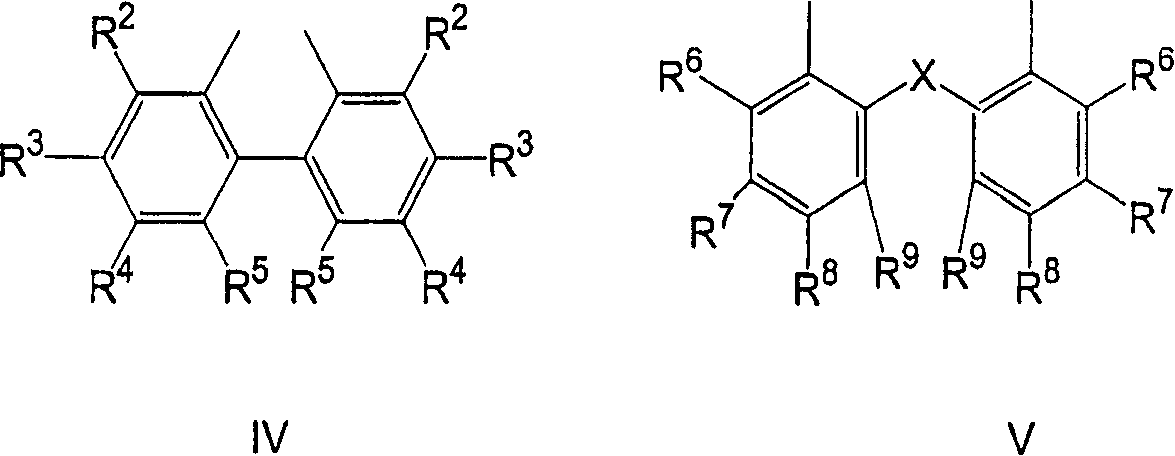
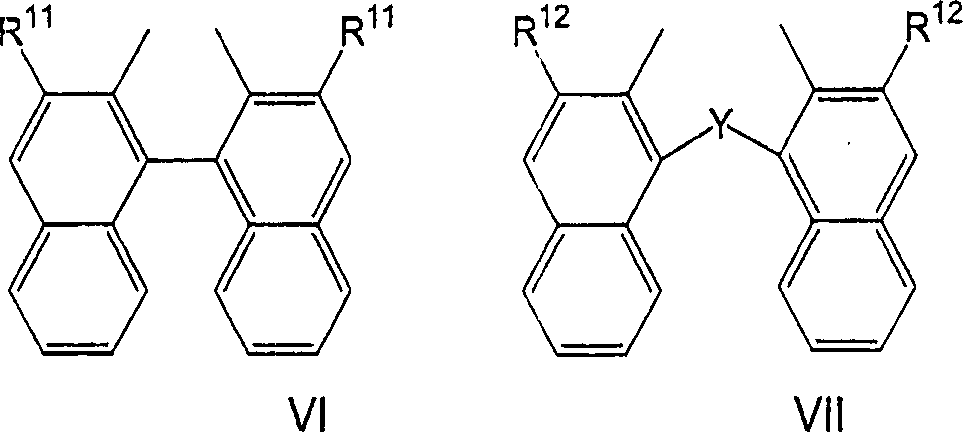
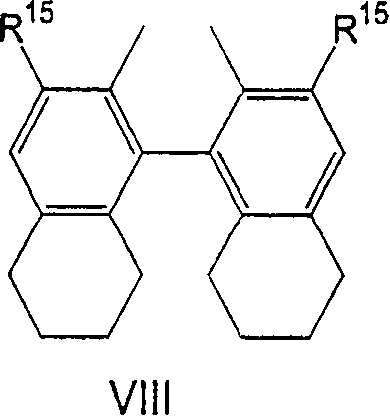
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
A cyanation reaction and catalyst technology, which is applied in the field of preparation of hydrocyanation reaction catalysts, can solve the problems of ligand degradation and increase in the amount of by-products formed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 illustrates the aqueous NiCl 2 Azeotropic drying of , followed by reduction using zero-valent iron, to produce active catalysts. Example 2 illustrates the aqueous NiCl 2 Simultaneous drying and reduction using zero-valent iron to produce active catalysts.
[0049] Example 1
[0050] A 250 mL round bottom flask was fitted with a condenser for liquid removal, ligand addition funnel, stirrer, septum port, thermocouple, pressure sensor, and vacuum throttle to control pressure. An electric heating mantle provides heat to the round bottom flask. All accessories are joined by vacuum-tight ground glass joints. Under nitrogen, 120 ml of fresh 3-pentenenitrile was added to the addition funnel. 1.4 g of 30 wt% NiCl 2 The aqueous solution is filled into a gas-tight syringe with a valve between the needle and the syringe. 120 grams of 3-pentenenitrile plus 16.24 grams of ligands with the above structure IX (wherein R 17 is isopropyl, R 18 is H and R 19 is methyl)...
Embodiment 2
[0053] A 250 mL round bottom flask was fitted with a condenser for liquid removal, a liquid addition funnel, a stirrer, a septum port, a thermocouple, a pressure transducer, and a vacuum throttle to control pressure. An electric heating mantle provides heat to the round bottom flask. All accessories are joined by vacuum-tight ground glass joints. Under nitrogen, 120 ml of fresh 3-pentenenitrile was added to the addition funnel. 1.44 g of 30 wt% NiCl 2 The aqueous solution is filled into a gas-tight syringe with a valve between the needle and the syringe. 120 grams of 3-pentenenitrile plus 16.49 grams of ligands with the above structure IX (wherein R 17 is isopropyl, R 18 is H and R 19 is methyl) plus 6.59 g of iron was added to the round bottom flask. The system was degassed by pumping and purging three times with nitrogen. The flask was heated with stirring. The pressure was adjusted to -0.1 psia (0.7 kPa). Insert the needle on the gas-tight syringe through the septu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com