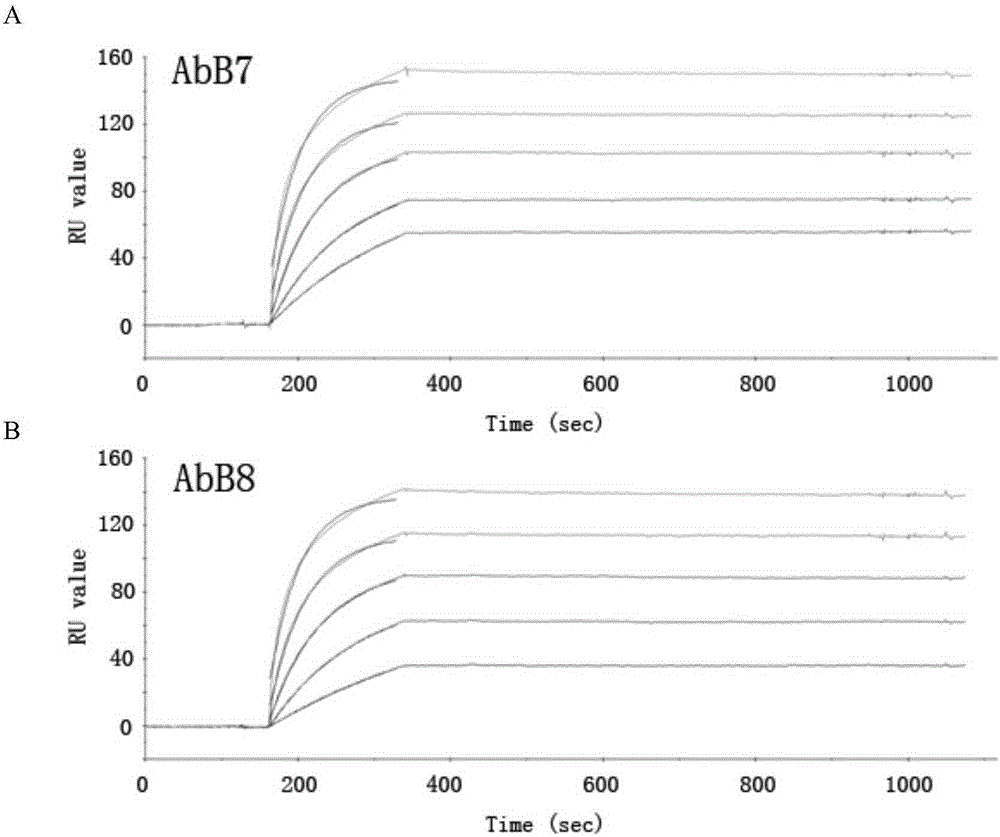
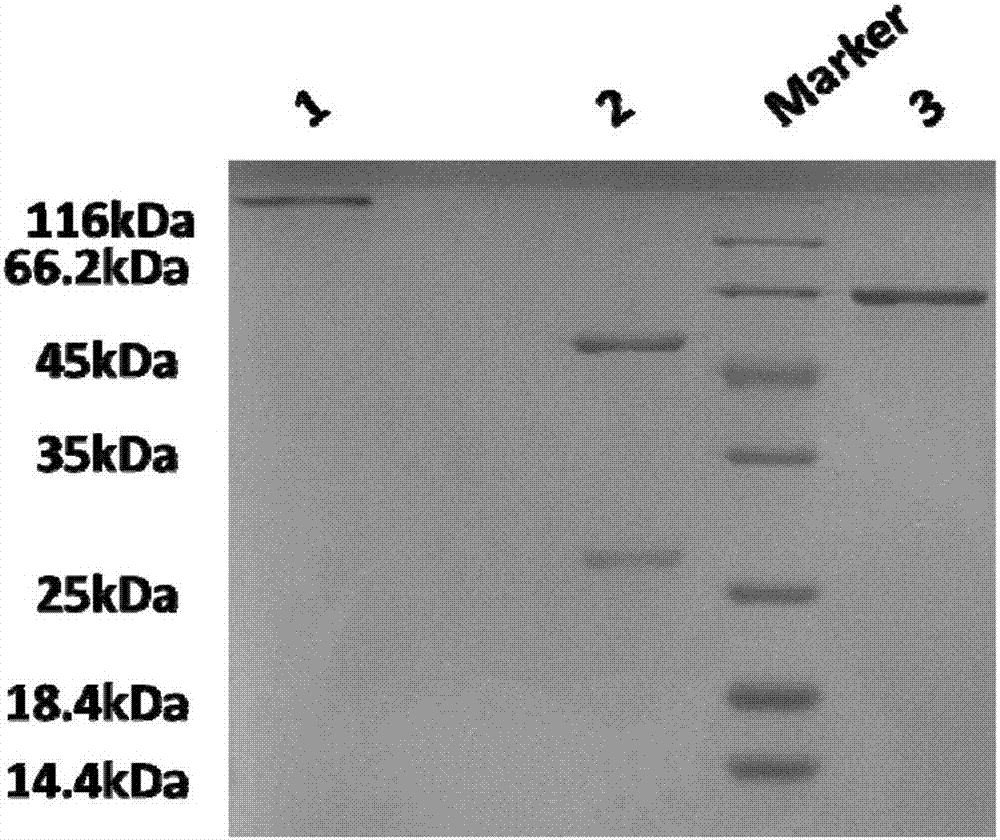
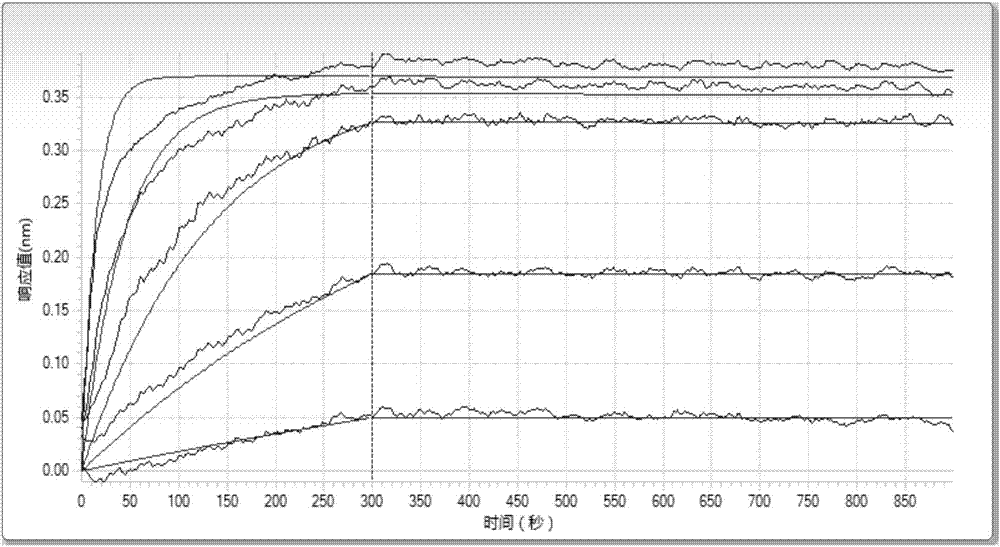
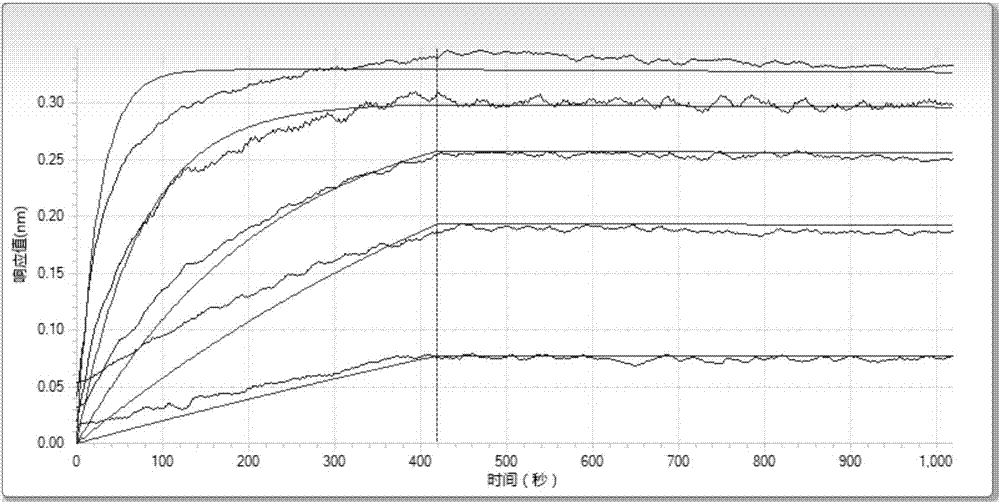
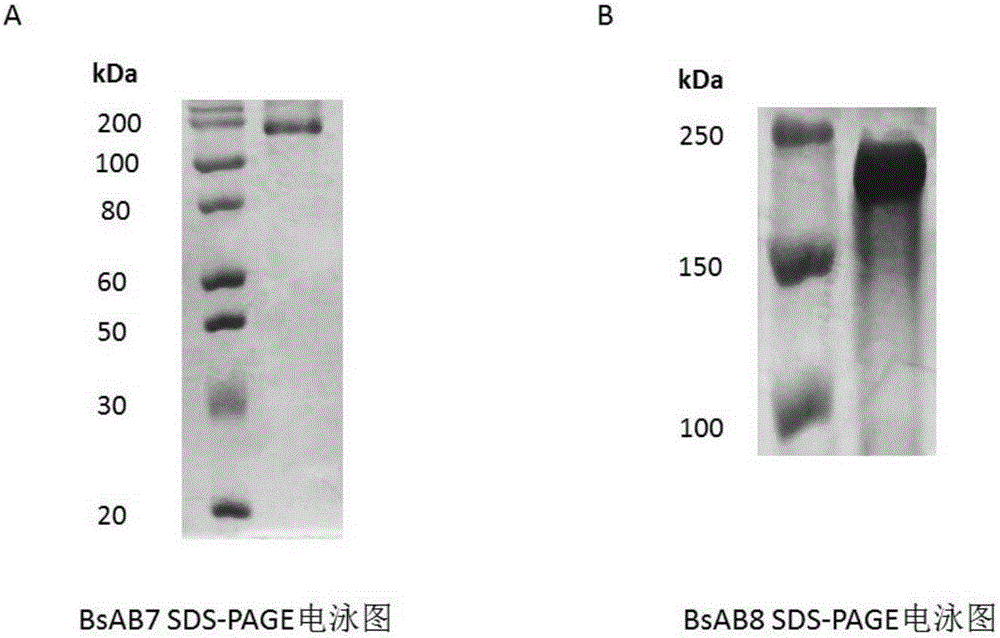
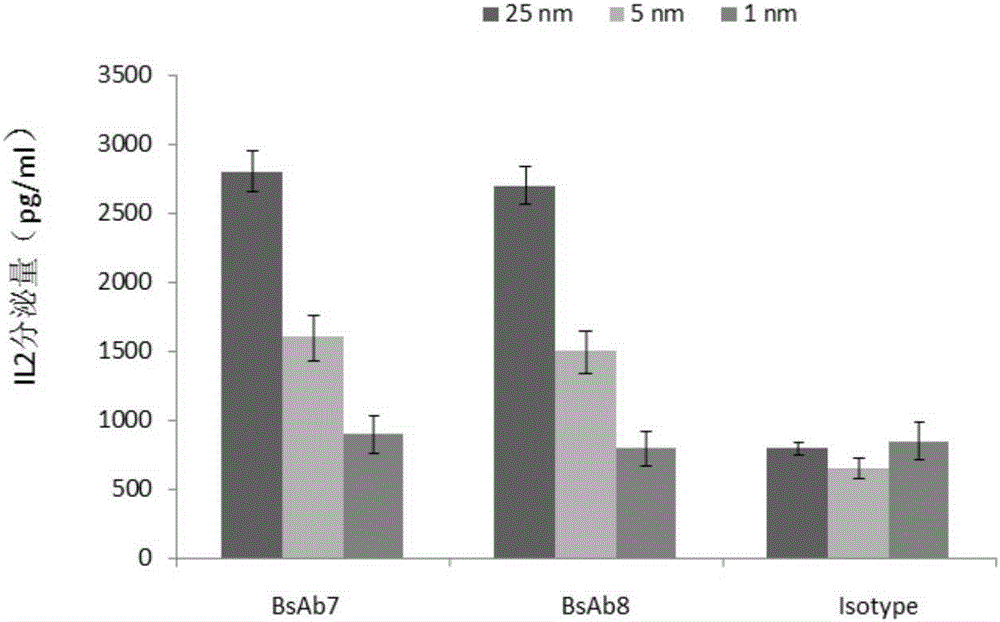
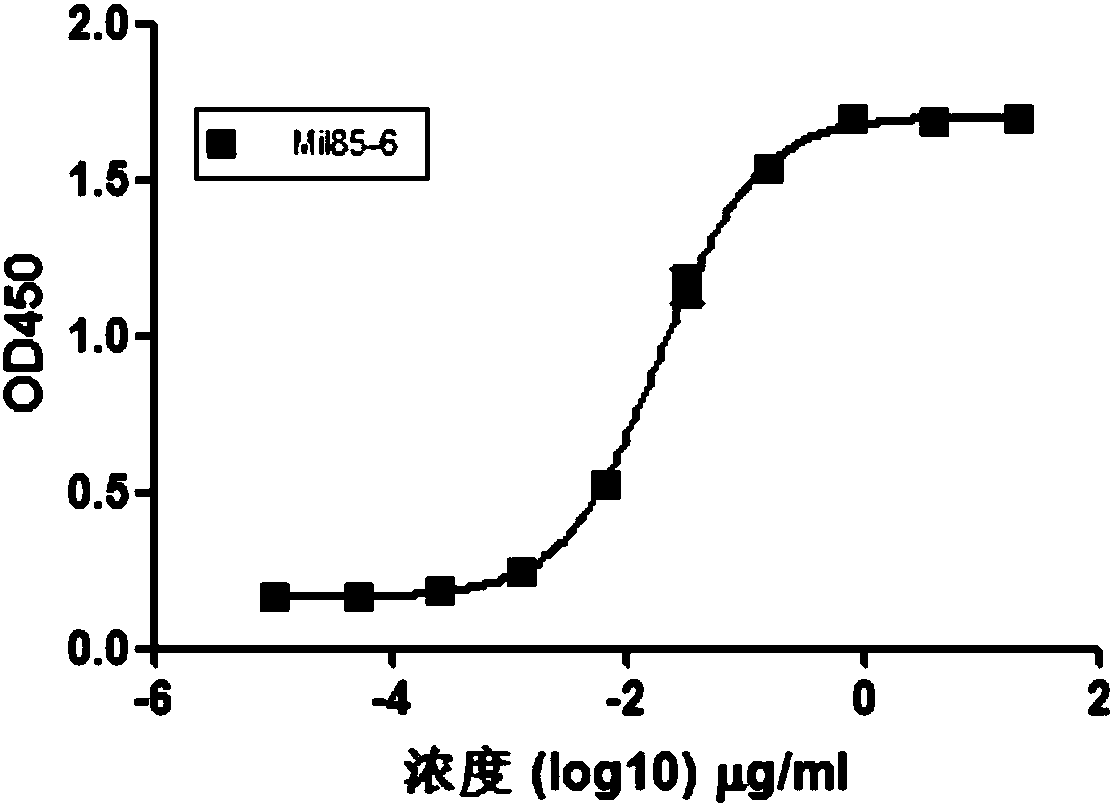
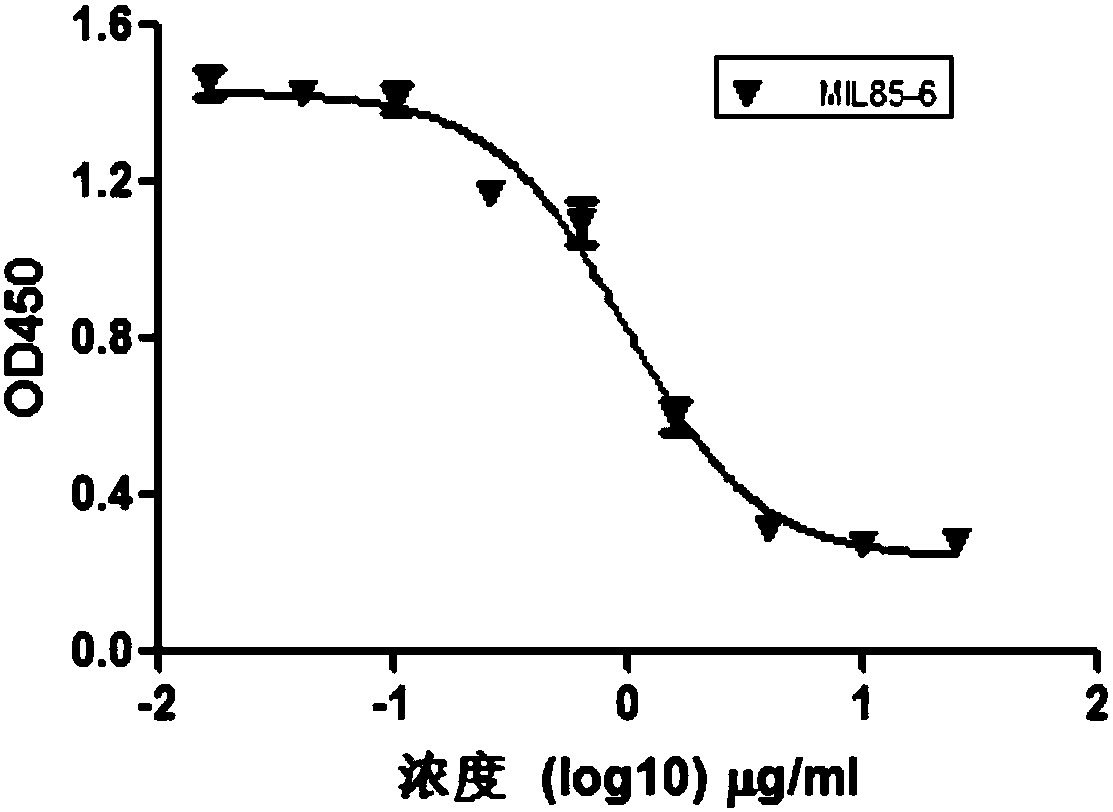
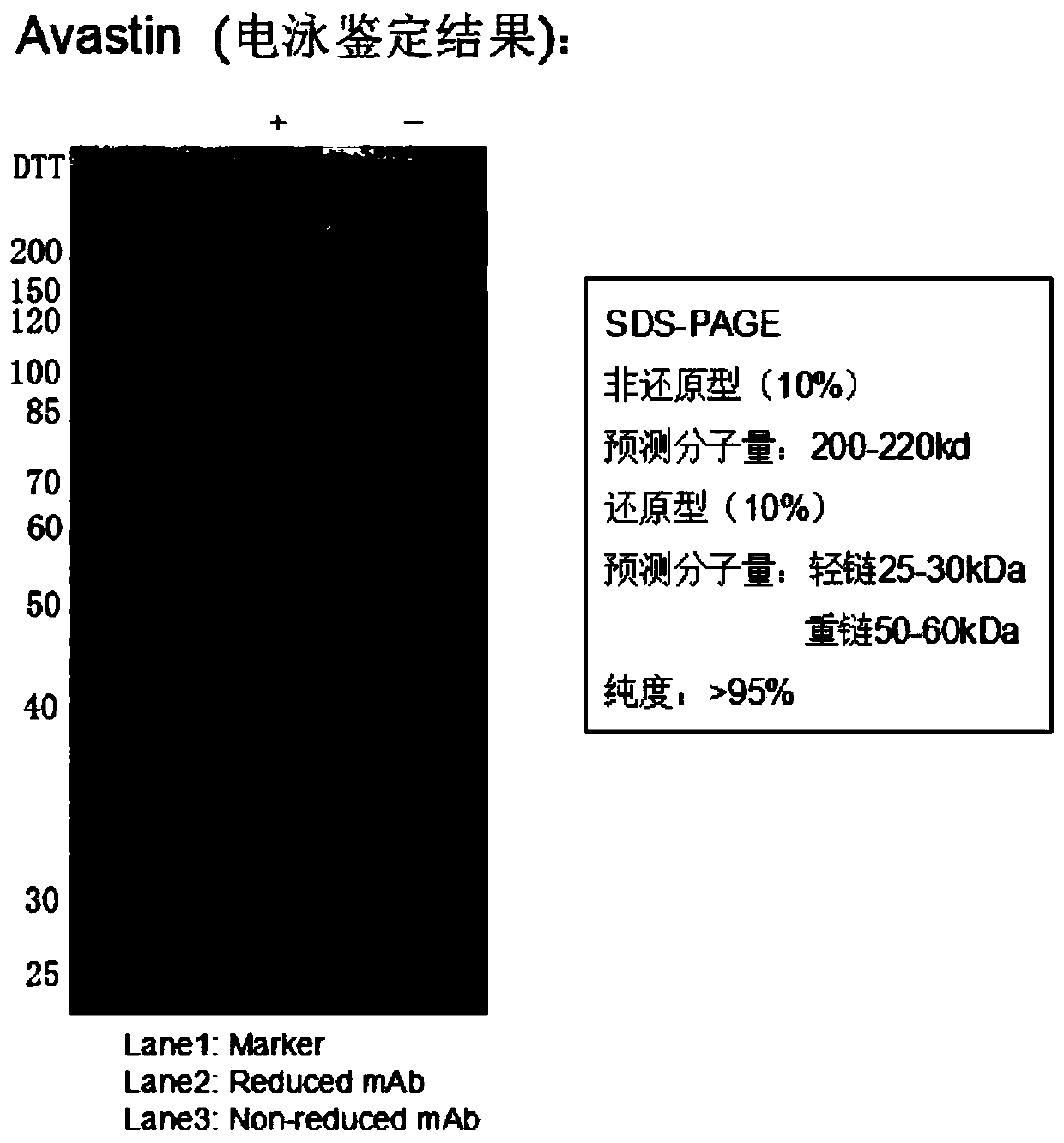
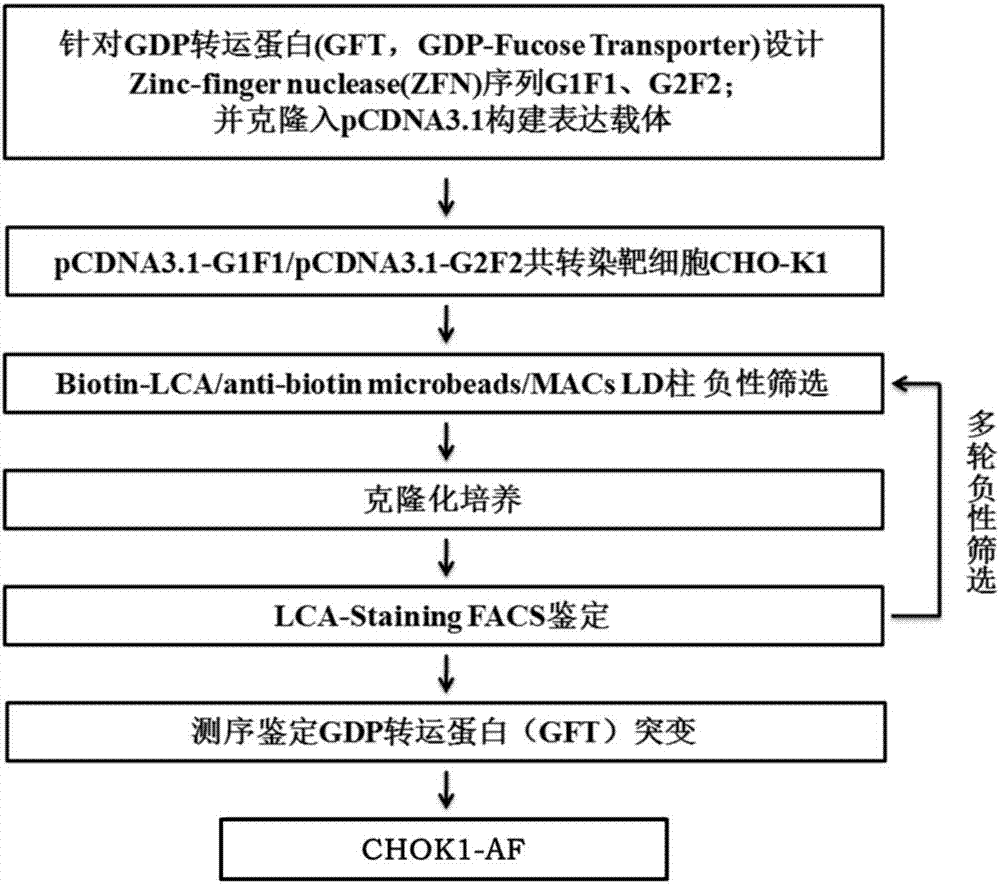
Patents
Literature
125 results about "Molecular Immunology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sub-discipline of immunology which investigates the molecular interaction involved in antigen recognition and processing, antibody-antigen interactions, cell-cell interactions, cell death, etc.
Anti-CTLA4-anti-PD-1 bifunctional antibody, pharmaceutical composition and use thereof
ActiveCN106967172ABinding blockRelief of immunosuppressionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyT lymphocyte
Belonging to the field of tumor treatment and molecular immunology, the invention relates to an anti-CTLA4-anti-PD-1 bifunctional antibody, a pharmaceutical composition and use thereof. Specifically, the anti-CTLA4-anti-PD-1 bifunctional antibody comprises: a PD-1 (programmed death-1) targeted first protein functional region, and a CTLA4 (cytotoxic T lymphocyte sociated antigen 4) targeted second protein functional region. The bifunctional antibody provided by the invention can well bind to CTLA4 and PD-1 specifically, specifically remove the immunosuppression of CTLA4 and PD-1 on the body, and activate T lymphocytes, thus having good application prospect.
Owner:AKESO PHARMA INC
Anti-PD-1 humanized monoclonal antibody and application thereof
ActiveCN105175544AHigh affinityPlay effectivelyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMolecular ImmunologyNucleotide
The invention discloses an anti-PD-1 humanized monoclonal antibody and application thereof, belonging to the technical field of molecular immunology. The anti-PD-1 humanized monoclonal antibody provided by the invention contains a light chain and a heavy chain, wherein the amino acid sequence of the light chain is as shown in SEQ ID NO.2, and the amino acid sequence of the heavy chain is as shown in SEQ ID NO.4 or SEQ ID NO.6. Meanwhile, the invention also provides a nucleotide sequence coding the light chain and heavy chain. The anti-PD-1 humanized monoclonal antibody provided by the invention has very high affinity with human PD-1 protein, and can interdict PD-1 / PD-L1 combination so as to promote T cell proliferation and IFN-gamma secretion.
Owner:ANHUI RUBIOX VISION BIOTECH
Anti-PD1 monoclonal antibody, pharmaceutical composition and uses thereof
ActiveCN106977602ABinding blockRelief of immunosuppressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMolecular ImmunologyAntigen Binding Fragment
The present invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-PD1 monoclonal antibody, a pharmaceutical composition and uses thereof. In particularly, the present invention relates to a monoclonal antibody or an antigen-binding fragment thereof, wherein the heavy chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:9-11, and / or the light chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:12-14. According to the present invention, the monoclonal antibody can well and specifically bind to PD1, can specifically release the immunosuppression of PD1 on body, and can activate T lymphocytes.
Owner:CTTQ AKESO (SHANGHAI) BIOMED TECH CO LTD
PDL-1 antibody, pharmaceutical composition thereof and application of PDL-1 antibody
ActiveCN107151269ABinding blockRelief of immunosuppressionAntibody mimetics/scaffoldsMicroorganism based processesMolecular ImmunologyHeavy chain
The invention belongs to the field of oncotherapy and molecular immunology, and relates to a PDL-1 antibody, a pharmaceutical composition thereof and application of the PDL-1 antibody, in particular to a monoclonal antibody of PDL-1 or an antigen binding fragment thereof. A variable region of the heavy chain of the monoclonal antibody comprises CDR of which the amino acid sequence is SEQ ID NO: 15-17; and a variable region of the light chain of the monoclonal antibody comprises CDR of which the amino acid sequence is SEQ ID NO: 18-20. The monoclonal antibody can be well combined to PDL-1 specifically, inhibition of PDL-1 to body immunity is relieved specifically, and T lymphocyte is activated.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
VEGF-resistant and PD-1-resistant difunctional antibody and application thereof
ActiveCN105175545AHybrid immunoglobulinsImmunoglobulins against growth factorsMolecular ImmunologyT cell
The invention discloses a VEGF-resistant and PD-1-resistant difunctional antibody and application thereof, belonging to the technical field of molecular immunology. The VEGF-resistant and PD-1-resistant difunctional antibody contains a light chain an a heavy chain, wherein the light chain has an amino acid sequence as shown in SEQ ID NO.2, and the heavy chain has an amino acid sequence as shown in SEQ ID NO.4 or SEQ ID NO.6. Meanwhile, the invention provides a gene for encoding the difunctional antibody and the application of the difunctional antibody. The difunctional antibody provided by the invention can be combined with PD-1 and VEGF, has very high affinity, can be used for effectively simulating T cells to secrete IL2 and induce T cells to secrete IFN-gamma and can also be used for remarkably inhibiting the growth of tumor of a mouse so as to have a huge potential in application to preparation of anti-cancer drugs.
Owner:ANHUI RUBIOX VISION BIOTECH
Anti-PD-L1 antibody as well as pharmaceutical composition and application of anti-PD-L1 antibody
ActiveCN106699891ABinding blockRelief of immunosuppressionBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenMolecular Immunology
The invention belongs to the field of oncotherapy and molecular immunology, and relates to an anti-PD-L1 antibody as well as a pharmaceutical composition and an application of the anti-PD-L1 antibody. Particularly, the invention relates to a monoclonal antibody or an antigen binding fragment of the monoclonal antibody, wherein a heavy chain variable region of the monoclonal antibody comprises a CDR, the amino acid sequence of the CDR is SEQ ID NO:1-3, and / or a light chain variable region of the monoclonal antibody comprises a CDR, and the amino acid sequence of the CDR is SEQ ID NO:4-6. The monoclonal antibody is specifically bound with PD-L1 well, specifically relieves immunosuppression of PD-1 on the organism, and activates the T iymphocyte.
Owner:BEIJING MABWORKS BIOTECH
Anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and application thereof
InactiveCN106632674ABinding blockRelief of immunosuppressionRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyHeavy chain
The invention belongs to the fields of oncotherapy and molecular immunology, and relates to an anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and an application thereof, in particular to a monoclonal antibody or an antigen binding fragment thereof. The heavy chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 13-15; and / or the light chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 16-18. The monoclonal antibody can be specifically bound with PD-1 very well, specifically remove immunosuppression of PD-1 on an organism and activate T lymphocytes.
Owner:ZEDA BIOPHARMACEUTICALS INC
Antigen epitope III of neutrality B cell of chicken IBDV VP2 protein and application thereof
The present invention belongs to molecular immunology field, mainly relating to chicken infectious bursal disease virus IBDV VP2 polypeptide fragment, more specificly relating to B cell antigen epitope polypeptide fragment in VP2 protein. The present invention also relates to IBDV VP2 protein neutrality and uses of B cell antigen epitope in preparing immunogen, immune animal, immune protection and uses for detecting antibody of resisting chicken infectious bursal disease virus IBDV or polypeptide of chicken infectious bursal disease virus IBDV. Animal is immunized by immunogen or vaccine designed according to B cell antigen epitope which can neutralize chicken IBDV VP2 protein and can produce antibody for neutralizing IBDV in vitro or vivo, so the immunogen can prevent virus infection to animal. Chicken IBDV VP2 protein neutralized B cell antigen epitope vaccine can prevent infection of chicken IBD classic toxic strain, superstrong toxic strain or / and variant toxic strain.
Owner:HENAN INST OF SCI & TECH
Antigen epitope for exciting human anti-tubercle bacillus protective immunoreaction and its use
InactiveCN1858059AHelp preventAids in healingAntibacterial agentsBacterial antigen ingredientsMolecular ImmunologyBCG vaccine
The present invention relates to molecular immunology technology, and aims at screening out antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, researching protective immunoreaction mechanism against tuberculosis, and further developing new type of concatenate polyepitope tuberculosis vaccine. The present invention provides one kind of antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, and the peptide contains the amino acid sequence selected from SEQ ID Nos. 2, 5, 10, 12, 14 and 15. The present invention also provides the screening process and use of the peptide. The present invention may be used in preventing and controlling tuberculosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
An Anti-ctla4 monoclonal antibody or its antigen binding fragments, pharmaceutical compositions and uses
ActiveUS20170216433A1Efficient activationAntibody mimetics/scaffoldsBiological material analysisMolecular ImmunologyTumor therapy
The present invention belongs to the fields of tumor therapy and molecular immunology, and provides an anti-CTLA4 monoclonal antibody or antigen binding fragment thereof, a pharmaceutical composition thereof and use thereof. The monoclonal antibody of the present invention can block the binding of CLTA4 to B7, relieve the immunosuppression on the body by CTLA4, and activate T lymphocytes.
Owner:AKESO BIOPHARMA
Specific B cell epitope polypeptide of NS1 protein of encephalitis B virus and use thereof
ActiveCN102206249AStrong specificityReduce false positive ratePeptidesGenetic engineeringMolecular ImmunologyScreening method
The invention discloses the sequence of a specific B cell epitope polypeptide of an NS1 protein of encephalitis B virus and the use of the epitope polypeptide in the diagnosis of encephalitis B virus and a screening method of specific B cell epitope polypeptide of NS1 protein of encephalitis B virus, and belongs to the field of molecular immunology. The amino sequence of the epitope polypeptide is represented by SEQ ID No.1 or SEQ ID No.2. The specific B cell epitope synthetic polypeptide of the JEV NS1 protein, the coupling antigen of the synthetic epitope polypeptide and the epitope fusion expression protein can be used for specifically detecting a JEV NS1 protein antibody generated in body which is immunized and infected. The epitope polypeptide can be used for diagnosis of JEV infection, evaluation on immunity effect and identification and diagnosis of immunity of inactivated vaccine and natural infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Monoclonal antibody of avian leukosis virus subgroup J surface protein and preparation method thereof
InactiveCN102304181ABright fluorescent signalNo fluorescenceImmunoglobulins against virusesTissue cultureDiseaseMolecular Immunology
The invention belongs to the cross technical field of molecular immunology and virology, and particularly relates to a hybridoma cell based on hybridoma type leukosis virus subgroup J isolated strain with preservation number of CGMCC (China General Microbiological Culture Collection) No. 5018, a high-specificity monoclonal antibody of avian leukosis virus subgroup J (ALV-J) surface protein (SU) secreted by the hybridoma cell and a preparation method of the monoclonal antibody. By using the high-specificity monoclonal antibody prepared by the invention, a technical support is provided for diagnosis, prevention and treatment of avian leukosis virus subgroup J disease, and the economic loss caused by avian leukosis virus subgroup J disease is effectively reduced.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Preparation and application of CpG DNA molecule anti-infection and immunity prepn
InactiveCN1692943ARaise the level of immune responseReduce plasmid usageSugar derivativesGenetic material ingredientsBiotechnologyMolecular Immunology
An anti-infectious immunopotentiator for pig, ox, yak, etc is prepared through artificially synthesizing the CpG oligonucleotide sequce able to excite the reproductive activity of immune cell, preparing the chitosan nanoparticles from deacetyl chitosan, and using said chitosan nanoparticles for molecular packaging of CpG oligonucleotide. It can be used to immunize the experimental animal by intramuscular injection or oral applialtion.
Owner:SICHUAN UNIV
Grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine
InactiveCN107760716AInducible propertiesImproving immunogenicityViral antigen ingredientsAntiviralsEscherichia coliDisease
The invention discloses a grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine, and belongs to the technical field of gene engineering and molecular immunology. The preparation method disclosed by the invention is characterized by comprising the steps of extracting viral genome RNA, performing reverse transcription to convert the viral genome RNA into cDNA, amplifying a corresponding DNA sequence out, constructing the DNA sequence to pcDNA-3.1(+) plasmid, converting Escherichia coli DH5alpha, screening out positive clone bacteria containing recombinant plasmid, culturing a lot of the positive bacteria and extracting recombinant plasmid S11-pcDNA3.1 contained in the bacteria. The recombinant plasmid is utilized as nucleic acid vaccine to perform intramuscular injection on the grass carps, and the nucleic acid vaccine enters the muscle cells and expresses VP35 protein of the grass carp reovirus in the muscle cells; thus, fish body immune cell proliferation is stimulated, antiviral related gene expression is up regulated, fish bodies are stimulated to generate antiviral antibodies, and capability of grass carps in resisting grass carp reovirus infection is effectively improved; furthermore, the grass carp reovirus S11 gene eukaryotic expression recombinant plasmid can be used for preventing a grass carp hemorragic disease caused by the grass carp reovirus in aquaculture.
Owner:HENAN NORMAL UNIV
O-type foot and mouth disease virus antigen epitope molecular mimic peptide and application thereof
InactiveCN101597327AAntigenicNon-toxicAntiviralsPeptidesVirulent characteristicsMolecular Immunology
The invention relates to the molecular immunology field. Although the existing foot-and-mouth disease weak poison vaccines and inactivated vaccines and other routine vaccines have good immunogenicity, but the vaccines have some unsafe factors such as reversion of virulence, incomplete virus blanching, the escape of live-virus from a preparation factory and the like so that people are motivated to find a more safe and effective FMD vaccine. In the molecular mimic peptide invention, phage display techniques are used to sieve effective FMDV epitopes to provide an O-type foot and mouth disease virus antigen epitope molecular mimic peptide and the invention also provides an application thereof in preparing immunogen and swine foot-and-mouth disease epitope peptide vaccine. The FMDV epitope peptide vaccine using the molecular mimic peptide of the invention has the antigenicity of target molecule without toxicity, is very safe and has low mass production cost and good economic effect and social effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for extracting exosome from chicken bile and application of exosome in immunology
InactiveCN102697812AEasy to maintain biological activityExtraction time is shortDigestive systemAntibody ingredientsDiseaseIntestinal structure
The invention belongs to the field of molecular immunology, and in particular relates to an exosome secreted and released from the epidermal cells of a chicken liver, gall bladder and bile duct, and a preparation method of the exosome. The exosome contains hepatogenic proteins, such as C-reactive protein, aspartate aminotransferase, and vitronectin, epithelium-derived mucin and proteins related to an immune reaction, such as immunoglobulin, and can be taken as a biological indicator for studying liver diseases and as an adjuvant for immune reaction for immune-regulation, and can also be takenas a carrier for treating the diseases of livers, gall bladders and intestines.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Anti-VEGF and anti-PD1 bispecific antibody with novel structure
ActiveCN110498857AStimulation functionGrowth inhibitionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyIFN-gamma secretion
The invention relates to an anti-vascular endothelial growth factor (VEGF) and anti-PD-1 bispecific antibody with a novel structure, and belongs to the technical field of molecular immunology. In a heavy-chain variable region of the antibody, CDR-H1 is an amino acid sequence shown as SEQ ID NO: 1, CDR-H2 is an amino acid sequence shown as SEQ ID NO: 2, and CDR-H3 is an amino acid sequence shown asSEQ ID NO: 3; and in a light-chain variable region of the antibody, CDR-L is an amino acid sequence shown as SEQ ID NO: 4. The bispecific antibody Ps3Vm can be effectively combined with PD-1 proteinand VEGF protein, can effectively compete with PDL-1 to be combined with PD-1 protein and compete with VEGF-A to be combined with VEGF protein, and can effectively stimulate T cell function and secrete cytokines IL-2 and IFN-gamma at the same time. An isotype control antibody cannot promote T cell proliferation and IL-2 and IFN-gamma secretion. In addition, the bispecific antibody Ps3Vm can also obviously inhibit tumor growth of mice.
Owner:ANHUI RUBIOX VISION BIOTECH
Streptococcus iniae vaccine and preparation and application thereof
InactiveCN101869705AEasy to makeImprove protectionAntibacterial agentsBacterial antigen ingredientsEscherichia coliMolecular Immunology
The invention relates to the field of the molecular immunology, in particular to a streptococcus iniae vaccine and preparation and application thereof. Particularly, the vaccine is expressed by a base sequence in a sequence table SEQ ID No.1. A preparation method comprises the following steps: using streptococcus iniae G26 as a template and using a primer F2 / R5 to carry out PCR; and connecting a PCR product with plasmids pBTA1 and converting colon bacillus DH5l alpha by the connecting solution to obtain a bacterial strain DH5 alpha / pTASP11, i.e. a bacterial strain of the streptococcus iniae vaccine, which expresses a protein encoded by the base sequence in the sequence table SEQ ID No.1. The application of the streptococcus iniae vaccine is that the obtained bacterial strain of the streptococcus iniae vaccine is cultured by an LB culture medium until OD600 is 0.8 and then is dissolved in PBS and the obtained vaccine preparation solution has effect on immune protection for the streptococcus iniae. The obtained vaccine of the invention has very simple preparation process and does not need any extraction and purification process. The protection efficiency of the streptococcus iniae vaccine aiming at streptococcus iniae infection reaches 100 percent.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Anti-CTLA4 and anti-PD-1 bispecific antibody and application thereof
PendingCN112300286AEliminate binding activityReduced activityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyIntravenous gammaglobulin
The invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-CTLA4 and anti-PD1 bispecific antibody and application thereof. Specifically, the anti-CTLA4 andanti-PD1 bifunctional antibody comprises a first protein functional region targeting PD-1 and a second protein functional region targeting CTLA4, wherein according to an EU numbering system, a heavy chain constant region of immunoglobulin contained in the bispecific antibody mutates at any two or three of a 234th site, a 235th site and a 237th site, and after mutation, the affinity constant of thebispecific antibody and Fc[gamma]RIIIa and / or C1q is reduced compared with that before mutation. The bifunctional antibody disclosed by the invention can be well and specifically combined with CTLA4and PD-1, specifically relieves the immunosuppression of the CTLA4 and the PD-1 on the body, activates T lymphocytes and has a good application prospect.
Owner:AKESO PHARMA INC
B-cell antigenic multi-epitope peptide linked in tandem in OmpU of vibrio mimicus, making method and application thereof
InactiveCN101747416AEasy accessAccurately obtainedAntibacterial agentsMicroorganism based processesDiseaseMolecular Immunology
The present invention relates to an antigenic B-cell multi-epitope peptide linked in tandem in the outer membrane protein(Omp) U gene of vibrio mimicus, a making method and an application thereof, which belong to the field of molecular immunology. The B-cell multi-epitope peptide linked in tandem can induce fish to make protective immunity response to vibrio mimicus infection. In the present invention, the amino acid sequence of the antigenic B-cell multi-epitope peptide linked in tandem in the OmpU is shown in SEQIDNO:10. The B-cell multi-epitope peptide linked in tandem in the OmpU of vibrio mimicus is made by a genetic engineering technique. The verification of immune blotting analysis, specific antibody detection and immune animal protective experiments shows that the peptide can elicit efficient and specific protective humoral immunity response to vibrio mimicus infection. The B-cell multi-epitope peptide linked in tandem can be used for the immune diagnosis and the immune prevention and treatment of aquatic animal ascitic diseases. The present invention has high social benefit and economic benefit.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Establishment of hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody
InactiveCN103773736AImmunoglobulins against virusesMicroorganism based processesMolecular ImmunologyHN Protein
The invention relates to establishment of a hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody, which belongs to the technical field of molecular immunology and virology. The purified HN protein expressed by duck NDV isolate SDO3 pronucleus is used as immunogen, one hybridoma cell strain capable of secreting duck NDV-resisting isolate monoclonal antibody is researched, and the preservation number is CCTCC C2013173. The NDV specificity monoclonal antibody secreted by the hybridoma cell strain cannot be specially combined with the NDV strain but can be specifically combined with NDV clinical wild strain, so that the NDV specificity monoclonal antibody can be used as an identification reagent to be used for identifying the NDV vaccine virus and clinical wild strain, and the specificity is strong; the sensitivity is 1: 212 HAU; the hybridoma cell strain has the advantage of high sensitivity.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Recombinant multi-epitope antigen of bovine coronavirus and application thereof
ActiveCN107033250AAvoid infectionImprove prokaryotic expression efficiencySsRNA viruses positive-senseBacteriaMolecular ImmunologyAgricultural science
The invention discloses a recombinant multi-epitope antigen of bovine coronavirus, also discloses application of the recombinant multi-epitope antigen of the bovine coronavirus in bovine coronavirus prevention ad diagnosis and belongs to the field of molecular immunology. An amino acid sequence of the recombinant multi-epitope antigen of the bovine coronavirus is a concatemer of a bovine coronavirus BCoV S protein epitope. The recombinant multi-epitope antigen, capable of causing a neutralizing antibody, of the bovine coronavirus serves as an immunogen or a vaccine, the neutralizing antibody oriented to the bovine coronavirus BCoV can be produced after the animal body is immunized, and the BCoV can be neutralized in vitro to prevent the animal body from virus infection. In addition, the recombinant multi-epitope antigen of the bovine coronavirus can also serve as a reagent for detecting a bovine coronavirus BCoV antibody or a polypeptide antibody.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI +2
Grass carp reovirus VP35 protein subunit vaccine, preparation method and applications thereof
ActiveCN108324936AEasy to prepareClear ingredientsViral antigen ingredientsAntiviralsAntigenMolecular Immunology
The invention discloses a grass carp reovirus VP35 protein subunit vaccine, a preparation method and applications thereof, and belongs to the technical field of genetic engineering and molecular immunology, wherein the technical scheme is that the gene sequence of the subunit vaccine protein is represented by a sequence table SEQ ID No.1. The invention further specifically discloses a preparationmethod of the grass carp reovirus VP35 protein subunit vaccine, and applications of the grass carp reovirus VP35 protein subunit vaccine as the anti-grass-carp-reovirus-infection vaccine in improvement of the survival rate of grass carps infecting the grass carp reovirus. According to the present invention, the grass carp reovirus VP35 protein subunit vaccine is the novel antigen protein vaccine,has characteristics of simple preparation method, clear component, safety and stability, and can induce the high non-specific and specific immune responses of grass carp bodies so as to obtain the good immune protection effect.
Owner:HENAN NORMAL UNIV
Anti-PD-1-anti-VEGFA bispecific antibodies, pharmaceutical compositions and uses thereof
PendingCN112830972AReduced activityEliminate binding activityHybrid immunoglobulinsImmunoglobulins against growth factorsMolecular ImmunologyIntravenous gammaglobulin
The invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-VEGFA-anti-PD-1 bispecific antibody and an application thereof. Specifically, the anti-VEGFA-anti-PD-1 bispecific antibody comprises a PD-1-targeting first protein functional region and a VEGFA-targeting second protein functional region, according to an EU numbering system, a heavy chain constant region of immune globulin contained in the bispecific antibody mutates at two sites of 234 and 235, and after mutation, the affinity constant of the bispecific antibody is reduced compared to the affinity constant of Fc [gamma] RI, Fc [gamma] RIIa, Fc [gamma] RIIIa and / or C1q before mutation. The bispecific antibody disclosed by the invention can be specifically combined with VEGFA and PD-1, specifically relieve immunosuppression of VEGFA and PD-1 on an organism and inhibit angiogenesis caused by tumors at the same time, and has a good application prospect.
Owner:AKESO BIOPHARMA
Antibody for resisting human epidermal growth factor receptor 2 (HER2), as well as medical composition and use of antibody
InactiveCN107446045AGood curative effectGood tumor suppressor effectCompounds screening/testingImmunoglobulins against cell receptors/antigens/surface-determinantsHigh cellAntigen
The invention belongs to the field of tumor treatment and the field of molecular immunology, and relates to an antibody for resisting human epidermal growth factor receptor 2 (HER2), as well as a medical composition and use of antibody, in particular to the antibody for resisting Her2 or an antigen binding fragment of the antibody for resisting Her2. The antibody for resisting Her2 comprises a first heavy chain and a second heavy chain, wherein according to the first heavy chain, a variable region of heavy chain (VH) of the first heavy chain comprises a complementarity-determining region (CDR) of which the amino acid sequence is SEQ ID No: 1-3, and the amino acid sequence of the CH of the first heavy chain is as shown in SEQ ID No: 7; according to the second heavy chain, a variable region of heavy chain (VH) of the second heavy chain comprises a complementarity-determining region (CDR) of which the amino acid sequence is SEQ ID No: 4-6, and the amino acid sequence of the CH of the first heavy chain is as shown in SEQ ID No: 8. Compared with the combination of two antibodies of trastuzumab and pertuzumab, the antibody disclosed by the invention has higher cell direct killing activity and higher antibody dependent cellmediated cytotoxicity (ADCC) activity; the complement-dependentcytotoxicity (CDC) activity of the two antibodies of trastuzumab and pertuzumab is equivalent to that of the antibody disclosed by the invention; the antibody disclosed by the invention adopts a structure of a normal antibody; and fucose is also knocked out, so that the ADCC activity is improved.
Owner:BEIJING MABWORKS BIOTECH
Prepn. and application tech. for pig interleukin-4 gene anti-diseases prepns.
InactiveCN1692942ARaise the level of immune responseImprove immunityGenetic material ingredientsAntiinfectivesDiseaseTibetan pig
An anti-infectious immunopotentiator for animals is prepared through cloning the interleukin-4 gene of Tibetan pig, configuring its eukaryon expression plasmid VTPIL-4, preparing chitosan nanoparticles from deacetyl chitosan, and using said chitosan nanoparticles for molecular packaging of plasmid VTPIL-4. It can be used for immunizing experimental animal by intramuscular injection.
Owner:SICHUAN UNIV
Neutral B cell antigen epitope polypeptide of encephalitis b virus E protein and uses thereof
InactiveCN101333246AAvoid infectionViral antigen ingredientsPeptidesMolecular ImmunologyJapanese encephalitis virus Antibody
The invention discloses a polypeptides epitope in Japanese encephalitis virus E protein neutralizing B cell, also discloses the application of the polypeptides epitope in the prevention, treatment and diagnosis of Japanese encephalitis, belonging to the field of molecular immunology. The amino acid sequence of the polypeptides epitope disclosed in the invention is shown in SEQ ID NO: 1 or SEQ ID NO: 2. After being used as immunogen or vaccine immune animal organisms, the JEV E protein neutralizing B cell polypeptides epitope of the invention can produce neutralizing antibody against Japanese encephalitis virus, and can neutralize the Japanese encephalitis virus in vivo or in vitro so as to prevent the virus infecting animal organisms. The polypeptides epitope of the invention can immunise animals if being self-connected or inter-connected with carriers; the anti-peptide antibodies or the anti-Japanese encephalitis virus antibodies produced after the immunising of animals can neutralize the Japanese encephalitis virus in vitro and in vivo and generate immune protection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
B cell antigen epitope polypeptide of encephalitis B virus E protein and uses thereof
The invention discloses a polypeptides epitope in Japanese encephalitis virus E protein neutralizing B cell, also discloses the application of the polypeptides epitope in the prevention, treatment and diagnosis of Japanese encephalitis, belonging to the field of molecular immunology. The amino acid sequence of the polypeptides epitope disclosed in the invention is selected from any of the amino acid shown in SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 5. After being used as immunogen or vaccine immune animal organisms, the JEV E protein neutralizing B cell polypeptides epitope of the invention can produce neutralizing antibody against Japanese encephalitis virus, and can neutralize the Japanese encephalitis virus in vivo or in vitro so as to prevent the virus infecting animal organisms. The polypeptides epitope of the invention or the conjugate of the polypeptides epitope can be used as the reagents for detecting the anti-Japanese encephalitis virus antibodies or anti-Japanese encephalitis virus polypeptide antibodies.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
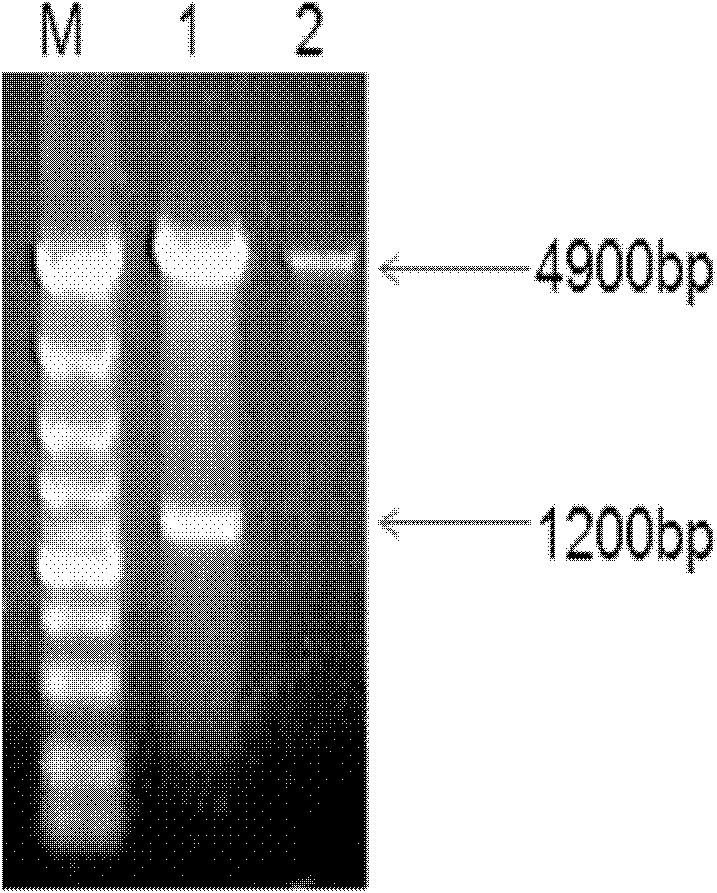
Monoclonal antibody of avian reticuloendotheliosis virus envelope protein and preparation method thereof
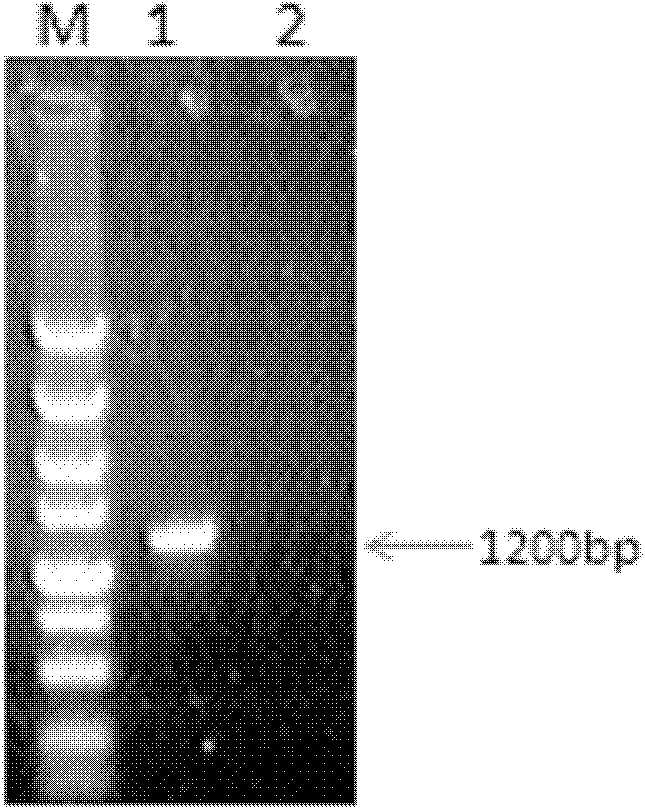
InactiveCN102304180APreserve the antigenic siteEliminate distractionsMicroorganism based processesImmunoglobulins against virusesAntigenBALB/c
The invention belongs to the cross technical field of molecular immunology and virology, and particularly relates to a monoclonal antibody of avian reticuloendotheliosis virus envelope protein. The monoclonal antibody is characterized in that 1200bp of genetic fragment in a relative conservation region of an avian reticuloendotheliosis virus (REV) envelope (env) gene is selected, and 400 amino acids expressed by the fragment comprise more than 90% of antigen sites of the REV, so that most of the antigen sites of the virus are retained and the interference caused by genovariation is excluded, prokaryotic expression product His-env fusion protein is obtained by utilizing the env gene containing the conservation genetic fragment, the Balb / C mouse is immunized by the fusion protein, and the monoclonal antibody of the REV ENV (envelope) protein is obtained through fusion of oncocyte SP2 / 0 and splenocyte of the immunized mouse, screening and cloning. The monoclonal antibody provides a technical support for diagnosis and prevention as well as scientific research of avian reticuloendotheliosis.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
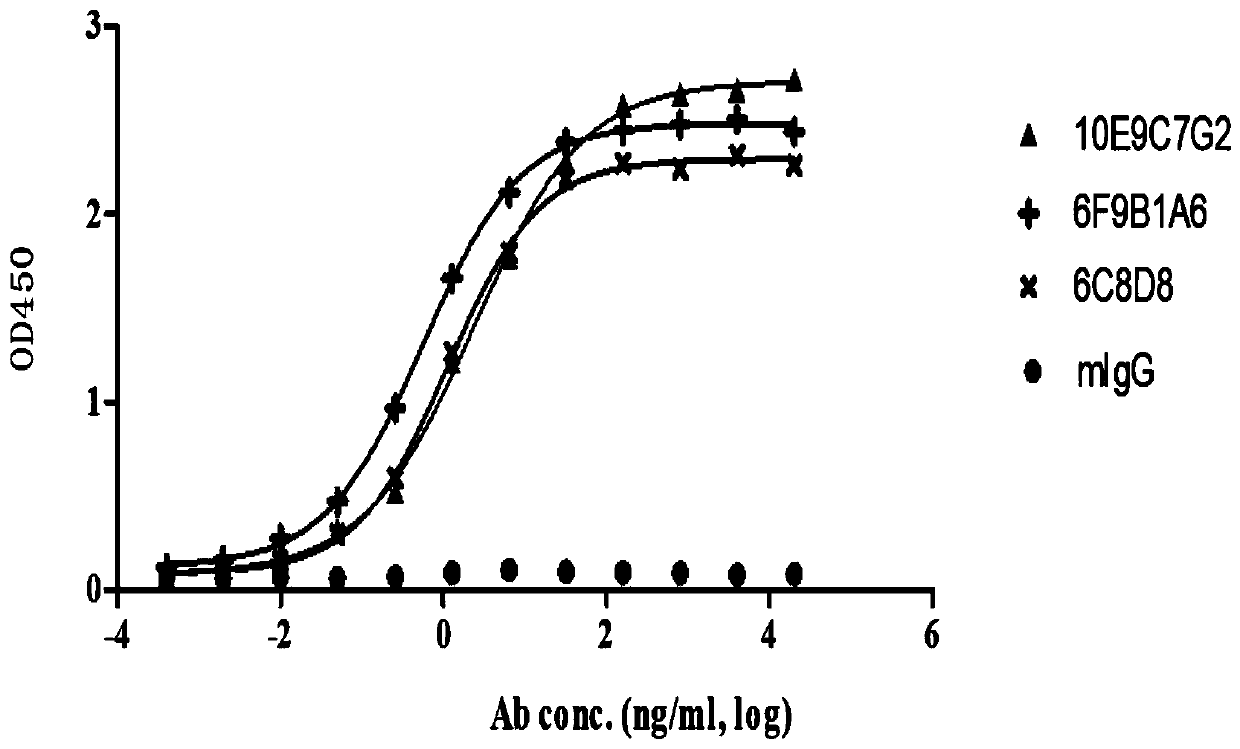
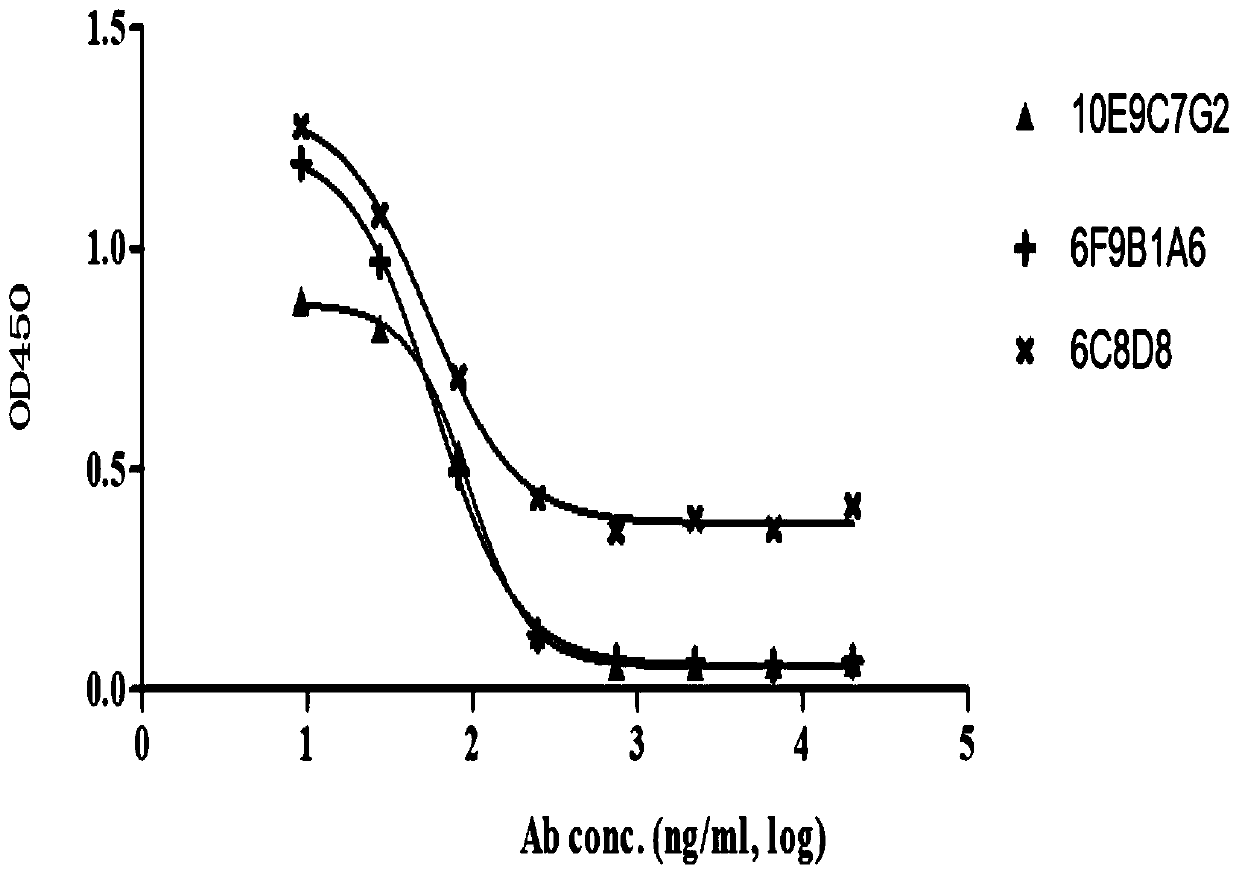
Group of PD-L1-resisting monoclonal antibodies and medical application thereof
ActiveCN110903391ACombined with effective blockingBinding blockImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunopotencyMolecular Immunology
The invention discloses a group of PD-L1-resisting monoclonal antibodies and a medical application thereof and belongs to the field of oncotherapy and molecular immunology. The invention specificallyrelates to the group of PD-L1-resisting monoclonal antibodies and the medical application thereof. According to the invention, the group of PD-L1-resisting monoclonal antibodies, having an excellent effect of blocking the interaction between PD-L1 and PD-1, are obtained through a hybridoma technology. Humanized modification and affinity maturation are successfully performed on the antibodies. Theantibodies provided by the invention show a great application prospect of preparing medicines for blocking and adjusting the function and level of the PD-L1 and significantly enhancing organism immunity especially medicines for treating cancer.
Owner:DONGDA BIOSCIENCE INC (SUZHOU)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com