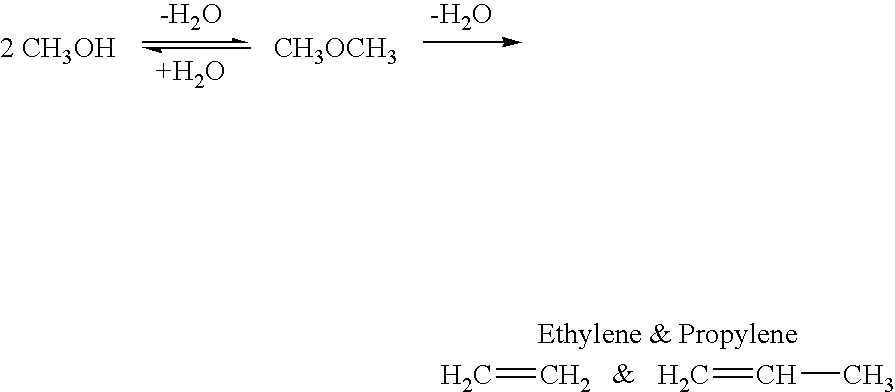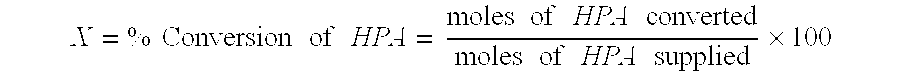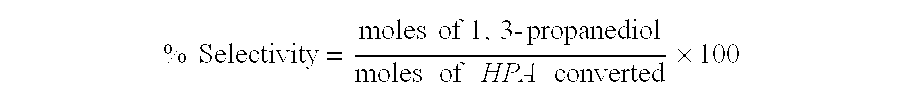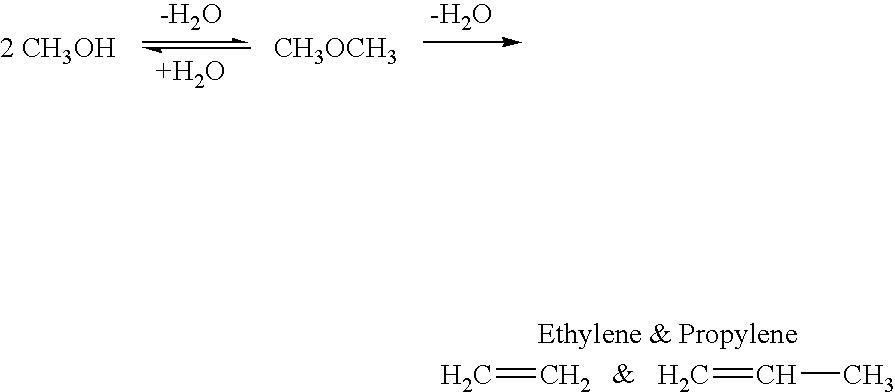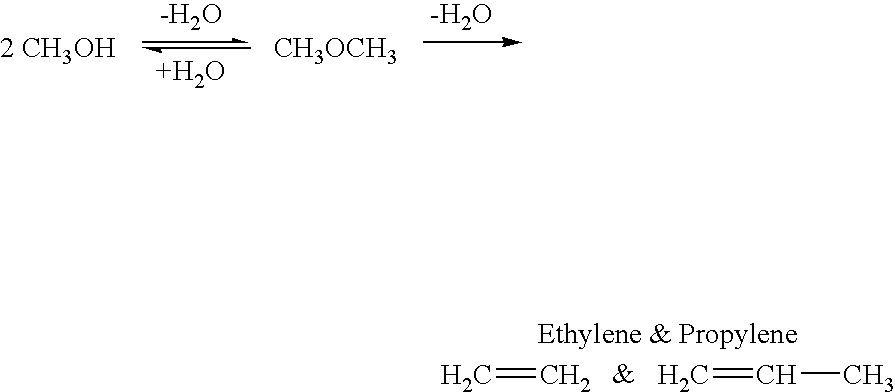Patents
Literature
1294results about "Preparation by oxygen reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
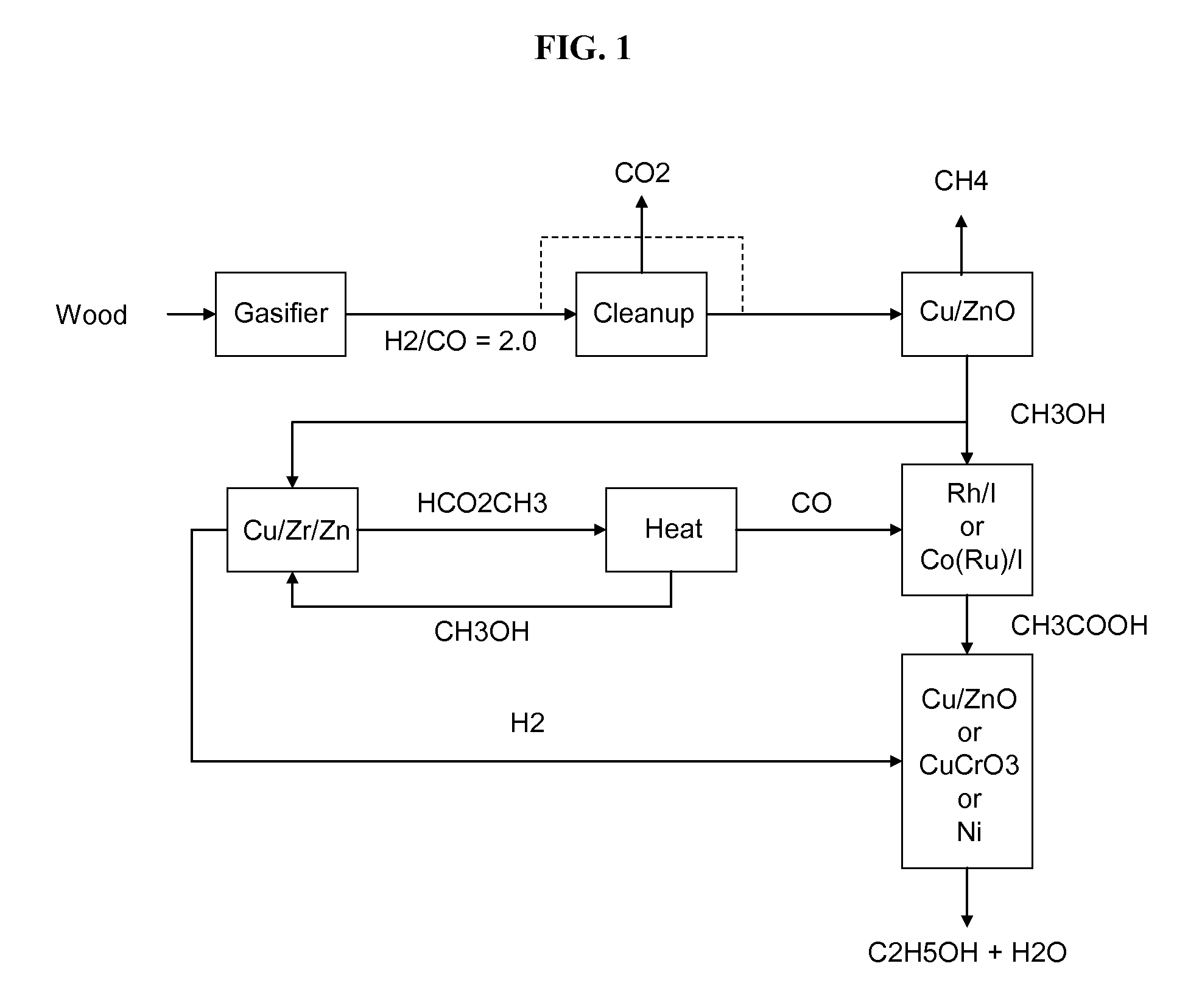
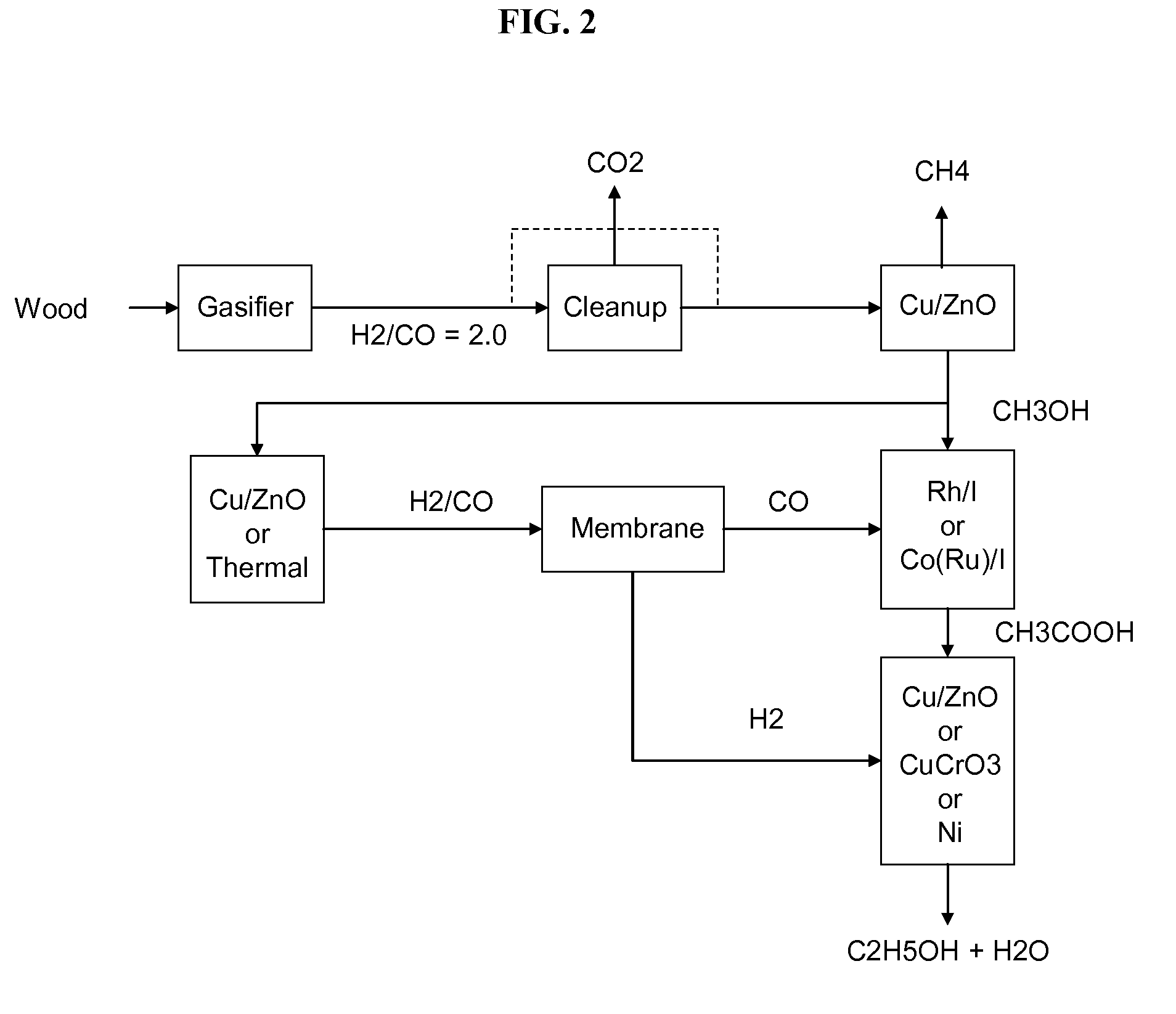
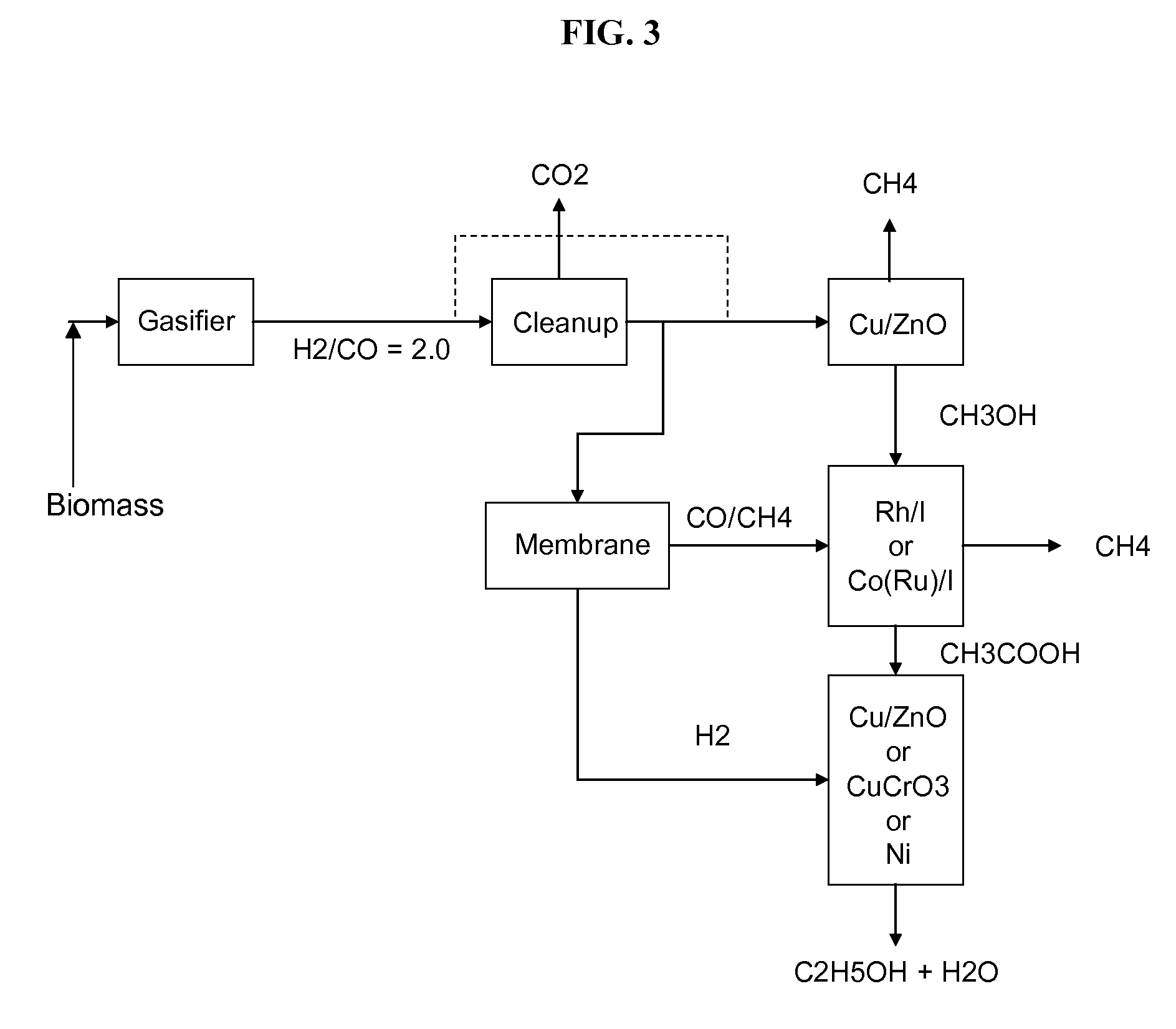
Methods and apparatus for selectively producing ethanol from synthesis gas
The invention provides methods and apparatus for selectively producing ethanol from syngas. As disclosed herein, syngas derived from cellulosic biomass (or other sources) can be catalytically converted into methanol, which in turn can be catalytically converted into acetic acid or acetates. Finally, the acetic acid or acetates can be reduced to ethanol according to several variations. In some embodiments, yields of ethanol from biomass can exceed 100 gallons per dry ton of biomass.
Owner:CELANESE INT CORP
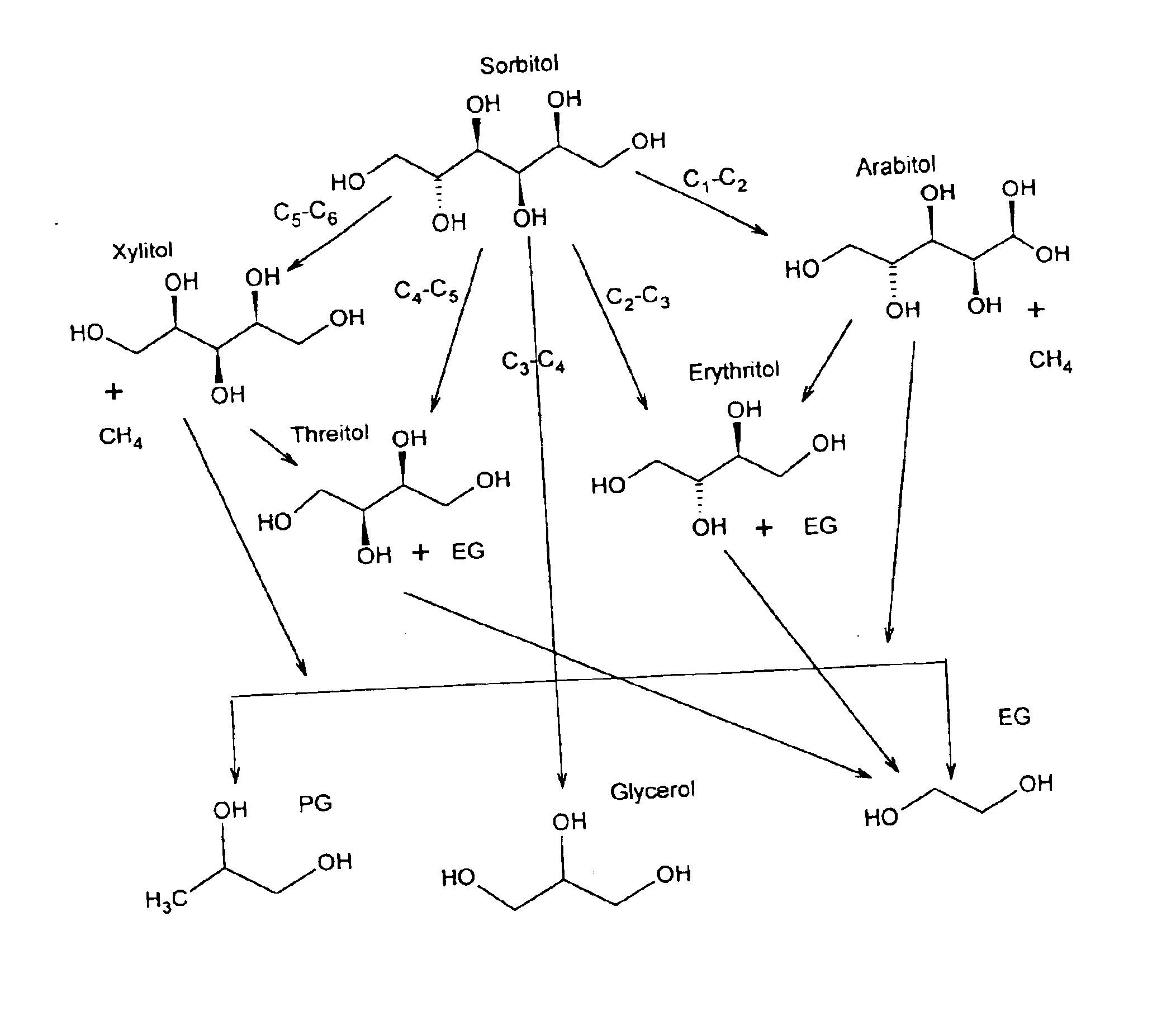
Hydrogenolysis of 6-carbon sugars and other organic compounds
InactiveUS6841085B2High selectivityHigh activityBiocideHydroxy compound active ingredientsHydrogenAlcohol sugars
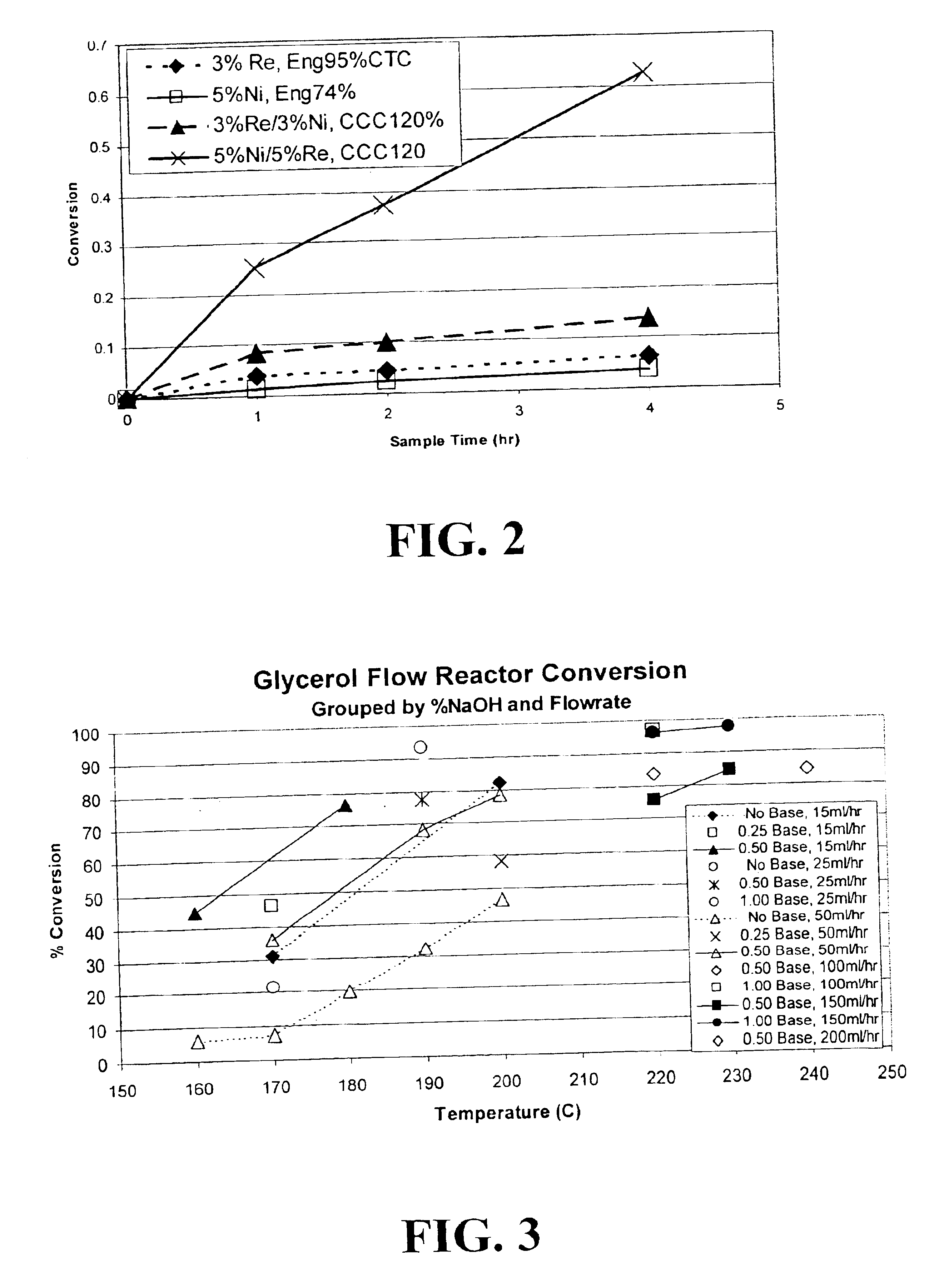
Methods for hydrogenolysis are described which use a Re-containing multimetallic catalyst for hydrogenolysis of both C—O and C—C bonds. Methods and compositions for reactions of hydrogen over a Re-containing catalyst with compositions containing a 6-carbon sugar, sugar alcohol, or glycerol are described. It has been surprisingly discovered that reaction with hydrogen over a Re-containing multimetallic catalyst resulted in superior conversion and selectivity to desired products such as propylene glycol.
Owner:MICHIGAN STATE UNIV +1
Method for producing alcohols by hydrogenation of carbonyl compounds
InactiveUS6486366B1Less amountHigh strengthSugar derivativesOrganic compound preparationCobaltPt element
A method for preparation of alcohols by catalytic hydrogenation of carbonyl compounds with hydrogen or hydrogen-containing gases in the presence of a hydrogenation catalyst of Raney type, where the catalyst is used in the form of hollow bodies, Preferred as catalytically active components are nickel, cobalt, copper, iron, platinum, palladium or ruthenium.
Owner:DEGUSSA AG
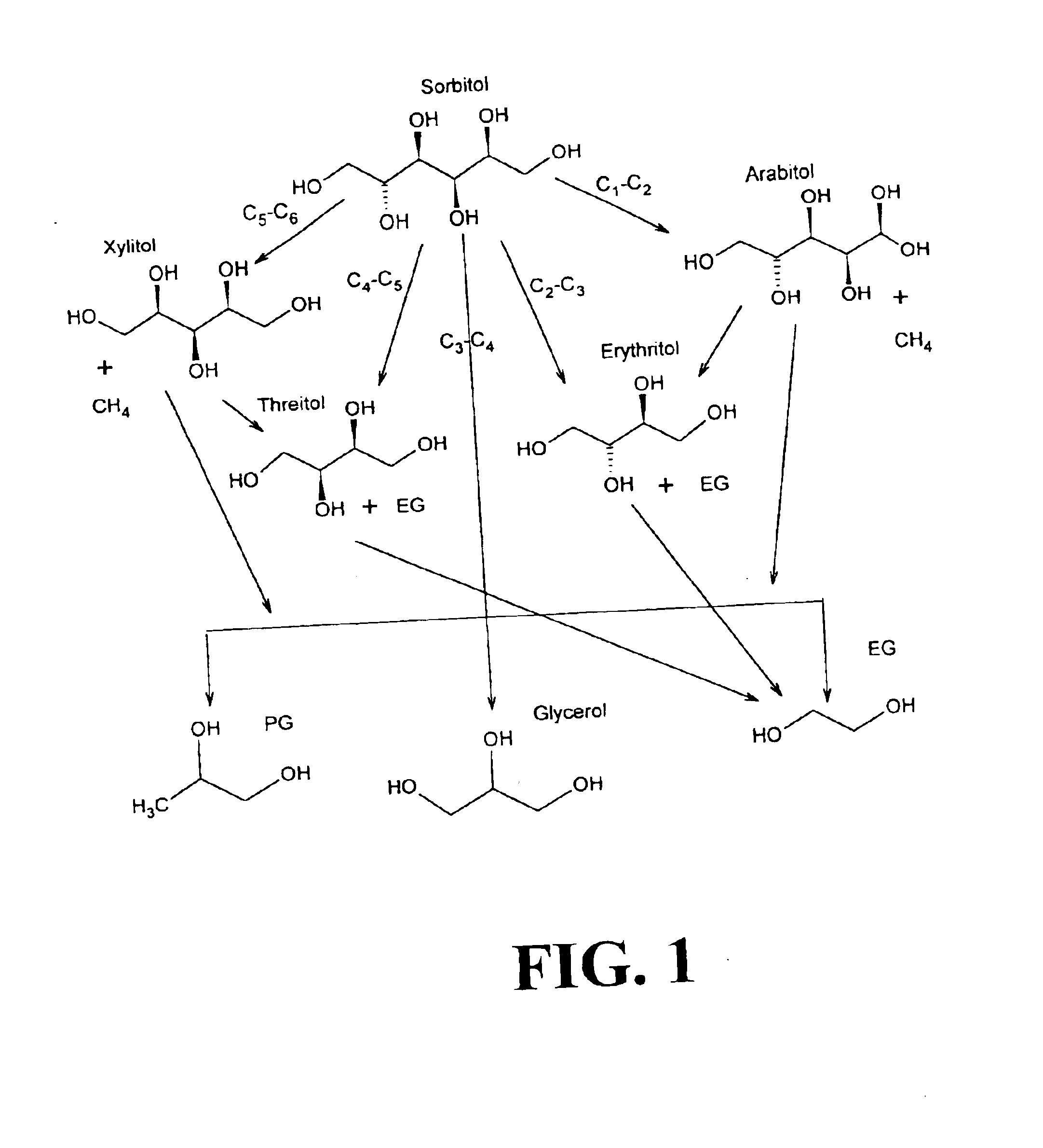
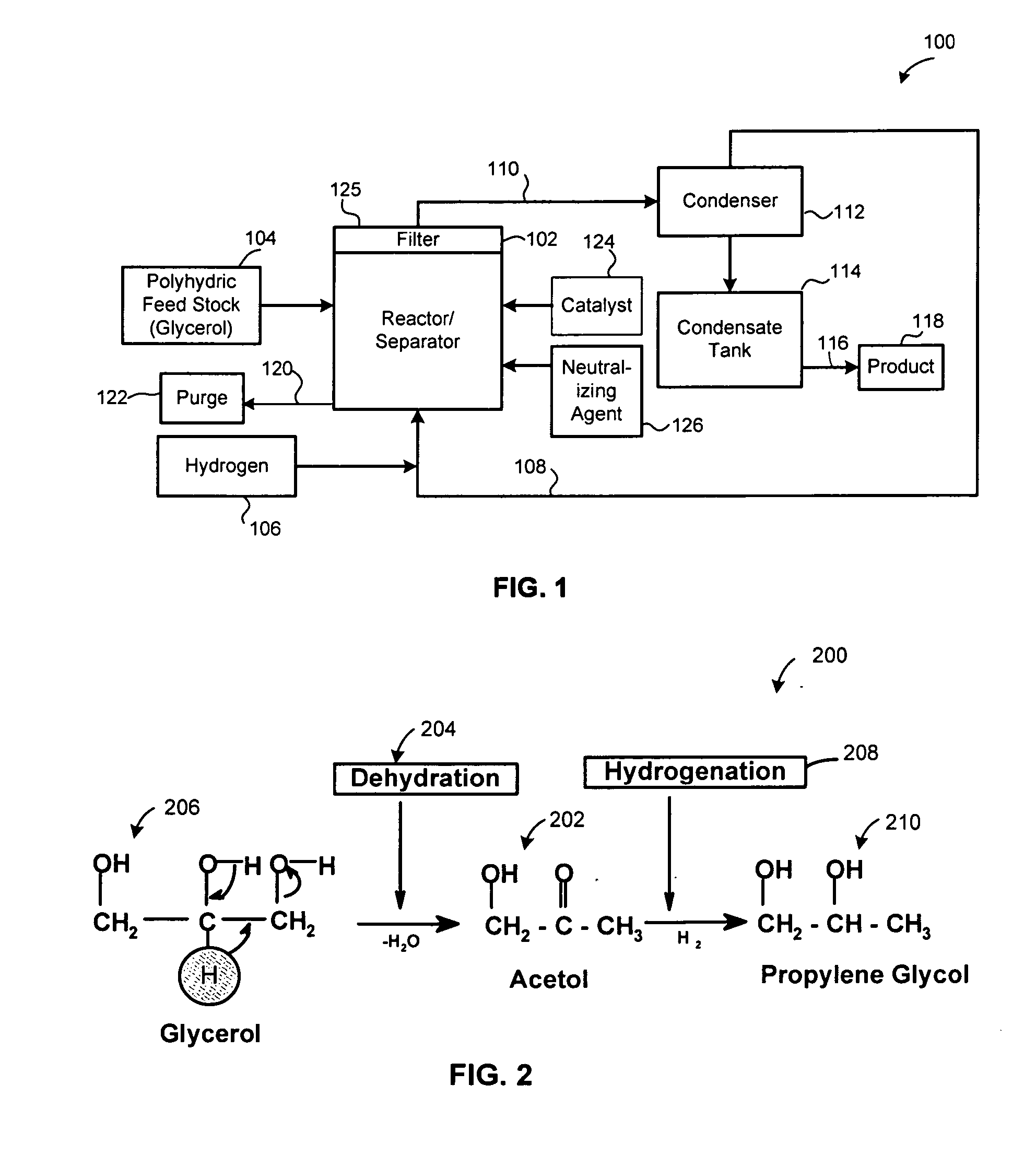
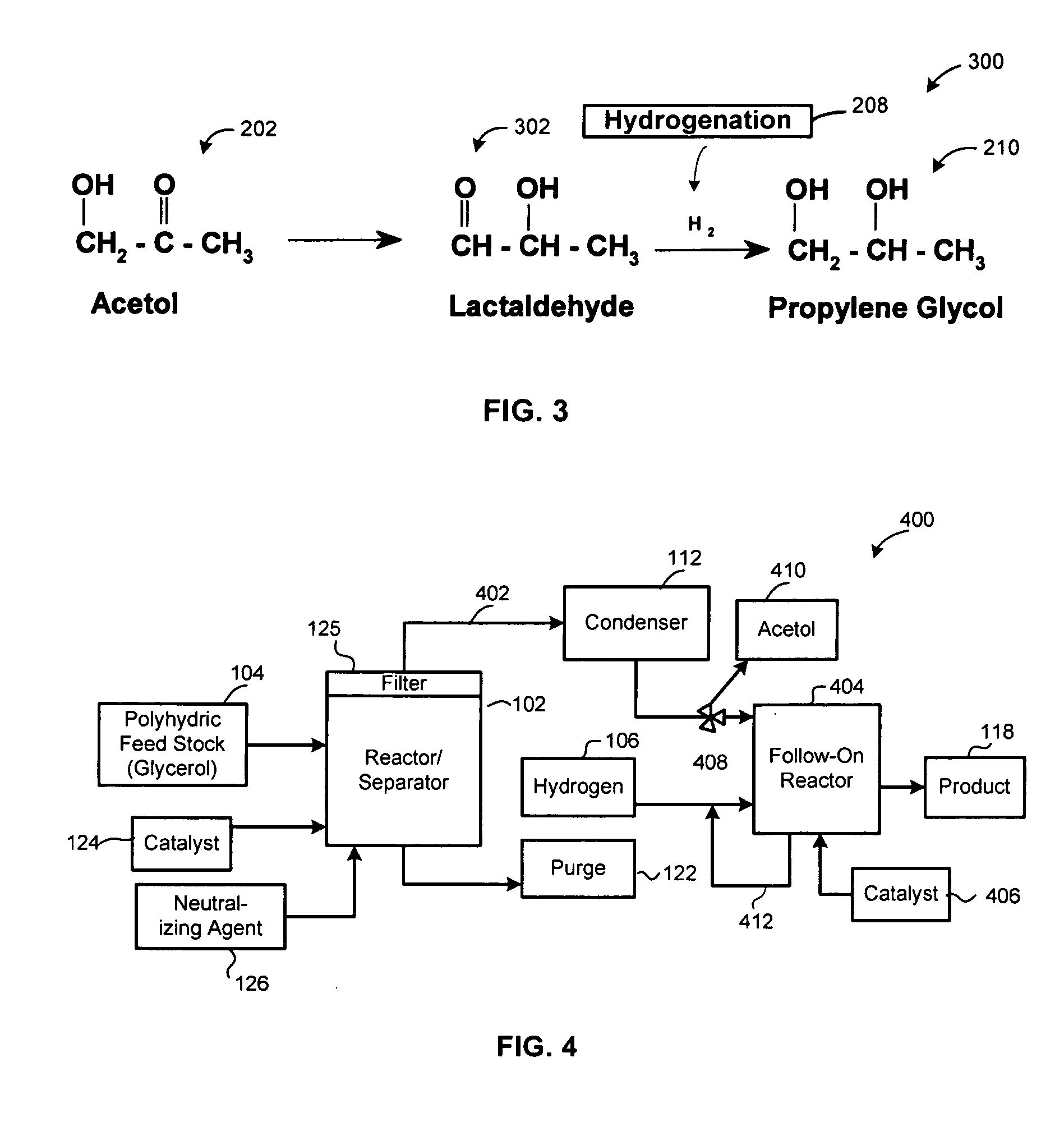
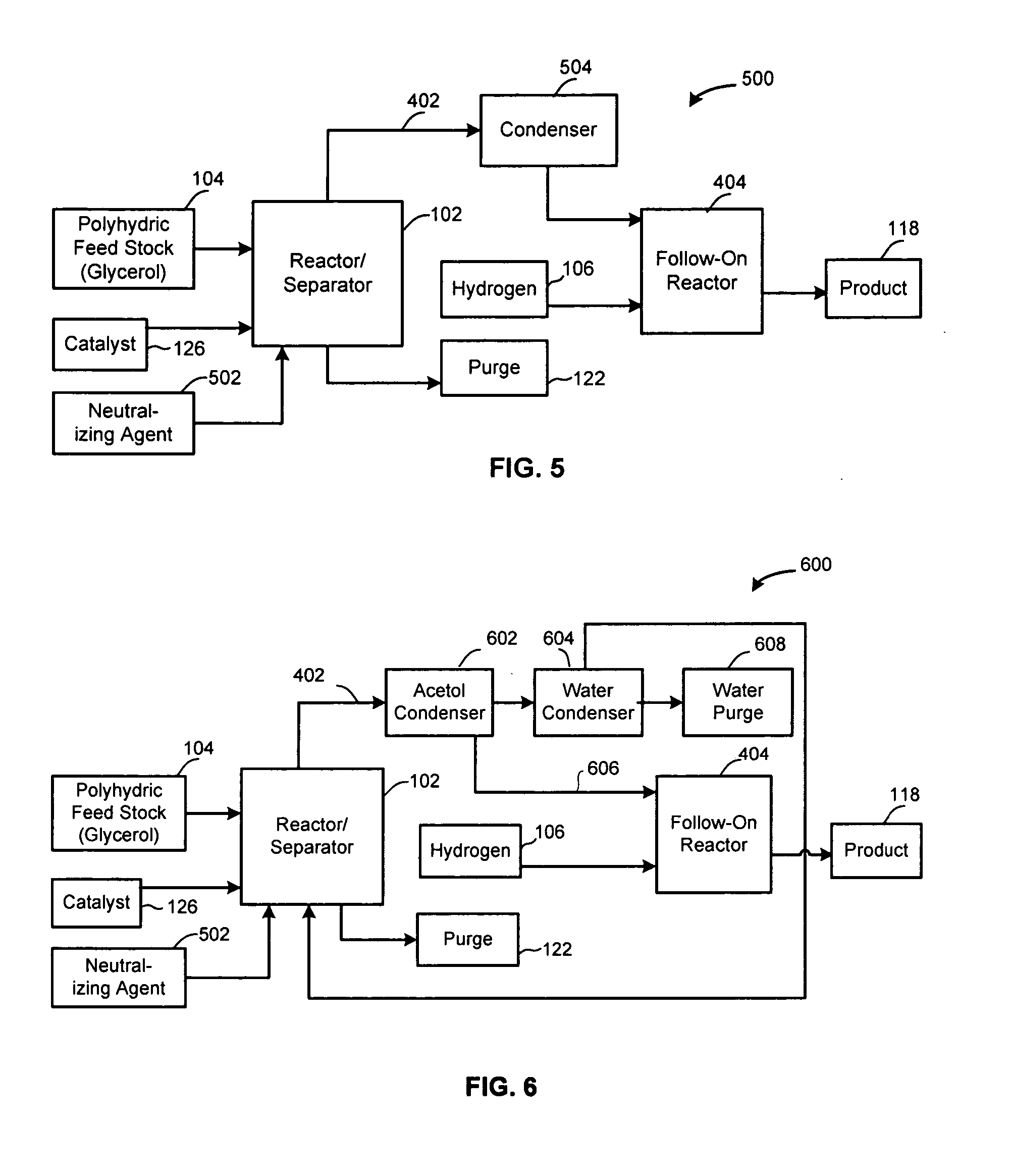
Method of producing lower alcohols from glycerol
ActiveUS20050244312A1High selectivityOrganic compound preparationOxygen compounds preparation by reductionAlcoholBoiling point
A reactive-separation process converts glycerin into lower alcohols, having boiling points less than 200° C., at high yields. Conversion of natural glycerin to propylene glycol through an acetol intermediate is achieved at temperatures from 150° to 250° C. at a pressure ranging from 1 and 25 bar. The preferred applications of the propylene glycol are as an antifreeze, deicing compound, or anti-icing compound. The preferred catalyst for this process in a copper-chromium powder.
Owner:RENEWABLE ALTERNATIVES LLC +1
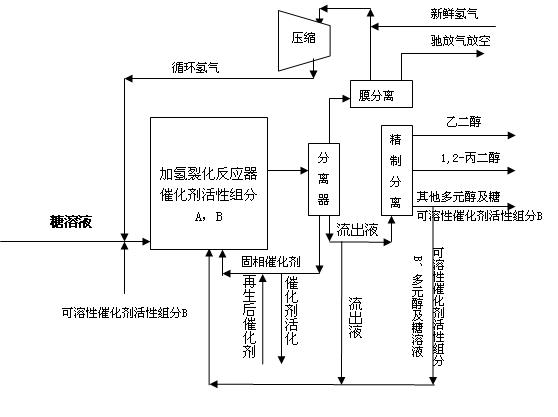
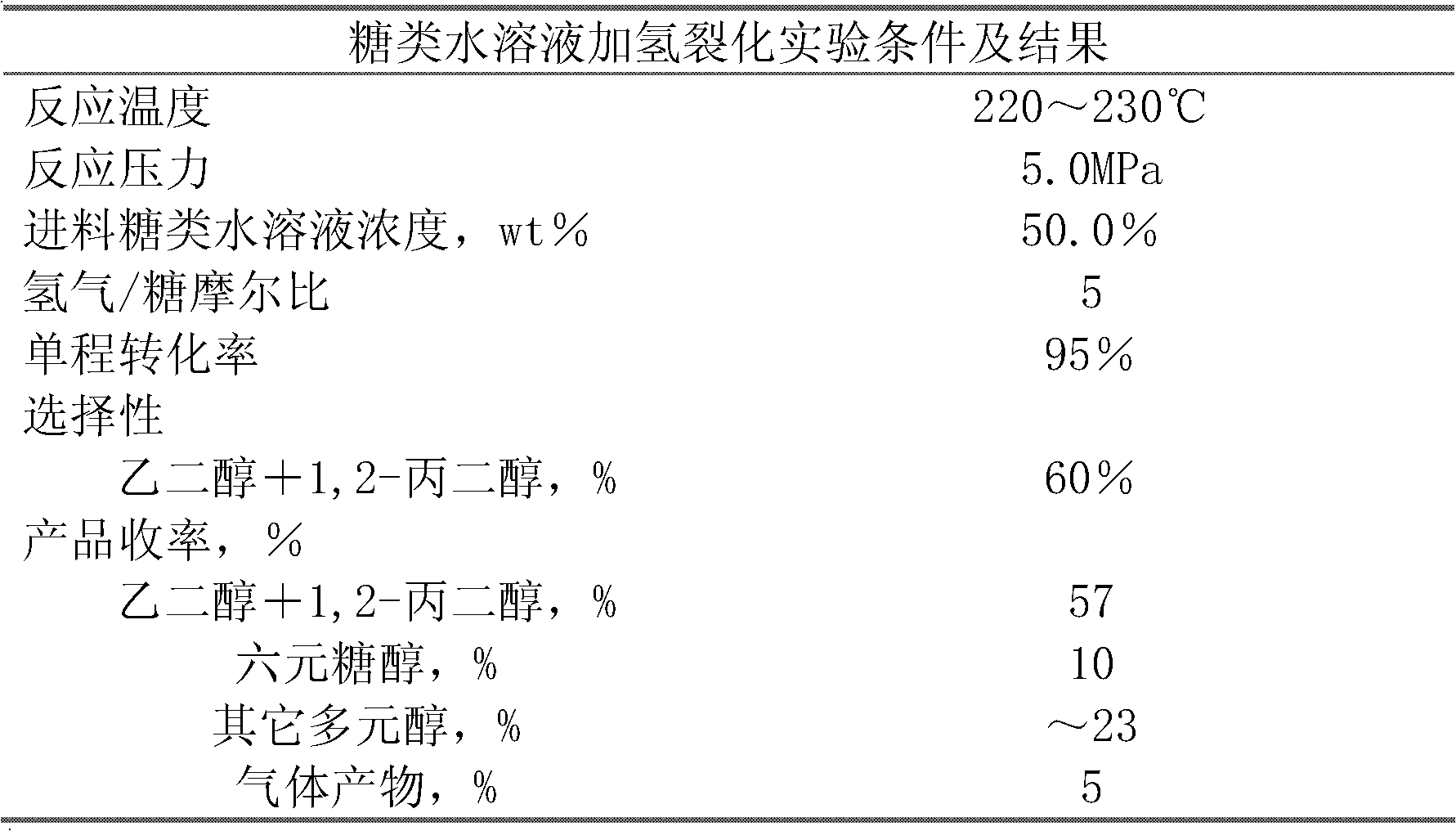
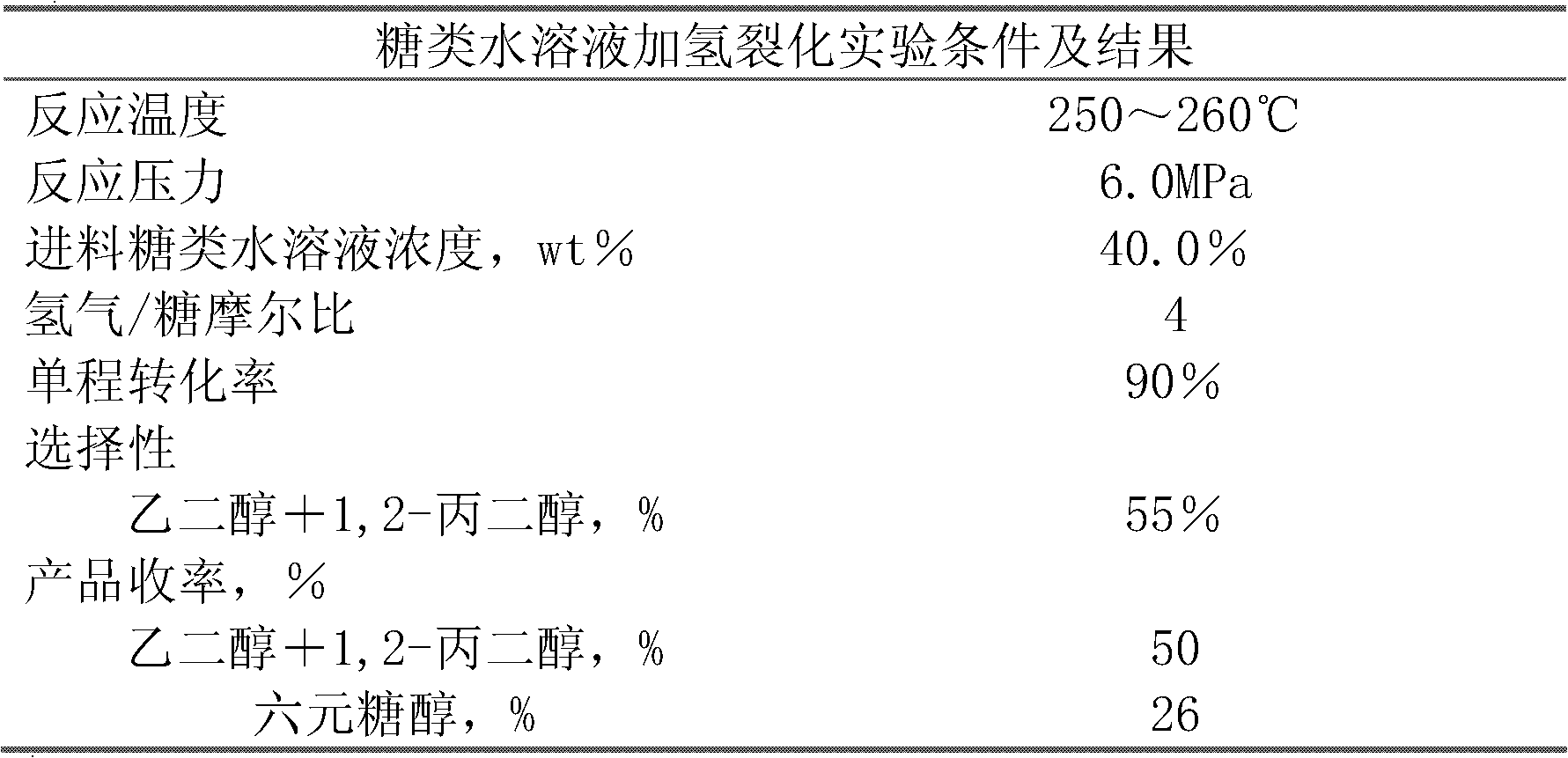
Method for producing ethylene glycol and 1,2-propylene glycol through continuous hydrocrackin of sugars
ActiveCN102643165ARealize continuous inputReduce consumptionOrganic compound preparationPreparation by OH group eliminationGas phaseOligosaccharide
The invention relates to a continuous reaction process flow for producing ethylene glycol and 1, 2-propylene glycol through continuous hydrocrackin of sugars and provides a method for producing the ethylene glycol and the 1,2-propylene glycol through continuous hydrocrackin of sugars. In the process flow, sugars (comprising one or more than two of sugar, glucose, fructose, xylose, soluble xylo-oligosaccharide and starch) are hydrocracked in the presence of a catalyst in a reactor and the hydrocracked product enters a separating system. Hydrogen gas in a gas phase is recycled by separating and recovering; a liquid phase product partially reflows to the reactor and other liquid phase products are refined and separated to form the ethylene glycol, the propylene glycol and other polyhydric alcohols; and concentrated soluble catalyst components in the refined and separated residual component liquid phase are partially returned to the reactor.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
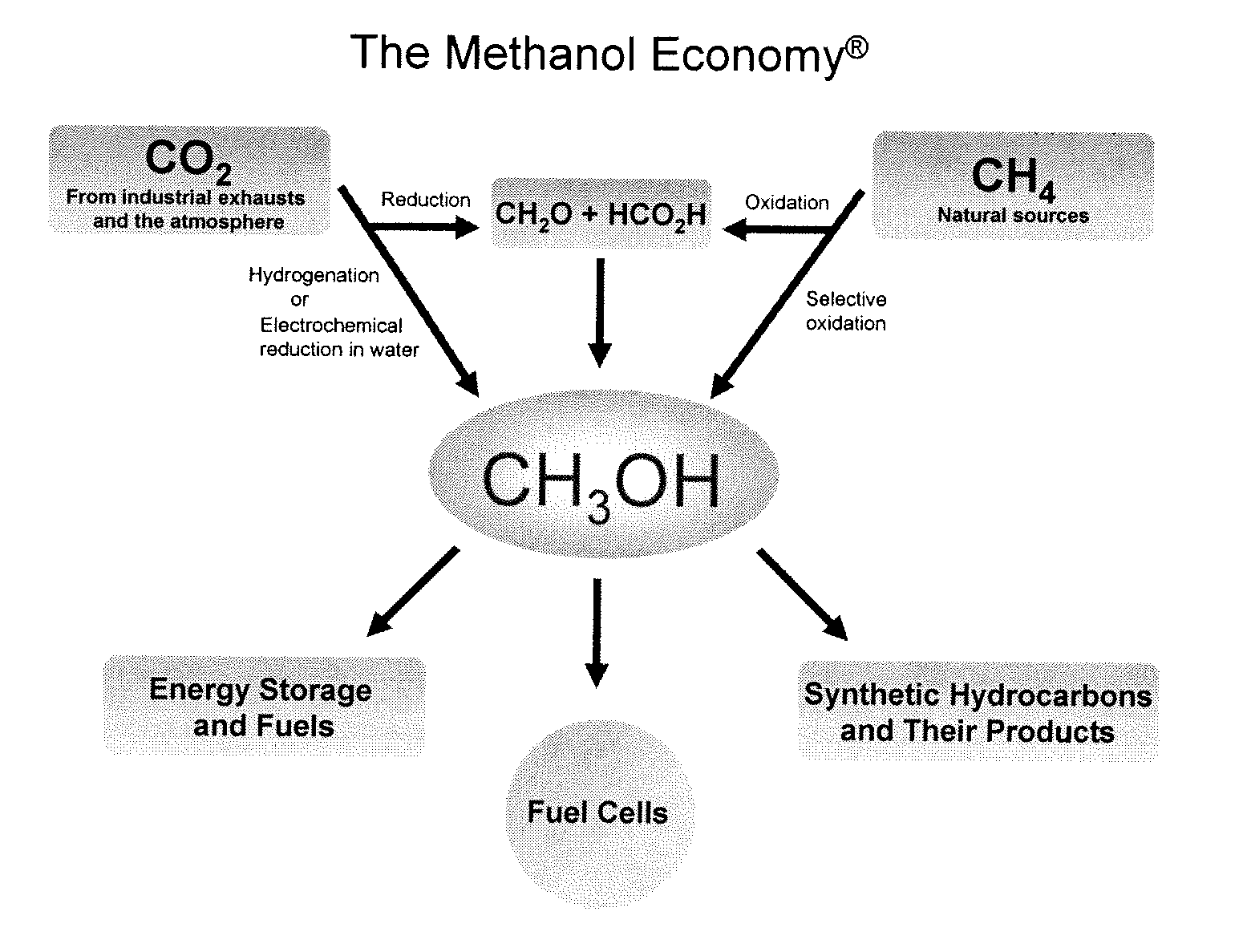
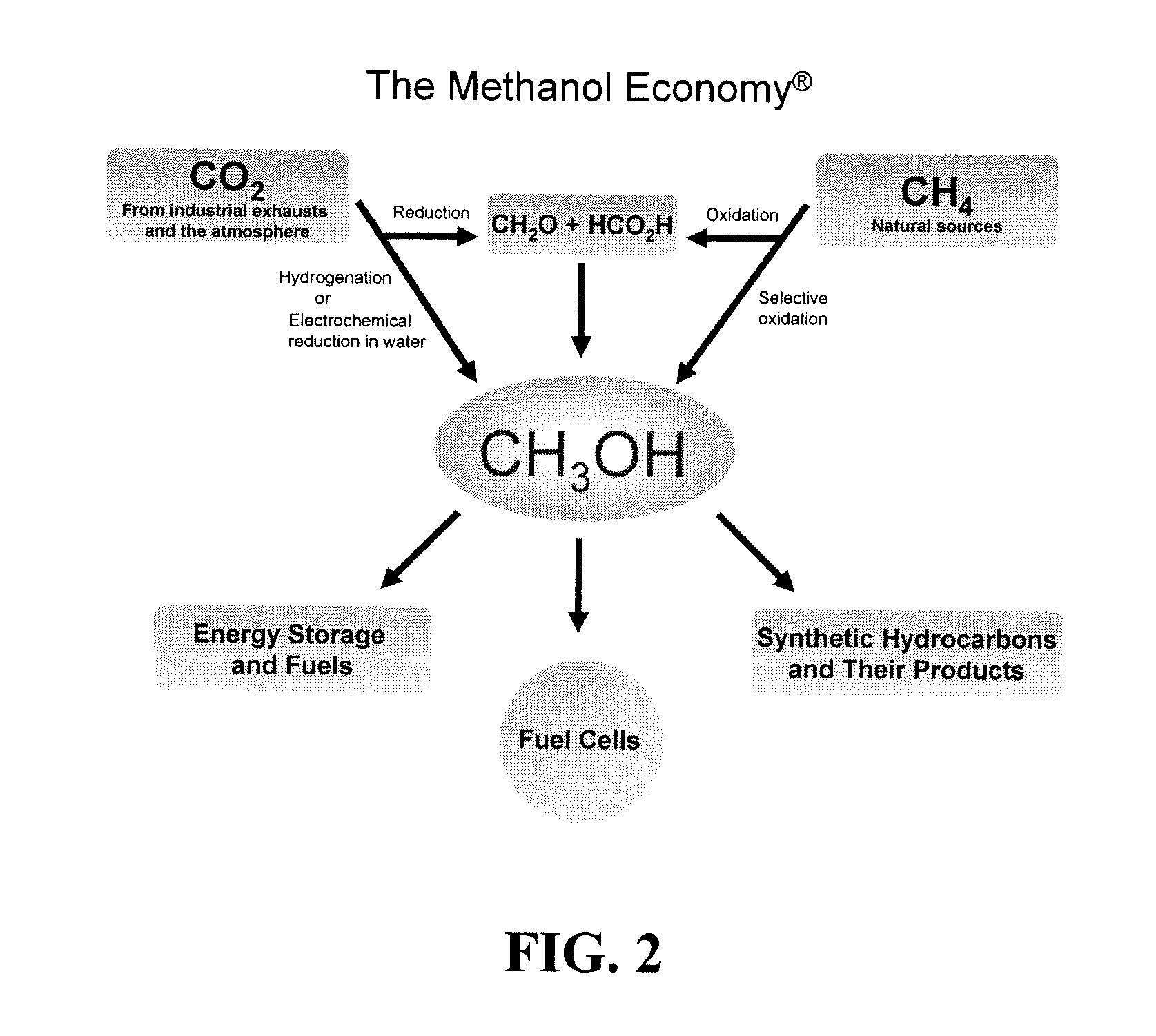
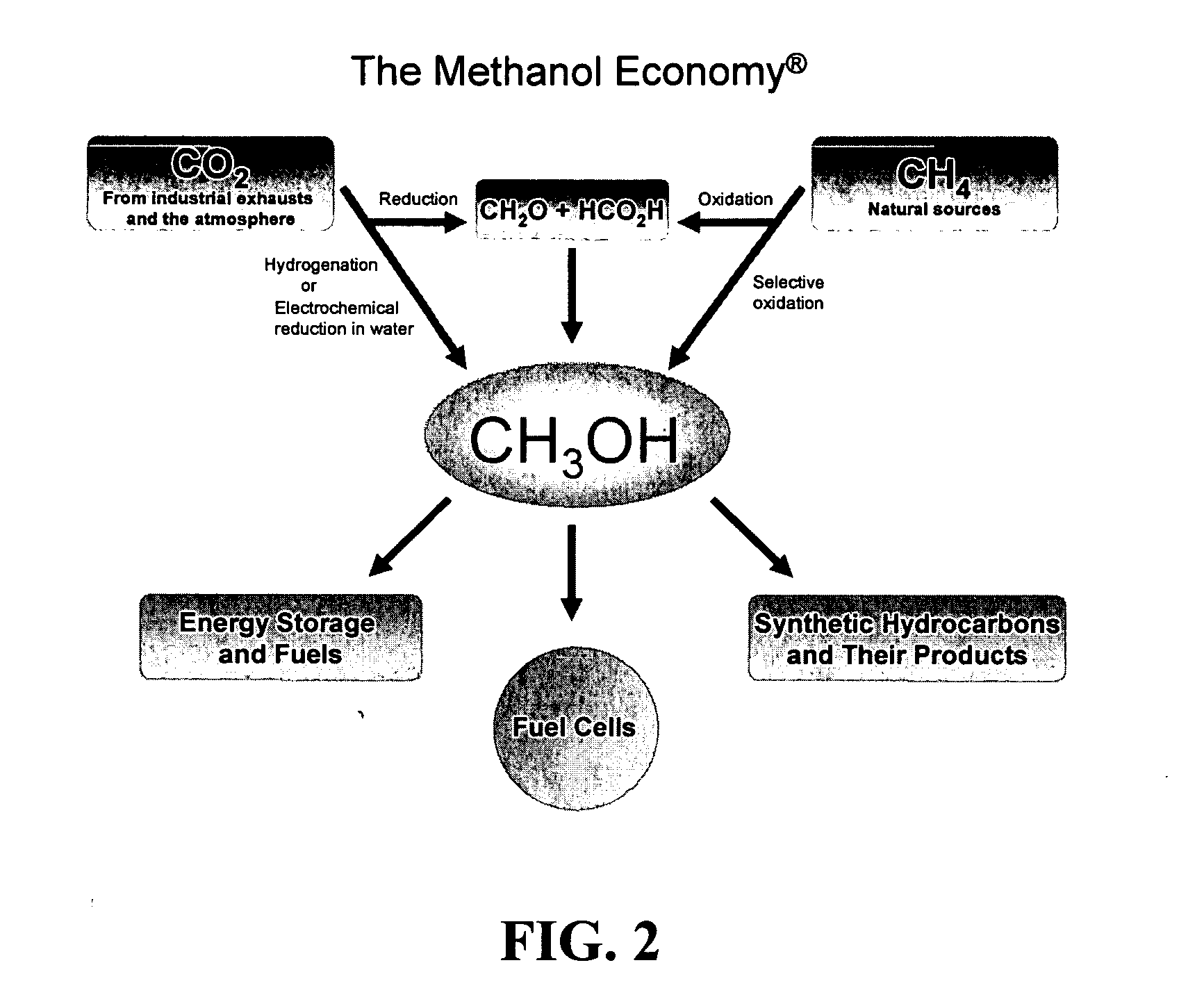
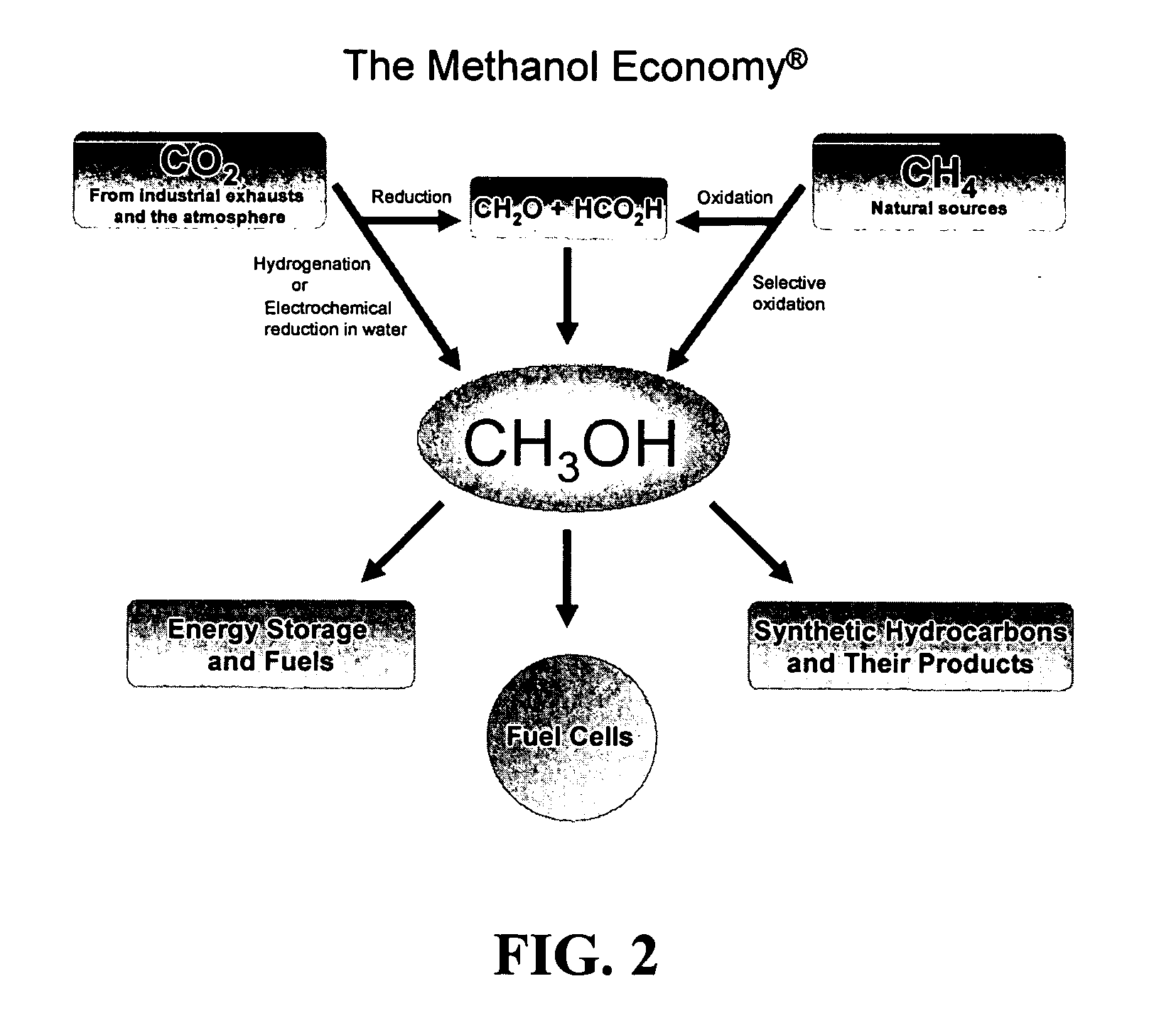
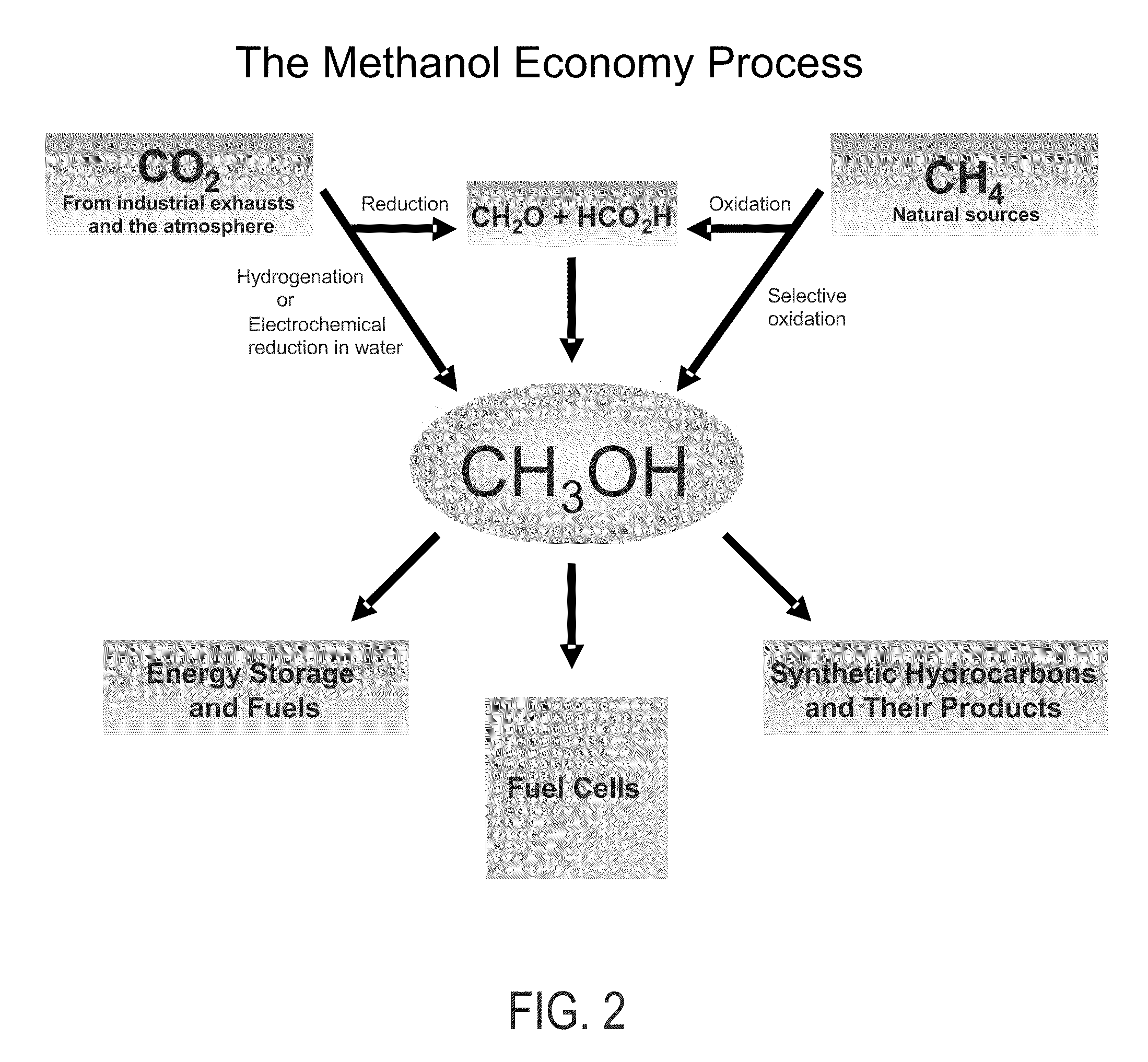
Efficient and selective chemical recycling of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS20070254969A1Avoid emissionsElectrolysis componentsOxygen compounds purification/separationElectrochemistryDimethyl ether
An efficient and environmentally beneficial method of recycling and producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by chemical or electrochemical reduction secondary treatment to produce essentially methanol, dimethyl ether and derived products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Efficient and selective conversion of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS20060235091A1Minimize or eliminate the disadvantages or dangers inherentElectrolysis componentsCarbon compoundsHydrogenFlue gas
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by electrochemical reduction produces formic acid acid and some formaldehyde and methanol mixtures. The formic acid can be used as source of carbon as well as hydrogen to produce methanol, dimethyl ether and other products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method for producing methanol, dimethyl ether, derived synthetic hydrocarbons and their products from carbon dioxide and water (moisture) of the air as sole source material
InactiveUS7378561B2High activityImprove surface activityOrganic compound preparationOrganic chemistry methodsNano structuringWater source
A method for producing methanol and dimethyl ether using the air as the sole source of materials is disclosed. The invention relates to a method for separating the water (i.e., the moisture in the air) and carbon dioxide content of atmospheric air for their use in the subsequent production of methanol, dimethyl ether and derived synthetic hydrocarbons as products. The method includes the conversion of carbon dioxide and water under conditions sufficient to produce methanol and / or dimethyl ether. Methanol and / or dimethyl ether can be used as fuel or fuel additives or further converted to synthetic hydrocarbons and their products. Carbon dioxide is captured on a suitable absorbent, preferentially polyethyleneimine supported on nano-structured fumed silica. The process can also involve hydrogenation with hydrogen produced by electrolysis of water obtained from the air or from any other water source. Methanol can be dehydrated to produce dimethyl ether or further processed to produce synthetic hydrocarbons, polymers, and products derived from them by other known methods.
Owner:UNIV OF SOUTHERN CALIFORNIA
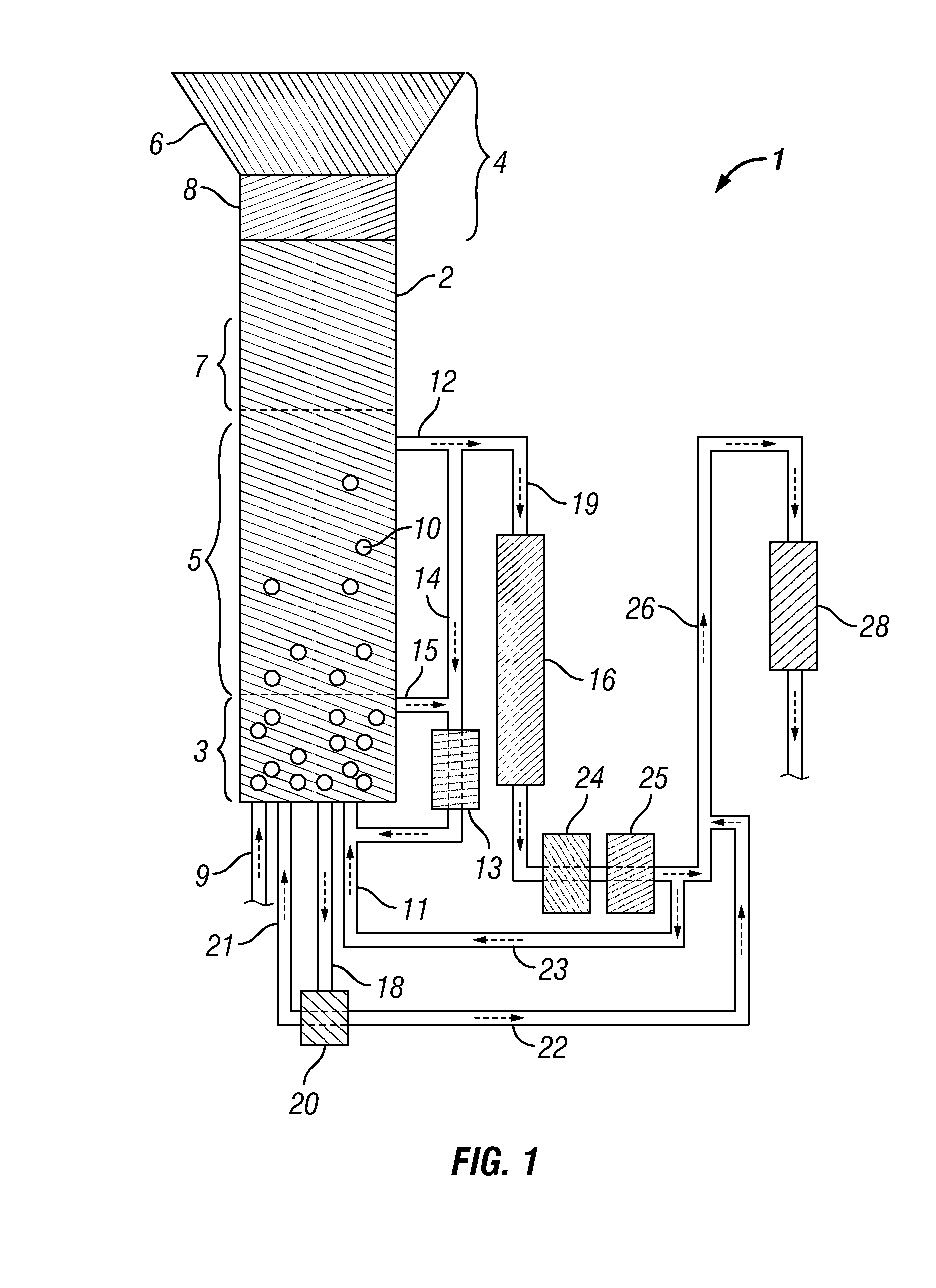
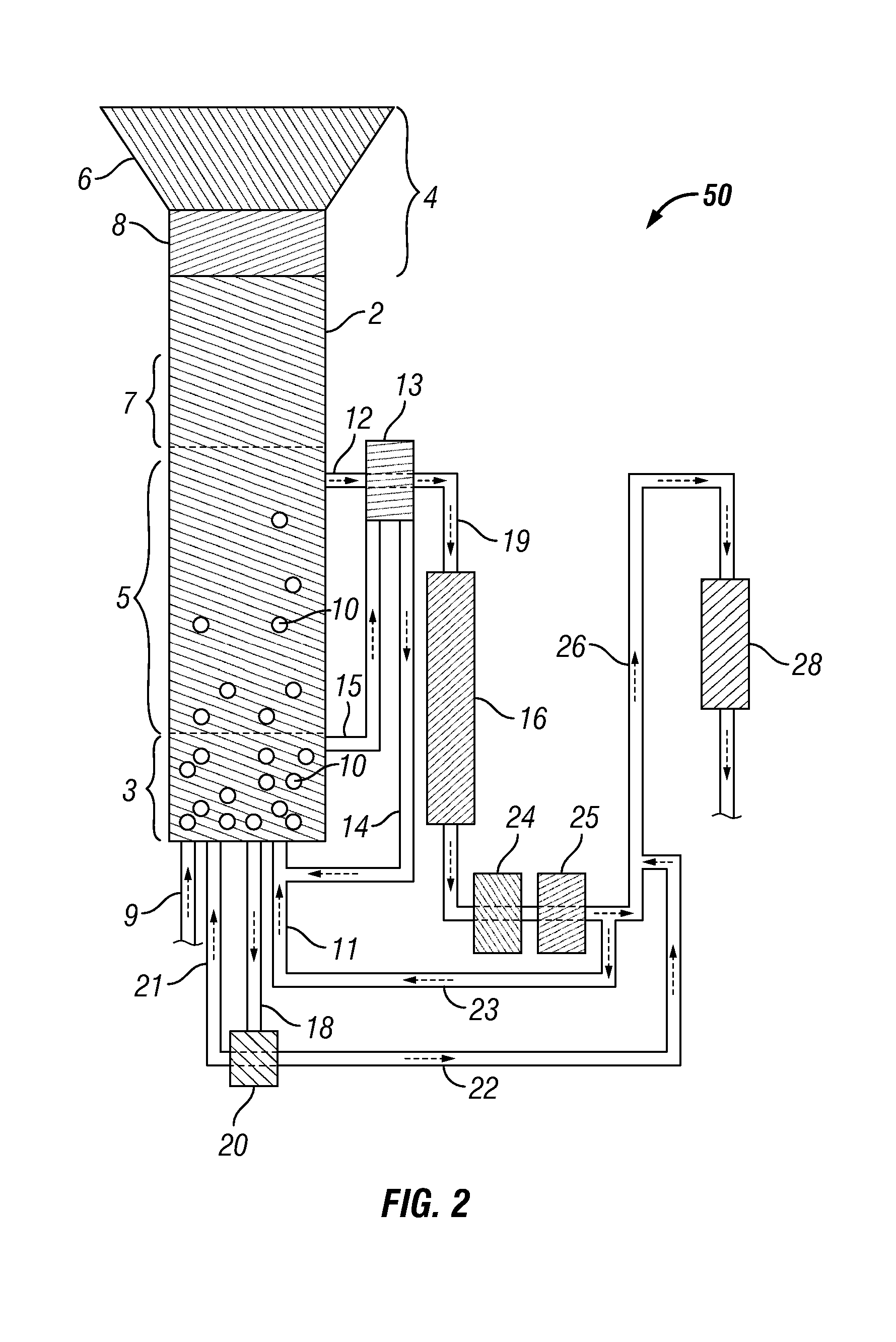
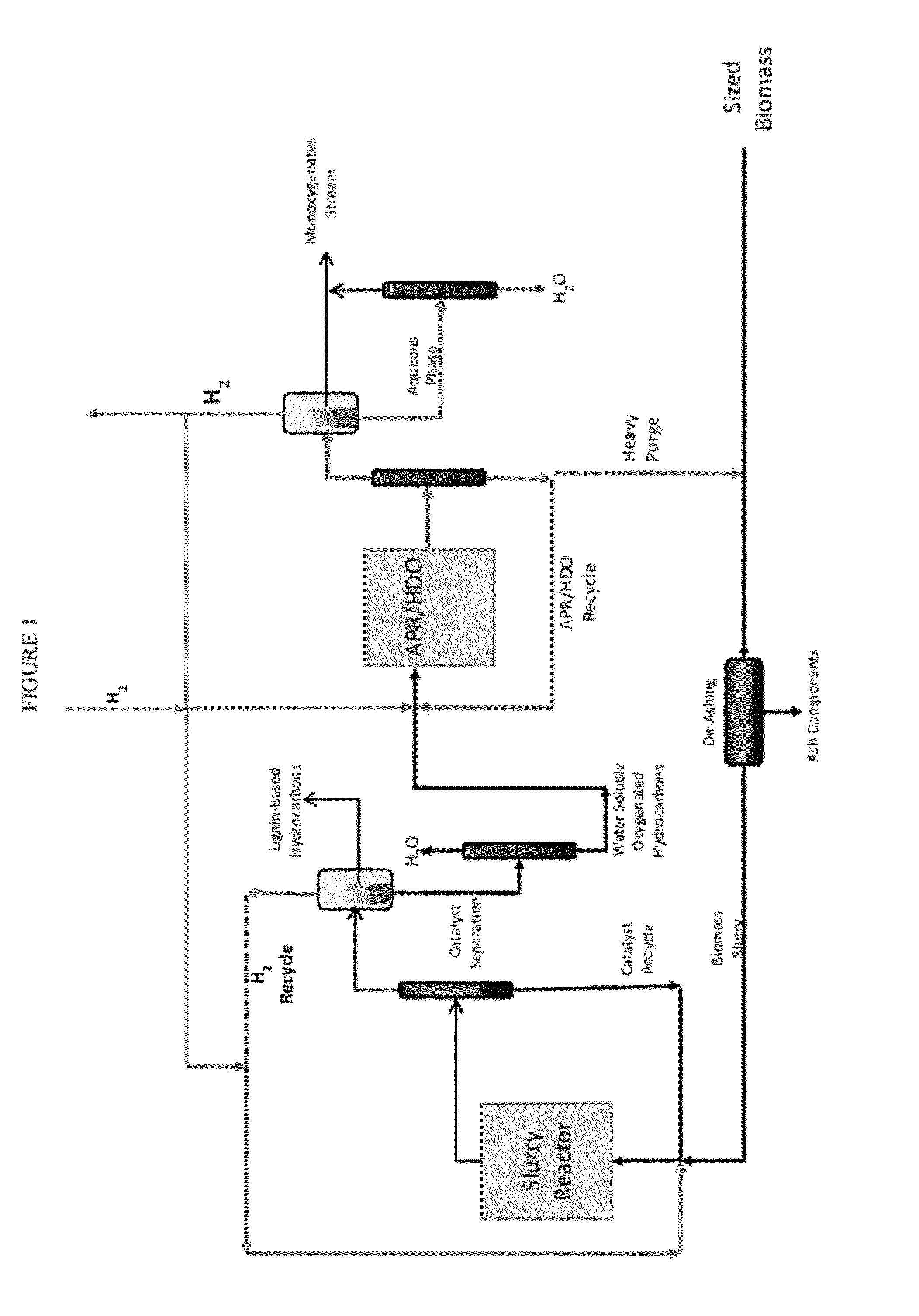
Methods for hydrothermal digestion of cellulosic biomass solids in the presence of a slurry catalyst and a digestible filter aid
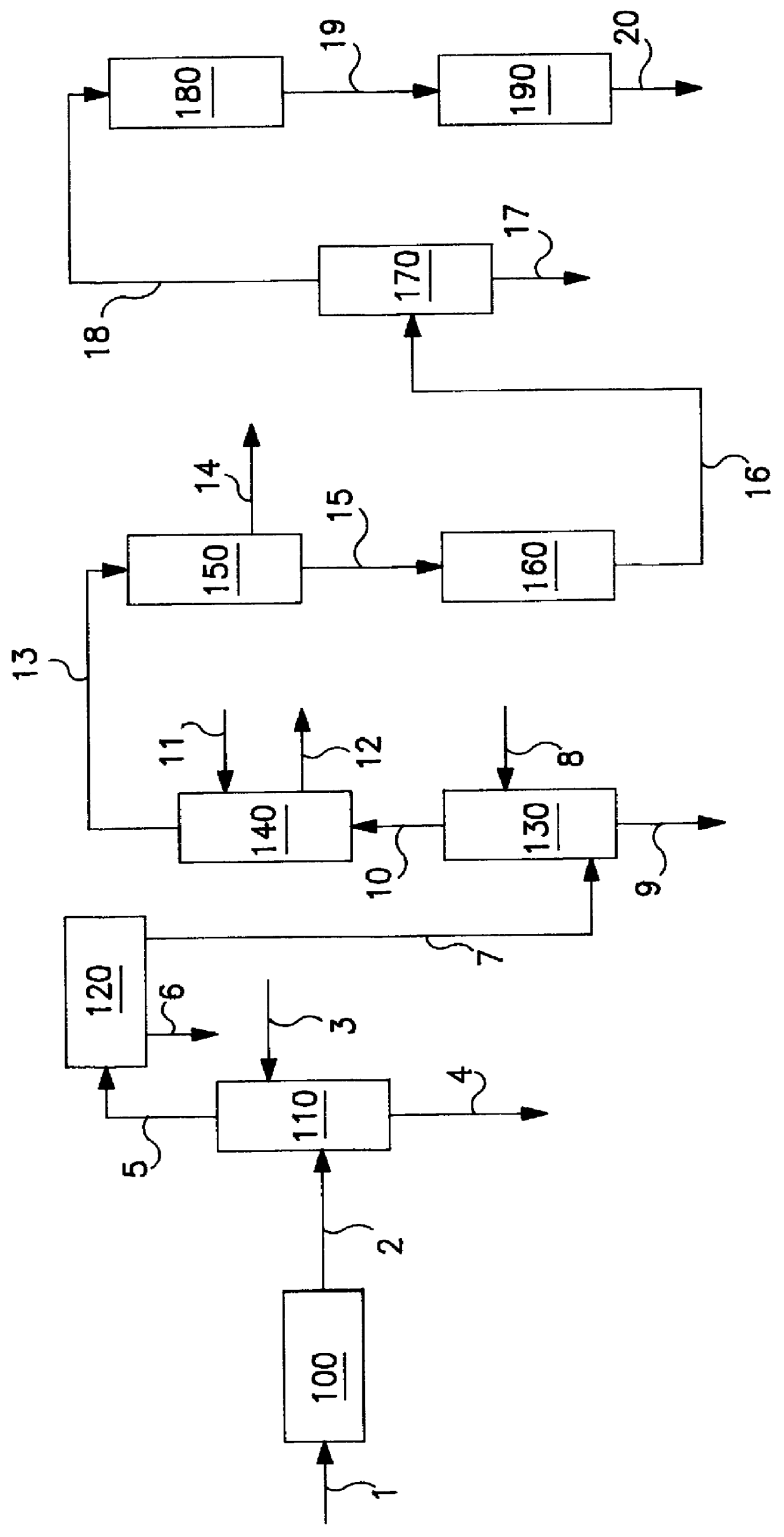
Digesting cellulosic biomass in the presence of a slurry catalyst may reduce degradation product formation, but catalyst distribution and retention can be problematic. Digestion methods can comprise: providing cellulosic biomass solids and a slurry catalyst capable of activating molecular hydrogen in a digestion unit; providing a digestible filter aid in the digestion unit; distributing the slurry catalyst within the cellulosic biomass solids using fluid flow; retaining at least a portion of the slurry catalyst in a fixed location using the digestible filter aid; heating the cellulosic biomass solids in the presence of the slurry catalyst, a digestion solvent, and molecular hydrogen, thereby forming a liquor phase comprising soluble carbohydrates; and performing a catalytic reduction reaction on the soluble carbohydrates within the digestion unit, thereby at least partially forming a reaction product comprising a triol, a diol, a monohydric alcohol, or any combination thereof in the digestion unit.
Owner:SHELL USA INC
Hydrothermal hydrocatalytic treatment of biomass
A method of hydrothermal hydrocatalytic treating biomass is provided. Lignocellulosic biomass is treated with a digestive solvent to form a pretreated biomass containing soluble carbohydrates. The pretreated biomass is contacted, with hydrogen at a temperature in the range of 150° C. to less than 300° C. in the presence of a pH buffering agent and a supported hydrogenolysis catalyst containing (a) sulfur, (b) Mo or W, and (c) Co, Ni or mixture thereof, incorporated into a suitable support, to form a plurality of oxygenated hydrocarbons.
Owner:SHELL OIL CO
Catalytic hydrogenation of 3-hydroxypropanal to 1,3-propanediol
InactiveUS6342646B1Improves performance and lifetimeImprove hydrogenation activityOrganic compound preparationOxygen compounds preparation by reductionAsymmetric hydrogenation1,3-Propanediol
The present invention provides an improved process for the hydrogenation of 3-hydroxypropanal to 1,3-propanediol which comprises purifying an aqueous solution of 3-hydroxypropanal by contacting said aqueous solution with a purifying agent prior to hydrogenation.
Owner:EI DU PONT DE NEMOURS & CO
Specific Branched Aldehydes, Alcohols, Surfactants, and Consumer Products Based Thereon
ActiveUS20100137649A1Promote degradationImprove solubilityOxygen-containing compound preparationPreparation by oxo-reaction and reductionHydrogenAlcohol
A process for preparing a detergent alcohol mixture comprising the steps of providing one or more poly-branched poly-olefins, wherein the poly-branched poly-olefins must contain one non-branched terminal olefin and one or more additional branched olefins in the molecule; hydroformylating said poly-branched poly-olefins to produce a poly-branched olefin containing aldehyde product with one or more olefins or mixture thereof; reducing the aldehyde product of step (b) in the presence of hydrogen and a hydrogenation catalyst to form a poly-branched detergent alcohol mixture; and removing said poly-branched alcohol mixture from said catalyst and branched aldehydes, alcohols and surfactants produced from the products of this process.
Owner:PROCTER & GAMBLE CO
Selective oxidative conversion of methane to methanol, dimethyl ether and derived products
ActiveUS20060235088A1Minimize or eliminate the disadvantages or dangers inherentPreparation by oxidation reactionsElectrolysis componentsFormate EstersDimethyl ether
The present invention relates to a method of producing methanol from a methane source by oxidizing methane under conditions sufficient to a mixture of methanol and formaldehyde while minimizing the formation of formic acid and carbon dioxide. The oxidation step is followed by treatment step in which formaldehyde is converted into methanol and formic acid which itself can further be converted into methanol via catalytic hydrogenation of intermediately formed methyl formate.
Owner:UNIV OF SOUTHERN CALIFORNIA
Hydrogenation of biochemical derived 1,3 -propanediol
InactiveUS7098368B2Organic compound preparationOxygen compounds purification/separationDownstream processingHydrogenation process
The invention discloses a hydrogenation process for removing impurities and controlling acid for use in downstream processing of biochemically-derived 1,3-propanediol. Preferably, the biochemically-derived 1,3-propanediol, before the contacting, has an initial color and, after the contracting, has a color that is lower than the initial color.
Owner:EI DU PONT DE NEMOURS & CO
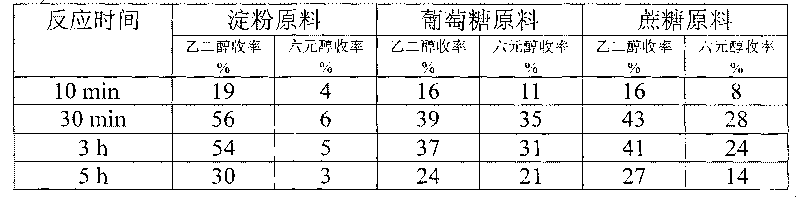
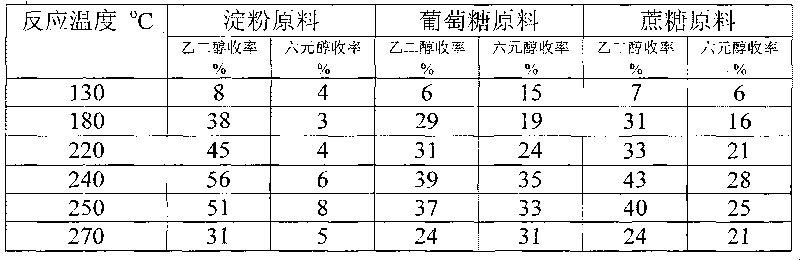
Method for preparing ethanediol from polyhydroxy compounds
ActiveCN101735014ARaw material resources are renewableMeet the requirements of sustainable developmentOrganic compound preparationCatalyst activation/preparationHydrogen pressureCobalt
The invention provides a method for preparing ethanediol from polyhydroxy compounds comprising starch, hemicellulose, cane sugar, glucose, fructose and fructosan. The method comprises the following steps of: taking the polyhydroxy compounds as reaction raw materials, and taking metals, carbides, nitrides, and phosphides of transition metals in families VIII, IX and X, such as ferrum, cobalt, nickel, ruthenium, rhodium, palladium, iridium, platinum, molybdenum and tungsten as catalytic active components to form a polymetallic catalyst; and performing further catalytic conversion under a hydrothermal condition that the temperature is 120 to 300 DEG C and the hydrogen pressure is 1 to 13 MPa to prepare the ethanediol from the polyhydroxy compounds with high efficiency, high selectivity and high yield. The method for preparing the ethanediol from the polyhydroxy compounds has the outstanding advantages of renewable raw materials, environment-friendly reaction process, and atom economical efficiency. Simultaneously, compared with other techniques taking biomasses as the raw materials, the method has the advantages of simple process and high yield.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing ethylene glycol from cellulose
ActiveCN101723802ALow costWide variety of sourcesOrganic compound preparationCatalyst activation/preparationHydrogen pressurePolyethylene glycol
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Methods and systems for processing lignin during hydrothermal digestion of cellulosic biomass solids
InactiveUS20140121419A1Easy to adaptOxygen-containing compound preparationOrganic compound preparationCelluloseAlcohol
Digestion of cellulosic biomass solids may be complicated by release of lignin therefrom. Methods for digesting cellulosic biomass solids may comprise: providing cellulosic biomass solids in a digestion solvent; at least partially converting the cellulosic biomass solids into a phenolics liquid phase comprising lignin, an aqueous phase comprising an alcoholic component derived from the cellulosic biomass solids, and an optional light organics phase; combining at least the phenolics liquid phase and the aqueous phase with one another, thereby forming a combined phase; and separating at least a portion of the alcoholic component from at least a portion of the combined phase.
Owner:SHELL OIL CO
Methods for production and processing of a glycol reaction product obtained from hydrothermal digestion of cellulosic biomass solids
InactiveUS20140121420A1Easy to adaptOxygen-containing compound preparationOrganic compound preparationCellulosePtru catalyst
Hydrothermal digestion of cellulosic biomass solids may be conducted such that a glycol reaction product is formed for subsequent processing. Processing of a glycol reaction product may include a drying operation conducted prior to condensation of the glycol reaction product into higher molecular weight compounds. Methods for digesting cellulosic biomass solids to form a glycol reaction product can comprise: providing cellulosic biomass solids and a slurry catalyst in a hydrothermal digestion unit, the slurry catalyst being capable of activating molecular hydrogen; heating the cellulosic biomass solids in the hydrothermal digestion unit in the presence of the slurry catalyst, a digestion solvent, and molecular hydrogen, thereby forming a liquor phase comprising soluble carbohydrates; and performing a first catalytic reduction reaction on the soluble carbohydrates within the hydrothermal digestion unit, thereby at least partially converting the soluble carbohydrates into a reaction product comprising a glycol.
Owner:SHELL OIL CO
Method and Systems for Procesing Lignin During Hydrothermal Digestion of Cellulosic Biomass Solids While Producing a Monohydric Alcohol Feed
InactiveUS20140121418A1Low viscosityOrganic compound preparationOxygen compounds preparation by reductionCelluloseAlcohol
Digestion of cellulosic biomass solids may be complicated by release of lignin therefrom. Methods for digesting cellulosic biomass solids may comprise: providing cellulosic biomass solids in the presence of a digestion solvent, molecular hydrogen, and a slurry catalyst capable of activating molecular hydrogen; at least partially converting the cellulosic biomass solids into a phenolics liquid phase comprising lignin, an aqueous phase comprising a glycol derived from the cellulosic biomass solids, and an optional light organics phase; wherein at least a portion of the slurry catalyst accumulates in the phenolics liquid phase as it forms; combining the glycol with the phenolics liquid phase, thereby forming a combined phase; and heating the combined phase in the presence of molecular hydrogen; wherein heating the combined phase reduces the viscosity of the phenolics liquid phase and transforms at least a portion of the glycol into a monohydric alcohol.
Owner:SHELL OIL CO
Hydroformylation process
InactiveUS6049011AAvoid the needReduce processingPreparation by oxo-reaction and reductionPreparation by hydrogenationEthyleneHydrocarbon
PCT No. PCT / EP96 / 00163 Sec. 371 Date Oct. 15, 1997 Sec. 102(e) Date Oct. 15, 1997 PCT Filed Jan. 17, 1996 PCT Pub. No. WO96 / 22265 PCT Pub. Date Jul. 25, 1996A dilute ethylene stream, e.g., one produced by steam cracking, is oxonated to yield propanal, without the need to separate other lower hydrocarbons.
Owner:EXXON CHEM PAT INC
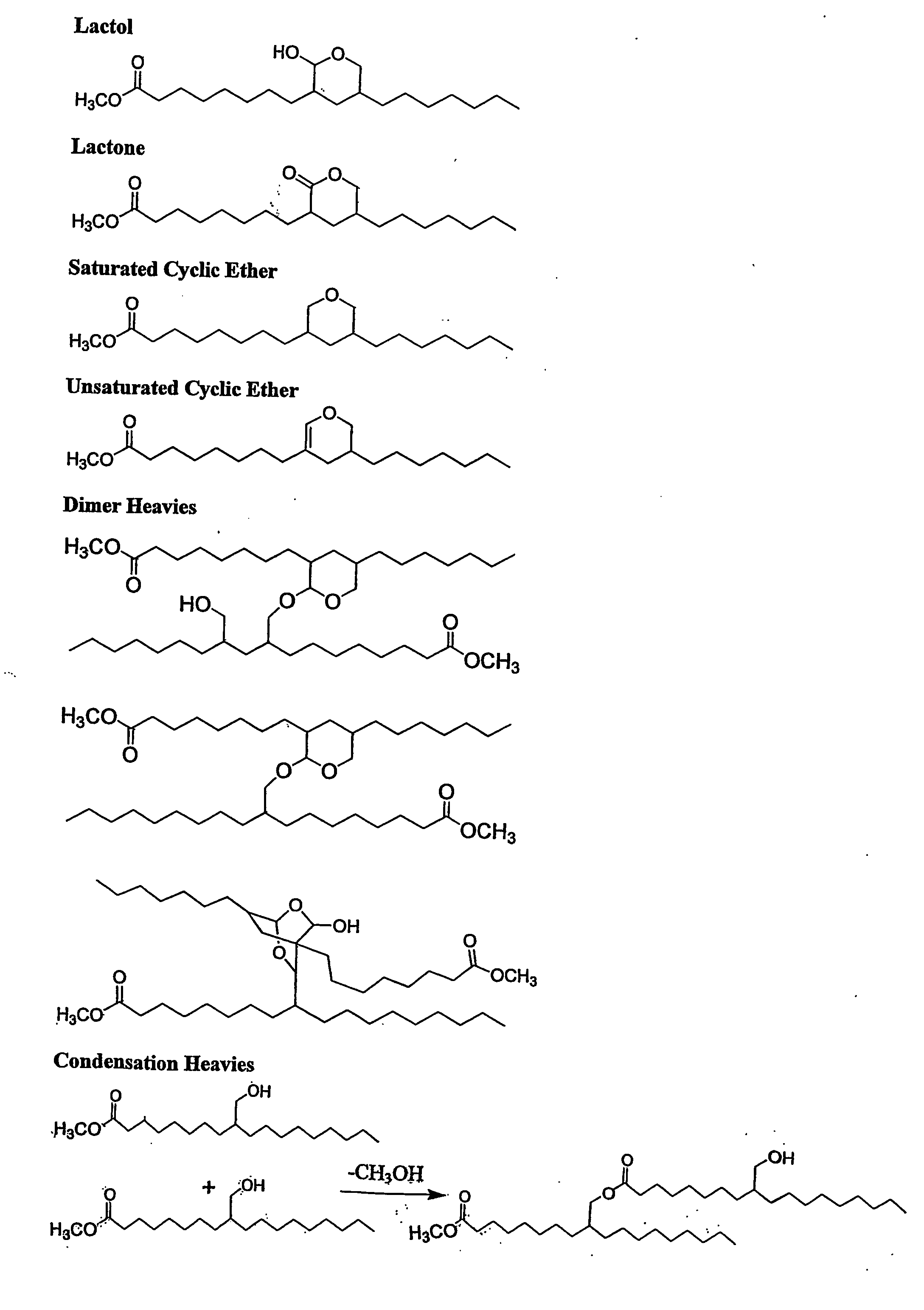
Aldehyde and alcohol compositions derived from seed oils
An aldehyde composition derived by hydroformylation of a transesterified seed oil and containing a mixture of formyl-substituted fatty acids or fatty acid esters having the following composition by weight: greater than about 10 to less than about 95 percent monoformyl, greater than about 1 to less than about 65 percent diformyl, and greater than about 0.1 to less than about 10 percent triformyl-substituted fatty acids or fatty acid esters, and having a diformyl to triformyl weight ratio of greater than about 5 / 1; preferably, greater than about 3 to less than about 20 percent saturates; and preferably, greater than about 1 to less than about 20 percent unsaturates. An alcohol composition derived by hydrogenation of the aforementioned aldehyde composition, containing a mixture of hydroxymethyl-substituted fatty acids or fatty acid esters having the following composition by weight: greater than about 10 to less than about 95 percent monoalcohol {mono(hydroxymethyl)}, greater than about 1 to less than about 65 percent diol {di(hydroxymethyl)}, greater than about 0.1 to less than about 10 percent triol, tri(hydroxmethyl)-substituted fatty acids or fatty acid esters; preferably greater than about 3 to less than about 35 percent saturates; and preferably, less than about 10 percent unsaturates. The alcohol composition can be converted into an oligomeric polyol for use in the manufacture of polyurethane slab stock flexible foams.
Owner:DOW GLOBAL TECH LLC
Efficient and selective conversion of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS7605293B2Minimize or eliminate the disadvantages or dangers inherentElectrolysis componentsCarbon compoundsHydrogenFlue gas
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by electrochemical reduction produces formic acid acid and some formaldehyde and methanol mixtures. The formic acid can be used as source of carbon as well as hydrogen to produce methanol, dimethyl ether and other products.
Owner:UNIV OF SOUTHERN CALIFORNIA
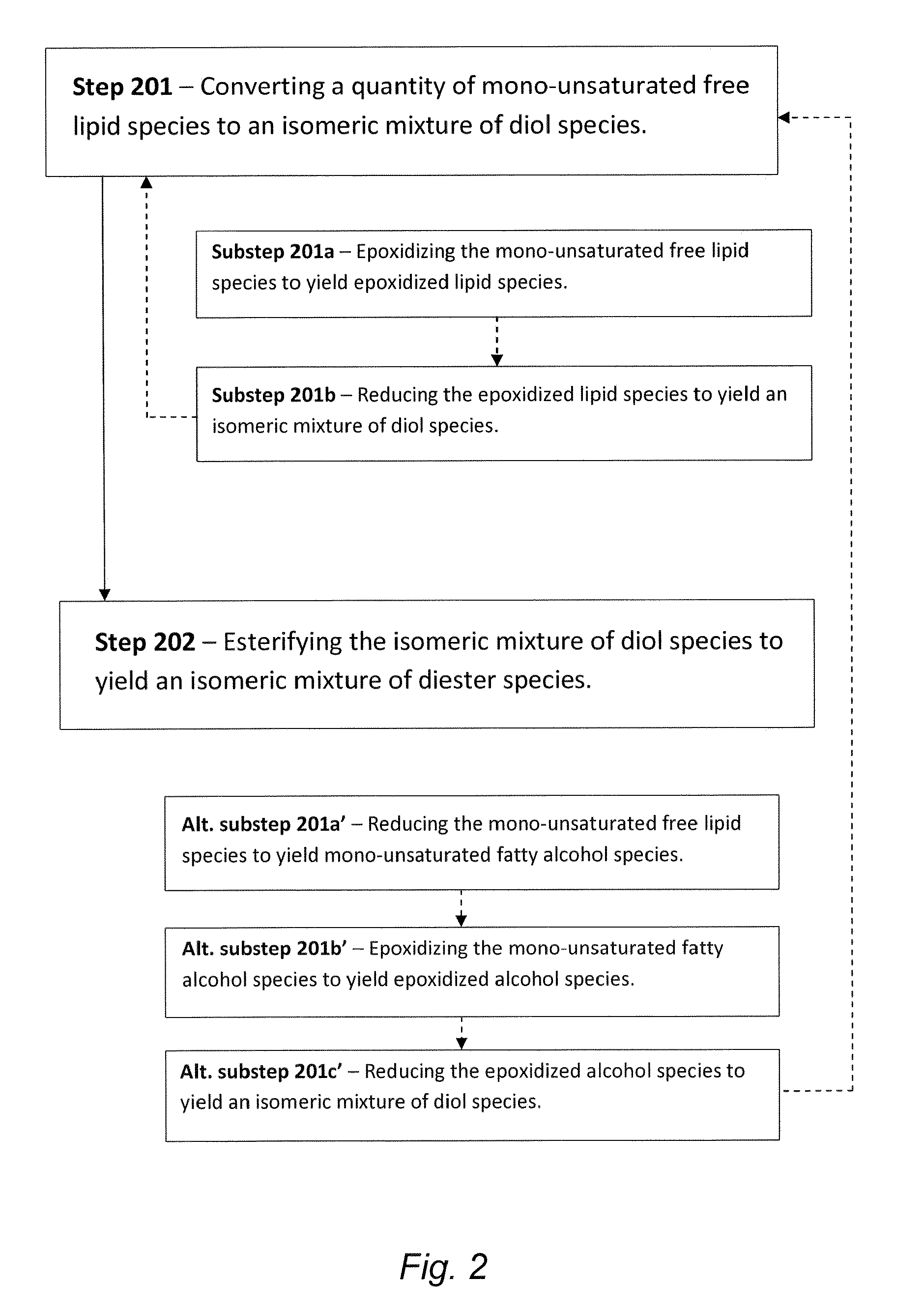
Synthesis of biolubricant esters from unsaturated fatty acid derivatives
The present invention is generally directed to diester-based lubricant compositions comprising one or more isomeric mixtures of diester species. The present invention is also directed to methods of making these and other similar lubricant compositions. In some embodiments, the methods for making such diester-based lubricants utilize a biomass precursor material from which mono-unsaturated free lipid species can be provided or otherwise generated, wherein such mono-unsaturated free lipid species arc converted to isomeric diol species en route to the synthesis of diester species for use as / in the diester-based lubricant compositions.
Owner:CHEVROU USA INC
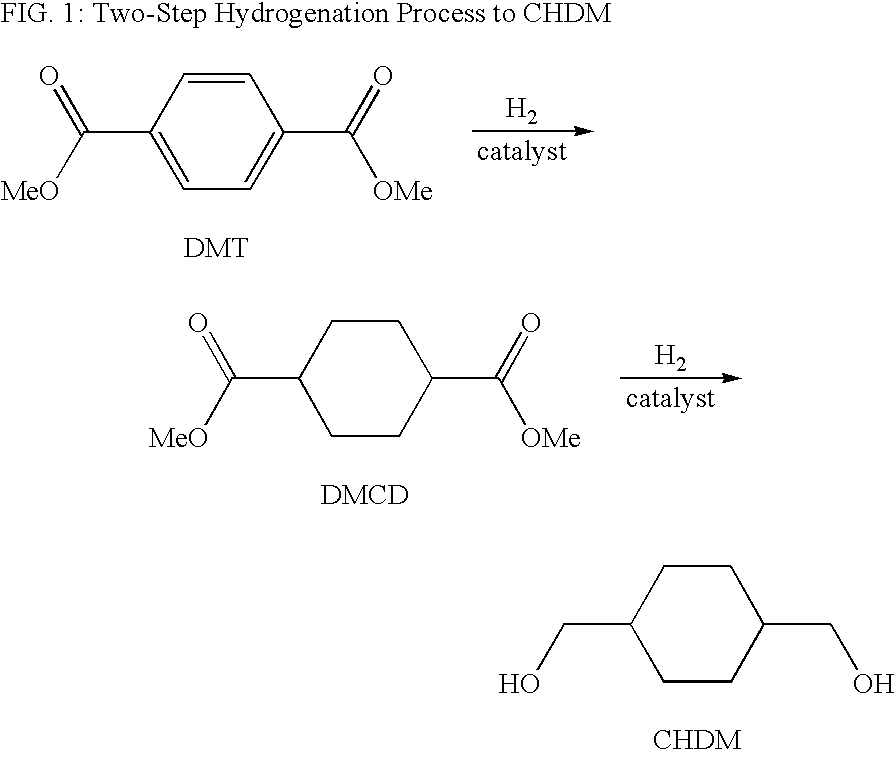
Process for a cyclohexanedimethanol using raney metal catalysts
InactiveUS6919489B1High trans contentOrganic compound preparationOxygen compounds preparation by reductionRheniumCyclohexanedimethanol
Disclosed is a process for a cyclohexanedimethanol by hydrogenation of a cyclohexane-dicarboxylate ester in the presence of a Raney metal catalyst doped with rhenium. The process is useful for the reparation of 1,4-cyclohexanedimethanol from dialkyl esters of 1,4-cyclohexanedicarboxylate or dialkyl terephthalates. When Raney nickel is used as the catalyst, the process produces CHDM having a high trans content.
Owner:EASTMAN CHEM CO
Method for producing ethylene glycol from polyhydroxy compound
ActiveUS20110046419A1High yieldHigh selectivityOxygen-containing compound preparationOrganic compound preparationIridiumHydrogen pressure
A method for producing ethylene glycol, including (a) adding a polyhydroxy compound and water to a sealed high-pressure reactor, (b) removing air and introducing hydrogen, and (c) allowing the polyhydroxy compound to react in the presence of a catalyst while stiffing. The catalyst includes a first active ingredient and a second active ingredient. The first active ingredient includes a transition metal of Group 8, 9, or 10 selected from iron, cobalt, nickel, ruthenium, rhodium, palladium, iridium, and platinum, and / or a mixture thereof. The second active ingredient includes a metallic state of molybdenum and / or tungsten, or a carbide, nitride, or phosphide thereof. The method is carried out at a hydrogen pressure of 1-12 MPa, at a temperature of 120-300° C. for not less than 5 min in a one-step catalytic reaction. The efficiency, selectivity, and the yield of ethylene glycol are high. The preparation process is simple and the materials used are renewable.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of preparing ethylene glycol from cellulose
ActiveUS7960594B2High yieldHigh selectivityOxygen-containing compound preparationOrganic compound preparationCelluloseIridium
A method for preparing ethylene glycol from cellulose uses the cellulose as the feed for the reaction. The cellulose conversion is performed over catalysts which are composed of the metallic state, carbides, nitrides, or phosiphides of molybdenum or tungsten, and metallic cobalt, nickel, ruthenium, rhodium, palladium, iridium, and platinum of the group 8, 9, or 10 transition metals. The catalytic conversion of cellulose is conducted at 120 to 300° C. and hydrogen pressure 1 to 12 MPa under the hydrothermal conditions to achieve the high efficiency, high selectivity, and high yield of ethylene glycol. Compared to the existing method of preparing ethylene glycol from ethylene, the method, using the renewable raw material for the reaction, is friendly to the environment, and has high atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Methods for preparing ethylene glycol from polyhydroxy compounds
ActiveUS20120172633A1High yieldHigh selectivityOxygen-containing compound preparationOrganic compound preparationHydrogen pressureHeteropoly acid
This invention provides methods for producing ethylene glycol from polyhydroxy compounds such as cellulose, starch, hemicellulose, glucose, sucrose, fructose, fructan, xylose and soluble xylooligosaccharides. The methods uses polyhydroxy compounds as the reactant, a composite catalyst having active components comprising one or more transition metals of Groups 8, 9, or 10, including iron, cobalt, nickel, ruthenium, rhodium, palladium, iridium, and platinum, as well as tungsten oxide, tungsten sulfide, tungsten hydroxide, tungsten chloride, tungsten bronze oxide, tungsten acid, tungstate, metatungstate acid, metatungstate, paratungstate acid, paratungstate, peroxotungstic acid, pertungstate, heteropoly acid containing tungsten. Reacting at a temperature of 120-300° C. and a hydrogen pressure of 1-13 MPa under hydrothermal conditions to accomplish one-step catalytic conversion. It realizes efficient, highly selective, high yield preparation of ethylene glycol and propylene glycol from polyhydroxy compounds. The advantage of processes disclosed in this invention include renewable raw material and high atom economy. At the same time, compared with other technologies that converts biomass raw materials into polyols, methods disclosed herein enjoy advantages including simple reaction process, high yield of targeted products, as well as easy preparation and low cost for the catalysts.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Tungsten carbide catalysts, their preparation and application in synthesis of ethylene glycol from cellulose
ActiveUS20100255983A1Improve efficiencyHigh selectivityOxygen-containing compound preparationOrganic compound preparationHydrogen pressureCobalt
Tungsten carbide catalysts are used in preparation of ethylene glycol by hydrogenating degradation of cellulose. The catalyst includes tungsten carbide as main catalytic active component, added with small amount of one or more transition metals such as nickel, cobalt, iron, ruthenium, rhodium, palladium, osmium, iridium, platinum, and copper as the second metal, supported on one or more porous complex supports such as active carbon, alumina, silica, titanium dioxide, silicon carbide, zirconium oxide, for conversion of cellulose to ethylene glycol. The catalyst realizes high efficiency, high selectivity, and high yield in the conversion of cellulose to ethylene glycol at the temperature of 120-300° C., hydrogen pressure of 1-10 MPa, and hydrothermal conditions. Compared to the existing industrial synthetic method of ethylene glycol using ethylene as feedstock, the invention has the advantages of using renewable raw material resources, environment friendly process, and excellent atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Organo-catalytic biomass deconstruction
ActiveUS20120167876A1Organic compounds purification/separation/stabilisationHydrocarbon from oxygen organic compoundsEnvironmental engineeringOrganocatalysis
Owner:VIRENT
Popular searches
Preparation by hydrogenolysis Carboxylic preparation from carbon monoxide reaction Preparation by oxygen reduction Animal repellants Bulk chemical production Other chemical processes Pharmaceutical non-active ingredients Metal/metal-oxides/metal-hydroxide catalysts Plant growth regulators Raney catalysts
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com