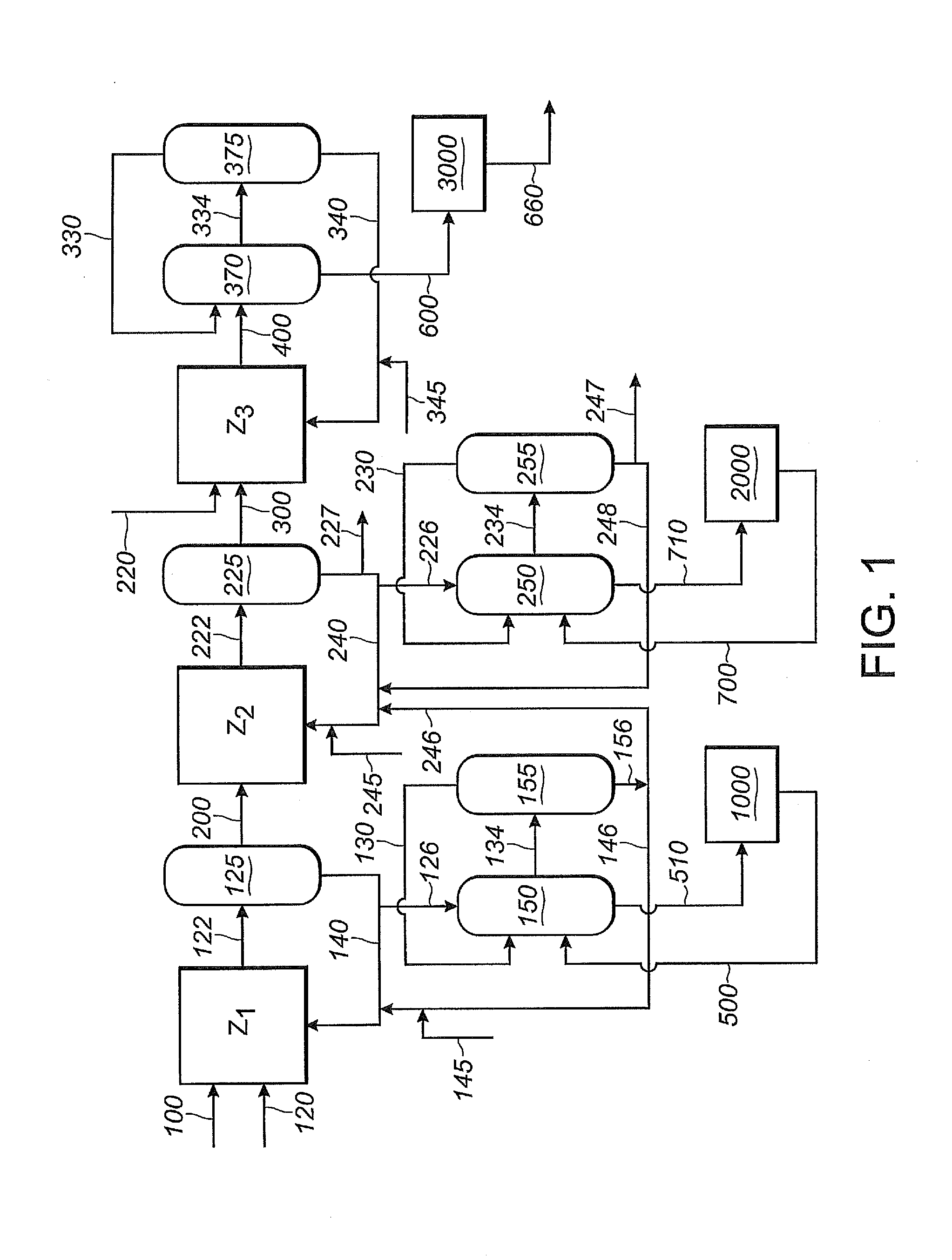
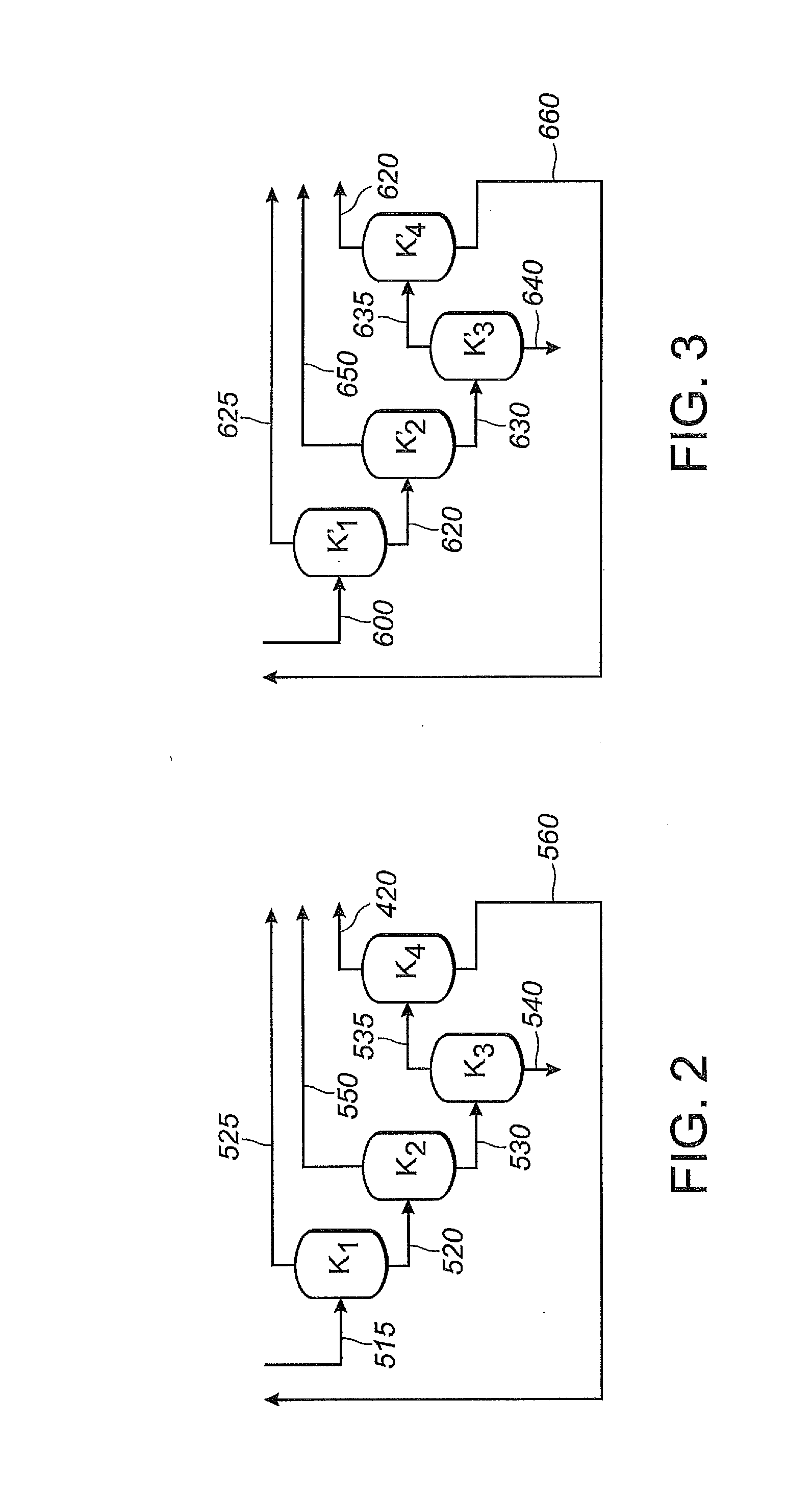
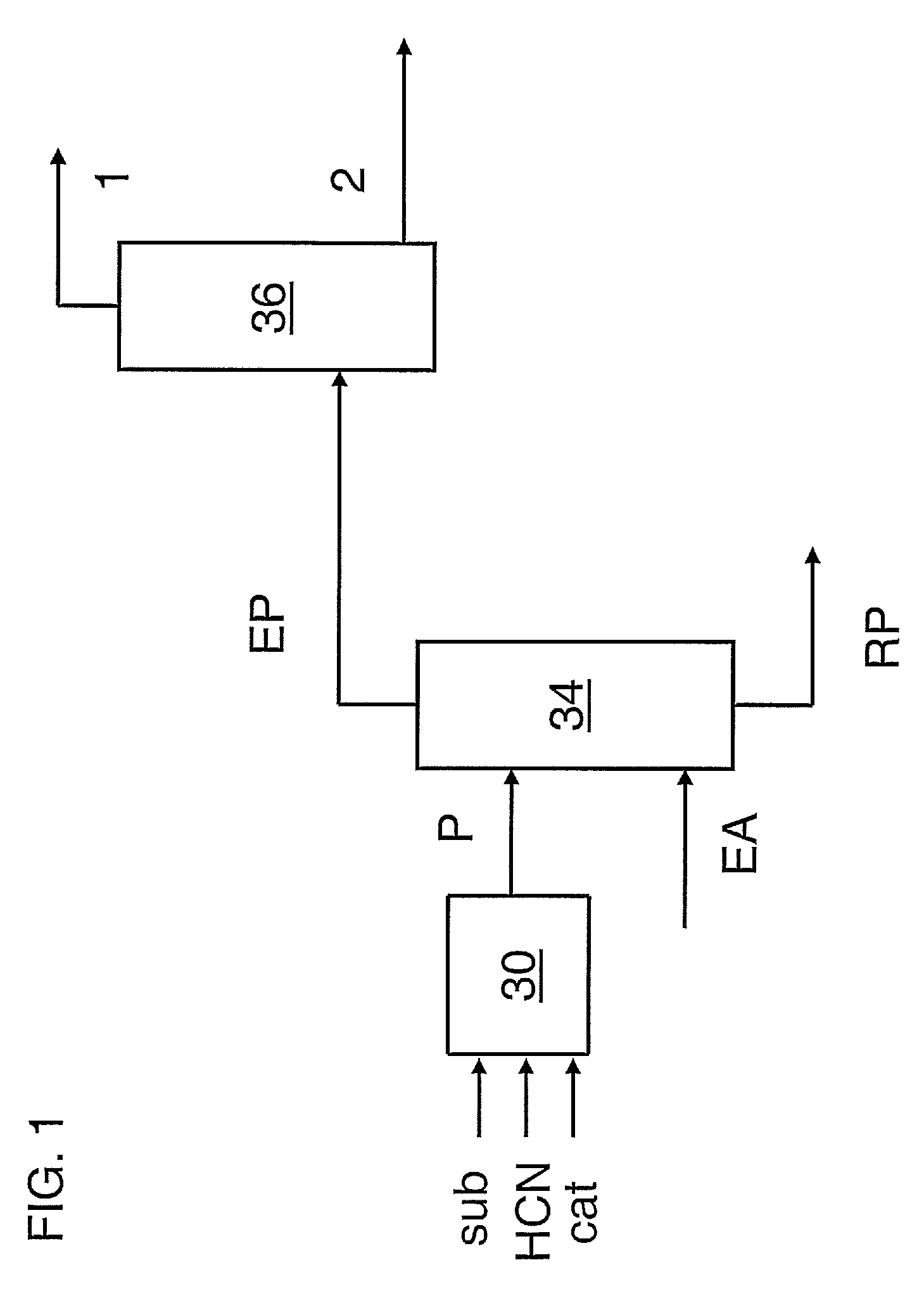
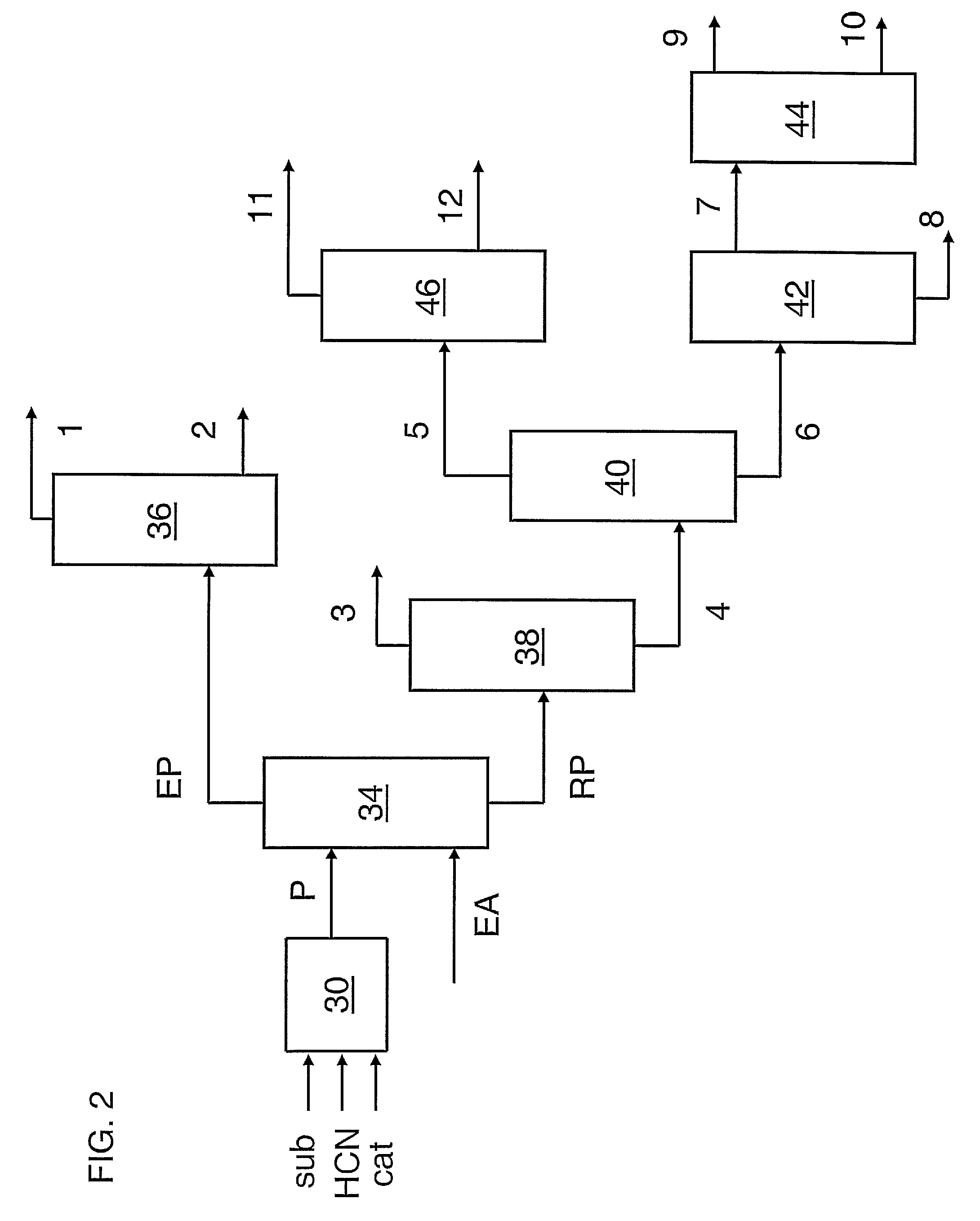
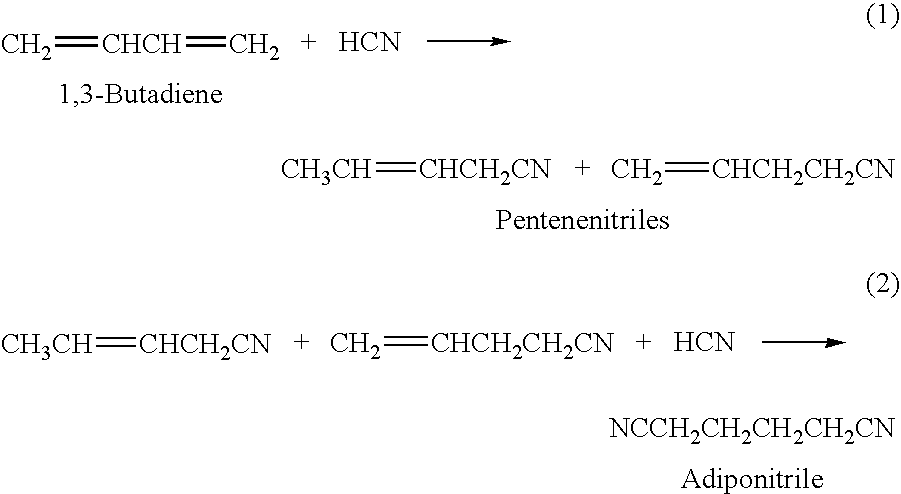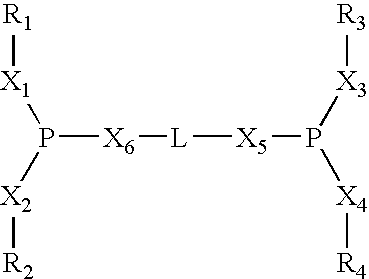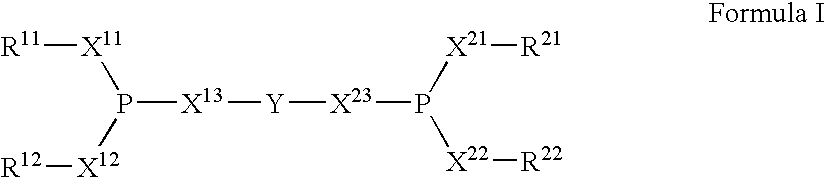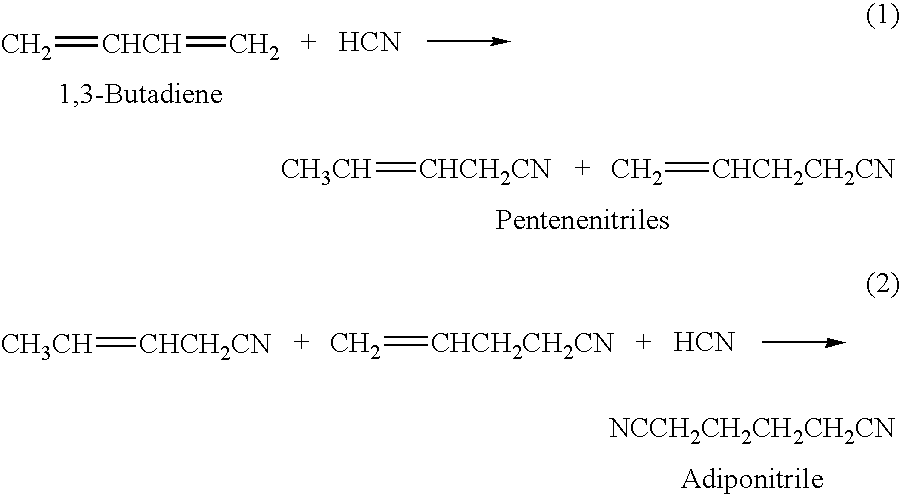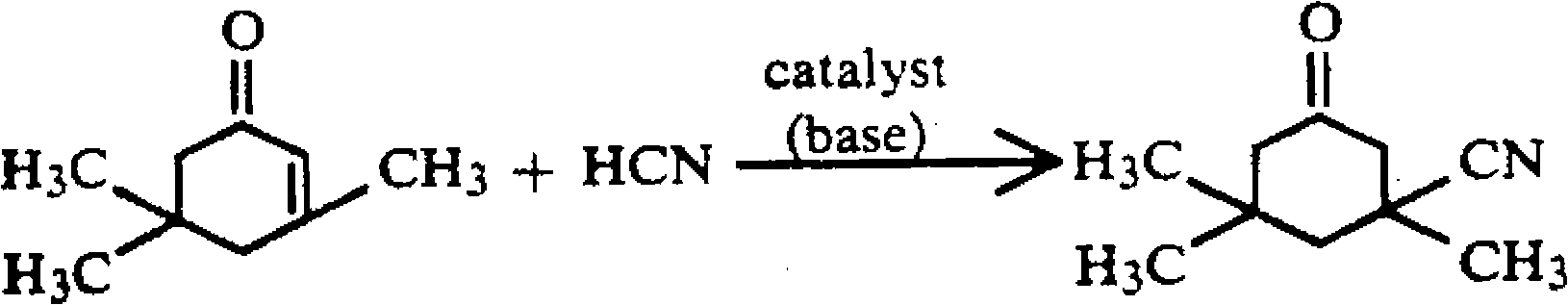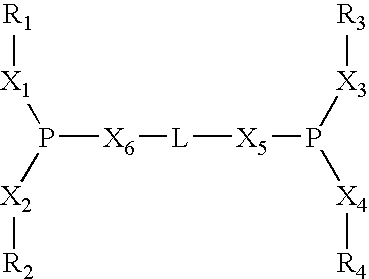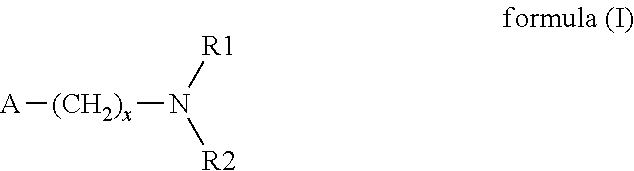Patents
Literature
196results about "Preparation by hydrogen cyanide addition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
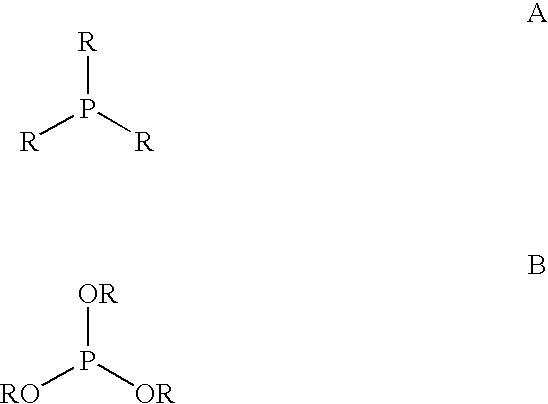
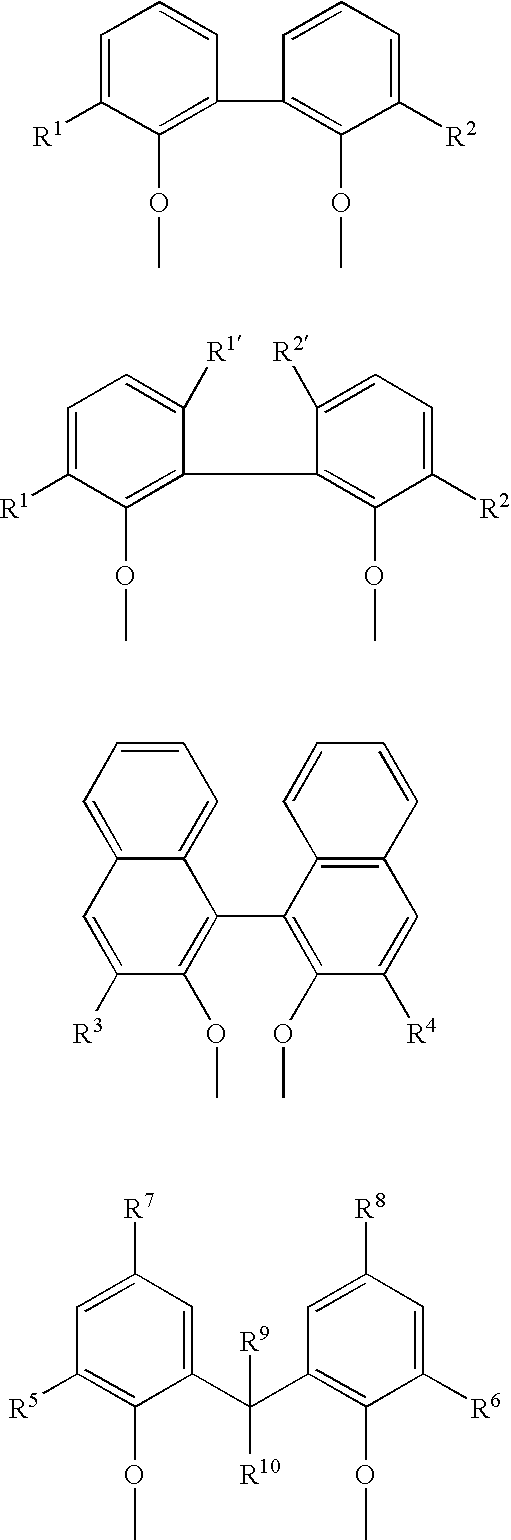
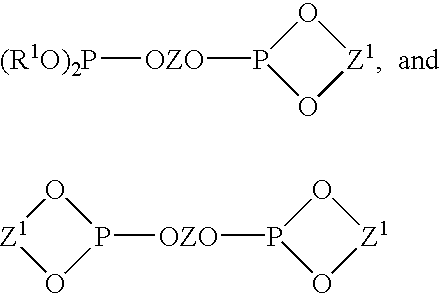
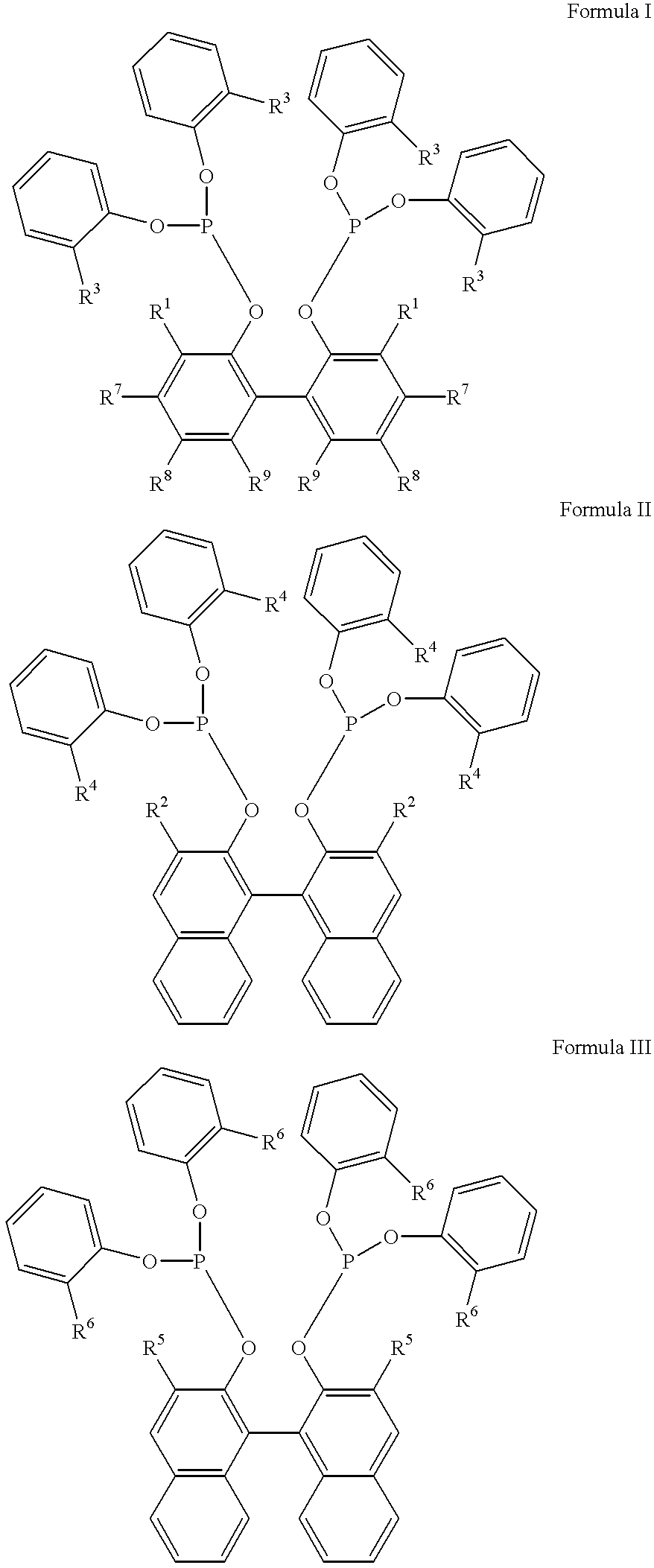
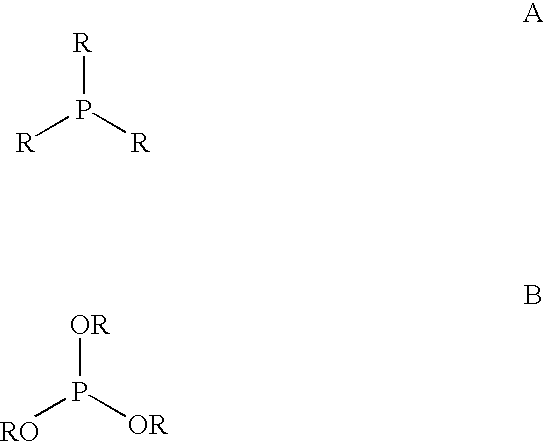
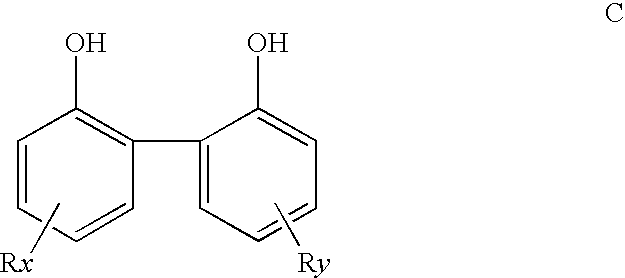
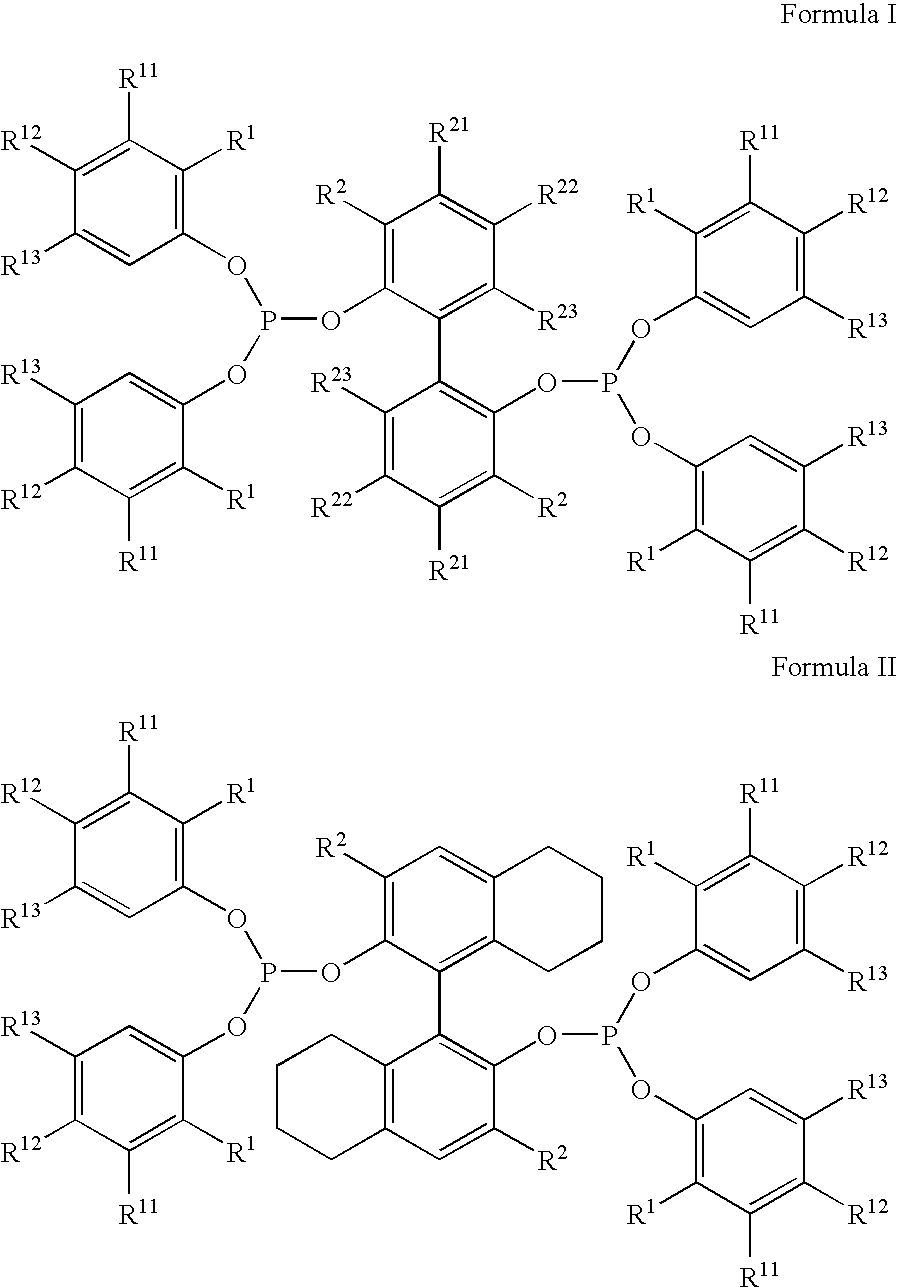
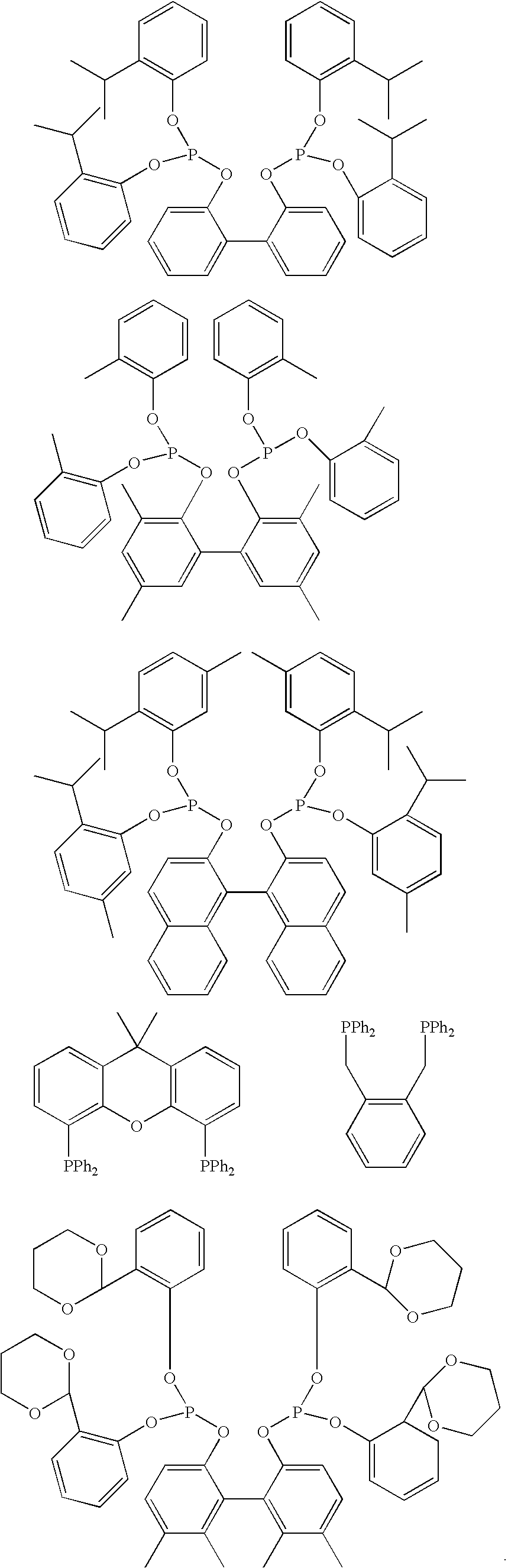
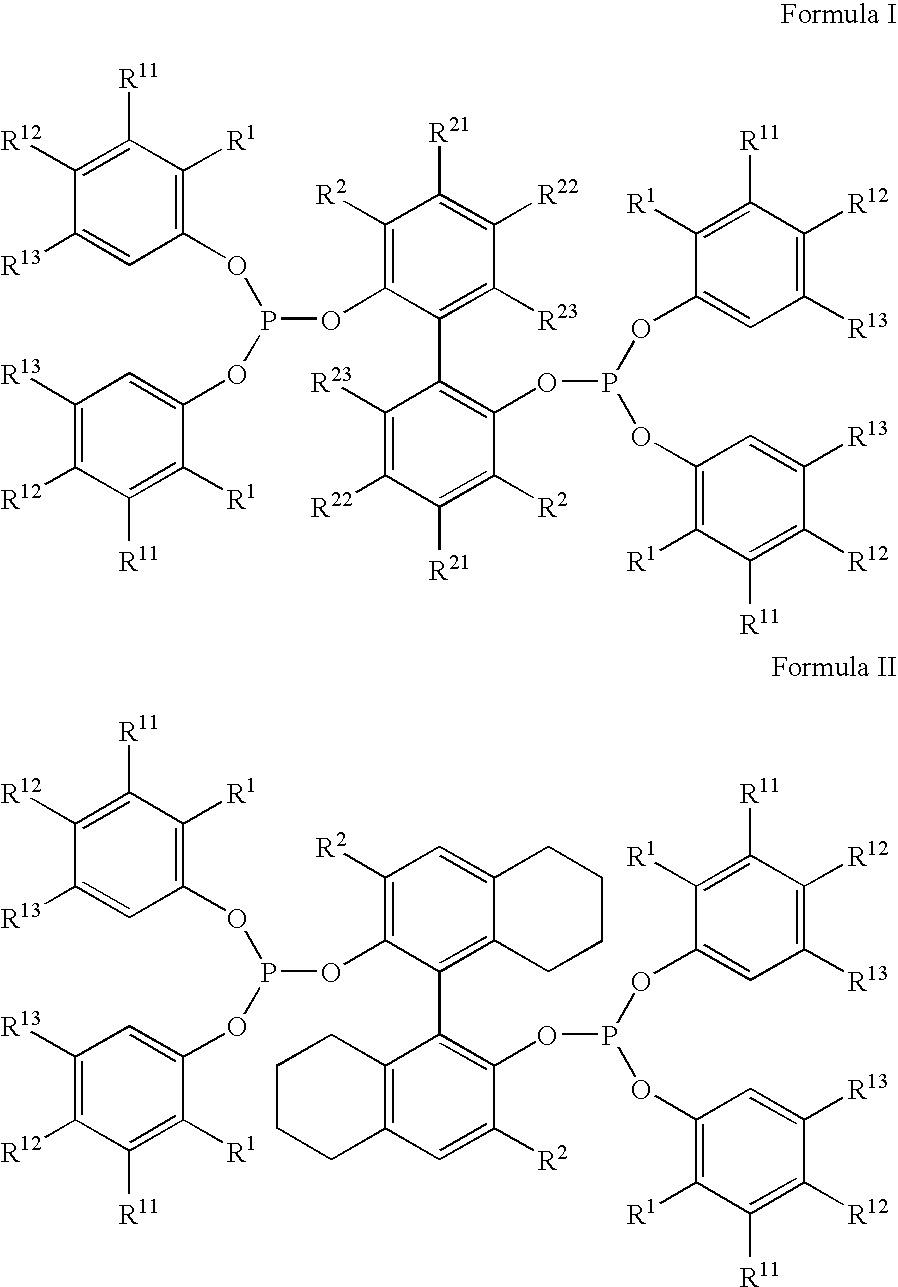
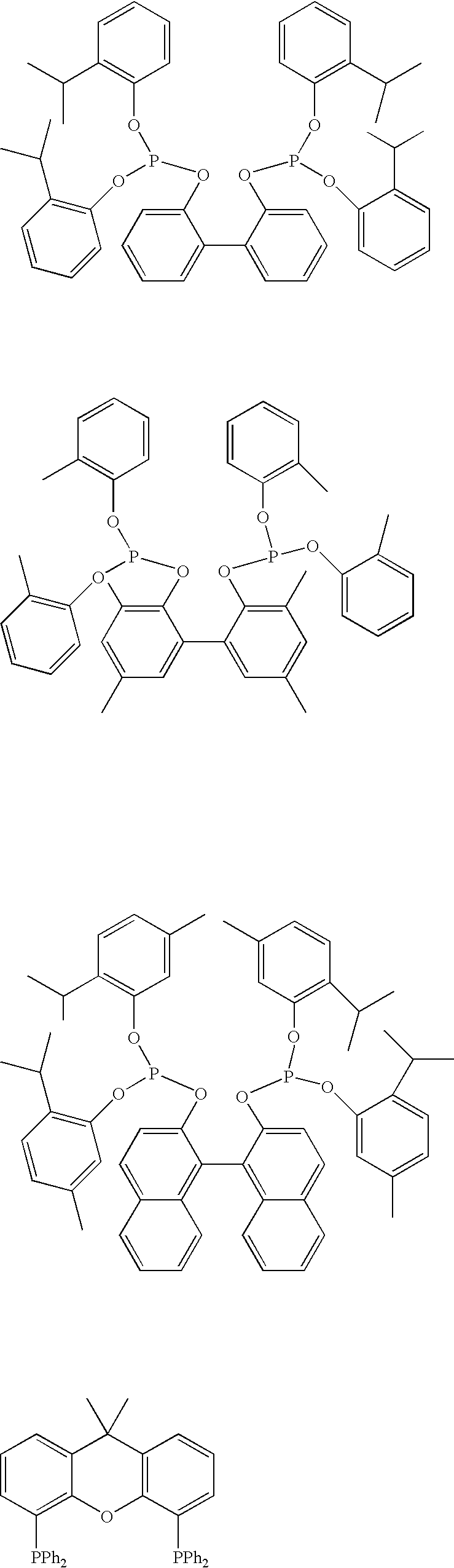
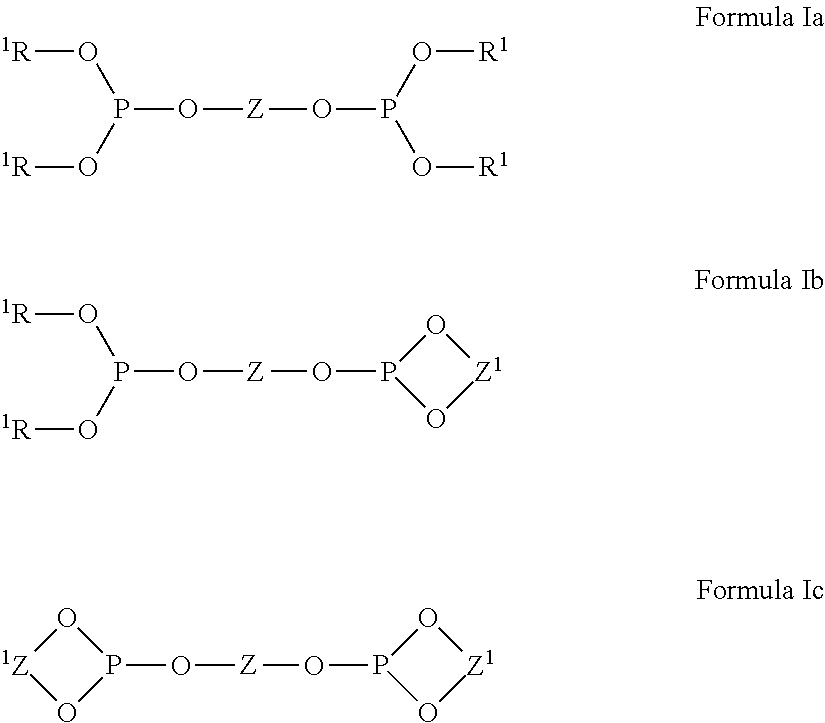
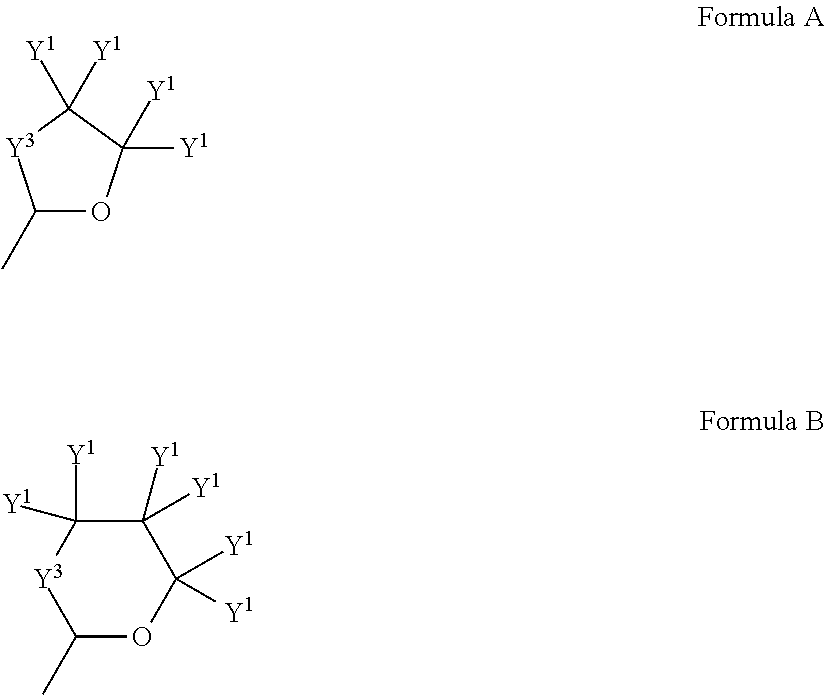
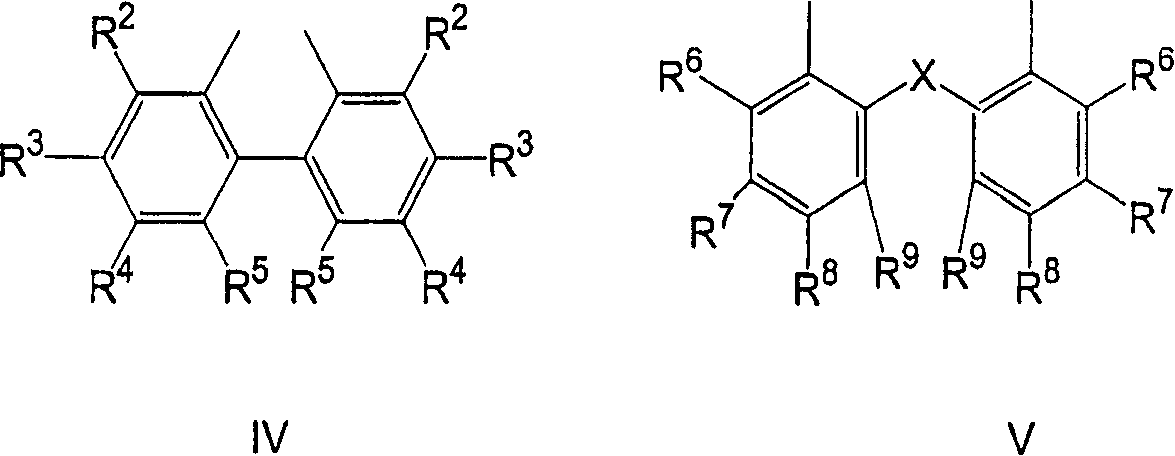
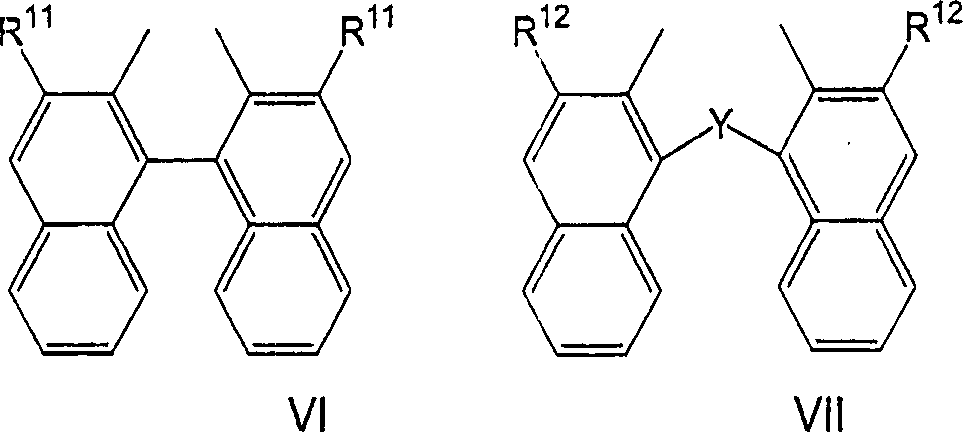
Multidentate phosphite ligands, catalytic compositions containing such ligands, and catalytic processes utilizing such catalytic compositions
InactiveUS6812352B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationOrtho position
Owner:INVISTA NORTH AMERICA S A R L
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
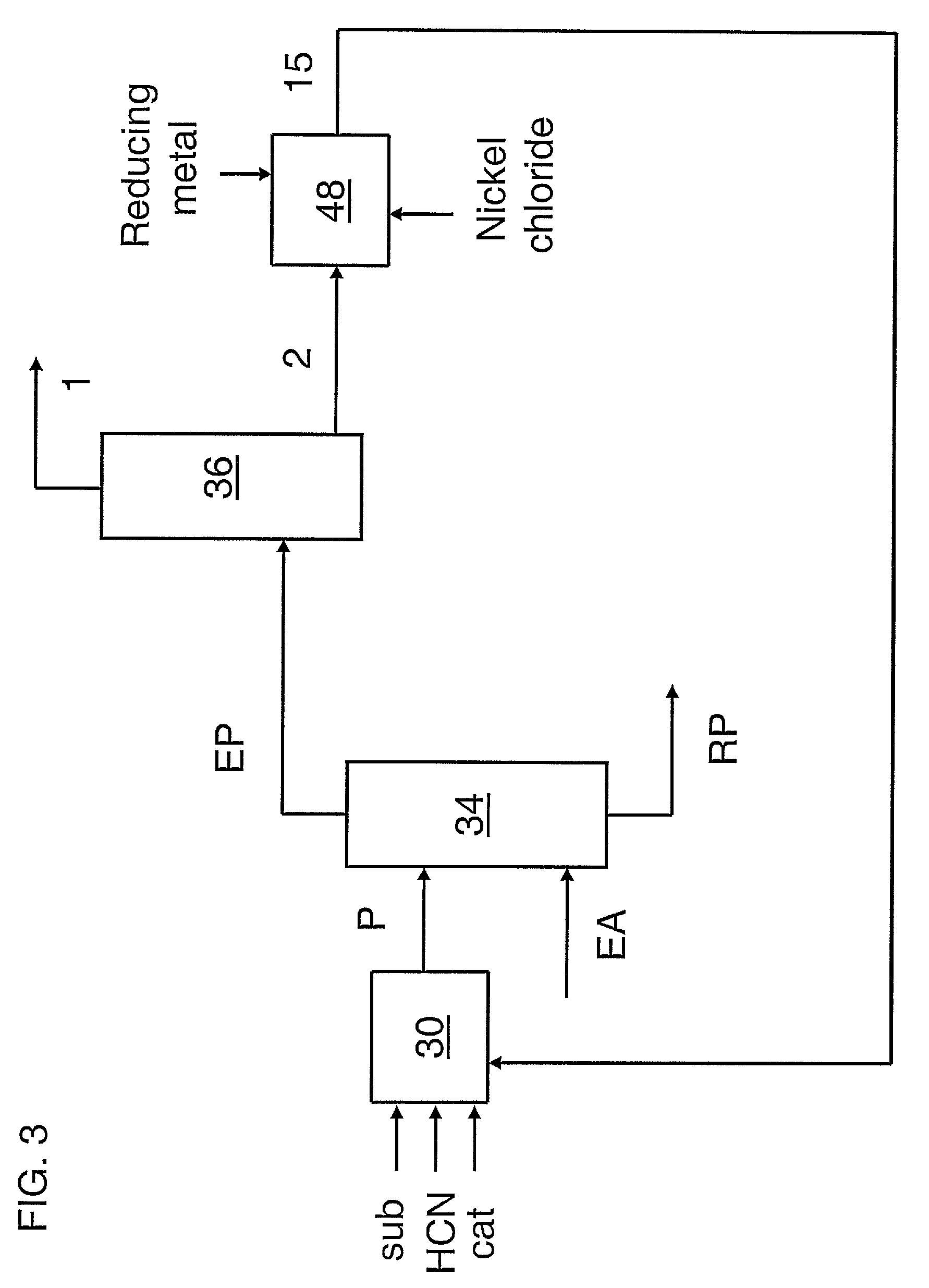
A process for preparing a hydrocyanation catalyst comprising contacting a bidentate phosphorous-containing ligand with a molar excess of nickel chloride in the presence of a nitrile solvent and a reducing metal which is more electropositive than nickel. Preferably, the nickel chloride is dried before use.
Owner:INVISTA NORTH AMERICA R L
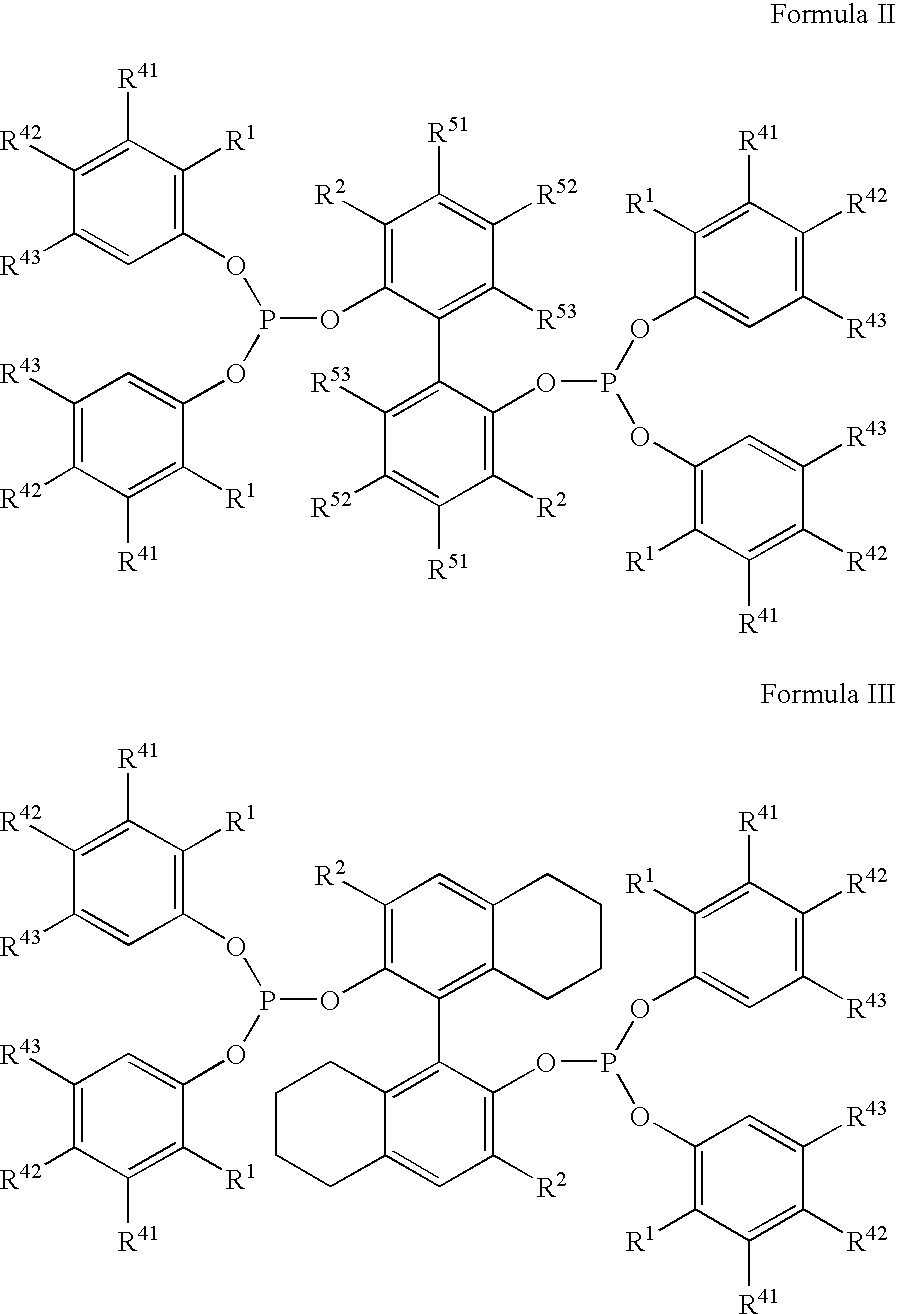
Multidentate phosphite ligands, catalytic compositions containing such ligands and catalytic processes utilizing such catalytic compositions
InactiveUS6380421B1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenOrtho position
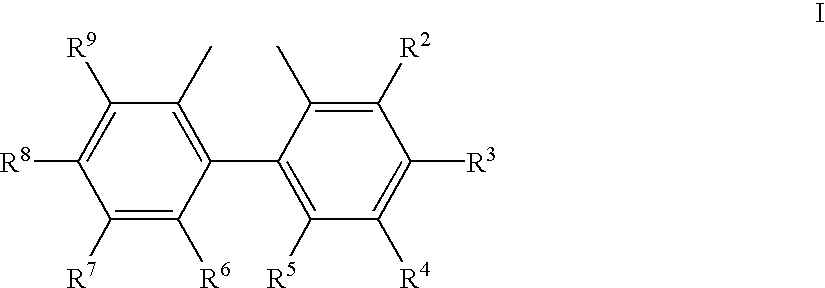
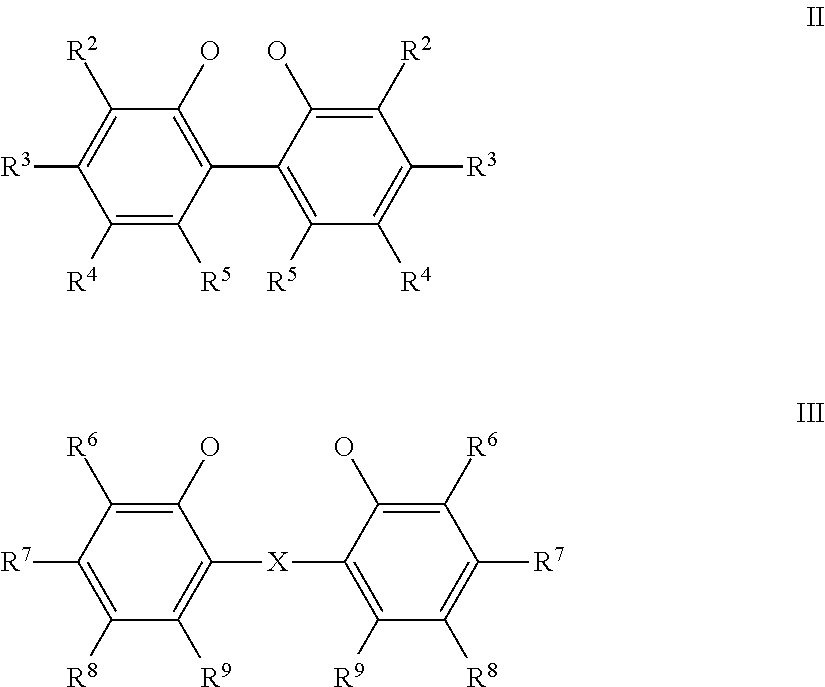
Hydrocyanation reactions employing multidentate phosphite ligands and multidentate phosphite ligands are disclosed. The ligands have phenyl containing substituents attached to the ortho position of the terminal phenol group and / or attached to the ortho position of the bridging group. Catalyst compositions havng such ligands achieve 97% or greater distribution in hydrocyanation.
Owner:INVISTA NORTH AMERICA R L
Supported bis(phosphorus) ligands and their use in the catalysis
InactiveUS6984604B2High selectivityHigh yieldOther chemical processesOrganic compound preparationIsomerizationOrganic compound
Supported bis(phosphorus) ligands are disclosed for use in a variety of catalytic processes, including the isomerization, hydrogenation, hydroformylation, and hydrocyanation of unsaturated organic compounds. Catalysts are formed when the ligands are combined with a catalytically active metal, such as nickel.
Owner:INVISTA NORTH AMERICA R L
Method for the synthesis of acrylonitrile from glycerol
InactiveUS20100048850A1Less heat releaseIncrease partial pressureOrganic compound preparationPreparation by hydrocarbon ammoxidationGas phaseGlycerol
The invention relates to a novel way to synthesize acrylonitrile from a renewable raw material and more particularly relates to a method for producing acrylonitrile by the ammoxidation of glycerol in gaseous phase. The method can be implemented in a single step, or the glycerol can be previously submitted to a dehydration step. The acrylonitrile thus obtained meets the requirements of green chemistry.
Owner:ARKEMA FRANCE SA
Multidentate phosphite ligands, catalytic compositions containing such ligands, and catalytic prosses utilizing such catalytic compositions
InactiveUS20030023110A1Organic compound preparationOrganic chemistry methodsIsomerizationOrtho position
Multidentate phosphite ligands are disclosed for use in reactions such as hydrocyanation and isomerization. The catalyst compositions made therefrom and the various catalytic processes which employ such multidentate phosphite ligands are also disclosed. In particular, the ligands have heteroatom-containing substituents on the carbon attached to the ortho position of the terminal phenol group.
Owner:INVISTA NORTH AMERICA R L
Nickel metal compositions and nickel complexes derived from basic nickel carbonates
ActiveUS20110196168A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDicarbonateMetallurgy
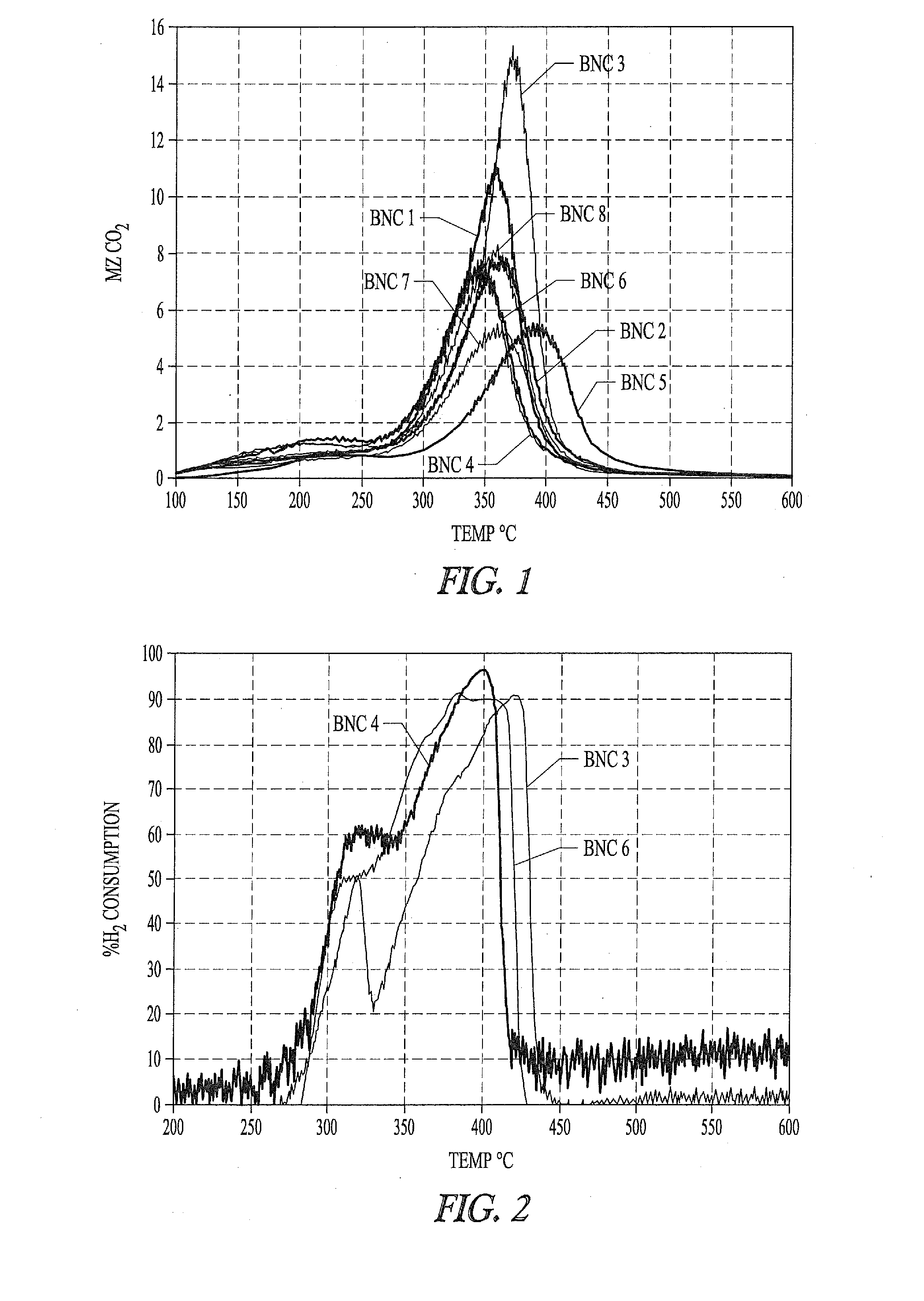
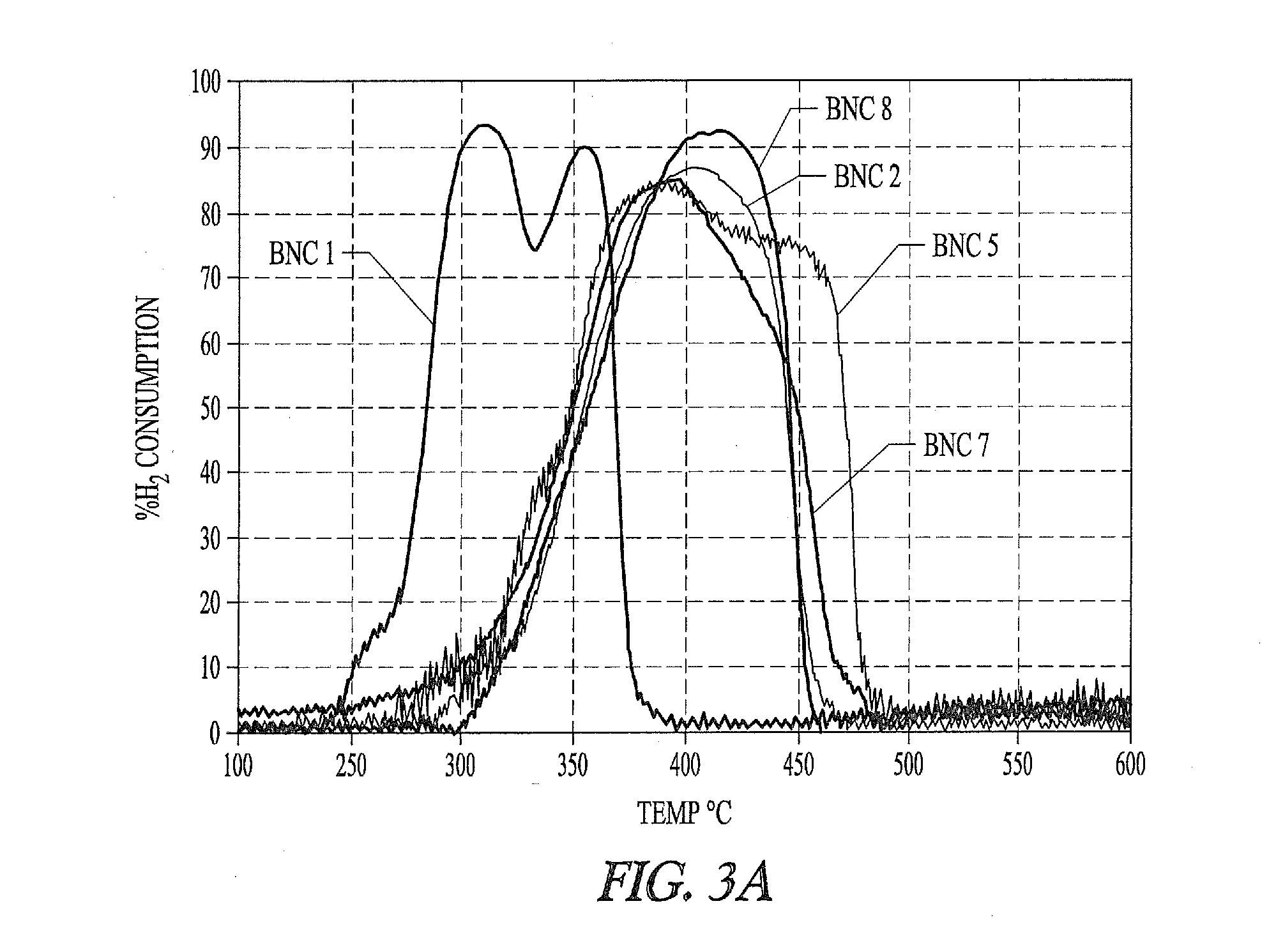
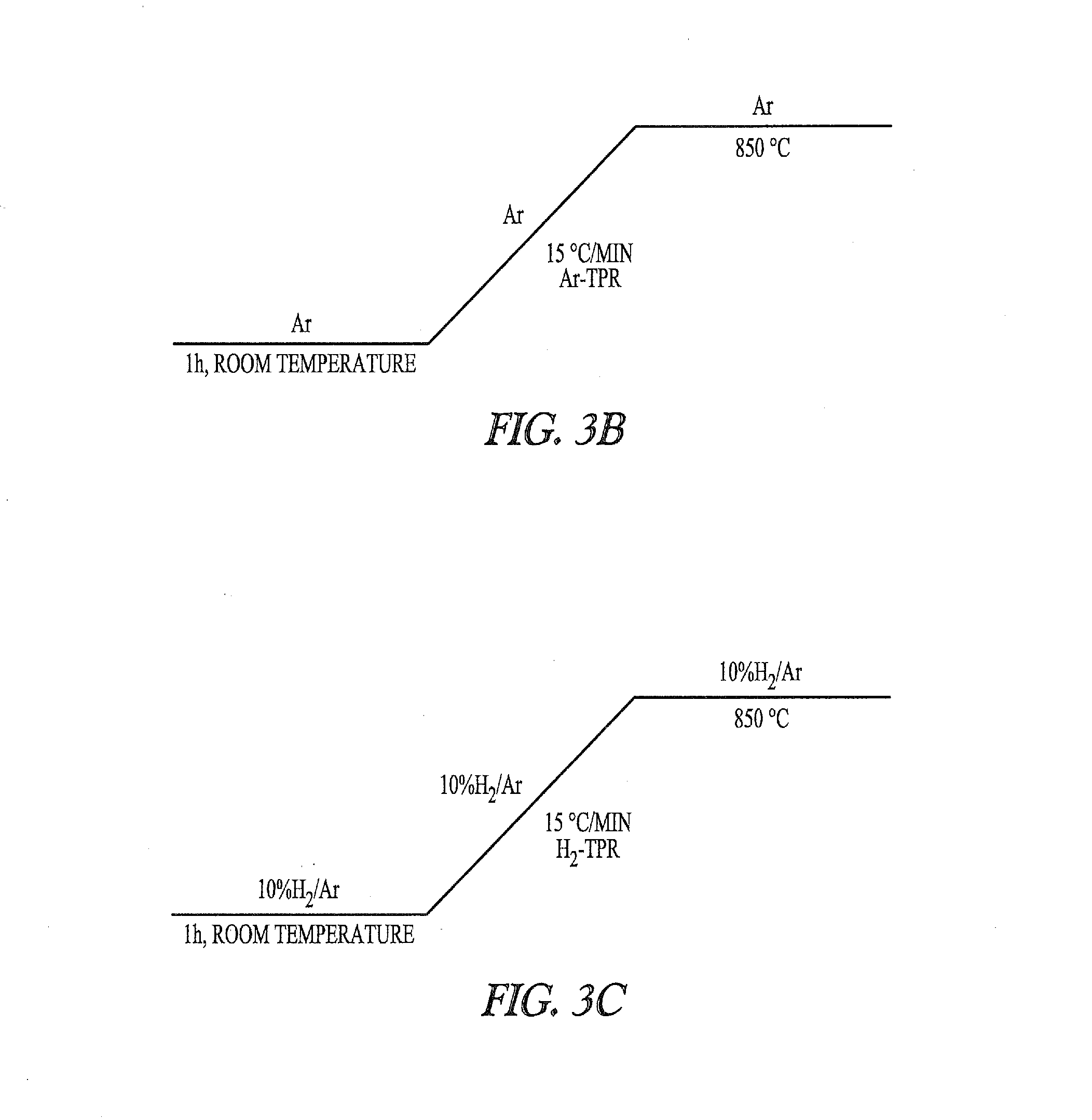
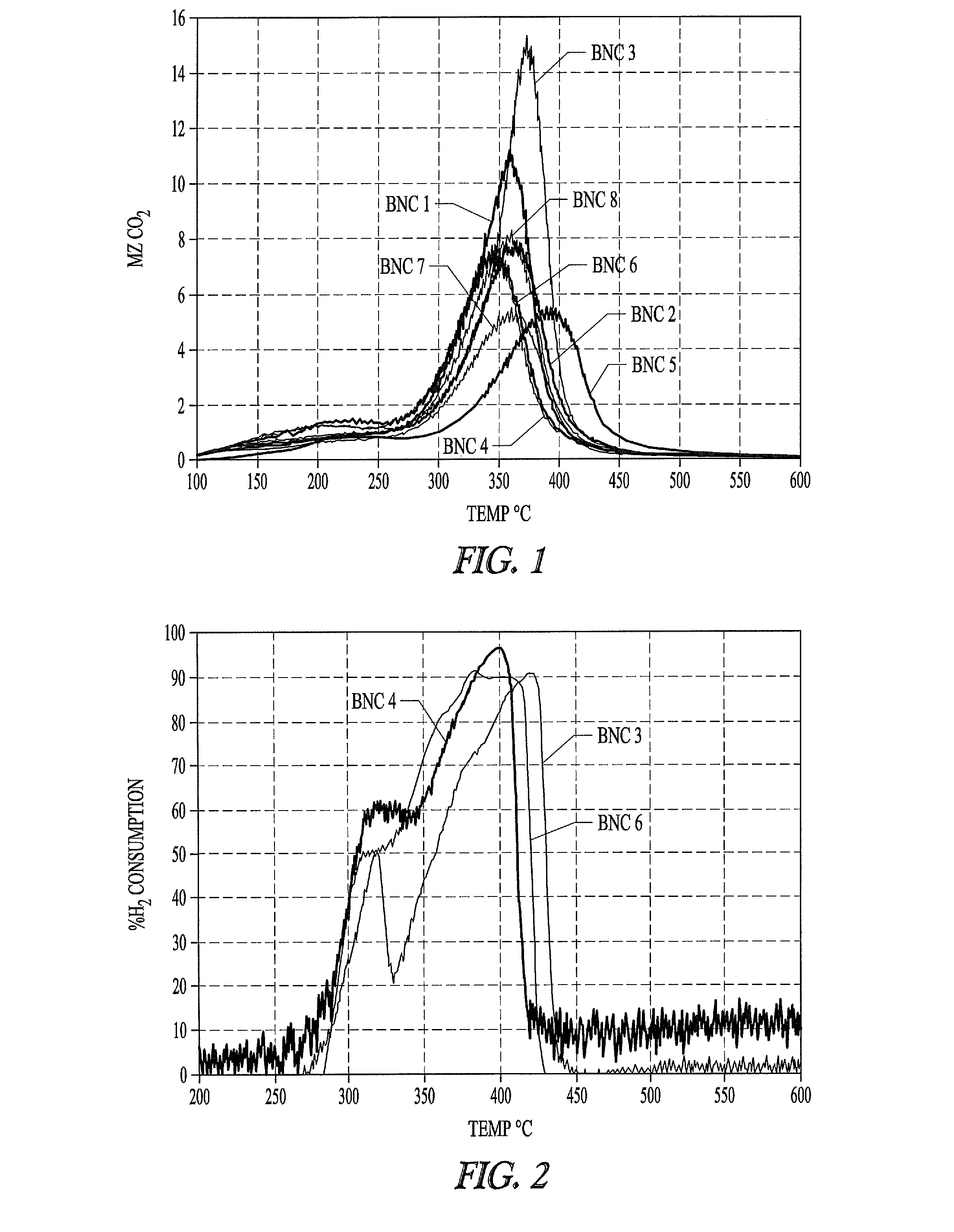
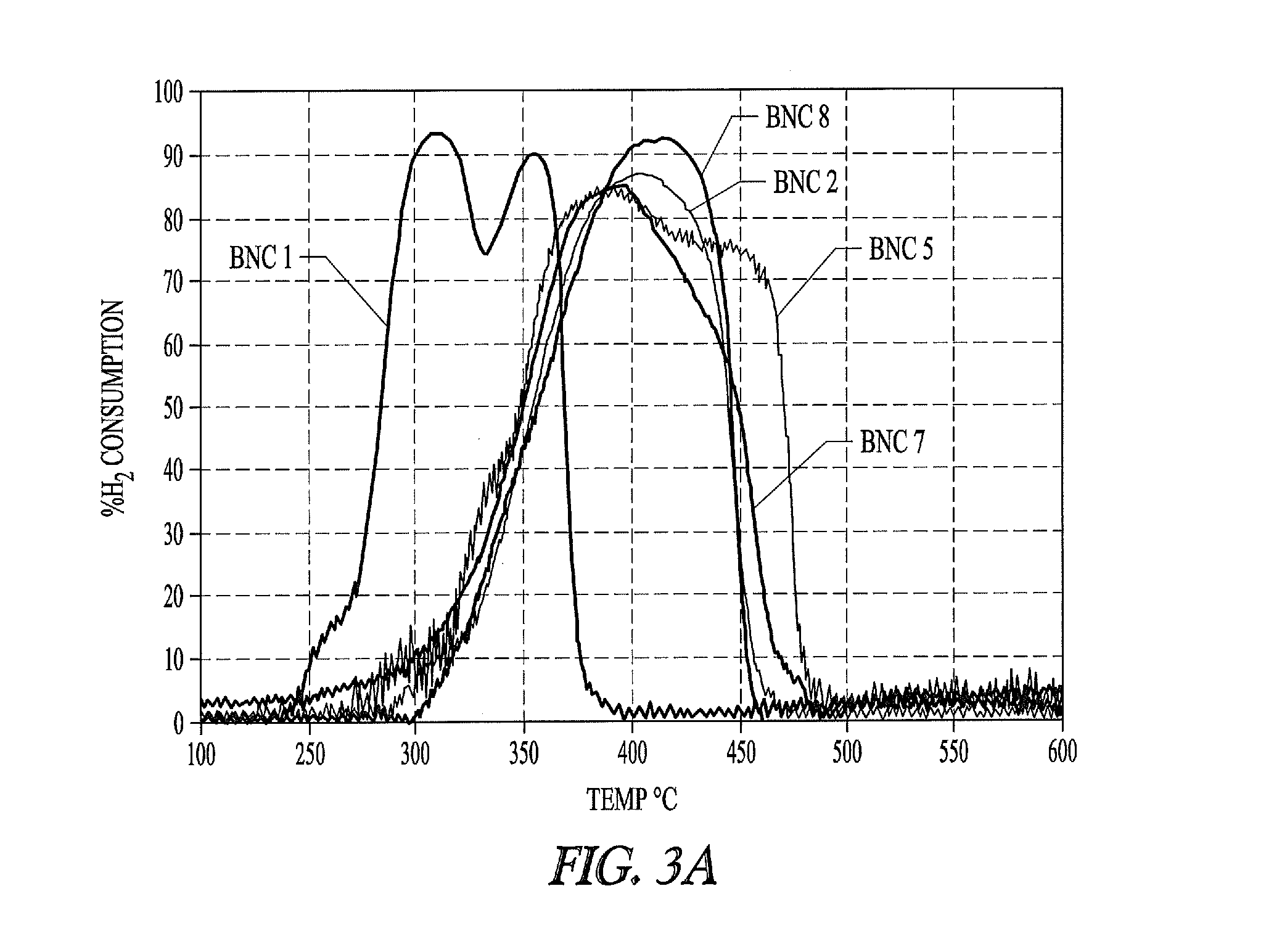
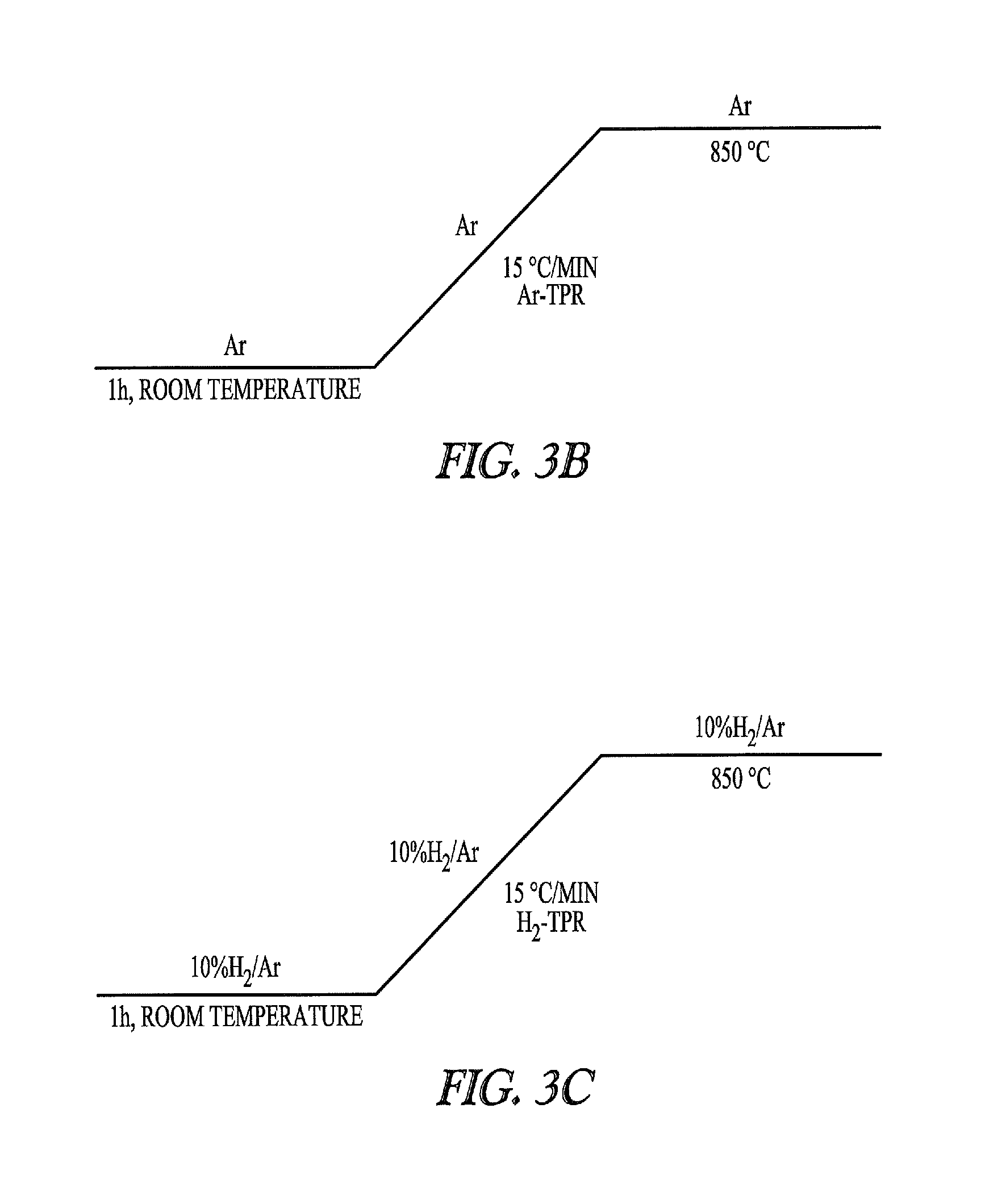
Nickel-metal-containing solids for use in manufacturing nickel metal complexes are disclosed. The nickel-metal-containing solids are made by reducing basic nickel carbonates. By varying the molar ratios of carbonates and bicarbonates to nickel salts, the methods provide basic nickel carbonates that produce superior nickel metal-containing solids that react more effectively with phosphorous-containing ligands. The phosphorous containing ligands can be both monodentate and bidentate phosphorous-containing ligands.
Owner:INV NYLON CHEM AMERICAS LLC
Multiphasic microchannel reactions
InactiveUS7118920B2Improved fluid separationHigh reactivityPreparation by oxidation reactionsOrganic chemistry methodsUnit operationPhase-transfer catalyst
Multiphasic reactions, especially those reactions using a phase transfer catalyst, are conducted in microchannel apparatus. Advantageously, these reactions can be conducted with two, planar microlayers of reactants in adjacent laminar flow streams. Microchannel apparatus and methods for conducting unit operations such as reactions and separations in microchannel apparatus is also described. Microchannel apparatus can provide advantages for controlling reactions and separating products, solvents or reactants in multiphase reactions.
Owner:BATTELLE MEMORIAL INST
Hydrocyanation of pentenenitriles
ActiveUS20090182164A1Organic compound preparationPreparation by hydrogen cyanide additionHydrogenHydrocyanation
The invention provides a hydrocyanation process to produce adiponitrile and other dinitriles having six carbon atoms, in the presence of catalyst composition comprising a zero-valent nickel and at least one bidentate phosphorus-containing ligand wherein the bidentate phosphorus-containing ligand gives acceptable results according to at least one protocol of the 2-Pentenenitrile Hydrocyanation Test Method.
Owner:INV NYLON CHEM AMERICAS LLC
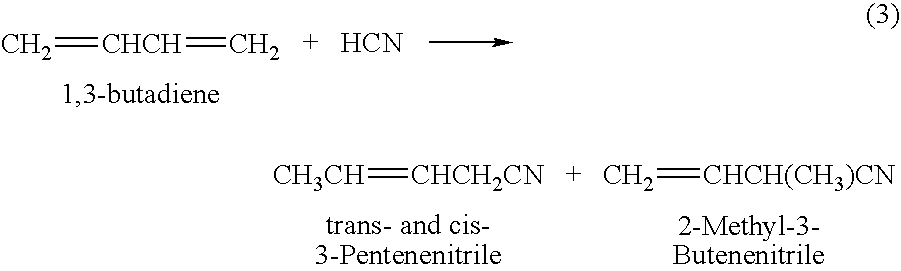
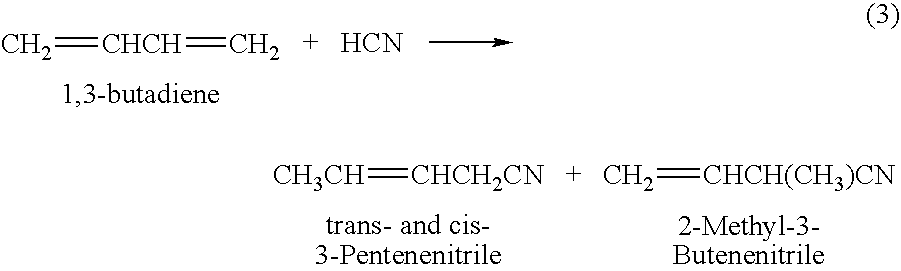
Method for producing 3-pentenenitrile
InactiveUS7541486B2Improve processing yieldReduce thermal stressOrganic compound preparationPreparation by hydrogen cyanide additionPtru catalystButadiene Dioxide
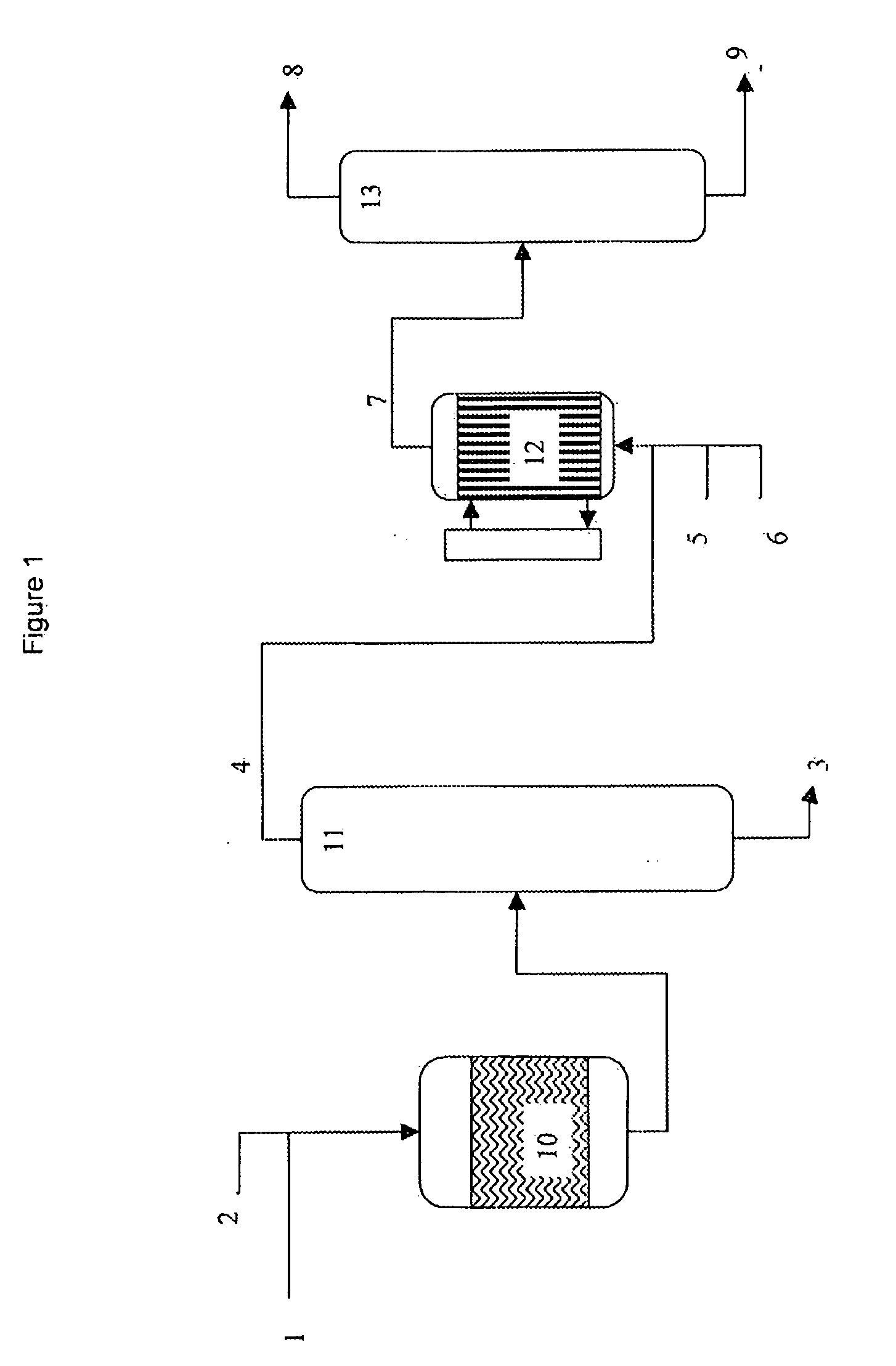
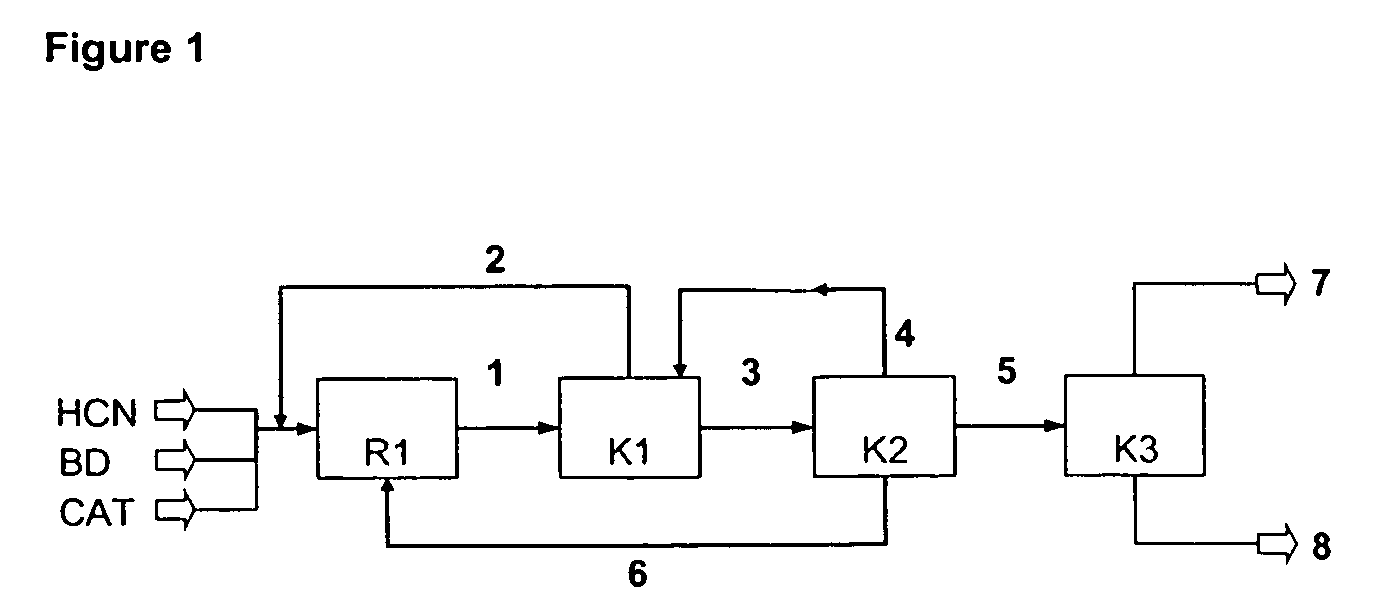
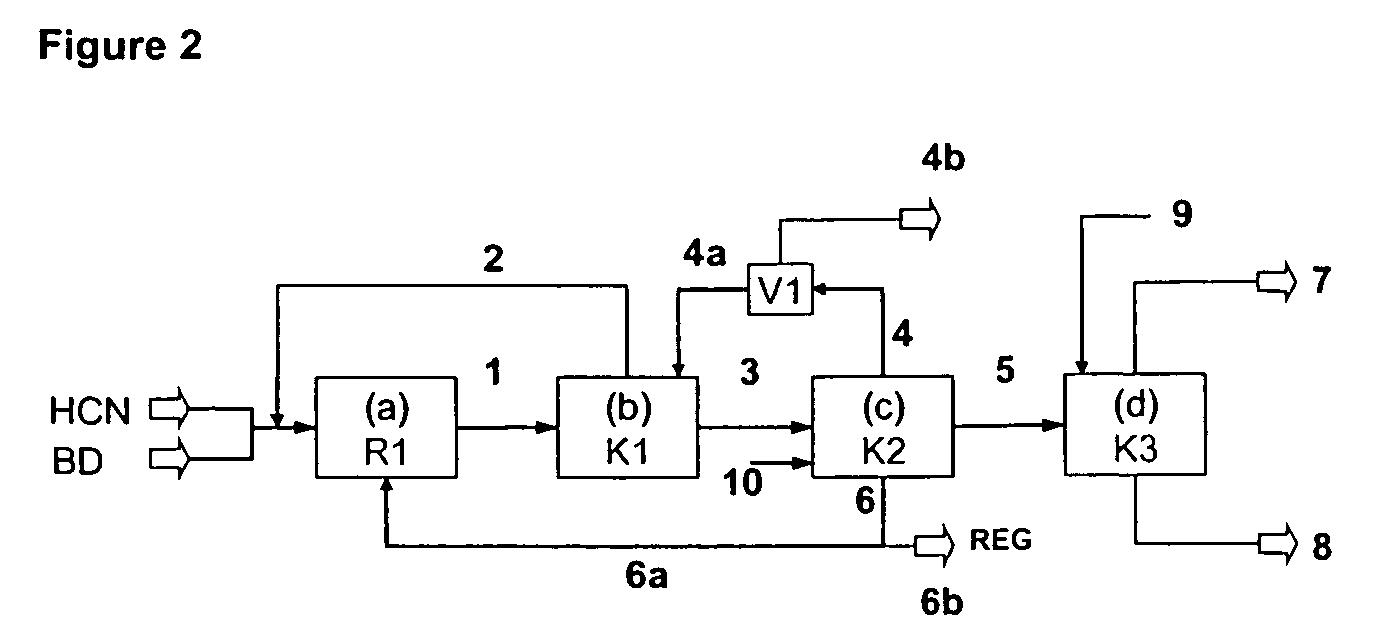
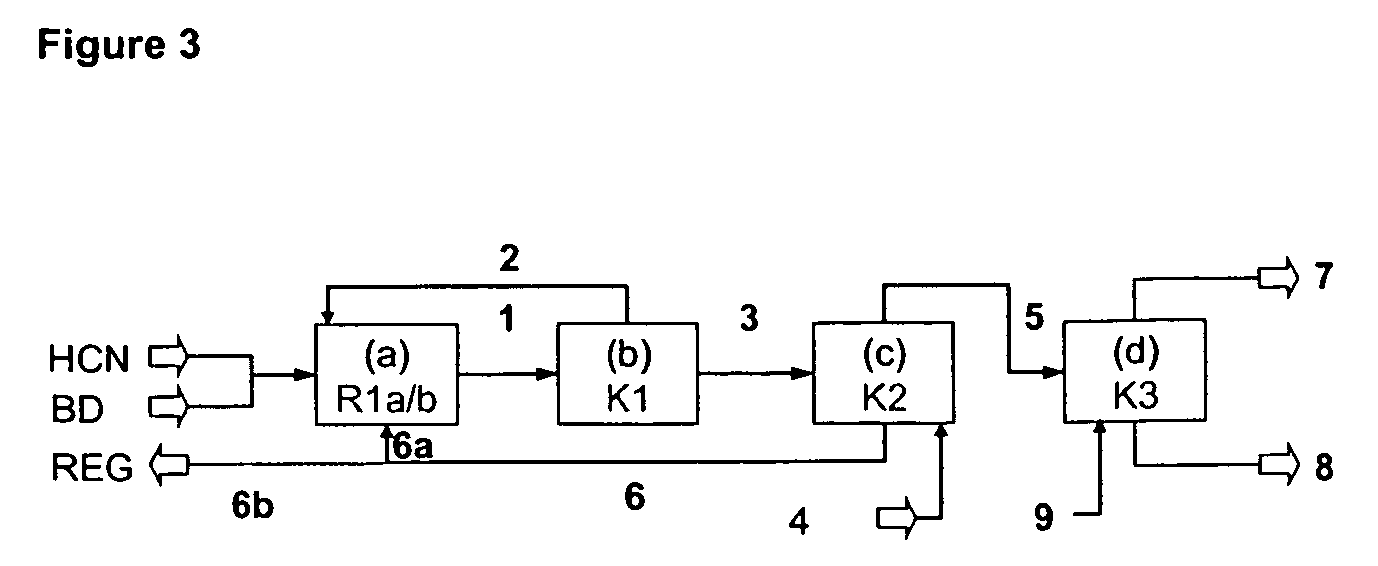
A process is described for preparing 3-pentenenitrile, characterized by the following process steps:(a) reacting 1,3-butadiene with hydrogen cyanide over at least one catalyst to obtain a stream 1 which comprises 3-pentenenitrile, 2-methyl-3-butenenitrile, the at least one catalyst and 1,3-butadiene,(b) distilling stream 1 in a column to obtain a high-1,3-butadiene stream 2 as the top product and a low-1,3-butadiene stream 3 as the bottom product which comprises 3-pentenenitrile, the at least one catalyst and 2-methyl-3-butenenitrile,(c) distilling stream 3 in a column to obtain a stream 4 as the top product which comprises 1,3-butadiene, a stream 5 which comprises 3-pentenenitrile and 2-methyl-3-butenenitrile at a side draw of the column, and a stream 6 as the bottom product which comprises the at least one catalyst,(d) distilling stream 5 to obtain a stream 7 as the top product which comprises 2-methyl-3-butenenitrile, and a stream 8 as the bottom product which comprises 3-pentenenitrile.
Owner:BASF AG
Hydrocyanation process with reduced yield losses
ActiveUS20080015381A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionNitriteHydrogen
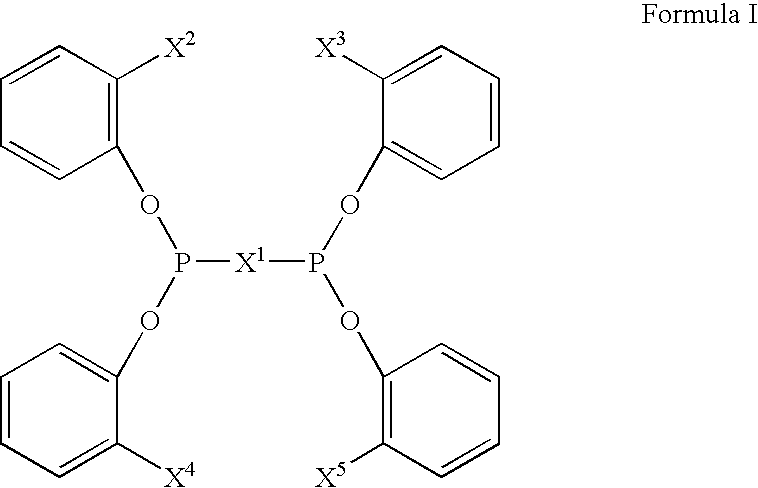
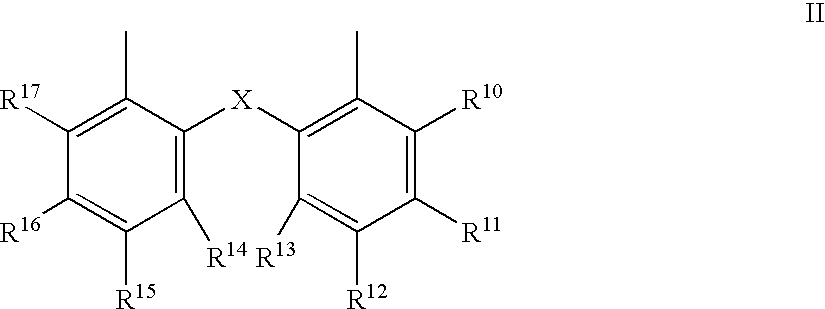
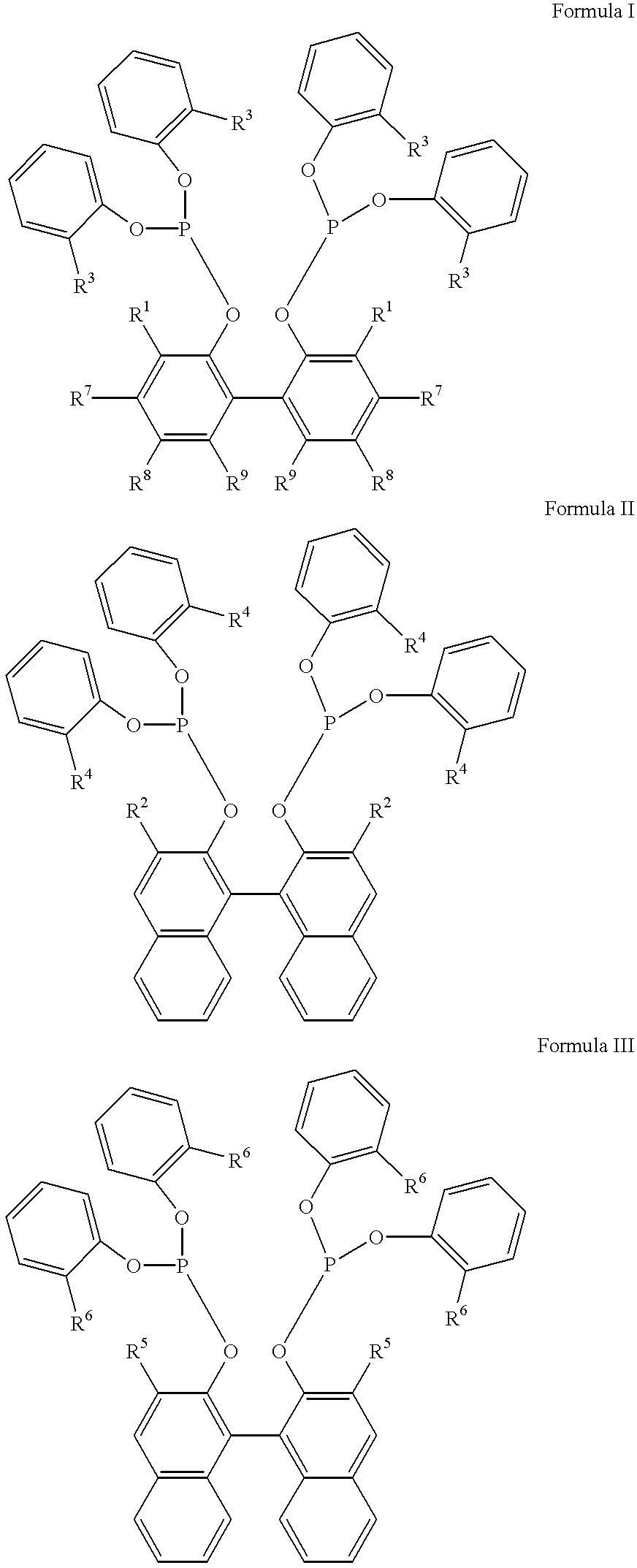
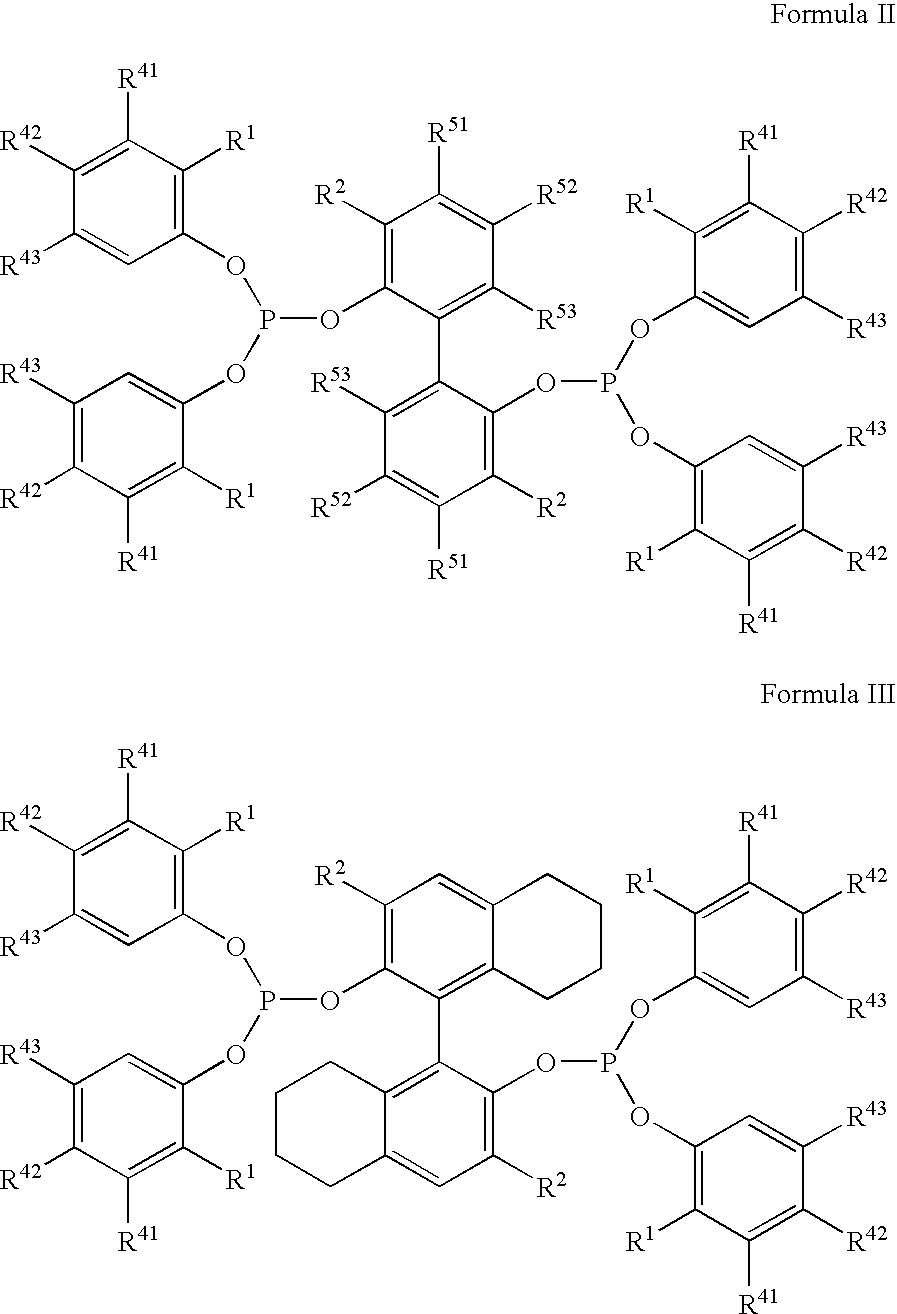
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitrites having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitrites, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitrites, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed)(molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one bidentate phosphite ligand; andwherein the bidentate phosphite ligand is selected from a member of the group represented by Formulas I and 11 as described herein.
Owner:INV NYLON CHEM AMERICAS LLC
Main-group metal-based asymmetric catalysts and applications thereof
InactiveUS6844448B2Lactams preparationCarbamic acid derivatives preparationTetradentate ligandNucleophile
The present invention relates to a method and catalysts for the stereoselective addition of a nucleophile to a reactive π-bond of a substrate. The chiral, non-racemic catalysts of the present invention constitute the first examples of catalysts for nucleophilic additions that comprise a main-group metal and a tri- or tetra-dentate ligand.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Process of synthesis of compounds having nitrile functions from ethylenically unsaturated compounds
InactiveUS7084293B2High selectivityReduce disadvantagesOrganic compound preparationGroup 8/9/10/18 element organic compoundsButadiene DioxideAlkyl nitrites
The present invention relates to a process of hydrocyanation of ethylenically unsaturated organic compounds to compounds having at least one nitrile function, particularly the hydrocyanation of diolefins such as butadiene, or of substituted olefins such as alkene nitrites such as pentenenitriles; the subject hydrocyanation is carried out in the presence of a catalytic system comprising a metallic element and mono- and pluri-dentate organophosphorus ligands.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation process with reduced yield losses
ActiveUS20080015382A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionHydrogenPhosphite ester
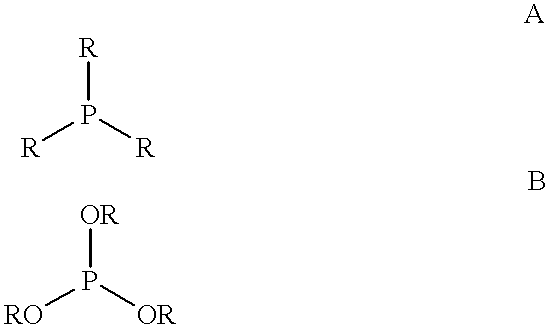
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitriles, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed) / (molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one multidentate phosphorus-containing ligand;wherein the multidentate phosphorus-containing ligand is selected from the group consisting of a phosphite, a phosphonite, a phosphinite, a phosphine, and a mixed phosphorus-containing ligand or a combination of such members; andwherein the multidentate phosphorus-containing ligand gives acceptable results according to at least one protocol of the 2-Pentenenitrile Hydrocyanation Test Method.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation process with reduced yield losses
ActiveUS7659422B2Investment exemptionSelective and efficient and stableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenHydrogen cyanide
A hydrocyanation process produces adiponitrile and other dinitriles having six carbon atoms. The process involves forming a reaction mixture in the presence of at least one Lewis acid. The reaction mixture includes ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition. The reaction mixture is continuously fed while controlling the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles. In the reaction product mixture, including adiponitrile, the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles is from about 0.2 / 1 to about 10 / 1. Included in the catalyst precursor composition is a zero-valent nickel and at least one bidentate phosphite ligand.
Owner:INV NYLON CHEM AMERICAS LLC
Process for the synthesis of glycolonitrile
ActiveUS20060160196A1Minimize glycolonitrile decompositionOrganic compound preparationPhosphorus organic compoundsGlycollic acidHCN poisoning
A process to prepare substantially pure glycolonitrile in an aqueous medium is provided by reacting hydrogen cyanide and formaldehyde. The formaldehyde feed stream is heated prior to reacting with hydrogen cyanide, resulting in an aqueous glycolonitrile solution with fewer impurities, especially less unreacted formaldehyde, than is obtained by other methods. The process enables production of an aqueous glycolonitrile solution that requires less post-reaction purification (if any at all) prior to enzymatically converting the glycolonitrile into glycolic acid.
Owner:THE CHEMOURS CO FC LLC
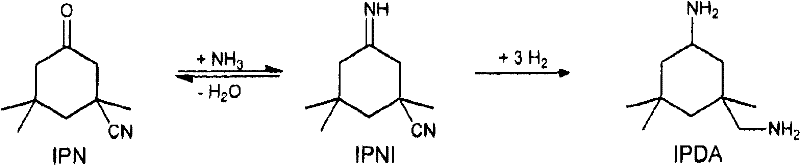
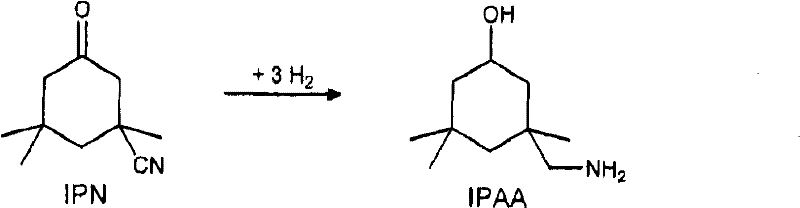
Process for preparing 3-aminomethyl-3,5,5-trimethylcyclohexylamine
InactiveCN102531916AIncrease concentrationOrganic compound preparationWater/sewage treatment by heatingIsophoroneAsymmetric hydrogenation
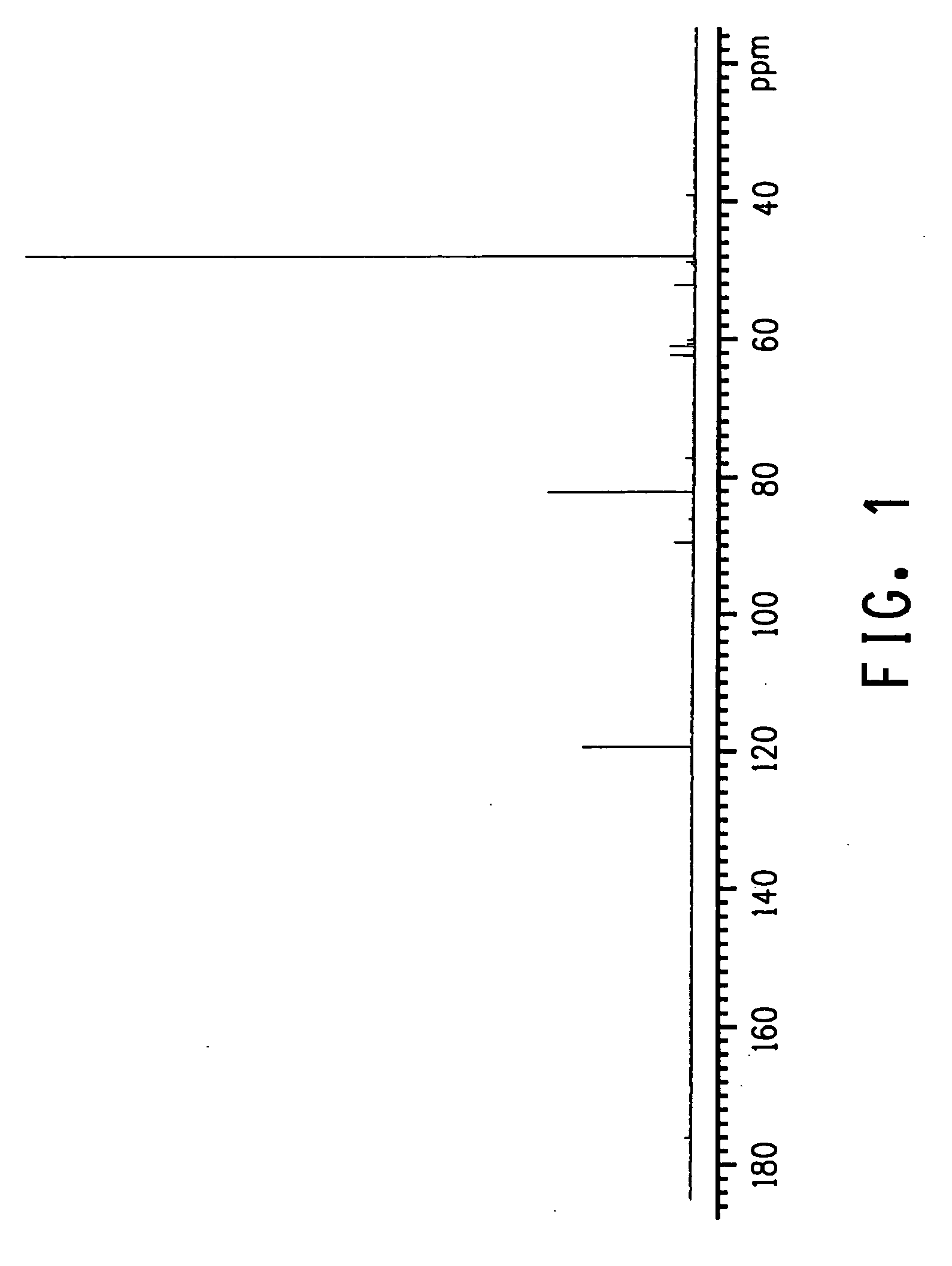
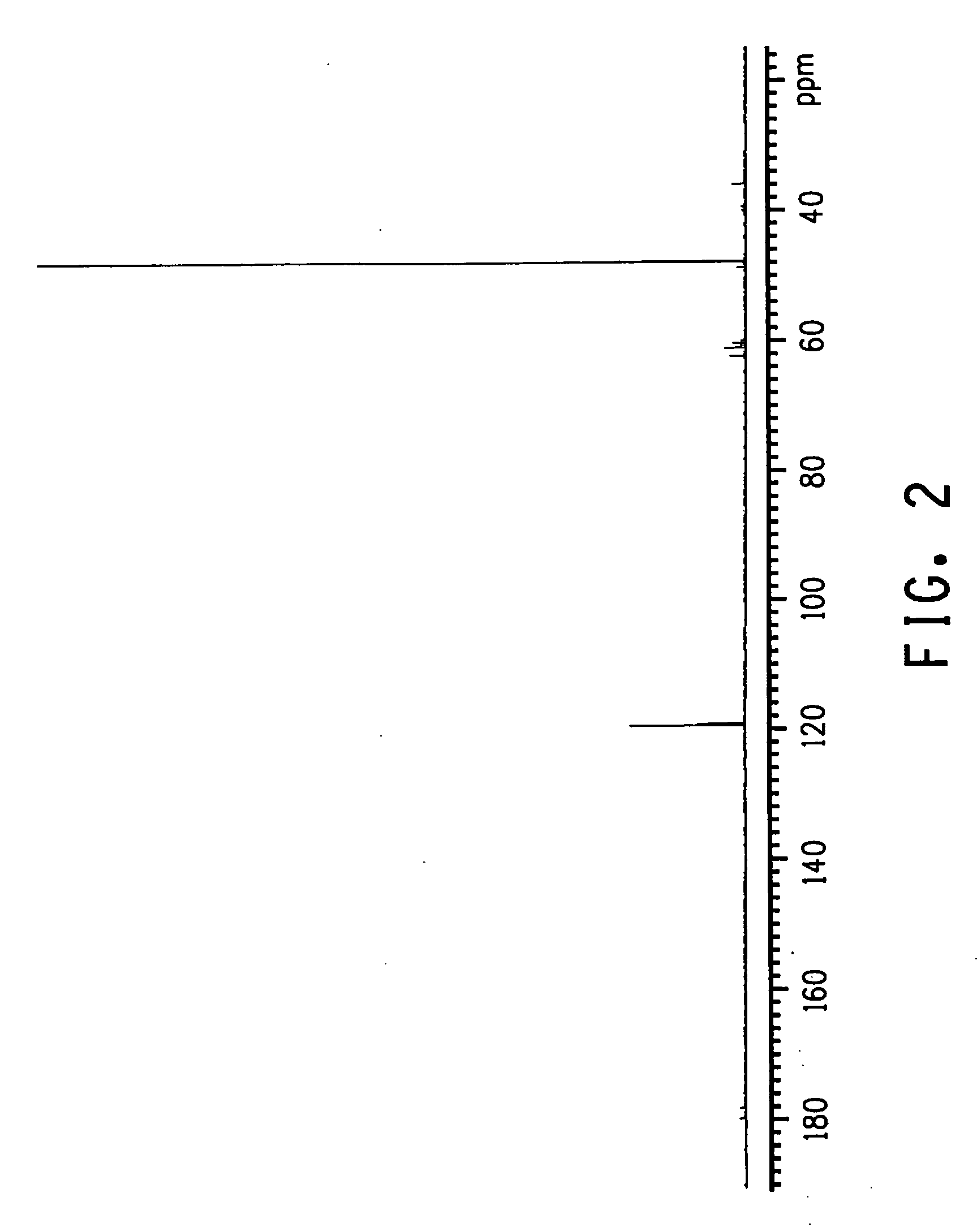
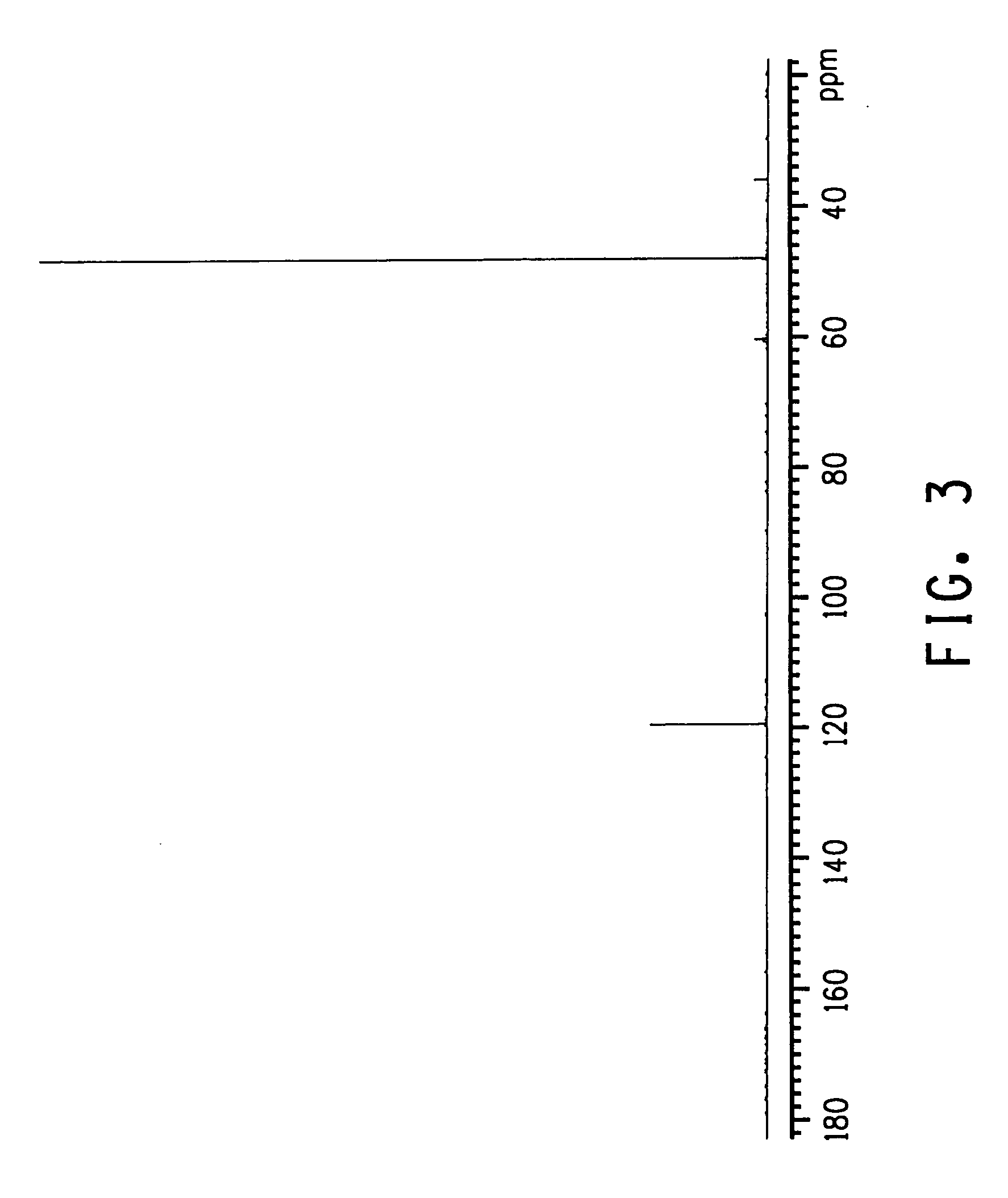
The invention relates to an improved process for preparing 3-aminomethyl-3,5,5-trimethylcyclohexylamine, referred to hereinafter as isophoronediamine or, in abbreviated form, IPDA, by: I. preparation of isophorone by catalyzed aldol condensations with acetone as reactant; II. Reaction of isophorone with HCN to form isophoronenitrile (IPN, 3-cyano-3,5,5-trimethylcyclohexanone); III. catalytic hydrogenation and / or catalytic reductive amination (also referred to as aminative hydrogenation) of 3-cyano-3,5,5-trimethylcyclohexanone, hereinafter called isophoronenitrile or, in abbreviated form, IPN, to give the isophoronediamine.
Owner:EVONIK DEGUSSA GMBH
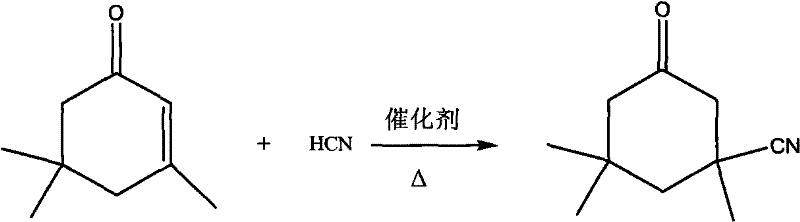
Method for preparing 3-cyan-3,5,5-trimethyl cyclohexanone
InactiveCN101851178AReduce manufacturing costIndustrialization is simplePreparation by hydrogen cyanide additionCyclohexanoneOrganic acid
The invention discloses a method for preparing 3-cyan-3,5,5-trimethyl cyclohexanone. The method comprises the following steps of: taking isophorone as a raw material and adding hydrocyanic acid into the isophorone to perform addition reaction at the high temperature of between 100 and 200 DEG C under the action of an alkali catalyst to produce a crude product of the 3-cyan-3,5,5-trimethyl cyclohexanone; reducing the temperature to 80 to 120 DEG C, adding at least one organic acid serving as a neutralizer and a stabilizer into the reaction mixture and adding an organic solvent or a mixed solvent of the organic solvent and water into the reaction mixture; performing crystallization by cooling and filtering the mixture so as to obtain the finished 3-cyan-3,5,5-trimethyl cyclohexanone. The method can greatly reduce the production cost and has simple industrialized process and easy operation.
Owner:四川省天然气化工研究院
Process of synthesis of compounds having nitrile functions from ethylenically unsaturated compounds
InactiveUS20060252955A1High selectivityReduce instabilityOrganic compound preparationPreparation by hydrogen cyanide additionIsomerizationHCN poisoning
A process of hydrocyanation of diolefins such as butadiene is carried out in the presence of a catalytic system comprising a transition metal and mono- and pluri-dentate organophosphorus ligands. The reaction medium containing branched nitrites subsequently is isomerized in the absence of hydrogen cyanide.
Owner:INV NYLON CHEM AMERICAS LLC
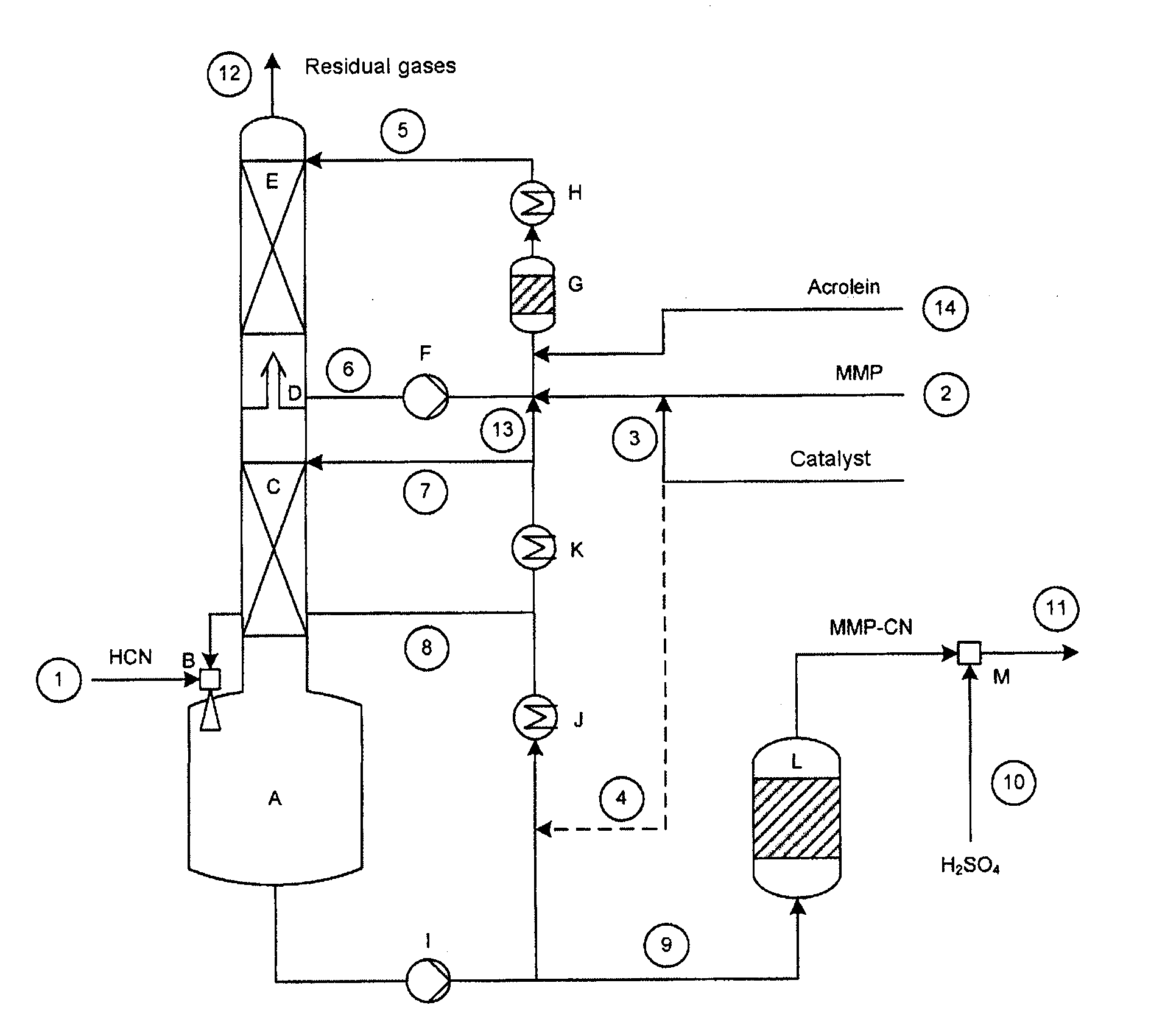
Method for the production of 2-hydroxy-4-(methylthio)butyronitrile from 3-(methylthio)propanal and hydrogen cyanide
ActiveUS20120215022A1Increase response rateLow byproduct formationOrganic compound preparationOrganic chemistry methodsCyanidePropynal
A method for the production of 2-hydroxy-4-(methylthio)butyronitrile having good storage stability in a multi-zone reactor, is provided. 3-methylmercaptopropionaldehyde is reacted with hydrogen cyanide in the presence of a base as catalyst in a main reaction zone of the multizone reactor to form a reaction mixture comprising the 2-hydroxy-4-(methylthio)butyronitrile, unreacted 3-methylmercaptopropionaldehyde, the catalyst and residual amounts of gaseous hydrogen cyanide. The residual gaseous hydrogen cyanide is removed from the main reaction zone to an absorption and post-reaction zone of the reactor which comprises a mixture of 3-methylmercaptopropionaldehyde and the catalyst; and the gaseous hydrogen cyanide is further reacted with the 3-methylmercaptopropionaldehyde in the absorption and post reaction zone. A molar ratio of hydrogen cyanide to 3-(methylthio)propanal in the main reaction zone is from 0.98 to 1.03.
Owner:EVONIK OPERATIONS GMBH
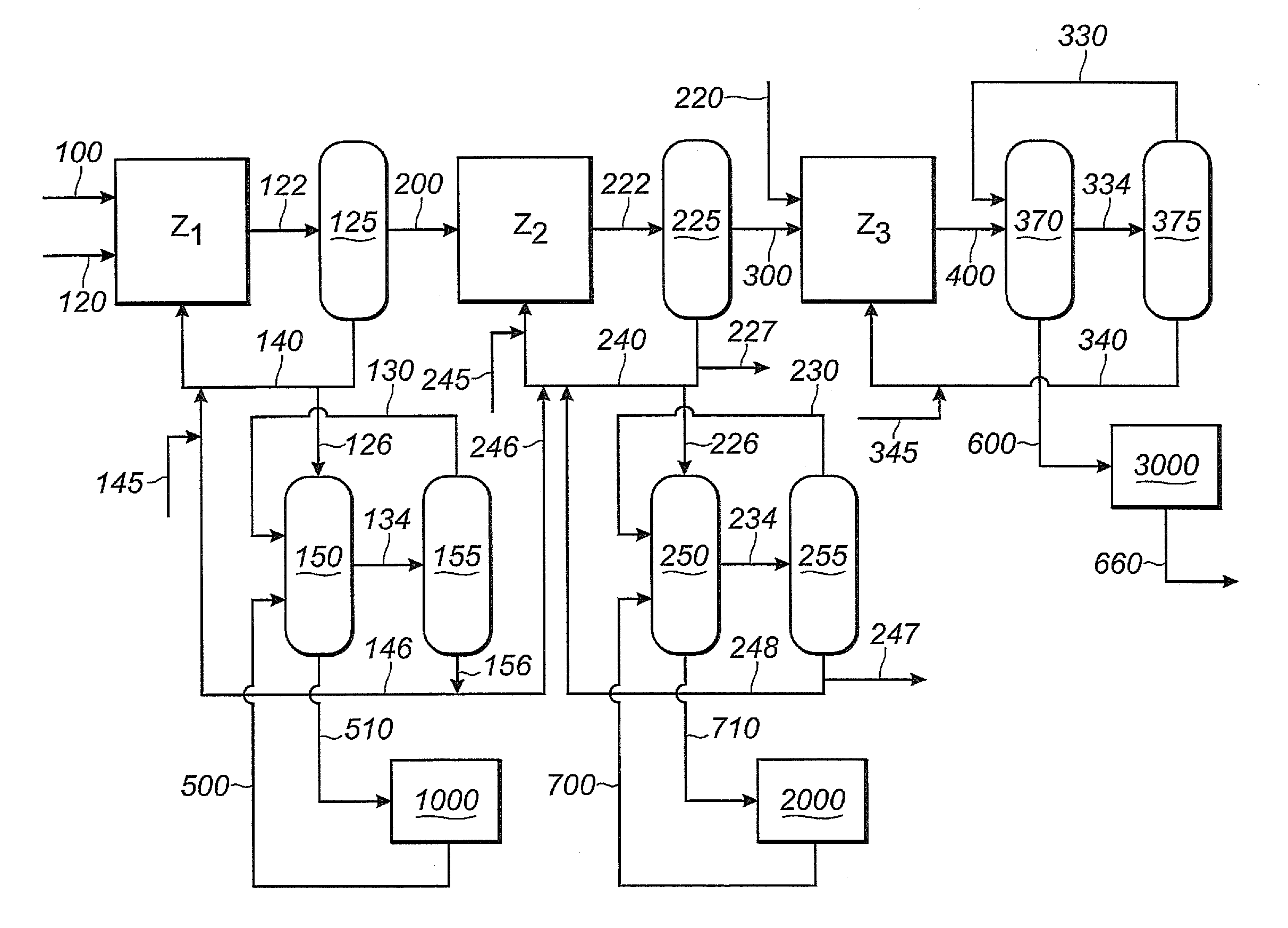
Process for making nitriles
ActiveUS20130211126A1Reduce nickel contentOrganic compound preparationChemical recyclingMethyl groupHydrogen cyanide
Adiponitrile is made by reacting 3-pentenenitrile with hydrogen cyanide. The 3-pentenenitrile is made by reacting 1,3-butadiene with hydrogen cyanide and by isomerizing 2-methyl-3-butenenitrile. The reaction of 1,3-butadiene with hydrogen cyanide to produce 3-pentenenitrile also produces small amounts of dinitrile compounds, including adiponitrile (ADN) and methylglutaronitrile (MGN). Methylglutaronitrile is removed to provide an adiponitrile-enriched stream, which is used in a catalyst purification step.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation of pentenenitriles
ActiveUS8088943B2Organic compound preparationPreparation by hydrogen cyanide additionHydrogenHydrocyanation
Owner:INV NYLON CHEM AMERICAS LLC
Metal-ligand catalyst formation
ActiveUS20130143730A1Excellent complex formation propertyForm is therefore complexOrganic-compounds/hydrides/coordination-complexes catalystsSynthetic resin layered productsSulfurMetallic Nickel
As described herein, nickel treated with sulfur provides a surprisingly effective source of nickel atoms for generating nickel-phosphorus-containing ligand complexes useful as hydrocyanation catalysts.
Owner:INV NYLON CHEM AMERICAS LLC
Use of chiral oxazoline
InactiveCN101099936AOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by hydrogen cyanide additionEthyl groupChirality
Owner:HEFEI UNIV OF TECH
Reactor for preparing isophorone nitrile and method for continuously preparing isophorone nitrile by adopting reactor
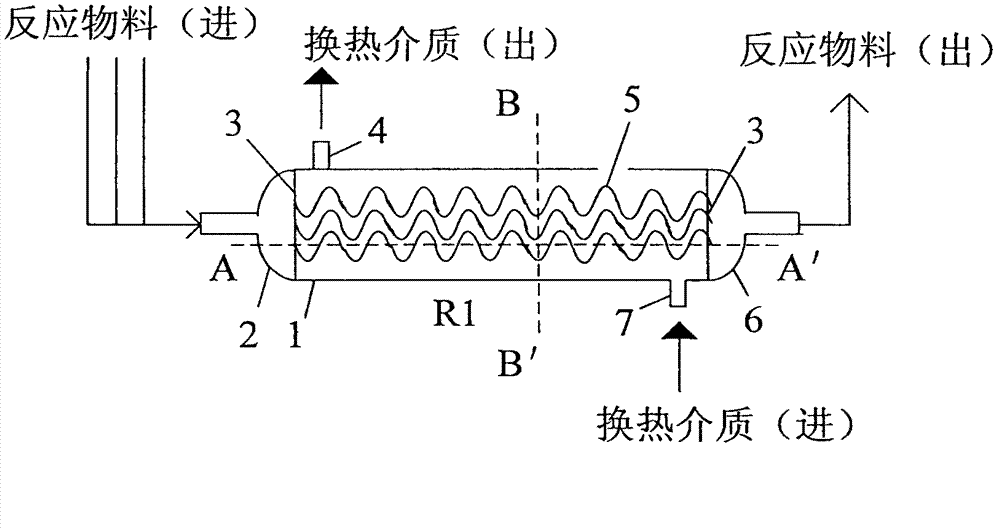
ActiveCN103301799AImprove heat transfer efficiencyImprove mass transfer efficiencyChemical/physical/physico-chemical stationary reactorsPreparation by hydrogen cyanide additionMicron scaleIsophorone
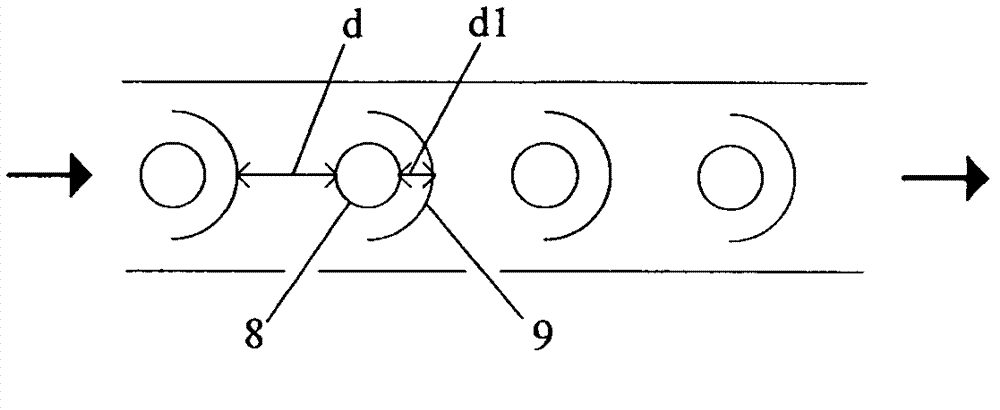
The invention provides a reactor for preparing isophorone nitrile and a method for continuously preparing the isophorone nitrile by adopting the reactor. A plurality of millimeter to micron-sized narrow regular reaction channels which are arranged in parallel are arranged in the reactor, and inner members are arranged in the reaction channels, so that the reactor has good heat and mass transfer effects, and the dwell time can be accurately controlled, thereby obtaining a high reaction conversion rate and a high product yield. Compared with a traditional batch reactor or a common continuous tubular reactor, the method has obvious advantages on many aspects of improvement in the reaction effect in preparation of the isophorone nitrile, safety in production, energy conservation, consumption reduction and the like.
Owner:WANHUA CHEM GRP CO LTD +1
Nickel compositions for preparing nickel metal and nickel complexes
ActiveUS20110311428A1Organic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsNickel saltPhysical chemistry
Nickel compositions for use in manufacturing nickel metal compositions, and specifically to methods of making basic nickel carbonates used to produce nickel metal compositions are disclosed. By varying the molar ratios of carbonates and bicarbonates to nickel salts, the methods provide basic nickel carbonates that produce superior nickel-containing solids that react more effectively with phosphorous-containing ligands. The phosphorous containing ligands can be both monodentate and bidentate phosphorous-containing ligands.
Owner:INV NYLON CHEM AMERICAS LLC
Nickel form for preparation of catalytic nickel-ligand complexes
ActiveUS20130144082A1Form is therefore complexAvoid wastingNickel organic compoundsGlass/slag layered productsParticulatesGram
A novel nickel particulate form is provided that efficiently forms a zero-valent nickel complex with a phosphorus-containing ligands in an organic liquid to form a hydrocyanation catalyst. Particles in the nickel particulate form comprise nickel crystallites. For example, the nickel particulate form can have a BET Specific Surface Area of at least about 1 m2 / gm; an average crystallite size less than about 20-25 nm, the nickel particulate form can have at least 10% of the crystallites in the nickel form can have can have a diameter (C10) of less than about 10 nm, and / or there are on average at least about 1015 surface crystallites per gram nickel. A ratio of BET SSA to C50 for the nickel particulate form can be at least about 0.1×109 m / gm and preferably at least about 0.4×109 m / gm. Methods of preparation and use are also provided.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation method for ethylenically unsaturated organic compounds
The invention concerns a hydrocyanation method for ethylenically unsaturated organic compounds in particular to obtain nitriles, and more particularly hydrocyanation of substituted diolefins or of olefins for producing dinitriles, and / or the isomerization of nitriles obtained by hydrocyanation. The invention more particularly concerns hydrocyanation catalysed by a nickel-based compound. Thus the catalyst used in said production method is treated in output with hydrogen cyanide to recover and re-dissolve the nickel precipitated in the form of nickel hydroxide. The method enables to regenerate and prolong the life span of a catalyst charge. Moreover, it enables to reduce pollution in the installations using said method.
Owner:RHODIA POLYAMIDE INTERMEDIATES
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
ActiveCN1735460AOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsChlorideSolvent
A process for preparing a hydrocyanation catalyst comprising contacting a bidentate phosphorous-containing ligand with nickel chloride in the presence of a nitrile solvent and a reducing metal which is more electropositive than nickel the nickel chloride being introduced as an aqueous solution and the water being removed concurrently with the reduction reaction by azeotropic distillation.
Owner:INVISTA TECHNOLOG IES S A R L
Preparation method of isophorone nitrile
ActiveCN102199109AShort processReduce consumptionPreparation by hydrogen cyanide additionIsophoroneMaterial consumption
The invention provides a preparation method of isophorone nitrile. According to the method, addition reaction between isophorone and hydrocyanic acid is performed to produce isophorone nitrile in the presence of a basic catalyst, wherein the basic catalyst is a basic anion exchange resin. The crude product prepared by the preparation method can be directly purified, the steps of catalyst treatment for separating the product from the catalyst thereby simplifying the preparation process, reducing equipment investment, shortening material process flow and lowering material consumption, and achieving more environmentally-friendly effect.
Owner:WANHUA CHEM GRP CO LTD +1
Popular searches
Catalytic reactions Catalyst activation/preparation Nickel halides Preparation by carbon monoxide reaction Metal/metal-oxides/metal-hydroxide catalysts Hydrogen cyanide preparation/purification/separation Nickel carbonates Chemical/physical/physico-chemical microreactors Preparation from carboxylic acid esters/lactones Withdrawing sample devices
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com