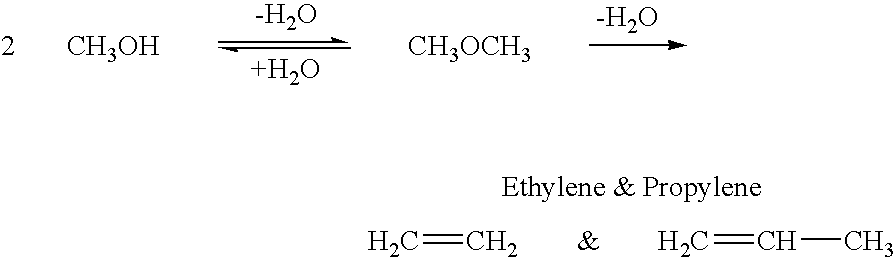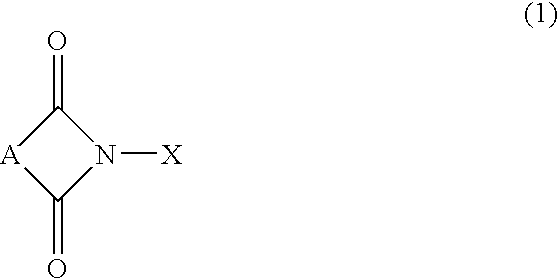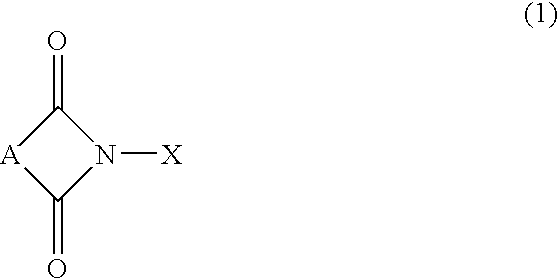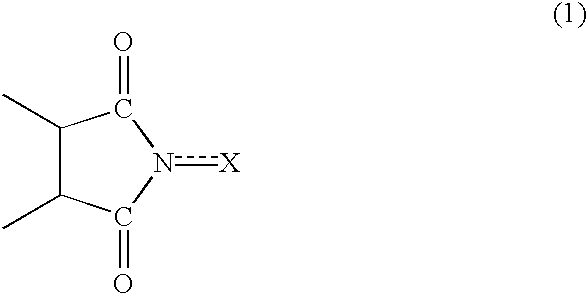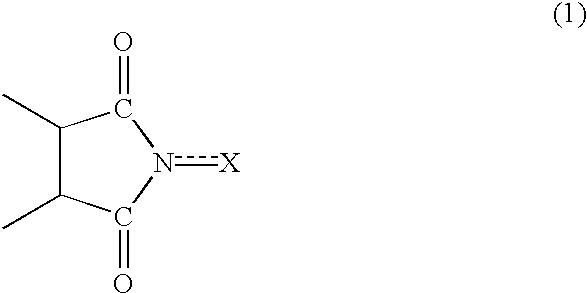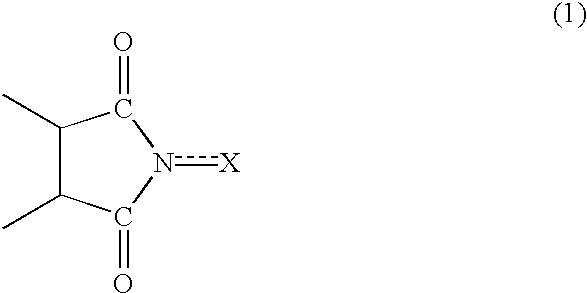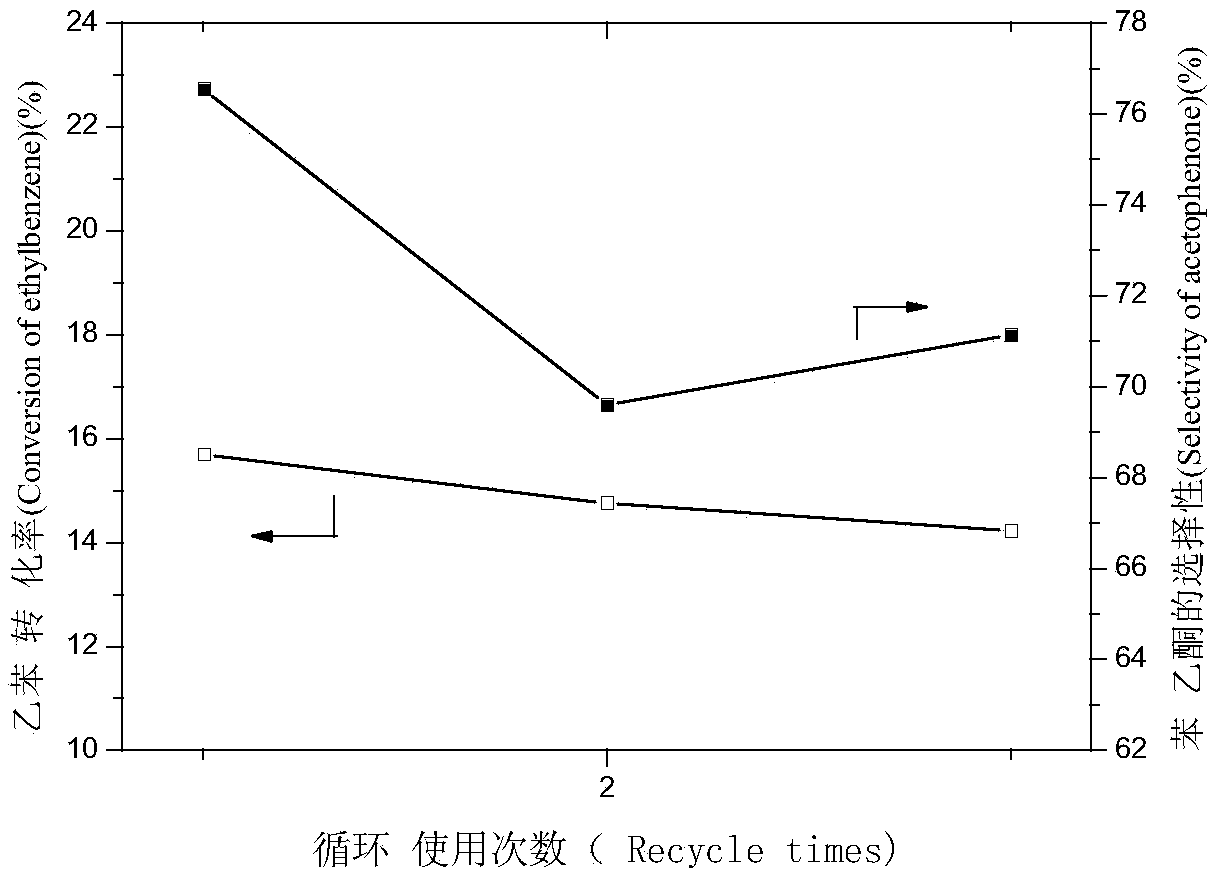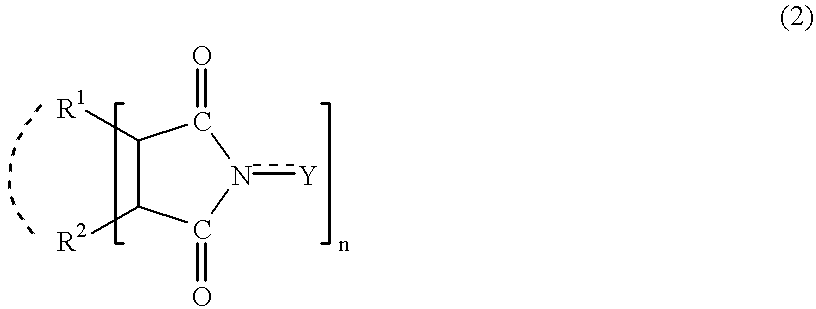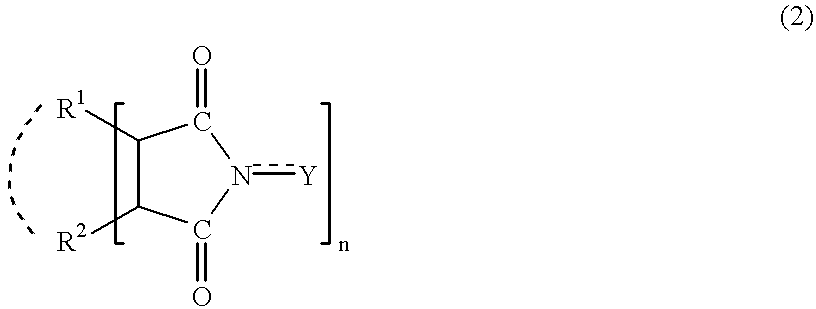Patents
Literature
1067results about "Preparation by oxidation reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
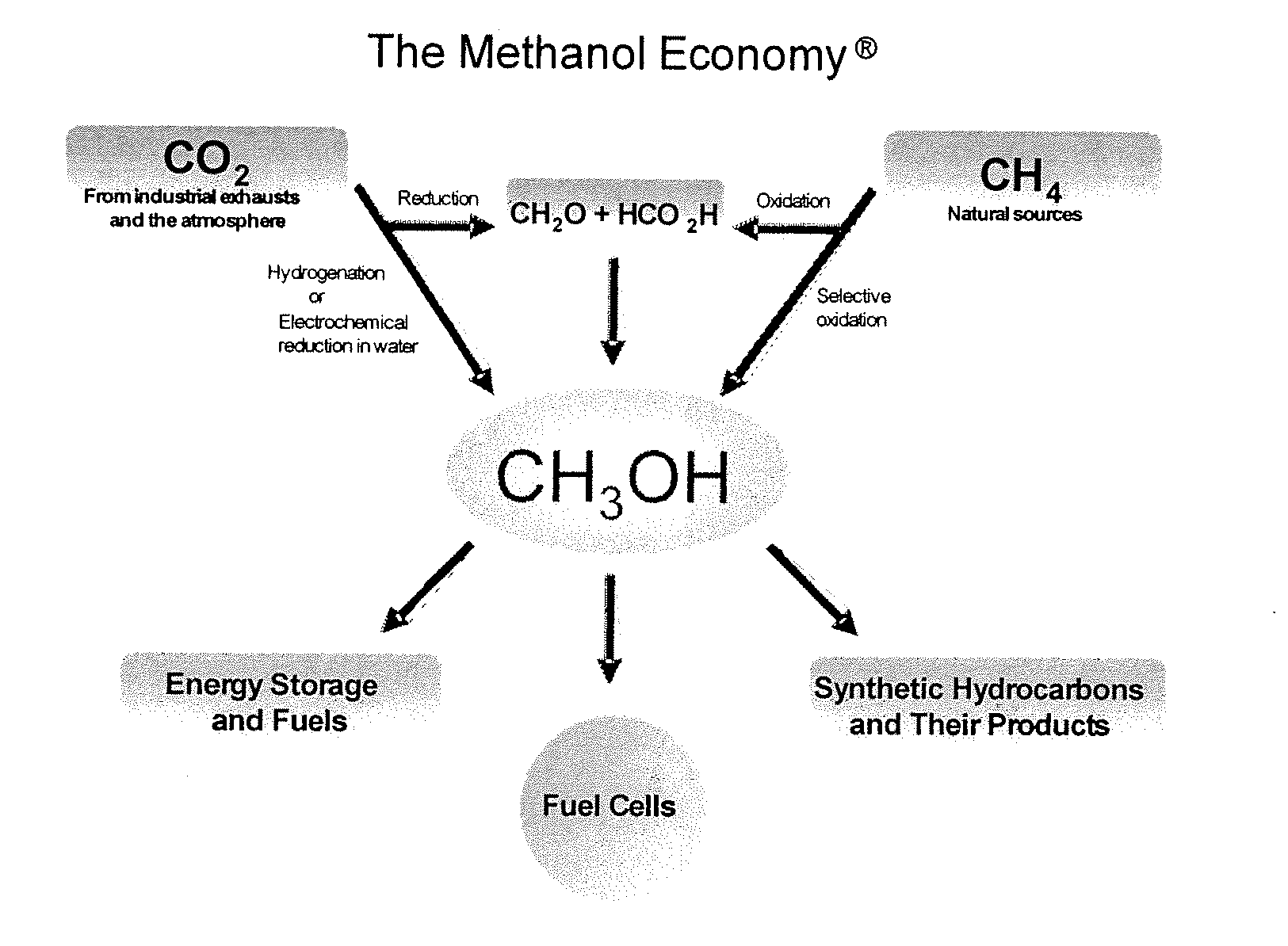
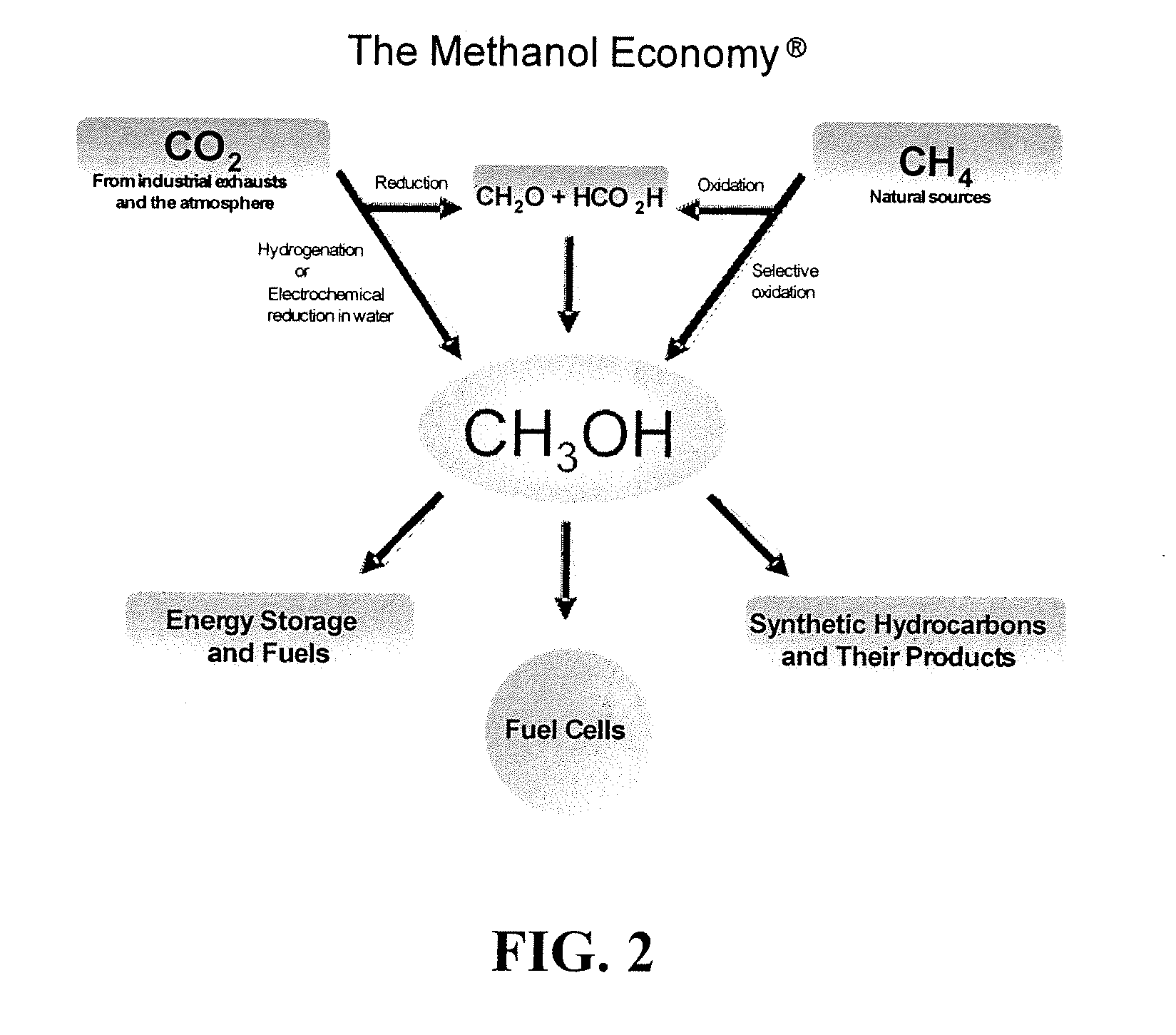
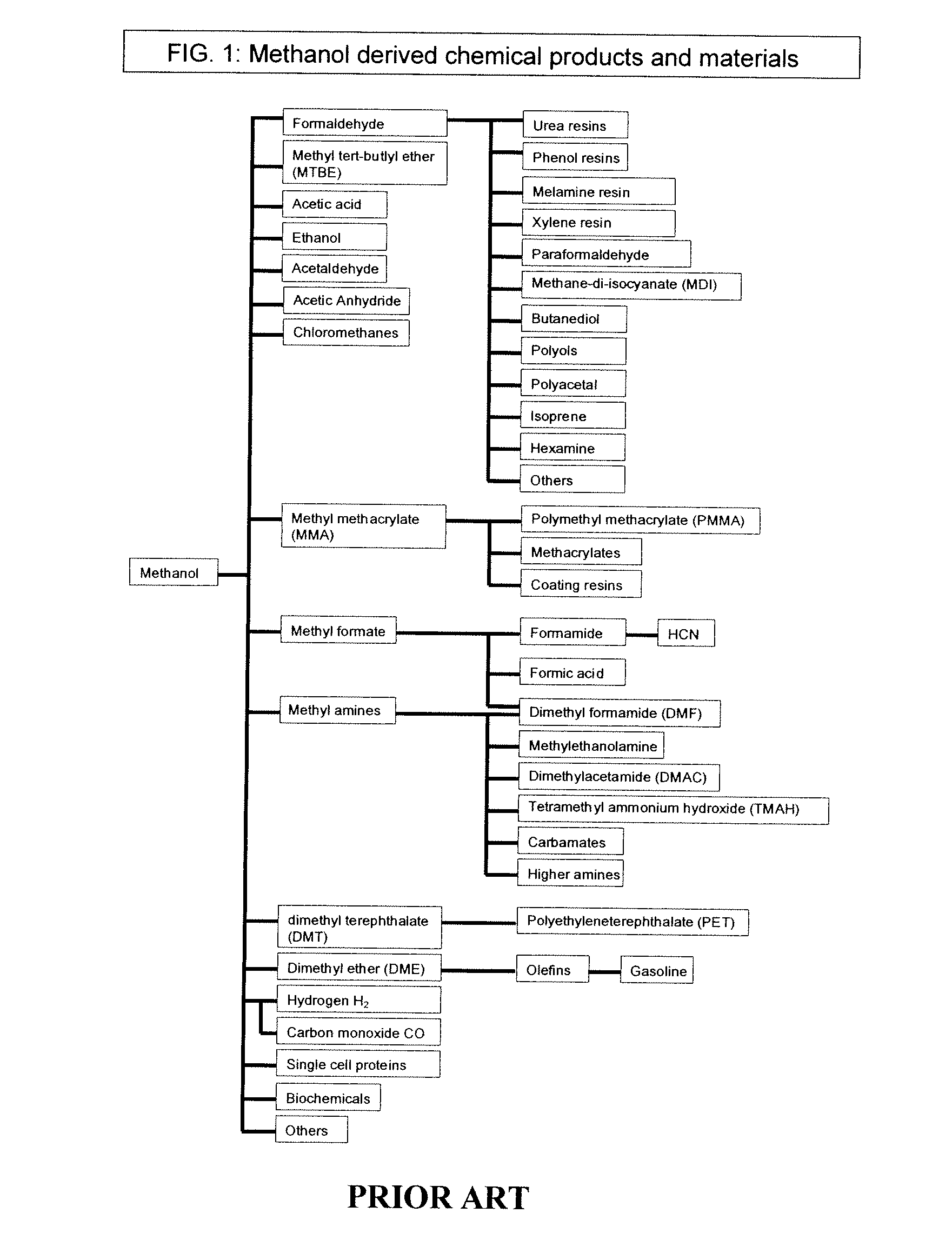
Selective oxidative conversion of methane to methanol, dimethyl ether and derived products
ActiveUS20060235088A1Minimize or eliminate the disadvantages or dangers inherentPreparation by oxidation reactionsElectrolysis componentsFormate EstersDimethyl ether
The present invention relates to a method of producing methanol from a methane source by oxidizing methane under conditions sufficient to a mixture of methanol and formaldehyde while minimizing the formation of formic acid and carbon dioxide. The oxidation step is followed by treatment step in which formaldehyde is converted into methanol and formic acid which itself can further be converted into methanol via catalytic hydrogenation of intermediately formed methyl formate.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method of hydrocarbon preservation and environmental protection
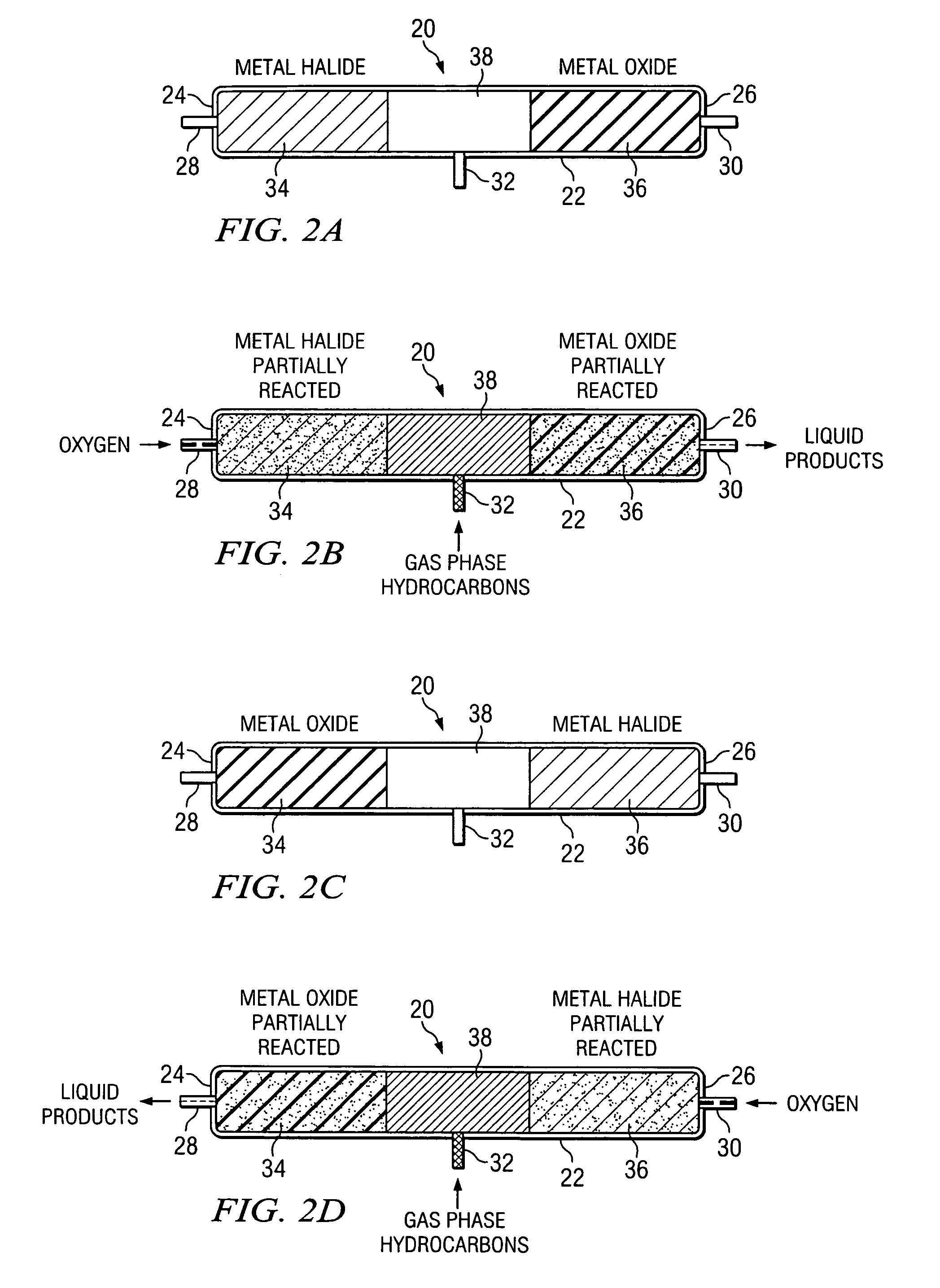
InactiveUS7019182B2Preparation by oxidation reactionsOxygen-containing compound preparationLiquid productGas phase
Owner:REACTION 35 LLC
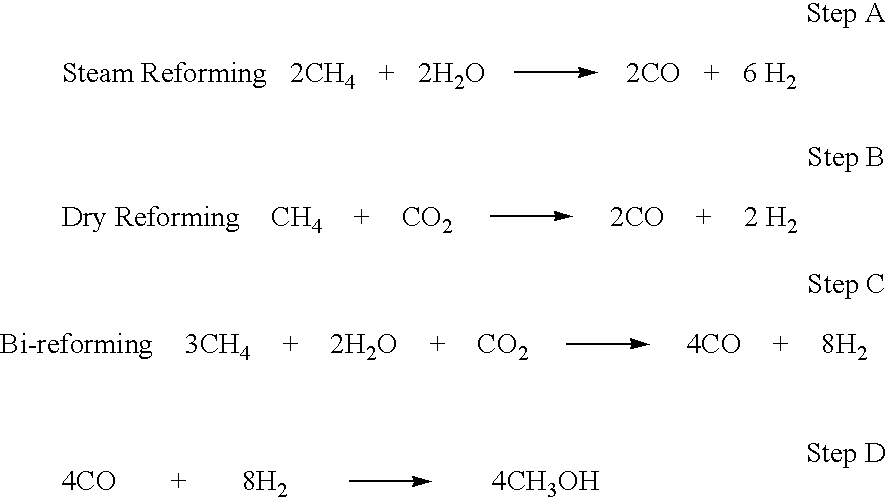
Conversion of carbon dioxide to methanol and/or dimethyl ether using bi-reforming of methane or natural gas
ActiveUS20080319093A1Raw materials are simplePromote recoveryPreparation by oxidation reactionsHydrogenCatalytic methodHydrogen
The invention discloses a method of converting carbon dioxide to methanol and / or dimethyl ether using any methane source or natural gas consisting of a combination of steam and dry reforming, in a specific ratio to produce a 2:1 molar ratio of hydrogen and carbon monoxide with subsequent conversion of the CO and H2 mixture exclusively to methanol and / or dimethyl ether. This method is termed the BI-REFORMING™ process. Dehydrating formed methanol allows producing dimethyl ether (DME) using any suitable catalytic method, including use of solid acid catalysts. When recycling formed water into the bi-reforming step the conversion of carbon dioxide with methane produces exclusively dimethyl ether without any by-product formation and complete utilization of hydrogen
Owner:UNIV OF SOUTHERN CALIFORNIA
Crystalline MWW-type titanosilicate catalyst for producing oxidized compound, production process for the catalyst, and process for producing oxidized compound by using the catalyst
InactiveUS20040092757A1Material nanotechnologyPreparation by oxidation reactionsDouble bondOxidizing agent
A crystalline titanosilicate catalyst which is usable as a catalyst in the oxidation reaction of a compound having a carbon-carbon double bond and at least one other functional group, a process for producing the catalyst, and a process for producing an oxidized compound by an oxidation reaction using the catalyst. It has been found that a crystalline titanosilicate having a structural code of MWW effectively functions as a catalyst in an oxidation reaction of a compound having a carbon-carbon double bond and at least one other functional group wherein the carbon-carbon double bond of the compound is oxidized by using a peroxide as an oxidizing agent, thereby to highly selectively provide an intended oxidized compound.
Owner:SHOWA DENKO KK
Conversion of carbon dioxide to dimethyl ether using bi-reforming of methane or natural gas
The invention provides for a method of forming dimethyl ether by bimolecular dehydration of methanol produced from a mixture of hydrogen and carbon dioxide obtained by reforming of methane, water and carbon dioxide in a ratio of about 3:2:1. Subsequent use of water produced in the dehydration of methanol in the bi-reforming process leads to an overall ratio of carbon dioxide to methane of about 1:3 to produce dimethyl ether.
Owner:UNIV OF SOUTHERN CALIFORNIA
Process for preparation of hydroperoxides
Owner:REPSOL QUIMICA
Method of separating imide compound
InactiveUS20020169331A1Efficient separationPreparation by oxidation reactionsOrganic compound preparationImideCompound a
A reaction product and an imide compound can be separated from a reaction mixture obtained by reacting a substrate in the presence of the imide compound having an imide unit represented by the following formula (1): wherein X represents an oxygen atom, a hydroxyl group or an acyloxy group by (A1) a solvent-crystallization step for crystallizing the imide compound with at least one solvent selected from the group consisting of a hydrocarbon, a chain ether and water, (A2) a cooling-crystallization step for crystallizing the reaction product by cooling, or (B) an extraction step for distributing the reaction product into a phase of a water-insoluble solvent and the imide compound into a phase of an aqueous solvent, respectively by using the aqueous solvent containing at least water and the water-insoluble solvent separable from the aqueous solvent. Further, the imide compound and the metal catalyst can be separated from a mixture containing the imide compound and the metal catalyst by (C) a solvent-crystallization step for crystallizing the imide compound by using a solvent for crystallization, (D) an absorption step for absorbing the metal catalyst by an absorption treatment, or (E) an extraction step for distributing the imide compound into a phase of a water-insoluble solvent and the metal catalyst into a phase of an aqueous solvent, respectively by using the aqueous solvent containing at least water and the water-insoluble solvent separable from the aqueous solvent.
Owner:DAICEL CHEM IND LTD
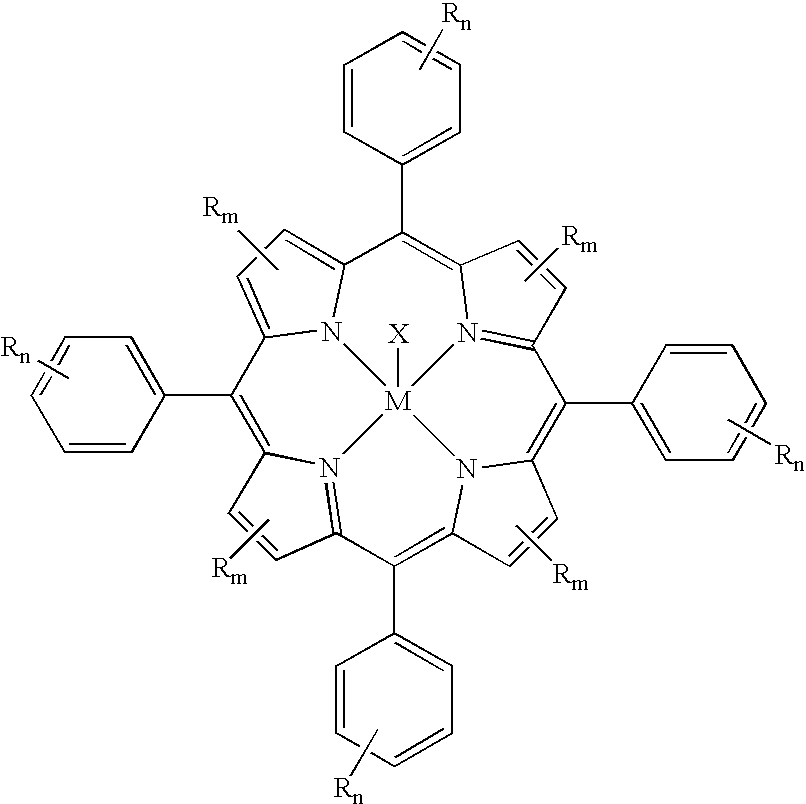
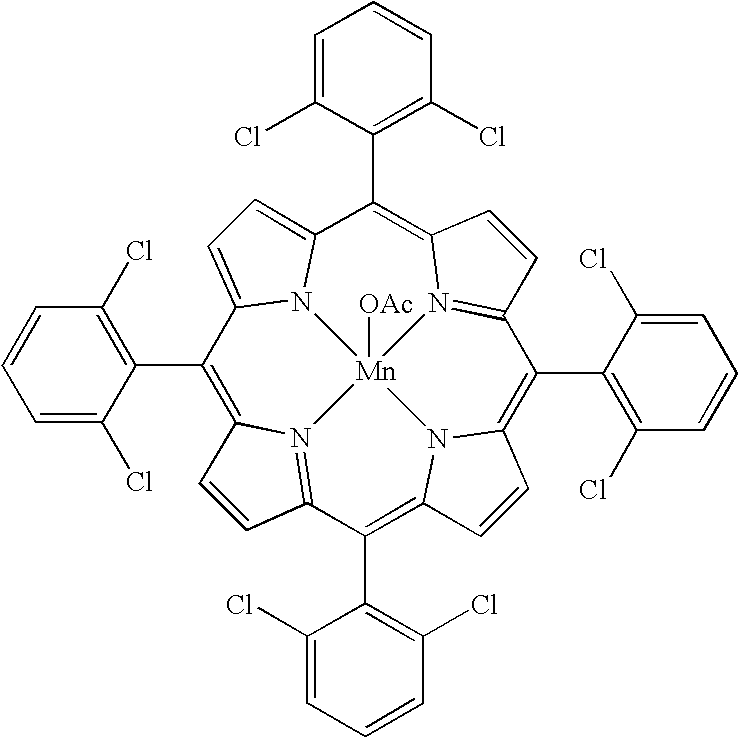
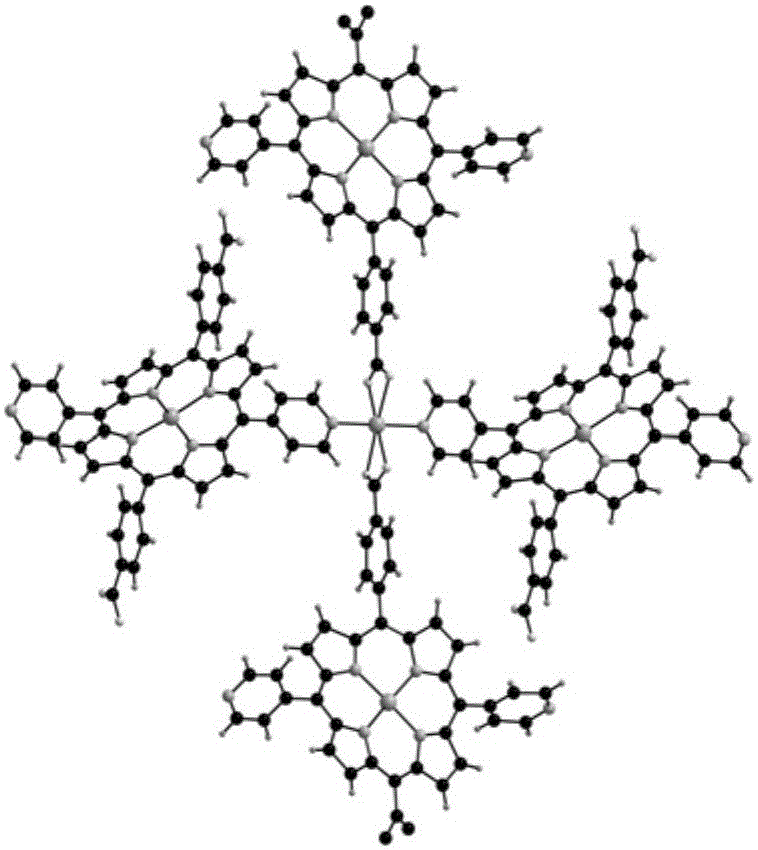
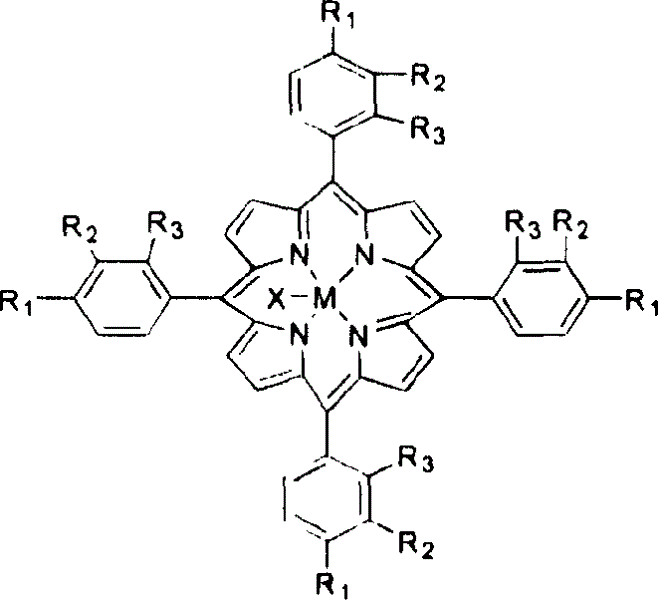
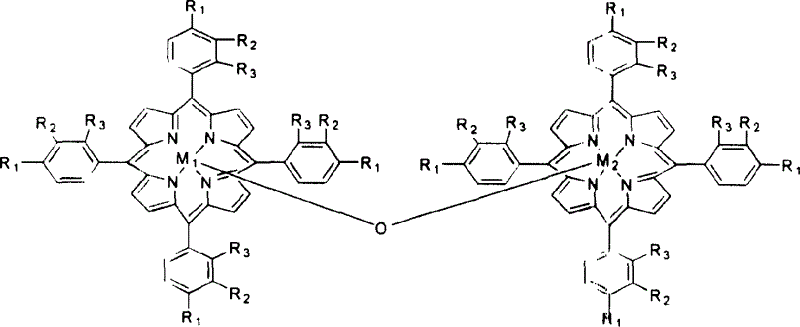
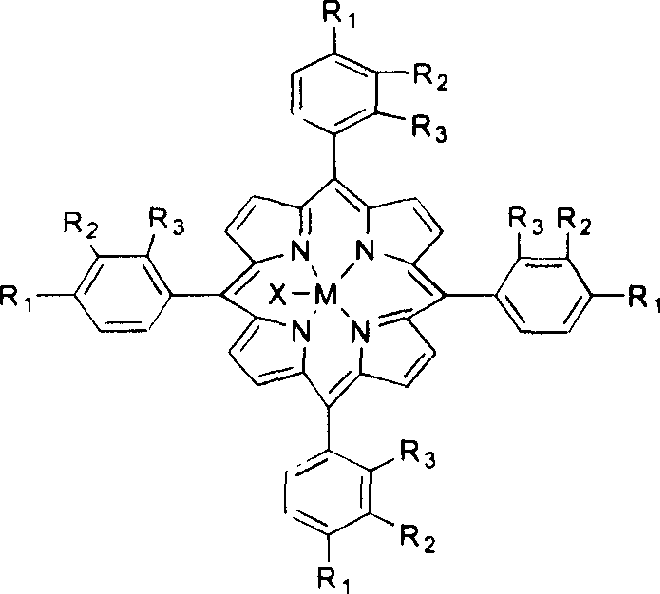
Method for atmospheric catalytic oxidation of cyclohexane by metalloporphyrin
InactiveCN1405131APreparation by oxidation reactionsOxygen compounds preparation by hydrocarbon oxidationCyclohexanoneReaction temperature
The invention relates to a new process in which under the catalytic action of metal porphyrin the cyclohexane can be selectively oxdated by air and made into cyclohexanol and cyclohexanone. Under the condition of 2-10 atm air, reaction temp. is 125-150 deg.C, it selects and uses micro-oxo double metal porphyrin and single metal porphyrin or their solid carrier as main catalyst, and uses transition metal salt or oxide as co-catalyst, the metal porphyrin can high-effectively and high-selectively catalyze oxidation of cyclohexane by using air in the biological concentratino as biological enzyme. The dose of metal porphyrin is small and said metal porphyrin can be repeatedly used, and said catalyst dose also is small, its catalystic effect is good, said catalyst can be used for making homogeneous catalysis, and after it is solid-carried, it also can be used for making heterogeneous catalysis.
Owner:HUNAN UNIV
Preparation method of monatomic dispersion catalyst with high catalytic performance
InactiveCN103566935AImprove catalytic performanceImprove stabilityPreparation by oxidation reactionsOrganic compound preparationAlkaneMicrosphere
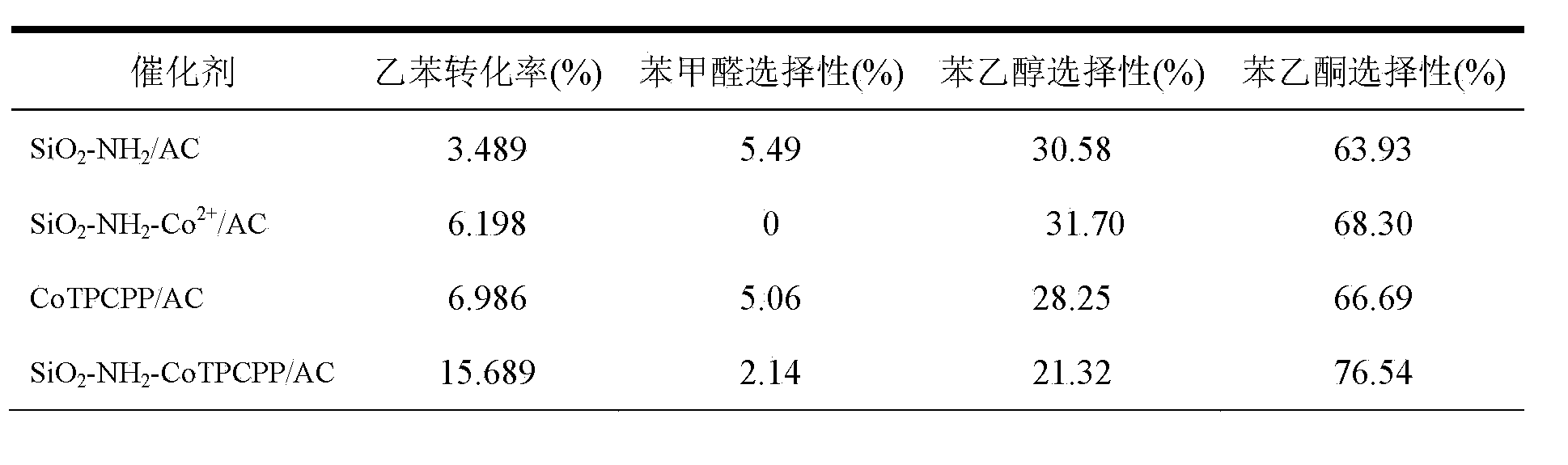
The invention belongs to the technical field of catalysis and relates to a method for preparing a monatomic cobalt catalyst by roasting silicon dioxide pellets and immobilizing metalloporphyrin. The method is realized by adopting the following technical scheme: namely stirring and dispersing nano silicon dioxide particles, dropwise adding 3-aminopropyl triethoxy silane, so as to obtain amino modified SiO2 pellets, then linking monocarboxyl metalloporphyrin (CoTPCPP) onto an amino modified SiO2 carrier through organic complexing, carbonizing porphyrin through high-temperature calcination in an inert atmosphere, and loading highly dispersed cobalt atoms onto the amino modified SiO2 pellets, thus an amino modified silicon dioxide pellet loaded monatomic cobalt catalyst is obtained. The monatomic dispersion catalyst has high catalytic activity and stability on a molecular oxygen selective ethylbenzene catalytic oxidation reaction in absence of solvent; meanwhile, a preparation method is simple, and product quality can be easily controlled, so that the monatomic dispersion catalyst is applicable to the fields of alkane selective oxidation reactions and synthesis of medical intermediates.
Owner:HUNAN UNIV
Multiphasic microchannel reactions
InactiveUS7118920B2Improved fluid separationHigh reactivityPreparation by oxidation reactionsOrganic chemistry methodsUnit operationPhase-transfer catalyst
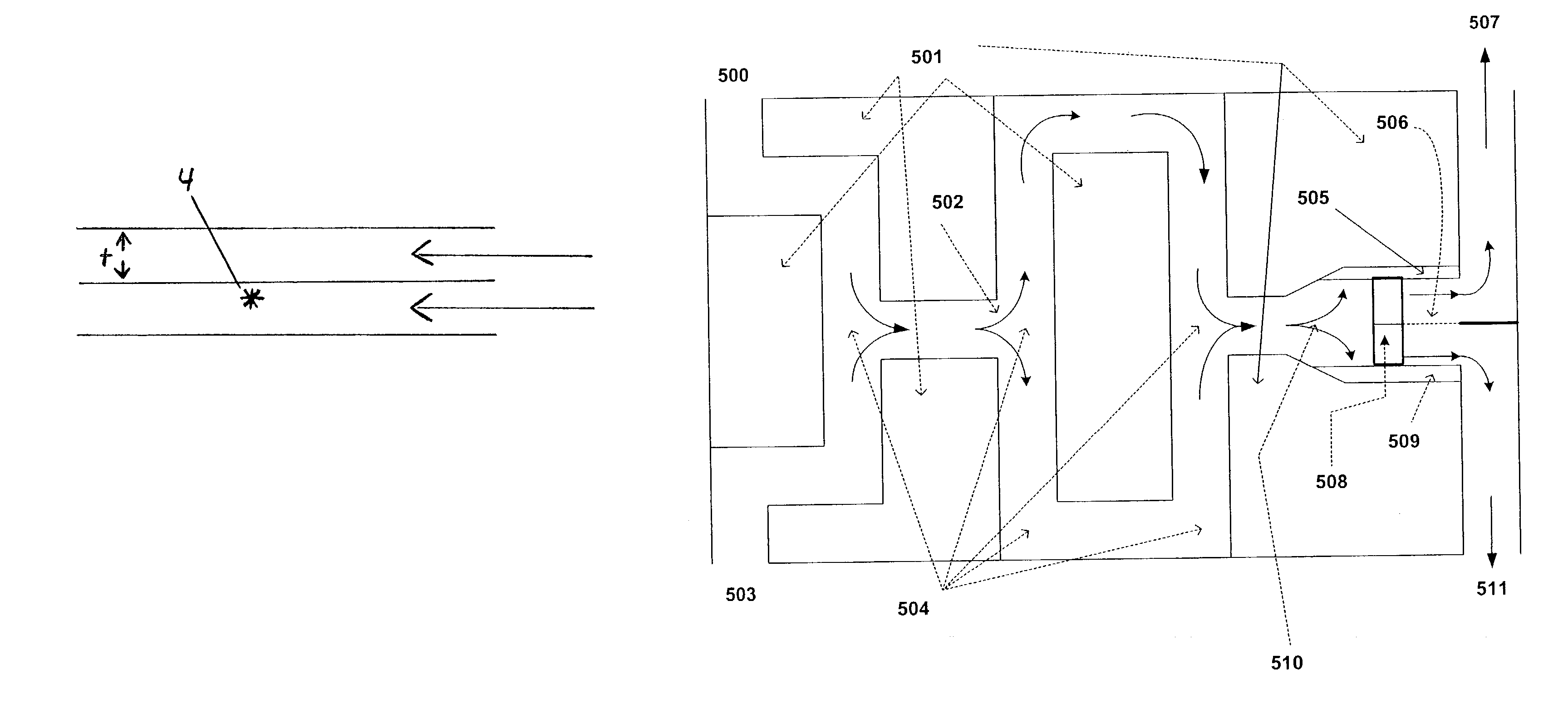
Multiphasic reactions, especially those reactions using a phase transfer catalyst, are conducted in microchannel apparatus. Advantageously, these reactions can be conducted with two, planar microlayers of reactants in adjacent laminar flow streams. Microchannel apparatus and methods for conducting unit operations such as reactions and separations in microchannel apparatus is also described. Microchannel apparatus can provide advantages for controlling reactions and separating products, solvents or reactants in multiphase reactions.
Owner:BATTELLE MEMORIAL INST
Adamantane derivatives and process for producing them
InactiveUS6392104B1Efficiently provideImprove isolationPreparation by oxidation reactionsOrganic compound preparationPtru catalystDouble bond
In the presence of an imide compound (e.g., N-hydroxyphthalimide) shown by the formula (2):wherein R1 and R2 independently represents a hydrogen atom, a halogen atom, an alkyl group, an aryl group, a cycloalkyl group; or R1 and R2 may bond together to form a double bond or an aromatic or non-aromatic ring; Y is O or OH and n=1 to 3;or the imide compound and a co-catalyst (e.g., a transition metal compound), an adamantane derivative having a functional group such as a nitro group, an amino group, a hydroxyl group, a carboxyl group, a hydroxymethyl group and an isocyanato group is oxidized with oxygen. According to the above method, an adamantane derivative having a hydroxyl group together with a functional group such as a nitro group, an amino group, a hydroxyl group, a carboxyl group, a hydroxymethyl group and an isocyanato group is efficiently obtained.
Owner:DAICEL CHEM IND LTD
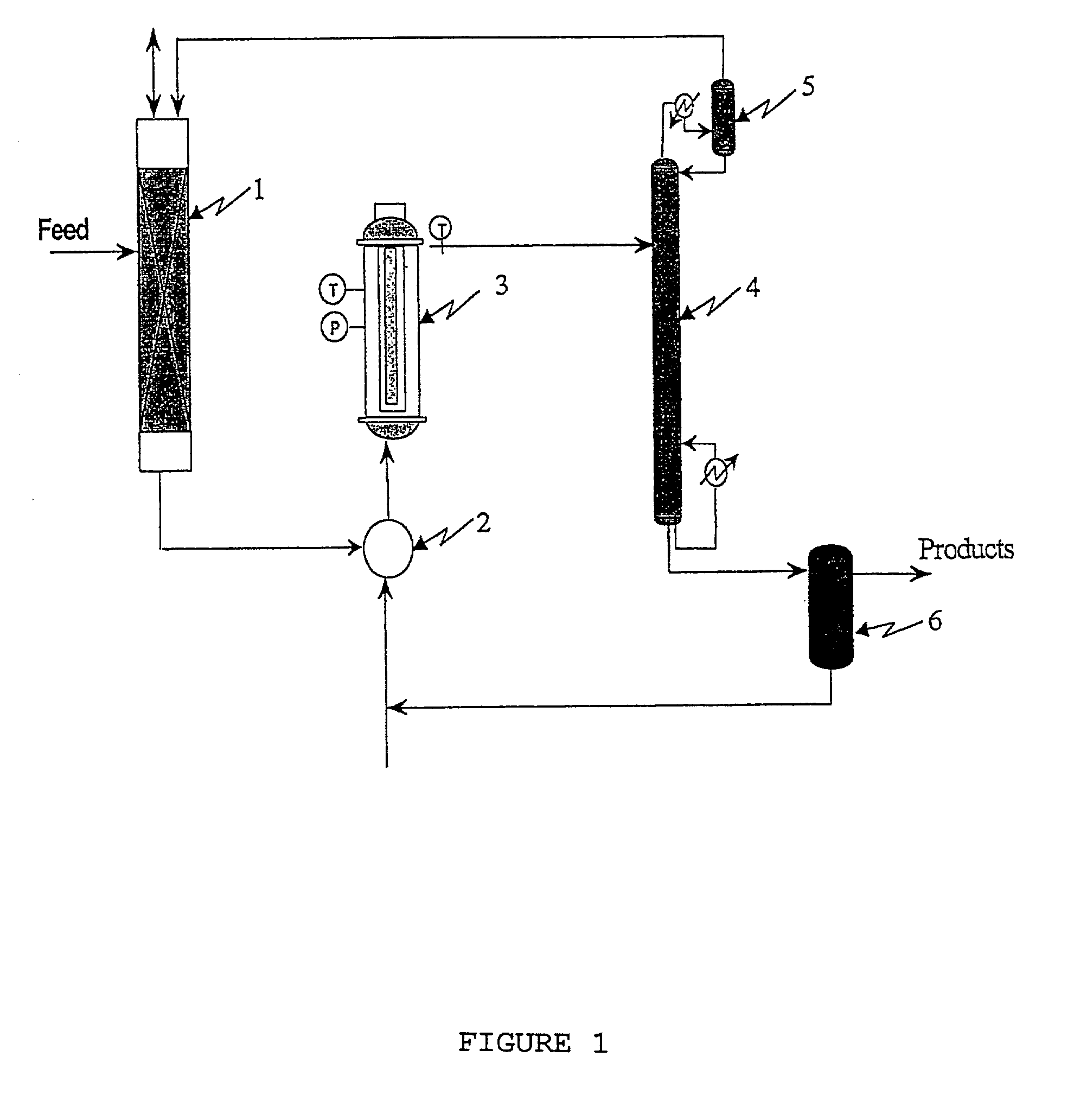
Process for the production of synthesis gas
InactiveUS20010006615A1Improve conversion yieldOxygen-containing compound preparationPreparation by oxidation reactionsSyngasThermodynamics
A process for the production of synthesis gas for obtaining compounds such as ammonia or methanol, in which hydrocarbons and steam are reacted first in a primary reforming section (11) and then-together with oxygen-in a secondary reforming section (12), thus obtaining CO, CO2, H2 and possibly N2 which are then fed to a carbon monoxide conversion section (13, 14), is distinguished by the fact of reacting hydrocarbons, steam and oxygen in an autothermal reforming section (20) provided in parallel with respect to other reforming sections (11, 12), and feeding the so produced CO, CO2, H2 and possibly N2 to the carbon monoxide conversion section (13, 14).
Owner:BADANO MARCO
Regenerative adsorbents of modified amines on solid supports
ActiveUS20160199810A1Facilitated reaction kineticsReduce volatilityPreparation by oxidation reactionsOxygen-containing compound preparationSorbentAdsorption desorption
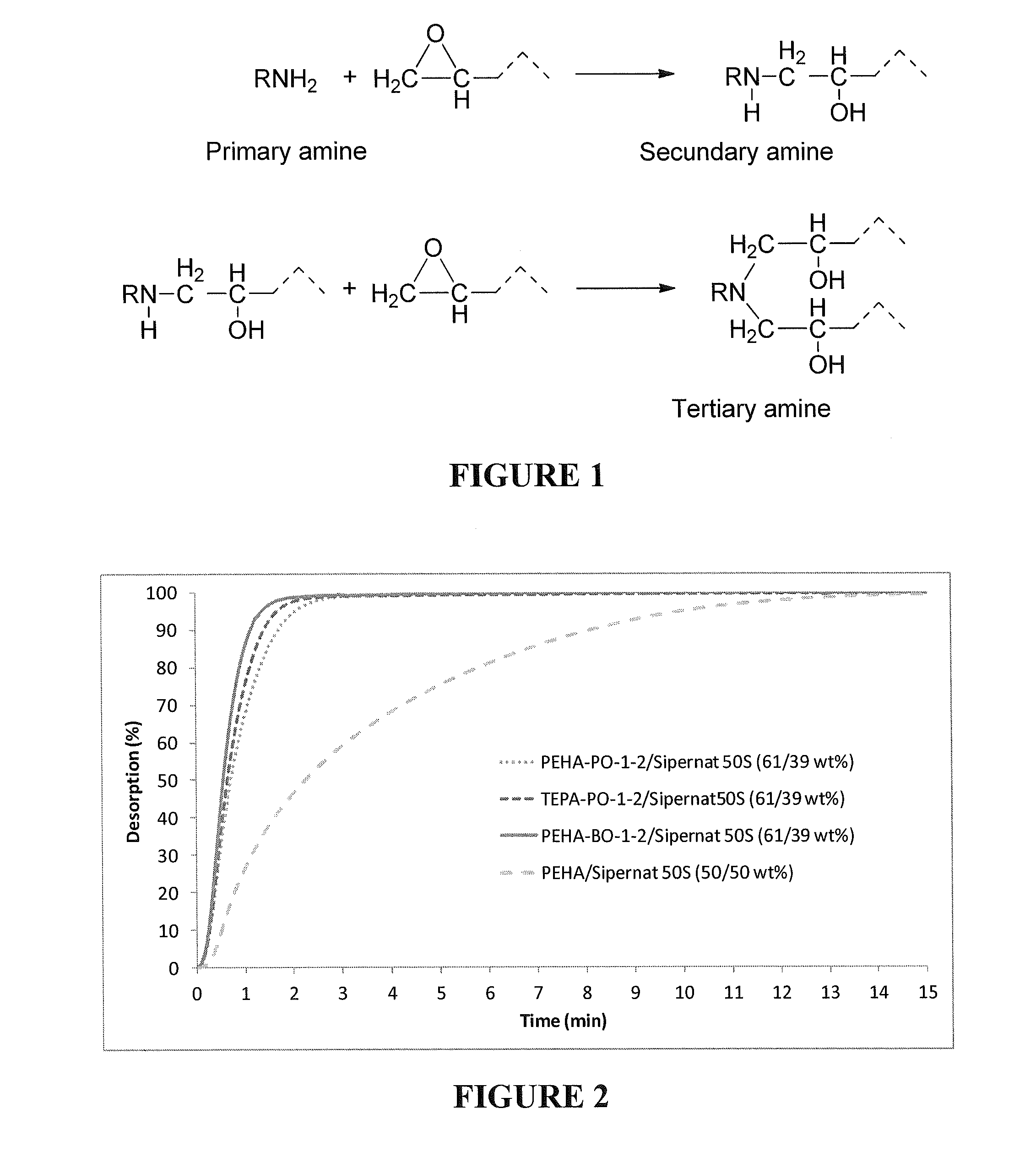
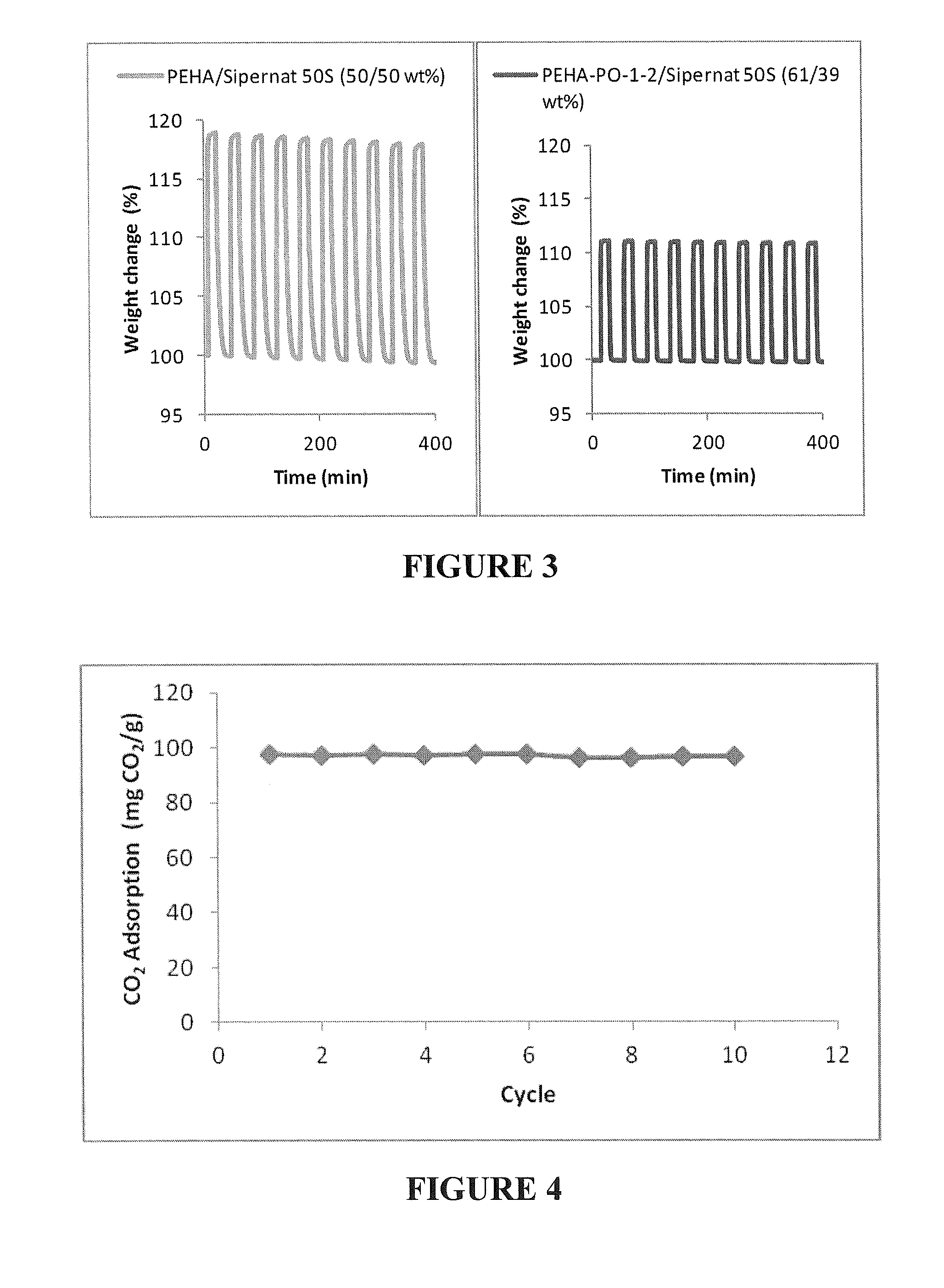
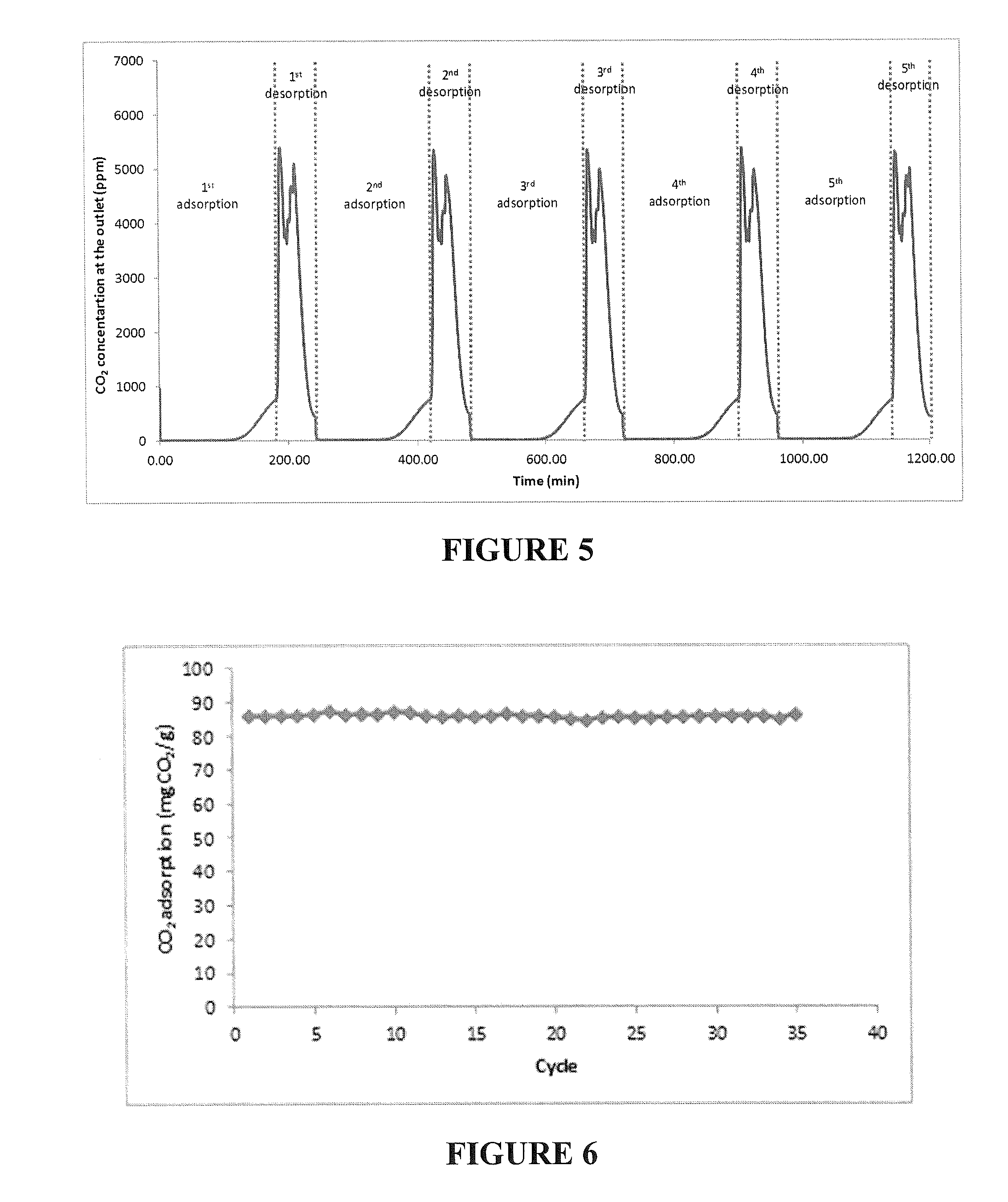
The invention relates to regenerative, solid sorbents for adsorbing carbon dioxide from a gas mixture, including air, with the sorbent including a modified polyamine and a solid support. The modified polyamine is the reaction product of an amine and an epoxide. The sorbent provides structural integrity, as well as high selectivity and increased capacity for efficiently capturing carbon dioxide from gas mixtures, including the air. The sorbent is regenerative, and can be used through multiple cycles of adsorption-desorption.
Owner:UNIV OF SOUTHERN CALIFORNIA
Catalyst for preparing methane by low-temperature oxidization of methane and preparation method and application thereof
InactiveCN101875016AReduce energy consumptionMiniaturizationPreparation by oxidation reactionsMolecular sieve catalystsMolecular sieveCopper oxide
The invention discloses a catalyst for preparing methane by low-temperature oxidization of methane and a preparation method and application thereof. The catalyst consists of a molecular sieve carrier and an active ingredient supported on the carrier; and the active ingredient comprises copper oxide or copper oxide and noble metal compound active ingredient, wherein the mass ratio of the molecular sieve to the copper in the copper oxide to the noble metal is 100:1-5:0-1. The molecular sieve serves as the carrier, and the preparation method for the catalyst comprises the following steps of: mixing a precursor copper acetate of the basic active ingredient or / and a metal precursor of the noble metal active ingredient in a certain ratio to prepare solution; and soaking the molecular sieve in the solution to allow the metals of the active ingredient to be supported on the carrier, and washing, drying and baking to prepare the catalyst. The catalyst can catalyze the methane oxidation at low temperature to prepare the methanol; a catalytic reaction system is simple; and the yield and selectivity of the target product methanol are high.
Owner:CHINA NAT OFFSHORE OIL CORP +1
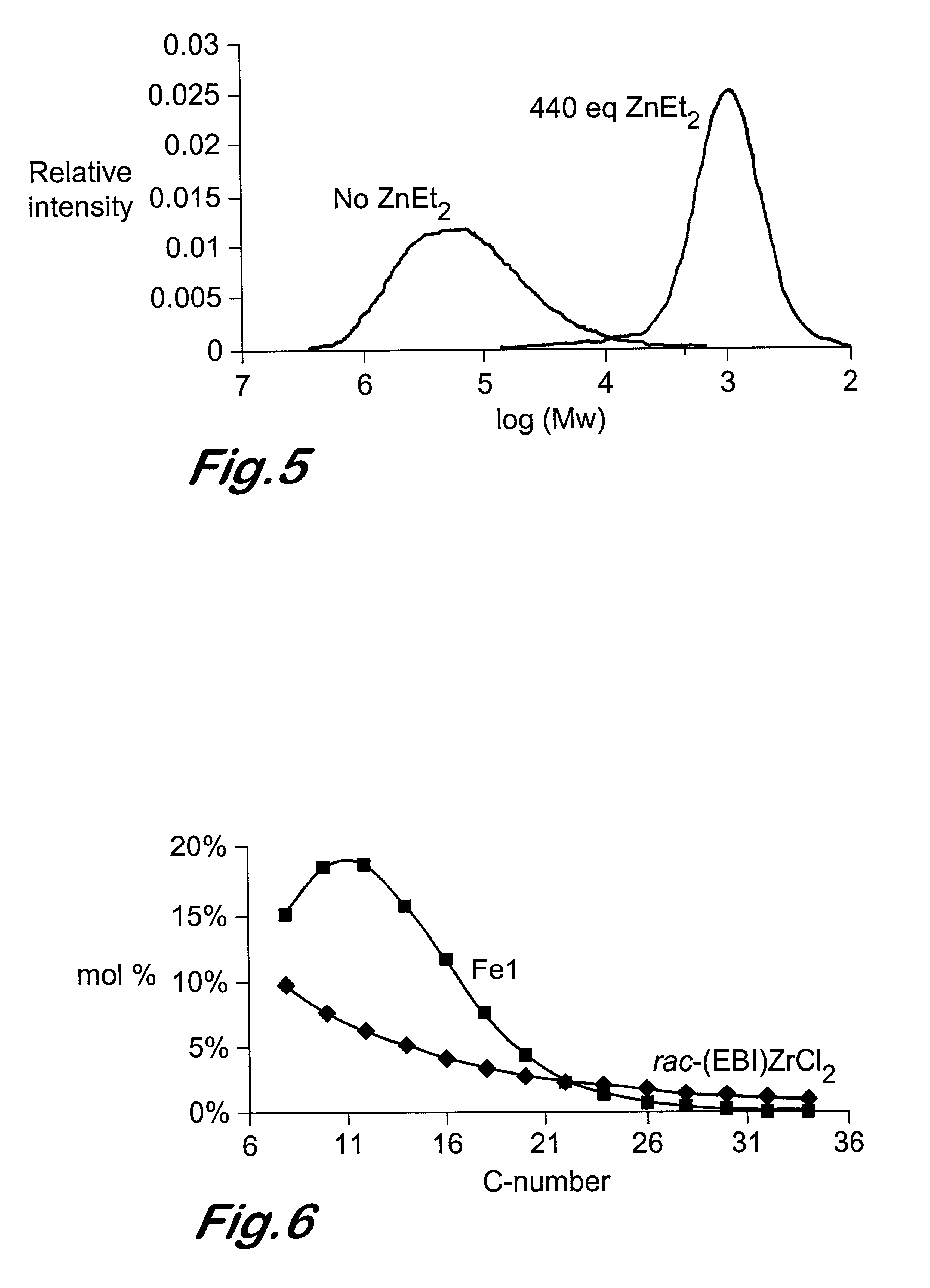
Chain growth reaction process
InactiveUS7087686B2Preparation by oxidation reactionsOxygen-containing compound preparationAlcoholLanthanide
A process is disclosed for the preparation of zinc alkyl chain growth products via a catalysed chain growth reaction of an alpha-olefin on a zinc alkyl, wherein the chain growth catalyst system employs a group 3-10 transition metal, or a group 3 main group metal, or a lanthanide or actinide complex, and optionally a suitable activator. The products can be further converted into alpha-olefins by olefin displacement of the grown alkyls as alpha-olefins from the zinc alkyl chain growth product, or into primary alcohols, by oxidation of the resulting zinc alkyl chain growth product to form alkoxide compounds, followed by hydrolysis of the alkoxides.
Owner:INEOS SALES (UK) LTD
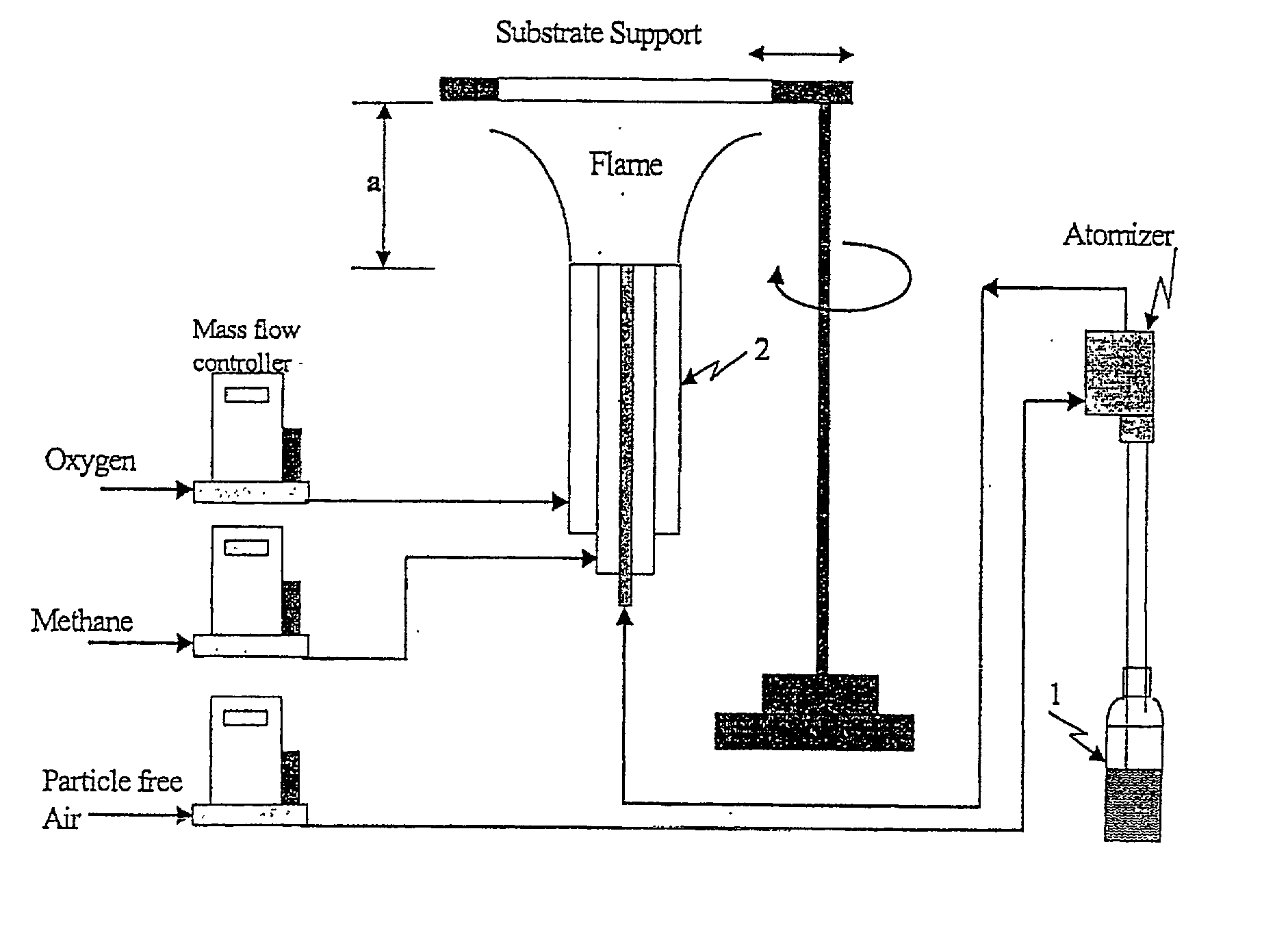
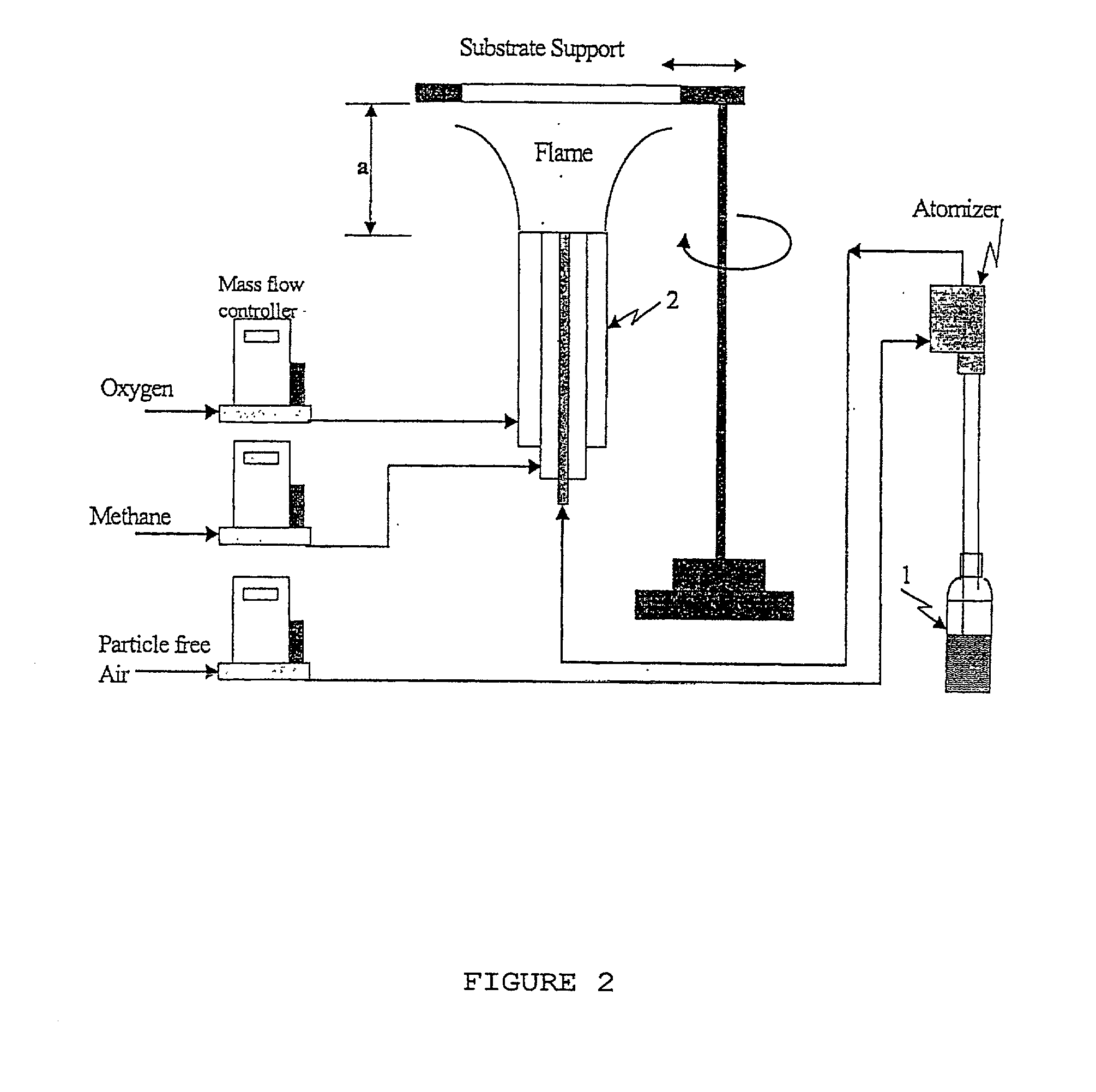
Process for photo-induced partial oxidation of organic chemicals to alcohols, ketones, and aldehydes using flame deposited non-structured photocatalysts
InactiveUS20020029955A1Preparation by oxidation reactionsMolten spray coatingPartial oxidationAlcohol
Organic molecules are partially oxidized in that the gas phase on supported and immobilized photocatalysts deposited having a nanostructure. the photocatalysts are semiconductors such as titanium dioxide and are preferentially coated onto a substrate by flame aerosol coating.
Owner:U S ENVIRONMENTAL PROTECTION AGCY
Catalyst for synthesizing benzaldehyde and benzyl alcohol from toluol, the preparation process and application thereof
InactiveCN1485131AEasy to separateEase of usePreparation by oxidation reactionsCarbonyl compound preparation by oxidationAlkaline earth metalBenzaldehyde
A catalyst of synthesizing benzaldehyde and benzyl alcohol by methylbenzene, its manufacturing method and application. The active component is zirconium, or transit metals, alkaline metal, alkaline-earth metals, metals of IIIAíóIVA and VA Group in the Periodic Table. The method comprises solving a zirconium compound or a zirconium compound and a compound of an element as the active component, adding a precipitator; drying, burning, pulverizing to particles. In reactions, a gas containing oxygen is the oxygen source, such as oxygen and air, an organic solution wouldn't be used. The catalyzing reaction is 180-195 degree C, the pressure is 0.8-1.2Mpa. when the conversion rate of methylbenzene is 13.0úÑ, the total selectivity of benzaldehyde and benzyl alcohol is 86.6úÑ. In the method, the catalyst is easy to be separated and could be recyclable, the reaction condition is mild.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Novel flow chart for preparing acetic acid, methanol and dimethyl ether from methane by non synthetic gas process
ActiveCN1640864APreparation by oxidation reactionsOrganic compound preparationAcetic acidGas composition
The new process of synthesizing acetic acid with methane consists of two reaction steps, including the first step of reacting methane, oxygen and hydrogen bromide or hydrobromic acid on catalyst to produce CH3Br, CO and H2O; and the second step of reacting CH3Br, CO and H2O on catalyst to prepare acetic acid. During the reaction of synthesizing methanol and dimethyl ether, the first step of oxybromizing reaction may have the reacting gas composition regulated and different catalyst adopted to reach the aim of creating CH3Br and H2O in high selectivity; and methanol and dimethyl ether may be then produced through hydrolysis on one other kind of catalyst. In the process of preparing acetic acid or methanol and dimethyl ether, HBr as the circular reaction medium is regenerated and returned to the reactor for reuse.
Owner:MICROVAST POWER SYST CO LTD
Preparation method and application of load type nano-gold catalyst
InactiveCN101829567AImprove loading efficiencyEasy to operatePreparation by oxidation reactionsCarbonyl compound preparation by oxidationHalloysiteGold particles
The invention discloses a preparation method and an application of a load type nano-gold catalyst. The preparation method comprises the following steps of: (1) adding 2.0g of halloysite nanotube carrier, 2.05 to 6.15mL of chlorogold acid solution with a concentration of 10g / L and 40 to 120mL of deionized water to a 250mL flask with three necks; (2) putting the flask into an oil bath with the temperature of 60 DEG C; adjusting the pH of the solution to 8 to 12 by using 4.0M ammonia water; then carrying out stirring reflux at 95 DEG C-105 DEG C for 1 hour; filtering; rinsing for 5 minutes by using 10 to 20mL of 4.0M ammonia water; washing twice with 15 to 20mL of hot water; drying for 1 to 2 hours at 100 DEG C; and finally roasting for 3 to 4 hours in air at 300 DEG C to obtain the load type nano-gold catalyst. The invention has the advantages of simple and convenient preparation method, uniformly dispersed gold particles and high loading efficiency. The catalyst provided by the invention can be used for cyclohexene oxidation of cyclonene and cyclohexenol, with the advantages of mild reaction condition, good activity and selectivity and less catalyst utilization quantity.
Owner:ZHEJIANG UNIV
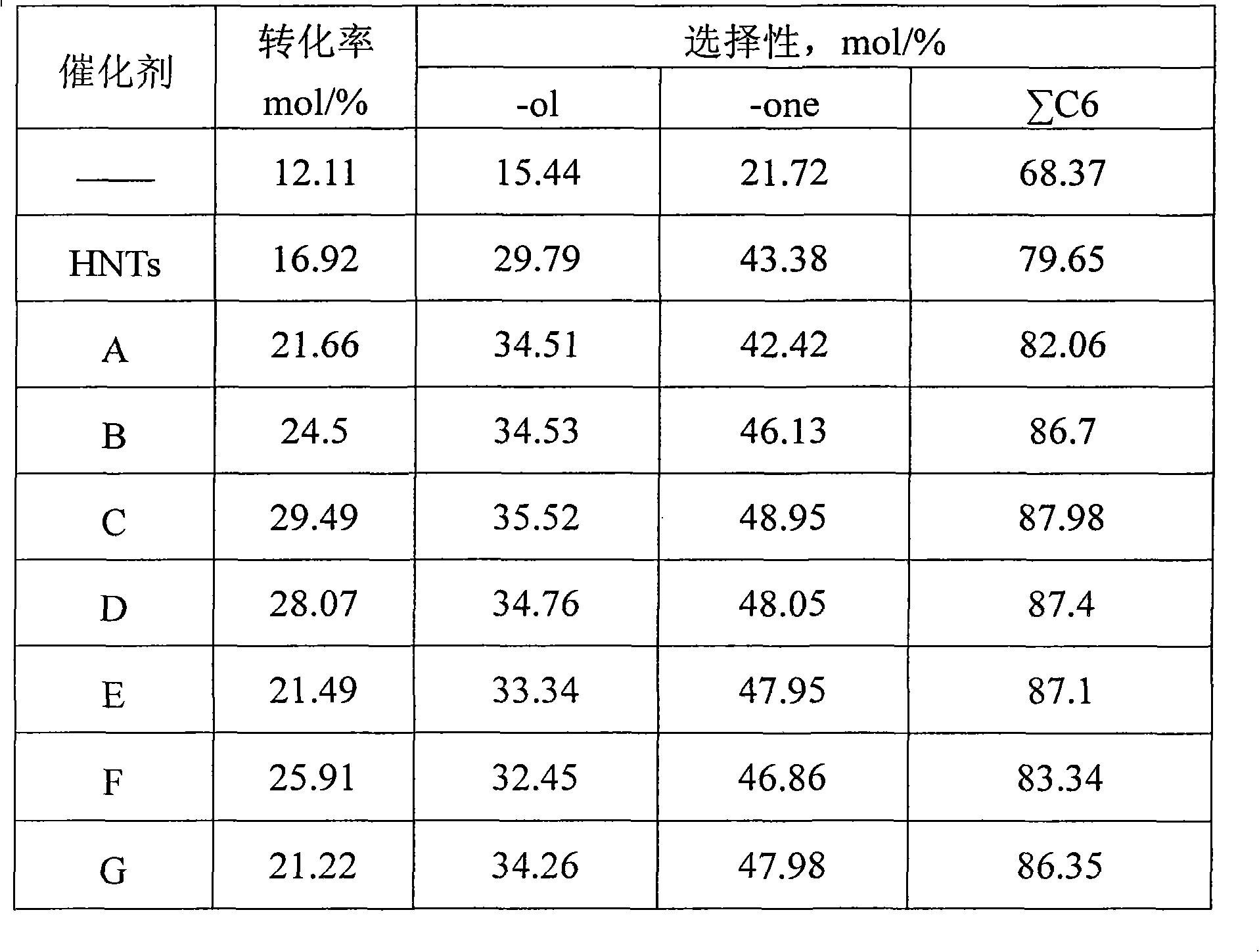
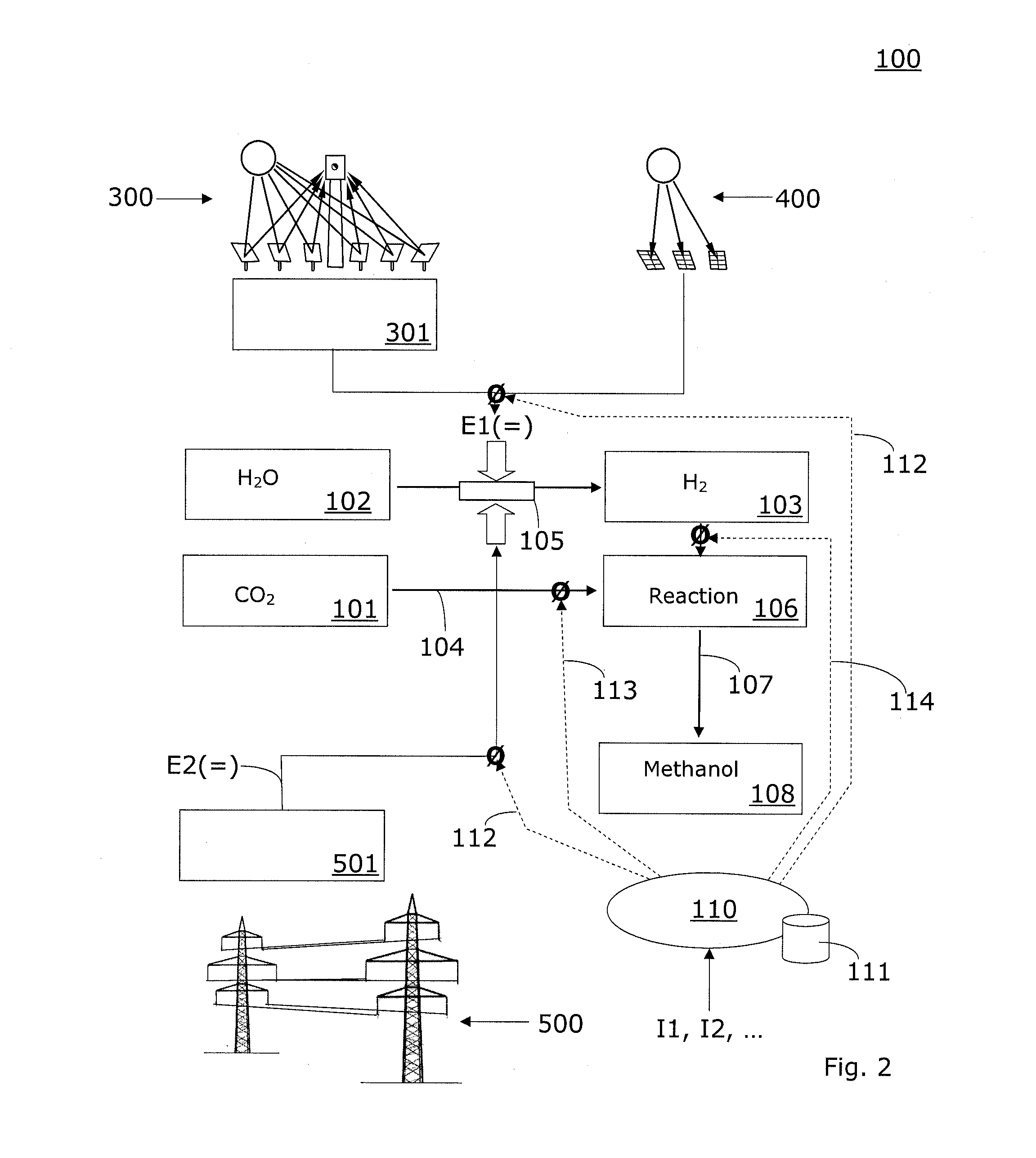
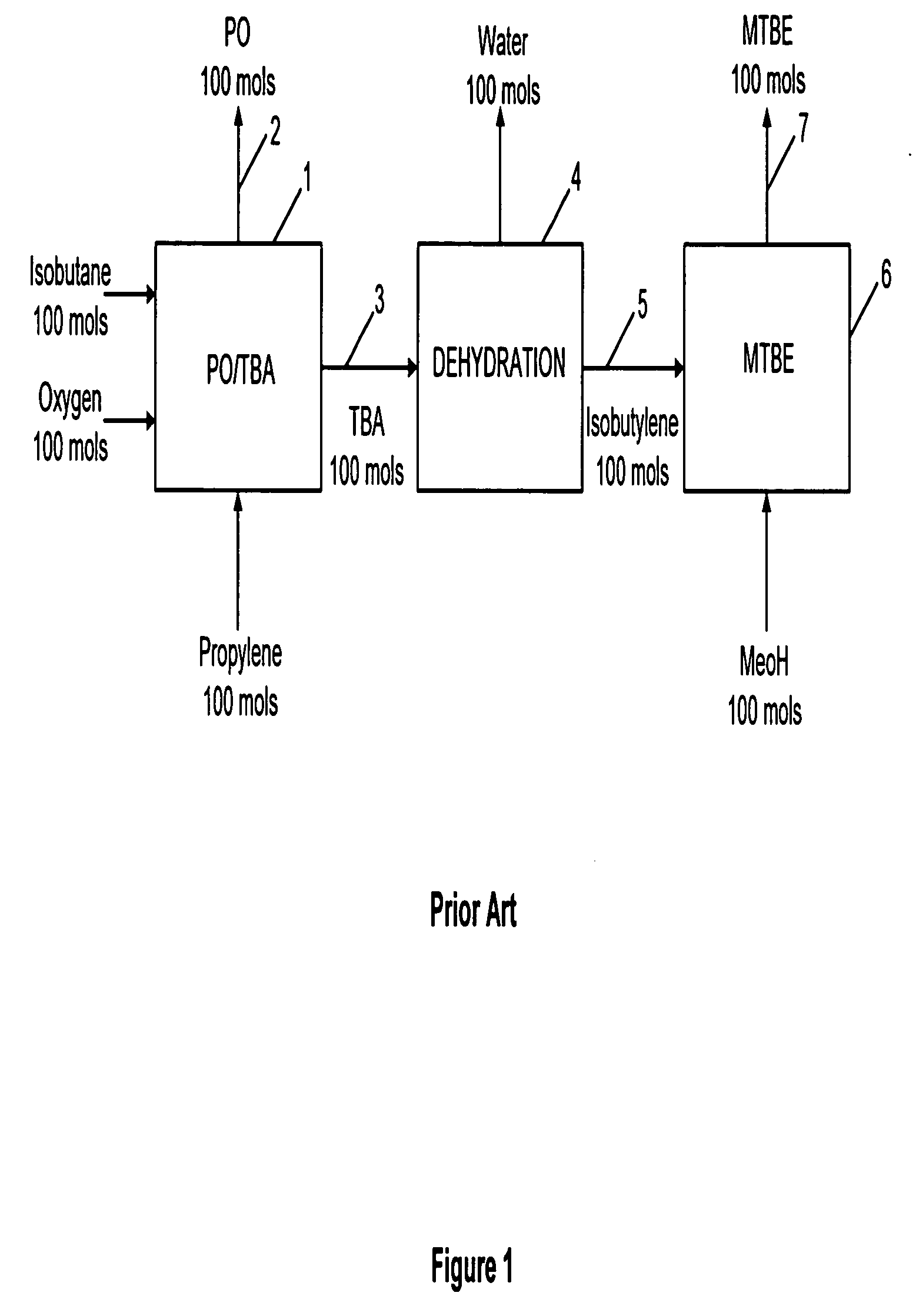
Method and system for providing a hydrocarbon-based energy carrier using a portion of renewably produced methanol and a portion of methanol that is produced by means of direct oxidation, partial oxidation, or reforming
InactiveUS20120226080A1Reduction of worldwide emissionImprove balanceOxygen-containing compound preparationPreparation by oxidation reactionsPartial oxidationWind power
Methanol is produced as a storable and transportable carbon-based energy carrier using at least two different methods. To produce a first methanol portion, water is electrolyzed, preferably using energy from renewable energy sources such as solar or wind power, to produce hydrogen. The hydrogen is then reacted with carbon dioxide to produce the first methanol portion. To produce a second methanol portion, methane is reacted with oxygen. A controller can advantageously be used to control the amount of chemicals in each reaction.
Owner:SILICON FIRE AG
Oxyfunctionalization of polyolefins
ActiveUS6953868B2Oxygen-containing compound preparationPreparation by oxidation reactionsPolymer sciencePolyolefin
The present invention is a method for oxyfunctionalizing, that is, introducing oxygen functionality to, a polyolefin such as polypropylene and poly(ethylene-alt-propylene). The polyolefin is contacted with an oxygen source such as a persulfate and catalytic amounts of a metal porphyrin complex under mild conditions to yield an oxyfunctionalized polymer that has a polydispersity that is very similar to that of the starting polymer.
Owner:RGT UNIV OF MINNESOTA
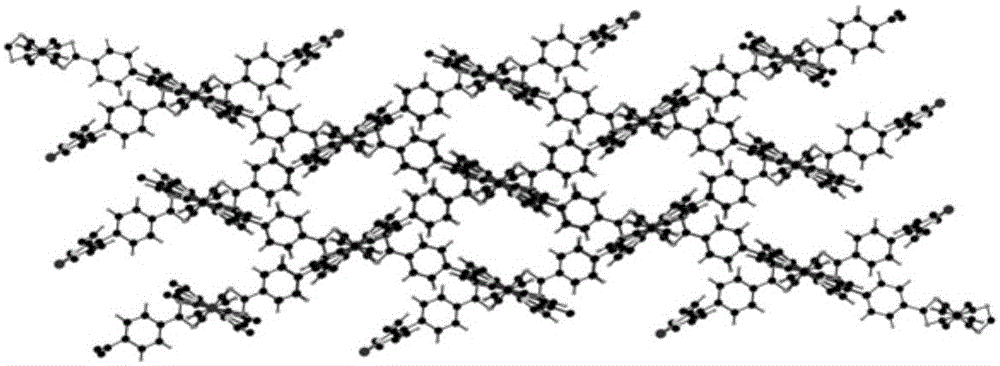
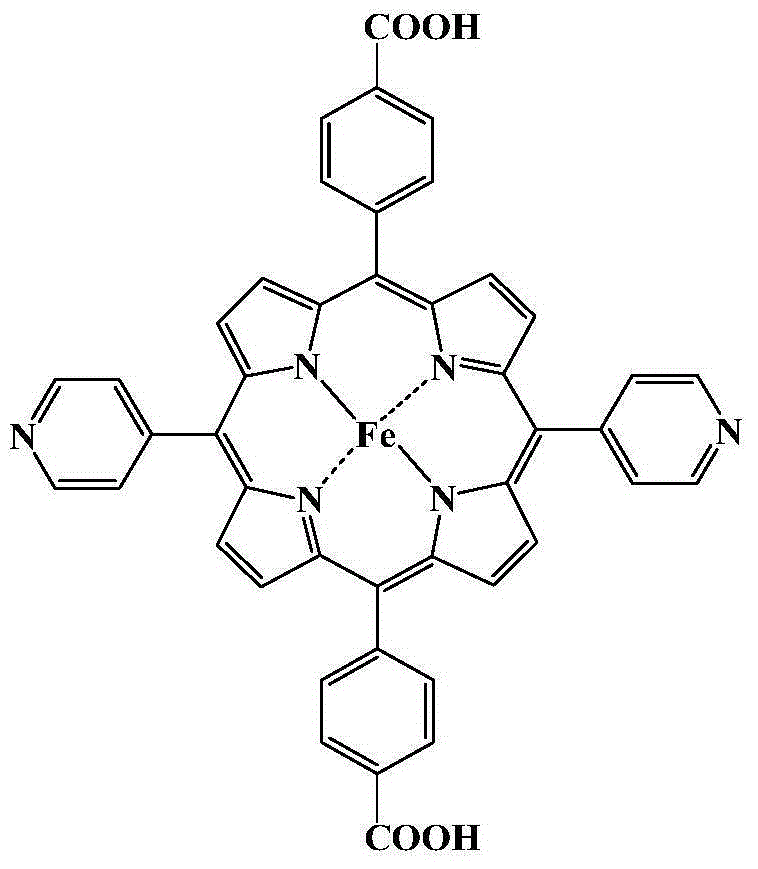
Metal organic framework material of Fe porphyrin ligand, preparation method therefor and application thereof
InactiveCN105061776ANovel structureImprove catalytic performancePreparation by oxidation reactionsOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidN dimethylformamide
The invention discloses a metal organic framework material of a Fe porphyrin ligand, a preparation method therefor and an application thereof, and belongs to the technical field of crystalline materials. A chemical formula of the Fe porphyrin ligand is FeL, wherein L is am organic ligand, namely 5, 15-bipyridine-10 and 20-dicarboxyphenyl ironporphyrin. In a closing condition, a crystal of the metal organic framework material is obtained by the organic ligand, namely 5, 15-bipyridine-10, 20-dicarboxyphenyl ironporphyrin and iron nitrate nonahydrate by virtue of thermal reaction in a solution of N,N-dimethylformamide and acetic acid. The metal organic framework material has an iron porphyrin catalytic property, and can be used for selective catalysis of cycloalkane.
Owner:BEIJING UNIV OF TECH
Load type nano-au catalyst and the preparing method
InactiveCN101036887AEasy to manufactureLow costPreparation by oxidation reactionsCatalyst carriersCyclohexanoneKetone
The present invention discloses a supported gold nano-particles catalyst and the preparation method of the same, which comprises Au, Ce, Zr, Al and Si; wherein Au is the main active component and has a mass percent of 0.1~4.0%; Ce and Zr are the assistant active component and has a mass percent of 3~57%, wherein the mol ratio of Ce to Zr is 1~10; Al and Si are the carrier and has a mass percent of 40~97%. The catalyst is prepared by a sequential immersion method, wherein the alumina or silicon oxide carrier is firstly impregnated by an aqueous liquid containing cerous nitrate and zirconium nitrate and then the gold component is impregnated. The present invention has the advantages of that: the preparation method is simple, the stability is excellent and the cost is low. For the reaction of preparing cyclohexanone and cyclohexanol by cyclohexane oxygenation, the catalyst of the present invention can obtain high ketone alcohol selectivity even under the condition of a high conversion, besides the peroxide content in the product is very low. For example, when the conversion of cyclohexane is 12.8%, the ketone alcohol selectivity is up to 92.6% and the peroxide content is only 0.7%.
Owner:ZHEJIANG UNIV
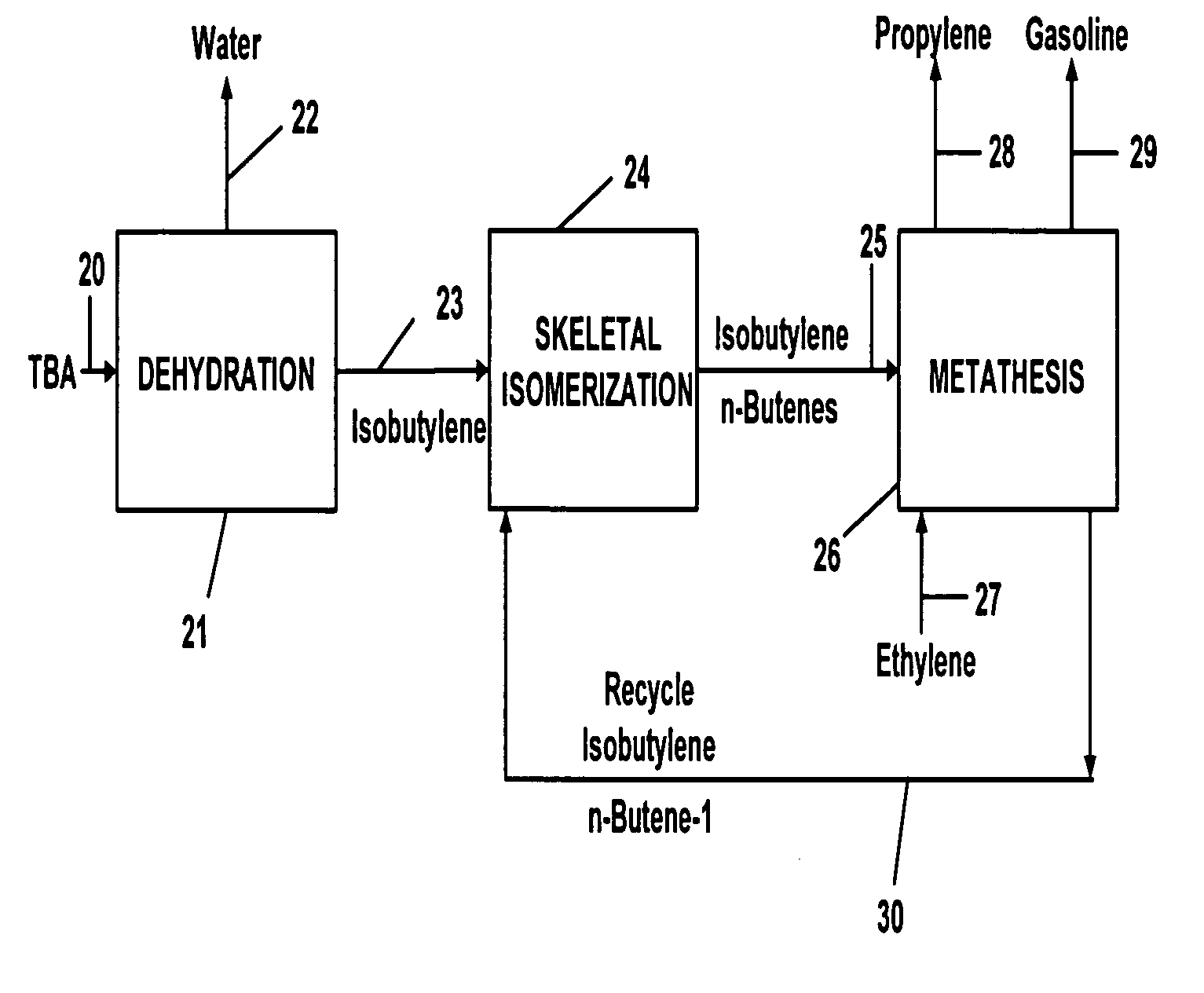
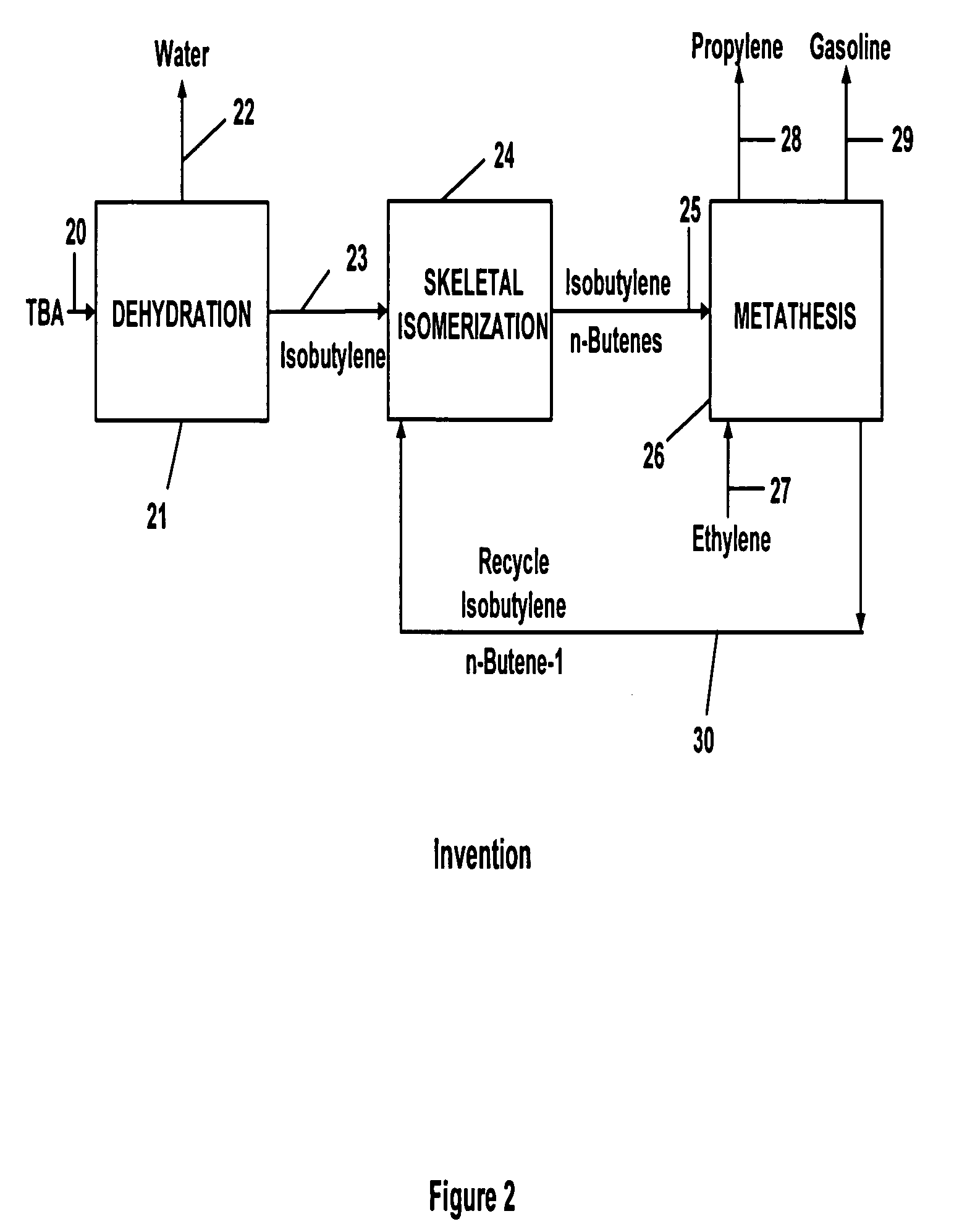
Propylene production
InactiveUS20050250969A1Versatile product mixHydrocarbon by isomerisationPreparation by oxidation reactionsIsomerizationGasoline
A method for forming propylene wherein ethylene is employed as a primary feed material in a combination of steps comprising dehydration, skeletal isomerization, and metathesis to form a versatile product mix that can contain one or more of propylene, gasoline, MTBE, and propylene oxide.
Owner:EQUSR CHEM LP
Preparation of new layered double hydroxides exchanged with osmate for asymmetric dihydroxylation of olefins to vicinal diols
InactiveUS6387033B1Facilitates oxygen transferHigh activityPreparation by oxidation reactionsCarboxylic acid esters preparationDiolAlkene
LDH-osmate of the formula [MII(1-x)MIIIx(OH)2][OsO42-]x / 2.zH2O wherein MII is a divalent cation selected from the group consisting of Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+ and Ca2+ and MIII is a trivalent ion selected from the group consisting of Al3+, Cr3+, Mn3+, Fe3+ and Co3+, and x is the mole fraction having integral value ranging from 0.2 to 0.33, and z is the number of water molecules and ranges from 1 to 4, useful as, a catalyst, and a process for the preparation thereof and use thereof to manufacture vicinal diols.
Owner:COUNCIL OF SCI & IND RES
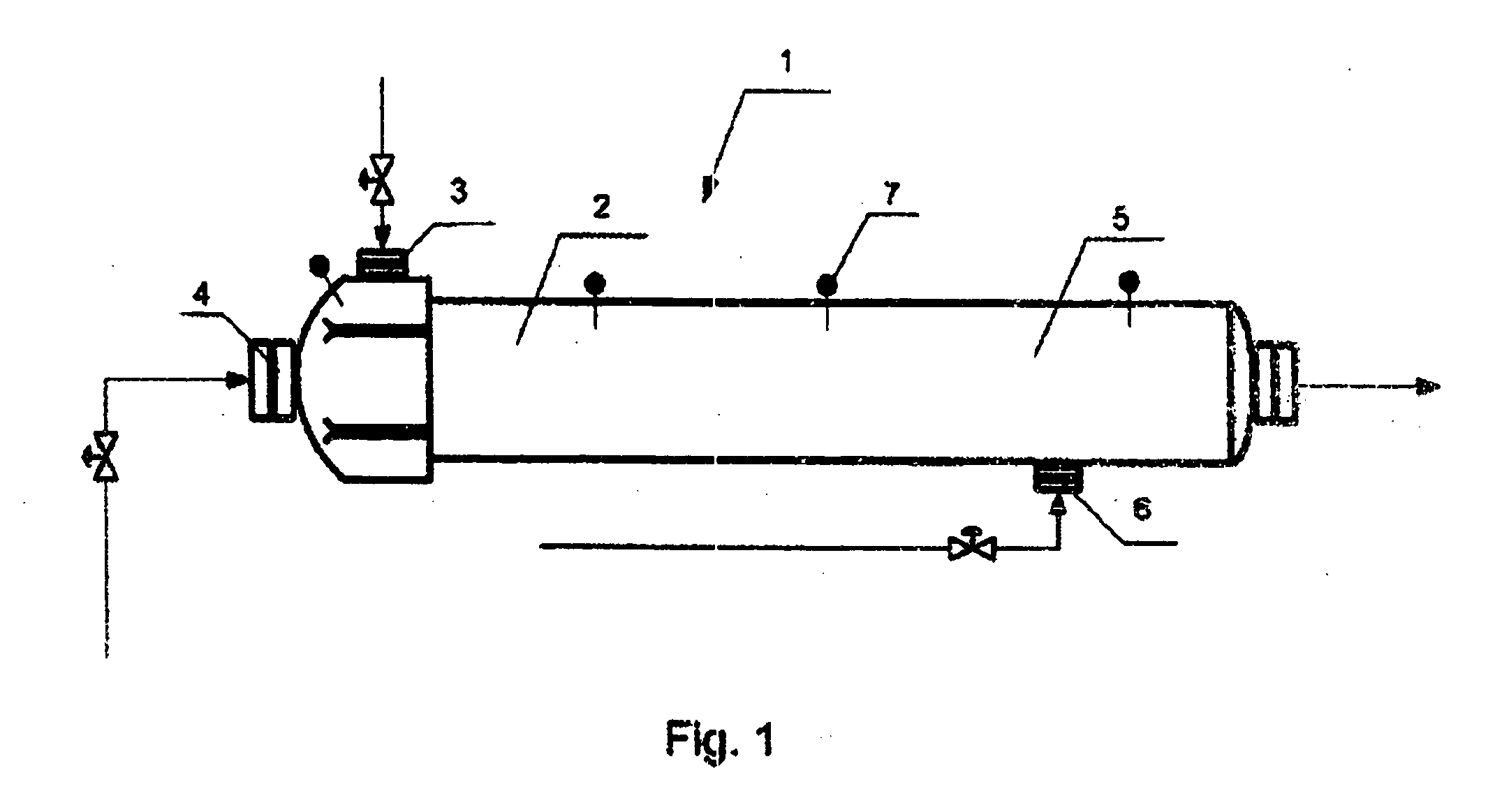
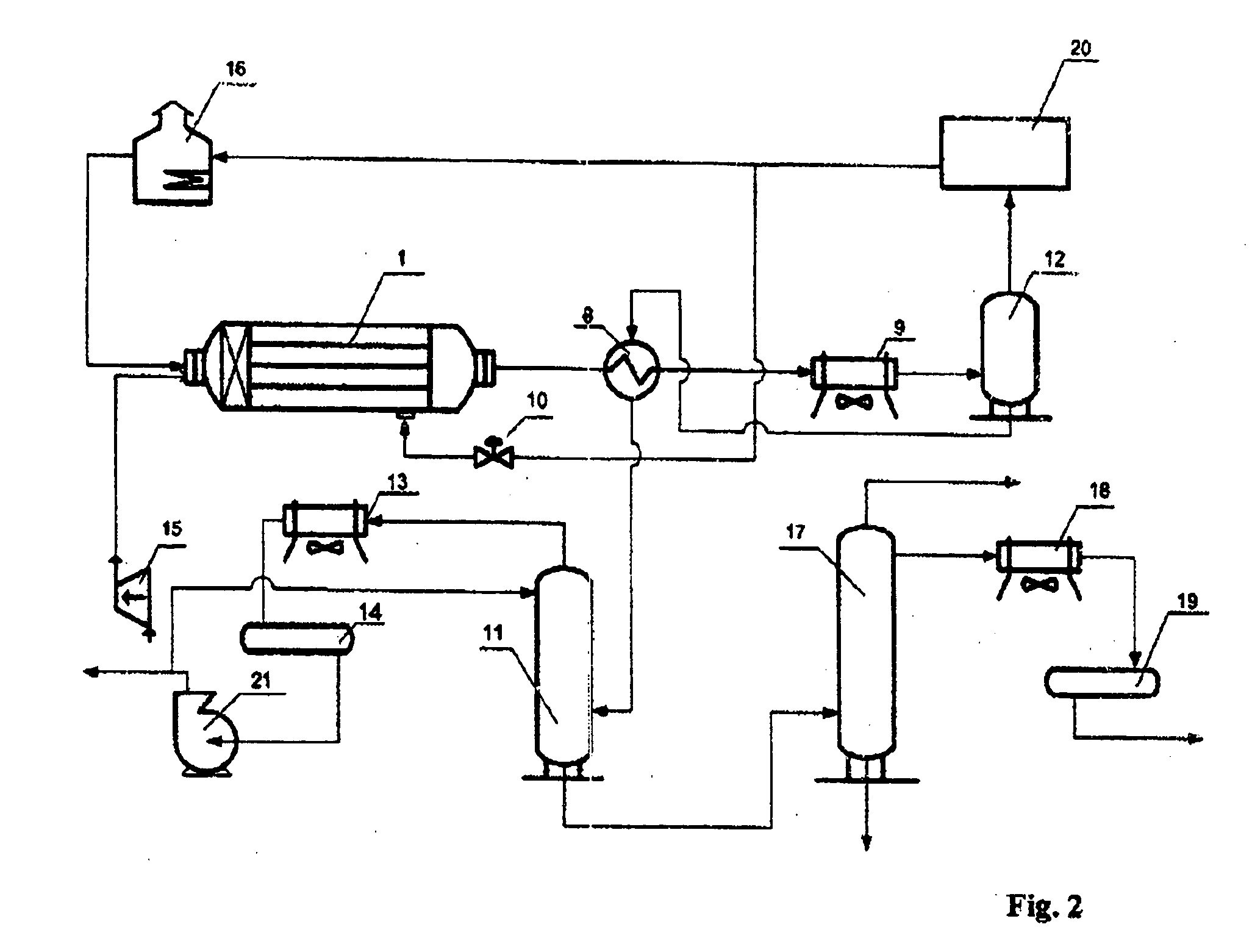
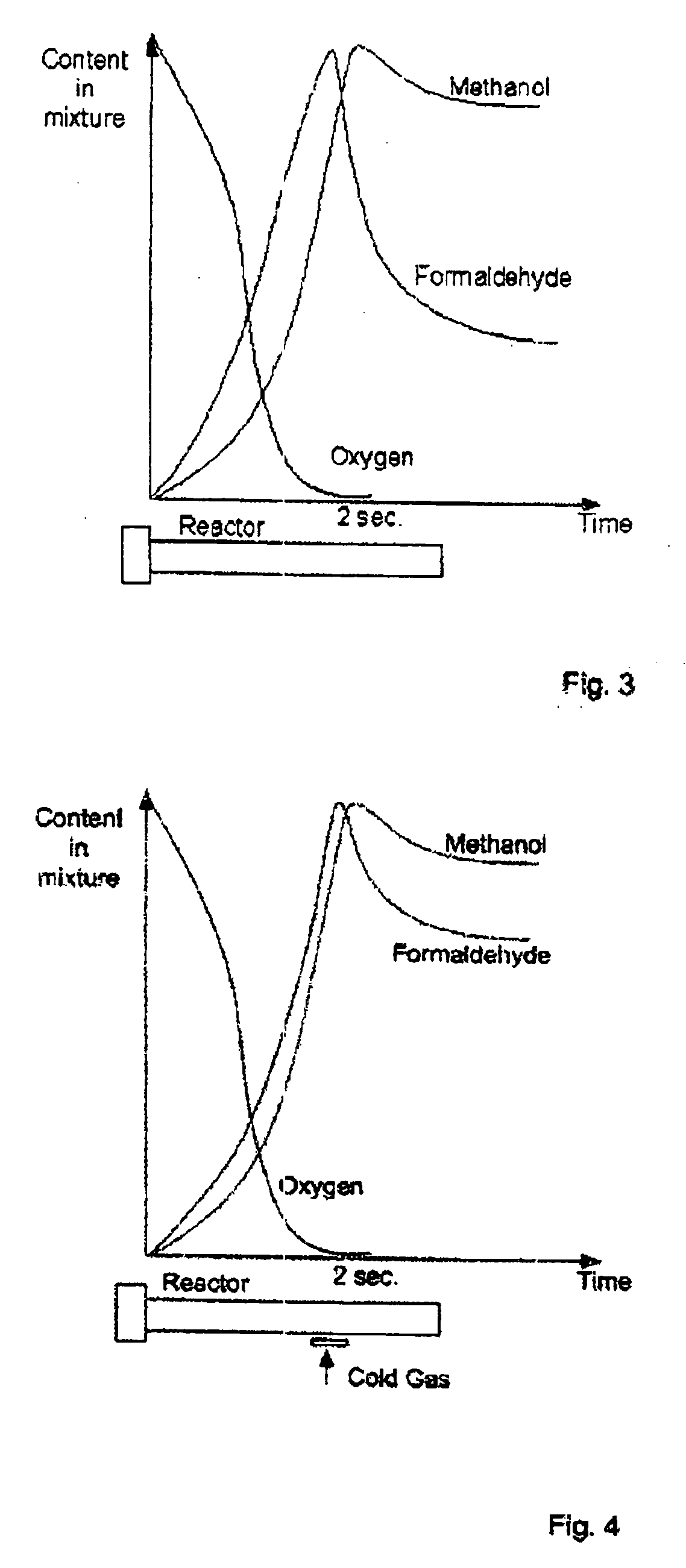
Method of and apparatus for producing methanol
ActiveUS20060035986A1Lower reaction temperaturePreparation by oxidation reactionsOrganic compound preparationOxygenHydrocarbon
Production of methanol includes supplying into a reactor a hydrocarbon-containing gas, supplying into the reactor an oxygen-containing as gas, carrying out in the reactor an oxidation of the heated hydrocarbon-containing gas by oxygen of the oxygen-containing gas, and supplying into the reactor a cold hydrocarbon-containing gas to be mixed directly with a mixture of the heated hydrocarbon-containing gas and the oxygen-containing gas at a later stage of the reaction to produce also formaldehyde.
Owner:GAS TECH
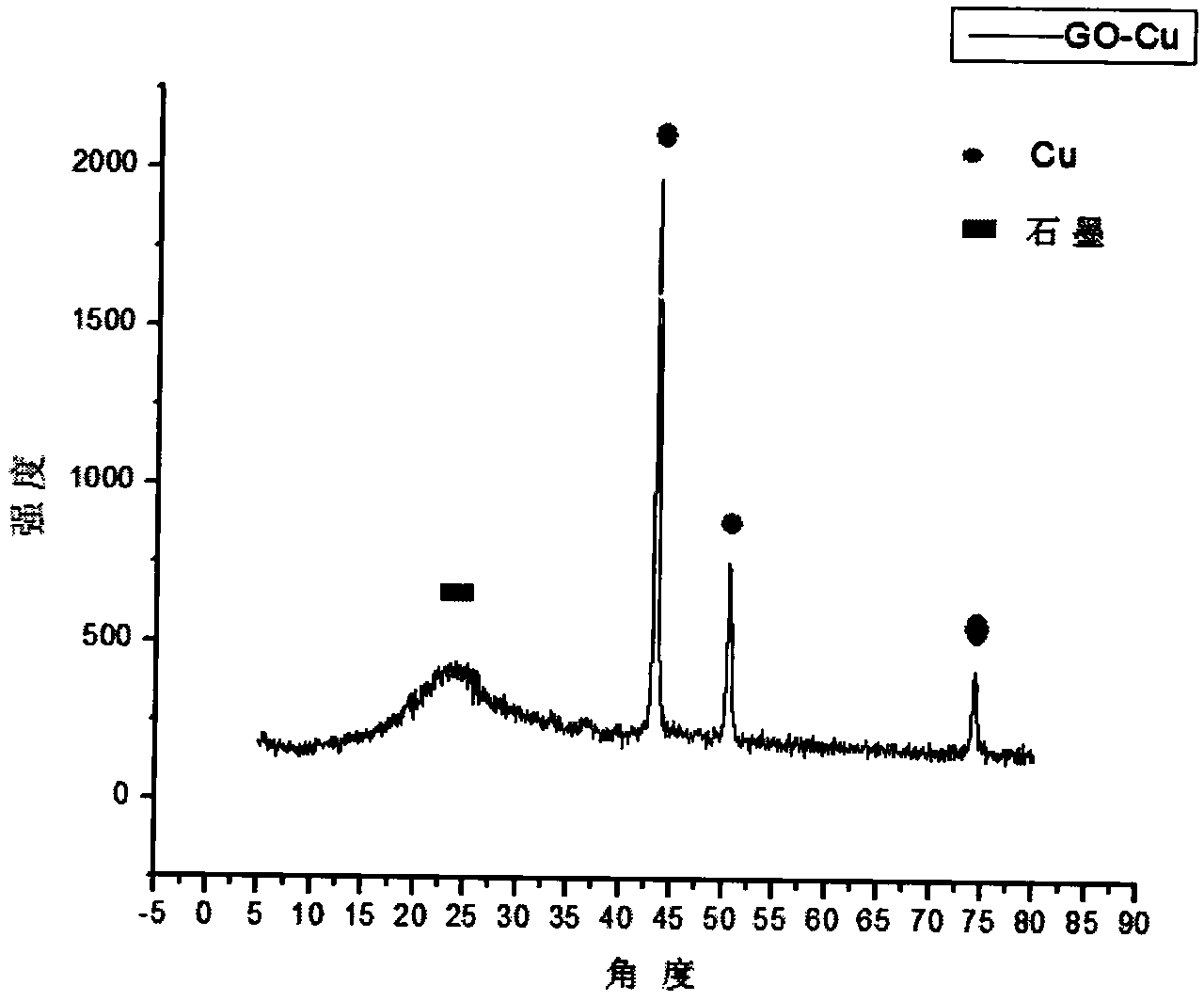
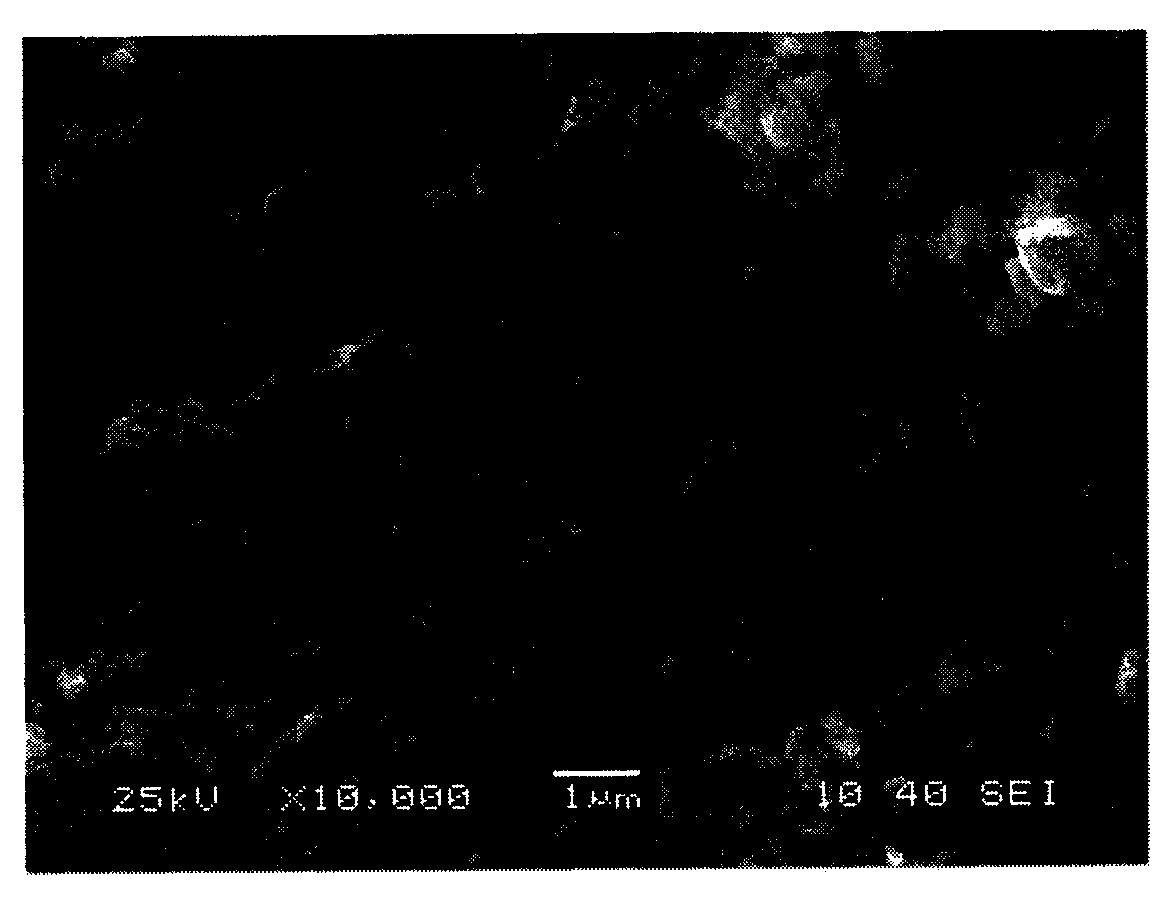
Graphene loaded metal nano composite material, and preparation method and application thereof
ActiveCN104028269ACheap methodEfficient methodPreparation by oxidation reactionsOrganic compound preparationSolventReducing agent
The invention belongs to the chemical field of nano materials, and in particular relates to a graphene loaded metal nano composite material, and a preparation method and application thereof. The invention discloses the graphene loaded metal nano composite material. Under the dispersion and bearing action of graphene, the composite material can be uniformly distributed; the size of the composite material is about 10-100nm. The composite material mainly consists of graphite and simple metal inorganic salt which serve as raw materials, water serving as a solvent, hydrazine hydrate serving as a reducing agent and a proper amount of a surfactant, and is reacted to obtain graphene loaded metal nano particles. The graphene loaded metal nano composite material has the advantages of low cost, high efficiency, energy conservation and the like, and embodiments excellent catalysis activity in benzyl oxidization, so that a low-cost, environment-friendly and high-efficiency nano composite material catalyst is supplied to industrial production.
Owner:NANJING TECH UNIV
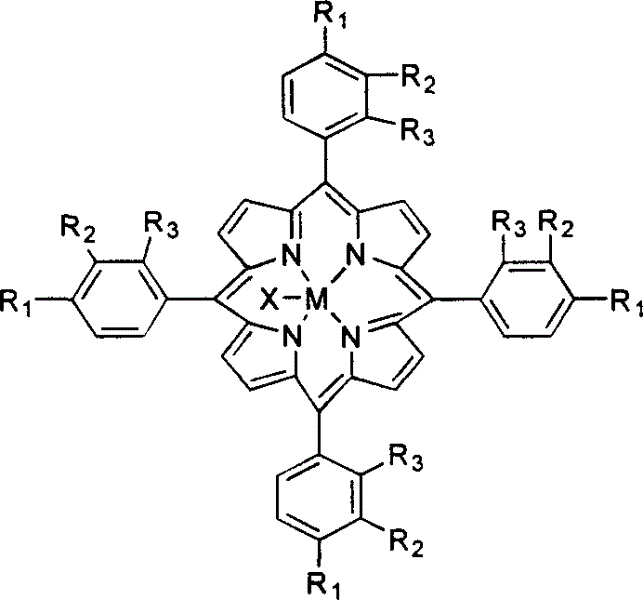
Method for catalytic conversion of alkyl cyclohexanol and alkyl cyclohexanone from air oxidized alkyl cyclohexane
InactiveCN1530357AControl deep oxidationReduce manufacturing costPreparation by oxidation reactionsOrganic compound preparationIsopropylcyclohexaneCyclohexanone
A process for selectively oxidizing alkyl cyclohexane by air under the catalysis of mu-oxygen bimetal porphyrin, single-metal porphyrin, or their solid carried substance to become alkyl cyclohexanol and alkyl cyclohexanone is disclosed. Said alkyl cyclohexane may be methyl cyclohexane, ethyl cyclohexane, isopropyl cyclohexane, etc. Its advantages are low cost, high catalytic performance, low reaction temp (less than 200 deg.C) and high transform rate.
Owner:HUNAN UNIV
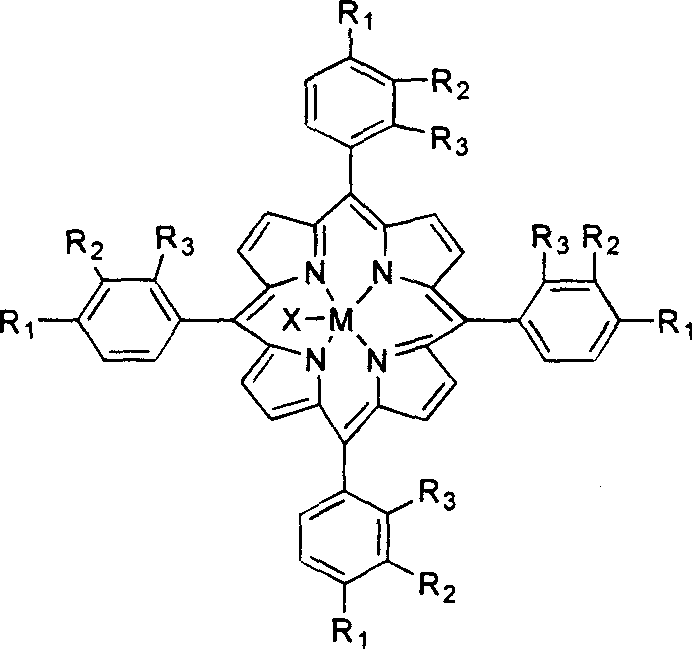
Process for preparing aldehyde and alcohol by selective catalysis air oxidation of toluene and substituted toluene
InactiveCN1521153AReduce dosageHigh catalytic efficiencyPreparation by oxidation reactionsOxygen compounds preparation by hydrocarbon oxidationBenzaldehydePorphyrin
The present invention is selectively catalytic air oxidation and substitution of toluene to form aldehyde and alcohol. Under the conditions of 2-10 atm air pressure, 25-180 deg.c temperature and 0.1-1 MPa reaction pressure, and in the presence of catalyst comprising module-metalloporphyrin oxide or solid carried metalloporphyrin as main catalyst and salt or oxide of transition metal as co-catalyst, toluene is oxidized with air into benzaldehyde and benzyl alcohol or oxidized and substituted into substituted benzaldehyde and substituted benzyl alcohol. The present invention has less catalyst consumption, high catalytic efficiency, low reaction temperature and pressure, easy-to-control oxidation depth, product with high purity and being easy separated. The catalyst may be used in homogeneous catalysis or solid carried for inhomogeneous catalysis.
Owner:HUNAN UNIV
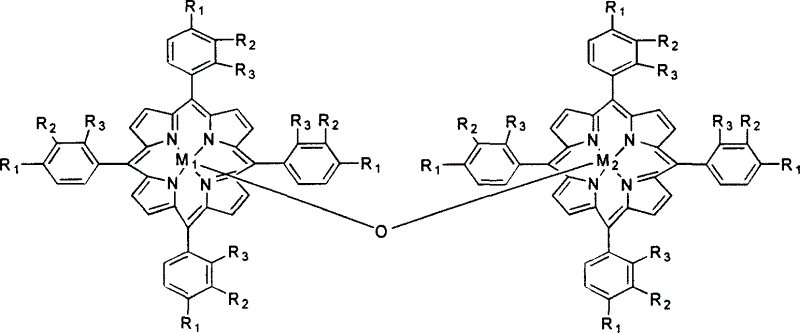
Catalyst oxdie cyclohexane process
InactiveCN1247501CReduce loadReduce concentrationPreparation by oxidation reactionsOxygen compounds preparation by hydrocarbon oxidationCyclohexanonePorphyrin
A process for preparing cyclohexanol, cyclohexanone and adipic acid by catalytic oxidization of cyclohexane under existance of metallic porphyrin catalyst and a certain temp and pressure through combination of different reactors is disclosed. Its advantages are high single-pass conversion rate of cyclohexane and total output rate, low energy consumption and cost, and no need of cyclohexanol or acetone solvent and cocatalyst.
Owner:CHINA PETROLEUM & CHEM CORP
Popular searches
Ethylene production Bio-feedstock Preparation by oxygen reduction Hydrocarbon oils treatment products Oxygen compounds preparation by reduction Preparation by aldehyde oxidation-reduction Liquid carbonaceous fuels Carboxylic compound preparation Electrolytic organic reduction Ether preparation by compound dehydration
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com