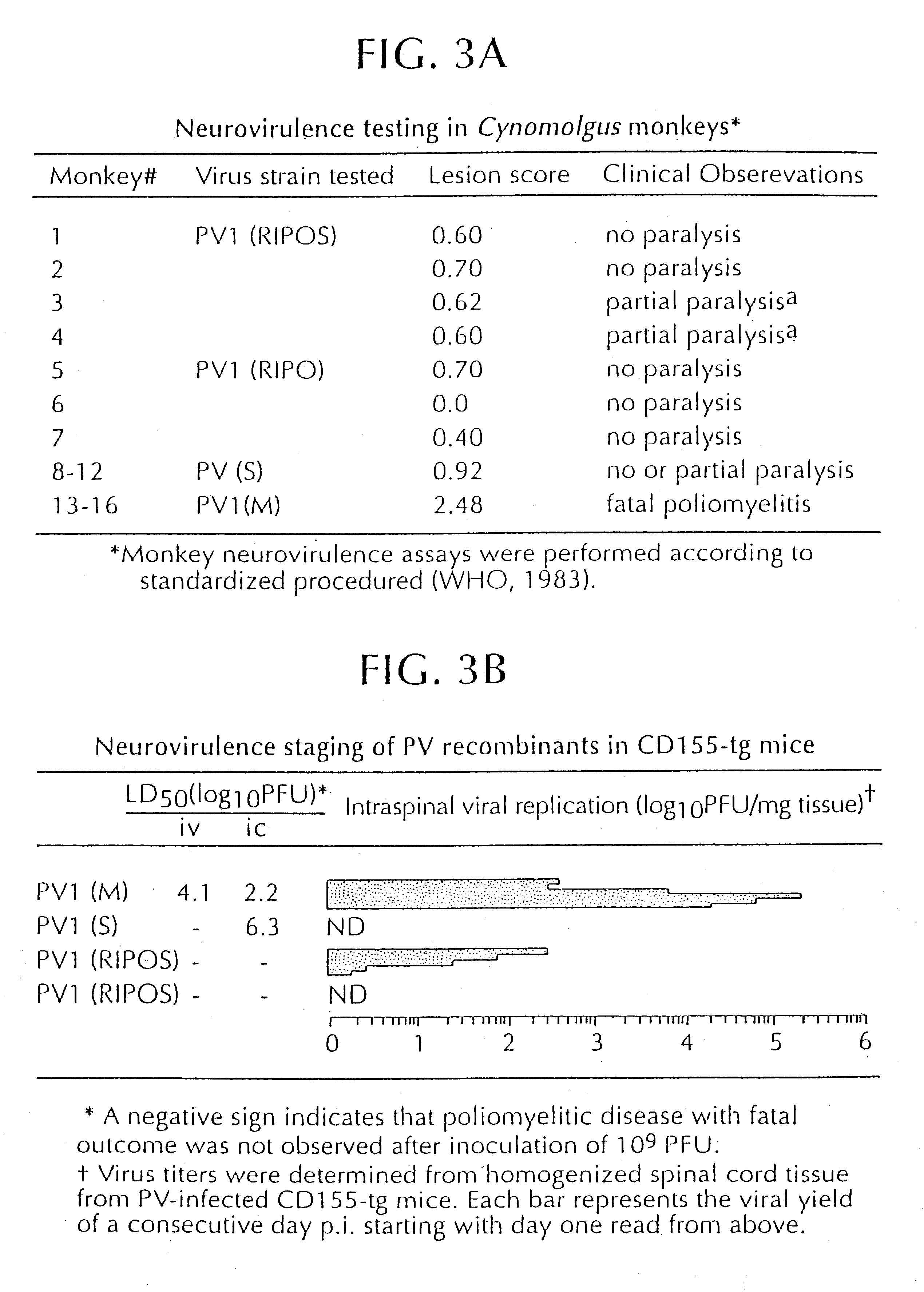
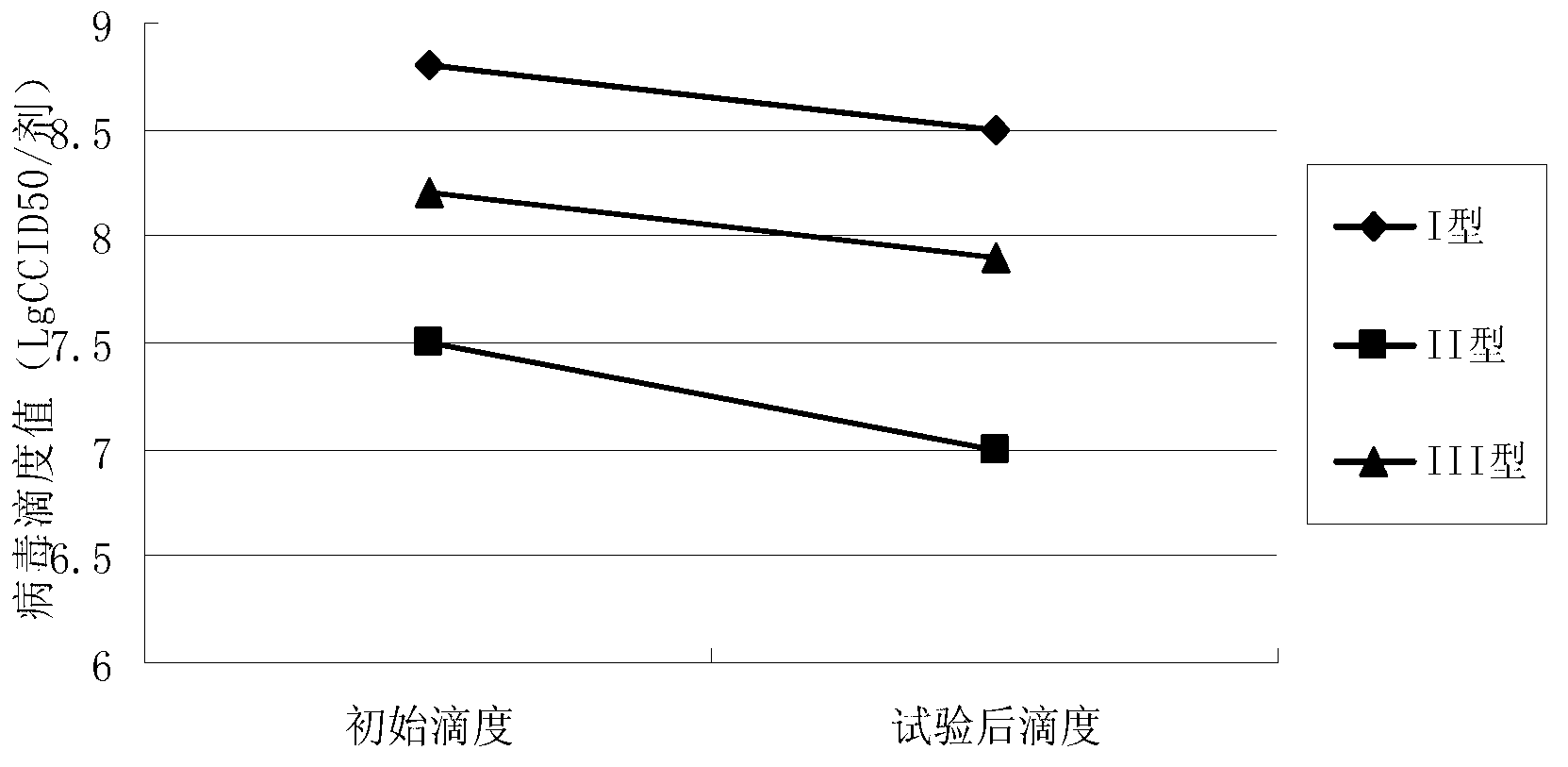
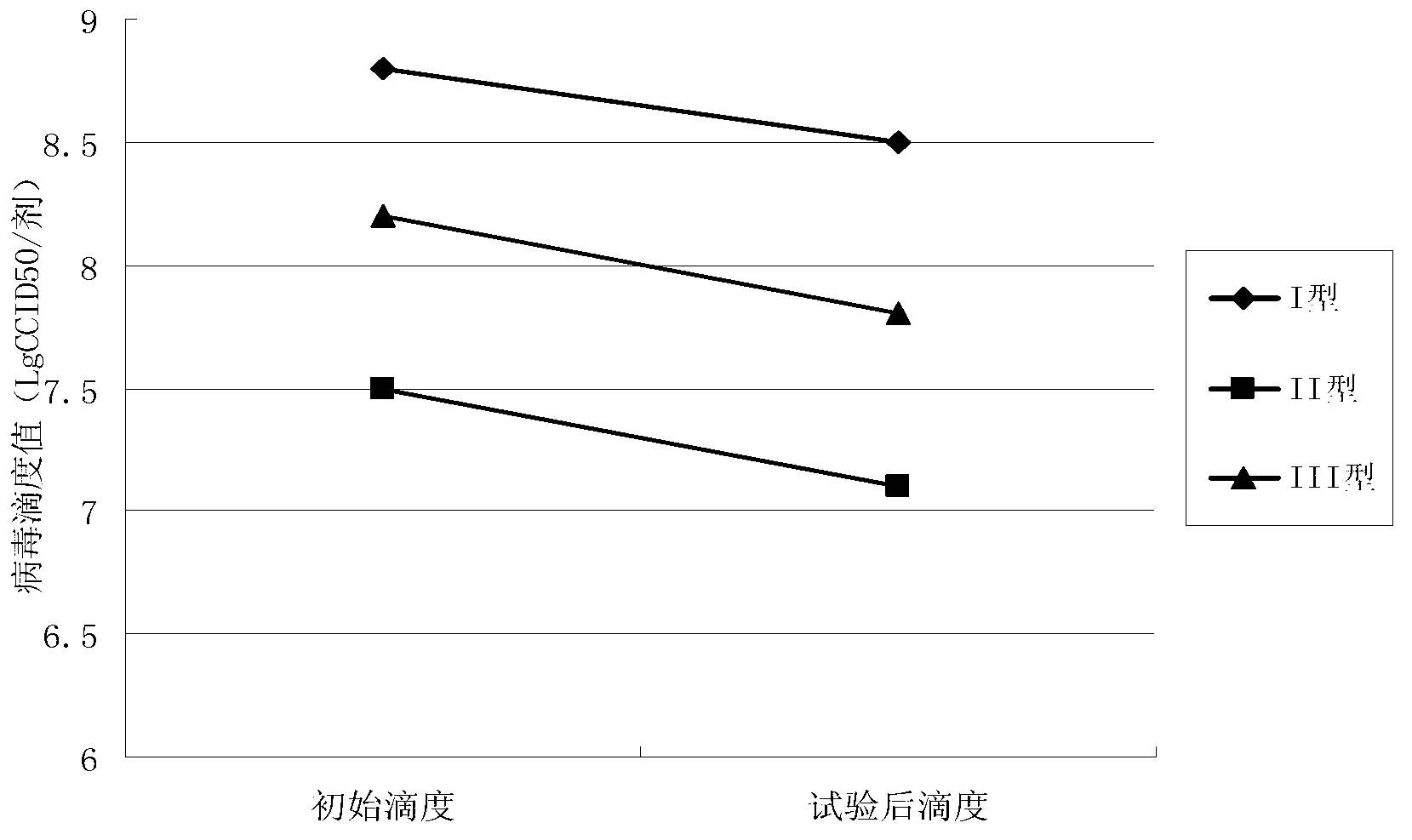
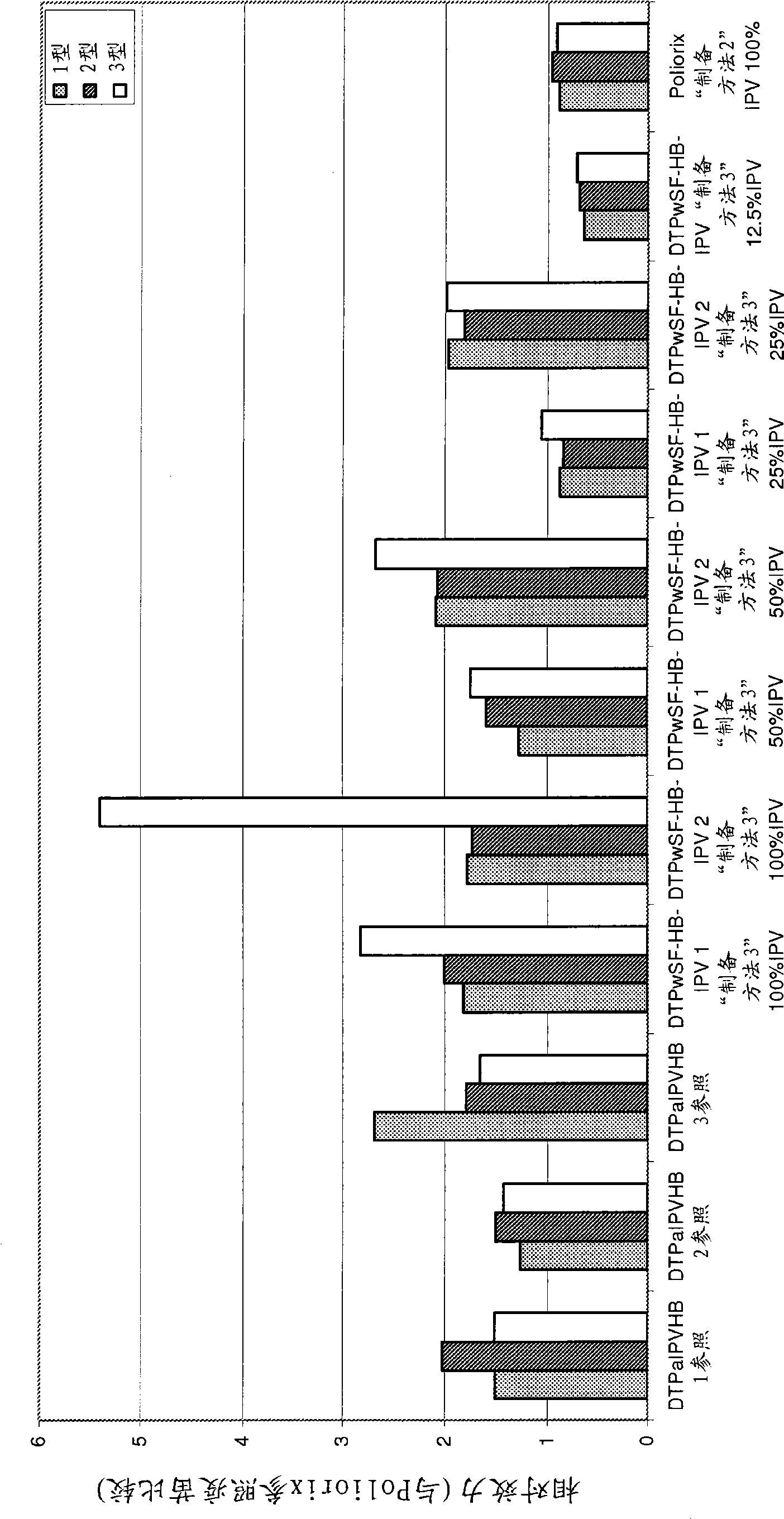
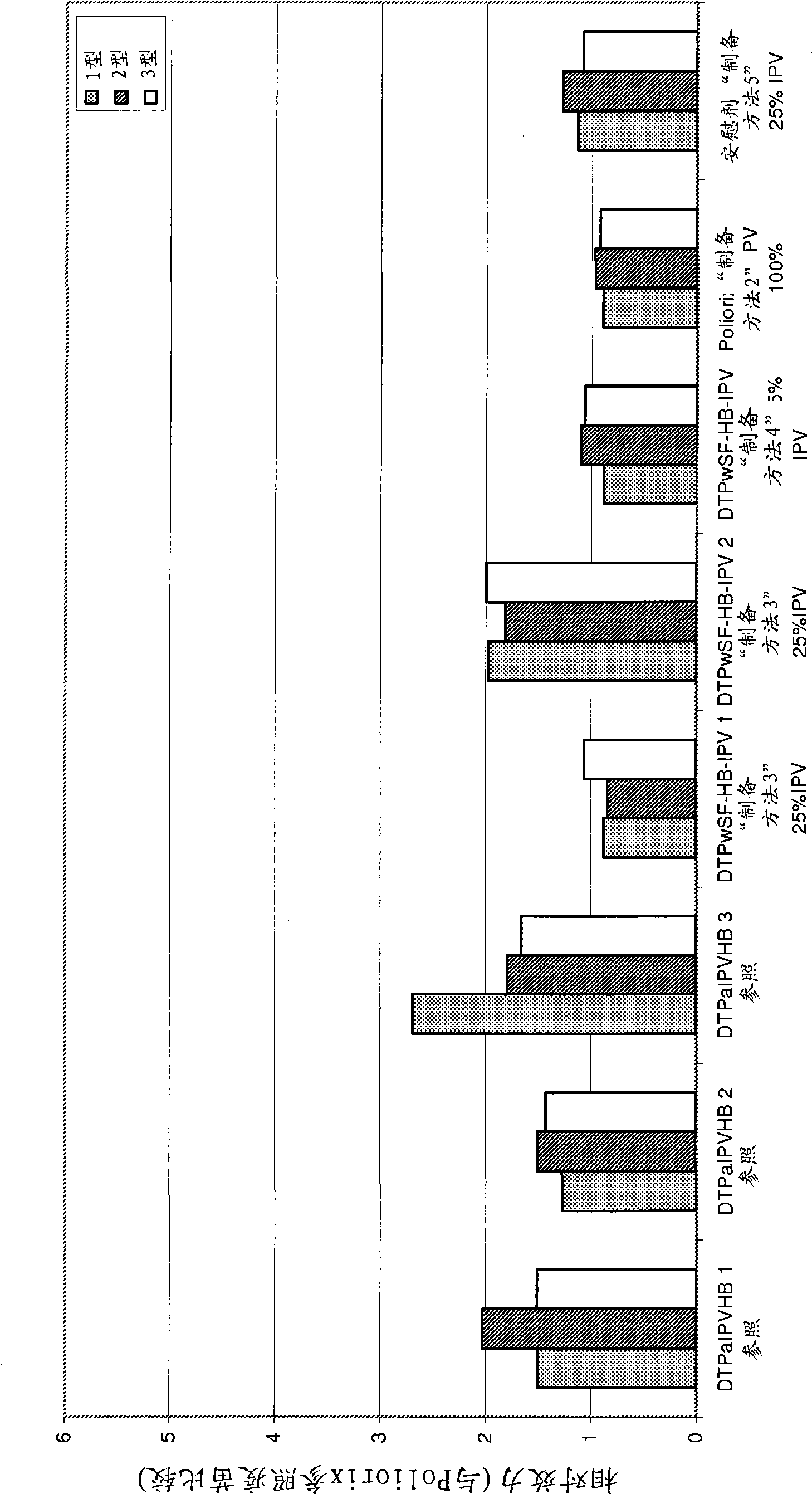
Patents
Literature
94 results about "Poliomyelitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A viral infection causing nerve injury which leads to partial or full paralysis.
Modulation of the poliovirus receptor function
InactiveUS20070041985A1Modulate and change physiological PVR functionUnderstanding of roleOrganic active ingredientsPeptide/protein ingredientsCell adhesionLymphatic Spread
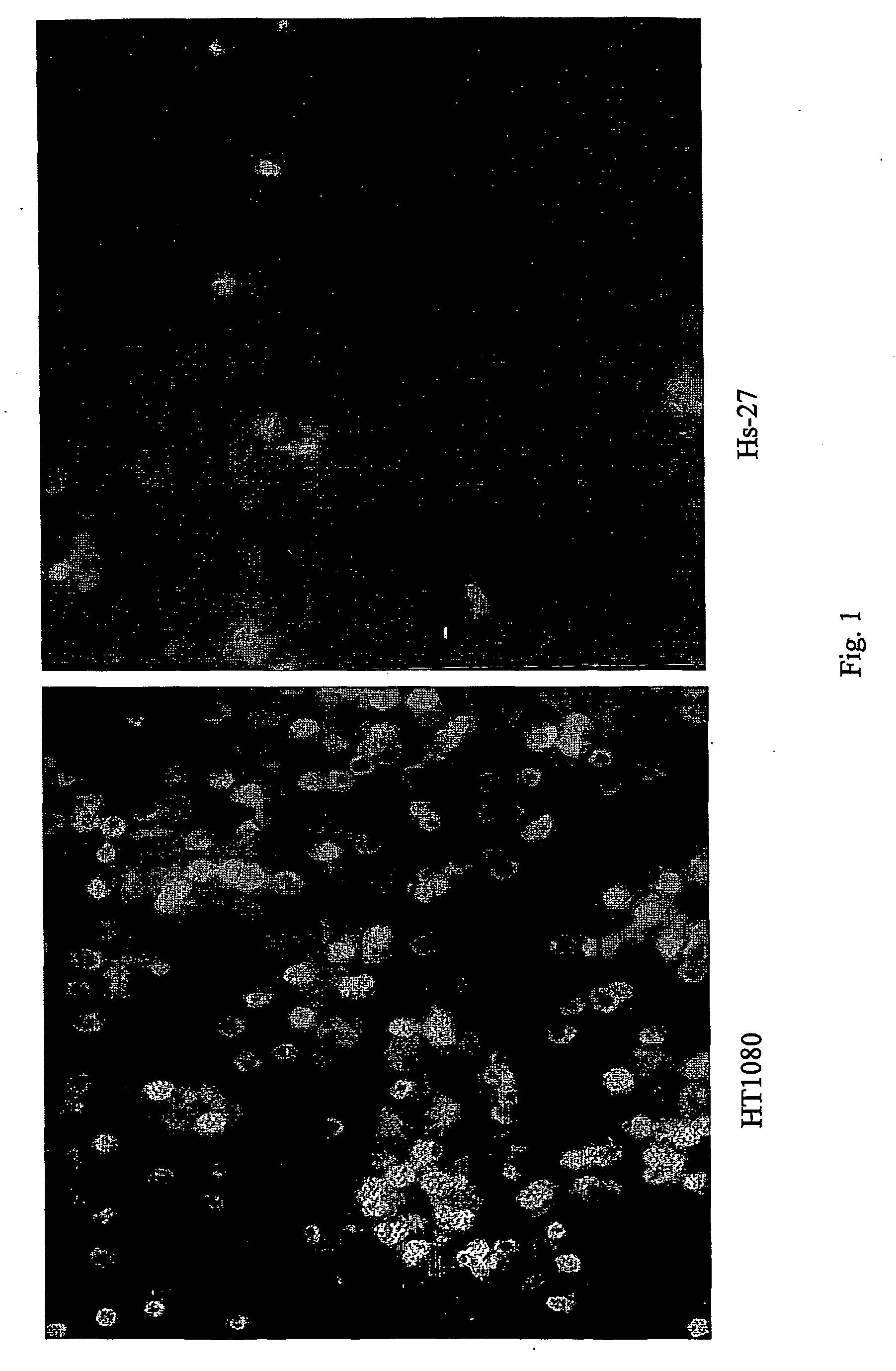
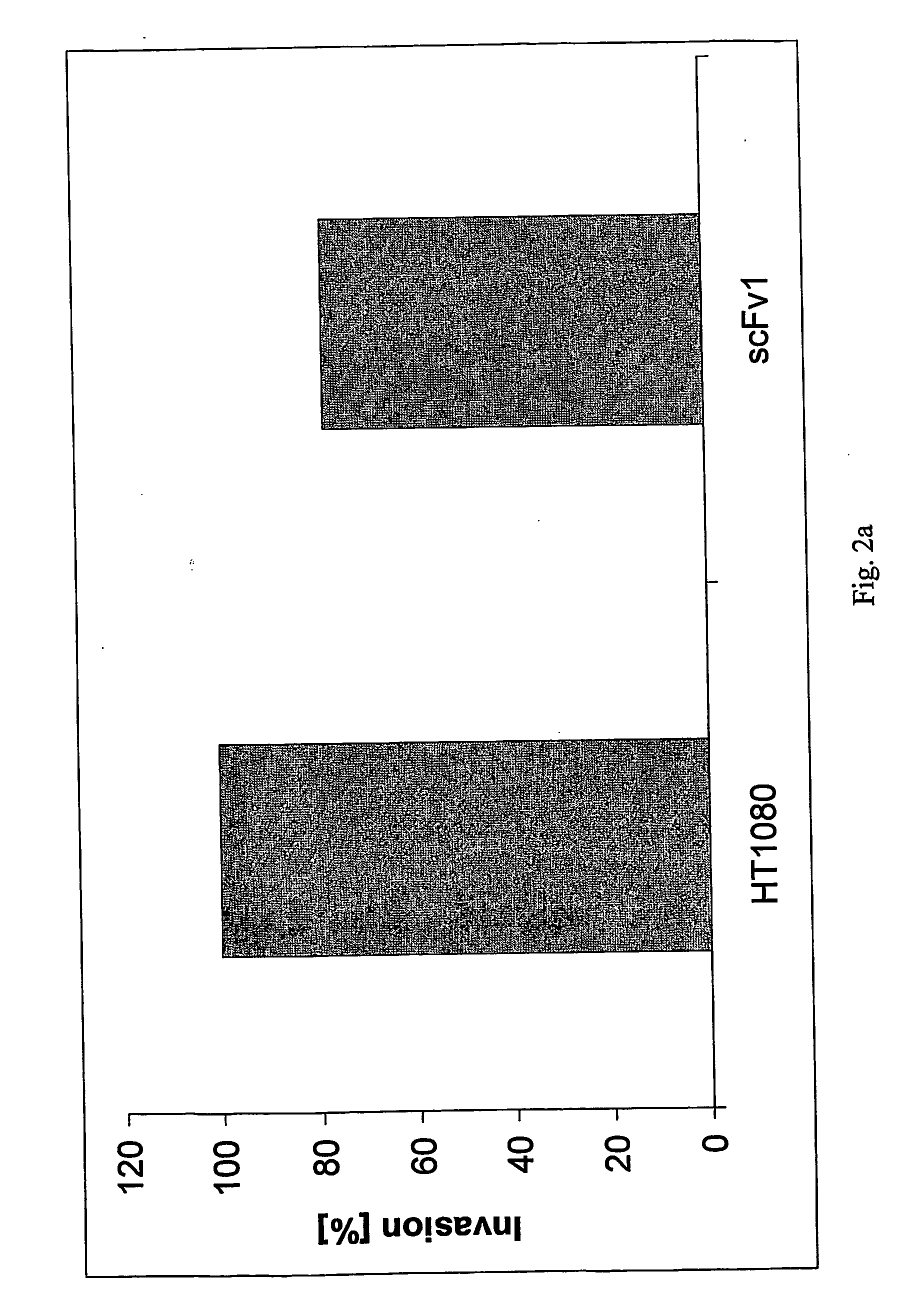
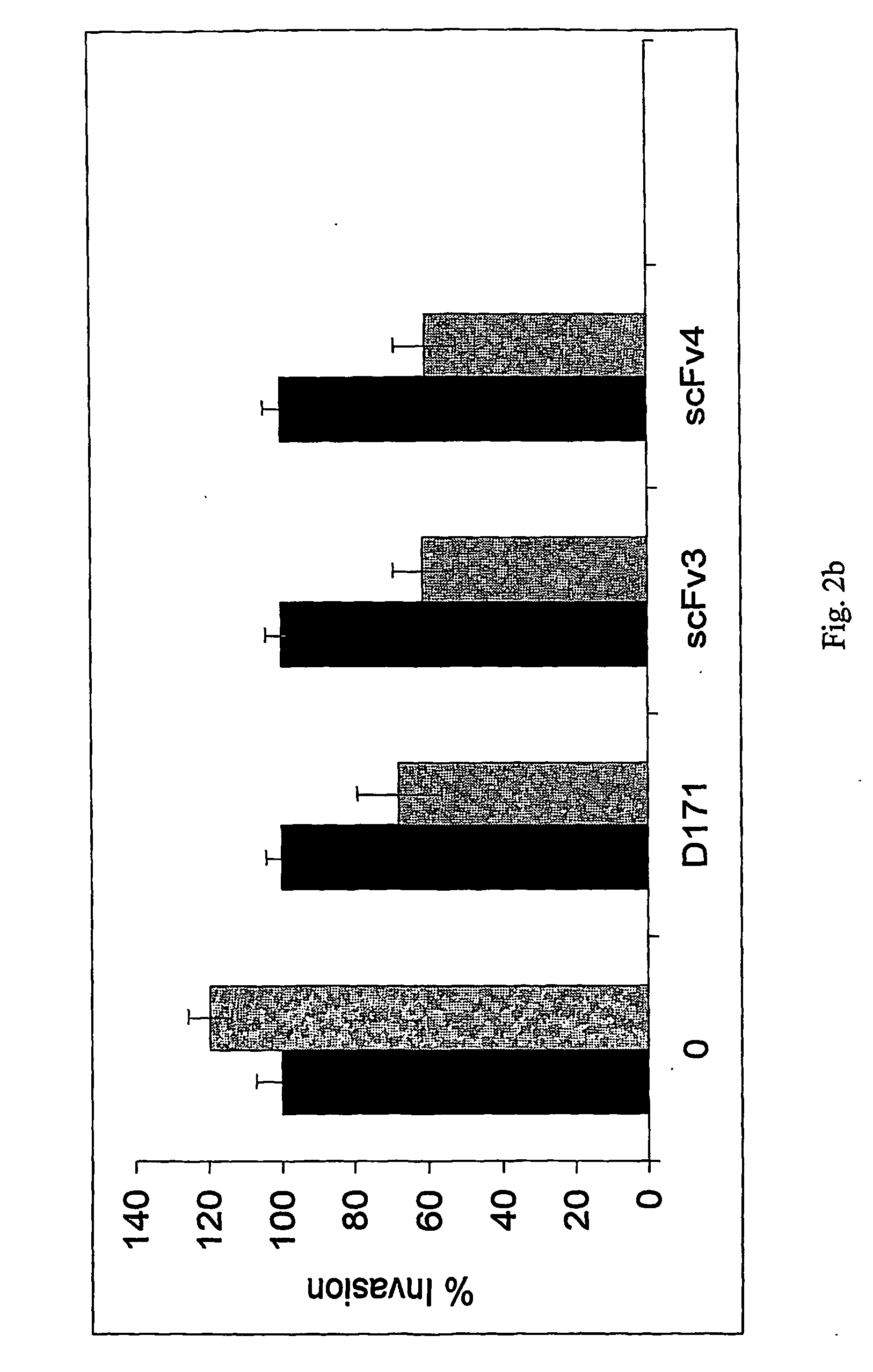
The present invention relates to the identification, the isolation and the use of molecules interfering with the function(s) mediated by the poliovirus receptor (PVR) on cells. The molecules can be used for the treatment of cells having a metastatic potential, metastasis and cancer. Further methods are provided that are useful for identifying and isolating molecules which have the capacity to modulate PVR mediated adhesion or invasion potential of cells.
Owner:TUFTS UNIV
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
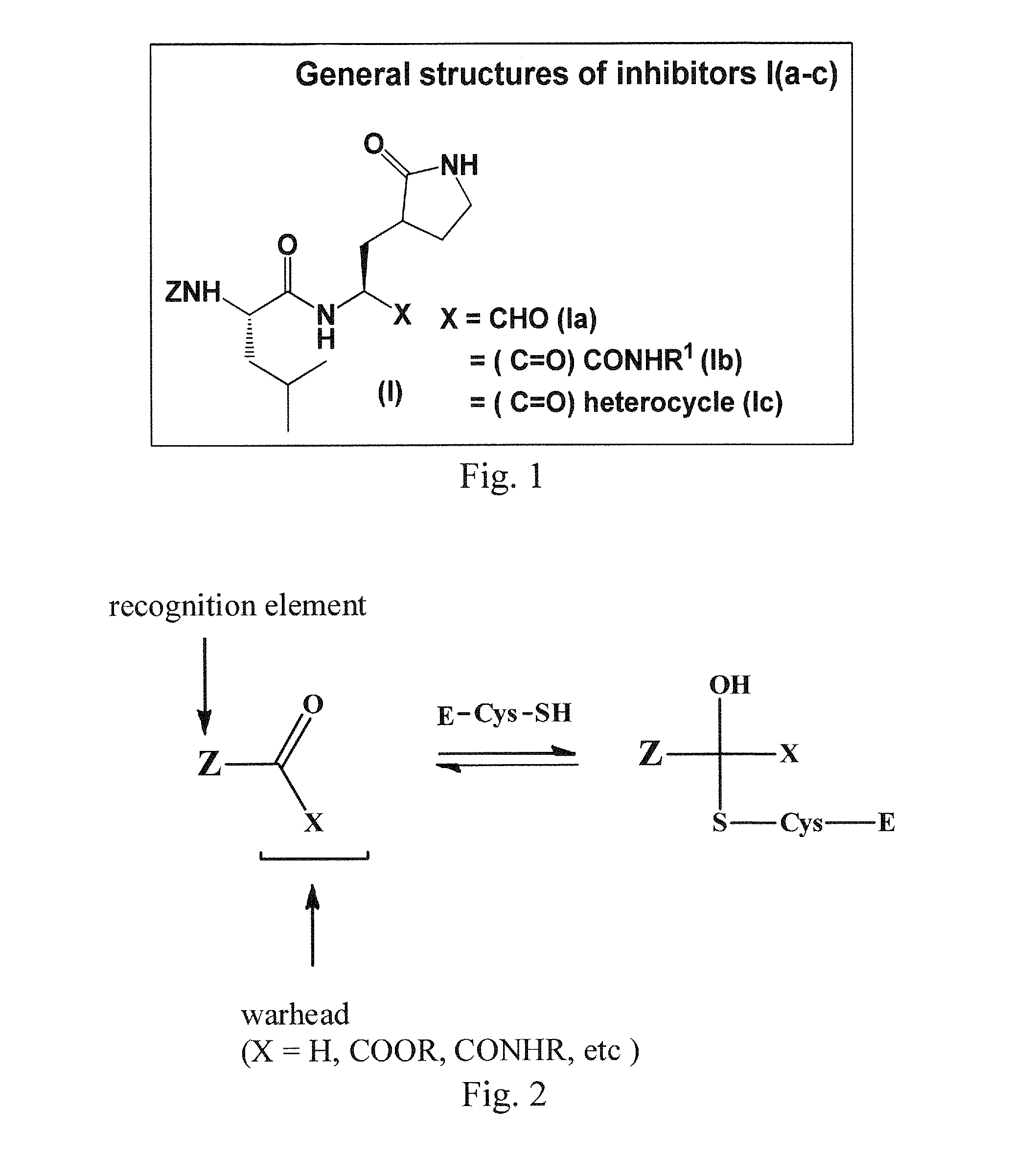
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Recombinant poliovirus for the treatment of cancer
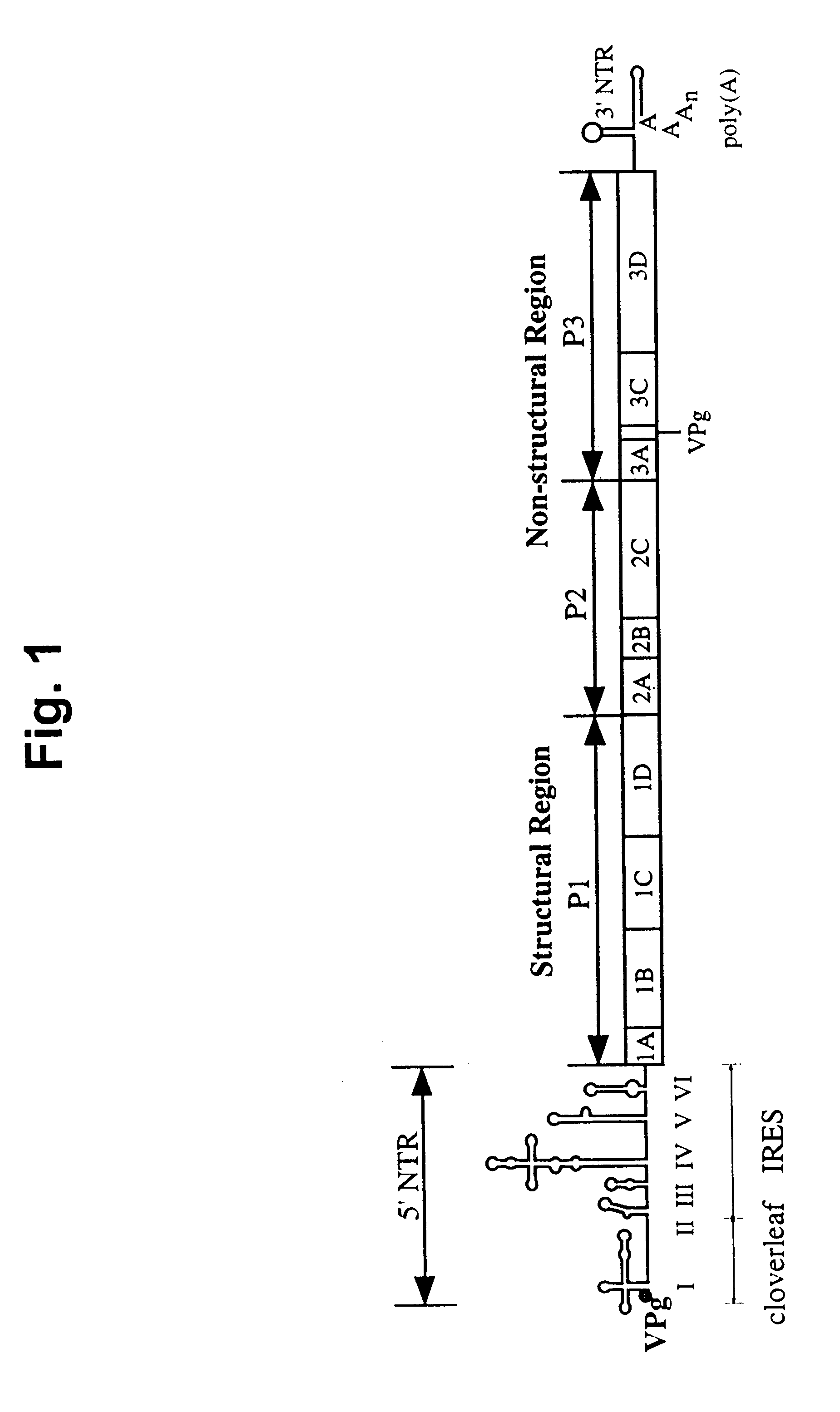
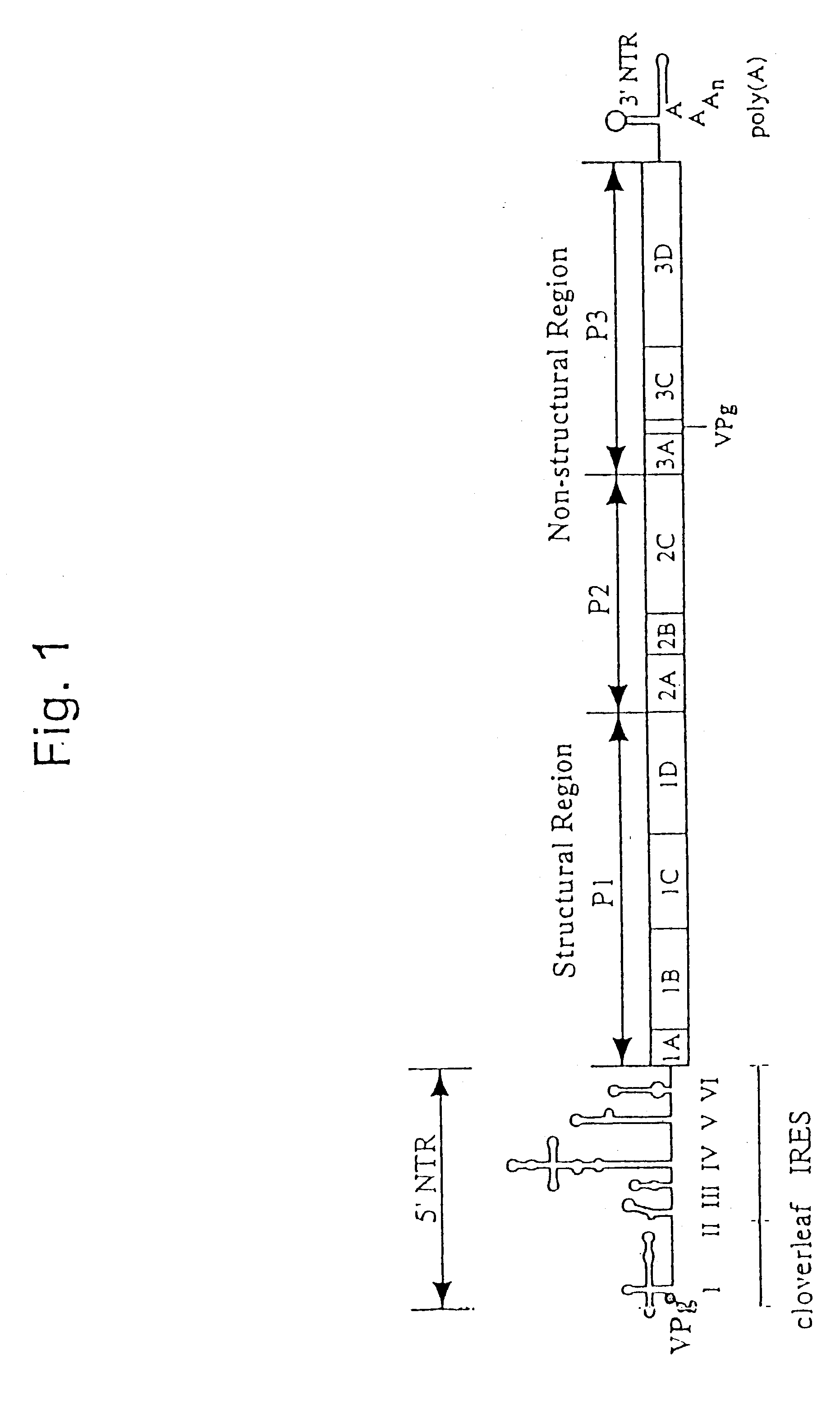
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:NEW YORK UNIV OF RES FOUND OF THE
Agents for the inhibition of virus replication through regulation of protein folding
The invention concerns agents for the treatment of acute and chronic infections with human and animal pathogenic viruses which assemble along the cell membrane and are released through budding on the surface of the cell. Hereunto count especially causative agents of infectious diseases such as AIDS, hepatitis, hemorrhagic fever, SARS, smallpox, measles, polio or the flu. The subjects of the invention are agents that contain inhibitors of the protein folding as active components. Hereunto count inhibitors of cellular folding enzymes (the enzymatic chaperones) as well as substances that disturb the folding of proteins through chemical chaperones. The following substance classes and their derivates belong thereunto: Geldanamycin, Deoxyspergualin, 4-PBA or Herbimycin A. Due to these agents the highly organised processes of the assembly and the proteolytical maturation of virus structure proteins is disturbed. As a result the release and production of infectious decendent viruses is prevented.
Owner:VIROLOGIK GMBH
Recombinant poliovirus for the treatment of cancer
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Multiple vaccination including Serogroup C Meningococcus
ActiveUS20100034847A1Accurate measurementAvoid potential suppression effectAntibacterial agentsBacterial antigen ingredientsVaccinationAdjuvant
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminium phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Pentacyclic triterpene enterovirus EV71 inhibitors, and medicinal compositions and medicinal use thereof
InactiveCN104840467AOrganic active ingredientsNervous disorderAcute hyperglycaemiaHand-foot-and-mouth disease
The invention relates to the pharmacy field, concretely relates to a use of a series of pentacyclic triterpene compounds as enterovirus EV71 inhibitors, and especially relates to an application in the preparation of medicines for preventing and treating EV71 infection induced hand-foot-and-mouth diseases and complications thereof, such as synanche, myocarditis, pulmonary edema, encephalitis, herpes, septicemia, hypertension, hyperglycemia, cognitive function disorder, poliomyelitis-like paralysis and many nerve system associated diseases. The invention also discloses medicinal compositions of the series of the pentacyclic triterpene compounds as enterovirus EV71 inhibitors.
Owner:CHINA PHARM UNIV
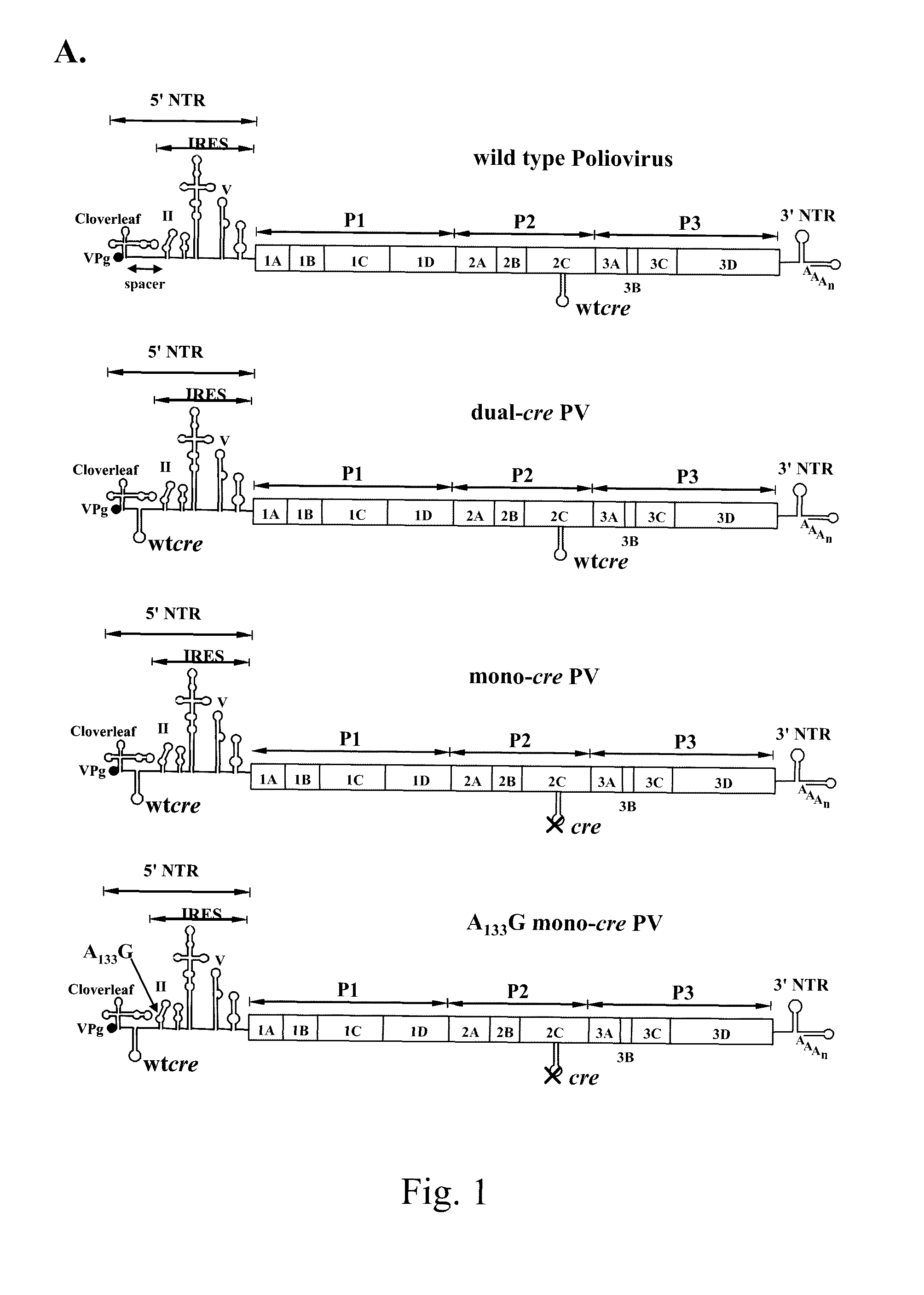
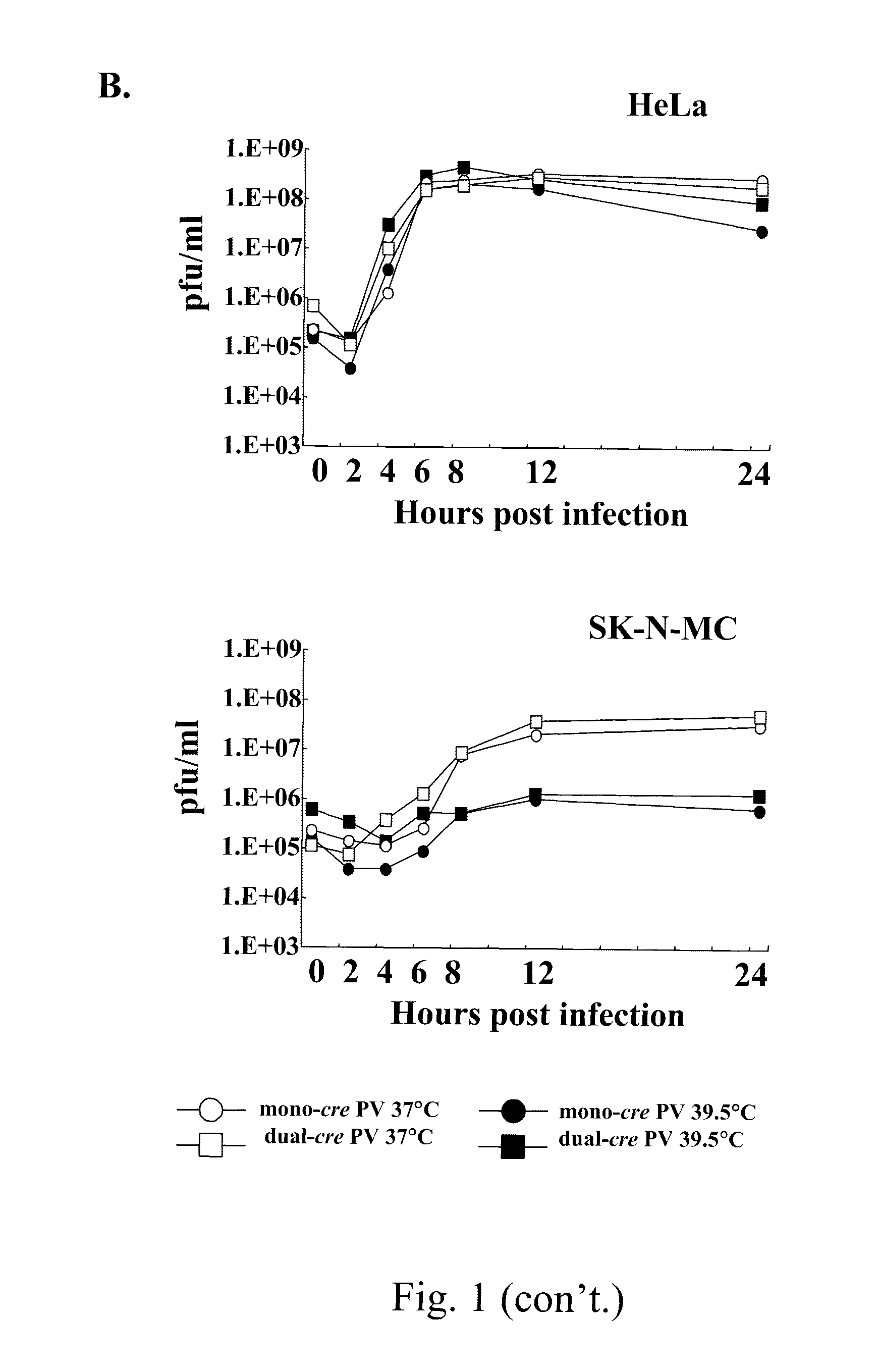
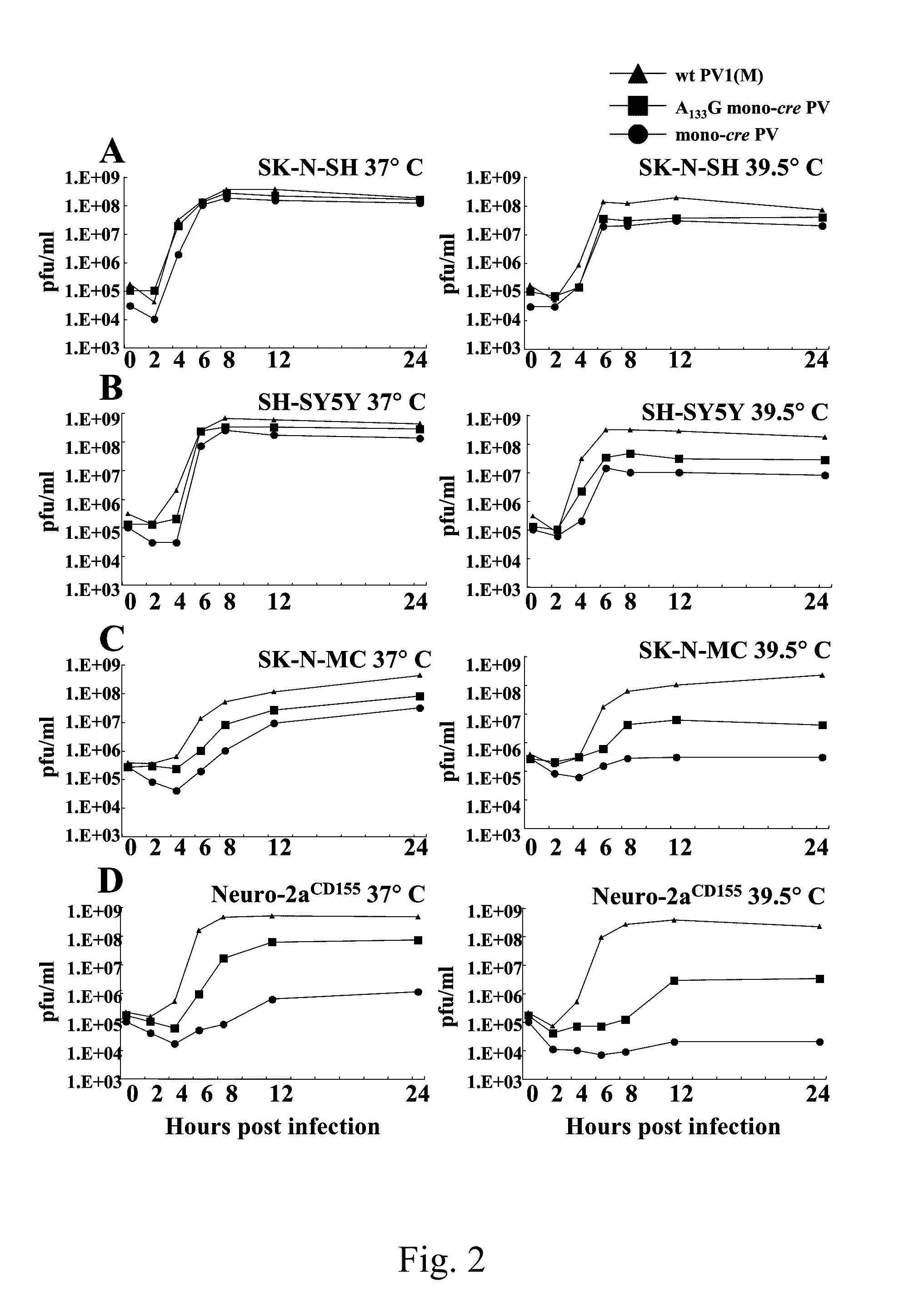
Attenuated poliovirus
ActiveUS8066983B2Enhanced replication propertyStably attenuatedBiocideSsRNA viruses positive-sensePoliomyelitisPoliovirus Receptor
A novel and stable attenuated poliovirus, which replicates in neuroblastoma cells, is produced by engineering an indigenous replication element (cre), into the 5′ non-translated genomic region and inactivating the native cre element located in the coding region of 2C (mono-crePV). The stably attenuated poliovirus replicates in a neuroblastoma model (Neuro-2aCD155 tumors) expressing CD155, the poliovirus receptor, and is effective for oncolytic treatment and cure of solid tumors, such as neuroblastoma.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
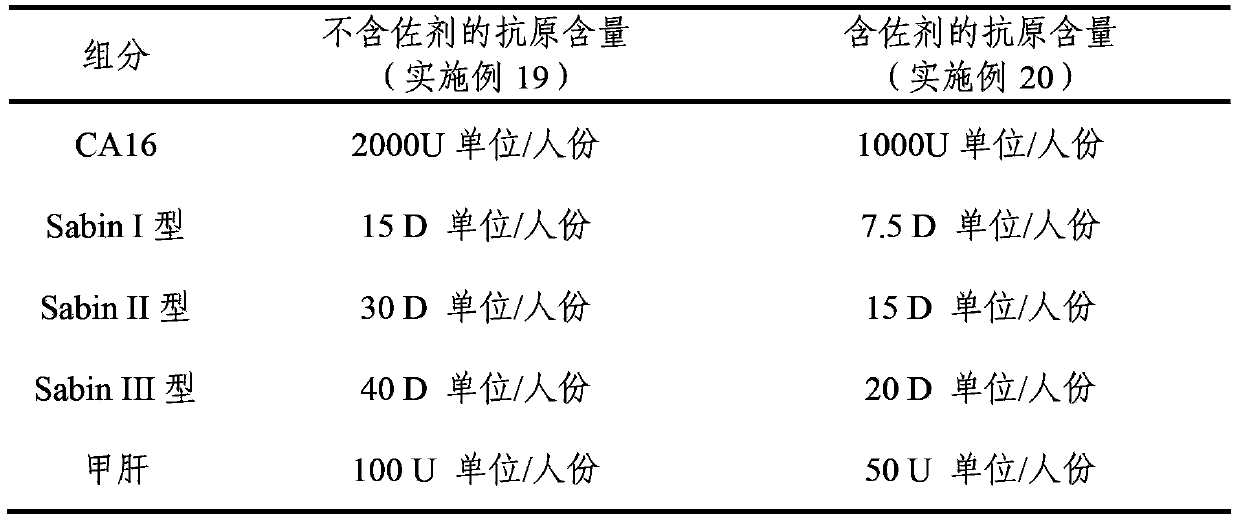
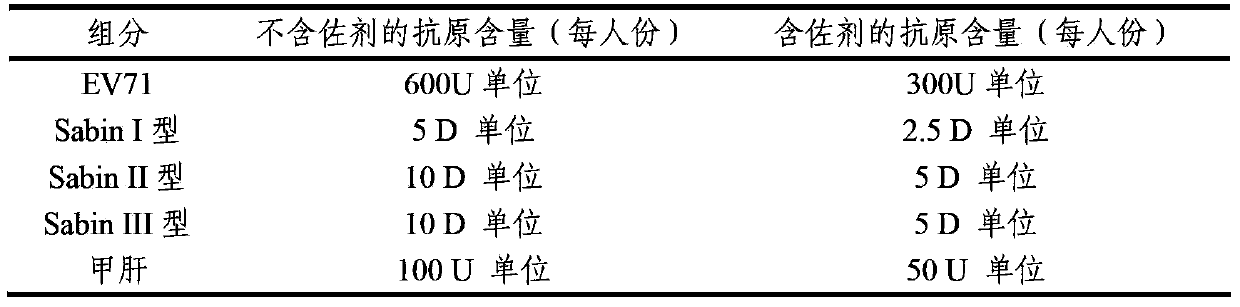
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
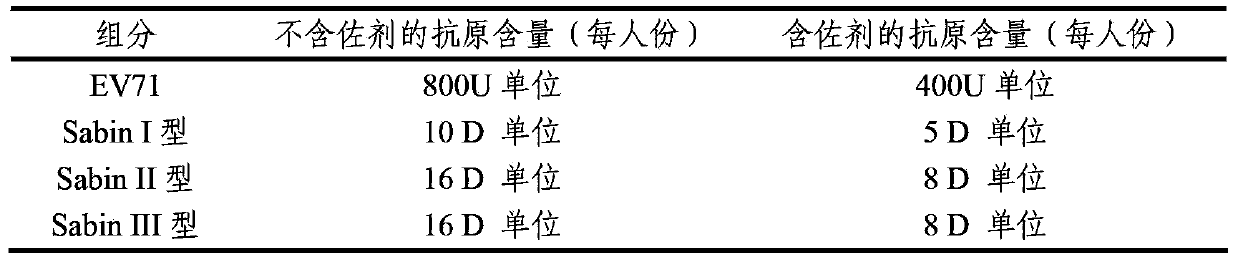
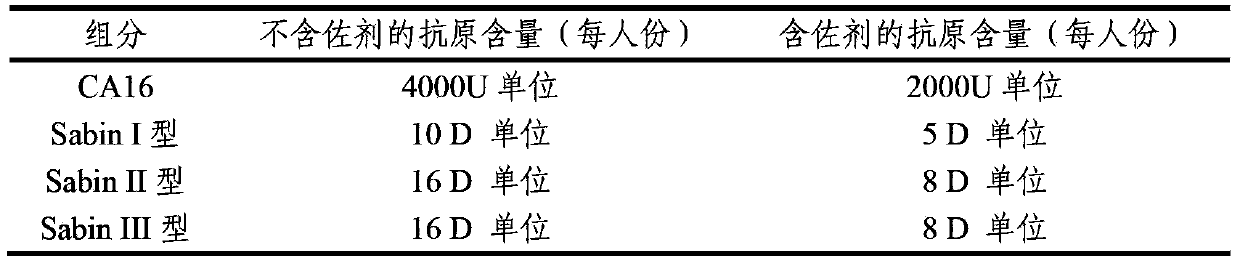
Multivalent immunogenic composition containing enterovirus antigens
ActiveCN103386126AImproving immunogenicityImprove securityBacterial antigen ingredientsViral antigen ingredientsHepatitis A AntigensTetanus toxoids
The invention provides a multivalent immunogenic composition containing enterovirus antigens. The composition comprises inactivated EV71 antigens and / or inactivated CA16 antigens, and inactivated polio antigens. The composition can further comprise antigens selected from hepatitis A antigens, hepatitis B antigens, acellular pertussis antigens, tetanus toxoid, diphtheria toxoid, Haemophilus influenzae type b capsular polysaccharide, and meningococcal polysaccharide antigens, as well as physiologically acceptable carriers combined with bacterial polysaccharide antigens. The invention also provides a preparation method of the composition. The composition can prevent invasion of a plurality of pathogens simultaneously without interference among the antigens, and the immunogenicity is no less than that of individually activated antigens. With the composition, vaccination processes are significantly simplified, and the vaccination efficiency is improved with reduced costs.
Owner:SINOVAC BIOTECH
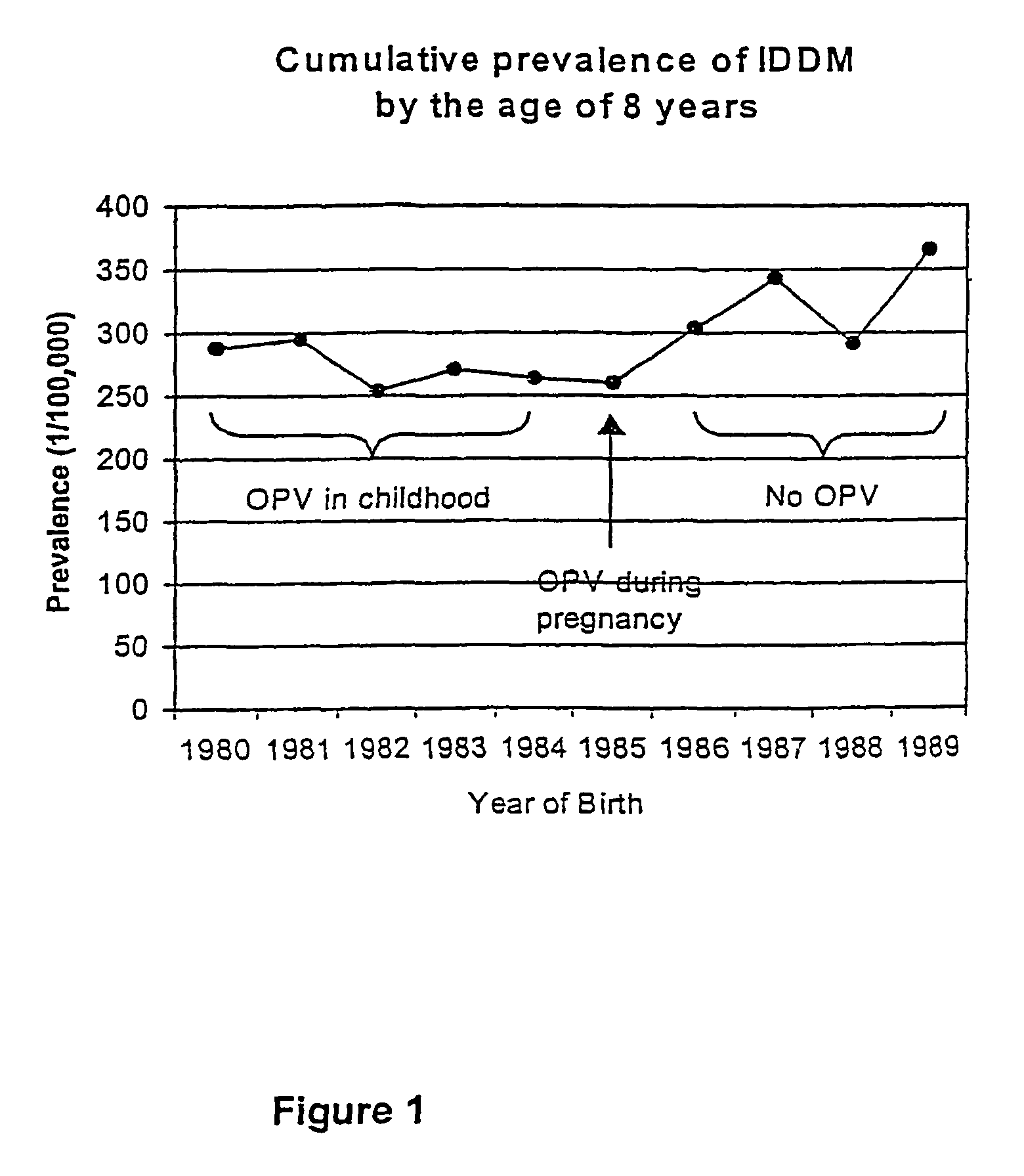
Prevention of Type 1 diabetes and other non-polio enterovirus diseases
InactiveUS20060240466A1Avoid harmful side effectHarmful side effectBiocideSsRNA viruses positive-senseDiseaseSide effect
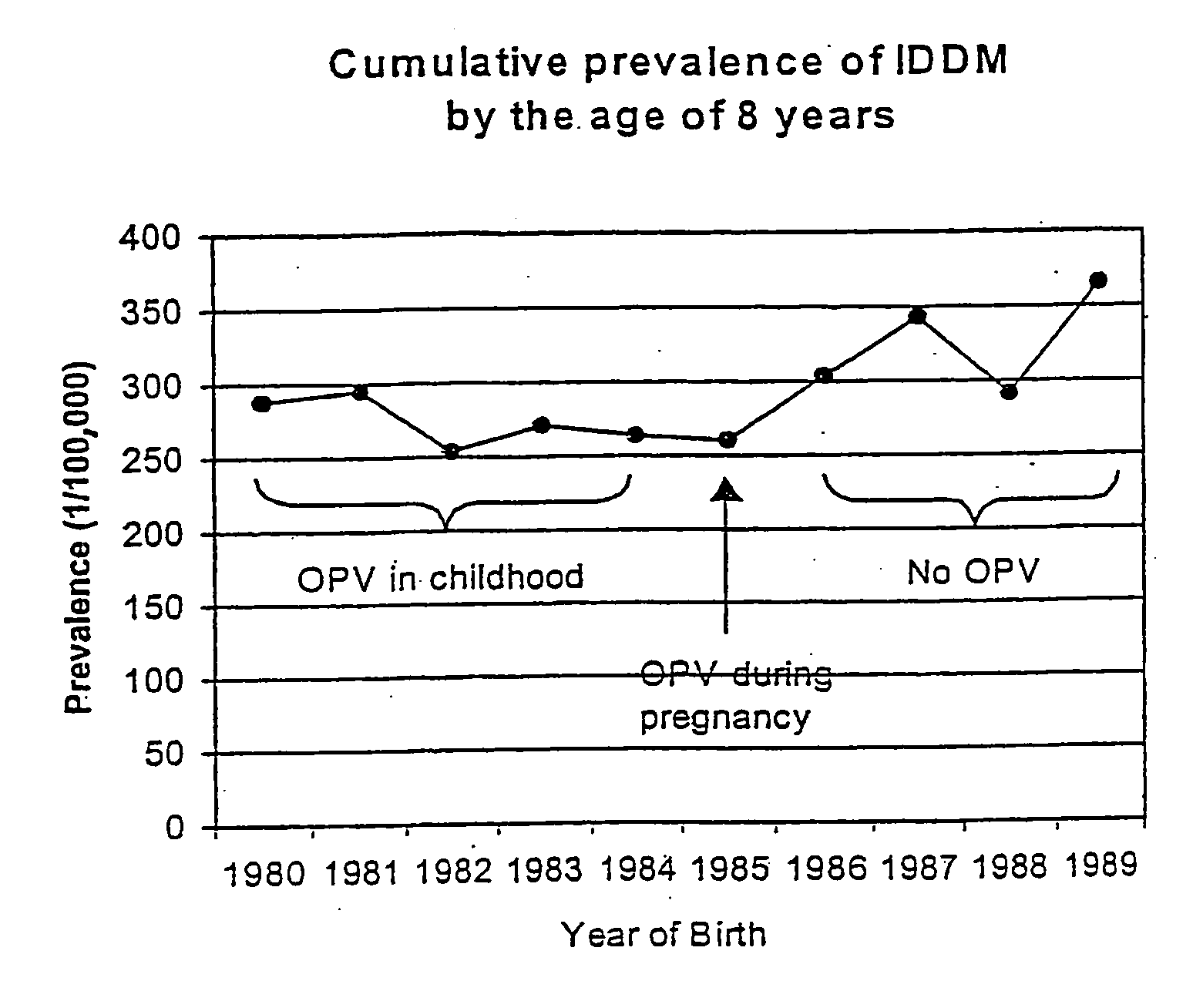
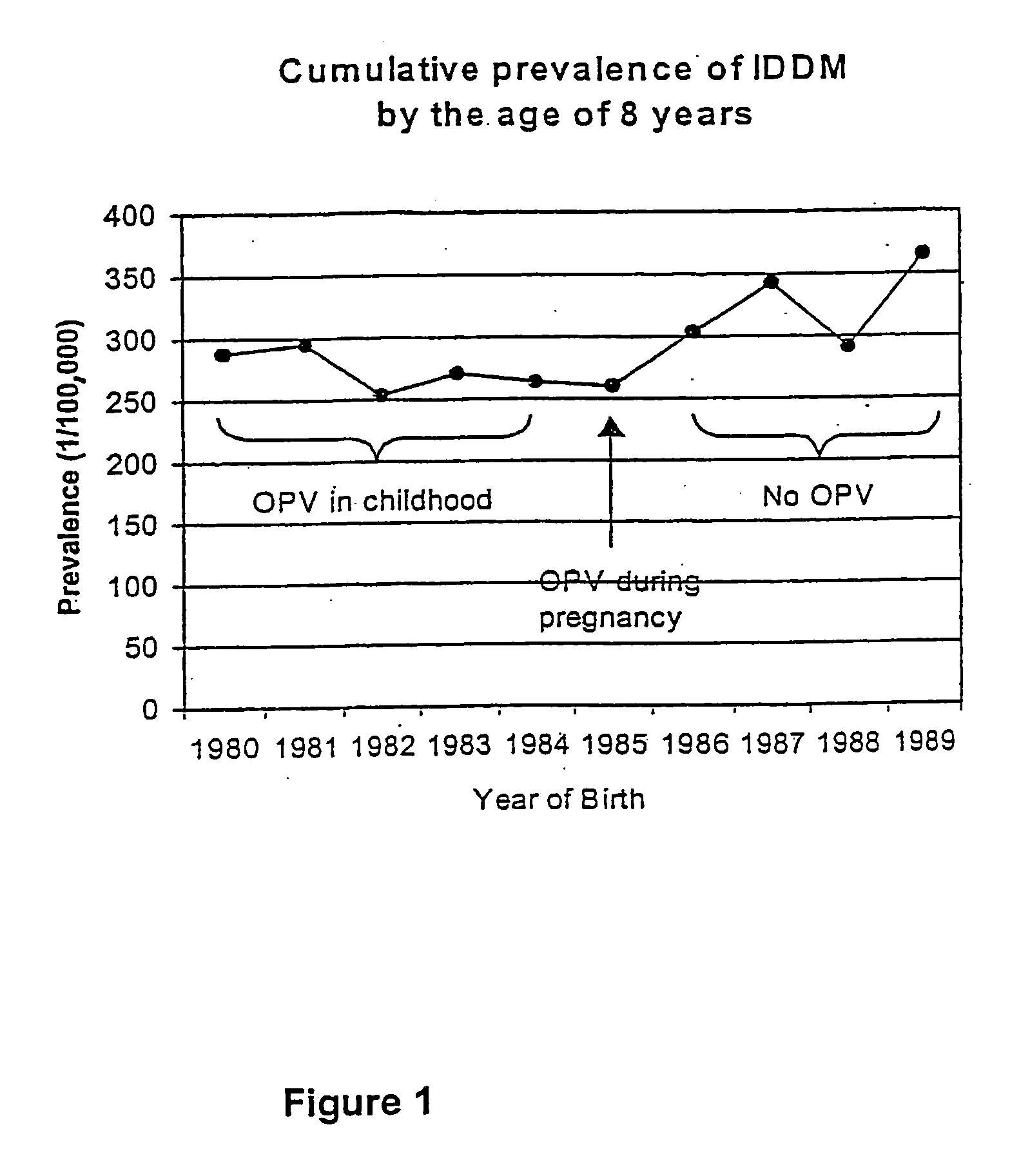
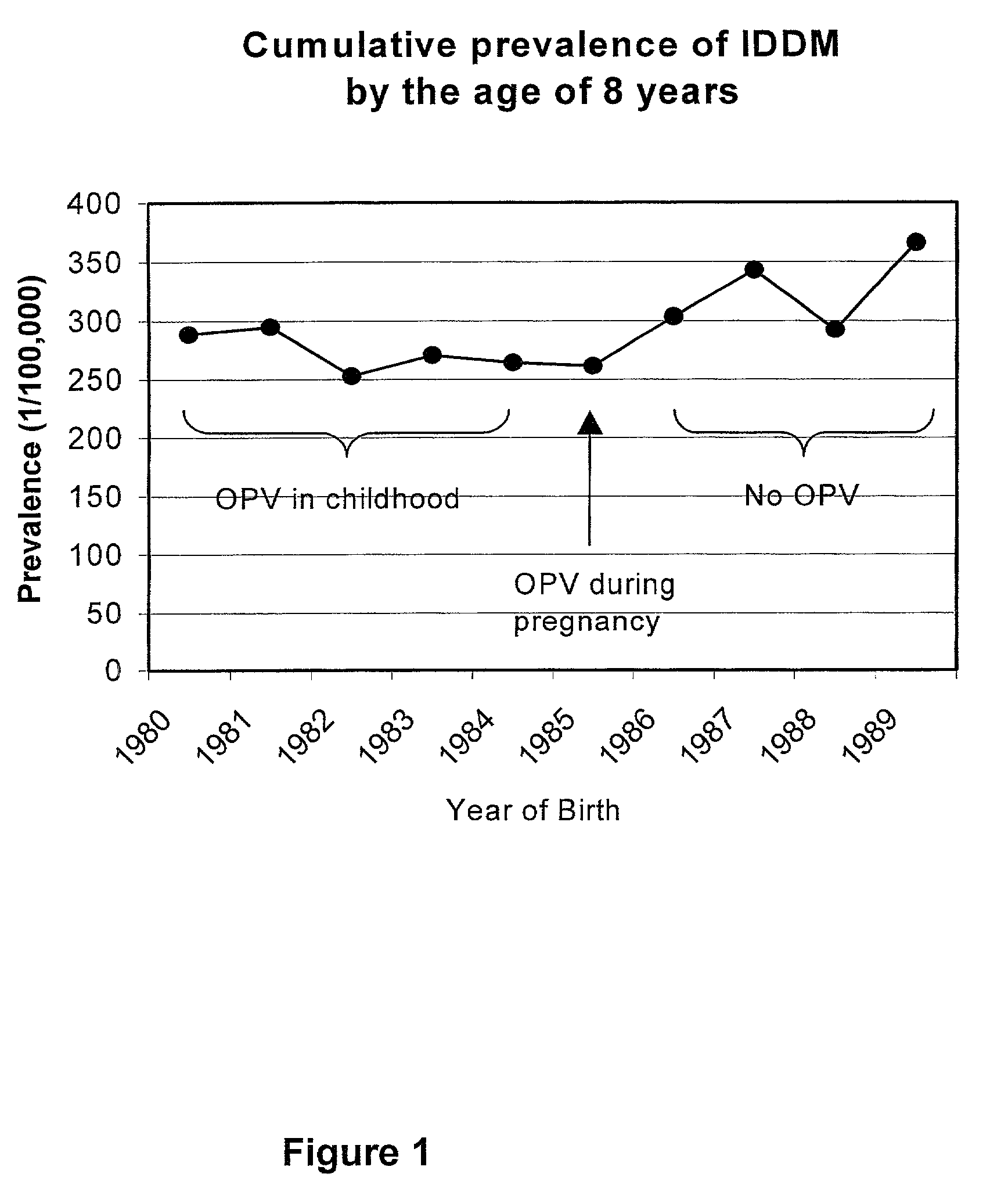
Live virus vaccines comprise attenuated viruses, while other vaccines comprise killed viruses or parts thereof. It has now been found that the immune response induced by oral poliovirus vaccine (OPV), which is a live vaccine, is cross-reactive with non-polio enteroviruses. OPV is therefore useful in the prevention of non-polio enterovirus diseases, especially Type 1 diabetes mellitus (IDDM). OPV is also useful in combination with killed / subunit non-polio enterovirus vaccines, whereby it prevents harmful side-effects of the killed / subunit vaccine by shifting the immune response from a harmful Th2-type response to a Th1 type response.
Owner:HYOTY HEIKKI +1
Multi-component vaccine comprising at least three antigens to protect against disease cased by Haemophilus influenzae
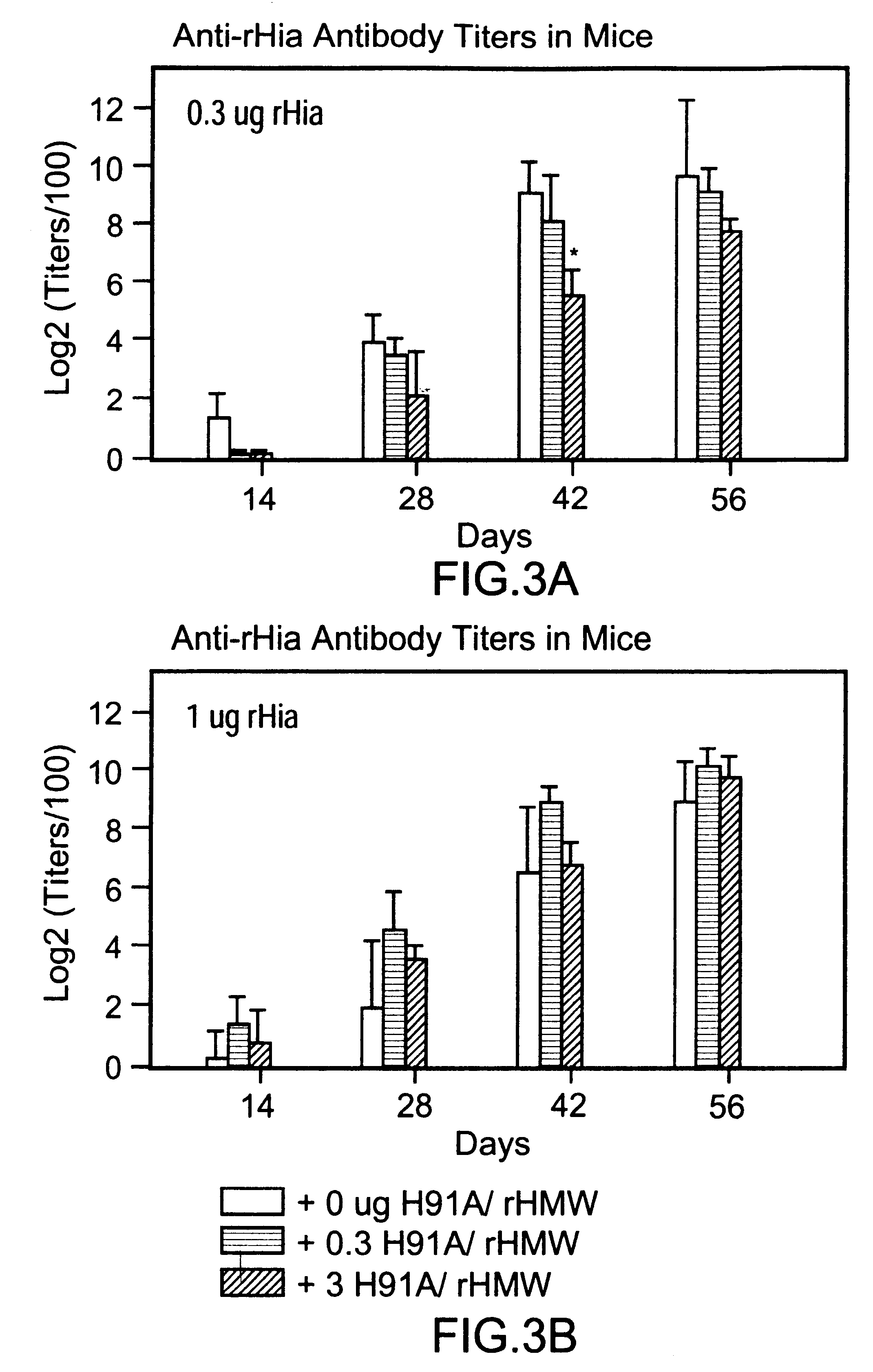
InactiveUS6342232B1No suppression of anti-rHMW responseStimulate immune responseAntibacterial agentsSenses disorderProteolysisDiphtheria vaccination
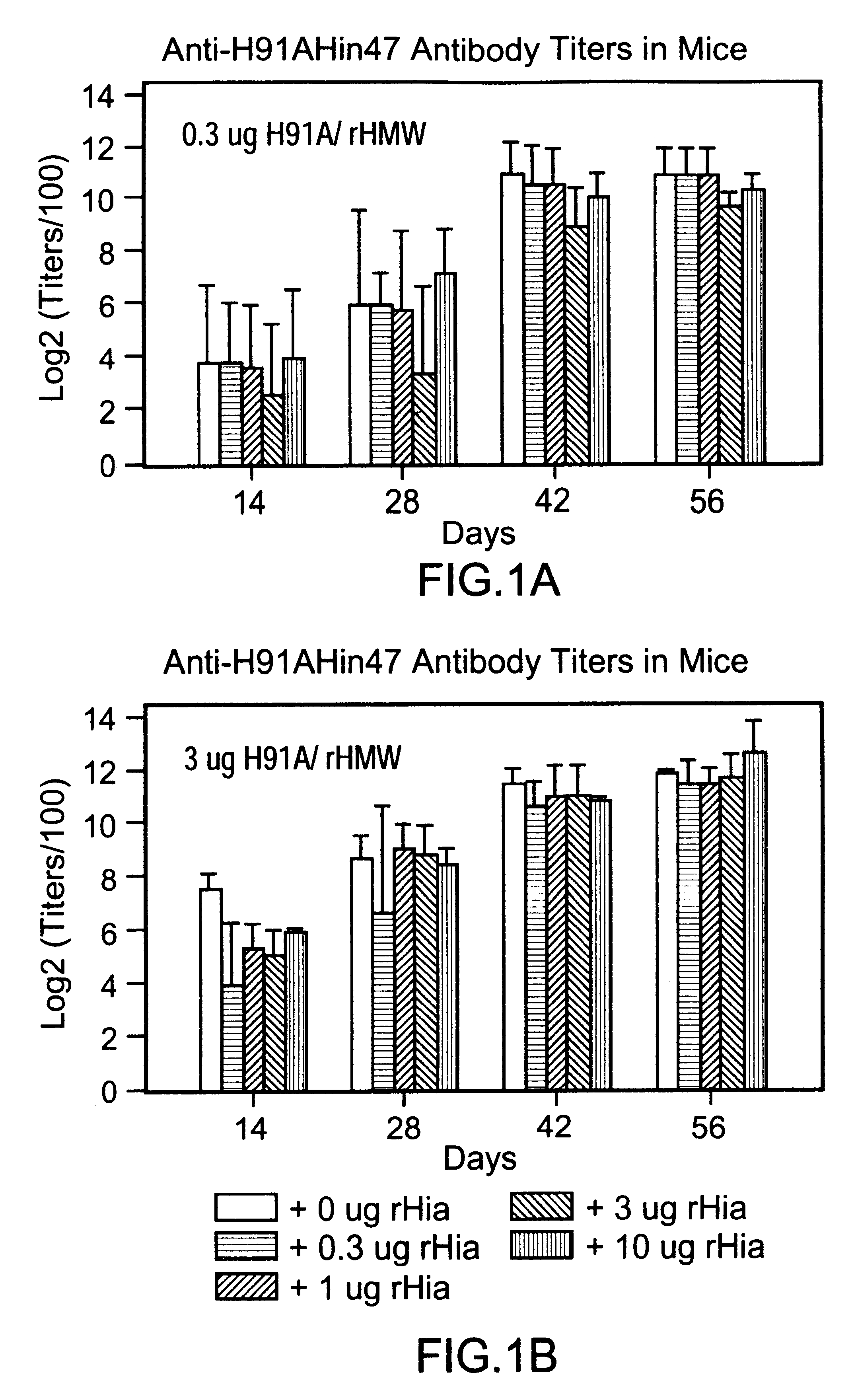
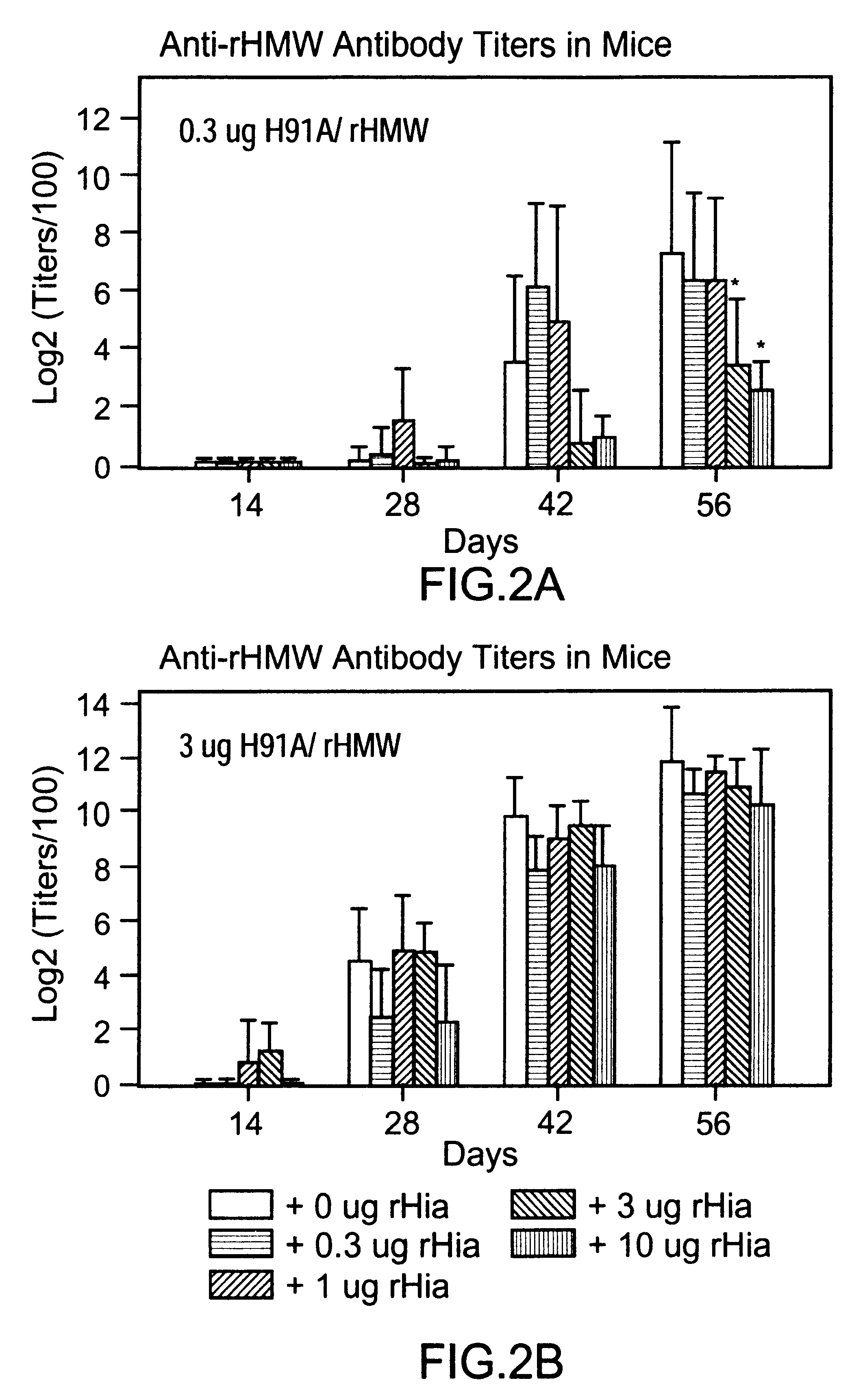
A multi-component immunogenic composition confers protection on an immunized host against infection caused by Haemophilus influenzae. Such composition comprises at least three different antigens of Haemophilus influenzae, two of which are adhesins. High molecular weight (HMW) proteins and Haemophilus influenzae adhesin (Hia) proteins of non-typeable Haemophilus influenzae comprise the adhesin components while the other antigen is a non-proteolytic analog of Hin47 protein. Each component does not impair the immunogenicity of the others. The Haemophilus vaccine may be combined with DTP component vaccines, which may contain inactivated poliovirus, including type 1, type 2 and / or type 3, and / or a conjugate of a capsular polysaccharide of Haemophilus influenzae and tetanus or diphtheria toxoid, including PRP-T, to provide a multi-valent component vaccine without impairment of the immunogenic properties of the other antigens.
Owner:AVENTIS PASTUER LTD
Gene recombinant vaccine for preventing enterovirus 71 infection and preparation method thereof
The invention discloses a gene recombinant vaccine for preventing enterovirus 71 (EV71) infection and a preparation method thereof. The inflection comprises multiple diseases related with the nervous system such as hand-foot-and-mouth disease, paralytic diseases of sterile meningitis, cephalitis and poliomyelitis and the like. Escherichia coli labile enterotoxin B subunit (LTB) is used as an immunological enhancement adjuvant, two fragments of linear neutralizing epitope SP55 and SP70 in EV71 virus coat protein VP1 are used as antigens, prokaryotic expression plasmids of LTB-SP55-SP70 fusion genes are constructed by using gene engineering technology, the plasmids are expressed in escherichia coli, and a recombinant expression product is purified for preparing the EV71 virus gene engineering vaccine.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Vaccine
InactiveUS20100040647A1Adequate and improved level of protectionAntibacterial agentsSsRNA viruses positive-senseDiseaseTetanus
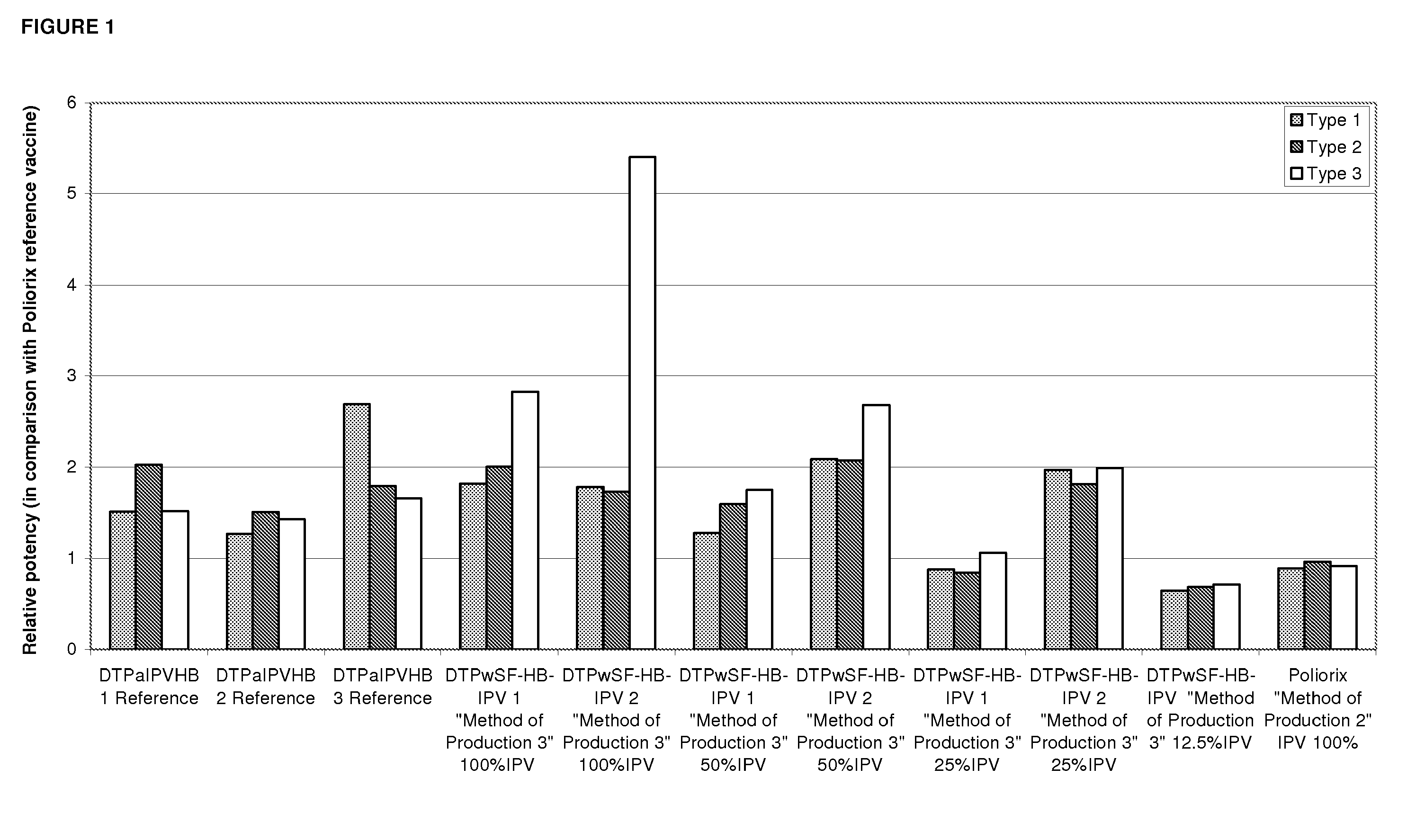
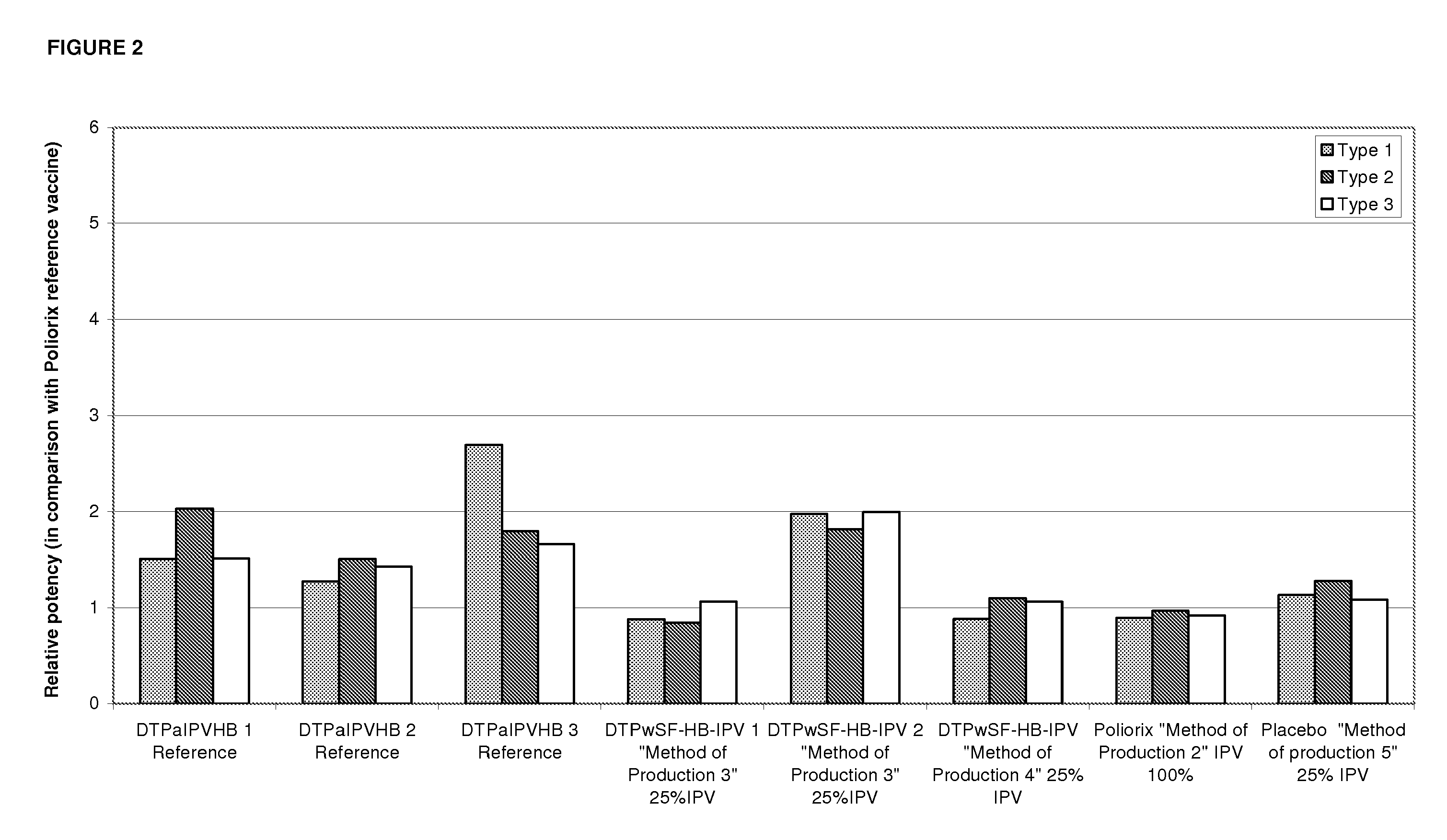
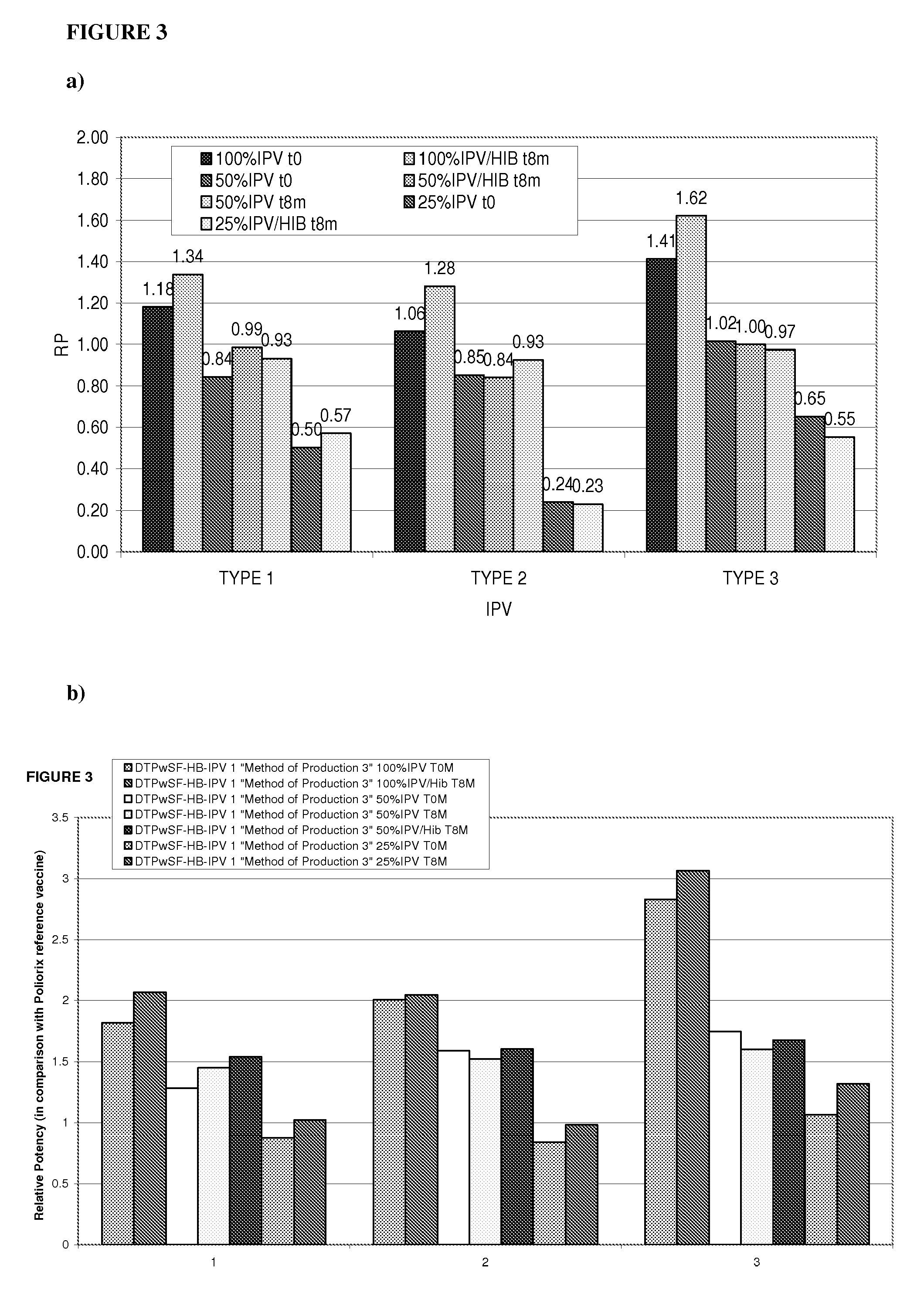
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus are provided
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vector for expressing protein of poliovirus sample particles and method for preparing poliovirus sample particles
ActiveCN104480143AAssembly influenceAffect expression imbalanceViral antigen ingredientsInactivation/attenuationDiseasePoliovirus
The invention discloses a vector for expressing protein of poliovirus sample particles and a method for preparing the poliovirus sample particles. The vector contains expression cassettes with the following structures: an arbitrary gene in poliovirus structural proteins VP0, VP1 and VP3 genes is located in the downstream of a promoter 1, and the other two genes are connected by virtue of a 2A sequence and are located in the downstream of a promoter 2, and directions of promoting expressions of the two promoters are opposite. The method for preparing the poliovirus sample particles comprises the following steps: transfecting the carrier to a corresponding host cell, culturing to obtain virus particles or recombinant baculovirus, and infecting the virus to the host cell if the recombinant baculovirus is obtained so as to obtain the virus sample particles. The poliovirus sample particles can be used for respectively inducing high-titer neutralization titer in a body, and can be used as a vaccine for preventing and treating poliovirus infected related diseases.
Owner:SOUTH CHINA UNITED VACCINE INST
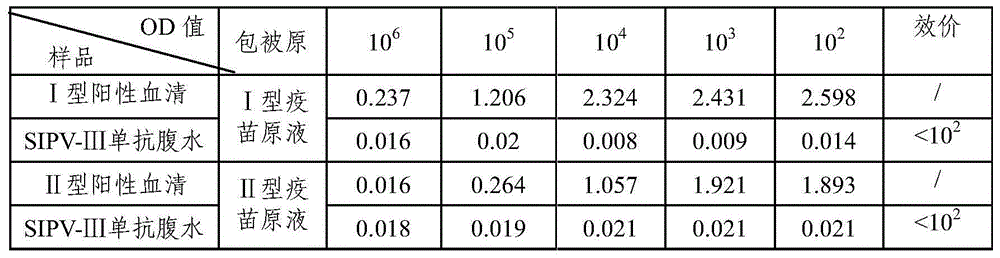
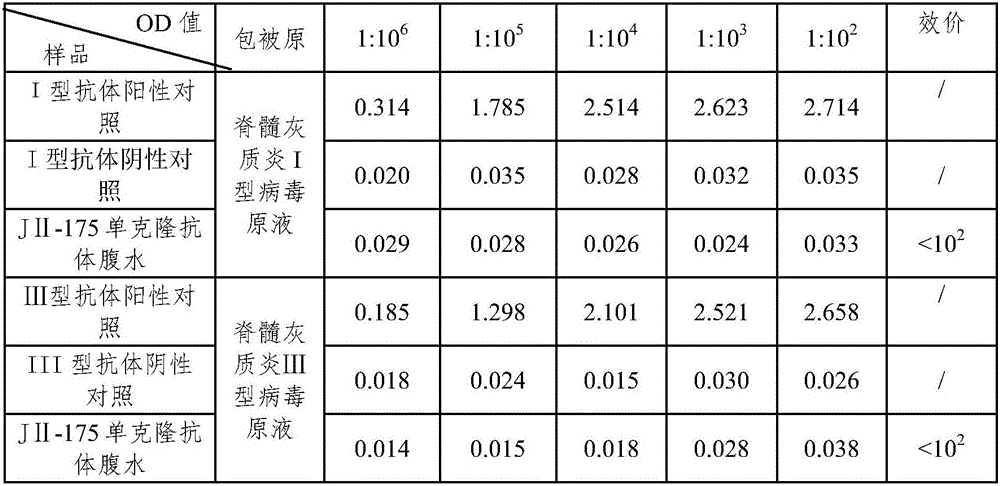
Sabin strain poliovirus type III monoclonal antibody and application thereof
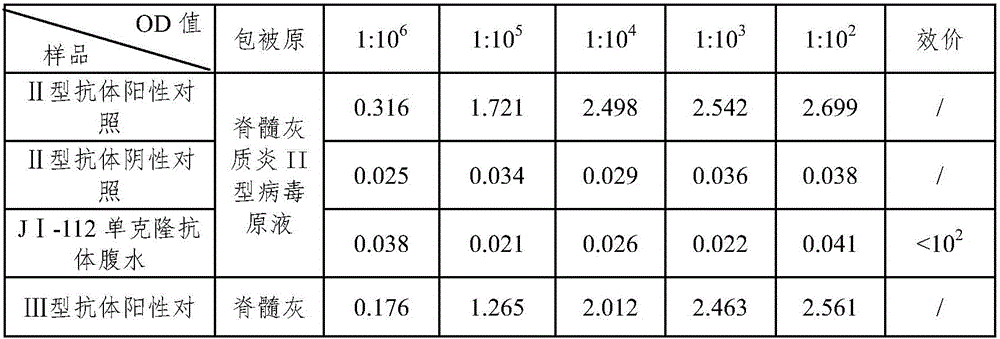
ActiveCN104371980AHigh titer reactivityHigh School and ValenceImmunoglobulins against virusesMicroorganism based processesEnterovirusPoliomyelitis
The invention provides a Sabin strain poliovirus type III monoclonal antibody and an application thereof, and belongs to the field of immunology. After a mouse is immunized and inoculated with Sabin strain poliovirus type III, mouse spleen cells are fused with mouse myeloma cells, a hybridoma cell strain producing the anti-Sabin strain poliovirus type III specific monoclonal antibody is screened and has the preservation number of CGMCC No.9233, and the secreted monoclonal antibody has high titer, has strong neutralizing activity, and can effectively block infection of the poliovirus type III; at the same time, the antibody can specifically distinguish the poliovirus type III from poliovirus type I, poliovirus type II and other various enteroviruses, has good specificity, and can be used for preparing poliovirus type III antigen content detection kits and antibody detection kits, also can be used for identification detection of the poliovirus type III, and has the broad application prospect.
Owner:SINOVAC BIOTECH
Prevention of type 1 diabetes and other non-polio enterovirus diseases
InactiveUS7972592B2Avoid harmful side effectHarmful side effectBiocideSsRNA viruses positive-senseDiseaseSide effect
Live virus vaccines comprise attenuated viruses, while other vaccines comprise killed viruses or parts thereof. It has now been found that the immune response induced by oral poliovirus vaccine (OPV), which is a live vaccine, is cross-reactive with non-polio enteroviruses. OPV is therefore useful in the prevention of non-polio enterovirus diseases, especially Type 1 diabetes mellitus (IDDM). OPV is also useful in combination with killed / subunit non-polio enterovirus vaccines, whereby it prevents harmful side-effects of the killed / subunit vaccine by shifting the immune response from a harmful Th2-type response to a Th1 type response.
Owner:HYOTY HEIKKI +1
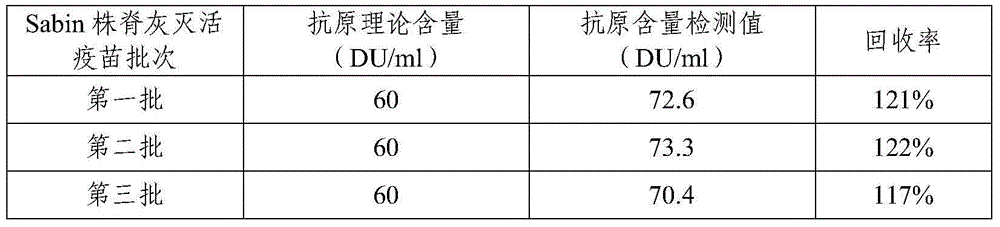
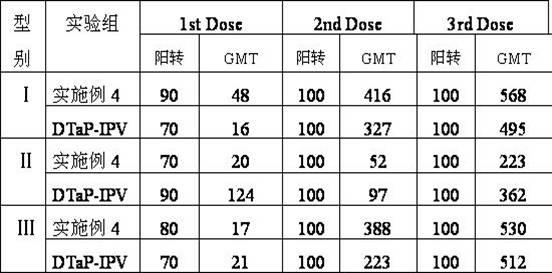
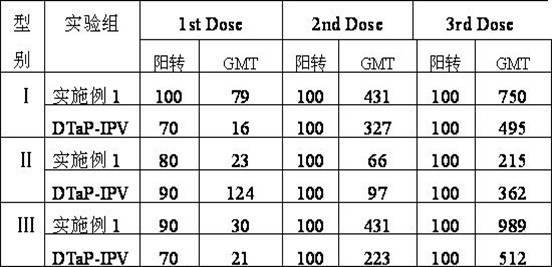
Combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin inactivated poliovirus and preparation thereof
InactiveCN102178949ASimplified and expanded immunization programsReduce the number of vaccinationsAntibacterial agentsAntiviralsDiseaseTetanus toxoids
The invention provides combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin (DTaP-sIPV) inactivated poliovirus and preparation thereof. The DTaP-sIPV is characterized in that each 100ml of the combined vaccine comprises the following components: 400-1800ug (PN) of acellular pertussis (AP) stock solution, 300-700Lf of tetanus toxoid (TT), 1000-2500Lf of diphtheria toxoid (DT), 126-154mg of Al(OH)<3>, 3000-6000DU of sIPV I, 5700-7100DU of sIPV II, 4500-9000DU of sIPV III, 765-935mg of NaCl, 0-600mg of 2-phenoxyethanol and the balance of H<2>O. Compared with the existing products, the DTaP-sIPV has the advantages of higher biological safety, better side reaction and the like; and the DTaP-sIPV has the beneficial effects of preventing a plurality of target diseases, reducing inoculating needles, simplifying immunization programs, improving inoculation rate, reducing opportunity of cross infection, being popular with a majority of parents and children, saving various expenses and facilitating smooth promotion of immunization plan.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Poliovirus type II monoclonal antibody and application thereof
ActiveCN105907724AHigh titer reactivityGood ability to neutralize virusImmunoglobulins against virusesMicroorganism based processesPoliomyelitisImmunologic function
The invention relates to a poliovirus type II monoclonal antibody and an application thereof, belonging to the field of immunology and the field of vaccines. In particular, the invention relates to a hybridoma cell strain generating the poliovirus type II monoclonal antibody, the monoclonal antibody produced by the hybridoma cell strain and applications of the hybridoma cell strain and the monoclonal antibody, wherein the collection number of the hybridoma cell strain is CGMCC No.12291.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Advanced oral poliovirus vaccine (Adv-OPV) deprived of the possibility of vaccine associated paralytic poliomyelities (VAPP)
The present invention relates to a novel recombinant Sabin type 1 poliovirus vector for the immunogenicity of neutralizing antibody against polioviral infection, which comprises: (a) a genomic nucleotide sequence of a parent Sabin type 1 poliovirus; (b) a nucleotide sequence encoding an additional polioviral cleavage site; and (c) a nucleotide sequence of a conformational epitope encoding a VP1 neutralizing epitope of poliovirus type 2 or 3 and linked to the nucleotide sequence of (b).
Owner:JW CREAGENE
Cold-adapted-viral-attenuation (cava) and novel attenuated poliovirus strains
A poliovirus (PV) strain was attenuated by a novel method of Cold-Adapted-Viral-Attenuation (CAVA). The resulting recombinant attenuated PV, CAVA-PV, shows wild- type replication at 30DEG C, but no substantial replication at 37DEG C. The inability to replicate at 37DEG C is defined by an inability to quantify virus during infection at this temperture by titration (infectious units), qPCR (viral RNA) or Electron Microscopy (visual signs of infection). CAVA-PV is genetically stable under production conditions and shows utility for use as the backbone to produce attenuated strains with the same antigenic profile as conventional vaccines by replacing the sequence coding for the capsid of CAVA-PV with sequences coding for capsids of different PV strains. Furthermore, mutations identified in CAVA-PV can be engineered into different, even wild-type and neurovirulent poliovirus background strains to obtain additional CAVA-PV strains.
Owner:JANSSEN VACCINES & PREVENTION BV
Prevention of Type 1 diabetes and other non-polio enterovirus diseases
InactiveUS7090855B1Harmful side effectAvoid harmful side effectSsRNA viruses positive-senseViral antigen ingredientsDiseaseSide effect
Live virus vaccines comprise attenuated viruses, while other vaccines comprise killed viruses or parts thereof. It has now been found that the immune response induced by oral poliovirus vaccine (OPV), which is a live vaccine, is cross-reactive with non-polio enteroviruses. OPV is therefore useful in the prevention of non-polio enterovirus diseases, especially Type 1 diabetes mellitus (IDDM). OPV is also useful in combination with killed / subunit non-polio enterovirus vaccines, whereby it prevents harmful side-effects of the killed / subunit vaccine by shifting the immune response from a harmful Th2-type response to a Th1 type response.
Owner:HYOTY HEIKKI +1
Tephromyelitis type I virus monoclonal antibody and application thereof
ActiveCN106119210AHigh titer reactivityGood ability to neutralize virusImmunoglobulins against virusesAntiviralsPoliomyelitisImmunologic function
The invention relates to a tephromyelitis type I virus monoclonal antibody and application thereof and belongs to the field of immunology and the field of vaccinology. More particularly, the invention relates to a tephromyelitis type I virus monoclonal antibody hybridoma cell strain with the preservation number of CGMCC (China General Microbiological Culture Collection Center) No.12292, a monoclonal antibody generated by the hybridoma cell strain and the application of the monoclonal antibody.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Modulation of the poliovirus receptor function
InactiveCN1777622AOrganic active ingredientsGenetic material ingredientsLymphatic SpreadCell adhesion
The present invention relates to the identification, isolation and use of molecules that interfere with functions mediated by the poliovirus receptor (PVR) on cells. The molecule can be used to treat cells with metastatic potential, metastases and cancer. The present invention further provides methods useful for identifying and isolating molecules capable of modulating PVR-mediated adhesion or invasive potential of cells.
Owner:TUFTS UNIV
Poliovirus vaccine for oral administration
ActiveCN103316335AImprove stabilityImprove securityViral antigen ingredientsAntiviralsPoliovirus VaccinesPhosphate
The invention provides a poliovirus vaccine for oral administration. The poliovirus vaccine contains poliovirus antigen, carbohydrate, phosphate and carboxylate. The oral poliovirus vaccine provided by the invention can be adopted to raise stability of the vaccine antigen component, has good stability at temperatures of 37 DEG C, 2-8 DEG C and 25 DEG C, has stable antigen activity, can be used to enhance gastric acid resistance after oral administration of the vaccine finished product, and has good immune response stimulation capability and good safety. The poliovirus vaccine for oral administration contains no other humanized or animal-based protein and has better safety. Meanwhile, the poliovirus vaccine requires low cost, and is suitable for industrial production of the product.
Owner:SINOVAC BIOTECH
Vaccine
InactiveCN101534854AIncrease doseIncreased level of protectionViral antigen ingredientsAgainst vector-borne diseasesDiseaseTetanus
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus (IPV) is provided, which can maintain an adequate or improved level of protection against polio.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Combination vaccine with whole cell pertussis
InactiveUS20110195087A1Safe wayLow costAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, whole cell pertussis and polio. The present invention also relates to inclusion of one or more antigens in the said combination vaccine, for protection against infections caused by Haemophilus influenzae. Hepatitis virus, and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
Attenuated polio viruses
ActiveUS20100158945A1Reduce infectivitySsRNA viruses positive-senseViral antigen ingredientsBase Pair MismatchCoding region
The invention provides an attenuated poliovirus which does not have a base pair mismatch in stem (a) or (b) of domain V of the 5′ non-coding region of its genome, wherein at least seven of the base pairs in stems (a) and (b) are U-A or A-U base pairs.
Owner:SEC OF STATE FOR HEALTH & SOCIAL CARE
Alcohol-based disinfectant
A virucidal disinfectant or antiseptic is disclosed comprising 45-65 wt % of at least one alcohol, and 0.05-0.5 wt % of at least one phosphonate. The pH should be adjusted to lie between 3 and 10. Such disinfectant / antiseptic can be used for disinfecting a living or a non-living object and exhibits activity against a broad range of microorganisms such as viruses, fungi and / or bacteria, in particular against naked viruses such as polio.
Owner:DR SCHUMACHER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com