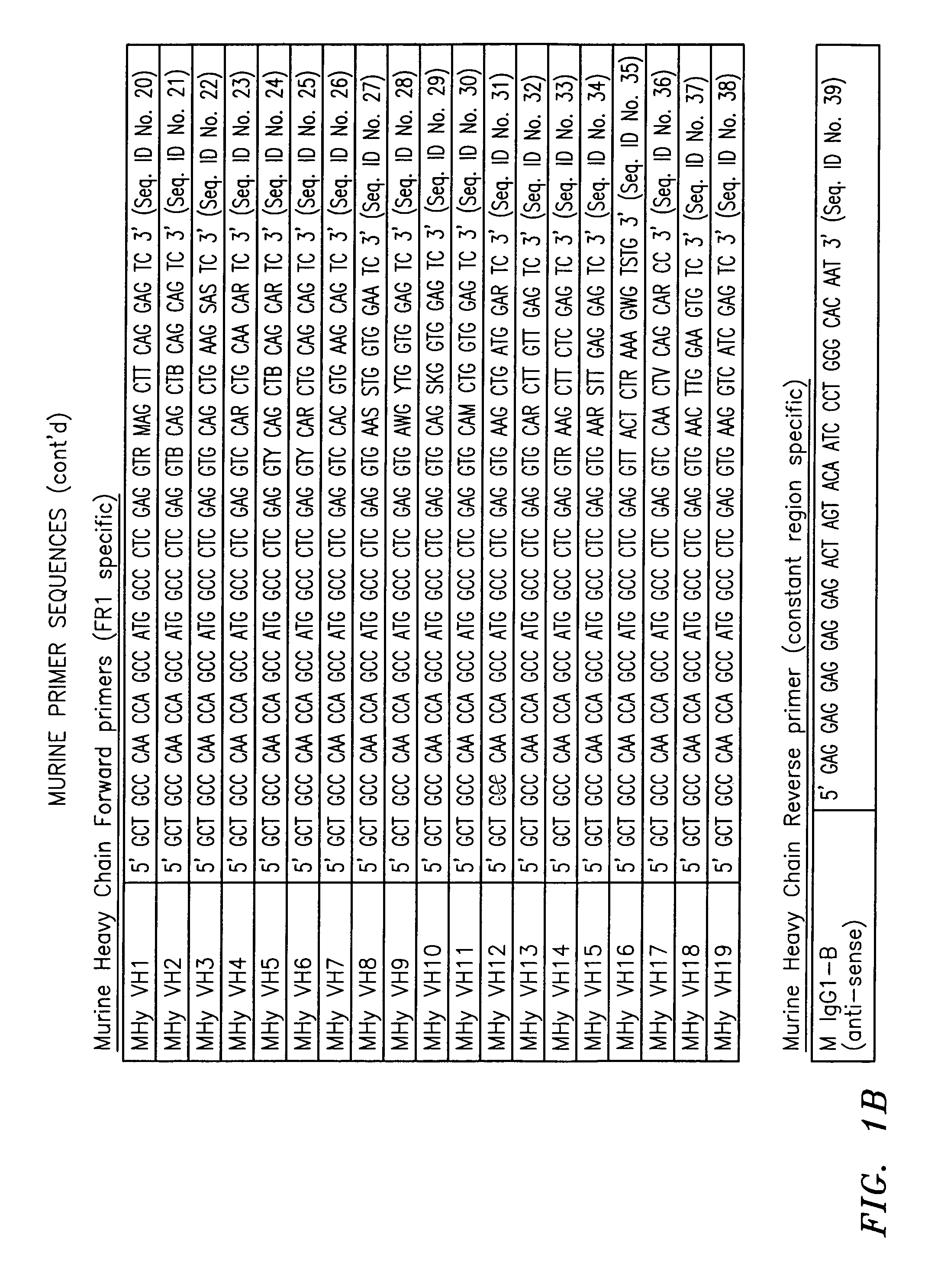
Patents
Literature
207 results about "Encephalitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An inflammation of the brain usually caused due to infection.
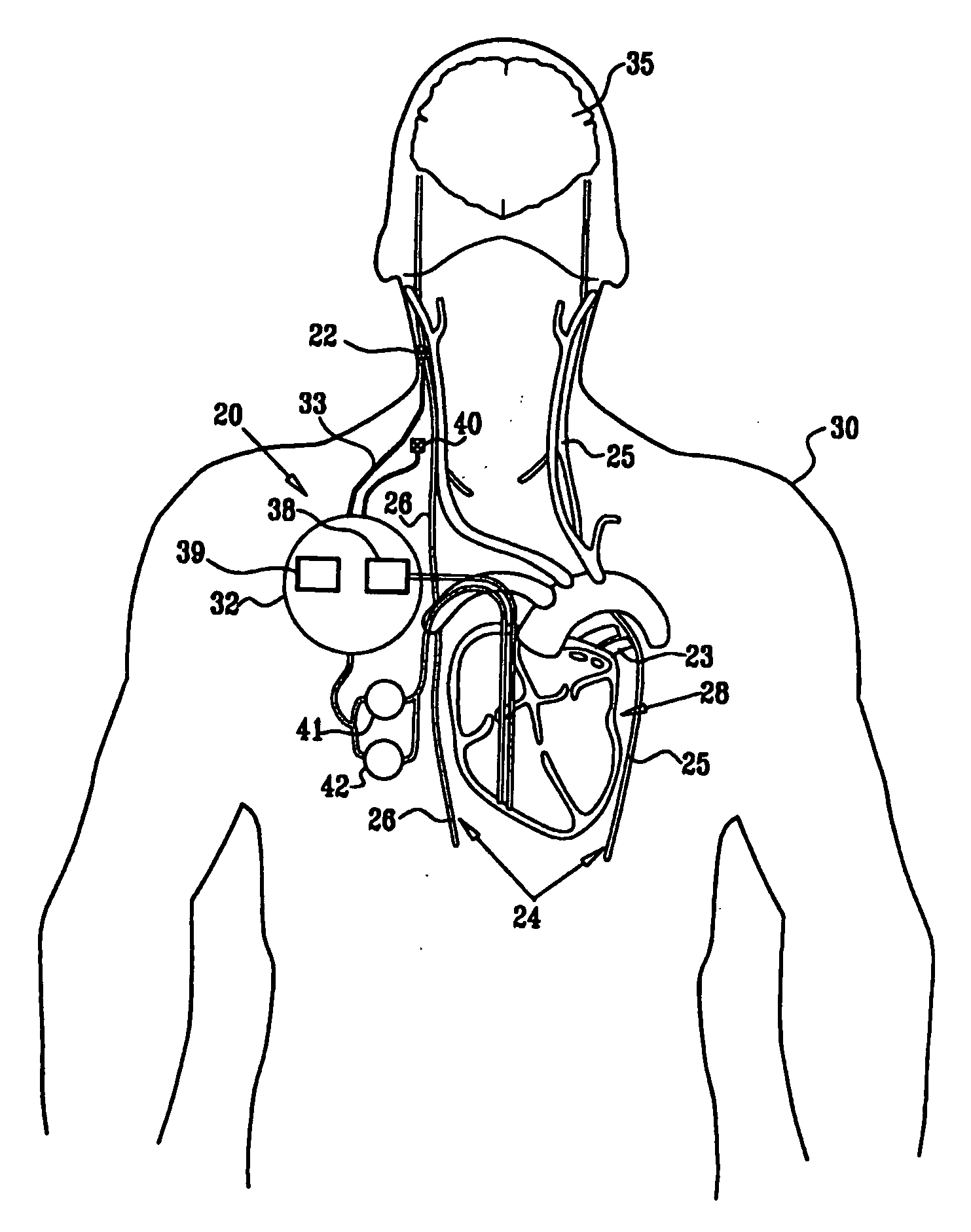
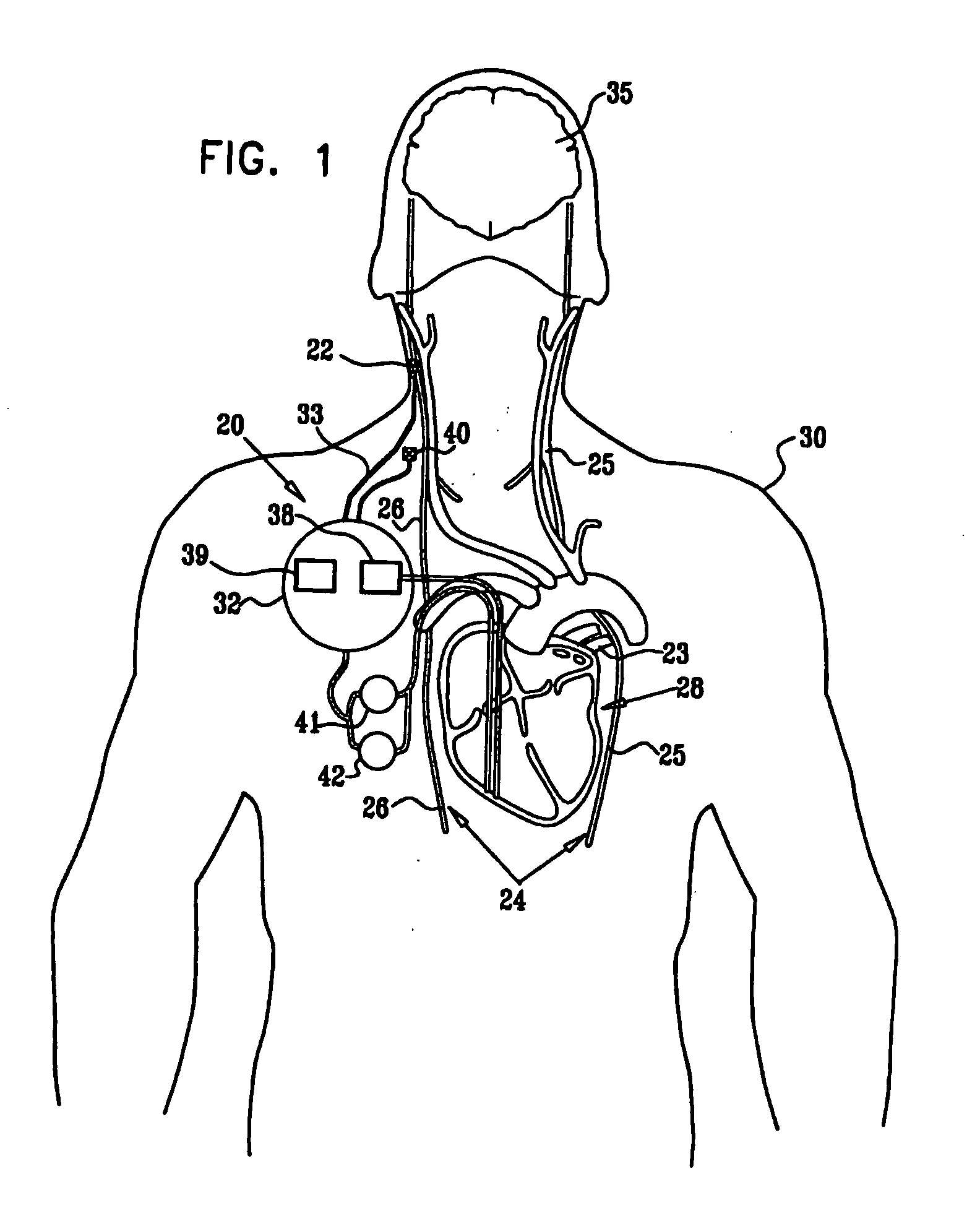
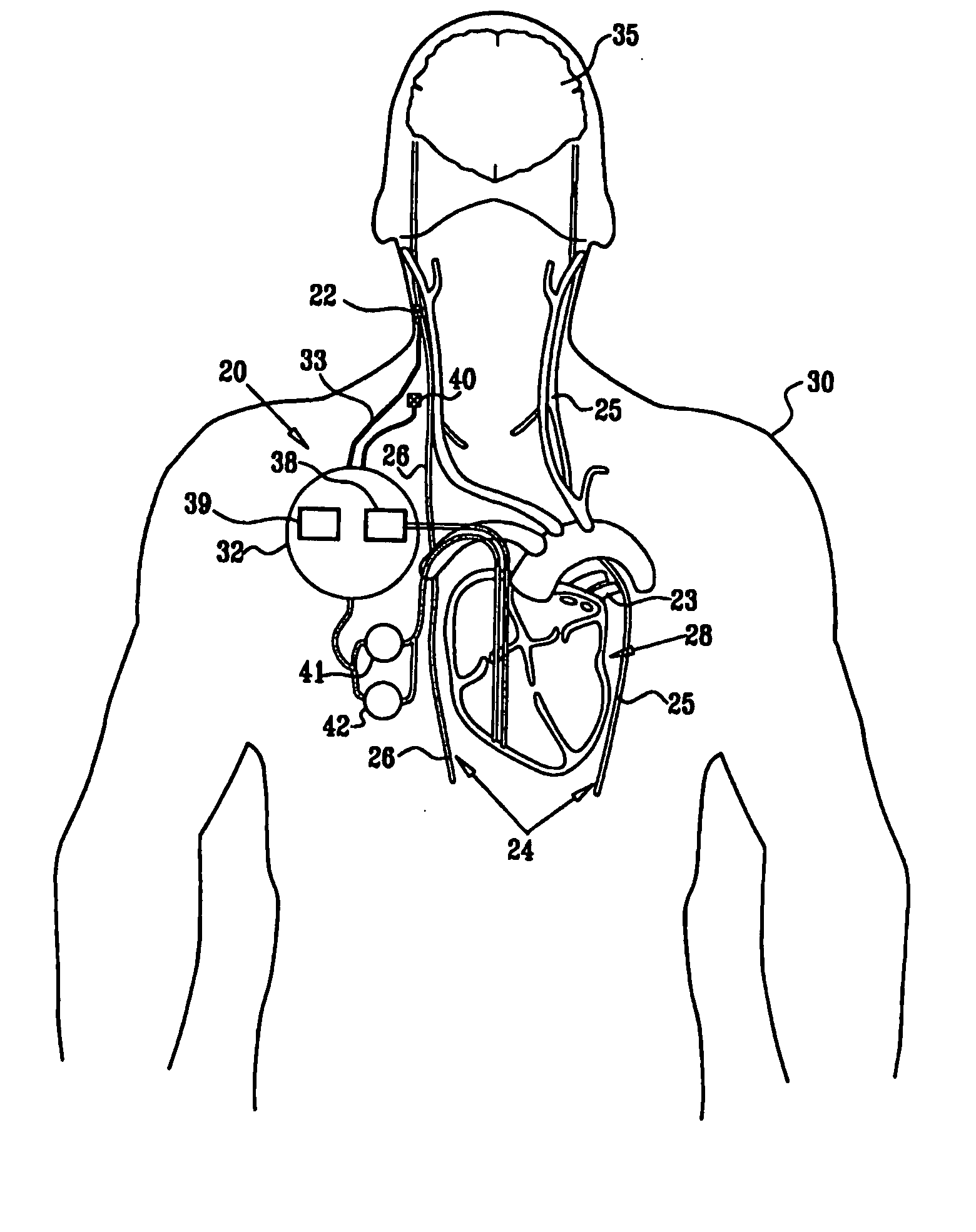
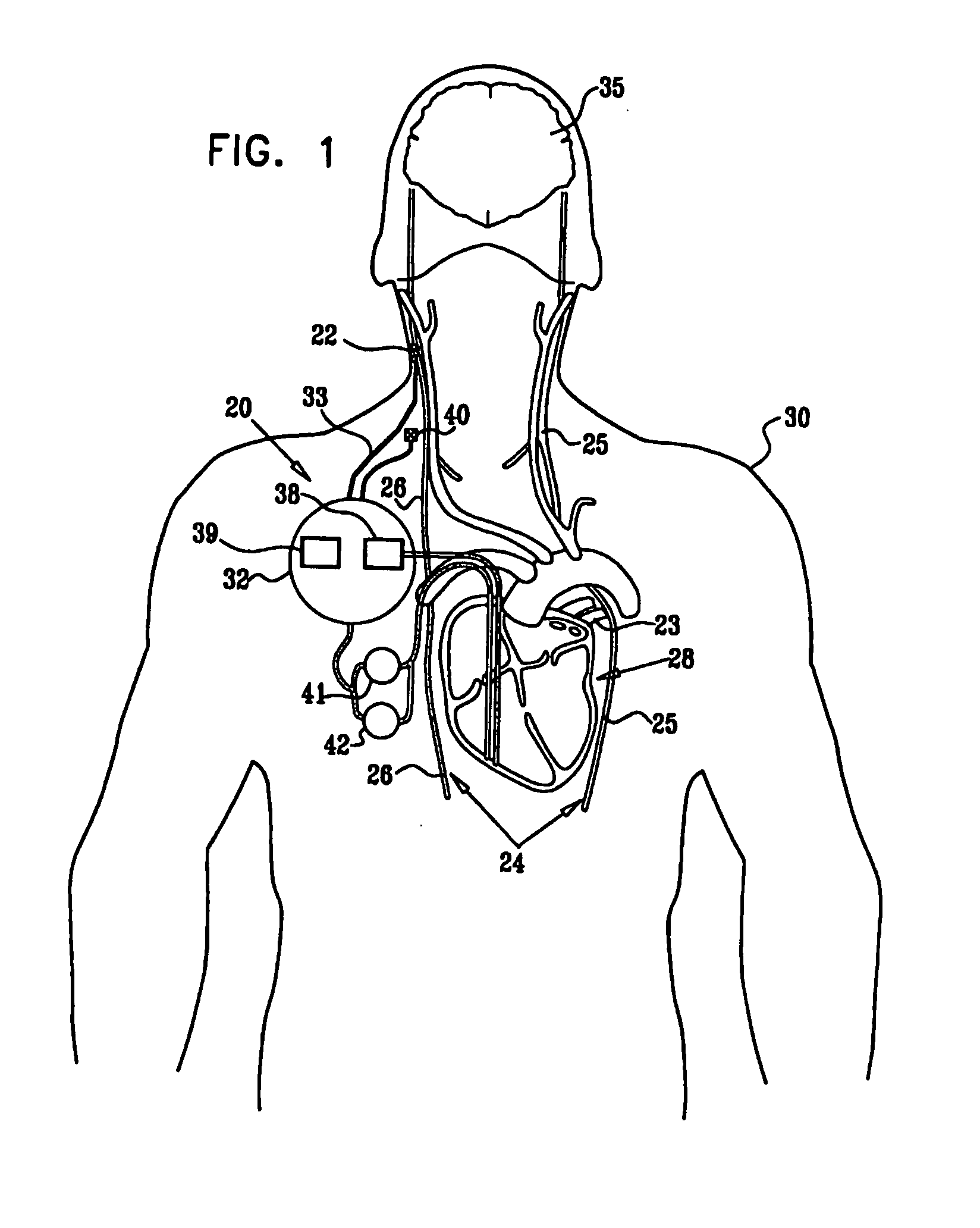
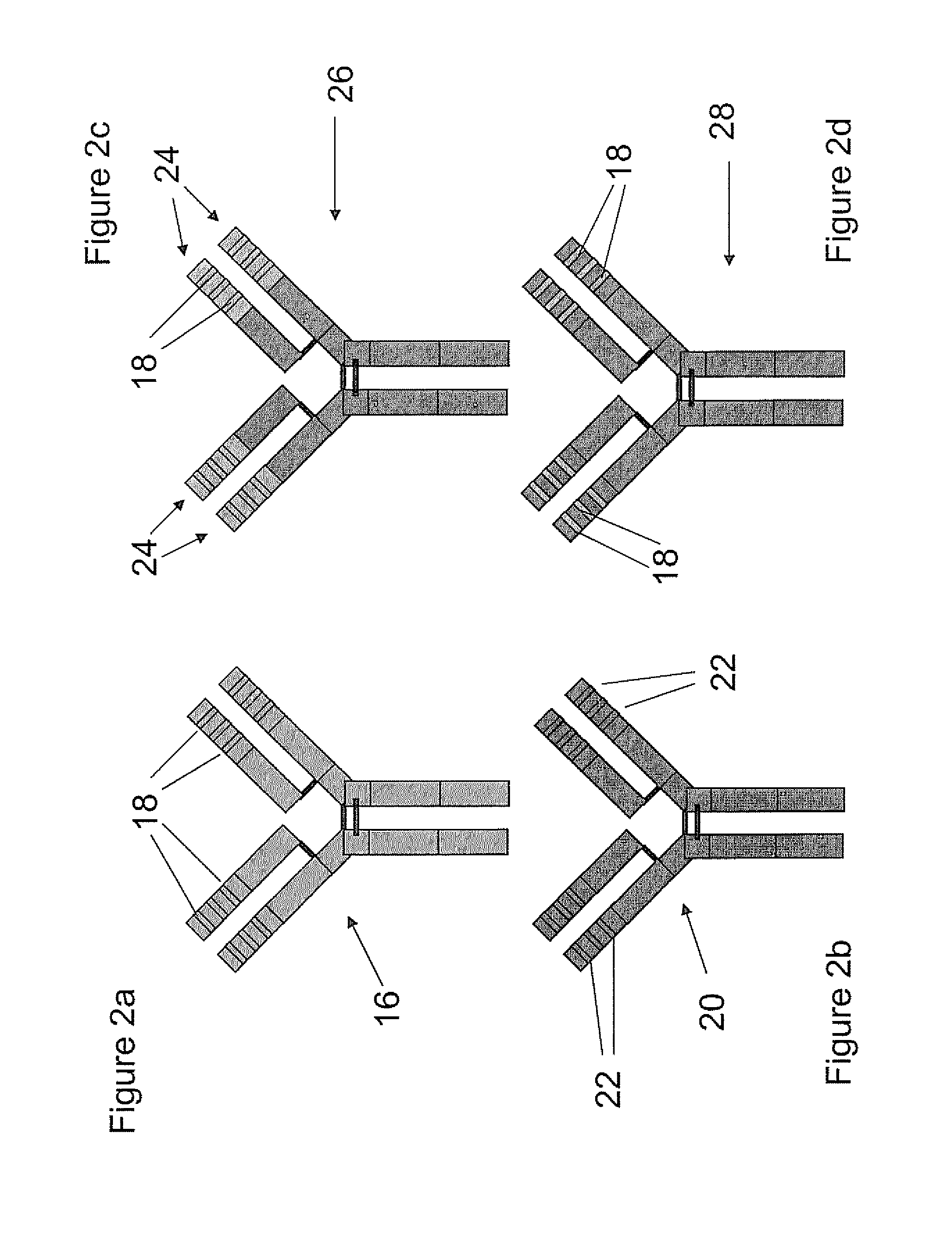
Combined parasympathetic stimulation and drug therapy
InactiveUS20080125843A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsMedical devicesNervous systemMyelitis
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
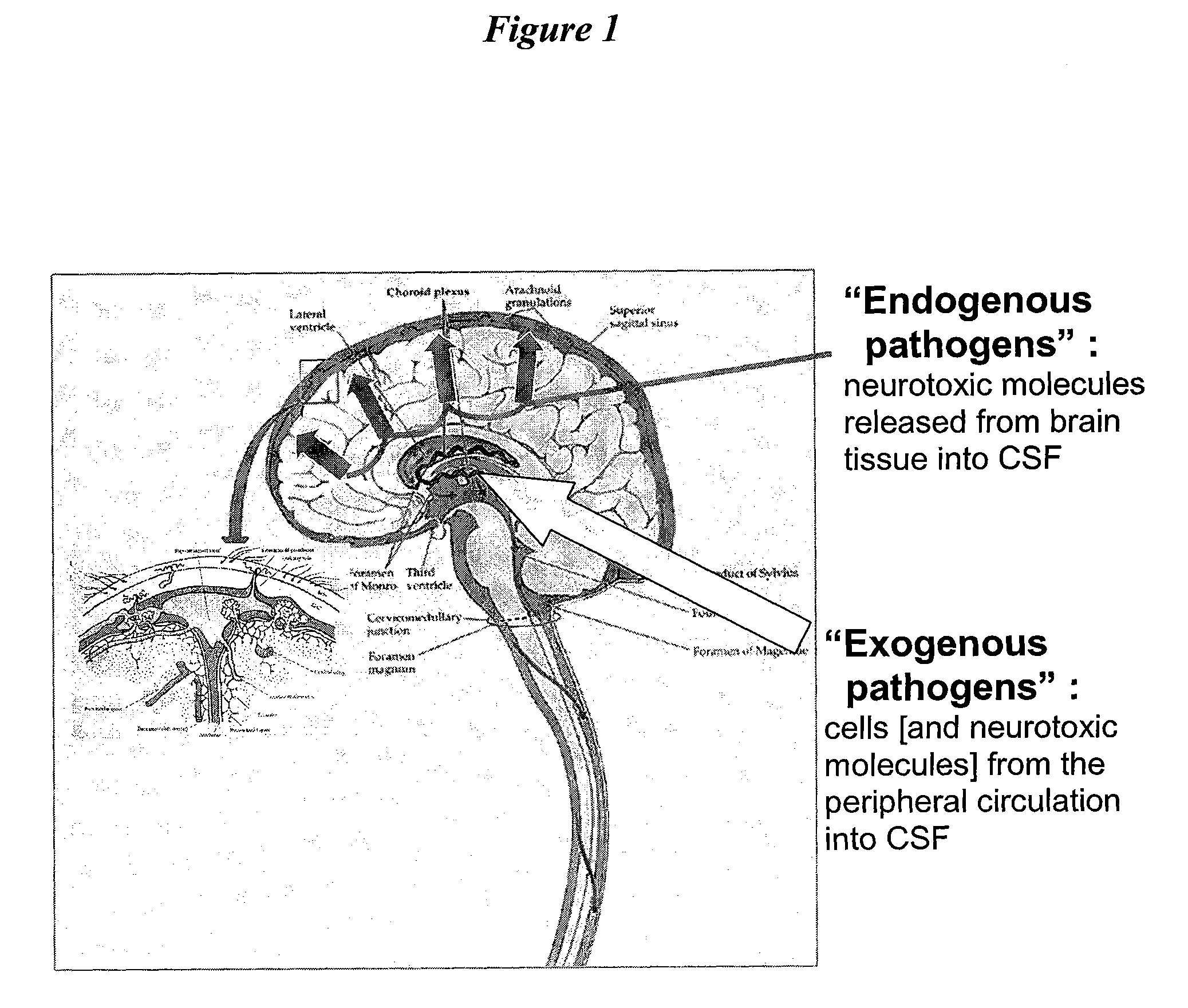
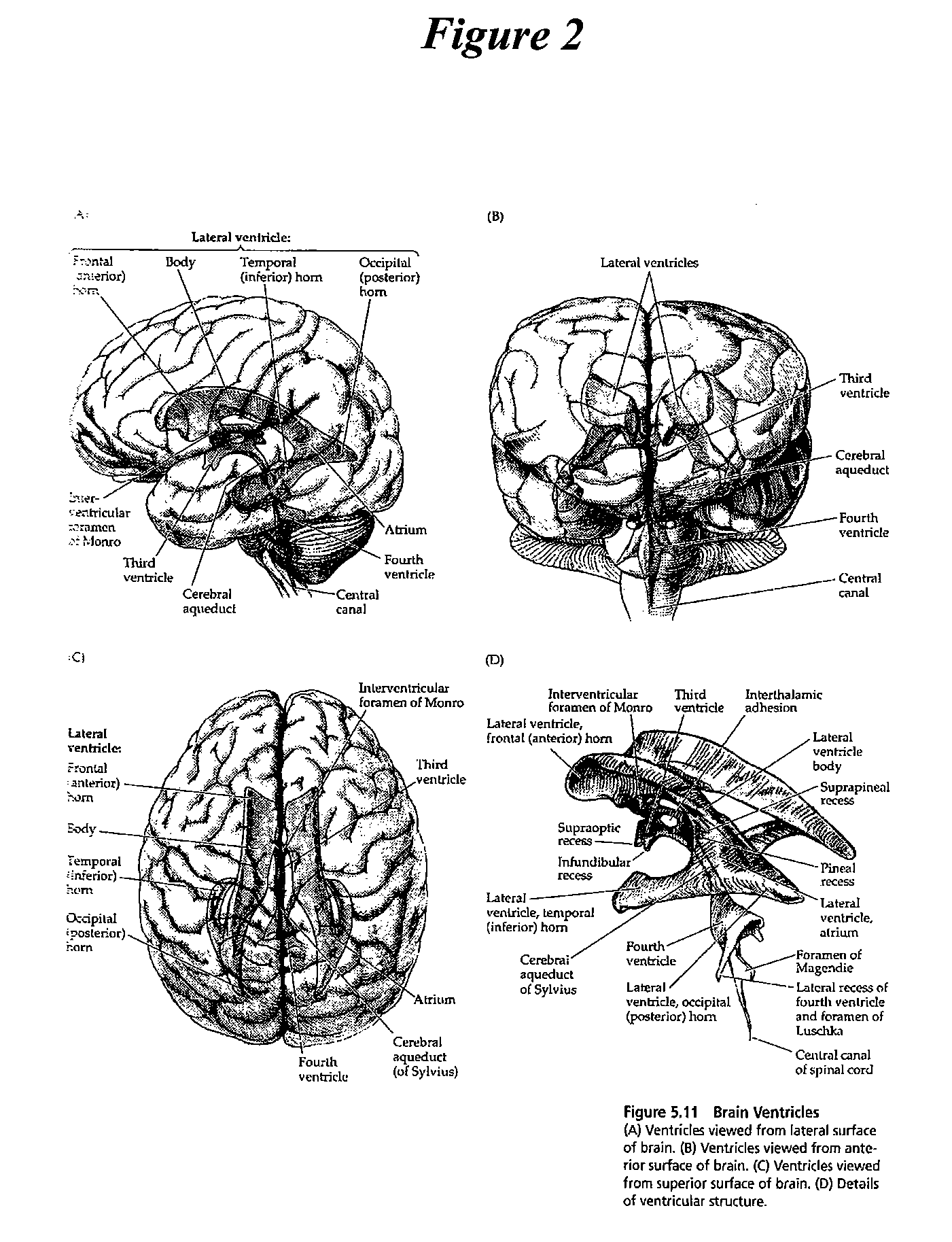
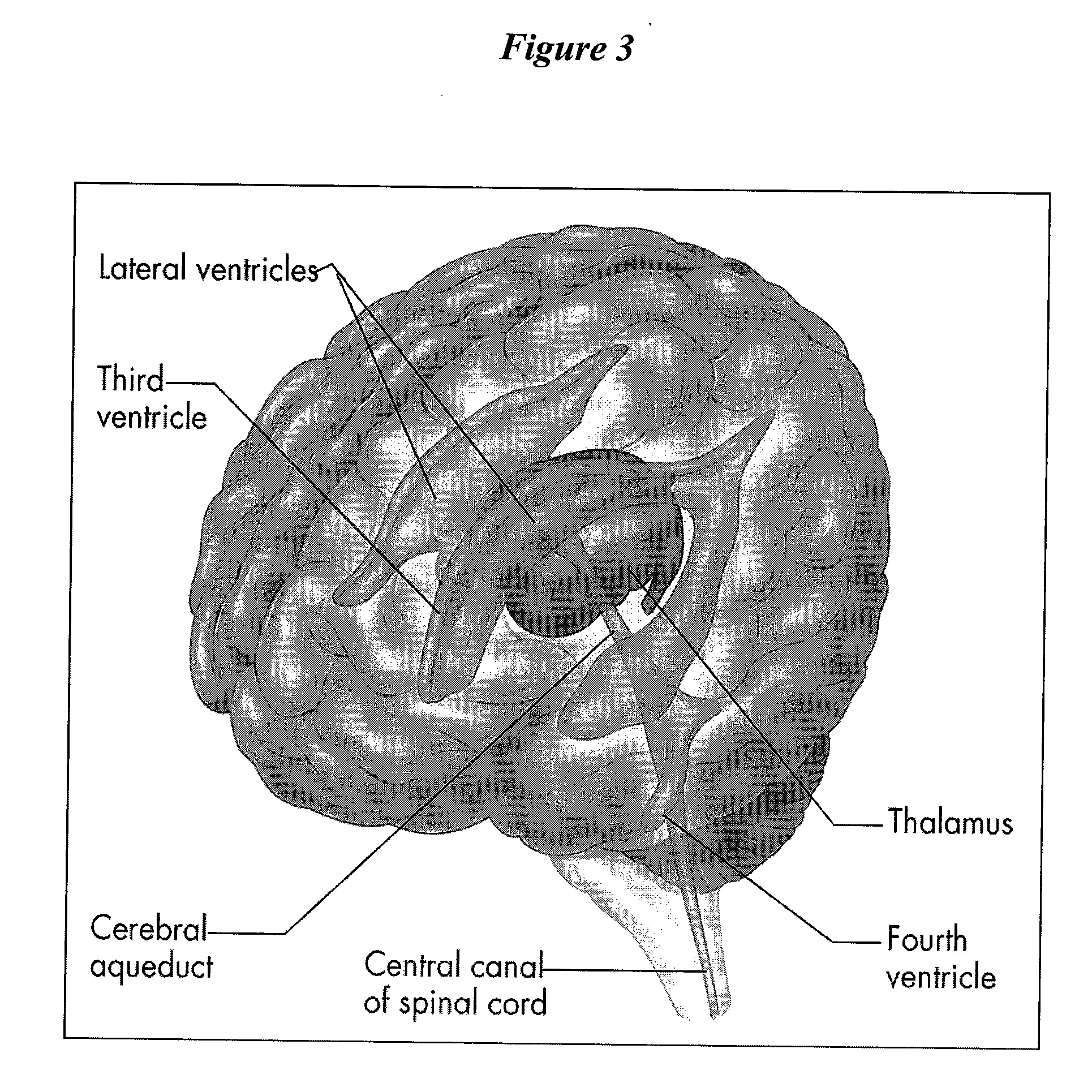
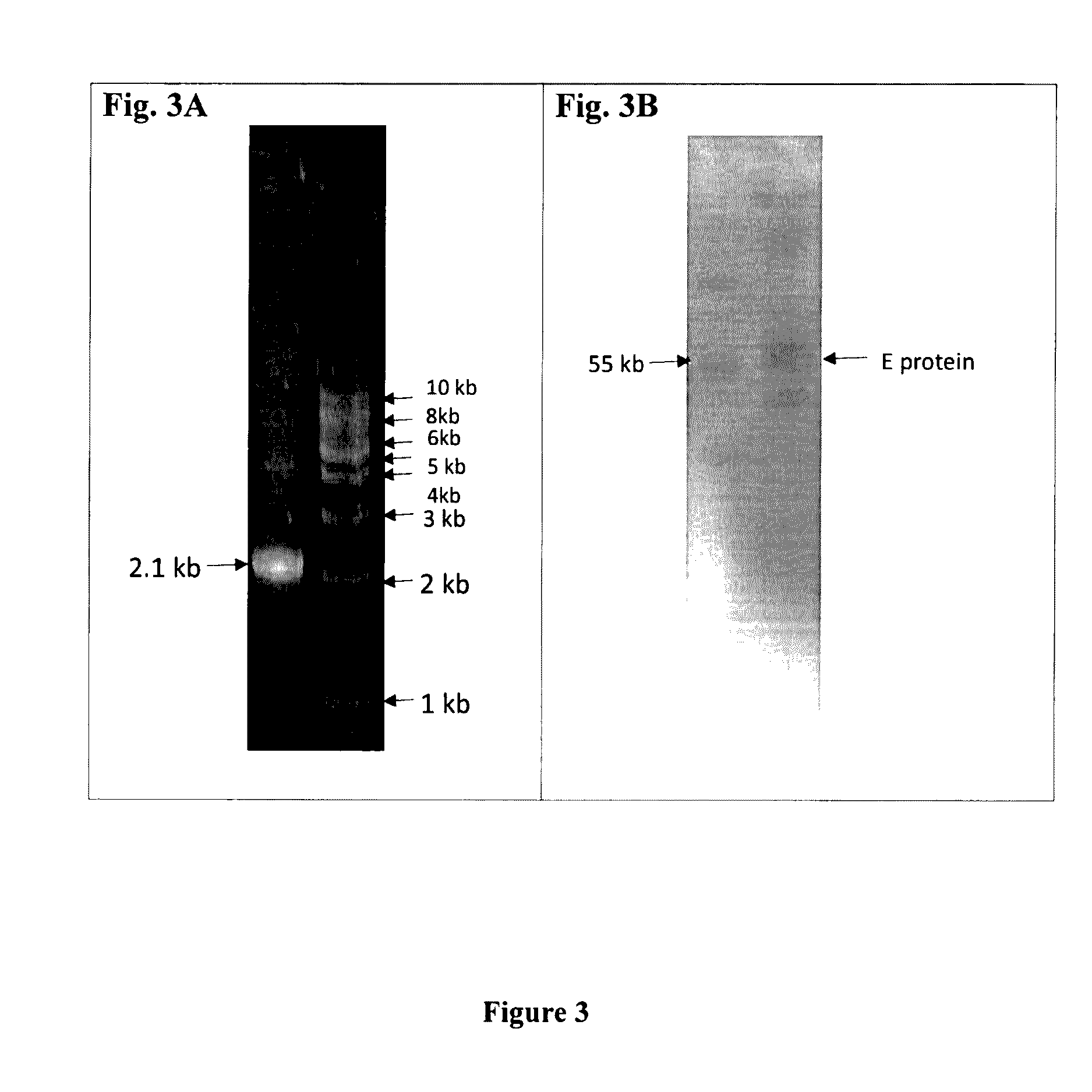
Cerebrospinal Fluid Purification System
The present invention provides methods and systems for conditioning cerebrospinal fluid (CSF). The methods provide for efficiently removing target compounds from CSF. The systems provide for a multilumen flow path and exchange of a majority volume portion of CSF in the CSF space. The removal and / or delivery of specific compounds can be tailored to the pathology of the specific disease. The removal is targeted and specific, for example, through the use of specific size-exclusion thresholds, antibodies against specific toxins, and other chromatographic techniques, as well as delivery and / or removal of targeted therapeutic agents. The invention finds use as a diagnostic, therapeutic and drug delivery platform for a variety of diseases affecting the CNS by accessing the CSF space. Exemplified disease conditions treatable by the present CSF processing systems and methods include, but are not limited to: Cerebral Vasospasm, Guillain Bane Syndrome, illustrating multi-lumen lumbar approach Alzheimer's, Parkinson's, Huntington's, Multiple Sclerosis, Amyotrophic Lateral Sclerosis, Spinal Cord Injury, Traumatic Brain Injury, Stroke, Cancer affecting the brain or spinal cord, Prion disease, Encephalitis from various causes, Meningitis from various causes, diseases secondary to enzymatic or metabolic imbalances, Biological Warfare, etc. For the first time, the present invention offers patients a disease-modifying, disruptive technology treatment platform that addresses the known disease pathogenesis of a number of neurologic conditions to which there are presently limited and ineffective treatment options.
Owner:NEUROFLUIDICS
Minimal-heart-rate reduction parasympathetic stimulation
ActiveUS20080275514A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsInternal electrodesMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Vaccine compositions
ActiveUS20170014502A1SsRNA viruses positive-sensePharmaceutical delivery mechanismPathogenImmunogenicity
The present disclosure provides vaccine compositions for prophylaxis and treatment of Zika virus infections comprising Zika virus antigens in immunogenic compositions, and in combination of Zika antigens with one or more arbovirus antigens such as Chikungunya virus and Japanese encephalitis virus antigens, methods of preparation and production of such compositions for use as vaccines for eliciting immune response in mammals against the above mentioned pathogens.
Owner:BHARAT BIOTECH INTERNATIONAL
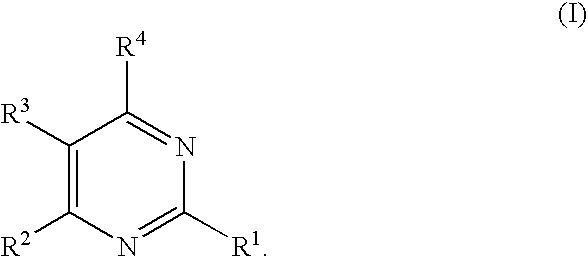
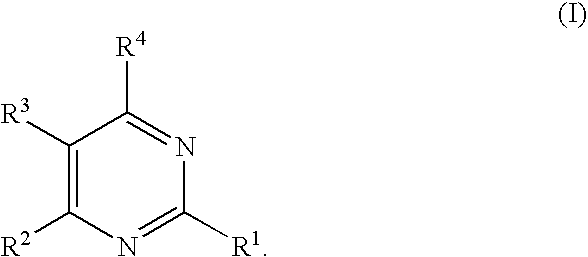
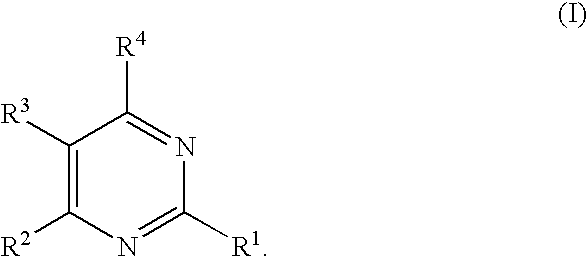
Substituted pyrimidines
Owner:MERCK SHARP & DOHME CORP
West nile vaccine
InactiveUS7153513B2Safe and effectiveSuitable for useAntibacterial agentsSsRNA viruses negative-senseEquidaeDisease
The present invention provides a safe and effective vaccine composition against West Nile virus disease. An immunogenically active component of West Nile virus or plasmid DNA, an adjuvant such as a metabolizable oil, and a pharmacologically acceptable carrier are formulated into an immunizing vaccine. The invention also provides a method for the prevention or amelioration of West Nile disease, such as encephalitis, in equidae by administering the vaccine composition herein set forth.
Owner:ZOETIS SERVICE LLC
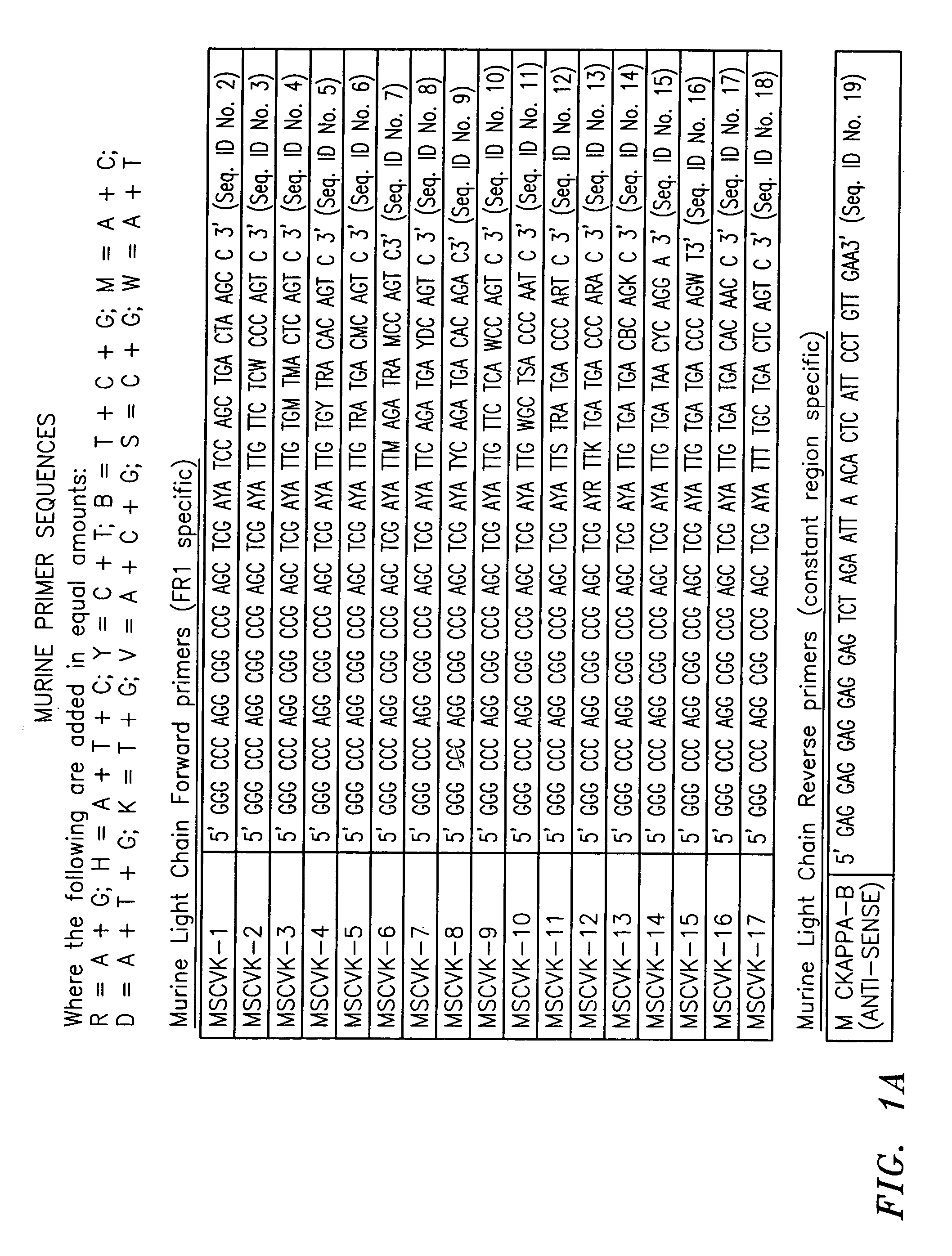
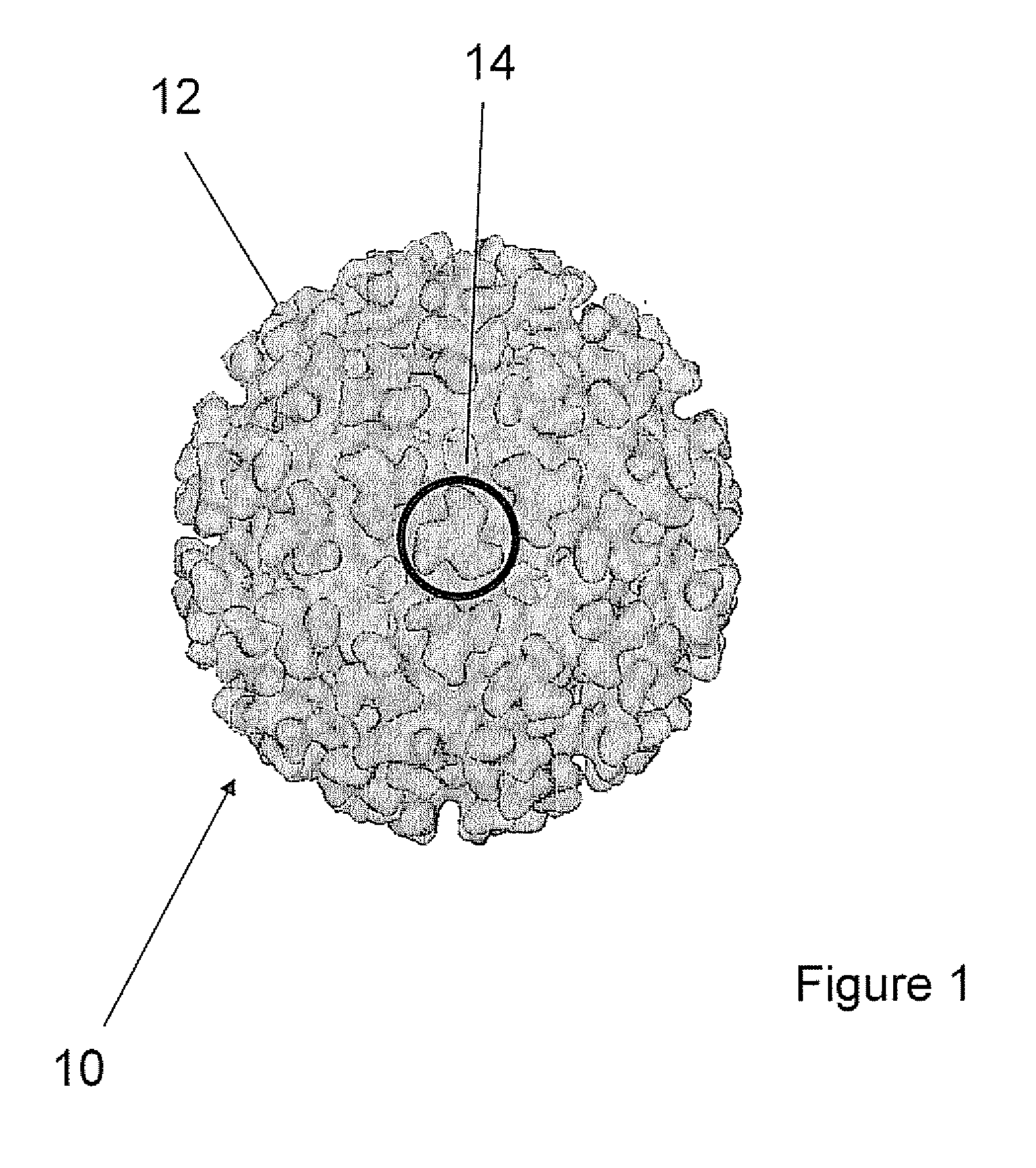
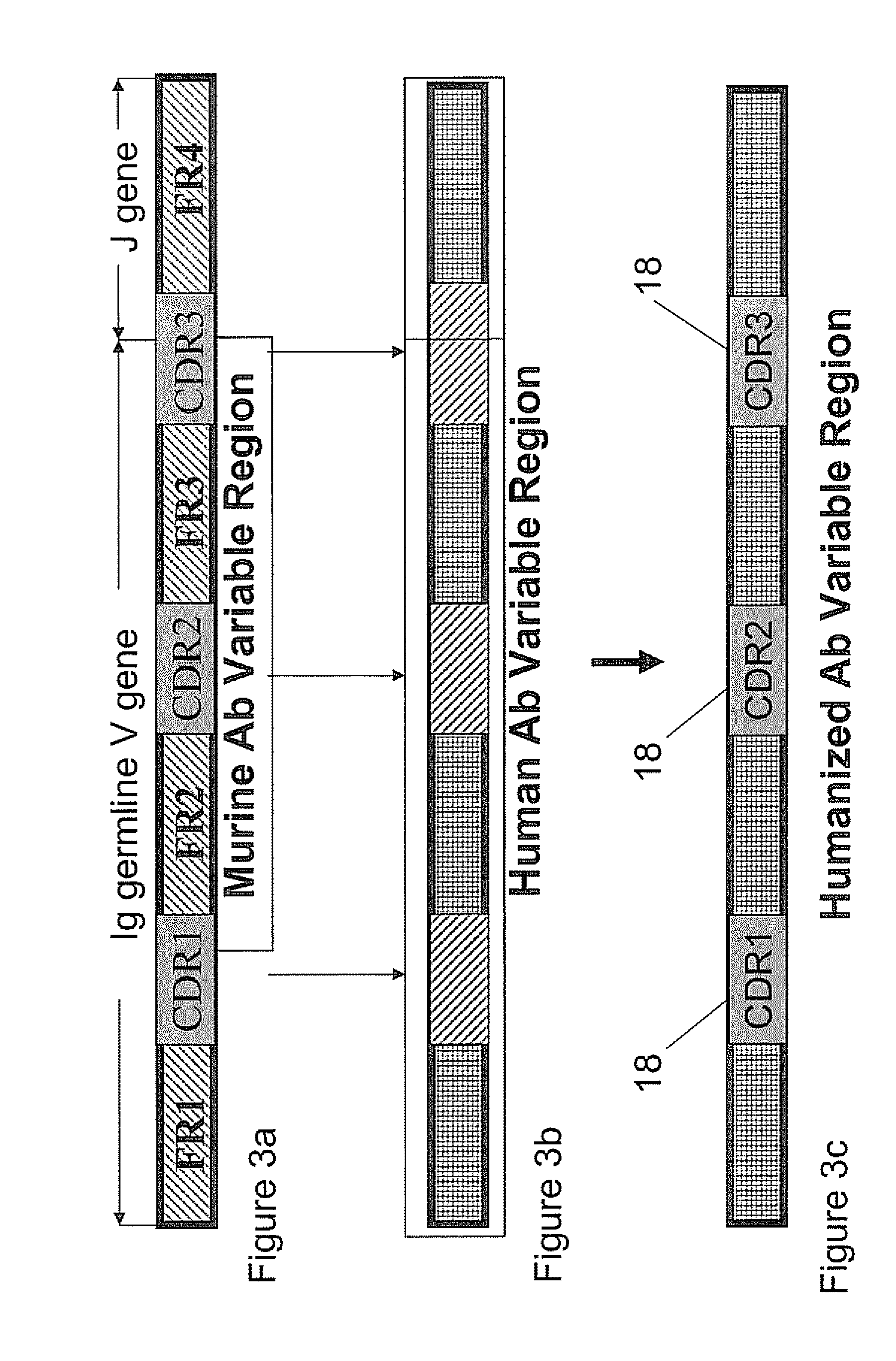
Humanized Anti-venezuelan equine encephalitis virus recombinant antibody
A CDR grafted humanized rAb comprises a human Ig framework having CDRs from murine mAb 1A4A1 VH and VL. DNA sequences and vectors incorporating such sequences are also provided as are pharmaceutical preparations and methods of using the humanized rAbs.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
hNT-neuron human neuronal cells to replace ganglion cells
Disclosed herein is the treatment of vision loss in a mammal by transplanting an effective amount of hNT-Neuron cells. The treatment can be accomplished by injecting the cells into the retinal area of the eye. Additionally, the cells can be injected into the visual cortex of the brain. Conditions to be treated are vision loss due to optic nerve damage, including glaucoma, optic nerve sheath meningioma and glioma, Graves' ophthalmopathy, benign or malignant orbital tumors, metastatic lesions, tumors arising from the adjacent paranasal sinuses or middle cranial fossa, giant pituitary adenomas, brain tumors or abscesses, cerebral trauma or hemorrhage, meningitis, arachnoidal adhesions, pseudotumor cerebri, cavernous sinus thrombosis, dural sinus thrombosis, encephalitis, space-occupying brain lesions, severe hypertensive disease or pulmonary emphysema.
Owner:LAYTON BIOSCI
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
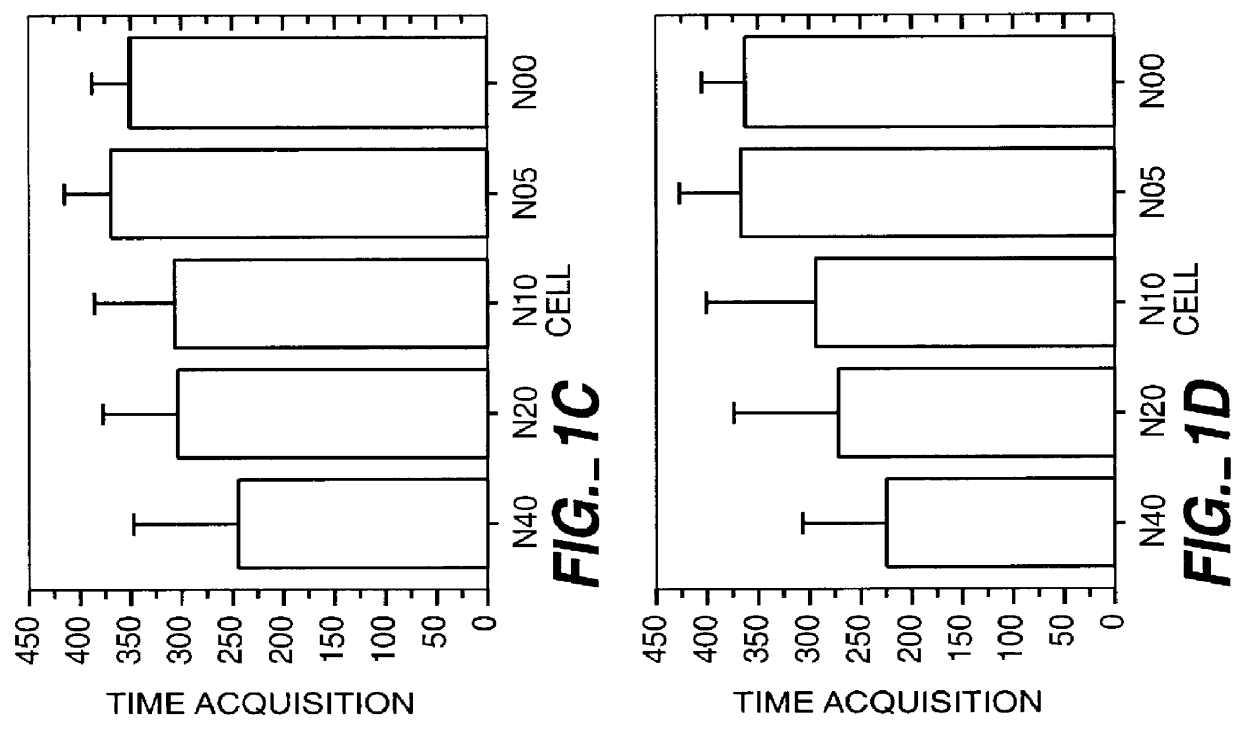
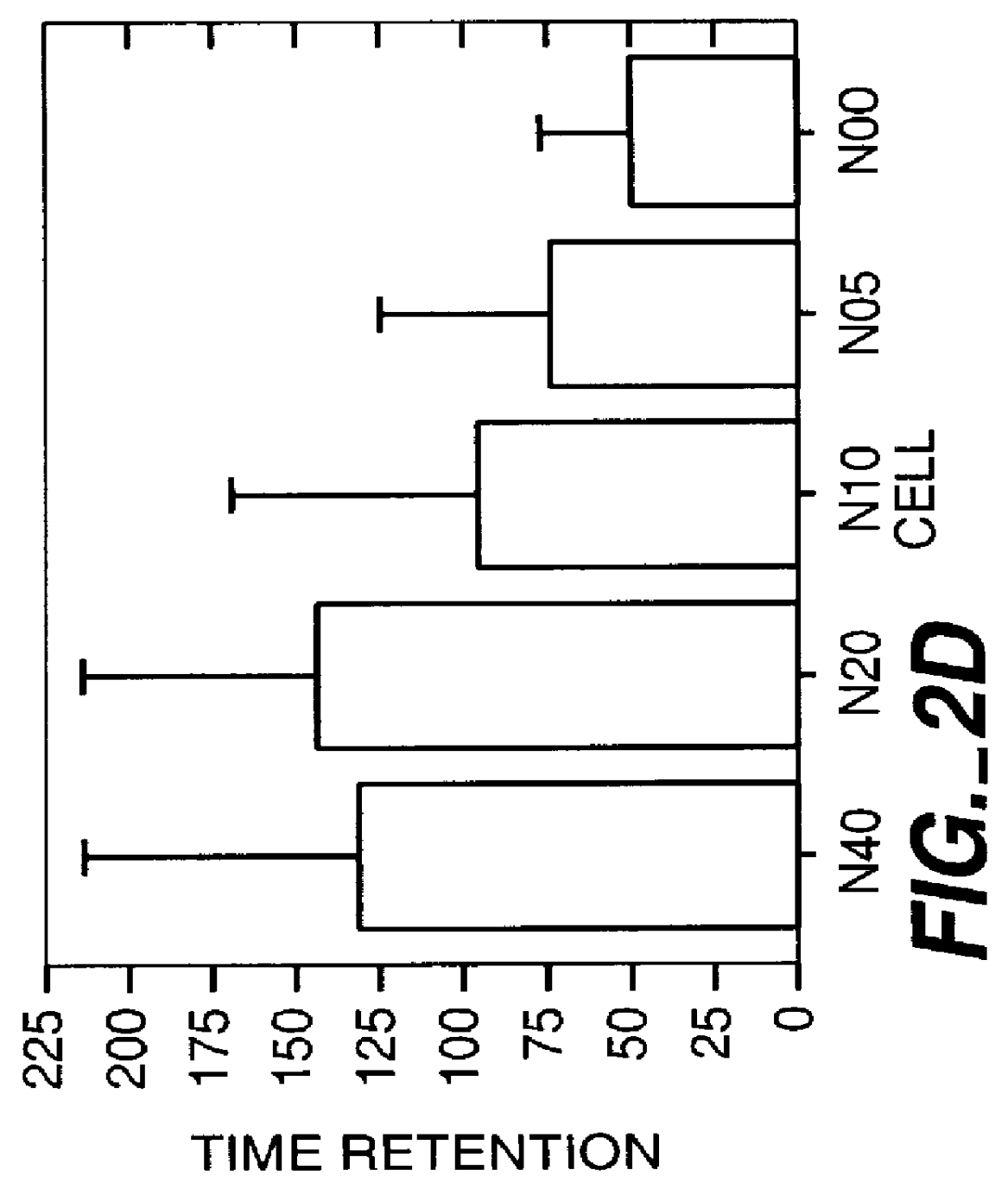
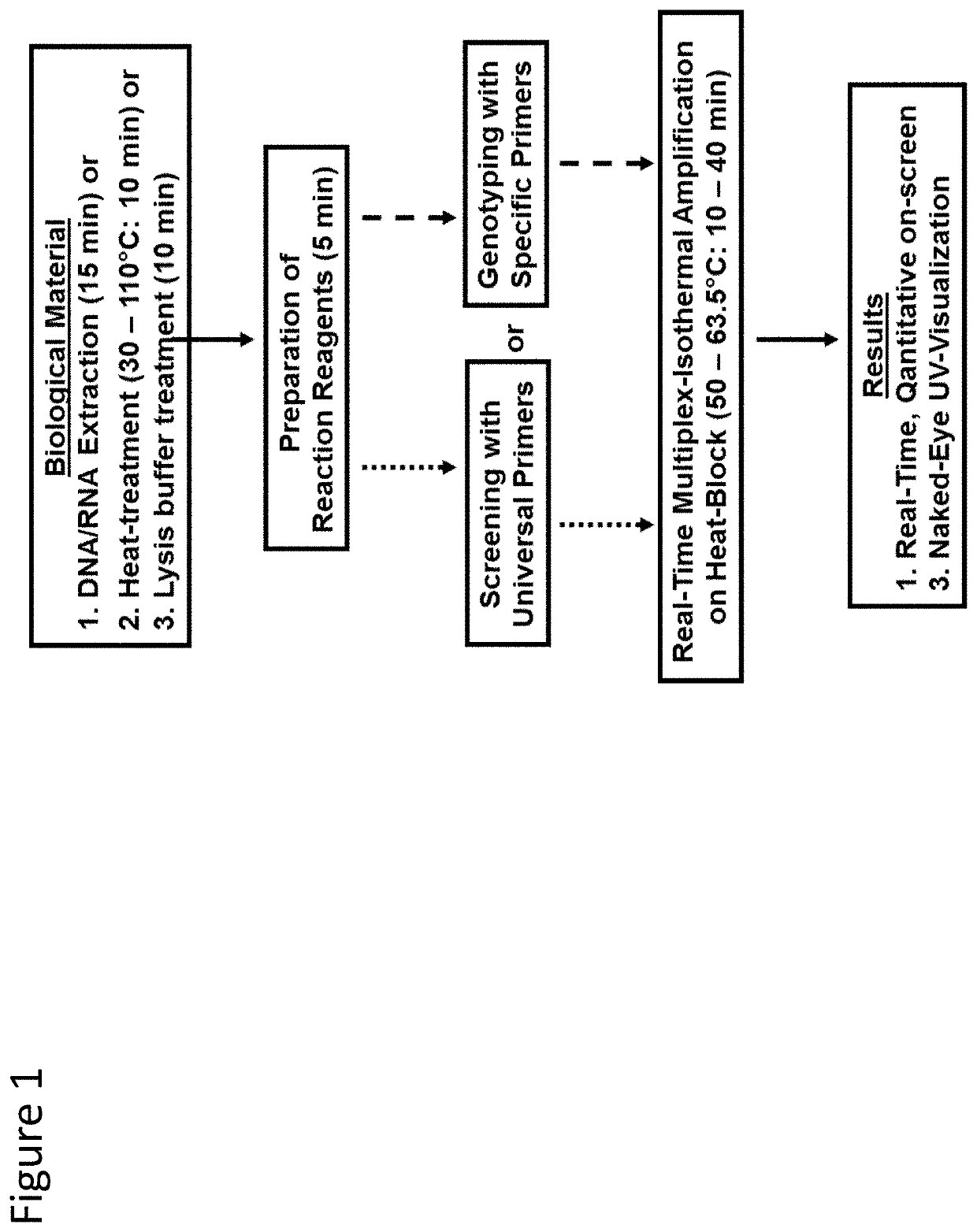
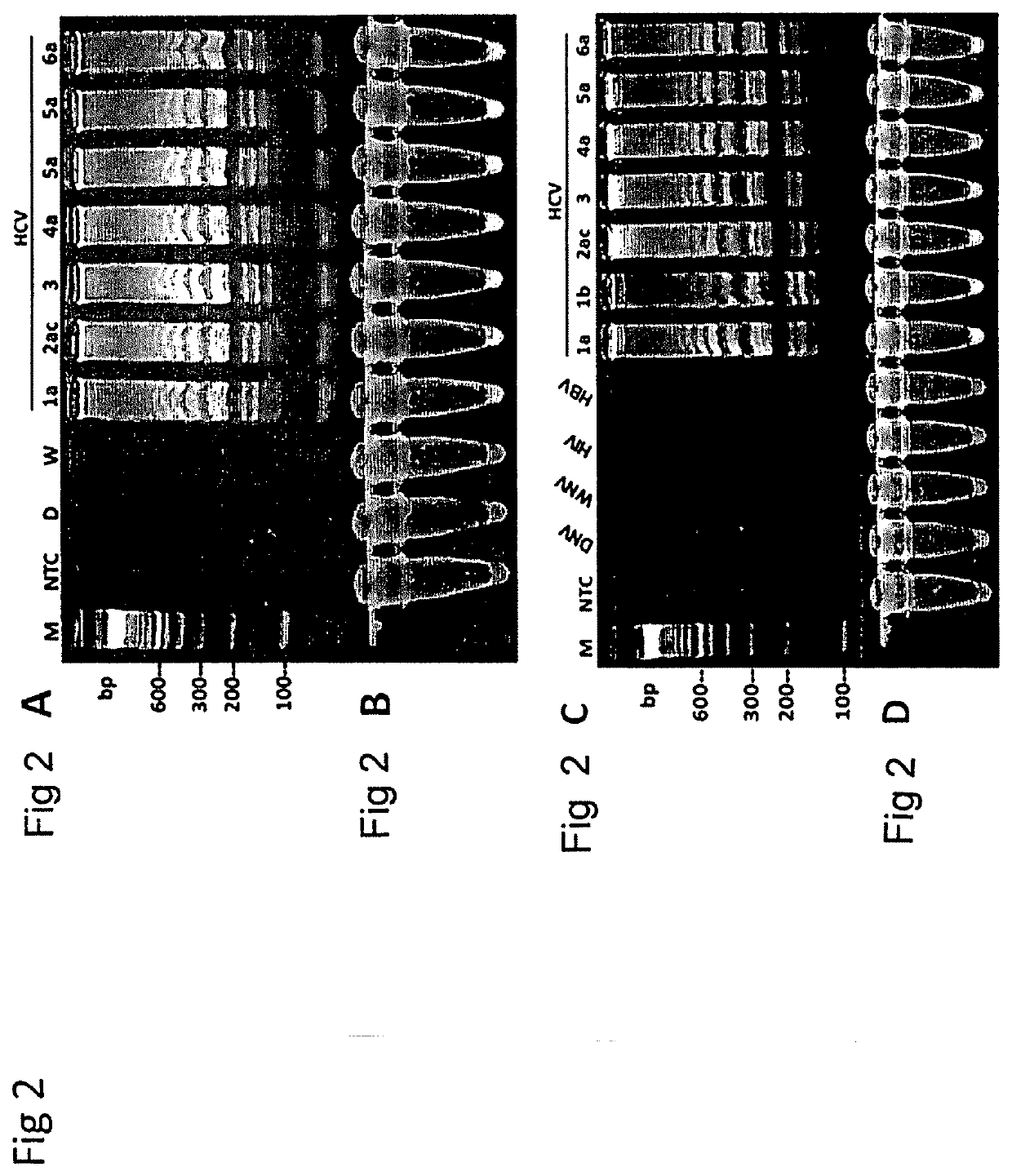
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
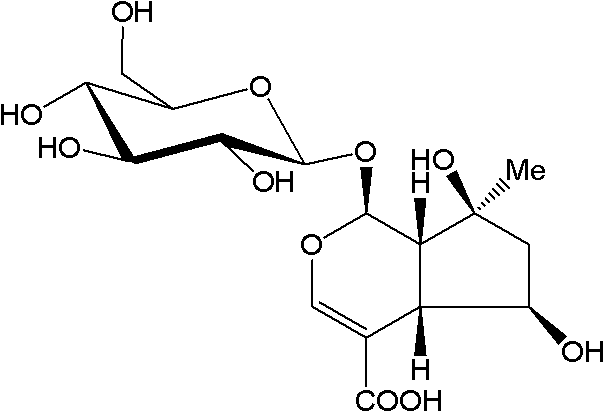
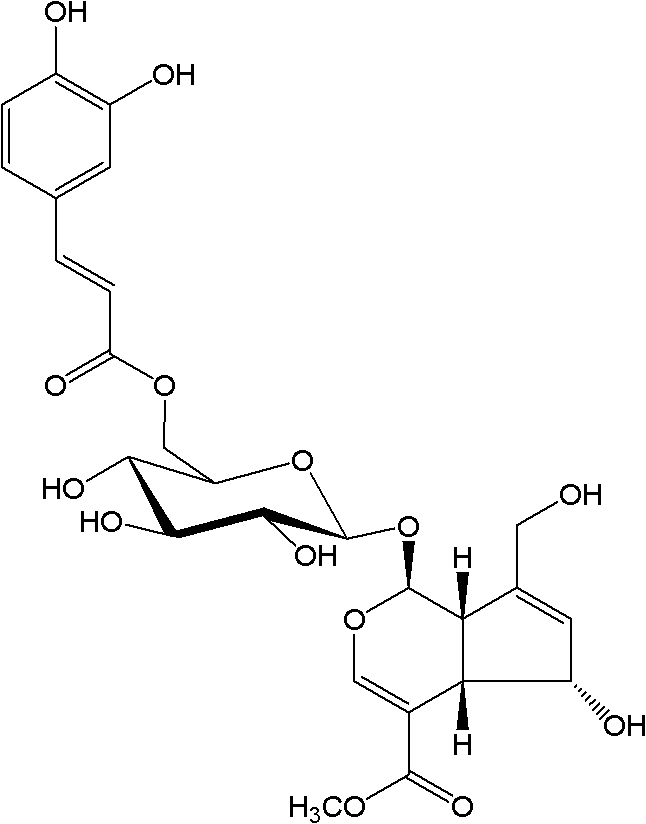
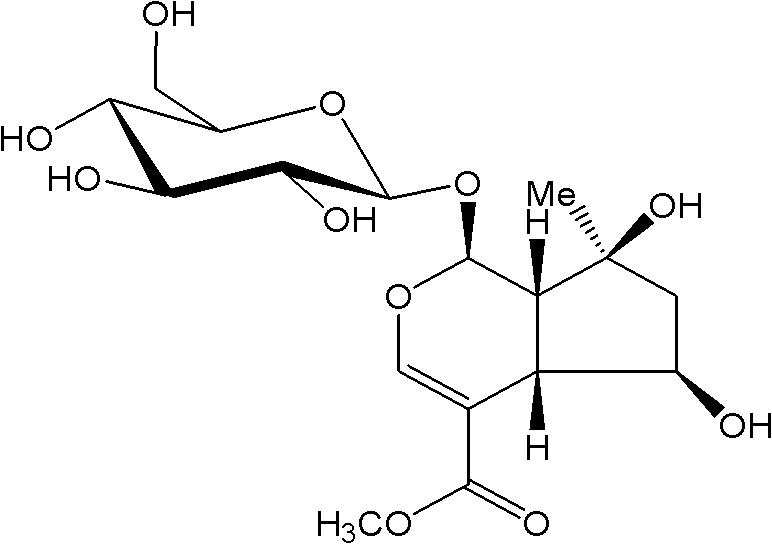
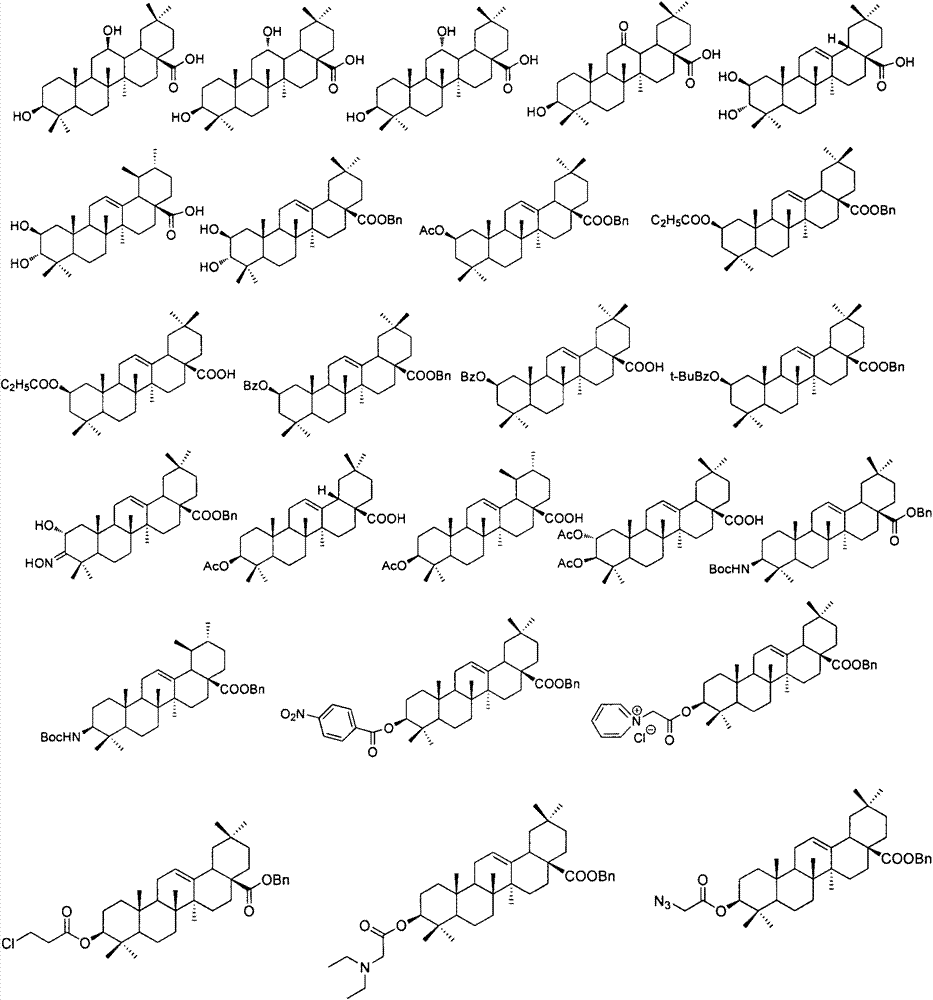
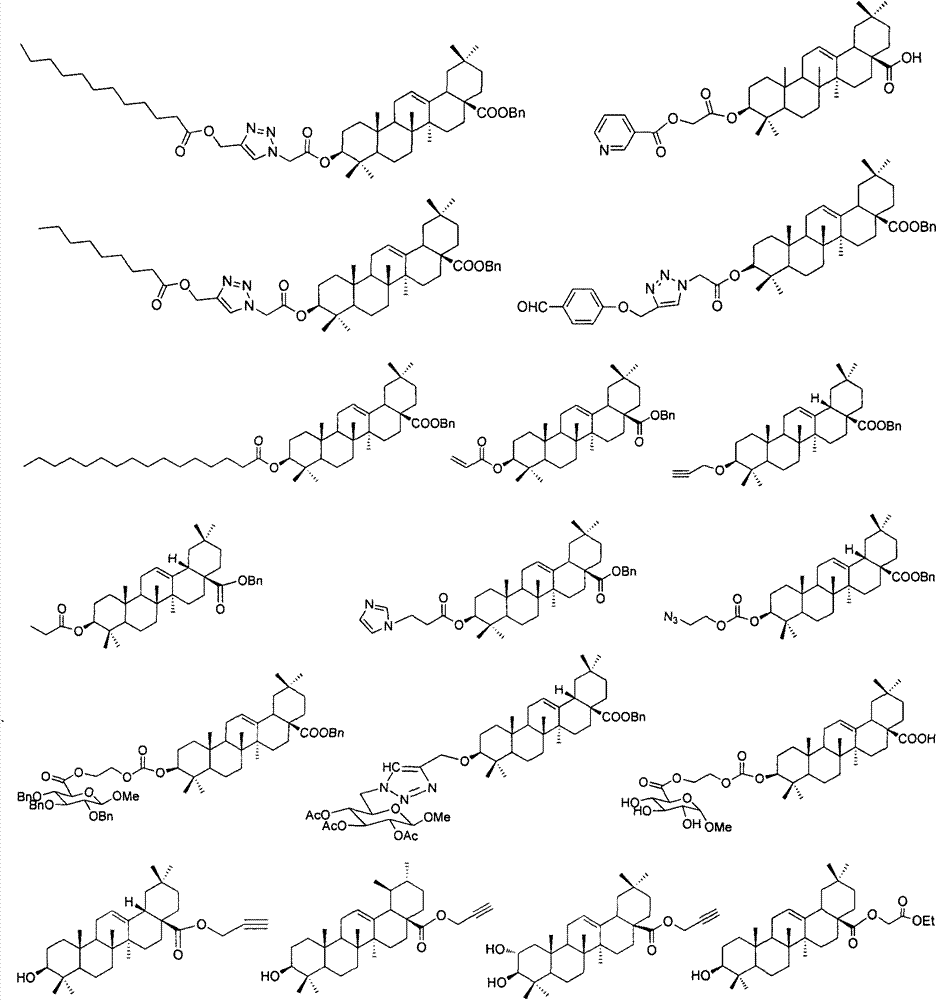
Substituted furo[2,3-b]pyridine derivatives
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
Artificial cpg single-stranded oligodeoxynucleotide and antiviral use thereof
The present invention provides a series of artificial CpG-containing single-stranded oligodeoxynucleotides (ODNs), each of which is consisted of single-stranded oligodeoxynucleotide DNA molecule containing one or more CpG(s), wherein said ODNs can stimulate human peripheral blood mononuclear cell (PBMC) to produce antiviral substances. These ODNs can protect the cells against the attack from virus, wherein said virus is preferably selected from the group consisted of influenza virus and single-stranded positive strand RNA virus such as SARS virus, hepatitis C virus, dengue virus and Japanese encephalitis virus. Moreover, the antiviral use of artificial CpG ODNs and its use for treating and preventing viral infection are also provided.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
Hsv-1 and hsv-2 vaccines and methods of use thereof
ActiveUS20110177125A1Reduce morbidityReducing pathogenesisSenses disorderNervous disorderVaccinationGenital ulcer
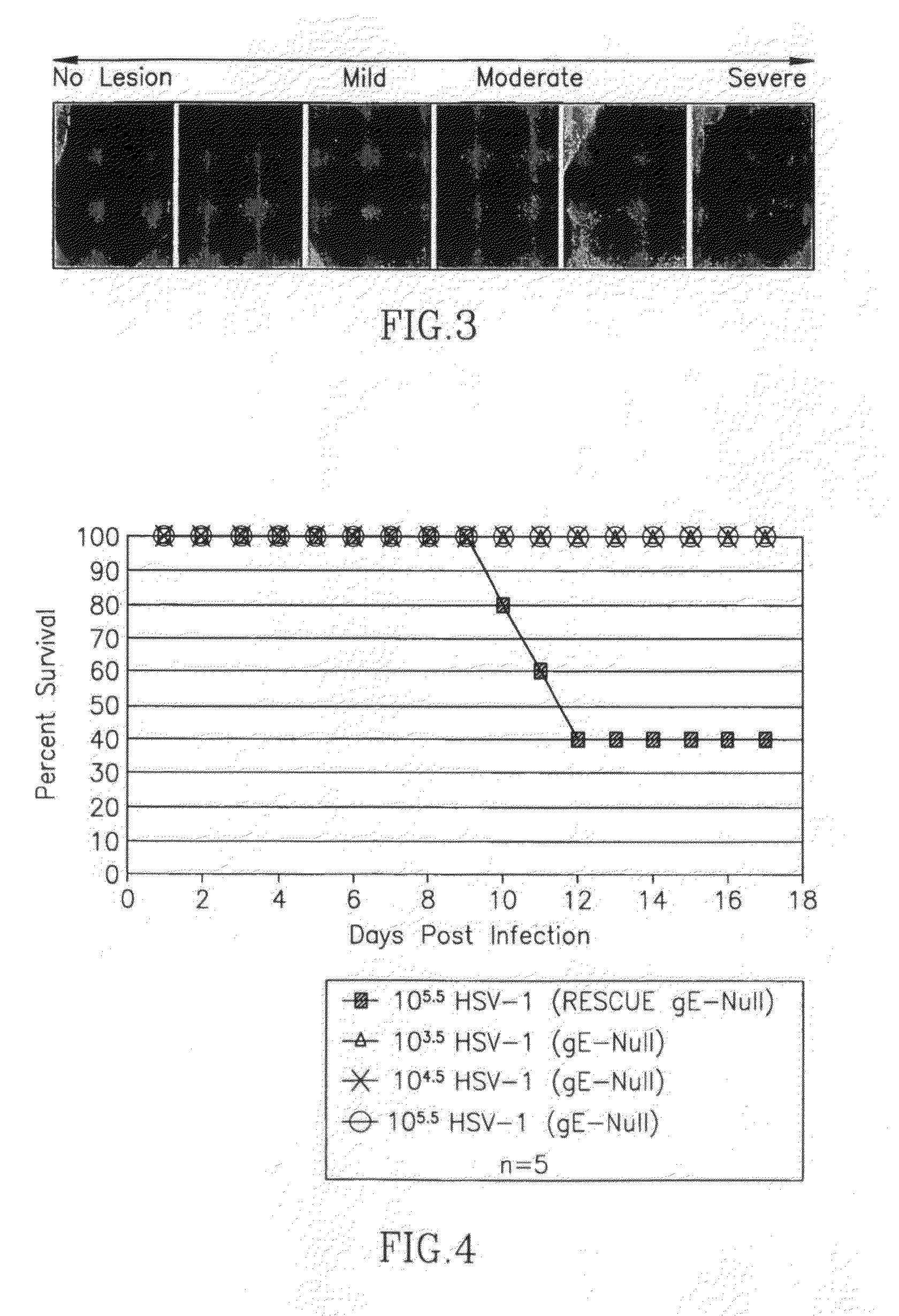
This invention provides methods of vaccinating a subject against a Herpes Simplex Virus (HSV) infection and disorders and symptoms associated with same, and impeding, inhibiting, reducing the incidence of, and suppressing HSV infection, neuronal viral spread, formation of zosteriform lesions, herpetic ocular disease, herpes-mediated encephalitis, and genital ulcer disease in a subject, comprising the step of contacting the subject with a mutant strain of the HSV, containing an inactivating mutation in a gene encoding a gE, gl, Us9, or other proteins.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
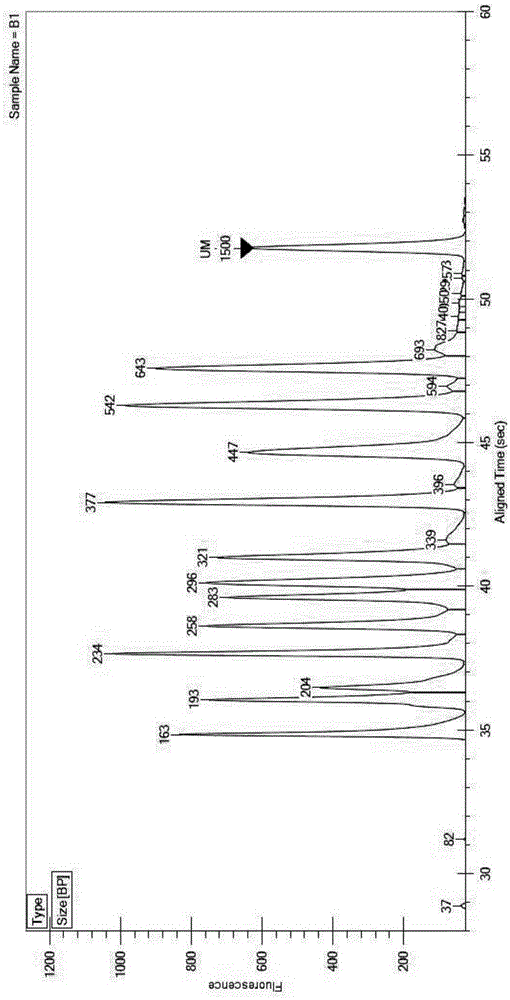
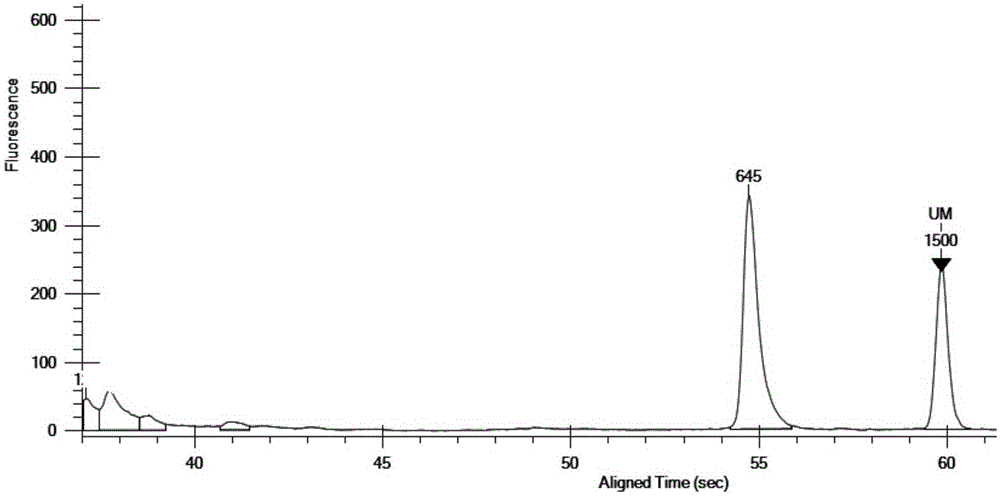
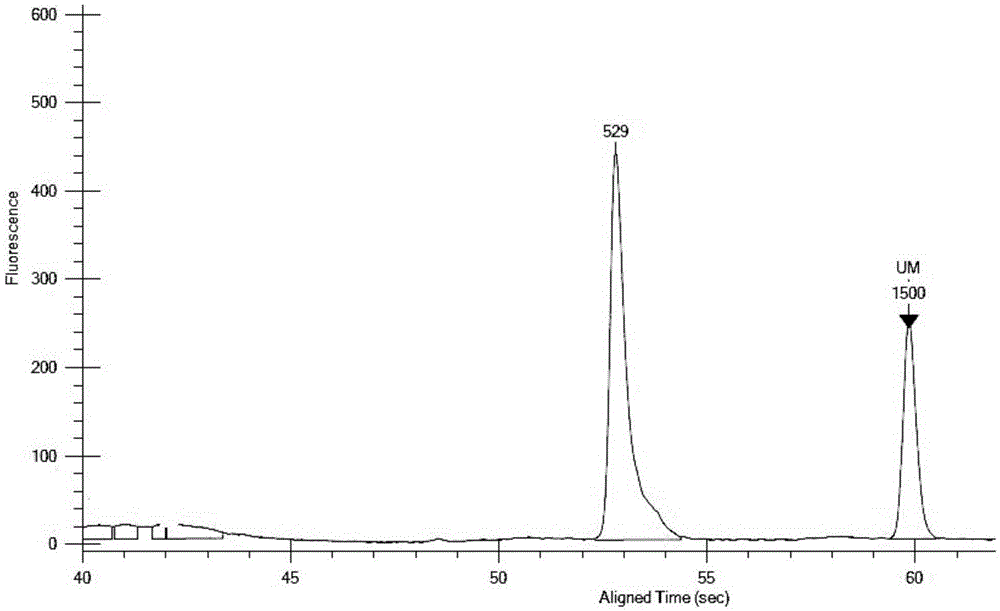
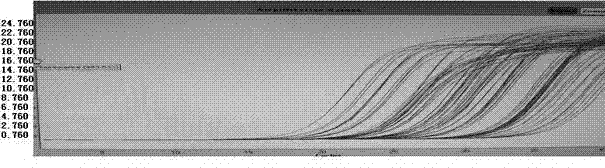
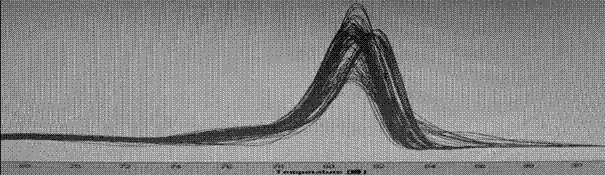
Multiplex PCR detection kit for 12 encephalitis virus nucleic acids and application thereof
ActiveCN105385787ASuitable for routine testingSuitable for emergency testingMicrobiological testing/measurementMicroorganism based processesEncephalitisMultiplex pcrs
The invention discloses a multiplex PCR detection kit for 12 encephalitis virus nucleic acids and application thereof. The multiplex PCR detection kit comprises primers used for amplifying specific gene sites of the following 12 encephalitis virus: JeV, HTLV1, WNV, HSV1, HSVII, HHV6, JCV, HTLV2, BBF, HCMV, EBV and VZV. The detection kit has the advantages of capacity of realizing multiplex detection, high sensitivity and rapidness and convenience in usage. Moreover, specific primer sequences are employed to guarantee reliability of detection results. A detection method is simple to operate and labor-saving and time-saving; detection flux is great, and cost for reagent consumables is low; nucleic acids extracted from encephalitis pathogen samples can be directly detected; and requirements on a detection platform and manual technique levels are low, so the method can be extensively popularized in conventional detection.
Owner:NANJING MOKOBIO BIOTECH +1
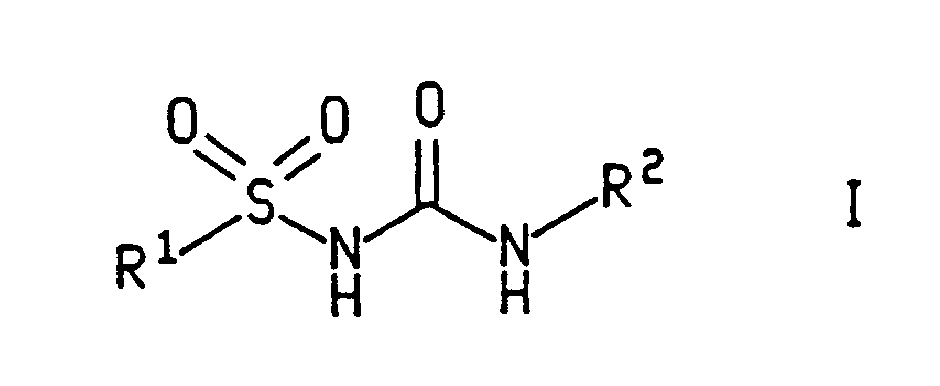
Sulfonyl urea derivatives and their use in control of interleukin-1 activity
A compound of formula (I) wherein R<1> and R<2> are as defined in the description, R<2> being an aromatic group, useful in the treatment and condition selected from the group consisting of the group meningitis and salpingitis, septic shock, disseminated intravascular coagulation, and / or adult respiratory distress syndrome, acute or chronic inflammation, arthritis, cholangitis, colitis, encephalitis, endocarditis, glomerulonephritis, hepatitis, myocarditis, pancreatitis, pericarditis, reperfusion injury, vasculitis, acute and delayed hypersensitivity, graft rejection, and graft-versus-host disease, auto-immune diseases including Type 1 diabetes mellitus and multiple sclerosis, periodonate diseases, interstitial pulmonary fibrosis, cirrhosis, systemic sclerosis, keloid formation tumors which produce IL-1 as an autocrine growth factor, cachexia, Alzeimer's disease, percussion injury, depression, atherosclerosis, osteoporosis in a mammal, including a human.
Owner:PFIZER INC
Anti-herpes simplex virus I-form medicament composition and uses thereof
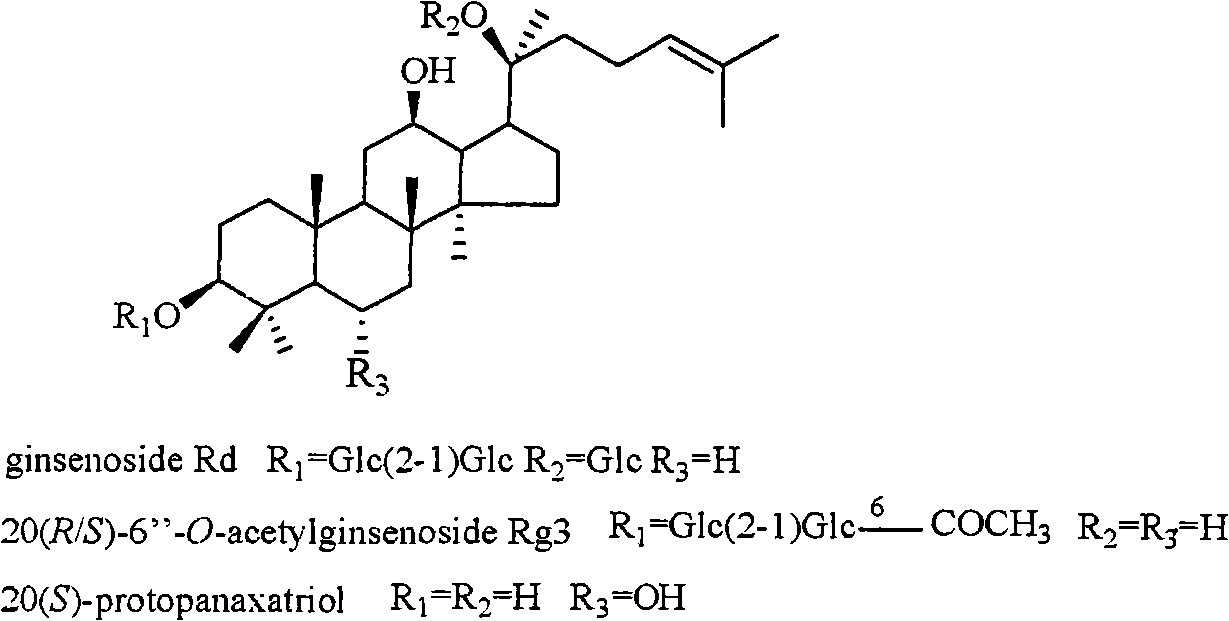
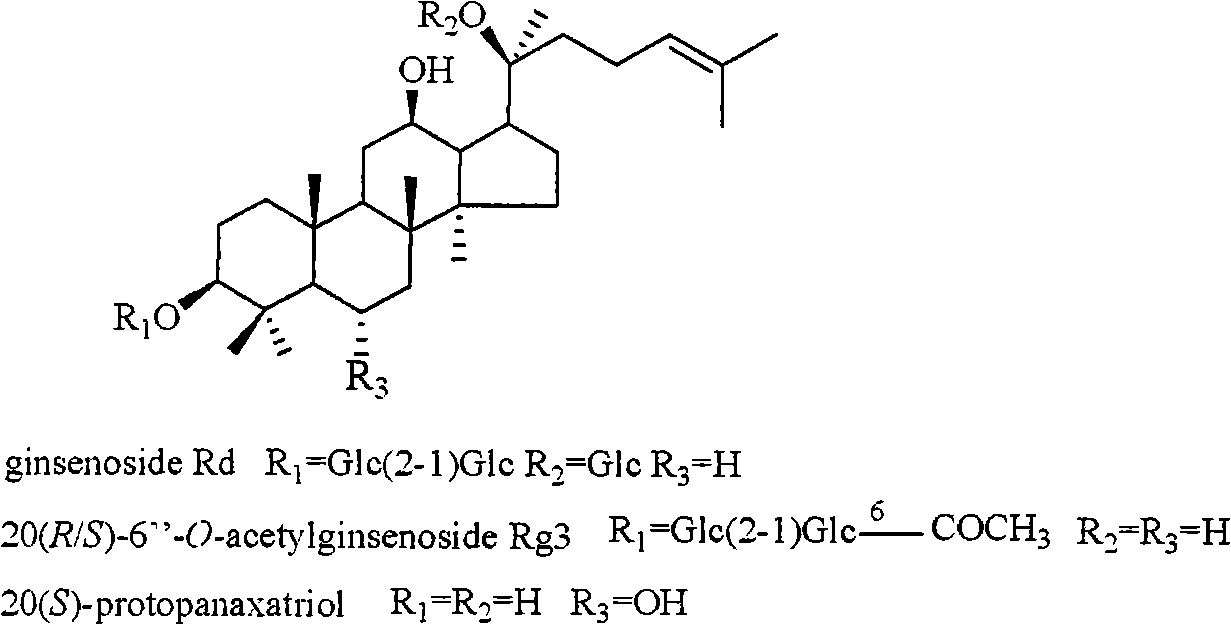
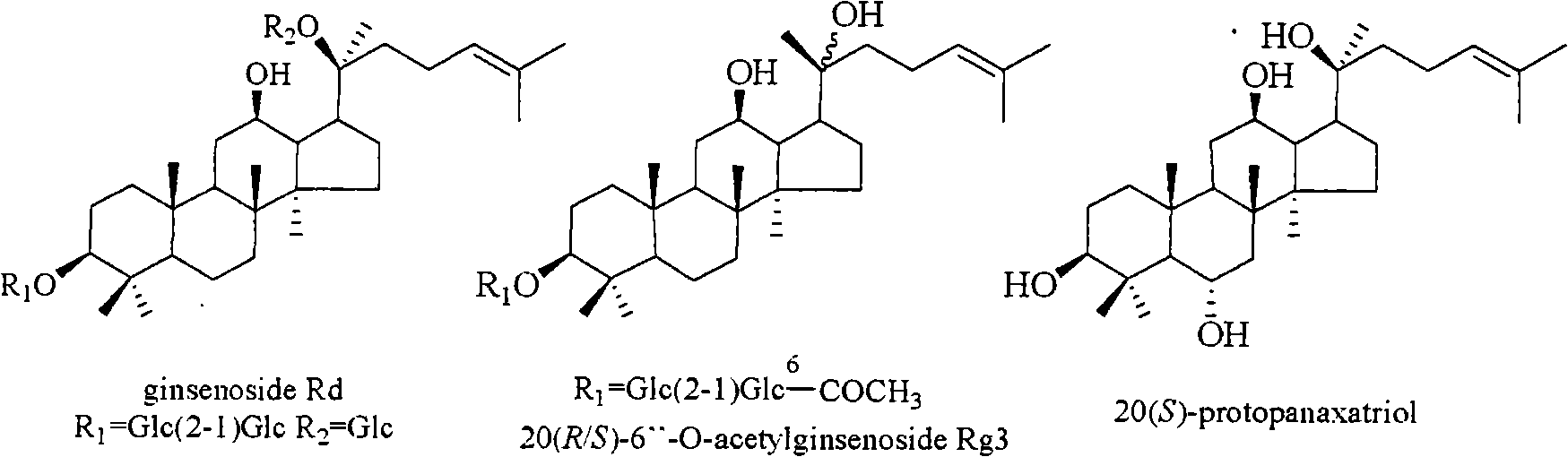
InactiveCN101322714AOrganic active ingredientsDigestive systemStructural formulaHerpes simplex disease
The invention relates to a pharmaceutical composition with dammarane-type tetracyclic triterpene compound as an active component and an application thereof to the pharmaceutical field. Panax notoginseng is separated to obtain three dammarane-type tetracyclic triterpene compounds as shown in the following structural formula, in vitro pharmacological experiments show that the pharmaceutical composition has good in vitro inhibitory activity for herpes simplex virus type I and can be applied to the preparation of medicines for resisting herpes simplex virus type I and is used for treating herpetic keratitis, encephalitis, pneumonia, ulcerative stomatitis, blister and the like caused by herpes simplex virus type I.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Medicine for treating dementia and preparing method thereof
InactiveCN1552415ASmall toxicityToxic and side effectsNervous disorderUnknown materialsSalvia miltiorrhizaRhizome
A Chinese medicine for treating chronic dementia, sanile dementia, vanular dementia and AD is prepared from 7 Chinese-medicinal materials including astragalus root, red sage root, Chuan-xiong rhizome, borneol, etc. Its advantages are high curative effect and quickly taking its effect.
Owner:TIANJIN TASLY PHARMA CO LTD
Compounds having virus resistance and composition thereof
ActiveCN103211829ATherapeuticPreventiveOrganic active ingredientsAntiviralsHuman papillomavirusViral infectious disease
The invention discloses compounds having virus resistance and a composition thereof. The composition comprises one or more of the 13 compounds comprising 6'-O-caffeoyl deacetyl asperulosidic acid methyl ester. The compounds having virus resistance and the composition thereof can be extracted from plants and can also be prepared by synthesis. The composition comprising the compounds having virus resistance can prevent and treat viral infection-caused diseases such as influenza, myocarditis, conjunctivitis, viral pneumonia, encephalitis, leukemia, hepatitis B, hepatitis C, AIDS, genitourinary system infection, EB virus infection, human papillomavirus infection and cytomegalovirus infection. The composition can be prepared into an oral preparation, an injection, a spray, a subcutaneous injection and an anus suppository.
Owner:樊向德 +1
Pentacyclic triterpene enterovirus EV71 inhibitors, and medicinal compositions and medicinal use thereof
InactiveCN104840467AOrganic active ingredientsNervous disorderAcute hyperglycaemiaHand-foot-and-mouth disease
The invention relates to the pharmacy field, concretely relates to a use of a series of pentacyclic triterpene compounds as enterovirus EV71 inhibitors, and especially relates to an application in the preparation of medicines for preventing and treating EV71 infection induced hand-foot-and-mouth diseases and complications thereof, such as synanche, myocarditis, pulmonary edema, encephalitis, herpes, septicemia, hypertension, hyperglycemia, cognitive function disorder, poliomyelitis-like paralysis and many nerve system associated diseases. The invention also discloses medicinal compositions of the series of the pentacyclic triterpene compounds as enterovirus EV71 inhibitors.
Owner:CHINA PHARM UNIV
Recombinant baby hamster kidney (BHK) cell line capable of expressing encephalitis B virus PrM/M-E protein and application thereof
ActiveCN102337248AFight infectionHigh expressionViral antigen ingredientsAntiviralsMicroorganismEncephalitis
The invention discloses a recombinant baby hamster kidney cell line (BHK 21) capable of stably expressing encephalitis B virus PrM / M-E protein, and more particularly, the recombinant cell line capable of expressing the PrM / M-E protein is BHK-JEV-ME and is preserved in the China General Microbiological Culture Collection Center (CGMCC) with the culture preservation number of CGMCC No. 5263. The invention also discloses a method for preparing the recombinant cell line and the application of the recombinant cell line in the preparation of encephalitis B prevention vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Immunologic diagnosis kit for detecting dengue virus NS1 antigen and application thereof
ActiveCN101726593AImprove featuresIncreased sensitivityMaterial analysisAgainst vector-borne diseasesBiotinSerotype
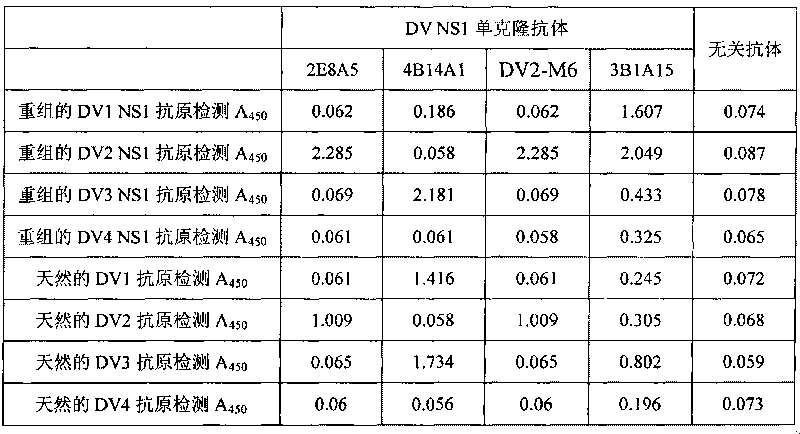
The invention discloses an immunologic diagnosis kit for detecting dengue virus NS1 antigen and application thereof. The kit comprises a capturing antibody and a detecting body combined with a marker, and the kit is characterized in that the capturing antibody consists of a monoclonal antibody 2E8A5, a monoclonal antibody DV2M6 and a monoclonal antibody 4B14A1, the detecting antibody is a monoclonal antibody 3B1A15, and the marker can be biotin, horseradish peroxidase, alkaline phosphatase, colloidal gold or fluorescein. The kit can specifically detect NS1 proteins of four serotype dengue viruses and does not have cross reaction with other viruses of flavivirus system, such as flavivirus and encephalitis B virus, the sensitivity of the kit is at least four times higher than that of detection of foreign commodity kits, and the kit can efficiently reduce the omission factor of the clinical application.
Owner:SOUTHERN MEDICAL UNIVERSITY
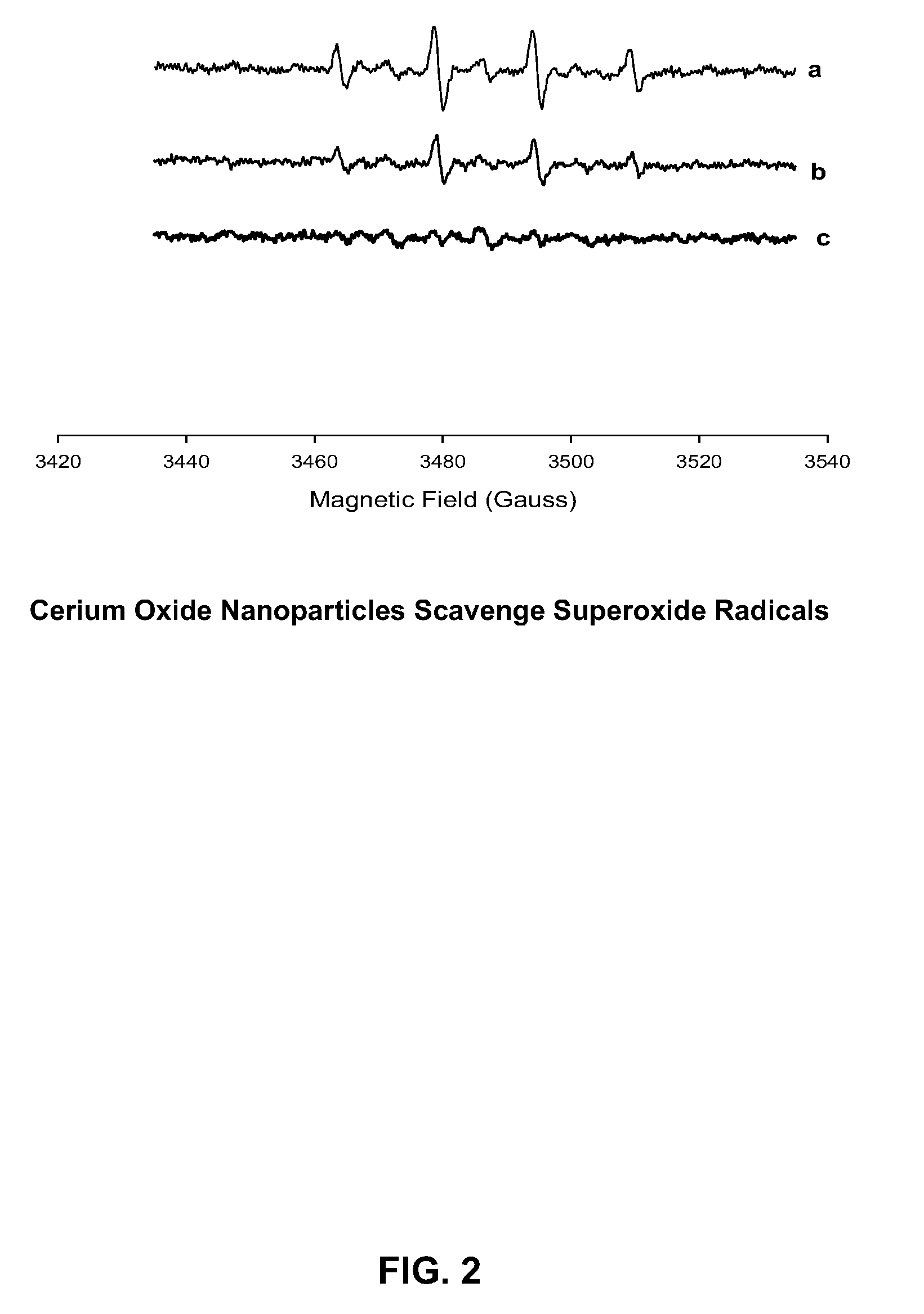
Cerium Oxide Nanoparticles for Treatment and Prevention of Alzheimer's Disease, Parkinson's Disease, and Disorders Associated with Free Radical Production and/or Mitochondrial Dysfunction
ActiveUS20090092671A1Reduce lossesDeter and prevent dopaminergic neuronal lossPowder deliveryBiocideMitochondrial diseaseLipid peroxidation
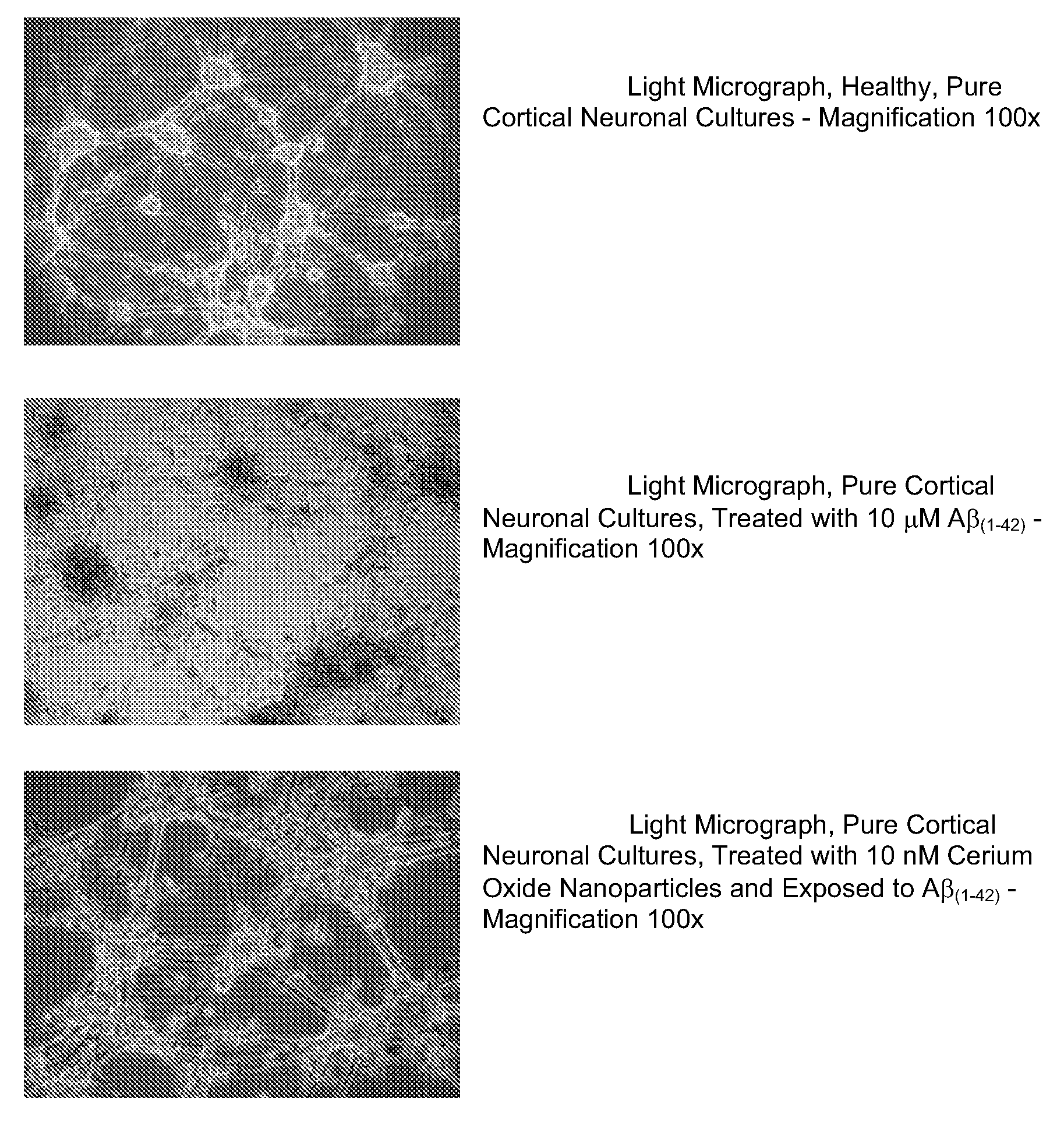
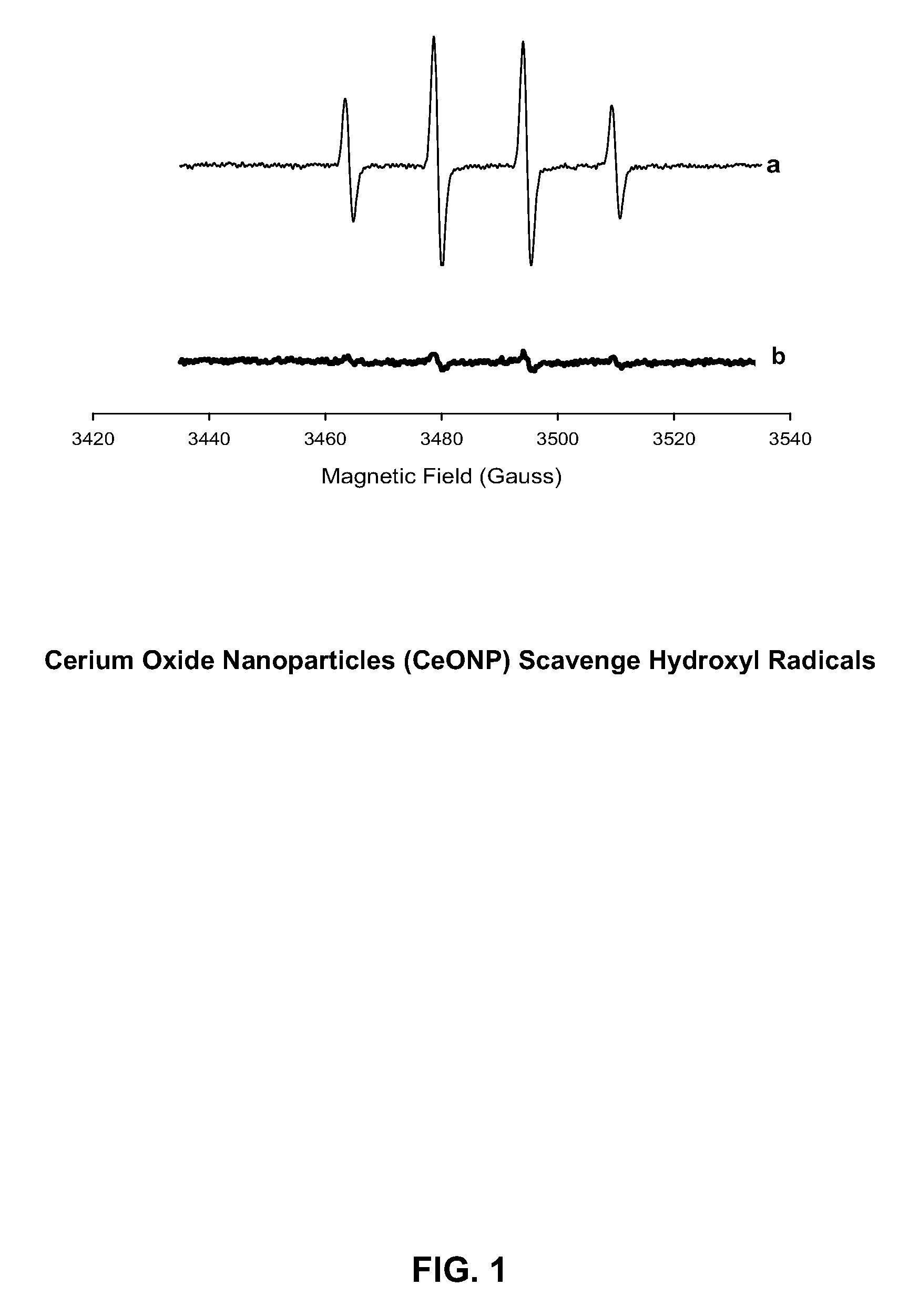
Cerium oxide nanoparticles (CeONP) can be used to treat or prevent neurodegenerative diseases, including for example Alzheimer's Disease, Parkinson's Disease, Huntington's Disease, AIDS-related dementia, ALS, progressive supranuclear palsy, and encephalitis, as well as mitochondrial diseases and diseases associated with mitochondrial damage. In particular, CeONP having an average size of about 2 nm to about 100 nm can be administered in an amount sufficient to block production of hydroxyl or superoxide radicals, block free radical production by Aβ(1-42), block Aβ(1-42)-induced neuronal death, block Aβ(1-42)-induced [Ca2+]i dysfunction in neurons, block Aβ(1-42)-induced lipid peroxidation, decrease loss of dopaminergic neurotransmission, or reduce mitochondrial dysfunction in a cell. CeONP can also be effective in treating conditions involving toxic exposures to compounds that induce mitochondrial dysfunction, such as rotenone, cyanide, carbon monoxide, polychlorinated biphenyls (PCBs) and other mitochondrial toxins.
Owner:EDWARD VIA VIRGINIA COLLEGE OF OSTEOPATHIC MEDICINE
Recombinant flaviviral constructs and uses thereof
InactiveUS20130243809A1Successfully expressingHigh insertion capacitySsRNA viruses positive-senseViral antigen ingredientsStructural proteinViral replication
A recombinant viral construct for expressing an exogenous polypeptide in a cell and uses thereof are provided. The recombinant viral constructs are derived from Japanese encephalitis virus (JEV). The recombinant viral constructs encodes a fusion protein, which includes an exogenous (i.e., non-JEV) polypeptide and a JEV non-structural protein 1 (JEV NS1) or a segment thereof. Particularly, the exogenous polypeptide is inserted into the carboxyl-terminus of the JEV NS1, and the production of the recombinant fusion protein does not affect viral replication. Upon infection a cell with such recombinant viral constructs, JEV particles comprising limited multiplicative virions (LMV) may be produced. Each LMV comprises the as-described JEV replicon. The JEV particles are useful in eliciting an immune response to the exogenous polypeptide in a host and thereby confer the host with protective immunization against the exogenous polypeptide.
Owner:NAT DEFENSE MEDICAL CENT
Early stage detection molecular marker of Angiostrongylus cantonensis disease and primer
ActiveCN103695532AImprove accuracyEarly treatmentMicrobiological testing/measurementFermentationDiseaseEncephalitis
The invention belongs to the field of biology, and relates to early stage detection molecular marker of early stage Angiostrongylus cantonensis disease, and primer and correlated kit thereof. The necessary composition of the diagnostic reagent contains: aca-miR-146a-5p sequence and three related primers (stem loop primer, upstream and downstream primer) designed according to the sequence design. The invention firstly provides an application of aca-miR-146a-5p in preparation for diagnosis of Angiostrongylus cantonensis disease. The diagnostic reagent can accurately and simply detect infection of Angiostrongylus cantonensis at early stage, and can distinguish between meningitis or encephalitis caused by Angiostrongylus cantonensis and other meningitis or encephalitis with good social and economic benefits.
Owner:SUN YAT SEN UNIV
Repair traditional Chinese medicine used for treating blood cell infected by flaviviridae
InactiveCN105920380AEffective preventionEffective therapeuticNervous disorderDigestive systemMedicinal herbsDisease
The invention relates to the field of traditional Chinese medicine, and discloses a repair traditional Chinese medicine used for treating blood cell infected by flaviviridae. The traditional Chinese medicine is prepared from: by weight, herba tubocapsici anomali 30g-80g, cymbopogon citratus 20g-50g, camellia flower 10g-30g, citrus aurantium flower 10g-30g, bitter gourd 20g-50g, mint 20g-50g, phellodendron amurense 10g-30g, cypress leaf 10g-30g, salix leaf 10g-30g, persimmon leaf 15g-35g, perilla nankinensis 10g-20g, radix sophorae flavescentis 30g-60g, eleusine indica 25g-80g, syzygium aromaticum 10g-30g, lonicera japonica 30g-80g, agastache rugosus 20g-50g, portulaca oleracea 10g-30g, glycyrrhiza 5g-15g, chrysanthemum 10g-35g, and foeniculum vulgare 10g-35g. The traditional Chinese medicine has cure effect of the diseases such as dengue, Zika virus, encephalitis, hepatitis and liver cancer infected by flaviviridae spreading, can treat and repair the blood cell from the source, has prophylaxis and treatment effect on flaviviridae diseases, is without any toxicity and side effects, and can improve the cure rate and cure efficiency of patients.
Owner:林家希
Humanized antibodies against the venezuelan equine encephalitis virus
InactiveUS20060024666A1Prevent and neutralize viral infectionFungiBacteriaHumanized antibodyViral infection
Owner:ALEXION PHARMA INC
Method for producing human diploid cell encephalitis B inactivated vaccine
InactiveCN102406927AImprove quality and safetyImprove quality stabilityViral antigen ingredientsAntiviralsUltrafiltrationDiploid cells
The invention relates to the technical field of biology, in particular to a method for producing a human diploid cell encephalitis B inactivated vaccine. The method sequentially comprises the following steps of: resuscitating and subculturing a human diploid cell strain; culturing the human diploid cell strain by using a multi-stage bioreactor; inoculating an encephalitis B virus strain, and performing virus multiplication culture; harvesting a virus culture solution; and inactivating the virus culture solution, performing ultrafiltration, purifying, adding a glycoprotein protective agent, and preparing the vaccine. Human diploid cells are used as a cell matrix, so that the vaccine is safe and is free from exogenous factor pollution; the human diploid cells are cultured by the bioreactor through stage-by-stage linear amplification, so that the large-scale production of the vaccine with low cost, high quality, safety and stability is easy to implement; and the titer of the virus harvested solution is subjected to sampling inspection every day, so that the high expression of the harvested virus culture is ensured, and production quality is ensured.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Novel technology for detecting viruses of arbovirus encephalitis
ActiveCN102199672AReduce usageHigh sensitivityMicrobiological testing/measurementEncephalitis VirusesVirus
The invention relates to a method capable of detecting common viruses of arbovirus encephalitis. The invention also relates to an amplification primer and an extension primer which are used for detecting the common viruses of arbovirus encephalitis, and a kit containing the primers.
Owner:BGI GENOMICS CO LTD +1
Antagonists of CB1 Receptor
The invention relates to an antagonist of CB1 receptor for use in the treatment of a pathologic condition or disorder selected from the group consisting of bladder and gastrointestinal disorders; inflammatory diseases; cardiovascular diseases; nephropathies; glaucoma; spasticity; cancer; osteoporosis; metabolic disorders; obesity; addiction, dependence, abuse and relapse related disorders; psychiatric and neurological disorders; neurodegenerative disorders; autoimmune hepatitis and encephalitis; pain; reproductive disorders and skin inflammatory and fibrotic diseases.
Owner:UNIV DE BORDEAUX +1
Attenuation of encephalitogenic alphavirus and uses thereof
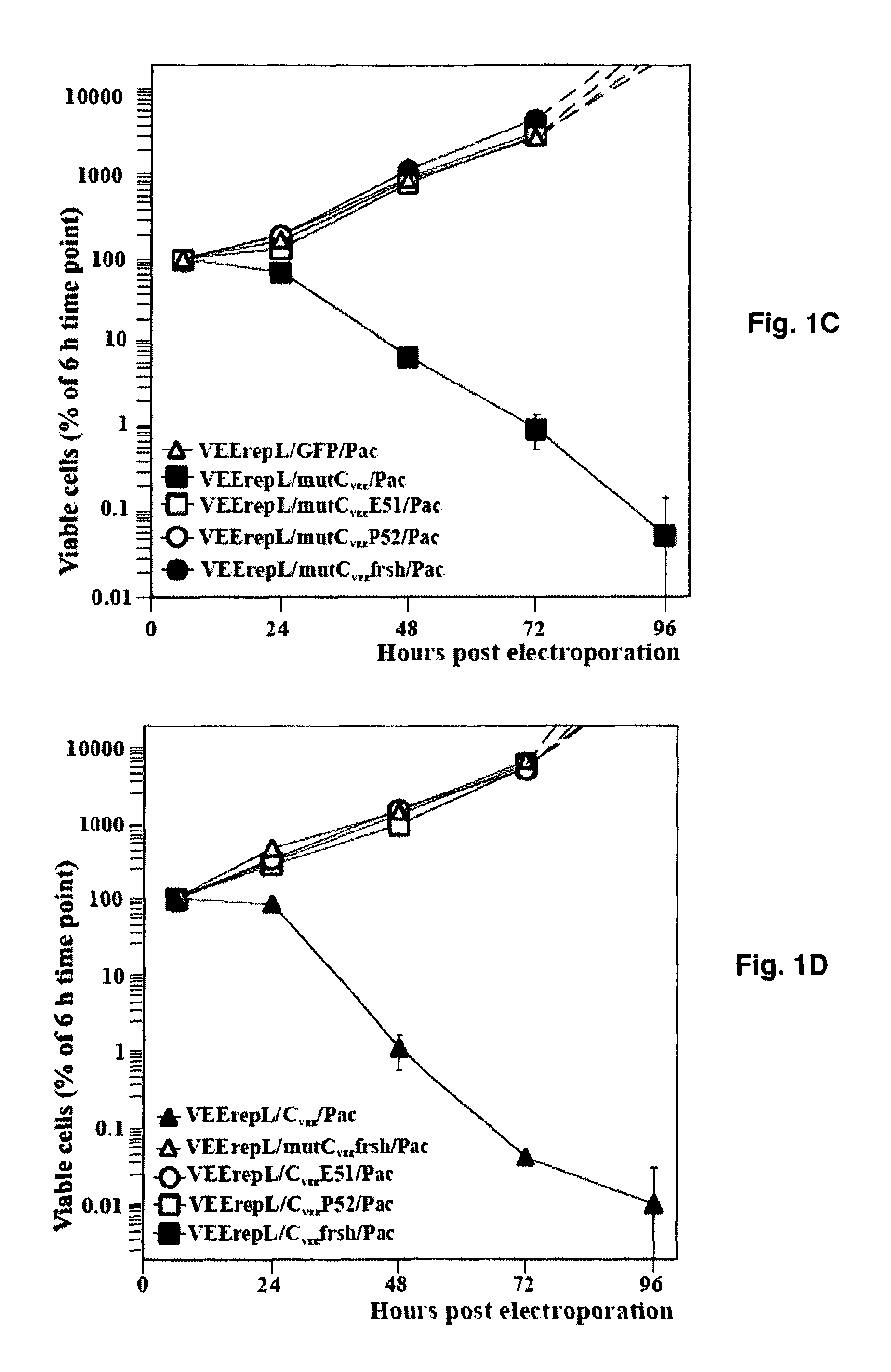
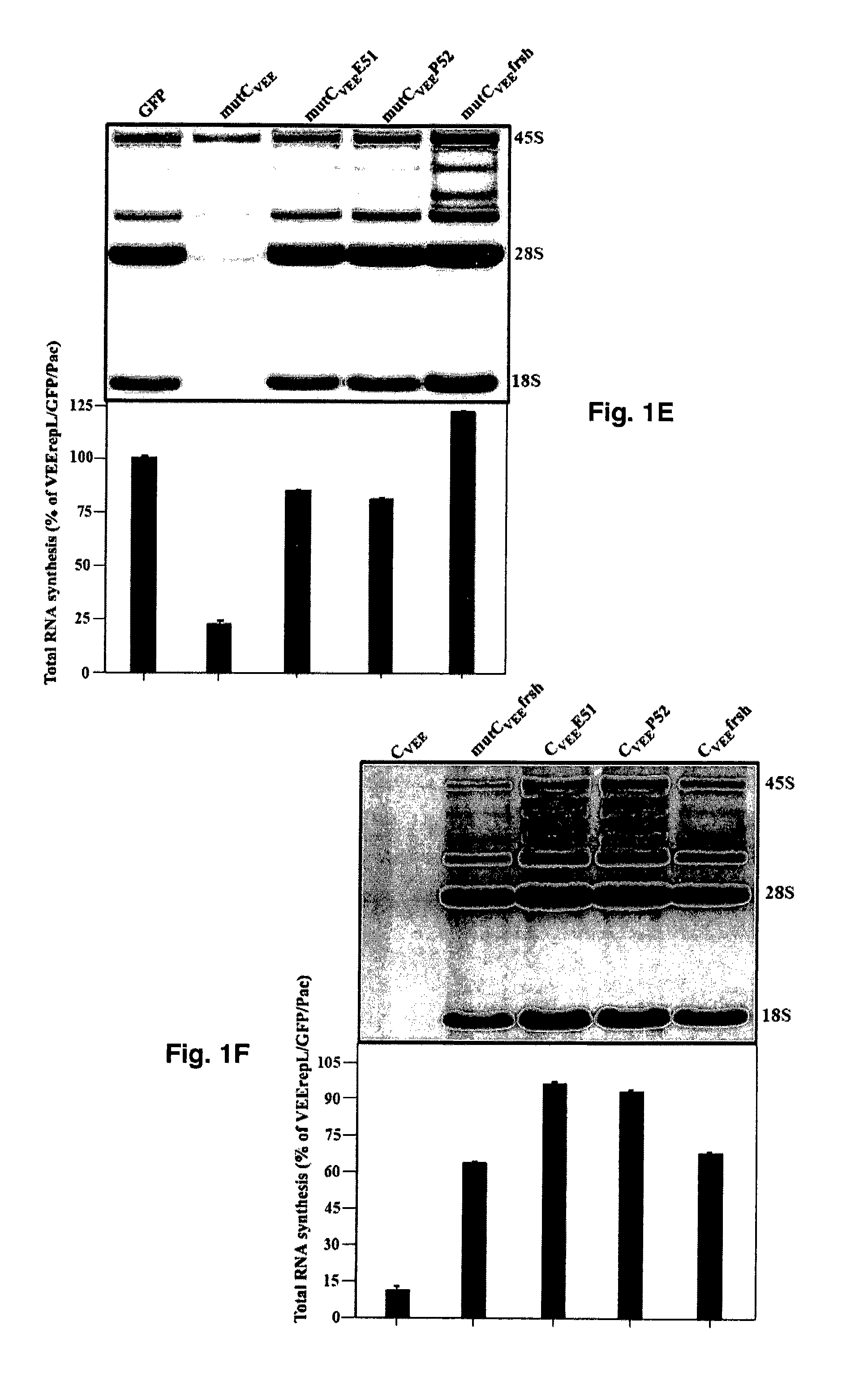
The present invention is drawn to generating attenuated and less cytopathic forms of New World alphaviruses that can be used in immunogenic compositions as vaccines against both Old and New World alphaviruses. In this regard, the present invention discloses that the N-terminal, ˜35-aa-long peptide of VEEV, EEEV and, most likely, of WEEV capsid proteins plays the most critical role in the downregulation of cellular transcription and development of cytopathic effect. The identified, VEEV-specific peptide, CVEE30-68, includes two domains with distinguished functions. The integrity of both domains determines not only the intracellular distribution of CVEE, but is also essential for direct capsid function in the inhibition of transcription. The replacement of the N-terminal fragment of CVEE by its SINV-specific counterpart in VEEV TC-83 genome does not affect virus replication in vitro, but makes it less cytopathic and more attenuated in vivo.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
A new method for the detection of yellow fever virus by fluorescent quantitative PCR and a PCR system for the detection of yellow fever virus
ActiveCN102277446AQuick QualificationQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceEncephalitisFluorescence
The invention discloses a novel fluorescence quantitative PCR (Polymerase Chain Reaction) detection method for yellow fever viruses and a yellow fever virus detection PCR system. The yellow fever virus detection PCR system comprises primers and probes, a Premix Ex Taq reaction liquid, and sterilized Tris water, wherein the primers and the probes are good in detection specificity and high in sensitivity, is very suitable to bouquet fever viruses, and has no cross reactions with the yellow fever viruses and encephalitis B viruses.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted furo[2,3-b]pyridine derivatives Substituted furo[2,3-b]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/3ed63723-40f5-4ba6-aa1b-8315990b9ddc/US20050272763A1-20051208-C00001.png)
![Substituted furo[2,3-b]pyridine derivatives Substituted furo[2,3-b]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/3ed63723-40f5-4ba6-aa1b-8315990b9ddc/US20050272763A1-20051208-C00002.png)
![Substituted furo[2,3-b]pyridine derivatives Substituted furo[2,3-b]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/3ed63723-40f5-4ba6-aa1b-8315990b9ddc/US20050272763A1-20051208-C00003.png)