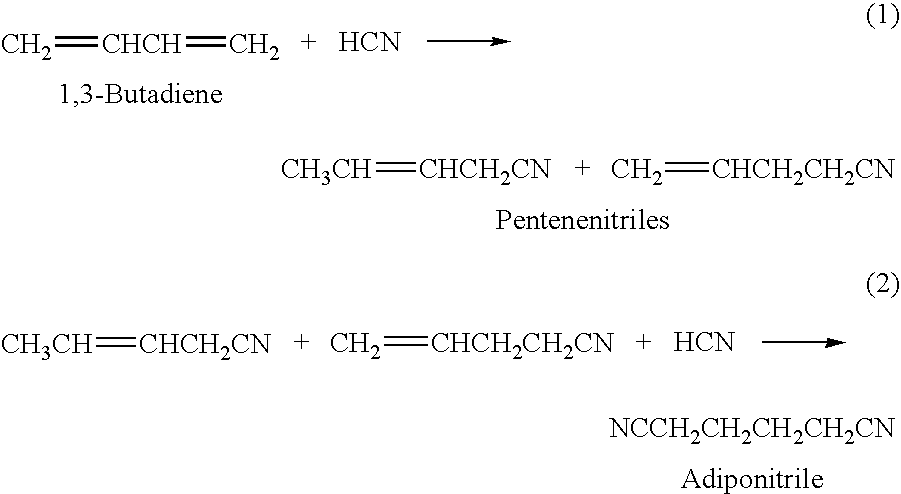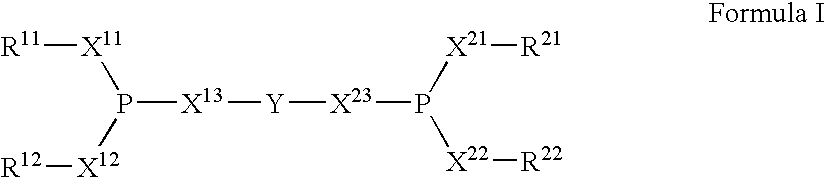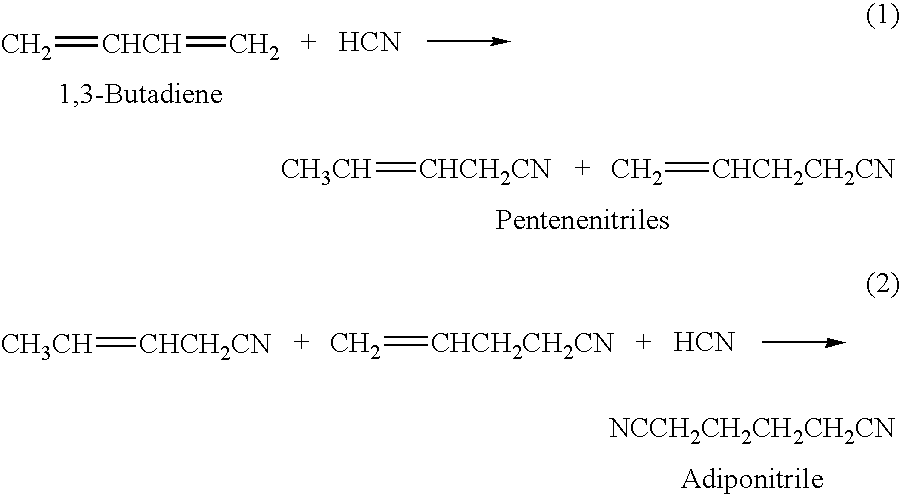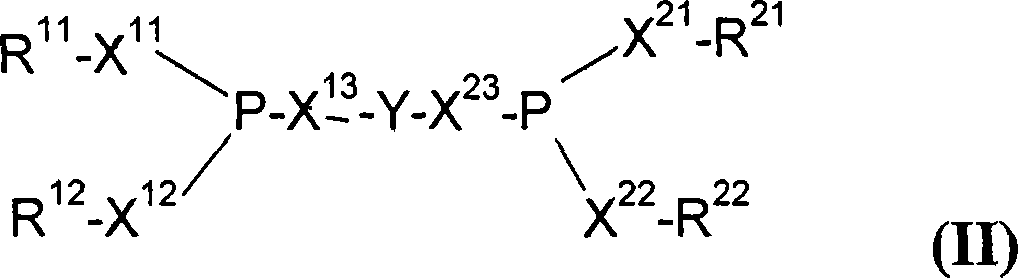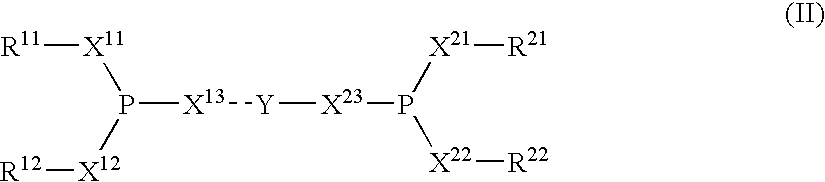Patents
Literature
94 results about "Hydrocyanation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
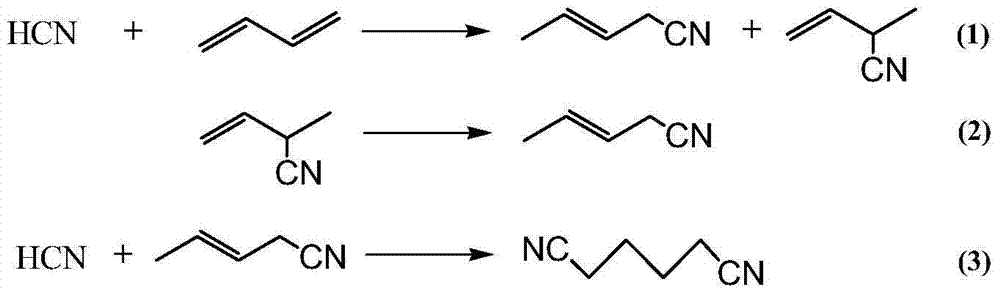
Hydrocyanation is, most fundamentally, the process whereby H⁺ and ⁻CN ions are added to a molecular substrate. Usually the substrate is an alkene and the product is a nitrile. When ⁻CN is a ligand in a transition metal complex, its basicity makes it difficult to dislodge, so, in this respect, hydrocyanation is remarkable. Since cyanide is both a good σ–donor and π–acceptor its presence accelerates the rate of substitution of ligands trans from itself, the trans effect. A key step in hydrocyanation is the oxidative addition of hydrogen cyanide to low–valent metal complexes. In hydrocyanation of unsaturated carbonyls addition over the alkene competes with addition over the carbonyl group.
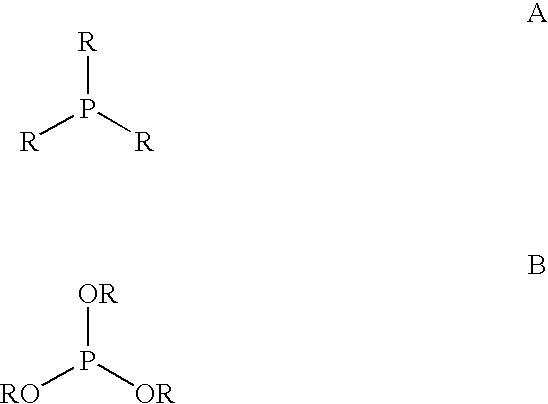
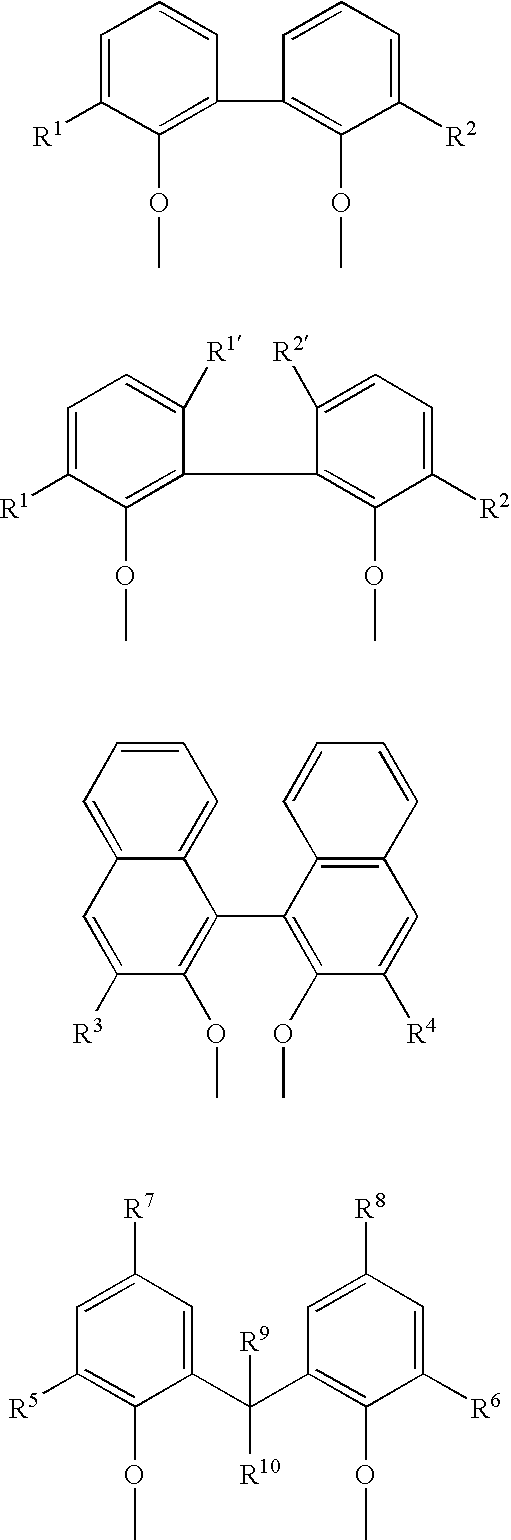
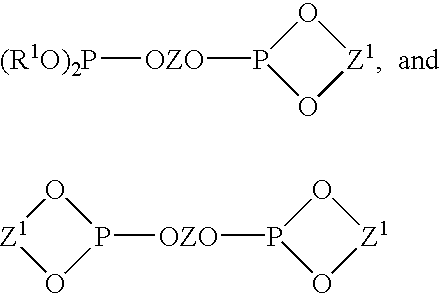
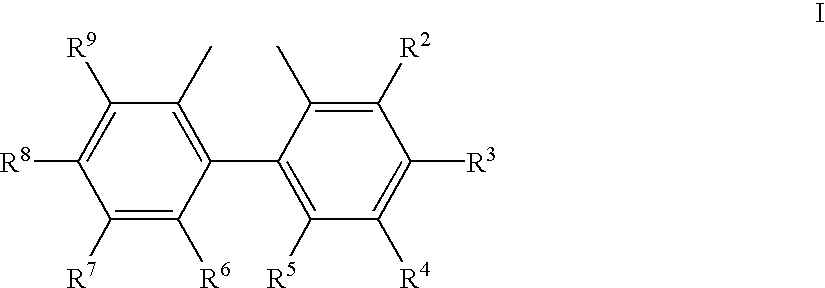
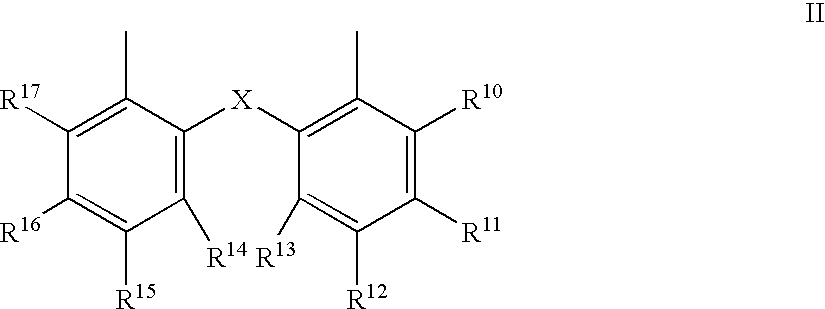
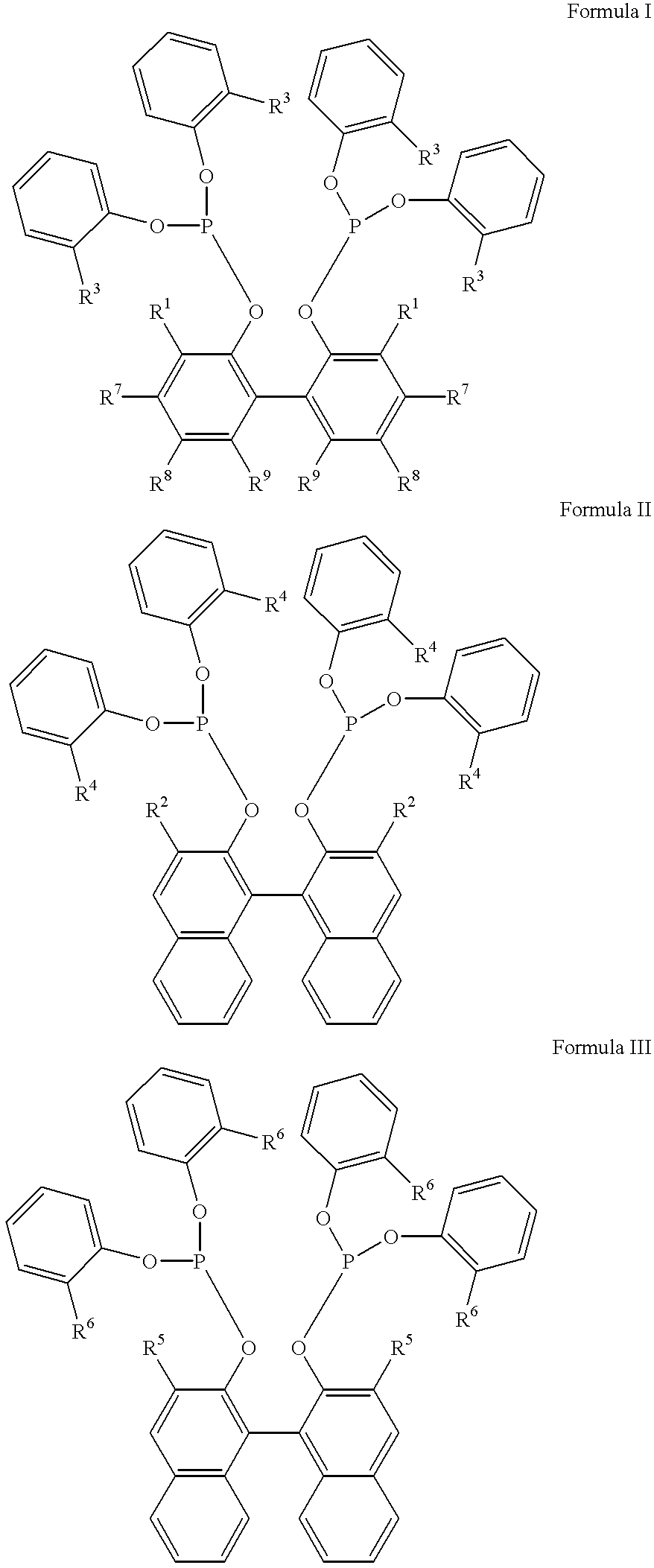
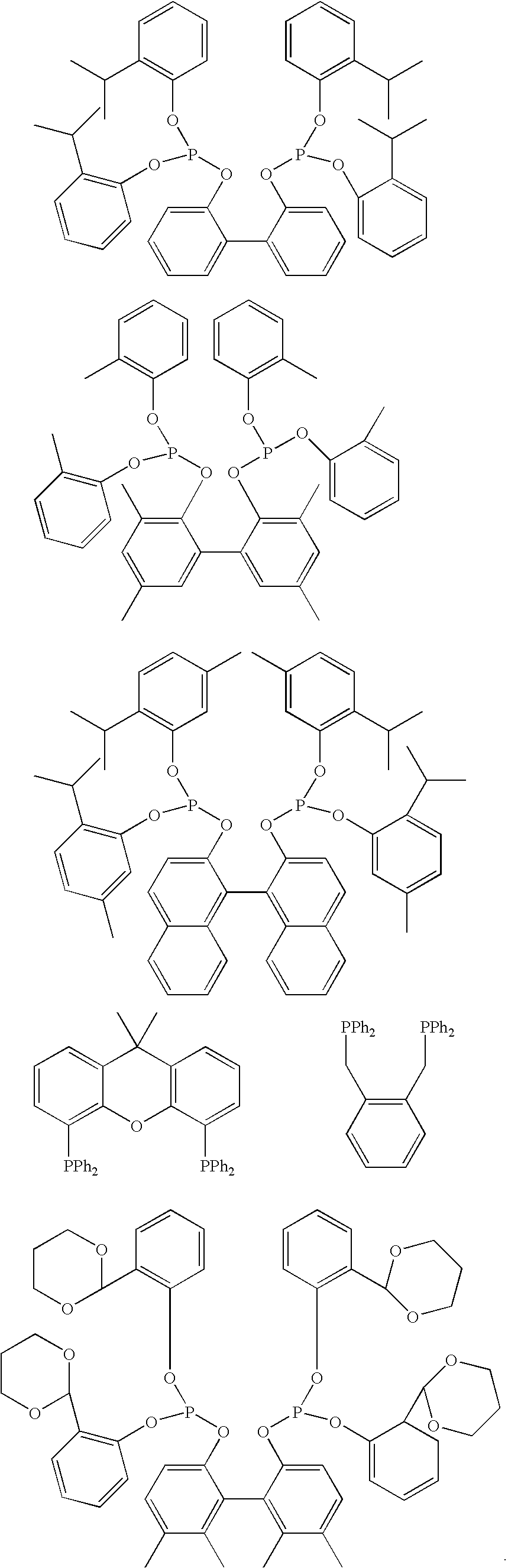
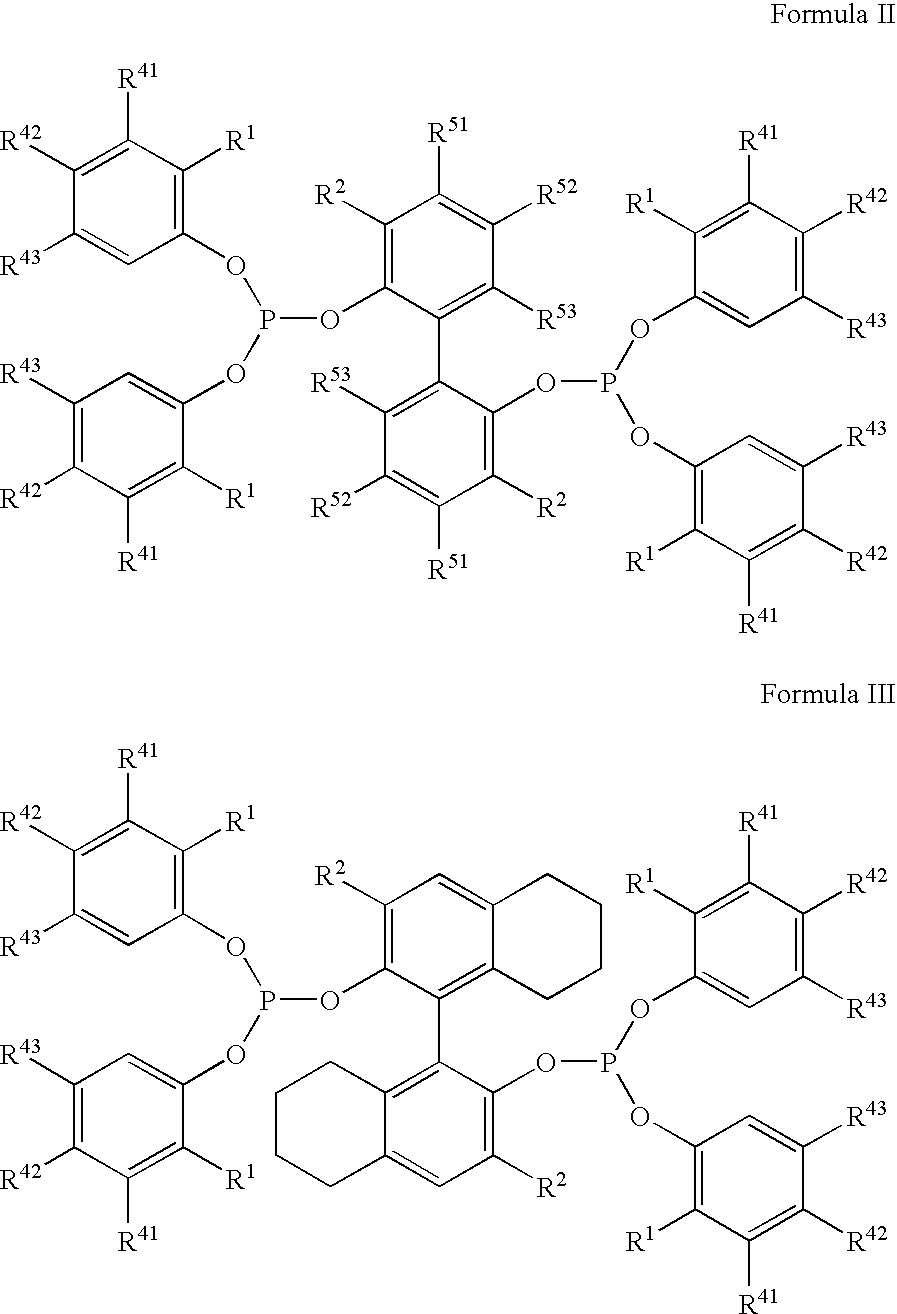
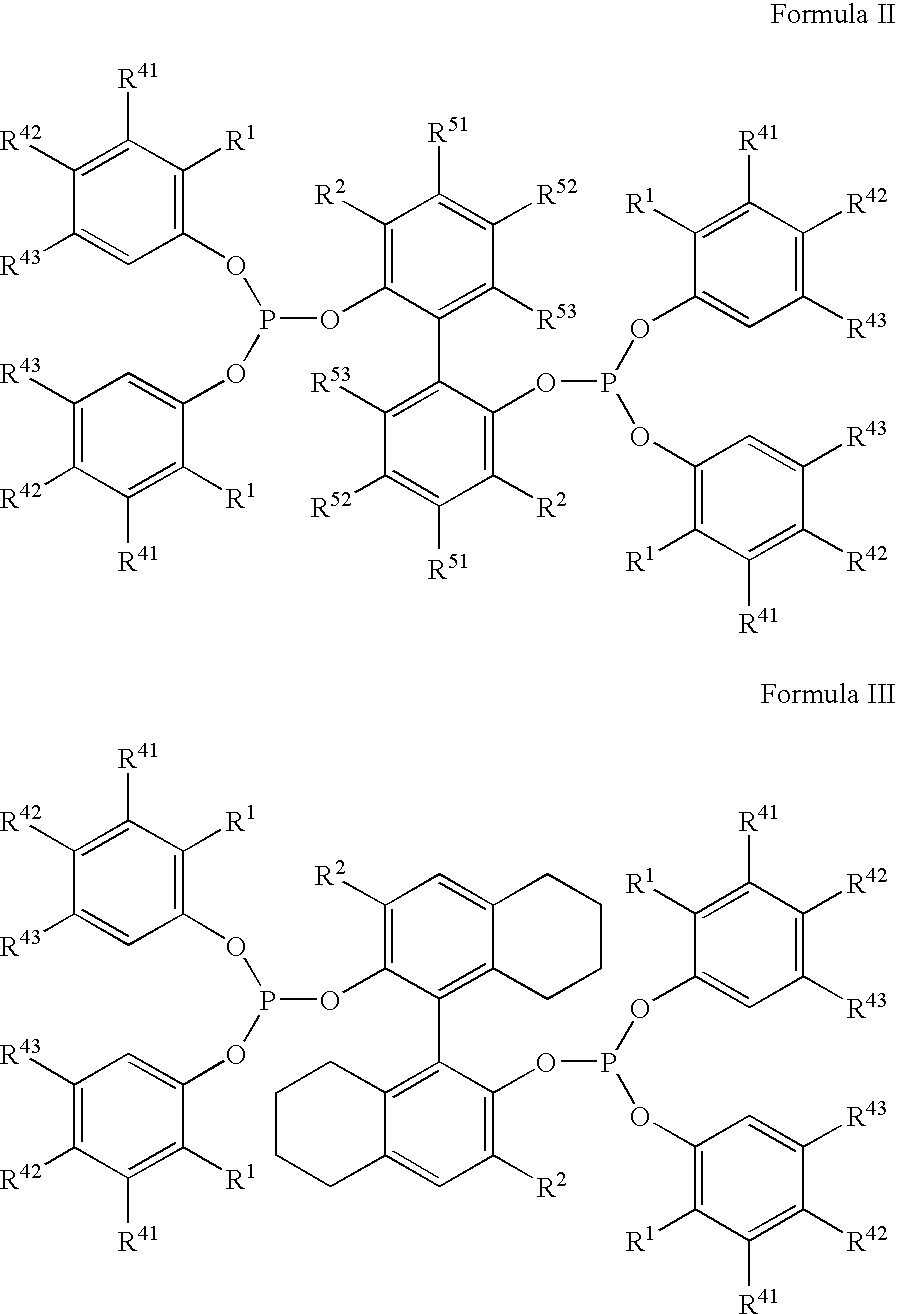
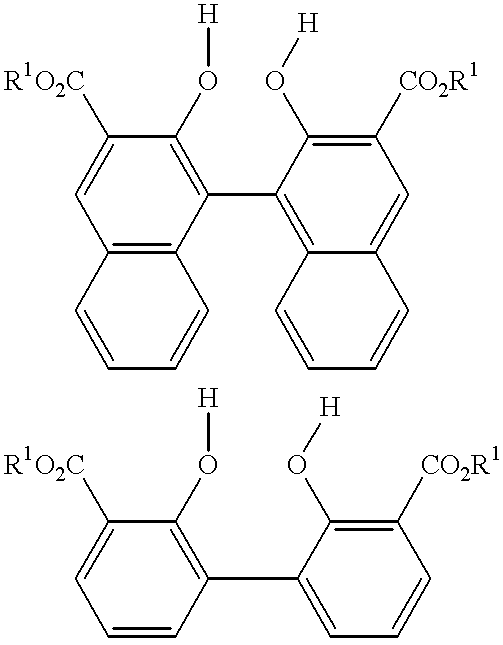
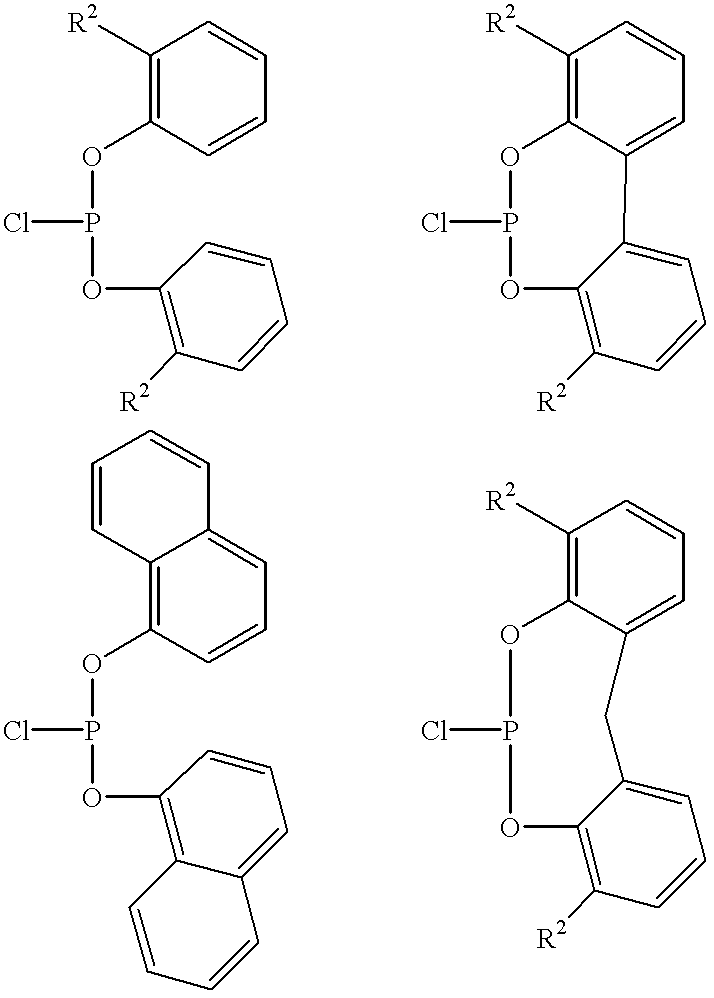
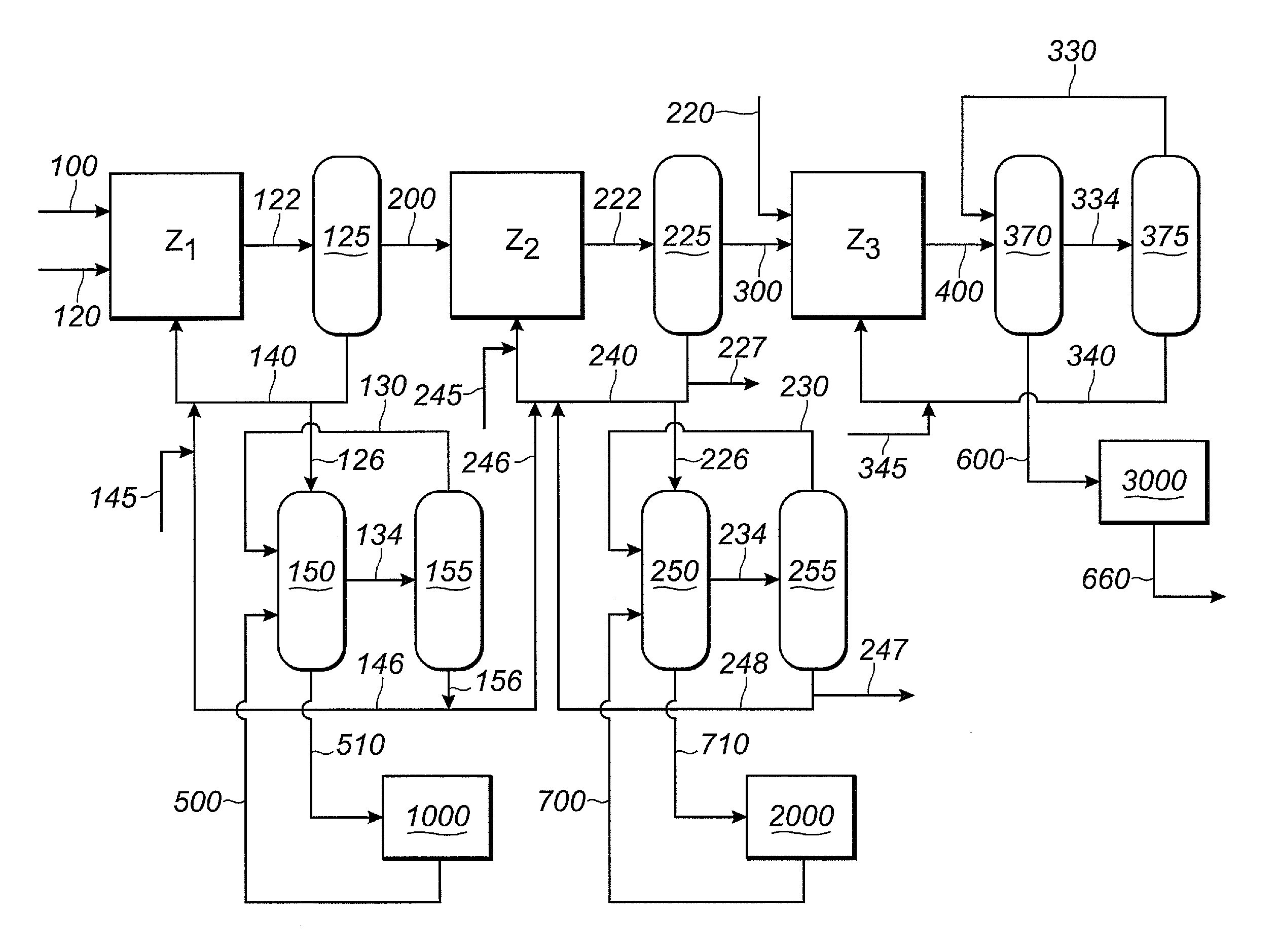
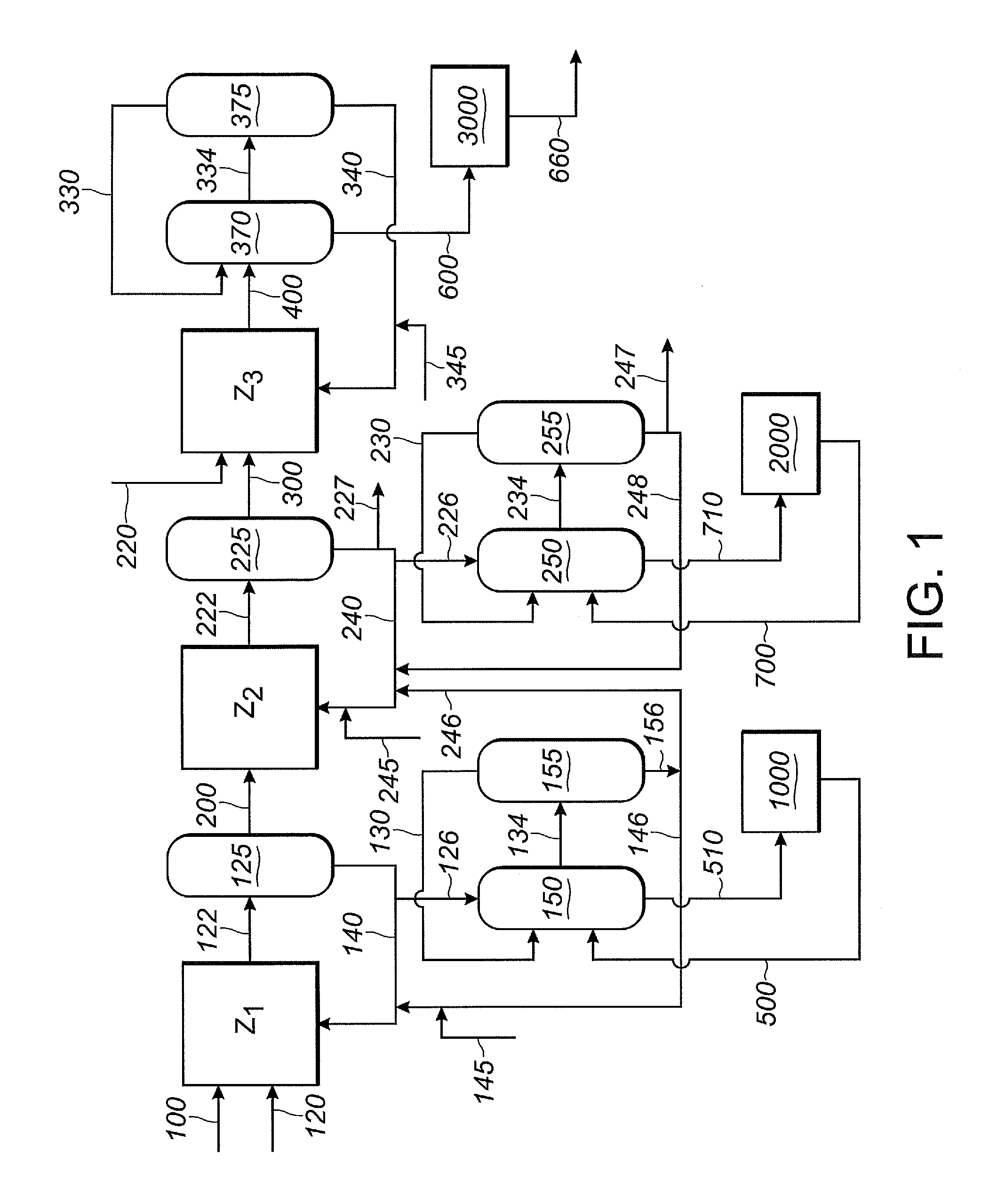
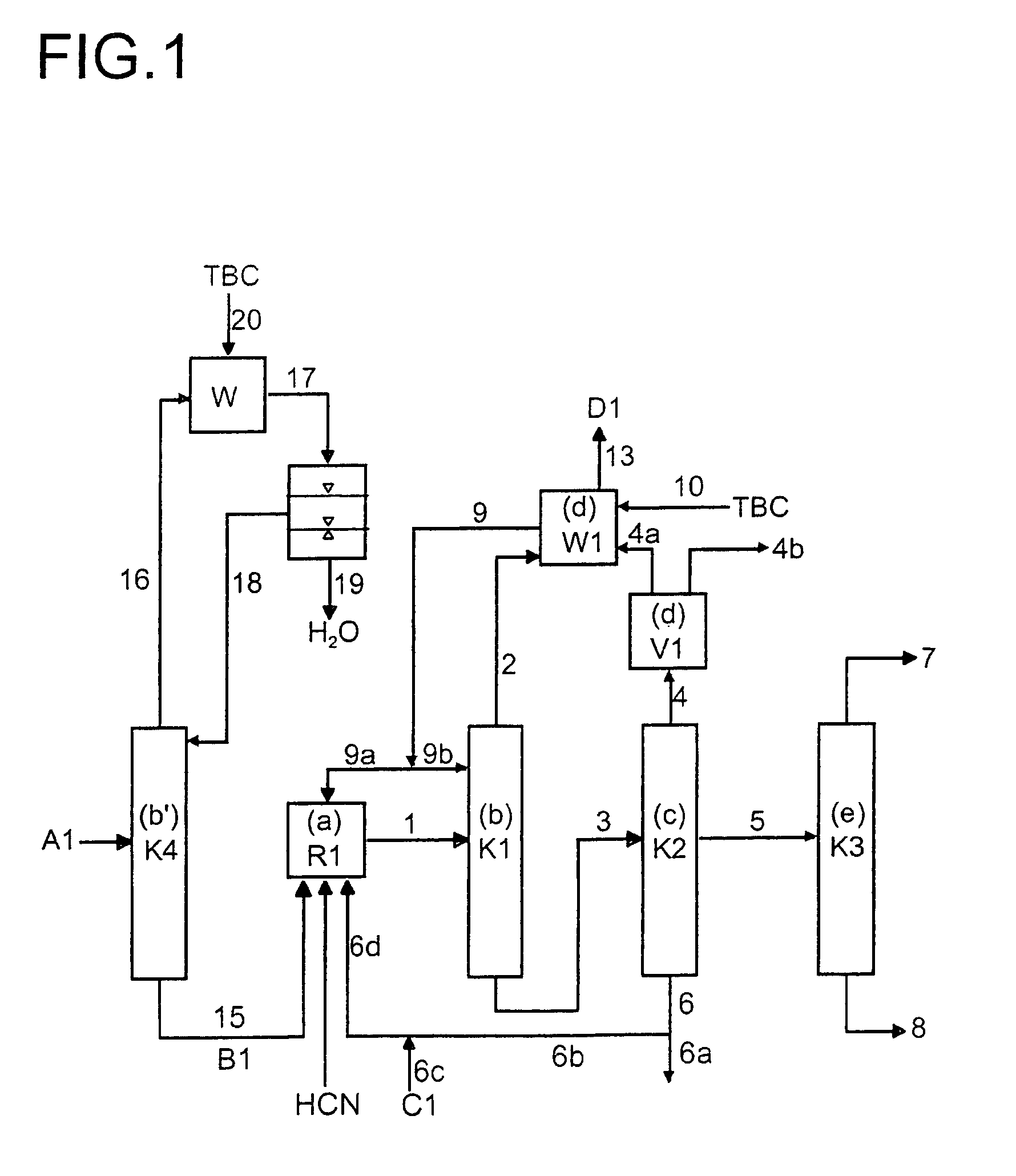
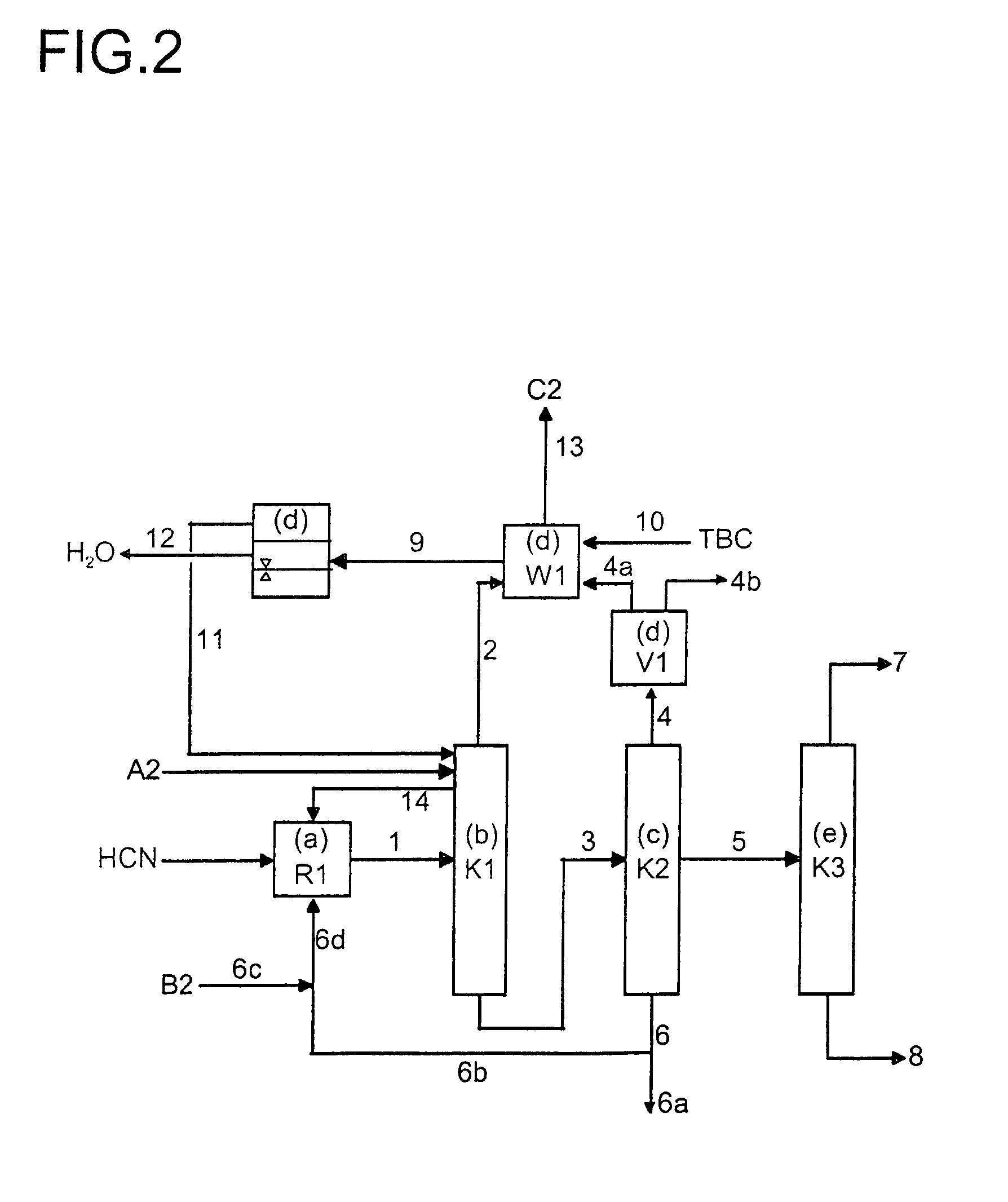
Multidentate phosphite ligands, catalytic compositions containing such ligands, and catalytic processes utilizing such catalytic compositions
InactiveUS6812352B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationOrtho position
Owner:INVISTA NORTH AMERICA S A R L
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
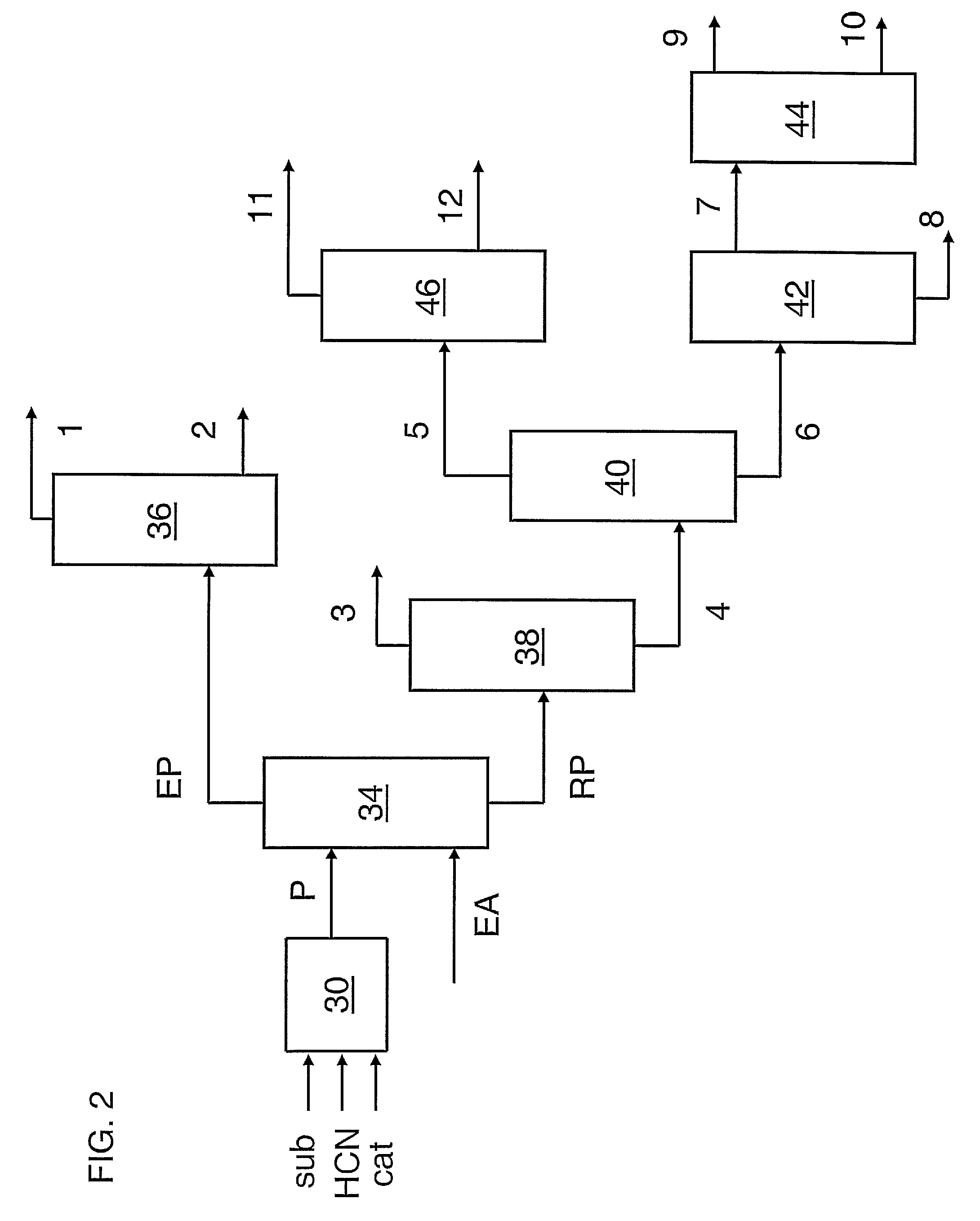
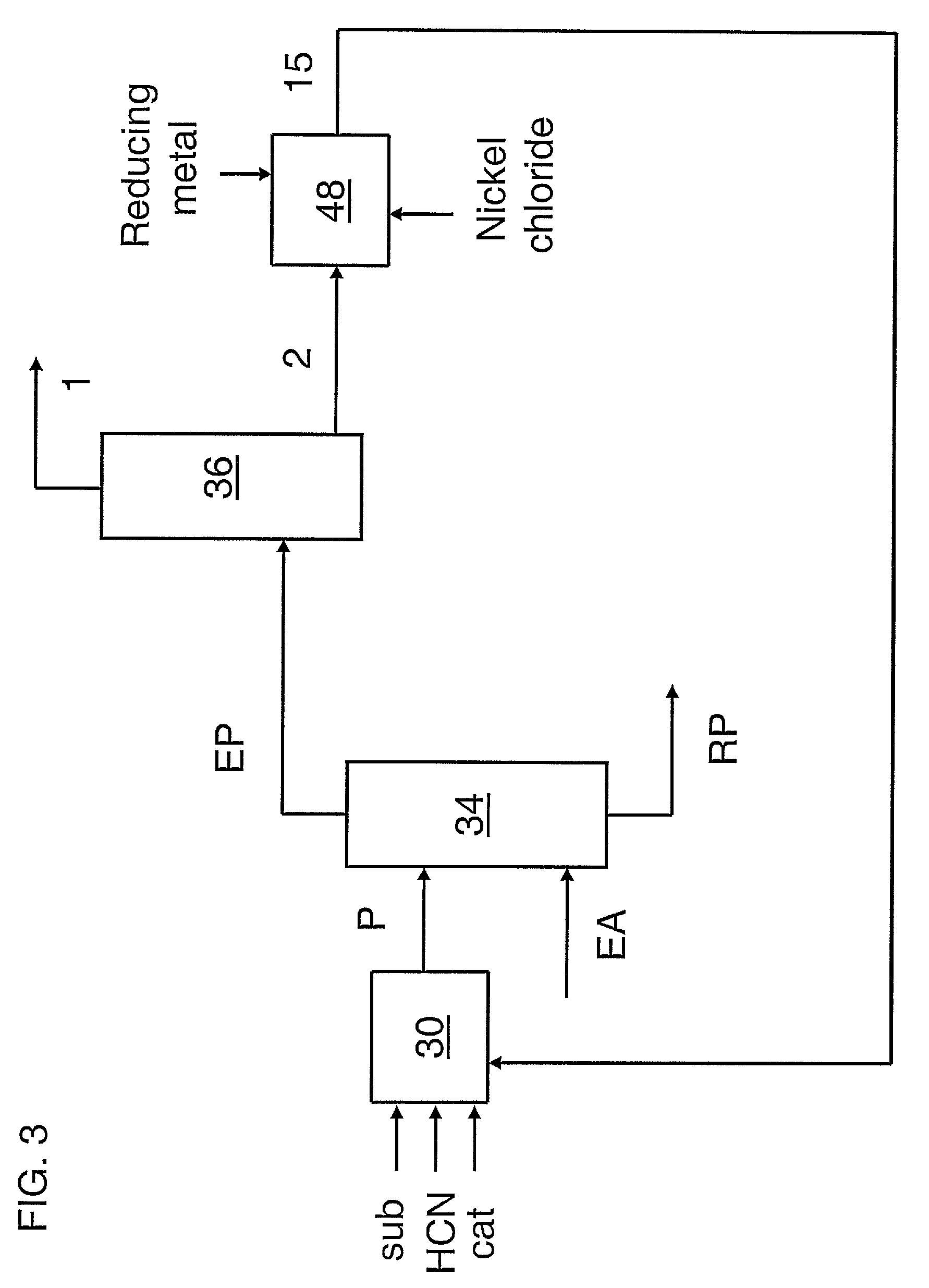
A process for preparing a hydrocyanation catalyst comprising contacting a bidentate phosphorous-containing ligand with a molar excess of nickel chloride in the presence of a nitrile solvent and a reducing metal which is more electropositive than nickel. Preferably, the nickel chloride is dried before use.
Owner:INVISTA NORTH AMERICA R L
Multidentate phosphite ligands, catalytic compositions containing such ligands and catalytic processes utilizing such catalytic compositions
InactiveUS6380421B1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenOrtho position
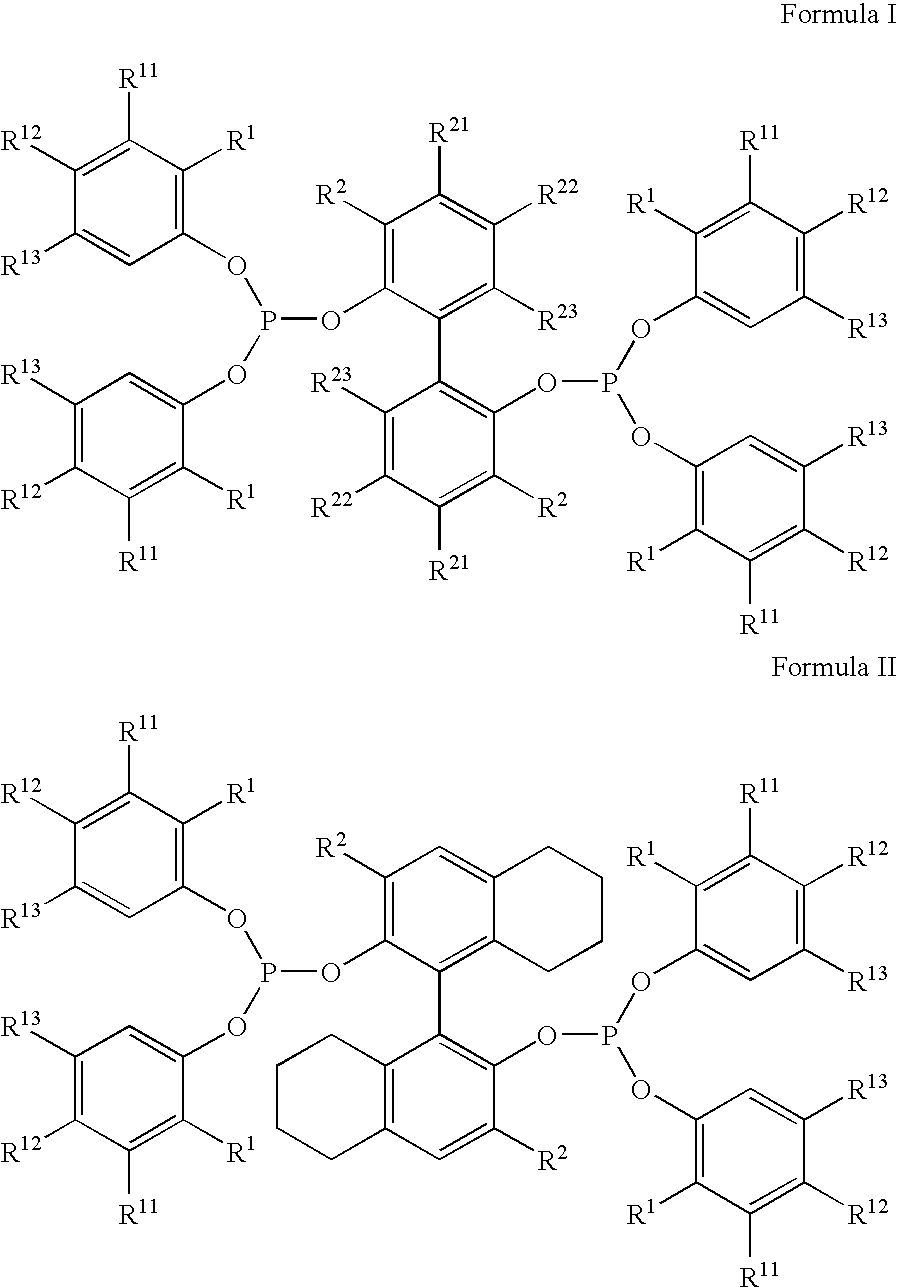
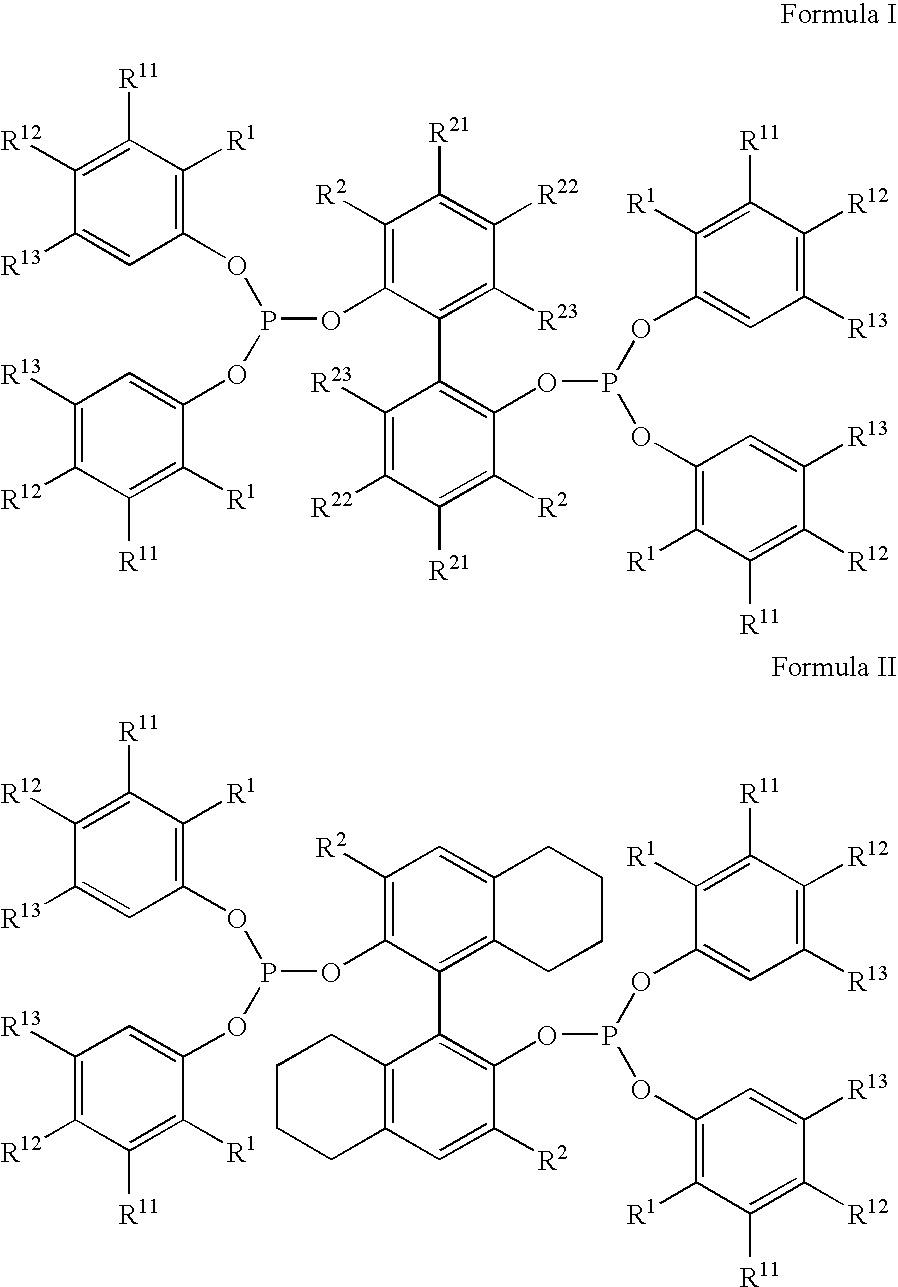
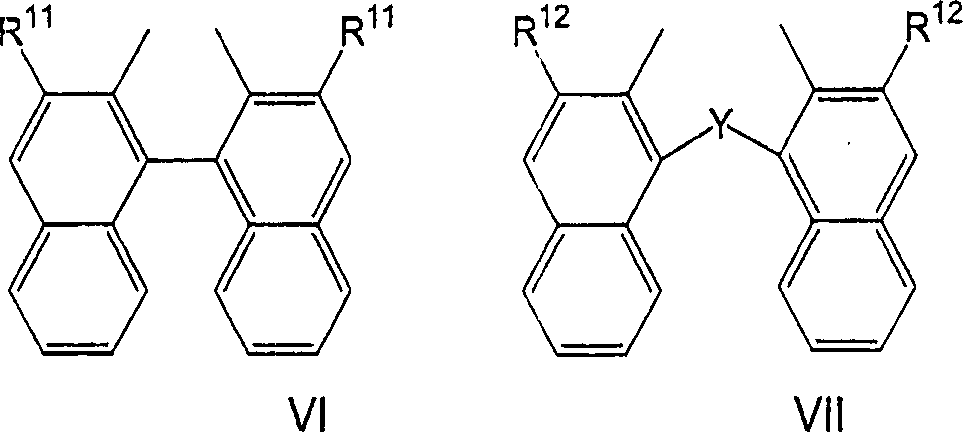
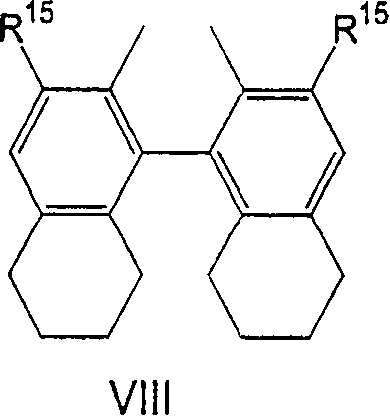
Hydrocyanation reactions employing multidentate phosphite ligands and multidentate phosphite ligands are disclosed. The ligands have phenyl containing substituents attached to the ortho position of the terminal phenol group and / or attached to the ortho position of the bridging group. Catalyst compositions havng such ligands achieve 97% or greater distribution in hydrocyanation.
Owner:INVISTA NORTH AMERICA R L
Supported bis(phosphorus) ligands and their use in the catalysis
InactiveUS6984604B2High selectivityHigh yieldOther chemical processesOrganic compound preparationIsomerizationOrganic compound
Supported bis(phosphorus) ligands are disclosed for use in a variety of catalytic processes, including the isomerization, hydrogenation, hydroformylation, and hydrocyanation of unsaturated organic compounds. Catalysts are formed when the ligands are combined with a catalytically active metal, such as nickel.
Owner:INVISTA NORTH AMERICA R L
Multidentate phosphite ligands, catalytic compositions containing such ligands, and catalytic prosses utilizing such catalytic compositions
InactiveUS20030023110A1Organic compound preparationOrganic chemistry methodsIsomerizationOrtho position
Multidentate phosphite ligands are disclosed for use in reactions such as hydrocyanation and isomerization. The catalyst compositions made therefrom and the various catalytic processes which employ such multidentate phosphite ligands are also disclosed. In particular, the ligands have heteroatom-containing substituents on the carbon attached to the ortho position of the terminal phenol group.
Owner:INVISTA NORTH AMERICA R L
Hydrocyanation of pentenenitriles
ActiveUS20090182164A1Organic compound preparationPreparation by hydrogen cyanide additionHydrogenHydrocyanation
The invention provides a hydrocyanation process to produce adiponitrile and other dinitriles having six carbon atoms, in the presence of catalyst composition comprising a zero-valent nickel and at least one bidentate phosphorus-containing ligand wherein the bidentate phosphorus-containing ligand gives acceptable results according to at least one protocol of the 2-Pentenenitrile Hydrocyanation Test Method.
Owner:INV NYLON CHEM AMERICAS LLC
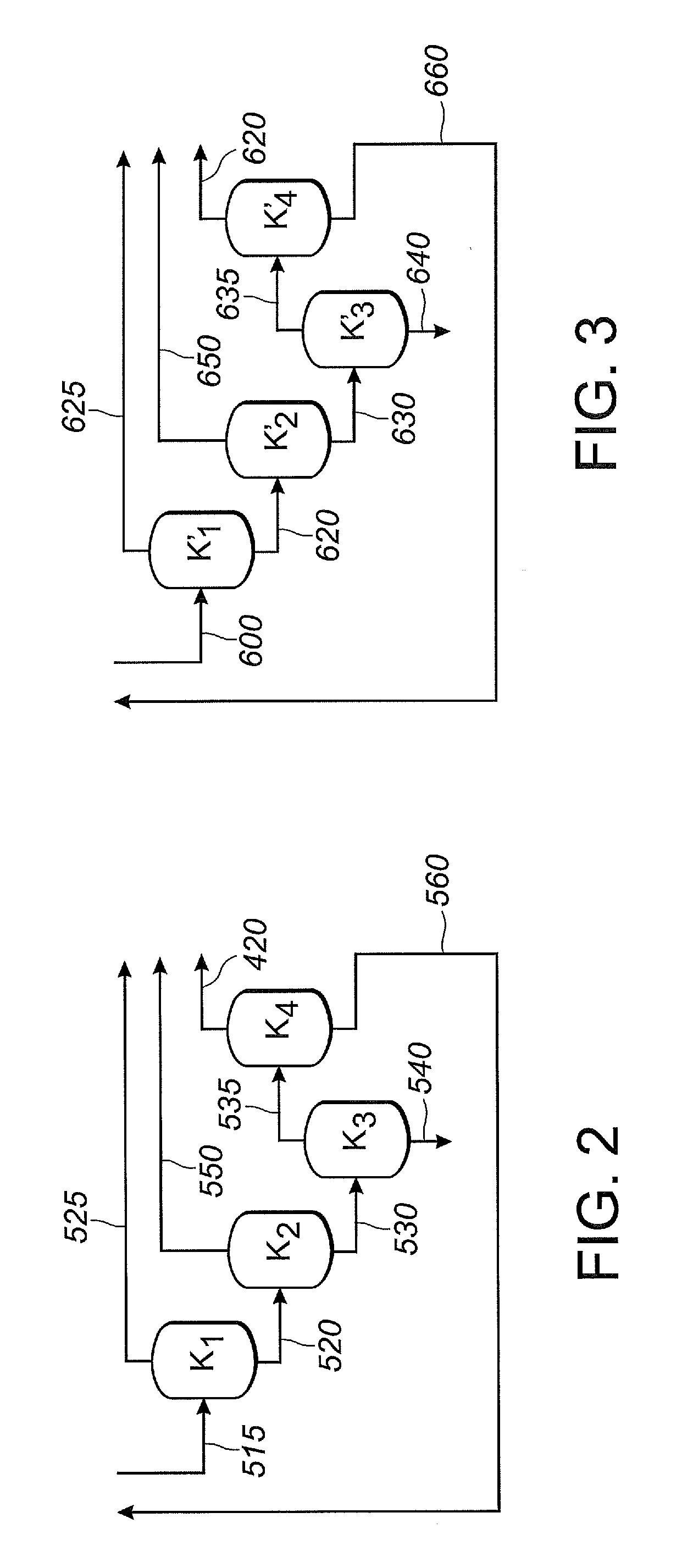
Hydrocyanation process with reduced yield losses
ActiveUS20080015381A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionNitriteHydrogen
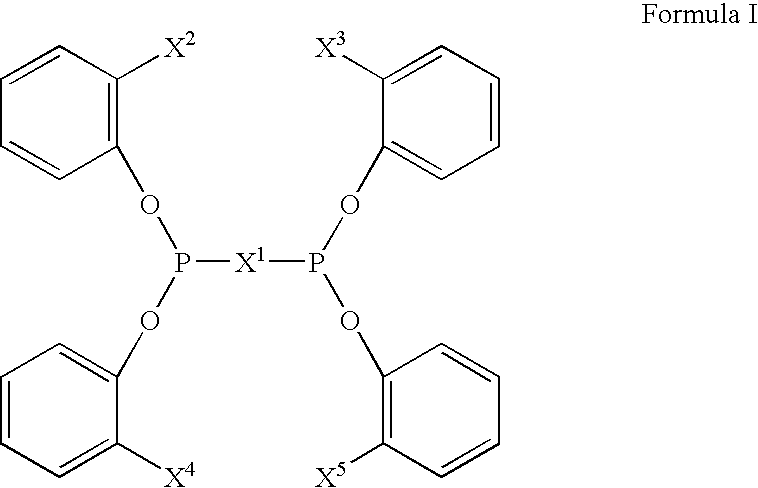
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitrites having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitrites, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitrites, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed)(molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one bidentate phosphite ligand; andwherein the bidentate phosphite ligand is selected from a member of the group represented by Formulas I and 11 as described herein.
Owner:INV NYLON CHEM AMERICAS LLC
Process of synthesis of compounds having nitrile functions from ethylenically unsaturated compounds
InactiveUS7084293B2High selectivityReduce disadvantagesOrganic compound preparationGroup 8/9/10/18 element organic compoundsButadiene DioxideAlkyl nitrites
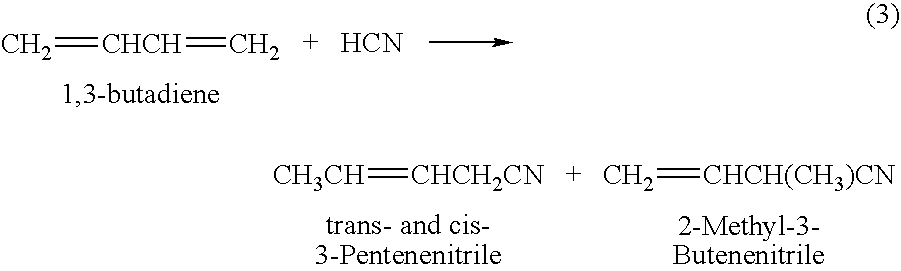
The present invention relates to a process of hydrocyanation of ethylenically unsaturated organic compounds to compounds having at least one nitrile function, particularly the hydrocyanation of diolefins such as butadiene, or of substituted olefins such as alkene nitrites such as pentenenitriles; the subject hydrocyanation is carried out in the presence of a catalytic system comprising a metallic element and mono- and pluri-dentate organophosphorus ligands.
Owner:INV NYLON CHEM AMERICAS LLC
Heteroarylic-arylic diphosphines as chiral ligands
InactiveUS6153758ACarboxylic acid esters preparationNickel organic compoundsCarbon–carbon bondDiphosphines
PCT No. PCT / EP97 / 06358 Sec. 371 Date Apr. 18, 1999 Sec. 102(e) Date Apr. 18, 1999 PCT Filed Nov. 14, 1997 PCT Pub. No. WO98 / 22484 PCT Pub. Date May 28, 1998Diphosphines of a mixed heteroarylic-arylic type, wherein the phosphine group carrying backbone is constituted by the interconnection of a five-atom heteroaromatic ring and a carbocyclic aromatic ring, forming an atropoisomeric chiral system with a C1 symmetry. Said chiral diphosphines are advantageously used as ligands for the formation of chiral complexes with transition metals, in particular Ru, Rh, Pd, Ir, Ni. The so-obtained chiral complexes are used as chiral complexes are used as chiral catalysts for stereocontrolled reactions, in particular diastereo and enantioselective reduction reactions, hydroformylation reactions, hydrosilylation reactions, hydrocyanation reactions, double-bond isomerisation reactions, other reactions of carbon-carbon bond formation.
Owner:CHEMI SPA
Hydrocyanation process with reduced yield losses
ActiveUS20080015382A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionHydrogenPhosphite ester
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitriles, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed) / (molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one multidentate phosphorus-containing ligand;wherein the multidentate phosphorus-containing ligand is selected from the group consisting of a phosphite, a phosphonite, a phosphinite, a phosphine, and a mixed phosphorus-containing ligand or a combination of such members; andwherein the multidentate phosphorus-containing ligand gives acceptable results according to at least one protocol of the 2-Pentenenitrile Hydrocyanation Test Method.
Owner:INV NYLON CHEM AMERICAS LLC
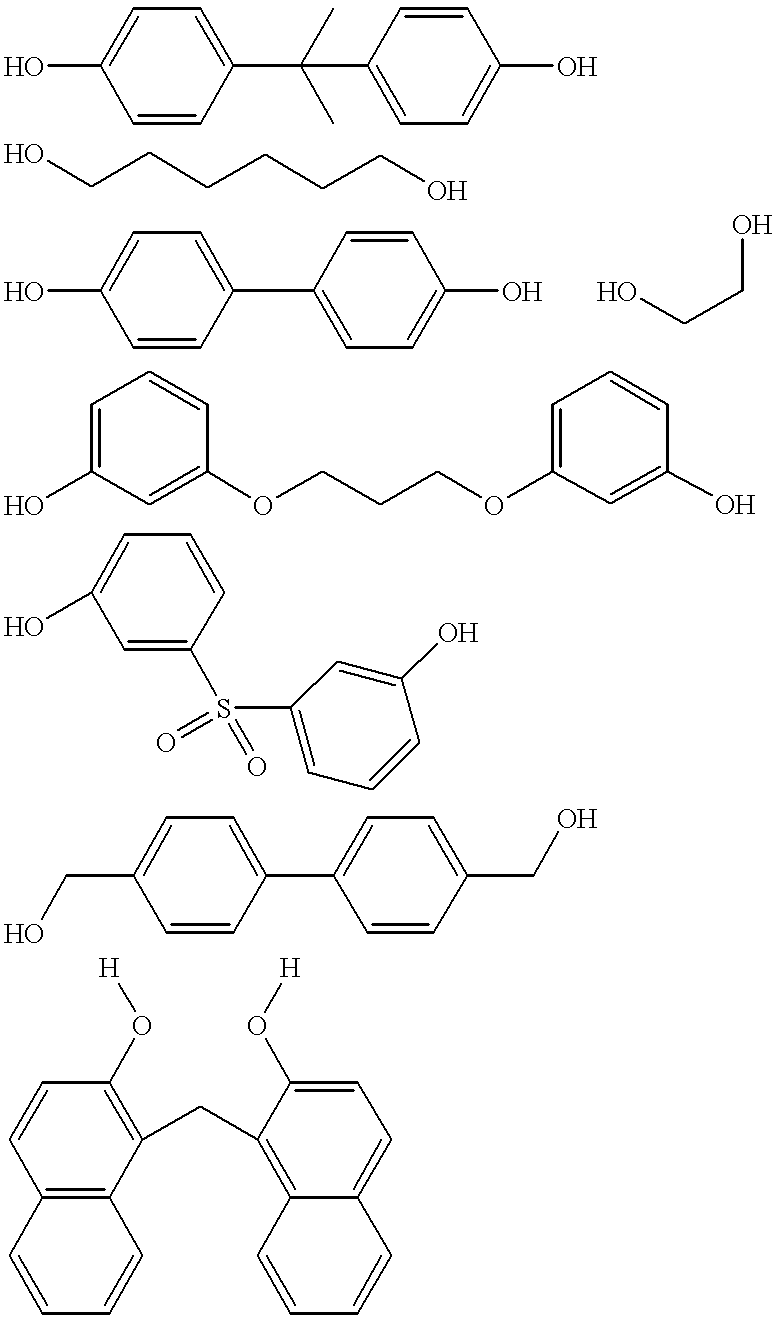
Polymeric phosphite composition and hydrocyanation of unsaturated organic compounds and the isomerization of unsaturated nitriles
InactiveUS6855799B2Reduce solubilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsIsomerizationOrganic compound
A polymeric composition, a process for producing the composition, and a process for using the composition in, for example, hydrocyanation or isomerization are disclosed. The composition comprises repeat units derived from (1) a carbonyl compound, a monomer, and phosphorochloridite; (2) phosphorus trichloride, a polyhydric alcohol, and an aromatic diol; or (3) combinations of (1) and (2) in which the monomer can be a polyhydric alcohol, an amine, combinations thereof. The composition can further comprise a Group VIII metal and optionally a Lewis acid. The composition can be produced by (1) contacting a carbonyl compound with the monomer to produce an intermediate and contacting the intermediate with phosphorochloridite; (2) contacting phosphorus trichloride with a second polyhydric alcohol under a condition sufficient to produce a phosphorus-containing polymer and contacting the phosphorus-containing polymer with an aromatic diol; or (3) contacting an N,N-dialkyl dichlorophosphoramidite with a second polyhydric alcohol to produce a polymer phosphoramidite, contacting the polymer phosphoramidite with an acid such as HCl to produce the phospphorus-containing polymer, which is then contacted with an aromatic diol. The composition can be used as catalyst, for example, for converting an unsaturated organic compound to a nitrile and isomerizing a nitrile.
Owner:INVISTA NORTH AMERICA R L
Hydrocyanation process with reduced yield losses
ActiveUS7659422B2Investment exemptionSelective and efficient and stableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenHydrogen cyanide
A hydrocyanation process produces adiponitrile and other dinitriles having six carbon atoms. The process involves forming a reaction mixture in the presence of at least one Lewis acid. The reaction mixture includes ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition. The reaction mixture is continuously fed while controlling the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles. In the reaction product mixture, including adiponitrile, the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles is from about 0.2 / 1 to about 10 / 1. Included in the catalyst precursor composition is a zero-valent nickel and at least one bidentate phosphite ligand.
Owner:INV NYLON CHEM AMERICAS LLC
Calcination and reduction process including a fluidizing bed reactor
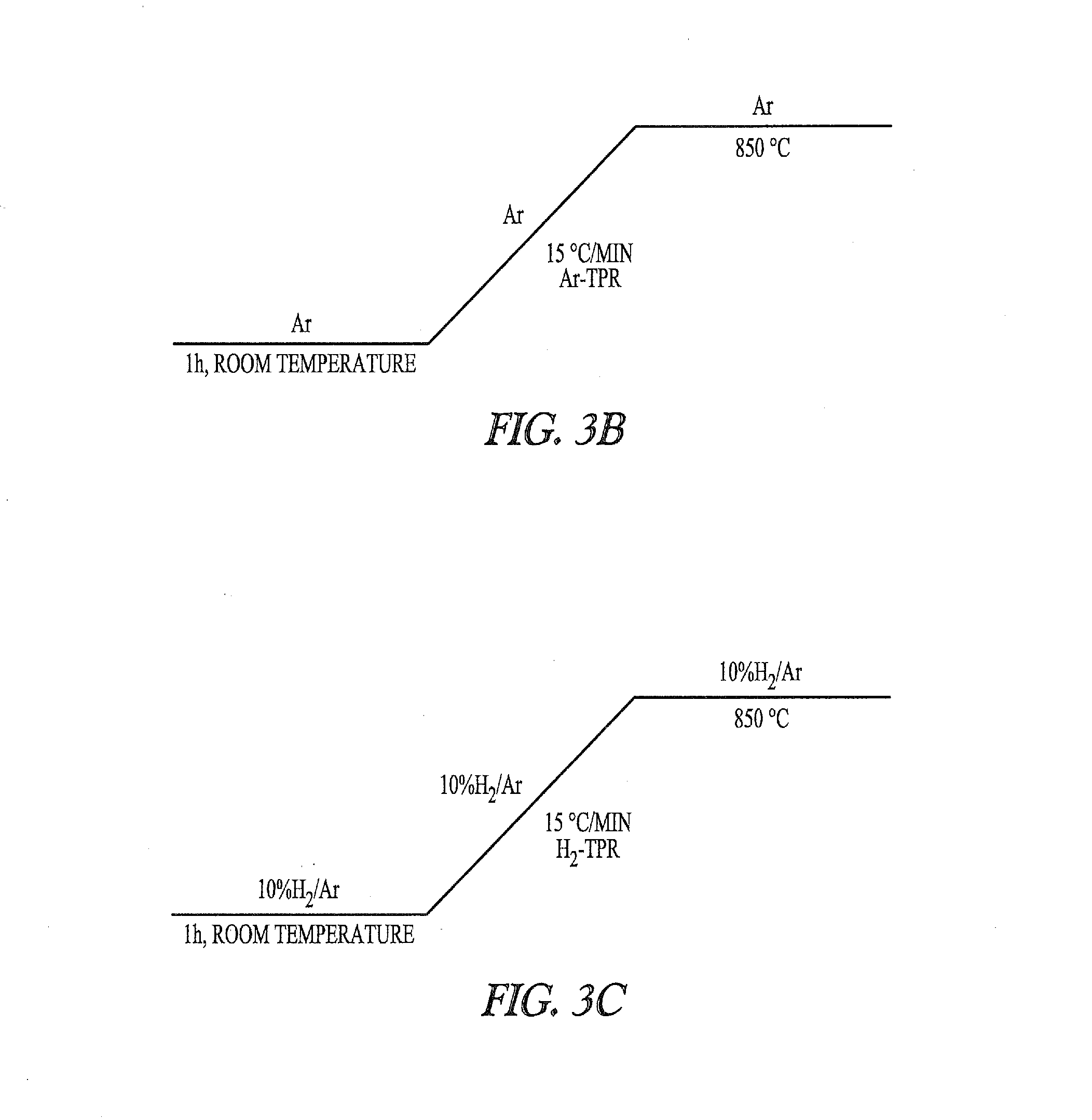
ActiveUS20130144079A1Organic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsPtru catalystFluidized bed
These disclosures relate to preparing nickel metal (Ni(0)) suited for use in catalyst systems, such as nickel complexes with phosphorus-containing ligands, useful to catalyze the hydrocyanation of ethylenically unsaturated compounds. The methods described herein can include use of steam during reduction of nickel.
Owner:INV NYLON CHEM AMERICAS LLC
Process of synthesis of compounds having nitrile functions from ethylenically unsaturated compounds
InactiveUS20060252955A1High selectivityReduce instabilityOrganic compound preparationPreparation by hydrogen cyanide additionIsomerizationHCN poisoning
A process of hydrocyanation of diolefins such as butadiene is carried out in the presence of a catalytic system comprising a transition metal and mono- and pluri-dentate organophosphorus ligands. The reaction medium containing branched nitrites subsequently is isomerized in the absence of hydrogen cyanide.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation of pentenenitriles
ActiveUS8088943B2Organic compound preparationPreparation by hydrogen cyanide additionHydrogenHydrocyanation
Owner:INV NYLON CHEM AMERICAS LLC
Metal-ligand catalyst formation
ActiveUS20130143730A1Excellent complex formation propertyForm is therefore complexOrganic-compounds/hydrides/coordination-complexes catalystsSynthetic resin layered productsSulfurMetallic Nickel
As described herein, nickel treated with sulfur provides a surprisingly effective source of nickel atoms for generating nickel-phosphorus-containing ligand complexes useful as hydrocyanation catalysts.
Owner:INV NYLON CHEM AMERICAS LLC
Separation of nickel(0) complexes and phosphorus-containing ligands from nitrile mixtures
InactiveCN1914216AImprove stabilityGood choiceOrganic compounds purification/separation/stabilisationOrganic-compounds/hydrides/coordination-complexes catalystsHydrocyanationNitrile
The invention relates to a method for the extractive separation of nickel(0) complexes and phosphorus-containing ligands and / or free phosphorus-containin g ligands from a reaction discharge of a hydrocyanation of unsaturated mononitries to dinitriles, by means of a hydrocarbon, the hydrocarbon and the reaction discharge being separated into two phases at a temperature T (DEG C). The invention is characterised in that the content of nickel(0) complexes and phosphorus-containing ligands and / or free phosphorus-containing ligands in the reaction discharge of the hydrocyanation is at least y wt%, depending on the temperature T, and a maximum of 60 wt% independently of the temperature T. The numerical value of the minimum content y is indicated by the equation y = 0.5 T + 20, T being a dimensionless numerical value.
Owner:BASF AG
Nickel form for preparation of catalytic nickel-ligand complexes
ActiveUS20130144082A1Form is therefore complexAvoid wastingNickel organic compoundsGlass/slag layered productsParticulatesGram
A novel nickel particulate form is provided that efficiently forms a zero-valent nickel complex with a phosphorus-containing ligands in an organic liquid to form a hydrocyanation catalyst. Particles in the nickel particulate form comprise nickel crystallites. For example, the nickel particulate form can have a BET Specific Surface Area of at least about 1 m2 / gm; an average crystallite size less than about 20-25 nm, the nickel particulate form can have at least 10% of the crystallites in the nickel form can have can have a diameter (C10) of less than about 10 nm, and / or there are on average at least about 1015 surface crystallites per gram nickel. A ratio of BET SSA to C50 for the nickel particulate form can be at least about 0.1×109 m / gm and preferably at least about 0.4×109 m / gm. Methods of preparation and use are also provided.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation method for ethylenically unsaturated organic compounds
The invention concerns a hydrocyanation method for ethylenically unsaturated organic compounds in particular to obtain nitriles, and more particularly hydrocyanation of substituted diolefins or of olefins for producing dinitriles, and / or the isomerization of nitriles obtained by hydrocyanation. The invention more particularly concerns hydrocyanation catalysed by a nickel-based compound. Thus the catalyst used in said production method is treated in output with hydrogen cyanide to recover and re-dissolve the nickel precipitated in the form of nickel hydroxide. The method enables to regenerate and prolong the life span of a catalyst charge. Moreover, it enables to reduce pollution in the installations using said method.
Owner:RHODIA POLYAMIDE INTERMEDIATES
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
ActiveCN1735460AOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsChlorideSolvent
A process for preparing a hydrocyanation catalyst comprising contacting a bidentate phosphorous-containing ligand with nickel chloride in the presence of a nitrile solvent and a reducing metal which is more electropositive than nickel the nickel chloride being introduced as an aqueous solution and the water being removed concurrently with the reduction reaction by azeotropic distillation.
Owner:INVISTA TECHNOLOG IES S A R L
Hydrocyanation of 2-pentenenitrile
ActiveUS20080015379A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenHydrocyanation
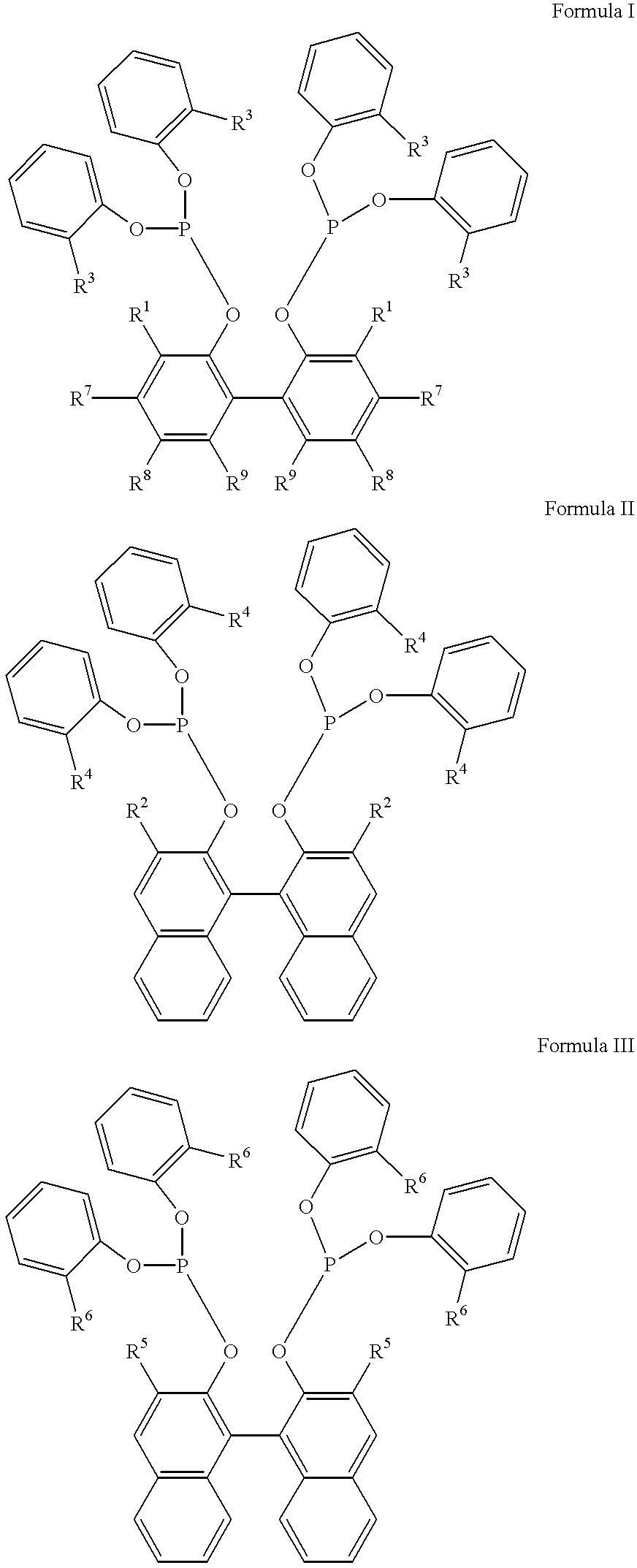
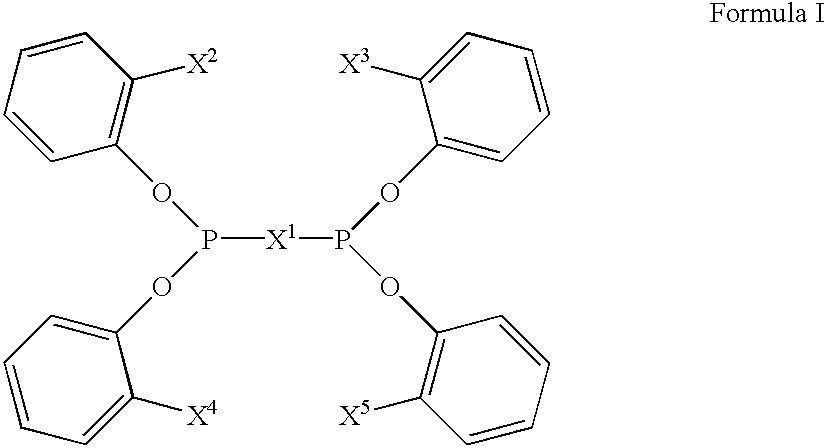
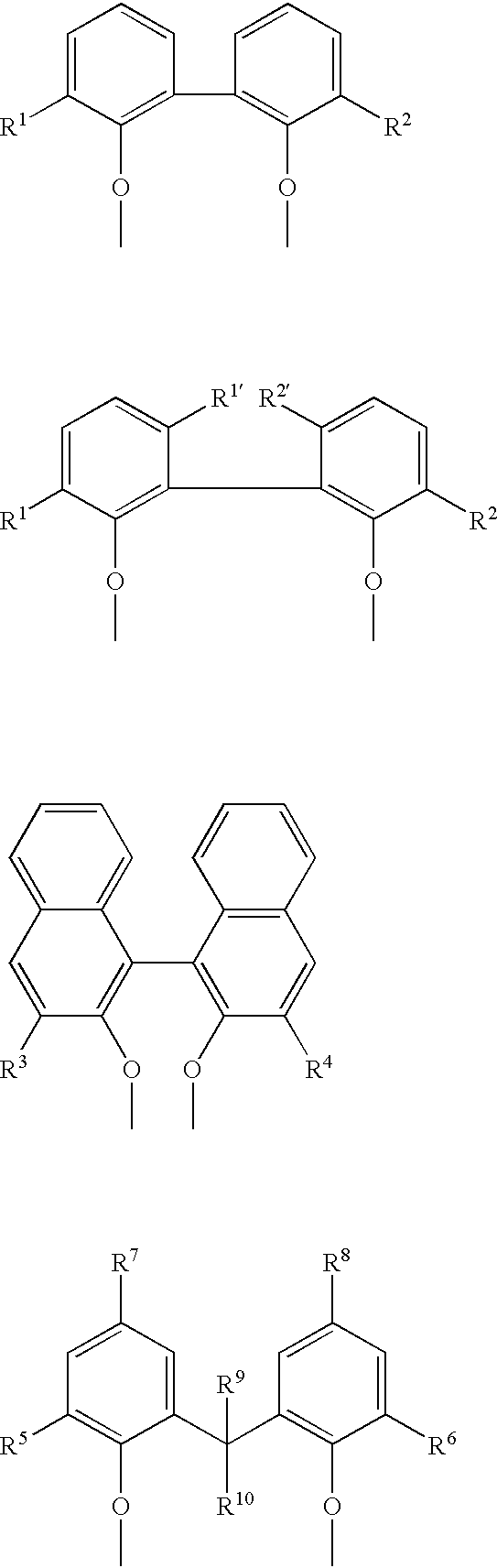
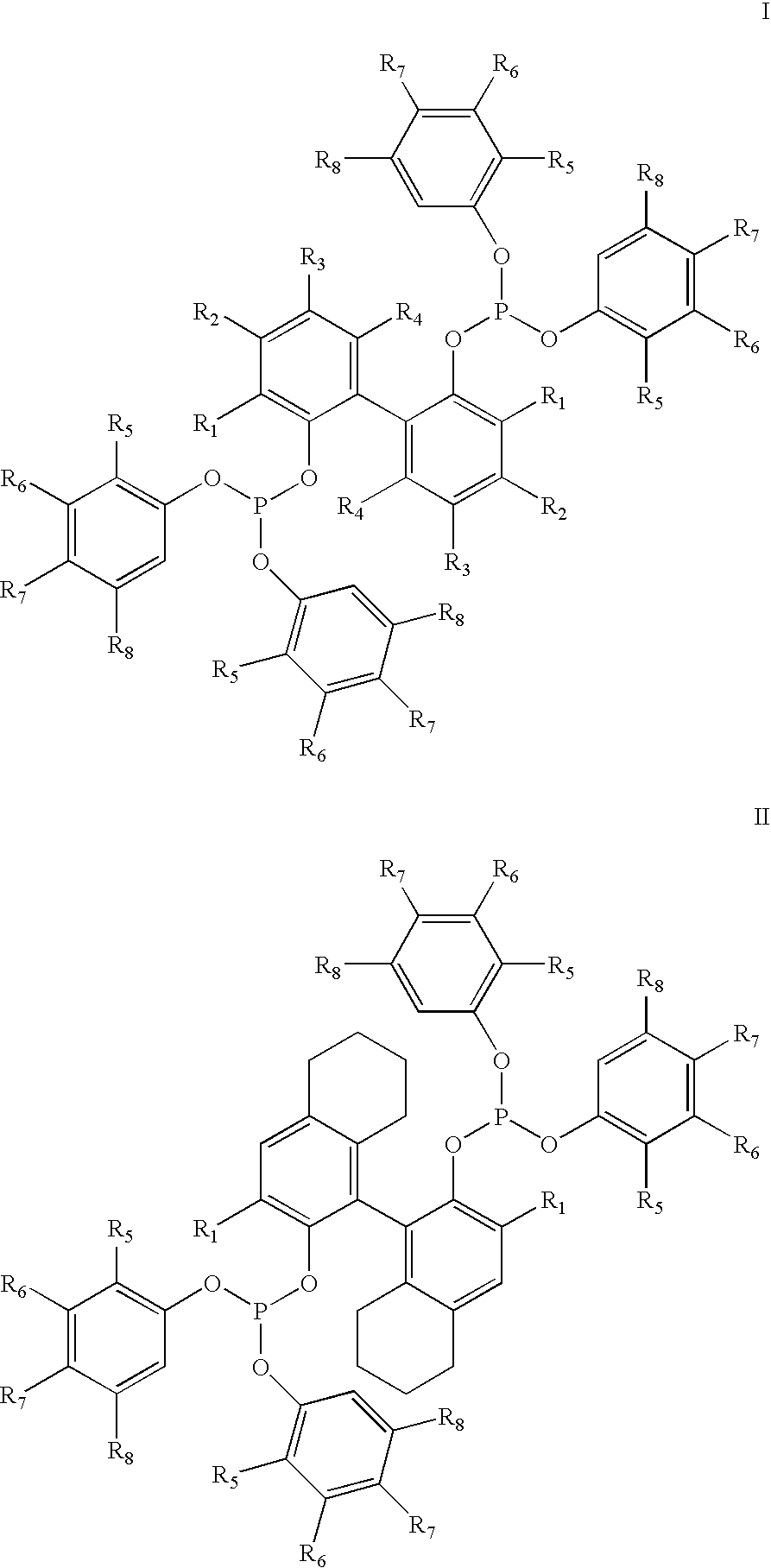
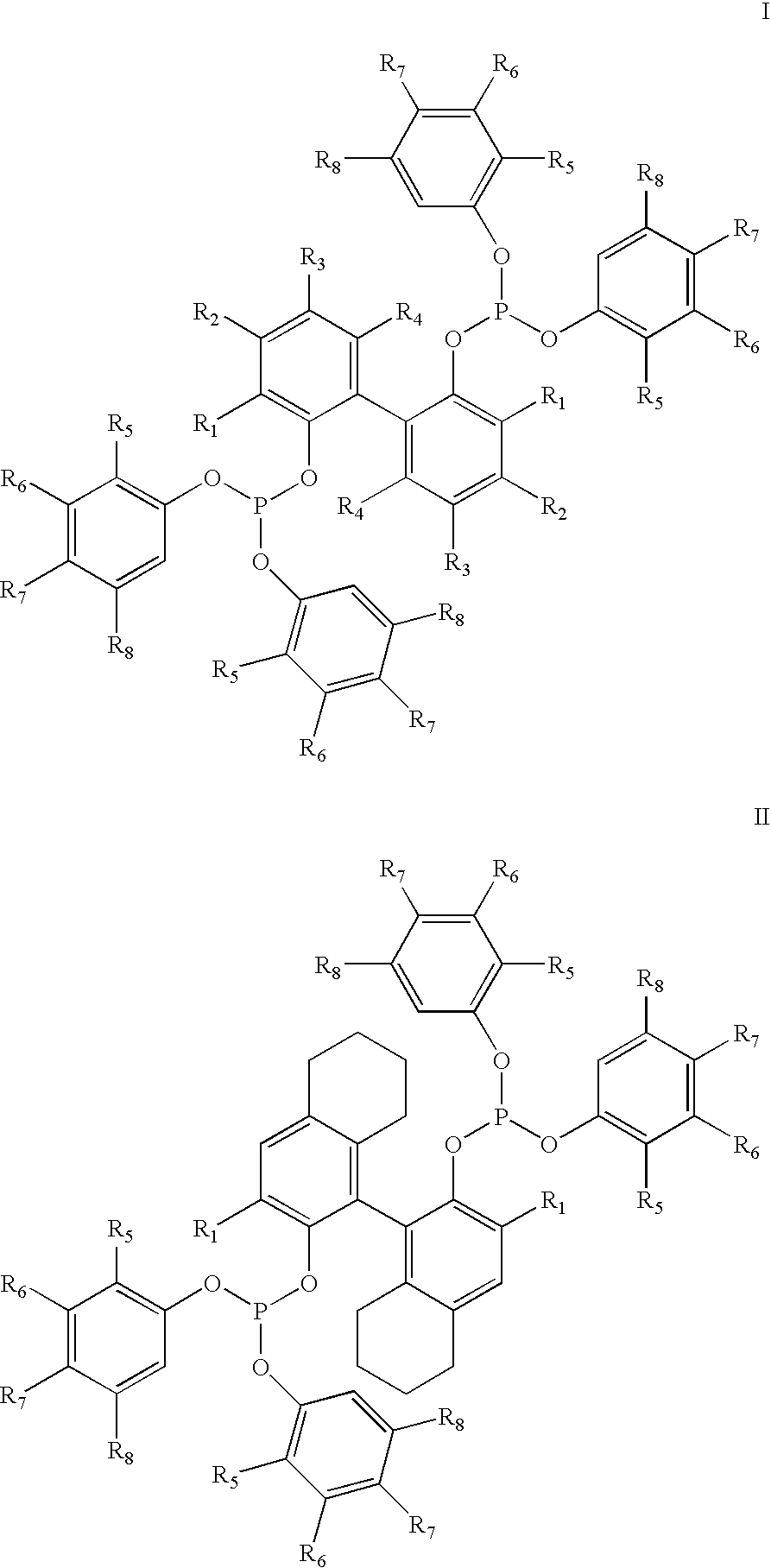
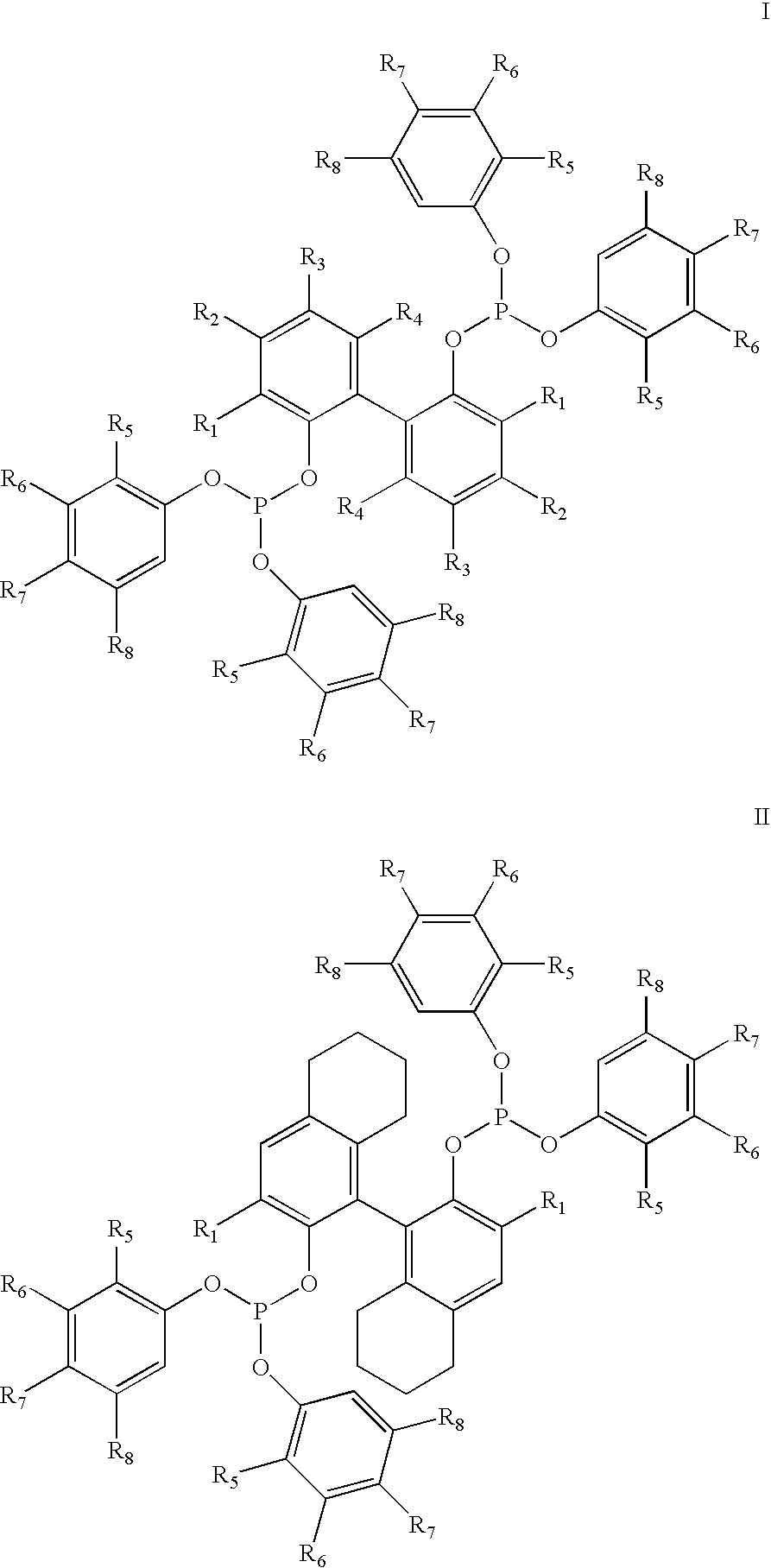
The invention provides a process for hydrocyanation, comprising: contacting 2-pentenenitrile with hydrogen cyamide at a temperature in the range of about 0° C. to about 150° C. in the presence of at least one Lewis acid promoter and a catalyst precursor composition, wherein the catalyst precursor composition comprises a zero-valent nickel and at least one bidentate phosphite ligand selected from a member of the group represented by Formula I and Formula II, in which all like reference characters have the same meaning, except as further explicitly limited:wherein R1 and R5 are independently selected from the group consisting of C1 to C5 hydrocarbyl; and R2, R3, R4, R6, R7 and R8 are independently selected from the group consisting of H and C1 to C4 hydrocarbyl.
Owner:INV NYLON CHEM AMERICAS LLC
Polymeric phosphite composition and hydrocyanation of unsaturated organic compounds and the isomerization of unsaturated nitriles
InactiveCN1390241AControl solubilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsIsomerizationOrganic compound
A polymeric composition, a process for producing the composition, and a process for using the composition in, for example, hydrocyanation or isomerization are disclosed. The composition comprises repeat units derived from (1) a carbonyl compound, a monomer, and phosphorochloridite; (2) phosphorus trichloride, a polyhydric alcohol, and an aromatic diol; or (3) combinations of (1) and (2) in which the nonomer can be a polyhydric alcohol, an amine, combinations thereof. The composition can further comprise a Group VIII metal and optionally a Lewis acid. The composition can be produced by (1) contacting a carbonyl compound with the monomer to produce an intermediate and contacting the intermediate with phosphorochloridite; (2) contacting phosphorus trichloride with a second polyhydric alcohol under a condition sufficient to produce a phosphorus-containing polymer and contacting the phosphorus-containing polymer with an aromatic diol; or (3) contacting an N, N-dialkyl dichlorophosphoramidite with a second polyhydric alcohol to produce a polymer phosphoramidite, contacting the polymer phosphoramidite with an acid such as HC1 to produce the phosphorus-containing polymer, which is then contacted with an aromatic diol. The composition can be used as catalyst, for example, for converting an unsaturated organic compound to a nitrile and isomerizing a nitrile.
Owner:INVISTA TECHNOLOG IES S A R L
Novel tridentate phosphine ligand and application of same to linear hydroformylation and similar reactions
ActiveCN102875599AEasy to synthesizeHigh yieldGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalysts1-OcteneFormylation reaction
The invention discloses a novel tridentate phosphine ligand and application of the same to linear hydroformylation and similar reactions. The tridentate phosphine ligand is applied to efficient high-selectivity hydroformylation for the first time at present and applicable to efficient high-selectivity isomerized hydroformylation, hydrocarboxylation, hydrocyanation, isomerized formylation, hydroaminomethylation and the similar reactions. During the linear hydroformylation, selectivity of the tridentate phosphine ligand is obviously higher than that of a corresponding bidentate phosphine ligand. Besides, compared with a corresponding quadridentate phosphine ligand, the tridentate phosphine ligand is approximate in selectivity, easier to synthesize and higher in yield and displays higher TON (turnover number). The novel tridentate phosphine ligand selectively converts 1-hexylene and 1-octylene into n-heptaldehyde (used for synthesis of orange essence and rose essence) and n-nonaldehyde (flavoring matter) and is of great significance to hydroformylation-based industrial production.
Owner:WUHAN UNIV
Process for making nitriles
ActiveUS20130150610A1Minimize consequencesOrganic compound preparationChemical recyclingHydrogen cyanideHydrocyanation
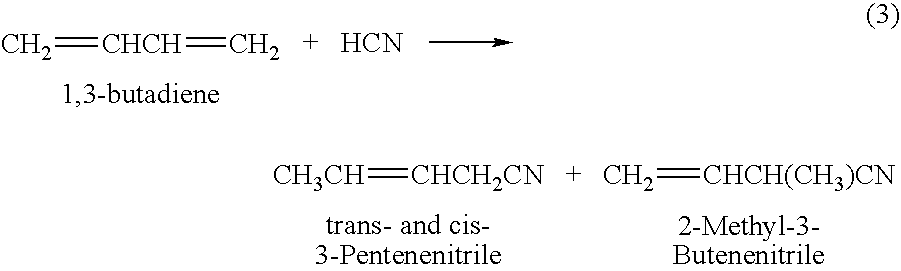
A hydrocyanation reaction is used to react 1,3-butadiene with hydrogen cyanide in the presence of a catalyst to produce pentenenitriles, as well as reaction byproducts, such as methylglutaronitrile (MGN). The effluent from the hydrocyanation reaction is distilled in a particular manner to produce a pentenenitrile-enriched stream, a catalyst-enriched stream and a stream enriched in methylglutaronitrile (MGN). At least a portion of the catalyst enriched stream may be recycled to the hydrocyanation reaction. 3-pentenenitrile may be recovered and, optionally, further reacted with HCN to make adiponitrile (ADN).
Owner:INV NYLON CHEM AMERICAS LLC
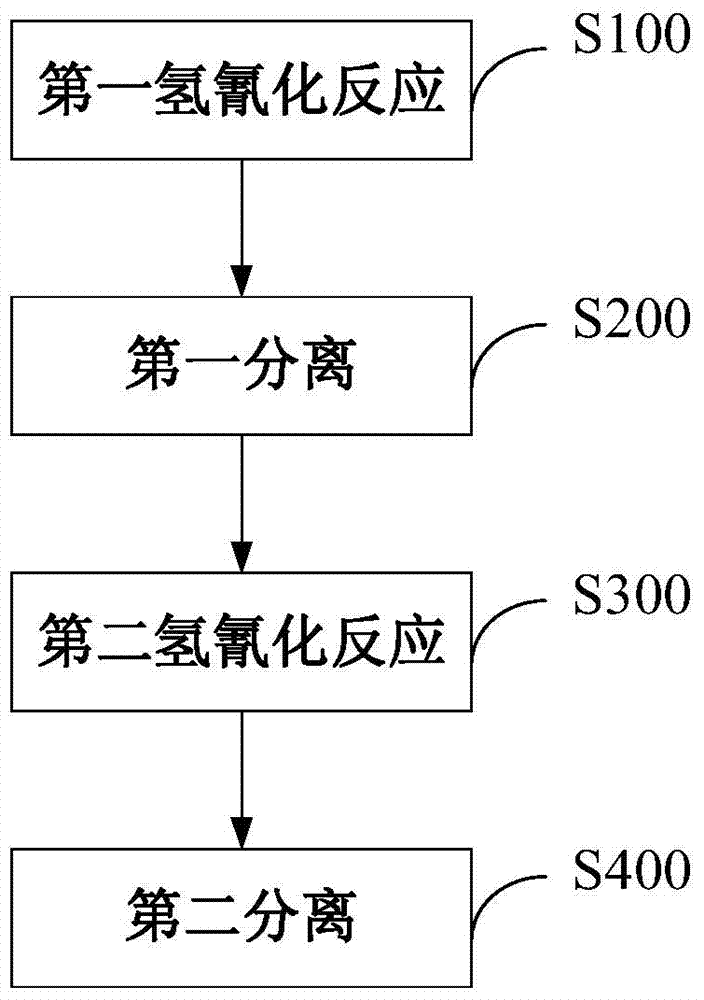
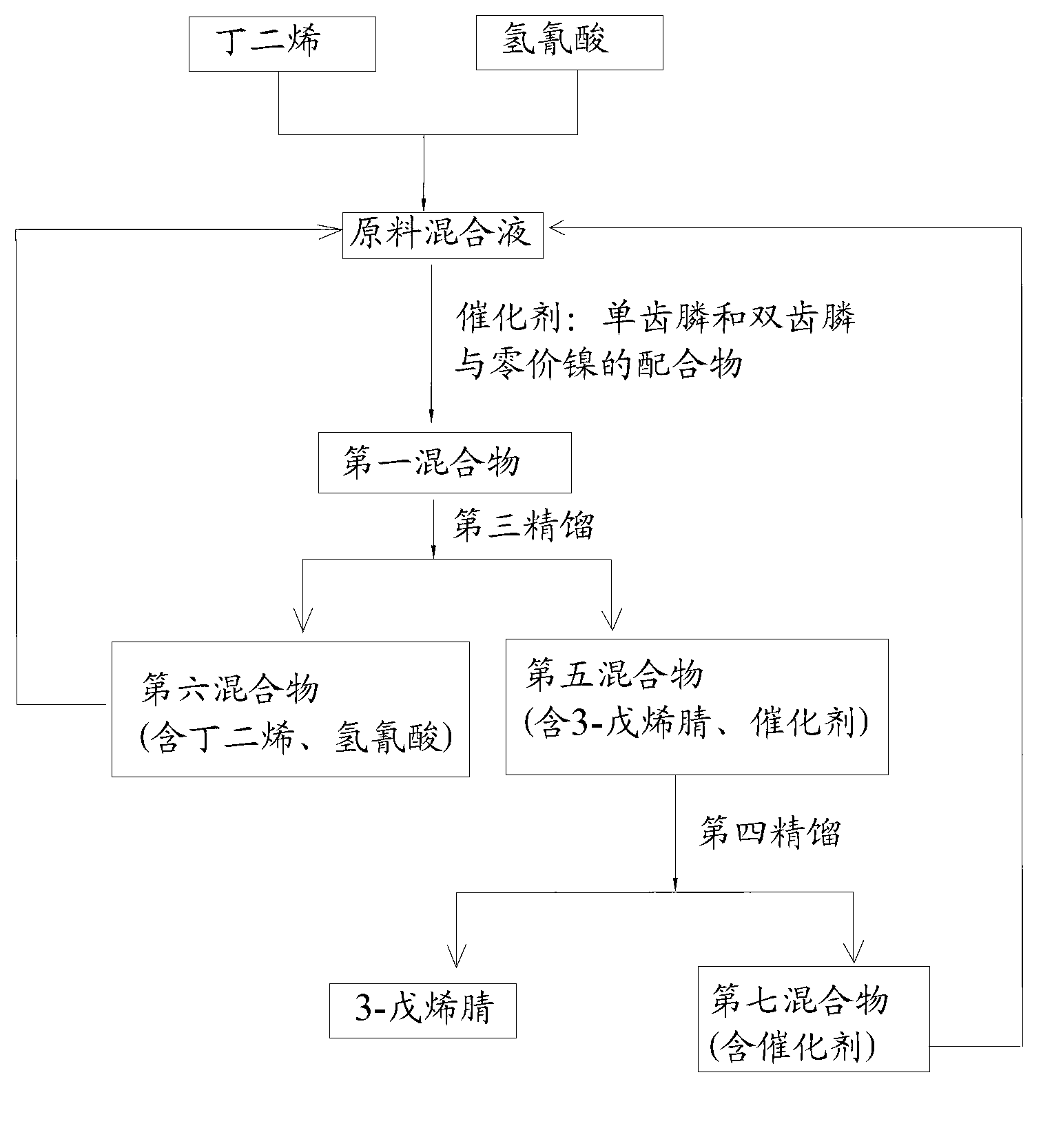
Method for preparing adiponitrile
ActiveCN103664691AHigh purityQuality improvementPreparation by hydrogen cyanide additionIsomerizationPhosphine
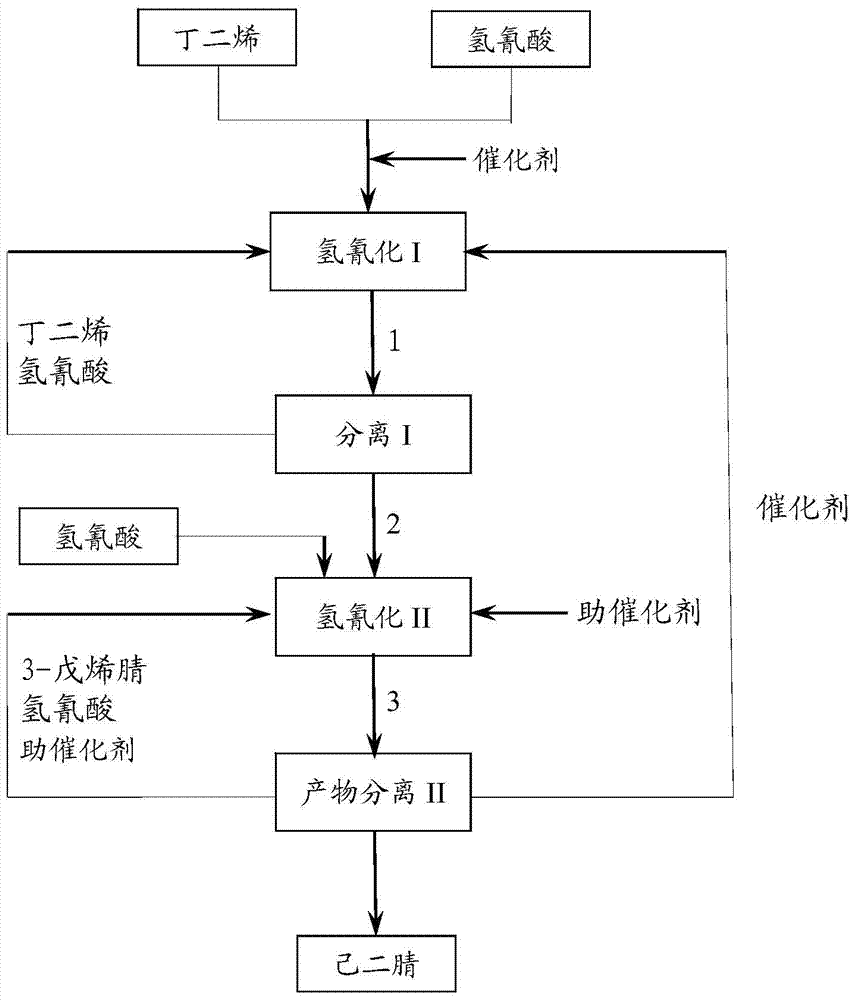
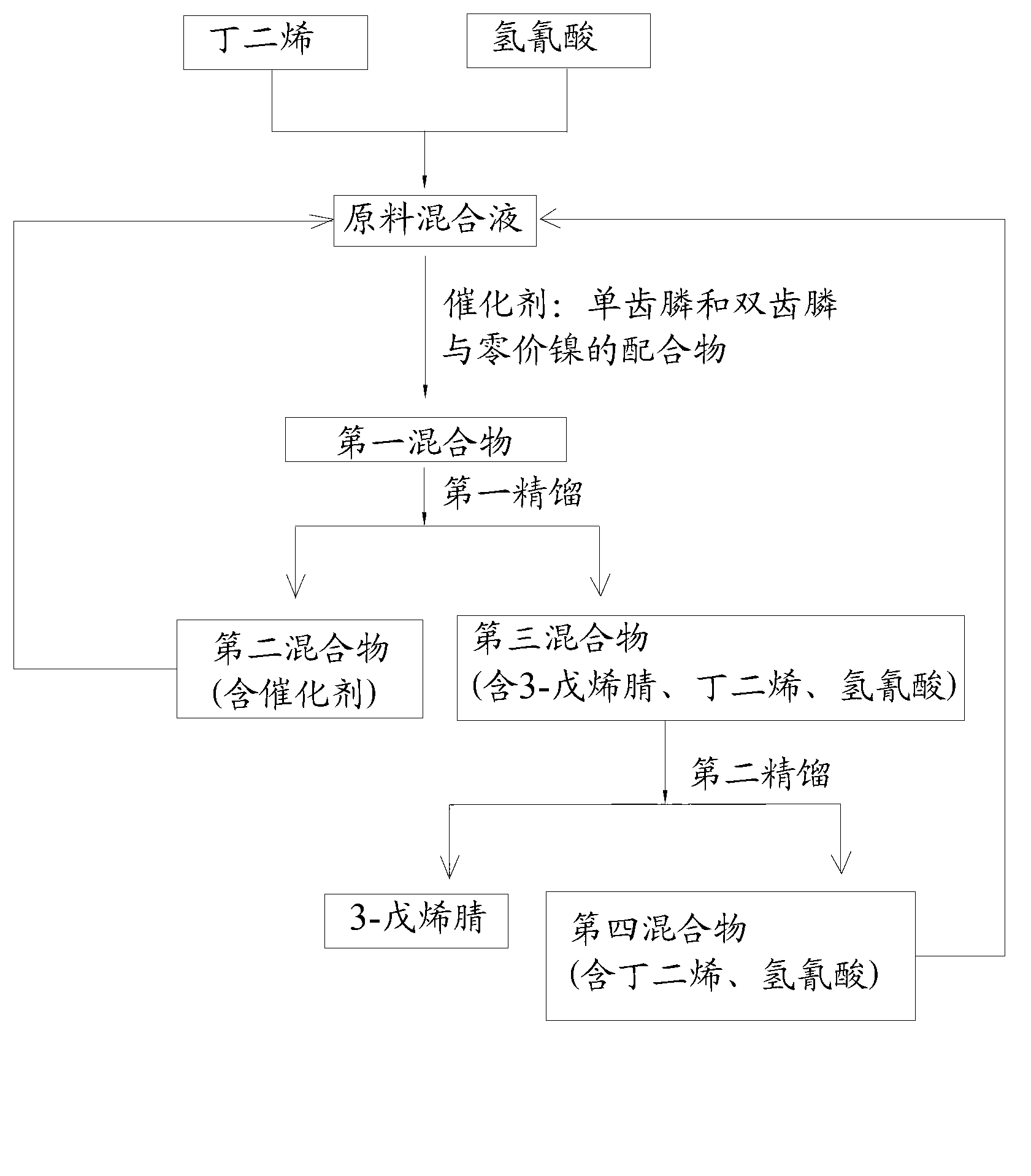
The invention discloses a method for preparing adiponitrile. The method comprises the following steps: performing a first hydrocyanation reaction on butadiene and first hydrocyanic acid under the action of a catalyst to obtain a first mixture; performing primary separation on each component in the first mixture to obtain a second mixture, residual butadiene and residual first hydrocyanic acid respectively; adding a co-catalyst into the second mixture, and introducing second hydrocyanic acid to obtain a third mixture containing the adiponitrile; performing secondary separation on the third mixture to obtain the adiponitrile, residual 3-pentenenitrile, residual second hydrocyanic acid, the reacted catalyst and the reacted co-catalyst, wherein the catalyst is a coordination compound consisting of a monodentate phosphine ligand, a bidentate phosphine ligand and zerovalent nickel, and the co-catalyst is Lewis acid. The method for preparing the adiponitrile has the advantages of small quantity of side products, no need of performing a 2M3BN isomerization reaction, simple process, low cost, high yield, good product quality, economical efficiency and environmental friendliness.
Owner:ANHUI ANQING SHUGUANG CHEM GRP +1
Preparation method of 3-pentenenitrile and preparation method of adiponitrile
ActiveCN103012197AEfficient preparationCheap manufacturingPreparation by hydrogen cyanide additionButadiene DioxidePhosphine
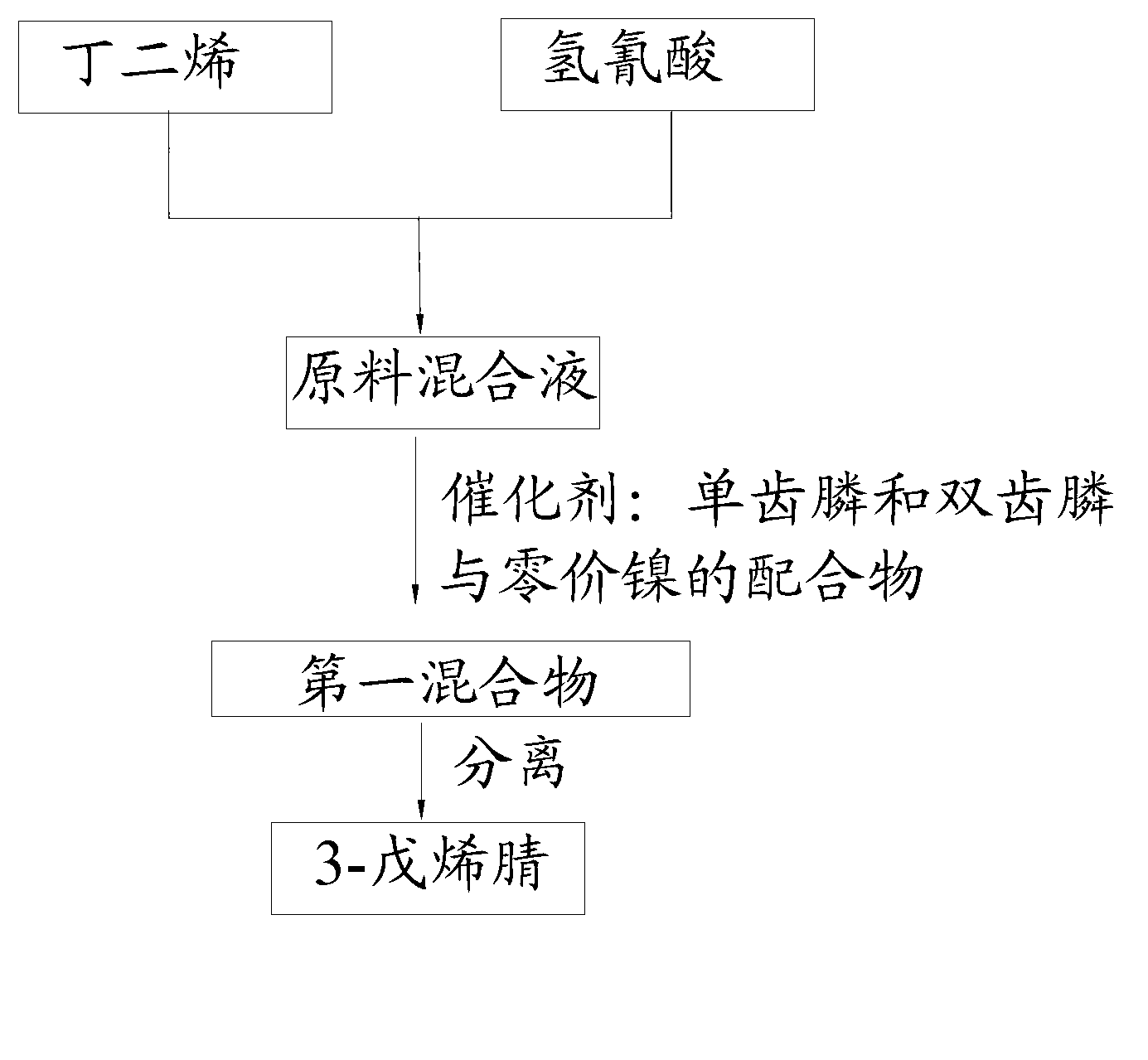
The invention provides a preparation method of 3-pentenenitrile and a preparation method of adiponitrile. The preparation method of 3-pentenenitrile comprises the following steps: 1, performing the hydrocyanation reaction on raw material mixed solution of butadiene and hydrocyanic acid under the action of a catalyst so as to obtain a first mixture containing the 3-pentenenitrile; and 2, carrying out separation on the first mixture to obtain the 3-pentenenitrile, wherein the catalyst is a coordination complex consisting of monodentate phosphine, bidentate phosphine and zero-valent nickel. By adopting the method, the 3-pentenenitrile can be effectively, efficiently and economically prepared by using the butadiene and the hydrocyanic acid as the raw materials; the integral process is safe and reliable; the flow is simple; and moreover, production cost and equipment investment can be obviously reduced.
Owner:ANHUI ANQING SHUGUANG CHEM GRP +1
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
ActiveCN100348322COrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsChlorideSolvent
A process for preparing a hydrocyanation catalyst comprising contacting a bidentate phosphorous-containing ligand with nickel chloride in the presence of a nitrile solvent and a reducing metal which is more electropositive than nickel the nickel chloride being introduced as an aqueous solution and the water being removed concurrently with the reduction reaction by azeotropic distillation.
Owner:INVISTA TECHNOLOG IES S A R L
Method of recovering fumed silica from spent potliner
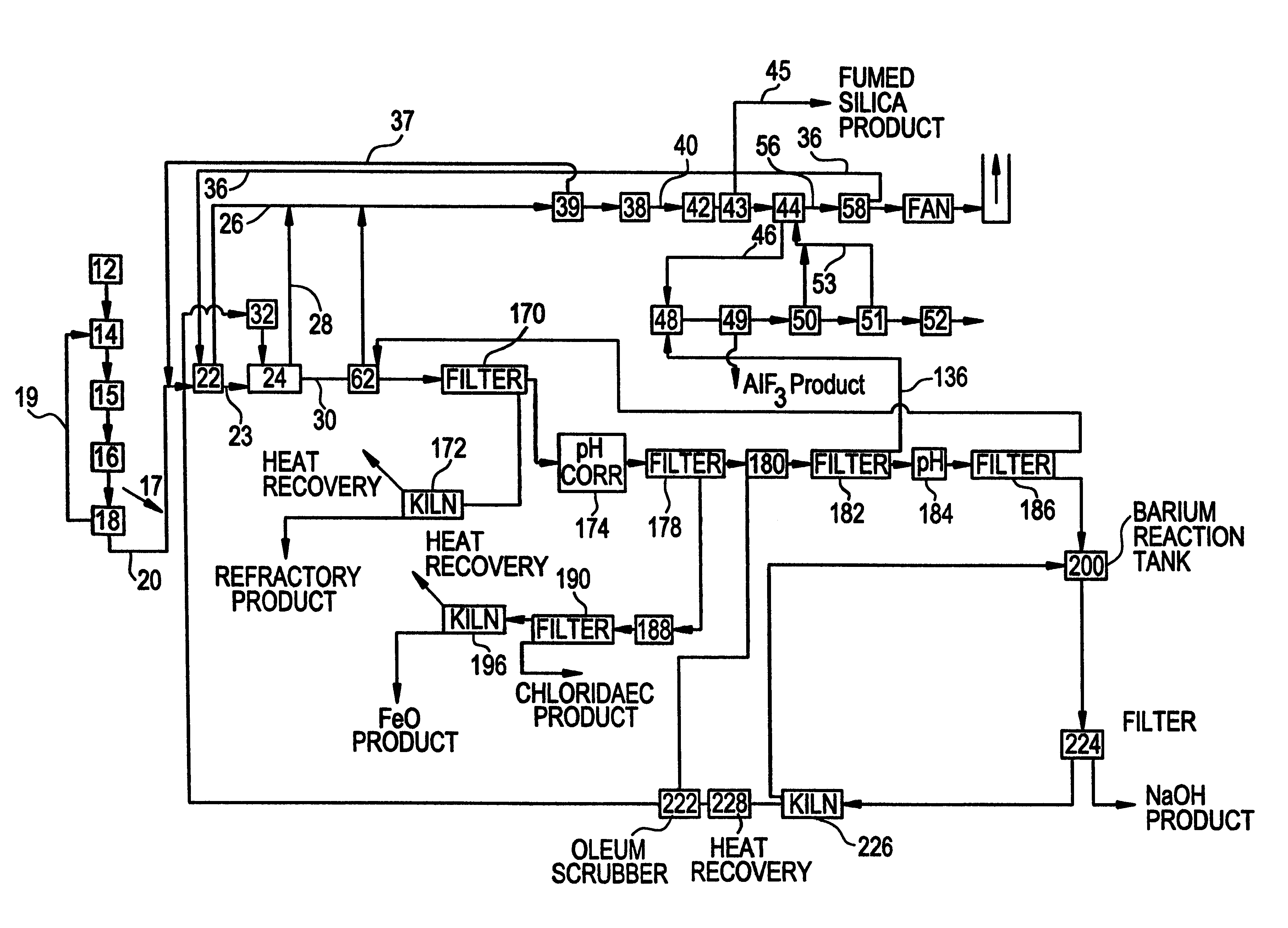
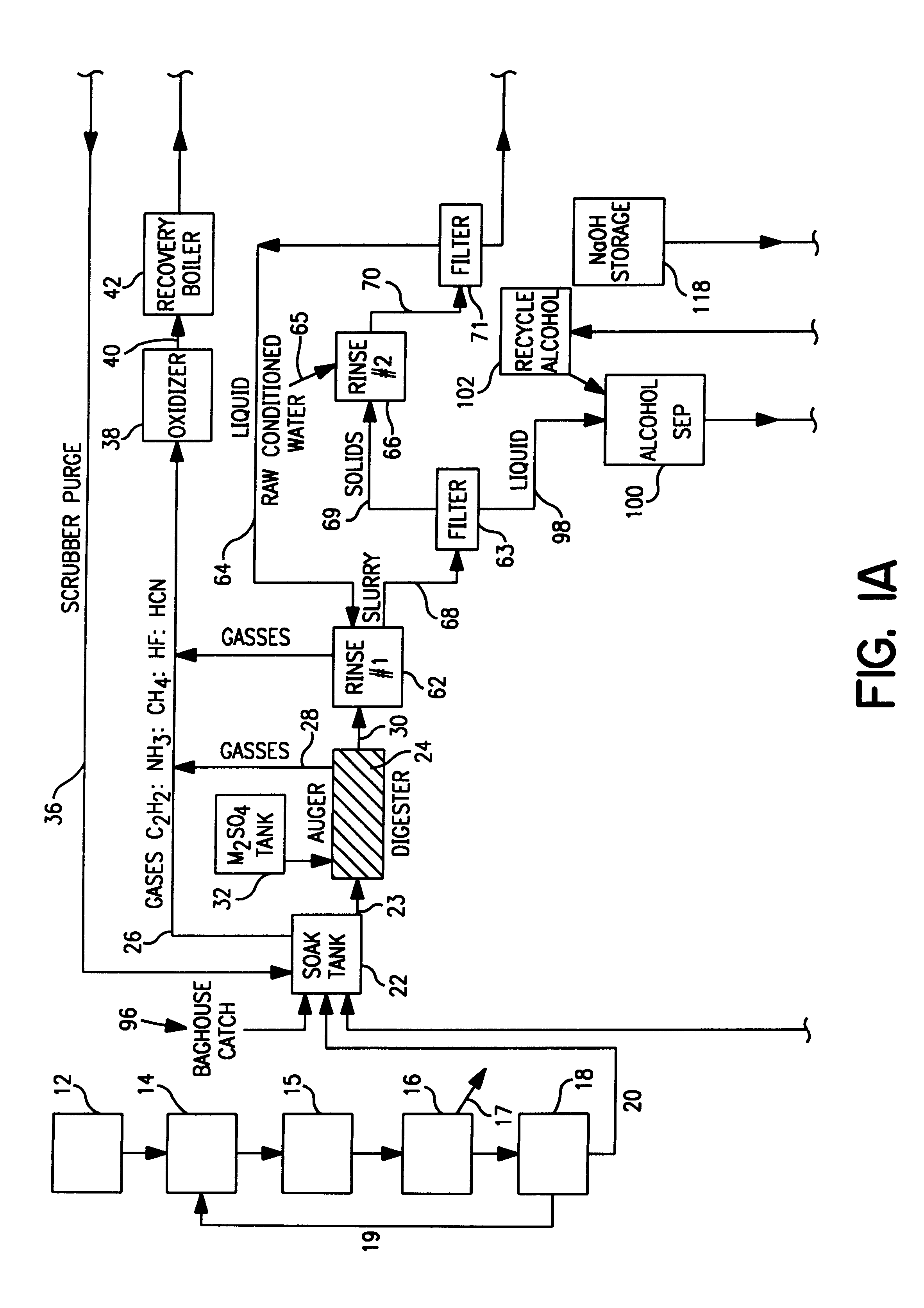
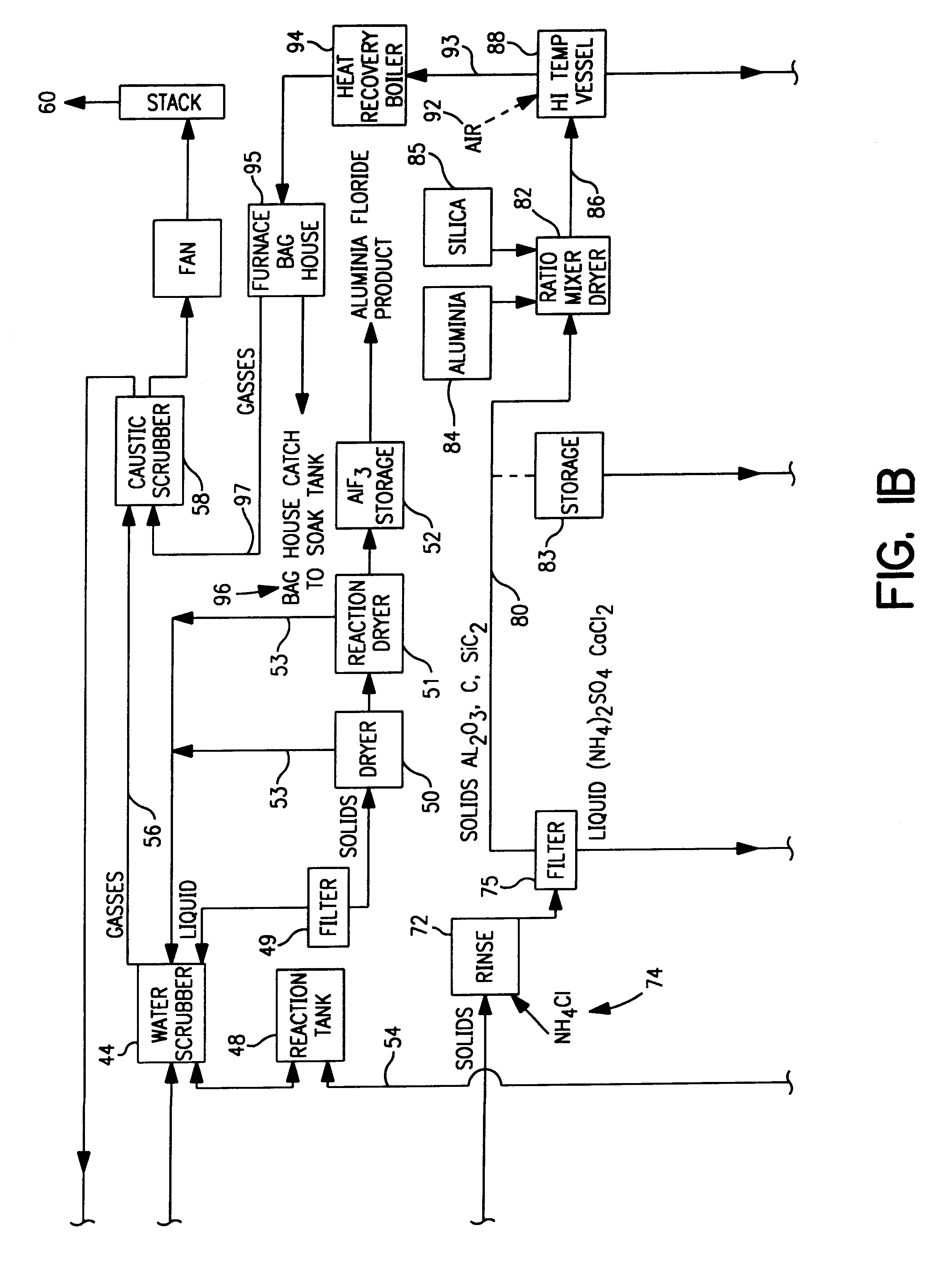
Spent potliner from an aluminum reduction cell is subject to an acid digest and the digest may be adjusted to produce a first gas component comprised of at least one material selected from the group consisting of silicon tetrafluoride, hydrogen fluoride, hydrogen cyanide gas and water vapor, and a slurry component comprised of at least one material selected from the group consisting of carbon, silica, alumina, and sodium, iron, calcium and magnesium compounds. The first gas component is removed from the digester and heated to a temperature sufficiently high to convert said silicon tetrafluoride to fumed silica and hydrogen fluoride. Thereafter, the fumed silica is separated from the hydrogen fluoride to recover fumed silica from spent potliner material.
Owner:GOLDENDALE ALUMINIUM CO
Process of hydrocyanation of unsaturated carboxylic acid derivatives
Owner:INVISTA NORTH AMERICA R L
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com