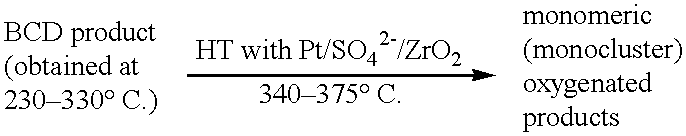Patents
Literature
19596 results about "Phenyl group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In organic chemistry, the phenyl group or phenyl ring is a cyclic group of atoms with the formula C₆H₅. Phenyl groups are closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl groups have six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl groups are chemically aromatic and have equal bond lengths between carbon atoms in the ring.
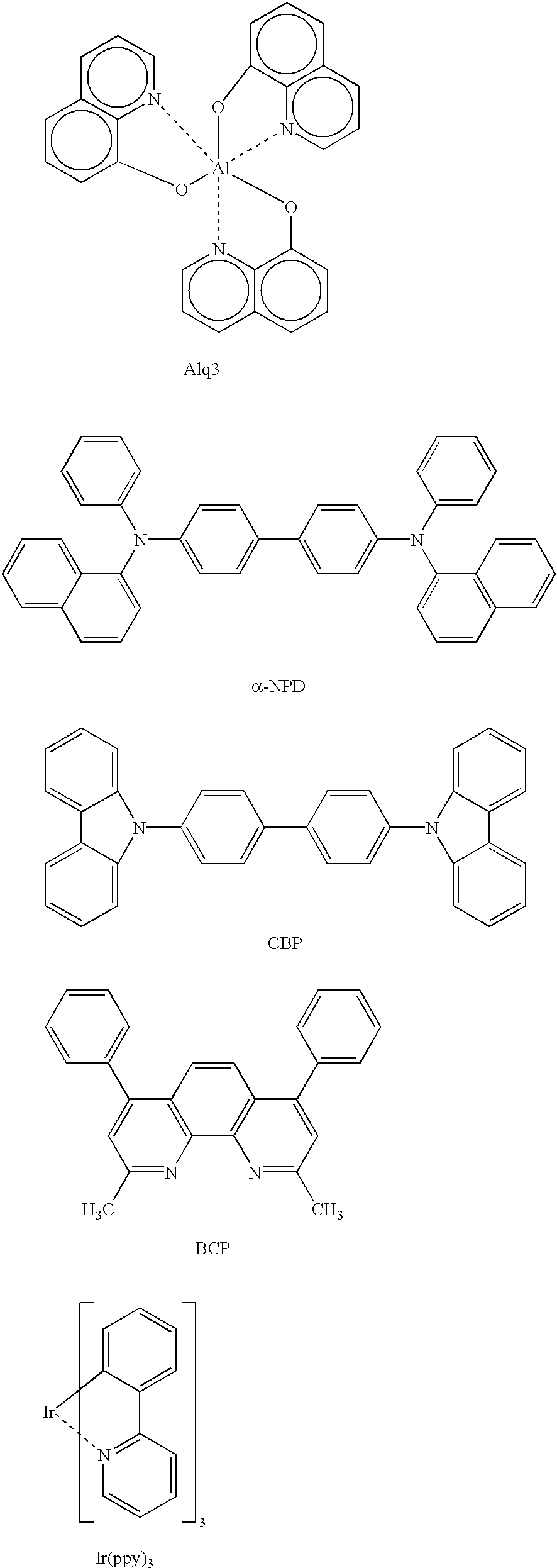
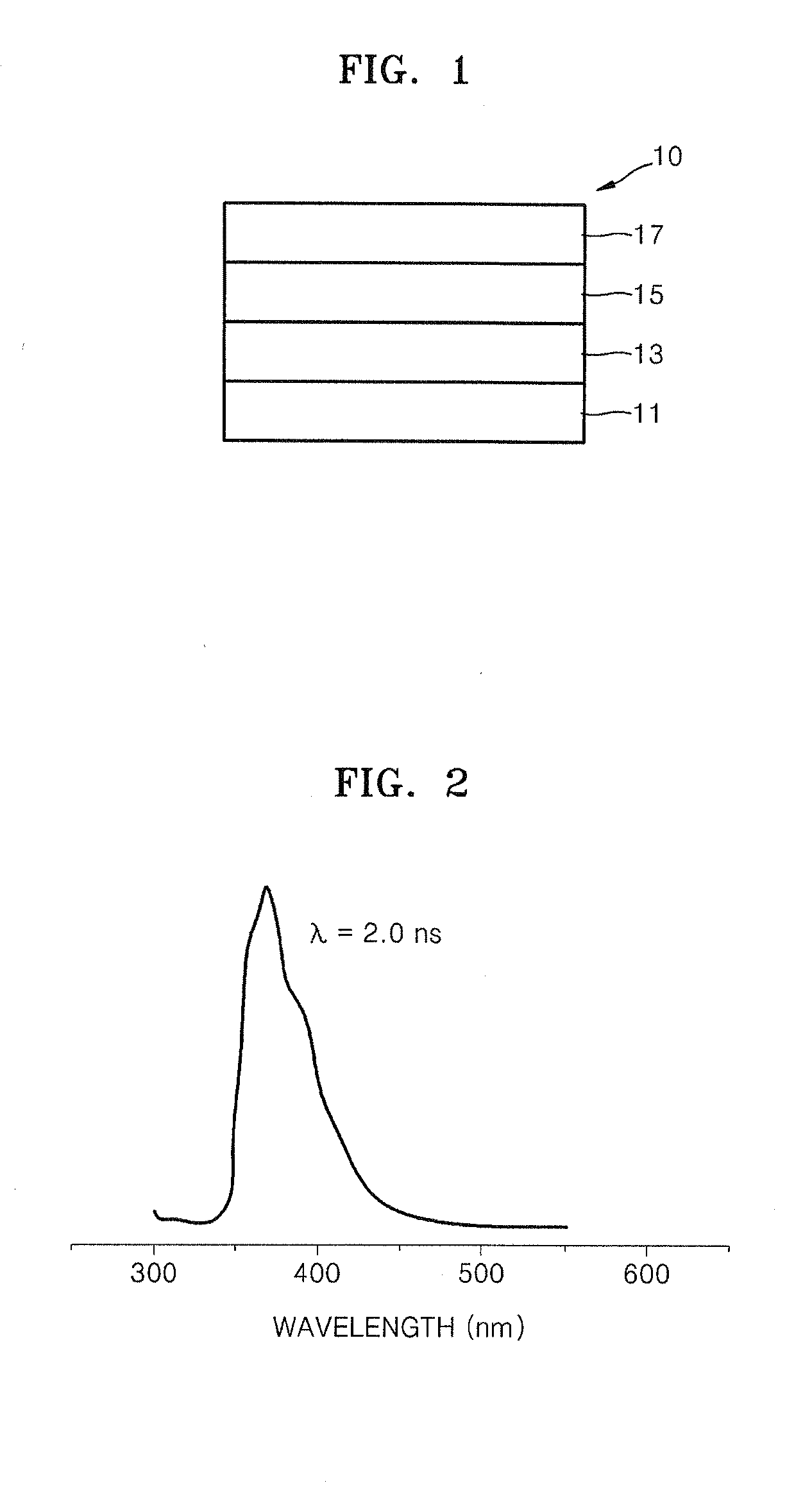
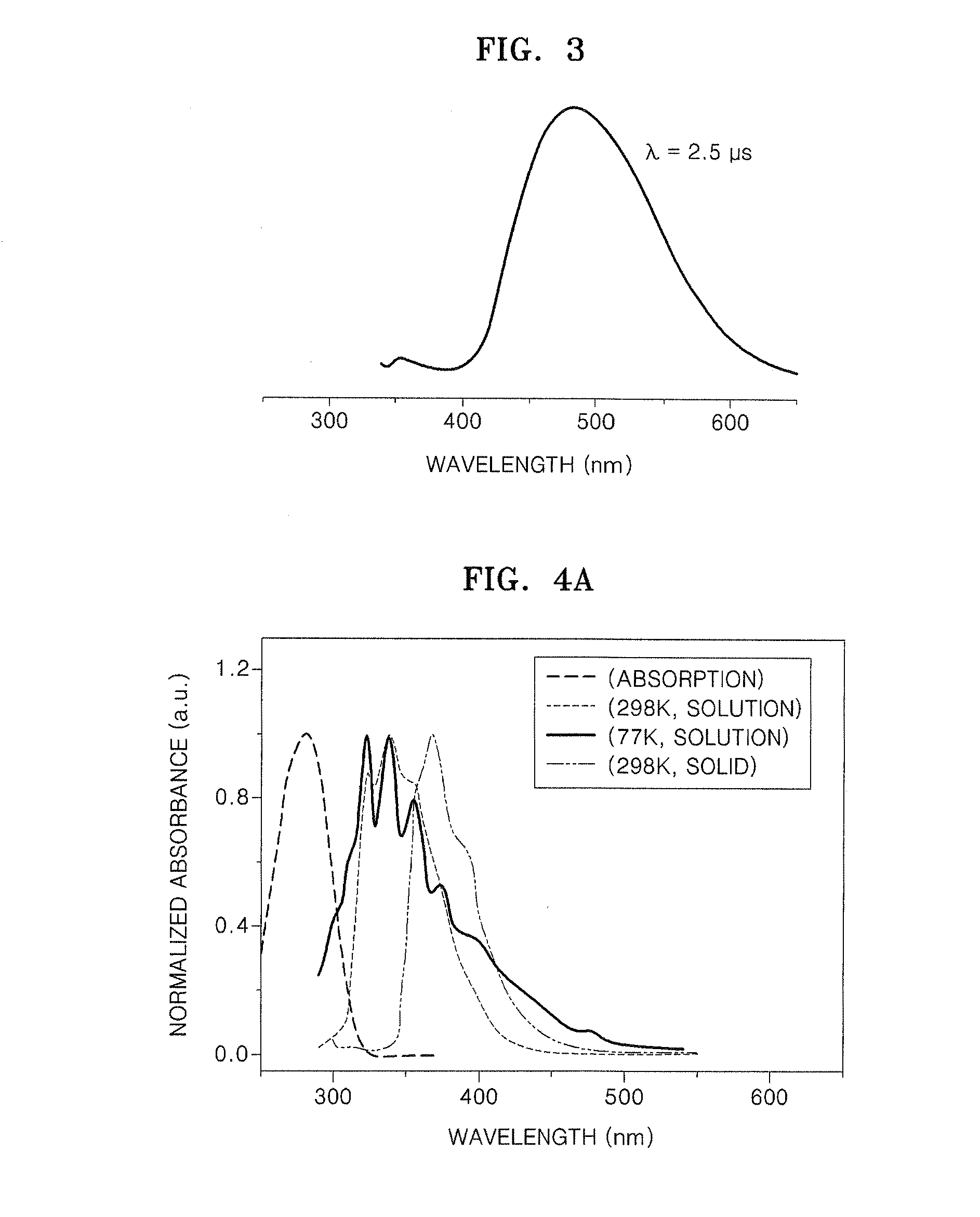
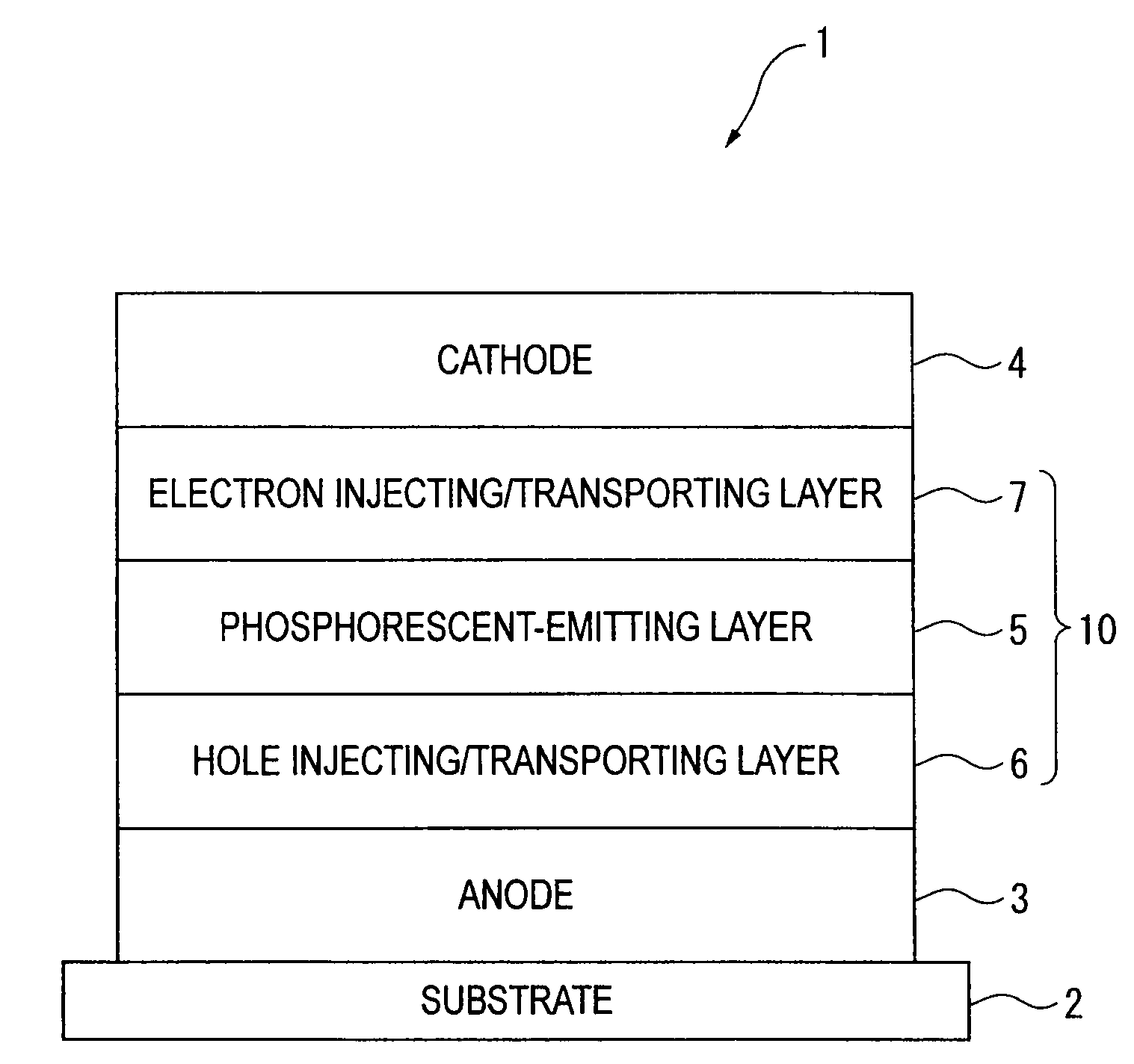
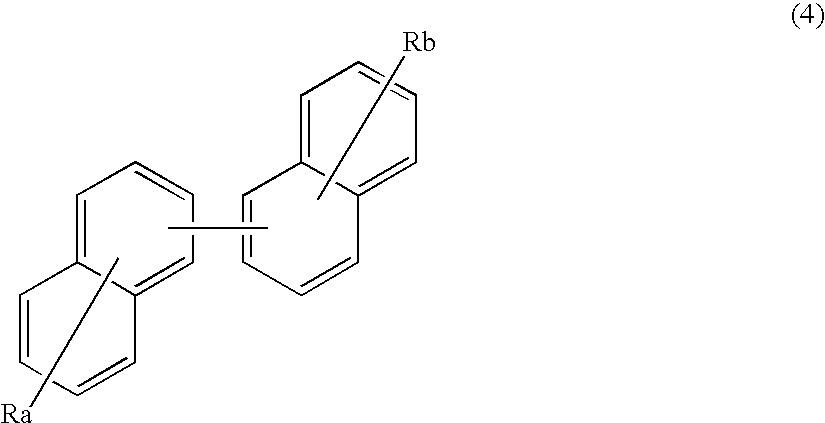
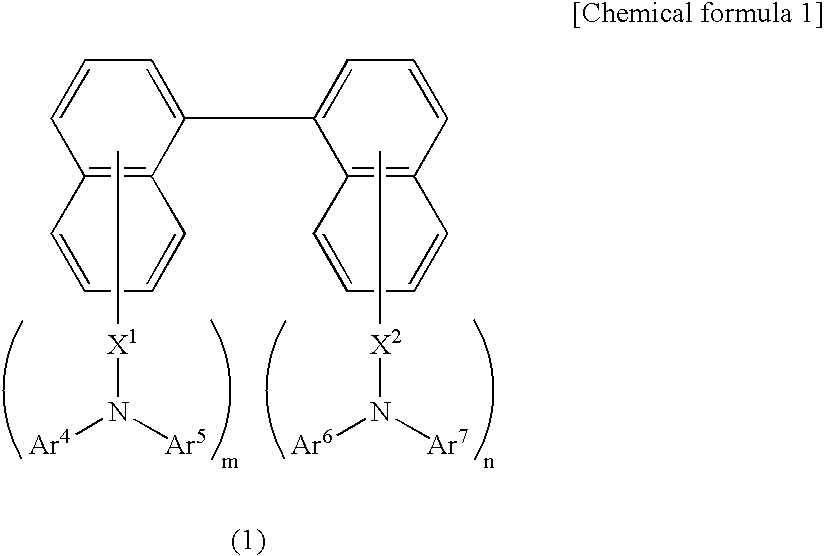
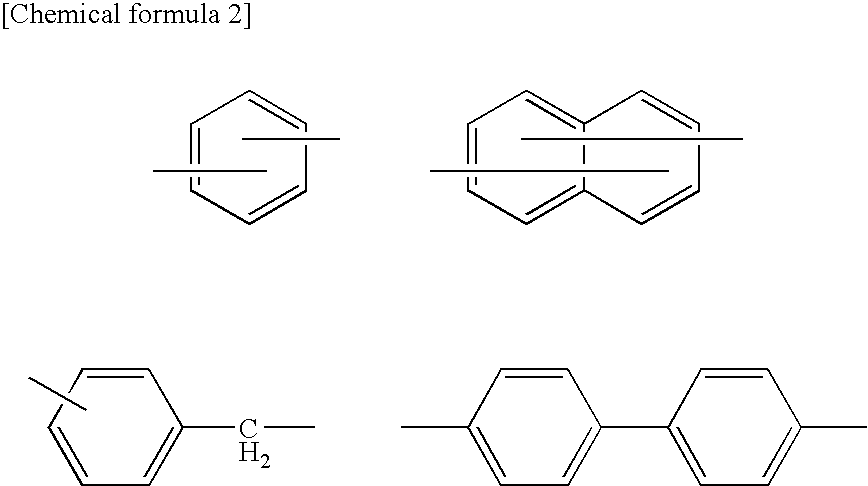
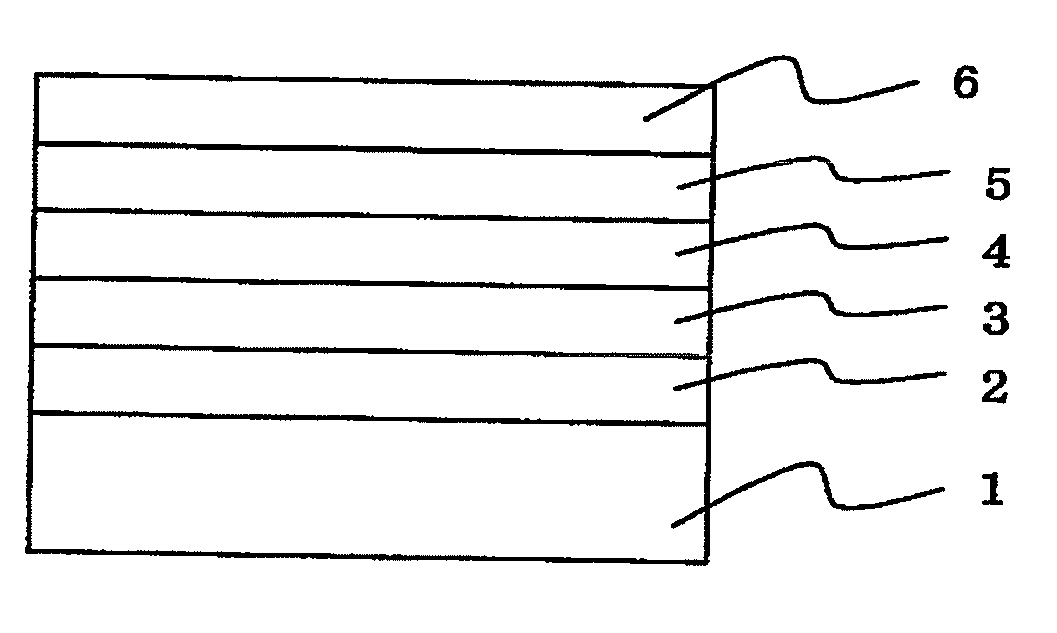
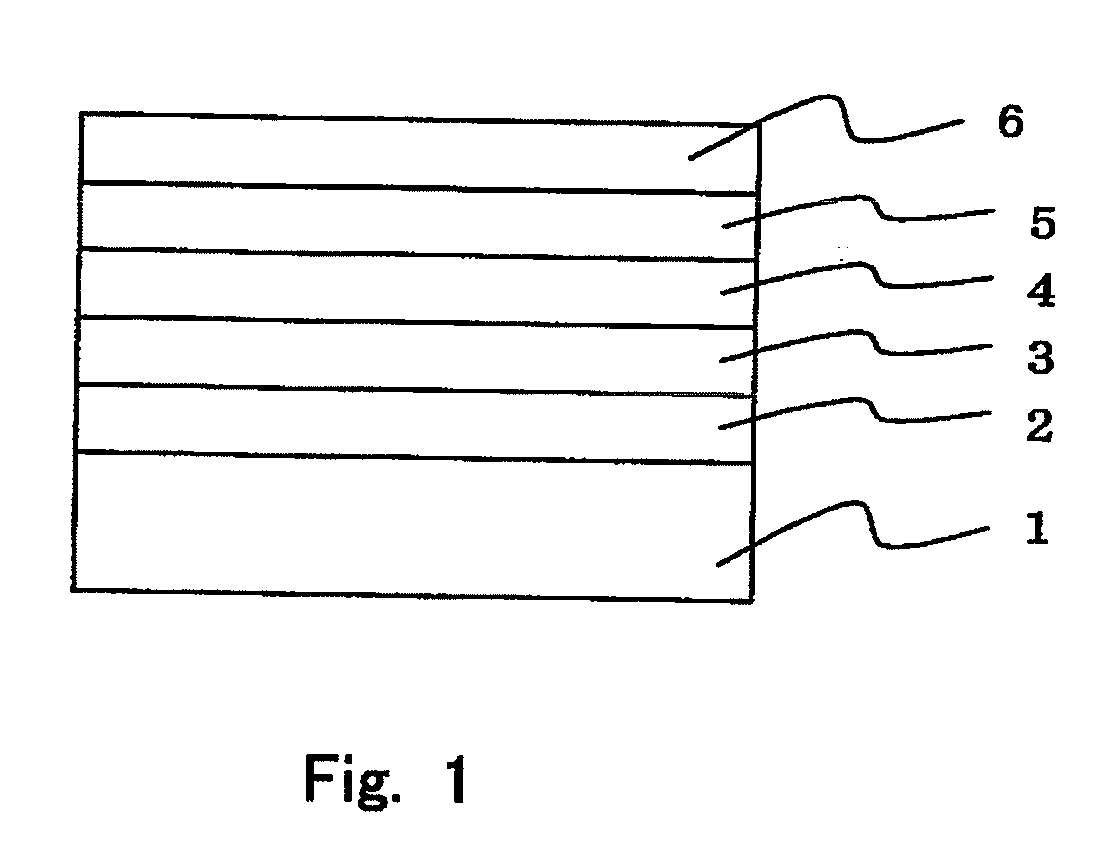
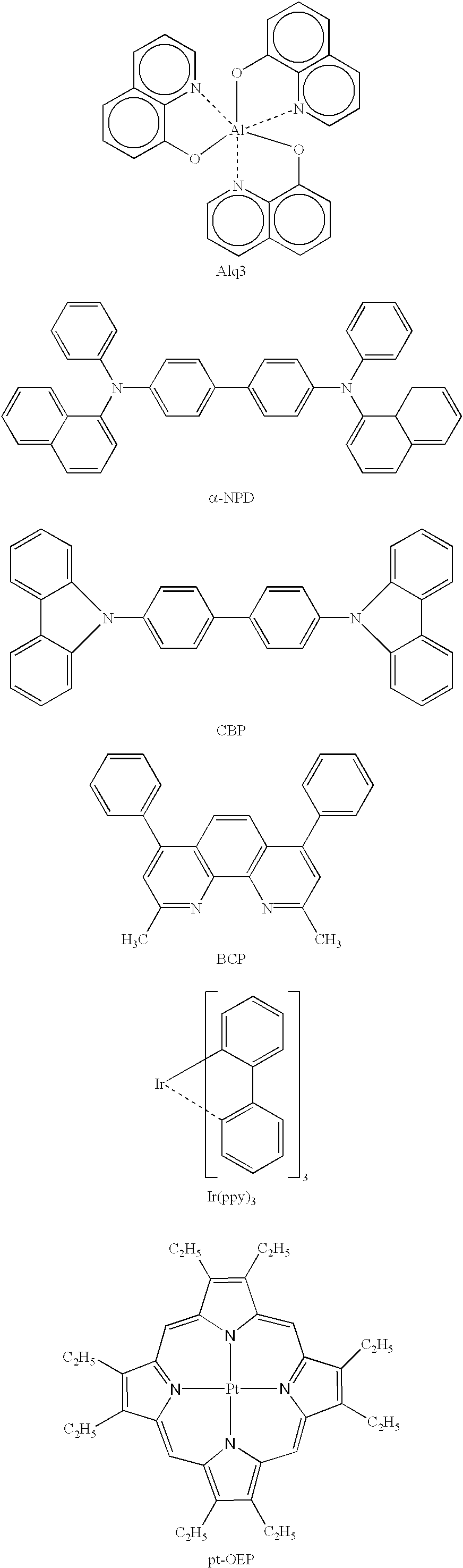
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090009065A1Improve efficiencyLong lastingSilicon organic compoundsDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
Owner:IDEMITSU KOSAN CO LTD
Organometallic compound and organic electroluminescence device employing the same
InactiveUS20130033172A1Indium organic compoundsDischarge tube luminescnet screensChemical structureOrganic electroluminescence
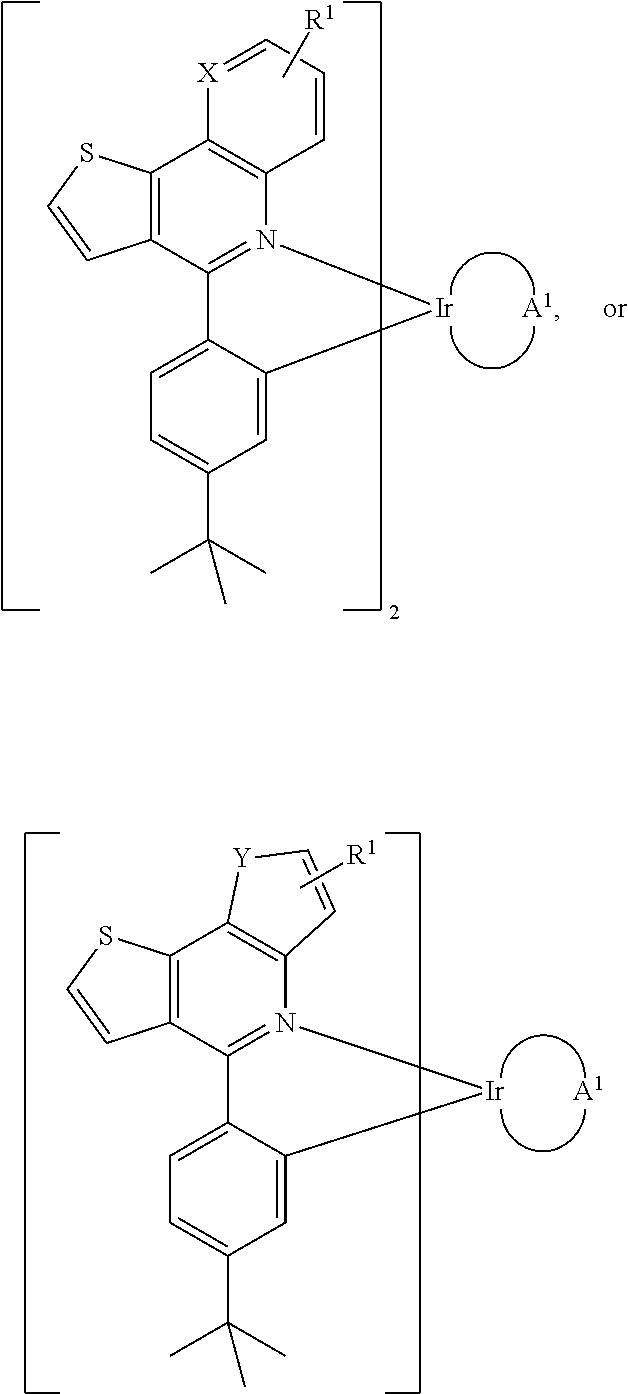
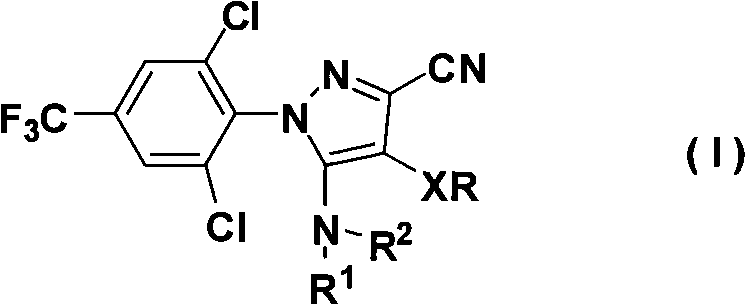
Organometallic compounds and organic electroluminescence devices employing the same are provided. The organometallic compound has a chemical structure represented below:wherein, X is C—H or N, Y is CH2 or NH; R1 is H, or C1-8 alkyl; and A1 is acetylacetone ligand, acetylacetone with phenyl group ligand, or derivatives thereof.
Owner:IND TECH RES INST
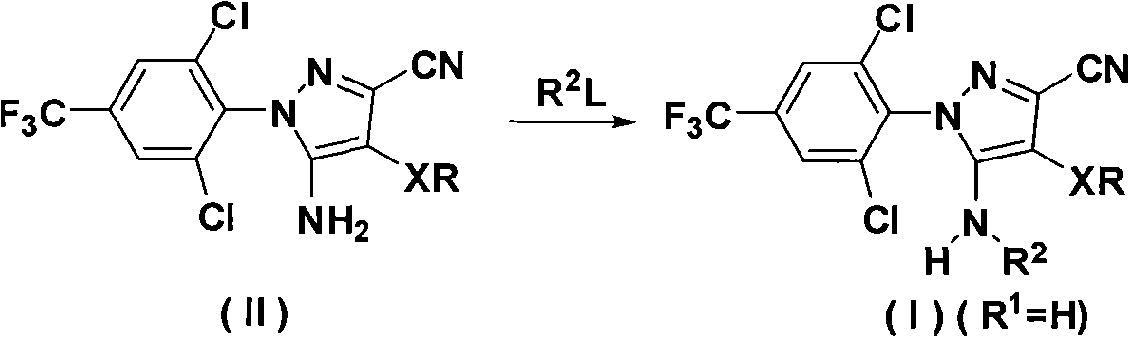
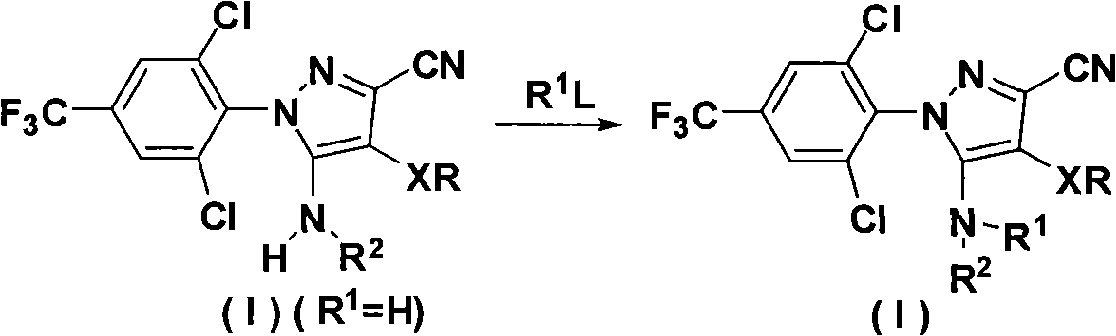
N-benz-3-substituted amino pyrazoles compounds with insecticidal activity
The invention discloses an N-phenyl-3-substitution amino-pyrazole compound which is shown in a formular (1) and has the insecticidal activity, and a preparation method thereof.
Owner:HUNAN CHEM RES INST
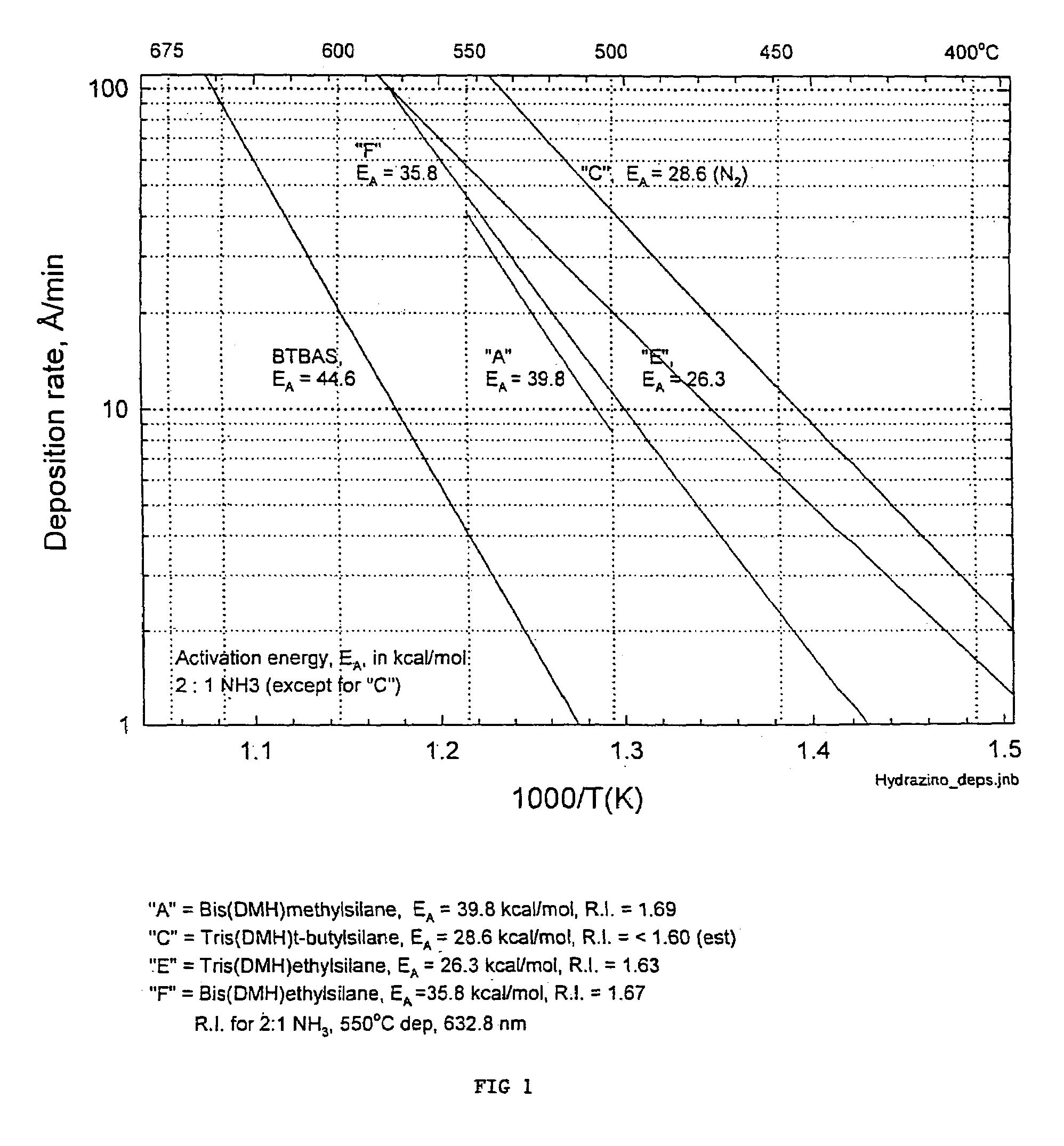
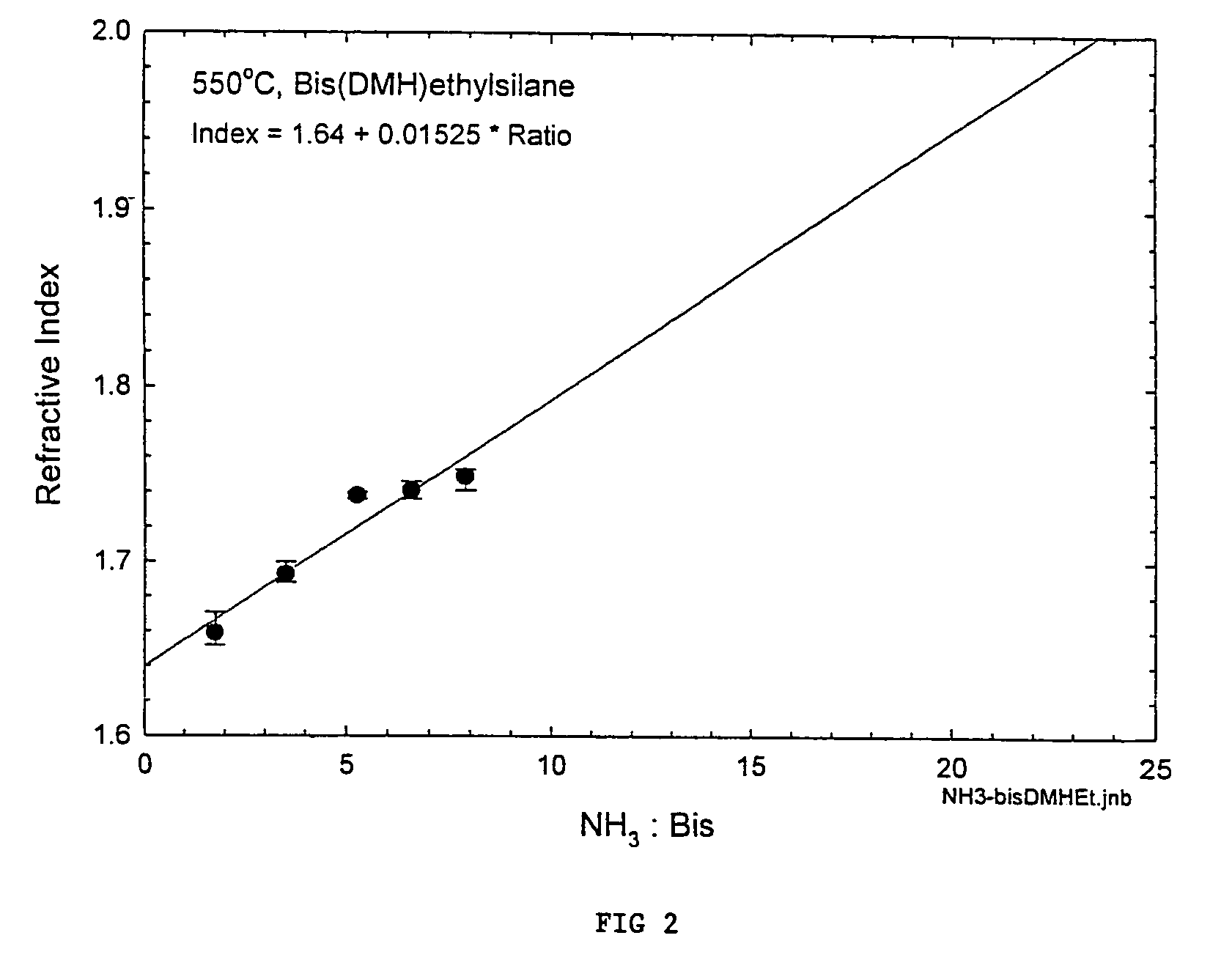
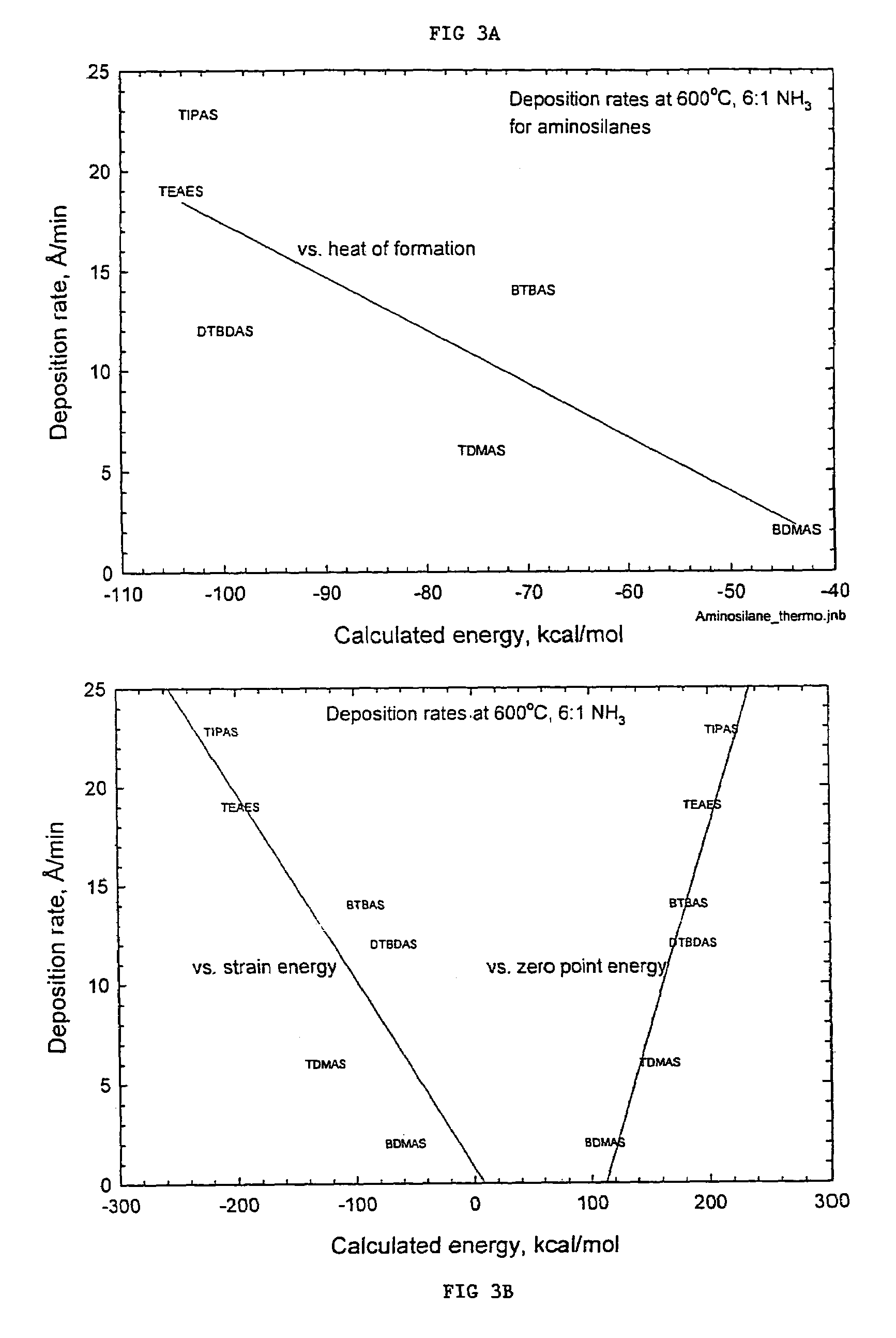
Precursors for depositing silicon containing films and processes thereof
Processes for precursors for silicon dielectric depositions of silicon nitride, silicon oxide and silicon oxynitride on a substrate using a hydrazinosilane of the formula:[R12N—NH]nSi(R2)4−nwhere each R1 is independently selected from alkyl groups of C1 to C6; each R2 is independently selected from the group consisting of hydrogen, alkyl, vinyl, allyl, and phenyl; and n=1–4. Some of the hydrazinosilanes are novel precursors.
Owner:VERSUM MATERIALS US LLC
Compositions for organic electroluminescent device and organic electroluminescent device
InactiveUS20060182993A1Reduce inactivationChange propertiesDischarge tube luminescnet screensDuplicating/marking methodsSolubilityHole injection layer
Disclosed are compositions for an organic electroluminescent device favorably used for forming a hole injection layer and a hole transport layer of the organic electroluminescent device by a wet film forming method. The compositions for the organic electroluminescent device, which are composite solutions prepared by dissolving hole transport materials such as aromatic diamine compounds and an electron acceptor such as tri(pentafluorophenyl)boron in a solvent that contains an ether solvent and / or an ester solvent whose water solubility at 25° C. is 1 weight % or less in the solvent, with a concentration of 10 weight % or higher in the compositions.
Owner:MITSUBISHI CHEM CORP
Cyclic azine derivatives, processes for producing these, and organic electroluminescent element containing these as component
ActiveUS20120214993A1Improve drivabilityAccelerate emissionsOrganic chemistryElectroluminescent light sourcesOrganic electroluminescenceOrganic compound
A cyclic azine compound represented by general formula (1):wherein each Ar1 represents an aromatic group, which is unsubstituted or substituted by a C1-4 alkyl group, a phenyl group or a pyridyl group; and A represents a group selected from those which are represented by general formulae (2) to (5), described in the description. The cyclic azine compound is useful for an organic compound layer of fluorescent or phosphorescent EL device.
Owner:TOSOH CORP +1
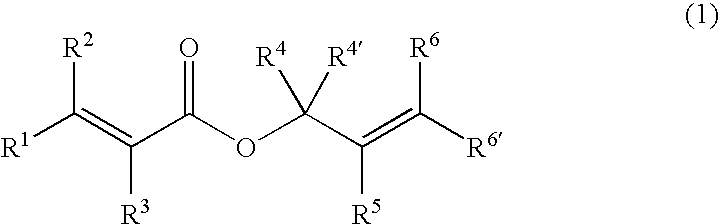
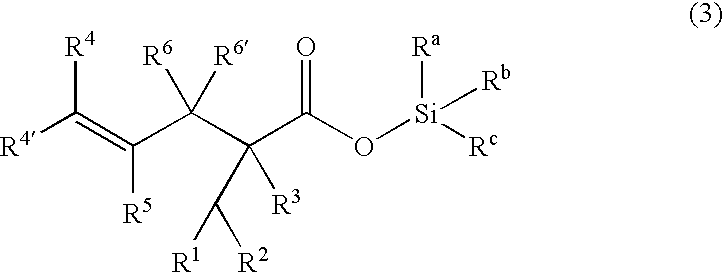
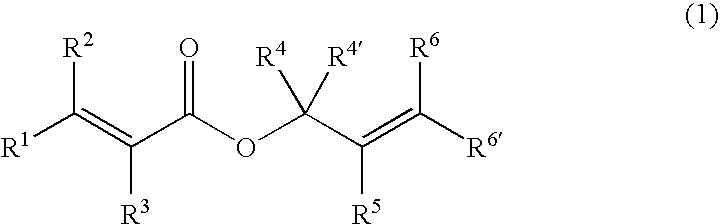
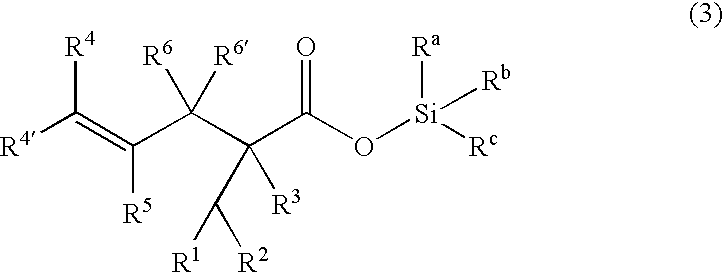
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS7307178B2Few stepsHigh yieldSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM CO LTD
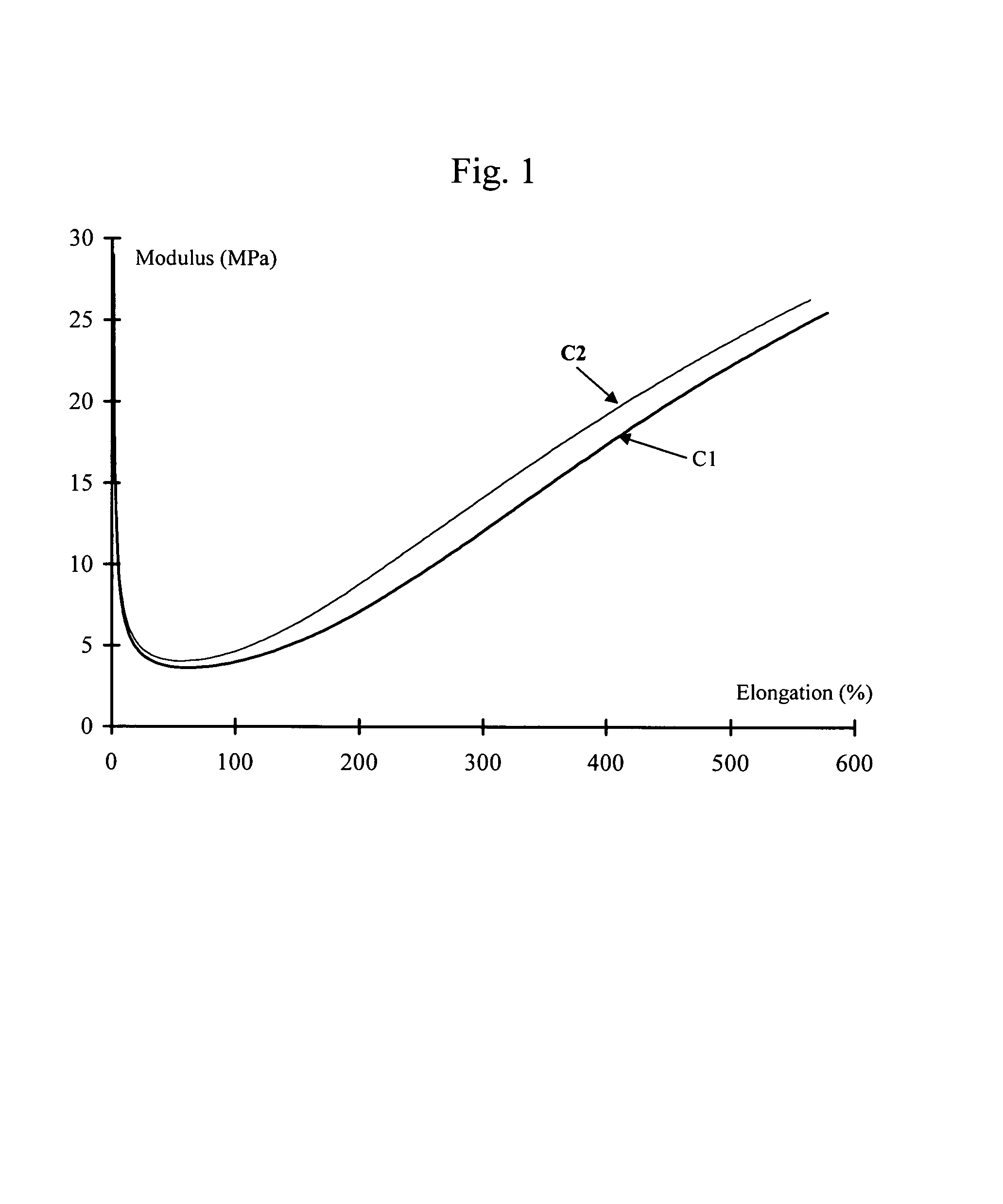
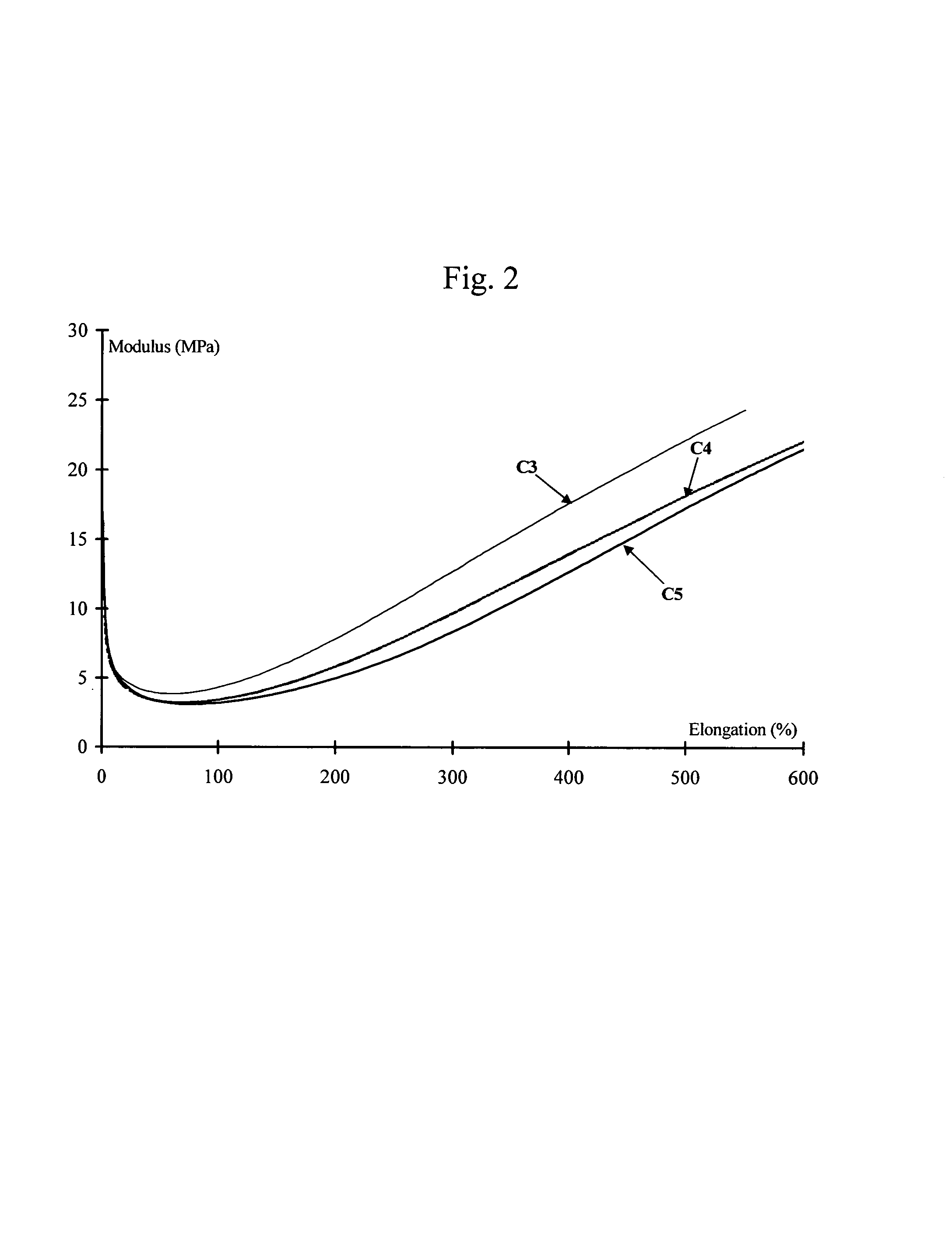
Tire and tread comprising a bis-alkoxysilane tetrasulfide as coupling agent
Owner:MICHELIN & CO CIE GEN DES ESTAB MICHELIN
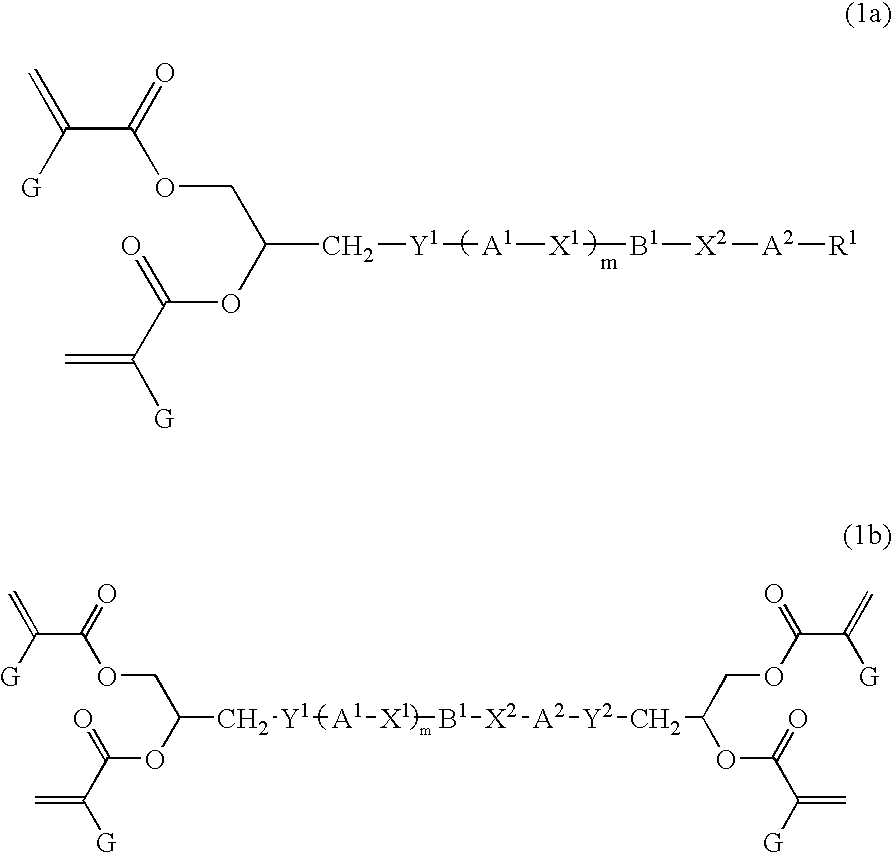
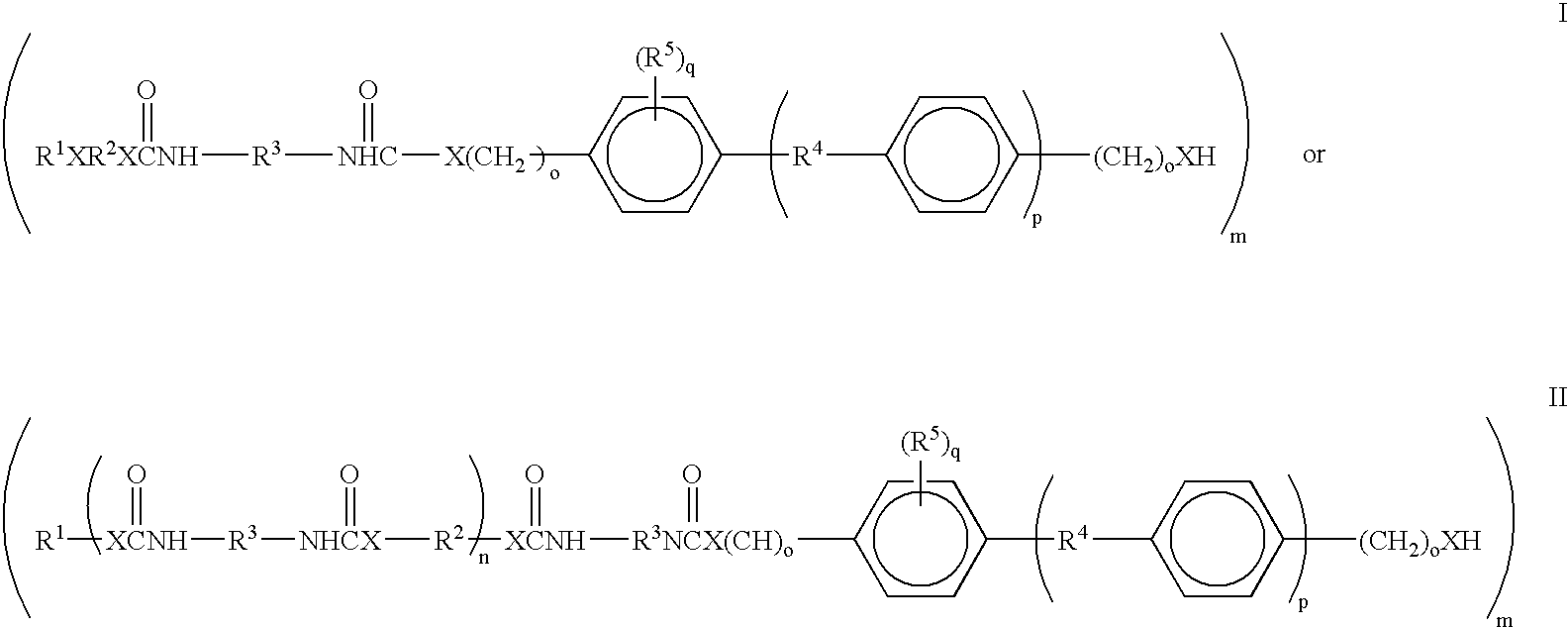
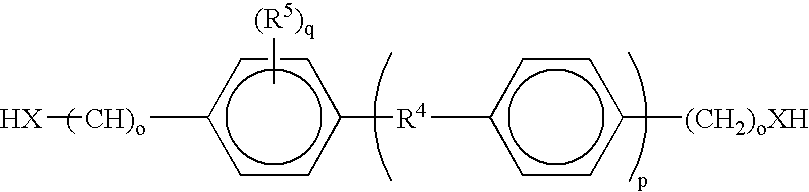
Liquid crystal polyfunctional acrylate derivative and polymer thereof
A compound represented by the following general formula (1a) or (1b), a liquid crystal composition containing the compound, and a polymer obtained by polymerizing the compound or the composition: wherein R1 represents a fluorine atom, an alkyl group or the like; Y1 and Y2 each independently represents alkylene or the like; A1, A2, B1 and B2 each independently represents 1,4-cyclohexylene, 1,4-phenylene or the like; X1 and X2 each independently represents a single bond, —COO—, —OCO— or the like; m represents 0, 1 or 2; and G represents hydrogen, methyl or the like.
Owner:JNC CORP +1
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS20050070729A1High yieldFew stepsSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM IND CO LTD
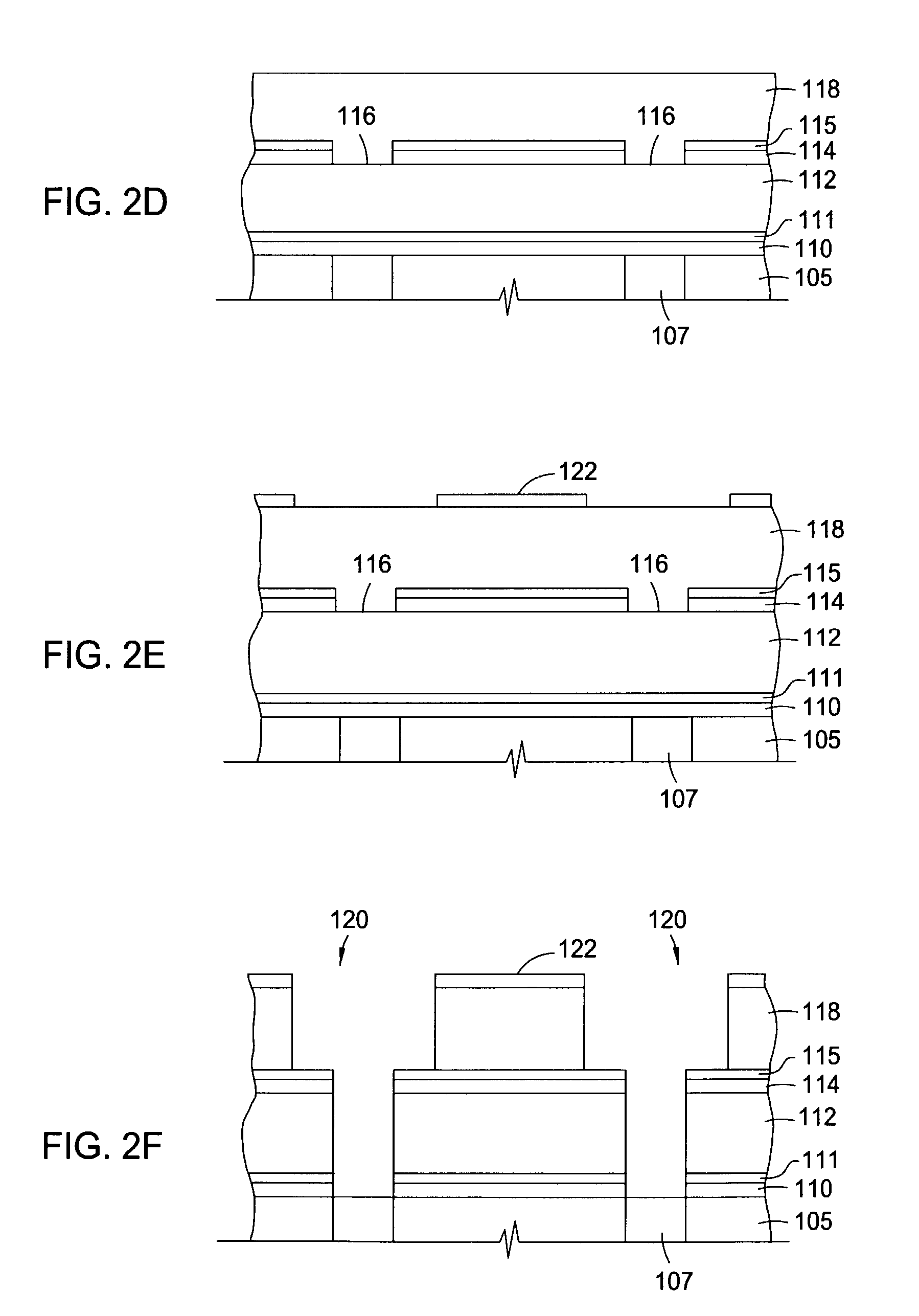
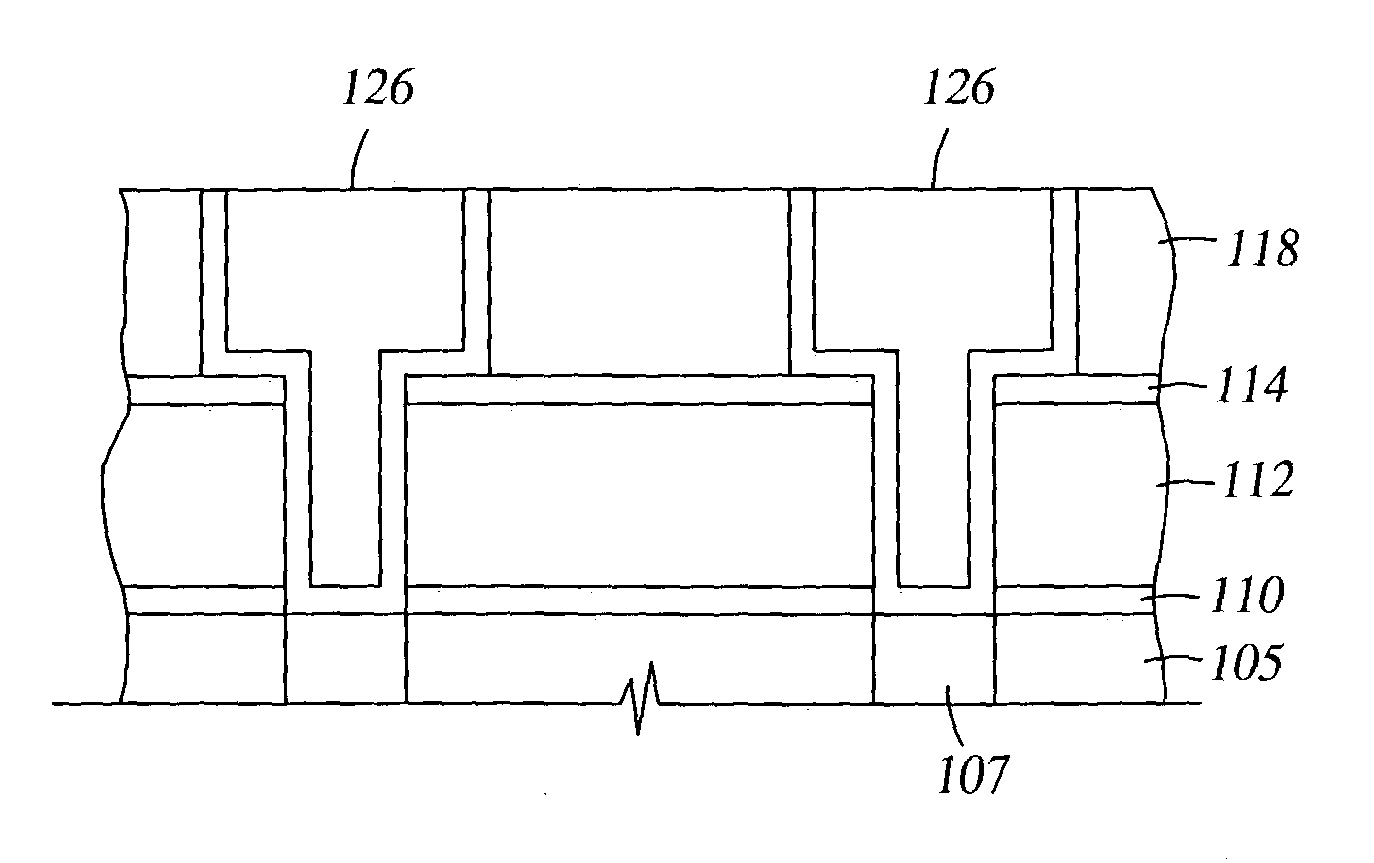
Two-layer film for next generation damascene barrier application with good oxidation resistance
InactiveUS7749563B2Low dielectric constantLiquid surface applicatorsSemiconductor/solid-state device manufacturingOxygenPhenyl group
A method is provided for processing a substrate including providing a processing gas comprising an organosilicon compound comprising a phenyl group to the processing chamber, and reacting the processing gas to deposit a low k silicon carbide barrier layer useful as a barrier layer in damascene or dual damascene applications with low k dielectric materials. A method is provided for depositing a silicon carbide cap layer that has substantially no phenyl groups attached to silicon atoms from a processing gas comprising an oxygen-free organosilicon compound on a low k silicon carbide barrier layer.
Owner:APPLIED MATERIALS INC
Process for applying a streamable epoxy adhesive
InactiveUS20050070634A1Low viscosityHigh strength bondAdhesive processes with adhesive heatingEpoxy resin adhesivesBENZYL ALCOHOL/WATERViscosity
The invention is a composition comprising applying to a substrate a stream of an adhesive comprising: one or more epoxy resins; one or more rubber modified epoxy resins; one or more toughening compositions comprising the reaction product of one or more isocyanate terminated prepolymers and one or more capping compounds having one or more phenolic, benzyl alcohol, aminophenyl, or, benzylamino groups wherein the reaction product is terminated with the capping compounds; one or more curing agents for epoxy resins and one or more catalysts which initiate cure at a temperature of about 100° C. or greater; and optionally; fillers adhesion promoters, wetting agents or rheological additives useful in epoxy adhesive compositions; wherein the adhesive composition has a viscosity at 45° C. of about 20 Pa.s to about 400 Pa.s. The composition can be used as an adhesive and applied as a stream using a high speed streaming process.
Owner:DOW GLOBAL TECH LLC
Metal coordination compound, luminescence device and display apparatus
InactiveUS20020094453A1Solid-state devicesSemiconductor/solid-state device manufacturingHydrogen atomNitrogen
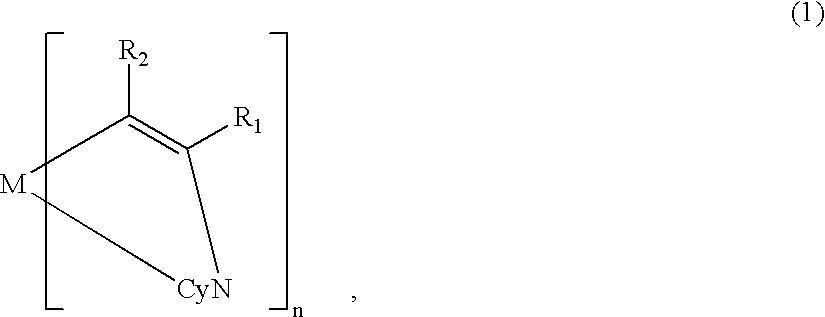
A metal coordination compound suitable as an organic material for a luminescent device is represented by the following formula (1): wherein M denotes Ir, Pt, Rh or Pd; n is 2 or 3; Y denotes an alkylene group having 2-6 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S- or -CO- and capable of including hydrogen atom which can be replaced with a linear or branched alkyl group which has 1-10 carbon atoms and is capable of including hydrogen atom which can be replaced with fluorine atom; and CyN denotes a cyclic group containing nitrogen atom connected to M and capable of having a substituent selected from the group consisting of halogen atom; nitro group; phenyl group; trialkylsilyl group having 1-8 carbon atoms; and a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including hydrogen atom which can be replaced with fluorine atom.
Owner:CANON KK
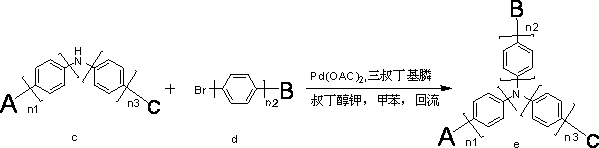
Organic electroluminescent material containing tertiary aromatic amine structure and preparation method and application thereof
InactiveCN102702075AImprove performanceImprove luminous efficiencyOrganic chemistrySolid-state devicesCarbazoleStructural formula
The invention discloses an organic electroluminescent material containing a tertiary aromatic amine structure. The organic electroluminescent material containing the tertiary aromatic amine structure is characterized in that: the structural formula is shown in the specifications; and in the structural formula, n1, n2 and n3 independently represent that the quantity of benzene ring is 0 or 1 respectively; a radical A represents a substituted carbazole radical; a radical B represents a structural radical containing substituted fluorenyl; and a structure C is a structure radical containing phenyl and substituted phenyl. The organic electroluminescent material is a fluorescent material which has high luminous efficiency; a result of the luminous efficiency in a solution can further indicate that the organic electroluminescent material which has high luminous efficiency and of which the brightness and performance can meet the industrial development can be applied to electroluminescent devices by serving as a luminous material or a luminous main body material or a transmission material. A synthesizing process has the advantages of reaction in two simple steps, easiness and convenience for operating, easiness for purifying, great increase in the industrial synthesizing yield, great reduction in cost, wide application, applicability to a plurality of materials for devices, and wide prospect. Meanwhile, a substituted radical is adjusted, so that the performance of the material further meets requirement of industrialization.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
Pharmaceutical compositions including N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide (Compound 1) and methods of using such compositions are described herein.
Owner:VERTEX PHARMA INC
Optical Film
A composition comprising a polymerizable compound of the formula (1) and a rod-shaped polymerizable liquid crystal compound: P2-E2-X2-B2-A2-(G2)t-Y-(G1)s-A1-B1-X1-E1-P1 (1) (in the formula (1), Y represents a di-valent group, s and t represent each independently an integer of 0 or 1, G1 and G2 when s and t are 1 represent each independently —CR1R2—, R1 and R2 represent each independently an alkyl group having 1 to 4 carbon atoms, halogen atom or hydrogen atom, A1 and A2 represent each independently a di-valent cyclic hydrocarbon group, di-valent heterocyclic group, methylenephenylene group, oxyphenylene group or thiophenylene group, B1 and B2 represent each independently a di-valent group, X1 and X2 represent each independently a di-valent group, E1 and E2 represent each independently an alkylene group having 2 to 25 carbon atoms, and P1 and P2 represent a hydrogen atom or polymerizable group, at least one of P1 and P2 being a polymerizable group.).
Owner:SUMITOMO CHEM CO LTD
Cinnamide compound
The present invention relates to a compound represented by Formula (I): (wherein Ar1 represents an imidazolyl group which may be substituted with 1 to 3 substituents; Ar2 represents a pyridinyl group, a pyrimidinyl group, or a phenyl group which may be substituted with 1 to 3 substituents; X1 represents (1) —C≡C— or (2) a double bond etc. which may be substituted; R1 and R2 represent, for example, a C1-6 alkyl group or C3-8 cycloalkyl group which may be substituted) or a pharmacologically acceptable salt thereof and to the use thereof as pharmaceutical agents. The object of the present invention is to find a therapeutic or preventive agent for diseases caused by Aβ. According to the present invention, a therapeutic or preventive agents for diseases caused by Aβ can be provided.
Owner:EISIA R&D MANAGEMENT CO LTD
2′-fluoronucleosides
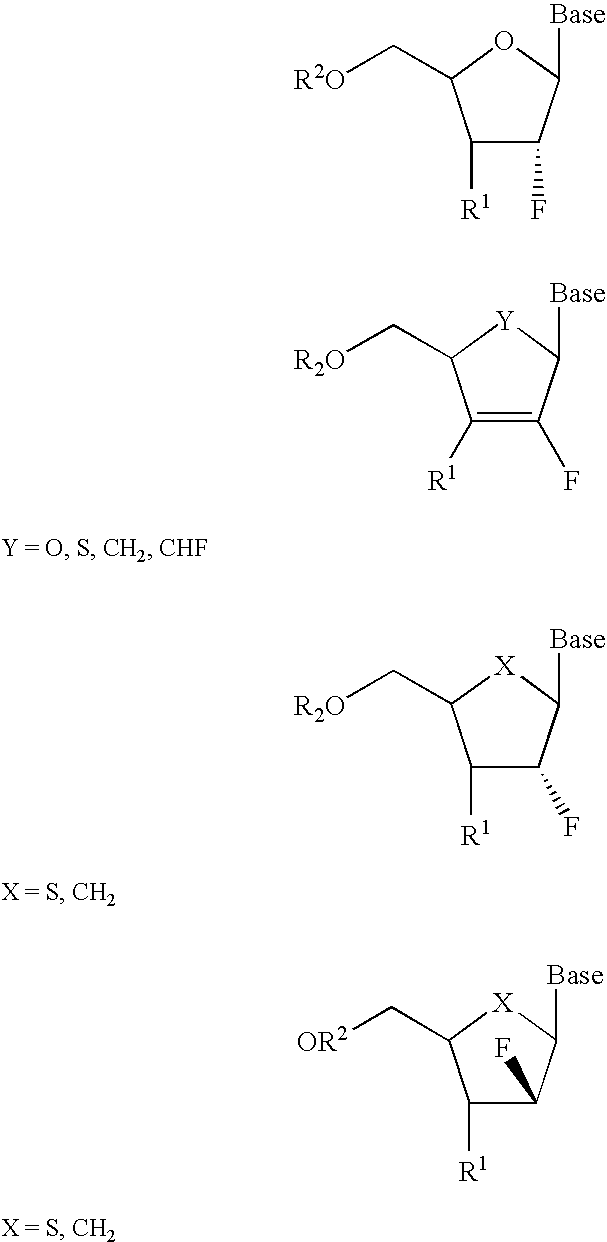
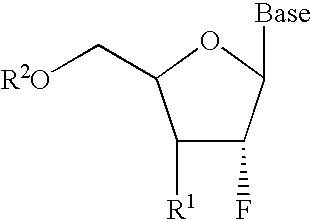
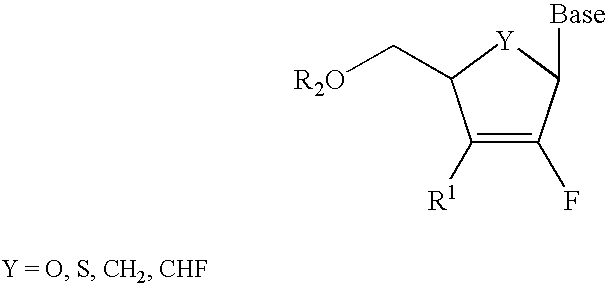
InactiveUS6911424B2Sure easyUseful in treatmentBiocideGroup 5/15 element organic compoundsPhosphoric Acid EstersPurine
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae: wherein[0001]Base is a purine or pyrimidine base;[0002]R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;[0003]R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; and[0004]R3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY
Method of improving stability in low k barrier layers
InactiveUS20040137756A1Decorative surface effectsSemiconductor/solid-state device detailsHydrogenPhenyl group
A method is provided for processing a substrate including providing a processing gas comprising hydrogen gas and an organosilicon compound comprising a phenyl group to the processing chamber, and reacting the processing gas to deposit a low k silicon carbide barrier layer useful as a barrier layer in damascene or dual damascene applications with low k dielectric materials.
Owner:APPLIED MATERIALS INC
Organometallic Complex, Light-Emitting Element, Light-Emitting Device, Electronic Device and Lighting Device
ActiveUS20120098417A1Improve emission efficiencyReduce power consumptionGroup 5/15 element organic compoundsSolid-state devicesIridiumHydrogen
Provided is a novel substance that can emit phosphorescence. Alternatively, provided is a novel substance with high emission efficiency. An organometallic complex in which a 4-arylpyrimidine derivative is a ligand and iridium is a central metal is provided. Specifically, an organometallic complex having a structure represented by a general formula (G1) is provided. In the general formula (G1), R1 represents a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms or a substituted or unsubstituted aryl group having 6 to 10 carbon atoms, R2 represents any of hydrogen, a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms, and a substituted or unsubstituted phenyl group, R3 represents hydrogen or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms, and Ar1 represents a substituted or unsubstituted arylene group having 6 to 10 carbon atoms.
Owner:SEMICON ENERGY LAB CO LTD
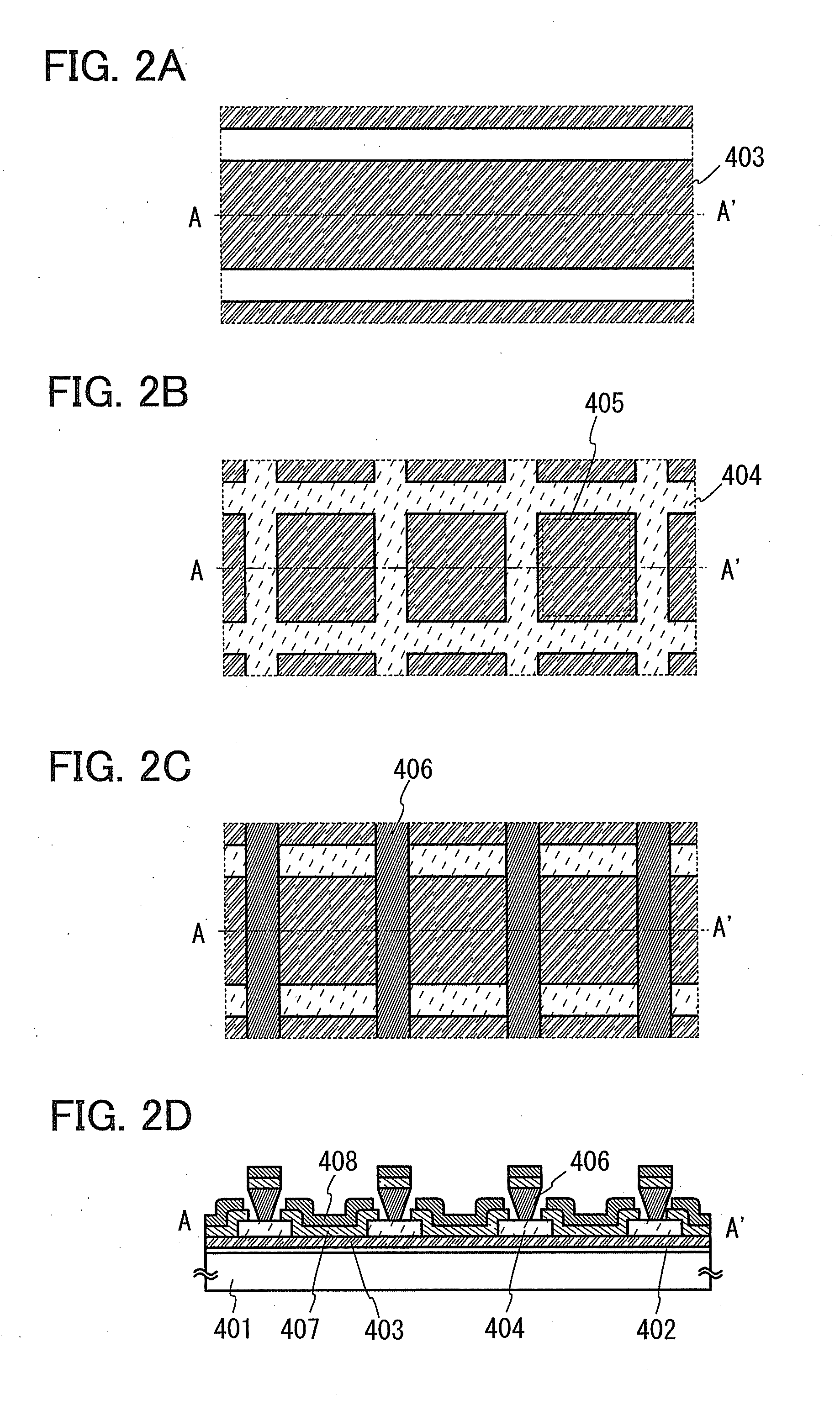
Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
InactiveUS20110064811A1Improve solubilityGood physical and chemical stabilityBiocidePowder deliveryFormamideOxoquinolines
The present invention relates to solid state forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide (Compound 1), pharmaceutical compositions thereof and methods therewith.
Owner:VERTEX PHARMA INC
Panchromatic photosensitizers and dye-sensitized solar cell using the same
InactiveUS20100258175A1Improve photoelectric conversion efficiencyImprove response efficiencyRuthenium organic compoundsElectrolytic capacitorsPhosphoric acidCarboxylic acid
Panchromatic photosensitizers having a Formula of ML1L2X were synthesized, wherein M comprises ruthenium atom; X is a monodentate anion; L1 is heterocyclic bidentate ligand having one of formulae listed below:wherein G2 has one of formulae listed below:and L2 is a tridentate ligand having a formula listed below:The substituents R1, R2, R3, R4, R5, R6, R7 of L1 and L2 are the same or different, and represent alkyl, alkoxy, alkylthio, alkylamino, halogenated alkyl, phenyl or substituted phenyl group, carboxylic acid or counter anion thereof, sulfonic acid or counter anion thereof, phosphoric acid or counter anion thereof, amino-group, halogens, or hydrogen. The above-mentioned photosensitizers are suitable to use as sensitizers for fabrication of high efficiency dye-sensitized solar cell.
Owner:NATIONAL TSING HUA UNIVERSITY
Metal coordination compound, luminescence device and display apparatus
A metal coordination compound suitable as an organic material for a luminescent device is represented by the following formula (1): wherein M denotes Ir, Pt, Rh or Pd; n is 2 or 3; R1 and R2 independently denote a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including hydrogen atom which can be replaced with fluorine atom; and CyN denotes a cyclic group containing nitrogen atom connected to M and capable of having a substituent selected from the group consisting of halogen atom; nitro group; phenyl group; trialkylsilyl group having 1-8 carbon atoms; and a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including hydrogen atom which can be replaced with fluorine atom.
Owner:CANON KK
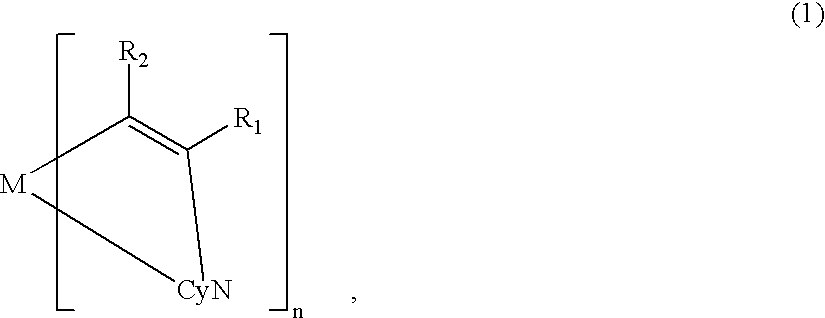
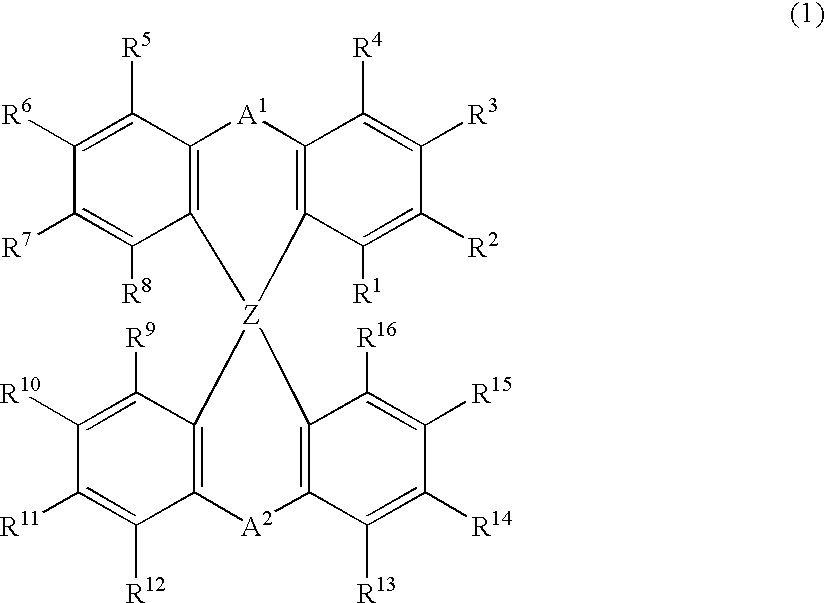
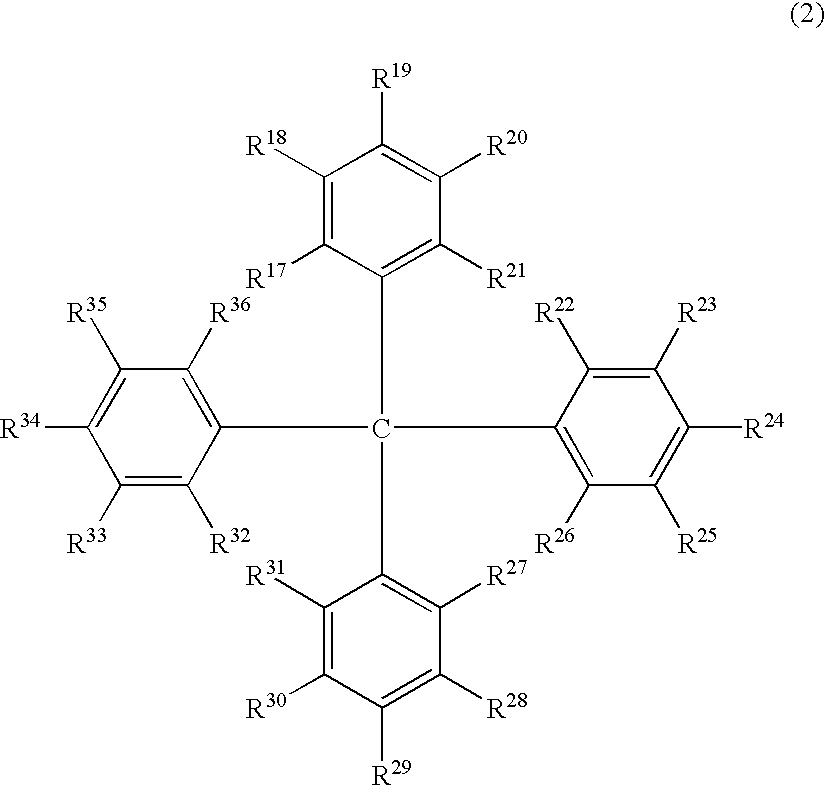
Luminescent element material and luminescent element comprising the same
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a least one type of compound denoted by (a) to (d) below. (a) A compound having a plurality of 1,7-phenanthroline skeletal structures (b) A benzoquinoline derivative (c) A spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. (d) A tetraphenylmethane derivative represented by general formula (2) R17 to R36 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. However, at least one of R17 to R36 is selected from substituents represented by general formula (3). -X-Ar (3) X is a single bond or is selected from the following, and Ar denotes a condensed aromatic ring or heteroaromatic ring. In the case where X is phosphorus oxide, then Ar represents an aromatic hydrocarbon or heteroaromatic ring. n is an natural number.
Owner:TORAY IND INC
12-Aryl prostaglandin analogs
Compounds comprisingare disclosed, wherein Y, A, X, R, D, and n are as described.A compound comprising a prostaglandin EP2 selective agonist wherein the ω-chain comprises a substituted phenyl, wherein at least one substituent consists of hydrocarbyl or non-linear hydroxyhydrocarbyl is also disclosed herein.Methods, compositions, and medicaments related thereto are also disclosed.
Owner:ALLERGAN INC
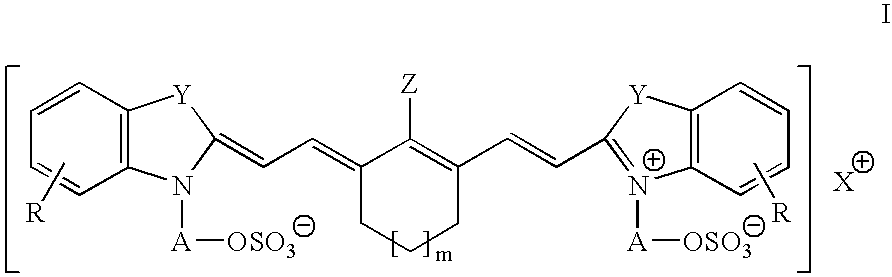
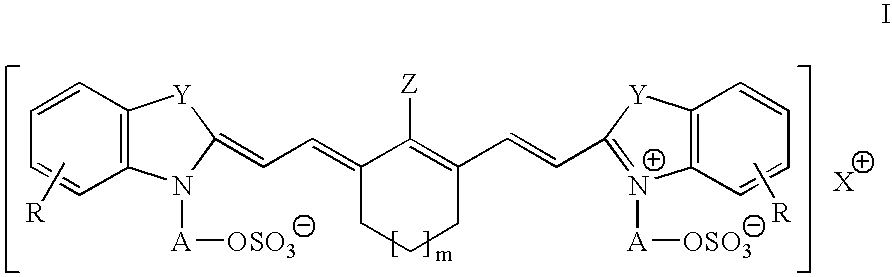
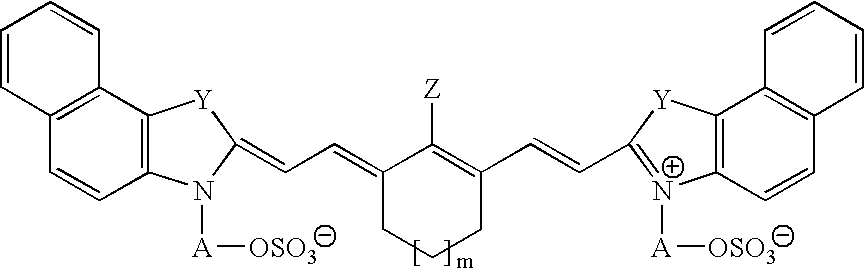
Infrared absorbing N-alkylsulfate cyanine compounds
Owner:KODAK POLYCHROME GRAPHICS
Two cyclic cinnamide compound
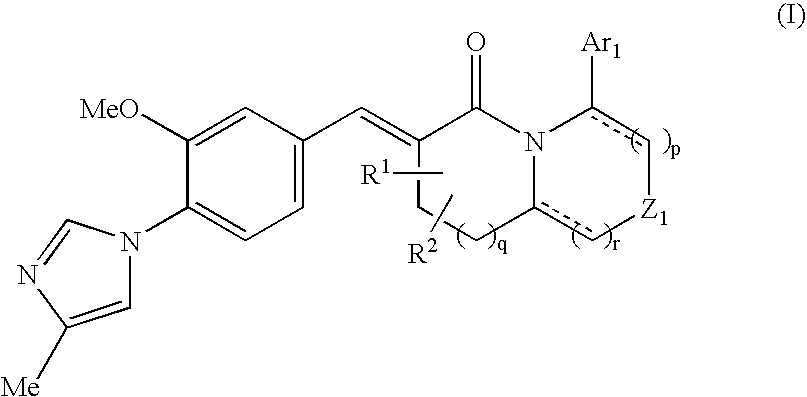
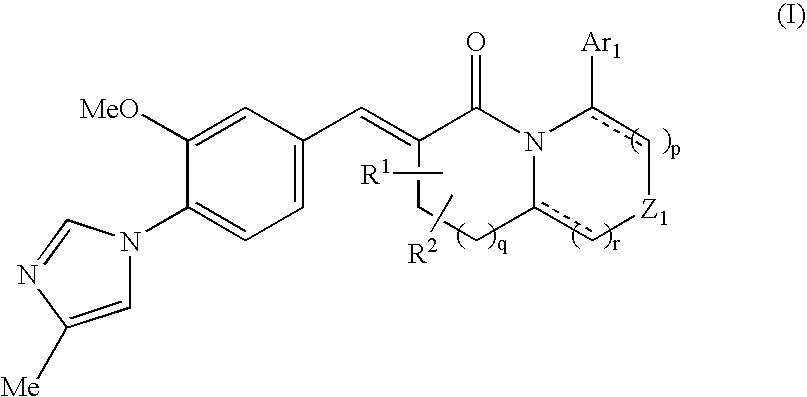
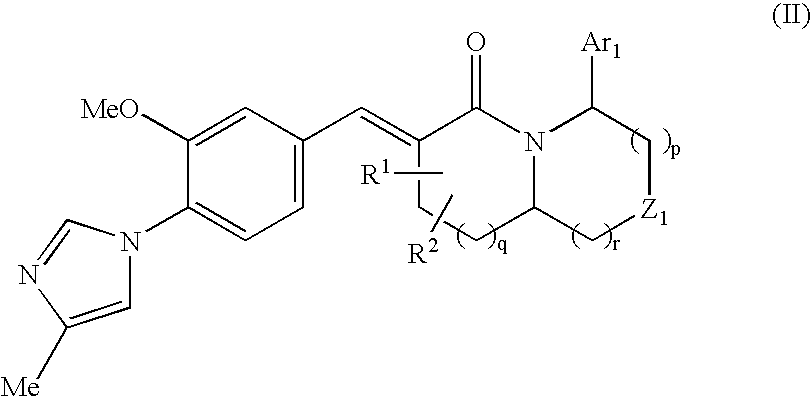
The present invention relates to a novel two cyclic cinnamide compound and a pharmaceutical agent comprising the compound as an active ingredient. The two cyclic cinnamide compound represented by the general formula (I): wherein represents a single bond or a double bond; Ar1 represents a phenyl group or pyridinyl group that may be substituted with 1 to 3 substituents; R1 and R2 each represent a C1-6 alkyl group, a hydroxyl group, or the like; Z1 represents a methylene group or vinylene group, which may be substituted with 1 or 2 substituents selected from Substituent Group A1, an oxygen atom, or an imino group that may be substituted with a substituent selected from Substituent Group A1; and p, q, and r each represent an integer of 0 to 2, which has an effect of reducing Aβ40 and Aβ42 production, and thus is particularly useful as a prophylactic or therapeutic agent for a neurodegenerative disease caused by Aβ such as Alzheimer's disease or Down's syndrome.
Owner:EISIA R&D MANAGEMENT CO LTD
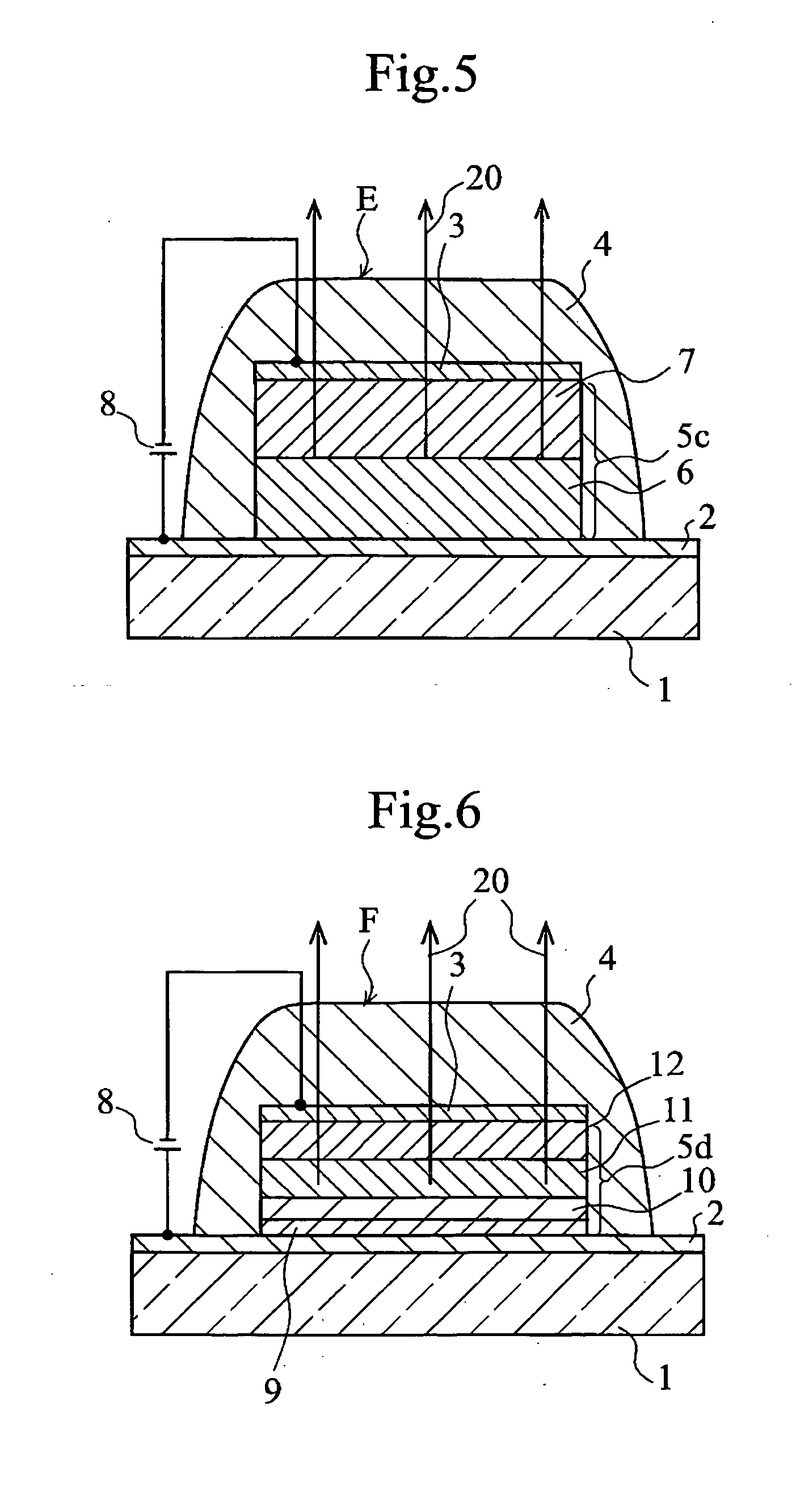
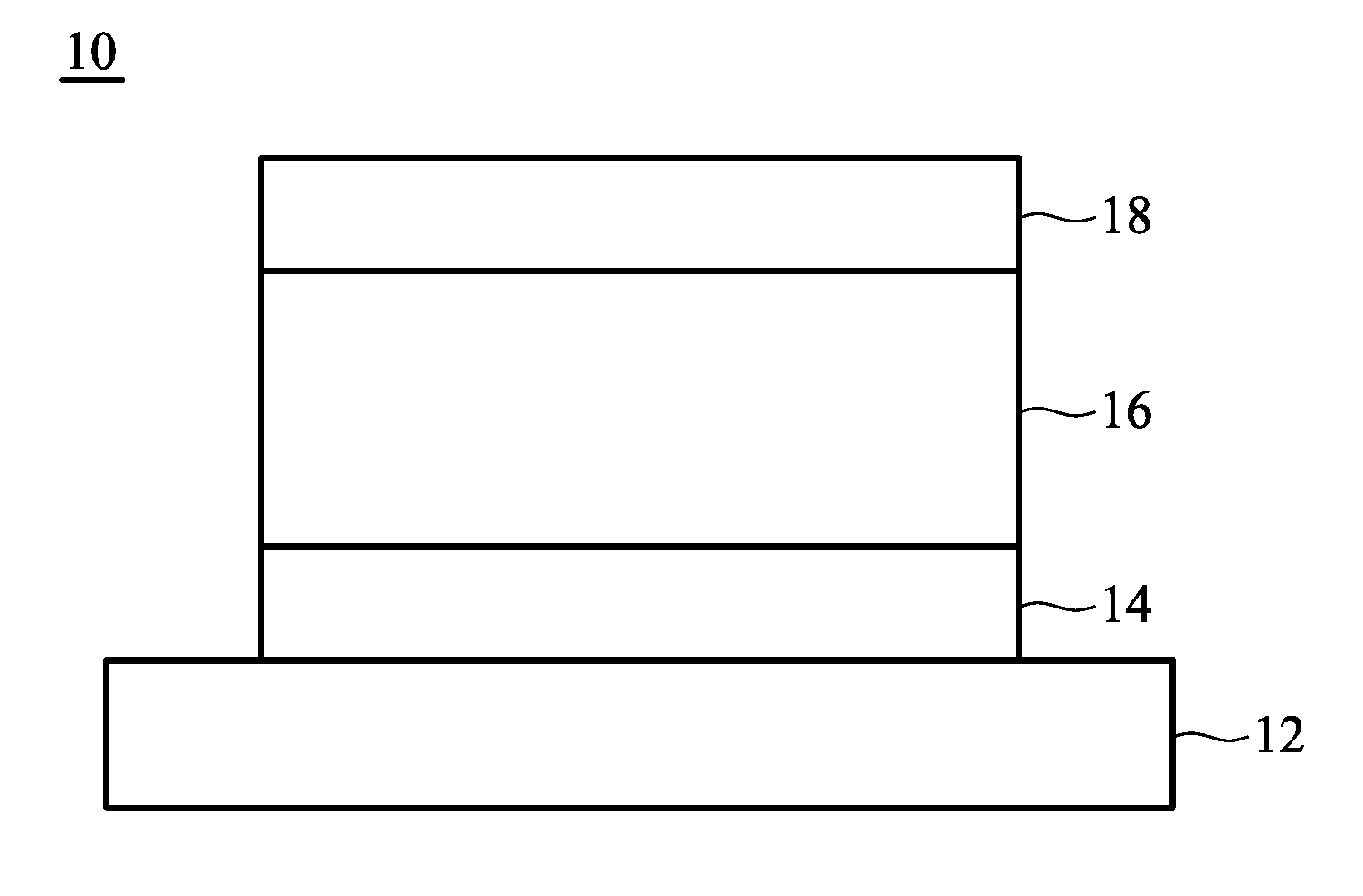
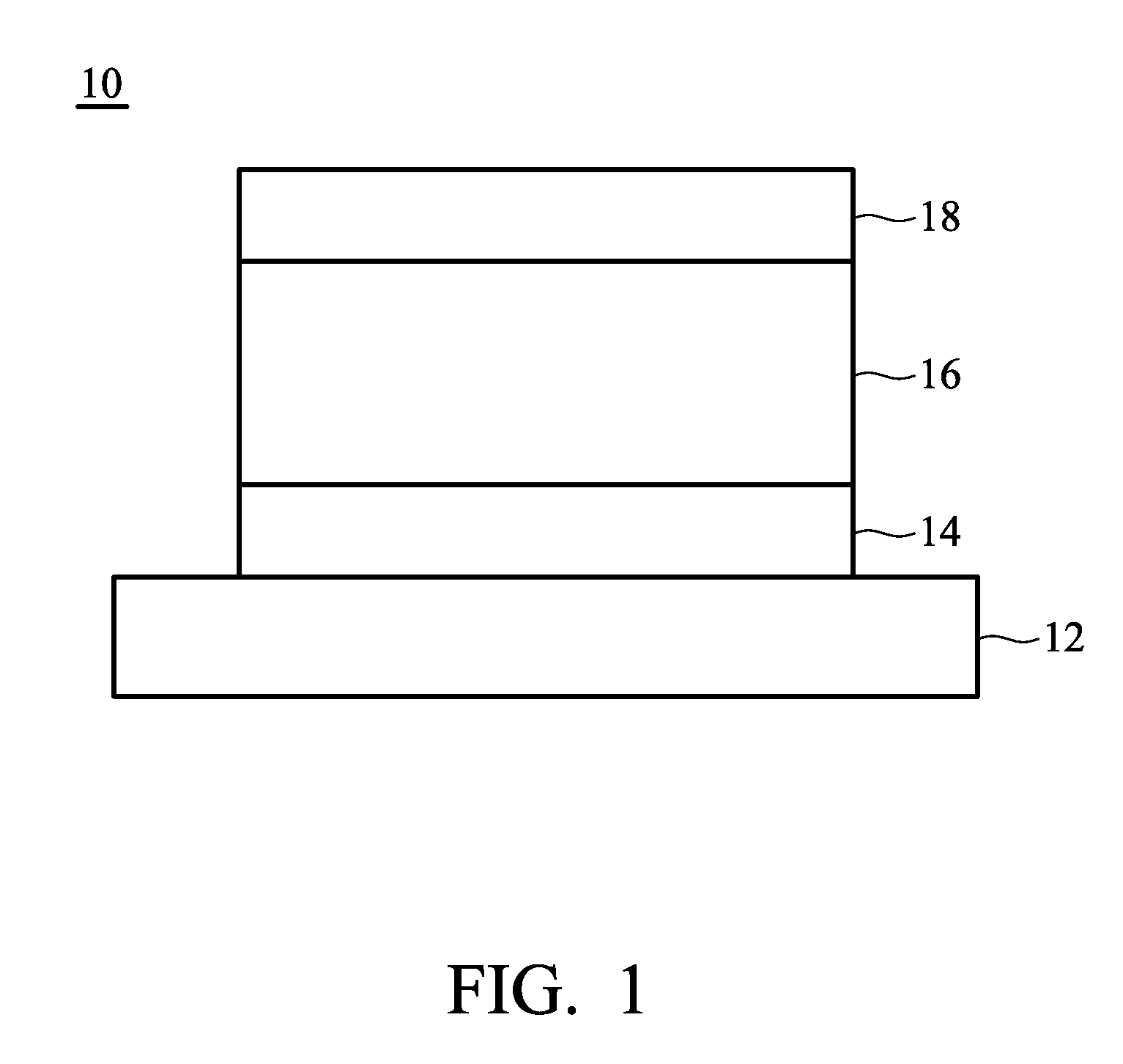
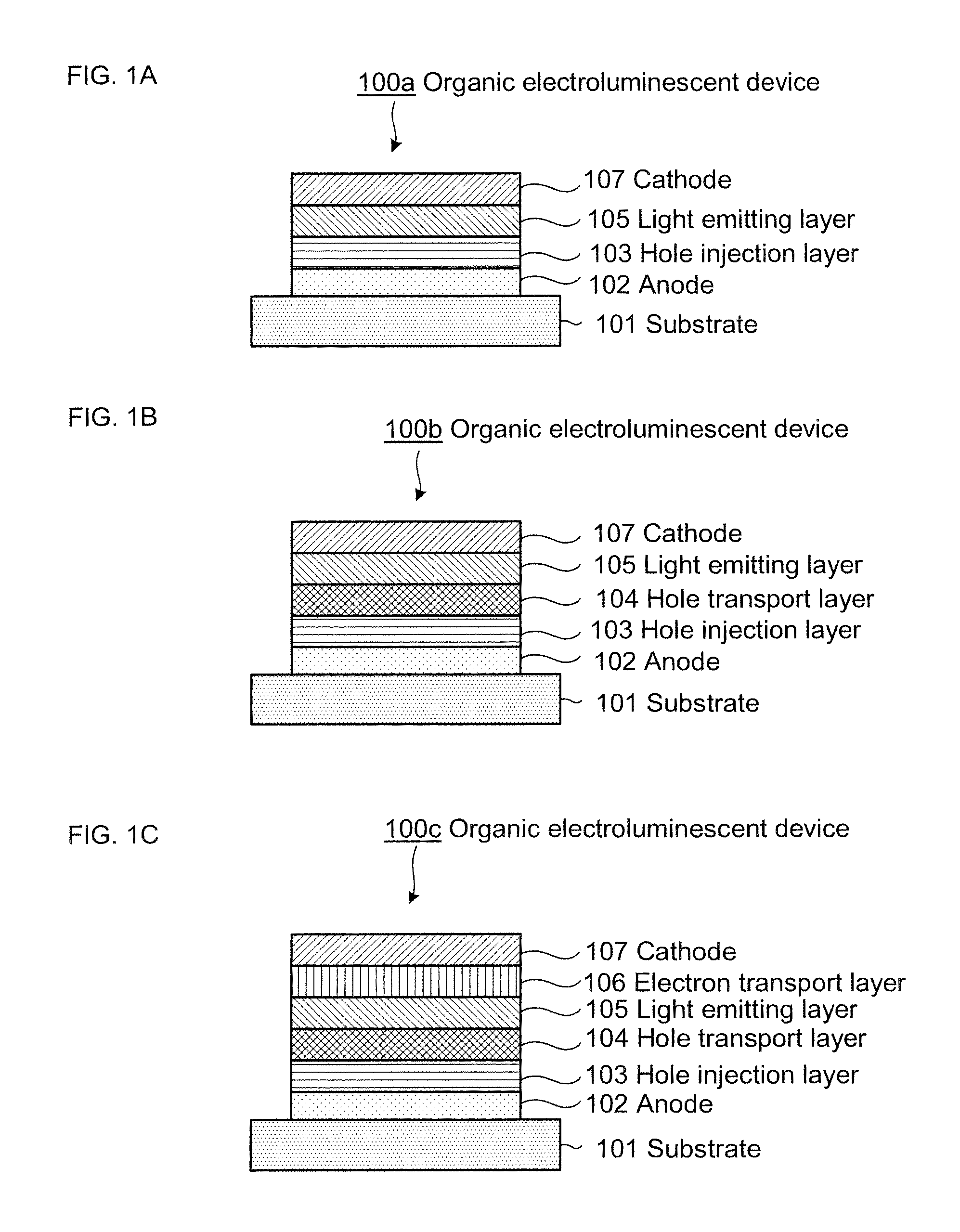
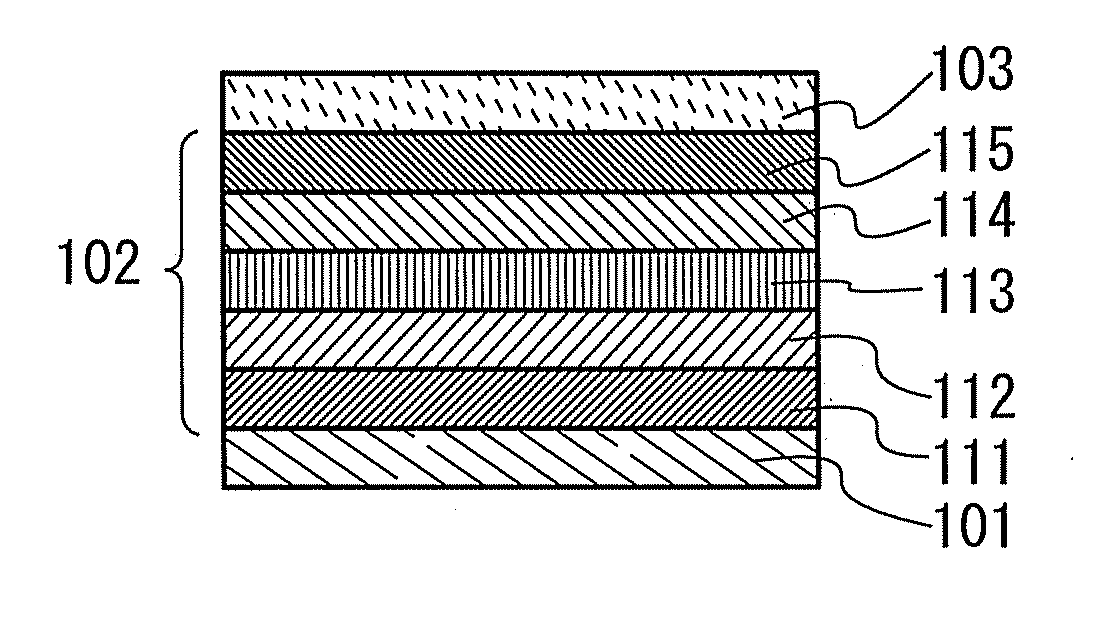
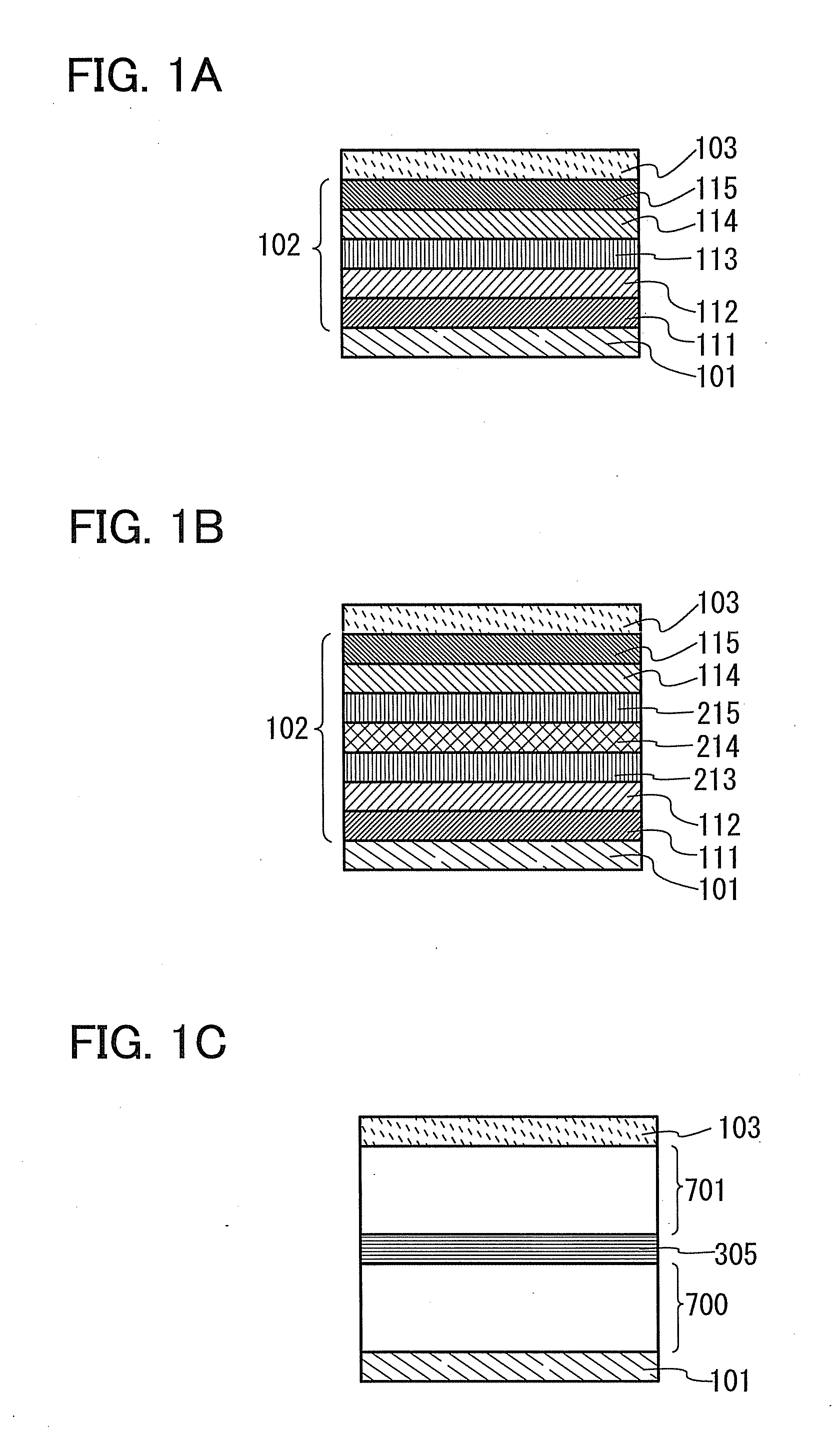
Organic electroluminescent element and lumiscent device or display including the same
InactiveUS20050158577A1Superior in luminanceImprove reliabilityDischarge tube luminescnet screensElectroluminescent light sourcesDisplay deviceHole transport layer
The present invention provides an organic electroluminescent element which is superior in luminance, reliability, and thermal stability and is capable of selectively emitting light with comparative long wavelengths such as red and good color purity and a light-emitting device or display device incorporated therewith. The organic electroluminescent element consists of a glass substrate (1), an anode (2), a hole transporting layer (10), an emitting layer (11), an electron transporting layer (12), and a cathode (3), which are sequentially laminated on top of the other. The emitting layer (11) is formed from a mixture composed of at least one species of the styryl compound represented by the general formula [I] below and a material with charge transporting capability. Y—CH═CH—X General formula [I](where X denotes an aryl group (such as phenyl group) which has a substituent group (such as cyano group and methyl group), and Y denotes a group having a skeleton of aminophenyl group or the like.)
Owner:JOLED INC
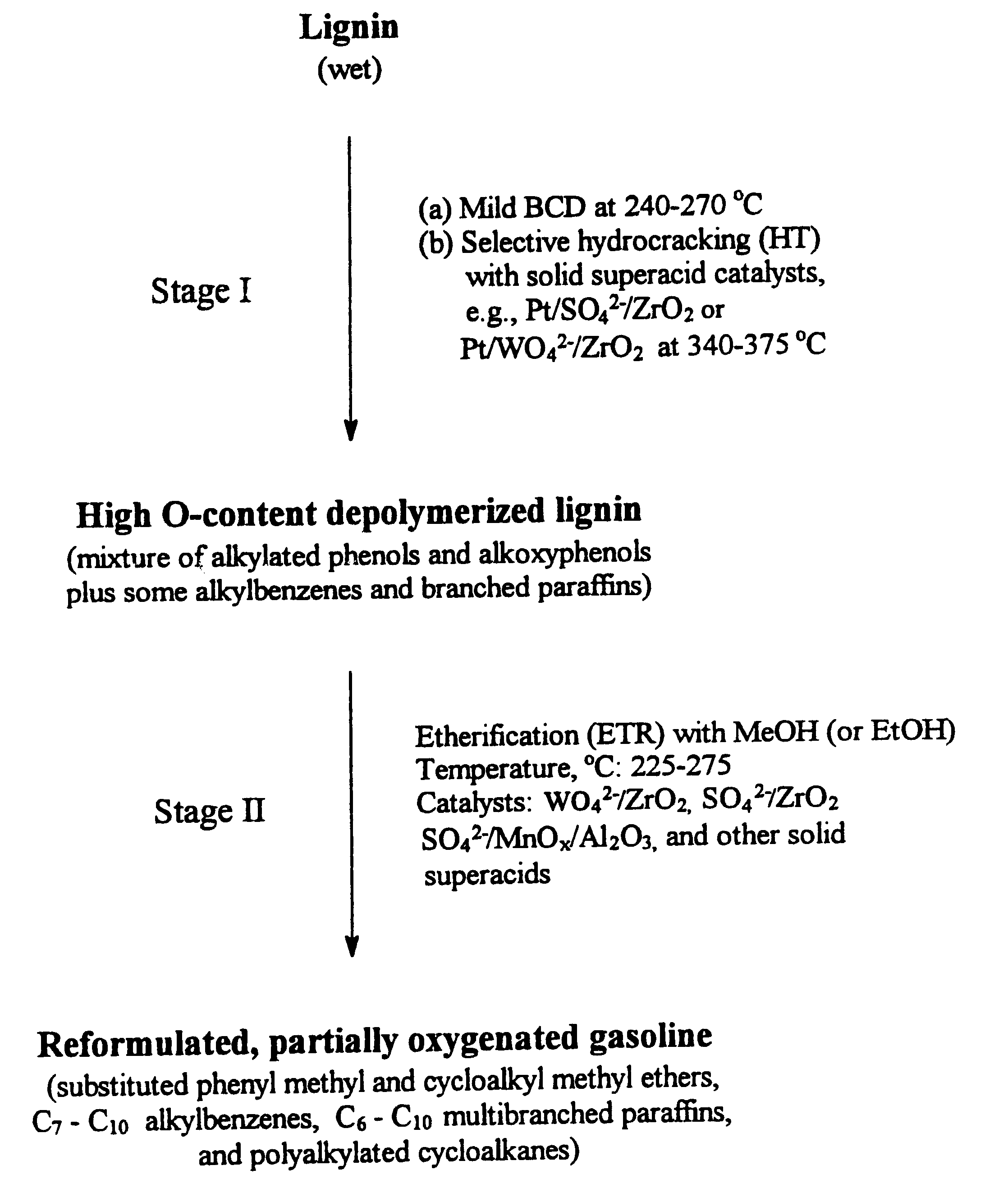
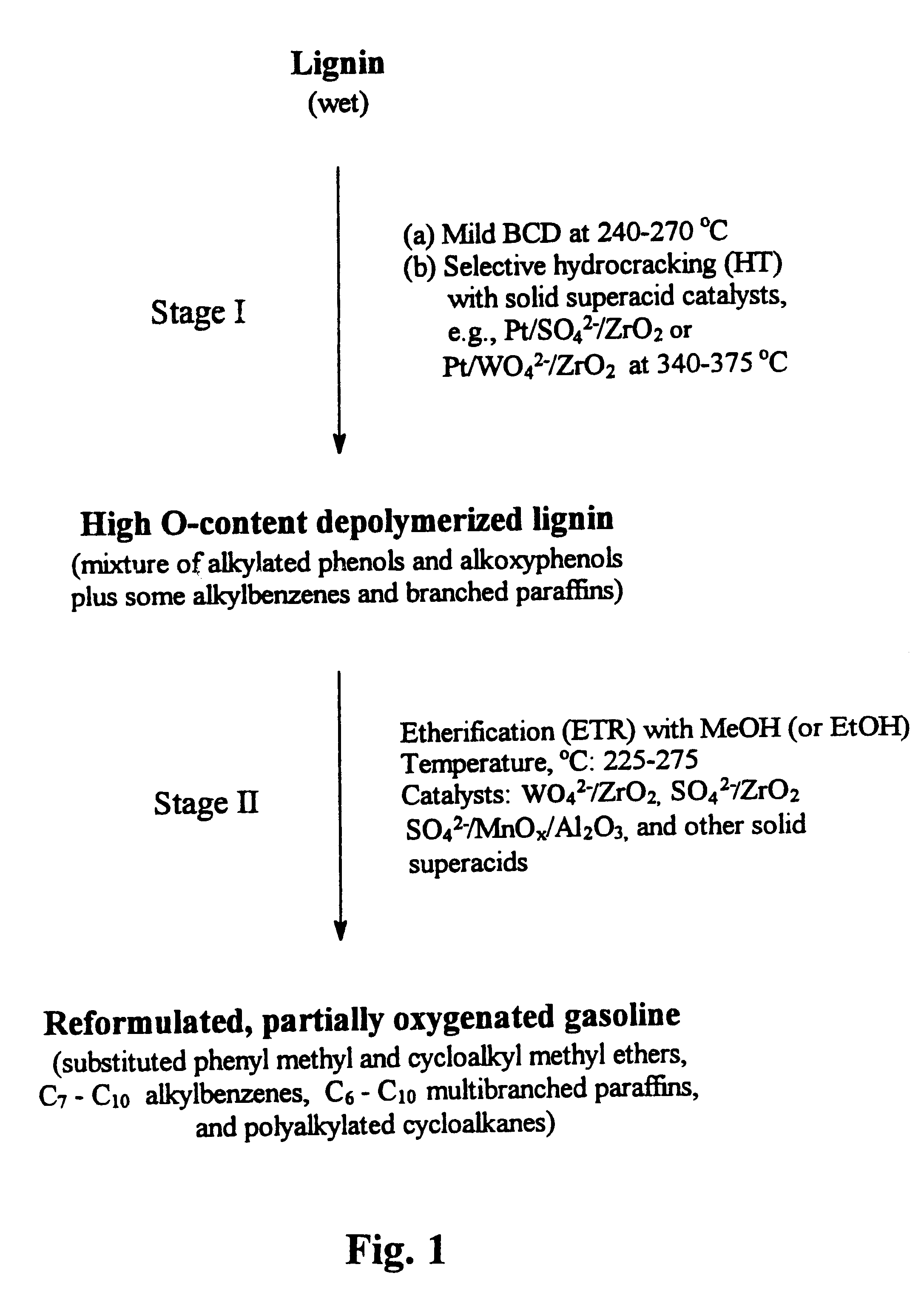
Process for conversion of lignin to reformulated, partially oxygenated gasoline
InactiveUS6172272B1Short reaction timeMaintain good propertiesOrganic compound preparationHydrocarbon from oxygen organic compoundsDepolymerizationMethyl group
A high-yield process for converting lignin into reformulated, partially oxygenated gasoline compositions of high quality is provided. The process is a two-stage catalytic reaction process that produces a reformulated, partially oxygenated gasoline product with a controlled amount of aromatics. In the first stage of the process, a lignin feed material is subjected to a base-catalyzed depolymerization reaction, followed by a selective hydrocracking reaction which utilizes a superacid catalyst to produce a high oxygen-content depolymerized lignin product mainly composed of alkylated phenols, alkylated alkoxyphenols, and alkylbenzenes. In the second stage of the process, the depolymerized lignin product is subjected to an exhaustive etherification reaction, optionally followed by a partial ring hydrogenation reaction, to produce a reformulated, partially oxygenated / etherified gasoline product, which includes a mixture of substituted phenyl / methyl ethers, cycloalkyl methyl ethers, C7-C10 alkylbenzenes, C6-C10 branched and multibranched paraffins, and alkylated and polyalkylated cycloalkanes.
Owner:ALLIANCE FOR SUSTAINABLE ENERGY +1
Carborane compound, organic light-emitting diode including the same and flat display device including organic light-emitting diode
ActiveUS20120319088A1Solve low luminous efficiencySolid-state devicesSemiconductor/solid-state device manufacturingPhysical chemistryDisplay device
A compound represented by Formula 1 below:(R1)a—CB—[Ar]n—CB—(R2)b <Formula 1>wherein CB denotes carborane, Ar is a substituted or unsubstituted phenylene group, and a detailed description of R1, R2, a, b, and n is provided in the detailed description. An organic light-emitting diode including an organic layer including the compound has high luminous efficiency.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


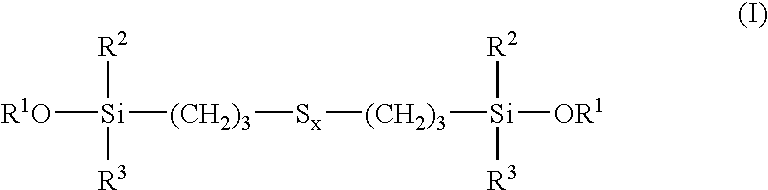
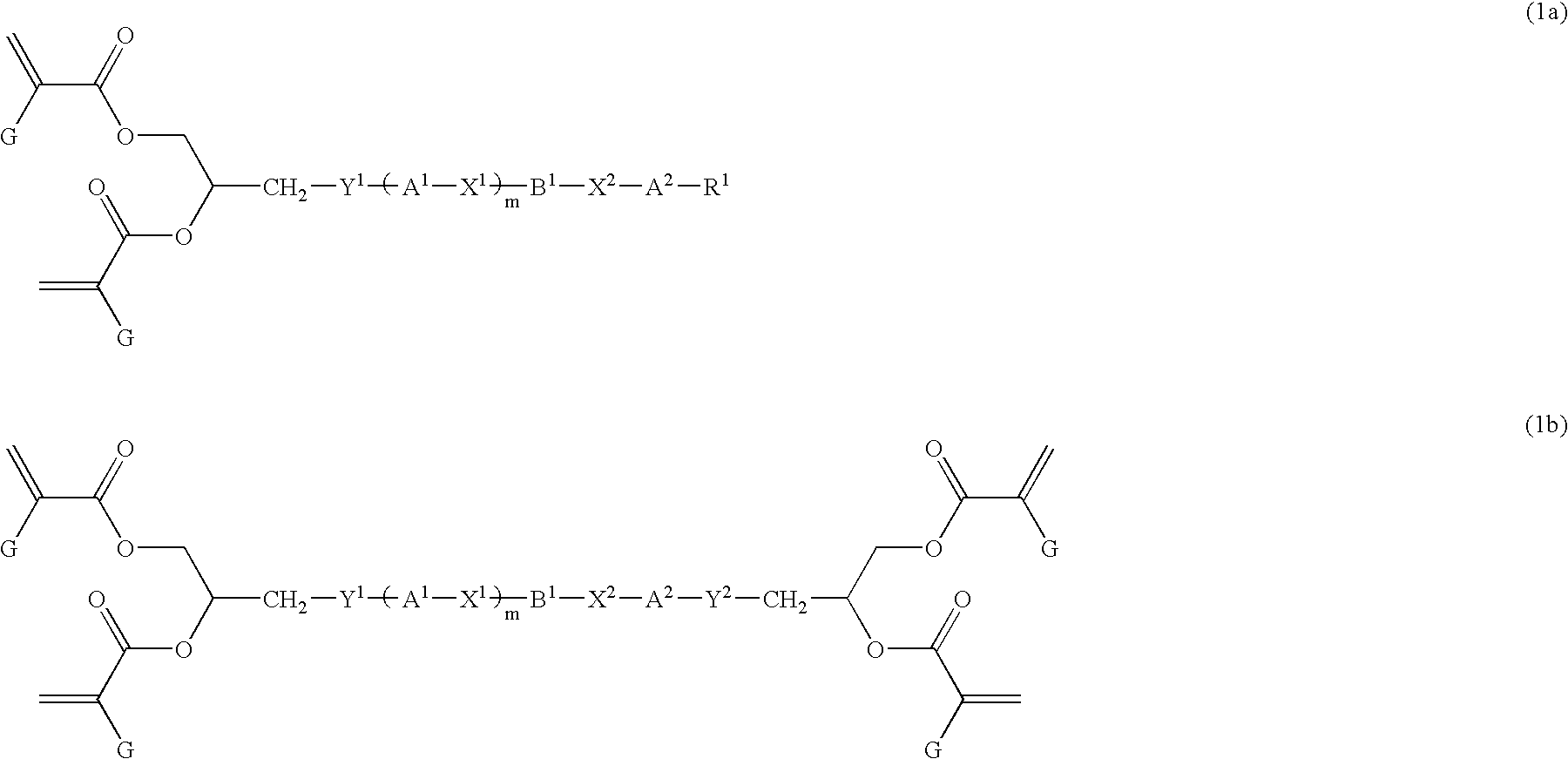

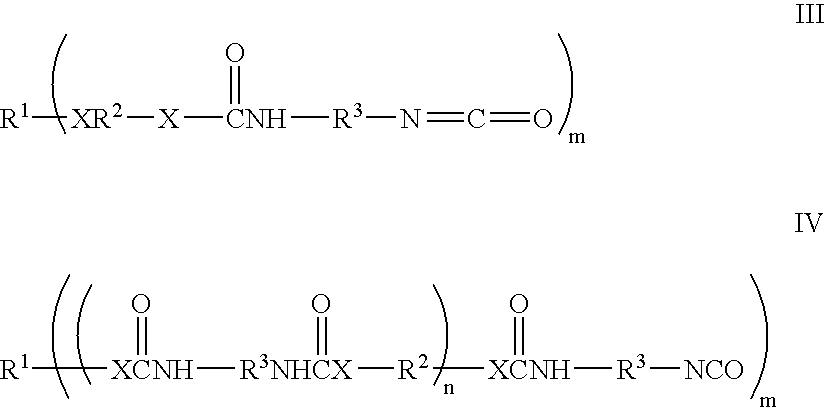



![Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7fc68549-2d37-4f83-89dd-f36e605466b3/US07553855-20090630-D00001.png)
![Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7fc68549-2d37-4f83-89dd-f36e605466b3/US07553855-20090630-D00002.png)
![Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7fc68549-2d37-4f83-89dd-f36e605466b3/US07553855-20090630-D00003.png)

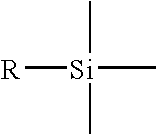
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00000.png)
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00001.png)
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00002.png)