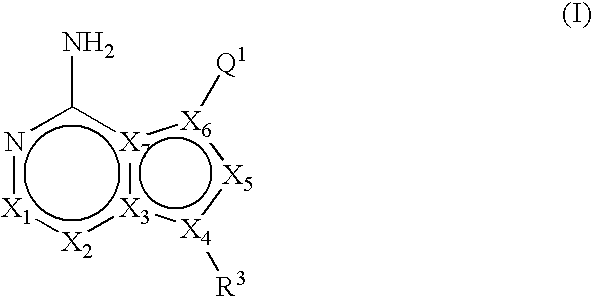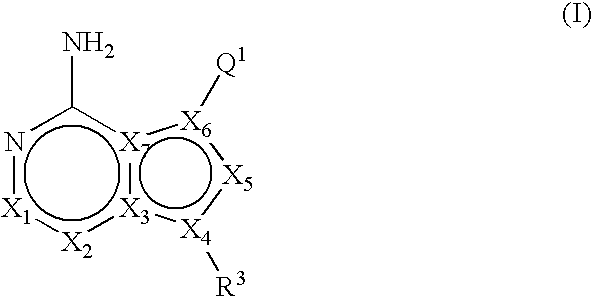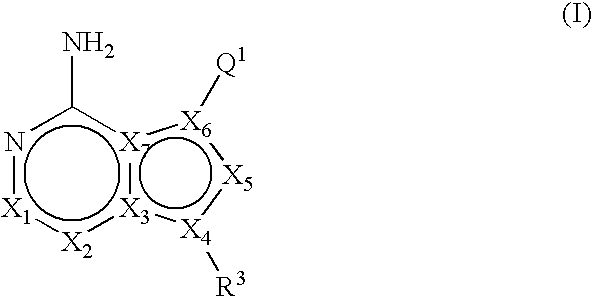Patents
Literature
16046 results about "Carcinosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carcinosis, or carcinomatosis, is disseminated cancer, forms of metastasis, whether used generally or in specific patterns of spread.
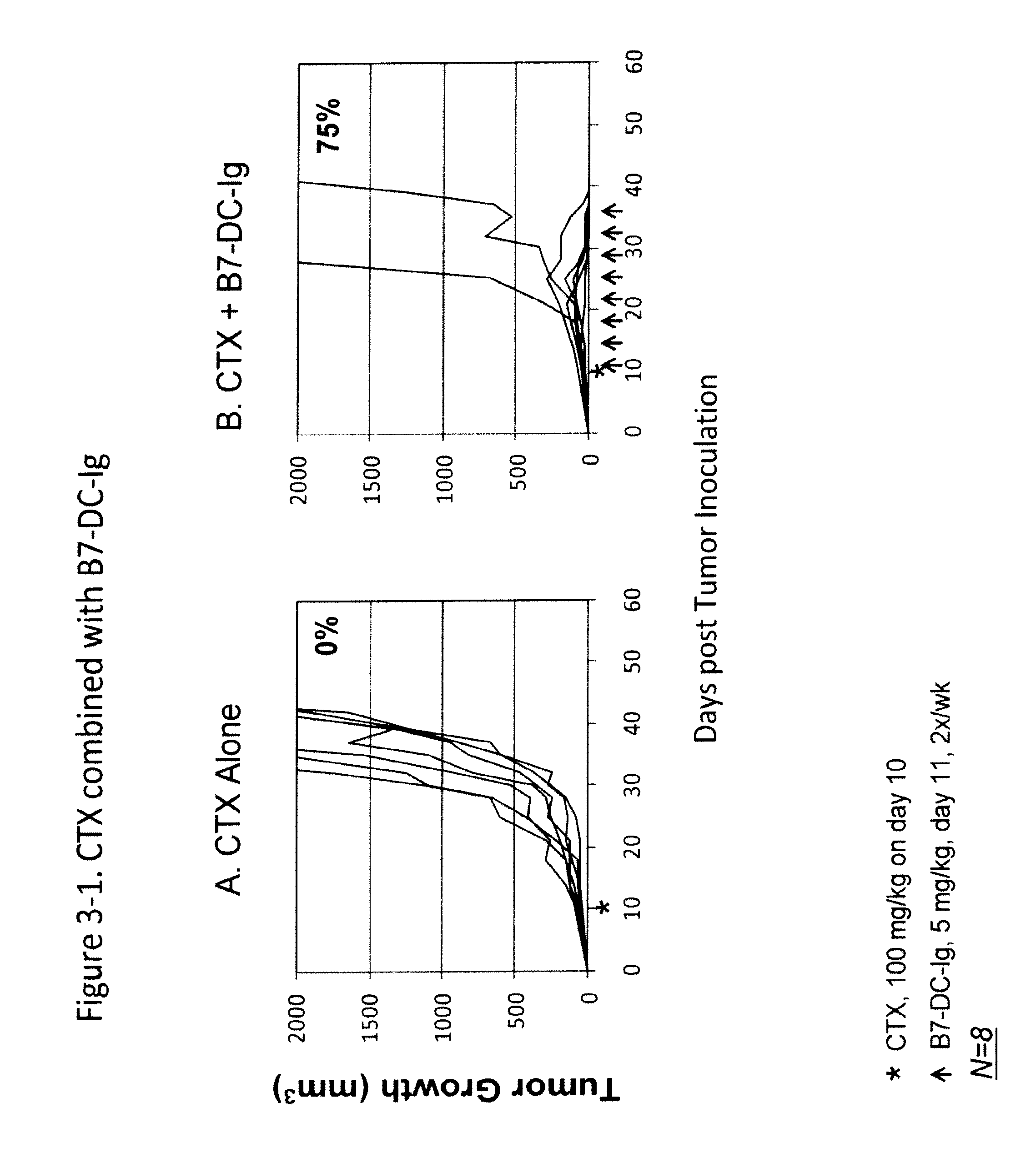
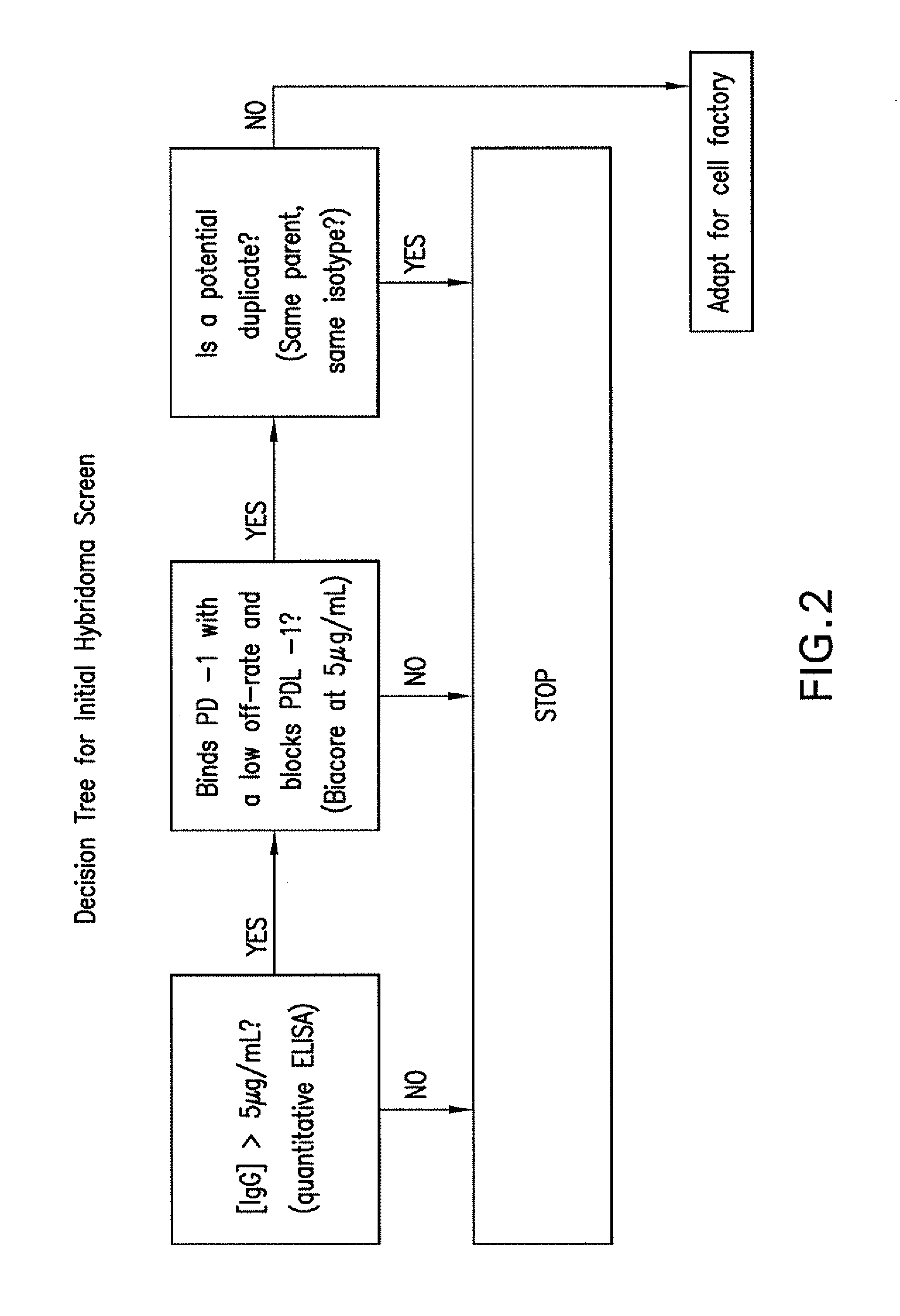
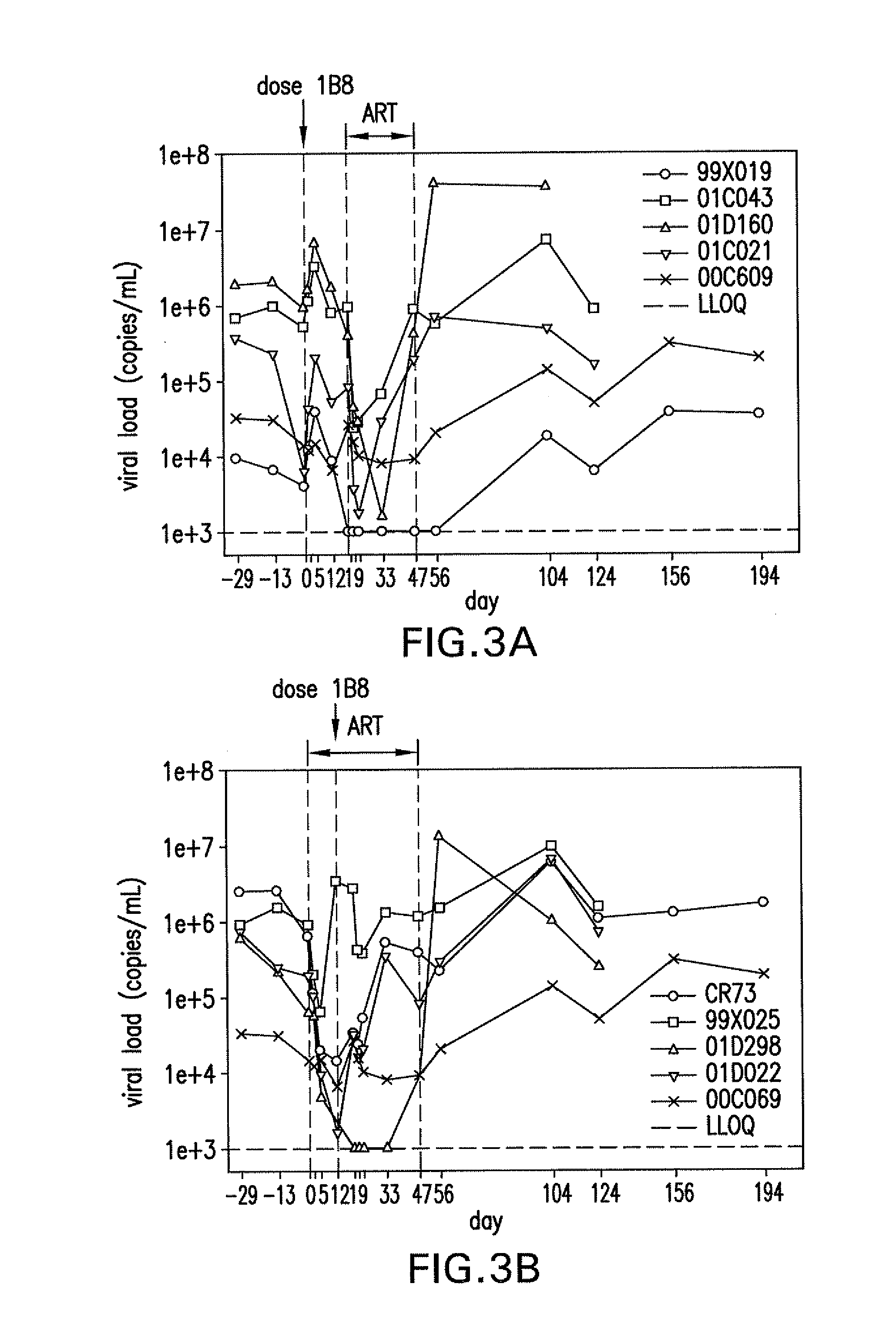
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114649A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsT cellInfective disorder
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease
Drug-Linker-Ligand Conjugates are disclosed in which a Drug is linked to a Ligand via a peptide-based Linker unit. In one embodiment, the Ligand is an Antibody. Drug-Linker compounds and Drug compounds are also disclosed. Methods for treating cancer, an autoimmune disease or an infectious disease using the compounds and compositions of the invention are also disclosed.
Owner:SEAGEN INC
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
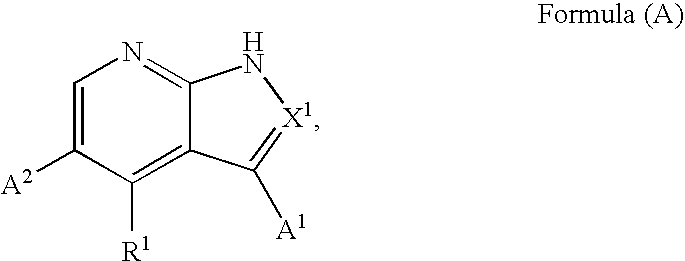
Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors
The present invention provides heteroaryl substituted pyrrolo[2,3-b]pyridines and heteroaryl substituted pyrrolo[2,3-b]pyrimidines that modulate the activity of Janus kinases and are useful in the treatment of diseases related to activity of Janus kinases including, for example, immune-related diseases, skin disorders, myeloid proliferative disorders, cancer, and other diseases.
Owner:INCYTE HLDG & INCYTE
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
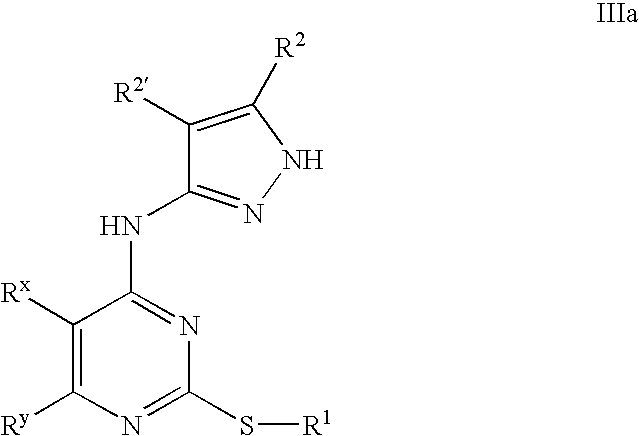
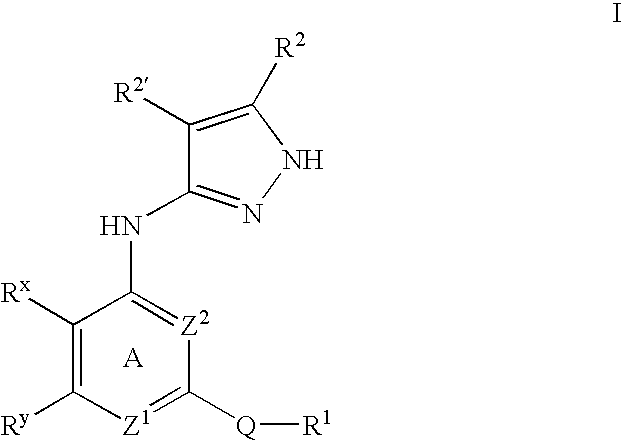
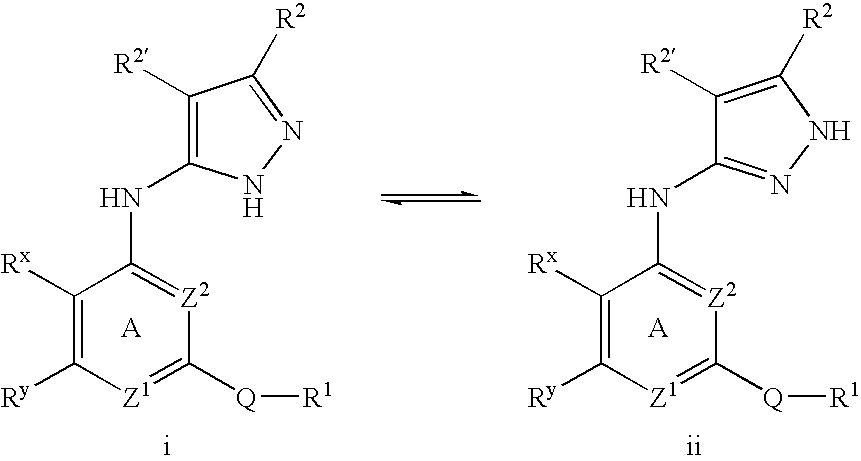
Pyrazole compounds useful as protein kinase inhibitors
This invention describes novel pyrazole compounds of formula IIIa: wherein R1 is T-Ring D, wherein Ring D is a 5-7 membered monocyclic ring or 8-10 membered bicyclic ring selected from aryl, heteroaryl, heterocyclyl or carbocyclyl; Rx, Ry, R2; and R2′ are as described in the specification. The compounds are useful as protein kinase inhibitors, especially as inhibitors of Aurora-2 and GSK-3, for treating diseases such as cancer, diabetes and Alzheimer's disease.
Owner:VERTEX PHARMA INC
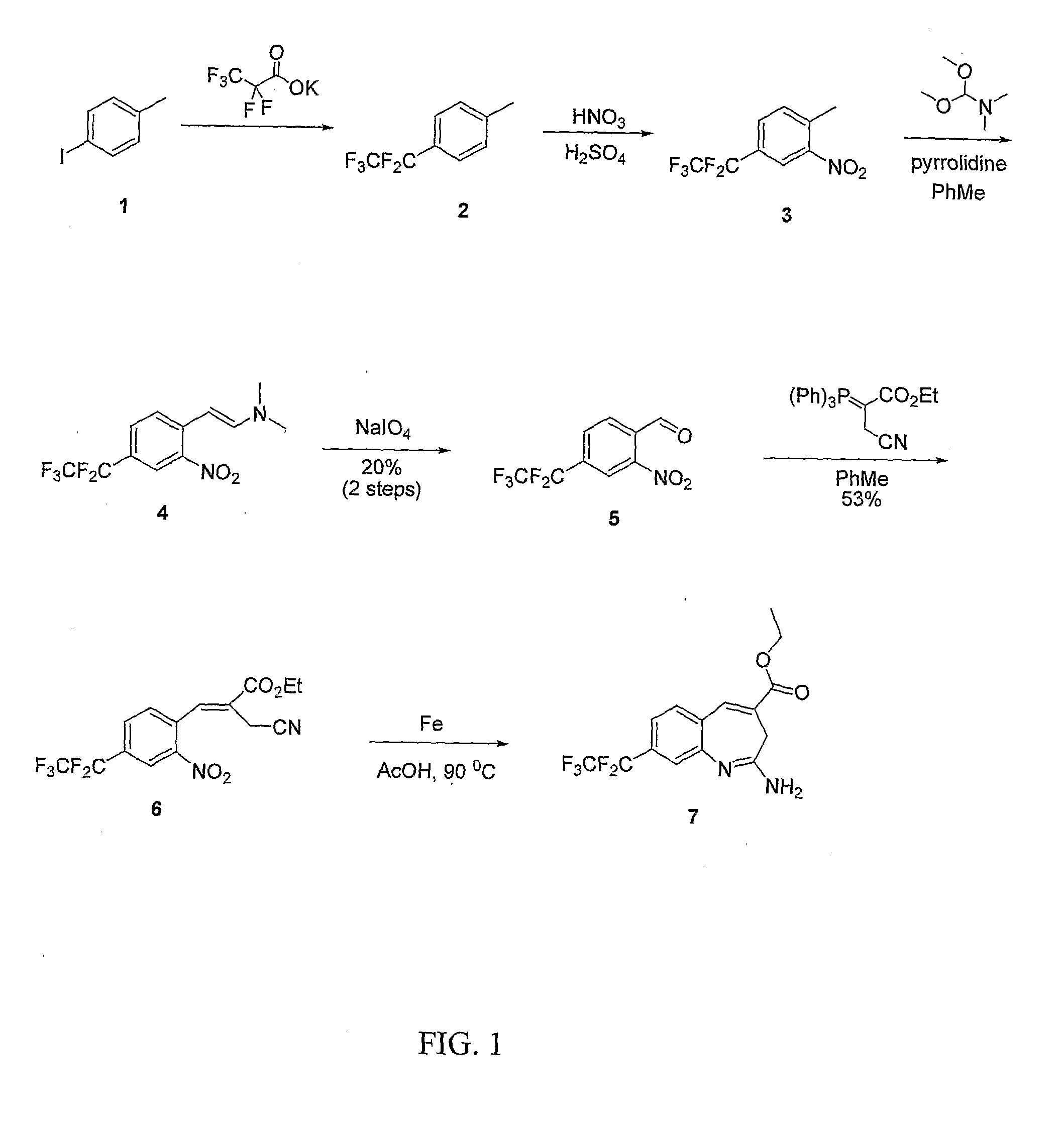
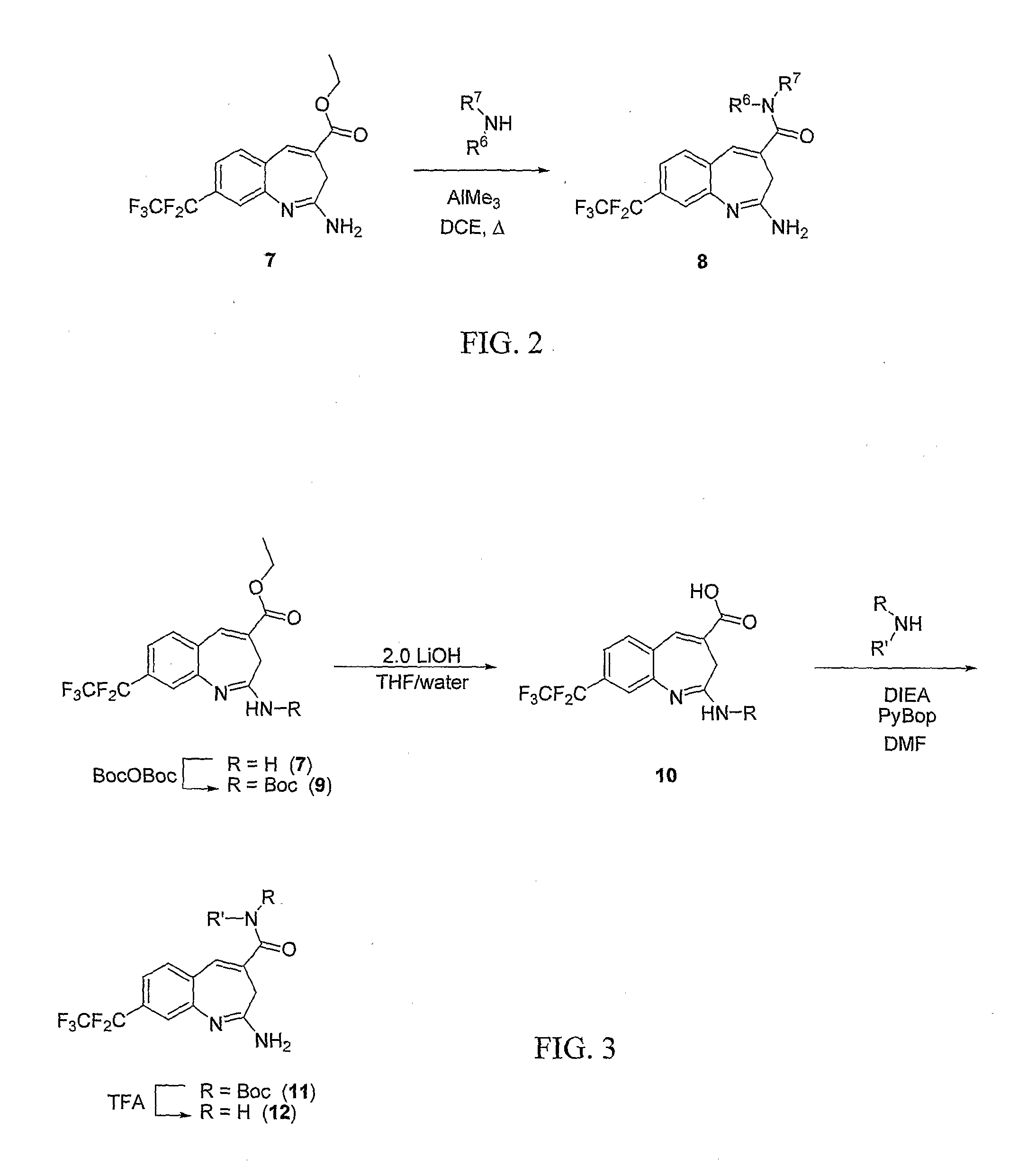
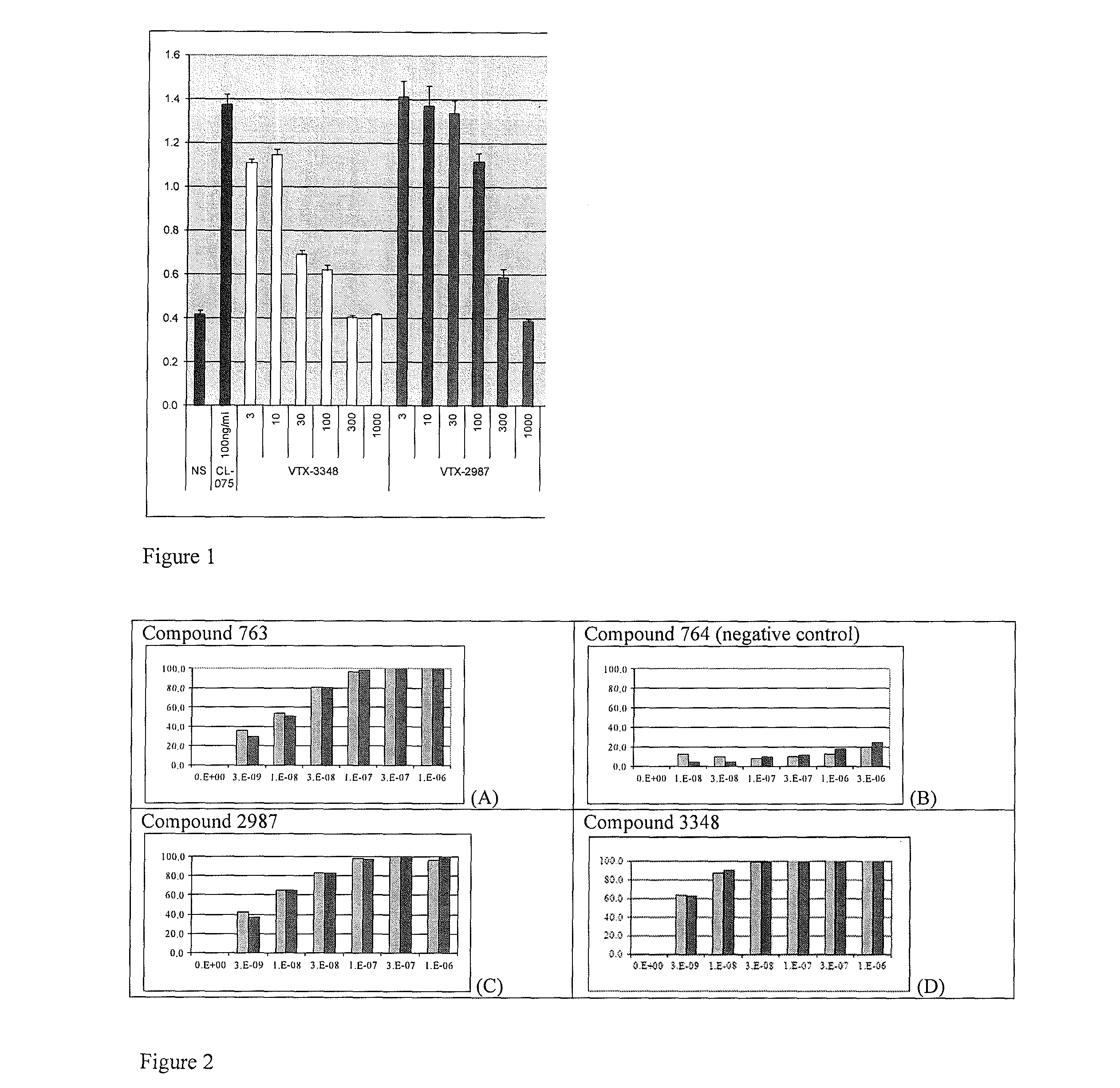
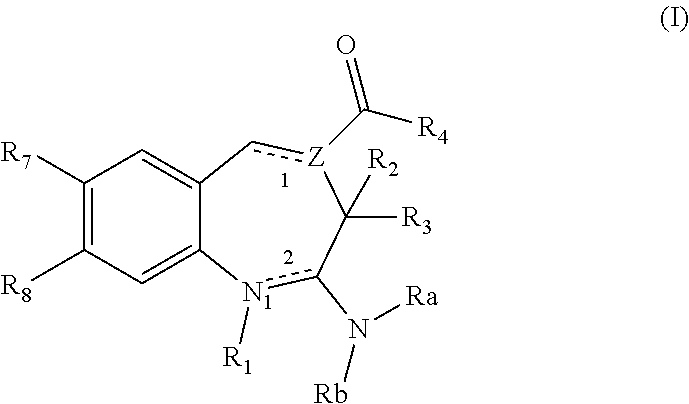
8-Substituted Benzoazepines as Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in the treatment of autoimmunity, inflammation allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer and immunodeficiency.
Owner:ARRAY BIOPHARMA
Substituted Benzoazepines As Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in treating or preventing disease, including cancer, autoimmune disease, fibrotic disease, cardiovascular disease, infectious disease, inflammatory disorder, graft rejection, or graft-versus-host disease.
Owner:ARRAY BIOPHARMA +1
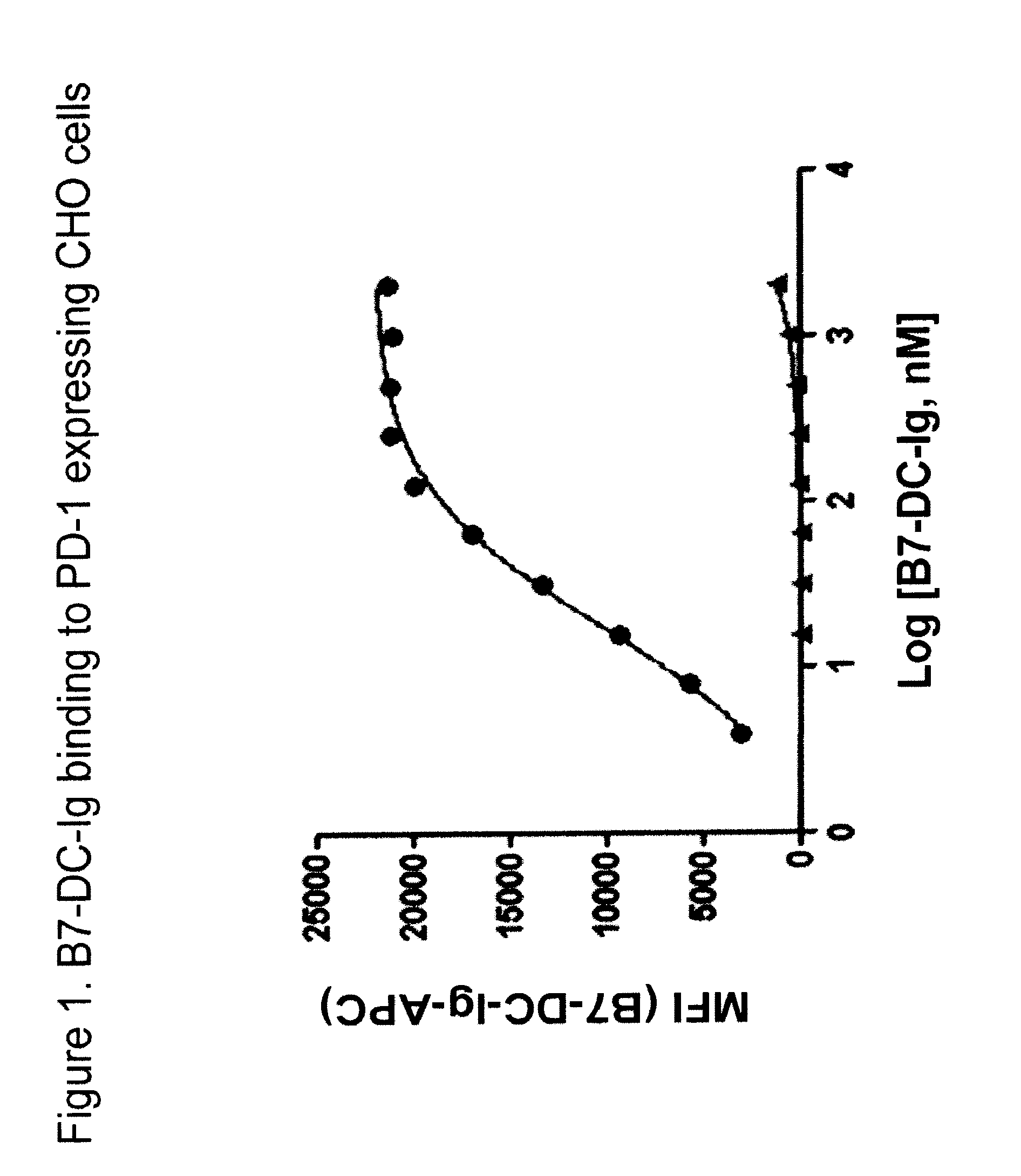
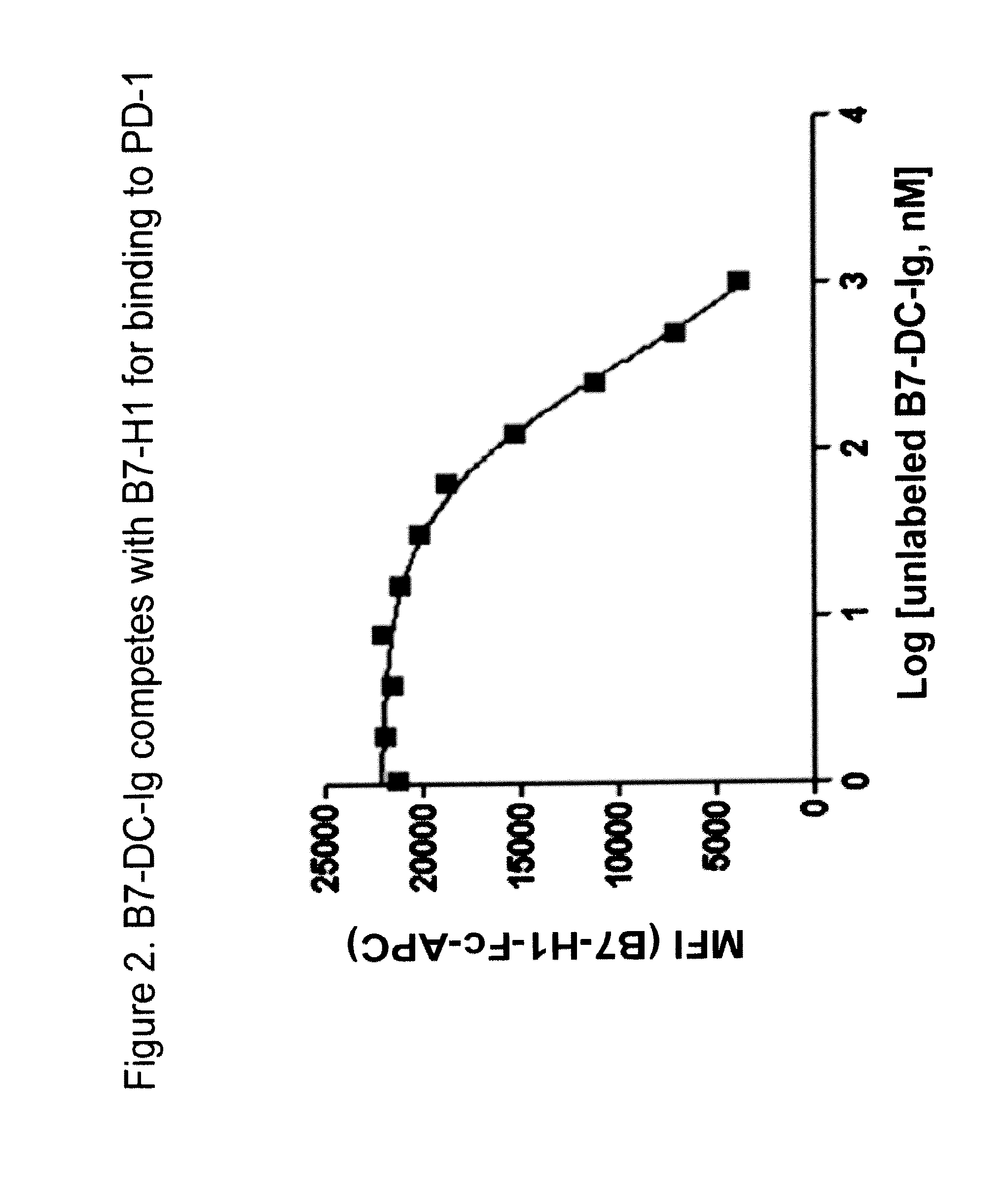
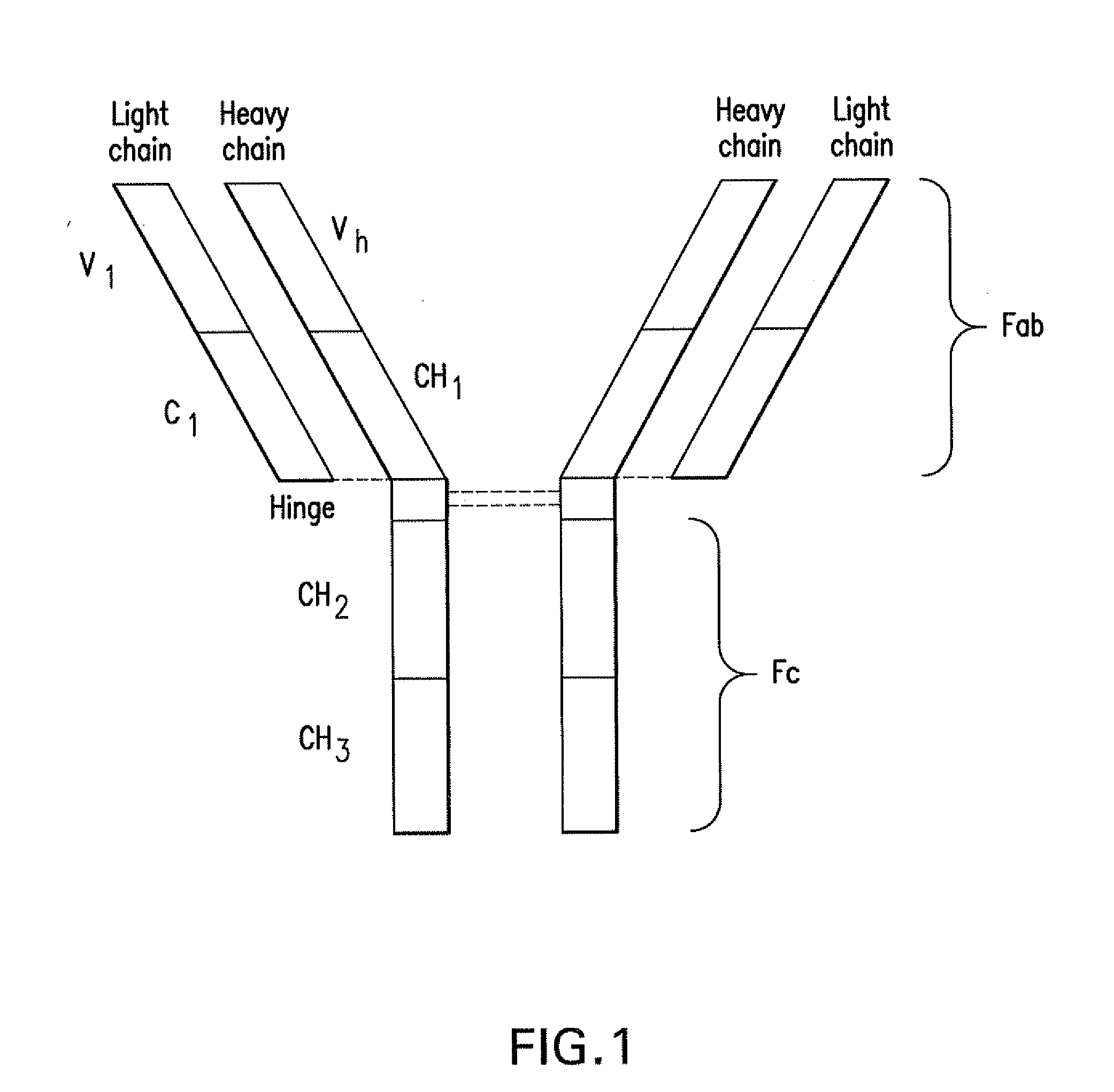
Pd-1 binding proteins
ActiveUS20110008369A1Enhance host anti-microbial immunityImprove immunityAntibody mimetics/scaffoldsImmunoglobulins against animals/humansPD-L1Host immunity
The present invention features PD-1 binding proteins, a subset of which inhibits binding of PD-L1 to the PD-1 receptor. These binding proteins can be employed to modulate the immune system through the manipulation of the PD-1 signaling pathway, enhancing host immunity to treat infections and cancer.
Owner:MERCK SHARP & DOHME LLC
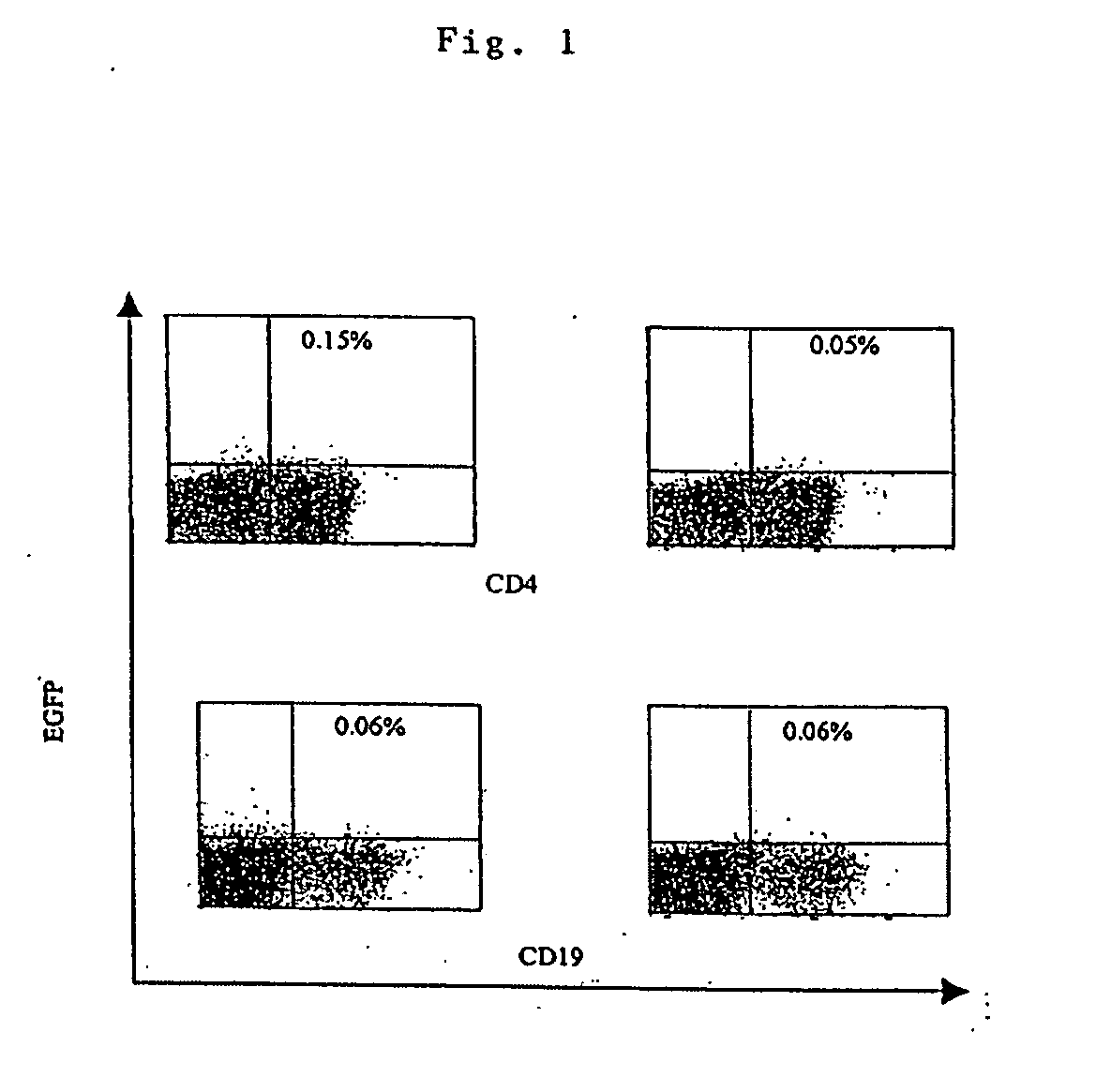
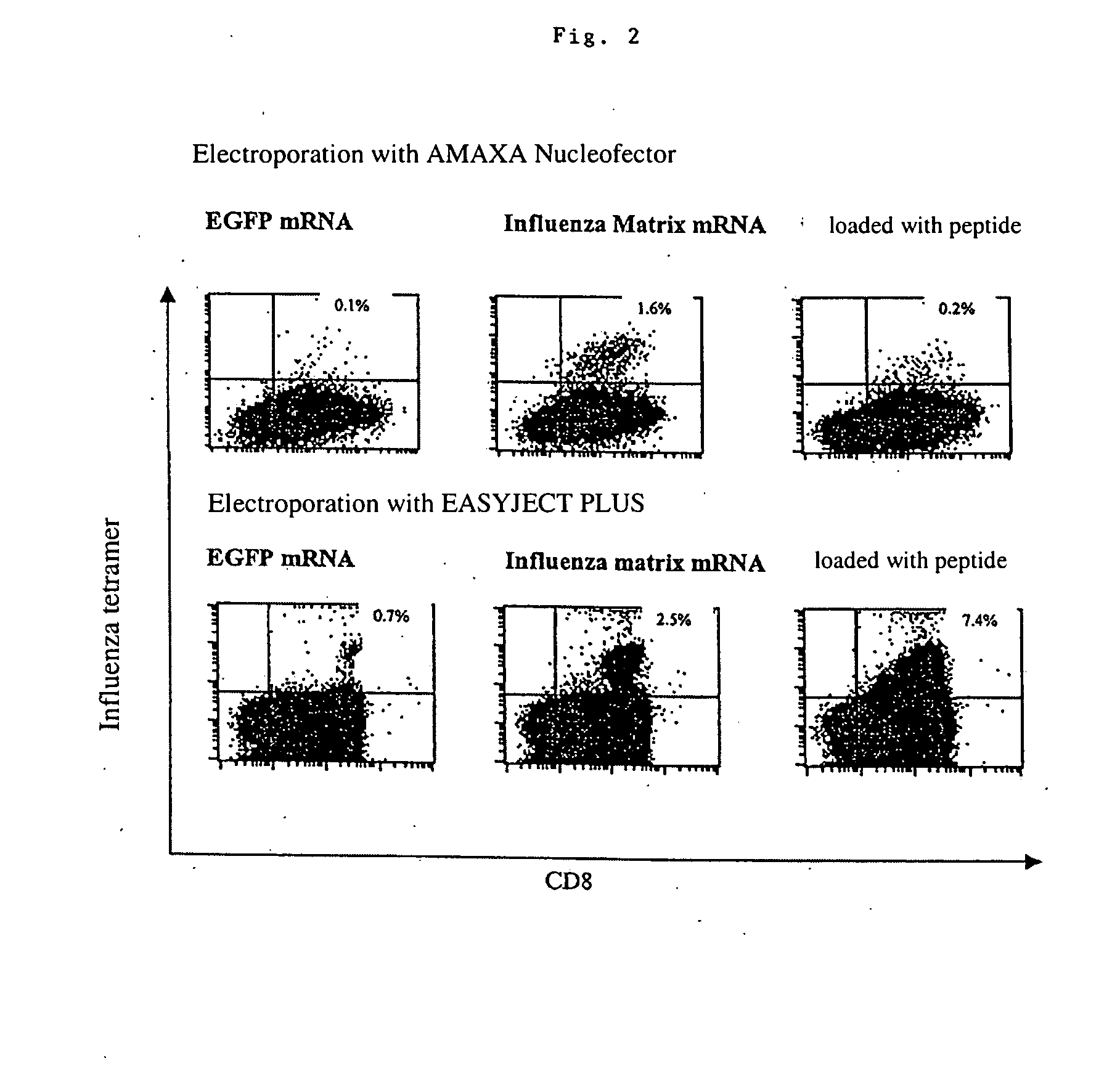
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
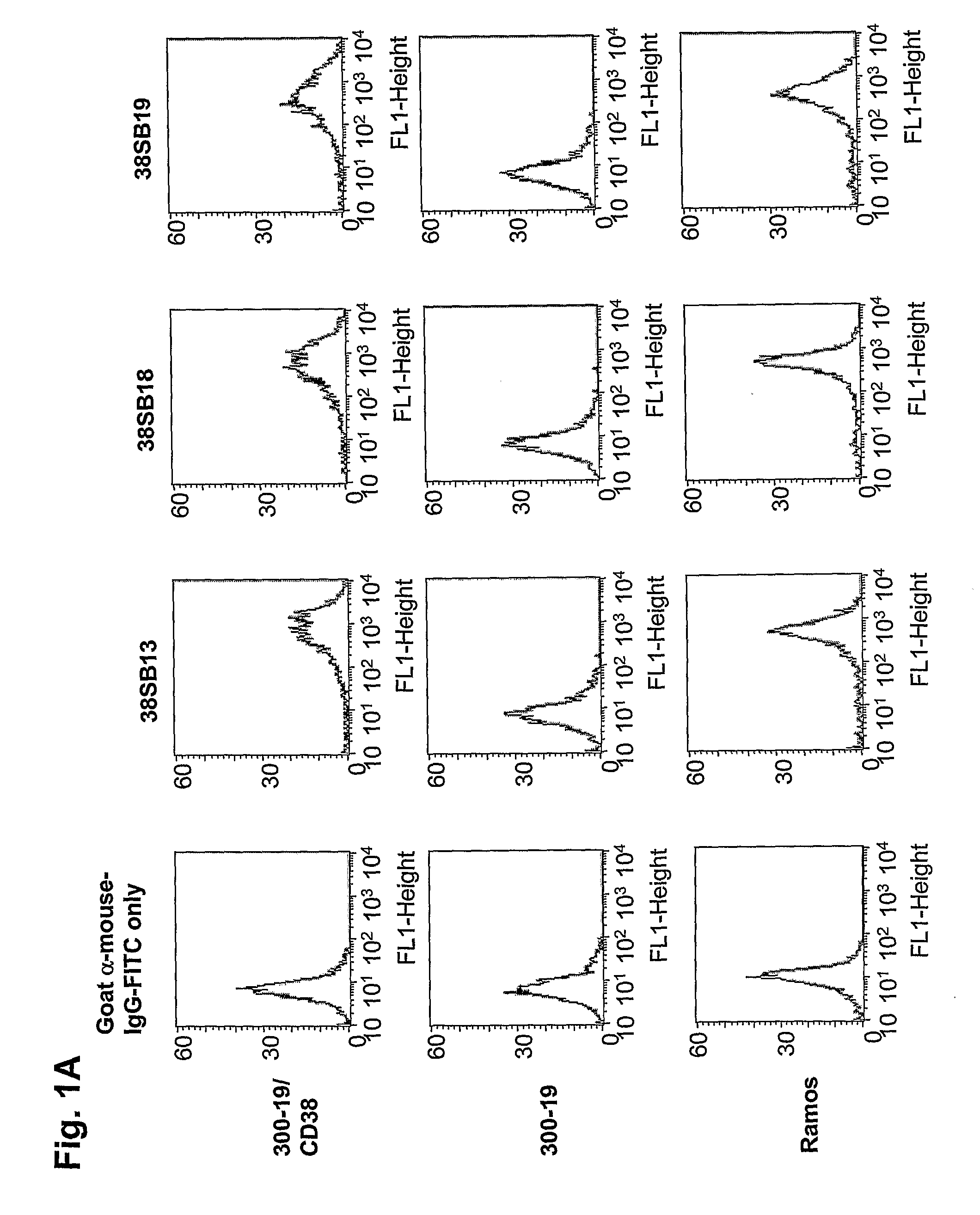
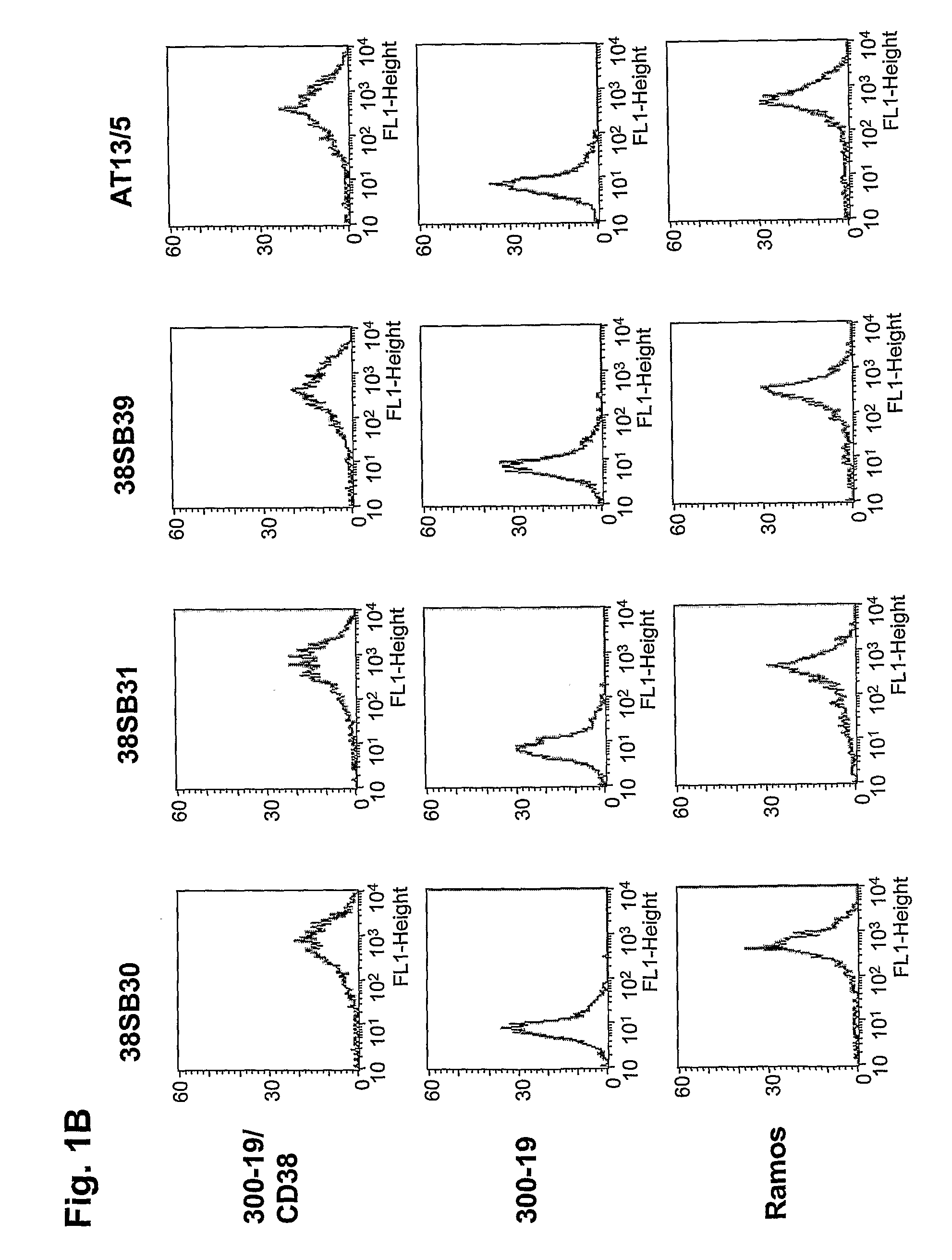
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
Rodent HER2 tumor model
The invention concerns HER<HIL><PDAT>2< / BOLD><PDAT>-transgenic non-human mammals, animal models for screening drug candidates for the treatment of diseases and disorders associated with the overexpression of HER<HIL><PDAT>2< / BOLD><PDAT>. In particular, the invention concerns animal models designed to test drug candidates for the treatment of HER<HIL><PDAT>2< / BOLD><PDAT>-overexpressing cancers, including breast cancer, that are not responding or poorly responding to current treatments.< / PTEXT>
Owner:SAN VALLEY SYST +1
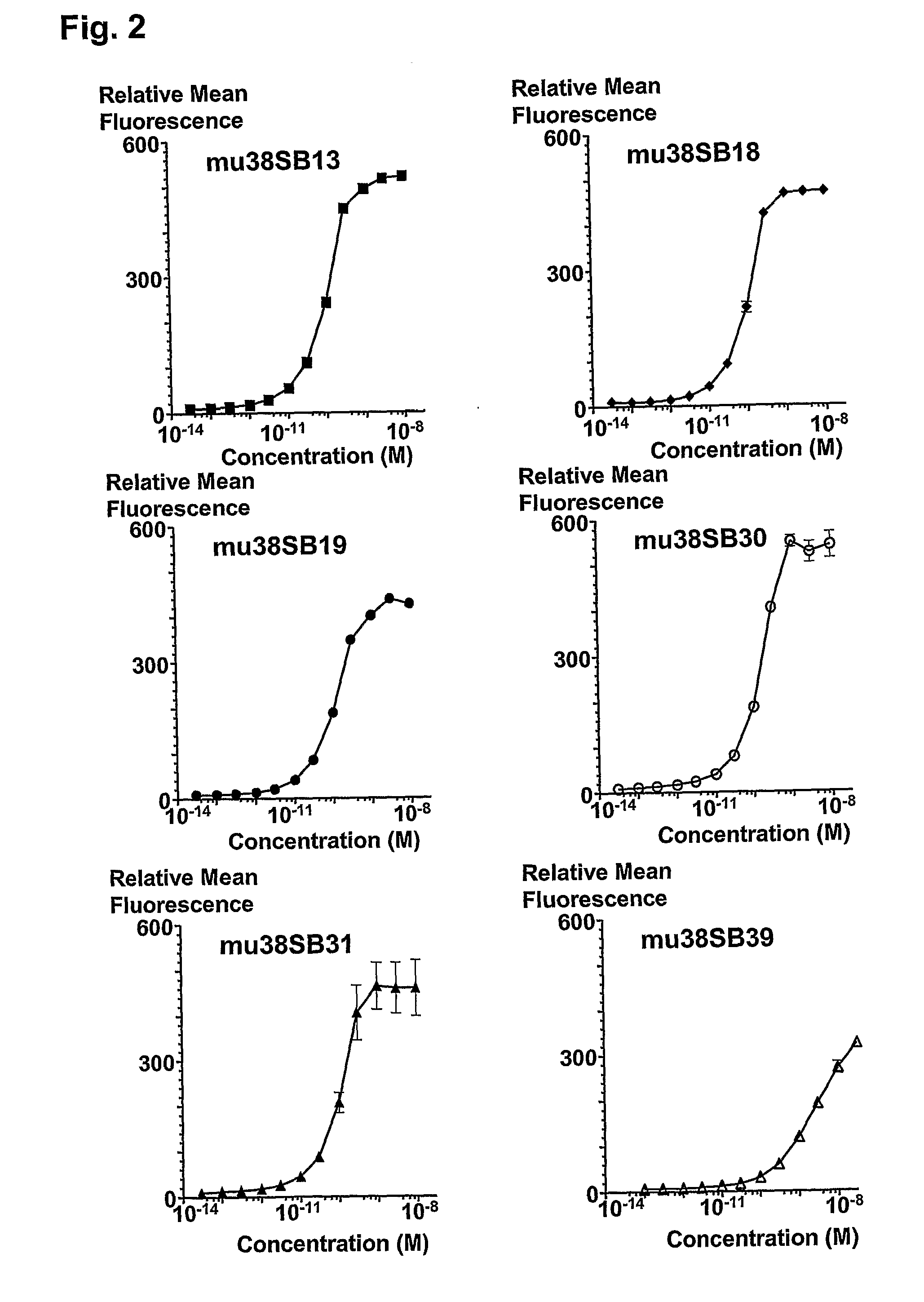
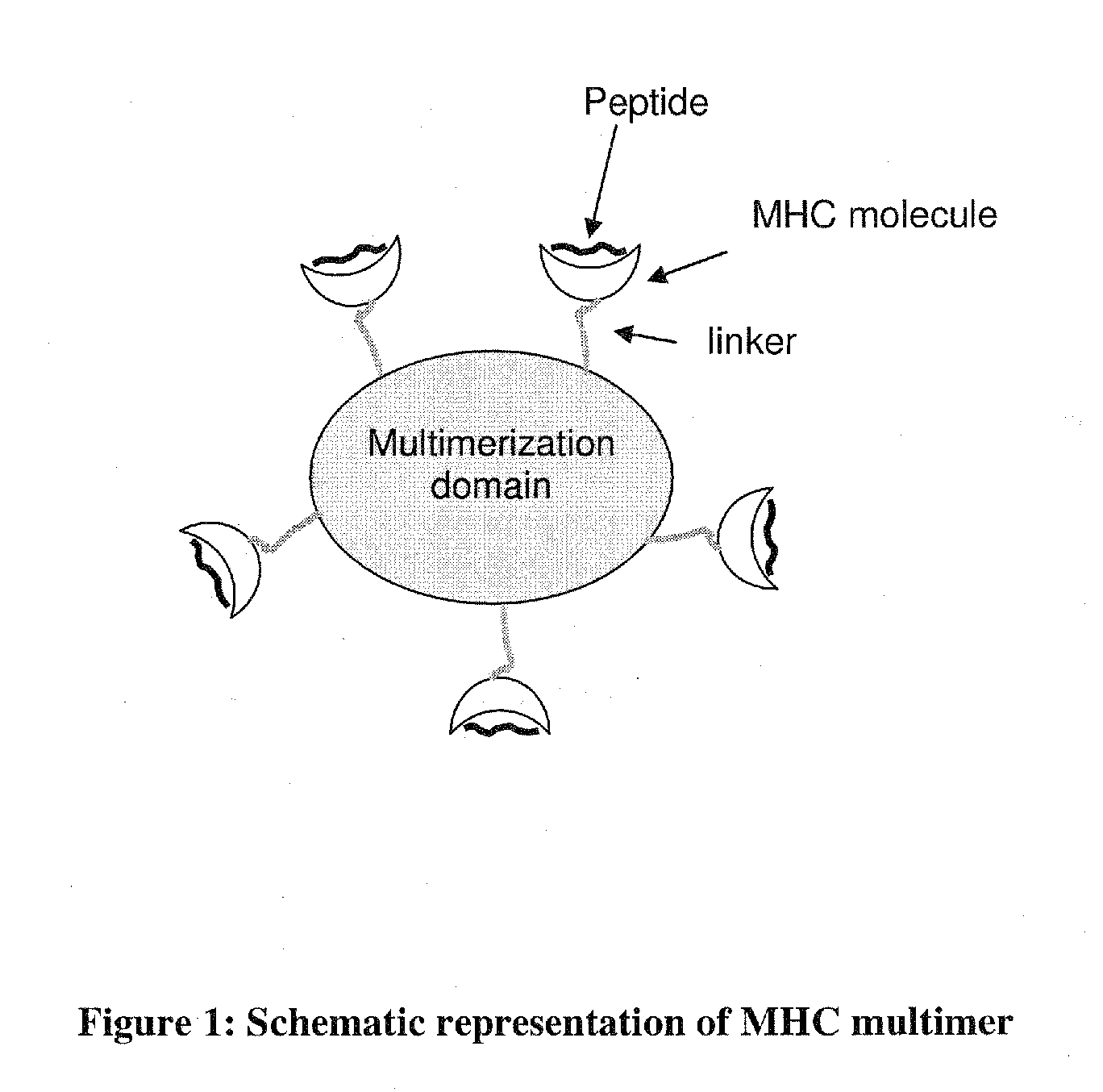
MHC Multimers in Cancer Vaccines and Immune Monitoring
InactiveUS20110318380A1Reduces infectious titerImprove efficacyPeptide/protein ingredientsImmunoglobulinsAntigenDisease
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising cancer antigenic peptides and uses there of.
Owner:AGILENT TECH INC
Degraded agonist antibody
InactiveUS20040242847A1Excellent antigen-binding propertyExcellent agonist activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAntiendomysial antibodies
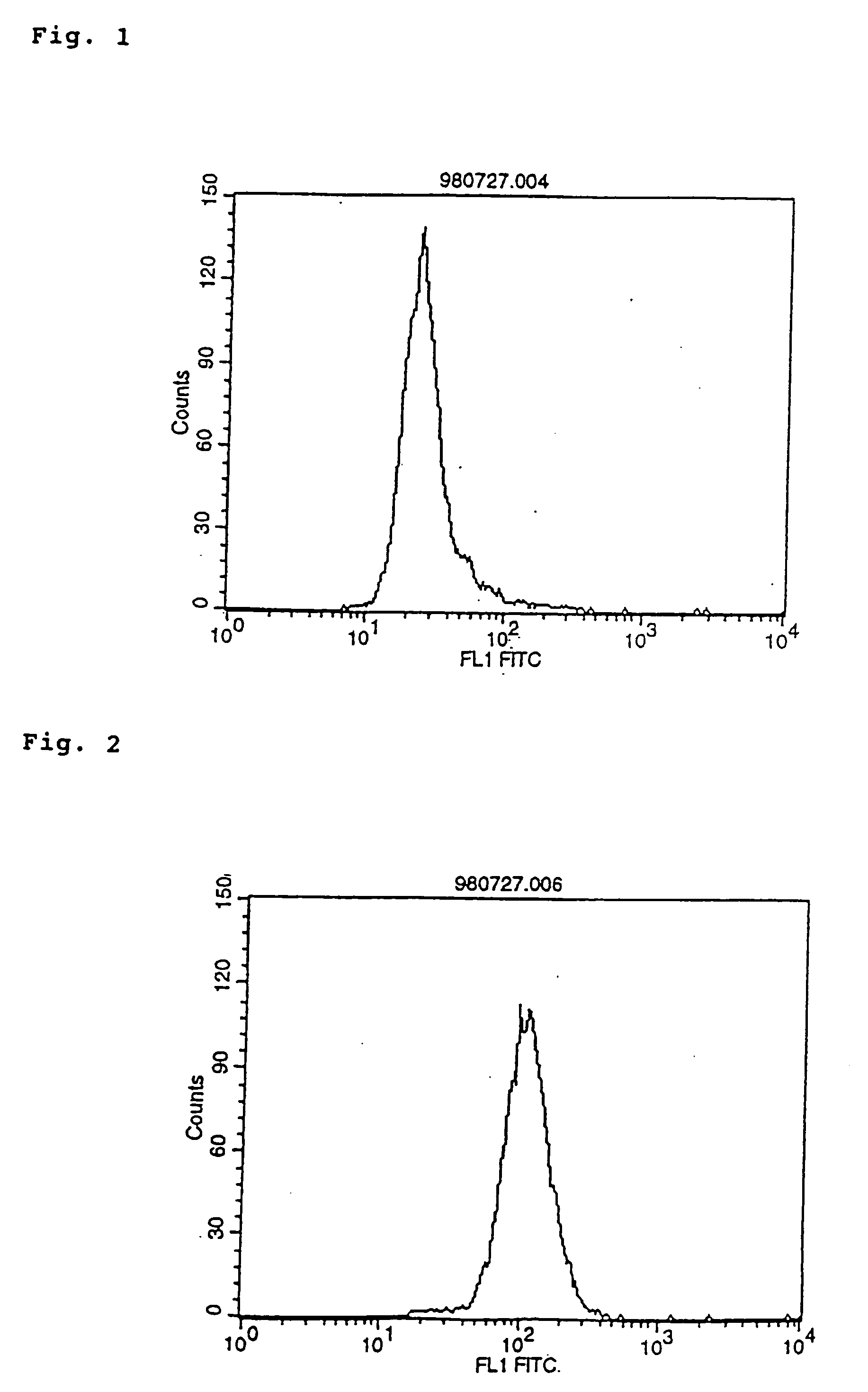
The invention relates to a modified antibody which contains two or more H chain V regions and two or more L chain V regions of monoclonal antibody and can transduce a signal into cells by crosslinking a cell surface molecule(s) to thereby serve as an agonist. The modified antibody can be used as a signal transduction agonist and, therefore, useful as a preventive and / or remedy for various diseases such as cancer, inflammation, hormone disorders and blood diseases.
Owner:CHUGAI PHARMA CO LTD
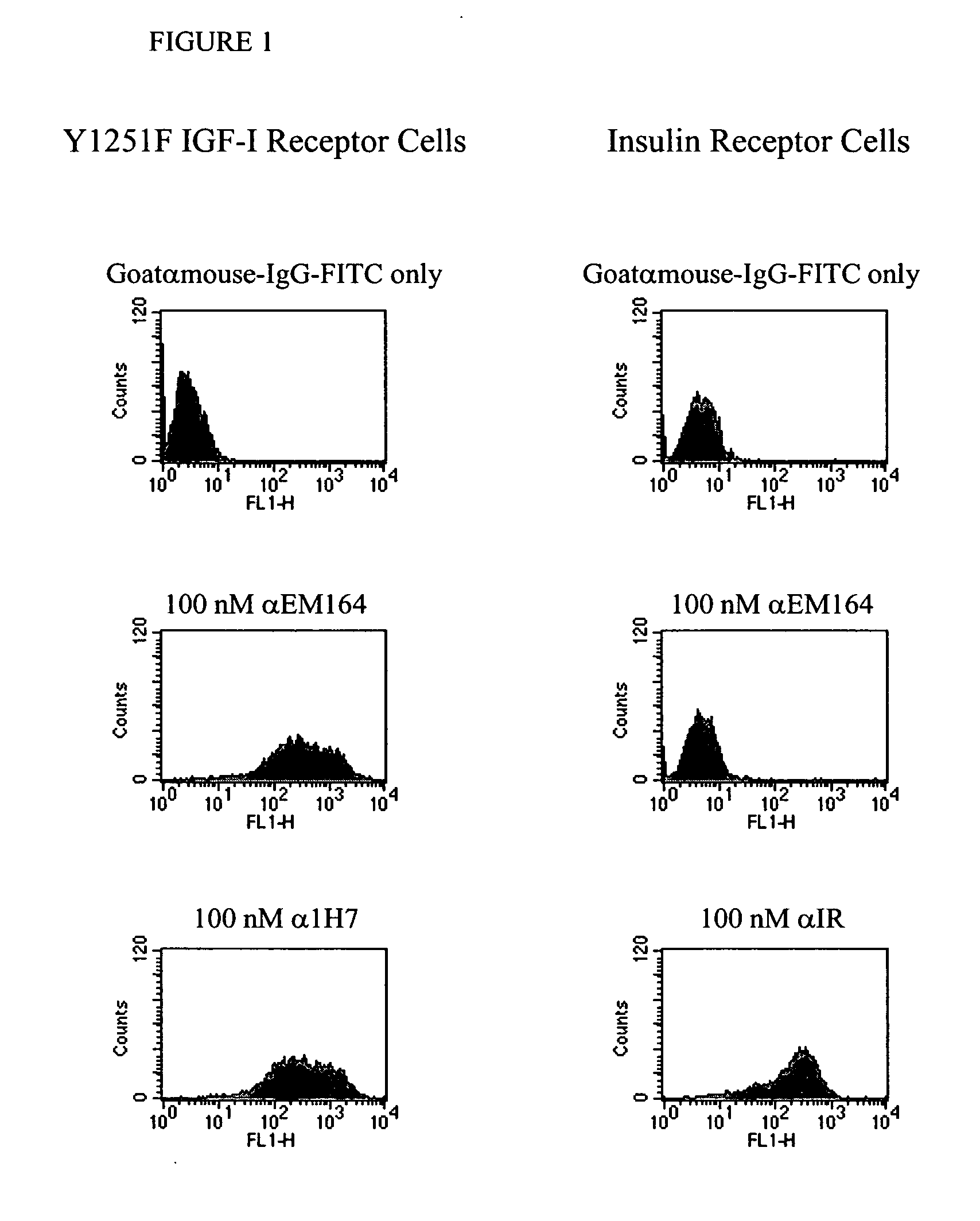
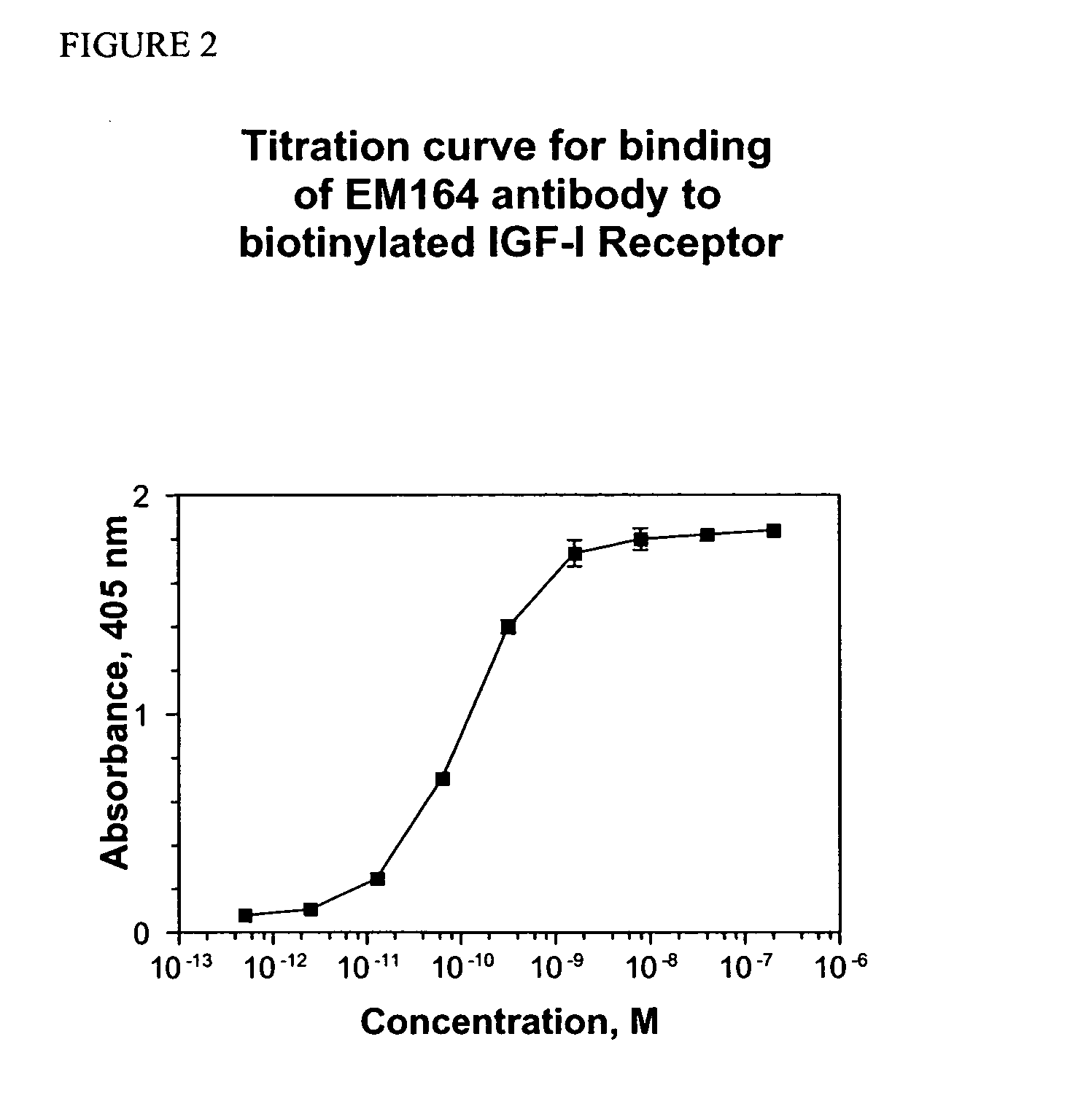
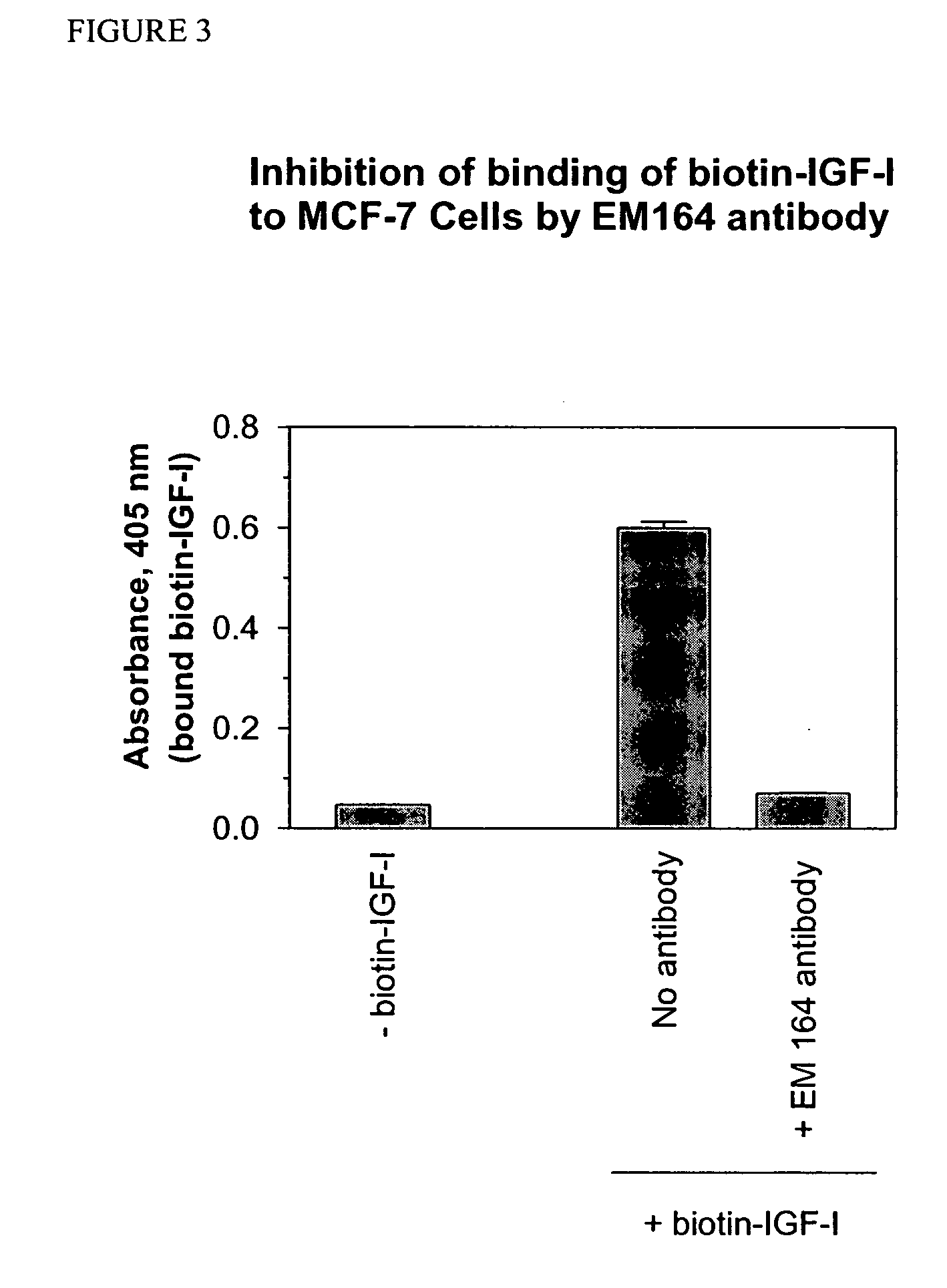
Anti-IGF-I receptor antibody
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to, and inhibit, insulin-like growth factor-I receptor, antagonize the effects of IGF-I, IGF-II and serum on the growth and survival of tumor cells, and which are substantially devoid of agonist activity. Said antibodies and fragments thereof may be used, optionally in conjunction with other therapeutic agents, in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma, prostate cancer and pancreatic cancer, and said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM') of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM' allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
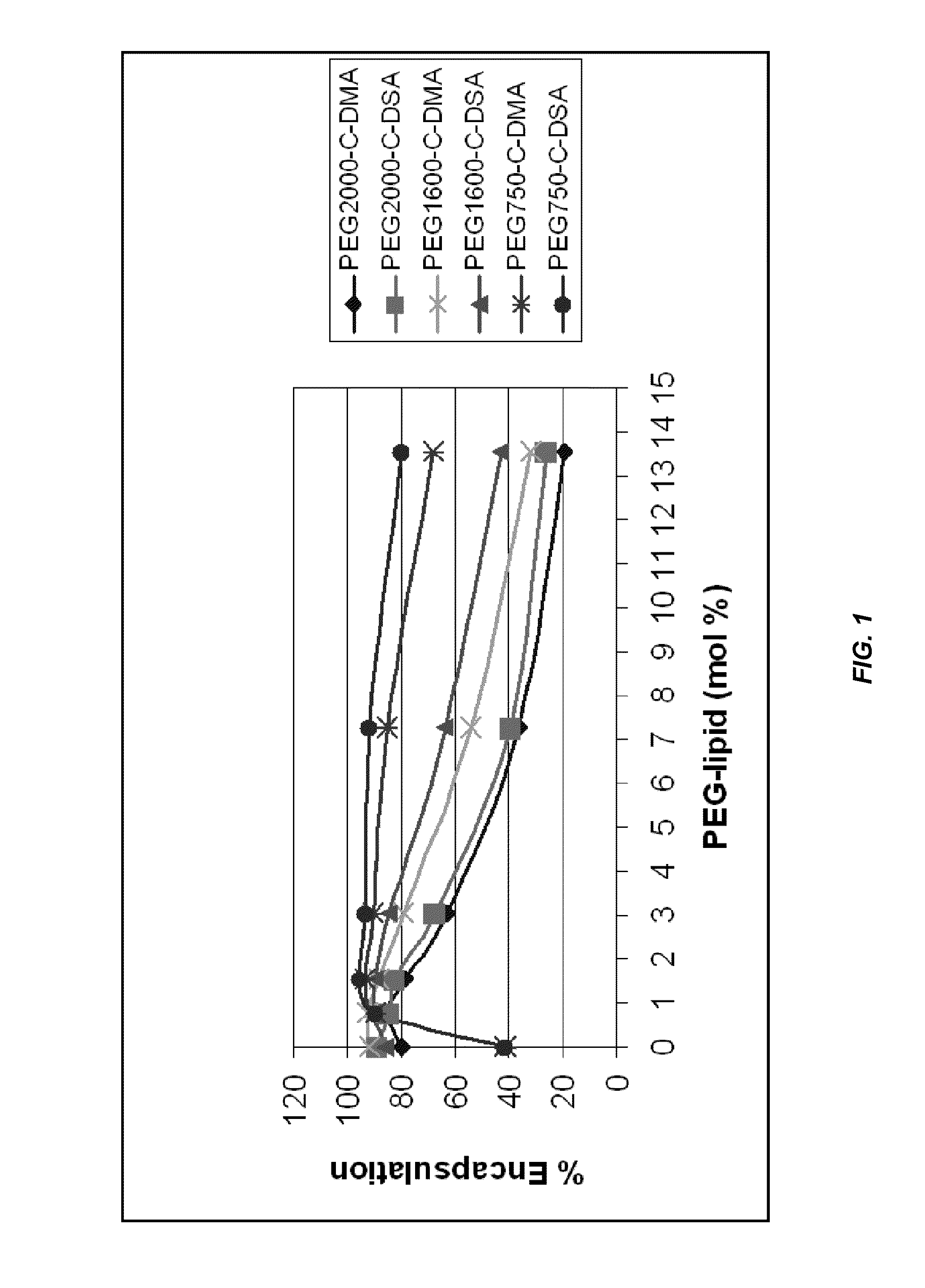
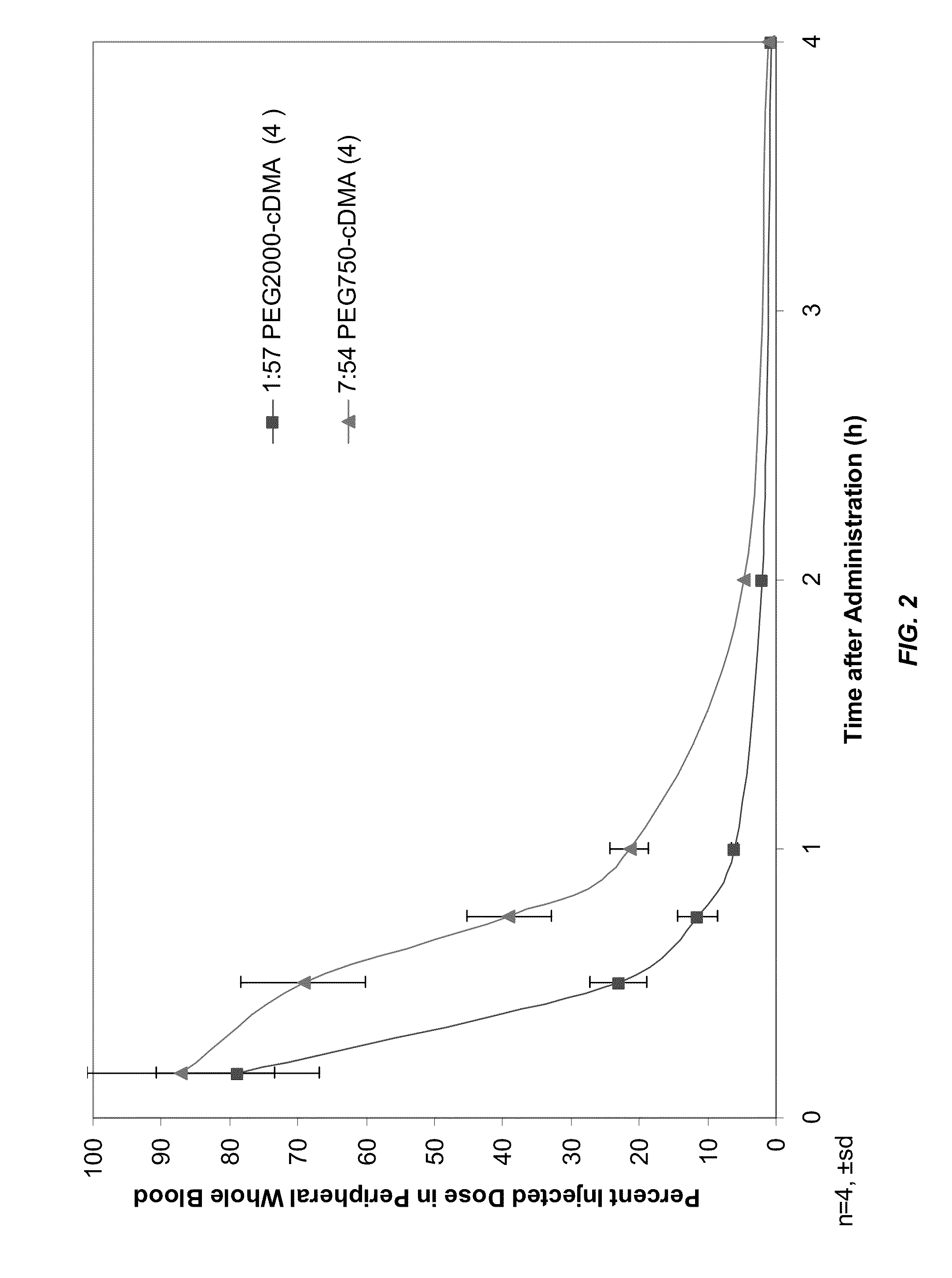
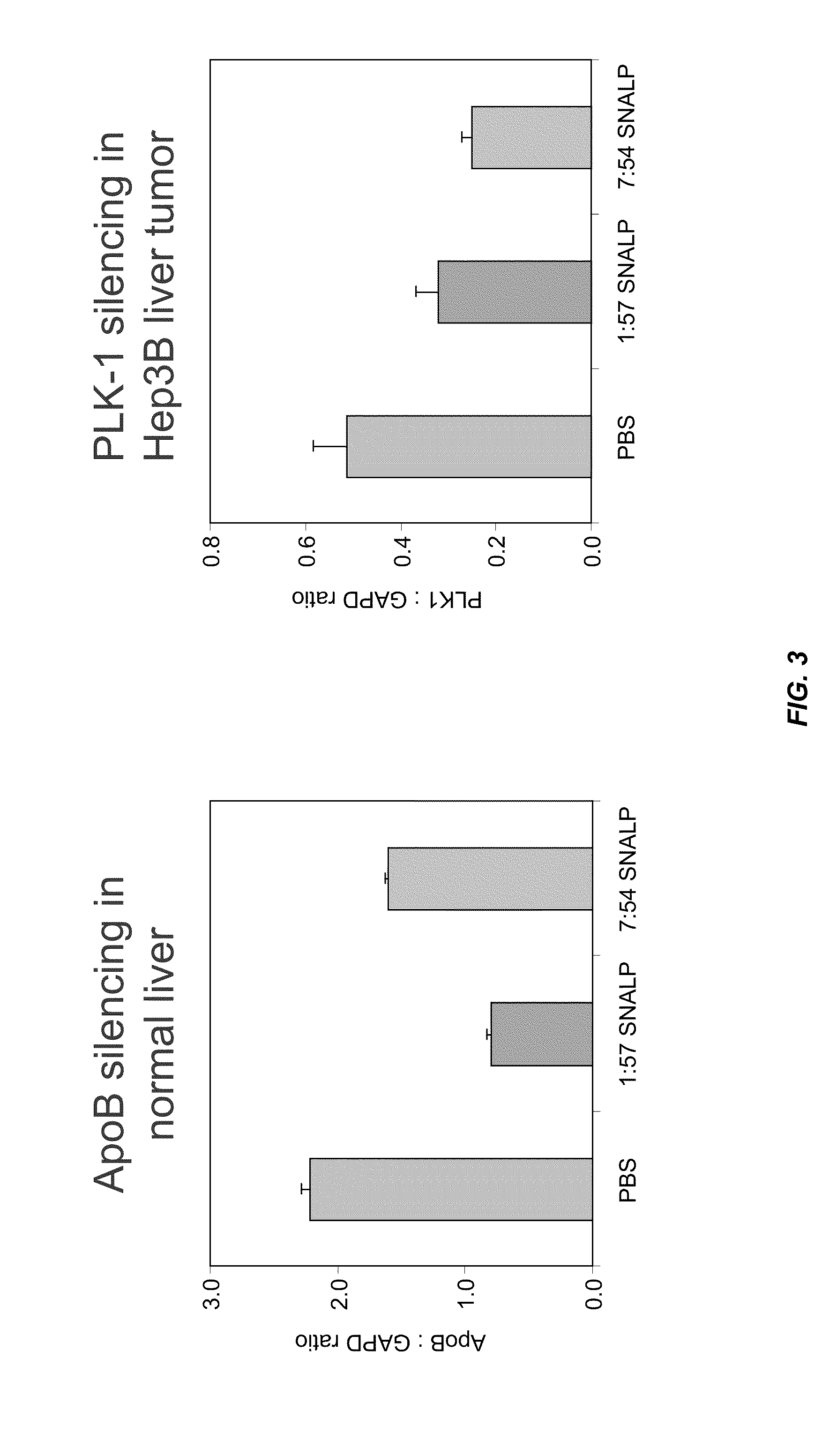
Novel lipid formulations for delivery of therapeutic agents to solid tumors
ActiveUS20110076335A1Improve effectivenessFavorable toxicity profileOrganic active ingredientsSpecial deliveryLipid formationLipid particle
The present invention provides novel, serum-stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides serum-stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (e.g., one or more interfering RNA molecules), methods of making the SNALP, and methods of delivering and / or administering the SNALP (e.g., for the treatment of cancer). In particular embodiments, the present invention provides tumor-directed lipid particles that preferentially target solid tumors. The tumor-directed formulations of the present invention are capable of preferentially delivering a payload such as a nucleic acid to cells of solid tumors compared to non-cancerous cells.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Therapy of cancer by insect cells containing recombinant baculovirus encoding genes
Provided are compositions and methods of use for insect cells comprising baculovirus encoding non-surface expressed proteins and peptides. The claimed invention particularly relates to compositions comprising insect cells containing baculovirus that express cytokines. Such compositions may be administered by, for example, direct intratumoral injection into tumors in mammals, resulting in tumor reduction or recission. Another aspect of the claimed invention concerns methods of promoting resistance to the reoccurence of tumors in mammals who have undergone such tumor recission. In a specific aspect of the claimed invention, the mammals are human subjects presenting with various forms of cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods and compositions for the treatment of persistent infections
ActiveUS20070122378A1Reduced activityReduce expressionAntibacterial agentsOrganic active ingredientsMicrobiologyPathology
The present invention provides methods and compositions for the treatment, prevention, or reduction of persistent infections, such as chronic infections, latent infections, and slow infections and cancer. The methods and compositions of the invention are also useful for the alleviation of one or more symptoms associated with such infections and cancer.
Owner:DANA FARBER CANCER INST INC +3
Compositions, formulations and kit with anti-sense oligonucleotide and anti-inflammatory steroid and/or obiquinone for treatment of respiratory and lung disesase
InactiveUS20070021360A1Decreased airwayOrganic active ingredientsBiocideDiseaseAntiendomysial antibodies
A pharmaceutical composition and formulations comprise preventative, prophylactic or therapeutic amounts of an oligo(s) anti-sense to a specific gene(s) or its corresponding mRNA(s), and a glucocorticoid and / or non-glucocorticoid steroid or a ubiquinone or their salts. The agents, composition and formulations are used for treatment of ailments associated with impaired respiration, bronchoconstriction, lung allergy(ies) or inflammation, and abnormal levels of adenosine, adenosine receptors, sensitivity to adenosine, lung surfactant and ubiquinone, such as pulmonary fibrosis, vasoconstriction, inflammation, allergies, allergic rhinitis, asthma, impeded respiration, lung pain, cystic fibrosis, bronchoconstriction, COPD, RDS, ARDS, cancer, and others. The present treatment is effectively administered by itself for conditions without known therapies, as a substitute for therapies exhibiting undesirable side effects, or in combination with other treatments, e.g. before, during and after other respiratory system therapies, radiation, chemotherapy, antibody therapy and surgery, among others. Each of the agents of this invention may be administered directly into the respiratory system so that they gain direct access to the lungs, or by other effective routes of administration. A kit comprises a delivery device, the agents and instructions for its use.
Owner:EPIGENESIS PHARMA LLC
6,6-Bicyclic ring substituted heterobicyclic protein kinase inhibitors
ActiveUS20060235031A1Treatment and/or prevention of hyperproliferative diseasesBiocideSenses disorderDiseasePTK Inhibitors
Compounds of the formula and pharmaceutically acceptable salts thereof, wherein X1, X2, X3, X4, X5, X6, X7, R1, and Q1 are defined herein, inhibit the IGF-1R enzyme and are useful for the treatment and / or prevention of hyperproliferative diseases such as cancer, inflammation, psoriasis, allergy / asthma, disease and conditions of the immune system, disease and conditions of the central nervous system.
Owner:ACERTA PHARMA BV
Ligands that have binding specificity for VEGF and/or EGFR and methods of use therefor
InactiveUS20070003549A1Not effectiveExtended half-lifeNervous disorderAntipyreticVascular endothelial growth factorCancer therapy
Disclosed are ligands that have binding specificity for vascular endothelial growth factor (VEGF), for epidermal growth factor receptor (EGFR), or for VEGF and EGFR. Also disclosed are methods of using these ligands. In particular, the use of these ligands for cancer therapy is described.
Owner:DORMANTIS LTD
HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS
The present invention provides heteroaryl substituted pyrrolo[2,3-b]pyridines and heteroaryl substituted pyrrolo[2,3-b]pyrimidines that modulate the activity of Janus kinases and are useful in the treatment of diseases related to activity of Janus kinases including, for example, immune-related diseases, skin disorders, myeloid proliferative disorders, cancer, and other diseases.
Owner:INCYTE HLDG & INCYTE
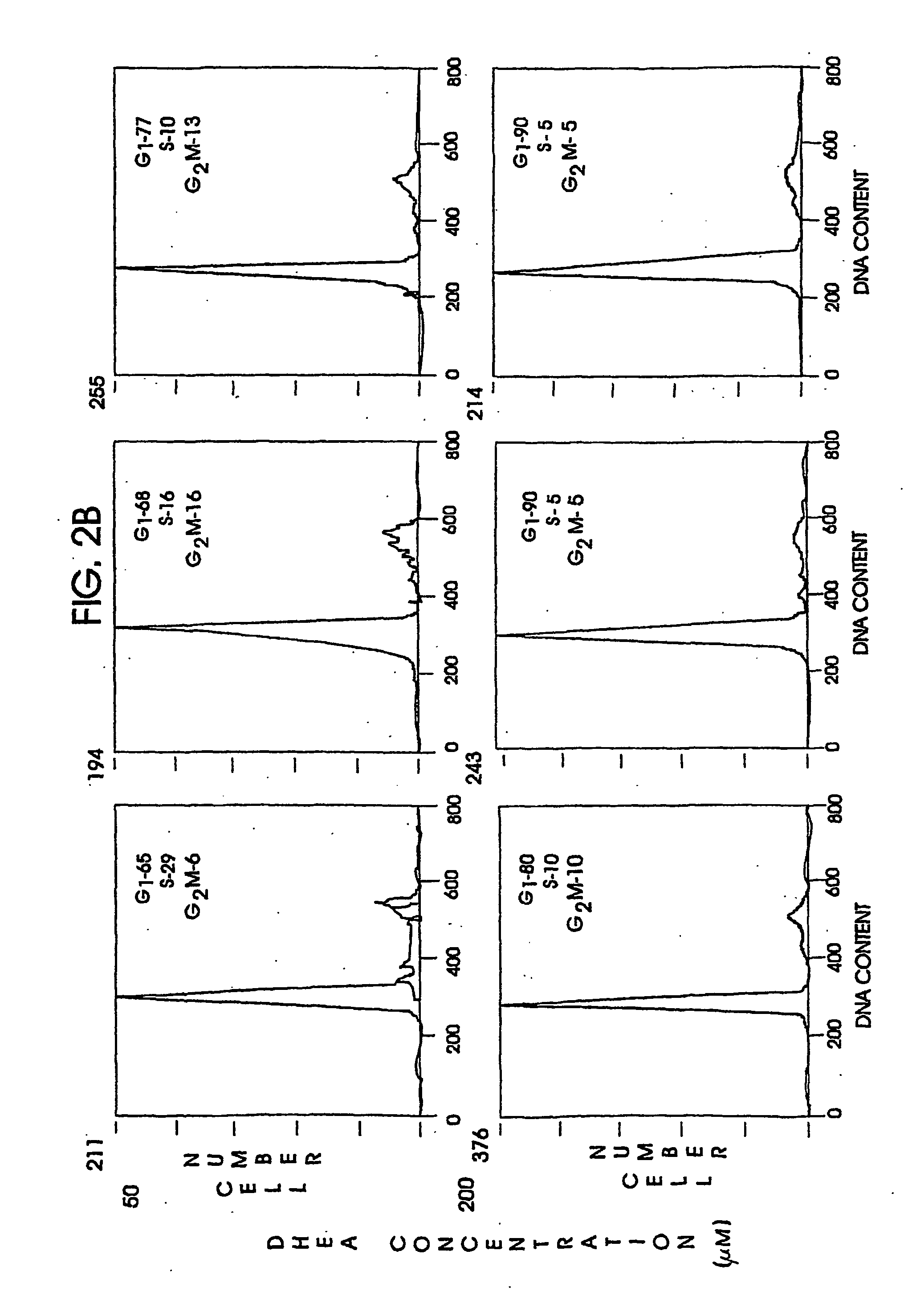
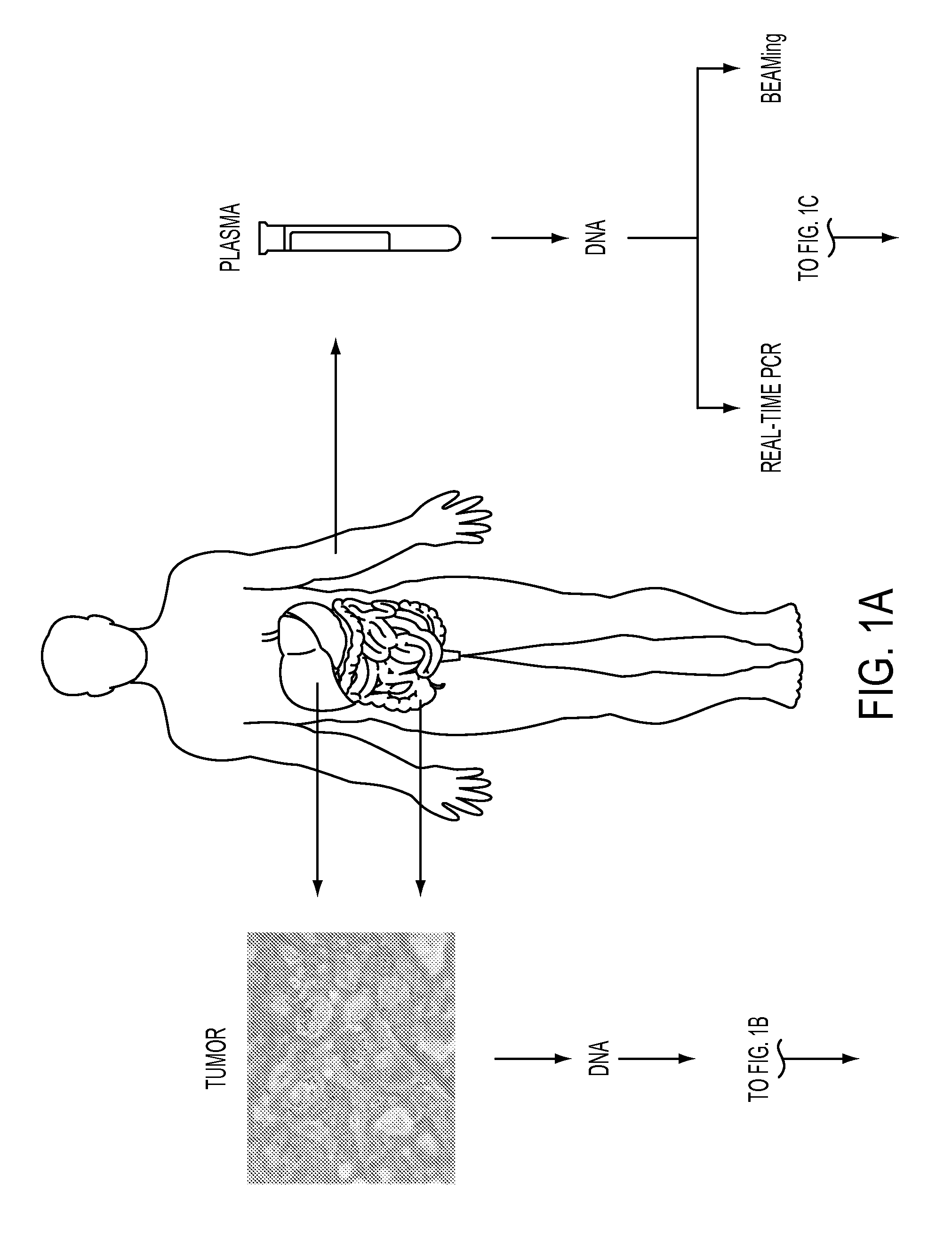
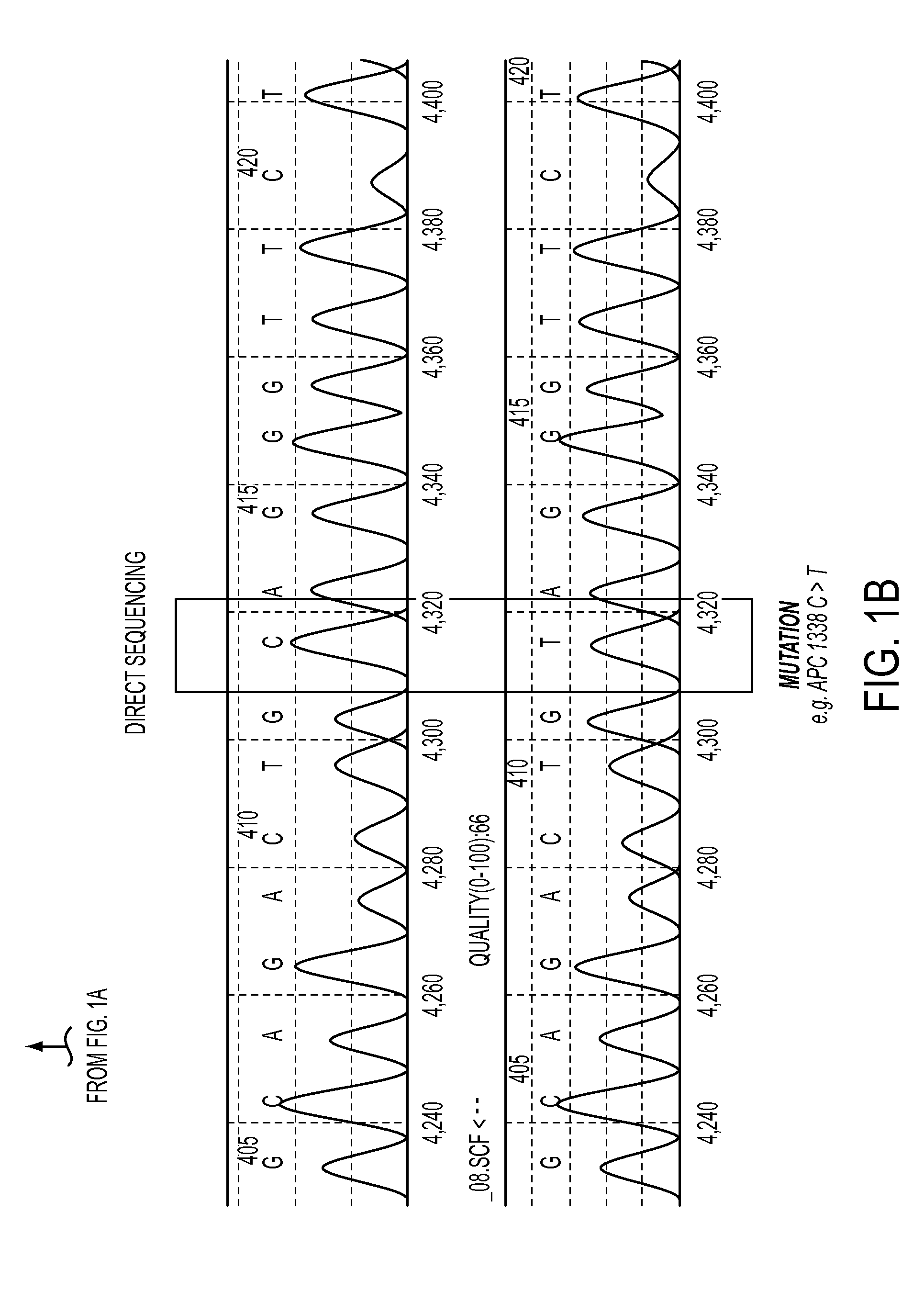
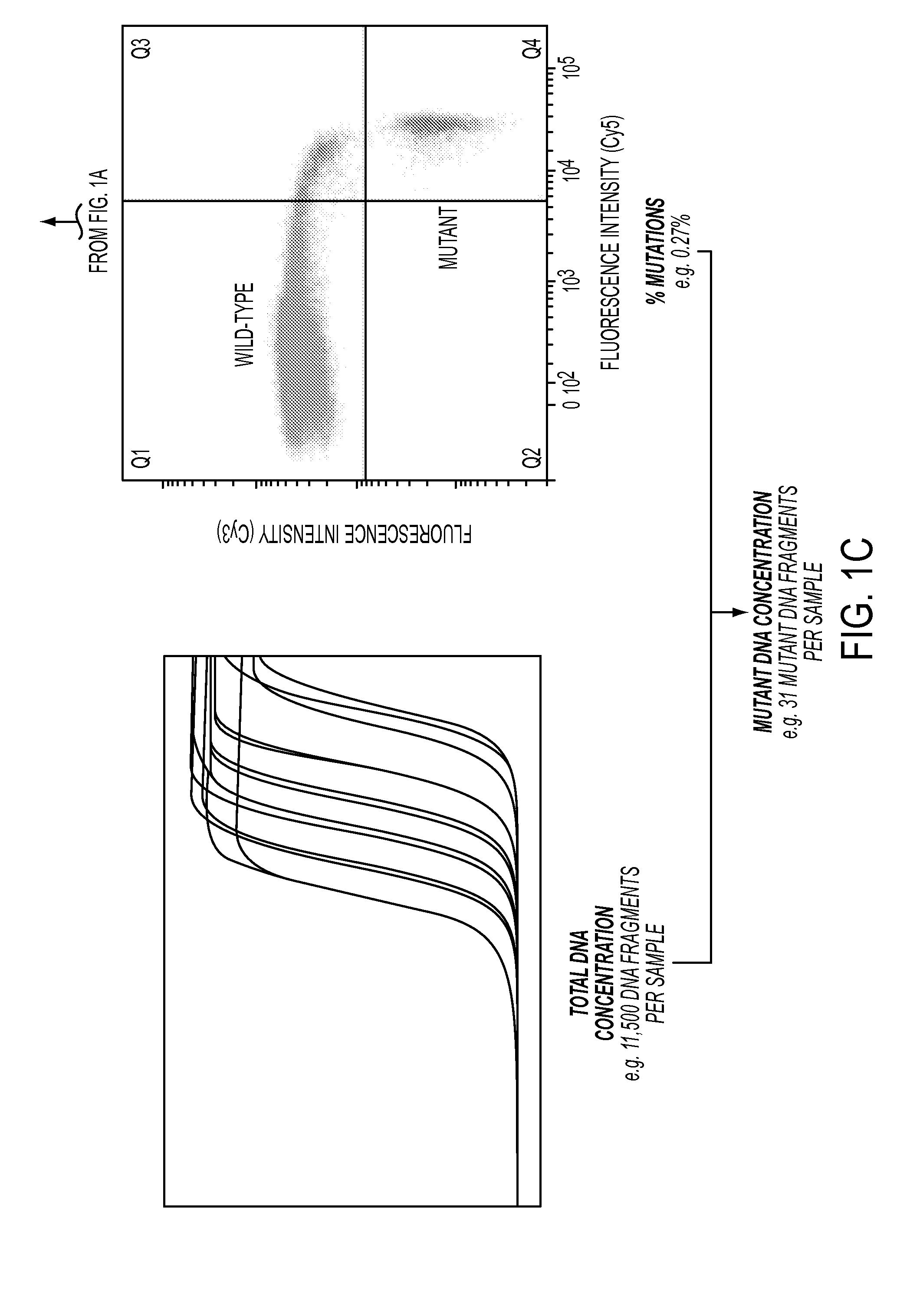
Circulating Mutant DNA to Assess Tumor Dynamics
DNA containing somatic mutations is highly tumor specific and thus, in theory, can provide optimum markers. However, the number of circulating mutant gene fragments is small compared to the number of normal circulating DNA fragments, making it difficult to detect and quantify them with the sensitivity required for meaningful clinical use. We apply a highly sensitive approach to quantify circulating tumor DNA (ctDNA) in body samples of patients. Measurements of ctDNA can be used to reliably monitor tumor dynamics in subjects with cancer, especially those who are undergoing surgery or chemotherapy. This personalized genetic approach can be generally applied.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Angiogenesis-modulating compositions and uses
InactiveUS20050054568A1Prevent angiogenesis driven pathologyUtility in treatmentOrganic active ingredientsSenses disorderCancerPathology diagnosis
Hedgehog agonists and antagonists can be used to regulate angiogenesis, and to prevent angiogenesis driven pathologies. Furthermore, hedgehog agonists and antagonists have utility in modulating tissue repair and in the treatment of many forms of cancer.
Owner:CURIS INC
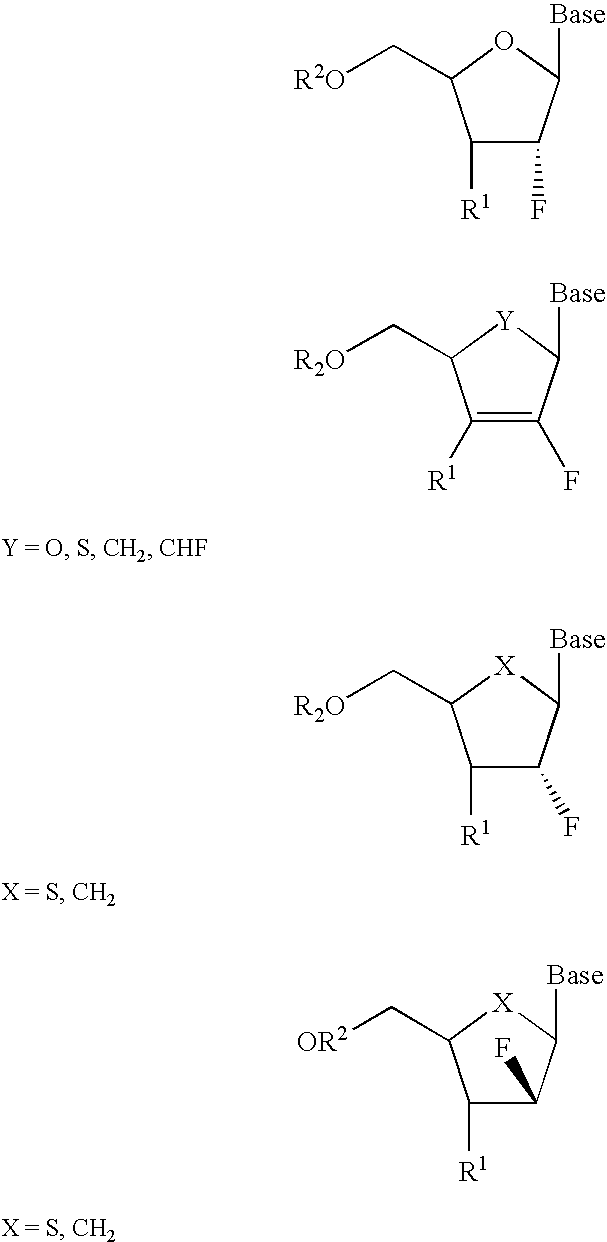
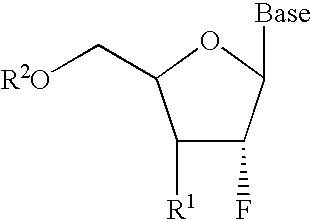
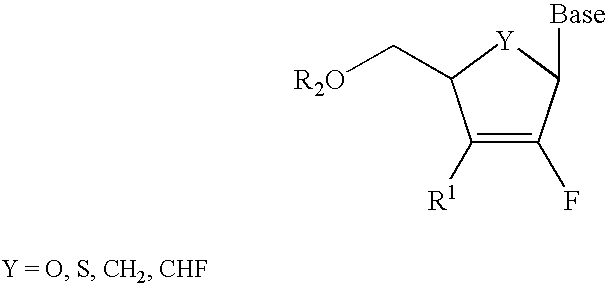
2′-fluoronucleosides
InactiveUS6911424B2Sure easyUseful in treatmentBiocideGroup 5/15 element organic compoundsPhosphoric Acid EstersPurine
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae: wherein[0001]Base is a purine or pyrimidine base;[0002]R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;[0003]R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; and[0004]R3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY
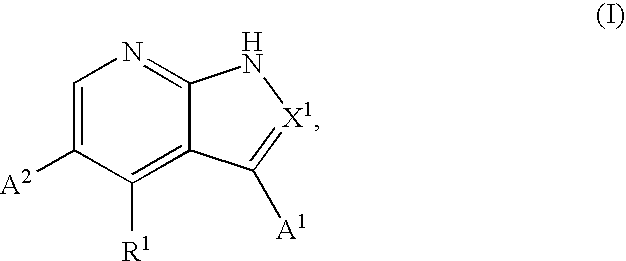
Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds
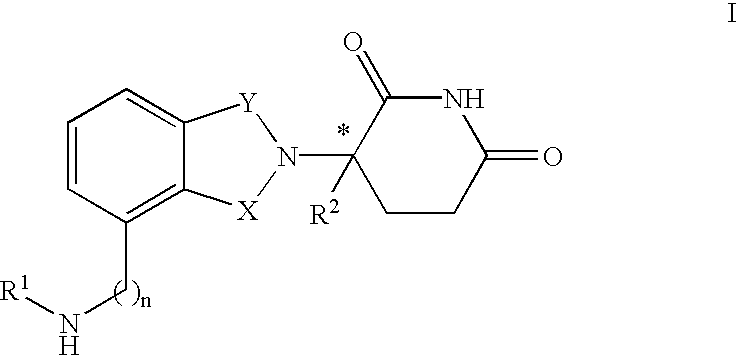
Compounds of Formula I-a and all pharmaceutically-acceptable forms thereof, are described herein. The variables R1, R2, R3, Z1, Q, and A shown in Formula I-a are defined herein. Pharmaceutical compositions containing one or more compounds of Formula I-a, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein. Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and / or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I-a effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and / or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases. Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity. Methods of treatment include administering a compound of Formula I-a as a single active agent or administering a compound of Formula I-a in combination with one or more other therapeutic agent. A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I-a under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.
Owner:GILEAD CONNENTICUT INC
Fused bicyclic mTOR inhibitors
ActiveUS20070112005A1Useful in treatmentBiocideNervous disorderDiscovery and development of mTOR inhibitorsChemistry
Compounds represented by Formula (I) or a pharmaceutically acceptable salt thereof, are inhibitors of mTOR and useful in the treatment of cancer.
Owner:OSI PHARMA INC
Substituted pyrrolopyridines and pyrazolopyridines as kinase modulators
Provided herein are substituted pyrrolopyridine heterocycles and substituted pyrazolopyridine heterocycles, pharmaceutical compositions comprising said heterocycles and methods of using said heterocycles in the treatment of disease. The heterocycles disclosed herein function as kinase modulators and have utility in the treatment of diseases such as cancer, allergy, asthma, inflammation, obstructive airway disease, autoimmune diseases, metabolic disease, infection, CNS disease, brain tumor, obesity, asthma, hematological disorder, degenerative neural disease, cardiovascular disease, or disease associated with angiogenesis, neovascularization, or vasculogenesis.
Owner:SGX PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
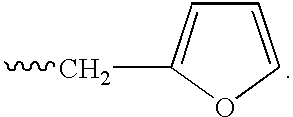
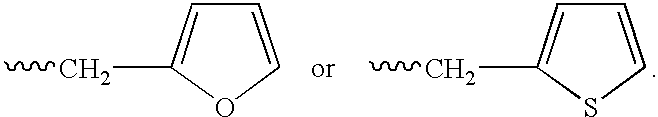
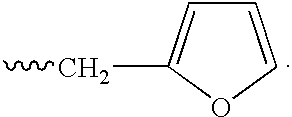
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka.patsnap.com/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00001.png)
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka.patsnap.com/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00002.png)
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka.patsnap.com/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00003.png)
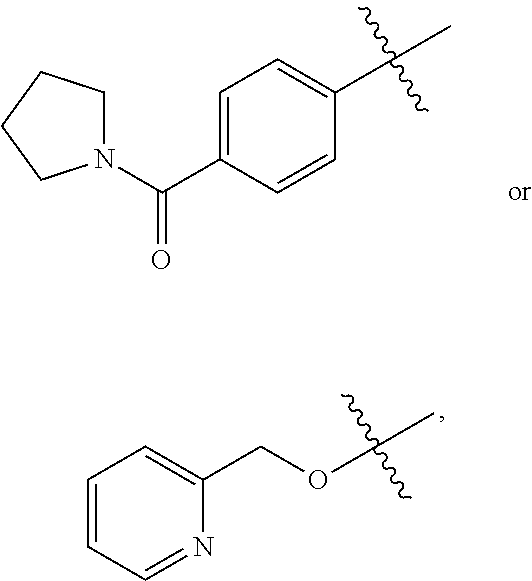









![HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS](https://images-eureka.patsnap.com/patent_img/9ca91ab9-beda-49e3-8a59-55336dec4de6/US20090181959A1-20090716-C00001.png)
![HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS](https://images-eureka.patsnap.com/patent_img/9ca91ab9-beda-49e3-8a59-55336dec4de6/US20090181959A1-20090716-C00002.png)
![HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS](https://images-eureka.patsnap.com/patent_img/9ca91ab9-beda-49e3-8a59-55336dec4de6/US20090181959A1-20090716-C00003.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00001.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00002.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00003.png)