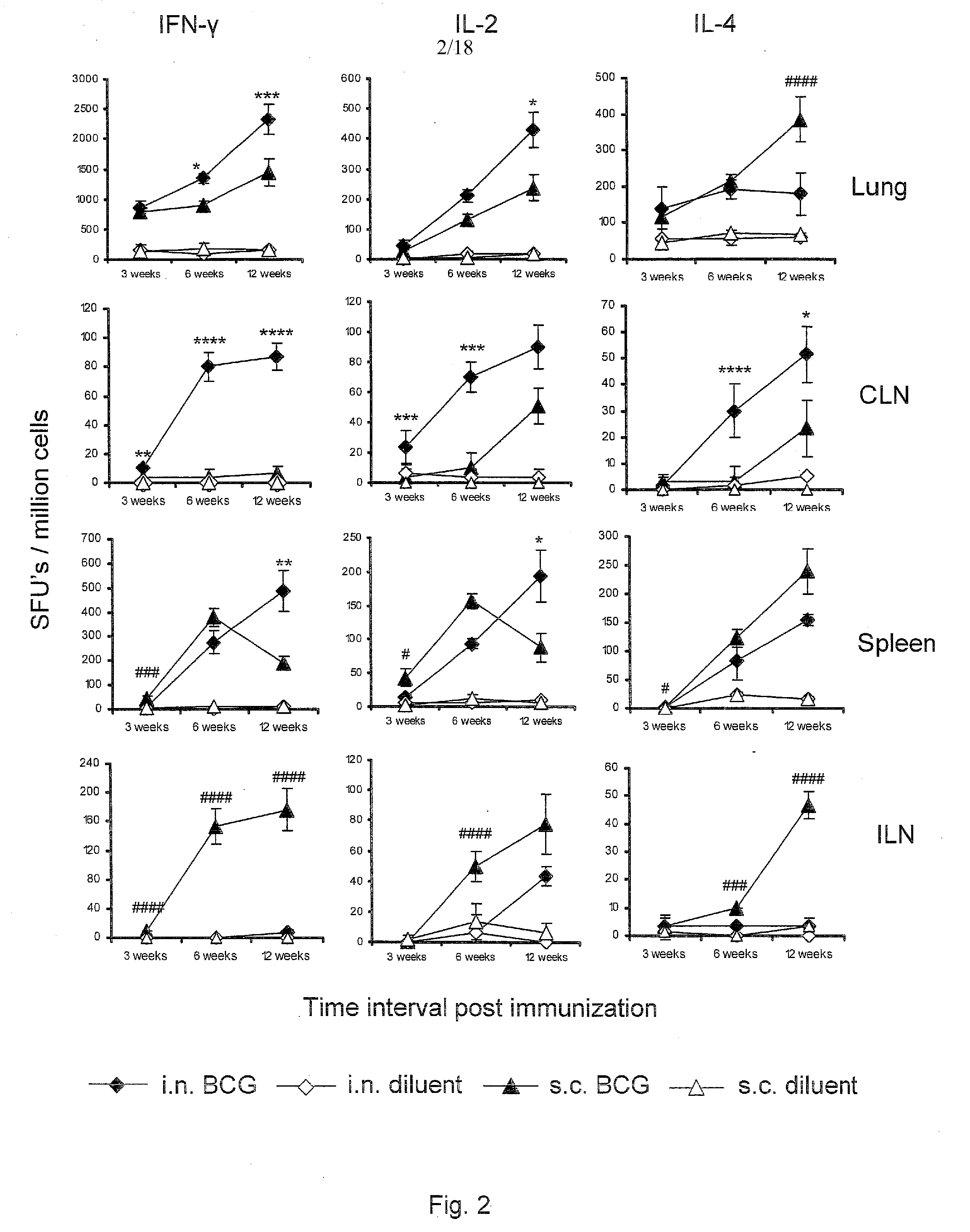
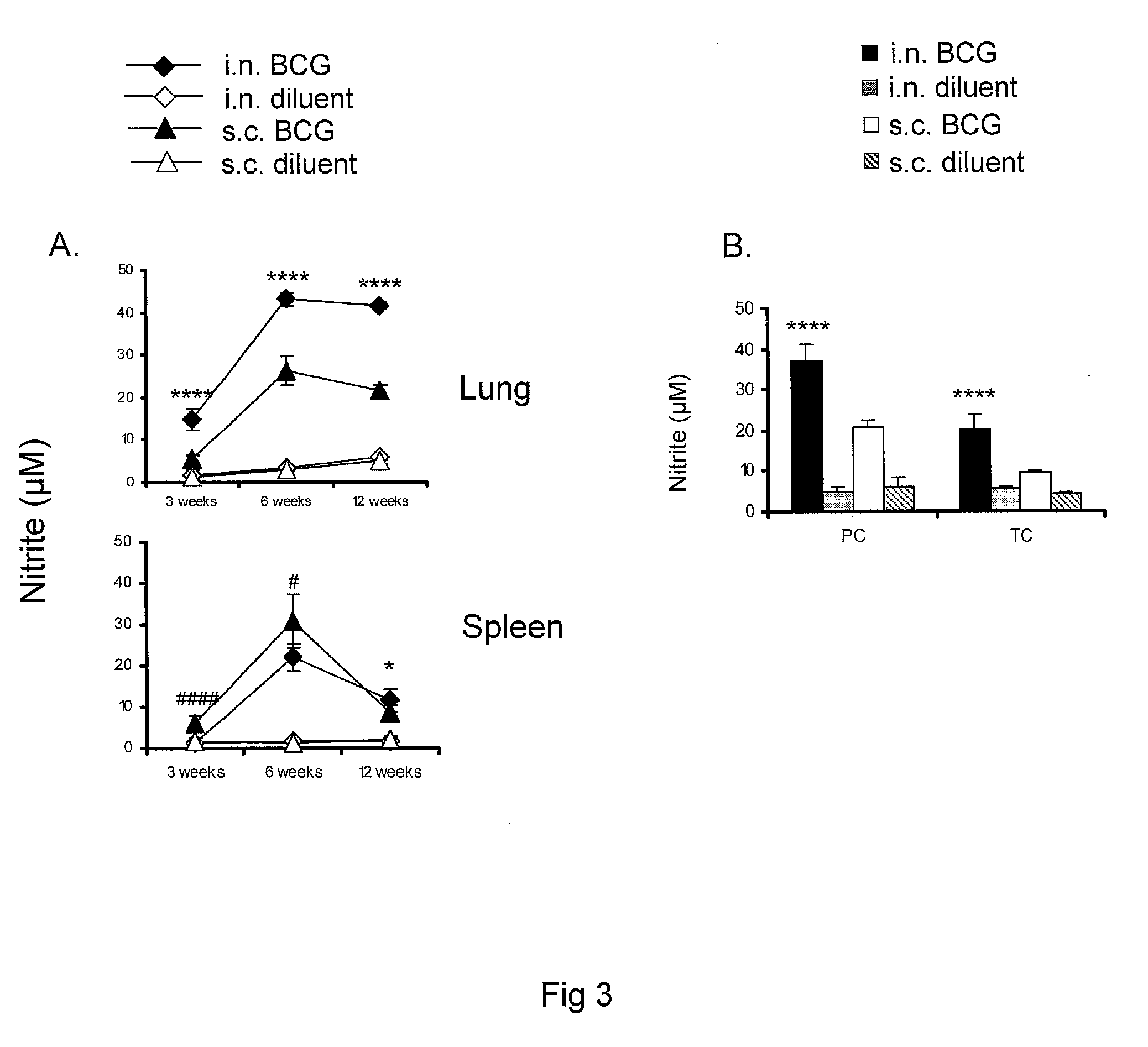
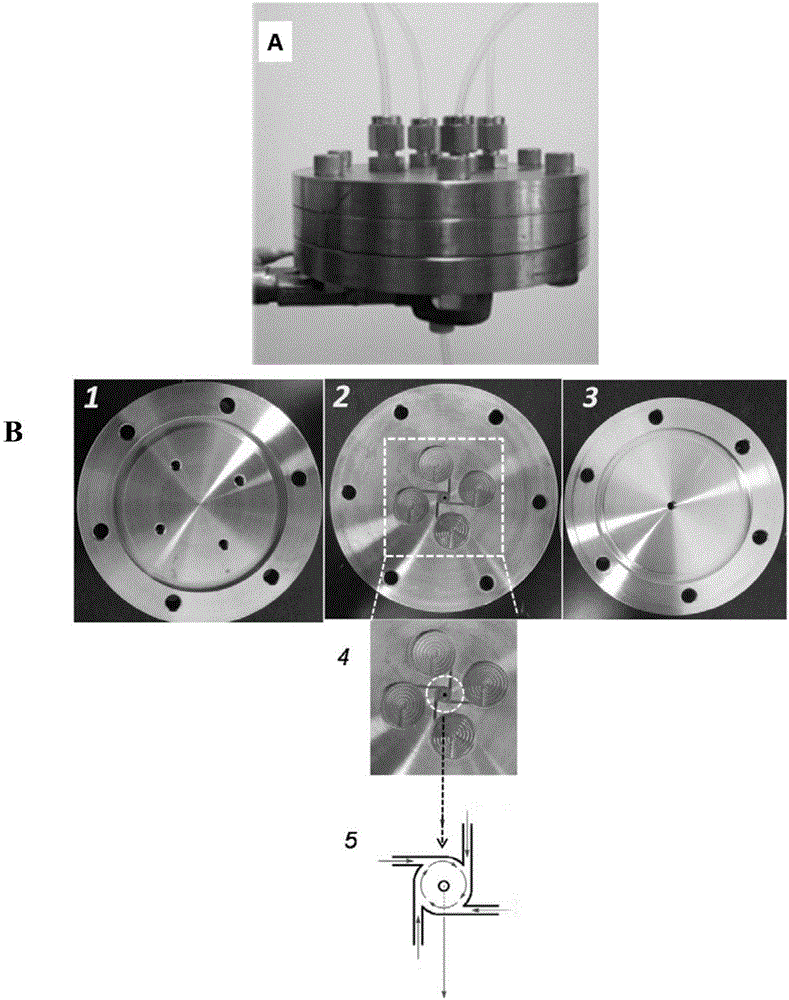
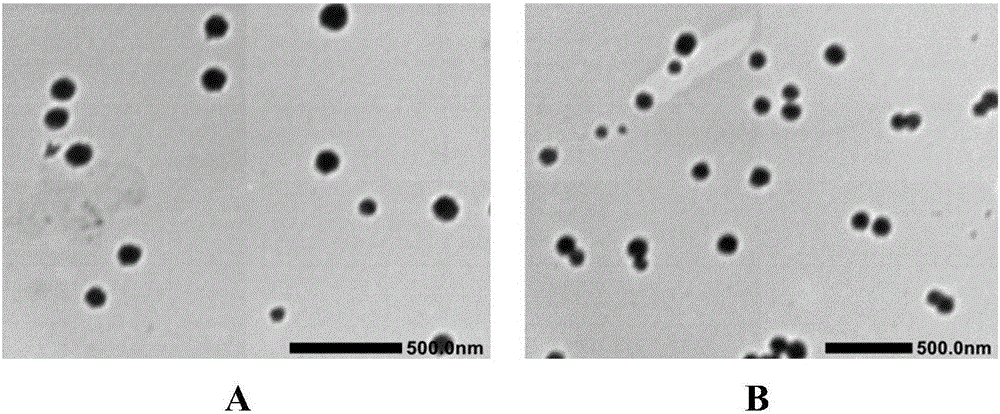
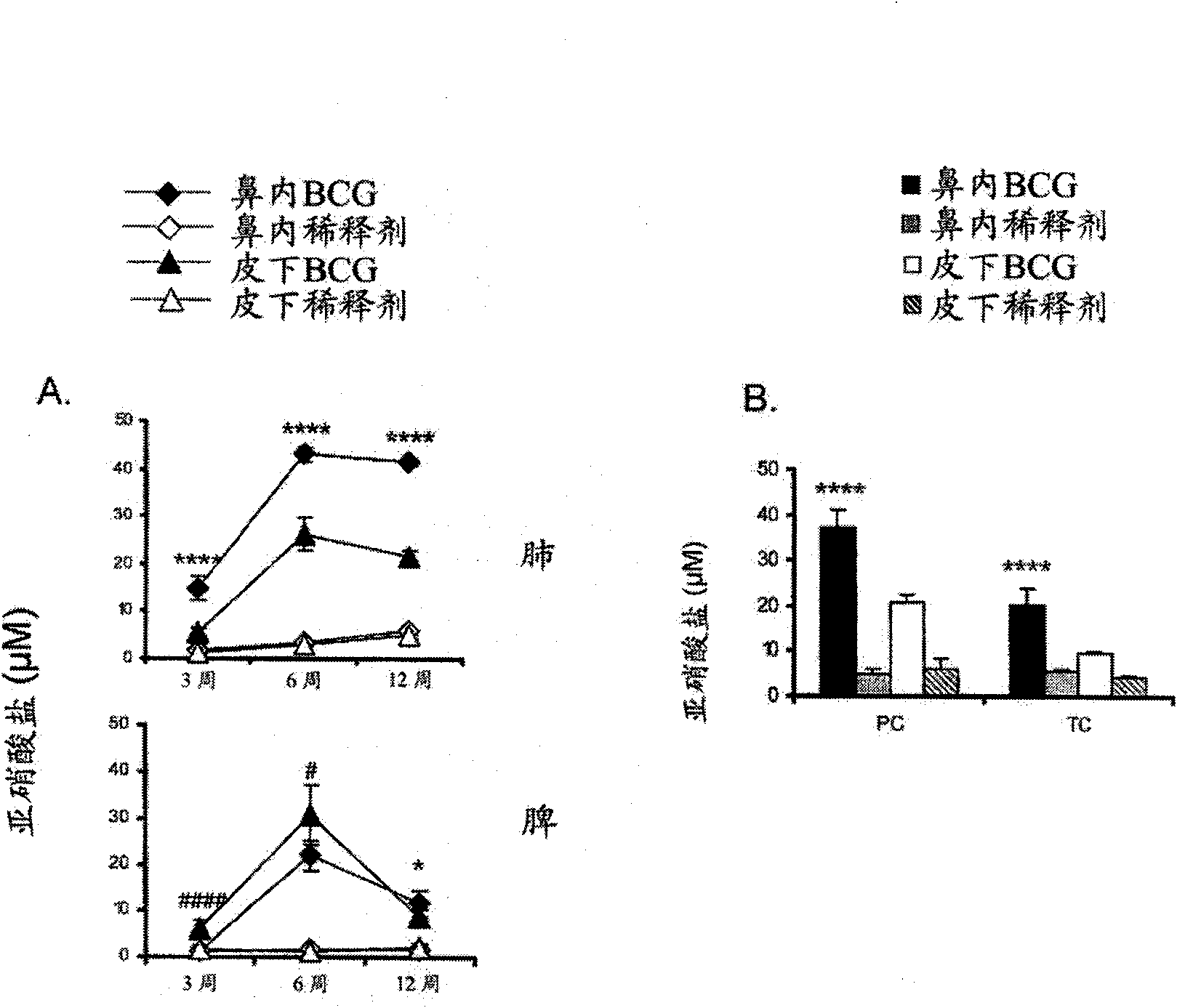
Patents
Literature
94 results about "BCG vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bacillus Calmette–Guérin (BCG) vaccine is a vaccine primarily used against tuberculosis (TB). In countries where tuberculosis or leprosy is common, one dose is recommended in healthy babies as close to the time of birth as possible. In areas where tuberculosis is not common, only children at high risk are typically immunized, while suspected cases of tuberculosis are individually tested for and treated. Adults who do not have tuberculosis and have not been previously immunized but are frequently exposed may be immunized as well. BCG also has some effectiveness against Buruli ulcer infection and other nontuberculous mycobacteria infections. Additionally it is sometimes used as part of the treatment of bladder cancer.
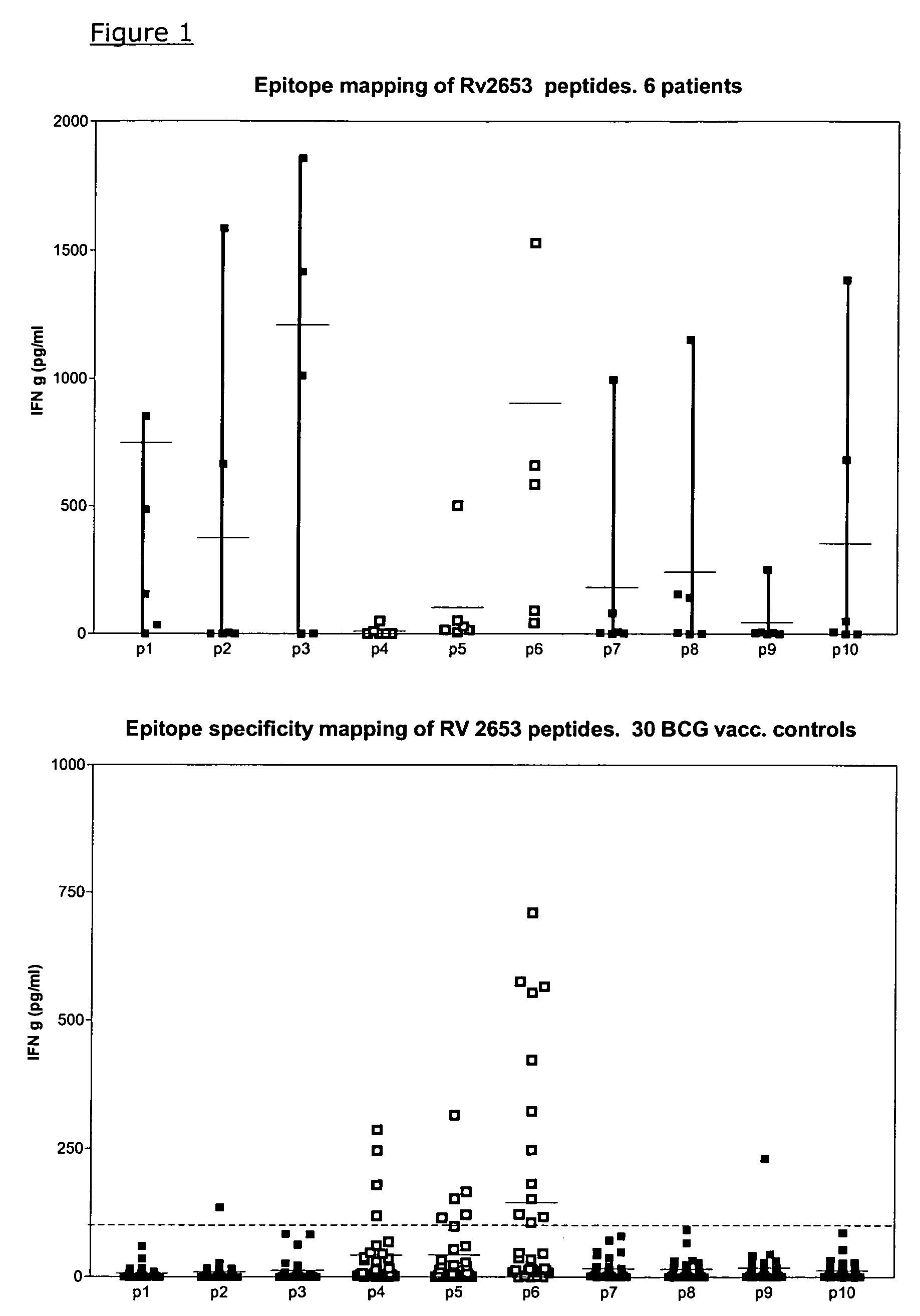
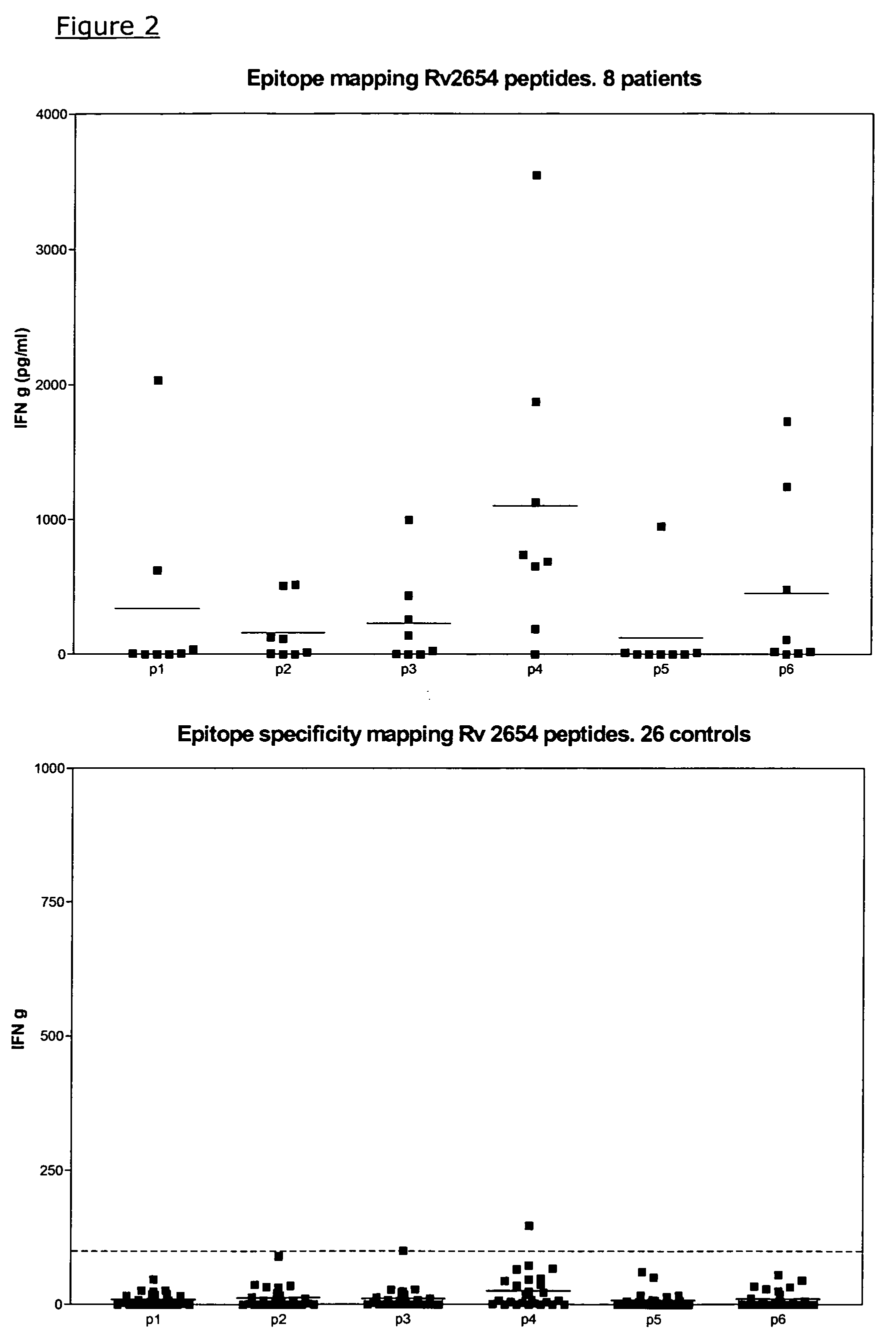
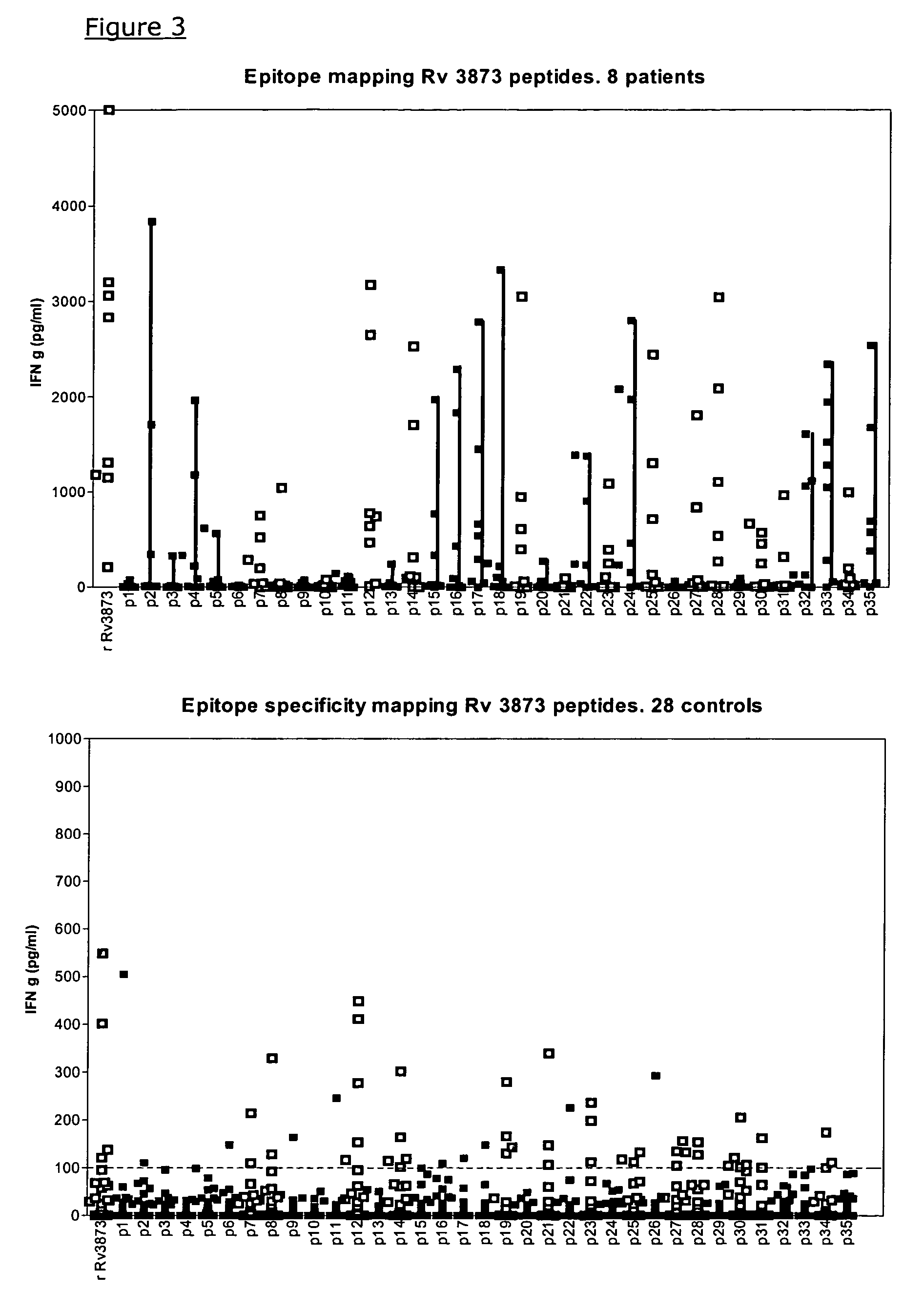
Specific epitope based immunological diagnosis of tuberculosis
ActiveUS20060115847A1Easy to identifyTesting is superfluousBacterial antigen ingredientsMicrobiological testing/measurementSkin allergy testPeripheral blood mononuclear cell
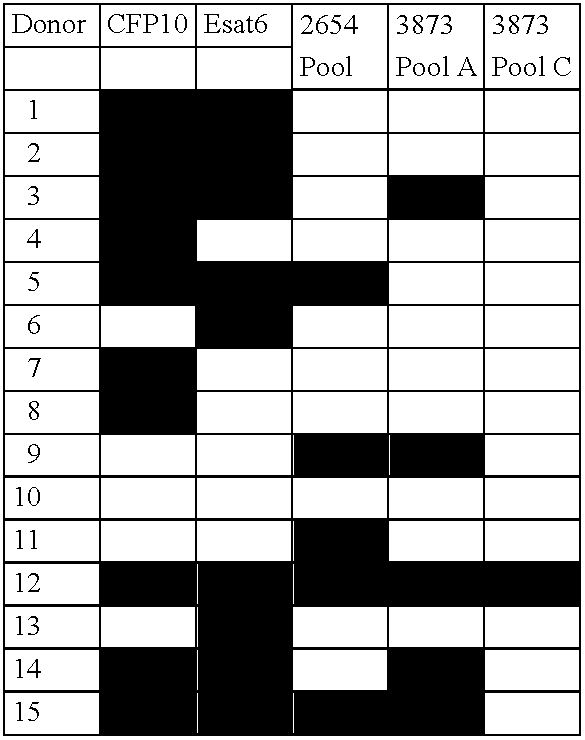
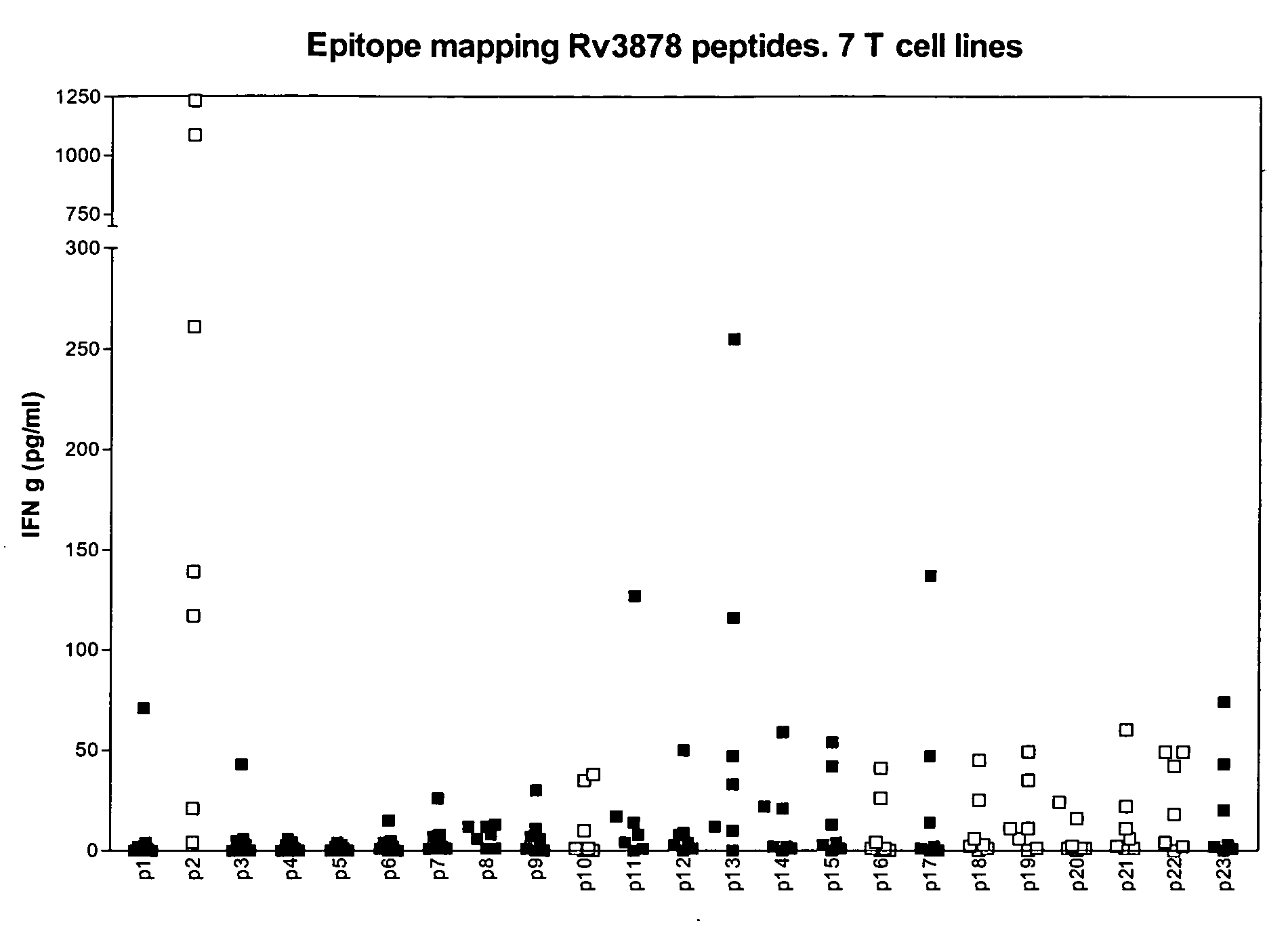
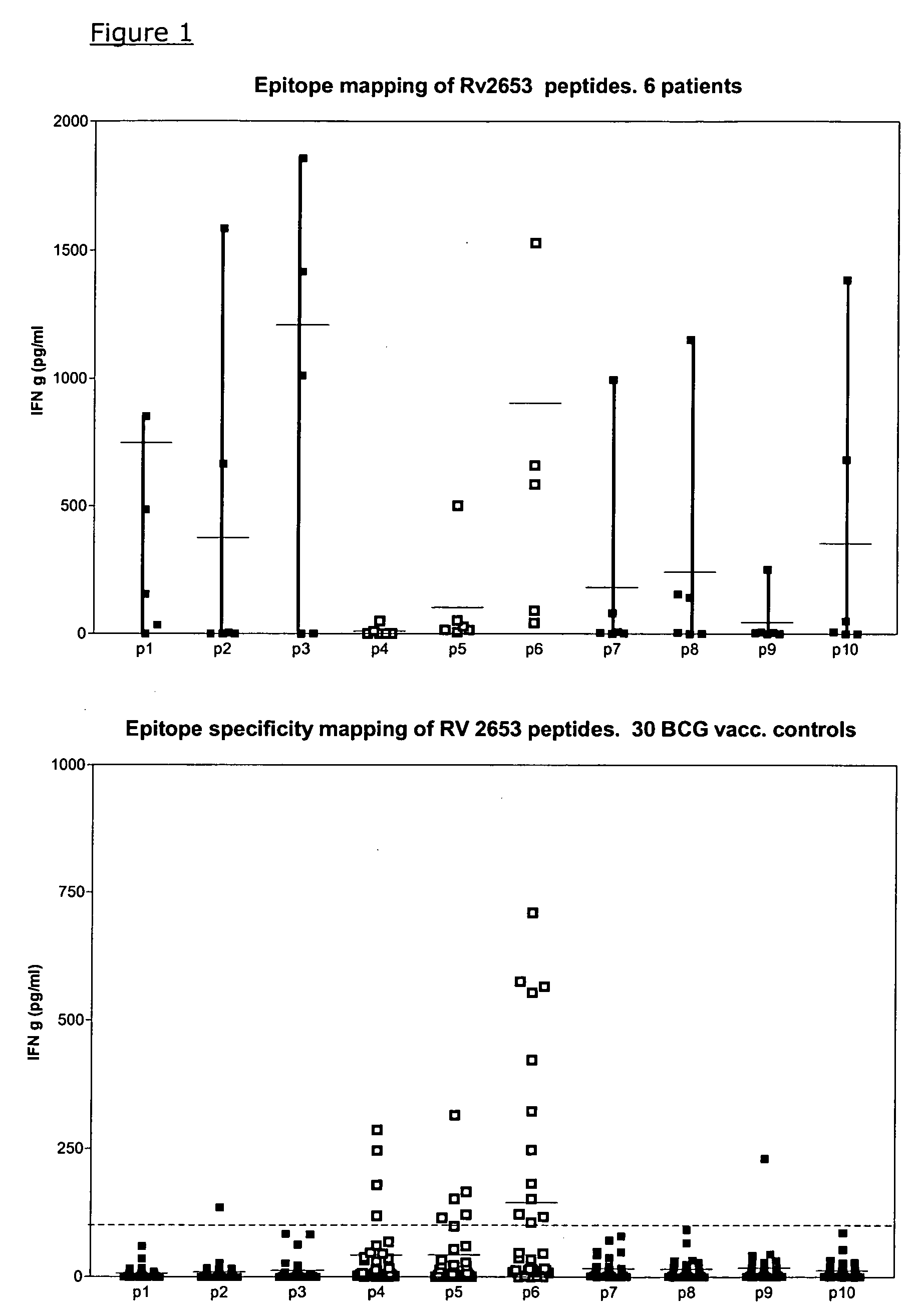
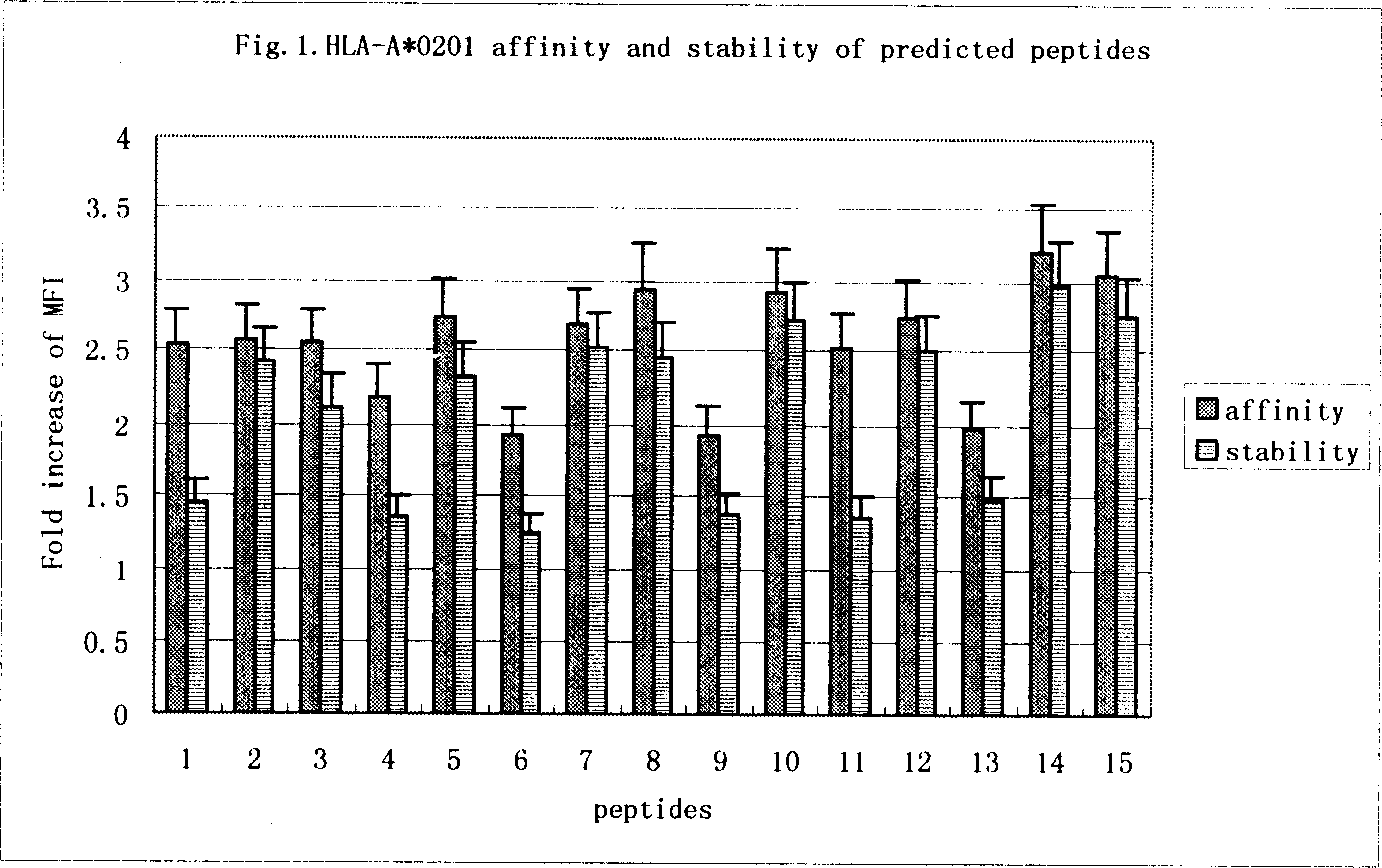
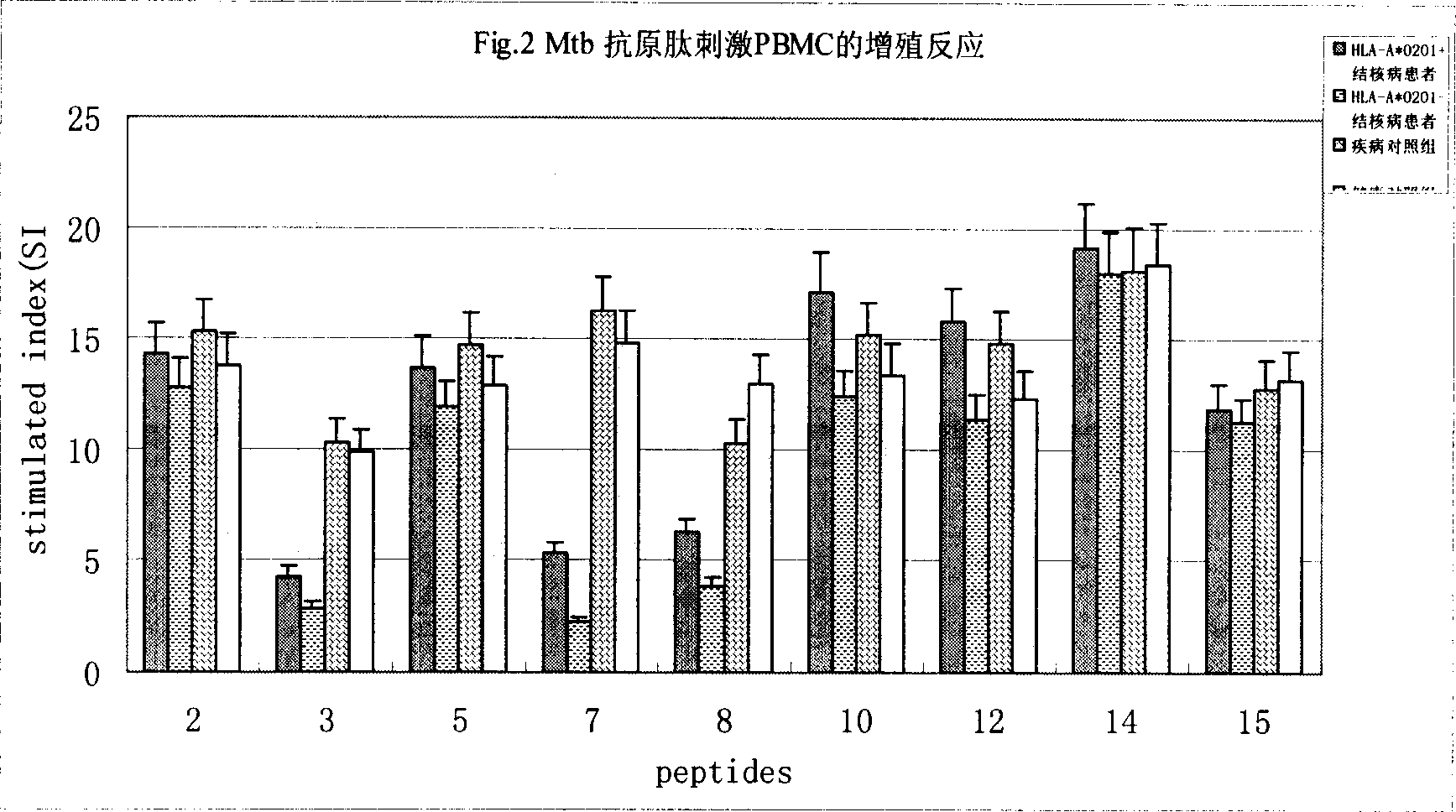
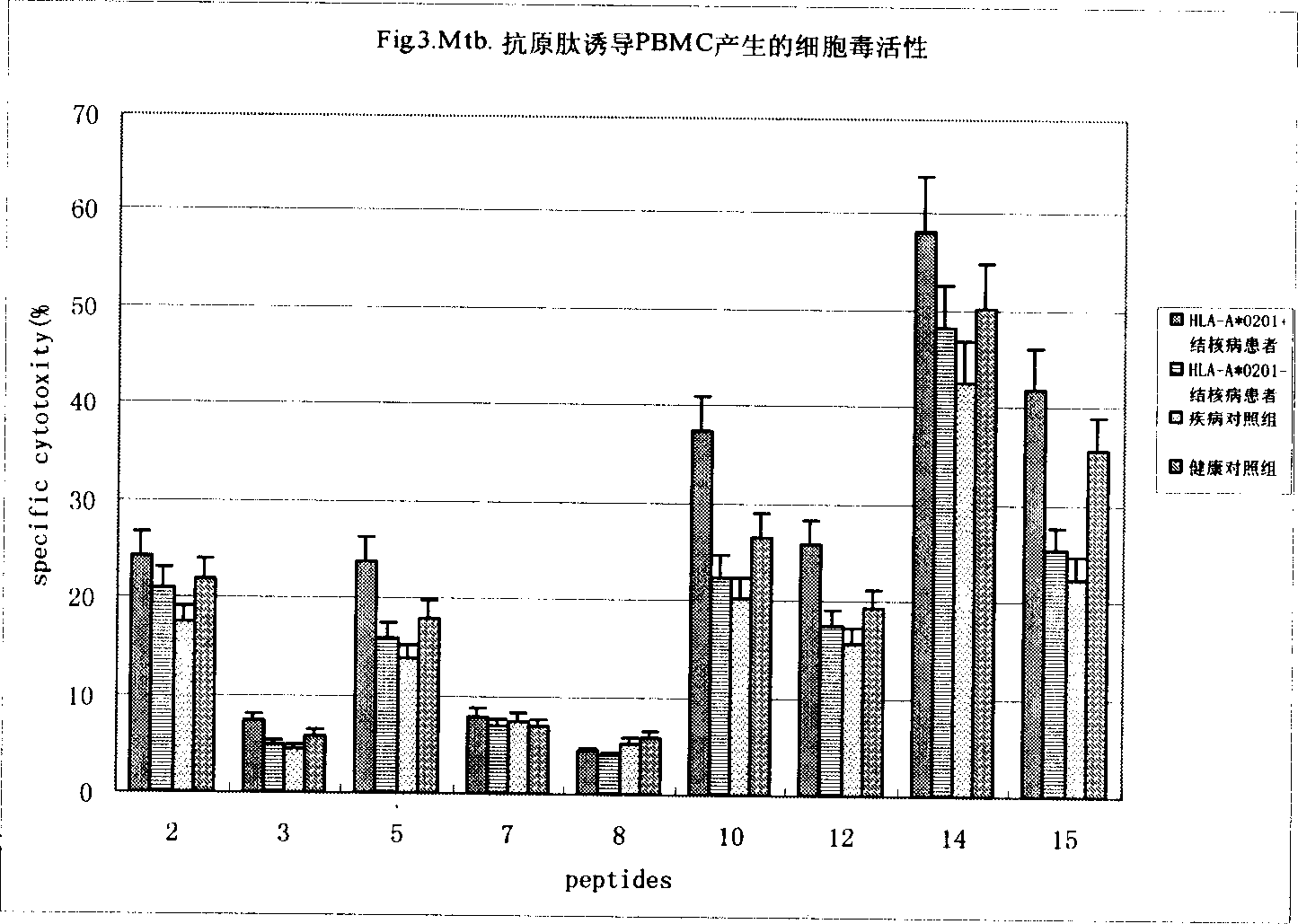
The currently used method for immunological diagnosis of tuberculosis infection, the tuberculin skin test, is problematic for a number of reasons; it has low specificity in BCG vaccinated individuals, a high interobserver variance and requires skill to be read and interpreted. Furthermore it requires an extra visit to the clinic to have the test read. Both people vaccinated with BCG and those exposed to non-tuberculosis mycobacteria give a positive skin test result similar to that seen in a TB infected individual. This also applies for purified protein derivative (PPD) when used in a blood cell based test. The present invention discloses the development of an immunological TB diagnostic tool based on a combination of epitopes from proteins encoded by regions of the M. Tuberculosis (M. tub.) genome, that are not present in the BCG vaccine strain or in the most common non-tuberculosis mycobacteria. Four recently characterized proteins with this diagnostic potential were selected. Peptides from these proteins were tested one by one with peripheral blood mononuclear cells from microscopy or culture confirmed TB patients as well as from healthy BCG vaccinated controls. Some combinations of peptides showed a sensitivity level comparable to the level seen with the two wellknown M. tuberculosisspecific proteins ESAT 6 and CFP 10. An epitope combination with these peptides combined with ESAT 6 and CFP 10 gave a sensitivity of 93%, representing a raise in sensitivity of about 26-33% compared to using ESAT6 or CFP 10 alone. The results from a panel of TB patients, using a collection of the new specific epitopes clearly demonstrates, that addition of other specific epitopes to the already known specific antigens, increases the sensitivity of a diagnostic assay based on cell mediated immune response.
Owner:STATENS SERUM INST
Polypeptide vaccine and vaccination strategy against mycobacterium
A vaccine is provided wherein a polypeptide or combination of peptides from M. tuberculosis is administered to a subject to elicit an immune response. The polypeptide vaccine is administered as part of a prime-boost strategy with BCG vaccine to increase the immunoprotection in a subject such that prevention or elimination of disease is achieved. Finally, a pharmaceutical package is provided that encompasses a polypeptide vaccine for M. tuberculosis that when administered to a subject elicits immunoprotection.
Owner:EMORY UNIVERSITY +1
Reagent and method for detecting active tuberculosis and tuberculosis dormant infection
The invention belongs to the biomedical detection field, in particular relates to a reagent and method used for detecting activity tuberculosis and latent tuberculosis infection; based on the genomic principle, the invention discloses a novel detection reagent for mycobacterium tuberculosis, containing protein or polypeptide which is represented by SEQ ID1-2, 4-5, 8-28; the method uses one or a plurality of SEQ ID 1-28 protein or polypeptide to contact T cells of a mycobacterium tuberculosis host, and detects cytokine released from the T cells; the method can detect the tuberculosis and latent infection effectively and is not interfered by BCG vaccine at the same time; the invention also discloses a diagnostic reagent kit and other application based on the protein or polypeptide and the method; compared with the T-SPOT of the prior art, the invention can improve detectable rate obviously under the condition that the specificity is not reduced; the reagent kit has cheap price, and the cost is 1 / 5 to 1 / 10 of that of the T-SPOT reagent, thus being beneficial to being popularized in the developing countries and poor areas.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
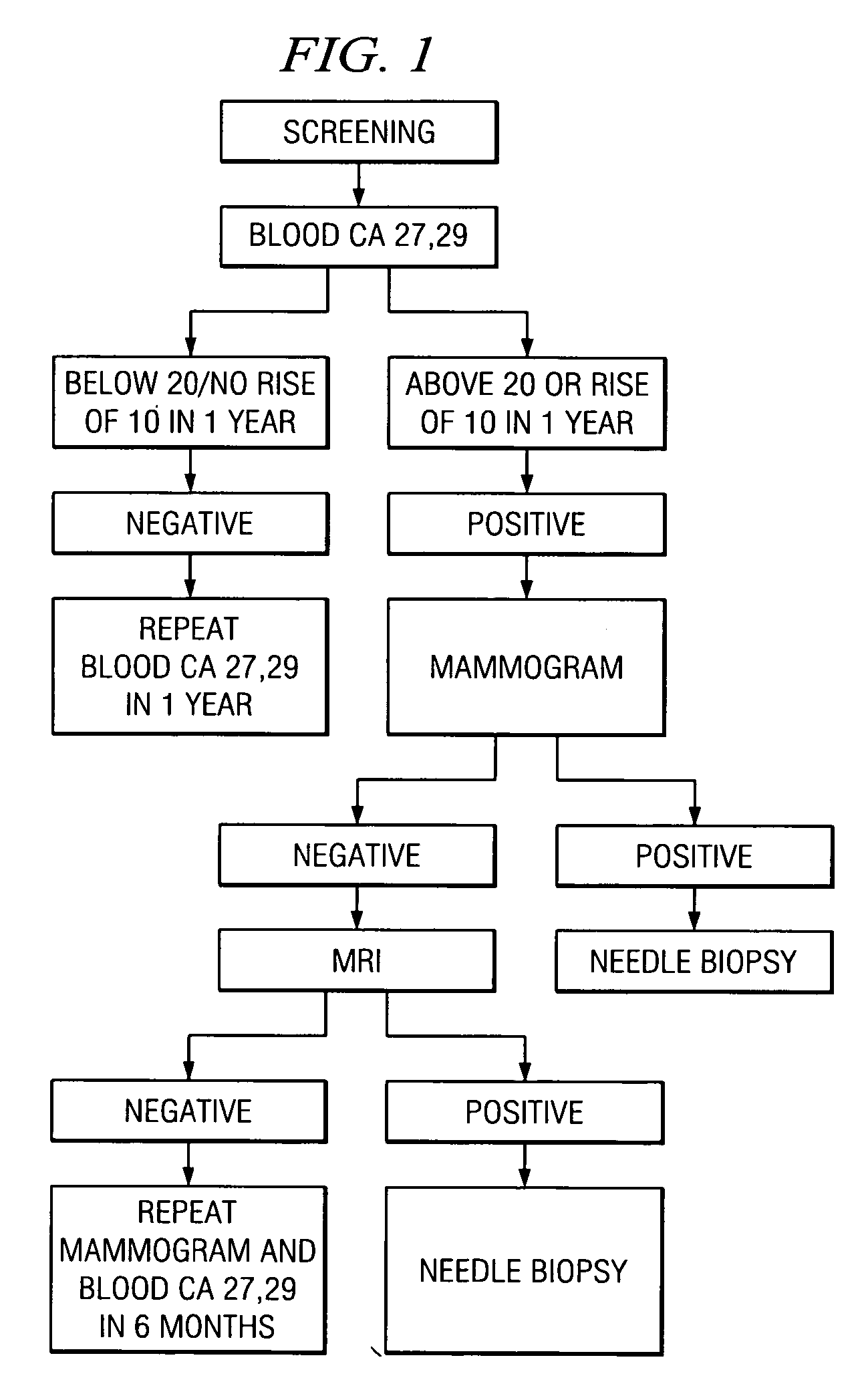
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
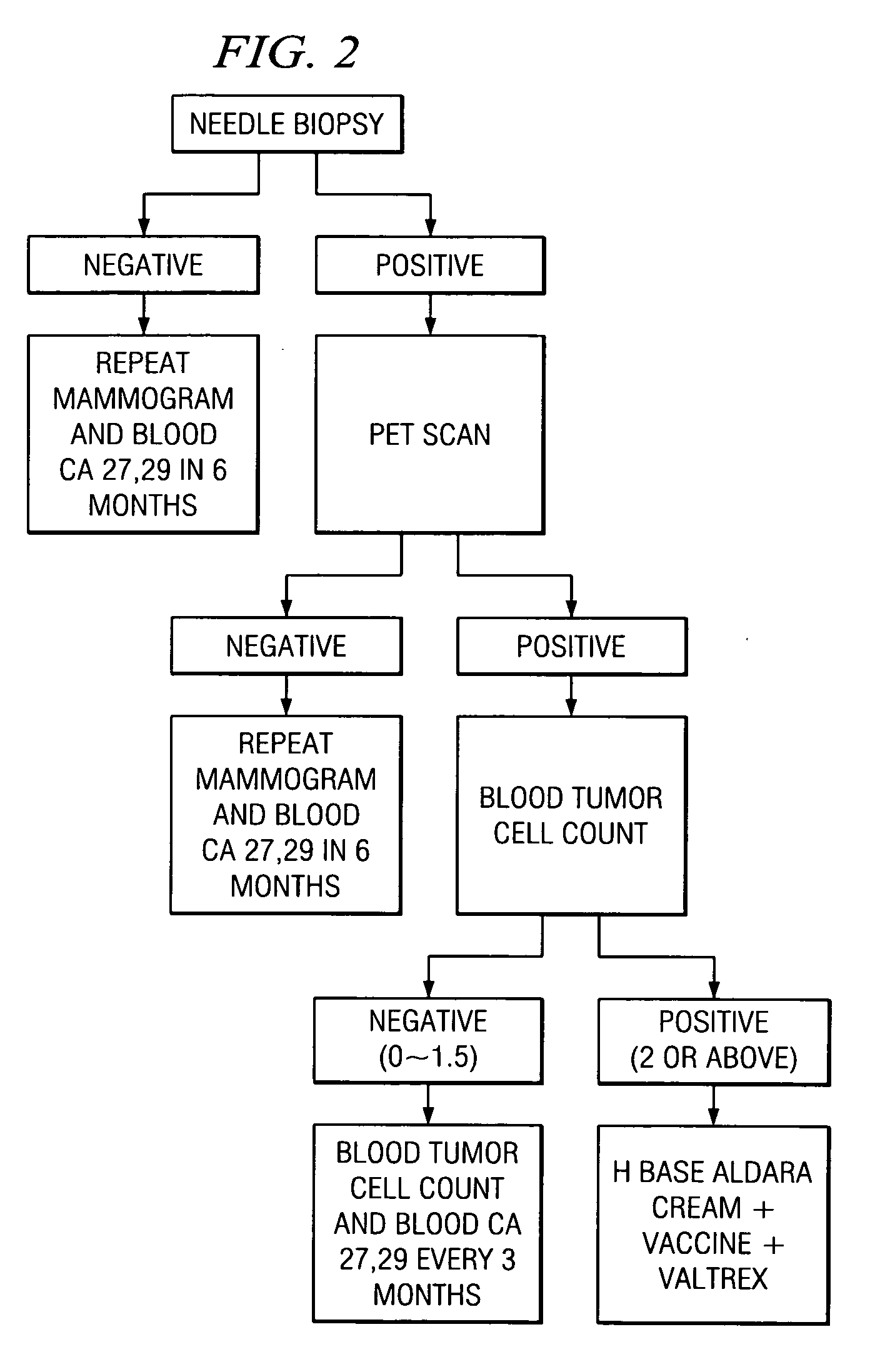
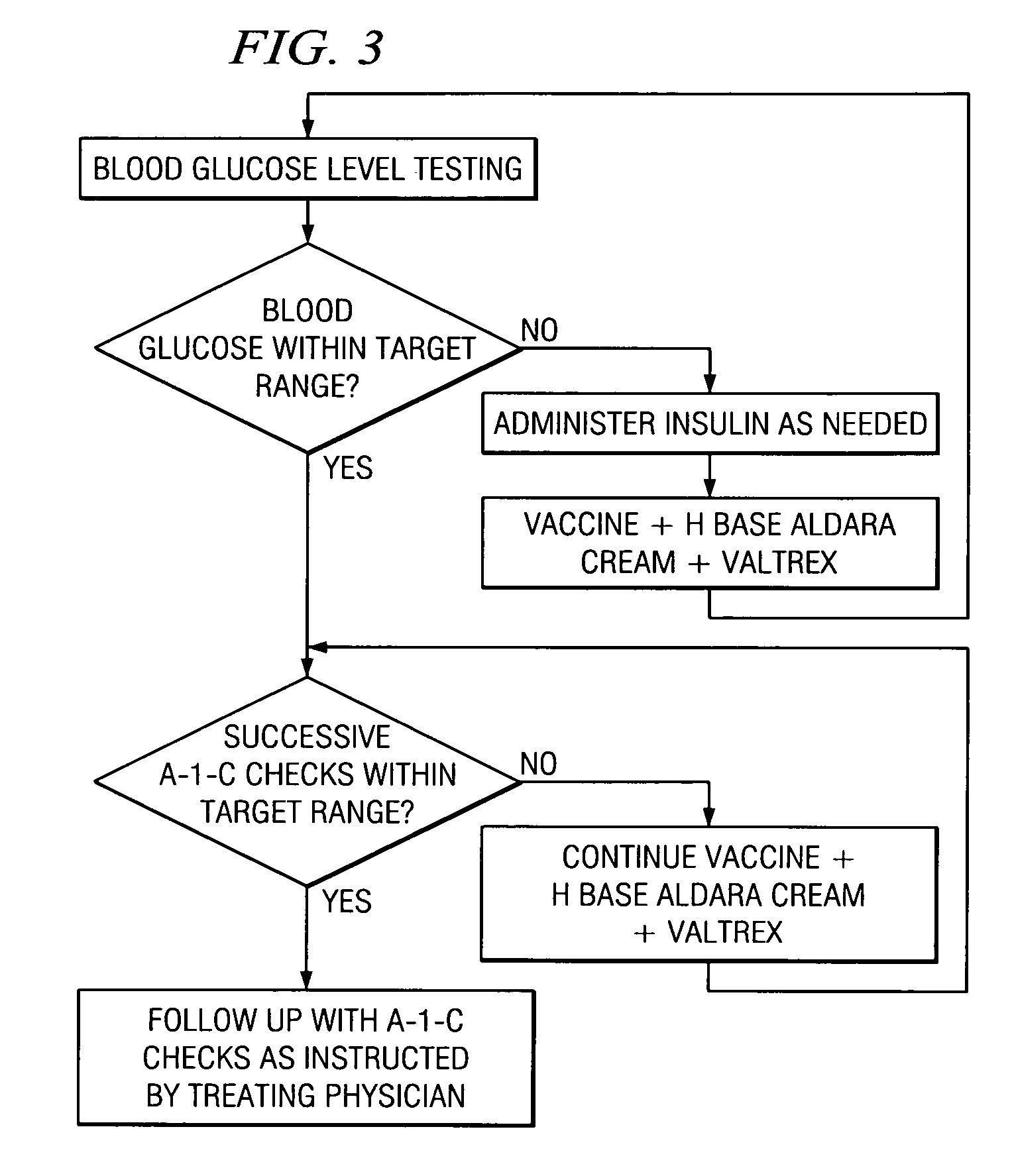
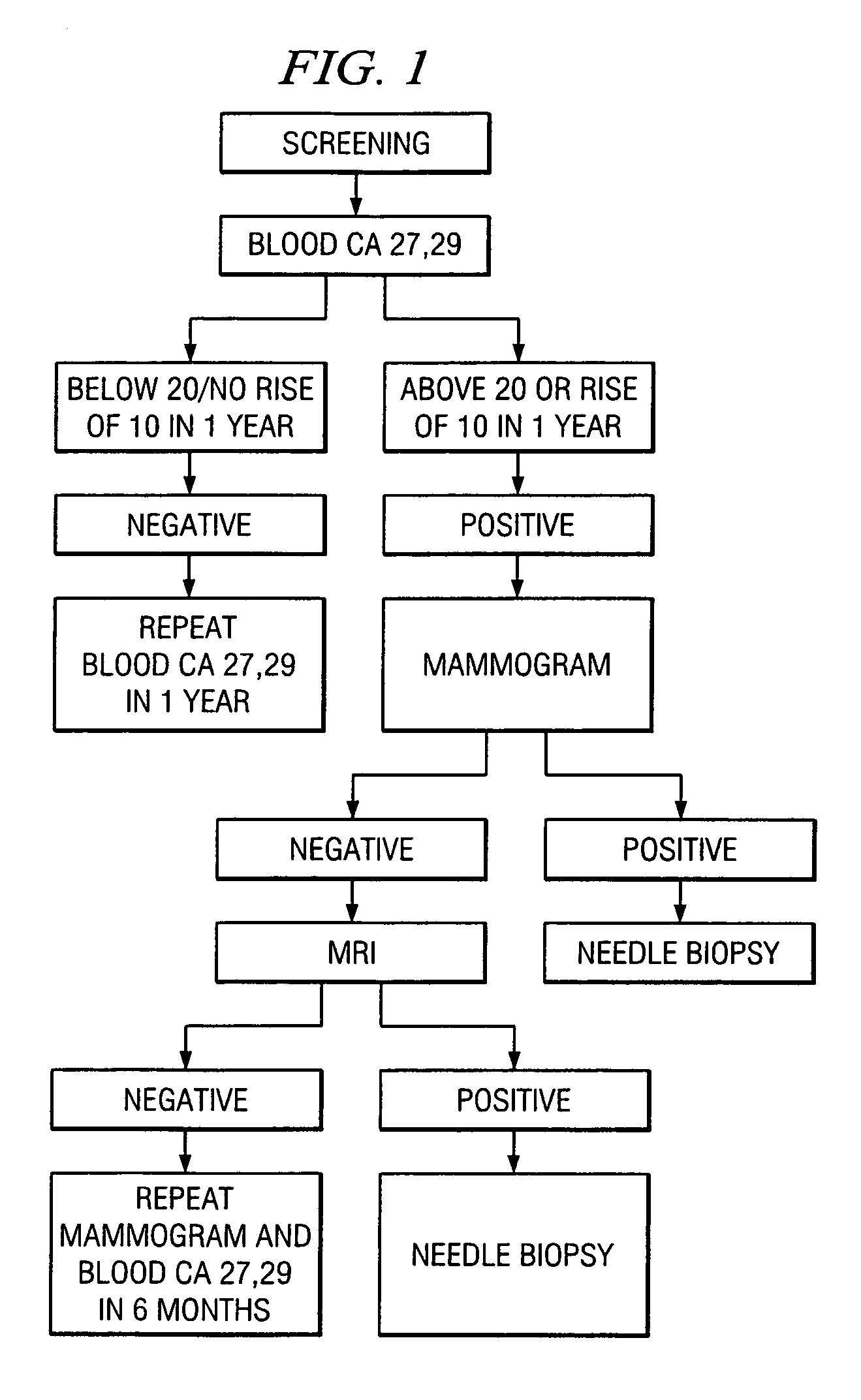
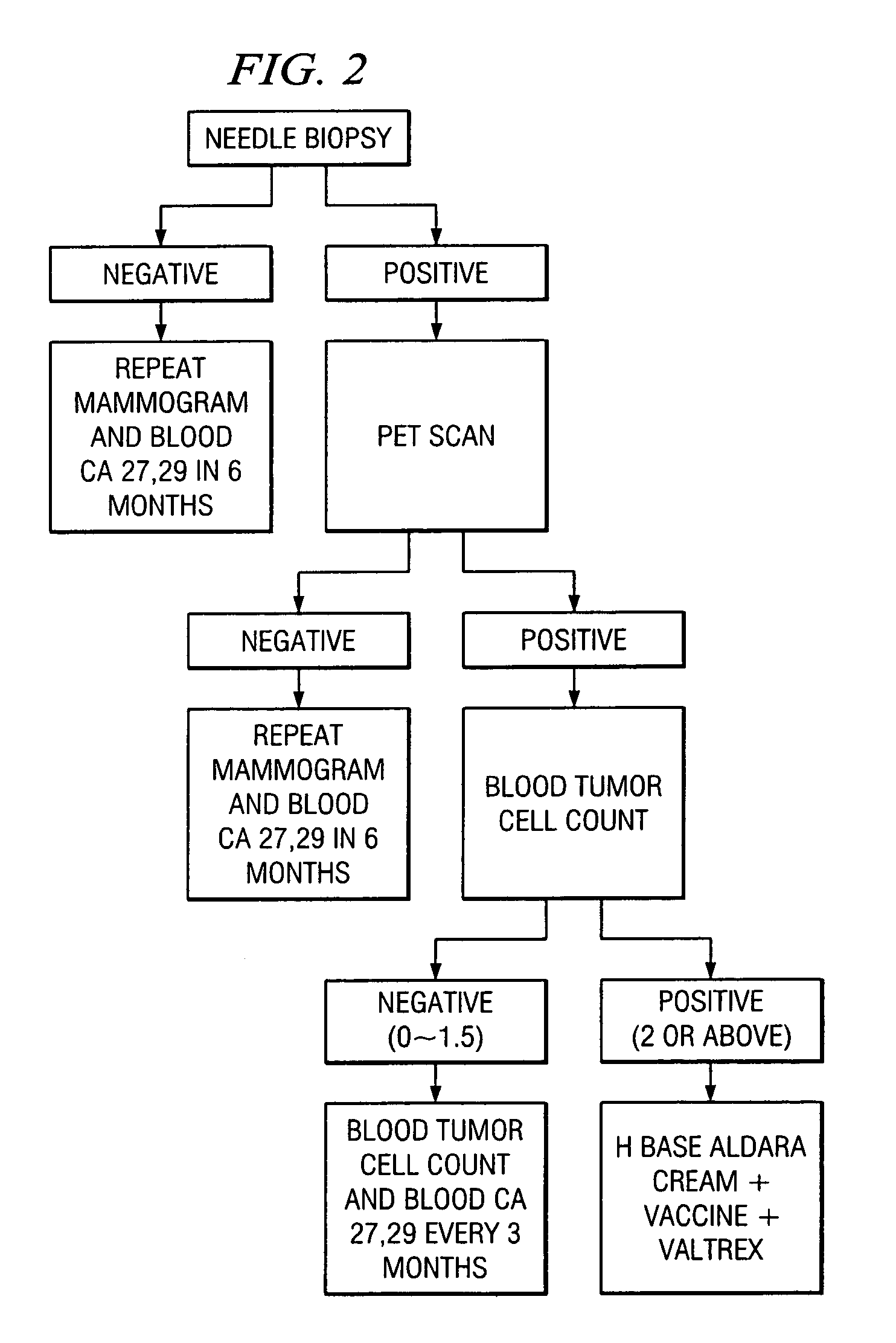
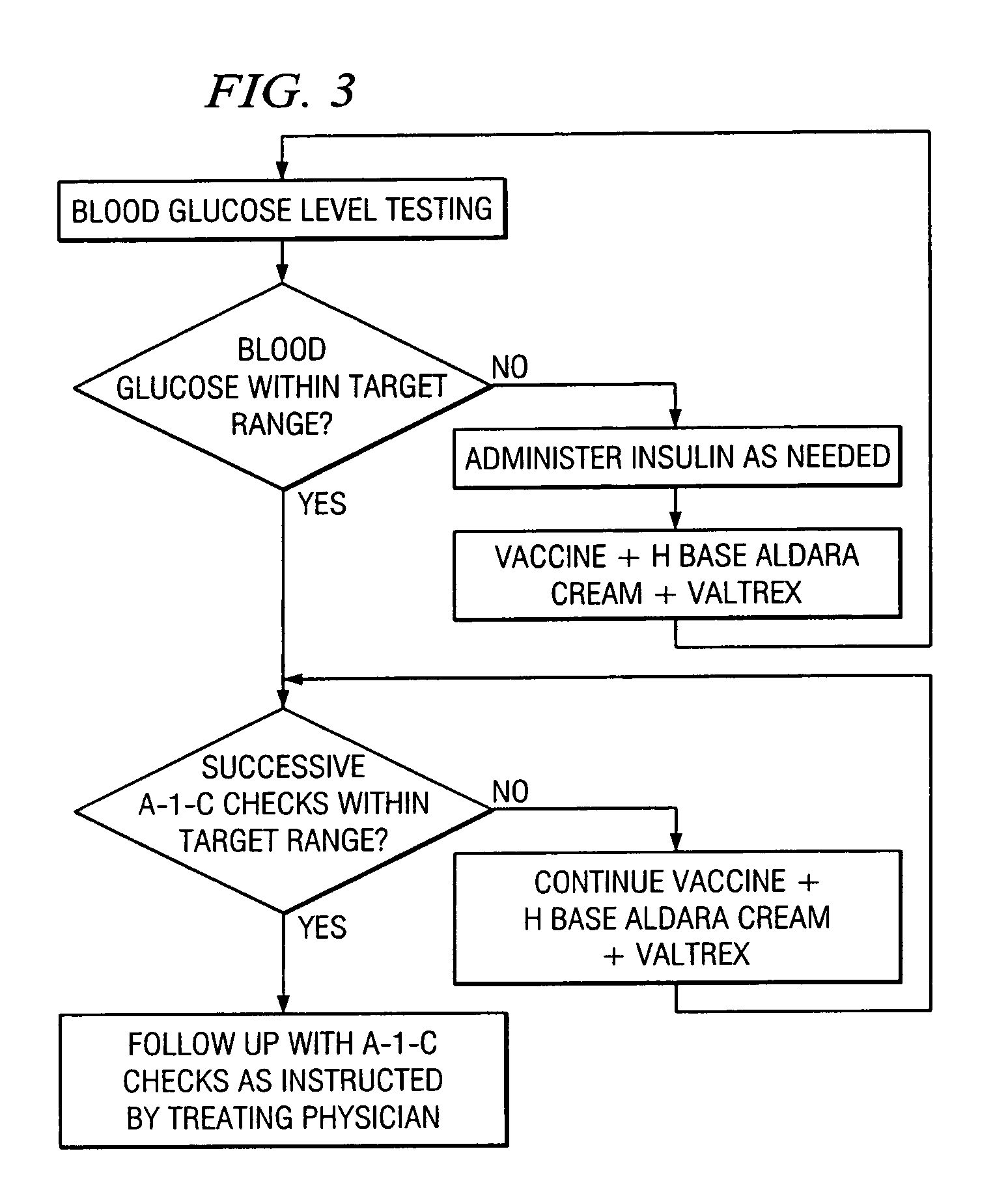
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering an needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA® (imiquimod) 5% cream with an equal amount of H base cream; administering a vaccine containing tumor necrosis factor, preferably the BCG vaccine; and orally administering VALTREX® (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:WOODWARD FAMILY LTD A PARTNERSHIP OF THE STATE OF TEXAS JOHN R WOODWARD GENERAL PARTNER +1
Recombinant protein vaccine for preventing and treating human prostata cancer
The recombinant protein vaccine is a fusion protein formed from connection of BCG vaccine heat shock protein 65 with 1-5 copies of human prostatic specific antigen cytotoxin T lymphocyte polyepitope,in which the single-copy polypeptide of said human prostatic specific antigen cytotoxin T lymphocyte polyepitope possesses amino acid sequence showed by SEQ ID NO:2, and the double single-copy polypeptide of the human prostatic specific antigen dcytotoxin T lymphocyte polyeptope possesses amino acid sequence showed by SEQ ID NO:4. After it is applied in human body, it can effectively prevent and cure carcinoma of prostate. Said invention also provides the gene for coding said two kinds of recombinant protein vaccines.
Owner:BEIJING HYDVAX BIOTECH
Antigen epitope for exciting human anti-tubercle bacillus protective immunoreaction and its use
InactiveCN1858059AHelp preventAids in healingAntibacterial agentsBacterial antigen ingredientsMolecular ImmunologyBCG vaccine
The present invention relates to molecular immunology technology, and aims at screening out antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, researching protective immunoreaction mechanism against tuberculosis, and further developing new type of concatenate polyepitope tuberculosis vaccine. The present invention provides one kind of antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, and the peptide contains the amino acid sequence selected from SEQ ID Nos. 2, 5, 10, 12, 14 and 15. The present invention also provides the screening process and use of the peptide. The present invention may be used in preventing and controlling tuberculosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering a needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA (TM) (imiquimod) 5% cream with an equal amount of H base cream (TM); administering a vaccine that induces production of tumor necrosis factor, preferably the BCG vaccine; and orally administering Valtrex (TM) (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:LES MEDECINS
Reagent for detecting tubercle bacillus infection in vitro and method thereof
ActiveCN101446585AValid in vitro assayOvercome the disadvantages of unsatisfactory effectImmunoglobulins against bacteriaBiological testingBCG vaccineT cell
The invention discloses a reagent for detecting tubercle bacillus infection in vitro and a method thereof. The reagent comprises M233 polypeptide represented by SEQ ID No.1; and cytokine released from T cells is detected by contacting the M233 polypeptide or an analog thereof with the T cells of a tubercle bacillus host to determine whether the T cells identify the M233 polypeptide or the analog thereof. The reagent has the advantages of high sensitivity, good specificity, being free from interference of BCG vaccine and non-tuberculosis mycobacteria vaccine, being capable of detecting active pulmonary tuberculosis patients, the patients with dormant infection and healthy persons who contact with mycobacterium nontuberculosis. The reagent and the method are especially applicable to detecting tuberculosis and / or dormant infection thereof for Chinese people.
Owner:GUANGZHOU RHFAY BIOTECH CO LTD
Specific epitope based immunological diagnosis of tuberculosis
ActiveUS7838013B2Easy to identifyTesting is superfluousBacterial antigen ingredientsMicrobiological testing/measurementBCG vaccineDiagnostic tools
The currently used method for immunological diagnosis of tuberculosis infection, the tuberculin skin test, is problematic for a number of reasons; it has low specificity in BCG vaccinated individuals, a high interobserver variance and requires skill to be read and interpreted. Furthermore it requires an extra visit to the clinic to have the test read. Both people vaccinated with BCG and those exposed to non-tuberculosis mycobacteria give a positive skin test result similar to that seen in a TB infected individual. This also applies for purified protein derivative (PPD) when used in a blood cell based test. The present invention discloses the development of an immunological TB diagnostic tool based on a combination of epitopes from proteins encoded by regions of the M. tuberculosis (M. tub.) genome, that are not present in the BCG vaccine strain or in the most common non-tuberculosis mycobacteria.
Owner:STATENS SERUM INST
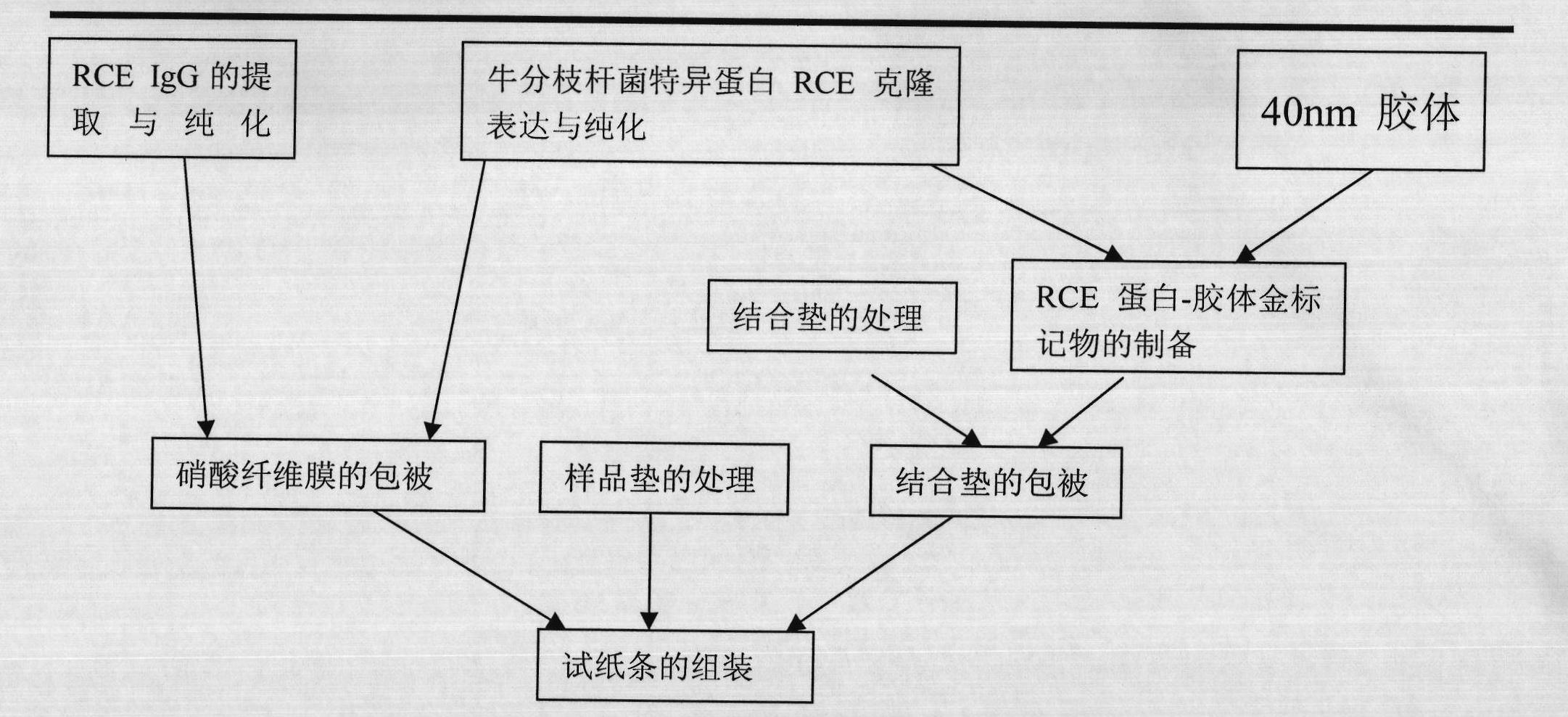
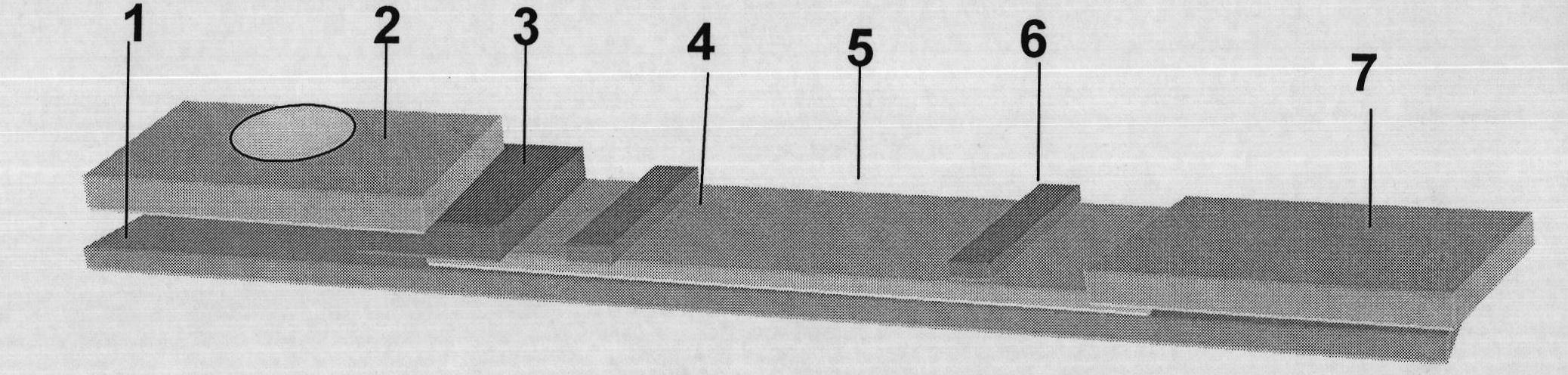
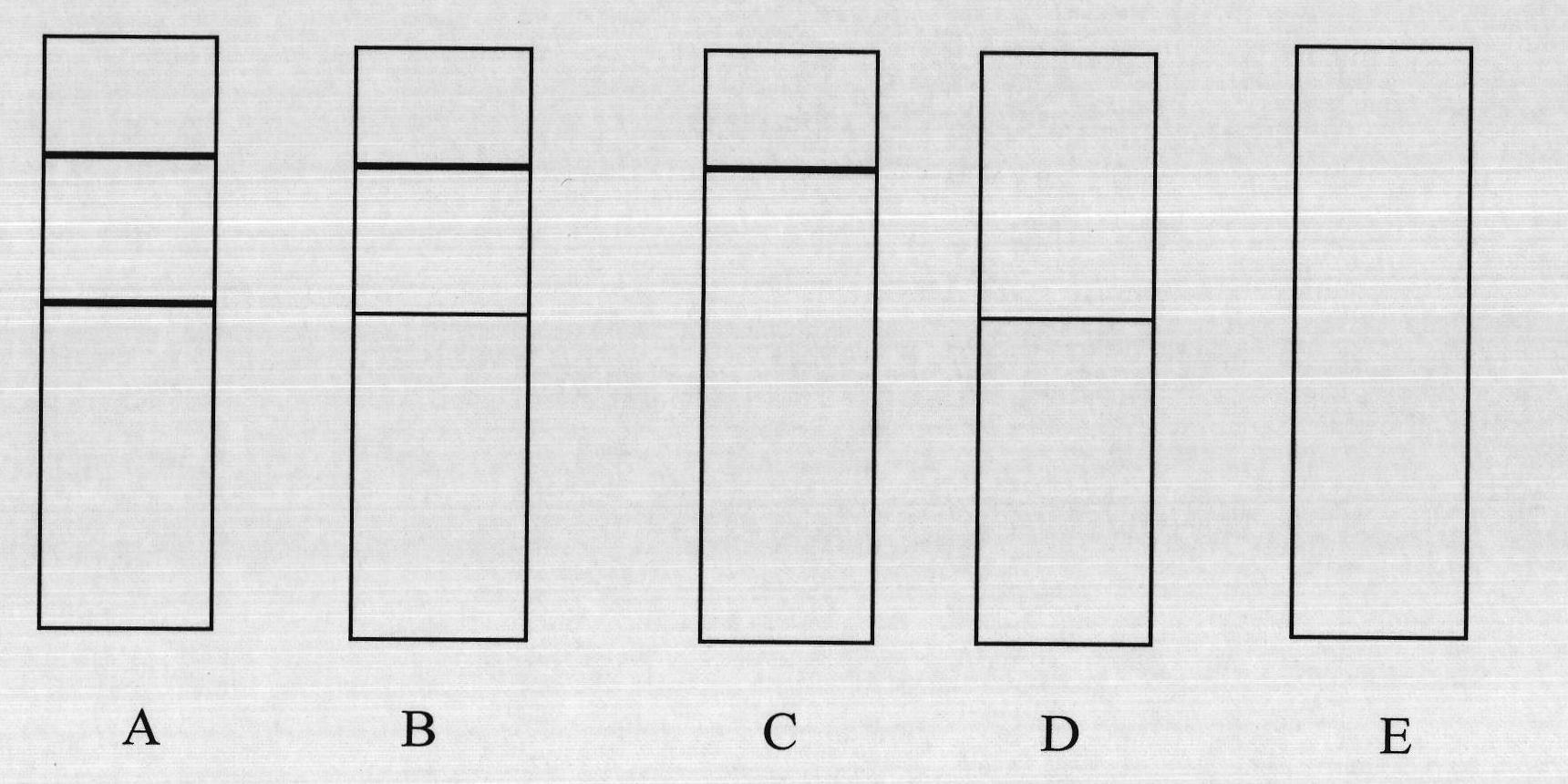
Bovine tuberculosis antibody identifying and detecting test strip prepared by applying Rv3872 novel fusion protein
InactiveCN101900727AWith identificationFunctionalBacteriaMicroorganism based processesAntigenNitrocellulose
The invention belongs to the technical field of animal infectious disease gene engineering and discloses a bovine tuberculosis antibody detecting immune colloidal gold test strip prepared by utilizing RV3872, ESAT6 and CFP10 fusion protein, and a preparation method and application. A colloidal gold immunochromatographic test strip is established by using the fusion protein as a colloidal gold labeled antigen and a capture antigen in a detection region of a nitrocellulose membrane. The detection of the bovine tuberculosis antibody by using test strip has prominent advantages of strong specificity and high sensitivity, and simultaneously bacillus calmette-guerin immunity and nontuberculosis mycobacteria infection can be identified and detected. The test strip comprises recombinant Escherichia coli BL21 / pET28a-MPBrce, and the strain expresses mycobacterium bovis RCE proteins and is preserved in the China Center for Type Culture Collection with the collection number of CCTCC No:M208244.
Owner:HUAZHONG AGRI UNIV
Polypeptide vaccine and vaccination strategy against mycobacterium
A vaccine is provided wherein a polypeptide or combination of peptides from M. tuberculosis is administered to a subject to elicit an immune response. The polypeptide vaccine is administered as part of a prime-boost strategy with BCG vaccine to increase the immunoprotection in a subject such that prevention or elimination of disease is achieved. Finally, a pharmaceutical package is provided that encompasses a polypeptide vaccine for M. tuberculosis that when administered to a subject elicits immunoprotection.
Owner:EMORY UNIVERSITY +1
Nanoparticle containing EV71VP1 protein and preparation method of nanoparticle
ActiveCN106421770AMorphological rulesRound shapeSsRNA viruses positive-senseViral antigen ingredientsNanoparticleAnionic polymers
The invention belongs to the technical field of medicine, and relates to a nanoparticle containing EV71VP1 protein and a preparation method of the nanoparticle. Specifically, the invention relates to a nanoparticle containing EV71 VP1 protein, an immunologic adjuvant, a cationic polymer and an anionic polymer, wherein the immunologic adjuvant is selected from one or more of TNF-alpha, CpG, bcg vaccine and flagellin. The invention further relates to a method for preparing the nanoparticle including immunogenic composition (such as vaccine) of the nanoparticle. The nanoparticle is used for preparing the immunogenic composition (such as vaccine) and for the nanoparticle or the immunogenic composition (such as vaccine) to initiate or enhance the immune response of subjects to EV71.
Owner:GUANGZHOU LIDE BIOMEDICINE TECH CO LTD
Proteins expressed by mycobacterium tuberculosis and not by bcg and their use as diagnostic reagents and vaccines
InactiveUS20070224122A1Avoid the build processReduce the severity of the diseaseCompounds screening/testingAntibacterial agentsOpen reading frameTuberculosis mycobacterium
The invention provides polypeptides encoded by open reading frames present in the genome of Mycobacterium tuberculosis but absent from the genome of BCG and diagnostic and prophylactic methodologies using these polypeptides.
Owner:NEW JERSEY UNIVESITY OF MEDICINE & DENTISTRY OF
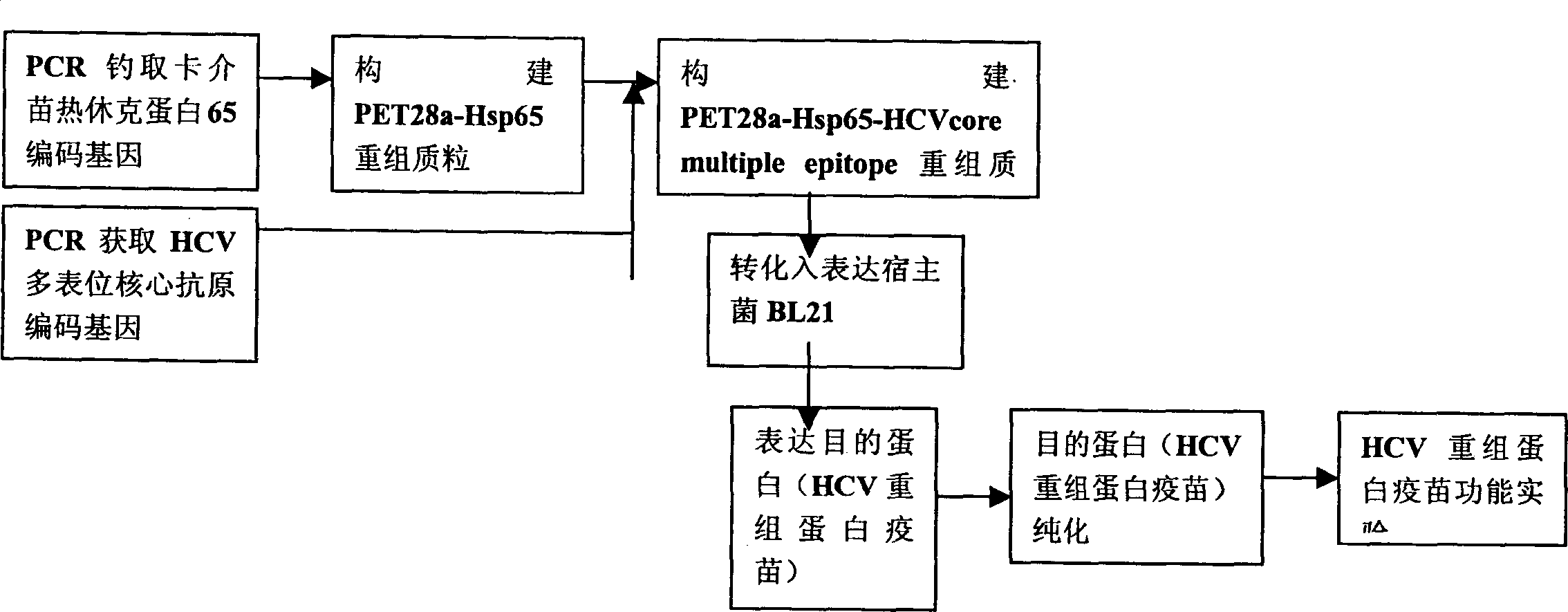
Vaccine of recombined albumen for preventing and treating infection of human C type hepatitis virus and its usage
A recombinant protein vaccine which is a fusion protein of BCG vaccine's heat shock protein 65 and the core antigen of multi-epitope hepatitis-C virus, its amino acid sequence and nucleotide sequencefor coding it, the expression carrier containing said nucleotide sequence, the host cell containing said expression carrier, the preparing process of said recombinant protein vaccine, the vaccine containing said recombinant protein for preventing and treating hepatitis C, and a method for detecting the activity of specifically killing T, lymphocytes by the hepatitis C induced by said vaccine and its cell model are disclosed.
Owner:BEIJING HYDVAX BIOTECH
Tuberculosis gene vaccine assembled by chitosan delivery system and preparation method and use thereof
InactiveCN101455846AConvenient inductionSafe and non-toxicAntibacterial agentsBacterial antigen ingredientsWhole bodyEukaryotic plasmids
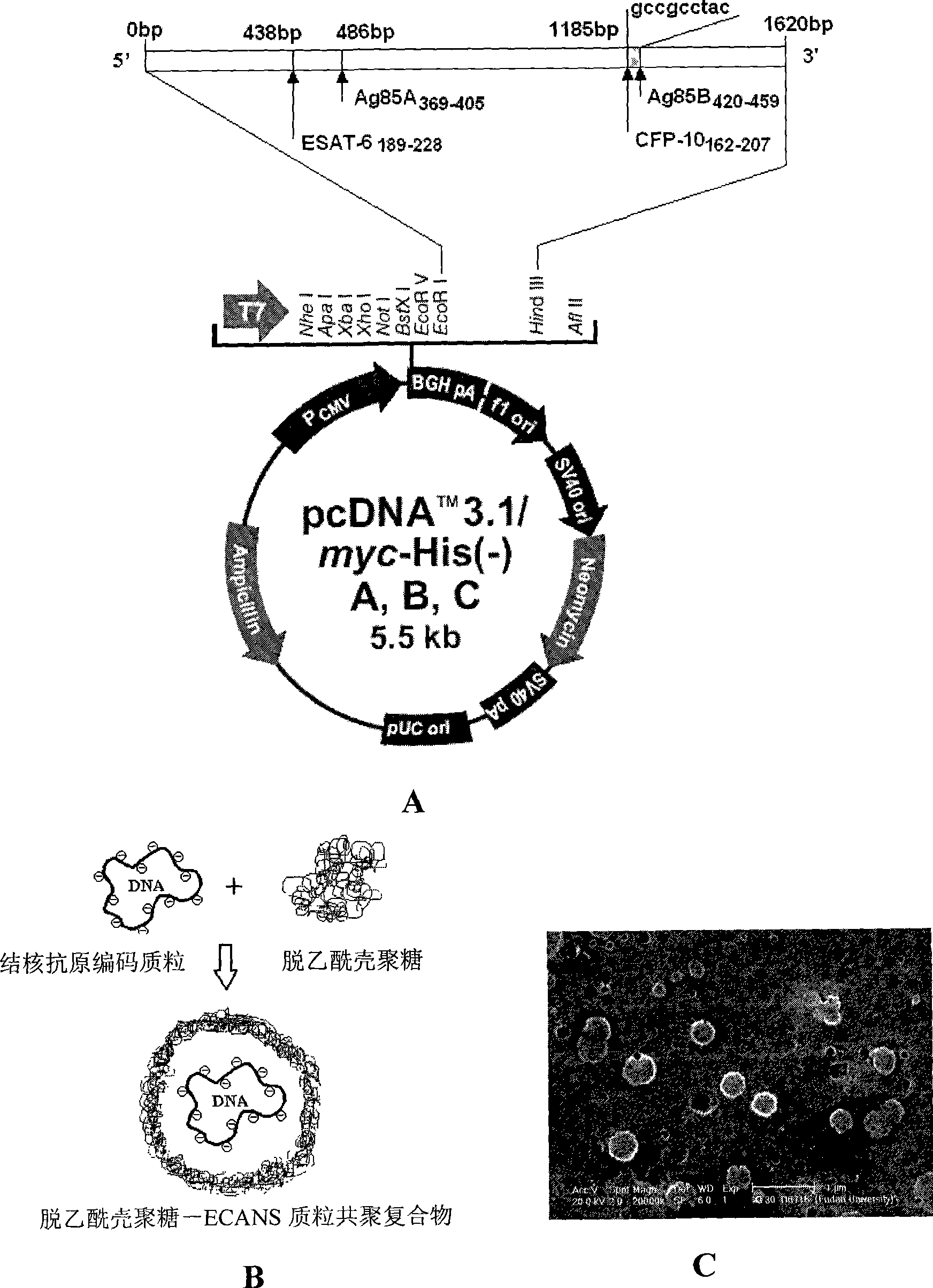
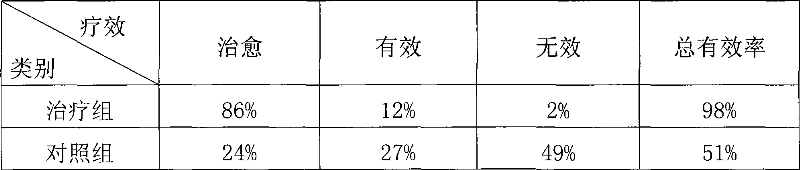
The invention relates to a tuberculosis gene vaccine assembled by a chitosan delivery system, which consists of chitosan and tuberculosis antigen encoding plasmids, wherein full-length genes of tubercle bacillus heat shock proteins HSP65 are inserted into the tuberculosis antigen encoding plasmids; and four T cell epitope genes EAST-6[189-228], Ag85A[369-405], CFP10[162-207] and Ag85B[420-459] from tubercle bacillus antigens are inserted into the HSP65 full-length genes. The invention also discloses application of the tuberculosis gene vaccine. By performing nasal drip of the gene vaccine on an immune mouse, the gene vaccine is proved to be capable of inducing response to special antibodies of a plurality of tuberculosis antigens, inducing killing response of locally strong tuberculosis special T cells of the whole body and lung mucous membranes, simultaneously inducing immunological response of Th1 which secretes high-level IFN gamma and have the effect which is obviously superior to the prior BCG vaccine, and is a superior vaccine for preventing and treating tuberculosis.
Owner:FUDAN UNIV
TB vaccine heat reversal protein65 and multiepi-position HER-2 Antigen fusion protein recombinat protein vaccine
The present invention discloses a recombinant protein vaccine which is a fusion protein prepared from the BCG vaccine heat-shock protein 65 and multi-epitope HER-2 antigen and can be used for effectively preventing and treating breast cancer. The gene for coding the said recombinant protein vaccine is also disclosed.
Owner:BEIJING HYDVAX BIOTECH
Medicinal composition for treating tuberculosis and its preparing process
InactiveCN101041024AImprove immunityAvoid the pain of surgeryAntibacterial agentsAmphibian material medical ingredientsMedicinal herbsToad Venom
Disclosed is a pharmaceutical composition for treating tuberculosis which is prepared from the following Chinese medicinal herbs (by weight ratio): Radix Ranunculi Ternati 1-3 weight parts, toad venoms 1-3 weight parts, rhubarb horsetails 1-3 weight parts, fritillary 1-3 weight parts, selfheal 1-3 weight parts, asparagus root 1-3 weight parts. The invention also discloses the process for preparing the pharmaceutical composition.
Owner:刘玉正
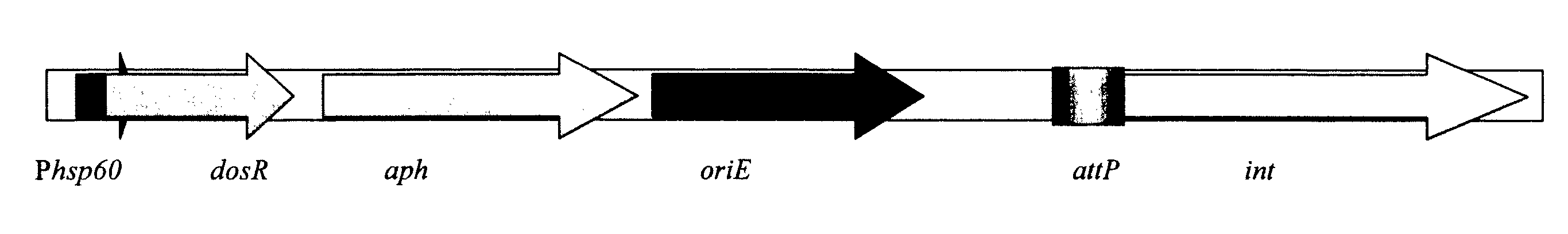
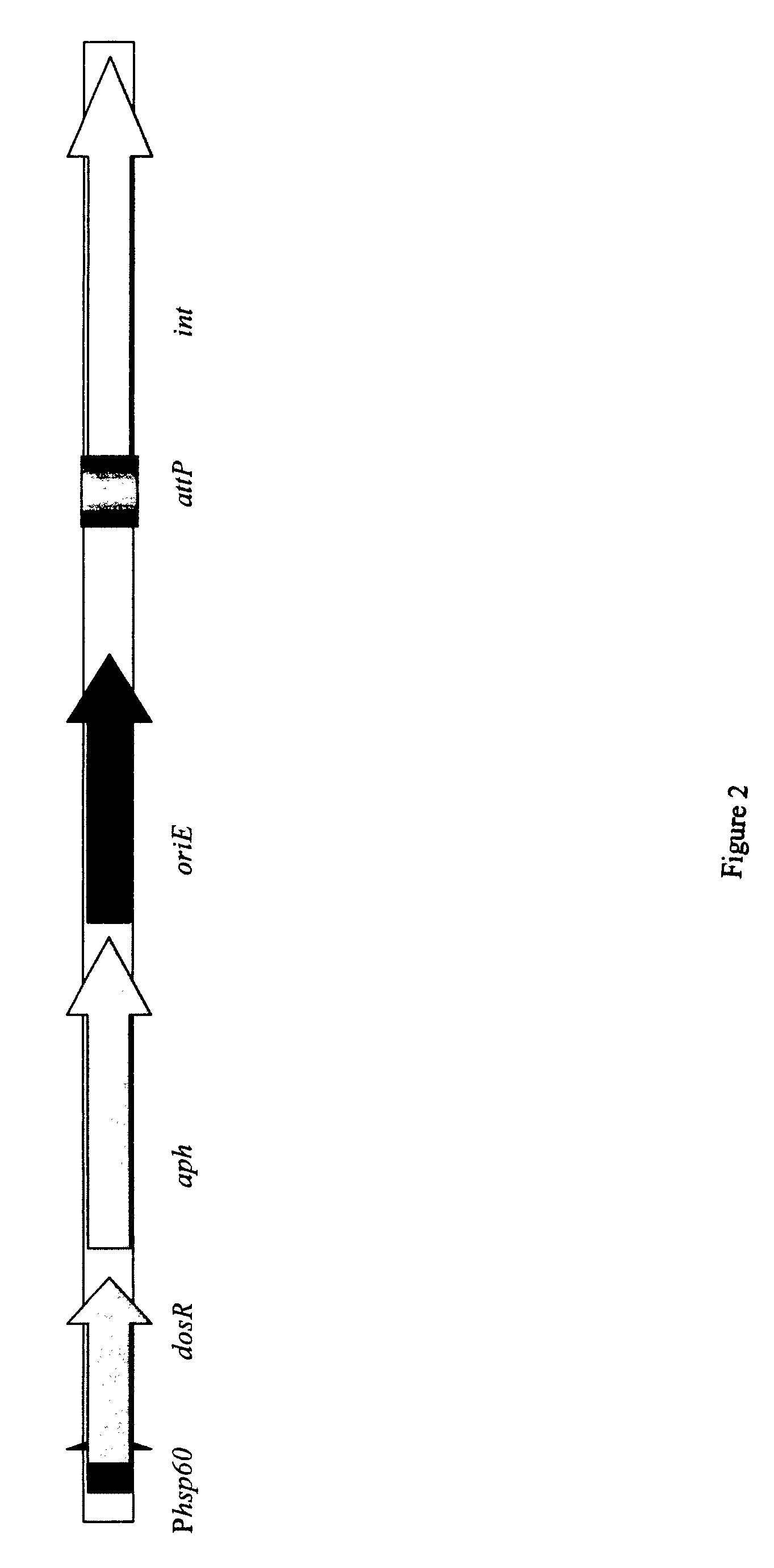
Generation of new bcg vaccine strains protecting against the establishment of latent mycobacterium tuberculosis infection and reactivation from the latent or persistent state
InactiveUS20090123492A1Ability of to to attenuatedShorten the progressBacterial antigen ingredientsSugar derivativesADAMTS ProteinsBCG vaccine
A vaccine for treating or preventing the establishment of latent tuberculosis infections is provided. The vaccine comprises a recombinant mycobacterium that overexpresses the transcription factor DosR, at a level sufficient to induce production of the dosR regulon genes or proteins. A host to whom the vaccine is administered mounts an immune response to the dosR regulon proteins and is thus protected from the establishment, persistence or reactivation of latent tuberculosis.
Owner:INT AIDS VACCINE INITIATIVE INC +1
Mycobacterium tuberculosis fusion protein and application thereof in induction of peripheral blood mononuclear cells to generate cytokines
ActiveCN105601747AIncreased sensitivityStrong specificityAntibacterial agentsBacterial antigen ingredientsAntigenPeripheral blood mononuclear cell
The invention discloses a mycobacterium tuberculosis fusion protein and an application thereof in induction of peripheral blood mononuclear cells (PBMCs) to generate cytokines. The fusion protein includes three proteins PPE41, ESAT-6 and PE25, and the proteins are connected through connecting peptides. Compared with present stimulants, the fusion protein provided by the invention has the advantages of efficient effect, strong sensitivity, high specificity and good stimulation effect. The fusion protein stimulates the PBMCs to generate a large amount of mycobacterium tuberculosis antigen specific IFN-gamma, IL-2, TNF-alpha and other tuberculosis related factors, and the above stimulation induction reaction is free from BCG vaccine interference. The fusion protein can effectively improve the tuberculosis detection rate and is of positive significance to control the tuberculosis. The fusion protein can be applied in researches of the tuberculosis pathopoiesis and immunoprophylaxis mechanisms and control of the tuberculosis as a stimulant.
Owner:SUN YAT SEN UNIV
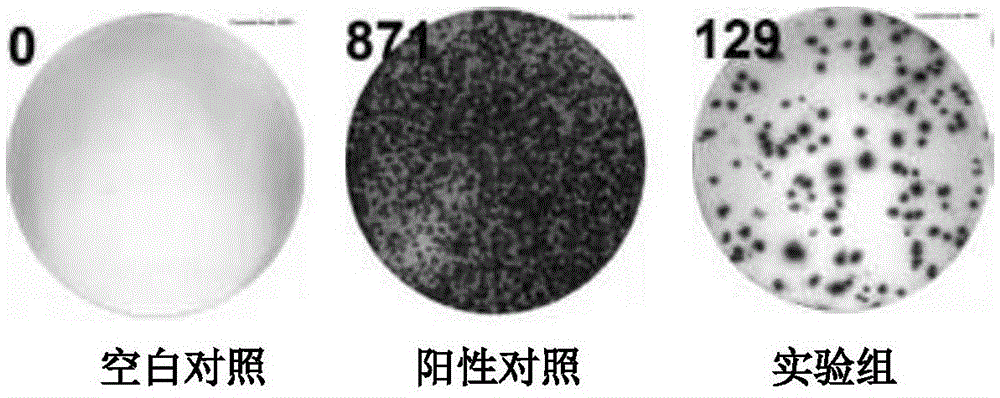
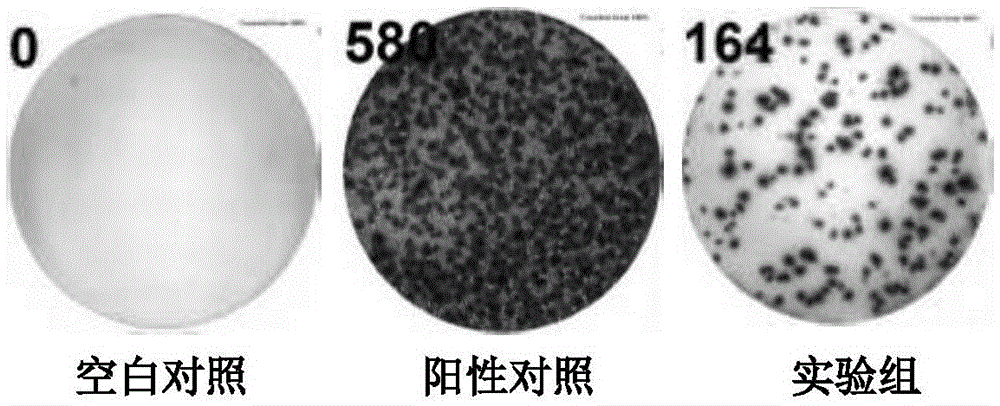
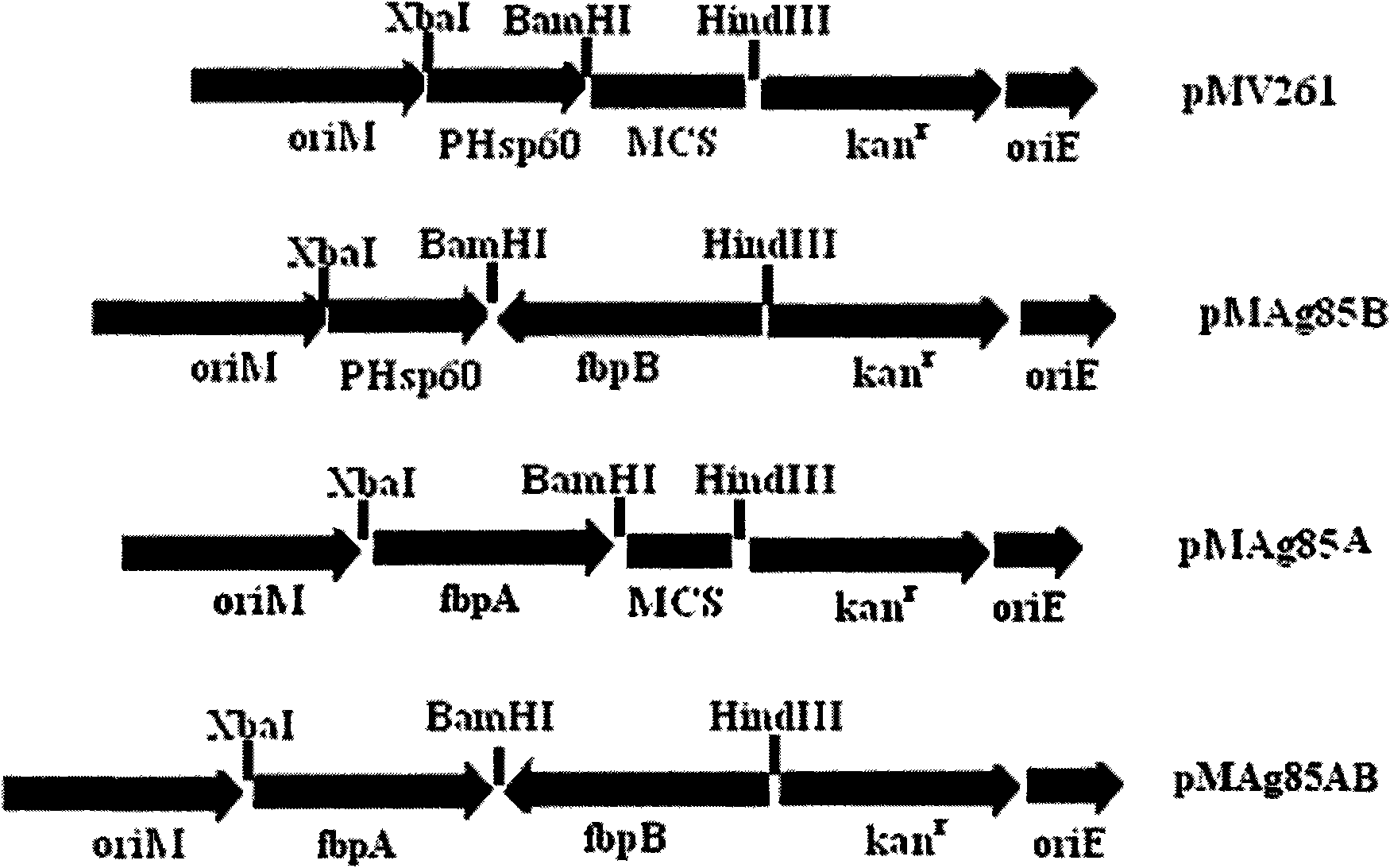
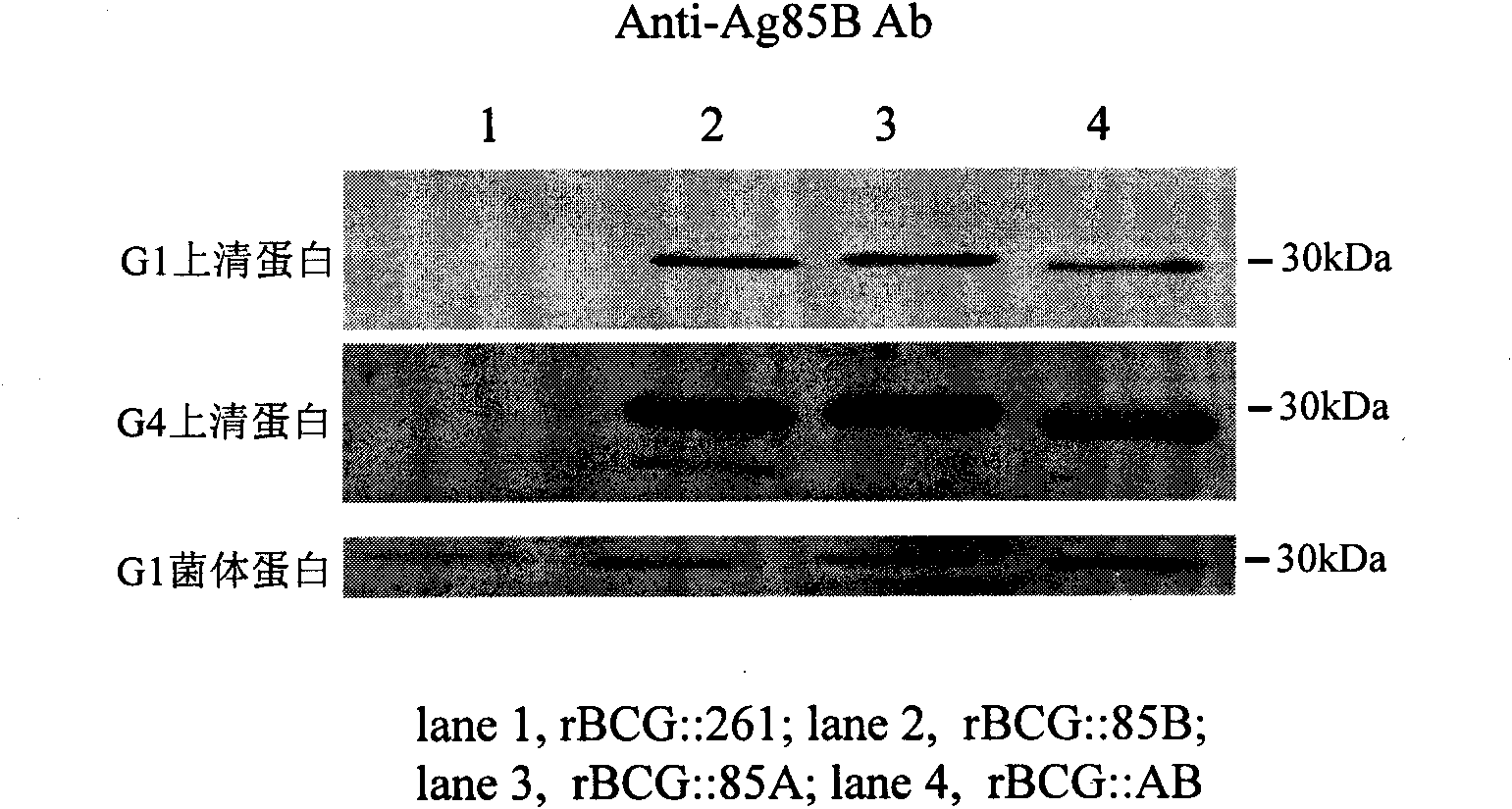
Recombination BCG vaccine rBCG::AB
InactiveCN101921802ASecurity unchangedChange safety featuresAntibacterial agentsBacterial antigen ingredientsEscherichia coliRecombinant escherichia coli
The invention provides a recombination BCG vaccine rBCG::AB for protecting against tubercle bacillus and a preparation method thereof. The preparation method comprises the following steps: firstly, amplifying full-length genes containing promoters and signal peptides of Ag85A and Ag85B proteins, and establishing a recombination colibacillus-mycobacteria shuttle expression plasmid after subcloning; and then, transforming the plasmid in a BCG vaccine to form the recombination BCG vaccine rBCG::AB which can simultaneously excessively express the Ag85A and Ag85B proteins. The recombination BCG vaccine rBCG::AB realizes the high-abundance overexpression of the Ag85A and Ag85B proteins in the culture filtrate of the recombination BCG vaccine. After the immunization of animals, the recombination BCG vaccine rBCG::AB can obviously induce cellular immune response to the Ag85A and Ag85B proteins and provide stable and persistent protection against infection, thereby overcoming the defects of short protection time, instable protection against adult tuberculosis and the like of the traditional BCG vaccine.
Owner:HUAZHONG UNIV OF SCI & TECH
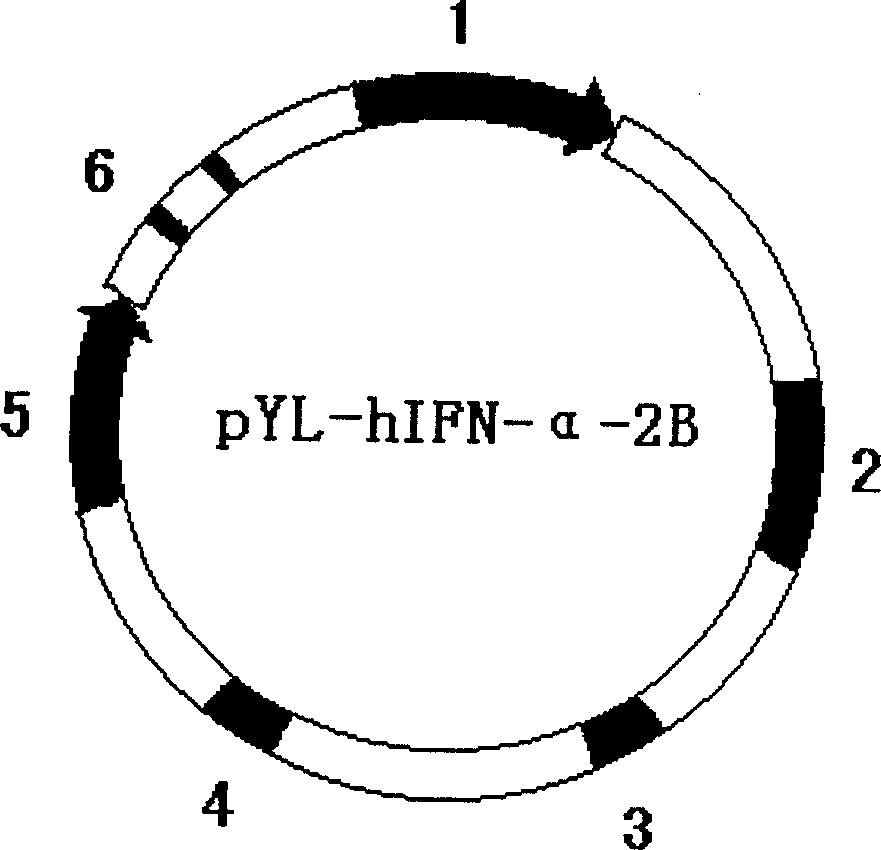
BCG vaccine strain of recombined interferon and its preparation process
The invention discloses a recombinant interferon--BCG Vaccine bacterial strain and its preparing method, transferring the composed pshuttle phIFN-alpha-2B into the BCG to compose Rbcg-hIFN-alpha-2B bacterial strain. Because it can secrete the interferon IFN-alpha-2B, it can reduce the usage of BCG as compared with that of the wild BCG on the condition of reaching the same and even increased immune effect, thus reducing toxic and side effect; and it can secrete cell factors at the properest time and part. Therefore, it lays the foundation of application to curing cystic tumors.
Owner:天津市泌尿外科研究所 +5
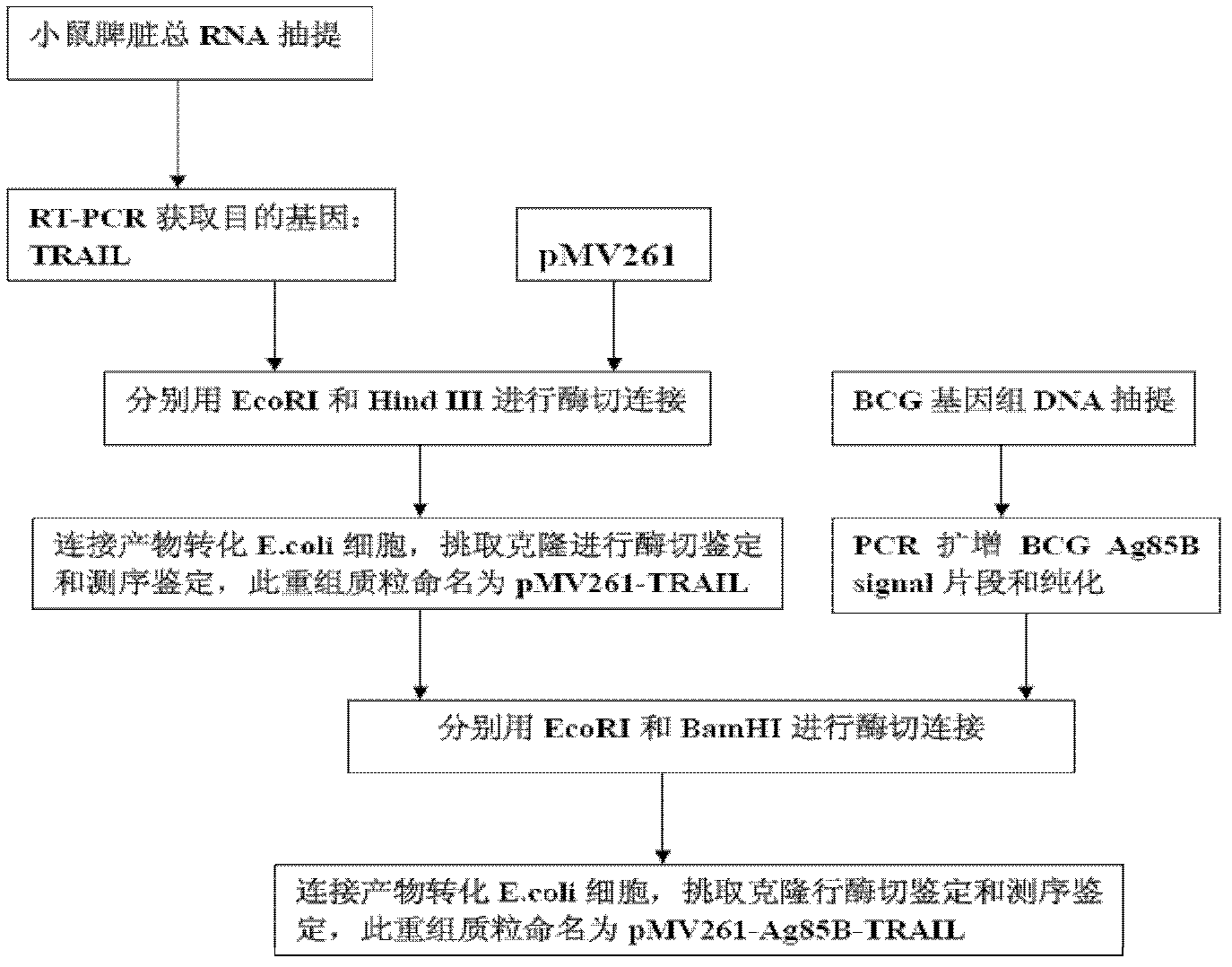
Construction and application of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG)
InactiveCN102327604AConnection direction is correctMeet the design requirementsAntibacterial agentsBacterial antigen ingredientsAntigenSide effect
The invention provides construction of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG), and relates to a shuttle expression vector comprising a signal peptide fragment of a major secretory antigen Ag85B of BCG and a gene fragment of a TRAIL and a construction method thereof. The obtained shuttle expression vector pMV261-Ag85B-TRAIL is used for constructing rBCGTRAIL, and can be applied to preparation of TRAIL rBCG for treating superficial bladder tumors, preventing postoperative recurrence thereof and preventing tuberculosis. The rBCG has dual functions of TRAIL and BCG, so that cooperative and synergistic actions of the TRAIL and BCG can be better brought into play; rBCG-TRAIL can secrete TRAIL, and the using amount of the rBCG-TRAIL can be lower than that of the BCG under the condition that the same or better immune effect is achieved, so that the toxic or side effect is reduced; and the rBCG-TRAIL can directly secrete TRAIL efficiently on a certain part, so that tumor cells can be killed in cooperation with the rBCG-TRAIL, and high cost caused by the use of a foreign cell factor is avoided.
Owner:沈周俊
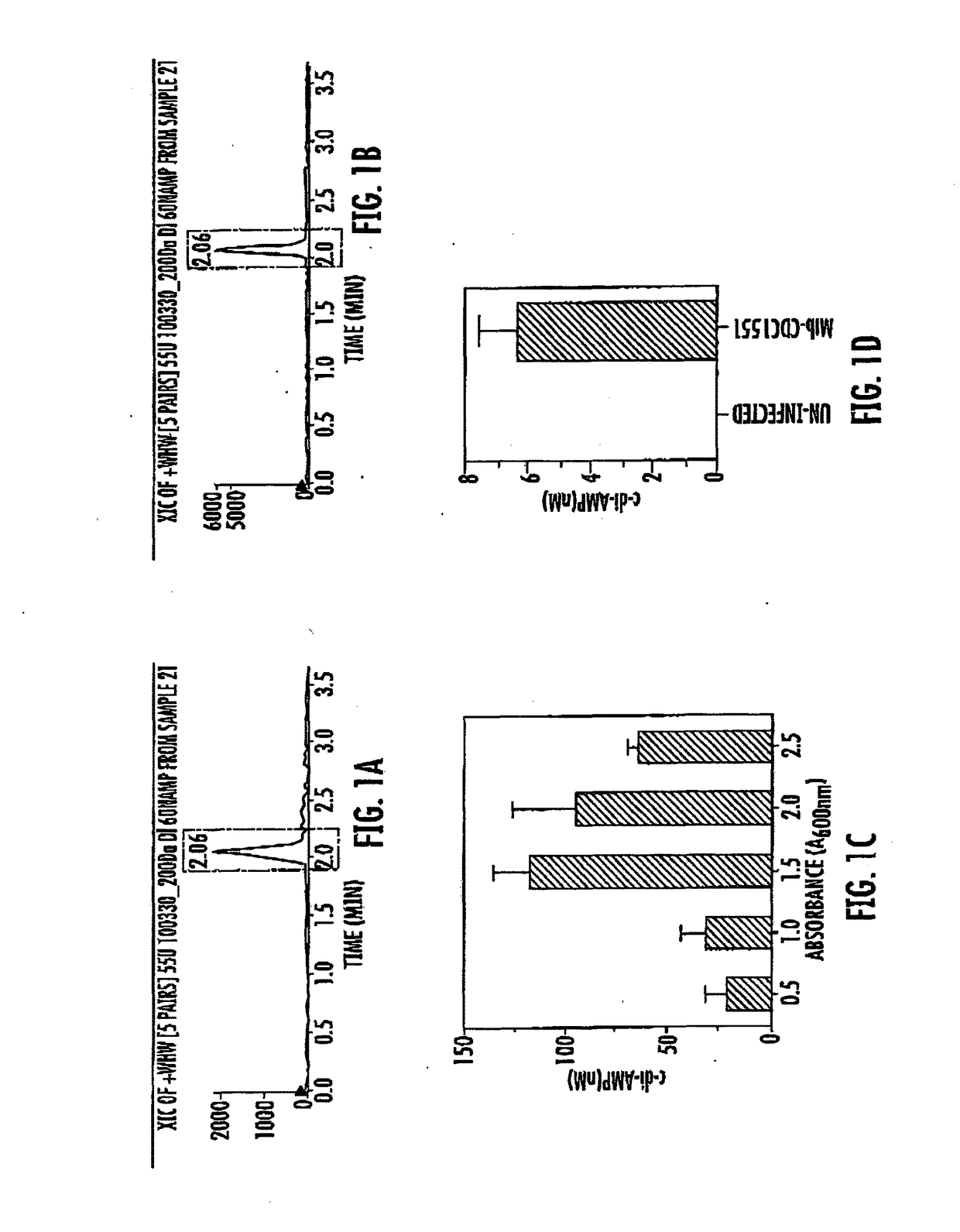
Bacteria over-expressing c-di-amp and therapeutic methods
ActiveUS20180028577A1Improving immunogenicityBacterial antigen ingredientsBacteria material medical ingredientsMycobacteriumHSP60
The present invention includes the discovery of a strain of Mycobacterium comprising an expression vector encoding a di-adenylate cyclase enzyme. The Mycobacterium is selected from the group consisting of Mycobacterium tuberculosis, Mycobacterium bovis, or a combination thereof and the preferred strain of Mycobacterium is BCG. The preferred expression vector is a mycobacterial expression vector including an hsp60 promoter and a DNA sequence of diadenylate cyclase (disA), or a functional part thereof. The strains of Mycobacterium are used in therapeutic applications including tuberculosis and cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Polypeptide vaccine and vaccination strategy against mycobacterium
A vaccine is provided wherein a polypeptide or combination of peptides from M. tuberculosis is administered to a subject to elicit an immune response. The polypeptide vaccine is administered as part of a prime-boost strategy with BCG vaccine to increase the immunoprotection in a subject such that prevention or elimination of disease is achieved. Finally, a pharmaceutical package is provided that encompasses a polypeptide vaccine for M. tuberculosis that when administered to a subject elicits immunoprotection.
Owner:U S GOVERNMENT DEPT OF HEALTH & HUMAN SERVICES SECRETARIAT
Bacillus coli-mycobacteria shuttling expression plasmid vector and its application in preparation of pathogenic microorganism vaccine
The invention relates to an Escherichia coli-mycobacterium shuttle vector plasmid suitable for secretory expression of pathogenic microorganism antigen and application thereof to preparation of a pathogenic microorganism vaccine. The Escherichia coli-mycobacterium shuttle vector plasmid is constructed based on an Escherichia coli-mycobacterium shuttle vector plasmid pBCG-2000; a modified mycobacterium tuberculosis heat shock protein 60 promoter regulation sequence with SEQ ID NO.1 as the sequence is inserted; a T4 transcription termination sequence which is artificially synthesized and has the sequence of SEQ ID NO.2 is inserted; a modified signal peptide sequence, with SEQ ID NO.3 as the sequence, of a BCG antigen gene 85b is inserted; and an artificial synthetic sequence with SEQ ID NO.4 as the sequence is added. The Escherichia coli-mycobacterium shuttle vector plasmid can be applied to the research of recombinant vaccines of resisting Toxophasma gondii, SARS coronaviruses and tubercle bacillus.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION +1
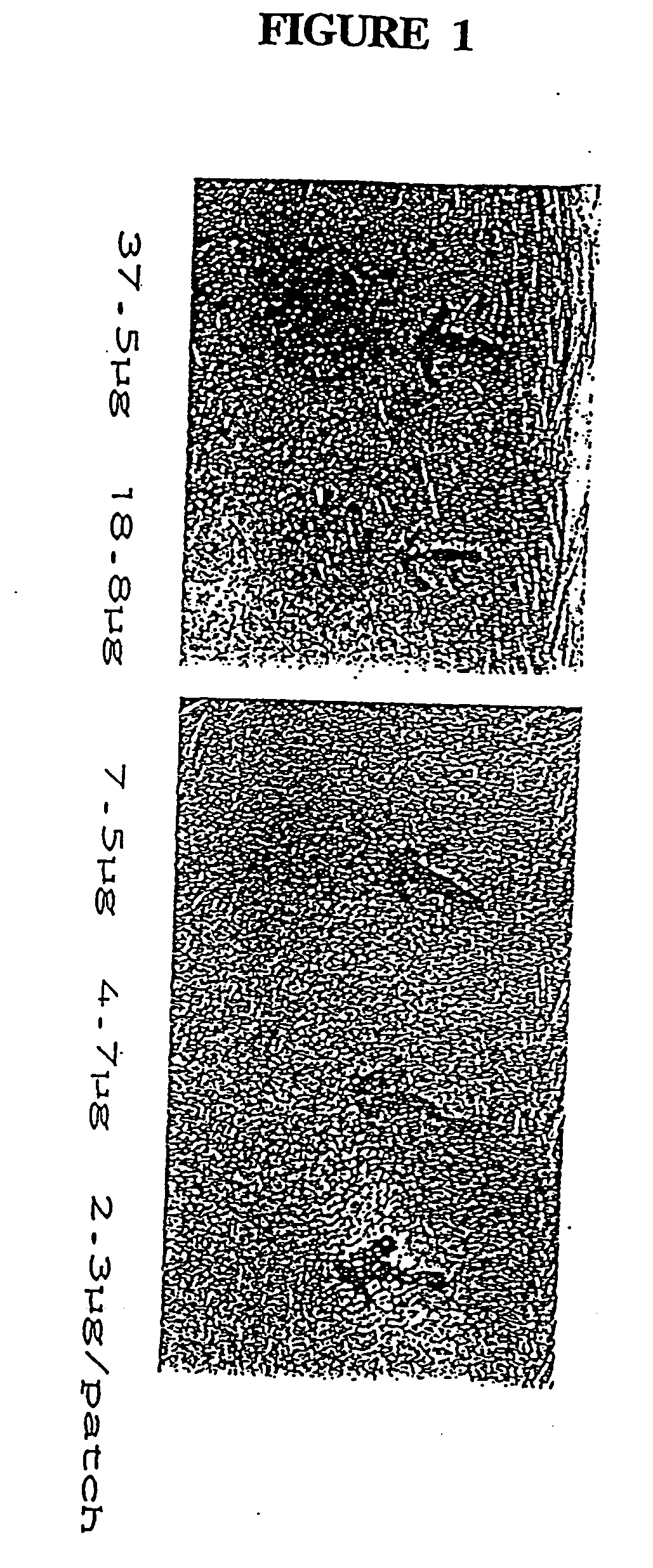
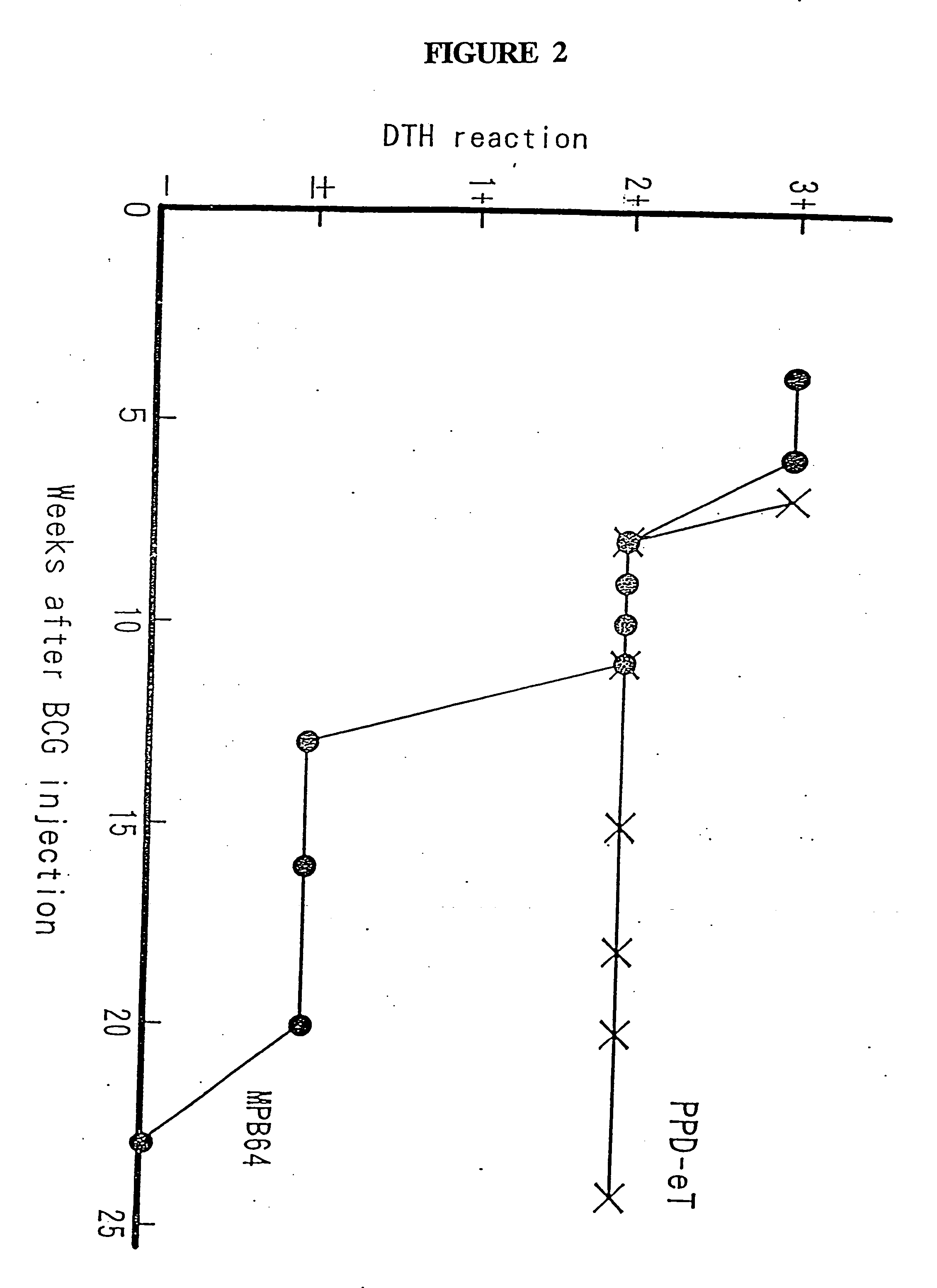
Two-stage type microneedle array patch capable of simultaneously realizing BCG vaccine inoculation and diagnosis, and preparation method thereof
PendingCN112023033AControl release timeEasy to operateCompounds screening/testingAntibacterial agentsBiotechnologyFreeze-drying
The invention discloses a two-stage type microneedle array patch capable of simultaneously realizing BCG vaccine inoculation and diagnosis. The two-stage type microneedle array patch comprises a microneedle array and a patch; the microneedle array is arranged on the patch; the microneedle comprises a soluble drug-loaded microneedle tip and an insoluble microneedle supporting end; the soluble drug-loaded microneedle tip is prepared by mixing a BCG vaccine, tubercle bacillus element microspheres and soluble auxiliary materials and performing freeze drying; and the tubercle bacillus element microspheres are prepared by combining slow-release auxiliary materials and tubercle bacillus elements. The BCG vaccine and the tubercle bacillus elements are gathered at the soluble microneedle drug-loaded tip at the same time; and the release time of the tubercle bacillus elements is controlled through a slow-release microsphere technology, so that two drug administration purposes of inoculating theBCG vaccine and diagnosing whether the BCG vaccine is successfully inoculated or not can be achieved through one-time drug administration. The invention further discloses a preparation method of the patch.
Owner:中山大学深圳
Methods and compositions for detection and diagnosis of infectious diseases
InactiveUS20050106107A1Easy to manageSensitive highUltrasonic/sonic/infrasonic diagnosticsCompounds screening/testingAntigenMedicine
Methods and compositions for the detection and diagnosis of infectious diseases are provided. In particular, efficient and sensitive methods and compositions for the detection of active mycobacterial disease are provided for distinguishing between individuals having active disease, and individuals who have been immunologically exposed, such as those infected with a mycobacterium but are without active disease, or those who have been vaccinated with BCG. The methods comprise topical application of antigen compositions for transdermal delivery.
Owner:JAPAN BCG LAB
SRNA marker for distinguishing mycobacterium tuberculosis and BCG vaccine and use thereof
ActiveCN110093432AEasy to trainEasy accessMicrobiological testing/measurementMicroorganism based processesBacteroidesFluorescence
The invention discloses an sRNA marker for distinguishing mycobacterium tuberculosis and a BCG vaccine and a use thereof. For solving the problem that mycobacterium tuberculosis infection and BCG vaccination are difficult to distinguish, the type of sRNA in mycobacterium tuberculosis is detected and screened by bacterial RNA sequencing firstly, a specific primer and kit capable of detecting the sRNA of mycobacterium tuberculosis are designed according to the screened target sRNA sequence, and a corresponding real-time fluorescence quantitative PCR detection method is established. After free sRNA is extracted from bacterial bodies and cDNA is synthesized, the content of sRNA is detected by the real-time fluorescent quantitative PCR method. The experiment proves that the method can be used for distinguishing the mycobacterium tuberculosis and the BCG vaccine, has the advantages of high sensitivity, good specificity, low cost, convenience in operation and the like, and is hopeful to further meet the clinical and laboratory needs for rapid diagnosis of the mycobacterium tuberculosis and the BCG vaccine.
Owner:HARBIN MEDICAL UNIVERSITY
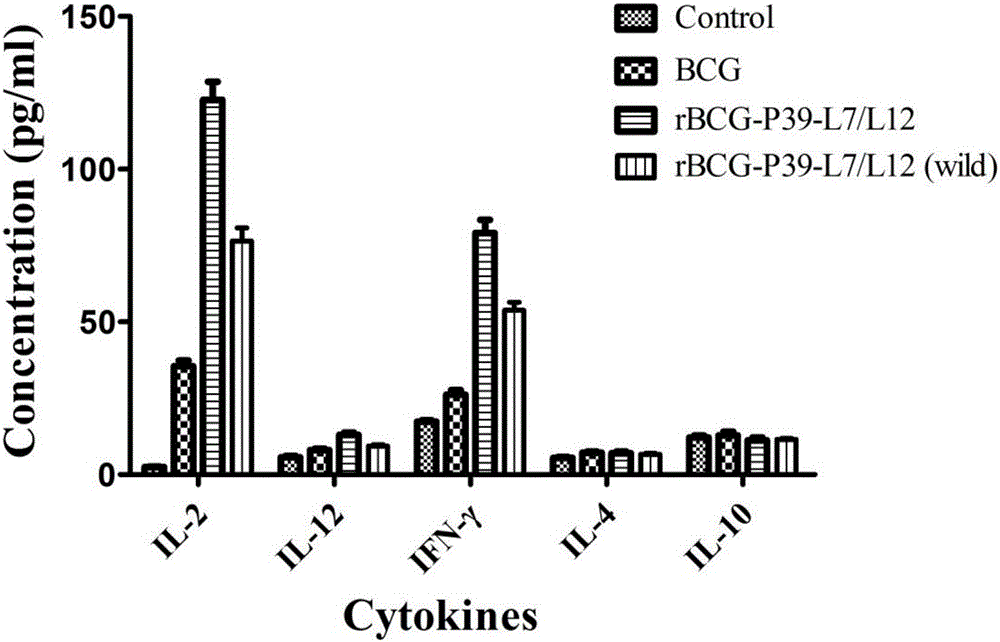
rBCG for expression of Br. Melitensis P39 and L7/L12 fusion gene and construction method thereof
ActiveCN106834331ASignificant immune adjuvant effectLow costAntibacterial agentsBacterial antigen ingredientsRibosomal proteinBCG vaccine
The invention provides rBCG for expression of Br. Melitensis P39 and L7 / L12 fusion gene. The rBCG is constructed by transferring an expression vector carrying codon-optimized Br. Melitensis P39 and L7 / L12 fusion gene into BCG. Brucellosis-generated cytoplasm binding protein PBP39 (coding gene is P39) and Brucellosis ribosomal protein L7 / L12 are both T-cell antigen. Bacillus Calmette-Guerin (BCG) vaccine is the only one commercial vaccine for preventing tuberculosis so far. The BCG vaccine has a remarkable immunologic adjuvant effect and is an exogenous gene expression host with good performance and high safety. By BCG expression of the codon-optimized Brucellosis P39 and L7 / L12 fusion gene, expression quantity of the target gene can be increased. The rBCG vaccine can simulate intracellur infection and parasitic characteristics of Brucellosis to more effectively induce body to generate immune response, can perform advantages of high safety, simple preparation, low cost, etc. of BCG as the expression host as well as the immunologic adjuvant effect of the BCG itself, and is expected to become a novel Brucellosis vaccine.
Owner:INNER MONGOLIA MEDICAL UNIV
Traditional Chinese medicine compound recipe for treating chronic hepatitis-B and preparation method thereof
InactiveCN101491574AEffective treatmentLower blood ALTDigestive systemAntiviralsChronic hepatitisBCG vaccine
The invention relates to a Chinese medicine compound prescription for treating chronic hepatitis B and a method for preparing the same. The Chinese medicine compound prescription consists of mile swertia, serissa foetida comm, astragalus root and radix salivae miltiorrhizae; the materials are decocted by water and extract to obtain a compound active extract. The Chinese medicine compound prescription has the protecting function on liver injury, can reduce the level of ALT and MDA of serum in liver, raise the activity of SOD of serum and has effect of treating hepatitis caused by bcg vaccine. The Chinese medicine compound prescription can be used for preparing a drug with the function of treating chronic hepatitis B.
Owner:BEIJING SUNHO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com