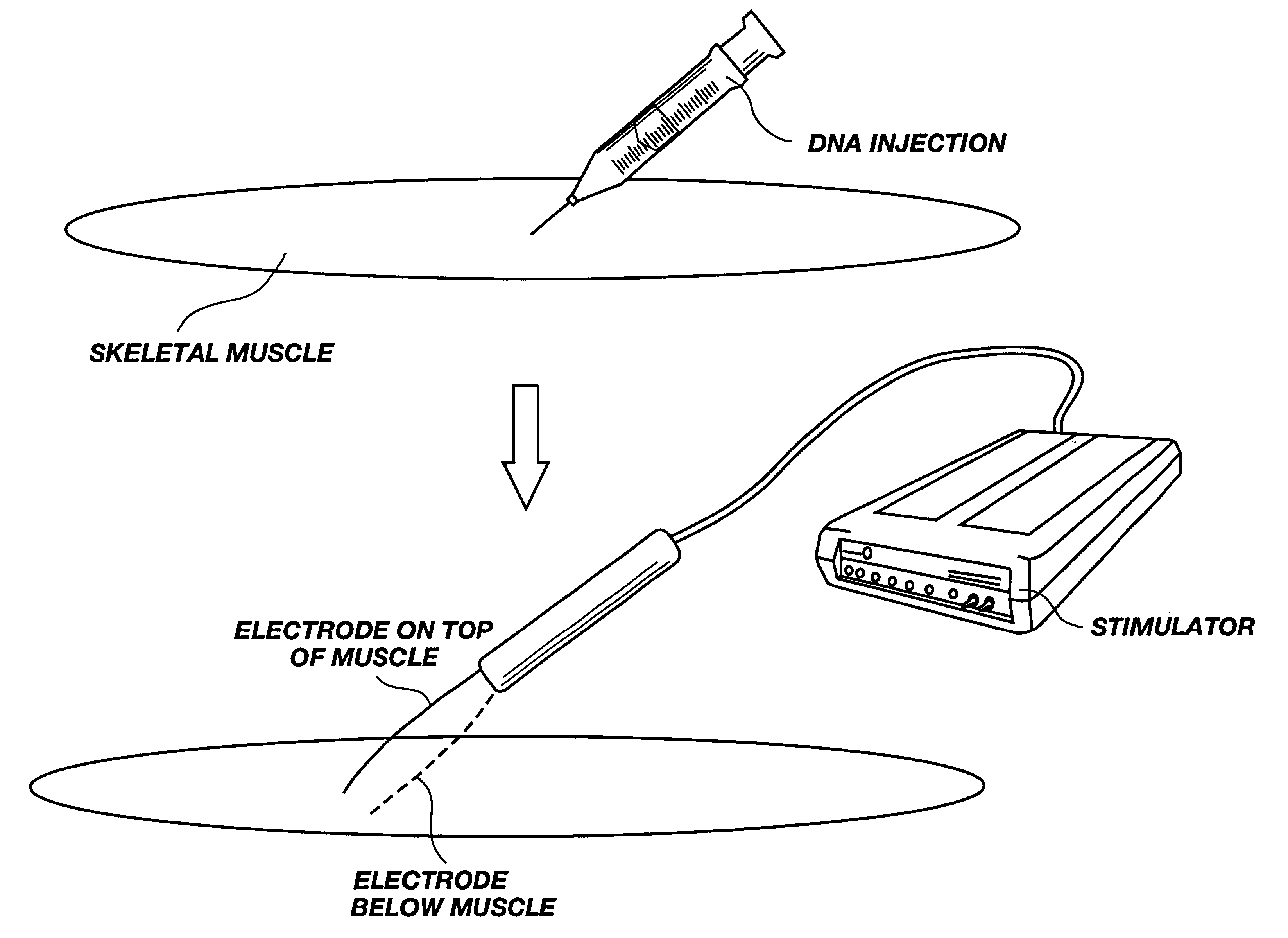
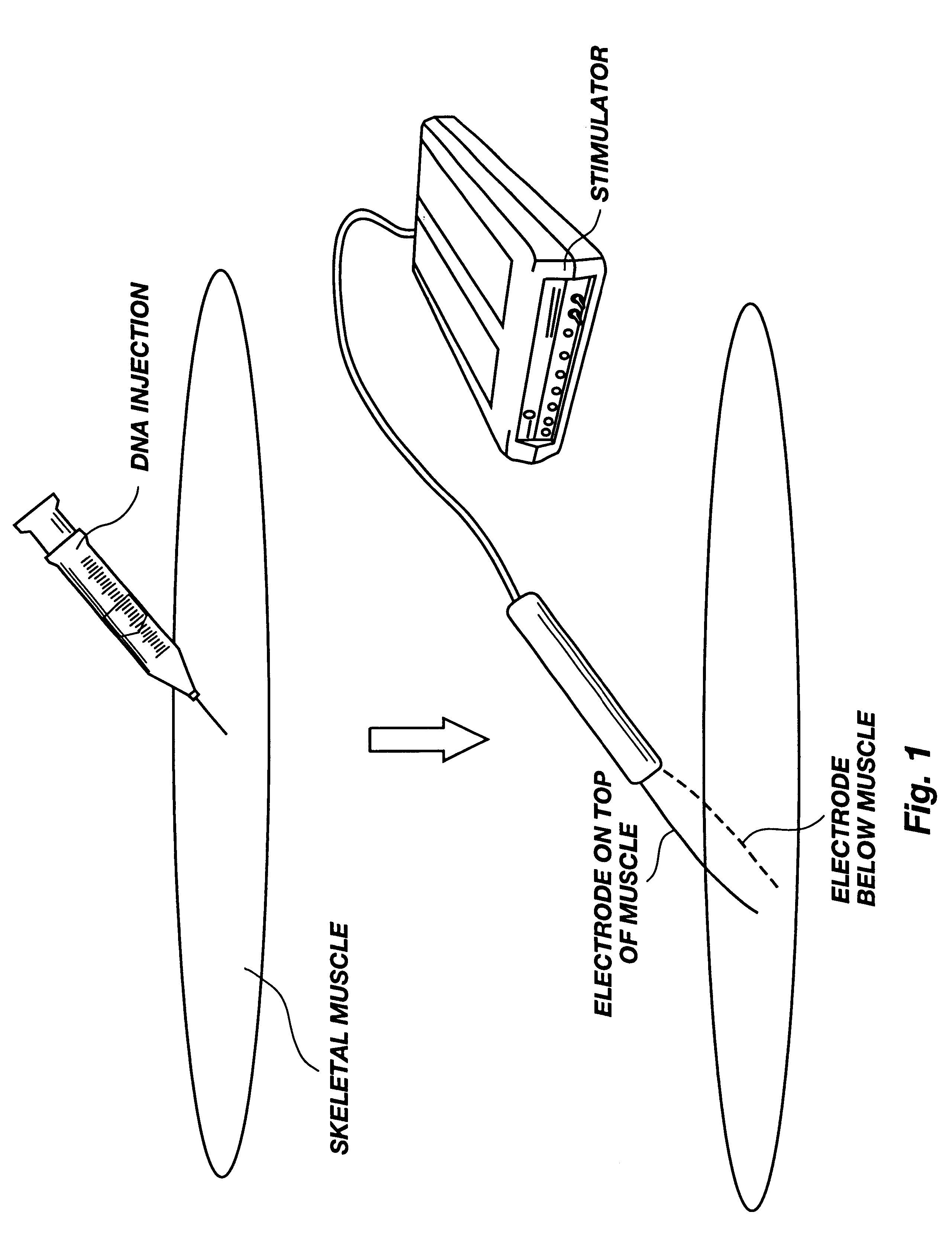
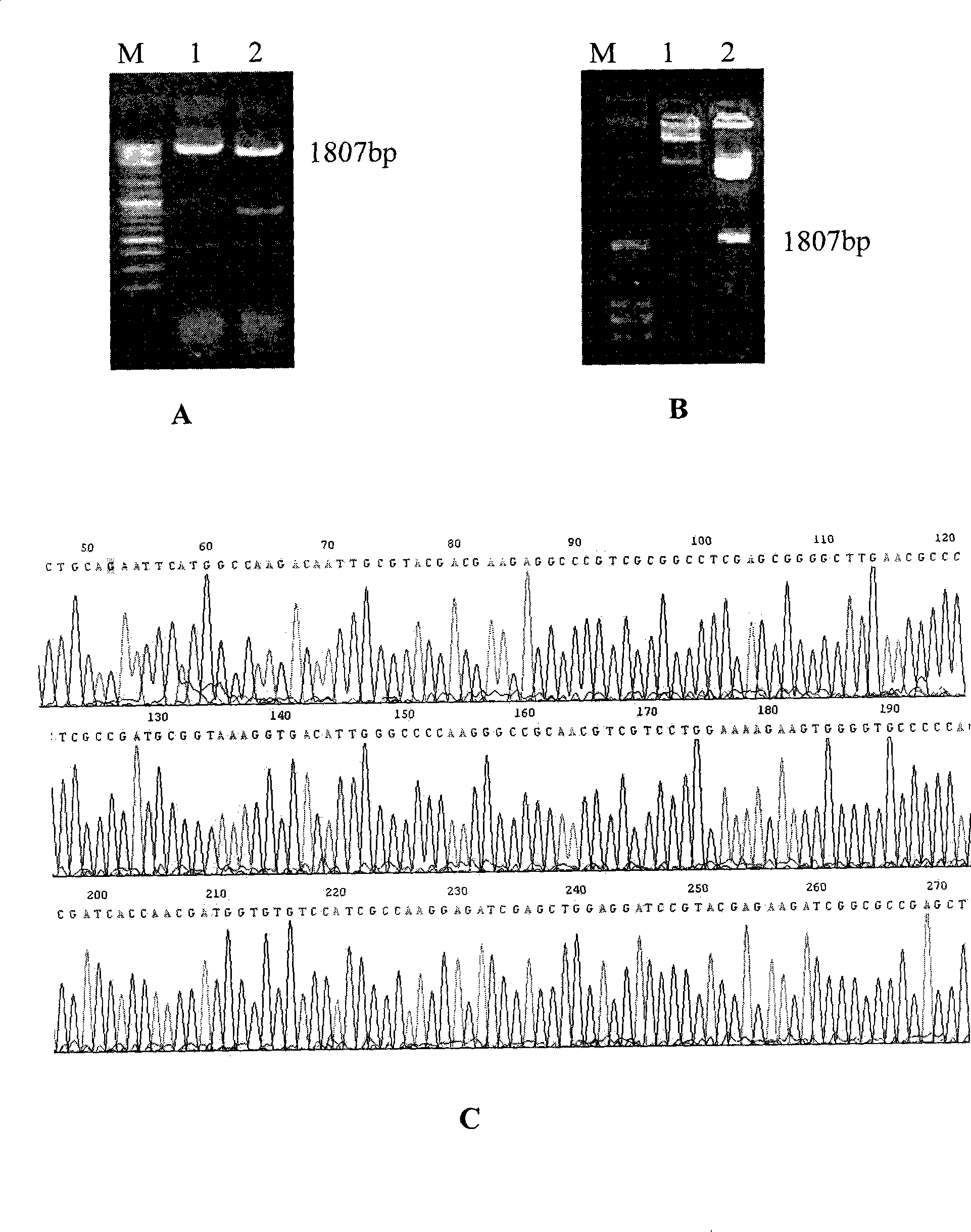
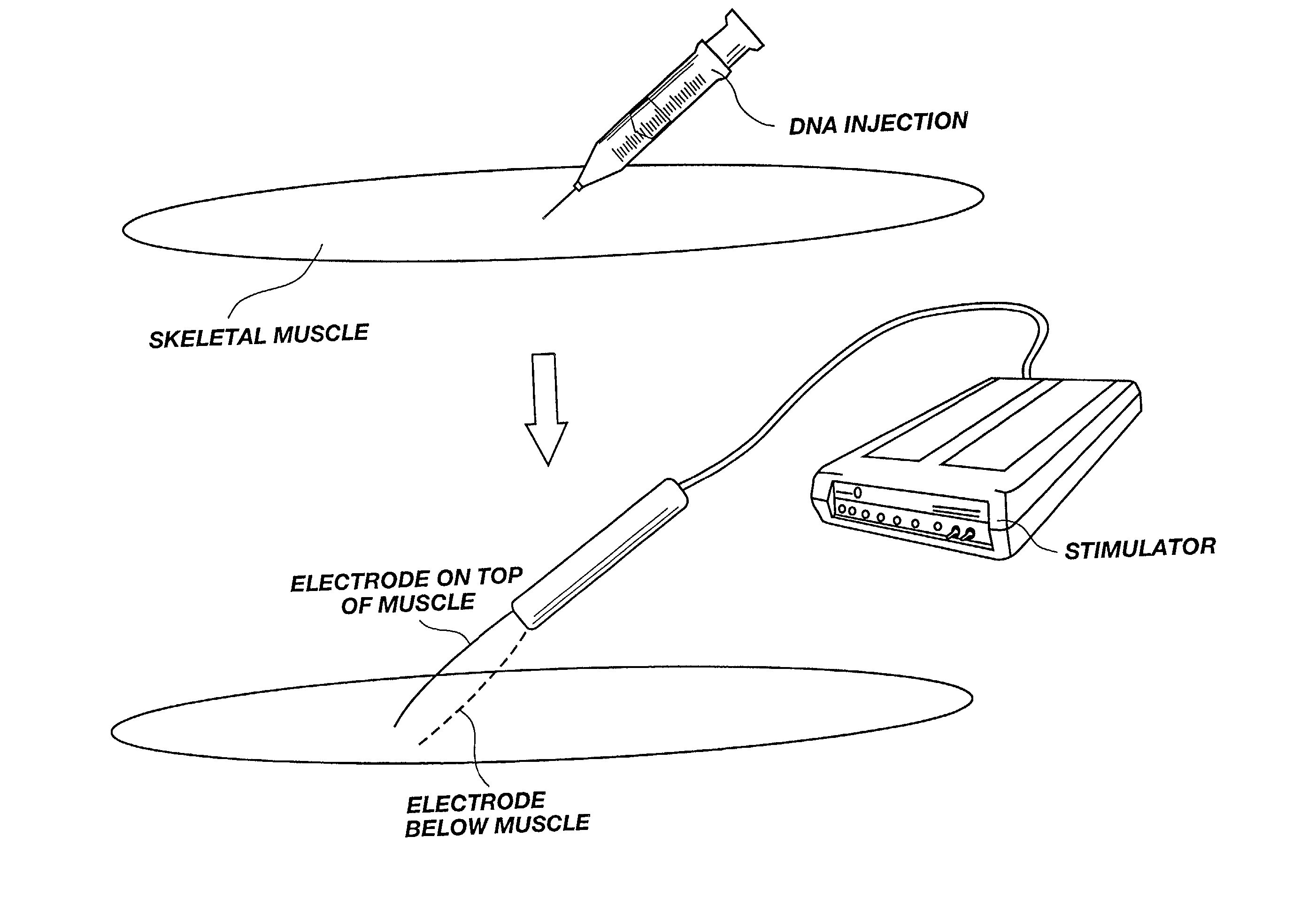
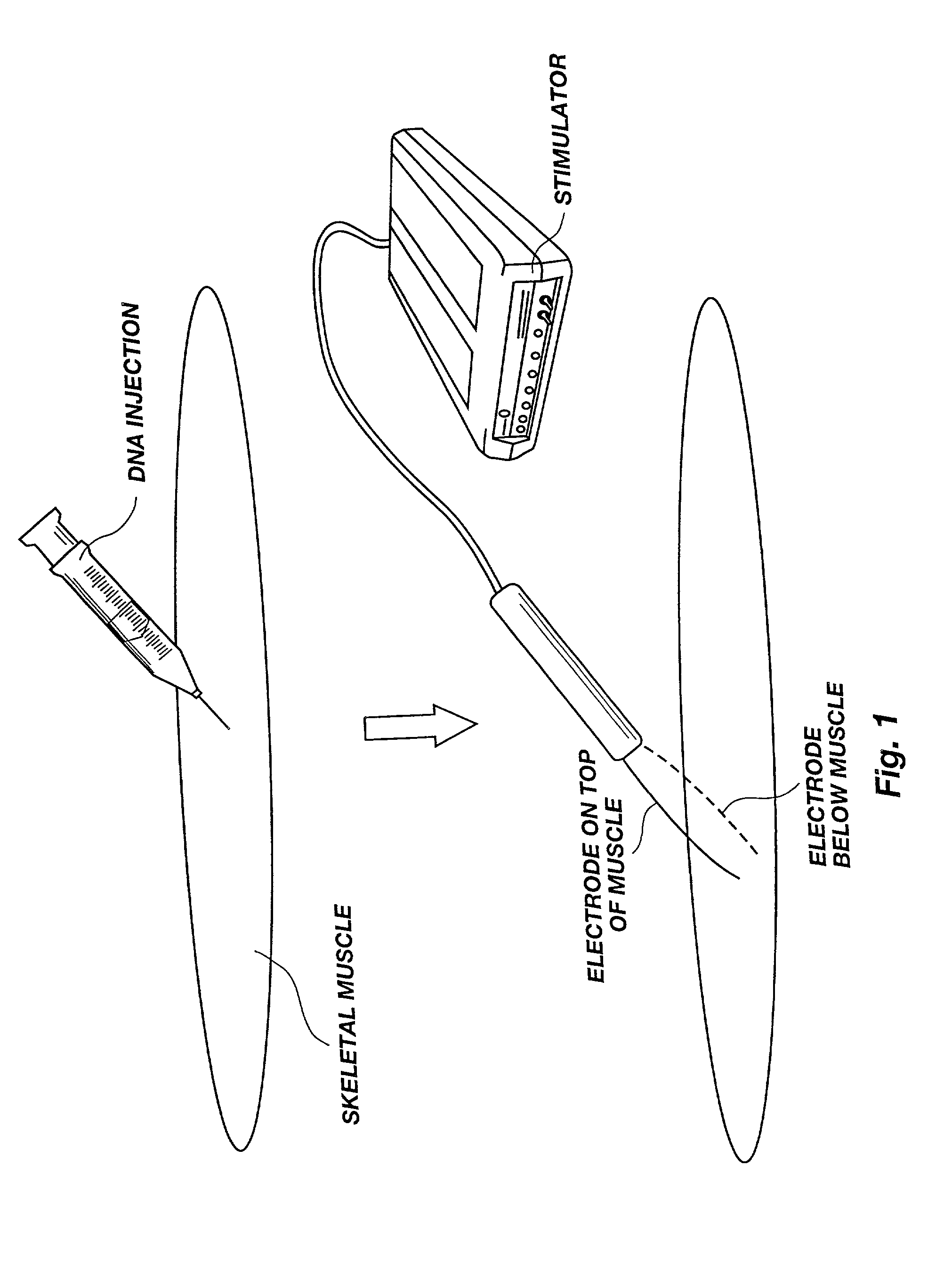
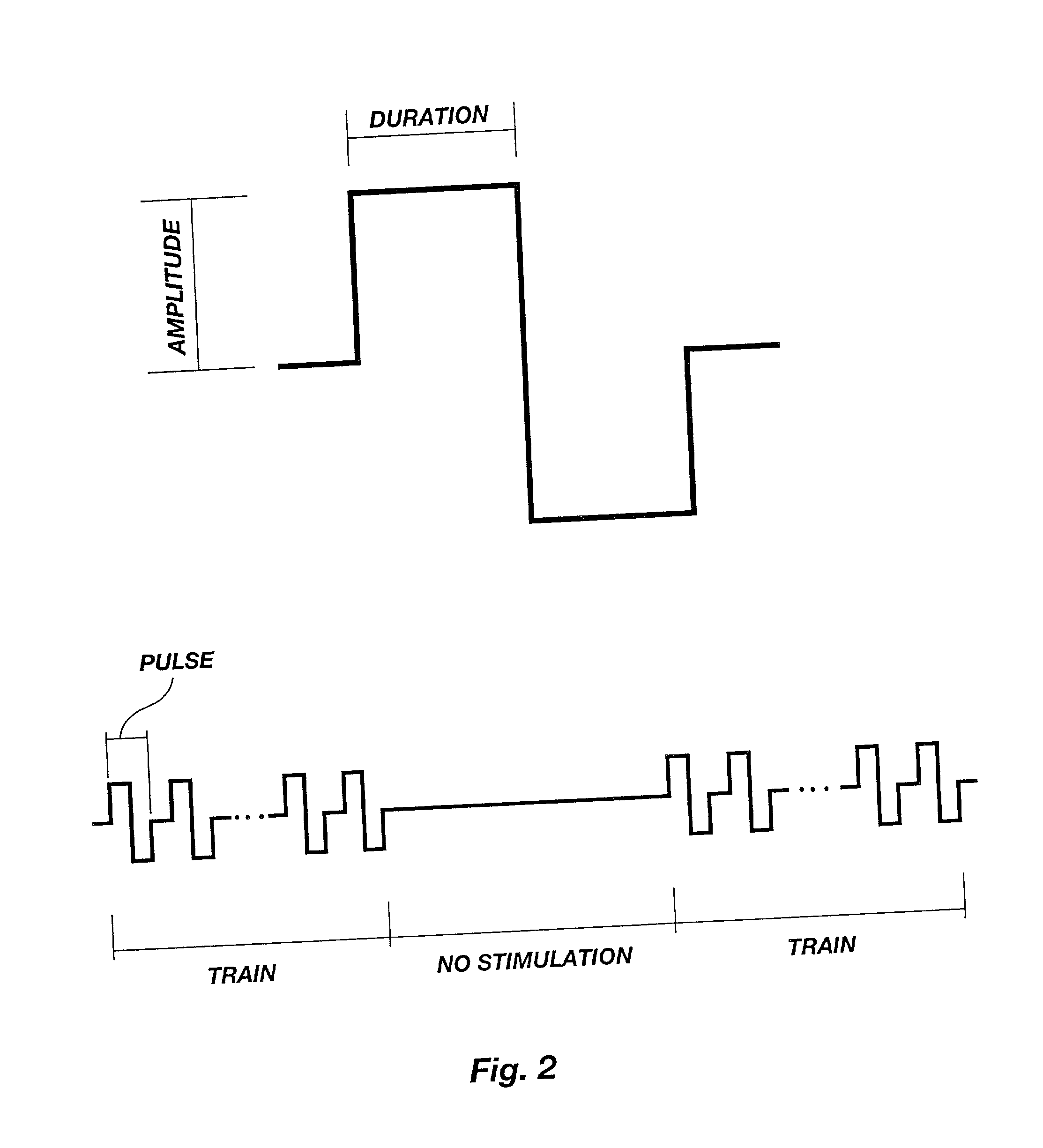
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
pH triggerable polymeric particles
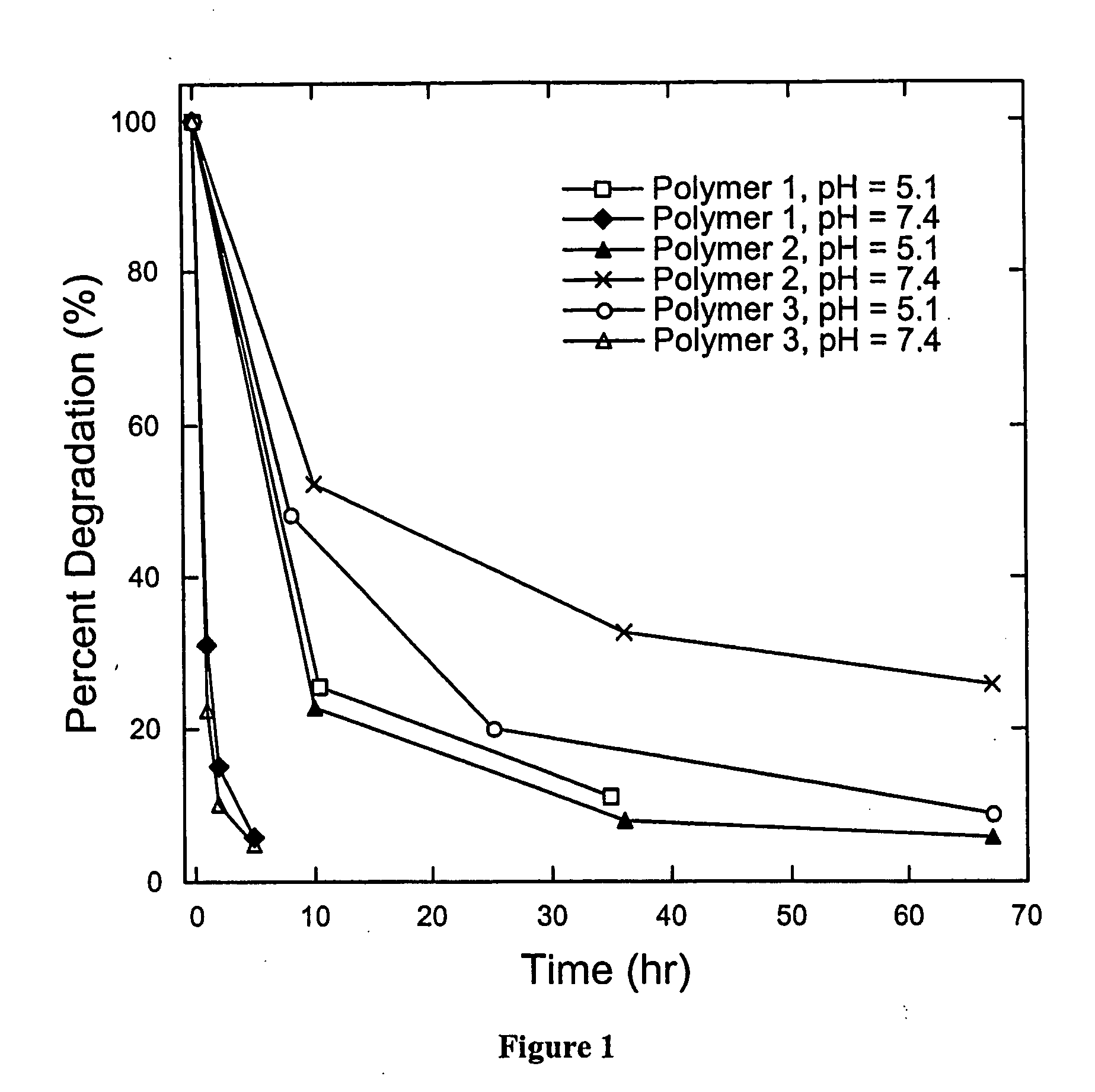
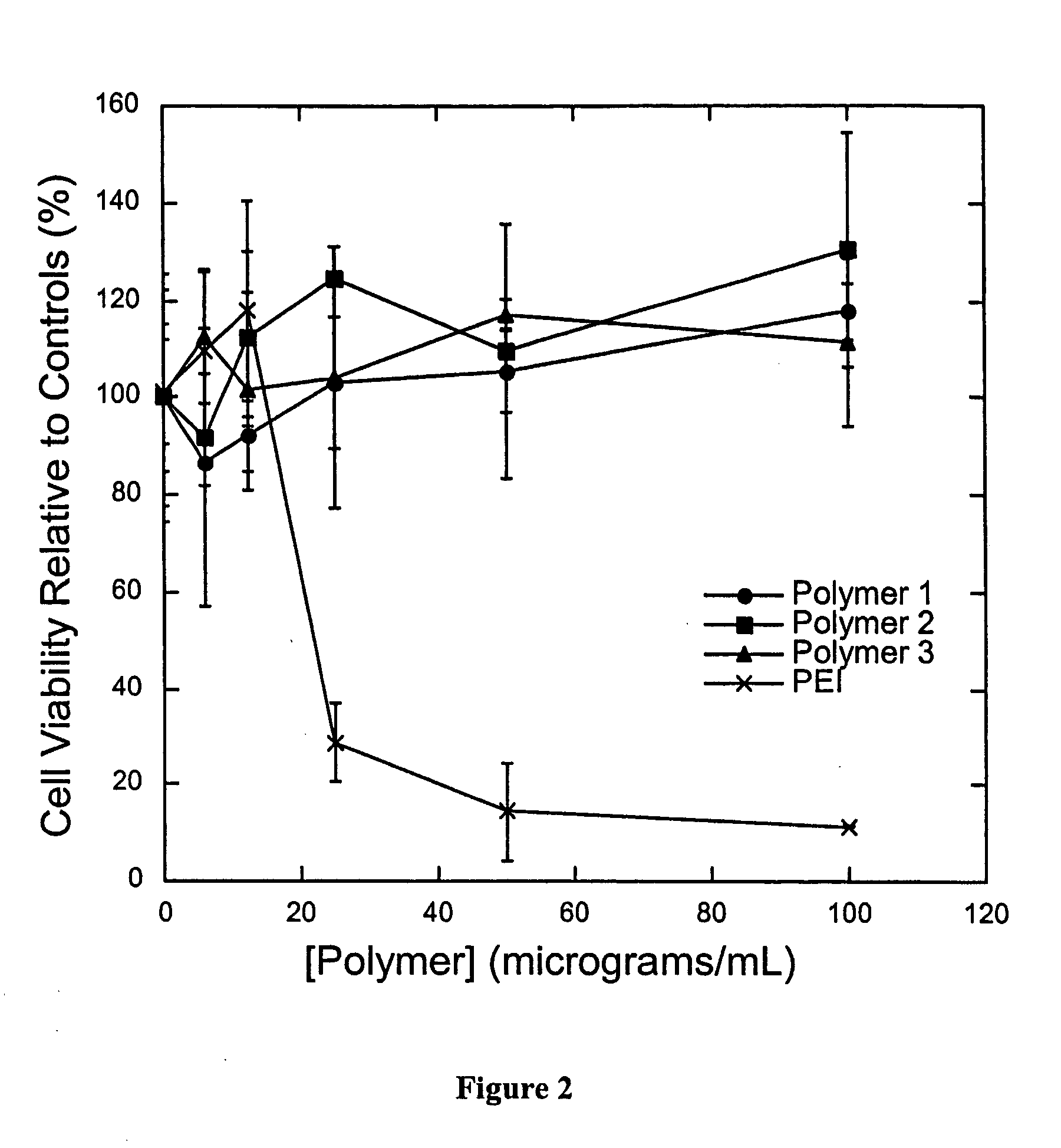
A drug delivery system comprising pH triggerable particles is described. The pH triggerable particles comprise and agent(s) to be delivered, which is encapsulated in a matrix comprising a pH trigger agent and a polymer. Agents including nucleic acids may be delivered intracellularly using the inventive pH triggerable particles. Upon exposure to an acidic environment such as the endosome or phagosome of a cell, the particles dissolve or disrupt due to protonation or an increase in solubility of the pH triggering agent. Pharmaceutical compositions and methods of preparing and administering these particles are also described. These particles may be particularly useful in genetic vaccination.
Owner:MASSACHUSETTS INST OF TECH
Method for increasing expression of rna-encoded proteins
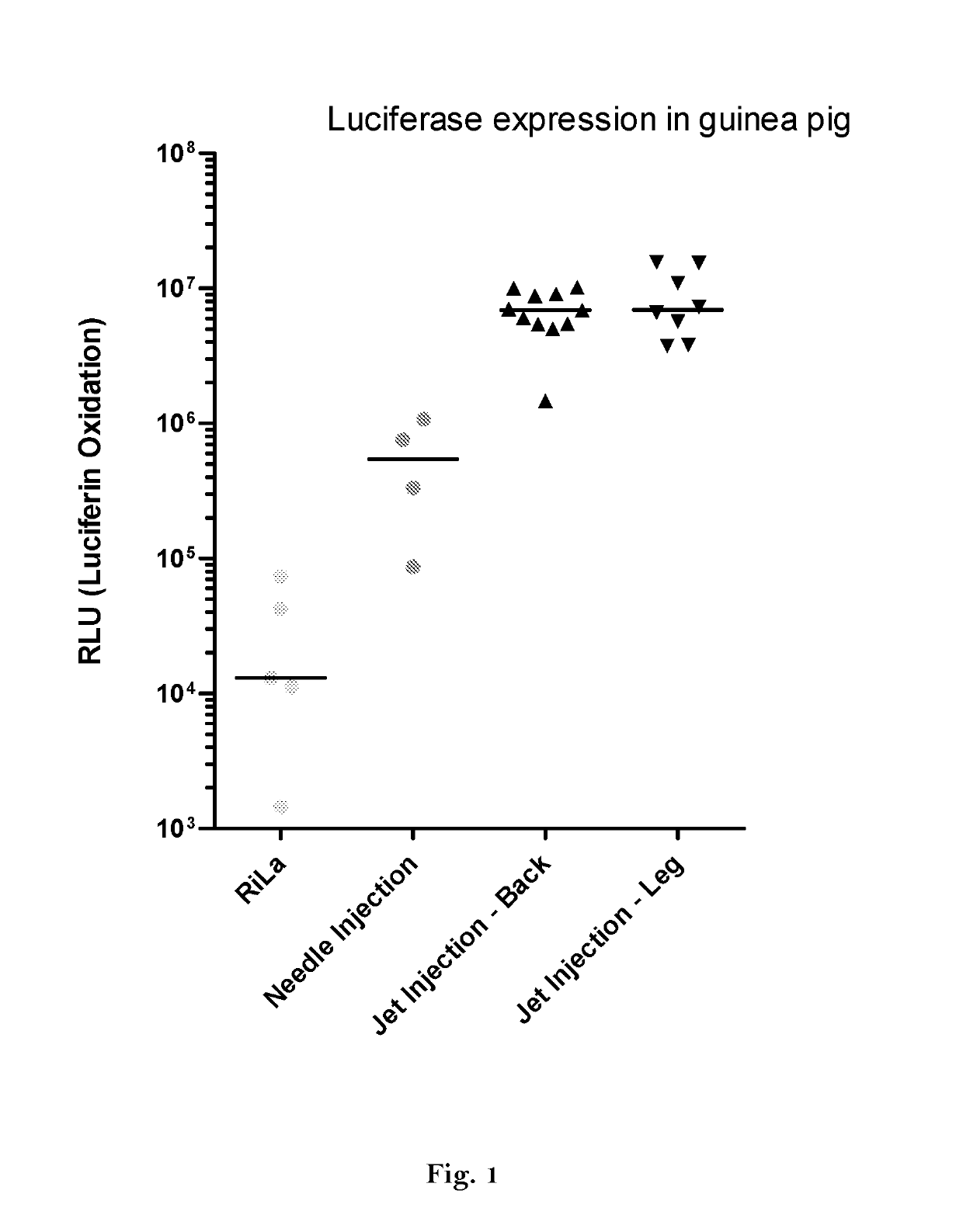
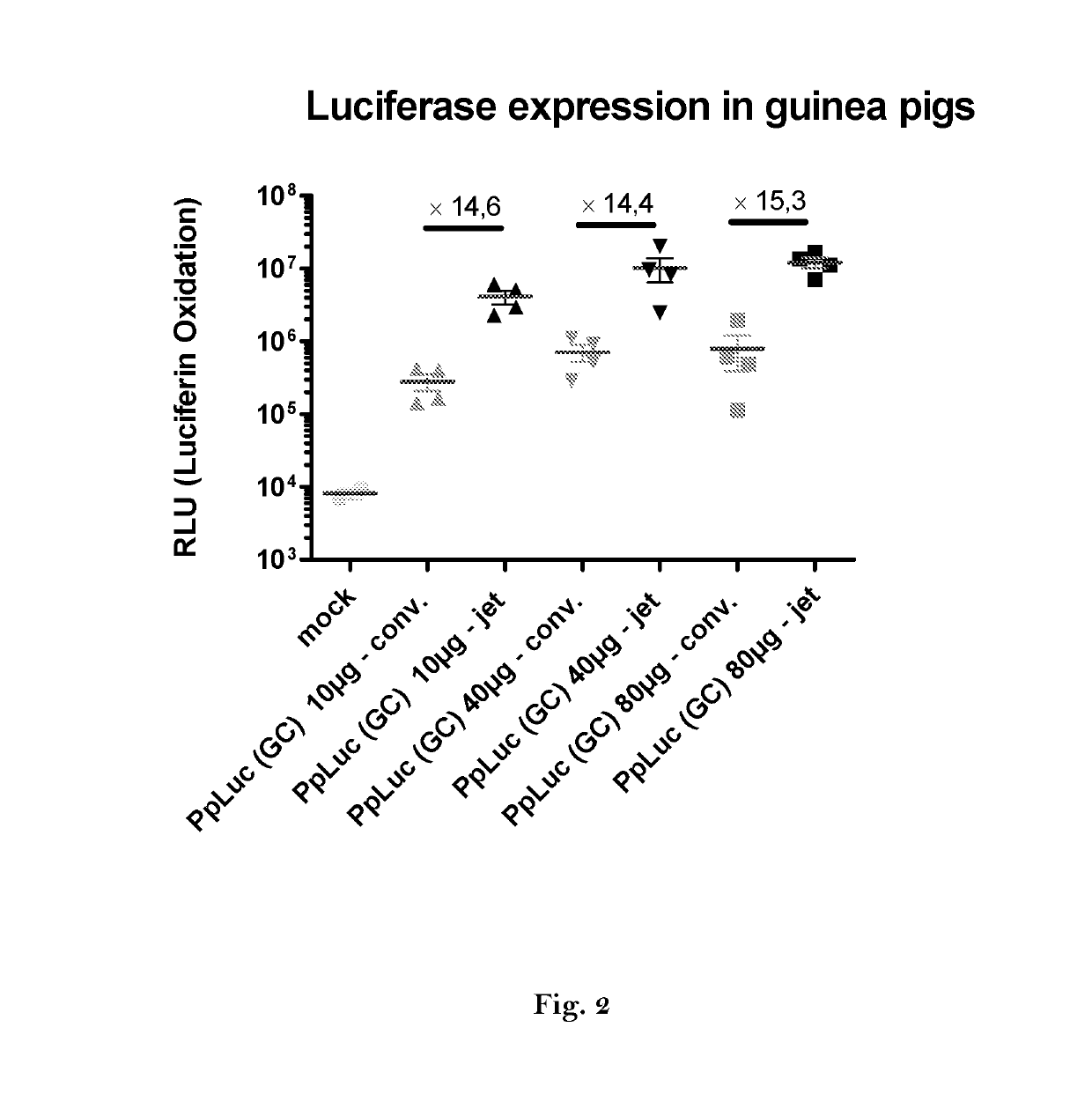
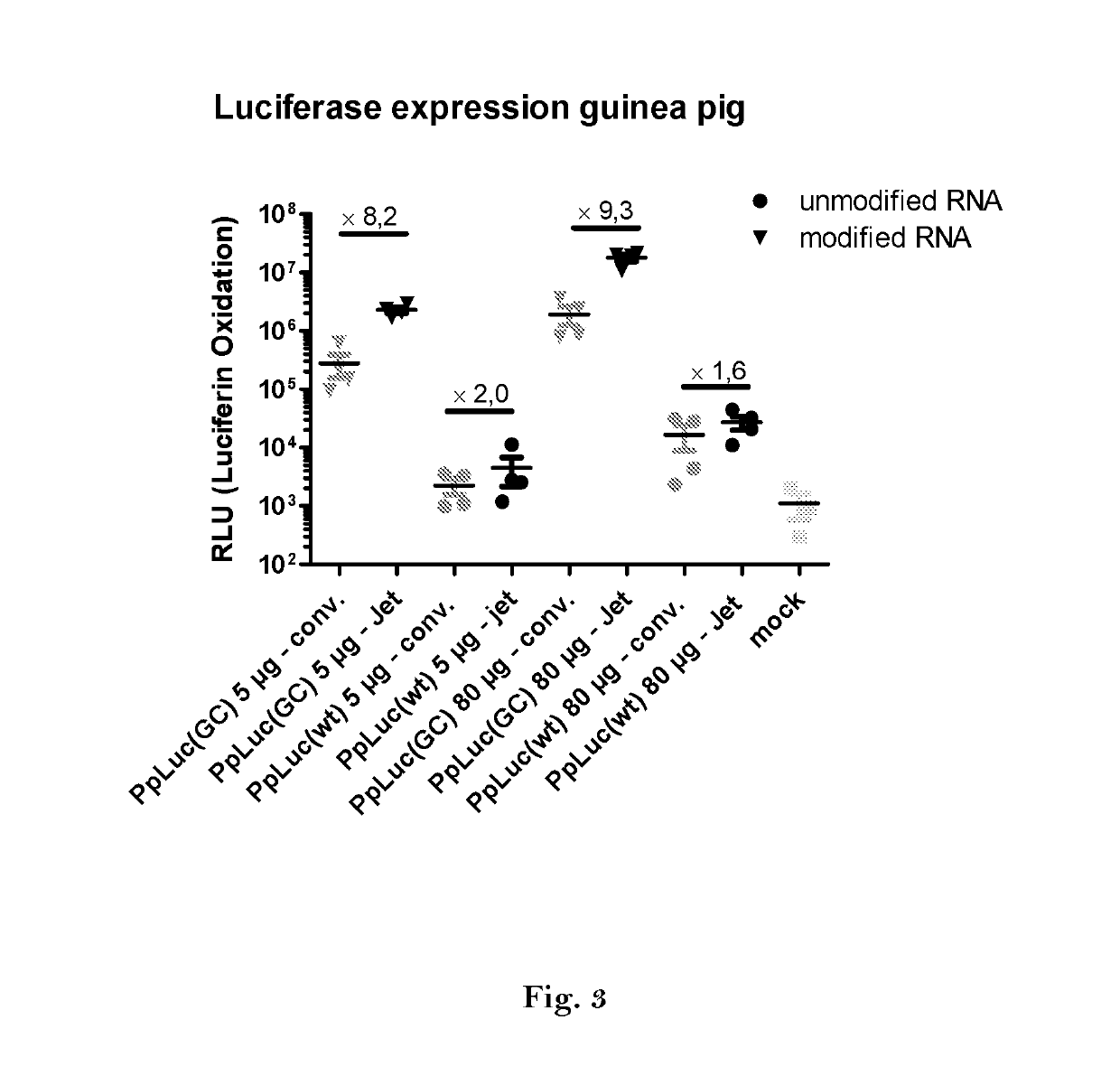
ActiveUS20160166710A1High expressionImprove securitySsRNA viruses negative-sensePeptide/protein ingredientsOpen reading frameJet injection
The invention relates to an RNA comprising at least one open reading frame (ORF) and comprising at least one modification, which increases the expression of the encoded peptide or protein. Furthermore, the invention relates to the medical use of such a modified RNA administered to a subject by jet injection. The invention relates further to a pharmaceutical composition and to a kit of parts comprising said modified RNA for administration by jet injection, preferably for use in the field of gene therapy and / or genetic vaccination. Additionally, the invention relates to a method for enhancing the (localized) expression of RNA-encoded peptides or proteins in the dermis or muscle (of a mammal) comprising administering the modified RNA by jet injection. And finally, the invention relates to a method of treatment comprising administering the modified RNA by jet injection to a subject in need thereof.
Owner:CUREVAC SE
Design principle for construction of expression constructs for gene therapy
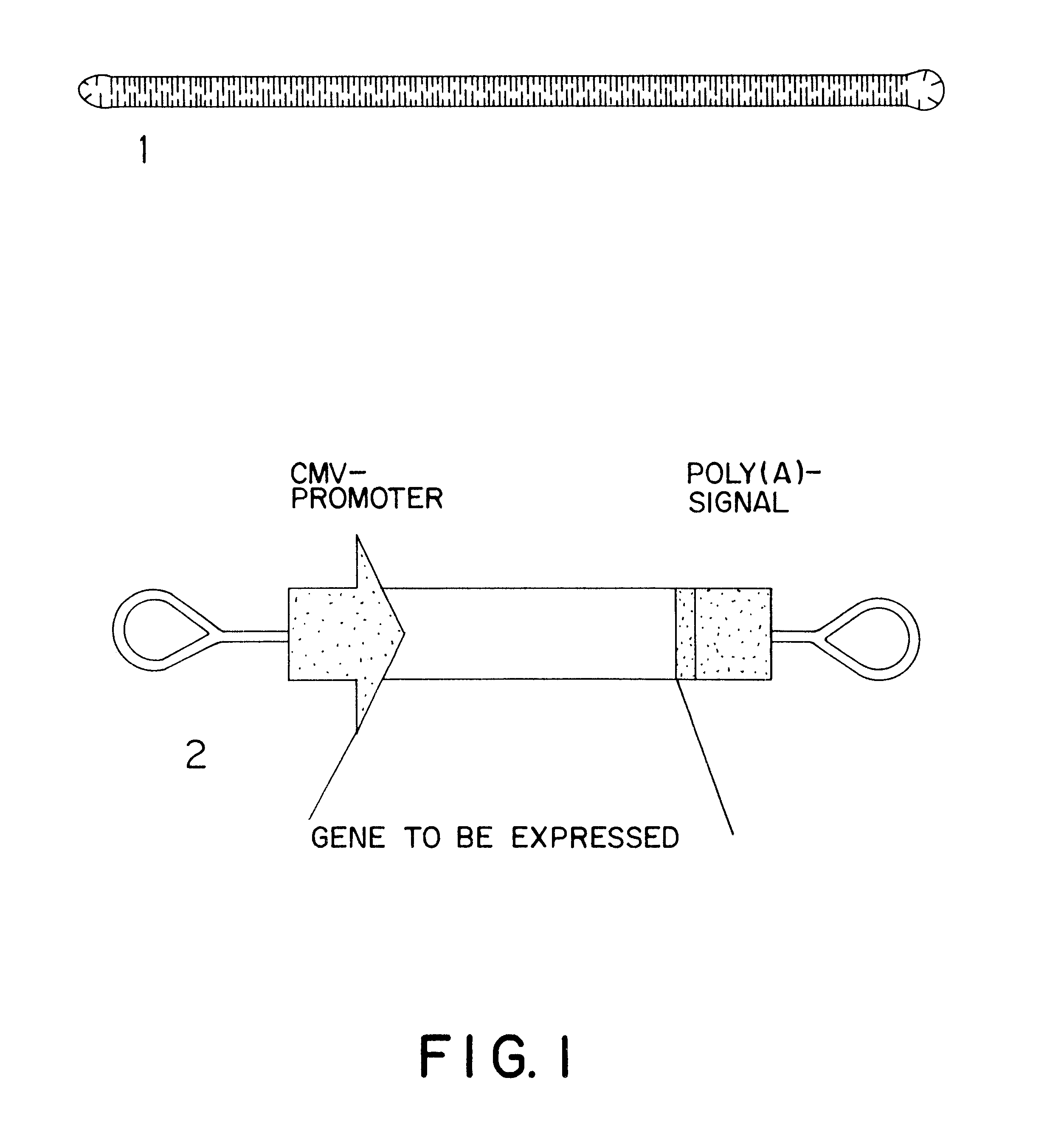
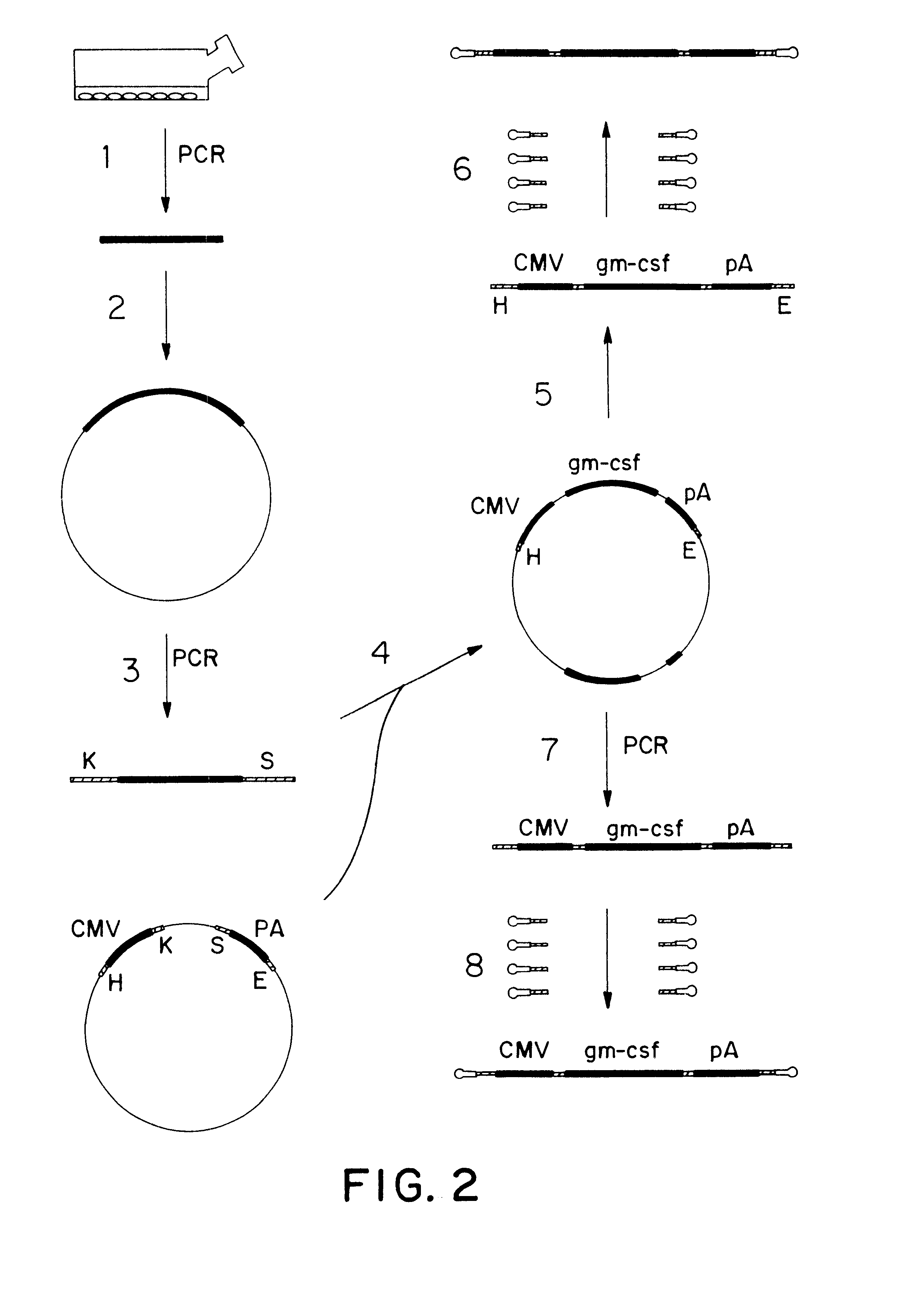
The invention concerns an expressible nucleic acid construct, which contains only the sequence information necessary for expressing a gene for RNA or protein synthesis. Expression constructs of this type can be used in gene therapy and genetic vaccination and avoid many of the risks associated with constructs today. The invention further concerns the possibility of improving the conveying of the construct into cells or tissue by covalent linkage of the construct, for example to particles or peptides.
Owner:MOLOGEN AG
Genetic vaccination device and process for forming an injection therefor
InactiveUS6951613B2Ampoule syringesGenetic material ingredientsGenetic MaterialsMembrane configuration
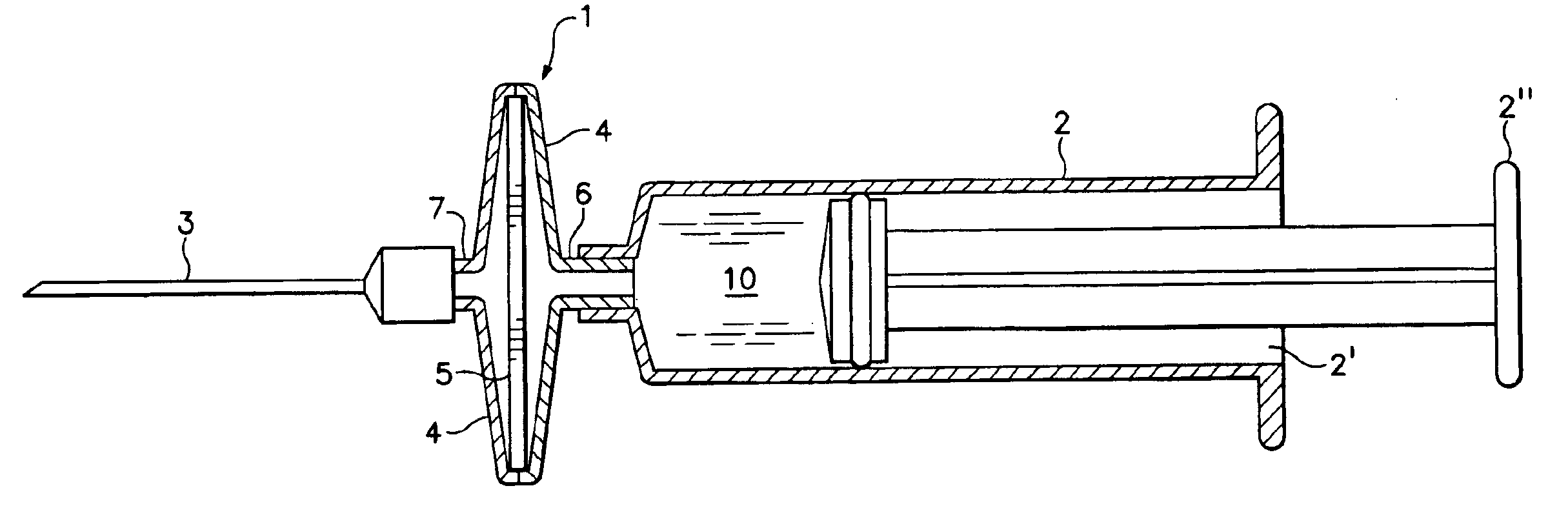
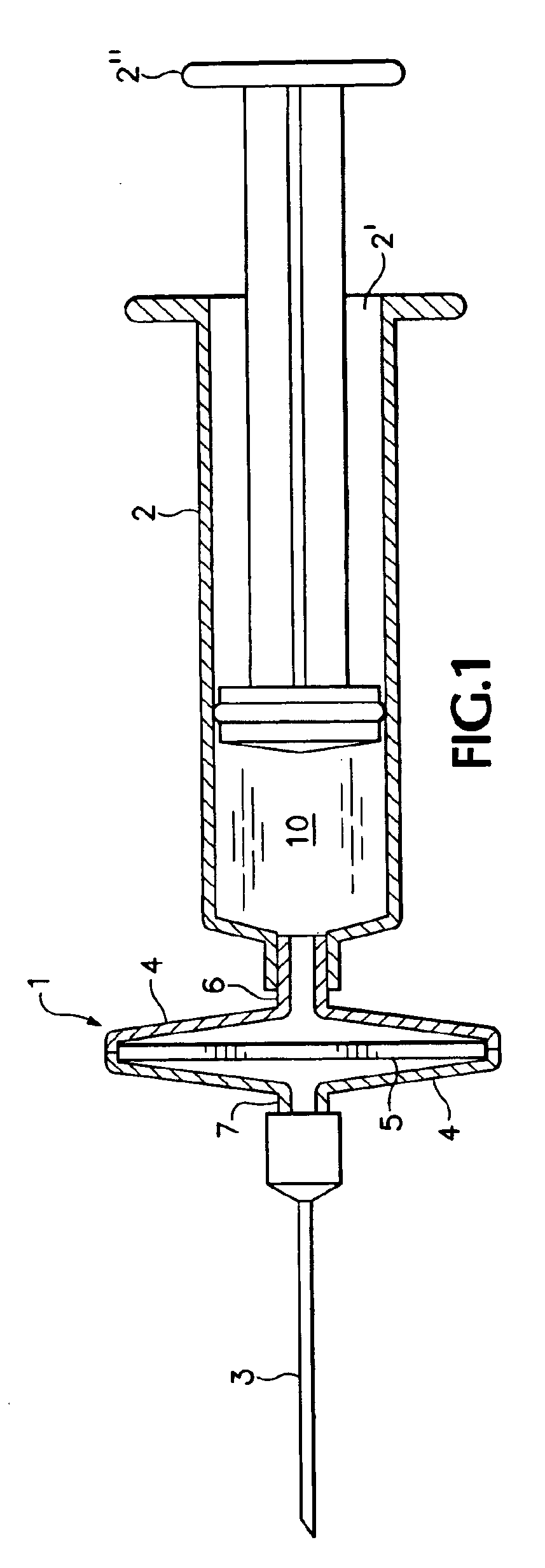
There is disclosed a genetic vaccination device and a process for forming an injection solution therefor, the device comprising a syringe and canula coupled to a membrane adsorber having genetic material adsorbed thereon, and the process comprising eluting the genetic material from the membrane adsorber so as to form an injection solution containing the genetic material.
Owner:SARTORIUS STEDIM BIOTECH GMBH
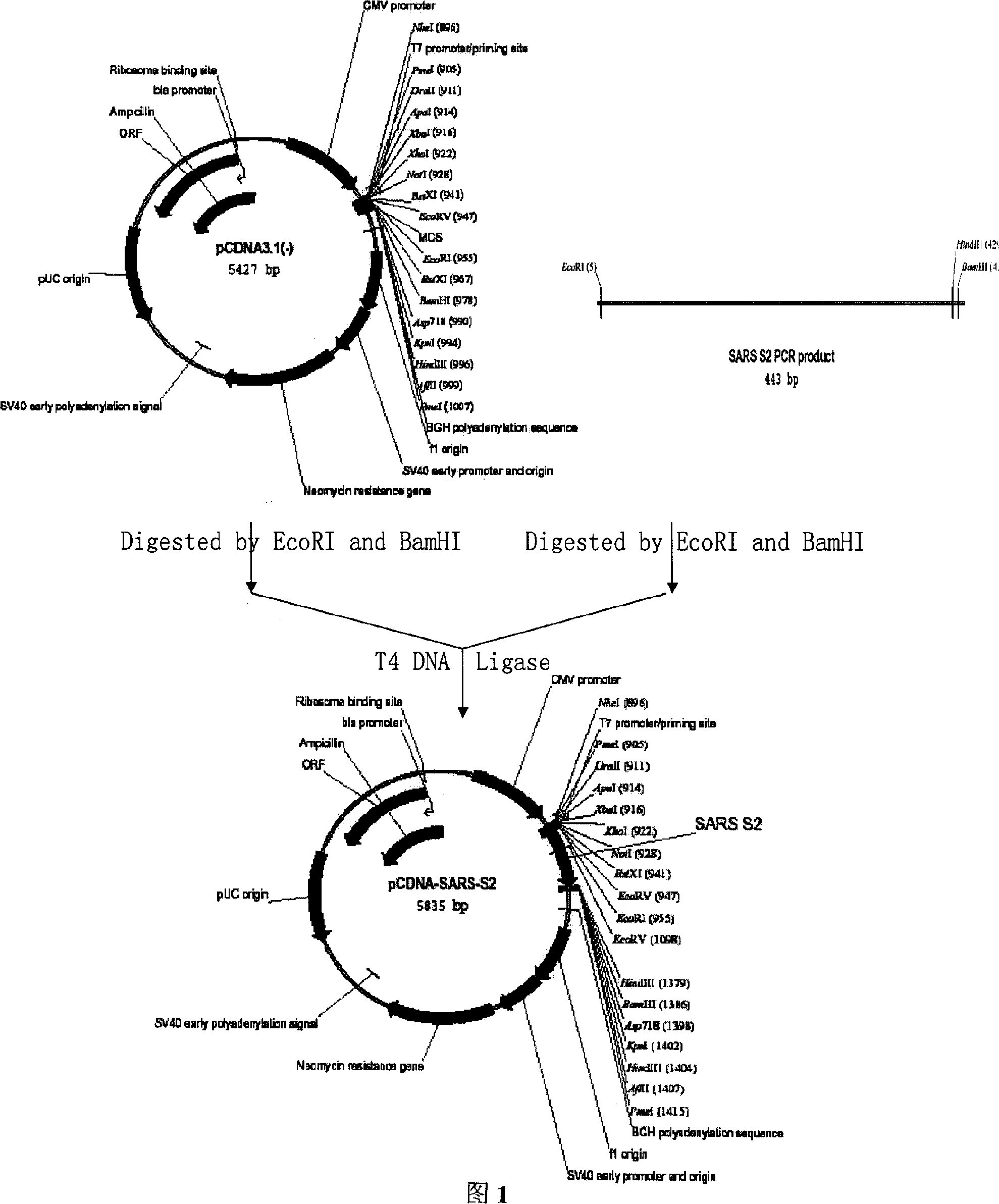
SARS vaccine of adenovirus carrier and preparation method, application of coronavirus S gene
InactiveCN1562365AEffective generationEffective induction ofGenetic material ingredientsAntiviralsGenetic engineeringOrganism
The invention pertains to biological genetic engineering field, specificly relating to SARS vaccine of adenovirus carrier and preparation method, application of related coronavirus S gene in preparation of the SARS vaccine for preventing SARS. Through a bioengineering means, the related coronary virus S gene and a defective adenovirus are recombined, which makes protective immunogen protein or polypeptide expressing in it. A genic vaccine that can arouse the mucosal immunogenicity is produced through amplifying training, purification, and preparation, which induces a immunity reaction in the respiratory mucosa, makes the organism produce the corresponding antibody, and prevents virus from infection. Compared with the traditional inactivated viral particles vaccine, the invention has a high safety; it is convenient to operate; it is not limited by the specific conditions such as intramuscular injection; and it has an extensive clinical application prospect.
Owner:SUN YAT SEN UNIV CANCER CENT
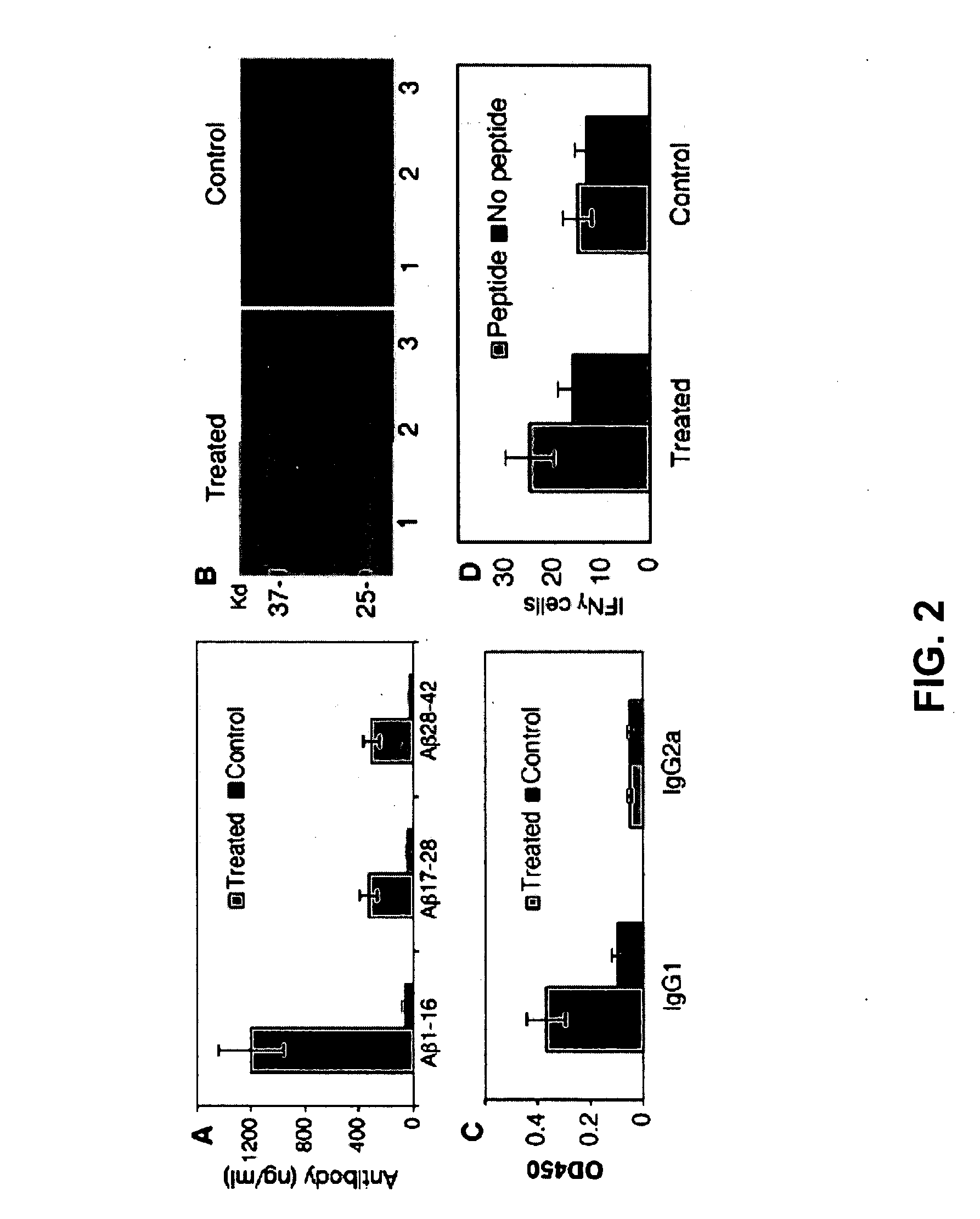
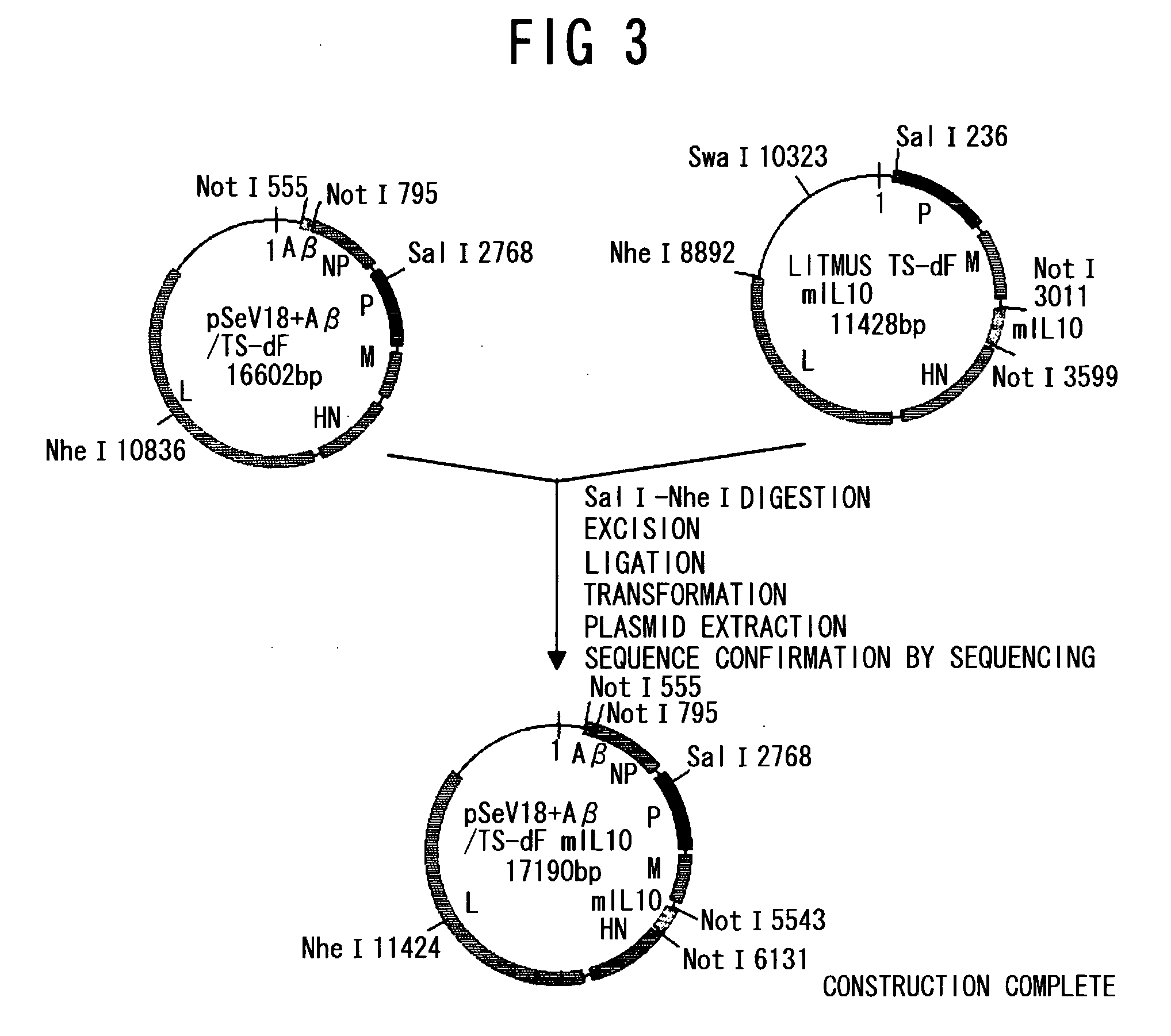
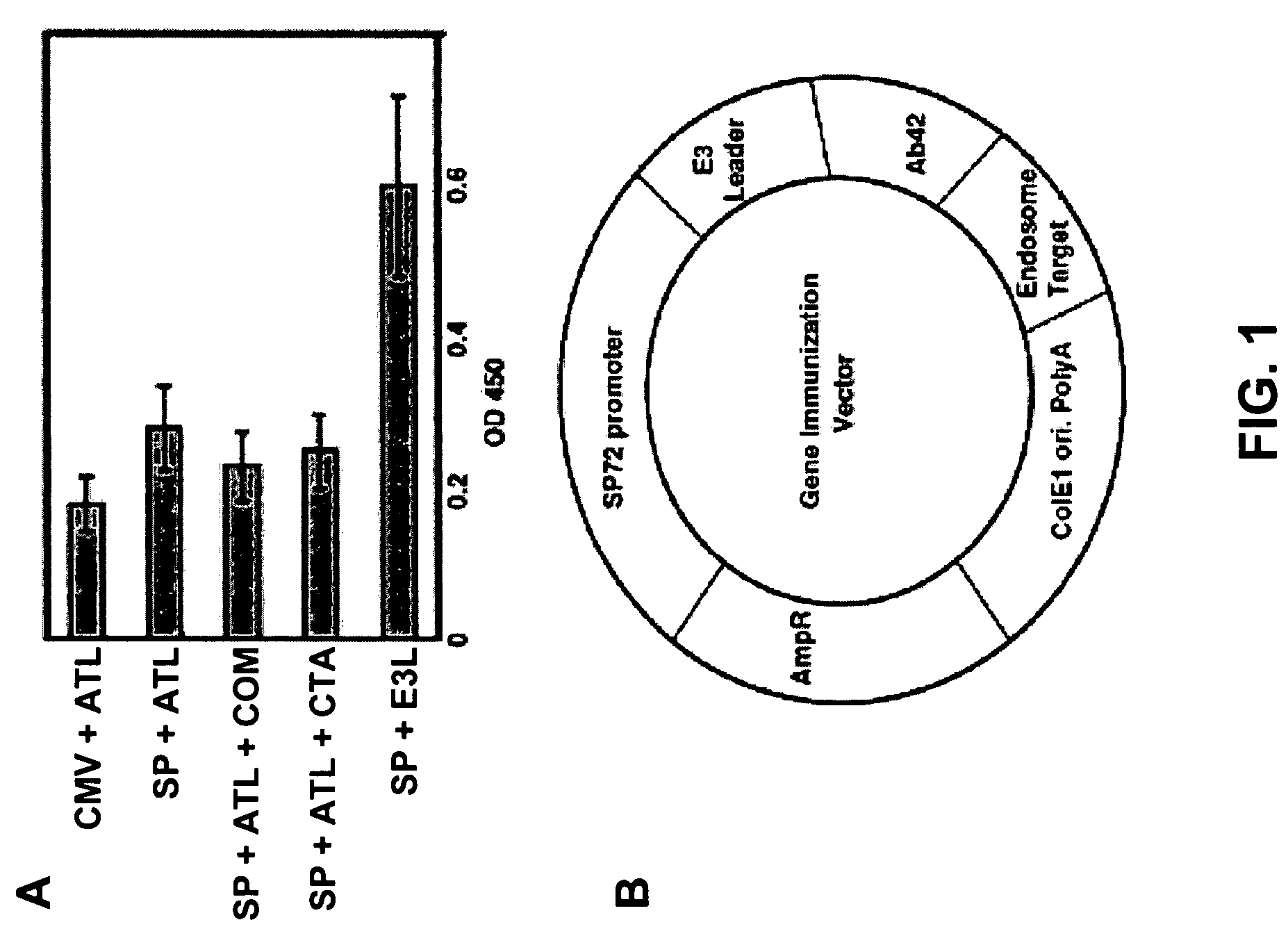
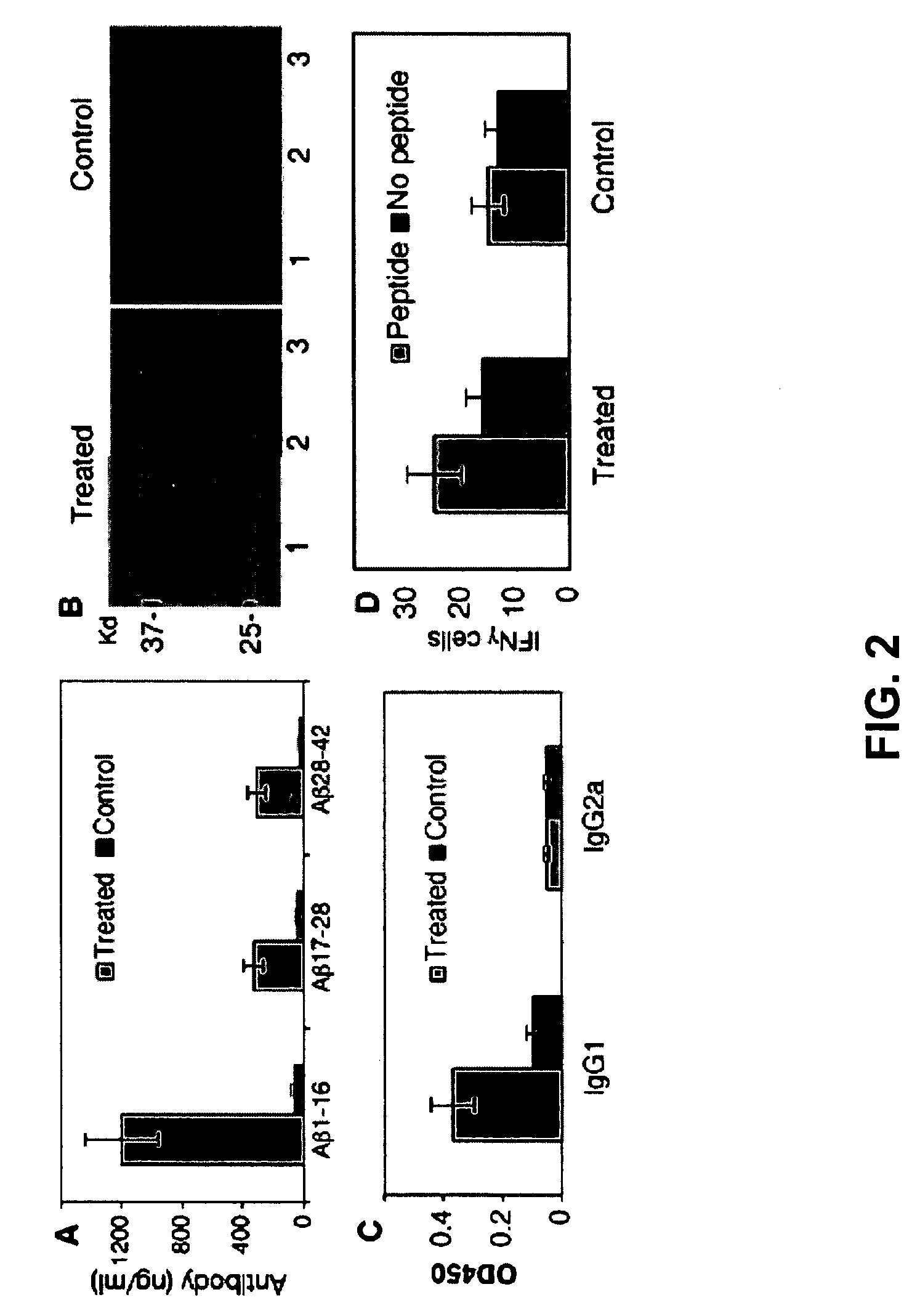
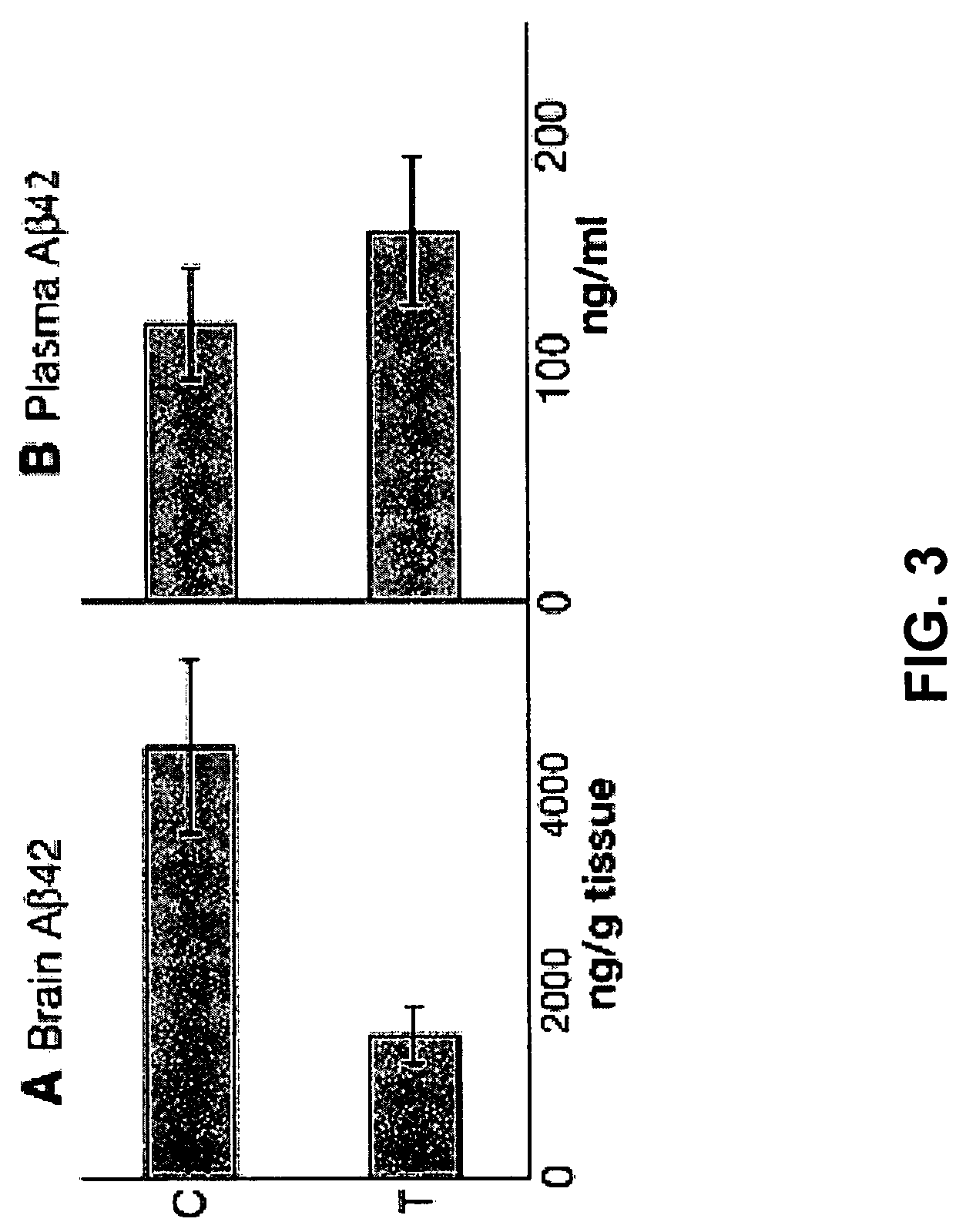
Amyloid beta gene vaccines
ActiveUS20070280953A1Improve translation initiation codon contextHigh expressionPolypeptide with localisation/targeting motifSugar derivativesVaccinationAmyloid beta
The invention generally concerns compositions and methods for genetic vaccination with amyloid beta (Aβ) protein. Such vaccines may provide effective treatment for neurodegenerative disease such as Alzheimer's disease. Vaccination methods are can be used to induce a Th2 type immune response directed to Aβ. This immune response id shown to substantially reduce Aβ concentration and Aβ plaque size in an Alzheimer's model system.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Epidermal mRNA vaccine
InactiveUS20200085852A1Easy to identifyFaster and strong attackOrganic active ingredientsWhole-cell/virus/DNA/RNA ingredientsDiseaseGenetic vaccine
The invention concerns the field of genetic vaccination, in particular RNA vaccines. The present invention provides an mRNA for use in the treatment and / or prevention of a disease, wherein the mRNA is administered to the epidermis. Furthermore, the invention provides compositions comprising the mRNA for epidermal administration or kits comprising the mRNA for epidermal administration. Moreover, the invention concerns the medical use of the mRNA or compositions comprising the mRNA, wherein the mRNA or compositions comprising the mRNA are administered to the epidermis.
Owner:CUREVAC AG
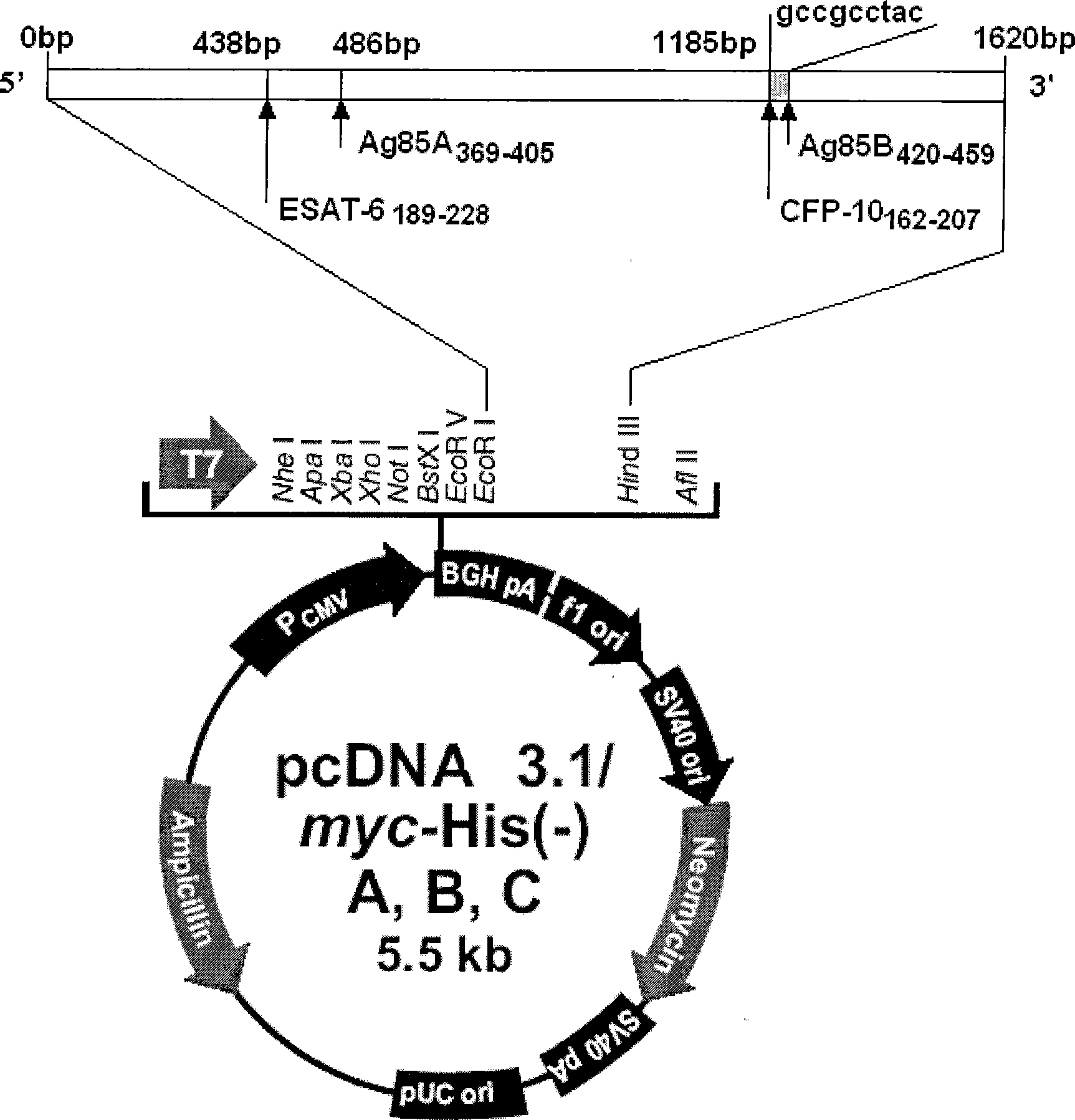
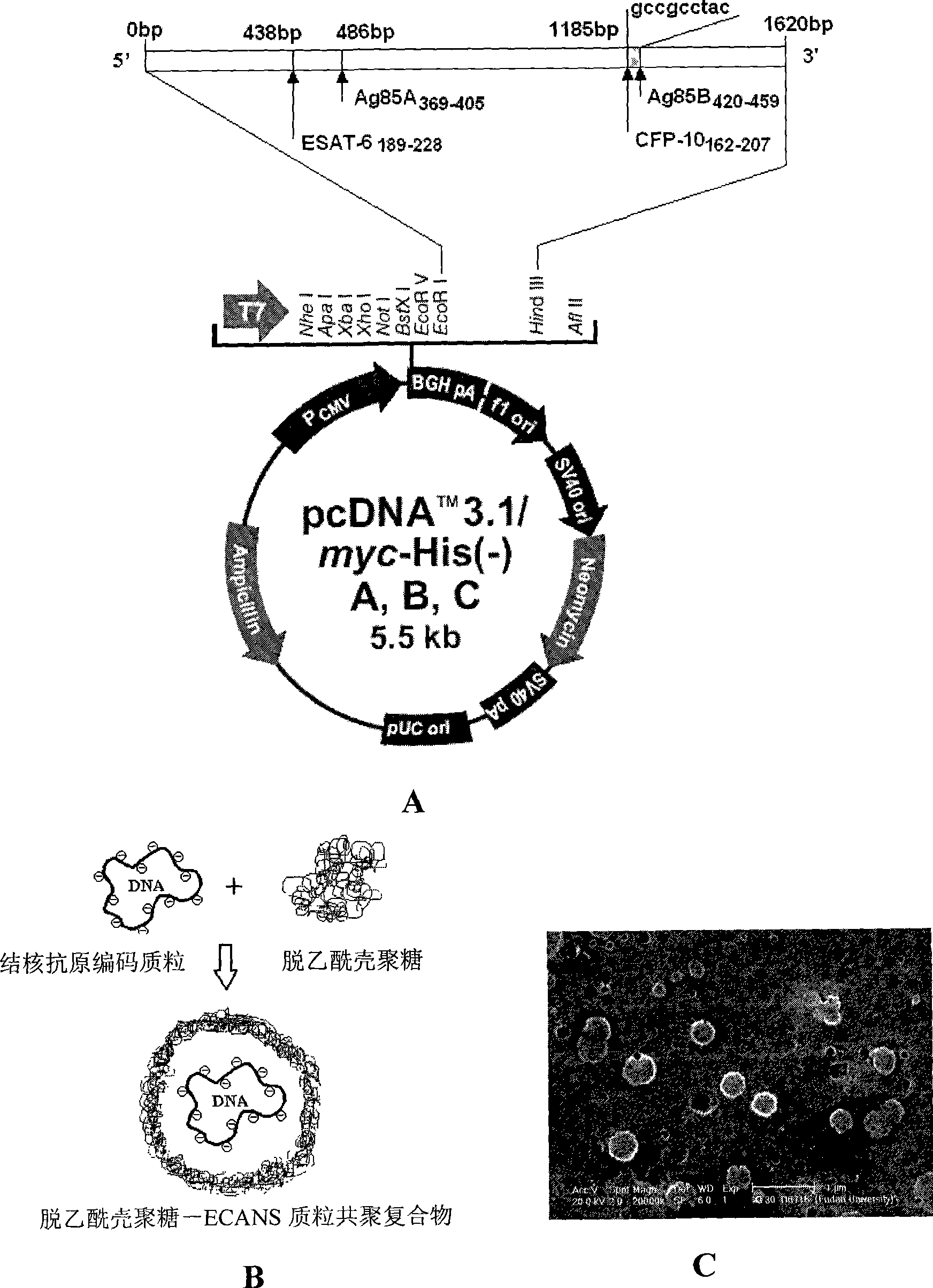
Tuberculosis gene vaccine based on T cell epitope as well as preparation method and use thereof
InactiveCN101451145AActivate immune responseDoes not affect the spatial structureAntibacterial agentsGenetic material ingredientsIntramuscular injectionTreating tuberculosis
The invention discloses a tuberculosis gene vaccine based on T cell epitopes, wherein a full-length gene, embedded with four T cell epitope polypeptide genes which come from mycobacterium tuberculosis antigen, of mycobacterium tuberculosis heat shock protein is inserted into a vector. The invention also discloses a method for preparing the vaccine, which comprises the following steps: four T cell epitope genes, namely EAST-6189-228, Ag85A369-405, CFP10162-207 and Ag85B420-459 which come from the mycobacterium tuberculosis antigen are inserted into an HSP65 full-length gene. The invention also discloses application of an ECANS tuberculosis gene vaccine. Through the intramuscular injection of the gene vaccine into an immune mouse, the experiment proves that the vaccine can induce a specific antibody which aims at a plurality of tuberculosis antigens to response, can induce stronger tuberculosis specific killing response, can induce Th1 immune response at the same time, secrete high-level IFN gamma, and is a good vaccine for preventing and treating tuberculosis.
Owner:FUDAN UNIV
Highly safe intranasally administrable gene vaccines for treating alzheimer's disease
InactiveUS20090170798A1Large scaleLow costSsRNA viruses negative-senseOrganic active ingredientsDiseaseNose
An objective of the present invention is to provide a safe and effective vaccine therapy for Alzheimer's disease. A minus strand RNA viral vector carrying amyloid gene was constructed, and administered intranasally to 24- to 25-months-old APP transgenic mice. The level of serum anti-A 42 antibody was determined and showed to be markedly higher than the control. The results of histological investigation showed that the administration of a vector of the present invention markedly reduced senile plaques in all of the frontal lobe, parietal lobe, and hippocampus. The brain A level was also markedly reduced. Furthermore, the administration of a vector of the present invention did not result in lymphocyte infiltration in the central nervous system.
Owner:DNAVEC CORP +1
Immune enhanced gene vaccine of hepatitis B virus core antigen and preparation method thereof
InactiveCN101884793AEnhance immune responseEfficient induction of antiviral replication abilityGenetic material ingredientsDigestive systemAnti virusHepatitis B virus core Antigen
The invention relates to the technical field of gene engineering, in particular to an immune enhanced gene vaccine of a hepatitis B virus core antigen (HBcAg). The vaccine is an immune stimulating complex (ISCOM) vaccine consisting of HBcAg and OX40 ligand (OX40L) dual-gene co-expression recombinant eukaryotic vector, saponin Quil A, cholesterol and lecithin. The vaccine can promote immune response of specific T cells of the HBcAg and efficiently induce the anti-virus replication capacity of immune cells, and has good application prospect in the field of hepatitis B virus infection resistance. The invention also relates to a preparation method for the vaccine with simple and convenient operation and low cost.
Owner:ARMY MEDICAL UNIV
Design principle for the construction of expression constructs for gene therapy
InactiveUS7074772B2Facilitated releaseEasier chemical linkingBiocideNanotechGenetic vaccineCell biology
The invention concerns an expressible nucleic acid construct, which contains only the sequence information necessary for expressing a gene for RNA or protein synthesis. Expression constructs of this type can be used in gene therapy and genetic vaccination and avoid many of the risks associated with constructs today. The invention further concerns the possibility of improving the conveying of the construct into cells or tissue by covalent linkage of the construct, for example to particles of peptides.
Owner:MOLOGEN AG
Duck plague virus gene deletion transfer vector and application thereof
InactiveCN104480142ADoes not affect immunogenicityMaintain immunogenicityMicroorganism based processesAntiviralsDiseaseTransfer vector
The invention aims at providing a duck plague virus gene deletion transfer vector and an application of the duck plague virus gene deletion transfer vector. The transfer vector is subjected to homologous recombination with duck plague virus to obtain duck plague virus gene deletion engineering strains which can be prepared into a high-safety and high-prevention-effect duck plague virus gene deletion vaccine. The duck plague virus gene deletion vaccine does not influence the virus immunogenicity while lowering down the virus pathogenicity; therefore, the prepared duck plague virus gene deletion vaccines are high in safety, able to remain the original virus immunogenicity, suitable for prevention and control for the current duck plague diseases in domestic, and beneficial for the cleaning treatment of duck plague in a duck farm; in addition, the recombined duck plague virus strains do not contain any exogenous genes; and compared with the similar duck plague virus gene vaccines reported in the prior art, the duck plague virus gene deletion vaccine has the advantage that no EGFP (Enhanced Green Fluorescent Protein), lacZ and other marker gene are contained, so that the bio-safety is improved.
Owner:QINGDAO VLAND BIOTECH INC
Immunogenic peptides for use in the prevention and/or treatment of infectious diseases, autoimmune diseases, immune responses to allofactors, allergic diseases, tumors, graft rejection and immune responses against viral vectors used for gene therapy or gene vaccination
ActiveUS10023847B2Limited degreeReduced activityVirusesPeptide/protein ingredientsVaccinationAutoimmune responses
Owner:IMCYSE
Method for preparing actinobacillus pleuropneumoniae (App) bacterial ghost and method for preparing subunit vaccine by loading pasteurella antigen with App bacterial ghost
InactiveCN101934072APrevention of swine pleuropneumoniaPrevention of PasteurellosisAntibacterial agentsBacterial antigen ingredientsAntigenPleuronectes pinnifasciatus
The invention discloses a method for preparing an actinobacillus pleuropneumoniae (App) bacterial ghost and a method for preparing a subunit vaccine by loading a pasteurella antigen with the App bacterial ghost. A recombinant swine App bacterial ghost is prepared by controllable double-cracking technology and a pasteurella protection gene is introduced into an App bacterial ghost carrier, so that swine pleuropneumonia and a pasteurella bigeminal gene vaccine for preventing and treating swine pasteurellosis and swine pleuropneumonia are obtained. The preparation of the bacterial ghost carrier and the application of the bacterial ghost carrier to the prevention and treatment of important animal epidemic diseases are realized and a method is provided for the research of a multi-geminal gene vaccine at the same time. An animal experiment indicates that the protection rates of the bigeminal vaccine on infectious swine pleuropneumonia and pasteurellosis are up to 99 percent and 99.2 percent respectively.
Owner:TIANJIN AGRICULTURE COLLEGE
Adeno-associated virus capsid protein gene, corresponding protein and application of protein
The invention discloses an adeno-associated virus capsid protein gene, a corresponding protein and the applications of the protein. The nucleotide sequence of the adeno-associated virus capsid protein gene is shown in SEQ ID NO:1 and the amino acid sequence of the protein coded by the gene is shown in SEQ ID NO:2. The adeno-associated virus vector packaged by the adeno-associated virus capsid protein can be used to effectively introduce foreign genes in cells outside the body or in the body and prepare the medicines related to gene therapy or gene vaccines.
Owner:SUN YAT SEN UNIV
Gene vaccine for anti SARS coronal virus and use thereof
InactiveCN1449826AAdvantage preventionAdvantageous therapeuticGenetic material ingredientsAntiviralsNucleotideIn vivo
The present invention discloses a gene vaccine for resisting SARS coronavirus and its application, including eucaryotic expressino plasmid containing total or partial gene fragment of total-length gene sequence of SARS coronavirus S protein. The nucleotide sequence of coded SARS coronavirus S protein can be operatively connected with a promotor, then it can express the complete or partial polypeptide of the coded SARS coronavirus S protein in vivo. The described gene vaccine can be used for immunizing host including human body and rodent, for example mouse to make it produce specific protective body fluid immune and cell immune to resist the infection of SARS coronavirus. Said invention is good in stability, convenient in production and low in cost.
Owner:WUHAN UNIV
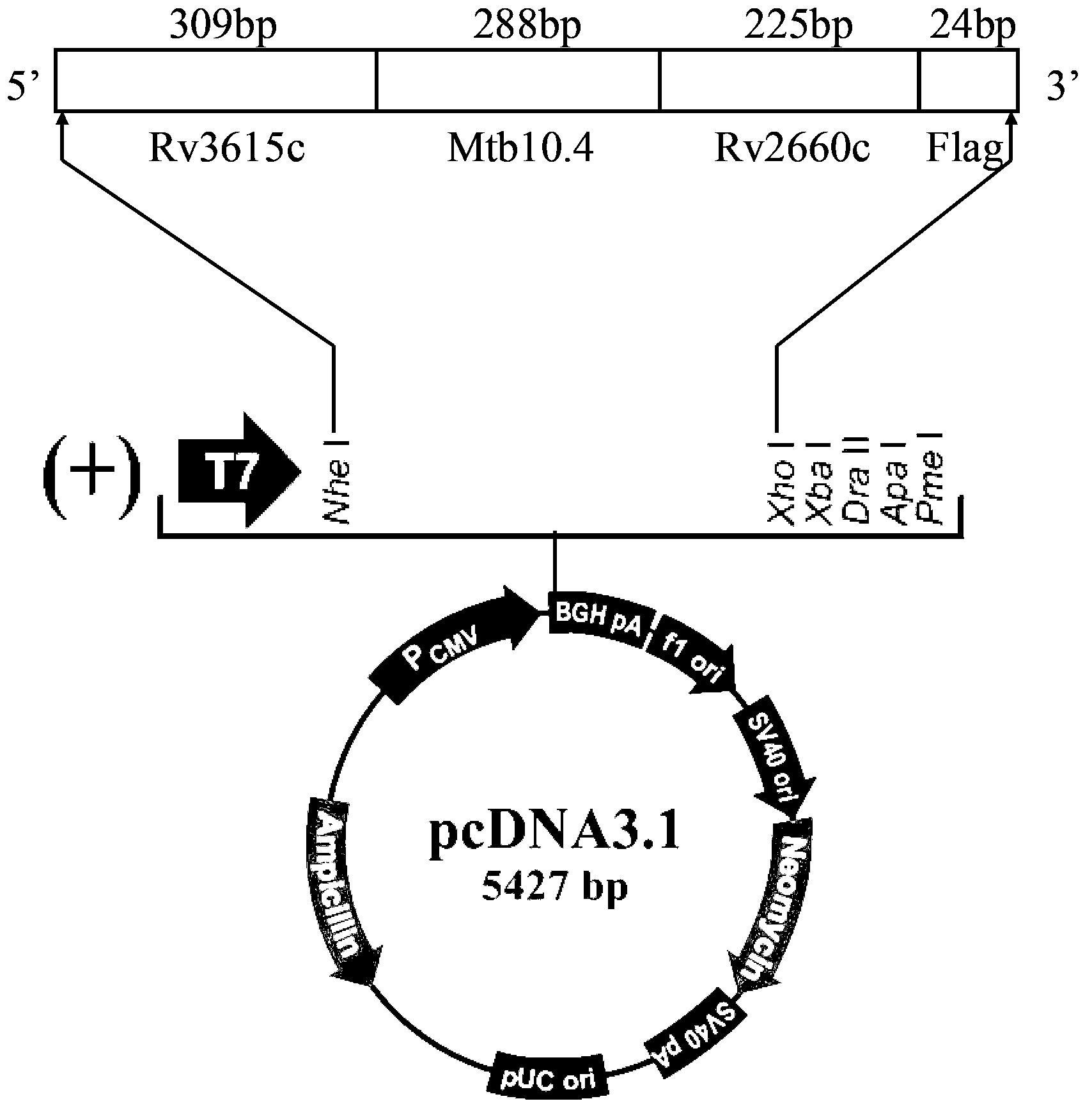
Three-antigen fusion gene vaccine of mycobacterium tuberculosis as well as preparation method and application of three-antigen fusion gene vaccine
The invention discloses a fusion protein gene which is formed by sequentially connecting an antigen gene Rv3615c, an antigen gene Mtb10.4 and an antigen gene Rv2660c in series from a 5' end to a 3' end. The invention also provides a three-antigen fusion gene vaccine of mycobacterium tuberculosis as well as a preparation method and application of the three-antigen fusion gene vaccine of the mycobacterium tuberculosis, wherein the three-antigen fusion gene vaccine is composed of a carrier, and a segment of the fusion protein gene is inserted to the carrier. Rat immunological experiments show that the three-antigen fusion gene vaccine of the mycobacterium tuberculosis can be used for inducing a better cellular immunological response. The three-antigen fusion gene vaccine of the mycobacterium tuberculosis, disclosed by the invention, can be used for inducing a specific killing response and a stronger CD4<+> and CD8<+>T cellular immune protection reaction having an important protection effect on mycobacterium tuberculosis infection, can be used for effectively resisting the mycobacterium tuberculosis infection, and plays a better protection effect on tuberculosis infected rats.
Owner:SUZHOU UNIV
Method for increasing expression of RNA-encoded proteins
ActiveUS10293060B2Improve securitySsRNA viruses negative-sensePeptide/protein ingredientsOpen reading frameJet injection
The invention relates to an RNA comprising at least one open reading frame (ORF) and comprising at least one modification, which increases the expression of the encoded peptide or protein. Furthermore, the invention relates to the medical use of such a modified RNA administered to a subject by jet injection. The invention relates further to a pharmaceutical composition and to a kit of parts comprising said modified RNA for administration by jet injection, preferably for use in the field of gene therapy and / or genetic vaccination. Additionally, the invention relates to a method for enhancing the (localized) expression of RNA-encoded peptides or proteins in the dermis or muscle (of a mammal) comprising administering the modified RNA by jet injection. And finally, the invention relates to a method of treatment comprising administering the modified RNA by jet injection to a subject in need thereof.
Owner:CUREVAC SE
Tuberculosis gene vaccine assembled by chitosan delivery system and preparation method and use thereof
InactiveCN101455846AConvenient inductionSafe and non-toxicAntibacterial agentsBacterial antigen ingredientsWhole bodyEukaryotic plasmids
The invention relates to a tuberculosis gene vaccine assembled by a chitosan delivery system, which consists of chitosan and tuberculosis antigen encoding plasmids, wherein full-length genes of tubercle bacillus heat shock proteins HSP65 are inserted into the tuberculosis antigen encoding plasmids; and four T cell epitope genes EAST-6[189-228], Ag85A[369-405], CFP10[162-207] and Ag85B[420-459] from tubercle bacillus antigens are inserted into the HSP65 full-length genes. The invention also discloses application of the tuberculosis gene vaccine. By performing nasal drip of the gene vaccine on an immune mouse, the gene vaccine is proved to be capable of inducing response to special antibodies of a plurality of tuberculosis antigens, inducing killing response of locally strong tuberculosis special T cells of the whole body and lung mucous membranes, simultaneously inducing immunological response of Th1 which secretes high-level IFN gamma and have the effect which is obviously superior to the prior BCG vaccine, and is a superior vaccine for preventing and treating tuberculosis.
Owner:FUDAN UNIV
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS20020038112A1Strong immune responseEfficient killing of transfected cellsBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:MATHIESEN IACOB +1
Mucosa M-cell targeted viral myocarditis gene vaccine and preparation method thereof
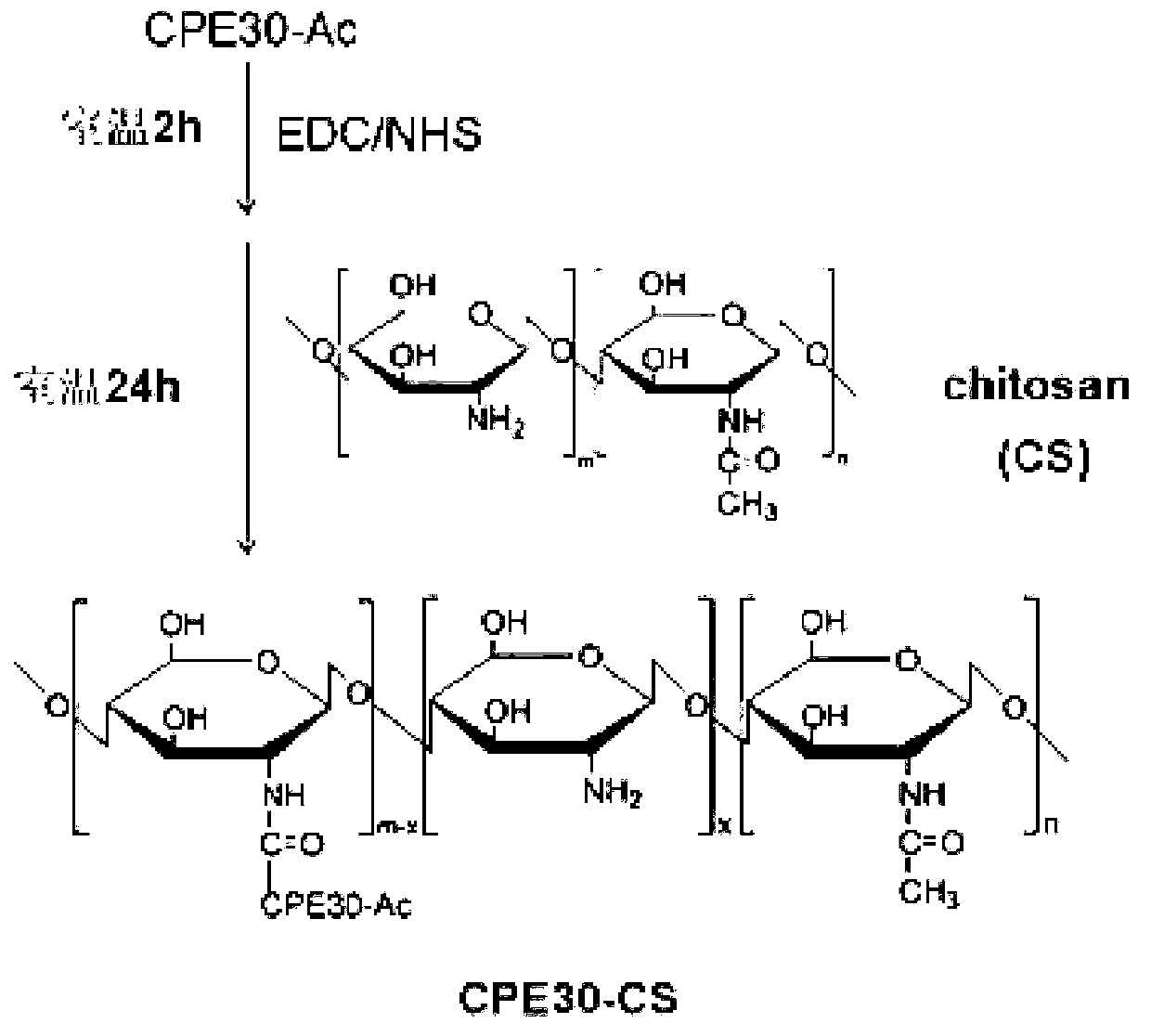
ActiveCN103341179AValid responseEfficiently induces a responseGenetic material ingredientsAntiviralsWhole bodyViral Myocarditis
The invention discloses a mucosa M-cell targeted viral myocarditis gene vaccine. The vaccine is prepared by compounding a mucosa delivery system capable of targeting mucosa M-cells and B3-type coxsackie virus antigen encoding plasmids through cross-linking copolymerization. The invention further discloses a preparation method of the gene vaccine. The invention further discloses a preparation method of the mucosa delivery system capable of targeting the mucosa M-cells, and the mucosa delivery system is acquired after stable amide-ester bonds are formed by carboxyl groups and amino groups in deacetylated chitosan, wherein the carboxyl groups in peptide fragment CPE30 are targeted by M-cells which are activated by using EDC (Dichloroethane) and NHS (N-Hydroxysuccinimide). By nasally dropping the gene vaccine onto immunized mice, the gene vaccine is proved to be capable of effectively inducing the response between specific antigen serum and mucoantibody, obviously enhancing the local specific T-cell killing ability of the whole body and gastrointestinal mucosa and significantly improving the ability of mice for resisting B3-type coxsackie virus, thereby being an excellent prophylactic vaccine for viral myocarditis.
Owner:SUZHOU UNIV
Immunogenic peptides for use in the prevention and/or treatment of infectious diseases, autoimmune diseases, immune responses to allofactors, allergic diseases, tumors, graft rejection and immune responses against viral vectors used for gene therapy or gene vaccination
ActiveUS20130259885A1Enhance immune responseLimited degreeVirusesPeptide/protein ingredientsVaccinationAutoimmune responses
The invention describes new peptides containing epitopes recognized by CD4+ natural killer T (NKT) cells for increasing activity for use in infectious diseases, autoimmune diseases, immune reaction to administration of allofactors, allergic diseases, therapy of tumors, prevention of graft rejection and prevention of immunization against viral proteins used in gene therapy or gene vaccination.
Owner:IMCYSE
Functional DNA clone for hepatitis C virus (HCV) and uses thereof
InactiveUS7235394B1Enabled dissectionImprove protectionSsRNA viruses positive-senseVectorsRNA SequenceGenome
The present invention relates to the determination of an authentic HCV genome RNA sequences, to construction of infectious HCV DNA clones, and to use of the clones, or their derivatives, in therapeutic, vaccine, and diagnostic applications. The invention is also directed to HCV vectors, e.g., for gene therapy of gene vaccines.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Amyloid beta gene vaccines
ActiveUS7479550B2High expressionIncrease initiativePolypeptide with localisation/targeting motifBiocideVaccinationAmyloid beta
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Gene vaccine vector, and preparation method and application thereof
ActiveCN104274839AImproving immunogenicityIncrease loadAerosol deliveryGenetic material ingredientsMicrobiologyStructural formula
The invention discloses a gene vaccine vector, and a preparation method and an application thereof; the gene vaccine vector is a supramolecular hydrogel formed through phosphatase catalysis of a small-molecular peptide; the small-molecular peptide has the structural formula represented by the formula (I), wherein when m=1, n=0, 1, 2 or 3; when n=1, m=1, 2 or 3. The gene vaccine vector after being loaded with DNA has the advantages of strong immunogenicity, large load capacity, no obvious toxicity and injectable immunity. The gene vaccine vector is mild in preparation conditions and simple in process.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Viral myocarditis gene vaccine, its preparation method and application
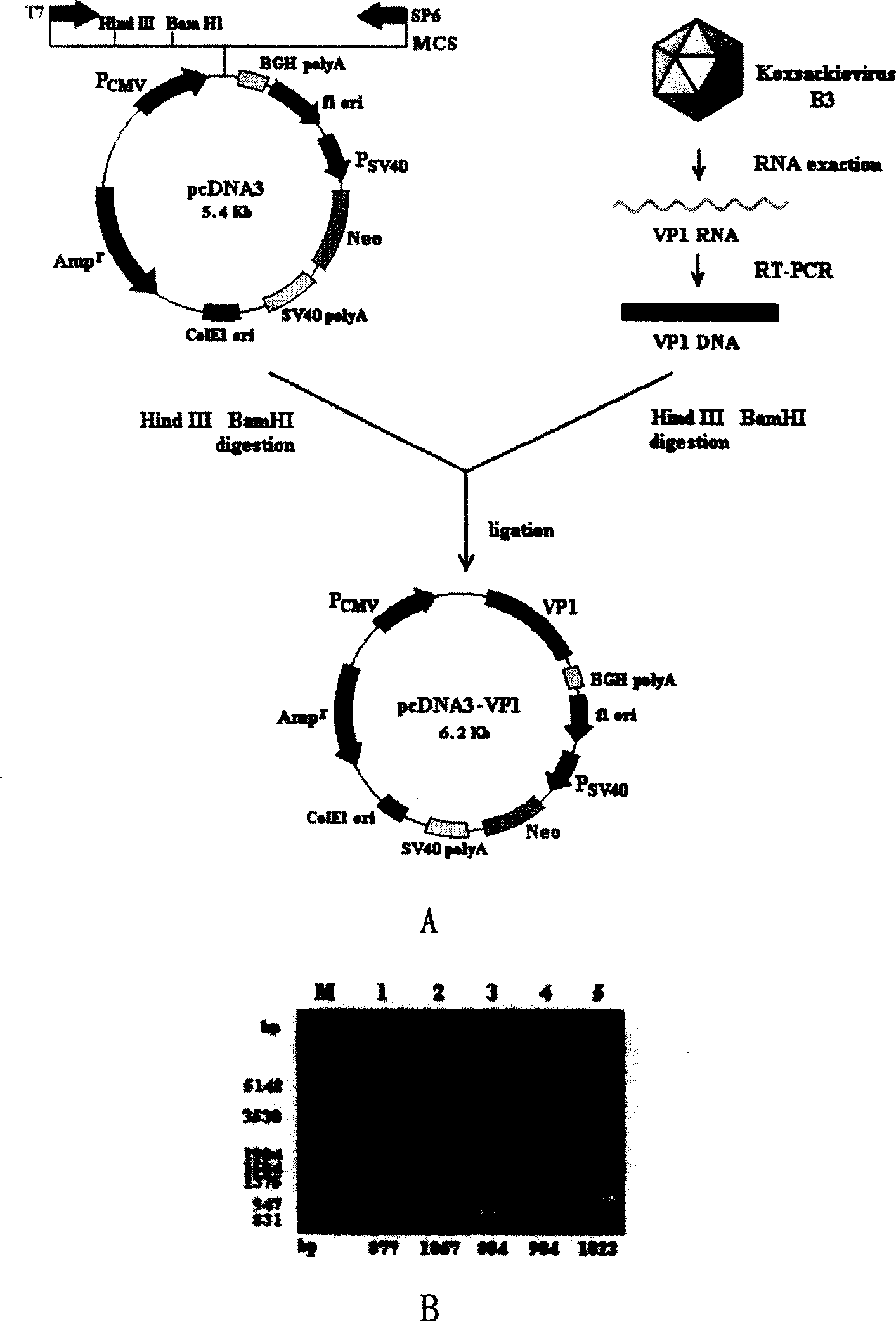
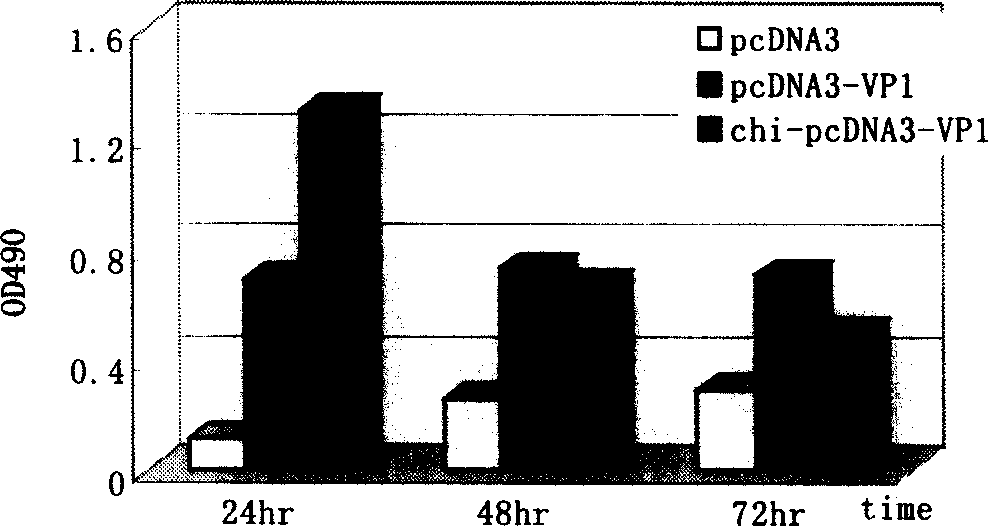
The present invention discloses a viral myocarditis gene vaccine, Said vaccine is constructed by using B3 type Coxsackie virus structural protein VPI gene and pcDNA carrier together, and its exterior is covered with a biological polysaccharide. It also discloses a method for preparing said gene vaccine and the application of said gene vaccine in preparation of medicine for preventing viral myocarditis.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
HPV (human papillomavirus) DNA (deoxyribonucleic acid) genetic typing method and kit thereof
InactiveCN103320533AHelp in early preventionEasy to operateMicrobiological testing/measurementMicroorganism based processesHuman papillomavirusTyping methods
The invention discloses an HPV(human papillomavirus) DNA (deoxyribonucleic acid) genetic typing method and a kit thereof. The method disclosed by the invention can be used for typing DNA of 46 types of HPVs, is simple to operate, is economical and practical and fast and efficient, and is combined with chip capillary electrophoresis testing to analyze the HPV DNA type with high throughput. When applied in the medical domain, the HPV / DNA genetic typing method is combined with the existing lamina ThinPrep cytology morphology diagnosis and is benefit to the early-stage prevention, prognosis and research of target gene vaccines for cervical cancer; and the method can be used for detecting multiple types and finding unknown types, thereby being especially suitable for research of pathogenesis and epidemiology of HPV-related diseases, such as hypopharyngeal carcinoma and head and neck cancer.
Owner:TSINGHUA UNIV +1
NDA vaccine eucaryon expression carrier and application in preparation of gene vaccine
The invention discloses a eucaryon expression vector of a DNA vaccine and the application. The eucaryon expression vector of DNA vaccine is a recombination pVAX1 eucaryon expression vector which is got by adding the internal ribosome in pVAX1 vector skeleton and entering the site coded sequence. The eucaryon expression vector may show the wide spectrum curing function for breast cancer, pancreatic cancer, restocarcinoma and multi-type malignancy solid tumor, which has good humoral immunity and cell-mediated immunity effect, and inhibits the growth of the tumor.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
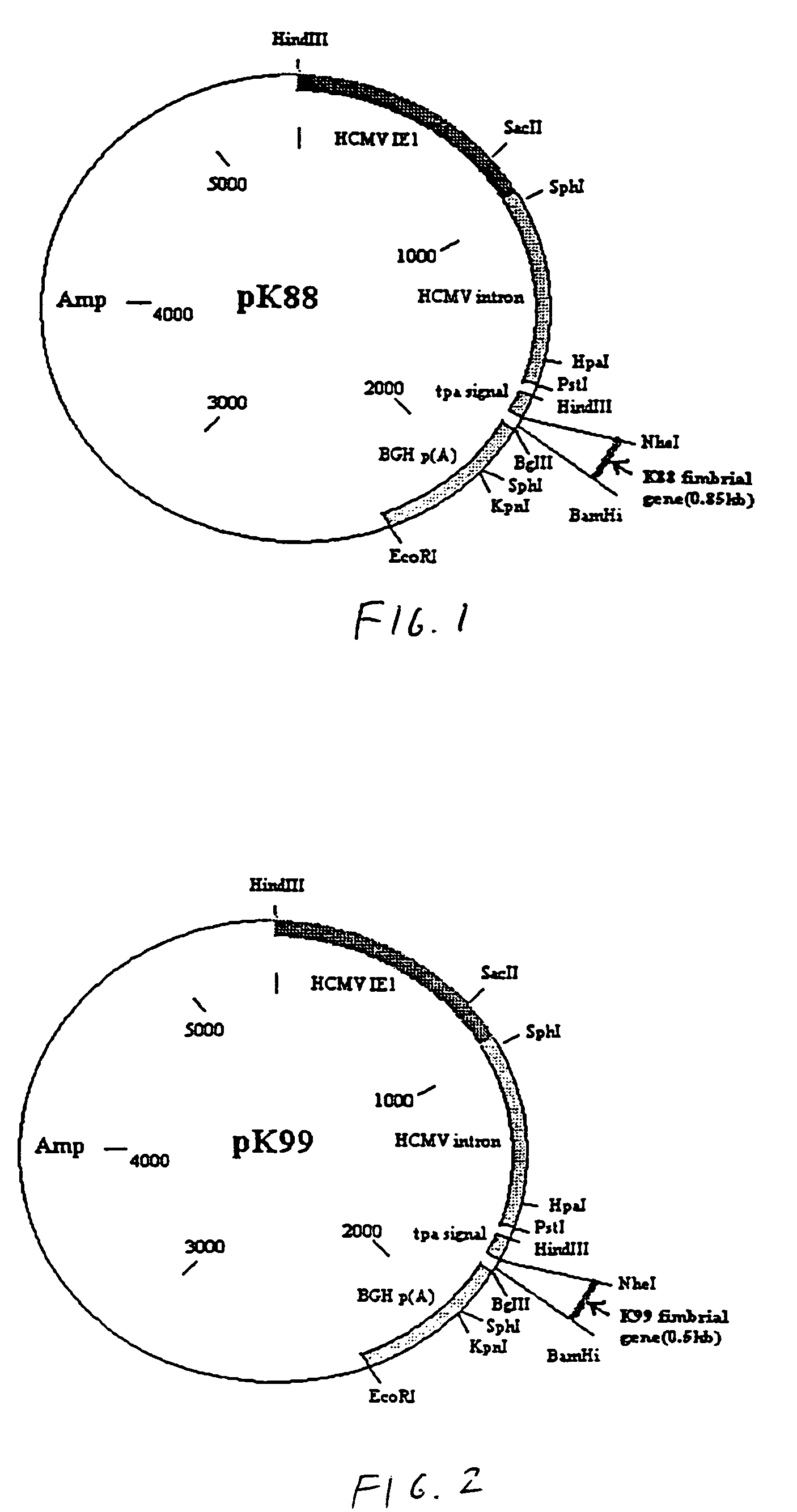
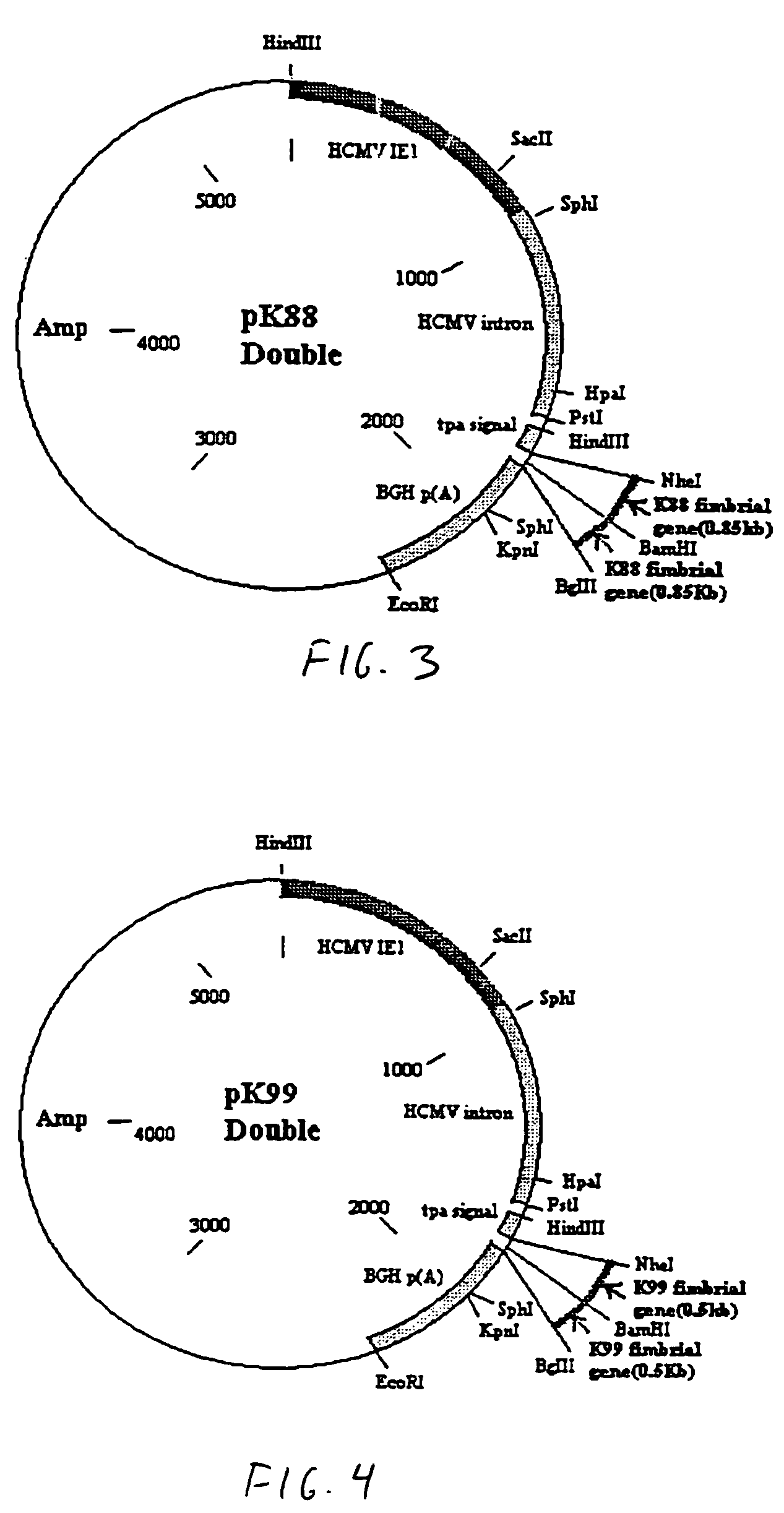
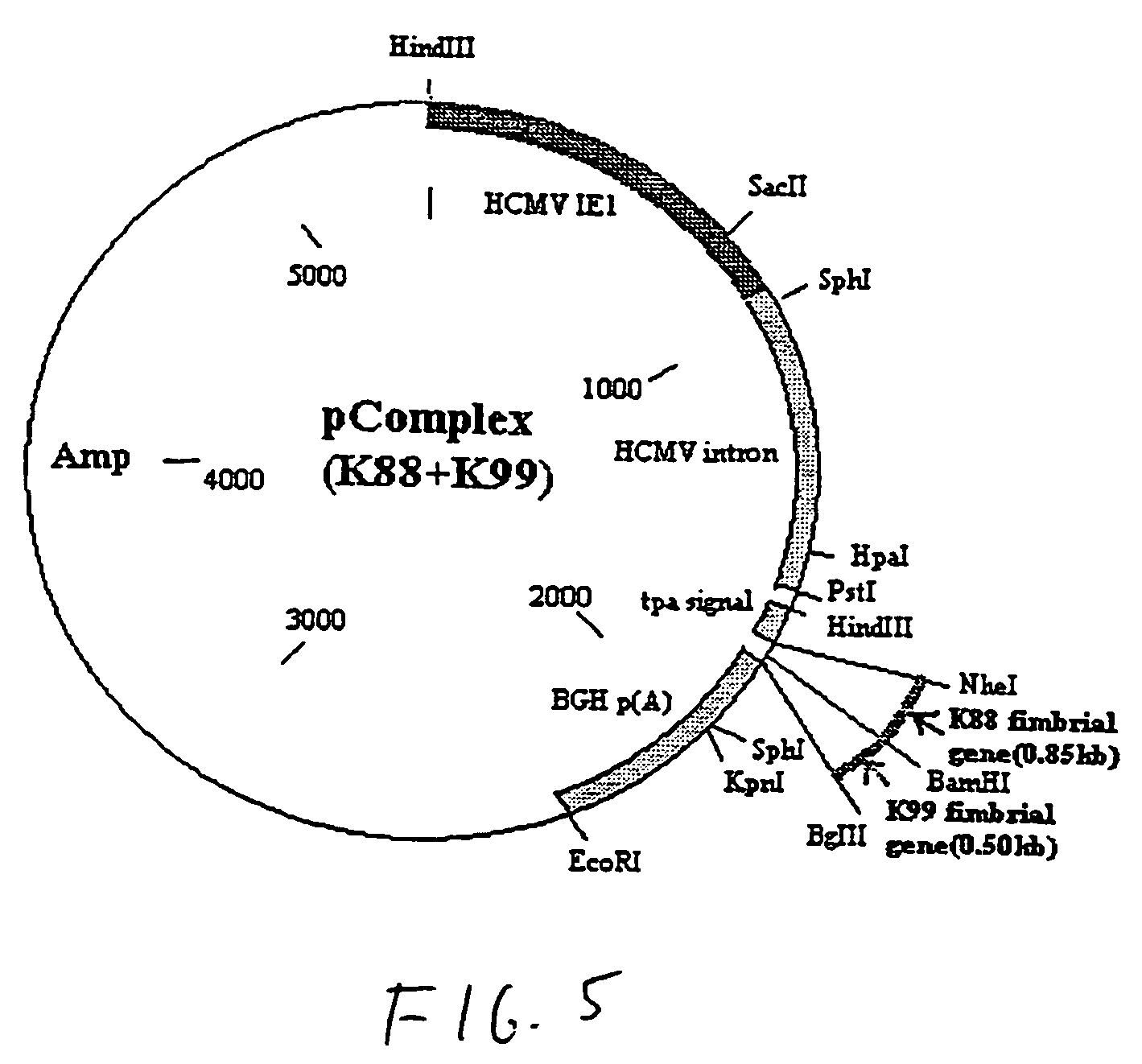
Genetic vaccines for the production of chicken egg-yolk antibodies against enterotoxigenic Escherichia coli and other pathogens
Genetic vaccine which comprises plasmid(s) containing genes coding for antigens of enterotoxigenic Escherichia coli (ETEC) strains is disclosed. Additionally, plasmids may consist of multiple copies of the same antigen (i.e. K88 or K99 fimbrial antigen) or multiple antigens (ie. K88 and K99 fimbrial antigens) and genetic adjuvants such as cytokines (IL-2, IL-4 & GM-CSF), costimulatory molecules (CD80 & CD86) or chemokines or immunostimulatory sequences. A method for isolating antibodies from chicken egg yolk for passive immunization of animals, as well as humans to control diarrhoeal diseases using the genetic vaccines is also disclosed.
Owner:UNIVERSITY OF MANITOBA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com