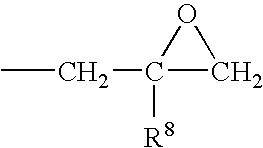
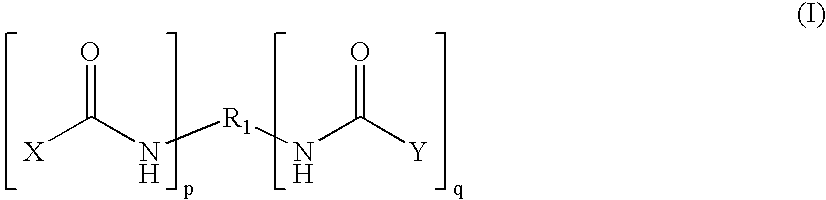
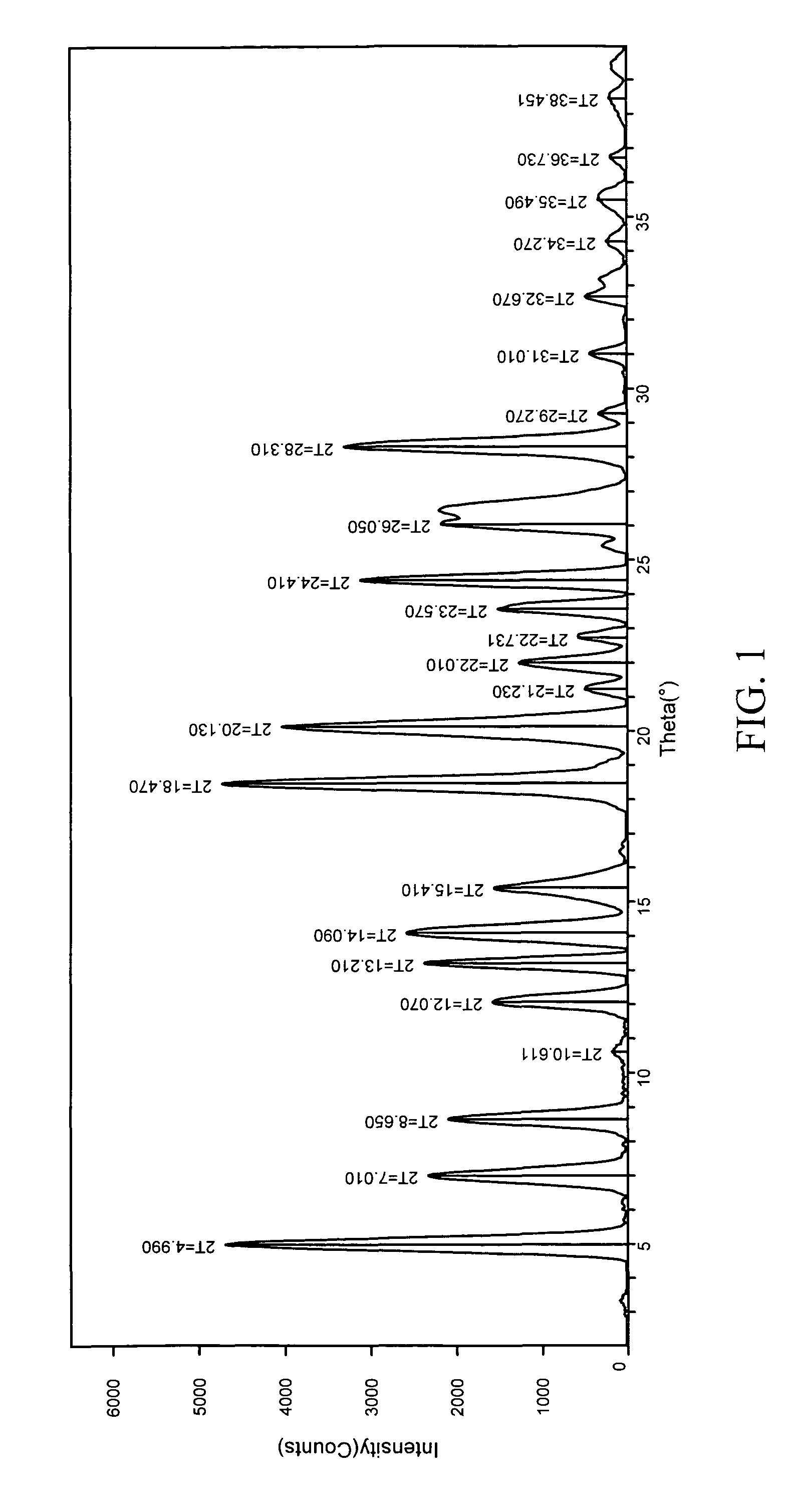
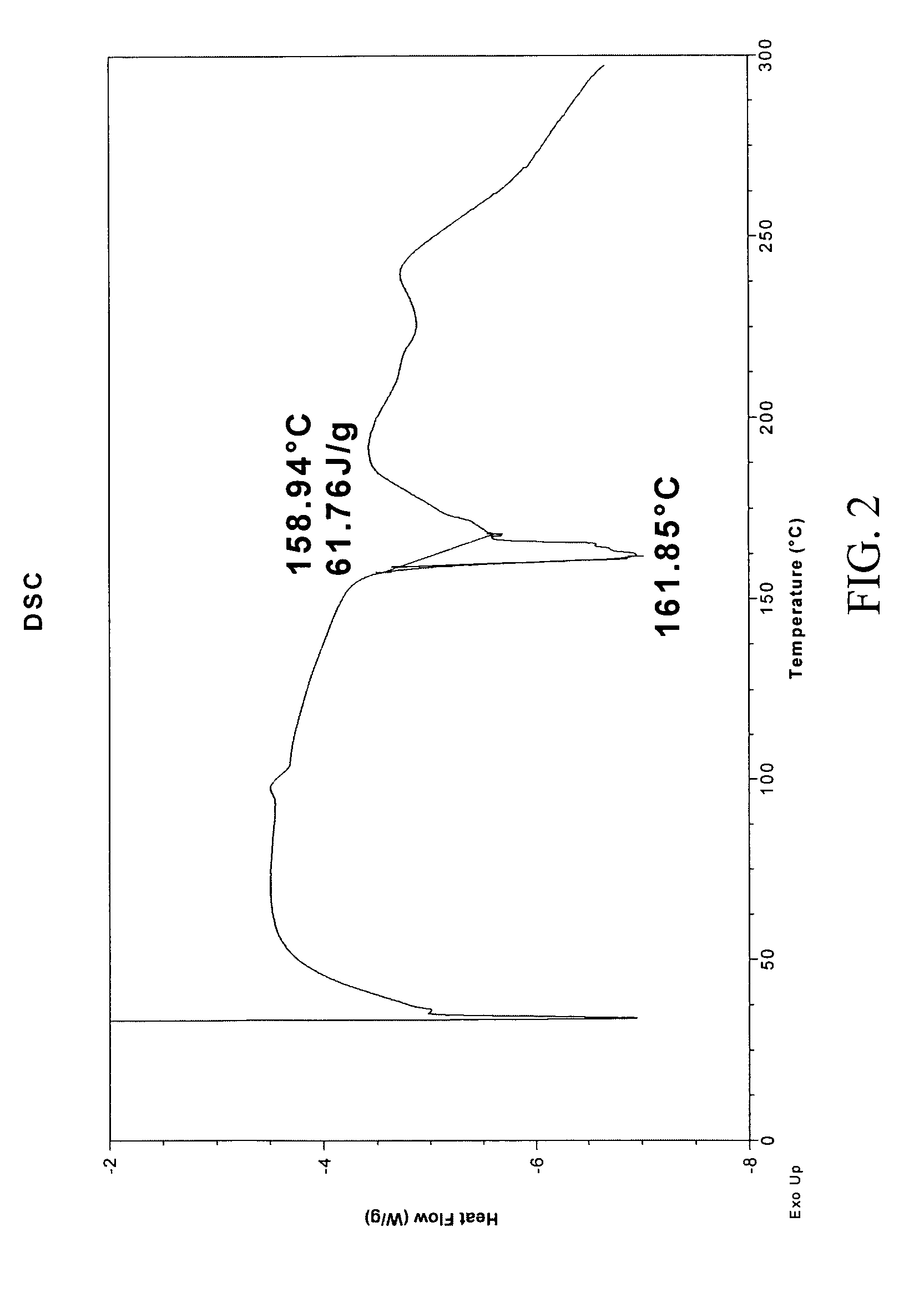
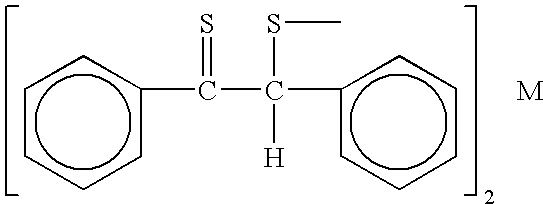
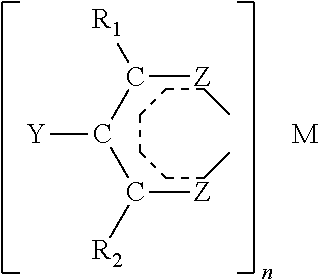
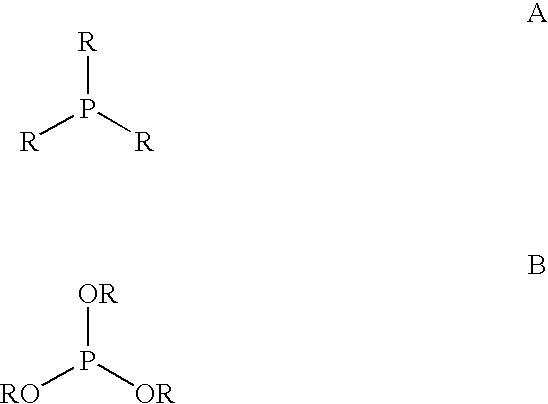
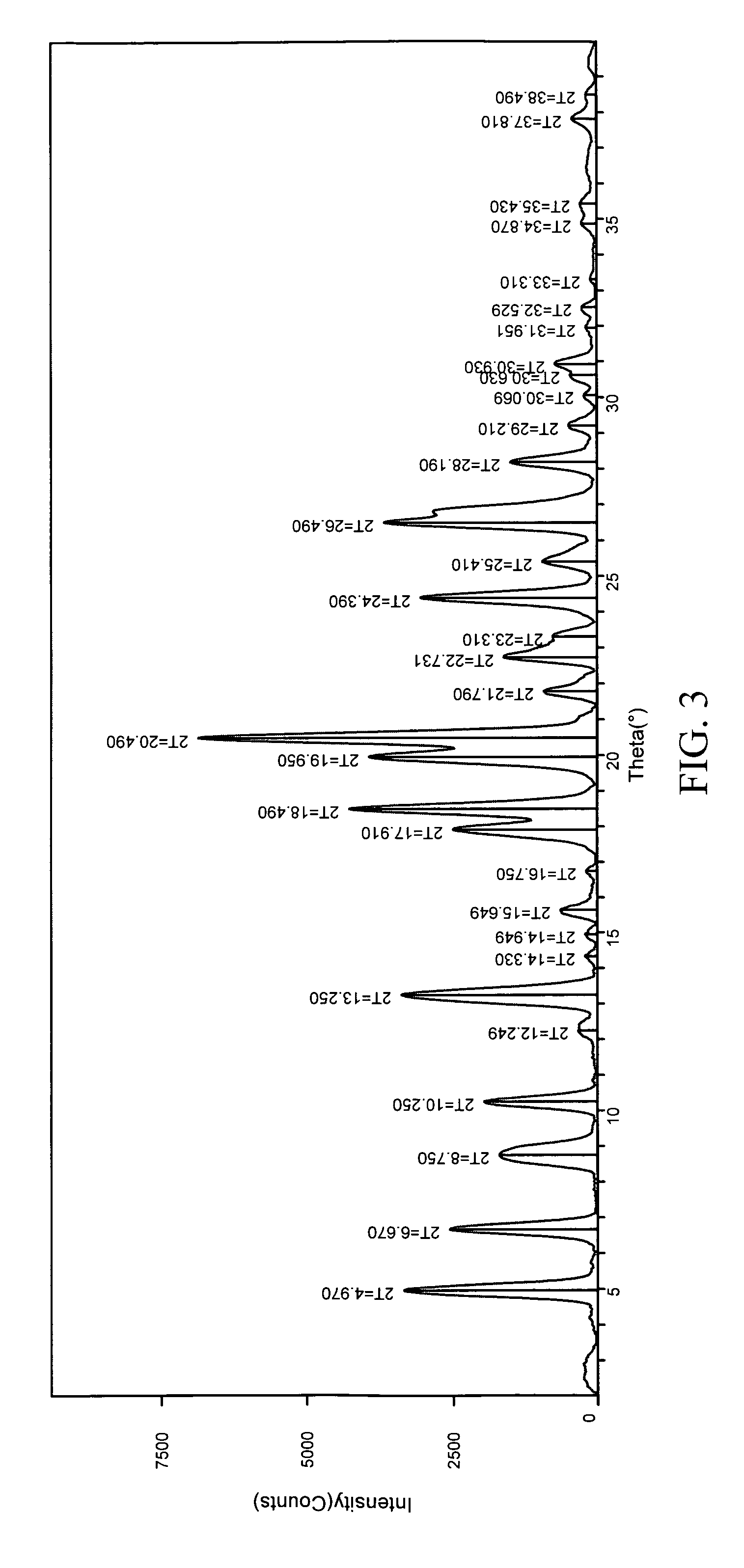
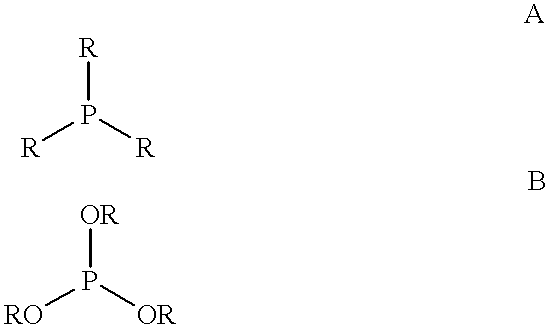
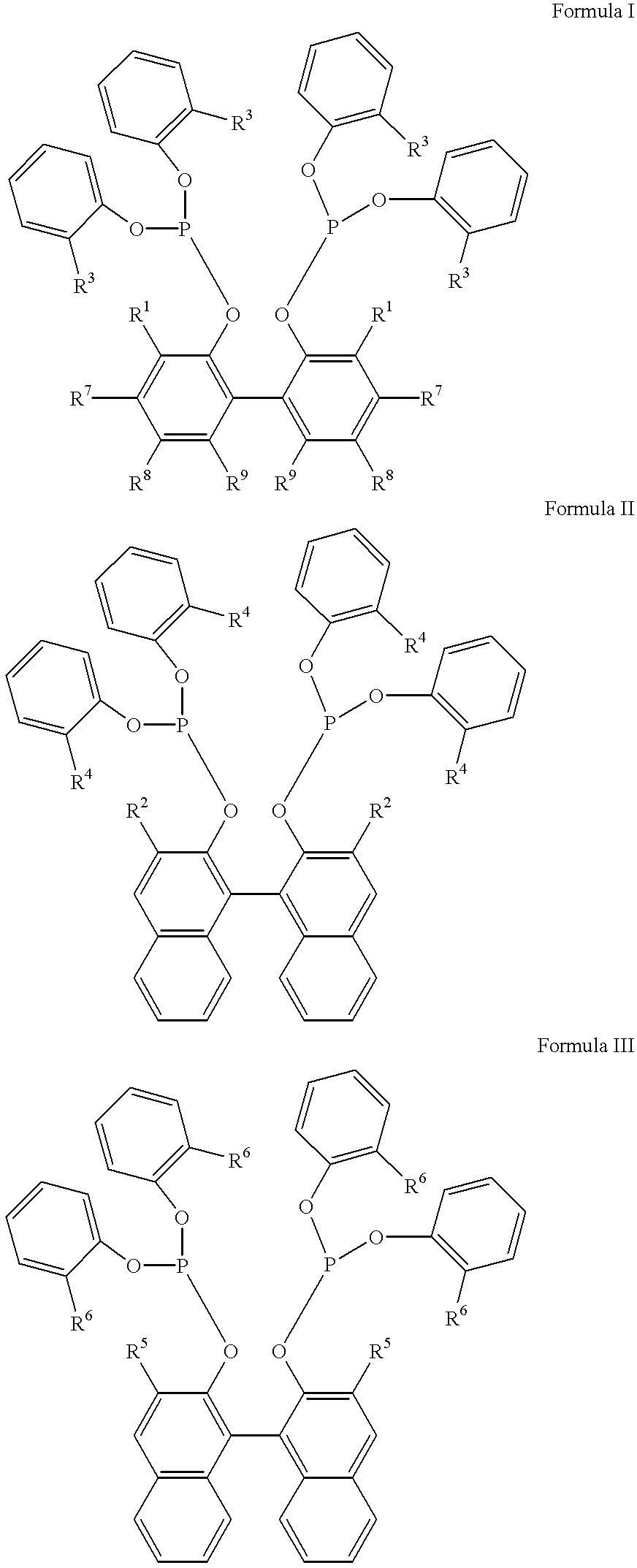
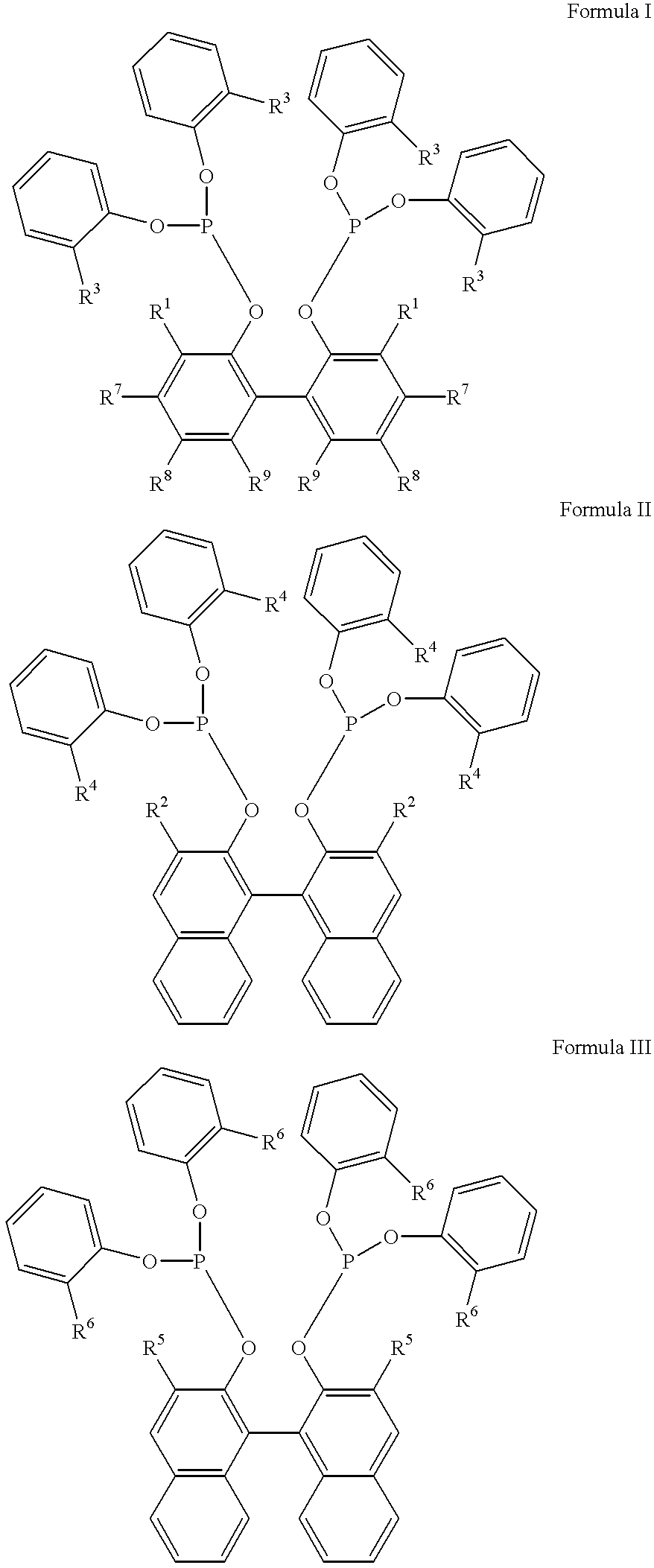
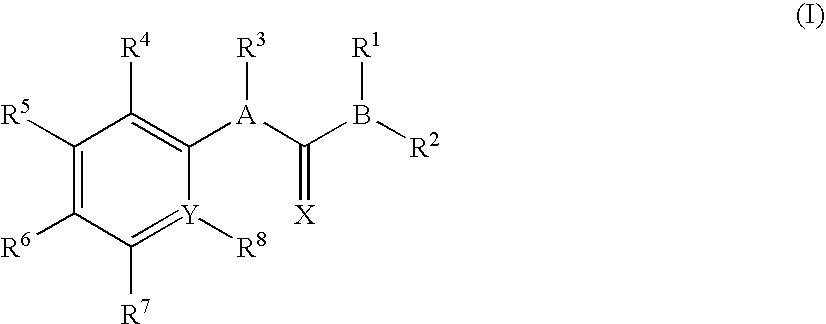
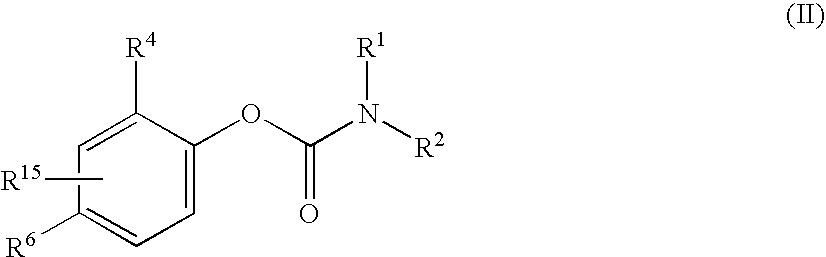
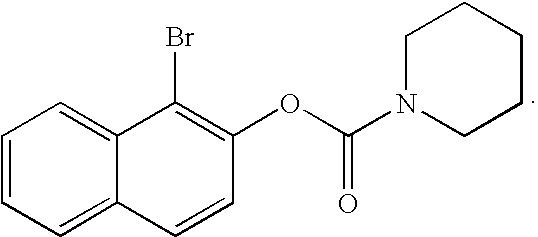
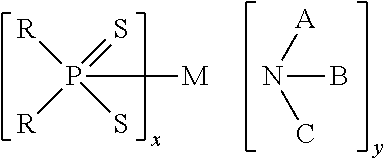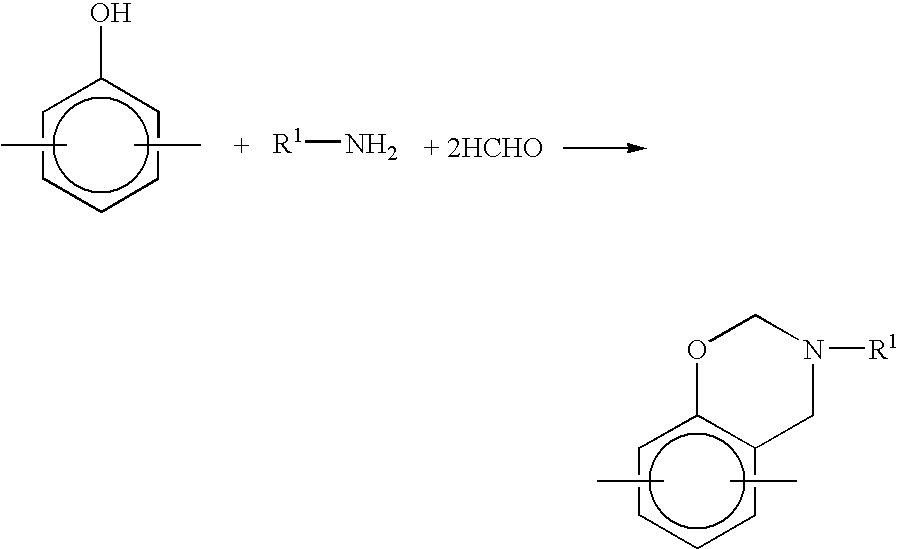Patents
Literature
26322 results about "Phenols" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, C₆H₅OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.
Precursor source mixtures
A precursor source mixture useful for CVD or ALD of a film comprising: at least one precursor composed of an element selected from the group consisting of Li, Na, K, Rb, Cs, Fr, Be, Mg, Ti, Zr, Hf, Sc, Y, La, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, P, Sb and Bi, to which is bound at least one ligand selected from the group consisting of hydride, alkyl, alkenyl, cycloalkenyl, aryl, alkyne, carbonyl, amido, imido, hydrazido, phosphido, nitrosyl, nitryl, nitrate, nitrile, halide, azide, alkoxy, siloxy, silyl, and halogenated, sulfonated or silyated derivatives thereof, which is dissolved, emulsified or suspended in an inert liquid selected from the group consisting of aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ethers, aldehydes, ketones, acids, phenols, esters, amines, alkylnitrile, halogenated hydrocarbons, silyated hydrocarbons, thioethers, amines, cyanates, isocyanates, thiocyanates, silicone oils, nitroalkyl, alkylnitrate, and mixtures thereof. The precursor source mixture may be a solution, emulsion or suspension and may consist of a mixture of solid, liquid and gas phases which are distributed throughout the mixture.
Owner:GLOBALFOUNDRIES INC
Toughened epoxy adhesive composition
ActiveUS20060276601A1Improved lap shear and impact peel strengthGood storage stabilityPolyureas/polyurethane adhesivesSynthetic resin layered productsElastomerEnd-group
The invention is an epoxy resin based adhesive composition comprising an epoxy resin and a compound comprising an elastomeric prepolymer residue selected from the group of a polyurethane, a polyurea and a polyurea polyurethane having isocyanate end groups, the isocyanate end groups of said prepolymer residue being capped by a capping compound selected from the group consisting of a primary aliphatic, cycloaliphatic, heteroaromatic and araliphatic amine, a secondary aliphatic, cycloaliphatic, aromatic, heteroaromatic and araliphatic amine, a thiol and an alkyl amide, said capping compound being bound to the end of the polymer chain of the elastomeric prepolymer in a manner such that the end to which it is bonded no longer has a reactive group. In addition to the capping compound defined above above, a capping compound selected from the group consisting of a phenol and a polyphenol can be used for capping the isocyanate end groups of the prepolymer residue
Owner:DOW GLOBAL TECH LLC
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Phenolic antiseptic compositions and methods of use
ActiveUS20060052452A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideDiphenyl etherSURFACTANT BLEND
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including an antiseptic selected from the group consisting of halogenated phenols, bisphenols, diphenyl ethers, anilides and derivatives thereof, and combinations thereof. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Inherently radiopaque bioresorbable polymers for multiple uses
Preferred embodiments of the present invention relate to polymeric medical devices, such as stents. More particularly, the compositions disclosed herein comprise halogen-containing phenol moeities, that may be used for medical devices and other uses whereby bioresorbable and radiopaque and physicomechanical properties are desired.
Owner:REVA MEDICAL LLC
Polycarbonate preparation process
Disclosed is a polycarbonate composition and process from making same, wherein introduction of a chain terminator and an acyl halide other than phosgene is after at least 25 percent of the hydroxyl groups in the dihydric phenol have been converted to chloroformate groups.
Owner:TRINSEO EURO GMBH
Catalytic antioxidants
The present invention is directed to lubricating oils exhibiting improved resistance to oxidation and deposit / sludge formation comprising a lubricant base oil and catalytic antioxidants comprising an effective amount of a) one or more polymetal organometallic compound; and, b) effective amounts of one or more substituted N,N′-diaryl-o-phenylenediamine compounds or c) one or more hindered phenol compounds or both, to a method for improving the antioxidancy and the resistance to deposit / sludge formation of formulated lubricating oil compositions by the addition thereto of an effective amount of the aforementioned catalytic antioxidants, and to an additive concentrate containing the aforementioned catalytic antioxidants.
Owner:EXXON RES & ENG CO
Storage stable isocyanate-reactive component containing vegetable oil-based polyol
The present invention provides an isocyanate-reactive component containing at least 10 wt. %, based on the weight of the isocyanate-reactive component, of a vegetable oil-based polyol, a nonionic emulsifier containing one of an aliphatic alcohol ethoxylate and an aliphatic phenol ethoxylate having a polymerized ethylene oxide content of at least 25 moles per equivalent of alcohol or phenol and a HLB value greater than 17, one or more non-vegetable oil-based polyols, one or more silicone surfactants, and optionally, water or other blowing agents, catalysts, pigments and fillers, wherein the isocyanate-reactive component is storage stable at temperatures of from −10° C. to 60° C. for at least three days. The inventive isocyanate-reactive component can be shipped and stored at normal shipping and storage temperatures and still produce acceptable foam on a daily basis whilst helping to satisfy polyurethane foam and elastomer producers' “green” requirements.
Owner:BAYER MATERIALSCIENCE AG
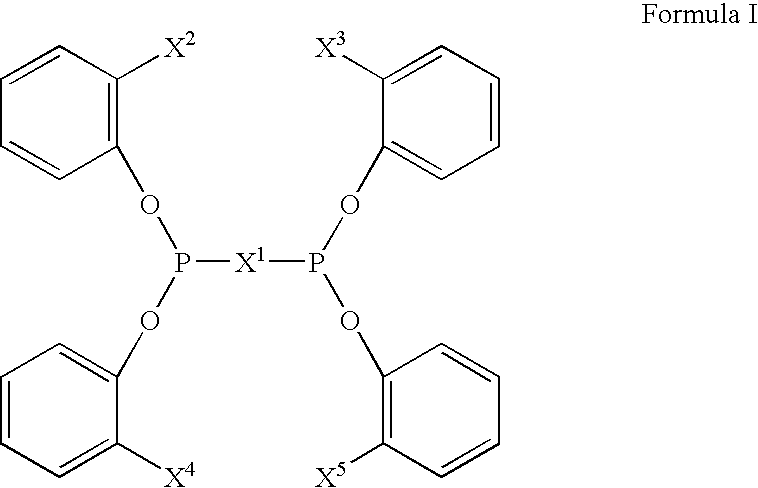
Multidentate phosphite ligands, catalytic compositions containing such ligands, and catalytic processes utilizing such catalytic compositions
InactiveUS6812352B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationOrtho position
Owner:INVISTA NORTH AMERICA S A R L
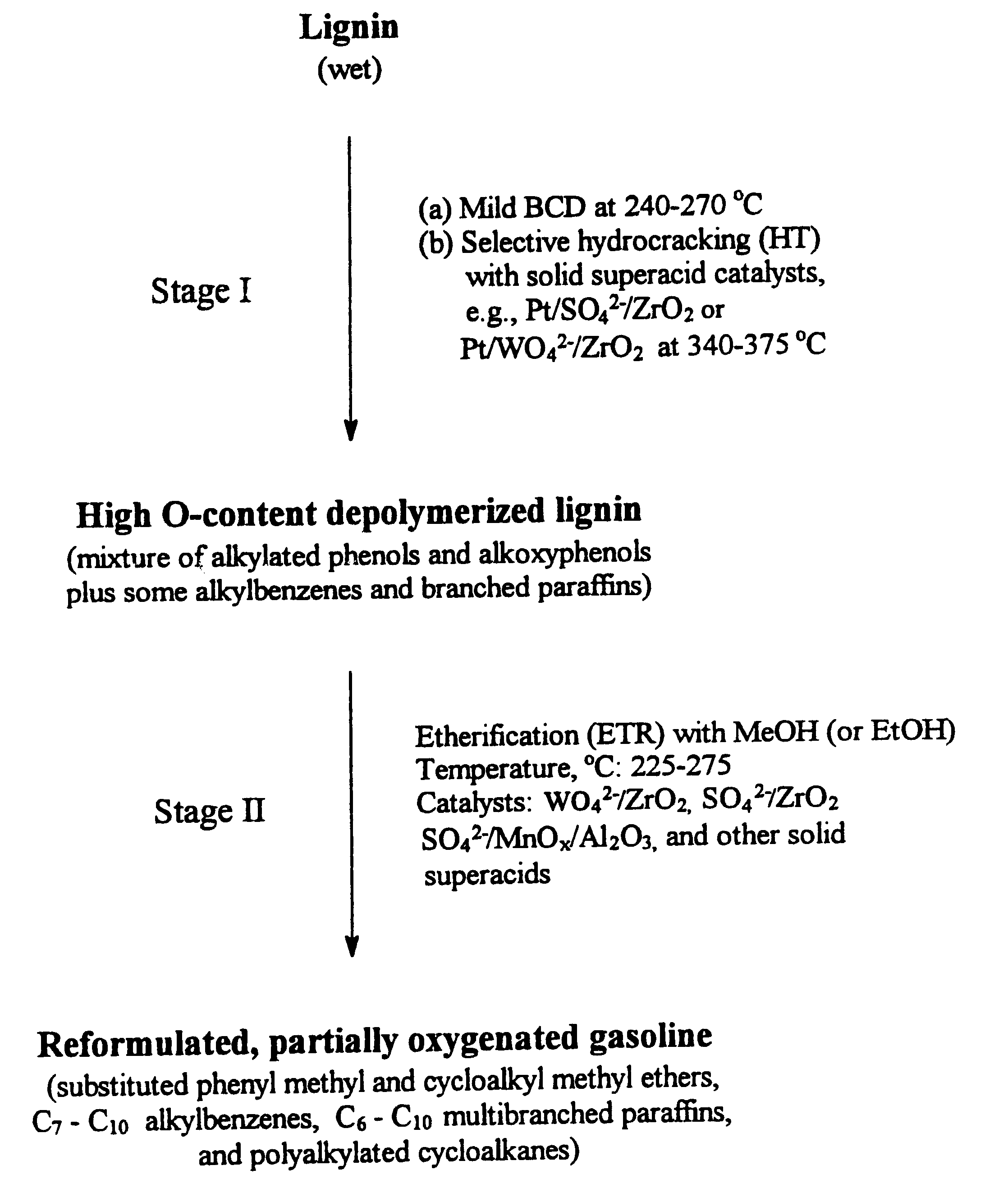
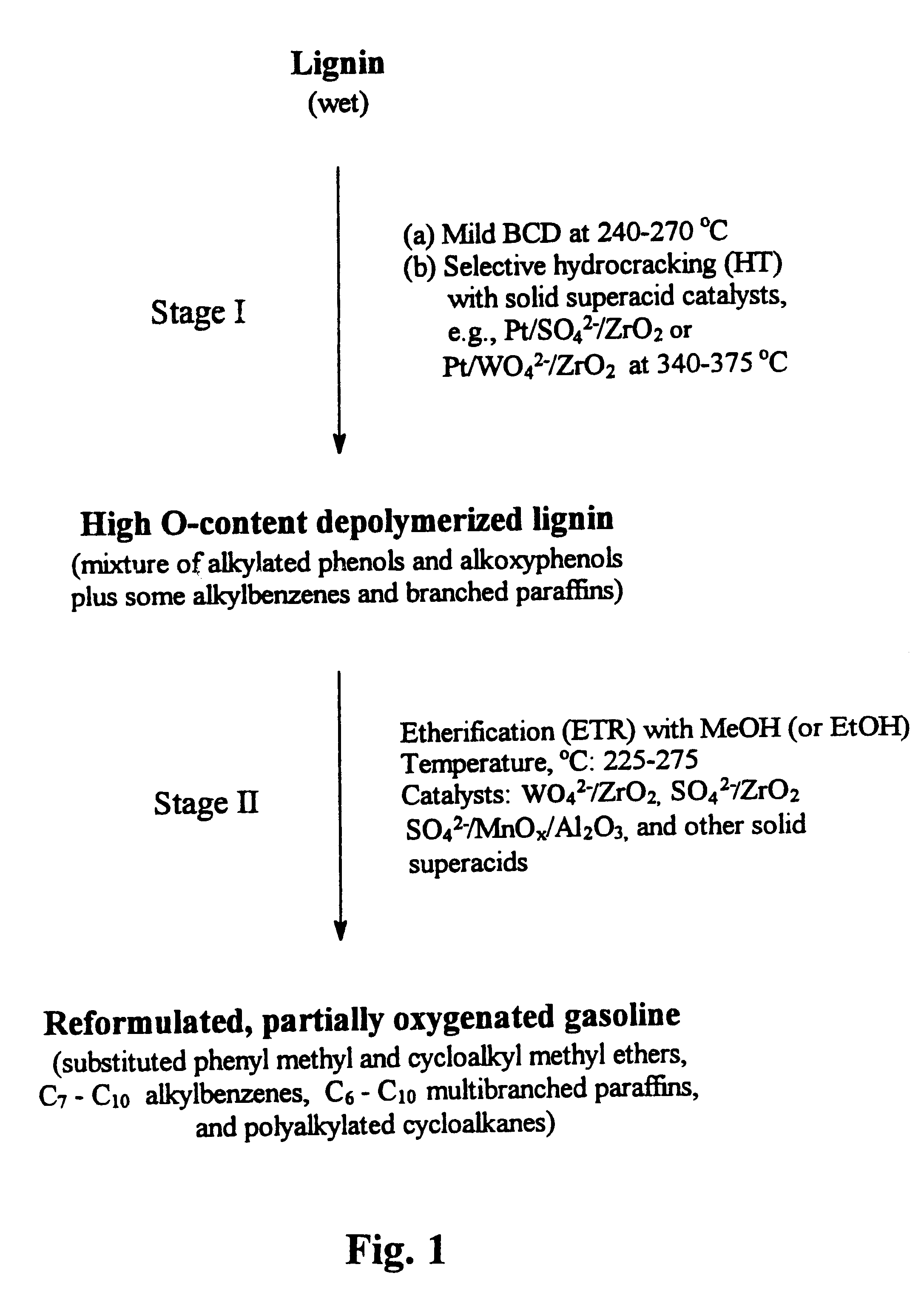
Process for conversion of lignin to reformulated, partially oxygenated gasoline
InactiveUS6172272B1Short reaction timeMaintain good propertiesOrganic compound preparationHydrocarbon from oxygen organic compoundsDepolymerizationMethyl group
A high-yield process for converting lignin into reformulated, partially oxygenated gasoline compositions of high quality is provided. The process is a two-stage catalytic reaction process that produces a reformulated, partially oxygenated gasoline product with a controlled amount of aromatics. In the first stage of the process, a lignin feed material is subjected to a base-catalyzed depolymerization reaction, followed by a selective hydrocracking reaction which utilizes a superacid catalyst to produce a high oxygen-content depolymerized lignin product mainly composed of alkylated phenols, alkylated alkoxyphenols, and alkylbenzenes. In the second stage of the process, the depolymerized lignin product is subjected to an exhaustive etherification reaction, optionally followed by a partial ring hydrogenation reaction, to produce a reformulated, partially oxygenated / etherified gasoline product, which includes a mixture of substituted phenyl / methyl ethers, cycloalkyl methyl ethers, C7-C10 alkylbenzenes, C6-C10 branched and multibranched paraffins, and alkylated and polyalkylated cycloalkanes.
Owner:ALLIANCE FOR SUSTAINABLE ENERGY +1
Liquid dispersion comprising dibenzylidene sorbital acetals and ethoxylated nonionic surfactants
InactiveUS6102999ALow viscosityInexpensive fluid dispersionOrganic chemistryTransportation and packagingPeristaltic pumpPolyolefin
This invention relates to a fluid dispersion of at least one dibenzylidene sorbitol acetal derivative. The sorbitol acetal derivative is useful as a clarifying agent for polyolefins and the inventive fluid dispersion permits improvements in the handling and processing of and mixing within the polyolefin composition. The inventive dispersion must be shelf stable, retain its nucleating effects, be compatible with polypropylene (and other polyolefins), and possess both short-term and long-term viscosities which permit acceptable transport through a standard polyolefin-manufacturing peristaltic pump. The preferred inventive dispersion thus comprises 3,4-DMDBS and at least one ethoxylated nonionic surfactant having an HLB of greater than about 8.5. Preferred surfactants include those selected from the group consisting essentially of ethoxylated sorbitan (C8-C22) monoesters and ethoxylated nonyl-phenol ethers. The inventive dispersion may be introduced within any polyolefin composition, preferably polypropylene, which may then be molded into any shape or form. A method of producing a polyolefin plastic utilizing the inventive dispersion is also provided.
Owner:MILLIKEN & CO
Delayed release pharmaceutical composition containing 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol
InactiveUS20050058706A1Good repeatabilityGood treatment effectOrganic active ingredientsBiocideHydrophobic polymerBULK ACTIVE INGREDIENT
A pharmaceutical formulation for delayed release of the active ingredient 3-(3-dimethylamino-1-ethyl-2-methylpropyl)phenol or a pharmaceutically acceptable salt thereof in a matrix containing between 1 and 80 wt. % of at least one pharmaceutically acceptable hydrophilic or hydrophobic polymer as a matrix forming agent and exhibiting in vivo the following release rate: 3 to 35% by weight (based on 100% by weight active ingredient) 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 0.5 hours; 5 to 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 1 hour; 10 to 75% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 2 hours; 15 to 82% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 3 hours; 30 to 97% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 6 hours; more than 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 12 hours; more than 70% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 18 hours, and more than 80% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 24 hours.
Owner:GRUNENTHAL GMBH
Pharmaceutical co-crystal compositions
InactiveUS20070026078A1Improve solubilityLow hygroscopicityBiocidePowder deliveryThioketoneHydroxamic acid
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
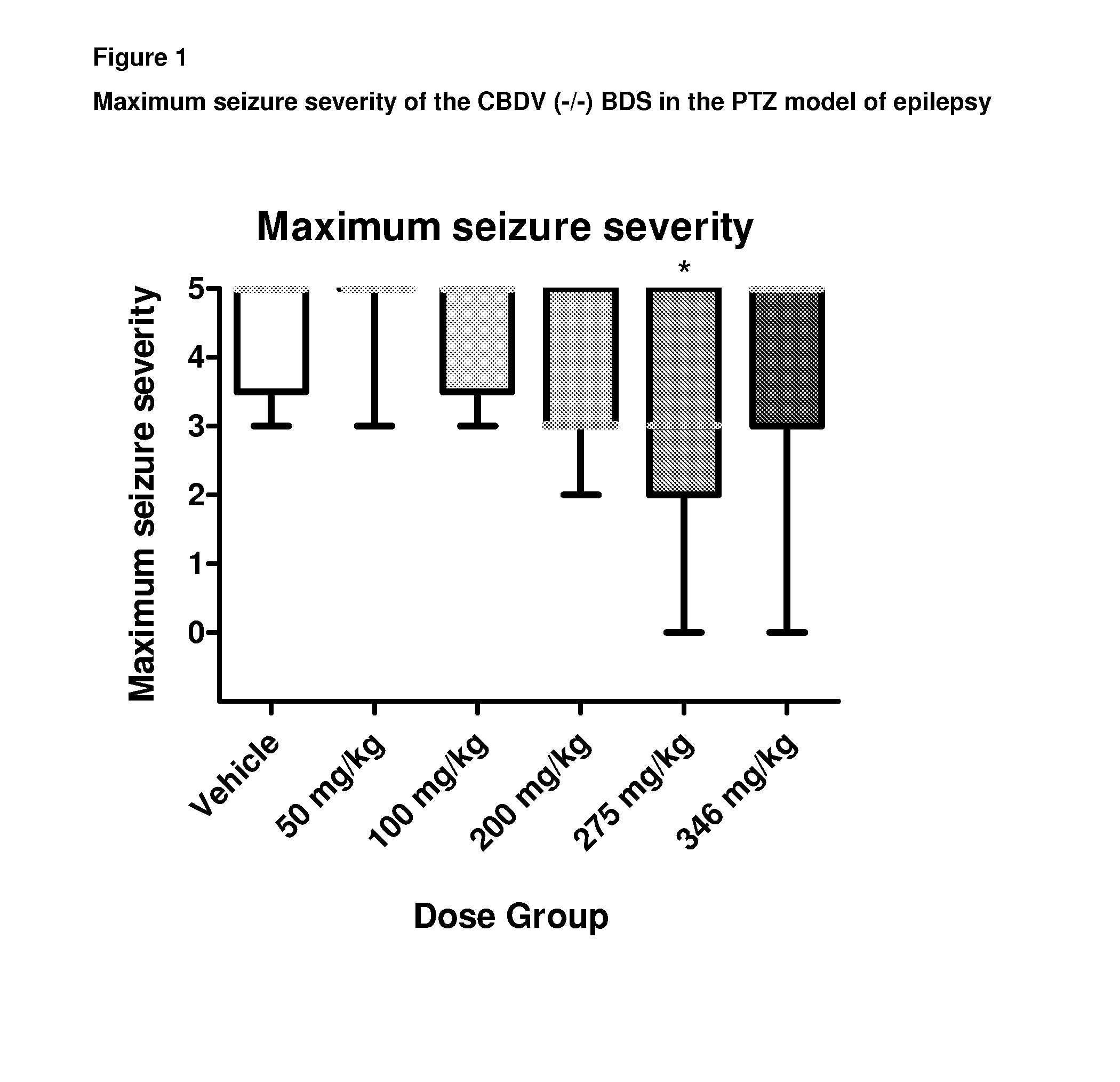
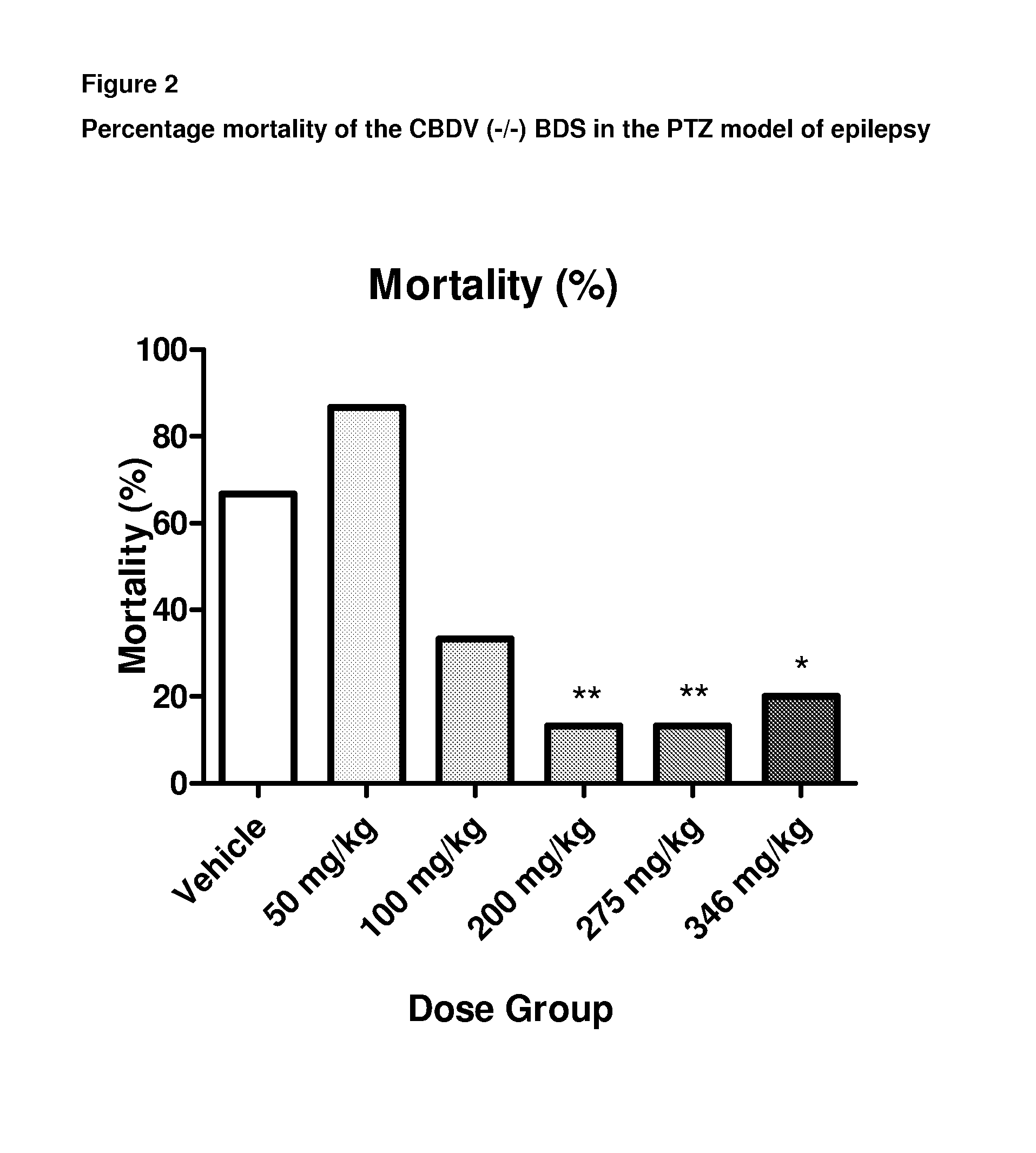
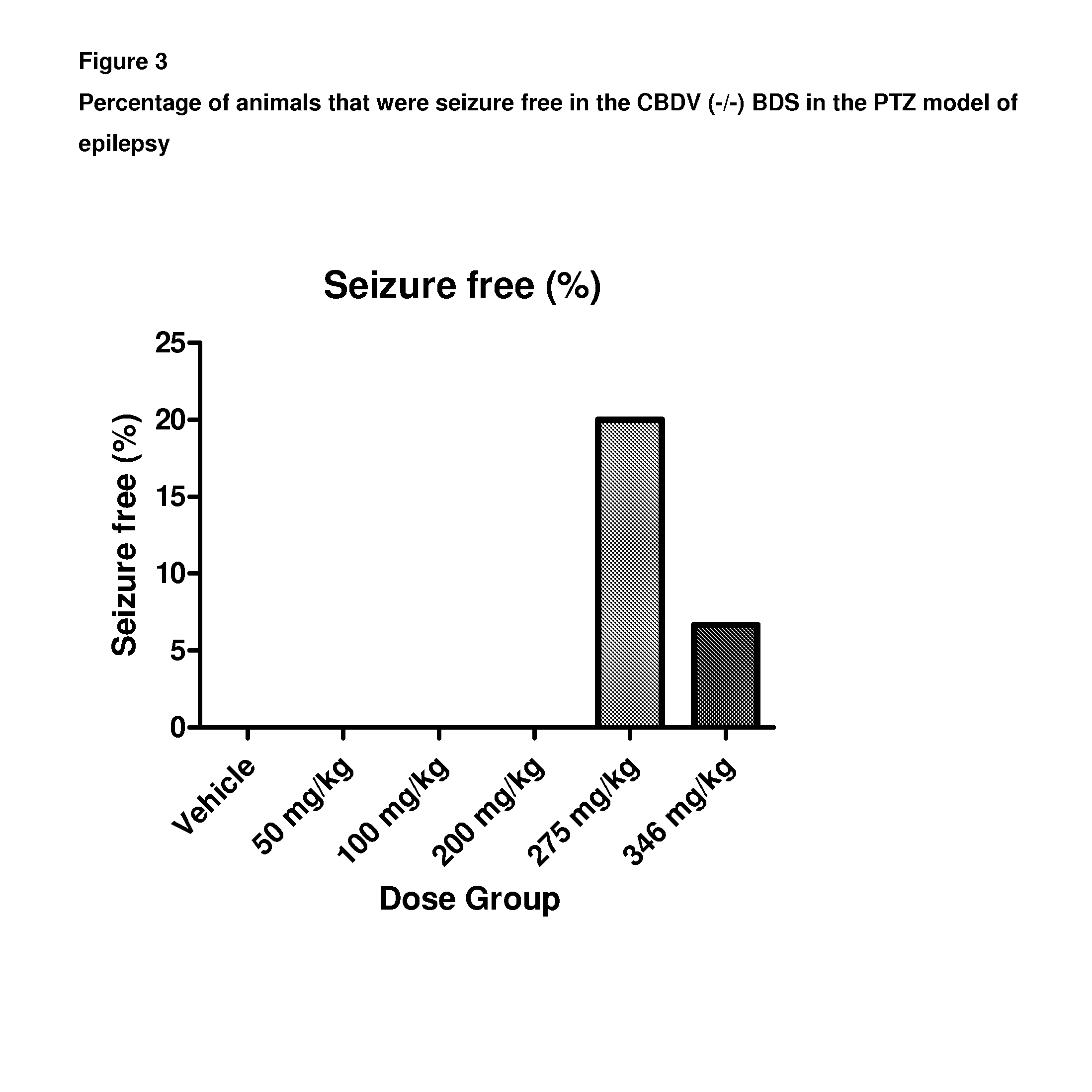
A pharmaceutical composition comprising the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD)
This invention relates to a pharmaceutical composition comprising or consisting essentially of the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD). The composition is particularly safe and efficacious for use in the treatment of neurological conditions, characterized by hyper-excitability of the central nervous system, convulsions or seizures such as occur in epilepsy. Preferably the CBDV and the CBD are present with at least one non-cannabinoid component of cannabis such as one or more terpenes or a terpene fraction. More particularly the composition further comprises one or more cannabichromene type compounds. Particularly cannabichromene propyl variant (CBCV) and / or cannabichromene (CBC). More particularly still the composition is absent or substantially absent of other cannabinoids, including in particular tetrahydrocannabinol (THC) and tetrahydrocannabivarin (THCV), which would normally be present in significant amounts in cannabis chemotypes bred to contain a significant amount of CBDV and / or CBD.
Owner:GW PHARMA LTD
Tocopheryl polyethylene glycol succinate powder and process for preparing same
InactiveUS20070184117A1Without compromising handling characteristicPowder deliveryOrganic active ingredientsPolymer scienceTocopherol polyethylene glycol succinate
A powdered tocopheryl polyethylene glycol succinate (TPGS™) having an average particle size of less than about 1000 microns. In one embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process that includes atomizing a fluidic tocopheryl polyethylene glycol succinate into an environment suitable for solidifying the atomized tocopheryl polyethylene glycol succinate. In another embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process of applying a force to a solid tocopheryl polyethylene glycol succinate starting material that is sufficient to produce a powdered product.
Owner:EASTMAN CHEM CO
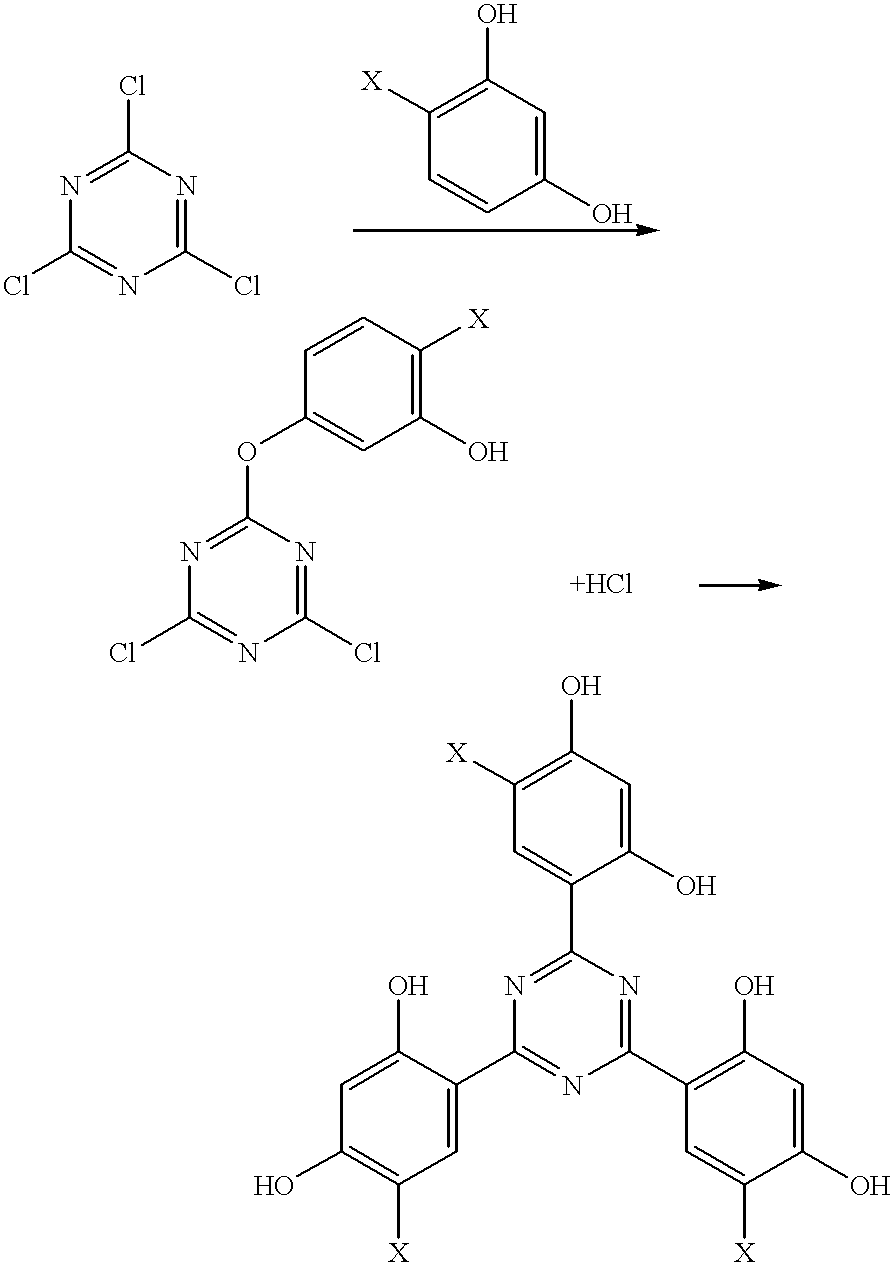
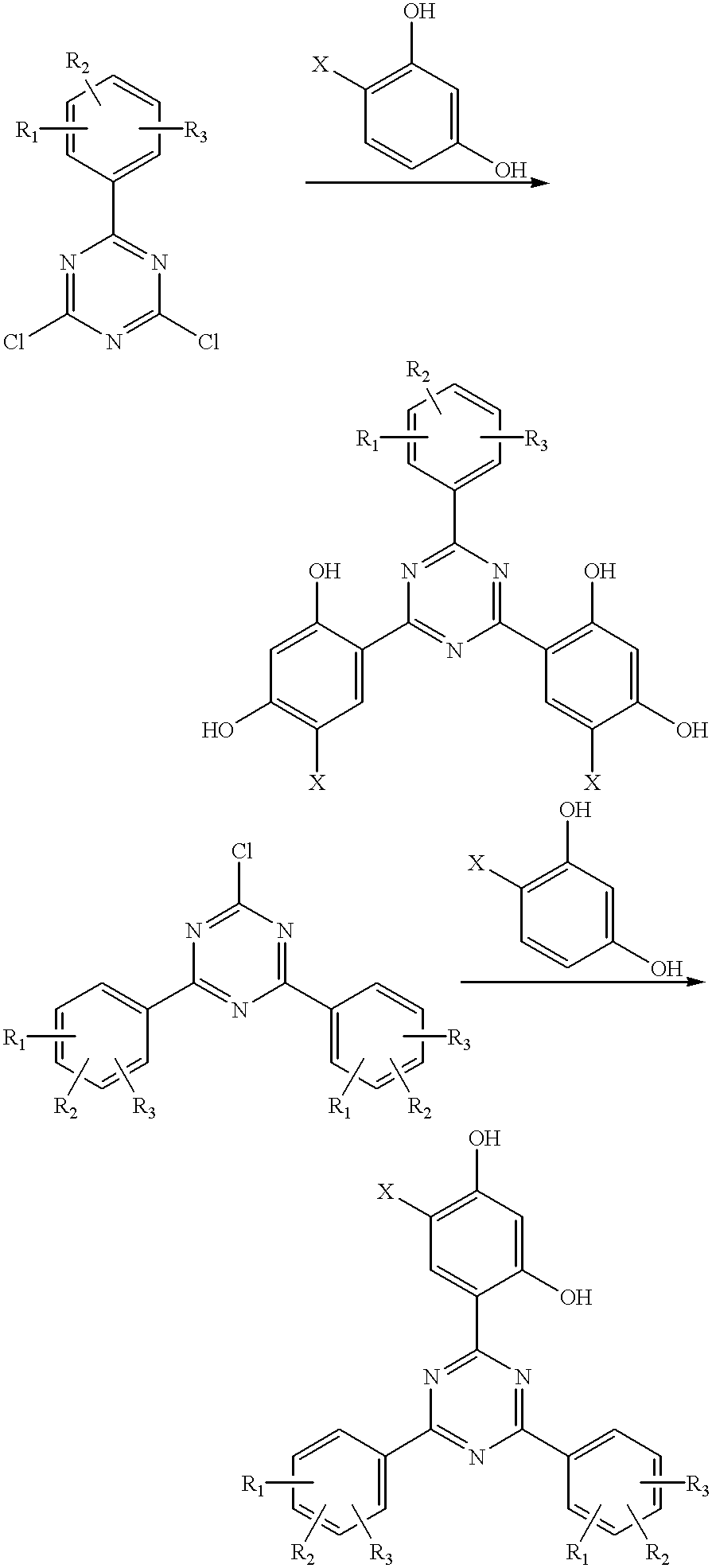
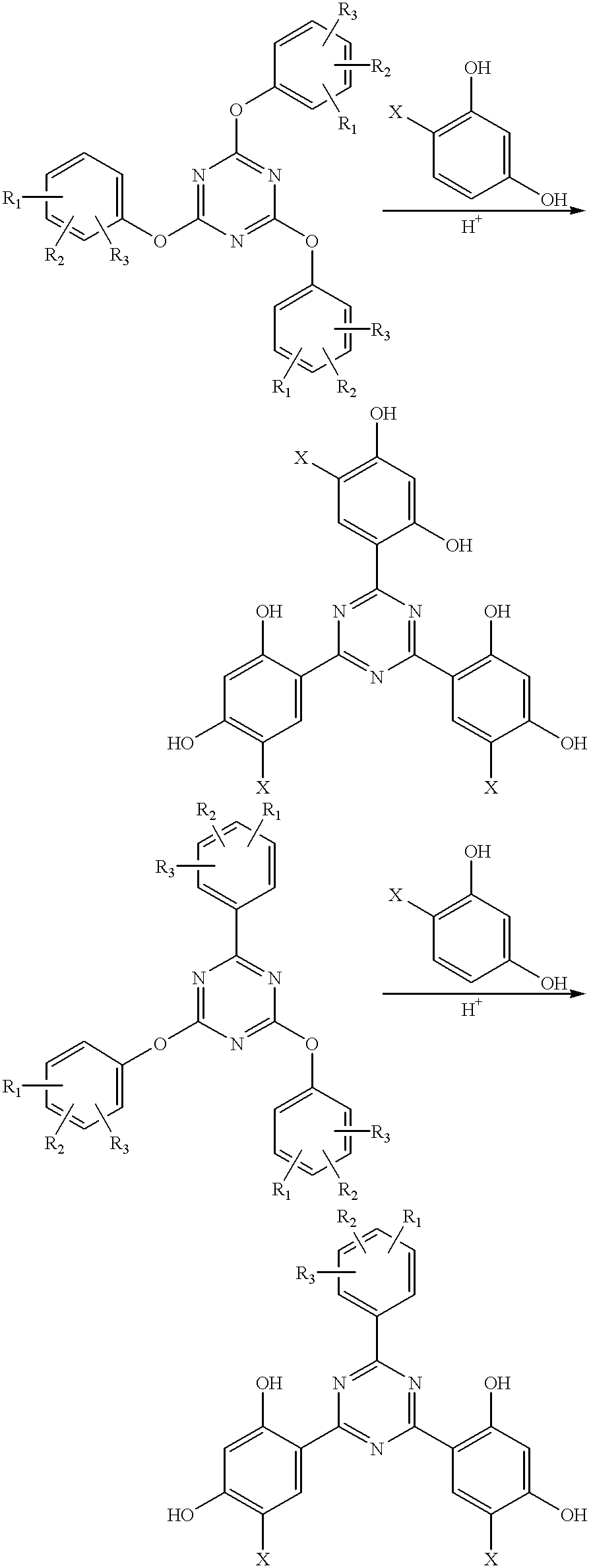
Methods for the preparation of tris-aryl-o-hydroxyphenyl-s-triazines
InactiveUS6242598B1Reduce in quantityPoor leaving groupOrganic chemistryLewis acid catalysisResorcinol
A process for preparing 2-(2,4-dihydroxyphenyl)-4.6-diaryl-s-triazines in three steps starting with cyanuric chloride is described. Step 1 involves the nucleophilic (basic) displacement of one chlorine atom with a phenolic moiety. Step 2 involves a Friedel-Crafts reaction using a Lewis acid catalyst (preferably aluminum chloride) to replace the remaining two chlorine atoms with aryl groups such as xylyl. Finally, step 3 involves replacing the phenolic moiety with resorcinol using either a Lewis acid or protic acid catalyst or combinations thereof. Some additional processes only peripherally related to the three-step process outlined above are also described for the preparation of various s-triazine compounds. The s-triazines prepared are useful as UV absorbers for the stabilization of organic substrates against the adverse effects of actinic light.
Owner:CIBA SPECIALTY CHEM CORP
Inherently radiopaque bioresorbable polymers for multiple uses
Preferred embodiments of the present invention relate to polymeric medical devices, such as stents. More particularly, the compositions disclosed herein comprise halogen-containing phenol moeities, that may be used for medical devices and other uses whereby bioresorbable and radiopaque and physicomechanical properties are desired.
Owner:REVA MEDICAL LLC
Lubricating oil for bearing
ActiveUS20060019840A1Improve performanceLiquid carbonaceous fuelsAdditivesGallic acid esterPHENOL LIQUID
Disclosed herein is a lubricating oil for bearings comprising (a) a diester represented by General Formula (1) wherein R1 and R2 are the same or different, and each represents a C3-C17 linear alkyl group; A represents a C2-C10 linear alkylene group or A represents a branched alkylene group consisting of a linear alkylene group, the linear alkylene group being the principal chain, and one or more alkyl groups (branches) bonded to the linear alkylene group, wherein the total number of carbon atoms of the linear alkylene group and the one or more alkyl groups is 3 to 10; with the proviso that when A is a branched alkylene group and has two or more alkyl groups, the two or more alkyl groups are not bonded to the same carbon atom; or a mixture of the diester with an additional base oil and (b) at least one member selected from the group consisting of phenol-based antioxidants and amine-based antioxidants, and optionally containing (c) at least one member selected from the group consisting of phosphorus-based compounds and aliphatic linear monocarboxylic acids, and further optionally containing (d) at least one member selected from the group consisting of benzotriazole-based compounds and gallic acid-based compounds.
Owner:NEW JAPAN CHEM CO
Quaternary Ammonium Salt of a Mannich Compound
ActiveUS20080052985A1Organic chemistryTransportation and packagingCompound aQuaternary ammonium cation
A quaternary ammonium salt detergent made from the reaction product of the reaction of: (a) Mannich reaction product having a tertiary amino group, said Mannich reaction product being prepared from the reaction of a hydrocarbyl-substituted phenol, an aldehyde, and amine; and (b) a quaternizing agent suitable for converting the tertiary amino group to a quaternary nitrogen and the use of such quaternary ammonium salt detergents in a fuel composition to reduce intake valve deposits.
Owner:THE LUBRIZOL CORP
Method for nucleotide detection
ActiveUS20120196758A1Prevent degradationMicrobiological testing/measurementLibrary screeningGallic acid esterNucleic acid detection
A method of inhibiting light-induced degradation of nucleic acids includes irradiating a portion of the nucleic acids in the presence of a detection solution comprising a polyphenolic compound. A method of detecting a nucleic acid having a fluorescent tag includes irradiating at least a portion of the nucleic acid with light of a suitable wavelength to induce a fluorescence emission and detecting the fluorescence emission. Optionally, the polyphenolic compound is gallic acid, a lower alkyl ester thereof, or mixtures thereof. A kit includes one or more nucleotides, an enzyme capable of catalyzing incorporation of the nucleotides into a nucleic acid strand and a polyphenolic compound suitable for preparing a detection solution.
Owner:ILLUMINA INC
Resin composition, method of its composition, and cured formulation
InactiveUS20060029811A1Improve flame retardant performanceMaintain good propertiesSemiconductor/solid-state device detailsSynthetic resin layered productsCarboxylic acidMicroparticle
It is an object of the present invention to provide a resin composition which can form cured formulations having various excellent properties such as an insulating property, thermal shock resistance, moldability / formability and strength, and exhibit an excellent appearance in which transparency is enhanced, a resin composition whose cured thin film has excellent flame retardancy, good mechanical property and heat resistance, a dispersing element containing an inorganic microfine particle which can give a flame retardancy to a resin, to which the inorganic microfine particle is added, and can reduce a hygroscopic property to the extent possible, a method for producing the same and a cured formulation obtained by using the resin composition. The present invention relates to a resin composition comprising a compound having at least one of a glycidyl group and / or an epoxy group and an inorganic microfine particle, a resin composition comprising three components of a phenolic compound, a compound having at least one of a glycidyl group and / or an epoxy group and an inorganic microfine particle, a flame retardant resin composition comprising a polyhydric phenol and an inorganic microfine particle, and a dispersing element containing an inorganic microfine particle obtained by a hydrolysis condensation reaction of alkoxide and / or metal carboxylate in a dispersion medium.
Owner:NIPPON SHOKUBAI CO LTD
Use of cocoa solids having high cocoa polyphenol content in tabletting compositions and capsule filling compositions
Disclosed and claimed are cocoa extracts, compounds, combinations thereof and compositions containing the same, such as polyphenols or procyanidins, methods for preparing such extracts, compounds and compositions, as well as uses for them, especially a polymeric compound of the formula An, wherein A is a monomer of the formula:wherein n is an integer from 2 to 18, such that there is at least one terminal monomeric unit A, and one or a plurality of additional monomeric units;R is 3-(alpha)-OH, 3-(beta)-OH, 3-(alpha)-O-sugar, or 3-(beta)-O-sugar;bonding between adjacent monomers takes place at positions 4, 6 or 8;a bond of an additional monomeric unit in position 4 has alpha or beta stereochemistry;X, Y and Z are selected from the group consisting of monomeric unit A, hydrogen, and a sugar, with the provisos that as to the at least one terminal monomeric unit, bonding of the additional monomeric unit thereto (the bonding of the additional monomeric unit adjacent to the terminal monomeric unit) is at position 4 and optionally Y=Z=hydrogen;the sugar is optionally substituted with a phenolic moiety, at any position on the sugar, for instance via an ester bond, andpharmaceutically acceptable salts or derivatives thereof (including oxidation products).
Owner:MARS INC
Electrical smoking system and method
InactiveCN1633247AReduce gaseous componentsIncandescent ignitionCigar manufactureIntegratorAcrylonitrile
An electric smoking system includes a cigarette including a cylindrical tobacco web partially filled with tobacco material to define a filled tobacco rod portion and an unfilled tobacco rod portion, and an electric lighter. The wrapper includes a filler of ammonium-containing compounds effective to reduce the gaseous constituents of the smoke produced during smoking. The system includes a pilot burner including at least one heating vane and a controller adapted to control heating of the heating vane. The lighter is configured to at least partially contain the cigarette such that the heater blade heats the heating region of the cigarette. Manipulating the controller to limit the heating of the heater blades to a predetermined temperature range which allows the delivery of the smoke generated when the portion of the tobacco rod is heated while at least reducing the amount of smoke present in the smoke as compared to smoking a cigarette having only calcium carbonate as filler. A gaseous component. The gaseous components that can be reduced include carbon monoxide, 1,3 butadiene, isoprene, acrolein, acrylonitrile, hydrogen cyanide, 0-toluidine, 2-naphthylamine, nitrogen oxide, benzene, NNN, Phenol, catechol, benzanthracene and benzopyrene.
Owner:PHILIP MORRIS PROD SA
Multidentate phosphite ligands, catalytic compositions containing such ligands and catalytic processes utilizing such catalytic compositions
InactiveUS6380421B1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenOrtho position
Hydrocyanation reactions employing multidentate phosphite ligands and multidentate phosphite ligands are disclosed. The ligands have phenyl containing substituents attached to the ortho position of the terminal phenol group and / or attached to the ortho position of the bridging group. Catalyst compositions havng such ligands achieve 97% or greater distribution in hydrocyanation.
Owner:INVISTA NORTH AMERICA R L
Method for producing benzoxazine resin
InactiveUS7041772B2Improve efficiencyPromote safe productionNatural resin processChemical/physical/physico-chemical stationary reactorsOrganic solventReaction system
The present invention discloses a process for producing a benzoxazine resin which comprises the steps of reacting a phenol compound, an aldehyde compound and a primary amine in the presence of an organic solvent to synthesize a benzoxazine resin and removing generated condensation water and the organic solvent from a system under heating and a reduced pressure, wherein a pressure in the reaction system at the time of removal is set to 260 mmHg or higher.
Owner:HITACHI CHEM CO LTD
Method of preparing a poly(arylene ether), and a poly(arylene ether) prepared thereby
A method of preparing a poly(arylene ether) includes oxidatively polymerizing a monohydric phenol in solution, concentrating the solution by removing a portion of the solvent to form a concentrated solution having a cloud point, Tcloud, and combining the concentrated solution with an anti-solvent to precipitate the poly (arylene ether), wherein the concentrated solution has a temperature of at least about (Tcloud-10° C.) immediately before it is combined with the anti-solvent. The method reduces the formation of undesirably fine particles in the product poly(arylene ether).
Owner:SHPP GLOBAL TECH BV
Emulsification type metal cutting liquor composition
InactiveCN101240218AImprove the lubrication effectImprove cooling effectAdditivesBase-materialsPhenolCutting fluid
Disclosed is an emulsifying metal-cutting-fluid composition comprising base oil or oily agent, mixed alcohol-amine, anionic surfactant, nonionic surfactant, antirust agent, copper alloy corrosion inhibitor, preservative and the like. The invention has a strong general usability, suitable for metal processing, particularly aluminum alloy metal processing with advantages of excellent lubricity, corrosion resistance, a low cost and being free of toxic or harmful substances such as nitrites and phenols, so as to keep the environment and operators away from harmfulness.
Owner:河北九熙新材料科技有限公司
Method of polishing a silicon-containing dielectric
InactiveUS7071105B2Other chemical processesSemiconductor/solid-state device manufacturingThiolHydroxamic acid
The invention is directed to a method of polishing a silicon-containing dielectric layer involving the use of a chemical-mechanical polishing system comprising (a) an inorganic abrasive, (b) a polishing additive, and (c) a liquid carrier, wherein the polishing composition has a pH of about 4 to about 6. The polishing additive comprises a functional group having a pKa of about 4 to about 9 and is selected from the group consisting of arylamines, aminoalcohols, aliphatic amines, heterocyclic amines, hydroxamic acids, aminocarboxylic acids, cyclic monocarboxylic acids, unsaturated monocarboxylic acids, substituted phenols, sulfonamides, thiols, salts thereof, and combinations thereof. The invention is further directed to the chemical-mechanical polishing system, wherein the inorganic abrasive is ceria.
Owner:CABOT MICROELECTRONICS CORP
Substituted Phenols as Active Agents Inhibiting Vegf Production
The present invention relates to methods, compounds, and compositions for inhibiting angiogenesis. More particularly, the present invention relates to methods, compounds, and compositions for inhibiting VEGF production.
Owner:PTC THERAPEUTICS INC
Natural resin formulations
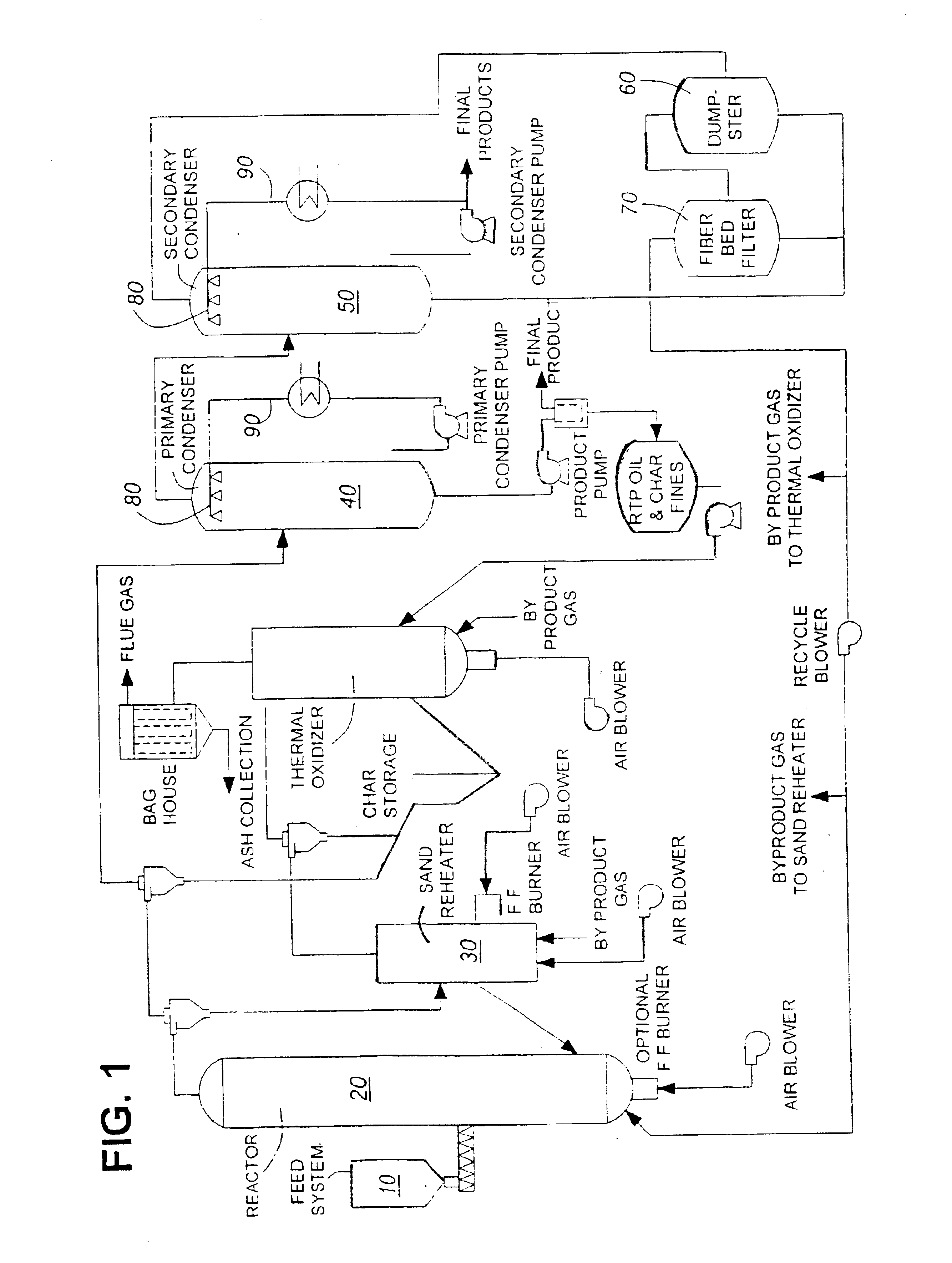
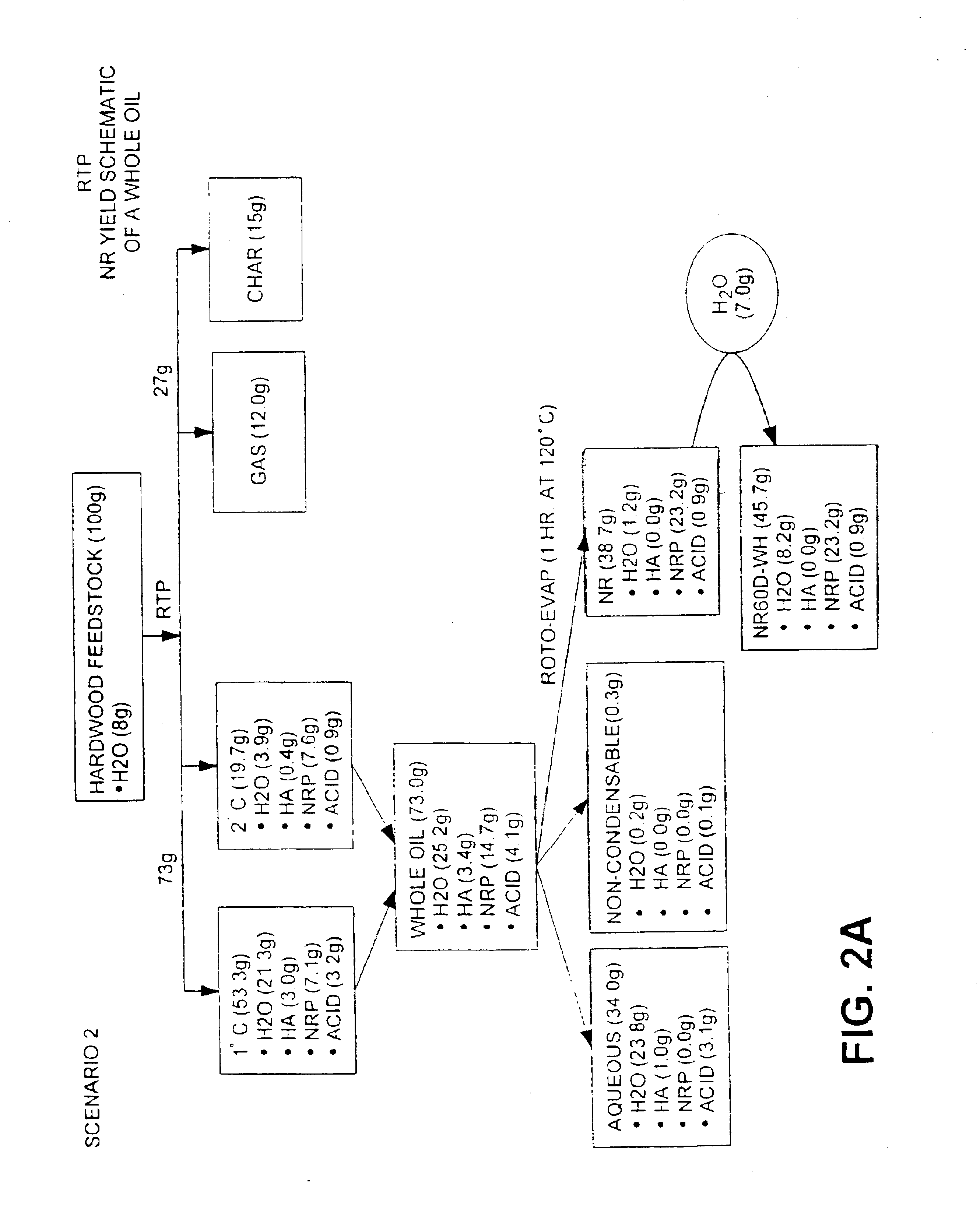
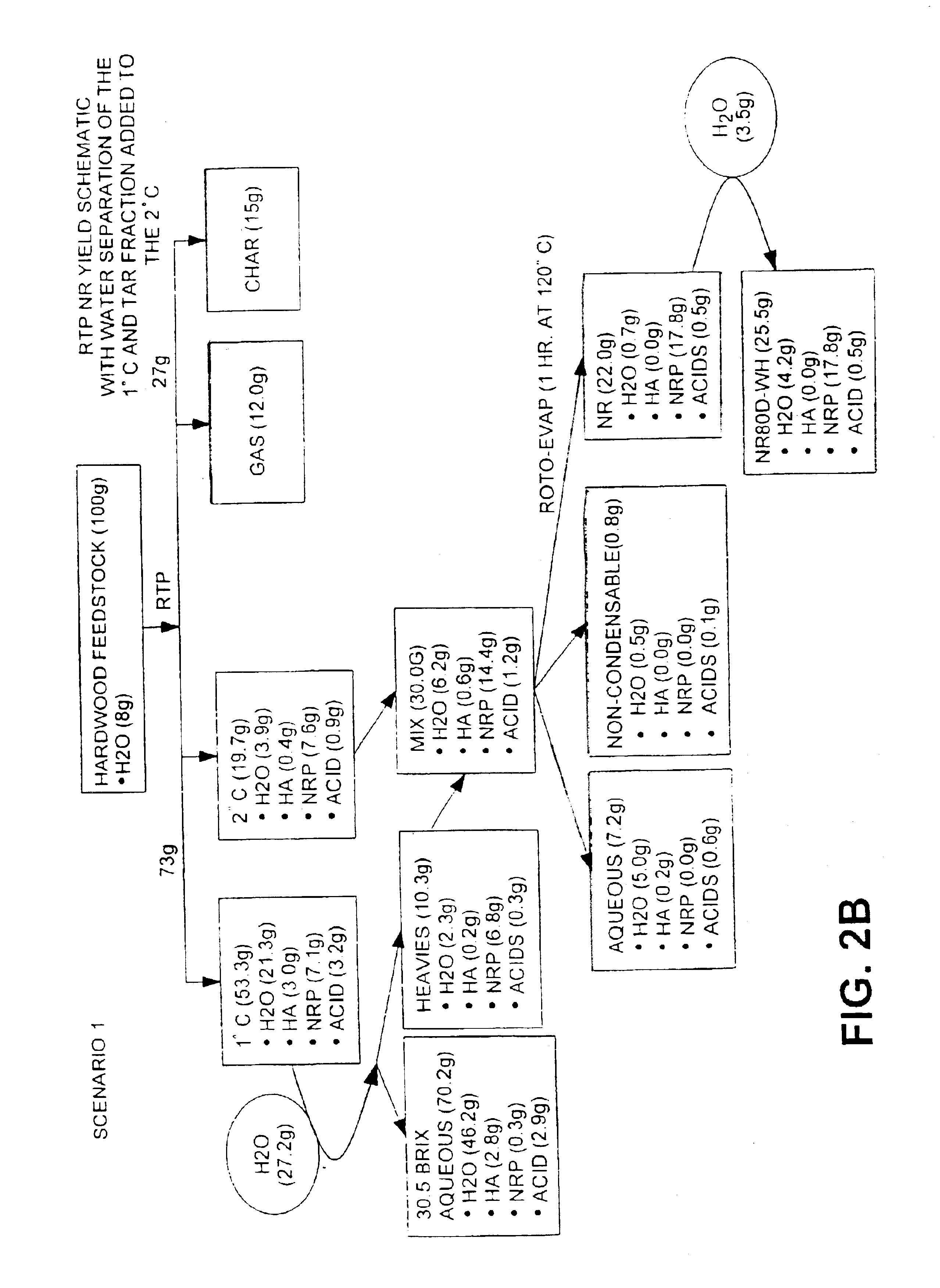
InactiveUS6844420B1Low viscosityImprove liquidityFatty oils/acids recovery from wasteBiofuelsNatural resinDistillation
This invention is directed to a method of preparing a natural resin by liquefying wood, bark, forest residues, wood industry residues, or other biomass using rapid destructive distillation (fast pyrolysis). Fast pyrolysis produces both vapors and char from biomass, and following removal of the char from the product vapors, a liquid pitch product is recovered and processed by distillation, evaporation, or a combination thereof, in order to obtain a natural resin which may be in either liquid or solid form. The natural resin comprises a total phenolic content from about 30% to about 80% (w / w), and is a highly-reactive ligninic compound that has been found to be suitable for use within resin formulations without requiring any further extraction or fractionation procedures. Resins comprising up to 60% natural resin have been prepared and tested in board production and found to exhibit similar properties associated with commercially available resins. The natural resin may substitute for phenol, or for both phenol and formaldehyde within phenol-containing resins. Similarly, the natural resin can replace a substantial part of the components within urea-containing resins.
Owner:ENSYN RENEWABLES
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com